Abstract
Gardenia jasminoides (GJ) is a widely used herbal medicine with anti-inflammatory properties, but its effects on the ORAI1 channel, which is important in generating intracellular calcium signaling for T cell activation, remain unknown. In this study, we investigated whether 70% ethanolic GJ extract (GJEtOH) and its subsequent fractions inhibit ORAI1 and determined which constituents contributed to this effect. Whole-cell patch clamp analysis revealed that GJEtOH (64.7% ± 3.83% inhibition at 0.1 mg/ml) and all its fractions showed inhibitory effects on the ORAI1 channel. Among the GJ fractions, the hexane fraction (GJHEX, 66.8% ± 9.95% at 0.1 mg/ml) had the most potent inhibitory effects in hORAI1-hSTIM1 co-transfected HEK293T cells. Chemical constituent analysis revealed that the strong ORAI1 inhibitory effect of GJHEX was due to linoleic acid, and in other fractions, we found that genipin inhibited ORAI1. Genipin significantly inhibited IORAI1 and interleukin-2 production in CD3/CD28-stimulated Jurkat T lymphocytes by 35.9% ± 3.02% and 54.7% ± 1.32% at 30 μM, respectively. Furthermore, the same genipin concentration inhibited the proliferation of human primary CD4+ T lymphocytes stimulated with CD3/CD28 antibodies by 54.9% ± 8.22%, as evaluated by carboxyfluorescein succinimidyl ester assay. Our findings suggest that genipin may be one of the active components of GJ responsible for T cell suppression, which is partially mediated by activation of the ORAI1 channel. This study helps us understand the mechanisms of GJ in the treatment of inflammatory diseases.
Keywords: CD4 positive T lymphocytes, Gardenia, Genipin, Interleukin-2, ORAI1 protein
INTRODUCTION
Gardenia jasminoides (GJ) is an evergreen shrub belonging to the family Rubiaceae that originated in Asian countries, and is commonly found in Korea, Japan, China, and India [1]. In Asia, the fruit of GJ (Gardenia fructus [GF]) is commonly used as a traditional herbal medicine, and is registered in the pharmacopeia of South Korea, Japan, and China [2,3]. GF is used therapeutically against disorders such as inflammation, jaundice, conjunctival congestion, angina, hypertension, and pathopyretic ulcers [1,4]. GF is well known for its anti-diabetic, anti-depressive, anti-oxidative, and anti-inflammatory activities [5]. GF and its chemical constituents show broad-spectrum anti-inflammatory activity; both the innate and adaptive immune systems mediate the inflammatory response. Recent studies have shown that GF and its chemical constituents suppress allergic inflammation in atopic dermatitis (AD) and asthma. Topical application of 70% ethanolic GF extract reduces AD symptoms in Dermatophagoides farinae-exposed NC/Nga mice [3]. GF and its active chemical constituent, geniposide, effectively inhibit histamine release from mast cells, which may drive the anti-allergic effects of GF [3]. Geniposide and genipin, an aglycon of geniposide, effectively alleviate the asthmatic inflammatory response in ovalbumin (OVA)-induced asthmatic model. Additionally, geniposide significantly ameliorates airway hyperresponsiveness, which is a major characteristic of asthma [2,6]. Although the therapeutic effects of GF in patients with allergic diseases have been elucidated, the early signal transduction events mediated by GF and its chemical constituents are unclear.
Generation of intracellular calcium signaling, stimulated by T cell receptor/high-affinity IgE receptor (FcεRI), is a key process in CD4+ T cells and mast cells [7]. The binding of an allergen to both receptors, which are coupled to Gq protein, results in activation of phospholipase C beta (PLCβ) [8]. The activated PLCβ consequently hydrolyzes the phosphodiester bond that links phosphorylated inositol with acylated glycerol moiety [8]. PIP2 cleavage then generates inositol 1,4,5-trisphosphate, which is soluble and can diffuse across the membrane, and diacylglycerol, which remains in the membrane [8].
IP3 binds to the IP3 receptor, which is located in endoplasmic reticulum (ER) Ca2+ stores. This can lead to depletion of ER calcium stores, causing an influx of calcium across the plasma membrane in a cascade called store-operated Ca2+ entry (SOCE) [9]. Intracellular calcium influx via the calcium release-activated calcium channel1 (ORAI1) is an important contributor for cell activation, not only in T cells, but also other immune cell types [10]. For these reasons, we investigated the effects of GF and its active chemical constituents on the calcium ion channel ORAI1. We also evaluated the possible correlations between inhibition of cytokine production in stimulated human CD4+ T cells and the rate of ORAI1 inhibition.
METHODS
Cell culture
Human embryonic kidney 293 T (HEK293T) and Jurkat T cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). HEK293T cells were cultured at 37°C and 10% CO2 in Dulbecco's modified Eagle's medium (DMEM; Welgene, Gyeongsan, Korea) supplemented with 10% fetal bovine serum (FBS; Welgene) and 1% penicillin/streptomycin (P/S; GE Healthcare, Chicago, IL, USA). Jurkat T cells were cultured in RPMI1640 (Gibco; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS and 1% P/S at 37°C in a 5% CO2 incubator.
Transient transfection
To measure the ORAI1 current, human ORAI1 (hORAI1) and STIM1 (hSTIM1) were co-transfected into HEK293T cells. Transfection was performed using Turbofect (Thermo Scientific, Waltham, MA, USA) according to the manufacturer's protocol. Green fluorescence protein (pEGFP-N1, Life Technologies) was transduced at a ratio of 10:1 to identify the transfected cells. For patch clamp assay, we combined 0.9 μg hORAI1 vector, 0.9 μg hSTIM1 vector, 0.2 μg pEGFP, and 4 μl Turbofect in serum-free DMEM (without FBS or P/S) and incubated the mixture for 15 min at room temperature (25°C). This mixture was added to the cell cultures and the cells were incubated for 24 h.
Preparation of 70% ethanolic extract of Gardenia jasminoides (GJEtOH)
Dried herb GJ was obtained from SAEROM Pharmaceutical Co. (Anseong, Korea). The dried GJ (20 g) was evenly ground, and then extracted using 70% ethanol at 65°C for 2 h. The extracted solution was filtered under reduced pressure (10–50 torr) using filter paper (Advantec, 150 mm, NO. 5B) and then concentrated under reduced pressure (at 40°C, 10–50 torr) to remove the ethanol. The concentrated extract was homogenized by lyophilization. The final yield of the GJ extract was 26.8%.
Isolation of GJ fraction
An extract was prepared using 20 g of dry GJ as described above, and 3.22 g extract was obtained, which constituted a 16.1% yield. To prepare the solvent fraction, dry GJ was dissolved in water and then mixed with n-hexane to obtain a separated n-hexane layer, which was collected as the n-hexane fraction (GJHEX). The remaining water layer was then re-mixed with ethyl acetate to obtain a separated ethyl acetate layer, which was collected as the GJ ethyl acetate fraction (GJEtOAc). The above procedure was repeated with butanol to obtain the GJ n-butyl alcohol fraction (GJBuOH). Finally, the GJ water fraction (GJH2O) was obtained from the last separated layer. The separated fraction layers were concentrated under reduced pressure (10–50 torr) to remove the solvent and then lyophilized. The obtained yields were as follows: 13.6% for GJHEX, 7.1% for GJEtOAc, 41.4% for GJBuOH, and 35.8 % for GJH2O.
Cytotoxicity assay
The cytotoxicity of the prepared extract and fractions was analyzed using CCK8 (Dojindo Laboratories, Kumamoto, Japan). Sample preparation and analysis were performed according to the protocol provided by the manufacturer. Jurkat T cells were seeded at 2 × 104 cells/well in 96-well plates. Then, GJEtOH at the concentration of 0.001 mg/ml–1 mg/ml, GJH2O at 0.001 mg/ml–0.1 mg/ml, GJBuOH at 0.001 mg/ml–0.1 mg/ml, GJEtOAc at 0.001 mg/ml–0.1 mg/ml, or GJHEX at 0.001 mg/ml–0.1 mg/ml were added to the wells, and the cells were cultured for 72 h. Then, 10 μl CCK-8 per 100 μl culture medium was added to the wells, and the cells were incubated for an additional 3 h. Absorbance was measured at 450 nm (BioTek Instruments Inc., Winooski, VT, USA).
Electrophysiology
The ORAI1 current (IORAI1) was measured using HEK293T cells transiently co-transfected with ORAI1 and STIM1. Current was recorded using Axopatch 200B (Molecular Devices, Sunnyvale, CA, USA) and Digidata 1440A (Molecular Devices) and analyzed using pCLAMP 10.4 (Molecular Devices), Origin 8 (Microcal, Northampth, MA, USA), and GraphPad prism 6 software (GraphPad, La Jolla, CA, USA). All recordings and analyses of whole-cell patch clamp assay of IORAI1 current were performed as reported previously [11,12].
Gas chromatography mass spectrometry (GC/MS)
GC/MS analysis was performed at the Korea Basic Science Institute (Western Seoul Center, SD301) using an Agilent 6890 Plus gas chromatographer equipped with a 5973N mass selective detector quadrupole mass spectrometer (Palo Alto, CA, USA). A DB-5MS capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness, 5% diphenyl-95% dimethylsiloxane phase) was obtained from J&W Scientific (Folsom, CA, USA). Chemical constituents were identified by comparing the obtained mass spectra with a built-in NIST library database. All recordings and GC/MS analyses were performed in accordance with previously reported protocols [12].
High performance liquid chromatography (HPLC)
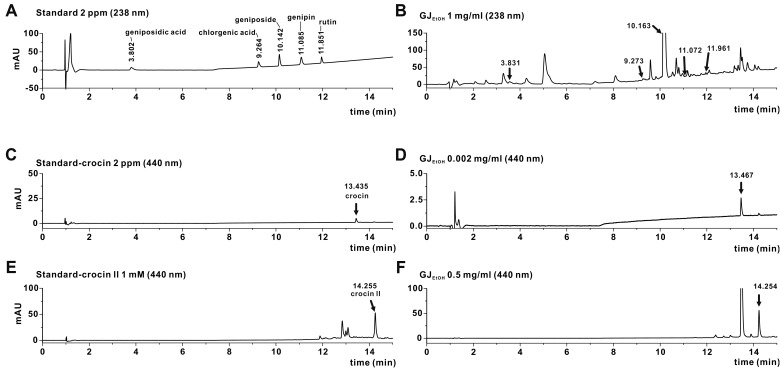
The components of FMEtOH were analyzed using HPLC (1290 Series; Agilent technologies, Santa Clara, CA, USA) at the Korea Basic Science Institute (Seoul, Korea). GJEtOH (10 mg/ml; 10 μl) was injected into a Poroshell 120 SB-C18 column (3.0 × 100 mm, 2.7 μm; Agilent technologies), separated, and detected of its chemical constituents at a wavelength of 238 nm and 440 nm. The temperature of the column was maintained at 30°C. The mobile phases were 0.1% formic acid (A) and 0.1% formic acid/acetonitrile (B) used at a flow rate of 0.5 ml/min. The gradient conditions were as follows: 5% B for 6 min; 5%–40% B for 10 min; 40%–90% B for 0.5 min; 90% B for 1.5 min; and equilibration with 5% B.
Interleukin-2 (IL-2) assay
Jurkat T cells were co-stimulated with antibodies against CD3 and CD28 (Peprotech, Rocky Hill, NJ, USA) to induce the secretion of IL-2. Briefly, 5 μg/ml anti-CD3 was added to a 96-well plate at 50 μl/well, which was then incubated for 3 h at 37°C and then washed three times with DPBS. Jurkat T cells were seeded at a density of 5 × 105 cells/well. Then, 2 μg/ml anti-CD28 per well (1 μl) was added to the wells, and the plate was incubated for 72 h at 37°C and 5% CO2. The culture supernatant was then collected and diluted 1:3, after which the total amount of IL-2 secreted by Jurkat T cells was measured using an IL-2 ELISA kit (Peprotech) per manufacturer’s instructions.
Isolation of human primary CD4+ T lymphocytes
All procedures using human blood were approved by the Institutional Review Board (IRB), Dongguk University College of Medicine (2017-07-003 IRB). Human peripheral blood samples were obtained from healthy voluntary donors after obtaining written informed consent from them. Peripheral blood mononuclear cells (PBMCs) were isolated via density gradient centrifugation using the Ficoll-Paque Plus medium (GE Healthcare, Chicago, IL, USA), after which the isolation of human naïve T lymphocytes was performed using a CD4+ T cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) following the manufacturer’s protocol and previously described methods [11].
T cell proliferation assay
To assess cellular proliferation, purified CD4+ T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) and analyzed as described previously [11].
Statistical analyses
All data are expressed as mean ± standard error of the mean (SEM) and were analyzed using GraphPad prism 6.0 (GraphPad) and Origin 8.0 (Microcal). Differences between two groups were analyzed using Student’s t-test, while those among multiple groups were analyzed using one-way analysis of variance (ANOVA); Bonferroni multiple comparison test was used for post-hoc analysis. p-value < 0.05 was considered statistically significant.
RESULTS
Inhibition of IORAI1 by GJEtOH and its fractions
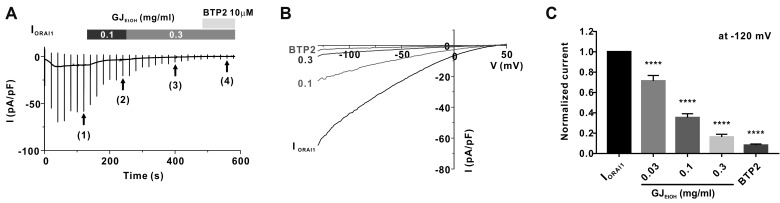
To assess whether GJEtOH and its fractions exerted inhibitory effects on the ORAI1 channel, we performed whole-cell patch clamp using ORAI1-STIM1-co-overexpressing HEK293T cells. As shown in Fig. 1A and B, after confirming the steady-state ORAI1 current (IORAI1) (Fig. 1A (1)), we treated the cells with 0.1 mg/ml (2) and 0.3 mg/ml (3) GJEtOH , which were added into the extracellular solution. At the end of the experiments, the cells were treated with 10 μM BTP2, which is a potent ORAI1 channel inhibitor, to confirm the basal current (Fig. 1A (4)). The inhibition rates of IORAI1 following treatment with each concentration of GJEtOH are summarized in Fig. 1C. These results suggest that GJEtOH effectively inhibited IORAI1 at a dose-dependent manner (28.6% ± 5.36%, 64.7% ± 3.83%, and 83.7% ± 2.68% at the concentrations of 0.03, 0.1, and 0.3 mg/ml, respectively, Fig. 1C, Supplementary Fig. 1).
Fig. 1. Inhibitory effects of 70% ethanolic extract of Gardenia jasminoides (GJEtOH) on ORAI1 current (IORAI1) in human STIM1 and ORAI1 co-transfected HEK293T cells.
(A) Representative chart trace recordings of IORAI1 and inhibition of IORAI1 activity by 0.1 and 0.3 mg/ml GJEtOH and 10 μM BTP2. (B) Related current (I)-voltage (V) relationship graph at steady-state IORAI1 (1), 0.1 (2), 0.3 (3) mg/ml GJEtOH and 10 μM BTP2 (4). (C) Graph shows inhibition of IORAI1 using 0.03, 0.1, and 0.3 mg/ml GJEtOH and 10 μM BTP2, compared with normalized currents at –120 mV (n = 11). ****p < 0.0001 vs. control.
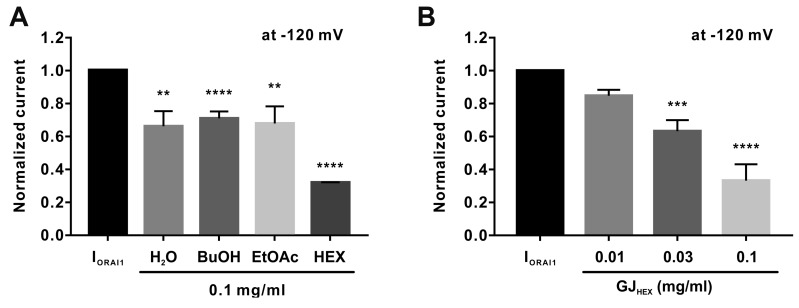
Next, to determine the components of GJEtOH that exerted inhibitory effects on the ORAI1 channel, we prepared four solvent fractions of GJEtOH according to solvent polarity. As shown in Fig. 2A, all fractions examined in this study exerted inhibitory effects on IORAI1. Among them, the inhibitory effects of 0.1 mg/ml hexane fraction on IORAI1 were more potent than those of other fractions at the same concentration. IORAI1 was concentration-dependently inhibited by 15.3% ± 3.62%, 36.8% ± 6.75%, and 66.8% ± 9.95% by 0.01, 0.03 and 0.1 mg/ml hexane fraction, respectively (Fig. 2B).
Fig. 2. Seventy percent ethanolic extract of Gardenia jasminoides (GJEtOH) solvent fractions inhibit ORAI1 current (IORAI1).
(A) Normalized columnar analysis of IORAI1 inhibition using 0.1 mg/ml water (H2O), butanol (BuOH), ethyl acetate (EtOAc), and hexane (HEX) fractions at –120 mV (n = 4). **p < 0.01 and ****p < 0.0001 vs. the control. (B) Different concentrations of hexane fraction result in varying rates of IORAI1 inhibition at −120 mV (n = 6). ***p < 0.001 and ****p < 0.0001 vs. control.
Analysis of the effects of chemical constituents of GJHEX on IORAI1
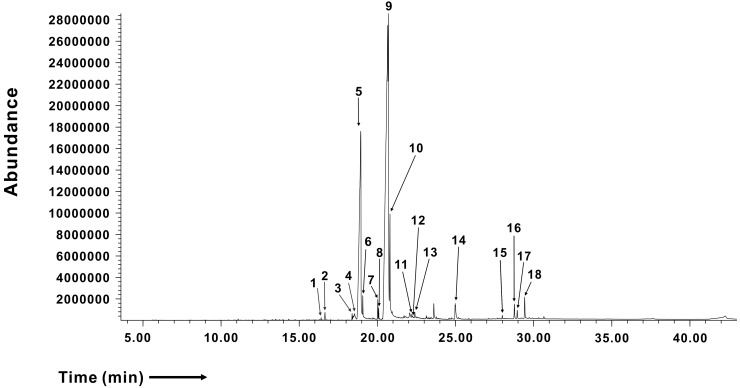
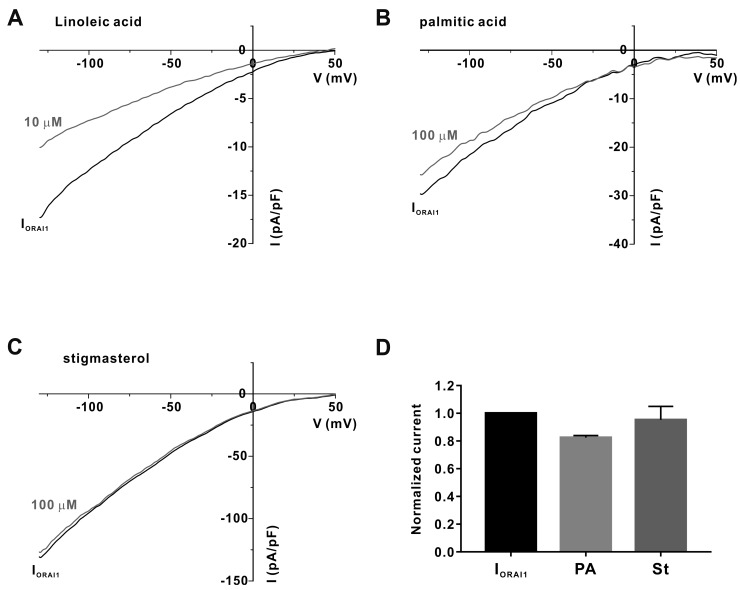
To determine the components of the hexane fraction of GJEtOH (GJHEX) that exerted inhibitory effects on IORAI1, we analyzed GJHEX using GC/MS (Fig. 3). By comparison with known compounds from the National Institute of Standards and Technology (NIST) database, we selected only the compounds having a minimum match quality of 95% (Table 1). Most of the constituents of GJHEX were fatty acids, terpenoids, tocopherols, and sterols. Linoleic acid and palmitic acid were the most common constituents of GJHEX, with their sums accounting for 85% of GJHEX. Stigmasterol, which is a plant sterol known for its anti-inflammatory activity [13,14], was also present in GJHEX at 0.34%. Recently, we reported that linoleic acid significantly inhibited IORAI1 and IL-2 cytokine production in Jurkat T lymphocytes [12] (Fig. 4A). Therefore, we evaluated whether other major compounds, such as palmitic acid and stigmasterol, exerted inhibitory effects on IORAI1. However, as shown in Fig. 4, none of these compounds showed inhibitory activity toward IORAI1.
Fig. 3. Chromatograms of hexane fractions of 70% ethanolic extract of Gardenia jasminoides (GJ) generated using gas chromatography mass spectrometry (GC/MS).
The peak number indicates putative identification of known compounds with matching degree of over 95%. Detailed list of chemical components of GJ is provided in Table 1.
Table 1.
GC/MS analysis of the hexane fractions of 70% ethanolic extract of Gardenia jasminoides
| Peak No. | RT | Compound | CAS | Area | Total (%) |
|---|---|---|---|---|---|
| 1 | 16.41 | Z-11-Tetradecenoic acid | 000000-00-0 | 5634276 | 0.105 |
| 2 | 16.65 | Myristic acid | 000544-63-8 | 16729458 | 0.313 |
| 3 | 18.39 | Methyl palmitate | 000112-39-0 | 6910718 | 0.129 |
| 4 | 18.54 | Hexadecenoic acid | 002416-20-8 | 32645561 | 0.611 |
| 5 | 18.94 | Palmitic acid | 000057-10-3 | 1276239350 | 23.87 |
| 6 | 19.07 | Ethyl palmitate | 000628-97-7 | 56682725 | 1.06 |
| 7 | 20.03 | 10,13-Octadecadienoic acid methyl ester | 056554-62-2 | 27866601 | 0.521 |
| 8 | 20.09 | Methyl elaidate | 001937-62-8 | 17308108 | 0.324 |
| 9 | 20.66 | Linoleic acid | 000060-33-3 | 3349190461 | 62.642 |
| 10 | 20.81 | Stearic acid | 000057-11-4 | 246685756 | 4.614 |
| 11 | 22.07 | Linoleic acid | 000060-33-3 | 22553772 | 0.422 |
| 12 | 22.21 | 14-Methyl-8-hexadecyn-1-ol | 064566-18-3 | 12230997 | 0.229 |
| 13 | 22.40 | Eicosanoic acid | 000506-30-9 | 12992515 | 0.243 |
| 14 | 24.99 | 14-Methyl-8-hexadecyn-1-ol | 064566-18-3 | 55209713 | 1.033 |
| 15 | 28.00 | Vitamin E | 000059-02-9 | 5470124 | 0.102 |
| 16 | 28.77 | Campesterol | 000474-62-4 | 32222261 | 0.603 |
| 17 | 28.95 | Stigmasterol | 000083-48-7 | 18199530 | 0.34 |
| 18 | 29.43 | β-Sitosterol | 000083-46-5 | 47871355 | 0.895 |
Fig. 4. Effects of linoleic acid, palmitic acid, and stigmasterol on ORAI1 current (IORAI1).
(A) Representative current (I)-voltage (V) relationship curve showing the potent inhibition of IORAI1 by 10 μM linoleic acid (n = 3). (B) Representative I-V relationship curve of IORAI1 inhibition by 100 μM palmitic acid (n = 3). (C) Representative I-V relationship curve of IORAI1 inhibition by 100 μM stigmasterol (n = 3). (D) Columnar statistical analysis summarizing the results of the effects of palmitic acid (PA) and stigmasterol (St) on IORAI1.
Analysis of the effects of chemical constituents of other GJ fractions on IORAI1
Although GJHEX exerted the strongest inhibitory effects on IORAI1, other fractions also showed inhibitory activity toward IORAI1. Therefore, we examined whether other known chemical constituents of GJ exhibited inhibitory activity toward the ORAI1 channel. First, we selected eight known active components of GJ mainly found in GJBuOH and GJEtOAc (Supplementary Fig. 2) [5]. We then used HPLC analysis to determine whether these constituents were present in GJEtOH (Fig. 5). We confirmed that seven of the eight previously reported chemical constituents existed in GJEtOH. We then performed a whole-cell patch clamp assay using these compounds at the concentration of 100 μM. Because all chemicals were dissolved in DMSO at DMSO concentrations of less than 1 μl/ml, for control experiments, we confirmed that 1 μl/ml DMSO showed no inhibitory effects on IORAI1 (Fig. 6A).
Fig. 5. Identification of chemical constituents of 70% ethanolic extract of Gardenia jasminoides (GJEtOH) using high performance liquid chromatography (HPLC).
(A, B) HPLC chromatograms of standard chemicals (A), including geniposidic acid, chlorogenic acid, geniposide, genipin, and rutin, and HPLC chromatograms of GJEtOH (B). The detection of chemical constituents in GJEtOH was performed by comparing the retention times of the UV spectral peak with those of the standard chemicals at 238 nm. (C, D) HPLC chromatograms of standard chemicals (C), including crocin and HPLC chromatograms of GJEtOH (D) detected at 440 nm. (E, F) HPLC chromatograms of standard chemicals (E), including crocin II and HPLC chromatograms of GJEtOH (F) detected at 440 nm.
Fig. 6. Concentration-dependent inhibitory effects of genipin on ORAI1 current (IORAI1) in STIM1 and ORAI1 co-transfected HEK293T cells.
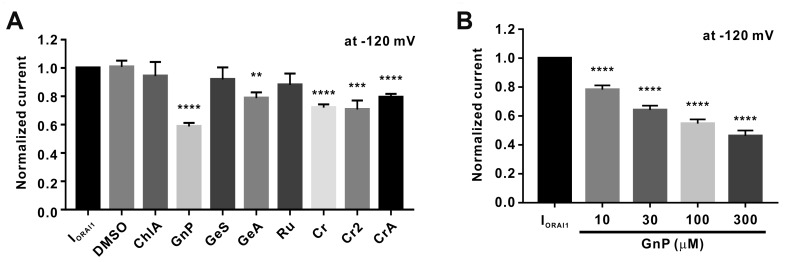
(A) Normalized columnar analysis of the effects of the nine chemical constituents of 70% ethanolic extract of Gardenia jasminoides (GJEtOH) on IORAI1 (n = 6). **p < 0.01, ***p < 0.001 and ****p < 0.0001 vs. the control. DMSO, dimethyl sulfoxide; ChlA, chlorogenic acid; GnP, genipin; GeS, geniposide; GeA, geniposidic acid; Ru, rutin; Cr, crocin; Cr2, crocin II; CrA, croceic acid. (B) Normalized histograms of IORAI1 inhibition by 10, 30, 100, and 300 μM genipin (n = 12). ****p < 0.0001 vs. the control.
As shown in Fig. 6A, genipin exerted the most potent effects on IORAI1, while geniposidic acid, crocin, and crocin II also showed slight, but statically significant, inhibitory effects on IORAI1. We next assessed the effects of different concentrations of genipin on IORAI1 activity. Genipin dose-dependently inhibited the activity of IORAI1 by 21.7% ± 2.93%, 35.9% ± 3.02%, 45.2% ± 2.82%, and 53.7% ± 3.63% at concentrations of 10, 30, 100, and 300 μM, respectively (Fig. 6B).
Effects of genipin on IL-2 secretion in the human T lymphocyte cell line (Jurkat T cells)
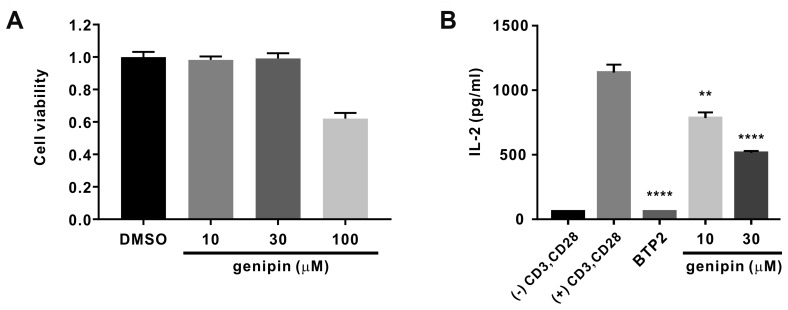
Prior to examining cytokine production and cell proliferation, we evaluated the effects of genipin in human T lymphocytes with the CCK-8 cytotoxicity assay. A 72-h treatment with genipin at various concentrations (up to over 100 μM) showed cytotoxic effects in human T lymphocytes (Fig. 7A). Based on these results, genipin concentrations at which 80% of the cells survived (< 100 μM) were consequently determined and used in subsequent experiments. Jurkat T cells were stimulated with 5 μg/ml anti-CD3 and 2 μg/ml anti-CD28 for 72 h. As shown in Fig. 7B, the production of IL-2 by Jurkat T cells was significantly inhibited by treatment with 10 μM BTP2. We also observed that genipin concentration-dependently inhibited IL-2 secretion by 31.1 ± 3.83 and 54.7 ± 1.32 at concentrations of 10 and 30 μM, respectively (Fig. 7B).
Fig. 7. Inhibition of interleukin-2 (IL-2) secretion by genipin in CD3/CD28 co-stimulated human T lymphocytes.
(A) Human naïve CD4+ T lymphocytes were treated with different concentrations of genipin for 72 h; same volume of solvent (dimethyl sulfoxide, DMSO) under the same conditions was used as vehicle control. Cell viability was assessed with a CCK-8 assay. Cell viability of genipin-treated groups was assessed relative to that of control cells normalized as 1 (n = 3). (B) IL-2 levels were measured in culture supernatants of CD3/CD28 co-stimulated Jurkat T cells at 72 h post-treatment with 10 and 30 μM genipin; treatment with 10 μM BTP2 was used as a positive control. Significance of IL-2 secretion inhibition by genipin was compared between the genipin plus CD3/CD28 co-stimulation treatment group and the CD3/CD28 co-stimulation only group (n = 3). **p < 0.01, ****p < 0.0001 vs. the control.
Genipin potently inhibits the proliferation of human primary T cells
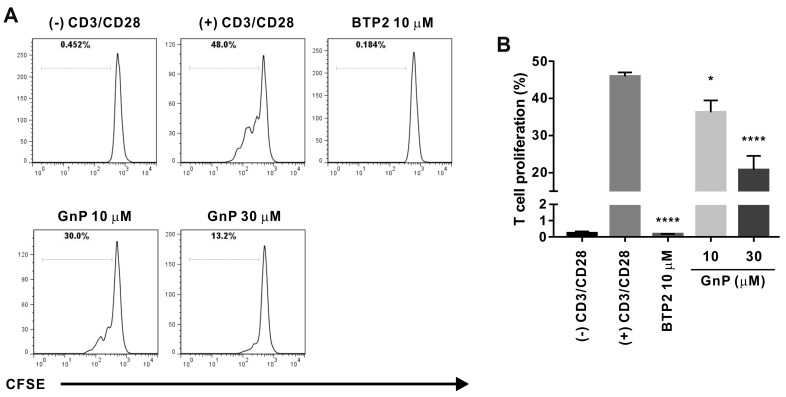
The stimulation of CD3/CD28 receptors of T cell can facilitate T cell expansion and differentiation that partially mimics stimulation by antigen-presenting cells [15]. Therefore, we assessed whether genipin could inhibit PBMC-derived naïve human CD4+ T cells induced to proliferate by CD3/CD28 co-stimulation. Flow-cytometric analysis of CFSE-labeled T cells showed that treatment with genipin dose-dependently suppressed T cell proliferation by 21.0 ± 6.94 and 54.9 ± 8.22 at concentrations of 10, and 30 μM, respectively (Fig. 8). The degree of the inhibitory effect of genipin on IL-2 production and T cell proliferation was slightly higher than that on ORAI1. Therefore, these results suggest that the underlying mechanisms of the suppressive effect of genipin on T cells by CD3/CD28 receptor stimulation might be mediated by the effects of IORAI1 inhibition by genipin.
Fig. 8. Genipin inhibits the proliferation of CD3/CD28 co-stimulated primary human CD4+ T lymphocytes.
(A) A representative plot shows division of human naïve CD4+ T cells as assessed using carboxyfluorescein succinimidyl ester (CFSE) dilution. Human naïve CD4+ T cells were stained with 1 μM CFSE, treated with 10, 30, and 100 μM genipin (GnP), and cultured in anti-CD3 hAb-coated plates with 2 μg/ml anti-CD28. Cell proliferation was assessed by flow cytometry after 72 h of CD3/CD28 receptor stimulation. Human naïve CD4+ T cells stimulated with anti-CD3 and anti-CD28 only were used as negative controls; those treated with 10 μM BTP2 and stimulated with anti-CD3 and anti-CD28, served as positive control. (B) Statistical analysis summarizing the results of three independent experiments and indicating the relative proportion of CD4+ T cells to the whole T cell population induced by CD3/CD28 co-stimulation. *p < 0.05, ****p < 0.0001 vs. the control.
DISCUSSION
Gardenia and its active chemical constituents, such as genipin, have been extensively studied [5]. In particular, numerous pharmacological studies have examined GF for its anti-inflammatory activity and therapeutic effects on patients with allergic diseases [2,3,6,16,17]. Most of these studies have examined how GF and its chemical constituents affect animal models of allergic disease, such as those modeling asthma or AD. The suppressive effects of GF and its constituents on transcription factors and cytokines related to allergic diseases have also been investigated. However, there have been no reports on the effects of GF on calcium ion channels, which are related to immune-cell activation. Engagement of antigen receptors triggers cascades of calcium-dependent signaling pathways in various immune cells including CD4+ T and mast cells. Among the various calcium channels, intracellular calcium signaling via the ORAI1 channel plays a crucial role not only in mast cell degranulation, but also in CD4+ T cell differentiation and cytokine production [18,19]. Therefore, we evaluated the inhibitory effects of GJEtOH and its active constituents on the ORAI1 channel and human CD4+ T cell activities relevant to inflammatory disease.
The effects of GJHEX and its chemical constituents on IORAI1
In the present study, we performed a whole-cell patch clamp assay to assess the inhibitory effects of GJEtOH and its solvent fractions on IORAI1 (Figs. 1 and 2). Our results indicate that SPHEX exerted the most potent inhibitory effects on IORAI1 in human ORAI1 and STIM1-overexpressing HEK293T cells (showing an inhibition of 66.8% ± 9.95% at a concentration of 0.1 mg/ml, Fig. 2B). Using GC/MS analysis, we then determined that 18 chemical constituents of GJHEX were responsible for IORAI1 inhibition (Fig. 3). Linoleic acid, an essential polyunsaturated omega-6 fatty acid, was shown to be the major component (62.64%) of GJHEX (Table 1), and it showed a potent inhibitory effect on IORAI1 (Fig. 3A) However, we did not perform further experiments in relation to this because our previous study had already shown that LA potently inhibits ORAI1 channel activity and, in turn, IL-2 production, induced by CD3/CD28 co-stimulation in human T lymphocytes [12].
We additionally used the whole-cell patch clamp assay to assess whether palmitic acid (the second most abundant component of GJHEX, 23.57%) and stigmasterol, another component of GJHEX known to have anti-inflammatory activity, inhibited ORAI1 channel activity. Our results show, however, that palmitic acid and stigmasterol did not exhibit any inhibitory activity toward IORAI1 (Fig. 4). Therefore, linoleic acid was the active chemical constituent of GJHEX that inhibited ORAI1 channel activity.
The inhibitory effects of genipin on IORAI1
Although GJHEX showed the most potent inhibitory effects on IORAI1, GJBuOH and GJEtOAc also showed inhibitory effects on IORAI1. Therefore, we analyzed GJEtOH for the presence of the eight active compounds previously reported to be present in GJBuOH and GJEtOAc [5]. Of the eight compounds, we detected seven in GJEtOH (Fig. 5) and analyzed them via whole-cell patch clamp assay to determine which of them inhibited IORAI1 activity (Fig. 6). Interestingly, five of these compounds showed statistically significant effects on IORAI1, with genipin showing the strongest inhibitory effects on IORAI1 activity (Fig. 6A). At concentrations of 10, 30, 100, and 300 μM, genipin showed dose-dependent inhibition of IORAI1 activity, but did not completely inhibit the activity of IORAI1 even when used at 300 μM (53.7% ± 3.63%) (Fig. 6B).
IL-2 production and cell proliferation inhibition by genipin in CD3/CD28-co-stimulated human T lymphocytes
Genipin, which is an aglycon of geniposide, shows anti-cancer, anti-fungal, anti-oxidative, anti-inflammatory, and hepatoprotective activities [20,21]. Recent studies have shown that genipin inhibits LPS- or sepsis-induced systemic inflammation, and can downregulate the activation of NFκB and the inflammasome, which are involved in the activity of the innate immune system [22-25]. In our present study, we have shown that genipin also exerts an inhibitory effect on CD3/CD28 co-stimulated T cells, which are components of the adaptive immune system.
Before examining the inhibitory effect of genipin on IL-2 production, the cytotoxic effect of genipin was measured, revealing a strong toxicity at genipin concentrations over 100 μM. Therefore, subsequent experiments were conducted at 10 and 30 μM genipin (Fig. 7A). Treatment with 10 and 30 μM genipin showed a statistically significant inhibitory effect on IL-2 production in CD3/CD28-co-stimulated Jurkat T cells (Fig. 7B). Although the inhibitory effect was weaker than that of 10 μM BTP2, the inhibitory effect on IL-2 production in CD3/CD28-co-stimulated Jurkat T cells was stronger than that on IORAI1 (35.9% ± 3.02% vs. 54.7% ± 1.32% at 30 μM). These results indicate that genipin-mediated inhibition of IL-2 production was likely due to the inhibition of ORAI1 activity.
It was reported that genipin shows anti-proliferative effects in various cancer cell lines [20]; therefore, we isolated primary human CD4+ naïve T cells from PBMCs that had been activated using co-stimulation with antibodies against CD3 and CD28. As shown in Fig. 8, genipin exhibited potent anti-proliferative effects on primary human CD4+ T cells (54.9% ± 8.22% inhibition at 30 μM), as reflected by the decreased rates of IL-2 production in Jurkat T cells.
In summary, we revealed that GJEtOH and its chemical constituent, genipin, showed inhibitory effects on the activity of the ORAI1 channel. We also confirmed that genipin inhibited IL-2 production and cell proliferation in human T cells induced by CD3/CD28 co-stimulation. To the best of our knowledge, this is the first study to show these effects of genipin in human primary CD4+ T cells. However, to evaluate the pharmacological activity of genipin in the context of allergic diseases, it is necessary to use mast cells or Th2 T cells, which are differentiated from CD4+ naïve T cells. This was not addressed in our present study, but will be the focus of our future study. Our findings provide important basis for understanding the mechanisms of the traditional herbal medicine GJ in the treatment of inflammatory diseases.
SUPPLEMENTARY MATERIALS
Supplementary data including two figures can be found with this article online at http://pdf.medrang.co.kr/paper/pdf/Kjpp/Kjpp2020-24-04-08-s001.pdf.
ACKNOWLEDGEMENTS
This research was supported by the Convergence of Conventional Medicine and Traditional 2 Korean Medicine R&D program funded by the Ministry of Health & Welfare (Korea) through the Korean Health Industry Development Institute (KHIDI) (grant no. HI16C0766) and also supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education of South Korea (no. NRF-2018R1A6A3A01012806).
Footnotes
Author contributions: J.H.N. and W.K.K. conceived and designed the study. H.J.K., Y.R.N., and J.H.W. performed the experiments, and acquired, analyzed, and interpreted data. H.J.K. and Y.R.N. drafted the manuscript. W.K.K. critically revised the manuscript for important intellectual content. J.H.N. and W.K.K. provided final approval of the version to be submitted. All authors have read and approved the final manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Liu H, Chen YF, Li F, Zhang HY. Fructus Gardenia (Gardenia jasminoides J. Ellis) phytochemistry, pharmacology of cardiovascular, and safety with the perspective of new drugs development. J Asian Nat Prod Res. 2013;15:94–110. doi: 10.1080/10286020.2012.723203. [DOI] [PubMed] [Google Scholar]
- 2.Deng Y, Guan M, Xie X, Yang X, Xiang H, Li H, Zou L, Wei J, Wang D, Deng X. Geniposide inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. Int Immunopharmacol. 2013;17:561–567. doi: 10.1016/j.intimp.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 3.Sung YY, Lee AY, Kim HK. The Gardenia jasminoides extract and its constituent, geniposide, elicit anti-allergic effects on atopic dermatitis by inhibiting histamine in vitro and in vivo. J Ethnopharmacol. 2014;156:33–40. doi: 10.1016/j.jep.2014.07.060. [DOI] [PubMed] [Google Scholar]
- 4.Koo HJ, Lim KH, Jung HJ, Park EH. Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. J Ethnopharmacol. 2006;103:496–500. doi: 10.1016/j.jep.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Xiao W, Li S, Wang S, Ho CT. Chemistry and bioactivity of Gardenia jasminoides. J Food Drug Anal. 2017;25:43–61. doi: 10.1016/j.jfda.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko JW, Shin NR, Park SH, Cho YK, Kim JC, Seo CS, Shin IS. Genipin inhibits allergic responses in ovalbumin-induced asthmatic mice. Int Immunopharmacol. 2017;53:49–55. doi: 10.1016/j.intimp.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Nam JH, Kim WK. The role of TRP channels in allergic inflammation and its clinical relevance. Curr Med Chem. 2020;27:1446–1468. doi: 10.2174/0929867326666181126113015. [DOI] [PubMed] [Google Scholar]
- 8.Litosch I. Decoding Gαq signaling. Life Sci. 2016;152:99–106. doi: 10.1016/j.lfs.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 9.Woo JS, Srikanth S, Gwack Y. Modulation of Orai1 and STIM1 by cellular factors. In: Kozak JA, Putney JW, editors. Calcium entry channels in non-excitable cells. CRC Press; Boca Raton (FL): 2018. pp. 73–92. [DOI] [PubMed] [Google Scholar]
- 10.Feske S, Wulff H, Skolnik EY. Ion channels in innate and adaptive immunity. Annu Rev Immunol. 2015;33:291–353. doi: 10.1146/annurev-immunol-032414-112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HJ, Nam YR, Kim EJ, Nam JH, Kim WK. Spirodela polyrhiza and its chemical constituent vitexin exert anti-allergic effect via ORAI1 channel inhibition. Am J Chin Med. 2018;46:1243–1261. doi: 10.1142/S0192415X18500659. [DOI] [PubMed] [Google Scholar]
- 12.Kim HJ, Woo J, Nam YR, Nam JH, Kim WK. Flos Magnoliae and its constituent linoleic acid suppress T lymphocyte activation via store-operated calcium entry. Am J Chin Med. 2019;47:1627–1641. doi: 10.1142/S0192415X19500836. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Youn K, Jun M. Major compounds of red ginseng oil attenuate Aβ25-35-induced neuronal apoptosis and inflammation by modulating MAPK/NF-κB pathway. Food Funct. 2018;9:4122–4134. doi: 10.1039/C8FO00795K. [DOI] [PubMed] [Google Scholar]
- 14.Gabay O, Sanchez C, Salvat C, Chevy F, Breton M, Nourissat G, Wolf C, Jacques C, Berenbaum F. Stigmasterol: a phytosterol with potential anti-osteoarthritic properties. Osteoarthritis Cartilage. 2010;18:106–116. doi: 10.1016/j.joca.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Trickett A, Kwan YL. T cell stimulation and expansion using anti-CD3/CD28 beads. J Immunol Methods. 2003;275:251–255. doi: 10.1016/S0022-1759(03)00010-3. [DOI] [PubMed] [Google Scholar]
- 16.Park SH, An JE, Jang S, Kim JY, Lee JW, Kim HK. Gardenia jasminoides extract without crocin improved atopic dermatitis-like skin lesions via suppression of Th2-related cytokines in Dfe-induced NC/Nga mice. J Ethnopharmacol. 2019;241:112015. doi: 10.1016/j.jep.2019.112015. [DOI] [PubMed] [Google Scholar]
- 17.Sung YY, Kim HK. Crocin ameliorates atopic dermatitis symptoms by down regulation of Th2 response via blocking of NF-κB/STAT6 signaling pathways in mice. Nutrients. 2018;10:1625. doi: 10.3390/nu10111625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srikanth S, Woo JS, Sun Z, Gwack Y. Immunological disorders: regulation of Ca2+ signaling in T lymphocytes. Adv Exp Med Biol. 2017;993:397–424. doi: 10.1007/978-3-319-57732-6_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holowka D, Wilkes M, Stefan C, Baird B. Roles for Ca2+ mobilization and its regulation in mast cell functions: recent progress. Biochem Soc Trans. 2016;44:505–509. doi: 10.1042/BST20150273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shanmugam MK, Shen H, Tang FR, Arfuso F, Rajesh M, Wang L, Kumar AP, Bian J, Goh BC, Bishayee A, Sethi G. Potential role of genipin in cancer therapy. Pharmacol Res. 2018;133:195–200. doi: 10.1016/j.phrs.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Habtemariam S, Lentini G. Plant-derived anticancer agents: lessons from the pharmacology of geniposide and its aglycone, genipin. Biomedicines. 2018;6:39. doi: 10.3390/biomedicines6020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Wang X, Ma C, Li Z, Chen H, Zhang Z, Li T. Genipin protects rats against lipopolysaccharide-induced acute lung injury by reinforcing autophagy. Int Immunopharmacol. 2019;72:21–30. doi: 10.1016/j.intimp.2019.03.052. [DOI] [PubMed] [Google Scholar]
- 23.Yu SX, Du CT, Chen W, Lei QQ, Li N, Qi S, Zhang XJ, Hu GQ, Deng XM, Han WY, Yang YJ. Genipin inhibits NLRP3 and NLRC4 inflammasome activation via autophagy suppression. Sci Rep. 2015;5:17935. doi: 10.1038/srep17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JS, Kim SJ, Lee SM. Genipin attenuates sepsis-induced immunosuppression through inhibition of T lymphocyte apoptosis. Int Immunopharmacol. 2015;27:15–23. doi: 10.1016/j.intimp.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 25.Nam KN, Choi YS, Jung HJ, Park GH, Park JM, Moon SK, Cho KH, Kang C, Kang I, Oh MS, Lee EH. Genipin inhibits the inflammatory response of rat brain microglial cells. Int Immunopharmacol. 2010;10:493–499. doi: 10.1016/j.intimp.2010.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.