Abstract
Surface disinfectants are regularly used in prophylactic and infection control measures. Concern has been raised whether residues of sub-inhibitory disinfectant concentrations may constitute a selective pressure and could contribute to the development of strains which are tolerant and/or resistant to biocides including antibiotics. The current study investigated whether Staphylococcus (S.) aureus ATCC® 29213™ and ATCC® 6538™ would change their growth characteristics and antimicrobial susceptibility profiles after prolonged treatment with sub-inhibitory concentrations of sodium hypochlorite (NaOCl). NaOCl is a fast-acting disinfectant with a broad-spectrum activity, inexpensive and widely used in healthcare and the food production industry. Minimum inhibitory concentration (MIC) for NaOCl was determined by broth macrodilution according to the guidelines for disinfectant efficacy testing provided by the German Veterinary Medical Society. Serial passages after 24 h and 72 h, respectively, in defined sub-inhibitory concentrations of NaOCl resulted in a number of phenotypic variants. Two of these variants, derived from S. aureus ATCC® 29213™, showed elevated MICs of oxacillin and were considered as in vitro-generated borderline oxacillin-resistant S. aureus (BORSA). Transmission electron microscopy revealed a significantly thickened cell wall in these isolates, a phenomenon that has also been described for Listeria monocytogenes after low-level exposure to NaOCl. Whole genome sequencing revealed an early stop codon in the gene coding for the GdpP protein and thereby abolishing the function of this gene. GdpP represents a phosphodiesterase that regulates gene expression, and loss of function of the GdpP protein has been described in association with borderline oxacillin resistance. Our findings suggest that a mutation in the GdpP protein gene and morphological changes of the cell wall were induced by repeated exposure to sub-lethal NaOCl concentrations, and most likely accounted for a BORSA phenotype in two variants derived from S. aureus ATCC® 29213™.
Keywords: Microbiology, Antibiotic resistant bacteria, Antimicrobial, Bacteriology, Health sciences, Staphylococcus, Veterinary medicine, Staphylococcus aureus, Sodium hypochlorite, Cell wall, Transmission electron microscopy, BORSA, GdpP
Microbiology; Antibiotic resistant bacteria; Antimicrobial; Bacteriology; Health sciences; Staphylococcus; Veterinary medicine; Staphylococcus aureus; Sodium hypochlorite; Cell wall; Transmission electron microscopy; BORSA; GdpP
1. Introduction
Exposure to low-level concentrations of biocides may trigger bacterial adaptive responses or resistance against antimicrobial agents in vitro (Thomas et al., 2000; Chapman, 2003; Karatzas et al., 2007; Randall et al., 2007; Meyer and Cookson, 2010). This has been studied extensively for triclosan, quaternary ammonium compounds, and chlorhexidine (reviewed in Kampf, 2018). Furthermore, antibacterial biocides at low concentrations induce horizontal gene transfer, thereby facilitating the spread of antimicrobial resistance genes between bacteria in vitro (Jutkina et al., 2018; Kampf, 2018). Inappropriate use of surface disinfectants (for example, incorrect concentration or excessive sub-inhibitory biocide residues on surfaces) may constitute a selective pressure contributing to the development of tolerant or resistant strains (Li et al., 1997; Russell, 2001, 2004; Huet et al., 2008). Sodium hypochlorite (NaOCl) is a strong oxidizing agent with a broad-spectrum antimicrobial efficacy against a wide range of bacteria, including multidrug-resistant bacteria (Köhler et al., 2018). NaOCl is available worldwide, is favorable in terms of cost and benefit and is used for a wide range of applications, for example disinfection of surfaces, laundry, and drinking water (Rutala and Weber, 1997; Anonymous, 2013; Pereira et al., 2015). In the European Economic Area/and or Switzerland, it is approved for use as a biocide for human hygiene, disinfection, veterinary hygiene, food and animal feeds, and drinking water (https://echa.europa.eu/de/substance-information/-/substanceinfo/100.028.790). Likely concentrations at which NaOCl (calculated as available chlorine) will be used as a product-type 3 for veterinary hygiene vary between 2,000 mg/L (0.2%) and 30,000 mg/L (3%) (Anonymous, 2017). Resistance to NaOCl is very uncommon (reviewed in Kampf, 2018). Despite this, a minimum inhibitory concentration (MIC) of 4,100 mg/L active chlorine (Salmonella spp., Staphylococcus (S.) aureus) and 8,200 mg/L (Enterococcus spp., Escherichia coli, Klebsiella pneumoniae) was proposed to determine resistance (reviewed in Kampf, 2018).
S. aureus is an important pathogen in human and veterinary medicine, livestock production, and food processing. S. aureus is tolerant to desiccation and survives for months on dry surfaces (Kramer et al., 2006; Kadariya et al., 2014). Only few studies exist regarding effects observed after low-level exposure of S. aureus to NaOCl (Kusumaningrum et al., 2003; Buzón-Durán et al., 2017; Ujimine et al., 2017). Recently, biofilm production by methicillin-resistant S. aureus (MRSA) induced by sub-inhibitory concentrations of NaOCl has been demonstrated (Buzón-Durán et al., 2017). The present study investigated whether repeated treatment of S. aureus by sub-inhibitory concentrations of NaOCl would have an effect on antimicrobial susceptibility, growth characteristics on sheep blood agar, and susceptibility to NaOCl.
2. Materials and methods
2.1. Bacterial strains and growth conditions
Experiments were conducted using S. aureus (SA) strains ATCC® 29213™ and ATCC® 6538™. Strain ATCC® 29213™ is the well characterized quality control strain for antimicrobial susceptibility testing (AST) (MIC determination and disk diffusion) recommended by EUCAST (Anonymous, 2019). According to ATCC® strain characteristics, it is susceptible to oxacillin (https://www.lgcstandards-atcc.org/products/all/29213.aspx?geo_country=de#characteristics) and exhibits weak β-lactamase activity (Anonymous, 2019). S. aureus ATCC® 6538™ is a control strain according to DIN 58959-9 (Anonymous, 1997) and the reference strain for disinfectant efficacy testing (Anonymous, 2019a). Both strains were obtained from the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures (DSMZ; Braunschweig, Germany). Cultures were grown overnight on Columbia sheep blood agar (CSA) containing 7% blood (Oxoid Deutschland GmbH/Thermo Scientific, Wesel, Germany) at 37 °C. AST was performed at a DIN EN ISO 15189 accredited diagnostic laboratory (amedes MVZ für Laboratoriumsdiagnostik und Mikrobiologie Halle/Leipzig GmbH, Halle, Germany) using VITEK®2 technology (bioMérieux Deutschland GmbH, Nürtingen, Germany) according to the EUCAST recommendations. Results were evaluated using clinical breakpoints given by EUCAST (http://www.eucast.org/clinical_breakpoints/).
2.2. Determination of minimum inhibitory concentrations for NaOCl
MIC values for NaOCl were determined following the “Guidelines for disinfectant testing”, provided by the German Veterinary Medical Society (DVG e.V.) (Anonymous, 2019a). The final concentrations tested were 0.01% (v/v), 0.02%, 0.03% and so on up to 0.1%, 0.25% and 0.5% NaOCl (CAS-No. 7681-52-9; AppliChem GmbH, Darmstadt, Germany; 10%–14% active chlorine according to the manufacturer). Each concentration was tested in duplicate at two independent occasions. In brief, screw cap tubes containing 2.5 mL double concentrated tryptic soy broth (TSB) were filled up with 2.5 mL of double concentrated NaOCl. Subsequently, 50 μL of S. aureus (1.4–1.9 × 109 colony-forming units (cfu)/mL) were added to each test tube. Preparations were mixed and incubated at 37 °C. After 72 h of incubation, turbidity was measured as an indicator of bacterial growth. The lowest concentration with absence of growth was interpreted as MIC.
2.3. Effect of sub-inhibitory NaOCl concentrations on growth characteristics, susceptibility to NaOCl, and antimicrobial susceptibility
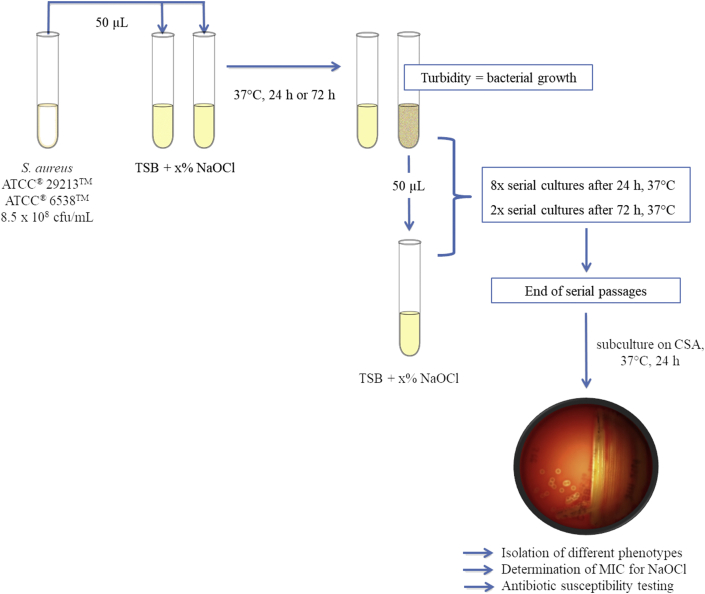
The following sub-inhibitory concentrations of NaOCl were arbitrarily defined: 0.01%, 0.005%, 0.0025%, 0.00125%, 0.0006%, and 0.0003%. Experiments were performed according to Karatzas et al. (2007), but modified in that we chose two different contact times, i.e. 24 h and 72 h. The latter incubation time corresponded to NaOCl MIC determination. Tubes were prepared and incubated at 37 °C as described above. The initial inoculum for both strains was 8.5 × 108 cfu/mL. From all tubes showing bacterial growth after the respective contact time, a 50 μL-aliquot was transferred to a freshly prepared tube containing the same NaOCl concentration and further incubated (Figure 1). This was repeated eight times after 24 h and twice after 72 h of incubation. From all tubes showing bacterial growth at the end of each experiment, aliquots were spread out on CSA using a 1 μL disposable loop. After 24 h of incubation at 37 °C, colony morphology was noted and colonies of different phenotype were separated on new CSA plates. Each of these isolates was subjected to NaOCl MIC determination (Figure 1) and AST (amedes MVZ für Laboratoriumsdiagnostik und Mikrobiologie Halle/Leipzig GmbH) as described above. AST was further performed after two subcultures on CSA. In addition, NaOCl MIC was determined after two serial passages in TSB. Both were intended to test whether any observed changes in the susceptibility patterns was robust. All isolates were stored at -80 °C for further investigation (CRYOBANK™ tubes; MAST Diagnostika GmbH, Reinfeld, Germany). As a control, the experiments using strains ATCC® 29213™ and ATCC® 6538™ as described above were additionally performed using TSB without any supplementation.
Figure 1.
Schematic presentation of the experimental setup. Both S. aureus ATCC® strains were inoculated into tryptic soy broth (TSB) containing different concentrations of NaOCl. Turbidity after the respective incubation time indicated bacterial growth. From turbid cultures, a 50 μL-aliquot was transferred to a freshly prepared tube containing the same NaOCl concentration and further incubated. This was repeated eight times after 24 h and twice after 72 h of incubation, respectively. At the end of each experiment, subcultures were prepared on CSA, colony morphology was noted and colonies of different phenotype were further investigated. x% indicates a NaOCl concentration of 0.01%, 0.005%, 0.0025%, 0.00125%, 0.0006%, and 0.0003%, respectively.
2.4. Macrorestriction analysis
Macrorestriction analysis was performed using the original S. aureus ATCC® 29213™ and two variant isolates (SA29213-A, SA29213-B) with altered phenotypes to confirm the clonal identity. SmaI and ApaI were used as restriction enzymes as described elsewhere (Murchan et al., 2003; Kadlec et al., 2009).
2.5. Agar disk diffusion
In addition, agar disk diffusion was performed using penicillin (10 U, Oxoid Deutschland GmbH/Thermo Scientific) according to CLSI standards for S. aureus ATCC® 29213™ and the two strains SA29213-A and SA29213-B (CLSI, 2019). The zone edges of the penicillin inhibition zones were evaluated according to the zone edge test (CLSI, 2019).
2.6. β-lactam susceptibility testing by broth microdilution
Additional broth microdilution was performed for S. aureus ATCC® 29213™ as well as its variants SA29213-A and SA29213-B according to CLSI standards (CLSI, 2019) using ampicillin, amoxicillin/clavulanic acid, penicillin, ceftiofur, cefquinome, cephalothin, cefazolin, cefotaxime, cefoperazone, and oxacillin.
2.7. Growth kinetics of S. aureus ATCC® 29213™ and its variants
A propagation assay was used to evaluate possible changes in the fitness of SA29213-A and SA29213-B compared to S. aureus ATCC® 29213™. Bacteria were grown from cryo-stocks on CSA at 37 °C for 24 h. A fresh overnight subculture (CSA, 37 °C) was used to perform growth kinetics. Cells were suspended in TSB resulting in an initial inoculum of 5.5 × 108 cfu/mL (S. aureus ATCC® 29213™), 5.8 × 108 cfu/mL (SA29213-A), and 6.1 × 108 cfu/mL (SA29213-B), respectively. Each suspension was used to inoculate 24 wells (100 μL/well) on a 96-well flat-bottom microtiter plate (Sarstedt AG & Co., Nümbrecht, Germany). The remaining wells were filled with TSB only and served as blank. The plate was incubated over a period of 6 h at 37 °C without shaking. Bacterial growth was measured every 15 min by recording the optical density at 595 nm using a Multiskan Ascent (Thermo Labsystems, Helsinki, Finland) microplate reader. Prior to recording, the plate was shaken for 3 s at 480 rpm. The assay was performed twice.
2.8. Transmission electron microscopy (TEM)
S. aureus ATCC®29213™, SA29213-A, and SA29213-B were grown at 37 °C overnight in TSB. Thereafter, cultures were centrifuged at 300 x g for 15 min and the supernatants were discarded. A total of 2.5 mL of glutaraldehyde (2.5% in 0.1 M sodium cacodylate buffer with 0.1 M sucrose, 1 mM CaCl2, pH 7.2) was added to the bacterial cell pellet, gently mixed and incubated for 1 h at room temperature. Thereafter, a 1.2 mL-aliquot of each preparation was transferred to a tube, and centrifuged (500 x g, 15 min) again. Supernatants were removed, pellets rinsed in phosphate-buffered saline (PBS; pH 7.4) at room temperature for 10 min. Centrifugation, removal of supernatants and rinsing in PBS two times for 10 min was repeated twice for each bacterial isolate. Prior to secondary fixation and embedding in resin 10 μL of each glutaraldehyde-fixed bacterial cell pellet were injected into a LUMITainer™ microcapsule (diameter 3.5 mm) (LUM GmbH, Berlin, Germany) for easier handling. Further processing for secondary fixation and embedding was conducted as follows: osmification with 1% OsO4 (in 0.1 M cacodylate buffer with 1.5% potassium hexacyanoferrate (II) trihydrate; Sigma-Aldrich, St. Louis MO, USA) for 2 h, rinsing in distilled water (five times for 7 min each), dehydration in ascending methanol including propylene oxide as intermedium (twice for 10 min each), and resin embedding in glycid ether 100™ (Serva, Heidelberg, Germany). Ultra-thin sections (50 nm) were cut and collected on uncoated 300 mesh nickel thin bar grids.
Thirty cells of each preparation with nearly equatorial cut surfaces were chosen to determine cell wall thickness. Digital TEM images were taken at a final magnification of ✕20.000 using a Zeiss EFTEM Libra 120™ (Zeiss, Oberkochen, Germany) and were analyzed with the TEM-based image analysis software (iTEM). For each cell, measurements were performed at five cell wall positions by two investigators, independently. To evaluate differences in cell wall thickness between the three strains, a linear mixed effects model was fitted to the data. The strain and an indicator of the person who took the measurements were treated as fixed covariates, and the model included random intercept for each cell. The model allowed for evaluator-specific variance of the random intercepts. The model was compared to other candidate models using likelihood ratio tests. The goodness of fit of the model was assessed by inspection of its residuals. Results are expressed as means ± standard deviation (SD). P-values were computed using the fitted model, treating the parental strain as a reference. Differences were regarded as significant for P < 0.05. Analyses were performed using R, version 3.4.1 (R Core Team, 2019). Mixed effects models were fitted using the R package nlme.
2.9. Whole genome sequencing and sequence analysis
S. aureus ATCC® 29213™ as well as its variants SA29213-A and SA29213-B were subjected to whole genome sequencing (WGS). For DNA extraction the QIAamp® DNA Mini Kit (QIAGEN, Hilden, Germany) was used with some modifications. To lyse the staphylococcal cell wall, 25 μL lysostaphin solution (0.1 mg/mL) was added to the cells prior to incubation for 25 min at 37 °C. After this, 75 μL TE buffer and 25 μL proteinase K (0.1 mg/L) were added and incubation continued for 25 min at 37 °C. 75 μL PBS and 2 μL RNAse (2 μg/μL) were added and slightly mixed. Then the protocol for the kit was followed starting with the addition of AL buffer. The Nextera XT library preparation kit (Illumina Inc., San Diego, USA) was used for the preparation of the WGS libraries according to the manufacturer's recommendations. The 2×300 bp paired-end sequencing in 40-fold multiplexes was performed on the Illumina MiSeq (Illumina Inc., San Diego, USA) platform. SPAdes 3.14.0 (Nurk et al., 2013) was used for de novo assembly of the raw reads. The sequence of S. aureus ATCC® 29213™ was used as reference to which the variants SA29213-A and SA29213-B were mapped with Bowtie2 (Langmead and Salzberg, 2012). The obtained sequences were annotated using RAST annotation server (Aziz et al., 2008) and compared to each other and with S. aureus ATCC® 29213™ reference (NZ_LHUS00000000). The nucleotide sequences were analyzed using Geneious v 11.1.4 (Biomatters, Auckland, New Zealand). Regarding the reduced oxacillin susceptibility, the sequences of the three strains in question were compared for the proteins GdpP, FemA, FemB, MgrA, MprF (using S. aureus NCTC 8325 (NCBI:txid93061) as a reference) and FemC (GlnR), FemD (GlmM), CcpA as well as the penicillin binding proteins (PBP) 1, 2, 3 and 4 (using S. aureus Mu50 (NCBI:txid158878) as a reference). Regarding the phenotypic differences in the hemolysis patterns of the three strains, sequences of the three strains in question were compared for the proteins of the bicomponent gamma-hemolysins (HlgA, HlgB and HlgC) (using S. aureus Mu50 (NCBI:txid158878) as a reference) as well as alpha-hemolysin (Hla) and delta-hemolysin (Hld) (using S. aureus NCTC8325 (NCBI:txid93061) as a reference).
3. Results
3.1. Phenotypic characteristics of S. aureus ATCC®29213™ and ATCC® 6538™
On CSA, S. aureus ATCC®29213™ grew in medium-sized, smooth, golden-yellow colonies with a narrow zone of complete hemolysis (Figure 2 A) and was susceptible to all antimicrobial agents tested except benzylpenicillin (Table 1). The MIC value determined for NaOCl was 0.07%. S. aureus ATCC®6538™ revealed medium-sized, smooth, golden-yellow colonies with a clear zone of complete hemolysis. The strain was susceptible to all antimicrobial agents tested (Table 1) and exhibited a NaOCl MIC value of 0.1%.
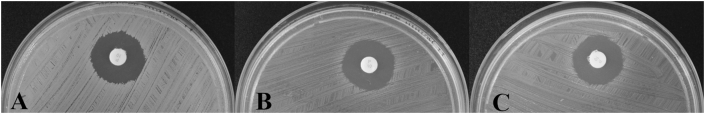
Figure 2.
A-C. Colony appearance of S. aureus grown on Columbia sheep blood agar for 24 h at 37°C. (A) Parental strain ATCC® 29213™. (B) Variant SA29213-A obtained after serial passages in 0.005% NaOCl. (C) Variant SA29213-B obtained after serial passages in 0.005% NaOCl.
Table 1.
MIC values of both ATCC® S. aureus strains and their variants obtained by Vitek®2 analysis.
| Antibiotic substance | Minimum inhibitory concentrations (mg/L) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
S. aureus strains and variants | ||||||||||||||
| ATCC® 29213™ | SA29213-A | SA29213-B | SA29213-C | SA29213-D | SA29213-E | SA29213-F | SA29213-G | SA29213-H | ATCC® 6538™ | SA6538-a | SA6538-b | SA6538-c | SA6538-d | |
| Benzylpenicillin | ≥0.5b | ≥0.5b | ≥0.5b | 0.25b | ≥0.5b | ≥0.5b | ≥0.5b | ≥0.5b | ≥0.5b | ≤0.03a | 0.06a | ≤0.03a | ≤0.03a | ≤0.03a |
| Oxacillin | ≤0.25a | ≥4b,∗ | ≥4b,∗ | ≤0.25a | ≤0.25a | 0.5a | ≤0.25a | ≤0.25a | 0.5a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a |
| Gentamicin | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a |
| Levofloxacin | 0.25a | ≤0.12a | ≤0.12a | ≤0.12a | 0.25a | 0.5a | ≤0.12a | ≤0.12a | ≤0.12a | ≤0.12a | 0.25a | ≤0.12a | ≤0.12a | ≤0.12a |
| Erythromycin | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a |
| Clindamycin | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a | ≤0.25a |
| Vancomycin | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | 1a | 1a | ≤0.5a | 1a |
| Teicoplanin | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a |
| Tetracycline | ≤1.0a | ≤1.0a | ≤1.0a | ≤1.0a | ≤1.0a | ≤1.0a | ≤1.0a | ≤1.0a | ≤1.0a | ≤1.0a | ≤1.0a | ≤1.0a | ≤1.0a | ≤1.0a |
| Tigecycline | ≤0.12a | ≤0.12a | ≤0.12a | ≤0.12a | ≤0.12a | ≤0.12a | ≤0.12a | ≤0.12a | ≤0.12a | ≤0.12a | ≤0.12a | ≤0.12a | ≤0.12a | ≤0.12a |
| Rifampicin | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a |
| Fosfomycin | ≤8a | 16a | ≤8a | ≤8a | ≤8a | ≤8a | ≤8a | ≤8a | ≤8a | ≤8a | ≤8a | ≤8a | ≤8a | ≤8a |
| Fusidic acid | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a | ≤0.5a |
| Mupirocin | ≤2a | ≤2a | ≤2a | ≤2a | ≤2a | ≤2a | ≤2a | ≤2a | ≤2a | ≤2a | ≤2a | ≤2a | ≤2a | ≤2a |
| Linezolid | 2a | 1a | 1a | 2a | 2a | 2a | 2a | 1a | 2a | 2a | 2a | 1a | 2a | 2a |
∗Both isolates were repeatedly tested and results for oxacillin varied between 2 mg/L (i.e. interpreted as susceptible) and ≥4 mg/L (i.e. interpreted as resistant).
Values interpreted as susceptible.
Values interpreted as resistant.
3.2. Effect of sub-inhibitory concentrations of NaOCl on growth characteristics, susceptibility to NaOCl, and antimicrobial susceptibility
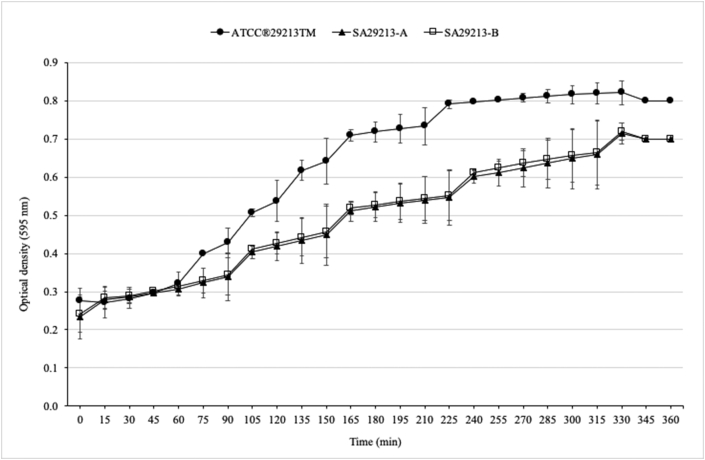
In experiments with serial passages after 24 h of incubation, growth of S. aureus ATCC® 29213™ occurred at 0.0003%, 0.0006%, 0.00125%, and 0.0025%. In contrast, growth also occurred in 0.005% NaOCl in experiments with serial passages after 72 h of incubation. Changes in colony morphology were visible in both experimental setups. Besides typical S. aureus ATCC® 29213™ colonies, eight different phenotypes with and without hemolysis were found (Table 2). Antimicrobial susceptibility profiles of all strains were identical except for oxacillin, levofloxacin, fosfomycin, and linezolid (Table 1). Strains SA29213-A (Figure 2 B) and SA29213-B (Figure 2 C) exhibited decreased susceptibility to oxacillin (MIC ≥4 mg/L) with an at least 16-fold increase in the MIC values compared to S. aureus ATCC® 29213™ (Table 1). Two subcultures on CSA were performed to prove whether these changes were reversible. MICs decreased to 2 mg/L (i.e. susceptible) after the first subculture but were consistent with the first result (i.e. resistant, ≥4 mg/L oxacillin) after the second subculture on CSA. The MICs determined for NaOCl ranged from 0.07% to 0.09% (Table 2). The agar disk diffusion test for S. aureus ATCC® 29213™, SA29213-A, and SA29213-B revealed a zone diameter of 20 mm for penicillin (Figure 3 A–C). Thus, all three strains were classified as penicillin resistant (CLSI, 2019). The corresponding zone edges were sharp, which indicated the presence of a β-lactamase (CLSI, 2019). Broth microdilution also confirmed that the original strain and the two variants were resistant to penicillin. The MICs of the two variants were 2 mg/L compared to 1 mg/L for the original strain. However, it should be noted that deviations of +/- one dilution step are within the acceptable range for AST (CLSI, 2018a). The oxacillin MIC of the original strain was 0.25 mg/L compared with 1 mg/L for both variants. This corresponded to an increase of the MIC by two dilution steps, although the variants would still be considered as susceptible (CLSI, 2019). A slight increase in the MICs was also seen for ampicillin, amoxicillin/clavulanic acid, ceftiofur, cephalothin, cefazolin, cefotaxime and cefoperazone, whereas no change was seen among the MICs for cefquinome (Supplementary Table 1). Growth curves of S. aureus ATCC® 29213™ and its two variants are shown in Figure 4. After a short lag phase, a distinct increase in cell density corresponding to an increase in optical density was noticed for S. aureus ATCC® 29213™. In contrast, growth of SA29213-A and SA29213-B seemed to be impaired as demonstrated by a less steep growth curve and lower cell densities. Figure 4 summarizes average values obtained from two independent growth experiments.
Table 2.
Phenotypic characteristics of S. aureus-derived variants obtained after serial passages in sub-inhibitory NaOCl concentrations.
| Strain | NaOCl concentration (v/v) | Serial passage after | Colony morphology on CSA | Variant | MIC value for NaOCl determined after |
||
|---|---|---|---|---|---|---|---|
| culture on CSA | 1st passage in TSB | 2nd passage in TSB | |||||
| ATCC® 29213TM | 0.005% | 72 h | tiny, yellow, narrow hemolysis | SA29213-A | 0.08% | 0.07% | 0.07% |
| 0.005% | 72 h | tiny, yellow, very narrow hemolysis | SA29213-B | 0.08% | 0.07% | 0.07% | |
| 0.005% | 72 h | medium-sized, lutescent, narrow hemolysis | SA29213-F | 0.08% | 0.09% | 0.08% | |
| 0.0025% | 72 h | small, lutescent, no hemolysis | SA29213-E | 0.08% | 0.08% | 0.07% | |
| 0.0006% | 72 h | convex, yellow, distinct hemolysis | SA29213-C | 0.08% | 0.08% | 0.07% | |
| 0.0025% | 24 h | medium-sized, yellow, narrow hemolysis | SA29213-H | 0.08% | 0.07% | 0.07% | |
| 0.0025% | 24 h | medium-sized, yellow, no hemolysis | SA29213-D | 0.08% | 0.08% | 0.08% | |
| 0.00125% | 24 h | small, yellow, weak hemolysis beneath the colony | SA29213-G | 0.07% | 0.07% | 0.09% | |
| ATCC® 6538TM | 0.01% | 24 h | tiny, yellowish, no hemolysis | SA6538-a | 0.1% | not done | not done |
| 0.005% | 24 h | small, yellow, no hemolysis | SA6538-b | 0.1% | not done | not done | |
| 0.01% | 72 h | small, convex, yellow, no hemolysis | SA6538-c | 0.25% | 0.1% | not done | |
| 0.0025% | 72 h | small, convex, yellowish, distinct hemolysis | SA6538-d | 0.25% | 0.1% | not done | |
At the end of each experiment (i.e. eight times serial subculture after 24 h and two serial subcultures after 72 h of incubation, respectively) subcultures were prepared on CSA from every tube showing bacterial growth. Cultures were grown at 37 °C for 24 h and colony morphology was assessed thereafter. Each colony with a different phenotype was separated on CSA. CSA – Columbia sheep blood agar (7% blood), NaOCl – sodium hypochlorite, MIC – minimum inhibitory concentration, TSB – tryptic soy broth.
Figure 3.
A-C. Results of the penicillin disk diffusion test. A – S. aureus ATCC® 29213™; B – variant SA29213-A; C – variant SA29213-B.
Figure 4.
Growth of S. aureus ATCC® 29213™ (filled circles), SA29213-A (filled triangles), and SA29213-B (open boxes) monitored by determining the optical densities at 595 nm in TSB at 37 °C without shaking. Experiments were performed in duplicate and error bars represent the standard deviation.
S. aureus ATCC® 6538™ grew at concentrations up to 0.01% NaOCl in both experimental setups. Colony morphology also varied (Table 2), but the antimicrobial susceptibility profiles of the four derived variants (Table 1) closely resembled that of the original strain S. aureus ATCC® 6538™. MICs for NaOCl were identical to the values determined initially (0.1 mg/L) with the exception of SA6538-c and SA6538-d. For both, the MIC was 2.5-times higher, but decreased to 0.1% after one passage in TSB (Table 2).
Control experiments performed in TSB did not result in changes of colony morphology for both S. aureus strains, ATCC® 29213™ and ATCC® 6538™. The antimicrobial susceptibility profiles revealed a slight increase in MICs e.g. for levofloxacin or erythromycin (Supplementary Table 2), but overall strains showed resistance patterns identical to the original strains. The MIC determined for NaOCl at the end of the experiments was 0.05% and 0.06%. Detailed results can be taken from Supplementary Table 2.
3.3. Macrorestriction analysis
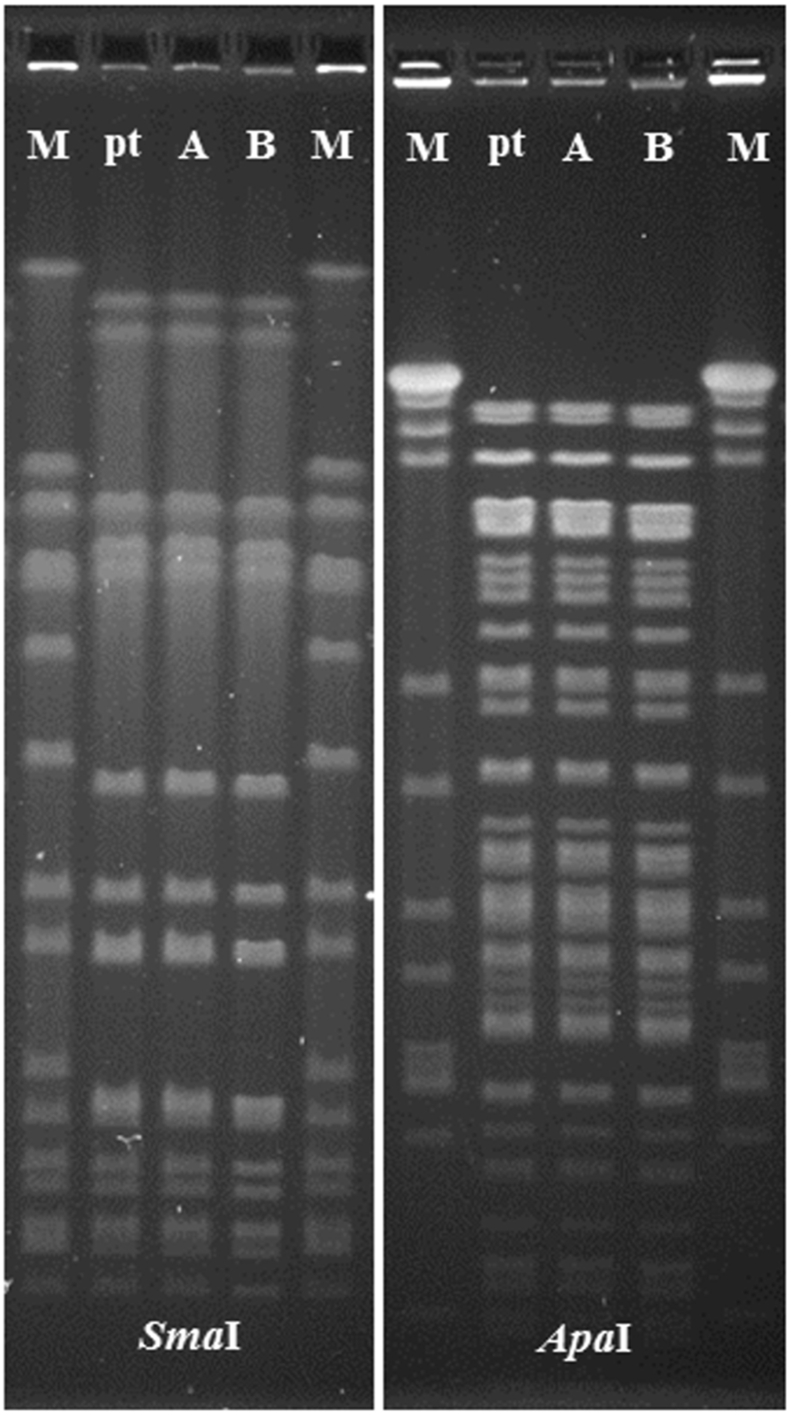
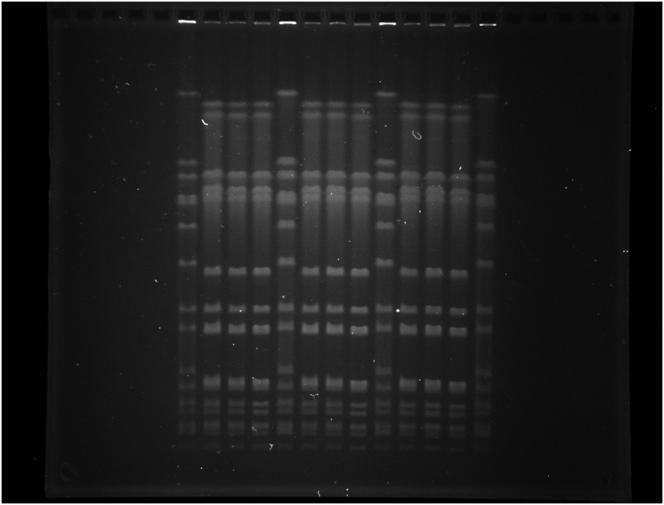
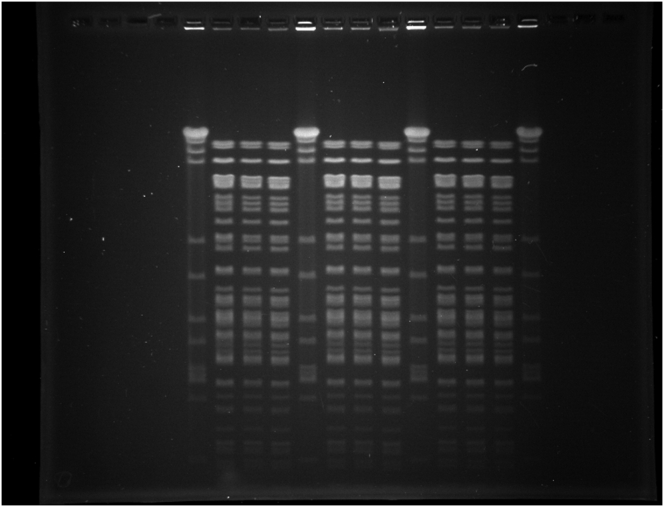
Macrorestriction analysis using SmaI and ApaI revealed indistinguishable pulsed-field gel electrophoresis (PFGE) patterns for S. aureus ATCC® 29213™ and variants SA29213-A and SA29213-B (Figure 5, Supplementary Figure 1 and Supplementary Figure 2).
Figure 5.
SmaI and ApaI fragment patterns obtained after PFGE analysis. M – SmaI-digested S. aureus strain NCTC 8325, pt – S. aureus ATCC® 29213™, A – variant SA29213-A, B – variant SA29213-B. The full, non-adjusted PFGE images of SmaI- and ApaI-digestions can be found as Supplementary Figure 1 (SmaI) and Supplementary Figure 2 (ApaI). Figure 5 was composed of the five lanes taken from the middle part of each gel image.
3.4. Transmission electron microscopy
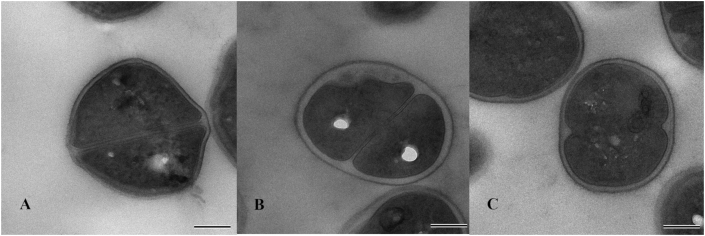
Morphological changes in S. aureus SA29213-A and SA29213-B were examined by TEM and compared to S. aureus ATCC® 29213™. TEM micrographs of representative cells are shown in Figure 6 A-C. The mean cell wall thickness of S. aureus ATCC® 29213™ was 18.96 nm (95% CI: 17.70–20.22). SA29213-A and SA29213-B exhibited a significantly thickened cell wall phenotype of 26.30 nm (95% CI: 25.04–27.56, P = 1.89 × 10−12) and 21.11 nm (95% CI: 19.85–22.37, P = 0.018), respectively.
Figure 6.
A-C. Comparison of cell wall thickness of SA29213-A and SA29213-B with the parental strain S. aureus ATCC® 29213TM. Thin-section micrographs of the following strains are shown: A – S. aureus ATCC® 29213™; B – variant SA29213-A; C – variant SA29213-B. Final magnification x20,000. Scale bar indicates 200 nm.
3.5. Sequence analysis
The analysis of the proteins associated with reduced oxacillin susceptibility revealed no differences in the amino acid (aa) sequences of FemA, FemB, FemC, FemD, MgrA, CcpA and MprF as well as the penicillin binding proteins (PBP) 1, 2, 3 and 4. However, compared to the original strain S. aureus ATCC®29213™, both variants, SA29213-A (bioproject PRJNA613990, acc. no. JAAVNA000000000) and SA29213-B (bioproject PRJNA613990, acc. no. JAAVMZ000000000), exhibited a change from Glutamin (Q) to a stop codon at position 139 in the 656 aa GdpP protein. Regarding the hemolysin proteins HlgA, HlgB, HlgC, Hla and Hld, no aa sequence differences between the original strain and the two variants were observed.
4. Discussion
S. aureus is an important opportunistic pathogen in humans, companion animals, and livestock. It survives on inanimate dry surfaces for months (Kramer et al., 2006), which highlights the necessity of proper surface disinfection measures. Exposure of bacteria to low concentrations of a disinfectant (e.g. inappropriate dilution, remnants of the disinfectant) might rapidly select for decreased susceptibility against this biocide or even to antibiotics (Loughlin et al., 2002; Thomas et al., 2005; SCENIHR, 2010). It has been demonstrated in vitro that a sub-lethal (often sub-inhibitory) biocide concentration decreases bacterial susceptibility to that biocide and modifies the antimicrobial susceptibility profile, but does not necessarily trigger clinical antimicrobial resistance (SCENIHR, 2009; SCENIHR, 2010).
In this study, the effect of sub-inhibitory concentrations of NaOCl on growth characteristics, susceptibility to NaOCl and antibiotic susceptibility patterns of two S. aureus strains was examined. Prolonged treatment with sub-inhibitory concentrations resulted in the development of colonies with noticeable differences in phenotype in that some colonies were smaller, discolored, and with impaired hemolysis. The characteristic color of S. aureus is mediated by carotenoids which are rapidly destroyed by NaOCl (Marshall and Wilmoth, 1981; Siems et al., 1999) resulting in chromogenic variants. Moreover, NaOCl stress has been associated with degradation of chromosomal DNA, alteration of proteins and disruption of the cell membrane (Aboualizadeh et al., 2017; Ujimine et al., 2017). It could be assumed that NaOCl-induced degradation of chromosomal DNA may be related to the morphological changes described here. There was no change in MICs to NaOCl in NaOCl-adapted strains except for two variants obtained from S. aureus ATCC® 6538™ (i.e. SA6538-c and SA6538-d). Both strains showed a 2.5-times decrease in susceptibility to NaOCl but this was reversible by one passage in TSB, suggesting a temporary and not genetically fixed adaptation process. Similar findings have been reported elsewhere (Bansal et al., 2018; Gao and Liu, 2014). Treatment of bacteria with sub-inhibitory concentrations of NaOCl might contribute to the emergence of antibiotic resistance (Molina-González et al., 2014; Obe et al., 2018). This is supported by our findings in that two phenotypic variants of S. aureus ATCC® 29213™, i.e. SA29213-A and SA29213-B, showed in vitro borderline-resistance against oxacillin (MIC of ≥4 mg/L and 2 mg/L). In accordance to others (Molina-González et al., 2014) this phenomenon was strain-dependent as it was not seen in isolates derived from S. aureus strain ATCC® 6538™. It should briefly be mentioned that MIC values for oxacillin determined by VITEK®2 technology distinctly varied from those determined by broth microdilution (Supplementary Table 1). This might be associated with the different testing methods used and has been previously described (Scholtzek et al., 2019). Macrorestriction analysis excluded a laboratory contamination with another S. aureus strain and confirmed the clonality of SA29213-A, SA29213-B, and S. aureus ATCC® 29213™. Borderline-resistant S. aureus (BORSA) are characterized by oxacillin MICs at or just above the susceptibility breakpoint (Chambers, 1997). Several mechanisms have been hypothesized to explain borderline resistance in mecA-negative S. aureus, including hyperproduction of a β-lactamase, amino acid substitutions in penicillin-binding proteins (PBPs), or mutations in regulating genes that lead to PBP4 overproduction (McDougal and Thornsberry, 1986; Tomasz et al., 1989; Argudín et al., 2018). Recently, plasmid-encoded methicillin resistance conferred by mecB has also been described in S. aureus (Becker et al., 2018). S. aureus ATCC® 29213™ is characterized as oxacillin-susceptible and exhibits oxacillin MICs ranging between 0.12 – 0.5 mg/L (CLSI, 2018b). Furthermore, a GenBank database search found no entries for methicillin-resistance genes in S. aureus ATCC® 29213™. Therefore, these genetically determined resistance mechanisms could be excluded in our strains. Another mechanism causing reduced susceptibility to oxacillin is the upregulation of multiple-drug efflux pumps as described for S. aureus after multiple-exposure to benzalkonium chloride (Huet et al., 2008). Unfortunately, this aspect could not further be investigated in our study. Borderline oxacillin resistance can be attributed to β-lactamase hyperproduction (McDougal and Thornsberry, 1986; Maalej et al., 2012; Hryniewicz and Garbacz, 2017; Scholtzek et al., 2019). S. aureus ATCC® 29213™ possesses the blaZ gene (GenBank accession number NZ_MOPB01000048) which encodes a penicillin-hydrolyzing class A β-lactamase. Penicillin agar disk diffusion with the interpretation of the zone edges confirmed the presence and the expression of a β-lactamase, not only in S. aureus ATCC® 29213™, but also in the two derived isolates tested. Interestingly, no differences in the zone diameters were seen between S. aureus ATCC® 29213™, SA29213-A and SA29213-B. Therefore, the expression of the β-lactamase is unlikely to be the reason of the reduced oxacillin susceptibility. As β-lactam antimicrobials interfere with bacterial cell wall synthesis, the decreased susceptibility to oxacillin of SA29213-A and SA29213-B might be associated with substantial reorganization of the staphylococcal cell wall, which was significantly thicker in the two variants as compared to S. aureus ATCC® 29213™. Heterogeneity of the cell wall thickness after NaOCl treatment was also found in TEM observations by Ujimine et al. (2017). Moreover, the decreased oxacillin susceptibility might also be associated with the mutation resulting in an early stop codon in the gene coding for the GdpP protein and thereby abolishing the function of this gene. GdpP represents a phosphodiesterase that regulates gene expression, and loss of function of the GdpP protein has been described in association with borderline oxacillin resistance (Griffiths and O'Neill, 2012). This protein comprises two functional domains: The GGDEF domain containing a diguanylate cyclase, which confers the capacity to synthesize the second nucleotide messenger cyclic di-GMP. The DHH domain harbors the phosphodiesterase characteristic catalytic DHH motif and imparts hydrolysis of cyclic di-AMP, a second messenger molecule which is involved in cell wall synthesis and homeostasis, and is essential for the growth and cell viability of many Gram-positive bacteria including S. aureus (Corrigan et al., 2011; Huynh and Woodward, 2016; Fahmi et al., 2017; Commichau et al., 2018). Impaired growth was also seen in both variants of S. aureus ATCC® 29213™. Cell wall changes after low-level exposure to NaOCl were also described for Listeria monocytogenes, another Gram-positive bacterial species (Gao and Liu, 2014; Bansal et al., 2018). Similar morphological and biological changes, including decreased growth rate, less or no hemolysis, have been described for vancomycin- and teicoplanin-resistant S. aureus, adaptive resistance to amikacin, and treatment of S. aureus with erythromycin and acriflavine (Nishino, 1975; Cui et al., 2003; McCallum et al., 2006; Kawai et al., 2009; Dai et al., 2012; Yuan et al., 2013). Our findings may suggest that a mutation in the gene for the GdpP protein and morphological changes of the cell wall induced by NaOCl most likely accounted for a borderline oxacillin resistance phenotype in the two S. aureus ATCC® 29213™ variants. Survival of such variant strains in certain environmental niches may constitute a possible threat to susceptible populations, particularly in healthcare.
Declarations
Author contribution statement
Stephanie Speck, Cindy Wenke, Andrea T. Feßler, Johannes Kacza, Anissa D. Scholtzek: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Franziska Geber: Performed the experiments; Analyzed and interpreted the data.
Dennis Hanke, Inga Eichhorn: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Stefan Schwarz, Uwe Truyen: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Maciej Rosolowski: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Saxon State Ministry of Social Affairs and Consumer Protection.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are thankful to Mario Reinhardt, Dana Rüster, Christina Speck and Vivian Hensel for their help in the laboratory and for preparing figures. We acknowledge support from the German Research Foundation (DFG) and Leipzig University within the program of Open Access Publishing.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary Figure 1.
Supplementary Figure 2.
References
- Aboualizadeh E., Bumah V.V., Masson-Meyers D.S., Eells J.T., Hirschmugl C.J., Enwemeka C.S. Understanding the antimicrobial activity of selected disinfectants against methicillin-resistant Staphylococcus aureus (MRSA) PloS One. 2017;12(10) doi: 10.1371/journal.pone.0186375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous, Deutsches Institut für Normung e.V., N. M. Beuth Verlag; 1997. DIN 58959-9. Anforderungen an den Einsatz von Kontrollstämmen zur Prüfung von Kulturmedien mit Beiblatt 1. [Google Scholar]
- Anonymous, DIN EN 901:2013-12 . German version; 2013. Chemicals Used for Treatment of Water Intended for Human Consumption - Sodium Hypochlorite. [Google Scholar]
- Anonymous . 2017. Regulation (EU) No 528/2012 Concerning the Making Available on the Market and Use of Biocidal Products. Active Chlorine Released from Sodium Hypochlorite Product-type 3 (Veterinary hygiene)https://echa.europa.eu/documents/10162/cd256d88-480e-3ff8-5aea-8d658d6e24a9 Cited 10 April 2020 January 2017. Available from: [Google Scholar]
- Anonymous, The European Committee on Antimicrobial Susceptibility Testing . 2019. Routine and Extended Internal Quality Control for MIC Determination and Disk Diffusion as Recommended by EUCAST.http://www.eucast.org Version 9.0, 2019. Available from: Cited 23 April 2019. [Google Scholar]
- Anonymous . 2019. DVG Guidelines for MIC.http://www.desinfektion-dvg.de/fileadmin/FG_Desinfektion/Dokumente/Fuer_Gutachter/Pruefrichtlinien/IV_MHK_7Nov20175.pdf Cited Available from: [Google Scholar]
- Argudín M.A., Roisin S., Nienhaus L., Dodémont M., de Mendonça R., Nonhoff C. Genetic diversity among Staphylococcus aureus isolates showing oxacillin and/or cefoxitin resistance not linked to the presence of mec genes. Antimicrob. Agents Chemother. 2018;62(7) doi: 10.1128/AAC.00091-18. e00091-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz R.K., Bartels D., Best A.A., DeJongh M., Disz T., Edwards R.A. The RAST Server: rapid annotations using subsystems technology. BMC Genom. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal M., Nannapaneni R., Sharma C.S., Kiess A. Listeria monocytogenes response to sublethal chlorine induced oxidative stress on homologous and heterologous stress adaptation. Front. Microbiol. 2018;9:2050. doi: 10.3389/fmicb.2018.02050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K., van Alen S., Idelevich E.A., Schleimer N., Seggewiß J., Mellmann A. Plasmid-encoded transferable mecB-mediated methicillin resistance in Staphylococcus aureus. Emerg. Infect. Dis. 2018;24(2):242–248. doi: 10.3201/eid2402.171074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzón-Durán L., Alonso-Calleja C., Riesco-Peláez F., Capita R. Effect of sub-inhibitory concentrations of biocides on the architecture and viability of MRSA biofilms. Food Microbiol. 2017;65:294–301. doi: 10.1016/j.fm.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Chambers H.F. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin. Microbiol. Rev. 1997;10(4):781–791. doi: 10.1128/cmr.10.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J.S. Disinfectant resistance mechanisms, cross-resistance, and co-resistance. Int. Biodeterior. Biodegrad. 2003;51:271–276. [Google Scholar]
- CLSI . 29th ed. vol 39. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2019. Performance Standards for Antimicrobial Susceptibility Testing. CLSI Supplement M100. [Google Scholar]
- CLSI . CLSI Standard VET01. fifth ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2018. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. [Google Scholar]
- CLSI . CLSI Supplement VET08. fourth ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2018. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. [Google Scholar]
- Commichau F.M., Heidemann J.L., Ficner R., Stülke J. Making and breaking of an essential poison: the cyclases and phosphodiesterases that produce and degrade the essential second messenger cyclic di-AMP in bacteria. J. Bacteriol. 2018;201 doi: 10.1128/JB.00462-18. e00462-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan R.M., Abbott J.C., Burhenne H., Kaever V., Gründling A. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog. 2011;7(9) doi: 10.1371/journal.ppat.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L., Ma X., Sato K., Okuma K., Tenover F.C., Mamizuka E.M. Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J. Clin. Microbiol. 2003;41(1):5–14. doi: 10.1128/JCM.41.1.5-14.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y., Zhou X., Ma X., Lu H., Li H. Misidentification of vancomycin-resistant Staphylococcus aureus as coagulase-negative Staphylococcus. J. Med. Microbiol. 2012;61(10):1454–1458. doi: 10.1099/jmm.0.045518-0. [DOI] [PubMed] [Google Scholar]
- Fahmi T., Port G.C., Cho K.H. c-di-AMP: an essential molecule in the signaling pathways that regulate the viability and virulence of Gram-positive bacteria. Genes (Basel) 2017;8(8):E197. doi: 10.3390/genes8080197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Liu C. Biochemical and morphological alteration of Listeria monocytogenes under environmental stress caused by chloramine-T and sodium hypochlorite. Food Contr. 2014;46:455–461. [Google Scholar]
- Griffiths J.M., O'Neill A.J. Loss of function of the GdpP protein leads to joint β-lactam/glycopeptide tolerance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2012;56:579–581. doi: 10.1128/AAC.05148-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet A.A., Raygada J.L., Mendiratta K., Seo S.M., Kaatz G.W. Multidrug efflux pump overexpression in Staphylococcus aureus after single and multiple in vitro exposures to biocides and dyes. Microbiology. 2008;154(Pt 10):3144–3153. doi: 10.1099/mic.0.2008/021188-0. [DOI] [PubMed] [Google Scholar]
- Huynh T.N., Woodward J.J. Too much of a good thing: regulated depletion of c-di-AMP in the bacterial cytoplasm. Curr. Opin. Microbiol. 2016;30:22–29. doi: 10.1016/j.mib.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryniewicz M.M., Garbacz K. Borderline oxacillin-resistant Staphylococcus aureus (BORSA -A more common problem than expected? J. Med. Microbiol. 2017;66:1367–1373. doi: 10.1099/jmm.0.000585. [DOI] [PubMed] [Google Scholar]
- Jutkina J., Marathe N.P., Flach C.F., Larsson D.G.J. Antibiotics and common antibacterial biocides stimulate horizontal transfer of resistance at low concentrations. Sci. Total Environ. 2018;616–617:172–178. doi: 10.1016/j.scitotenv.2017.10.312. [DOI] [PubMed] [Google Scholar]
- Kadariya J., Smith T.C., Thapaliya D. Staphylococcus aureus and staphylococcal food-borne disease: an ongoing challenge in public health. BioMed Res. Int. 2014;2014:827965. doi: 10.1155/2014/827965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadlec K., Ehricht R., Monecke S., Steinacker U., Kaspar H., Mankertz J. Diversity of antimicrobial resistance pheno- and genotypes of methicillin-resistant Staphylococcus aureus ST398 from diseased swine. J. Antimicrob. Chemother. 2009;64(6):1156–1164. doi: 10.1093/jac/dkp350. [DOI] [PubMed] [Google Scholar]
- Kampf G. Springer Nature Switzerland AG; Cham, Switzerland: 2018. Antiseptic Stewardship; p. 694. [Google Scholar]
- Karatzas K.A., Webber M.A., Jorgensen F., Woodward M.J., Piddock L.J., Humphrey T.J. Prolonged treatment of Salmonella enterica serovar Typhimurium with commercial disinfectants selects for multiple antibiotic resistance, increased efflux and reduced invasiveness. J. Antimicrob. Chemother. 2007;60(5):947–955. doi: 10.1093/jac/dkm314. [DOI] [PubMed] [Google Scholar]
- Kawai M., Yamada S., Ishidoshiro A., Oyamada Y., Ito H., Yamagishi J. Cell-wall thickness: possible mechanism of acriflavine resistance in methicillin-resistant Staphylococcus aureus. J. Med. Microbiol. 2009;58(Pt 3):331–336. doi: 10.1099/jmm.0.004184-0. [DOI] [PubMed] [Google Scholar]
- Köhler A.T., Rodloff A.C., Labahn M., Reinhardt M., Truyen U., Speck S. Efficacy of sodium hypochlorite against multidrug-resistant Gram-negative bacteria. J. Hosp. Infect. 2018 Nov;100(3):e40–e46. doi: 10.1016/j.jhin.2018.07.017. [DOI] [PubMed] [Google Scholar]
- Kramer A., Schwebke I., Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumaningrum H.D., Paltinaite R., Koomen A.J., Hazeleger W.C., Rombouts F.M., Beumer R.R. Tolerance of Salmonella Enteritidis and Staphylococcus aureus to surface cleaning and household bleach. J. Food Protect. 2003;66(12):2289–2295. doi: 10.4315/0362-028x-66.12.2289. [DOI] [PubMed] [Google Scholar]
- Li X.-Z., Nikaido H., Williams K.E. Silver-resistant mutants of Escherichia coli display active efflux of Ag+ and are deficient in porins. J. Bacteriol. 1997;179:6127–6132. doi: 10.1128/jb.179.19.6127-6132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin M.F., Jones M.V., Lambert P.A. Pseudomonas aeruginosa cells adapted to benzalkonium chloride show resistance to other membrane-active agents but not to clinically relevant antibiotics. J. Antimicrob. Chemother. 2002;49(4):631–639. doi: 10.1093/jac/49.4.631. [DOI] [PubMed] [Google Scholar]
- Maalej S.M., Rhimi F.M., Fines M., Mnif B., Leclercq R., Hammami A. Analysis of borderline oxacillin-resistant Staphylococcus aureus (BORSA) strains isolated in Tunisia. J. Clin. Microbiol. 2012;50(10):3345–3348. doi: 10.1128/JCM.01354-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J.H., Wilmoth G.J. Pigments of Staphylococcus aureus, a series of triterpenoid carotenoids. J. Bacteriol. 1981 Sep;147(3):900–913. doi: 10.1128/jb.147.3.900-913.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum N., Karauzum H., Getzmann R., Bischoff M., Majcherczyk P., Berger-Bächi B. In vivo survival of teicoplanin-resistant Staphylococcus aureus and fitness cost of teicoplanin resistance. Antimicrob. Agents Chemother. 2006;50(7):2352–2360. doi: 10.1128/AAC.00073-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal L.K., Thornsberry C. The role of beta-lactamase in staphylococcal resistance to penicillinase-resistant penicillins and cephalosporins. J. Clin. Microbiol. 1986;23(5):832–839. doi: 10.1128/jcm.23.5.832-839.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B., Cookson B. Does microbial resistance or adaptation to biocides create a hazard in infection prevention and control? J. Hosp. Infect. 2010;76(3):200–205. doi: 10.1016/j.jhin.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Molina-González D., Alonso-Calleja C., Alonso-Hernando A., Capita R. Effect of sub-lethal concentrations of biocides on the susceptibility to antibiotics of multi-drug resistant Salmonella enterica strains. Food Contr. 2014;40:329–334. [Google Scholar]
- Murchan S., Kaufmann M.E., Deplano A., de Ryck R., Struelens M., Elsberg Zinn C. Harmonization of pulsed-field gel electrophoresis protocols for epidemiological typing of strains of methicillin-resistant Staphylococcus aureus: a single approach developed by consensus in 10 European laboratories and its application for tracing the spread of related strains. J. Clin. Microbiol. 2003;41(4):1574–1585. doi: 10.1128/JCM.41.4.1574-1585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino T. An electron microscopic study of antagonism between cephalexin and erythromycin in Staphylococcus aureus. Jpn. J. Microbiol. 1975;19:53–63. doi: 10.1111/j.1348-0421.1975.tb00847.x. [DOI] [PubMed] [Google Scholar]
- Nurk S., Bankevich A., Antipov D., Gurevich A., Korobeynikov A., Lapidus A. Assembling genomes and mini-metagenomes from highly 800 chimeric reads. J. Comput. Biol. 2013;20:714–737. doi: 10.1089/cmb.2013.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obe T., Nannapaneni R., Sharma C.S., Kiess A. Homologous stress adaptation, antibiotic resistance, and biofilm forming ability of Salmonella enterica serovar Heidelberg ATCC8326 on different food-contact surfaces following exposure to sublethal chlorine concentrations. Poultry Sci. 2018;97(3):951–961. doi: 10.3382/ps/pex346. [DOI] [PubMed] [Google Scholar]
- Pereira S.S., Oliveira H.M., Turrini R.N., Lacerda R.A. Disinfection with sodium hypochlorite in hospital environmental surfaces in the reduction of contamination and infection prevention: a systematic review. Rev. Esc. Enferm. USP. 2015;49(4):681–688. doi: 10.1590/S0080-623420150000400020. [DOI] [PubMed] [Google Scholar]
- Randall L.P., Cooles S.W., Coldham N.G., Penuela E.G., Mott A.C., Woodward M.J. Commonly used farm disinfectants can select for mutant Salmonella enterica serovar Typhimurium with decreased susceptibility to biocides and antibiotics without compromising virulence. J. Antimicrob. Chemother. 2007;60(6):1273–1280. doi: 10.1093/jac/dkm359. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2019. R: A Language and Environment for Statistical Computing.https://www.R-project.org Available from: Cited 11 June 2019. [Google Scholar]
- Russell A.D. Mechanisms of bacterial insusceptibility to biocides. Am. J. Infect. Contr. 2001;29:259–261. doi: 10.1067/mic.2001.115671. [DOI] [PubMed] [Google Scholar]
- Russell A.D. Bacterial adaptation and resistance to antiseptics, disinfectants and preservatives is not a new phenomenon. J. Hosp. Infect. 2004;57(2):97–104. doi: 10.1016/j.jhin.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Rutala W.A., Weber D.J. Uses of inorganic hypochlorite (bleach) in health-care facilities. Clin. Microbiol. Rev. 1997;10(4):597–610. doi: 10.1128/cmr.10.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks) 19 January 2009. Assessment of the Antibiotic Resistance Effects of Biocides.http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_021.pdf Available from: Cited 13 May 2019. [Google Scholar]
- SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks) 17 March 2010. Research Strategy to Address the Knowledge Gaps on the Antimicrobial Resistance Effects of Biocides.http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_028.pdf Available from: Cited 13 May 2019. [Google Scholar]
- Scholtzek A.D., Hanke D., Walther B., Eichhorn I., Stöckle S.D., Klein K.S. Molecular characterization of equine Staphylococcus aureus isolates exhibiting reduced oxacillin susceptibility. Toxins (Basel) 2019;11(9):E535. doi: 10.3390/toxins11090535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siems W.G., Sommerburg O., van Kuijk F.J. Lycopene and beta-carotene decompose more rapidly than lutein and zeaxanthin upon exposure to various pro-oxidants in vitro. Biofactors. 1999;10(2-3):105–113. doi: 10.1002/biof.5520100204. [DOI] [PubMed] [Google Scholar]
- Thomas L., Maillard J.Y., Lambert R.J., Russell A.D. Development of resistance to chlorhexidine diacetate in Pseudomonas aeruginosa and the effect of a "residual" concentration. J. Hosp. Infect. 2000;46(4):297–303. doi: 10.1053/jhin.2000.0851. [DOI] [PubMed] [Google Scholar]
- Thomas L., Russell A.D., Maillard J.Y. Antimicrobial activity of chlorhexidine diacetate and benzalkonium chloride against Pseudomonas aeruginosa and its response to biocide residues. J. Appl. Microbiol. 2005;98(3):533–543. doi: 10.1111/j.1365-2672.2004.02402.x. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Drugeon H.B., de Lencastre H.M., Jabes D., McDougall L., Bille J. New mechanism for methicillin resistance in Staphylococcus aureus: clinical isolates that lack the PBP 2a gene and contain normal penicillin-binding proteins with modified penicillin-binding capacity. Antimicrob. Agents Chemother. 1989;33(11):1869–1874. doi: 10.1128/aac.33.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujimine S., Tone S., Saito M., Yamada S. Intracellular morphological changes in Staphylococcus aureus induced by treatment with sodium hypochlorite. Med. Mol. Morphol. 2017;50(3):178–184. doi: 10.1007/s00795-017-0159-6. [DOI] [PubMed] [Google Scholar]
- Yuan W., Hu Q., Cheng H., Shang W., Liu N., Hua Z. Cell wall thickening is associated with adaptive resistance to amikacin in methicillin-resistant Staphylococcus aureus clinical isolates. J. Antimicrob. Chemother. 2013;68(5):1089–1096. doi: 10.1093/jac/dks522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.