Abstract
Background
Small viral reservoirs are found predominantly in HIV-1 controllers and individuals treated during acute/early HIV-1 infection. However, other HIV+ individuals could naturally also harbour low viral reservoirs.
Methods
We screened 451 HIV-1-infected treated-individuals with suppressed plasma viremia for at least 3 years and stored cryopreserved peripheral blood mononuclear cells (PBMCs). Total HIV-DNA was analysed in PBMCs with ddPCR. Individuals with <50 HIV-DNA copies/106 PBMCs constitute the ‘Low Viral Reservoir Treated’ cohort (LoViReT). Longitudinal samples were obtained from 12 chronically treated LoViReT and compared to 13 controls (>50 HIV-DNA copies/106 PBMCs) to analyse total HIV-DNA, T-cell and NK-cell populations, HIV-1 specific antibodies, and plasma inflammation markers.
Findings
We found that 9.3% of the individuals screened had <50 HIV-DNA copies/106 PBMCs. At least 66% initiated cART during the chronic phase of HIV-1 infection (cp-LoViReT). Cp-LoViReT harboured lower levels of HIV-DNA before cART and after treatment introduction the decays were greater compared to controls. They displayed a marked decline in quantity and avidity in HIV-specific antibodies after initiation of cART. Cp-LoViReT had fewer CD8+ TTM and TEMRA in the absence of cART, and higher CD8+ TN after 18 months on therapy.
Interpretation
Treated chronically HIV-1-infected LoViReT represent a new phenotype of individuals characterized by an intrinsically reduced viral reservoir, less impaired CD8+ T-cell compartment before cART, and low circulating HIV-1 antigens despite being treated in the chronic phase of infection. The identification of this unique group of individuals is of great interest for the design of future eradication studies.
Funding
MSD Spain
Keywords: HIV reservoir, HIV latency, total HIV-DNA, immunophenotyping, HIV-specific antibodies
PANEL 1: RESEARCH IN CONTEXT.
Evidence before this study
We searched PubMed using the terms “HIV reservoir”, “HIV DNA”, or “HIV latency”, and “low”, with no restrictions by date or language, for studies that assess HIV-1-infected individuals with a very low reservoir. We found evidence of low viral reservoirs, mostly in individuals treated during acute/early infection, elite controllers and post-treatment controllers. However, we also found 4 studies that described treated chronically infected individuals with a low HIV reservoir and correlated low viral reservoirs with clinical parameters and/or viral rebound if therapy is interrupted.
Added value of this study
We demonstrate that 9% of HIV-1-infected individuals can harbour low levels of HIV-DNA (LoViReT individuals), with 66% of them treated during the chronic phase of infection. Ours is the first study to assess the kinetics of reservoirs in chronically HIV-1-infected individuals harbouring low levels of total HIV-1 DNA. We found a combination of intrinsically low reservoir with enhanced decay of latency after initiation of treatment. In addition, the LoViReT individuals had a less impaired CD8+ T compartment before initiation of treatment and lower amounts of circulating antigens after 5 years on treatment.
Implications of all the available evidence
Our results reveal a novel phenotype of chronically treated individuals characterized by a naturally reduced and more ART-sensitive viral reservoir. Therefore, LoViReT individuals are of particular interest when designing approaches to eradicate HIV, since their smaller reservoir and other characteristics might represent an advantage over individuals with a larger reservoir. Further studies on the host and viral parameters of these individuals are warranted to identify factors that are potentially involved in these phenomena. Such studies should contribute to the development of more rational HIV remission strategies based on novel concepts.
Alt-text: Unlabelled box
1. Introduction
The implementation of combination antiretroviral therapy (cART) to treat HIV-1 infection has substantially improved the life expectancy of HIV-1-infected individuals. However, cART does not completely eliminate HIV-1, which persists as a latent infection mainly in resting CD4+ T cells, thus leading to rapid relapse of viremia if therapy is interrupted [1]. Consequently, there is emerging interest in developing safe and affordable curative strategies to eliminate the need for lifelong therapy while improving the health of people living with HIV and reducing the risk of viral transmission to uninfected individuals [2], [3], [4].
A wide variety of strategies currently focus on eradicating HIV-1 with the aim of reducing the latent viral reservoir to undetectable levels [5]. Therefore, factors related to the size, distribution, and stability of the viral reservoir are continuously being investigated. It has been postulated that the amount of HIV-1 DNA is a predictor of disease progression in primary infection [6] and during the natural course of HIV-1 infection [7]. Lower levels of HIV-1 DNA have been observed, mostly in elite controllers, who spontaneously control viral replication [8], as well as in post-treatment controllers [9], and allogeneic stem cell transplant recipients [10], [11], [12], [13].
Various studies suggest that early initiation of cART is an important factor in reducing the size of the viral reservoir [14,15], especially if initiated at Fiebig stage I [16]. Unfortunately, individuals are rarely treated during the acute phase, since most new diagnoses of HIV-1 infection are made at the chronic stage, when the reservoirs are more stable [17]. Eradication strategies need to be effective in the vast majority of treated chronically HIV-1-infected individuals. Several studies have described treated chronically infected individuals with low or even undetectable levels of total HIV-1 DNA [18], [19], [20]. However, no retrospective data have been reported on the joint proportion of individuals who achieve a low reservoir after initiation of treatment in both the acute and the chronic phases. Furthermore, the factors involved in achieving these low latency levels have not been investigated in depth.
In this study, we screened the total HIV-1 DNA reservoir in 451 treated HIV-1-infected individuals with suppressed plasma viremia for at least 3 years and stored cryopreserved peripheral blood mononuclear cells (PBMCs) to establish the Low Viral Reservoir Treated cohort (LoViReT). We aimed to study the kinetics of these decreased reservoirs and to analyse associated clinical and immunological factors. To do so, we focused on a subset of LoViReT individuals who initiated treatment in the chronic phase of the infection (cp-LoViReT) in order to identify strategies that could be applied in the vast majority of treated HIV-1-infected individuals.
2. Methods
2.1. Study participants
We retrospectively screened 451 HIV-1-infected subjects undergoing regular follow-up at Hospital Germans Trias i Pujol (n=319) and Hospital Clinic (n=132) in Barcelona. We included individuals under suppressive cART with undetectable viremia (HIV-RNA <50 copies/ml) for at least 3 years and with available cryopreserved PBMCs. Demographic and clinical data were collected from the clinical database. The characteristics of the study participants were similar to those of previously reported cohorts of individuals with low HIV reservoirs in terms of sex, age, and time with infection [18], [19], [20]. Treated chronically infected participants were defined as individuals with more than 6 months between acquisition of HIV-1 and initiation of treatment. All subjects provided their signed informed consent to participate in the study. The study was approved by the Ethics Committee at both recruiting hospitals (reference #: PI-014-083).
2.2. Quantification of proviral HIV-1 DNA
The size of the proviral reservoir was measured in PBMCs for screening and in purified CD4+ peripheral T cells for the longitudinal analysis (Miltenyi Biotech) by droplet digital polymerase chain reaction (ddPCR), as previously reported, without modifications [21]. Cells were resuspended in lysis buffer at a concentration of 5 × 104 cells/µl, which consisted of UltraPure® DNAse-RNAse–free water (Gibco, Invitrogen) containing 10 mM Tris-HCl (pH=9•0), 0•1% Triton x-100 (Sigma), and 400 μg/ml Proteinase K (Ambion). Two different primer sets per subject (5′LTR and Gag [21]) were used to circumvent potential mismatches in the viral sequences that could prevent efficient amplification. In addition, the RPP30 [21] housekeeping gene was measured in parallel to normalize sample input. PBMCs from HIV-negative donors were used as negative controls and assayed in each plate to set the positive/negative threshold. The number of those negative control wells was the same as that of the replicates for each sample. Raw ddPCR data were analysed using the QX100™ Droplet Reader and the software application QuantaSoft v.1.6 (Bio-Rad, Hercules, CA, USA).
2.3. Viral tropism
Viral coreceptor tropism was determined on proviral DNA by sequencing of HIV-1 gp120 hypervariable region 3 (V3). Tropism was predicted using the Geno2Pheno algorithm (GENAFOR, Bonn, Germany), with a false-positive rate (FPR) of 10% (non–R5-tropism: FPR ≤10%) [22].
2.4. Immunophenotyping and activation markers in peripheral CD4+, CD8+, and NK cells
For T-cell immunophenotyping, cells were incubated with Fixable Viability Stain 780 (APC H7), CD3 (SK7), CD4 (RPA-T4), and CD8 (SK1) monoclonal antibodies. T-cell maturation was based on the expression of CD45RA (HI100), and CD197/CCR7 (G043H7) was analysed in cryopreserved PBMCs in order to define naïve (CD45RA+CCR7+, TN), central memory (CD45RA–CCR7+, TCM), effector (CD45RA–CCR7–, TEF), and effector memory RA+ cells (CD45RA+CCR7–, TEMRA). CD27 (O323) was used to differentiate effector cells into effector memory cells (CD27–CD4+ or CD27+CD8+, TEM), and transitional memory cells (CD27+CD4+ or CD27–CD8+, TTM). CD4 and CD8 T cells were also analysed for expression of HLA-DR (L243) and CD38 (HIT2) to define activated cells (HLA-DR+CD38+); expression of CD279/PD-1 (EG12.2H7) was analysed to measure exhausted cells (CD279+). TIM-3 (F38-2E2), LAG-3 (11C3C65), CD32 (FUN-2), and CD2 (RPA-2.10) markers were also measured in CD4+ T cells as potential surrogate markers of the viral reservoir through analysis of CD4+TIM-3+ CD4+LAG3+, CD4+CD32+, CD4+CD32bright (to avoid potential B-cell contamination), and CD4+CD2bright cells. The gating strategy is shown in Suppl. Fig. 2.
For NK immunophenotyping, cells were incubated with the appropriate monoclonal antibodies and Zombie Aqua™ for 30 min at 4°C, washed, and fixed with 4% PFA. The following monoclonal antibodies were used for surface staining: CD3 (UCHT1), CD14 (M5E2), and CD19 (SJ25C1) for the lineage negative cells and CD45 (HI30), CD16 (3G8), CD56 (NCAM16.2), CD69 (FN50), NKp46/CD335 (9E2), NKP30/CD337 (AF29-4D12), NKG2A/CD159a (REA110), NKG2C/CD159c (134591), NKB1 (DX9), KIR2D/CD158a (NKVFS1), KIR3DL1/DL2/CD158e/k (REA970), CD161(DX12), and CXCR5/CD185 (MU5UBEE) for NK cell phenotyping as previously reported [23].
Samples were acquired in a BD LSRFortessa or LSRII flow cytometer (BD Bioscience) and analysed using FlowJo software (Tree Star). T cells were analysed with automated detection of marker cutoffs based on fluorescence-minus-one controls (OurFlow platform using R packages).
2.5. Viral inhibition by CD8+ T and NK cells
PBMCs were thawed and cultured overnight in RPMI 1640 containing GlutaMAX, 20% FCS, penicillin (10 IU/ml), and streptomycin (10 μg/ml). Analyses were performed as previously described [24]. Briefly, CD4+ T cells were purified by positive selection with antibody-coated magnetic beads (EasySep Human CD4 Positive Selection, StemCell Technologies). The elution containing CD4-depleted PBMCs was split and used for purification of CD8+ T-cells and NK cells by negative selection (EasySep Human CD8+ Cell Enrichment, EasySep Human NK Enrichment, StemCell Tecnhologies). Cells were separated using a RoboSep instrument (Stemcell Technology).
CD4+ T cells were activated for 3 days in the presence of 4 μg/ml PHA-L (Roche) and 100 IU/ml IL-2 (Human IL-2 IS, premium grade, Miltenyi Biotech). During this period, NK cells were cultured in the presence of IL-15 at 0•1 ng/ml and CD8+ T cells in the absence of cytokines. After 3 days of culture, living activated CD4+ T cells were seeded in a 96-U-well plate (106 cells/ml in triplicate) alone or in the presence of CD8+ T cells or NK cells (1:1 ratio) and then exposed to HIV-1 BaL (CCR5 tropic strain) (10 ng p24/ml). After spinoculation (1200 x g for 1 h at room temperature), cells were cultured for 1 h at 37°C and washed before culture in the presence of IL-2 (100 IU/ml). Culture supernatants were removed, and the media culture was replenished at day 3. Levels of p24 in culture supernatants were analysed at day 3 after infection for co-culture with NK cells and at day 7 after infection for co-cultures with CD8+ T-cells using an ELISA p24 assay (HIV-1 p24 ELISA kit, XpressBio).
2.6. Quantification of inflammation biomarkers
Concentrations of the pro-inflammatory or homeostatic cytokines IL-2, IL-6, IL-7, IL-10, IL-27, and IP10, as well as the coagulation biomarker D-dimer, were quantified in plasma using a bead-based multiplex immunoassay (ProcartaPlex, eBioscience) according to the manufacturer's recommendations. Measurements were performed using a Luminex 100 instrument (Luminex Corp., Austin, TX, USA) and analysed using a standard curve for each cytokine.
2.7. HIV antibody quantification
Specific HIV-1 antibodies were measured in plasma samples using the HIV-1/2 VITROS assay (Ortho Clinical Diagnostics, Rochester, NY, USA), a low sensitive (LS) version of the VITROS anti-HIV-1 assay, and a limiting antigen avidity assay (Lag-Avidity) (Sedia, Portland, OR, USA), as previously described [25].
2.8. Statistical analysis
The clinical characteristics of the study population were presented as percentages for categorical variables and as median and interquartile range (IQR) for continuous variables. The association between clinical parameters with low levels of cell-associated total HIV-1 DNA were analysed using univariate and multivariate logistic regression models; the odds ratio (OR), p-value, and C-index are reported. Variables associated with the LoViReT status (p<0•1) were considered candidates for the final model. The multivariate model was built using a stepwise procedure and by selecting the variables associated with LoViReT. The parameters assessed include gender, age at diagnosis, mode of infection, AIDS events, maximum viral load reported, CD4 nadir, time since HIV-1 diagnosis, viral blips (<500 copies/ml), detectable plasma viral load (pVL; any value above limit of detection), time with detectable pVL, tropism, and the accumulated pVL score calculated as the area of the positive pVL plot over time and normalized (divided) by the follow-up time. The model was validated internally using a 10‐fold cross‐validation, and predictive ability was assessed using the C-index (equivalent to the AUC under the ROC curve).
In the longitudinal analysis, clinical differences between groups were assessed using the Mann-Whitney test in the case of continuous variables and the Fisher exact test in the case of categorical variables. Clinical variables were matched between LoViReT subjects and controls using the random forest algorithm in order to avoid confounding factors associated with low HIV-1 DNA levels. Random forest [26] is a popular machine learning algorithm based on ensembles of Classification and Regression Trees (CART) algorithm (regression and classification trees), which provide good predictive performance and low overfitting. In addition to the prediction model, the algorithm provides a proximity measure matrix between observations that can be used in matching [27]. In order to produce balanced treatment and control groups in terms of all relevant clinical factors, we selected a set of controls based on the RF proximity measure. Differences within groups were assessed using the Wilcoxon signed-rank test.
The analyses were performed with R (v3.4) and GraphPad (v5.01).
3. Results
3.1. Characteristics of the screening population
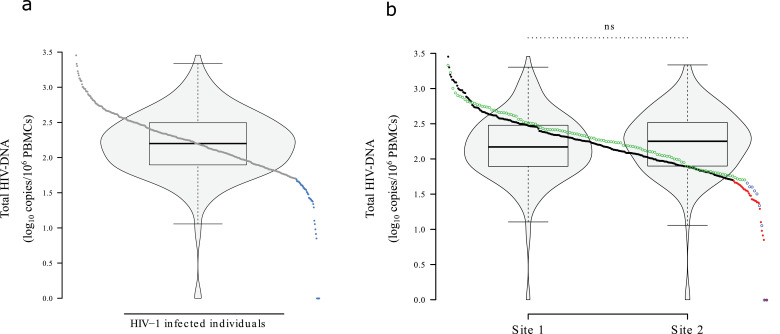
We analysed samples from 451 individuals with more than 3 years under suppressive cART (Suppl. Fig. 1). Their characteristics are summarized in Table 1. Log10 proviral HIV-1 DNA in PBMCs was normally distributed (Fig. 1a). Median HIV-1 DNA was 158•5 copies/106 PBMCs (IQR, 79-313).
Table 1.
Clinical characteristics of the subjects included in the study
| Characteristic | N (%) | Median [IQR] |
|---|---|---|
| Male sex | 377 (83•6) | |
| Region of origin | ||
| Spain | 236 (81) | |
| Europe | 17 (6) | |
| America | 34 (12) | |
| Africa | 4 (1) | |
| Age at diagnosis | 31 [27-37] | |
| Mode of infection | ||
| MSM | 252 (58) | |
| Heterosexual | 87 (20) | |
| Injecting drug use | 55 (13) | |
| Other | 40 (9) | |
| AIDS events | ||
| Yes | 46 (10) | |
| No | 405 (90) | |
| Zenith viral load (log10 copies/ml plasma) | 4•9 [4•3-5•3] | |
| CD4 nadir (cells/µl) | 273 [159-358] | |
| Time since HIV diagnosis (years) | 13 [7-19] | |
| Viral blips | 0 [0-1] | |
| Virological failures | 0 [0-2] | |
| Tropism | ||
| R5-tropic | 329 (73) | |
| Non-R5 or dual | 93 (21) | |
| Viral subtype | ||
| B | 403 (96) | |
| Non B | 19(4) | |
| Total HIV-1 DNA (copies/106 PBMCs) | 158•5 [78•7-313•4] | |
| At the time of HIV-1 DNA measurements | ||
| Age | 46 [41-51] | |
| Time suppressed (years) | 5•6 [4-8] | |
| CD4 T cells (cells/µl) | 676 [495-891] | |
| CD8 T cells (cells/µl) | 766 [567-1022] | |
| CD4/CD8 ratio | 0.9 [0•6-1•2] |
Fig. 1.
Total HIV-1 DNA.
(a) Total HIV-1 DNA of 451 individuals after screening with ddPCR. Subjects with <50 copies/106 PBMCs are shown in light blue. (b) Comparative distribution of total HIV-1 DNA between the 2 recruiting centres. Subjects with <50 copies/106 PBMCs are shown in blue and red respectively to the each site. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
A total of 42 individuals were under the 10th percentile (<50 HIV-1 DNA copies/106 PBMC) (9•3%), including 4 (0•9%) who had levels below the limit of detection for the 2 sets of primers used. They were all included in the “LoViReT” (Low Viral Reservoir Treated Individuals) Cohort. The distribution of total HIV-1 DNA and proportion of LoViReT individuals were similar between the 2 recruiting centres (Fig. 1b). The clinical histories of LoViReT subjects confirmed that 28 individuals were treated in the chronic phase of the infection (>6 months since acquisition) and were defined as chronic phase LoViReT subjects (cp-LoViReT). Out of the remaining LoViReT subjects, 6 were treated in the acute stage, and 8 lacked proven information of their time with infection at initiation of cART (Suppl. Fig. 1).
3.2. Factors related to LoViReT status
Clinical, immunological, and virological data were collected to search for factors predicting whether the subject would belong to the LoViReT cohort (Table 2).
Table 2.
Clinical associations with low levels of total HIV-1 DNA (LoViReT status)
| Clinical variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR [95% CI] | p-value& | OR [95% CI] | p-value& | |
| Gender (Male) | 0•981 | |||
| Age at diagnosis | 0•642 | |||
| Mode of infection | 0•782 | |||
| AIDS events | 0•749 | |||
| Maximum viral load reported log10 | 0•67 [0•51-0•89] | 0•007 | 0•66 [0•49-0•89] | 0•0069 |
| CD4 nadir (multiples of 100) | 1•47 [1•20-1•81] | <0•001 | 1•46 [1•19-1•81] | 0•0003 |
| Time since HIV diagnosis | 0•177 | |||
| Viral blips (<500 copies/ml) | 0•64 [0•34-1•05] | 0•083 | ||
| Detectable pVL (>50 copies/ml) | 0•72 [0•55-0•90] | 0•002 | 0•79 [0•60-0•98] | 0•0286 |
| Time with detectable pVL (years) | 0•76 [0•58-0•94] | 0•008 | ||
| Tropism | 0•394 | |||
| Accumulated viral load score | 0•78 [0•62-0•95] | 0.013 | ||
| At the time of HIV-1 DNA measurements | ||||
| Age | 0•531 | |||
| Time suppressed (years) | 0•902 | |||
| CD4 T cells (multiples of 100) | 0•648 | |||
| CD8 T cells (multiples of 100) | 0•246 | |||
| % CD4 T cells | 0•344 | |||
| % CD8 T cells | 0•262 | |||
| CD4/CD8 ratio | 0•217 | |||
p-values based on likelihood ratio test
In the univariate analysis, being in the LoViReT cohort was associated with a lower maximal reported pVL (p=0•0065), higher CD4+ T-cell nadir (p=0•002), lower number of viral blips (p=0•08), fewer detectable pVLs defined as any pVL >50 copies/ml (p=0•0021), and lower time with detectable pVL (p=0•0078) (Table 2). In the multivariate regression model, only a lower maximal reported pVL, higher CD4+ nadir, and fewer detectable pVLs remained significantly associated with LoViReT status (Table 2). Using the c-index method to evaluate the predictive capacity of the multivariate model, we observed a goodness of fit of 0•72 after cross-validation. This is considered a moderate predictive capacity.
Thus, LoViReT individuals seemed to have lower peaks of pVL, a higher CD4+ T-cell nadir, and fewer detectable pVLs during their clinical follow-up. However, this multivariate model had limited predictive capacity.
Longitudinal analysis of HIV-1 DNA reservoirs: For further analyses, we focused on the cp-LoViReT population, since this cohort is less well characterized (Suppl. Fig.1). Thus, we aimed to unravel whether the cp-LoViReT reservoir decreased as a consequence of cART or whether these subjects had lower reservoirs before initiation of cART. According to sample availability, we selected a subgroup of 12 cp-LoViReT and compared them with 13 treated chronically infected matched controls (>50 HIV-1 DNA copies/106 PBMCs) to perform a retrospective longitudinal reservoir analysis in purified CD4+ T cells. We previously ensured that there were no differences in clinical parameters between cp-LoViReT and controls (Suppl. Table 1).
Total HIV-1 DNA was measured before cART and in several samples after initiation of cART (median: 4 [IQR 4-5] samples per individual) (Fig. 2a). All participants had detectable HIV-1 DNA before therapy, thus minimizing technical primer-mismatch hybridization issues. Fig. 2a shows the dynamics of total HIV-1 DNA in all the samples analysed. We observed a phase of faster decay during the first 18 months on cART, followed by a slower decay.
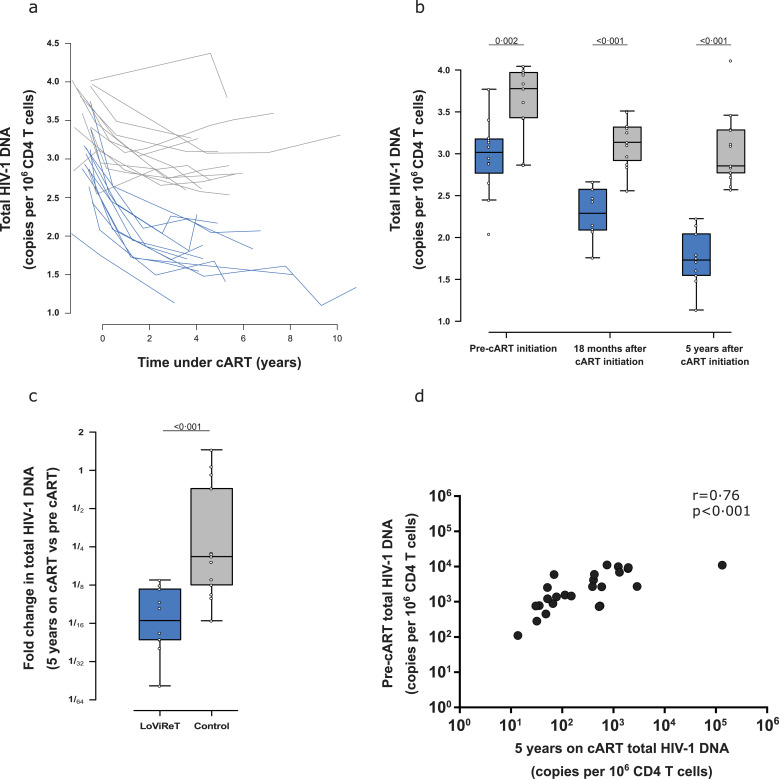
Fig. 2.
Longitudinal measure of total HIV-1 DNA in CD4+ T cells by ddPCR.
(a) Decay in total HIV-1 DNA before and after initiation of cART. The light grey box indicates the period under cART. (b) Box plot of total HIV-1 DNA at 3 different time points: pre-cART, 18 months after initiation of cART, and 5 years after initiation of cART. (c) Fold change decay in the sample before initiation of treatment and after 5 years of cART. (d) Spearman correlation for total HIV-1 DNA pre-cART and after 5 years of cART.
When we analysed the time points separately, we observed that before initiation of cART, cp-LoViReT already harboured significantly lower levels of HIV-1 DNA (p=0•002) (Fig. 2b). The differences observed before cART were independent from pVL, since no statistically significant differences were observed between the groups (Suppl. Table 1).
After 18 months of treatment, a pronounced decline in HIV-1 DNA was observed in all those studied, although median values were lower in cp-LoViReT than in controls (p<0•001). Finally, after 5 years on cART, samples from both groups showed the greatest differences within the study (p<0•001). cp-LoViReT reached a median of 54 HIV-1 DNA copies/106 CD4+ T cells, which is comparable to the values we observed in the initial PBMC screening.
When we compared the decay in the HIV reservoir after 5 years on cART with before cART, we observed a significantly greater decrease in cp-LoViReT subjects than in controls (16- vs. 5-fold respectively, p<0•001) (Fig. 2c). In addition, a significant positive correlation was observed between these 2 time points (rho=0•76, p<0•001) (Fig. 2d).
We observed that cp-LoViReT individuals had low viral reservoirs before initiation of cART, as well as enhanced decay during treatment.
Characterization of T-cell subsets, activation, exhaustion, and reservoir surrogate markers: Immunophenotyping of T cells was performed in the samples used for the longitudinal reservoir analysis. As expected, we found a significant increase in the frequency of CD4+ T cells and a significant decrease in the frequency of CD8+ T cells after initiation of cART, thus leading to a significant recovery of the CD4/CD8 T-cell ratio. No differences were observed between cp-LoViReT and the control groups over time (data not shown).
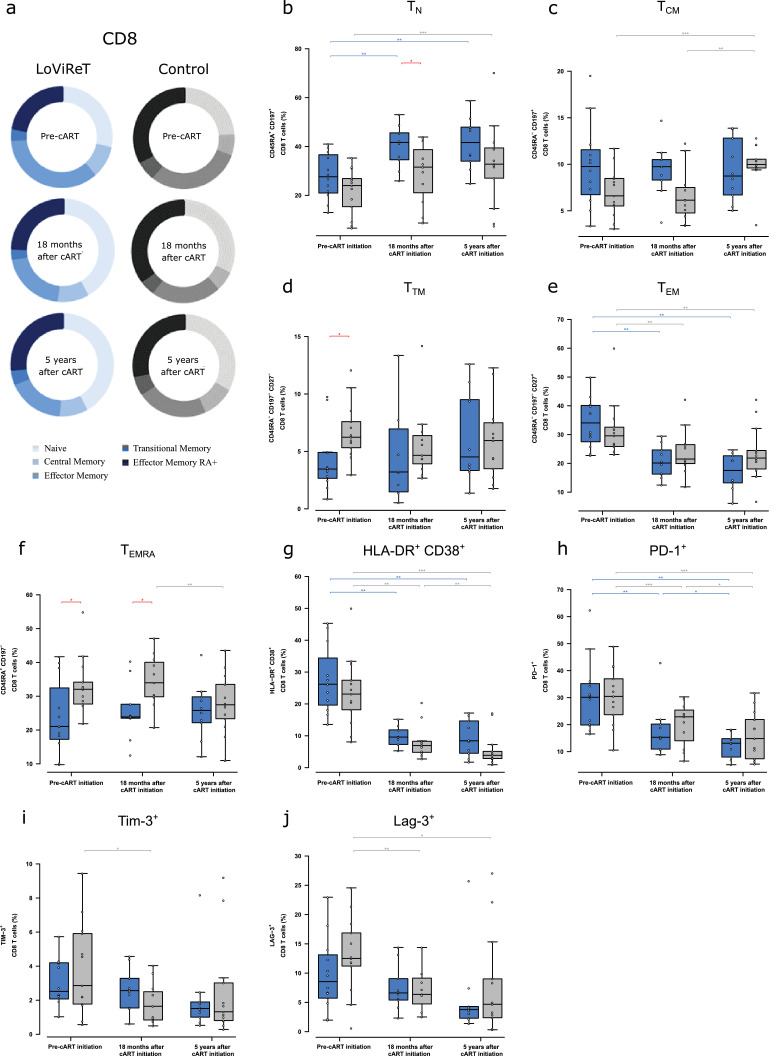
Analysis of maturation subsets in CD8+ T cells revealed the expected significant increase in the frequency of TN cells after initiation of therapy in both groups, with similar rates of increase; however, the frequency was higher at month 18 in cp-LoViReT than in controls (p=0•043) (Fig. 3a,b). Although we did not observe significant differences between the groups in the frequency of TCM (Fig. 3c), we did detect a lower frequency of TTM and TEMRA cells before initiation of cART in cp-LoViReT than in controls (p=0•011 for TTM and p=0•016 for TEMRA). This association disappeared when cART was introduced (Fig. 3d–f). The correlation between HIV-DNA and these cellular markers was assayed at each time point, although we did not find statistically significant correlations (data not shown).
Fig. 3.
Analysis of maturation subsets, activation, exhaustion, and surrogate markers in the reservoir in CD8+ T cells.
(a) Median CD8+ T-cell values for the frequency of the maturation subsets (naïve, central memory, effector memory, transitional memory, and effector memory RA+) in controls and cp-LoViReT at 3 different time points: pre-cART, 18 months after initiation of cART, and 5 years after initiation of cART. Maturation stages were defined based on the combination of CD45RA, CD197 (CCR7), and CD27. (b) CD45RA+CD197+ (Naive), (c) CD45RA–CD197+ (central memory), (d) CD45RA–CD197–CD27– (transitional memory), (e) CD45RA–CD197–CD27+ (effector memory), and (f) CD45RA+CD197– (effector memory RA+). (g) CD8 activation levels (HLA-DR+CD38+) in both study groups over time. (h-j) CD8+ T-cell exhaustion markers including PD-1 (CD279), TIM-3, and LAG-3. The cp-LoViReT group is depicted in blue and the control group in grey. Intragroup statistically significant differences are depicted with a light blue or grey line for LoViReT and controls respectively; statistically significant differences between groups are depicted with a red line.
Activated (CD38+HLA-DR+) and PD-1+ CD8+ T cells decreased significantly over time in both groups (Fig. 3g,h). However, no significant differences between groups were observed. Values for the other exhaustion markers analysed, TIM-3 and LAG-3, were similar in both groups (Fig. 3i,j).
In CD4+ T cells, we did not observe any significant difference between cp-LoViReT and controls over time in any T-cell maturation subset (Suppl. Fig. 3a-f). Activation, defined as co-expression of CD38+HLA-DR+, and PD-1 expression levels decreased overtime in both groups with no intergroup differences (Suppl. Fig. 3g,h). Similarly, we did not find significant intergroup differences in the reservoir surrogate markers TIM-3+, CD2bright, CD32, and LAG-3+ (Suppl. Fig. 3i–l).
Additionally, immunophenotyping of NK cells was performed in 11 samples before initiation of cART and after 5 years on cART. In this case, we analysed the frequencies of NK cells and their different activator/inhibitor receptors with a putative role in HIV control. No differences were found between cp-LoViReT and controls in the frequency of NK cells (Suppl. Fig. 4a) or in the expression of the C-type lectin-like receptors (NKG2) (Suppl. Fig. 4b-e), killer immunoglobulin-like receptors (KIR) (Suppl. Fig. 4f-g), activator natural cytotoxicity receptors (NCR) (Suppl. Fig. 4h-i), and CD161 (Fig Suppl. 4j). However, a statistically significant decrease in CD69 and CXCR5 was observed for cp-LoViReT once they started cART, with similar trends observed in the controls (Suppl. Fig. 4k-l).
In summary, we observed changes in CD8+ T-cell maturation in cp-LoViReT with higher levels of TN in combination with lower TTM and TEMRA CD8+ T cells before initiation of cART. This observation was not associated with changes in activation or exhaustion of CD8+ T cells in this group.
3.3. CD4+ susceptibility and viral inhibition by CD8+ and NK cells
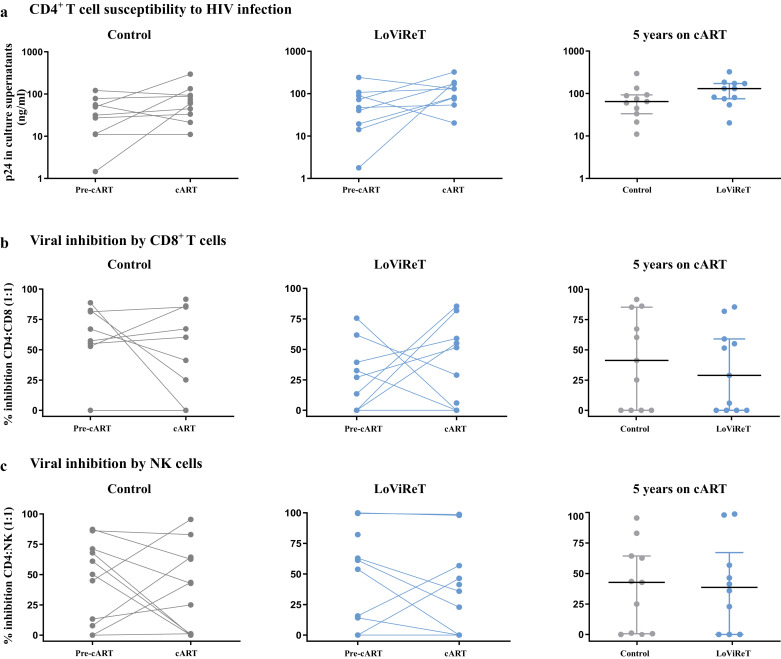
To evaluate the functionality of the T cells and NK cells in both groups of individuals, we analysed the susceptibility of the target cells to HIV-1 infection and viral inhibition by CD8 and NK cells in the same samples as those used for NK immunophenotyping. Thus, we selected 11 cp-LoViReT and 11 controls with available samples before initiation of cART and after 5 years on cART. We pulsed autologous CD4+ T cells from each individual with a laboratory-adapted R5- viral strain for 7 days (Fig. 4a). We observed that CD4+ T cells from cp-LoViReT and controls were susceptible to HIV-1 infection and that there were no significant differences between groups before initiation of cART or after 5 years on cART.
Fig. 4.
CD4+ T cell susceptibility to HIV and viral inhibition by CD8+ T and NK cells.
PBMC samples before initiation of cART and after 5 years on cART were used to analyse the susceptibility of CD4+ T cells to HIV BaL (CCR5 tropic strain) (10 ng p24/ml) (a). We also measured the ex vivo ability of CD8+ T cells (b) and NK cells (c) to inhibit superinfected autologous CD4+ T cells at a 1:1 ratio. The cp-LoViReT group is depicted in blue and the control group in grey.
Autologous CD8+ T cells were also tested to analyse the suppression of viral replication (Fig. 4b); no significant differences between cp-LoViReT and controls were observed. High variability in the inhibition percentage was recorded in all the samples assayed; this could be explained by the limitation arising from the use of frozen cells in this assay.
Similarly, we did not find significant differences in the percentage of inhibition by autologous NK cells between groups before initiation of treatment or after 5 years on cART (Fig. 4c).
CD4+ T cells from cp-LoViReT were perfectly susceptible to HIV infection, with no signs of distinct CD8 and NK cytotoxic activities compared with control individuals.
3.4. Inflammatory marker levels in cp-LoViReT
We measured soluble plasma pro-inflammatory or homeostatic cytokines (IL-2, IL-6, IL-7, IL-10, IL-27, IP10) and the coagulation factor D-dimer in plasma samples from 8 cp-LoViReT and 9 controls before cART and after 5 years on cART (selected according to sample availability). We did not find statistically significant differences either over time or between groups (Suppl. Table 2).
3.5. HIV-1 specific antibodies
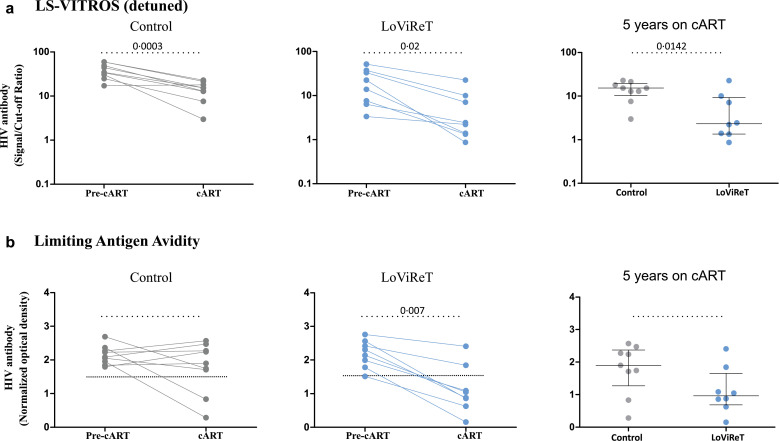
To determine whether low reservoirs could be associated with humoral responses to HIV-1, we analysed HIV-1-specific antibodies in plasma samples before initiation of cART and after 5 years on cART in 8 cp-LoViReT and 9 controls for whom plasma samples were available. We observed a decrease in HIV-1-specific antibody levels in both groups after initiation of cART (Fig. 5a). This decay was greater in cp-LoViReT than in controls (Fig. 5a).
Fig. 5.
Measurement of HIV-1-specific antibodies.
Plasma samples before initiation of cART and after 5 years of cART were tested for HIV-1-specific antibody levels using a detuned version of the HIV-1 VITROS assay (a) and a limiting antigen avidity assay (b). The dotted line represents the HIV-1 antibody assay diagnostic cut-off level used to classify individuals as HIV-1-positive or -negative. The p-values between groups were assessed using the Mann-Whitney test.
We also measured the avidity of antibodies using a limiting antigen avidity assay. We observed trend toward a more pronounced reduction in antibody avidity in cp-LoViReT when cART was initiated, with most individual levels falling below the cut off seen in acute HIV-1-infected subjects (Fig. 5b).
Therefore, HIV-1 antibody quantity and quality were more markedly diminished upon initiation of cART in cp-LoViReT.
4. Discussion
Latent infection of CD4+ T cells is established very early after infection and is the major obstacle to curing HIV-1 infection [28]. A low HIV-1 DNA reservoir has been associated with early treatment [14] and better clinical outcome [29]. Nevertheless, the size of the HIV-1 DNA reservoir is not necessarily associated with time to viral rebound in subjects treated early after infection and who discontinued their cART [16,29]. The global frequency of HIV-1-infected individuals harbouring low levels of HIV-1 DNA and the determinants of these levels remain unknown.
In our study, we found that 9•3% of the individuals screened had HIV-1 DNA levels below 50 copies/106 PBMCs (LoViReT subjects). The proportion of LoViReT individuals was similar in the 2 recruiting centres, indicating that our data might be extrapolated to other clinical sites with similar subject populations and viral subtypes. Previous studies in treated chronically infected individuals found that 28% and 19% of individuals had HIV-1 DNA levels below 150 copies/106 PBMCs and 66 copies/106 PBMCs, respectively [18,19]. While both studies revealed greater amounts of individuals with low HIV-1 DNA levels, the differences observed with our results could be explained by their higher cut-off for defining low HIV-1 DNA. Reanalysis of our dataset after applying their cut-offs revealed that 33% and 19% of individuals, respectively, had a “low reservoir”, thus confirming this hypothesis. We maintained our cut-off of 50 copies/106 PBMCs because it represents the 10th percentile of the screened population.
Various clinical parameters were associated with LoViReT status, including a higher CD4+ nadir, lower maximum pVL, and fewer detectable pVLs. Nevertheless, the model is not strong enough to identify LoViReT individuals, suggesting that other viral or immunological factors might be involved. The same 3 factors have been found to be closely associated with harbouring low levels of HIV-1 reservoir [18,19,30,31], probably because a higher CD4+ T-cell nadir could prevent repopulation of the immune system with expanded latently infected lymphocytes. Moreover, a lower pVL peak might have prevented massive seeding of CD4+ T cells by HIV-1. Similarly, fewer detectable pVLs during the course of the infection might have prevented replenishment of the reservoir.
Previous studies have reported the association between early treatment and low viral reservoirs [14,32,33]. However, only individuals treated in Fiebig I-IV (between 1 week and 1 month after infection) were capable of achieving low levels of total HIV-1 DNA after more than 3 years on treatment [14,15]. Surprisingly, if early treatment is excluded as the main cause of a remarkably low reservoir, 66% of the LoViReT subjects were treated more than 6 months after acquisition of HIV-1 (cp-LoViReT). Since eradication strategies need to be effective in the vast majority of treated chronically infected individuals, it would be of considerable interest to know the mechanism responsible for the low reservoir detected in this group of individuals.
Data on the kinetics of HIV-1 reservoirs have been reported in treated chronically infected individuals [34], [35], [36] harbouring standard levels of total HIV-1 DNA or treated during primary infection [15,37]. However, to our knowledge, this is the first study to address the kinetics of reservoirs in chronically HIV-1-infected individuals harbouring low levels of total HIV-1 DNA, including during the pre-cART period. Our results provide the first evidence that cp-LoViReT have intrinsically lower levels of total HIV-1 DNA before starting treatment, despite having the same levels of plasma viremia as controls. Besides, once cART is introduced, their reservoir seems to be more ART-sensitive, since decay of total HIV-1 DNA is faster in cp-LoViReT. We also proved that their CD4+ T cells were susceptible to infection; therefore, the low reservoir observed is probably due to factors that do not severely impact the viral replication cycle in the cells. Thus, we cannot exclude the possibility that cp-LoViReT might have more linear unintegrated DNA, which decays faster than integrated DNA in the presence of cART [38,39]. These parameters will be analysed in future prospective studies. Additionally, and consistent with other authors, we found a positive correlation between pre-treatment levels of total HIV-1 DNA and levels after 5 years on cART, thus demonstrating the importance of certain features before initiation of cART, such as host factors and/or the immune system, in determining subsequent reservoir size [17,40].
A naturally low level of cell-associated HIV-1 DNA could result in a more preserved immune system. cp-LoViReT had fewer CD8+ TTM and TEMRA in the absence of cART, with frequencies similar to those of HIV-1-negative individuals, suggesting a less impaired CD8+ T-cell compartment. HIV-1 infection disrupts T-cell subset homeostasis, with a dramatic decrease in the frequency of CD8+ TN cells and massive expansion of CD8+ TTM cells in infected individuals treated during both the acute phase and the chronic phase [41]. Furthermore, after 18 months on therapy, the differences in CD8+ TN cells between cp-LoViReT and controls become statistically significant, with the result that CD8+ TN cells were more numerous than in controls. The higher proportion of CD8+ TN, also observed in elite controllers, may suggest shared preservation of thymic function [42]. However, in LoViReT individuals, the preserved CD8+ T-cell compartment does not seem to be associated with an enhanced cytotoxic capacity of their CD8+ T cells or NK cells, in contrast to HIV-1 controllers for CD8+ T cells [43] and post-treatment controllers for NK cells [44]. Further exploration with fresh cells, more participants, and assessment of the frequency of HIV-specific cells will be needed to fully elucidate this point.
Finally, consistent with the kinetics of total HIV-1 DNA, the marked decline in the quantity and avidity of HIV-1-specific antibodies in cp-LoViReT during cART indicates a lower amount of circulating HIV-1 antigen. Our findings are in line with results in treated chronically infected individuals from Keating et al [45], who reported an association between declining antibody levels during cART and lower levels of antigen production, better viral control, and lower systemic viral burdens. cp-LoViReT represent a new phenotype of individuals characterized by low intrinsic total HIV-1 DNA, better immune preservation, and low circulating HIV-1 antigens despite being treated in the chronic phase of the infection. Levels of proviral DNA while on cART in some individuals of the well-characterized post-treatment and elite controller cohorts were as low as those of LoViReT individuals, with median levels of 1.7 and 1.5 log10 copies per million PBMCs, respectively [46]; however, we can rule out an overlap between cohorts. LoViReT had high viral loads during chronic infection before initiation of treatment. This finding differs from the main factors associated with elite and post-treatment controller cohorts. In addition, various studies suggest that having low amounts of HIV-1 DNA does not prevent early and consistent viral rebound if therapy is interrupted in infected individuals receiving long-term treatment [20,47,48]. Therefore, a very low reservoir is not necessarily associated with spontaneous control of viral replication, as might be the case in elite controllers or post-treatment controllers. However, when attempting to identify combined approaches toward finding a cure for HIV-1 infection, cp-LoViReT appear to be excellent research participants, since they have better preserved immune cell populations and start from a smaller reservoir, which might be an advantage over individuals with a larger reservoir.
Our study was subject to a series of limitations. HIV-1 DNA was only measured in peripheral blood, whereas larger HIV-1 reservoirs are known to be found in lymph nodes and gut-associated lymphoid tissues. However, as this was a retrospective analysis, it was not possible to have access to those samples. The full size of the HIV-1 reservoir was only evaluated in total HIV-1 DNA, thus enabling all forms of cellular HIV-1 DNA (unintegrated linear, 1-LTR and 2-LTR circular forms, and integrated forms) to be quantified. Other methods of studying the HIV reservoir, such as assessment of replication-competent or -defective virions and quantification of the inducible reservoir at the RNA level, could prove useful. However, the quantification of replicative or intact virus in individuals with very low viral reservoirs might be challenging, since they represent less than 10% of total HIV-DNA [49].
In addition, we were unable to identify a molecular mechanism that could explain the limited reservoir in LoViReT individuals. Future research will be needed to unravel whether this cohort shares a common molecular mechanism that decreases the ability of the virus to establish latency.
In conclusion, we found that some HIV-1-infected individuals have low levels of total HIV-1 DNA (LoViReT subjects) and that most were treated in the chronic phase of the infection. Moreover, they have a less compromised CD8+ T-cell compartment and lower HIV-1-specific antibody levels, probably as a result of lower amounts of circulating antigens. Among those who started treatment in the chronic phase, these are perfect candidates for the study of combined approaches to finding a cure for HIV-1, since we proved that cp-LoViReT are characterized by a viral reservoir that is intrinsically reduced before initiation of cART and enhanced decay after initiation of treatment.
Declaration of Competing Interest
B.C. declares that outside the submitted work has received grants from Gilead, ViiV Healthcare and MSD; received consultancy fees from MSD; and a shareholder of AlbaJuna Therapeutics and AELIX therapeutics. M.G-C. declares educational/consultancy fees from BMS, Pierre Fabre, Roche, Takeda, and AstraZeneca outside the submitted work. A.S-C has received research grants from MSDAVENIR and personal fees from MSD, ViiV healthcare and Janssen, outside the submitted work. J.B. is the founder and CEO and a shareholder of AlbaJuna Therapeutics and receives grants from MSD and Grifols outside the submitted work. J.M-P. holds an institutional grant and has received educational/consultancy fees from Merck; outside the submitted work, he has received fees from AbiVax, AstraZeneca, Gilead Sciences, Grifols, Janssen, and ViiV Healthcare. All the other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank the IGTP Cytometry Core Facility and staff (MA Fernandez and G Requena) for their contribution to this publication.
Founding sources
This research was sponsored in part by Grifols and by Merck Sharp & Dohme España, S.A. (IISP 54925). The funding organizations had no input in the design of the study; in the collection, analyses, or interpretation of the data; writing of the manuscript; or in the decision to submit the study for publication. CG is supported by the PhD fellowship of the Spanish Ministry of Education, Culture and Sport (FPU15/03698). NH received a post-doctoral grant from the Jaqueline Beytout Foundation. FG received the support of “José María Segovia de Arana” contracts (2019) and MMT from the NIH (R01AI143457).
Authors’ Contributions
J.M-P. and M.S. conceived and designed the study; C.G., M.J., V.G-S., V.M., N.H., and M.S. performed the experiments; C.G., V.U., M.J., V.G-S., V.M., N.H., and M.S. analysed the data; C.G., J.D., M.J., V.G-S., V.M., N.H., M.M-T., A.S-C,. J.B, J.M-P., and M.S. interpreted the results; B.C., L.L., F.G. contributed with patient samples and clinical data. C.G. and M.S. wrote the paper. All the authors read, reviewed, and approved the final paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.102830.
Contributor Information
Javier Martinez-Picado, Email: jmpicado@irsicaixa.es.
Maria Salgado, Email: msalgado@irsicaixa.es.
Appendix. Supplementary materials
References
- 1.Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 2.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. (80-) [DOI] [PubMed] [Google Scholar]
- 3.Deeks SG, Autran B, Berkhout B, Benkirane M, Cairns S, Chomont N. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol. 2012;12:607–614. doi: 10.1038/nri3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katlama C, Deeks SG, Autran B, Martinez-Picado J, van Lunzen J, Rouzioux C. Barriers to a Cure: New concepts in targeting and eradicating HIV-1 reservoirs. Lancet. 2013;381:2109–2117. doi: 10.1016/S0140-6736(13)60104-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margolis DM. Towards an HIV Cure: a View of a Developing Field. J Infect Dis. 2017;215:S109–S110. doi: 10.1093/infdis/jiw633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avettand-Fènoël V, Boufassa F, Galimand J, Meyer L, Rouzioux C, ANRS SEROCO Cohort Study Group HIV-1 DNA for the measurement of the HIV reservoir is predictive of disease progression in seroconverters whatever the mode of result expression is. J Clin Virol. 2008;42:399–404. doi: 10.1016/j.jcv.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Goujard C, Bonarek M, Meyer L, Bonnet F, Chaix M-L, Deveau C. CD4 cell count and HIV DNA level are independent predictors of disease progression after primary HIV type 1 infection in untreated patients. Clin Infect Dis. 2006;42:709–715. doi: 10.1086/500213. [DOI] [PubMed] [Google Scholar]
- 8.Graf EH, Mexas AM, Yu JJ, Shaheen F, Liszewski MK, Di Mascio M. Elite suppressors harbor low levels of integrated HIV DNA and high levels of 2-LTR circular HIV DNA compared to HIV+ patients on and off HAART. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sáez-Cirión A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C. Post-Treatment HIV-1 Controllers with a Long-Term Virological Remission after the Interruption of Early Initiated Antiretroviral Therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hütter G, Nowak D, Mossner M, Ganepola S, Müßig A, Allers K. Long-Term Control of HIV by CCR5 Delta32/Delta32 Stem-Cell Transplantation. N Engl J Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 11.Duarte RF, Salgado M, Sánchez-Ortega I, Arnan M, Canals C, Domingo-Domenech E. CCR5 Δ32 homozygous cord blood allogeneic transplantation in a patient with HIV: a case report. Lancet HIV. 2015;2:e236–e242. doi: 10.1016/S2352-3018(15)00083-1. [DOI] [PubMed] [Google Scholar]
- 12.Salgado M, Kwon M, Gálvez C, Badiola J, Nijhuis M, Bandera A. Mechanisms That Contribute to a Profound Reduction of the HIV-1 Reservoir After Allogeneic Stem Cell Transplant. Ann Intern Med. 2018;169:674. doi: 10.7326/M18-0759. [DOI] [PubMed] [Google Scholar]
- 13.Gupta RK, Abdul-Jawad S, McCoy LE, Mok HP, Peppa D, Salgado M. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature. 2019;568:244–248. doi: 10.1038/s41586-019-1027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buzon MJ, Martin-Gayo E, Pereyra F, Ouyang Z, Sun H, Li JZ. Long-Term Antiretroviral Treatment Initiated at Primary HIV-1 Infection Affects the Size, Composition, and Decay Kinetics of the Reservoir of HIV-1-Infected CD4 T Cells. J Virol. 2014;88:10056–10065. doi: 10.1128/jvi.01046-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laanani M, Ghosn J, Essat A, Melard A, Seng R, Gousset M. Impact of the timing of initiation of antiretroviral therapy during primary HIV-1 infection on the decay of cell-associated HIV-DNA. Clin Infect Dis. 2015;60:1715–1721. doi: 10.1093/cid/civ171. [DOI] [PubMed] [Google Scholar]
- 16.Colby DJ, Trautmann L, Pinyakorn S, Leyre L, Pagliuzza A, Kroon E. Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat Med. 2018;24:923–926. doi: 10.1038/s41591-018-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parisi SG, Sarmati L, Andreis S, Scaggiante R, Cruciani M, Ferretto R. Strong and persistent correlation between baseline and follow-up HIV-DNA levels and residual viremia in a population of naïve patients with more than 4 years of effective antiretroviral therapy. Clin Microbiol Infect. 2015;21:288. doi: 10.1016/j.cmi.2014.10.009. .e5-7. [DOI] [PubMed] [Google Scholar]
- 18.Fourati S, Flandre P, Calin R, Carcelain G, Soulie C, Lambert-Niclot S. Factors associated with a low HIV reservoir in patients with prolonged suppressive antiretroviral therapy. J Antimicrob Chemother. 2014;69:753–756. doi: 10.1093/jac/dkt428. [DOI] [PubMed] [Google Scholar]
- 19.Cuzin L, Pugliese P, Saune K, Allavena C, Ghosn J, Cottalorda J. Levels of intracellular HIV-DNA in patients with suppressive antiretroviral therapy. AIDS. 2015;29:1665–1671. doi: 10.1097/QAD.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 20.Calin R, Hamimi C, Lambert-Niclot S, Carcelain G, Bellet J, Assoumou L. Treatment interruption in chronically HIV-infected patients with an ultralow HIV reservoir. AIDS. 2016;30:761–769. doi: 10.1097/QAD.0000000000000987. [DOI] [PubMed] [Google Scholar]
- 21.Morón-López S, Puertas MC, Gálvez C, Navarro J, Carrasco A, Esteve M. Sensitive quantification of the HIV-1 reservoir in gut-associated lymphoid tissue. PLoS One. 2017;12 doi: 10.1371/journal.pone.0175899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raymond S, Delobel P, Mavigner M, Ferradini L, Cazabat M, Souyris C. Prediction of HIV Type 1 Subtype C Tropism by Genotypic Algorithms Built From Subtype B Viruses. JAIDS J Acquir Immune Defic Syndr. 2010;53:167–175. doi: 10.1097/QAI.0b013e3181c8413b. [DOI] [PubMed] [Google Scholar]
- 23.Gondois-Rey F, Chéret A, Mallet F, Bidaut G, Granjeaud S, Lécuroux C. A Mature NK Profile at the Time of HIV primary infection is associated with an early response to cART. Front Immunol. 2017;8:54. doi: 10.3389/fimmu.2017.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sáez-Cirión A, Shin SY, Versmisse P, Barré-Sinoussi F, Pancino G. Ex vivo T cell-based HIV suppression assay to evaluate HIV-specific CD8+ T-cell responses. Nat Protoc. 2010;5:1033–1041. doi: 10.1038/nprot.2010.73. [DOI] [PubMed] [Google Scholar]
- 25.Keating SM, Hanson D, Lebedeva M, Laeyendecker O, Ali-Napo NL, Owen SM. Lower-sensitivity and avidity modifications of the vitros anti-HIV 1+2 assay for detection of recent HIV infections and incidence estimation. J Clin Microbiol. 2012;50:3968–3976. doi: 10.1128/JCM.01454-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breiman L. Random Forests. Mach Learn. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 27.Zhao P, Su X, Ge T, Fan J. Propensity score and proximity matching using random forest. Contemp Clin Trials. 2016;47:85–92. doi: 10.1016/j.cct.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitney JB, Hill AL, Sanisetty S, Penaloza-Macmaster P, Liu J, Shetty M. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014;512:74–77. doi: 10.1038/nature13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams JP, Hurst J, Stöhr W, Robinson N, Brown H, Fisher M. HIV-1 DNA predicts disease progression and post-treatment virological control. Elife. 2014;3:e03821. doi: 10.7554/eLife.03821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgard M, Boufassa F, Viard JP, Garrigue I, Ruffault A, Izopet J. Factors influencing peripheral blood mononuclear cell-associated HIV-1 DNA level after long-term suppressive antiretroviral therapy in 236 patients. AIDS. 2009;23:2165–2171. doi: 10.1097/QAD.0b013e32833032d4. [DOI] [PubMed] [Google Scholar]
- 31.Boulassel MR, Chomont N, Pai NP, Gilmore N, Sékaly RP, Routy JP. CD4 T cell nadir independently predicts the magnitude of the HIV reservoir after prolonged suppressive antiretroviral therapy. J Clin Virol. 2012;53:29–32. doi: 10.1016/j.jcv.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Plana M, García F, Gallart T, Tortajada C, Soriano A, Palou E. Immunological benefits of antiretroviral therapy in very early stages of asymptomatic chronic HIV-1 infection. AIDS. 2000;14:1921–1933. doi: 10.1097/00002030-200009080-00007. [DOI] [PubMed] [Google Scholar]
- 33.Bart PA, Rizzardi GP, Tambussi G, Chave JP, Chapuis AG, Graziosi C. Immunological and virological responses in HIV-1-infected adults at early stage of established infection treated with highly active antiretroviral therapy. AIDS. 2000;14:1887–1897. doi: 10.1097/00002030-200009080-00002. [DOI] [PubMed] [Google Scholar]
- 34.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 35.Viard J-P, Burgard M, Hubert J-B, Aaron L, Rabian C, Pertuiset N. Impact of 5 years of maximally successful highly active antiretroviral therapy on CD4 cell count and HIV-1 DNA level. AIDS. 2004;18:45–49. doi: 10.1097/00002030-200401020-00005. [DOI] [PubMed] [Google Scholar]
- 36.Stanoeva KR, König A, Fukuda A, Kawanami Y, Kuwata T, Satou Y. Total HIV-1 DNA Dynamics and Influencing Factors in Long-Term ART-Treated Japanese Adults: A Retrospective Longitudinal Analysis. J Acquir Immune Defic Syndr. 2018;78:239–247. doi: 10.1097/QAI.0000000000001662. [DOI] [PubMed] [Google Scholar]
- 37.Strain MC, Little SJ, Daar ES, Havlir DV, Günthard HF, Lam RY. Effect of Treatment, during Primary Infection, on Establishment and Clearance of Cellular Reservoirs of HIV-1. J Infect Dis. 2005;191:1410–1418. doi: 10.1086/428777. [DOI] [PubMed] [Google Scholar]
- 38.Koelsch KK, Liu L, Haubrich R, May S, Havlir D, Günthard HF. Dynamics of Total, Linear Nonintegrated, and Integrated HIV‐1 DNA In Vivo and In Vitro. J Infect Dis. 2008;197:411–419. doi: 10.1086/525283. [DOI] [PubMed] [Google Scholar]
- 39.Murray AJ, Kwon KJ, Farber DL, Siliciano RF. The Latent Reservoir for HIV-1: How immunologic memory and clonal expansion contribute to HIV-1 Persistence. J Immunol. 2016;197:407–417. doi: 10.4049/jimmunol.1600343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parisi SG, Andreis S, Mengoli C, Scaggiante R, Ferretto R, Manfrin V. Baseline Cellular HIV DNA Load Predicts HIV DNA Decline and Residual HIV Plasma Levels during Effective Antiretroviral Therapy. J Clin Microbiol. 2012;50:258. doi: 10.1128/JCM.06022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Breton G, Chomont N, Takata H, Fromentin R, Ahlers J, Filali-Mouhim A. Programmed death-1 is a marker for abnormal distribution of naive/memory T cell subsets in HIV-1 infection. J Immunol. 2013;191:2194–2204. doi: 10.4049/jimmunol.1200646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groves KC, Bibby DF, Clark DA, Isaksen A, Deayton JR, Anderson J. Disease Progression in HIV-1-Infected Viremic Controllers. J Acquir Immune Defic Syndr. 2012;61:407–416. doi: 10.1097/QAI.0b013e318269c414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sáez-Cirión A, Lacabaratz C, Lambotte O, Versmisse P, Urrutia A, Boufassa F. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007;104:6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott-Algara DDCAVCJ-SBFLOHLS-CARC Post-treatment controllers have particular NK Cells With High Anti-HIV Capacity: VISCONTI Study - CROI Conference. Proceedings of the Conference Retroviruses Opportunistic Infect.; Seattle (WA); EEUU; 2015. p. 23. [Google Scholar]
- 45.Keating SM, Pilcher CD, Jain V, Lebedeva M, Hampton D, Abdel-Mohsen M. HIV antibody level as a marker of HIV persistence and low-level viral replication. J Infect Dis. 2017:72–81. doi: 10.1093/infdis/jix225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avettand-Fènoël V, Hocqueloux L, Ghosn J, Cheret A, Frange P, Melard A. Total HIV-1 DNA, a marker of viral reservoir dynamics with clinical implications. Clin Microbiol Rev. 2016;29:859–880. doi: 10.1128/CMR.00015-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castagna A, Muccini C, Galli L, Bigoloni A, Poli A, Spagnuolo V. Analytical treatment interruption in chronic HIV-1 infection: Time and magnitude of viral rebound in adults with 10 years of undetectable viral load and low HIV-DNA (APACHE study) J Antimicrob Chemother. 2019;74:2039–2046. doi: 10.1093/jac/dkz138. [DOI] [PubMed] [Google Scholar]
- 48.Pannus P, Rutsaert S, De Wit S, Allard SD, Vanham G, Cole B. Rapid viral rebound after analytical treatment interruption in patients with very small HIV reservoir and minimal on-going viral transcription. J Int AIDS Soc. 2020;23:e25453. doi: 10.1002/jia2.25453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruner KM, Wang Z, Simonetti FR, Bender AM, Kwon KJ, Sengupta S. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature. 2019;566:120–125. doi: 10.1038/s41586-019-0898-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.