Summary
Bariatric surgery is an effective treatment option for patients with type 2 diabetes mellitus (T2DM) and obesity. This study aims to compare the effects of Roux‐en‐Y gastric bypass (RYGB) and sleeve gastrectomy (SG) on remission of T2DM. MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials were searched for studies published between database inception and 21 November 2019. A meta‐analysis, using a random effects model, was performed to calculate relative risk (RR) of T2DM remission between the groups in randomized controlled trials (RCTs). Of 2650 records identified, 12 records from 10 different RCTs were finally included. The studies comprised 705 patients with follow‐up from 1 to 5 years. The remission rate of T2DM at 1 year was higher among those undergoing RYGB (156/276, 57%) compared with those undergoing SG (128/275, 47%), RR (95% CI) 1.20 (1.00‐1.45), P = .047, I 2 = 24.9%, moderate‐quality evidence. Among studies with 2‐ to 5‐year follow‐up, there was no difference in remission rates between the RYGB (132/263, 50%) and SG (121/266, 46%) groups, RR 1.06 (0.94‐1.20), P = .34, I 2 = 0.0%, low‐quality evidence. RYGB resulted in a higher rate of T2DM remission compared with SG after 1 year. The T2DM remission rates did not differ in studies with 2‐ to 5‐year follow‐up.
Keywords: Roux‐en‐Y gastric bypass, sleeve gastrectomy, systematic review, type 2 diabetes mellitus
Abbreviations
- BMI
body mass index
- CI
confidence interval
- RCT
randomized controlled trial
- RR
relative risk
- RYGB
Roux‐en‐Y gastric bypass
- SD
standard deviation
- SG
sleeve gastrectomy
- T2DM
type 2 diabetes mellitus
1. INTRODUCTION
As a direct consequence of the obesity epidemic, the prevalence of obesity‐related comorbidities, including type 2 diabetes mellitus (T2DM), has increased. Between 1980 and 2014, the number of adults with diabetes increased fourfold, from 108 million to 422 million.1 For the individual, T2DM is associated with reduced quality of life, stigma, increased medical expenses, and risk of diabetes‐related microvascular complications.2, 3 In addition, T2DM and its complications impose a substantial burden on society in terms of increased health care costs, reduced productivity, either inability to work or work absenteeism, and lost productive capacity because of early mortality.4
Bariatric surgery is an effective treatment option for patients with obesity and T2DM, and the superiority of surgery over medical care for glycaemic control has been demonstrated in several randomized controlled trials (RCTs) and cohort studies.5, 6, 7, 8, 9, 10 The American Diabetes Association recommends bariatric surgery “as an option to treat T2DM in appropriate surgical candidates with BMI ≥ 40 kg/m2 (BMI ≥ 37.5 kg/m2 in Asian Americans), regardless of the level of glycemic control or complexity of glucose‐lowering regimens, and in adults with BMI 35.0–39.9 kg/m2 (32.5–37.4 kg/m2 in Asian Americans) when hyperglycemia is inadequately controlled despite lifestyle and optimal medical therapy”.11 In 2016, more than 100 000 patients with diabetes underwent bariatric surgery, whereof the majority received Roux‐en‐Y gastric bypass (RYGB) or sleeve gastrectomy (SG).12, 13
Improved glycaemic control after bariatric surgery is mainly explained by weight loss. However, even before changes in body weight occur, insulin and glucose levels improve—indicating that some of the hypoglycaemic effects of bariatric surgery may be independent of weight loss.14 Caloric restriction after surgery is thought to be a driver of this early improved glycaemic homeostasis, but the anatomical alterations of the gut and intestines postoperatively may also be important contributors. RYGB and SG are similar in that both procedures reduce the size of the stomach, but only RYGB includes a bypass of the duodenum and the proximal small intestine. Thus, particularly after RYGB, there is a rapid delivery of undigested food to the small intestine with subsequent increased release of the gut‐derived insulin stimulating the hormone glucagon‐like peptide‐1 (GLP‐1). Moreover, some studies indicate that exclusion of duodenal nutrient exposure is responsible for the weight‐loss–independent effects on glucose homeostasis immediately after RYGB,15, 16, 17, 18 but the results are inconsistent.19 Bariatric surgery is also accompanied by changes in other gut‐derived and pancreatic‐derived hormones20 and changes in the microbial composition,21 which directly or indirectly may influence glycaemic control.
With bariatric surgery considered a highly effective treatment of T2DM in patients with obesity, a comparison of the effectiveness of the two most commonly performed bariatric procedures on T2DM remission is vital. A recent systematic review of RCTs found diabetes remission rates to favour RYGB at 1, 3, and 5 years, but not significantly.22 However, this review failed to include two important RCTs,23, 24 including the 1‐year results from the landmark STAMPEDE trial. The meta‐analysis was also flawed by duplicate publications, and it did not exclude studies of variants of gastric bypass, such as banded and mini gastric bypass. Another systematic review of RCTs from 2017 suggested that RYGB and SG were equally effective in resolving T2DM in patients with obesity25 but that further studies were required. Reviews including both RCTs and retrospective studies have concluded that RYGB is associated with better resolution and control of T2DM than SG,26, 27, 28 while one review found no difference between the procedures.29 However, some important trials were missing from these reviews, and two larger RCTs have recently been published.30, 31
1.1. Objectives
This systematic review and meta‐analysis seeks to compare, through the results available from RCTs, the effects of RYGB and SG on remission of T2DM in patients eligible for bariatric surgery.
2. METHODS
2.1. Protocol and registration
The protocol of this systematic review was submitted to PROSPERO on 7 October 2019 (see Data S1 for the complete protocol).
2.2. Eligibility criteria
We included RCTs of adults (18 years and older) eligible for bariatric surgery, comparing the effects of RYGB and SG on remission of T2DM. Studies where either all or some of the study participants had T2DM were included, with studies with follow‐up of less than 1 year excluded.
2.3. Information sources and search strategy
We performed the search in cooperation with health science librarians with expertise in systematic review searching, using medical subject headings (MeSH) and text words related to T2DM, bariatric surgery, RYGB, and SG (see Table S1 for the complete search strategy). MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov were searched. The literature search was limited to English language and humans. Articles published ahead of print were evaluated, but protocols were not included. The search was restricted to studies published between database inception to 21 November 2019.
2.4. Data collection
The literature search results were uploaded to an Internet‐based review program (www.covidence.org) that facilitates review literature screening and cooperation among the reviewers. Two of the authors (HB and DH) independently screened the abstracts sourced by the search against the inclusion criteria. Full texts for all papers meeting the inclusion criteria were subsequently obtained. Any uncertainty or disagreement between the reviewers was resolved through discussion with a third party (JH).
2.5. Data extraction
We extracted the following data from each study: authors, publication year, country, trial registration number, study design and duration, study population characteristics (number of patients with T2DM, BMI, HbA1c levels), T2DM remission criteria, percentage of participants with diabetes remission in the RYGB and SG groups, and the primary outcomes of the trial. If results according to different remission criteria were given, the Buse's consensus group criteria were used if available.32
2.6. Risk of bias within individual studies
Two reviewers (HB and DH) independently evaluated risk of bias within the individual studies using the Cochrane collaborations tool.33 The risk of selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases were judged as high, low, or unclear. The reviewers resolved uncertainty and disagreement through discussion and consensus with a third party (JH).
2.7. Statistical analysis
T2DM remission rates from the individual studies were used to calculate a relative risk (RR) of remission with a 95% confidence interval (CI). Random‐effect models were used to adjust for possible variations in baseline risk between the trials. RRs were calculated according to the intention to treat principle where missing cases were treated as nonremission of T2DM. The studies were grouped into studies with short‐term (1‐year) follow‐up and studies with medium‐term (2‐ to 5‐year) follow‐up. When results from multiple time points in the time frame 2 to 5 years were reported from the same cohort, results from the latest time point were reported. The Cochrane Q test was used to assess between‐study heterogeneity, and the magnitude of heterogeneity was evaluated by the I 2 statistics. I 2 values of 25%, 50%, and 75% were regarded as low, moderate, and high heterogeneity, respectively.34 Sensitivity analyses and subgroup analyses were subsequently performed. Publication bias was assessed by visual inspection of funnel plots and the Egger test.
Statistical analyses were performed using Review manager 5.3 and STATA/MP 14.2. P values less than .05 were considered statistically significant.
2.8. Quality of evidence
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) was used to assess the overall quality of evidence for the included RCTs.35 Evidence was downgraded from “high quality” by one level in case of serious (or by two for very serious) study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias.
3. RESULTS
3.1. Study selection
The literature search identified 2650 unique citations, whereof 2625 were excluded because of irrelevant content, duplication, or non‐English language (Figure 1). Of the 25 studies that were full‐text screened, 13 did not meet the inclusion criteria either because follow‐up time was too short,36, 37, 38, 39 the authors did not differentiate between improvement and remission of T2DM,40 other bariatric procedures were performed (mini gastric bypass, banded RYGB, and metabolic gastric bypass),41, 42, 43, 44 the study was not an RCT,45 no patients in the SG‐group had T2DM,46 or the study included a subpopulation of an already included RCT.47 We detected two studies that had been performed in the same study population,48, 49 and of these, only the study referring to a clinical trial registry number was included.48 Finally, 12 articles of 10 different RCTs,5, 23, 24, 30, 31, 48, 50, 51, 52, 53, 54, 55 where of four had data on remission at different lengths of follow‐up, were included in the systematic review.
Figure 1.
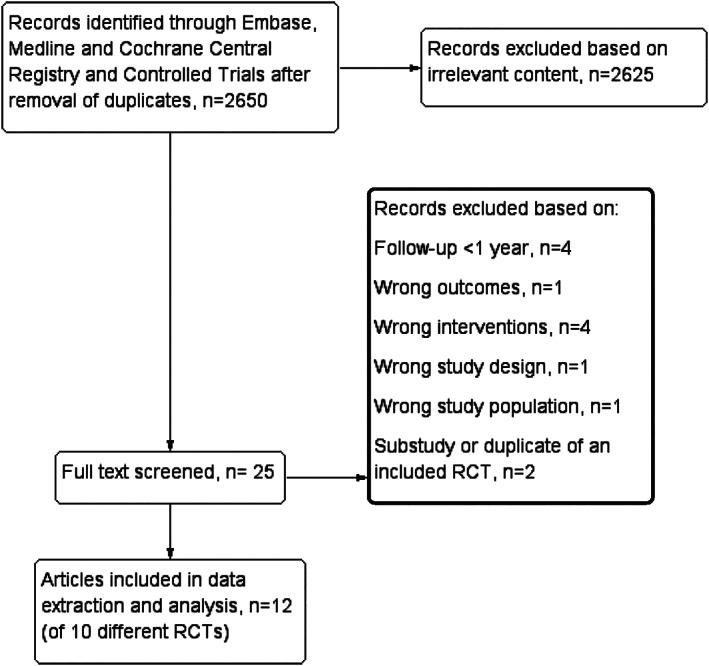
Flow diagram
3.2. Study characteristics
The 10 studies comprised 705 patients with T2DM, with the majority including patients with BMI ≥ 35 kg/m2 (Table 1). However, the STAMPEDE trial and two studies from Chinese populations5, 23, 54, 55 also included patients with BMI < 35 kg/m2, where of one55 had only recruited patients with BMI ≤ 35 kg/m2. Half of the patients (n = 352) underwent RYGB, while the remaining half (n = 353) underwent SG. Five trials5, 23, 24, 31, 54, 55 included only patients with T2DM, and these five had complete remission of T2DM (HbA1c < 6.0% or HbA1c ≤ 6.0%) as the primary or secondary endpoint. The remaining trials had weight loss as the main outcome and remission of T2DM as a secondary or exploratory outcome. However, in two trials,30, 50 only data on partial T2DM remission were given. Length of follow‐up was in the time frame 1 to 5 years, and four trials5, 23, 30, 51, 52, 53 included data on both short‐ and medium‐term follow‐up. Sample sizes ranged from 10 to 120 patients, with all but two studies51, 52, 53 single‐centre studies, and all the studies were performed in either Europe, Asia, or the United States. One30 of the two authors contacted in order to clarify data responded to this request.
Table 1.
Characteristics of the included trials
| Authors | Registration | Country | Study Design | Duration | Percent of Patients with T2DM | Number of Patients with T2DM | Age, ya | BMI, kg/m2 a | HbA1c, %a | T2DM Remission Criteria | Primary Endpoint |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hofsø 2019 31 | NCT01778738 | Norway | Single‐centre, triple‐blinded RCT comparing RYGB and sleeve | 1 year | 100 |
RYGB: 54 Sleeve: 55 |
RYGB : 48.2 (8.9) Sleeve: 47.1 (10.2) | RYGB : 42.4 (5.4) Sleeve: 42.1 (5.3) | RYGB : 7.6 (6.8‐8.5) b Sleeve: 7.9 (6.9‐9.9) | HbA1c ≤ 6% with no pharmacologic therapy | T2DM remission |
| Kalinowski 2017 48 | NCT01806506 | Poland | Single‐centre, nonblinded, RCT comparing RYGB and sleeve | 1 year | 36.1 |
RYGB: 14 Sleeve: 12 |
RYGB : 43.9 (10.8) c Sleeve: 44.9 (10.6) c |
RYGB : 48.6 (5.4) c Sleeve: 46.1 (5.9) c |
RYGB : 6.3 (0.9) c Sleeve: 6.4 (1.3) c |
HbA1c < 6% and fasting glucose <5.6 mmol/L with no pharmacologic therapy | Weight loss |
| Kehagias 2011 50 | ‐ | Greece | Single‐centre, double‐blinded RCT, comparing RYGB and sleeve | 3 years | 16.7 |
RYGB: 5 Sleeve: 5 |
RYGB : 36.0 (8.4) c Sleeve: 33.7 (9.9) c |
RYGB : 45.8 (3.7) c Sleeve: 44.9 (3.4) c |
RYGB : NR Sleeve: NR |
Fasting plasma glucose <126 mg/dL or 2‐h plasma glucose <200 mg/dL during OGTT or no pharmacologic therapy | Weight loss |
| Keidar 2013 24 | NCT00667706 | Israel | Single‐centre, nonblinded RCT comparing RYGB and sleeve | 1 year | 100 |
RYGB: 22 Sleeve: 19 |
RYGB : 51.5 (8.3) Sleeve: 47.7 (11.7) |
RYGB : 42.0 (4.8) Sleeve: 42.5 (5.2) |
RYGB : 7.70 (1.3) Sleeve: 8.34 (1.8) |
Cessation of glucose lowering medication and normald HbA1c and glucose levels | HbA1c level change |
| Peterli 2013 51 and 2018 52 | NCT00356213 | Switzerland | Multicentre, nonblinded RCT comparing RYGB and sleeve | 1 and 5 years | 24.9 |
RYGB: 28 Sleeve: 26 |
RYGB : 42.1 (11.2) c Sleeve: 43.0 (11.1) c |
RYGB : 44.2 (5.3) c Sleeve: 43.6 (5.2) c |
RYGB: 7.2 (6.4‐8.0) SG: 7.6 (6.8‐8.4) |
1 year: No pharmacologic therapy | Weight loss |
| 5 years: HbA1c < 6% and fasting glucose <100 mg/dL (<5.6 mmol/L) with no pharmacologic therapy for at least 1 year | |||||||||||
| Ruiz‐Tovar 2018 30 | NCT03467646 | Spain | Single‐centre, nonblinded RCT comparing RYGB, sleeve and one‐anastomosis gastric bypass | 1 and 5 years | 31.7 |
RYGB: 59 Sleeve: 61 |
RYGB : 45.0 (11.3) c Sleeve: 43.9 (10.9) c |
RYGB : 45.3 (3.2) c Sleeve: 46.5 (3.4) c |
RYGB : NR Sleeve: NR |
Plasma glucose below 110 mg/dL (6.1 mmol/L) and HbA1c below 6.5% with no pharmacologic therapy | Weight loss |
| Salminen 2018 53 | NCT00793143 | Finland | Multi‐centre, nonblinded RCT comparing RYGB and sleeve | 1 and 5 years | 42.1 |
RYGB: 49 Sleeve: 52 |
RYGB : 48.4 (9.3) c Sleeve: 48.5 (9.6) c |
RYGB: 47.4 (45.8‐49.0) SG: 46.3 (44.7‐47.9) |
RYGB : 7.2 (7.0‐7.5) SG : 7.2 (7.0‐7.5) |
1 year: No pharmacologic therapy | Weight loss |
| 5 years: HbA1c < 6% and fasting glucose <100 mg/dL (<5.6 mmol/L) with no pharmacologic therapy for at least 1 year | |||||||||||
| Schauer 2012 23 and 2017 5 | NCT00432809 | USA | Single‐centre, nonblinded, RCT comparing intensive medical therapy alone or intensive medical therapy combined with either RYGB or sleeve gastrectomy | 1 and 5 years | 100 |
RYGB: 50 Sleeve: 50 |
RYGB : 48.3 (8.4) Sleeve: 47.9 (8.0) |
RYGB : 37.0 (3.3) Sleeve: 36.2 (3.9) |
RYGB: 9.3 (1.5) SG: 9.5 (1.7) |
HbA1c ≤ 6% without pharmacologic therapy | Glycated haemoglobin level ≤ 6.0% with or without the use of pharmacologic therapy |
| Tang 2016 54 | ‐ | China | Single‐centre, nonblinded, RCT comparing RYGB and sleeve | 2 years | 100 |
RYGB: 40 Sleeve: 40 |
RYGB : 40.4 (12.3) Sleeve: 36.6 (8.0) |
RYGB : 37.8 (5.6) Sleeve: 38.4 (8.6) |
RYGB : 7.4 (1.8) SG : 7.4 (1.8) |
HbA1c < 6% and fasting glucose <100 mg/dL (<5.6 mmol/L) with no pharmacologic therapy for at least 1 year | Partial remission and complete remission of T2DM |
| Yang 2015 55 | ‐ | China | Single‐centre, nonblinded, RCT comparing RYGB and sleeve gastrectomy | 3 years | 100 |
RYGB: 32 Sleeve: 32 |
RYGB : 41.4 (9.3) Sleeve: 40.4 (9.4) |
RYGB : 32.3 (2.4) Sleeve: 31.8 (3.0) |
RYGB: 8.9 (1.3) SG: 8.5 (1.2) |
HbA1c < 6.0% and fasting glucose <7.0 mmol/L with no pharmacologic therapy | T2DM remission |
Abbreviations: BMI, body mass index; NR, not reported; OGTT, oral glucose tolerance test; RCT, randomized controlled trial; RYGB, Roux‐en‐Y gastric bypass; SG, sleeve gastrectomy, T2DM, type 2 diabetes mellitus.
Mean (SD) or (95% CI) unless otherwise stated.
Median (interquartile range).
For the total population—not only the patients with T2DM.
Level not reported.
3.3. Risk of bias
Risk of bias within the individual studies is shown in Figure 2. All studies, except for one,54 had adequately described the method of randomization. Methods of allocation concealment were properly described in half5, 23, 31, 50, 51, 52, 53 of the included trials, and only two studies31, 50 were blinded (participants and study personnel), whereof one31 was triple blinded (participants, study personnel, and data analyser). All included studies were regarded as having low risk of attrition bias. Four studies were either not registered50, 54, 55 or registered after completion of the trial30 and were thus regarded as having high risk of reporting bias. One study24 was considered to be at high risk of other biases because of deviation from the study protocol reported on Clinical Trials (NCT00667706). A summary of the percentage of trials that were at low, unclear, and high risk of bias for the different domains is shown in Figure 3.
Figure 2.
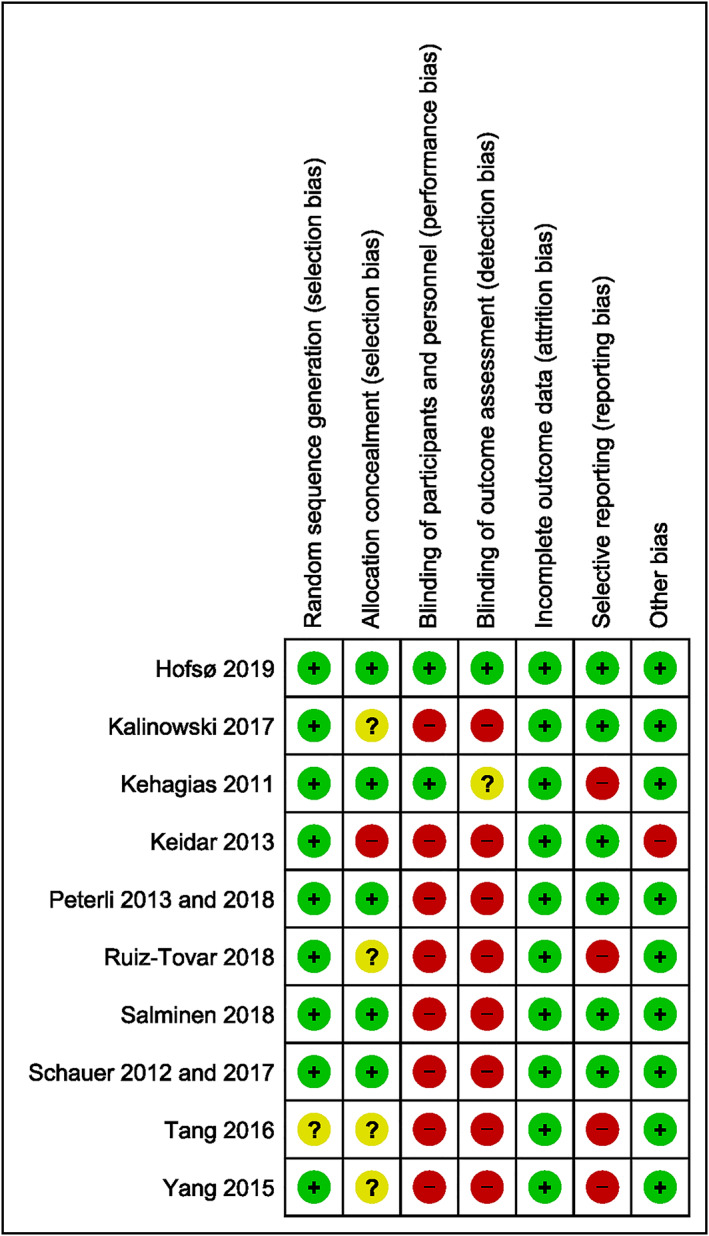
Risk of bias summary
Figure 3.
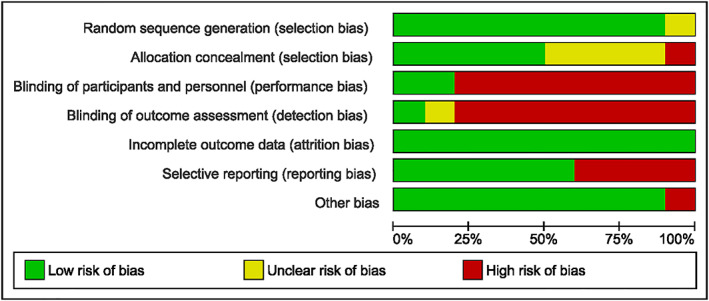
Risk of bias graph
3.4. Results from meta‐analyses
3.4.1. Short‐term follow‐up
Among the seven studies that compared the effects of RYGB and SG on remission of T2DM at 1 year, the remission rate was higher among patients undergoing RYGB (156/276, 57%) compared with those undergoing SG (128/275, 47%), RR (95% CI) 1.20 (1.00‐1.45), P = .047, and the heterogeneity between the studies was low (I 2 = 24.9%, P = .239, moderate‐quality evidence) (Figure 4, Table 2).
Figure 4.
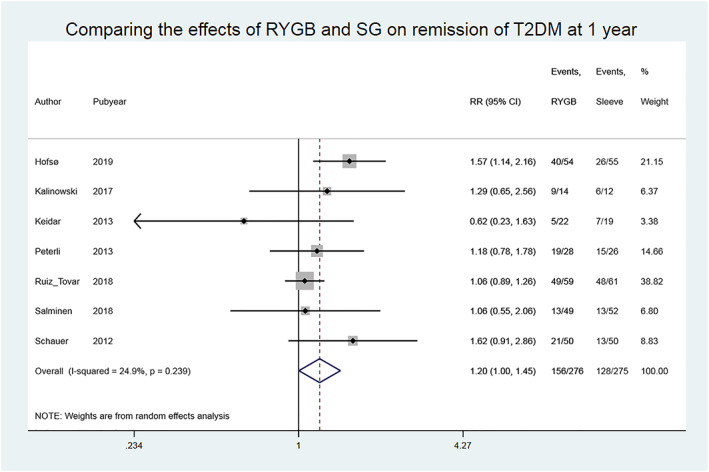
Comparing the effects of Roux‐en‐Y gastric bypass (RYGB) and sleeve gastrectomy (SG) on remission of type 2 diabetes mellitus (T2DM) in studies with short‐term follow‐up
Table 2.
Quality of evidence and summary of findings
| Certainty Assessment | Summary of Findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Participants (Studies) Follow‐Up | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Overall Certainty of Evidence | Study Event Rates, % | Relative Effect (95% CI) | Anticipated Absolute Effects | ||
| With SG | With RYGB | Risk with SG | Risk Difference with RYGB | ||||||||
| Remission of T2DM (follow‐up: 1 year) | |||||||||||
| 551 (7 RCTs) | Seriousa | Not serious | Not serious | Not serious | None | Moderate | 128/275 (46.5%) | 156/276 (56.5%) | RR 1.20 (1.00 to 1.45) | 465 per 1000 | 93 more per 1000 (from 0 fewer to 209 more) |
| Remission of T2DM (follow‐up: range 2 to 5 years) | |||||||||||
| 529 (7 RCTs) | Seriousb | Not serious | Not serious | Seriousc | None | Low | 121/266 (45.5%) | 132/263 (50.2%) | RR 1.06 (0.94 to 1.20) | 455 per 1000 | 27 more per 1000 (from 27 fewer to 91 more) |
Abbreviations: CI, confidence interval; RCT, randomized controlled trial; RR, risk ratio; RYGB, Roux‐en‐Y gastric bypass; SG, sleeve gastrectomy, T2DM, type 2 diabetes mellitus.
See risk of bias figure.
See risk of bias figure.
The 95% CI overlaps no effect.
When excluding the two studies24, 30 with high risk of bias in three domains, the effect estimate increased, RR (95% CI) 1.38 (1.12‐1.70), P = .0023, in favour of RYGB. One of the studies30 with high risk of bias was heavily weighted (38.8%), and removal of this study from the analysis resulted in an effect estimate of 1.33 (1.09‐1.64), P = .0056. In addition, after restricting the analysis to studies that only included patients with T2DM,23, 24, 31 the effect estimate (RR) increased: 1.39 (0.94‐2.07), P = .10.
There was no indication of publication bias as the funnel plot was satisfactory, and the Egger test indicated that there was no small‐study effect (P = .62).
3.4.2. Medium‐term follow‐up
Seven studies reported data on remission of T2DM 2 to 5 years after bariatric surgery. The overall estimate showed that the remission rates were 132/263 (50%) in the RYGB group and 121/266 (46%) in the SG group, with no significant between‐group difference, RR (95% CI) 1.06 (0.94‐1.20), P = .34, and no heterogeneity between the studies, I 2 = 0.0%, P = .70, low‐quality evidence (Figure 5, Table 2).
Figure 5.
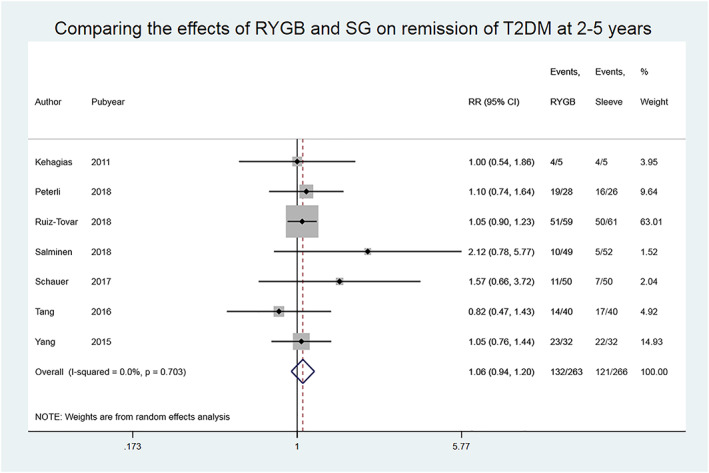
Comparing the effects of Roux‐en‐Y gastric bypass (RYGB) and sleeve gastrectomy (SG) on remission of type 2 diabetes mellitus (T2DM) in studies with medium‐term follow‐up
Excluding the studies judged as being at high risk of bias in at least three domains did not alter the overall effect estimate: 1.19 (0.89‐1.60), P = .25. Three studies included only patients with T2DM, and a subgroup analysis of these studies provided an effect estimate of 1.03 (0.79‐1.34), P = .830.
There was no indication of publication bias as the funnel plot was satisfactory, and the Egger test indicated no small‐study effect (P = .26).
4. DISCUSSION
4.1. Summary of main results
The current systematic review and meta‐analysis compared the effect of RYGB and SG on remission of T2DM in patients with overweight or obesity. Ten different RCTs were included, and the estimates showed that the likelihood of remission was 20% higher among patients undergoing RYGB (57%) compared with SG (47%) 1 year after surgery, but the remission rates did not differ significantly between the surgical procedures 2 to 5 years after surgery. Taking into account the absolute risk difference of 93 more remissions per 1000 surgeries after RYGB shown in the present meta‐analysis and recent estimates of approximately 100 000 patients with diabetes undergoing bariatric surgery per year globally,12, 13 favouring RYGB over SG could result yearly in 9300 more cases with short‐term remission of diabetes.
4.2. Quality of evidence
We consider the quality of evidence from this systematic review to be moderate (short‐term follow‐up) to low (medium‐term follow‐up). The quality of evidence for studies with medium‐term follow‐up was downgraded for serious imprecision because of confidence intervals of the absolute effects being wide. Also, quality of evidence was downgraded one level for risk of bias as we considered risk of bias to be serious both for studies with short‐ and medium‐term follow‐up. Only one of the included trials was judged as having low risk of bias in all domains, and lack of blinding was the main culprit for down‐rating the studies. When studies with higher risk of bias were excluded from the analysis, the effect estimate increased in favour of RYGB. It is worrisome that the study by Ruiz‐Tovar et al,30 which was heavily weighted in our analyses, was registered in a clinical trial registry 2 weeks prior to publication of the results. The authors presented limited data on baseline characteristics and patient selection, and without the exact details as to how this study was conducted, it is difficult to assess potential biases.
4.3. Agreements and disagreements with other reviews
In contrast with our results, a recently published meta‐analysis of RCTs22 reported no significant difference in the 1‐year remission rate of T2DM among patients undergoing RYGB compared with those undergoing SG. However, in this review, the landmark STAMPEDE trial23 and the RCT by Keidar et al were not included in the meta‐analysis of studies with 1‐year follow‐up.24, 54 This review also differed from the present review considering that other variants of gastric bypass, such as banded and mini gastric bypass,41, 42 were included, and understandably, it could not include the recently published results from the Oseberg‐trial.
Partly in contrast with our findings, another recent systematic review25 of RCTs concluded that there was no difference between RYGB and SG in T2DM resolution 1 to 5 years post surgery. However, this meta‐analysis did not include the results from the STAMPEDE trial23 and the study by Tang et al.54
Our findings partly support the results of a previous meta‐analysis of 18 randomized and nonrandomized studies,28 which reported that patients undergoing RYGB had higher short‐ to long‐term odds (95% CI) (1.49 (1.04‐2.12), P = .03) of T2DM resolution than patients undergoing SG.
4.4. Strengths and limitations
The present review was based on a broad literature search performed in cooperation with an expert librarian, and it is unlikely that important trials were overlooked. However, the number of studies included in the meta‐analyses were low, and the pooled estimates should be interpreted with caution. This review included studies of patients with similar characteristics (patients with T2DM eligible for bariatric surgery), and this is reflected in the low heterogeneity between the studies. Because of the low number of studies included in the meta‐analyses, we cannot exclude the possibility of publication bias. The definitions of remission of T2DM varied slightly between studies, some studies reported only data on partial remission, and seemingly small differences in glycaemic thresholds used to define diabetes remission may impact the proportions achieving remission, making comparisons between studies difficult.
5. CONCLUSION
The results from this systematic review show that RYGB resulted in a higher remission rate of T2DM after 1 year compared with SG. In studies with medium‐term follow‐up, there were no differences in T2DM remission rates between the two procedures; however, larger studies with longer term follow‐up are warranted.
AUTHOR CONTRIBUTIONS
H.B., D.H., J.K.H., and J.H. wrote the manuscript. H.B. was responsible for the statistical analyses. All authors critically participated in data interpretation, reviewed the manuscript for intellectual content, and approved the final version of the manuscript.
CONFLICT OF INTEREST
No conflict of interest was declared.
FUNDING
Vestfold Hospital Trust.
Supporting information
Data S1. Supporting information
Table S1. Supporting information
ACKNOWLEDGEMENTS
We are grateful to Mariann Mathiesen at the Medical Libraries at Vestfold Hospital Trust for performing the systematic search, and we thank Matthew McGee for proofreading the manuscript.
Borgeraas H, Hofsø D, Hertel JK, Hjelmesæth J. Comparison of the effect of Roux‐en‐Y gastric bypass and sleeve gastrectomy on remission of type 2 diabetes: A systematic review and meta‐analysis of randomized controlled trials. Obesity Reviews. 2020;21:e13011 10.1111/obr.13011
REFERENCES
- 1. WHO . Diabetes. https://www.who.int/news-room/fact-sheets/detail/diabetes. Published 2018. Updated October 30. 2018. .
- 2. Lean ME, Leslie WS, Barnes AC, et al. Primary care‐led weight management for remission of type 2 diabetes (DiRECT): an open‐label, cluster‐randomised trial. Lancet. 2018;391(10120):541‐551. [DOI] [PubMed] [Google Scholar]
- 3. Coleman KJ, Haneuse S, Johnson E, et al. Long‐term microvascular disease outcomes in patients with type 2 diabetes after bariatric surgery: evidence for the legacy effect of surgery. Diabetes Care. 2016;39(8):1400‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American DA. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917‐928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—5‐year outcomes. N Engl J Med. 2017;376(7):641‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Courcoulas AP, Belle SH, Neiberg RH, et al. Three‐year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg. 2015;150(10):931‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Halperin F, Ding SA, Simonson DC, et al. Roux‐en‐Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1‐year results of a randomized clinical trial. JAMA Surg. 2014;149(7):716‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ikramuddin S, Korner J, Lee WJ, et al. Roux‐en‐Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the diabetes surgery study randomized clinical trial. JAMA ‐ Journal of the American Medical Association. 2013;309(21):2240‐2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jakobsen G, Småstuen M, Sandbu R, et al. Association of bariatric surgery vs medical obesity treatment with long‐term medical complications and obesity‐related comorbidities. JAMA. 2018;319(3):291‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hofso D, Nordstrand N, Johnson LK, et al. Obesity‐related cardiovascular risk factors after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur. 2010;163(5):735‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American Diabetes Association . 7. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(Suppl 1):S65‐S72. [DOI] [PubMed] [Google Scholar]
- 12. Welbourn R, Hollyman M, Kinsman R, et al. Bariatric surgery worldwide: baseline demographic description and one‐year outcomes from the Fourth IFSO Global Registry Report 2018. Obes Surg. 2019;29(3):782‐795. [DOI] [PubMed] [Google Scholar]
- 13. Angrisani L, Santonicola A, Iovino P, et al. IFSO Worldwide Survey 2016: primary, endoluminal, and revisional procedures. Obes Surg. 2018;28(12):3783‐3794. [DOI] [PubMed] [Google Scholar]
- 14. Batterham RL, Cummings DE. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care. 2016;39(6):893‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Korner J, Bessler M, Cirilo LJ, et al. Effects of Roux‐en‐Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90(1):359‐365. [DOI] [PubMed] [Google Scholar]
- 16. Laferrere B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux‐en‐Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30(7):1709‐1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morinigo R, Lacy AM, Casamitjana R, Delgado S, Gomis R, Vidal J. GLP‐1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg. 2006;16(12):1594‐1601. [DOI] [PubMed] [Google Scholar]
- 18. le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243(1):108‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kindel TL, Martins PJ, Yoder SM, et al. Bypassing the duodenum does not improve insulin resistance associated with diet‐induced obesity in rodents. Obesity (Silver Spring). 2011;19(2):380‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meek CL, Lewis HB, Reimann F, Gribble FM, Park AJ. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides. 2016;77:28‐37. [DOI] [PubMed] [Google Scholar]
- 21. Debédat J, Amouyal C, Aron‐Wisnewsky J, Clément K. Impact of bariatric surgery on type 2 diabetes: contribution of inflammation and gut microbiome? Semin Immunopathol. 2019;41(4):461‐475. [DOI] [PubMed] [Google Scholar]
- 22. Lee Y, Doumouras AG, Yu J, et al. Laparoscopic sleeve gastrectomy versus laparoscopic Roux‐en‐Y gastric bypass: a systematic review and meta‐analysis of weight loss, comorbidities, and biochemical outcomes from randomized controlled trials. Ann Surg. 2019;1. [DOI] [PubMed] [Google Scholar]
- 23. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567‐1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keidar A, Hershkop KJ, Marko L, et al. Roux‐en‐Y gastric bypass vs sleeve gastrectomy for obese patients with type 2 diabetes: a randomised trial. Diabetologia. 2013;56(9):1914‐1918. [DOI] [PubMed] [Google Scholar]
- 25. Osland E, Yunus RM, Khan S, Memon B, Memon MA. Diabetes improvement and resolution following laparoscopic vertical sleeve gastrectomy (LVSG) versus laparoscopic Roux‐en‐Y gastric bypass (LRYGB) procedures: a systematic review of randomized controlled trials. Surg Endosc Other Interv Tech. 2017;31(4):1952‐1963. [DOI] [PubMed] [Google Scholar]
- 26. Zhang Y, Wang J, Sun X, et al. Laparoscopic sleeve gastrectomy versus laparoscopic Roux‐en‐Y gastric bypass for morbid obesity and related comorbidities: a meta‐analysis of 21 studies. [Erratum appears in Obes Surg. 2015 Jan;25(1):27 Note: Ju, Wang [corrected to Wang, Ju]; Cao, Zhanguo [corrected to Cao, Zhangou]; Xinsheng, Xu [corrected to Xu, Xinsheng]; Daquan, Liu [corrected to Liu, Daquan]; Xiangyang, Xin [corrected to Xin, Xiangyang]; PMID: 25287532]. Obes Surg. 2015;25(1):19‐26. [DOI] [PubMed] [Google Scholar]
- 27. Yu J, Zhou X, Li L, et al. The long‐term effects of bariatric surgery for type 2 diabetes: systematic review and meta‐analysis of randomized and non‐randomized evidence. Obes Surg. 2015;25(1):143‐158. [DOI] [PubMed] [Google Scholar]
- 28. Li JF, Lai DD, Lin ZH, Jiang TY, Zhang AM, Dai JF. Comparison of the long‐term results of Roux‐en‐Y gastric bypass and sleeve gastrectomy for morbid obesity: a systematic review and meta‐analysis of randomized and nonrandomized trials. Surgical Laparoscopy, Endoscopy and Percutaneous Techniques. 2014;24(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 29. Yip S, Plank LD, Murphy R. Gastric bypass and sleeve gastrectomy for type 2 diabetes: a systematic review and meta‐analysis of outcomes. Obes Surg. 2013;23(12):1994‐2003. [DOI] [PubMed] [Google Scholar]
- 30. Ruiz‐Tovar J, Carbajo MA, Jimenez JM, et al. Long‐term follow‐up after sleeve gastrectomy versus Roux‐en‐Y gastric bypass versus one‐anastomosis gastric bypass: a prospective randomized comparative study of weight loss and remission of comorbidities. Surg Endosc. 2019;33(2):401‐410. [DOI] [PubMed] [Google Scholar]
- 31. Hofsø D, Fatima F, Borgeraas H, et al. Gastric bypass versus sleeve gastrectomy in patients with type 2 diabetes (Oseberg): a single‐centre, triple‐blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(12):912‐924. [DOI] [PubMed] [Google Scholar]
- 32. Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care. 2009;32(11):2133‐2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Barros F, Setubal S, Martinho JM, Monteiro AB. Early endocrine and metabolic changes after bariatric surgery in grade III morbidly obese patients: a randomized clinical trial comparing sleeve gastrectomy and gastric bypass. Metab Syndr Relat Disord. 2015;13(6):264‐271. [DOI] [PubMed] [Google Scholar]
- 37. Helmio M, Victorzon M, Ovaska J, et al. SLEEVEPASS: a randomized prospective multicenter study comparing laparoscopic sleeve gastrectomy and gastric bypass in the treatment of morbid obesity: preliminary results. Surg Endosc. 2012;26(9):2521‐2526. [DOI] [PubMed] [Google Scholar]
- 38. Helmio M, Victorzon M, Ovaska J, et al. Comparison of short‐term outcome of laparoscopic sleeve gastrectomy and gastric bypass in the treatment of morbid obesity: a prospective randomized controlled multicenter SLEEVEPASS study with 6‐month follow‐up. Scand J Surg. 2014;103(3):175‐181. [DOI] [PubMed] [Google Scholar]
- 39. Peterli R, Wolnerhanssen B, Peters T, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux‐en‐Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250(2):234‐241. [DOI] [PubMed] [Google Scholar]
- 40. Zhang Y, Zhao H, Cao Z, et al. A randomized clinical trial of laparoscopic Roux‐en‐Y gastric bypass and sleeve gastrectomy for the treatment of morbid obesity in China: a 5‐year outcome. Obes Surg. 2014;24(10):1617‐1624. [DOI] [PubMed] [Google Scholar]
- 41. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146(2):143‐148. [DOI] [PubMed] [Google Scholar]
- 42. Murphy R, Clarke MG, Evennett NJ, et al. Laparoscopic sleeve gastrectomy versus banded Roux‐en‐Y gastric bypass for diabetes and obesity: a prospective randomised double‐blind trial. Obes Surg. 2018;28(2):293‐302. [DOI] [PubMed] [Google Scholar]
- 43. Casajoana A, Pujol J, Garcia A, et al. Predictive value of gut peptides in T2D remission: randomized controlled trial comparing metabolic gastric bypass, sleeve gastrectomy and greater curvature plication. Obes Surg. 2017;27(9):2235‐2245. [DOI] [PubMed] [Google Scholar]
- 44. Nemati R, Lu J, Dokpuang D, Booth M, Plank LD, Murphy R. Increased bile acids and FGF19 after sleeve gastrectomy and Roux‐en‐Y gastric bypass correlate with improvement in type 2 diabetes in a randomized trial. Obes Surg. 2018;28(9):2672‐2686. [DOI] [PubMed] [Google Scholar]
- 45. Viana EC, Araujo‐Dasilio KL, Miguel GPS, et al. Gastric bypass and sleeve gastrectomy: the same impact on IL‐6 and TNF‐α. Prospective Clinical Trial. Obesity Surgery. 2013;23(8):1252‐1261. [DOI] [PubMed] [Google Scholar]
- 46. Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide‐YY levels after Roux‐en‐Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247(3):401‐407. [DOI] [PubMed] [Google Scholar]
- 47. Kashyap SR, Bhatt DL, Wolski K, et al. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care. 2013;36(8):2175‐2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kalinowski P, Paluszkiewicz R, Wroblewski T, et al. Ghrelin, leptin, and glycemic control after sleeve gastrectomy versus Roux‐en‐Y gastric bypass‐results of a randomized clinical trial. Surg Obes Relat Dis. 2017;13(2):181‐188. [DOI] [PubMed] [Google Scholar]
- 49. Paluszkiewicz R, Kalinowski P, Wroblewski T, et al. Improvement of metabolic parameters after laparoscopic sleeve gastrectomy (LSG) versus Roux‐En‐Y gastric bypass (RYGB)—early results of a prospective randomized trial. Obes Surg. 2011;21(8):983‐984. [Google Scholar]
- 50. Kehagias I, Karamanakos SN, Argentou M, Kalfarentzos F. Randomized clinical trial of laparoscopic Roux‐en‐Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI<50 kg/m2 . Obes Surg. 2011;21(11):1650‐1656. [DOI] [PubMed] [Google Scholar]
- 51. Peterli R, Borbely Y, Kern B, et al. Early results of the swiss muliticentre bypass or sleeve study (SMBOSS): A prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux‐en‐Y gastric bypass. Annals of Surgery. 2013;258(5):690‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Peterli R, Wölnerhanssen B, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux‐en‐y gastric bypass on weight loss in patients with morbid obesity: the SM‐BOSS randomized clinical trial. JAMA. 2018;319(3):255‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Salminen P, Helmio M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux‐en‐Y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA. 2018;319(3):241‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tang Q, Sun Z, Zhang N, et al. Cost‐effectiveness of bariatric surgery for type 2 diabetes mellitus: a randomized controlled trial in China. Medicine (Baltimore). 2016;95(20):e3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang J, Wang C, Cao G, et al. Long‐term effects of laparoscopic sleeve gastrectomy versus roux‐en‐Y gastric bypass for the treatment of Chinese type 2 diabetes mellitus patients with body mass index 28‐35 kg/m(2). BMC Surg. 2015;15(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information
Table S1. Supporting information


