Abstract
Neuronal dendrites are highly branched and specialized compartments with distinct structures and secretory organelles (e.g., spines, Golgi outposts), and a unique cytoskeletal organization that includes microtubules of mixed polarity. Dendritic membranes are enriched with proteins, which specialize in the formation and function of the post-synaptic membrane of the neuronal synapse. How these proteins partition preferentially in dendrites, and how they traffic in a manner that is spatiotemporally accurate and regulated by synaptic activity are long-standing questions of neuronal cell biology. Recent studies have shed new insights into the spatial control of dendritic membrane traffic, revealing new classes of proteins (e.g., septins) and cytoskeleton-based mechanisms with dendrite-specific functions. Here, we review these advances by revisiting the fundamental mechanisms that control membrane traffic at the levels of protein sorting and motor-driven transport on microtubules and actin filaments. Overall, dendrites possess unique mechanisms for the spatial control of membrane traffic, which might have specialized and co-evolved with their highly arborized morphology.
Keywords: dendrites, axons, microtubules, actin, septins, kinesins, myosins, adaptor proteins, scaffolds, sorting, membrane traffic
1. Introduction
Named after “dendron”, the Greek word for “tree”, dendrites are highly branched processes that neurons develop for connecting and communicating with axons. Most neurons generate only a single axon, but form multiple dendrites with complex geometries and morphologies, which differ depending on their three-dimensional position (i.e., apical, basal) and neuronal cell type (Benavides-Piccione et al., 2006; DeFelipe and Jones, 1988; Fiala and Harris, 1999; Koester and O’Leary, 1992; McAllister, 2000; Spruston, 2008). Dendrites are compartments with distinct membrane organelle and protein content, and develop the highly specialized dendritic spines, which form synapses and regulate synaptic strength (Hanus and Ehlers, 2008; Hering and Sheng, 2001; Horton and Ehlers, 2004; Nusser, 2012; Rochefort and Konnerth, 2012). In contrast to axons, whose morphogenesis has been extensively studied, it is less understood how dendrites acquire their structural and biochemical identity. As axonal morphogenesis precedes dendritic outgrowth, dendrites have been often viewed as structures made by default, so to speak. Recent advances, however, amend this view, showing that dendrite-specific mechanisms account for the structural and biochemical identity of dendrites. Here, we review the molecular players and mechanisms that spatially control membrane traffic in dendrites focusing on protein sorting in secretory and endocytic organelles, and transport on the microtubule and actin cytoskeleton (Figure 1).
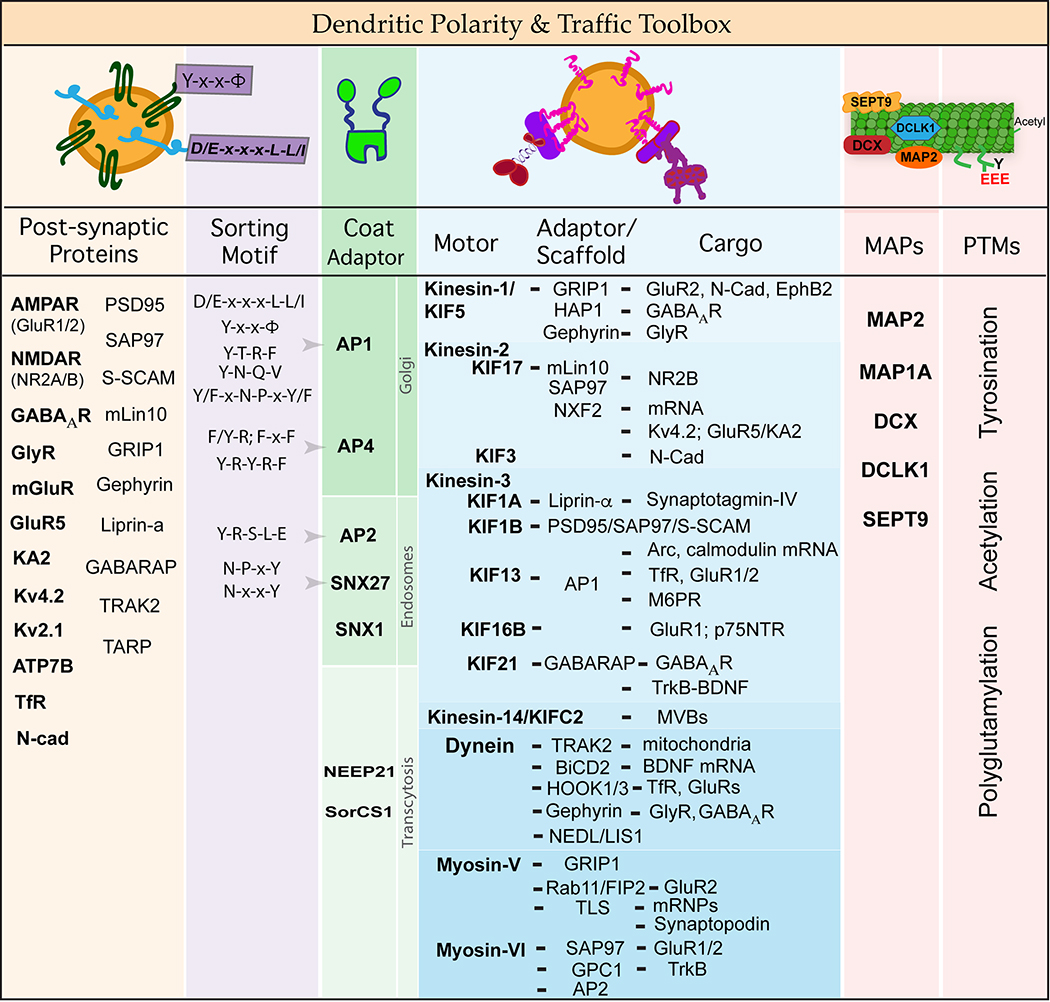
Figure 1.
Dendritic polarity and traffic toolbox. Dendrites are enriched with post-synaptic membrane proteins including the AMPA (GluR1/2, GluR2/3) and NMDA (NR1, NR2A/B) receptors of the excitatory synapse, the GABAA and glycine receptors (GlyR) of the inhibitory synapse, kainate receptors such as GluR5 and KA2, potassium (Kv4.2, Kv2.1) and copper (ATP7B) ion channels, and the transferrin receptor (TfR). Dendritic localization and targeting of these receptors and channels involves interaction with adaptor and scaffold proteins (e.g., PSD95, SAP97, GRIP1, mLin10, Liprin-α, GABARAP), which interface with membrane- and cytoskeleton-based mechanisms of intracellular traffic. Specificity in the trafficking of dendritic membrane proteins is achieved during sorting at the trans-Golgi and endosomes, transport by kinesin, dynein and myosin motors, and additional guidance from microtubule-associated proteins (MAPs) and post-translational modifications (PTMs) of the cytoskeleton which provide spatial cues. This figure summarizes and tabulates the sorting motifs that interact with membrane coat adaptors, the cytoskeletal motors and their interactions with adaptor/scaffold and cargo proteins, and the MAPs and PTMs that function in dendritic membrane polarity and traffic.
2. Dendrite morphogenesis: cytoskeletal and membrane organization
Dendrite outgrowth and arborization have been well documented dating back to the historic observations of Camillo Golgi and Santiago Ramon y Cajal, which were made in histological preparations, and the seminal studies of Gary Banker in living cultured neurons (Banker, 2018; Bentivoglio et al., 2019; Garcia-Lopez et al., 2010). Observations of neurons in vivo and in vitro show that dendrites often form and grow after the development of an axon (Barnes and Polleux, 2009; Dotti et al., 1988; Funahashi et al., 2014; Tahirovic and Bradke, 2009). Dendritic outgrowth is characterized by an inherent asymmetry as the apical dendrite extends longer and terminates into a branched pattern (apical tuft), while basal dendrites undergo continuous branching forming trees of diverse lengths and terminal branches (Fiala and Harris, 1999; Lefebvre et al., 2015; McAllister, 2000; Spruston, 2008). In pyramidal neurons that migrate radially to the cortical plate, the apical dendrite is generated from the leading process with cues from the extracellular matrix (Funahashi et al., 2014; Kon et al., 2017; Sakakibara and Hatanaka, 2015). However, in a subset of migrating neurons of the developing hippocampus that lack a leading process, and in cultured neurons in vitro, the apical dendrite appears to arise stochastically from one of the undifferentiated neurites of the cell body (Funahashi et al., 2014; Kon et al., 2017; Sakakibara and Hatanaka, 2015). During growth, both apical and basal dendrites taper off as their shaft becomes thinner with increasing distance from the cell body (Fiala and Harris, 1999; Lefebvre et al., 2015; McAllister, 2000). This is a salient characteristic of dendrites as axons do not taper off. Dendrites also exhibit a self-avoidance phenomenon termed tiling, which is the ability to grow and branch into areas without overlapping due to repulsive dendrite-to-dendrite cues (Grueber and Sagasti, 2010; Lefebvre et al., 2015). Dendrite outgrowth and branching are highly dynamic as growing dendrites elongate, shrink, cease to grow and remodel their branched arbors, all of which are influenced by extracellular factors and synaptic activity (Lohmann and Wong, 2005; Maletic-Savatic et al., 1999; McAllister, 2000; Spruston, 2008). Knowledge of how dendrite dynamics are extrinsically and intrinsically controlled has been steadily advancing. Studies in genetically tractable organisms such as D. melanogaster and C. elegans have yielded important insights and were reviewed previously (Dong et al., 2015; Jan and Jan, 2010; Puram and Bonni, 2013; Sundararajan et al., 2019; Yogev and Shen, 2017).
At a rudimentary intrinsic level, dendrite development is mechanistically dependent on the microtubule and actin cytoskeleton, which drives neurite formation, and exocytosis, which supplies membrane for dendritic growth (Conde and Caceres, 2009; Kennedy and Ehlers, 2011; Konietzny et al., 2017; Penazzi et al., 2016). The spatial organization of microtubules and actin, which are structurally polarized polymers, is critical for dendrite morphogenesis, influencing neurite formation and the directionality of membrane traffic. Early observations using electron microscopy techniques showed that undifferentiated neurites and the distal regions of mature dendrites contain uniformly oriented microtubules with their plus-ends facing away (plus-endout) from the cell body (Baas et al., 1989; Baas et al., 1988; Baas and Lin, 2011). In contrast, proximal and mid regions of the dendritic shaft contained microtubules with mixed orientation, suggesting that minus-end-out microtubules are added during the development of dendrites (Baas et al., 1989; Baas et al., 1988; Wang et al., 1996). Recent studies have confirmed the presence of a microtubule network of mixed orientation in dendritic shafts, but microtubules with minus-end-out orientation were also found in immature neurites and distal dendrites (Kollins et al., 2009; Yau et al., 2016). Moreover, dendritic microtubules appear to organize in bundles of opposite unidirectional orientation that stretch along the dendritic shaft, resembling the microtubules of the ciliary axoneme that function like double-track railways for intraflagellar transport (Stepanek and Pigino, 2016; Tas et al., 2017). In contrast to mammalian dendrites, microtubules in Drosophila and C. elegans dendrites are predominately orientated with their minus-ends-out (Rolls, 2011).
In coordination with microtubules, the actin cytoskeleton promotes the growth and shape of dendrites (Konietzny et al., 2017; Lei et al., 2016). Prior to dendrite differentiation, waves of actin polymerization propagate from the cell body to the tip of neurites, widening the shafts of neurites and thereby enabling more microtubule growth and membrane traffic (Flynn et al., 2009; Ruthel and Banker, 1999; Winans et al., 2016). Actin polymerization drives chiefly the formation of lamellipodia and filopodia, which are membrane protrusions that give rise to neurites, dendritic branches and spines, and shape the growth cones at the tips of growing dendrites (Flynn, 2013; Gallo, 2013; Miller and Suter, 2018; Omotade et al., 2017). Lamellipodial protrusions are generated from branched actin filaments and filopodia from bundles of linear actin filaments, which are in part made by remodeling of branched actin (Pollard et al., 2000; Svitkina, 2018). Polymerization of branched and linear actin filaments provide a physical force for neurite membrane outgrowth, which is also aided by microtubules pushing against the plasma membrane by sliding against neighboring microtubules and/or linear actin bundles (Del Castillo et al., 2019; Flynn, 2013; Miller and Suter, 2018). These cytoskeletal interactions are critical not only for the formation of nascent neurites, but also the branching of dendrites and the advance of their growth cones.
In contrast to axonal morphogenesis, which early on is characterized by the formation of periodic circumferential actin rings that connect to the membrane via spectrin, dendrites do not develop this membrane-associated periodic skeleton (MPS) until they are fully matured (Han et al., 2017; Unsain et al., 2018; Zhong et al., 2014). However, only a subset of dendrites contains MPS with circumferential actin rings (Bar et al., 2016; Han et al., 2017; He et al., 2016; Sidenstein et al., 2016; Zhong et al., 2014). Somatodendritic membranes have a polygonal lattice-like actin-spectrin array, which resembles the membrane skeleton of erythrocytes (Han et al., 2017; He et al., 2016; Zhong et al., 2014).
Dendritic growth is dependent on the secretory pathway. Studies in Drosophila and mammalian neurons have demonstrated that defects in ER-to-Golgi traffic diminish membrane supply to dendrites without affecting axon growth (Horton et al., 2005; Ye et al., 2007). Consistent with these findings, dendrites possess ribosome-bound ER (rough ER), smooth ER, ER exit sites, ER-to-Golgi intermediate compartment (ERGIC) and Golgi membranes (Hanus and Ehlers, 2016; Kennedy and Ehlers, 2006; Ramirez and Couve, 2011; Wu et al., 2017). In contrast, axons contain predominately smooth ER with a tubular appearance and are largely devoid of rough ER and Golgi, although the latter have been observed in the axons of sensory neurons (Luarte et al., 2018; Merianda et al., 2009; Terasaki, 2018). ER-derived vesicles traffic locally or undergo long-range transport, which is directed to the ER-to-Golgi intermediate compartment (ERGIC) and Golgi membranes, or alternatively traffic to recycling endosomes and the plasma membrane bypassing the Golgi (Bowen et al., 2017; Hanus and Ehlers, 2008; Hanus et al., 2014; Krijnse-Locker et al., 1995).
Golgi membranes are found in dendrites and localize preferentially at branch points. These Golgi outposts have been shown to promote the nucleation of acentrosomal microtubules (Horton et al., 2005; Ori-McKenney et al., 2012). Dendritic branch growth and retraction correlate with the presence and absence of Golgi outposts, respectively (Ye et al., 2007). Notably, the Golgi apparatus of the cell body localizes at the base of the long apical dendrite, which further underscores the role of Golgi-derived vesicle traffic in dendritic growth (Wu et al., 2015). Recent evidence indicates that dendrites contain a more extensive Golgi satellite membrane system, which glycosylates dendritic proteins en route to endosomes (Mikhaylova et al., 2016).
Recycling endosomes and endolysosomes are distributed along the dendritic shafts and spines, regulating the traffic, localization and turnover of post-synaptic proteins (Lazo et al., 2013; Park et al., 2006; Satoh et al., 2008; van der Sluijs and Hoogenraad, 2011; Winckler et al., 2018). Lysosomes localize to and fuse with dendritic spines in response to synaptic activity, which promotes synaptic plasticity through the turnover of synaptic membrane proteins and remodeling of the extracellular matrix by metalloproteinases (Goo et al., 2017; Padamsey et al., 2017; van Bommel et al., 2019). Interestingly, lysosomes of highly acidic pH localize predominately in proximal dendrites and the cell body, where the bulk degradation occurs, and degradation of dendritic cargo involves trafficking of endosomes from distal to proximal dendrites (Kulkarni and Maday, 2018; Yap et al., 2018). Taken together with the distinct features of the dendritic cytoskeleton, this higher-order organization of secretory and endocytic organelles is indicative of dendrite-specific mechanisms for the spatial control of the cytoskeleton and membrane traffic.
3. Sorting of dendritic membrane proteins in secretory and endocytic organelles
Segregation of membrane proteins into distinct organelle domains and vesicular carriers is the first step in establishing specific intracellular routes and destinations. Sorting of membrane proteins during exit from the trans-Golgi network was first discovered for proteins targeted to the apical and basolateral domains of polarized epithelia, and subsequently found to be conserved in hippocampal neurons, where epithelial basolateral proteins largely sort into dendrites (Dotti et al., 1991; Dotti and Simons, 1990; Jareb and Banker, 1998; Simons and Wandinger-Ness, 1990). Sorting of apical proteins into axons has been controversial, but several axonal proteins traffic to the apical membrane of epithelial cells (Anderson et al., 2005; Dotti et al., 1991; Jareb and Banker, 1998; Yeaman et al., 1997). Conservation between the basolateral and somatodendritic, and potentially apical and axonal trafficking pathways pointed to specific sorting determinants (e.g., amino acid motifs, glycan residues) and mechanisms (Maeder et al., 2014; Mellman and Nelson, 2008; Mostov et al., 2003; Rodriguez-Boulan and Powell, 1992). Over the years, a diversity of membrane adaptor proteins has been identified to selectively interact with membrane proteins, mediating their clustering and packaging into distinct vesicles during exit from the Golgi complex and endolysosomal organelles (Bonifacino, 2014; Guardia et al., 2018; Nakatsu and Ohno, 2003).
During exit from the Golgi complex, dendritic membrane proteins are sorted from their axonal counterparts by clathrin adaptor proteins (Figures 1 and 2). From the existing five heterotetrameric clathrin adaptor proteins (APs), AP-1, AP-3 and AP-4 function at the trans-Golgi network (Bonifacino, 2014). Axonal and dendritic membrane proteins contain dileucine (D/E-x-x-x-L-L/I; x, any amino acid) and tyrosine-based (Y-x-x-Φ; Φ, bulky hydrophobic residue) motifs in their cytoplasmic tails, which are recognized by APs that compete for binding to these motifs with differential affinities (Dwyer et al., 2001; Farias et al., 2012; Jain et al., 2015; Li et al., 2016; Margeta et al., 2009; Nakatsu et al., 2004; Salazar et al., 2005; Zhou et al., 2016). Presence of a proline residue upstream of dileucine motifs has been shown to increase the affinity of AP-3 over AP-1 (Rodionov et al., 2002). Dendritic membrane proteins are primarily sorted by AP-1, which is required for the dendritic polarity of the N-methyl-D-aspartate (NMDA) receptor subunits NR2A/NR2B, the metabotropic glutamate receptor 1 mGluR1, the potassium voltage-gated channel Kv2.1 and copper transporter ATP7B (Farias et al., 2012; Jain et al., 2015; Zhou et al., 2016). Interestingly, the dendritic localization of post-synaptic AMPA (α-amino-3-hydroxy5-methyl-4-isoxazolepropionic acid) receptors is not mediated by AP-1. Instead, it requires AP-4 which associates with the GluR1-GluR4 subunits indirectly by interacting with transmembrane AMPA receptor regulatory proteins (TARPs) through an unconventional phenylalanine- and tyrosine-rich motif (YRYRF) (Matsuda et al., 2008; Yap et al., 2003). Additionally, AP-4 recognizes motifs with FR and FTF residues, which are present in the cytoplasmic tails of olfactory receptors (Yap et al 2003). In C. elegans, which does not express AP-4, these receptors are sorted by AP-1 (Dwyer et al., 2001). AP1-binding motifs are also critical for the dendritic polarity of the Coxsackie and adenovirus receptor (CAR; YNQV), the transferrin receptor (TfR; YTRF) and the low-density lipoprotein receptor (LDLR; FxNPxY), which is not expressed in neurons and interacts with AP-1 indirectly (Brown et al., 1997; Chen et al., 1990; Collawn et al., 1990; Farias et al., 2012; Silverman et al., 2001; West et al., 1997).
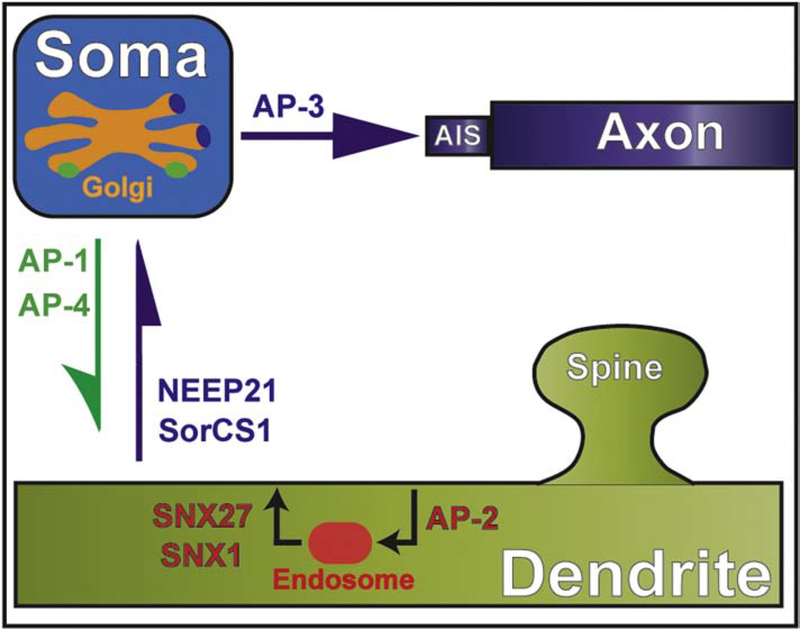
Figure 2.
Sorting of somatodendritic and axonal proteins by membrane adaptors. Schematic shows the major compartments of a neuronal cell and the clathrin adaptors (AP-1, AP-3, AP-4) involved in the sorting of somatodendritic and axonal proteins in the trans-Golgi of the cell body. Adaptors proteins (NEEP21, SorCS1) that mediate the sorting of axonal proteins for retrieval from dendrites (transcytosis) and the endocytic recycling of dendritic membrane proteins (AP-2, SNX27, SNX1) are also outlined.
Dendritic membrane polarity relies on protein sorting not only in the trans-Golgi network en route to the plasma membrane, but also in endosomes. In the endocytic pathway, dendritic proteins are retained by recycling back to the plasma membrane and axonal membrane proteins are selectively sorted for return to the axon, a trafficking pathway known as transcytosis (Lasiecka and Winckler, 2011; van der Sluijs and Hoogenraad, 2011). Endosomes, which are broadly distributed along the dendritic shaft, every two microns and within 300 nm of the plasma membrane, recycle the bulk of dendritic membrane proteins (e.g., AMPA, NMDA and G-proteincoupled receptors) (Choy et al., 2014). These endosomes associate with the retromer (Vps26/29/35), a membrane coat complex that recognizes and sorts proteins into tubular membrane domains for trafficking to plasma membrane or the Golgi complex, averting movement to degradative lysosomes (Choy et al., 2014; Gallon and Cullen, 2015). Recognition of specific proteins by the retromer complex is mediated by the sorting nexins (SNXs), which contain phospoinositide- and protein-binding domains and interact directly with the retromer (Gallon and Cullen, 2015).
The sorting nexin SNX27 is enriched in dendrites and functions as retromer adaptor for dendritic membrane proteins. SNX27 has an N-terminal PDZ (PSD95, Dlg, Zo1) domain, which recognizes the C-terminal PDZ-binding motifs of a number of post-synaptic receptors and channels including the β2-adrenegic and AMPA receptors that recycle to the dendritic shaft membrane via retromer-associated endosomes (Halff et al., 2019; Hussain et al., 2014; Lauffer et al., 2010; Loo et al., 2014; Lunn et al., 2007). Phosphorylation of serine and threonine residues at the C-terminal end of the PDZ-binding motifs of NMDA receptors enhances binding to SNX27 (Clairfeuille et al., 2016). SNX27 also contains a FERM (band4.1-ezrin-radixinmoesin)-like domain that recognizes the N-P-x-Y motif of activated tropomyosin-related kinase A (TrkA) receptors with a phosphorylated tyrosine at the Y0 position (Ghai et al 2013). In addition to SNX27, the sorting nexin SNX1 has been shown to regulate the recycling of the metabotropic glutamate receptor mGluR1, and SNX16 is implicated in the tubulation of neuronal late endosomes (Sharma et al., 2018; Wang et al., 2019).
Removal of axonal membrane proteins from the somatodendritic compartment through transcytosis is critical for maintaining dendritic membrane identity and regulating the axon-dendrite balance of neuronal proteins in a developmental and physiological context. In dendritic endosomes, selective sorting of axon-destined proteins has been reported for neuronal proteins including the pre-synaptic cell adhesion molecules L1/NgCAM and neurexin, the cannabinoid receptor type 1 (CB1R) and neurotrophin receptors (Ascano et al., 2009; Bel et al., 2009; Leterrier et al., 2006; Wisco et al., 2003). In the secretory pathway, L1/NgCAM is first targeted to dendrites through the AP-binding cytoplasmic motif YRSLE, which also functions as a motif for endocytosis from the plasma membrane by AP-2 (Yap et al., 2008a). Transcytosis of L1/NgCAM to the axon depends on a glycine and serine rich sequence of its cytoplasmic tail and a second signal in its extracellular domain (Sampo et al., 2003; Yap et al., 2008a). It is unclear how these crosstalk with the YRSLE motif for sequential targeting to dendrites and then the axon. However, phosphorylation of the tyrosine of YRSLE suggests a mechanism by which the dendritic signal might become weaker or rendered inactive in a spatiotemporally controlled manner (Schaefer et al., 2002; Wisco et al., 2003).
L1/NgCAM transcytosis begins with sorting in dendritic recycling endosomes, where NgCAM segregates into motile tubular-vesicular carriers of distinct lipid composition that divert away from traffic to the lysosome (Yap et al., 2008b). The precise mechanism of this sorting event is not known, but it requires NEEP21 (neuron-enriched endosomal protein of 21 kD), which belongs to the NEEP21/Calcyon/P19 family of endosomal sorting proteins that interact with APs and dynein (Lasiecka et al., 2014; Shi et al., 2018; Shi et al., 2017; Yap et al., 2008b). Interestingly, NEEP21 is also involved in the recycling of AMPA receptors to the dendritic plasma membrane (Alberi et al., 2005; Steiner et al., 2005; Steiner et al., 2002), raising the question of how NEEP21 distinguishes between cargo that recycles back to the dendritic membrane and transcytotic cargo destined for the axon.
Similar to the adaptor-like function of NEEP21, SorCS1 (sortillin-related CNS expressed 1) is involved in the endosomal sorting of neurexin for transcytosis to the axon (Ribeiro et al., 2019). SorCS1 functions during the transition of neurexin from early endosomes to the recycling endosome, interacting with the Rab11-interacting protein Rip11/FIP5, which has been shown to recruit kinesin-2 motors to tubular endosomes in non-neuronal cells (Ribeiro et al., 2019). Axonal localization of Caspr2 (contactin-associated protein 2), another cell adhesion molecule of the neurexin family, is also dependent on endocytosis from the dendritic plasma membrane and regulated by phosphorylation by protein kinase C (PKC) (Bel et al., 2009). Endocytosis of the neurotrophin receptor TrkA and the cannabinoid receptor type 1 (CB1R) from the plasma membrane of the cell body is also required for anterograde transport into axons (Ascano et al., 2009; Leterrier et al., 2006). TrkA receptors move toward the axon in Rab11-positive carriers that egress from the recycling endosome (Ascano et al., 2009). Notably, the adaptor sortilin is involved in the anterograde axonal transport of neurotrophin receptors, and transcytosis of inactivated TrkA receptors is regulated by phosphorylation (Vaegter et al., 2011; Yamashita et al., 2017).
While transcytosis provides a mechanism for the exit and re-routing of axonal proteins from the somatodendritic compartment, clearance of axonal proteins can also be achieved by selective degradation in dendrites. For example, axonal localization of the Akt protein kinase depends on degradation by the dendritic ubiquitin proteasome system (Yan et al., 2006). This raises the possibility of axonal membrane proteins targeted to the lysosome for degradation through mechanisms that involve ubiquitination and sorting by the endosomal sorting complexes required for transport (ESCRT) machinery (Vietri et al., 2019). The ESCRT machinery is critical for dendritic morphology, but has yet to be implicated in the sorting of dendritic proteins (Sadoul et al., 2018). Interestingly, the axonal localization of the voltage gated sodium channel Nav2.1 involves elimination from the somatodendritic compartment by endocytosis (Fache et al., 2004). However, it is unknown whether this occurs through sorting into degradative endolysosomes and/or transcytosis, which take place in parallel with selective retention in the axon by anchoring to the AIS (Garrido et al., 2003). Taken together with sorting in the secretory pathway, endosomal and transcytotic sorting mechanisms contribute to the maintenance of a biochemically distinct somatodendritic compartment.
4. Selection and transport of somatodendritic cargo by cytoskeletal motors and adaptor scaffold proteins
Following protein sorting, membrane vesicles move to the destination of their cargo through selective interactions with molecular motors (kinesins, dynein, myosins; Figure 3) and spatial cues from cytoskeletal polymers, which contain proteins and post-translational modifications that modulate motor-cargo motility (Atherton et al., 2013; Bodakuntla et al., 2019; Burute and Kapitein, 2019; Janke and Kneussel, 2010; Venkatesh et al., 2020). Long-distance movement from the cell body to dendrites or axons, takes place on microtubules and is driven by kinesins, a large family of motors that move generally toward the plus ends of microtubules, and dynein, a minus end-directed motor (Hirokawa et al., 2010; Reck-Peterson et al., 2018; Verhey and Hammond, 2009). Thus, navigation of neuronal traffic occurs primarily on microtubules, while actin filaments and unconventional myosins provide a more localized control by intercepting and redirecting traffic at specific regions (e.g., base of dendritic spines, axon initial segment; AIS). Much work has been devoted to elucidating the itineraries and selective interactions of kinesins with neuronal cargo, and more recently, a new wave of research has been advancing our understanding of how microtubule-associated proteins and posttranslational modifications provide a traffic code for the spatial guidance of kinesin/dynein-driven transport (Atherton et al., 2013; Bodakuntla et al., 2019; Burute and Kapitein, 2019; Hirokawa et al., 2010; Kelliher et al., 2019; Park and Roll-Mecak, 2018).
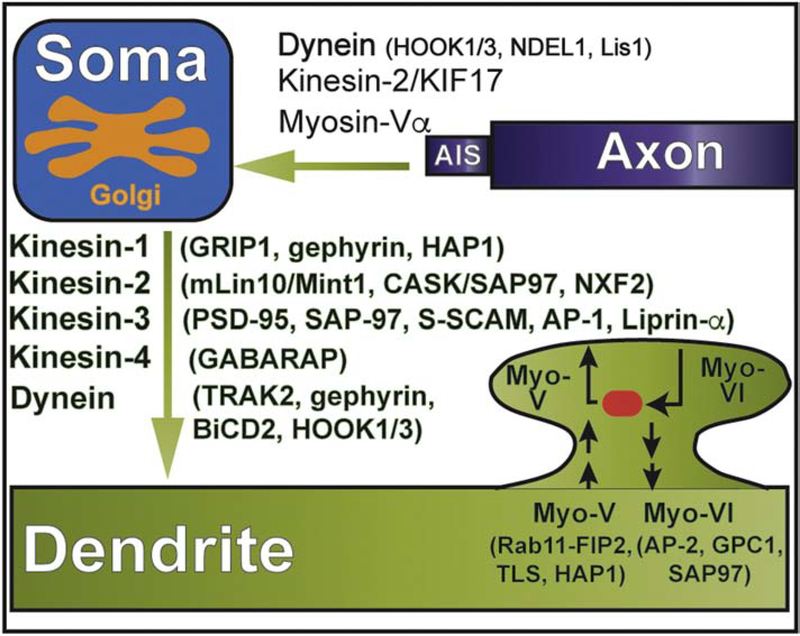
Figure 3.
Cytoskeletal motors and adaptors in the polarized and local traffic of dendritic proteins. Schematic shows the microtubule (kinesin, dynein) and actin motors (myosins, myo) involved in the retrieval of somatodendritic proteins from the axon initial segment and transport from the cell body to dendrites. In addition, the myosin motors that drive membrane traffic in dendritic spines are shown. Kinesin motors are summarized under their respective families (e.g. kinesin-1) and the adaptor/scaffold proteins that mediate interaction with dendritic cargo are provided in parentheses.
Selective transport of membrane cargo from the cell body to axons and/or dendrites depends on the activity and inherent axonodendritic preference of microtubule motors, which is modulated by their interaction with cargo and microtubules, and regulated by post-translational modifications (Hirokawa et al., 2010; Reck-Peterson et al., 2018; Verhey and Hammond, 2009). Inducible coupling of kinesin motor domains to peroxisomes of the cell body showed that only motors of the kinesin-3 (KIF1A, KIF1B, KIF1C) and kinesin-4 (KIF21A, KIF21B) subfamilies move potently into dendrites in addition to axons (Lipka et al., 2016). Dynein is biased toward dendrites, which is primarily due to microtubules with minus ends pointing outward in dendrites (Kapitein et al., 2010). Despite an inherent selectivity of their motor domains for axons, kinesin-1 (KIF5A, KIF5C) and kinesin-2 (KIF17) transport a variety of post-synaptic receptors in dendrites (Heisler et al., 2014; Setou et al., 2002; Wong-Riley and Besharse, 2012). This is achieved by interactions with cargo and microtubules, which alter the axonal preference of motor domains (see below).
Cargo-binding is a major determinant for the activation and directionality of microtubule motors, although the mechanistic basis of this phenomenon is poorly understood. Motor-cargo binding is often mediated by adaptor and scaffold proteins, which couple cargo to more than one motor and can selectively regulate motors by promoting dimerization or relieving autoinhibition (Cross and Dodding, 2019; Fu and Holzbaur, 2014). Association of kinesin-1/KIF5 with the cargo adaptor and scaffolding protein GRIP1 (glutamate receptor-interacting protein 1) bestows a dendritic directionality (Setou et al., 2002), overriding the preferential interaction of kinesin-1 with axonal microtubules, which has been attributed to microtubule organization, stability and MAPs (Balabanian et al., 2017; Farias et al., 2015; Hammond et al., 2010; Monroy et al., 2018; Nakata et al., 2011; Pan et al., 2019). GRIP1 contains multiple PDZ (PSD95/SAP90/DLG/ZO-1) domains that interact simultaneously with the C-terminal tails of N-cadherin and GluR2, which sort together into the same vesicular carriers for transport to dendrites (Heisler et al., 2014). Thereby, GRIP1 functions as an adaptor that links GluR2 to N-cadherin, and altogether to kinesin-1. GRIP1 is also a kinesin-1 adaptor for the post-synaptic ephrin receptor tyrosine kinase EphB2; GRIP1 is required for proper EphB2 traffic to dendrites and dendritic growth (Hoogenraad et al., 2005). Through a similar mode of cargo-adaptor coupling, kinesin-1 transports glycine receptors, which are linked by gephyrin, a post-synaptic scaffolding protein of the inhibitory synapse, and GABAA receptors, which bind kinesin-1 through the huntingtin-associated protein-1 (HAP-1) (Maas et al., 2009; Rathgeber et al., 2015; Twelvetrees et al., 2010).
The kinesin-2 motor KIF17 transports the NMDA receptor NR2B with the adaptor scaffolding proteins mLin10/Mint1 and CASK/SAP97 (Guillaud et al., 2003; Guillaud et al., 2008; Jeyifous et al., 2009; Setou et al., 2000). The C-terminal tail of KIF17 binds the PDZ1 domain of mLin10/Mint1 subunit, which in turn interacts with NR2B via the mLin7 subunit of the mLin10/mLin2/mLin7 scaffold complex (Guillaud et al., 2008; Setou et al., 2000). Association of KIF17 with mLin10/Mint1 is regulated by the Ca2+/calmodulin-dependent protein kinase II (CaMKII) and septin 9 (SEPT9), a member of the family of septin GTPases (Bai et al., 2016; Guillaud et al., 2008). Phosphorylation of the C-terminal tail of KIF17 by CaMKII results in dissociation of mLin10/Mint1, and SEPT9 competes with mLin10/Mint1 for binding to the KIF17 tail (Guillaud et al., 2008). Interestingly, the CASK/SAP-97 adaptor functions in a specialized mode of NR2B transport, which begins in the ER with NMDA receptor subunits sorting from their AMPA counterparts for transport to Golgi outposts, bypassing the Golgi complex of the cell body (Jeyifous et al., 2009). In addition to NR2B, kinesin-2/KIF17 is responsible for the dendritic transport of the potassium channel Kv4.2, the kainate receptor GluR5 and mRNAs, which bind KIF17 through the RNA export factor NXF2 and the RNA-binding protein FMRP (Fragile X mental retardation protein) (Chu et al., 2006; Kayadjanian et al., 2007; Takano et al., 2007). Notably, KIF17 mediates the transport of GluR5 only in distal dendrites and in complex with the kainate receptor KA2, indicating that kinesin motors are specialized not only for specific cargo but also regions of the somatodendritic compartment (Kayadjanian et al., 2007).
Spatial control of kinesin-2/KIF17 localization and function can be exerted by actin filaments and coupling to other motors such as dynein, which have been shown to restrict forward movement of KIF17 in the AIS (Franker et al., 2016). Alternatively, KIF17 may take over transport of cargo from dynein at specific regions (e.g., dendritic branch points) owing to changes in microtubule orientation. This may occur through cargo hand-off or selective activation by a cargo adaptor that is bound to both kinesin and dynein (Fu and Holzbaur, 2014).
While KIF17 has emerged as the predominate kinesin-2 motor that mediates transport of dendritic membrane proteins and mRNA, a recent study revealed that the cargo-binding tails of the heteromeric kinesin-2 motors KIF3A/B and KIF3A/C interact selectively with somatodendritic membrane cargo (Yang et al., 2019). The identity of this cargo remains unknown, but KIF3A has been reported to interact with N-cadherin in a manner that is upregulated by synaptic activity through phosphorylation of the C-terminal tail of KIF3A by protein kinase A (PKA) and CaMKII (Ichinose et al., 2015).
A number of kinesin-3 motor domains (KIF1A, KIF1B, KIF1C) and cargo-binding tails (KIF1A, KIF1Bβ) move into the somatodendritic compartment, albeit not exclusively (Jenkins et al., 2012; Lipka et al., 2016; Yang et al., 2019). Kinesin 3 motors are unique in their ability to move persistently over long distances upon cargo-induced dimerization (Soppina et al., 2014), and thus may enable cargo to move in the highly branched dendrites faster and more efficiently. Consistent with this possibility, dendritic arborization but not outgrowth is impaired in Drosophila larvae by mutating a residue that abrogates the dimerization of Unc-104/KIF1A (Kern et al., 2013). KIF1A interacts with the scaffolding protein liprin-α and and co-traffics in dendrites with vesicles containing synaptotagmin-IV and the low-density lipoprotein receptor (LDL-R), which localizes to the somatodendritic compartment of hippocampal neurons (Jenkins et al., 2012; McVicker et al., 2016; Shin et al., 2003). In parallel, KIF1A mediates the axonal transport of synaptic vesicle precursors, which involves a direct interaction with the Rab3 guanine nucleotide exchange factor DENN/MADD (differentially expressed in normal and neoplastic cells/MAP kinase activating death domain) and release of Unc-104/KIF1A autoinhibition by the small GTPase Arl8 (Niwa et al., 2016; Niwa et al., 2008). KIF1Bα has been shown to interact with the post-synaptic scaffold proteins PSD-95, SAP-97 and S-SCAM (synaptic scaffolding molecule), all of which contain PDZ domains that interact with post-synaptic receptors and ion channels (Mok et al., 2002). Moreover, the KIF1Bβ isoform associates with ribonucleoprotein particles, mediating the transport of dendritically localized mRNAs that encode for calmodulin and Arc (activity-regulated cytoskeleton-associated protein) (Charalambous et al., 2013).
The kinesin-3 motors KIF13A and KIF13B associate preferentially with endosomes that contain the transferrin receptor (TfR), which is highly polarized in dendrites and co-traffics with AMPA receptors (Bentley and Banker, 2015). KIF13A interacts directly with β1-adaptin of the AP-1 adaptor complex, which mediates the sorting of dendritic proteins (Nakagawa et al., 2000). Although KIF13A has a preference for somatodendritic cargo and transports the mannose-6phosphate receptor from the Golgi to the plasma membrane, it is unknown if KIF13A associates with dendritically destined proteins that are sorted in the trans-Golgi and/or endosomes by AP-1 (Nakagawa et al., 2000).
KIF16B, a kinesin-3 motor involved in the transcytosis of TfR in epithelial cells, contains a PX domain that binds strongly to phosphatidylinositol-3-phosphate, which is enriched in early endosomes (Perez Bay et al., 2013; Pyrpassopoulos et al., 2017). Notably, KIF16B localizes to early endosomes of the somatodendritic compartment and mediates the dendritic transport of endosomes carrying GluR1 and the neurotrophin receptor p75NTR (Farkhondeh et al., 2015). Interestingly, the somatodendritic localization and function of KIF16B is dependent on two coiled-coil domains of its stalk domain, which bind and inhibit the motor domain, suggesting that this autoinhibition is selectively relieved in the somatodendritic compartment (Farkhondeh et al., 2015).
Similar to kinesin-3, the kinesin-4 motor domains of KIF21A and KIF21B also move into dendrites (Lipka et al., 2016). The full-length KIF21B is a somatodendritic motor, which is more enriched in dendrites than the cell body, while KIF21A has no polarized distribution (Marszalek et al., 1999). In hippocampal neurons, KIF21B colocalizes with the γ2 subunit of GABAA receptors at non-synaptic sites and is required for GABAA receptor delivery to the cell surface (Labonte et al., 2014). KIF21B forms a complex with the GABAA receptor and the GABA receptor-associated protein (GABARAP), which interacts with gephyrin, the N-ethylmaleimidesensitive factor (NSF) and the E3 ubiquitin ligase tripartite-motif containing 3 (TRIM3; Genau and Behrends, 2016; Kittler et al., 2001; Kneussel et al., 2000; Labonte et al 2014). KIF21B motility is modulated by TRIM3 through an unknown mechanism that may involve a conformational change in KIF21B upon mono-ubiquitination (Labonte et al., 2013). KIF21B, GABARAP and TRIM3 provide an alternative mode of GABAA transport to kinesin-1 and HAP-1 (Nakajima et al., 2012; Twelvetrees et al., 2010). Notably, TRIM3 is part of an endosomal complex termed cytoskeleton-associated recycling or transport complex (CART), which includes the endosomal adaptor hrs (hepatocyte growth factor-regulated kinase substrate), actinin-4 and myosin V (Yan et al., 2005). Hence, transport of GABAA receptors by KIF21B might be more compatible with a hand-off to myosin-V and a transition from the microtubules of the dendritic shaft to the actin filaments of dendritic spines (Yan et al., 2005). In addition to GABAA receptors, KIF21B transports signaling endosomes with BDNF-bound TrkB receptors, bestowing a retrograde bias toward the cell body which is further enhanced by synaptic activity (Ghiretti et al., 2016). The retrograde bias of KIF21B indicates a preference for dendritic microtubules oriented with their plus ends towards the cell body. It is unclear how KIF21B establishes such preference, but it might be due to its C-terminal WD40 domain which associates preferentially with the GTP-bound lattice of microtubules and regulates the organization and dynamics of dendritic microtubules (Muhia et al., 2016; van Riel et al., 2017).
Dynein directs cargo from the cell body to dendrites (Kapitein et al., 2010), but recognition of somatodendritic cargo by dynein is much less understood than kinesins. The microtubule minus-directed kinesin KIFC2 is implicated in the dendritic transport of multivesicular body-like organelles, but does not appear to target cargo into dendrites (Lipka et al., 2016; Saito et al., 1997). In dynein-mediated transport, cargo specificity is achieved through adaptor proteins, which activate dynein by promoting dynein-dynactin interaction, recruiting two dynein dimers per dynactin complex and/or relieving the autoinhibitory conformation of dynein (Reck-Peterson et al., 2018). Discovery of dynein-activating adaptors is fairly recent and limited to a small number of proteins, which have in common a coiled coil domain and binding sites for the dynein intermediate light chain and factors that further promote or regulate dynein association with cargo (Reck-Peterson et al., 2018). From the known dynein adaptors, TRAK2 (trafficking protein, kinesin binding 2/Milton) is a dendrite-specific adaptor for mitochondria (van Spronsen et al., 2013). TRAK2 steers mitochondria to dendrites and is required for dendritic outgrowth, while TRAK1 mediates mitochondrial transport in axons (van Spronsen et al., 2013). Bicaudal-D2 (BicD2) is required for local synthesis of BDNF in dendrites and thus, may transport untranslated BDNF mRNA in dendrites (Oe et al., 2016). Furthermore, gephyrin appears to mediate the dynein-driven transport of post-synaptic glycine and GABAA receptors by interacting with the dynein light chains 1 and 2 (Fuhrmann et al., 2002; Maas et al., 2006).
In addition to their functions in dendritic trafficking, dynein adaptors are critical for the retrieval of somatodendritic proteins from the axon. The adaptors HOOK1 and HOOK3 are required for the dynein-driven retrieval of TfR from axons, which sustains the somatodendritic polarity of TfR (Guo et al., 2016). Notably, HOOK1/3 are effectors of Rab5, which has been shown to regulate the somatodendritic distribution of TfR and glutamate receptors (Guo et al., 2016). Reversal of somatodendritic cargo during axonal entry is also dependent on the dynein regulator NDEL1 and its binding partner Lis1, which are anchored to the AIS by ankyrin G (Kuijpers et al., 2016). NudE, the Drosophila homolog of NDEL1, is required for the transport and localization of Golgi outposts in dendrites, which is critical for proper branching (Arthur et al., 2015). In agreement, dynein mutants shift the branching patterns of dendrites from distal tips to regions proximal to the cell body (Aguirre-Chen et al., 2011; Satoh et al., 2008; Zheng et al., 2008). Dynein-driven targeting of Golgi outposts to dendrites is complemented by an autoinhibition of kinesin-1, which prevents movement of Golgi outposts into the axons of Drosophila neurons (Kelliher et al., 2018).
Microtubule motor adaptors not only scaffold and specify the interactions of motors with their respective cargo, but also provide a mechanism for a particular cargo to switch between kinesin- and dynein-driven motility (Cross and Dodding, 2019; Fu and Holzbaur, 2014; Olenick and Holzbaur, 2019; Reck-Peterson et al., 2018). A growing number of adaptor and scaffold proteins have been discovered to associate with both dynein and kinesin motors, coordinating movements of opposing directionality (Cross and Dodding, 2019). Phosphorylation of cargo adaptors/scaffolds by upstream signaling pathways has been shown to activate a cargo-bound motor over another (Cross and Dodding, 2019; Olenick and Holzbaur, 2019). For example, phosphorylation of the JNK-interacting protein (JIP) scaffold switches axonal transport of the amyloid precursor protein (APP) from retrograde dynein-driven to anterograde kinesin-driven movement (Fu and Holzbaur, 2013). In addition to phosphorylation, cargo adaptors can also trigger motor switching upon sumoylation or ubiquitination and interaction with small GTPases (Cross and Dodding, 2019; Olenick and Holzbaur, 2019). This type of coordination might be particularly necessary in the spatial guidance of membrane traffic in dendrites, where cargo is likely to transition frequently between microtubules of opposite polarity (Liot et al., 2013). However, we have little knowledge of how somatodendritic cargo coordinate their movement on dendritic microtubules. Studies of the huntingtin scaffold protein, which appears to mediate the dendritic transport of β-actin mRNA and TrkB receptors, indicate that somatodendritic cargo may indeed switch directionality through cargo adaptor and scaffold proteins (Caviston and Holzbaur, 2009; Liot et al., 2013; Ma et al., 2011). Huntingtin interacts with the adaptor HAP1, which associates with both kinesin-1 and dynactin (Engelender et al., 1997; McGuire et al., 2006). Notably, phosphorylation of huntingtin is posited to function as a molecular switch for anterograde transport (Colin et al., 2008). More work is required to determine how somatodendritic cargo employ cargo adaptor and scaffold proteins for directional movement in the geometrically complex dendrites.
5. Spatial guidance of membrane traffic by the neuronal cytoskeleton
Directional specificity in intracellular transport relies on selective motor-cargo interactions, but ultimately requires regulation of motor-cargo motility by cytoskeletal determinants such as microtubule post-translational modifications (PTMs) and microtubule-associated proteins (MAPs) which provide critical spatial cues (Figure 4). Microtubules comprise a heterogeneous network of polymers with a diversity of α-/β-tubulin isotypes and PTMs that include: i) removal/presence of the terminal tyrosine residue of α-tubulin (detyrosination/tyrosination), ii) addition/removal of an acetyl moiety at the Lysine40 amino acid of α-tubulin (acetylation/deacetylation), iii) addition/removal of side chains comprising of one or multiple glutamates to glutamic residues of the C-terminal tails of α-/β-tubulin (mono- or polyglutamylation/deglutamylation), and iv) removal/presence of the penultimate (Δ2) or last two glutamates (Δ3) of tubulin’s C-terminal tail (Magiera et al., 2018; Park and Roll-Mecak, 2018). Because the C-terminal tails of tubulin, which project away from the microtubule lattice, provide a binding interface for many motors and MAPs, PTMs have a direct impact on motor-cargo motility (Atherton et al., 2013; Janke and Kneussel, 2010; Magiera et al., 2018). Taken together with a mosaic landscape of MAPs, which is characterized by differential MAP distribution and enrichment on distinct subsets of microtubules, PTMs and MAPs comprise a regulatory code - the tubulin and MAP code - for membrane traffic (Atherton et al., 2013; Bodakuntla et al., 2019; Janke and Kneussel, 2010; Park and Roll-Mecak, 2018; Verhey and Gaertig, 2007).
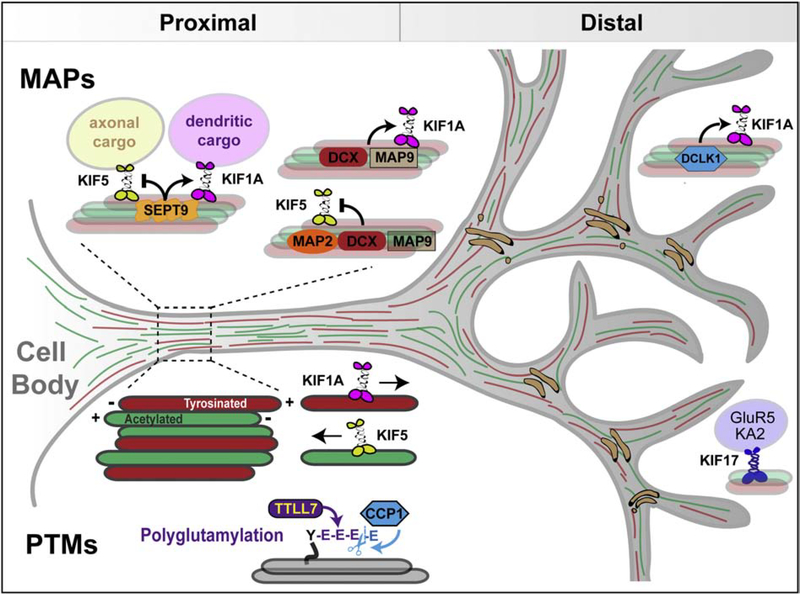
Figure 4.
Spatial control of dendritic membrane traffic by MAPs and microtubule PTMs. Schematic depicts dendritic MAPs and microtubule PTMs with roles in the regulation of kinesin-driven membrane traffic. SEPT9 and DCLK1 localize in proximal and distal dendrites, respectively. SEPT9 impedes traffic of axonal cargo of kinesin-1 into dendrites, while it promotes the anterograde transport of dendritic cargo of kinesin-3. DCLK1, DCX and MAP9 also promote the microtubule binding and motility of kinesin-3. In vitro motility assays indicate that MAP2, DCX and MAP9 inhibit kinesin-1 motility, but it is unknown whether these MAPs can impede entry of kinesin-1 and its axonal cargo into dendrites. Dendrites contain tyrosinated and acetylated microtubules, which are oriented with their plus-ends away and toward the cell body, respectively. The kinesin-3/KIF1A motor associates preferentially with tyrosinated plus-end out microtubules and kinesin-1/KIF5 interacts with acetylated plus-end in microtubules. Dendritic microtubules are also modified with short glutamate chains, which are maintained by the tubulin glutamylase TTLL7 (tubulin tyrosine like ligase 7) and the deglutamylase CCP1 (cytosolic carboxypeptidase 1). Note that kinesin-2/KIF17 transports the kainate receptors GluR5 and KA2 in distal dendrites, which is indicative of a spatial specificity in dendritic motor-cargo traffic.
Dendritic microtubules are predominately tyrosinated, acetylated and polyglutamylated (Hammond et al., 2010; Ikegami et al., 2006; Konishi and Setou, 2009; Tas et al., 2017). Additionally, dendritic microtubules are uniquely enriched with the microtubule-binding proteins MAP1A, MAP2, doublecortin, doublecortin-like kinase 1 (DCLK1) and SEPT9 (Bernhardt and Matus, 1984; Karasmanis et al., 2018; Lipka et al., 2016; Monroy et al., 2019; Schoenfeld et al., 1989; Shiomura and Hirokawa, 1987). Acetylation, a PTM that marks long-lived “stable” microtubules, is found in both axons and dendrites, but dendrites appear to be particularly enriched with tyrosinated microtubules (Hammond et al., 2010; Konishi and Setou, 2009; Szyk et al., 2014). The somatodendritic polarity of the tubulin tyrosine ligase-like 7 (TTLL7) enzyme indicates that short chain polyglutamylation is dominant in dendrites (Ikegami et al., 2006; Mukai et al., 2009; van Dijk et al., 2007). Notably, formation of MAP2-positive neurites occurs concomitantly with accumulation of TTLL7 and polyglutamylated β-tubulin, whose presence is critical for the outgrowth of MAP2-positive neurites (Ikegami et al., 2006). Recent work revealed that acetylated microtubules are predominately oriented with their plus ends toward the cell body, while tyrosinated microtubule plus ends point away from the cell body (Tas et al., 2017).
On the surface of dendritic microtubules, PTMs co-exist with MAPs such as MAP1A and MAP2 (Shiomura and Hirokawa, 1987). Interestingly, microtubule binding of these MAPs is differentially modulated by PTMs. For example, long polyglutamyl chains reduce the affinity of MAP2 without affecting MAP1A (Bonnet et al., 2001). Hence, PTM gradients across the length of a dendrite may result in local enrichments of MAPs. SEPT9 and DCLK1 are more enriched in proximal and distal regions of dendrites, respectively, but is unknown whether this is due to a gradient of a PTM (Karasmanis et al., 2018; Lipka et al., 2016; Shin et al., 2013). In the axons of sensory neurons, however, there is a distal-to-proximal gradient of microtubule tyrosination, which biases and promotes microtubule-dynein binding at axonal terminals (Nirschl et al., 2016). Furthermore, MAPs have been observed to compete with one another for microtubule binding (Monroy et al., 2018; Spiliotis et al., 2008). Hence, mutualistic and competitive interactions between PTMs and MAPs are likely to result in spatial patterns of unique PTM and MAP combinations, which in turn locally control membrane traffic.
The discovery of the AIS as a vesicular sieve provided a dominant paradigm for local cytoskeleton-based control of membrane traffic and its broader impact on neuronal polarity (Huang and Rasband, 2018; Leterrier, 2018; Song et al., 2009). Together with the pre-axonal exclusion zone (PAEZ), the AIS excludes and reverses the traffic of somatodendritic vesicles and organelles. The PAEZ comprises a segment of the microtubule cytoskeleton in the interface of the neuronal cell body with the AIS, which is devoid of rough ER and Golgi membranes, and vesicles with somatodendritic proteins such as AMPA receptors and TfR (Farias et al., 2015). Microtubules of the PAEZ are highly acetylated and preferentially bound by kinesin-1/KIF5, whose motor domain moves selectively into axons (Farias et al., 2015). Coupling of somatodendritic proteins to kinesin-1/KIF5 induced transport on PAEZ microtubules and entry into axons, both of which were dependent on microtubule acetylation (Farias et al., 2015). Consistent with observations from a number of studies, acetylation is a microtubule PTM that favors kinesin-1-driven transport (Reed et al., 2006). Although the underlying mechanism is unknown, acetylated microtubules are preferentially bundled and have protofilaments with weaker lateral contacts, both of which may favor the binding and mechanochemistry of kinesin-1 (Balabanian et al., 2017; Eshun-Wilson et al., 2019). Alternatively, acetylated microtubules could favor the accumulation of specific MAPs such members of the MAP7 family, which promote the recruitment and activation of kinesin-1, and the axonal entry of kinesin-1 cargo (Chaudhary et al., 2019; Hooikaas et al., 2019; Monroy et al., 2018; Pan et al., 2019; Tymanskyj et al., 2018).
While PAEZ microtubules select against somatodendritically destined vesicles, the AIS cytoskeleton reverses sometodendritc cargo out of the axon. This reversal involves synergy between microtubules and actin filaments (Arnold, 2009). The AIS cytoskeleton consists of a patchy network of short actin filament, which are predominately oriented with their barbed ends toward the cell body, and bundles of parallel microtubules, which are organized by the microtubule-binding protein TRIM46 (tripartite motif containing protein 46) with their minus ends facing the cell body (Harterink et al., 2019; Jones et al., 2014; van Beuningen et al., 2015; Watanabe et al., 2012). Upon entering the AIS, vesicles with somatodendritic proteins are halted on actin filaments by myosin-Vα, which orients them toward the cell body and enables engagement with dynein (Al-Bassam et al., 2012; Kapitein et al., 2013; Watanabe et al., 2012). Powered by NDEL, which is anchored to the AIS membrane skeleton by ankyrin-G, dynein moves somatodendritic cargo back into the cell body (Kuijpers et al., 2016). It is not well understood how somatodendritic cargo is handed off between kinesin, myosin-Vαand dynein, but cargo adaptors/scaffolds that bind to multiple motors may mediate these switches with input from signaling kinases. Interestingly, the adaptor and scaffold protein GRIP1 that directs kinesin-1 into dendrites has been shown to interact with myosin-VI in a phospho-regulated manner, and dynein reversal requires NDEL1 phoshorylation by the cyclin-dependent kinase 5 (CDK5) (Klinman et al., 2017; Lv et al., 2015).
The polarity of somatodendritic proteins was long thought to depend on their exclusion and reversal by the AIS cytoskeleton, but recent work indicates that vesicle trafficking is also sorted during entry into dendrites. Similar to the sorting of somatodenditic vesicles at axonal entry, axonal cargo of kinesin-1 stall and reverse directionality during entry into dendrites (Karasmanis et al., 2018). By contrast, dendritic cargo of kinesin-3 moves with an anterograde bias (Karasmanis et al., 2018). In parallel and consistent with these findings, rigor mutants of kinesin-1/KIF5 and kinesin-3/KIF1A were independently discovered to associate preferentially with the acetylated plus-end-in and tyrosinated plus-end-out microtubules of dendrites, respectively (Tas et al., 2017). Moreover, the directional biases of kinesin-1/KIF5 and kinesin3/KIF1A, and their cargos, arise from modulation of their motility by SEPT9 and DCLK1, which are specifically enriched in dendrites, and possibly other MAPs such as doublecortin and MAP9 (Karasmanis et al., 2018; Lipka et al., 2016; Monroy et al., 2020).
SEPT9 belongs to the septin family of GTPases, which associate with subsets of microtubules and actin filaments, and membrane domains of micron-scale curvature (Bridges et al., 2016; Cannon et al., 2019; Spiliotis, 2018). SEPT9 bundles microtubules through the repeat motifs of a structurally disordered domain and suppresses microtubule catastrophe (Bai et al., 2013; Nakos et al., 2019a; Nakos et al., 2019b). In dendrites SEPT9 localizes to microtubule bundles proximal to the cell body and maintains the polarity of neuronal traffic by impeding the motility of axonal cargo of kinesin-1/KIF5 and enhancing the anterograde movement of dendritic cargo of kinesin-3/KIF1A (Karasmanis et al., 2018). In vitro (cell-free) motility assays showed that SEPT9 reduces the binding and motility of the kinesin-1/KIF5 motor domain on microtubules, while it has the opposite effect on kinesin-3/KIF1A (Karasmanis et al., 2020). In agreement, SEPT9 depletion resulted in loss of the intrinsic axonal polarity of the kinesin-1/KIF5 motor domain, which entered dendrites (Karasmanis et al., 2018). Interestingly, SEPT9 does not affect the microtubule motility of kinesin-2/KIF17, but impacts its association with cargo (Bai et al., 2016). By modulating cargo-motor binding in proximal dendrites, SEPT9 may provide a spatial bias in the cargo selection of kinesin-2/KIF17, which mediates the transport of kainate receptors that localize specifically in distal dendrites (Kayadjanian et al., 2007). It is unknown how SEPT9 becomes enriched in microtubules of proximal dendrites, but post-translational modifications such as polyglutamylation could play a role. Notably, septins associate with polyglutamylated microtubules and have been implicated in the generation and maintenance of short polyglutamylated side-chains (Froidevaux-Klipfel et al., 2015; Spiliotis et al., 2008).
Sorting of vesicle traffic on dendritic microtubules involves additional MAPs. DCLK1 is a dendritic MAP that associates preferentially with dendritic microtubules and interacts with the motor domain of kinesin-3/KIF1 (Lipka et al., 2016). Although DCLK1 does not impact kinesin-1/KIF5, it is required for the transport of kinesin-3/KIF1 cargo (Lipka et al., 2016). Dendritic entry of the dense core vesicle marker neuropeptide Y (NPY), which is mediated by KIF1A and KIF1C, is markedly reduced in DCLK1-depleted neurons (Lipka et al., 2016). Similarly, knockdown of DCLK1 and MAP1A, but not MAP1B or MAP2, diminish the somatodendritic polarity of peroxisomes mobilized by chemically inducible coupling to KIF1C (Lipka et al., 2016). Doublecortin has also been shown to impact the dendritic traffic of KIF1A and its cargo VAMP2 (Liu et al., 2012). In vitro cell-free motility assays indicate that MAP9 also promotes the motility of kinesin-3, while doublecortin and MAP2 were found to interfere with the microtubule binding of kinesin-1/KIF5 (Monroy et al., 2020). However, it is unknown whether doublecortin and MAP2 function like SEPT9 in impeding the entry of axonal cargo of kinesin-1/KIF5 into dendrites. Overall, MAPs appear to function combinatorially for the spatial control of membrane traffic in dendrites.
In parallel with their direct effects on the motility of motor-cargo, MAPs are also likely to impact membrane traffic through regulation of microtubule organization and dynamics. Recent work has revealed that the motility of kinesin-1/KIF5 and kinesin-3/KIF1A are influenced by the spacing and plus end dynamics of microtubules, respectively (Conway et al., 2014; Guedes-Dias et al., 2019). Kinesin-1/KIF5 motors run slower and shorter distances on microtubules that are closely packed (Conway et al., 2014). Conversely, microtubules that are spaced apart facilitate motility and relieve the inhibitory effects of MAPs by enhancing access to the microtubule surface (Conway et al., 2014). As the length of the projection domains of MAPs have been implicated in microtubule spacing (Chen et al., 1992), MAPs may differentially impact motor-cargo motility depending on how closely they bundle microtubules. In addition to spacing, the nucleotide-bound status and dynamicity of microtubules can impact motor affinity and movement. Kinesin and dynein motors have differential affinities for the growing GTP-bound plus-ends. Dynein is recruited to microtubule plus ends through dynactin, which interacts with the plus-end binding and tracking protein EB1 (Duellberg et al., 2014; Jha et al., 2017; Ligon et al., 2006; Moughamian and Holzbaur, 2012). Kinesin-2/KIF17 has similarly reported to interact with EB1 and kinesin-1/KIF5 with GTP-bound tubulin, while kinesin-3/KIF1A has a weak affinity for GTP-bound microtubules and detaches from distal microtubule ends (Guedes-Dias et al., 2019; Jaulin and Kreitzer, 2010; Nakata et al., 2011). Hence, MAPs that modulate microtubule dynamics are likely to impact the attachment and dissociation of motor-cargo complexes, and thereby, the directionality of movement in dendrites. Similarly, modulation of the physical and dynamic properties of microtubules by PTMs is also likely to impact motor-driven transport.
6. Spatiotemporal and synaptic control of membrane traffic into dendritic spines
Dendritic spines, the bulbous protrusions that synapse with axons, constitute a specialized compartment of paramount physiological significance for the central nervous system (Hering and Sheng, 2001; Martinez-Cerdeno, 2017; Rochefort and Konnerth, 2012). Spatiotemporal control of membrane trafficking from the dendritic shaft to spines and back, and within the spine compartment itself, is essential for the development and plasticity of synapses, and their modulation by neuronal activity (Choquet and Triller, 2013; Hotulainen and Hoogenraad, 2010; Kennedy and Ehlers, 2011; Lei et al., 2016; Nakahata and Yasuda, 2018). Dendritic spines are pleomorphic in shape and size, but they are invariably connected to the dendritic shaft by a narrow ~100 nm-wide neck (Hering and Sheng, 2001; Rochefort and Konnerth, 2012). Dendritic spines contain mainly smooth ER, endosomes and actin filaments, and are supplied with lysosomes, microtubules and mRNA from the dendritic shaft (Dent, 2017; Konietzny et al., 2017; Lei et al., 2016). On the spine membrane, glutamate receptors and post-synaptic scaffold proteins are organized in microdomains, clustering at synaptic contacts (post-synaptic density) and diffusing laterally in the perisynaptic membrane (Adrian et al., 2014; Choquet and Triller, 2013; Hruska et al., 2018; Kim and Sheng, 2009). Spine membrane proteins traffic to and recycle from endosomes, which requires regulation of membrane traffic locally within the spine (Kennedy and Ehlers, 2011; van der Sluijs and Hoogenraad, 2011) (Figure 5A). However, post-synaptic proteins are also delivered through exocytosis of vesicles and organelles that enter spines from the dendritic shaft (Dent, 2017; Hanus and Ehlers, 2008; Padamsey et al., 2017; Wagner et al., 2011). The latter events rely on mechanisms that position and/or target membrane organelles in close proximity to spines, and microtubule entry into spines (Figure 5B).
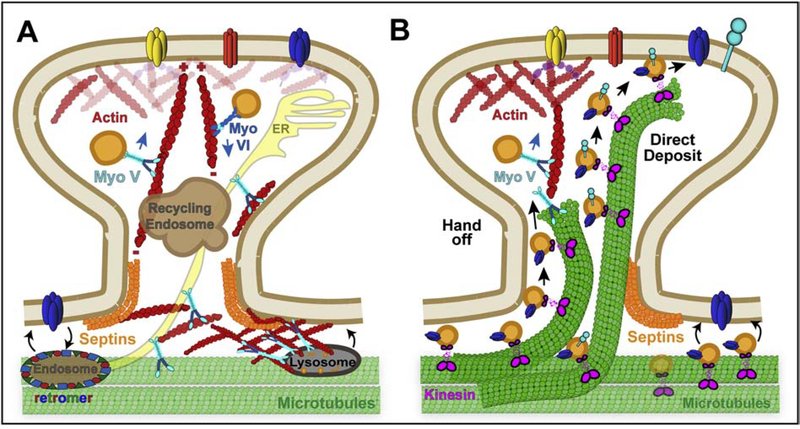
Figure 5.
Membrane traffic in dendritic spines. (A) The actin motors myosin V and VI mediate the transport of post-synaptic cargo toward and away from the dendritic spine head, respectively. At the base of the dendritic spines and along the dendritic shaft, endolysosomes are entrapped and anchored to actin patches by myosin V, which provides a mechanism for fusion with the spine membrane in response to synaptic activity. Dendritic membrane proteins recycle through retromer-positive endosomes, which localize along the dendritic shaft membrane. (B) Dynamic microtubules that enter into dendritic spines deliver vesicles to the spine membrane by either a handoff mechanism, which involves vesicle switching from microtubules to actin filaments, or direct delivery of microtubule plus-end-associated vesicles to the membrane. Microtubule-dependent transport of vesicles also occurs in the dendritic shaft, where vesicles with post-synaptic receptors are delivered by microtubule motors to synapses and membrane sites of the dendritic shaft.
In dendritic spines, endosomal membrane traffic occurs along branched and linear actin filaments, whose dynamics underlie spine formation and plasticity (Hotulainen and Hoogenraad, 2010; Konietzny et al., 2017; Lei et al., 2016). The processive unconventional myosins V and VI, which move to the barbed fast-growing and pointed slow-growing ends of actin filaments, respectively, mediate the transport of endocytic vesicles in dendritic spines (Hammer and Sellers, 2011; Wells et al., 1999). In hippocampal neurons, myosin Vb is recruited by Rab11 and Rab11-FIP2 to recycling endosomes that contain GluR2 receptors, mobilizing them into the dendritic spine in response to activation of NMDA receptors (Wang et al., 2008). Mechanistically, Ca2+ ions trigger a conformational change in myosin Vb, which promotes interaction with Rab11-FIP2, leading to the release of GluR2 receptors into the spine membrane for the long-term potentiation (LTP) of synapses (Wang et al., 2008). In Purkinje neurons, myosin Va mediates the movement of smooth ER tubules into dendritic spines, which occurs in response to metabotropic glutamate receptor (mGluR) activation and results in the release of calcium from the ER, promoting mGluR-dependent long-term depression (LTD) (Wagner et al., 2011). ER tubules are not present in most of the dendritic spines of pyramidal neurons, but a subset of spines contains a specialized ER structure termed the spine apparatus (SA) (Spacek, 1985; Spacek and Harris, 1997). The SA consists of laminar stacks of ER membranes, which localize to the neck and base of spines, and marked by the protein synaptopodin, which in turn is essential for SA formation (Deller et al., 2003; Deller et al., 2000). Synaptopodin interacts with myosin V and is markedly diminished from dendritic spines upon expression of a dominant negative myosin V (Konietzny et al., 2019). Translocation of mRNA into dendritic spines is also mediated by myosin Va, which associates with messenger ribonuclear protein (mRNP) complexes in a Ca2+-dependent manner; myosin Va interacts with translocated in liposarcoma (TLS), an mRNA-binding protein that colocalizes with RNA-transporting granules and actin filaments (Yoshimura et al., 2006). Hence, myosin V appears to specialize in the transport of endosomal and ER membranes as well as mRNAs from the dendritic shaft into spines, which involves transitioning of cargo transport from the microtubules of the dendritic shaft to the actin filaments of spines. As myosin V interacts with microtubules (Ali et al., 2007; Zimmermann et al., 2011), switching between microtubules and actin filaments, it is uniquely suited for transitioning cargo movement from microtubules to actin filaments at the interface of the dendritic shaft with spines.
In contrast to myosin V, which mediates entry into spines, myosin VI functions mainly in the opposite direction, driving the internalization of post-synaptic membrane proteins and exit of endosomes from dendritic spines. Immunoprecipitations from dendritic membrane extracts revealed that myosin VI interacts with the clathrin adaptor AP-2, the GluR1 and GluR2 subunits of AMPA receptors and the scaffold protein SAP97 (synapse-associated protein-97), which controls the post-synaptic density of glutamate receptors (Buss et al., 2001; Osterweil et al., 2005; Wu et al., 2002). Importantly, myosin VI is required for AMPA-induced internalization of GluR1, which is critical for LTD (Osterweil et al., 2005). Consistent with a role in egress from spine membranes, chemically induced coupling of myosin VI to Rab11 results in expulsion of recycling endosomes into dendritic shafts, diminishing PSD95 and GluR localization in dendritic spines (Esteves da Silva et al., 2015). In addition to glutamate receptors, myosin VI facilitates the internalization of the brain derived neurotropic factor (BDNF) receptor TrkB (tropomyosinrelated kinase B) (Yano et al., 2006). Myosin VI forms a complex with TrkB and glypican-1 (Gpc1), which functions as an adaptor between myosin VI and the cytoplasmic tail of TrkB (Yano et al., 2006).
In dendritic shafts, transport of membrane organelles, vesicles and mRNAs takes place on microtubules and has been likened to conveyor belts or sushi-belts, as referred to in the case of mRNA transport, which can supply spines with material in transit (Doyle and Kiebler, 2011; Hanus et al., 2014; Hoerndli et al., 2015a). Long-range movement along microtubules enables transient interactions with the base of dendritic spines, but growing evidence suggests that these are not entirely stochastic. Dendrites appear to possess structures and mechanisms that spatially control and bias membrane traffic into spines.
Recent work showed that actin meshworks provide an obstacle in microtubule-dependent transport, entrapping membrane organelles at the base of dendritic spines and shaft synapses – synapses that form by axon terminals making contact with dendritic shafts instead of spines (Bucher et al., 2020). Dendritic actin patches are stationary and stable, consisting of branched and linear actin, and unlike axonal actin patches, do not appear to originate from endosomal membranes (van Bommel et al., 2019). Endolysosomal organelles, however, are embedded in actin patches, which slow down and pause the microtubule-dependent movement of lysosomes and peroxisomes (van Bommel et al., 2019). Notably, lysosome stalling at actin patches depends on myosin V, while myosin VI is not involved (van Bommel et al., 2019). Anchoring of lysosomes to actin patches by myosin V can provide a mechanism for lysosome stalling and fusion with dendritic membrane in response to synaptic stimulation. Myosin V and its calmodulin light chains are conformationally sensitive to Ca2+ concentrations (Krementsov et al., 2004). Thus, upon synaptic activation, accumulation of myosin V at dendritic actin patches and its mobility into dendritic spines are regulated by the flux of Ca2+ ions, which occurs through NMDA receptors and lysosomes themselves; Ca2+ ions are released from lysosomes upon synaptic stimulation (Goo et al., 2017). Synaptic activity induces trafficking of lysosomes into dendritic spines and their fusion with dendritic membranes (Goo et al., 2017; Padamsey et al., 2017). Upon lysosome exocytosis, release of the proteolytic enzyme cathepsin B enhances the activity of the matrix metalloproteinase 9 (MMP9), which in turn impacts synaptic strength by remodeling the extracellular matrix (Padamsey et al., 2017). Thus, actin patches may provide a mechanism for directing lysosomes into dendritic spines in response to synaptic activity.
Interestingly, synaptic activity has been shown to restrict the long-range movement of post-ER secretory carriers, which was attributed to the phosphorylation of kinesin-2/KIF17 by the Ca2+/calmodulin-dependent protein kinase II (CaMKII) (Hanus et al., 2014). Because peroxisomes that are artificially coupled to kinesin-2/KIF17 stall at dendritic actin patches (van Bommel et al., 2019), it is possible that dendritic actin patches restrict post-ER membrane traffic in response to synaptic activity.
Currently, it is unknown how dendritic actin patches are strategically positioned to entrap endolysosomes at the base of dendritic spines and shaft synapses. A combination of physical and signaling cues from the dendritic membrane may spatiotemporally instruct the formation of actin patches. At the base of dendritic spines, the saddle-like membrane curvature between the shaft and spine neck membranes, is enriched with septins that recognize and assemble preferentially on micron-scale membrane curvatures (Bridges et al., 2016; Ewers et al., 2014; Tada et al., 2007; Xie et al., 2007). Interestingly, this septin localization is regulated by phosphorylation, which shifts septins from the base to the head of the spine, where SEPT7 interacts with PSD95 (Yadav et al., 2017). Septins impede the diffusion of AMPA receptors (Ewers et al., 2014), but can also play a role in the assembly of actin patches and their interaction with microtubules (Hu et al., 2012). New studies indicate that WAVE, an actin nucleation promoting factor, recognizes saddle curvatures of the membrane in complex with IRS53p, a membrane curvature-sensing protein (Pipathsouk et al., 2019). Therefore, assembly of actin patches at the base of dendritic spines could be spatially controlled by curvature-sensing actin-binding proteins and further reinforced by actin-microtubule crosslinking factors. Synaptic activity is likely to exert a temporal control over the assembly of actin patches through signaling kinase pathways (e.g., calmodulin- and cAMP-dependent protein kinases), which regulate actin assembly and potentially the localization and functions of septins and WAVE (Bucher et al., 2020; Hotulainen and Hoogenraad, 2010; Lei et al., 2016; Schatzle et al., 2018).
Actin patches provide a “hand-off” mechanism for directing membrane traffic from the microtubules of the dendritic shaft to the actin filaments of spines, however recent evidence points to a “direct-deposit” mode of vesicle delivery to spine membranes, which results from microtubule entry into spines (Dent, 2017). This mode of membrane traffic relies on the capture of dynamic microtubule plus ends that grow from the dendritic shaft to the neck and/or head of spines (Figure 5B).
EM observations of microtubules in dendritic spines were initially met with skepticism, but microtubule entry into dendritic spines has been extensively and reproducibly reported in living neurons (Hu et al., 2011; Hu et al., 2008; Jaworski et al., 2009; Kapitein et al., 2011; Merriam et al., 2011; Merriam et al., 2013; Schatzle et al., 2018). Microtubule plus ends invade a subset of dendritic spines at steady state, and the number and duration of these events increase with synaptic activity, chemically induced long-term potentiation and activation of TrkB receptors by BDNF (Hu et al., 2011; Hu et al., 2008; Merriam et al., 2011; Merriam et al., 2013). Conversely, induction of NMDA-dependent long-term depression diminishes MT entry into spines and results in the dissociation of the microtubule plus end-binding protein 3 (EB3), which accumulates on microtubule bundles of the dendritic shaft through a direct interaction with MAP2 (Kapitein et al., 2011). Microtubule invasions correlate with enhanced delivery of post-synaptic proteins (e.g., PSD95) and enlargement of the spine (Hu et al., 2011; Merriam et al., 2011). Notably, the kinesin 3 motor KIF1A and synaptotagmin-IV-containing vesicles traffic synchronously with growing microtubules into dendritic spines, and delivered directly by microtubules to the spine membrane (McVicker et al., 2016). Consistent with these observations in murine pyramidal neurons, kinesin-mediated delivery of AMPA receptors has been reported in C elegans neurons (Hoerndli et al., 2013; Hoerndli et al., 2015b). Moreover, microtubule plus ends were recently found to deliver synaptic vesicle precursors driven by kinesin-3/KIF1A to axonal pre-synaptic sites (Guedes-Dias et al., 2019).
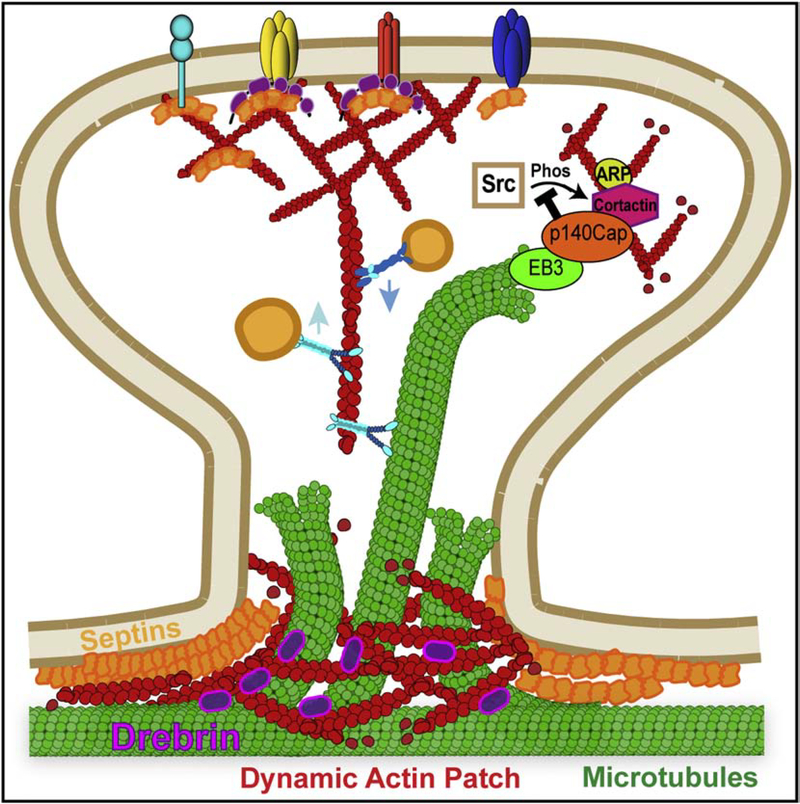
How are microtubule plus ends targeted and captured by dendritic spines? Microtubule invasions into spines are dependent on actin filaments and correlate with enhanced actin polymerization in response to synaptic calcium flux (Merriam et al., 2013; Schatzle et al., 2018). Guidance of microtubule plus end entry into spines correlates with the presence of dynamic actin filaments at the base of spines (Figure 6), which involves a cortactin-dependent remodeling of actin in response to synaptic activity (Schatzle et al., 2018). Mechanistically, microtubule-actin crosslinking proteins such as drebrin could mediate the physical capture of microtubules by actin (Merriam et al., 2013). Interestingly, a positive feedback loop appears to exist between the actin filaments of dendritic spines and entry of microtubule plus ends as the latter can stimulate actin assembly (Rodriguez et al., 2003). For example, the microtubule plus end-binding protein EB3 associates with p140Cap/SNIP, which in turn interacts with cortactin that promotes actin assembly in dendritic spines (Jaworski et al., 2009) (Figure 6). As new mechanisms of actin polymerization from microtubule plus ends have emerged (Henty-Ridilla et al., 2016), microtubule-dependent remodeling of actin in dendritic spines warrants further investigation. Overall, microtubule-actin crosstalk appears to be critical for the spatial control of membrane traffic to dendritic spines by either a hand-off or direct-deposit mechanism.
Figure 6.
Actin-microtubule crosstalk in dendritic spines. Microtubule plus ends are captured at the base of dendritic spines by dynamic patches of actin filaments and possibly other cytoskeletal elements such as septins, which are enriched at the membrane curvature along the base and neck of dendritic spines. Microtubule-actin crosstalk is mediated by proteins such as drebrin, which interacts with both actin and microtubules, and myosin V, which can diffuse on the microtubule lattice and thereby, could transition vesicular cargo from microtubules to actin filaments. Notably, microtubules have the capacity to regulate the actin organization of dendritic spines through proteins that associate with microtubule plus ends. For example, p140Cap/SNIP associates with the microtubule plus-end protein EB3 and interacts with cortactin that promotes Arp2/3-induced assembly of branched actin filaments. Microtubule plus ends with p140Cap/SNIP can promote actin assembly by inhibiting the Src kinase, which is a negative regulator of cortactin.
7. Looking into the future: open questions and perspectives
Despite significant advances in understanding the intrinsic and extrinsic mechanisms underlying dendritic morphogenesis and plasticity, dendrites remain less studied and understood than axons. New advances have begun to shed light on the organization and navigation of membrane traffic in dendrites, but questions abound on nearly every aspect of dendritic development and homeostasis. Looking into the future, we conclude by considering a number of critical questions in the following areas of dendritic membrane traffic:
Dendrite Morphogenesis. As axonal morphogenesis precedes the outgrowth of dendrites, dendritic polarity has been presumed to commence as a consequence of the sorting functions of the AIS. Dendrite-selective cargo, however, traffic preferentially to minor neurites prior to the formation of AIS in the major longest neurite - the presumptive axon (Petersen et al., 2014). While the PAEZ is posited to mediate this early sorting (Farias et al., 2015), hitherto unknown effectors and mechanisms are likely to function in parallel with the PAEZ and AIS. Discovering a septin-based mechanism of vesicle sorting in proximal dendrites, which excludes axonal motor-cargo, underscores the existence of dendrite-based mechanisms of neuronal polarity (Karasmanis et al., 2018). We nevertheless have little understanding of the early stages of dendrite morphogenesis. The intrinsic machineries underlying dendrite-specific features such as extensive branching, dendritic spine formation and a unique organization of ER and Golgi membranes are poorly understood. Similarly, the apical-basal differentiation of dendrites has been little studied. Apical dendrites form in cultured neurons in vitro and therefore, asymmetry among dendrites is driven by intrinsic mechanisms that are yet to be discovered (Horton et al., 2006).
Protein Sorting. Transcytosis and the existence of non-canonical organelles (e.g., ER spine apparatus, Golgi satellites and outposts) and trafficking pathways (e.g., ER to recycling endosome and plasma membrane) involve mechanisms of protein sorting that elude our current understanding. For proteins that undergo transcytosis, it is unclear how their axonal and somatodendritic sorting motifs are selectively inhibited or activated in a region-specific manner, and recognized by competing adaptors. The sorting mechanisms that divert transcytotic axonally-destined cargo or signaling receptors/complexes from degradative endolysosomes are poorly understood. Given the presence of an extensive ER network in dendrites, continuous synthesis of axonal proteins poses the question of whether transcytosis is the main mechanism of clearance or targeting for degradation in lysosomes occurs by sorting in the ER, Golgi outposts, endosomes and/or multivesicular bodies. Alternatively, are there sorting mechanisms that exclude axonal membrane proteins from dendritic ER exit sites or package them into vesicles, which are destined to the Golgi complex of the cell body instead of the local Golgi outposts? Lastly, how dendritic proteins are sorted for trafficking directly from the ER/ERGIC to recycling endosomes or the plasma membrane remains unexplored.
Cytoskeleton-dependent transport. Over the last 30 years, much effort has been expended on elucidating motor-cargo specificity, and how cytoskeletal motors function and are regulated. Although substantial progress has been made in these areas, how cargo and adaptor scaffold proteins alter the motile properties of motors is little understood. For example, it is unknown how the GRIP1 cargo adaptor/scaffold alters the axonal selectivity of kinesin-1/KIF5, enabling entry of kinesin-1/KIF5-GRIP1-cargo into dendrites. Is this effect due to conformational changes in the kinesin motor or the result of a new set of interactions between the GRIP1-bound motor and axonal or dendritic MAPs? The discovery of dynein-activating adaptors, several of which also interact with kinesins, has elevated the importance of cargo adaptors/scaffolds in dictating the directionality and run lengths of cargo, and coordinating the activity of multiple motors. In parallel, we have begun to scratch the surface of the tubulin and MAP codes that regulate traffic on the surface of dendritic microtubules. Understanding how combinations of PTMs and MAPs instruct the itineraries of dendritic motor-cargo looms as a new frontier. In light of new evidence indicating that dendritic microtubules resemble multilane highways of unidirectional orientation, it is imperative to determine: i) which MAPs associate with microtubule bundles of particular orientation, ii) how motors and their cargo switch between microtubules of opposing orientations, and iii) how microtubule organization and membrane traffic transitions through dendritic branch points. The latter pose many questions about the continuity, directionality and regulation of membrane traffic at dendritic branch junctions.
Dendritc spines. The width and area of dendritic spines, which are not much larger than the diffraction limit of light microscopy, have posed limitations in the study of membrane traffic in dendritic spines. Recent technological advances in time-lapse super-resolution microscopy usher in a new era of spine research. Microtubule entry into dendritic spines has also raised new questions about membrane traffic in and out of spines. For example, the mechanisms of microtubule capture and entry, and their regulation by neuronal activity, are poorly understood. It is also unknown how microtubules interface with actin filaments, and how membrane traffic may transition from microtubules to actin and vice versa in dendritic spines.
Future research in these areas will transform our current understanding of membrane traffic in dendrites, and provide new insights into the pathogenesis of neurological diseases that arise from defects in neuronal trafficking. Autism spectrum disorders, intellectual disabilities and neuropsychiatric disorders are all characterized by alterations in neuronal dendritic trees and spines (Kulkarni and Firestein, 2012). Mutations in clathrin adaptor proteins have been reported in patients with intellectual disabilities, epileptic encephalopathy and spastic paraplegia (Sanger et al., 2019). Epileptic phenotypes result directly from defects in kinesin-1/KIF5-mediated transport of GABAA receptors (Nakajima et al., 2012). Similarly, defects in the somatodendritic transport of serotonin (5-hydroxytryptamine, 5-HT1A) receptors by kinesin-3/KIF13A and NMDA receptors (NR2B) by kinesin-2/KIF17 are linked to the pathogenesis of anxiety and schizophrenia, respectively (Alsabban et al., 2019; Zhou et al., 2013). In addition to motors, MAPs and PTMs are also implicated in disease-causing alterations of membrane traffic. In Alzheimer’s disease, amyloids cause an abnormal accumulation of hyperphosphorylated tau in the somatodendritic compartment, which in turn results in loss of dendritic spines; tau is a critical component of post-synaptic excitotoxicity (Ittner and Ittner, 2018). In summary, more work is needed to unravel how defects in membrane traffic contribute to the abnormal development and degeneration of dendrites. Future studies will advance our knowledge of dendritic membrane traffic in health and disease, and may lead to new therapeutic avenues for the treatment of neurological disorders.
Highlights.
Dendrites are highly branched and specialized compartments with distinct membrane and cytoskeletal organization.
How post-synaptic proteins partition and traffic preferentially into dendrites is a fundamental question of basic neuroscience.
Recent advances have shed new insights into the spatial control of dendritic membrane traffic.
Dendrite-specific mechanisms promote and underlie the axonodendritic polarity of neuronal membrane traffic, which might have co-evolved with their highly arborized morphology.
Acknowledgments
We thank Dr. Eva Karasmanis (UC San Diego/HHMI) for valuable feedback and comments on the manuscript, and Dr. Bettina Winckler (University of Virginia) for advice. We apologize for the inadvertent omission of any references and work from colleagues in this field of study. The authors were supported by NIH/NIGMS grant GM097664 to E.T.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adrian M, Kusters R, Wierenga CJ, Storm C, Hoogenraad CC, Kapitein LC, 2014. Barriers in the brain: resolving dendritic spine morphology and compartmentalization. Front Neuroanat 8, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre-Chen C, Bulow HE, Kaprielian Z, 2011. C. elegans bicd-1, homolog of the Drosophila dynein accessory factor Bicaudal D, regulates the branching of PVD sensory neuron dendrites. Development 138, 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Bassam S, Xu M, Wandless TJ, Arnold DB, 2012. Differential trafficking of transport vesicles contributes to the localization of dendritic proteins. Cell Rep 2, 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberi S, Boda B, Steiner P, Nikonenko I, Hirling H, Muller D, 2005. The endosomal protein NEEP21 regulates AMPA receptor-mediated synaptic transmission and plasticity in the hippocampus. Mol Cell Neurosci 29, 313–319. [DOI] [PubMed] [Google Scholar]
- Ali MY, Krementsova EB, Kennedy GG, Mahaffy R, Pollard TD, Trybus KM, Warshaw DM, 2007. Myosin Va maneuvers through actin intersections and diffuses along microtubules. Proc Natl Acad Sci U S A 104, 4332–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsabban AH, Morikawa M, Tanaka Y, Takei Y, Hirokawa N, 2019. Kinesin Kif3b mutation reduces NMDAR subunit NR2A trafficking and causes schizophrenia-like phenotypes in mice. EMBO J 39, e101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E, Maday S, Sfakianos J, Hull M, Winckler B, Sheff D, Folsch H, Mellman I, 2005. Transcytosis of NgCAM in epithelial cells reflects differential signal recognition on the endocytic and secretory pathways. J Cell Biol 170, 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold DB, 2009. Actin and microtubule-based cytoskeletal cues direct polarized targeting of proteins in neurons. Sci Signal 2, pe49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur AL, Yang SZ, Abellaneda AM, Wildonger J, 2015. Dendrite arborization requires the dynein cofactor NudE. J Cell Sci 128, 2191–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascano M, Richmond A, Borden P, Kuruvilla R, 2009. Axonal targeting of Trk receptors via transcytosis regulates sensitivity to neurotrophin responses. J Neurosci 29, 11674–11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton J, Houdusse A, Moores C, 2013. MAPping out distribution routes for kinesin couriers. Biol Cell 105, 465–487. [DOI] [PubMed] [Google Scholar]
- Baas PW, Black MM, Banker GA, 1989. Changes in microtubule polarity orientation during the development of hippocampal neurons in culture. J Cell Biol 109, 3085–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Deitch JS, Black MM, Banker GA, 1988. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci U S A 85, 8335–8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Lin S, 2011. Hooks and comets: The story of microtubule polarity orientation in the neuron. Dev Neurobiol 71, 403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Bowen JR, Knox TK, Zhou K, Pendziwiat M, Kuhlenbaumer G, Sindelar CV, Spiliotis ET, 2013. Novel septin 9 repeat motifs altered in neuralgic amyotrophy bind and bundle microtubules. J Cell Biol 203, 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Karasmanis EP, Spiliotis ET, 2016. Septin 9 interacts with kinesin KIF17 and interferes with the mechanism of NMDA receptor cargo binding and transport. Mol Biol Cell 27, 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanian L, Berger CL, Hendricks AG, 2017. Acetylated Microtubules Are Preferentially Bundled Leading to Enhanced Kinesin-1 Motility. Biophys J 113, 1551–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker G, 2018. The Development of Neuronal Polarity: A Retrospective View. J Neurosci 38, 1867–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar J, Kobler O, van Bommel B, Mikhaylova M, 2016. Periodic F-actin structures shape the neck of dendritic spines. Sci Rep 6, 37136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes AP, Polleux F, 2009. Establishment of axon-dendrite polarity in developing neurons. Annu Rev Neurosci 32, 347–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bel C, Oguievetskaia K, Pitaval C, Goutebroze L, Faivre-Sarrailh C, 2009. Axonal targeting of Caspr2 in hippocampal neurons via selective somatodendritic endocytosis. J Cell Sci 122, 3403–3413. [DOI] [PubMed] [Google Scholar]
- Benavides-Piccione R, Hamzei-Sichani F, Ballesteros-Yanez I, DeFelipe J, Yuste R, 2006. Dendritic size of pyramidal neurons differs among mouse cortical regions. Cereb Cortex 16, 9901001. [DOI] [PubMed] [Google Scholar]
- Bentivoglio M, Cotrufo T, Ferrari S, Tesoriero C, Mariotto S, Bertini G, Berzero A, Mazzarello P, 2019. The Original Histological Slides of Camillo Golgi and His Discoveries on Neuronal Structure. Front Neuroanat 13, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley M, Banker G, 2015. A Novel Assay to Identify the Trafficking Proteins that Bind to Specific Vesicle Populations. Curr Protoc Cell Biol 69, 13 18 11–13 18 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt R, Matus A, 1984. Light and electron microscopic studies of the distribution of microtubule-associated protein 2 in rat brain: a difference between dendritic and axonal cytoskeletons. J Comp Neurol 226, 203–221. [DOI] [PubMed] [Google Scholar]
- Bodakuntla S, Jijumon AS, Villablanca C, Gonzalez-Billault C, Janke C, 2019. Microtubule-Associated Proteins: Structuring the Cytoskeleton. Trends Cell Biol 29, 804–819. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, 2014. Adaptor proteins involved in polarized sorting. J Cell Biol 204, 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet C, Boucher D, Lazereg S, Pedrotti B, Islam K, Denoulet P, Larcher JC, 2001. Differential binding regulation of microtubule-associated proteins MAP1A, MAP1B, and MAP2 by tubulin polyglutamylation. J Biol Chem 276, 12839–12848. [DOI] [PubMed] [Google Scholar]
- Bowen AB, Bourke AM, Hiester BG, Hanus C, Kennedy MJ, 2017. Golgi-independent secretory trafficking through recycling endosomes in neuronal dendrites and spines. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges AA, Jentzsch MS, Oakes PW, Occhipinti P, Gladfelter AS, 2016. Micron-scale plasma membrane curvature is recognized by the septin cytoskeleton. J Cell Biol 213, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MD, Banker GA, Hussaini IM, Gonias SL, VandenBerg SR, 1997. Low density lipoprotein receptor-related protein is expressed early and becomes restricted to a somatodendritic domain during neuronal differentiation in culture. Brain Res 747, 313–317. [DOI] [PubMed] [Google Scholar]
- Bucher M, Fanutza T, Mikhaylova M, 2020. Cytoskeletal makeup of the synapse: Shaft versus spine. Cytoskeleton (Hoboken) 77: 55–64. [DOI] [PubMed] [Google Scholar]
- Burute M, Kapitein LC, 2019. Cellular Logistics: Unraveling the Interplay Between Microtubule Organization and Intracellular Transport. Annu Rev Cell Dev Biol 35, 29–54 [DOI] [PubMed] [Google Scholar]
- Buss F, Arden SD, Lindsay M, Luzio JP, Kendrick-Jones J, 2001. Myosin VI isoform localized to clathrin-coated vesicles with a role in clathrin-mediated endocytosis. EMBO J 20, 3676–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon KS, Woods BL, Crutchley JM, Gladfelter AS, 2019. An amphipathic helix enables septins to sense micrometer-scale membrane curvature. J Cell Biol 218, 1128–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston JP, Holzbaur EL, 2009. Huntingtin as an essential integrator of intracellular vesicular trafficking. Trends Cell Biol 19, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charalambous DC, Pasciuto E, Mercaldo V, Pilo Boyl P, Munck S, Bagni C, Santama N, 2013. KIF1Bbeta transports dendritically localized mRNPs in neurons and is recruited to synapses in an activity-dependent manner. Cell Mol Life Sci 70, 335–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary AR, Lu H, Krementsova EB, Bookwalter CS, Trybus KM, Hendricks AG, 2019. MAP7 regulates organelle transport by recruiting kinesin-1 to microtubules. J Biol Chem 294, 10160–10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kanai Y, Cowan NJ, Hirokawa N, 1992. Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature 360, 674–677. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Goldstein JL, Brown MS, 1990. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem 265, 3116–3123. [PubMed] [Google Scholar]
- Choquet D, Triller A, 2013. The dynamic synapse. Neuron 80, 691–703. [DOI] [PubMed] [Google Scholar]
- Choy RW, Park M, Temkin P, Herring BE, Marley A, Nicoll RA, von Zastrow M, 2014. Retromer mediates a discrete route of local membrane delivery to dendrites. Neuron 82, 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu PJ, Rivera JF, Arnold DB, 2006. A role for Kif17 in transport of Kv4.2. J Biol Chem 281, 365–373. [DOI] [PubMed] [Google Scholar]
- Clairfeuille T, Mas C, Chan AS, Yang Z, Tello-Lafoz M, Chandra M, Widagdo J, Kerr MC, Paul B, Merida I, Teasdale RD, Pavlos NJ, Anggono V, Collins BM, 2016. A molecular code for endosomal recycling of phosphorylated cargos by the SNX27-retromer complex. Nat Struct Mol Biol 23, 921–932. [DOI] [PubMed] [Google Scholar]
- Colin E, Zala D, Liot G, Rangone H, Borrell-Pages M, Li XJ, Saudou F, Humbert S, 2008. Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. EMBO J 27, 2124–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collawn JF, Stangel M, Kuhn LA, Esekogwu V, Jing SQ, Trowbridge IS, Tainer JA, 1990. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell 63, 1061–1072. [DOI] [PubMed] [Google Scholar]
- Conde C, Caceres A, 2009. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci 10, 319–332. [DOI] [PubMed] [Google Scholar]
- Conway L, Gramlich MW, Ali Tabei SM, Ross JL, 2014. Microtubule orientation and spacing within bundles is critical for long-range kinesin-1 motility. Cytoskeleton (Hoboken) 71, 595–610. [DOI] [PubMed] [Google Scholar]
- Cross JA, Dodding MP, 2019. Motor-cargo adaptors at the organelle-cytoskeleton interface. Curr Opin Cell Biol 59, 16–23. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Jones EG, 1988. Cajal on the cerebral cortex: an annotated translation of the complete writings. Oxford: University Press. [Google Scholar]
- Del Castillo U, Norkett R, Gelfand VI, 2019. Unconventional Roles of Cytoskeletal Mitotic Machinery in Neurodevelopment. Trends Cell Biol 29, 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deller T, Korte M, Chabanis S, Drakew A, Schwegler H, Stefani GG, Zuniga A, Schwarz K, Bonhoeffer T, Zeller R, Frotscher M, Mundel P, 2003. Synaptopodin-deficient mice lack a spine apparatus and show deficits in synaptic plasticity. Proc Natl Acad Sci U S A 100, 10494–10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deller T, Merten T, Roth SU, Mundel P, Frotscher M, 2000. Actin-associated protein synaptopodin in the rat hippocampal formation: localization in the spine neck and close association with the spine apparatus of principal neurons. J Comp Neurol 418, 164–181. [DOI] [PubMed] [Google Scholar]
- Dent EW, 2017. Of microtubules and memory: implications for microtubule dynamics in dendrites and spines. Mol Biol Cell 28, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Shen K, Bulow HE, 2015. Intrinsic and extrinsic mechanisms of dendritic morphogenesis. Annu Rev Physiol 77, 271–300. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Parton RG, Simons K, 1991. Polarized sorting of glypiated proteins in hippocampal neurons. Nature 349, 158–161. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Simons K, 1990. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell 62, 63–72. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA, 1988. The establishment of polarity by hippocampal neurons in culture. J Neurosci 8, 1454–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M, Kiebler MA, 2011. Mechanisms of dendritic mRNA transport and its role in synaptic tagging. EMBO J 30, 3540–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duellberg C, Trokter M, Jha R, Sen I, Steinmetz MO, Surrey T, 2014. Reconstitution of a hierarchical +TIP interaction network controlling microtubule end tracking of dynein. Nat Cell Biol 16, 804–811. [DOI] [PubMed] [Google Scholar]
- Dwyer ND, Adler CE, Crump JG, L’Etoile ND, Bargmann CI, 2001. Polarized dendritic transport and the AP-1 mu1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron 31, 277–287. [DOI] [PubMed] [Google Scholar]
- Engelender S, Sharp AH, Colomer V, Tokito MK, Lanahan A, Worley P, Holzbaur EL, Ross CA, 1997. Huntingtin-associated protein 1 (HAP1) interacts with the p150Glued subunit of dynactin. Hum Mol Genet 6, 2205–2212. [DOI] [PubMed] [Google Scholar]
- Eshun-Wilson L, Zhang R, Portran D, Nachury MV, Toso DB, Lohr T, Vendruscolo M, Bonomi M, Fraser JS, Nogales E, 2019. Effects of alpha-tubulin acetylation on microtubule structure and stability. Proc Natl Acad Sci U S A 116, 10366–10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves da Silva M, Adrian M, Schatzle P, Lipka J, Watanabe T, Cho S, Futai K, Wierenga CJ, Kapitein LC, Hoogenraad CC, 2015. Positioning of AMPA ReceptorContaining Endosomes Regulates Synapse Architecture. Cell Rep 13, 933–943. [DOI] [PubMed] [Google Scholar]
- Ewers H, Tada T, Petersen JD, Racz B, Sheng M, Choquet D, 2014. A Septin-Dependent Diffusion Barrier at Dendritic Spine Necks. PLoS One 9, e113916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fache MP, Moussif A, Fernandes F, Giraud P, Garrido JJ, Dargent B, 2004. Endocytotic elimination and domain-selective tethering constitute a potential mechanism of protein segregation at the axonal initial segment. J Cell Biol 166, 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias GG, Cuitino L, Guo X, Ren X, Jarnik M, Mattera R, Bonifacino JS, 2012. Signal-mediated, AP-1/clathrin-dependent sorting of transmembrane receptors to the somatodendritic domain of hippocampal neurons. Neuron 75, 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias GG, Guardia CM, Britt DJ, Guo X, Bonifacino JS, 2015. Sorting of Dendritic and Axonal Vesicles at the Pre-axonal Exclusion Zone. Cell Rep 13, 1221–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkhondeh A, Niwa S, Takei Y, Hirokawa N, 2015. Characterizing KIF16B in neurons reveals a novel intramolecular “stalk inhibition” mechanism that regulates its capacity to potentiate the selective somatodendritic localization of early endosomes. J Neurosci 35, 50675086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Harris KM, 1999. Dendrite Structure In: Stuart G, Spruston N, Hausser M (Eds.), Dendrites. Oxford University Press, New York. [Google Scholar]
- Flynn KC, 2013. The cytoskeleton and neurite initiation. Bioarchitecture 3, 86–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn KC, Pak CW, Shaw AE, Bradke F, Bamburg JR, 2009. Growth cone-like waves transport actin and promote axonogenesis and neurite branching. Dev Neurobiol 69, 761–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franker MA, Esteves da Silva M, Tas RP, Tortosa E, Cao Y, Frias CP, Janssen AFJ, Wulf PS, Kapitein LC, Hoogenraad CC, 2016. Three-Step Model for Polarized Sorting of KIF17 into Dendrites. Curr Biol 26, 1705–1712. [DOI] [PubMed] [Google Scholar]
- Froidevaux-Klipfel L, Targa B, Cantaloube I, Ahmed-Zaid H, Pous C, Baillet A, 2015. Septin cooperation with tubulin polyglutamylation contributes to cancer cell adaptation to taxanes. Oncotarget 6, 36063–36080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu MM, Holzbaur EL, 2013. JIP1 regulates the directionality of APP axonal transport by coordinating kinesin and dynein motors. J Cell Biol 202, 495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu MM, Holzbaur EL, 2014. Integrated regulation of motor-driven organelle transport by scaffolding proteins. Trends Cell Biol 24, 564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann JC, Kins S, Rostaing P, El Far O, Kirsch J, Sheng M, Triller A, Betz H, Kneussel M, 2002. Gephyrin interacts with Dynein light chains 1 and 2, components of motor protein complexes. J Neurosci 22, 5393–5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi Y, Namba T, Nakamuta S, Kaibuchi K, 2014. Neuronal polarization in vivo: Growing in a complex environment. Curr Opin Neurobiol 27, 215–223. [DOI] [PubMed] [Google Scholar]
- Gallo G, 2013. Mechanisms underlying the initiation and dynamics of neuronal filopodia: from neurite formation to synaptogenesis. Int Rev Cell Mol Biol 301, 95–156. [DOI] [PubMed] [Google Scholar]
- Gallon M, Cullen PJ, 2015. Retromer and sorting nexins in endosomal sorting. Biochem Soc Trans 43, 33–47. [DOI] [PubMed] [Google Scholar]
- Garcia-Lopez P, Garcia-Marin V, Freire M, 2010. The histological slides and drawings of cajal. Front Neuroanat 4, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido JJ, Fernandes F, Moussif A, Fache MP, Giraud P, Dargent B, 2003. Dynamic compartmentalization of the voltage-gated sodium channels in axons. Biol Cell 95, 437–445. [DOI] [PubMed] [Google Scholar]
- Genau HM, Behrends C, 2016. GABARAP proteins as scaffolds in localized TIAM1-RAC1 signaling. Mol Cell Oncol 3, e1027440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai R, Bugarcic A, Liu H, Norwood SJ, Skeldal S, Coulson EJ, Li SS, Teasdale RD, Collins BM, 2013. Structural basis for endosomal trafficking of diverse transmembrane cargos by PX-FERM proteins. Proc Natl Acad Sci U S A 110, E643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiretti AE, Thies E, Tokito MK, Lin T, Ostap EM, Kneussel M, Holzbaur ELF, 2016. Activity-Dependent Regulation of Distinct Transport and Cytoskeletal Remodeling Functions of the Dendritic Kinesin KIF21B. Neuron 92, 857–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goo MS, Sancho L, Slepak N, Boassa D, Deerinck TJ, Ellisman MH, Bloodgood BL, Patrick GN, 2017. Activity-dependent trafficking of lysosomes in dendrites and dendritic spines. The Journal of Cell Biology 216, 2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber WB, Sagasti A, 2010. Self-avoidance and tiling: Mechanisms of dendrite and axon spacing. Cold Spring Harb Perspect Biol 2, a001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia CM, De Pace R, Mattera R, Bonifacino JS, 2018. Neuronal functions of adaptor complexes involved in protein sorting. Curr Opin Neurobiol 51, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes-Dias P, Nirschl JJ, Abreu N, Tokito MK, Janke C, Magiera MM, Holzbaur ELF, 2019. Kinesin-3 Responds to Local Microtubule Dynamics to Target Synaptic Cargo Delivery to the Presynapse. Curr Biol 29, 268–282 e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaud L, Setou M, Hirokawa N, 2003. KIF17 dynamics and regulation of NR2B trafficking in hippocampal neurons. J Neurosci 23, 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaud L, Wong R, Hirokawa N, 2008. Disruption of KIF17-Mint1 interaction by CaMKIIdependent phosphorylation: a molecular model of kinesin-cargo release. Nat Cell Biol 10, 19–29. [DOI] [PubMed] [Google Scholar]
- Guo X, Farias GG, Mattera R, Bonifacino JS, 2016. Rab5 and its effector FHF contribute to neuronal polarity through dynein-dependent retrieval of somatodendritic proteins from the axon. Proc Natl Acad Sci U S A 113, E5318–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halff EF, Szulc BR, Lesept F, Kittler JT, 2019. SNX27-Mediated Recycling of Neuroligin-2 Regulates Inhibitory Signaling. Cell Rep 29, 2599–2607 e2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer JA 3rd, Sellers JR, 2011. Walking to work: roles for class V myosins as cargo transporters. Nat Rev Mol Cell Biol 13, 13–26. [DOI] [PubMed] [Google Scholar]
- Hammond JW, Huang CF, Kaech S, Jacobson C, Banker G, Verhey KJ, 2010. Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol Biol Cell 21, 572–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Zhou R, Xia C, Zhuang X, 2017. Structural organization of the actin-spectrin-based membrane skeleton in dendrites and soma of neurons. Proc Natl Acad Sci U S A 114, E6678E6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus C, Ehlers MD, 2008. Secretory outposts for the local processing of membrane cargo in neuronal dendrites. Traffic 9, 1437–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus C, Ehlers MD, 2016. Specialization of biosynthetic membrane trafficking for neuronal form and function. Curr Opin Neurobiol 39, 8–16. [DOI] [PubMed] [Google Scholar]
- Hanus C, Kochen L, Tom Dieck S, Racine V, Sibarita JB, Schuman EM, Ehlers MD, 2014. Synaptic control of secretory trafficking in dendrites. Cell Rep 7, 1771–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harterink M, Vocking K, Pan X, Soriano Jerez EM, Slenders L, Freal A, Tas RP, van de Wetering WJ, Timmer K, Motshagen J, van Beuningen SFB, Kapitein LC, Geerts WJC, Post JA, Hoogenraad CC, 2019. TRIM46 Organizes Microtubule Fasciculation in the Axon Initial Segment. J Neurosci 39, 4864–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Zhou R, Wu Z, Carrasco MA, Kurshan PT, Farley JE, Simon DJ, Wang G, Han B, Hao J, Heller E, Freeman MR, Shen K, Maniatis T, Tessier-Lavigne M, Zhuang X, 2016. Prevalent presence of periodic actin-spectrin-based membrane skeleton in a broad range of neuronal cell types and animal species. Proc Natl Acad Sci U S A 113, 60296034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler FF, Lee HK, Gromova KV, Pechmann Y, Schurek B, Ruschkies L, Schroeder M, Schweizer M, Kneussel M, 2014. GRIP1 interlinks N-cadherin and AMPA receptors at vesicles to promote combined cargo transport into dendrites. Proc Natl Acad Sci U S A 111, 5030–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henty-Ridilla JL, Rankova A, Eskin JA, Kenny K, Goode BL, 2016. Accelerated actin filament polymerization from microtubule plus ends. Science 352, 1004–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering H, Sheng M, 2001. Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci 2, 880–888. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Niwa S, Tanaka Y, 2010. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron 68, 610–638. [DOI] [PubMed] [Google Scholar]
- Hoerndli FJ, Kallarackal AJ, Maricq AV, 2015a. Mobile AMPARs are required for synaptic plasticity. Channels (Austin) 9, 230–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerndli FJ, Maxfield DA, Brockie PJ, Mellem JE, Jensen E, Wang R, Madsen DM, Maricq AV, 2013. Kinesin-1 regulates synaptic strength by mediating the delivery, removal, and redistribution of AMPA receptors. Neuron 80, 1421–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerndli FJ, Wang R, Mellem JE, Kallarackal A, Brockie PJ, Thacker C, Madsen DM, Maricq AV, 2015b. Neuronal Activity and CaMKII Regulate Kinesin-Mediated Transport of Synaptic AMPARs. Neuron 86, 457–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenraad CC, Milstein AD, Ethell IM, Henkemeyer M, Sheng M, 2005. GRIP1 controls dendrite morphogenesis by regulating EphB receptor trafficking. Nat Neurosci 8, 906915. [DOI] [PubMed] [Google Scholar]
- Hooikaas PJ, Martin M, Muhlethaler T, Kuijntjes GJ, Peeters CAE, Katrukha EA, Ferrari L, Stucchi R, Verhagen DGF, van Riel WE, Grigoriev I, Altelaar AFM, Hoogenraad CC, Rudiger SGD, Steinmetz MO, Kapitein LC, Akhmanova A, 2019. MAP7 family proteins regulate kinesin-1 recruitment and activation. J Cell Biol 218, 1298–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton AC, Ehlers MD, 2004. Secretory trafficking in neuronal dendrites. Nat Cell Biol 6, 585–591. [DOI] [PubMed] [Google Scholar]
- Horton AC, Racz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD, 2005. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron 48, 757–771. [DOI] [PubMed] [Google Scholar]
- Horton AC, Yi JJ, Ehlers MD, 2006. Cell type-specific dendritic polarity in the absence of spatially organized external cues. Brain Cell Biol 35, 29–38. [DOI] [PubMed] [Google Scholar]
- Hotulainen P, Hoogenraad CC, 2010. Actin in dendritic spines: connecting dynamics to function. The Journal of Cell Biology 189, 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska M, Henderson N, Le Marchand SJ, Jafri H, Dalva MB, 2018. Synaptic nanomodules underlie the organization and plasticity of spine synapses. Nat Neurosci 21, 671682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Bai X, Bowen JR, Dolat L, Korobova F, Yu W, Baas PW, Svitkina T, Gallo G, Spiliotis ET, 2012. Septin-driven coordination of actin and microtubule remodeling regulates the collateral branching of axons. Curr Biol 22, 1109–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Ballo L, Pietila L, Viesselmann C, Ballweg J, Lumbard D, Stevenson M, Merriam E, Dent EW, 2011. BDNF-induced increase of PSD-95 in dendritic spines requires dynamic microtubule invasions. J Neurosci 31, 15597–15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Viesselmann C, Nam S, Merriam E, Dent EW, 2008. Activity-dependent dynamic microtubule invasion of dendritic spines. J Neurosci 28, 13094–13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CY, Rasband MN, 2018. Axon initial segments: structure, function, and disease. Ann N Y Acad Sci 1420, 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain NK, Diering GH, Sole J, Anggono V, Huganir RL, 2014. Sorting Nexin 27 regulates basal and activity-dependent trafficking of AMPARs. Proc Natl Acad Sci U S A 111, 11840–11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose S, Ogawa T, Hirokawa N, 2015. Mechanism of Activity-Dependent Cargo Loading via the Phosphorylation of KIF3A by PKA and CaMKIIa. Neuron 87, 1022–1035. [DOI] [PubMed] [Google Scholar]
- Ikegami K, Mukai M, Tsuchida J, Heier RL, Macgregor GR, Setou M, 2006. TTLL7 is a mammalian beta-tubulin polyglutamylase required for growth of MAP2-positive neurites. J Biol Chem 281, 30707–30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner A, Ittner LM, 2018. Dendritic Tau in Alzheimer’s Disease. Neuron 99, 13–27. [DOI] [PubMed] [Google Scholar]
- Jain S, Farias GG, Bonifacino JS, 2015. Polarized sorting of the copper transporter ATP7B in neurons mediated by recognition of a dileucine signal by AP-1. Mol Biol Cell 26, 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YN, Jan LY, 2010. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci 11, 316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C, Kneussel M, 2010. Tubulin post-translational modifications: encoding functions on the neuronal microtubule cytoskeleton. Trends Neurosci 33, 362–372. [DOI] [PubMed] [Google Scholar]
- Jareb M, Banker G, 1998. The polarized sorting of membrane proteins expressed in cultured hippocampal neurons using viral vectors. Neuron 20, 855–867. [DOI] [PubMed] [Google Scholar]
- Jaulin F, Kreitzer G, 2010. KIF17 stabilizes microtubules and contributes to epithelial morphogenesis by acting at MT plus ends with EB1 and APC. J Cell Biol 190, 443–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J, Kapitein LC, Gouveia SM, Dortland BR, Wulf PS, Grigoriev I, Camera P, Spangler SA, Di Stefano P, Demmers J, Krugers H, Defilippi P, Akhmanova A, Hoogenraad CC, 2009. Dynamic Microtubules Regulate Dendritic Spine Morphology and Synaptic Plasticity. Neuron 61, 85–100. [DOI] [PubMed] [Google Scholar]
- Jenkins B, Decker H, Bentley M, Luisi J, Banker G, 2012. A novel split kinesin assay identifies motor proteins that interact with distinct vesicle populations. J Cell Biol 198, 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyifous O, Waites CL, Specht CG, Fujisawa S, Schubert M, Lin EI, Marshall J, Aoki C, de Silva T, Montgomery JM, Garner CC, Green WN, 2009. SAP97 and CASK mediate sorting of NMDA receptors through a previously unknown secretory pathway. Nat Neurosci 12, 1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha R, Roostalu J, Cade NI, Trokter M, Surrey T, 2017. Combinatorial regulation of the balance between dynein microtubule end accumulation and initiation of directed motility. EMBO J 36, 3387–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SL, Korobova F, Svitkina T, 2014. Axon initial segment cytoskeleton comprises a multiprotein submembranous coat containing sparse actin filaments. J Cell Biol 205, 67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang RS, Fölsch H 2011. ARH cooperates with AP-1B in the exocytosis of LDLR in polarized epithelial cells. J Cell Biol 193, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein LC, Schlager MA, Kuijpers M, Wulf PS, van Spronsen M, MacKintosh FC, Hoogenraad CC, 2010. Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr Biol 20, 290–299. [DOI] [PubMed] [Google Scholar]
- Kapitein LC, van Bergeijk P, Lipka J, Keijzer N, Wulf PS, Katrukha EA, Akhmanova A, Hoogenraad CC, 2013. Myosin-V opposes microtubule-based cargo transport and drives directional motility on cortical actin. Curr Biol 23, 828–834. [DOI] [PubMed] [Google Scholar]
- Kapitein LC, Yau KW, Gouveia SM, van der Zwan WA, Wulf PS, Keijzer N, Demmers J, Jaworski J, Akhmanova A, Hoogenraad CC, 2011. NMDA receptor activation suppresses microtubule growth and spine entry. J Neurosci 31, 8194–8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasmanis EP, Phan CT, Angelis D, Kesisova IA, Hoogenraad CC, McKenney RJ, Spiliotis ET, 2018. Polarity of Neuronal Membrane Traffic Requires Sorting of Kinesin Motor Cargo during Entry into Dendrites by a Microtubule-Associated Septin. Dev Cell 46, 204–218 e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayadjanian N, Lee HS, Pina-Crespo J, Heinemann SF, 2007. Localization of glutamate receptors to distal dendrites depends on subunit composition and the kinesin motor protein KIF17. Mol Cell Neurosci 34, 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher MT, Saunders HA, Wildonger J, 2019. Microtubule control of functional architecture in neurons. Curr Opin Neurobiol 57, 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher MT, Yue Y, Ng A, Kamiyama D, Huang B, Verhey KJ, Wildonger J, 2018. Autoinhibition of kinesin-1 is essential to the dendrite-specific localization of Golgi outposts. J Cell Biol 217, 2531–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Ehlers MD, 2006. Organelles and trafficking machinery for postsynaptic plasticity. Annu Rev Neurosci 29, 325–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Ehlers MD, 2011. Mechanisms and function of dendritic exocytosis. Neuron 69, 856–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern JV, Zhang YV, Kramer S, Brenman JE, Rasse TM, 2013. The kinesin-3, unc-104 regulates dendrite morphogenesis and synaptic development in Drosophila. Genetics 195, 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Sheng M, 2009. The postsynaptic density. Curr Biol 19, R723–724. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Rostaing P, Schiavo G, Fritschy JM, Olsen R, Triller A, Moss SJ, 2001. The subcellular distribution of GABARAP and its ability to interact with NSF suggest a role for this protein in the intracellular transport of GABA(A) receptors. Mol Cell Neurosci 18, 13–25. [DOI] [PubMed] [Google Scholar]
- Klinman E, Tokito M, Holzbaur ELF, 2017. CDK5-dependent activation of dynein in the axon initial segment regulates polarized cargo transport in neurons. Traffic 18, 808–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Haverkamp S, Fuhrmann JC, Wang H, Wassle H, Olsen RW, Betz H, 2000. The gamma-aminobutyric acid type A receptor (GABAAR)-associated protein GABARAP interacts with gephyrin but is not involved in receptor anchoring at the synapse. Proc Natl Acad Sci U S A 97, 8594–8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester SE, O’Leary DD, 1992. Functional classes of cortical projection neurons develop dendritic distinctions by class-specific sculpting of an early common pattern. J Neurosci 12, 1382–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins KM, Bell RL, Butts M, Withers GS, 2009. Dendrites differ from axons in patterns of microtubule stability and polymerization during development. Neural Dev 4, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon E, Cossard A, Jossin Y, 2017. Neuronal Polarity in the Embryonic Mammalian Cerebral Cortex. Front Cell Neurosci 11, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konietzny A, Bar J, Mikhaylova M, 2017. Dendritic Actin Cytoskeleton: Structure, Functions, and Regulations. Front Cell Neurosci 11, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konietzny A, Gonzalez-Gallego J, Bar J, Perez-Alvarez A, Drakew A, Demmers JAA, Dekkers DHW, Hammer JA 3rd, Frotscher M, Oertner TG, Wagner W, Kneussel M, Mikhaylova M, 2019. Myosin V regulates synaptopodin clustering and localization in the dendrites of hippocampal neurons. J Cell Sci 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi Y, Setou M, 2009. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat Neurosci 12, 559–567. [DOI] [PubMed] [Google Scholar]
- Krementsov DN, Krementsova EB, Trybus KM, 2004. Myosin V: regulation by calcium, calmodulin, and the tail domain. J Cell Biol 164, 877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krijnse-Locker J, Parton RG, Fuller SD, Griffiths G, Dotti CG, 1995. The organization of the endoplasmic reticulum and the intermediate compartment in cultured rat hippocampal neurons. Mol Biol Cell 6, 1315–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers M, van de Willige D, Freal A, Chazeau A, Franker MA, Hofenk J, Rodrigues RJ, Kapitein LC, Akhmanova A, Jaarsma D, Hoogenraad CC, 2016. Dynein Regulator NDEL1 Controls Polarized Cargo Transport at the Axon Initial Segment. Neuron 89, 461–471. [DOI] [PubMed] [Google Scholar]
- Kulkarni VA, Firestein BL, 2012. The dendritic tree and brain disorders. Mol Cell Neurosci 50, 10–20. [DOI] [PubMed] [Google Scholar]
- Kulkarni VV, Maday S, 2018. Neuronal endosomes to lysosomes: A journey to the soma. J Cell Biol 217, 2977–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonte D, Thies E, Kneussel M, 2014. The kinesin KIF21B participates in the cell surface delivery of gamma2 subunit-containing GABAA receptors. Eur J Cell Biol 93, 338–346. [DOI] [PubMed] [Google Scholar]
- Labonte D, Thies E, Pechmann Y, Groffen AJ, Verhage M, Smit AB, van Kesteren RE, Kneussel M, 2013. TRIM3 regulates the motility of the kinesin motor protein KIF21B. PLoS One 8, e75603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasiecka ZM, Winckler B, 2011. Mechanisms of polarized membrane trafficking in neurons - focusing in on endosomes. Mol Cell Neurosci 48, 278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasiecka ZM, Yap CC, Katz J, Winckler B, 2014. Maturational conversion of dendritic early endosomes and their roles in L1-mediated axon growth. J Neurosci 34, 14633–14643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffer BE, Melero C, Temkin P, Lei C, Hong W, Kortemme T, von Zastrow M, 2010. SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J Cell Biol 190, 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo OM, Gonzalez A, Ascano M, Kuruvilla R, Couve A, Bronfman FC, 2013. BDNF regulates Rab11-mediated recycling endosome dynamics to induce dendritic branching. J Neurosci 33, 6112–6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre JL, Sanes JR, Kay JN, 2015. Development of dendritic form and function. Annu Rev Cell Dev Biol 31, 741–777. [DOI] [PubMed] [Google Scholar]
- Lei W, Omotade OF, Myers KR, Zheng JQ, 2016. Actin cytoskeleton in dendritic spine development and plasticity. Curr Opin Neurobiol 39, 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier C, 2018. The Axon Initial Segment: An Updated Viewpoint. J Neurosci 38, 21352145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier C, Laine J, Darmon M, Boudin H, Rossier J, Lenkei Z, 2006. Constitutive activation drives compartment-selective endocytosis and axonal targeting of type 1 cannabinoid receptors. J Neurosci 26, 3141–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Merrill Sean A., Jorgensen Erik M., Shen K, 2016. Two Clathrin Adaptor Protein Complexes Instruct Axon-Dendrite Polarity. Neuron 90, 564–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon LA, Shelly SS, Tokito MK, Holzbaur EL, 2006. Microtubule binding proteins CLIP-170, EB1, and p150Glued form distinct plus-end complexes. FEBS Lett 580, 1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liot G, Zala D, Pla P, Mottet G, Piel M, Saudou F, 2013. Mutant Huntingtin alters retrograde transport of TrkB receptors in striatal dendrites. J Neurosci 33, 6298–6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka J, Kapitein LC, Jaworski J, Hoogenraad CC, 2016. Microtubule-binding protein doublecortin-like kinase 1 (DCLK1) guides kinesin-3-mediated cargo transport to dendrites. EMBO J 35, 302–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JS, Schubert CR, Fu X, Fourniol FJ, Jaiswal JK, Houdusse A, Stultz CM, Moores CA, Walsh CA, 2012. Molecular basis for specific regulation of neuronal kinesin-3 motors by doublecortin family proteins. Mol Cell 47, 707–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann C, Wong RO, 2005. Regulation of dendritic growth and plasticity by local and global calcium dynamics. Cell Calcium 37, 403–409. [DOI] [PubMed] [Google Scholar]
- Loo LS, Tang N, Al-Haddawi M, Dawe GS, Hong W, 2014. A role for sorting nexin 27 in AMPA receptor trafficking. Nat Commun 5, 3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luarte A, Cornejo VH, Bertin F, Gallardo J, Couve A, 2018. The axonal endoplasmic reticulum: One organelle-many functions in development, maintenance, and plasticity. Dev Neurobiol 78, 181–208. [DOI] [PubMed] [Google Scholar]
- Lunn ML, Nassirpour R, Arrabit C, Tan J, McLeod I, Arias CM, Sawchenko PE, Yates JR 3rd, Slesinger PA, 2007. A unique sorting nexin regulates trafficking of potassium channels via a PDZ domain interaction. Nat Neurosci 10, 1249–1259. [DOI] [PubMed] [Google Scholar]
- Lv K, Chen L, Li Y, Li Z, Zheng P, Liu Y, Chen J, Teng J, 2015. Trip6 promotes dendritic morphogenesis through dephosphorylated GRIP1-dependent myosin VI and F-actin organization. J Neurosci 35, 2559–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Savas JN, Yu MS, Culver BP, Chao MV, Tanese N, 2011. Huntingtin mediates dendritic transport of beta-actin mRNA in rat neurons. Sci Rep 1, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas C, Belgardt D, Lee HK, Heisler FF, Lappe-Siefke C, Magiera MM, van Dijk J, Hausrat TJ, Janke C, Kneussel M, 2009. Synaptic activation modifies microtubules underlying transport of postsynaptic cargo. Proc Natl Acad Sci U S A 106, 8731–8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas C, Tagnaouti N, Loebrich S, Behrend B, Lappe-Siefke C, Kneussel M, 2006. Neuronal cotransport of glycine receptor and the scaffold protein gephyrin. J Cell Biol 172, 441451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeder CI, Shen K, Hoogenraad CC, 2014. Axon and dendritic trafficking. Curr Opin Neurobiol 27, 165–170. [DOI] [PubMed] [Google Scholar]
- Magiera MM, Singh P, Gadadhar S, Janke C, 2018. Tubulin Posttranslational Modifications and Emerging Links to Human Disease. Cell 173, 1323–1327. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K, 1999. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 283, 1923–1927. [DOI] [PubMed] [Google Scholar]
- Margeta MA, Wang GJ, Shen K, 2009. Clathrin adaptor AP-1 complex excludes multiple postsynaptic receptors from axons in C. elegans. Proc Natl Acad Sci U S A 106, 1632–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek JR, Weiner JA, Farlow SJ, Chun J, Goldstein LS, 1999. Novel dendritic kinesin sorting identified by different process targeting of two related kinesins: KIF21A and KIF21B. J Cell Biol 145, 469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Cerdeno V, 2017. Dendrite and spine modifications in autism and related neurodevelopmental disorders in patients and animal models. Dev Neurobiol 77, 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Miura E, Matsuda K, Kakegawa W, Kohda K, Watanabe M, Yuzaki M, 2008. Accumulation of AMPA receptors in autophagosomes in neuronal axons lacking adaptor protein AP-4. Neuron 57, 730–745. [DOI] [PubMed] [Google Scholar]
- McAllister AK, 2000. Cellular and molecular mechanisms of dendrite growth. Cereb Cortex 10, 963–973. [DOI] [PubMed] [Google Scholar]
- McGuire JR, Rong J, Li SH, Li XJ, 2006. Interaction of Huntingtin-associated protein-1 with kinesin light chain: implications in intracellular trafficking in neurons. J Biol Chem 281, 3552–3559. [DOI] [PubMed] [Google Scholar]
- McVicker DP, Awe AM, Richters KE, Wilson RL, Cowdrey DA, Hu X, Chapman ER, Dent EW, 2016. Transport of a kinesin-cargo pair along microtubules into dendritic spines undergoing synaptic plasticity. Nat Commun 7, 12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Nelson WJ, 2008. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol 9, 833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merianda TT, Lin AC, Lam JS, Vuppalanchi D, Willis DE, Karin N, Holt CE, Twiss JL, 2009. A functional equivalent of endoplasmic reticulum and Golgi in axons for secretion of locally synthesized proteins. Mol Cell Neurosci 40, 128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam EB, Lumbard DC, Viesselmann C, Ballweg J, Stevenson M, Pietila L, Hu X, Dent EW, 2011. Dynamic microtubules promote synaptic NMDA receptor-dependent spine enlargement. PLoS One 6, e27688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam EB, Millette M, Lumbard DC, Saengsawang W, Fothergill T, Hu X, Ferhat L, Dent EW, 2013. Synaptic regulation of microtubule dynamics in dendritic spines by calcium, F-actin, and drebrin. J Neurosci 33, 16471–16482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhaylova M, Bera S, Kobler O, Frischknecht R, Kreutz MR, 2016. A Dendritic Golgi Satellite between ERGIC and Retromer. Cell Rep 14, 189–199. [DOI] [PubMed] [Google Scholar]
- Miller KE, Suter DM, 2018. An Integrated Cytoskeletal Model of Neurite Outgrowth. Front Cell Neurosci 12, 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok H, Shin H, Kim S, Lee JR, Yoon J, Kim E, 2002. Association of the kinesin superfamily motor protein KIF1Balpha with postsynaptic density-95 (PSD-95), synapseassociated protein-97, and synaptic scaffolding molecule PSD-95/discs large/zona occludens-1 proteins. J Neurosci 22, 5253–5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy BY, Sawyer DL, Ackermann BE, Borden MM, Tan TC, Ori-McKenney KM, 2018. Competition between microtubule-associated proteins directs motor transport. Nat Commun 9, 1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy BY, Tan TC, Oclaman JM, Han JS, Simo S, Niwa S, Nowakowski DW, McKenney RJ, Ori-McKenney KM, 2020. A Combinatorial MAP Code Dictates Polarized Microtubule Transport. Dev Cell 53, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K, Su T, ter Beest M, 2003. Polarized epithelial membrane traffic: conservation and plasticity. Nat Cell Biol 5, 287–293. [DOI] [PubMed] [Google Scholar]
- Moughamian AJ, Holzbaur EL, 2012. Dynactin is required for transport initiation from the distal axon. Neuron 74, 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhia M, Thies E, Labonte D, Ghiretti AE, Gromova KV, Xompero F, Lappe-Siefke C, Hermans-Borgmeyer I, Kuhl D, Schweizer M, Ohana O, Schwarz JR, Holzbaur ELF, Kneussel M, 2016. The Kinesin KIF21B Regulates Microtubule Dynamics and Is Essential for Neuronal Morphology, Synapse Function, and Learning and Memory. Cell Rep 15, 968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai M, Ikegami K, Sugiura Y, Takeshita K, Nakagawa A, Setou M, 2009. Recombinant mammalian tubulin polyglutamylase TTLL7 performs both initiation and elongation of polyglutamylation on beta-tubulin through a random sequential pathway. Biochemistry 48, 1084–1093. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Setou M, Seog D, Ogasawara K, Dohmae N, Takio K, Hirokawa N, 2000. A novel motor, KIF13A, transports mannose-6-phosphate receptor to plasma membrane through direct interaction with AP-1 complex. Cell 103, 569–581. [DOI] [PubMed] [Google Scholar]
- Nakahata Y, Yasuda R, 2018. Plasticity of Spine Structure: Local Signaling, Translation and Cytoskeletal Reorganization. Front Synaptic Neurosci 10, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Yin X, Takei Y, Seog DH, Homma N, Hirokawa N, 2012. Molecular motor KIF5A is essential for GABA(A) receptor transport, and KIF5A deletion causes epilepsy. Neuron 76, 945–961. [DOI] [PubMed] [Google Scholar]
- Nakata T, Niwa S, Okada Y, Perez F, Hirokawa N, 2011. Preferential binding of a kinesin-1 motor to GTP-tubulin-rich microtubules underlies polarized vesicle transport. J Cell Biol 194, 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu F, Ohno H, 2003. Adaptor protein complexes as the key regulators of protein sorting in the post-Golgi network. Cell Struct Funct 28, 419–429. [DOI] [PubMed] [Google Scholar]
- Nakatsu F, Okada M, Mori F, Kumazawa N, Iwasa H, Zhu G, Kasagi Y, Kamiya H, Harada A, Nishimura K, Takeuchi A, Miyazaki T, Watanabe M, Yuasa S, Manabe T, Wakabayashi K, Kaneko S, Saito T, Ohno H, 2004. Defective function of GABAcontaining synaptic vesicles in mice lacking the AP-3B clathrin adaptor. J Cell Biol 167, 293302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakos K, Radler MR, Spiliotis ET, 2019a. Septin 2/6/7 complexes tune microtubule plus-end growth and EB1 binding in a concentration- and filament-dependent manner. Mol Biol Cell 30, 2913–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakos K, Rosenberg M, Spiliotis ET, 2019b. Regulation of microtubule plus end dynamics by septin 9. Cytoskeleton (Hoboken) 76, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirschl JJ, Magiera MM, Lazarus JE, Janke C, Holzbaur EL, 2016. alpha-Tubulin Tyrosination and CLIP-170 Phosphorylation Regulate the Initiation of Dynein-Driven Transport in Neurons. Cell Rep 14, 2637–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa S, Lipton DM, Morikawa M, Zhao C, Hirokawa N, Lu H, Shen K, 2016. Autoinhibition of a Neuronal Kinesin UNC-104/KIF1A Regulates the Size and Density of Synapses. Cell Rep 16, 2129–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa S, Tanaka Y, Hirokawa N, 2008. KIF1Bbeta- and KIF1A-mediated axonal transport of presynaptic regulator Rab3 occurs in a GTP-dependent manner through DENN/MADD. Nat Cell Biol 10, 1269–1279. [DOI] [PubMed] [Google Scholar]
- Nusser Z, 2012. Differential subcellular distribution of ion channels and the diversity of neuronal function. Curr Opin Neurobiol 22, 366–371. [DOI] [PubMed] [Google Scholar]
- Oe S, Miki H, Nishimura W, Noda Y, 2016. Mechanism of the Dendritic Translation and Localization of Brain-derived Neurotrophic Factor. Cell Struct Funct 41, 23–31. [DOI] [PubMed] [Google Scholar]
- Olenick MA, Holzbaur ELF, 2019. Dynein activators and adaptors at a glance. J Cell Sci 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omotade OF, Pollitt SL, Zheng JQ, 2017. Actin-based growth cone motility and guidance. Mol Cell Neurosci 84, 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori-McKenney KM, Jan LY, Jan YN, 2012. Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron 76, 921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterweil E, Wells DG, Mooseker MS, 2005. A role for myosin VI in postsynaptic structure and glutamate receptor endocytosis. J Cell Biol 168, 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padamsey Z, McGuinness L, Bardo SJ, Reinhart M, Tong R, Hedegaard A, Hart ML, Emptage NJ, 2017. Activity-Dependent Exocytosis of Lysosomes Regulates the Structural Plasticity of Dendritic Spines. Neuron 93, 132–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Cao Y, Stucchi R, Hooikaas PJ, Portegies S, Will L, Martin M, Akhmanova A, Harterink M, Hoogenraad CC, 2019. MAP7D2 Localizes to the Proximal Axon and Locally Promotes Kinesin-1-Mediated Cargo Transport into the Axon. Cell Rep 26, 1988–1999 e1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Roll-Mecak A, 2018. The tubulin code in neuronal polarity. Curr Opin Neurobiol 51, 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD, 2006. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron 52, 817–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penazzi L, Bakota L, Brandt R, 2016. Microtubule Dynamics in Neuronal Development, Plasticity, and Neurodegeneration. Int Rev Cell Mol Biol 321, 89–169. [DOI] [PubMed] [Google Scholar]
- Perez Bay AE, Schreiner R, Mazzoni F, Carvajal-Gonzalez JM, Gravotta D, Perret E, Lehmann Mantaras G, Zhu YS, Rodriguez-Boulan EJ, 2013. The kinesin KIF16B mediates apical transcytosis of transferrin receptor in AP-1B-deficient epithelia. EMBO J 32, 2125–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen JD, Kaech S, Banker G, 2014. Selective microtubule-based transport of dendritic membrane proteins arises in concert with axon specification. J Neurosci 34, 4135–4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipathsouk A, Brunetti RM, Town JP, Breuer A, Pellett PA, Marchuk K, Ngoc-Han TT, Krummel MF, Stamou DS, Weiner OD, 2019. WAVE complex self-organization templates lamellipodial formation. bioRxiv 836585; doi: 10.1101/836585. [DOI] [Google Scholar]
- Pollard TD, Blanchoin L, Mullins RD, 2000. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu Rev Biophys Biomol Struct 29, 545–576. [DOI] [PubMed] [Google Scholar]
- Puram SV, Bonni A, 2013. Cell-intrinsic drivers of dendrite morphogenesis. Development 140, 4657–4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrpassopoulos S, Shuman H, Ostap EM, 2017. Adhesion force and attachment lifetime of the KIF16B-PX domain interaction with lipid membranes. Mol Biol Cell 28, 3315–3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez OA, Couve A, 2011. The endoplasmic reticulum and protein trafficking in dendrites and axons. Trends Cell Biol 21, 219–227. [DOI] [PubMed] [Google Scholar]
- Rathgeber L, Gromova KV, Schaefer I, Breiden P, Lohr C, Kneussel M, 2015. GSK3 and KIF5 regulate activity-dependent sorting of gephyrin between axons and dendrites. Eur J Cell Biol 94, 173–178. [DOI] [PubMed] [Google Scholar]
- Reck-Peterson SL, Redwine WB, Vale RD, Carter AP, 2018. The cytoplasmic dynein transport machinery and its many cargoes. Nat Rev Mol Cell Biol 19, 382–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ, 2006. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol 16, 2166–2172. [DOI] [PubMed] [Google Scholar]
- Ribeiro LF, Verpoort B, Nys J, Vennekens KM, Wierda KD, de Wit J, 2019. SorCS1mediated sorting in dendrites maintains neurexin axonal surface polarization required for synaptic function. PLoS Biol 17, e3000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort NL, Konnerth A, 2012. Dendritic spines: from structure to in vivo function. EMBO Rep 13, 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov DG, Honing S, Silye A, Kongsvik TL, von Figura K, Bakke O, 2002. Structural requirements for interactions between leucine-sorting signals and clathrin-associated adaptor protein complex AP3. J Biol Chem 277, 47436–47443. [DOI] [PubMed] [Google Scholar]
- Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, WatermanStorer CM, 2003. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol 5, 599–609. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Powell SK, 1992. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol 8, 395–427. [DOI] [PubMed] [Google Scholar]
- Rolls MM, 2011. Neuronal polarity in Drosophila: sorting out axons and dendrites. Dev Neurobiol 71, 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthel G, Banker G, 1999. Role of moving growth cone-like “wave” structures in the outgrowth of cultured hippocampal axons and dendrites. J Neurobiol 39, 97–106. [DOI] [PubMed] [Google Scholar]
- Sadoul R, Laporte MH, Chassefeyre R, Chi KI, Goldberg Y, Chatellard C, Hemming FJ, Fraboulet S, 2018. The role of ESCRT during development and functioning of the nervous system. Semin Cell Dev Biol 74, 40–49.28811263 [Google Scholar]
- Saito N, Okada Y, Noda Y, Kinoshita Y, Kondo S, Hirokawa N, 1997. KIFC2 is a novel neuron-specific C-terminal type kinesin superfamily motor for dendritic transport of multivesicular body-like organelles. Neuron 18, 425–438. [DOI] [PubMed] [Google Scholar]
- Sakakibara A, Hatanaka Y, 2015. Neuronal polarization in the developing cerebral cortex. Front Neurosci 9, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar G, Craige B, Love R, Kalman D, Faundez V, 2005. Vglut1 and ZnT3 co-targeting mechanisms regulate vesicular zinc stores in PC12 cells. J Cell Sci 118, 1911–1921. [DOI] [PubMed] [Google Scholar]
- Sampo B, Kaech S, Kunz S, Banker G, 2003. Two distinct mechanisms target membrane proteins to the axonal surface. Neuron 37, 611–624. [DOI] [PubMed] [Google Scholar]
- Sanger A, Hirst J, Davies AK, Robinson MS, 2019. Adaptor protein complexes and disease at a glance. J Cell Sci 132. [DOI] [PubMed] [Google Scholar]
- Satoh D, Sato D, Tsuyama T, Saito M, Ohkura H, Rolls MM, Ishikawa F, Uemura T, 2008. Spatial control of branching within dendritic arbors by dynein-dependent transport of Rab5-endosomes. Nat Cell Biol 10, 1164–1171. [DOI] [PubMed] [Google Scholar]
- Schaefer AW, Kamei Y, Kamiguchi H, Wong EV, Rapoport I, Kirchhausen T, Beach CM, Landreth G, Lemmon SK, Lemmon V, 2002. L1 endocytosis is controlled by a phosphorylation-dephosphorylation cycle stimulated by outside-in signaling by L1. J Cell Biol 157, 1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzle P, Esteves da Silva M, Tas RP, Katrukha EA, Hu HY, Wierenga CJ, Kapitein LC, Hoogenraad CC, 2018. Activity-Dependent Actin Remodeling at the Base of Dendritic Spines Promotes Microtubule Entry. Curr Biol 28, 2081–2093.e2086. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, McKerracher L, Obar R, Vallee RB, 1989. MAP 1A and MAP 1B are structurally related microtubule associated proteins with distinct developmental patterns in the CNS. J Neurosci 9, 1712–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setou M, Nakagawa T, Seog DH, Hirokawa N, 2000. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science 288, 1796–1802. [DOI] [PubMed] [Google Scholar]
- Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, Hirokawa N, 2002. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature 417, 83–87. [DOI] [PubMed] [Google Scholar]
- Sharma R, Gulia R, Bhattacharyya S, 2018. A Critical Role for Sorting Nexin 1 in the Trafficking of Metabotropic Glutamate Receptors. J Neurosci 38, 8605–8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Hines T, Bergson C, Smith D, 2018. Coupling of microtubule motors with AP-3 generated organelles in axons by NEEP21 family member calcyon. Mol Biol Cell 29, 2055–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Muthusamy N, Smith D, Bergson C, 2017. Dynein binds and stimulates axonal motility of the endosome adaptor and NEEP21 family member, calcyon. Int J Biochem Cell Biol 90, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin E, Kashiwagi Y, Kuriu T, Iwasaki H, Tanaka T, Koizumi H, Gleeson JG, Okabe S, 2013. Doublecortin-like kinase enhances dendritic remodelling and negatively regulates synapse maturation. Nat Commun 4, 1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Wyszynski M, Huh KH, Valtschanoff JG, Lee JR, Ko J, Streuli M, Weinberg RJ, Sheng M, Kim E, 2003. Association of the kinesin motor KIF1A with the multimodular protein liprin-alpha. J Biol Chem 278, 11393–11401. [DOI] [PubMed] [Google Scholar]
- Shiomura Y, Hirokawa N, 1987. Colocalization of microtubule-associated protein 1A and microtubule-associated protein 2 on neuronal microtubules in situ revealed with double-label immunoelectron microscopy. J Cell Biol 104, 1575–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidenstein SC, D’Este E, Bohm MJ, Danzl JG, Belov VN, Hell SW, 2016. Multicolour Multilevel STED nanoscopy of Actin/Spectrin Organization at Synapses. Sci Rep 6, 26725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MA, Kaech S, Jareb M, Burack MA, Vogt L, Sonderegger P, Banker G, 2001. Sorting and directed transport of membrane proteins during development of hippocampal neurons in culture. Proc Natl Acad Sci U S A 98, 7051–7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Wandinger-Ness A, 1990. Polarized sorting in epithelia. Cell 62, 207–210. [DOI] [PubMed] [Google Scholar]
- Song AH, Wang D, Chen G, Li Y, Luo J, Duan S, Poo MM, 2009. A selective filter for cytoplasmic transport at the axon initial segment. Cell 136, 1148–1160. [DOI] [PubMed] [Google Scholar]
- Soppina V, Norris SR, Dizaji AS, Kortus M, Veatch S, Peckham M, Verhey KJ, 2014. Dimerization of mammalian kinesin-3 motors results in superprocessive motion. Proc Natl Acad Sci U S A 111, 5562–5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spacek J, 1985. Three-dimensional analysis of dendritic spines. II. Spine apparatus and other cytoplasmic components. Anat Embryol (Berl) 171, 235–243. [DOI] [PubMed] [Google Scholar]
- Spacek J, Harris KM, 1997. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J Neurosci 17, 190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliotis ET, 2018. Spatial effects - site-specific regulation of actin and microtubule organization by septin GTPases. J Cell Sci 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliotis ET, Hunt SJ, Hu Q, Kinoshita M, Nelson WJ, 2008. Epithelial polarity requires septin coupling of vesicle transport to polyglutamylated microtubules. J Cell Biol 180, 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N, 2008. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci 9, 206–221. [DOI] [PubMed] [Google Scholar]
- Steiner P, Alberi S, Kulangara K, Yersin A, Sarria JC, Regulier E, Kasas S, Dietler G, Muller D, Catsicas S, Hirling H, 2005. Interactions between NEEP21, GRIP1 and GluR2 regulate sorting and recycling of the glutamate receptor subunit GluR2. EMBO J 24, 2873–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner P, Sarria JC, Glauser L, Magnin S, Catsicas S, Hirling H, 2002. Modulation of receptor cycling by neuron-enriched endosomal protein of 21 kD. J Cell Biol 157, 1197–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanek L, Pigino G, 2016. Microtubule doublets are double-track railways for intraflagellar transport trains. Science 352, 721–724. [DOI] [PubMed] [Google Scholar]
- Sundararajan L, Stern J, Miller DM 3rd, 2019. Mechanisms that regulate morphogenesis of a highly branched neuron in C. elegans. Dev Biol 451, 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina T, 2018. The Actin Cytoskeleton and Actin-Based Motility. Cold Spring Harb Perspect Biol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyk A, Deaconescu AM, Spector J, Goodman B, Valenstein ML, Ziolkowska NE, Kormendi V, Grigorieff N, Roll-Mecak A, 2014. Molecular basis for age-dependent microtubule acetylation by tubulin acetyltransferase. Cell 157, 1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T, Simonetta A, Batterton M, Kinoshita M, Edbauer D, Sheng M, 2007. Role of Septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr Biol 17, 1752–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahirovic S, Bradke F, 2009. Neuronal polarity. Cold Spring Harb Perspect Biol 1, a001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano K, Miki T, Katahira J, Yoneda Y, 2007. NXF2 is involved in cytoplasmic mRNA dynamics through interactions with motor proteins. Nucleic Acids Res 35, 2513–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tas RP, Chazeau A, Cloin BMC, Lambers MLA, Hoogenraad CC, Kapitein LC, 2017. Differentiation between Oppositely Oriented Microtubules Controls Polarized Neuronal Transport. Neuron 96, 1264–1271 e1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M, 2018. Axonal endoplasmic reticulum is very narrow. J Cell Sci 131. [DOI] [PubMed] [Google Scholar]
- Twelvetrees AE, Yuen EY, Arancibia-Carcamo IL, MacAskill AF, Rostaing P, Lumb MJ, Humbert S, Triller A, Saudou F, Yan Z, Kittler JT, 2010. Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin. Neuron 65, 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymanskyj SR, Yang BH, Verhey KJ, Ma L, 2018. MAP7 regulates axon morphogenesis by recruiting kinesin-1 to microtubules and modulating organelle transport. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsain N, Stefani FD, Caceres A, 2018. The Actin/Spectrin Membrane-Associated Periodic Skeleton in Neurons. Front Synaptic Neurosci 10, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaegter CB, Jansen P, Fjorback AW, Glerup S, Skeldal S, Kjolby M, Richner M, Erdmann B, Nyengaard JR, Tessarollo L, Lewin GR, Willnow TE, Chao MV, Nykjaer A, 2011. Sortilin associates with Trk receptors to enhance anterograde transport and neurotrophin signaling. Nat Neurosci 14, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beuningen SFB, Will L, Harterink M, Chazeau A, van Battum EY, Frias CP, Franker MAM, Katrukha EA, Stucchi R, Vocking K, Antunes AT, Slenders L, Doulkeridou S, Sillevis Smitt P, Altelaar AFM, Post JA, Akhmanova A, Pasterkamp RJ, Kapitein LC, de Graaff E, Hoogenraad CC, 2015. TRIM46 Controls Neuronal Polarity and Axon Specification by Driving the Formation of Parallel Microtubule Arrays. Neuron 88, 1208–1226. [DOI] [PubMed] [Google Scholar]
- van Bommel B, Konietzny A, Kobler O, Bär J, Mikhaylova M, 2019. F-actin patches associated with glutamatergic synapses control positioning of dendritic lysosomes. The EMBO Journal 38, e101183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluijs P, Hoogenraad CC, 2011. New insights in endosomal dynamics and AMPA receptor trafficking. Semin Cell Dev Biol 22, 499–505. [DOI] [PubMed] [Google Scholar]
- van Dijk J, Rogowski K, Miro J, Lacroix B, Edde B, Janke C, 2007. A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol Cell 26, 437–448. [DOI] [PubMed] [Google Scholar]
- van Riel WE, Rai A, Bianchi S, Katrukha EA, Liu Q, Heck AJ, Hoogenraad CC, Steinmetz MO, Kapitein LC, Akhmanova A, 2017. Kinesin-4 KIF21B is a potent microtubule pausing factor. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Spronsen M, Mikhaylova M, Lipka J, Schlager MA, van den Heuvel DJ, Kuijpers M, Wulf PS, Keijzer N, Demmers J, Kapitein LC, Jaarsma D, Gerritsen HC, Akhmanova A, Hoogenraad CC, 2013. TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron 77, 485–502. [DOI] [PubMed] [Google Scholar]
- Venkatesh K, Mathew A, Koushika SP, 2020. Role of actin in organelle trafficking in neurons. Cytoskeleton (Hoboken) 77, 97–109. [DOI] [PubMed] [Google Scholar]
- Verhey KJ, Gaertig J, 2007. The tubulin code. Cell Cycle 6, 2152–2160. [DOI] [PubMed] [Google Scholar]
- Verhey KJ, Hammond JW, 2009. Traffic control: regulation of kinesin motors. Nat Rev Mol Cell Biol 10, 765–777. [DOI] [PubMed] [Google Scholar]
- Vietri M, Radulovic M, Stenmark H, 2019. The many functions of ESCRTs. Nat Rev Mol Cell Biol 21, 25–42. [DOI] [PubMed] [Google Scholar]
- Wagner W, Brenowitz SD, Hammer JA 3rd, 2011. Myosin-Va transports the endoplasmic reticulum into the dendritic spines of Purkinje neurons. Nat Cell Biol 13, 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yu W, Baas PW, Black MM, 1996. Microtubule assembly in growing dendrites. J Neurosci 16, 6065–6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhao Z, Rodal AA, 2019. Higher-order assembly of Sorting Nexin 16 controls tubulation and distribution of neuronal endosomes. J Cell Biol 218, 2600–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Edwards JG, Riley N, Provance DW Jr., Karcher R, Li XD, Davison IG, Ikebe M, Mercer JA, Kauer JA, Ehlers MD, 2008. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell 135, 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Al-Bassam S, Miyazaki Y, Wandless TJ, Webster P, Arnold DB, 2012. Networks of polarized actin filaments in the axon initial segment provide a mechanism for sorting axonal and dendritic proteins. Cell Rep 2, 1546–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AL, Lin AW, Chen LQ, Safer D, Cain SM, Hasson T, Carragher BO, Milligan RA, Sweeney HL, 1999. Myosin VI is an actin-based motor that moves backwards. Nature 401, 505–508. [DOI] [PubMed] [Google Scholar]
- West AE, Neve RL, Buckley KM, 1997. Identification of a somatodendritic targeting signal in the cytoplasmic domain of the transferrin receptor. J Neurosci 17, 6038–6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans AM, Collins SR, Meyer T, 2016. Waves of actin and microtubule polymerization drive microtubule-based transport and neurite growth before single axon formation. Elife 5, e12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winckler B, Faundez V, Maday S, Cai Q, Guimas Almeida C, Zhang H, 2018. The Endolysosomal System and Proteostasis: From Development to Degeneration. J Neurosci 38, 9364–9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisco D, Anderson ED, Chang MC, Norden C, Boiko T, Folsch H, Winckler B, 2003. Uncovering multiple axonal targeting pathways in hippocampal neurons. J Cell Biol 162, 13171328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley MT, Besharse JC, 2012. The kinesin superfamily protein KIF17: one protein with many functions. Biomol Concepts 3, 267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Nash JE, Zamorano P, Garner CC, 2002. Interaction of SAP97 with minus-enddirected actin motor myosin VI. Implications for AMPA receptor trafficking. J Biol Chem 277, 30928–30934. [DOI] [PubMed] [Google Scholar]
- Wu Y, Whiteus C, Xu CS, Hayworth KJ, Weinberg RJ, Hess HF, De Camilli P, 2017. Contacts between the endoplasmic reticulum and other membranes in neurons. Proc Natl Acad Sci U S A 114, E4859–e4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YK, Fujishima K, Kengaku M, 2015. Differentiation of apical and basal dendrites in pyramidal cells and granule cells in dissociated hippocampal cultures. PLoS One 10, e0118482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Vessey JP, Konecna A, Dahm R, Macchi P, Kiebler MA, 2007. The GTPbinding protein Septin 7 is critical for dendrite branching and dendritic-spine morphology. Curr Biol 17, 1746–1751. [DOI] [PubMed] [Google Scholar]
- Yadav S, Oses-Prieto JA, Peters CJ, Zhou J, Pleasure SJ, Burlingame AL, Jan LY, Jan YN, 2017. TAOK2 Kinase Mediates PSD95 Stability and Dendritic Spine Maturation through Septin7 Phosphorylation. Neuron 93, 379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita N, Joshi R, Zhang S, Zhang ZY, Kuruvilla R, 2017. Phospho-Regulation of Soma-to-Axon Transcytosis of Neurotrophin Receptors. Dev Cell 42, 626–639 e625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Guo L, Wang Y, 2006. Requirement of dendritic Akt degradation by the ubiquitin-proteasome system for neuronal polarity. J Cell Biol 174, 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Sun W, Kujala P, Lotfi Y, Vida TA, Bean AJ, 2005. CART: an Hrs/actinin4/BERP/myosin V protein complex required for efficient receptor recycling. Mol Biol Cell 16, 2470–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Bostick Z, Garbouchian A, Luisi J, Banker G, Bentley M, 2019. A novel strategy to visualize vesicle-bound kinesins reveals the diversity of kinesin-mediated transport. Traffic 20, 851–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H, Ninan I, Zhang H, Milner TA, Arancio O, Chao MV, 2006. BDNF-mediated neurotransmission relies upon a myosin VI motor complex. Nat Neurosci 9, 1009–1018. [DOI] [PubMed] [Google Scholar]
- Yap CC, Digilio L, McMahon LP, Garcia ADR, Winckler B, 2018. Degradation of dendritic cargos requires Rab7-dependent transport to somatic lysosomes. J Cell Biol 217, 31413159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap CC, Murate M, Kishigami S, Muto Y, Kishida H, Hashikawa T, Yano R, 2003. Adaptor protein complex-4 (AP-4) is expressed in the central nervous system neurons and interacts with glutamate receptor delta2. Mol Cell Neurosci 24, 283–295. [DOI] [PubMed] [Google Scholar]
- Yap CC, Nokes RL, Wisco D, Anderson E, Folsch H, Winckler B, 2008a. Pathway selection to the axon depends on multiple targeting signals in NgCAM. J Cell Sci 121, 15141525. [DOI] [PubMed] [Google Scholar]
- Yap CC, Wisco D, Kujala P, Lasiecka ZM, Cannon JT, Chang MC, Hirling H, Klumperman J, Winckler B, 2008b. The somatodendritic endosomal regulator NEEP21 facilitates axonal targeting of L1/NgCAM. J Cell Biol 180, 827–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau KW, Schatzle P, Tortosa E, Pages S, Holtmaat A, Kapitein LC, Hoogenraad CC, 2016. Dendrites In Vitro and In Vivo Contain Microtubules of Opposite Polarity and Axon Formation Correlates with Uniform Plus-End-Out Microtubule Orientation. J Neurosci 36, 10711085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan YN, 2007. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell 130, 717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C, Le Gall AH, Baldwin AN, Monlauzeur L, Le Bivic A, Rodriguez-Boulan E, 1997. The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK cells. J Cell Biol 139, 929–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev S, Shen K, 2017. Establishing Neuronal Polarity with Environmental and Intrinsic Mechanisms. Neuron 96, 638–650. [DOI] [PubMed] [Google Scholar]
- Yoshimura A, Fujii R, Watanabe Y, Okabe S, Fukui K, Takumi T, 2006. Myosin-Va facilitates the accumulation of mRNA/protein complex in dendritic spines. Curr Biol 16, 23452351. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Wildonger J, Ye B, Zhang Y, Kita A, Younger SH, Zimmerman S, Jan LY, Jan YN, 2008. Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat Cell Biol 10, 1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, He J, Zhou R, Lorenzo D, Babcock HP, Bennett V, Zhuang X, 2014. Developmental mechanism of the periodic membrane skeleton in axons. Elife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Niwa S, Guillaud L, Tong Y, Hirokawa N, 2013. A molecular motor, KIF13A, controls anxiety by transporting the serotonin type 1A receptor. Cell Rep 3, 509–519. [DOI] [PubMed] [Google Scholar]
- Zhou X, Zeng J, Ouyang C, Luo Q, Yu M, Yang Z, Wang H, Shen K, Shi A, 2016. A novel bipartite UNC-101/AP-1 μ1 binding signal mediates KVS-4/Kv2.1 somatodendritic distribution in Caenorhabditis elegans. FEBS Letters 590, 76–92. [DOI] [PubMed] [Google Scholar]
- Zimmermann D, Abdel Motaal B, Voith von Voithenberg L, Schliwa M, Okten Z, 2011. Diffusion of myosin V on microtubules: a fine-tuned interaction for which E-hooks are dispensable. PLoS One 6, e25473. [DOI] [PMC free article] [PubMed] [Google Scholar]