Abstract
The fall armyworm, Spodoptera frugiperda, is a species native to the Americas and has spread to many countries in Africa and Asia in recent years. Proactive actions for potential invasion of S. frugiperda to China coordinated by government agencies and agricultural extension systems resulted in timely detection in January 2019 in Yunnan province neighboring onto Myanmar. The extensive monitoring in southern provinces of China since February 2019 resulted in dynamic tracking of S. frugiperda spreading to 13 provincial regions in China within 4 months by May 10, 2019, which is crucial for timely management actions in the fields. The first detections of S. frugiperda (corn strain) in China were confirmed using cytochrome oxidase subunit 1 (CO1) and triosephosphate isomerase (Tpi) genes molecular marker method. In addition to S. frugiperda, larvae of three other noctuid species with similar morphological appearance (S. litura, S. exigua and Mythimna separata) can occur simultaneously and cause similar damage in cornfields in southern China. Thus, we can use both morphological and molecular marker methods to compare larval stages of four noctuid species. Further, we discuss the risk of potential spread of invasive S. frugiperda to other regions and impact on corn production in China.
Keywords: corn, molecular identification, Mythimna separata, Spodoptera exigua, Spodoptera frugiperda, Spodoptera litura
Introduction
Fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae), is one of the most devastating pests of corn in the Americas. S. frugiperda is a polyphagous pest with a broad range of hosts, over 80 plant species, and it is highly migratory (Pogue, 2002; CABI, 2016). It is known that S. frugiperda feeds on corn leaves, tassel and ears during the larval stage, which causes serious damage to corn (Sena et al., 2003). In Brazil, S. frugiperda attacks on corn reduced the yield up to 34%, causing annual losses of 400 million US dollars (Sena et al., 2003; Figueiredo et al., 2005; Lima et al., 2010). It is native to tropical and sub‐tropical areas in America but has spread rapidly across almost all sub‐Saharan countries after it was first detected in western and central Africa in early 2016, and 2018 in Uganda (Goergen et al., 2016; Otim et al., 2018). Then it was confirmed in several Asian countries such as India (Mallapur et al., 2018), Sri Lanka, Thailand, Yemen and Myanmar (CABI, 2019) as well as Bangladesh (Farmer, 2019) in 2018.
When S. frugiperda invasion was confirmed in India in July 2018, we submitted a report “Rapid spread of crop‐devastating fall armyworm in Africa and invasion in India” to the government agencies of Ministry of Agriculture and Rural Affairs (MARA), China in August 2018, and then published a review paper “Potential invasion of the crop‐devastating insect pest fall armyworm Spodoptera frugiperda in China” in Plant Protection in November 2018 (Guo et al., 2018). When S. frugiperda was confirmed in Myanmar in December 2018, the notification was issued by MARA for field monitoring in Yunnan and Guangxi provinces neighboring Myanmar for timely detection of S. frugiperda. The first suspected detection of S. frugiperda in China was confirmed based on morphological methods in January 2019 [(China National Agro‐Tech Extension and Service Center (NATESC), 2019a; International Plant Protection Convention (IPPC, 2019)] in Yunnan province adjacent to Myanmar. It was also confirmed by methods using molecular makers (Zhang et al., 2019).
The females lay eggs on abaxial leaf surfaces of host leaves and an individual adult can lay 100 to 200 eggs per egg mass (Kumela et al., 2018). Immediately after hatching, neonates start feeding on the leaf which leads to complete skeletonization (Brévault et al., 2018). Duration of larval stage (usually six instars) is about 14 days during the summer and may prolong to 30 days during cold weather and the last instar moves onto the ground and pupates (Sparks, 1979). The adult of S. frugiperda has strong flight ability and has spread rapidly in Africa, India and other Asian countries (Early et al., 2018).
To control S. frugiperda, an effective identification method is needed because similar species like S. litura (Fabricius), S. exigua (Hübner) and Mythimna separata (Walker) also occur simultaneously in cornfields. In our previous report, the morphological differences among the four noctuid species were described (Guo et al., 2019). However, the morphological characteristics of these pests and the damage symptoms they cause on corn leaves are very similar, and sometimes this may lead to misidentification with other noctuid species, especially for the 1st and 2nd instars. Also, egg masses of the three Spodoptera species are very difficult to distinguish based on morphological characteristics. In addition, S. frugiperda consists of “rice” and “corn” subpopulations and differs in host plant distribution without difference in morphological features (Pashley, 1986; Pashley et al., 1987; Nagoshi et al., 2017). Molecular identification methods could be more accurate than the morphological identification. With the rapid advancement in molecular biology techniques, various biological macromolecules containing evolutionary information have been used as molecular markers, which are widely used in species identification and phylogenetic analysis (Tautz et al., 2002). Mitochondrial DNA (mtDNA) has many advantages for intraspecific identifications due to maternal inheritance, high copy number, and both conservative and high‐mutation rate DNA segments (Mauro et al., 2006). Currently, the cytochrome oxidase subunit 1 (CO1) gene has been used as a standard universal sequence used to elucidate the species uncertainty in morphological taxonomy (Paul et al., 2003; Murray & Prowell, 2005; Solovyeva et al., 2014) and this technology has been adapted to distinguish S. frugiperda (Nagoshi, 2007a; Nagoshi, 2007b; Kergoat et al., 2012; Cock et al., 2017). In addition, polymorphisms in triosephosphate isomerase (Tpi) gene (Knowles, 1991) helps to identify specific host strains and the efficiency of this gene during species identification has been explained in many reports (Nagoshi, 2010, 2012; Nagoshi et al., 2017).
In this study, we reported the initial detections and sequential spread of S. frugiperda in 13 provincial regions (Yunnan, Guangxi, Guangdong, Guizhou, Hunan, Hainan, Fujian, Zhejiang, Hubei, Sichuan, Jiangxi, Chongqing, and Henan) of China within 4 months by May 10, 2019 and confirmation of the species using molecular markers based on CO1 and Tpi genes. We also compared molecular differences among four similar noctuid larvae of S. frugiperda, S. exigua, S. litura and M. separata from cornfields.
Materials and methods
Initial detections of S. frugiperda in China
On January 3, 2019, NATESC (2019b) of MARA published a notification to provincial Plant Protection Stations nationwide in China for proactive actions for potential invasion of S. frugiperda into China, with special requirement to set up key monitoring sites for all year‐round monitoring in the four southern provinces (i.e., Yunnan, Guangxi, Guangdong, Hainan) and other regions with high invasion risks. The major monitoring methods were: (1) on‐farm monitoring by national agricultural extension agencies, National Crop Pest Monitoring and Forecasting Regional Stations (>1000 in total), with special attention in winter cornfields in Yunnan and Guangxi starting January 2019, in the southern provinces starting February 2019, and nationwide starting April 2019; (2) adult trapping using black light traps and vertical‐pointing searchlight traps in the southern provinces starting February 2019 and 220 counties of 26 provinces since April 2019; (3) sex pheromone trapping using equipment confirmed by NATESC on key host crops in the four southern provinces since February 2019. NATESC published a standardized method “Methods of Investigation and Forecast for Fall Armyworm in 2019 (Preliminary)” in March 2019 for data collection in China (NATESC, 2019c).
Sample collection and molecular identification and analysis of CO1 and Tpi genes
In January, 2019 the first suspected S. frugiperda infestation was detected in a cornfield in Jiangcheng county (of Pu'er City), Yunnan province; we surveyed S. frugiperda on winter corn in Pu'er and Dehong (Dehong Dai and Jingpo Autonomous prefecture) of Yunnan province during January 12–24 and confirmed the S. frugiperda invasion by morphological identification. The S. litura, M. separata and S. exigua larvae, as well as suspected S. frugiperda larvae were collected from a winter cornfield in Dehong, Yunnan province (24.42°N, 98.60°E) on January 20, 2019. Totally, 250–350 larvae were collected from Yunnan province. The collected larvae were transported to the laboratory and reared in a controlled environment chamber at 27±1 °C, with 60%–70% relative humidity (RH), a 14:10 L:D photoperiod.
The CO1 primer of S. frugiperda, M. separata (Mehrdad et al., 2006) and S. exigua (Hebert et al., 2003) were designed based on previous reports. Total DNA was extracted from whole body of 4th instars using a DNA kit (Tianmoboi, China) and stored at −20 °C for further analysis. Twenty individuals from each species were selected and genomic DNA was extracted from every individual larva (n = 20). Polymerase chain reaction (PCR) amplifications were carried out in a volume of 20 µL reaction containing: 10 µL of Super HF PCR Master Mix (HeroGen Biotechnology, USA), 2 µL primers (Forward and Reverse), 1 µL DNA template and 7 µL sterile H2O. S. litura primers were designed according to the sequence from the National Center for Biotechnology Information (NCBI) (GenBank No. KF022223.1). On other hand, based on single‐nucleotide polymorphisms at e4183 site the Tpi gene was selected for analysis and this site explains strain differentiation. In females, the Tpi e4183 site possesses either a C or T nucleotide, which can differentiate the two S. frugiperda strains. The corn strain possesses C nucleotide (Tpi e4183‐C) and rice strain with T (Tpi e4183‐R). These Tpi genes are likely to be Z‐linked in Lepidoptera (Yasukochi et al., 2006). However, there are more possibilities of overlap between Tpi genes in male genomic DNA during PCR product sequencing, which possess both C and T nucleotides at e4183 site (Nagoshi, 2010; Nagoshi et al., 2017). To overcome this problem, primer design and methods were followed according to Nagoshi et al. (Fig. 1) (Nagoshi, 2010; Nagoshi et al., 2017). Twenty larvae were selected randomly, and DNA was isolated. All specific primer details are mentioned in Table 1. Amplifications were performed in Techne‐5000 (UK) and programmed according to the following conditions: initial denaturation at 94 °C for 5 min; 30 amplification cycles consisting of 1 min at 94 °C, 30 s at 50 °C, 45 s of extension at 72 °C, and final extension at 72 °C for 10 min.
Figure 1.
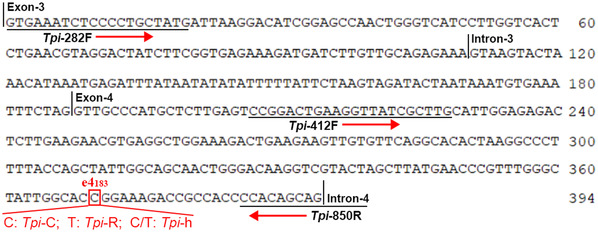
Triosephosphate isomerase (Tpi) gene mapping at e4183 polymorphic site for a C or T nucleotide. Polymerase chain reaction amplification was done using primers Tpi‐282F and Tpi‐850R. DNA sequencing was performed using primer Tpi‐412F, which initiates in the same exon as e4183. [Correction added on 12 September 2019, after first online publication: Figure 1 has been corrected to include a missing ‘C’ in the sequence.]
Table 1.
Selected species‐specific cytochrome oxidase subunit 1 and triosephosphate isomerase (Tpi) primers
| Species | Primer | Sequence (5′–3′) |
|---|---|---|
| Spodoptera frugiperda/Mythimna separata | Forward | ATTCAACCAATCATAAAGATATTGG |
| Reverse | TAAACTTCTGGATGTCCAAAAAATCA | |
| S. exigua | Forward | GGTCAACAAATCATAAAGATATTGG |
| Reverse | TAAACTTCAGGGTGACCAAAAAATCA | |
| S. litura | Forward | ACAAATCATAAAGATATTGGAACATTA |
| Reverse | TAAACTTCGGGATGTCCAAAAAATCA | |
| Tpi‐282F | – | GGTGAAATCTCCCCTGCTATG |
| Tpi‐850R | – | AATTTTATTACCTGCTGTGG |
| Tpi‐412F | – | CCGGACTGAAGGTTATCGCTTG |
Sequencing and species identification
The PCR products from four noctuid species were outsourced to Shenggong Biotec (Shanghai, China) for sequencing. The assembled sequences from four samples were subjected to molecular identification using the online NCBI Basic Local Alignment Search tool (BLAST) (https://www.ncbi.nlm.nih.gov/). The assembled nucleotide using the CO1 method was submitted to NCBI (GenBank No. MK790611). A phylogenetic tree was constructed using the maximum likelihood method (MEGA 7.0 software, USA) with 1000 replications.
Results
The initial detection of S. frugiperda in China
Larvae detections
The first suspected S. frugiperda infestation was detected in a cornfield in Jiangcheng county, Yunnan province on January 11, 2019. Additional field surveys on wide range areas in Yunnan province confirmed the presence of S. frugiperda larvae in cornfields in three cities or autonomous prefectures (Pu'er, Dehong and Baoshan) of Yunnan province by the end of January 2019. There was an average of 0.05–0.20 larvae per corn plant and maximum two larvae per plant. There were six cities or autonomous prefectures in Yunnan with S. frugiperda larvae in cornfields by the end of February 2019 (Fig. 2). By April 2019, there were 63 counties from 12 cities or autonomous prefectures in Yunnan with the infestation of larvae detected on 5200 ha of corn fields and 3700 ha in sugarcane fields, with highest larvae infestation of 100% corn plants and up to 1.5–2.0 larvae per plant in selected fields in Longchuan county.
Figure 2.
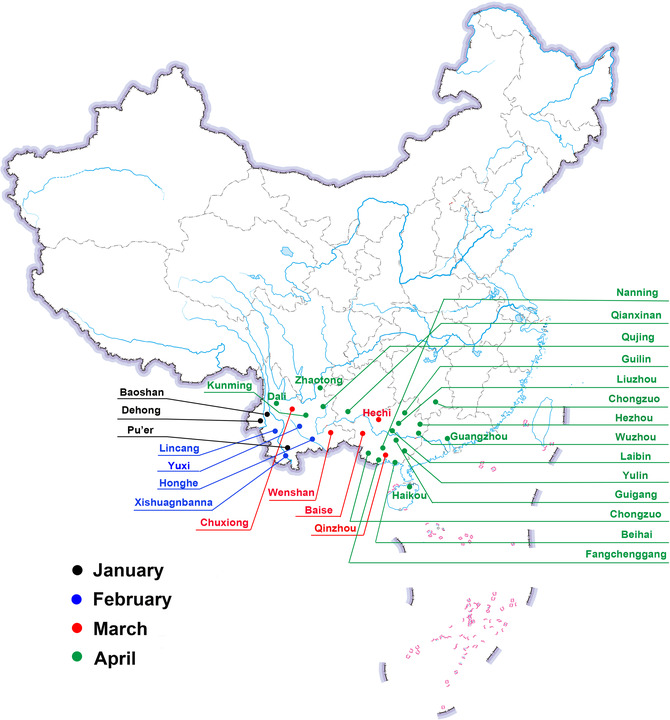
Map showing the location of Spodoptera frugiperda in China by April 30, 2019. The dots in black, blue, red and green represent areas where S. frugiperda were found in respective months of 2019. GS(2019)3426.
By the end of April 2019, there were six provincial regions in China with S. frugiperda larvae detected (Fig. 2); Guangxi Zhuang Autonomous Region was the second provincial area with S. frugiperda larvae detected in two cities (Chongzuo and Baise) by April 2019. The highest larvae infestation was 50% of corn plants in selected cornfields. Guangdong was the third province with S. frugiperda larvae detected in Zengcheng and Conghua districts of Guangzhou City. In late April 2019 the S. frugiperda larvae were detected in the fourth and fifth provincial regions of Guizhou (Qianxinan Prefecture) and Hunan province (Chenzhou City). On April 30, 2019 the S. frugiperda larvae were detected in Haikou of Hainan as the sixth province, which is the major region of winter research nurseries for corn breeding in China. By May 10, 2019, it was also found in seven additional provincial regions (Fujian, Zhejiang, Hubei, Sichuan, Jiangxi, Chongqing and Henan) resulting in 13 provincial regions in total within four months (Table 2).
Table 2.
The severity of Spodoptera frugiperda infestations in 13 provinces in China by May 10, 2019 (NATESC, 2019e, f)
| Provincial region | Month of first detection | Insect stage | No. of cities | No. of counties | Crop type | Infestation and crop acreage |
|---|---|---|---|---|---|---|
| Yunnan | January | Larvae | 15 | 112 | Corn | 20 000 ha, up to 3 larvae per plant and normally 1%–10% plants infested. |
| April | Larvae | 3 | 3 | Sugarcane | 3680 ha, up to 40% plants infested in Longchuan county. | |
| Guangxi | March | Adults | 3 | 6 | – | – |
| April | Larvae | 14 | 65 | Corn/sugarcane | Corn: 46 600 ha, up to 6 larvae per plant and normally 5%–9% plants infested. | |
| Guangdong | April | Larvae | 7 | 16 | Corn | 187 ha, up to 0.2 larvae per plant and 10%–20% plants infested. |
| Guizhou | April | Larvae | 6 | 21 | Corn | 1200 ha, up to 0.4 larvae per plant and normally 9%–11% plants infested. |
| Hunan | April | Larvae | 6 | 12 | Corn | 1300 ha, up to 1.2 larvae per plant and normally 5%–41% plants infested. |
| Hainan | April | Larvae | – | 18 | Corn | 30 ha, up to 0.7 larvae per plant and 80% plants infested in Wanning county. |
| Fujian | May | Larvae | 6 | 9 | Corn | 6.6 ha, up to 1.2 larvae per plant and 20%–40% plants infested. |
| Zhejiang | May | Larvae | 2 | 2 | Corn | 5.3 ha, up to 0.02–0.1 larvae per plant and 1%–10% plants infested. |
| Hubei | May | Larvae | 2 | 1 | Corn | 333 ha, up to 0.2–0.6 larvae per plant and 9.5%–18% plants infested in Tongshan county. |
| Sichuan | May | Larvae | 1 | 1 | Corn | <1 ha, up to 0.1 larvae per plant and 1.3% plants infested in Xichang City. |
| Jiangxi | May | Larvae | 1 | 1 | Corn | 33 ha, up to 0.3 larvae per plant and 3%–30% plants infested in Ganzhou City. |
| Chongqing | May | Larvae | 1 | 1 | Corn | 2 ha, up to 0.6 larvae per plant and up to 50% plants infested in Beibei District. |
| Henan | May | Larvae | 1 | 1 | Corn | <1 ha in Xinyang City. |
Adult detections
In Yunnan from February 19 to April 1, 2019 in Ruili of Dehong, the total number of moths per sex pheromone traps and sticky‐card traps with food bait were 32 and 159, respectively. There were fewer moths, only 20, detected using vertical‐pointing searchlight traps from January to March from Jiangcheng of Pu'er. In Guangxi, the first S. frugiperda moth was detected in Hechi of Yizhou on March 11, 2019 (Table 2). Subsequently, more moths were trapped in Hechi (Du'an and Donglan counties), Baise (Youjiang and Tianlin counties) and Qinzhou (Pubei county) before larvae were detected in these regions.
Molecular identification and phylogenetic analysis
The sequences obtained from our samples by CO1 marker were subjected to NCBI BLAST, the results showed positive hits and the species were identified as S. frugiperda, S. litura, S. exigua and M. separata. Especially, S. frugiperda from Yunnan of China belonged to a haplotype that showed 100% match with that of India (GenBank No. MH753324‐27 and MH190445). In addition, other lepidopterans showed sequence similarity above 90% when comparing with S. frugiperda (Fig. 3). Based on polymorphisms at Tpi e4183 site, 15 larval samples out of 20 belonged to the corn strain (Tpi e4183‐ C) and five samples showed heterozygous polymorphisms (Tpi‐h e4183 C and T).
Figure 3.
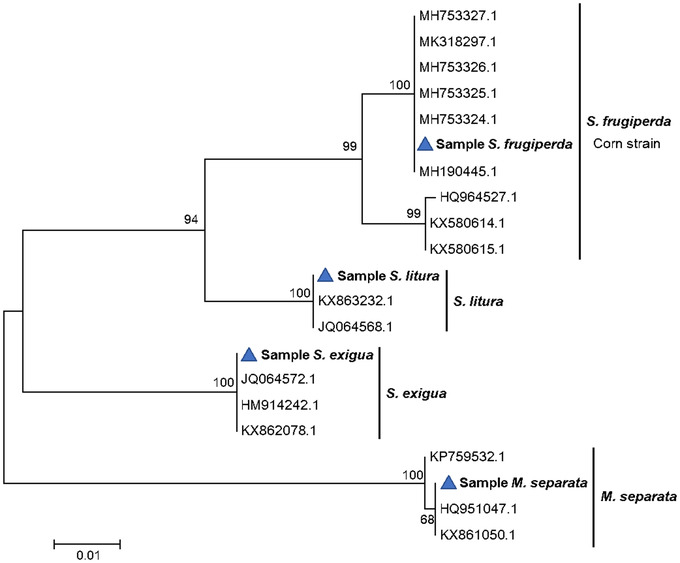
The phylogenetic tree was constructed using cytochrome oxidase subunit 1 (CO1) sequences from four Lepidoptera noctuid species. Maximum likelihood method was adopted based on the Tamura‐Nei model (Tamura & Nei, 1993). The nucleotide sequences were aligned using the Clustal W program and the evolutionary analyses were conducted in MEGA 7.0 (Kumar et al., 2016). The triangle indicates the samples collected from China used for this study.
Discussion
The exotic S. frugiperda has been detected in some countries in Africa and Asia in recent years. The spread or invasion of S. frugiperda is not only related to global trade and transport, but also strong long‐distance migration ability of the S. frugiperda moths. Proactive actions for potential invasion of S. frugiperda to China coordinated by government agencies and agricultural extension systems resulted in rapid detection of the first larvae on January 11, 2019 in a cornfield in Yunnan province near Myanmar. Scientists, especially those from the Chinese Academy of Agricultural Sciences (CAAS), provided timely and important support on the invasive pest alert, migration forecasting, monitoring and identification methods, and pest management techniques (Guo et al., 2018; Guo et al., 2019; Wu et al., 2019; Zhang et al., 2019). The extensive monitoring in the southern provinces resulted in dynamic tracking of the S. frugiperda spreading in China, which is crucial for coordinating management of this pest in new locations. It was detected in >260 counties of 13 provinces within 4 months by May 10, 2019, suggesting it is spreading rapidly.
Wu et al. (2019) simulated the migration patterns from Myanmar to spread to Yunnan and Guangxi. Their flight toward other provinces in southern China (Guizhou, Guangdong, Hainan and Hunan Provinces) was also predicted. If prevention and control is not timely, the pest will be able to spread toward the main corn producing areas of China and even reach the Korean peninsula and Japan in June or July of 2019. Currently, S. frugiperda management mainly relies on the use of chemical insecticides and Bacillus thuringiensis (Bt) corn (Cook et al., 2004). However, with the repeated use of pesticides and highly adaptable capabilities of this species, S. frugiperda can develop resistance to those chemicals (Yu, 1991, Gutiérrez‐Moreno et al., 2019) and to the plants expressing a Cry1A or Cry1F Bt protein (Farias et al., 2014; Huang et al., 2014; Omoto et al., 2016). It is therefore essential to use an integrated pest management (IPM) approach to manage S. frugiperda and other Lepidoptera pests in corn. In March 2019, NATESC (2019d) published the IPM Guide on Fall Armyworm in China (2019 Preliminary Version), which will be updated based on current studies. Based on the experiences in North and South America (Huang et al., 2014; Burtet et al., 2017) and South Africa (Botha et al., 2019), Bt corn plants can be highly effective on S. frugiperda when utilized as part of an IPM program after registration for cultivation in China in the future. The use of pyramided traits expressing effective Bt proteins on S. frugiperda with different sites of action (Burtet et al., 2017; Marques et al., 2019) should be prioritized and then deployed with appropriate refuge, resistance monitoring, and grower education. In southern China, three similar noctuid larvae of native lepidopteran species (S. litura, S. exigua and M. separata) are often found in the same field and each species can cause considerable damage on important agricultural crops, such as corn, rice, wheat, cotton and vegetables (Jiang et al., 2011). However, on‐farm identification of larvae of these pests using morphological characteristics is challenging, and often results in misidentifications, especially for early instars. Moreover, the traditional morphological classification requires a high level of knowledge and experience by researchers or extension specialists. Therefore, a more accurate and timely identification method is needed to differentiate these species and that can be achieved through molecular characterization. In this research, four noctuid larvae of lepidopteran pests from cornfields with three of them belonging to the same genus were collected and identified by both morphology and molecular methods. From the morphological identification, we can separate S. frugiperda from the other three species (S. litura, S. exigua and M. separata). Molecular methods were used to further confirm the morphological identification results (Fig. 3). Fast and accurate molecular methods are very important for timely identifying and distinguishing the larvae with similar morphology in cornfields and designing the control measures on newly invaded species. Meanwhile, the molecular methods may help greatly to understand its distant migratory and invasion pattern around the globe.
Three native noctuid species (S. litura, S. exigua and M. separata) are important pests infesting corn in China. The invasive S. frugiperda has the potential to become a hugely important pest of corn, if not the most important pest of corn at least in certain regions of China. It is particularly problematic for corn plants because female moths oviposit on corn leaves and larvae have strong serrated mandibles that allow them to feed on most corn tissues (Brown & Dewhurst, 1975; Pogue, 2002). The cannibalistic nature of S. frugiperda larvae also is an important factor because it allows them to dominate interspecific competitors and reduce intraspecific rivals (Chapman et al., 2000). In addition, larvae usually bore into corn whorls or ears and develop under protected conditions, which allows them to avoid direct exposure to insecticides. This greatly increases the chance of survival for S. frugiperda during insecticidal sprays and low‐dose exposure to insecticides probably has contributed to their resistance to several classes of insecticides (Adamczyk et al., 1999; Gutiérrez‐Moreno et al., 2019).
Two S. frugiperda strains were identified, one preferentially infests corn and the other preferentially infests rice (Pashley et al., 1985). The rice strain also prefers to infest millet and grass and the corn strain prefers corn and sorghum (Nagoshi & Meagher, 2004a,b). Both strains can infest corn and are morphologically indistinguishable (Pashley, 1986). According to strain identification results, only corn strain moths were discovered in Yunnan province, China. The invasion path of S. frugiperda is likely India, Myanmar then Yunnan province, China, after which they migrated to the eastern and northern regions of China. This possibility is due to its long‐distance flight ability and adaptability to environments. Supporting our hypothesis, migration of S. frugiperda inside the American states and its adaptability to different climatic conditions are well documented (Raulston & Sparks, 1986). Sequencing results of CO1 are consistent with the sequence of S. frugiperda found in India, and this result strongly supports our hypothesis on the invasion path to China. China as the second largest corn growing region in the world has not yet approved commercial planting of Bt corns. As a new invasive species, it will take time to sort out the spread potential, migratory dynamics, and ultimately the impact this moth will have on crops in China. As a new invasion species, S. frugiperda will enhance its reproductive capacity and spreading performance as part of its life history strategy (Johnson, 1987). Hence, if S. frugiperda is not well controlled, it particularly may have a serious impact on corn production in China. In addition, there are other major pests of corn in China, including Ostrinia furnacalis (He et al., 2003), Conogethes punctiferalis (Lu et al., 2010) and M. separata. A sustainable and effective IPM strategy to mitigate the impact of these insect pests of corn, especially S. frugiperda, is urgently needed.
Disclosure
The authors declare they have no conflict interests.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2018YFD020062) and the Modern Agricultural Industry Technology System (CARS‐02).
The copyright for this article was changed on May 18 after original online publication.
References
- Adamczyk, J.J. , Leonard, B.R. and Graves J.B. (1999) Toxicity of selected insecticides to fall armyworms (Lepidoptera: Noctuidae) in laboratory bioassay studies. Florida Entomologist, 82, 230–236. [Google Scholar]
- Botha, A.S. , Erasmus, A. , du Plessis, H. and Van den Berg, J. (2019) Efficacy of Bt maize for control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in South Africa. Journal of Economic Entomology, 10.1093/jee/toz048. [DOI] [PubMed] [Google Scholar]
- Brévault, T. , Ndiaye, A. , Badiane, D. , Bal, A.M. , Sembène, M. , Silvie, P . et al (2018) First records of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), in Senegal. Entomologia Generalis, 37, 129–142. [Google Scholar]
- Brown, E.S. and Dewhurst C.F. (1975) The genus Spodoptera (Lepidoptera, Noctuidae) in Africa and the Near East. Bulletin of Entomological Research, 65, 221–262. [Google Scholar]
- Burtet, L.M. , Bernardi, O. , Melo, A.A. , Pes, M.P , Strahl, T.T. and Guedes, J.V. (2017) Managing fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), with Bt maize and insecticides in southern Brazil. Pest Management Science, 73, 2569–2577. [DOI] [PubMed] [Google Scholar]
- CABI (2016) Datasheet Spodoptera frugiperda (fall armyworm). Invasive Species Compendium, http://www.cabi.org/isc/datasheet/29810 (accessed April 26, 2019). [Google Scholar]
- CABI (2019) Datasheet Spodoptera frugiperda (fall armyworm). Invasive Species Compendium, https://www.cabi.org/isc/datasheet/29810#94987198-9f50-4173-8bbd-30bd93840e73?tdsourcetag=s_pcqq_aiomsg (accessed April 26, 2019). [Google Scholar]
- Chapman, J.W. , Williams, T. , Martínez, A.M. , Cisneros, J. , Caballero, P. , Cave, R.D. et al (2000) Does cannibalism in Spodoptera frugiperda (Lepidoptera: Noctuidae) reduce the risk of predation? Behavioral Ecology and Sociobiology, 48, 321–327. [Google Scholar]
- Cock, M.J.W. , Beseh, P.K. , Buddie, A.G. , Cafáet, G. and Crozier, J. (2017) Molecular methods to detect Spodoptera frugiperda in Ghana, and implications for monitoring the spread of invasive species in developing countries. Scientific Reports, 10.1038/s41598-017-04238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, D.R. , Leonard, B.R. and Gore, J. (2004) Field and laboratory performance of novel insecticides against armyworms (Lepidoptera: Noctuidae). Florida Entomologist, 87, 433–439. [Google Scholar]
- Early, R. , Pablo, G.M. , Murphy, S.T. and Day, R. (2018) Forecasting the global extent of invasion of the cereal pest Spodoptera frugiperda, the fall armyworm. NeoBiota, 40, 25–50. [Google Scholar]
- Farias, J.R. , Andow, D.A. , Horikoshi, R.J. , Sorgatto, R.J. , Fresia, P. , Santos, C.D . et al (2014) Field‐evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Protection, 64, 150–158. [Google Scholar]
- Farmer, B. (2019) Fall armyworm marches on as pest that devastated African crops spreads in Asia. The Telegraph, https://www.telegraph.co.uk/news/2019/01/09/fall-armyworm-marches-pest-devastated-african-crops-spreads/ (accessed April 26, 2019). [Google Scholar]
- Figueiredo, M.L.C. , Penteado‐Dias, A.M. and Cruz, I. (2005) Danos provocados por Spodoptera frugiperda na produção de matéria seca e nos rendimentos de grãos, na cultura do milho. Sete Lagoas, MG: Embrapa, CNPMS, 130 https://ainfo.cnptia.embrapa.br/digital/bitstream/CNPMS/18881/1/Com_130.pdf (accessed May 30, 2019). [Google Scholar]
- Goergen, G. , Kumar, P.L. , Sankung, S.B. , Togola, A. and Tamò, M. (2016) First report of outbreaks of the fall armyworm Spodoptera frugiperda (J.E. Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in west and central Africa. PLoS ONE, 11, e0165632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, J.F. , Jing, D.P. , Tai, H.K. , Zhang, A.H. , He, K.L. and Wang, Z.Y. (2019) Morphological characteristics of Spodoptera frugiperda in comparison with three other lepidopteran species with similar injury characteristics and morphology in cornfields. Plant Protection, 45, 7–12. [Google Scholar]
- Guo, J.F. , Zhao, J.Z. , He, K.L. , Zhang, F. and Wang, Z.Y. (2018) Potential invasion of the crop‐devastating insect pest fall armyworm Spodoptera frugiperda to China. Plant Protection, 44, 1–10. [Google Scholar]
- Gutiérrez‐Moreno, R.G. , Mota‐Sanchez, D. , Blanco, C.A. , Whalon, M.E. , Terán‐Santofimio, H. , Rodriguez‐Maciel, J.C . et al (2019) Field‐evolved resistance of the fall armyworm (Lepidoptera: Noctuidae) to synthetic insecticides in Puerto Rico and Mexico. Journal of Economic Entomology, 112, 792–802. [DOI] [PubMed] [Google Scholar]
- He, K.L. , Wang, Z.Y. , Zhou, D.R. , Wen, L.P. , Song, Y.Y. and Yao, Z.Y. (2003) Evaluation of transgenic Bt corn for resistance to the Asian corn borer (Lepidoptera: Pyralidae). Journal of Economic Entomology, 96, 935–940. [DOI] [PubMed] [Google Scholar]
- Hebert, P.D.N , Cywinska, A. , Ball S.L. and deWaard, J.R. (2003) Biological identifications through DNA barcodes. Proceedings of the Royal Society of London. Series B: Biological Sciences, 270, 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, F.N. , Qureshi, J.A. , Meagher, R.L. , Reisig, D.D. , Head, G.P. , Andow, D.A . et al (2014) Cry1F resistance in fall armyworm Spodoptera frugiperda: single gene versus pyramided Bt maize. PLoS ONE, 9, e112958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPPC (2019) First detection of fall armyworm in China. https://www.ippc.int/en/news/first-detection-of-fall-armyworm-in-china/ (accessed March 28, 2019).
- Jiang, X. , Luo, L. and Zhang, L. (2011) Regulation of migration in Mythimna separata (Walker) in China: a review integrating environmental, physiological, hormonal, genetic, and molecular factors. Environmental Entomology, 40, 516–533. [DOI] [PubMed] [Google Scholar]
- Johnson, S.J. (1987) Migration and the life history strategy of the fall armyworm, Spodoptera frugiperda in the western hemisphere. International Journal of Tropical Insect Science, 8, 543–549. [Google Scholar]
- Kergoat, G.J. , Prowell, D.P. , Le Ru, B.P. , Mitchell, A. , Dumas, P. , Clamens, A.L . et al (2012) Disentangling dispersal, vicariance and adaptive radiation patterns: a case study using armyworms in the pest genus Spodoptera (Lepidoptera: Noctuidae). Molecular Phylogenetics and Evolution, 65, 855–870. [DOI] [PubMed] [Google Scholar]
- Knowles, J.R. (1991) Enzyme catalysis: not different, just better. Nature, 350, 121–124. [DOI] [PubMed] [Google Scholar]
- Kumar, S. , Stecher, G. and Tamura, K. (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumela, T. , Simiyu, J. , Sisay, B. , Likhayo, P. , Mendesil, E. , Gohole, L . et al (2018) Farmers' knowledge, perceptions, and management practices of the new invasive pest, fall armyworm (Spodoptera frugiperda) in Ethiopia and Kenya. International Journal of Pest Management, 65, 1–9. [Google Scholar]
- Lima, M.S. , Silva, P.S.L. , Oliveira, O.F. , Silva, K.M.B. and Freitas, F.C.L. (2010) Corn yield response to weed and fall armyworm controls. Planta Daninha, 28, 103–111. [Google Scholar]
- Lu, J.Q , Wang, Z.Y. , He, K.L. and Liu, Y. (2010) Research history, progresses and prospects in the yellow peach moth, Conogethes punctiferalis . Plant Protection, 36, 31–38. [Google Scholar]
- Mallapur, C.P. , Naik, A.K. , Hagari, S. , Prabhu, S.T. and Patil, P.K. (2018) Status of alien pest fall armyworm, Spodoptera frugiperda (J.E. Smith) on maize in Northern Karnataka. Journal of Entomology and Zoology Studies, 6, 432–436. [Google Scholar]
- Marques, L. M. , Santos, A.C. , Castro, B.A. , Moscardini, V.F. , Rosseto, J. , Silva, O.A.B.N . et al (2019) Assessing the efficacy of Bacillus thuringiensis (Bt) pyramided proteins Cry1F, Cry1A.105, Cry2Ab2, and Vip3Aa20 expressed in Bt maize against Lepidopteran pests in Brazil. Journal of Economic Entomology, 112, 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro, D.S. , Gower, D.J. , Zardoya, R. and Wilkinson, M. (2006) A hotspot of gene order rearrangement by tandem duplication and random loss in the vertebrate mitochondrial genome. Molecular Biology and Evolution, 23, 227. [DOI] [PubMed] [Google Scholar]
- Mehrdad, H. , Janzen, D.H. , Burns, J.M. , Hallwachs, W. and Hebert, P.D.N. (2006) DNA barcodes distinguish species of tropical Lepidoptera. Proceedings of the National Academy of Sciences USA, 103, 968–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, D. and Prowell, D.P. (2005) Molecular phylogenetics and evolutionary history of the neotropical satyrine subtribe Euptychiina (Nymphalidae: Satyrinae). Molecular Phylogenetics and Evolution, 34, 67–80. [DOI] [PubMed] [Google Scholar]
- Nagoshi, R.N. (2010) The fall armyworm triose phosphate isomerase (Tpi) gene as a marker of strain identity and interstrain mating. Annals of the Entomological Society of America, 103, 283–292. [Google Scholar]
- Nagoshi, R.N. (2012) Improvements in the identification of strains facilitate population studies of fall armyworm subgroups. Annals of the Entomological Society of America, 105, 351–358. [Google Scholar]
- Nagoshi, R.N. and Meagher, R.L. (2004a) Seasonal distribution of fall armyworm (Lepidoptera: Noctuidae) host strains in agricultural and turf grass habitats. Environmental Entomology, 33, 881–889. [Google Scholar]
- Nagoshi, R.N. and Meagher, R.L. (2004b) Behavior and distribution of the two fall armyworm host strains in Florida. Florida Entomologist, 87, 440–449. [Google Scholar]
- Nagoshi, R.N. , Koffi, D. , Agboka, K. , Tounou, K.A. , Banerjee, R. , Jurat‐Fuentes, J.L. et al (2017) Comparative molecular analyses of invasive fall armyworm in Togo reveal strong similarities to populations from the eastern United States and the Greater Antilles. PLoS ONE, 12, e0181982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi, R.N. , Silvie, P. , Meagher, R.L. , Lopez, J. and Machado, V. (2007a) Identification and comparison of fall armyworm (Lepidoptera: Noctuidae) host strains in Brazil, Texas, and Florida. Annals of the Entomological Society of America, 100, 394–402. [Google Scholar]
- Nagoshi, R.N. , Silvie, P. and Meagher, R.L. (2007b) Comparison of haplotype frequencies differentiate fall armyworm (Lepidoptera: Noctuidae) corn‐strain populations from Florida and Brazil. Journal of Economic Entomology, 100, 954–961. [DOI] [PubMed] [Google Scholar]
- NATESC (2019a) Fall armyworn, the major pest has invaded in Yunnan, the field scouting and monitoring for this pest to start immediately within China. https://www.natesc.org.cn/Html/2019_01_29/28092_151760_2019_01_29_457209.html (accessed April 26, 2019).
- NATESC (2019b) Notification for prevention and precaution of invasion and damage of fall armyworm. https://www.natesc.org.cn/Html/2019_01_03/28092_52304_2019_01_03_456907.html (accessed April 26, 2019).
- NATESC (2019c) Methods of investigation and forecast for fall armyworm in 2019 (Preliminary). https://www.natesc.org.cn/Html/2019_03_01/28092_52304_2019_03_01_457510.html (accessed April 26, 2019)
- NATESC (2019d) Technical guideline for fall armyworm control in 2019 (Preliminary). https://www.natesc.org.cn/Html/2019_03_13/2_1878_2019_03_13_457664.html (accessed April 26, 2019).
- NATESC (2019e) Recent occurrence and infection of Spodoptera frugiperda . https://www.natesc.org.cn/Html/2019_05_06/28092_151760_2019_05_06_458341.html (accessed May 25, 2019).
- NATESC (2019f) Spodoptera frugiperda invaded 13 provinces and infested spring corn. https://www.natesc.org.cn/Html/2019_05_14/28092_151760_2019_05_14_458487.html?from=singlemessage&isappinstalled=0 (accessed May 25, 2019).
- Omoto, C. , Bernardi, O. , Salmeron, E. , Sorgatto, R.J. , Dourado, P.M. , Crivellari, A . et al (2016) Field‐evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest Management Science, 72, 1727–1736. [DOI] [PubMed] [Google Scholar]
- Otim, M.H. , Tay, W.T. , Walsh, T.K. , Kanyesigye, D. , Adumo, S. , Abongosi, J . et al (2018) Detection of sister‐species in invasive populations of the fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) from Uganda. PLoS ONE, 13, e0194571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashley, D.P. (1986) Host‐associated genetic differentiation in fall armyworm (Lepidoptera: Noctuidae): a sibling species complex? Annals of Entomological Society of America, 79, 898–904. [Google Scholar]
- Pashley, D.P. , Johnson, S.J. and Sparks, A.N. (1985) Genetic population structure of migratory moths: the fall armyworm (Lepidoptera: Noctuidae). Annals of the Entomological Society of America, 78, 756–762. [Google Scholar]
- Pashley, D.P. , Sparks, T.C. and Quisenberry, S.S. (1987) Two fall armyworm strains feed on corn, rice and bermudagrass. Louisiana Agriculture Louisiana Agricultural Experiment Station, 30, 8–9. [Google Scholar]
- Paul, D.N. , Ratnasingham, S. and Dewaard, J.R. (2003) Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proceedings Biological sciences, 270, S96–S99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue, G.M. (2002) A world revision of the genus Spodoptera Guenée (Lepidoptera: Noctuidae). Memoirs of the American Entomological Society, 43, 1–202. [Google Scholar]
- Raulston, J.R. and Sparks, A.N. (1986) Fall armyworm distribution and population dynamics in the southeastern states. Florida Entomologist, 69, 468–487. [Google Scholar]
- Sena Jr, D.G. , Pinto, F.A.C. , Queriroz, D.M. and Viana, P.A. (2003) Fall armyworm damaged maize plant identification using digital images. Biosystems Engineering, 85, 449–454. [Google Scholar]
- Solovyeva, E.N. , Poyarkov, N.A. , Dunayev, E.A. , Nazarow, R.A. , Lebedev, V.S. and Bannikova, A.A. (2014) Phylogenetic relationships and subgeneric taxonomy of toad‐headed agamas Phrynocephalus (Reptilia, Squamata, Agamidae) as determined by mitochondrial DNA sequencing. Doklady Biological Sciences Proceedings of the Academy of Sciences of the USSR Biological Sciences, 455, 119–124. [DOI] [PubMed] [Google Scholar]
- Sparks, A.N. (1979) A review of the biology of the fall armyworm. Florida Entomologist, 62, 82–87. [Google Scholar]
- Tamura, K. and Nei, M. (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution, 10, 512–526. [DOI] [PubMed] [Google Scholar]
- Tautz, D. , Arctander, P. , Minwlli, A. , Thomas, R.H. and Vogler, A.P. (2002) DNA points the way ahead in taxonomy. Nature, 418, 479–479. [DOI] [PubMed] [Google Scholar]
- Wu, Q.L. , Jiang, Y.Y. and Wu, K.M. (2019) Analysis of migration routes of the fall armyworm Spodoptera frugiperda (J.E. Smith) from Myanmar to China. Plant Protection, 45, 1–6. [Google Scholar]
- Yasukochi, Y. , Ashakumary, L.A. , Baba, K. , Yoshido, A. and Sahara, K. (2006) A second‐generation integrated map of the silkworm reveals synteny and conserved gene order between lepidopteran insects. Genetics, 173, 1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, S.J. (1991) Insecticide resistance in the fall armyworm, Spodoptera frugiperda (J.E. Smith). Pesticide Biochemistry and Physiology, 39, 84–91. [Google Scholar]
- Zhang, L. , Jin, M.H. , Zhang, D.D. , Jiang, Y.Y. , Liu, J. , Wu, K.M . et al (2019) Molecular identification of invasive fall armyworm Spodoptera frugiperda in Yunnan province. Plant Protection, 45, 19–24. [Google Scholar]


