Abstract
Allergen immunotherapy is a cornerstone in the treatment of allergic children. The clinical efficiency relies on a well‐defined immunologic mechanism promoting regulatory T cells and downplaying the immune response induced by allergens. Clinical indications have been well documented for respiratory allergy in the presence of rhinitis and/or allergic asthma, to pollens and dust mites. Patients who have had an anaphylactic reaction to hymenoptera venom are also good candidates for allergen immunotherapy. Administration of allergen is currently mostly either by subcutaneous injections or by sublingual administration. Both methods have been extensively studied and have pros and cons. Specifically in children, the choice of the method of administration according to the patient's profile is important. Although allergen immunotherapy is widely used, there is a need for improvement. More particularly, biomarkers for prediction of the success of the treatments are needed. The strength and efficiency of the immune response may also be boosted by the use of better adjuvants. Finally, novel formulations might be more efficient and might improve the patient's adherence to the treatment. This user's guide reviews current knowledge and aims to provide clinical guidance to healthcare professionals taking care of children undergoing allergen immunotherapy.
Keywords: Allergy, immune regulation, immunotherapy, tolerance
| Table of contents | |
|---|---|
| Introduction to the Allergen Immunotherapy User's Guide | 4 |
| 1. An Overview of Allergen‐Specific Immunotherapy Mechanisms, Applications and Biomarkers | 5 |
| 2. Differences in Mechanistic and Clinical Responses to Allergen Immunotherapy Between Adults and Children | 12 |
| 3. Biomarkers for Allergen Immunotherapy: Antibody Responses and Digital Health Systems | 18 |
| 4. Preventive Effects of Allergen Immunotherapy on Allergic Diseases | 24 |
| 5. What is the Evidence for AIT in the Treatment of Allergic Rhinitis in Children? | 29 |
| 6. What is the Evidence on AIT for the Prevention and Treatment of Asthma in Children? | 35 |
| 7. Prescribing Immunotherapy to Pollens in Children: Influence of Geographical and Sociological Diversity | 39 |
| 8. Prescribing Immunotherapy to House Dust Mites in Children | 46 |
| 9. Prescribing Immunotherapy to Furry Animals and Less Common Aeroallergen in Children | 50 |
| 10. What is the Evidence for Venom Immunotherapy in Children? | 57 |
| 11. Subcutaneous or Sublingual Allergen Immunotherapy for Allergic Rhinitis and Asthma in Children? | 61 |
| 12. Practical Aspects of Using Allergen Immunotherapy in Children | 69 |
| 13. Adjuvants in Allergen‐Specific Immunotherapy | 75 |
| I References | 81 |
Introduction to the Allergen Immunotherapy User's Guide
Immunotherapy is the only specific and disease‐modifying treatment for allergic conditions. It is the only therapy that has demonstrated the capacity not only to improve symptoms, reduce the need for medications, but also to induce specific tolerance beyond the duration of the treatment and to prevent the development of new allergic conditions. Allergen immunotherapy (AIT) in children can be indicated in rhinitis, asthma, food, and venom allergies. This user's guide provides a detailed up‐to‐date overview of AIT for inhalant allergens and insect venom in children, with a focus on the practical implications for research and clinical practice.
A review on the newest concepts on the mechanisms of action of AIT is provided by C. Akdis. As a key event, T regulatory cells that release IL‐10, TGF‐b, and other molecules are induced by AIT and contribute to a suppressive milieu. B‐cell responses induced by AIT, as well as other cells with suppressive functions, also play an important role. The result is the reduction of allergic inflammation and related symptoms.
The role of adjuvants to enhance AIT clinical efficacy is reviewed by M. Shamji and S. Durham with a focus on the desired properties, that is, a robust safety profile, strong immunogenicity and reduced allergenicity and unwanted reactions.
The preventive effects of AIT on allergic diseases are reviewed by S. Halken. Her review highlights the evidence on an asthma preventive effect in children with pollen‐induced allergic rhinitis treated with AIT, mainly for birch and grass. This protective effect lasts at least 2 years from AIT completion.
A molecular approach, traditionally called “component‐resolved diagnosis” (CRD), has become an important tool not only in the diagnosis of allergic diseases but also for the correct indication of AIT. P. Matricardi, a leading expert in this area, reviews the usefulness of CRD to improve decision making around AIT at an individual level. The implementation of digital health systems to improve diagnosis and management of allergic patients in relation to AIT is also covered.
The evidence on AIT for the treatment of allergic rhinitis (AR) and asthma in children is reviewed by G. Roberts and P. Rodríguez del Rio, respectively. For AR, there is good quality evidence for particular AIT products as an effective treatment for children and adolescents. However, evidence for some products is heterogeneous or lacking. When prescribing AIT in children, the evidence behind particular AIT products is of utmost importance, along with patients’ characteristics, family preference, and the clinician's own experience and resources. In asthma, the evidence supporting AIT in children is weaker than in adults although promising, especially regarding the ability of AIT to reduce asthma symptoms and medication use, both during and after AIT.
Evidence on AIT to the main inhalant allergens including pollens (D. Barber and M. Álvaro‐Lozano), house dust mites (C. Riggioni and M. Álvaro‐Lozano), animal dander (P. Comberiati and M. Vazquez‐Ortiz), and hymenoptera venoms (G. Sturm) has been reviewed. In the chapter on pollen AIT, the different European pollen scenarios based on the continent geographic diversity are presented. These heterogeneous and often complex pollinosis phenotypes require region‐specific approaches to diagnostic and intervention strategies. In Mediterranean dry areas, patients show the most complex profiles with multiple sensitizations and overlapping pollen seasons, which make both appropriate diagnosis and treatment challenging. In such regions, a molecular approach helps differentiate between clinically relevant genuine sensitizations from cross‐reactivity, and thus, it can be an invaluable tool to inform AIT prescription. House dust mite (HDM) allergens are the most relevant inducers of allergic diseases worldwide. Hence, AIT to HDM is one of the most useful tools for treating HDM‐induced respiratory disease when indicated. The clinical efficacy of HDM AIT in AR is well established regarding reduction in symptoms and medication use, especially in children experiencing moderate‐to‐severe AR despite appropriate pharmacotherapy. Regarding HDM‐induced asthma in children, the benefits of AIT are also well documented, particularly in children with persistent asthma, normal lung function, and concomitant AR. There is very limited high‐quality evidence to support the use of AIT to less common aero‐allergens, such as animal dander, molds, and cockroaches, especially in the pediatric population. Hymenoptera stings are the second leading cause of anaphylactic reactions in childhood. Venom AIT protects a high percentage of honeybee allergic patients and vespid‐allergic patients. Nevertheless, further studies investigating the clinical effectiveness and the optimal duration of VIT in children are needed.
From a day‐to‐day perspective, A. Muraro and S. Arasi provide an overview on the practical aspects related to the use of AIT in children. The importance of using only standardized products with documented evidence of clinical efficacy is highlighted.
Subcutaneous (SCIT) and sublingual (SLIT) AIT is reviewed by O. Cavkaytar and A. Eifan. Efficacy and safety of the two modalities are compared based on evidence from randomized head‐to‐head trials in children. Although there is good quality evidence on the efficacy and safety for both SLIT and SCIT in well‐selected children with allergic rhinitis and well‐controlled asthma to pollen and house dust mites, more research regarding specific outcomes and the head‐to‐head comparison is needed directly comparing the two routes in children. The difficulties of conducting double‐blind placebo‐controlled RCTs in children are also discussed.
In summary, this users’ guide provides an up‐to‐date overview on key aspects of AIT in children with implications for clinical practice. Clinical decision making should be informed by the newest evidence on the indications, products, and expected clinical outcomes of AIT. Precision medicine, including a molecular approach and e‐health technology, might improve health outcomes in children receiving AIT. Future research in children should include high‐quality RCT to help elucidate current knowledge and evidence gaps regarding AIT use in children.
Guest Editors:
Montserrat Alvaro‐Lozano; malvaro@sjdhospitalbarcelona.org
Marta Vazquez‐Ortiz; marta.vazquez.ortiz@gmail.com
1 An Overview of Allergen‐Specific Immunotherapy Mechanisms, Applications and Biomarkers
Corresponding author
Cezmi A. Akdis
Swiss Institute of Allergy and Asthma Research (SIAF), University of Zürich, Herman‐Burchard‐Strasse 9, CH‐7265 Davos Wolfgang, Switzerland.
Tel.+41‐81‐4100848; Fax: +41‐81 4100840
e‐mail: akdisac@siaf.uzh.ch
Abstract
Allergen‐specific immunotherapy (AIT) is an allergen tolerance‐inducing treatment for allergic diseases such as allergic rhinitis, asthma, and food and venom allergies. AIT aims to induce allergen‐specific regulatory T (Treg) cells and their suppressor cytokines such as IL‐10, TGF‐β, and surface molecules such as CTLA‐4 and PD1, all of which form a suppressive milieu. Modulation of T‐ and B‐cell responses, antibody isotypes and functional limitation of mast cells, eosinophils as well as basophils cumulatively end up with induction of a long‐term allergen‐specific immune tolerance. AIT limits allergic inflammation, and in turn the symptoms of allergy, decreases disease severity and medication requirements, and also prevents new sensitizations. Although having limitations, such as patient adherence, efficacy, and life‐threatening side effects, AIT is still the only treatment that offers the possibility of long‐term cure. Extensive efforts nourished by massive progression in the area of cellular and molecular allergology have led to development of novel administration routes of AIT and production of innovative biologic products. All of the mentioned efforts aim to improve AIT to overcome possible drawbacks in standardization, safety, efficacy, compliance, treatment duration, and also related high costs. Precision/personalized medicine, a hot topic of medicine, may also contribute to success of AIT by better definition of disease endotypes, particularly an AIT responsive endotype and by directing ideally selected patients to the best custom‐tailored therapy option.
Abbreviations
AIT – Allergen‐specific immunotherapy
AD – Atopic dermatitis
AR – Allergic rhinitis
Breg – B regulatory
CRD – Component‐resolved diagnosis
DC – Dendritic cells
EPIT – Epicutaneous immunotherapy
Ig – Immunoglobulin
IL – Interleukin
ILC – Innate lymphoid cells
ILIT – Intralymphatic immunotherapy
NAC – Nasal allergen challenge
NK – Natural killer
SCIT – Subcutaneous immunotherapy
SLIT – Sublingual immunotherapy
TGF – Transforming growth factor
Th – T helper
Treg – T regulatory
VIT – Venom immunotherapy
Introduction
The high prevalence and morbidity of atopic diseases such as allergic rhinitis (AR), asthma, atopic dermatitis (AD), and food and venom allergies have led to a demand for the development of disease‐modifying therapy strategies which target the underlying pathomechanisms. In allergen‐specific immunotherapy (AIT), induction of immune tolerance to allergens in question is the main issue, which is intended to be long‐lasting. Both conventional routes of AIT recognized as subcutaneous (SCIT) and sublingual (SLIT) in selected cases of AR, asthma, and venom allergies have been utilized successfully for many years. Besides, food allergies and their treatment with different modes of immunotherapies are on the agenda, especially for pediatric population. Decreases in quality of life of both patients and their parents, and difficulties in strict avoidance measures and elimination diets, together with accidental exposures and anxiety of forthcoming reactions tend to put AIT also into the prime scene as a requirement for food allergy management.1, 2, 3, 4, 5 Furthermore, several human studies evaluating the possible contribution of AIT in AD have been conducted. Studies in mouse models report induction of regulatory T (Treg) cells by AIT in AD models. However, there is a need for systematic data review to define AIT as a new indication for AD in future.6, 7
AIT decreases allergic inflammation and in turn the symptoms of allergy, disease severity, and medication requirements. AIT also has protective effects on new sensitizations, progression of AR into asthma, and also on asthma severity.8, 9, 10, 11, 12, 13 There is still room for improvement of AIT within the perspectives of safety, efficacy, and adherence in daily practice and by development and utilization of modified allergens and/or by application of AIT via novel routes. This review summarizes immune mechanisms underlying AIT and recent developments in the field.
Allergic inflammation and type 2 immunity
Allergy can be defined as immunoglobulin (Ig) E‐dependent hypersensitivity reaction to environmental antigens, under the influence of immune and tissue microenvironments, in genetically predisposed individuals.14, 15 This understanding should be revisited in the nomenclature task forces, because except for anaphylaxis alone with the involvement of cardiac, respiratory, and vascular systems without any visible inflammation, a type 2 tissue inflammation with T cells, B cells, basophils, and other cells in all of the allergic diseases appear with tissue involvement (Figure 1). Several food allergens, drugs, and insect venoms can induce clinical symptoms only with anaphylaxis without tissue inflammation in IgE‐sensitized individuals. In addition to allergens, epithelial integrity, barrier dysfunction, and diversity of microbiota play key roles in the shaping of immune response toward sensitization.16, 17 Allergens captured, internalized, and processed by dendritic cells (DCs) in skin or mucosal surfaces are consequently presented to naïve CD4+ T cells in the regional lymph nodes. Following presentation of the T‐cell epitope peptides of protein allergens together with costimulatory molecules, naïve T cells with certain antigen specificity differentiate into T helper (Th) 2 cells with the capacity to produce and secrete Th2‐type cytokines such as interleukin (IL)‐4, IL‐5, IL‐9, IL‐13, and IL‐31 in atopic individuals18 (Figure 1). These cytokines are known to contribute to allergic inflammation. IL‐4 and IL‐13 activate B cells to class switch to IgE; they also play a role in T cell, eosinophil migration to allergic tissues. IL‐5 acts on activation, recruitment, and survival of eosinophils; IL‐13 contributes to maturation of epithelia, production of mucus as well as smooth muscle contraction and extracellular matrix generation, and IL‐31 contributes to itch. IL‐4 and IL‐13 open tight junction barrier and cause barrier leakiness.19 IL‐9 contributes to general allergic phenotype by enhancing all of the aspects, such as IgE and eosinophilia.20 A new and potent cell subset, type 2 innate lymphoid cells (ILC) contribute to allergic inflammation in asthma, AD, and AR by enhancing the activity of Th2 cells, eosinophils, and their cytokines. Thymic stromal lymphopoietin (TSLP), IL‐25, and IL‐33 are epithelium‐derived cytokines that can be rapidly released following an allergic trigger. Th2 cells can also produce these cytokines. They have partially overlapping functions to target and activate type 2 ILCs. Upon activation, ILC2s produce IL‐5 and IL‐13 that contribute to recruitment and survival of eosinophils, mucus hypersecretion, airway inflammation, and bronchial hyper‐reactivity. IL‐25 can directly and indirectly contribute to house dust mite–induced asthma exacerbations.20, 21, 22
Figure 1.
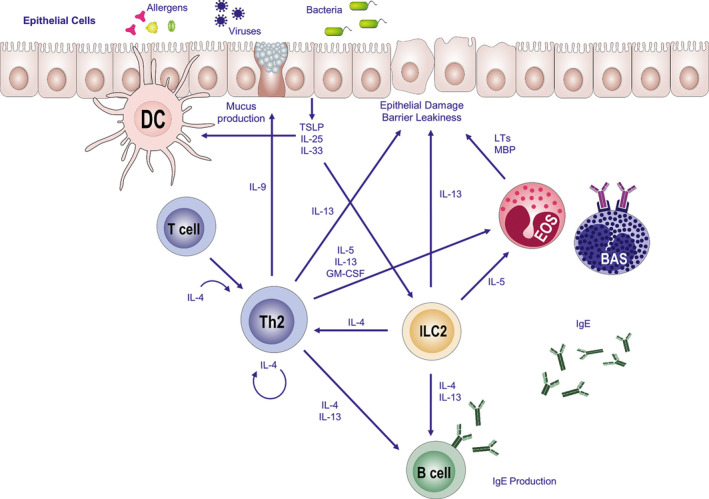
Inicolortiation of allergic immune responses. Dendritic cells, the professional antigen‐presenting cells uptake and process allergens and present allergen peptides to naïve CD4+ T cells. Naïve CD4+ T cells differentiate to Th2 cells with the existence of IL‐4 and produce the cytokines IL‐4, IL‐5, IL‐9, and IL‐13, namely type 2 cytokines. Consequently, B cells produce IgE which binds to specific Fcε receptors on basophils and mast cells, the effector cells of allergic inflammation. This occurrence is termed sensitization. Upon encountering the same allergen for the second time, immediate degranulation of these effector cells leads to release and production of histamine and leukotrienes, all of which give rise to immediate hypersensitivity reactions. IL‐9 induces mucus production, while IL‐13 and eosinophil products such as major basic protein can induce barrier leakiness. ILC2 contributes to allergic inflammation by type 2 cytokine production. The epithelium‐derived cytokines: TSLP, IL‐25, and IL‐33 can also be produced by Th2 cells and can activate ILC2. IL‐25 activates DC. (BAS: basophils, DC: dendritic cells, EOS: eosinophils, ILC: innate lymphoid cells, LT: leukotriene, MBP: major basic protein, Th2: T helper type 2 cells, TSLP: thymic stromal lymphopoietin.)
Allergen‐specific IgE that is produced by B cells binds to high‐affinity IgE receptors (Fcε) present on mast cells, basophils, and eosinophils, the effector cells of allergy. Once IgE is bound to Fcε receptors, these sensitized effector cells are activated upon the re‐exposure to that specific allergen, which then immediately release their pre‐formed mediators such as histamine, proteoglycans, tryptase, and chymase, located in their granules, followed by production and release of biogenic mediators as proteases, histamine, leukotrienes as well as cytokines, all of which underlie the allergic, type‐1 hypersensitivity reactions.15, 23
The latest advances both in allergology, immunology, and biomedicine have contributed to better definition of already heterogenetic allergic diseases with respect to underlying molecular mechanisms. Novel terms as phenotype, endotype, theratype, regiotype, and efforts to discover novel biomarkers arose, all of which are under intense investigation for better understanding of allergic diseases as well as for shedding light to the development of better therapeutic strategies.24 Biomarkers are molecules that can be used in disease diagnosis, patient selection as well as for monitorization of therapy success. Phenotype defines the morphology and clinical characteristics of a disease together with unique responses to therapy, with no concentration on underlying pathogenesis. Endotype defines the cellular or molecular pathological process in relation with the molecular mechanism underlying a subgroup of diseases. Regiotype defines regional differences in allergens and environment. Theratype is used for definition of clinical responders to a particular therapy option.15, 25, 26 Together with these innovative terms in the area, one may consider that better description of disease endotypes could lead to better definition of the underlying pathogenesis, which in turn will permit the development of novel therapy regimens to be precision tailored to individual patients.
Allergen‐specific immunotherapy and immune tolerance in allergic disorders
Immune tolerance
Immune tolerance in allergy, the induction of long‐term unresponsiveness to allergens either in natural exposure settings or in in vivo challenges is an active immune response status formed by a complex network of immune cells, tissues, and mediators. In immune tolerance, changes in allergen‐specific memory T‐ and B‐cell responses diminished IgE as well as enhanced IgG4 production from B cells, and downregulation of mast cell and basophil activation thresholds occur as a net result of allergen exposure or subcutaneous and sublingual AIT, all of which end up with suppression of allergic symptoms (Figure 2). Immune tolerance is a prerequisite for limitation of reactions against either self or microbial antigens and allergens, for prevention of chronic inflammation and also tissue destruction.27
Figure 2.
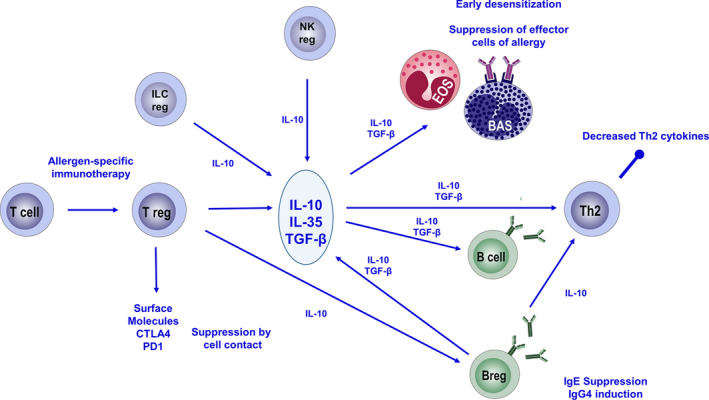
Immune regulation of allergic immune responses as a consequence of AIT. Allergen‐specific immunotherapy‐induced Treg cells that produce IL‐10, TGF‐β, and IL‐35 and also express surface molecules as CTLA4 and PD1 all of which contribute to suppression. Treg cells suppress Th2 cells, basophils, and eosinophils and also induce allergen‐specific Breg cells. The suppressive milieu limits production of IgE and induces production of IgG4 from B cells. Breg cells, NKreg cells, and ILCreg cells contribute to induction and maintenance of allergen‐specific tolerance. (BAS: basophils, EOS: eosinophils, ILCreg: regulatory innate lymphoid cells, NKreg: regulatory natural killer cells, Treg: regulatory T cells.)
Regulatory T cells and AIT
Data obtained from both human and mouse studies revealed important contributions of Treg cells in induction and maintenance of immune tolerance.6, 28, 29 Increase of allergen‐specific Treg cells and reduction in frequency of Th2 cells during AIT, as well as in natural high‐dose exposure studies as such in beekeepers, were revealed.30 Treg cells form a specific subset of CD4+ T cells and are best known with their suppressive properties by production of cytokines as IL‐10 and TGF‐β and also by utilization of inhibitory surface molecules such as CTLA4 and PD1.15, 31, 32, 33, 34 Adoptive transfer of Treg cells has protective effects in a number of T‐cell–mediated disease murine models.35 AIT upregulates the activated allergen‐specific Treg cells, while downregulating dysfunctional allergen‐specific Treg cell subsets (Figure 3) . Following a successful AIT course, correction of previously dysregulated Treg cellular responses is associated with improved clinical scores.32When frequency of allergen‐reactive T‐cell subsets and their cytokine productions were investigated in peripheral blood mononuclear cells of AR patients receiving AIT, after treatment, allergen‐reactive IL‐5+IL‐13+CD27‐CD161+CD4+ cells and ST2+CD45RO+CD4+ cells were decreased, in comparison with placebo. Especially, in AIT responders, significant reductions in allergen‐reactive ST2+CD45RO+CD4+ cells were observed, which might be a candidate biomarker for treatment follow‐up.36 Recently, a detailed allergen‐specific T‐cell study reported a significant increase in the numbers of Der p 1‐specific FOXP3+ Helios+ CD25+ CD127‐ Treg cells after 30 weeks. As an interesting finding, ILT3+ Treg cells displayed compromised suppressive function and low FOXP3 expression and this subset substantially decreased from baseline after 3 years of AIT. In addition, Der p 1‐specific IL‐10 and IL‐22 responses have increased after 30 weeks, but only IL‐10+ Der p 1‐specific Treg cells remained present at high frequency after 3 years of AIT. Increased number of FOXP3+ Helios+ and IL‐10+ and decreased ILT3+ Treg cell responses correlated with improved allergic symptoms.32IL‐35, an anti‐inflammatory cytokine produced by both Breg and Treg cells, can act as an inducer of both cell populations with immunosuppressive capacity. Dysregulated IL‐35 inducible Treg cells in patients with AR were restored in response to AIT.37
Figure 3.
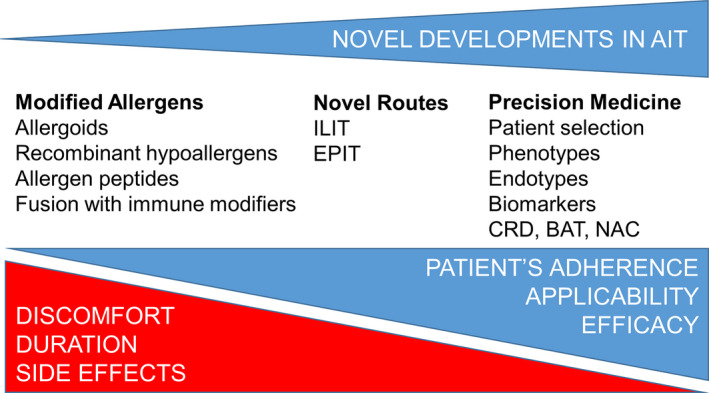
Contribution of novel developments in AIT. AIT is the only option to establish a long‐term, medication‐free cure of allergic diseases. Utilization of modified allergens aims increased efficacy and limitation of side effects such as risk of anaphylaxis, helps for better and longer presentation of the allergen peptides, with no binding to IgE present in the patients. ILIT decreases the number of injections required, the total received allergen dose, and also the therapy duration. EPIT does not require injections; therefore, it is more patient‐friendly. Both routes increase patient adherence to therapy. Precision medicine contributes to AIT by better characterization of patients, selection of custom‐tailored therapy per patient, and monitorization of therapy success by biomarkers. (ILIT: intralymphatic immunotherapy, EPIT: epicutaneous immunotherapy, CRD: component‐resolved diagnosis, BAT: basophil activation test, NAC: nasal allergen challenge.)
Contribution of regulatory B cells to immune tolerance
Besides Treg cells, contribution of other cell subsets to the establishment and maintenance of immune tolerance is being elucidated nowadays. Suppressor B cells producing IL‐10 were termed as regulatory B (Breg) cells and contribute to protection against chronic inflammatory conditions by production of IL‐10, transforming growth factor (TGF)‐β, and IL‐35. A potential role for Breg cells in induction of tolerance during AIT was attributed, which was linked with diminished IgE and increased IgG4 production, together with increased IL‐10 production from allergen‐specific T and B cells.38, 39, 40 The CD27+ naive fraction of IL‐10‐producing Breg cells are specifically confined to IgG4 production.38 Transfection of naive B cells only with IL‐10 is sufficient to induce a regulatory phenotype, Breg cells with IL‐10 and IL‐1 receptor antagonist expression, with suppressive capacities of IgE and dendritic cells.41
Tolerogenic dendritic cells
Dendritic cells, a heterogeneous group of antigen‐presenting cells, have central roles in initiation of immune responses or establishment of tolerance to allergens. The tolerogenic subtype of DCs (tDCs) have the capacity to promote induction of Treg cells from naïve T cells and can also stimulate the expansion of the existing Treg cell populations.42, 43, 44 Monocyte‐derived tDCs of healthy individuals were demonstrated to favor differentiation of allergen‐specific Treg cells and in turn suppress T‐cell responses. In mouse models, tDCs were revealed to inhibit allergic airway inflammation.45, 46 Better understanding of DCs with tolerogenic properties may contribute to the development of new therapy regimens, not only in allergy and asthma, but also autoimmunity and organ transplantation.
Natural killer cells with regulatory roles
Natural killer (NK) cells, a subset of lymphocytes, have potential cytotoxicity against tumor cells and virus‐infected cells. They contribute to inflammation and also immune regulation by their production of cytokines in resemblance with Th cell subsets.47 The IL‐10‐producing NK cell subset was revealed and termed as NK regulatory cells, which have capacity to limit antigen‐specific T‐cell responses.48 The role of NK cells in AIT remains to be elucidated. NK‐T cells also have not been studied in the course of AIT, but the triggering of HR2 on inducible NK‐T cells and suppression of their activity represents an important immune regulatory mechanism against lipid antigens that are constituents of allergen extracts.49
Innate lymphoid cells in allergy
Innate lymphoid cells (ILCs), a recently discovered group of lymphocytes, which lack specific antigen receptors, play roles in both allergic and non‐allergic inflammatory diseases. ILC2s contribute to allergic inflammation by production of Th2 type cytokines, by promotion of mucus production, eosinophilia, and mast cell accumulation in allergic diseases.50 AIT was revealed to inhibit seasonal increases in peripheral ILC2s.51 Induction of IL‐10+ ILCregs from ILC2s by retinoic acid was recently demonstrated in human nasal tissues as well as lung tissues of house dust mite‐induced mouse model of type 2 lung inflammation.52 Another study proposed important contribution of ILCregs in intestinal regulation, by secretion of IL‐10.53CD40‐ligand expressing ILC3s collaborate with B cells for the induction of immature translational Breg cells as one of the mechanisms of induction of Breg cells.
These regulatory cell populations form a suppressive milieu, which ends up with a slow decrease in production of allergen‐specific IgE, and early switch of B cells to produce IgG4 and as a consequence, increase of IgG4 antibodies, which is a non‐inflammatory isotype within the perspective of allergic disorders. Due to both dominance of inhibitory cytokines and a drop in levels of IgE, an increased threshold of mast cell and basophil activation for degranulation is established, all of which is termed as early desensitization. As a result of induction of allergen‐specific tolerance, Th2 cells and their relevant cytokines such as IL‐4, IL‐5, and IL‐13 as well as T‐cell proliferation are suppressed.15, 54, 55 Downregulation of Th2 key regulator: GATA3 and upregulation of Treg transcription factor: FoxP3 was revealed, which were in correlation with sustained protection following AIT in a mouse model.56 Decrease in tissue localized mast cells and eosinophils form the late desensitization.
Allergen‐specific immunotherapy and unmet needs in clinical practice
Allergen‐specific immunotherapy mainly relies on induction of a long‐term allergen‐specific immune tolerance.57, 58, 59, 60 It is reportedly well accepted that AIT is the disease modifying, most rational modality of treatment for allergic disorders, especially for AR, allergic asthma, and in insect venom allergy.10, 61, 62, 63, 64 Short‐ and long‐term safety concerns, efficacy, and comparative potency against conventional pharmacotherapy, eagerness of patients, and their families to initiate and thereafter compliance of them for long‐term treatment durations in addition to compatibility and ease of application in real daily‐life, as well as treatment costs and associated deliverables such as loss of school / work days appear as both triggering and limiting factors for AIT. Efforts to improve and overcome these issues include allergen standardization, development of less allergenic and more immunogenic allergen molecules, and methods of easy introduction of these newly developed allergen molecules to the immune system in a cheaper, safer, painless route within a very short duration without any unwanted adverse effects.65, 66 While AIT is widely available in Europe, due to limited availability of the guidelines and reimbursement problems, a great number of patients who will possibly benefit from AIT do not have access to this therapy option.67
Questions can be easily raised when considering AIT especially in pediatric patients. “When to start, with which allergen, for how long, by which route?” are among the first couple of questions, which may be expanded as “Should we start AIT at the beginning of atopic march?”, “Should we repeat AIT courses within the following years?”. Should one allergen or multiple allergens be used especially in polysensitized patients? Should the duration of treatment be just until observation of improvement of symptoms or longer? SCIT or SLIT or possible other novel routes? Administration of AIT at home or at clinic? Daily or monthly or annually or just once?
Undoubtedly, one of the most striking key points in AIT is patient selection. Correct patient selection increases the success of AIT. As heterogeneity in patient‐dependent factors, including sensitization patterns, efficiency of environmental avoidance measures, conjunction of triggering insults such as infections, microbiome characteristics, epithelial barrier functions, environmental pollution in addition to endotype, phenotype, and associated comorbidities of the patient's active disease, and adherence to prescribed treatments are major key points during AIT.
SCIT is the most widely accepted method of AIT, which has a story started at the beginning of 19th century. Several meta‐analyses have shown the efficacy of SCIT especially in AR and asthma patients.68, 69 However, anaphylaxis related with SCIT arose as a safety concern for both patients and clinicians. The other major drawbacks of SCIT include monthly repeated injections, which may be a real limiting factor for pediatric patients. There is a necessity of SCIT to be administered in a clinical setting with experienced personnel in the management of adverse reactions, including anaphylaxis.67 On the other hand, SLIT appeared as an alternative route, where allergen extracts were introduced to oral mucosal surfaces. Although SLIT is administered at home settings and seems like, it is a user‐friendly method, especially in pediatric age group, long‐term treatment duration decreases adherence to treatment.70, 71, 72, 73, 74 Several meta‐analyses have shown that SLIT is effective for AR as well as asthma and has been proven to be a safe route of administration.2, 3, 4, 69, 75, 76, 77, 78, 79, 80, 81, 82, 83 There is clear evidence for effectiveness of both SCIT and SLIT.84 Superiority of one mode of administration over the other could not be consistently demonstrated. Both routes induce comparable IgG4 production, allergen‐specific tolerance, and basophil suppression. There was a trend toward favoring SCIT for symptom and medication scores. SCIT has an early onset of action and very early desensitization effect. More robust increase of IgG4 and decrease of IgE are also observed in SCIT compared to SLIT.54, 74 In SCIT, while DCs present in the skin uptake the allergens, tolerogenic oral mucosal or tonsillar DCs uptake the allergens in SLIT. These cells are known with their continuous upregulated expressions of FcεRI, MHC I and MHC II, in addition to costimulatory molecules CD40, CD80, and CD86, which contribute to tolerance induction.74
EAACI guidelines for AIT and detailed meta‐analyses reported last years represent key and most updated documents in the area.2, 3, 10, 64, 66, 67, 69, 85, 86, 87, 88, 89, 90, 91, 92, 93 It is a well‐known fact that patients with moderate‐to‐severe AR may have predisposition to develop asthma. According to a retrospective real‐world analysis, SLIT with grass pollen is capable of reducing the need for AR as well as asthma medications.94 Another retrospective real‐world study of birch pollen AIT have verified significantly reduced medication intake in AR and asthma patients in up to 6 years of follow‐up, which is accompanied by significantly reduced risk of new‐onset asthma.95 Duration of AIT is an important question nowadays. Three years of SCIT or SLIT was proved to be clinically successful for AR, which modulated allergic immune responses toward a 2‐3 years‐sustained tolerant state, following termination of the therapy.96
Food allergy is a result of tolerance loss to common dietary antigens. The range of affected disease can vary from skin manifestations to gastrointestinal and respiratory symptoms and even to life‐threatening anaphylaxis. Although avoidance which forms the responsible dietary allergens is the classical main preventive measure, risk of anaphylaxis due to accidental exposures arises the need for further interventions. In this point, oral food allergen immunotherapy appears as a promising tool for the management of food allergy. Administration of gradually increasing doses of the culprit allergen induces a tolerant state during oral food allergen immunotherapy. Several trials have reported promising results especially with peanut, cow's milk, and egg, especially in pediatric age group.97, 98, 99, 100, 101, 102
Clonal mast cell disorders (cMCD) encompass monoclonal mast cell activation syndrome and systemic mastocytosis. The patients with cMCD have increased risk of severe anaphylaxis following bee stings. The intensified risk of developing anaphylaxis could be linked to increased numbers of mast cells together with increased levels of IgE. Lifelong venom immunotherapy (VIT) which is found to be safe and effective in the treatment of bee venom allergies has been also recommended in patients with cMCD. A recent single‐center study reported VIT as a safe and effective therapy for cMCD patients in which, venom‐specific IgG4 levels were increased which have been proposed as a biomarker to monitor the clinical efficacy of VIT.103
Novel interventions in allergen‐specific immunotherapy
To overcome the drawbacks listed above, studies on design of novel AIT vaccines aiming to increase the efficacy limit the possible side effects and risk of anaphylaxis and also decrease both the amounts of allergens applied and the durations of AIT have been focused. Allergoids, recombinant hypoallergens, immunogenic allergen peptides, adjuvants that are stimulators of innate immune system, allergens fused with immune modifiers and peptide transporting proteins are among these efforts.104 Trials investigating grass, ragweed, tree pollen, and house dust mite allergoid utilization in AIT have revealed success.105, 106 Alternative routes for administration of AIT allergens have been projected for improvement of both safety and efficacy. Intralymphatic and epicutaneous routes are lately pursued novel routes of AIT. Both routes are promising in grass pollen allergy, in which less number of required interventions and lower total doses of allergens are administered. Although there are increasing numbers of intralymphatic immunotherapy(ILIT) trials, there is currently not enough evidence for its routine use, and there are no authorized allergen extracts commercially available for this application route.107 In ILIT, antigen injection directly into lymph nodes enhances direct presentation of antigens and rapid generation of local tolerogenic T‐ and B‐cell responses together with limitation of IgE‐mediated reactions.33, 108 ILIT could be accepted as a safe and patient‐friendly approach; however, ultrasound‐guided injections in ILIT require experienced and skilled approach, and also more trials with extended follow‐up periods are requisite for assessment of long‐term tolerance potential of this novel route.106, 109 The development of epicutaneous immunotherapy (EPIT) comes from the knowledge that epidermis lacks blood vessels, but comprises great numbers of antigen‐presenting cells, which permits local presentation of antigens, while preventing systemic reactions to allergens.110 In other words, increased allergen presentation to immune system through highly rich number of epidermal antigen‐presenting cells in the skin is facilitated by EPIT. Patches with absorbed allergens get into contact with skin for several hours and support better comfort and compliance of patients due to non‐involvement of needles and injections.106, 111 EPIT is a safe and patient‐friendly approach in AIT; however, there still are requirements for studies to define optimal regimens.
Biomarkers for monitoring the clinical efficacy of allergen immunotherapy
Despite the advances in AIT in recent years, some treated patients do not benefit from AIT. There is an increasing need for the discovery of prognostic and predictive biomarkers that will improve the selection of patients, who will best respond to AIT and help to tailor therapy regimens.107
According to an EAACI position paper in 2017, potential biomarkers for monitorization of the clinical efficacy of AIT were summarized as follows: a) IgE (total IgE, sIgE/total IgE) b) subclasses of IgG (allergen‐specific IgG, IgG1 and sIgG4, sIgE/IgG4 ratio) c) IgE serum inhibitory activity for IgE (IgE‐FAB), d) basophil activation, e) chemokines and cytokines, f) cell markers such as Tregs, Bregs, and DCs, and g) in vivo biomarkers including provocation tests.112, 113
IgE antibodies delineate type‐1 allergic diseases as well as atopy. Especially, allergen‐specific IgE have been used to ascertain initiators of IgE‐mediated allergic symptoms. Indebt investigation of specific IgE levels has been gaining importance day by day, due to component‐resolved diagnosis (CRD), which allows detailed molecular profiling of the specific IgE repertoire of allergic patients.114 Instead of allergen extracts, CRD utilizes allergen molecules, which have potential to improve analytical test sensitivity as well as supplying information about possible cross‐reactivity. CRD supports more precise diagnosis before initiation of AIT, especially in polysensitized patients.115 When specificity, sensitivity, and predictive values of CRD are taken into account, it is clear that there is a gap. The results of a systematic review summarizing 11 studies revealed high specificity and low sensitivity in selected components of food allergens: cow's milk, shrimp, hen's egg, hazelnut, and peanut. Given that patients enrolled to the studies were suspected for having allergy, the positive predictive values were claimed to be high, whereas negative predictive values were lower than expected. Robust studies for further accumulation of evidence are required before a definite conclusion could be raised.116
Nasal brushing, sponges, or swabs are relatively noninvasive methods to detect nasal‐specific IgE.117 Measurement of specific IgE in secretions could be useful in monitoring circulation and local IgE response to AIT. Mucosal synthesis of specific IgE can occur in the absence of systemic atopy in AR.118
The basophil activation test evaluates the biologic relevance of novel allergen components to be used in AIT. Activation of mast cells and basophils following IgE cross‐linking leads to degranulation of these cells and degranulated basophils upregulate expression of CD63. Basophil activation test has capacity to identify the IgE‐sensitizing allergen as well as the allergenic potential of the specific IgE antibodies.109
Nasal allergen challenge (NAC) is a clinical approach for diagnosis of AR as well as for confirmation of clinical relevance of allergens by clinical reproduction of AR. NAC has potential to confirm the allergic origin of the symptoms as well as the possible allergen to be utilized in AIT. During the course of treatment, NAC could be utilized for evaluation of nasal mucosal response that in turn could inform the patients’ response to AIT. NAC has capacity to detect small differences between treatments. This test is used as an important efficacy parameter in AIT clinical trials.107, 119
Conclusions
During the course of a successful immunotherapy, regulatory cell populations including Tregs, Bregs, as well as ILCs are induced, all of which produce regulatory cytokines such as IL‐10, TGF‐β, and IL‐35.27, 30, 37, 39, 40, 52 The induction and maintenance of allergen‐specific tolerance is an active immune response state which leads to unresponsiveness to allergens and a gradual decrease in symptoms of allergic disorders. High‐dose allergen exposure models and beekeepers have taught us a lot about the mechanisms underlying the establishment and maintenance of allergen tolerance. AIT shares the same basics with these models and is being utilized for cure of allergic diseases. Novel routes of administration as well as development of hypoallergenic but immunogenic peptides are important milestones for increased efficacy and limited side effects. Discovery of novel biomarkers of allergic inflammation together with progression of diagnosis will both help for better definition of disease mechanisms and better selection of patients for best‐tailored therapy options.
Longitudinal birth cohorts taught us about atopic march and natural history of allergic disorders, in addition to understanding of impacts of genes, epigenome, microbiota, and barrier dysfunction in the development of allergic disorders. All of these have increased our understanding for the underlying mechanisms and management of allergic disorders. It has been clearer in the last years that instead of palliative symptomatic solutions, more long‐lasting etiology‐ and pathogenesis‐oriented approaches are necessary. Actually, in today's practice there are no urgent and rigid rules to start AIT. In general, patient's symptoms are expected to be severe enough for AIT decision. After a few symptom‐full seasons; such as, a season with marked symptoms that cannot be controlled by medication in asthma or having two bad allergy seasons with long symptom durations and long‐term medication requirements, or allergies and medication requirements all around the year promote the prescription of AIT.
Conflict of interest
The authors declare financial support for several research grants, but no direct conflict of interest with this review article.
Financial support
The Lab of authors M.A. and C.A.A. has been supported by Swiss National Science Foundation and Christine Kühne Center for Allergy Research and Education, Davos, and research grants from Allergopharma Reinbek, Germany; Actelion Basel, Switzerland; Idorsia Basel, Switzerland; Scibase Stockholm, Sweden.
2 Differences in Mechanistic and Clinical Responses to Allergen Immunotherapy Between Adults and Children
Corresponding author:
Dr Mohamed H. Shamji
Immunomodulation and Tolerance Group
Allergy & Clinical Immunology
Inflammation, Repair and Development
National Heart & Lung Institute, Imperial College London
1st Floor, Room 111, Sir Alexander Fleming Building
South Kensington Campus
London SW7 2AZ, United Kingdom
Tel: +44 (0) 20 75941673, Mobile: +44 (0) 7872850369.
Email: m.shamji@imperial.ac.uk
Abstract
Allergen‐specific immunotherapy (AIT) remains to be the only disease‐modifying therapy for the treatment of IgE‐mediated diseases in adults and children. It involves the repeated administration of allergen extracts to an allergic individual to provide long‐term relief of symptoms and improvement in the quality of life. AIT is recommended in seasonal allergic rhinoconjunctivitis and allergic asthma and also in patients suffering from perennial allergy. Unlike symptomatic anti‐allergic medications, AIT has been shown to modify the underlying immune response providing ultimately long‐term clinical benefits. Two routes of administration of AIT, subcutaneous (SCIT) or sublingual (SLIT), are currently used in the clinic and have both been illustrated to have long‐term clinical benefit for the treatment of allergic rhinitis. While there is a substantial amount of evidence indicating the efficacy and long‐term maintenance of AIT in adults, limited studies have been performed in children.
Further to this, extensive in vitro studies have been invested in unraveling the mechanism of action of AIT, though many unanswered questions remain. These studies are mainly performed in adult subjects, with evidence in children lacking. Whether or not the mechanism of action of AIT is the same in children as in adults also remains to be fully identified. Exploring the benefits of AIT in children will not only provide a therapeutic approach for the treatment of allergic rhinitis but also provide the possibility of intervening the early phase of disease progression, like in the case of asthma.
Abbreviations
AIT – Allergen‐specific immunotherapy
AR – Allergic rhinitis
BRB – Bregs Regulatory B cells
HDM – House dust mite
IgE – Immunoglobulin E
IgG – Immunoglobulin G
iTr – Inducible T regulatory cells
SCIT – Subcutaneous allergen‐specific immunotherapy
SLIT – Sublingual allergen‐specific immunotherapy
Tfh – T follicular helper cells
Tfr – T follicular regulatory cells
Introduction
Allergic rhinitis (AR), asthma, and food allergy constitute major IgE‐mediated allergic disorders in children and adults, with an increasing prevalence worldwide. While most patients benefit from avoidance strategies and symptomatic drug treatment, a significant proportion still have persistent symptoms and are at risk of severe and life‐threatening allergic reactions. Allergen‐specific immunotherapy (AIT) is currently accepted as the only clinically effective treatment for IgE‐mediated allergic diseases and is recommended to those who do not respond to common avoidance strategies and pharmacotherapies.120 Displaying a disease‐modifying effect, AIT is known to provide a long‐term clinical benefit that may persist for several years after cessation of treatment.81, 121 To date, AIT is well‐accepted and routinely prescribed worldwide, both in adults and in pediatric population to tackle mainly allergic rhinitis and well‐controlled asthma, but also a growing number in food allergies. This review will thoroughly discuss our current understanding of the clinical and immunologic effects of AIT in children and adults. Moreover, novel approaches of AIT to enhance safety and efficacy will be considered.
Mechanisms of allergic rhinitis
AR involves a biphasic reaction consisting of the early phase of the immediate reaction (occurring within minutes) and late‐phase reaction (occurring 6‐12 hours following allergen exposure). One of the significant components of the immediate response following allergen exposure in a sensitized individual is the degranulation of basophils and mast cells as a result of allergen‐IgE cross‐linking the high‐affinity IgE receptor (FcεRI).122 Degranulation of these effector cells results in the release of mediators (ie, histamine) that induce early symptoms of allergic rhinitis such as sneezing and rhinorrhea.123 Following the early‐phase response, the late‐phase response is characterized by prolonged symptoms that include nasal blockage. Pro‐inflammatory cytokines such as IL‐4 and IL‐13 are released by mast cells and cause upregulation in adhesion molecules, facilitating the infiltration of basophils, eosinophils, and T lymphocytes into the lining of the nasal mucosa.124
Moreover, chemokines such as TARC, eotaxin, RANTES, and MCP‐4 also serve as chemoattractants for eosinophils, basophils, and T lymphocytes.125, 126, 127 Recruitment of T lymphocytes and their differentiation toward a Th2 phenotype results in the release of more cytokines (IL‐3, IL‐4, and IL‐13) promoting IgE production by plasma cells.128 Finally, other novel subsets of T cells that include T follicular helper (Tfh) cells129 and allergen‐specific Th2 (Th2A) cells130 are reported as a key driver of allergen‐induced inflammation.11, 131 The recruitment wave of these effector cells from lymphatic tissue and circulating blood into the local target organ marks the late‐phase response of allergic reaction.
Allergen immunotherapy
AIT is a safe and clinically effective treatment in carefully selected patients with aeroallergen‐induced IgE‐mediated disease such as AR with and without asthma. AR affects 10‐15% of children and 26% of adults in the UK with an overall prevalence of 23% in Europe. Typical symptoms include runny nose, itchy eyes, nasal congestions, symptoms of allergic conjunctivitis. Quality of life of those affected is significantly partly due to lack of sleep. Pharmacotherapy such as non‐sedating oral, topical intranasal antihistamines, and intranasal corticosteroids is effective in the majority of adults and children with AR.132 AIT is indicated in those who do not respond to conventional pharmacotherapy.
AIT inhibits the occurrence of seasonal and perennial symptoms to the sensitizing allergen, the need for rescue pharmacotherapy intake, and improves quality of life. Treated patients experience more number of “well days” than “hell days”. AIT for AR can be administered either by the subcutaneous (SCIT) or by the sublingual (SLIT) route. Both SCIT and SLIT have been shown effective in allergic rhinitis, generally within 2‐4 months of initiating treatment and may be given pre/co‐seasonally for short‐term benefit. Indirect comparisons have suggested that immunotherapy is more effective than anti‐allergic drugs. In view of potential side effects, cost, and the necessary patient commitment, the long‐term benefit is an essential consideration for the recommendation of immunotherapy over standard pharmacotherapy.
Several clinical studies within the past decades have revealed that protracted AIT treatment between three and five years yields a long‐term clinical benefit, with clear evidence in both adults and children being well‐documented.
Subcutaneous allergen immunotherapy (SCIT)
SCIT involves weekly up‐dosing injections, followed by monthly maintenance injections for at least three years.133, 134 In view of occasional systemic allergic side effects, SCIT requires administration in a specialist allergy clinic with access to resuscitative measures. While SCIT is effective in mediating long‐term clinical benefit, it is associated with poor compliance, with approximately only 25% of patients completing a 3‐year SCIT treatment.135 Studies previously conducted on SCIT revealed that the primary cause of non‐compliance was the inconvenience related to injections, as well as the cost of treatment.135
SCIT is highly effective for the treatment of AR, in patients with seasonal pollinosis136 as well as patients suffering from perennial allergy and sensitivity to house dust mite132 (Table 1). A randomized controlled clinical trial involving grass pollen–allergic patients showed that SCIT treatment was associated with improved quality of life and a 30% and 44% reduction in seasonal symptoms and the need for anti‐allergic medication during the pollen season, respectively.137 In addition to this, a similar observation was observed in SCIT for the treatment of allergic asthma in adults, though it was found to be more effective in seasonal asthma, compared to perennial asthma.138, 139, 140 The long‐term clinical benefit of SCIT has been shown across various studies. In a 7‐year trial where patients with severe seasonal allergic rhinitis received either SCIT to grass pollen extract or placebo, it was shown that total symptom scores were significantly lower in immunotherapy groups compared to control group.141, 142 The trial also showed that total symptom scores and rescue medication intake remained low for at least three years following cessation of treatment.133 In addition to this, other studies involving Parietaria 143 and ragweed144 SCIT confirmed that long‐term clinical benefit of SCIT could be achieved following 3‐year treatment.
TABLE 1.
AIT Studies in adults for the treatment of allergic rhinitis with or without asthma
| Study (Year) | Age | AIT Mode (Disease) | Duration | Clinical results | Immunological results |
|---|---|---|---|---|---|
| Varney et al (1991) | Adult | Grass SCIT (Rhinitis) | 1 mo |
↓ Medication use ↓ Total SS ↓ VAS scores |
‐ |
| Creticos et al (1996) | Adult | Ragweed SCIT (Asthma) | 24 mo |
↓ Total SS ↓ SPT to ragweed ↓ BHR |
↑ Ragweed sIgG |
| Frew et al (2006) | Adult | Grass SCIT (Rhinitis) | 12 mo |
↓ SMS ↑ QoL |
‐ |
| O’Hehir et al (2009) | Adult | HDM SLIT (Rhinitis) | 24 mo |
↓ Rhinitis SS ↓ Asthma SS ↑ QoL |
↓ Ag‐induced T cell division ↓ IL‐5, TGF‐β ↑ IL‐10, IFN‐γ ↑ HDM sIgG4 |
| James et al (2011) | Adult | Grass SCIT (Rhinitis) | 24 mo | ↓ SMS |
↓ Grass sIgG1 ↓ Grass sIgG4 Maintained inhibitory activity of IgG |
| Durham et al (2012) | Adult | Grass SLIT (Rhinitis) | 36 mo |
↓ RTSS ↓ Daily medication score |
‐ |
| Bergmann et al (2014) | Adult | HDM SLIT (Rhinitis) | 12 mo |
↓ SS ↓ Rescue medication score |
‐ |
| Demoly et al (2016) | Adult | HDM SLIT (Rhinitis) | 12 mo |
↓ SMS ↑ QoL |
‐ |
| Scadding et al (2017) | Adult | Grass SCIT and SLIT (Rhinitis) | 24 mo |
No reduction in TNSS No change in RQLQ No change in VAS ↓ Early and Late skin response |
↑ Grass sIgG4 (SCIT/SLIT) ↓ Grass sIgE (SCIT) No change in grass sIgE (SLIT) |
Abbreviations: BHR, bronchial hypersensitivity; ICS, inhaled cortcosteroid; QoL, quality of life; RTSS, rhinoconjunctivitis symptom score; SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy; SMS, Symptom and Medication Score; SPT, skin prick test; SS, Symptom Score; TNSS, Total Nasal Symptom Score; VAS, Visual Analogue Scale.
While there is a vast amount of evidence for the clinical efficacy of SCIT in adults, the evidence is lacking in pediatric patients (Table 2). AIT is currently recommended in children with moderate‐to‐severe seasonal allergic rhinitis and well‐controlled asthma. Currently, there is modest evidence for clinical efficacy of continuous SCIT in children suffering from seasonal allergic rhinitis to grass pollen and perennial allergic rhinitis to house dust mites.10 A prospective randomized controlled trial of pollen immunotherapy, the Preventive Allergy Treatment (PAT) Study, in children suffering from season allergic rhinitis investigated the development of asthma for ten years. This study revealed that significantly less SCIT‐treated subjects developed asthma at 10‐year follow‐up.145 This study also yielded evidence that SCIT may reduce the onset of new allergen sensitization in children.146, 147 It is important to note that there are currently no studies investigating the long‐term clinical efficacy of SCIT in perennial AR in children (Figure 4).
TABLE 2.
AIT studies in children for allergic rhinitis with or without asthma
| Study (Year) | Age | AIT Mode (Disease) | Duration | Clinical results | Immunological results |
|---|---|---|---|---|---|
| Des Roches et al (1991) | Children | HDM SCIT (Rhinitis) | 36 mo | ↓ Occurrence in new sensitization | ‐ |
| Pajno et al (2001) | Children | HDM SCIT (Rhinitis/Asthma) | 36 mo | ↓ Occurrence in new sensitization | ‐ |
|
Möller et al (2002) – PAT Study |
Children | Grass and/or birch pollen SCIT (Rhinitis/Asthma) | 36 mo |
↓ BHR ↓ conjunctivitis VAS score ↓ asthma VAS score |
‐ |
| Follow up of PAT Study: Niggemann et al (2006) | Children | Grass and/or birch pollen SCIT (Rhinitis/Asthma) | 36 mo |
↓ asthma Improvement in CPT |
‐ |
|
The GAP trial: Valovirta et al (2011 & 2018) |
Children | Grass SLIT (Rhinitis/Asthma) | 36 mo |
↓ asthma symptoms ↓ medication use ↓ RTSS ↓ Grass SPT |
↓ total IgE ↓ Grass sIgE |
| Mosbech et al (2014 & 2015) | Adolescent/Adult | HDM SLIT (Rhinitis/Asthma) | 12 mo | ↓ ICS | ‐ |
| Nolte et al (2016) | Adolescent/Adult | HDM SLIT (Rhinitis) | 52 wk |
↓ Total Rhinitis SS ↓ Daily symptom and medication score ↓ VAS score |
‐ |
| Okubo et al (2017) | Adolescent/Adult | HDM SLIT | 12 mo |
↓ Total SS ↓ QoL |
‐ |
| Masuyama et al (2018) | Children | HDM SLIT | 12 mo | ↓ Rhinitis SMS |
↑ HDM sIgE followed by a decline ↑ HDM sIgG4 |
Abbreviations: BHR, bronchial hypersensitivity; CPT, conjunctival provocation test; ICS, inhaled cortcosteroid; QoL, quality of life; RTSS, rhinoconjunctivitis symptom score; SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy; SMS, Symptom and Medication Score; SPT, Skin Prick Test; SS, Symptom Score; TNSS, Total Nasal Symptom Score; VAS, Visual Analogue Scale.
Figure 4.
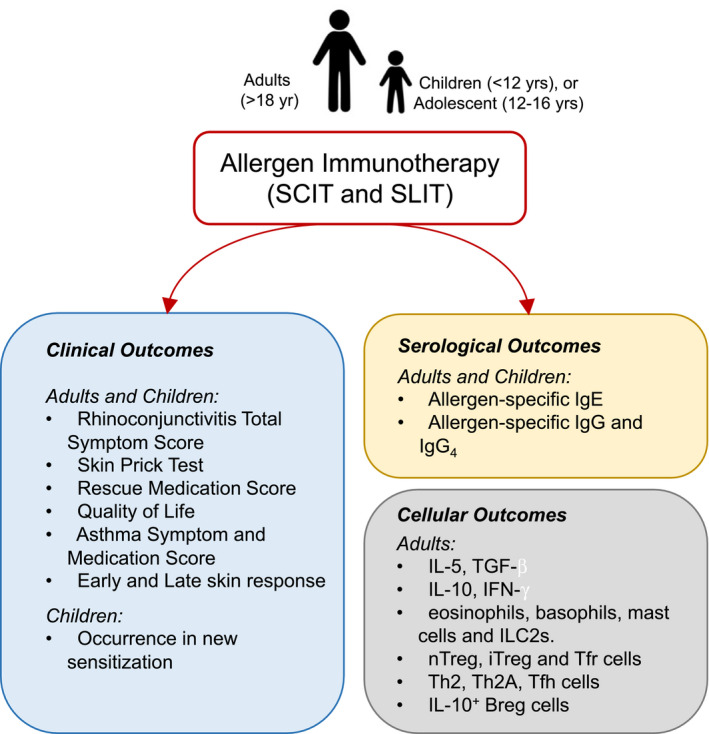
Mechanistic and clinical responses of allergen immunotherapy (AIT) on adults and children. Allergen immunotherapy, administered as subcutaneous (SCIT) or sublingual (SLIT), is associated with various clinical outcomes in adults in children. This is also accompanied by modulation in serological readouts that includes IgE and IgG in both adults and children. The majority of cellular outcomes of AIT have been based on studies in adults. Whether the same cellular modulation is found in children is yet to be fully identified. ILC2s: Group 2 innate lymphoid cells, nTreg: natural regulatory T cells, iTreg: inducible regulatory T cells, Tfr: T follicular regulatory cells, Th2A: allergen‐specific Th2 cells; Tfh: T follicular helper cells, Breg: regulatory B cells.
Figure 5.
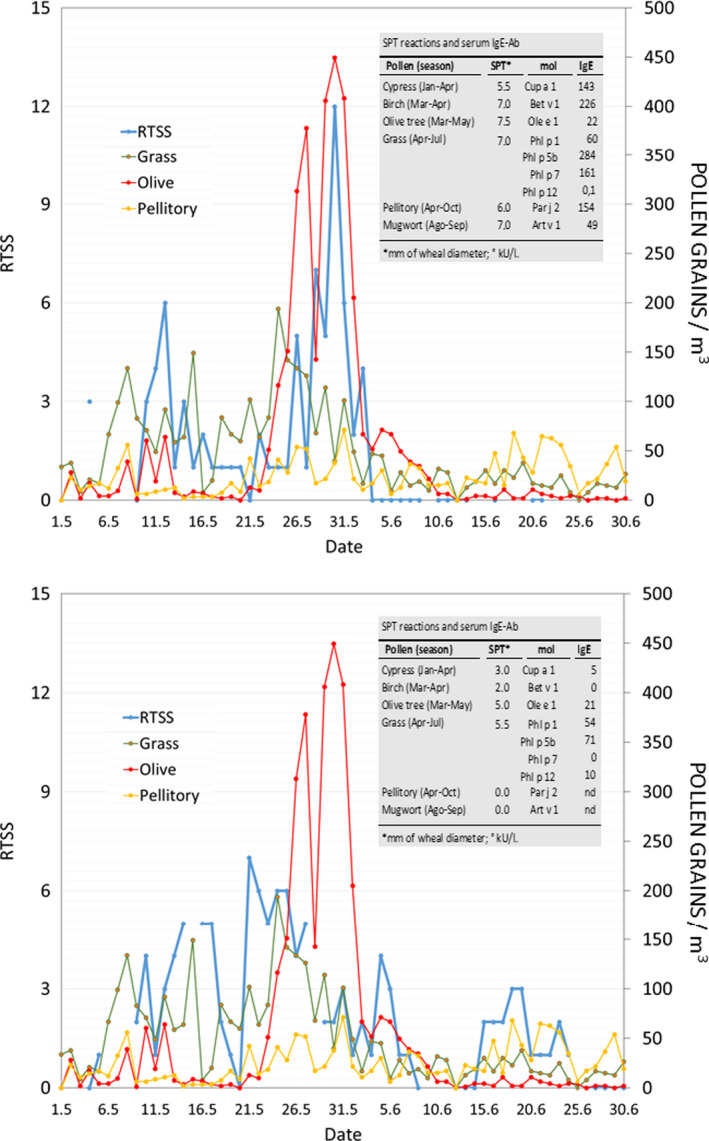
Trajectories of pollen counts vs symptoms ‐ Symptom severity vs pollen counts in 2 patients with allergic rhinitis. Data on severity of symptoms (collected using a smartphone app) are reported as the Rhinoconjunctivitis Total Symptom Score (RTSS) (A, Patient 1; B, Patient 2). Pollen counts (grains/m3) were obtained from the pollen station of Ascoli Piceno. Data on skin prick test reactions to pollen extracts and on serum IgE levels against major allergenic molecules are shown. We also measured the following serum IgE levels (kUA/L) against pollen extracts: Patient 1, cypress 143, birch 226, olive tree 122, grass 404, pellitory 191, mugwort 96; Patient 2, cypress 2, birch 9, olive tree 24, grass 157 (Reused with the permission from Bianchi A, et al J Investig Allergol Clin Immunol. 2016246).
Sublingual allergen immunotherapy (SLIT)
SLIT involves daily administration of either drops or tablets placed under the tongue. It is effective and has a safer profile such that it may be self‐administered in the patient's home.134, 148 Typical side effects of SLIT include itching, lip and tongue swelling that occurs in up to 50% of participants, with systemic reactions being infrequent. In addition to this, SLIT has been associated with poor compliance, with only approximately 12% patients completing a 3‐year SLIT treatment.135 The poor compliance is likely due to SLIT facing similar problems with other conventional pharmacotherapy regimens.149
Grass SLIT is effective in reducing clinical symptoms resulting in improved quality of life. Its efficacy was shown to be comparable for both polysensitized and monosensitized AR patients and with or without allergic asthma. There is a growing number of evidence illustrating that a 3‐year SLIT treatment can induce disease‐modifying effects with the tolerogenic benefit that persists for at least two years following cessation of the treatment.150, 151 Despite the positive outlook on SLIT treatment for the treatment of grass pollen allergy, the GRASS Trial, which was a randomized, double‐blind, placebo‐controlled trial, showed that two years of grass pollen SLIT was not effective in improving nasal response to allergen challenge at 3‐year follow‐up, compared to placebo.152 Several large trials of SLIT tablets have also been performed in adults with perennial rhinitis and IgE sensitivity to house dust mites.153, 154, 155 Though the majority of participants were polysensitized and had mild asthma comorbidity, clinical efficacy and safety readouts were not impacted. All the trials provided evidence of efficacy in rhinitis with approximately 25% reduction in combined symptom medication scores compared to the placebo group. In addition to this, house dust mite SLIT tablet has also been shown to be effective in reducing the need for inhaled corticosteroids.156
Meta‐analysis and a systematic review of 60 studies showed that SLIT treatment was clinically effective for the treatment of seasonal and perennial allergic rhinitis, though the evidence was found to be less convincing in children compared to adults.81, 157, 158 Studies involving pediatric subjects who suffer from HDM perennial allergic rhinitis showed that HDM SLIT tablets were well tolerated and resulted in improvement in HDM‐induced rhinitis symptoms.72, 159, 160 In addition to assessing the efficacy of SLIT in reducing AR symptoms, its effect on asthma development has also been assessed in pediatric subject groups. Similar to the PAT study involving SCIT, the Grazax Asthma Prevention (GAP) trial illustrated that 5‐year grass SLIT treatment significantly reduced the risk of experiencing asthma symptoms or the need for asthma medication, though no difference in the primary outcome (ie, onset of asthma) was observed.58, 161 The trial also showed approximately 30% and 27% reduction in grass allergic rhinoconjunctivitis symptoms and rescue medication, respectively. This was also accompanied by reduced total IgE, grass pollen‐specific IgE and skin prick test reactivity in the treated group, compared to placebo. Despite these observations, more clinical trials are required to confirm and validate the efficacy and long‐term clinical benefits of AIT in children.
Mechanisms of action of allergen immunotherapy
The mechanism of action of AIT has been shown to involve several immunologic pathways, requiring the interplay between both the innate and adaptive immune response and with the primary goal in restoring immune tolerance to allergens. This is achieved by modulation of both early‐ (decrease in the number of effector cells such as mast cells, basophils, and eosinophils and type 2 innate lymphoid cells51, 162, 163, 164, 165) and late‐ (induction of regulatory T‐ and B‐cell responses,38, 166, 167, 168, 169, 170 and regulation of allergen‐specific antibodies171) phase allergic response. While the mechanisms of action of AIT have been studied extensively in adults, limited studies have been performed in children (Figure 1).
Effect of AIT on the innate immune response
Within the innate immune response, basophils and mast cells play a crucial role in mediating allergic responses. AIT has been demonstrated to induce early desensitization of both basophils and mast cells, ultimately resulting in suppression in their ability to respond to allergen‐IgE cross‐linking,172 eventually resulting in a decrease in tissue infiltration and release of mediators by basophils and mast cells. The mechanism to which a successful inhibition of mast cells and basophils can be achieved has been studied thoroughly, with studies illustrating that IgG antibodies produced during AIT inhibit basophil activation and intracellular histamine release.173, 174 Successful AIT treatment (both SCIT and SLIT) is associated with the induction of blocking antibodies IgG4 that can compete with IgE for allergen binding, resulting in the prevention of mast cell and basophil activation and degranulation. A more recent investigation, however, has also revealed that both IgG3 and IgG2 were also responsible for the suppression of FcεRI‐mediated basophil responsiveness. Furthermore, in a study involving venom immunotherapy patients, it was shown that AIT could lead to the upregulation of histamine type 2 receptor, which can, in turn, suppress FcεRI‐mediated basophil responsiveness,172 though further validation is required to elucidate the underlying mechanism fully.
In addition to its effects on basophils and mast cells, AIT has also been shown to modulate dendritic cells (DCs) and innate lymphoid cells (ILCs). DCs are specialized antigen‐presenting cells that play a role in initiating and sustaining allergic inflammation. DCs are also known to support the induction of tolerance through the regulation of T‐cell responses. The pivotal role of DCs was reflected in a recent study illustrating that classical DCs are involved in transporting sublingual antigens to the lymph nodes hence inducing antigen‐specific regulatory T cells.175 In addition to this, in vitro stimulation of DCs with vitamin A metabolite, retinoic acid, was shown to cause DCs to acquire a tolerogenic phenotype characterized by the expression of IL‐10, TGF‐β, and IL‐27.176 In addition to the effect of AIT on DCs, AIT has also been illustrated to act on ILCs. A subset of ILCs, known as group 2 ILCs (ILC2s), plays a vital role in mediating allergic inflammation, and this was evident from their rapid induction in the peripheral blood following cat allergen provocation test.165
Additionally, a cross‐sectional study involving grass pollen SCIT‐treated patients illustrated elevated levels of ILC2 in grass pollen–allergic patients compared to healthy control during the grass pollen season and that this seasonal increase is inhibited following SCIT treatment.51 This observation was confirmed in a more recent study involving HDM SCIT patients.177 Like DCs, retinoic acid‐skewed ILCs have also been shown to possess a tolerogenic phenotype, characterized by the expression of IL‐10.
Effect of AIT on adaptive immune response
AIT has been shown to induce regulatory T and B cells with immunoregulatory capacity. SCIT has been demonstrated to stimulate the local expansion of natural FOXP3+CD25+ Tregs in the nasal mucosa of treated patients.178 SLIT is associated with epigenetic changes in Tregs that includes hypomethylation of the FOXP3 promoter region and that this is responsible for the suppressive function of Tregs.179 Moreover, grass and birch pollen immunotherapy has been associated with the induction of IL‐10‐producing Tregs.180, 181 The generation and increase of another subset of inducible Tregs such as IL‐35‐inducible Tregs (iTR35) were demonstrated in patients who underwent grass pollen SLIT.169 The underlying mechanism of AIT on the adaptive immune response is also reflected in its actions on other cells that includes allergen‐specific Th2 (Th2A) cells, a novel subset of cells characterized by high expression of CD161 and CD49d, in addition to the classical Th2‐related surface markers.182 It was demonstrated that this novel subset of cells is significantly reduced following grass pollen immunotherapy183 and following oral peanut immunotherapy.184 Long‐term SCIT and SLIT studies to grass pollen showed that clinical improvement was associated with a decrease in allergen‐specific CRTh2+CCR4+CD27‐CD4+ Th2 cells.152 Finally, other novel subsets of T cells such as Tfh cells or T follicular regulatory (Tfr) cells have been linked to the maintenance of peripheral tolerance following AIT. While a recent study showed that impairment of Tfr cells may contribute to aberrant production of IgE antibodies in patients with allergic rhinitis and that AIT improves this defect,185 further validation is required.
A growing number of evidences point toward the role of regulatory B (Breg) cells in mediating AIT efficacy. Bregs are cells with immunosuppressive capacity, as demonstrated by their ability to release IL‐10 and enhance IgG4 production.186 It has been shown in a previous study that an increase in the frequency of IL‐10‐producing Breg cells was observed in both bee venom‐allergic patients who are undergoing AIT treatment as well as non‐allergic beekeepers exposed to a high dose of the allergen.38 This evidence suggests the potential role of Bregs in the initiation and maintenance of immune tolerance induced by AIT; however, further validation is needed.
Novel approaches
AIT is currently considered as the only clinically effective treatment of IgE‐mediated allergic diseases displaying long‐term clinical benefits. Despite the progression of research to improve the efficacy of AIT, it is still associated with drawbacks that pose a major problem for the use of AIT. This includes adverse effects such as anaphylaxis and poor patient compliance due to the long treatment regime involved in AIT. These drawbacks highlight the need for the development of novel therapeutic strategies to improve efficiency with reduced side effects. The use of adjuvants, in combination with AIT, to enhance their immunogenicity and reducing allergenicity and unwanted reactions, has been studied extensively. This includes the use of aluminum hydroxide, microcrystalline tyrosine, monophospholipid A (ie, TLR‐4 agonist187, 188, 189 and TLR‐9 agonist190), and calcium phosphate, which have yielded positive results in adults, though less is known about their use in children.
Efforts are being invested in identifying novel approaches of AIT that may provide a better therapeutic approach for the treatment of allergic disease. While conventional AIT uses purified whole‐allergen extracts191 or recombinant allergens,192 more recent studies have attempted the use of other molecules, though many of these need further validation in a clinical trial. This includes the use of biologics (ie, anti‐IgE) in combination with AIT, which has been shown to provide a safer profile and maintained effectiveness in children.193, 194 One promising candidate that has surfaced in more recent years, however, is the use of short linear peptides as SCIT. A recent randomized, double‐blind, placebo‐controlled trial involving grass pollen–allergic patients illustrated that a 3‐week short course of adjuvant‐free hydrolysates of Lolium perenne peptide (LPP) immunotherapy over 4 visits is safe and well tolerated.40, 195, 196 The study also illustrated that peptide AIT reduces combined symptom and rescue medication scores throughout the pollen season in the active group compared to placebo. Follow‐up mechanistic study demonstrated that the mechanism behind the efficacy of peptide AIT involves immune modulation of T‐ and B‐cell compartments.197 Whether the efficacy of peptide AIT can also be translated in pediatric subjects remains to be validated.
Conflict of interest
The authors declare that there is no conflict of interest in relation to this article.
Conclusions
Extensive clinical and research studies have been invested in the past decades to elucidate the mechanism of action, safety, and efficacy of AIT for allergic rhinitis in adults and children. Clinical trials have shown the potential role of AIT not only as a long‐term disease‐modifying treatment for allergic rhinitis, but also as a preventative measure in both respiratory and food allergy. Exploring the benefits of AIT in children will not only provide a therapeutic approach for the treatment of allergic rhinitis but also provide the possibility of intervening the early phase of disease progression. This was reflected in several studies of pediatric subjects where AIT treatment resulted in delayed onset of asthma. Despite the progression, many unanswered questions remain especially in pediatric subjects, highlighting the unmet need in AIT and the development of well‐designed trials, with the key aim in delivering personalized medicine. A deeper understanding of the underlying mechanisms of action of AIT will improve not only the current therapeutic strategies but also forward novel development. In parallel to this, an improved diagnostic tool may allow a more precise diagnosis for better AIT prescription. Future longitudinal, prospective, and well‐designed clinical trials are awaited to validate the current strategies of AIT further and to assess and investigate novel approaches of AIT.
3 Biomarkers for Allergen Immunotherapy: Antibody Responses and Digital Health Systems
Corresponding author
Paolo Maria Matricardi
Dept. of Paediatric Pneumology and Immunology and Intensive Care Medicine
Charitè Medical University
Augustenburgerplatz, 1
13353 Berlin, Germany
+49 30 450 566 406
+49 30 450 566 931
Email: paolo.matricardi@charite.de
Abstract
Several studies have demonstrated that serum IgE antibodies to certain allergenic molecules can be used as biomarkers for the prescription of allergen immunotherapy (AIT). Individual sensitization profiles may provide significant information for A) the detection of genuine sensitization to an allergen source (eg, Phl p 1 for Phleum pratense); B) a potential diagnosis of oral allergy syndrome (eg, Phl p 12; profilin); C) the risk assessment of allergic asthma (eg, Phl p 7); or D) the prediction of side effects (eg, polymolecular sensitization including Phl p 12 for SLIT). Recent studies in mite‐allergic patients also suggest that the IgE sensitization profile and local (nasal) IgG4 responses to specific allergenic molecules may be useful in predicting the efficacy of AIT. Further studies are needed to validate this interesting hypothesis. However, the analysis of serological biomarkers alone is not sufficient to complete a diagnostic workup in allergic patients. Linking test results to the individual clinical phenotype is fundamental and has so far often been performed using laborious paper diaries. Mobile health technologies now offer valuable tools to assess the clinical relevance of serological test results. The combined use of molecular biomarkers (component‐resolved diagnostics) and prospective monitoring of patient symptoms via e‐Diary will likely chaperon allergy practice into the new era of precision medicine.
Keywords
Allergen, Allergen Immunotherapy, Allergic rhinitis, Biomarker, Diagnosis, Digital Health, Immunoglobulin E, Immunoglobulin G, Monitoring, Prediction, Safety.
Introduction
Allergen immunotherapy
To date, allergen immunotherapy (AIT) represents the only curative path for allergic diseases, as it targets the underlying antigen‐specific immunologic mechanisms. Since the first use of pollen extract in a therapeutic approach, performed by Noon more than 100 years ago,198 AIT has evolved exceedingly. Allergen content, vehicles and adjuvants, route and schedule of administration have been improved according to study outcomes. Parallelly, production and documentation requirements have become significantly more complex.199 Albeit the conventional routes of administration remain subcutaneous immunotherapy (SCIT), sublingual immunotherapy (SLIT), and oral immunotherapy (OIT), new approaches, such as epicutaneous and intralymphatic applications, are under investigation.200, 201 Moreover, AIT has been used in a wide range of allergic disorders and their different constellations, such as allergic rhinitis, allergic conjunctivitis, and allergic asthma as well as food, venom, and drug allergies.2 Despite this long history and the broad range of possible applications, the role of AIT as a therapeutic approach to allergic diseases remains strongly debated with only a minority of eligible patients receiving this kind of treatment.
Definitions of biomarkers
In a rigid definition, biologic markers are molecules that initiate physiologic or pathologic phenomena.202 They reflect an objectively quantifiable measure of disease expression, severity, and/or response to therapy. Generally, biomarkers are restricted to molecules measured in blood and/or other body fluids. Alternatively, though anatomical and structural parameters obtained through imaging, functional, histologic, and cellular tests, as well as genetic polymorphisms, RNA expression, or the assessment of clinical disease severity with standardized tools can also serve as biomarkers.203 They can be beneficial in many different settings, especially in diagnostic processes and disease staging, identifying patients who will benefit from the treatment, monitoring disease trends, treatment efficacy and its side effects, predicting long‐lasting protection, and thus improving acceptance and compliance.
Focus on respiratory allergies and specific antibodies to allergen molecules
A crucial (and still unmet) step to implement AIT in clinical settings is the identification of objective biomarkers in order to initiate and monitor AIT in the context of precision medicine.2 An EAACI Task Force on “Biomarkers for monitoring the clinical efficacy of allergen Immunotherapy” concluded that those biomarkers should be classified into seven domains.112 The first two domains being IgE (total IgE, specific IgE, and specific IgE/total IgE ratio) and IgG subclasses (sIgG1 and sIgG4, including the specific IgE/IgG4 ratio), while the other domains include IgE serum inhibitory activity, basophil activation, cytokines and chemokines, and cellular and in vivo markers.112 Considering this diversity, the present review focuses comprehensively on two aspects: the diagnostic relevance of antibody responses in the serum and their relevance for clinical practice, facilitated by mobile health technologies.
Antibodies as diagnostic biomarkers
Sensitization to species‐specific and cross‐reactive molecules as diagnostic biomarkers
Grass pollen allergy represents a typical example for the use of allergen‐specific IgE as a biomarker, not only for an etiological confirmation of the presumptive diagnosis but also for the subsequent prescription of AIT. Patients with symptoms of allergic rhinitis during the grass pollen season and a positive SPT/IgE response to grass pollen extracts are further investigated in order to detect serum IgE antibodies to Phl p 1, Phl p 2, Phl p 5, Phl p 7, Phl p 11, and Phl p 12. The identification of IgE antibodies toward one or more of the molecules Phl p 1, Phl p 2, Phl p 5, and/or Phl p 11 is then followed by the prescription of grass pollen‐AIT. The presence of IgE to Phl p 12 (profilin), however, is followed by an investigation of a potential oral allergy syndrome (OAS) including SPT/IgE assays with other pollen, fruit, or vegetable extracts. Furthermore, the identification of IgE to Phl p 7 alerts the doctor of a greater severity of disease including a higher risk of asthma.115
The impact of molecular assays on doctors’ decisions
Distinguishing a potentially underlying co‐sensitization from cross‐sensitization presents a major advantage of CRD in allergology.204, 205 A study among 651 Italian children with moderate‐to‐severe pollen‐related allergic rhinitis focused on this aspect. In the sera of a significant number of patients with a positive clinical history for pollen allergy and concordant SPT results (ranging from 69% for mugwort allergic children to 10% of those with allergic reactions to grass pollen), no specific IgE antibodies to the major expected allergens were detectable. After considering also the CRD results, the SPT‐based decision on specific immunotherapy prescription or composition was adapted in 277 (42%) or 315 (48%) children according to the European or American approach, respectively.206 This study demonstrates a high effect of CRD on the prescription and composition of AIT, especially in geographic areas with frequent polysensitization to airborne allergens. Similar conclusions were reached in a study of sensitization patterns to grass and olive pollen allergen molecules in 1263 Spanish patients. All subjects suffered from seasonal allergic rhinitis and positive SPT responses to grass and olive pollens. Based on a traditional diagnostic approach, 922 (73%) patients would have received an AIT prescription with both, grass and olive pollen. Based on additional IgE results obtained by CRD, though, the investigators changed the composition of AIT in 56.8% of the patients.207 As a consequence of the so‐called “molecular spreading” process, subjects with allergic multimorbidities are more likely sensitized to a multitude of allergen molecules.208 While being less responsive to allergen immunotherapy, these subjects are easily identifiable by serum IgE testing with microarray technology and might benefit from a more comprehensive anti‐IgE treatment.209 To our best knowledge, specific trials on the impact of anti‐IgE therapy in extremely polysensitized asthma patients still need to be performed.
Antibodies as biomarkers predicting efficacy and safety
The heterogeneity of the molecular sensitization profile
A cross‐sectional study done in Italy with 176 children suffering from grass pollen allergy demonstrated the vast amount of additional information that molecular diagnostic tests can provide compared to extract‐based diagnostic tests. In terms of quality, conventional ELISA based on the allergenic extract of Timothy grass generated similar profiles for each patient. Albeit exhibiting different degrees of amplitude, all sera were positive for Timothy grass. When using a molecular assay, the alleged homogeneity was replaced by a remarkable diversity of responses. Overall, a series of 39 different IgE sensitization profiles to grass pollen molecules could be observed.210 Another cross‐sectional study with 1120 children even found 82 different profiles.211 Both studies included patients sensitized to only one molecule (in most cases Phl p 1), as well as those sensitized to 5 of the examined 8 allergenic molecules. Differences in the molecular sensitization profile were also linked to clinical phenotypes. Phl p 7 for instance served as a reliable biomarker for asthma and could be associated with a greater severity of seasonal allergic rhinitis.211 By contrast, Phl p 12 served as a biomarker of oral allergy syndrome (OAS).211 A German study group among 101 adult patients with pollinosis compared molecular IgE results to P pratense molecules with nasal and conjunctival provocation tests. The group described not only a substantial heterogeneity in sensitization profiles, but also a positive correlation between the number of recognized molecules and the likelihood of a positive provocation test result. Interestingly, none of these IgE profiles matched exactly the composition of a previously published component‐resolved specific immunotherapy containing Phl p 1, Phl p 2, Phl p 5a/b, and Phl p 6.212 Similarly, the individual sensitization profiles of 119 patients with house dust mite allergy from the German MAS cohort were extremely heterogeneous at the age of 20 years, with 27 subjects responding to only one molecule (monomolecular profile), 50 subjects responding to 2 to 4 molecules (oligomolecular profile), and 42 subjects producing IgE to 5 or more of the 12 tested molecules.213
The heterogeneity of the AIT preparations
A consistent composition combined with a stable potency related to clinical efficacy is the major requirement for a standardization of allergenic extracts. However, the Monograph on Allergen Products, a European regulation, permits a wide range of variation.214 The representation of individual allergenic molecules in an extract can vary from 50% to 200% as measured by IgE inhibition tests.214 A relatively simple allergen extract containing, for example, only 3 major allergenic proteins can thus have different batches with different allergen content, ranging from very low to very high, including all intermediate possibilities. Reflecting these regulations, allergen extracts from different manufacturers differ to a great extent in their molecular composition and potency, although they represent the same allergen sources. This underlines that allergenic extract standardization remains difficult, if not impossible.215, 216, 217 Translating this problem into the daily clinical practice, divergent SPT wheal reactions for the same allergen species in the same patient elicited by different allergen extracts are not surprising.214, 216 The variability of in vitro IgE test results to extracts of the same allergen source can in part similarly be attributed to the extract composition.218 Standardization of allergen extracts both for diagnosis and for therapy hence remains an issue in allergology.
Matching and mismatching between IgE molecular sensitization profiles and AIT composition
A relevant and yet unanswered question is whether sensitization profiles affect the individual outcomes of AIT. Despite the significant interest for patient‐tailored immunotherapy,219, 220 there is still no product available in the commercial market. Whether patients with different sensitization profiles respond differently to the same allergen‐specific immunotherapy or not remains to be answered. A theoretical approach was presented in a study that compared all possible combinations between the molecular sensitization profiles of 176 study participants and the molecular profile210 of an allergen AIT preparation previously clinically tested for Timothy grass pollen allergy.221 Five categories of molecular matching/mismatching between the patients’ IgE sensitization profile and the molecular composition of an AIT preparation have been exhaustively analyzed and described (Table 1)210:
Table 1.
Molecular matching and mismatching in AIT
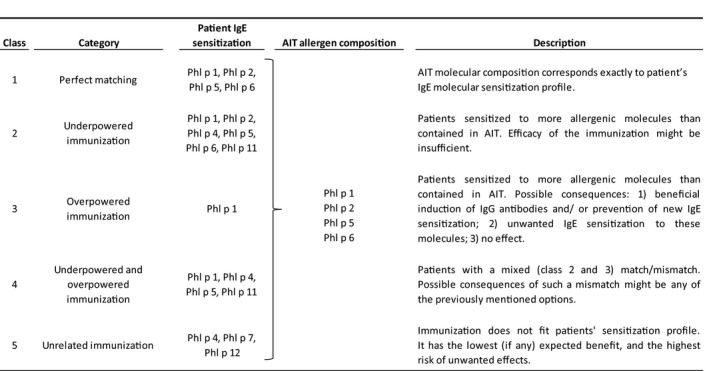
Class 1: Perfect matching—The molecular composition of the AIT corresponds exactly to the patient's IgE molecular sensitization profile.
Class 2: Underpowered immunization—Some patients were sensitized to more allergenic molecules than the molecules contained in the immunotherapy. In this case, the efficacy of the immunization might be insufficient.
Class 3: Overpowered immunization—The immunotherapy preparation contains more molecules than the individual patient's sensitization profile. Three consequences might result: a beneficial induction of IgG antibodies and/or prevention of new IgE sensitization,221 an unwanted IgE sensitization to these molecules,222 or no effect at all.223, 224
Class 4: Underpowered/overpowered immunization—This category includes patients with a mixed (type 1 and 2) match/mismatch. The expected effects of such a mismatch might be any of the previously mentioned options.
Class 5: Unrelated immunization—In this case, the immunotherapy preparation does not match the sensitization profile of the patient. Consequently, this category is associated with the lowest expected benefit as well as the highest risk of unwanted effects.
Yet, there has not been an analysis with enough statistical power to test the above‐mentioned combinations of matching/mismatching between IgE results and immunotherapy outcomes. Given the synergistic role of individual molecules in triggering IgE‐mediated degranulation of mast cells, the general immune response to specific immunotherapy might be rather linked to the overall concentration of IgE antibodies directed against an allergenic source than to the nature of the individual molecules that they recognize. The effect of multiple sensitizations on the efficacy of AIT has been examined at extract level.225, 226, 227 For example, a study has shown that SLIT for Dermatophagoides pteronyssinus and/or Dermatophagoides farinae improved nasal symptoms and rescue medication scores equally well in polysensitized and monosensitized patients with allergic rhinitis.228
Antibodies as biomarkers predicting AIT efficacy and safety
Allergenic molecules have been used in various clinical studies to monitor changes in the specific antibody repertoire of treated patients and have shown good results.229, 230 In a recent study, the efficacy of SLIT in mite‐allergic patients was proven to be highly influenced by the IgE sensitization profile before the start of AIT. Among the full set of house dust mite (HDM)‐allergic participants including all IgE variabilities, no efficacy of a HDM‐SLIT could be shown.231 If, by contrast, only patients with IgE to Der p 1 or Der p 2 had been included in the analysis, a positive outcome would have resulted.231 These conclusions therefore seem to confirm a lower efficacy of AIT in patients with stronger molecular spreading, as previously theorized (“mismatch type 1, underpowered immunization”; Table 1).210, 232 This recent study emphasizes that the use of molecular assays for the prediction and monitoring of AIT efficacy is a promising approach. However, more investigation is needed to consider IgE antibodies as a predictive biomarker of efficacy.233 Interestingly, it has also been shown that a co‐sensitization to Phl p 5 and Phl p 12 predicts the incidence of side effects during AIT.234
IgE antibodies as biomarkers in monitoring the response to AIT
Previous reviews and a position paper on biomarkers for AIT have mostly shown disadvantages of the use of IgE antibody levels in monitoring the response to AIT.167 IgE concentrations tend to increase during the up‐dosing phase of the treatment and decline years after. Yet, no correlation with the clinical response to AIT has been found at an individual level. It is still debated whether the ratio between specific and total IgE may be used as a parameter to predict and monitor AIT efficacy with a need for more studies on this matter. Moreover, the units used to quantify the two parameters (total and specific IgE) are not necessarily comparable. It is also unknown whether the concentration and/or specific activity of IgE antibodies are more significant at local level (eg, in the nasal mucosa or in the nasal secretions) or in the serum.112, 167
IgG antibodies as biomarkers in monitoring the response to AIT
The genesis of blocking IgG antibodies plays an essential role in inducing protection against IgE‐induced allergic reactions.235 Their blocking capacity is defined as competition with IgE for the same epitopes with a resulting inhibition of degranulating cell activation.236, 237 IgG1 antibodies, produced mostly upon natural exposure to allergens, may play a protective role, and their dimension and persistence are still debated. A recent study investigating the immunomodulatory effects of a prophylactic sublingual house dust mite (HDM) immunotherapy in HDM‐sensitized, yet non‐allergic, children could show a broader epitope repertoire of IgG antibodies in treated children versus those receiving placebo.238 Interestingly, this was not the case for IgE or IgG4 antibodies. Nevertheless, an artificial, repeated exposure to allergens, such as the administration of AIT, has also repeatedly been shown to induce allergen‐specific IgG4 antibodies. The blocking capacity of IgG4 antibodies has been postulated as one of the major mechanisms of AIT in respiratory allergies and IgE‐FAB assays allow the assessment of their inhibitory function at individual level.237, 239, 240 While the sole increase in serum‐specific IgG4 levels could not be confirmed as a biomarker of AIT efficacy at the individual patient level, functional assays have shown promising results in identifying responders to treatment,237, 240 especially in the maintenance phase of HDM SCIT.240 Further, an increase in concentration of IgG4 antibodies against the offending allergen measured at local (nasal) level has been reported to predict the efficacy of grass pollen immunotherapy.241 Although the testing of IgG4 antibodies is still considered irrelevant for the assessment of AIT efficacy in clinical practice, it might bring an advantage for monitoring both the molecular composition of the AIT preparation and the patient's compliance.167
Molecular Allergology and Digital Health: from guidelines to clinical practice
IgE sensitization and its clinical relevance
Establishing, even with extreme precision, genuine IgE sensitization profiles to a pollen is not sufficient for AIT prescription. A causal association between exposure to the suspected allergen and allergic symptoms must be established before the planning and administration of a long‐term, demanding treatment such as AIT. The occurrence of symptoms within the pollen season is normally considered a sufficient condition to start AIT.201 However, the etiological diagnosis of seasonal AR is difficult in polysensitized patients living in geographic areas with overlapping pollination seasons.205, 227 Nasal challenge tests or controlled exposure in a pollen chamber is thus useful to demonstrate that the contact with a pollen is able to trigger allergic symptoms.201 The implementation of these tests, though, is mostly limited to ENT specialists and few centers where they have to be performed under safe conditions.
Symptoms monitoring, pollen trajectories, and electronic diaries
Prospectively collected data are likely more reliable than a retrospective clinical history, which may refer to a time point several months before the doctor′s visit. The precise matching of retrospectively collected clinical information to exposure data is very difficult. By contrast, the prospectively assessed symptom load can be easily matched to the daily pollen concentration. Moreover, changes over time in disease severity can be longitudinally matched to parallel variations in allergen exposure. Hence, a clinical‐environmental diary can be very helpful in demonstrating the clinical relevance of a positive serological biomarker. In this respect, standardized digital questionnaires may serve as a facilitating tool to collect symptom data as a marker of disease activity.
The recording of symptom scores (SS) or symptom medication scores (SMS) has been proven successful in clinical trials regarding the efficacy and safety of AIT for many decades.242 During the last 15 years, several different scores were developed to measure the severity of allergic rhinitis (AR). Different disease severity scores tend to give similar results at population level but can often produce heterogeneous slopes in individual patients.243 A consensus has been recently found on a “Combined Symptom Medication Score” (CSMS) that takes into account the impact of medication on symptom manifestation during the pollen season.89 Clinical diaries have been long documented on paper, requiring considerable efforts, time, and calculations for their interpretation. Therefore, the use of paper symptom diaries was often sporadic in routine clinical practice.244 Recently, the use of Internet‐based information platforms and the electronic format of disease severity scores have made the monitoring of respiratory allergies much easier and user‐friendly.245
Trajectories of allergic symptoms: objective diagnostic biomarkers?
In the last decade, several groups have accumulated experience in the use of mobile health (mHealth) technologies to monitor allergic rhinitis and wheezing disorders in childhood.245, 246, 247, 248, 249, 250 While algorithms have been tested to help clinicians improve the treatment of allergic rhinoconjunctivitis and select potential candidate patients for an immunotherapy,249 other systems aim at assessing individual disease severity over time,250 which can also be a useful tool for measuring AIT efficacy. Our group, on the other hand, described cases of patients sensitized to multiple seasonal allergens with overlapping pollination periods, in which the selection of the eliciting allergen source for a targeted AIT prescription was difficult to impossible. A diagnostic solution had been offered by asking patients to register their symptoms on a daily basis during the spring season using a smartphone with a dedicated electronic clinical diary app. This type of apps record the individual trajectories of disease severity via different symptom scores (eg, the Rhinoconjunctivitis Total Symptom Score (RTSS)) and allows their comparison with the trajectories of locally relevant pollen counts.245, 246
To show the potential use of prospective digital symptom recording, Bianchi et al. described two clinical cases in which SPT with allergenic extracts was positive (wheal diameter >3 mm) in both patients for many pollen including olive and timothy grass, whose pollination period in Ascoli, Italy, is between April and June.246 Representing a common scenario in this region, molecular IgE results (UniCAP, Phadia, Sweden) in both cases provided evidence of co‐sensitization (cutoff ≥0·35 kU/L) not only to the major allergenic molecules of olive (Ole e 1) and timothy (Phl p 1, Phl p 5), but also to cross‐reacting molecules (Phl p 12, profilin; Phl p 7, polcalcin).246 However, when comparing the RTSS and the pollen count trajectories of the individual patients, the peaks of symptom severity clearly differed. While the time of most severe symptoms of the first patient coincided with the peak of olive pollen counts, the second patient experienced more severe symptoms during the peak period of grass pollen (Figure 1). Based on these data the attending pediatrician changed her initial idea of AIT prescription for olive pollen in the first patient and for grass pollen in the second one. The prospective and consistent recording of nasal and conjunctival symptoms during the pollination period has therefore contributed fundamentally to the identification of the triggering pollen (olive pollen in the first case; grass pollen in the second).246
Conclusions
The use of in vitro antibody assays as biomarkers for allergen immunotherapy in the area of respiratory allergies varies in relevance according to the target of investigation. Testing IgE antibodies specific for genuine and cross‐reactive molecules can be essential to consider a particular allergen source as the putative cause of allergic symptoms. A causal association between allergen exposure and symptoms may then be demonstrated by individual prospective symptom and exposure monitoring over time. This challenging data collection and interpretation has recently been significantly facilitated by mobile health technologies.
Conflict of interest
P. M. Matricardi is a consultant for Hycor, Euroimmun, and Novartis; has received research funding from the Deutsche Forschungsgemeinschaft (DFG; grant no. MA‐4740/1‐1), Hycor Biomedical, and Euroimmun and reagents for research from Thermo Fisher; and receives speaker's fees from Euroimmun, Thermo Fisher Scientific, Stallergenes‐Greer, and HAL Allergy.
4 Preventive Effects of Allergen Immunotherapy on Allergic Diseases
Corresponding author
Susanne Halken, Hans Christian Andersen Children's Hospital, Odense University Hospital, Odense, Denmark Email susanne.halken@rsyd.dk Tel. +45 65415967
Abstract
Allergen immunotherapy (AIT) is a disease‐modifying treatment for IgE‐mediated allergic disease with effects beyond cessation of AIT that may include important preventive effects. The European Academy of Allergy and Clinical Immunology (EAACI) published a clinical practice guideline for AIT including the prevention of development of allergic diseases. AIT reduces allergic rhinitis (AR) symptoms and concomitant medication need in patients with AR and pollen allergy. Additionally, there is now good evidence for an asthma preventive effect for AIT in children with AR and pollen allergy, primarily birch and grass. This has not been demonstrated in other populations nor with other allergens such as house dust mites. This effect is well documented to last until 2 years post‐AIT. Some studies suggest that it may last longer depending on which asthma‐outcome parameters are applied. There is no sufficient evidence for AIT for prevention in healthy individuals nor in populations with other allergic conditions. Currently, the indications for AIT for prevention of allergic disease are linked to those for treatment of pollen AR. As the asthma preventive effect may reduce the burden and costs of the disease, the AR severity at which AIT becomes indicated in children and adolescents with AR and pollen allergy may be reduced. The preventive potential of AIT should be included in decision making for AIT and should be discussed with the patients. The potential for prevention of new allergic conditions in other populations needs to be investigated further.
Keywords
Allergen immunotherapy, allergic diseases, allergy, atopy, prevention, sensitization, asthma, allergic rhinitis; atopic dermatitis, atopic eczema
Abbreviations
AD – Atopic dermatitis (atopic eczema)
AIT – Allergen immunotherapy
AR – Allergic rhinitis / allergic rhinoconjunctivitis
ARIA – Allergic Rhinitis and its Impact of Asthma
HDM – House dust mite
RCT – Randomized controlled trial
SCIT – Subcutaneous immunotherapy
SLIT – Sublingual immunotherapy
SR – Systematic Review
Introduction
Allergic diseases are among the commonest chronic diseases and include atopic eczema/dermatitis (AD), asthma, allergic rhinitis / rhinoconjunctivitis (AR), food allergy, and venom allergy.251, 252, 253, 254, 255 Allergic diseases can cause a considerable burden to individuals with impaired quality of life256 and an economic burden to the patients and the society.257, 258 They frequently start in early childhood, continue throughout adulthood, and are characterized by a high degree of comorbidity.251 Family history of atopy is a risk factor for development of allergic disease, and children with sensitization and/or early manifestation (eg, AD and food allergy) or later manifestation (eg, AR) of atopic diseases have a higher risk for development of other allergic manifestations, such as asthma.259, 260, 261 In addition, childhood AD and AR are strongly associated with persistence and severity of allergic asthma into adulthood.262, 263
Allergen immunotherapy (AIT) has been used for the treatment of allergic diseases for more than 100 years. There is good evidence for the clinical efficacy of AIT for AR, allergic asthma, and moderate‐to‐severe venom allergy.10, 69, 91 These may even lead to a sustained reduction in symptoms and requirement for symptomatic treatment beyond cessation of AIT.10, 69, 91 Furthermore, AIT has the potential to induce immunologic changes that result in immune modification.88, 264 It also has a potential preventive effect88, 265, 266, 267 in reducing the risk of developing asthma in children with AR.268, 269, 270, 271 Accordingly, AIT has recently been considered as a possible preventive strategy in the treatment of allergic diseases.
This review is based on the European Academy of Allergy and Clinical Immunology (EAACI) Systematic Review (SR)90 and Guideline on Allergen Immunotherapy for prevention of allergic disease published in 2017.87 The aim is to provide an overview and to discuss the current evidence for the use of AIT for the prevention of the development i) the first allergic disease, ii) further allergic comorbidities in those with established allergic disease, and iii) the development of new allergic sensitizations.
AIT for prevention of allergic diseases
Possible strategies for using AIT in the prevention of allergic diseases
Strategies to prevent development of a new allergic disease by AIT may vary for different populations and at different stages in life. Such preventive strategies need to be pursued for different scenarios. For example used in the EAACI Guideline are:
Those planning pregnancy to take measures such as AIT to reduce the risk of their child becoming allergic.
Healthy infants, children, and adolescents/adults.
Infants and young children with early manifestations of allergy such as AD.
Older children / adolescents who manifest allergic disease such as AR.
If AIT is to be recommended for the prevention of allergic diseases, evidence is required that there is a relevant and substantial beneficial effect on clinical outcomes for the individual. Furthermore, safety aspects related to AIT, the severity and discomfort/risk of the disease to be avoided, quality of life, patient experience, and health economics should be evaluated. Thus, an optimal balance between benefits, harms, costs, and other possible disadvantages should be achieved.
What is the evidence for using AIT for the prevention of allergic diseases?
For those planning a pregnancy, to reduce the risk of their child becoming allergic: There is only one very low quality, case‐control study. This compared children of at least one allergic parent whose parents did or did not receive AIT at least nine months before birth.272 This study found a significantly lower risk for developing any allergic disease and asthma in children of allergic parents after AIT compared with the controls. The authors hypothesized that AIT in allergic parents might reduce the risk of allergies in their offspring but this requires further investigation. Currently, there is not sufficient evidence for or against using AIT for allergic adults for the prevention of allergic disease in their offspring.
For healthy individuals: Only two randomized controlled trials (RCTs) investigated the effect of AIT in healthy individuals on the risk for development of their first allergic disease. A large (n = 111), low risk of bias RCT273 found no preventive effect of oral house dust mite (HDM) AIT on AD, wheeze, and food allergy among infants with a family history of allergic diseases at 1 year of age, whereas a small (n = 29), high risk of bias study,274 reported a reduced risk of developing symptoms of pollinosis among asymptomatic adults sensitized to Japanese cedar pollen in the SLIT group. Thus, there is currently no good evidence for or against the use of AIT for the prevention of the development of first allergic disease in healthy individuals.
For infants and young children with early manifestations such as AD: A recent SR90 found only one low risk of bias RCT. It investigated the effects of 12 months of daily SLIT with a mixture of HDM, cat, and grass allergens for the prevention of asthma and new sensitizations in 12‐ to 30‐month‐old children (n = 50) with AD and food sensitization.275 Due to lack of a priori immunologic changes indicating that the treatment was not delivering sufficient allergen transmucosally to trigger immunologic recognition, recruitment was interrupted and the trial reduced to a pilot study status. After 48‐month follow‐up, no differences in asthma prevalence or new sensitization to aeroallergens were seen between the two groups.275 It can be concluded that there is not sufficient evidence for AIT for the prevention of the development of a first allergic disease in individuals with AD at present. More studies are needed.
Older children/adolescents with manifest allergic disease such as AR: The EAACI SR90 identified six RCTs of varying quality investigating the preventive effect up to two years post‐AIT with one or more of grass, birch, Parietaria, and/or HDM allergens on the development of new asthma in individuals with AR. The SR and meta‐analysis90 demonstrated a significant preventive effect of AIT on the development of new asthma up to two years post‐AIT in patients with AR. Subgroup analyses showed that AIT with either SLIT or SCIT was beneficial for those aged <18 years but not ≥18 years and for pollen AIT only. For HDM AIT, there was a non‐statistically significant impact despite an OR of 0.20, which may be because the groups were too small. The results are supported by four large‐scale, real‐life, retrospective, non‐randomized studies71, 94, 95, 276 based on German and French longitudinal prescription databases. These all report a preventive effect of AIT with birch / grass pollen allergen on the progression from AR to asthma.
For the long‐term preventive effect, that is, two or more years post‐AIT, the EAACI SR90 identified two high risk of bias SCIT RCTs145, 277 in patients with AR and birch/grass and HDM allergy. Both showed a significantly lower risk for developing asthma in the SCIT groups as compared to the controls, up to seven,145, 278, 279 and two years post‐AIT.277 Asthma was diagnosed by clinical criteria by the investigators. A large recently published low risk of bias RCT, the pediatric Grazax Asthma Prevention (GAP) study,58, 280 investigated the effect of a three‐year course of grass SLIT tablets on the development of asthma in 812 children (age 5‐12 years at inclusion) with AR and grass pollen allergy but without any signs of asthma. This study58 failed to demonstrate the preventive effect of AIT on the development of asthma as defined by very strict a priori criteria including reversibility to beta‐2‐agonists (OR = 0.91; 95% CI [0.58 to 1.41])58, 280 during treatment and 2‐year follow‐up post‐AIT. However, the number of subjects with asthma symptoms or asthma medication usage was significantly lower in the SLIT group compared to the placebo group during the entire five‐year trial period, the two‐year post‐AIT follow‐up, and at the end of the five‐year trial period (OR 0.66; 95% CI 0.45 to 0.97; p < 0.036). In this study, the preventive effect appeared to be strongest for the youngest children.58 Two high risk of bias non‐randomized studies, including one with grass pollen SCIT270, 271 and one with HDM SCIT,281 in children with AR also suggested a long‐term effect up to 12 years post‐AIT. As published in the SR,90 the meta‐analysis showed no overall evidence of reduction in the risk of developing asthma long‐term, at least two years post‐AIT. However, there was a high degree of heterogeneity and the negative result was due to one RCT with very strict diagnostic criteria for the primary outcome parameter but where there was a significant reduction in asthma symptoms and/or medication.3 Thus, there are indications that there may be a long‐term preventive effect on the development of asthma symptoms and the use of asthma medication. Further confirmatory studies are needed.
What is the evidence for using AIT for the prevention of new allergic sensitizations
The EAACI SR identified six RCTs investigating the effects of AIT on the risk of developing new sensitizations, up to two years post‐AIT. There were of varying quality, used with varying allergens and formulations and showed inconsistent results.273, 282, 283, 284, 285, 286 One low risk of bias RCT273 looked at oral HDM AIT for healthy infants at high risk of developing allergic disease. It found a significant reduction in sensitization to any common allergen in the active group compared with the placebo group after 1 year but no difference in HDM sensitization.273 The other two low risk of bias RCTs found no effect of SLIT on the development of new sensitizations in adult patients allergic to peach282 post‐AIT and after SLIT with grass pollen or HDM extract in monosensitized children.283 Another three RCTs of moderate to high risk of bias284, 285, 286 found a significantly lower incidence of new sensitizations among children and adults with AR treated with SLIT285, 286 and SCIT284 as compared to controls. In the EAACI SR,90 meta‐analysis showed an overall reduction in the risk of allergic sensitization but the sensitivity analyses, excluding the two high risk of bias studies,285, 286 failed to confirm this risk reduction.90 Due to the high degree of heterogeneity, the results should be interpreted cautiously.
For a long‐term effect at least two years post‐AIT, the SR identified two moderate287 to high risk of bias RCTs. There was no preventive effect of SCIT among children with moderate‐to‐severe asthma followed into adulthood287 and in adults with AR three years post‐AIT288 treated with a mixture of aeroallergens and grass pollen allergens, respectively. Another high risk of bias RCT277 found less new sensitizations among AR patients (>5 years old) treated with HDM SCIT compared with controls two years post‐AIT.277
Thus, there is currently no good evidence for or against preventive effect of AIT on the development of new sensitizations in any populations.
Safety
The safety issues of AIT for prevention have not been separately investigated. This applies to safety of AIT related to the indication and contraindications of AR.3, 10 SCIT is occasionally associated with allergic side effects and should therefore be administered in a specialist setting. Fatalities are very rare and have not been reported with the use of SLIT. Though the EAACI SR251 was not designed for the investigation of safety, it included 7 studies (6 SLIT and 1 SCIT) that reported either no increase in generalized itching or no major systemic side effects. In a recent meta‐analysis about the efficacy of grass pollen SLIT tablet in AR,289 no episodes of anaphylaxis was reported, despite seven treatment‐related severe adverse events treated with adrenaline being reported in the RCTs. In recent real‐life clinical studies of AIT, less severe systemic reactions were reported with SLIT than with SCIT, although the overall rate of adverse reactions is similar in SCIT and SLIT.290, 291 The safety profile for prevention is regarded as being similar to that of AIT for treatment of AR.
Using AIT for the prevention of the development of new allergic disease or sensitization requires the use of products and schedules with a high level of safety, especially in healthy individuals. However, if AIT is indicated due to treatment of an already existing allergic disease, and the preventive effect can be regarded as an additional effect meaning that the safety profile should be considered within that context.
Products, SCIT or SLIT, and schedules
The products, doses, and AIT schedules used in the AIT prevention trials vary. No high‐quality trials compared different AIT products, SLIT drops versus tablets, or pre/co‐seasonal versus perennial AIT in children. However, there is a subgroup analysis in the EAACI SR90 and two lower quality, real‐life non‐randomized AIT treatment studies based on large German longitudinal prescription databases.71, 276 These suggest that SCIT and SLIT with grass and birch pollen allergens are both effective and that AIT for three or more years tended to have a stronger preventive effect than AIT for less than three years.
The current evidence does not allow us to identify whether or not SCIT and SLIT are superior in efficacy. The choice therefore depends on availability, patients / family's preferences, safety, costs, routes, schedules, and patient adherence to the AIT treatment.
When evaluating the studies that address the potential of AIT for prevention, the significant heterogeneity can be explained by the different study design, study population, products, and schedules used. Therefore, there is no “class‐effect” in AIT and an individual product‐based evaluation of the evidence for efficacy is recommended before treatment with a specific product is initiated.2, 3, 61, 87, 92, 267, 292
Clinical implications and discussion
Many children with AR and pollen allergy benefit from AIT with reduced AR symptoms and need for medication. Currently, AIT is recommended for the treatment of patients with moderate‐to‐severe pollen‐induced AR not optimally controlled on antihistamines and nasal corticosteroids.3 Additionally there is good documented evidence for an asthma preventive effect for AIT in children with AR and pollen allergy, primarily birch and grass. This is not yet the case for other populations nor other allergens such as HDM. This effect is well documented to last until 2 years post‐AIT. Some studies suggest that it may last longer depending on which asthma‐outcome parameter and study design is applied. This longer‐term preventive effect has been demonstrated only for asthma symptoms and/or medication and not if more strict criteria, such as demonstrated reversibility to inhalation of a β‐2 agonist, are applied. It remains to be determined as to which asthma‐outcome parameter is most relevant clinically, a diagnosis based on demonstrated reversibility or on symptoms and medication use.
This asthma preventive effect has been demonstrated in RCTs that included children with a history of AR, documented pollen allergy and the need for medication often for at least one previous season. Therefore, it can be assumed that they had persistent symptoms. However, there are no exact data on severity with children and adolescents included in these prevention studies not necessarily fulfilling the proper severity indications for AIT for the treatment of AR.293 In the most recent RCT (GAP study) including children older than 5 years, it appeared that the preventive effect was strongest for the youngest children.58 Consequently, it may be that in the future the indication for AIT in children with AR can be extended to milder disease. Furthermore, some patients with less severe AR may prefer AIT to reduce medication use and avoid side effects of other treatments, to obtain long‐term efficacy and/or to obtain the asthma preventive effect.
None of the studies on the prevention of the development of asthma in AR included preschool children. Hence, there is currently no good evidence for AIT for prevention in this age group.
Since the indication for AIT for the prevention of asthma is linked to the indication for treatment of AR, the products, schedules, and doses used should have proven effectiveness for AR with the relevant allergen product. Therefore, only those products with proven preventive capacities in the clinical documentation and in line with the indication for AR should be considered for use in allergy prevention.
The development of new sensitizations may impose a higher risk for the development of further symptomatic allergies. So it might be relevant to prevent the development of new sensitizations. At present, there is no good evidence for a preventive effect of AIT on the development of new sensitizations.
As described in the EAACI AIT Guideline for prevention, there are many gaps in the current evidence. Crucially, there is a lack of evidence regarding patient selection, such as optimal age and characteristics for preventive AIT, and for the optimal allergen preparation, mode, and duration of AIT administration. There is also a need to define standardized relevant outcomes including asthma and quality of life for future studies, which should be followed by an expert panel comprising academia, industry and regulatory authorities. Importantly, future studies should address the cost‐effectiveness of such preventive strategies.
Conclusion
Apart from the well‐documented clinical effect of AIT for the treatment of AR and pollen allergy, there is now also good evidence that AIT can prevent development of new asthma in children with AR and pollen allergy, especially grass and birch, at least up to two years post‐AIT. Currently, there is no evidence for an allergy preventive effect of AIT for other populations and allergens.
Currently, the indications and contraindications for AIT for prevention of allergic disease are linked to those for treatment of pollen AR. This is documented IgE‐mediated disease caused by pollen allergens and not sufficiently controlled by antihistamines and nasal corticosteroids. Meanwhile, the asthma preventive effect may in the future reduce the level of severity of AR required for the indication of AIT in children and adolescents with AR and pollen allergy.
Before initiating AIT, a patient‐centered discussion is required with the patient / family covering the beneficial effects on controlling AR symptoms as well as the asthma preventive effect, disadvantages, potential harms, patients’ preferences, patients’ adherence, and costs.
Box 1: Key points.
AIT for preventing the development of new asthma in children with AR
-
Children/adolescents with moderate‐to‐severe AR and grass/birch pollen allergy, not sufficiently controlled despite relevant pharmacotherapy, benefit from treatment with AIT with a sustained reduction in AR symptoms and medication. Additionally, there:
-
☐
is a reduced risk for development of new asthma up to two years post‐AIT (moderate‐to‐high quality evidence) with SCIT or SLIT
-
☐
are some data suggest that this benefit may persist after two years post‐AIT as regards asthma symptoms and medication use
-
☐
A beneficial preventive effect of AIT has not yet been sufficiently documented for other populations or other allergens such as house dust mite allergens.
AIT for three or more years seems to have a stronger asthma preventive effect than AIT for less than three years
The current evidence does not identify whether SCIT or SLIT is superior in terms of efficacy, so this choice depends on availability, patients / family's’ preferences, safety, costs, routes, schedules, and patients adherence to the AIT treatment and on the clinical documentation of the respective AIT product in clinical trials.
There is currently no good evidence for the use of AIT in preschool children for prevention.
Only products registered with the indication for AR (e.g. pollen allergy at present and maybe HDM in the future) should be considered for use in allergy prevention
Using AIT for preventive purposes should include all normal safety recommendations as for treatment of AR
AIT should only be prescribed by those with the competencies to administer it; for SCIT and first dose SLIT resuscitation equipment should be on site with trained staff available.
The indication and initiation of AIT should always be preceded by a patient‐centered discussion with the patient / family; this should consider the possible benefits, harms, disadvantages, costs, preferential route of AIT (SCIT vs SLIT) based on the individual patient's profile, preferences, and considerations for AIT adherence
AIT is the only treatment option for IgE‐mediated allergic diseases with disease‐modifying capacities. Therefore, AIT may in the future be considered in patients with milder AR, as AIT might modify the natural disease history, including the long‐term effect in AR and the preventive effect on the development of asthma; this is not achievable with pharmacotherapy
Authors’ contribution
S Halken drafted the manuscript, and A Muraro, G Roberts, O Pfaar, E Angier, and S Arasi critically discussed and reviewed the draft. All the authors satisfied the international Vancouver authorship criteria.
Conflict of interest
S. Halken reports personal fees and non‐financial support from ALK‐Abelló, outside the submitted work; G. Roberts has a patent issued: “Use of sublingual immunotherapy to prevent the development of allergy in at risk infants”; and his university has received payments for the activities he has undertaken giving expert advice to ALK, and presenting at company symposia for ALK, Allergen Therapeutics, and Meda, and serving as a member of an Independent Data Monitoring Committee for Merck outside of this work; Dr. Pfaar reports grants and personal fees from ALK‐Abelló, grants and personal fees from Allergopharma, grants and personal fees from Stallergenes Greer, grants and personal fees from HAL Allergy Holding B.V./HAL Allergie GmbH, grants and personal fees from Bencard Allergie GmbH/Allergy Therapeutics, grants and personal fees from Lofarma, grants from Biomay, grants from Nuvo, grants from Circassia, grants and personal fees from ASIT Biotech Tools S.A., grants and personal fees from Laboratorios LETI/LETI Pharma, personal fees from MEDA Pharma/MYLAN, grants and personal fees from Anergis S.A., personal fees from Mobile Chamber Experts (a GA2LEN Partner), personal fees from Indoor Biotechnologies, grants from Glaxo Smith Kline, personal fees from Astellas Pharma Global, outside the submitted work. E. Angier reports being Secretary of Primary Care Interest Group EAACI. ALK conference SOSA meeting 2015. Previous paid advisory board one each for MEDA 2012, Stallergenes, 2012, Schering Plough 2009, and one paid lecture by MEDA; S. Arasi has nothing to disclose; A. Muraro reports speaker's fees from Aimmune DVB, Mylan, Nestlè outside the submitted work.
5 What is the Evidence for AIT in the Treatment of Allergic Rhinitis in Children?
Corresponding author
Professor Graham Roberts, Paediatric Allergy and Respiratory Medicine (MP803), Clinical & Experimental Sciences & Human Development in Health Academic Units University of Southampton Faculty of Medicine & University Hospital Southampton, Southampton, UK.
Email: g.c.roberts@soton.ac.uk
Tel. 0044 (0)2381206160
Abstract
Despite good pharmacotherapy and avoidance, many children and adolescents continue to have troublesome symptoms from rhinoconjunctivitis. In this review, we use the EAACI Allergen Immunotherapy (AIT) for Rhinoconjunctivitis Guideline to examine the evidence for AIT in children and adolescents. To illustrate the evidence, two cases are considered. While there is good evidence that AIT is an effective treatment strategy for children and adolescents with AR, the evidence base is heterogeneous. Not all products and formulations have been shown to be effective. Before treatment is commenced with a specific AIT product, it is recommended that clinicians evaluate the evidence for its efficacy. In general, AIT for rhinoconjunctivitis has a good safety profile but there are differences for SCIT and SLIT. For individual children and adolescents, there are often a number of potential approaches. Choosing the most appropriate one will depend on the available effective products, experience of the clinic, characteristics of the patient, and the family's preference. Evidence gaps remain especially around optimum dosing schedules and the long‐term clinical effectiveness of AIT after an AIT course.
Abbreviations
AIT – Allergen immunotherapy
AR – Allergic rhinoconjunctivitis
SMD – Standardized mean difference
SLIT – Sublingual immunotherapy
SCIT – Subcutaneous immunotherapy
EPIT – Epicutaneous immunotherapy
ILIT – Intralymphatic immunotherapy
HDM – House dust mite
Introduction
Allergic rhinitis (AR) is an atopic condition affecting the upper airways, often with associated conjunctival disease. Typical symptoms include nasal congestion, sneezing, nasal itching, and watery nasal discharge.294 Symptoms can be seasonal or perennial. Diagnosis is confirmed by evidence of allergen‐specific IgE sensitization, with exposure to the index allergen correlating with symptoms. Other triggers of rhinitis symptoms include smoke, cold air, dust, and viral infections. Allergic rhinitis is one of the most prevalent atopic diseases, affecting around 15% of adolescents.295 Symptomatic allergic rhinitis is associated with reduced quality of life and impaired school performance,296 and in the adult population, costs due to lost work productivity are higher than those incurred by asthma.293 Treatment centers around allergen avoidance, which can reduce symptoms but is not always feasible,297 and pharmacotherapy. Medications used include oral and topical antihistamines, nasal corticosteroids, and leukotriene receptor antagonists.293, 294 Nasal saline irrigation is also a treatment option in children, which is safe, effective, and under‐utilized.298 These treatments may have side effects, and symptoms may promptly recur upon cessation of administration. Allergen immunotherapy (AIT) offers an alternative treatment approach that leads to desensitization and long‐term benefits even after treatment is discontinued.3
This review is based on the European Academy of Allergy and Clinical Immunology allergic rhinoconjunctivitis immunotherapy guideline published in 2018. The literature review was updated for the review.
General considerations
AIT should only be considered in children who remain significantly symptomatic despite optimizing medical therapy and avoidance measures3; symptoms should interfere with daily life or sleep293 (Box 1). Alternatively, it can be considered in less severe cases to take advantage of the potential disease‐modifying effects to prevent asthma.87 There should be evidence of allergen‐specific sensitization with either positive SPT or specific IgE. Correct identification of the responsible allergen is key to ensure the correct product is used.10 Some driving allergens are easy to identify as they are strongly seasonal, examples being tree and grass pollen. Where the driving allergen is perennial, such as animal dander or house dust mite, identifying the key allergen is more difficult. Absolute contraindications include severe asthma, active malignancy or active uncontrolled systemic autoimmune conditions, and initiation of treatment during pregnancy3 (Box 2). Relative contraindications in the pediatric population include partially controlled asthma, primary and secondary immunodeficiencies, and a history of serious systemic reactions to AIT.
Box 1. General indications in children and adolescents.
AIT should be considered when all of these criteria are met:
• Symptoms strongly suggestive of AR, with or without conjunctivitis
• There is evidence of IgE sensitization (positive SPT and/or serum‐specific IgE) to one or more clinically relevant allergen
• Experience moderate‐to‐severe symptoms which interfere with usual daily activities or sleep despite regular and appropriate pharmacotherapy and/or avoidance strategies
• AIT may also be considered in less severe AR where a patient wishes to take advantage of its long‐term effect on AR and potential to prevent asthma with grass pollen AIT
• Standardized AIT products with evidence of efficacy in the clinical documentation should be used
Box 2. General contraindications in children and adolescents.
Absolute contraindications:
• Severe asthma
• Active malignancy
• Active uncontrolled systemic autoimmune conditions
• Pregnancy (initiation)
Relative contraindications:
• Partially controlled asthma
• Primary and secondary immunodeficiencies
• History of serious systemic reactions to AIT.
We will discuss the current evidence on the efficacy of AIT for AR, using two hypothetical cases as a framework.
Tom, 14, and Rosie, 7, have both been referred for consideration of AIT. They have severe allergic rhinitis and both remain symptomatic despite maximal pharmacotherapy with good adherence to treatment. Tom is symptomatic in the late spring and early summer months and is sensitized to grass pollen and house dust mite (HDM). Rosie suffers from perennial symptoms, is sensitized to HDM, and also suffers from asthma.
Is there evidence that AIT would be of benefit for Tom and Rosie?
A recent large meta‐analysis concluded that there is substantial evidence for the efficacy of AIT in AR, with significant reduction in standardized mean differences for symptoms and medication scores combined (SMD −0.49, 95% CI −0.69, −0.30).10 The majority of studies included only adult participants, resulting in overall weaker recommendations for the use of AIT in children and adolescents due to a lack of evidence.3 There is still, however, evidence of benefit within this age group. When combining SLIT and SCIT studies together SMD for symptom scores in children was −0.25 (95% CI −0.46, −0.05) showing evidence of benefit; SMD for medication scores was 0.21 (95% CI −0.42, 0.01), suggesting there was a benefit; and SMD for combined medication and symptom score was −0.85 (95% CI −1.52, −0.17), showing a beneficial effect of AIT.10 From a health‐system perspective, SLIT and SCIT are considered cost‐effective in England, treatment cost within both the pediatric and adult population being under the cost‐effectiveness threshold of £20000 per QALY (quality‐adjusted life years) as set by NICE (National Institute for Health and Care Excellence).3
Evidence therefore suggests that AIT will be of benefit to Tom and Rosie. Is there any evidence to help us decide between subcutaneous and sublingual immunotherapy for our patients?
Evidence for SCIT
Seasonal allergic rhinitis (Box 3)
Box 3. Recommendations for immunotherapy for children and adolescents with AR driven by seasonal allergens.
• Continuous SCIT – short‐term benefit (Grade B)
• Pre‐ and pre‐/co‐seasonal SCIT – short‐term benefit (Grade B)
• Modified (allergoids) and unmodified allergen SCIT extracts – short‐term benefit (Grade B)
• Continuous grass pollen SCIT – short‐ and long‐term benefit (Grade B)
• Pre‐/co‐seasonal or continuous SLIT – short‐term benefit (Grade A)
• SLIT with tablets for pollens – short term benefit (Grade A)
• SLIT aqueous solutions for pollens – short‐term benefit (Grade A in children)
• Continuous grass pollen SLIT tablets or SLIT solution – long‐term benefit (Grade A)
Early studies on SCIT for seasonal allergens recruited small mixed adult and pediatric populations. These double‐blind, randomized studies, using pre‐ and co‐seasonal SCIT to grass pollens, demonstrated an improvement in symptom scores among the treated groups compared to placebo.299, 300 A larger study subsequently recruited birch and/or grass ‐pollen‐allergic children aged 6‐14, randomizing them to either continuous SCIT or placebo for three years.278 At termination of treatment, a significant improvement was seen in both rhinitis and conjunctival scores in the SCIT group, a long‐term beneficial effect which persisted at both the five‐ and ten‐year follow‐up (two and seven years off treatment).145, 279 Other studies have also reported long‐term benefits to SCIT, with an ongoing reduction in AR symptoms 12 years after discontinuation of treatment.270 There is therefore moderate evidence for the benefit of SCIT, either continuous, pre‐ or pre‐/co‐seasonal, in the treatment of childhood seasonal AR, with some evidence for long‐term benefit. Evidence in adults is stronger and based on a larger number of studies.10 Although there is no specific pediatric evidence on optimum dosing schedule, in an adult head‐to‐head trial of perennial versus pre‐seasonal SCIT to grass pollen, perennial treatment showed greater improvement in symptom scores after three years.301
Perennial allergic rhinitis (Box 4)
Box 4. Recommendations for immunotherapy for children and adolescents with AR driven by perennial allergens.
• Continuous SCIT – short‐term benefit (Grade C)
• Both modified (allergoids) and unmodified allergen SCIT extracts – short‐term benefit (Grade B)
• SLIT with tablets for HDM – short‐term benefit (Grade A) and long‐term benefit (Grade C)
• SLIT aqueous solutions for HDM cannot be recommended for short‐term benefit
High‐quality exclusive pediatric data are lacking on the use of SCIT to treat perennial allergic rhinitis. Recommendations are based on adult studies, which are few and heterogeneous. These small randomized placebo‐controlled studies assessed the effect of HDM SCIT, administered for either one or two years, and demonstrated a reduction in AR symptom and medication scores.302, 303 A larger recent adult study showed an improvement in AR symptoms and medication use after a year of HDM allergoid SCIT.304 Continuous SCIT is therefore recommended for perennial AR due to HDM in adults; recommendation for use in children is weaker.10 Evidence for efficacy for HDM SCIT in children is extrapolated from adult results and from small non‐placebo‐controlled studies demonstrating benefit of SCIT compared to pharmacotherapy alone; significant reduction in rhinitis scores was seen after three years and small additional benefits noted if treatment was extended for a further two years.305, 306, 307
There is good evidence that Tom may benefit from SCIT for his seasonal AR (Grade B for benefit on therapy and after discontinuation) (Figure 7); however, evidence for the benefit of HDM SCIT for Rosie is less robust (Grade C for benefit on therapy).
Figure 7.
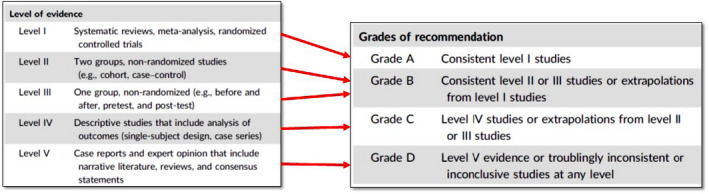
Linkage between level of evidence and grade of recommendation
Figure 6.
Electronic diaries for respiratory allergies—the screenshots are examples of electronic clinical diary APPs dedicated to allergic rhinitis and asthma: allergy monitor, MASK‐Air, Pollen.
Evidence for slit
Seasonal AR (Box 3)
Early small pediatric placebo‐controlled trials demonstrated the effectiveness of pre‐ and co‐seasonal administration of grass pollen allergoid SLIT308, 309 with improvement in rhinitis, chest, and eye symptom scores. Subsequent larger randomized blinded studies confirmed the beneficial effect of pollen SLIT, with noted improvements in rescue medication use as well as symptoms.58, 310, 311, 312, 313, 314 SLIT was beneficial in different populations when administered pre‐/ co‐seasonally and was safe and well tolerated. Continuous SLIT administration was also found to be effective.58, 315 In a direct comparison of continuous versus pre‐co‐seasonal regimens, no significant differences were noted between the protocols.316 Long‐term benefit has been found with reduction in symptom scores two years after cessation of grass pollen SLIT.58, 317 For seasonal AR to grass pollen, there is evidence of benefit with SLIT using either tablet or droplet formulations.58, 308, 309, 310, 311, 312, 313 Evidence suggests improved efficacy of treatment when grass pollen SLIT is administered for three years.58, 315, 317 In summary, there is evidence to strongly recommend the use of SLIT for seasonal AR, either continuously or pre‐co‐seasonally and either in aqueous or in tablet form, and a minimum of three years of treatment is recommended.3 The majority of SLIT studies have, however, been performed with grass pollen allergens, and there is only limited mixed adult and pediatric evidence for effectiveness of other seasonal allergens, with studies on birch pollen tablets, tree pollen drops, and cedar pollen extract demonstrating benefit.314, 318, 319
Perennial AR (Box 4)
Compared with seasonal AR, there is less evidence of benefit of SLIT to perennial allergens, namely HDM. The formulation used is particularly important. In two large mixed adult and pediatric trials, the use of HDM SLIT tablets was effective in reducing rhinitis symptoms after a year of administration,159, 160 with improvements also seen in medication use and quality of life.79 Pooled data on adolescents from these trials showed that treatment with HDM SLIT tablets resulted in a 22% decrease in rhinitis symptoms and that it was safe and well tolerated.320 However, the aqueous formulation of HDM extract has very limited evidence of benefit, with the majority of pediatric studies failing to find improvement in symptom/ medication scores.321, 322, 323, 324 Long‐term efficacy of HDM SLIT has only been assessed in adults, where sustained benefit is seen a year after cessation of treatment.153 In summary, there is only evidence for the effectiveness of tablet SLIT in perennial AR, with treatment lasting at least one year.3
When pooling all the pediatric SLIT data studies together, a meta‐analysis demonstrated a clear benefit to SLIT in reducing both symptoms scores (SMD −0.42, 95% CI −0.63, −0.21) and medication scores (SMD −0.6, 95% CI −1.12, −0.07).10
Both Tom and Rosie are therefore likely to benefit from SLIT (Grade A for benefit during and after therapy for seasonal allergen); however, in Rosie's case, only a HDM SLIT tablet formulation with evidence of efficacy should be used (grade A for benefit on therapy and grade C after cessation for HDM tablet). A decision is made and Rosie will be given AIT in the form of HDM SLIT tablets. How can we decide between SCIT or SLIT for Tom?
SLIT or SCIT
When deciding whether to use SLIT or SCIT for the treatment of childhood AR initial decisions should be based on the existing evidence and on ensuring a treatment modality is chosen which has demonstrated benefit for the allergen in question. A meta‐analysis has demonstrated that both SLIT and SCIT are effective routes of administration, with SMD for symptoms and medication scores of −0.51 (95% CI −0.77, −0.26) for SCIT and −0.47 (95% CI −0.81, −0.12) for SLIT.10 Decisions should therefore be based on patient co‐factors, local availability, individual preference, and safety. Both SCIT and SLIT have been associated with systemic allergic adverse reactions but with very different characteristics. SCIT may cause some mild local side effects but is also associated with an approximate 1:2,000 risk of a moderate‐to‐severe systemic side effect per injection.3 In contrast, SLIT usually causes local oral side effects for a few days or weeks on initiation but is very rarely associated with moderate or severe systemic side effects.3 So consideration needs to be given to the balance between efficacy and safety with immunotherapy (Figure 8). From a health‐system perspective, studies have shown mixed results and we are unable to conclude whether SLIT or SCIT is more cost‐effective.325 It is important therefore to discuss the pros and cons of the different treatment options with the patient and family. This is summarized in Figure 9 and will be explored in greater depth in a later article in the series. Whichever modality is chosen, current recommendation is for a minimum of three years of treatment with AIT.3
Figure 8.
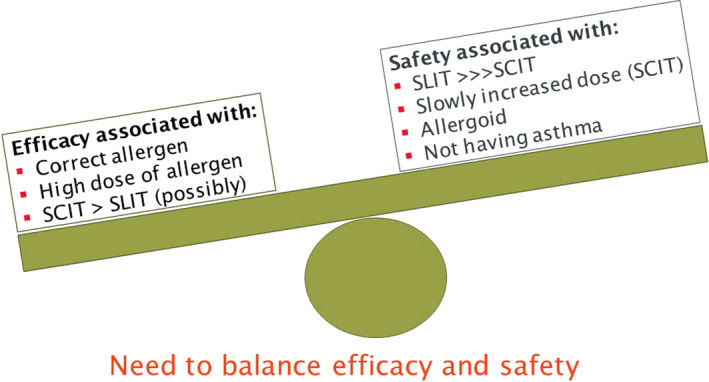
Balance between efficacy and safety with immunotherapy
Figure 9.
An approach to deciding the best AIT approach for different children and adolescents with allergic rhinoconjunctivitis. Reproduced from Roberts G, et al, Allergy. 20183.
Following discussion, Tom opts for SCIT as his family is worried about adherence to daily SLIT.
Other routes of administration
There has been developing interest in using alternative treatment delivery strategies. Epicutaneous immunotherapy (EPIT) using grass pollen allergens has shown moderate benefits111, 326 when administered pre‐pollen season. A significant reduction in AR symptoms was seen for the initial and subsequent pollen season, with no effect on medication scores, and side effects included significant local reactions. In the one published pediatric trial, EPIT to grass pollen reduced AR symptoms and antihistamine use compared to placebo327 but numbers were small. The intralymphatic (ILIT) route has shown efficacy in small trials, including in adolescent patients, with an improvement in self‐recorded symptoms after only three injections.328, 329, 330 However, the majority of evidence comes from small pilot studies. Some have reported very high rates of systemic side effects331 and one double‐blind placebo‐controlled trial demonstrated no benefit.332 There is therefore currently not enough evidence to support the use of either EPIT or ILIT for AR.
Patient factors
Rosie is 7 and has asthma for which she takes inhaled corticosteroids. Will these factors prevent her from receiving AIT?
Co‐existing asthma
Mild‐to‐moderate well‐controlled asthma is not a contraindication to AIT.3, 145, 315 Many of the published AR AIT study enrolled patients with co‐existing asthma and improvement in asthma symptoms is often seen following treatment. Recent adult studies have enrolled participants with both poorly controlled allergic asthma and AR and demonstrated a reduction in exacerbations among the HDM SLIT‐treated groups.333 Equivalent pediatric studies are currently underway. Currently, however, severe or uncontrolled asthma remains a contraindication for AIT.3 For Tom, there is evidence that a three‐year course of AIT can help prevent the future development of asthma, and this should be a consideration when deciding whether to commence children with troublesome AR on AIT.87 Readers are directed to companion articles in this series on the role of AIT in asthma and in allergy prevention.
Patient age
The majority of pediatric trials involved older school‐age children and adolescents, with children aged five and over participating in a number of larger trials.310, 311 Children younger than five are seldom included in randomized trials due to concerns regarding the potential safety of AIT in this age group and about the ability to make an accurate diagnosis of allergic rhinitis in this age group. Young children are also likely to struggle with the repeated injections required for SCIT. SLIT does appear to be safe in this age group,334, 335, 336 with a similar side effect profile and rates to older children. However, adherence can be poor, with nearly half discontinuing treatment within the first few months in one trial.337 Decision to start AIT in these younger age groups is therefore at the discretion of the treating physician and child's family, and age is not per se a contraindication.3
Tom is polysensitized—sensitized to both grass and HDM. Is this a contraindication?
Polysensitization
Polysensitization is common, with over 50% of patients with a respiratory allergy sensitized to two or more allergens.338, 339 It is important to differentiate between the polysensitized but mono‐allergic patient, where symptoms are due to only one allergen, from the polysensitized and polyallergic patient, for whom two or more allergens are driving the disease process.338, 339 For the mono‐allergic individual, single allergen immunotherapy is effective irrespective of sensitization status, with similar treatment effects seen in mono‐ and polysensitized patients,340 and therefore, AIT is recommended.3 Studies have demonstrated that AIT is safe in polysensitized and polyallergic children.339, 341 For polyallergic individuals, immunotherapy with a multiple allergen mixture has mixed evidence of efficacy, with studies demonstrating either no effect,342 or mixed effects with limited added benefit compared to mono‐therapy.341, 343, 344, 345 Further complicating the picture is the large number of different allergen mixtures used worldwide, most of which have not been evaluated.339 With no definitive evidence on best practice, approaches to the polyallergic patient vary according to local protocol. These include the use of a single allergen formulation targeting the most clinically troublesome allergy; a mixture of two homologous allergens if the responsible allergens are biologically related (eg, birch/hazel/nut mixture) or separate AIT to non‐related allergens administered at least 30 minutes apart.3, 339
Conclusion
There is good evidence that AIT is an effective treatment strategy for children with AR that remain symptomatic despite optimizing pharmacotherapy and allergen avoidance. However, the evidence is heterogeneous and not all products and formulations have been shown to be effective. Therefore, before commencing treatment with a specific product, it is recommended that clinicians evaluate the evidence for its efficacy.3 For individual patients, there are often a number of potential approaches. Choosing the most appropriate one will depend on the available effective products, experience of the clinic, characteristics of the patient, and the family's preference. Evidence gaps remain, in particular around optimum dosing schedules and long‐term clinical effectiveness of AIT, and further research in these areas is warranted.
Author contributions
CA initially drafted the manuscript which was edited by GR. The other authors reviewed and edited the manuscript. All the authors have reviewed and approved the final version.
Conflict of interest
Oliver Pfaar reports grants and personal fees from ALK‐Abelló, grants and personal fees from Allergopharma, grants and personal fees from Stallergenes Greer, grants and personal fees from HAL Allergy Holding B.V./HAL Allergie GmbH, grants and personal fees from Bencard Allergie GmbH/Allergy Therapeutics, grants and personal fees from Lofarma, grants from Biomay, grants from Nuvo, grants from Circassia, grants and personal fees from ASIT Biotech Tools S.A., grants and personal fees from Laboratorios LETI/LETI Pharma, personal fees from Novartis Pharma, personal fees from MEDA Pharma, grants and personal fees from Anergis S.A., personal fees from Mobile Chamber Experts (a GA2LEN Partner), personal fees from Pohl‐Boskamp, personal fees from Indoor Biotechnologies, grants from Glaxo Smith Kline, and personal fees from Astellas Pharma Global, outside the submitted work. Graham Roberts's University has received fees from ALK‐Abello and AllergoPharma for consultancy work he has undertaken outside of this work. Oliver Pfaar, Susanne Halken, Antonella Muraro, and Graham Roberts are authors of the EAACI Rhinoconjunctivitis AIT guideline.
6 What is the evidence on AIT for the prevention and treatment of asthma in children?
Corresponding author:
Pablo Rodríguez del Río, Allergy Department, Hospital Infantil Niño Jesús, Madrid, Spain
Email: prrio@yahoo.es
Abstract
Allergic asthma is the most frequent phenotype among asthmatic children. Allergen immunotherapy (AIT) is a highly attractive therapeutic approach to allergic asthma due to its disease‐modifying effect and short‐ and long‐term efficacy. However, the evidence supporting AIT in children is weaker than in adults. We performed a pragmatic review of current evidence from a disease‐severity perspective point.
Few studies have analyzed the role of AIT in preventing asthma onset in children with allergic rhinitis. However, their results are promising, especially as regard the ability of grass AIT to decrease the risk of asthma symptoms or asthma‐medication use, both during and after AIT treatment.
Regarding mild‐to‐moderate asthma, several double‐blind placebo‐controlled randomized trials (DBPCRTs) have provided evidence on the efficacy of AIT, reporting a reduction in symptom score, asthma‐medication score, or a combination of the two. However, disparities in outcome definitions and the heterogeneity of interventions complicate analyses of pooled data and prevent robust conclusions from being drawn. As for severe asthma in children, very few studies have been conducted due to the relative to absolute contraindication of AIT. Recently, omalizumab was successfully used as pre‐co‐treatment; alongside other strategies, this could be a means of expanding the number of candidates for AIT.
New trial designs using sets of common outcomes or enhancing the use of pragmatic clinical trials could help overcome the difficulties facing DBPCRTs in asthmatic children.
Key words
Asthma, children, pediatric allergy, immunotherapy, AIT
Introduction/Background
Asthma is a complex disease in which the identification of several pheno‐ and endotypes in adults346 is starting to be used as a driver for therapeutic interventions, especially in severe asthma.347 As most adult asthmatics had onset in childhood,348 further insights into pediatric asthma may help minimize the population‐wide impact of this disease.
Asthma is the most frequent chronic disease in children,349 and substantial efforts have been made to determine its different clinical patterns or phenotypes.350 Mere sensitization to airborne and food allergens, and, particularly, when clinically relevant allergy is established, have been found to be risk factors of future and more severe asthma in later stages in life.351, 352, 353 Studies with a mechanistic focus have revealed two main endotypes: the less frequent T2‐low asthma, characterized by the presence of neutrophils in sputum and higher expression of IL‐17, and the more common T2‐high asthma, affecting from 50%354to 70%355 of children according to the selection of endotype‐biomarker, associated with such changes as peripheral and sputum eosinophilia, high serum IgE, and elevated fractional exhaled nitric oxide.356
Therefore, both from a mechanistic and phenotypical point of view, childhood asthma is often an allergy‐driven condition and thus should be approached from an etiological perspective by employing immunotherapy, the only treatment shown to modify the natural course of the disease. However, the paucity of clinical trials on allergen immunotherapy in asthmatic children,357 the contradictory results from meta‐analyses showing insufficient358 versus good efficacy,69 and the positive results of some real‐life studies95 depict an unclear scenario in which a pragmatic interpretation of the evidence on AIT efficacy in pediatric asthma could be useful in guiding clinical decisions.
AIT efficacy for asthma prevention
The evidence gathered on the natural course of respiratory allergy has enabled the identification of subjects at risk who might be good candidates for preventive interventions. Research to date has shown that family history of atopy increases the risk of allergy in newborns,359 that sensitization can precede clinical manifestations by up to 5 years,208 and that allergic rhinitis is a precursor of asthma.260 In a very innovative study, Zolkipli et al.360 randomized 111 infants with family history of atopy to receive either an oral HDM extract for 12 months or placebo, to evaluate its preventive effect on allergen sensitization and onset of allergic diseases. Unfortunately, the results only showed effectiveness in avoiding sensitization to any allergen, but not in preventing HDM sensitization or allergy diseases such as eczema, wheeze, or food allergy. So far, probably due to the multifactorial origin of asthma, the only interventions generally accepted as being effective for primary and secondary prevention are difficult to implement and mostly consist of reducing exposure to smoke, pollution, and dampness.361, 362
According to the best available evidence,90 the role of AIT in asthma prevention is limited to secondary prevention, minimizing the risk of disease onset in children with allergic rhinitis. Adding to existing data for birch, grass, and mites suggesting this protective effect despite the various methodological limitations of these studies,145, 285 Valovirta et al.58 recently published a large double‐blind placebo‐controlled randomized trial (DBPCRT) on the protective effect of high‐dose sublingual immunotherapy with a grass‐pollen tablet in allergic children without asthma to avoid the onset of asthma. A total of 812 children (5–12 years) were randomized in a 1:1 ratio, receiving active treatment or placebo for 3 years + 2 years of follow‐up without treatment. For the primary outcome, consisting of a stringent asthma definition mostly based on spirometric evidence of reversible bronchoconstriction, the intervention showed no efficacy. However, for the highly relevant secondary efficacy outcomes (ie, asthma symptoms, medication use, and a combination of these), the treatment was efficacious during active treatment and follow‐up. The authors also analyzed the number of patients needed to treat (NNT) to prevent 1 additional child from suffering asthma symptoms and using asthma medication during the 2 years of follow‐up. Interestingly, the NNT was 6 and 20 for children 5 and 12 years of age, respectively, suggesting that the treatment offers added efficacy in younger patients due to their greater tendency to present asthma symptoms and use medication.
Inherent limitations of retrospective studies notwithstanding, two large real‐life studies using data gathered from prescription databases also evidenced the efficacy of grass tablets71 and birch AIT (both SLIT and SCIT)95 in decreasing the risk of asthma‐medication use in children and adults with allergic rhinitis.
AIT efficacy in mild‐to‐moderate asthma
The largest body of evidence on AIT for asthma draws its data from children with mild‐to‐moderate asthma. Several meta‐analyses and systematic reviews support the use of AIT in asthmatic children,69, 139, 363 though others point to a lack of consistent findings justifying its use.358, 364 Despite such discrepancy, all reported significant methodological difficulties in pooling data, affecting their ability to arrive at robust conclusions. An absence of validated364 and/or clinically meaningful outcomes358 and the inability to include all suitable studies in statistical analyses due to unavoidable outcome differences69, 363 are among the most relevant hurdles mentioned.
Illustrating some of the previously mentioned issues, in the most recent EAACI meta‐analysis,69 only 6 out of 13 studies meeting the inclusion criteria established by the authors provided data that could be pooled and qualified for quantitative synthesis of efficacy. These 6 trials (Table 2) differ substantially in the allergens studied (2 grass, 3 HDM, 1 Alternaria), the intervention duration (6 months to 3 years), route of administration (SLIT and SCIT), and in the AIT product (all different, though two studies used an HDM extract from the same company, one in tablet form (according to the authors),365 and the other in drops.366
Table 2.
Detailed information on design and outcome definition in clinical trials including only children in the 2017 EAACI meta‐analysis (DHAMI2017)
| Author, year | Participant characteristics | Intervention: extract and treatment duration | Definition of primary outcomes and secondary asthma‐related outcomes |
|---|---|---|---|
| HUI 2014 (A) | 90 mild to moderate HDM asthmatics, 5–14 y | SCIT, Alutard SQ (D.pt), 36 months | Primary outcome: not clearly stated |
| Mean daily dose of ICS: scheduled reductions every 3 months if asthma controlled (no asthma symptoms over the previous 6 months) | |||
| SS: daytime symptoms: 0–3 (0 for no symptoms to 3 for enduring symptoms affecting routine activity); nighttime symptoms: 0–4 (0 for no symptoms to 4 for sleeplessness) | |||
| Other variables: PEF | |||
| ROBERTS 2006 (A, B) | 39 moderate to severe grass‐pollen asthmatics, 3–16 y | SCIT, Alutard SQ (grass pollen), 2 y | Primary outcome: asthma symptom‐medication score |
| SS: for 5 variables (wheezing, coughing, shortness of breath, tight chest, and breathing problems while exercising), score 0–3 for each symptom (0 for no symptoms to 3 for severe symptoms) | |||
| MS: SABA: 0.5 points/dose; Budesonide: 50 μg 0.5 points/dose; Salmeterol: “budesonide score doubled”; Oral prednisolone (dose not stated): 20 points/day | |||
| Other variables: bronchial provocation test, allergen titration, lung function, sputum eosinophilia, and FeNO | |||
| LUE 2006 (A, B) | 20 mild to moderate HDM asthmatics, 6–12 y | SLIT, Staloral (D.pt, D. far), 6 months | Primary outcome: not clearly stated |
| SS: daytime symptoms: 0–3 (0 for no symptoms to 3 for severe symptoms); nighttime symptoms: 0–3 (0 for no symptoms to 3 for severe symptoms) | |||
| MS: budesonide, SABA, oral prednisolone as rescue medication (recorded). Details of scoring system not provided | |||
| Other variables: FEV1 and PEF variation | |||
| PHAM‐THI 2007 (A) | 111 mild to moderate HDM asthmatics, 5–15 y | SLIT, “Stallergenes tablets” (D.pt, D.far), 18 months | Primary outcome: SS (nocturnal and diurnal) and number of asthma‐free days |
| SS: daytime symptoms: 0–3 (0 for no symptoms to 3 for severe symptoms); nighttime symptoms: 0–3 (0 for no symptoms to 3 for severe symptoms) | |||
| MS: SABA, Budesonide and prednisone tablets allowedDetails of scoring system not provided | |||
| Other variables: use of inhaled corticosteroids, asthma‐free days (when SS = 0 and MS = 0), QoL, PEF | |||
| STELMACH 2008 (A) | 50 mild to moderate grass pollen asthmatics, 6–17 y | SLIT, Staloral (grass pollen), 2 y | Primary outcome: SS and MS reductions |
| SS: daytime symptoms: 0–3 (0 for no symptoms to 3 for symptoms that affected two or more daily activities); nocturnal awakenings: 0–3 (0 for no symptoms to 3 for disturbed sleep all or most of the night) | |||
| Medication score: use of SABA 0–3 (0 if not used, 3 for more than 3 times used a day) | |||
| Other variables: FEV1, FEF25‐75%, PEF | |||
| KUNA 2011 (B) | 50 mild to moderate alternaria asthmatics, 5–18 y | SCIT, Alergopharma (Alternaria), 3 y | Primary outcome: combined symptom medication score (sum of symptom and medication scores) |
| SS: for 3 variables (cough, wheeze, and dyspnea), score 0–3 (0 for no symptoms to 3 for severe, hard‐to‐tolerate symptoms interfering with daily activities and/or sleeping) | |||
| MS: SABA 1 point (unclear if per use or per day), budesonide 6 points, Montelukast: 6 points, 5 mg. Prednisolone: 4 points | |||
| Other variables: asthma symptoms VAS, QoL |
A, included in symptom score forest plot; B, included in medication score forest plot; D. f, Dermatophagoides farinae; D. pt, Dermatophagoides pteronyssinus; FEF 25–75%, forced expiratory flow at 25–75%; FeNO, fraction of exhaled nitric oxide; FEV1, forced expiratory volume in 1 s; ICS, inhaled corticosteroids; LABA, long‐acting beta agonist; MS, medication score; PEF, peak expiratory flow; QoL, quality of life; SABA, short‐acting beta agonist; SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy; SPT, skin‐prick test; SS, symptom score; y, years, HDM, house dust mite.
Regarding the outcome selected to reflect efficacy, none used exacerbation‐related or asthma‐control outcomes as a primary end‐point, this despite current recommendations in the EMA guidelines for the development of asthma treatment products.367 It can be argued that these 2 clinically meaningful variables may not be sufficiently sensitive for detecting changes in mild‐to‐moderate asthmatic subjects because exacerbations in these patients occur less frequently than in severe asthmatics. However, the preferred primary outcome of efficacy is usually some sort of symptom score (SS), medication score (MS), or a combination of these (Table 2). Great discrepancy is seen in the definitions of these two variables, likely as a result of the lack of standardized, validated SS and MS. For the SS, Roberts et al.368 evaluate 5 items and address difficulty with exercise, a proven marker of uncontrolled asthma in children,369 while Kuna et al.370 only use 3 clinical variables and do not include exercise performance. Among authors distinguishing between day‐ and nighttime symptoms, Lue et al.366 use the same 4‐point scale without offering a detailed description of what should be considered mild or severe, while Stelmach et al.371 describe severity of daytime symptoms as the impairment of daily activities and for night symptoms use nocturnal awakenings as the main rating criterion. There is a similar lack of consensus for MS; indeed, some studies do not clearly report the scoring system used365, 366 or different points are assigned for the same drug throughout all studies (Table 2). Though these differences do not impact the internal consistency of each trial, they are detrimental to the value of their pooled data.
In light of the limitations of meta‐analyses, approaches focusing on single‐product studies may hold promise. The pivotal study by Virchow et al.,333 conducted in a population of 834 adults, showed the efficacy of a sublingual house dust mite (HDM) tablet in reducing the risk of moderate‐to‐severe asthma exacerbations compared to placebo. Similarly, a previous study using the same product156 offers evidence of its efficacy in reducing the use of inhaled corticosteroids in mild‐to‐moderate asthmatic patients. The findings of these two studies were sufficient to be included in the GINA guidelines as an add‐on therapy for mite allergic asthma,372 a milestone not achieved before.
One of the most widely cited classical studies of pediatric patients showing no effect of AIT on asthma is that of Adkinson et al.,342 in which 121 children with moderate‐to‐severe asthma were randomized to receive active SCIT or placebo. The results failed to demonstrate an improvement in the primary outcome (medication score) and in the secondary end‐points (symptom score and methacholine sensitivity). A potential explanation for this result is that in most cases the subjects received mixtures containing up to 7 allergens, which likely prevented the individual therapeutic doses for each of them from being reached. Another large DBPCRT showing no efficacy is the one carried out by Pham‐Thi et al.365 in a population of 111 HDM asthmatic children undergoing high‐dose SLIT. After 18 months, actively treated subjects did not exhibit significant improvement compared to children receiving placebo in terms of symptom scores, use of medication, or lung function. In the words of the authors, factors which may have precluded significant differences were the underpowered nature of the study and the inclusion of patients with very mild asthma.
Acknowledging that negative results can be attributable to a lack of effect of therapy, most DBPCRTs confirm the efficacy of AIT in different asthma outcomes. The steroid‐sparing effect of AIT was proven in the study by Zielen et al.373 conducted in a cohort of 65 HDM GINA II and GINA III asthmatic children. Subjects included in this research received subcutaneous immunotherapy over a 2‐year period with an allergoid containing 7 and 6 μg of Der p 1 and Der p 2, respectively, per maintenance dose, with actively treated subjects experiencing a significant dose reduction of inhaled fluticasone; this steroid‐sparing effect was later supported by the findings of Hui et al.374 In two different DBPCRTs performed in grass‐allergic asthmatic children over 2 years, the use of high‐dose AIT, that is, SLIT371 and SCIT,368 proved its efficacy to reduce symptom scores among the active group compared to placebo.
In sum, although several other DBPCRTs provide evidence of efficacy in reducing symptom scores308, 321, 366, 370, 374, 375, 376 asthma‐medication scores,321, 366, 370, 377 or the combined symptoms and medication score,65, 308, 368, 378 the evidence base on AIT for pediatric allergic mild‐to‐moderate asthma is not optimal.
AIT efficacy in severe asthma
It has been found that AIT produces the best results in patients with more severe allergic rhinitis,379 and the same could be expected with asthma. However, evidence concerning AIT efficacy in severe asthma is quite limited because asthma is one of the most frequently reported risk factors for systemic reactions with SCIT,65, 380 especially when uncontrolled.381, 382 This experience gathered from clinical trials and real‐life reports consistent with these findings were included in clinical guidelines, leading severe asthma to become an absolute contraindication for AIT administration for several years. These have since been revised, and currently, only uncontrolled asthma is an absolute contraindication, while controlled severe asthma has been downgraded to a relative contraindication.383
Due to the difficulties in performing clinical trials in children and the relative to absolute contraindication of AIT in severe asthma, very few studies have been carried out in this field. One noteworthy exception supporting the efficacy of AIT in this complex population is the work of Tsai et al.,384 who randomized 40 moderate‐to‐severe persistent asthmatic children (5–14 years) to receive an HDM extract subcutaneously or standard therapy (controls). After 6 months of follow‐up, no systemic reactions were recorded, and although both groups significantly improved compared to baseline, only actively treated subjects experienced greater improvement in symptom and medication scores (p < 0.01).
To minimize risk, different treatment approaches have been proposed for these patients,383 such as the use of allergoids, which have a safer profile than natural depot extracts,65 or sublingual immunotherapy, due to its extraordinarily good safety profile.385
However, more attention has been given to omalizumab as pre‐co‐treatment. Massanari et al.386published a DBPCRT to evaluate the effect of omalizumab on the tolerability of SCIT in 248 adults with at least moderate persistent allergic asthma according to GINA. Fewer patients on omalizumab experienced systemic reactions compared to those receiving placebo (13.5% vs 26.2%, p = 0.017), and treated patients experienced fewer respiratory‐related systemic reactions (6 vs 24 out of 17 and 32 systemic reactions in active vs placebo, respectively), lending further evidence of the protective effect of omalizumab, though without providing any information on the efficacy of AIT.
Omalizumab was successfully used in a cohort of 17 pediatric patients (7–18 years) with severe uncontrolled allergic asthma,387 enabling 5 patients to resume AIT after the therapy was discontinued due to adverse events, and the 12 remaining subjects safely started a new treatment course. Additional evidence supporting this protective effect was obtained from a study of 6 severe persistent asthma patients (11–21 years) in whom short‐term use of omalizumab (1 year) facilitated the tolerance of a high‐dose SCIT HDM extract.388
Interestingly, after 1 year of use, omalizumab was discontinued and patients remained on HDM SCIT for a total of 25.5 months, with 5 patients experiencing a highly relevant clinical improvement in baseline severity and medication use.
Currently, the insufficient evidence on AIT in pediatric severe asthma does not allow it to be recommended in daily practice. However, when used alone or in combination with omalizumab, AIT has been successfully administered by some authors, thereby encouraging further research.
Discussion
Childhood is the most vulnerable stage of life, and several ethical, economic, and methodological issues affecting all fields of medicine have limited the number of randomized controlled trials in this population,389 resulting in a meager set of efficacy data. Pediatric investigation plans390 have been created to address this situation, though application in the field of AIT has sparked criticism.391 Among other measurements, these plans establish that pediatric trials should include a placebo group and be performed only after efficacy and safety in adults has been shown. The delay that these added trials would provoke when approving a treatment with proven efficacy in adult populations and the withholding of disease‐modifying treatment from the placebo group are the main obstacles to replicating the way the best evidence is built for adults.
Some authors suggest the use of head‐to‐head, non‐inferiority studies in children to avoid use of a placebo group64 and thus lessen these difficulties. In different areas of medicine, the creation of a core outcome set (COS) has been proposed to avoid heterogeneity.390
These proposals aim to define a number of outcomes that must be measured and reported in all trials focusing on specific conditions. Doing so would facilitate data pooling from different trials while improving knowledge with minimum waste and intervention. Though some initiatives have been undertaken in the field of asthma,393, 394 no AIT‐focused proposals have emerged thus far. Another potential alternative for improving evidence while bypassing the burdens of DBPCRT is to upgrade the relevance of pragmatic studies in guidelines, as these studies are being successfully used in other areas of asthma.395, 396 Such real‐world research is undertaken in less stringently selected populations than in randomized controlled trials and provide a measure of intervention effectiveness that could complement evidence from more conventional studies previously conducted in adults.
Although the available data support the efficacy of AIT in pediatric asthma, this knowledge base would benefit from greater robustness. Several factors such as underpowered studies, heterogeneity of efficacy outcomes, lack of clear clinical significance of end‐points, and the use of different products are the main limitations hindering the clinical value of pooled analyses. Given the great challenges posed by the prospect of conducting large randomized placebo‐controlled trials in pediatric populations, defining and universally implementing a set of common efficacy outcomes may be advantageous to future grouped analyses, and pragmatic clinical trials designed for the pediatric population may further help fill in the gaps in current evidence.
Conflict of interest
P. Rodriguez del Rio received research funding from the Health Research Fund of Carlos III Health Institute, Foundation for Biomedical Research of the Niño Jesus University Children's Hospital, and Spanish Society of Allergology and Clinical Immunology Foundation and reports honoraria for consultancy and/or advisory board and/or lectures from ALK‐Abello, FAES Pharma, LETI Pharma, Merck, Aimmune, Allergy Therapeutics, MEDA Pharma, and Novartis. R. Bazire has nothing to disclose in relation to this article.
7 Prescribing Immunotherapy to Pollens in Children: Influence of Geographical and Sociological Diversity
Corresponding author
Domingo Barber Hernandez, School of Medicine, Institute for Applied Molecular Medicine (IMMA), Universidad CEU San Pablo, Madrid, Spain Email: domingo.barberhernandez@ceu.es
Abstract
Geographic diversity across Europe is reflected in heterogeneous and complex pollen allergy phenotypes, which hinder the implementation of harmonized intervention strategies. Moreover, sociological aspects—such as different health insurance systems and associated reimbursement policies—strongly influence prescription approaches. In the Northern and Central areas of Europe, patients usually have a relatively simple phenotype compared to Southern European patients, especially in Mediterranean dry areas. Here, patients show the most complex profiles, which makes both diagnosis and intervention strategies complex. In the last years, an increased repertoire of new diagnosis tools that have shed light on allergic phenotypes have been developed and are being progressively incorporated in daily clinical practice. However, the relatively high cost and the lack of insurance coverage in many countries create the need to define optimized use of molecular tools and associated diagnosis algorithms. Food allergies associated with previous pollen sensitization are in progression and have a clear geographic distribution.
Here, we review the European scenario of pollen allergies, describing relevant pollen gradients, the effect on pollen‐allergic patients and the recommended allergy practices for diagnosis and treatment. It should be considered as a helpful tool for the daily management of pediatric pollen allergy.
There is a need of developing better intervention strategies for polysensitized patients living in Southern European regions as well as to determine the role of allergen immunotherapy as a potential preventive tool in respiratory allergy and related food allergies.
Introduction
Pollen sensitization of allergic patients usually starts early in life. Pollen‐allergic respiratory symptoms appear normally after other allergic manifestations (food allergy, atopic dermatitis, etc.). Pollens, together with mites, are the leading respiratory allergic sensitizers. The induction of sensitization by pollen allergenic species will depend on the local pollen exposure repertoire, and the sensitization profile will be, in most cases, increasingly complex during the life of the allergic patient, both in number of allergen sources recognized and in the number of allergens recognized in the allergen source.208 Early intervention during childhood with allergen‐specific immunotherapy (AIT), based on its prevention potential, has a unique positioning within pharmacological intervention options; however, AIT intervention will be influenced by the pollen exposome of the area, as well as different sociological aspects with marked differences between Northern and Southern Europe.
In probably one of the most comprehensive molecular sensitization studies performed so far,397, 398, 399 where a significant proportion of pediatric patients was included, there were no significant differences in the complexity of sensitization phenotypes between adults and children. One interesting take‐home message arising from these studies is that, in southern areas, sensitization complexity was much lower than perceived, with clear dominance of grasses and olive allergy in most of the territory. Only in the Mediterranean border and semi‐desertic areas (as Murcia and Alicante), patients displayed sensitization profiles with no clear pollen dominance. This last area is the only area with a very complex sensitization profile and multiple relevant pollen species, such as Salsola, Parietaria, Cupressus, Artemisia, or Platanus.400, 401
Allergic patients living in Northern and Central Europe present as well a complex sensitization profile. Usually, grasses and birch pollen allergy dominate the allergy sensitization landscape, but in contrast to grasses and olive pollen, which have coincident pollen seasons, grasses and birch have differentiated pollen seasons. Moving to Central Europe and especially to the East, other allergies—in particular Ambrosia, Artemisia, and ash tree pollen (which is almost identical to olive)—appear. In fact, in a study similar to the previous ones (Barber et al unpublished), allergic patients from Vienna presented sensitization clusters not very different to the ones found in Spain. However, perceived complexity might vary for different reasons. One of the most confounding factors is the sensitization prevalence to pan‐allergens, especially profilin and polcalcin. Sensitization to polcalcin is normally lower than 10% in all studied areas. However, sensitization to profilin ranks from 5% to 60%, being strongly associated with the intensity of grass pollen counts in the area.397 In the last decades, a progressive increase in profilin prevalence among pediatric patients has been reported.402, 403 Interestingly, clinical reactivity to profilin is a good model to understand how differential exposure levels to pollens may lead to a diversity of clinical phenotypes, with effect in T‐cell reactivity,404 barrier disruption,405 and systemic biomarker signatures.406 We need to understand how these differences in allergen exposure levels affect pediatric patients, and how early intervention may stop disease progression to severe phenotypes later in life. A better understanding of these dynamics will allow new biomarker strategies to define AIT intervention.25
Following adequate diagnosis algorithms, and a combination of in vivo and in vitro methods—which may include molecular allergology tools—are mandatory for deciding AIT intervention strategy in pediatric‐allergic patients407 . A comprehensive clinical guide for allergy diagnosis has already been published.115 Properly diagnosing children will lead, with a careful anamnesis, to the determination of clinically relevant allergies, which should constitute the basis for AIT prescription. We will thus analyze the different aspects for AIT formula selection in base to known geographic differences across Europe.
Clinical aspects to consider for prescribing AIT to pollens
AIT of pollens in children aims to reduce rhinoconjunctivitis and asthma during and after AIT discontinuation. In the last years, extensive clinical trials programs performed in adults and children with sublingual tablets have allowed to establish that the effect (around 30% reduction in symptoms and medication scores) is similar in both age groups,408 that it is allergen‐specific, and that a vaccine with a particular allergen has no clinical effect in allergic symptoms caused by unrelated species.409 There is as well clinical evidence on the tolerability of two simultaneous vaccines.410 With regard to secondary prevention in allergic children, this has been clearly proved in a prospective five‐year study, where grass‐allergic children suffering from rhinitis were treated sublingually aiming to prevent asthma.58 It has been recently elucidated the sequence of immunologic mechanisms associated with early, persistent, and sustained effect, providing scientific support for continuous administration of AIT for at least three consecutive years.411 Briefly, early effect is linked to desensitization mechanisms of effector cells. A progressive development and fixation of a memory T‐cell regulatory response are linked to long‐term and sustained effect (at least two years after AIT cessation).
The EAACI Guidelines on Allergen Immunotherapy3 in allergic rhinoconjunctivitis summarize the above‐mentioned evidence and recommend best AIT practices. Documented evidence supports single‐allergen vaccines when possible, or the use of two simultaneous vaccines. The duration of the therapy, either SLIT or SCIT, is recommended to be three years to achieve sustained effect. This aspect has been recently reviewed as well by Penagos et al.96
For regulatory reasons, clinical trials on pediatric patients are performed after first evidence in adults; thus, the evidence arising from pediatric trials is lower. However, combined experience performed on the trials for grass sublingual tablet registration both in adults and in children supports similar clinical effect and underlying mechanisms.412
In the last two decades, multiple big clinical trials have been performed both in adults and in children supporting the global clinical development of allergen immunotherapy tablets. The joined analysis arising from these studies supports efficacy in allergic rhinitis both in adults and in children. Moreover, a significant proportion of patients included were polysensitized with similar clinical effect.413 This fact indicates that selecting clinically relevant allergens is pivotal for AIT clinical benefit. The same authors state that SLIT tablet therapy has superior or similar efficacy compared to conventional pharmacotherapy for seasonal rhinitis.
A comprehensive view of the body of evidence of pollen AIT is summarized in a recent publication on the pharmaceutical development of the first globally registered AIT product.414
Major allergen sensibilization and AIT selection
Major allergens of an allergenic pollen are defined as the protein components that sensitize most of allergic patients to that pollen. In most of the pollen species (grasses, birch, olive, etc.), major allergens sensitize more than 90% of the allergic patients. These proteins are normally the most abundant proteins in the pollen extract. The biologic standardization procedure for allergy vaccines manufacturing uses a serum pool obtained by mixing a limited serum repertoire of allergic patients. The sIgE of major allergens dominates the sIgE content of the pool. Based on specific potency assays, using this pool, a biologic potency is assigned to a vaccine. In addition, the content of the major allergen is measured.
The clinical development program of a vaccine is adjusted to major allergen dose. In many cases, minor allergens will be pan‐allergens (profilins, polcalcins, nsLTPs, glucanases, etc.). Patients sensitized only to species‐specific minor allergens are rare and no clinical evidence of the effect of AIT on them is available. Moreover, dose of minor allergen can greatly vary from batch to batch of the same manufacturer or between different manufacturers. Barber et al397 described that in areas of very high olive pollen exposure, 5% of the patients were monosensitized to Ole e 7. The ratio of Ole e 1 (the major olive allergen) and Ole e 7 in a vaccine might be between 2,2 and 50,417 meaning a high variability in the administered dose, that is mostly adjusted to major allergen dose.
For all these reasons, patients that are not sensitized to the major allergen of a particular allergen source should not be considered eligible for AIT, unless products controlled in minor allergen dose are available.
Simple sensitization areas
As previously mentioned, in an important part of European territory a limited number of pollen species dominate clinical allergic pollen phenotypes. This is the case of areas dominated by grass pollen, the combination of grass and birch pollen or grass and olive. In epidemiological studies performed in Spain,400 in the areas dominated by grass and olive pollinosis, patients sensitized only to grasses, olive, or the combination of both cover up to 95% of pollen‐allergic patients. It seems that the immune systems of allergic patients do not react to other pollens present at much lower pollen count levels.
Clinical trials performed with allergic patients resident in these areas support the equal efficacy level of single‐allergen vaccines in mono‐ or polysensitized patients.3 In fact, the molecular profile analysis of 1905 patients included in pivotal clinical trials for grass tablet immunotherapy registration in the United States revealed that 85% of the patients included in the trial were polysensitized.96
As a consequence, simple diagnosis/therapy algorithms (Figures 10, 11, 12) can be suggested for the diagnosis/treatment of pollen‐allergic patients resident in these areas.
Figure 10.
Suggested algorithm for areas with high exposure to grasses and birch pollen
Figure 11.
Suggested algorithm for areas with high exposure to grasses and olive pollen. Different major and specific major allergens for Parietaria, Cypress, Artemisia, Salsola, Platanus acerifolia, and Ragweed are included in the algorism, as representative of frequent local pollens.
Figure 12.
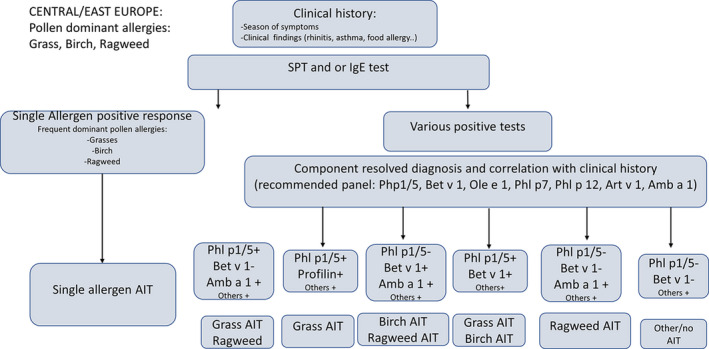
Suggested algorithm for areas with three clinically relevant pollen species
Extreme exposure areas
In single‐allergen‐dominated areas, there are spots of extremely high exposure. These areas have been extensively studied in the case of olive and grass pollen.397, 404, 405, 418 Patients exposed to very high pollen levels show a more severe clinical phenotype with particular clinical characteristics. Two areas in Spain—Jaen/Cordoba for olive pollen and Extremadura for grasses—have been identified so far. The best intervention strategy for this type of severe phenotypes is not established. Affected patients have particular clinical characteristics, such as severe profilin‐induced food reactions or sensitization to Ole e 7 as markers for clinical severity.
Other extreme exposed areas can be identified, as Sicily for pellitory, some areas of Finland for birch, or Barcelona for Platanus acerifolia. Specific patient characteristics need to be further investigated.
Complex sensitization areas
While in many areas of Europe pollens such as grasses, birch, or olive dominate pollen exposome, in other areas there is no clear pollen dominating allergic sensitization. These areas are normally relatively dry (southeastern part of Spain, southern Italy, or southern Greece, for example), and patients show complex and variable sensitization profiles.400 As opposed to the other areas, it seems that, in the absence of a dominant pollen, patients get sensitized to multiple pollens that are present at relatively low concentrations. As a consequence, patients are often polysensitized to multiple pollens and pan‐allergens. In an epidemiological molecular study, it was demonstrated that patients sensitized simultaneously to both pan‐allergens (profilin and polcalcin), duplicated the real number of sensitizations to major independent allergens, and showed more years of disease evolution.398
There is a lack of clinical trials performed in these types of patients. Therefore, no clinical recommendation for AIT intervention can be provided.
It is known that allergy in children will start with a limited number of sensitizations.232 Early intervention studies and their potential effect in stopping allergic spreading might be an obvious target for clinical trials in pediatric‐allergic patients living in these areas.
Pollen food–allergic phenotypes
A significant proportion of children with pollen respiratory allergy develop food‐related allergy. Mastrorilli et al419 described that 24% of children with pollen allergy presented simultaneously food allergy associated with profilins, PR10, and LTPs. Moreover, a significant proportion presented simultaneous sensitization to more than one pan‐allergen with a more severe phenotype. These authors proposed five classes of patients with different combinations of sensitization to the three pan‐allergens families. As a consequence, a careful evaluation of food allergy phenotype in pediatric patients is advisable.
PR10 and Profilins
Food allergies linked to pollinosis dominate food allergy phenotypes in food‐allergic adults. Cross‐reactive allergens between pollen and foods trigger these phenotypes115, 419
Three protein families, PR10, profilins, and non‐specific lipid transfer proteins (LTPs), are responsible of these phenotypes. PR10 allergy is triggered by previous sensitization to birch pollen major allergen Bet v 1. Profilin is present in all pollens, but food allergy mediated by profilin is strongly associated with grass pollen counts and is increasingly relevant with the increase of grass pollen exposure levels.397, 403 The evidence of the potential role of AIT as a therapeutic or preventive strategy for PR10 or profilin‐mediated pollen food allergy syndrome in children is inconclusive. There is a lack of adequate clinical trials focused on pediatric patients with pollen‐associated food allergy.
Non‐specific Lipid Transfer proteins (LTPs)
LTP allergy is an increasingly common allergic syndrome.416 It affects Southern European countries to a greater extent, but its prevalence is also increasing in North and Central Europe.420 Peach LTP, Pru p 3, is the leading LTP sensitizer. There are cross‐reactive pollen food LTPs,421 and, in fact, in areas with high exposure to cross‐reactive pollen LTPs, such as Artemisia in Canary islands or Platanus acerifolia in Barcelona, LTP‐allergic subjects present a more complex phenotype.422
Sensitization prevalence in pediatric patients is higher than in adults. In an epidemiological survey performed in pollen‐allergic patients,397, 398 the prevalence of sensitization to LTPs in children was 22%, duplicating the prevalence in adults. If we consider that pollen allergy prevalence is around 20‐25%, these numbers mean that up to 5% of children living in the south of Europe are sensitized to LTPs.
The cross‐reactivity of Pru p 3 with pollen LTPs, and its high prevalence, recommends that SPT (skin prick test) Pru p 3 diagnosis could be routinely tested in respiratory allergy. This would allow to identify Pru p 3‐sensitized children that cross‐react to other pollens with cross‐reactive LTPs, as Artemisia or Platanus. Art v 3 or Pla a 3 sensitization in the absence of Art v 1 or Pla a 1 or Pla a 2 recognition is normally associated with Pru p 3 sensitization. Pru p 3 sensitization in the absence of food allergy should be considered a mere risk factor and should not lead to food avoidance measures. There is a commercially available peach diagnostic with a good sensitivity/specificity profile available, that is a Pru p 3‐enriched peach peel extract with very low content of other allergens (PR10 and profilin).398
In principle, intervention strategies in LTP food‐mediated allergy should be based on Pru p 3 intervention. There are successful clinical trials in LTP allergy using a Pru p 3‐quantified peach extract vaccine.422, 423 Moreover, Pru p 3 vaccines have a clinical benefit in other LTP‐mediated reactions (such as peanut Ara h 9).423 This vaccine is commercially available in a limited number of countries. Availability and accepted indication of AIT extremely vary across Europe, from Countries such as the UK, where AIT is publicly funded generally only for severe hay fever impacting significantly on quality of life despite maximum medication treatment, to other countries such as Spain where AIT is extensively used on a named patient basis in milder cases. Finding an adequate balance in allergen extract regulation across Europe is urgently needed..92, 424
Sociological aspects in the prescription of AIT to pollens
There are fundamental differences in the health reimbursement systems for AIT across Europe. While in Northern/Central Europe, AIT is practically fully covered, in Southern countries this coverage greatly varies from one country to another. In general, AIT is only partially covered. As a consequence, the option of using simultaneously two individual vaccines in polysensitized patients is normally not feasible.
Trying to overcome this fact, an interesting approach has been a discussion following the Delphi method to explore consensus based on expert opinions, carried out by a panel of sixty‐two Spanish allergists. Using this approach, a clear consensus was met in the diagnosis and intervention strategies for selecting AIT approach dealing with two main Spanish pollen sources (grasses and olive). Interestingly, for real polysensitized patients (those sensitized to more than two major allergens of non‐related species), it was not possible to reach a consensus, exemplifying the difficulties of intervention approaches with this type of patients.425
As an outcome of this work, the panel reached consensus in several points: 1. SPT is not enough for accurate diagnosis. 2. Molecular diagnosis is useful when the relevant molecules are available. 3. AIT should be always evidence‐driven. 4. Clinical records and knowledge on allergen exposure in each area are essential to select AIT composition. 5. Do not mix more than three components in a single vaccine. 6. Each vaccine should have its own clinical evidence.
Subcutaneous or sublingual?
Current scientific evidence supports both administration routes. In a recent study, a direct comparison between the best documented subcutaneous and sublingual vaccines for grass allergy demonstrated similar efficacy.152 Moreover, in this study, two years of administration for any of the two administration routes were not enough to induce sustained benefit, supporting three years of administration, as previously mentioned. Sublingual AIT, however, shows a better safety profile. This fact is a clear advantage for pediatric administration, although adherence—especially in adolescents—is questioned.
Election between the two routes should be based on sociological aspects, on the documentation level and allergen availability in the vaccines (which vary from one allergen to another) and the potential foreseen compliance of the patient.
Selection algorithms
These algorithms should be used together with other tools. When possible, a detailed knowledge of local aerobiology is advisable. Molecular epidemiological studies, if available, can identify the relevant pollens in the area.400 E‐Diaries246 have been as well proposed to discriminate clinical relevant from clinically irrelevant sensitizations in genuinely polysensitized patients, in particular for non‐overlapping pollens. On the other hand, nasal/bronchial/conjunctival provocation tests could be helpful to discriminate overlapping pollens, but there is limited access to quality extract for provocation in many Countries, and multiple testing in pediatric patients might not be feasible.
In Figures 10, 11, 12, 13, potential algorithms for AIT formula election are shown. As previously discussed, for AIT selection sensitization to major allergens and link to clinical sensitization relevance is needed.
Figure 13.
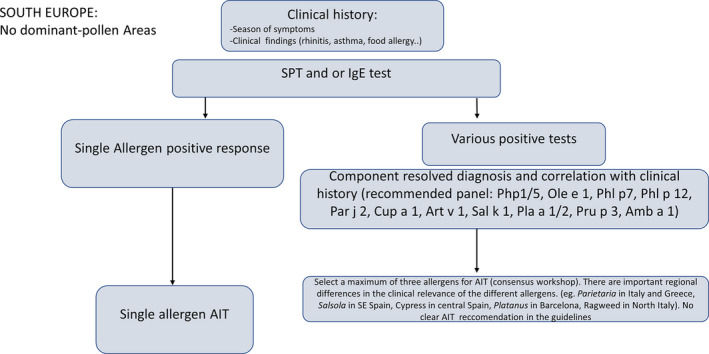
Suggested algorithm for areas with no dominant pollens. Whenever possible a maximum of two allergens should be formulated. There is a consensus of a working group (37) supporting the use of three allergens, but there is no clinical evidence supporting this practice.
Figures 10 and 11 show the simplest pollen areas. In these areas, there are always one or two dominant pollen allergies. Even if the patient is sensitized to other allergens, these pollens will dominate clinical phenotypes and should be the therapeutic preferred option.
In Figure 12, an area with three dominant pollens is exemplified. Selection criteria for AIT are more complex, and clinical anamnesis should guide the selection, especially if pollen seasons are not coincident. The same criteria could be applied to other areas with three dominant pollens, such as central areas of Spain, for example, Madrid, with grass, olive, and Cypress.
Figure 13 reflects a different scenario. In these areas, there are multiple potential pollens, and it is difficult to identify a clinically dominant sensitization. Molecular diagnosis tools are needed, and optimum intervention approaches are to be defined. There is a strong local variability. For example, Platanus allergy is very relevant in Barcelona, while Salsola is the leading allergy in Alicante or Murcia. Parietaria is probably the most relevant pollen clinically in many parts of Italy and Greece. Adaptation of diagnosis algorithms to each particular region is recommended. Epidemiological studies and building of sensitization maps400 are recommended as valuable tools to identify relevant local allergens.
These algorithms are set to provide orientation. More detailed use of molecular diagnosis and associated algorithms strategies can be consulted in EAACI Molecular Allergology User's Guide.25 In general, the clinical relevance of a pollen will be linked to the duration and intensity of the pollen season. Besides, pollen sensitization should always be evaluated in the overall sensitization profile of the patients. It should be considered that other sensitizers—especially perennial allergens such as mites, Alternaria or epithelia—often dominate children allergic phenotypes.
Conclusions
The clinical management of pollen‐allergic patients presents many common features, as well as marked differences caused by the intensity, duration, and complexity of the different pollen gradients.
There is no easy way of predicting the interaction between different pollen gradients and allergic patients in a particular territory, meaning that sensitization epidemiological studies as the one performed in Spain397, 398 would be needed to understand sensitization dynamics.
In the last years, different epidemiological studies, focused on sensitization patterns, have shed light on this complexity, allowing the identification of different allergic phenotypes.
While most clinical trials have been focused on main pollen allergies in patients resident in areas with relatively simple phenotypes, there is a need to explore best intervention approaches in polysensitized patients living mostly in Mediterranean areas.
Food allergies associated with previous pollen sensitization seem to be in progression, both in prevalence and in severity. AIT intervention may be a logical strategy to prevent food allergy development. Specific food allergy prevention studies should be carried out in pediatric patients
Early intervention strategies, with a focus on secondary prevention, appear as the most promising option for allergy disease management
With the generalization of optimized diagnosis strategies using molecular allergology, we have started to understand patient sensitization dynamics and set the basis for future research.
The diversity of allergic phenotypes makes these types of allergy an unattractive business case for pharmaceutical companies. Researcher‐driven collaborative studies are probably the best option for improving etiological management of complex allergic patients.
Conflict of interest
D. Barber declares to receive scientific consultancy fees from ALK‐Abello A/S and Aimmune Therapeutics; M Alvaro Lozano declares that there is no conflict of interest in relation to this article.
Key considerations for clinical practice.
Consider AIT only for clinically relevant allergic sensitizers
If possible, use single allergen vaccines
If single allergen vaccines cannot cover clinically relevant allergens, consider a maximum of three allergens.
Select products with documented clinical evidence (safety/efficacy)
Maintain the therapy for three years
Routes of administration should be considered based on sociological aspects (compliance, etc.)
Use the adequate diagnosis algorithms including molecular diagnosis
8 Prescribing Immunotherapy to House Dust Mites in Children
Corresponding Author
Dr. Montserrat Alvaro‐Lozano,
Hospital Sant Joan de Déu Barcelona, Passeig de Sant Joan de Déu, 2, 08950 Esplugues de Llobregat, Barcelona
Tel: 932 53 21 00
malvaro@sjdhospitalbarcelona.org
Abstract
House dust mite (HDM) allergens are the most relevant inducers of allergic disease worldwide. Furthermore, HDM is the most relevant persistent allergen in childhood. Allergen immunotherapy (AIT) to HDM is one of the most useful tools available for treating respiratory disease due to HDM allergy. It is a long‐time treatment option for patients suffering from allergic respiratory disease; however, it remains underused in the pediatric population. To improve knowledge on immunotherapy, the European Academy of Allergy and Clinical Immunology has been working on guidelines to homogenize recommendations with regard to AIT in children and adults.
The aim of this review was to highlight the importance of HDM allergy in children and to summarize current evidence of HDM AIT for the treatment and prevention of allergic disease in childhood.
The clinical efficacy of HDM AIT in allergic rhinitis is well established. Broad evidence has shown that it results in a reduction of symptoms and medication use, especially in children experiencing moderate‐to‐severe symptoms despite appropriate pharmacotherapy. The benefits of AIT in allergic asthma are also well known, being particularly suitable for patients with persistent asthma, normal lung function, and concomitant allergic rhinitis. No evidence suggests AIT worsens eczema or induces more frequent exacerbations.
Allergen standardization is of the upmost importance in pediatric population to ensure effective and safe therapies. The outcomes for future clinical trials need to be clearly defined and standardized. With the use of precision medicine and e‐health knowledge, AIT could be prescribed in a more individualized manner, hence improving adherence.
Abbreviations
AA – allergic asthma
AD – atopic dermatitis
AIT – allergen immunotherapy
AR – allergic rhinoconjunctivitis
ARIA – Allergic Rhinitis and its Impact on Asthma
DBPC – double‐blind placebo control
EAACI – European Academy of Allergy and Clinical Immunology
GINA – Global initiative for asthma guidelines
HDM – house dust mites
QoL – quality of life
SCIT – subcutaneous immunotherapy
SLIT – sublingual immunotherapy
SPT – skin prick test
Key words
House dust mite, allergen immunotherapy, asthma, rhinitis, children
Introduction
House dust mite (HDM) allergens are the most relevant inducers of allergic disease worldwide. Mite allergens sensitize genetically predisposed individuals and induce symptoms such as allergic rhinoconjunctivitis (AR), allergic asthma (AA), and atopic dermatitis (AD).426, 427 The most relevant HDM allergens worldwide are the fecal pellets of species Dermatophagoides pteronyssinus, Dermatophagoides farinae, Euroglyphus maynei, and Blomia tropicalis.426
HDM allergy has a profound impact in pediatric patients and their families’ quality of life (QoL). A meta‐analysis shows that in patients with AR and AA, QoL is more affected in individuals with HDM perennial allergy than in those suffering from seasonal pollen allergies.427 HDM allergy also comes with an important economic burden in direct costs for disease management and indirect costs due to high levels of absenteeism.428
Advances in the knowledge of mite molecular biology, a better understanding of proteins allergenic properties and cross‐reactivities, provide key information for the accurate diagnoses and immunotherapeutic approaches that can target HDM allergy.426
Allergen immunotherapy (AIT) is a long‐time treatment option for patients suffering from allergic respiratory disease. However, it remains underused in the pediatric population. Children with allergic respiratory disease that is not controlled by standard pharmacotherapy, including biologics, are an important unmet need in everyday clinical practice. AIT could benefit some of these patients, though comprehensive guidelines that standardize and promote AIT use are needed.
The European Academy of Allergy and Clinical Immunology (EAACI) has been working on guidelines to standardize recommendations with regards to AIT in children and adults.3, 83 Furthermore, recent ARIA guidelines review pathways for the prescription of AIT and highlight the importance of individualized care, persistence of symptoms despite appropriate medications, identification of relevant allergens, and good‐quality, efficacious extracts.429
New approaches in respiratory allergic diseases such as observing daily symptoms with mobile technology can help us understand intra‐individual variability of allergic multimorbidity and aid in the diagnosis and treatment of AR and AA.430
The aim of this review was to highlight the importance of HDM allergy in children and to summarize current evidence of HDM AIT for the treatment and prevention of allergic disease in childhood.
The importance of HDM allergy in childhood
The World Health Organization and the International Union of Immunological Societies (WHO/IUIS) allergen nomenclature subcommittee currently includes up to 65 D. pteronyssinus and D. farinae allergens, as well as 14 allergens from Blomia tropicalis.431 The serodominant groups are 1, 2, and 23 fecal allergens for Dermatophagoides species.432
It is known that individual allergen components have specific characteristics that allow them to contribute in different ways to the overall allergenicity of the mite.426 The main example is the group 1 allergens of Dermatophagoides spp. named Der p 1 and Der f 1 for D. pteronyssinus and D. farinae, respectively. These allergens are cysteine proteases that can become enzymatically active and gain the ability to act as adjuvants in Th2 inflammatory response.432
Longitudinal studies confirm that HDM sensitization occurs prior to polysensitization. Subjects with the broadest IgE sensitization have significantly higher risk of mite‐related AR and asthma than unsensitized participants. Also, sIgE to Der p 1 or Der p 23 at age 5 years or less predicted asthma at school age.213
The moment in life a patient becomes sensitized is also important. It has been proven that earlier sensitization to HDM, specifically before 2 years of age, is associated with loss of lung function at school age. The stronger the degree of exposure to the HDM allergen, the more likely the child will develop airway hyper‐responsiveness. No such effects have been reported with seasonal allergens.433, 434
The degree of sensitization is also relevant for different allergic diseases. Asthmatic children sensitized to HDM recognized more allergens than non‐asthmatic children with HDM allergy. This is seen when comparing children with HDM‐allergic asthma and HDM‐allergic children suffering exclusively from allergic rhinitis. IgE levels to Der p 1, Der p 2, and Der p 23 were significantly higher in asthmatic children than in children without asthma.435
Globally, it is known that over 80% of HDM‐allergic patients are sensitized to group 1 and 2 allergens from D. pteronyssinus and D. farinae species. Thus, it has been suggested that a mixture of the two extracts made using both feces and bodies can be appropriate in a large scale.436 A more personalized approach would be ideal, but is hard to achieve due to higher costs in studies and production. The proper understanding of HDM sensitization patterns in different individuals will allow a more precise diagnosis and help customize the immunotherapy that will best target each child's needs.
Allergy prevention and HDM AIT
It is known that children with AR have an increased risk of developing asthma 437 and that AD and AR in childhood correlate with AA persisting into adulthood.262 Thus, the potential role of AIT in preventing the progression of allergic disease has been an area of interest for decades.
Studies assessing the long‐term effectiveness of AIT in children with AR indicate that AIT might reduce the risk of developing asthma. However, the preventive role of AIT has been stablished for patients suffering seasonal pollen‐driven AR but not for those with HDM‐driven disease.87
For AR children sensitized to HDM, few studies have evaluated long‐term efficacy and possible prevention of asthma. Longitudinal trials have been carried out using D. farinae AIT drops in a Chinese cohort; however, they are heterogeneous and lack clear outcomes.438
One of the most important weaknesses of most prevention trials is the lack of concrete parameters by which asthma outcomes will be measured. Studies to date are heterogeneous and focus on demonstration of reversibility, active symptoms, or use of medication. In order to safely and effectively prescribe HDM AIT in children, the outcomes for future clinical trials need to be clearly defined and standardized.87
The potential preventive role of HDM AIT in healthy individuals at risk of developing new allergic disease has also been studied. In a proof‐of‐concept trial, oral AIT to HDM given in infancy did prevent sensitization to other allergens.439 Nonetheless, no effect was seen in sensitization to HDM itself and on the development of allergic diseases such as eczema, wheeze, or food allergy.273
A recent study showed that patients treated preventively with HDM AIT had higher IgG4 epitope diversity to HDM allergens compared to placebo with no change in IgE diversity. These suggest a possible benefit in immunomodulation for preventive AIT.238
More robust clinical studies are needed to stablish a true prevention pattern. To date, there is no evidence to recommend HDM AIT for the prevention of a first allergic disease in healthy individuals.87
HDM AIT routes of administration in children
HMD AIT products are commercially available via subcutaneous (SCIT) or sublingual (SLIT) administration. The later can be either drops or tablets. Alternative routes such as intralymphatic or epicutaneous have not been tested in a pediatric population.3
AIT can be used in precision medicine as it takes into account the different sensitizations and multimorbidty of each individual child. Indirect evidence can support which patient profile could benefit the most, from AIT such as those with more severe and persistent allergy that can affect school performance and educational milestones.429
Allergen standardization is of upmost importance in pediatric population to ensure effective and safe therapies. It is a prerequisite for the production of reagents for allergen‐specific diagnosis and intervention in allergic diseases to guarantee potency, consistency in composition, and stability.88, 266
Recently, the Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines released evidence‐based, simple care pathways for allergen immunotherapy prescription. It states that AIT should be prescribed only by specialists and needs to be individualized, based on the relevance of the allergen and the persistence of symptoms despite adequate base medication.430 Extracts used must be of good quality and proven efficacy.92
To date, there is only one SLIT HDM tablet approved in Europe, for adults aged 18 to 65 years. Important recommendations for its use are as follows: (i) the patient should not have had a severe asthma exacerbation within the last 3 months of AIT initiation; (ii) in patients with asthma and experiencing an acute respiratory tract infection, initiation of treatment should be postponed; (iii) AIT is not indicated for the treatment of acute exacerbations and patients must be informed of the need to seek medical attention; (iv) HDM AIT should initially be used as an add‐on therapy to controller treatment and reduction in asthma controllers should be performed gradually according to management guidelines.83, 440
Several HDM SCIT products are available. There is important heterogeneity between them in allergen extracts, potency, adjuvants, and efficacy. General recommendations when prescribing HDM SCIT in children include the following: i) check prescription profile of AIT and match with patients age and concomitant allergies, ii) make sure the patient asthma is well controlled and assess with lung function test prior to initiating SCIT, iii) a minimum of 30‐minute observation in the office is mandatory, iv) provider must have proper conditions to administer SCIT, manage severe bronchospasm and anaphylaxis.83
The standard course of both HDM SCIT and SLIT in children is 3 years; little advantages are achieved with longer therapies.3, 307
HDM AIT for treatment of allergic rhinitis in children
AIT is the only current treatment available that can have disease‐modifying effect; this aspect is of upmost importance in children with AR. There is broad evidence of the clinical efficacy of HDM AIT in treating AR. Both SCIT and SLIT have been proven to reduce symptoms and medication use.3 See Table 3: Considerations in clinical practice.
Table 3.
Key considerations for clinical practice
|
HDM AIT should be considered in children experiencing moderate‐to‐severe symptoms which interfere with usual daily activity or sleep despite regular and appropriate pharmacotherapy.3 AIT is not recommended for infectious or non‐allergic rhinitis.429
A meta‐analysis by EAACI highlights important heterogeneity in effectiveness between products.10 Thus, product‐specific evaluation should be made prior to prescription.92
SCIT using unmodified or modified allergen extracts is recommended for the treatment of perennial HDM AR in children and provides short‐term benefits.10 Most data available are extrapolated from adult studies.303, 441 In the case of HDM AIT, more studies are needed to evaluate long‐term effectiveness in children as well as exclusively pediatric randomized, placebo‐controlled data.10
HDM SLIT tablet is recommended for the treatment of AR in adolescents and adults.154 There is low risk of systemic side effects and studies have low heterogeneity.10 There is strong evidence with regard to short‐term efficacy up to one year.159 Longer treatment can be beneficial but is associated with more side effects.155 Long‐term benefit up to 3 years has been proven in adults; a confirmatory pediatric study is needed.153
There is no enough evidence to recommend SLIT aqueous drops in AR treatment.442 This can be due to the different allergen content and the volume administered in the drops.10
For HDM AIT prescription, it is recommended to use single allergen species or a mixture of well‐documented homologous allergens from the same biologic family.3 The use of mixtures of allergens of non‐related biologic families is not recommended.92 One small study using dual SLIT (HDM and grass pollen) in children showed efficacy in treating patients with a variety of sensitivities.443
There are limited trials that use HDM AIT in preschool children, currently the age for beginning HDM AIT is 5 years. The diagnosis of AR is more difficult to make at an early age and special consideration can be given to children with clear HDM‐driven AR age 2‐5 in specific cases. This could change in the future if better prevention trials are carried out.3
Anti‐IgE therapy has been used to reduce symptoms and improve safety profile of AIT. There is no clear recommendation with regard to children with HDM allergy but could be used in severe cases of AR and asthma. The appropriate duration of the anti‐IgE therapy is unclear.194
When evaluating a polysensitized patient, the first step is to determine if the allergy symptoms are triggered by a single allergen (mono‐allergic) or by multiple (polyallergic). In the case of mono‐allergic patients, HDM AIT can be prescribed with a favorable outcome. In polyallergic patients, efficacy is limited specially if the allergens triggering the symptoms are not biologically related.3 No studies have shown benefits in AIT to multiple allergens in children.
HDM AIT for treatment of asthma in children
There is limited evidence that evaluates safety and efficacy in HDM AIT for AA. Most studies were originally designed for AR and retrospectively analyze the impact on a subgroup of patients with asthma.430
Multimorbidity is common in allergic disease, over 85% of patients with asthma also have AR, and this AR also increases the severity of asthma. A benefit of AIT is that it can act on ocular, nasal, and lung symptoms at the same time proving an integrated approach. ARIA guidelines also encourage the use of HDM SLIT as add‐on therapy in patients with AA and concomitant moderate‐severe AR not controlled by pharmacotherapy. However, they state that in patients with AR there is no clear evidence that progression to AA will be prevented.429
There is substantial evidence recommending the use of SLIT drops in AA as add‐on treatment to decrease symptoms and medication use in children which could potentially lead to a steroid sparing effect 69, 83 The Global Initiative for Asthma Guidelines (GINA) states the beneficial role of AIT in asthma in cases where allergic sensitization plays a major role. SLIT can be added in patients with AR and persistent asthma with FEV1 >70%. This recommendation is made for patients over 12 years of age who advance to Step 3 therapy.372
In a systematic review published by EAACI, it is confirmed in the subgroup analysis, that SCIT is effective in children with controlled HDM‐driven allergic asthma as add‐on treatment for reduction of symptoms and medication scores. However, there is important heterogeneity in HDM SCIT studies including double‐blind placebo control (DBPC) and non‐DBPC which use different extracts or allergoids, different delivery systems such as lysosome‐encapsulated allergen and different end‐points.69, 444
A basic principle in AIT prescription is the fact that uncontrolled asthma is a contraindication for AIT and children's lung function should be monitored before beginning an AIT treatment.383 Patients with moderate asthma are likely to be the ones that benefit more from AIT; however, this needs confirmation.83, 429
There is lack of evidence that supports that HDM SCIT or SLIT decreases exacerbations in children, improves lung function, or improves airway hyper‐reactivity.69, 83
HDM AIT in atopic dermatitis in children
Several small trials and one multicenter study give insight into HDM SCIT on AD. Patients initiated SCIT for respiratory allergy and a secondary outcome was the improvement of their AD.445 No evidence was seen that AIT worsened AD or induced more frequent exacerbations.446 This evidence was analyzed in a systematic review where SCIT had moderate evidence as treatment for AD.447 Some trials have also seen benefit with HDM SLIT.448
In an AD European consensus, HDM AIT is considered an option in selected cases but no clear indication is given. Hypothetically, patients with a positive atopy patch test and corresponding history of eczema flares may be candidates for AIT with the eliciting allergen. Nonetheless, larger trials needed to confirm efficacy are needed.449
Conclusions
HDM AIT is one of the most useful tools in treating allergic respiratory disease in children. With the use of precision medicine and e‐health knowledge, AIT can be prescribed in a more individualized manner and improve adherence.
Due to the efforts of international allergy societies, there are now guidelines for the safe and effective use of HDM AIT in children. However, it is important to highlight the need for product‐based evaluation on an individual basis for all AIT prescriptions. Furthermore, there are still important knowledge gaps and there is an urgent need for more robust evidence in pediatric population (see Table 4: Knowledge gaps and future research needs).
Table 4.
Knowledge GAPS and future research needs
|
Conflicts of interest
Authors declare no conflict of interest with regard to this article.
9 Prescribing immunotherapy to furry animals and less common aeroallergen in children
Corresponding author
Marta Vazquez‐Ortiz, Department of Paediatrics, Imperial College London, London, United
Kingdom, Email: m.vazquez-ortiz@imperial.ac.uk
Abstract
Allergen‐specific immunotherapy (AIT) has proven effective to treat respiratory allergy (ie, allergic rhinitis and asthma) both in children and in adults. Even though AIT has been used in clinical practice for over a century targeting different aeroallergens, its clinical efficacy and safety have been demonstrated in appropriate clinical trials only for selected pollens and house dust mites. In contrast, there is very limited high‐quality evidence to support the use of AIT to less common aeroallergens, such as furry animals, molds, and cockroach, especially in the pediatric population, and data cannot be extrapolated from trials targeting other allergens. Indeed, there is agreement that the efficacy of AIT should not be considered as a class effect, but proven for every single product.
The purpose of this review was to summarize high‐quality evidence from double‐blind placebo‐controlled randomized controlled trials on the effectiveness and safety of AIT for furry animals, molds, and cockroach, with a specific focus on studies including children and adolescents.
Keywords
Allergen; Allergy; Allergic rhinitis; Asthma; Cat; Children; Cockroach; Dog; Furry Animals; Immunotherapy; Molds.
Abbreviations
AIT – allergen‐specific immunotherapy
AR – allergic rhinitis
DBPCRCT – double‐blind, placebo‐controlled, randomized controlled trial
Key consideration for Clinical practice.
-
Aeroallergen avoidance and drug therapy are the mainstays of treatment for proven IgE‐mediated respiratory allergy (allergic rhinitis and asthma). However, complete aeroallergen avoidance cannot be achieved with current environmental control measures. Allergen‐specific immunotherapy (AIT) can be a potential add‐on treatment option when conventional management strategies are unfeasible or ineffective, and specific IgE sensitization plus a clear relationship between clinical symptoms and exposure to that particular allergen has been identified. Nonetheless, the evidence supporting the use of AIT for furry animals, molds, and cockroach in children is poor, and data on cost‐effectiveness are lacking. Specifically:
Limited high‐quality evidence supports the use of AIT for cat allergy, mainly the use of subcutaneous immunotherapy in adult patients.
There is no clear clinical evidence of AIT effectiveness for dog allergy, likely due to the lack of standardized extracts and variability in sensitization profiles.
There is insufficient evidence on the efficacy and safety of AIT to other furry animals, such as horses, rodents, and rabbits.
Low strength evidence supports the use of AIT for mold allergy, mainly the use of subcutaneous immunotherapy with Alternaria extracts. Data for taxa of fungi other than Alternaria and Cladosporium are non‐existent.
Clinical efficacy of AIT for cockroach is currently under investigation. Lack of standardized cockroach extracts for AIT due to the lack of immunodominant allergen(s) and highly variable sensitization profiles of allergic patients.
Knowledge gaps and future research.
There is a need for double‐blind, placebo control randomized trials, using standardized allergen extracts, common clinical outcomes scoring systems, and adequately powered sample sizes including children and adults, assessing both clinical efficacy and cost‐effectiveness of AIT for furry animals, molds, and cockroaches.
Introduction
As children tend to spend most of their time in indoor environments, increasing exposure to indoor aeroallergens other than house dust mites, such as animal dander, molds, and cockroach, can significantly contribute to the development of allergic sensitization and persistent respiratory allergy.450, 451, 452 Allergen avoidance and pharmacotherapy represent the mainstays of treatment for proven IgE‐mediated respiratory allergy. However, complete aeroallergen avoidance cannot be achieved with current environmental control measures.450, 451, 452 Additionally, a significant number of patients continue to experience allergic symptoms on exposure to these inhalant allergens despite effective pharmacological therapy. Where conventional management strategies are unfeasible or ineffective, allergen‐specific immunotherapy (AIT) can be a potential treatment option.266
AIT is a recognized effective therapy for respiratory allergy both in children and adults, with a unique potential ability to modify the natural course of the disease and induce long‐term clinical benefits, that can persist for years after discontinuation of treatment.3, 90 Despite these advantageous features, there are a number of unmet needs regarding AIT, specifically in terms of discrepancy in allergen standardization methods, heterogeneity in study design (ie, patient selection, sources and types of allergenic extracts used, administration protocols, symptom and outcome scoring systems, duration of treatment), safety aspects, preventive effects, adherence, and cost‐effectiveness.453
In addition, it should be noted that the majority of high‐quality randomized controlled trials have proven the efficacy and safety of AIT for pollens and house dust mite allergy.10, 69 Therefore, meta‐analyses results tend to reflect the positive outcomes of AIT for these selected aeroallergens and should be interpreted with caution as for the extrapolation to other inhalant allergen sources. In this regard, there is agreement that the efficacy of AIT should not be considered as a class effect, but more correctly identified for every single product.292
Despite being used in clinical practice for many years, there is limited high‐quality evidence supporting the use of AIT to less common aeroallergen sources, such as furry animals, molds, and cockroach, especially in children.266, 450, 451, 452 The purpose of this review is to summarize such evidence in the pediatric population, by focusing on higher order data from double‐blind placebo‐controlled randomized controlled trials (DBPCRCTs) (Tables 5, 6, 7).
Table 5.
Summary of double‐blind placebo‐controlled‐randomized controlled trials of allergen immunotherapy to furry animals in the pediatric age
| Author | Allergen | Participants n (age, y) | Allergic disease | AIT type | Target maintenance dose/major allergen content | Treatment duration | Main efficacy results | Main safety results |
|---|---|---|---|---|---|---|---|---|
| Valovirta (1984 – 1986)486 | Dog | 27 (5‐18 y) | Asthma | SCIT | ● 100000 SQ/every 4 weeks● Dog major allergen NR | 1 year | † Conjunctival provocation test and s‐IgG‡ Symptom scores, s‐IgE and allergen‐specific BPT | No significant difference in mild reactions between the groups |
| Sundin and Hedlin (1986)458, 462 | Cat or Dog | 41 (8‐47 y) | Asthma | SCIT | Children● 80000 SQ /every 4 weeks● Cat Fel d 1 3.4 μg● Dog albumin 60.8 μgAdults● 100000 SQ/every 4 weeks ● Cat Fel d 1 4.3 μg● Dog albumin 76 μg | 1 year | † Allergen‐specific BPT in the cat AIT treated group. † SPT and s‐IgG and s‐IgG4 in both groups.‡ Allergen‐specific and histamine BPT in the dog AIT‐treated group.Histamine BPT within the cat AIT‐treated group compared to baseline, but not within the dog AIT treated. (No comparison active vs placebo reported for this outcome.) | AIT group:GR mild in all 12 children during the initial build‐up phase. 2 adults stopped AIT, one in the active group, and one in the placebo group due to GR |
| Haugaard (1992)459 | Cat and/or Dog | 24 (13‐48 y) | Asthma | SCIT | ● 100000 BU /every 4‐6 weeks● Cat Fel d 1 200 μg● Dog major allergen NR | 1 year (first 5 months included a placebo control group) | ‡ Allergen‐specific and histamine BPT at 5 monthsSignificant improvement in the Allergen‐specific and histamine BPTs within the cat AIT treated group at 12 months compared to baseline, but not within the dog AIT‐treated group. | AIT group: GR in 4 patients (ie, 1 anaphylactic reaction and 1 severe asthma attack during build‐up; and 2 mild asthma attack during maintenance)LR in 25% of all injections. |
| Alvarez‐Cuesta (1994)461 | Cat | 28 (15‐65 y) | Asthma and ARC | SCIT | ● 40 BU /every 4 weeks● Cat Fel d 1 13.2 μg | 1 year | † Short‐term symptom and medication scores, SPT, conjunctival provocation test, and allergen‐specific BPT.Methacholine BPT unchanged in the active group; worsen in the placebo group (no comparison active vs placebo reported for this outcome | AIT group LR in 7 subjects (1.5% of all administered doses)GR in 3 subjects treated with epinephrine (0.41% of all administered doses) |
| Alvarez‐Cuesta (2007) 466 | Cat | 33 (16‐51 y) | ARC ± Asthma | SLIT | 2 drops/dailyof vial containing Cat Fel d 1 0.51 μg/ml | 1 year | † Total and Nasal Symptoms Scores, peak expiratory flow on natural exposure challenge and SPT | No local or systemic adverse reactions experienced by either group |
AIT: allergen‐specific immunotherapy; ARC: allergic rhinoconjunctivitis; BPT: bronchial provocation test; BU: biologic units; GR: generalized reactions; LR: local reactions; NR: not reported; SCIT: subcutaneous allergen immunotherapy; SLIT: sublingual allergen immunotherapy; SPT: skin prick test; s‐IgE (specific IgE); s‐IgG (specific IgG); SQ: standardized quality units.
†: Statistically significant improvements (p < 0.05) in the AIT vs. control groups
‡: No significant difference in the AIT vs. control groups.
Table 6.
Summary of double‐blind placebo‐controlled‐randomized controlled trials of allergen immunotherapy to molds in the pediatric age
| Author | Allergen | Participants n (age, y) | Allergic Disease | AIT type | Target Maintenance dose/Major allergen content | Treatment Duration | Main Efficacy Results | Main Safety Results |
|---|---|---|---|---|---|---|---|---|
| Horst (1990)486 | Alternaria alternata | 24 (5‐56 y) | ARC ± Asthma | SCIT | ● 2000 BU/every 2 weeks● Alt a 11.6 μg | 1 year | † Symptoms score, medication score, allergen‐specific nasal challenges, SPT, s‐IgG. | AIT group: GR mild in 2 subjects |
| Tabar (2008)486 | Alternaria alternata | 28 (7‐29 y) | ARC ± Asthma | SCIT | ● 1670 UBE/every 4 weeks● Alt a 10.1 μg | 1 year | † Respiratory symptoms (after 6 months), peak expiratory flow and Asthma Subjective Severity. ‡ Symptoms (respiratory, nasal, ocular), medication scores, and rhinitis subjective severity after 12 months. | AIT group: GR mild in 2 subjects |
| Kuna (2011) 492 | Alternaria alternata | 50 (5‐18 y) | ARC ± Asthma | SCIT | ● 5000 TU/every 4‐6 weeks● Alt a 18 μg | 3 years | † Symptom‐medication score;Asthma/ARC‐related quality of life at year 2 and year 3. † s‐IgG4 level at year 3. † allergen‐specific nasal challenge 1 year after the end of the study.‡ Symptom‐medication score at year 1. | AIT group:LR mild in 7 subjects.GR mild in 1 subjectPlacebo group:GR Mild in 2 subjects |
| Tabar (2019) 492 | Purified Alt a 1 (Alternaria alternata) | 111 (12‐44 y) | ARC ± Asthma | SCIT | High Dose● Alt a 10.37 μg /every 4 weeks Low Dose● Alt a 10.2 mcg mcg/every 4 weeks | 2 years (first year included a placebo control group) | † Medication score for the high‐dose SCIT after 1 year† SPT, s‐IgG4/s‐IgE ratio in both high‐ and low‐dose active groups.‡ Symptom‐medication score for the low‐dose SCIT after 1 year | AIT group:13 (5.4%) immediate LR172 (71.7%) late LR38 (15.8%) GR mild (grade 1 World Allergy Organization Grading System)17 (7.1%) GR moderate (grade 2 World Allergy Organization Grading System)No significant between‐group differences in the incidence of adverse reactions |
| Cortellini (2010)489 | Alternaria alternata | 27 (14‐42 y) | ARC ± Asthma | SLIT | ● 5 drops of the 10000 RU vial/every other days; ● Alt a 1 1.5 μg/ml in the 10000 RU vial (≈ 0.4 μg/dose) | 10 months | † Symptoms score, medication score, and SPT. s‐IgE increased in the AIT group ‡ s‐IgG | AIT group: LR mild in 1 subject |
| Dreborg (1986)489 | Cladosporium herbarum | 30 (5‐17 y) | Asthma | SCIT | ● 100000 BU/every 4 weeks● major allergen NR | 10 months | † Medication scores, allergen‐specific conjunctival and bronchial sensitivity ‡ Symptom scores (eye, nose, and bronchial) and peak expiratory flow rates after 6 months | AIT group:LR in 4 subjectsGR in 13 of 16 subjects |
| Malling (1986)489 | Cladosporium herbarum | 22 (16‐54 y) | Asthma | SCIT | ● 100000 BU/every 4 weeks ● major allergen NR | 5‐7 months | † Symptom‐medication scores | AIT group: LR in 8 subjectsGR, asthma deterioration in all 11 actively treated subjects during the build‐up, and 3 anaphylactic reactions. |
AIT: allergen‐specific immunotherapy; ARC: allergic rhinoconjunctivitis; BU: biologic units; GR: generalized reactions; LR: local reactions; NR: not reported; SCIT: subcutaneous allergen immunotherapy; SLIT: sublingual allergen immunotherapy; SPT: skin prick test; s‐IgE (specific IgE); s‐IgG (specific IgG); RU: standardized in radioallergosorbent test units; TU: total units; UBE: unidades biológicas equivalents.
†: Statistically significant improvements (p < 0.05) in the AIT vs. control groups.
‡: No significant difference in the AIT vs. control groups.
Table 7.
Summary of double‐blind placebo‐controlled‐randomized controlled trials of allergen immunotherapy to cockroach in the pediatric age
| Author | Allergen | Participants n (age, y) | Allergic disease | AIT type | Target maintenance dose/major allergen content | Treatment Duration | Main efficacy results | Main safety results |
|---|---|---|---|---|---|---|---|---|
| Wood (2014)500, 502 | Cockroach | 89 (5‐17 y) | ARC or Asthma (mild) | SLIT | High Dose● 7370 BAU (0.84 mL)/twice daily● Bla g 1 202 μg/dose ● Bla g 216.8 μg/doseLow dose● 3685 BAU (0.42 mL)/daily● Bla g 1 50 μg/dose ● Bla g 24.2 μg/dose | 3 months(no clinical response assessed, only biomarkers) | † Increase in cockroach s‐IgE level for both the high and low‐dose active group † Increase in cockroach s‐IgG levelin the high‐dose active group‡ s‐IgG and s‐IgG4 for the low‐dose active group‡ s‐IgG4 for the high‐dose active group) ‡ blocking antibody response for both high and low‐dose active groups | AIT group:LR in 6 subjects receiving low dose LR in 1 subject receiving high dosePlacebo group:LR in 1 subject |
AIT: allergen‐specific immunotherapy; ARC: allergic rhinoconjunctivitis; BAU: bioequivalent allergy units; GR: generalized reactions; LR: local reactions; SLIT: sublingual allergen immunotherapy; s‐IgE (specific IgE); s‐IgG (specific IgG).
†: Statistically significant improvements (p < 0.05) in the AIT vs. control groups.
‡: No significant difference in the AIT vs. control groups.
Methods
MEDLINE/PubMed was searched using the following subject headings, “rhinitis,” “rhinoconjunctivitis,” “conjunctivitis,” “asthma,” “allergy,” “immunotherapy,” “allergen immunotherapy,” in combination with one of the following terms, “cat,” “dog,” “horse,” “pet,” “furry animal,” “animal dander,” “mold,” “fungus,” “fungi,” “Alternaria,” “Cladosporium,” and “cockroach.” The search strategy was limited to human studies on immunotherapy from any period, that were randomized, and included a controlled population. Articles cited in selected studies were also included after being reviewed for appropriateness. When no or very limited evidence was found in children, evidence available in adults was reported.
Immunotherapy for allergy to furry animals
Over the past decades, the prevalence of sensitization to furry animals, in particular to cats and dogs, has increased in Westernized societies, where household pet ownership is common.450 In these countries, specific IgE sensitization to cat or dog can affect over 25% of the atopic population and it tends to progressively increase with age.454, 455 Sensitization to major allergens from cat (Fel d 1) or dog (Can f 1) during early childhood increases the risk of developing allergic respiratory symptoms in adolescence, with multiple sensitizations to cat or dog allergens conferring the highest risk.455
Environmental avoidance of furry animals may not be emotionally acceptable to pet‐owning households and cannot be completely achieved given the ubiquitous presence of such allergens also in homes, schools, and public environments where no pets are present.456 For patients with allergic respiratory diseases driven by animal dander, who are not well controlled on adequate allergen avoidance and pharmacotherapy, current guidelines and consensus documents recommend considering AIT with standardized extracts of documented quality.3, 450 However, reproducible evidence of clinical efficacy for dog AIT is missing and high‐quality studies showing the effectiveness of cat AIT are very limited.
Cat allergy
There are a few DBPCRCTs on AIT to cat allergy, which have mainly evaluated subcutaneous immunotherapy (SCIT) in adult patients and have shown mixed results in terms of clinical and immunologic efficacy by using different allergen extracts and administrations regimens.457
Compared with placebo, SCIT with cat extract has demonstrated to induce significant improvement in patient‐reported symptom‐medication scores and/or in allergen‐specific provocation tests (conjunctival, nasal, and bronchial), but not in non‐specific bronchial reactivity, in adults with asthma and allergic rhinitis (AR) after 12 months of treatment.457, 458, 459, 460, 461 Only 2 DBPCRCTs have evaluated the effect of SCIT in children and adolescents with asthma and cat allergy, showing significant improvement in allergen‐specific bronchial reactivity after 12 months of active treatment 458, 459 (Table 5). Regarding immunologic changes, SCIT with cat extract has demonstrated to induce a reduction in skin test reactivity and a dose‐dependent increase in allergen‐specific IgG and IgG4, with an optimal maintenance dose containing 15 μg of Fel d 1 462, 463, 464
Only 2 high‐quality studies have examined the effect of sublingual immunotherapy (SLIT) with cat extract.465, 466 Nelson et al. 465 included 41 adults and reported no significant difference in symptom scores and nasal blockage index after 105 days of active SLIT compared with placebo. In a more recent DBPCRCT, Alvarez‐Cuesta et al.466 examined 50 monosensitized adults to cat dander, also including one adolescent, and found a significant reduction in total and nasal symptoms scores and skin test reactivity in the active SLIT group (who received a cumulative dose of 17.1 μg of Fel d 1) compared with placebo after 1 year of treatment.
There are limited safety data on AIT for cat allergy. This is due to the limited number of studies and the heterogeneity in allergenic products and protocols of administration used. As for other aeroallergens, systemic reactions were more frequently during SCIT than SLIT, affecting both children and adults.457 Although the majority of the systemic reactions were mild, anaphylactic reactions requiring epinephrine occurred during SCIT with cat extract (Table 5).
Recently, to improve the safety and efficacy profile of AIT in patients with cat allergy, new approaches such as using Fel d 1 T‐cell epitopes and alternative routes of delivery have been attempted. Of note, none of these experimental studies have included pediatric subjects. Intradermal injections of Fel d 1 T‐cell epitopes seemed at first a promising approach, with long‐term clinical benefit.467 However, in a recent phase III trial, the same immunotherapy product failed to demonstrate significant benefit compared with placebo in cat allergic adult patients.468 In the first human DBPCRCT, 3 intralymphatic injections of recombinant Fel d 1 at increasing doses 4 weeks apart showed a good safety profile and significantly increased nasal tolerance to cat extract and cat‐specific IgG4 levels in 12 adults with AR due to cat allergy.328 Nevertheless, intralymphatic immunotherapy remains an experimental treatment.
Dog allergy
Available high‐quality studies on AIT in patients with dog allergy are limited to 3 DBPCRCTs, which have evaluated the effect of SCIT with dog extracts in children and adults with asthma and have reported poor results in terms of clinical efficacy 469 (Table 5).
Valovirta et al. 470, 471 looked at 27 children and adolescents with asthma and dog allergy. After 1 year of treatment, those patients in the active SCIT group reported a significant increase in the conjunctival tolerance to dog extract and the dog‐specific IgG level, but no difference in allergen‐specific bronchial reactivity and symptom scores compared with placebo.
Sundin et al.458, 462 examined the clinical and immunologic effects of SCIT with cat or dog dander extracts in 41 children and adult with cat‐ or dog‐induced asthma. After 12 months of treatment, the 7 patients in the dog allergen‐treated group had a significant improvement in immunologic parameters, but no difference in symptom score and bronchial reactivity compared with placebo. Conversely, the parallel cat allergen‐treated arm showed a clinically significant reduction in cat‐specific bronchial reactivity as well as significant immunologic responses compared with placebo. Following the first year of AIT, the trial was unblinded, and 4 participants in the placebo group with dog allergy were offered SCIT for 3 years. In the final 11 patients receiving SCIT with dog extract, no significant improvements were noted after 2 and 3 years compared with the measurements recorded after 1 year of treatment, as well as in the long‐term follow‐up 5 years after the completion of the study.472, 473, 474 The authors concluded there was no clear clinical evidence to support the efficacy of SCIT with dog extract, although the study was underpowered to detect a difference in the predefined end‐points and there was no placebo group after the first year of study with which to compare follow‐up results.
A subsequent trial by Haugaard et al.459 evaluated whether the lack of efficacy of SCIT with dog extract could be due to concomitant cat allergy, for which patients were not receiving AIT in previous studies. This study enrolled 24 adolescents and adults with asthma, of which 12 were allergic only to cat, 2 were allergic only to dog, and 10 had both cat and dog allergy. The latter group was treated with both cat and dog extracts. However, participants with dog allergy, who received 12 months of SCIT with dog extract with or without cat dander extracts, reported no reduction in dog‐specific and unspecific bronchial reactivity compared with placebo.459
Regarding safety, overall SCIT with dog extract was well tolerated in these studies, with a reported rate of systemic reactions between 0% and 0.3%.469
The lack of clinical efficacy of AIT for dog allergy has been attributed to the poor quality of the allergenic extracts used in earlier studies and to the variability in sensitization patterns to dog allergens.469 So far, there is paucity of evidence regarding the optimal maintenance dose of AIT to dog required to induce clinical benefits. In a recent study, Lent et al. used a new acetone‐precipitated dog extract for SCIT and showed a dose‐dependent change in immunologic parameters, with the dose containing 15 μg Can f 1 per 0.5 mL maintenance dose producing the most relevant response. However, no significant changes in symptoms scores were reported after this AIT.475 Despite Can f 1 being a major dog allergen, it is not always the immunodominant allergen in individual patients. Recently, a prostatic protein found only in male dog urine, named Can f 5, has been identified as a new major allergen, with some dog allergic individuals being selectively sensitized only to this component.469
Other furry animals
Currently, there is insufficient evidence on the efficacy and safety of AIT to other furry animals such as horse, rodents, and rabbits. Available evidence is limited to a few open and small studies of SCIT for horse [34] and laboratory animal allergy [35], mainly including adults, whereas high‐quality studies are lacking.
Immunotherapy for mold allergy
Fungal allergy is a relatively common condition among individuals with respiratory allergy. The estimated prevalence in Europe is about 5% based on skin tests sensitizations to molds, with a large geographic variation between Nordic and Mediterranean countries.478 In the latter, prevalence can be as high as 20% among patients attending an allergy clinic for respiratory symptoms.479
There is a well‐documented relationship between sensitization and exposure to fungi from the genera Alternaria, Cladosporium, Penicillium, and Aspergillus and risk of developing and worsening of allergic respiratory diseases. Moreover, patients allergic to molds tend to have a more severe course of asthma or AR.451 Alternaria is the fifth most frequent sensitizing aeroallergen in Europe, after house dust mites, grass pollen, cat, and birch pollen.478
There is limited high‐quality evidence of clinical efficacy of AIT in mold allergy, which has addressed only Alternaria and Cladosporium allergy. Some of the published studies have major limitations, such as open design,480, 481 lack of placebo control group,480, 481, 482, 483, 484 lack of long‐term follow‐ups,483, 484, 485, 486, 487, 488, 489 and use of allergen extracts without proper standardization.488, 489 The difficulty in producing standardized allergenic extracts is due to complexity, variability, and stability of fungal allergen sources, which significantly hampers the use of AIT for mold‐allergic patients.451 Recently, Di Bona et al. have conducted a systematic review which included 7 RCTs on AIT with Alternaria (3 without a placebo control group) and 2 RCTs on AIT with Cladosporium, comprising a total of 268 patients (99 adults and 169 children). By using the GRADE approach, these authors have found low strength evidence supporting the use of AIT with fungal extracts for the treatment of respiratory allergy, due to inconsistencies in trial results, small sample sizes (median 27 patients per trial), and moderate‐to‐high risk of bias in almost all the studies evaluated.490
Alternaria alternata
There currently are 5 DBPCRCTs evaluating the efficacy of SCIT for respiratory allergy to Alternaria, 4 of which also included children and adolescents (Table 6).
Horst et al. 485 used a standardized extract in 24 patients (5‐56 years) monosensitized to Alternaria with AR, with or without asthma. After 1 year of SCIT with a maintenance dose containing 1.6 μg of the major Alternaria allergen Alt a 1 (corresponding to a cumulative Alt a 1 dose of 47.1 μg per year), the actively treated group showed significant improvements in symptoms (rhinoconjunctivitis and asthma) and medication scores, tolerance to Alternaria‐specific nasal challenge, and immunologic parameters compared with placebo.
In a subsequent study, Tabar et al.486 used a different standardized extract containing a lower concentration of Alt a 1 (maintenance dose with 0.1 μg of Alt a 1, corresponding to a cumulative Alt a 1 dose of 1.23 μg per year) in 28 patients (7‐29 years) monosensitized to Alternaria with mild AR and asthma. They found no significant differences in symptoms and severity of rhinoconjunctivitis and need for medications between the two groups throughout the 1‐year study period. Although respiratory symptoms and peak expiratory flow significantly improved in the actively treated group compared with placebo after 6 months, these differences lacked significance after 12 months of treatment. However, the underpowered sample size, the very low maintenance dose of Alt a 1 in the extract used, and the mild nature of allergic diseases at baseline could have contributed to the overall lack of efficacy of SCIT in this study.
The landmark SCIT trial with Alternaria extract was conducted by Kuna et al.,370 who used a high‐dose depot extract (cumulative Alt a 1 dose of 65.6 μg per year) for 3 years in 50 children and adolescents only allergic to Alternaria and with moderate‐to‐severe allergic rhinoconjunctivitis and/or intermittent or mild‐to‐moderate asthma. They showed SCIT to be effective in improving symptom‐medication scores and related quality of life progressively from the second year of treatment onward. This could suggest that studies with longer treatment duration (as recommended by Guidelines 3, 266) might have shown better efficacy results.
Prieto et al. 491 were the first to evaluate the efficacy and safety of SCIT with purified natural Alt a 1 in 39 adults with respiratory allergy associated with Alternaria exposure. After 1 year of treatment, with a maintenance dose of 0.2 μg/month (corresponding to a cumulative Alt a 1 dose of 2.7 μg per year), the active treatment group reported a significant increase in allergen‐specific IgG4, but showed no significant improvement in bronchial hyper‐responsiveness to direct and indirect stimuli, and in markers of allergic airway inflammation compared to placebo.491 Considering the good safety profile of this SCIT product, the authors recently evaluated the efficacy and safety of a higher maintenance dose of purified Alt a 1 (ie, 0.37 μg, corresponding to a cumulative Alt a 1 dose of 4.99 mg/year) compared to either the previously tested dose or placebo in 111 subjects (aged 12‐44 years) with allergic rhinoconjunctivitis with or without mild‐moderate controlled asthma and monosensitized to Alternaria.492 After 1 year of treatment, the per‐protocol analysis showed a significant improvement for the primary outcome (ie, patient‐reported nasal‐ocular symptom‐medication score) in the high‐dose, but not in the low‐dose active group, compared with placebo. An intention‐to‐treat analysis was only possible for the secondary outcome (ie, Alt a 1 specific‐IgG4/IgE ratio) which was significantly increased in both active groups, with the high‐dose group reporting greater results. Of note, the safety profile was good and similar across the 3 treatment groups.492
With regard to SLIT, there currently is only one DBPCRCT, which used a standardized extract in 27 patients (aged 14‐42 years) with Alternaria‐related moderate‐to‐severe AR plus intermittent asthma. The intervention lasted 10 months (corresponding to a cumulative dose of 60 μg of Alt a 1), with the SLIT‐treated group showing a significant reduction in symptoms, medication intake, and skin test reactivity compared with the placebo group.487
Regarding safety, both SCIT and SLIT with Alternaria extracts were generally well tolerated in these studies, with some mild general adverse reactions in the SCIT‐treated groups.490
Cladosporium herbarum
Two DBPCRCTs conducted more than 30 years ago have studied the efficacy of SCIT with Cladosporium extract in asthmatic children and adults.488, 489 Despite showing some level of improvement in respiratory reactivity 488 and symptom‐medication scores 489 after active treatment, the rate of systemic adverse reactions was significant in both studies. Notably, in the study by Malling et al.,489 all 11 actively treated subjects had episodes of asthma during the AIT build‐up phase, with 3 of them reporting anaphylactic reactions.
Immunotherapy for cockroach allergy
Cockroach sensitization is a significant contributor to asthma morbidity among children living in low‐income urban areas of the United States, who are exposed to high levels of cockroach allergens in their homes, as compared to non‐sensitized or non‐exposed children.493 Cockroach allergen exposures and sensitization based on skin prick testing have also been reported in some European urban populations, with some studies suggesting a clinical relevance of such sensitization in children.494, 495 However, a recent study using serum IgE‐component testing showed that isolated true sensitization to cockroach‐specific molecules was rare in Central Europe (0.6% of cases).496
Home‐based environmental control interventions have proven to be useful in reducing the exposure to cockroach allergens and associated asthma morbidity.497 However, such control strategies are difficult to maintain, and exposure may continue in other indoor environments such as schools.493 In this regard, cockroach AIT could potentially make a difference in the treatment of asthmatic patients who are sensitized and exposed to high levels of cockroach allergens. However, the lack of cockroach immunodominant allergen(s) and the highly variable sensitization profiles to cockroach antigens among individual patients have hindered the production of standardized extracts for AIT that contain all the relevant allergens,498 hampering the potential of AIT to provide full clinical benefit.
There currently is only one DBPCRCT on the clinical efficacy of AIT for cockroach allergy, which used SCIT with aqueous crude extracts from American cockroach in 42 adults with asthma, AR, or both. After 1 year of treatment, the active group showed a significant increase in cockroach‐specific IgG4 and an improvement in both non‐specific bronchial reactivity and symptoms scores, but not in medication scores, compared with placebo.499
More recently, Wood et al. 500 reported the results from 4 phase I/II pilot studies, which were designed to assess the safety and immunologic responses related to SLIT and SCIT with German cockroach extract. Two of the studies focusing on SLIT had a randomized, double‐blind design and one of them included children with perennial AR, mild asthma, or both (Table 7). Overall, SCIT was found to be immunologically more effective than SLIT, especially in cockroach‐specific IgG4 and blocking antibody responses, and considered to be more likely to produce clinical benefit. The subsequent phase III study on clinical efficacy of SCIT for cockroach allergy is currently ongoing.501
Conclusion
At present, there are limited high‐quality data to support the use of AIT for furry animals, molds, and cockroach, both in children and in adults with respiratory symptoms due to these allergens. The available evidence on the effectiveness of AIT with cat extract is based on a few DBPCRCTs with SCIT, which included a limited number of patients, primarily adults. These studies showed mixed clinical and immunologic efficacy outcomes and used different allergen products and administration protocols. Very low‐quality evidence supports the use of SLIT with cat extract. High‐quality studies have failed to confirm AIT effectiveness for dog allergy. Of note, most such studies on cat and dog AIT were conducted over 20‐30 years ago. To date, there are no high‐quality studies addressing the efficacy and safety of AIT to other furry animals such as horses, rodents, and rabbits.
With regard to AIT for mold allergy, low‐quality evidence supports the effectiveness and safety of SCIT for Alternaria‐related respiratory allergy in children and adults, whereas very low‐quality data support the use of SLIT with Alternaria extracts and SCIT with Cladosporium extracts. AIT for cockroach allergy has demonstrated to induce significant immunologic improvements, although clinical efficacy is currently suggested only by an individual study, which does not permit a conclusive evaluation. There is a strong need for large, well‐designed DBPCRCTs, using consistently standardized allergen extracts, common clinical outcomes scoring systems, and properly powered sample sizes including both children and adults before a clear recommendation can be made on AIT for all these aeroallergens sources. Efforts should also be made to assess cost‐effectiveness of AIT, which is currently not known.
Future research on immunotherapy to furry animal, molds, and cockroach should take into account the molecular complexity of these allergens, and work toward safer and more effective products tailored to individual patients’ sensitization profiles.
Conflict of interest
The authors declare there is no conflict of interest in relation to this article.
10 What is the Evidence for Venom Immunotherapy in Children?
Corresponding author:
Gunter Sturm, MD, PhD
Department of Dermatology and Venerology
Medical University of Graz
Auenbruggerplatz 8
8036 Graz
Austria
Phone: +43/316/385‐80318
Fax: +43/316/385‐12466
Email: gunter.sturm@medunigraz.at
Abstract
Up to 95% of the general population is stung at least once in their lifetime by a Hymenoptera species. Hymenoptera stings are the second leading cause of anaphylactic reactions in childhood. Hymenoptera venom allergy presents in the form of large local reactions (LLRs) and systemic sting reactions (SSR). The only causal treatment is venom immunotherapy (VIT), which protects up to 84% of patients allergic to honeybee venom and up to 96% of patients treated with vespid venom from further SSR. Honeybee venom allergy is more frequently seen in children but severe SSR are less common than in adults.
VIT is indicated in children (>5 years) following a systemic sting reaction that exceeds generalized skin symptoms. In children with only cutaneous symptoms, VIT is not routinely performed. However, decision on VIT has to be made individually concerning special conditions, for example, beekeeping parents or concomitant diseases or medication.
It has been shown by several studies that the VIT protocols and venom doses used for adults can be used for children as well: Independent of the up‐dosing protocol used, patients should be treated with a maintenance dose of 100 μg for at least 5 years. The long‐term immune tolerance induced by VIT is greater in children compared to adults, and only 5% relapsed up to 20 years after stopping VIT. However, further studies investigating the clinical effectiveness and the optimal duration of VIT in children are needed.
Introduction
Hymenoptera stings are the major cause of anaphylaxis in adults in Europe and North America502, 503, 504, 505 and the second leading cause of anaphylactic reactions in childhood, after food allergy.504 Studies indicate that 56.6 to 94.5% of the general population has been stung at least once in their lifetime by a Hymenoptera species.506 Hymenoptera venom allergy (HVA) is generally caused by stings of vespids of the genera Vespula (Vespula germanica and Vespula vulgaris), Vespa (Vespa crabro; hornet), and Polistes and of apids of the genera Apis (Apis mellifera; honeybee) and Bombus (eg, bombus terrestris; bumblebee).507 In central and northern Europe, stings from vespids and honeybees are the most prevalent, whereas stings from Polistes and Vespula are more frequent than honeybee stings in the Mediterranean area.508 Data from the European network of severe allergic reactions indicate that 70.6% of insect sting anaphylaxis was caused by wasps (Vespula spp.), 23.4% by honeybees, and 4.1% by hornets.504 The clinical presentations of Hymenoptera venom allergy are large local reactions (LLRs) at the sting site and systemic sting reactions (SSR). In general, a LLR has been defined as a swelling exceeding a diameter of 10 cm that lasts for more than 24 hours.508 In SSR, mild symptoms usually manifest as generalized skin symptoms including flushing, urticaria, and angioedema. Typically, dizziness, dyspnea, and nausea are examples for moderate reactions, while anaphylactic shock and loss of consciousness and/or cardiac or respiratory arrest all define severe SSR.509 Severe reactions are life‐threatening and have been attributed to fatalities. Although the reported frequency of fatalities is low with 0.03 to 0.48/million inhabitants/year,506 Hymenoptera sting mortality may have been underestimated due to unrecognized stings in unexplained causes of death. The mortality rate of children is unknown but probably lower than in adults.
All patients with HVA are advised to carry an emergency kit comprising of H1‐antihistamines, corticosteroids, and depending on their previous SSR, an adrenaline autoinjector.61 The only treatment that can potentially prevent further systemic sting reactions is venom immunotherapy (VIT), which is reported to be effective in 77‐84% of patients treated with honeybee venom and in 91‐96% of patients treated with vespid venom.510, 511 A systematic review for the European Academy of Allergy and Clinical Immunology (EAACI) allergen immunotherapy guidelines confirmed that VIT is effective in reducing subsequent SSR in both children and adults and that this treatment modality can have a significant beneficial impact on disease‐specific quality of life (QoL).91
Clinical aspects
The rate of self‐reported SSR in European epidemiological studies is up to 3.4%,512, 513 while LLRs occur in 0.9 to 20.5%514, 515 of children stung by a Hymenoptera species. The prevalence of 2.8% of asymptomatic sensitization has been reported in an Italian study, evaluating questionnaires and skin‐prick tests in primary schoolchildren.516 In 68% of children with a history of allergic reactions to insect stings, skin symptoms were the only clinical manifestation and systemic reactions rarely affected the cardio‐circulatory system.517 Risk factors for severe systemic reactions after Hymenoptera stings have been reported previously: Graif et al. investigated a population of adolescents aged 13‐14 years and found that children with atopic diseases (asthma, allergic rhinitis, atopic eczema) had a significantly higher rate of severe reactions than non‐atopic. Compared to 24.8% of non‐atopic children, 36.9% of children with an atopic disease reported an allergic reaction to an insect sting. Therefore, it was concluded that atopic diseases should be considered as risk factors for reactions of any severity.518 These results were confirmed by Yavuz et al. who found that severe reactions were related to mild eosinophilia and concomitant atopic diseases. Female sex was a risk factor as well; however, the study comprised of 76 children of whom 75% were boys.519 Differences between children and adults are listed in Table 8.
Table 8.
Clinical differences between children and adults with insect venom allergy
Indications and Contraindications
According to the guidelines on allergen immunotherapy of the EAACI, VIT is indicated in children following a systemic allergic reaction exceeding generalized skin symptoms with a documented sensitization to the venom of the culprit insect.61 In general, routine diagnostic investigation of HVA is based on the patient's personal history including the classification of the type and grade of reaction, skin testing, and/or the detection of specific IgE (sIgE) antibodies in serum520 and/or the basophil activation test (BAT).61 Component‐resolved diagnosis (CRD) was considered to significantly improve the diagnosis of insect venom allergy. However, it is still evolving and currently of limited use.521, 522 The diagnostic tools in children are not different from those used in adults.523
Children with previously mild SSR, which were limited to the skin, do not necessarily require VIT517, 524 since it has been shown that children with this type of reaction have only a 10% risk of re‐developing systemic reactions.523 However, particular situations of increased risk of re‐stings should be considered when deciding on VIT. Special conditions (eg, if parents are beekeepers, concomitant diseases, or medication) may justify VIT also in cases of mild SSR.525 For a summary, see Figure 14.
Figure 14.
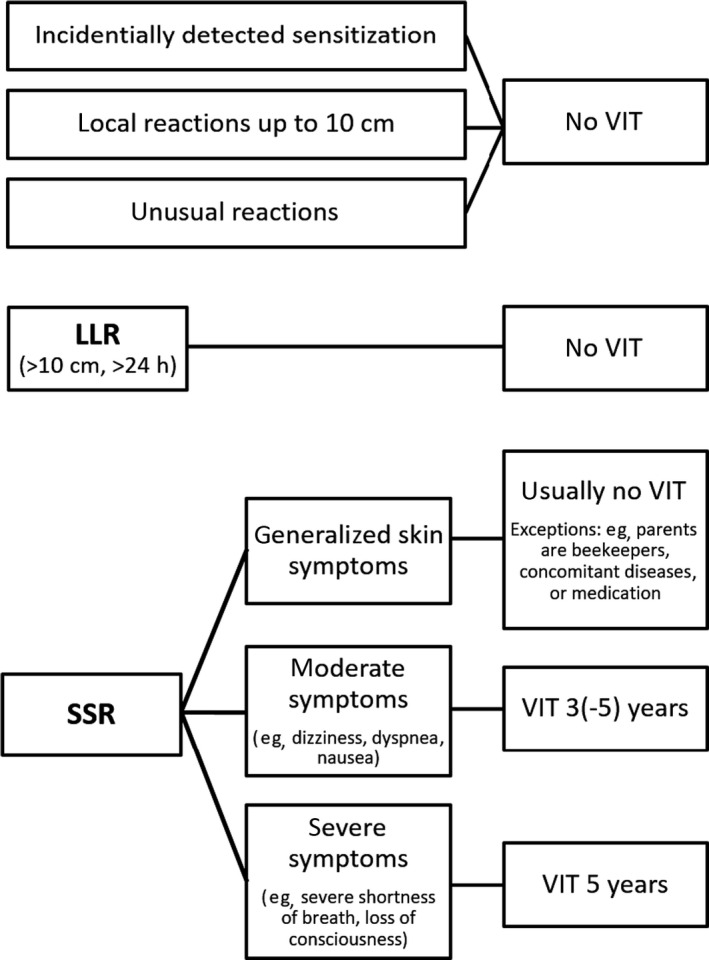
Recommendations for venom immunotherapy (VIT) for children. VIT is recommended in children who suffered from systemic reactions exceeding generalized skin symptoms (adapted from Sturm et al.522). LLR: large local reaction; SSR: systemic sting reactions
In contrast to former assumptions, an important number of children do not outgrow allergic reactions to insect stings. The risk of a subsequent SSR in patients who do not receive VIT is significantly higher in those with a history of a moderate to severe reaction than in those with a previous mild (cutaneous) reaction.526 If VIT is considered, special conditions have to be considered. However, most of the recommendations originate from adult populations: Cardiovascular and organ‐specific autoimmune disease must be stable and ACE inhibitor and/or beta‐blocker treatment may be continued if required, after informing the patient about potential risks. Malignant disease, multisystem autoimmune disease, and age <5 years are contraindications to VIT. However, successful VIT in children under four years has been reported.527 Therefore, if the SSR were severe, VIT may also be performed in children <5 years, when the child is likely to be cooperative.61
Treatment protocols
VIT consists of an up‐dosing phase and a maintenance phase, which is necessary to ensure a persistent effect of VIT. Different up‐dosing protocols are available: The conventional protocol, where venom preparations are administered weekly with increasing venom doses until the maintenance dose of 100 μg is reached in, at the earliest, 15 weeks, can be administered in outpatient clinics.528 With rush528, 529, 530, 531, 532 and ultra‐rush533, 534, 535, 536 protocols, maintenance dose is reached much faster: Using rush protocols, patients receive several injections with increasing venom doses on consecutive days until the maintenance dose is reached after 5 days. With the ultra‐rush protocol, patients reach the maintenance dose within a few hours, after receiving several injections. Both rush and ultra‐rush protocols are only performed in hospitals during an inpatient stay. That these protocols are also safe for children has been demonstrated in several studies.537, 538, 539, 540, 541 Cluster protocols (several injections per day, usually 1‐2 weeks apart) are another alternative to conventional protocols.542, 543 In general, conventional protocols appear to be best tolerated, while rush and ultra‐rush protocols are more frequently associated with side effects.544
The recommended starting dose for up‐dosing protocols ranges from 0.001 to 0.1 μg. However, it has been shown that a starting dose of 1 μg is safe in adults and children.545 The recommended interval for the maintenance dose is 4‐6 weeks for aqueous preparations and 6‐8 weeks for depot preparations.525 Two studies demonstrated an efficacy of 50 μg maintenance doses in children and therefore favor the use of reduced doses to improve safety and decrease treatment costs.546, 547 However, given the clear dose dependency of VIT,548 the fact that children tolerate 100 μg doses well leads to the recommendation to use a maintenance dose of 100 μg in pediatric patients as well.538, 539, 540, 549 A dose of 200 μg is recommended for patients (usually adults) who did not tolerate a sting challenge or a field sting while on 100 μg maintenance VIT.548
Systemic side effects and their risk factors
Side effects are generally mild and respond well to anti‐allergic treatment.383, 550 VIT‐related side effects in children have been shown to be at least as high as in adults.538 The most important risk factor is treatment with honeybee venom: It has been reported that there is a 3.1‐ to 6‐fold higher risk for systemic side effects due to treatment with honeybee venom compared to vespid venom.532, 544, 545 Rapid dose increase during the up‐dosing phase is an established risk factor as well.544, 551 Whether mastocytosis and/or elevated tryptase levels are a risk factor has been controversially discussed in the past.544, 552, 553, 554 Although the debate is ongoing, patients treated with ACE inhibitors and beta‐blockers are not considered to have a higher risk of adverse events.544, 555, 556 Importantly, severe initial sting reactions,544, 556, 557 positive skin tests at low test concentrations, and high specific‐IgE levels554, 556, 557 are not regarded as risk factors for adverse events.
Duration of VIT
Termination after about one or two years leads to a relapse in 22‐27% of cases, both adults and children,558, 559 and several studies conclude that a minimum of a five‐year treatment is superior for long‐term effectiveness.560, 561, 562, 563 Fiedler et al. reported that among 40 children treated with mean 3‐year VIT, 50% developed another systemic sting reaction at a median follow‐up of 13 years.564 Therefore, it is suggested that even in pediatric patients, VIT duration should be at least 5 years.565
Effectiveness
VIT induced long‐term protection in most children: 84.4% of patients treated with honeybee venom and 94.1% of patients treated with vespid venom were fully protected at accidental re‐stings: 32 of 54 of patients treated with bee venom and 17 of 34 patients treated with vespid venom were re‐stung by the respective venom.527 These findings confirm previous studies that patients treated with honeybee venom are at higher risk for treatment failure and relapse compared to those, who were treated with vespid venom.560, 566, 567 Eighty‐nine percent of adult patients treated with honeybee VIT tolerated sting challenge already one week after reaching the maintenance dose. Those patients, who were not protected with 100 μg of venom, tolerated sting challenges immediately after reaching the increased maintenance dose of 200 μg.568 Whether this applies for children as well remains to be investigated.
Golden et al. reported that in children with systemic reactions with cardiovascular or respiratory involvement, the risk of re‐developing anaphylaxis was 32% in untreated children compared to 1‐3% of those treated with VIT.526 In another study, 62% of allergic, untreated children tolerated subsequent stings, whereas 18% developed severe systemic reactions.569
Most effectiveness data are obtained during VIT, and there are only few reports on the outcome following VIT withdrawal for more than five years: 7‐7.5% of patients treated with vespid venom relapsed after 7 to 10 years,560, 567 while 15.8% had re‐sting reactions after stopping honeybee VIT.560 In children, the long‐term effect is superior compared to adults since only 5% with moderate to severe reactions relapsed after up to 20 years after stopping VIT.526
Conclusions
Honeybee venom allergy is more frequently seen in children but severe systemic reactions are less common than in adults.523 Treatment with VIT is recommended in children who suffered from systemic reactions exceeding generalized skin symptoms.61 In children with only cutaneous systemic reactions, VIT is not routinely performed61, 524, 570 since it has been shown that children with this type of reaction have only a 10% risk of re‐developing systemic reactions.523 However, some children may have an increased risk of re‐stings (eg, children of beekeepers) and VIT may be considered in these patients even in cases of skin symptoms alone.525 In general, VIT appears to be more effective in children compared to adults524, 560, 571 and it rapidly reduces the risk of future systemic sting reactions from 50‐70% without VIT to 1‐2% under VIT.566
Summary
Children with HVA are diagnosed and treated in much the same way as adults. In contrast to former assumptions, an important number of children do not outgrow allergic reactions to insect stings.526 Due to a lack of studies including a sufficient number of pediatric patients, most recommendations for children are derived from adults. It has been shown that the common used up‐dosing protocols are safe in children and that the long‐term immune tolerance induced by VIT is greater than in adults. However, most effectiveness data are obtained during VIT, and therefore, there is still a gap in the evidence for the clinical effectiveness of VIT in children and the optimal duration of VIT. In addition, the effect of VIT on health‐related quality of life should be investigated further.
Conflict of interest
G. Sturm reports grants from ALK Abello, personal fees from Novartis , personal fees from Bencard, personal fees from Stallergens, personal fees from HAL, personal fees from Allergopharma, personal fees from Mylan, outside the submitted work. L. Arzt‐Gradwohl has nothing to disclose in relation to this article.
11 Subcutaneous or Sublingual Allergen Immunotherapy for Allergic Rhinitis and Asthma in Children?
Corresponding author
Dr Aarif O. Eifan, MD
Allergy and Clinical Immunology,
Royal Brompton Hospitals NHS Foundation Trust,
NHLI, Imperial College London,
Dovehouse St, London SW3 6LY
Abstract
There is an increasing prevalence of allergic rhinitis and asthma induced by common allergens in children. Allergen immunotherapy (AIT) has been used over 100 years and has been shown to be effective in the treatment of allergic respiratory disease. Pharmacotherapy with topical corticosteroids is effective in the control of symptoms with improvement of lung function in allergic asthmatics and rhinitis but it does not modify the natural course of the disease. Allergen immunotherapy, involving the administration of increasing concentrations of allergen extract, is currently accepted as the only treatment modality that has the potential to modify the course of allergic disease.
This review exclusively focuses on AIT in children with allergic rhinitis and/or asthma and discusses the efficacy and safety of subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT) in children based on randomized controlled trials. Efficacy and safety of SCIT versus SLIT were compared based on evidence presented from randomized head‐to‐head comparative trials performed in children. Overall, based on currently published studies, there is an acceptable evidence of efficacy and safety for both SLIT and SCIT in well‐selected children with allergic rhinitis and well‐controlled asthma sensitized to pollen and house dust mite. More research on optimal regimen, efficacy, and safety of allergen immunotherapy in children is needed as definitive studies are lacking.
Keywords
Allergy, asthma, dust mite, immunotherapy, rhinitis, subcutaneous, sublingual
Clinical vignette
An 8‐year‐old boy has a history of nasal blockage all year around for the last 4 years. He has persistent clear‐watery nasal drainage and becomes troublesome with constant sneezing, nasal itching, and cough during the autumn and winter seasons. He developed shortness of breath and wheezing episodes at least 3 times a year for the last 2 years. He misses 5‐7 of school days per year due to asthma and rhinitis symptoms. He has been on daily oral antihistamines, nasal steroids spray, and intermittent use of inhaled corticosteroids and salbutamol as needed with little benefit. There is no history of hospital admission or systemic steroid use. He has one sibling who has atopic dermatitis and has maternal atopic history. Allergy testing was positive for house dust mite only. Dust mite prevention measures were taken with little improvement. How should this case be managed?
Introduction
Allergic rhinitis and asthma are currently the most common chronic respiratory disease in childhood worldwide with increasing prevalence rates reported in both developed and underdeveloped countries.293, 572 Pharmacotherapy with inhaled and nasal corticosteroids is effective in the control of symptoms with improvement of lung function in allergic asthmatics and rhinitis, but it does not modify the natural course of the disease.573, 574 Allergen immunotherapy (AIT), involving the administration of increasing concentrations of allergen extract, is currently accepted as the only treatment modality that has the potential to modify the course of allergic disease.293 Allergen immunotherapy has been shown to reduce allergic rhinitis (AR) and asthma symptoms and medication usage, prevent asthma development in patients younger than 18 years of age with grass pollen–induced AR, and increase quality of life, with a sustained long‐term effect.3, 87, 242, 575 Recent GINA guideline suggests AIT to be considered in mild‐moderate well‐controlled asthmatics as an intervention in management of asthma.576
Subcutaneous immunotherapy (SCIT) has been shown to be effective in reducing asthma symptoms scores, medication usage, and improve allergen‐specific and non‐specific bronchial hyper‐responsiveness.139 Similarly, it has been shown to be effective in controlling seasonal and perennial AR symptoms and reduction of medication use.136 This route of administration can occasionally be associated with adverse events including severe reactions which may be life‐threatening, hence needs to be administered in a specialist setting.136 Sublingual immunotherapy (SLIT) appears to be associated with a lower incidence of systemic reactions. Long‐term, SLIT results in the reduction and in a long‐term treatment as an adjunct to pharmacotherapy results in the reduction of both duration and dose of nasal and inhaled corticosteroids, and successful discontinuation along with an improvement of rhinitis and asthma symptoms, and lung functions.81, 577, 578 Sublingual immunotherapy is commonly associated with local adverse events including itching and swelling in the mouth, which occasionally might persist for several weeks. Otherwise, SLIT in both AR and asthmatics has a very good safety profile in post‐marketing surveillance of large cohorts and clinical trials involving children.70, 579
Both SCIT and SLIT have been shown to have disease‐modifying effect for both seasonal and perennial allergen‐induced asthma. Two years of treatment with grass pollen SCIT in a randomized double‐blind placebo‐controlled trial was associated with a substantial reduction in asthma symptoms and medication requirement, and bronchial hyper‐reactivity compared to placebo.368 A significant steroid‐sparing effect of AIT in asthmatic children was observed after 2 years of house dust mite (HDM)‐SCIT treatment when compared to pharmacological therapy in randomized control trial (RCT).373 Several previous double‐blind, placebo‐controlled trials of sublingual grass pollen, and HDM tablet immunotherapy156, 580 produced similar results showing persistent improvement in rhinitis and asthma symptoms and reduction in inhaled corticosteroids usage. There is evidence that both SCIT145 and SLIT58, 581 can prevent asthma development and progression in children. The aim of this review was to compare the efficacy and safety of SCIT and SLIT in children with asthma and/or AR based on published data. For this purpose, search engines were used to search for Cochrane meta‐analysis and systematic reviews as well as head‐to‐head RCTs investigating the efficacy of AIT for AR and asthma in children.
Allergen Immunotherapy in Allergic Asthma
Evidence from cochrane meta‐analyses for allergic asthma in children
There are two recent Cochrane reviews that compared AIT with placebo in asthmatic patients. Abramson et al139 assessed the effect of allergen SCIT in asthmatics. A total of 88 trials were included comprising of both adults’ and children pooled data. There was no subanalysis performed for the pediatric age group in this meta‐analysis. Overall, a significant reduction in asthma symptoms with standard mean difference (SMD) (‐0.59; 95% CI, −0.83 to −0.35) and medication requirement (SMD, −0.53; 95% CI, −0.80 to −0.27) was found in subjects receiving SCIT compared to controls with significant heterogeneity between studies (I 2 = 90% and I 2 = 66.9%, respectively). A subanalysis found significant reductions in symptom for both seasonal asthma (SMD, −0.61; 95% CI, −0.87 to −0.35) and perennial asthma (SMD, −0.48; 95% CI, −0.96 to −0.0). Bronchial hyper‐reactivity which has been recognized as a hallmark of asthma was reduced following allergen SCIT (SMD −0.35; 95% CI, −0.59 to −0.11). Normansell et al582 evaluated the efficacy of SLIT in asthmatics, which included 52 studies published up to March 2015 meeting the inclusion criteria with a total of 5077 participants to compare. Of the 52 studies, 25 recruited children only. There was a general trend that suggested SLIT being more efficacious compared to placebo, with reduction in bronchial hyper‐reactivity (SMD, 0.69; 95% CI, −0.04 to 1.43) but the quality of evidence was found to be low. Authors concluded that selected studies used unvalidated outcomes including symptoms and medication scores contributing to the low quality of evidence.
Adverse events reported in these Cochrane reviews were extracted for both local and systemic reactions in relation to SCIT139 or SLIT582 compared to the corresponding placebo. Local reactions were reported in 16 trials and 32 trials reported systemic reactions comparing allergen SCIT with placebo in asthmatics. The relative risk (RR) was 1.4 (95% CI, 0.97 to 2.02) for local reactions and 2.45 (95% CI; 1.91 to 3.13) for systemic reaction. The incidence of systemic reactions per patient was estimated at 5% to 7% with 0.06% to 1.01% estimated incidence per injection.139 Authors conclude that allergen SCIT in asthmatics is not without risk of significant systemic reaction. On the other hand, SLIT adverse events in asthmatics were reported in 22 studies comprising of 2560 participants comparing with corresponding placebo.582 In this SLIT Cochrane review that assessed adverse events using risk difference (RD) analysis, serious adverse events were uncommon in asthmatics treated with allergen SLIT (RD 0.0012; 95% CI, −0.0077 to 0.0102) with no difference with placebo.
Evidence from systematic reviews and meta‐analyses for allergic asthma in children
Recent comprehensive systematic review conducted by Dhami et al69 on efficacy and safety of allergen SCIT and SLIT compared to placebo for allergic asthma identified 98 RCTs in both children and adults. Total of 30 RCTs involving allergen SCIT and 30 allergen SLIT were analyzed for clinical efficacy in children. Allergen extracts included in the analyses were grass, trees, HDM, cat, dog, molds, latex, and weeds. Improvement of symptoms scores was observed for both SCIT and SLIT compared to placebo (SMD, −0.58; 95% CI, −1.17 to −0.01). When compared to SLIT in children, SCIT was found to be more efficacious in controlling asthma symptoms (SMD, −1.64; 95% CI, −2.51 to −0.78). Allergen immunotherapy in asthmatic children was found to improve medication requirement compared to placebo (SMD, −0.49; 95% CI, −0.98 to 0.00) (Figure 15). On subanalyzing individual allergens, both AIT‐HDM (SMD, −2.10; 95% CI, −3.29 to 0.91) and tree pollen (SMD, −1.08; 95% CI, −1.79 to −0.37) were found to be more efficacious in perennial and seasonal asthma, respectively. On indirect comparison, subgroup analysis of AIT safety found that there was a higher risk of local or systematic reaction for SCIT RR = 2.22, (95% CI, 1.48 to 3.33) than SLIT RR = 1.49, (95% CI, 1.13 to 1.98). Authors concluded that both SCIT and SLIT reduce symptoms and medication scores in allergic asthma with modest increased risk of adverse events.69
Figure 15.
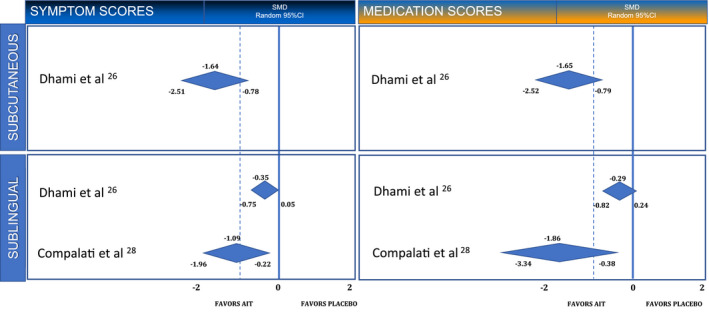
Summary of systematic reviews and meta‐analyses on SCIT and SLIT for allergic asthma. AIT, Allergen immunotherapy
Rice et al577 recently reported results of a thorough systematic review using grading scheme that extracted data from 28 RCTs and 12 non‐RCTs conducted in children with allergic asthma for clinical efficacy and safety of AIT (25 SCIT trials, 15 SLIT). They found a moderate Strength of Evidence (SOE) that SCIT reduces long‐term asthma medication requirement; however, they reported that SLIT reduces medication requirement with low SOE compared to controls. There was insufficient data from SCIT or SLIT to grade asthma symptoms as studies included did not use validated questionnaire.577 Local and systemic reactions were reported more frequently in both SCIT and SLIT. Authors concluded that in asthmatic children, SCIT is effective in reducing asthma medication use and that adverse events are common in both SCIT and SLIT, but anaphylaxis is reported rarely.
Lin et al583 conducted systematic reviews on SLIT only for the treatment of allergic asthma and rhinitis. After identifying 8156 potentially relevant trials, a total of 63 SLIT‐RCTs with 5131 participants were included for the comprehensive systematic review. Twenty trials (n = 1814 patients) included children only while 17 included both adults and children, and 26 enrolled adults only. The majority of studies evaluated efficacy of SLIT on HDM‐induced asthma. The strength of evidence that SLIT is efficacious in reducing asthma symptoms in children was found to be high with more than 60% of studies reporting greater than 40% improvement compared to controls. There was a moderate grade evidence showing SLIT decreases medication requirement in asthmatics when compared to control. Local adverse events were more frequent in SLIT patients compared to placebo with rare systemic adverse events. There were no fatal reactions or death in SLIT‐treated patients. Authors concluded that SLIT is associated with reduction in asthma symptoms and medication use.
Compalati et al systematically reviewed the efficacy of HDM‐SLIT in perennial respiratory allergic diseases.584 A total of eight RCTs (n = 220 children) were included to analyze SLIT efficacy on HDM‐induced asthma symptoms. There was a significant reduction in asthma symptom (SMD, −1.09; 95% CI −1.96 to −0.22) and medication requirement (SMD, −1.86; 95% CI, −3.34 to −0.38) (Figure 15). Authors concluded that SLIT in HDM allergy is effective in children with prospect of modifying the natural history of allergic diseases.
All reviews recommended the need of large homogeneous trials with head‐to‐head studies comparing SLIT and SCIT in children.
Evidence from head‐to‐head SCIT vs SLIT trials in children with allergic asthma
Head‐to‐head RCTs comparing efficacy and safety of SLIT versus SCIT in children with asthma are very limited. There are only 3 RCTs conducted in children with allergic asthma that compared head‐to‐head the efficacy and safety of SCIT versus SLIT306, 585, 586, 587 (Table 9). Of these, 2 were open label306, 585, 587 and one was double‐dummy placebo‐controlled trial.586
Table 9.
Overview of head‐to‐head trials comparing SCIT and SLIT in pediatric allergic asthma and allergic rhinitis
| Study | Study design | Patients (n) | Age (Mean:SD) | Allergen extract | Allergen extract, manufacturer | Cumulative 1 year dose | Duration | Clinical Results for Allergic Asthma | Efficacy on asthma symptom and medication use | Clinical Results for AR | Efficacy on AR symptom and medication use |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eifan AO et al 29,30 | RCT, no placebo |
16 SCIT 16 SLIT 16 Control |
SCIT (7.0 ± 1.8) SLIT (6.5 ± 1.6) Control (7.6 ± 2.0) |
Der p, Der f 1 : 1 mixture |
SLIT; drops, ALK‐ABELLO SCIT; ALUTARD SQ, ALK‐ABELLO |
SLIT; 73 876.8 STU SCIT; 1131540 SQ‐U |
3 Year | Both SCIT and SLIT leads to reduction in asthma symptoms, medication usage, nasal and skin reactivity with sustained long term clinical improvement. | SCIT=SLIT>control |
SCIT leads to reduction in rhinitis symptoms. Both SCIT and SLIT leads to reduction in medication usage when compared to control group |
SCIT>SLIT>control for ARSS SCIT=SLIT for ARMS |
| Yukselen A31 | RCT, double‐dummy, placebo |
10 SCIT 10 SLIT 10 Placebo |
SCIT (10.9 ± 3.2) SLIT (9.2 ± 3.4) Placebo (10.1 ± 2.7) |
Der p, Der f 1 : 1 mixture |
SLIT drops (NovoHelisen Oral, Allergopharma) SCIT (NovoHelisen Depot, Allergopharma) |
SLIT; 173,733 TU SCIT; 43,770 TU |
1 Year | Both SCIT and SLIT leads to reduction in asthma symptoms, medication usage when compared to baseline. SCIT was more effective in reducing asthma symptoms compared to controls. | SCIT>SLIT>placebo |
SCIT leads to reduction in allergic rhinitis and medication requirement compared to placebo. SLIT more effective in reducing allergic rhinitis and medication requirement compared to placebo. |
SCIT>SLIT>placebo |
| Keles S et al32 | RCT, no placebo |
11 SCIT 13 SLIT 14 SCITPlusSLIT 12 Control |
SCIT (7.1 ± 1.8) SLIT (8.6 ± 2.1) SCITPlusSLIT (8.2 ± 1.4) Control (7.9 ± 2.8) |
Der p, Der f 1 : 1 mixture |
SLIT; drops, ALK‐ABELLO SCIT; ALUTARD SQ, ALK‐ABELLO |
SLIT;27150.7 STU SCIT; 1231540 SQ – U SCITPlusSLIT; 331540 SQ‐U and 21600 STU |
1.5 Years | Both SCIT and SLIT leads to reduction in asthma symptoms, medication usage, nasal and skin reactivity. Similar effect between SCITPlusSLIT regime and SCIT only. |
SCITPlusSLIT=SCIT SCIT>SLIT>control |
SCITPlusSLIT reduces rhinitis symptoms SCIT only and SCITPlusSLIT reduces medicine requirement when compared to control |
SCITPlusSLIT >SCIT SCIT=SLIT=control for AR symptoms. SCITPlusSLIT=SCIT>SLIT>control for AR medication requirement |
ARMS: Allergic Rhinitis Medication Score; ARSS: Allergic Rhinitis Symptom Score; Der f: Dermatophagoides farinae; Der p: Dermatophagoides pteronyssinus; RCT: randomized controlled study; SCIT: subcutaneous immunotherapy; SLIT: sublingual immunotherapy.
Eifan et al585 included 48 children with mild‐to‐moderate HDM‐allergic asthma in this randomized open‐label trial. After one year of treatment, there was significant reduction in asthma symptoms, medication requirement, and skin reactivity to HDM in both SCIT and SLIT compared to controls. This clinical efficacy was maintained in both SCIT and SLIT after 3 years of treatment with persistent reduction in asthma symptoms and medication use.306 There was no difference when head‐to‐head comparison was made between SCIT and SLIT regarding clinical efficacy. Two cases of systemic reaction were observed in the SCIT group during up dosing phase with one needing adrenaline administration. Otherwise, there were no adverse reactions observed in the SLIT or control group during the study.
Yukselen et al586 randomized 30 children with mild‐to‐moderate HDM‐induced asthma in double‐blind, double‐dummy single‐centered trial comparing SLIT versus SCIT. Compared with controls, a significant reduction in medication requirement was observed in the SCIT‐treated group only but not SLIT. There was no difference when head‐to‐head comparison was made between SCIT and SLIT regarding clinical efficacy. No systemic adverse reactions were reported in any of the groups. Keles et al587 conducted an open‐labeled randomized prospective study comparing 4 arms of treatment. They compared a novel mode of treatment which includes combination of SCIT for updosing followed by SLIT for maintenance (SCIT plus SLIT), SCIT only, SLIT only, and control group in mild‐moderate HDM‐induced asthmatic children. There was a significant reduction in asthma symptoms and medication use in SCIT, SCIT plus SLIT, and SLIT groups compared to controls.
Allergen immunotherapy in allergic rhinitis
Evidence from cochrane meta‐analyses for allergic rhinitis in children
There are 2 Cochrane meta‐analysis published that compared the efficacy of AIT with placebo in AR patients.81, 136 Radulovic et al81 published their findings in 2010, in which they performed a subgroup analysis of 15 SLIT‐RCTs comprising of 1392 treated AR children. In this subanalysis, the efficacy of sublingual drops and tablets in seasonal rhinitis (grass pollen, weed, and trees) using different regimens including pre‐, co‐seasonally, or whole year and the efficacy of SLIT with HDM were pooled and analyzed. The authors showed a significant reduction in AR symptoms (SMD, −0.52; 95% CI, −0.94 to −0.10) and medication requirements (SMD, −0.16; 95% CI, −0.32 to 0.00) in SLIT‐treated patients compared to placebo. Studies were found to be homogenous for medication score (I 2 = 36%) and a substantial heterogeneity for symptom scores (I 2 = 92%). There were no further Cochrane reviews on efficacy and safety of SLIT on AR published thereafter. Calderon et al136 did not perform subgroup analysis in children in the Cochrane meta‐analysis on SCIT for AR as there were no RCTs of SCIT conducted exclusively in children with AR prior to meta‐analysis.
Evidence from systematic reviews and meta‐analysis for allergic rhinitis in children
In the most recent meta‐analysis by Dhami et al,10 both SCIT and SLIT were found to improve AR symptoms compared to placebo (SMD, −0.65; 95% CI, −0.86 to −0.43) and (SMD, −0.48; 95% CI, −0.61 to −0.36), respectively. However, significant heterogeneity was reported in SCIT (I 2 = 62%) and SLIT (I 2 = 69%) studies mainly due to different regimens, dose, and extract used. On subgroup analysis, 12 SCIT and SLIT studies were performed in children with AR. Allergen immunotherapy was found to reduce AR symptoms (SMD, −0.25; 95% CI, −0.46 to −0.05, I 2 = 54%) with mild benefit on reducing medication requirement (SMD, −0.021; 95% CI, −0.42 to 0.01, I 2 = 25%). When comparing modes of treatments in children with AR, SLIT had strong evidence of efficacy over placebo in reduction of rhinitis symptoms and medication requirement (SMD, −0.42; 95% CI, −0.62 to −0.21, I 2 = 0% and SMD, −0.59; 95% CI, −1.12 to −0.07, I 2 = 38%). It is important to note that in this subanalysis, only five clinical trials were involved (2 HDM, 2 grass pollen, 1 Parietaria judaica pollen) with 183 patients in the SLIT active treatment arm and 183 patients in the placebo group. Interestingly, no subgroup analysis was performed on the effect of SCIT on AR symptom and medication scores in patients younger than 18 years of age, although there are head‐to‐head studies comparing SCIT and SLIT with active pharmacotherapy in pediatric age.306, 585, 586, 587
In a systematic review and meta‐analysis performed by Dretzke et al,84 the effect of AIT on seasonal rhinitis was assessed by indirectly comparing SCIT versus SLIT. On subgroup analysis in children, there were no differences between SCIT and SLIT for reduction of AR symptom, medication requirements, and improvement in quality of life. Lin et al583 analyzed 12 studies involving 1065 rhinitis children comparing SLIT and placebo by grading the strength of evidence as high, moderate, low, and insufficient based on the risk of bias and the magnitude of effect in the involved studies. Authors reported a moderately strong evidence supporting SLIT as efficacious in improving rhinitis symptoms and medication use in children, but it is important to note that a meta‐analysis could not be conducted as the outcomes in different studies were reported heterogeneously.583
In another meta‐analysis performed by Di Bona et al involving both children and adult data, the efficacy of SCIT, SLIT tablet, and SLIT drops for grass pollen–induced seasonal AR was compared indirectly. SCIT was found to be significantly effective for symptom improvement (SMD, −0.92; 95% CI, −1.26 to 0.58) and medication requirement (SMD, −0.58; 95% CI, −0.86 to −0.30).588 The authors reported a higher degree of evidence for SCIT efficacy in terms of symptoms and medication requirement when compared to a moderate to low degree of evidence of efficacy for SLIT drops (SMD, 95% CI, −0.25; −0.45 to −0.05 and −0.37; −0.74 to −0.00, respectively) and SLIT tablets (SMD, 95% CI, −0.40; −0.54 to −0.57 and −0.30; 0.44 to −0.16, respectively).
Compalati et al584 analyzed 5 RCTs comprising of 235 children comparing the effect of HDM‐SLIT and placebo in AR children. They found no difference from placebo in perennial rhinitis symptom reduction and medication requirement. Authors suggested that significant SLIT efficacy found only for asthma symptoms but not for rhinitis might be due to insufficient sample size of subgroup analysis. Kim et al363 included the studies published until 2012 comparing SCIT and SLIT with placebo and found a moderate to strong degree of benefit of SCIT in reducing rhinitis medication requirement with weak to strong degree of benefit for rhinitis symptoms to SLIT. Authors concluded that due to few numbers of studies and some inconsistent results, the strength of evidence was low to support superiority of SCIT over SLIT in reducing AR symptoms and medication use.
Evidence from head‐to‐head SCIT vs SLIT trials in children with allergic rhinitis
There are several head‐to‐head RCTs comparing the efficacy and safety of SCIT versus SLIT involving either only adults or adults, children, and adolescents with AR.152, 589, 590, 591, 592 In this review, RCTs that included children only306, 585, 586, 587 will be discussed in detail (Table 9).
Eifan et al585 compared the efficacy and safety of SCIT versus SLIT with the control group in a prospective randomized trial of HDM‐sensitized rhinitis children. This was the first prospective study comparing the efficacy of SCIT and SLIT in a single center in children. At the end of first year, AR symptoms decreased by more than half in both SCIT and SLIT compared to controls (p = 0.01 and p = 0.03, respectively) suggesting that both SCIT and SLIT are effective for controlling AR. Allergic rhinitis medication requirement was significantly reduced only in SLIT. Karakoc‐Aydıner et al306 re‐assessed at the end of 3 years of treatment and found persistent reduction in AR symptoms in both modes of AIT compared to controls. Significant reduction of AR medication requirement in both SCIT and SLIT groups was observed compared to controls at the end of 3 years of treatment. There was no difference in clinical efficacy between SCIT and SLIT when compared head to head at the end of 3 years. The main limitation of these prospective studies in children was the low number of participants in each treatment arm and the absence of a placebo group. This is mainly due to ethical reasons, as it is difficult to design long‐term SCIT involving trials using double‐blind placebo control methodology in pediatric age group. Nevertheless, the methodology used in recent trials allowed to make a thorough comparison of the two immunotherapy modalities with that of pharmacotherapy alone in a prospectively randomized manner for the 3 years of treatment in pediatric patients for the first time.
Yukselen et al586 reported the first double‐blind placebo‐controlled trial comparing head‐to‐head SCIT and SLIT in HDM‐AR with/or without asthma. Standardized SLIT drops (D.pt. and D.f; 50:50, mite extracts, Novo‐Helisen Oral, Allergopharma) were taken at increased doses daily for 12 weeks and the maintenance dose was 3 times/week as 28 drops of 1000 TU/ml of was self‐administered at home. Standardized D.pt. and D.f. (50/50) mite extracts (Novo‐Helisen Depot, Allergopharma) were used for SCIT (Table 9). Induction phase continued 12 weeks with weekly injections followed by the maintenance phase with repeated injections every four weeks. The cumulative dose for SLIT and SCIT is presented in Table 9. Allergic rhinitis symptoms and medication requirement decreased significantly in SCIT but not in SLIT group when compared to placebo. Authors concluded that HDM‐SCIT was more effective in reducing AR compared to HDM‐SLIT. Keles et al587 introduced a novel regime of SCIT plus SLIT group in which SCIT was administered in the build‐up phase and SLIT in the maintenance phase and compared head to head with SCIT only, SLIT only, and controls. At the end of one year of treatment, they found AR symptoms were improved in 88%, 36%, and 94% in SCIT only, SLIT only, and SCIT plus SLIT groups, respectively compared to controls with similar efficacy for medication requirement. There was no significant change at 4 months and 1st year of treatment in SLIT only group in terms of both AR symptoms and AR medication requirement suggesting that building up with injections and then maintenance via sublingual route might augment the efficacy of AIT in maintaining the control of rhinitis symptoms. Therefore, authors concluded that the successive administration of SCIT and SLIT is more efficacious than SLIT alone in controlling rhinitis symptoms.
In summary, these results of head‐to‐head comparison in children with AR and asthma showed that SCIT is superior to SLIT in symptom reduction and medication use in the first year of treatment but this difference disappears at the end of three years of treatment. Administration of SLIT in the maintenance phase following SCIT in induction phase might be an option to augment the efficacy of SLIT particularly for the first year of treatment.
Evidence of safety and tolerability of SCIT versus SLIT in children
In the head‐to‐head SCIT versus SLIT trials, local adverse events were reported in both SCIT‐ and SLIT‐treated children with similar numbers seen in placebo group but resulted in withdrawal in active SLIT group due to troublesome symptoms586 (Table 10). There were systemic adverse events involving the respiratory system occurred in the active SCIT group during the induction phase585 leading to treatment discontinuation. No systemic symptoms reported in the SLIT group.
Table 10.
Adverse events reported in head‐to‐head prospective randomized controlled studies including children and adolescents with allergic rhinitis and asthma
| Study | Allergen extract | Patients (n) | Age | Drop‐out | Treatment duration | |||
|---|---|---|---|---|---|---|---|---|
| Build‐up | Maintenance | |||||||
| Eifan AO29,30 | Der p, Der f |
16 SCIT 16 SLIT 16 Control |
SCIT (7.0 ± 1.8) SLIT (6.5 ± 1.6) Control 7.6 ± 2.0) |
2 patients (SCIT, SR) |
3 years | Two grade 3 & 4 systemic reactions, one local swelling of 7 cm | None | None |
| Yukselen A31 | Der p, Der f |
10 SCIT 10 SLIT 10 Placebo |
SCIT (10.9 ± 3.2) SLIT (9.2 ± 3.4) Placebo (10.1 ± 2.7) |
1 Patient (active SLIT, LR) |
2 years |
Local reaction in 2 active & 2 placebo |
None |
Local reaction, 3 active, 2 placebo |
| Keles S32 | Der p, Der f |
11 SCIT 13 SLIT 14 SCITPlusSLIT 12 Control |
SCIT (7.1 ± 1.8) SLIT (8.6 ± 2.1) SCITPlusSLIT (8.2 ± 1.4) Control (7.9 ± 2.8) |
2 Patient (SCIT) | 1 year | Two grade 3 systemic reaction | None |
None None in SCITPlusSLIT |
AR: Allergic rhinitis; Der f: dermatophagoides farinae; Der p : dermatophagoides pteronyssinus; HDM: house dust mite; IT: immunotherapy; LR: local reaction; SCIT: subcutaneous immunotherapy; SLIT: sublingual immunotherapy; SR: systemic reaction.
In a recent large‐scale study, the adverse systemic reactions of AIT in children and adolescents were assessed via a prospective survey among physicians.593 Since this was an observational, non‐interventional study, the mode of treatments using AIT (SCIT, SLIT drops, and SLIT tablets) was not proportionally selected. A total of 19699 SCIT and 131550 SLIT doses were administered of which the estimated frequency of systemic side effects with SCIT and SLIT was 0.11% and 0.004%, respectively. Ninety percent of SCIT reactions were seen with natural extracts and during induction phase, and nearly 80% of reactions with SLIT were seen with SLIT drops involving mostly respiratory system. Overall only three cases were defined as anaphylaxis and all were due to SCIT. The discontinuation rate due to adverse reactions in children was found to be one in five with no differences in between the two AIT modalities.593
In a pooled double‐blind placebo‐controlled analysis of safety of 5‐grass pollen tablet, 2 phase‐III studies included 312 children and adolescents.594 There was no serious drug‐related adverse event in both active drug and placebo groups. However, 4.5% in active and 1.3% in placebo group prematurely discontinued from the study due to adverse events. In another multicenter open‐label study, the adherence rate for children and adolescents taking SLIT grass pollen tablet was reported as 97.1% after 1 month, 92.1% after first season, 92% after 2 years, and 83.1% in intention‐to‐treat analysis after 3 years of treatment in a real‐life setting.595 There are exist some safety data about SCIT or SLIT separately; however, large‐scale head‐to‐head comparative studies for SCIT and SLIT are needed in pediatric patients in order to evaluate adverse events in relation to adherence and its impact on clinical efficacy.
Recommendation and unmet needs
The child described in the vignette presents with typical perennial severe rhinitis symptoms and co‐existing mild intermittent asthma. Treatment with dust mite prevention and long‐term pharmacotherapy had little benefit in controlling his symptoms and improving his quality of life including school attendance. According to the current international recommendations, allergen immunotherapy should be considered in cases where pharmacotherapy alone is not effective. Sublingual or subcutaneous immunotherapy are to be considered in this child's management as current guidelines87, 596 agree that AIT (SCIT or SLIT) is not contraindicated in children with mild‐to‐moderate well‐controlled allergic asthma with co‐existing allergic rhinitis and should be considered in controlled HDM driven asthma.
When selecting either SCIT or SLIT, the main points to be considered should be the availability of products, cost, safety and convenience. Currently, both mode both treatments are widely available in local hospitals in most European countries and UK. Sublingual allergen immunotherapy has been shown to be safe and convenient, allowing patient's self‐administration with a consequent reduction of the indirect costs. Both, SCIT and SLIT are effective in treating allergic rhinitis with or without asthma induced by aeroallergen and to be recommended for a minimum duration of 3 years.
There remains an unmet need to perform an adequately powered study in children to evaluate the optimum duration, regime of treatment and starting age, as well as the preventive effects of different modes of treatment including tablets, SCIT and SLIT drops, and age of treatment.
Conclusion
There is an acceptable evidence that treatment of allergic asthma and rhinitis with AIT is proved to be effective and clinically safe in studies with limited sample size. Quality of evidence in children studies was found to be moderate‐high for SCIT and low‐moderate for SLIT. The relative efficacy of SCIT and SLIT with different allergens remains to be determined. It is important to note that the results of the meta‐analysis should be interpreted with caution as the quality of evidence being limited to small studies, substantial heterogeneity of study protocol and an overall low grade of evidence that does not allow firm conclusions. Further studies in children and adolescents with sound methodological quality, adequate sample size, and outcome observations lasting longer than 2 years are needed to address remaining uncertainties.
Conflict of interest
The authors declare that there is no conflict of interest in relation to this article.
12 Practical Aspects of Using Allergen Immunotherapy in Children
Corresponding author
Stefania ArasiMD, PhD
Pediatric Allergology Unit,
Bambino Gesù Hospital (IRCCS),
Piazza S. Onofrio, 00161 Rome, Italy
Email: stefania.arasi@yahoo.it
Joint first authorship
A Muraro and S Arasi join the first authorship
Abstract
Allergen immunotherapy (AIT) is currently a well‐established treatment in the clinical management of allergic respiratory diseases and is considerably relevant for the pediatric population in light of the AIT potential to modify the natural history of the disease. AIT should be offered to any child with moderate‐severe allergic rhinitis starting from 5 years of age, further to an adequate risk‐benefit assessment which includes adherence. An earlier age and mild disease could be considered based on an individual evaluation. Both subcutaneous AIT and sublingual have a good efficacy and safety profile with safer outcomes for SLIT compared to SCIT. Only standardized products with documented evidence of clinical efficacy should be used. Patients and families should be appropriately informed of the AIT protocols and possible side effects as well as of the duration of 3 up to 5 years. A summary of key evidence with relevance to clinical practice is presented as well as knowledge gaps and future research needs.
Keywords
allergen‐specific immunotherapy; allergic rhinitis; children; IgE‐mediated allergic diseases; prevention; sublingual immunotherapy; subcutaneous immunotherapy
Abbreviations
AIT – Allergen immunotherapy
AR – allergic rhinitis
SCIT – subcutaneous immunotherapy
SLIT – sublingual immunotherapy
WAO – World Allergy Organization
Impact statement
Allergen immunotherapy is currently the only active treatment for IgE‐mediated respiratory diseases. AIT has a unique ability to modify the natural history of the disease; this is of considerable relevance especially in childhood. A critical pragmatic approach is provided with a specific focus on the pediatric population.
Introduction
Allergen immunotherapy (AIT) is recognized as a clinically effective and safe therapy for allergic respiratory diseases. It has a unique ability to modify the natural history of the disease. For this reason, childhood has been proposed as the best time window for intervention, both in terms of treatment and prevention. AIT was introduced in clinical practice more than one century ago by Noon.198 Unfortunately, it was initially performed with allergen extracts of poor quality and definition. Over the years, AIT has evolved in several aspects: from the allergen content to vehicle and adjuvant, from the route and schedule of administration to the production, distribution, and documentation.89, 199 Altogether, these changes have led to a remarkable improvement both in the efficacy and in safety profile of the treatment. Today, AIT is accepted and routinely prescribed worldwide in respiratory allergies.
In clinical practice, the conventional routes of administration are currently only subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT). Others, such as the epicutaneous and intralymphatic routes, are under investigation.3 SCIT is usually administered as a depot adsorbed on aluminum hydroxide or tyrosine. Both unmodified and modified extracts may be used. SCIT schedules are heterogeneous, as they differ in the number of injections per visit, number of visits per week, and the rapidity with which the patient reaches the maintenance dose. Depending on the specific product, conventional SCIT schedules involve at least one injection up to three per week during a build‐up phase that lasts a variable number of weeks, followed by a maintenance phase, during which injections are given every two up to six weeks over a period of years. The use of SLIT is more recent: The first report in a randomized clinical trial dates back to 1986597(that is 75 years later the first SCIT report 198), and the first use of SLIT in tablet form was reported in 2001. After those early trials, SLIT has rapidly gained scientific credibility and broad clinical application. SLIT may be administered either as fast‐dissolving tablets or drops to be retained under the tongue for at least one minute and then swallowed. In seasonal allergic rhinitis (AR), SLIT is recommended to be taken either continuously or pre‐/co‐seasonally, starting at least two months (better four months) before the commencing of the pollen season.3
When to consider AIT?
Indications ‐ AIT is recommended in allergic rhinitis/conjunctivitis with/without allergic asthma, with an evidence of specific IgE sensitization toward clinically relevant inhalant allergens.3 Since it is an allergen‐specific treatment, the identification of the allergen(s) driving patient's symptoms is the first imperative step in order to select correctly the allergen product to be used for the specific patient. Especially in polysensitized patients, the first‐line tests (ie, skin prick test and levels of IgE toward allergen extracts) may be insufficient to clearly identify the key inhalant allergen driving allergic symptoms. Molecular diagnostics may help in discriminating primary sensitizations from cross‐reactions and, therefore, in selecting the allergen(s) to be used. Nasal or conjunctival provocation testing has been proposed to prove in loco the clinical relevance of the allergic systemic sensitization. However, they are currently mainly confined to research setting. Only standardized AIT products with documented clinical evidence of efficacy should be used when available 10, 325 (‘product‐specific evaluation’) as there is no class‐effect in AIT.3, 267
In terms of indications, the severity of AR symptoms represents a crucial point. Currently, AIT is recommended in patients with moderate‐to‐severe AR symptoms according to ARIA classification.598 However, AIT may also be considered in patients with less severe allergic rhinitis who wants to take advantage of its long‐term effects, including the potential of AIT in preventing asthma.87, 90, 325
The use of AIT in asthma has been a matter of debate owing to the lack of trials designed to investigate AIT in asthma, especially in pediatric population. Most data derive from studies designed for AR in which some participants also suffered from asthma,69 and some clinically relevant aspects (eg, pulmonary function, intake of inhaled steroids, rate of exacerbation, and coexistence of infections) were not always assessed nor reported. Based on current literature, uncontrolled and severe asthma remains an absolute contraindication to the prescription of AIT.325, 383 Some studies specifically designed for asthma, although in adults, found that AIT can decrease the need of inhaled corticosteroids for asthma control and, more importantly, that AIT can lower the rate of asthma exacerbations.156, 333, 373 Furthermore, the most recent Global Initiative for Asthma Management document included, for the first time, SLIT as a possible add‐on therapeutic option in asthma treatment of adults with concomitant AR to house dust mites.599
Concerning the age, there is limited evidence in preschoolers.334, 335, 600 It has been established by expert consensus that AIT in a child can be started at 5 years of age for safety reasons However, AIT can be considered in children younger than 5 years of age on individual basis. This should take into account several factors, including the effect on the quality of life, the expected acceptance, and adherence to AIT.
In order to schematically provide a guide to healthcare professionals delivering AIT to children with allergic respiratory diseases, practical considerations and a procedural algorithm are shown in Table 11 and Figure 16, respectively.
Table 11.
Practical considerations for healthcare professionals delivering AIT to children with allergic respiratory diseases
| Training and facilities | ● Expertise in the diagnosis and differential diagnoses of AR and allergic asthma by history and supporting SPT or specific IgE testing. |
| ● Training in recognition and management of severe allergic reactions including anaphylaxis. | |
| ● Availability of equipment and trained personal to manage severe allergic reactions. | |
| ● Training in administration of specific AIT products. | |
| ● Facilities to observe patient for at least 30 minutes with SCIT injections and initial dose of SLIT. | |
| Assessing patient and deciding on best approach | ● Effective communication with patients and his/her family about practicalities of AIT, expected benefits and potential adverse effects. |
| ● Identification of clinical contraindications to AIT. | |
| ● Select an AIT product with documented evidence for efficacy and safety, for the patient's specific presentation, wherever possible. | |
| Undertaking AIT | ● Start AIT with SLIT for seasonal allergic diseases at least 2, and preferably 4, months before the pollen season. For SCIT, the updosing period should be finalized before start of the relevant season. |
| ● Preferably start AIT for perennial allergic disease when allergen exposure is lowest and avoidance measures are in place. | |
| ● Dose reductions (usually 50%) or split doses for adverse effects, intercurrent illness, or delayed dosing as recommended by Summary of products for SCIT. | |
| ● Dose interruption with oral lesions and other issues as recommended by Summary of products characteristics for SLIT. | |
| ● Facilities to regularly follow‐up patient promoting adherences to therapy and watching for adverse effects. |
Adapted from Roberts et al.3
Figure 16.
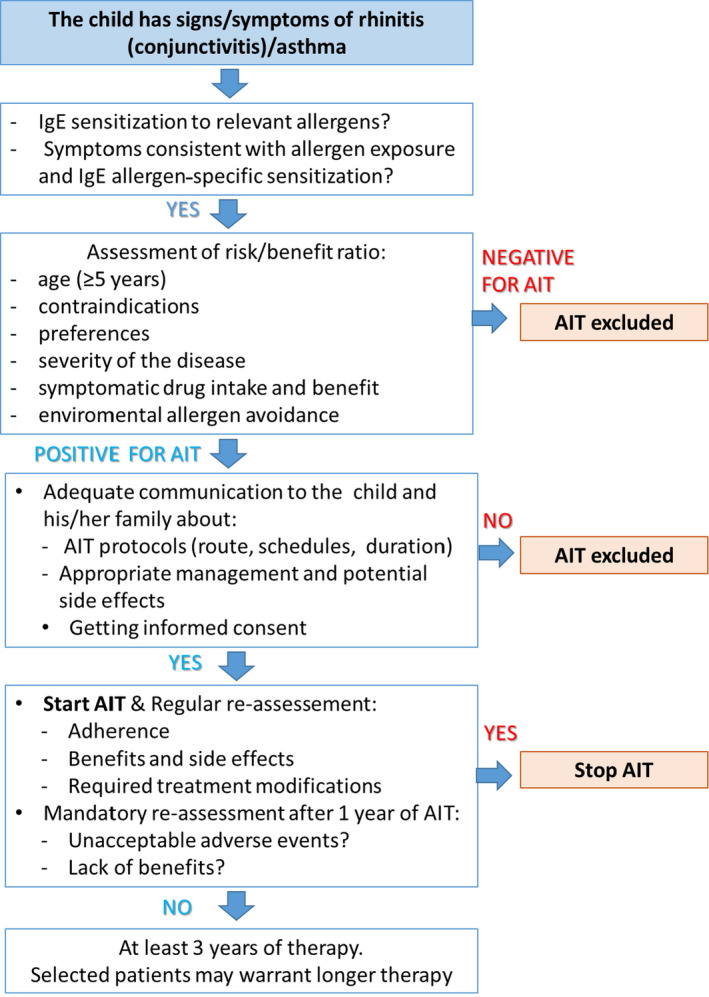
Algorithm on the clinical approach of allergen immunotherapy (AIT) in children suffering from allergic respiratory diseases.
Contraindications ‐ Before commencing AIT, clinicians should carefully evaluate any patient‐related absolute or relative contraindication (Table 12). This should include children's and caregivers’ preference and likely adherence given the need for 3–5 years of treatment. Therefore, any medical or social condition that might prevent patients from attending frequent clinical visits or taking SLIT drops daily represents an absolute contraindication. Although most data come from case reports and case series of severe adverse events and not on higher quality studies, uncontrolled asthma is recognized as an absolute contraindication as well as any active not controlled severe systemic autoimmune disorder or active malignant neoplasia. A careful revision of the summary of product characteristics is mandatory in order to consider specific contraindications for individual preparations.
Table 12.
Current contraindications to AIT in children
| Absolute contraindications |
|
| Relative contraindications |
|
Efficacy
There are many studies investigating the efficacy and safety of AIT in the medical literature.10, 601 Nevertheless, the interpretation of current evidence remains challenging because of the heterogeneity among studies. The results of individual studies are difficult to compare because studies have used different populations, different methods (eg, diagnostic criteria; allergens, formulation, and strength of products used; schedules; dose; route of administration; duration of the intervention), and different outcomes. Additionally, many studies have small sample size and miss adjustment for confounders. Furthermore, not all AIT products in current use have sufficient data to support their efficacy in clinical practice. For all these reasons, the current guidelines recommend strongly an individual product‐based evaluation of the evidence for efficacy before treatment with a specific product is initiated.3, 267
AIT is a good example of the need for stratification and, therefore, proposed as potential model for the so‐called “precision medicine”. This fits the expression: “the right treatment for the right patient at the right time”. Therefore, the correct identification of the key allergen(s) driving the symptoms is pivotal likewise the identification of good responders to the treatment. In this perspective, specific relevant factors able to affect AIT efficacy should be evaluated carefully.
Especially in areas with a high biologic complexity, such as the Mediterranean one, a major problem is the high number of polyallergic patients, even in the pediatric population. Recently, the European guidelines3 have suggested that polysensitized patients who are polyallergic for taxonomically related homologous allergens can be recommended to receive either a single allergen or a mixture of homologous allergens from that biologic family that covers all the major allergens. Patients who are polyallergic for non‐homologous allergens may be recommended to start AIT with either the allergen responsible for most of their AR symptoms or separate treatment with the two clinically most important allergens.
Adherence to the treatment is another important issue able to affect the efficacy of such a long treatment working on the immunologic system. Therefore, it is an obligatory element to be considered before commencing AIT (see above “Contraindications”).
Other allergen factors may relevantly influence AIT efficacy, such as standardization of allergen products; allergenic mixture; and specific allergens. When possible standardized allergen products should be used both for diagnosis and for AIT in order to select properly both the eligible patient and the most suitable and effective treatment. In terms of allergen mixtures, the European Medicines Agency (EMA) recommends to mix only homologous allergens (that are usually taxonomically related) and to not include allergens with enzymatic activities (eg, house dust mites).602 For some less common allergens, the so‐called orphan allergens data are overall lacking and clinical decision should be taken on individual basis.
For how long should AIT be prescribed?
Currently available data suggest that AIT (both routes SCIT and SLIT) should be used for 3 to 5 years in children to achieve a significant clinical efficacy. This may also modify the clinical history of allergic respiratory diseases and prevent its evolution. Therefore, the recommended duration for either SCIT or SLIT is at least 3 years 3, 80, 148, 603 and probably five years for venom.61
A clinical improvement (both in terms of symptoms decrease and in terms of drug intake) can be reasonably expected already in the first year of therapy. Several causes should be considered when the treatment fails, such as wrong diagnosis, too short duration of therapy, inadequate dosage, inadequate adherence.363 In this context, patients treated with SLIT, who take the dose at home, should be evaluated every 3‐ 6 months in order to: verify the clinical benefit of SLIT, to ensure the adherence to treatment, to consider when indicated stopping AIT treatment. Once a clinical benefit is ascertained, SLIT should be continued for a period of at least three years. After three years of treatment, AIT can be prolonged for additional 2 or more years, based on the outcomes of the treatment upon informed decision of the family and the patient. Therefore, the total duration of AIT (ie, 3 to 5 years or even more) must be established on individual basis. Given the potential of AIT to modify the natural history of the disease, it might be more profitable to commence AIT in the first phase rather than in the late course of the allergic disease when non‐reversible damages are present and the progression cannot be modified. Relief of allergic symptoms and long‐lasting efficacy are two goals that can be obtained in allergic children. Namely in order to achieve successful results, it is important to begin AIT during childhood when bronchial asthma is often less severe, and children show one or few sensitizations.604
Safety
The safety profile of both SCIT and SLIT in the pediatric population has been thoroughly evaluated in most of the clinical studies.80, 605 It has been shown that both SCIT and SLIT are safe and well‐tolerated treatments in children with AR and adequately controlled allergic asthma.
SCIT‐In the subcutaneous route, most reactions are local reactions at the injections site: redness, itching, or swelling.10 In children, local reactions in the arm(s) could be particularly uncomfortable. Local measures (eg, cooling or topical glucocorticoids) or oral antihistamines are helpful for these reactions. In case of enlarged local reaction (redness/swelling >10 cm in diameter), clinicians should consider to adapt the next dose according to the summary of product characteristics. Systemic reactions (eg, asthma, angioedema, generalized urticarial, anaphylaxis) have been described in around 2% of all SCIT patients. Although very rarely, fatal or near‐fatal systemic reactions due to SCIT have been reported.606For this reason, all SCIT injections should be administered by experienced and trained healthcare professional in clinical setting with all facilities to attend any severe adverse event, including severe anaphylaxis.3, 10, 607 Over 80% of adverse reactions occur within 30 minutes after SCIT injection.65, 593 Therefore, it is recommended to perform clinical observation of the patient for at least 30 minutes after the injection in the clinic.3
SLIT: only one appears to have a better safety profile than SCIT; reported systemic adverse events are generally fewer and less severe. Possibly this difference may be explained, at least in part, by the amount of immunologically active allergen. In fact, although the total amount of allergen in SLIT is usually classically 50‐ to 100‐fold the doses used for SCIT, sublingually administered allergens are diluted and flushed away by saliva, so that the actual amounts of allergens that finally penetrated the mucosa and encountered antigen‐presenting cells including dendritic cells have to be estimated much lower than the initially administered amounts.608In addition, tonsils may play a role in induction of allergen‐specific T‐cell tolerance as demonstrated by in vivo existence of Bet v 1‐specific Treg cells in human tonsils609and dendritic cells have been shown to be more tolerogenic in the oral mucosa than in the skin.610, 611 Anaphylaxis was described anecdotically and no fatality, due to SLIT which has been reported in about three decades of clinical use. A high frequency of local adverse reactions has been reported with SLIT usually limited to the oral mucosa.603 However, other reactions have been recorded with SLIT, such as asthma, urticaria, abdominal pain.603 A risk for eosinophil esophagitis (EoE) has been recognized and currently EoE should be considered a contraindication for SLIT. It is recommended to observe patients for at least 30 minutes after only the first dose by staff able to manage anaphylaxis in clinic. Other SLIT doses (that are the majority) are administered—usually daily—outside the clinical setting, without medical supervision. Therefore, specific instruction should be provided to patients regarding the management of adverse reactions (mostly local), unplanned interruptions in treatment, and situations when SLIT should be temporarily withheld (eg, oropharyngeal infections, acute gastro‐enteritis, asthma exacerbations).
In order to standardize the system of reporting AIT adverse events, the World Allergy Organization (WAO) position papers recommend a consistent use of systemic Reaction Grading and Classification 607and SLIT Local Reactions Grading System.612 Even when AIT is suitable for children with allergen rhinitis and well‐controlled mild‐to‐moderate allergic asthma, absolute and relative contraindication plus risk factors (Table 13) should be always considered.
Table 13.
Risk factors for systemic reactions during AIT
|
Adapted from Pfaar et al.267
Any window for prevention?
In the era of the so‐called “allergic pandemic”, prevention represents one of the major concerns, especially in pediatrics. It is known that the clinical expression of respiratory allergies tends to change over time, according to a “natural history”, the so‐called “atopic march”. In the typical sequence, allergic rhinitis often precedes the onset of asthma and, therefore, it can be considered a risk factor for the development of allergic asthma 613, 614
In addition, there is often the tendency to develop new sensitivities along time.615 Therefore, as AIT is the only disease‐modifying treatment in allergic diseases, the potential preventing effects of AIT have been suggested and investigated for the prevention not only of the development of allergic comorbidities in patients with established allergic diseases, but also the development of first allergic disease in not‐sensitized children and pregnant mothers and in still healthy children with allergic sensitization. Certainly, alongside efficacy, another pivotal issue to be considered is the safety profile, especially in the context of prevention in healthy individuals. However, there is overall lack of evidence on this topic. The recent European guidelines suggest that a three‐year‐long course of subcutaneous or sublingual AIT can be recommended for children and adolescents with moderate‐to‐severe AR due to grass or birch pollen in order to prevent the onset of allergic asthma for up to two years post‐AIT cessation in addition to its sustained effect on AR symptoms and medication.278, 285, 581, 605, 616 A few trials suggest a preventive effect on the onset of asthma symptoms and medication use as long as 10 years of follow‐up.58, 145, 285 However, data are scarce for AR triggered by house dust mites (with just one study in adults) or other allergens different from grass/birch.285, 605, 617 Because of inconsistent results, at this moment AIT cannot be recommended for the prevention of new sensitizations, nor in patients with allergic rhinitis and/or asthma nor in healthy individuals.87, 273, 285, 618 There is lack of evidence also about the role of AIT in preventing the onset of allergic diseases in individuals with early‐life atopic manifestation (eg, atopic eczema and food allergy) and in healthy subjects (with or without atopic sensitization).273, 275 However, there are good data providing evidence for the prevention of the development of asthma in children with allergic rhinitis and pollen allergy up to 2 years after the end of the treatment.58, 145, 278 Further well‐designed clinical trials are needed to better clarify the value of AIT as disease‐modifying treatment in the prevention of allergic diseases
Current gaps and future perspectives
Several studies have evaluated AIT; however, there is broad heterogeneity among them, which underlines the importance of improvement in harmonization of clinical trial design.64 This heterogeneity affects the robustness of the current evidence. For instance, studies differ in the populations evaluated. This deserves some consideration. For instance, children with atopic heredity have a higher risk of developing allergic disease(s). Furthermore, children with IgE sensitization and/or early manifestations of atopic diseases (eg, atopic dermatitis and food allergy) have a higher risk for developing other allergic manifestations (eg, asthma).619, 620, 621 The age of the study population is another pivotal factor as the phenotypic expression may change with age and some manifestations may even disappear spontaneously.10, 620 To compare the results of individual studies is made more difficult because of the heterogeneity not only of the population investigated, but also of methods of analysis, outcomes (eg, diagnostic criteria; allergens, formulation, and strength of products used; schedules; dose; route of administration; duration of the intervention), and outcomes’ report.622 Furthermore, many studies have small sample size and missing adjustment for confounders. The regimen of administration and the amount of the maintenance dose as well the protocols of administration are not standardized; c) the description and classification of side effects is variable among studies; quality of life and evaluation of health economics are overall missing. Moreover, the content of major allergen(s) remains largely variable among manufacturers, the availability of AIT products differ among countries and not all AIT products used provide sufficient data to support their efficacy in clinical practice. Therefore, an individual product‐based evaluation of the evidence for efficacy is strongly recommended before treatment with a specific product is initiated.3, 267 To recognize the gaps in the current evidence is a preliminary and mandatory phase in order to stimulate in the near future the development of longitudinal, prospective, well‐designed studies with the final goal of a “precision medicine/prevention”, tailored on the specific individual characteristic of the patient.264
Conflict of interest
Antonella Muraro reports speaker's fee for ALK, Stallergenes, Mylan, Nestlè. Susanne Halken was member of Steering Committee for the Grazax Asthma Prevention (GAP) study, published 2017; paid by ALK‐Abelló for participation in meetings only; since 2018 country coordinator (DK) and involved in a multicenter RCT (MT‐11) on HDM SLIT (Acarizax) for HDM allergic asthma in children; only study expenses were paid by the company.
Oliver Pfaar reports grants and personal fees from ALK‐Abelló, grants and personal fees from Allergopharma, grants and personal fees from Stallergenes Greer, grants and personal fees from HAL Allergy Holding B.V./HAL Allergie GmbH, grants and personal fees from Bencard Allergie GmbH/Allergy Therapeutics, grants and personal fees from Lofarma, grants from Biomay, grants from Nuvo, grants from Circassia, grants and personal fees from ASIT Biotech Tools S.A., grants and personal fees from Laboratorios LETI/LETI Pharma, personal fees from MEDA Pharma/MYLAN, grants and personal fees from Anergis S.A., personal fees from Mobile Chamber Experts (a GA2LEN Partner), personal fees from Indoor Biotechnologies, grants from Glaxo Smith Kline, personal fees from Astellas Pharma Global, outside the submitted work.
Other authors have no conflict of interest to declare.
13 Adjuvants in Allergen‐Specific Immunotherapy
Corresponding Author:
Dr Mohamed H. Shamji
Immunomodulation and Tolerance Group, Allergy & Clinical Immunology
Inflammation, Repair and Development, National Heart & Lung Institute, Imperial College London, 1st Floor, Room 111, Sir Alexander Fleming Building, South Kensington Campus
London SW7 2AZ, United Kingdom
Tel: +44 (0) 20 75943476
Emails: m.shamji@imperial.ac.uk
Joint first authorship
M Kirtland and J A Layhadi join the first authorship
Abstract
Allergen‐specific immunotherapy (AIT), administered subcutaneously (SCIT) or sublingually (SLIT), is an effective treatment for IgE‐mediated allergic diseases, in particular, rhinoconjunctivitis with or without asthma. Long‐term treatment with AIT is associated with persistent clinical benefits following discontinuation. Although AIT is the only disease‐modifying treatment, it is associated with several challenges that include poor patient compliance due to the protracted duration of treatment to induce a state of tolerance and the potential risk of anaphylaxis.
The clinical efficacy and safety of AIT can be enhanced when it is administered in combination with adjuvants. Characteristics of the ideal adjuvant include a robust safety profile, enhancing immunogenicity, and an ability to absorb allergens in a depot, hence reducing allergenicity and severe unwanted reactions. To date, aluminum hydroxide, microcrystalline tyrosine, monophospholipid A, and calcium phosphate are used as adjuvants for AIT. However, while aluminum hydroxide remains the most widely used adjuvant in AIT, it is also associated with a significant unwanted immunologic response such as induction of type II pro‐allergic inflammation, highlighting the current unmet needs of other novel adjuvants in AIT in adults. An even more significant unmet need is observed in children due to the lack of long‐term clinical studies. This review focuses on the mechanisms of action of current adjuvants used in conjunction with AIT in adults and children and how they can be either beneficial or detrimental to health. Moreover, novel adjuvants that are in the early stages of experimental medicine or clinical trials are thoroughly reviewed.
Abbreviations
AIT – Allergen‐specific Immunotherapy
AA – Alum Aluminum
APC – Antigen‐presenting cells
CaP – Calcium phosphate
DCs – Dendritic cells
HDM – House dust mite
MCT – Microcrystalline tyrosine
MPL – Monophosphoryl lipid A
SCIT – Subcutaneous allergen immunotherapy
SLIT – Sublingual allergen immunotherapy
TLR – Toll‐like receptors
VLP – Virus‐like particles
Introduction
Allergen‐specific immunotherapy (AIT) administered either by subcutaneous (SCIT) or by the sublingual (SLIT) route, is the only disease‐modifying therapy with long‐lasting clinical benefit in patients with allergic rhinitis with or without asthma.150, 623, 624, 625 Persistence in the clinical benefit of AIT is associated with the induction of allergen‐specific tolerance and is achieved by regulation of the innate and adaptive immune compartments. This can involve deletion and/or anergy of TH2 and allergen‐specific TH2 (TH2A) cells resulting in immune deviation toward a TH1‐mediated response,626, 627 or their suppression through the induction of T and B regulatory cells.169, 178, 628, 629 Moreover, regulation of B cells during AIT results in the induction of allergen‐specific IgG, IgG4, or IgA blocking antibodies, which can attenuate IgE‐mediated FcεRI and FcεRII pro‐allergic responses.167, 630
Since the inception of AIT by Leonard Noon in 1911, numerous approaches to enhance the safety and efficacy of AIT have been developed, resulting in the treatment regimens used in current clinical practice. A vital component of these regimens are adjuvants, a group of compounds that can be co‐administered with the allergen extract to enhance antigen‐specific immune responses for poorly immunogenic therapies and confer safety. The use of adjuvants in conjunction with AIT induces a potent and longer‐lasting immune response to AIT, which can be achieved at a shorter treatment duration.631 Adjuvants can enhance the efficiency of AIT and allow the administration of lower doses.
Currently, four adjuvant compounds are clinically used in adjunct to AIT: aluminum hydroxide (alum), microcrystalline tyrosine (MCT), monophosphoryl lipid A (MPL), and calcium phosphate (CaP). While alum, MCT, and CaP are known to modulate antigen presentation, MPL is thought to act as a direct immunomodulatory agent. In this review, we evaluate the current use of these adjuvants in conjunction with AIT in adults and children. We explore the mechanisms of action of these adjuvants and how they can be either beneficial or detrimental to health. Finally, novel adjuvants that are in the early stages of experimental research or clinical trials will also be thoroughly reviewed (Table 14).
Table 14.
Adjuvants currently used in AIT
| Adjuvants | Mechanisms of action | Advantages | Disadvantages | Level of evidence |
|---|---|---|---|---|
| Aluminum hydroxide | ‐ Depot effect | ‐ Widely used as an adjuvant | ‐ Formation of granuloma at administration site | Phase IV |
| (alum) | ‐ Inflammasome activation (NALP3/NLRP3) | ‐ Highly efficacious | ‐ Induction of TH2 immune response | |
| ‐ Enhance antigen uptake and presentation to APCs | ‐ Long‐term safety (ie neurotoxic effects) | |||
| ‐ Potential acute and long‐term toxicity | ||||
| ‐ Lack of biodegradability | ||||
| Monophosphoryl lipid A (MPL) | ‐ TLR‐4 agonist that acts as a direct immunomodulator | ‐ Immunomodulatory properties | ‐ Expensive | Phase II/III |
| ‐ Enhance allergen uptake | ‐ Well tolerated in children and adults in short‐course treatment | ‐ Transient and local side effects at injection site | ||
| Microcrystalline tyrosine (MCT) | ‐ Depot effect | ‐ Biodegradable | ‐ Cannot be used in tyrosine metabolism disorders | Phase I/II |
| ‐ Inflammasome activation | ‐ Safe and well tolerated | |||
| ‐ Highly efficacious | ||||
| Calcium phosphate | ‐ Depot effect | ‐ Naturally present and biocompatible | ‐ Local side effects at site of administration | Small number of human studies |
| (CaP) | ‐ Inflammasome activation (NALP3) | ‐ Well tolerated by most patients | ‐ Lower adjuvant efficacy compared to alum |
Aluminum salts
Aluminum salts (alum) remain the most prevalently used form of adjuvant in vaccines and AIT formulations, with its first documented use dating back to the early 20th century,632 followed by a successful application in humans in the 1930s.633 Alum then remained the only adjuvant used in human medicines for approximately 70 years.634, 635 The history of alum as an adjuvant in AIT spans an equal period of time, with its first use in an allergy setting demonstrating an enhanced rate of sensitization to ragweed pollen in guinea pigs that were administered with the allergen precipitated with alum.636 Interestingly, this did not interfere with the de‐sensitizing properties of AIT, due to the capacity of the adjuvant to limit rapid absorption and this depot effect highlighted a potential therapeutic use. Today, the adoption of alum in AIT varies depending on the location. In the United States, formulations for AIT containing alum are rarely adopted, with the allergen often administered in a soluble form. However, in Europe, the majority of AIT products for SCIT contain allergen adsorbed to alum.637 The clear advantage of alum in SCIT preparations is the enhancement of safety through a limitation in the rate of systemic exposure.638 Outside of the context of AIT, alum has an equally excellent safety record,639 although recently the long‐term consequences of continuous alum exposure have been brought into question—primarily regarding its lack of biodegradability within humans. Another cause for concern with alum is its widespread use despite a lack of full knowledge of its mechanisms of action. The first observation of these mechanisms was made in the 1950s with the observation of granuloma formations containing antibody‐producing cells at the site of administration.640 Further studies have since tried to identify several key factors mediating alum activity, although the full mechanisms continue to remain elusive.
The induction of a robust adaptive immune response following provocation requires the involvement of antigen‐presenting cells (APCs) becoming activated. Alum has been observed to enhance both antigen uptake and presentation in human APCs,641 with a similar observation in mice of prolonged antigen accumulation and presentation within dendritic cells (DCs).642 However, these findings remain a subject of controversy as a lack of both antigen presentation and activation following alum stimulation in mouse DCs has also been observed.643
It has long been observed that alum is a robust inducer of a TH2‐mediated response, which is arguably counter‐intuitive for AIT. A fundamental discrepancy in these observations is that many studies were conducted in animal sensitization models. While this is undoubtedly insightful, the primary outcome of AIT is de‐sensitization and so more studies investigating alum as an adjuvant in de‐sensitization would be more enlightening. Nonetheless, the potential for TH2 stimulation was first observed to be regulated by IL‐4 and IL‐1,644 although IL‐1 was subsequently found not to be required.645 Additionally, in the absence of IL‐4646 or IL‐13,647 alum favored induction to a TH1 response, although IL‐5 is still induced. In addition to the promotion of TH2 cells, the potential of alum to promote antigen‐specific IgE and IgG1 has also been an area of interest. De‐sensitization models have also observed promotion of IgE and IL‐4 responses in mice treated with alum and antigen.648, 649 While the mechanisms of TH2 induction are not yet fully understood, it has been demonstrated that IL‐18650 and natural killer T cells651 can mediate this alum response (Figure 17).
Figure 17.
Comparison of the actions of alum and MCT. Alum administration leads to immediate activation of the inflammasome and recruitment of APCs. Inflammasome activation can lead to granuloma formation and the formation of a depot for sustained release. Downstream of this, enhanced antigen presentation by APCs promotes activation of Th1 and Th2 cells. Additionally, activated eosinophils can prime naïve B cells to produce IgE and IgG. MCT administration leads to inflammasome and depot formation but not a granuloma as it is biodegradable. Mechanisms are largely similar to alum with preferential induction of Th1 over Th2 and IgG over IgE.
A crucial final mechanism in alum‐mediated responses is in the induction of inflammasome and, in particular, the role of NALP3/NLRP3. This essential protein within the inflammasome can be activated as a consequence of lysosomal degradation following alum uptake,652 resulting in the production of IL‐1, IL‐13, and IL‐18.653 While NLRP3 has been shown to play a role in inducing a TH2 response, a study has revealed that alum can induce TH2 responses independently of NLRP3.654 The role of the inflammasome in antibody production is also controversial, with studies advocating that NLRP3‐deficient mice have either functional655 or dysfunctional antigen‐specific antibody production to alum.656 Other mediators induced by alum also include uric acid,657 DNA released from necrotic cells,658 HMGB1, calreticulin, and HSP70.659, 660
While the mechanisms by which alum is induced require further investigations, its potency as an adjuvant is evident. It is more efficacious than other adjuvants such as tyrosine661 and calcium phosphate.662 Additionally, the safety conferred is beneficial, and alum is utilized in AIT preparations in Europe although not in the United States.637
The primary concerns with aluminum are the potential neurotoxic effects following continuous administration during development as part of routine vaccinations,663 and its lack of biodegradability. A rat model of immunotherapy assessing retention of aluminum revealed little to no systemic exposure, although a high level of retention at the dose site up to 180 days was observed. More recently, a study comparing SCIT formulations has observed that alum accumulated in the bones of rats following a single injection. It was suggested, however, that were this translated to humans as a full SCIT regimen, the accumulation of alum may lack clinical relevance.664 In the context of pediatric administration, where additional alum containing vaccines are likely to be administered, this proposed lack of clinical relevance may not apply. There was also an interesting observation of differences in plasma levels despite SCIT formulations containing comparable (1 mg/mL vs 1.13 mg/mL) alum concentrations suggesting that the manufacturing process may be a key determinant of systemic alum release. Whether this has long‐term consequences is uncertain as aluminum is non‐toxic and non‐essential for biologic processes.665 A separate study reported persistent itching nodules as a rare (<1%) side effect of injections containing an aluminum adjuvant, potentially leading to the development of contact hypersensitivity to aluminum.666
It is currently the case that alum remains the most common adjuvant for AIT regimes, despite the inconclusive knowledge on its mechanism of action and safety considerations over long‐term use and accumulation. Furthermore, it remains a concern that there are comparatively few studies directly comparing AIT formulations containing alum and those without, with studies primarily highlighting the benefits of alum versus alternative adjuvants. One such study, however, identified no clear difference in efficacy or safety between allergen extracts that were adsorbed to aluminum hydroxide and those that were not.667 Future recommendations among the scientific community differ between recommending its adoption637 or increasing the rate of research into alternatives so that it can be discontinued.638 Despite this, research into alum use in AIT continues with investigations into its effects in allergoid preparations where its activity was unaffected668 or determining new optimum allergen: adjuvant ratios.669 It seems the case that until an adjuvant with similar if not enhanced characteristics becomes widely available, it is likely here to stay.
Monophosphoryl Lipid A and other Toll‐like receptor agonists
Toll‐like receptors (TLRs) are essential components of innate immunity and live, or attenuated whole organism‐based vaccines are robust at stimulating them to provide an immune response. Unlike alum, MCT, and CaP, TLR agonists act as immunomodulators that directly activate innate immunity, leading to subsequent induction of the adaptive immune system. TLRs are predominately present on APCs but are also present on most cells of the immune system. They are capable of recognizing extracellular stimuli such as bacterial lipopeptides or the genetic material of bacteria or viruses that have entered the intracellular compartment670 (Figure 18). This initiates a downstream signaling response, through either the adaptor proteins MyD88 or TRIF, leading to the production of inflammatory cytokines through the activation of transcription factors such as NFκB, AP‐1, or IRF3/7.671
Figure 18.
Toll‐like Receptors. TLRs can be classed as recognizing extracellular stimuli (TLR2/1, TLR2/6, TLR4, TLR5) or intracellular stimuli (TLR3, TLR7, TLR8, TLR9). Recruitment of the adaptor protein MyD88 for all TLRs except TLR3, which signals through TRIF, leads to activation of transcription factors NFκB, AP‐1, and interferon response factors (IRFs) 3 and 7. This leads to the production of inflammatory cytokines, regulatory cytokines such as IL‐10 and type I interferons depending on the stimulus.
There is currently one adjuvant in clinical use for AIT that targets TLRs—monophosphoryl lipid A (MPL), which targets TLR4. MPL is a component of lipopolysaccharide (LPS) isolated from the bacterial species Salmonella Minnesota R595 that has been de‐toxified for administration to animals and humans.672 It is utilized for AIT in the formulation Pollinex Quattro®, a formulation of allergoids for grass or birch pollen adsorbed to MCT and administered with MPL. The compound is well tolerated and efficacious in a pre‐seasonal short course of AIT in both adults187, 188 and children.673, 674 The compound is also under ongoing investigation in clinical trials for uses outside of grass SCIT. Outside of SCIT, MPL containing SLIT has revealed efficacy in reducing nasal challenge scores in a phase I/IIa trial.675 There was also a promising phase IIb trial where efficacy has been demonstrated in allergoids to either ragweed189 or birch.676 While it is certainly clear that MPL containing AIT regimens are beneficial against placebo, there is yet to be a direct comparison with AIT lacking the agonist and it remains unclear if MPL is providing more efficacy than the previous Pollinex‐R formulation that contained only allergen and tyrosine, and a comparative study would be needed to evaluate this. Interestingly, in the phase I/IIa trial with MPL as an adjuvant in SLIT, a dose‐dependent effect of MPL was documented, with increases of specific IgG and smaller IgE increases than those on lower doses of MPL.675 The most recent phase III clinical trial data on birch pollen for Pollinex Quattro, however, revealed no difference in in‐season symptom scores between treatment and placebo arms, despite differences in immunoglobulin production between the groups, raising further questions about the efficacy of MPL in AIT.
The primary mechanisms of MPL are the induction of TH1‐mediated response to the allergen,677, 678 which is arguably more favorable to AIT than the TH2 response induced by alum. Additionally, there is a desirable induction of IgG1 and IgG4 over IgE.672, 674, 675, 676, 679 Upstream of this response, MPL has also been observed to enhance allergen uptake680 as well as activating T and B cells,681 including T regulatory cells674 and reducing DC‐mediated TH2 development.682 There are concerns with the use of MPL however, as TLR4 activation can promote TH2 responses, as well as TH1 and expression of TLR4 is observed to be enhanced in the nose during symptomatic seasonal allergic rhinitis. TLR4 agonists can enhance cytokine release for both TH1 and TH2 mediators following allergen challenge683 and so arguably the route of administration may need to be a key consideration.
Interestingly, MPL has primarily been used with MCT or derivatives and not alum. Alum has been previously shown not to require TLR signaling and co‐administration may enhance the efficacy of AIT. In a mouse model, co‐administration of alum with a TLR4 agonist reduced TH2‐mediated responses while maintaining efficacy,684 suggesting a potential future use of alum/TLR adjuvants.
Outside of TLR4, other agonists targeting alternative TLRs are being investigated, with compounds targeting TLR9 and TLR7 making it to a clinical trial for use in immunotherapy regimens.
TLR9 agonists
TLR9 recognizes bacterial DNA (Figure 18) and has been investigated for potential immune‐modulating activity when administered as an adjuvant in AIT. Clinical trials for AIT have not progressed beyond phase II despite promising data. The TLR9 agonist oligodeoxyribonucleotide immunostimulatory sequence of DNA (AIC) was administered in conjunction with ragweed allergen before the season and reduced nasal symptom scores, IL‐4+ basophils, and IgE production.190 This form of therapy was observed to have an increase of T regulatory cells within the nasal mucosa,685 a property also exhibited by TLR4. Other trials have investigated the use of A‐type CpG molecules that have been packaged into virus‐like particles (VLPs) for stability. The phase I/IIa trial with CYT003‐QbG10 revealed the efficacy and safety of the compound in association with enhanced IgG and transient increases in IgE.686 The phase IIb trial also demonstrated efficacy in reducing symptom and medication scores.687 Within food allergy, nanoparticles containing CpG/peanut have been administered to mice via oral AIT and have revealed efficacy in decrease TH2 responses and enhancing IgG2a while decreasing IgE/IgG1 levels.688 Further trials and investigations in humans may yield TLR9 as a promising adjuvant for AIT, due to its strong TH1‐inducing anti‐allergic capacity.
TLR7
TLR7 primarily recognizes single‐strand (ss) RNA from viruses but can also be targeted therapeutically with a class of small molecules called imidazoquinolines. Compounds from this group, such as imiquimod, are currently used therapeutically in certain circumstances to target melanoma. A novel imidazoquinoline compound, GSK2245035, has been demonstrated to suppress allergic activity in in vitro human assays689 and has been well tolerated in early‐phase clinical trials.690, 691
Another agonist—AZD8848 has demonstrated a capacity to reduce allergic symptoms to allergen challenge in allergic rhinitis.692 When administered before allergen challenge, this was again demonstrated with a promotion of interferons to reduce allergic responsiveness.693 Interestingly, an antedrug approach was utilized to limit systemic exposure of this IFN response, although this was shown to be insufficient following multiple doses.694 The capacity of TLR7 to robustly suppress allergic responses is desirable; however, limiting systemic effects remains priority before it may become a viable adjuvant.
Microcrystalline tyrosine
Microcrystalline tyrosine (MCT) was initially developed for use in the treatment of allergy with AIT. Superior for its biodegradable nature, MCT is an ideal adjuvant with a depot effect, greater safety profile and has been associated with an ability to enhance the immune response, with comparative efficacy to alum.665, 695, 696 The high efficacy of MCT was also demonstrated in other models that include influenza vaccine in a ferret model.697 Unlike MPL, MCT does not involve the activation of TLR4 pathway,648 but instead signals through a similar downstream pathway as alum, involving a caspase‐dependent secretion of IL‐1β from cultured human monocytes648 (Figure 17). Despite mechanistic similarities with alum, studies in mice showed that MCT has a better safety profile and induced fewer anaphylactic reactions, IL‐4, and IgE production. Furthermore, MCT was able to enhance IgG production698 and increase production of TH1 cytokines (IFN‐γ) and the immunomodulatory cytokine IL‐10.648
A study evaluating the induction of IgG4 following AIT using a non‐adjuvanted US product (Hollister‐Stier®, Spokane, WA, US) was reported as more potent in inducing IgG4 antibodies when compared to European products with MCT (Tyrosine®, Allergy Therapeutics, UK) or alum (Novo‐Helisen®, AllergoPharma, Germany).699 The study, however, involved only patients who did not have adverse side effects, ruling out any conclusions on the safety profiles of the different adjuvants. Finally, preclinical and clinical studies in humans have confirmed that MCT is safe as an adjuvant whereby no genotoxicity, teratogenicity, mutagenicity, or carcinogenicity was observed. A good local and systemic tolerability in adults was also observed.700
Calcium Phosphate
The use of calcium phosphate (CaP) as an adjuvant in vaccines against various infectious diseases dates back to the 1980s.701 Studies have revealed that CaP is well tolerated in humans and possess a better efficacy than alum when used as part of a booster vaccine for diphtheria.702 Since then, CaP has been approved as an adjuvant by the World Health Organization, and in the context of AIT, CaP is used as a depot adjuvant, though to a much lesser extent than alum.696 CaP is currently marketed in Europe as a component of SCIT allergy vaccines in combination with certain allergen extracts such as grass pollen and mite.703
Human studies illustrated that CaP can adsorb antigens, does not induce production of IgE and can increase production of IgG.702 The ability of CaP to adsorb antigens meant that they facilitate the uptake by phagocytic cells and can enhance immunogenicity of protein allergens, while favoring strong IgG responses. For this reason, CaP has been proposed as a potential replacement to aluminum‐based adjuvants. Moreover, it has recently been speculated that CaP particles promote a more balanced immune response, when compared to alum which often introduce a predominantly TH2 immune response.702 CaP has also been shown to induce the NALP3 inflammasome, resulting in the secretion of IL‐1β and IL‐18 cytokines. Despite their promising properties, early animal studies showed contradicting findings in which CaP induced local adverse effects and lower adjuvant efficacy when compared to alum.662 Further studies are undoubtedly required to further validate the safety of CaP.
Novel Adjuvants
Nanoparticles
In more recent years, various studies have been underway to identify novel adjuvants with better efficacy and safety than the adjuvants currently used in AIT. One example includes nanoparticles that can be utilized as an adjuvant or delivery system in AIT. Utilizing nanoparticles will provide an extra layer of protection for the allergen from degradation therefore achieving high concentration at the site of action and increasing immunogenic properties. To complement this, they can prevent allergen recognition by IgE from basophils or mast cells, reducing allergenicity and thus the risk of adverse events.704 The potential use of nanoparticles as adjuvants was also illustrated in animal models of oral immunotherapy against food allergen.705, 706
Virus‐Like Particles
Other candidates being evaluated as adjuvants for AIT include virus‐like particles (VLPs) and liposomes. VLPs, generated through high copy numbers of a viral capsid protein, can conjugate to allergens which can then be recognized as pathogen‐associated molecular pattern (PAMPs) by the human innate immune system.696 A previous study involving the administration of a Der p 1 VLP conjugates to healthy subjects resulted in the induction of specific IgG.707 A similar observation was also seen when conjugates of house dust mite (HDM) allergen and VLP were administered into HDM subjects.686 More recently, VLPs containing TLR‐9 agonist QbG10 was shown to improve asthma symptoms and reduce medication use in a double‐blind and randomized study,708 highlighting the idea that allergen may not be needed altogether for AIT. While VLP application in AIT has been shown to be well tolerated in humans,709 ongoing clinical trials involving peanut allergy vaccine are still underway to further validate their efficacy.
Liposomes
Fewer studies have been invested in evaluating the use of liposomes as adjuvants in AIT. Composed of lipid bilayers, liposomes, like nanoparticles, can encapsulate allergen and act as an adjuvant and delivery system.700 Despite disappointing observations in previous studies for the use of liposomes in AIT, a more recent randomized, double‐blind, placebo‐controlled trial showed that house dust mite allergen extract encapsulated in liposomes was efficient in increasing specific IgG, IgG1, and IgG4 blocking antibodies in patients with allergic asthma.710 This finding was also accompanied by a reduction in numbers of eosinophils in local target organs as well as improved clinical scores in patients receiving active treatment. However, further studies to evaluate the safety profile of liposomes are still required to warrant its use as a potential adjuvant in AIT.
Probiotics
The majority of studies involving adjuvants currently used in AIT and novel candidates have been performed in murine models and human adult subjects. Less is known about their use in children, especially the safety and efficacy aspect. The use of several immunopotentiators and their effect when administered with AIT has previously been studied in children with little success. Intradermal administration of Bacillus Calmette‐Guérin (BCG) in parallel with SLIT in children who are asthmatics to mite allergens did not yield any positive clinical impact.711 A lack of clinical improvement was also seen when children with HDM allergy were treated with steroids with or without vitamin D3 during SCIT treatment.712 Further studies are still required to also identify if probiotics such as lactic acid bacteria, which have been shown to decrease atopic dermatitis in children, can be used as adjuvants to treat allergic patients.713 More recently, a double‐blind placebo‐controlled trial to investigate the co‐administration of probiotics and peanut oral immunotherapy in peanut‐allergic children reported the treatment to be efficacious, through improved peanut‐specific IgE levels and IgG4 levels and sustained unresponsiveness as shown through skin prick tests.714 A study investigating the role of oral Clostridium butyricum administration as part of SCIT to treat allergic asthma revealed an enhanced suppression of TH2 cytokines and IgE serum levels as well as promoting B regulatory cells.715 Interestingly, comparisons were made against conventional AIT and the bacterium alone, and it was observed the combination of the two provided the most effective treatment, with no adverse safety concerns. While encouraging, study numbers utilizing this approach are limited and the successful use of probiotics in AIT would depend on the optimization of the route of administration and selection of a suitable bacterial strain, as strain specificity has shown to be important.716
Conclusions
While allergen‐specific immunotherapy remains to be the only curative treatment of allergic diseases, several drawbacks that include long treatment duration and risk of anaphylaxis persist. To tackle this issue, allergy research has focused on the development of potent adjuvants that can enhance or modulate the immune response with lower dose allergen administration. In recent years, a significant development on novel adjuvants with great potential and superiority beyond the conventionally used alum has surfaced. Other adjuvants, currently used in the market (MCT, MPL, and CaP) as well as novel adjuvants (nanoparticles, liposomes, and VLPs), have shown promising features that include either greater efficacy in modulating the immune response, reducing unwanted adverse reactions or facilitating allergen delivery to allow shorter updosing phases. Despite these advances, there is still a lack of one ideal adjuvant that fulfill all of the aforementioned criteria, highlighting the need for further research to continue this search.
Conflict of interest
Dr. Shamji reports grants and personal fees from ASIT Biotech.sa, grants from ALK, grants from Regeneron, grants from Merck, grants from Immune Tolerance Network, grants from ASIT Biotech.sa, personal fees from ALK, personal fees from Allergopharma, outside the submitted work. The rest of the authors declare that there is no conflict of interest in relation to this article.
Taken from EAACI allergic rhinoconjunctivitis allergen immunotherapy guidelines. Short‐term benefit refers to when on immunotherapy while long‐term benefit refers to effect after immunotherapy stopped.
Taken from EAACI allergic rhinoconjunctivitis allergen immunotherapy guidelines. Short‐term benefit refers to when on immunotherapy while long‐term benefit refers to effect after immunotherapy stopped.
Acknowledgments
We thank Ms Theresa Lipp for English language revision.
Acknowledgements
We are grateful to David Gonzalez de Olano for his critical review of the manuscript and his insightful comments. The authors also wish to thank O Shaw for revising the text for issues related to the English language. We also thank Stallergenes Greer for their support of the English language review.
Alvaro‐Lozano M, Akdis CA, Akdis, M , Alviani C, Angier E, Arasi S, Arzt‐Gradwohl L, Barber D, Bazire R, Cavkaytar O, Comberiati P, Dramburg S, Durham SR, Eifan AO, Forchert L, Halken S, Kirtland M, Kucuksezer UC, Layhadi JA, Matricardi PM, Muraro A, Ozdemir C, Pajno GB, Pfaar O, Potapova E, Riggioni C, Roberts G, Rodríguez del Río P, Shamji MH, Sturm GJ, Vazquez‐Ortiz M. EAACI Allergen Immunotherapy User's Guide. Pediatr Allergy Immunol. 2020: 31(Suppl. 25): 1‐101. 10.1111/pai.13189
References
- 1. Pajno GB, Castagnoli R, Antonella M, et al. Allergen Immunotherapy for IgE‐Mediated Food Allergy: there is a measure in everything to a proper proportion of therapy. Pediatr Allergy Immunol 2019;30(4):415‐422. [DOI] [PubMed] [Google Scholar]
- 2. Pajno GB, Fernandez‐Rivas M, Arasi S, et al. EAACI Guidelines on allergen immunotherapy: IgE‐mediated food allergy. Allergy. 2018;73(4):799‐815. [DOI] [PubMed] [Google Scholar]
- 3. Roberts G, Pfaar O, Akdis CA, et al. EAACI Guidelines on Allergen Immunotherapy: Allergic rhinoconjunctivitis. Allergy. 2018;73(4):765‐798. [DOI] [PubMed] [Google Scholar]
- 4. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE‐mediated food allergy: a systematic review and meta‐analysis. Allergy. 2017;72(8):1133‐1147. [DOI] [PubMed] [Google Scholar]
- 5. Rigbi NE, Goldberg MR, Levy MB, Nachshon L, Golobov K, Elizur A. Changes in patient quality of life during oral immunotherapy for food allergy. Allergy. 2017;72(12):1883‐1890. [DOI] [PubMed] [Google Scholar]
- 6. Shin JU, Kim SH, Noh JY, et al. Allergen‐specific immunotherapy induces regulatory T cells in an atopic dermatitis mouse model. Allergy. 2018;73(9):1801‐1811. [DOI] [PubMed] [Google Scholar]
- 7. Ridolo E, Martignago I, Riario‐Sforza GG, Incorvaia C. Allergen immunotherapy in atopic dermatitis. Exp Rev Clin Immunol. 2018;14(1):61‐68. [DOI] [PubMed] [Google Scholar]
- 8. Cardona V, Luengo O, Labrador‐Horrillo M. Immunotherapy in allergic rhinitis and lower airway outcomes. Allergy. 2017;72(1):35‐42. [DOI] [PubMed] [Google Scholar]
- 9. Demoly P, Kleine‐Tebbe J, Rehm D. Clinical benefits of treatment with SQ house dust mite sublingual tablet in house dust mite allergic rhinitis. Allergy. 2017;72(10):1576‐1578. [DOI] [PubMed] [Google Scholar]
- 10. Dhami S, Nurmatov U, Arasi S, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: A systematic review and meta‐analysis. Allergy. 2017;72(11):1597‐1631. [DOI] [PubMed] [Google Scholar]
- 11. Di Bona D, Plaia A, Leto‐Barone MS, La Piana S, Macchia L, Di Lorenzo G. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: a systematic review. Allergy. 2017;72(5):691‐704. [DOI] [PubMed] [Google Scholar]
- 12. Schmitt J, Wustenberg E, Kuster D, Mucke V, Serup‐Hansen N, Tesch F. The moderating role of allergy immunotherapy in asthma progression: Results of a population‐based cohort study. Allergy. 2020;75:596‐602. [DOI] [PubMed] [Google Scholar]
- 13. Morjaria JB, Caruso M, Rosalia E, Russo C, Polosa R. Preventing progression of allergic rhinitis to asthma. Curr Allergy Asthma Rep. 2014;14(2):412. [DOI] [PubMed] [Google Scholar]
- 14. Platts‐Mills TAE, Schuyler AJ, Erwin EA, Commins SP, Woodfolk JA. IgE in the diagnosis and treatment of allergic disease. J Allergy Clin Immunol. 2016;137(6):1662‐1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kucuksezer UC, Ozdemir C, Akdis M, Akdis CA. Precision/Personalized Medicine in Allergic Diseases and Asthma. Arch Immunol Ther Exp. 2018;66(6):431‐442. [DOI] [PubMed] [Google Scholar]
- 16. Wang M, Tan G, Eljaszewicz A, et al. Laundry detergents and detergent residue after rinsing directly disrupt tight junction barrier integrity in human bronchial epithelial cells. J Allergy Clin Immunol. 2019; 143(5):1892‐1903. [DOI] [PubMed] [Google Scholar]
- 17. Katsoulis K, Ismailos G, Kipourou M, Kostikas K. Microbiota and asthma: Clinical implications. Respir Med. 2019;146:28‐35. [DOI] [PubMed] [Google Scholar]
- 18. Breiteneder H, Diamant Z, Eiwegger T, et al. Future research trends in understanding the mechanisms underlying allergic diseases for improved patients care. Allergy. 2019;74:2293‐2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sugita K, Steer CA, Martinez‐Gonzalez I, et al. Type 2 innate lymphoid cells disrupt bronchial epithelial barrier integrity by targeting tight junctions through IL‐13 in asthmatic patients. J Allergy Clin Immunol. 2018;141(1):300‐310. [DOI] [PubMed] [Google Scholar]
- 20. Akdis M, Aab A, Altunbulakli C, et al. Interleukins (from IL‐1 to IL‐38), interferons, transforming growth factor beta, and TNF‐alpha: Receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138(4):984‐1010. [DOI] [PubMed] [Google Scholar]
- 21. Claudio E, Wang H, Kamenyeva O, Tang W, Ha HL, Siebenlist U. IL‐25 Orchestrates Activation of Th Cells via Conventional Dendritic Cells in Tissue to Exacerbate Chronic House Dust Mite‐Induced Asthma Pathology. J Immunol. 2019;203:2319‐2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ozdemir C, Kucuksezer UC, Akdis M, Akdis CA. The concepts of asthma endotypes and phenotypes to guide current and novel treatment strategies. Expert Rev Respir Med. 2018;12(9):733‐743. [DOI] [PubMed] [Google Scholar]
- 23. Moon TC, Befus AD, Kulka M. Mast cell mediators: their differential release and the secretory pathways involved. Front Immunol. 2014;5:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agache I, Akdis CA. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J Clin Investig. 2019;130:1493‐1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eguiluz‐Gracia I, Tay TR, Hew M, et al. Recent developments and highlights in biomarkers in allergic diseases and asthma. Allergy. 2018;73(12):2290‐2305. [DOI] [PubMed] [Google Scholar]
- 26. Muraro A, Lemanske RF Jr, Castells M, et al. Precision medicine in allergic disease‐food allergy, drug allergy, and anaphylaxis‐PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma and Immunology. Allergy. 2017;72(7):1006‐1021. [DOI] [PubMed] [Google Scholar]
- 27. van de Veen W, Stanic B, Wirz OF, Jansen K, Globinska A, Akdis M. Role of regulatory B cells in immune tolerance to allergens and beyond. J Allergy Clin Immunol. 2016;138(3):654‐665. [DOI] [PubMed] [Google Scholar]
- 28. Smaldini PL, Trejo F, Cohen JL, Piaggio E, Docena GH. Systemic IL‐2/anti‐IL‐2Ab complex combined with sublingual immunotherapy suppresses experimental food allergy in mice through induction of mucosal regulatory T cells. Allergy. 2018;73(4):885‐895. [DOI] [PubMed] [Google Scholar]
- 29. Bachmann MF, Kundig TM. Allergen‐specific immunotherapy: is it vaccination against toxins after all? Allergy. 2017;72(1):13‐23. [DOI] [PubMed] [Google Scholar]
- 30. Meiler F, Zumkehr J, Klunker S, Ruckert B, Akdis CA, Akdis M. In vivo switch to IL‐10‐secreting T regulatory cells in high dose allergen exposure. J Exp Med. 2008;205(12):2887‐2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lou W, Wang C, Wang Y, Han D, Zhang L. Responses of CD4(+) CD25(+) Foxp3(+) and IL‐10‐secreting type I T regulatory cells to cluster‐specific immunotherapy for allergic rhinitis in children. Pediatr Allergy Immunol. 2012;23(2):140‐149. [DOI] [PubMed] [Google Scholar]
- 32. Boonpiyathad T, Sokolowska M, Morita H, et al. Der p 1‐specific regulatory T‐cell response during house dust mite allergen immunotherapy. Allergy. 2019;74:976‐985. [DOI] [PubMed] [Google Scholar]
- 33. Zaleska A, Eiwegger T, Soyer O, et al. Immune regulation by intralymphatic immunotherapy with modular allergen translocation MAT vaccine. Allergy. 2014;69(9):1162‐1170. [DOI] [PubMed] [Google Scholar]
- 34. Soyer OU, Akdis M, Ring J, et al. Mechanisms of peripheral tolerance to allergens. Allergy. 2013;68(2):161‐170. [DOI] [PubMed] [Google Scholar]
- 35. Palomares O, Martin‐Fontecha M, Lauener R, et al. Regulatory T cells and immune regulation of allergic diseases: roles of IL‐10 and TGF‐beta. Genes Immun. 2014;15(8):511‐520. [DOI] [PubMed] [Google Scholar]
- 36. Ihara F, Sakurai D, Yonekura S, et al. Identification of specifically reduced Th2 cell subsets in allergic rhinitis patients after sublingual immunotherapy. Allergy. 2018;73(9):1823‐1832. [DOI] [PubMed] [Google Scholar]
- 37. Layhadi JA, Eguiluz‐Gracia I, Shamji MH. Role of IL‐35 in sublingual allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2019;19(1):12‐17. [DOI] [PubMed] [Google Scholar]
- 38. van de Veen W, Stanic B, Yaman G, et al. IgG4 production is confined to human IL‐10‐producing regulatory B cells that suppress antigen‐specific immune responses. J Allergy Clin Immunol. 2013;131(4):1204‐1212. [DOI] [PubMed] [Google Scholar]
- 39. van de Veen W. The role of regulatory B cells in allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2017;17(6):447‐452. [DOI] [PubMed] [Google Scholar]
- 40. Mosges R, Koch AF, Raskopf E, et al. Lolium perenne peptide immunotherapy is well tolerated and elicits a protective B‐cell response in seasonal allergic rhinitis patients. Allergy. 2018;73(6):1254‐1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stanic B, van de Veen W, Wirz OF, et al. IL‐10‐overexpressing B cells regulate innate and adaptive immune responses. J Allergy Clin Immunol. 2015;135(3):771‐780. [DOI] [PubMed] [Google Scholar]
- 42. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245‐252. [DOI] [PubMed] [Google Scholar]
- 43. Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat Immunol. 2010;11(8):647‐655. [DOI] [PubMed] [Google Scholar]
- 44. Gordon JR, Ma Y, Churchman L, Gordon SA, Dawicki W. Regulatory dendritic cells for immunotherapy in immunologic diseases. Front Immunol. 2014;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun W, Wei JW, Li H, Wei FQ, Li J, Wen WP. Adoptive cell therapy of tolerogenic dendritic cells as inducer of regulatory T cells in allergic rhinitis. International forum of allergy & rhinology. 2018;8(11):1291‐1299. [DOI] [PubMed] [Google Scholar]
- 46. Aragao‐Franca LS, Rocha VCJ, Cronemberger‐Andrade A, et al. Tolerogenic Dendritic Cells Reduce Airway Inflammation in a Model of Dust Mite Triggered Allergic Inflammation. Allergy Asthma Immunol Res. 2018;10(4):406‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Deniz G, Akdis M, Aktas E, Blaser K, Akdis CA. Human NK1 and NK2 subsets determined by purification of IFN‐gamma‐secreting and IFN‐gamma‐nonsecreting NK cells. Eur J Immunol. 2002;32(3):879‐884. [DOI] [PubMed] [Google Scholar]
- 48. Deniz G, Erten G, Kucuksezer UC, et al. Regulatory NK cells suppress antigen‐specific T cell responses. J Immunol. 2008;180(2):850‐857. [DOI] [PubMed] [Google Scholar]
- 49. Ferstl R, Frei R, Barcik W, et al. Histamine receptor 2 modifies iNKT cell activity within the inflamed lung. Allergy. 2017;72(12):1925‐1935. [DOI] [PubMed] [Google Scholar]
- 50. Kortekaas Krohn I, Shikhagaie MM, Golebski K, et al. Emerging roles of innate lymphoid cells in inflammatory diseases: Clinical implications. Allergy. 2018;73(4):837‐850. [DOI] [PubMed] [Google Scholar]
- 51. Lao‐Araya M, Steveling E, Scadding GW, Durham SR, Shamji MH. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2014;134(5):1193‐1195. [DOI] [PubMed] [Google Scholar]
- 52. Morita H, Kubo T, Ruckert B, et al. Induction of human regulatory innate lymphoid cells from group 2 innate lymphoid cells by retinoic acid. J Allergy Clin Immunol. 2019;143:2190‐2201.e9. [DOI] [PubMed] [Google Scholar]
- 53. Wang S, Xia P, Chen Y, et al. Regulatory Innate Lymphoid Cells Control Innate Intestinal Inflammation. Cell. 2017;171(1):201‐216. [DOI] [PubMed] [Google Scholar]
- 54. Ozdemir C, Kucuksezer UC, Akdis M, Akdis CA. Mechanisms of Aeroallergen Immunotherapy: Subcutaneous Immunotherapy and Sublingual Immunotherapy. Immunology and allergy clinics of North America. 2016;36(1):71‐86. [DOI] [PubMed] [Google Scholar]
- 55. Kucuksezer UC, Ozdemir C, Akdis M, Akdis CA. Mechanisms of immune tolerance to allergens in children. Korean journal of pediatrics. 2013;56(12):505‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mondoulet L, Dioszeghy V, Busato F, et al. Gata3 hypermethylation and Foxp3 hypomethylation are associated with sustained protection and bystander effect following epicutaneous immunotherapy in peanut‐sensitized mice. Allergy. 2019;74(1):152‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. James LK, Shamji MH, Walker SM, et al. Long‐term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. 2011;127(2):509‐516. [DOI] [PubMed] [Google Scholar]
- 58. Valovirta E, Petersen TH, Piotrowska T, et al. Results from the 5‐year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. J Allergy Clin Immunol. 2018;141(2):529‐538. [DOI] [PubMed] [Google Scholar]
- 59. Marogna M, Spadolini I, Massolo A, Canonica GW, Passalacqua G. Long‐lasting effects of sublingual immunotherapy according to its duration: a 15‐year prospective study. J Allergy Clin Immunol. 2010;126(5):969‐975. [DOI] [PubMed] [Google Scholar]
- 60. Marogna M, Bruno M, Massolo A, Falagiani P. Long‐lasting effects of sublingual immunotherapy for house dust mites in allergic rhinitis with bronchial hyperreactivity: A long‐term (13‐year) retrospective study in real life. Int Arch Allergy Immunol. 2007;142(1):70‐78. [DOI] [PubMed] [Google Scholar]
- 61. Sturm GJ, Varga EM, Roberts G, et al. EAACI guidelines on allergen immunotherapy: Hymenoptera venom allergy. Allergy. 2018;73(4):744‐764. [DOI] [PubMed] [Google Scholar]
- 62. Arzt L, Bokanovic D, Schrautzer C, et al. Immunological differences between insect venom‐allergic patients with and without immunotherapy and asymptomatically sensitized subjects. Allergy. 2018;73(6):1223‐1231. [DOI] [PubMed] [Google Scholar]
- 63. Huang Y, Wang C, Wang X, Zhang L, Lou H. Efficacy and safety of subcutaneous immunotherapy with house dust mite for allergic rhinitis: A Meta‐analysis of Randomized Controlled Trials. Allergy. 2019;74(1):189‐192. [DOI] [PubMed] [Google Scholar]
- 64. Pfaar O, Alvaro M, Cardona V, Hamelmann E, Mosges R, Kleine‐Tebbe J. Clinical trials in allergen immunotherapy: current concepts and future needs. Allergy. 2018;73(9):1775‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Calderon MA, Vidal C, Rodriguez Del Rio P, et al. European Survey on Adverse Systemic Reactions in Allergen Immunotherapy (EASSI): a real‐life clinical assessment. Allergy. 2017;72(3):462‐472. [DOI] [PubMed] [Google Scholar]
- 66. Bonertz A, Roberts G, Slater JE, et al. Allergen manufacturing and quality aspects for allergen immunotherapy in Europe and the United States: An analysis from the EAACI AIT Guidelines Project. Allergy. 2018;73(4):816‐826. [DOI] [PubMed] [Google Scholar]
- 67. Ryan D, Gerth van Wijk R, Angier E, et al. Challenges in the implementation of the EAACI AIT guidelines: A situational analysis of current provision of allergen immunotherapy. Allergy. 2018;73(4):827‐836. [DOI] [PubMed] [Google Scholar]
- 68. Viswanathan RK, Busse WW. Allergen immunotherapy in allergic respiratory diseases: from mechanisms to meta‐analyses. Chest. 2012;141(5):1303‐1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dhami S, Kakourou A, Asamoah F, et al. Allergen immunotherapy for allergic asthma: A systematic review and meta‐analysis. Allergy. 2017;72(12):1825‐1848. [DOI] [PubMed] [Google Scholar]
- 70. Ozdemir C, Yazi D, Gocmen I, et al. Efficacy of long‐term sublingual immunotherapy as an adjunct to pharmacotherapy in house dust mite‐allergic children with asthma. Pediatr Allergy Immunol. 2007;18(6):508‐515. [DOI] [PubMed] [Google Scholar]
- 71. Zielen S, Devillier P, Heinrich J, Richter H, Wahn U. Sublingual immunotherapy provides long‐term relief in allergic rhinitis and reduces the risk of asthma: A retrospective, real‐world database analysis. Allergy. 2018;73(1):165‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Masuyama K, Okamoto Y, Okamiya K, et al. Efficacy and safety of SQ house dust mite sublingual immunotherapy‐tablet in Japanese children. Allergy. 2018;73(12):2352‐2363. [DOI] [PubMed] [Google Scholar]
- 73. Pfaar O. Sublingual immunotherapy with house dust mite tablets in children‐The evidence‐based journey of allergen immunotherapy proceeds. Allergy. 2018;73(12):2271‐2273. [DOI] [PubMed] [Google Scholar]
- 74. Ozdemir C, Kucuksezer UC, Akdis M, Akdis CA. Under the skin or under the tongue: differences and similarities in mechanisms of sublingual and subcutaneous immunotherapy. Immunotherapy. 2013;5(11):1151‐1158. [DOI] [PubMed] [Google Scholar]
- 75. Abramowicz M, Kruszewski J, Chcialowski A. Evaluation of the placebo effect in the trials of allergen immunotherapy effectiveness: meta‐analysis of randomized and placebo‐controlled trials. Postepy dermatologii i alergologii. 2018;35(6):620‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Berings M, Karaaslan C, Altunbulakli C, et al. Advances and highlights in allergen immunotherapy: On the way to sustained clinical and immunologic tolerance. J Allergy Clin Immunol. 2017;140(5):1250‐1267. [DOI] [PubMed] [Google Scholar]
- 77. Calderon MA, Bousquet J, Canonica GW, et al. Guideline recommendations on the use of allergen immunotherapy in house dust mite allergy: Time for a change? J Allergy Clin Immunol. 2017;140(1):41‐52. [DOI] [PubMed] [Google Scholar]
- 78. Larenas‐Linnemann D, Luna‐Pech JA. What you should not miss from the systematic reviews and meta‐analyses on allergen‐specific immunotherapy in 2017. Curr Opin Allergy Clin Immunol. 2018;18(3):168‐176. [DOI] [PubMed] [Google Scholar]
- 79. Okamoto Y, Fujieda S, Okano M, Yoshida Y, Kakudo S, Masuyama K. House dust mite sublingual tablet is effective and safe in patients with allergic rhinitis. Allergy. 2017;72(3):435‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pajno GB, Bernardini R, Peroni D, et al. Clinical practice recommendations for allergen‐specific immunotherapy in children: the Italian consensus report. Italian journal of pediatrics. 2017;43(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Radulovic S, Calderon MA, Wilson D, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev 2010. (12):CD002893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Romantsik O, Tosca MA, Zappettini S, Calevo MG. Oral and sublingual immunotherapy for egg allergy. Cochrane Database Syst Rev. 2018;4:CD010638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Agache I, Lau S, Akdis CA, et al. EAACI guidelines on allergen immunotherapy: House dust mite‐driven allergic asthma. Allergy. 2019;74:855‐873. [DOI] [PubMed] [Google Scholar]
- 84. Dretzke J, Meadows A, Novielli N, Huissoon A, Fry‐Smith A, Meads C. Subcutaneous and sublingual immunotherapy for seasonal allergic rhinitis: a systematic review and indirect comparison. J Allergy Clin Immunol. 2013;131(5):1361‐1366. [DOI] [PubMed] [Google Scholar]
- 85. Muraro A, Roberts G, Halken S, et al. EAACI guidelines on allergen immunotherapy: Executive statement. Allergy. 2018;73(4):739‐743. [DOI] [PubMed] [Google Scholar]
- 86. Agache I. EAACI guidelines on allergen immunotherapy‐Out with the old and in with the new. Allergy. 2018;73(4):737‐738. [DOI] [PubMed] [Google Scholar]
- 87. Halken S, Larenas‐Linnemann D, Roberts G, et al. EAACI guidelines on allergen immunotherapy: Prevention of allergy. Pediatric Allergy Immunol. 2017;28(8):728‐745. [DOI] [PubMed] [Google Scholar]
- 88. Jutel M, Agache I, Bonini S, et al. International Consensus on Allergen Immunotherapy II: Mechanisms, standardization, and pharmacoeconomics. J Allergy Clin Immunol. 2016;137(2):358‐368. [DOI] [PubMed] [Google Scholar]
- 89. Pfaar O, Demoly P, Gerth van Wijk R, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy. 2014;69(7):854‐867. [DOI] [PubMed] [Google Scholar]
- 90. Kristiansen M, Dhami S, Netuveli G, et al. Allergen immunotherapy for the prevention of allergy: A systematic review and meta‐analysis. Pediatr Allergy Immunol. 2017;28(1):18‐29. [DOI] [PubMed] [Google Scholar]
- 91. Dhami S, Zaman H, Varga EM, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta‐analysis. Allergy. 2017;72(3):342‐365. [DOI] [PubMed] [Google Scholar]
- 92. Bonertz A, Roberts GC, Hoefnagel M, et al. Challenges in the implementation of EAACI guidelines on allergen immunotherapy: A global perspective on the regulation of allergen products. Allergy. 2018;73(1):64‐76. [DOI] [PubMed] [Google Scholar]
- 93. Larenas‐Linnemann DES, Antolin‐Amerigo D, Parisi C, et al. National clinical practice guidelines for allergen immunotherapy: An international assessment applying AGREE‐II. Allergy. 2018;73(3):664‐672. [DOI] [PubMed] [Google Scholar]
- 94. Devillier P, Molimard M, Ansolabehere X, et al. Immunotherapy with grass pollen tablets reduces medication dispensing for allergic rhinitis and asthma: a retrospective database study in France. Allergy. 2019;74:1317‐1326. [DOI] [PubMed] [Google Scholar]
- 95. Wahn U, Bachert C, Heinrich J, Richter H, Zielen S. Real‐world benefits of allergen immunotherapy for birch pollen‐associated allergic rhinitis and asthma. Allergy. 2019;74(3):594‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Penagos M, Eifan AO, Durham SR, Scadding GW. Duration of Allergen Immunotherapy for Long‐Term Efficacy in Allergic Rhinoconjunctivitis. Current treatment options in allergy. 2018;5(3):275‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Reier‐Nilsen T, Michelsen MM, Lodrup Carlsen KC, et al. Feasibility of desensitizing children highly allergic to peanut by high‐dose oral immunotherapy. Allergy. 2019;74(2):337‐348. [DOI] [PubMed] [Google Scholar]
- 98. Bluemchen K, Eiwegger T. Oral peanut immunotherapy How much is too much? How much is enough? Allergy. 2019;74(2):220‐222. [DOI] [PubMed] [Google Scholar]
- 99. Kawamoto N, Kaneko H, Kawamoto M, et al. Oral immunotherapy with antigenicity‐modified casein induces desensitization in cow's milk allergy. Allergy. 2020;75:197‐200. [DOI] [PubMed] [Google Scholar]
- 100. Machinena A, Lozano J, Piquer M, et al. Oral immunotherapy protocol for hen's egg allergic children: Improving safety. Pediatr Allergy Immunol. 2019;30:760‐763. [DOI] [PubMed] [Google Scholar]
- 101. Martin‐Munoz MF, Belver MT, Alonso Lebrero E, et al. Egg oral immunotherapy in children (SEICAP I): Daily or weekly desensitization pattern. Pediatr Allergy Immunol. 2019;30(1):81‐92. [DOI] [PubMed] [Google Scholar]
- 102. Yucel E, Sipahi Cimen S, Varol S, Suleyman A, Ozdemir C, Tamay ZU. Red meat desensitization in a child with delayed anaphylaxis due to alpha‐Gal allergy. Pediatr Allergy Immunol. 2019;30:771‐773. [DOI] [PubMed] [Google Scholar]
- 103. Jarkvist J, Salehi C, Akin C, Gulen T. Venom immunotherapy in patients with clonal mast cell disorders: IgG4 correlates with protection. Allergy 2020;75:169‐177. [DOI] [PubMed] [Google Scholar]
- 104. Jutel M, Akdis CA. Novel immunotherapy vaccine development. Curr Opin Allergy Clin Immunol. 2014;14(6):557‐563. [DOI] [PubMed] [Google Scholar]
- 105. Huser C, Dieterich P, Singh J, et al. A 12‐week DBPC dose‐finding study with sublingual monomeric allergoid tablets in house dust mite‐allergic patients. Allergy. 2017;72(1):77‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gunawardana NC, Durham SR. New approaches to allergen immunotherapy. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2018;121(3):293‐305. [DOI] [PubMed] [Google Scholar]
- 107. Pfaar O, Lou H, Zhang Y, Klimek L, Zhang L. Recent developments and highlights in allergen immunotherapy. Allergy. 2018;73(12):2274‐2289. [DOI] [PubMed] [Google Scholar]
- 108. Martinez‐Gomez JM, Johansen P, Erdmann I, Senti G, Crameri R, Kundig TM. Intralymphatic injections as a new administration route for allergen‐specific immunotherapy. Int Arch Allergy Immunol. 2009;150(1):59‐65. [DOI] [PubMed] [Google Scholar]
- 109. Hoffmann HJ, Valovirta E, Pfaar O, et al. Novel approaches and perspectives in allergen immunotherapy. Allergy. 2017;72(7):1022‐1034. [DOI] [PubMed] [Google Scholar]
- 110. Senti G, von Moos S, Kundig TM. Epicutaneous allergen administration: is this the future of allergen‐specific immunotherapy? Allergy. 2011;66(6):798‐809. [DOI] [PubMed] [Google Scholar]
- 111. Senti G, von Moos S, Tay F, Graf N, Johansen P, Kundig TM. Determinants of efficacy and safety in epicutaneous allergen immunotherapy: summary of three clinical trials. Allergy. 2015;70(6):707‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Shamji MH, Kappen JH, Akdis M, et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: an EAACI Position Paper. Allergy. 2017;72(8):1156‐1173. [DOI] [PubMed] [Google Scholar]
- 113. Eguiluz‐Gracia I, Testera‐Montes A, Gonzalez M, et al. Safety and reproducibility of nasal allergen challenge. Allergy. 2019. [DOI] [PubMed] [Google Scholar]
- 114. Jones SM, Sicherer SH, Burks AW, et al. Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults. J Allergy Clin Immunol. 2017;139(4):1242‐1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Matricardi PM, Kleine‐Tebbe J, Hoffmann HJ, et al. EAACI Molecular Allergology User's Guide. Pediatr Allergy Immunol. 2016;27(Suppl 23):1‐250. [DOI] [PubMed] [Google Scholar]
- 116. Flores Kim J, McCleary N, Nwaru BI, Stoddart A, Sheikh A. Diagnostic accuracy, risk assessment, and cost‐effectiveness of component‐resolved diagnostics for food allergy: A systematic review. Allergy. 2018;73(8):1609‐1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hamizan A, Alvarado R, Rimmer J, et al. Nasal mucosal brushing as a diagnostic method for allergic rhinitis. Allergy and asthma proceedings. 2019;40(3):167‐172. [DOI] [PubMed] [Google Scholar]
- 118. Rondon C, Eguiluz‐Gracia I, Shamji MH, et al. IgE Test in Secretions of Patients with Respiratory Allergy. Curr Allergy Asthma Rep. 2018;18(12):67. [DOI] [PubMed] [Google Scholar]
- 119. Auge J, Vent J, Agache I, et al. EAACI Position paper on the standardization of nasal allergen challenges. Allergy. 2018;73(8):1597‐1608. [DOI] [PubMed] [Google Scholar]
- 120. Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(1 Suppl):S1‐S55. [DOI] [PubMed] [Google Scholar]
- 121. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63(Suppl 86):8‐160. [DOI] [PubMed] [Google Scholar]
- 122. Taylor JA, Karas JL, Ram MK, Green OM, Seidel‐Dugan C. Activation of the high‐affinity immunoglobulin E receptor Fc epsilon RI in RBL‐2H3 cells is inhibited by Syk SH2 domains. Mol Cell Biol. 1995;15(8):4149‐4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Naclerio R. Clinical manifestations of the release of histamine and other inflammatory mediators. J Allergy Clin Immunol. 1999;103(3 Pt 2):S382‐S385. [DOI] [PubMed] [Google Scholar]
- 124. Pawankar R, Mori S, Ozu C, Kimura S. Overview on the pathomechanisms of allergic rhinitis. Asia Pac Allergy. 2011;1(3):157‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lilly CM, Nakamura H, Kesselman H, et al. Expression of eotaxin by human lung epithelial cells: induction by cytokines and inhibition by glucocorticoids. J Clin Invest. 1997;99(7):1767‐1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Li L, Xia Y, Nguyen A, et al. Effects of Th2 cytokines on chemokine expression in the lung: IL‐13 potently induces eotaxin expression by airway epithelial cells. J Immunol. 1999;162(5):2477‐2487. [PubMed] [Google Scholar]
- 127. Sekiya T, Miyamasu M, Imanishi M, et al. Inducible expression of a Th2‐type CC chemokine thymus‐ and activation‐regulated chemokine by human bronchial epithelial cells. J Immunol. 2000;165(4):2205‐2213. [DOI] [PubMed] [Google Scholar]
- 128. Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S73‐S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Varricchi G, Harker J, Borriello F, Marone G, Durham SR, Shamji MH. T follicular helper (Tfh) cells in normal immune responses and in allergic disorders. Allergy. 2016;71(8):1086‐1094. [DOI] [PubMed] [Google Scholar]
- 130. Kwok WW, Roti M, Delong JH, et al. Direct ex vivo analysis of allergen‐specific CD4+ T cells. J Allergy Clin Immunol. 2010;125(6):1407‐1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wambre E, Van Overtvelt L, Maillere B, et al. Single cell assessment of allergen‐specific T cell responses with MHC class II peptide tetramers: methodological aspects. Int Arch Allergy Immunol. 2008;146(2):99‐112. [DOI] [PubMed] [Google Scholar]
- 132. Calderon MA, Penagos M, Sheikh A, Canonica GW, Durham SR. Sublingual immunotherapy for allergic conjunctivitis: Cochrane systematic review and meta‐analysis. Clin Exp Allergy. 2011;41(9):1263‐1272. [DOI] [PubMed] [Google Scholar]
- 133. Durham SR, Walker SM, Varga EM, et al. Long‐term clinical efficacy of grass‐pollen immunotherapy. N Engl J Med. 1999;341(7):468‐475. [DOI] [PubMed] [Google Scholar]
- 134. Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131(5):1288‐1296. [DOI] [PubMed] [Google Scholar]
- 135. Kiel MA, Roder E, Gerth van Wijk R, Al MJ., Hop WC, Rutten‐van Molken MP. Real‐life compliance and persistence among users of subcutaneous and sublingual allergen immunotherapy. J Allergy Clin Immunol. 2013;132(2):353‐360. [DOI] [PubMed] [Google Scholar]
- 136. Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;1:CD001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Frew AJ, Powell RJ, Corrigan CJ, Durham SR, Group UKIS. Efficacy and safety of specific immunotherapy with SQ allergen extract in treatment‐resistant seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117(2):319‐325. [DOI] [PubMed] [Google Scholar]
- 138. Abramson M, Puy R, Weiner J. Immunotherapy in asthma: an updated systematic review. Allergy. 1999;54(10):1022‐1041. [DOI] [PubMed] [Google Scholar]
- 139. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;8:CD001186. [DOI] [PubMed] [Google Scholar]
- 140. Creticos PS, Reed CE, Norman PS, et al. Ragweed immunotherapy in adult asthma. N Engl J Med. 1996;334(8):501‐506. [DOI] [PubMed] [Google Scholar]
- 141. Varney VA, Gaga M, Frew AJ, Aber VR, Kay AB, Durham SR. Usefulness of immunotherapy in patients with severe summer hay fever uncontrolled by antiallergic drugs. BMJ. 1991;302(6771):265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Walker SM, Varney VA, Gaga M, Jacobson MR, Durham SR. Grass pollen immunotherapy: efficacy and safety during a 4‐year follow‐up study. Allergy. 1995;50(5):405‐413. [DOI] [PubMed] [Google Scholar]
- 143. Ariano R, Kroon AM, Augeri G, Canonica GW, Passalacqua G. Long‐term treatment with allergoid immunotherapy with Parietaria. Clinical and immunologic effects in a randomized, controlled trial. Allergy. 1999;54(4):313‐319. [DOI] [PubMed] [Google Scholar]
- 144. Naclerio RM, Proud D, Moylan B, et al. A double‐blind study of the discontinuation of ragweed immunotherapy. J Allergy Clin Immunol. 1997;100(3):293‐300. [DOI] [PubMed] [Google Scholar]
- 145. Jacobsen L, Niggemann B, Dreborg S, et al. Specific immunotherapy has long‐term preventive effect of seasonal and perennial asthma: 10‐year follow‐up on the PAT study. Allergy. 2007;62(8):943‐948. [DOI] [PubMed] [Google Scholar]
- 146. Pajno GB, Barberio G, De Luca F, Morabito L, Parmiani S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six‐year follow‐up study. Clin Exp Allergy. 2001;31(9):1392‐1397. [DOI] [PubMed] [Google Scholar]
- 147. Des Roches A, Paradis L, Menardo JL, Bouges S, Daures JP, Bousquet J. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. VI. Specific immunotherapy prevents the onset of new sensitizations in children. J Allergy Clin Immunol. 1997;99(4):450‐453. [DOI] [PubMed] [Google Scholar]
- 148. Canonica GW, Cox L, Pawankar R, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J. 2014;7(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Jutel M, Bartkowiak‐Emeryk M, Breborowicz A, et al. Sublingual immunotherapy (SLIT)–indications, mechanism, and efficacy: Position paper prepared by the Section of Immunotherapy, Polish Society of Allergy. Ann Agric Environ Med. 2016;23(1):44‐53. [DOI] [PubMed] [Google Scholar]
- 150. Durham SR, Emminger W, Kapp A, et al. SQ‐standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129(3):717‐725. [DOI] [PubMed] [Google Scholar]
- 151. Didier A, Malling HJ, Worm M, Horak F, Sussman GL. Prolonged efficacy of the 300IR 5‐grass pollen tablet up to 2 years after treatment cessation, as measured by a recommended daily combined score. Clin Transl Allergy. 2015;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Scadding GW, Calderon MA, Shamji MH, et al. Effect of 2 Years of Treatment With Sublingual Grass Pollen Immunotherapy on Nasal Response to Allergen Challenge at 3 Years Among Patients With Moderate to Severe Seasonal Allergic Rhinitis: The GRASS Randomized Clinical Trial. JAMA. 2017;317(6):615‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Bergmann KC, Demoly P, Worm M, et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts in adults with allergic rhinitis. J Allergy Clin Immunol. 2014;133(6):1608‐1614. [DOI] [PubMed] [Google Scholar]
- 154. Mosbech H, Canonica GW, Backer V, et al. SQ house dust mite sublingually administered immunotherapy tablet (ALK) improves allergic rhinitis in patients with house dust mite allergic asthma and rhinitis symptoms. Ann Allergy Asthma Immunol. 2015;114(2):134‐140. [DOI] [PubMed] [Google Scholar]
- 155. Demoly P, Emminger W, Rehm D, Backer V, Tommerup L, Kleine‐Tebbe J. Effective treatment of house dust mite‐induced allergic rhinitis with 2 doses of the SQ HDM SLIT‐tablet: Results from a randomized, double‐blind, placebo‐controlled phase III trial. J Allergy Clin Immunol. 2016;137(2):444‐451. [DOI] [PubMed] [Google Scholar]
- 156. Mosbech H, Deckelmann R, de Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double‐blind, placebo‐controlled trial. J Allergy Clin Immunol. 2014;134(3):568‐575. [DOI] [PubMed] [Google Scholar]
- 157. Penagos M, Compalati E, Tarantini F, et al. Efficacy of sublingual immunotherapy in the treatment of allergic rhinitis in pediatric patients 3 to 18 years of age: a meta‐analysis of randomized, placebo‐controlled, double‐blind trials. Ann Allergy Asthma Immunol. 2006;97(2):141‐148. [DOI] [PubMed] [Google Scholar]
- 158. Penagos M, Passalacqua G, Compalati E, et al. Metaanalysis of the efficacy of sublingual immunotherapy in the treatment of allergic asthma in pediatric patients, 3 to 18 years of age. Chest. 2008;133(3):599‐609. [DOI] [PubMed] [Google Scholar]
- 159. Nolte H, Bernstein DI, Nelson HS, et al. Efficacy of house dust mite sublingual immunotherapy tablet in North American adolescents and adults in a randomized, placebo‐controlled trial. J Allergy Clin Immunol. 2016;138(6):1631‐1638. [DOI] [PubMed] [Google Scholar]
- 160. Okubo K, Masuyama K, Imai T, et al. Efficacy and safety of the SQ house dust mite sublingual immunotherapy tablet in Japanese adults and adolescents with house dust mite‐induced allergic rhinitis. J Allergy Clin Immunol. 2017;139(6):1840‐1848. [DOI] [PubMed] [Google Scholar]
- 161. Valovirta E, Berstad AK, de Blic J, et al. Design and recruitment for the GAP trial, investigating the preventive effect on asthma development of an SQ‐standardized grass allergy immunotherapy tablet in children with grass pollen‐induced allergic rhinoconjunctivitis. Clin Ther. 2011;33(10):1537‐1546. [DOI] [PubMed] [Google Scholar]
- 162. Nouri‐Aria KT, Pilette C, Jacobson MR, Watanabe H, Durham SR. IL‐9 and c‐Kit+ mast cells in allergic rhinitis during seasonal allergen exposure: effect of immunotherapy. J Allergy Clin Immunol. 2005;116(1):73‐79. [DOI] [PubMed] [Google Scholar]
- 163. Furin MJ, Norman PS, Creticos PS, et al. Immunotherapy decreases antigen‐induced eosinophil cell migration into the nasal cavity. J Allergy Clin Immunol. 1991;88(1):27‐32. [DOI] [PubMed] [Google Scholar]
- 164. Wilson DR, Nouri‐Aria KT, Walker SM, et al. Grass pollen immunotherapy: symptomatic improvement correlates with reductions in eosinophils and IL‐5 mRNA expression in the nasal mucosa during the pollen season. J Allergy Clin Immunol. 2001;107(6):971‐976. [DOI] [PubMed] [Google Scholar]
- 165. Doherty TA, Scott D, Walford HH, et al. Allergen challenge in allergic rhinitis rapidly induces increased peripheral blood type 2 innate lymphoid cells that express CD84. J Allergy Clin Immunol. 2014;133(4):1203‐1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42(4):607‐612. [DOI] [PubMed] [Google Scholar]
- 167. Shamji MH, Durham SR. Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J Allergy Clin Immunol. 2017;140(6):1485‐1498. [DOI] [PubMed] [Google Scholar]
- 168. Scadding GW, Calderon MA, Bellido V, et al. Optimisation of grass pollen nasal allergen challenge for assessment of clinical and immunological outcomes. J Immunol Methods. 2012;384(1–2):25‐32. [DOI] [PubMed] [Google Scholar]
- 169. Shamji MH, Layhadi JA, Achkova D, et al. Role of IL‐35 in sublingual allergen immunotherapy. J Allergy Clin Immunol. 2019;143(3):1131‐1142. [DOI] [PubMed] [Google Scholar]
- 170. O'Hehir RE, Gardner LM, de Leon MP, et al. House dust mite sublingual immunotherapy: the role for transforming growth factor‐beta and functional regulatory T cells. Am J Respir Crit Care Med. 2009;180(10):936‐947. [DOI] [PubMed] [Google Scholar]
- 171. Francis JN, James LK, Paraskevopoulos G, et al. Grass pollen immunotherapy: IL‐10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J Allergy Clin Immunol. 2008;121(5):1120‐1125. [DOI] [PubMed] [Google Scholar]
- 172. Novak N, Mete N, Bussmann C, et al. Early suppression of basophil activation during allergen‐specific immunotherapy by histamine receptor 2. J Allergy Clin Immunol. 2012;130(5):1153‐1158. [DOI] [PubMed] [Google Scholar]
- 173. Shamji MH, Layhadi JA, Scadding GW, et al. Basophil expression of diamine oxidase: a novel biomarker of allergen immunotherapy response. J Allergy Clin Immunol. 2015;135(4):913‐921. [DOI] [PubMed] [Google Scholar]
- 174. MacGlashan D Jr, Hamilton RG. Parameters determining the efficacy of CD32 to inhibit activation of FcepsilonRI in human basophils. J Allergy Clin Immunol. 2016;137(4):1256‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Tanaka Y, Nagashima H, Bando K, et al. Oral CD103(‐)CD11b(+) classical dendritic cells present sublingual antigen and induce Foxp3(+) regulatory T cells in draining lymph nodes. Mucosal Immunol. 2017;10(1):79‐90. [DOI] [PubMed] [Google Scholar]
- 176. Dawicki W, Li C, Town J, Zhang X, Gordon JR. Therapeutic reversal of food allergen sensitivity by mature retinoic acid‐differentiated dendritic cell induction of LAG3(+)CD49b(‐)Foxp3(‐) regulatory T cells. J Allergy Clin Immunol. 2017;139(5):1608‐1620. [DOI] [PubMed] [Google Scholar]
- 177. Fan DC, Wang XD, Wang CS, Wang Y, Cao FF, Zhang L. Suppression of Immunotherapy on Group 2 Innate Lymphoid Cells in Allergic Rhinitis. Chin Med J (Engl). 2016;129(23):2824‐2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Radulovic S, Jacobson MR, Durham SR, Nouri‐Aria KT. Grass pollen immunotherapy induces Foxp3‐expressing CD4+ CD25+ cells in the nasal mucosa. J Allergy Clin Immunol. 2008;121(6):1467‐1472. [DOI] [PubMed] [Google Scholar]
- 179. Syed A, Garcia MA, Lyu SC, et al. Peanut oral immunotherapy results in increased antigen‐induced regulatory T‐cell function and hypomethylation of forkhead box protein 3 (FOXP3). J Allergy Clin Immunol. 2014;133(2):500‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Suarez‐Fueyo A, Ramos T, Galan A, et al. Grass tablet sublingual immunotherapy downregulates the TH2 cytokine response followed by regulatory T‐cell generation. J Allergy Clin Immunol. 2014;133(1):130‐138. [DOI] [PubMed] [Google Scholar]
- 181. Jutel M, Akdis M, Budak F, et al. IL‐10 and TGF‐beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003;33(5):1205‐1214. [DOI] [PubMed] [Google Scholar]
- 182. Wambre E, DeLong JH, James EA, LaFond RE, Robinson D, Kwok WW. Differentiation stage determines pathologic and protective allergen‐specific CD4+ T‐cell outcomes during specific immunotherapy. J Allergy Clin Immunol. 2012;129(2):544‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Wambre E, DeLong JH, James EA, et al. Specific immunotherapy modifies allergen‐specific CD4(+) T‐cell responses in an epitope‐dependent manner. J Allergy Clin Immunol. 2014;133(3):872‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Wambre E, Bajzik V, DeLong JH, et al. A phenotypically and functionally distinct human TH2 cell subpopulation is associated with allergic disorders. Sci Transl Med. 2017;9(401):eaam9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Yao Y, Wang ZC, Wang N, et al. Allergen immunotherapy improves defective follicular regulatory T cells in patients with allergic rhinitis. J Allergy Clin Immunol. 2019;144(1):118‐128. [DOI] [PubMed] [Google Scholar]
- 186. van de Veen W, Wirz OF, Globinska A, Akdis M. Novel mechanisms in immune tolerance to allergens during natural allergen exposure and allergen‐specific immunotherapy. Curr Opin Immunol. 2017;48:74‐81. [DOI] [PubMed] [Google Scholar]
- 187. Drachenberg KJ, Wheeler AW, Stuebner P, Horak F. A well‐tolerated grass pollen‐specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy. 2001;56(6):498‐505. [DOI] [PubMed] [Google Scholar]
- 188. DuBuske LM, Frew AJ, Horak F, et al. Ultrashort‐specific immunotherapy successfully treats seasonal allergic rhinoconjunctivitis to grass pollen. Allergy Asthma Proc. 2011;32(6):466. [DOI] [PubMed] [Google Scholar]
- 189. Patel P, Holdich T, Fischer von Weikersthal‐Drachenberg KJ, Huber B. Efficacy of a short course of specific immunotherapy in patients with allergic rhinoconjunctivitis to ragweed pollen. J Allergy Clin Immunol. 2014;133(1):121‐129. [DOI] [PubMed] [Google Scholar]
- 190. Creticos PS, Schroeder JT, Hamilton RG, et al. Immunotherapy with a ragweed‐toll‐like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355(14):1445‐1455. [DOI] [PubMed] [Google Scholar]
- 191. Bousquet J, Lockey R, Malling HJ, et al. Allergen immunotherapy: therapeutic vaccines for allergic diseases. World Health Organization. American academy of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 1998;81(5):401‐405. [DOI] [PubMed] [Google Scholar]
- 192. Valenta R, Niespodziana K, Focke‐Tejkl M, et al. Recombinant allergens: what does the future hold? J Allergy Clin Immunol. 2011;127(4):860‐864. [DOI] [PubMed] [Google Scholar]
- 193. Rolinck‐Werninghaus C, Hamelmann E, Keil T, et al. The co‐seasonal application of anti‐IgE after preseasonal specific immunotherapy decreases ocular and nasal symptom scores and rescue medication use in grass pollen allergic children. Allergy. 2004;59(9):973‐979. [DOI] [PubMed] [Google Scholar]
- 194. Larenas‐Linnemann D, Wahn U, Kopp M. Use of omalizumab to improve desensitization safety in allergen immunotherapy. J Allergy Clin Immunol. 2014;133(3):937. [DOI] [PubMed] [Google Scholar]
- 195. Mosges R, Kasche EM, Raskopf E, et al. A randomized, double‐blind, placebo‐controlled, dose‐finding trial with Lolium perenne peptide immunotherapy. Allergy. 2018;73(4):896‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Shamji MH, Ceuppens J, Bachert C, et al. Lolium perenne peptides for treatment of grass pollen allergy: A randomized, double‐blind, placebo‐controlled clinical trial. J Allergy Clin Immunol. 2018;141(1):448‐451. [DOI] [PubMed] [Google Scholar]
- 197. Sharif H, Singh I, Kouser L, et al. Immunologic mechanisms of a short‐course of Lolium perenne peptide immunotherapy: A randomized, double‐blind, placebo‐controlled trial. J Allergy Clin Immunol. 2019;144(3):738‐749. [DOI] [PubMed] [Google Scholar]
- 198. Noon L. Prophylactic inoculation against hay fever. The Lancet. 1911;177(4580):1572‐1573. [Google Scholar]
- 199. Calderón M, Cardona V, Demoly P. One hundred years of allergen immunotherapy European Academy of Allergy and Clinical Immunology celebration: review of unanswered questions. Allergy. 2012;67(4):462‐476. [DOI] [PubMed] [Google Scholar]
- 200. Esposito S, Isidori C, Pacitto A, et al. Epicutaneous immunotherapy in rhino‐conjunctivitis and food allergies: a review of the literature. J Transl Med 2018;16:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Hellkvist L, Hjalmarsson E, Kumlien Georén S, et al. Intralymphatic immunotherapy with 2 concomitant allergens, birch and grass: A randomized, double‐blind, placebo‐controlled trial. J Allergy Clin Immunol. 2018;142:1338‐1341. [DOI] [PubMed] [Google Scholar]
- 202. Aj JA, Colburn WA, DeGruttola VG, et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89‐95. [DOI] [PubMed] [Google Scholar]
- 203. Robinson WH, Lindstrom TM, Cheung RK, Sokolove J. Mechanistic biomarkers for clinical decision making in rheumatic diseases. Nat Rev Rheumatol. 2013;9(5):267‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Lyons JJ, Milner JD. Primary atopic disorders. J Exp Med. 2018;215(4):1009‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Valenta R, Twaroch T, Swoboda I. Component‐resolved diagnosis to optimize allergen‐specific immunotherapy in the Mediterranean area. J Investig Allergol Clin Immunol. 2007;17(Suppl 1):36‐40. [PubMed] [Google Scholar]
- 206. Stringari G, Tripodi S, Caffarelli C, et al. The effect of component‐resolved diagnosis on specific immunotherapy prescription in children with hay fever. Journal of Allergy and Clinical Immunology. 2014;134(1):75‐81. [DOI] [PubMed] [Google Scholar]
- 207. Moreno C, Justicia JL, Quiralte J, et al. Olive, grass or both? Molecular diagnosis for the allergen immunotherapy selection in polysensitized pollinic patients. Allergy. 2014;69(10):1357‐1363. [DOI] [PubMed] [Google Scholar]
- 208. Hatzler L, Panetta V, Lau S, et al. Molecular spreading and predictive value of preclinical IgE response to Phleum pratense in children with hay fever ‐ ScienceDirect. J Allergy Clin Immunol. 2012;130(4):894‐901. [DOI] [PubMed] [Google Scholar]
- 209. Asero Riccardo . Disappearance of severe oral allergy syndrome following omalizumab treatment. ‐ PubMed ‐ NCBI. Eur Ann Allergy. Clin Immunol. 2017;49:143‐144. [PubMed] [Google Scholar]
- 210. Tripodi S, Frediani T, Lucarelli S. Molecular profiles of IgE to Phleum pratense in children with grass pollen allergy: Implications for specific immunotherapy. (J Allergy. Clin Immunol. 2012;129:834‐839. [DOI] [PubMed] [Google Scholar]
- 211. Cipriani F, Mastrorilli C, Tripodi S, et al. Diagnostic relevance of IgE sensitization profiles to eight recombinant Phleum pratense molecules. Allergy. 2018;73(3):673‐682. [DOI] [PubMed] [Google Scholar]
- 212. Darsow U, Brockow K, Pfab F, et al. Allergens. Heterogeneity of molecular sensitization profiles in grass pollen allergy–implications for immunotherapy?. Clin Exp Allergy 2014;44(5):778‐786. [DOI] [PubMed] [Google Scholar]
- 213. Posa D, Perna S, Resch Y, et al. Evolution and predictive value of IgE responses toward a comprehensive panel of house dust mite allergens during the first 2 decades of life. Journal of Allergy and Clinical Immunology. 2017;139(2):541‐549. [DOI] [PubMed] [Google Scholar]
- 214. European Directorate for the Quality of Medicines (EDQM) . Allergen products— pro‐ducta allergenica. European Pharma‐copoeia 6th ed Strasbourg: Council of Europe. 2010;679‐680.
- 215. Focke M, Marth K, Valenta R. Molecular composition and biological activity of commercial birch pollen allergen extracts. Eur J Clin Invest. 2009;39(5):429‐436. [DOI] [PubMed] [Google Scholar]
- 216. Løwenstein H. Characterization and standardization of allergen extracts. Chem Immunol Allergy. 2014;100:323‐332. [DOI] [PubMed] [Google Scholar]
- 217. Focke M, Marth K, Flicker S, Valenta R. Heterogeneity of commercial timothy grass pollen extracts. Clin Exp Allergy. 2008;38(8):1400‐1408. [DOI] [PubMed] [Google Scholar]
- 218. Wojtalewicz N, Goseberg S, Kabrodt K, Schellenberg I. Six years of INSTAND e. V. sIgE proficiency testing. Allergo J Int. 2017;26(2):43‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Cromwell O, Matricardi PM, Fiebig H. Patient‐tailored recombinant allergen products–mission impossible? Arb Paul Ehrlich Inst Bundesinstitut Impfstoffe Biomed Arzneim Langen Hess. 2009;96:210‐217. [PubMed] [Google Scholar]
- 220. Cromwell O, Häfner D, Nandy A. Recombinant allergens for specific immunotherapy. J Allergy Clin Immunol. 2011;127:865‐872. [DOI] [PubMed] [Google Scholar]
- 221. Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen‐specific immunotherapy with recombinant grass pollen allergens. Journal of Allergy and Clinical Immunology. 2005;116(3):608‐613. [DOI] [PubMed] [Google Scholar]
- 222. Movérare R, Elfman L, Vesterinen E, Metso T, Haahtela T. Development of new IgE specificities to allergenic components in birch pollen extract during specific immunotherapy studied with immunoblotting and Pharmacia CAP System. Allergy. 2002;57(5):423‐430. [DOI] [PubMed] [Google Scholar]
- 223. Asero R. Lack of de novo sensitization to tropomyosin in a group of mite‐allergic patients treated by house dust mite‐specific immunotherapy. Int Arch Allergy Immunol. 2005;137(1):62‐65. [DOI] [PubMed] [Google Scholar]
- 224. Asero R. Pollen specific immunotherapy is not a risk factor for de novo sensitization to cross‐reacting allergens in monosensitized subjects. J Investig Allergol Clin Immunol. 2006;16(4):253‐257. [PubMed] [Google Scholar]
- 225. Calderón MA, Cox L, Casale TB, Moingeon P, Demoly P. Multiple‐allergen and single‐allergen immunotherapy strategies in polysensitized patients: Looking at the published evidence. Journal of Allergy and Clinical Immunology. 2012;129(4):929‐934. [DOI] [PubMed] [Google Scholar]
- 226. Bahceciler NN, Galip N, Cobanoglu N. Multiallergen‐specific immunotherapy in polysensitized patients: where are we? Immunotherapy. 2013;5(2):183‐190. [DOI] [PubMed] [Google Scholar]
- 227. Ciprandi G, Melioli G, Passalacqua G, Canonica GW. Immunotherapy in polysensitized patients: new chances for the allergists? Ann Allergy Asthma Immunol. 2012;109(6):392‐394. [DOI] [PubMed] [Google Scholar]
- 228. Lee J‐E, Choi Y‐S, Kim M‐S, et al. Efficacy of sublingual immunotherapy with house dust mite extract in polyallergen sensitized patients with allergic rhinitis. Ann Allergy Asthma Immunol. 2011;107(1):79‐84. [DOI] [PubMed] [Google Scholar]
- 229. Gadermaier E, Staikuniene J, Scheiblhofer S, et al. Recombinant allergen‐based monitoring of antibody responses during injection grass pollen immunotherapy and after 5 years of discontinuation. Allergy. 2011;66(9):1174‐1182. [DOI] [PubMed] [Google Scholar]
- 230. Rossi RE, Monasterolo G, Coco G, Silvestro L, Operti D. Evaluation of serum IgG4 antibodies specific to grass pollen allergen components in the follow up of allergic patients undergoing subcutaneous and sublingual immunotherapy. Vaccine. 2007;25(5):957‐964. [DOI] [PubMed] [Google Scholar]
- 231. Chen K‐W, Zieglmayer P, Zieglmayer R, et al. Selection of house dust mite–allergic patients by molecular diagnosis may enhance success of specific immunotherapy. Journal of Allergy and Clinical Immunology. 2019;143(3):1248‐1252. [DOI] [PubMed] [Google Scholar]
- 232. Matricardi PM. Allergen‐specific immunoprophylaxis: Toward secondary prevention of allergic rhinitis? Pediatr Allergy Immunol. 2014;25(1):15‐18. [DOI] [PubMed] [Google Scholar]
- 233. Posa D, Hofmaier S, Arasi S, Matricardi PM. Natural Evolution of IgE Responses to Mite Allergens and Relationship to Progression of Allergic Disease: a Review. Curr Allergy Asthma Rep. 2017;17(5):28. [DOI] [PubMed] [Google Scholar]
- 234. Sastre J, Rodríguez F, Campo P, Laffond E, Marín A, Alonso MD. Adverse reactions to immunotherapy are associated with different patterns of sensitization to grass allergens. Allergy. 2015;70(5):598‐600. [DOI] [PubMed] [Google Scholar]
- 235. Hofmaier S, Comberiati P, Matricardi PM. Immunoglobulin G in IgE‐mediated allergy and allergen‐specific immunotherapy. Eur Ann Allergy Clin Immunol. 2014;46:6‐11. [PubMed] [Google Scholar]
- 236. Shamji MH, Francis JN. Measurement of Allergen‐Specific Inhibitory Antibody Activity. Methods Mol Biol. 2019;2020:33‐43. [DOI] [PubMed] [Google Scholar]
- 237. Huber S, Lang R, Steiner M, et al. Does clinical outcome of birch pollen immunotherapy relate to induction of blocking antibodies preventing IgE from allergen binding? A pilot study monitoring responses during first year of AIT. Clin Transl Allergy. 2018;8(8):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Ponce M, Schroeder F, Bannert C, et al. Preventive sublingual immunotherapy with House Dust Mite extract modulates epitope diversity in pre‐school children. Allergy. 2019;74:780‐787. [DOI] [PubMed] [Google Scholar]
- 239. Shamji MH, Wilcock LK, Wachholz PA, Dearman RJ, Kimber I, Wurtzen PA. The IgE‐facilitated allergen binding (FAB) assay: validation of a novel flow‐cytometric based method for the detection of inhibitory antibody responses. J Immunol Methods. 2006;20(317):71‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Feng M, Su Q, Lai X, et al. Functional and Immunoreactive Levels of IgG4 Correlate with Clinical Responses during the Maintenance Phase of House Dust Mite Immunotherapy. J Immunol. 2018;200:3897‐3904. [DOI] [PubMed] [Google Scholar]
- 241. Shamji MH, Kappen J, Abubakar‐Waziri H, et al. Nasal allergen‐neutralizing IgG4 antibodies block IgE‐mediated responses: Novel biomarker of subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2019;143:1067‐1076. [DOI] [PubMed] [Google Scholar]
- 242. Bousquet J, Pfaar O, Togias A, et al. 2019 ARIA Care pathways for allergen immunotherapy. Allergy. 2019;74:2087‐2102. [DOI] [PubMed] [Google Scholar]
- 243. Florack J, Brighetti MA, Perna S, et al. Comparison of six disease severity scores for allergic rhinitis against pollen counts a prospective analysis at population and individual level. Pediatr Allergy Immunol. 2016;27(4):382‐390. [DOI] [PubMed] [Google Scholar]
- 244. Kelly MD, Young DY, Lane NM, Shames RS. Validation of electronic versus paper subject diaries. Journal of Allergy and Clinical Immunology. 2004;113(2):S320. [Google Scholar]
- 245. Pizzulli A, Perna S, Florack J, et al. The impact of telemonitoring on adherence to nasal corticosteroid treatment in children with seasonal allergic rhinoconjunctivitis. Clin Exp Allergy. 2014;44(10):1246‐1254. [DOI] [PubMed] [Google Scholar]
- 246. Bianchi A, Tsilochristou O, Gabrielli F, Tripodi S, Matricardi PM. The Smartphone: A Novel Diagnostic Tool in Pollen Allergy? J Investig Allergol Clin Immunol. 2016;26(3):204‐207. [DOI] [PubMed] [Google Scholar]
- 247. Pizzulli A, Perna S, Bennewiz A, et al. The impact of nasal aspiration with an automatic device on upper and lower respiratory symptoms in wheezing children: a pilot case‐control study. Ital J Pediatr. 2018;44(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Bédard A, Basagaña X, Anto JM, et al. Mobile technology offers novel insights into the control and treatment of allergic rhinitis: The MASK study. J Allergy Clin Immunol. 2019;144:135‐143. [DOI] [PubMed] [Google Scholar]
- 249. Courbis AL, Murray RB, Arnavielhe S, et al. Electronic Clinical Decision Support System for allergic rhinitis management: MASK e‐CDSS. Clin Exp Allergy. 2018;48:1640‐1653. [DOI] [PubMed] [Google Scholar]
- 250. Bousquet J, Hellings PW, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) Phase 4 (2018): Change management in allergic rhinitis and asthma multimorbidity using mobile technology. J Allergy Clin Immunol. 2019;143:864‐879. [DOI] [PubMed] [Google Scholar]
- 251. Christiansen ES, Kjaer HF, Eller E, et al. The prevalence of atopic diseases and the patterns of sensitization in adolescence. Pediatr Allergy Immunol. 2016;27:847‐853. [DOI] [PubMed] [Google Scholar]
- 252. Henriksen L, Simonsen J, Haerskjold A, et al. Incidence rates of atopic dermatitis, asthma, and allergic rhinoconjunctivitis in Danish and Swedish children. J Allergy Clin Immunol. 2015;136:360‐366. [DOI] [PubMed] [Google Scholar]
- 253. Tejedor Alonso MA, Moro MM, Mugica Garcia MV. Epidemiology of anaphylaxis. Clin Exp Allergy. 2015;45:1027‐1039. [DOI] [PubMed] [Google Scholar]
- 254. Patil VK, Kurukulaaratchy RJ, Venter C, et al. Changing prevalence of wheeze, rhinitis and allergic sensitisation in late childhood: findings from 2 Isle of Wight birth cohorts 12 years apart. Clin Exp Allergy. 2015;45:1430‐1438. [DOI] [PubMed] [Google Scholar]
- 255. Nwaru BI, Hickstein L, Panesar SS, et al. The epidemiology of food allergy in Europe: a systematic review and meta‐analysis. Allergy 2014;69:62‐75. [DOI] [PubMed] [Google Scholar]
- 256. Muraro A, Dubois AE, DunnGalvin A, et al. Flokstra‐de Blok BM. EAACI Food Allergy and Anaphylaxis Guidelines. Food allergy health‐related quality of life measures. Allergy. 2014;69:845‐853. [DOI] [PubMed] [Google Scholar]
- 257. Zuberbier T, Lotvall J, Simoens S, Subramanian SV, Church MK. Economic burden of inadequate management of allergic diseases in the European Union: a GA(2) LEN review. Allergy. 2014;69:1275‐1279. [DOI] [PubMed] [Google Scholar]
- 258. Bahadori K, Doyle‐Waters MM, Marra C, et al. Economic burden of asthma: a systematic review. BMC Pulm Med. 2009;9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Guerra S, Sherrill DL, Martinez FD, Barbee RA. Rhinitis as an independent risk factor for adult‐onset asthma. J Allergy Clin Immunol. 2002;109:419‐425. [DOI] [PubMed] [Google Scholar]
- 260. Burgess JA, Walters EH, Byrnes GB, et al. Childhood allergic rhinitis predicts asthma incidence and persistence to middle age: a longitudinal study. J Allergy Clin Immunol. 2007;120:863‐869. [DOI] [PubMed] [Google Scholar]
- 261. Rochat MK, Illi S, Ege MJ, et al. Allergic rhinitis as a predictor for wheezing onset in school‐aged children. J Allergy Clin Immunol. 2010;126:1170‐1175. [DOI] [PubMed] [Google Scholar]
- 262. Martin PE, Matheson MC, Gurrin L, et al. Childhood eczema and rhinitis predict atopic but not nonatopic adult asthma: a prospective cohort study over 4 decades. J Allergy Clin Immunol. 2011;127:1473‐1479. [DOI] [PubMed] [Google Scholar]
- 263. Arasi S, Porcaro F, Cutrera R, Fiocchi AG. Severe Asthma and Allergy: A Pediatric Perspective. Front Pediatr. 2019;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Pfaar O, Bonini S, Cardona V, et al. Perspectives in allergen immunotherapy: 2017 and beyond. Allergy. 2018;73(Suppl 104):5‐23. [DOI] [PubMed] [Google Scholar]
- 265. Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558‐562. [DOI] [PubMed] [Google Scholar]
- 266. Jutel M, Agache I, Bonini S, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol 2015;136:556‐568. [DOI] [PubMed] [Google Scholar]
- 267. Pfaar O, Bachert C, Bufe A, et al. Guideline on allergen‐specific immunotherapy in IgE‐mediated allergic diseases: S2k Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Society for Pediatric Allergy and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA), the Austrian Society for Allergy and Immunology (OGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Dermatology (DDG), the German Society of Oto‐ Rhino‐Laryngology, Head and Neck Surgery (DGHNO‐KHC), the German Society of Pediatrics and Adolescent Medicine (DGKJ), the Society for Pediatric Pneumology (GPP), the German Respiratory Society (DGP), the German Association of ENT Surgeons (BV‐HNO), the Professional Federation of Paediatricians and Youth Doctors (BVKJ), the Federal Association of Pulmonologists (BDP) and the German Dermatologists Association (BVDD). Allergo J Int. 2014;23:282‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Jacobsen L, Nuchel PB, Wihl JA, Lowenstein H, Ipsen H. Immunotherapy with partially purified and standardized tree pollen extracts. IV. Results from long‐term (6‐year) follow‐up. Allergy. 1997;52:914‐920. [DOI] [PubMed] [Google Scholar]
- 269. Nieto A, Wahn U, Bufe A, et al. Allergy and asthma prevention 2014. Pediatr Allergy Immunol. 2014;25:516‐533. [DOI] [PubMed] [Google Scholar]
- 270. Eng PA, Borer‐Reinhold M, Heijnen IA, Gnehm HP. Twelve‐year follow‐up after discontinuation of preseasonal grass pollen immunotherapy in childhood. Allergy. 2006;61:198‐201. [DOI] [PubMed] [Google Scholar]
- 271. Eng PA, Reinhold M, Gnehm HP. Long‐term efficacy of preseasonal grass pollen immunotherapy in children. Allergy. 2002;57:306‐312. [DOI] [PubMed] [Google Scholar]
- 272. Bozek A, Jarzab J, Bednarski P. The effect of allergen‐specific immunotherapy on offspring. Allergy Asthma Proc. 2016;37:59‐63. [DOI] [PubMed] [Google Scholar]
- 273. Zolkipli Z, Roberts G, Cornelius V, et al. Randomized controlled trial of primary prevention of atopy using house dust mite allergen oral immunotherapy in early childhood. J Allergy Clin Immunol. 2015;136:1541‐1547. [DOI] [PubMed] [Google Scholar]
- 274. Yamanaka K, Shah SA, Sakaida H, et al. Immunological parameters in prophylactic sublingual immunotherapy in asymptomatic subjects sensitized to Japanese cedar pollen. Allergol Int. 2015;64:54‐59. [DOI] [PubMed] [Google Scholar]
- 275. Holt PG, Sly PD, Sampson HA, et al. Prophylactic use of sublingual allergen immunotherapy in high‐risk children: a pilot study. J Allergy Clin Immunol. 2013;132:991‐993. [DOI] [PubMed] [Google Scholar]
- 276. Schmitt J, Schwarz K, Stadler E, Wustenberg EG. Allergy immunotherapy for allergic rhinitis effectively prevents asthma: Results from a large retrospective cohort study. J Allergy Clin Immunol. 2015;136:1511‐1516. [DOI] [PubMed] [Google Scholar]
- 277. Song W, Lin X. Chai R. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014;28:300‐302. [PubMed] [Google Scholar]
- 278. Moller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT‐study). J Allergy Clin Immunol. 2002;109:251‐256. [DOI] [PubMed] [Google Scholar]
- 279. Niggemann B, Jacobsen L, Dreborg S, et al. Five‐year follow‐up on the PAT study: specific immunotherapy and long‐term prevention of asthma in children. Allergy. 2006;61:855‐859. [DOI] [PubMed] [Google Scholar]
- 280. Valovirta E, Berstad AK, de BJ, et al. Design and recruitment for the GAP trial, investigating the preventive effect on asthma development of an SQ‐standardized grass allergy immunotherapy tablet in children with grass pollen‐induced allergic rhinoconjunctivitis. Clin Ther 2011;33:1537‐1546. [DOI] [PubMed] [Google Scholar]
- 281. Peng H, Li CW, Lin ZB, Li TY. Long‐term efficacy of specific immunotherapy on house dust mite‐induced allergic rhinitis in China. Otolaryngol Head Neck Surg. 2013;149:40‐46. [DOI] [PubMed] [Google Scholar]
- 282. Garcia BE, Gonzalez‐Mancebo E, Barber D, Martin S, Tabar AI. Diaz de Durana AM, Garrido‐Fernandez S, Salcedo G, Rico P, Fernandez‐Rivas M. Sublingual immunotherapy in peach allergy: monitoring molecular sensitizations and reactivity to apple fruit and Platanus pollen. J Investig Allergol Clin Immunol. 2010;20:514‐520. [PubMed] [Google Scholar]
- 283. Szepfalusi Z, Bannert C, Ronceray L, et al. Preventive sublingual immunotherapy in preschool children: first evidence for safety and pro‐tolerogenic effects. Pediatr Allergy Immunol. 2014;25:788‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Pifferi M, Baldini G, Marrazzini G, et al. Benefits of immunotherapy with a standardized Dermatophagoides pteronyssinus extract in asthmatic children: a three‐year prospective study. Allergy. 2002;57:785‐790. [DOI] [PubMed] [Google Scholar]
- 285. Marogna M, Tomassetti D, Bernasconi A, et al. Preventive effects of sublingual immunotherapy in childhood: an open randomized controlled study. Ann Allergy Asthma Immunol. 2008;101:206‐211. [DOI] [PubMed] [Google Scholar]
- 286. Marogna M, Spadolini I, Massolo A, Canonica GW, Passalacqua G. Randomized controlled open study of sublingual immunotherapy for respiratory allergy in real‐life: clinical efficacy and more. Allergy. 2004;59:1205‐1210. [DOI] [PubMed] [Google Scholar]
- 287. Limb SL, Brown KC, Wood RA, Eggleston PA, Hamilton RG, Adkinson NF Jr. Long‐term immunologic effects of broad‐spectrum aeroallergen immunotherapy. Int Arch Allergy Immunol. 2006;140:245‐251. [DOI] [PubMed] [Google Scholar]
- 288. Dominicus R. 3‐years’ long‐term effect of subcutaneous immunotherapy (SCIT) with a high‐dose hypoallergenic 6‐grass pollen preparation in adults. Eur Ann Allergy Clin Immunol. 2012;44:135‐140. [PubMed] [Google Scholar]
- 289. Di BD, Plaia A, Leto‐Barone MS, La PS, Di LG. Efficacy of Grass Pollen Allergen Sublingual Immunotherapy Tablets for Seasonal Allergic Rhinoconjunctivitis: A Systematic Review and Meta‐analysis. JAMA Intern Med. 2015;175:1301‐1309. [DOI] [PubMed] [Google Scholar]
- 290. Calderon MA, Vidal C, Del Rodriguez RP, et al. European Survey on Adverse Systemic Reactions in Allergen Immunotherapy (EASSI): a real‐life clinical assessment. Allergy. 2017;72:462‐472. [DOI] [PubMed] [Google Scholar]
- 291. Del Rodriguez RP, Vidal C, Just J, et al. The European Survey on Adverse Systemic Reactions in Allergen Immunotherapy (EASSI): A paediatric assessment. Pediatr Allergy Immunol. 2017;28:60‐70. [DOI] [PubMed] [Google Scholar]
- 292. Bachert C, Larche M, Bonini S, et al. Allergen immunotherapy on the way to product‐based evaluation‐a WAO statement. World Allergy Organ J. 2015;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Brozek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines‐2016 revision. J Allergy Clin Immunol. 2017;140(4):950‐958. [DOI] [PubMed] [Google Scholar]
- 294. Greiner AN, Hellings PW, Rotiroti G, Scadding GK. Allergic rhinitis. Lancet (London, England). 2011;378(9809):2112‐2122. [DOI] [PubMed] [Google Scholar]
- 295. Ait‐Khaled N, Pearce N, Anderson HR, Ellwood P, Montefort S, Shah J. Global map of the prevalence of symptoms of rhinoconjunctivitis in children: The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Allergy. 2009;64(1):123‐148. [DOI] [PubMed] [Google Scholar]
- 296. Walker S, Khan‐Wasti S, Fletcher M, Cullinan P, Harris J, Sheikh A. Seasonal allergic rhinitis is associated with a detrimental effect on examination performance in United Kingdom teenagers: case‐control study. J Allergy Clin Immunol. 2007;120(2):381‐387. [DOI] [PubMed] [Google Scholar]
- 297. Sheikh A, Hurwitz B. Nurmatov U, van Schayck CP. House dust mite avoidance measures for perennial allergic rhinitis. The Cochrane database of systematic Reviews. 2010;7:Cd001563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Head K, Snidvongs K, Glew S, et al. Saline irrigation for allergic rhinitis. Cochrane Database Syst Rev. 2018;6:Cd012597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Weyer A, Donat N, L'Heritier C, et al. Grass pollen hyposensitization versus placebo therapy. I. Clinical effectiveness and methodological aspects of a pre‐seasonal course of desensitization with a four‐grass pollen extract. Allergy. 1981;36(5):309‐317. [DOI] [PubMed] [Google Scholar]
- 300. Bousquet J, Hejjaoui A, Skassa‐Brociek W, et al. Double‐blind, placebo‐controlled immunotherapy with mixed grass‐pollen allergoids. I. Rush immunotherapy with allergoids and standardized orchard grass‐pollen extract. J Allergy Clin Immunol. 1987;80(4):591‐598. [DOI] [PubMed] [Google Scholar]
- 301. Tworek D, Bochenska‐Marciniak M, Kuprys‐Lipinska I, Kupczyk M, Kuna P. Perennial is more effective than preseasonal subcutaneous immunotherapy in the treatment of seasonal allergic rhinoconjunctivitis. American journal of rhinology & allergy. 2013;27(4):304‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Dokic D, Schnitker J, Narkus A, Cromwell O, Frank E. Clinical effects of specific immunotherapy: a two‐year double‐blind, placebo‐controlled study with a one year follow‐up. Prilozi. 2005;26(2):113‐129. [PubMed] [Google Scholar]
- 303. Varney VA, Tabbah K, Mavroleon G, Frew AJ. Usefulness of specific immunotherapy in patients with severe perennial allergic rhinitis induced by house dust mite: a double‐blind, randomized, placebo‐controlled trial. Clin Exp Allergy. 2003;33(8):1076‐1082. [DOI] [PubMed] [Google Scholar]
- 304. Pfaar O, Nell MJ, Boot JD, et al. A randomized, 5‐arm dose finding study with a mite allergoid SCIT in allergic rhinoconjunctivitis patients. Allergy. 2016;71(7):967‐976. [DOI] [PubMed] [Google Scholar]
- 305. Song Y, Long J, Wang T, Xie J, Wang M, Tan G. Long‐term efficacy of standardised specific subcutaneous immunotherapy in children with persistent allergic rhinitis due to multiple allergens including house dust mites. The Journal of laryngology and otology. 2018;132(3):230‐235. [DOI] [PubMed] [Google Scholar]
- 306. Karakoc‐Aydiner E, Eifan AO, Baris S, et al. Long‐Term Effect of Sublingual and Subcutaneous Immunotherapy in Dust Mite‐Allergic Children With Asthma/Rhinitis: A 3‐Year Prospective Randomized Controlled Trial. J Investig Allergol Clin Immunol. 2015;25(5):334‐342. [PubMed] [Google Scholar]
- 307. Arroabarren E, Tabar AI, Echechipia S, Cambra K, Garcia BE, Alvarez‐Puebla MJ. Optimal duration of allergen immunotherapy in children with dust mite respiratory allergy. Pediatr Allergy Immunol. 2015;26(1):34‐41. [DOI] [PubMed] [Google Scholar]
- 308. Caffarelli C, Sensi LG, Marcucci F, Cavagni G. Preseasonal local allergoid immunotherapy to grass pollen in children: a double‐blind, placebo‐controlled, randomized trial. Allergy. 2000;55(12):1142‐1147. [DOI] [PubMed] [Google Scholar]
- 309. Pajno GB, Vita D, Parmiani S, Caminiti L, La Grutta S, Barberio G. Impact of sublingual immunotherapy on seasonal asthma and skin reactivity in children allergic to Parietaria pollen treated with inhaled fluticasone propionate. Clin Exp Allergy. 2003;33(12):1641‐1647. [DOI] [PubMed] [Google Scholar]
- 310. Wahn U, Tabar A, Kuna P, et al. Efficacy and safety of 5‐grass‐pollen sublingual immunotherapy tablets in pediatric allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2009;123(1):160‐166. [DOI] [PubMed] [Google Scholar]
- 311. Bufe A, Eberle P, Franke‐Beckmann E, et al. Safety and efficacy in children of an SQ‐standardized grass allergen tablet for sublingual immunotherapy. J Allergy Clin Immunol. 2009;123(1):167‐173. [DOI] [PubMed] [Google Scholar]
- 312. Halken S, Agertoft L, Seidenberg J, et al. Five‐grass pollen 300IR SLIT tablets: efficacy and safety in children and adolescents. Pediatr Allergy Immunol. 2010;21(6):970‐976. [DOI] [PubMed] [Google Scholar]
- 313. Blaiss M, Maloney J, Nolte H, Gawchik S, Yao R, Skoner DP. Efficacy and safety of timothy grass allergy immunotherapy tablets in North American children and adolescents. J Allergy Clin Immunol. 2011;127(1):64‐71. [DOI] [PubMed] [Google Scholar]
- 314. Valovirta E, Jacobsen L, Ljorring C, Koivikko A, Savolainen J. Clinical efficacy and safety of sublingual immunotherapy with tree pollen extract in children. Allergy. 2006;61(10):1177‐1183. [DOI] [PubMed] [Google Scholar]
- 315. Bufe A, Ziegler‐Kirbach E, Stoeckmann E, et al. Efficacy of sublingual swallow immunotherapy in children with severe grass pollen allergic symptoms: a double‐blind placebo‐controlled study. Allergy. 2004;59(5):498‐504. [DOI] [PubMed] [Google Scholar]
- 316. Stelmach I, Kaluzinska‐Parzyszek I, Jerzynska J, Stelmach P, Stelmach W, Majak P. Comparative effect of pre‐coseasonal and continuous grass sublingual immunotherapy in children. Allergy. 2012;67(3):312‐320. [DOI] [PubMed] [Google Scholar]
- 317. Ott H, Sieber J, Brehler R, et al. Efficacy of grass pollen sublingual immunotherapy for three consecutive seasons and after cessation of treatment: the ECRIT study. Allergy. 2009;64(9):1394‐1401. [DOI] [PubMed] [Google Scholar]
- 318. Nony E, Bouley J, Le Mignon M, et al. Development and evaluation of a sublingual tablet based on recombinant Bet v 1 in birch pollen‐allergic patients. Allergy. 2015;70(7):795‐804. [DOI] [PubMed] [Google Scholar]
- 319. Okamoto Y, Okubo K, Yonekura S, et al. Efficacy and safety of sublingual immunotherapy for two seasons in patients with Japanese cedar pollinosis. Int Arch Allergy Immunol. 2015;166(3):177‐188. [DOI] [PubMed] [Google Scholar]
- 320. Matsuoka T, Bernstein DI, Masuyama K, et al. Pooled efficacy and safety data for house dust mite sublingual immunotherapy tablets in adolescents. Pediatr Allergy Immunol. 2017;28(7):661‐667. [DOI] [PubMed] [Google Scholar]
- 321. Bahceciler NN, Isik U, Barlan IB, Basaran MM. Efficacy of sublingual immunotherapy in children with asthma and rhinitis: a double‐blind, placebo‐controlled study. Pediatr Pulmonol. 2001;32(1):49‐55. [DOI] [PubMed] [Google Scholar]
- 322. Hirsch T, Sahn M, Leupold W. Double‐blind placebo‐controlled study of sublingual immunotherapy with house dust mite extract (D.pt.) in children. Pediatr Allergy Immunol. 1997;8(1):21‐27. [DOI] [PubMed] [Google Scholar]
- 323. Marcucci F, Sensi L, Frati F, et al. Effects on inflammation parameters of a double‐blind, placebo controlled one‐year course of SLIT in children monosensitized to mites. Allergy. 2003;58(7):657‐662. [DOI] [PubMed] [Google Scholar]
- 324. de Bot CM, Moed H, Berger MY, et al. Sublingual immunotherapy not effective in house dust mite‐allergic children in primary care. Pediatr Allergy Immunol. 2012;23(2):150‐158. [DOI] [PubMed] [Google Scholar]
- 325. Asaria M, Dhami S, van Ree R, et al. Health economic analysis of allergen immunotherapy for the management of allergic rhinitis, asthma, food allergy and venom allergy: A systematic overview. Allergy. 2018;73(2):269‐283. [DOI] [PubMed] [Google Scholar]
- 326. Senti G, von Moos S, Tay F, et al. Epicutaneous allergen‐specific immunotherapy ameliorates grass pollen‐induced rhinoconjunctivitis: A double‐blind, placebo‐controlled dose escalation study. J Allergy Clin Immunol. 2012;129(1):128‐135. [DOI] [PubMed] [Google Scholar]
- 327. Agostinis F, Forti S, Di Berardino F. Grass transcutaneous immunotherapy in children with seasonal rhinoconjunctivitis. Allergy. 2010;65(3):410‐411. [DOI] [PubMed] [Google Scholar]
- 328. Senti G, Crameri R, Kuster D, et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol. 2012;129(5):1290‐1296. [DOI] [PubMed] [Google Scholar]
- 329. Patterson AM, Bonny AE, Shiels WE 2nd, Erwin EA. Three‐injection intralymphatic immunotherapy in adolescents and young adults with grass pollen rhinoconjunctivitis. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2016;116(2):168‐170. [DOI] [PubMed] [Google Scholar]
- 330. Hylander T, Larsson O, Petersson‐Westin U, et al. Intralymphatic immunotherapy of pollen‐induced rhinoconjunctivitis: a double‐blind placebo‐controlled trial. Respir Res. 2016;17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Lee SP, Choi SJ, Joe E, et al. A Pilot Study of Intralymphatic Immunotherapy for House Dust Mite, Cat, and Dog Allergies. Allergy, asthma & immunology research. 2017;9(3):272‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Witten M, Malling HJ, Blom L, Poulsen BC, Poulsen LK. Is intralymphatic immunotherapy ready for clinical use in patients with grass pollen allergy? J Allergy Clin Immunol. 2013;132(5):1248‐1252. [DOI] [PubMed] [Google Scholar]
- 333. Virchow JC, Backer V, Kuna P, et al. Efficacy of a House Dust Mite Sublingual Allergen Immunotherapy Tablet in Adults With Allergic Asthma: A Randomized Clinical Trial. JAMA. 2016;315(16):1715‐1725. [DOI] [PubMed] [Google Scholar]
- 334. Fiocchi A, Pajno G, La Grutta S, et al. Safety of sublingual‐swallow immunotherapy in children aged 3 to 7 years. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2005;95(3):254‐258. [DOI] [PubMed] [Google Scholar]
- 335. Agostinis F, Tellarini L, Canonica GW, Falagiani P, Passalacqua G. Safety of sublingual immunotherapy with a monomeric allergoid in very young children. Allergy. 2005;60(1):133. [DOI] [PubMed] [Google Scholar]
- 336. Rienzo VD, Minelli M, Musarra A, et al. Post‐marketing survey on the safety of sublingual immunotherapy in children below the age of 5 years. Clin Exp Allergy. 2005;35(5):560‐564. [DOI] [PubMed] [Google Scholar]
- 337. Pajno GB, Caminiti L, Crisafulli G, et al. Adherence to sublingual immunotherapy in preschool children. Pediatr Allergy Immunol. 2012;23(7):688‐689. [DOI] [PubMed] [Google Scholar]
- 338. Migueres M, Davila I, Frati F, et al. Types of sensitization to aeroallergens: definitions, prevalences and impact on the diagnosis and treatment of allergic respiratory disease. Clinical and translational allergy. 2014;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Demoly P, Passalacqua G, Pfaar O, Sastre J, Wahn U. Management of the polyallergic patient with allergy immunotherapy: a practice‐based approach. Allergy, asthma, and clinical immunology : official journal of the Canadian Society of Allergy and Clinical Immunology. 2016;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Nelson H, Blaiss M, Nolte H, Wurtz SO, Andersen JS, Durham SR. Efficacy and safety of the SQ‐standardized grass allergy immunotherapy tablet in mono‐ and polysensitized subjects. Allergy. 2013;68(2):252‐255. [DOI] [PubMed] [Google Scholar]
- 341. Ciprandi G, Cadario G, Di Gioacchino GM, et al. Sublingual immunotherapy in children with allergic polysensitization. Allergy and asthma proceedings. 2010;31(3):227‐231. [DOI] [PubMed] [Google Scholar]
- 342. Adkinson NF Jr, Eggleston PA, Eney D, et al. A controlled trial of immunotherapy for asthma in allergic children. The New England journal of medicine. 1997;336(5):324‐331. [DOI] [PubMed] [Google Scholar]
- 343. Li L, Guan K. Effect on quality of life of the mixed house dust mite/weed pollen extract immunotherapy. Asia Pacific allergy. 2016;6(3):168‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Nelson HS. Multiallergen immunotherapy for allergic rhinitis and asthma. J Allergy Clin Immunol. 2009;123(4):763‐769. [DOI] [PubMed] [Google Scholar]
- 345. Passalacqua G. The use of single versus multiple antigens in specific allergen immunotherapy for allergic rhinitis: review of the evidence. Curr Opin Allergy Clin Immunol. 2014;14(1):20‐24. [DOI] [PubMed] [Google Scholar]
- 346. Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy. 2012;42(5):650‐658. [DOI] [PubMed] [Google Scholar]
- 347. Campo P, Rodríguez F, Sánchez‐García S, et al. Phenotypes and endotypes of uncontrolled severe asthma: new treatments. J Investig Allergol Clin Immunol. 2013;23(2):76‐88. [PubMed] [Google Scholar]
- 348. Bisgaard H, Bønnelykke K. Long‐term studies of the natural history of asthma in childhood. J Allergy Clin Immunol. 2010;126(2):187‐197. [DOI] [PubMed] [Google Scholar]
- 349. Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355(21):2226‐2235. [DOI] [PubMed] [Google Scholar]
- 350. Just J, Saint Pierre P, Amat F, et al. What lessons can be learned about asthma phenotypes in children from cohort studies? Pediatr Allergy Immunol. 2015;26(4):300‐305. [DOI] [PubMed] [Google Scholar]
- 351. Iordanidou M, Loukides S, Paraskakis E. Asthma phenotypes in children and stratified pharmacological treatment regimens. Expert Rev Clin Pharmacol. 2017;10(3):293‐303. [DOI] [PubMed] [Google Scholar]
- 352. Alduraywish SA, Standl M, Lodge CJ, et al. Is there a march from early food sensitization to later childhood allergic airway disease? Results from two prospective birth cohort studies. Pediatr Allergy Immunol. 2017;28(1):30‐37. [DOI] [PubMed] [Google Scholar]
- 353. Fitzpatrick AM, Baena‐Cagnani CE, Bacharier LB. Severe asthma in childhood: recent advances in phenotyping and pathogenesis. Curr Opin Allergy Clin Immunol. 2012;12(2):193‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Licari A, Castagnoli R, Brambilla I, et al. Asthma Endotyping and Biomarkers in Childhood Asthma. Pediatr Allergy Immunol Pulmonol. 2018;31(2):44‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Trung Z, Peters C, Goldman N, Chipps BE. Overlap of atopic, eosinophilic, and TH2‐high asthma phenotypes in a general population with current asthma. Ann Allergy Asthma Immunol. 2016;116(1):37‐42. [DOI] [PubMed] [Google Scholar]
- 356. Licari A, Manti S, Castagnoli R, et al. Immunomodulation in pediatric asthma. Front Pediatr. 2019;7:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Papadopoulos NG, Čustović A, Cabana MD, et al. Pediatric asthma: An unmet need for more effective, focused treatments. Pediatr Allergy Immunol. 2019;30(1):7‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. van de Griendt E‐J, Tuut MK, de Groot H, Brand PLP. Applicability of evidence from previous systematic reviews on immunotherapy in current practice of childhood asthma treatment: a GRADE (Grading of Recommendations Assessment, Development and Evaluation) systematic review. BMJ Open. 2017;7(12):e016326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Illi S, Weber J, Zutavern A, et al. Perinatal influences on the development of asthma and atopy in childhood. Ann Allergy Asthma Immunol. 2014;112(2):132‐139. [DOI] [PubMed] [Google Scholar]
- 360. Zolkipli Z, et al. Randomized controlled trial of primary prevention of atopy using house dust mite allergen oral immunotherapy in early childhood. J Allergy Clin Immunol. 2015;136:1541‐1547. [DOI] [PubMed] [Google Scholar]
- 361. Carlsen KH, Carlsen KCL. Primary and secondary prevention of asthma. En: Global atlas of Asthma. EAACI 2013. Akdis CA and Agache I.; p. 124‐126.
- 362. Tanno LK, Haahtela T, Calderon MA, Cruz A, Demoly P. Joint Allergy Academies. Implementation gaps for asthma prevention and control. Respir Med. 2017;130:13‐19. [DOI] [PubMed] [Google Scholar]
- 363. Kim JM, Lin SY, Suarez‐Cuervo C, et al. Allergen‐specific immunotherapy for pediatric asthma and rhinoconjunctivitis: a systematic review. Pediatrics. junio de. 2013;131(6):1155‐1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Normansell R, Kew KM, Bridgman A‐L. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2015(8):CD011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Pham‐Thi N, Scheinmann P, Fadel R, Combebias A, Andre C. Assessment of sublingual immunotherapy efficacy in children with house dust mite‐induced allergic asthma optimally controlled by pharmacologic treatment and mite‐avoidance measures. Pediatr Allergy Immunol. 2007;18(1):47‐57. [DOI] [PubMed] [Google Scholar]
- 366. Lue K‐H, Lin Y‐H, Sun H‐L, Lu K‐H, Hsieh J‐C, Chou M‐C. Clinical and immunologic effects of sublingual immunotherapy in asthmatic children sensitized to mites: a double‐blind, randomized, placebo‐controlled study. Pediatr Allergy Immunol. 2006;17(6):408‐415. [DOI] [PubMed] [Google Scholar]
- 367. EMA 2015 . CHMP/EWP/2922/01 Rev.1 Guideline on the clinical investigation of medicinal products for the treatment of asthma.
- 368. Roberts G, Hurley C, Turcanu V, Lack G. Grass pollen immunotherapy as an effective therapy for childhood seasonal allergic asthma. J Allergy Clin Immunol. 2006;117(2):263‐268. [DOI] [PubMed] [Google Scholar]
- 369. Hedlin G. Management of severe asthma in childhood–state of the art and novel perspectives. Pediatr Allergy Immunol. 2014;25(2):111‐121. [DOI] [PubMed] [Google Scholar]
- 370. Kuna P, Kaczmarek J, Kupczyk M. Efficacy and safety of immunotherapy for allergies to Alternaria alternata in children. J Allergy Clin Immunol. 2011;127(2):502‐508. [DOI] [PubMed] [Google Scholar]
- 371. Stelmach I, Kaczmarek‐Woźniak J, Majak P, Olszowiec‐Chlebna M, Jerzynska J. Efficacy and safety of high‐doses sublingual immunotherapy in ultra‐rush scheme in children allergic to grass pollen. Clin Exp Allergy. 2009;39(3):401‐408. [DOI] [PubMed] [Google Scholar]
- 372. Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention, 2018. Available from: https://ginasthma.org/wp-content/uploads/2018/03/WMS-FINAL-GINA-2018-Appendix_v1.3.pdf
- 373. Zielen S, Kardos P, Madonini E. Steroid‐sparing effects with allergen‐specific immunotherapy in children with asthma: a randomized controlled trial. J Allergy Clin Immunol. 2010;126(5):942‐949. [DOI] [PubMed] [Google Scholar]
- 374. Hui Y, Li L, Qian J, Guo Y, Zhang X, Zhang X. Efficacy analysis of three‐year subcutaneous SQ‐standardized specific immunotherapy in house dust mite‐allergic children with asthma. Exp Ther Med. 2014;7(3):630‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Ippoliti F, De Santis W, Volterrani A, et al. Immunomodulation during sublingual therapy in allergic children. Pediatr Allergy Immunol. 2003;14(3):216‐221. [DOI] [PubMed] [Google Scholar]
- 376. Niu C‐K, Chen W‐Y, Huang J‐L, Lue K‐H, Wang J‐Y. Efficacy of sublingual immunotherapy with high‐dose mite extracts in asthma: a multi‐center, double‐blind, randomized, and placebo‐controlled study in Taiwan. Respir Med. 2006;100(8):1374‐1383. [DOI] [PubMed] [Google Scholar]
- 377. Price JF, Warner JO, Hey EN, Turner MW, Soothill JF. A controlled trial of hyposensitization with adsorbed tyrosine Dermatophagoides pteronyssinus antigen in childhood asthma: in vivo aspects. Clin Allergy. 1984;14(3):209‐219. [DOI] [PubMed] [Google Scholar]
- 378. Drachenberg KJ, Urban E, Pröll S, Woroniecki SR. Sublingual specific immunotherapy for adults and children: a post‐marketing surveillance study. Allergol Immunopathol (Madr). 2004;32(2):76‐81. [DOI] [PubMed] [Google Scholar]
- 379. Howarth P, Malling H‐J, Molimard M, Devillier P. Analysis of allergen immunotherapy studies shows increased clinical efficacy in highly symptomatic patients. Allergy. 2012;67(3):321‐327. [DOI] [PubMed] [Google Scholar]
- 380. Schiappoli M, Ridolo E, Senna G, et al. A prospective Italian survey on the safety of subcutaneous immunotherapy for respiratory allergy. Clin Exp Allergy. 2009;39(10):1569‐1574. [DOI] [PubMed] [Google Scholar]
- 381. Update CSM. Desensitising vaccines. Br Med J (Clin Res Ed). 1986;293(6552):948. [PMC free article] [PubMed] [Google Scholar]
- 382. Bernstein DI, Wanner M, Borish L, Liss GM. Immunotherapy Committee, American Academy of Allergy, Asthma and Immunology. Twelve‐year survey of fatal reactions to allergen injections and skin testing: 1990‐2001. J Allergy Clin Immunol. 2004;113(6):1129‐1136. [DOI] [PubMed] [Google Scholar]
- 383. Pitsios C, Demoly P, Bilò MB, et al. Clinical contraindications to allergen immunotherapy: an EAACI position paper. Allergy. 2015;70(8):897‐909. [DOI] [PubMed] [Google Scholar]
- 384. Tsai T‐C, Lu J‐H, Chen S‐J, Tang R‐B. Clinical efficacy of house dust mite‐specific immunotherapy in asthmatic children. Pediatr Neonatol. 2010;51(1):14‐18. [DOI] [PubMed] [Google Scholar]
- 385. Calderón MA, Simons FER, Malling H‐J, Lockey RF, Moingeon P, Demoly P. Sublingual allergen immunotherapy: mode of action and its relationship with the safety profile. Allergy. 2012;67(3):302‐311. [DOI] [PubMed] [Google Scholar]
- 386. Massanari M, Nelson H, Casale T, et al. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. J Allergy Clin Immunol. 2010;125(2):383‐389. [DOI] [PubMed] [Google Scholar]
- 387. Stelmach I, Majak P, Jerzyńska J, Bojo M, Cichalewski Ł, Smejda K. Children with severe asthma can start allergen immunotherapy after controlling asthma with omalizumab: a case series from Poland. Arch Med Sci. 2015;11(4):901‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Lambert N, Guiddir T, Amat F, Just J. Pre‐treatment by omalizumab allows allergen immunotherapy in children and young adults with severe allergic asthma. Pediatr Allergy Immunol. 2014;25(8):829‐832. [DOI] [PubMed] [Google Scholar]
- 389. Joseph PD, Craig JC, Caldwell PHY. Clinical trials in children. Br J Clin Pharmacol. 2015;79(3):357‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. The European Parliament Council . Regulation (EC) No 1901/2006 on medicinal products for paediatric use and amending Regulation (EEC) No 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No 726/2004, 2006: Official Journal of the European Union.
- 391. Rose K, Kopp MV. Pediatric investigation plans for specific immunotherapy: Questionable contributions to childhood health. Pediatr Allergy Immunol. 2015;26(8):695‐701. [DOI] [PubMed] [Google Scholar]
- 392. Williamson PR, Altman DG, Bagley H, et al. The COMET Handbook: version 1.0. Trials. 2017;18(Suppl 3):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59‐99. [DOI] [PubMed] [Google Scholar]
- 394. Sinha IP, Gallagher R, Williamson PR, Smyth RL. Development of a core outcome set for clinical trials in childhood asthma: a survey of clinicians, parents, and young people. Trials. 2012;13:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Price D, Hillyer EV, van der Molen T. Efficacy versus effectiveness trials: informing guidelines for asthma management. Curr Opin Allergy Clin Immunol. 2013;13(1):50‐57. [DOI] [PubMed] [Google Scholar]
- 396. Jacques L, Bakerly ND, New JP, Svedsater H, Lay‐Flurrie J, Leather DA. Effectiveness of fluticasone furoate/vilanterol versus fluticasone propionate/salmeterol on asthma control in the Salford Lung Study. J Asthma. 2019;56(7):748‐757. [DOI] [PubMed] [Google Scholar]
- 397. Barber D, Torre F, Feo F, et al. Understanding patient sensitization profiles in complex pollen areas: a molecular epidemiological study. Allergy. 2008;63:1550‐1558. [DOI] [PubMed] [Google Scholar]
- 398. Barber D, de la Torre F, Lombardero M, et al. Component‐resolved diagnosis of pollen allergy based on skin testing with profilin, polcalcin and lipid transfer protein pan‐allergens. Clinical et Experimental Allergy. 2009;39:1764‐1773. [DOI] [PubMed] [Google Scholar]
- 399. Cuesta‐Herránz J, Barber D, Blanco C, et al. Differences among Pollen‐Allergic Patients with and without Plan Food Allergy. Int Arch Allergy Immunol. 2010;153:182‐192. [DOI] [PubMed] [Google Scholar]
- 400. Barber D, Díaz‐Perales A, Villalba M, Chivato T. Challenges for allergy diagnosis in regions with complex pollen exposures. Curr Allergy Asthma Rep. 2015;15:496. [DOI] [PubMed] [Google Scholar]
- 401. Di Fraia M, Arasi S, Castelli S, et al. A new molecular multiplex IgE assay for the diagnosis of pollen allergy in Mediterranean countries: A validation study. Clin Exp Allergy. 2019;49:341‐349. [DOI] [PubMed] [Google Scholar]
- 402. Asero R, Tripoldi S. Dondi A et al.: Prevalence and clinical relevance of IgE sensitization to profilin in childhood. A multicenter study. Int Arch Allergy Immunol. 2015;168:25‐31. [DOI] [PubMed] [Google Scholar]
- 403. Rodríguez Del Río P, Díaz‐Perales A, Sánchez‐García S, et al. Profilin, a Change in the Paradigm. J Investig Allergol Clin Immunol. 2018;28:1‐12. [DOI] [PubMed] [Google Scholar]
- 404. Lund G, Brand S, Ramos T, et al. Strong and frequent T‐cell responses to the minor allergen Phl p 12 in Spanish patients IgE‐sensitized to Profilins. Allergy. 2018;73:1013‐1021. [DOI] [PubMed] [Google Scholar]
- 405. Rosace D, Gomez‐Casado C, Fernandez P, et al. Profilin‐mediated food‐induced allergic reactions are associated with oral epithelial remodeling. J Allergy Clin Immunol. 2019;143:681‐690. [DOI] [PubMed] [Google Scholar]
- 406. Obeso D, Mera‐Berriatua L, Rodríguez‐Coira J, et al. Multi‐omics analysis points to altered platelet functions in severe food‐associated respiratory allergy. Allergy. 2018;73:2137‐2149. [DOI] [PubMed] [Google Scholar]
- 407. Matricardi PM, Dramburg S, Potapova E, et al. Molecular diagnosis for allergen immunotherapy. J Allergy Clin Immunol. 2019;143:831‐843. [DOI] [PubMed] [Google Scholar]
- 408. Kaur A, Skoner D, Ibrahim J, et al. Effect of grass sublingual tablet immunotherapy is similar in children and adults: A Bayesian approach to design pediatric sublingual immunotherapy trials. J Allergy Clin Immunol. 2018;141:1744‐1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Ellis AK, Tenn MW, Steacy LM, et al. Lack of effect of Timothy grass pollen sublingual immunotherapy tablet on birch pollen‐induced allergic rhinoconjunctivitis in an environmental exposure unit. Ann Allergy Asthma Immunol. 2018;120:495‐503. [DOI] [PubMed] [Google Scholar]
- 410. Maloney J, Berman G, Gagnon R, et al. Sequential Treatment Initiation with Timothy Grass and Ragweed Sublingual Immunotherapy Tablets Followed by Simultaneous Treatment Is Well Tolerated. J Allergy Clin Immunol Pract. 2016;4:301‐309. [DOI] [PubMed] [Google Scholar]
- 411. Varona R, Ramos T, Escribese MM, et al. Persistent regulatory T‐cell response 2 years after 3 years of grass tablet SLIT: Links to reduced eosinophil counts, sIgE levels, and clinical benefit. Allergy. 2019;74:349‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Nolte H, Maloney J. The global development and clinical efficacy of sublingual tablet immunotherapy for allergic diseases. Allergol Int. 2018;67:301‐308. [DOI] [PubMed] [Google Scholar]
- 413. Nolte M, Barber D, Maloney J, et al. Timothy specific IgE levels are associated with efficacy and safety of timothy grass sublingual immunotherapy tablet. Ann Allergy Asthma Immunol 2015;15:509‐515. [DOI] [PubMed] [Google Scholar]
- 414. Barber D, Rico P, Blanco C, Fernandez‐Rivas M, et al. GRAZAX®: a sublingual immunotherapy vaccine for Hay fever treatment: from concept to commercialization. Hum Vaccin Immunother. 2019;15(12):2887‐2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Barber D, Moreno C, Ledesma A, et al. Degree of olive pollen exposure and sensitization patterns. Clinical implications. J Investig Allergol Clin Immunol. 2007;17:11‐16. [PubMed] [Google Scholar]
- 416. Salcedo G, Sánchez‐Monge R, Barber D, Díaz‐Perales A. Plant non‐specific lipid transfer proteins: An interface between plan defence and human allergy. Biochem Biophys Acta. 2007;781‐791. [DOI] [PubMed] [Google Scholar]
- 417. Díaz‐Perales A, Lombardero M, Sánchez‐Monge R, et al. Lipid‐transfer proteins as potential plant panallergens: cross‐reactivity among proteins of Artemisia pollen, Castanea nut and Rosaceae fruits, with different IgE‐binding capacities. Clin Exp Allergy. 2000;30:1403‐1410. [DOI] [PubMed] [Google Scholar]
- 418. Plaza MP, Alcazar P, Galán C. Correlation between airborne Olea europaea pollen concentrations and levels of the major allergen Ole e 1 in Córdoba, Spain, 2012–2014. Int J Biometeorol. 2016;60:1841‐1847. [DOI] [PubMed] [Google Scholar]
- 419. Mastrorilli C, Tripodi S, Caffarelli C, et al. Endotypes of pollen‐food syndrome in children with seasonal allergic rhinoconjunctivitis: a molecular classification. Allergy. 2016;71:1181‐1191. [DOI] [PubMed] [Google Scholar]
- 420. Skypala IJ, Cecchi L, Shamji MH, et al. Lipid Transfer Protein allergy in the United Kingdom: Characterization and comparison with a matched Italian cohort. Allergy 2019;74:1341‐1350. 10.1111/all.13747 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Palacín A, Gómez‐Casado C, Rivas LA, et al. Graph based study of allergen cross‐reactivity of plant lipid transfer proteins (LTPs) using microarray in a multicenter study. PLoS ONE. 2012;7e50799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Fernández‐Rivas M, Garrido Fernández S, et al. Randomized double‐blind, placebo‐controlled trial of sublingual immunotherapy with a Pru p 3 quantified peach extract. Allergy. 2009;64(6):876‐883. [DOI] [PubMed] [Google Scholar]
- 423. Gomez F, Bogas G, Gonzalez M, et al. The clinical and immunological effects of Pru p 3 sublingual immunotherapy on peach and peanut allergy in patients with systemic reactions. Clin Exp Allergy. 2017;47:339‐350. [DOI] [PubMed] [Google Scholar]
- 424. Bonertz A, Roberts G, Slater J, et al. Allergen manufacturing and quality aspects for allergen immunotherapy in Europe and the United States: An analysis from the EAACI AIT Guidelines Project. Allergy 2018;7:816‐826. [DOI] [PubMed] [Google Scholar]
- 425. Vidal C, Enrique E, Gonzalo A, et al. Diagnosis and allergen immunotherapy treatment of polysensitised patients with respiratory allergy in Spain: an Allergists’ Consensus. Expert Clinical Participants. Clin Transl Allergy. 2014;7(4):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. Sánchez‐Borges M, Fernández‐Caldas E, Thomas W, et al. International consensus (ICON) on: clinical consequences of mite hypersensitivity, a global problem. World Allergy Organ J. 2017;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427. Linneberg A, Dam Petersen K, Hahn‐Pedersen J, et al. Burden of allergic respiratory disease: a systematic review. Clin Mol Allergy. 2016 Sep;28(14):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428. Tavakoli H, FitzGerald JM, Chen W, et al. Ten‐year trends in direct costs of asthma: a population‐based study. Allergy. 2017 Feb;72(2):291‐299. [DOI] [PubMed] [Google Scholar]
- 429. Bousquet J, Pfaar O, Togias A, et al. ARIA Care pathways for allergen immunotherapy. Allergy 2019;74:2087‐2102. [DOI] [PubMed] [Google Scholar]
- 430. Bousquet J, Devillier P, Anto JM, et al. Daily allergic multimorbidity in rhinitis using mobile technology: A novel concept of the MASK study. Allergy. 2018;73(8):1622‐1631. [DOI] [PubMed] [Google Scholar]
- 431. WHO/IUIS Allergen Nomenclature Sub‐Committee . Allergen Nomenclature. http://www.allergen.org. Accessed 15 September 2019.
- 432. Thomas WR. Hierarchy and molecular properties of house dust mite allergens. Allergol Int. 2015;64:304‐311. [DOI] [PubMed] [Google Scholar]
- 433. Illi S, von Mutius E, Lau S, et al. Perennial allergen sensitization early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763‐770. [DOI] [PubMed] [Google Scholar]
- 434. Lodge CJ, Lowe AJ, Gurrin LC, et al. House dust mite sensitization in toddlers predicts current wheeze at age 12 years. J Allergy Clin Immunol. 2011;128:782‐788. [DOI] [PubMed] [Google Scholar]
- 435. Resch Y, Michel S, Kabesch M, et al. Different IgE recognition of mite allergen components in asthmatic and nonasthmatic children. J Allergy Clin Immunol. 2015;136:1083‐1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436. Batard T, Baron‐Bodo V, Martelet A, et al. Patterns of IgE sensitization in house dust mite‐allergic patients: implications for allergen immunotherapy. Allergy. 2016;71:220‐229. [DOI] [PubMed] [Google Scholar]
- 437. Rochat MK, Illi S, Ege MJ, et al. Allergic rhinitis as a predictor for wheezing onset in school ‐aged children. J Allergy Clin Immunol. 2010;126(1170–5):19. [DOI] [PubMed] [Google Scholar]
- 438. Yao HB, Lu JF. Long‐term clinical effect and safety observation of sublingual dermatophagoides farinae drop in preschool and school‐age children with allergic rhinitis]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017 Mar 5;31(5):377‐381. [DOI] [PubMed] [Google Scholar]
- 439. Inal A, Altintas DU, Yilmaz M, et al. Prevention of new sensitizations by specific immunotherapy in children with rhinitis and/or asthma monosensitized to house dust mite. J Investig Allergol Clin Immunol. 2007;17:85‐91. [PubMed] [Google Scholar]
- 440. Summary of product characteristics . Acarizax 12 SQ‐HDM oral lyophilisate. European Medicines Agency. https://mricts-mrpeu/Human/Downloads/DE_H_1947_001_FinalSPCpdf. 2016.
- 441. Dokic D, Schnitker J, Narkus A, Cromwell O, Frank E. Clinical effects of specific immunotherapy: a two‐year double‐blind, placebo‐controlled study with a one year follow‐up. Makedon Akad Na Nauk Umet Oddelenie Za Bioloshki Meditsinski Nauki Pril. 2005;26:113‐129. [PubMed] [Google Scholar]
- 442. de Bot CMA, Moed H, Berger MY, et al. Sublingual immunotherapy not effective in house dust mite‐allergic children in primary care. Pediatr Allergy Immunol. 2012;23:151‐159. [DOI] [PubMed] [Google Scholar]
- 443. Swamy RS, Reshamwala N, Hunter T, et al. Epigenetic modifications and improved regulatory T‐cell function in subjects undergoing dual sublingual immunotherapy. J Allergy Clin Immunol. 2012;130:215‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444. Calderón MA, Casale TB, Demoly P. Validation of Patient‐Reported Outcomes for Clinical Trials in Allergic Rhinitis. A Systematic Review. J Allergy Clin Immunol Pract. 2019 2019,;7(5):1450‐1461. [DOI] [PubMed] [Google Scholar]
- 445. Werfel T, Breuer K, Rueff F, et al. Usefulness of specific immunotherapy in patients with atopic dermatitis and allergic sensitization to house dust mites: a multi‐centre, randomized, dose‐response study. Allergy. 2006;61:202‐205. [DOI] [PubMed] [Google Scholar]
- 446. Novak N, Bieber T, Hoffmann M, et al. Efficacy and safety of subcutaneous allergen‐specific immunotherapy with depigmented polymerized mite extract in atopic dermatitis. J Allergy Clin Immunol. 2012;130:925‐931. [DOI] [PubMed] [Google Scholar]
- 447. Bae JM, Choi YY, Park CO, Chung KY, Lee KH. Efficacy of allergen‐specific immunotherapy for atopic dermatitis: a systematic review and meta‐analysis of randomized controlled trials. J Allergy Clin Immunol. 2013;132:110‐117. [DOI] [PubMed] [Google Scholar]
- 448. Pajno GB, Caminiti L, Vita D, et al. Sublingual immunotherapy in mite sensitized children with atopic dermatitis: a randomized, double‐blind, placebo‐controlled study. J Allergy Clin Immunol. 2007;120:164‐170. [DOI] [PubMed] [Google Scholar]
- 449. Wollenberg S, Barbarot T, et al. Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II A. J Eur Acad Dermatol Venereol. 2018 Jun;32(6):850‐878. [DOI] [PubMed] [Google Scholar]
- 450. Dávila I, Domínguez‐Ortega J, Navarro‐Pulido A, et al. Consensus document on dog and cat allergy. Allergy. 2018;73:1206‐1222. [DOI] [PubMed] [Google Scholar]
- 451. Larenas‐Linnemann D, Baxi S, Phipatanakul W, Portnoy JM. Environmental Allergens Workgroup. Clinical evaluation and management of patients with suspected fungus sensitivity. J Allergy Clin Immunol Pract. 2016;4:405‐414. [DOI] [PubMed] [Google Scholar]
- 452. Portnoy J, Chew GL, Phipatanakul W, et al. Environmental assessment and exposure reduction of cockroaches: a practice parameter. J Allergy Clin Immunol 2013;132:802‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453. Comberiati P, Marseglia GL, Barberi S, Passalacqua G, Peroni DG. Allergen‐Specific Immunotherapy for Respiratory Allergy in Children: Unmet Needs and Future Goals J Allergy Clin Immunol Pract. 2017;5(4):946‐950. [DOI] [PubMed] [Google Scholar]
- 454. Konradsen JR, Fujisawa T, van Hage M, et al. Allergy to furry animals: New insights, diagnostic approaches, and challenges. J Allergy Clin Immunol. 2015;135(3):616‐625. [DOI] [PubMed] [Google Scholar]
- 455. Asarnoj A, Hamsten C, Wadén K, et al. Sensitization to cat and dog allergen molecules in childhood and prediction of symptoms of cat and dog allergy in adolescence: A BAMSE/MeDALL study. J Allergy Clin Immunol. 2016;137(3):813‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456. Portnoy J, Kennedy K, Sublett J, et al. Environmental assessment and exposure control: a practice parameter—furry animals. Ann Allergy Asthma Immunol. 2012;108:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457. Dhami S, Agarwal A. Does evidence support the use of cat allergen immunotherapy? Curr Opin Allergy Clin Immunol. 2018;18(4):350‐355. [DOI] [PubMed] [Google Scholar]
- 458. Sundin B, Lilja G, Graff‐Lonnevig V, et al. Immunotherapy with partially purified and standardized animal dander extracts. I. Clinical results from a double‐blind study on patients with animal dander asthma. J Allergy Clin Immunol. 1986;77:478‐487. [DOI] [PubMed] [Google Scholar]
- 459. Haugaard L, Dahl R. Immunotherapy in patients allergic to cat and dog dander. I. Clinical results. Allergy. 1992;47:249‐254. [DOI] [PubMed] [Google Scholar]
- 460. Varney VA, Edwards J, Tabbah K, Brewster H, Mavroleon G, Frew AJ. Clinical efficacy of specific immunotherapy to cat dander: a double‐blind placebo‐controlled trial. Clin Exp Allergy. 1997;27:860‐867. [PubMed] [Google Scholar]
- 461. Alvarez‐Cuesta E, Cuesta‐Herranz J, Puyana‐Ruiz J, Cuesta‐Herranz C, Blanco‐Quiros A. Monoclonal antibody‐standardized cat extract immunotherapy: risk‐benefit effects from a double‐blind placebo study. J Allergy Clin Immunol. 1994;93:556‐566. [DOI] [PubMed] [Google Scholar]
- 462. Hedlin G, Graff‐Lonnevig V, Heilborn H, et al. Immunotherapy with cat‐ and dog‐dander extracts. II. In vivo and in vitro immunologic effects observed in a 1‐year double‐blind placebo study. J Allergy Clin Immunol. 1986;77:488‐496. [DOI] [PubMed] [Google Scholar]
- 463. Ewbank PA, Murray J, Sanders K, Curran‐Everett D, Dreskin S, Nelson HS. A double‐blind, placebo‐controlled immunotherapy dose response study with standardized cat extract. J Allergy Clin Immunol. 2003;111:155‐161. [DOI] [PubMed] [Google Scholar]
- 464. Nanda A, O'Connor M, Anand M, et al. Dose dependence and time course of the immunologic response to administration of standardized cat allergen extract. J Allergy Clin Immunol. 2004;114:1339‐1344. [DOI] [PubMed] [Google Scholar]
- 465. Nelson HS, Oppenheimer J, Vatsia GA, Buchmeier A. A double‐blind, placebo‐controlled evaluation of sublingual immunotherapy with standardized cat extract. J Allergy Clin Immunol. 1993;92:229‐236. [DOI] [PubMed] [Google Scholar]
- 466. Alvarez‐Cuesta E, Berges Gimeno P, Mancebo EG, Fernandez‐Caldas E, Cuesta‐Herranz J, Casanovas M. Sublingual immunotherapy with a standardized cat dander extract: evaluation of efficacy in a double blind placebo controlled study. Allergy. 2007;62:810‐817. [DOI] [PubMed] [Google Scholar]
- 467. Patel D, Couroux P, Hickey P, et al. Fel d1‐derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: a randomized, placebo‐controlled study. J Allergy Clin Immunol. 2012;131:103‐109. [DOI] [PubMed] [Google Scholar]
- 468. Circassia . Circassia announces top‐line results from cat allergy phase III study. Oxford: Circassia Pharmaceuticals PLC; 2016; Available from: http:// www.circassia.com/media/press-releases/circassia-announces-top-line-results-from-cat-allergy-phase-iii-study/. [Accessed March 2019] [Google Scholar]
- 469. Smith DM, Coop CA. Dog allergen immunotherapy: past, present, and future. Ann Allergy Asthma Immunol. 2016;116(3):188‐189. [DOI] [PubMed] [Google Scholar]
- 470. Valovirta E, Koivikko A, Vanto T, Viander M, Ingeman L. Immunotherapy in allergy to dog: a double‐blind clinical study. Ann Allergy. 1984;53:85‐88. [PubMed] [Google Scholar]
- 471. Valovirta E, Viander M, Koivikko A, Vanto T. Ingeman L. Immunotherapy in allergy to dog. Immunologic and clinical findings of a double‐blind study. Ann Allergy. 1986;57:173‐179. [PubMed] [Google Scholar]
- 472. Lilja G, Sundin B, Graff‐Lonnevig V, et al. Immunotherapy with cat‐ and dog dander extracts. IV. Effects of 2 years of treatment. J Allergy Clin Immunol. 1989;83:37‐44. [DOI] [PubMed] [Google Scholar]
- 473. Hedlin G, Graff‐Lonnevig V, Heilborn H, et al. Immunotherapy with cat‐ and dog‐dander extracts. V. Effects of 3 years of treatment. J Allergy Clin Immunol. 1991;87:955‐964. [DOI] [PubMed] [Google Scholar]
- 474. Hedlin G, Heilborn H, Lilja G, et al. Long‐term follow‐up of patients treated with a three‐year course of cat or dog immunotherapy. J Allergy Clin Immunol. 1995;96:879‐885. [DOI] [PubMed] [Google Scholar]
- 475. Lent AM, Harbeck R, Strand M, et al. Immunologic response to administration of standardized dog allergen extract at differing doses. J Allergy Clin Immunol. 2006;118:1249‐1256. [DOI] [PubMed] [Google Scholar]
- 476. Fernández‐Távora L, Rico P, Martín S. Clinical experience with specific immunotherapy to horse dander. J Investig Allergol Clin Immunol. 2002;12:29‐33. [PubMed] [Google Scholar]
- 477. Wahn U, Siraganian RP. Efficacy and specificity of immunotherapy with laboratory animal allergen extracts. J Allergy Clin Immunol. 1980;65:413‐421. [DOI] [PubMed] [Google Scholar]
- 478. Bousquet PJ, Chinn S, Janson C, Kogevinas M, Burney P, Jarvis D. Geographical variation in the prevalence of positive skin tests to environmental aeroallergens in the European Community Respiratory Health Survey I. Allergy. 2007;62:301‐309. [DOI] [PubMed] [Google Scholar]
- 479. D'Amato G, Chatzigeorgiou G, Corsico R, et al. Evaluation of the prevalence of skin prick test positivity to Alternaria and Cladosporium in patients with suspected respiratory allergy. A European multicenter study promoted by the Subcommittee on Aerobiology and Environmental Aspects of Inhalant Allergens of the European Academy of Allergology and Clinical Immunology. Allergy. 1997;52:711‐716. [DOI] [PubMed] [Google Scholar]
- 480. Cantani A, Businco E, Maglio A. Alternaria allergy: a three‐year controlled study in children treated with immunotherapy. Allergol Immunopathol (Madrid). 1988;16:1‐4. [PubMed] [Google Scholar]
- 481. Bernardis P, Agnoletto M, Puccinelli P. Injective versus sublingual immunotherapy in Alternaria tenuis allergic patients. J Invest Allergol Clin Immunol. 1996;6:55‐62. [PubMed] [Google Scholar]
- 482. Pozzan M, Milani M. Efficacy of sublingual specific immunotherapy in patients with respiratory allergy to Alternaria alternata: a randomised, assessor‐blinded, patient‐reported outcome, controlled 3‐year trial. Curr Med Res Opin. 2010;26(12):2801‐2806. [DOI] [PubMed] [Google Scholar]
- 483. Criado Molina A, Guerra Pasadas F, Daza Muñoz JC, et al. Immunotherapy with an oral Alternaria extract in childhood asthma. Clinical safety and efficacy and effects on in vivo and in vitro parameters.. Allergol Immunopathol (Madr) 2002;30:319. [DOI] [PubMed] [Google Scholar]
- 484. Kiliç M, Altintaş DU, Yilmaz M, Bingöl‐Karakoç G, Burgut R, Güneşer‐Kendirli S. Evaluation of efficacy of immunotherapy in children with asthma monosensitized to Alternaria. Turk J Pediatr. 2011;53:285‐294. [PubMed] [Google Scholar]
- 485. Horst M, Hejjaoui A, Horst V. A double blind, placebo –controlled rush immunotherapy with standardized Alternaria extract. J Allergy Clin Immunol. 1990;85:460‐472. [DOI] [PubMed] [Google Scholar]
- 486. Tabar AI, Lizaso MT, Garcıa BE, et al. Double‐blind, placebo‐controlled study of Alternaria alternata immunotherapy: clinical efficacy and safety. Pediatr Allergy Immunol. 2008;19(1):67‐75. [DOI] [PubMed] [Google Scholar]
- 487. Cortellini G, Spadolini I, Patella V, et al. Sublingual immunotherapy for Alternaria‐induced allergic rhinitis: a randomized placebo‐controlled trial. Ann Allergy Asthma Immunol. 2010;105(5):382‐386. [DOI] [PubMed] [Google Scholar]
- 488. Dreborg S, Agrell B, Foucard T, Kjellman NI, Koivikko A, Nilsson S. A double‐blind, multicenter immunotherapy trial in children, using a purified and standardized Cladosporium herbarum preparation, I: clinical results. Allergy. 1986;41:131‐140. [DOI] [PubMed] [Google Scholar]
- 489. Malling HJ, Dreborg S, Weeke B. Diagnosis and immunotherapy of mould allergy, V: clinical efficacy and side effects of immunotherapy with Cladosporium herbarum. Allergy. 1986;41:507‐519. [DOI] [PubMed] [Google Scholar]
- 490. Di Bona D, Frisenda F, Albanesi M, Di Lorenzo G, Caiaffa MF, Macchia L. Efficacy and safety of allergen immunotherapy in patients with allergy to molds: A systematic review. Clin Exp Allergy. 2018;48(11):1391‐1401. [DOI] [PubMed] [Google Scholar]
- 491. Prieto L, Palacios R, Aldana D, et al. Effect of allergen‐specific immunotherapy with purified Alt a1 on AMP responsiveness, exhaled nitric oxide and exhaled breath condensate pH: a randomized double blind study. Allergy Asthma. Clin Immunol. 2010;16(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492. Tabar AI, Prieto L, Alba P, et al. Double‐blind, randomized, placebo‐controlled trial of allergen‐specific immunotherapy with the major allergen Alt a 1. J Allergy Clin Immunol 2019;144:216‐223.e3. [DOI] [PubMed] [Google Scholar]
- 493. Bassirpour G, Zoratti E. Cockroach allergy and allergen‐specific immunotherapy in asthma: potential and pitfalls. Curr Opin Allergy Clin Immunol. 2014;14(6):535‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494. Do DC, Zhao Y, Gao P. Cockroach allergen exposure and risk of asthma. Allergy. 2016;71(4):463‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495. La Grutta S, Cibella F, Passalacqua G, et al. Association of Blattella germanica sensitization with atopic diseases in pediatric allergic patients. Pediatr Allergy Immunol. 2011;22(5):521‐527. [DOI] [PubMed] [Google Scholar]
- 496. Panzner P, Vachová M, Vlas T, Vítovcová P, Brodská P, Malý M. Cross‐sectional study on sensitization to mite and cockroach allergen components in allergy patients in the Central European region. Clin Transl Allergy. 2018;4(8):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497. Morgan WJ, Crain EF, Gruchalla RS, et al. Inner‐City Asthma Study Group. Results of a home‐based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068‐1080. [DOI] [PubMed] [Google Scholar]
- 498. Glesner J, Filep S, Vailes LD, et al. Allergen content in German cockroach extracts and sensitization profiles to a new expanded set of cockroach allergens determine in vitro extract potency for IgE reactivity. J Allergy Clin Immunol. 2019;143(4):1474‐1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499. Srivastava D, Gaur SN, Arora N, Singh BP. Clinico‐immunological changes postimmunotherapy with Periplaneta americana. Eur J Clin Invest. 2011;41:879‐888. [DOI] [PubMed] [Google Scholar]
- 500. Wood RA, Togias A, Wildfire J, et al. Development of cockroach immunotherapy by the Inner‐City Asthma Consortium. J Allergy Clin Immunol. 2014;133:846‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501. Cockroach Immunotherapy in Children and Adolescents (CRITICAL) . ClinicalTrials.gov Identifier: NCT03541187Accessed date: March 2019. https://clinicaltrials.gov/ct2/show/NCT03541187
- 502. Wood RA, Camargo CA Jr, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461‐467. [DOI] [PubMed] [Google Scholar]
- 503. Worm M, Eckermann O, Dolle S, et al. Triggers and treatment of anaphylaxis: an analysis of 4,000 cases from Germany. Austria and Switzerland. Deutsches Arzteblatt international. 2014;111(21):367‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504. Worm M, Moneret‐Vautrin A, Scherer K, et al. First European data from the network of severe allergic reactions (NORA). Allergy. 2014;69(10):1397‐1404. [DOI] [PubMed] [Google Scholar]
- 505. Xu YS, Kastner M, Harada L, Xu A, Salter J, Waserman S. Anaphylaxis‐related deaths in Ontario: a retrospective review of cases from 1986 to 2011. Allergy, asthma, and clinical immunology. 2014;10(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506. Antonicelli L, Bilo MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr Opin Allergy Clin Immunol. 2002;2(4):341‐346. [DOI] [PubMed] [Google Scholar]
- 507. de Graaf DC, Aerts M, Danneels E, Devreese B. Bee, wasp and ant venomics pave the way for a component‐resolved diagnosis of sting allergy. J Proteomics. 2009;72(2):145‐154. [DOI] [PubMed] [Google Scholar]
- 508. Bilo BM, Rueff F, Mosbech H, Bonifazi F, Oude‐Elberink JN. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60(11):1339‐1349. [DOI] [PubMed] [Google Scholar]
- 509. Muraro A, Roberts G, Clark A, et al. The management of anaphylaxis in childhood: position paper of the European academy of allergology and clinical immunology. Allergy. 2007;62(8):857‐871. [DOI] [PubMed] [Google Scholar]
- 510. Muller U, Helbling A, Berchtold E. Immunotherapy with honeybee venom and yellow jacket venom is different regarding efficacy and safety. J Allergy Clin Immunol. 1992;89(2):529‐535. [DOI] [PubMed] [Google Scholar]
- 511. Rueff F, Vos B, Elberink JO, et al. Predictors of clinical effectiveness of Hymenoptera venom immunotherapy. Clin Exp Allergy. 2014. [DOI] [PubMed] [Google Scholar]
- 512. Bilo BM, Bonifazi F. Epidemiology of insect‐venom anaphylaxis. Curr Opin Allergy Clin Immunol. 2008;8(4):330‐337. [DOI] [PubMed] [Google Scholar]
- 513. Jennings A, Duggan E, Perry IJ, Hourihane JO. Epidemiology of allergic reactions to hymenoptera stings in Irish school children. Pediatr Allergy Immunol. 2010;21(8):1166‐1170. [DOI] [PubMed] [Google Scholar]
- 514. Graif Y, Romano‐Zelekha O, Livne I, Green MS, Shohat T. Allergic reactions to insect stings: results from a national survey of 10,000 junior high school children in Israel. J Allergy Clin Immunol. 2006;117(6):1435‐1439. [DOI] [PubMed] [Google Scholar]
- 515. Quercia O, Incorvaia C, Marseglia GL, et al. Prevalence and incidence of reactions to insect stings in children: a reappraisal. Minerva Pediatr. 2014;66(4):257‐260. [PubMed] [Google Scholar]
- 516. Novembre E, Cianferoni A, Bernardini R, et al. Epidemiology of insect venom sensitivity in children and its correlation to clinical and atopic features. Clin Exp Allergy. 1998;28(7):834‐838. [DOI] [PubMed] [Google Scholar]
- 517. Schuberth KC, Lichtenstein LM, Kagey‐Sobotka A, Szklo M, Kwiterovich KA, Valentine MD. An epidemiologic study of insect allergy in children. I. Characteristics of the disease. J Pediatr. 1982;100(4):546‐551. [DOI] [PubMed] [Google Scholar]
- 518. Graif Y, Romano‐Zelekha O, Livne I, Green MS, Shohat T. Increased rate and greater severity of allergic reactions to insect sting among schoolchildren with atopic diseases. Pediatr Allergy Immunol. 2009;20(8):757‐762. [DOI] [PubMed] [Google Scholar]
- 519. Yavuz ST, Sahiner UM, Buyuktiryaki B, et al. Clinical features of children with venom allergy and risk factors for severe systemic reactions. Int Arch Allergy Immunol. 2013;160(3):313‐321. [DOI] [PubMed] [Google Scholar]
- 520. Sainte‐Laudy J, Sabbah A, Drouet M, Lauret MG, Loiry M. Diagnosis of venom allergy by flow cytometry. Correlation with clinical history, skin tests, specific IgE, histamine and leukotriene C4 release. Clin Exp Allergy. 2000;30(8):1166‐1171. [DOI] [PubMed] [Google Scholar]
- 521. Arzt L, Bokanovic D, Schrautzer C, et al. Questionable diagnostic benefit of the commercially available panel of bee venom components. Allergy. 2017;72(9):1419‐1422. [DOI] [PubMed] [Google Scholar]
- 522. Sturm GJ, Arzt‐Gradwohl L, Varga EM. Diagnosis and treatment of Hymenoptera venom allergy. Allergy. 2019;74:2016‐2018. [DOI] [PubMed] [Google Scholar]
- 523. Golden DB. Insect allergy in children. Curr Opin Allergy Clin Immunol. 2006;6(4):289‐293. [DOI] [PubMed] [Google Scholar]
- 524. Valentine MD, Schuberth KC, Kagey‐Sobotka A, et al. The value of immunotherapy with venom in children with allergy to insect stings. N Engl J Med. 1990;323(23):1601‐1603. [DOI] [PubMed] [Google Scholar]
- 525. Bonifazi F, Jutel M, Bilo BM, Birnbaum J, Muller U. Prevention and treatment of hymenoptera venom allergy: guidelines for clinical practice. Allergy. 2005;60(12):1459‐1470. [DOI] [PubMed] [Google Scholar]
- 526. Golden DB, Kagey‐Sobotka A, Norman PS, Hamilton RG, Lichtenstein LM. Outcomes of allergy to insect stings in children, with and without venom immunotherapy. N Engl J Med. 2004;351(7):668‐674. [DOI] [PubMed] [Google Scholar]
- 527. Stritzke AI, Eng PA. Age‐dependent sting recurrence and outcome in immunotherapy‐treated children with anaphylaxis to Hymenoptera venom. Clin Exp Allergy. 2013;43(8):950‐955. [DOI] [PubMed] [Google Scholar]
- 528. Golden DB, Valentine MD, Kagey‐Sobotka A, Lichtenstein LM. Regimens of Hymenoptera venom immunotherapy. Ann Intern Med. 1980;92(5):620‐624. [DOI] [PubMed] [Google Scholar]
- 529. Yunginger JW, Paull BR, Jones RT, Santrach PJ. Rush venom immunotherapy program for honeybee sting sensitivity. J Allergy Clin Immunol. 1979;63(5):340‐347. [DOI] [PubMed] [Google Scholar]
- 530. Gillman SA, Cummins LH, Kozak PP Jr, Hoffman DR. Venom immunotherapy: comparison of “rush” vs “conventional” schedules. Ann Allergy. 1980;45(6):351‐354. [PubMed] [Google Scholar]
- 531. Laurent J, Smiejan JM, Bloch‐Morot E, Herman D. Safety of Hymenoptera venom rush immunotherapy. Allergy. 1997;52(1):94‐96. [DOI] [PubMed] [Google Scholar]
- 532. Sturm G, Kranke B, Rudolph C, Aberer W. Rush Hymenoptera venom immunotherapy: a safe and practical protocol for high‐risk patients. J Allergy Clin Immunol. 2002;110(6):928‐933. [DOI] [PubMed] [Google Scholar]
- 533. van der Zwan JC, Flinterman J, Jankowski IG, Kerckhaert JA. Hyposensitisation to wasp venom in six hours. Br Med J (Clin Res Ed). 1983;287(6402):1329‐1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534. Bernstein JA, Kagen SL, Bernstein DI, Bernstein IL. Rapid venom immunotherapy is safe for routine use in the treatment of patients with Hymenoptera anaphylaxis. Ann Allergy. 1994;73(5):423‐428. [PubMed] [Google Scholar]
- 535. Birnbaum J, Charpin D, Vervloet D. Rapid Hymenoptera venom immunotherapy: comparative safety of three protocols. Clin Exp Allergy. 1993;23(3):226‐230. [DOI] [PubMed] [Google Scholar]
- 536. Roll A, Hofbauer G, Ballmer‐Weber BK, Schmid‐Grendelmeier P. Safety of specific immunotherapy using a four‐hour ultra‐rush induction scheme in bee and wasp allergy. J Investig Allergol Clin Immunol. 2006;16(2):79‐85. [PubMed] [Google Scholar]
- 537. Steiss JO, Jodicke B, Lindemann H. A modified ultrarush insect venom immunotherapy protocol for children. Allergy Asthma Proc. 2006;27(2):148‐150. [PubMed] [Google Scholar]
- 538. Stoevesandt J, Hosp C, Kerstan A, Trautmann A. Safety of 100 microg venom immunotherapy rush protocols in children compared to adults. Allergy, asthma, and clinical immunology : official journal of the Canadian Society of Allergy and Clinical Immunology. 2017;13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539. Confino‐Cohen R, Rosman Y, Goldberg A. Rush Venom Immunotherapy in Children. The journal of allergy and clinical immunology In practice. 2017;5(3):799‐803. [DOI] [PubMed] [Google Scholar]
- 540. Nittner‐Marszalska M, Cichocka‐Jarosz E, Malaczynska T, et al. Safety of Ultrarush Venom Immunotherapy: Comparison Between Children and Adults. J Investig Allergol Clin Immunol. 2016;26(1):40‐47. [PubMed] [Google Scholar]
- 541. Birnbaum J, Ramadour M, Magnan A, Vervloet D. Hymenoptera ultra‐rush venom immunotherapy (210 min): a safety study and risk factors. Clin Exp Allergy. 2003;33(1):58‐64. [DOI] [PubMed] [Google Scholar]
- 542. Malling HJ, Djurup R, Sondergaard I, Weeke B. Clustered immunotherapy with Yellow Jacket venom. Evaluation of the influence of time interval on in vivo and in vitro parameters. Allergy. 1985;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 543. Tarhini H, Knani J, Michel FB, Bousquet J. Safety of venom immunotherapy administered by a cluster schedule. J Allergy Clin Immunol. 1992;89(6):1198‐1199. [DOI] [PubMed] [Google Scholar]
- 544. Rueff F, Przybilla B, Bilo MB, et al. Predictors of side effects during the buildup phase of venom immunotherapy for Hymenoptera venom allergy: the importance of baseline serum tryptase. J Allergy Clin Immunol. 2010;126(1):105‐111. [DOI] [PubMed] [Google Scholar]
- 545. Roumana A, Pitsios C, Vartholomaios S, Kompoti E, Kontou‐Fili K. The safety of initiating Hymenoptera immunotherapy at 1 microg of venom extract. J Allergy Clin Immunol. 2009;124(2):379‐381. [DOI] [PubMed] [Google Scholar]
- 546. Konstantinou GN, Manoussakis E, Douladiris N, et al. A 5‐year venom immunotherapy protocol with 50 mug maintenance dose: safety and efficacy in school children. Pediatr Allergy Immunol. 2011;22(4):393‐397. [DOI] [PubMed] [Google Scholar]
- 547. Houliston L, Nolan R, Noble V, et al. Honeybee venom immunotherapy in children using a 50‐mug maintenance dose. J Allergy Clin Immunol. 2011;127(1):98‐99. [DOI] [PubMed] [Google Scholar]
- 548. Rueff F, Wenderoth A, Przybilla B. Patients still reacting to a sting challenge while receiving conventional Hymenoptera venom immunotherapy are protected by increased venom doses. J Allergy Clin Immunol. 2001;108(6):1027‐1032. [DOI] [PubMed] [Google Scholar]
- 549. Kohli‐Wiesner A, Stahlberger L, Bieli C, Stricker T, Lauener R. Induction of specific immunotherapy with hymenoptera venoms using ultrarush regimen in children: safety and tolerance. Journal of allergy. 2012;2012:790910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550. Livingston MG, Livingston HM. Monoamine oxidase inhibitors. An update on drug interactions. Drug Saf. 1996;14(4):219‐227. [DOI] [PubMed] [Google Scholar]
- 551. Mosbech H, Muller U. Side‐effects of insect venom immunotherapy: results from an EAACI multicenter study. European Academy of Allergology and Clinical Immunology. Allergy. 2000;55(11):1005‐1010. [DOI] [PubMed] [Google Scholar]
- 552. Bonadonna P, Zanotti R, Caruso B, et al. Allergen specific immunotherapy is safe and effective in patients with systemic mastocytosis and Hymenoptera allergy. J Allergy Clin Immunol. 2008;121(1):256‐257. [DOI] [PubMed] [Google Scholar]
- 553. Bonadonna P, Gonzalez‐de‐Olano D, Zanotti R, et al. Venom immunotherapy in patients with clonal mast cell disorders: efficacy, safety, and practical considerations. The journal of allergy and clinical immunology In practice. 2013;1(5):474‐478. [DOI] [PubMed] [Google Scholar]
- 554. Korosec P, Ziberna K, Silar M, et al. Immunological and clinical factors associated with adverse systemic reactions during the build‐up phase of honeybee venom immunotherapy. Clin Exp Allergy. 2015;45(10):1579‐1589. [DOI] [PubMed] [Google Scholar]
- 555. Muller UR, Haeberli G. Use of beta‐blockers during immunotherapy for Hymenoptera venom allergy. J Allergy Clin Immunol. 2005;115(3):606‐610. [DOI] [PubMed] [Google Scholar]
- 556. Stoevesandt J, Hain J, Stolze I, Kerstan A, Trautmann A. Angiotensin‐converting enzyme inhibitors do not impair the safety of Hymenoptera venom immunotherapy build‐up phase. Clin Exp Allergy. 2014;44(5):747‐755. [DOI] [PubMed] [Google Scholar]
- 557. Lockey RF, Turkeltaub PC, Olive ES, Hubbard JM. Baird‐Warren IA, Bukantz SC. The Hymenoptera venom study. III: Safety of venom immunotherapy. J Allergy Clin Immunol. 1990;86(5):775‐780. [DOI] [PubMed] [Google Scholar]
- 558. Golden DB, Johnson K, Addison BI, Valentine MD, Kagey‐Sobotka A, Lichtenstein LM. Clinical and immunologic observations in patients who stop venom immunotherapy. J Allergy Clin Immunol. 1986;77(3):435‐442. [DOI] [PubMed] [Google Scholar]
- 559. Randolph CC, Reisman RE. Evaluation of decline in serum venom‐specific IgE as a criterion for stopping venom immunotherapy. J Allergy Clin Immunol. 1986;77(6):823‐827. [DOI] [PubMed] [Google Scholar]
- 560. Lerch E, Muller UR. Long‐term protection after stopping venom immunotherapy: results of re‐stings in 200 patients. J Allergy Clin Immunol. 1998;101(5):606‐612. [DOI] [PubMed] [Google Scholar]
- 561. Keating MU, Kagey‐Sobotka A, Hamilton RG, Yunginger JW. Clinical and immunologic follow‐up of patients who stop venom immunotherapy. J Allergy Clin Immunol. 1991;88(3 Pt 1):339‐348. [DOI] [PubMed] [Google Scholar]
- 562. Golden DB, Kwiterovich KA, Kagey‐Sobotka A, Valentine MD, Lichtenstein LM. Discontinuing venom immunotherapy: outcome after five years. J Allergy Clin Immunol. 1996;97(2):579‐587. [DOI] [PubMed] [Google Scholar]
- 563. Golden DB, Kwiterovich KA, Kagey‐Sobotka A, Lichtenstein LM. Discontinuing venom immunotherapy: extended observations. J Allergy Clin Immunol. 1998;101(3):298‐305. [DOI] [PubMed] [Google Scholar]
- 564. Fiedler C, Miehe U, Treudler R, Kiess W, Prenzel F. Long‐Term Follow‐Up of Children after Venom Immunotherapy: Low Adherence to Anaphylaxis Guidelines. Int Arch Allergy Immunol. 2017;172(3):167‐172. [DOI] [PubMed] [Google Scholar]
- 565. Bilo MB, Pravettoni V, Bignardi D, et al. Hymenoptera Venom Allergy: Management of children and adults in clinical practice. J Investig Allergol Clin Immunol. 2019;29:180‐205. [DOI] [PubMed] [Google Scholar]
- 566. Golden DB, Kagey‐Sobotka A, Lichtenstein LM. Survey of patients after discontinuing venom immunotherapy. J Allergy Clin Immunol. 2000;105(2 Pt 1):385‐390. [DOI] [PubMed] [Google Scholar]
- 567. Reisman RE. Duration of venom immunotherapy: relationship to the severity of symptoms of initial insect sting anaphylaxis. J Allergy Clin Immunol. 1993;92(6):831‐836. [DOI] [PubMed] [Google Scholar]
- 568. Goldberg A, Confino‐Cohen R. Bee venom immunotherapy ‐ how early is it effective? Allergy. 2010;65(3):391‐395. [DOI] [PubMed] [Google Scholar]
- 569. Lange J, Cichocka‐Jarosz E, Marczak H, et al. Natural history of Hymenoptera venom allergy in children not treated with immunotherapy. Ann Allergy Asthma Immunol. 2016;116(3):225‐229. [DOI] [PubMed] [Google Scholar]
- 570. Dhami S, Nurmatov U, Varga EM, et al. Allergen immunotherapy for insect venom allergy: protocol for a systematic review. Clin Transl Allergy. 2015;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571. Graft DF, Schuberth KC, Kagey‐Sobotka A, et al. Assessment of prolonged venom immunotherapy in children. J Allergy Clin Immunol. 1987;80(2):162‐169. [DOI] [PubMed] [Google Scholar]
- 572. The Global Asthma . Report 2014. Auckland, New Zealand: Global Asthma Network; 2014. [Google Scholar]
- 573. Guilbert TW, Morgan WJ, Zeiger RS, et al. Long‐term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354(19):1985‐1997. [DOI] [PubMed] [Google Scholar]
- 574. Murray CS, Woodcock A, Langley SJ, et al. Secondary prevention of asthma by the use of inhaled fluticasone propionate in wheezy INfants (IFWIN): double‐blind, randomised, controlled study. Lancet. 2006;368(9537):754‐762. [DOI] [PubMed] [Google Scholar]
- 575. Eifan AO, Shamji MH, Durham SR. Long‐term clinical and immunological effects of allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2011;11(6):586‐593. [DOI] [PubMed] [Google Scholar]
- 576. GINA Executive Committee . Global Initiative for Asthma; Global Strategy for Asthma Management and Prevention. Bethesda, MA: National Heart, Lung and Blood Institute, National Institute of Health; 2018. [Google Scholar]
- 577. Rice JL, Diette GB, Suarez‐Cuervo C, et al. Allergen‐Specific Immunotherapy in the Treatment of Pediatric Asthma: A Systematic Review. Pediatrics. 2018;141:e20173833. [DOI] [PubMed] [Google Scholar]
- 578. Calamita Z, Saconato H, Pelá AB, Atallah AN. Efficacy of sublingual immunotherapy in asthma: systematic review of randomized‐clinical trials using the Cochrane Collaboration method. Allergy. 2006;61(10):1162‐1172. [DOI] [PubMed] [Google Scholar]
- 579. Di Rienzo V, Pagani A, Parmiani S, et al. Post‐marketing surveillance study on the safety of sublingual immunotherapy in pediatric patients. Allergy. 1999;54:1110‐1113. [DOI] [PubMed] [Google Scholar]
- 580. Bufe A, Eberle P, Franke‐Beckmann E, et al. Safety and efficacy in children of an SQ‐standardized grass allergen tablet for sublingual immunotherapy. J Allergy Clin Immunol. 2009;123:167‐173. [DOI] [PubMed] [Google Scholar]
- 581. Novembre E, Galli E, Landi F, et al. Co‐seasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2004;114:851‐857. [DOI] [PubMed] [Google Scholar]
- 582. Normansell R, Kew KM, Bridgman AL. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2015;28(8):CD011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583. Lin SY, Erekosima N, Kim JM, et al. Sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: a systematic review. JAMA. 2013 Mar 27;309(12):1278‐1288. [DOI] [PubMed] [Google Scholar]
- 584. Compalati E, Passalacqua G, Bonini M, et al. The efficacy of sublingual immunotherapy for house dust mites respiratory allergy: results of a GA2LEN meta‐analysis. Allergy. 2009;64(11):1570‐1579. [DOI] [PubMed] [Google Scholar]
- 585. Eifan AO, Akkoc T, Yildiz A, et al. Clinical efficacy and immunological mechanisms of sublingual and subcutaneous immunotherapy in asthmatic/ rhinitis children sensitized to house dust mite: an open randomized controlled trial. Clin Exp Allergy. 2010;40:922‐932. [DOI] [PubMed] [Google Scholar]
- 586. Yukselen A, Kendirli SG, Yilmaz M, et al. Effect of one‐year subcutaneous and sublingual immunotherapy on clinical and laboratory parameters in children with rhinitis and asthma: a randomized, placebo controlled, double‐blind, double‐dummy study. Int Arch Allergy Immunol. 2012;157(3):288‐298. [DOI] [PubMed] [Google Scholar]
- 587. Keles S, Karakoc‐Aydiner E, Ozen A, et al. A novel approach in allergen specific immunotherapy: combination of sublingual and subcutaneous routes. J Allergy Clin Immunol. 2011;128:808‐815. [DOI] [PubMed] [Google Scholar]
- 588. Di Bona D, Plaia A, Leto‐Barone MS, La Piana S, Di Lorenzo G. Efficacy of subcutaneous and sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a meta‐analysis‐based comparison. J Allergy Clin Immunol. 2012;130(5):1097‐1107. [DOI] [PubMed] [Google Scholar]
- 589. Khinchi MS, Poulsen LK, Carat F, Andre C, Hansen AB, Malling HJ. Clinical efficacy of sublingual and subcutaneous birch pollen allergen‐specific immunotherapy: a randomized, placebo‐controlled, double‐blind, double‐dummy study. Allergy. 2004;59(1):45‐53. [DOI] [PubMed] [Google Scholar]
- 590. Mungan D, Misirligil Z, Gurbuz L. Comparison of the efficacy of subcutaneous and sublingual immunotherapy in mite‐sensitive patients with rhinitis and asthma–a placebo controlled study. Ann Allergy Asthma Immunol. 1999;82(5):485‐490. [DOI] [PubMed] [Google Scholar]
- 591. Mauro M, Russello M, Incorvaia C, Gazzola GB, Di Cara G, Frati F. Comparison of efficacy, safety and immunologic effects of subcutaneous and sublingual immunotherapy in birch pollinosis: a randomized study. Eur Ann Allergy Clin Immunol. 2007;39(4):119‐122. [PubMed] [Google Scholar]
- 592. Piazza I, Bizzaro N. Humoral response to subcutaneous, oral, and nasal immunotherapy for allergic rhinitis due to Dermatophagoides pteronyssinus. Ann Allergy. 1993;71(5):461‐469. [PubMed] [Google Scholar]
- 593. Rodriguez Del Rio P, Vidal C, Just J, et al. The European Survey on Adverse Systemic Reactions in Allergen Immunotherapy (EASSI): A paediatric assessment. Pediatr Allergy Immunol. 2017;28(1):60‐70. [DOI] [PubMed] [Google Scholar]
- 594. Didier A, Bons B. Safety and tolerability of 5‐grass pollen tablet sublingual immunotherapy: pooled analysis and clinical review. Expert Opin Drug Saf. 2015;14(5):777‐788. [DOI] [PubMed] [Google Scholar]
- 595. Kiotseridis H, Arvidsson P, Backer V, Braendholt V, Tunsäter A. Adherence and quality of life in adults and children during 3‐years of SLIT treatment with Grazax‐a real life study. NPJ Prim Care Respir Med. 2018;28(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596. Agache I, Lau S, Akdis CA, Smolinska S, Bonini M, et al. EAACI Guidelines on Allergen Immunotherapy: House dust mite‐driven allergic asthma. Allergy. 2019;74(5):855‐873. [DOI] [PubMed] [Google Scholar]
- 597. Scadding GK, Brostoff J. Low dose sublingual therapy in patients with allergic rhinitis due to house dust mite. Clin Allergy. 1986;16(5):483‐491. [DOI] [PubMed] [Google Scholar]
- 598. Bousquet J, Schünemann HJ, Samolinski B, et al. Allergic Rhinitis and its Impact on Asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol. 2012;130(5):1049‐1062. [DOI] [PubMed] [Google Scholar]
- 599. 2019 hgow‐cuG–m‐P‐G‐wpAM.
- 600. Shao J, Cui YX, Zheng YF, et al. Efficacy and safety of sublingual immunotherapy in children aged 3‐13 years with allergic rhinitis. Am J Rhinol Allergy. 2014;28(2):131‐139. [DOI] [PubMed] [Google Scholar]
- 601. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: a systematic overview of systematic reviews. Clin Transl Allergy. 2017;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602. Agency EMAGoAPPaQILPbEM, 2019) ECBhweeedeGdlSgWpasMA.
- 603. Arasi S, Passalacqua G, Caminiti L, Crisafulli G, Fiamingo C, Pajno GB. Efficacy and safety of sublingual immunotherapy in children. Expert Rev Clin Immunol. 2016;12(1):49‐56. [DOI] [PubMed] [Google Scholar]
- 604. Pajno GB. Allergen immunotherapy in early childhood: between Scylla and Charybdis!. Clin Exp Allergy. 2005;35(5):551‐553. [DOI] [PubMed] [Google Scholar]
- 605. Calderon MA, Gerth van Wijk R, Eichler I, et al. Perspectives on allergen‐specific immunotherapy in childhood: an EAACI position statement. Pediatr Allergy Immunol. 2012;23(4):300‐306. [DOI] [PubMed] [Google Scholar]
- 606. Amin HS, Liss GM, Bernstein DI. Evaluation of near‐fatal reactions to allergen immunotherapy injections. J Allergy Clin Immunol. 2006;117(1):169‐175. [DOI] [PubMed] [Google Scholar]
- 607. Cox L, Larenas‐Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol. 2010;125(3):569‐574. [DOI] [PubMed] [Google Scholar]
- 608. Moingeon P. Update on immune mechanisms associated with sublingual immunotherapy: practical implications for the clinician. J Allergy Clin Immunol Pract. 2013;1(3):228‐241. [DOI] [PubMed] [Google Scholar]
- 609. Kücüksezer UC, Palomares O, Rückert B, et al. Triggering of specific Toll‐like receptors and proinflammatory cytokines breaks allergen‐specific T‐cell tolerance in human tonsils and peripheral blood. J Allergy Clin Immunol. 2013;131(3):875‐885. [DOI] [PubMed] [Google Scholar]
- 610. Lawrence MG, Steinke JW, Borish L. Basic science for the clinician: Mechanisms of sublingual and subcutaneous immunotherapy. Ann Allergy Asthma Immunol. 2016;117(2):138‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611. Schulten V, Tripple V, Aasbjerg K, et al. Distinct modulation of allergic T cell responses by subcutaneous vs. sublingual allergen‐specific immunotherapy. Clin Exp Allergy. 2016;46(3):439‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612. Passalacqua G, Baena‐Cagnani CE, Bousquet J, et al. Grading local side effects of sublingual immunotherapy for respiratory allergy: speaking the same language. J Allergy Clin Immunol. 2013;132(1):93‐98. [DOI] [PubMed] [Google Scholar]
- 613. Tham EH, Leung DY. Mechanisms by Which Atopic Dermatitis Predisposes to Food Allergy and the Atopic March. Allergy Asthma Immunol Res. 2019;11(1):4‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614. Hill DA, Spergel JM. The atopic march: Critical evidence and clinical relevance. Ann Allergy Asthma Immunol. 2018;120(2):131‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615. Matricardi PM. Molecular profile clustering of IgE responses and potential implications for specific immunotherapy. Curr Opin Allergy Clin Immunol. 2013;13(4):438‐445. [DOI] [PubMed] [Google Scholar]
- 616. Möller C, Dreborg S, Lanner A, Björkstén B. Oral immunotherapy of children with rhinoconjunctivitis due to birch pollen allergy. A double blind study. Allergy. 1986;41(4):271‐279. [DOI] [PubMed] [Google Scholar]
- 617. Grembiale RD, Camporota L, Naty S, Tranfa CM, Djukanovic R, Marsico SA. Effects of specific immunotherapy in allergic rhinitic individuals with bronchial hyperresponsiveness. Am J Respir Crit Care Med. 2000;162(6):2048‐2052. [DOI] [PubMed] [Google Scholar]
- 618. García BE, González‐Mancebo E, Barber D, et al. Sublingual immunotherapy in peach allergy: monitoring molecular sensitizations and reactivity to apple fruit and Platanus pollen. J Investig Allergol Clin Immunol. 2010;20(6):514‐520. [PubMed] [Google Scholar]
- 619. Khan SJ, Dharmage SC, Matheson MC, Gurrin LC. Is the atopic march related to confounding by genetics and early‐life environment? A systematic review of sibship and twin data. Allergy. 2018;73(1):17‐28. [DOI] [PubMed] [Google Scholar]
- 620. Tran MM, Lefebvre DL, Dharma C, et al. Predicting the atopic march: Results from the Canadian Healthy Infant Longitudinal Development Study. J Allergy Clin Immunol. 2018;141(2):601‐607. [DOI] [PubMed] [Google Scholar]
- 621. Gough H, Grabenhenrich L, Reich A, et al. Allergic multimorbidity of asthma, rhinitis and eczema over 20 years in the German birth cohort MAS. Pediatr Allergy Immunol. 2015;26(5):431‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622. Pfaar O, Kleine‐Tebbe J, Hörmann K, Klimek L. Allergen‐specific immunotherapy: which outcome measures are useful in monitoring clinical trials? Immunol Allergy Clin North Am. 2011;31(2):289‐309. [DOI] [PubMed] [Google Scholar]
- 623. Dahl R, Kapp A, Colombo G, et al. Efficacy and safety of sublingual immunotherapy with grass allergen tablets for seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;118(2):434‐440. [DOI] [PubMed] [Google Scholar]
- 624. Didier A, Worm M, Horak F, et al. Sustained 3‐year efficacy of pre‐ and coseasonal 5‐grass‐pollen sublingual immunotherapy tablets in patients with grass pollen‐induced rhinoconjunctivitis. J Allergy Clin Immunol. 2011;128(3):559‐566. [DOI] [PubMed] [Google Scholar]
- 625. Durham SR, Emminger W, Kapp A, et al. Long‐term clinical efficacy in grass pollen‐induced rhinoconjunctivitis after treatment with SQ‐standardized grass allergy immunotherapy tablet. J Allergy Clin Immunol. 2010;125(1):131‐138. [DOI] [PubMed] [Google Scholar]
- 626. Durham SR, Ying S, Varney VA, et al. Grass pollen immunotherapy inhibits allergen‐induced infiltration of CD4+ T lymphocytes and eosinophils in the nasal mucosa and increases the number of cells expressing messenger RNA for interferon‐gamma. J Allergy Clin Immunol. 1996;97(6):1356‐1365. [DOI] [PubMed] [Google Scholar]
- 627. Ebner C, Siemann U, Bohle B, et al. Immunological changes during specific immunotherapy of grass pollen allergy: reduced lymphoproliferative responses to allergen and shift from TH2 to TH1 in T‐cell clones specific for Phl p 1, a major grass pollen allergen. Clin Exp Allergy. 1997;27(9):1007‐1015. [DOI] [PubMed] [Google Scholar]
- 628. Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn‐Schmid B, Ebner C. Sublingual immunotherapy induces IL‐10‐producing T regulatory cells, allergen‐specific T‐cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007;120(3):707‐713. [DOI] [PubMed] [Google Scholar]
- 629. Scadding GW, Shamji MH, Jacobson MR, et al. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3‐expressing cells and elevated allergen‐specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E‐facilitated allergen binding to B cells. Clin Exp Allergy. 2010;40(4):598‐606. [DOI] [PubMed] [Google Scholar]
- 630. Wachholz PA, Soni NK, Till SJ, Durham SR. Inhibition of allergen‐IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J Allergy Clin Immunol. 2003;112(5):915‐922. [DOI] [PubMed] [Google Scholar]
- 631. Awate S, Babiuk LA, Mutwiri G. Mechanisms of action of adjuvants. Front Immunol. 2013;4:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632. Glenny AT, Pope CG, Waddington H, Wallace U. Immunological notes. XVII–XXIV. Journal of Pathology and Bacteriology. 1926;29(1):31‐40. [Google Scholar]
- 633. Glenny AT, Barr M. The precipitation of diphtheria toxoid by potash alum. The Journal of Pathology and Bacteriology. 1931;34(2):131‐138. [Google Scholar]
- 634. Di Pasquale A, Preiss S, Tavares Da Silva F, Garcon N. Vaccine Adjuvants: from 1920 to 2015 and Beyond. Vaccines 2015;3(2):320‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635. Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19(12):1597‐1608. [DOI] [PubMed] [Google Scholar]
- 636. Harrison WT. Effect of Alum‐Precipitated Ragweed Pollen Extract on Guinea Pigs. Public Health Rep. 1934;49(14):462‐464. [Google Scholar]
- 637. Mahler V, Esch RE, Kleine‐Tebbe J, et al. Understanding differences in allergen immunotherapy products and practices in North America and Europe. J Allergy Clin Immunol. 2019;143(3):813‐828. [DOI] [PubMed] [Google Scholar]
- 638. Jensen‐Jarolim E. Aluminium in Allergies and Allergen immunotherapy. The World Allergy Organization journal. 2015;8(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639. Hogenesch H. Mechanism of immunopotentiation and safety of aluminium adjuvants. Front Immunol. 2012;3:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640. White RG, Coons AH, Connolly JM. Studies on antibody production. III. The alum granuloma. J Exp Med. 1955;102(1):73‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641. Mannhalter JW, Neychev HO, Zlabinger GJ, Ahmad R, Eibl MM. Modulation of the human immune response by the non‐toxic and non‐pyrogenic adjuvant aluminium hydroxide: effect on antigen uptake and antigen presentation. Clin Exp Immunol. 1985;61(1):143‐151. [PMC free article] [PubMed] [Google Scholar]
- 642. Ghimire TR, Benson RA, Garside P, Brewer JM. Alum increases antigen uptake, reduces antigen degradation and sustains antigen presentation by DCs in vitro. Immunol Lett. 2012;147(1–2):55‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643. Sun H, Pollock KG, Brewer JM. Analysis of the role of vaccine adjuvants in modulating dendritic cell activation and antigen presentation in vitro. Vaccine. 2003;21(9–10):849‐855. [DOI] [PubMed] [Google Scholar]
- 644. Grun JL, Maurer PH. Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: the role of endogenous interleukin 1 in proliferative responses. Cell Immunol. 1989;121(1):134‐145. [DOI] [PubMed] [Google Scholar]
- 645. Schmitz N, Kurrer M, Kopf M. The IL‐1 receptor 1 is critical for Th2 cell type airway immune responses in a mild but not in a more severe asthma model. Eur J Immunol. 2003;33(4):991‐1000. [DOI] [PubMed] [Google Scholar]
- 646. Brewer JM, Conacher M, Satoskar A, Bluethmann H, Alexander J. In interleukin‐4‐deficient mice, alum not only generates T helper 1 responses equivalent to Freund's complete adjuvant, but continues to induce T helper 2 cytokine production. Eur J Immunol. 1996;26(9):2062‐2066. [DOI] [PubMed] [Google Scholar]
- 647. Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombacher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen‐specific Th2 responses in the absence of IL‐4‐ or IL‐13‐mediated signaling. Journal of immunology. 1999;163(12):6448‐6454. [PubMed] [Google Scholar]
- 648. Leuthard DS, Duda A, Freiberger SN, et al. Microcrystalline Tyrosine and Aluminum as Adjuvants in Allergen‐Specific Immunotherapy Protect from IgE‐Mediated Reactivity in Mouse Models and Act Independently of Inflammasome and TLR Signaling. Journal of immunology. 2018;200(9):3151‐3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649. de la Torre MV, Baeza ML, Najera L, Zubeldia JM. Comparative study of adjuvants for allergen‐specific immunotherapy in a murine model. Immunotherapy. 2018;10(14):1219‐1228. [DOI] [PubMed] [Google Scholar]
- 650. Pollock KG, Conacher M, Wei XQ, Alexander J, Brewer JM. Interleukin‐18 plays a role in both the alum‐induced T helper 2 response and the T helper 1 response induced by alum‐adsorbed interleukin‐12. Immunology. 2003;108(2):137‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651. Shah HB, Devera TS, Rampuria P, Lang GA, Lang ML. Type II NKT cells facilitate Alum‐sensing and humoral immunity. J Leukoc Biol. 2012;92(4):883‐893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652. Hornung V, Bauernfeind F, Halle A, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9(8):847‐856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653. Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453(7198):1122‐1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654. McKee AS, Munks MW, MacLeod MK, et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. Journal of immunology. 2009;183(7):4403‐4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655. Franchi L, Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide‐mediated IL‐1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38(8):2085‐2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656. Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. Journal of immunology. 2008;181(1):17‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657. Kool M, Soullie T, van Nimwegen M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205(4):869‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658. Marichal T, Ohata K, Bedoret D, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17(8):996‐1002. [DOI] [PubMed] [Google Scholar]
- 659. Svensson A, Sandberg T, Siesjo P, Eriksson H. Sequestering of damage‐associated molecular patterns (DAMPs): a possible mechanism affecting the immune‐stimulating properties of aluminium adjuvants. Immunol Res. 2017;65(6):1164‐1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660. Wang Y, Rahman D, Lehner T. A comparative study of stress‐mediated immunological functions with the adjuvanticity of alum. The Journal of biological chemistry. 2012;287(21):17152‐17160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661. Hebert J, Small P. Comparison of alum‐precipitated aqueous extracts and modified ragweed tyrosine adsorbate vaccine in the treatment of ragweed hay fever. Annals of allergy. 1988;60(3):226‐230. [PubMed] [Google Scholar]
- 662. Goto N, Kato H, Maeyama J, et al. Local tissue irritating effects and adjuvant activities of calcium phosphate and aluminium hydroxide with different physical properties. Vaccine. 1997;15(12–13):1364‐1371. [DOI] [PubMed] [Google Scholar]
- 663. Tomljenovic L, Shaw CA. Mechanisms of aluminum adjuvant toxicity and autoimmunity in pediatric populations. Lupus. 2012;21(2):223‐230. [DOI] [PubMed] [Google Scholar]
- 664. Weisser K, Goen T, Oduro JD, Wangorsch G, Hanschmann KO, Keller‐Stanislawski B. Aluminium from adjuvanted subcutaneous allergen immunotherapeutics in rats is mainly detected in bone. Allergy. 2020;75:215‐217. [DOI] [PubMed] [Google Scholar]
- 665. McDougall SA, Heath MD, Kramer MF, Skinner MA. Analysis of aluminium in rat following administration of allergen immunotherapy using either aluminium or microcrystalline‐tyrosine‐based adjuvants. Bioanalysis. 2016;8(6):547‐556. [DOI] [PubMed] [Google Scholar]
- 666. Bergfors E, Trollfors B, Inerot A. Unexpectedly high incidence of persistent itching nodules and delayed hypersensitivity to aluminium in children after the use of adsorbed vaccines from a single manufacturer. Vaccine. 2003;22(1):64‐69. [DOI] [PubMed] [Google Scholar]
- 667. Mosbech H, Malling HJ, Biering I, et al. Immunotherapy with yellow jacket venom. A comparative study including three different extracts, one adsorbed to aluminium hydroxide and two unmodified. Allergy. 1986;41(2):95‐103. [DOI] [PubMed] [Google Scholar]
- 668. Heydenreich B, Bellinghausen I, Lund L, et al. Adjuvant effects of aluminium hydroxide‐adsorbed allergens and allergoids ‐ differences in vivo and in vitro. Clin Exp Immunol. 2014;176(3):310‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669. Hauswald B, Wolf H, Becker F, Becker S, Schnitker J, Wustenberg E. Tolerability of a new fast updosed immunologically enhanced subcutaneous immunotherapy formulation with an optimized allergen to adjuvant ratio under routine practice conditions: a noninterventional observational study. J Investig Allergol Clin Immunol. 2013;23(7):471‐477. [PubMed] [Google Scholar]
- 670. O'Neill LA, Golenbock D, Bowie AG. The history of Toll‐like receptors ‐ redefining innate immunity. Nature Rev Immunol. 2013;13(6):453‐460. [DOI] [PubMed] [Google Scholar]
- 671. Kawasaki T, Kawai T. Toll‐like receptor signaling pathways. Front Immunol. 2014;5:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672. Wheeler AW, Marshall JS, Ulrich JT. A Th1‐inducing adjuvant, MPL, enhances antibody profiles in experimental animals suggesting it has the potential to improve the efficacy of allergy vaccines. Int Arch Allergy Immunol. 2001;126(2):135‐139. [DOI] [PubMed] [Google Scholar]
- 673. Rosewich M, Girod K, Zielen S, Schubert R, Schulze J. Induction of Bronchial Tolerance After 1 Cycle of Monophosphoryl‐A‐Adjuvanted Specific Immunotherapy in Children With Grass Pollen Allergies. Allergy, asthma & immunology research. 2016;8(3):257‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674. Rosewich M, Schulze J, Eickmeier O, et al. Tolerance induction after specific immunotherapy with pollen allergoids adjuvanted by monophosphoryl lipid A in children. Clin Exp Immunol. 2010;160(3):403‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675. Pfaar O, Barth C, Jaschke C, Hormann K, Klimek L. Sublingual allergen‐specific immunotherapy adjuvanted with monophosphoryl lipid A: a phase I/IIa study. Int Arch Allergy Immunol. 2011;154(4):336‐344. [DOI] [PubMed] [Google Scholar]
- 676. Worm M, Higenbottam T, Pfaar O, et al. Randomized controlled trials define shape of dose response for Pollinex Quattro Birch allergoid immunotherapy. Allergy. 2018;73(9):1812‐1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677. Rosewich M, Lee D, Zielen S. Pollinex Quattro: an innovative four injections immunotherapy in allergic rhinitis. Human vaccines & immunotherapeutics. 2013;9(7):1523‐1531. [DOI] [PubMed] [Google Scholar]
- 678. Puggioni F, Durham SR, Francis JN. Monophosphoryl lipid A (MPL) promotes allergen‐induced immune deviation in favour of Th1 responses. Allergy. 2005;60(5):678‐684. [DOI] [PubMed] [Google Scholar]
- 679. Mothes N, Heinzkill M, Drachenberg KJ, et al. Allergen‐specific immunotherapy with a monophosphoryl lipid A‐adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy‐induced blocking antibodies. Clin Exp Allergy. 2003;33(9):1198‐1208. [DOI] [PubMed] [Google Scholar]
- 680. Deifl S, Kitzmuller C, Steinberger P, et al. Differential activation of dendritic cells by toll‐like receptors causes diverse differentiation of naive CD4+ T cells from allergic patients. Allergy. 2014;69(12):1602‐1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681. Ismaili J, Rennesson J, Aksoy E, et al. Monophosphoryl lipid A activates both human dendritic cells and T cells. Journal of immunology. 2002;168(2):926‐932. [DOI] [PubMed] [Google Scholar]
- 682. Kuipers H, Hijdra D, de Vries VC, et al. Lipopolysaccharide‐Induced Suppression of Airway Th2 Responses Does Not Require IL‐12 Production by Dendritic Cells. J Immunol. 2003;171(7):3645. [DOI] [PubMed] [Google Scholar]
- 683. Ekman AK, Adner M, Cardell LO. Toll‐like receptor 7 activation reduces the contractile response of airway smooth muscle. Eur J Pharmacol. 2011;652(1–3):145‐151. [DOI] [PubMed] [Google Scholar]
- 684. Bortolatto J, Mirotti L, Rodriguez D, Gomes E, Russo M. Adsorption of Toll‐Like Receptor 4 Agonist to Alum‐Based Tetanus Toxoid Vaccine Dampens Pro‐T Helper 2 Activities and Enhances Antibody Responses. Journal of immunology research. 2015;2015:280238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685. Asai K, Foley SC, Sumi Y, et al. Amb a 1‐immunostimulatory oligodeoxynucleotide conjugate immunotherapy increases CD4+CD25+ T cells in the nasal mucosa of subjects with allergic rhinitis. Allergology international : official journal of the Japanese Society of Allergology. 2008;57(4):377‐381. [DOI] [PubMed] [Google Scholar]
- 686. Senti G, Johansen P, Haug S, et al. Use of A‐type CpG oligodeoxynucleotides as an adjuvant in allergen‐specific immunotherapy in humans: a phase I/IIa clinical trial. Clin Exp Allergy. 2009;39(4):562‐570. [DOI] [PubMed] [Google Scholar]
- 687. Klimek L, Willers J, Hammann‐Haenni A, et al. Assessment of clinical efficacy of CYT003‐QbG10 in patients with allergic rhinoconjunctivitis: a phase IIb study. Clin Exp Allergy. 2011;41(9):1305‐1312. [DOI] [PubMed] [Google Scholar]
- 688. Srivastava KD, Siefert A, Fahmy TM, Caplan MJ, Li XM, Sampson HA. Investigation of peanut oral immunotherapy with CpG/peanut nanoparticles in a murine model of peanut allergy. J Allergy Clin Immunol. 2016;138(2):536‐543. [DOI] [PubMed] [Google Scholar]
- 689. Biggadike K, Ahmed M, Ball DI, et al. Discovery of 6‐Amino‐2‐{[(1S)‐1‐methylbutyl]oxy}‐9‐[5‐(1‐piperidinyl)pentyl]‐7,9‐dihydro‐8H‐pu rin‐8‐one (GSK2245035), a Highly Potent and Selective Intranasal Toll‐Like Receptor 7 Agonist for the Treatment of Asthma. J Med Chem. 2016;59(5):1711‐1726. [DOI] [PubMed] [Google Scholar]
- 690. Tsitoura D, Ambery C, Price M, et al. Early clinical evaluation of the intranasal TLR7 agonist GSK2245035: Use of translational biomarkers to guide dosing and confirm target engagement. Clin Pharmacol Ther. 2015;98(4):369‐380. [DOI] [PubMed] [Google Scholar]
- 691. Ellis AK, Tsitoura DC, Quint D, Powley W, Lee LA. Safety and pharmacodynamics of intranasal GSK2245035, a TLR7 agonist for allergic rhinitis: A randomized trial. Clin Exp Allergy. 2017;47(9):1193‐1203. [DOI] [PubMed] [Google Scholar]
- 692. Greiff L, Cervin A, Ahlstrom‐Emanuelsson C, et al. Repeated intranasal TLR7 stimulation reduces allergen responsiveness in allergic rhinitis. Respir Res. 2012;13:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693. Greiff L, Ahlstrom‐Emanuelsson C, Alenas M, et al. Biological effects and clinical efficacy of a topical Toll‐like receptor 7 agonist in seasonal allergic rhinitis: a parallel group controlled phase IIa study. Inflammation research : official journal of the European Histamine Research Society [et al]. 2015;64(11):903‐915. [DOI] [PubMed] [Google Scholar]
- 694. Delaney S, Biffen M, Maltby J, et al. Tolerability in man following inhalation dosing of the selective TLR7 agonist, AZD8848. BMJ open respiratory research. 2016;3(1):e000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695. Bell AJ, Heath MD, Hewings SJ, Skinner MA. The adsorption of allergoids and 3‐O‐desacyl‐4’‐monophosphoryl lipid A (MPL(R)) to microcrystalline tyrosine (MCT) in formulations for use in allergy immunotherapy. J Inorg Biochem. 2015;152:147‐153. [DOI] [PubMed] [Google Scholar]
- 696. Klimek L, Schmidt‐Weber CB, Kramer MF, Skinner MA, Heath MD. Clinical use of adjuvants in allergen‐immunotherapy. Exp Rev Clin Immunol. 2017;13(6):599‐610. [DOI] [PubMed] [Google Scholar]
- 697. Heath MD, Swan NJ, Marriott AC, et al. Comparison of a novel microcrystalline tyrosine adjuvant with aluminium hydroxide for enhancing vaccination against seasonal influenza. BMC Infect Dis. 2017;17(1):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698. Baldrick P, Richardson D, Wheeler AW. Review of L‐tyrosine confirming its safe human use as an adjuvant. Journal of applied toxicology. 2002;22(5):333‐344. [DOI] [PubMed] [Google Scholar]
- 699. Park KH, Lee SC, Son YW, et al. Different Responses in Induction of Allergen Specific Immunoglobulin G4 and IgE‐Blocking Factors for Three Mite Subcutaneous Immunotherapy Products. Yonsei Med J. 2016;57(6):1427‐1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700. Zubeldia JM, Ferrer M, Davila I, Justicia JL. Adjuvants in Allergen‐Specific Immunotherapy: Modulating and Enhancing the Immune Response. J Investig Allergol Clin Immunol. 2019;29(2):103‐111. [DOI] [PubMed] [Google Scholar]
- 701. Gupta RK, Siber GR. Adjuvants for human vaccines–current status, problems and future prospects. Vaccine. 1995;13(14):1263‐1276. [DOI] [PubMed] [Google Scholar]
- 702. Masson JD, Thibaudon M, Belec L, Crepeaux G. Calcium phosphate: a substitute for aluminum adjuvants? Expert review of vaccines. 2017;16(3):289‐299. [DOI] [PubMed] [Google Scholar]
- 703. Lin Y, Wang X, Huang X, Zhang J, Xia N, Zhao Q. Calcium phosphate nanoparticles as a new generation vaccine adjuvant. Expert review of vaccines. 2017;16(9):895‐906. [DOI] [PubMed] [Google Scholar]
- 704. Pohlit H, Bellinghausen I, Frey H, Saloga J. Recent advances in the use of nanoparticles for allergen‐specific immunotherapy. Allergy. 2017;72(10):1461‐1474. [DOI] [PubMed] [Google Scholar]
- 705. Brotons‐Canto A, Gamazo C, Martin‐Arbella N, et al. Evaluation of nanoparticles as oral vehicles for immunotherapy against experimental peanut allergy. Int J Biol Macromol. 2018;110:328‐335. [DOI] [PubMed] [Google Scholar]
- 706. Gamazo C, Garcia‐Azpiroz M, Souza Reboucas J, Gastaminza G, Ferrer M, Irache JM. Oral immunotherapy using polymeric nanoparticles loaded with peanut proteins in a murine model of fatal anaphylaxis. Immunotherapy. 2017;9(15):1205‐1217. [DOI] [PubMed] [Google Scholar]
- 707. Kundig TM, Senti G, Schnetzler G, et al. Der p 1 peptide on virus‐like particles is safe and highly immunogenic in healthy adults. J Allergy Clin Immunol. 2006;117(6):1470‐1476. [DOI] [PubMed] [Google Scholar]
- 708. Beeh KM, Kanniess F, Wagner F, et al. The novel TLR‐9 agonist QbG10 shows clinical efficacy in persistent allergic asthma. J Allergy Clin Immunol. 2013;131(3):866‐874. [DOI] [PubMed] [Google Scholar]
- 709. Kundig TM, Klimek L, Schendzielorz P, Renner WA, Senti G, Bachmann MF. Is The Allergen Really Needed in Allergy Immunotherapy? Current treatment options in allergy. 2015;2(1):72‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710. Basomba A, Tabar AI, de Rojas DH, et al. Allergen vaccination with a liposome‐encapsulated extract of Dermatophagoides pteronyssinus: a randomized, double‐blind, placebo‐controlled trial in asthmatic patients. J Allergy Clin Immunol. 2002;109(6):943‐948. [DOI] [PubMed] [Google Scholar]
- 711. Arikan C, Bahceciler NN, Deniz G, et al. Bacillus Calmette‐Guerin‐induced interleukin‐12 did not additionally improve clinical and immunologic parameters in asthmatic children treated with sublingual immunotherapy. Clin Exp Allergy. 2004;34(3):398‐405. [DOI] [PubMed] [Google Scholar]
- 712. Majak P, Rychlik B, Stelmach I. The effect of oral steroids with and without vitamin D3 on early efficacy of immunotherapy in asthmatic children. Clin Exp Allergy. 2009;39(12):1830‐1841. [DOI] [PubMed] [Google Scholar]
- 713. Kalliomaki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4‐year follow‐up of a randomised placebo‐controlled trial. Lancet. 2003;361(9372):1869‐1871. [DOI] [PubMed] [Google Scholar]
- 714. Tang ML, Ponsonby AL, Orsini F, et al. Administration of a probiotic with peanut oral immunotherapy: A randomized trial. J Allergy Clin Immunol. 2015;135(3):737‐744. [DOI] [PubMed] [Google Scholar]
- 715. Liu J, Chen FH, Qiu SQ, et al. Probiotics enhance the effect of allergy immunotherapy on regulating antigen specific B cell activity in asthma patients. Am J Transl Res. 2016;8(12):5256‐5270. [PMC free article] [PubMed] [Google Scholar]
- 716. de Roock S, van Elk M, van Dijk ME, et al. Lactic acid bacteria differ in their ability to induce functional regulatory T cells in humans. Clin Exp Allergy. 2010;40(1):103‐110. [DOI] [PubMed] [Google Scholar]


