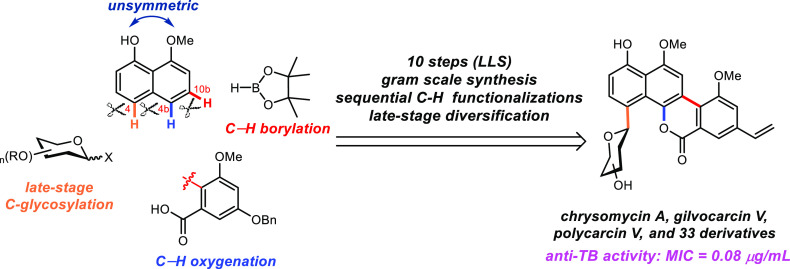
Abstract
Tuberculosis (TB) is a life-threatening disease resulting in an estimated 10 million new infections and 1.8 million deaths annually, primarily in underdeveloped countries. The economic burden of TB has been estimated as approximately 12 billion USD annually in direct and indirect costs. Additionally, multi-drug-resistant (MDR) and extreme-drug-resistant (XTR) TB strains resulting in about 250 000 deaths annually are now widespread, increasing pressure on the identification of new anti-TB agents that operate by a novel mechanism of action. Chrysomycin A is a rare C-aryl glycoside first discovered over 60 years ago. In a recent high-throughput screen, we found that chrysomycin A has potent anti-TB activity, with minimum inhibitory concentration (MIC) = 0.4 μg/mL against MDR-TB strains. However, chrysomycin A is obtained in low yields from fermentation of Streptomyces, and the mechanism of action of this compound is unknown. To facilitate the mechanism of action and preclinical studies of chrysomycin A, we developed a 10-step, scalable synthesis of the isolate and its two natural congeners polycarcin V and gilvocarcin V. The synthetic sequence was enabled by the implementation of two sequential C–H functionalization steps as well as a late-stage C-glycosylation. In addition, >10 g of the advanced synthetic intermediate has been prepared, which greatly facilitated the synthesis of 33 new analogues to date. The structure–activity relationship was subsequently delineated, leading to the identification of derivatives with superior potency against MDR-TB (MIC = 0.08 μg/mL). The more potent derivatives contained a modified carbohydrate residue which suggests that further optimization is additionally possible. The chemistry we report here establishes a platform for the development of a novel class of anti-TB agents active against drug-resistant pathogens.
Short abstract
We report a 10-step and scalable synthesis of chrysomycin A, a potent anti-TB C-aryl glycoside. The synthesis highlights sequential C-H functionalizations and the late-stage diversification.
Introduction
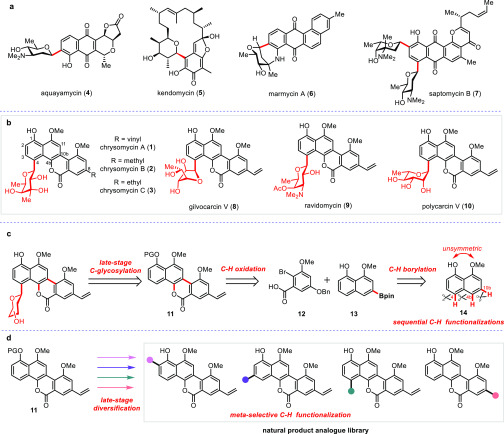
Tuberculosis (TB) has become the number one life-threatening infectious disease, whose treatment is further complicated by the emergence of drug-resistant strains.1 Over the past four decades, only two new drugs, bedaquiline2 and delamanid,3 have been approved by the FDA and EMA, respectively, for the treatment of MDR-TB. Considering the high attrition rate in clinical trials, more effective anti-TB drug candidates with distinct molecular scaffolds are in urgent need. Chrysomycin A (1) is an antitumor antibiotic first isolated from Streptomyces A-419 in 1955 as a mixture with chrysomycin B (2).4 Recently, we rediscovered chrysomycin A (1) and its natural congeners from mining of a 10K actinobacteria genome sequences5 and found that chrysomycin A showed promising antimicrobial activity against a number of Gram-positive strains and the MDR-TB strain with a minimum inhibitory concentration (MIC) of 0.4 μg/mL (see Tables S1 and S2). Kumar and co-workers also independently reported that chrysomycin A showed inhibitory activity against M. tb strains.6 Congeneric chrysomycin C (3) was isolated from Streptomyces sporoverrucosus in 2013.7 Chrysomycins A–C belong to the gilvocarcin family of C-aryl glycosides, which are a unique class of C-glycosilyated natural products and exhibit various important biological activities.8 For instance, aquayamycin (4, Figure 1a), produced by Streptomyces misawanensis, displays notable antimicrobial activity against Gram positive bacteria.9 Kendomycin (5) is a potent endothelin receptor antagonist and a calcitonin receptor agonist.10 Marmycin A (6) and saptomycin B (7) show significant cytotoxicity against several tumor cell lines.11,12 Gilvocarcin V (8), closely related to chrysomycin A, exhibits potent antitumor and antibacterial activity.13 The sugar moieties are mainly located at the ortho-position of phenol in most C-glycosilyated natural products, which are often biogenetically attached through an initial O-glycosylation followed by a Fries-like rearrangement mediated by various glycosyltransferases.14 Notably, chrysomycins possess the uncommon and unique para-substituted sugar moiety, and more investigations are required to fully elucidate the biological function of these rare para-substituted C-glycosides.
Figure 1.
C-aryl glycoside natural products and synthetic plans for chrysomycin A and its analogues. (a) Representative bioactive C-aryl glycosides. (b) Structures of gilvocarcin family natural products. (c) Our bond disconnections of chrysomycin A. In our retrosynthetic analysis of chrysomycin A, both the sugar moiety and vinyl group were assembled at the late stage. The core structure of the chromophore was constructed through sequential regioselective C–H functionalizations. (d) Late-stage diversification of the natural product at multiple sites.
To our knowledge, no successful total synthesis of chrysomycins has been reported since the original discovery of these natural products 60 years ago. The Hart group attempted to synthesize chrysomycin B with a strategy to form the lactone in the presence of carbohydrate moiety at the C4 position but without success.15 Attracted by the fascinating structure and potent anti-TB activity of chrysomycin A, we set out to accomplish its total synthesis that would serve as a sustainable source and platform for a structure–activity relationship study, which may facilitate the discovery of potential lead compounds for the treatment of TB. We aim to develop a scalable synthesis which will then allow for diverse late-stage transformations including direct C–H functionalization to rapidly access a wide range of analogues.
The landmark syntheses of other C8-vinyl gilvocarcin family members (Figure 1b) gilvocarcin V (8)16 and ravidomycin (9)17 were accomplished by the Suzuki group, and recently polycarcin V (10)18 was synthesized by Minehan and co-workers. Their synthetic strategies all relied on the early-stage glycosylation prior to the construction of the chromophore backbone. However, these previous synthetic works all suffered from a long linear sequence (18–30 steps) which was not amenable for the preparation of diverse analogues. As this family of natural products is only distinguished by variation of side chains at C4 and C8, we aimed to develop a highly convergent synthetic route, which would allow late-stage installation of various substituents at both C4 and C8 positions and thus facilitate the derivatization of this family of natural products. We anticipate that this family of natural products could be accessed from 11 via late-stage C4 Friedel–Crafts type C-glycosylation, although this transformation could be conceivably challenging due to the fact that regioselectivity normally favors C2-selective C-glycosylation.19,20
Results and Discussion
Retrosynthetic Analysis of Chrysomycin A
Inspired by recent advances in natural product synthesis using multiple C–H activation reactions,21−28 we envision that the synthesis of 11 could be drastically simplified by adopting sequential regioselective C–H functionalizations of the unsymmetric monomethylated naphthalenediol 14 (Figure 1c). The C10b instead of C3 functionality (Figure 1c) would be built by intermolecular C–H borylation, while the C4b instead of C11 functionality would be installed by carboxyl-group-directed intramolecular C–H oxygenation (Figure 1c). Finally, late-stage and direct diversification of chrysomycin A via C–H functionalizations or other transformations would provide a more effective approach than de novo synthesis to generate various natural productlike analogues for a subsequent structure–activity relationship (SAR) study in order to generate potential lead compounds for drug discovery (Figure 1d).29,30 Notably, the site-selective C–H functionalizations of complex molecules may also open the door to install various functional groups that are inaccessible with the traditional synthetic route.
Synthesis of the Aglycon 21
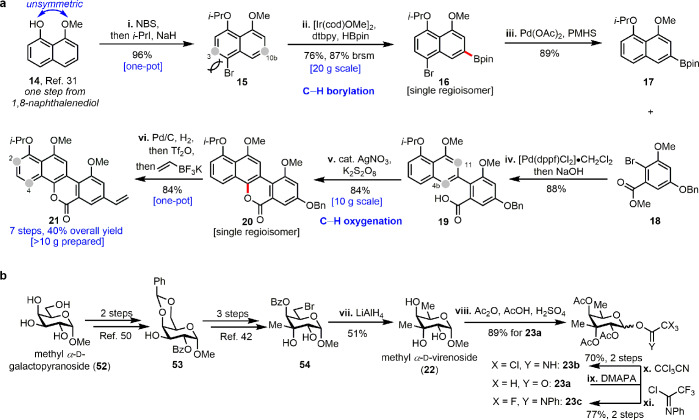
Our synthesis began with the C–H borylation at C10b (Figure 2a). Due to the asymmetric structure of 14, we were able to install a removable bromine atom as a blocking group at C4 to constrict C3-borylation. Bromide 15 was prepared smoothly in 96% yield (one-pot bromination and protection) from 14 that was generated by monomethylation of the commercially available 1,8-naphthalenediol.31 We then explored the selective catalytic borylation/dehalogenation sequence.32 A commonly used boron source B2pin2 was first applied to the iridium-catalyzed C–H borylation33 reaction in THF, but this condition gave no reaction. Only 4% of the desired 16 was obtained when the boron source was changed to HBpin. To our delight, the reaction was significantly improved when conducted in hexane even on a 20 g scale (76%, 87% yield brsm).34 Remarkably, we also obtained a single regioisomer for this C–H borylation. The following Pd-catalyzed chemoselective hydrodebromination proceeded smoothly by employing fluoride-activated PMHS32 to deliver the requisite boronate ester 17 in 89% yield.
Figure 2.
Concise and scalable syntheses of aglycon and glycosyl donors. (a) Regioselective C–H functionalizations enabled scalable preparation of the aglycon 21. (b) Synthesis of glycosyl donors 23a–c. Reagents and conditions are as follows: (i) NBS, MeCN, r.t.; i-PrI, NaH, DMF, 0 to 70 °C. (ii) [Ir(cod)OMe]2, dtbpy, HBpin, hexane, 80 °C. (iii) Pd(OAc)2, KF, PMHS, THF, H2O, r.t. (iv) [Pd(dppf)Cl2]·CH2Cl2, KOH, MTBE/H2O, 80 °C, then 40% NaOH (aq). (v) K2S2O8, AgNO3, MeCN/H2O, 50 °C. (vi) Pd/C, H2, MeOH, r.t.; Tf2O, Et3N, CH2Cl2, −78 °C; potassium vinyltrifluoroborate, [Pd(dppf)Cl2]·CH2Cl2, Et3N, n-PrOH, reflux. (vii) LiAlH4, THF, 50 °C. (viii) Ac2O, AcOH, H2SO4, r.t. (ix) DMAPA, THF, 20 °C. (x) CCl3CN, DBU, 4 Å MS, CH2Cl2, r.t. (xi) N-Phenyltrifluoroacetimidoyl chloride, K2CO3, acetone, r.t.
With 17 in hand, our attention was turned to the construction of the aglycon (Figure 2a). Suzuki–Miyaura cross-coupling using boronate ester 17 and bromide 18(35) followed by hydrolysis of the methyl ester with 40% aqueous NaOH in one pot afforded acid 19 in 88% yield. With respect to the regioselectivity of the remote C–H oxygenation, both α- and β-oxygenation of 2-(naphthalen-2-yl)benzoic acid have been reported.36,37 Encouraged by initial investigations using Cu(OAc)2 and [PhCO2]2 in HFIP38 to cyclize 19, where C4b instead of C11 was specifically oxidized to generate ca. 10% of the desired product 20, we changed the oxidant to the much cheaper K2S2O839 and obtained 20 in 57% yield. The yield was further improved to 84% on the gram scale by the addition of a catalytic amount of AgNO3.39 At this point, the core of aglycon was fully constructed. The following functional group transformations assembled the vinyl group at C8: removal of the benzyl protecting group by hydrogenolysis, subsequent installation of the triflate, and Suzuki–Miyaura coupling employing potassium vinyltrifluoroborate40 in one pot were achieved in 84% yield. Over 10 g of 1-O-isopropyldefucogilvocarcin V (21) was obtained in 40% overall yield from 1,8-naphthalenediol, which demonstrated the efficiency and scalability of this synthetic route compared to the previously reported approach.41
Syntheses of Glycosyl Donors
The carbohydrate portion of chrysomycin A is virenose, a branched-chained sugar which has rarely existed in natural products. The synthesis of methyl α-d-virenoside (22) was first accomplished in 1980.42 We modified and improved the previous synthesis to efficiently generate 22 in good yield (Figure 2b).42,50 Upon treatment with AcOH/Ac2O/H2SO4, 22 underwent acetolysis to provide tetraacetate 23a(43) as a mixture of anomers (α/β = 1/4) in 89% yield. The other two glycosyl donors trichloroacetimidate 23b and N-phenyltrifluoroacetimidate 23c(44) were also synthesized from 23a (Figure 2b).
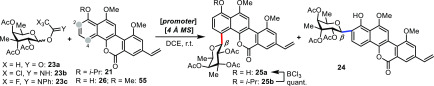
Survey of Reaction Conditions for the C-Glycosylation Reaction
After the two major building blocks for chrysomycin A were all obtained, the synthesis was then centered on the crucial late-stage C-glycosylation reaction. In early 1990s, Daves and co-workers first examined a late-stage C-glycosylation model for the gilvocarcin family natural product; however, they obtained a 1/1 ratio of α- and β-anomers.19,20 In our case, challenges lay not only in the stereoselectivity of the anomeric center but also in the regioselectivity complicated by stereoelectronic effects of the fully elaborated chromophore. Daves’ conditions were initially applied to the aglycon 21 (Table 1, entry 1) but afforded the C2-glycosylated product 24 exclusively with the removal of the i-Pr group (41% yield). The C4-glycosylated product 25 could only be isolated in a trace amount when excess aglycon 21 (relative to 23a) and less promoter (3.0 equiv of SnCl4) were used (Table 1, entry 2). The initial screening of various promoters did not improve the reaction outcome (Table 1, entries 3–6). Interestingly, in the SnCl4-promoted reaction (Table 1, entry 7), 26 and 25a were obtained where we speculated that the i-Pr group of both 21 and 25b were removed under this condition. Alternatively, the i-Pr group of 25b could be further deprotected with BCl3 to afford 25a in quantitative yield (Figure 3). When 4 Å MS was added to the reaction solution, we observed a significant improvement in the regioselectivity of C4-glycosylation. Higher loading of 4 Å MS was positively correlated to the improved regioselectivity and prevented the undesired removal of the i-Pr group in both 21 and the C4-glycosylated product 25b (Table 1, entries 8–13). The optimized reaction was carried out with 20.0 wt equiv of 4 Å MS to afford excellent regioselectivity (25/24 > 95/5) and 50% yield of 25 (Table 1, entry 13). Further screening of various glycosyl donors indicated that acetate 23a was a better glycosyl donor than 23b and 23c (Table 1, entries 15–19). Notably, glycosylation turned out to be sluggish when the i-Pr group was replaced by the methyl group, presumably because the methyl group is more stable toward the deprotection condition (Table 1, entry 20). To further investigate the selectivity of C2 glycosylation, defucogilvocarcin V (26) was directly subjected to the conditions used in entries 1 and 7, which afforded 37% yield of 24 and a trace of 25, respectively, as a single regioisomer (Table 1, entries 21 and 22).
Table 1. Optimization of the C-Glycosylation Reaction.
| entrya | D/A | promoter (equiv) | T (°C) | 4 Å MS (wt equiv) | 25/24b | yield of 25c (%) |
|---|---|---|---|---|---|---|
| 1d | 23a/21 | SnCl4 (9.0) | 25 | 0 | 0/100 | 41e |
| 2 | 23a/21 | SnCl4 (3.0) | 25 | 0 | <5 | |
| 3 | 23a/21 | Et2O·BF3 (3.0) | 25 | 4.0 | 0 | |
| 4 | 23a/21 | TMSOTf (3.0) | 25 | 4.0 | <5 | |
| 5 | 23a/21 | Cp2ZrCl2 (3.0), AgClO4 (3.0) | 25 | 4.0 | 0 | |
| 6 | 23a/21 | SnCl4 (3.0), AgClO4 (3.0) | 25 | 4.0 | 60/40 | 14 |
| 7 | 23a/21 | SnCl4 (3.0) | 25 | 4.0 | 72/28 | 25 |
| 8 | 23a/21 | SnCl4 (3.0) | 15 | 4.0 | 52/48 | <15 |
| 9 | 23a/21 | SnCl4 (3.0) | 35 | 4.0 | 55/45 | 10 |
| 10f | 23a/21 | SnCl4 (3.0) | 25 | 4.0 | 0 | |
| 11g | 23a/21 | SnCl4 (3.0) | 25 | 4.0 | 63/37 | 11 |
| 12 | 23a/21 | SnCl4 (3.0) | 25 | 12.0 | 81/19 | 30 |
| 13 | 23a/21 | SnCl4(3.0) | 25 | 20.0 | >95/5 | 50 |
| 14h | 23a/21 | SnCl4 (3.0) | 25 | 4.0 | 50/50 | 11 |
| 15 | 23b/21 | SnCl4 (3.0) | 25 | 20.0 | 90/10 | 26 |
| 16 | 23b/21 | TMSOTf (3.0) | 25 | 20.0 | <5 | |
| 17 | 23b/21 | TMSOTf (0.4) | 25 | 20.0 | <5 | |
| 18 | 23c/21 | SnCl4 (3.0) | 25 | 20.0 | >95/5 | 41 |
| 19 | 23c/21 | SnCl4 (0.5) | 25 | 20.0 | >95/5 | 11i |
| 20 | 23a/55 | SnCl4 (3.0) | 25 | 12.0 | <5j | |
| 21d | 23a/26 | SnCl4 (9.0) | 25 | 0 | 0/100 | 37e |
| 22 | 23a/26 | SnCl4 (3.0) | 25 | 4.0 | 0/100 | <5e |
Conditions: 23 (1.0 equiv), aglycon (3.0 equiv), 4 Å MS, promoter (3.0 equiv), solvent (0.017 M), r.t.
Ratio determined by 1H NMR of the crude reaction mixture.
Combined yield of 25a and 25b.
1.0 equiv of aglycon, 4.0 equiv of 23a, and 9.0 equiv of SnCl4 were used.
Isolated yield of 24.
DCE/THF or MeCN was used as solvent.
Concentration = 0.034 M.
1.0 equiv of aglycon, 3.0 equiv of 23a, and 3.0 equiv of SnCl4 were used.
α-25b was also obtained in 19% yield.
Deprotection of 1-hydroxyl group did not happen. D/A = donor/acceptor.
Figure 3.
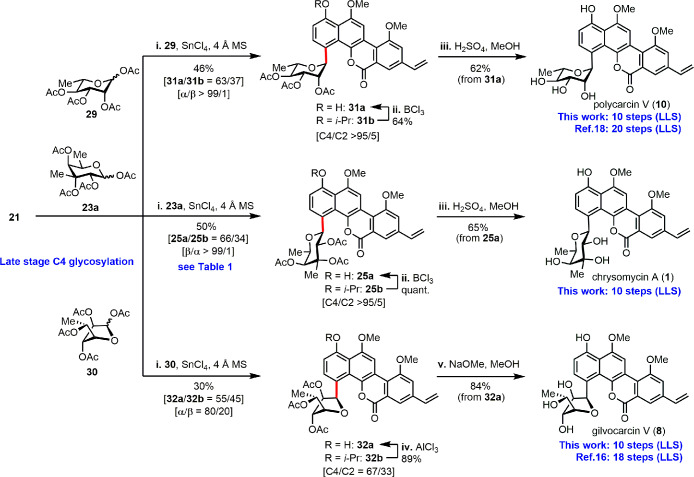
Total syntheses of chrysomycin A, polycarcin V, and gilvocarcin V through late-stage C-glycosylation. Reagents and conditions are as follows: (i) SnCl4, 4 Å MS, DCE, r.t. (ii) BCl3, CH2Cl2, −20 °C. (iii) 1.5 M H2SO4 in MeOH, 70 °C. (iv) AlCl3, CH2Cl2, −20 °C. (v) NaOMe, MeOH, r.t.
Based on the above-mentioned results, a plausible mechanism of this SnCl4-promoted C-glycosylation reaction is depicted in Scheme S1. There are three proposed reaction pathways to account for the observed different regioselectivities. The regioselective formation of C2-glycosylated product 24 from 26 (Table 1, entries 21 and 22) probably proceeds via the O- to C-glycoside rearrangement sequence that was previously proposed by Suzuki and co-workers16,45 (path A). When much excess of SnCl4 (9.0 equiv) was applied (entry 1), the i-Pr group of 21 was quickly removed to form 26, which underwent path A as well. Comparing entry 7 with entry 22, we speculate that the C2-glycosylated product 24 could be generated via path B, in which the protected aglycon 21 attacks oxonium ion 27, followed by Friedel–Crafts-type C2-glycosylation to yield intermediate 28. After deprotection of the i-Pr group, 24 is obtained. In contrast, C4-glycosylated products 25 may only be formed via Friedel–Crafts-type C4-glycosylation of 21 (path C), which is sterically favored compared to C2-glycosylation. In this process, 4 Å MS blocked the removal of the i-Pr group of 21 by SnCl4 (as observed) and thus presumably increased the proportion of the Friedel–Crafts reaction to afford a better yield of 25. In addition, the C4-glycosylation was found to be highly β-selective due to the neighboring group participation effect.
Synthesis of Chrysomycin A, Polycarcin V, and Gilvocarcin V
Finally, triacetate 25a was subjected to the global deacetylation. Saponification of 25a using diverse bases unexpectedly led to a complex of mono- or diacetate (Table S4). Fortunately, acid prompted deacetylation using 1.5 M of H2SO4 in MeOH cleanly afforded the desired chrysomycin A (1) in 65% yield (Figure 3). Overall, the total synthesis of chrysomycin A was accomplished in 10 steps (longest linear sequence, LLS) from the commercially available 1,8-naphthalenediol.
We next adapted our highly convergent and flexible synthetic route to prepare the other two gilvocarcin family members polycarcin V (10) and gilvocarcin V (8) (Figure 3). Using l-rhamnose tetraacetate (29) and d-fucofuranose tetraacetate (30) to react with chromophore 21, C4-glycosylation products 31 and 32 were provided, respectively. Upon treatment with H2SO4/MeOH, 31a was converted to polycarcin V (10) in 62% yield. Considering that the acidic conditions would cause anomerization and/or ring expansion of d-fucofuranose,15 the basic condition using NaOMe in MeOH was applied for the synthesis of gilvocarcin V (8). As a result, polycarcin V and gilvocarcin V were also synthesized in 10 steps (LLS). Compared to the previously reported total syntheses, 20-step for polycarcin V,18 18-step for gilvocarcin V,16 our work further demonstrated the remarkable efficiency of the sequential C–H functionalizations and late-stage C-glycosylation strategy.
Late-Stage Diversification of Chrysomycin A
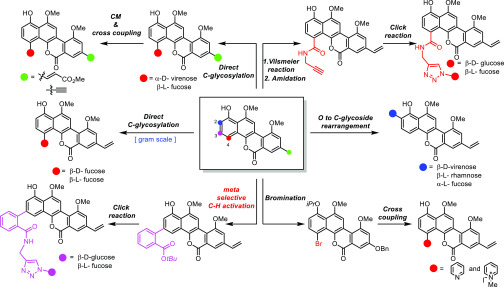
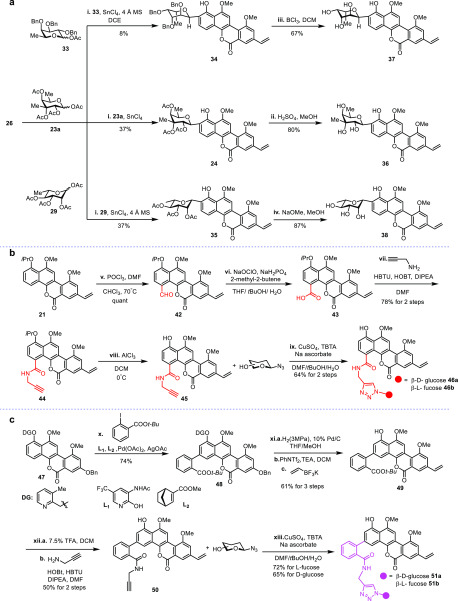
This established synthetic route enabled us to further extensively explore SAR. A series of analogues of chrysomycin A were prepared through various late-stage functionalizations at C2, C3, C4, and C8 positions (Figure 4). O- to C-glycoside rearrangement was applied to install three different sugar units at the C2 position selectively (Figure 5a). Using 23a, 33, and 29 to react with defucogilvocarcin V (26), C2-glycosylation products 24, 34, and 35 were provided, respectively. Removal of the acyl group in 24 and 35 by acidic and basic conditions offered 36 and 38, and upon treatment with BCl3, 34 was converted to 37 in 67% yield. Olefin cross-metathesis and Sonogashira coupling were used to provide C8 alkynyl (39) and C8 methyl acrylate (40) analogues, respectively (Scheme S2). To increase the cell membrane permeability, positively charged lipophilic C4-noncarbohydrate analogue (41) was synthesized by the cross-coupling reaction46 (Scheme S3). As for C4 functionalization, two commercially available 6-deoxy hexoses d- and l-fucose were installed regio- and stereoselectively by late-stage C-glycosylation to compare the anti-TB activity of these two enantiomers (Scheme S4). Naturally abundant natural sugars such as glucose and galactose were utilized as glycosyl donor candidates, but unfortunately none of them succeeded even with numerous attempts (Table S5). As an alternative strategy, we decided to incorporate a propargyl amide group into C4 as a handle to install various azide sugars through “click chemistry” (Figure 5b). Based on this design, the formyl group was first installed at the C4 position by Vilsmeier–Haack reaction to afford 42, followed by Pinnick oxidation to afford carboxylic acid intermediate 43. After amidation and deprotection, propygyl amide intermediate 45 was obtained. Finally, using “click chemistry”, we introduced d-glucose and l-fucose, respectively.
Figure 4.
Methods used for late-stage diversification of chrysomycin A.
Figure 5.
Late-stage diversification of chrysomycin A. (a) Synthesis of the C2 glycosylated derivatives. (b) Synthesis of the C4 hybrid derivatives. (c) Synthesis of the C3 hybrid derivatives via meta-selective C–H functionalization. Reagents and conditions are as follows: (i) SnCl4, 4 Å MS, DCE, r.t. (ii) 1.5 M H2SO4 in MeOH, 70 °C. (iii) BCl3, CH2Cl2, −78 °C. (iv) NaOMe, MeOH, r.t. (v) POCl3, DMF, CHCl3, 70 °C. (vi) NaOClO, NaH2PO4, 2-methyl-2-butene, THF/tBuOH/H2O, r.t. (vii) 2-Propynylamine, HBTU, HOBt, DIPEA, DMF, r.t. (viii) AlCl3, DCM, r.t. (ix) CuSO4, TBTA, sodium ascorbate, DMF/tBuOH/H2O, r.t. (x) tert-butyl 2-iodobenzoate, L1, L2, Pd(OAc)2, AgOAc, r.t. (xi) Pd/C, H2(3 MPa), THF/MeOH, r.t.; PhNTf2, TEA, DCM, .r.t.; potassium vinyltrifluoroborate, [Pd(dppf)Cl2]·CH2Cl2, Et3N, n-PrOH, reflux. (xii) 7.5% TFA, DCM, r.t.; prop-2-yn-1-amine, HOBT, HBTU, DIPEA, DMF, r.t. (xiii) β-Glycosyl azides, CuSO4, TBTA, Na ascorbate, DMF/tBuOH/H2O, r.t.
We then turned our attention to the challenging C3 functionalization (Figure 5c). To the best of our knowledge, there are no methods reported for the selective meta-C-glycosylation on a phenol substrate. Moreover, in general very few conventional chemistries are available for the direct installation of any functional groups on the phenol meta position. Therefore, we planned to introduce a handle first via a meta-selective C–H functionalization strategy. Initial attempts on C–H borylation and other nondirected C–H activation methods were unsuccessful (Table S6).47,48 Fortunately, when a pyridine directing group was introduced,49 a benzoic acid ester was successfully installed at the C3 position regioselectively to afford 48 in 74% yield. With this desired meta functionalized intermediate in hand, both the directing group and benzyl group were removed simultaneously by hydrogenolysis. After selective triflation and Suzuki coupling, 49 was generated in 61% yield. Then, propargyl amide 50 was obtained through deprotection and amidation in 50% overall yield. Finally, the Cu(I)-catalyzed “click” reaction afforded the desired C3 analogues 51a and 51b smoothly.
Study of Structure–Activity Relationship
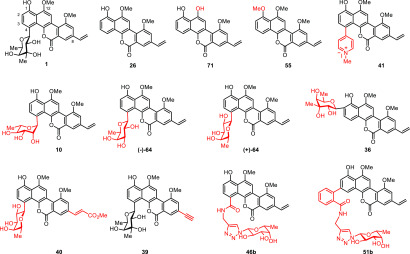
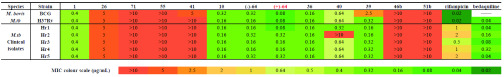
More than 30 natural product analogues were synthesized and evaluated for antituberculosis activity against a panel of pathogens including the wild-type M. tb H37Rv strain and five clinically isolated rifampicin-resistant M. tb strains (Hr 1–5) (Table S1). We also used rifampicin, the widely applied first-line anti-TB drug, and bedaquiline, the latest second-line anti-TB drug, as positive control substrates. As a result, 6 compounds (10, (−)-64, (+)-64, 36, 40, 39) showed promising antituberculosis activity (MIC < 1 μg/mL) against M. tb H37Rv and five rifampicin-resistant M. tb strains (Hr1–5) (Table 2). The sugar moiety was found to play an important role in the bioactivity, as seen in defucogilvocarcin V (26) (>10-fold loss of activity). Methylation of the 1-OH or demethylation of the 12-OMe was also found to be detrimental to activity. Polycarcin V (10), which showed an MIC of 0.16 μg/mL against M. tb, was more potent than chrysomycin A (1). A further change of the sugar portion to d- or l-fucose was also beneficial to activity. Interestingly, the β-l-fucosyl analogue (+)-64 showed better activity than the β-d-fucosyl one (−)-64. Remarkably, (+)-64, the most active analogue, had 5-fold potency enhancement against M. tb compared to chrysomycin A (1). The C2-glycosylated analogue 36 displayed an MIC of 0.16 μg/mL against M. tb. Even more interestingly, comparing to bedaquiline, 36 showed comparable activity and inhibited the growth of five rifampicin-resistant M. tb strains (Hr 1–5) with the same MIC values (0.16 μg/mL), suggesting that the sugar moiety at the C2 position may play an important role to overcome the drug resistance. The C8-substituents were found to exert significant influence on the biological activity. For example, the C8-ethynyl analogue 39 exhibited comparable anti-TB activity to chrysomycin A (1) despite higher MIC against M. bovis BCG. (E)-C8-methyl acrylate analogue 40 was less potent than C8-vinyl analogue (+)-64. C4-pyridinium salt analogue 41, C4 hybrid analogues 46b, and C3 hybrid analogues 51b lost activity. Overall, it is interesting and encouraging to note that both 36 and (+)-64 display significant activity against these challenging rifampicin- or bedaquiline-resistant strains, which may offer promising drug leads for the treatment of MDR-TB.
Table 2. MIC Values (μg/mL) for Chrysomycin A and Its Analogues against Various Mycobacterial Strains.
Conclusion
In summary, the 10-step and gram-scale total synthesis of chrysomycin A has been accomplished in a highly convergent manner. Key features of the synthesis include two sequential C–H activation reactions and a late-stage C-glycosylation. The synthesis described in Figure 2a has provided >10 g of the advanced aglycon 21. We furthermore demonstrated the late-stage diversification of natural products as a powerful strategy to generate natural product derivatives for drug discovery.51 Accordingly, various late-stage diversification reactions, including the meta-selective C–H functionalization, have been applied to generate 33 new analogues efficiently. Extensive evaluation of the anti-TB activity of these compounds shed a light on the SAR of chrysomycin A and offered a more potent derivative (+)-64 (5-fold enhancement) that showed a potential to overcome the drug resistance. We can envision that adapting the chemistry reported herein to create additional derivatives with optimized structures may help elucidate the mode of action and address an important area of unmet medical need.
Acknowledgments
We thank Dr. Benke Hong, Dr. Hiu Chun Lam, and Prof. Seth Herzon (Yale University) for helpful discussions. Financial support from the National Natural Science Foundation of China (21625201, 21961142010, 21661140001, 91853202, and 21521003 to X. Lei; 31430002 and 31720103901 to L. Zhang), the National Key Research and Development Program of China (2017YFA0505200 to X. Lei), the Beijing Outstanding Young Scientist Program (BJJWZYJH01201910001001 to X. Lei), and Peking-Tsinghua Center for Life Science is gratefully acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.0c00122.
Experimental procedures, new compound characterization data, and biological evaluation data (PDF)
Author Contributions
# F. Wu, J. Zhang, and F. Song contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- World Health Organization . Global tuberculosis report 2018. http://www.who.int/tb/.
- Kakkar A. K.; Dahiya N. Bedaquiline for the treatment of resistant tuberculosis: Promises and pitfalls. Tuberculosis 2014, 94, 357–362. 10.1016/j.tube.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Gler M. T.; Skripconoka V.; Sanchez-Garavito E.; Xiao H.; Cabrera-Rivero J. L.; Vargas-Vasquez D. E.; Gao M.; Mohamed Awad M. B.; Park S.-K.; Shim T. S.; et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N. Engl. J. Med. 2012, 366, 2151–2160. 10.1056/NEJMoa1112433. [DOI] [PubMed] [Google Scholar]
- Strelitz F.; Flon H.; Asheshov I. N. Chrysomycin: a new antibiotic substance for bacterial viruses. J. Bacteriol. 1955, 69, 280–283. 10.1128/JB.69.3.280-283.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For our initial anti-TB activity evaluation of chrysomycin A, see the Supporting Information.
- Muralikrishnan B.; Dan V. M.; Vinodh J. S.; Jamsheena V.; Ramachandran R.; Thomas S.; Dastager S. G.; Kumar K. S.; Lankalapalli R. S.; Kumar R. A. Anti-microbial activity of chrysomycin A produced by Streptomyces sp. against Mycobacterium tuberculosis. RSC Adv. 2017, 7, 36335–36339. 10.1039/C7RA05576E. [DOI] [Google Scholar]
- Jain S. K.; Pathania A. S.; Parshad R.; Raina C.; Ali A.; Gupta A. P.; Kushwaha M.; Aravinda S.; Bhushan S.; Bharate S.; Viswakarma R. A. Chrysomycins A–C, antileukemic naphthocoumarins from Streptomyces sporoverrucosus. RSC Adv. 2013, 3, 21046–21053. 10.1039/c3ra42884b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K.; Ando Y.; Matsumoto T.; Suzuki K. Total synthesis of aryl C-glycoside natural products: strategies and tactics. Chem. Rev. 2018, 118, 1495–1598. 10.1021/acs.chemrev.7b00380. [DOI] [PubMed] [Google Scholar]
- Sezaki M.; Hara T.; Ayukawa S.; Takeuchi T.; Okami Y.; Hamada M.; Nagatsu T.; Umezawa H. Studies on a new antibiotic pigment, aquayamycin. J. Antibiot. 1968, 21, 91–97. 10.7164/antibiotics.21.91. [DOI] [PubMed] [Google Scholar]
- Funahashi Y.; Kawamura N.; Ishimaru T.. 19-membered ring compound, its production and use thereof. JP patent JP08231551[A296090], 1996.
- Martin G. D.; Tan L. T.; Jensen P. R.; Dimayuga R. E.; Fairchild C. R.; Raventos-Suarez C.; Fenical W. Marmycins A and B, cytotoxic pentacyclic C-glycosides from a marine sediment-derived actinomycete related to the genus Streptomyces. J. Nat. Prod. 2007, 70, 1406–1409. 10.1021/np060621r. [DOI] [PubMed] [Google Scholar]
- Abe N.; Enoki N.; Nakakita Y.; Uchida H.; Nakamura T.; Munekata M. Novel antitumor antibiotics, saptomycins. J. Antibiot. 1993, 46, 1536–1549. 10.7164/antibiotics.46.1536. [DOI] [PubMed] [Google Scholar]
- Nakano H.; Matsuda Y.; Ito K.; Ohkuba S.; Morimoto M.; Tomita F. Gilvocarcins, new antitumor antibiotics. J. Antibiot. 1981, 34, 266–270. 10.7164/antibiotics.34.266. [DOI] [PubMed] [Google Scholar]
- Kharel M. K.; Pahari P.; Shepherd M. D.; Tibrewal N.; Nybo S. E.; Shaaban K. A.; Rohr J. Angucyclines: Biosynthesis, mode-of-action, new natural products, and synthesis. Nat. Prod. Rep. 2012, 29, 264–325. 10.1039/C1NP00068C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart D. J.; Merriman G. H.; Young D. G. Synthesis of C-aryl glycosides related to the chrysomycins. Tetrahedron 1996, 52, 14437–14458. 10.1016/0040-4020(96)00876-9. [DOI] [Google Scholar]
- Hosoya T.; Takashiro E.; Matsumoto T.; Suzuki K. Total synthesis of the gilvocarcins. J. Am. Chem. Soc. 1994, 116, 1004–1015. 10.1021/ja00082a023. [DOI] [Google Scholar]
- Futagami S.; Ohashi Y.; Imura K.; Hosoya T.; Ohmori K.; Matsumoto T.; Suzuki K. Total synthesis of ravidomycin: revision of absolute and relative stereochemistry. Tetrahedron Lett. 2000, 41, 1063–1067. 10.1016/S0040-4039(99)02235-2. [DOI] [Google Scholar]
- Cai X.; Ng K.; Panesar H.; Moon S. J.; Paredes M.; Ishida K.; Hertwech C.; Minehan T. G. Total synthesis of the antitumor natural product polycarcin V and evaluation of its DNA binding profile. Org. Lett. 2014, 16, 2962–2965. 10.1021/ol501095w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok D. I.; Outten R. A.; Huhn R.; Daves G. D. Jr 8-Ethyl-1-methoxybenzo [d] naphtho [1, 2-b] pyran-6-one C-glycosides by acid-catalyzed glycosylation. J. Org. Chem. 1988, 53, 5359–5361. 10.1021/jo00257a032. [DOI] [Google Scholar]
- Farr R. N.; Kwok D. I.; Daves G. D. Jr 8-Ethenyl-1-hydroxy-4-. beta.-D-ribofuranosylbenzo [d] naphtho [1, 2-b] pyran-6-one and 8-ethenyl-1-hydroxy-4-(2′-deoxy-. beta.-D-ribofuranosyl) benzo [d] naphtho [1, 2-b] pyran-6-one. Synthetic C-glycosides related to the gilvocarcin, ravidomycin, and chrysomycin antibiotics. J. Org. Chem. 1992, 57, 2093–2100. 10.1021/jo00033a034. [DOI] [Google Scholar]
- Abrams D. J.; Provencher P. A.; Sorensen E. J. Recent applications of C–H functionalization in complex natural product synthesis. Chem. Soc. Rev. 2018, 47, 8925–8967. 10.1039/C8CS00716K. [DOI] [PubMed] [Google Scholar]
- Bedell T. A.; Hone G. A.; Valette D.; Yu J. Q.; Davies H. M.; Sorensen E. J. Rapid construction of a benzo-fused indoxamycin core enabled by site-selective C– H functionalizations. Angew. Chem., Int. Ed. 2016, 55, 8270–8274. 10.1002/anie.201602024. [DOI] [PubMed] [Google Scholar]
- Gutekunst W. R.; Baran P. S. C–H functionalization logic in total synthesis. Chem. Soc. Rev. 2011, 40, 1976–1991. 10.1039/c0cs00182a. [DOI] [PubMed] [Google Scholar]
- Feng Y.; Holte D.; Zoller J.; Umemiya S.; Simke L. R.; Baran P. S. Total synthesis of verruculogen and fumitremorgin A enabled by ligand-controlled C–H borylation. J. Am. Chem. Soc. 2015, 137, 10160–10163. 10.1021/jacs.5b07154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C.; Rangasamy E.; Liang C.; Sakamoto J.; More K. L.; Chi M. Cover picture: excellent stability of a lithium-ion-conducting solid electrolyte upon reversible Li+/H+ exchange in aqueous solutions. Angew. Chem., Int. Ed. 2015, 54, 1–6. 10.1002/anie.201410930. [DOI] [PubMed] [Google Scholar]
- Wang D. H.; Yu J. Q. Highly convergent total synthesis of (+)-lithospermic acid via a late-stage intermolecular C– H olefination. J. Am. Chem. Soc. 2011, 133, 5767–5769. 10.1021/ja2010225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A. D.; Chepiga K. M.; Yamaguchi J.; Itami K.; Davies H. M. Concise syntheses of dictyodendrins A and F by a sequential C–H functionalization strategy. J. Am. Chem. Soc. 2015, 137, 644–647. 10.1021/ja512059d. [DOI] [PubMed] [Google Scholar]
- Hong B.; Li C.; Wang Z.; Chen J.; Li H.; Lei X. Enantioselective total synthesis of (−)-Incarviatone A. J. Am. Chem. Soc. 2015, 137, 11946–11949. 10.1021/jacs.5b08551. [DOI] [PubMed] [Google Scholar]
- Robles O.; Romo D. Chemo- and site-selective derivatizations of natural products enabling biological studies. Nat. Prod. Rep. 2014, 31, 318–334. 10.1039/C3NP70087A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shugrue C. S.; Miller S. J. Applications of nonenzymatic catalysts to the alteration of natural products. Chem. Rev. 2017, 117, 11894–11951. 10.1021/acs.chemrev.7b00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. A.; Sherwood T. C.; Ross A. G. Total syntheses of dalesconol A and B. Angew. Chem., Int. Ed. 2010, 49, 5146–5150. 10.1002/anie.201002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasundara C. R.; Unold J. M.; Oppenheimer J.; Smith M. R. III; Maleczka R. E. Jr A catalytic borylation/dehalogenation route to o-fluoro arylboronates. Org. Lett. 2014, 16, 6072–6075. 10.1021/ol5028738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros A.; Fernández R.; Lassaletta J. M. Functional group directed C–H borylation. Chem. Soc. Rev. 2014, 43, 3229–3243. 10.1039/C3CS60418G. [DOI] [PubMed] [Google Scholar]
- Preshlock S. M.; Ghaffari B.; Maligres P. E.; Krska S. W.; Maleczka R. E. Jr; Smith M. R. III High-throughput optimization of Ir-catalyzed C–H borylation: a tutorial for practical applications. J. Am. Chem. Soc. 2013, 135, 7572–7582. 10.1021/ja400295v. [DOI] [PubMed] [Google Scholar]
- Sharif E. U.; O’Doherty G. A. Regioselective bromination: an approach to the D-ring of the gilvocarcins. Heterocycles 2014, 88, 1275–1285. 10.3987/COM-13-S(S)90. [DOI] [Google Scholar]
- Ramirez N. P.; Bosque I.; Gonzalez-Gomez J. C. Photocatalytic dehydrogenative lactonization of 2-arylbenzoic acids. Org. Lett. 2015, 17, 4550–4553. 10.1021/acs.orglett.5b02269. [DOI] [PubMed] [Google Scholar]
- Li Y.; Ding Y. J.; Wang J. Y.; Su Y. M.; Wang X. S. Pd-Catalyzed C–H lactonization for expedient synthesis of biaryl lactones and total synthesis of cannabinol. Org. Lett. 2013, 15, 2574–2577. 10.1021/ol400877q. [DOI] [PubMed] [Google Scholar]
- Gallardo-Donaire J.; Martin R. Cu-catalyzed mild C (sp2)–H functionalization assisted by carboxylic acids en route to hydroxylated arenes. J. Am. Chem. Soc. 2013, 135, 9350–9353. 10.1021/ja4047894. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Gulevich A. V.; Gevorgyan V. General and practical carboxyl-group-directed remote C-H oxygenation reactions of arenes. Chem. - Eur. J. 2013, 19, 15836–15840. 10.1002/chem.201303511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander G. A.; Rivero M. R. Suzuki cross-coupling reactions of potassium alkenyltrifluoroborates. Org. Lett. 2002, 4, 107–109. 10.1021/ol0169729. [DOI] [PubMed] [Google Scholar]
- Jung M. E.; Jung Y. H. Total Synthesis of the Aglycone of the 8-Methyl Benzonaphthopyrone Antibiotics, Gilvocarcin M, Virenomycin M, and Albacarcin M. Tetrahedron Lett. 1988, 29, 2517–2520. 10.1016/S0040-4039(00)86100-6. [DOI] [Google Scholar]
- Yoshimura J.; Hong N.; Sato K. I. Synthesis of methyl β-D-virenoside. Chem. Lett. 1980, 9, 1131–1132. 10.1246/cl.1980.1131. [DOI] [Google Scholar]
- Kitamura K.; Ando Y.; Matsumoto T.; Suzuki K. Synthesis of the pluramycins: two designed anthrones as enabling platforms for flexible bis-C-glycosylation. Angew. Chem., Int. Ed. 2014, 53, 1258–1261. 10.1002/anie.201308016. [DOI] [PubMed] [Google Scholar]
- Yu B.; Sun J. Glycosylation with glycosyl N-phenyltrifluoroacetimidates (PTFAI) and a perspective of the future development of new glycosylation methods. Chem. Commun. 2010, 46, 4668–4679. 10.1039/c0cc00563k. [DOI] [PubMed] [Google Scholar]
- Matsumoto T.; Katsuki M.; Suzuki K. New approach to C-aryl glycosides starting from phenol and glycosyl fluoride. Lewis acid-catalyzed rearrangement of O-glycoside to C-glycoside. Tetrahedron Lett. 1988, 29, 6935–6938. 10.1016/S0040-4039(00)88479-8. [DOI] [Google Scholar]
- Yarlagadda V.; Samaddar S.; Paramanandham K.; Shome B. R.; Haldar J. Membrane disruption and enhanced inhibition of cell-wall biosynthesis: A synergistic approach to tackle vancomycin-resistant bacteria. Angew. Chem., Int. Ed. 2015, 54, 13644–13649. 10.1002/anie.201507567. [DOI] [PubMed] [Google Scholar]
- Vora H. U.; Silvestri A. P.; Engelin C. J.; Yu J. Q. Rhodium(II)-catalyzed nondirected oxidative alkenylation of arenes: Arene loading at one equivalent. Angew. Chem., Int. Ed. 2014, 53, 2683–2686. 10.1002/anie.201310539. [DOI] [PubMed] [Google Scholar]
- Wang P.; Verna P.; Xia G.; Shi J.; Qiao J. X.; Tao S.; Cheng P. T. W.; Poss M. A.; Farmer M. E.; Yeung K. S.; Yu J. Q. Ligand-accelerated non-directed C–H functionalization of arenes. Nature 2017, 551, 489–493. 10.1038/nature24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P.; Farmer M. E.; Huo X.; Jain P.; Shen P.-X.; Ishoey M.; Brader J. E.; Wisniewski S. R.; Eastgate M. D.; Yu J. Q. Iterative exponential growth synthesis and assembly of uniform diblock copolymers. J. Am. Chem. Soc. 2016, 138, 9269–9276. 10.1021/jacs.6b04966. [DOI] [PubMed] [Google Scholar]
- Raju R.; Castillo B. F.; Richardson S. K.; Thakur M.; Severins R.; Kronenberg M.; Howell A. R. Synthesis and evaluation of 3 ″-and 4 ″-deoxy and-fluoro analogs of the immunostimulatory glycolipid, KRN7000. Bioorg. Med. Chem. Lett. 2009, 19, 4122–4125. 10.1016/j.bmcl.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a recent review on the late-stage diversification of natural products, see:Hong B.; Luo T.; Lei X.. Late-stage diversification of natural products. ACS Cent. Sci. 2020, in press. 10.1021/acscentsci.9b00916 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.