Abstract
Aims
Obesity and hepatic fat accumulation diminish hepatic insulin clearance, which can cause hyperinsulinaemia. Sodium/glucose‐cotransporter 2 inhibitors (SGLT2‐is) improve insulin resistance and hyperinsulinaemia by weight loss via increased urinary glucose excretion in type 2 diabetes. However, there are few reports of the influence of SGLT2‐is on hepatic insulin clearance. We examined the impact of an SGLT2‐i on hepatic insulin clearance and explored the clinical influence associated with changes in hepatic insulin clearance via an SGLT2‐i and the mechanism of the effects of SGLT2‐i.
Materials and methods
Data were analysed from 419 patients with type 2 diabetes controlled by diet and exercise. Patients received a placebo or the SGLT2‐i tofogliflozin (TOFO) (placebo: n = 56; TOFO: n = 363) orally once daily for ≥24 weeks. Hepatic insulin clearance was calculated from the ratio of areas under the curve (AUC) of C‐peptide and insulin levels derived from oral meal tolerance test data (C‐peptide AUC0‐120 min/insulin AUC0‐120 min: HICCIR). The correlation of HICCIR via the SGLT2‐i with other clinical variables was analysed using multivariate analysis.
Results
HICCIR was significantly increased via TOFO at week 24. Furthermore, with TOFO insulin and triglyceride (TG) levels were significantly reduced (P < 0.001) and β‐hydroxybutyrate (BHB) was significantly elevated (P < 0.001). Changes in HICCIR were significantly correlated with changes in TG and BHB via TOFO.
Conclusions
Increased HICCIR was significantly associated with reduced TG via TOFO and contributed to the greater increase in BHB compared with placebo in addition to the correction of hyperinsulinaemia.
1. INTRODUCTION
Inhibition of renal glucose reabsorption by SGLT2 inhibitors (SGLT2‐is) results in glycaemic improvement via an insulin‐independent mechanism.1 Calorie loss by urinary glucose excretion (UGE) via SGLT2‐is leads to weight loss, improved insulin sensitivity and correction of hyperinsulinaemia.2, 3 Epidemiological studies showed that inappropriate hyperinsulinaemia is a risk factor for atherosclerosis.4 In a meta‐analysis in which blood insulin levels were reported, it was concluded that hyperinsulinaemia is a risk factor for cardiovascular disease.5 Diminished hepatic insulin clearance resulting from obesity6 and hepatic fat accumulation7 are among the causes of inappropriate hyperinsulinaemia and insulin resistance. Therefore, regulating hepatic insulin clearance and correcting hyperinsulinaemia are important therapeutic targets in patients with type 2 diabetes and insulin resistance. SGLT2‐is were reported to increase circulating levels of β‐hydroxybutyrate (BHB).8 An increase in BHB via SGLT2‐is was associated with decreases in the plasma insulin level and the insulin‐to‐glucagon ratio.8, 9 Therefore, the impact of SGLT2‐is on hepatic insulin clearance, which is one of the regulators of insulin levels, may be attributed to increases in BHB via SGLT2‐is. However, reports of the evaluation of the impact of SGLT2‐is on hepatic insulin clearance and other influences related to hepatic insulin clearance changes via SGLT2‐is are scarce. Therefore, the aim of this study was to investigate the impact of an SGLT2‐i on hepatic insulin clearance and to explore its linkage with simultaneous changes in clinical parameters via SGLT2‐i in drug‐naive patients with type 2 diabetes enrolled in Phase 2 and 3 studies of tofogliflozin (TOFO).
2. MATERIALS AND METHODS
This was a pooled analysis to evaluate the impact on hepatic insulin clearance by SGLT2 inhibition from two prospective Phase 2 and 3 studies (Table S1; see Supporting Information). The duration of these studies was at least 24 weeks and enrolled patients with type 2 diabetes not taking glucose‐lowering agents to compare a placebo with different doses of TOFO. The CSG003JP study (placebo, TOFO 10, 20 and 40 mg monotherapy) was a 24‐week randomized, double‐blind, placebo‐controlled, combined Phase 2 and 3 study.10 The CSG004JP study (TOFO 20 and 40 mg monotherapy) was a 52‐week randomized‐controlled, open‐label, Phase 3 study.11 Details of those studies were previously reported.10, 11 Data from the 24‐week core treatment period in each study were included in this pooled analysis.
All studies were conducted in accordance with the Declaration of Helsinki and International Council for Harmonisation (ICH) Good Clinical Practice. The protocols were reviewed and approved by the institutional review board of each participating centre. All patients provided written informed consent to participate in the study before enrolment.
In this post‐hoc study to investigate the association of hepatic insulin clearance leading to a shift in insulin levels, BHB and triglyceride (TG), we focused on clinical indicators [e.g. liver enzymes, lipids, incretin, index related to fatty liver (FL), insulin resistance/sensitivity] and performed the analysis as described below.
Baseline characteristics of study participants are shown in Table S1 and S2 (see Supporting Information). Laboratory variables measured at baseline were glycated haemoglobin (HbA1c), fasting plasma glucose (FPG), fasting C‐peptide, fasting insulin (fasting IRI), fasting glucagon, glucagon‐like peptide‐1 (GLP‐1), homeostatic model assessment of insulin resistance (HOMA‐IR) scores, 24‐variable HOMA for β‐cell function and insulin sensitivity (iHOMA2: iHOMA2%β and iHOMA2%S),12 the Matsuda index {10 000/square root of [fasting glucose(mg/dL) × fasting insulin(μU/mL) × mean glucose × mean insulin]} by the mealtolerance test (MTT) 0–120min,13 adiponectin, free fatty acids (FFA), BHB, hepatic enzymes [aspartate aminotransferase (AST), alanine aminotransferase (ALT) and γ‐glutamyl transpeptidase (γGTP)], serum lipids (LDL‐C, HDL‐C and TG), and the estimated glomerular filtration rate calculated from serum creatinine (eGFR). A standardized MTT was performed at baseline and week 24. Postprandial glucose, IRI and C‐peptide were evaluated as glucose area under the curve (AUC)0–120min, IRI AUC0‐120min and C‐peptide AUC0‐120min, respectively. Hepatic insulin clearance was calculated as the ratio of AUC of C‐peptide and IRI derived from MTT data (C‐peptide AUC0‐120 min/IRI AUC0‐120 min: HICCIR).14 C‐peptide and IRI are co‐secreted in equimolar amounts from the pancreas to the portal vein. IRI but not C‐peptide is partially metabolized in the liver and flows into the peripheral circulation. On the other hand, C‐peptide flows into the peripheral circulation without being metabolized in the liver. Therefore, we could evaluate hepatic insulin clearance as the HICCIR.15 In addition, assessments at baseline and 24 weeks after administration of TOFO or placebo included body weight, body mass index (BMI) and waist circumference. As in our previous report,16 the presence of FL was estimated using a cut‐off value based on the FL index (FLI) (e0.953 × loge (TG) + 0.139*BMI + 0.718*loge (γGTP) + 0.053 × waist circumference – 15.745)/(1 + e0.953 × loge (TG) + 0.139 × BMI + 0.718 × loge (γGTP) + 0.053 × waist circumference – 15.745) × 100.17 An FLI ≥35 for males and ≥ 20 for females determined the presence of FL, while an FLI <35 for males and < 20 for females ruled out FL according to a report targeting over 30 000 Asians.18 In this report, the FLI had excellent discriminative ability to detect patients with ultrasonographic FL (positive likelihood ratio 3.12 for males and 4.43 for females).
To assess the influence of the changes in HICCIR via TOFO, study participants receiving TOFO were divided into quartiles (Q) according to the percentage change from baseline in HICCIR (%△HICCIR) at week 24 after administration of TOFO (Q1‐Q4) (Table 1). The group with the lowest value of %△HICCIR was designated as the Q1 group and the group with the highest such value was designated as the Q4 group.
Table 1.
Baseline characteristics according to quartiles of percentage change in HICCIR at week 24 after administration of tofogliflozin
| Quartile 1(%Δ HICCIR a < +5.5) | Quartile 2(+5.5 ≤ %Δ HICCIR a < +20.5) | Quartile 3(+20.5 ≤ %Δ HICCIR a < +35.5) | Quartile 4(+35.5 ≤ %Δ HICCIR a) | P | |
|---|---|---|---|---|---|
| N | 76 | 76 | 77 | 76 | |
| Age (years) | 55.1 (11.5) | 59.6 (9.8) | 58.1 (9.3) | 58.1 (9.8) | 0.048 |
| Sex, men/women, n (%) | 60 (78.9)/16 (21.1) | 44 (57.9)/32 (42.1) | 45 (58.4)/32 (41.6) | 50 (65.8)/26 (34.2) | 0.02 |
| Tofogliflozin, n (%) 10/20/40 mg | 9 (11.8)/20 (26.3)/47 (61.8) | 16 (21.1)/21 (27.6)/39 (51.3) | 11 (14.3)/29 (37.7)/37 (48.1) | 9 (11.8)/28 (36.8)/39 (51.3) | 0.35 |
| Fatty liver indexb | 43.5 (30.1) | 44.7 (25.4) | 50.0 (27.8) | 44.1 (27.6) | 0.44 |
| Fatty liver, n (%) | 43 (56.6) | 51 (67.1) | 55 (71.4) | 45 (59.2) | 0.20 |
| Duration of diabetes (years) | 5.8 (5.4) | 5.8 (5.3) | 6.4 (6.7) | 5.4 (4.9) | 0.73 |
| eGFR (mL/min/1.73 m2) | 85.3 (20.4) | 83.3 (19.7) | 85.8 (19.3) | 84.8 (17.2) | 0.86 |
| Body weight (kg) | 71.3 (16.8) | 67.1 (12.5) | 67.0 (12.2) | 67.6 (13.5) | 0.18 |
| Waist circumference (cm) | 90.0 (13.1) | 88.7 (8.5) | 89.4 (9.8) | 88.5 (10.0) | 0.80 |
| BMI (kg/m2) | 25.4 (4.4) | 25.3 (3.4) | 25.5 (4.0) | 25.2 (4.5) | 0.95 |
| HbA1c (%) | 8.3 (1.0) | 7.9 (0.7) | 8.1 (0.8) | 7.9 (0.8) | 0.01 |
| Fasting plasma glucose (mg/dL) | 166.8 (41.7) | 156.3 (22.7) | 162.3 (34.3) | 155.3 (30.5) | 0.11 |
| Fasting insulin (pmol/L) | 49.4 (38.7) | 54.6 (31.5) | 49.8 (31.8) | 60.2 (60.1) | 0.36 |
| Fasting C‐peptide (pmol/L) | 474.3 (221.4) | 487.4 (208.5) | 460.0 (196.7) | 464.3 (205.3) | 0.85 |
| Fasting glucagon (pmol/L) | 20.1 (5.4) | 19.8 (4.9) | 19.3 (4.4) | 20.0 (5.3) | 0.74 |
| Glucose AUC0‐120min (mg/dL·2 h) | 526.3 (123.3) | 505.8 (80.6) | 513.9 (91.6) | 499.4 (87.8) | 0.36 |
| Insulin AUC0‐120min (pmol/L·2 h) | 279.5 (160.4) | 373.9 (209.0) | 401.9 (279.5) | 439.2 (297.6) | <0.001 |
| C‐peptide AUC0‐120min (pmol/L·2 h) | 1897.9 (675.4) | 2157.1 (799.5) | 2203.2 (845.8) | 2163.9 (797.3) | 0.06 |
| Glucagon AUC0‐120min (pmol/L·2 h) | 41.4 (10.3) | 40.3 (9.5) | 39.6 (8.1) | 40.3 (10.1) | 0.71 |
| HICCIR c | 7.6 (2.0) | 6.5 (1.9) | 6.6 (2.4) | 6.0 (2.2) | <0.001 |
| Total GLP‐1 AUC0‐120min (pmol/L·2 h) | 19.2 (15.2) | 17.7 (7.2) | 18.8 (6.6) | 18.5 (7.4) | 0.81 |
| Active GLP‐1 AUC0‐120min (pmol/L·2 h) | 4.6 (5.2) | 4.0 (1.9) | 4.4 (1.9) | 4.1 (1.9) | 0.61 |
| HOMA‐IRd | 3.4 (2.6) | 3.5 (2.2) | 3.3 (2.1) | 3.9 (4.0) | 0.62 |
| iHOMA2%Se | 140.9 (97.0) | 119.6 (79.5) | 131.3 (79.4) | 129.2 (84.8) | 0.50 |
| iHOMA2%βe | 29.2 (19.5) | 33.2 (16.3) | 31.7 (23.7) | 36.1 (24.9) | 0.25 |
| Matsuda index | 5.1 (3.3) | 4.1 (2.6) | 4.3 (2.7) | 4.2 (2.7) | 0.13 |
| LDL‐C (mg/dL) | 118.7 (32.9) | 126.2 (26.0) | 129.6 (34.4) | 124.8 (28.9) | 0.17 |
| HDL‐C (mg/dL) | 58.7 (17.0) | 58.1 (18.1) | 59.6 (15.2) | 60.2 (17.4) | 0.88 |
| Ln‐TG (ln[mg/dL]) | 4.8 (0.7) | 4.9 (0.5) | 5.0 (0.6) | 4.9 (0.6) | 0.10 |
| AST (IU/L) | 25.4 (9.5) | 25.8 (8.8) | 27.5 (11.2) | 24.7 (7.8) | 0.31 |
| ALT (IU/L) | 29.6 (18.4) | 28.9 (14.1) | 30.7 (18.9) | 27.0 (14.2) | 0.57 |
| ɤGTP (IU/L) | 50.2 (76.5) | 45.1 (41.0) | 52.5 (55.0) | 43.4 (34.4) | 0.70 |
| Fasting free fatty acid (mmol/L) | 0.59 (0.22) | 0.59 (0.21) | 0.59 (0.24) | 0.59 (0.20) | 0.999 |
| Adiponectin (μg/mL) | 7.0 (3.1) | 7.5 (3.9) | 7.6 (4.0) | 6.9 (3.1) | 0.55 |
| Ln‐β‐hydroxybutyrate (ln[μmol/L]) | 4.2 (0.9) | 3.8 (0.7) | 3.9 (0.8) | 3.8 (0.6) | <0.01 |
Note: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUC, area under the curve; BMI, body mass index; eGFR, estimated glomerular filtration rate; GLP‐1, glucagon‐like peptide 1; ɤGTP, ɤ‐glutamyl transpeptidase; HbA1c, glycated haemoglobin.Data are expressed as mean (SD).Analyses were performed by one‐way analysis of variance or Fisher’s exact test across groups.
Percentage change from baseline in HICCIR.
Fatty liver index (e0.953 × ln[TG] + 0.139 × BMI + 0.718 × ln(ɤGTP) + 0.053 × waist circumference – 15.745)/(1 + e0.953 × ln[TG] + 0.139 × BMI + 0.718 × ln[ɤGTP] + 0.053 × waist circumference – 15.745) × 100.
C‐peptide AUC0‐120min/insulin AUC0‐120min ratio.
Homeostatic model assessment insulin resistance.
From the iHOMA2 model.
2.1. Meal tolerance test
Details of the protocol for the MTT were previously described.16 In short, after fasting for at least 10 h, participants attended the medical institution participating in the clinical trials and underwent an MTT with a test meal. The test meal provided 314 kcal/1314 kJ (5.45 g protein, 73.05 g carbohydrate and 0 g lipids).
2.2. Statistical analysis
The adjusted assessment of HICCIR was analysed using an analysis of covariance (ANCOVA) model with the group (placebo or TOFO) as a fixed effect and their baseline values as covariates. Similarly, changes in the other variables with the participant in fasting and postprandial states were analysed using an ANCOVA model. To assess the association of TOFO dosage with changes in variables, the adjusted variables were analysed using an ANCOVA model with the group (TOFO 10, 20 or 40 mg) as a fixed effect and baseline values of each group as covariates. The difference from baseline to week 24 was analysed by a one‐sample t‐test. The correlation analysis at week 24 was performed using Pearson product–moment correlation coefficients. We also performed multivariate analyses to explore clinical variables that might independently correlate with changes in ln‐transformed BHB (ln‐BHB) and %△HICCIR from baseline to week 24 in the participants receiving TOFO. All variables initially identified based on clinical considerations were used as explanatory variables in these multivariate analyses (Table 3, and Tables S5‐S7; see Supporting Information). The model was simplified using a stepwise method by increasing and decreasing variables that were P < 0.05. The characteristics of study participants were summarized with appropriate descriptive statistics (means ± SD for continuous variables and counts and percentages for categorical variables). Differences in the baseline assessments between the placebo and TOFO groups were analysed by the unpaired t‐test or the Fisher’s exact test. Baseline differences in the assessments between quartiles according to %△HICCIR at week 24 after administration of TOFO were analysed by one‐way analysis of variance (ANOVA) or Fisher’s exact test across groups. The adjusted changes in variables, specifically, HbA1c, FPG, glucose AUC0‐120min by MTT, body weight, waist circumference, fasting FFA, adiponectin, LDL‐C, HDL‐C, TG, hepatic enzymes and FLI, according to quartiles of %△HICCIR at week 24 after administration of TOFO were analysed using an ANCOVA model with the groups (quartiles) as a fixed effect and their baseline values as covariates. From those analyses, we evaluated P values corresponding to the groups using the F‐test and simultaneously performed t‐tests using estimated least squares means in each group based on these models of analysis. Analyses also were performed by ANOVA across quartiles for the following variables: fasting IRI, fasting C‐peptide, fasting glucagon, IRI AUC0‐120min, C‐peptide AUC0‐120min, glucagon AUC0‐120min, HICCIR, total GLP‐1 AUC0‐120min, active GLP‐1 AUC0‐120min, Matsuda index, iHOMA2%S and iHOMA2%β. The proportion of FL was analysed by Fisher’s exact test. All HbA1c values are presented using NGSP units. The (two‐sided) significance level for each test was 0.05. All data were analysed by SAS release 9.3 software (SAS Institute, Cary, NC, USA).
Table 3.
Factors that might independently correlate with change in ln‐β‐hydroxybutyrate at week 24
| Factors that might independently correlate with Δln‐β‐hydroxybutyrate at week 24 | Regression coefficient | P |
|---|---|---|
| %Δ fasting free fatty acid (increase 10%) | 0.10 | <0.001 |
| %Δ HICCIR a (increase 10%) | 0.04 | 0.02 |
| %Δ iHOMA2%Sb (increase 10%) | 0.03 | <0.001 |
| Δ ln‐TG (increase 0.1 ln[mg/dL]) | −0.05 | <0.001 |
Note: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUC, area under the curve; eGFR, estimated glomerular filtration rate; GLP‐1, glucagon‐like peptide 1; TG, triglyceride.Factors remained through stepwise variable selection with P < 0.05.Potential baseline predictors were age, sex, duration of diabetes, change in glycated haemoglobin and ln‐transformed TG from baseline to week 24 and the percentage change in the following variables from baseline to week 24: eGFR, body weight, waist circumference, fasting plasma glucose, glucose AUC0‐120min, active GLP‐1 AUC0‐120min, HICCIR, adiponectin, fasting free fatty acid, AST, ALT, ɤGTP, iHOMA2Sb and iHOMA2βb.
C‐peptide AUC0‐120min/insulin AUC0‐120min ratio.
From the iHOMA2 model.
3. RESULTS
HICCIR and serum BHB were significantly increased in the TOFO group (P < 0.001 vs. baseline, P < 0.001 vs. placebo) at week 24 (Table S3; see Supporting Information). In addition, significant reductions in FPG, HbA1c, glucose AUC0‐120min, IRI AUC0‐120min and C‐peptide AUC0‐120min by MTT, body weight, waist circumference, hepatic enzymes and FLI were observed (P < 0.001 vs. baseline, P < 0.001 vs. placebo) in the TOFO group at week 24. In addition, a significant reduction in ln‐TG was observed (P < 0.001 vs. baseline, P < 0.01 vs. placebo). As to changes in other variables from baseline to week 24, fasting IRI and fasting C‐peptide were significantly decreased in the TOFO group compared with the placebo group (P < 0.001) (Table S3; see Supporting Information). Furthermore, with TOFO, iHOMA2%β (P < 0.001 vs. baseline) was significantly increased as was iHOMA2%S (P < 0.001 vs. baseline, P < 0.01 vs. placebo) (Table S3; see Supporting Information). We analysed the effects on variables via TOFO at week 24 according to dosage (Table S4; see Supporting Information). HICCIR increased and ln‐TG decreased significantly from baseline in each dosage group, but there were no statistically significant changes across dosage groups. A significant increase in ln‐BHB was observed in each dosage group with significant differences across groups.
When the participants receiving TOFO were divided into quartiles according to %△HICCIR at week 24 after administration of TOFO, there were no significant differences in baseline characteristics across groups in BMI, insulin resistance/sensitivity index, FPG, hepatic enzymes, FLI, serum lipids, eGFR and C‐peptide AUC0‐120min using MTT but there were significant differences in age, sex ratio, HbA1c, ln‐BHB, HICCIR and IRI AUC0‐120min using MTT (Table 1). As to changes in variables according to quartiles of %△HICCIR at week 24 after administration of TOFO, in the order of Q4‐Q1 greater reductions in the percentage changes (%△) in body weight, fasting IRI, IRI AUC0‐120min, C‐peptide AUC0‐120min using MTT and FLI were observed (Table 2). In addition, there was a significant difference across quartiles in the amount of change in ln‐TG (Q1: –0.06; Q2: –0.15; Q3: –0.23; Q4: –0.20, P = 0.01) (Table 2). Furthermore, the degree of change in ln‐BHB, and %△iHOMA2%S and %△Matsuda index significantly increased in the order of Q4‐Q1 (Table 2). Correlation analysis indicated a significant negative relationship between %△HICCIR and change in ln‐TG (r = −0.23) (Figure 1A), ln‐BHB and ln‐TG (r = −0.27) (Figure 1D) at week 24 after administration of TOFO. On the other hand, significant positive relationships between %△HICCIR and %△active GLP‐1 AUC0‐120min by MTT (r = 0.15) or change in ln‐BHB (r = 0.28) were observed (Figure 1B and 1C). In addition, significant correlations were observed between changes in ln‐BHB and indicators related to plasma glucose, insulin levels, fasting FFA, eGFR and FLI (Table S5; see Supporting Information). Multivariate analysis indicated that the change in ln‐BHB was independently correlated with %△HICCIR, %△fasting FFA, %△iHOMA2%S and change in ln‐TG (Table 3). In addition, %△HICCIR was independently correlated with %△iHOMA2%S, %△eGFR, %△active GLP‐1 AUC0‐120min, and change in HbA1c, ln‐BHB and ln‐TG by univariate and multivariate analyses (Tables S6 and S7; see Supporting Information).
Table 2.
Changes in variables according to quartiles of percentage change in HICCIR at week 24 after administration of tofogliflozin
| Variables | Quartile 1(%Δ HICCIR a < +5.5) | Quartile 2(+5.5 ≤ %Δ HICCIR a < +20.5) | Quartile 3(+20.5 ≤ %Δ HICCIR a < +35.5) | Quartile 4(+35.5 ≤ %Δ HICCIR a) | P |
|---|---|---|---|---|---|
| N | 76 | 76 | 77 | 76 | |
| Fasting insulin (%) | −5.5 (3.3) | −24.9 (3.3)*** | −23.7 (3.3)*** | −31.1 (3.3)*** | <0.001 |
| Fasting C‐peptide (%) | −5.3 (2.7)* | −10.7 (2.7)*** | −10.0 (2.7)*** | −9.8 (2.7)*** | 0.49 |
| Fasting glucagon (%) | 0.59 (2.08) | −2.18 (2.08) | 1.53 (2.07) | −2.10 (2.08) | 0.48 |
| Insulin AUC0‐120min (%) | 17.4 (2.2)*** | −10.2 (2.2)*** | −19.4 (2.1)*** | −30.6 (2.2)*** | <0.001 |
| C‐peptide AUC0‐120min (%) | 11.3 (2.3)*** | 1.8 (2.3) | 3.3 (2.3) | 3.4 (2.3) | 0.02 |
| Glucagon AUC0‐120min (%) | −4.1 (1.7)* | −2.3 (1.7) | −2.3 (1.7) | −3.7 (1.7)* | 0.81 |
| HICCIR b (%) | −4.4 (1.0)*** | 13.5 (1.0)*** | 28.2 (1.0)*** | 50.1 (1.0)*** | <0.001 |
| Total GLP‐1 AUC0‐120min (%) | 7.2 (4.3) | 6.5 (4.3) | 2.1 (4.3) | 4.2 (4.3) | 0.83 |
| Active GLP‐1 AUC0‐120min (%) | 24.1 (5.1)*** | 27.6 (5.1)*** | 28.9 (5.0)*** | 41.4 (5.1)*** | 0.09 |
| Matsuda index (%) | 32.9 (5.6)*** | 64.6 (5.6)*** | 70.2 (5.6)*** | 92.7 (5.6)*** | <0.001 |
| iHOMA2%Sc (%) | 27.7 (5.9)*** | 53.3 (5.9)*** | 51.1 (5.8)*** | 64.6 (5.9)*** | <0.001 |
| iHOMA2%βc (%) | 65.8 (7.3)*** | 26.1 (7.3)*** | 32.2 (7.3)** | 19.9 (7.3)** | <0.001 |
| HbA1cd (%) | −0.88 (0.06)*** | −0.80 (0.06)*** | −0.74 (0.06)*** | −0.73 (0.06)*** | 0.23 |
| Fasting plasma glucosed (mg/dL) | −32.9 (1.9)*** | −33.2 (1.9)*** | −30.6 (1.9)*** | −32.4 (1.9)*** | 0.77 |
| Glucose AUC0‐120min d (mg/dL·2 h) | −112.3 (5.6)*** | −110.0 (5.6)*** | −98.5 (5.6)*** | −103.7 (5.6)*** | 0.29 |
| Body weight (%) | −3.4 (0.3)*** | −4.8 (0.3)*** | −4.5 (0.3)*** | −4.9 (0.3)*** | <0.01 |
| Waist circumference (%) | −2.7 (0.5)*** | −2.3 (0.5)*** | −2.8 (0.5)*** | −3.0 (0.5)*** | 0.69 |
| eGFR (%) | 4.4 (1.7)** | −1.2 (1.7) | 0.2 (1.6) | −0.9 (1.7) | 0.07 |
| LDL‐C (%) | 1.3 (1.9) | −1.2 (1.9) | 0.9 (1.9) | −0.4 (1.9) | 0.77 |
| HDL‐C (%) | 2.9 (1.6) | 5.1 (1.6)** | 7.9 (1.6)*** | 10.5 (1.6)*** | <0.01 |
| ln‐TG (ln[mg/dL]) | −0.06 (0.04) | −0.15 (0.04)*** | −0.23 (0.04)*** | −0.20 (0.04)** | 0.01 |
| AST (%) | −9.5 (2.1)*** | −10.5 (2.1)*** | −6.3 (2.1)** | −7.6 (2.1)*** | 0.49 |
| ALT (%) | −14.3 (2.6)*** | −20.1 (2.6)*** | −19.4 (2.6)*** | −20.5 (2.6)*** | 0.29 |
| ɤGTP (%) | −18.8 (2.9)*** | −21.9 (2.9)*** | −20.0 (2.8)*** | −19.0 (2.9)*** | 0.86 |
| Fatty liver indexe (%) | −18.3 (2.9)*** | −27.6 (2.9)*** | −29.2 (2.9)*** | −31.2 (2.9)*** | 0.01 |
| Fatty liver at week 24, n (%) | 37 (48.7) | 35 (46.1) | 44 (57.1) | 32 (42.1) | 0.30 |
| Fasting free fatty acid (%) | 17.6 (5.6)** | 22.3 (5.6)*** | 30.7 (5.6)*** | 34.6 (5.6)*** | 0.13 |
| Adiponectin (%) | 11.4 (2.2)*** | 13.3 (2.2)*** | 12.0 (2.2)*** | 12.6 (2.2)*** | 0.94 |
| ln‐β‐hydroxybutyrate (ln[μmol/L]) | 0.43 (0.09)*** | 0.61 (0.09)*** | 0.75 (0.09)*** | 0.82 (0.09)*** | 0.02 |
Note: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUC, area under the curve; BMI, body mass index; eGFR, estimated glomerular filtration rate; GLP‐1, glucagon‐like peptide 1; ɤGTP, ɤ‐glutamyl transpeptidase; HbA1c, glycated haemoglobin; TG, triglycerides.Data are expressed as mean (standard error).Analyses were performed by ANOVA across quartiles for the following variables; fasting insulin, fasting C‐peptide, fasting glucagon, insulin AUC0‐120min, C‐peptide AUC0‐120min, glucagon AUC0‐120min, HICCIR, total GLP‐1 AUC0‐120min, active GLP‐1 AUC0‐120min, Matsuda index, iHOMA2S and iHOMA2β.Data are expressed as least squares mean (standard error).Analyses were performed by ANCOVA adjusted baseline values for the following variables: HbA1c, fasting plasma glucose, glucose AUC0‐120min, body weight, waist circumference, fasting free fatty acid, adiponectin, LDL‐C, HDL‐C, ln‐transformed TG, AST, ALT, ɤGTP, fatty liver index and ln‐transformed β‐hydroxybutyrate.Proportion of fatty liver was analysed by Fisher’s exact test.*P < 0.05, **P < 0.01, ***P < 0.001 vs. baseline (t‐test for least squares mean).
Percentage change from baseline in HICCIR.
C‐peptide AUC0‐120min/insulin AUC0‐120min ratio.
From the iHOMA2 model.
HbA1c, fasting plasma glucose and glucose AUC0‐120min were evaluated as absolute changes from baseline to week 24.
Fatty liver index (e0.953 × ln[TG] + 0.139 × BMI + 0.718 × ln(ɤGTP) + 0.053 × waist circumference – 15.745)/(1 + e0.953 × ln[TG] + 0.139 × BMI + 0.718 × ln[ɤGTP] + 0.053 × waist circumference – 15.745) × 100.
Figure 1.
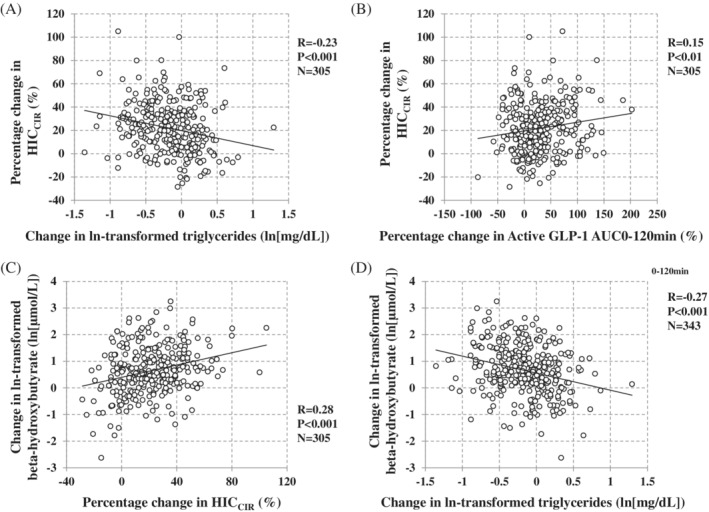
Correlations between percentage change in HICCIR and variables and between ln‐β‐hydroxybutyrate and variables after 24 weeks of administration of tofogliflozin. A,B, Correlation between percentage change in HICCIR and change in ln‐transformed triglycerides and percentage change in active GLP‐1 AUC0‐120min by a meal tolerance test at week 24. C,D, Correlations between ln‐β‐hydroxybutyrate and HICCIR and change in ln‐transformed triglycerides. Correlation analysis was performed using Pearson correlation coefficient. HICCIR, C‐peptide AUC0‐120min/insulin AUC0‐120min ratio; GLP‐1, glucagon‐like peptide‐1
4. DISCUSSION
To the best of our knowledge, this study is the first to evaluate the impact of changes in HICCIR via SGLT2 inhibition and to explore the linkage of changes in HICCIR with that of TG and BHB via an SGLT2‐i in drug‐naïve patients with type 2 diabetes.
Insulin resistance caused by weight gain and visceral fat accumulation may lead to hyperinsulinaemia, which might prompt not only further weight gain but also cardiometabolic disorders associated with the risk of cardiovascular disease.19 A decline in hepatic insulin clearance is one of the causes of hyperinsulinaemia.20 In a study of obese individuals without diabetes, it was shown that the insulin clearance rate might decrease before the insulin secretion rate increases as a compensatory mechanism when insulin sensitivity decreases.21 Furthermore, a decline in hepatic insulin clearance was observed from the early stages of type 2 diabetes.22 In particular, the presence of FL23 and type 2 diabetes24 decreased hepatic insulin clearance. Therefore, optimization of hepatic insulin clearance is considered useful to prevent various metabolic disorders, including glucose intolerance and complications caused by inappropriate hyperinsulinaemia. Previously, reports that various interventions might influence hepatic insulin clearance have been scant. Weight reduction25 and antidiabetic agents such as a biguanide26 and thiazolidinediones27 increased hepatic insulin clearance whereas sulfonylurea28 decreased hepatic insulin clearance. Results of the current study indicated that SGLT2 inhibition significantly increased HICCIR at week 24 (Table S3; see Supporting Information). However, identifying patients who tended to have increased HICCIR via SGLT2‐i was difficult in performing this study. This is because the baseline characteristics according to quartiles of %△HICCIR via TOFO were very similar across the groups except for baseline HICCIR, age, sex ratio, HbA1c and ln‐BHB (Table 1). In addition, the mechanism by which SGLT2‐is induced the increases in hepatic insulin clearance has not been clarified. However, of interest is a report that excess carbohydrate intake elevated plasma TG concentration and reduced insulin clearance.29 Data have shown a significant negative correlation between diet‐induced changes in fasting plasma TG concentration and changes in insulin clearance.29, 30 Fasting plasma VLDL‐TG concentration reflects hepatic TG content,31 and it has been speculated that the increase in intrahepatic TG accumulation associated with the intake of a high‐carbohydrate diet is directly linked to decreased hepatic insulin clearance. The decreased hepatic insulin clearance because of a high‐carbohydrate diet may be associated with reduced expression of the glycoprotein carcinoembryonic antigen‐related cell adhesion molecule 1 (CEACAM1) through such a high‐carbohydrate diet.30 CEACAM1 promotes hepatic insulin endocytosis and hepatic insulin clearance and, hence, maintains systemic insulin sensitivity.32 The present study indicated that there was a significant negative correlation between the %△HICCIR and the change in ln‐TG after TOFO administration (Figure 1A). In addition, increased %△HICCIR or a decrease in change in ln‐TG via TOFO was significantly correlated with the increase in serum BHB reflecting lipolysis associated with decreased insulin levels (Figure 1C and 1D). These independent correlations among HICCIR, TG and BHB were revealed by multivariate analysis (Table 3). FLI also improved significantly in the order of Q4‐Q1 after TOFO administration (Table 2). These results provided support that calorie loss due to UGE by the SGLT2‐i has an effect similar to that of a carbohydrate‐restricted diet, and the improvement of FLI or TG via TOFO in the order of Q4‐Q1 suggested that TOFO increases HICCIR through reduction of intrahepatic TG and improvement in FL. We also previously indicated that the influence of FL was a stronger contributor than obesity to reduced hepatic insulin clearance.16 Therefore, SGLT2 inhibition might normalize the inappropriate hyperinsulinaemia in type 2 diabetes by correcting disorders of hepatic insulin clearance through improvement of FL, including reduction of intrahepatic TG content along with plasma TG concentration. Further investigation will be needed to clarify the mechanism by which SGLT2‐is increases hepatic insulin clearance. Interestingly, there was a significant positive correlation between %△HICCIR and %△active GLP‐1 AUC0‐120min but not total GLP‐1 AUC0‐120min via TOFO (Figure 1B). In addition, multivariate analysis indicated that the increase in %△HICCIR was independently correlated with the increase in %△active GLP‐1 AUC0‐120min (Table S7; see Supporting Information). Although it is difficult to clarify this mechanism in the present study, a previous study indicated that dapagliflozin administration reduced the serum level of soluble dipeptidyl peptidase‐4 (sDPP‐4) in patients with type 2 diabetes with non‐alcoholic FL disease33; therefore, a decrease in serum sDPP‐4 via an SGLT2‐i may be partly involved.
A previous study that examined the association between hepatic insulin clearance and FPG levels in participants with type 2 diabetes and impaired glucose tolerance revealed no significant correlation between hepatic insulin clearance and FPG levels.34 That report suggests that changes in hepatic insulin clearance do not necessarily correlate with plasma glucose levels. In fact, multivariate analysis showed that changes in fasting glucose and Δglucose AUC0‐120min using MTT were not independent factors (Table S7; see Supporting Information). On the other hand, multivariate analysis also showed that the decrease in HbA1c via TOFO was independently correlated with the decrease in percentage change in HICCIR (Tables S6 and S7; see Supporting Information). We could not clarify the mechanism of this association in the current study. A future prospective study will be needed.
Interestingly, euglycaemic ketoacidosis was reported as a specific side effect of SGLT2‐is.35 Although details of the mechanism of this side effect have not been clarified, the increased HICCIR levels leading to the decreased blood insulin, which occurred independently of the decrease in blood glucose accompanied by the administration of SGLT2‐i, may be partly responsible for this side effect. To support this hypothesis, the present study showed a significant positive relationship between an increase in %△HICCIR and BHB via TOFO (Figure 1C). Furthermore, an independent relationship between those factors was observed in the multivariate analysis (Table 3). However, an elevation of BHB via an SGLT2‐i also has been suggested to be a benefit. Recent studies suggested that BHB might be cardioprotective because BHB is freely taken up by the heart and is oxidized in priority to FFA and that in the myocardium, the use of ketone bodies might be energy efficient through improving oxygen consumption.36
As mentioned above, in general, decreases in hepatic insulin clearance are because of a metabolic abnormality such as obesity or FL but genetic factors may be involved in high hepatic insulin clearance values.22 In recent years, genome‐wide association studies have revealed several single nucleotide polymorphisms related to susceptibility to type 2 diabetes. It was reported that common variants of the solute carrier family 30 member 8 gene (SLC30A8) increase the susceptibility to type 2 diabetes.37 Zinc (Zn) is required for the formation of the insulin hexamer, and Zn transporter‐8 (ZnT8) is a transporter that leads Zn from the cytosol to the intragranular spaces in insulin. SLC30A8 encodes ZnT8. It became clear that hepatic insulin clearance was increased in human carriers of rs13266634, which is a major risk allele of SLC30A8.38 Thus, future study is needed to clarify how the increase in hepatic insulin clearance related to the administration of an SGLT2‐i affects metabolism in patients with type 2 diabetes who have this risk allele.
The current study has some limitations. First, it was an integrated analysis that included prospective studies of participants with limited information on their baseline characteristics. In addition, the number of participants receiving each dosage of TOFO was limited and unbalanced for a sufficient evaluation of dosage response related to variables. Secondly, insulin sensitivity was not evaluated using insulin‐clamp methods. Thirdly, HICCIR was a surrogate estimate of hepatic insulin clearance, not a direct measurement of hepatic insulin clearance. Therefore, we could not investigate precisely the biological and pathological effects via TOFO on hepatic insulin clearance or insulin sensitivity. Fourthly, it is debatable whether weight loss and improved insulin sensitivity are primary antidiabetic actions of SGLT2‐is or effects secondary to the improved glycaemic control via SGLT2‐is. Unfortunately, we could not elucidate this point sufficiently in the current study. Finally, we have not been able to examine genes among participants that would affect hepatic insulin clearance.
In conclusion, this study indicated that an SGLT2‐i might improve inappropriate hyperinsulinaemia by increasing HICCIR. Also suggested was that increases in HICCIR via the SGLT2‐i was related to decreases in plasma TG concentration (reflecting reduction of intrahepatic TG content and improving FL) and elevated plasma BHB levels via the SGLT2‐i. Inappropriate hyperinsulinaemia is associated with various adverse events, including cardiovascular events. On the other hand, SGLT2‐is have been shown to have a protective effect on heart and kidney and to suppress some of these adverse events. Dysregulation of hepatic insulin clearance is one of the important pathophysiologies observed in various conditions in which there are metabolic abnormalities, including type 2 diabetes, obesity and FL. Therefore, our study clarified how an SGLT2‐i affected HICCIR and indicated that the relationship between changes in HICCIR and other clinical parameters, including BHB via an SGLT2‐i, is important for a partial understanding of the mechanism of the multifaceted effect of this drug, including its cardioprotective or renal protective effect revealed in recent trials.39 Further studies to clarify the mechanism of changes in hepatic insulin clearance and impact of such changes via an SGLT2‐i on the clinical course should be conducted.
Supporting information
Figure S1 Glucose, insulin and C‐peptide after a meal tolerance test in participants receiving placebo. (A) Glucose, (B) Insulin, (C) C‐peptide. Data are expressed as mean (standard deviation). Comparisons between baseline and week 24 were performed using a paired t‐test. n.s. not significant; ** p < 0.01 vs. baseline. Solid line, baseline; dotted line, at week 24
Figure S2. Glucose, insulin and C‐peptide after a meal tolerance test in participants receiving tofogliflozin. (A) Glucose, (B) Insulin, (C) C‐peptide. Data are expressed as mean (standard deviation). Comparisons between baseline and week 24 were performed using a paired t‐test. n.s. not significant; ** p < 0.01, *** p < 0.001 vs. baseline. Solid line, baseline; dotted line, at week 24
Supplemental Table 1 Integrated analysis of two clinical studies
Supplemental Table 2: Baseline characteristics in comparison of administration of tofogliflozin with placebo
Supplemental Table 3: Change in variables in comparison of administration of tofogliflozin with placebo from baseline to week 24
Supplemental Table 4: Change in focused variables for comparison of dosages of tofogliflozin administered from baseline to week 24
Supplemental Table 5: Correlations between change in ln‐beta‐hydroxybutyrate and changes in variables at week 24
Supplemental Table 6: Correlations between percentage change in HICCIR and changes in variables at week 24
Supplemental Table 7: Factors that might independently correlate with percentage change in HICCIR #1 at week 24
ACKNOWLEDGMENTS
We sincerely acknowledge all participants in the TOFO studies.
Matsubayashi Y, Yoshida A, Suganami H, et al. Association of increased hepatic insulin clearance and change in serum triglycerides or β‐hydroxybutyrate concentration via the sodium/glucose‐cotransporter 2 inhibitor tofogliflozin. Diabetes Obes Metab. 2020;22:947–956. 10.1111/dom.13980
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13980.
REFERENCES
- 1. Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8(8):495‐502. [DOI] [PubMed] [Google Scholar]
- 2. Merovci A, Solis‐Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124(2):509‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium‐glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124(2):499‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Despres JP, Lamarche B, Mauriege P, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334(15):952‐957. [DOI] [PubMed] [Google Scholar]
- 5. Ruige JB, Assendelft WJ, Dekker JM, Kostense PJ, Heine RJ, Bouter LM. Insulin and risk of cardiovascular disease: a meta‐analysis. Circulation. 1998;97(10):996‐1001. [DOI] [PubMed] [Google Scholar]
- 6. Marini MA, Frontoni S, Succurro E, et al. Differences in insulin clearance between metabolically healthy and unhealthy obese subjects. Acta Diabetol. 2014;51(2):257‐261. [DOI] [PubMed] [Google Scholar]
- 7. Kotronen A, Juurinen L, Tiikkainen M, Vehkavaara S, Yki‐Jarvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology. 2008;135(1):122‐130. [DOI] [PubMed] [Google Scholar]
- 8. Prattichizzo F, De Nigris V, Micheloni S, La Sala L, Ceriello A. Increases in circulating levels of ketone bodies and cardiovascular protection with SGLT2 inhibitors: Is low‐grade inflammation the neglected component? Diabetes Obes Metab. 2018;20(11):2515‐2522. [DOI] [PubMed] [Google Scholar]
- 9. Ferrannini E, Baldi S, Frascerra S, et al. Shift to Fatty Substrate Utilization in Response to Sodium‐Glucose Cotransporter 2 Inhibition in Subjects Without Diabetes and Patients With Type 2 Diabetes. Diabetes. 2016;65(5):1190‐1195. [DOI] [PubMed] [Google Scholar]
- 10. Kaku K, Watada H, Iwamoto Y, et al. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter‐2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined Phase 2 and 3 randomized, placebo‐controlled, double‐blind, parallel‐group comparative study. Cardiovasc Diabetol. 2014;13:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tanizawa Y, Kaku K, Araki E, et al. Long‐term safety and efficacy of tofogliflozin, a selective inhibitor of sodium‐glucose cotransporter 2, as monotherapy or in combination with other oral antidiabetic agents in Japanese patients with type 2 diabetes mellitus: multicenter, open‐label, randomized controlled trials. Expert Opin Pharmacother. 2014;15(6):749‐766. [DOI] [PubMed] [Google Scholar]
- 12. Hill NR, Levy JC, Matthews DR. Expansion of the homeostasis model assessment of beta‐cell function and insulin resistance to enable clinical trial outcome modeling through the interactive adjustment of physiology and treatment effects: iHOMA2. Diabetes Care. 2013;36(8):2324‐2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462‐1470. [DOI] [PubMed] [Google Scholar]
- 14. Pivovarova O, Bernigau W, Bobbert T, et al. Hepatic insulin clearance is closely related to metabolic syndrome components. Diabetes Care. 2013;36(11):3779‐3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Polonsky KS, Rubenstein AH. C‐peptide as a measure of the secretion and hepatic extraction of insulin. Pitfalls and limitations. Diabetes. 1984;33(5):486‐494. [DOI] [PubMed] [Google Scholar]
- 16. Matsubayashi Y, Yoshida A, Suganami H, et al. Role of fatty liver in the association between obesity and reduced hepatic insulin clearance. Diabetes Metab. 2018;44(2):135‐142. [DOI] [PubMed] [Google Scholar]
- 17. Bedogni G, Bellentani S, Miglioli L, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang BL, Wu WC, Fang KC, et al. External validation of fatty liver index for identifying ultrasonographic fatty liver in a large‐scale cross‐sectional study in Taiwan. PloS one. 2015;10(3):e0120443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuniga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bonora E, Zavaroni I, Coscelli C, Butturini U. Decreased hepatic insulin extraction in subjects with mild glucose intolerance. Metabolism: clinical and experimental. 1983;32(5):438‐446. [DOI] [PubMed] [Google Scholar]
- 21. Jung SH, Jung CH, Reaven GM, Kim SH. Adapting to insulin resistance in obesity: role of insulin secretion and clearance. Diabetologia. 2018;61(3):681‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Watada H, Tamura Y. Impaired insulin clearance as a cause rather than a consequence of insulin resistance. Journal of diabetes investigation. 2017;8(6):723‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bril F, Lomonaco R, Orsak B, et al. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Hepatology (Baltimore, Md). 2014;59(6):2178‐2187. [DOI] [PubMed] [Google Scholar]
- 24. Ohashi K, Komada H, Uda S, et al. Glucose Homeostatic Law: Insulin Clearance Predicts the Progression of Glucose Intolerance in Humans. PloS one. 2015;10(12):e0143880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Henry RR, Brechtel G, Griver K. Secretion and hepatic extraction of insulin after weight loss in obese noninsulin‐dependent diabetes mellitus. J Clin Endocrinol Metab. 1988;66(5):979‐986. [DOI] [PubMed] [Google Scholar]
- 26. Morin‐Papunen L, Vauhkonen I, Koivunen R, Ruokonen A, Martikainen H, Tapanainen JS. Metformin versus ethinyl estradiol‐cyproterone acetate in the treatment of nonobese women with polycystic ovary syndrome: a randomized study. J Clin Endocrinol Metab. 2003;88(1):148‐156. [DOI] [PubMed] [Google Scholar]
- 27. Osei K, Gaillard T, Schuster D. Thiazolidinediones increase hepatic insulin extraction in African Americans with impaired glucose tolerance and type 2 diabetes mellitus. A pilot study of rosiglitazone Metabolism: clinical and experimental. 2007;56(1):24‐29. [DOI] [PubMed] [Google Scholar]
- 28. Osei K, Rhinesmith S, Gaillard T, Schuster D. Metabolic effects of chronic glipizide gastrointestinal therapeutic system on serum glucose, insulin secretion, insulin sensitivity, and hepatic insulin extraction in glucose‐tolerant, first‐degree relatives of African American patients with type 2 diabetes: new insights on mechanisms of action. Metabolism: clinical and experimental. 2003;52(5):565‐572. [DOI] [PubMed] [Google Scholar]
- 29. Lundsgaard AM, Sjoberg KA, Hoeg LD, et al. Opposite Regulation of Insulin Sensitivity by Dietary Lipid Versus Carbohydrate Excess. Diabetes. 2017;66(10):2583‐2595. [DOI] [PubMed] [Google Scholar]
- 30. Bojsen‐Moller KN, Lundsgaard AM, Madsbad S, Kiens B, Holst JJ. Hepatic Insulin Clearance in Regulation of Systemic Insulin Concentrations‐Role of Carbohydrate and Energy Availability. Diabetes. 2018;67(11):2129‐2136. [DOI] [PubMed] [Google Scholar]
- 31. Mittendorfer B, Yoshino M, Patterson BW, Klein S. VLDL Triglyceride Kinetics in Lean, Overweight, and Obese Men and Women. J Clin Endocrinol Metab. 2016;101(11):4151‐4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DeAngelis AM, Heinrich G, Dai T, et al. Carcinoembryonic antigen‐related cell adhesion molecule 1: a link between insulin and lipid metabolism. Diabetes. 2008;57(9):2296‐2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aso Y, Kato K, Sakurai S, et al. Impact of dapagliflozin, an SGLT2 inhibitor, on serum levels of soluble dipeptidyl peptidase‐4 in patients with type 2 diabetes and non‐alcoholic fatty liver disease. Int J Clin Pract. 2019;73:e13335. [DOI] [PubMed] [Google Scholar]
- 34. Metabolic Contrasts Between Youth and Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes: I . Observations Using the Hyperglycemic Clamp. Diabetes Care. 2018;41(8):1696‐1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meyer EJ, Gabb G, Jesudason D. SGLT2 Inhibitor‐Associated Euglycemic Diabetic Ketoacidosis: A South Australian Clinical Case Series and Australian Spontaneous Adverse Event Notifications. Diabetes Care. 2018;41(4):e47‐e49. [DOI] [PubMed] [Google Scholar]
- 36. Ferrannini E, Mark M, Mayoux E. CV Protection in the EMPA‐REG OUTCOME Trial: A "Thrifty Substrate" Hypothesis. Diabetes Care. 2016;39(7):1108‐1114. [DOI] [PubMed] [Google Scholar]
- 37. Saxena R, Voight BF, Lyssenko V, et al. Genome‐wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science (New York, NY) 2007;316(5829):1331–1336. [DOI] [PubMed] [Google Scholar]
- 38. Tamaki M, Fujitani Y, Hara A, et al. The diabetes‐susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J Clin Invest. 2013;123(10):4513‐4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mahaffey KW, Jardine MJ, Bompoint S, et al. Canagliflozin and Cardiovascular and Renal Outcomes in Type 2 Diabetes and Chronic Kidney Disease in Primary and Secondary Cardiovascular Prevention Groups: Results from the Randomized CREDENCE Trial. Circulation. 2019;140:739‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Glucose, insulin and C‐peptide after a meal tolerance test in participants receiving placebo. (A) Glucose, (B) Insulin, (C) C‐peptide. Data are expressed as mean (standard deviation). Comparisons between baseline and week 24 were performed using a paired t‐test. n.s. not significant; ** p < 0.01 vs. baseline. Solid line, baseline; dotted line, at week 24
Figure S2. Glucose, insulin and C‐peptide after a meal tolerance test in participants receiving tofogliflozin. (A) Glucose, (B) Insulin, (C) C‐peptide. Data are expressed as mean (standard deviation). Comparisons between baseline and week 24 were performed using a paired t‐test. n.s. not significant; ** p < 0.01, *** p < 0.001 vs. baseline. Solid line, baseline; dotted line, at week 24
Supplemental Table 1 Integrated analysis of two clinical studies
Supplemental Table 2: Baseline characteristics in comparison of administration of tofogliflozin with placebo
Supplemental Table 3: Change in variables in comparison of administration of tofogliflozin with placebo from baseline to week 24
Supplemental Table 4: Change in focused variables for comparison of dosages of tofogliflozin administered from baseline to week 24
Supplemental Table 5: Correlations between change in ln‐beta‐hydroxybutyrate and changes in variables at week 24
Supplemental Table 6: Correlations between percentage change in HICCIR and changes in variables at week 24
Supplemental Table 7: Factors that might independently correlate with percentage change in HICCIR #1 at week 24


