Summary
Purpose:
To examine associations of KRAS mutation with tumor-deposit status and overall survival in colorectal cancer (CRC) patients.
Methods:
This retrospective cohort study included patients with incidental CRC diagnosed during 2010–2014 and recorded statuses of KRAS and tumor deposit in the National Cancer Database of the U.S. Multivariable logistic regression and time-varying Cox regression analyses were used.
Results:
We included 45,761 CRC patients with KRAS status (24,027[52.5%] men, 24,240 [53.0%] <65 years old, 17,338 [37.9%] with KRAS mutation). Adjusted for microsatellite instability, age, pathologic stage and tumor grade, KRAS mutation (versus wild-type) was associated with tumor-deposit presence (odds ratio=1.11, 95% CI 1.02–1.20). KRAS mutation was also linked to worse overall survival of CRC patients regardless of tumor-deposit status (adjusted Hazard ratio [HR]=1.20, 95% CI 1.07–1.33 for CRC with tumor deposits, and adjusted HR=1.24, 95% CI 1.14–1.35 for CRC without) or tumor-stage (adjusted HR=1.32, 95% CI 1.14–1.54 for early-stage and adjusted HR=1.18, 95% CI 1.10–1.27 for late-stage). Microsatellite instability was associated with better overall survival in CRC without tumor-deposit (adjusted HR=0.89, 95% CI 0.79–0.99), but not in CRC with tumor-deposit (adjusted HR=1.12, 95% CI 0.97–1.30).
Conclusion:
KRAS mutation is independently associated with tumor-deposit presence, and a worse overall survival in CRC patients.
Keywords: Colorectal cancer, biomarker, survival rate, KRAS, microsatellite instability
Introduction
Colorectal cancer (CRC) is the third most common cancer, as well as a leading cause of cancer death, in the United States [1]. Kirsten rat sarcoma viral oncogene homolog (KRAS) is a protooncogene that plays an important role in the development and treatment of CRC. Its mutation occurs in approximately 30 to 45% of CRC, and mostly in codon 12 or 13 [2–7]. The patients with KRAS mutation are unlikely to benefit from anti-EGFR (epidermal growth factor receptor) therapy, which should thus be applied to only the CRC with wild type KRAS as recommended by the National Comprehensive Cancer Network (NCCN) guidelines [8]. However, the association between KRAS mutation and patients’ survival remained controversial. A clinical trial showed that KRAS mutation was linked to worse survivals in CRC patients [9], while other reports failed to show any prognostic value of KRAS [10, 11]. Nonetheless, the NCCN guidelines for Treatment of Colon/Rectal Cancer suggested that all patients with metastatic CRC should be treated with the detection of KRAS mutations [8].
The tumor node metastasis (TNM) staging system of the American Joint Committee on Cancer (AJCC) is widely used for staging CRC patients and guiding adjuvant therapies. The 7th and 8th editions of the AJCC TNM staging systems (AJCC 7 in 2010; AJCC8 in 2018) classified extranodal tumor deposits (TDs) that lack regional lymph node metastasis as N1c in TNM staging system [12, 13]. The presence of TDs was associated with shorter decreased disease-free survival (DFS) and overall survival (OS) [14, 15]. TDs-positive CRC patients appeared to have worse DFS than those with higher N stages when treated as stage III disease [16]. However, our recent work also showed the upstaging node-negative CRC with TD (N0 to N1c) led to more chemotherapy and 43% more all-cause mortality.[17] Therefore, the factors associated with survivals of TD-positive and TD-negative CRC are largely unknown. The associations of KRAS status with TD status and OS of CRC are also unclear.
In this retrospective cohort study, we aimed to investigate association of KRAS mutation with TD status and that with OS of CRC using a large cancer-database.
Methods
The National Cancer Database (NCDB), established in 1989, is a nationwide, facility-based, clinical-surveillance based cancer dataset in the U.S. It is the largest clinical cancer registry in the world and a joint program sponsored by the Commission on Cancer of the American College of Surgeons and American Cancer Society [18]. The NCDB extracts data through all available components of the medical record by certified tumor registrars at all cancer centers accredited by the American College of Surgeon’s Commission on Cancer. A rigorous program has been implemented to ensure data quality. Scholars have used NCDB to investigate colorectal, breast and lung cancers [19–23]. An Institutional Review Board (IRB) review was exempt for this study due to the use of publicly available, deidentified, existing database (Exempt category 4).
In this retrospective cohort study on the 2017-release of NCDB (followed through Dec. 2014), the end point was the OS which was modeled using multivariable Cox regression. We also examined the factors linked to TD status.
No cancer specific survivals could be assessed due to the lack of data on the death cause in the NCDB [18]. The inclusion criteria were all incident CRC cases diagnosed during 2010–2014, with data of KRAS status, which became part of the NCDB (as Site-specific factor 9) for CRC in 2010. The data for surgical resection of primary tumor were extracted from NCDB as described before [16, 17]. We included the following factors in the univariate regression analyses: age, sex, tumor location (colon versus rectum), microsatellite instability (MSI) status, KRAS status, pathologic tumor stage (the 7th AJCC staging manual, according to the data item TNM_EDITION_NUMBER), tumor grade (high versus low), race, Charlson-Deyo score, chemotherapy status, and radiotherapy status. Charlson-Deyo score is the sum of the comorbid score derived from the entries in the Charlson Comorbidity Score Mapping Table according to the patient’s primary and secondary disease codings [18]. A factor will be included in multivariable regression analyses if its P in the univariate analysis was less than 0.10. Specifically, MSI status was classified as stable/low (codes 20 and 40) and unstable/high (codes 50 and 60) because of its potential predictive value for chemotherapy outcome [24]. TD status was classified as positive, negative and not available based on the Site-specific factor 4 [16, 17]. The race was classified as non-Hispanic (NH) White, NH Black, Hispanic and Others according to the data items of race and Hispanic ethnicity. Chemotherapy statuses were classified as received if chemotherapy data-item was coded as chemotherapy (not otherwise specified) administered, single-agent chemotherapy or multiagent chemotherapy administered as first course therapy (codes 1, 2 and 3); otherwise as classified not received (codes 0, 86 and 87). Radiotherapy statuses were classified as received if a radiotherapy was indicated (codes 1–5), and classified as not received if no radiotherapy indicated (code 0).
We conducted statistical analyses using Stata (version 15, StataCorp LLC, College Station, TX). The Pearson’s Chi-square test and logistic regression models were used to assess potential associations. Multivariable Cox regression models with time-varying covariates were used for survival analyses, including the factors that had a P value less than 0.10 in univariate Cox regression models. Only the factors with significant time-variance were included as time-varying covariate. The likelihood ratio test was used to examine the differences between the models with and without potential factor interactions. All P values were two-sides, with P<0.05 as statistically significant.
Results
Among the 59,592 CRC cases with known KRAS status in the NCDB, 11,392 (19.1%) had no surgical resections of the CRC, and were excluded because the pathologic assessment of their tumor deposit status was not possible. Several factors were associated with KRAS status (Table 1), including age, sex, tumor location, MSI status, TD status, pathologic tumor stage, tumor grade, race and chemotherapy status.
Table 1.
Baseline characteristics of resected incident, colorectal cancers with known KRAS status in National Cancer Database diagnosed during 2010–2014
| KRAS statusa | ||||||
|---|---|---|---|---|---|---|
| Wild Type, n (%) | Mutated, n (%) | P value | ||||
| Total | 28423 | (62) | 17338 | (38) | 45761 | |
| Age | 0.016 | |||||
| <65 year | 14931 | (53) | 9309 | (54) | 24240 | |
| 65+ year | 13492 | (47) | 8029 | (46) | 21521 | |
| Sex | <0.001 | |||||
| Male | 15159 | (53) | 8868 | (51) | 24027 | |
| Female | 13264 | (47) | 8470 | (49) | 21734 | |
| Tumor deposit | 0.001 | |||||
| None | 19126 | (68) | 11368 | (66) | 30494 | |
| Yes | 7076 | (25) | 4536 | (27) | 11612 | |
| NA | 1835 | (7) | 1200 | (7) | 3035 | |
| Microsatellite Instability (MSI) | <0.001 | |||||
| Stable/Low | 9116 | (81) | 5533 | (86) | 14649 | |
| Unstable/High | 2112 | (19) | 905 | (14) | 3017 | |
| Location | 0.006 | |||||
| Colon | 24778 | (87) | 15266 | (88) | 40044 | |
| Rectum | 3645 | (13) | 2072 | (12) | 5717 | |
| Tumor stage | <0.001 | |||||
| I-II | 8806 | (33) | 4575 | (28) | 13381 | |
| III-IV | 17837 | (67) | 11722 | (72) | 29559 | |
| Tumor grade | <0.001 | |||||
| Low | 19470 | (72) | 13016 | (79) | 32486 | |
| High | 7696 | (28) | 3501 | (21) | 11197 | |
| Charlson-Deyo Score | 0.55 | |||||
| 0 | 20811 | (73) | 12775 | (74) | 33586 | |
| 1 | 5752 | (20) | 3452 | (20) | 9204 | |
| 2 | 1860 | (7) | 1111 | (6) | 2971 | |
| Race | <0.001 | |||||
| NH White | 21722 | (79) | 12316 | (73) | 34038 | |
| NH Black | 2816 | (10) | 2506 | (15) | 5322 | |
| Hispanic | 1795 | (7) | 1260 | (8) | 3055 | |
| Others | 1218 | (4) | 716 | (4) | 1934 | |
| Received chemotherapy | <0.001 | |||||
| No | 7743 | (29) | 4169 | (26) | 11912 | |
| Yes | 18747 | (71) | 12013 | (74) | 30760 | |
| Received radiotherapy | 0.098 | |||||
| No | 24490 | (87) | 15024 | (88) | 39514 | |
| Yes | 3649 | (13) | 2133 | (12) | 5782 | |
MSI, microsatellite instability; NH, Non-Hispanic; P, Chi-square test for associations; NA, not available;
Sum of the subtotals may not be equal to the grand total due to missing data in some strata.
Multivariable logistic regression analyses were conducted to identified the factors independently linked to tumor deposit status and CRC OS. We found that MSI (Unstable/High vs Stable/Low), KRAS (Mutated vs Wild type), Age (65+ vs <65 year), Pathologic stage (III-IV vs I-II) and Tumor grade (High vs Low) were associated with presence of tumor deposit (versus absence), but not Charlson-Deyo Score, tumor location or sex (n=15,229, Table 2). In subgroup analyses, KRAS mutations were linked to TD status in early-stage CRC (stage I-II, n=5,769, P=0.005), but not in late-stage CRC (stage III-IV, n=9,460, P=0.10).
Table 2.
Factors associated with tumor deposit status (present vs absent) of incident colorectal cancers in National Cancer Database diagnosed during 2010–2014
| OR | 95% CI | P | |
|---|---|---|---|
| All tumor | |||
| MSI (Unstable/High vs Stable/Low) | 0.84 | (0.75 to 0.95) | 0.004 |
| KRAS (Mutated vs Wild type) | 1.11 | (1.02 to 1.20) | 0.015 |
| Location (Rectal vs Colonic) | 1.14 | (0.99 to 1.30) | 0.064 |
| Age (65+ vs <65 year) | 0.85 | (0.78 to 0.92) | <0.001 |
| Pathologic stage (III-IV vs I-II) | 14.44 | (12.51 to 16.66) | <0.001 |
| Tumor grade (High vs Low) | 1.58 | (1.45 to 1.73) | <0.001 |
| Early stage tumor (stage I-II) | |||
| MSI (Unstable/High vs Stable/Low) | 0.87 | (0.61 – 1.25) | 0.454 |
| KRAS (Mutated vs Wild type) | 1.49 | (1.13 – 1.97) | 0.005 |
| Location (Rectal vs Colonic) | 1.13 | (0.77 – 1.65) | 0.545 |
| Age (65+ vs <65 year) | 0.71 | (0.54 – 0.95) | 0.020 |
| Tumor grade (High vs Low) | 1.83 | (1.30 – 2.57) | 0.001 |
| Late stage tumor (stage III-IV) | |||
| MSI (Unstable/High vs Stable/Low) | 0.84 | (0.74 – 0.95) | 0.004 |
| KRAS (Mutated vs Wild type) | 1.08 | (0.99 – 1.17) | 0.100 |
| Location (Rectal vs Colonic) | 1.14 | (0.98 – 1.31) | 0.080 |
| Age (65+ vs <65 year) | 0.86 | (0.79 – 0.94) | 0.001 |
| Tumor grade (High vs Low) | 1.57 | (1.43 – 1.72) | <0.001 |
P of multivariable logistic regression analysis; MSI, microsatellite instability; OR, odds ratio; CI, confidence intervals. Race, sex and Charlson-Deyo Score were not linked to tumor deposit status.
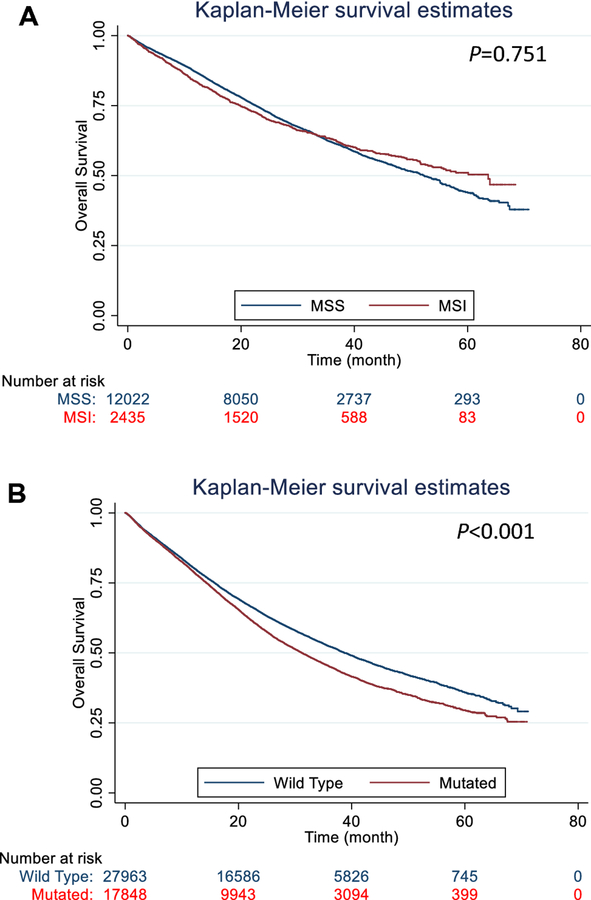
Univariate Cox regression analysis and log-rank test both showed that KRAS mutation (versus wild-type) was linked to a worse OS of the CRCs, but no association between MSI status and OS (Figure). The multivariate Cox regression analyses also showed that the prognostic values of MSI status differed by tumor deposit status (Table 3). Given the association of MSI and KRAS statuses, we included the interaction of the two in a Cox regression model, and found no statistical significance (P=0.627 and 0.434 in the TD negative and positive groups, respectively). The likelihood ratio test also showed no significant differences between the models with and without such an interaction (P=0.626 and 0.432 in the TD negative and positive groups, respectively). In the CRC with or without tumor-deposits, KRAS mutation (versus Wild type) was independently linked to a worse OS (n=8,110, P=0.008 for with tumor deposits, and n=2,618, P=0.004 for without); However, in the CRC with insufficient or no applicable data of tumor deposit status, KRAS mutation (versus Wild type) was linked to a better OS (n=457, P=0.039). Among the patients without tumor resection, KRAS mutation (versus Wild type) was also associated with a worse OS (Table 3, n=156, P <0.001). We further conducted multivariable Cox regression analyses by tumor pathologic stage, and found KRAS status was linked to OS in early and late stage CRCs (Table 4, P<0.001).
Figure.
Kaplan-Meier plots of the incident colorectal cancers diagnosed during 2010–2014 in the National Cancer Database that had a known KRAS status. A. The status of microsatellite instability (MSI) was not associated with OS of the colorectal cancers (n=14,457, Log-rank test P=0.751). B. The KRAS mutation (versus wild type) was associated with a worse OS of the colorectal cancers (n=45,811, Log-rank test P<0.001).
Table 3.
Factors associated with overall survival of incident colorectal cancers in National Cancer Database diagnosed during 2010–2014 by tumor deposit status
| HR | 95% CI | Pa | |
|---|---|---|---|
| Tumor-deposits absent in resected tumorb | |||
| MSI (Unstable/High vs Stable/Low) | 0.89 | (0.79 – 0.997) | 0.044 |
| KRAS (Mutated vs Wild type) | 1.24 | (1.14 – 1.35) | <0.001 |
| Tumor-deposits present in resected tumorc | |||
| MSI (Unstable/High vs Stable/Low) | 1.12 | (0.97 – 1.30) | 0.124 |
| KRAS (Mutated vs Wild type) | 1.20 | (1.07 – 1.33) | 0.001 |
| No tumor resectiond | |||
| MSI (Unstable/High vs Stable/Low) | 0.51 | (0.24 – 1.08) | 0.078 |
| KRAS (Mutated vs Wild type) | 2.07 | (1.33 – 3.22) | 0.001 |
MSI, microsatellite instability.
P of multivariate Cox regression analyses adjusted for age, tumor grade, pathologic stage, Charlson-Deyo score, chemotherapy status, radiotherapy status and race.
Age, pathologic stage and chemotherapy status included as time-varying covariates.
Tumor grade and chemotherapy status included as time-varying covariates.
Chemotherapy status included as time-varying covariate.
Table 4.
Factors associated with overall survival of incident colorectal cancers in National Cancer Database diagnosed during 2010–2014 by tumor pathologic stage
| HR | 95% CI | Pa | |
|---|---|---|---|
| Stage I-IIb | |||
| MSI (Unstable/High vs Stable/Low) | 0.92 | (0.77 – 1.11) | 0.397 |
| KRAS (Mutated vs Wild type) | 1.32 | (1.14 – 1.54) | <0.001 |
| Tumor deposit (Present vs absent) | 1.28 | (1.09 – 1.51) | 0.002 |
| Stage III-IVc | |||
| MSI (Unstable/High vs Stable/Low) | 0.98 | (0.89 – 1.08) | 0.689 |
| KRAS (Mutated vs Wild type) | 1.18 | (1.10 – 1.27) | <0.001 |
| Tumor deposit (Present vs absent) | 1.42 | (1.35 – 1.50) | <0.001 |
MSI, microsatellite instability.
P of multivariate Cox regression analyses adjusted for age, tumor grade, pathologic stage, Charlson-Deyo score, chemotherapy status, radiotherapy status and race.
Chemotherapy status included as time-varying covariate.
Tumor grade, age and chemotherapy status included as time-varying covariates.
Discussion
Here we showed that KRAS mutation was more frequent in TDs-positive CRC in a multivariable model. Adjusted to many prognostic and therapeutic factors, KRAS mutation was still associated with worse OS of the CRC with or without TD, and worse OS of early- and late-stage CRC. But the association of MSI with OS differed by tumor-deposit status in CRC patients.
The TNM/AJCC 7th and 8th Editions define tumor cells present in peri-colorectal adipose tissue and lymph drainage sites without residual lymph-node tissue as TD. It may represent a completely replaced lymph node, venous invasion or discontinuous tumor spread.[12, 13] TDs are found linked to several aggressive histologic features [25–28]. The RAS family of oncogenes was one of the first to be identified as mutated in human cancer [29]. Ras functions downstream of EGFR and the KRAS gene product (Kras protein) passes EGFR mitogenic signals from cell surface into various EGFR effectors in the cell nucleus. These EGFR signals play an important role in CRC development, progression and metastasis, through apoptotic, angiogenic and invasion pathways [30, 31]. Considering our finding that KRAS mutation was associated with TD presence and worse CRC OS, it is possible that KRAS mutation also involves in the TD development and CRC progression. Additional studies of molecular and cell biology are warranted to examine such a role of KRAS mutation.
Recently, Andreyev et al. found that, compared with wild type, KRAS mutations is linked to higher possibility of CRC recurrence and patient death [2]. Studies also show that patients with KRAS mutation in curable CRC had a shorter OS or shorter recurrence free survival in those with early CRC [9, 32], while recent studies found no significant prognostic value of KRAS mutation status [10, 11]. Therefore, the prognostic values of KRAS mutations in TDs-positive CRC appear still not well defined. In this study of a large, nationally represented U.S. population, KRAS mutation in CRC was independently associated with worse OS regardless of TD or tumor-resection status, or tumor pathologic stage.
MSI refers to the hypermutable state of cells caused by impaired DNA mismatch repair (MMR). The NCCN and European Society for Medical Oncology (ESMO) guidelines recommended universal testing of MSI/MMR for CRC [8, 33]. A previous study showed deficient MMR status was associated with improved DFS in the patients treated with surgery alone and no survival benefits in patients treated with fluorouracil (FU)-based regimens [24, 34]. Sinicrope et al revealed that deficient MMR in stage II CRC was also linked to better survivals among the recipients of adjuvant FOLFOX (folinic acid, fluorouracil, and oxaliplatin) therapy [35, 36]. The multivariable regression analysis in this cohort shows MSI was independently associated with better OS in CRC without tumor-deposit, but not in CRC with tumor-deposit. It suggests that MSI status may play a role in the prognostication of patients with TD-negative CRC, while no plausible explanation for the differential prognostic association of MSI in TD-negative and TD-positive CRC. TD as a pathology feature may be helpful to stratify CRC patients for MSI testing.
Several strengths of the study are noteworthy. We used the NCDB, which is a widely used and validated large cancer database [18]. Several clinical prognostic and therapeutic factors were also included in the survival analysis, including Charlson-Deyo Score, radiotherapy status, race and chemotherapy status. Inclusion of these models are expected to reduce, if not eliminate, the potential biases associated with those factors. Moreover, the large sample size of the study lent us the advantages of more statistical power and inclusion of more covariates in analyses. Further, to our knowledge this is the first to show that the KRAS mutations were associated with worse OS in patients without CRC resection. Finally, we included several factors as time varying covariates due to violating constant proportional hazard assumption. Many studies did not check whether the proportional hazard is constant in their Cox regression models. They thus may report the uncorrected HR.
The current study had several limitations. First, this retrospective study might have a selection bias despite the adjustment of many covariates. Second, about 11.5% of the CRC cases in the NCDB had data on KRAS mutation and were included in the study. There thus may be a potential selection bias due to the lack of universal KRAS testing. A population-based study is needed to confirm our findings. Third, anti-EGFR therapy was associated with KRAS status, but not captured in any known population-based cancer databases. Additional validation studies thus are needed. Finally, the interobserver variations in the detection TD, MSI and KRAS may exist. Future works using a centralized laboratory are needed to address this issue.
Conclusions
In this NCDB-based cohort, KRAS mutation is independently associated with the tumor deposit presence in CRC, and a worse OS in CRC regardless of tumor-deposit status or tumor stage. Therefore, KRAS mutation may play a role in the development of tumor deposits in CRC and serve as a target for CRC treatment, besides guiding anti-EGFR treatment.
Acknowledgement
The study was in part supported by an Initiative for Multidisciplinary Research Teams (IMRT) award from Rutgers University, Newark, NJ (to N.G. and L.Z.), the U.S. National Institute of Health (R01 AT010243 to N.G.), the National Natural Science Foundation of China (No. 81872387 to M.Z.) and China Scholarship Council (to M.Z.). The funders have no roles in the study design or manuscript preparation.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
No conflict of interest is declared by any of the authors.
We cannot share the patient-level data which are confidential and were obtained with review and approval from the National Cancer Database and the American College of Surgeons.
Bibliography
- 1.Siegel RL, Miller KD, Jemal A. (2020) Cancer statistics, 2020. CA: a cancer journal for clinicians; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Andreyev HJ, Norman AR, Cunningham D, Oates JR, Clarke PA. (1998) Kirsten ras mutations in patients with colorectal cancer: the multicenter “RASCAL” study. J Natl Cancer Inst. 90: 675–84. [DOI] [PubMed] [Google Scholar]
- 3.De Roock W, Claes B, Bernasconi D, et al. (2010) Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 11: 753–62. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein SD, Sayegh R, Christensen S, Swalsky PA. (1993) Genotypic classification of colorectal adenocarcinoma. Biologic behavior correlates with K-ras-2 mutation type. Cancer. 71: 3827–38. [DOI] [PubMed] [Google Scholar]
- 5.McDermott U, Longley DB, Johnston PG. (2002) Molecular and biochemical markers in colorectal cancer. Ann Oncol. 13 Suppl 4: 235–45. [DOI] [PubMed] [Google Scholar]
- 6.Nagata Y, Abe M, Kobayashi K, et al. (1990) Glycine to aspartic acid mutations at codon 13 of the c-Ki-ras gene in human gastrointestinal cancers. Cancer Res. 50: 480–2. [PubMed] [Google Scholar]
- 7.Shaw P, Tardy S, Benito E, Obrador A, Costa J. (1991) Occurrence of Ki-ras and p53 mutations in primary colorectal tumors. Oncogene. 6: 2121–8. [PubMed] [Google Scholar]
- 8.NCCN. (2018) NCCN Clinical Practice Guidelines in Oncology: Colon cancer v1.2018. National Comprehensive Cancer Network. [DOI] [PubMed] [Google Scholar]
- 9.Maughan TS, Adams RA, Smith CG, et al. (2011) Addition of cetuximab to oxaliplatin-based firstline combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 377: 2103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth AD, Tejpar S, Delorenzi M, et al. (2010) Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60–00 trial. J Clin Oncol. 28: 466–74. [DOI] [PubMed] [Google Scholar]
- 11.Ogino S, Meyerhardt JA, Irahara N, et al. (2009) KRAS mutation in stage III colon cancer and clinical outcome following intergroup trial CALGB 89803. Clin Cancer Res. 15: 7322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song JS, Chang HJ, Kim DY, et al. (2011) Is the N1c category of the new American Joint Committee on cancer staging system applicable to patients with rectal cancer who receive preoperative chemoradiotherapy? Cancer. 117: 3917–24. [DOI] [PubMed] [Google Scholar]
- 13.Amin MB, Greene FL, Edge SB, et al. (2017) The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 67: 93–9. [DOI] [PubMed] [Google Scholar]
- 14.Puppa G, Maisonneuve P, Sonzogni A, et al. (2007) Pathological assessment of pericolonic tumor deposits in advanced colonic carcinoma: relevance to prognosis and tumor staging. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 20: 843–55. [DOI] [PubMed] [Google Scholar]
- 15.Lo DS, Pollett A, Siu LL, Gallinger S, Burkes RL. (2008) Prognostic significance of mesenteric tumor nodules in patients with stage III colorectal cancer. Cancer. 112: 50–4. [DOI] [PubMed] [Google Scholar]
- 16.Mayo E, Llanos AA, Yi X, Duan SZ, Zhang L. (2016) Prognostic Value of Tumor Deposit and Perineural Invasion Status in Colorectal Cancer Patients: a SEER-Based Population Study. Histopathology. 69: 230–8. [DOI] [PubMed] [Google Scholar]
- 17.Chavali LB, Llanos AAM, Yun JP, Hill SM, Tan XL, Zhang L. (2017) Radiotherapy for Patients With Resected Tumor Deposit-Positive Colorectal Cancer. Archives of pathology & laboratory medicine. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Boffa DJ, Rosen JE, Mallin K, et al. (2017) Using the National Cancer Database for Outcomes Research: A Review. JAMA oncology. 3: 1722–8. [DOI] [PubMed] [Google Scholar]
- 19.Shaikh T, Handorf EA, Meyer JE, Hall MJ, Esnaola NF. (2018) Mismatch Repair Deficiency Testing in Patients With Colorectal Cancer and Nonadherence to Testing Guidelines in Young Adults. JAMA oncology. 4: e173580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pezzi TA, Schwartz DL, Mohamed ASR, et al. (2018) Barriers to Combined-Modality Therapy for Limited-Stage Small Cell Lung Cancer. JAMA oncology. 4: e174504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joshi SS, Handorf E, Strauss D, et al. (2018) Treatment Trends and Outcomes for Patients With Lymph Node-Positive Cancer of the Penis. JAMA oncology. 4: 643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodman CR, Seagle BL, Friedl TWP, et al. (2018) Association of Circulating Tumor Cell Status With Benefit of Radiotherapy and Survival in Early-Stage Breast Cancer. JAMA oncology. 4: e180163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adam MA, Turner MC, Sun Z, et al. (2018) The appropriateness of 30-day mortality as a quality metric in colorectal cancer surgery. American journal of surgery. 215: 66–70. [DOI] [PubMed] [Google Scholar]
- 24.Sargent DJ, Marsoni S, Monges G, et al. (2010) Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 28: 3219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prabhudesai A, Arif S, Finlayson CJ, Kumar D. (2003) Impact of microscopic extranodal tumor deposits on the outcome of patients with rectal cancer. Diseases of the colon and rectum. 46: 1531–7. [DOI] [PubMed] [Google Scholar]
- 26.Tateishi S, Arima S, Futami K, et al. (2005) A clinicopathological investigation of “tumor nodules” in colorectal cancer. Surg Today. 35: 377–84. [DOI] [PubMed] [Google Scholar]
- 27.Yamano T, Semba S, Noda M, et al. (2015) Prognostic significance of classified extramural tumor deposits and extracapsular lymph node invasion in T3–4 colorectal cancer: a retrospective single-center study. BMC Cancer. 15: 859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagayoshi K, Ueki T, Nishioka Y, et al. (2014) Tumor deposit is a poor prognostic indicator for patients who have stage II and III colorectal cancer with fewer than 4 lymph node metastases but not for those with 4 or more. Diseases of the colon and rectum. 57: 467–74. [DOI] [PubMed] [Google Scholar]
- 29.Simanshu DK, Nissley DV, McCormick F. (2017) RAS Proteins and Their Regulators in Human Disease. Cell. 170: 17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lockhart AC, Berlin JD. (2005) The epidermal growth factor receptor as a target for colorectal cancer therapy. Semin Oncol. 32: 52–60. [DOI] [PubMed] [Google Scholar]
- 31.Ciardiello F, Tortora G. (2001) A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 7: 2958–70. [PubMed] [Google Scholar]
- 32.Hutchins G, Southward K, Handley K, et al. (2011) Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol. 29: 1261–70. [DOI] [PubMed] [Google Scholar]
- 33.Van Cutsem E, Cervantes A, Adam R, et al. (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Annals of oncology : official journal of the European Society for Medical Oncology. 27: 1386–422. [DOI] [PubMed] [Google Scholar]
- 34.Jover R, Zapater P, Castells A, et al. (2006) Mismatch repair status in the prediction of benefit from adjuvant fluorouracil chemotherapy in colorectal cancer. Gut. 55: 848–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaanan A, Shi Q, Taieb J, et al. (2018) Role of Deficient DNA Mismatch Repair Status in Patients With Stage III Colon Cancer Treated With FOLFOX Adjuvant Chemotherapy: A Pooled Analysis From 2 Randomized Clinical Trials. JAMA Oncol. 4: 379–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinicrope FA, Foster NR, Thibodeau SN, et al. (2011) DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst. 103: 863–75. [DOI] [PMC free article] [PubMed] [Google Scholar]