Abstract
Patients with a current diagnosis of breast cancer are enjoying dramatic cure rates and survivorship secondary to an increase in awareness, earlier detection, and more effective therapies. Although strategies such as Breast Cancer Awareness Month in October focus on early detection, lifestyle changes are seldom discussed other than dietary concerns and physical activity. Lifestyle modifications centered on diet and exercise have been demonstrated to affect overall disease-free survival in breast cancer. Since the early 2000s, the role of the human gut microbiota and its relation to breast cancer has become a major area of interest in the scientific and medical community. We live and survive owing to the symbiotic relationship with the microorganisms within us: the human microbiota. Scientific advances have identified a subset of the gut microbiota: the estrobolome, those bacteria that have the genetic capability to metabolize estrogen, which plays a key role in most breast cancers. Recent research provides evidence that the gut microbiome plays a substantial role in estrogen regulation. Gut microbiota diversity appears to be an essential component of overall health, including breast health. Future research attention should include a more extensive focus on the role of the human gut microbiota in breast cancer.
Keywords: breast cancer survivorship, estrobolome, human gut microbiota, inflammation, lifestyle, metabolic syndrome, microbiome, whole food plant-based diet
INTRODUCTION
In 2020, nearly 260,000 women in the US will receive a diagnosis of breast cancer, and more than 40,000 will die due to the disease.1,2 Breast cancer is the most common malignancy among women, affecting 2.4 million women and responsible for more than 500,000 deaths worldwide.3 In 2015, there were an estimated 3.4 million breast cancer survivors in the US.4 This number increases yearly. Most breast cancer patients survive disease free for many years, making survivorship a major health issue. The American College of Surgeons has mandated that survivorship care plans become an integral component of their accreditation. This has prompted the appearance of a plethora of articles addressing lifestyle issues that positively affect many chronic comorbidities, a decrease in recurrence, and an increase in overall survival.4,5 Lifestyle recommendations, although inadequately addressed, also contribute to prolonged survival.5,7 Such recommendations are generally focused on diet and exercise, but the developing awareness of the influence of the human gut microbiota on survival and overall health creates the need to expand that focus to encompass diet, exercise, and the microbiome.
GUT MICROBIOTA AND HEALTH
The human microbiome is composed of trillions of microorganisms living inside and outside the human body. Often used interchangeably, the terms microbiome and microbiota are, in fact, distinctive. The microbiome defines the collection of the genomes that the microbiota (the bacterial population) possess. The microbiota are now considered an “essential organ” and have been associated with overall health and chronic disease.5,8,9 Bacteria, fungi, protozoa, yeast, and viruses comprise up to 90% of the human cellular population.10 These organisms, until recently, were the unrecognized “organ system” responsible for most of our immunity. The microbiota rely on us, and in turn, we rely on them, representing a truly symbiotic relationship. An imbalance of healthy and derogatory bacteria can lead to uncontrolled processes resulting in the development of chronic conditions, including cancer.5,11,12 Results of recent investigations have suggested that specific hormones, particularly estrogen, and the gut microbiome might act synergistically in the development of obesity, type 2 diabetes mellitus (T2DM), and cancer.5,12
Metabolic syndrome is characterized by central obesity, T2DM, hypercholesterolemia, insulin resistance/hyperinsulinemia, and hypertension (Table 1). Metabolic syndrome is the result of lifestyle choices that are modifiable by healthy changes.13–17 Recommendations addressing lifestyle adjustments that influence breast cancer survivorship are evidence based.5,7 The underlying reasons for their effectiveness are multifactorial, poorly understood, and often vague. In this report, we address potential factors that play a major role in breast cancer survival, particularly those that are influenced by the gut microbiota. The potential ability to manipulate our gut microbiota through lifestyle recommendations may positively affect survival.
Table 1.
Characteristics of the Metabolic Syndrome
| • Obesity (central) |
| • Hypertension |
| • T2DM (often related to obesity) |
| • Hypercholesterolemia (↓HDL, ↑Triglycerides) |
| • Hyperinsulinemia (insulin resistance) |
Since 2010, the recognition of the gut microbiota on human health has been monumental, as demonstrated by the number of medical publications in well-respected, peer-reviewed journals.18 We are beginning to understand that a large portion of our immunity resides in the human gut microbiota and that this ecologic system, in and of itself, is a unique “organ.” The roughly 37 trillion cells in an average human are far surpassed by the nearly 100 trillion bacterial cells, which account for an estimated 2.25 to 2.7 kg (5–6 lb) of an average human’s weight.19 Furthermore, a human’s DNA is outnumbered by the DNA of these microbes by a factor of 100 to 800.20 The role of the gut microbiota and their effect on dysbiosis (alterations in gut diversity) needs further investigation and may identify potential links in the development of cancer.21,22 The recently discovered estrobolome—those bacteria that are the subset of the microbiota possessing genetic traits responsible for estrogen metabolism and degradation—plays an important role in the development and/or progression of breast cancer.23
There is little literature addressing the influence of the human gut microbiota on long-term breast cancer survivorship. We address how lifestyle and the gut microbiota influence these concerns here and in Appendix 1.
DISCUSSION
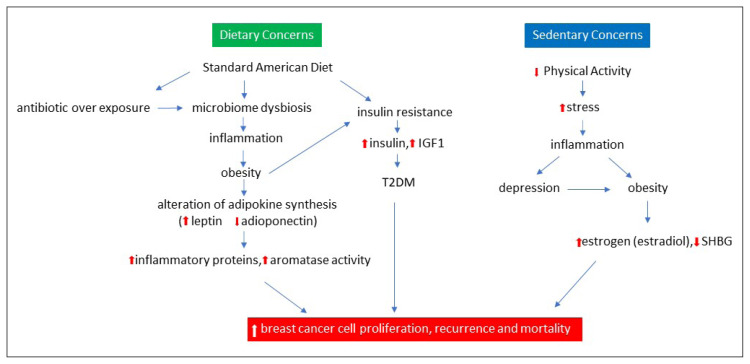
Breast cancer survivorship is on the rise.24,25 Nearly 90% of patients survive at least 5 years after diagnosis. Breast cancer is no longer considered an acute disease; rather, it is now a chronic condition.7,26 This creates an opportunity to improve the lives of survivors through lifestyle choices. The Western lifestyle (a diet high in sugar and fat, low in fiber, and minimal activity), however, puts one at risk of breast cancer. What has been referred to as the standard American diet results in obesity, insulin resistance, dysbiosis, and inflammation.5 Insulin also stimulates the synthesis of insulin growth factor-1 (IGF-1), linked to tumor growth and metastasis. Both estrogen and IGF-1-mediated signaling are increased in obese postmenopausal women.27 “Cross-talk” between such pathways represents an important link to tumor progression. Obesity leads to a pathway of subclinical inflammation; adipose tissues contribute to insulin resistance as well as cancer development and progression. Activated macrophages in adipose tissues in obese individuals produce proinflammatory mediators. Obesity leads to insulin resistance, an increased level of insulin, IGF-1, a decrease in adiponectin (the fat-burning hormone), and an increase in leptin (the satiety hormone).27,28 Leptin promotes angiogenesis, whereas adiponectin inhibits the same.28 Obesity has been associated with an increased risk of postmenopausal breast cancer in addition to multiple metabolic disorders.5 Several biologic mechanisms may contribute to a worsened prognosis of obese patients with breast cancer. This is at least in part the result of the presence of comorbidities in patients with breast cancer (Figure 1).17,29,30
Figure 1.
Impact of lifestyle on breast cancer.
Although the diagram separates diet and inactivity, they are intimately connected, leading to an increase in obesity, T2DM, and ultimately an increase in inflammation.
IGF1 = insulin growth factor 1; SHGB = steroid hormone-binding globulin; T2DM = type 2 diabetes mellitus.
Metabolic syndrome is a cluster of conditions that predict an increased risk of cardiovascular disease and T2DM.30 Although major attention regarding this syndrome has focused on cardiovascular disease risks, results of recent studies suggest that the metabolic syndrome also plays an independent role in increasing the risk factors for breast cancer.31 Yet, the conditions that define this syndrome are modifiable by lifestyle changes. A well-recognized risk factor for breast cancer and recurrence, obesity may also be substantially influenced by our gut microbiome.32 In addition to its capacity to store lipid, adipose tissue should be viewed as an active endocrine and metabolic organ. The human gut microbiome is intimately associated with obesity.19
A major factor underlying the increased risk of hormone receptor-positive breast cancers in obese women is an elevated estrogen level, which is related to increased adipose tissue mass and the production/storage of multiple inflammatory mediators.5 Such proinflammatory molecules have been linked to tumor progression and the upregulation of aromatase (the enzyme responsible for the conversion of testosterone to estrogenic compounds and unwanted byproducts of cholesterol metabolism).27 Obesity also leads to insulin resistance, hyperinsulinemia, and impaired glucose tolerance. High levels of fasting insulin in patients with breast cancer have been associated with distant recurrence, metastases, and increased mortality. Insulin has been implicated in cancer progression by virtue of its mitogenic, antiapoptotic, and proangiogenic properties.
Physical inactivity promotes stress, inflammation, and psychological issues such as depression, which are influenced by the gut microbiome. Obesity and self-image concerns may further contribute to depression. Physical activity after breast cancer decreases cancer recurrence by 24%, decreases the risk of breast cancer-related death by 34%, and decreases all-cause mortality by 41%.33 Observational evidence suggests a primary reduction in breast cancer between 30% to 50% with regular physical activity.34 Even exercise such as walking for 30 minutes a day, 5 times per week, may appreciably affect overall health. Physical activity guidelines for health in the US have recently been updated and have concluded that a sedentary lifestyle may be responsible for up to 10% of premature deaths.35–37 Although the “Physical Activity Guidelines for Americans” report is noteworthy and applauded, there was absolutely no mention of the role of lifestyle or its promotion as an interventional strategy in the article by Giroir and Wright.35 The article did not specifically address cancer survivorship or issues regarding the composition of the gut microbiota, which have been identified as playing a major role in fitness and survival.38 However, it did note the importance of physical activity for overall health. Additional studies have demonstrated that the adoption of a healthy lifestyle after a breast cancer diagnosis may decrease mortality rates by up to 50%. This can be accomplished if patients adhere to the adoption of a high fruit/vegetable diet (4–5 servings per day) coupled with regular physical activity (30 minutes/5 times per week).39
Lifestyle medicine, as it relates to breast cancer survivorship, relies on 3 major pillars: diet, physical activity, and stress management. Stress management is outside the scope of this article; it is extensively discussed elsewhere.5,7,40,41 There remains considerable debate regarding the association of diet, physical activity, and cancer prevention.42 Medical advances in treating chronic conditions have seen a revolution since the turn of the 21st century. Perhaps 2 of the most important advances have been 1) the identification of epigenetics (turning genes on and off) and the modification of gene expression as opposed to the alteration of the genetic code itself, which can be influenced by lifestyle,5,7,43–45 and 2) the recognition that the human gut microbiome plays a major role in overall health.46–48 We believe the identification of the human gut microbiome equals the importance of the discovery of aspirin, antibiotics, and vaccines. Our bodies are inhabited by trillions of microorganisms that are vital to our survival, the majority of which reside in the gastrointestinal tract, particularly in the large intestine.18,49,50 The exact number/ratio of human cells vs gut microorganisms and the ratio of their DNA has been debated.51 Further investigation is needed to resolve these figures. One fact is indisputable: the role of the gut microbiota is indeed important and regulates our well-being.52
The human microbiota represent the constellation of microorganisms that inhabit our bodies. The complex interactions of the gut microbiota remain beyond our understanding, regardless of numerous advances in genomic profiling.53,54 More than 50% of our gut microbiota refuses to be cultured or identified outside the body using current technologies.8,53,55
Nearly 90% of our gut bacteria are composed of 2 major phyla: Bacteroidetes and Firmicutes.46,53,56 These phyla and their ratios have been extensively studied; however, our understanding of them and their interactions remains elusive.32,47,57 Improper ratios of thousands of species have been linked to the development of multiple chronic conditions and account for more than 80% of all chronic maladies.5–7,44,53,58,59 When the ratios are optimal, these gut microbiota provide valuable services (energy production through the fermentation of foods, synthesis of vitamins, the building of amino acids, and a general oversight of the immune system halting infections), keeping chronic conditions at bay and preventing disease.
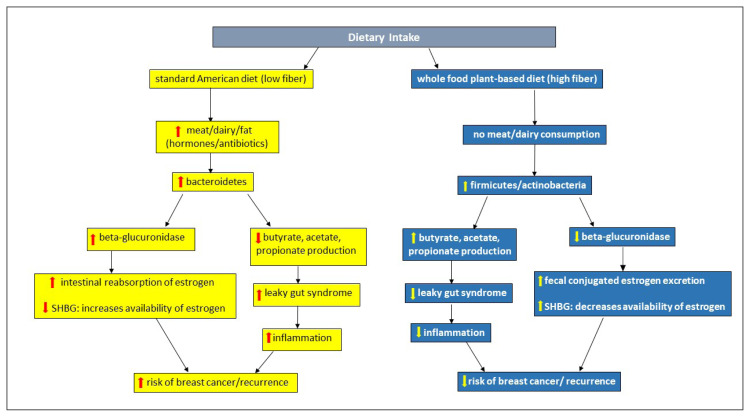
Diet plays an integral role in the complex interrelationship between the human gut microbiota, estrogen metabolism, and its influence on breast cancer recurrence as well as metastatic potential. The standard American diet results in the increased propagation of unhealthy bacteria, which contain high levels of β-glucuronidase. This enzyme is responsible for deconjugating estrogen and returning it to the circulatory system, thus raising its availability to further fuel estrogen-responsive cancers. This diet results in a decrease in the production of short-chain fatty acids (SCFA; butyrate, propionate, and acetate), which play a major role in the prevention of “leaky gut syndrome.” This syndrome is responsible for the flow of harmful inflammatory products into the circulatory system, influencing the development and recurrence of breast cancer. Inflammatory proteins promote insulin resistance and support leptin, which influences carcinogenesis.60 Insulin binds steroid hormone-binding globulin (SHBG), increasing estrogen availability, promoting higher estrogen levels, and contributing to breast carcinogenesis.27,61 Adiponectin levels are decreased, resulting in insulin resistance and increased levels of IGF-1, which promote cell proliferation.27
In contrast, a whole-food, plant-based diet (especially one high in fiber) results in the promotion of “healthy” microbiota. By decreasing the activity of β-glucuronidase, circulating estrogen levels are minimized, and SGBH is increased along with the fecal excretion of estrogen. As SCFAs are increased, they protect the colonic mucosa from developing leaky gut syndrome, decrease inflammation, and potentially lower the risk of breast cancer.5,27,48,62–65 Estrogen is conjugated in the liver and excreted into the gastrointestinal tract; estrogen is deconjugated by bacterial glucuronidase and is reabsorbed as free estrogen into the bloodstream. Multiple bacteria are involved in this process; however, which bacteria are high producers of β-glucuronidase remain controversial (Figure 2).66,67
Figure 2.
Dietary influence on gut microbiota/microbiome and estrogen metabolism.
SHBG = steroid hormone-binding globulin.
The current literature regarding the gut microbiota is confusing and contradictory as a result of 2 factors. First, only recently have we acquired technologies that effectively identify an important, albeit small, portion of the microbiota. Second, there appears to be a disconnect that surrounds the interaction or interactions of these bacteria. What is currently understood are the major bacteria and their phyla, which are summarized as follows. The phylum Firmicutes includes the genera Lactobacillus and Clostridium (various subtypes), the phylum Bacteroidetes includes the genera Prevetella and Bacteroides, and the phylum Actinobacteria includes the genus Bifidobacterium. These are believed to be the major producers of SCFAs that result in a decreased breast cancer risk, recurrence, and mortality. There is evidence that 1 or another of the phyla in the gastrointestinal tract may be responsible for the majority of SCFA production. It appears that the primary producers of butyrate (the major colonic epithelial protector) are Firmicutes.68 Bacteroidetes may increase propionate, another beneficial SCFA, although this has been less extensively studied.69
Firmicutes and Bacteroidetes are the major colonic phyla responsible for the metabolism of fiber and polyphenols. Multiple studies have reached different conclusions on the impact of these phyla, particularly as they relate to obesity (a major risk factor for breast cancer).19,49,50,67,70 Leaky gut syndrome and the inflammation associated with it may well be minimized by the consumption of a high-fiber diet, leading to the production of SCFAs and intestinal alkaline phosphatase.71 Intestinal alkaline phosphatase is a protein of the intestinal epithelium that plays a major role in gut endothelial integrity. Along with SCFAs, intestinal alkaline phosphatase strengthens the tight junctions of the colonic mucosa, decreasing the leakage of harmful pathogens and their carcinogenic potential.5,20,72–74 Chronic inflammation may be promoted by the gut microbiota through its influence on self-proliferation and apoptosis.5,66,74–76
Nonalcoholic fatty liver disease (NAFLD) affects nearly one-fourth of the global population.77,78 This presence of fat in the liver (hepatic steatosis) is a diagnosis based on exclusion of other causes such as excessive alcohol consumption. Regardless of our poor understanding of its etiology, NAFLD is of great importance and a major cause of mortality, not only owing to the condition itself but also as a harbinger of malignancies, including breast cancer.77 This association results from the fact that NAFLD is associated with metabolic syndrome.79,80 Components of metabolic syndrome and its association with breast cancer have been described and documented in numerous publications.81–83 The influence of NALFD on extrahepatic carcinogenesis and mortality has also been noted.84 The association is poorly understood; however, multiple hypotheses to explain a carcinogenic link have been put forth.85 The common link appears to be an inflammatory state, fueled by hyperinsulinemia and resulting in tumor proliferation.60
Inflammation, obesity, T2DM, and chronic conditions such as cancer share common pathways, which are influenced by the human gut microbiota.57,86–90 This complex and intricate system affects numerous distant organ systems.91 The idea that our microbial “friends” aid and participate in the promotion of our health is hardly a new concept; in fact, such recognition dates to the 20th century.18 Similarly, the association of inflammation and cancer has long been recognized owing to the work of Virchow.92–94 Inflammation plays a role in most chronic conditions and, if uncontrolled, leads to chronic processes that promote tumorigenesis, from initiation to metastasis.95
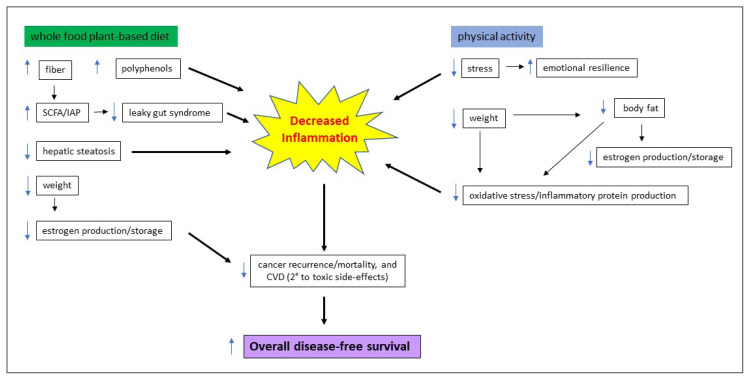
In the diverse human gut microbiota exists a subset of bacteria that possess the genetic capability to metabolize estrogen: the estrobolome. These microbes favor fiber as their primary source of energy. When a high-fiber diet is consumed, the estrobolome increases the metabolism of estrogen and thus its elimination from the body. Because nearly 70% of breast cancers are estrogen fueled, a high-fiber diet contributes to estrogen elimination, robbing breast cancer cells of a major fuel source. The “commonsense” recommendation to increase dietary fiber in the setting of breast cancer decreases inflammation. The increased consumption of fiber and polyphenols, readily available from a whole-food, plant-based diet, contributes to an overall increase in breast cancer survival.5,7,96–98 The benefits of lifestyle recommendations in the setting of breast cancer are summarized in Figure 3.
Figure 3.
Benefits of lifestyle recommendations for overall disease-free survival in breast cancer.
CVD = cardiovascular disease; IAP = intestinal alkaline phosphatase; SCFA = short-chain fatty acids.
Our gut microbiota are not only subject to our dietary intake but also are influenced by multiple prescription drugs and over-the-counter medications. The Western population seeks a cure for multiple conditions with a drug prescription. This has led to a nation that relies on a “pill for every ill,” and such an ideology may affect the gut microbiome (Appendix 2).
The Western world lives in a state of chronic inflammation largely due to the standard American diet and low physical activity, both of which are modifiable. The gut microbiota are a major conduit in the inflammatory process. Our immune system can only be challenged to a certain degree. When overcome by oxidative stressors and chronic inflammation, we may no longer be capable of responding to immunosuppressive conditions; the development of malignancies is the result.99
Our sedentary lifestyle, the link between the gut microbiota and obesity, is well known.5,100 Overweight has now become a pandemic with serious psychosocial ramifications.101 Obesity is also an inflammatory state that promotes immune responses and carcinogenesis.5,102,103 Carcinogenesis is fueled by the development of obesity because fatty tissues (particularly in the midsection) are largely responsible for the promotion and storage of numerous proteins that promote inflammation and estrogen production/storage, fueling most breast cancers.5,52
Diabetes, now a pandemic, is recognized not just as a metabolic condition but an inflammatory process as well.91,104,105 The interaction of diabetes, obesity, and carcinogenesis is well known.91,92 Understanding the role of the human gut microbiome in breast cancer survival, obesity, and the comorbidity of diabetes should be a focus of further research. Interventional strategies need to be identified starting with the promotion of a healthy lifestyle.
Emotional resilience (our response and recovery from a considerable life-altering event) and the ability to deal with such stressful issues (acute and chronic) are also influenced by the gut microbiota. In particular, depression is an underaddressed concern in women with a diagnosis of breast cancer.106–110 The gut microbiota play an important role in our ability to deal with emotional concerns because they are responsible for the production of multiple neurotransmitters, including γ-aminobutyric acid, norepinephrine, serotonin, and dopamine.111–113 The human gut is responsible for the production of nearly 90% of the neurologic regulators of chemicals that affect our emotions.
A whole-food, plant-based diet contributes to the favorable ratio of Firmicutes/Bacteroidetes. Fat and dairy consumption increases Bacteroidetes, whereas plant and fiber consumption increases Prevotella, Akkermansia, and other favorable bacteria.114–117 Most research to date has addressed only the bacterial population of the gut, and the investigation of the other microorganisms has received little attention. The complex interactions of these inhabitants have yet to be discovered but are certain to exist. The gut microbiota may be our most powerful endocrine regulator, because it affects nearly all distant organs and their appropriate functions.118,119 There exists a cross-talk between our immune system and the microbiota with the constant exchange of signals, serving as an alarm for immune system activation. The population of our bacterial occupants is affected by dietary choices. Our gut microbiota represent an individual genetic “fingerprint” of each of us, as unique individuals, with no 2 being alike (Sidebar 1: The Lifecycle of the Gut Microbiota).
Sidebar 1. The Lifecycle of the Gut Microbiota.
The gut microbiota is established at birth as the infant passes through the birth canal and becomes exposed to the vaginal flora. In those born by cesarean section, such an early exposure to the human microbiota is forfeited. Long-term consequences on the future health of such individuals is influenced by their microbiota habitants.1 Additionally, the breast-fed child is also exposed to additional bacteria, especially from colostrum, which is rich in bifidobacteria and further adds to the colonization of the newborn gut. Lack of early colonization of the young gut has been documented to result in a number of future chronic conditions.2 The inhabitants of the young gut microbiota appear to play a significant role in the establishment of early onset immunity.3,4 As the impact of the gut microbiota becomes further unraveled, we are starting to realize that as we age our microbiota “ages” as well; a concept not previously recognized. Each human cell has a natural life cycle with apoptosis (cell death) as their final destination. Currently, we hardly possess a full understanding of the complete life cycle of the nearly 100 trillion gut microorganisms that occupy our gastrointestinal tract. Despite lack of such information, we do understand that the gut microbiota is also subject to an aging process.5,6 Telomere length, which decreases with age, has been recognized as a bio-marker of aging and its association with the development of malignancies has been noted.5–9 Bacterial DNA also possess telomeres, which decrease in length with aging, resulting in a reduced lifespan.
Because of our newly acquired understanding of the importance of the gut microbiota, recent attention has also been directed to the importance of educating health care practitioners and patients regarding a healthy lifestyle. This must begin with a focus on nutrition education—the food sources that feed and promote a healthy gut microbiota.7 As with all living organisms, the health of the microbiota is determined by the quality of the nutrients consumed. There exists a significant lack of nutritional education in medical schools and post-graduate training. As such we are ill-prepared to provide basic and much needed nutritional advice for the prevention and reversal of chronic conditions. Recent publications have called attention to this important matter.7,10–12 Ultimately a healthy, high-fiber, whole food plant-based diet, combined with an active lifestyle, reduces the risks of comorbidities, improves health, and improves breast cancer survivorship.
Sidebar References
- 1.Odamaki T, Kato K, Sugahara H, et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiology. 2016;16:90. doi: 10.1186/s12866-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arboleya S, Watkins C, Stanton C, Ross RP. Gut Bifidobacteria Populations in Human Health and Aging. Front Microbiol. 2016 Aug 19;7:1204. doi: 10.3389/fmicb.2016.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urbaniak C, Burton JP, Reid G. Breast, Milk and Microbes: A Complex Relationship that Does Not End with Lactation. 2012 doi: 10.2217/WHE.12.23. [DOI] [PubMed] [Google Scholar]
- 4.Toscano M, DeGrandi R, et al. Role of the Human Breast Milk- Associated Microbiota on the Newborns Immune System: A Mini Review. Front Microbiol. 2017;8:2100. doi: 10.3389/fmicb.2017.02100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boccardi V, Paolisso G, Mecocci P. Nutrition and lifestyle in healthy aging: the telomerase challenge. Aging. 2016;8(1):12–15. doi: 10.18632/aging.100886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werner CM, Hecksteden A, Morsch A, et al. Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur Heart J. 2018;40(1):34–46. doi: 10.1093/eurheartj/ehy585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodai BI, Nakata TE, Wong WT, et al. Lifestyle Medicine: A Brief Review of Its Dramatic Impact on Health and Survival. The Permanente Journal. 2018;22:17–025. doi: 10.7812/TPP/17-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodai BI, Tuso P. Breast cancer survivorship: A comprehensive review of long-term medical issues and lifestyle recommendations. Perm J. 2015 Spring;19(2):48–79. doi: 10.7812/TPP/14-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telemerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013 Oct;14(11):1112–1120. doi: 10.1016/S1470-2045(13)70366-8. [DOI] [PubMed] [Google Scholar]
- 10.Abbasi J. Medical Students Around the World Poorly Trained in Nutrition. JAMA. Published online October 31, 2019. [DOI] [PubMed]
- 11.Devries S, Willett W, Bonow RO. Nutrition education in medical school, residency training, and practice. JAMA. 2019 Mar 21;321(14):1351–2. doi: 10.1001/jama.2019.1581. [DOI] [PubMed] [Google Scholar]
- 12.Rahman V. Time to revamp nutrition education for physicians. Perm J. 2019;23:19.052. doi: 10.1812/TPP/19.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
CONCLUSION
Breast cancer is now a chronic condition and is no longer an acute disease with the mediocre cure rates of decades ago. As such, there is time to intervene and provide healthy lifestyle recommendations that affect long-term, disease-free survival. Dietary recommendations are of major importance as they influence the gut microbiota, a major factor in increasing immunologic strength. Additionally, management of breast cancer survivors is now recognized as a new subspecialty. More health care programs are emerging that address long-term issues related to this ever-expanding population. Most individuals with breast cancer far exceed a 5-year disease-free survival. Dietary and lifestyle interventions can play a major role in furthering this success. Interventions include alterations that reshape the human gut microbiota through dietary recommendations and increasing the microbial diversity, which can substantially affect long-term health, particularly in breast cancer.
Much of the information published about the microbiome in well-respected, peer-reviewed journals reports contradictory conclusions, strengthening the need for further research. Regardless, there is evidence that the gut microbiome plays an important role in breast cancer survival. We remain in a place of ignorance regarding our understanding of not only the gut microbiota but also their complex interactions. A deeper understanding of the microbiota will result in the development of biomarkers for breast cancer and other cancers, perhaps allowing for even earlier diagnosis and a further increase in survival. It is time to implement changes in our lifestyle to further avert a fast-approaching disastrous course.
The composition of our microbiota depends on our lifestyle and may possibly become the most important factor in health maintenance. It is obvious that lifestyle patterns, both dietary and activity concerns, influence the gut microbiota’s flora in complex ways. Consumption of a poor diet and a sedentary lifestyle have a substantial impact on our gut microbiota. As physicians, it is our duty to promote and educate ourselves and our patients regarding the dramatic impact of lifestyle on overall health. Providing such guidance could well replace numerous future surgeries and prescriptions.
Acknowledgments
Kathleen Louden, ELS, of Louden Health Communications performed a primary copyedit.
Appendix 1. Adverse effects of currents treatments for breast cancer and the gut microbiome
Cardiovascular Concerns
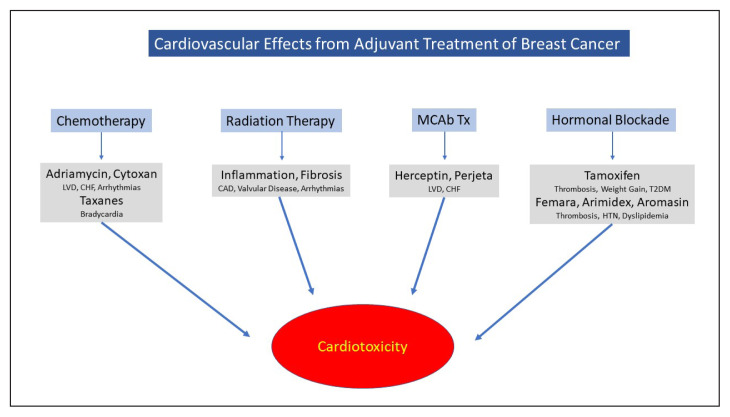
Cardiovascular disease (CVD) is the current leading cause of mortality in women in the United States.1–3 Although mortality rates for CVD have declined in recent years, this decline has waned.4 Recent information appears conflicting regarding long-term toxicities of current regimens employed to treat breast cancer.5 Survival following breast cancer has increased in the past decades, and as such, increase the risk of death from cardiovascular events simply due to aging. Cardiac events exceed the risk of death from breast cancer or its recurrence.1,2,6–8 Currently employed chemotherapeutic agents may result in future chronic cardiovascular complications.3,9,10 Targeted biologic therapies have assumed a prominent role in the treatment of breast cancer portend short- and long-term cardiotoxic effects.11–13 Radiation therapy, as an adjunctive treatment in breast conserving therapy (BCT), has been proven equally effective compared with modified radical mastectomy, and results in reduced recurrence and mortality.14–20 As radiotherapy is undergoing a rapid evolution, (i.e. alterations in schedules of administration, radiation exposure, dosage, length of therapy, etc), we anticipate potential cardiovascular effects will diminish. However, many current survivors had been subjected to more intensive and invasive radiotherapies years ago and, thus their cardiovascular adverse effects may only be peaking at 10–20 years post exposure. As such, cardiovascular adverse effects are more likely to occur in this aging population.21–26 (Figure 1)
Figure 1.
Cardiovascular health is influenced by the bacteria that reside in our gastrointestinal tract.27 The Standard American Diet may promote the development of CVD by influencing the growth and altering the ratios of good/bad bacteria.28 A major concern is the recognition that the consumption of red and processed meats results in an increase in all-cause mortality. Such foods are high in L-carnitine and lead to elevated serum levels of trimethylamine which are hepatically converted to trimethylamine oxide (TMAO) through the action of the gut microbiota. Red meat intake reduction may decrease TMAO formation, inhibiting atherogenesis by the down-regulation of the macrophagic uptake of oxidized endothelial cells. Additionally, this may minimize damage to the colonic endothelial barrier decreasing the development of the “leaky gut” syndrome.29,30 The association of TMAO production appears to be gut-microbiota dependent.27,31,32
Bone Health Concerns
Breast cancer survivors are at an increased risk of developing osteoporosis, as an adverse effect of current therapies, as well as increased longevity. As nearly 75% of breast cancers are estrogen driven, the use of aromatase inhibitors (AIs; anastrozole, letrozole and aromasin) in estrogen receptor positive breast cancers have been markedly effective in decreasing recurrence,6,33 however AIs can result in a substantial and often rapid decrease in bone mineral density and contribute to an increased risk of fracture.34,35 Osteoporosis, the destruction of the bony matrix, is a condition often unrecognized until a fracture event. Risk factors for the development of osteoporosis have been identified and aggressive interventions for prevention are needed.36–41 Multiple prescription drugs, especially for minimal indications such as gastrointestinal symptoms related to acid reflux and psychotropic drugs for depression also promote the development of osteoporosis. These, among other frequently prescribed medications, account for more than 100 million prescriptions annually.42–49 Recent studies have established an association between the human gut microbiota and bone metabolism.50–52 The exact mechanism of bone metabolism regulation by the gut microbiota is unclear, however multiple pathways have been proposed. These include influencing the immune and endocrine systems as well as potential interference with the absorption of calcium.50,53–55 A healthier microbiota could be beneficial in maintaining bone health of breast cancer survivors.
Hormonal Blockade Concerns
The majority of breast cancers are endogenous estrogen driven,56–62 a favorable characteristic associated with less aggressive disease. More than 30 years of data demonstrate the effect of hormonal blockade in decreasing death rates.9,63,64 Strong evidence suggests overall disease-free survival in patients adhering to long-term hormonal blockade. Unfortunately, a substantial number of patients who would benefit from such therapeutic interventions do not avail themselves of this proven recommendation. Rates of compliance to a 5-year regimen only approach 30% to 70%.65–67 Previous studies have demonstrated a higher recurrence rate and an increase in mortality in those discontinuing the recommended 5-year regimen.68,69 More recent studies suggest that a 10-year protocol may result in a further increase in overall disease-free survival.70–72 Adherence to a five-year regimen appears to be beyond many patient’s capabilities; asking them to adhere to twice that number of years, as previously recommended, presents an even further challenge.6 Lifestyle recommendations, particularly dietary changes, may be beneficial for patients who cannot tolerate the adverse-effects nor adhere to such long-term anti-estrogenic therapies.
The gut microbiome is intricately involved in the regulation of estrogen levels. A dysbiotic environment plays a crucial role in circulating estrogens. Within the nearly 100 trillion microbes that inhabit us, there exists a subset of these colonizers known to possess the genetic capability to influence estrogen metabolism, the “estrobolome.”73,74 Although systemic estrogen modulation is beyond the scope of this article, the reader is directed to several comprehensive reviews of this issue.6,74,75
Thromboembolic Concerns
Cancer, regardless of tissue origin, is a prothrombotic state,76–79 well recognized since the German physician Virchow originally identified the association of cancer and inflammation in 1863.80–82 The second leading cause of death in patients diagnosed with a malignancy is, in fact, a thromboembolic event.78,83 Patients with a cancer diagnosis have a 4- to7-fold increased risk of a thromboembolic event compared with those without cancer.84,85 An important adverse effect of anti-estrogenic therapy is an increase in the risk of thromboembolic events. Tamoxifen, the earliest and most frequently prescribed hormonal blocker increases the risk of such an episode by1% to 2%.86–90 The human gut microbiome directly increases the potential of a thromboembolic event through its role in the generation of trimethylamine and in the up-regulation of platelet production, increasing the risk of thrombosis. The association between TMAO (oxidized trimethylamine) production and the consumption of foods high in L-carnitine (red meat), and choline (poultry, fish and dairy) is well known. TMAO production is easily influenced by modifying our diet decreasing thrombogenesis.6,30,33,91,92
Emotional Concerns
Depression and anxiety, so prevalent in the modern world, are major risk factors affecting health. Depression is projected to be the second-largest health care burden within the next few years.93 Depression is particularly relevant in the setting of breast cancer as it is often unrecognized, under addressed, and inadequately treated.6,30,94,95 Depression has also been associated with an increase in CVD.96 Undiagnosed, depression can diminish treatment adherence, resulting in inferior outcomes.97 Intensified screening, earlier interventions, and “cancer-specific depression”counseling has become available.98,99 The role of the gut microbiome is now recognized as a contributor to depression and to overall mental health.100 This is the result of the gut microbiota’s influences on the levels of circulating chemicals that directly influence mood and affect. The role of diet, as it affects depression and anxiety is an area of intense research as current evidence supports such an association.101–103
References
- 1.Robertson RM. Women and cardiovascular disease: the risks of misperception and the need for action. Circulation. 2001 May 15;103(19):2318–20. doi: 10.1161/01.CIR.103.19.2318. [DOI] [PubMed] [Google Scholar]
- 2.Canto JG, Kiefe CI. Age-specific analysis of breast cancer versus heart disease mortality in women. Am J Cardiol. 2014 Jan 15;113(2):410–1. doi: 10.1016/j.amjcard.2013.08.055. [DOI] [PubMed] [Google Scholar]
- 3.Mehta LS, Watson KE, Barac S, et al. Cardiovascular Disease and Breast Cancer: Where these Entities Intersect A Scientific Statement from the American Heart Association. Circulation. 2018;137 doi: 10.1161/Cir.00000000000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sidney S, Quesenberry CP, Jr, Jaffe MG, et al. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol. 2016 Aug 1;1(5):594–9. doi: 10.1001/jamacardio.2016.1326. [DOI] [PubMed] [Google Scholar]
- 5.Weberspals J, Jansen L, Muller OJ, et al. Long-term heart-specific mortality among 347476 breast cancer patients treated with radiotherapy or chemotherapy: a registry-based cohort study. European Heart Journal. p. ehy167. [DOI] [PubMed]
- 6.Bodai BI, Tuso P. Breast cancer survivorship: A comprehensive review of long-term medical issues and lifestyle recommendations. Perm J. 2015 Spring;19(2):48–79. doi: 10.7812/TPP/14-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz PN, Beck ML, Stava C, Vassilopoulou-Sellin R. Health profiles in 5836 long-term cancer survivors. Int J Cancer. 2003 Apr 20;104(4):488–95. doi: 10.1002/ijc.10981. [DOI] [PubMed] [Google Scholar]
- 8.Yeh ET, Tong AT, Lenihan DJ, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004 Jun 29;109(25):3122–31. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]
- 9.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005 May 14–20;365(9472):1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 10.Bird BR, Swain SM. Cardiac toxicity in breast cancer survivors: review of potential cardiac problems. Clin Cancer Res. 2008 Jan 1;14(1):14–24. doi: 10.1158/1078-0432.CCR-07-1033. [DOI] [PubMed] [Google Scholar]
- 11.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002 Feb 1;20(3):719–26. doi: 10.1200/JCO.20.3.719. [DOI] [PubMed] [Google Scholar]
- 12.Barroso-Sousa R, Santana IA, Testa L, de Melo Gagliato D, Mano MS. Biological therapies in breast cancer: common toxicities and management strategies. Breast. 2013 Dec;22(6):1009–18. doi: 10.1016/j.breast.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Gabric ID. Cardiotoxicity to biological cancer therapy. Cardiologia Croatia. 2017;12(1) Vlejara. https://doi.org/10.158361ccar2017.16. [Google Scholar]
- 14.Nattinger AB, Gottlieb MS, Veum J, Yahnke D, Goodwin JS. Geographic variation in the use of breast-conserving treatment for breast cancer. N Engl J Med. 1992 Apr 23;326(17):1102–7. doi: 10.1056/NEJM199204233261702. [DOI] [PubMed] [Google Scholar]
- 15.Albain KS, Green SR, Lichter AS, et al. Influence of patient characteristics, socioeconomic factors, geography, and systemic risk on the use of breast-sparing treatment in women enrolled in adjuvant breast cancer studies: an analysis of two intergroup trials. J Clin Oncol. 1996 Nov;14(11):3009–17. doi: 10.1200/JCO.1996.14.11.3009. [DOI] [PubMed] [Google Scholar]
- 16.Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998 Feb;16(2):441–52. doi: 10.1200/JCO.1998.16.2.441. [DOI] [PubMed] [Google Scholar]
- 17.Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomized trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 2000 May 20;355(9217):1757–70. doi: 10.1016/S0140-6736(00)02263-7. [DOI] [PubMed] [Google Scholar]
- 18.Rutqvist LE, Rose C, Cavallin-Ståhl E. A systematic overview of radiation therapy effects in breast cancer. Acta Oncol. 2003;42(5–6):532–45. doi: 10.1080/02841860310014444. [DOI] [PubMed] [Google Scholar]
- 19.Clarke M, Collins R, Darby S, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005 Dec 17;366(9503):2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 20.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomized trials. Lancet. 2011 Nov 12;378(9804):1707–16. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Recht A. Which breast cancer patients should really worry about radiation-induced heart disease—and how much? J Clin Oncol. 2006 Sep 1;24(25):4058–61. doi: 10.1200/JCO.2006.07.7909. [DOI] [PubMed] [Google Scholar]
- 22.Borger JH, Hooning MJ, Boersma LJ, et al. Cardiotoxic effects of tangential breast irradiation in early breast cancer patients: the role of irradiated heart volume. Int J Radiat Oncol Biol Phys. 2007 Nov 15;69(4):1131–8. doi: 10.1016/j.ijrobp.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 23.Doyle JJ, Neugut AI, Jacobson JS, et al. Radiation therapy, cardiac risk factors, and cardiac toxicity in early-stage breast cancer patients. Int J Radiat Oncol Biol Phys. 2007 May 1;68(1):82–93. doi: 10.1016/j.ijrobp.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Luini A, Gatti G, Zurrida S, et al. The evolution of the conservative approach to breast cancer. Breast. 2007 Apr;16(2):120–9. doi: 10.1016/j.breast.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Witteles RM. Radiation therapy for breast cancer: buyer beware. J Am Coll Cardiol. 2011 Jan 25;57(4):453–4. doi: 10.1016/j.jacc.2010.08.637. [DOI] [PubMed] [Google Scholar]
- 26.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013 Mar 14;368(11):987–98. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 27.Koeth RA, Want Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imamura F, O’Connor L, Ye Z, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015 Jul 21;351:h3576. doi: 10.1136/bmj.h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuso P, Ismail MH, Ha BP, et al. Nutritional update for physicians: plant-based diets. Perm J. 2013 Sprint;17(2):61–6. doi: 10.7812/TPP/12-085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuso P, Stoll SR, Li WW. A Plant-based diet, atherogenesis, and coronary artery disease prevention. Perm J. 2015 Winter;19(1):62–7. doi: 10.7812/TPP/14-036.Epnb2014Nov24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbasi J. TMAO and heart disease: The New Red Meat Risk? JAMA. doi: 10.1001/jama.2019.3910. Published online. May 23, 2019. [DOI] [PubMed] [Google Scholar]
- 32.Wu-Kai W, Chen C-G, Liu P-Y, Paynood S, et al. Identification of TMAO – producer phenotype and host-diet-gut dysbiosis by carnitine challenge test in human and germ-free mice. Gut. 2018 doi: 10.1139/gutjnl-2018-317155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bodai BI, Nakata TE, Wong WT, et al. Lifestyle Medicine: A Brief Review of Its Dramatic Impact on Health and Survival. The Permanente Journal. 2018;22:17–025. doi: 10.7812/TPP/17-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabaglio M, Sun Z, Price KN, et al. BIG 1–98 Collaborative and International Breast Cancer Study Groups Bone fractures among postmenopausal patients with endocrine-responsive early breast cancer treated with 5 years of letrozole or tamoxifen in the BIG 1–98 trial. Ann Oncol. 2009 Sep;20(9):1489–98. doi: 10.1093/annonc/mdp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coleman RE, Banks LM, Girgis SI, et al. Reversal of skeletal effects of endocrine treatments in the Intergroup Exemestane Study. Breast Cancer Res Treat. 2010 Nov;124(1):153–61. doi: 10.1007/s10549-010-1121-7. [DOI] [PubMed] [Google Scholar]
- 36.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005 Dec;115(2):3318–25. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ralston SH. Genetic determinants of osteoporosis. Curr Opin Rheumatol. 2005 Jul;17(4):475–9. doi: 10.1097/01.bor.0000166385.62851.92. [DOI] [PubMed] [Google Scholar]
- 38.Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. 2005 Feb;194(2 Suppl):S3–11. doi: 10.1016/j.ajog.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 39.Chen Z, Maricic M, Bassford TL, et al. Fracture risk among breast cancer survivors: results from the Women’s Health Initiative Observational Study. Arch Intern Med. 2005 Mar 14;165(5):552–8. doi: 10.1001/archinte.165.5.552. [DOI] [PubMed] [Google Scholar]
- 40.US Preventive Services Task Force. Screening for osteoporosis: US preventive services task force recommendation statement. Ann Intern Med. 2011 Mar 1;154(5):356–64. doi: 10.7326/0003-4819-154-5-201103010-00307. [DOI] [PubMed] [Google Scholar]
- 41.Body JJ. Increased fracture rate in women with breast cancer: a review of the hidden risk. BMC Cancer. 2011 Aug 29;11:384. doi: 10.1186/1471-2407-11-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanis JA, McCloskey EV. Risk factors in osteoporosis. Maturitas. 1998 Nov 16;30(3):229–33. doi: 10.1016/S0378-5122(98)00090-5. [DOI] [PubMed] [Google Scholar]
- 43.Williams JW, Jr, Mulrow CD, Chiquette E, Noël PH, Aguilar C, Cornell J. A systematic review of newer pharmacotherapies for depression in adults: evidence report summary. Ann Intern Med. 2000 May 2;132(9):743–56. doi: 10.7326/0003-4819-132-9-200005020-00011. [DOI] [PubMed] [Google Scholar]
- 44.Bliziotes MM, Eshleman AJ, Zhang XW, Wiren KM. Neurotransmitter action in osteoblasts: expression of a functional system for serotonin receptor activation and reuptake. Bone. 2001 Nov;29(5):477–86. doi: 10.1016/S8756-3282(01)00593-2. [DOI] [PubMed] [Google Scholar]
- 45.Battaglino R, Fu J, Späte U, et al. Serotonin regulates osteoclast differentiation through its transporter. J Bone Miner Res. 2004 Sep;19(9):1420–31. doi: 10.1359/JBMR.040606. [DOI] [PubMed] [Google Scholar]
- 46.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006 Dec 27;296(24):2947–53. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 47.Roux C, Briot K, Gossec L, et al. Increase in vertebral fracture risk in postmenopausal women using omeprazole. Calcif Tissue Int. 2009 Jan;84(1):13–9. doi: 10.1007/s00223-008-9188-4. [DOI] [PubMed] [Google Scholar]
- 48.Targownik LE, Leslie WD, Davison KS, et al. CaMos Research Group The relationship between proton pump inhibitor use and longitudinal change in bone mineral density: a population-based study from the Canadian Multicentre Osteoporosis Study (CaMos) Am J Gastroenterol. 2012 Sep;107(9):1361–9. doi: 10.1038/ajg.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rizzoli R, Cooper C, Reginster JY, et al. Antidepressant medications and osteoporosis. Bone. 2012 Sep;51(3):606–13. doi: 10.1016/j.bone.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 50.Xu X, Jia X, Mo L, et al. Intestinal Microbiota: a potential target for the treatment of postmenopausal osteoporosis. Bone Research. 2017;5:17046. doi: 10.1038/boneres.2017..46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan J, Herzog JW, Tsang K, et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci USA. 2016;113:E7554–e7563. doi: 10.1073/pnas.1607235113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guss JD, Horsfield MW, Fontenele FF, et al. Alterations to the gut microbiome impair bone strength and tissue material properties. J Bone Miner Res. 2017;32:1343–1353. doi: 10.1002/jbmr.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zupan J, Jeras M, Marc J. Osteoimmunology and the influence of pro-inflammatory cytokines on osteoclasts. Biochem Med (Zagreb) 2013;23:43–63. doi: 10.11613/BM.2013.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geuking MB, Koller Y, Rupp S, et al. The interplay between the gut microbiota and the immune system. Gut Microbes. 2014;5:411–418. doi: 10.4161/gmic.29330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crotti TN, Dharmapatni AA, Alias E, et al. Osteoimmunology: major and costimulatory pathway expression associated with chronic inflammatory induced bone loss. J Immunol Res. 2015;2015 doi: 10.1155/2015/281287. 281287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998 Nov 26;339(22):1609–18. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 57.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999 May;17(5):1474–81. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 58.Fabian CJ, Kimler BF. Selective estrogen-receptor modulators for primary prevention of breast cancer. J Clin Oncol. 2005 Mar 10;23(8):1644–55. doi: 10.1200/JCO.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 59.Kennecke HF, Ellard S, O’Reilly S, Gelmon KA. New guidelines for treatment of early hormone-positive breast cancer with tamoxifen and aromatase inhibitors. B C Med J. 2006 Apr;48(3):121–6. [Google Scholar]
- 60.Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9(1):R6. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Montemurro F, Aglietta M. Hormone receptor-positive early breast cancer: controversies in the use of adjuvant chemotherapy. Endocr Relat Cancer. 2009 Dec;16(4):1091–102. doi: 10.1677/ERC-09-0033. [DOI] [PubMed] [Google Scholar]
- 62.Lumachi F, Brunello A, Maruzzo M, Basso U, Basso SM. Treatment of estrogen receptor-positive breast cancer. Cure Med Chem. 2013;20(5):596–604. doi: 10.2174/092986713804999303. [DOI] [PubMed] [Google Scholar]
- 63.Tamoxifen for early breast cancer: an overview of the randomized trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998 May 16;351(9114):1451–67. doi: 10.1016/S0140-6736(97)11423-4. [DOI] [PubMed] [Google Scholar]
- 64.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,679 early-stage breast cancer patients. J Clin Oncol. 2010 Sep 20;28(27):4120–8. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003 Feb 15;21(4):602–6. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 66.Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012 Jul;134(2):459–78. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2014 Jul 20;32(21):2255–69. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geiger AM, Thwin SS, Lash TL, et al. Recurrences and second primary breast cancers in older women with initial early-stage disease. Cancer. 2007 Mar 1;109(5):966–74. doi: 10.1002/cncr.22472. [DOI] [PubMed] [Google Scholar]
- 69.Yood MU, Owusu C, Buist DS, et al. Mortality impact on less-than-standard therapy in older breast cancer patients. J Am Coll Surg. 2008 Jan;206(1):66–75. doi: 10.1016/j.jamcollsurg.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 70.Cuzick J, Sestak L, Baum M, et al. ATAC/LATTE Investigators Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10 year analysis of the ATAC trial. Lancet Oncol. 2010 Dec;11(12):1135–41. doi: 10.1016/S1470-2045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- 71.Davies C, Pan H, Godwin J, et al. Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013 Mar 9;381(9869):805–16. doi: 10.1016/S0140-6736(12)61963-1. DOI: http://dx.doi.org/10.1016/S0140-6736(12)61963-1. Erratum in: Lancet 2013 Mar 9; 381(9869): 804. DOI: http://dx.doi.org/10.1016/S0140-6736(13)60252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gray RG, Rea D, Handley K, et al. aTTOM Collaborative Group aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6953 women with early breast cancer [ASCP Annual Meeting Abstracts] J Clin Oncol. 2013;31(15_suppl May 20):5. [Google Scholar]
- 73.Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe. 2011;10(4):324–35. doi: 10.1016/j.chom.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kwa M, Plottel CS, Blaser MJ, et al. The Intestinal Microbiome and Estrogen Receptor-Positive Female Breast Cancer. 2016 doi: 10.1093/jnci/djw029. JNCI108(8):djw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: Physiological and clinical implications. maturitas. 2017;103:45–53. doi: 10.1016/j.maturitas.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 76.Turken S, Siris E, Seldin D, Flaster E, Hyman G, Lindsay R. Effects of tamoxifen on spinal bone density in women with breast cancer. J Natl Cancer Inst. 1989 Jul 19;81(14):1086–8. doi: 10.1093/jnci/81.14.1086. [DOI] [PubMed] [Google Scholar]
- 77.Love RR, Mazess RB, Barden HS, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992 Mar 26;326(13):852–6. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 78.Letai A, Kuter DJ. Cancer, coagulation, and anticoagulation. Oncologist. 1999;4(6):443–9. [PubMed] [Google Scholar]
- 79.Vehmanen L, Elomaa I, Blomqvist C, Saarto T. Tamoxifen treatment after adjuvant chemotherapy has opposite effects on bone mineral density in premenopausal patients depending on menstrual status. J Clin Oncol. 2006 Feb 1;24(4):675–80. doi: 10.1200/JCO.2005.02.3515. [DOI] [PubMed] [Google Scholar]
- 80.Green KB, Silverstein RL. Hypercoagulability in cancer. Hematol Oncol Clin North Am. 1996 Apr;10(2):449–530. doi: 10.1016/S0889-8588(05)70349-X. . [PubMed] 388. [DOI] [PubMed] [Google Scholar]
- 81.Rickles FR, Levine MN. Venous thromboembolism in malignancy and malignancy in venous thromboembolism. Haemostasis. 1998;28(Suppl 3):43–9. doi: 10.1159/000022404. [DOI] [PubMed] [Google Scholar]
- 82.Kumar DR, Hanlin E, Glurich I, Mazza JJ, Yale SH. Virchow’s contribution to the understanding of thrombosis and cellular biology. Clin Med Res. 2010 Dec;8(3–4):168–72. doi: 10.3121/cmr.2009.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Donati MB. Cancer and thrombosis. Haemostasis. 1994 Mar-Apr;24(2):128–31. doi: 10.1159/000217092. [DOI] [PubMed] [Google Scholar]
- 84.Falanga A, Zacharski L. Deep vein thrombosis in cancer: the scale of the problem and approaches to management. Ann Oncol. 2005 May;16(5):696–701. doi: 10.1093/annonc/mdi165. [DOI] [PubMed] [Google Scholar]
- 85.Mandalà M, Tondini C. Adjuvant therapy in breast cancer and venous thromboembolism. Thromb Res. 2012 Oct;130(Suppl 1):S66–70. doi: 10.1016/j.thromres.2012.08.280. [DOI] [PubMed] [Google Scholar]
- 86.Cummings FJ, Gray R, Davis TE, et al. Tamoxifen versus placebo: double-blind adjuvant trial in elderly women with stage II breast cancer. NCI Monogr. 1986;(1):119–23. [PubMed] [Google Scholar]
- 87.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998 Sep 16;90(18):1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 88.Braithwaite RS, Chlebowski RT, Lau J, George S, Hess R, Col NF. Meta-analysis of vascular and neoplastic events associated with tamoxifen. J Gen Intern Med. 2003 Nov;18(11):937–47. doi: 10.1046/j.1525-1497.2003.20724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vogel VG, Costantino JP, Wickerham DL, et al. National Surgical Adjuvant Breast and Bowel Project (NSABP) Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006 Jun 21;295(23):2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 90.Vogelvang TE, van der Mooren MJ, Mijatovic V, Kenemans P. Emerging selective estrogen receptor modulators: special focus on effects on coronary heart disease in postmenopausal women. Drugs. 2006;66(2):191–221. doi: 10.2165/00003495-200666020-00005. [DOI] [PubMed] [Google Scholar]
- 91.Zhu W, Gregory JC, Org E, et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperactivity and Thrombosis Risk. Cell. 2017;165(1):111–24. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu W, Want Z, Tang WH, et al. Gut Microbe-Generated Trimethylamine N-Oxide From Dietary Choline is Prothrombotic in Subjects. Circulation. 2017;135:1167–73. doi: 10.1161/CIRCUALTIONAHA.116.025338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat Med. 1990 Nov;4(11):1241–3. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 94.Fann JR, Thomas-Rich AM, Katon WJ, et al. Major depression after breast cancer: a review of epidemiology and treatment. Gen Hosp Psychiatry. 2008 Mar-Apr;30(2):112–26. doi: 10.1016/j.genhosppsych.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 95.Mitchell AJ, Chan M, Bhatti H, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011 Feb;12(2):160–74. doi: 10.1016/S1470-2045(11)70002-X. [DOI] [PubMed] [Google Scholar]
- 96.Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: A clinical review. Eur Heart J. 2014 Jun 1;35(21):1365–72. doi: 10.1093/eurheartj/eht462. [DOI] [PubMed] [Google Scholar]
- 97.Reich M, Lesur A, Perdrizet-Chevallier C. Depression, quality of life and breast cancer: a review of the literature. Breast Cancer Res Treat. 2008 Jul;110(1):9–17. doi: 10.1007/s10549-007-9706-5. [DOI] [PubMed] [Google Scholar]
- 98.Strong V, Waters R, Hibberd C, et al. Management of depression for people with cancer (SMaRT oncology 1): a randomised trial. Lancet. 2008 Jul 5;372(9632):40–8. doi: 10.1016/S0140-6736(08)60991-5. [DOI] [PubMed] [Google Scholar]
- 99.Fann JR, Berry DL, Wolpin S, et al. Depression screening using the Patient Health Questionnaire-9 administered on a touch screen computer. Psychooncology. 2009 Jan;18(1):14–22. doi: 10.1002/pon.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clarke G, Stilling RM, Kennedy PJ, et al. Minireview: Gut microbiota: the neglected endocrine organ. Mol Endocrinol. 2014 Aug;28(8):1221–38. doi: 10.1210/me.2014-1108. Epub 2014 Jun 3. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jacka FN, Pasco JA, Mykldefun A, et al. Association of Western and traditional diets with depression and anxiety in women. AmJ psychiatry. 2010;169(3):305–11. doi: 10.1176/appi.ajp.2009.09060881. [DOI] [PubMed] [Google Scholar]
- 102.Lassale C, Batty GD, Baghdadli P, et al. Healthy dietary indices and risks of depressive outcomes. Mol Psychiatry. 2018 doi: 10.1038/s41380-018-0237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berk M, Jacka FN. Diet and Depression-From Confirmation to Implementation. JAMA. 2019;321(9):842–3. doi: 10.1001/jama.2019.0273. [DOI] [PubMed] [Google Scholar]
Appendix 2. The impact of common pharmacologic interventions on the gut microbiota and breast cancer
Antibiotics
Recent concerns have been raised that the ongoing, indiscriminate use of antibiotics may result in an increase in the incidence and fatality of breast cancer.1 Lifesaving antibiotics are one of the most effective therapeutics since their initial identification by Ehrlich and Fleming in the early 20th century.2 Undoubtedly, saving countless lives, these drugs may also have become a significant threat to our future. Overprescribing, a common daily practice, disrupts the normal flora of the gut microbiota and may contribute to disease.1,3 In 2015 nearly 300 million antibiotic prescriptions were dispensed in the US; nearly one-third lacked a proper indication.4,5 Antibiotics, by destroying pathogens also disrupt healthy bacteria and contribute to a state of dysbiosis.6 Furthermore, resistance to antibiotics develops as bacterial genes evolve and the growth of multidrug resistant pathogens emerge.7 In addition to overprescribing, antibiotics enter our diet through meat and dairy products that contain high levels of antibiotics used prophylactically in animals. In fact, most antibiotics produced in the US (18.4 million pounds) are utilized by the agricultural industry.8 Antibiotics, given in early childhood, also have a profound influence on the development of future obesity.9 Antibiotics have a definitive impact on the gut microbiota, although their exact interference requires further investigation.10,11 Antibiotic use and its relation to breast cancer development has been postulated, as these drugs may disrupt the phytochemical metabolic pathways that influence the development and progression of breast cancer.1
Proton-Pump Inhibitors
Nearly 150 million prescriptions for proton-pump inhibitors are written annually in the US to treat gastrointestinal complaints, in particular, reflux.12,13 The majority of such prescriptions are proton pump inhibitors, which inhibit the gastric delivery of acids.14 Anti-reflux medications, first introduced in the 1980s, contribute to decreasing the diversity of the gut microbiota. Many of these medications are “over the counter” and are used for prolonged periods, without demonstrable benefit, and beyond professional control.13 Bacteria originating in the oral cavity may be altered by these medications and contribute to dysbiosis in the distal gastrointestinal tract.14–16 Such drugs also interfere with breast cancer survival as they affect bone metabolism leading to the development of osteoporosis and fragility.17,18 As our understanding of the gut microbiome expands, the influence of commonly prescribed medications and the role they play in the development of disease progression deserves attention.19,20
Antidepressants
Antidepressants are yet another overprescribed medication in the US. Nearly 10% of our population consume such drugs on a regular basis.17,21,22 These prescriptions have increased nearly 65% since 2010 and women are twice as likely to be prescribed such medications.23 Each year millions of prescriptions are written; more than one-third are inappropriately dispensed, without evidence of efficacy. Mental health issues, beyond depression, and their relationship to the gut microbiome, are receiving increased attention.24 Evidence is accumulating that the gut microbiota communicates with the central nervous system influencing human behavior. The gut microbiota not only synthesizes, but also respond to neurotransmitters that affect our mental health.25,26
Polypharmacy
Consideration must also be given to the issue of polypharmacy—the simultaneous prescription of multiple drugs which is increasing in the aging population.27 It has long been known that as the number of drugs prescribed rises, so do potential interactions, often negating or potentiating effects of one on the other or even resulting in adverse events. Polypharmacy needs to be recognized as a growing problem as many malignancies are also age related.27–29 As the gut microbiota becomes further characterized, newer targeted therapies may be developed that affect overall disease-free breast cancer survival and long-term cure.30 Multiple other drugs affect the microbiotic ecology which are beyond the scope of the current manuscript but are reviewed in-depth and are available.31,32
References
- 1.Velicer CM, Heckbert, Lampe JW, et al. JAMA. 2004;291(7):827–35. doi: 10.1001/jama.291.7.827. [DOI] [PubMed] [Google Scholar]
- 2.Aminov R. A Brief History of the Antibiotic Era: Lessons Learned and Challenges for the Future. Front Microbiol. 2010 doi: 10.389/fmicb.2010.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies J, Davies J. Origins and Evolution of Antibiotic Residence. Microbiota Mol Biol Rev. 2010;74(3):417–33. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Outpatient Antibiotic Prescriptions – United States. 2014. cdc.gov/antibiotic-use/community/pdfs/annual report-2015.pdf.
- 5.Fleming-Dutra K, et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits. JAMA. 2018;315(17):1864–73. doi: 10.1001/jama.2016.4151. [DOI] [PubMed] [Google Scholar]
- 6.Becuttini S, Taur Y, Pamer EG. Antibiotic Induced Changes in the Intestinal Microbiota and Disease Trends. Molec Med. 2016;22(6):458–78. doi: 10.1016/j.molmed.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perera M, Perera I. Fire in the Forest: Adverse Effects of Antibiotics on the Healthy Human Gut Microbiome. Int J Med Rev. 2018;5(1):19–26. [Google Scholar]
- 8.Trends in U.S. Antibiotic Use. 2018 pewtrusts.org/antibiotic-resistance-project. [Google Scholar]
- 9.Bailey LC, Forrest CB, Zhang P, et al. JAMA Pediatr. 2014;168(11):1063–9. doi: 10.1001/jamapediatrics.2014.1539. [DOI] [PubMed] [Google Scholar]
- 10.Francino MR. Antibiotics and the Human Gut Microbiome: Dysbiosis and Accumulation of Resistance. Front Microbiol. 2015:1543. doi: 10.3389/fmicb.2015.01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ianiro G, Tilq H, Gasbarrini A. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut. 2016;65:1906–15. doi: 10.1136/gutjnl-2016-312297. [DOI] [PubMed] [Google Scholar]
- 12.Targownik LE, Leslie WD, Davison KS, et al. CaMos Research Group The relationship between proton pump inhibitor use and longitudinal change in bone mineral density: a population-based study from the Canadian Multicentre Osteoporosis Study (CaMos) Am J Gastroenterol. 2012 Sep;107(9):1361–9. doi: 10.1038/ajg.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melgar S, Nieuwdorp M. Are Proton Pump Inhibitors Affecting Intestinal Microbiota health? Gastroenterolgy. 2015;149:848–63. doi: 10.1053/j.gastro.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 14.Imhann F, Bonder MJ, Vila AV. Proton pump inhibitors affect the microbiome. Gut. 2016;65:740–8. doi: 10.1136/gutjni-2015-33310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reveles KR, Ryan CN, Chan L, Cosimi RA, Haynes WL. Proton pump inhibitor use associated with changes in gut microbiota composition. Gut. 2018 Jul;67(7):1369–1370. doi: 10.1136/gutjnl-2017-315306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takagi T, Naito Y, Inoue R, et al. The influence of long-term use of proton pump inhibitors on the gut microbiota: an age-sex-matched case-controlled study. J Clin BiochemNutr. 2018;62(1):100–5. doi: 10.3164/jcbn.17-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodai BI, Tuso P. Breast cancer survivorship: A comprehensive review of long-term medical issues and lifestyle recommendations. Perm J. 2015 Spring;19(2):48–79. doi: 10.7812/TPP/14-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaezi MF, yang XY, Howden CW. Complications of proton pump inhibitor therapy. Gastroenterology. 2017;152:35–48. doi: 10.1053/j.gastro.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 19.Lynch SV, Pederson O. the human intestinal microbiome in health and in disease. N Engl J Med. 2016;375:2369–79. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 20.Takanishi T, naito Y, Inoue R. The influence to long-term use of proton pump inhibitors on the gut microbiota: an age-sex-matched-case-controlled study. J Con Biochem Nutr. 2018;62(7):100–5. doi: 10.3164/jcbn.17-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams JW, Jr, Mulrow CD, Chiquette E, Noël PH, Aguilar C, Cornell J. A systematic review of newer pharmacotherapies for depression in adults: evidence report summary. Ann Intern Med. 2000 May 2;132(9):743–56. doi: 10.7326/0003-4819-132-9-200005020-00011. [DOI] [PubMed] [Google Scholar]
- 22.Diem SJ, Blackwell TL, Stone KL, et al. Use of antidepressants and rates of hip bone loss in older women: the study of osteoporotic fractures. Arch Intern Med. 2007 Jun 25;167(12):1240–5. doi: 10.1001/archinte.167.12.1240. [DOI] [PubMed] [Google Scholar]
- 23.Winerman L. By the numbers: antidepressants use on the rise. Am Psych Assoc. 2017;10(45):120. [Google Scholar]
- 24.Valles-Colomer M, Falony G, Darzi Y, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol. 2019 Apr;4(4):623–632. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- 25.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behavior. Nat Rev Neurosci. 2012;13:701–12. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 26.Galland L. The gut Microbiome and the Brain. J med food. 2014;17(12):1261.72. doi: 10.1089/jmf.2014.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maher RL, Hanlon JT, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1) doi: 10.1517/14740338.2013.827660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panebianco C, Andriulli A, Panzienza V. Pharmacomicrobiomics: exploiting the drug-microbiota interactions in anticancer therapies. Microbiome. 2018;6:92. doi: 10.1186/s40168-018-0483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rea D, Coppola G, Palma G, Barbieri A, Luciano A, Del Prete P, Rossetti S, Berretta M, Facchini G, Perdonà S, Turco MC, Arra C. Microbiota effects on cancer: from risks to therapies. Oncotarget. 2018 Apr 3;9(25):17915–17927. doi: 10.18632/oncotarget.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: A new clinical frontier. Gut. 2016 Feb;65(2):330–9. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bastard QL, AI-Ghalith GA, Gregorre M, et al. systematic review: human gut dysbiosis induced by non-antibiotic prescription medication. Ailment Pharmacol Ther. 2018;47:332–45. doi: 10.111/apt.14451. [DOI] [PubMed] [Google Scholar]
- 32.Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623–8. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.Breast cancer facts and figures 2017–2018. Atlanta GA: American Cancer Society; 2017. [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017 Jan;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990–2015. A systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017 Apr 1;3(4):524–48. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Angelis R, Tavilla A, Verdecchia A, et al. Breast cancer survivors in the United States: geographic variability and time trends, 2005–2015. Cancer. 2009 May 1;115(9):1954–66. doi: 10.1002/cncr.24217. [DOI] [PubMed] [Google Scholar]
- 5.Bodai BI, Nakata TE, Wong WT, et al. Lifestyle Medicine: A Brief Review of Its Dramatic Impact on Health and Survival. The Permanente Journal. 2018;22:17–025. doi: 10.7812/TPP/17-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford ES, Bergmann MM, Kröger J, Schienkiewitz A, Weikert C, Boeing H. Healthy living is the best revenge: findings from the European Prospective Investigation Into Cancer and Nutrition-Potsdam study. Arch Intern Med. 2009 Aug 10;169(15):1355–62. doi: 10.1001/archinternmed.2009.237. [DOI] [PubMed] [Google Scholar]
- 7.Bodai BI, Tuso P. Breast cancer survivorship: A comprehensive review of long-term medical issues and lifestyle recommendations. Perm J. 2015 Spring;19(2):48–79. doi: 10.7812/TPP/14-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hullar MAJ, Burnett-Hartman AN, Lampe JW. Gut Microbes, Diet, and Cancer. Cancer treatment and research. 2014;159:377–399. doi: 10.1007/978-3-642-38007-5_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang B, Yao M, Lv L, Ling Z, Li L. The human microbiota in health and disease. Engineering. 2017 Feb;3(1):71–82. [Google Scholar]
- 10.Turnbaugh PJ, Ley RE, Hamady M, et al. The human genome project. Nature. 2007;449:804–10. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeisel SH, Warrier M. Trimethylamine N-Oxide, The Microbiome, and Heart and Kidney Disease. Ann Rev Nutr. 2017;37:157–81. doi: 10.1146/annurev-nutr-071816-064732. Epub2017 Jul 17. [DOI] [PubMed] [Google Scholar]
- 12.Chen KL, Madak-Erdogan Z. Estrogen and Microbiota Crosstalk: Should We pay Attention? Trends in Endocrinology and Metabolism. 2016;27(11):752–5. doi: 10.1016/j.tem.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Kaaks R. Nutrition, hormones and breast cancer. Is insulin the missing link? Cancer Causes Control. 1996;7:605–25. doi: 10.1007/BF00051703. [DOI] [PubMed] [Google Scholar]
- 14.Peairs KS, Barone BB, Snyder CF, et al. Diabetes mellitus and breast cancer outcomes: A systematic review and meta-analysis. J Clin Oncol. 2011;29:40–6. doi: 10.1200/JCO.2009.27.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erickson K, Patterson RE, Flatt SW, et al. Clinically defined type 2 diabetes and prognosis in early-stage breast cancer. J Clin Oncol. 2011;29:40–6. doi: 10.1200/JCO.2010.29.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Censi A, Gennri A. Insulin breast cancer connections confirmatory data set the stage for better care. J Clin Oncol. 2011;29(1):7–10. doi: 10.1200/JCO.2010.32.3022. [DOI] [PubMed] [Google Scholar]
- 17.Wani B, Aziz SA, Ganaie MA, Mir MH. Metabolic syndrome and breast cancer risk. Indian J Med Paediatr Oncol. 2017;38:434–9. doi: 10.4103/ijmpo.ijmpo_168_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belkaid Y, Hand TW. Role of the Microbiota in Immunity and Inflammation. Cell. 2014;157:121–41. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology; human gut microbes associated with obesity. Nature. 2006b;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 20.Xuan C, Shamonki JM, Chung A, DiNome ML, Chung M, Sieling PA, et al. Microbial Dysbiosis Is Associated with Human Breast Cancer. PLoS ONE. 2014;9(1):e83744. doi: 10.1371/journal.pone.0083744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Research. 2011;22:292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castellarin M, Warren R, Freeman JD, Dreolini L, Krzywinski M, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Research. 2012;22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams S. Estrobolome disparities may lead to developing biomarkers that could mitigate cancer risk. J Natl Cancer Inst. 2016;108(8):djw130. doi: 10.1093/jnci/djw130. [DOI] [Google Scholar]
- 24.Weingart SN, Brown E, Bach PB, et al. NCCN task force report: oral chemotherapy. J Natl Compr Canc Netw. 2008 Mar;6(Suppl 3):S1–14. [PubMed] [Google Scholar]
- 25.Bedard PL, Siu LL. Tilting the balance of dose modification for oral anticancer drugs? J Clin Oncol. 2014 May 20;32(15):1537–9. doi: 10.1200/JCO.2014.55.2372. [DOI] [PubMed] [Google Scholar]
- 26.Cancer stat facts: Female breast cancer [Internet] National Cancer Institute Surveillance, Epidemiology, and End Results Program; [cited 2017 Jun 17]. https://seer.cancer.gov/statfacts/html/breast.html. [Google Scholar]
- 27.Sinicrope FA, Dannenberg AJ. Obesity and breast cancer prognosis: Weight of the evidence. J Clin Oncol. 2011 Jan 1;29(1):4–7. doi: 10.1200/JCO.2010.32.1752. [DOI] [PubMed] [Google Scholar]
- 28.Jarde T, Perrier S, Vasson MP, et al. Molecular mechanisms of leptin and adiponectin in breast cancer. Euro J Cancer. 2011;42:33–43. doi: 10.1016/j.ejca.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Hauner D, Hauner H. Metabolic Syndrome and Breast Cancer: Is There a Link? Breast Care. 2014;9:277–81. doi: 10.1159/000365951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ode E. Historical perspectives of the metabolic syndrome. Dermatol. 2018;36(1):3–8. doi: 10.1016/j.clindermatol.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Gezen G, Roach EC, Kizilarslanogly MC, et al. Metabolic syndrome and breast cancer: an overview. JBUON. 2012;17(2):223–9. [PubMed] [Google Scholar]
- 32.Komaroff AL. The microbiome and risk for obesity and diabetes. JAMA. 2017 Jan 24;317(4):355–356. doi: 10.1001/jama.2016.20099. [DOI] [PubMed] [Google Scholar]
- 33.Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28(3):753–65. doi: 10.1007/s12032-010-9536-x.Epub2010Apr22. [DOI] [PubMed] [Google Scholar]
- 34.Friedenreich CM, Orenstein MR. Physical activity in cancer prevention: etiologic evidence and biological mechanisms. J Nutr. 2002;132(11 Suppl):3456S–3464S. doi: 10.1093/jn/132.11.3456S. [DOI] [PubMed] [Google Scholar]
- 35.Giroir BP, Wright DW. Physical activity guidelines for health and prosperity in the United States. JAMA. 2018;320(19):1971–2. doi: 10.1001/jama.2018.16998. [DOI] [PubMed] [Google Scholar]
- 36.Patterson R, McNamara E, Tanoio M, et al. Sedentary behavior and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol. 2018;33(9):811–829. doi: 10.1007/s10654-018-0380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang L, Cao C, Kantor E, et al. Trends in Sedentary Behavior Among the US Population, 2001–2016. JAMA. 2019;321(16):1587–97. doi: 10.001/jama.2019.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulsen JA, Placek TS, Carter SJ, et al. Gut microbiota composition associated with alterations in cardiorespiratory fitness and psychosocial outcomes among breast cancer survivors. Support Cancer Care. 2017;25(5):1563–70. doi: 10.1007/s00520-016-3568.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pierce JP, Stefanick ML, Flatt SW, et al. Greater survival after breast cancer in physically active women with high vegetable/fruit intake regardless of obesity. J Clin Oncol. 2007;25(17):2345/51. doi: 10.1200//jco2006.08.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antoni MH, Lehman JM, Kilbourn KM, et al. Cognitive-Behavioral Stress Management Intervention Decreases the Prevalence of Depression and Enhances Benefit Finding Among Women Under Treatment for Early-Stage Breast Cancer. Health Psych. 2001;20(1):20–32. doi: 10.1037//0278-6133.20.1.20. [DOI] [PubMed] [Google Scholar]
- 41.Cramer H, Lauche R, Paul A, et al. Mindfulness-based stress reduction for breast cancer – a systemic review and meta-analysis. Curr Oncol. 2012;19(5):e343–52. doi: 10.3747/co.19.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marmot M. Diet, Cancer, and NCD prevention. Lancet Oncol. 2018;19:863–4. doi: 10.1016/S1470-2045(18)30382-6. [DOI] [PubMed] [Google Scholar]
- 43.Ornish D, Magbauna MJ, Weidner G, et al. Changes in prostate gene expression in men undergoing an intensive nutrition and lifestyle intervention. Proc Natl Acad Sci USA. 2008 Jun 17;105(24):8369–74. doi: 10.1073/pnas.0803080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ornish D. Intensive lifestyle changes and health reform. Lancet Oncol. 2009 Jul;10(7):638–9. doi: 10.1016/S1470-2045(09)70175-5. [DOI] [PubMed] [Google Scholar]
- 45.Ellsworth DL, Croft DT, Weyandt J, et al. Intensive cardiovascular risk reduction induces sustainable changes in expression of genes and pathways important to vascular function. Circulation: Cardiovascular Genetics. 2014 Apr 7;2:151–60. doi: 10.1161/circgenetics.113.000121. [DOI] [PubMed] [Google Scholar]
- 46.Tojo R, Suarez A, Clemente MG, et al. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol. 2014;20:15163–15176. doi: 10.3748/wjg.v20.i41.15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koliada A, Syzenko G, Mosieko V, et al. Association between body mass index and Firmicutes/Bacteriodes ratio in an adult Ukranian Population. BMC Microbiology. 2017;17:120. doi: 10.1186/512866-017-1027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zitvogel L, Galluzzi L, Viaud S, et al. Cancer and the gut microbiota: an unexpected link. Sci Transl Med. 2015;7(271):271ps1. doi: 10.1126/scitranslmed.3010473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duncan SH, Lobley GE, Holtrop G, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes. 2008;32:1720–1724. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- 50.Jandhyala SM, Talukday R, Subramanyam C, et al. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016;14(8):e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016 Apr 20;8(1):42. doi: 10.1186/s13073-016-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graf D, DiCagno R, Fak F, et al. Contribution of the diet to the composition of the human gut microbiota. Microbial Ecology in Health and Disease. 2015;26:26164. doi: 10.3402/mehd.v26.26164. Doi.org/10.3402/mehd.v26.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol. 1996;4:430–435. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 56.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–30. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006 Dec 21;444(7122):1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 58.Ford ES, Bergmann MM, Boeing H, Li C, Capewell S. Healthy lifestyle behaviors and all-cause mortality among adults in the United States. Prev Med. 2012 Jul;55(1):23–7. doi: 10.1016/j.ypmed.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hyman MA, Ornish D, Roizen M. Lifestyle medicine: treating the causes of diseases. Altern Ther Health Med. 2009 Nov-Dec;15(6):12–4. [PubMed] [Google Scholar]
- 60.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khan S, Shukla S, Sinha S, et al. Role of adipokines and cytokines in obesity-associated breast cancer: therapeutic targets. Cytokine Growth Factor Rev. 2013;24:503–13. doi: 10.1016/j.cytogfr.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 62.Kwa M, Plottel CS, Blaser MJ, et al. The Intestinal Microbiome and Estrogen Receptor-Positive Female Breast Cancer. 2016 doi: 10.1093/jnci/djw029. JNCI108(8):djw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skar V, Skar AG, Midtvedt T, et al. Beta-glucuronidase-producing bacteria in bile from the common bile duct in patients treated with endoscopic papillotomy for gallstone disease. Scand J Gastroenterol. 1986 Mar;21(2):253–6. doi: 10.3109/00365528609034656. [DOI] [PubMed] [Google Scholar]
- 64.den Besten, Gijs, van Eunene K, Groen AK, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–40. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shapira I, Sutlan K, Lee A, et al. Evolving Concepts: How Diet and the Intestinal Microbiome Act as modulators of Breast Malignancy. ISRN Oncology. 2013 doi: 10.1155/2013/693920. Article ID 693920. doi.org/10.1155.2013/693920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dabek M, McCrae SI, Stevens VJ, et al. Distribution of beta-glucuronidase and beta-glucuronidase activity of the beta-glucuronide gene gus in human colonic bacteria. FEMS Microbiol Ecol. 2008;66:487–95. doi: 10.1111/j.1574-6941.2008.00520.x. [DOI] [PubMed] [Google Scholar]
- 67.Fernandez M, Reina-Perez I, Strogra JM, et al. Breast Cancer and Its Relationship with the Microbiota. Int J Environ Res Public Health. 2018;15:1747. doi: 10.3390/ijerph15081747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vital M, Chuang Howe A, Tiedje JM. Revealing the Bacterial Butyrate Synthesis Pathways by Analyzing (Meta)genomic Data. mBio. 2014 Apr;5(2):e00889–14. doi: 10.1128/mBio.00889-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang J, Tan Q, Fu Q, et al. Gastrointestinal microbiome and breast cancer: Correlations, mechanisms and potential implications. Breast Cancer. 2017;24:220–8. doi: 10.1007/s12282-016-0734-z. [DOI] [PubMed] [Google Scholar]
- 70.Luu TH, Michel C, Bard JM, et al. Intestinal Proportion of Blautia sp. is Associated with Clinical Stage and Histoprognostic Grade in Patients with Early-Stage Breast Cancer. Nutr Cancer. 2017 Feb-Mar;69(2):267–275. doi: 10.1080/01635581.2017.1263750. [DOI] [PubMed] [Google Scholar]
- 71.Fawley J, Gourlay DM. Intestinal alkaline phosphatase: A summary of its role in clinical disease. J Surg Res. 2016;202(1):225–34. doi: 10.1016/j.jss.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ashida H, Ogawa M, Kim M, et al. Bacteria and host interactions in the gut epithelial barrier. Nat Chem Biol. 2011;8:36–45. doi: 10.1038/nchembio.741. [DOI] [PubMed] [Google Scholar]
- 73.Pabst O. New concepts in the generation and functions of IgA. Nat Rev Immunol. 2012;12:821–832. doi: 10.1038/nri3322. [DOI] [PubMed] [Google Scholar]
- 74.Rubin R. High-fiber diet might protect against range of conditions. JAMA. 2019 May 7;321(17):1653–1655. doi: 10.1001/jama.2019.2539. [DOI] [PubMed] [Google Scholar]
- 75.Schwabe R, Jobin C. The microbiome and cancer. Nat Tev Cancer. 2013;13:800–12. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vogtmann E, Goedert JJ. Epidemiologic studies of the human microbiome and cancer. Br J Cancer. 2016;114:237–42. doi: 10.1038/bjc.2015.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanna C, Rosso C, Marlett M, et al. Non-alcoholic fatty liver disease and extra-hepatic cancers. Int J Mol Sci. 2016;17:717. doi: 10.3390/ijms17050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Younossi ZM, Keening AB, Abdelatit D, et al. Global epidemiology of no-alcohol fatty liver disease meta-analytic assessment of prevalence, incidence and outcomes. Hepatology. 2016;68:73–83. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 79.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:547–64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 80.Chacko KR, Reinus J. Extrahepatic complications of nonalcoholic fatty liver disease. Clin Liver Dis. 2016;20:387–401. doi: 10.1016/j.cld.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 81.Rosanto V, Bosetti F, Abagnato CA, et al. Metabolic syndrome and the risk of breast cancer in postmenopausal women. Ann Oncol. 2011;22:2687–92. doi: 10.1093/annonc/mdr025. [DOI] [PubMed] [Google Scholar]
- 82.Esposito K, Chiodini P, Coaloa P, et al. Metabolic syndrome and the risk of cancer. Diabetes Care. 2012;35:2402–11. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berrino F, Villarini A, Traina A, et al. Metabolic Syndrome and breast cancer prognosis. Breast Cancer Res Treat. 2014;147:159–65. doi: 10.1007/s10549-014-3076-6. [DOI] [PubMed] [Google Scholar]
- 84.Brown JC, Harhay MO, Harhay MN. Nonalcoholic fatty liver disease and mortality among cancer survivors. Cancer Epidemiol. 2017;48:104–9. doi: 10.1016/j.canep.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nseir W, Abu-Rahmeh Z, Tsipis A, et al. Relationship between Non-Alcoholic Fatty Liver Disease and Breast Cancer. Isr Med Assoc J. 2017 Apr;19(4):242–245. [PubMed] [Google Scholar]
- 86.Larsen N, Vogensen FK, van den Berg FW, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010 Feb 5;5(2):e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Windey K, De Preter V, Verbeke K. Relevance of protein fermentation to gut health. Mol Nutr Food Res. 2012 Jan;56(1):184–96. doi: 10.1002/mnfr.201100542. [DOI] [PubMed] [Google Scholar]
- 88.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013 Apr 25;368(17):1575–84. doi: 10.1056/nejmoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rajendhran J, Shanker M, Dinakaren V, Rathinavel A, Gunasekaran P. Contrasting circulating microbiome in cardiovascular disease patients and healthy individuals. Int J Cardiol. 2013 Oct 12;168(5):5118–20. doi: 10.1016/j.ijcard.2013.07.232. [DOI] [PubMed] [Google Scholar]
- 90.Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: A new clinical frontier. Gut. 2016 Feb;65(2):330–9. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goldfine AB, Shoelson SE. Therapeutic approaches targeting inflammation for diabetes and associated cardiovascular risk. J Clin Invest. 2017 Jan 3;127(1):83–93. doi: 10.1172/JCI88884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Balkwill F, Mantorani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9225):539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 93.Heidland A, Klassen A, Rutkowski P, et al. The contribution of Rudolf Virchow to the concept of inflammation. J Nephrol. 2006;(Suppl 10):5102–9. [PubMed] [Google Scholar]
- 94.Landskron G, De la Fuente M, Thuwajit P, et al. Chronic Inflammation and Cytokines in the Tumor Microenvironment. J Immunol Research. 2014 doi: 10.1155/2014/149185. 149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hunter P. The inflammation theory of disease. EMBO Reports. 2012;11:968–70. doi: 10.1038/embor.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tuso P, Ismail MH, Ha BP, et al. Nutritional update for physicians: Plant-based diets. Perm J. 2013 Spring;17(2):61–6. doi: 10.7812/TPP/12-085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rowland IR. Toxicological implications of the normal microflora. In: Tannock GW, editor. Medical importance of the normal microflora. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1999. [Google Scholar]
- 98.Roopchard DE, Carmody RN, Kuhn P, et al. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and attenuate High Fat diet-Induced metabolic Syndrome. Diabetes. 2015;64:2547–58. doi: 10.2337/db14-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reuter S, Subash CG, Chaturuedi MM, et al. Oxidative Stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010;49(11):1603–16. doi: 10.1016/J.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wolf KJ, Lorenz RG. Gut microbiota and obesity. Curr Obes Rep. 2012 Mar 1;1(1):1–8. doi: 10.1007/s13679-011-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Apovian CM. The obesity epidemic-understanding the disease and the treatment. N Engl J Med. 2016;374:177–9. doi: 10.1056/NEJMe1514957. [DOI] [PubMed] [Google Scholar]
- 102.Cancello R, Clément K. Review article: Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG. 2006 Oct;113(10):1141–7. doi: 10.1111/j.1471-0528.2006.01004.x. [DOI] [PubMed] [Google Scholar]
- 103.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Ann Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 104.Klonoff DC. The increasing incidence of diabetes in the 21st century. J Diabetes Sci Technol. 2009 Jan;3(1):1–2. doi: 10.1177/193229680900300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005 May;115(5):1111–9. doi: 10.1172/jci25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mitchell AJ, Chan M, Bhatti H, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011 Feb;12(2):160–74. doi: 10.1016/S1470-2045(11)70002-X. [DOI] [PubMed] [Google Scholar]
- 107.Fann JR, Berry DL, Wolpin S, et al. Depression screening using the Patient Health Questionnaire-9 administered on a touch screen computer. Psychooncology. 2009 Jan;18(1):14–22. doi: 10.1002/pon.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Berk M, Jacka FN. Diet and Depression- From Confirmation to Implementation. JAMA. 2019;321(9):842–3. doi: 10.1001/jama.2019.0273. [DOI] [PubMed] [Google Scholar]
- 109.Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 110.Dash S, Clarke G, Berk M, Jacka FN. The gut microbiome and diet in psychiatry: focus on depression. Curr Opin Psychiatry. 2015 Jan;28(1):1–6. doi: 10.1097/YCO.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 111.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behavior. Nat Rev Neurosci. 2012;13:701–12. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 112.Gillard L. The Gut Microbiome and the Brain. J Med Food. 2014;17(12):1261–72. doi: 10.1089/jmf.2014.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Valles-Colomer M, Falony G, Darzi Y, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol. 2019 Apr;4(4):623–632. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- 114.Dao MC, Everard A, Aron-Wisnewsky J. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 115.Anhê FF, Pilon G, Roy D, et al. Triggering Akkermansia with dietary polyphenols: A new weapon to combat the metabolic syndrome? [published correction appears in Gut Microbes. doi: 10.1136/gutjnl-2014-307142] Gut Microbes. 2016;7(2):146–153. doi: 10.1080/19490976.2016.1142036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cani PD, de Vos WM. Next-generation beneficial microbes: The case of Akkermanisa muciniphila. Front Microbiol. 2018;8:1765. doi: 10.3389/fmicb.2017.01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Geerlings SY, Kostopoulos I, de Vos WM, Belzer C. Akkermansia muciniphila in the Human Gastrointestinal Tract: When, Where, and How? Microorganisms. 2018 Jul 23;6(3) doi: 10.3390/microorganisms6030075. pii: E75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Evans JM, Morris LS, Marchesi JR. The gut microbiome- the role of a virtual organ in the endocrinology of the host. J Endocrinol. 2013;218:R37–R47. doi: 10.1530/JOE-13-0131. [DOI] [PubMed] [Google Scholar]
- 119.Clarke G, Stilling RM, Kennedy PJ, et al. Minireview: Gut microbiota: the neglected endocrine organ. Mol Endocrinol. 2014 Aug;28(8):1221–38. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]