Abstract
Antimicrobial resistance (AMR) is a growing threat to global public health, and the overuse of antibiotics in animals has been identified as a major risk factor. With high levels of international trade and direct connectivity to the aquatic environment, shrimp aquaculture may play a role in global AMR dissemination. The vast majority of shrimp production occurs in low‐ and middle‐income countries, where antibiotic quality and usage is widely unregulated, and where the integration of aquaculture with family livelihoods offers many opportunities for human, animal and environmental bacteria to come into close contact. Furthermore, in shrimp growing areas, untreated waste is often directly eliminated into local water sources. These risks are very different to many other major internationally‐traded aquaculture commodities, such as salmon, which is produced in higher income countries where there are greater levels of regulation and well‐established management practices. Assessing the true scale of the risk of AMR dissemination in the shrimp industry is a considerable challenge, not least because obtaining reliable data on antibiotic usage is very difficult. Combating the risks associated with AMR dissemination is also challenging due to the increasing trend towards intensification and its associated disease burden, and because many farmers currently have no alternatives to antibiotics for preventing crop failure. In this review, we critically assess the potential risks the shrimp industry poses to AMR dissemination. We also discuss some of the possible risk mitigation strategies that could be considered by the shrimp industry as it strives for a more sustainable future in production.
Keywords: antibiotics, antimicrobial resistance, aquaculture, international trade, low and middle income countries, shrimp
Introduction
Antimicrobial resistance (AMR) is the term used to describe microbial organisms that can resist the effects of drugs and chemicals designed to kill them. It is now widely regarded one of the greatest risks to human and animal health, and the current rate of spread is such that by 2050 an estimated 10 million people globally will die from resistant infections, with an associated economic cost of £60 trillion (O'Neill 2016). For many years, research into AMR was principally focused on human health, but more recently, much interest has been directed to the agricultural and environmental sectors, due to the intrinsic interlinkages between humans, animals and the environment (the ‘One Health’ notion). Furthermore, almost 70% of total antibiotic use is in livestock and this consumption is projected to rise by 67% by 2030 (Van Boeckel et al. 2015). This is a major concern since the overuse of antibiotics has been identified as the single most important factor driving the rise in resistant infections (CDC, 2013). Although antibiotic usage in humans in high‐income countries appears to be gradually reducing (Klein et al. 2018), it remains high in some food production sectors, most notably poultry and swine (Van Boeckel et al. 2015). In low and middle income countries (LMICs), antibiotic consumption in both humans and animals is increasing (Van Boeckel et al. 2015; Klein et al. 2018), although precise data is often difficult to obtain since usage is widely unregulated and indiscriminate, with antibiotics freely available ‘over the counter’ and little capacity for enforcing regulations and surveillance. Controlling contagion, through improving sanitation, access to clean water and governance, has also been shown as key to controlling AMR dissemination (Collignon et al. 2018), and since these factors are frequently associated with LMICs, this further emphasizes the significant risks of antibiotic usage in animal production in these countries.
Aquaculture (the farming of aquatic organisms) is considered one of the most sustainable sources of animal protein and is the fastest growing food sector, yet in order to meet global demand, production must further expand by 50% by 2050 (FAO, 2014). Currently, aquaculture accounts for around 10% of all animal protein consumed globally, with this figure expected to increase by 50% by 2030 (The World Bank, 2013). In 2016, 93% of aquaculture production took place in LMICs (89% in Asia; FAO, 2016a), and although much of the produce is consumed domestically, there is a huge international export market. As in agriculture, aquaculture is thought to have high levels of antibiotic use, although exact figures for much of the industry are hard to confidently establish due to the lack of surveillance. In terms of risk to AMR dissemination, aquaculture is of particular concern in terms of the propensity for emergence, persistence and transmission within the aqueous environment, which is already known to be an important reservoir of clinically relevant antimicrobial resistance genes (Marti et al. 2014). In addition, the relative lack of infrastructure in terms of waste treatment in many LMICs means that any treatments (including antibiotics) and microbial matter (including resistant bacteria) from ponds are often released directly into the aquatic environment. Transmission to people may also be enhanced through direct contact with pond water and livestock; an estimated 18 million people work within the primary aquaculture industry globally (FAO, 2016b). Despite these significant risk factors, aquaculture has thus far received relatively little attention in terms of infectious disease and AMR research.
In this review, we evaluate current understanding of the role of the shrimp aquaculture industry in the emergence and dissemination of AMR. Many other forms of aquaculture, notably finfish, share many of the same risks for AMR (Henriksson et al. 2017), but we focus on shrimp aquaculture (used collectively to refer to the farming of shrimp and prawn species) for the following reasons. Firstly, shrimp has a large, growing, global market and is traded extensively around the world. Secondly, the farming of crustaceans brings somewhat different risks and challenges compared to fish species, which in the context of AMR mainly relates to the fact that shrimp do not have an acquired immune system and can therefore be more susceptible to pathogens, often leading to a greater need for antimicrobial treatments (Smith et al. 2003; Hauton & Smith 2007). Thirdly, the more primitive immune system in shrimp does not respond to vaccination, which in the fin fish industry has paid great dividends in terms of reducing disease and antimicrobial usage (Sommerset et al. 2005; Gudding & Van Muiswinkel 2013). Finally, most shrimp production (87% in 2016) occurs in Asia (FAO, 2016a) and this is an area that has been identified as posing a very significant risk to the global burden of AMR (Chereau et al. 2017). In this review, we assess the existing evidence for the emergence and dissemination of AMR in this sector, and review the drivers behind antimicrobial use in shrimp aquaculture worldwide. We also discuss possible risk mitigation strategies, aimed at promoting the prudent use of antibiotics and/or reducing the necessity for them, in order to increase the sustainability of the shrimp industry and minimize its contribution to the growing global AMR problem.
The global shrimp industry
In 2016, 67% of global shrimp production was farmed and 33% wild‐captured (FAO, 2016a). Marine shrimp farming makes up the vast majority of production, and mainly involves two penaeid shrimp species, Penaeus vannamei (Whiteleg shrimp) and Penaeus monodon (Giant tiger prawn). The shrimp industry began to develop on a large scale in coastal areas of South East Asia in the 1970s, and although Peneaus monodon was the preferred species for many years, its susceptibility to disease has led to a preference for farming Litopenaeus vannamei, which has constituted the largest shrimp industry growth. Freshwater prawn farming has also been gradually increasing, and here the two main species are Macrobrachium rosenbergii (Giant river prawn) Macrobrachium nipponense (Oriental river prawn; Fig. S1). Shrimp farming has been a traditional way of life for many years, through trapping larvae from rivers or seas and cultivating them. A large proportion of shrimp farmers across the globe remain as small holders, with extensive pond systems (large ponds, with low animal densities), and shrimp farming is often integrated with other types of crops and livestock production, with a view to making the most efficient usage of the land and minimising the economic risks associated with monoculture and disease (Fig. 1). However, in many established shrimp‐producing countries, including Thailand, Vietnam and China, intensive shrimp farming is increasing. In many cases, this includes the use of enclosed recirculating aquaculture systems (RAS) as they require less water and have improved biosecurity in order to reduce disease risk. Intensification of shrimp production has largely been possible due to the adoption of new technologies, such as improved feed formulations and the hatchery production of specific pathogen‐free (SPF) post‐larvae from breeding adults (broodstock; Kumar & Engle 2016). Broodstock are typically obtained from the wild but a reduction in numbers and increased prevalence of disease in wild stock has led to the development of broodstock domestication in some countries, which commonly offer SPF animals (mainly Litopenaeus vannamei) that are certified as free from a number of common diseases.
Figure 1.
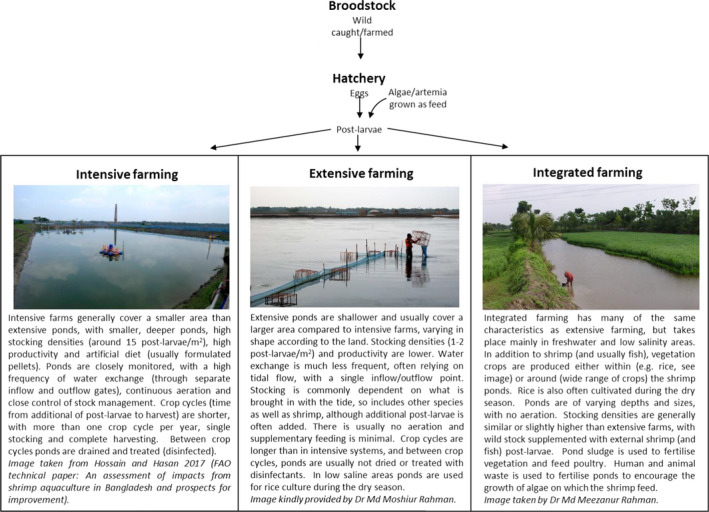
Shrimp farming. Typical shrimp farming process, showing characteristics of the different types of production: intensive, extensive and integrated. Images are of ponds in Bangladesh (credits shown).
One of the main disadvantages of captive broodstock breeding programmes is that, without very careful management, a reduced genetic diversity can result, increasing the risks associated with disease susceptibility. Disease is a significant constraint on the development and sustainability of shrimp and prawn culture and has had devastating impacts on entire geographical regions of the industry. Shrimp are affected by a range of bacterial, viral, fungal and parasitic pathogens and until recently the highest profile problems have been caused by viral diseases, notably White Spot Syndrome Virus (WSSV); cumulative losses to this pathogen alone were estimated in 2012 to be more than USD 6 billion (Lightner et al. 2012). Bacterial diseases are also a major problem. For example, it is now recognized that acute hepatopancreatic necrosis disease (AHPND), is associated with strains of bacterial Vibrio species carrying an insect toxin plasmid (Han et al. 2015; Restrepo et al. 2018). Infections cause up to 100% mortality in post‐larvae shrimp, and losses due to this pathogen were estimated at more than USD 1 billion in Asia at the end of 2013 (FAO, 2013). This increasing disease burden not only threatens the national GDP of these LMICs but also has a huge impact on the livelihoods of those who depend on subsistence farming. To illustrate this, an infection in Vietnam in 2006 resulted in the death of 50% of the 2 billion shrimp post‐larvae cultivated, and this affected an estimated 14,000 households (Anh et al. 2010).
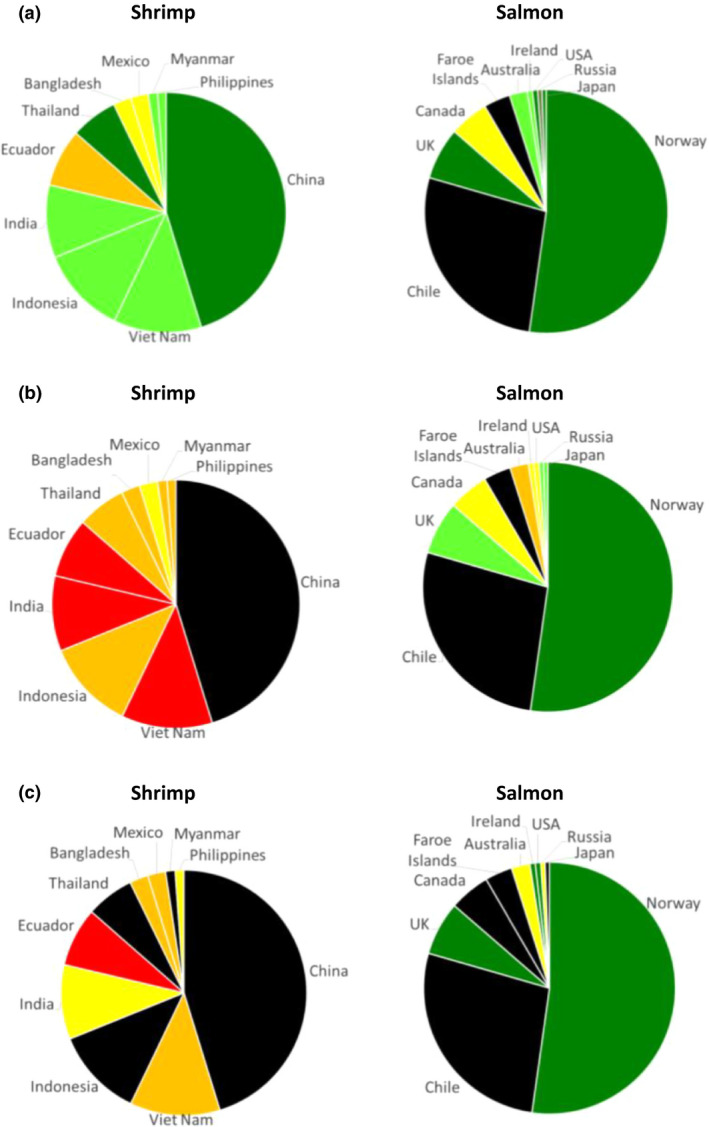
Despite the high disease burden, shrimp was the most highly traded food commodity by value for many years, until it was overtaken in 2016 by salmon (Fig. 2a). In this year, these two products alone represented 32.5% of the aquaculture export industry (USD 23.5 billion exports for salmon and USD 22.9 billion for shrimp; FAO, 2016a). Although the international market value of these commodities is very similar, the nature of these industries differs greatly. Salmon are exported and traded between high‐income countries (HICs), whereas the majority of shrimp production takes place in LMICs and is exported to HICs (Figs 2b and S2). The growth of both the shrimp and salmon industries has been largely supported by the intensification of production, however, the increased levels of disease associated with this intensification has placed an ever‐growing importance on the containment of both known and emerging pathogens. Between 1987 and 2013, the Norwegian aquaculture industry (which is predominantly salmon production) increased from 300,000 tonnes to 1.2 million tonnes; the use of vaccination against major pathogens and the availability of water of high quality is considered to have supported the containment of the disease through this intensification, since antibiotic use was reduced by 99% during this same period (Norwegian Ministry of Health and Care Services, 2015). In contrast, the reduction in the rate of growth of the shrimp industry is largely due to problems with disease, and antimicrobial agents (e.g. disinfectants, fungicides) have become an integral part of many shrimp farming‐operating procedures. Improved water quality, better hygiene and stricter farm biosecurity measures would undoubtedly go a long way to addressing the problems with disease in shrimp production, and therefore reduce the need for antibiotic usage. Levels of sanitation infrastructure and governance in the main shrimp‐producing countries are also very low (Figs 3a–c and S3), and, without the option of vaccination, there is limited scope for prophylaxis. Due to the nature of shrimp farming, most shrimp‐producing countries also have a warmer climate that lends itself to greater pathogenic burden and an increased number of vectors such as insects and migratory birds, which have been shown to play an important role in both disease and AMR dissemination (Fig. 3d; Ahlstrom et al. 2018; Onwugamba et al. 2018).
Figure 2.
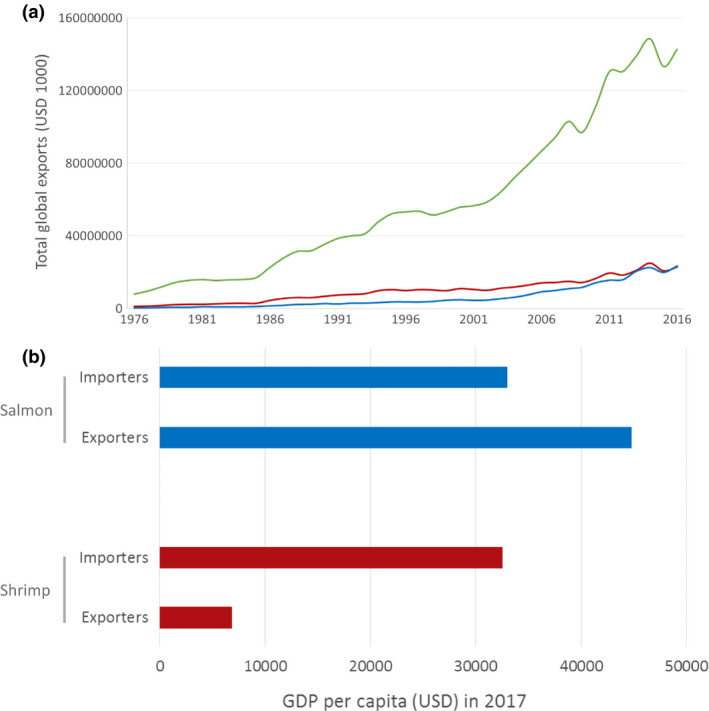
The global shrimp industry. (a) Shrimp and salmon are the highest internationally‐traded seafood commodities by value. In 2016, salmon overtook shrimp as the highest value traded commodity. Data, obtained from the FAO's FishStat J Global Fishery and Aquaculture Commodity Statistics Dataset, shows the total global market value of exports over time. (b) Weighted average GDP per capita of the top 10 shrimp and salmon exporting and importing countries. Weighted averages were calculated by taking the sum of imports/exports (data from the FAO's FishStat J Global Fishery and Aquaculture Commodity Statistics Dataset) for the top 10 countries and calculating each country's fraction of this. This fraction was then multiplied by the GDP per capita for that country (World Bank databank; 2015 data) to obtain the weighted average score. Total aquaculture (green); Shrimp (red); Salmon (blue).
Figure 3.
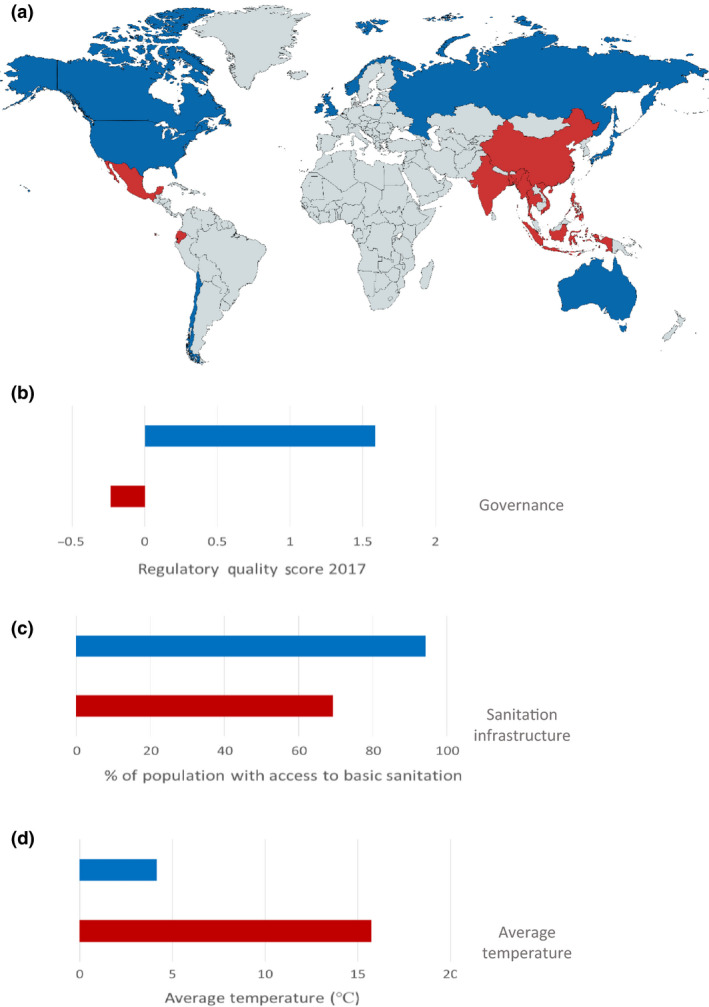
Selected comparisons between the top 10 shrimp and salmon producing countries. (a) Global geographical distributions of the top 10 shrimp (red) and salmon (blue) producing countries. Data obtained from the FAO's FishStat J Global Fishery and Aquaculture Production Statistics Dataset were used to produce the map with www.mapchart.net. (b–d) Differences between shrimp (red) and salmon (blue) producing countries in terms of (b) governance (taken as mean Regulatory Quality Score in 2017; regulatory quality is one of the six dimensions of governance used in the World Governance Indicators Project: http://info.worldbank.org/governance/WGI/#home), (c) sanitation infrastructure (World Bank Databank 2015 data: People using at least basic sanitation services (% of population)) and (d) climate: average monthly temperature between 1991–2015 (World Bank Climate Change Knowledge Portal).
Antibiotic usage in shrimp aquaculture
A wide range of antibiotics and other antimicrobials, including heavy metals, fungicides and antiparasitics, are used in shrimp aquaculture, with many reports describing the use of clinically relevant antibiotics, particularly in hatcheries (Holmstrom et al. 2003; Uddin & Kader 2006; Thuy et al. 2011; Mostafa Shamsuzzaman & Kumar Biswas 2012; Ali et al. 2016; Chi et al. 2017; Hinchliffe et al. 2018). However, as is the case in most LMICs, accurate antimicrobial sales and usage data are difficult to obtain since antibiotics are freely available over the counter without a veterinary prescription, and can be of varying quality (Tran et al. 2018). Many shrimp farmers do not have easy access to professionals or facilities for accurate disease diagnosis, and obtain advice on treatments from farm supply shops, neighbouring farmers, government representatives, non‐governmental organizations such as Worldfish (https://www.worldfishcenter.org/) or drug manufacturers/vendors, who are also known to provide financial incentives (Pham et al. 2015). Collating information on antibiotic sales and usage is also difficult, due to considerable differences between farm types, sizes, species, climate, geography, disease risk, water supply, etc., even within one country. Further, antibiotic usage may fluctuate greatly from year to year, even in the same regions, due to climate and disease outbreaks. This high level of variation in antibiotic usage is reflected in inconsistent reports from farmer surveys (Lyle‐Fritch et al. 2006; Thuy et al. 2011; Rico et al. 2013; Tuševljak et al. 2013; Ali et al. 2016; Liu et al. 2017; Thi Kim Chi et al. 2017), and this situation is familiar across the aquaculture world, with reviews on antibiotic usage in general aquaculture demonstrating the scale and complexity of this issue (Vignesh et al. 2011; Done et al. 2016; Muziasari et al. 2016; Mo et al. 2017; Lozano et al. 2018).
Antibiotics are typically applied by mixing with feed or, less commonly, directly to the water. The direct application of antibiotics only to the individual animals requiring treatment (therapy) is not practical, so treatments are typically considered to be metaphylactic (where the whole population of animals, including both infected and uninfected animals are treated). True prophylactic use in aquaculture, where the animals are treated in the absence of disease, is considered rare in farms but more common in hatcheries (Smith 2008, 2012; Zhang et al. 2011). A recent study on Bangladesh shrimp hatcheries reported the use of up to 80 kg antibiotics per production cycle in a single hatchery (Hinchliffe et al. 2018).
The limited scope for immunoprophylaxis results in antibiotics currently being the most common method for preventing and treating disease in shrimp aquaculture, despite many of the most devastating diseases in recent years having been viral. Thus, although we cannot estimate the potential losses saved by the use of antimicrobials, antibiotic usage in shrimp aquaculture is likely to be inappropriate and inefficient, due to limited diagnostic capacity, poor regulation of quality and usage and poor application (in terms of antibiotic selection, doses, methods, timing, etc.). Generally, antibiotic usage (and antimicrobial use more widely) is thought to be higher in intensive rearing systems where there is a greater need for chemical agents to clean and maintain shrimp pond hygiene, as well as to prevent or treat disease.
Antimicrobial resistance in the shrimp industry
The ability of AMR genes to pass between bacteria, a process known as horizontal gene transfer, or HGT, is thought to underlie the rapid rise in resistant pathogens seen across the globe (Martinez et al. 2015). This process can occur between unrelated bacterial species (Li et al. 2018), meaning that resistance genes (which confer antibiotic resistance to the bacteria carrying them) present in non‐pathogenic, environmental bacteria (many species of which are unidentified) can be transferred to animal or human pathogenic bacteria and pose a threat to animal and human health. This risk is most likely to occur in environments where non‐pathogenic and pathogenic bacteria are brought into close contact, such as animal/human guts and environments where animal/human waste is present, such as waste water treatment plants, agricultural soils and in surface water run‐off from land applied with organic fertilization. Resistance gene dissemination is further exacerbated by the presence of antimicrobial compounds, which select for the expression and transfer of resistance genes, even at low concentrations (Wistrand‐Yuen et al. 2018). There is evidence to support the transfer of AMR genes from clinically relevant pathogens to aquatic organisms, with studies demonstrating that fish and shellfish pathogens have acquired resistance genes within mobile genetic elements (MGEs; segments of DNA that are easily transferred from one bacterium to another, e.g. transposons and plasmids) with very high similarity to those previously recovered from clinical bacterial isolates, demonstrating likely shared recent origins (McIntosh et al. 2008). An increasing number of publications have described the genetic basis of AMR in aquaculture environments, and a comprehensive review on this subject has been recently published (see Miller & Harbottle, 2018). Thus, the presence of resistance genes in MGEs in fish pathogens (including zoonotic pathogens) demonstrates the potential for AMR dissemination to animal/human/environmental bacteria through HGT.
Considering the size and international nature of the shrimp industry, there is very little information on AMR in shrimp farming environments. This is surprising as these farms are potential AMR dissemination hotspots for many reasons: stocking densities are often very high, which brings with it associated high bacterial densities and raised stress levels for shrimp, which in turn makes the stock more susceptible to infection; high levels of disease incidence; the widespread usage of chemicals; the release of untreated waste into the local environment; and high levels of unprotected human contact with water/animals. The large quantities of shrimp that are then transported and consumed both domestically and internationally make it likely that this industry poses a significant risk to global AMR dissemination (Fig. 4). Although many of these risk factors can be applied to a large proportion of the aquaculture industry in general, one of the AMR risks that is particularly pertinent to shrimp farming is the high level of traditional integrated farming practices, where human and livestock/poultry waste is used to fertilize ponds in order to promote algal growth within the pond which the shrimp then feed on. This is not only common but also promoted by many governments as being more environmentally and economically sustainable, however it increases the prevalence of human and zoonotic pathogens in the shrimp pond. Furthermore, the heavy use of antibiotics in livestock and poultry increases the levels and variety of antibiotic residues present in the pond, increasing the selection pressure. In these farming systems, sludge (sediment) from ponds is often used to feed the animals or fertilize vegetable or rice fields, which are subsequently also used for human and animal consumption. Thus, an environment is created which favours the emergence and spread of AMR genes, and this is supported by reports identifying high levels of resistance in these systems (Suzuki & Hoa 2012). Indeed, in one study in an integrated fish farming, it was shown that there was a significant increase in resistance across a crop cycle, from levels of 1–5% of bacteria to 80–100% in 2 months, and particularly high levels of resistance in human‐associated Enterococcus bacteria (Petersen et al. 2002).
Figure 4.
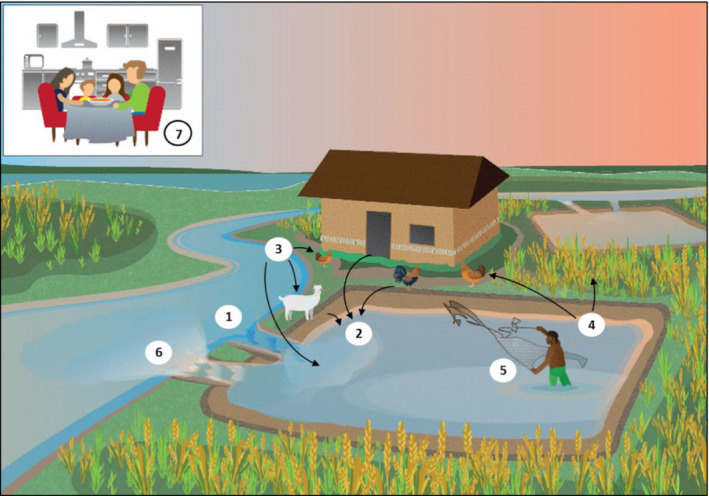
Ways in which shrimp farming poses a potential risk to AMR dissemination for a typical integrated small‐scale shrimp farm. 1. Fresh water is sourced from local water sources and mixed with saline water, in order to reach the desired salinity. These water supplies may be polluted with antibiotics, resistant bacteria and human/animal pathogens. 2. Human and animal waste, containing human/animal pathogens, is often used to fertilize the pond to encourage the growth of algae on which the shrimp feed. 3. Unregulated use of antibiotics, which can be added directly to the pond water and/or to the animals/humans living there. 4. Pond sediment/sludge containing antibiotics and resistant bacteria/pathogens is used as fertilizer for chicken feed and crops, both of which are eaten by the humans. 5. Humans have direct contact with pond water, sediment/sludge and shrimp/other animals. 6. Waste (water and sediment) is released directly back into local water sources, often untreated. 7. Shrimp (and associated bacteria/antibiotic residues) are transported and consumed internationally.
The impact of resistant infections on shrimp health is largely unknown, since few farmers have access to diagnostic services to identify the pathogen causing the disease, let alone the resistance levels. In 1994, mass mortality of shrimp larvae due to antimicrobial resistance was reported in Indian hatcheries, where 70–90% of larvae died from infection with strains of Vibrio harveyi that were highly resistant to a number of drugs, two of which (cotrimoxazole and chloramphenicol) had been used prophylactically. The lack of resistant Vibrio in the input sea water and shrimp eggs led the authors to conclude that the long‐term use of these drugs had led to a build‐up of resistant bacteria in the larval rearing tanks (Karunasagar et al. 1994). This was also supported by a separate study by the same researchers, who tested water samples from six hatcheries in India and found that larval tanks were predominantly populated by Vibrio species, with very high levels of resistance to many different antibiotics (Otta et al. 2001).
With the tripartite agreement from the WHO, FAO and OIE focused on addressing the problem of AMR on a global scale, there is an increasing drive towards better regulation of antimicrobial usage. In line with this, all of the top 10 shrimp‐producing countries had AMR action plans under development as of July 2018, according to the WHO AMR self‐assessment survey, which has set out to collect data from each country annually in order to monitor and evaluate the global AMR action plan (Fig. 5). Implementation of these action plans is, however, more of a challenge, and progress towards this is far less advanced (Table S1). When compared to the salmon industry, shrimp‐producing countries show much less progress in developing national monitoring systems for both antimicrobial use and AMR in animals and the environment (Fig. 5 and Table S1). Over a decade ago, the FAO's consultation on ‘Antimicrobial Use in Aquaculture and Antimicrobial Resistance’ identified that there was a need for more national and regional data on AMR, antimicrobial residues and antimicrobial usage, as well as for more knowledge on the spread of AMR genes from aquatic and fish bacteria to human pathogens (WHO, FAO and OIE, 2006). This is still the case today, with only a few studies having looked at AMR in the shrimp farming industry. A summary of the published studies describing evidence for AMR in bacteria associated with farmed shrimp species is provided in Table S2. It is very difficult to compare the results of the different studies due to considerable variation in the type of sample analysed (e.g. farmed/retail shrimp, pond water, sediment samples), the shrimp species involved, bacterial species characterized, antibiotics assessed and the methods used to characterize AMR. These variations can sometimes be explained by examining the purpose of the study: those that investigated AMR in human pathogens associated with shrimp (commonly Vibrio and Aeromonas species) often tested the susceptibility to antibiotics that are used to treat human infections, whereas those that aimed to assess the effect of AMR on the efficacy of the main antibiotics used in aquaculture usually tested the susceptibility to those antibiotics. Thus, the direct comparison between studies is not possible due to the differences in methods and interpretative criteria deployed. Another important issue is that 10 of the 37 studies highlighted in Table S2 that tested for phenotypic resistance did not state whether they used standardized methods and interpretative criteria for susceptibility testing (CLSI or EUCAST, see section below on Standardization of surveillance of antimicrobial usage and AMR in shrimp aquaculture). Further, of those that did, many cited the standard but did not provide evidence that they had followed the well‐defined interpretative criteria. The use of standardized methods is essential to ensure results between different studies are properly comparable (Schwarz et al. 2010). It is also important to avoid conflation acquired with intrinsic resistance, since many organisms have natural low susceptibility to certain classes of antibiotics. For instance, many Vibrio are intrinsically resistant to first generation penicillins (Chiou et al. 2015). A number of human pathogens are also intrinsically resistant to colistin, and, like many of the polymyxins, colistin binds to the surface of plastics and does not diffuse well through agar, meaning that results from many of the typical disc diffusion methods are meaningless (World Health Organization, 2018).
Figure 5.
Progress on AMR governance. Data from the WHO AMR self‐assessment survey, in which a multi‐sectoral group from each country is asked to submit annual responses to a questionnaire on AMR and antimicrobial use, in order to monitor progress and identify areas for action. Data shows country responses for the top 10 shrimp‐ and salmon‐producing countries (year two data, 2017–2018, published on 18th July 2018) for the topics below. Countries rate themselves from A (no action being taken; red) to E (best practice; dark green). Responses for each category are shown, taken directly from the self‐assessment survey. Black indicates no response given. Pie chart proportions indicate relative levels of production (in metric tonnes) for each country. OIE: World Organisation for Animal Health. (a) No response (black); No national AMR action plan (red); National AMR action plan under development (orange); National AMR action plan developed (yellow); National AMR action plan approved by government that reflects Global Action Plan objectives, with an operational plan and monitoring arrangements (light green); National AMR action plan has funding sources identified, is being implemented and has relevant sectors involved with a defined monitoring and evaluation process in place) (dark green). (b) No response (black); No national plan or system for monitoring sales/use of antimicrobials in animals (red); Plan agreed for monitoring quantities of antimicrobials sold for/used in animals, based on OIE standards (orange); Data collected and reported on total quantity of antimicrobials sold for/used in animals and their intended type of use (therapeutic or growth promotion) (yellow); On a regular basis, data is collected and reported to the OIE on the total quantity of antimicrobials sold for/used in animals nationally, by antimicrobial (light green); class, by species (aquatic or terrestrial), method of administration, and by type of use (therapeutic or growth promotion) (dark green) Data on antimicrobials used under veterinary supervision in animals are available at farm level, for individual animal species). (c) No response (black); No national plan for a system of monitoring of AMR is available (red); National plan for monitoring AMR but capacity (including laboratory) for surveillance and reporting data on AMR is lacking (orange); Some AMR data is collected locally but may not use a standardised approach and lacks national coordination and/or quality management (yellow); Priority pathogenic/ commensal bacterial species have been identified for surveillance (light green). Data systematically collected and reported on levels of resistance in at least 2 of those bacterial species, involving a laboratory that follows quality management processes, e.g. proficiency testing; National system of surveillance of AMR established for priority pathogens and for relevant commensal bacteria which follows quality assurance processes in line with intergovernmental standards (dark green). Laboratories that report for AMR surveillance follow quality assurance processes).
Dissemination of resistance through shrimp farms and hatcheries
High levels of antimicrobial resistant bacteria have been reported in the environment local to shrimp farms (Sundaramanickam et al. 2015; Rocha et al. 2016), and AMR genes have been shown to persist in the environment close to aquaculture sites even after antibiotic usage has ceased (Tamminen et al. 2011). This is particularly concerning since many of the pathogens present in shrimp ponds and hatcheries (e.g. Vibrio and Aeromonas species) are endemic to the surrounding marine and estuarine environments, meaning that resistant bacteria released into these environments have the potential to become the predominant strains. This risk is compounded by the high levels of antibiotic pollution in the environment surrounding shrimp aquaculture facilities, with growing evidence of the role of farms themselves in this pollution, through the release of water and pond sediments (Akinbowale et al. 2006; Hoa et al. 2011; Hossain et al. 2017, 2018; Lai et al. 2018). Inflowing local water sources can also be heavily polluted with antimicrobial compounds and resistant bacteria. For example, in China it was reported that the AMR genes present in shrimp farm input water correlated with that of the pond water, both in their abundance and temporal patterns (Su et al. 2017). Resistant bacteria can also enter shrimp farms through products added to the ponds, such as shrimp feed and probiotics. In intensive and semi‐intensive shrimp farming, pellet feeds containing animal‐ and plant‐based products are common, however their composition varies and antibiotic compounds are often added in order to preserve the feed in hot climates. To our knowledge, no studies have investigated the carriage of AMR genes in shrimp feed, although multidrug‐resistant pathogens have been detected in fish feed, with one study reporting 132 unique AMR genes and four mobile genetic elements in five fishmeal products (Han et al. 2017; Antunes et al. 2018; Novais et al. 2018). Extensive shrimp farming is more commonly associated with home‐made feeds mixed with antibiotics, however one survey of Vietnamese farmers found that many did not know the appropriate method of use, or dose, of the antibiotics incorporated, and sometimes even did not know the name of the antibiotic being used (Le & Munekage 2004). Concerns have also been raised over the accuracy and quality of the probiotics sold to shrimp (and other aquaculture) farmers, with studies reporting bacterial content different from those listed on the label, and carrying resistance to multiple antimicrobials (Noor Uddin et al. 2015; Uma & Rebecca 2018). Unconsumed feed and probiotics also contribute to antibiotic‐and AMR‐rich waste feed and faeces released into the local environment, where they may be taken up by wild organisms (Thuy et al. 2011). For example, it is thought that 95% of consumed oxytetracycline is released into the surrounding environment through faeces (Serrano 2005). Due to the high levels of untreated waste containing antimicrobial residues and resistant bacteria discharged by shrimp farming into the local environment, it is also possible that human populations living downstream of these facilities may be directly exposed. A recent study by Shen et al. (2018) looked at levels of Escherichia coli carrying the mcr‐1 gene conferring resistance to colistin in stool samples from more than 5000 individuals across China, and found that higher levels of mcr‐1 correlated with living in aquaculture‐intense areas. The use of colistin in animals is now banned in China, but used to be orally administered to pigs and poultry and is poorly absorbed, thus the authors suggested that these levels could be due to faecal contamination of the aquatic environment, where colistin is highly stable.
Given the nature of the shrimp farming environment, many shrimp farms are at, or close to, sea level, and flooding poses a huge threat to farmers’ livelihoods. It also provides a means by which resistant bacteria and antibiotic residues present in the ponds and aquatic environment can be disseminated over an enormous area very quickly. Increasing global temperatures have also been suggested to play a role in increasing AMR (MacFadden et al. 2018), presumably due to increased temperatures favouring bacterial growth. Indeed, a higher prevalence of V. parahaemolyticus was detected in shrimp sold at Chinese domestic markets during the summer months (Yang et al. 2017).
Another important factor contributing towards AMR emergence and dissemination in the shrimp farming environment is co‐selection with other antimicrobials, such as anti‐fungicides, anti‐parasitics and heavy metals (Seiler & Berendonk 2012). If these are present, selection for resistance genes can take place at lower antibiotic concentrations (Murray et al. 2018). High levels of heavy metals, commonly lead, cadmium, chromium, copper and zinc, have been detected in shrimp and pond water (Seiler & Berendonk 2012; He et al. 2016; Sarkar et al. 2016). Many heavy metals adsorb to particulate matter and are concentrated in the pond bed sediments where the shrimp reside and feed. Antibiotics are also known to persist in pond sediments as when adsorbed to them they degrade more slowly (Thuy et al. 2011). Thus, shrimp pond sediments may be a hub for AMR emergence and dissemination, and indeed these have been shown to contain higher levels of antibiotics and antibiotic‐resistant bacteria than pond water (Le & Munekage 2004; Le et al. 2005). Other sources of aquaculture pollution also promote the growth of micro‐organisms, and therefore increase the risk of AMR dissemination, in the local environment, such as organic enrichment with nitrates or phosphates (Anh et al. 2010).
One of the main direct routes for AMR dissemination to humans in the shrimp industry is likely to be through human contact with resistant bacteria present in the farm environment. Compared to salmon farming, shrimp farming occurs in countries where aquaculture is labour‐intensive; ten times more people are employed per tonne of production in the main shrimp‐producing country (China; 78 people per 1000 tonnes) compared to the main salmon‐producing country (Norway; 6 people per 1000 tonnes; FAO, 2016b). Thus, human exposure levels are considered to be very high in shrimp farming, largely due to a greater prevalence of traditional farming methods. For example, small‐scale farm workers frequently wade through ponds to feed, assess and harvest animals, and most live in close proximity to ponds and other animals on the farm who are exposed to the same pathogens and AMR genes. Many more people are also exposed through shrimp processing and onward sale. In agricultural settings, a number of studies have now shown that humans in direct contact with animals (e.g. farm workers) have increased levels of AMR genes and resistant bacteria associated with the farm animals and the antibiotics they were being exposed to (Marshall & Levy 2011). Reports of this in aquaculture are very limited, with one study showing that people working within Korean aquaculture had a higher faecal carriage of resistant bacteria than control restaurant employees (Shin & Cho 2013), and a second identifying quinolone resistance genes in marine bacteria in a Chilean salmon aquaculture area that were identical to those in urinary tract E. coli pathogens from humans living close by, suggestive of HGT (Tomova et al. 2015). There are also bacterial infections associated with shrimp handling, for example, fish‐handler's disease, commonly caused by infection of cuts in the skin with Mycobacterium species, which are rarely serious but can develop into deeper tissue infection or sepsis. Although there are no reports of resistance being a problem in these infections, they provide a route by which resistance genes can potentially enter the human body.
Human consumption of shrimp is another route by which AMR genes from shrimp can be transferred into human pathogens, since any resistant bacteria associated with shrimp are brought into close contact with human gut bacteria (Hoelzer et al. 2017). A number of human pathogens are associated with shrimp, such as Vibrio parahaemolyicus, one of the most important causes of food‐borne bacterial infectious diarrhoea in South East Asia (Nair et al. 2007; Li et al. 2014), and Vibrio vulnificus, a serious human pathogen carrying a very high case fatality rate (Jones & Oliver 2009). Resistance genes have been detected in shrimp products destined for human consumption (Table S2), with one study associating high levels of the colistin resistance mcr‐1 gene with consumption of meat, pork, mutton and aquaculture products (Shen et al. 2018). Consumption of resistant strains of these bacteria not only poses a threat to humans through their inability to be treated, but also raises the levels of resistance genes in the gut, increasing the likelihood of dissemination.
Risk mitigation strategies
In order to be effective, measures put in place aimed at limiting the risks of AMR dissemination must be appropriate for each geographical, social, political and cultural context (World Health Organization, 2015; Kakkar et al. 2018). There are a number of key areas that the industry as a whole, or at least individual countries or areas within them, could consider. As with all AMR action plans, the principal means by which the risk of AMR dissemination in the shrimp industry might be mitigated is through reducing contagion and promoting the prudent use of antimicrobials. Indeed, controlling contagion is essential for the shrimp industry irrespective of the risks of AMR, due to the ever‐increasing burden of disease that threatens the future of the industry and the livelihoods of those within it.
Regulate antibiotic sales/usage
For effective prevention and better control over the use of antimicrobial agents in aquaculture, national and international level regulatory frameworks on the sales, use and quality of antimicrobial agents are required. All of the top 10 shrimp‐producing countries do have regulations in place for the use of antibiotics in aquaculture, but there is a huge disconnect between regulation and enforcement. In an ideal world, veterinarians, or other licensed fish health professionals, would prescribe antimicrobials, and licensing would be enforced and guided by qualified fisheries and animal health professionals. This is not the case in most shrimp‐producing countries, and imports to the EU and US are regularly rejected due to testing positive for prohibited antibiotic residues or residues above the maximum residue levels (MRLs; Fig. 5). For example, between 1st October 2014 and 30th September 2015, 32% of sampled shrimp imports from peninsular Malaysia to the US tested positive for chloramphenicol and/or nitrofuran residues, antimicrobial compounds that are banned for use in food production in Malaysia (Fig. 6). This led to the FDA imposing an Import Alert on shrimp products from Malaysia in April 2016 (FDA Import Alert #16‐136). Similarly, there has recently been a growing concern within the EU over the level of antibiotic use in the Indian shrimp farming, and, accordingly, the rate of testing has been increased from 10 to 50 per cent (Behera 2018). However, although export restrictions undoubtedly help to reduce antibiotic usage, they only detect antibiotic usage immediately prior to harvesting, and do not apply pressure to hatcheries and shrimp destined for domestic markets. It is also worth noting that the balance of trade is shifting from a bipolar Global South to North, to South‐South trading, upsetting the current trade‐related and buyer‐led forms of regulation (Belton et al. 2018). Despite significant progress in the development of AMR action plans, a significant barrier to reducing antibiotic usage in the major shrimp‐producing countries is the lack of resources to implement the regulations that are already in place.
Figure 6.
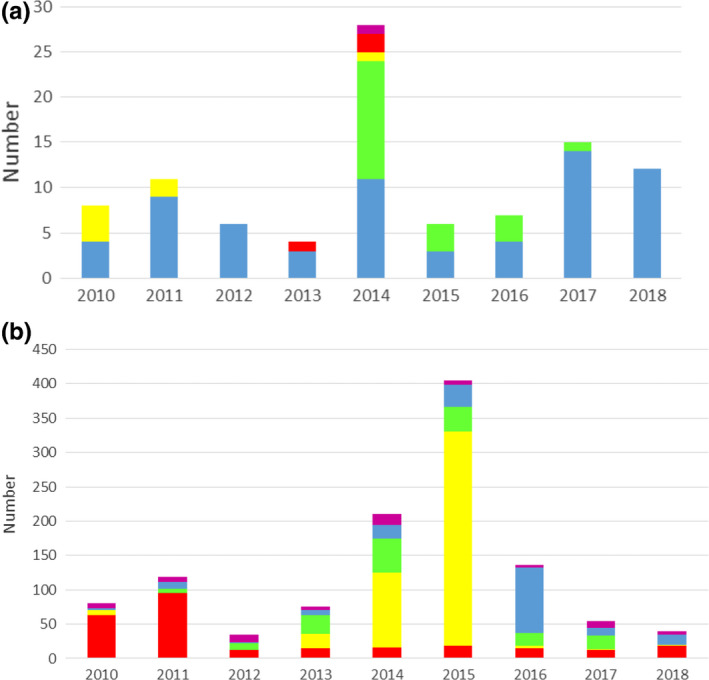
Shrimp export rejections due to the presence of residues of prohibited antibiotics or those exceeding the maximum residual levels permitted. Number of border rejections due to antibiotic residue hazards in (a) EU (data taken from EU's Rapid Alert System for Food and Feed portal on 03/12/2018, analysing search results from 01/01/2010 to 31/10/2018, filtering only shrimp border rejections due to antibiotic residues). India (blue); Vietnam (green); Bangladesh (yellow); China (red); Myanmar (purple). (b) USA (data taken from FDA's Refusal Entry Database, from 01/01/2010 – 31/10/2018, filtering only shrimp border rejections due to antibiotic residues. Data on total number of samples tested each year were not readily available from either database. China (red); Malaysia (yellow); Vietnam (green); India (blue); Others (purple)
Alternatives to antimicrobials
There is currently limited scope for prophylaxis through vaccination in shrimp, due to their inability to raise an adaptive immune response, although the increasing evidence for innate immune memory may offer future possibilities (Milutinović & Kurtz 2016). Alternative options have focussed on stimulating shrimp immunity and maintaining a healthy shrimp and pond microbiome (a term given to describe the suite of microorganisms in a particular environment), with pond water bacterioplankton composition having been suggested as a potential indicator of shrimp health status (Zhang et al. 2014). A number of immune stimulants are now available for use in shrimp (Barman & Nen 2015), although there is limited data on their effectiveness. Probiotics are widely used, and prebiotics have also shown some efficacy in shrimp. As an example, Litopenaeus vannamei fed on a diet with supplementary poly‐b‐hydroxybutyrate (PHB) had increased growth and feed conversion rates, and showed improved immunity (Duan et al. 2017). Similar positive results were seen with PHB in Macrobrachium rosenbergii larvae (Nhan et al. 2010). Many farmers also reportedly use herbal medicines (Chi et al. 2017), and these have been shown to exhibit antimicrobial effects. Examples here include two essential oils from the Madagascan plant, Cinnamosma fragrans, which had effects similar to erythromycin in lowering bacterial count and increasing survival rates of Penaeus monodon larvae in hatcheries (Randrianarivelo et al. 2010). The identity of the active ingredients of many herbal remedies, however, are unknown and some may be toxic to humans or the environment. In addition, it is highly likely that resistance will ultimately develop to any antimicrobial compound present.
One of the most promising alternative treatment options to replace antibiotics is phage therapy. Bacteriophages (referred to as ‘phages’) are viruses that infect and kill bacteria, so theoretically they could be used to treat infections and reduce levels of resistant bacteria. However, in order for this to be effective, the infecting/resistant pathogen must be identified, and this diagnostic capacity is not widely available in the shrimp industry. One option that has been suggested for shrimp aquaculture is to target bacteria which are known to colonize shrimp ponds and cause infections in both shrimp and humans, for example, phages against Vibrio harveyi have been isolated from shrimp hatcheries and shown to increase survival of larvae. In one study, phage therapy over a period of 17 days increased the survival of Penaeus mondon larvae from 17 to 86%, whereas antibiotic treatment survival rates were 40% (Vinod et al. 2006). However, it must be noted that phages have also been shown to be vectors for the dissemination of AMR genes, since they can transfer pieces of DNA from one bacterial host to another (Subirats et al. 2016). Other compounds specifically targeting Vibrio bacteria are also available and could be potential treatments for shrimp Vibrio infections, as well as more general methods such as quorum‐sensing inhibitors that interfere with bacterial cell–cell communication (Defoirdt et al. 2011).
Improved disease diagnosis
Accurate diagnosis of disease allows for the selection of appropriate antimicrobials, and avoidance of antibiotics critical for use in human health. This would prevent the use of antibiotics for viral infections, but the administration of medicines in these circumstances may in fact be reasonable given the suite of possible pathogen interactions at any one time. For example, WSSV infection has been shown to increase susceptibility to other bacterial pathogens (Phuoc et al. 2008). Yet, many shrimp‐producing countries do not have the resources to provide farmers with easy and affordable access to veterinary officers or diagnostic facilities, and so farmers have no choice but to self‐diagnose and self‐treat. Better diagnostic support and access would allow farmers to receive advice not only on the best treatment, but also on the best application method, dose and length of treatment, all of which would help to reduce unnecessary antimicrobial usage. In line with this, our own work with WorldFish in Bangladesh is supporting the development of in‐country skills sets for accurate pathogen and disease diagnosis. We are doing this through facilitating the use of a combination of molecular (largely PCR‐based methods, but also more comprehensive sequencing approaches, e.g. MinION sequencing) and histopathology techniques. The associated research in this programme of work is also seeking to better understand the assemblages of microorganisms associated with shrimp infections and disease states – the so‐called pathobiome, as many diseases in shrimp (and finfish) aquaculture appear to be polysyndromic in nature and are not caused by a single pathogen (Stentiford et al. 2017 ).
Advances in next generation‐sequencing technologies have transformed human health infectious disease diagnosis, and offer much potential for transfer to the aquaculture sector. Whole genome‐sequencing (WGS) of bacterial isolates not only facilitates accurate diagnosis, but also allows for improved monitoring of disease epidemiology, as well as the detection and surveillance of AMR genes and associated mobile genetic elements (Bayliss et al. 2017). In addition, research into shrimp transcriptomics shows potential for applications in health monitoring and the development of immune stimulants or treatments to common infections (Rodriguez‐Anaya et al. 2018). As more of the shrimp industry moves towards selective breeding of broodstock, these new technologies also offer much scope for genomic‐assisted selection, for example, through identification of genes encoding greater tolerance to specific pathogens or stress (Guppy et al. 2018). With such potential impact, it is no surprise that much of the current scientific research into shrimp aquaculture is focusing on the application of these technologies, although the capacity to implement any outcomes from this research remains limited in most shrimp‐producing countries, as most laboratories do not have the expertise, staffing or equipment to conduct such studies. Laboratory capacity and expertise is improving, however, through initiatives such as the UK government‐funded Fleming Fund (https://www.flemingfund.org/), and technological advances such as the development of the portable MinION sequencer (https://nanoporetech.com/products/minion) are increasing the accessibility of these applications to the shrimp industry. Further, pond‐side Genedrive platform PCR‐based diagnostics, that are straightforward enough for farmers to use, are also becoming a reality, and these have recently been deployed in commercial farm settings across regions in Thailand (Minardi et al. 2019).
Improved hygiene and farm management
Better management practices (BMPs) for shrimp farming are often based upon the FAO's Code of Conduct for Responsible Fisheries (FAO, 1995), with the aim of standardising management practices. Usually, BMPs are adapted for the various types of shrimp production (e.g. intensive/extensive), and thereby aim to reduce the risk of disease and environmental degradation specific to these production systems. For example, in terms of antimicrobial usage, large vertically integrated companies may be able to put in place advanced biosecurity systems to limit the spread of disease both within and between their ponds, but this relative control may be offset by higher stocking densities and greater perceived risks relative to loss values. Both may act as drivers for higher disease risk and greater usage of antimicrobials. In contrast, subsistence farmers may find control much harder to achieve. In these cases, farmers may also be less risk averse, especially if practising polyculture where losses to shrimp crop may be offset by other products (e.g. rice, fish; Hinchliffe et al. 2018; Little et al. 2018). In all production types, proper management of the aquaculture environment in the terms of parameters such as feed rates, dissolved oxygen, stocking densities, movement restrictions, water treatment and biological control could go a long way in avoiding excessive use of antimicrobial agents. Thus, one of the routes to reducing unnecessary antimicrobial usage is to encourage farmers to adopt BMPs. A recent report by Suzuki & Hoang Nam 2018 reported that in Vietnamese shrimp farming, which consists mainly of small‐scale farms, receiving training and appropriate information increased BMP adoption, and that BMP adoption reduced disease outbreaks.
Increased species diversity
A general shift in shrimp farming from Penaeus mondon to Litopenaeus vannamei in the last decade is considered to have helped the expansion of the industry. This is because Litopenaeus vannamei has a higher feed conversion rate and can tolerate a wider range of salinities, allowing inland farming where there is lower salinity. Importantly for antibiotic usage, it also has a higher tolerance to disease, with higher hatchery survival rates (Kumar & Engle 2016). Since pathogens often affect one species more than another (e.g. Penaeus monodon is thought to be more susceptible to WSSV than Litopenaeus vannamei; Tuyen et al. 2014), it could be argued that the industry would benefit from a wider variety of species, and a lower disease burden would reduce the need for antimicrobials. Interestingly, genetically improved species have been successfully introduced to tilapia farming (Kumar & Engle 2016), so there could be scope for selected breeding of new shrimp species to reduce the disease burden in shrimp.
Better regulation of feed and probiotic manufacture and sales
The use of medicated feed is widespread in aquaculture. Avoiding overuse of feed is thus a relatively straightforward way to reduce environmental contamination with antimicrobial residues and resistant bacteria, since up to 30% of feed is unconsumed (Cabello et al. 2013). Inappetence is a typical symptom of infections in aquatic animals, so providing medicated feed can be of questionable benefit unless very carefully managed (Ranjan et al. 2017). Regulations on feed used in aquaculture are in place in some countries, for example the Vietnamese National Fisheries Quality Assurance and Veterinary Directorate (NAVIQAVED) attempts to test and control feed quality across the industry. However, the large number of feeds available on the market makes this very difficult, with more than 100 types of feed produced domestically and many more imported from across the world (Anh et al. 2010).
Probiotics are now widely used in shrimp aquaculture to stimulate immunity and improve feed digestion and water quality, despite scepticism from the scientific community over their efficacy. A few studies have suggested that administration of probiotics to Litopenaeus vannamei post‐larvae can improve growth and feed efficiency, increase expression of immune related genes and even protect against infection by WSSV (Chai et al. 2016; Miandare et al. 2016). However, reports identifying discrepancies in probiotic contents and labelling, and the presence of resistant bacteria, show the need for better regulation of their contents and improvements in the information provided for the farmer on their specific purpose, dosage and correct application measures (Noor Uddin et al. 2015). Thus, as for medicated feed, avoiding excess use of probiotics is necessary to reduce any risk of contamination with unknown and potentially resistant bacteria.
Farmer education
Many of the major shrimp‐producing countries offer a range of courses on aquaculture management for farmers to attend, ranging from government/non‐governmental organisation workshops to university degrees. However, surveys conducted on farmers’ knowledge of antibiotic use have suggested that they have a limited understanding about the purpose of antibiotics, their appropriate use, dose and method of application (Holmstrom et al. 2003; Mostafa Shamsuzzaman & Kumar Biswas 2012; Pham et al. 2015). For example, one survey of Vietnamese farmers found that, despite 45% of respondents believing that antibiotics had no effect, 86% still used them, and 12 of the 23 antibiotics being used were listed as critically important by the WHO (Pham et al. 2015). In a separate study, 27% of Thai shrimp farmers surveyed were using antibiotics to treat viral diseases such as white spot syndrome (Holmstrom et al. 2003). In line with these reports, the WHO AMR self‐assessment survey found that training and professional education on AMR in the farming sector was very poor in the top 10 shrimp farming countries, although the high‐income salmon farming countries had much to improve on too (Table S1).
Certification
Aquaculture certification and labelling programmes have become the primary tool to address sustainability issues in farmed seafood, which includes promoting prudent antimicrobial usage. Such schemes are already in place, with the majority of all certified aquaculture production covered through the Aquaculture Stewardship Council (ASC), Global Aquaculture Alliance Best Aquaculture Practice (GAABAP), Global G.A.P. and Friend of the Sea. To increase audit efficiency and reduce farmer administrative workload, the three leading aquaculture certification programmes, ASC, GAABAP and Global G.A.P. have now drafted and agreed combined checklists for farms that seek more than one certificate, however the growth in the number of certification schemes has increased confusion. As a result, the Global Sustainable Seafood Initiative (GSSI) has been created, tasked with developing a global benchmarking tool to measure and compare certification schemes and standards performance. Despite this progress on certification, one of its main disadvantages is that it is largely focused on the export market, and has limited influence on the production of shrimp destined for the domestic market and hatcheries, where certification is less valued. Further, the costs associated with becoming certified, and the requirement for book keeping and auditing, are more accessible to larger farms, whereas the majority of shrimp farmers, in many countries, are family‐run smallholders.
Standardization of surveillance of antimicrobial usage and AMR in shrimp aquaculture
Over a decade ago, the WHO/FAO/OIE's consultation on ‘Antimicrobial Use in Aquaculture and Antimicrobial Resistance’ identified that there was a need for both more national and regional data on AMR, antimicrobial residues and antimicrobial usage, as well as for more knowledge on the spread of AMR genes from aquatic and fish bacteria to human pathogens (World Health Organization, Food and Agriculture Organization of the United Nations and World Organization for Animal Health, 2006). Improved surveillance has been identified as key to reducing antimicrobial usage across all human and animal health sectors, and is an integral part of national AMR action plans. Surveillance of antimicrobial usage and resistance would cover the entire shrimp industry and would not be restricted to products destined for export. However, like most LMICs, the main shrimp‐producing countries are still at a very early stage in the development of any type of AMR/AMU surveillance system (Fig. 5), and effective surveillance will be very difficult to achieve without first gaining tighter control of antibiotic sales.
Standardization of the methods used to both measure sensitivity to antibiotics and then interpret the resultant antimicrobial sensitivity testing (AST) data (Alderman & Smith 2001; World Organization for Animal Health, 2018) is also crucial. It is strongly recommended that Clinical and Laboratory Standards Institutes (CLSI; or other equivalent international standards, e.g. EUCAST) are used for this type of testing. It is also very important that appropriate quality assurance processes are in place to ensure the integrity of AST data. For bacteria isolated from aquatic animals, the creation of CLSI standards for antimicrobial disk diffusion testing and determination of minimal inhibitory concentrations by broth micro‐ and microdilution are important steps in this process (CLSI, 2006, 2014a,2014b). It is recognized that further work is required here to develop these provisional standards, particularly with the types of pathogens that affect tropical species such as shrimp.
There are major efforts underway (e.g. via the UK Government‐funded Fleming Fund) to support LMICs to improve their laboratory capacity for the surveillance of AMR and antibiotic usage. Although most of this will understandably be used to improve surveillance in clinical settings, it is also recognized that this should encompass animal (including aquaculture) production in these countries. As an example, the Fleming Fund and UK Government have recently provided funding to support the development of a new Food and Agriculture Organisation of the United Nations (FAO) designated International Reference Centre for AMR, run through the UK agencies of the Department for Environment, Food and Rural Affairs ‐ Veterinary Medicines Directorate (VMD), Centre for Environment Fisheries and Aquaculture Science (CEFAS) and the Animal and Plant Health Agency (APHA). The centre will seek to improve the balance of the one‐health approach and take the steps needed to improve AMR surveillance in the animal and environmental sectors. Specifically, the centre will provide technical assistance, training and quality assurance to support an increase in AMR surveillance in low‐ and middle‐income countries across Asia and Africa.
Conclusions
There is a growing movement to reduce the use of antimicrobials in food animals in general, but many of the practical solutions put forward, such as increasing the price of antibiotics and capping the amount used per animal, rely on governments having the means to implement and enforce regulations and assess their efficacy, which is not the case in most shrimp‐producing countries (Van Boeckel et al. 2017). One of the main barriers to better regulation is that the Competent Authorities responsible for their implementation in many shrimp‐producing countries are very understaffed and under resourced. As a result, there is often a paucity of officials compared to the number of farms, and a lack of the knowledge required for accurate diagnosis of the disease conditions. This makes long term programmes (e.g. surveillance) difficult to implement and become effective. To this end, a phased approach to creating the surveillance networks required for LMIC governments to effectively achieve reductions in inappropriate antimicrobial usage has recently been suggested (Schar et al. 2018). Another barrier to effective antibiotic sales and usage regulation and surveillance in many shrimp‐producing countries is the informal medicines supply network, in which the sale of substandard and counterfeit antibiotics is a problem (Zellweger et al. 2017). It is unrealistic, and unfair, to expect farmers in LMICs to reduce antibiotic usage without major investments in farm hygiene, biosecurity and associated diagnostic/veterinary services (UN Interagency Coordination Group on Antimicrobial Resistance, 2019). In many shrimp‐producing countries, the majority of diagnostic and prescriptive services are provided by a largely untrained and unregulated paraveterinary sector, since there are simply not enough trained veterinary officers (Hinchliffe 2019).
Thus, the infrastructure for the central control of the regulatory, diagnostic and surveillance systems does not exist or is insufficient in many shrimp‐producing countries. Adopting as models the systems that work in high income countries may not be practical in the timescales that are required to reduce the global burden of AMR (Cox et al. 2017). Solutions could therefore look towards more self‐regulatory processes. This could include a greater involvement of extension officers, such as those employed by WorldFish and other international non‐governmental organisations, for delivering training and educational programmes (https://www.worldfishcenter.org/). Raising farmer awareness of better management practices should also improve farm hygiene and biosecurity, thereby reducing contagion and AMR dissemination. More generally, increased community awareness of AMR and the risks of antibiotic misuse could promote a degree of self‐policing, of both farmers and the local antibiotic suppliers, which in shrimp farming communities are often untrained local farm shop owners (Hinchliffe 2019). Aquaculture could usefully learn from the human health sector, which is facing the same issues in LMICs in terms of lack of resources for regulation and surveillance of antibiotic sales and usage. In some LMICs, antibiotic stewardship programmes in the human drug sector have been focusing on the drug suppliers, for example the accredited drug dispensing outlet (ADDO) programme, which began in Tanzania and has now been extended to other African countries and Bangladesh (Rutta et al. 2015). This programme of accreditation for local drug shops, which includes training and signing up to codes of conduct, has seen significant improvements in dispensing practices, although it has been noted that future efforts should also include public engagement, in order to reduce public demand for these drugs (Embrey et al. 2016).
Since AMR and inappropriate antibiotic use are true ‘One Health’ issues, that do not respect differences in human/animal sector boundaries, it seems sensible that these sectors both learn from and work with each other, in order to effect maximal understanding and impact. In the context of AMR, local actions have international consequences, thus national governments, international agencies/organisations and the private sector need to co‐ordinate their efforts, knowledge and resources if we are to reduce the risk that the shrimp industry poses to global AMR dissemination. This review has highlighted some of the socioeconomic, political and cultural aspects of this risk, and has outlined some of the major challenges that must be overcome in order to mitigate it. In doing so, we have demonstrated the paucity of information available on the use of antimicrobials, the prevalence of AMR in shrimp and human pathogens, as well as on the types of resistance mechanisms carried by these species in shrimp aquaculture. As a result, it is currently very difficult to accurately assess the risks posed by the use of antimicrobials in this rapidly growing sector, which includes a wide range of production systems at the interface between the aquatic and terrestrial environments. Given the complexity of the shrimp aquaculture environment in terms of potential sources of AMR genes and antibiotic residues, and the means by which these are disseminated, it is therefore vital to gather more available data on AMR and antibiotic sales/usage in shrimp aquaculture in order to establish where future efforts aiming to reduce this are best placed. Equally important, however, is the need for wider public engagement and a better understanding of the social, economic and political limitations to achieving these goals in each country. A co‐ordinated approach, between experts in the aquaculture, clinical, environmental and social disciplines, from both the private and public sectors, will be essential.
Data availability statement
The research data supporting this publication are provided within this paper.
Supporting information
Figure S1. Shrimp production.
Figure S2. Top 10 shrimp and salmon importing and exporting countries in 2016.
Figure S3. Top 10 shrimp and salmon producing countries in 2016.
Table S1. Selected data from the WHO AMR self‐assessment survey (a subset of which is shown in Fig. 5).
Table S2. Evidence for antimicrobial resistance in bacteria associated with farmed shrimp.
Acknowledgements
This work was supported by a BBSRC‐funded Daphne Jackson Fellowship (KT), a BBSRC‐Newton Babba grant (‘Novel molecular approaches for advancing prediction and mitigation of disease outbreaks in aquaculture for small scale farmers’; CRT, DB, and MMR; ref BB/N00504X/1) and an ESRC grant under the cross UK Research Council ‘Tacking Antimicrobial Resistance’ programme (‘Production without medicalisation: a pilot intervention in global protein production’; SH and CRT; ref ES/P004008/1). MMR is supported by the CGIAR Research Program on Fish Agri‐food Systems (FISH).
Contributor Information
Kelly Thornber, Email: k.thornber@exeter.ac.uk.
Charles R. Tyler, Email: c.r.tyler@exeter.ac.uk.
References
- Ahlstrom CA, Bonnedahl J, Woksepp H, Hernandez J, Olsen B, Ramey AM (2018) Acquisition and dissemination of cephalosporin‐resistant E. coli in migratory birds sampled at an Alaska landfill as inferred through genomic analysis. Scientific Reports 8 (1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinbowale OL, Peng H, Barton MD (2006) Antimicrobial resistance in bacteria isolated from aquaculture sources in Australia. Journal of Applied Microbiology 100: 1103–1113. [DOI] [PubMed] [Google Scholar]
- Alderman DJ, Smith P (2001) Development of draft protocols of standard reference methods for antimicrobial agent susceptibility testing of bacteria associated with fish diseases. Aquaculture 196: 211–243. [Google Scholar]
- Ali H, Rico A, Murshed‐e‐Jahan K, Belton B (2016) An assessment of chemical and biological product use in aquaculture in Bangladesh. Aquaculture 454: 199–209. [Google Scholar]
- Anh PT, Kroeze C, Bush SR, Mol APJ (2010) Water pollution by intensive brackish shrimp farming in south‐east Vietnam: causes and options for control. Agricultural Water Management 97: 872–882. [Google Scholar]
- Antunes P, Campos J, Mourão J, Pereira J, Novais C, Peixe L (2018) Inflow water is a major source of trout farming contamination with Salmonella and multidrug resistant bacteria. Science of the Total Environment 642: 1163–1171. [DOI] [PubMed] [Google Scholar]
- Barman D, Nen P (2015) Immunostimulants for aquaculture health management. Journal of Marine Science: Research & Development 03(03). 10.4172/2155-9910.1000134. [DOI] [Google Scholar]
- Bayliss SC, Verner‐Jeffreys DW, Bartie KL, Aanensen DM, Sheppard SK, Adams A et al (2017) The promise of whole genome pathogen sequencing for the molecular epidemiology of emerging aquaculture pathogens. Frontiers in Microbiology 8: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera N (2018) EU ambassador rules out possibility of a ban on Indian shrimp imports, Business Standard. [Cited 7 Dec 2018.] Available from URL: https://www.business-standard.com/article/economy-policy/eu-ambassador-rules-out-possibility-of-a-ban-on-indian-shrimp-imports-118092500916_1.html
- Belton B, Bush SR, Little DC (2018) Not just for the wealthy: rethinking farmed fish consumption in the Global South. Global Food Security 16: 85–92. [Google Scholar]
- Cabello FC, Godfrey HP, Tomova A, Ivanova L, Dölz H, Millanao A et al (2013) Antimicrobial use in aquaculture re‐examined: Its relevance to antimicrobial resistance and to animal and human health. Environmental Microbiology 15: 1917–1942. [DOI] [PubMed] [Google Scholar]
- CDC (2013) Antibiotic resistance threats in the United States, 2013. Current, 114 pp. https://doi.org/CS239559-B.
- Chai PC, Song XL, Chen GF, Xu H, Huang J (2016) Dietary supplementation of probiotic Bacillus PC465 isolated from the gut of Fenneropenaeus chinensis improves the health status and resistance of Litopenaeus vannamei against white spot syndrome virus. Fish and Shellfish Immunology 54: 602–611. [DOI] [PubMed] [Google Scholar]
- Chereau F, Opatowski L, Tourdjman M, Vong S (2017) Risk assessment for antibiotic resistance in South East Asia. BMJ 358: j3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi TTK, Clausen JH, Van PT, Tersbøl B, Dalsgaard A (2017) Use practices of antimicrobials and other compounds by shrimp and fish farmers in Northern Vietnam. Aquaculture Reports 7: 40–47. [Google Scholar]
- Chiou J, Li R, Chen S (2015) CARB‐17 family of β‐lactamases mediates intrinsic resistance to penicillins in Vibrio parahaemolyticus . Antimicrobial Agents and Chemotherapy 59: 3593–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI (2006) VET‐03 Methods for Antimicrobial Disk Susceptibility Testing of Bacteria Isolated From Aquatic Animals, 1st Edition. [Cited 28 Jan 2019.] Available from URL: https://clsi.org/standards/products/veterinary-medicine/documents/vet03/
- CLSI (2014a) Performance Standards for Antimicrobial Susceptibility Testing of Bacteria Isolated From Aquatic Animals; Second Informational Supplement.
- CLSI (2014b) VET‐04 Methods for Broth Dilution Susceptibility Testing of Bacteria Isolated From Aquatic Animals, 2nd Edition. [Cited 28 Jan 2019.] Available from URL: https://clsi.org/standards/products/veterinary-medicine/documents/vet04/
- Collignon P, Beggs JJ, Walsh TR, Gandra S, Laxminarayan R (2018) Anthropological and socioeconomic factors contributing to global antimicrobial resistance: a univariate and multivariable analysis. The Lancet Planetary Health 2: e398–e405. [DOI] [PubMed] [Google Scholar]
- Cox JA, Vlieghe E, Mendelson M, Wertheim H, Ndegwa L, Villegas MV et al (2017) Antibiotic stewardship in low‐ and middle‐income countries: the same but different? Clinical Microbiology and Infection 23: 812–818. [DOI] [PubMed] [Google Scholar]
- Defoirdt T, Sorgeloos P, Bossier P (2011) Alternatives to antibiotics for the control of bacterial disease in aquaculture. Current Opinion in Microbiology 14: 251–258. [DOI] [PubMed] [Google Scholar]
- Done HY, Venkatesan AK, Halden RU (2016) Erratum to: does the recent growth of aquaculture create antibiotic resistance threats different from those associated with land animal production in agriculture? The AAPS Journal 18: 1583. [DOI] [PubMed] [Google Scholar]
- Duan Y, Zhang Y, Dong H, Zheng X, Wang Y, Li H et al (2017) Effect of dietary poly‐β‐hydroxybutyrate (PHB) on growth performance, intestinal health status and body composition of Pacific white shrimp Litopenaeus vannamei (Boone, 1931). Fish and Shellfish Immunology 60: 520–528. [DOI] [PubMed] [Google Scholar]
- Embrey M, Vialle‐Valentin C, Dillip A, Kihiyo B, Mbwasi R, Semali IA et al (2016) Understanding the role of accredited drug dispensing outlets in Tanzania's health system. PLoS One 11: e0164332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (1995) Code of Conduct for Responsible Fisheries. Food & Agriculture Organization. ISBN 92‐5‐103834‐5.
- FAO (2013) FAO Fisheries and Aquaculture Report No. 1053 Report of the FAO/MARD Technical Workshop on Early Mortality Syndrome (EMS) or Acute Hepatopancreatic Necrosis Syndrome (AHPNS) of Cultured Shrimp (under TCP/VIE/3304). FAO Fisheries and Aquaculture Report No. 1053. Rome, p. 54.
- FAO (2014) The state of world fisheries and aquaculture, Food and Agriculture Oraganization of the United Nations. doi: 92‐5‐105177‐1.
- FAO (2016a) Fishstat J: Global Fishery and Aquaculture Production Statistics.
- FAO (2016b) The state of world fisheries and aquaculture 2016: Contributing to food security and nutrition for all, The State of World Fisheries and Aquaculture 2016. 10.1056/NEJM199312023292302 [DOI]
- Gudding R, Van Muiswinkel WB (2013) A history of fish vaccination: science‐based disease prevention in aquaculture. Fish and Shellfish Immunology 35: 1683–1688. [DOI] [PubMed] [Google Scholar]
- Guppy JL, Jones DB, Jerry DR, Wade NM, Raadsma HW, Huerlimann R et al (2018) The state of “Omics” research for farmed Penaeids: advances in research and impediments to industry utilization. Frontiers in Genetics 9: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JE, Tang KF, Tran LH, Lightner DV (2015) Photorhabdus insect‐related (Pir) toxin‐like genes in a plasmid of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) of shrimp. Diseases of Aquatic Organisms 113: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Wang J, Zhao Z, Chen J, Lu H, Liu G (2017) Fishmeal application induces antibiotic resistance gene propagation in mariculture sediment. Environmental Science and Technology 51: 10850–10860. [DOI] [PubMed] [Google Scholar]
- Hauton C, Smith VJ (2007) Adaptive immunity in invertebrates: a straw house without a mechanistic foundation. BioEssays 29: 1138–1146. [DOI] [PubMed] [Google Scholar]
- He Y, Jin L, Sun F, Hu Q, Chen L (2016) Antibiotic and heavy‐metal resistance of Vibrio parahaemolyticus isolated from fresh shrimps in Shanghai fish markets, China. Environmental Science and Pollution Research 23: 15033–15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson PJG, Rico A, Troell M, Klinger DH, Buschmann AH, Saksida S et al (2017) Unpacking factors influencing antimicrobial use in global aquaculture and their implication for management: a review from a systems perspective. Sustainability Science 13: 1105–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchliffe S (2019) Production without medicalisation: report from workshop on AMR, One Health and Aquaculture, Dhaka, Bangladesh. February 12‐13th 2019.
- Hinchliffe S, Butcher A, Rahman MM (2018) The AMR problem: demanding economies, biological margins, and co‐producing alternative strategies. Palgrave Communications 4: 142. [Google Scholar]
- Hoa PT, Managaki S, Nakada N, Takada H, Shimizu A, Anh DH et al (2011) Antibiotic contamination and occurrence of antibiotic‐resistant bacteria in aquatic environments of northern Vietnam. Science of the Total Environment 409: 2894–2901. [DOI] [PubMed] [Google Scholar]
- Hoelzer K, Wong N, Thomas J, Talkington K, Jungman E, Coukell A (2017) Antimicrobial drug use in food‐producing animals and associated human health risks: what, and how strong, is the evidence? BMC Veterinary Research 13: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrom K, Graslund S, Wahlstrom A, Poungshompoo S, Bengtsson B‐E, Kautsky N (2003) Antibiotic use in shrimp farming and implications for environmental impacts and human health. International Journal of Food Science and Technology 38: 255–266. [Google Scholar]
- Hossain A, Nakamichi S, Habibullah‐Al‐Mamun M, Tani K, Masunaga S, Matsuda H (2017) Occurrence, distribution, ecological and resistance risks of antibiotics in surface water of finfish and shellfish aquaculture in Bangladesh. Chemosphere 188: 329–336. [DOI] [PubMed] [Google Scholar]
- Hossain A, Nakamichi S, Habibullah‐Al‐Mamun M, Tani K, Masunaga S, Matsuda H (2018) Occurrence and ecological risk of pharmaceuticals in river surface water of Bangladesh. Environmental Research 165: 258–266. [DOI] [PubMed] [Google Scholar]
- Jones MK, Oliver JD (2009) Vibrio vulnificus: disease and pathogenesis. Infection and Immunity 77: 1723–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar M, Chatterjee P, Chauhan AS, Grace D, Lindahl J, Beeche A et al (2018) Antimicrobial resistance in South East Asia: time to ask the right questions. Global Health Action 11: 1483637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunasagar I, Pai R, Malathi GR, Karunasagar I (1994) Mass mortality of Penaeus monodon larvae due to antibiotic‐resistant Vibrio harveyi infection. Aquaculture 128: 203–209. [Google Scholar]
- Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra S, Levin SA et al (2018) Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proceedings of the National Academy of Sciences 115: E3463–E3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G, Engle CR (2016) Technological advances that led to growth of shrimp, salmon, and tilapia farming. Reviews in Fisheries Science and Aquaculture 24: 136–152. [Google Scholar]
- Lai WW‐P, Lin Y‐C, Wang Y‐H, Guo YL, Lin AY‐C (2018) Occurrence of emerging contaminants in aquaculture waters: cross‐contamination between aquaculture systems and surrounding waters. Water, Air, and Soil Pollution 229: 249 10.1007/s11270-018-3901-3. [DOI] [Google Scholar]
- Le TX, Munekage Y (2004) Residues of selected antibiotics in water and mud from shrimp ponds in mangrove areas in Viet Nam. Marine Pollution Bulletin 49: 922–929. [DOI] [PubMed] [Google Scholar]
- Le TX, Munekage Y, Kato SI (2005) Antibiotic resistance in bacteria from shrimp farming in mangrove areas. Science of the Total Environment 349: 95–105. [DOI] [PubMed] [Google Scholar]
- Li Y, Xie X, Shi X, Lin Y, Qiu Y, Mou J et al (2014) Southern coastal. Emerging Infectious Diseases 20(4): 2012–2015. 10.3201/eid2004.130744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Dechesne A, He Z, Madsen JS, Nesme J, Sørensen SJ et al (2018) Estimating the transfer range of plasmids encoding antimicrobial resistance in a wastewater treatment plant microbial community. Environmental Science and Technology Letters 5: 260–265. [Google Scholar]
- Lightner DV, Redman RM, Pantoja CR, Tang KF, Noble BL, Schofield P et al (2012) Historic emergence, impact and current status of shrimp pathogens in the Americas. Journal of Invertebrate Pathology 110: 174–183. [DOI] [PubMed] [Google Scholar]
- Little DC, Young JA, Zhang W, Newton RW, Al Mamun A, Murray FJ (2018) Sustainable intensification of aquaculture value chains between Asia and Europe: a framework for understanding impacts and challenges. Aquaculture 493: 338–354. [Google Scholar]
- Liu X, Steele JC, Meng XZ (2017) Usage, residue, and human health risk of antibiotics in Chinese aquaculture: a review. Environmental Pollution 223: 161–169. [DOI] [PubMed] [Google Scholar]
- Lozano I, Díaz NF, Muñoz S, Riquelme C (2018) Antibiotics in Chilean aquaculture: a review In: Savic S. (ed) Antibiotic Use in Animals. 10.5772/intechopen.71780. [DOI] [Google Scholar]
- Lyle‐Fritch LP, Romero‐Beltrán E, Páez‐Osuna F (2006) A survey on use of the chemical and biological products for shrimp farming in Sinaloa (NW Mexico). Aquacultural Engineering 35: 135–146. [Google Scholar]
- MacFadden DR, McGough SF, Fisman D, Santillana M, Brownstein JS (2018) Antibiotic resistance increases with local temperature. Nature Climate Change 8: 510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall BM, Levy SB (2011) Food animals and antimicrobials: impacts on human health. Clinical Microbiology Reviews 24: 718–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti E, Variatza E, Balcazar JL (2014) The role of aquatic ecosystems as reservoirs of antibiotic resistance. Trends in Microbiology 22: 36–41. [DOI] [PubMed] [Google Scholar]
- Martinez JL, Coque TM, Baquero F (2015) What is a resistance gene? Ranking risk in resistomes. Nature Reviews. Microbiology 13: 116–123. [DOI] [PubMed] [Google Scholar]
- McIntosh D, Cunningham M, Ji B, Fekete FA, Parry EM, Clark SE et al (2008) Transferable, multiple antibiotic and mercury resistance in Atlantic Canadian isolates of Aeromonas salmonicida subsp. salmonicida is associated with carriage of an IncA/C plasmid similar to the Salmonella enterica plasmid pSN254. The Journal of Antimicrobial Chemotherapy 61: 1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miandare HK, Yarahmadi P, Abbasian M (2016) Immune related transcriptional responses and performance of Litopenaeus vannamei post‐larvae fed on dietary probiotic PrimaLac® . Fish and Shellfish Immunology 55: 671–678. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harbottle H (2018) Antimicrobial drug resistance in fish pathogens. Antimicrobial Resistance in Bacteria From Livestock and Companion Animals 6: 501–520. [DOI] [PubMed] [Google Scholar]
- Milutinović B, Kurtz J (2016) Immune memory in invertebrates. Seminars in Immunology 28: 328–342. [DOI] [PubMed] [Google Scholar]
- Minardi D, Bateman KS, Kuzdzal A, Stone M, Avant J, Condliffe R et al (2019) Testing of a pond‐side molecular diagnostic tool for the detection of white spot syndrome virus in shrimp aquaculture. Journal of the World Aquaculture Society 50: 18–33. [Google Scholar]
- Mo WY, Chen Z, Leung HM, Leung AOW (2017) Application of veterinary antibiotics in China's aquaculture industry and their potential human health risks. Environmental Science and Pollution Research 24: 8978–8989. [DOI] [PubMed] [Google Scholar]
- Mostafa Shamsuzzaman M, Kumar Biswas T (2012) Aqua chemicals in shrimp farm: a study from south‐west coast of Bangladesh. Egyptian Journal of Aquatic Research 38: 275–285. [Google Scholar]
- Murray AK, Zhang L, Yin X, Zhang T, Buckling A, Snape J, Gaze WH (2018) Novel insights into selection for antibiotic resistance in complex microbial communities. MBio 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muziasari WI, Pärnänen K, Johnson TA, Lyra C, Karkman A, Stedtfeld RD et al (2016) Aquaculture changes the profile of antibiotic resistance and mobile genetic element associated genes in Baltic Sea sediments. FEMS Microbiology Ecology 92: fiw052. [DOI] [PubMed] [Google Scholar]
- Nair GB, Ramamurthy T, Bhattacharya SK, Dutta B, Takeda Y, Sack DA (2007) Global dissemination of Vibrio parahaemolyticus serotype O3:K6 and its serovariants. Clinical Microbiology Reviews 20(1): 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nhan DT, Wille M, De Schryver P, Defoirdt T, Bossier P, Sorgeloos P (2010) The effect of poly β‐hydroxybutyrate on larviculture of the giant freshwater prawn Macrobrachium rosenbergii . Aquaculture 302: 76–81. [Google Scholar]
- Noor Uddin GM, Larsen MH, Christensen H, Aarestrup FM, Phu TM, Dalsgaard A (2015) Identification and antimicrobial resistance of bacteria isolated from probiotic products used in shrimp culture. PLoS One 10: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwegian Ministry of Health and Care Services (2015) National Strategy Against Antimicrobial Resistance 2015‐2020.
- Novais C, Campos J, Freitas AR, Barros M, Silveira E, Coque TM et al (2018) Water supply and feed as sources of antimicrobial‐resistant Enterococcus spp. in aquacultures of rainbow trout (Oncorhyncus mykiss), Portugal. Science of the Total Environment 625: 1102–1112. [DOI] [PubMed] [Google Scholar]
- O'Neill J (2016) Tackling drug‐resistant infections globally: final report and recommendations. Review on Antimicrobial Resistance: 84 pp. 10.1016/j.jpha.2015.11.005. [DOI] [Google Scholar]
- Onwugamba FC, Fitzgerald JR, Rochon K, Guardabassi L, Alabi A, Kühne S et al (2018) The role of “filth flies” in the spread of antimicrobial resistance. Travel Medicine and Infectious Disease 22: 8–17. [DOI] [PubMed] [Google Scholar]
- Otta SK, Karunasagar I, Karunasagar I (2001) Bacteriological study of shrimp, Penaeus Monodon fabricius, hatcheries in India. Journal of Applied Ichthyology 17: 59–63. [Google Scholar]
- Petersen A, Andersen JS, Kaewmak T, Somsiri T, Dalsgaard A (2002) Impact of integrated fish farming on antimicrobial resistance in a pond environment. Applied and Environmental Microbiology 68: 6036–6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham DK, Chu J, Do NT, Brose F, Degand G, Delahaut P et al (2015) Monitoring antibiotic use and residue in freshwater aquaculture for domestic use in Vietnam. EcoHealth 12: 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuoc LH, Corteel M, Nauwynck HJ, Pensaert MB, Alday‐Sanz V, Van den Broeck W et al (2008) Increased susceptibility of white spot syndrome virus‐infected Litopenaeus vannamei to Vibrio campbellii . Environmental Microbiology 10: 2718–2727. [DOI] [PubMed] [Google Scholar]
- Randrianarivelo R, Danthu P, Benoit C, Ruez P, Raherimandimby M, Sarter S (2010) Novel alternative to antibiotics in shrimp hatchery: effects of the essential oil of Cinnamosma fragrans on survival and bacterial concentration of Penaeus monodon larvae. Journal of Applied Microbiology 109: 642–650. [DOI] [PubMed] [Google Scholar]
- Ranjan A, Sahu NP, Gupta S (2017) Prospects of medicated feed in aquaculture. Nutrition & Food Science International Journal 3(4): 555617. [Google Scholar]
- Restrepo L, Bayot B, Arciniegas S, Bajaña L, Betancourt I, Panchana F et al (2018) PirVP genes causing AHPND identified in a new Vibrio species (Vibrio punensis) within the commensal Orientalis clade. Scientific Reports 8(1): 13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico A, Phu TM, Satapornvanit K, Min J, Shahabuddin AM, Henriksson PJG et al (2013) Use of veterinary medicines, feed additives and probiotics in four major internationally traded aquaculture species farmed in Asia. Aquaculture 412–413: 231–243. [Google Scholar]
- Rocha RS, Sousa OV, Vieira RHSF (2016) Multidrug‐resistant Vibrio associated with an estuary affected by shrimp farming in Northeastern Brazil. Marine Pollution Bulletin 105(1): 337–340. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Anaya LZ, Casillas‐Hernández R, Lares‐Jiménez LF, Gonzalez‐Galaviz JR (2018) Next‐generation sequencing technologies and the improvement of aquaculture sustainability of Pacific white shrimp (Litopenaeus vannamei). Scientific Reports 115: 15612. [Google Scholar]
- Rutta E, Liana J, Embrey M, Johnson K, Kimatta S, Valimba R et al (2015) Accrediting retail drug shops to strengthen Tanzania's public health system: an ADDO case study. Journal of Pharmaceutical Policy and Practice 8: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar T, Alam MM, Parvin N, Fardous Z, Chowdhury AZ, Hossain S et al (2016) Assessment of heavy metals contamination and human health risk in shrimp collected from different farms and rivers at Khulna‐Satkhira region, Bangladesh. Toxicology Reports 3: 346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schar D, Sommanustweechai A, Laxminarayan R, Tangcharoensathien V (2018) Surveillance of antimicrobial consumption in animal production sectors of low‐ and middle‐income countries: optimizing use and addressing antimicrobial resistance. PLoS Medicine 15: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S, Silley P, Simjee S, Woodford N, van Duijkeren E, Johnson AP et al (2010) Editorial: assessing the antimicrobial susceptibility of bacteria obtained from animals. Journal of Antimicrobial Chemotherapy 65: 601–604. [DOI] [PubMed] [Google Scholar]
- Seiler C, Berendonk TU (2012) Heavy metal driven co‐selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Frontiers in Microbiology 3: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P (2005) Responsible use of antibiotics in aquaculture, Igarss 2005. 10.1007/s13398-014-0173-7.2. [DOI]
- Shen Y, Zhou H, Xu J, Wang Y, Zhang Q, Walsh TR et al (2018) Anthropogenic and environmental factors associated with high incidence of mcr‐1 carriage in humans across China. Nature Microbiology 3: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HH, Cho SH (2013) Prevalence of antimicrobial resistance in Escherichia coli strains isolated from fishery workers. Osong Public Health and Research Perspectives 4: 72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P (2008) Antimicrobial resistance in aquaculture the use of antimicrobials in aquaculture. Revue Scientifique et Technique (International Office of Epizootics) 27: 243–264. [PubMed] [Google Scholar]
- Smith P (2012) Antibiotics in aquaculture; reducing the use and maintaining the efficacy In: Austin B. (ed) Infectious Diseases in Aquaculture, pp. 161–189. Woodhead, Cambridge. [Google Scholar]
- Smith VJ, Brown JH, Haut C (2003) Immunostimulation in crustaceans: does it really protect\against infection? Fish & Shellfish Immunology 15: 71–90. [DOI] [PubMed] [Google Scholar]
- Sommerset I, Krossøy B, Biering E, Frost P (2005) Vaccines for fish in aquaculture. Expert Review of Vaccines 4(1): 89–101. [DOI] [PubMed] [Google Scholar]
- Stentiford G, Sritunyalucksana K, Flegel TW, Williams BAP, Withyachumnarnkul B, Itsathitphaisarn O, Bass D (2017) New paradigms to help solve the global aquaculture disease crisis. PLoS Pathogens 13(2): e1006160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Liu S, Hu X, Xu X, Xu W, Xu Y et al (2017) Occurrence and temporal variation of antibiotic resistance genes (ARGs) in shrimp aquaculture: ARGs dissemination from farming source to reared organisms. Science of the Total Environment 607–608: 357–366. [DOI] [PubMed] [Google Scholar]
- Subirats J, Sànchez‐Melsió A, Borrego CM, Balcázar JL, Simonet P (2016) Metagenomic analysis reveals that bacteriophages are reservoirs of antibiotic resistance genes. International Journal of Antimicrobial Agents 48: 163–167. [DOI] [PubMed] [Google Scholar]
- Sundaramanickam A, Kumar PS, Kumaresan S, Balasubramanian T (2015) Isolation and molecular characterization of multidrug‐resistant halophilic bacteria from shrimp farm effluents of Parangipettai coastal waters. Environmental Science and Pollution Research 22: 11700–11707. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Hoa PTP (2012) Distribution of quinolones, sulfonamides, tetracyclines in aquatic environment and antibiotic resistance in Indochina. Frontiers in Microbiology 3: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Hoang Nam V (2018) Better management practices and their outcomes in shrimp farming: evidence from small‐scale shrimp farmers in Southern Vietnam’. Aquaculture International 26: 469–486. [Google Scholar]
- Tamminen M, Karkman A, Lõhmus A, Muziasari WI, Takasu H, Wada S et al (2011) Tetracycline resistance genes persist at aquaculture farms in the absence of selection pressure. Environmental Science and Technology 45: 386–391. [DOI] [PubMed] [Google Scholar]
- The World Bank (2013) Fish to 2030: Prospects for fisheries and aquaculture, The World Bank. Agriculture and Environmental Services. doi: 83177‐GLB.
- Thuy HTT, Nga LP, Loan TTC (2011) Antibiotic contaminants in coastal wetlands from Vietnamese shrimp farming. Environmental Science and Pollution Research 18: 835–841. [DOI] [PubMed] [Google Scholar]
- Tomova A, Ivanova L, Buschmann AH, Rioseco ML, Kalsi RK, Godfrey HP et al (2015) Antimicrobial resistance genes in marine bacteria and human uropathogenic Escherichia coli from a region of intensive aquaculture. Environmental Microbiology Reports 7: 803–809. [DOI] [PubMed] [Google Scholar]
- Tran KC, Tran MP, Phan TV, Dalsgaard A (2018) Quality of antimicrobial products used in white leg shrimp (Litopenaeus vannamei) aquaculture in Northern Vietnam. Aquaculture 482: 167–175. [Google Scholar]
- Tuševljak N, Dutil L, Rajić A, Uhland FC, McClure C, St‐Hilaire S et al (2013) Antimicrobial use and resistance in aquaculture: findings of a globally administered survey of aquaculture‐allied professionals. Zoonoses and Public Health 60: 426–436. [DOI] [PubMed] [Google Scholar]
- Tuyen NX, Verreth J, Vlak JM, de Jong MCM (2014) Horizontal transmission dynamics of White spot syndrome virus by cohabitation trials in juvenile Penaeus monodon and P. vannamei . Preventive Veterinary Medicine 117: 286–294. [DOI] [PubMed] [Google Scholar]
- Uddin SA, Kader MA (2006) The use of antibiotics in shrimp hatcheries in Bangladesh. Journal of Fisheries and Aquatic Science 1(1): 64–67. [Google Scholar]
- Uma A, Rebecca G (2018) Antibiotic resistance in bacterial isolates from commercial probiotics used in aquaculture. International Journal of Current Microbiology and Applied Sciences 7(01): 1737–1743. [Google Scholar]
- UN Interagency Coordination Group on Antimicrobial Resistance (2019) Draft recommendations of the ad hoc interagency coordination group on antimicrobial resistance. WHO. [Cited 21 May 2019.] Available from URL: https://www.who.int/antimicrobial-resistance/interagency-coordination-group/Draft_IACG_recommendations_for_public_discussion_290119.pdf?ua=1
- Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP et al (2015) Global trends in antimicrobial use in food animals. Proceedings of the National Academy of Sciences 112: 5649–5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boeckel TP, Glennon EE, Chen D, Gilbert M, Robinson TP, Grenfell BT et al (2017) Reducing antimicrobial use in food animals. Science 357: 1350–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignesh R, Karthikeyan BS, Periyasamy N, Devanathan K (2011) Antibiotics in aquaculture: an overview. South Asian Journal 1: 114–120. [Google Scholar]
- Vinod MG, Shivu MM, Umesha KR, Rajeeva BC, Krohne G, Karunasagar I et al (2006) Isolation of Vibrio harveyi bacteriophage with a potential for biocontrol of luminous vibriosis in hatchery environments. Aquaculture 255: 117–124. [Google Scholar]
- Wistrand‐Yuen E, Knopp M, Hjort K, Koskiniemi S, Berg OG, Andersson DI (2018) Evolution of high‐level resistance during low‐level antibiotic exposure. Nature Communications 9(1): 1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2015) Global Action Plan on Antimicrobial Resistance, pp. 1–28. WHO Press, Geneva. ISBN 978 92 4 150976 3. [Google Scholar]
- World Health Organization (2018) The detection and reporting of colistin resistance.
- World Health Organization, Food and Agriculture Organization of the United Nations and World Organization for Animal Health (2006) Antimicrobial Use in Aquaculture and Antimicrobial Resistance. Report of a Joint FAO/OIE/WHO Expert Consultation on Antimicrobial Use in Aquaculture and Antimicrobial Resistance. AT PA WORLD HEALTH ORGANIZATION IS IS, p. 95.
- World Organization for Animal Health (2018) Development and harmonisation of national antimicrobial resistance surveillance and monitoring programmes for aquatic animals. Aquatic Animal Health Code, Chapter 6.4. [Cited 1 Feb 2019.] Available from URL: http://www.oie.int/index.php?xml:id=171&L=0&htmfile=chapitre_antibio_development_harmonisation.htm
- Yang Y, Xie J, Li H, Tan S, Chen Y, Yu H (2017) Prevalence, antibiotic susceptibility and diversity of Vibrio parahaemolyticus isolates in seafood from South China. Frontiers in Microbiology 8: 2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger RM, Carrique‐Mas J, Limmathurotsakul D, Day NPJ, Thwaites GE, Baker S et al (2017) A current perspective on antimicrobial resistance in Southeast Asia. Journal of Antimicrobial Chemotherapy 72: 2963–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YB, Li Y, Sun XL (2011) Antibiotic resistance of bacteria isolated from shrimp hatcheries and cultural ponds on Donghai Island, China. Marine Pollution Bulletin 62: 2299–2307. [DOI] [PubMed] [Google Scholar]
- Zhang D, Wang X, Xiong J, Zhu J, Wang Y, Zhao Q et al (2014) Bacterioplankton assemblages as biological indicators of shrimp health status. Ecological Indicators 38: 218–224. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Shrimp production.
Figure S2. Top 10 shrimp and salmon importing and exporting countries in 2016.
Figure S3. Top 10 shrimp and salmon producing countries in 2016.
Table S1. Selected data from the WHO AMR self‐assessment survey (a subset of which is shown in Fig. 5).
Table S2. Evidence for antimicrobial resistance in bacteria associated with farmed shrimp.
Data Availability Statement
The research data supporting this publication are provided within this paper.


