Abstract
Understanding the mechanisms behind neurodifferentiation in adults will be an important milestone in our quest to identify treatment strategies for cognitive disorders observed during our natural ageing or disease. It is now clear that the maturation of neural stem cells to neurones, fully integrated into neuronal circuits requires a complete remodelling of cellular metabolism, including switching the cellular energy source. Mitochondria are central for this transition and are increasingly seen as the regulatory hub in defining neural stem cell fate and neurodevelopment. This review explores our current knowledge of metabolism during adult neurodifferentiation.
Keywords: Metabolic switch, Neural stem cells, Neural progenitor cells, Adult neurogenesis, Metabolism, Mitochondria
Introduction
The potential for pluripotent cells to undergo both replication and differentiation into highly specialised tissues is the central defining feature of multicellular organisms. It also serves as a mean to maintain and repair themselves, and loss of this cellular renewal capacity has long been seen as a hallmark of the natural ageing process [1]. Quiescent stem cells have been identified in a wide range of adult tissues, capable of renewing and replenishing various organs, but the brain has long been exempt from this observation, with neural neogenesis believed to be exclusive to the developing embryo, ceasing shortly after birth [2–4] (for a comprehensive historical review [5]). Early indications of mitotic cells in rat brains were reported already at the turn of the last century [6, 7], and by the late 1960s Joseph Altman provided more evidence of cells undergoing active proliferation in the dentate gyrus, the olfactory bulb, and the neocortex of adult rats, cats, and guinea pigs [8–12]. Additional reports suggested neurogenesis in a range of animals, including songbirds [13–15], macaque [16], and humans [17, 18]. Major neurodifferentiation is now accepted to occur in specific niches of the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the hippocampal dentate gyrus [19–23], and no or little post-natal neurogenesis in cortical neurons [24–26], although there is still a debate around adult neurogenesis [27–29]. However, the presence of quiescent neural stem cells (NSCs) questions their purpose, what regulates their activation and differentiation, and whether there is a connection between NSCs and the natural cognitive decline observed during human ageing. This review attempts to summarise our current understanding of what metabolic factors define and regulate neurodifferentiation in the adult brain.
The brain is a highly complex organ with mostly specialised cells, where neurons form a large interconnected network with synaptic activity, which is embedded in a complex set of glial cells. Astrocytes, a type of glial cells [30], are considered to play a supportive and protective role that provides the structural basis for our brain, as well as modulating synaptic transmissions [31, 32], provide energy for neurons [33], secretion and absorption of neurotransmitters, and can drive circadian behaviour [34]. Our ability to learn and adapt means also that this network is not static, but that interactions need to be maintained and new ones formed or replaced. This neuronal plasticity means cells will require different metabolic profiles during a variety of stages and understanding these requirements might allow us to improve their cellular function.
The developing brain follows a well-organised protocol during embryogenesis [35–38], and is considered to be predominantly metabolically glycolytic [39]. During adolescence the developing brain of both rats and humans has been shown to induce a metabolic shift from fatty acid oxidation to glucose-based metabolism [39–41], and by the time we are adults, our brains consume around 25% of our glucose intake, despite accounting for only ~ 2% of our body weight. At the same time, ~ 20% of our inhaled oxygen is used in the brain [39, 42–44], demonstrating a huge bioenergetic requirement, which is mostly satisfied by oxidative phosphorylation (OXPHOS) [45].
Nevertheless, the regions identified as containing NSCs have been shown to remain glycolytic [46], suggesting that although the purpose of embryonic and adult NSCs is very different, the mechanisms of differentiation might be similar. Structurally, the SVZ in the adult brain lines the lateral ventricles, separated by a layer of ependymal cells [46]. In rodents the neural precursors in the SVZ have been shown to form interneurones and astrocytes that will migrate to the olfactory bulb [47, 48]. Migration in humans is still debated, and relocation to the striatum or cortex have also been reported [49, 50]. The SGZ forms a narrow layer between the granule cell layer and hilus of the dentate gyrus in the hippocampus and is accepted to be one of the stem-cell-containing niches of the adult brain [51]. What exactly triggers neurogenesis is not fully established, but proliferation of NSCs in the SVZ and SGZ has, for instance, been observed as a consequence of ischemic stroke, leading to cell migration towards the lesion to contribute to repair [52, 53]. Thus, the SVZ and SGZ microenvironments are thought to provide the appropriate conditions for NSCs to proliferate, while also allowing for the differentiation into the relevant neurons via several rounds of asynchronous proliferation. This transformation has to undergo several distinct stages, where (a) the NSC has to exit its quiescent state, (b) proliferate, (c) migrate to its appropriate location, (d) terminally differentiate, and (e) integrate into the existing neuronal circuits [54, 55]. Each step is highly regulated and involves hormones, growth factors, neurotransmitters, and environmental factors. Additionally, diseases states or the genetic composition can influence greatly neurogenesis. Together these factors will inevitably affect the intracellular metabolic state, driving the different stages of neurogenesis.
Energy metabolism in the brain
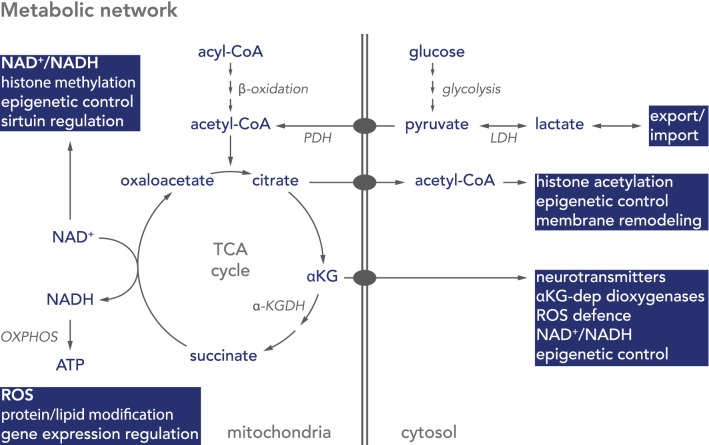
Glucose metabolism begins with glucose entering the cell and being converted into pyruvate during glycolysis. During aerobic respiration, pyruvate enters mitochondria and is decarboxylated to acetyl-CoA by the pyruvate dehydrogenase complex (PDH), followed by condensation with oxaloacetate to citrate in the tricarboxylic acid (TCA) cycle (Fig. 1). In a series of reactions, citrate is then decarboxylated back to oxaloacetate, releasing carbon dioxide and reducing nicotinamide (NAD) and flavin (FAD) adenine dinucleotides to NADH and FADH2, respectively. The oxidation of these redox co-factors in the mitochondrial electron transport chain (ETC) leads to the formation of an electrochemical gradient across the inner mitochondrial membrane, which is used to drive an ATP synthase to form ATP from ADP and molecular phosphate (Pi) (Fig. 1) [56]. This oxidative phosphorylation (ETC + ATP synthase = OXPHOS) is dependent on oxygen, with approximately 80% of inhaled oxygen reduced to water at the final step of the ETC [42].
Fig. 1.
Schematic diagram of metabolic pathways important in neurogenesis. In astrocytes glucose is metabolised to pyruvate via glycolysis, metabolised by lactate dehydrogenase (LDH), and exported as lactate. Neurons take up astrocyte-derived lactate to convert it back to pyruvate. Pyruvate is imported into mitochondria and converted by the pyruvate dehydrogenase complex (PDH) to acetyl-CoA, which enters the citric acid (TCA) cycle. Acetyl-CoA is also generated by breakdown of fatty acids (acyl-CoA) during β-oxidation. The TCA cycle condenses oxaloacetate with acetyl-CoA to form citrate, which either acts as precursor for cytosolic acetyl-CoA or is metabolised in the TCA cycle to α-ketoglutarate (αKG). Mitochondrial NADH is oxidised by NADH-ubiquinone oxidoreductase (complex I) of the oxidative phosphorylation system (OXPHOS), while β-oxidation- or TCA cycle-derived FADH2 reduces ubiquinone via the electron transfer flavoprotein-ubiquinone oxidoreductase (ETF-QO) or succinate-ubiquinone oxidoreductase (complex II), respectively
In the absence of oxygen pyruvate does not enter mitochondria but is converted to lactic acid by lactate dehydrogenase (LDH) (Fig. 1) [57]. This glycolytic metabolism of glucose is much less energy efficient, but some cells nevertheless rely predominantly on glycolysis to maintain their ATP pool, even in the presence of oxygen. This phenomenon is termed aerobic glycolysis and is important to provide precursors of essential biosynthesis pathways, such as purine and pyrimidines, amino acids, and triglycerides, which derive from intermediates of glycolysis and the pentose phosphate pathways [58–60]. In these cells the pyruvate kinase PKM2 regulates the last step within glycolysis, dephosphorylating phosphoenolpyruvate to pyruvate. By inactivating PKM2 intermediates of glycolysis accumulate and are preferentially channelled into other pathways [61]. This aerobic glycolysis is an important feature in cancer biology, where dividing cells rapidly need to increase their biomass [62]. Additionally, cataplerosis of the TCA cycle also diverts a number of intermediates to other pathways, reducing the contribution to energy production [63].
Besides glucose and lactate, ketone bodies represent an alternative fuel for neurons during fasting or extended exercise periods, reaching even 60–70% of the total energy supply for the brain [64]. Under low blood glucose levels adipocytes perform ketogenesis, releasing ketone bodies in the form of acetoacetate and b-hydroxybutyrate (BHB) into the bloodstream, which can cross the blood–brain barrier and be imported via MCT2 into neurons [65]. Additionally, unlike neurons, astrocytes are capable to perform ketogenesis providing with ketone bodies to neighbouring neurons [66]. Hence, neurons can shift from the oxidation of carbohydrates or lactate to ketone bodies in a transition process known as ‘G-to-K switch’ [67]. Evidence stems from mice either placed on intermittent fasting or high-ketone body containing diet, demonstrating the use of ketone bodies in the brain [67]. The effect of ketone bodies on neurogenesis is less clear, but has been correlated with improved synaptic plasticity, spatial learning, memory and cognition [68, 69]. Additionally, BHB has been shown to increase the expression of the brain-derived neurotrophic factor BDNF, and thereby promoting cellular resistance [70]. In contrast, prolonged hypothalamic exposure to ketone bodies stimulates hypothalamic neuropeptides and dysregulation of glucose homeostasis [71].
Astrocytes have a high glycolytic activity and are the main consumers of glucose in the brain, while only consuming around 20% of oxygen absorbed by the brain [39, 42–44]. In contrast, neurons are the main oxygen consumers, with high metabolic rate. This is achieved by instead of feeding the TCA cycle and utilising OXPHOS in mitochondria, astrocytes convert pyruvate to lactic acid via LDH and export it into the extra-cellular matrix via the monocarboxylate transporter, MCT4, where it is taken up by neurons via MCT2. There, lactate is converted back to pyruvate and used as fuel in mitochondria [72–75] or as signalling molecule [33, 76]. Astrocytes thus act as a buffer, safeguarding neurons from fluctuating blood glucose levels by continuously fuelling lactate to neurons. And indeed, neurons have been shown to have low glycolytic activity [77] and preferentially use lactate [75, 78], although both glucose and lactate have been shown to be able to stimulate oxidative metabolism in mature neurons [75, 79]. The energetic buffer capacity of astrocytes is further supported by the observation that unlike neurons, astrocytes have the capacity to store glycogen, which is broken down back to glucose by glycogenolysis upon synaptic activity [74, 80].
Metabolism in neural stem and progenitor cells
Hence, stem cells are generally considered to be glycolytic [81–83], and in agreement NSCs in the SGZ and SVZ are considered to have predominately glycolytic activity. This glycolytic nature of stem cells had for long been attributed to a combination of their cellular demands and a hypoxic microenvironment, but it has become clear that stem cells are indeed capable of using OXPHOS, but require glycolysis to maintain stemness rather than being an adaptation to its environment [84–88]. On the other hand, the oxidation of fatty acids has also been suggested to function as fuel source in NSCs, especially during NSC activation [89–94], and this activation of NSCs is dependent on a functional ETC and OXPHOS [95, 96]. Furthermore, the ability to adapt their metabolic state seems to be essential in NSCs for proliferation and differentiation [97–99]. Growing evidence suggests that mitochondria play a central and driving role in this transition, not only by providing ATP but also by regulating individual steps during neuronal differentiation, such as managing the cellular redox state, intracellular signalling pathways, or the epigenetic state of the cell [100–103]. Accordingly, to accommodate the changing metabolic demands of activation and differentiation to a neuronal lineage, NSCs need to switch their metabolism from a predominantly glycolytic to one that utilises mitochondrial OXPHOS.
The metabolic switch in neurogenesis
A number of reports have demonstrated that the transition from NSC to a neuronal lineage is accompanied by increased mitochondrial biogenesis, as well as the downregulation of glycolysis and fatty acid oxidation pathways (Fig. 2) [90, 91, 93, 95, 97, 99, 104, 105]. For instance, the progression from pluripotent progenitor cell to neuron is characterised by a strong reduction in glycolysis-related proteins, such as hexokinase 2 (HK2) and isoform A of lactate dehydrogenase (LDHA), which metabolises the reduction of pyruvate to lactate. Additionally, a switch from PKM2 to its constitutively active isoform PKM1 and an upregulation of OXPHOS-related genes has been observed [97, 99].
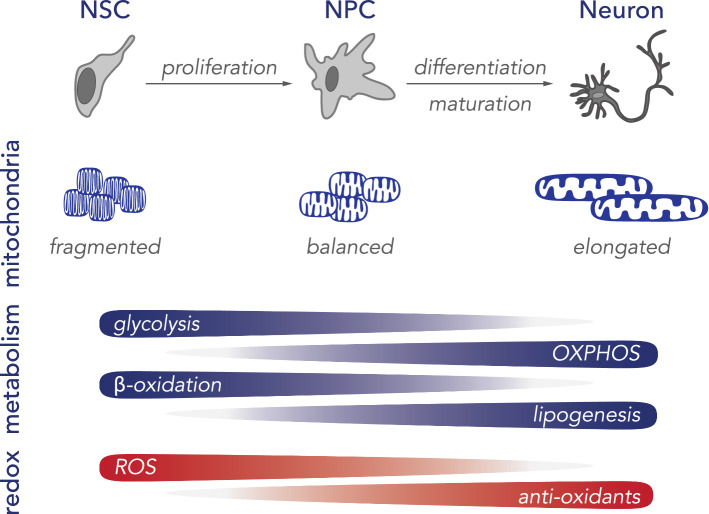
Fig. 2.
Schematic diagram of neuronal differentiation. During neurogenesis, neural stem cells (NSC) need to proliferate to neural progenitor cells (NPC) and differentiate into mature neurons. This progression is accompanied by several shifts, including the activation and proliferation of mitochondria, a transition from a glycolytic to aerobic metabolism, relying on oxidative phosphorylation (OXPHOS) for ATP synthesis. Additionally, fatty acid metabolism shifts from energy metabolism to de novo lipogenesis. The increased metabolic activity of the cells is accompanied by reactive oxygen species (ROS), which is countered by the activation of oxidative stress response genes to reduce overall ROS levels
Several factors have been shown to be important during this transition. For instance, the TP53-inducible glycolysis and apoptosis regulator TIGAR was shown to increase during brain development and neuronal development [106]. TIGAR inhibits glycolysis, directing the pathway into the pentose phosphate shunt, and is also proposed to regulate HK2 activity. Silencing in NSCs resulted in reduced neuronal differentiation and decreased expression of neuronal markers, such as β-III tubulin, microtubule-associated protein 2 (MAP2), glial fibrillary acidic protein (GFAP), Ngn1, and NeuroD1. Moreover, TIGAR expression was accompanied by decreased lactate production and increased expression of the neuron-specific lactate dehydrogenase B (LDHB), thus, shifting towards pyruvate production and mitochondrial metabolism [106]. Downregulation of HK2 and LDHA is required for neurodifferentiation, and overexpression of either HK2 or LDHA in NPCs blocks neurodifferentiation and promotes astrocyte formation, thus guiding differentiation away from a neuronal towards a glial profile [97].
Directionality of LDH seems to be predominantly regulated by substrate/product concentrations, although LDHA is proposed to prefer the conversion of pyruvate to lactate, while LDHB catalyses the reverse reaction [107]. LDHA is regulated by the transcription factors c-MYC and the hypoxia inducible factor HIF1α, but a recent study, using a neuroepithelial stem cell (NES) model of the autophagy adaptor Sequestosome 1 (SQSTM1/p62), suggested that upregulation of LDHA was independent of both c-Myc and HIF1α, arguing for additional pathways [99]. P62 is a cytosolic, multi-domain protein, considered to be involved in, among others, selective autophagy [108]. Patients with bi-allelic null mutations in p62 present with childhood- or adolescence-onset neurodegenerative disorder, characterised by progressive gait abnormalities, ataxia, dysarthria, dystonia, vertical gaze palsy, and cognitive decline [109, 110]. Loss of p62 in NES cells resulted in a dramatic increase of LDHA expression, which correlated with deficient neurodifferentiation [99]. This increase was absent in patient-derived fibroblasts, suggesting that the upregulation of LDHA might be stem cell-specific [99]. NES cells are a proliferative neural stem cell line that displays a high differentiation potential to various neuronal subtypes and glial cells [111]. Although patient-derived NES cells arrested proliferation upon neuronal induction, they failed to fully differentiate into neurons, as indicated by lower levels of the neuronal markers β-III tubulin and HuC/D. However, this did not seem to be due to the absence of mitochondrial-specific autophagy, but rather due to the cells’ failure to fully commit to a more aerobic metabolic profile as indicated by an inability to upregulate genes important for increased mitochondrial function, such as OXPHOS-specific genes. This could be partially rescued using the antioxidant N-acetylcysteine (NAC), suggesting an important role for p62 in oxygen sensing or reactive oxygen species (ROS) management [99]. Roles for p62 in oxidative stress management [112], hypoxia [113], and more recently, redox state management [114], have previously been suggested, with p62 shown to regulate the nuclear transcription factor erythroid 2-related factor 2 (NRF2). Under oxidative stress conditions p62 stabilises NRF2 by preventing its KEAP1-mediated degradation, which then translocates to the nucleus to bind to and activate upstream promoter regions of genes involved in inflammatory or antioxidant responses [115–119]. Two oxygen-sensitive cysteine residues in p62 have recently been suggested to activate autophagy in response to oxidative stress [114]. P62 is therefore potentially central to coordinating redox state and protein homeostasis in neurogenesis [99].
Support comes from rodent models deficient of p62, which present with several neurological phenotypes, ranging from memory loss to behavioural abnormalities to the accumulation of Tau tangles [120–123]. Specifically, p62 was shown to be important for neuronal cell survival and development in rats [121], while the deletion of p62 rescued the NSC pool in the SVZ and dental gyrus of autophagy-deficient FIP 200-KO mice, demonstrating an important role for p62 in the regulation of neuronal development, probably by regulating intracellular superoxide levels [124].
Redox state and ROS
Oxygen levels are crucial in determining cell fate [125], not only by regulating specific gene expression profiles through transcription factors such as HIF1α, but also by directly influencing enzymatic reactions [126]. In this respect, mitochondria are the largest consumers of oxygen, reducing it to water in the ETC in a coordinated fashion. Reactive oxygen species (ROS) are reduced forms of molecular oxygen that are predominantly formed during the transition of electrons in the ETC in mitochondria, or in peroxisomes. During OXPHOS a small percentage of molecular oxygen is reduced to superoxide (·O−2) due to electron leakage at complexes I or III of the mitochondrial respiratory chain (Fig. 3) [127]. Further dismutation or reduction can lead to a number of different radical oxygen species, including hydrogen peroxide (H2O2), which can leak out of mitochondria and act as a signalling molecule [128, 129]. Thus theoretically, ROS levels act as a function of mitochondrial respiration. However, multiple factors influence ROS levels, including oxygen availability, redox states of the redox co-factors NADH, FADH2, or ubiquinone, activities of antioxidant factors, such as glutathiones or superoxide dismutases, mitochondrial morphology, as well as mutations in OXPHOS subunits [130], and ROS levels are therefore much more complicated. Nevertheless, ROS can spontaneously react with a range of biological materials, including lipids, proteins, or nucleic acids, altering their function, and under stress situations ROS levels can lead to severe cellular damage, induce apoptosis and cell death, and has been suggested to be a part of various pathologies and the natural ageing process [131, 132].
Fig. 3.
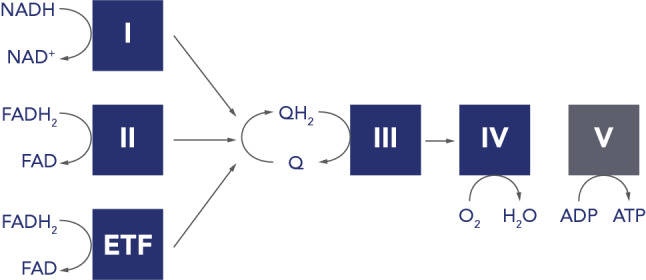
Schematic diagram of the reduction of ubiquinone. The complete oxidation of pyruvate generates 4 NADH molecules and 1 FADH2, which are oxidised on complex I (I; NADH: ubiquinone oxidoreductase) and complex II (II; succinate: ubiquinone oxidoreductase) of the mitochondrial electron transport chain, respectively, while reducing ubiquinone (Q) to ubiquinol (QH2). In contrast, one round of β-oxidation also forms 4 NADH, but 2 FADH2. The second FADH2 is oxidised by the electron transfer flavoprotein: ubiquinone oxidoreductase (ETF), which also contributes to the QH2 pool. Additionally, the two mitochondrial dehydrogenases glycerol 3-phosphate dehydrogenase and dihydroorotate dehydrogenase (both not shown) can contribute to the QH2 pool as part of glycerol metabolism and the de novo pyrimidine biosynthesis pathway, respectively. QH2 is oxidised at complex III (coenzyme Q: cytochrome c oxidoreductase). Oxygen is reduced to water at complex IV (IV; cytochrome c oxidase). An ATPase synthase (V) synthesises ATP from ADP at Pi)
ROS levels are known to be able to influence and activate various gene expression profiles [133] and have been suggested to be important for stem cell fate [134]. For instance, culturing NPCs in the presence of antioxidants improves neurodifferentiation [135, 136], and regulation of ROS levels has been suggested to be important for proliferation of hippocampal NPCs in mouse embryos [137]. Additionally, an age-dependent reduction in NRF2 expression has been linked to a reduced capacity of NPCs to regenerate in the SVZ of rats [138], but not in the SGZ of the dentate gyrus [139]. Nevertheless, NRF2 activity was important in both niches for linage commitment of NPCs to either a neuronal or glial fate. Further, studies in mice suggest that NRF2 levels can affect mitochondrial membrane potential and OXPHOS function, with loss of NRF2 associated with increased glycolytic activity and reduced substrate availability for mitochondrial respiration [140]. Thus, reduced NRF2 levels correlate with the age-associated reduction in NSCs in murine brains [138], and recent data suggest that this is caused by an inflammatory response in the ageing brain, linked to increased quiescence to protect the NSC pool [141]. The authors were also able to reactivate these old NSCs, suggesting a potential treatment target for age-associated neurodegeneration [141].
In general, quiescent NSCs are thought to have higher ROS levels, which gradually decrease during the progenitor stage, with low levels of ROS reported in mature neurons [128]. A recent study in mice suggested that NSCs require a spike in ROS levels before committing to proliferation and that this induction is prior to the redirection of cellular lipid metabolism to lipogenesis or induction of mitochondrial biogenesis [142]. Interestingly, the authors also suggested that NSCs can shift between different levels of proliferation induction, depending on ROS levels, without fully committing to enter neurogenesis [142]. It is therefore likely that multiple signals have to come together to initiate neurogenesis.
Oxygen levels vary across the brain, allowing for differential responses across neuronal niches. HIF1α is a main sensor of cellular oxygen, and upon low oxygen concentrations is stabilised, leading to the activation of a hypoxia response gene expression program [143–146]. Under hypoxic conditions the hydroxylation of HIF by α-ketoglutarate-dependent dioxygenases is suppressed, leading to the stabilisation of HIF1α, translocation to the nucleus and activation of gene expression. α-ketoglutarate (αKG, also known as 2-oxoglutarate) is a TCA cycle intermediate that can be converted to the neurotransmitter glutamate [146], thus firmly linking energy metabolism and oxygen sensing to neuronal function and potentially neurogenesis [147]. Recently, chronic mild hypoxia has been linked to adult neurogenesis in the hippocampus [148], while the deletion of HIF1α in mouse neural cells led to hydrocephaly, reduced number of NSCs and impaired spatial memory [149]. Along this line, work on stroke patients suggests that ischemia leads to the proliferation of NSCs in the SVZ and SGZ of the dentate gyrus, migration towards the lesion, and integration into the damaged area [52, 53]. Thus, oxygen sensing is clearly an important feature during neurogenesis, and its regulation is not only required for NSCs to exit their quiescent state but also to commit to their final cell linage. In agreement, oxygen regulates stemness via Wnt/β-catenin signalling [150], although this has been suggested to be independent HIF1α [151].
Lipid metabolism and neurogenesis
Long chain saturated fatty acids contain almost double the energy compared to glucose, and the brain consists of the second highest lipid content in the body after adipocytes. Despite this, fatty acid oxidation is low in the brain [152]. This is in contrast to other tissues with high energy demand, such as the heart, which utilise fatty acids as energy source, and the brain presumably requires fatty acids for lipid biosynthesis, rather than as energy source. While glucose is readily absorbed by cells from the blood, fatty acid transport is coupled to albumin, which does not cross the blood brain barrier, limiting lipid availability in the brain [153]. Nevertheless, the brain requires large amounts of lipids for membrane formation, which cannot be explained by de novo lipogenesis alone, and specific transporters for the uptake of essential fatty acids into the brain have since been identified [154–157]. There are several indications that astrocytes use β-oxidation as fuel source, allowing glucose to be predominantly used to support neurons [153, 158], but as β-oxidation releases acetyl-CoA there might be a metabolic compartmentalisation in neurons that does not favour lipids as energy source. For instance, acetyl-CoA, can either enter the TCA cycle, or be used for histone acetylation to regulate gene expression. It can form ketone bodies, which are used as energy source in neurons during starvation or enter the mevalonate pathway to generate farnesyl-pyrophosphate, which is important for the biosynthesis of the redox co-factor ubiquinone, sterol, cholesterol, heme A, dolichols, or the prenylation of proteins (Fig. 1) (For review see [158]). There are several other factors arguing against the use of fatty acids as energy source in neurons. First of all, the brain often requires fast bursts of ATP, which cannot be achieved by β-oxidation; further, β-oxidation has a higher oxygen requirement, which constitutes an increased risk for neurons to become hypoxic; and thirdly, the breakdown of fatty acids increases the FADH2/NADH ratio, due to FAD reduction at the electron transfer flavoprotein-ubiquinone oxidoreductase (ETF-QO), leading to increased competition for the ubiquinone pool in the inner mitochondrial membrane (Fig. 3). This, in turn, can depolarise mitochondria and increase the risk for ROS production at complex I of the ETC.
Nevertheless, as mentioned above, the glycolytic activity of NSCs is used to drive the synthesis of biomaterials for cell sustainability, with high expression of regulatable PKM2 [61], and NSCs in the SVZ of adult mouse brains have been shown to use β-oxidation for energy production [91, 92]. The increased bioenergetic demand during neuronal differentiation is accompanied by a switch to a more glucose-based metabolism, with a down-regulation of fatty acid oxidation. Simultaneously, de novo lipogenesis is upregulated [94, 153]. NSCs express a number of different fatty acid binding proteins (FBPs), with FBP5 and 7 important for NPC differentiation and migration [153, 159–161]. This correlates with an increase in lipid synthesis through fatty acid synthase during maturation to allow for increased membrane lipid synthesis [104]. This shift is regulated by the thyroid hormone responsive protein SPOT14, which is highly expressed in NSCs [90] and inhibits the fatty acid synthase FASN [104]. Additionally, metabolic analysis of embryonic NPCs demonstrated an increase in long chain fatty acids, citrate, cholesterol synthesis and decreased acyl-carnitines, suggesting fatty acid synthesis and membrane remodelling [105]. Thus, de novo lipogenesis is an important requirement for NSCs to exit their quiescent state and initiate proliferation. Interestingly, lipid droplets secreted from glial niche cells in Drosophila melanogaster have been shown to protect NSCs from exogenous ROS and enter proliferation [162], suggesting a further function of glial cells in regulating neurogenesis.
Mitochondrial morphology during neurodifferentiation
The increase in mitochondrial abundance and function is accompanied by an increase in mitochondrial DNA levels [163, 164], mitochondrial gene expression [165], and activity [166]. A reduced neuronal differentiation could be observed in culture by inhibiting mitochondrial translation with chloramphenicol [105, 165]. In addition, the expression of two master regulators of mitochondrial biogenesis, the peroxisome proliferator-activated receptor gamma co-activator 1-alpha (PGC1α) and the oestrogen-related receptor gamma (ERRγ), precedes increased mitochondrial abundance [97, 164]. This activation of mitochondria is marked by a remodelling and replacing the mitochondrial network [167].
Exercise is a well-known activator of mitochondrial biogenesis and has been suggested to trigger adult neurogenesis [168, 169]. However, overexpression of PGC1α in skeletal muscle had no effect on age-associated decline of NSCs [170], despite improved muscle performance and increased levels of the neuroplasticity promoting brain-derived neurotrophic factor, BDNF [168]. Thus, the exact mechanism is not yet clear. Nevertheless, the activation of stem cells coincides with changes in mitochondrial morphology from rounded small mitochondria, with dense and compact cristae in quiescent NSCs, to a more open and structured network [167]. These changes are believed to be fundamental to neurogenesis [91, 133, 171–173], and are possibly regulated by ROS signalling [133]. The importance of mitochondrial fusion and fission is well established, with numerous models, as well as mutations in patients with mitochondrial disease, demonstrating that disrupting either process can lead to severe consequences in the brain [174]. Additionally, the selective removal of mitochondria via autophagy, also termed mitophagy, has been shown to be an important contributor to cellular metabolism [175–177]. For instance, reduced expression of pro-apoptotic factors in murine NSCs resulted in reduced neurogenesis [178–180], while levels of the neuronal development regulator, NOTCH, have been suggested to be regulated by autophagy [181].
Many of the factors described above are essential for cell survival and the described mechanisms often require models with cell-type specific disruption of the factor in question. Although these studies have provided important information regarding the mechanisms of neurogenesis, it is likely that many essential genes will have similar effects, and thus the importance of, for instance, mitochondrial function in driving neurogenesis remains to be established. An indication that this is indeed the case stems from a mouse model with deficient proof-reading ability of the mitochondrial DNA polymerase γ, POLG [182, 183]. These mice present with increased mtDNA mutations, an age-associated decline in mitochondrial function, and a progressive aging phenotype [182, 184]. Ahlqvist and colleagues demonstrated that increased mtDNA mutation loads correlated with a reduction in NSCs in the SZV of adult mice, reduced self-renewal capacity, and decreased mitochondrial respiration [185]. Interestingly, self-renewal could be partially restored by NAC treatment, suggesting that ROS or the cellular redox state is important for NSC self-renewal [185]. Indeed, an increase in mtDNA mutations, together with reduced mitochondrial function, has been observed in brains during the natural ageing process in humans [186], and the prospect that modulating mitochondrial function and/or redox state to improve NSC renewal is enticing. These findings are supported by the observation that supplementation of somatic stem cell cultures with NAD+ improves cell survival and mitochondrial function [187, 188].
The requirement of NSCs to control their ROS and redox states suggests that oxygen might have negative effects in conjunction with mitochondrial disease [126]. In this regard, two mouse models with mitochondrial disease, could be rescued by reducing breathing oxygen levels [189, 190], although just increasing HIF1α had no effect [191]. Neural cells were not investigated in these models, but it will be interesting to determine whether hypoxia treatment has a positive effect on neurogenesis in combination with a mild mitochondrial dysfunction [192].
Conclusions
Several pathologies have been correlated with impaired neuronal differentiation, and the age-associated decline in cognitive function has been attributed to reduced neurogenesis with age [184]. For example, the autism spectrum disorder (ASD) has been linked to impaired neuronal development, since patient-derived reprogrammed cells showed impaired neuronal maturation [193], while a reduction of neurogenesis and impaired differentiation into neurons was reported in epileptic patients [194, 195]. Interestingly, reduced adult hippocampal neurogenesis has recently been observed in patients with Alzheimer’s disease [23], and mitochondrial dysfunction is a key feature in Alzheimer’s pathology [196], further pointing to an important connection between neurogenesis and mitochondria.
Over a century ago Santiago Ramon y Cajal wrote that in the brain "everything may die, nothing may be regenerated. It is for the science of the future to change, if possible, this harsh decree" [4]. The identification of adult neurogenesis has provided us with the possibility, but we are only at the beginning of understanding what factors are involved in the proliferation and differentiation of neuronal stem cells. Metabolism clearly plays an important part in this development and it is also clear that an elaborate network of factors, ranging from metabolites, oxygen, and transcription factors is required to carefully regulate quiescence, proliferation, and differentiation. The prospect that combinations of redox state modifiers, oxygen levels, and diet [197] has already shown to have positive effects on activating NSCs, brings hope to that the future indeed will be able to change this "harsh decree".
Acknowledgements
Open access funding provided by Karolinska Institute. AW is supported by the Swedish Research Council [2016-02179], the Knut and Alice Wallenberg Foundation [KAW 2013.0026]. AW is a Ragnar Söderberg Fellow in Medicine (M77/13).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Camilla Maffezzini and Javier Calvo-Garrido contributed equally to this work.
Contributor Information
Anna Wredenberg, Email: anna.wredenberg@ki.se.
Christoph Freyer, Email: christoph.freyer@ki.se.
References
- 1.Kirkwood TBL. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Kölliker A. Handbuch der gewebelehre des menschen. Für aerzte und studirende. Leipzig: W. Engelmann; 1855. [Google Scholar]
- 3.His W. Die Entwickelung des menschlichen Gehirns während der ersten Monate, Untersuchungsergebnisse. Leipzig: Hirzel; 1904. [Google Scholar]
- 4.Cajal SRY, DeFelipe J, Jones EG. Cajal's degeneration and regeneration of the nervous system. Oxford: Oxford University Press; 1991. [Google Scholar]
- 5.Gross CG. Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci. 2000;1:67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton A. The division of differentiated cells in the central nervous system of the white rat. J Comp Neurol. 1901;11:297–320. doi: 10.1002/cne.910110403. [DOI] [Google Scholar]
- 7.Allen E. The cessation of mitosis in the central nervous system of the albino rat. Baltimore: Waverley Press; 1912. [Google Scholar]
- 8.Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135:1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- 9.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 10.Altman J, Das GD. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1966;126:337–389. doi: 10.1002/cne.901260302. [DOI] [PubMed] [Google Scholar]
- 11.Altman J. Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anat Rec. 1963;145:573–591. doi: 10.1002/ar.1091450409. [DOI] [PubMed] [Google Scholar]
- 12.Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 13.Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burd GD, Nottebohm F. Ultrastructural characterization of synaptic terminals formed on newly generated neurons in a song control nucleus of the adult canary forebrain. J Comp Neurol. 1985;240:143–152. doi: 10.1002/cne.902400204. [DOI] [PubMed] [Google Scholar]
- 15.Paton JA, Nottebohm FN. Neurons generated in the adult brain are recruited into functional circuits. Science. 1984;225:1046–1048. doi: 10.1126/science.6474166. [DOI] [PubMed] [Google Scholar]
- 16.Gould E, Reeves AJ, Fallah M, et al. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci USA. 1999;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 18.Curtis MA, Kam M, Nannmark U, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 19.Gage FH, Coates PW, Palmer TD, et al. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci. 1995;92:11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy NS, Wang S, Jiang L, et al. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat Med. 2000;6:271–277. doi: 10.1038/73119. [DOI] [PubMed] [Google Scholar]
- 21.Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- 22.Spalding KL, Bergmann O, Alkass K, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nat Med. 2019;25:554–560. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- 24.Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001;294:2127–2130. doi: 10.1126/science.1065467. [DOI] [PubMed] [Google Scholar]
- 25.Spalding KL, Bhardwaj RD, Buchholz BA, et al. Retrospective birth dating of cells in humans. Cell. 2005;122:133–143. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 26.Apple DM, Fonseca RS, Kokovay E. The role of adult neurogenesis in psychiatric and cognitive disorders. Brain Res. 2017;1655:270–276. doi: 10.1016/j.brainres.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 27.Sorrells SF, Paredes MF, Cebrian-Silla A, et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555:377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boldrini M, Fulmore CA, Tartt AN, et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22:589–599.e5. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kempermann G, Gage FH, Aigner L, et al. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell. 2018;23:25–30. doi: 10.1016/j.stem.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 31.Piet R, Vargová L, Syková E, et al. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc Natl Acad Sci USA. 2004;101:2151–2155. doi: 10.1073/pnas.0308408100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pascual O, Casper KB, Kubera C, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 33.Magistretti PJ, Allaman I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat Rev Neurosci. 2018;19:235–249. doi: 10.1038/nrn.2018.19. [DOI] [PubMed] [Google Scholar]
- 34.Brancaccio M, Edwards MD, Patton AP, et al. Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science. 2019;363:187–192. doi: 10.1126/science.aat4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malatesta P, Appolloni I, Calzolari F. Radial glia and neural stem cells. Cell Tissue Res. 2008;331:165–178. doi: 10.1007/s00441-007-0481-8. [DOI] [PubMed] [Google Scholar]
- 36.Delgado-Esteban M, García-Higuera I, Maestre C, et al. APC/C-Cdh1 coordinates neurogenesis and cortical size during development. Nat Commun. 2013;4:2879. doi: 10.1038/ncomms3879. [DOI] [PubMed] [Google Scholar]
- 37.Paridaen JT, Huttner WB. Neurogenesis during development of the vertebrate central nervous system. EMBO Rep. 2014;15:351–364. doi: 10.1002/embr.201438447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertipaglia C, Gonçalves JC, Vallee RB. Nuclear migration in mammalian brain development. Semin Cell Dev Biol. 2018;82:57–66. doi: 10.1016/j.semcdb.2017.11.033. [DOI] [PubMed] [Google Scholar]
- 39.Goyal MS, Hawrylycz M, Miller JA, et al. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 2014;19:49–57. doi: 10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hawkins RA, Williamson DH, Krebs HA. Ketone-body utilization by adult and suckling rat brain in vivo. Biochem J. 1971;122:13–18. doi: 10.1042/bj1220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vannucci SJ, Simpson IA. Developmental switch in brain nutrient transporter expression in the rat. Am J Physiol Endocrinol Metab. 2003;285:E1127–E1134. doi: 10.1152/ajpendo.00187.2003. [DOI] [PubMed] [Google Scholar]
- 42.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 43.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Jain V, Langham MC, Wehrli FW. MRI estimation of global brain oxygen consumption rate. J Cereb Blood Flow Metab. 2010;30:1598–1607. doi: 10.1038/jcbfm.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36:587–597. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bond AM, Ming G-L, Song H. Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell. 2015;17:385–395. doi: 10.1016/j.stem.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marshall CAG, Suzuki SO, Goldman JE. Gliogenic and neurogenic progenitors of the subventricular zone: who are they, where did they come from, and where are they going? Glia. 2003;43:52–61. doi: 10.1002/glia.10213. [DOI] [PubMed] [Google Scholar]
- 48.Bonaguidi MA, Wheeler MA, Shapiro JS, et al. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ernst A, Alkass K, Bernard S, et al. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 50.Sanai N, Nguyen T, Ihrie RA, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gonçalves JT, Schafer ST, Gage FH. Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell. 2016;167:897–914. doi: 10.1016/j.cell.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 52.Marques BL, Carvalho GA, Freitas EMM, et al. The role of neurogenesis in neurorepair after ischemic stroke. Semin Cell Dev Biol. 2019 doi: 10.1016/j.semcdb.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 53.Dillen Y, Kemps H, Gervois P, et al. Adult neurogenesis in the subventricular zone and its regulation after ischemic stroke: implications for therapeutic approaches. Transl Stroke Res. 2019;145:573. doi: 10.1007/s12975-019-00717-8. [DOI] [PubMed] [Google Scholar]
- 54.Li G, Fang L, Fernández G, Pleasure SJ. The ventral hippocampus is the embryonic origin for adult neural stem cells in the dentate gyrus. Neuron. 2013;78:658–672. doi: 10.1016/j.neuron.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Almeida AS, Vieira HLA. Role of cell metabolism and mitochondrial function during adult neurogenesis. Neurochem Res. 2017;42:1787–1794. doi: 10.1007/s11064-016-2150-3. [DOI] [PubMed] [Google Scholar]
- 56.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 57.Semenza GL. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J. 2007;405:1–9. doi: 10.1042/BJ20070389. [DOI] [PubMed] [Google Scholar]
- 58.Schurr A. Glycolysis paradigm shift dictates a reevaluation of glucose and oxygen metabolic rates of activated neural tissue. Front Neurosci. 2018;12:700. doi: 10.3389/fnins.2018.00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tong X, Zhao F, Thompson CB. The molecular determinants of de novo nucleotide biosynthesis in cancer cells. Curr Opin Genet Dev. 2009;19:32–37. doi: 10.1016/j.gde.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Wyngene L, Vandewalle J, Libert C. Reprogramming of basic metabolic pathways in microbial sepsis: therapeutic targets at last? EMBO Mol Med. 2018;10:1. doi: 10.15252/emmm.201708712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Israelsen WJ, van der Heiden MG. Pyruvate kinase: function, regulation and role in cancer. Semin Cell Dev Biol. 2015;43:43–51. doi: 10.1016/j.semcdb.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem. 2002;277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 64.Morris AAM. Cerebral ketone body metabolism. J Inherit Metab Dis. 2005;28:109–121. doi: 10.1007/s10545-005-5518-0. [DOI] [PubMed] [Google Scholar]
- 65.Le Foll C. Hypothalamic fatty acids and ketone bodies sensing and role of FAT/CD36 in the regulation of food intake. Front Physiol. 2019;10:1036. doi: 10.3389/fphys.2019.01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le Foll C, Levin BE. Fatty acid-induced astrocyte ketone production and the control of food intake. Am J Physiol Regul Integr Comp Physiol. 2016;310:R1186–R1192. doi: 10.1152/ajpregu.00113.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mattson MP, Moehl K, Ghena N, et al. Intermittent metabolic switching, neuroplasticity and brain health. Nat Rev Neurosci. 2018;19:63–80. doi: 10.1038/nrn.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murray AJ, Knight NS, Cole MA, et al. Novel ketone diet enhances physical and cognitive performance. FASEB J. 2016;30:4021–4032. doi: 10.1096/fj.201600773R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hernandez AR, Hernandez CM, Campos K, et al. A ketogenic diet improves cognition and has biochemical effects in prefrontal cortex that are dissociable from hippocampus. Front Aging Neurosci. 2018;10:391. doi: 10.3389/fnagi.2018.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sleiman SF, Henry J, Al-Haddad R, et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife. 2016;5:560. doi: 10.7554/eLife.15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carneiro L, Geller S, Hébert A, et al. Hypothalamic sensing of ketone bodies after prolonged cerebral exposure leads to metabolic control dysregulation. Sci Rep. 2016;6:34909. doi: 10.1038/srep34909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dringen R, Gebhardt R, Hamprecht B. Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res. 1993;623:208–214. doi: 10.1016/0006-8993(93)91429-v. [DOI] [PubMed] [Google Scholar]
- 73.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown AM, Baltan Tekkök S, Ransom BR. Energy transfer from astrocytes to axons: the role of CNS glycogen. Neurochem Int. 2004;45:529–536. doi: 10.1016/j.neuint.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 75.Wyss MT, Jolivet R, Buck A, et al. In vivo evidence for lactate as a neuronal energy source. J Neurosci. 2011;31:7477–7485. doi: 10.1523/JNEUROSCI.0415-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang J, Ruchti E, Petit J-M, et al. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci. 2014;111:12228–12233. doi: 10.1073/pnas.1322912111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herrero-Mendez A, Almeida A, Fernández E, et al. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 78.Bouzier-Sore A-K, Voisin P, Canioni P, et al. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J Cereb Blood Flow Metab. 2003;23:1298–1306. doi: 10.1097/01.WCB.0000091761.61714.25. [DOI] [PubMed] [Google Scholar]
- 79.McKenna MC, Hopkins IB, Carey A. Alpha-cyano-4-hydroxycinnamate decreases both glucose and lactate metabolism in neurons and astrocytes: implications for lactate as an energy substrate for neurons. J Neurosci Res. 2001;66:747–754. doi: 10.1002/jnr.10084. [DOI] [PubMed] [Google Scholar]
- 80.Vilchez D, Ros S, Cifuentes D, et al. Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat Neurosci. 2007;10:1407–1413. doi: 10.1038/nn1998. [DOI] [PubMed] [Google Scholar]
- 81.Prigione A, Fauler B, Lurz R, et al. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells. 2010;28:721–733. doi: 10.1002/stem.404. [DOI] [PubMed] [Google Scholar]
- 82.Folmes CDL, Nelson TJ, Martinez-Fernandez A, et al. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011;14:264–271. doi: 10.1016/j.cmet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kondoh H, Lleonart ME, Nakashima Y, et al. A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid Redox Signal. 2007;9:293–299. doi: 10.1089/ars.2006.1467. [DOI] [PubMed] [Google Scholar]
- 84.Zhang J, Khvorostov I, Hong JS, et al. UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J. 2011;30:4860–4873. doi: 10.1038/emboj.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sánchez-Aragó M, García-Bermúdez J, Martínez-Reyes I, et al. Degradation of IF1 controls energy metabolism during osteogenic differentiation of stem cells. EMBO Rep. 2013;14:638–644. doi: 10.1038/embor.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takubo K, Nagamatsu G, Kobayashi CI, et al. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell. 2013;12:49–61. doi: 10.1016/j.stem.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Flores A, Schell J, Krall AS, et al. Lactate dehydrogenase activity drives hair follicle stem cell activation. Nat Cell Biol. 2017;19:1017–1026. doi: 10.1038/ncb3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maryanovich M, Zaltsman Y, Ruggiero A, et al. An MTCH2 pathway repressing mitochondria metabolism regulates haematopoietic stem cell fate. Nat Commun. 2015;6:7901–7909. doi: 10.1038/ncomms8901. [DOI] [PubMed] [Google Scholar]
- 89.Codega P, Silva-Vargas V, Paul A, et al. Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron. 2014;82:545–559. doi: 10.1016/j.neuron.2014.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shin J, Berg DA, Zhu Y, et al. Single-cell RNA-seq with waterfall reveals molecular cascades underlying adult neurogenesis. Cell Stem Cell. 2015;17:360–372. doi: 10.1016/j.stem.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stoll EA, Makin R, Sweet IR, et al. Neural stem cells in the adult subventricular zone oxidize fatty acids to produce energy and support neurogenic activity. Stem Cells. 2015;33:2306–2319. doi: 10.1002/stem.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hamilton LK, Dufresne M, Joppé SE, et al. Aberrant lipid metabolism in the forebrain niche suppresses adult neural stem cell proliferation in an animal model of Alzheimer's disease. Cell Stem Cell. 2015;17:397–411. doi: 10.1016/j.stem.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 93.Llorens-Bobadilla E, Zhao S, Baser A, et al. Single-cell transcriptomics reveals a population of dormant neural stem cells that become activated upon brain injury. Cell Stem Cell. 2015;17:329–340. doi: 10.1016/j.stem.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 94.Hamilton LK, Fernandes KJL. Neural stem cells and adult brain fatty acid metabolism: lessons from the 3xTg model of Alzheimer's disease. Biol Cell. 2018;110:6–25. doi: 10.1111/boc.201700037. [DOI] [PubMed] [Google Scholar]
- 95.Beckervordersandforth R, Ebert B, Schäffner I, et al. Role of mitochondrial metabolism in the control of early lineage progression and aging phenotypes in adult hippocampal neurogenesis. Neuron. 2017;93:560–573.e6. doi: 10.1016/j.neuron.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cabello-Rivera D, Sarmiento-Soto H, López-Barneo J, Muñoz-Cabello AM. Mitochondrial complex I function is essential for neural stem/progenitor cells proliferation and differentiation. Front Neurosci. 2019;13:664. doi: 10.3389/fnins.2019.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zheng X, Boyer L, Jin M, et al. Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation. Elife. 2016;5:859. doi: 10.7554/eLife.13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khacho M, Slack RS. Mitochondrial activity in the regulation of stem cell self-renewal and differentiation. Curr Opin Cell Biol. 2017;49:1–8. doi: 10.1016/j.ceb.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 99.Calvo-Garrido J, Maffezzini C, Schober FA, et al. SQSTM1/p62-directed metabolic reprogramming is essential for normal neurodifferentiation. Stem Cell Rep. 2019;12:696–711. doi: 10.1016/j.stemcr.2019.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wellen KE, Hatzivassiliou G, Sachdeva UM, et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mlody B, Lorenz C, Inak G, Prigione A. Energy metabolism in neuronal/glial induction and in iPSC models of brain disorders. Semin Cell Dev Biol. 2016;52:102–109. doi: 10.1016/j.semcdb.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 102.Yao B, Christian KM, He C, et al. Epigenetic mechanisms in neurogenesis. Nat Rev Neurosci. 2016;17:537–549. doi: 10.1038/nrn.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khacho M, Harris R, Slack RS. Mitochondria as central regulators of neural stem cell fate and cognitive function. Nat Rev Neurosci. 2019;20:34–48. doi: 10.1038/s41583-018-0091-3. [DOI] [PubMed] [Google Scholar]
- 104.Knobloch M, Braun SMG, Zurkirchen L, et al. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature. 2013;493:226–230. doi: 10.1038/nature11689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Agostini M, Romeo F, Inoue S, et al. Metabolic reprogramming during neuronal differentiation. Cell Death Differ. 2016;23:1502–1514. doi: 10.1038/cdd.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou W, Zhao T, Du J, et al. TIGAR promotes neural stem cell differentiation through acetyl-CoA-mediated histone acetylation. Cell Death Dis. 2019;10:198. doi: 10.1038/s41419-019-1434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Valvona CJ, Fillmore HL, Nunn PB, Pilkington GJ. The Regulation and function of lactate dehydrogenase a: therapeutic potential in brain tumor. Brain Pathol. 2016;26:3–17. doi: 10.1111/bpa.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lamark T, Svenning S, Johansen T. Regulation of selective autophagy: the p62/SQSTM1 paradigm. Essays Biochem. 2017;61:609–624. doi: 10.1042/EBC20170035. [DOI] [PubMed] [Google Scholar]
- 109.Haack TB, Ignatius E, Calvo-Garrido J, et al. Absence of the autophagy adaptor SQSTM1/p62 causes childhood-onset neurodegeneration with ataxia, dystonia, and gaze palsy. Am J Hum Genet. 2016;99:735–743. doi: 10.1016/j.ajhg.2016.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Muto V, Flex E, Kupchinsky Z, et al. Biallelic SQSTM1 mutations in early-onset, variably progressive neurodegeneration. Neurology. 2018;91:e319–e330. doi: 10.1212/WNL.0000000000005869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Falk A, Falk A, Koch P, et al. Capture of neuroepithelial-like stem cells from pluripotent stem cells provides a versatile system for in vitro production of human neurons. PLoS ONE. 2012;7:e29597. doi: 10.1371/journal.pone.0029597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jiang T, Harder B, Rojo de la Vega M, et al. p62 links autophagy and Nrf2 signaling. Free Radic Biol Med. 2015;88:199–204. doi: 10.1016/j.freeradbiomed.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rantanen K, Pursiheimo JP, Hogel H, et al. p62/SQSTM1 regulates cellular oxygen sensing by attenuating PHD3 activity through aggregate sequestration and enhanced degradation. J Cell Sci. 2013;126:1144–1154. doi: 10.1242/jcs.115667. [DOI] [PubMed] [Google Scholar]
- 114.Carroll B, Otten EG, Manni D, et al. Oxidation of SQSTM1/p62 mediates the link between redox state and protein homeostasis. Nat Commun. 2018;9:256. doi: 10.1038/s41467-017-02746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Copple IM, Lister A, Obeng AD, et al. Physical and functional interaction of sequestosome 1 with Keap1 regulates the Keap1-Nrf2 cell defense pathway. J Biol Chem. 2010;285:16782–16788. doi: 10.1074/jbc.M109.096545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fan W, Tang Z, Chen D, et al. Keap1 facilitates p62-mediated ubiquitin aggregate clearance via autophagy. Autophagy. 2010;6:614–621. doi: 10.4161/auto.6.5.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jain A, Lamark T, Lamark T, et al. p62/SQSTM1Is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Komatsu M, Komatsu M, Kurokawa H, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 119.Lau A, Wang XJ, Zhao F, et al. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol Cell Biol. 2010;30:3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Durán A, Serrano M, Leitges M, et al. The atypical PKC-interacting protein p62 is an important mediator of RANK-activated osteoclastogenesis. Dev Cell. 2004;6:303–309. doi: 10.1016/s1534-5807(03)00403-9. [DOI] [PubMed] [Google Scholar]
- 121.Joung I, Kim HJ, Kwon YK. p62 modulates Akt activity via association with PKCζ in neuronal survival and differentiation. Biochem Biophys Res Commun. 2005;334:654–660. doi: 10.1016/j.bbrc.2005.06.138. [DOI] [PubMed] [Google Scholar]
- 122.Ramesh Babu J, Lamar Seibenhener M, Peng J, et al. Genetic inactivation of p62 leads to accumulation of hyperphosphorylated tau and neurodegeneration. J Neurochem. 2008;106:107–120. doi: 10.1111/j.1471-4159.2008.05340.x. [DOI] [PubMed] [Google Scholar]
- 123.Kwon J, Han E, Bui C-B, et al. Assurance of mitochondrial integrity and mammalian longevity by the p62–Keap1–Nrf2–Nqo1 cascade. EMBO Rep. 2012;13:150–156. doi: 10.1038/embor.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang C, Chen S, Yeo S, et al. Elevated p62/SQSTM1 determines the fate of autophagy-deficient neural stem cells by increasing superoxide. J Cell Biol. 2016;212:545–560. doi: 10.1083/jcb.201507023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim D-Y, Rhee I, Paik J. Metabolic circuits in neural stem cells. Cell Mol Life Sci. 2014;71:4221–4241. doi: 10.1007/s00018-014-1686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mootha VK, Chinnery PF. Oxygen in mitochondrial disease: can there be too much of a good thing? J Inherit Metab Dis. 2018;41:761–763. doi: 10.1007/s10545-018-0210-3. [DOI] [PubMed] [Google Scholar]
- 127.Jastroch M, Divakaruni AS, Mookerjee S, et al. Mitochondrial proton and electron leaks. Essays Biochem. 2010;47:53–67. doi: 10.1042/bse0470053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Le Belle JE, Orozco NM, Paucar AA, et al. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8:59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bigarella CL, Liang R, Ghaffari S. Stem cells and the impact of ROS signaling. Development. 2014;141:4206–4218. doi: 10.1242/dev.107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Panieri E, Santoro MM. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis. 2016;7:e2253–e2253. doi: 10.1038/cddis.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Murphy MP, Holmgren A, Larsson N-G, et al. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011;13:361–366. doi: 10.1016/j.cmet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kauppila TES, Kauppila JHK, Larsson N-G. Mammalian mitochondria and aging: an update. Cell Metab. 2017;25:57–71. doi: 10.1016/j.cmet.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 133.Khacho M, Clark A, Svoboda DS, et al. Mitochondrial dynamics impacts stem cell identity and fate decisions by regulating a nuclear transcriptional program. Cell Stem Cell. 2016;19:232–247. doi: 10.1016/j.stem.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 134.Liang R, Ghaffari S. Stem cells, redox signaling, and stem cell aging. Antioxid Redox Signal. 2014;20:1902–1916. doi: 10.1089/ars.2013.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shaban S, El-Husseny MWA, Abushouk AI, et al. Effects of antioxidant supplements on the survival and differentiation of stem cells. Oxid Med Cell Longev. 2017;2017:5032102–5032116. doi: 10.1155/2017/5032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kärkkäinen V, Pomeshchik Y, Savchenko E, et al. Nrf2 regulates neurogenesis and protects neural progenitor cells against Aβ toxicity. Stem Cells. 2014;32:1904–1916. doi: 10.1002/stem.1666. [DOI] [PubMed] [Google Scholar]
- 137.Yoneyama M, Kawada K, Gotoh Y, et al. Endogenous reactive oxygen species are essential for proliferation of neural stem/progenitor cells. Neurochem Int. 2010;56:740–746. doi: 10.1016/j.neuint.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 138.Corenblum MJ, Ray S, Remley QW, et al. Reduced Nrf2 expression mediates the decline in neural stem cell function during a critical middle-age period. Aging Cell. 2016;15:725–736. doi: 10.1111/acel.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ray S, Corenblum MJ, Anandhan A, et al. A role for Nrf2 expression in defining the aging of hippocampal neural stem cells. Cell Transplant. 2018;27:589–606. doi: 10.1177/0963689718774030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Holmström KM, Baird L, Zhang Y, et al. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol Open. 2013;2:761–770. doi: 10.1242/bio.20134853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kalamakis G, Brüne D, Ravichandran S, et al. Quiescence modulates stem cell maintenance and regenerative capacity in the aging brain. Cell. 2019;176:1407–1419.e14. doi: 10.1016/j.cell.2019.01.040. [DOI] [PubMed] [Google Scholar]
- 142.Adusumilli VS, Walker TL, Overall RW, et al. Redox potential defines functional states of adult hippocampal stem cells. bioRxiv. 2019;7:606186. doi: 10.1101/606186. [DOI] [Google Scholar]
- 143.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Maxwell PH, Pugh CW, Ratcliffe PJ. Inducible operation of the erythropoietin 3′ enhancer in multiple cell lines: evidence for a widespread oxygen-sensing mechanism. Proc Natl Acad Sci USA. 1993;90:2423–2427. doi: 10.1073/pnas.90.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Carmeliet P, Dor Y, Herbert JM, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 146.Schödel J, Ratcliffe PJ. Mechanisms of hypoxia signalling: new implications for nephrology. Nat Rev Nephrol. 2019;15:641–659. doi: 10.1038/s41581-019-0182-z. [DOI] [PubMed] [Google Scholar]
- 147.Francis KR, Wei L. Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death Dis. 2010;1:e22–e22. doi: 10.1038/cddis.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sun C, Fu J, Qu Z, et al. Chronic mild hypoxia promotes hippocampal neurogenesis involving Notch1 signaling in epileptic rats. Brain Res. 2019;1714:88–98. doi: 10.1016/j.brainres.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 149.Tomita S, Ueno M, Sakamoto M, et al. Defective brain development in mice lacking the Hif-1alpha gene in neural cells. Mol Cell Biol. 2003;23:6739–6749. doi: 10.1128/mcb.23.19.6739-6749.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mazumdar J, O'Brien WT, Johnson RS, et al. O2 regulates stem cells through Wnt/β-catenin signalling. Nat Cell Biol. 2010;12:1007–1013. doi: 10.1038/ncb2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Candelario KM, Shuttleworth CW, Cunningham LA. Neural stem/progenitor cells display a low requirement for oxidative metabolism independent of hypoxia inducible factor-1alpha expression. J Neurochem. 2013;125:420–429. doi: 10.1111/jnc.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Edmond J. Energy metabolism in developing brain cells. Can J Physiol Pharmacol. 1992;70(Suppl):S118–S129. doi: 10.1139/y92-253. [DOI] [PubMed] [Google Scholar]
- 153.Knobloch M. The role of lipid metabolism for neural stem cell regulation. Brain Plast. 2017;3:61–71. doi: 10.3233/BPL-160035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Edmond J. Essential polyunsaturated fatty acids and the barrier to the brain: the components of a model for transport. J Mol Neurosci. 2001;16:181–193. doi: 10.1385/JMN:16:2-3:181. [DOI] [PubMed] [Google Scholar]
- 155.Nguyen LN, Ma D, Shui G, et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 156.Ben-Zvi A, Lacoste B, Kur E, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Betsholtz C. Lipid transport and human brain development. Nat Genet. 2015;47:699–701. doi: 10.1038/ng.3348. [DOI] [PubMed] [Google Scholar]
- 158.Schönfeld P, Reiser G. Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain. J Cereb Blood Flow Metab. 2013;33:1493–1499. doi: 10.1038/jcbfm.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kurtz A, Zimmer A, Schnütgen F, et al. The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development. 1994;120:2637–2649. doi: 10.1242/dev.120.9.2637. [DOI] [PubMed] [Google Scholar]
- 160.Watanabe A, Toyota T, Owada Y, et al. Fabp7 maps to a quantitative trait locus for a schizophrenia endophenotype. PLoS Biol. 2007;5:e297. doi: 10.1371/journal.pbio.0050297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Matsumata M, Sakayori N, Maekawa M, et al. The effects of Fabp7 and Fabp5 on postnatal hippocampal neurogenesis in the mouse. Stem Cells. 2012;30:1532–1543. doi: 10.1002/stem.1124. [DOI] [PubMed] [Google Scholar]
- 162.Bailey AP, Koster G, Guillermier C, et al. Antioxidant role for lipid droplets in a stem cell niche of Drosophila. Cell. 2015;163:340–353. doi: 10.1016/j.cell.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Facucho-Oliveira JM, St John JC. The relationship between pluripotency and mitochondrial DNA proliferation during early embryo development and embryonic stem cell differentiation. Stem Cell Rev Rep. 2009;5:140–158. doi: 10.1007/s12015-009-9058-0. [DOI] [PubMed] [Google Scholar]
- 164.O'Brien LC, Keeney PM, Bennett JP. Differentiation of human neural stem cells into motor neurons stimulates mitochondrial biogenesis and decreases glycolytic flux. Stem Cells Dev. 2015;24:1984–1994. doi: 10.1089/scd.2015.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vayssière JL, Cordeau-Lossouarn L, Larcher JC, et al. Participation of the mitochondrial genome in the differentiation of neuroblastoma cells. Vitro Cell Dev Biol. 1992;28A:763–772. doi: 10.1007/bf02631065. [DOI] [PubMed] [Google Scholar]
- 166.Chen C-T, Hsu S-H, Wei YH. Upregulation of mitochondrial function and antioxidant defense in the differentiation of stem cells. Biochim Biophys Acta. 2010;1800:257–263. doi: 10.1016/j.bbagen.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 167.Rastogi A, Joshi P, Contreras E, Gama V. Remodeling of mitochondrial morphology and function: an emerging hallmark of cellular reprogramming. Cell Stress. 2019;3:181–194. doi: 10.15698/cst2019.06.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wrann CD, White JP, Salogiannnis J, et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18:649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Duzel E, van Praag H, Sendtner M. Can physical exercise in old age improve memory and hippocampal function? Brain. 2016;139:662–673. doi: 10.1093/brain/awv407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Karlsson L, González-Alvarado MN, Motalleb R, et al. Constitutive PGC-1α overexpression in skeletal muscle does not protect from age-dependent decline in neurogenesis. Sci Rep. 2019;9:12320. doi: 10.1038/s41598-019-48795-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen H, Chan DC. Critical dependence of neurons on mitochondrial dynamics. Curr Opin Cell Biol. 2006;18:453–459. doi: 10.1016/j.ceb.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 172.Homem CCF, Steinmann V, Burkard TR, et al. Ecdysone and mediator change energy metabolism to terminate proliferation in Drosophila neural stem cells. Cell. 2014;158:874–888. doi: 10.1016/j.cell.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 173.Steib K, Schäffner I, Jagasia R, et al. Mitochondria modify exercise-induced development of stem cell-derived neurons in the adult brain. J Neurosci. 2014;34:6624–6633. doi: 10.1523/JNEUROSCI.4972-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chan DC. Mitochondrial dynamics and its involvement in disease. Annu Rev Pathol. 2019;15:2129252597. doi: 10.1146/annurev-pathmechdis-012419-032711. [DOI] [PubMed] [Google Scholar]
- 175.Guan J-L, Simon AK, Prescott M, et al. Autophagy in stem cells. Autophagy. 2013;9:830–849. doi: 10.4161/auto.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kaur J, Debnath J. Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol. 2015;16:461–472. doi: 10.1038/nrm4024. [DOI] [PubMed] [Google Scholar]
- 177.Boya P, Codogno P, Rodriguez-Muela N. Autophagy in stem cells: repair, remodelling and metabolic reprogramming. Development. 2018;145:dev146506. doi: 10.1242/dev.146506. [DOI] [PubMed] [Google Scholar]
- 178.Vázquez P, Arroba AI, Cecconi F, et al. Atg5 and Ambra1 differentially modulate neurogenesis in neural stem cells. Autophagy. 2012;8:187–199. doi: 10.4161/auto.8.2.18535. [DOI] [PubMed] [Google Scholar]
- 179.Lv X, Jiang H, Li B, et al. The crucial role of Atg5 in cortical neurogenesis during early brain development. Sci Rep. 2014;4:6010. doi: 10.1038/srep06010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xi Y, Dhaliwal JS, Ceizar M, et al. Knockout of Atg5 delays the maturation and reduces the survival of adult-generated neurons in the hippocampus. Cell Death Dis. 2016;7:e2127–e2127. doi: 10.1038/cddis.2015.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wu X, Fleming A, Ricketts T, et al. Autophagy regulates Notch degradation and modulates stem cell development and neurogenesis. Nat Commun. 2016;7:10533. doi: 10.1038/ncomms10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Trifunovic A, Wredenberg A, Falkenberg M, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 183.Kujoth GC, Hiona A, Pugh TD, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 184.Bratic A, Larsson N-G. The role of mitochondria in aging. J Clin Investig. 2013;123:951–957. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ahlqvist KJ, Hämäläinen RH, Yatsuga S, et al. Somatic progenitor cell vulnerability to mitochondrial DNA mutagenesis underlies progeroid phenotypes in Polg mutator mice. Cell Metab. 2012;15:100–109. doi: 10.1016/j.cmet.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 186.Theurey P, Pizzo P. The aging mitochondria. Genes (Basel) 2018;9:22. doi: 10.3390/genes9010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gomes AP, Price NL, Ling AJY, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang H, Ryu D, Wu Y, et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- 189.Jain IH, Zazzeron L, Goli R, et al. Hypoxia as a therapy for mitochondrial disease. Science. 2016;352:54–61. doi: 10.1126/science.aad9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ast T, Meisel JD, Patra S, et al. Hypoxia rescues frataxin loss by restoring iron sulfur cluster biogenesis. Cell. 2019;177:1507–1521.e16. doi: 10.1016/j.cell.2019.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ferrari M, Jain IH, Goldberger O, et al. Hypoxia treatment reverses neurodegenerative disease in a mouse model of Leigh syndrome. Proc Natl Acad Sci. 2017;114:E4241–E4250. doi: 10.1073/pnas.1621511114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Freyer C, Larsson N-G. Is energy deficiency good in moderation? Cell. 2007;131:448–450. doi: 10.1016/j.cell.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 193.Schafer ST, Paquola ACM, Stern S, et al. Pathological priming causes developmental gene network heterochronicity in autistic subject-derived neurons. Nat Neurosci. 2019;22:243–255. doi: 10.1038/s41593-018-0295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hattiangady B, Rao MS, Shetty AK. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis. 2004;17:473–490. doi: 10.1016/j.nbd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 195.Hattiangady B, Shetty AK. Decreased neuronal differentiation of newly generated cells underlies reduced hippocampal neurogenesis in chronic temporal lobe epilepsy. Hippocampus. 2010;20:97–112. doi: 10.1002/hipo.20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Perez Ortiz JM, Swerdlow RH. Mitochondrial dysfunction in Alzheimer's disease: role in pathogenesis and novel therapeutic opportunities. Br J Pharmacol. 2019;176:3489–3507. doi: 10.1111/bph.14585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Stangl D, Thuret S. Impact of diet on adult hippocampal neurogenesis. Genes Nutr. 2009;4:271–282. doi: 10.1007/s12263-009-0134-5. [DOI] [PMC free article] [PubMed] [Google Scholar]