Abstract
Nanocomposite hydrogels consist of polymeric network embedded with functional nanoparticles or nanostructures, which not only contribute to the enhanced mechanical properties but also exhibit the bioactivities for regulating cell behavior. Bisphosphonates (BPs) are capable of coordinating with various metal ions and modulating bone homeostasis. Thanks to the inherent dynamic properties of metal–ligand coordination bonds, BP-based nanocomposite hydrogels possess tunable mechanical properties, highly dynamic structures, and the capability to mediate controlled release of encapsulated therapeutic agents, thereby making them highly versatile for various biomedical applications. This review presents the comprehensive overview of recent developments in BP-based nanocomposite hydrogels with an emphasis on the properties of embedded nanoparticles (NPs) and interactions between hydrogel network and NPs. Furthermore, various challenges in the biomedical applications of these hydrogels are discussed to provide an outlook of potential clinical translation.
Keywords: Bisphosphonates, Metal–ligand coordination, Nanocomposite hydrogels
Graphical abstract
Highlights
-
•
Varieties and physicochemical properties of BPs, especially in coordination chemistry are highlighted.
-
•
The correlation between the nanostructures and highly dynamic properties of BP-based nanocomposite hydrogels are discussed.
-
•
Diverse biomedical applications of BP-based nanocomposite hydrogels are summarized.
1. Introduction
Nanocomposite hydrogels are three-dimensional (3D) polymeric network embedded with nanoparticles or nanostructures, which help reinforce the hydrogel structure and provide desirable bioactive functions. In the past few decades, nanocomposite hydrogels have experienced unprecedented development in various fields of biomedical engineering, including drug delivery [[1], [2], [3]], wound healing [[4], [5], [6], [7]], osteogenesis [[8], [9], [10], [11]], biosensors [12,13]. In nanocomposite hydrogels, the nanoparticles or nanostructures are embedded in the hydrogel network by either covalent bonds [[14], [15], [16], [17]] or noncovalent bonds including meta–ligand coordination bonds [[18], [19], [20], [21]], hydrogen bonds [22,23], van der Waals interactions [24] and electrostatic interactions [25,26]. Great advances have been made in the development of nanocomposite hydrogels. However, the comprehensive understanding on the fundamental principles and biomedical applications of BP-based nanocomposite hydrogels remains to be further explored. In this review, we focus on the fabrication, characterization and biomedical applications of BP-based nanocomposite hydrogels.
BPs are primary agents for the treatment of various bone diseases. The varieties of substituting side chain on the central carbon atom lead to the structural diversities of BPs. Due to the pair of phosphate groups, BPs possess the capacity of binding metal ions via coordination bonds, thereby forming self-assembled nanoparticles and nanostructures. These nanoparticles and nanostructures lay the structural basis of BP-based nanocomposite hydrogels. Meanwhile, BPs are important bioactive molecules with the anti-bone resorption and anti-tumor activities for clinical treatments. Moreover, many metal ions participate in various biological activities in human body. For example, magnesium ion (Mg2+) is required in the integrin binding and cell attachment and can therefore regulate the phenotypic polarization of macrophages [[27], [28], [29]]. Mg2+ ion is reported to induce the alternative polarization (M2) of host macrophages to modulate host responses to the biomedical implants [30,31]. Therefore, BP-based nanocomposite hydrogels have the potential to immunomodulate the recruited immune cells, which are important to the success of biomedical implants. Through metal–ligand coordination bonds, self-assembled bioactive BP-based NPs are tightly embedded into hydrogel networks. Based on the different methods of incorporating BP NPs into hydrogel network, BP-based nanocomposite hydrogels can be divided into two categories, for the ex-situ one, the NPs are pre-fabricated and then embedded into the hydrogel networks, whereas for the in-situ one, the NPs are formed and embedded into hydrogel networks during the gelation process. Furthermore, BP-based nanocomposite hydrogels have desirable properties, including tunable mechanical properties, self-healing, injectability, and stimuli-responsive release of loaded cargo molecules. Because of the excellent properties, BP-based nanocomposite hydrogels have been applied in various biomedical fields, including drug delivery, tissue regeneration, wound healing, and bioprinting.
We hope this review can help introduce the basic principles and attractive properties of BP-based nanocomposite hydrogels to researchers from different field so as to further expand the applications of BP-based nanocomposite hydrogels.
2. Introduction of bisphosphonates
2.1. Varieties of bisphosphonates
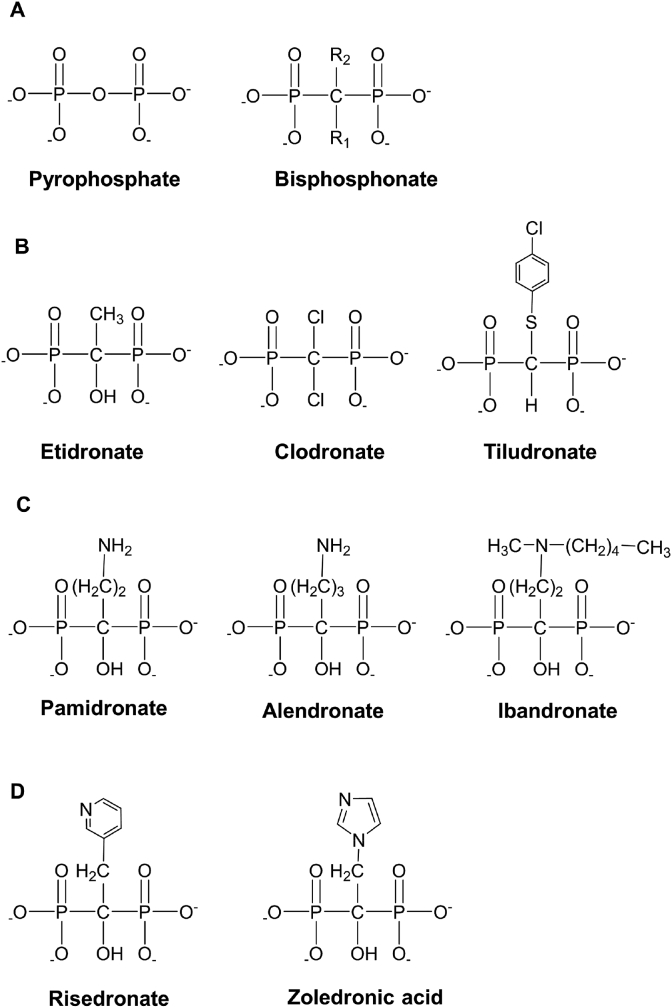
BPs are a family of chemicals used for treating bone diseases and have experienced significant development in recent years. About 30 years ago, Fleisch et al. found that pyrophosphate in plasma and urine has an inhibiting effect on ectopic calcification [32]. However, pyrophosphate is ineffective in oral administration and is rapidly deactivated by enzymatic hydrolysis after injection [33]. It was later found that replacing the P–O–P group in the pyrophosphate structure with the P–C–P group can increase the stability of the obtained derivative, which was designated as BP, against hydrolysis and change its biological properties and toxicity (Fig. 1A) [34]. Compared with pyrophosphate, BPs have the characteristic nonhydrolyzable carbon [35]. The two unique side chains surrounding the nonhydrolyzable carbon are the R1 and R2 groups [36]. The R1 side chain is related to the bone affinity, which is significantly enhanced when the R1 side chain is a hydroxyl group. The R2 side chain determines the anti-bone resorption activity of BPs [37]. Based on the diversities of the two side chains, the structures of BPs vary widely, and they experience three generations of development. The first generation of BPs includes those without nitrogen, which include etidronate, clodronate, and tiludronate (Fig. 1B). The second generation of BPs includes functional groups with nitrogen, including pamidronate, alendronate, and ibandronate (Fig. 1C). The third generation of BPs possesses the nitrogen-containing heterocyclic structures, including risedronate and zoledronate (Fig. 1D).
Fig. 1.
The structures of the three generations of BPs. (A) The general structure of pyrophosphate and BPs. (B) The first generation of BPs without nitrogen-containing side chains. (C) The second generation of BPs with the nitrogen-containing functional groups. (D) The third generation of BPs possessing the nitrogen-containing heterocyclic structures.
2.2. Chemical properties of bisphosphonates
Coordination bonds are a special type of covalent bond that require one atom (A) of the two bonding atoms to have a lone pair of electrons, and the other atom (B) has an “empty orbit” to accept the lone pair of electrons [38]. Atom A is called a ligand, while atom B is called a central atom or ion. In BP structures, there are two characteristic phosphonate groups bound to the center carbon that provide lone pairs of electrons. Recently, Zhang et al. reported a practical approach to prepare bisphosphonate–metal (BP–Metal) nanoparticles (NPs). Pamidronate can coordinate with various divalent cations of alkaline earth metals and some transition metals in the fourth period [39]. The sizes of self-assembled BP–Metal NPs are listed in Table 1. Moreover, the similar structures between pyrophosphates and BPs result in their similar coordination constants to various ions. The coordination constants between pyrophosphates and metal ions, which can be used as the reference for estimating those of BPs, are given in Table 2.
Table 1.
Size and polydispersity index (PDI) of the pamidronate–metal nanoparticles (NPs) [39].
| Metal | size (nm) | PDI |
|---|---|---|
| Mg2+ | 235 ± 33 | 1.06 |
| Ca2+ | 300 ± 70 | 1.17 |
| Sr2+ | 400 ± 82 | 1.12 |
| Ba2+ | 431 ± 100 | 1.16 |
| Mn2+ | 374 ± 75 | 1.18 |
| Fe2+ | 361 ± 79 | 1.12 |
| Co2+ | 180 ± 26 | 1.06 |
| Ni2+ | 326 ± 71 | 1.13 |
Table 2.
Radius of metal ions and coordination constants with pyrophosphate (an analog of BP) [39].
| Metal | Radii (pm) | lgβ |
|---|---|---|
| Mg2+ | 65 | 5.7 |
| Ca2+ | 99 | 5.0 |
| Sr2+ | 113 | 4.9 |
| Ba2+ | 135 | 4.6 |
| Mn2+ | 80 | 4.6 |
| Fe2+ | 76 | 4.7 |
| Co2+ | 74 | 6.1 |
| Ni2+ | 72 | 7.4 |
BPs can be dissociated from the coordinated ions under external stimuli. The first order dissociation constant of BP (pKa1) is near 1, indicating the strong BP acidity [40]. For nitrogen–containing BPs, the amino groups provide additional protonation sites while also increasing the acidity of phosphate groups [41]. According to Hard–Soft–Acid–Base theory, hard acids have a strong affinity towards hard bases, while soft acids have a strong affinity towards soft bases. The BPs are hard bases, and metal ions are hard acids. In acidic solutions, the hydrogen ions are much harder than metal ions, which can compete with metal ions and sequester them out of BP–Metal NP s, thereby breaking the coordinination bonds between the BPs and metal ions. Moreover, the strong chelating power of ethylenediaminetetraacetic acid (EDTA) breaks the coordination bonds between the BPs and metal ions after incubation with the 1 × 10−3 M of EDTA solution.
2.3. Bioactivities of bisphosphonates and their molecular mechanism
2.3.1. Anti-bone resorption activity and mechanism
BP has been recognized as one of the most effective bone resorption inhibitors. When BPs bind to bone hydroxyapatite, the two-step process of hydroxyapatite dissolution into “amorphous” calcium phosphate, which then transforms into hydroxyapatite, is inhibited [42]. The mechanism of anti-bone resorption activities has been investigated at different levels. At the cellular level, BPs directly change the morphology of osteoclasts, prevent osteoclast maturation, and inhibit the functions of osteoclasts [43]. BPs can be adsorbed to the mineral binding sites of osteoclasts and interfere with the attachment of osteoclasts to the mineralized bone tissue, and this leads to changes in osteoclasts ultrastructure [44].
At the biochemical level, the first generation of BPs (non-nitrogen) can be turned into the analogs of adenosine triphosphate (ATP), which is internalized by the osteoclasts [45]. Due to the non-hydrolysable carbon of the BPs, the analogs of ATP are not easily metabolized within cells [46]. The accumulation of BP-derived ATP analogs inhibits mitochondrial ADP/ATP translocase, thereby triggering osteoclastic apoptosis [47]. Unlike the first generation of BPs, the second and third generations (nitrogen-containing) inactivate the farnesyl pyrophosphate synthase (FPPS), which is a crucial enzyme in the mevalonate pathway that regulates the synthesis of cholesterol, isoprenoid lipids, and other sterols [48,49]. The production of isoprenoid, such as farnesyl pyrophosphate (FFP) and geranylgeranyl pyrophosphate (GGPP), is essential for posttranslational modifications of the signaling proteins, including Ras, Rho, and Rac [[50], [51], [52], [53], [54]]. The lack of these key signaling proteins leads to a loss of bone resorption activity and osteoclastic apoptosis.
2.3.2. Anti-tumor activity and mechanism
Besides the anti-bone resorption activity, BPs can also directly inhibit tumor cell migration and proliferation by inducing cell apoptosis [55]. Furthermore, bone contains abundant growth factors, including insulin-like and transforming growth factors, which are necessary for tumor cell growth and can be released from bone during bone resorption. After inhibiting bone resorption, the release of cancer-related growth factors is impeded [56]. In addition, BPs can exert anti-tumor activities by inhibiting the recruitment of tumor-associated macrophages (TAMs) to tumors, tumor-associated angiogenesis, and tumor-self seeding and by stimulating γ, δ-T cell cytotoxicity [[57], [58], [59]].
2.4. Clinical applications
The root cause of osteoporosis is the abnormal metabolism of bone caused by several factors (old age, disease, medicine, etc.), which reduces bone mass over the whole body, abnormally changes bone microstructure, and increases bone brittleness. Inhibiting bone absorption and promoting bone formation are the basic principles to treat osteoporosis [60]. Etidronate inhibits abnormal calcification and excessive bone absorption in the human body and reduces the bone conversion rate, thereby increasing bone density [61]. The second and third generations of BPs (nitrogen-containing) have also been utilized to treat osteoporosis. Alendronate is the primary drug to treat osteoporosis in postmenopausal women. It can effectively prevent and reduce the risks of subsequent spinal, non-spinal, hip, and wrist fractures [62]. Risedronate can effectively reduce the risks of vertebral, non-vertebral, and hip fractures in postmenopausal women [63].
Bone metastasis is a disease caused by relocation of primary malignant tumor cells from other tissues to bone [64]. Prostate, breast, lung, kidney, rectal, pancreatic, gastric, colon, and ovarian cancers are the most common malignant tumors accompanied by bone metastasis [65]. When tumor cells reach bone marrow via blood flow, tumor cells can destroy bone tissue interacting with osteoblasts, osteoclasts, and bone matrix cells, release a variety of growth factors stored in bone tissue, and make tumor cells proliferate and form metastasis [66].
3. BP-based nanocomposite hydrogels
3.1. Design and characterization of BP-based nanocomposite hydrogels
Metal–ligand coordination is widely used to prepare macromolecular hydrogels [67], where the metallic core can be either metal ions or inorganic nanoparticles (NPs). Among the various coordinating ligands, BPs are ideal candidates to prepare biomedical hydrogels due to their high affinity to various metal ions [[68], [69], [70]], excellent biocompatibility and bioactivity [[71], [72], [73]], and strong stability under physiological environments [39]. BPs can be introduced into the polymeric hydrogel network by co-polymerization or conjugation onto the polymer backbone. The BP-grafted polymers can be directly crosslinked by various metal ions, and the obtained hydrogels also exhibit highly dynamic properties due to the reversible nature of metal–ligand coordination bond [[74], [75], [76]]. However, the doping of either ex-situ or in-situ NPs can further stabilize hydrogel network and endow hydrogels with more unique properties. This review focus on the design and characterization of BP-based nanocomposite hydrogels.
3.1.1. Synthesis of BP-grafted polymers
BPs have been conjugated to various biocompatible polymers, including bioinert poly (ethylene glycol) (PEG) and bioactive hyaluronate (HA). Lopez-Perez et al. reported the conjugation of BPs to the ends of star-shaped PEG [74], where the number and molecular weight of arms can be easily tuned to evaluate the effects of the macromolecular architecture on hydrogel formation. However, the average modification degree of bisphosphonates is relatively low as the star-shaped PEG only possess 4 or 8 free chain-ends. Shi et al. developed a conjugation strategy based on the thiol-ene reaction [77,78], where the BP moieties are conjugated to the polymer backbone via photo-initiator and ultraviolet (UV) light to result in a brush-like and multivalent HA-BP polymer. Our own group adopted a mild conjugation strategy based on the Michael-type addition reaction to attach the BP moieties to the polymer backbone. The Michael-type addition reaction can be performed in a phosphate buffer (PB) at a pH of 8.0 without any additional treatment, yielding an BP-grafted polymer (HA-BP) with one BP moiety conjugated to each methacryloyl group on the polymer backbone [79].
3.1.2. Preparation of BP-based nanocomposite hydrogels
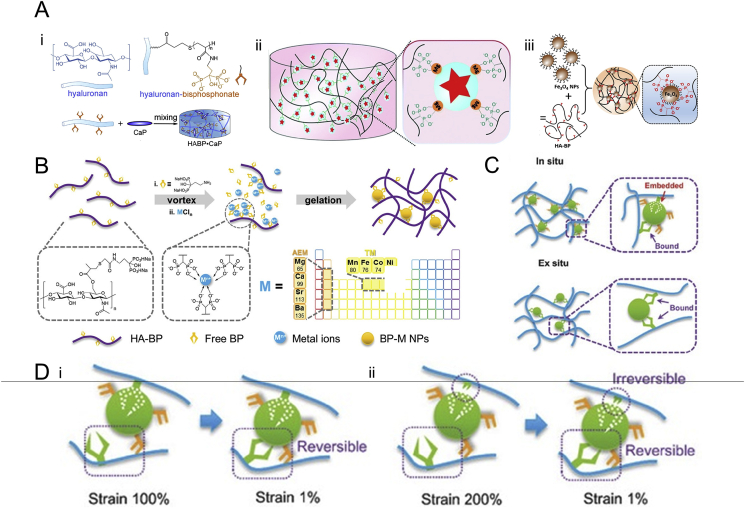
Nanocomposite hydrogels consist of a hydrated polymeric network embedded with functional NPs or nanostructures. The integration of various nanostructures can enhance the mechanical properties and supply various bioactive factors [80]. In general, embedded NPs or nanostructures can be formed either ex-situ or in-situ. The hydrogels embedded with ex-situ NPs can be prepared by simply mixing the HA-BP solution and inorganic NPs, and the coordinating ligands are only attached to the surface of the inorganic NPs (Fig. 2A).
Fig. 2.
Schematic illustration for the preparation and nanostructures of BP-based nanocomposite hydrogels. (A) The BP-based nanocomposite hydrogels embedded with ex-situ NPs: i) CaP NPs, reproduced with permission from Ref. [80], copyright 2014, Elsevier, ii) MgSiO3 NPs, reproduced with permission from Ref. [77], copyright 2016, Royal Society of Chemistry, iii) Fe3O4 NPs, reproduced with permission from Ref. [81], copyright 2019, American Chemistry Society. (B) The BP-based nanocomposite hydrogels embedded with in-situ NPs formed via self-assembly during the gelation process. Reproduced with permission from Ref. [39], copyright 2019, John Wiley and Sons. (C) For hydrogels embedded with ex-situ NPs, the grafted-BP moieties attach onto the surface of nanoparticles; for hydrogels embedded with in-situ NPs, some of the grafted-BP moieties entrap into the center of nanoparticles. Reproduced with permission from Ref. [79], copyright 2017, John Wiley and Sons. (D) The linkage between the attached grafted-BP moieties and the BP–Metal NPs is fully reversible, whereas the linkage between the entrapped grafted-BP moieties and the BP–Metal NPs is not reversible and cannot be restored autogenously. Reproduced with permission from Refs. [79], copyright 2017, John Wiley and Sons.
Nejadnik et al. reported a nanocomposite hydrogel formed using HA-BP and calcium phosphate (CaP) [80], which displays good self-healing properties and adhesiveness to mineral surfaces. The introduction of BP groups on the polymer backbones significantly enhances the storage modulus of the hydrogels and endows the hydrogels with shear-thinning and self-healing properties. Shi et al. reported nanocomposite hydrogels doped with either magnesium silicate (MgSiO3) [77] or magnetic nanoparticles (Fe3O4) [81]. The hydrogels formed by HA-BP and MgSiO3 NPs exhibited self-healing and pH-responsive properties. The hydrogels showed the gel-sol-gel transition under alternating shear strains of 1% and 400% and can rapidly self-heal to one piece after cutting. This feature can be attributed to the reversible bonding of grafted-BP on the surface of NPs. The weak bonding can be broken under high shear strain and then recover the initial binding state when the external shear forces are removed, thereby leading to the “gel–sol–gel” transition under alternating high and low shear strain. The ex-situ NPs have a higher probability of interacting with each other, thereby resulting in significant potential aggregation and even precipitation. Therefore, the modification degree of the coordinating moieties, the concentrations of polymers and NPs and the mixing methods need to be carefully adjusted to reduce NP aggregation.
Our group developed a series of BP-based nanocomposite hydrogels crosslinked by the BP–Mg NPs self-assembled in-situ (Fig. 2B). Our own work first reported a covalently crosslinked hydrogel stabilized using BP–Mg NPs [82]. The BP–Mg NPs are formed by simply photopolymerizing the mixture solution containing methacrylated hyaluronic acid (MeHA), acryloyl-modified pamidronate (Ac-BP), and MgCl2 solution. The Ac-BP and Mg2+ self-assembled into NPs bearing surface acryloyl groups, which functioned as crosslinkers to connect the MeHA network during photo-curing. We then utilized the in-situ assembled BP-cation NPs to fabricate supramolecular hydrogels [79,83]. The hydrogels are formed by the mixing of HA-BP, Ac-BP, and MgCl2, where the coordination between the BPs and Mg2+ drives the in-situ assembly of BP–Mg2+ NPs. The mechanical properties and stability of these hydrogels are much higher than those embedded with ex-situ NPs. Furthermore, our own work expanded the types of metal ions to alkaline earth metals and several transition metals [39]. Based on the different natures of the metal ions, the in-situ NPs have different sizes and interaction affinities with the HA-BP polymer backbones, leading to tunable and wide spectrum mechanical properties for such hydrogels.
3.1.3. Nanostructures of BP-based nanocomposite hydrogels
The nanostructures of BP-based nanocomposite hydrogels depend on multiple factors, including the doping of ex-situ or in-situ NPs, the type of BPs used, ionic radii and other intrinsic properties of metal ions. For the hydrogels embedded with ex-situ NPs, the BP moieties grafted on polymers can only form coordination bonds with the NP surfaces (Fig. 2C) [77,80,84]. Although scanning electron microscopy (SEM) confirmed the ex-situ NPs binding to the hydrogel matrix, the weak and unstable coordination bond on the NP surfaces generally results in weak hydrogel mechanical properties. The typical storage modulus of these hydrogels is lower than 1000 Pa, which is less ideal for some biomedical applications, especially where biomechanical loading is significant. Moreover, the BP groups are covalently grafted to the polymers and have limited release to the surrounding environment until the degradation of hydrogel structure.
The BP-based in-situ nanocomposite hydrogels possess a polymeric network with densely packed BP–Metal NPs. A portion of the BP moieties grafted on the polymer backbones (grafted-BP) are entrapped in the interior of the in-situ self-assembled BP–Metal NPs during the gelation process of these hydrogels (Fig. 2C) [79]. The entrapping of grafted-BP inside the in-situ NPs not only facilitates the gelation process of hydrogels but also stabilizes the hydrogel network. This enhances the interactions between the BP NPs and the hydrogel polymeric network, thereby enhancing the mechanical properties of the hydrogels. Meanwhile, some of the grafted BPs are weakly attached to the surface of BP NPs, contributing to the dynamic properties of these hydrogels. The existence of two types of connections between the BP moieties and metallic NPs is confirmed by testing the hydrogels with alternating high and low oscillatory shear strain. The storage modulus of the hydrogels can be fully recovered under 1% and 100% alternating shear strain but cannot recover to the initial values after application of 200% shear strain, and this reveals the existence of irreversible and reversible BP–Metal interactions (Fig. 2D). Moreover, the size and distribution of BP-Metal NPs are related to the ionic radius and other intrinsic properties of metal ions [39]. With the increasing ionic radius of alkaline earth metals (Mg2+, Ca2+, Sr2+, Ba2+), the chance of coordination binding of BPs and metal ions increases gradually, and the size and polydispersity of BP–Metal NPs also increases gradually. For the transition metals (Mn2+, Fe2+, Co2+, Ni2+) in the fourth period, the ionic radius of these metal ions is similar. However, the BP-Co NPs have abnormally small size and narrow distribution, which are only half of the values of NPs formed by other transition metal ions [39]. These phenomena may be attributed to the intrinsic property of metal ions, such as the ability to give away lone pairs.
3.2. Physicochemical properties of BP-based nanocomposite hydrogels
This section focuses on the physical and chemical properties of BP-based nanocomposite hydrogels, which are crosslinked and stabilized from the multifunctional BP–Metal NPs. The inherent dynamic and responsive properties of the coordination bonds endow these hydrogels with unique characteristics, which can be useful for biomedical applications.
3.2.1. Tunable mechanical properties
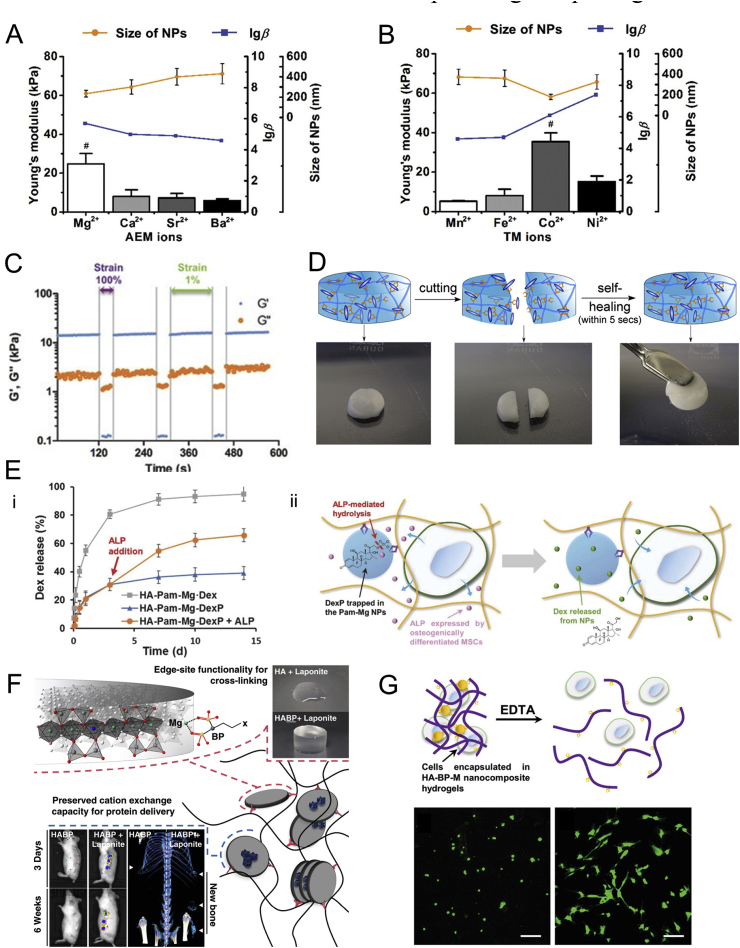
The mechanical properties of BP-based ex-situ nanocomposite hydrogels can be easily tuned by various factors (Fig. 3A and B), including the concentration of BP-grafted polymers and embedded NPs, the substitution degree of grafted-BP moieties and external environment (salt concentration, pH, etc.) [77,80,81]. Moreover, the mechanical properties are also determined by the strength of interaction between embedded NPs and polymeric network. Therefore, the broad types of embedded NPs also contribute to the tunable mechanical properties of these hydrogels. As for the BP-based in-situ nanocomposite hydrogels, the storage modulus and Young's modulus of these hydrogels are generally ten times higher than that of traditional nanocomposite hydrogels [79], while the mechanical properties of these hydrogels can still be easily tuned by adjusting various parameters, including the concentration of bisphosphonates and metal ions [79], the modification degree of BP moieties onto the polymer backbones [79], and the type of metal ions [39]. Especially, the interfacial strength between the fillers and the network is affected by the filler size and the interfacial interactions [85,86]. As mentioned in section 1.2, the sizes of the BP–Metal NPs are highly correlated to the ionic radius and other inherent properties of the metal ions. Therefore, the mechanical properties of these hydrogels can be tuned through the carefully selecting the type and dosage of metal ions.
Fig. 3.
Physicochemical properties of BP-based nanocomposite hydrogels. The mechanical properties of BP-based nanocomposite hydrogels can be tuned from the type of (A) alkaline earth metal ions or (B) some of the transition metals in the fourth period. Reproduced with permission from Ref. [39], copyright 2019, John Wiley and Sons. (C) The excellent shear-thinning properties of BP-based nanocomposite hydrogels. Reproduced with permission from Refs. [79], copyright 2017, John Wiley and Sons. (D) The excellent self-healing properties of BP-based nanocomposite hydrogels. Reproduced with permission from Ref. [80], copyright 2014, Elsevier. (E) Stimuli-responsive releasing kinetics of encapsulated drugs by the enzymatic removal of phosphate groups on the pro-drugs (DexP). (F) The capacity of Laponite to bind the bone inducing growth factor, BMP-2, for enhanced localized efficacy in vivo. (G) Stimuli-responsive releasing of encapsulated MSCs after incubation with EDTA solution. Reproduced with permission from Ref. [39], copyright 2019, John Wiley and Sons.
3.2.2. Excellent dynamic properties and injectability
The dynamic nature of the BP–Metal coordination bonds endows the BP-based nanocomposite hydrogels with excellent shear-thinning and self-healing properties [39,77,[79], [80], [81],83]. The hydrogels exhibit significant shear-thinning behaviors and show “gel–sol–gel” transitions under alternating high and low shear strains, which is attributed to the reversible bonding of the grafted-BP on the surface of NPs (Fig. 3C). Meanwhile, the storage modulus for these hydrogels can recover to nearly 100% of the initial values after cycles of the “gel–sol–gel” transition. The self-healing properties of these hydrogels are further revealed from the rapid re-integration of hydrogels after cutting within several minutes (Fig. 3D). Furthermore, the excellent shear-thinning and self-healing properties contribute to the rapid adaption of the injected hydrogels to irregular molds [39,79]. Our own work further demonstrated the dynamic diffusion of metal ions inside the hydrogels by stacked HA-BP–Co and HA-BP–Ni hydrogels as evidenced by the increasingly blurred boundaries between the top and bottom hydrogel layers with time [39].
3.2.3. Biocompatibility and controlled release of various cargos
The embedded NPs and polymeric network in BP-based nanocomposite hydrogels show low cytotoxicity to encapsulated cells. This ensures the BP-based nanocomposite hydrogels as a desirable 3D cell culture system [39,79], where the majority of encapsulated MSCs remain viable after up to 14 days of cell culture. The embedded NPs enable easy encapsulation of bioactive drugs or pro-drugs inside the hydrogel network. Shi et al. reported a BP-based nanocomposite hydrogel doped with the porous and hollow-like structure of MgSiO3 NPs loaded with anti-cancer drug (doxorubicin), which release the encapsulated doxorubicin under acidic tumor microenvironment [77]. Zhang et al. reported a bioactive BP-based nanocomposite hydrogel exhibiting regeneration-specific release of encapsulated dexamethasone via positive feedback (Fig. 3E) [83]. The enzyme-associated removal of phosphate groups for pro-drugs significantly expedites the release due to a lack of interactions between the dephosphorylated drugs and the BP–Metal NPs. Kim et al. developed a self-assembling hydrogel based on the interaction between bisphosphonate and positively charged Laponite nanoclays, and the obtained hydrogel mediates sustained release of either positively or negatively charged model proteins by the unique charge distribution of Laponite (negative surface charge and positive edge charge) (Fig. 3F) [87]. Moreover, the encapsulated MSCs can also be released from the hydrogels and remain viable upon the incubation with ethylenediaminetetraacetic acid (EDTA) [39], which is a chelating agent that can compete with metal ions inside the BP–Metal NPs. The hydrogels undergo a gel–sol transition after incubation with 1 × 10−3 M of EDTA for 1 h and completely release the encapsulated MSCs (Fig. 3G). Most of the released MSCs can adhere to the cell culture dish and show normal spreading morphologies.
4. Biomedical applications of BP-based nanocomposite hydrogels
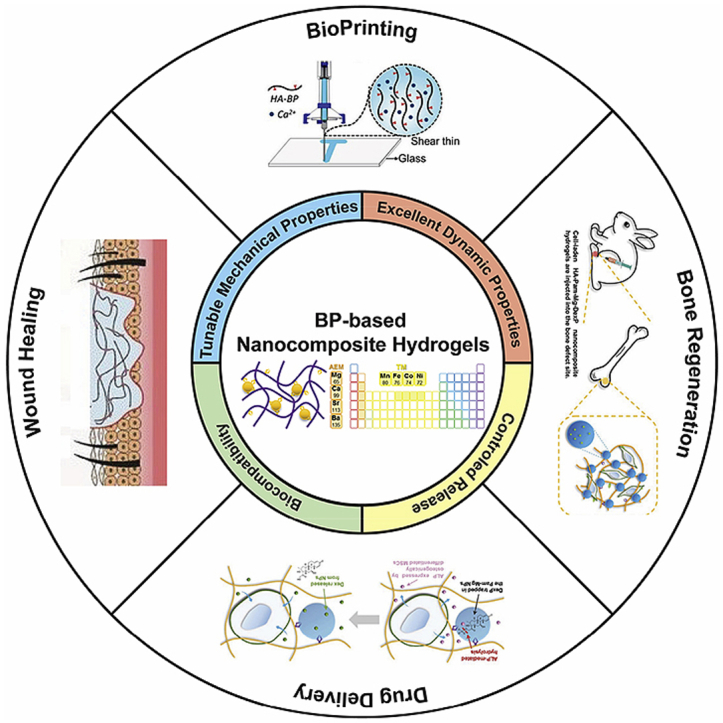
As introduced in section 2.2, the BP-based nanocomposite hydrogels stabilized by the dynamic BP–Metal coordination have excellent physiochemical properties, such as self-healing, injectability, biocompatibility, and stimuli-responsiveness. The unique highly tunable and stimuli-responsive properties of metal–ligand coordination endows the BP-based hydrogels with both affinity-mediated and biomarker-triggered drug delivery for biomedical applications, especially tissue regeneration, which cannot be easily achieved by other supramolecular interactions, such as hydrogen bond and electrostatic interactions [88]. Moreover, the metal–ligand coordination-based hydrogels can serve as highly tunable platforms to customize patient-specific drug dosage and drug combination, thereby enhancing the therapeutic outcomes [89]. These merits make the BP-based nanocomposite hydrogels highly effective biomaterials for a wide array of biomedical applications including drug delivery and tissue regeneration (Fig. 4). We summarized the functions and biomedical applications of representative BP-based nanocomposite hydrogels to provide a comprehensive overview (Table 3).
Fig. 4.
Schematic illustration of the interdependent relationship between the physicochemical properties of BP-based nanocomposite hydrogels and their biomedical applications. Reproduced with permission from Ref. [78], copyright 2017, American Chemistry Society. Reproduced with permission from Refs. [91], copyright 2018, John Wiley and Sons.
Table 3.
Summary of the key attributes and biomedical applications of representative BP-based nanocomposite hydrogels.
| Polymer backbone | Metal source | Key attributes | Biomedical applications | Reference |
|---|---|---|---|---|
| PEG | Ca2+ | Fast stress relaxation, Self-healing | Regenerative medicaine, Bioprinting | [74] |
| Gelatin | Bioactive glass | Adhesion, Robustness, | Bone regeneration | [90] |
| HA | MgSiO3 NPs | pH-responsiveness, Self-healing | Drug delivery | [77] |
| HA | Ag+ | Injectability, Antimicrobial properties | Wound healing | [91] |
| HA | Ca2+ | Shear-thinning, Self-healing | 3D printing | [78] |
| HA | Mg2+ | Injectability, Osteoinductivities | Drug delivery, Bone regeneration | [79,82,83] |
4.1. Drug delivery
Due to the non-covalent BP–Metal interactions, BPs function not only as a crosslinker of the hydrogels but also as the drug carrier or drug itself. Various metal ions such as magnesium ions (Mg2+) can regulate cellular behaviors and stimulate bone regeneration [[92], [93], [94], [95]]. However, the excessive and burst release of Mg2+ from carrier biomaterials may lead to cytotoxicity and bone loss [96,97]. Zhang et al. reported an HA-BP–Mg nanocomposite hydrogel where the release rate of Mg2+ can be carefully controlled [98]. The covalent immobilization of BP–Mg NPs within such hydrogel avoids the burst release of Mg2+ at the initial stages. Furthermore, the acidified microenvironment and the competitive binding between the BP and calcium ions within the body accelerate the release of Mg2+ in the later stages, and this helps sustain the release of Mg2+ over long periods at bone defect sites to enhance the osteogenesis of mesenchymal stem cells (MSCs) and in situ bone regeneration.
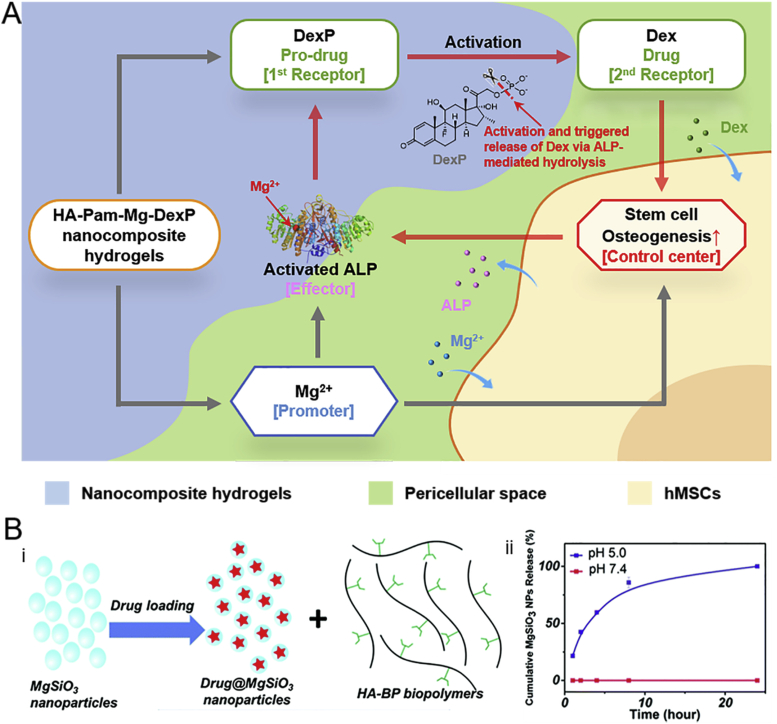
Dexamethasone (Dex) is a glucocorticoid that promotes osteogenic differentiation and is used for enhancing bone regeneration [[99], [100], [101]]. However, the simultaneous delivery of these therapeutic agents together with bioactive ions using the same vehicle remains a challenge [102]. Recently, Zhang et al. described a BP-based injectable nanocomposite hydrogel composed of pamidronate-magnesium (Pam–Mg) NPs for the simultaneous controlled release of Mg2+ and Dex (Fig. 5A) [83]. The Dex phosphate (DexP), a pro-drug of Dex, was loaded in the BP-based nanocomposite hydrogels, and the phosphate–Mg interactions reduced the burst release of DexP from the hydrogel. The Mg2+ released from the hydrogel is expected to promote osteogenic differentiation of hMSCs and elevate alkaline phosphatase (ALP) activity [103]. The expression of ALP then catalyzes the dephosphorylation of DexP, which accelerates the release of Dex at bone defect sites and further promotes osteogenesis. This positive feedback circuit not only achieved localized triggered delivery of Dex but also enhanced the bone regeneration in an animal model.
Fig. 5.
(A) Schematic illustration of the simultaneous release of Dex and Mg2+ from HA -BP–Mg nanocomposite hydrogel to enhance stem cell osteogenesis. The expression of ALP catalyzes the dephosphorylation of DexP, which accelerates the release of Dex at bone defect sites. (B) i) Drug loading and ii) drug release of MgSiO3 NPs from the colloidal hydrogel. When the NPs are injected into the acidic microenvironment, the MgSiO3 NPs are released due to the protonation of BP and the disruption of chelation with Mg2+. Reproduced with permission from Refs. [77], copyright 2016, Royal Society of Chemistry.
The carrier-based delivery and triggered release at the target site reduces the toxicity [104]. A self-healing colloidal hydrogel based on MgSiO3 NPs (drug carrier) and BP-grafted polymer (HA-BP) has been described for targeted drug delivery at tumor-specific microenvironments (Fig. 5B) [77]. Doxorubicin, an anti-cancer drug, was loaded in hydrogel stabilized by the coordination between BP-grafted polymer and MgSiO3 NPs. When the nanogel is injected into the acidic microenvironment, the MgSiO3 NPs are released due to the protonation of BP and the breaking of chelation with Mg2+ and kill human breast cancer cells (MCF-7) through cellular uptake.
4.2. Tissue regeneration
As detailed in section 1.4, the common clinical use of BPs is for treating bone diseases, especially osteoporosis, because BPs have a high affinity to calcium ion (Ca2+) and bone minerals [105]. In addition, other BP-binding cations, such as Ag+, also play important roles in various biochemical processes [79,91]. More importantly, with the aforementioned physicochemical properties, the BP-based nanocomposite hydrogels are suitable 3D scaffolds for regenerative medicine applications.
4.2.1. Biomineralization
Mineralization plays an important role in bone growth [106]. Yang et al. reported a novel hybrid hydrogel formed simply by mixing three solutions, namely BP and hydrazide dually modified polymer solution, aldehyde-derived polymer solution and Ca2+ solution [107]. The clusters prepared from coordination between BP and Ca2+ served as nuclei for further ion recruitment to promote biomineralization.
4.2.2. Bone regeneration
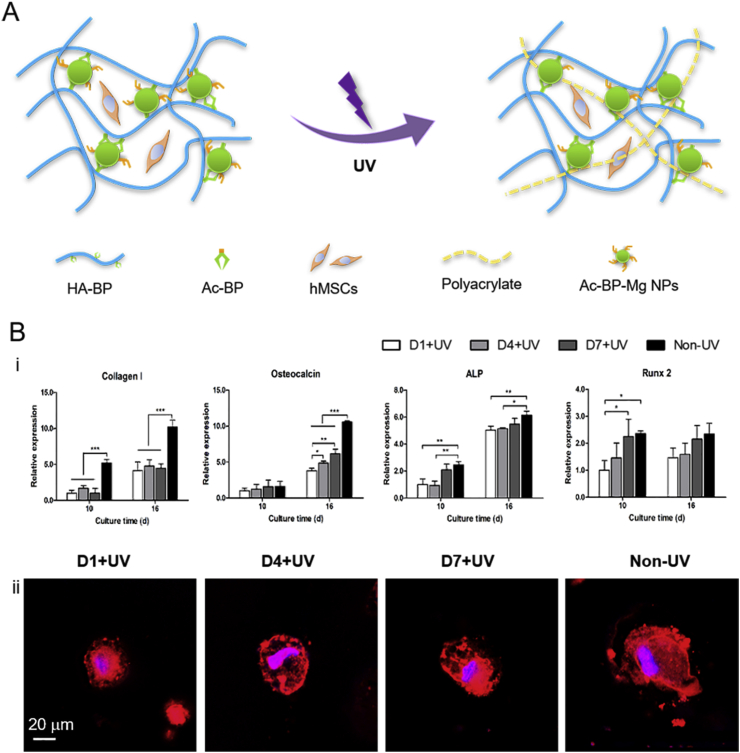
Smart hydrogels that can respond to external stimuli, such as light and magnetic fields, to achieve spatiotemporal control hold potential applications in cell differentiation and tissue regeneration [[108], [109], [110]]. Shi et al. reported a magnetic nanocomposite hydrogel formed by Fe3O4 NPs and HA-BP. The dynamic coordination bonds between BP and Fe3+ on the NP surfaces functioned as reversible crosslinks which provide hydrogels with dynamic properties such as shear thinning and injectability [81]. When the hydrogel was injected subcutaneously into rat, no abscesses was observed in tissues near the injection site. Furthermore, fibroblasts began migrating and proliferating inside the hydrogel. These findings suggest the Fe3O4 nanocomposite hydrogel holds great potential for tissue regeneration. Zhang et al. reported a hydrogel crosslinked by self-assembled BP–Mg NPs to study osteogenic differentiation of hMSCs (Fig. 6A). The acrylate groups on the NP surfaces can further polymerize under UV light illumination to create microenvironments with strengthened stiffness. Compared with cells in an earlier stiffened hydrogel, the delayed introduction of the strengthened stiffness enhanced cell spreading and osteogenic differentiation (Fig. 6B). These results show the structure of the hydrogels can significantly influence osteogenic differentiation and bone regeneration [79].
Fig. 6.
(A) Illustration of the fabrication of BP–Mg nanocomposite hydrogel with spatiotemporal controlled stiffness. (B) Cell response to matrix stiffening. i) fluorescence image of nuclei (blue) and F-actin (red) of hMSCs on day 16. ii) Relative gene expression of osteogenic biomarkers on day 10 and day 16. Reproduced with permission from Ref. [79], copyright 2017, John Wiley and Sons.
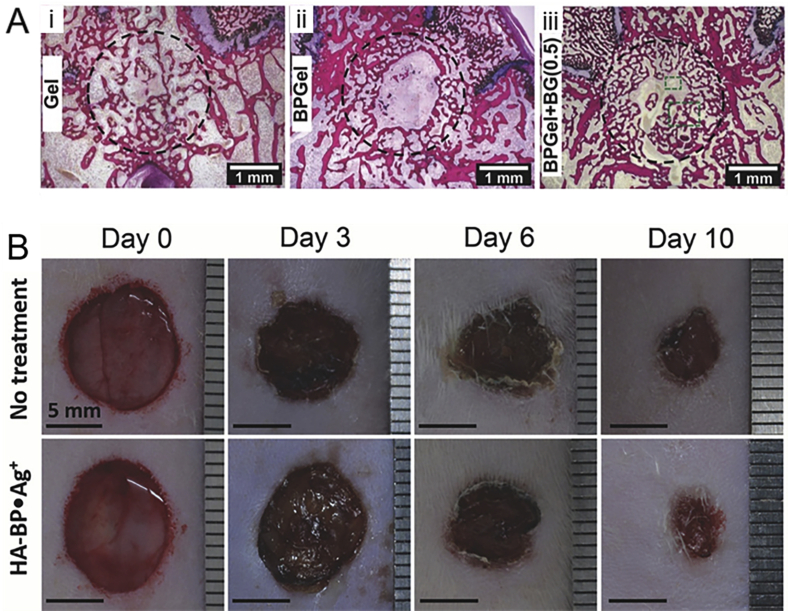
The composite hydrogels containing inorganic fillers like hydroxyapatite particles [111] and silicate glasses [112] not only promote mechanical properties but also enhance cell differentiation and bone regenerative capacity. A nanocomposite colloidal gel was made from BP-functionalized gelatin and bioactive glass for bone defect repair [90]. Vascularized bone formed abundantly inside the defect of osteoporotic rats due to the angiogenic effect of bioactive glass (Fig. 7A). In addition, the composite hydrogels demonstrate significant antiosteoporosis effect, which will further promote bone regeneration.
Fig. 7.
(A) Representative H&E stained histological images of bone formation in i) gel, ii) BP gel, and iii) BP-Bioglass (BG) nanocomposite gel. Vascularized bone formed abundantly inside the defect of osteoporotic rats due to the angiogenic effect of bioactive glass. (B) Images of wounds after BP-Ag nanocomposite hydrogel filled into wounds defect on day 0, day 3, day 6, day 10. The BP–Ag nanocomposite hydrogel displayed antimicrobial activities to both Gram-positive and Gram-negative microorganisms due to the antibacterial properties of silver, which prevents infections in wound healing. Reproduced with permission from Ref. [90], copyright 2017, John Wiley and Sons. Reproduced with permission from Refs. [91], copyright 2018, John Wiley and Sons.
4.2.3. Wound healing
Skin damage often generates irregularly shaped full-size defects that require additional treatment [113,114]. Shi et al. described a dynamically crosslinked moldable hydrogel based on silver ion (Ag+) and HA-BP to fill wound beds without pre-molding [91]. The BP–Ag nanocomposite hydrogel displayed antimicrobial activities to both Gram-positive and Gram-negative microorganisms due to the antibacterial properties of silver, which prevents infections in wound healing. Furthermore, treatment in rat wound models shows more complete epithelium layer regeneration, a lower rate of remaining wounds, and larger blood vessel formation compared with the control group (Fig. 7B). These findings suggest the promising potential of the dynamic coordination crosslinking hydrogels for regenerative wound healing.
4.3. Other applications
4.3.1. Bioprinting
Hydrogel with a similar structure to extracellular matrix can be a promising form of bioink for three-dimensional bioprinting [115]. However, it is difficult to maintain both the fluidity during injection and the robust mechanical properties after solidification while achieving a biocompatible environment. A recent study reported the preparation of hydrogel with both dynamic coordination and covalent crosslinks with two-step 3D bioprinting [78]. First, the polymer derivatives that contain BP and acrylamide groups are mixed with CaCl2 solution to form composite hydrogel connected by the chelation between BP and calcium ion. The reversible interactions enable shear-thinning behavior to permit the extrusion cell-laden hydrogel from a syringe. Photopolymerization was then applied to further strengthen the printed hydrogel. Importantly, the entire fabrication process does not affect the viability of cells encapsulated in the printed hydrogel.
4.3.2. Adhesion
Nejadnik et al. tested the adhesion between the BP nanocomposite hydrogel and the mineral surface containing CaP NPs [80]. The rheology showed that there is a cohesive failure mode when removing the hydrogel from the mineral coated titanium, and this suggests significant adhesion strength between the CaP NPs and BP on the hydrogel surface. Furthermore, the hydrogel adhesion on the mineral coated surface is significantly higher compared with the adhesion on the mineral-free surface, thereby demonstrating its potential applications as a bone adhesive.
5. Conclusions and perspectives
BP-based nanocomposite hydrogels are promising platforms to be integrated with other functional moieties for various biomedical applications. In this review, we have presented the state-of-the-art progresses in this field. The development of BPs is first summarized by classifying BPs according to the type of substituent side chains and associated physicochemical properties. We next review the different fabrication methods and the resultant properties of BP-based nanocomposite hydrogels. Finally, the diverse biomedical applications based on the unique properties of BP-based nanocomposite hydrogels are summarized. Although great achievements have been made in the development of BP-based nanocomposite hydrogels, the in-depth investigation on the material chemistry and structure and their biomedical applications remain to be further pursued. Herein, we attempt to discuss the key issues to be addressed in the evolution of BP-based nanocomposite hydrogels.
-
1.
Molecular thermodynamics and kinetics of metal–ligand coordination have been extensively studied in small molecular metal complexes. However, few studies have examined the shifts of association constants and kinetic rates in hydrated polymeric environments. It is important to investigate how the coordinating properties of BPs are affected by the properties of polymer backbones, including grafted electro-donating or electro-withdrawing side groups, acidity or alkalinity, and chain rigidity. These interactions of metal–ligand complex and polymers need to be further studied for rational design of BP-based nanocomposite hydrogels with tunable and highly dynamic properties.
-
2.
The tunable mechanical properties and highly dynamic properties of BP-based nanocomposite hydrogels are mainly contributed to the variability of metal ions and BPs. Therefore, it is of great significance to further fine tailor the structures of BPs and the composition of BP-Metal NPs to precisely manipulate the hydrogel properties (such as mechanical strength, injectability and biocompatibility).
-
3.
BP-based nanocomposite hydrogels are versatile carriers of therapeutic agents to mediate the localized delivery and regeneration-specific release. However, current studies on the design of enzyme-associated “smart” release of dephosphorylated pro-drugs are still limited to a few drug types and trigger mechanisms. By broadening scope of enzymes, pro-drugs and cells, BP-based nanocomposite hydrogels will be capable of mediating “smart” delivery of diverse therapeutic drugs via various responsive mechanism (positive feedback, negative feedback/on-demand release, tandem release, etc.).
-
4.
For the clinical translation of BP-based nanocomposite hydrogels, their biocompatibility should be fully evaluated via long term in vivo studies. High local concentration of metal ions and ligands, which could arise from rapid hydrogel degradation, may affect endogenous biochemical processes. Moreover, the degradation products can be released into systemic circulation and transported to distal organs. Therefore, the toxicity, biodegradation, biodistribution, clearance, and log-term fate of BP-based nanocomposite hydrogels and their degradation products have to be further investigated. The joint effort from biomaterial researchers and clinicians is required for the further development of the BP-based nanocomposite hydrogels with more unique functionalities and expanded biomedical applications.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The work was fully supported by Theme-based Research grant (Ref. T13-402/17-N) from the Research Grants Council of the Hong Kong Special Administrative Region. This research was supported by an Innovation Technology Fund (TCFS, GHP/011/17SZ), Hong Kong. This work was supported by National Natural Science Foundation of China (81772354, 81572137), Clinical Innovation Research Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory (2018GZR0201002), Guangzhou University Innovation and Entrepreneurship Education Project (2019PT104), National Key R&D Program of China (2016YFC1100100) to Zhi-Yong Zhang.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Zhi-Yong Zhang, Email: drzhiyong@126.com.
Liming Bian, Email: lbian@cuhk.edu.hk.
References
- 1.Hanafy N.A.N., Leporatti S., El-Kemary M.A. Mucoadhesive hydrogel nanoparticles as smart biomedical drug delivery system. Appl. Sci. Basel. 2019;9(5) [Google Scholar]
- 2.Hu Y., Niemeyer C.M. Designer DNA-silica/carbon nanotube nanocomposites for traceable and targeted drug delivery. J. Mater. Chem. B. 2020;8(11):2250–2255. doi: 10.1039/c9tb02861g. [DOI] [PubMed] [Google Scholar]
- 3.Liu C., Han J.L., Pei Y.X., Du J. Aptamer functionalized DNA hydrogel for wise-stage controlled protein release. Appl. Sci. Basel. 2018;8(10) [Google Scholar]
- 4.Deng H.L., Yu Z.P., Chen S.G., Fei L.T., Sha Q.Y., Zhou N., Chen Z.T., Xu C. Facile and eco-friendly fabrication of polysaccharides-based nanocomposite hydrogel for photothermal treatment of wound infection. Carbohydr. Polym. 2020;230 doi: 10.1016/j.carbpol.2019.115565. [DOI] [PubMed] [Google Scholar]
- 5.Dharmalingam K., Bordoloi D., Kunnumakkara A.B., Anandalakshmi R. Preparation and characterization of cellulose-based nanocomposite hydrogel films containing CuO/Cu2O/Cu with antibacterial activity. J. Appl. Polym. Sci. 2020 [Google Scholar]
- 6.GhavamiNejad A., Park C.H., Kim C.S. In situ synthesis of antimicrobial silver nanoparticles within antifouling zwitterionic hydrogels by catecholic redox chemistry for wound healing application. Biomacromolecules. 2016;17(3):1213–1223. doi: 10.1021/acs.biomac.6b00039. [DOI] [PubMed] [Google Scholar]
- 7.Olad A., Eslamzadeh M., Katiraee F., Mirmohseni A. Evaluation of in vitro anti-fungal properties of allicin loaded ion cross-linked poly (AA-co-AAm)/PVA/Cloisite 15A Nanocomposite hydrogel films as wound dressing materials. J. Polym. Res. 2020;27(4) [Google Scholar]
- 8.Cui N., Han K., Li M., Wang J.L., Qian J.M. Pure polylysine-based foamy scaffolds and their interaction with MC3T3-E1 cells and osteogenesis. Biomed. Mater. 2020;15(2) doi: 10.1088/1748-605X/ab5cfc. [DOI] [PubMed] [Google Scholar]
- 9.Makvandi P., Ali G.W., Della Sala F., Abdel-Fattah W.I., Borzacchiello A. Hyaluronic acid/corn silk extract based injectable nanocomposite: a biomimetic antibacterial scaffold for bone tissue regeneration. Mat. Sci. Eng. C-Mater. 2020;107 doi: 10.1016/j.msec.2019.110195. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y.H., Chen M.J., Dai Z.B., Cao H.L., Li J., Zhang W.A. Sustained protein therapeutics enabled by self-healing nanocomposite hydrogels for non-invasive bone regeneration. Biomater. Sci. Uk. 2020;8(2):682–693. doi: 10.1039/c9bm01455a. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y., Gu Z.P., Liu J., Huang K.Q., Liu G.T., Wu J. Arginine based poly (ester amide)/hyaluronic acid hybrid hydrogels for bone tissue Engineering. Carbohydr. Polym. 2020;230 doi: 10.1016/j.carbpol.2019.115640. [DOI] [PubMed] [Google Scholar]
- 12.Muya F.N., Baker P.G.L., Iwuoha E.I. Electrocatalysis-Us; 2020. Polysulfone Hydrogel Nanocomposite Alkaline Phosphatase Biosensor for the Detection of Vanadium. [Google Scholar]
- 13.Singh N., Ali A., Rai P., Ghori I., Sharma A., Malhotra B.D., John R. Dual-modality microfluidic biosensor based on nanoengineered mesoporous graphene hydrogels. Lab Chip. 2020;20(4):760–777. doi: 10.1039/c9lc00751b. [DOI] [PubMed] [Google Scholar]
- 14.Chacko R.T., Ventura J., Zhuang J.M., Thayumanavan S. Polymer nanogels: a versatile nanoscopic drug delivery platform. Adv. Drug Deliv. Rev. 2012;64(9):836–851. doi: 10.1016/j.addr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moughton A.O., Hillmyer M.A., Lodge T.P. Multicompartment block polymer micelles. Macromolecules. 2012;45(1):2–19. [Google Scholar]
- 16.Ryu J.H., Chacko R.T., Jiwpanich S., Bickerton S., Babu R.P., Thayumanavan S. Self-Cross-Linked polymer nanogels: a versatile nanoscopic drug delivery platform. J. Am. Chem. Soc. 2010;132(48):17227–17235. doi: 10.1021/ja1069932. [DOI] [PubMed] [Google Scholar]
- 17.Schluter A.D., Halperin A., Kroger M., Vlassopoulos D., Wegner G., Zhang B.Z. Dendronized polymers: molecular objects between conventional linear polymers and colloidal particles. ACS Macro Lett. 2014;3(10):991–998. doi: 10.1021/mz500376e. [DOI] [PubMed] [Google Scholar]
- 18.Annabi N., Tamayol A., Uquillas J.A., Akbari M., Bertassoni L.E., Cha C., Camci-Unal G., Dokmeci M.R., Peppas N.A., Khademhosseini A. 25th anniversary article: rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014;26(1):85–124. doi: 10.1002/adma.201303233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai T.J., Wang C.P., Wang Y.Q., Xu W., Hu J.J., Cheng Y.Y. A nanocomposite hydrogel with potent and broad-spectrum antibacterial activity. ACS Appl. Mater. Interfaces. 2018;10(17):15163–15173. doi: 10.1021/acsami.8b02527. [DOI] [PubMed] [Google Scholar]
- 20.Wang W., Zhang Y.Y., Liu W.G. Bioinspired fabrication of high strength hydrogels from non-covalent interactions. Prog. Polym. Sci. 2017;71:1–25. [Google Scholar]
- 21.Wang W.T., Fan X.Q., Li F.H., Qiu J.J., Umair M.M., Ren W.C., Ju B.Z., Zhang S.F., Tang B.T. Magnetochromic photonic hydrogel for an alternating magnetic field-responsive color display. Adv. Opt. Mater. 2018;6(4) [Google Scholar]
- 22.Liu R.Q., Liang S.M., Tang X.Z., Yan D., Li X.F., Yu Z.Z. Tough and highly stretchable graphene oxide/polyacrylamide nanocomposite hydrogels. J. Mater. Chem. 2012;22(28):14160–14167. [Google Scholar]
- 23.Pan C.G., Liu L.B., Chen Q., Zhang Q., Guo G.L. Tough, stretchable, compressive novel polymer/graphene oxide nanocomposite hydrogels with excellent self-healing performance. ACS Appl. Mater. Interfaces. 2017;9(43):38052–38061. doi: 10.1021/acsami.7b12932. [DOI] [PubMed] [Google Scholar]
- 24.Si H.J., Luo H.L., Xiong G.Y., Yang Z.W., Raman S.R., Guo R.S., Wan Y.Z. One-step in situ biosynthesis of graphene oxide-bacterial cellulose nanocomposite hydrogels. Macromol. Rapid Commun. 2014;35(19):1706–1711. doi: 10.1002/marc.201400239. [DOI] [PubMed] [Google Scholar]
- 25.Kumar A., Jaiswal M. Design and in vitro investigation of nanocomposite hydrogel based in situ spray dressing for chronic wounds and synthesis of silver nanoparticles using green chemistry. J. Appl. Polym. Sci. 2016;133(14) [Google Scholar]
- 26.Zhang T.T., Zuo T., Hu D.N., Chang C.Y. Dual physically cross-linked nanocomposite hydrogels reinforced by tunicate cellulose nanocrystals with high toughness and good self-recoverability. ACS Appl. Mater. Interfaces. 2017;9(28):24230–24237. doi: 10.1021/acsami.7b06219. [DOI] [PubMed] [Google Scholar]
- 27.Jang H.L., Jin K., Lee J., Kim Y., Nahm S.H., Hong K.S., Nam K.T. Revisiting whitlockite, the second most abundant biomineral in bone: nanocrystal synthesis in physiologically relevant conditions and biocompatibility evaluation. ACS Nano. 2014;8(1):634–641. doi: 10.1021/nn405246h. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J., Ma X.Y., Lin D., Shi H.S., Yuan Y., Tang W., Zhou H.J., Guo H., Qian J.C., Liu C.S. Magnesium modification of a calcium phosphate cement alters bone marrow stromal cell behavior via an integrin-mediated mechanism. Biomaterials. 2015;53:251–264. doi: 10.1016/j.biomaterials.2015.02.097. [DOI] [PubMed] [Google Scholar]
- 29.Zaveri T.D., Lewis J.S., Dolgova N.V., Clare-Salzler M.J., Keselowsky B.G. Integrin-directed modulation of macrophage responses to biomaterials. Biomaterials. 2014;35(11):3504–3515. doi: 10.1016/j.biomaterials.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang M., Yu Y.M., Dai K., Ma Z.Y., Liu Y., Wang J., Liu C.S. Improved osteogenesis and angiogenesis of magnesium-doped calcium phosphate cement via macrophage immunomodulation. Biomater. Sci. Uk. 2016;4(11):1574–1583. doi: 10.1039/c6bm00290k. [DOI] [PubMed] [Google Scholar]
- 31.Kang H.M., Yang B.G., Zhang K.Y., Pan Q., Yuan W.H., Li G., Bian L.M. Immunoregulation of macrophages by dynamic ligand presentation via ligand-cation coordination. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-09733-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleisch H., Russel R.G.G., Straumann F. Effect of pyrophosphate on hydroxyapatite and its implications in calcium homeostasis. Nature. 1966;212(5065):901–903. doi: 10.1038/212901a0. [DOI] [PubMed] [Google Scholar]
- 33.Drake M.T., Clarke B.L., Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008;83(9):1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell R.G.G. Bisphosphonates: the first 40 years. Bone. 2011;49(1):2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 35.Lazar A.C., Pacurar M., Campian R.S. Bisphosphonates in bone diseases treatment. Rev. Chim-Bucharest. 2017;68(2):246–249. [Google Scholar]
- 36.Van Acker H.H., Anguille S., Willemen Y., Smits E.L., Van Tendeloo V.F. Bisphosphonates for cancer treatment: mechanisms of action and lessons from clinical trials. Pharmacol. Therapeut. 2016;158:24–40. doi: 10.1016/j.pharmthera.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Russell R.G.G. Bisphosphonates - from bench to bedside. Ann Ny Acad Sci. 2006;1068:367–401. doi: 10.1196/annals.1346.041. [DOI] [PubMed] [Google Scholar]
- 38.Tiekink E.R.T. Supramolecular assembly based on "emerging" intermolecular interactions of particular interest to coordination chemists. Coord. Chem. Rev. 2017;345:209–228. [Google Scholar]
- 39.Zhang K.Y., Yuan W.H., Wei K.C., Yang B.G., Chen X.Y., Li Z., Zhang Z.Y., Bian L.M. Highly dynamic nanocomposite hydrogels self-assembled by metal ion-ligand coordination. Small. 2019;15(15) doi: 10.1002/smll.201900242. [DOI] [PubMed] [Google Scholar]
- 40.Galezowska J., Gumienna-Kontecka E. Phosphonates, their complexes and bio-applications: a spectrum of surprising diversity. Coord. Chem. Rev. 2012;256(1–2):105–124. [Google Scholar]
- 41.Matczak-Jon E., Videnova-Adrabinska V. Supramolecular chemistry and complexation abilities of diphosphonic acids. Coord. Chem. Rev. 2005;249(21–22):2458–2488. [Google Scholar]
- 42.Jobke B., Milovanovic P., Amling M., Busse B. Bisphosphonate-osteoclasts: changes in osteoclast morphology and function induced by antiresorptive nitrogen-containing bisphosphonate treatment in osteoporosis patients. Bone. 2014;59:37–43. doi: 10.1016/j.bone.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 43.Sato M., Grasser W., Endo N., Akins R., Simmons H., Thompson D.D., Golub E., Rodan G.A. Bisphosphonate action - alendronate localization in rat bone and effects on osteoclast ultrastructure. J. Clin. Invest. 1991;88(6):2095–2105. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui Y., Zhu T., Li D., Li Z., Leng Y., Ji X., Liu H., Wu D., Ding J. Bisphosphonate-functionalized scaffolds for enhanced bone regeneration. Adv. Healthcare Mater. 2019;8(23) doi: 10.1002/adhm.201901073. [DOI] [PubMed] [Google Scholar]
- 45.Carano A., Teitelbaum S.L., Konsek J.D., Schlesinger P.H., Blair H.C. Bisphosphonates directly inhibit the bone-resorption activity of isolated avian osteoclasts invitro. J. Clin. Invest. 1990;85(2):456–461. doi: 10.1172/JCI114459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frith J.C., Monkkonen J., Auriola S., Monkkonen H., Rogers M.J. The molecular mechanism of action of the antiresorptive and antiinflammatory drug clodronate - evidence for the formation in vivo of a metabolite that inhibits bone resorption and causes osteoclast and macrophage apoptosis. Arthritis Rheum. 2001;44(9):2201–2210. doi: 10.1002/1529-0131(200109)44:9<2201::aid-art374>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 47.Lehenkari P.P., Kellinsalmi M., Napankangas J.P., Ylitalo K.V., Monkkonen J., Rogers M.J., Azhayev A., Vaananen H.K., Hassinen I.E. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol. Pharmacol. 2002;61(5):1255–1262. doi: 10.1124/mol.61.5.1255. [DOI] [PubMed] [Google Scholar]
- 48.Amin D., Cornell S.A., Gustafson S.K., Needle S.J., Ullrich J.W., Bilder G.E., Perrone M.H. Bisphosphonates used for the treatment of bone disorders inhibit squalene synthase and cholesterol-biosynthesis. J. Lipid Res. 1992;33(11):1657–1663. [PubMed] [Google Scholar]
- 49.Amin D., Cornell S.A., Perrone M.H., Bilder G.E. 1-hydroxy-3-(methylpentylamino)-propylidene-1,1-bisphosphonic acid as a potent inhibitor of squalene synthase. Arzneim. Forsch. 1996;46(8):759–762. [PubMed] [Google Scholar]
- 50.Konstantinopoulos P.A., Karamouzis M.V., Papavassiliou A.G. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat. Rev. Drug Discov. 2007;6(7):540–555. doi: 10.1038/nrd2221. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura K., Fujita A., Murata T., Watanabe G., Mori C., Fujita J., Watanabe N., Ishizaki T., Yoshida O., Narumiya S. Rhophilin, a small GTPase Rho-binding protein, is abundantly expressed in the mouse testis and localized in the principal piece of the sperm tail. FEBS Lett. 1999;445(1):9–13. doi: 10.1016/s0014-5793(99)00087-3. [DOI] [PubMed] [Google Scholar]
- 52.Ridley A.J., Paterson H.F., Johnston C.L., Diekmann D., Hall A. The small gtp-binding protein rac regulates growth-factor induced membrane ruffling. Cell. 1992;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 53.Zerial M., Stenmark H. Rab GTPases in vesicular transport. Curr. Opin. Cell Biol. 1993;5(4):613–620. doi: 10.1016/0955-0674(93)90130-i. [DOI] [PubMed] [Google Scholar]
- 54.Zhang F.L., Casey P.J. Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 55.Coleman R., Gnant M., Morgan G., Clezardin P. Effects of bone-targeted agents on cancer progression and mortality. Jnci-J. Natl. Canc. 2012;104(14):1059–1067. doi: 10.1093/jnci/djs263. [DOI] [PubMed] [Google Scholar]
- 56.Weilbaecher K.N., Guise T.A., McCauley L.K. Cancer to bone: a fatal attraction. Nat. Rev. Canc. 2011;11(6):411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clezardin P. Bisphosphonates' antitumor activity: an unravelled side of a multifaceted drug class. Bone. 2011;48(1):71–79. doi: 10.1016/j.bone.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 58.Gnant M., Clezardin P. Direct and indirect anticancer activity of bisphosphonates: a brief review of published literature. Canc. Treat Rev. 2012;38(5):407–415. doi: 10.1016/j.ctrv.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Hamilton E., Clay T.M., Blackwell K.L. New perspectives on zoledronic acid in breast cancer: potential augmentation of anticancer immune response. Canc. Invest. 2011;29(8):533–541. doi: 10.3109/07357907.2011.605413. [DOI] [PubMed] [Google Scholar]
- 60.Chapurlat R.D., Delmas P.D. Drug insight: bisphosphonates for postmenopausal osteoporosis. Nat. Clin. Pract. Endocrinol. Metabol. 2006;2(4):211–219. doi: 10.1038/ncpendmet0121. quiz following 238. [DOI] [PubMed] [Google Scholar]
- 61.Eastell R., Walsh J.S., Watts N.B., Siris E. Bisphosphonates for postmenopausal osteoporosis. Bone. 2011;49(1):82–88. doi: 10.1016/j.bone.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 62.Wells G.A., Cranney A., Peterson J., Boucher M., Shea B., Robinson V., Coyle D., Tugwell P. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Db Syst Rev. 2008;1 doi: 10.1002/14651858.CD001155.pub2. [DOI] [PubMed] [Google Scholar]
- 63.Wells G., Cranney A., Peterson J., Boucher M., Shea B., Robinson V., Coyle D., Tugwell P. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Db Syst Rev. 2008;1 doi: 10.1002/14651858.CD004523.pub3. [DOI] [PubMed] [Google Scholar]
- 64.Gdowski A.S., Ranjan A., Vishwanatha J.K. Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trials. J. Exp. Clin. Canc. Res. 2017;36 doi: 10.1186/s13046-017-0578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Macedo F., Ladeira K., Pinho F., Saraiva N., Bonito N., Pinto L., Goncalves F. Bone metastases: an overview. Onco Rev. 2017;11(1):321. doi: 10.4081/oncol.2017.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Casimiro S., Ferreira A.R., Mansinho A., Alho I., Costa L. Molecular mechanisms of bone metastasis: which targets came from the bench to the bedside? Int. J. Mol. Sci. 2016;17(9) doi: 10.3390/ijms17091415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Webber M.J., Appel E.A., Meijer E.W., Langer R. Supramolecular biomaterials. Nat. Mater. 2016;15(1):13–26. doi: 10.1038/nmat4474. [DOI] [PubMed] [Google Scholar]
- 68.Bouhsina S., Buglyo P., Aad E.A., Aboukais A., Kiss T. Formation of oligonuclear complexes between copper(II) and 1-hydroxyethane-1,1-diphosphonic acid. Inorg. Chim. Acta. 2004;357(1):305–310. [Google Scholar]
- 69.Deluchat V., Bollinger J.C., Serpaud B., Caullet C. Divalent cations speciation with three phosphonate ligands in the pH-range of natural waters. Talanta. 1997;44(5):897–907. doi: 10.1016/s0039-9140(96)02136-4. [DOI] [PubMed] [Google Scholar]
- 70.Kubicek V., Kotek J., Hermann P., Lukes I. Aminoalkylbis(phosphonates): their complexation properties in solution and in the solid state. Eur. J. Inorg. Chem. 2007;2:333–344. [Google Scholar]
- 71.Galezowska J. Interactions between clinically used bisphosphonates and bone mineral: from coordination chemistry to biomedical applications and beyond. ChemMedChem. 2018;13(4):289–302. doi: 10.1002/cmdc.201700769. [DOI] [PubMed] [Google Scholar]
- 72.Galezowska J., Czapor-Irzabek H., Chmielewska E., Kafarski P., Janek T. Aminobisphosphonates based on cyclohexane backbone as coordinating agents for metal ions. Thermodynamic, spectroscopic and biological studies. New J. Chem. 2018;42(10):7723–7736. [Google Scholar]
- 73.Ossipov D.A. Bisphosphonate-modified biomaterials for drug delivery and bone tissue engineering. Expet Opin. Drug Deliv. 2015;12(9):1443–1458. doi: 10.1517/17425247.2015.1021679. [DOI] [PubMed] [Google Scholar]
- 74.Lopez-Perez P.M., da Silva R.M.P., Strehin I., Kouwer P.H.J., Leeuwenburgh S.C.G., Messersmith P.B. Self-healing hydrogels formed by complexation between calcium ions and bisphosphonate-functionalized star-shaped polymers. Macromolecules. 2017;50(21):8698–8706. doi: 10.1021/acs.macromol.7b01417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu L., Li X.Y., Shi X.T., Wang Y.J. Injectable alendronate-functionalized GelMA hydrogels for mineralization and osteogenesis. RSC Adv. 2018;8(40):22764–22776. doi: 10.1039/c8ra03550d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sanchez-Fernandez M.J., Immers M.R., Lanao R.P.F., Yang F., Bender J.C.M.E., Mecinovic J., Leeuwenburgh S.C.G., van Hest J.C.M. Alendronate-functionalized poly(2-oxazoline)s with tunable affinity for calcium cations. Biomacromolecules. 2019;20(8):2913–2921. doi: 10.1021/acs.biomac.9b00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi L., Han Y., Hilborn J., Ossipov D. Smart'' drug loaded nanoparticle delivery from a self-healing hydrogel enabled by dynamic magnesium-biopolymer chemistry. Chem. Commun. 2016;52(74):11151–11154. doi: 10.1039/c6cc05565f. [DOI] [PubMed] [Google Scholar]
- 78.Shi L.Y., Carstensen H., Holzl K., Lunzer M., Li H., Hilborn J., Ovsianikov A., Ossipov D.A. Dynamic coordination chemistry enables free directional printing of biopolymer hydrogel. Chem. Mater. 2017;29(14):5816–5823. [Google Scholar]
- 79.Zhang K.Y., Feng Q., Xu J.B., Xu X.Y., Tian F., Yeung K.W.K., Bian L.M. Self-assembled injectable nanocomposite hydrogels stabilized by bisphosphonate-magnesium (Mg2+) coordination regulates the differentiation of encapsulated stem cells via dual crosslinking. Adv. Funct. Mater. 2017;27(34) [Google Scholar]
- 80.Nejadnik M.R., Yang X., Bongio M., Alghamdi H.S., Van den Beucken J.J.J.P., Huysmans M.C., Jansen J.A., Hilborn J., Ossipov D., Leeuwenburgh S.C.G. Self-healing hybrid nanocomposites consisting of bisphosphonated hyaluronan and calcium phosphate nanoparticles. Biomaterials. 2014;35(25):6918–6929. doi: 10.1016/j.biomaterials.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 81.Shi L.Y., Zeng Y.Q., Zhao Y., Yang B., Ossipov D., Tai C.W., Dai J.W., Xu C.G. Biocompatible injectable magnetic hydrogel formed by dynamic coordination network. ACS Appl. Mater. Interfaces. 2019;11(49):46233–46240. doi: 10.1021/acsami.9b17627. [DOI] [PubMed] [Google Scholar]
- 82.Zhang K., Lin S., Feng Q., Dong C., Yang Y., Li G., Bian L. Nanocomposite hydrogels stabilized by self-assembled multivalent bisphosphonate-magnesium nanoparticles mediate sustained release of magnesium ion and promote in-situ bone regeneration. Acta Biomater. 2017;64:389–400. doi: 10.1016/j.actbio.2017.09.039. [DOI] [PubMed] [Google Scholar]
- 83.Zhang K.Y., Jia Z.F., Yang B.G., Feng Q., Xu X., Yuan W.H., Li X.F., Chen X.Y., Duan L., Wang D.P., Bian L.M. Adaptable hydrogels mediate cofactor-assisted activation of biomarker-responsive drug delivery via positive feedback for enhanced tissue regeneration. Adv. Sci. 2018;5(12) doi: 10.1002/advs.201800875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shi L.Y., Ding P.H., Wang Y.Z., Zhang Y., Ossipov D., Hilborn J. Self-healing polymeric hydrogel formed by metal-ligand coordination assembly: design, fabrication, and biomedical applications. Macromol. Rapid Commun. 2019;40(7) doi: 10.1002/marc.201800837. [DOI] [PubMed] [Google Scholar]
- 85.Ganesan V., Jayaraman A. Theory and simulation studies of effective interactions, phase behavior and morphology in polymer nanocomposites. Soft Matter. 2014;10(1):13–38. doi: 10.1039/c3sm51864g. [DOI] [PubMed] [Google Scholar]
- 86.Adnan A., Sun C.T., Mahfuz H. A molecular dynamics simulation study to investigate the effect of filler size on elastic properties of polymer nanocomposites. Compos. Sci. Technol. 2007;67(3–4):348–356. [Google Scholar]
- 87.Kim Y.H., Yang X., Shi L., Lanham S.A., Hilborn J., Oreffo R.O.C., Ossipov D., Dawson J.I. Bisphosphonate nanoclay edge-site interactions facilitate hydrogel self-assembly and sustained growth factor localization. Nat. Commun. 2020;11(1):1365. doi: 10.1038/s41467-020-15152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mura S., Nicolas J., Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013;12(11):991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 89.Webber M.J., Langer R. Drug delivery by supramolecular design. Chem. Soc. Rev. 2017;46(21):6600–6620. doi: 10.1039/c7cs00391a. [DOI] [PubMed] [Google Scholar]
- 90.Diba M., Camargo W.A., Brindisi M., Farbod K., Klymov A., Schmidt S., Harrington M.J., Draghi L., Boccaccini A.R., Jansen J.A., van den Beucken J., Leeuwenburgh S.C.G. Composite colloidal gels made of bisphosphonate-functionalized gelatin and bioactive glass particles for regeneration of osteoporotic bone defects. Adv. Funct. Mater. 2017;27(45) [Google Scholar]
- 91.Shi L.Y., Zhao Y.N., Xie Q.F., Fan C.X., Hilborn J., Dai J.W., Ossipov D.A. Moldable hyaluronan hydrogel enabled by dynamic metal-bisphosphonate coordination chemistry for wound healing. Adv. Healthcare Mater. 2018;7(5) doi: 10.1002/adhm.201700973. [DOI] [PubMed] [Google Scholar]
- 92.Yoshizawa S., Brown A., Barchowsky A., Sfeir C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014;10(6):2834–2842. doi: 10.1016/j.actbio.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 93.Yu Y.Q., Jin G.D., Xue Y., Wang D.H., Liu X.Y., Sun J. Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017;49:590–603. doi: 10.1016/j.actbio.2016.11.067. [DOI] [PubMed] [Google Scholar]
- 94.Lakhkar N.J., Lee I.H., Kim H.W., Salih V., Wall I.B., Knowles J.C. Bone formation controlled by biologically relevant inorganic ions: role and controlled delivery from phosphate-based glasses. Adv. Drug Deliv. Rev. 2013;65(4):405–420. doi: 10.1016/j.addr.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 95.Wong H.M., Zhao Y., Tam V., Wu S.L., Chu P.K., Zheng Y.F., To M.K.T., Leung F.K.L., Luk K.D.K., Cheung K.M.C., Yeung K.W.K. In vivo stimulation of bone formation by aluminum and oxygen plasma surface-modified magnesium implants. Biomaterials. 2013;34(38):9863–9876. doi: 10.1016/j.biomaterials.2013.08.052. [DOI] [PubMed] [Google Scholar]
- 96.Serre C.M., Papillard M., Chavassieux P., Voegel J.C., Boivin G. Influence of magnesium substitution on a collagen-apatite biomaterial on the production of a calcifying matrix by human osteoblasts. J. Biomed. Mater. Res. 1998;42(4):626–633. doi: 10.1002/(sici)1097-4636(19981215)42:4<626::aid-jbm20>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 97.Wong H.M., Chu P.K., Leung F.K.L., Cheung K.M.C., Luk K.D.K., Yeung K.W.K. Engineered polycaprolactone-magnesium hybrid biodegradable porous scaffold for bone tissue engineering. Prog. nat. Sci. Mater. Int. 2014;24(5):561–567. [Google Scholar]
- 98.Zhang K.Y., Lin S.E., Feng Q., Dong C.Q., Yang Y.H., Li G., Bian L.M. Nanocomposite hydrogels stabilized by self-assembled multivalent bisphosphonate-magnesium nanoparticles mediate sustained release of magnesium ion and promote in-situ bone regeneration. Acta Biomater. 2017;64:389–400. doi: 10.1016/j.actbio.2017.09.039. [DOI] [PubMed] [Google Scholar]
- 99.Feng Q., Wei K.C., Lin S.E., Xu Z., Sun Y.X., Shi P., Li G., Bian L.M. Mechanically resilient, injectable, and bioadhesive supramolecular gelatin hydrogels crosslinked by weak host-guest interactions assist cell infiltration and in situ tissue regeneration. Biomaterials. 2016;101:217–228. doi: 10.1016/j.biomaterials.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 100.Jaiswal N., Haynesworth S.E., Caplan A.I., Bruder S.P. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J. Cell. Biochem. 1997;64(2):295–312. [PubMed] [Google Scholar]
- 101.Cheng S.L., Yang J.W., Rifas L., Zhang S.F., Avioli L.V. Differentiation OF human bone-marrow osteogenic stromal cells IN vitro - induction OF the osteoblast phenotype BY dexamethasone. Endocrinology. 1994;134(1):277–286. doi: 10.1210/endo.134.1.8275945. [DOI] [PubMed] [Google Scholar]
- 102.Zhu M.L., Wei K.C., Lin S.E., Chen X.Y., Wu C.C., Li G., Bian L.M. Bioadhesive polymersome for localized and sustained drug delivery at pathological sites with harsh enzymatic and fluidic environment via supramolecular host-guest complexation. Small. 2018;14(7) doi: 10.1002/smll.201702288. [DOI] [PubMed] [Google Scholar]
- 103.Todorovic T., Vujanovic D. The influence of magnesium on the activity of some enzymes (AST, ALT, ALP) and lead content in some tissues. Magnes. Res. 2002;15(3–4):173–177. [PubMed] [Google Scholar]
- 104.Tiwari G., Tiwari R., Sriwastawal B., Bhati L., Pandey S., Pandey P., Bannerjee S.K. Drug delivery systems: an updated review. Int. J. Pharma. Invest. 2012;2(1):2–11. doi: 10.4103/2230-973X.96920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang L., Zhang M., Yang Z.M., Xu B. The first pamidronate containing polymer and copolymer. Chem. Commun. 2006;(26):2795–2797. doi: 10.1039/b605365c. [DOI] [PubMed] [Google Scholar]
- 106.Leeuwenburgh S.C.G., Jansen J.A., Mikos A.G. Functionalization of oligo(poly(ethylene glycol)fumarate) hydrogels with finely dispersed calcium phosphate nanocrystals for bone-substituting purposes. J. Biomater. Sci. Polym. Ed. 2007;18(12):1547–1564. [PubMed] [Google Scholar]
- 107.Yang X., Akhtar S., Rubino S., Leifer K., Hilborn J., Ossipov D. Direct "click" synthesis of hybrid bisphosphonate-hyaluronic acid hydrogel in aqueous solution for biomineralization. Chem. Mater. 2012;24(9):1690–1697. [Google Scholar]
- 108.Cezar C.A., Kennedy S.M., Mehta M., Weaver J.C., Gu L., Vandenburgh H., Mooney D.J. Biphasic ferrogels for triggered drug and cell delivery. Adv. Healthcare Mater. 2014;3(11):1869–1876. doi: 10.1002/adhm.201400095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gao F., Xie W.S., Miao Y.Q., Wang D., Guo Z.H., Ghosal A., Li Y.S., Wei Y., Feng S.S., Zhao L.Y., Fan H.M. Magnetic hydrogel with optimally adaptive functions for breast cancer recurrence prevention. Adv. Healthcare Mater. 2019;8(14):14. doi: 10.1002/adhm.201900203. [DOI] [PubMed] [Google Scholar]
- 110.Kim J.I., Chun C., Kim B., Hong J.M., Cho J.K., Lee S.H., Song S.C. Thermosensitive/magnetic poly(organophosphazene) hydrogel as a long-term magnetic resonance contrast platform. Biomaterials. 2012;33(1):218–224. doi: 10.1016/j.biomaterials.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 111.Wang H.A., Bongio M., Farbod K., Nijhuis A.W.G., van den Beucken J., Boerman O.C., van Hest J.C.M., Li Y.B., Jansen J.A., Leeuwenburgh S.C.G. Development of injectable organic/inorganic colloidal composite gels made of self-assembling gelatin nanospheres and calcium phosphate nanocrystals. Acta Biomater. 2014;10(1):508–519. doi: 10.1016/j.actbio.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 112.Diba M., Wang H.N., Kodger T.E., Parsa S., Leeuwenburgh S.C.G. Highly elastic and self-healing composite colloidal gels. Adv. Mater. 2017;29(11):8. doi: 10.1002/adma.201604672. [DOI] [PubMed] [Google Scholar]
- 113.Xu Z.P., Shi L.Y., Yang M.Y., Zhang H.P., Zhu L.J. Fabrication of a novel blended membrane with chitosan and silk microfibers for wound healing: characterization, in vitro and in vivo studies. J. Mater. Chem. B. 2015;3(17):3634–3642. doi: 10.1039/c5tb00226e. [DOI] [PubMed] [Google Scholar]
- 114.Shin Y.C., Shin D.M., Lee E.J., Lee J.H., Kim J.E., Song S.H., Hwang D.Y., Lee J.J., Kim B., Lim D., Hyon S.H., Lim Y.J., Han D.W. Hyaluronic acid/PLGA core/shell fiber matrices loaded with EGCG beneficial to diabetic wound healing. Adv. Healthcare Mater. 2016;5(23):3035–3045. doi: 10.1002/adhm.201600658. [DOI] [PubMed] [Google Scholar]
- 115.Billiet T., Vandenhaute M., Schelfhout J., Van Vlierberghe S., Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 2012;33(26):6020–6041. doi: 10.1016/j.biomaterials.2012.04.050. [DOI] [PubMed] [Google Scholar]