Abstract
Frailty is a clinical state of vulnerability to stressors resulting from cumulative alterations in multiple physiological and molecular systems. Frailty assessment in patients with chronic disease is useful for identifying those who are at increased risk for poor clinical and patient reported outcomes. Due to biobehavioral changes purported to cause both frailty and certain chronic lung diseases, patients with lung disease appear susceptible to frailty and prone to developing it decades earlier than community dwelling healthy populations. Herein, we review the literature and potential pathobiological mechanisms underpinning associations between Frailty in lung disease and age, sex, comorbidity and symptom burden, severity of lung disease, inflammatory biomarkers, various clinical parameters, body composition measures, and physical activity levels. We also propose a multipronged program of future research focused on improving the accuracy and precision of frailty measurement in lung disease, identifying blood-based biomarkers and measures of body composition for frailty, determining whether sub-phenotypes of frailty with distinct pathobiology exist, and developing personalized interventions that target the specific underlying mechanisms causing frailty.
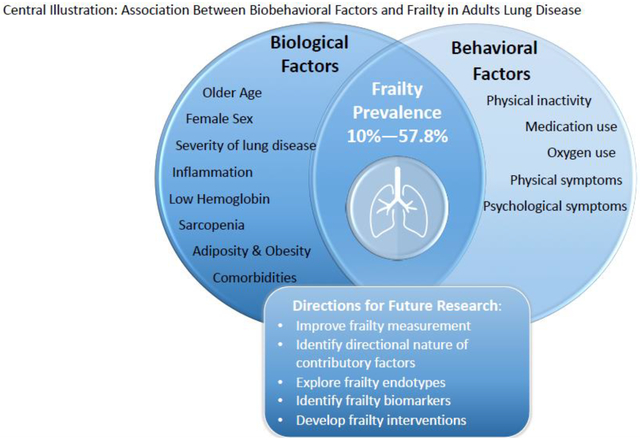
Graphical Abstract
Frailty, a concept originally developed in the geriatric literature, is a clinical state of vulnerability to stressors resulting from cumulative alterations in multiple physiological and molecular systems1 and increases the risk for poor clinical and patient reported outcomes. The putative underlying mechanisms of frailty may be attributed to age-related or disease-related changes or a combination of both.2 The cellular hallmarks of aging include stem cell exhaustion, altered intercellular communication, genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, and cellular senescence. These cellular mechanisms coupled with low physical activity level and malnutrition are thought to contribute to pathobiological changes (i.e., chronic inflammation, immune senescence, endocrine dysfunction, and sarcopenia) that lead to frailty.3 Similarly, some disease processes are associated with biobehavioral factors (e.g. inflammation, malnutrition, physical inactivity, sarcopenia) that predispose patients to frailty.2
While there is a general consensus on the conceptual definition of frailty, there continues to be considerable debate over how to operationalize it. There are two general concepts of frailty: the “physical” or “phenotypic” frailty and the “deficit accumulation” or “frailty index.4” The phenotypic model considers frailty to be a biological syndrome with distinct clinical manifestations. Several instruments have been developed to operationalize the frailty phenotype (e.g. Short Physical Performance Battery [SPPB], Clinical Frailty Scale, etc.), but the Fried Frailty Phenotype (FFP) is the most well-known and widely used. Components of the FFP include weight loss, weakness, exhaustion, slow walking speed, and low physical activity; patients are considered frail when three of more of these clinical manifestations are observed.5 Frailty is also conceptualized as an accumulation of deficits and is most commonly operationalized using the Frailty Index (FI), a mathematical model that calculates a ratio based on the number of deficits present (i.e., clinical symptoms, functional impairments, laboratory findings, disabilities, and comorbidities) relative to the total number of items assessed.6 Amongst the numerous available measures, there is no gold standard for assessing frailty. The most suitable frailty instrument should be determined by the needs or goals of the study or clinical setting. For example, if a short and simple tool to screen patients for frailty and predict who is at risk for adverse outcomes is the goal, the FI may be the most appropriate measure. Alternatively, the FFP or SPPB may be more suitable for those interested in researching the underlying mechanisms of frailty to identify modifiable targets for future interventions or to test the efficacy of an intervention to treat frailty.7
It is becoming increasingly evident that there is an interplay between frailty and chronic disease; frailty impacts chronic disease, and conversely chronic disease increases the risk for frailty.8 This seems to be the case in patients with chronic lung disease, a burdensome set of diseases that affects hundreds of millions of people and is the third leading cause of death and disability worldwide.9 In addition to a heightened risk of death, lung disease causes severe symptom burden, substantial health care resource utilization, and reduced quality of life.10 It can impact people across the lifespan, but predominantly affects older adults.9 For several reasons, patients with lung disease appear susceptible to frailty. For example, there are biological hallmarks of some chronic lung disease that mirror the underlying mechanisms associated with frailty, such as chronic systemic inflammation.11,12 Furthermore, the debilitating symptoms of lung disease, which include not only respiratory symptoms, but also fatigue, anxiety, depression, and sleep disturbances,13 may also increase the risk for frailty. These symptoms are thought to share similar biobehavioral mechanisms that lead to frailty—they are associated with inflammation,14–17 can reduce nutritional intake or appeteite18–20 and physical activity levels,21–23 which all cause ma lnutrition and sarcopenia.24 Finally, patients with lung disease are typically older and may experience age-related biological changes that also parallel the pathobiological changes believed to be associated with frailty. Due to both the disease-related and age-related changes that affect patients with lung disease, this population is at increased risk for developing frailty. Patients with lung disease who are frail experience worse clinical outcomes, including higher hospitalization and readmissions rates, prolonged hospitalizations, disability, worse patient reported outcomes, and higher mortality rates compared to patients who are not frail.25,11,26–32
Frailty is a dynamic condition that can improve with targeted interventions. However, without intervention, frailty will likely worsen, and patients will continue to be at greater risk for adverse outcomes. Understanding the pathobiology driving frailty in lung disease is critically important because it could strengthen early detection of patients at risk for becoming frail and highlight novel targets for intervention to prevent or ameliorate frailty. In turn, interventions to improve frailty could potentially also mitigate the adverse outcomes we are witnessing in this population. The aim of this review is to identify the associated biobehavioral factors and underlying pathobiological mechanisms of frailty that have been studied in lung disease and highlight areas for future research.
Frailty in Lung Disease
Investigations into frailty in lung disease are relatively young and generally limited to patients with either chronic obstructive pulmonary disease (COPD) or interstitial lung disease (ILD). COPD and ILD comprise the two most prevalent groups of end stage lung disease and, as a result, are the most common indications for lung transplantation.33 Less is known about frailty in patients with cystic fibrosis (CF) and pulmonary vascular disease (PVD). This is due to either exclusion of patients with CF and PVD from some studies or underrepresentation of patients with these diagnoses in existing studies. In studies of lung transplant candidates with a mix of lung diseases, for example, patients with CF and PVD represented only 2%−11% and 8%−9% of study samples, respectively.11,31,34 With those caveats, across chronic lung disease populations, frailty appears to be highly prevalent (6% to 76%).35,36,26,37 The reported variability in the prevalence of frailty in lung disease is a result of variation in sample sizes, distribution of sample characteristics (i.e. age, sex, severity of lung disease), and definitions and measures used to study frailty.
The causal factors driving frailty in lung disease are not yet well established. Much of what is known is limited to associations. Studies suggest that biobehavioral factors are likely relevant in the development of frailty in patients with lung disease, but future research in this area is critical for the development of effective interventions to prevent or reverse frailty. We aimed to highlight and discuss these biobehavioral factors to provide further insight into the pathobiological mechanism of frailty in lung disease and stimulate ideas for future research.
Biological Factors Associated with Frailty in Lung Disease
Age and Frailty
Older age was commonly associated with frailty in lung disease.
In studies of lung disease, the prevalence of and risk for frailty increase with age (see Table 1),29,38,39,35,40,41,27,28,42 a pattern similar to that observed in the community dwelling general population.43 However, frailty appears to develop in younger patients with lung disease at higher rates than is observed in the general population. For example, the prevalence of frailty among patients with advanced lung disease in the 5th and 6th decade of life ranges from 12% to 45%.11,26,31,36,38,39,41 This prevalence is similar to what is observed in community dwelling older populations in their 9th decade of life (15.7% of adults 80–84 years of age and 26.1% of adults >85 years of age).43 One emerging theory proposes that the pathobiology of accelerated aging may manifest as lung disease as well as frailty.
Table 1.
Studies of Age and Frailty in Lung Disease
| Chronological Age Older age was commonly associated with frailty in lung disease | |
|---|---|
| Patient Population | Finding |
| Older adults, some of whom had spirometrically defined lung disease | Age increased across the 3 levels of frailty (70.8±3.8 not-frail, 71.9±4.3 pre-frail, 73.8±4.2 frail, p<0.001)1 |
| COPD | Age was associated with higher odds of frailty (OR 1.05, 95% CI 1.02–1.08 per year)2 |
| COPD | Frail patients were older compared with those who were pre-frail or not frail (67.4±8.1 not frail, 69±9.6 pre-frail, 72.6±10 frail, p<0.001)3 |
| ILD | Age predicted frailty (every decade increase in age associated with an increase in FI by 0.044; R2 = 0.42, p = 0.04)4 |
| COPD | Age was associated with higher odds of frailty (OR 1.07, p<0.001)5 |
| COPD | Frail patients were older compared to those who were not (61.1±10.1 not-frail vs 72.6±5.8 frail, p=0.021)6 |
| COPD | Age was not significantly associated with higher odds of frailty (aOR 1.1, p=0.1)7 |
| COPD | Age tended to increase as frailty severity worsened (mild, moderate, severe) (69.4±9.4 years not-frail; 70.3±6.9 years mild frailty; 71.6±11.5 years moderate frailty; 75.6±6.7 years severe frailty, p=0.118)8 |
| COPD | Older age was not significantly associated with higher odds of frailty (OR 1.65, 95% CI .82–3.33)9 |
| ILD | Differences in age were not significant among those who were frail compared to those who were not (mean age 59±7 years not-frail vs 57±8 years frail, p-value NR)10 |
| Lung transplant candidates | Differences in age were not significant among those who were frail compared to those who were not (SPPB: 59 (50–65) frail vs. 59 (50–65) not frail, p=0.86; FFP 58 (46–63) frail vs. 59 (50–65) not frail, p=0.11)11 |
| Lung transplant candidates | Differences in age were not significant among those who were frail compared to those who were not (57.5 years [IQR 51–62.8] not frail vs 56 years [IQR 51.8–61.5], p =0.95)12 |
| COPD | Frail patients were younger compared to those who were not (mean age 63.7 ± 8.2 frail vs 67.1 ± 7.1 not-frail,p <0.001)13 |
| Biological Aging There is insufficient study of the relationship between frailty and markers of biological aging in lung disease | |
| ILD | Telomere length was not correlated with FI (r=0.04, p=0.57)14 |
COPD= chronic obstructive pulmonary disease; FI= frailty index; ILD= interstitial lung disease; IQR= interquartile range; NR= not reported; OR= odds ratio
Insufficient study of the relationship between markers of biological aging and frailty in lung disease.
Biological aging is a result of an accumulation of genetic and epigenetic modifications that lead to progressive cell deterioration, impaired tissue function, and decreased ability to maintain homeostasis.44 Nine molecular and cellular hallmarks of aging (e.g., mitochondrial dysfunction, immune senescence, telomere attrition) have been proposed and their role in the development of frailty has been studied in other populations,3 but not in the lung disease population. One such pathway involves mitochondrial dysfunction. Inside cells, mitochondria generate energy in the form of adenosine triphosphate by oxidizing glucose. Accordingly, pre-frail elderly have decreased skeletal muscle mitochondrial function,45 while slowness, exhaustion, shrinking, and frailty have been associated with lower mitochondrial copy numbers.46 Mitochondrial dysfunction and other cellular stressors can also result in the generation of reactive oxygen species and oxidative stress.47 Oxidative stress, in turn, has been associated with frailty in multiple studies.48 Indeed, oxidative stress was proposed as the basis of aging as far back as the 1950s.49 However, the direct mechanistic link between oxidative stress and aging has been questioned, as promotion of reactive oxygen species, through exercise or calorie restriction, can reduce aging, while certain antioxidants have been linked to aging pathology.50
Mitochondria are thought to have originated as prokaryotic organisms that were engulfed by eukaryotes, with whom they now live in symbiosis. As foreign organisms, however, they have their own genomes, and release of this mitochondrial DNA (mtDNA) and other mitochondrial components can trigger systemic inflammation through multiple damage-associated pattern receptors (toll-like receptor 9 [TLR9], formyl peptide receptor 1[FPR1], and cyclic GMP-AMP synthase [cGAS]). Activation of these receptors leads to increases in systemic inflammatory mediators linked to aging, including interleukin-6 (IL-6), interleukin 1 beta (IL1β), and interferons.51 In the context of lung transplantation, higher levels of donor mtDNA are linked to the development of primary graft dysfunction, while in a mouse model of lung transplantation, deletion of FPR1 reduced allograft injury.52 However, the contribution of mitochondrial dysfunction to frailty in lung disease remains unknown.
The role of cellular senescence in the development of frailty has also been studied in other populations. Satellite cells are muscle-resident stem cells that can activate to replenish muscle tissue following injury. However, with age, satellite cells become senescent. Senescence is a state of growth arrest that is likely important in preventing cancer development, and senescent satellite cells generally apoptose. Failure of autophagy and resulting mitochondrial dysfunction has been proposed as a potential mechanism driving satellite cell senescence.53
Senescence of multiple cell types has been associated with aging pathologies. These cells can be identified with p16, p21, and senescence-associated β-galactosidase markers, and adopt the senescence-associated secretory phenotype (SASP), a DNA-damage response associated with production of inflammatory mediators such as IL-6, plasminogen activator inhibitor-1 [PAI-1], tumor necrosis factor-alpha (TNFα), and insulin-like growth factor (IGF) binding proteins.54 It is worth noting that cellular senescence is a prominent feature of chronic lung allograft dysfunction following lung transplantation.55 Further, SASP component proteins are directly linked to frailty, including in lung transplant candidates.11 Transfer of senescent cells to mice results in physical dysfunction and impaired endurance. This frailty-like phenotype induced by senescent cells could be reversed with dasatinib and quercetin, a combination of “senolytics.” Senolytics are a class of drugs which selectively kill senescent cells by disabling the senescence associated anti-apoptotic pathways that protect senescent cells from their pro-apoptotic microenvironment. In mice, dasatinib and quercertin were successful at clearing senescent cells, alleviating the senescent cell induced frailty-like phenotype and increasing longevity.56 Future study of the therapeutic benefit of senolytics in reversing frailty in humans is needed.
Telomere dysfunction, referred to as telomeropathies, has been linked to accelerated biological aging57 (i.e. cell senescence) and is associated with muscular dystrophy.58 In adulthood, the most common manifestation of telomeropathies is lung disease, specifically idiopathic pulmonary fibrosis.59 The association between telomeropathies and frailty in lung disease has not been adequately studied. Patients with lung disease may be susceptible to frailty through this mechanism, given what we know about telomeropathies—that it causes accelerated biological aging, has been linked to muscular dystrophy, and commonly manifest as lung disease. However, only one small study of patients with pulmonary fibrosis has explored this relationship, and researchers reported no significant association between short peripheral blood telomeres and frailty.25 It may be that telomere-related pathology may only become apparent when telomeres reach a critical length, resulting in organ failure rather than frailty. Further studies with larger sample sizes are needed to test whether telomere shortening in satellite cells predicts the development of sarcopenia and frailty in both lung disease and the general population.
Biomarkers for Frailty
Research aimed at identifying robust biomarkers for frailty in the general population is ongoing. Circulating markers of frailty studied in various disease and aging populations include inflammatory markers (e.g., C-reactive protein [CRP], IL-6, TNFa), hormones (e.g., insulin-like growth factor-1 [IGF1]), mitochondrial DNA, and certain routine clinical lab parameters (e.g., hemoglobin, glomerular filtration rate, albumin).60 The study of these biomarkers as they relate to frailty in lung disease is in its infancy. (see Table 2).
Table 2.
Studies of Biomarkers for Frailty in Lung Disease
| Systemic inflammation Frailty is generally associated with inflammatory biomarkers in lung disease | |
|---|---|
| Patient Population | Finding |
| COPD | Frail patients had greater inflammation compared with those who were not (CRP 4.1±1.6 vs 3.2±2.8, p=0.021; Fibrinogen 3.6±1.3 vs 3.4±1.3, p=0.028)13 |
| Lung transplant candidates | Frail patients had higher levels of IL-6 (SPPB, p = 0.10; FFP, p = 0.003) and tumor necrosis factor receptor 1 (SPPB, p = 0.001; FFP, p = 0.001) compared to those who were not11 |
| Lung transplant candidates | No significant differences in analyzed cytokines (IL-10, IL-6, IL-2, IL-7, Il-12, IL-23, IL-18) among moderately frail compared to severely frail patients15 |
| Hemoglobin Lower hemoglobin levels are associated with frailty in lung disease | |
| ILD | Frail patients had a lower hemoglobin level (g/L) compared to those who were not(142±17 not-frail vs 137±19 frail, p <0.05)10 |
| Lung transplant candidates | Frail subjects had a lower hemoglobin level (g/dl) compared to those who were not (SPPB: 12.0±1.9 frail vs 13.4±2.0 not frail, p= 0.004; FFP: 12.5±1.8 frail vs 13.2±2.0 not frail, p=0.001)11 |
| Creatinine There is insufficient study of the relationship between frailty and creatinine levels in lung disease; inconsistent findings to date | |
| ILD | Frail patients had a lower creatinine level (umol/L) compared to those who were not (70±18 frail vs 83±15 not frail, p<0.05)10 |
| Lung transplant candidates | Differences in creatinine level were not significant among those who were frail compared to those who were not (SPPB: 0.9±0.5 frail vs 0.8±0.2 not frail, p=0.83; FFP: 0.8±0.3 frail vs 0.8±0.2 not frail, p=0.62)11 |
| Albumin There is insufficient study of the relationship between frailty and albumin in lung disease, but findings to date support a relationship | |
| ILD | Frail patients had lower albumin levels compared with those who were pre-frail or not frail (43±4 not frail, 44±4 pre-frail, 40±4 frail, p<0.05)10 |
| Leptin There is insufficient study of the relationship between frailty and leptin in lung disease, but findings to date support a relationship | |
| Lung transplant candidates | Frail patients tended to have lower leptin levels compared to those who were not (SPPB, p = 0.33; FFP, p = 0.08)11 |
| Insulin-like growth factor 1 (IGF-1) There is insufficient study of the relationship between frailty and IGF-1 in lung disease, but findings to date support a relationship | |
| Lung transplant candidates | Frail patients tended to have lower levels of IGF-1 compared to patients who were not (SPPB, p = 0.03; FFP, p = 0.16)11 |
COPD= chronic obstructive pulmonary disease; CRP= c-reactive protein; FFP= fried frailty phenotype; ILD= interstitial lung disease; IQR= interquartile range; SPPB= short physical performance battery
Frailty is generally associated with inflammatory biomarkers in lung disease.
Inflammation is a central pillar in the modern concept of aging and the development of age related diseases. In this framework, repetitive inflammatory insults, whether they be infectious, nutritional, or abnormal proteins, lead to activation of pro-inflammatory cytokine cascades.61 Over time, the cumulative impact of activated inflammatory pathways leads to biological aging and age-related diseases and conditions, such as frailty. Inflammaging is the term used to describe this pro-inflammatory state associated with aging.62
Multiple studies have correlated levels of pro-inflammatory cytokines with various frailty phenotypes in several disease and aging populations. IL-6 is produced early in inflammatory states and leads to production of numerous acute phase proteins, including CRP and fibrinogen.63 Increased IL-6 levels are associated with frailty in the elderly and are negatively correlated with grip strength and gait speed, two significant components of multiple frailty phenotype constructs.33,64
Systemic inflammation may very well play a role in the development of frailty in lung disease. Increased plasma levels of IL-6 mediate some of the association between lower forced expiratory volume in 1 second (FEV1) and shorter six minute walk distance (6MWD) in patients with COPD.65 Frail patients with COPD tend to have greater inflammation, as indicated by higher levels of circulating CRP and fibrinogen compared to patients with lung disease who are not frail.36 Lung transplant candidates who are frail tend to have higher levels of IL-6 and tumor necrosis factor receptor-1 (TNF-1) than non-frail candidates.11 IL-6 also likely plays a role in negative outcomes of frail patients with pulmonary disease. Higher IL-6 levels correlate with worse clinical outcomes and greater health care resource utilization following lung transplant surgery, including more severe primary graft dysfunction (a form of acute lung injury following allograft implantation), prolonged need for invasive mechanical ventilation, increasing intensive care unit length of stay, and increased mortality.66,67 However, it is unclear whether the role of systemic inflammation in frailty is causal, compensatory, or an epiphenomenon.36
The concept of inflammaging is more complicated than evaluating single cytokines. Cardoso et al developed a multi-tiered panel of proposed biomarkers for frailty, highlighted by several key inflammatory proteins, namely IL-6, C-X-C chemokine 10 (CXCL-10), fractalkine (CX3CL1), and long pentraxin-3 (PTX3). A panel of biomarkers may provide added value in identifying overall physiological decline contributing to onset of frailty.68 Orthogonal relationships between biomarkers associated with frailty suggests there may be mechanistically distinct subtypes or endotypes. A recent study by Marzetti et al of community-dwelling older adults demonstrated that elevated levels of a panel of inflammatory biomarkers, including P-selectin, CRP, and interferon-γ-inducible protein 10 (IP-10), discriminated patients with physical frailty and sarcopenia from non-sarcopenic, non-frail subjects.69 Only one study of patients with lung disease examined a panel of biomarkers for frailty. In this study, a panel of cytokines (IL-2, IL-6, IL-7, IL-10, IL-12p70, IL-18, IL-23) was not significantly associated with frailty severity.70 However, this was a small study of only 31 subjects with moderate or severe frailty by the Clinical Frailty Scale. Consequently, the contribution of a panel of cytokines to frailty could not be assessed and differences between moderately and severely frail patients may have been difficult to determine because of the small sample size. Future studies are needed to understand better the role of systemic inflammation in the development of frailty in patients with lung disease.
Insufficient study of the relationship between clinical parameters and frailty in lung disease.
Few studies have examined clinical parameters that serve as biomarkers of frailty in lung disease. Anemia, characterized by low hemoglobin levels, leads to signs and symptoms of frailty, namely fatigue, exhaustion, and weakness. Older adults and patients with chronic disease, such as lung disease, are at increased risk for developing anemia and its accompanying debilitating symptoms, which may contribute to the development of frailty.71,72 Conversely, anemia may result from chronic inflammation, a condition referred to as anemia of chronic disease, or malnutrition leading to iron-deficiency anemia.71 As previously described, inflammation and malnutrition are putative mechanism of frailty and therefore, anemia may occur concurrently with or after the development of frailty. Two studies of patients with lung disease revealed that patients who were frail had lower hemoglobin levels compared to those who were not.11,26 From these studies, we see that a relationship between anemia and frailty exists, but we cannot determine whether the role of anemia is causal or an epiphenomenon.
Creatinine is another clinical parameter associated with frailty. However, the underlying mechanisms thought to explain this relationship are different, depending on whether creatinine is low or high. Lower creatine could be reflective of sarcopenia in the frail population.26 Sarcopenia is a syndrome characterized by the progressive and generalized loss of skeletal muscle mass, strength, and function73 and is considered a fundamentally important component of frailty. In the setting of low muscle mass, serum creatinine levels are reduced.74 In a study of patients with ILD, frail patients had significantly lower serum creatinine levels compared to non-frail patients. Conversely, frailty is also seen in patients with high creatinine levels. The literature on frailty in the chronic kidney disease (CKD) population is robust and highlights the high prevalence of frailty in this population.75 The physiological changes associated with CKD, namely its proinflammatory state, may explain the high prevalence rates of frailty in CKD. However, in a study of lung transplant candidates, no difference in creatinine levels was observed between frail and non-frail candidates.11 However, it is important to note that patients with impaired renal function are often not listed for lung transplantation and therefore, patients with high creatinine may not have been included in the study sample. In summary, the relationship between creatinine and frailty is less clear in the lung disease population. Patients with both low and high creatinine are at risk for being frail. The mechanisms by which a patient develops frailty could be through sarcopenia, in which case, the patient may exhibit low creatinine, or inflammation, which may be the case in patients with CKD. Creatinine may be a useful biomarker for frailty and may highlight some of the underlying pathobiological mechanisms of frailty, but future work is needed in this area.
Only one study to date of lung transplant candidates found that hypoalbuminemia was associated with frailty.26 Although albumin is often considered a nutrition-related biomarker, hypoalbuminemia is no longer recommended for identifying malnutrition.76 Instead, it is thought to better reflect the severity of the inflammatory response.77 In another study, lower levels of leptin, a measure of adipose tissue mass, and IGF-1, an important mediator of growth hormones, were associated with frailty.11 The evidence linking these biomarkers to frailty in lung disease is limited, but these finding are consistent with the theory that frailly results from alterations in multiple physiological and molecular systems.1
Biomarkers are key in advancing our understanding of pathobiological mechanisms driving frailty. Research is ongoing to identify and understand individual and panels of biomarkers for frailty and to understand the directional relationship between these factors.
Body Composition and Frailty
Sarcopenia is associated with frailty in lung disease.
A core focus of geriatric medicine is the preservation of physical functioning and prevention of disability. In the 1990s, studies of frailty and sarcopenia emerged roughly in parallel; both aimed to elucidate the causes of disability. Originally coined by Rosenberg,78 the term “sarcopenia” was meant to reflect the loss of muscle mass that accompanies aging. In the ensuing decades, consensus statements have advanced the conceptual definition of sarcopenia. Sarcopenia is now defined as abnormally low lean muscle mass combined with reduced muscle strength or function.79,80
Because most operational definitions of frailty incorporate deficits in functioning (i.e., slowness, weakness), sarcopenia has long been considered to be a cardinal physical feature of frailty.81–85 The mechanisms by which sarcopenia develops include protein and micronutrient deficiencies, chronic inflammation, mitochondrial dysfunction, satellite cell senescence, endocrine dysregulation, and disuse atrophy—all of which are also associated with frailty.81,82 Specifically, certain inflammatory biomarkers associated with frailty, such as IL-6 and TNFa, can directly induce muscle catabolism.86,87
A robust and diverse body of literature has examined the association between various operational measures of sarcopenia—including imaging, functional, and strength indices—and outcomes in lung disease. For example, sarcopenia is prevalent in adults with COPD and is associated with reduced functional performance, exercise capacity, health-related quality of life and higher risk of mortality.88–92 In separate studies, reduced quadriceps strength and cross-sectional area were associated with increased risk of mortality.93,94 Shorter 6MWD was independently associated with mortality in adults with ILD.95,96 Reduced quadriceps strength was also associated with increased length of hospital stay following lung transplant surgery.97 Relatively few studies, however, have specifically evaluated the relationship between sarcopenia and frailty. In a population of lung transplant candidates with a mix of lung diseases, sarcopenia, quantified by whole-body dual X-ray absorptiometry (DXA), was both prevalent (46%) and associated with frailty.98,99 In the same population, frailty was also associated with markedly reduced 6MWD. In studies of patients with COPD, frailty was also associated with sarcopenia; skeletal muscle mass index was measured with either bioelectrical impedance analysis or DXA and sarcopenia was defined according to the consensus European Working Group on Sarcopenia in Older People criteria or the Asian Working Groups for Sarcopenia criteria.38,42 (see Table 3).
Table 3.
Studies of Body Composition and Frailty in Lung Disease
| Sarcopenia Sarcopenia is associated with frailty in lung disease | |
|---|---|
| Patient Population | Finding |
| Lung transplant candidates | Lean muscle mass was negatively correlated with frailty (by both the SPPB and FFP) (SPPB, r=−0.15; FFP, r=−0.21, p<0.05)11 |
| COPD | Frail patients had reduced SMI (8.1±1.8) and a higher proportion were sarcopenic (23.9%) compared with robust patients (SMI 8.6±1.9, p=0.002; 1.2% sarcopenic, p<0.001)3 |
| COPD | Sarcopenia was associated with higher odds of frailty (aOR 29.5, p=0.02)7 |
| Adiposity & Obesity Frailty is associated with adiposity and generally associated with obesity (measured by waist circumference and BMI) in patients with lung disease | |
| Lung transplant patients | Every 20 cm2 increase in VAT area was associated with 50% increased odds of frailty in subjects with high VAT (95% CI 1.2–1.9, p < .001), and 10% decreased odds of frailty in subjects with low VAT (95% CI 0.7–1.04, p = .12)16 |
| COPD | Higher waist circumference was associated with higher odds of frailty (aOR 1.3, p=0.01)7 |
| COPD | Frail patients had a larger waist circumference compared with those who were not (106±13 vs 104±11, p=0.041)13 |
| COPD | Frail patients had a higher BMI compared with those who were not (29±7 vs 28±5, p=0.038)13 |
| Older adults, some of whom had spirometrically defined lung disease | Frail patients had a higher BMI compared with those who were pre-frail or not frail (mean BMI: 26±3.7 not-frail, 26.6±4.0 pre-frail, 27.5±4.6 frail, p<0.001)1 |
| COPD | Differences in BMI were not significant among those who were frail compared to those who were pre-frail or robust (27.2±5.2 robust vs 27.8±6.5 pre-frail vs 27.9±7.6, p=0.71)3 |
| ILD | Differences in BMI were not significant among those who were frail compared to those who were not (mean BMI 28±4 not-frail vs 27±4 frail)10 |
| COPD | Differences in BMI were not significant among those who were frail-disabled compared with those who were not frail (mean BMI: 29±4.7 not-frail vs. 29±5.3 frail-disabled, p=0.849)2 |
| COPD | Differences in BMI were not significant across frailty severity categories (28.7±5.5 not-frail, 28.6±4.4 mildly frail, 28.5±8.4 moderately frail, 28.3±3.8 severely frail, p=0.996)8 |
| Lung transplant candidates | Differences in BMI were not significant among those who were frail compared with those who were not (24.5 (IQR 20.8–27.6) not-frail vs. 26.2 (IQR 22.4–29.8) frail, p=0.14)12 |
| COPD | Differences in BMI were not significant among those who were frail compared to those who were pre-frail or robust (27.9±4.9 not-frail vs 29.3±5.1 pre-frail vs 27.9±5.1 frail, p=0.345)6 |
| ILD | BMI was not significantly correlated with frailty (r=0.10, p=0.36)17 |
| Lung transplant candidates | Differences in BMI were not significant among those who were frail by the SPPB compared to those who were not (26.2±7.5 frail vs 26.1±4.5 not frail, p=0.93). However, frail patients by the FFP had a higher BMI compared to those who were not frail (24.1±4.6 frail vs 26.0±4.8 not frail, p=0.001)11 |
| Chronic lung disease | Differences in BMI were not significant among those who were frail compared to those who were not (data not presented in article)18 |
BMI= body mass index; COPD= chronic obstructive pulmonary disease; FFP= fried frailty phenotype; ILD= interstitial lung disease; IQR= interquartile range; SPPB= short physical performance battery; SMI= skeletal muscle mass; VAT= visceral adipose tissue
Adiposity and obesity are generally associated with frailty with lung disease.
While afforded less attention, obesity and adiposity are associated with frailty. Obesity—especially central—is associated with risk of subsequent frailty in community dwelling older adults.100,101 In particular, a higher amount of abdominal visceral adipose tissue is associated with frailty.102 In lung disease, the association between visceral adipose tissue mass and frailty was evaluated by different imaging modalities in two distinct cohorts of lung transplant candidates with a mix of lung diseases. In one cohort, adiposity was quantified by bioelectrical impedance assay and, in the other, through cross-sectional CT imaging. Across the entire study population, the relationship between visceral adipose tissue and frailty was not linear; patients with both low and high visceral adipose tissue were at increased risk for frailty. In subjects with high adiposity, increasing amounts of visceral adipose tissue were associated with higher odds of frailty. Whereas in patients with low visceral adipose tissue, increasing amounts of visceral adipose tissue were associated with lower odds of frailty. These findings suggest that mechanisms of frailty may differ by visceral adipose tissue. Also interesting, is that compared to frail patients with low visceral adipose tissue, patients with high visceral adipose tissue had lower grip strength,103 a finding that suggests a condition referred to as sarcopenic obesity, described below. A similar link between central obesity, measured as waist circumference, and frailty was identified in studies of patients with COPD; high waist circumference was associated with frailty.36,42 (see Table 3)
Obesity and adiposity may contribute to frailty through inflammation, cellular senescence, and sarcopenia. In obesity, visceral adipose tissue is characterized by a proinflammatory phenotype, featuring adipose tissue macrophages, increased CD8+ T cells, and IL-6 and TNFa production.104 Further adiposity is also associated with inflammatory “adipokines” and other cytokines, such as IL-6 and monocyte chemotactic protein-1.105,106 Senescent pre-adipocytes produce a senescence-associated secretory phenotype characterized by increased production of proinflammatory cytokines IL-6, chemokine ligand 1, monocyte chemoattractant protein-1, and TNFα, inducing an inflammatory phenotype locally and systemically.56 Elevated levels of many of these adipokines have been observed in frail adults. Cellular senescence in obese and aged adipose tissue decreases grip strength and gait speed in mice56 and, interestingly, inhibition of cellular senescence improves 6MWD and gait speed in patients with pulmonary fibrosis.107 Finally, obesity is associated with reduced grip strength, possibly due to intra-muscular lipid deposition and resultant impaired muscle function.108,109
Sarcopenic obesity may be associated with frailty in lung disease.
Aging and chronic disease contribute to the preferential loss of lean muscle as well as the gain of fat,110–112 resulting in a condition referred to as sarcopenic obesity.113 The causes of sarcopenic obesity are believed to be multifactorial, including an interplay between biological aging (i.e. cellular senescence), inflammation, sedentary lifestyle, unhealthy dietary habits, insulin resistance, and oxidative stress.114 Patients with lung disease are at increased risk for these biobehavioral changes that lead to sarcopenic obesity, which may in turn, increase this population’s risk for developing frailty. In studies of lung disease and frailty, where body mass index (BMI) was used as a measure of obesity, patients who were frail often maintained a high BMI,11,26,27,35,37–39,115 indicating that fail patients are more often overweight or obese. (see Table 3) These findings suggest that frailty in lung disease may not present in the typical manner—as a loss of weight— as described by the phenotypic theory.5 Instead, sarcopenic obesity may be a prevalent driver of frailty in lung disease.
Comorbid Disease and Frailty
Individual comorbidities and multimorbidity are associated with frailty in lung disease. Multimorbidity is common in patients with lung disease and is associated with increased risk for frailty, regardless of how frailty is operationalized.27,35,36,38,39 In studying comorbidities of patients with lung disease individually, those with arthritis,28,29 hypertension,28,29,35 depression,29 history of myocardial infarction,29 diabetes,28,29 stroke,29 kidney disease,29 liver disease,28 and history of cancer42 were more likely to be frail. These findings are consistent with the underlying theory of the frailty index, which suggests that the more deficits (symptoms, signs, disabilities, diseases, and laboratory measures) individuals have, the higher the likelihood of frailty.6 Frailty and comorbidity may cause or exacerbate each other and may result in a synergistic effect that gives rise to the adverse outcomes we observe among frail patients with chronic disease.28 (see Table 4)
Table 4.
Study of Comorbidities and Frailty in Lung Disease
| Individual Comorbidities and Multimorbidity are associated with frailty in lung disease | |
|---|---|
| Patient Population | Finding |
| Older adults, some of whom had spirometrically defined lung disease | The prevalence of arthritis increased across the 3 levels of frailty (42.9% not-frail, 54.8% pre-frail, 69.9% frail): (p<0.001)1 |
| COPD | Arthritis was associated with higher odds of frailty (OR 2.34, p <0.05)9 |
| Older adults, some of whom had spirometrically defined lung disease | The prevalence of depression increased across the 3 levels of frailty (4% not-frail, 17.1% pre-frail, 37.8% frail) (p<0.001)1 |
| Older adults, some of whom had spirometrically defined lung disease | The prevalence of myocardial infarction history increased across the 3 levels of frailty (9.1% not-frail, 12.9% pre-frail, 20.1% frail)1 |
| Older adults, some of whom had spirometrically defined lung disease | The prevalence of diabetes increased across the 3 levels of frailty (7.9% not-frail, 12.1% pre-frail, 18.7% frail) (p<0.001)1 |
| COPD | Diabetes was associated with higher odds of frailty (OR 4.86, p <0.05)9 |
| Older adults, some of whom had spirometrically defined lung disease | The prevalence of stroke history increased across the 3 levels of frailty (2.3% not-frail, 4.9% pre-frail, 8.6% frail) (p<0.001)1 |
| Older adults, some of whom had spirometrically defined lung disease | The prevalence of heart failure increased across the 3 levels of frailty (1.5% not-frail, 2.8% pre-frail, 7.2% frail) (p<0.001)1 |
| Older adults, some of whom had spirometrically defined lung disease | The prevalence of kidney disease increased across the 3 levels of frailty (1.3% not-frail, 2.7% pre-frail, 5.3% frail) (p<0.001)1 |
| COPD | Hypertension was associated with higher odds of frailty (OR 2.25, 95% CI 1.14–4.45)2 |
| Older adults, some of whom had spirometrically defined lung disease | The prevalence of hypertension increased across the 3 levels of frailty (39.4% not-frail, 47.8% pre-frail, 52.2% frail) (p<0.001)1 |
| COPD | Hypertension was associated with higher odds of frailty (OR 2.73, p <0.05)9 |
| COPD | Cancer was associated with higher odds of frailty (aOR 45.8, p=0.02)7 |
| ILD | Differences in MoCA scores were not significant among those who were frail compared to those who were not (MoCA score: 26±3 not-frail vs 26±4 frail)10 |
| COPD | Liver disease was associated with higher odds of frailty (OR 4.21, p <0.05)9 |
| COPD | Multimorbidity was associated with frailty (data not presented in article) (p=0.004)3 |
| COPD | ≥2 comorbidities was associated with higher odds of frailty (OR 2.35, 95% CI 1.14–4.81)2 |
| COPD | Mild, moderate, and severely frail patients had a higher comorbidity burden compared with those who were not frail (2.3±1.4 mild, 1.2±1.1 moderate, 2.4±1.2 severe vs 1.3±1.2 not frail, p <0.001)8 |
| COPD | Comorbidity burden (% >4 comorbidities) increased across the 3 levels of frailty (2% not-frail vs 42.6% pre-frail vs 50% frail, p=0.001)6 |
| COPD | Greater number of comorbidities was not significantly associated with higher odds of frailty (unfit) (≥3 comorbidities, OR 1.37, p=0.603; 2 comorbidities OR 2.00 p=0.192)5 |
COPD= chronic obstructive pulmonary disease; CI= confidence interval; ILD= interstitial lung disease; OR= odds ratio; MoCA= Montreal cognitive assessment
Severity of Lung Disease and Frailty
Severity of lung disease is generally associated with frailty.
In studies of lung transplant candidates with a mix of lung diseases (COPD, ILD, CF, PVD), frailty status did not differ significantly by underlying diagnosis (see Table 5).11,31,34 However, patients with CF and PVD are not well represented in studies of frailty in lung disease, only constituting 2%−11% and 8%−9% of study samples, respectively.11,31,34 It may not be that certain lung diagnoses increase the likelihood of developing frailty, but rather, that severity of lung disease is a more significant contributor of frailty. In a majority of studies, frailty was associated with worse lung function,29,30,36–38,41,116 greater number of exacerbations of lung disease,27,35,38,40,116 more advanced Global Initiative for Chronic Obstructive Lung Disease (GOLD) group35,38,116 (grouping based on symptoms, spirometry results, and exacerbation risk117), and use of supplemental oxygen (see Table 5).25,26 In the lung transplant candidate population, patients who were frail had a higher lung allocation score (LAS) than candidates who were not frail (see Table 5).11,31 The LAS is a measure that represents the severity of each candidate’s illness and his or her chance of surviving for one year following lung transplant. Patients with a higher LAS are considered more urgently in need of transplant and are prioritized for organ allocation in the countries that utilize the LAS system.118
Table 5.
Studies of Severity of Illness and Frailty in Lung Disease
| Lung Function Generally, frailty was associated with worse lung function | |
|---|---|
| Patient Population | Finding |
| COPD | Frail patients had poorer lung function compared to those who were not frail (FEV1 % predicted: 53±18 frail vs 60±19 not-frail,, p<0.001; FVC % predicted: 81±21 frail vs. 89±20 not-frail, p <0.001)13 |
| COPD | Frail patients had lower FEV1 % predicted (46.3±20.1) compared with those who were pre-frail (49±20.8) or not frail (57±22.4) (p<0.001)3 |
| ILD | Lower FEV1 (r= −0.22, p=0.045) was negatively correlated with frailty, and FVC (r= −0.21, p=0.06) and DLCO (r= −0.21, p=0.06) tended to be negatively correlated with frailty17 |
| ILD | FVC, (r=−0.27, p=0.003) FEV1 (r=−0.27, p=0.002), and DLCO (r= −0.31, p=0.001) were negatively correlated with frailty4 |
| COPD | Patients with severe-COPD had a 10-fold increased risk of frailty compared with patients with normal lung function (OR 10.0, 95% CI: 3.84–26.30, p < .001)19 |
| COPD | Frail patients had lower FEV1 (1.40±0.49 vs 1.91±0.53, p<0.05) and DLCO (8.99±4.59 vs 13.14±4.76, p <0.05) compared with patients who were robust, but did not have significantly lower FEV1 % predicted, FEV1/FVC, and TLC20 |
| ILD | Frail patients tended to have a lower FVC% predicted compared with those who were not frail (62±19 not-frail vs 54±15 frail (p-value NR).10 |
| COPD | Frail patients tended to have a lower FEV1% predicted and FVC % predicted compared to those who were pre-frail or not frail (FEV1% predicted: 49.8±14.5 not-frail vs 51.1±17.1 pre-frail vs 43±13.8 frail, p=0.269; FVC % predicted: (71.4±16.7 not frail, 66.7±19.1 pre-frail, 56.8±17.1 frail, p=0.084)6 |
| COPD | Degree of respiratory tract obstruction (FEV1 % predicted) was not significantly associated with higher odds of frailty (severe obstruction OR 1.39, p=0.572; moderate obstruction OR 0.42, p=0.079 compared to mild obstruction)5 |
| Lung transplant candidates | Lung function was not significantly different among those who were frail compared with those who were not by both the SPPB and FFP (SPPB: FEV1 1.4 (1.1–1.7) frail vs 1.3 (0.8–1.9) not frail, p=0.49; FVC 1.7 (1.4–2.5) frail vs 2.2 (1.6–2.7) not-frail, p=0.12) (FFP: FEV1 1.3 (0.8–1.7) frail vs 1.3 (0.8–1.8) not frail, p=0.58; FVC 1.9 (1.4–2.4) frail vs 2.0 (1.5–2.6, p=0.16) not-frail)11 |
| COPD | FEV1 % predicted was not associated with frailty (aOR 1.0, p=0.3)7 |
| Number of respiratory exacerbations per year Greater number of exacerbations in the previous year was associated with frailty in lung disease | |
| COPD | Frail patients reported higher number of exacerbations in the previous year (mean: 3.1±4.0) compared with those who were pre-frail (2.8±3.9) or robust patients (2.1±2.3) (p<0.001)3 |
| COPD | ≥2 exacerbations in the past 12 months was associated with higher odds of frailty (OR 1.73, 95% CI 1.07–2.78)2 |
| COPD | Patients with moderate-to-severe frailty had more exacerbations in the previous year compared with patients who were not frail (0.6 vs 0.2, p=0.042)8 |
| COPD | Frequent exacerbations of symptomatic COPD was associated with a 4.4-fold higher odds of frailty (OR 4.4, 95% CI: 1.41–14.04, p <.011)19 |
| COPD | Exacerbations were associated with higher odds of frailty (unfit) (OR 1.89, p=0.040)5 |
| COPD | Differences in frequency of exacerbations (%>2 in the past year) were not significant among those who were frail compared with those who were pre-frail or not frail (62.5% not-frail vs 58.4% pre-frail vs 41.7% frail, p=0.520)6 |
| GOLD Group Frailty was associated with more advanced GOLD group | |
| COPD | GOLD group B (low exacerbation risk, more symptoms) and D (high exacerbation risk, more symptoms) were associated with higher odds of frailty (OR 5.64, 95% CI 1.83–17.33, compared to GOLD group A (low exacerbation risk, less symptoms) and C (high exacerbation risk, less symptoms)2 |
| COPD | GOLD group D (high exacerbation risk, more symptoms) was associated with higher odds of frailty (OR 8.3, 95% CI: 3.25–20.96, p < .001, compared to patients without COPD)19 |
| Supplemental oxygen use Frailty was generally associated with use of supplemental oxygen | |
| ILD | Use of supplemental oxygen was positively correlated with frailty (oxygen use at rest r=0.22, p=0.04; on exertion r=0.29, p=0.006; nocturnal r=0.24, p=0.03)17 |
| ILD | Use of supplemental oxygen was higher among frail patients compared to those who were not (65% not-frail vs 92% frail, p <0.05)10 |
| ILD | Oxygen use was not a significant predictor of frailty (r value NR, p=0.36)4 |
COPD= chronic obstructive pulmonary disease; DLCO= Diffusing capacity of the lungs for carbon monoxide; FEV1 % predicted= forced expiratory volume; FFP= fried frailty phenotype; FVC= forced vital capacity; ILD= interstitial lung disease; IQR= interquartile range; NR= not reported; SPPB= short physical performance battery; TLC= total lung capacity;
There are physiological changes associated with worsening lung disease that may contribute to the pathobiological mechanisms associated with frailty. Chronic hypoxia can generate low grade systemic inflammation which can then provoke muscle apoptosis and protein degradation, increasing the risk for developing skeletal muscle dysfunction, a condition that manifest as decreased muscle strength and endurance and increased muscle fatiguability.119 Although speculative, it is plausible that the resultant skeletal muscle dysfunction may manifest later as sarcopenia, and frailty. Hypoxia can also directly inhibit cellular pathways that affect smooth muscle function and can lead to reduced muscle mass (sarcopenia). Oxidative stress is common in patients with lung disease and can further impair skeletal muscle contractility.120 Other factors involved in the genesis of skeletal muscle dysfunction and sarcopenia include disuse atrophy, corticosteroid use, and malnutrition,120 all of which are common in patients with progressively worsening lung disease. Decreased physical activity is a risk factor for exacerbations and hospitalizations,121 all of which contribute to disuse atrophy and increase the risk for developing skeletal muscle dysfunction, sarcopenia, and frailty. Corticosteroids are often prescribed for patients with more advanced stages of lung disease and who experience exacerbations. A side effect of this class of medication is muscle wasting, due to activation of pathways that lead to decreased protein synthesis and increased protein degradation,122 which can result in sarcopenia.
Malnutrition is also a contributory factor of frailty. It is defined as an acute, subacute, or chronic state of nutrition, in which a combination of varying degrees of overnutrition or undernutrition with or without inflammatory activity lead to a change in body composition and diminished function.123 A vicious cycle seems to exist between malnutrition and disease severity, as progression of disease leads to further malnourishment and malnutrition causes further worsening of disease.
Lung disease can impact nutritional intake and absorption and is associated with increased resting energy expenditure.124 Dyspnea is the most common symptom in patients with lung disease. Chewing and swallowing food alter breathing patterns and in patients with lung disease, this can cause greater dyspnea and oxygen desaturation. As a result, patients with lung disease may reduce their oral intake to avoid this experience. Further, restrictive and obstructive lung diseases contribute to increased resting energy expenditure—in patients with lung disease, the respiratory muscles are required to generate larger forces to expand the thoracic cage while working at either a mechanical disadvantage due to hyperinflation or against densely fibrotic lungs. Therefore, breathing requires increased energy expenditure, resulting in hypermetabolism. An increased metabolic rate coupled with reduced nutritional intake places the patient at increased risk for malnutrition.124 Patients with cystic fibrosis are at further risk for developing malnutrition because they often suffer from malabsorption issues due to exocrine pancreatic insufficiency. A mutation of the cystic fibrosis transmembrane conductance regulator protein impairs the pancreas from releasing into the small intestine the enzymes necessary for digestion and absorption of fat, protein, and fat-soluble vitamins, which increases the risk for malnutrition in this patient population.125
In summary, patients with lung disease are inherently at risk for developing frailty. Patients with lung disease suffer from chronic hypoxia, dyspnea, and disease related factors (i.e. malabsorption in cystic fibrosis) that increase their risk for skeletal muscle dysfunction, sarcopenia, malnutrition, and chronic inflammation.
Sex and Frailty
Female sex is generally associated with frailty in lung disease.
Consistent with studies in other disease and aging populations, the prevalence of frailty is commonly, but not uniformly, reported higher in females compared to males with lung disease (see Table 6).25,29,31,34,36,38 Mechanisms to explain the sex differences in frailty in lung disease were not discussed. However, in other disease and aging populations, this observation has been attributed to biological differences in chronic inflammation, sex hormones, body composition, and disabling conditions that increase the risk for developing frailty.126 Further, the sex differences in frailty corroborates the male-female health-survival paradox, that is that females may live longer than males, but with greater levels of disability, more co-morbidities, and poorer self-rated health.127
Table 6.
Studies of Sex and Frailty in Lung Disease
| Sex The prevalence offrailty was generally higher in females with lung disease than in males | |
|---|---|
| Patient Population | Finding |
| COPD | Females had a higher frailty score (0.17 ± 0.09) compared with males (0.14 ± 0.09) (p <0.001)13 |
| ILD | Females had a higher frailty score (0.238, IQR 0.120–0.358) and greater prevalence of frailty (56%) compared with males (0.167, IQR 0.071–0.286; prevalence 42%)14 |
| Lung transplant candidates | Frail patients tended to be female (63% female vs 37% male frail, p-value NR)21 |
| Older adults, some of whom had spirometrically defined lung disease | Frequency of females increased across the 3 levels of frailty (53.3% females not-frail, 60.3% pre-frail, 69.9% frail, p<0.001)1 |
| COPD | Frailty tended to be more common among females compared with males (29.7% females vs 22.8% males, p=0.08)3 |
| Lung transplant candidates | Females were more commonly frail than males (63% females vs 39% males, p = 0.02)12 |
| ILD | No significant differences in frailty by sex (15% females vs 85% males not-frail vs 29% females vs 71% males frail, p-value NR)10 |
| COPD | No significant differences in frailty by sex (80% males not-frail vs 79% males frail-disabled, p=0.884)2 |
| ILD | Sex was not correlated with frailty (r= NR, p=0.81)17 |
| COPD | Female sex was not significantly associated with frailty (OR 0.84, 95% CI 0.50–1.42, p >0.05, compared to males)9 |
| Chronic lung disease | No significant differences in 3 levels of frailty (not frail, pre-frail, frail) by sex (p-value NR)18 |
COPD= chronic obstructive pulmonary disease; ILD= interstitial lung disease; IQR= interquartile range; NR= not reported; OR= odds ratio
Several studies did not find a relationship between female sex and frailty status (see Table 6).11,26,35,37,41,115 The inconsistency in identifying an association between female sex and frailty across studies may be due to variation in study sample characteristics. Several studies were limited by small, homogenous samples that lacked relatively equal representation of males and females.26,35,37 In summary, there is much to be learned about the role of sex in the development of frailty in lung disease and the underlying mechanisms that explain this relationship.
Behavioral Factors Associated with Frailty in Lung Disease
Symptom Severity and Frailty
Greater symptom severity is associated with frailty in lung disease.
Patients with lung disease who are frail appear to experience more severe physical and psychological symptoms (see Table 7). Overall, patients with COPD who were frail reported worse respiratory related symptoms, using COPD Assessment Test, a well-validated self-reported questionnaire that measures the impact of COPD symptoms (e.g. cough, phlegm, chest tightness, breathlessness, sleep, energy) on patients’ overall health.30,35,36,38,40 Dyspnea appears to be the strongest predictor of frailty in patients with lung disease. This finding corroborates with the previously discussed finding that frailty is associated with worsening lung function. Dyspnea was most often assessed using a version of the Medical Research Council dyspnea scale, a five level rating scale based on a patient’s perception of dyspnea severity and its impact on daily living.128 Other self-reported measures of dyspnea that have been studied, including the University of California San Diego Shortness of Breath Questionnaire and Baseline Dyspnea Index, found a similar association between dyspnea and frailty.30,37,41 Fatigue also was significantly associated with frailty.37,38 This is not surprising given that studies measured frailty using the FFP and fatigue is defining component of this measure. There was also a trend towards higher levels of self-reported depressive symptoms and anxiety in patients with lung disease who were frail compared to patients who were not.26,38–40 (see Table 7)
Table 7.
Studies of Symptoms and Frailty in Lung Disease
| COPD Assessment Test (CAT) Patients with COPD who were frail reported higher COPD Assessment Test scores, a self-reported questionnaire that measures the impact of COPD symptoms (e.g. cough, phlegm, chest tightness, breathlessness, sleep, energy) on patients ‘ overall health | |
|---|---|
| Patient Population | Finding |
| COPD | Frail patients reported worse COPD related symptoms compared to those who were not frail (median CAT score 28 [IQR 24–32] vs 18 [IQR 13–23], p <0.001)13 |
| COPD | Frail patients reported worse COPD related symptoms (mean CAT score 25±7.9) compared to pre-frail (20.2±7.2) and robust patients (13.3±5.6) (p<0.001)3 |
| COPD | Worse COPD related symptoms (CAT score ≥10) was associated with higher odds of frailty (OR 4.65, 95% CI 1.86–11.63)2 |
| COPD | Frail patients reported worse COPD related symptoms compared with those who were not frail or pre-frail (mean CAT score: 13.2±5.6 not frail, 20.2±7.8 pre-frail, 25±7.9, p<0.001)3 |
| COPD | Frail patients reported worse COPD related symptoms compared with those who were robust or pre-frail (mean CAT score: 5.8±4.7 robust, 7.3±5.6 pre-frail, 15.2±9.1 frail, p<0.05)20 |
| COPD | Worse COPD related symptoms (CAT score) was associated with higher odds of frailty (unfit) (OR 1.21, p<0.001)5 |
| COPD | Frail patients reported worse COPD related symptoms compared with patients who were not (mean CAT score: 18.4±9.3 frail vs 11.4±5.7 not-frail, p=0.021)6 |
| Dyspnea Worse dyspnea scores were associated with frailty across all studies | |
| COPD | Frail patients reported more severe dyspnea compared with those who were not frail (dyspnea measured by the mMRC) (median mMRC score: 3 [IQR 2–4] frail vs 2 [IQR 1–3] not-frail, p<0.001)13 |
| COPD | Frail patients reported more severe dyspnea compared with patients who were pre-frail or not frail (mean mMRC score: not-frail: 2.4±1.1, pre-frail: 3.2±1.0, frail: 4±0.9, p<0.001)3 |
| COPD | Severe dyspnea (≥2 mMRC dyspnea scale) was associated with higher odds of frailty (OR 5.75, 95% CI 2.79–11.85)2 |
| COPD | Moderately-to-severely frail patients reported more severe dyspnea compared to those who were not frail (%MRC grade 4: 84.2% moderately/severely frail vs 19.9% not frail, p<0.001)8 |
| ILD | Severe dyspnea (measured using the University of California San Diego Shortness of Breath Questionnaire) was correlated with frailty (unadjusted r=0.62, p<0.001, adjusted for age, sex, and pack-years r=0.031, 95% CI 0.020–0.042)17 |
| ILD | Severe dyspnea (measured using the University of California San Diego Shortness of Breath Questionnaire) was the strongest predictor of frailty (r = 0.65, p < 0.001) and only independent predictor of frailty (0.034 increase in Frailty Index per 10-point increase in dyspnea score; R2 =0.37; p <0.001)4 |
| COPD | Shortness of breath was the strongest predictor of frailty (OR 3.98, p<0.05)9 |
| COPD | Dyspnea (measured using the MRC) worsened across the 3 levels of frailty (p<0.001); there was a threefold increase in frailty prevalence among patients with severe dyspnea (an MRC score of 5) compared with patients who reported less severe dyspnea (score of 3) (62.1% vs 21.0%, p<0.001)3 |
| COPD | Frail patients reported more severe dyspnea compared with robust and pre-frail patients (using the BDI) (9.8±1.8 robust, 9.5±1.7 pre-frail, 6±2.5 frail, p<0.05)20 |
| COPD | Prevalence of severe dyspnea (mMRC >2) increased across the 3 levels of frailty (%: 16.7% not-frail vs 34.7% pre-frail vs 83.3% frail, p=0.001)6 |
| Fatigue Fatigue was associated with frailty | |
| ILD | Fatigue was correlated with frailty (measurement NR) (r=NR, p=0.04)17 |
| COPD | Frail patients reported higher levels of fatigue (measured with the Chronic Respiratory Questionnaire) compared with patients who were pre-frail or not frail(17.6±4.3 not frail, 13.8±5.2 pre-frail, 10.9±4.6 frail, p<0.001)3 |
| Anxiety Anxiety was generally associated with frailty | |
| COPD | Frail patients reported higher levels of anxiety (mean HADS score 8.3±5.2) compared to pre-frail (6.8±4.4) and robust patients (5.2±3.4) (p<0.001)3 |
| COPD | Differences in self-reported anxiety were not significant among those who were frail compared to those who were pre-frail or not frail (% HADS ≥11: 8.3% not-frail, 14.1% prefrail, 8.3% frail, p=0.907)6 |
| Depressive symptoms Generally, depression was associated with frailty | |
| COPD | Frail patients reported more severe depression compared to pre-frail and not frail patients (mean HADS score: 4.1±2.7 robust, 6.3±3.7 pre-frail, 8.2±4.0 frail, p<0.001)3 |
| ILD | Differences in depression (measured by DMI) were not significant among those who were frail compared to those who were not (DMI score: 6±6 not-frail vs 10±8 frail)10 |
| COPD | Depression (measured by Center for Epidemiological Studies Depression scale) was not significantly associated with higher odds of frailty (OR 1.05, p=0.114)5 |
| COPD | Frail patients tended to have a higher prevalence of depression compared to patients who were pre-frail or not frail, (% HADS ≥11: 4.2% not-frail, 10% prefrail, 16.7% frail, p=0.477)6 |
| Older adults, some of whom had spirometrically defined lung disease | The prevalence of depression increased across the 3 levels of frailty (4% not-frail, 17.1% pre-frail, 37.8% frail) (p<0.001)1 |
BDI= baseline dyspnea index; COPD= chronic obstructive pulmonary disease; DMI= depression in the medically ill; HADS= hospital anxiety and depression scale; ILD= interstitial lung disease; IQR= interquartile range; NR= not reported; OR= odds ratio; MRC= modified research council
Physical and psychological symptoms examined in these studies (i.e., dyspnea, fatigue, depression, and anxiety) are associated with inflammation14–17,129 and can reduce appetite18–20 and physical activity level,21–23 potentially leading to sarcopenia and malnutrition,24 which are known drivers of frailty. Thus, symptoms may initiate the cascade of behavioral and pathobiological changes involved in the development of frailty. The contributory role of symptoms to the development of frailty has yet to be examined in lung disease. This is an important area of study as symptoms may serve as a novel target for intervention to prevent or reverse frailty.
Physical Inactivity and Frailty
Physical inactivity is associated with frailty in lung disease.
Physical inactivity has been recognized as a driver of frailty. Physical inactivity can lead to loss of muscle mass and strength (sarcopenia), deconditioning, dependency, and ultimately frailty.42 Physical activity level is an important component of the frailty phenotype and a defining component of the FFP. In studies of lung disease, frail patients reported lower weekly energy expenditure and less time in moderate activity compared to patients who were not frail.38 (see Table 8) Patients with lung disease are at increased risk for decreased physical activity levels due to the pathobiological changes and debilitating symptoms that accompany this progressive disease and impact functional capacity and activity.
Table 8.
Study of Physical Activity and Frailty in Lung Disease
| Physical Inactivity was associated with frailty | |
|---|---|
| Patient Population | Finding |
| COPD | Frail patients reported lower weekly energy expenditure (257.2 kcal) and less time in moderate activity (73.6 min/week) compared to pre-frail (1110.5 kcal and 322.6 min/week) and robust patients (1878.4 kcal and 532.8 min/week) (both p <0.001), measured using the Minnesota Leisure-Time Physical Activity3 |
COPD= chronic obstructive pulmonary disease
Pulmonary rehabilitation is one of the most effective interventions in pulmonary medicine. It is a supervised program that provides patients with exercise training and education with the aim of improving exercise capacity, functioning, strength, and health related quality of life.38,89 Across a variety of chronic lung diseases, completion of pulmonary rehabilitation yields better MRC dyspnea scores, exercise performance, physical activity levels and health status.38 In one study of lung transplant candidates, pulmonary rehabilitation improved frailty scores.130 However, patients who are frail are at greatest risk for program non-completion.89 High symptom burden, exacerbations, and more frequent hospitalizations may explain the higher risk for non-completion associated with frailty. Given the effectiveness of pulmonary rehabilitation on improving physical activity levels and the improved outcomes observed after completion of this intervention, it would be beneficial to identify ways to improve adherence to pulmonary rehabilitation in effort to prevent or reverse frailty in lung disease.
Cigarette Smoking and Frailty
Inconsistency in the relationship between cigarette smoking and frailty.
Cigarette smoking is an important modifiable lifestyle factor that is associated with frailty in community dwelling older adults.131 Inflammation may be the underlying pathobiological mechanism linking smoking and frailty.132 However, in studies of lung disease, the relationship between pack year history and smoking status (current and former smoker vs. never smoked) and frailty was not consistent. Two studies reported a non-significant higher prevalence of frailty among patients with longer pack year histories,36,39 while a majority of studies found no difference in frailty by pack year history26,27,37,41 or smoking status (current vs former).27,38,40 In one study, the risk for frailty was paradoxically increased in former smokers compared to current smokers (see Table 9).35 The researchers of this study speculated that patients with extreme frailty, impaired health status, and greater number of exacerbations, may be more likely quit smoking due to poor health status, although these factors were not controlled for in the analysis. Overall, smoking history or status does not seem to be a significant factor driving frailty in lung disease. This lack of association may be due to the fact that a majority of studies in lung disease included patients who were either current or former smokers with a long pack year history and therefore, smoking may not differentiate those at risk for frailty in a population with high prevalence of exposure to smoking. Or, is it possible that the impact of lung disease overwhelmed the relative contribution of smoking to the development of frailty.
Table 9.
Studies of Smoking and Frailty in Lung Disease
| Smoking Relationship between pack year history and smoking status and frailty was inconsistent | |
|---|---|
| Patient Population | Finding |
| COPD | Frail patients tended to have a longer smoking history compared with those who were not (mean pack year history: 42±26 frail vs. 40±25 not-frail, p=0.487)13 |
| Older adults, some of whom had spirometrically defined lung disease | Proportion of current smokers increased across the 3 levels of (%current smokers: 10.2% not-frail, 13.3% pre-frail, 13.4% frail, p=0.049)1 |
| COPD | Differences in smoking status (current smoker, former smoker, never smoked) were not significant among those who were frail compared to those who were not frail (% current smoker, former smoker, or never smoked: robust patients 9.8, 84.1, and 6.1; pre-frail, 18.7, 75.0, and 6.3; frail, 15.3, 77.0, and 7.7, p=0.45)3 |
| ILD | Differences in smoking history were not significant among those who were frail compared to those who were not (median pack years: 10 years [IQR 0, 26] not frail vs 10 years [IQR 0, 25] frail, p-value NR)10 |
| COPD | Smoking cessation was associated with higher odds of frailty (OR 2.37, 95% CI 1.10–5.28, reference: current smokers)2 |
| COPD | Differences in smoking status (active smoker) or smoking history (pack years) were not significant among those who were not frail or mildly, moderately, severely frail (active smoking: 37% not frail, 35% mild frailty, 27.8% moderate frailty, 31.6% severely frail, p=0.908; mean pack year history: 55.1±28.5 not-frail; 55.3±33.1 mild frailty; 55.4±27.6 moderate frailty; 56.9±25.7 severe frailty, p=0.973)8 |
| ILD | Smoking history (pack years) was not significantly correlated with frailty (r=0.09, p=0.41)17 |
| ILD | Smoking history (pack years) was not significantly correlated with frailty (r=0.06, p=0.56)4 |
| COPD | Smoking status (ex-smoker) tended to be associated with lower odds of frailty (unfit) (OR 0.45, p=0.089)5 |
| COPD | Differences in smoking history (pack years) were not significant among those who were frail compared to those who were pre-frail or not frail (mean pack years 49.4±26.3 not-frail vs 60.7±25.2 pre-frail vs 60.6±25.1 frail, p=0.146)6 |
COPD= chronic obstructive pulmonary disease; ILD= interstitial lung disease; NR= not reported; OR= odds ratio
Medication Use and Frailty
Medication use is associated with frailty in lung disease.
There is evidence to suggest that polypharmacy, or the use of multiple medications, is associated with frailty in chronic disease and aging populations, including those with lung disease.36,40,133,134 (see Table 10). In other disease populations, patients who were frail were more likely to exhibit changes in pharmacokinetics and pharmacodynamic response to drugs and were at higher risk for adverse drug reactions.135 The interaction between medications and frailty in lung disease is understudied. Researchers of one study reported that frail patients were more likely to have a medication adverse reaction requiring a dose reduction, suggesting that frailty may be associated with greater medication side effects and intolerance, but this analysis was underpowered and did not reach statistical significance.25 Furthermore, medications have sides effects that can impact physical activity levels, induce muscle wasting, and alter appetite leading to nutritional deficiencies and malnutrition. These side effects may increase the risk for developing frailty.40 The bidirectional relationship between medications and frailty and the suboptimal responses to medications need to be further explored through research, as both have important implications for patient management.
Table 10.
Studies of Medication Use and Frailty in Lung Disease
| Medication Use Frailty was associated with polypharmacy and generally associated with individual classes of medications commonly used to treat lung disease | |
|---|---|
| Patient Population | Finding |
| COPD | Frail patients reported taking more medications compared to those who were not frail (frail 8 [IQR 8–11] vs not-frail 5 [IQR 3–7],p <0.001)13 |
| COPD | Number of medications was associated with higher odds of frailty (unfit) (OR 1.60, p=0.007)5 |
| ILD | Frail patients more commonly used corticosteroids compared with those who were not frail (55% not-frail vs 79%% frail, p <0.05)10 |
| COPD | Use of corticosteroids was not significantly associated with higher odds of frailty (aOR 225.3, p=0.1)7 |
| Lung transplant candidates | Differences in corticosteroid use were not significant among those who were frail compared to those who were not (41% vs 39%, p = 0.78)12 |
| ILD | Patients treated with ILD-specific medications had a higher frailty score compared to untreated patients (0.244 vs. 0.191, p=0.005)14 |
| ILD | Frail patients were more likely to have a MAR resulting in dose reductions (odds ratio 11.3, p=0.04914 |
COPD= chronic obstructive pulmonary disease; ILD= interstitial lung disease; IQR= interquartile range; OR= odds ratio
Summary and Directions for Future Research
At this point we know that the prevalence of frailty in lung disease is high and seems to develop in patients at a younger age than what is typically seen in the community dwelling general population. Frailty tends to be more common in females with lung disease.25,29,31,34,36,38 Individuals with lung disease who are frail have a greater number of comorbidities,27,35,36,38,39 report taking more medications,36,40 have worse severity of lung disease (i.e. worse lung function,29,30,36–38,41,116 more advanced GOLD group35,38,116), and endure greater symptom burden. Early work aiming to define the underlying mechanisms of frailty in lung disease have identified associations between frailty and inflammatory biomarkers,11,36,70 clinical parameters (i.e. hemoglobin,11,26 creatinine,11,26 albumin,26 leptin,11 IGF-1 levels11), body composition measures (i.e. sarcopenia,11,38,42 obesity,29,36,42 adiposity103), and reduced physical activity levels. Importantly, the directional relationship between these factors and frailty has not been determined. Finally, frailty is associated with worse clinical and patient reported outcomes.25,11,26–32
Future research should address several key areas to deepen our understanding of frailty in lung disease and aid in our ability to better measure, prevent, and reverse it. No measure has been specifically developed to assess frailty in adults with lung disease and, of the existing measures, there is much debate with respect to which measure best assesses frailty in this population.4 The FI and FFP were the two most common measures used in studies of frailty in lung disease. The FI may be appropriate if the goal is to predict adverse outcomes but may be less so as for intervention studies since the FI is less responsive to change. Physical frailty measures, such as the FFP and SPPB, may be more appropriate if the goal is to identify the underlying mechanisms of frailty that could serve as targets for interventions or as outcome measures for interventions themselves.7 However, some components of the FFP, such as low activity or exhaustion, may be confounded by lung disease.11,136 Refining the FFP by substituting the Minnesota Leisure Time Physical Activity, the operational measure for low physical activity, with the Duke Activity Status Index, has been shown to improve the construct and predictive validity of frailty assessments in adults with lung disease.136 The SPPB may be a useful alternative measure of physical frailty in lung disease that has been shown to outperform the FFP in identifying risk for adverse outcomes in patients with advanced lung disease who are listed for lung transplantation, including delisting and both wait list and post-transplantmortality.11,137 Further study on the use of the SPPB in this population compared to other popular measures is needed. Alternatively, given the worldwide prevalence of lung disease, another solution may be to develop novel measures of frailty that are less confounded by lung disease.
Understanding why some patients with lung disease are at risk for developing frailty—and doing so at younger ages than what is seen in community dwelling older adults--is fundamentally important.26,26,35–37,43 Further, the prevalence of and morbidity and mortality from respiratory diseases disproportionately affects racial and ethnic minority groups and persons of lower socioeconomic status.138 Yet, few studies in lung disease have been conducted to explore the relationship between frailty and these important demographic and socioeconomic factors. Research in this area could highlight resources needed to prevent or reverse frailty in this population.
Defining the contributory factors of frailty in lung disease will advance our understanding of the basic pathobiology of frailty and also identify areas for intervention.139 Patients with advanced lung disease suffer from a high symptom burden that includes not only respiratory related symptoms (e.g. dyspnea, cough), but also fatigue, pain, anxiety, depression, sleep disturbances, reduced appetite, and changes in weight. Carefully designed studies aimed at defining the direction and magnitude of the relationship between frailty and symptoms are needed. Symptoms may contribute to the biobehavioral changes (i.e. inflammation, malnutrition, sarcopenia, decreased physical inactivity, reduced nutritional intake) that are believed to play a role in the development of frailty. Conversely, frailty may worsen symptoms. Considering both frailty and symptoms concurrently is important, as improvements in one may lead to improvements in the other.139 Symptoms may serve as a novel target to incorporate in future frailty interventions, but further research is needed to better understand the relationship between symptoms and frailty in lung disease.
The study of frailty in lung disease is generally limited to patients with either COPD or ILD and under evaluated in other chronic lung disease populations, such as cystic fibrosis and pulmonary vascular disease. This is a major limitation to our current understanding of frailty in lung disease and a direction for future study. Further, whether the causes of frailty are universal across heterogeneous lung diseases or whether sub-phenotypes of frailty (i.e., endotypes) with distinct pathobiology exist remains unknown. This work has important implications for understanding the pathobiology of frailty in diverse lung diseases and for developing personalized interventions that target the specific underlying mechanisms causing frailty. The most clinically relevant short-term areas of interest appear to be the identification of blood-based biomarkers (i.e pro inflammatory cytokines, antithrombin deficiency) and measures of body composition. Advances in the field of -omics suggests that evaluating panels of biomarkers rather than biomarkers in isolation, may provide added value.68 Although less immediately clinically relevant, more advanced assessments of immune dysfunction would also be valuable. We should also consider systems biology approaches to identify pathobiological mechanisms underlying frailty in lung disease. For example, considering combinations of multiple measures, such as biomarkers and imaging, may give us a more comprehensive understanding of the underlying mechanisms of frailty in lung disease. Finally, we need better predictive models that combine both clinical data and biomarkers for frailty to determine incremental benefit of measuring biomarkers in the prediction of clinical outcomes.
The microbiome is another potential biomarker for frailty. There is an expanding body of knowledge about the impact of the microbiome on inflammation and the association of gut microbial dysbiosis with muscle wasting, impaired grip strength, and slowed gait speed, important components of the frailty phenotype construct. Further research is needed to assess the causal relationship between organ-specific microbial composition and frailty, including the role of inflammatory pathways as mediators of this relationship.140 This is especially relevant to pulmonary disease given the rapidly increasing understanding of the role of the lung microbiome in lung disease.141–147
The overall goal of identifying and understanding the contributory factors and pathobiological mechanisms of frailty in lung disease is to develop effective interventions aimed to reverse or prevent frailty in this population, and in turn, increase patients’ likelihood for successful outcomes and improved quality of life. Pulmonary rehabilitation is the most effective intervention thus far in improving frailty in the lung disease population,38 however barriers to adherence and access need to be addressed. Nutrition related interventions have also been found to be effective for treating frailty in the community dwelling older adult population,148 but this has been less studied in the lung disease population. There is a need for not only effective interventions to prevent or reverse frailty in lung disease, but interventions need to be delivered in innovative ways to overcome some of barriers to adherence and access. The use of technology to deliver in-home based interventions to patients is a promising approach to overcome barriers related to adherence. Pharmacological therapy also may be promising approach to target frailty. There is some preliminary evidence that pharmacological therapy using senolytic agents, drugs that selectively induce senescent cell apoptosis, are effective at improving physical function (i.e. 6MWD, 4-meter gait speed, and chair stands) in patients with idiopathic pulmonary fibrosis.107 but further research is needed. Future research needs to focus on the discovery of pathways leading to frailty, modifiable protein targets, and manufacturing therapeutics for these targets. Finally, although there is evolving evidence that interventions can improve frailty scores, additional studies are needed to understand how such interventions affect clinical and patient reported outcomes in the lung disease population.
In conclusion, in patients with lung disease, frailty increases the risk for poor clinical and patient reported outcomes. Understanding the biobehavioral and pathobiological mechanisms driving frailty in lung disease will better position our field to develop and test the effectiveness of frailty interventions.
Acknowledgments
All authors have read the journal’s authorship agreement and policy on disclosure of potential conflicts of interest. John Greenland, MD, PhD has the following financial relationships to disclose: Scientific Advisory Board, Boehringer Ingelheim, Theravance, Genetech, Atara and research funding from bioMérieux, ThermoFisher. All other authors have no potential conflicts of interest to disclose. Brittany Koons, PhD, RN is supported by the NewCourtland Center for Transitions and Health (Individualized Care for At Risk Older Adults T32NR009356) at the University of Pennsylvania School of Nursing and the Robert Wood Johnson Foundation Future of Nursing Scholar program. John Greenland, MD, PhD, is supported by a career development award IK2CX001034 from the Clinical Sciences Research & Development Service of the VA Office of Research and Development. Jonathan Singer, MD, MS is supported by the National Heart, Lung, and Blood Institute (R01HL134851).
Abbreviations:
- 6MWD
six minute walk distance
- BMI
body mass index
- cGAS
cyclic GMP-AMP synthase
- CF
Cystic fibrosis
- CKD
Chronic kidney disease
- COPD
Chronic obstructive pulmonary disease
- CRP
C-reactive protein
- CX3CL1
fractalkine
- CXCL-10
C-X-C chemokine 10
- DXA
dual X-ray absorptiometry
- FEV1
forced expiratory volume in 1 second
- FFP
Fried frailty phenotype
- FI
Frailty Index
- FPR1
formyl peptide receptor 1
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- IGF
insulin-like growth factor
- ILD
Interstitial lung disease
- IL1β
interleukin 1 beta
- IL-6
interleukin-6
- IP-10
interferon-γ-inducible protein 10
- LAS
lung allocation score
- mtDNA
mitochondrial DNA
- PAI-1
plasminogen activator inhibitor-1
- PVD
Peripheral vascular disease
- PTX3
long pentraxin-3
- SASP
secretory phenotype
- SPPB
Short physical performance battery
- TLR9
toll-like receptor 9
- TNFα
tumor necrosis factor-alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005(31):pe24. [DOI] [PubMed] [Google Scholar]
- 2.Bottiger BA, Nicoara A, Snyder LD, et al. Frailty in the End-Stage Lung Disease or Heart Failure Patient: Implications for the Perioperative Transplant Clinician. Journal of cardiothoracic and vascular anesthesia. 2019;33(5):1382–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer JP, Lederer DJ, Baldwin MR. Frailty in Pulmonary and Critical Care Medicine. Ann Am Thorac Soc. 2016;13(8):1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobashigawa J, Dadhania D, Bhorade S, et al. Report from the American Society of Transplantation on frailty in solid organ transplantation. Am J Transplant. 2019;19(4):984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology Series A, Biological sciences and medical sciences. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 6.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. The journals of gerontology Series A, Biological sciences and medical sciences. 2007;62(7):722–727. [DOI] [PubMed] [Google Scholar]
- 7.Sternberg SA, Wershof Schwartz A, Karunananthan S, Bergman H, Mark Clarfield A. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59(11):2129–2138. [DOI] [PubMed] [Google Scholar]
- 8.Marengoni A, Vetrano DL, Manes-Gravina E, Bernabei R, Onder G, Palmer K. The Relationship Between COPD and Frailty: A Systematic Review and Meta-Analysis of Observational Studies. Chest. 2018;154(1):21–40. [DOI] [PubMed] [Google Scholar]
- 9.Forum of International Respiratory Societies. The Global Impact of Respiratory Disease – Second Edition. https://www.who.int/gard/publications/The_Global_Impact_of_Respiratory_Disease.pdf. Published 2017. Accessed.
- 10.Ding B, Small M, Bergstrom G, Holmgren U. COPD symptom burden: impact on health care resource utilization, and work and activity impairment. International journal of chronic obstructive pulmonary disease. 2017;12:677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer JP, Diamond JM, Gries CJ, et al. Frailty Phenotypes, Disability, and Outcomes in Adult Candidates for Lung Transplantation. American journal of respiratory and critical care medicine. 2015;192(11):1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budinger GRS, Kohanski RA, Gan W, et al. The Intersection of Aging Biology and the Pathobiology of Lung Diseases: A Joint NHLBI/NIA Workshop. The journals of gerontology Series A, Biological sciences and medical sciences. 2017;72(11):1492–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miravitlles M, Ribera A. Understanding the impact of symptoms on the burden of COPD. Respir Res. 2017;18(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irwin MR, Olmstead R, Carroll JE. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol Psychiatry. 2016;80(1):40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T. Inflammation in Fear-and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology. 2017;42(1):254–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baxter LC. Appetite Changes in Depression. Am J Psychiatry. 2016;173(4):317–318. [DOI] [PubMed] [Google Scholar]
- 19.Norden J, Gronberg AM, Bosaeus I, et al. Nutrition impact symptoms and body composition in patients with COPD. Eur J Clin Nutr. 2015;69(2):256–261. [DOI] [PubMed] [Google Scholar]
- 20.Shalit N, Tierney A, Holland A, et al. Factors that influence dietary intake in adults with stable chronic obstructive pulmonary disease. 2016;73(5):455–462. [Google Scholar]
- 21.Hiles SA, Lamers F, Milaneschi Y, Penninx B. Sit, step, sweat: longitudinal associations between physical activity patterns, anxiety and depression. Psychol Med. 2017;47(8):1466–1477. [DOI] [PubMed] [Google Scholar]
- 22.Kim I, Park C, Bronas U, et al. Impact of Sleep Disturbance on Next-Day Physical Activity In Chronic Obstructive Pulmonary Disease. American Journal of Respiratory Critical care Medicine Web site. https://www.atsjournals.org/doi/pdf/10.1164/ajrccm-conference.2018.197.1_MeetingAbstracts.A3248. Published 2018. Accessed September 15, 2019. [Google Scholar]
- 23.Penninx BW. Depression and cardiovascular disease: Epidemiological evidence on their linking mechanisms. Neurosci Biobehav Rev. 2017;74(Pt B):277–286. [DOI] [PubMed] [Google Scholar]
- 24.Simsek H, Meseri R, Sahin S, et al. Prevalence of sarcopenia and related factors in community-dwelling elderly individuals. Saudi Med J. 2019;40(6):568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guler SA, Kwan JM, Leung JM, Khalil N, Wilcox PG, Ryerson CJ. Functional aging in fibrotic interstitial lung disease: The impact of frailty on adverse health outcomes. Eur Respir J. 2019. [DOI] [PubMed] [Google Scholar]
- 26.Montgomery E, Macdonald PS, Newton PJ, et al. FRAILTY AS A PREDICTOR OF MORTALITY IN PATIENTS WITH INTERSTITIAL LUNG DISEASE REFERRED FOR LUNG TRANSPLANTATION. Transplantation. 2019. [DOI] [PubMed] [Google Scholar]
- 27.Bernabeu-Mora R, Garcia-Guillamon G, Valera-Novella E, Gimenez-Gimenez LM, Escolar-Reina P, Medina-Mirapeix F. Frailty is a predictive factor of readmission within 90 days of hospitalization for acute exacerbations of chronic obstructive pulmonary disease: a longitudinal study. Ther Adv Respir Dis. 2017;11(10):383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SK, Richardson CR, Holleman RG, Larson JL. Frailty in people with COPD, using the National Health and Nutrition Evaluation Survey dataset (2003–2006). Heart & lung : the journal of critical care. 2013;42(3):163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaz Fragoso CA, Enright PL, McAvay G, Van Ness PH, Gill TM. Frailty and respiratory impairment in older persons. Am J Med. 2012;125(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusunose M, Oga T, Nakamura S, Hasegawa Y, Nishimura K. Frailty and patient-reported outcomes in subjects with chronic obstructive pulmonary disease: are they independent entities? BMJ Open Respir Res. 2017;4(1):e000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson ME, Vakil AP, Kandel P, Undavalli C, Dunlay SM, Kennedy CC. Pretransplant frailty is associated with decreased survival after lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2016;35(2):173–178. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy CC, Novotny PJ, LeBrasseur NK, Wise RA, Sciurba FC, Benzo RP. Frailty and Clinical Outcomes in Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc. 2019;16(2):217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2016 Annual Data Report: Lung. Am J Transplant. 2018;18 Suppl 1:363–433. [DOI] [PubMed] [Google Scholar]
- 34.Layton AM, Armstrong HF, Baldwin MR, et al. Frailty and maximal exercise capacity in adult lung transplant candidates. Respiratory medicine. 2017;131:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ierodiakonou D, Kampouraki M, Poulonirakis I, et al. Determinants of frailty in primary care patients with COPD: the Greek UNLOCK study. BMC Pulm Med. 2019;19(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gale NS, Albarrati AM, Munnery MM, et al. Frailty: A global measure of the multisystem impact of COPD. Chron Respir Dis. 2018;15(4):347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guler SA, Kwan JM, Winstone TA, et al. Severity and features of frailty in systemic sclerosis-associated interstitial lung disease. Respiratory medicine. 2017;129:1–7. [DOI] [PubMed] [Google Scholar]
- 38.Maddocks M, Kon SS, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71(11):988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medina-Mirapeix F, Bernabeu-Mora R, Gimenez-Gimenez LM, Escolar-Reina P, Gacto-Sanchez M, de Oliveira-Sousa SL. Physical frailty characteristics have a differential impact on symptoms as measured by the CAT score: an observational study. Health and quality of life outcomes. 2018;16(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen PJ, Yang KY, Perng WC, Lin KC, Wang KY. Effect of dyspnea on frailty stages and related factors in Taiwanese men with COPD. International journal of chronic obstructive pulmonary disease. 2018;13:2463–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milne KM, Kwan JM, Guler S, et al. Frailty is common and strongly associated with dyspnoea severity in fibrotic interstitial lung disease. Respirology. 2017;22(4):728–734. [DOI] [PubMed] [Google Scholar]
- 42.Limpawattana P, Putraveephong S, Inthasuwan P, Boonsawat W, Theerakulpisut D, Chindaprasirt J. Frailty syndrome in ambulatory patients with COPD. International journal of chronic obstructive pulmonary disease. 2017;12:1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guan C, Niu H. Frailty assessment in older adults with chronic obstructive respiratory diseases. Clin Interv Aging. 2018;13:1513–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fougere B, Boulanger E, Nourhashemi F, Guyonnet S, Cesari M. Chronic Inflammation: Accelerator of Biological Aging. The journals of gerontology Series A, Biological sciences and medical sciences. 2017;72(9):1218–1225. [DOI] [PubMed] [Google Scholar]
- 45.Andreux PA, van Diemen MPJ, Heezen MR, et al. Mitochondrial function is impaired in the skeletal muscle of pre-frail elderly. Sci Rep. 2018;8(1):8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ashar FN, Moes A, Moore AZ, et al. Association of mitochondrial DNA levels with frailty and all-cause mortality. J Mol Med (Berl). 2015;93(2):177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kausar S, Wang F, Cui H. The Role of Mitochondria in Reactive Oxygen Species Generation and Its Implications for Neurodegenerative Diseases. Cells. 2018;7(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soysal P, Isik AT, Carvalho AF, et al. Oxidative stress and frailty: A systematic review and synthesis of the best evidence. Maturitas. 2017;99:66–72. [DOI] [PubMed] [Google Scholar]
- 49.Harman D Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. [DOI] [PubMed] [Google Scholar]
- 50.Ristow M, Schmeisser S. Extending life span by increasing oxidative stress. Free Radic Biol Med. 2011;51(2):327–336. [DOI] [PubMed] [Google Scholar]
- 51.Jang JY, Blum A, Liu J, Finkel T. The role of mitochondria in aging. J Clin Invest. 2018;128(9):3662–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scozzi D, Ibrahim M, Liao F, et al. Mitochondrial damage-associated molecular patterns released by lung transplants are associated with primary graft dysfunction. Am J Transplant. 2019;19(5):1464–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia-Prat L, Martinez-Vicente M, Perdiguero E, et al. Autophagy maintains stemness by preventing senescence. Nature. 2016;529(7584):37–42. [DOI] [PubMed] [Google Scholar]
- 54.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123(3):966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naikawadi RP, Green G, Jones KD, et al. Telomere Dysfunction Drives Chronic Lung Allograft Dysfunction Pathology. bioRxiv. 2019:746768. [Google Scholar]
- 56.Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Everaerts S, Lammertyn EJ, Martens DS, et al. The aging lung: tissue telomere shortening in health and disease. Respir Res. 2018;19(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tichy ED, Sidibe DK, Tierney MT, et al. Single Stem Cell Imaging and Analysis Reveals Telomere Length Differences in Diseased Human and Mouse Skeletal Muscles. Stem Cell Reports. 2017;9(4):1328–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greenland JR. Where the Chromosome Ends: Telomeres and Cytomegalovirus Risk in Lung Transplant Recipients. Am J Respir Crit Care Med. 2019;199(3):265–267. [DOI] [PubMed] [Google Scholar]
- 60.Saedi AA, Feehan J, Phu S, Duque G. Current and emerging biomarkers of frailty in the elderly. Clin Interv Aging. 2019;14:389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nature reviews Endocrinology. 2018;14(10):576–590. [DOI] [PubMed] [Google Scholar]
- 62.Fedarko NS. The biology of aging and frailty. Clin Geriatr Med. 2011;27(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harbor perspectives in biology. 2014;6(10):a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing research reviews. 2016;31:1–8. [DOI] [PubMed] [Google Scholar]
- 65.Baldi S, Jose PE, Bruschi C, et al. The mediating role of cytokine IL-6 on the relationship of FEV(1) upon 6-minute walk distance in chronic obstructive pulmonary disease. International journal of chronic obstructive pulmonary disease. 2014;9:1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hall DJ, Baz M, Daniels MJ, et al. Immediate postoperative inflammatory response predicts long-term outcome in lung-transplant recipients. Interactive cardiovascular and thoracic surgery. 2012;15(4):603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verleden SE, Martens A, Ordies S, et al. Immediate post-operative broncho-alveolar lavage IL-6 and IL-8 are associated with early outcomes after lung transplantation. Clinical transplantation. 2018;32(4):e13219. [DOI] [PubMed] [Google Scholar]
- 68.Cardoso AL, Fernandes A, Aguilar-Pimentel JA, et al. Towards frailty biomarkers: Candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res Rev. 2018;47:214–277. [DOI] [PubMed] [Google Scholar]
- 69.Marzetti E, Picca A, Marini F, et al. Inflammatory signatures in older persons with physical frailty and sarcopenia: The frailty “cytokinome” at its core. Experimental gerontology. 2019;122:129–138. [DOI] [PubMed] [Google Scholar]
- 70.Stanjek-Cichoracka A, Wozniak-Grygiel E, Laszewska A, Zembala M, Ochman M. Assessment of Cytokines, Biochemical Markers of Malnutrition and Frailty Syndrome Patients Considered for Lung Transplantation. Transplantation proceedings. 2019;51(6):2009–2013. [DOI] [PubMed] [Google Scholar]
- 71.Portillo K, Martinez-Rivera C, Ruiz-Manzano J. Anaemia in chronic obstructive pulmonary disease. Does it really matter? Int J Clin Pract. 2013;67(6):558–565. [DOI] [PubMed] [Google Scholar]
- 72.Sarkar M, Rajta PN, Khatana J. Anemia in Chronic obstructive pulmonary disease: Prevalence, pathogenesis, and potential impact. Lung India. 2015;32(2):142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cederholm T, Jensen GL, Correia M, et al. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. Clin Nutr. 2019;38(1):1–9. [DOI] [PubMed] [Google Scholar]
- 74.Thongprayoon C, Cheungpasitporn W, Kashani K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in critically ill patients. J Thorac Dis. 2016;8(5):E305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frailty Delgado C. and CKD: Chicken or the Egg? Clinical journal of the American Society of Nephrology : CJASN. 2019;14(11):1554–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Z, Pereira SL, Luo M, Matheson EM. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients. 2017;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J Parenter Enteral Nutr. 2012;36(3):275–283. [DOI] [PubMed] [Google Scholar]
- 78.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 Suppl):990s–991s. [DOI] [PubMed] [Google Scholar]
- 79.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and ageing. 2010;39(4):412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(5):547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morley JE. Sarcopenia in the elderly. Fam Pract. 2012;29 Suppl 1:i44–i48. [DOI] [PubMed] [Google Scholar]
- 82.Morley JE, Malmstrom TK. Frailty, sarcopenia, and hormones. Endocrinology and metabolism clinics of North America. 2013;42(2):391–405. [DOI] [PubMed] [Google Scholar]
- 83.Morley JE, Vellas B, Abellan van Kan G, et al. Frailty consensus: a call to action. Journal of the American Medical Directors Association. 2013;14(6):392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cooper C, Dere W, Evans W, et al. Frailty and sarcopenia: definitions and outcome parameters. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012;23(7):1839–1848. [DOI] [PubMed] [Google Scholar]
- 85.Cruz-Jentoft AJ, Landi F, Topinkova E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Current opinion in clinical nutrition and metabolic care. 2010;13(1):1–7. [DOI] [PubMed] [Google Scholar]
- 86.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol (1985). 2005;98(3):911–917. [DOI] [PubMed] [Google Scholar]
- 87.Llovera M, Lopez-Soriano FJ, Argiles JM. Effects of tumor necrosis factor-alpha on muscle-protein turnover in female Wistar rats. J Natl Cancer Inst. 1993;85(16):1334–1339. [DOI] [PubMed] [Google Scholar]
- 88.Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. The American journal of clinical nutrition. 2005;82(1):53–59. [DOI] [PubMed] [Google Scholar]
- 89.Jones SE, Maddocks M, Kon SS, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax. 2015;70(3):213–218. [DOI] [PubMed] [Google Scholar]
- 90.Schols AM, Buurman WA, Staal van den Brekel AJ, Dentener MA, Wouters EF. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax. 1996;51(8):819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sergi G, Coin A, Marin S, et al. Body composition and resting energy expenditure in elderly male patients with chronic obstructive pulmonary disease. Respir Med. 2006;100(11):1918–1924. [DOI] [PubMed] [Google Scholar]
- 92.Soler-Cataluña JJ, Sánchez-Sánchez L, Martínez-García MÁ, Sánchez PR, Salcedo E, Navarro M. Mid-Arm Muscle Area Is a Better Predictor of Mortality Than Body Mass Index in COPD. Chest. 2005;128(4):2108–2115. [DOI] [PubMed] [Google Scholar]
- 93.Swallow EB, Reyes D, Hopkinson NS, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 2007;62(2):115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marquis K, Debigaré R, Lacasse Y, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(6):809–813. [DOI] [PubMed] [Google Scholar]
- 95.Caminati A, Bianchi A, Cassandro R, Mirenda MR, Harari S. Walking distance on 6-MWT is a prognostic factor in idiopathic pulmonary fibrosis. Respir Med. 2009;103(1):117–123. [DOI] [PubMed] [Google Scholar]
- 96.Lederer DJ, Arcasoy SM, Wilt JS, D’Ovidio F, Sonett JR, Kawut SM. Six-minute-walk distance predicts waiting list survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174(6):659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rozenberg D, Singer LG, Herridge M, et al. Evaluation of Skeletal Muscle Function in Lung Transplant Candidates. Transplantation. 2017;101(9):2183–2191. [DOI] [PubMed] [Google Scholar]
- 98.Singer JP, Peterson ER, Snyder ME, et al. Body composition and mortality after adult lung transplantation in the United States. Am J Respir Crit Care Med. 2014;190(9):1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Singer JP, Diamond JM, Gries C, et al. Frailty phenotypies, disability, and outcomes in adult candidates for lung transplantation. Am J Respir Crit Care Med. 2015;192(11):1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stenholm S, Strandberg TE, Pitkala K, Sainio P, Heliovaara M, Koskinen S. Midlife obesity and risk of frailty in old age during a 22-year follow-up in men and women: the Mini-Finland Follow-up Survey. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(1):73–78. [DOI] [PubMed] [Google Scholar]
- 101.Garcia-Esquinas E, Jose Garcia-Garcia F, Leon-Munoz LM, et al. Obesity, fat distribution, and risk of frailty in two population-based cohorts of older adults in Spain. Obesity (Silver Spring). 2015;23(4):847–855. [DOI] [PubMed] [Google Scholar]
- 102.Hawkins KL, Zhang L, Ng DK, et al. Abdominal obesity, sarcopenia, and osteoporosis are associated with frailty in men living with and without HIV. AIDS. 2018;32(10):1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Anderson MR, Kolaitis NA, Gao Y, et al. A nonlinear relationship between visceral adipose tissue and frailty in adult lung transplant candidates. Am J Transplant. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–920. [DOI] [PubMed] [Google Scholar]
- 105.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2003;100(12):7265–7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. The Journal of clinical endocrinology and metabolism. 1998;83(3):847–850. [DOI] [PubMed] [Google Scholar]
- 107.Justice JN, Nambiar AM, Tchkonia T, et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stenholm S, Sallinen J, Koster A, et al. Association between obesity history and hand grip strength in older adults--exploring the roles of inflammation and insulin resistance as mediating factors. The journals of gerontology Series A, Biological sciences and medical sciences. 2011;66(3):341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Choi SJ, Files DC, Zhang T, et al. Intramyocellular Lipid and Impaired Myofiber Contraction in Normal Weight and Obese Older Adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2016;71(4):557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kyle UG, Genton L, Hans D, et al. Total body mass, fat mass, fat-free mass, and skeletal muscle in older people: cross-sectional differences in 60-year-old persons. Journal of the American Geriatrics Society. 2001;49(12):1633–1640. [DOI] [PubMed] [Google Scholar]
- 111.Chung JY, Kang HT, Lee DC, Lee HR, Lee YJ. Body composition and its association with cardiometabolic risk factors in the elderly: a focus on sarcopenic obesity. Archives of gerontology and geriatrics. 2013;56(1):270–278. [DOI] [PubMed] [Google Scholar]
- 112.Newman AB, Lee JS, Visser M, et al. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82(4):872–878; quiz 915–876. [DOI] [PubMed] [Google Scholar]
- 113.Cederholm T, Barazzoni R, Austin P, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36(1):49–64. [DOI] [PubMed] [Google Scholar]
- 114.Polyzos SA, Margioris AN. Sarcopenic obesity. Hormones (Athens). 2018;17(3):321–331. [DOI] [PubMed] [Google Scholar]
- 115.Mittal N, Raj R, Islam EA, Nugent K. The Frequency of Frailty in Ambulatory Patients With Chronic Lung Diseases. J Prim Care Community Health. 2016;7(1):10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Valenza MC, Torres-Sanchez I, Cabrera-Martos I, Rodriguez-Torres J, Gonzalez-Jimenez E, Munoz-Casaubon T. Physical Activity as a Predictor of Absence of Frailty in Subjects With Stable COPD and COPD Exacerbation. Respiratory care. 2016;61(2):212–219. [DOI] [PubMed] [Google Scholar]
- 117.Patel AR, Patel AR, Singh S, Singh S, Khawaja I. Global Initiative for Chronic Obstructive Lung Disease: The Changes Made. Cureus. 2019;11(6):e4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.United Network for Organ Sharing. Questions and anwsers for transplant professionals about lung allocation
- 119.Jeffery Mador M, Bozkanat E. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. Respiratory Research. 2001;2(4):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: cause, effects, and disease progression. International journal of chronic obstructive pulmonary disease. 2011;6:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shin KC. Physical activity in chronic obstructive pulmonary disease: clinical impact and risk factors. Korean J Intern Med. 2018;33(1):75–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hasselgren PO, Alamdari N, Aversa Z, Gonnella P, Smith IJ, Tizio S. Corticosteroids and muscle wasting: role of transcription factors, nuclear cofactors, and hyperacetylation. Curr Opin Clin Nutr Metab Care. 2010;13(4):423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.American Society for Parenteral and Enteral Nutrition. Definitions: Malnutrition. https://www.nutritioncare.org/Guidelines_and_Clinical_Resources/Toolkits/Malnutrition_Toolkit/Definitions/. Published 2015. Accessed.
- 124.Congleton J The pulmonary cachexia syndrome: aspects of energy balance. Proc Nutr Soc. 1999;58(2):321–328. [DOI] [PubMed] [Google Scholar]
- 125.Culhane S, George C, Pearo B, Spoede E. Malnutrition in cystic fibrosis: a review. Nutr Clin Pract. 2013;28(6):676–683. [DOI] [PubMed] [Google Scholar]
- 126.Gordon EH, Hubbard RE. Do sex differences in chronic disease underpin the sex-frailty paradox? Mech Ageing Dev. 2019;179:44–50. [DOI] [PubMed] [Google Scholar]
- 127.Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE. Sex differences in frailty: A systematic review and meta-analysis. Exp Gerontol. 2017;89:30–40. [DOI] [PubMed] [Google Scholar]
- 128.Hsu KY, Lin JR, Lin MS, Chen W, Chen YJ, Yan YH. The modified Medical Research Council dyspnoea scale is a good indicator of health-related quality of life in patients with chronic obstructive pulmonary disease. Singapore Med J. 2013;54(6):321–327. [DOI] [PubMed] [Google Scholar]
- 129.Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14(4):217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Singer JP, Soong A, Bruun A, et al. A mobile health technology enabled home-based intervention to treat frailty in adult lung transplant candidates: A pilot study. Clin Transplant. 2018;32(6):e13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kojima G, Iliffe S, Jivraj S, Liljas A, Walters K. Does current smoking predict future frailty? The English longitudinal study of ageing. Age Ageing. 2018;47(1):126–131. [DOI] [PubMed] [Google Scholar]
- 132.Kojima G, Iliffe S, Walters K. Smoking as a predictor of frailty: a systematic review. BMC Geriatr. 2015;15:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gutierrez-Valencia M, Izquierdo M, Cesari M, Casas-Herrero A, Inzitari M, Martinez-Velilla N. The relationship between frailty and polypharmacy in older people: A systematic review. Br J Clin Pharmacol. 2018;84(7):1432–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ying H, McAdams-DeMarco M, Segev DL. Polypharmacy and Frailty in Kidney Transplant Recipients.[abstract]. Am J Transplant Web site. https://atcmeetingabstracts.com/abstract/polypharmacy-and-frailty-in-kidney-transplant-recipients/. Published 2017. Accessed October 29, 2017. [Google Scholar]
- 135.McAdams-DeMarco MA, Law A, Tan J, et al. Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation. 2015;99(4):805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Baldwin MR, Singer JP, Huang D, et al. Refining Low Physical Activity Measurement Improves Frailty Assessment in Advanced Lung Disease and Survivors of Critical Illness. Ann Am Thorac Soc. 2017;14(8):1270–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Singer JP, Diamond JM, Anderson MR, et al. Frailty phenotypes and mortality after lung transplantation: A prospective cohort study. Am J Transplant. 2018;18(8):1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Celedon JC, Roman J, Schraufnagel DE, Thomas A, Samet J. Respiratory health equality in the United States. The American thoracic society perspective. Ann Am Thorac Soc. 2014;11(4):473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Denfeld QE, Winters-Stone K, Mudd JO, Hiatt SO, Lee CS. Identifying a Relationship Between Physical Frailty and Heart Failure Symptoms. J Cardiovasc Nurs. 2018;33(1):E1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ticinesi A, Nouvenne A, Cerundolo N, et al. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients. 2019;11(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Charlson ES, Diamond JM, Bittinger K, et al. Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. American journal of respiratory and critical care medicine. 2012;186(6):536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dickson RP, Singer BH, Newstead MW, et al. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nature microbiology. 2016;1(10):16113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.O’Dwyer DN, Dickson RP, Moore BB. The Lung Microbiome, Immunity, and the Pathogenesis of Chronic Lung Disease. Journal of immunology. 2016;196(12):4839–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Segal LN, Alekseyenko AV, Clemente JC, et al. Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome. 2013;1(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Segal LN, Clemente JC, Tsay JC, et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nature microbiology. 2016;1:16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Simon-Soro A, Sohn MB, McGinniss JE, et al. Upper Respiratory Dysbiosis with a Facultative-Dominated Ecotype in Advanced Lung Disease and Dynamic Change after Lung Transplant. Annals of the American Thoracic Society. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Salisbury ML, Han MK, Dickson RP, Molyneaux PL. Microbiome in interstitial lung disease: from pathogenesis to treatment target. Current opinion in pulmonary medicine. 2017;23(5):404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Puts MTE, Toubasi S, Andrew MK, et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing. 2017;46(3):383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
REFERENCES
- 1.Vaz Fragoso CA, Enright PL, McAvay G, Van Ness PH, Gill TM. Frailty and respiratory impairment in older persons. Am J Med. 2012;125(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ierodiakonou D, Kampouraki M, Poulonirakis I, et al. Determinants of frailty in primary care patients with COPD: the Greek UNLOCK study. BMC Pulm Med. 2019;19(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maddocks M, Kon SS, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71(11):988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milne KM, Kwan JM, Guler S, et al. Frailty is common and strongly associated with dyspnoea severity in fibrotic interstitial lung disease. Respirology. 2017;22(4):728–734. [DOI] [PubMed] [Google Scholar]
- 5.Chen PJ, Yang KY, Perng WC, Lin KC, Wang KY. Effect of dyspnea on frailty stages and related factors in Taiwanese men with COPD. International journal of chronic obstructive pulmonary disease. 2018;13:2463–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medina-Mirapeix F, Bernabeu-Mora R, Gimenez-Gimenez LM, Escolar-Reina P, Gacto-Sanchez M, de Oliveira-Sousa SL. Physical frailty characteristics have a differential impact on symptoms as measured by the CAT score: an observational study. Health and quality of life outcomes. 2018;16(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Limpawattana P, Putraveephong S, Inthasuwan P, Boonsawat W, Theerakulpisut D, Chindaprasirt J. Frailty syndrome in ambulatory patients with COPD. International journal of chronic obstructive pulmonary disease. 2017;12:1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernabeu-Mora R, Garcia-Guillamon G, Valera-Novella E, Gimenez-Gimenez LM, Escolar-Reina P, Medina-Mirapeix F. Frailty is a predictive factor of readmission within 90 days of hospitalization for acute exacerbations of chronic obstructive pulmonary disease: a longitudinal study. Ther Adv Respir Dis. 2017;11(10):383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SK, Richardson CR, Holleman RG, Larson JL. Frailty in people with COPD, using the National Health and Nutrition Evaluation Survey dataset (2003–2006). Heart & lung : the journal of critical care. 2013;42(3):163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montgomery E, Macdonald PS, Newton PJ, et al. FRAILTY AS A PREDICTOR OF MORTALITY IN PATIENTS WITH INTERSTITIAL LUNG DISEASE REFERRED FOR LUNG TRANSPLANTATION. Transplantation. 2019. [DOI] [PubMed] [Google Scholar]
- 11.Singer JP, Diamond JM, Gries CJ, et al. Frailty Phenotypes, Disability, and Outcomes in Adult Candidates for Lung Transplantation. American journal of respiratory and critical care medicine. 2015;192(11):1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson ME, Vakil AP, Kandel P, Undavalli C, Dunlay SM, Kennedy CC. Pretransplant frailty is associated with decreased survival after lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2016;35(2):173–178. [DOI] [PubMed] [Google Scholar]
- 13.Gale NS, Albarrati AM, Munnery MM, et al. Frailty: A global measure of the multisystem impact of COPD. Chron Respir Dis. 2018;15(4):347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guler SA, Kwan JM, Leung JM, Khalil N, Wilcox PG, Ryerson CJ. Functional aging in fibrotic interstitial lung disease: The impact of frailty on adverse health outcomes. Eur Respir J. 2019. [DOI] [PubMed] [Google Scholar]
- 15.Stanjek-Cichoracka A, Wozniak-Grygiel E, Laszewska A, Zembala M, Ochman M. Assessment of Cytokines, Biochemical Markers of Malnutrition and Frailty Syndrome Patients Considered for Lung Transplantation. Transplantation proceedings. 2019;51(6):2009–2013. [DOI] [PubMed] [Google Scholar]
- 16.Anderson MR, Kolaitis NA, Gao Y, et al. A nonlinear relationship between visceral adipose tissue and frailty in adult lung transplant candidates. Am J Transplant. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guler SA, Kwan JM, Winstone TA, et al. Severity and features of frailty in systemic sclerosis-associated interstitial lung disease. Respiratory medicine. 2017;129:1–7. [DOI] [PubMed] [Google Scholar]
- 18.Mittal N, Raj R, Islam EA, Nugent K. The Frequency of Frailty in Ambulatory Patients With Chronic Lung Diseases. J Prim Care Community Health. 2016;7(1):10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valenza MC, Torres-Sanchez I, Cabrera-Martos I, Rodriguez-Torres J, Gonzalez-Jimenez E, Munoz-Casaubon T. Physical Activity as a Predictor of Absence of Frailty in Subjects With Stable COPD and COPD Exacerbation. Respiratory care. 2016;61(2):212–219. [DOI] [PubMed] [Google Scholar]
- 20.Kusunose M, Oga T, Nakamura S, Hasegawa Y, Nishimura K. Frailty and patient-reported outcomes in subjects with chronic obstructive pulmonary disease: are they independent entities? BMJ Open Respir Res. 2017;4(1):e000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Layton AM, Armstrong HF, Baldwin MR, et al. Frailty and maximal exercise capacity in adult lung transplant candidates. Respiratory medicine. 2017;131:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]


