Abstract
Reductions of glutamate acid decarboxylase (GAD67) and subsequent GABA levels have been consistently observed in neuropsychiatric disorders like schizophrenia and depression, but it has remained unclear how GABAergic dysfunction contributes to different symptoms of the diseases. To address this issue, we investigated male mice haplodeficient for GAD67 (GAD67+/GFP mice), which showed a reduced social interaction, social dominance and increased immobility in the forced swim test. No differences were found in rotarod performance and sensorimotor gating. We also addressed potential effects of social deprivation, which is known, during early life, to affect GABAergic function and induces behavioral abnormalities similar to the symptoms found in psychiatric disorders. Indeed, social isolation of GAD67+/GFP mice provoked increased rearing activity in the social interaction test and hyperlocomotion on elevated plus maze. Since GABA closely interacts with the dopaminergic, serotonergic and cholinergic neurotransmitter systems, we investigated GAD67+/GFP and GAD67+/+ mice for morphological markers of the latter systems and found increased tyrosine hydroxylase (TH)-IR fiber densities in CA1 of dorsal hippocampus. By contrast, no differences in numbers and densities of TH-positive neurons of the midbrain dopamine regions, serotonin (5-HT) neurons of the raphe nuclei, or choline acetyltransferase (ChAT)-expressing neurons of basal forebrain and their respective terminal fields were observed. Our results indicate that GAD67 haplodeficiency impairs sociability and increases vulnerability to social stress, provokes depressive-like behavior and alters the catecholaminergic innervation in brain areas associated with schizophrenia. GAD67+/GFP mice may provide a useful model for studying the impact of GABAergic dysfunction as related to neuropsychiatric disorders.
Electronic supplementary material
The online version of this article (10.1007/s00429-020-02087-6) contains supplementary material, which is available to authorized users.
Keywords: GAD67, Hippocampus, Schizophrenia, Social interaction, Social isolation, Dopamine
Introduction
A corticolimbic GABAergic dysfunction has been implicated in the pathophysiology of neuropsychiatric disorders like schizophrenia and major depressive disorder (MDD) (Benes and Berretta 2001; Fatemi et al. 2005). The most consistent findings in post-mortem brain studies of schizophrenia patients are reduced numbers of parvalbumin (PARV)-containing GABAergic interneurons and a decreased expression of the 67-kDa isoform of the GABA-synthesizing enzyme glutamate acid decarboxylase (GAD67) in cerebral cortex and hippocampus (Akbarian et al. 1995; Guidotti et al. 2000; Hashimoto et al. 2003; Lewis et al. 2005). In contrast to GAD67, only inconsistent findings in schizophrenia are reported for the 65-kDa (GAD65) isoform (Guidotti et al. 2000; Glausier et al. 2015; de Jonge et al. 2017). Besides GABAergic dysfunction, alterations of other neurotransmitter pathways in different brain areas have been reported (Brisch et al. 2014). Regarding the dopaminergic (DAergic) system, a subcortical mesolimbic hyperactivity and prefrontal mesocortical hypoactivity were postulated (Meltzer and Stahl 1976; Howes and Kapur 2009). Also, alterations of the serotonergic (5-HT) system of the raphe nuclei and dysfunctions of the cholinergic system in the basal forebrain were found to be involved in schizophrenia (Raedler et al. 2007; Geyer and Vollenweider 2008). Schizophrenia is further characterized by heterogeneous clinical symptoms including social withdrawal, avolition, hyperactivity, depressive symptoms, deficits in sensorimotor gating and increased risk for aggression (Andreasen et al. 1990; Braff et al. 2001). However, due to the etiological heterogeneity of schizophrenia the underlying molecular and cellular pathomechanisms remain largely unknown. It is assumed that schizophrenia develops through complex interactions between multiple genes and environmental factors (Lewis and Levitt 2002). In accordance with the “two-hit” hypothesis (Bayer et al. 1999), the combination of a genetically predisposition (the first hit) and an early life adverse event (the second hit) profoundly affects brain development and subsequent adult behavior, which may contribute to the occurrence of psychiatric disorders (Fone and Porkess 2008; Pietropaolo et al. 2008). Previous studies showed that post-weaning social isolation in combination with a pharmacological downregulation of the GABAergic function could act as a potential second hit (Lim et al. 2012; Gaskin et al. 2016). However, the separate role of the commonly observed GAD67 deficit as a vulnerability factor and its direct behavioral effects remain unclear. Mouse mutants deficient for GAD67 and GAD65 provide an opportunity to address specific roles of the GABAergic system in brain development and function. Since, there is little evidence for a role of GAD65 similar to that of GAD67 in human schizophrenia and null mutation of the GAD65 gene in mice develop a phenotype of increased anxiety and pathological fear memory reminiscent of posttraumatic stress disorder (Müller et al. 2015), we focused on GAD67 deficiency in mice. Homozygous GAD67 mutants die shortly after birth due to a severe cleft palate (Asada et al. 1997). In contrast, GAD67+/GFP mice, carrying a non-functional allele of the GAD67 gene due to a knock-in for the green fluorescence protein (GFP), are viable and show normal gross brain morphology (Tamamaki et al. 2003). These mice have been widely used for the identification of GABAergic neurons (Marowsky et al. 2005; Brown et al. 2008). In addition, GAD67+/GFP mice display 36% reduction of GAD67 expression and 16% reduction of GABA levels in the brain of young adult (see also supplementary information and Fig. S1), whereas the level of GAD65 remains unchanged (Tamamaki et al. 2003; Wang et al. 2009). GAD67+/GFP mice are also reported to show an increased vulnerability to maternal and fetal stress (Uchida et al. 2011) in association with a region specific loss of parvalbumin (PARV)-positive GABAergic neurons, similar to that observed in psychiatric patients (Uchida et al. 2014). Therefore, GAD67+/GFP knock-in mice are appropriate tools to investigate the implications of GABAergic dysfunction as found in neuropsychiatric disorders. In the present study, behavioral and morphological consequences of GAD67 haplodeficiency were investigated that are potentially relevant for schizophrenia pathogenesis. Our data reveal that GAD67 haplodeficiency alone results in impaired social interaction and increases depression-like behavior. GAD67 haplodeficiency in combination with postweaning social isolation additionally provoked an increase of locomotor behavior. Further, deficiency of GAD67-mediated GABA synthesis results in an increased (TH)-positive fiber density in the hippocampus, suggesting an alteration of the catecholaminergic, presumably dopaminergic, system as a vulnerability factor downstream of the GABAergic hypofunction.
Materials and methods
Animals
Thirteen-to-eighteen-week-old male heterozygous GAD67–GFP knock-in C57BL/6 mice (GAD67+/GFP mice) in which GFP is inserted into the GAD67 gene (Tamamaki et al. 2003) and their wild-type control siblings (GAD67+/+) were examined in this study. Genotypes were determined with allele-specific PCR at the time of weaning as described previously (Janitzky et al. 2011). After weaning (postnatal day 21) mice from each genotype were either left in littermate groups of 2–3 or assigned to social isolation by individually housing (Makrolon cages measuring l/w/h: 27 × 21 × 14 cm, environmental enrichment with nesting material) until the beginning of the experiments. All animals were kept in a temperature, humidity, and light-controlled environment (22–25 °C, 55%, 12 h light–dark cycle, with light on at 06:00 a.m.) with free access to food and tap water. Isolated animals had only visual, auditory and olfactory contact with other isolation reared and group-housed mice. Behavioral experiments were performed during the active phase and body weight was measured before each experiment. All experiments were carried out in accordance with the European Communities Council Directive (2010/63/EU) and approved by the local authorities of Sachsen-Anhalt/Germany (AZ: 42502-2-1134 UniMD).
Social interaction test in the open field
The social interaction test was performed using pairs of unfamiliar strain mates (isolated GAD67+/GFP (n = 9) and GAD67+/+ (n = 8) and group-housed GAD67+/GFP (n = 9) and GAD67+/+ (n = 10)). Mice were tested in a light gray upwardly open plastic arena (l/w/h: 85 × 85 × 30 cm) with a homogenous, shadow free and bright neon-illumination (300 lx). Each experiment was recorded with a ceiling mounted camera (Panasonic CCTV Camera; Mod. WVBL200/6) and analyzed using Videomot2 video-tracking software (TSE, Homburg, Germany). At the beginning of each 10-min trial, two unfamiliar mice from the same genotype and housing condition (group housed or isolated) were placed into opposite corners of the open field, recorded, and automatically analyzed by the software for various parameters (Table 1). Analysis of distance traveled was carried out as a sign of motor activity, anxiety like behavior as time spent in center region. Afterwards, the coded video sequences were manually analyzed for a second set of parameters (Table 1). Data of the individual pairs were used for statistics. With self-compounded software, using fuzzy logic, collected raw data were additionally processed to compensate weaknesses of the image recognition software (Wolf et al. 2018).
Table 1.
Social interaction test in the open field
| Analyzed parameters during the experiment | |
|---|---|
| 1 | Time spent in contact in percent of experimental time [%]2 |
| 2 | Number of contacts [n]2 |
| 3 | Time spent in aggressivity in percent of experimental time [%]2 |
| 4 | Number of aggressive contacts2 |
| 5 | Mean distance between the two mice [cm]1 |
| 6 | Active social interaction, in percent of experimental time [%] during a movement speed higher than 3 cm/s1 |
| 7 | Passive social interaction, in percent of experimental time [%] during a movement speed lower than 1 cm/s1 |
| 8 | Sniffing [n]2 |
| 9 | Anogenital sniffing [n]2 |
| 10 | Following [n]2 |
| 11 | Rearing [n]2 |
| 12 | Leaning [n]2 |
| 13 | Grooming [n]2 |
| 14 | Distance traveled [m]1 |
| 15 | Time spent in the center or periphery of the open field in percent of experimental time [%]1 |
Automatically (1) and manually (2) analyzed parameters in the social interaction test
Social dominance tube test
To test social dominance a custom-made tube (transparent acrylic glass, 35 cm long and 3 cm diameter) was used. Each animal (group-housed GAD67+/GFP (n = 10) and GAD67+/+ (n = 10) and isolated GAD67+/GFP (n = 10) and GAD67+/+ (n = 10)) was given a pre-competition habituation period of three training trials on each of three consecutive days. Driven by food deprivation (85% of normal weight) the animals run through the tube to a food reward in a goal box (l/w/h: 15 × 9 × 9 cm). For each trial, animals were alternated in the direction that they ran through the tube. During the experiments one GAD67+/GFP and one unfamiliar GAD67+/+mouse from same housing condition were placed at opposite ends of the tube, in a head-to-head direction and released. A mouse was declared a “winner” when its opponent backed out with all four paws of the tube. The maximal test time was set to 2 min. Each mouse was tested for a total of seven times on two consecutive days, each time against an unfamiliar opponent (70 trails). The number of wins and losses for each genotype was determined.
Rotarod test
To assess mild neurological deficits such as impaired motor learning and coordination in rodents we used a mouse rotarod (model 47600, Ugo Basile, Comerio, Italy). One day prior the experiments mice (isolated GAD67+/GFP (n = 12) and GAD67+/+ (n = 12) and group-housed GAD67+/GFP (n = 12) and GAD67+/+ (n = 12)) were familiarized with the rotarod at fixed speed modes of 4 and 10 rpm for 1 min, respectively. At start of the experiments, mice underwent three rotarod trials per day on three consecutive days with accelerating speed from minimum of 4 to 40 rpm over the course of each 300 s (5 min) trial. Each mouse was given an intertrial interval of 30 min. The amount of time before the mouse fell from the rod was measured.
Forced swim test
The Porsolt forced swim test (FST) was conducted in a modified version with only one swim session. Mice (isolated GAD67+/GFP (n = 20) and GAD67+/+ (n = 15) and group-housed GAD67+/GFP (n = 13) and GAD67+/+ (n = 11)) were placed individually in a transparent acrylic glass cylinder (diameter 14 cm, height 21 cm) filled to a depth of 15 cm with tap water (23 °C). Each session was investigated for latency to first occurrence of immobility, time spent immobile, swimming and climbing. Only the last 4 min of the 6 min test session were analyzed by a trained observer blind to genotype. Immobility was defined as the cessation of limb movements except minor movement necessary to keep the animals head above water. Swimming was registered when large forepaw movements displaced the body around the cylinder. Climbing was defined when vigorous movements with forepaws out of the water, usually directed against the wall of the cylinder, were observed.
Elevated plus maze
The maze consisted of a plus-shaped gray wooden platform elevated 75 cm above the floor with a center (5 cm × 5 cm) connecting the four arms (25 cm × 5 cm). Two of the opposing arms were enclosed by 35-cm-high walls (closed arms), whereas the other two arms had thin 0.3-cm-high ledges (open arms). All arms were lit by shadow free neon-illumination (mean light intensity 100 lx). At the beginning of the test, individual mice (isolated GAD67+/GFP (n = 18) and GAD67+/+ (n = 15) and group-housed GAD67+/GFP (n = 15) and GAD67+/+ (n = 14)) were placed in the center of the maze and allowed to explore for 10 min. Number of entries with all paws into the arms and the center as well as time spent on different positions were analyzed using a top-mounted camera and custom-made software. After finishing each session, mice were transferred back to their home cages and the maze was thoroughly wiped clean.
Prepulse inhibition of acoustic startle response
Isolated GAD67+/GFP (n = 19) and GAD67+/+ (n = 16) and group-housed GAD67+/GFP (n = 14) and GAD67+/+ (n = 20) mice served for the experiments. The startle apparatus consists of a sound-attenuating chamber (l/w/h: 50 × 40 × 45 cm, illuminated by a 5 W cold light, with a movement sensitive piezo-accelerometer platform (Startle-Messsystem, University of Tübingen, Germany) on which a wire mesh test cage (l/w/h: 8 × 5 × 5 cm) was fixed. Movement-induced voltage changes were amplified (Piezo-Amp System, University of Tübingen, Germany) and digitized by data acquisition processor board DAP1200a (Microstar, Bellevue, WA) within a computer and recorded using the software program DAPview Plus® (Microstar Laboratories). Startle amplitude was calculated as the difference between peak-to-peak voltage during a time window 80 ms after stimulus onset and the 80 ms before stimulus onset. The force values F (N) were converted to acceleration values a = F/m (m/s2), which represent the startle amplitudes of the animal. Thus, the results given as acceleration values are independent from animal weight (m). Acoustic stimuli and steady background noise (65 dB) were performed by the signal synthesizer software SigGen-PC 1.44 (Waldmann, Tübingen, Germany), generated by a stimulus generation processor board ASPI ELF-31 (Medav, Uttenreuth, Germany), amplified (WPA-600; Sony, Tokyo, Japan) and presented by a speaker located in a distance of 21 cm from the center of test platform. Each startle session consisted of 85 trials and was started with an acclimation period of ten non-stimulus trials (NOSTIM) followed by three PULSE-ALONE (113 dB SPL) trials. These trials were not included in the analysis. During the acquisition period, four different types of stimuli (Table 2) were presented in a pseudo-random order with an intertrial interval of 20 s.
Table 2.
Parameters of acoustic startle response (ASR) and prepulse inhibition (PPI) tests
| Stimulus type | Description |
|---|---|
| 1. NOSTIM | No stimulus, white noise at 65 dB sound pressure level (SPL) |
| 2. PREPULSE | Prepulse alone, sine wave, 10 kHz, 75 dB SPL, duration 20 ms, rise and fall time 0.4 ms |
| 3. PULSE-ALONE | Startle stimulus alone, white noise at 93, 107 or 113 dB SPL, duration 20 ms, without rise and fall time |
| 4. PREPULSE + PULSE | Startle stimulus at 93, 107 or 113 dB SPL with preceding PREPULSE at 75 dB SPL, interstimulus interval (ISI) 100 ms |
Acoustic startle stimuli presented during the prepulse inhibition experiments
Preparation of the brains for histological analyses
Group-housed male GAD67+/GFP (n = 6) and GAD67+/+ (n = 6) mice were deeply anesthetized with an overdose of sodium pentobarbital (180 mg/kg i.p.; Merial GmbH, Hallbergmoos, Germany) and perfused transcardially with 50 ml of 0.9% saline followed by 150 ml of a mixture of 4% paraformaldehyde and 15% saturated picric acid in 0.1 M phosphate buffer pH 7.4 (PB). After decapitation, brains were rapidly dissected from the skull and postfixed for additional 5 h in the same fixative followed by 24 h storage in 20% sucrose at 4 °C. Subsequently, the brains were shock-frozen at − 40 °C in solid carbon dioxid cooled isopentane and stored at − 80 °C. The brains were cut coronally with a cryostat into four corresponding series of 40-µm-thick sections. The series were stained for 1. Nissl substance, 2. tyrosine hydroxylase (TH), 3. serotonin (5-HT) and 4. choline acetyltransferase (ChAT).
Immunohistochemistry
For cell counting and measurement of fiber densities, the free-floating sections were stained using immunofluorescence technique against TH, 5-HT and ChAT as described elsewhere (Nullmeier et al. 2014). The following primary antibodies were used (see also supplementary Figs. S2-4): rabbit TH polyclonal antibody (dilution: 1:1000; ab112; Abcam, Cambridge, UK), rabbit 5-HT polyclonal antibody (dilution: 1:15000; catalog number: 20080; ImmunoStar, Hudson, WI, USA) and goat ChAT polyclonal antibody (dilution: 1:100; Cat. #AB144P, Millipore, Billerica, MA, USA). Unspecific bindings of polyclonal antibodies were blocked before incubation using 10% bovine serum albumin (BSA) in phosphate buffer (0.1 M, pH 7.4) and 10% of corresponding normal serum from the host of the secondary antibody. Secondary antibodies (biotinylated rabbit anti-goat IgG (BA-5000) for ChAT and biotinylated goat anti-rabbit IgG (BA-1000) for 5-HT and TH; Vector Laboratories, Burlingame, CA, USA) were used in a dilution of 1:200. Antibodies were visualized by incubation with Cy3 conjugated avidin (dilution 1:1000; Cod. no. 003-160-083; Jackson ImmunoResearch Laboratories, Baltimore Pike, PA, USA). All sections were stained in parallel to ensure comparability and minimize experimental variability. After staining, the sections were mounted on glass slides (Super Frost Plus, ThermoScientific, Germany) and coverslipped.
Microscopy
Cell counting, area measurements and analysis of fiber density were carried out manually on coded glass slides. For microscopy and photographs, a Zeiss AxioImager.Z2 fluorescence microscope equipped with an AxioCam MRm Rev. 3.1 camera (Zeiss, Germany) and a 5× (area measurement) or 40 × objective (analyses of cell numbers and fiber densities) were used. The software Axiovision 4.8.1® (Zeiss, Germany) served for image analysis and scaled measurements.
Cell counting and area measurement
The number of ChAT-immunoreactive (IR) neurons was analyzed in the septum from bregma 1.10 mm to bregma 0.26 mm (Paxinos and Franklin 2004). The septum was subdivided into medial septum (MS, also including the horizontal and vertical diagonal band of Broca and the lambdoid septal zone) and the lateral septum (LS, also including the intermediary and ventral parts of the lateral septal nucleus). Investigation of the number of TH-positive neurons in the midbrain was carried out from bregma − 2.46 mm to bregma − 3.52 mm. The substantia nigra (SN) and ventral tegmental area (VTA) in the right hemisphere were subdivided in substantia nigra pars compacta (SNC), substantia nigra pars reticularis (SNR), substantia nigra pars lateralis (SNL) and VTA. Numbers of 5-HT-containing neurons and area measurements of the raphe nuclei were performed from bregma − 4.04 mm to bregma − 5.02 mm. Only the dorsal nucleus raphe (DR), median (MnR) and paramedian nucleus raphe (PMnR) were investigated. For each staining, all sections containing the area of interest were investigated and only cell bodies with clear neuronal shape and well-defined nucleus were counted. Additionally, neuron diameters were estimated and used for Abercrombie correction of neuron number (Abercrombie 1946). The Nissl-stained series were used to identify anatomical landmarks (Paxinos and Franklin 2004).
Measurement of fiber density
Measurement of ChAT, TH and 5-HT-IR fiber density was performed in dorsal hippocampus, dentate gyrus (DG) and amygdala of left hemisphere. Hippocampal CA1 and CA3 regions were divided into three layers: stratum oriens (Or), stratum radiatum (Rad) and stratum lacunosum moleculare (LMol) and dentate gyrus (DG) into two layers: stratum moleculare (Mol) and stratum multiforme (ML). Measurements of dorsal hippocampus and DG started at bregma − 1.8 mm. From five consecutive slices, one image of each layer was taken, respectively. The left amygdala was divided into four subfields: lateral amygdala (La); basolateral amygdala (BLA); central amygdala nucleus, capsular part (CeC); central amygdala nucleus, medial division (CeM). Measurements started at bregma − 0.94 mm and from three consecutive slices one image of each subfield was taken. For the analysis of the scaled microphotographs, the software ImageJ (version 1.47, https://imagej.nih.gov/ij/) was used. According to previously published methods (Panther et al. 2012), fiber densities were estimated with a calibrated grid (mesh size of 10 µm) and a square (length of one side (L) was 80 µm). The number of planes (P = 9) which correspond to the vertical and horizontal lines of the grid and the margin of the square, were represented in two sets of planes (2 × P). The area (A) was calculated by A = L × 2 × P = 1440 µm2. All intersections (n) of fibers with the grid were counted within the square. There is a relationship between the number of points per unit area (PA) and the specific line length per unit volume (LV): PA = 1/2 × LV. If LV = 2 × PA (µm/µm3) and PA = n/A (counts/µm2) the estimated length within a volume (fiber density) was gained.
Statistical analysis
Behavioral tests of social interaction, rotarod, FST, EPM, ASR and PPI were analyzed by 2 × 2 two-way analyses of variance (ANOVAs) using GENOTYPE (GAD67+/GFP vs GAD67+/+) and HOUSING (isolation vs group housed) as the between-subject factors. Additional within-subject factors (e.g. trials or PULSE-ALONE intensities) were also included according the nature of the considered variables. Post hoc analyses were performed using pairwise comparisons with Bonferroni correction. For analysis of social dominance, a binary logistic model was used. Data (wins/losses) were first analyzed for an interaction of GENOTYPE × HOUSING. If no significance was achieved, GENOTYPE and HOUSING were analyzed as main effects without interaction. For histological analysis, volume was calculated by using area and section thickness. Counted neuron numbers were quantified using Abercrombie’s method (Abercrombie 1946) and then calculated from volume (mm3) × density (n/mm3). Data were normally distributed (Shapiro–Wilk test). Each brain area was analyzed, respectively, using repeated-measures ANOVAs with LAYER or REGION (two to four levels, depending on the investigated brain area) as within-subject factors and GENOTYPE (two levels: GAD67+/GFP and GAD67+/+) as between-subject factor. Post hoc analyses were performed using unpaired t tests for unequal variances (Welch’s test), and adjusted p values were obtained via Bonferroni–Holm method. The VTA was investigated using an unpaired t test, respectively. Data are presented as means ± SEM. Alpha level was set at 0.05 for all main and interaction effects. The software package SPSS (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp) was used for statistical analysis.
Results
Alteration of social behavior in GAD67+/GFP mice
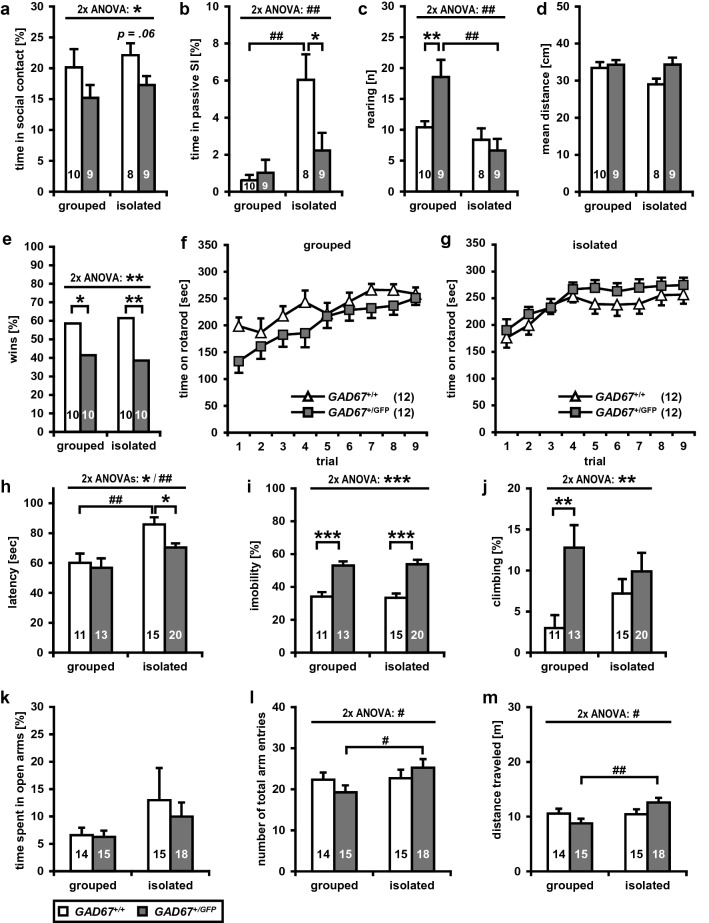
In the social interaction test (SI) pairs of male GAD67+/GFP and GAD67+/+ from same genotype and housing condition, respectively, were analyzed for different parameters (see Table 1, Fig. 1a–d). A two-way multivariate ANOVA (between-subject factors: GENOTYPE and HOUSING) revealed significant interactions of GENOTYPE × HOUSING for passive social interaction (F(1, 32) = 5.94, p < 0.05) and rearing (F(1, 32) = 6.38, p < 0.05). GENOTYPE showed a significant difference in time spent in social contact (F(1, 32) = 4.74, p < 0.05), revealing a reduction in social contacts for GAD67+/GFP mice (Fig. 1a). Only trends towards a significant main effect for passive social interaction (F(1, 32) = 3.86, p = 0.058) and mean distance between the animals (F(1, 32) = 3.72, p = 0.063) were found. Also, HOUSING revealed significant differences. Isolated mice from both genotypes showed an increased time spent in passive social interaction (F(1, 32) = 14.61, p < 0.01, Fig. 1b), higher numbers in sniffing (F(1, 32) = 45.68, p < 0.001) and anogenital sniffing behavior (F(1, 32) = 4.81, p < 0.05), lower rearing activity (F(1, 32) = 12.70, p < 0.01, Fig. 1c), increased grooming (F(1, 32) = 13.73, p < 0.01), and a higher number of aggressive contacts (F(1, 32) = 6.92, p < 0.05). Post hoc analyses for isolated GAD67+/GFP mice, compared with isolated GAD67+/+ mice, showed a trend towards a lower time spent in social contact (t = − 2.02, df = 15, p = 0.062, Fig. 1a) and significantly less time spent in passive social interaction (t = − 2.32, df = 15, p < 0.05, Fig. 1b), indicating that post-weaning social isolation affects social behavior in GAD67+/GFP mice. Group-housed GAD67+/GFP mice, compared with group-housed GAD67+/+mice, displayed only an increased rearing activity (t = 2.88, df = 17, p = 0.01, Fig. 1c). Thus, male GAD67+/GFP mice show a significant reduction in social interaction, particularly obvious in the socially isolated group. Further, isolated GAD67+/+mice, compared with group-housed GAD67+/+, spent significantly more time in passive social interaction (t = 3.86, df = 7.63, p < 0.01, Fig. 1b), showed an increased sniffing (T = 6.18, df = 16, p < 0.001) and grooming behavior (T = 2.45, df = 16, p < 0.05). Isolated GAD67+/GFP mice, compared with group-housed GAD67+/GFP, presented a reduced rearing activity (T = -3.54, df = 16, p < 0.01, Fig. 1c), an increased sniffing (t = 3.84, df = 16, p < 0.01) and grooming behavior (t = 2.79, df = 16, p < 0.05). With regard to distance moved (Fig. 1d) and time spent in unprotected center zone of the open field, no significant main effects for GENOTYPE or HOUSING were found.
Fig. 1.
GAD67+/GFP mice exhibit a subset of negative symptom-like behavioral deficits. a–d In the social interaction test a genotype had a significant main effect on time spent in social contact. However, only isolated GAD67+/GFP mice, compared to isolated GAD67+/+, showed a trend towards a lower time spent in social contact. b Social isolation had an effect on passive social interaction. Socially isolated GAD67+/+ mice spent significantly more time in passive social interaction than group-housed GAD67+/+ mice. Isolated GAD67+/GFP showed a significantly lower passive social interaction than socially isolated GAD67+/+ mice. c Social isolation had a significant effect on rearing activity. Isolated GAD67+/GFP mice revealed a lower rearing activity than group-housed GAD67+/GFP. Group-housed GAD67+/GFP showed a significantly higher rearing activity compared with group-housed GAD67+/+. d There were no differences in locomotor activity. e In social dominance tube test isolated and grouped GAD67+/GFP lost significantly more bouts, than GAD67+/+ mice. f, g Genotype and housing showed no effect on rotarod performance. h–j In the forced swim test significant main effects for genotype and housing were found for latency to appearance of first immobility period. h Isolated GAD67+/GFP, compared to isolated GAD67+/+ mice showed a significantly reduced latency. Interestingly, social isolation increased the latency of GAD67+/+ mice. iGAD67+/GFP exhibit a significantly increased time spent in immobility, independent from housing condition. j The genotype showed an effect on climbing activity. Group-housed GAD67+/GFP mice revealed a significantly higher climbing activity, compared to GAD67+/+. k–m In the elevated plus maze test, no differences were found for k time spent in open arms. l The number of total arm entries was dependent on the housing condition. Social isolation significantly increased the number of total arms entries, of GAD67+/GFP mice. m Social isolation had an effect on locomotor activity on EPM. Social isolation significantly increased the distance traveled in GAD67+/GFP mice. Statistics: Animal number n is indicated in parentheses or plot bars. Data are presented as mean ± SEM. The social interaction test, forced swim test, elevated plus maze rotarod were analyzed using two-way multivariate analyses of variance (MANOVA) or repeated measures ANOVA with GENOTYPE (two levels: GAD67+/+ and GAD67+/GFP) and HOUSING (two levels: group housed and isolated) as between-subject factors. Post hoc analyses were performed using unpaired t tests. The social dominance tube test was analyzed using a binary logistic model followed by Wald Chi-square test. *p < 0.05, **p < 0.01, ***p < 0.001 vs. GAD67+/+ mice. #p < 0.05, ##p < 0.01 vs. group-housed mice
Decreased social dominance in GAD67+/GFP mice
GABA, dopamine, and serotonin under various conditions are associated with aggressive behavior (Miczek et al. 1994; Narvaes and Martins de Almeida 2014). Therefore, the social dominance tube test was performed by pairing group-housed or socially isolated male mice of both genotypes. We found that GAD67+/GFP mice lost significantly more bouts, independent from housing condition (Fig. 1e, binary logistic model followed by Wald Chi-square test, GENOTYPE effect: Wald-χ2(1) = 7.18, Exp(B) = 0.39 (95% confidence interval (CI) = 0.20–0.78), p = 0.007; HOUSING effect: Wald-χ2(1) = 0.12, Exp(B) = 0.89 (95% CI = 0.45–1.75), p > 0.05; HOUSING and GENOTYPE interaction: Wald-χ2 (1) = 0.24, Exp(B) = 1.27 (95% CI = 0.49–3.30), p > 0.05, demonstrating that GAD67+/GFP mice are less dominant than wild-type mice when paired against each other. Exploring group-housed mice, GAD67+/GFP mice lost more bouts then GAD67+/+ mice (χ2 (1) = 4.11, p < 0.05, Fig. 1e). Additionally, this decrease in social dominance was found in socially isolated GAD67+/GFP mice (χ2 (1) = 7.31, p < 0.01, Fig. 1e).
Motor coordination is not impaired in GAD67+/GFP mice
To determine possible alterations in motor coordination and balance of GAD67+/GFP mice, compared to GAD67+/+, the rotarod test was used. All mice showed learning of the rotarod and reached a stable level of performance within 3 days (Fig. 1f, g). A three-way repeated-measures ANOVA (within-subject factor: TRIAL, between-subject factors: HOUSING and GENOTYPE) revealed no significant interaction and no main effect for GENOTYPE (n.s.). HOUSING only showed a trend towards to an increase in rotarod performance for isolated mice (F(1, 44) = 3.64, p = 0.063).
Increased depressive-like behavior in GAD67+/GFP mice
To investigate the effects of GAD67 haplodeficiency and social isolation on depressive-like behavior male isolated and group-housed GAD67+/GFP and GAD67+/+ mice were analyzed in the forced swim test (see Fig. 1h–j). A two-way multivariate ANOVA (between-subject factors: GENOTYPE and HOUSING) showed no significant interactions (n.s.). GENOTYPE revealed, that GAD67+/GFP exhibit a significantly reduced latency to appearance of first immobility (F(1, 55) = 6.23, p < 0.05, Fig. 1h), increased time spent in immobility (F(1, 55) = 50.67, p < 0.001, Fig. 1i), reduced time spent swimming (F(1, 55) = 58.61, p < 0.001) and increased climbing behavior (F(1, 55) = 7.48, p < 0.01, Fig. 1j). HOUSING showed that isolated GAD67+/GFP and GAD67+/+ mice had a significantly higher latency to appearance of first immobility period (Fig. 1h) compared with group-housed mice (F(1, 55) = 14.04, p < 0.001). Post hoc tests demonstrated a decreased latency to first immobility period (Fig. 1h) for isolated GAD67+/GFP (t = − 2.79, df = 22.51, p < 0.05), compared with isolated GAD67+/+ mice. This difference was not found for animals held in groups (n.s.). Group-housed and socially isolated GAD67+/GFP mice showed a comparable latency to first immobility period (n.s.). However, social isolation increased latency to first immobility in GAD67+/+ mice (t = 3.03, df = 22.90, p < 0.01, Fig. 1h). Most important, for time spent in immobility (Fig. 1i) post hoc analyses showed that isolated (t = 5.43, df = 32.59, p < 0.001), but also group housed (t = 5.26, df = 21.2, p < 0.001) GAD67+/GFP spent significantly more time in immobility compared to GAD67+/+ mice, respectively. Social isolation did not affect time in immobility (Fig. 1i) of GAD67+/GFP (n.s.) and GAD67+/+ mice (n.s.). For swimming, post hoc analyses revealed that isolated (t = − 5.6, df = 32.64, p < 0.001), and group-housed (t = − 5.98, df = 19.76, p < 0.001) GAD67+/GFP mice spent less time in active swimming, compared to GAD67+/+ mice. Isolation rearing did not impair swimming activity of GAD67+/GFP (n.s.) and GAD67+/+ mice (n.s.). With regard to climbing (Fig. 1j), post hoc tests revealed a significantly increased activity for group-housed GAD67+/GFP (t = 3.09, df = 18.65, p < 0.01), compared with group-housed GAD67+/+ mice. Isolated GAD67+/+ mice showed a trend towards a decreased climbing activity compared with group-housed GAD67+/+ mice (t = 1.79, df = 23.95, p = 0.087). Thus, isolated and group-housed GAD67+/GFP mice, respectively, show a higher depression like behavior compared with GAD67+/+ mice.
Social isolation influences the activity of GAD67+/GFP on the elevated plus maze
The effects of GAD67 haplodeficiency and isolation rearing on anxiety-related behavior were investigated on the elevated plus maze (EPM, see Fig. 1k–m). A two-way multivariate ANOVA revealed no significant interactions of HOUSING and GENOTYPE (n.s) or main effects of GENOTYPE (n.s). HOUSING did not affect the number of open arm entries (n.s.) or the time spent in open arms (n.s., Fig. 1k). However, significant effects of HOUSING were found for number of total arms entries (F(1, 58) = 5.67, p < 0.05, Fig. 1l), distance traveled (F(1, 58) = 6.62, p < 0.05, Fig. 1m) and closed arms entries (F(1, 58) = 5.28, p < 0.05). Also, the ratio of number of open arm entries to the number of total arm entries, a measure of anxiety-related behavior independent of locomotor activity, was calculated, but no HOUSING and GENOTYPE effects were found (n.s.). On closer examination of possible isolation rearing effects, isolated GAD67+/GFP, compared with group-housed GAD67+/GFP mice, showed an increase of total arms entries (t = 2.59, df = 31, p < 0.05, Fig. 1l), distance traveled (t = 3.01, df = 31, p < 0.01, Fig. 1m), and closed arms entries (t = 2.55 df = 31, p < 0.05), whereas isolated and group-housed GAD67+/+ showed no significant housing differences (n.s.). Thus, as a sign of an increased locomotor activity on EPM, post-weaning social isolation results in significantly more arm entries and an increased distance traveled of isolated GAD67+/GFP mice.
No alteration in prepulse inhibition of acoustic startle response
The acoustic startle response (ASR) and prepulse inhibition of acoustic startle (PPI) were analyzed at three different intensities (93, 107, 113 dB SPL) in male GAD67+/GFP and GAD67+/+ mice kept either in groups or social isolation (see Table 2, Fig. 2a–d). The three-way repeated-measures ANOVAs (within-subject factor: INTENSITY, between-subject factors: HOUSING and GENOTYPE) revealed no significant interactions or main effects for ASR and PPI, respectively. Thus, a heterozygous deletion of the GAD67 gene and social isolation do not affect neither the startle reflex responses nor the degree of PPI.
Fig. 2.
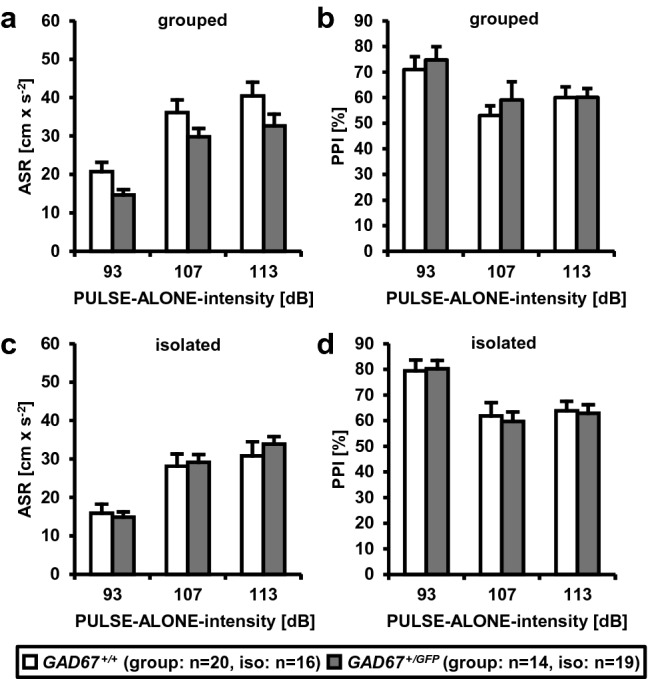
GAD67+/GFP mice show no deficits in prepulse inhibition of acoustic startle reflex. a, c Acoustic startle response (ASR) and b, d prepulse inhibition (PPI) of group-housed and socially isolated GAD67+/+ and GAD67+/GFP mice. No significant interactions or main effects were found, respectively. Data are presented as mean ± SEM. ASR and PPI were analyzed using three-way repeated-measures ANOVAs (within-subject factor: INTENSITY (three levels: 93, 107 and 113 dB), between-subject factors: GENOTYPE (two levels: GAD67+/+ and GAD67+/GFP) and HOUSING (two levels: group housed and isolated)
Tyrosine hydroxylase-IR neurons in substantia nigra/VTA
A hallmark of neuropsychiatric disorders is the disturbance of the dopaminergic system. Therefore, we analyzed GAD67+/GFP and GAD67+/+ mice for differences in neuronal numbers, volumes and neuronal densities of TH-IR neurons in SN subregions SNC, SNR and SNL, respectively (see Table 3). Two-way repeated-measures ANOVAs (within-subject factor: REGION; between-subject factor: GENOTYPE) revealed no significant interactions or main effects (n.s.). Additionally, no genotype differences were found in neuron number, volume and neuron density of VTA (n.s.; Welch’s tests). Thus, GAD67+/GFP and GAD67+/+ mice present no morphological differences in the investigated monoaminergic regions of the midbrain (see also supplementary Fig. S5).
Table 3.
Quantification of TH-IR neurons and fiber densities in GAD67+/+ and GAD67+/GFP mice
| TH-IR neurons and fiber densities | Statistical analysis | ||||||
|---|---|---|---|---|---|---|---|
| TH-IR neurons | Region | Layer | GAD67+/+ (n = 6) | GAD67+/GFP (n = 6) | REGION/LAYER × GENOTYPE | Effect of GENOTYPE | Post hoc |
| Neuron number [n] | SNC | 494.21 ± 28.55 | 465.96 ± 23.49 | – | |||
| SNR | 41.26 ± 2.61 | 45.77 ± 5.74 | F(1.04, 10.44) = 0.68, n.s | F(1, 10) = 0.44, n.s | – | ||
| SNL | 14.06 ± 1.10 | 11.28 ± 1.08 | – | ||||
| Volume [mm3] | SNC | 0.08 ± 0.00 | 0.08 ± 0.01 | – | |||
| SNR | 0.15 ± 0.01 | 0.14 ± 0.01 | F(2, 20) = 2.61, n.s | F(1, 10) = 2.31, n.s | – | ||
| SNL | 0.01 ± 0.00 | 0.01 ± 0.00 | – | ||||
| Density [n/mm3] | SNC | 6077.67 ± 239.37 | 6031.82 ± 214.57 | – | |||
| SNR | 269.47 ± 11.50 | 334.70 ± 33.74 | F(1.30, 13.03) = 0.12, n.s | F(1, 10) = 0.01, n.s | – | ||
| SNL | 1185.79 ± 61.12 | 1137.79 ± 120.15 | – | ||||
| Neuron number [n] | VTA | 283.46 ± 26.88 | 281.23 ± 10.20 | – | n.s. (Welch’s test) | ||
| Volume [mm3] | VTA | 0.07 ± 0.00 | 0.06 ± 0.00 | n.s. (Welch’s test) | |||
| Density [n/mm3] | VTA | 3977.40 ± 259.93 | 4344.88 ± 114.01 | n.s. (Welch’s test) | |||
| TH-IR fiber densities [μm/μm3] | |||||||
| Dorsal hippocampus | CA1 | Or | 0.06 ± 0.00 | 0.07 ± 0.00 | F(2, 20) = 0.02, n.s | F(1, 10) = 20.85, p < 0.01 | p < 0.001 |
| Rad | 0.08 ± 0.01 | 0.09 ± 0.01 | p < 0.05 | ||||
| LMol | 0.09 ± 0.00 | 0.10 ± 0.01 | p < 0.05 | ||||
| CA3 | Or | 0.08 ± 0.01 | 0.09 ± 0.02 | F(2, 20) = 0.30, n.s | F(1, 10) = 1.35, n.s | – | |
| Rad | 0.11 ± 0.02 | 0.12 ± 0.02 | – | ||||
| LMol | 0.09 ± 0.01 | 0.09 ± 0.01 | – | ||||
| DG | Mol | 0.06 ± 0.00 | 0.08 ± 0.02 | F(1, 10) = 0.27, n.s | F(1, 10) = 6.29, p < 0.05 | n.s | |
| ML | 0.09 ± 0.02 | 0.10 ± 0.01 | n.s | ||||
| Amygdala | BLA | 0.36 ± 0.01 | 0.37 ± 0.01 | F(3, 30) = 0.56, n.s | F(1, 10) = 0.11, n.s | – | |
| CeC | 0.32 ± 0.02 | 0.30 ± 0.01 | – | ||||
| CeM | 0.51 ± 0.01 | 0.50 ± 0.01 | – | ||||
| La | 0.25 ± 0.01 | 0.25 ± 0.01 | – | ||||
Coronal brains slices of GAD67+/+ and GAD67+/GFP mice were investigated for densities of tyrosine hydroxylase (TH)-immunoreactive (IR) neurons in subregions of substantia nigra (SN) and ventral tegmental area (VTA) and densities of TH-IR fibers in the dorsal hippocampus and amygdala. Values represent mean ± SEM. Differences between GAD67+/+ and GAD67+/GFP littermates were evaluated using repeated-measures ANOVAs with LAYER or REGION (two to four levels, depending on the investigated brain region) as the within-subject factors and GENOTYPE (two levels: GAD67+/+ and GAD67+/GFP) as the between-subject factor. The interactions and main effects of GENOTYPE are shown. Post hoc analyses were performed using unpaired t tests (Welch’s test) with Bonferroni–Holm adjustment. Neuron densities in the VTA were analyzed using unpaired t test (Welch’s test). For abbreviations, see list
Tyrosine hydroxylase-positive fibers in dorsal hippocampus and amygdala
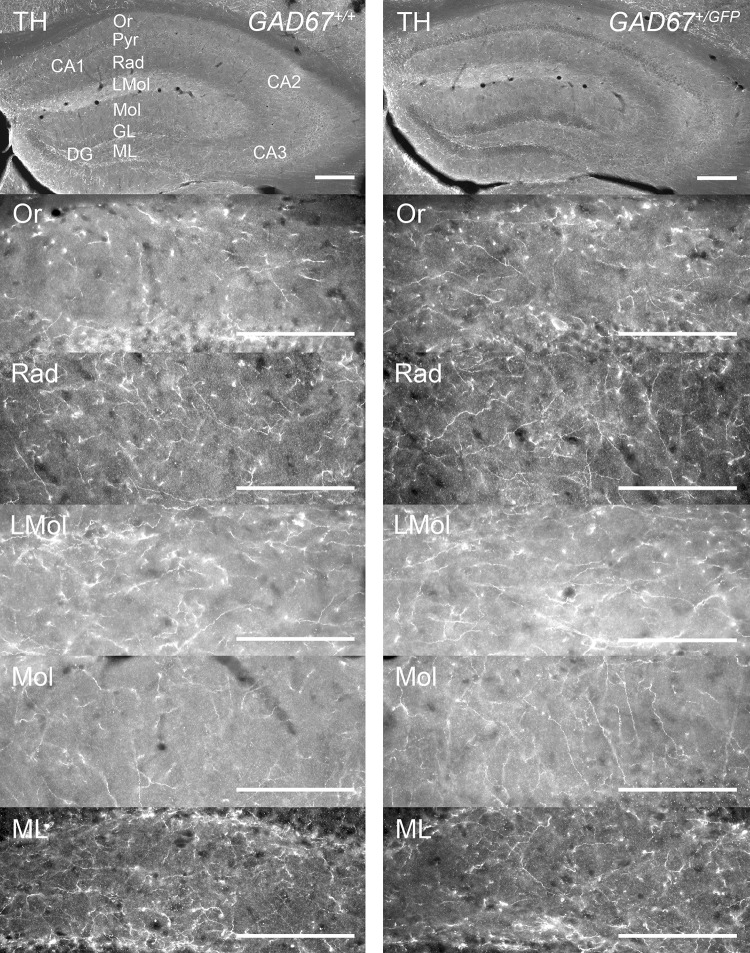
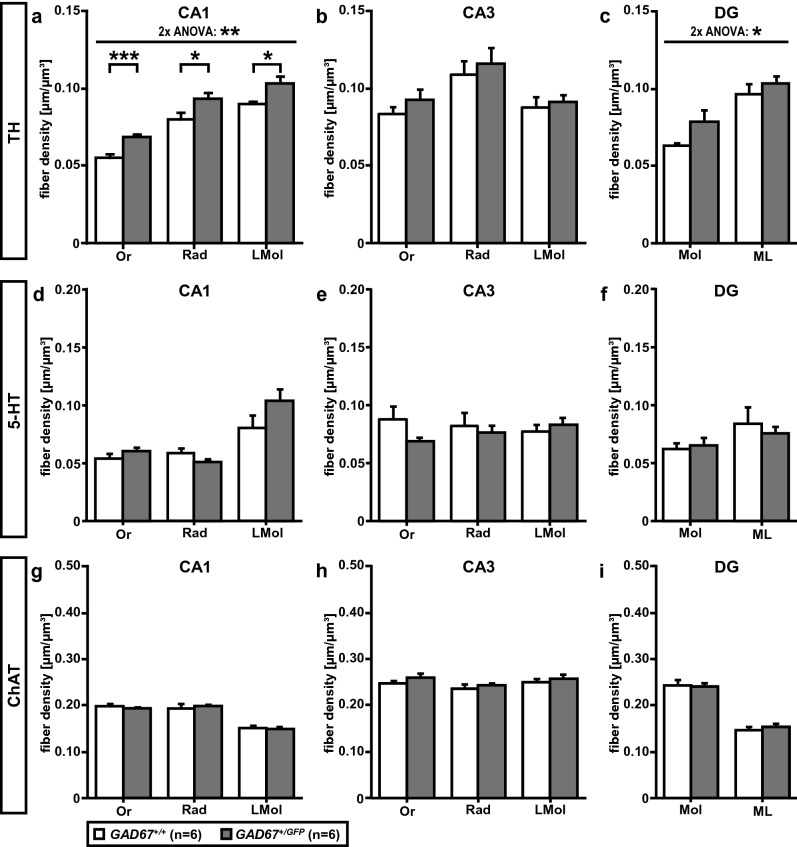
To study the impact of GAD67 haplodeficiency on monoaminergic innervation of dorsal hippocampus, we estimated the densities of TH-IR fibers in three layers of CA1, CA3 (Or, Rad, LMol) and two layers of DG (Mol, ML), respectively (Table 3; Figs. 3, 4a–c). For CA1 (Fig. 4a) a two-way repeated-measures ANOVA showed no interaction of LAYER × GENOTYPE (n.s.) but a significant main effect of GENOTYPE (F(1, 10) = 20.85, p < 0.01). Post hoc analyses showed that GAD67+/GFP, compared to GAD67+/+ mice, have a significantly higher density of TH-IR fibers in Or (t = 6.48, df = 8.42, p < 0.001), Rad (t = 2.54, df = 9.90, p < 0.05), and LMol (t = 3.02, df = 5.95, p < 0.05) of CA1. With reference to dorsal CA3 (Fig. 4b) a two-way repeated-measures ANOVA showed no significant interactions or main effects (n.s.). Investigating the dorsal DG (Fig. 4c), no interaction (n.s.) but a significant main effect of GENOTYPE (F(1, 10) = 6.29, p < 0.05) was found. However, the post hoc analyses did not reach statistical significance (n.s.). In the amygdala TH-IR fiber densities were estimated in: LA, BLA, CeC, and CeM (Table 3) as described above. The two-way repeated-measures ANOVA revealed no significant interaction or main effect (n.s.). Thus, GAD67+/GFP and GAD67+/+ mice demonstrate a higher TH-IR fiber density in dorsal hippocampal CA1, but no differences in other investigated subfields of dorsal hippocampus and amygdala.
Fig. 3.
Distribution of TH-IR fibers in dorsal hippocampus of GAD67+/+ and GAD67+/GFP mice. Microphotographs of coronal sections on the top give an overview. From top down images from CA1 and DG layers (CA1: Or, Rad, LMol; DG: Mol, ML) of each genotype are shown. The exemplary images are
taken from the same mouse, respectively. Scale bar, 100 µm. For abbreviations, see list
Fig. 4.
Tyrosine hydroxylase (TH)-, serotonin (5-HT)- and choline acetyltransferase (ChAT)-positive fiber densities in the dorsal hippocampal formation of GAD67+/+ and GAD67+/GFP mice. a–c For TH-IR fiber densities a significant main effect of GENOTYPE was found in a CA1 and c DG, but not b CA3. a For GAD67+/GFP the post hoc tests showed a significantly higher density of TH-IR fibers in all investigated layers of CA1 (Or, Rad and LMol). c For DG the alpha adjusted post hoc tests did not reach statistical significance. Investigating d–f 5-HT- and g–i ChAT-positive fiber densities, no differences between GAD67+/+ and GAD67+/GFP mice (n.s.) were found. Statistics: data are reported as mean ± SEM. Two-way repeated measures ANOVAs were performed using LAYER (three levels in dorsal CA1 and CA3: Or, Rad, Lm or two levels in DG: Mol, Hil) as within-subject factor and GENOTYPE (two levels: GAD67+/+ and GAD67+/GFP) as between-subject factor. Post hoc analyses were carried out using unpaired t tests with Bonferroni–Holm adjustment. For abbreviations, see list. *p < 0.05; **p < 0.01; ***p < 0.001
Serotonin-IR neurons in the raphe nuclei
Absolute neuron numbers, volumes, and neuronal densities of 5-HT-IR neurons were estimated in different raphe nuclei: DR, MnR and PMnR (Table 4). The two-way repeated-measures ANOVAs (within-subject factor: REGION; between-subject factor: GENOTYPE) revealed no significant interaction or main effects (n.s.). GAD67+/GFP and GAD67+/+ mice show no differences in the investigated raphe nuclei (see also supplementary Fig. S6).
Table 4.
Quantification of 5-HT-IR neurons and fiber densities in GAD67+/+ and GAD67+/GFP mice
| 5-HT-IR neurons and fiber densities | Statistical analysis | ||||||
|---|---|---|---|---|---|---|---|
| 5-HT-IR neurons | Region | Layer | GAD67+/+ (n = 6) | GAD67+/GFP (n = 6) | REGION/LAYER × GENOTYPE | Effect of GENOTYPE | Post hoc |
| Neuron number [n] | DR | 1812.13 ± 100.10 | 1841.95 ± 49.85 | – | |||
| MnR | 368.19 ± 17.21 | 344.92 ± 23.38 | F(1.06, 10.60) = 0.20, n.s | F(1, 10) = 0.01, n.s | – | ||
| PMnR | 153.17 ± 6.13 | 132.97 ± 9.52 | – | ||||
| Volume [mm3] | DR | 0.09 ± 0.00 | 0.10 ± 0.00 | – | |||
| MnR | 0.03 ± 0.00 | 0.03 ± 0.00 | F(2, 20) = 1.70, n.s | F(1, 10) = 0.08, n.s | – | ||
| PMnR | 0.06 ± 0.00 | 0.06 ± 0.00 | – | ||||
| Density [n/mm3] | DR | 19643.03 ± 846.27 | 19120.55 ± 467.40 | – | |||
| MnR | 12348.37 ± 819.33 | 11293.96 ± 791.83 | F(2, 20) = 0.32, n.s | F(1, 10) = 0.91, n.s | – | ||
| PMnR | 2615.53 ± 118.65 | 2370.43 ± 197.58 | – | ||||
| 5-HT-IR fiber densities [μm/μm]3 | |||||||
| Dorsal hippocampus | CA1 | Or | 0.05 ± 0.01 | 0.06 ± 0.00 | F(1.19, 11.90) = 3.25, n.s | F(1, 10) = 2.55, n.s | – |
| Rad | 0.06 ± 0.01 | 0.05 ± 0.00 | – | ||||
| LMol | 0.08 ± 0.02 | 0.10 ± 0.02 | – | ||||
| CA3 | Or | 0.09 ± 0.03 | 0.07 ± 0.01 | F(1.33, 13.33) = 4.34, p < 0.05 | F(1, 10) = 0.11, n.s | – | |
| Rad | 0.08 ± 0.03 | 0.08 ± 0.01 | – | ||||
| LMol | 0.07 ± 0.02 | 0.08 ± 0.01 | – | ||||
| DG | Mol | 0.06 ± 0.01 | 0.07 ± 0.02 | F(1, 10) = 0.95, n.s | F(1, 10) = 0.00, n.s | – | |
| ML | 0.08 ± 0.03 | 0.08 ± 0.01 | – | ||||
| Amygdala | BLA | 0.21 ± 0.01 | 0.20 ± 0.01 | F(3, 30) = 1.26, n.s | F(1, 10) = 0.02, n.s | – | |
| CeC | 0.19 ± 0.01 | 0.18 ± 0.01 | – | ||||
| CeM | 0.21 ± 0.00 | 0.20 ± 0.01 | – | ||||
| La | 0.18 ± 0.02 | 0.20 ± 0.01 | – | ||||
GAD67+/+ and GAD67+/GFP mice were investigated for differences in densities of serotonin (5-HT)-immunoreactive (IR) neurons in the raphe nuclei and densities of 5-HT-IR fibers in dorsal hippocampus and amygdala. Values represent mean ± SEM. Differences between in GAD67+/+ and GAD67+/GFP littermates were evaluated using repeated-measures ANOVAs with LAYER or REGION (two to four levels, depending on the investigated brain region) as the within-subject factors and GENOTYPE (two levels: GAD67+/+and GAD67+/GFP) as the between-subject factor. The interactions and main effects of GENOTYPE are shown. Post hoc analyses were performed using unpaired t tests (Welch’s test) with Bonferroni–Holm adjustment. For abbreviations, see list
Serotonin-positive fibers in dorsal hippocampus and amygdala
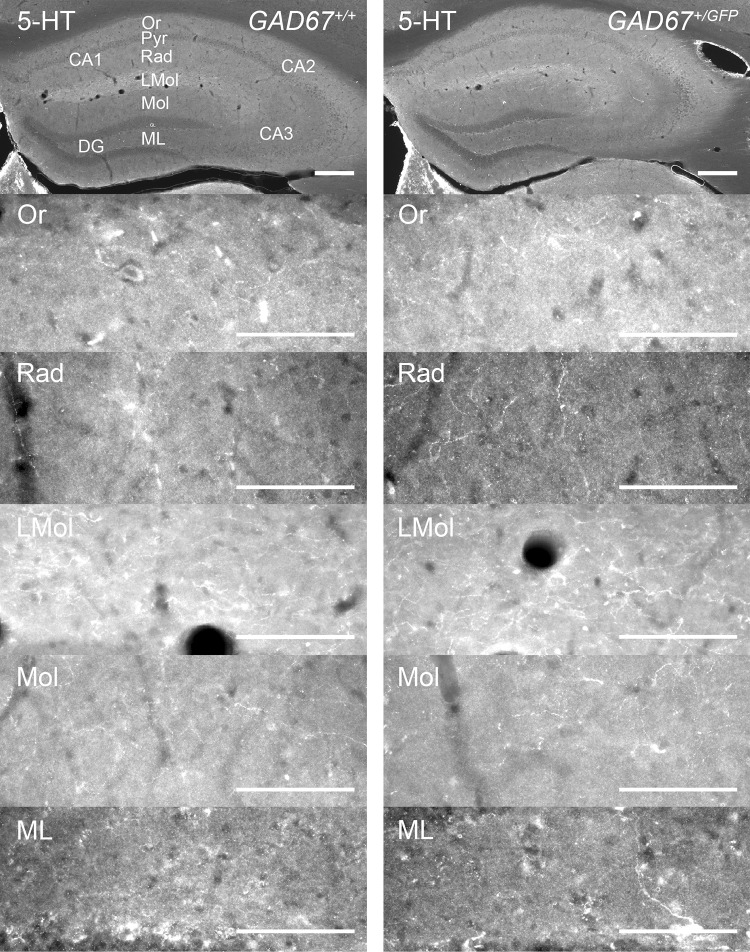
We further analyzed if GAD67+/GFP and GAD67+/+ mice show differences in densities of 5-HT-IR fibers in layers of dorsal hippocampal CA1, CA3 (Or, Rad, LMol) and DG (Mol, ML), respectively (Table 4; Figs. 4d–f and 5). For CA1 and DG (Fig. 4d, f) two-way repeated-measures ANOVAs (within-subject factor: LAYER; between-subject factor: GENOTYPE) revealed no significant interactions or main effects (n.s.). With reference to CA3 (Fig. 4e) the two-way repeated-measures ANOVA showed a significant interaction of LAYER and GENOTYPE (F(1.33, 13.33) = 4.34, p < 0.05) but no main effect for GENOTYPE (n.s.). Additionally, in the amygdala (Table 4) no significant interaction or main effect was found (ANOVA, n.s). Therefore, GAD67+/GFP and GAD67+/+ mice show comparable 5-HT-IR fiber densities in the investigated subregions of dorsal hippocampus and amygdala.
Fig. 5.
Microphotographs of coronal sections showing the distribution of 5-HT-IR fibers in dorsal hippocampus of GAD67+/+ and GAD67+/GFP mice. Images on the top give an overview. From top down images from CA1 and DG layers (CA1: Or, Rad, LMol; DG: Mol, ML) of genotype line are shown. The exemplary images are
taken from the same mouse, respectively. Scale bar, 100 µm. For abbreviations, see list
Choline acetyltransferase-IR neurons in septal region
To determine if GAD67+/GFP and GAD67+/+ littermates show differences in markers of the cholinergic system, we investigated both genotypes for neuronal numbers, volumes and neuronal densities of ChAT-positive neurons in medial (MS) and lateral (LS) septal region (see Table 5). For neuron number a two-way repeated-measures ANOVA (within-subject factor: REGION; between-subject factor: GENOTYPE) showed a significant interaction of REGION and GENOTYPE (F(1, 10) = 9.32, p < 0.05), but no significant effect of GENOTYPE (n.s.). Further, we examined both genotypes for differences in subregion volume and neuronal densities of MS and LS. Two-way repeated-measures ANOVAs (within-subject factor: REGION; between-subject factor: GENOTYPE) showed no significant interactions or main effects (n.s.), respectively. Therefore, GAD67+/GFP and GAD67+/+ littermates show comparable cholinergic markers in the investigated septal subregions (see also supplementary Fig. S7).
Table 5.
Quantification of ChAT-IR neurons and fiber densities in GAD67+/+ and GAD67+/GFP mice
| ChAT-IR neurons and fiber densities | Statistical analysis | ||||||
|---|---|---|---|---|---|---|---|
| ChAT-IR neurons | Region | Layer | GAD67+/+ (n = 6) | GAD67+/GFP (n = 6) | REGION/LAYER × GENOTYPE | Effect of GENOTYPE | Post hoc |
| Neuron number [n] | MS | 968.16 ± 61.90 | 1185.23 ± 54.63 | F(1, 10) = 9.32, p < 0,05 | F(1, 10) = 2.57, n.s | – | |
| LS | 755.67 ± 49.84 | 745.63 ± 42.49 | – | ||||
| Volume [mm3] | MS | 0.19 ± 0.02 | 0.22 ± 0.01 | F(1, 10) = 0.32, n.s | F(1, 10) = 1.51, n.s | – | |
| LS | 0.27 ± 0.03 | 0.30 ± 0.02 | – | ||||
| Density [n/mm3] | MS | 5312.56 ± 369.73 | 5331.96 ± 219.38 | F(1, 10) = 0.56, n.s | F(1, 10) = 0.17, n.s | – | |
| LS | 2857.53 ± 221.14 | 2576.45 ± 225.76 | – | ||||
| ChAT-IR fiber densities [μm/μm]3 | |||||||
| Dorsal hippocampus | CA1 | Or | 0.20 ± 0.01 | 0.19 ± 0.01 | F(2, 20) = 0.89, n.s | F(1, 10) = 0.04, n.s | – |
| Rad | 0.19 ± 0.02 | 0.20 ± 0.01 | – | ||||
| LMol | 0.15 ± 0.01 | 0.15 ± 0.01 | – | ||||
| CA3 | Or | 0.25 ± 0.01 | 0.26 ± 0.02 | F(2, 20) = 0.49, n.s | F(1, 10) = 1.61, n.s | – | |
| Rad | 0.24 ± 0.01 | 0.24 ± 0.01 | – | ||||
| LMol | 0.25 ± 0.01 | 0.26 ± 0.02 | – | ||||
| DG | Mol | 0.24 ± 0.03 | 0.24 ± 0.01 | F(1, 10) = 0.56, n.s | F(1, 10) = 0.00, n.s | – | |
| ML | 0.15 ± 0.02 | 0.15 ± 0.02 | – | ||||
| Amygdala | BLA | 0.59 ± 0.26 | 0.58 ± 0.12 | F(3, 30) = 0.22, n.s | F(1, 10) = 0.15, n.s | – | |
| CeC | 0.20 ± 0.01 | 0.19 ± 0.02 | – | ||||
| CeM | 0.38 ± 0.03 | 0.35 ± 0.03 | – | ||||
| La | 0.56 ± 0.25 | 0.55 ± 0.18 | – | ||||
GAD67+/+ and GAD67+/GFP mice were investigated for differences in densities of choline acetyltransferase (Chat)-IR neurons in the septal area and densities of Chat-IR fibers in dorsal hippocampus and amygdala. Values represent mean ± SEM. Differences between GAD67+/+ and GAD67+/GFP littermates were evaluated using repeated-measures ANOVAs with LAYER or REGION (two to four levels, depending on the investigated brain region) as the within-subject factors and GENOTYPE (two levels: GAD67+/+ and GAD67+/GFP) as the between-subject factor. The interactions and main effects of GENOTYPE are shown. Post hoc analyses were performed using unpaired t tests (Welch’s test) with Bonferroni–Holm adjustment. For abbreviations, see list
Choline acetyltransferase-positive fibers in dorsal hippocampus and amygdala
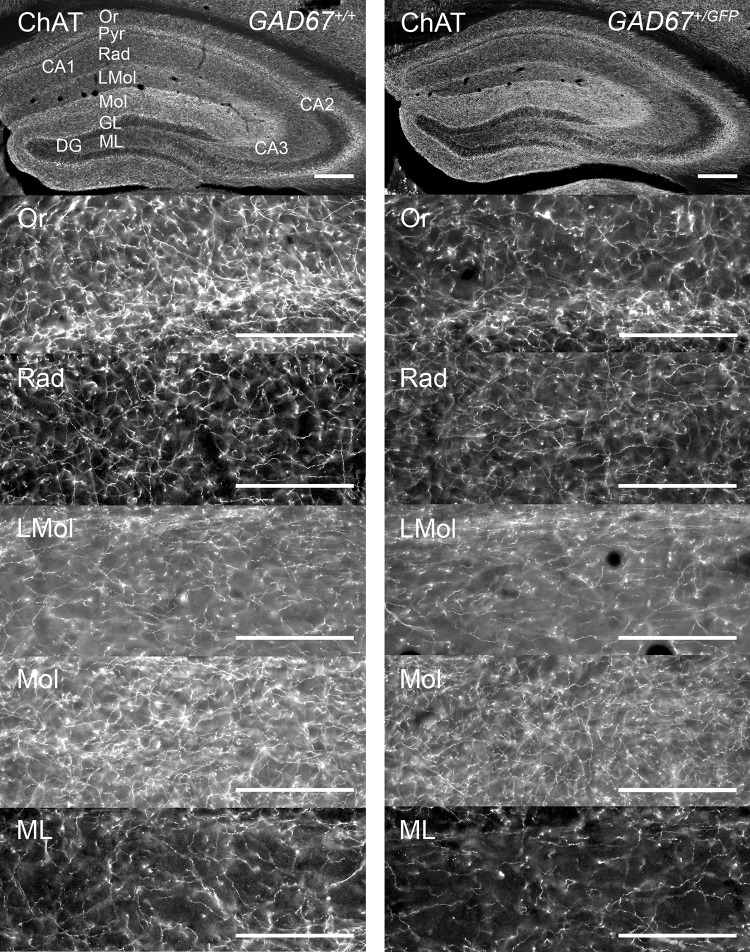
To investigate the cholinergic fiber systems of both genotypes, we estimated the densities of ChAT-IR fibers in dorsal hippocampal layers of CA1, CA3 (Or, Rad, LMol) and DG (Mol, ML), respectively (Table 5, Figs. 4g–i, 6). However, the two-way repeated-measures ANOVAs (within-subject factor: LAYER; between-subject factor: GENOTYPE) revealed no interactions or main effects (n.s), respectively (Fig. 4g–i). Additionally, in the amygdala (Table 5) no significant interactions or main effect (ANOVA, n.s.) were found. Thus, GAD67+/GFP and GAD67+/+ mice show no differences in ChAT-IR fiber densities in the investigated subregions of dorsal hippocampus and amygdala.
Fig. 6.
Distribution of ChAT-IR fibers in dorsal hippocampus of GAD67+/+ and GAD67+/GFP mice. Microphotographs of coronal sections on the top give an overview. From top down images from layers (CA1: Or, Rad, LMol; DG: Mol, ML) of each genotype are shown. The exemplary images are
taken from the same mouse, respectively. Scale bar, 100 µm. For abbreviations, see list
Discussion
In this study, we address the potential contribution of deficits in GAD67-mediated GABA synthesis in mice to behavioral and morphological phenotypes reminiscent of schizophrenia pathology. Our data reveal a profound alteration in GAD67+/GFP mice in the socio-emotional domain, including an altered vulnerability to social deprivation and a selective catecholaminergic, presumably dopaminergic, hyperinnervation of area CA1 in the dorsal hippocampus.
Social behavior, locomotor activity, anxiety and sensorimotor gating in GAD67+/GFP mice
Negative symptoms like social withdrawal, avolition and depressive-like behavior are common patterns of human schizophrenia (Samsom and Wong 2015) and also apparent in animal models (Ellenbroek and Cools 2000; Matrisciano et al. 2013). Complex social behaviors and emotional processing such as anxiety are depending on different cerebral cortical areas, the hippocampus and the amygdala (Bicks et al. 2015). On the other hand, motivation and execution of motor plans are processed along parallel cortico-striatal-thalamic-cortical loops (Haber 2003). Alterations in those complex circuits could affect social behavior, locomotor activity, anxiety and other behavioral traits (Guillin et al. 2007). The social interaction test in the open field allows analyzing spontaneous induced behavior largely independent from external influences (File and Seth 2003). Our behavioral analysis showed that GAD67+/GFP mice spent less time in social contact, marker of a profound disturbance in social interaction. Importantly, this alteration was independent from general motor activity, represented by a comparable distance moved of both genotypes in the open field. Our findings are in accordance with data from our previous study investigating sociability in GAD67+/GFP mice using the three-chamber social preference test (Sandhu et al. 2014). Additionally, GAD67+/GFP and GAD67+/+ mice where tested in the tube test for social dominance. This test was originally developed to analyze social hierarchy in mice through the measurement of aggression (Lindzey et al. 1961), which is known to be modulated by GABAergic function (Miczek et al. 2003). Independent from grouped and isolated housing conditions, GAD67+/GFP retreated more frequently than GAD67+/+ mice suggesting a reduced social dominance. This finding is supported by data from our previous study, showing decreased aggressive like behavior of GAD67+/GFP mice in the resident intruder test (Sandhu et al. 2014). However, we found no genotype differences in the number of aggressive contacts in the social interaction test, which may reflect different forms of social behavior and aggression in these two tasks (Nelson and Chiavegatto 2000).
Social behavior in rodents is highly complex and greatly depends on olfaction (Schultz and Tapp 1973). In our previous study, we found that male GAD67+/GFP mice show a reduced preference for female mice and a reduced sensitivity to social and non-social odors, maybe reflecting an altered olfactory sense. As demonstrated by expression of the immediate early gene c-Fos we found an unaltered activation of the olfactory bulb in GAD67+/GFP mice, but decreased neuronal activation in amygdala, bed nucleus of stria terminalis, medial preoptic area and lateral septum. It can be assumed, that GAD67+/GFP mice show a disturbance in detection or, more importantly, processing of olfactory stimuli in downstream circuits relevant for social behavior (Sandhu et al. 2014). GAD67+/GFP mice additionally showed altered non-social behavior, an increased rearing activity, in the social interaction test. This could be result of an impaired exploratory motor behavior and habituation of exploration in a novel environment, also reported for the DISC1 animal model of schizophrenia (Walsh et al. 2012). The social interaction test in the open field additionally allowed us to investigate the effects of post-weaning isolation rearing. Isolated male mice of both genotypes showed increased investigation of the unfamiliar test partner shown by higher numbers in sniffing, anogenital sniffing and aggressive contacts. This could be more result of the social isolation rearing (Shoji and Mizoguchi 2011) rather than of GAD67 haplodeficiency. Isolated mice also displayed an increased passive social behavior, which is seen when animals are sitting or lying close to each other, but without direct interaction (Sams-Dodd 1995). Additionally, we found lower rearing activity and increased repetitive self-grooming in isolated GAD67+/GFP and GAD67+/+ mice. Our current results reveal that independent from genotype social isolation rearing increases stress and additionally alters specific aspects of explorative and social behavior. The latter is supported by the finding, that GAD67 haplodeficiency itself provokes higher corticosterone levels and enhances maternal and fetal stress vulnerability (Uchida et al. 2011). However, it is important to mention that not only genetic manipulations or social isolation stress could compromise the behavioral phenotype of an animal model. Additionally, the choice of the genetic background strain might influence the outcome of different tests paradigms. This is important, since it was shown, that the genetic background strain influences locomotor activity and anxiety like behavior (Bothe et al. 2005; Voikar et al. 2005).
Negative symptoms of schizophrenia-like poor social drive are commonly associated with depressive-like behavior (Häfner et al. 1999). To analyze depressive-like behavior, we investigated GAD67+/GFP and GAD67+/+ mice in the forced swim test (FST) which is based on the assumption that immobility reflects a measure of behavioral despair (Borsini and Meli 1988). GAD67+/GFP mice showed increased time spent in immobility, indicating an increase of depressive-like behavior. It was shown that the administration of low doses GABA or GABA agonists can ameliorate forced swimming induced depressive-like behavior and are able to potentiate the effect of antidepressants (Borsini et al. 1988; Aley and Kulkarni 1989). On the other hand, GABA antagonists like picrotoxin increase the immobility in FST (Poncelet et al. 1987). Therefore, we suggest GAD67 haplodeficiency provokes depressive-like behavior as found in neuropsychiatric diseases. Social isolation only resulted in higher latency to the first immobility period but did not affect immobility in the FST in general. This is in line with studies (Yates et al. 1991; Hall et al. 1998; Simpson et al. 2012) showing that social isolation in rodents has no effect on immobility in the FST or can promote despair-like immobility only when isolated in a short period of time during early brain development (17–21 day-old animals). Interestingly, group-housed GAD67+/GFP, compared to GAD67+/+ mice, showed an increased climbing behavior in the FST, which is reported to be a predictor of an increased motor activity in this test (Lino-de-Oliveira et al. 2005; Vieira et al. 2008). Therefore, we suggest that GAD67+/GFP show an increased motor activity in FST, possible correlate of the positive symptom domain of schizophrenia (Jones et al. 2011).
Comorbid anxiety disorders are present in more than one third of patients with schizophrenia (Pokos and Castle 2006). The elevated plus maze (EPM) is commonly used as a behavioral assay to study anxiety like behavior in animal models (Walf and Frye 2007). However, our results reveal no differences in time spent in the open arms or locomotor activity between GAD67+/GFP and GAD67+/+ mice. This is further supported the study of Smith (2018) reporting no differences in anxiety related behavior of GAD67+/GFP on EPM. Modulation of the GABAergic system was shown to affect anxiety like behavior on EPM. GABAA receptor agonists like diazepam or chlordiazepoxide, increase the proportion of time spent in open arms on EPM, whereas GABAA-receptor antagonists like picrotoxin reduce this measure (Lister 1987; Rodgers et al. 1992). It is possible that the decrease in GAD67 is not significant enough to provoke alterations of anxiety like behavior in GAD67+/GFP mice. This assumption is partially supported by the finding, that GAD67+/GFP mice showed no alterations in exploring the unprotected center area in social interaction task in the open field. Therefore, GAD67 haplodeficiency alone or in combination with social isolation rearing did not affect anxiety related behavior of GAD67+/GFP mice, as demonstrated in the EPM and open-field test (Sandhu et al. 2014; Smith 2018). Thus, GAD67+/GFP mice may not be a suitable model to investigate the anxiety-related symptom domain of neuropsychiatric disorders.
Reductions of GAD67 and PARV in mouse cerebral cortex and hippocampus are associated with novelty-induced hyperlocomotion (Belforte et al. 2010) considered to be a sign of schizophrenia-like behavior in human and mutant mice (Laviola et al. 2006). However, spontaneous locomotor activity and motor coordination in social interaction and rotarod test were not affected in GAD67+/GFP mice, indicating preserved motor functions. Our findings are supported in part by the work of Smith (2018). This study also showed no genotype differences in EPM test, but showed a mild hyperactivity of GAD67+/GFP mice in the open field may be result of a longer duration of testing. Social isolation stress induced higher locomotor activity and total arm entries of isolated GAD67+/GFP mice on EPM, compared to group-housed GAD67+/GFP. This effect was not significant between isolated and group-housed GAD67+/+ mice. Since it was shown that postweaning social isolation affects GABAergic function (Hickey et al. 2012; Lim et al. 2012) and increases locomotor behavior on EPM (Abramov et al. 2004; Voikar et al. 2005), it is likely that the decrease of GAD67 alone is not significant enough to alter locomotor activity in GAD67+/GFP mice. Therefore, we suggest that the additional exposure to social isolation stress as a “second hit” increases the vulnerability of a GAD67 haplodeficiency in GAD67+/GFP mice, which results in impaired locomotor activity on the EPM. By contrast, spontaneous locomotor activity of isolated GAD67+/GFP was not increased in the social interaction test in the open field.
It is unlikely that social interaction or aggression is reduced in GAD67+/GFP mice because of a general deficit in sensorimotor function. Since several neuropsychiatric diseases including schizophrenia are accompanied by deficits in sensory information-processing (Braff et al. 2001), we analyzed GAD67+/GFP mice and controls for their startle response (ASR) and prepulse inhibition (PPI). However, we found no genotype differences, which suggest that GAD67 haplodeficiency has no influence on ASR and PPI. By contrast, mice lacking GAD67 primarily in PARV-IR neurons show a reduction in PPI indicating the important role of these neurons in sensorimotor gating (Fujihara et al. 2015). It is possible, that the reduction of GAD67 and consequently GABA in GAD67+/GFP mice is too small to cause sensorimotor gating deficits (Kolata et al. 2018). Since social isolation is used to model deficient sensorimotor gating in schizophrenia (Varty et al. 2006), we assumed that isolation rearing disrupts sensorimotor gating in our mice. Interestingly, neither GAD67+/GFP nor GAD67+/+ mice showed ASR or PPI deficits as a result of post-weaning isolation housing. In consequence, we varied the parameters for PPI measurement. First, we increased the interval between prepulse and pulse to 400 ms and, second, varied the prepulse intensities (70, 75 and 80 dB SPL). Additionally, no genotype or housing differences were found (data not shown). We conclude that social isolation does not affect startle activity or sensorimotor gating in GAD67+/GFP and GAD67+/+ mice, which is in line with other studies showing that social isolation during the critical developmental period has no effect on sensorimotor gating (Pietropaolo et al. 2008; Kulesskaya et al. 2011). However, PPI deficiency could be compromised by the duration of social isolation housing (Tueting et al. 2008), resulting in a comparatively strong decrease of GABA expression levels in GAD67+/+ control mice. Thus, long-term social isolation in adulthood could obscure the PPI deficits (Tueting et al. 2008) in GAD67+/GFP mice compared to GAD67+/+, which could explain the negative findings in the present study.
Tyrosine hydroxylase-IR neurons and fibers
Dopaminergic dysfunction in association with GAD67 deficiency is implicated in the pathophysiology of schizophrenia and associated with alterations in the hippocampus and amygdala (Laviolette 2007; Lodge and Grace 2011b; Brisch et al. 2014). Therefore, we were interested if GAD67 haplodeficiency is accompanied by alterations of the dopaminergic system. Tyrosine hydroxylase (TH) catalyzes the first and rate-determining step of the catecholamine biosynthesis and is expressed in all catecholaminergic neurons. Therefore, antibodies against TH-positive structures denote dopaminergic as well as noradrenergic neurons and fibers, but with regional differences (Asan 1993). We used tyrosine hydroxylase (TH)-immunoreactivity (IR) as a marker for dopamine since it is known to predominantly represent mesencephalic DAergic input in hippocampal CA1 of mice (Walling et al. 2012; for discussion see Nullmeier et al. 2014). In the amygdala, regionally different DAergic and noradrenergic innervation patterns are described and TH-IR fibers are found to be predominantly dopaminergic afferent fibers (Asan 1997). GAD67+/GFP mice, compared to GAD67+/+, showed no difference in density of TH-IR neurons and volume of substantia nigra (SN) and ventral tegmental area (VTA). However, we found that GAD67+/GFP mice exhibit a significantly higher density of TH-IR fibers in CA1 subfield of dorsal hippocampus, which can be interpreted as a hypercatecholaminergic, presumably hyperdopaminergic, innervation. Additionally, we investigated both genotypes for differences in TH-IR fiber density in the amygdala, but could not find any differences in the investigated subdivisions (Asan 1997).
It has been shown, that the dorsal hippocampus is crucial for spatial and long-term memories (Lodge and Grace 2011b; Brisch et al. 2014; Ragland et al. 2017) and modulates anxiogenic effects in the social interaction test (File et al. 1998). Additionally, dopamine D1-receptors in dorsal hippocampus were reported to mediate social learning and social behaviors in mice (Matta et al. 2017). Thus, it is possible, that the deficits in social interaction of GAD67+/GFP mice are consequence of a dysfunction of the hippocampal dopaminergic system. However, we only found alterations in dorsal hippocampal CA1, but not in the amygdala and dopaminergic midbrain regions. It is likely, that the higher density of TH-IR fibers in CA1 of GAD67+/GFP mice is induced locally by a GABAergic deficit in the hippocampus.
GAD67 reduction in the hippocampus is expected to lead to hyperactivity of midbrain dopamine neurons via a polysynaptic pathway (Kalkman and Loetscher 2003; Lodge and Grace 2011b). This appears to be mediated especially by a dysregulation of PARV-containing neurons (Lodge and Grace 2011a). A recent study, investigating GAD67 haplodeficiency showed that maternal stress postnatally decreases especially the density of PARV-positive GABAergic neurons in prefrontal cortex, hippocampus and somatosensory cortex of GAD67+/GFP mice (Uchida et al. 2014). As a consequence, GABAergic dysfunction in hippocampus could lead to alterations in the dopaminergic pathways and subsequently social behavior, as found in the present study. Additionally, it can be assumed that alterations of local GABAergic circuits in VTA could provoke dopaminergic alterations. Thus, hyperdopaminergic innervation in association with a disturbance in social (Sandhu et al. 2014) and depressive-like behavior may mimic important aspects of neuropsychiatric disorders like schizophrenia (Lisman et al. 2008; Grace 2012).
Serotonin-IR and choline acetyltransferase-IR neurons and fibers
The serotonergic and cholinergic systems are found to be involved in schizophrenia (Raedler et al. 2007; Geyer and Vollenweider 2008) and are in close interaction with the DAergic and GABAergic neurotransmitter systems (Kapur and Remington 1996; Scarr et al. 2013). Therefore, we were interested if GAD67 haplodeficiency is accompanied by alterations in both systems. However, GAD67+/GFP and GAD67+/+ mice showed no differences in the density of the 5-HT positive neurons and volumes of the investigated raphe nuclei. The same applied to the density of ChAT-IR neurons and volumes of the septal nuclei. Additionally, we found no alterations in the densities of 5-HT-IR and ChAT-IR fibers in hippocampus and amygdala of GAD67+/GFP mice. However, our data will only provide a descriptive morphological analysis and until now, impairments of the serotonergic or cholinergic system in GAD67+/GFP mice have not been reported in the literature. Therefore, at the moment, it is difficult to make clear statements about the impact of GAD67 haplodeficiency on both neurotransmitter systems and vice versa.
GAD67+/GFP as a mouse model of schizophrenia and major depressive disorder
Schizophrenia and major depressive disorder (MDD) are considered two distinct neuropsychiatric diseases. However, there is an overlap between negative symptoms of schizophrenia and certain depressive symptoms like anhedonia, avolition and social withdrawal (Siris et al. 1988). Additionally, both disorders were shown to share alterations in biological markers of GABAergic transmission. Similar to schizophrenia, reductions of cortical GAD67 expression and alterations in GABAA and GABAB receptor levels were reported in MDD (Fatemi et al. 2005; Abdallah et al. 2015; Fogaca and Duman 2019). In contrast to schizophrenia, cortical GABA levels appear mostly decreased in MDD (Sanacora et al. 1999; Tayoshi et al. 2010; Luscher and Fuchs 2015). It is suggested that this reduction of GABA in MDD could not only result from decreased levels of GAD67, but could also result from a reduction in the density of specific GABA interneuron subclasses (reviewed in Fogaca and Duman 2019). This is supported by studies showing reduced volumes of prefrontal cortex and hippocampus (MacQueen et al. 2008; Savitz and Drevets 2009) and decreased cortical densities of calbindin- and somatostatin-positive GABAergic interneurons in MDD patients (Rajkowska et al. 2007; Sibille et al. 2011; Luscher and Fuchs 2015). PARV-positive interneurons play an important role in cognitive function, emotional response and social interaction (Ferguson and Gao 2018). However, the expression of parvalbumin (PARV) and density of PARV-positive interneurons mostly appear unaltered in MDD, which is in contrast to schizophrenia (reviewed in Fogaca and Duman 2019). On the other hand, rodent studies showed that the exposure to chronic stress or social isolation cause reductions of PARV-positive neurons in prefrontal cortex and hippocampus (Czeh et al. 2015, 2018; Todorovic et al. 2019). That maternal stress, in addition to a heterozygous deletion of GAD67, diminishes neurogenesis of GABAergic neurons was also shown in GAD67+/GFP mice. Consequently, this postnatally results in a decreased density of PARV-positive interneurons in hippocampus, prefrontal and somatosensory cortex of GAD67+/GFP mice (Uchida et al. 2014; Wang et al. 2018), similar to that found in human schizophrenia. Therefore, prenatal and social isolation stress could disturb the function of specific interneuron subpopulations and mechanism underlying the control of behaviors related to mood and emotion in neuropsychiatric disorders like schizophrenia and MDD. GAD67+/GFP mice may provide a useful model for studying the impact of a heterozygous deletion of GAD67 on an enhanced vulnerability to prenatal and social isolation stress.
Conclusion
Cerebral cortical and hippocampal GAD67 reductions are consistently reported in schizophrenia and other neuropsychiatric disorders (Akbarian et al. 1995; Guidotti et al. 2000; Benes and Berretta 2001; Hashimoto et al. 2003; Lewis et al. 2005). The present results suggest that GAD67 haplodeficiency in GAD67+/GFP mice provokes profound disturbances in social behavior, social dominance and depressive-like behavior, which may reflect negative symptoms found in human schizophrenia and symptoms of MDD. In addition, our findings indicate that GAD67 haplodeficiency and social isolation stress may have additive influences on risks for developing schizophrenia or MDD (Fone and Porkess 2008). GAD67 haplodeficiency is further accompanied by a selectively increased density of TH-positive fibers in dorsal hippocampal CA1 suggesting an alteration of the dopaminergic system upstream to GABAergic dysfunction. GAD67+/GFP mice are useful to model GABAergic hypofunction as disposition for the development of neuropsychiatric disorders like schizophrenia and MDD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Open Access funding provided by Projekt DEAL. The experiments were conducted with the technical equipment of the Institute of Anatomy of the University of Magdeburg, Germany and the Institute of Molecular and Cellular Anatomy at Ulm University, Germany. This work was partially financed by grants from the Deutsche Forschungsgemeinschaft (SFB779 TP B05 to O.S. and H.S.). We gratefully thank A. Kröber, S. Vorwerk and J. Andratschke for excellent technical assistance. The authors reported no financial interests or potential conflicts of interest.
Abbreviations
- 5-HT
Serotonin
- ASR
Acoustic startle response
- BLA
Basolateral amygdala
- CA1-3
Hippocampal cornu ammonis regions
- CeC
Central amygdaloid nucleus, capsular part
- CeM
Central amygdaloid nucleus, medial division
- ChAT
Choline acetyltransferase
- CI
Confidence interval
- CPu
Caudate–putamen
- DA
Dopamine
- DG
Dentate gyrus
- DR
Dorsal nucleus raphe
- EPM
Elevated plus maze
- Exp(B)
Odds ratio
- FST
Forced swim test
- GAD
Glutamic acid decarboxylase
- GAD67+/+
Wild type
- GAD67+/GFP
GAD67–GFP Knock-in mice
- GL
Granule cell layer of dentate gyrus
- IR
Immunoreactivity
- La
Lateral amygdala
- LMol
Stratum lacunosum moleculare
- LS
Medial septum
- MDD
Major depressive disorder
- ML
Multiform layer of dentate gyrus
- MnR
Median raphe nucleus
- Mol
Stratum moleculare of dentate gyrus
- MS
Medial septum
- NAc
Nucleus accumbens
- NMDA-R
N-methyl-d-aspartate receptors
- Or
Stratum oriens
- PARV
Parvalbumin
- PFC
Prefrontal cortex
- PMnR
Paramedian raphe nucleus
- PPI
Prepulse inhibition of acoustic startle response
- Py
Stratum pyramidale
- Rad
Stratum radiatum
- SEM
Standard error of the mean
- SN
Substantia nigra
- SNC
Substantia nigra pars compacta
- SNL
Substantia nigra pars lateralis
- SNR
Substantia nigra pars reticulata
- TH
Tyrosine hydroxylase
- VTA
Ventral tegmental area
Funding
This work was partially financed by grants from the Deutsche Forschungsgemeinschaft (SFB779 TP B05 to O.S. and H.S.).
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interests or potential conflicts of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. The experimental animal procedures were approved by the local authorities of Sachsen-Anhalt/Germany (AZ: 42502–2-1134 UniMD) and carried out in accordance with the European Communities Council Directive (2010/63/EU). No human participants were used in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sven Nullmeier, Email: sven.nullmeier@uni.ulm.de.
Christoph Elmers, Email: christoph.elmers@web.de.
Wolfgang D’Hanis, Email: wolfgang.dhanis@med.ovgu.de.
Kiran Veer Kaur Sandhu, Email: kv.sandhu7@gmail.com.
Oliver Stork, Email: oliver.stork@ovgu.de.
Yuchio Yanagawa, Email: yuchio@gunma-u.ac.jp.
Patricia Panther, Email: patricia.panther@uniklinik-ulm.de.
Herbert Schwegler, Email: herbert.schwegler@web.de.
References
- Abdallah CG, Jackowski A, Sato JR, Mao X, Kang G, Cheema R, Coplan JD, Mathew SJ, Shungu DC. Prefrontal cortical GABA abnormalities are associated with reduced hippocampal volume in major depressive disorder. Eur Neuropsychopharmacol. 2015;25(8):1082–1090. doi: 10.1016/j.euroneuro.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Abramov U, Raud S, Kõks S, Innos J, Kurrikoff K, Matsui T, Vasar E. Targeted mutation of CCK2 receptor gene antagonises behavioural changes induced by social isolation in female, but not in male mice. Behav Brain Res. 2004;155(1):1–11. doi: 10.1016/j.bbr.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52(4):258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Aley KO, Kulkarni SK. GABA-mediated modification of despair behavior in mice. Naunyn Schmiedebergs Arch Pharmacol. 1989;339(3):306–311. doi: 10.1007/bf00173583. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Swayze VW, Tyrrell G, Arndt S. Positive and negative symptoms in schizophrenia A critical reappraisal. Arch Gen Psychiatry. 1990;47(7):615–621. doi: 10.1001/archpsyc.1990.01810190015002. [DOI] [PubMed] [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci USA. 1997;94(12):6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asan E. Comparative single and double immunolabelling with antisera against catecholamine biosynthetic enzymes: criteria for the identification of dopaminergic, noradrenergic and adrenergic structures in selected rat brain areas. Histochemistry. 1993;99(6):427–442. doi: 10.1007/BF00274095. [DOI] [PubMed] [Google Scholar]
- Asan E. Ultrastructural features of tyrosine-hydroxylase-immunoreactive afferents and their targets in the rat amygdala. Cell Tissue Res. 1997;288(3):449–469. doi: 10.1007/s004410050832. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Falkai P, Maier W. Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the "two hit hypothesis". JPsychiatrRes. 1999;33(6):543–548. doi: 10.1016/S0022-3956(99)00039-4. [DOI] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13(1):76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25(1):1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Bicks LK, Koike H, Akbarian S, Morishita H. Prefrontal cortex and social cognition in mouse and man. Front Psychol. 2015;6:1805. doi: 10.3389/fpsyg.2015.01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsini F, Mancinelli A, D'Aranno V, Evangelista S, Meli A. On the role of endogenous GABA in the forced swimming test in rats. Pharmacol Biochem Behav. 1988;29(2):275–279. doi: 10.1016/0091-3057(88)90156-6. [DOI] [PubMed] [Google Scholar]
- Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology. 1988;94(2):147–160. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG (2005) Behavioral differences among fourteen inbred mouse strains commonly used as disease models. Comp Med 55(4):326–334. https://www.ncbi.nlm.nih.gov/pubmed/16158908 [PubMed]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156(2–3):234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Brisch R, Saniotis A, Wolf R, Bielau H, Bernstein HG, Steiner J, Bogerts B, Braun K, Jankowski Z, Kumaratilake J, Henneberg M, Gos T. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front Psychiatry. 2014;5:47. doi: 10.3389/fpsyt.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, McKenna JT, Winston S, Basheer R, Yanagawa Y, Thakkar MM, McCarley RW. Characterization of GABAergic neurons in rapid-eye-movement sleep controlling regions of the brainstem reticular formation in GAD67-green fluorescent protein knock-in mice. Eur J Neurosci. 2008;27(2):352–363. doi: 10.1111/j.1460-9568.2008.06024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Vardya I, Varga Z, Febbraro F, Csabai D, Martis LS, Hojgaard K, Henningsen K, Bouzinova EV, Miseta A, Jensen K, Wiborg O. Long-term stress disrupts the structural and functional integrity of GABAergic neuronal networks in the medial prefrontal cortex of rats. Front Cell Neurosci. 2018;12:148. doi: 10.3389/fncel.2018.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Varga ZK, Henningsen K, Kovacs GL, Miseta A, Wiborg O. Chronic stress reduces the number of GABAergic interneurons in the adult rat hippocampus, dorsal-ventral and region-specific differences. Hippocampus. 2015;25(3):393–405. doi: 10.1002/hipo.22382. [DOI] [PubMed] [Google Scholar]
- de Jonge JC, Vinkers CH, Hulshoff Pol HE, Marsman A. GABAergic mechanisms in schizophrenia: linking postmortem and in vivo studies. Front Psychiatry. 2017;8:118–118. doi: 10.3389/fpsyt.2017.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek BA, Cools AR. Animal models for the negative symptoms of schizophrenia. Behav Pharmacol. 2000;11(3–4):223–233. doi: 10.1097/00008877-200006000-00006. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Stary JM, Earle JA, Araghi-Niknam M, Eagan E (2005) GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. SchizophrRes 72(2–3):109–122. http://www.ncbi.nlm.nih.gov/pubmed/15560956 [DOI] [PubMed]
- Ferguson BR, Gao W-J. PV interneurons: critical regulators of E/I balance for prefrontal cortex-dependent behavior and psychiatric disorders. Front Neural Circuits. 2018;12:37–37. doi: 10.3389/fncir.2018.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Kenny PJ, Ouagazzal AM. Bimodal modulation by nicotine of anxiety in the social interaction test: role of the dorsal hippocampus. Behav Neurosci. 1998;112(6):1423–1429. doi: 10.1037/0735-7044.112.6.1423. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463(1–3):35–53. doi: 10.1016/S0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Fogaca MV, Duman RS. Cortical GABAergic dysfunction in stress and depression: new insights for therapeutic interventions. Front Cell Neurosci. 2019;13:87. doi: 10.3389/fncel.2019.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32(6):1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Fujihara K, Miwa H, Kakizaki T, Kaneko R, Mikuni M, Tanahira C, Tamamaki N, Yanagawa Y. Glutamate decarboxylase 67 deficiency in a subset of GABAergic neurons induces schizophrenia-related phenotypes. Neuropsychopharmacology. 2015;40(10):2475–2486. doi: 10.1038/npp.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin PL, Toledo-Rodriguez M, Alexander SP, Fone KC. Down-regulation of hippocampal genes regulating dopaminergic, GABAergic, and glutamatergic function following combined neonatal phencyclidine and post-weaning social isolation of rats as a neurodevelopmental model for schizophrenia. Int J Neuropsychopharmacol. 2016;19(11):62. doi: 10.1093/ijnp/pyw062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Vollenweider FX. Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci. 2008;29(9):445–453. doi: 10.1016/j.tips.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Glausier JR, Kimoto S, Fish KN, Lewis DA. Lower glutamic acid decarboxylase 65-kDa isoform messenger RNA and protein levels in the prefrontal cortex in schizoaffective disorder but not schizophrenia. Biol Psychiatry. 2015;77(2):167–176. doi: 10.1016/j.biopsych.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Dopamine system dysregulation by the hippocampus: implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology. 2012;62(3):1342–1348. doi: 10.1016/j.neuropharm.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57(11):1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Guillin O, Abi-Dargham A, Laruelle M. Neurobiology of dopamine in schizophrenia. Int Rev Neurobiol. 2007;78:1–39. doi: 10.1016/S0074-7742(06)78001-1. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26(4):317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Häfner H, Löffler W, Maurer K, Hambrecht M, Wad H. Depression, negative symptoms, social stagnation and social decline in the early course of schizophrenia. Acta Psychiatr Scand. 1999;100(2):105–118. doi: 10.1111/j.1600-0447.1999.tb10831.x. [DOI] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GF, Pert A. The effects of social isolation on the forced swim test in Fawn hooded and Wistar rats. J Neurosci Methods. 1998;79(1):47–51. doi: 10.1016/S0165-0270(97)00155-6. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23(15):6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey AJ, Reynolds JN, Beninger RJ. Post-weaning social isolation and subchronic NMDA glutamate receptor blockade: effects on locomotor activity and GABA signaling in the rat suggest independent mechanisms. Pharmacol Biochem Behav. 2012;101(2):231–238. doi: 10.1016/j.pbb.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35(3):549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janitzky K, Schwegler H, Krober A, Roskoden T, Yanagawa Y, Linke R. Species-relevant inescapable stress differently influences memory consolidation and retrieval of mice in a spatial radial arm maze. Behav Brain Res. 2011;219(1):142–148. doi: 10.1016/j.bbr.2010.12.032. [DOI] [PubMed] [Google Scholar]
- Jones CA, Watson DJ, Fone KC. Animal models of schizophrenia. Br J Pharmacol. 2011;164(4):1162–1194. doi: 10.1111/j.1476-5381.2011.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkman HO, Loetscher E. GAD(67): the link between the GABA-deficit hypothesis and the dopaminergic- and glutamatergic theories of psychosis. J Neural Transm (Vienna) 2003;110(7):803–812. doi: 10.1007/s00702-003-0826-8. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G. Serotonin-dopamine interaction and its relevance to schizophrenia. Am J Psychiatry. 1996;153(4):466–476. doi: 10.1176/ajp.153.4.466. [DOI] [PubMed] [Google Scholar]
- Kolata SM, Nakao K, Jeevakumar V, Farmer-Alroth EL, Fujita Y, Bartley AF, Jiang SZ, Rompala GR, Sorge RE, Jimenez DV, Martinowich K, Mateo Y, Hashimoto K, Dobrunz LE, Nakazawa K. Neuropsychiatric phenotypes produced by GABA reduction in mouse cortex and hippocampus. Neuropsychopharmacology. 2018;43(6):1445–1456. doi: 10.1038/npp.2017.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesskaya N, Rauvala H, Voikar V. Evaluation of social and physical enrichment in modulation of behavioural phenotype in C57BL/6J female mice. PLoS ONE. 2011;6(9):e24755. doi: 10.1371/journal.pone.0024755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Gaudino C, Marino R, Keller F. Paradoxical effects of prenatal acetylcholinesterase blockade on neuro-behavioral development and drug-induced stereotypies in reeler mutant mice. Psychopharmacology. 2006;187(3):331–344. doi: 10.1007/s00213-006-0426-z. [DOI] [PubMed] [Google Scholar]
- Laviolette SR. Dopamine modulation of emotional processing in cortical and subcortical neural circuits: evidence for a final common pathway in schizophrenia? Schizophr Bull. 2007;33(4):971–981. doi: 10.1093/schbul/sbm048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Lim AL, Taylor DA, Malone DT. A two-hit model: behavioural investigation of the effect of combined neonatal MK-801 administration and isolation rearing in the rat. J Psychopharmacol. 2012;26(9):1252–1264. doi: 10.1177/0269881111430751. [DOI] [PubMed] [Google Scholar]
- Lindzey G, Winston H, Manosevitz M. Social dominance in inbred mouse strains. Nature. 1961;191(4787):474–476. doi: 10.1038/191474a0. [DOI] [PubMed] [Google Scholar]
- Lino-de-Oliveira C, Lima TCMD, Carobrez AdP. Structure of the rat behaviour in the forced swimming test. Behav Brain Res. 2005;158(2):243–250. doi: 10.1016/j.bbr.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31(5):234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92(2):180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Developmental pathology, dopamine, stress and schizophrenia. Int J Dev Neurosci. 2011;29(3):207–213. doi: 10.1016/j.ijdevneu.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 2011;32(9):507–513. doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Fuchs T. GABAergic control of depression-related brain states. Adv Pharmacol. 2015;73:97–144. doi: 10.1016/bs.apha.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Yucel K, Taylor VH, Macdonald K, Joffe R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol Psychiatry. 2008;64(10):880–883. doi: 10.1016/j.biopsych.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48(6):1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Matrisciano F, Tueting P, Dalal I, Kadriu B, Grayson DR, Davis JM, Nicoletti F, Guidotti A. Epigenetic modifications of GABAergic interneurons are associated with the schizophrenia-like phenotype induced by prenatal stress in mice. Neuropharmacology. 2013;68:184–194. doi: 10.1016/j.neuropharm.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta R, Tiessen AN, Choleris E. The role of dorsal hippocampal dopamine D1-type receptors in social learning, social interactions, and food intake in male and female mice. Neuropsychopharmacology. 2017;42(12):2344–2353. doi: 10.1038/npp.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Stahl SM. The dopamine hypothesis of schizophrenia: a review. Schizophr Bull. 1976;2(1):19–76. doi: 10.1093/schbul/2.1.19. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Weerts E, Haney M, Tidey J. Neurobiological mechanisms controlling aggression: preclinical developments for pharmacotherapeutic interventions. Neurosci Biobehav Rev. 1994;18(1):97–110. doi: 10.1016/0149-7634(94)90040-X. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, De Bold JF (2003) Neurosteroids, GABAA receptors, and escalated aggressive behavior. Horm Behav 44(3):242–257. https://www.ncbi.nlm.nih.gov/pubmed/14609546 [DOI] [PubMed]
- Müller I, Caliskan G, Stork O. The GAD65 knock out mouse - a model for GABAergic processes in fear- and stress-induced psychopathology. Genes Brain Behav. 2015;14(1):37–45. doi: 10.1111/gbb.12188. [DOI] [PubMed] [Google Scholar]
- Narvaes R, Martins de Almeida RM. Aggressive behavior and three neurotransmitters: dopamine, GABA, and serotonin—a review of the last 10 years. Psychol Neurosci. 2014;7(4):601–607. doi: 10.3922/j.psns.2014.4.20. [DOI] [Google Scholar]
- Nelson RJ, Chiavegatto S. Aggression in knockout mice. ILAR J. 2000;41(3):153–162. doi: 10.1093/ilar.41.3.153. [DOI] [PubMed] [Google Scholar]
- Nullmeier S, Panther P, Frotscher M, Zhao S, Schwegler H. Alterations in the hippocampal and striatal catecholaminergic fiber densities of heterozygous reeler mice. Neuroscience. 2014;275:404–419. doi: 10.1016/j.neuroscience.2014.06.027. [DOI] [PubMed] [Google Scholar]
- Panther P, Nullmeier S, Dobrowolny H, Schwegler H, Wolf R. CPB-K mice a mouse model of schizophrenia? Differences in dopaminergic, serotonergic and behavioral markers compared to BALB/cJ mice. Behav Brain Res. 2012;230(1):215–228. doi: 10.1016/j.bbr.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates: compact. 2. San Diego: Elsevier Academic Press; 2004. [Google Scholar]
- Pietropaolo S, Singer P, Feldon J, Yee BK. The postweaning social isolation in C57BL/6 mice: preferential vulnerability in the male sex. Psychopharmacology. 2008;197(4):613–628. doi: 10.1007/s00213-008-1081-3. [DOI] [PubMed] [Google Scholar]
- Pokos V, Castle D. Prevalence of comorbid anxiety disorders in schizophrenia spectrum disorders: a literature review. Curr Psychiatry Rev. 2006;2(3):285–307. doi: 10.2174/157340006778018193. [DOI] [Google Scholar]
- Poncelet M, Martin P, Danti S, Simon P, Soubrie P. Noradrenergic rather than GABAergic processes as the common mediation of the antidepressant profile of GABA agonists and imipramine-like drugs in animals. Pharmacol Biochem Behav. 1987;28(3):321–326. doi: 10.1016/0091-3057(87)90447-3. [DOI] [PubMed] [Google Scholar]
- Raedler TJ, Bymaster FP, Tandon R, Copolov D, Dean B. Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry. 2007;12(3):232–246. doi: 10.1038/sj.mp.4001924. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Layher E, Hannula DE, Niendam TA, Lesh TA, Solomon M, Carter CS, Ranganath C. Impact of schizophrenia on anterior and posterior hippocampus during memory for complex scenes. Neuroimage Clin. 2017;13:82–88. doi: 10.1016/j.nicl.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, O'Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology. 2007;32(2):471–482. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Lee C, Shepherd JK. Effects of diazepam on behavioural and antinociceptive responses to the elevated plus-maze in male mice depend upon treatment regimen and prior maze experience. Psychopharmacology. 1992;106(1):102–110. doi: 10.1007/BF02253596. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. Automation of the social interaction test by a video-tracking system: behavioural effects of repeated phencyclidine treatment. J Neurosci Methods. 1995;59(2):157–167. doi: 10.1016/0165-0270(94)00173-E. [DOI] [PubMed] [Google Scholar]
- Samsom JN, Wong AH. Schizophrenia and depression co-morbidity: what we have learned from animal models. Front Psychiatry. 2015;6:13. doi: 10.3389/fpsyt.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, Berman RM, Charney DS, Krystal JH. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56(11):1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- Sandhu KV, Lang D, Muller B, Nullmeier S, Yanagawa Y, Schwegler H, Stork O. Glutamic acid decarboxylase 67 haplodeficiency impairs social behavior in mice. Genes Brain Behav. 2014;13(4):439–450. doi: 10.1111/gbb.12131. [DOI] [PubMed] [Google Scholar]
- Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33(5):699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarr E, Gibbons AS, Neo J, Udawela M, Dean B. Cholinergic connectivity: it's implications for psychiatric disorders. Front Cell Neurosci. 2013;7:55. doi: 10.3389/fncel.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz EF, Tapp JT. Olfactory control of behavior in rodents. Psychol Bull. 1973;79(1):21–44. doi: 10.1037/h0033817. [DOI] [PubMed] [Google Scholar]
- Shoji H, Mizoguchi K. Aging-related changes in the effects of social isolation on social behavior in rats. Physiol Behav. 2011;102(1):58–62. doi: 10.1016/j.physbeh.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Sibille E, Morris HM, Kota RS, Lewis DA. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol. 2011;14(6):721–734. doi: 10.1017/S1461145710001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J, Bree D, Kelly JP. Effect of early life housing manipulation on baseline and drug-induced behavioural responses on neurochemistry in the male rat. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37(2):252–263. doi: 10.1016/j.pnpbp.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Siris SG, Adan F, Cohen M, Mandeli J, Aronson A, Casey E. Postpsychotic depression and negative symptoms: an investigation of syndromal overlap. Am J Psychiatry. 1988;145(12):1532–1537. doi: 10.1176/ajp.145.12.1532. [DOI] [PubMed] [Google Scholar]
- Smith KM. Hyperactivity in mice lacking one allele of the glutamic acid decarboxylase 67 gene. Atten Defic Hyperact Disord. 2018;10(4):267–271. doi: 10.1007/s12402-018-0254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467(1):60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Tayoshi S, Nakataki M, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S, Iga J, Ueno S, Harada M, Ohmori T. GABA concentration in schizophrenia patients and the effects of antipsychotic medication: a proton magnetic resonance spectroscopy study. Schizophr Res. 2010;117(1):83–91. doi: 10.1016/j.schres.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Todorovic N, Micic B, Schwirtlich M, Stevanovic M, Filipovic D. Subregion-specific protective effects of fluoxetine and clozapine on parvalbumin expression in medial prefrontal cortex of chronically isolated rats. Neuroscience. 2019;396:24–35. doi: 10.1016/j.neuroscience.2018.11.008. [DOI] [PubMed] [Google Scholar]
- Tueting P, Pinna G, Costa E. Homozygous and heterozygous reeler mouse mutants. In: Fatemi SH, editor. Reelin glycoprotein. New York: Springer; 2008. pp. 291–309. [Google Scholar]
- Uchida T, Oki Y, Yanagawa Y, Fukuda A. A heterozygous deletion in the glutamate decarboxylase 67 gene enhances maternal and fetal stress vulnerability. Neurosci Res. 2011;69(4):276–282. doi: 10.1016/j.neures.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Uchida T, Furukawa T, Iwata S, Yanagawa Y, Fukuda A. Selective loss of parvalbumin-positive GABAergic interneurons in the cerebral cortex of maternally stressed Gad1-heterozygous mouse offspring. Transl Psychiatry. 2014;4:e371. doi: 10.1038/tp.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varty GB, Powell SB, Lehmann-Masten V, Buell MR, Geyer MA. Isolation rearing of mice induces deficits in prepulse inhibition of the startle response. Behav Brain Res. 2006;169(1):162–167. doi: 10.1016/j.bbr.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Vieira C, De Lima TCM, Carobrez AD, Lino-De-Oliveira C. Frequency of climbing behavior as a predictor of altered motor activity in rat forced swimming test. Neurosci Lett. 2008;445(2):170–173. doi: 10.1016/j.neulet.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Voikar V, Polus A, Vasar E, Rauvala H. Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 2005;4(4):240–252. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2(2):322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling SG, Brown RA, Miyasaka N, Yoshihara Y, Harley CW. Selective wheat germ agglutinin (WGA) uptake in the hippocampus from the locus coeruleus of dopamine-beta-hydroxylase-WGA transgenic mice. Front Behav Neurosci. 2012;6:23. doi: 10.3389/fnbeh.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J, Desbonnet L, Clarke N, Waddington JL, O'Tuathaigh CM. Disruption of exploratory and habituation behavior in mice with mutation of DISC1: an ethologically based analysis. J Neurosci Res. 2012;90(7):1445–1453. doi: 10.1002/jnr.23024. [DOI] [PubMed] [Google Scholar]
- Wang T, Sinha AS, Akita T, Yanagawa Y, Fukuda A. Alterations of GABAergic neuron-associated extracellular matrix and synaptic responses in Gad1-heterozygous mice subjected to prenatal stress. Front Cell Neurosci. 2018;12:284. doi: 10.3389/fncel.2018.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kakizaki T, Sakagami H, Saito K, Ebihara S, Kato M, Hirabayashi M, Saito Y, Furuya N, Yanagawa Y. Fluorescent labeling of both GABAergic and glycinergic neurons in vesicular GABA transporter (VGAT)-venus transgenic mouse. Neuroscience. 2009;164(3):1031–1043. doi: 10.1016/j.neuroscience.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Wolf R, Dobrowolny H, Nullmeier S, Bogerts B, Schwegler H. Effects of neonatal excitotoxic lesions in ventral thalamus on social interaction in the rat. Eur Arch Psychiatry Clin Neurosci. 2018;268(5):461–470. doi: 10.1007/s00406-017-0781-2. [DOI] [PubMed] [Google Scholar]
- Yates G, Panksepp J, Ikemoto S, Nelson E, Conner R. Social isolation effects on the “behavioral despair” forced swimming test: effect of age and duration of testing. Physiol Behav. 1991;49(2):347–353. doi: 10.1016/0031-9384(91)90055-S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.