Abstract
Major depressive disorder with comorbid sleep disturbance has been associated with negative outcomes, including lower rates of treatment response and a greater likelihood of depressive relapse compared to those without sleep disturbance. However, little, if any, research has been conducted to understand why such negative treatment outcomes occur when sleep disturbance is present. In this conceptual review, we argue that the relationship of sleep disturbance and negative treatment outcomes may be mediated by alterations in neural reward processing in individuals with blunted trait-level reward responsivity. We first briefly characterize sleep disturbance in depression, discuss the nature of reward processing impairments in depression, and summarize the sleep/reward relationship in healthy human subjects. We then introduce a novel Integrated Sleep and Reward model of the course and maintenance of major depressive disorder and present preliminary evidence of sleep and reward interaction in unipolar depression. Finally, we discuss limitations of the model and offer testable hypotheses and directions for future research.
Keywords: sleep disturbance, depression, reward processing
Introduction
Major Depressive Disorder (MDD) remains one of the leading causes of disability worldwide, with a 12-month prevalence of approximately 5% (Ormel, Kessler, & Schoevers, 2019). In the search for effective and long-lasting treatments, considerable research has explored the underlying mechanisms of MDD with the goal of translating these insights into targeted treatments. Over the past several decades, research has shown that sleep disturbance, considered a core feature of MDD, may become severe enough to exist as a comorbid condition rather than a mere symptom of the disorder (Harvey, 2001). Thus, sleep disturbances may represent a causal mechanism that could shed light on the pathophysiology of MDD. In a comprehensive review of the ways in which sleep and circadian rhythm disturbance may serve a mechanistic function in mood disorders, Harvey (2011) detailed how sleep disturbance is key to the regulation of affect, connected to deficits in neurocognitive functioning, associated with suicidality, and linked with a poorer illness course. The extant literature repeatedly associates sleep disturbance with greater rates of non-remission to MDD treatment, a longer time to recovery, and greater rates of relapse and of incident depression (Franzen & Buysse, 2008). Conversely, there is burgeoning research showing that improved sleep disturbance is associated with significant reductions in depressive symptoms (Cunningham & Shapiro, 2018).
Despite these findings, little research has been conducted to understand why negative treatment outcomes accompany sleep disturbance, or how treatment of disturbed sleep may affect depression severity. It is possible that on a behavioral and/or neurobiological level, sleep disturbance is influencing other core features of MDD leading to poorer treatment outcomes. Further, in addition to understanding the mechanisms underlying MDD, a greater understanding of the links between sleep disturbance and poor treatment outcome can inform the development of more effective treatment strategies for individuals with MDD and significant sleep disturbance.
Although sleep disturbance has been linked with other fundamental aspects of MDD, only recently have we begun to see the emergence of theoretical models that attempt to explain how sleep disturbance may affect these processes. Palagini, Bastien, Marazziti, Ellis and Riemann (2019) recently proposed that insomnia and associated sleep loss may be a principal regulator of numerous systems involved in MDD. In addition to other subsystems in MDD, the authors note associations of insomnia and dysregulation of neural reward processing, also widely considered a core deficit of MDD (Admon & Pizzagalli, 2015). In the present review, we argue for the relative importance of these reward pathways and expand the discussion of the complex relationships between sleep and reward function to encompass distinct constructs that may be differentially impacted by sleep disruption. We also argue that, although sleep-mediated deficits in reward function may play an important role in the onset of MDD, they may be more strongly implicated in the maintenance of MDD and its resistance to traditional treatments, and may only be significant contributors to negative outcomes in individuals whose trait-level reward responsivity is low. In the following sections we will (1) briefly characterize sleep disturbance in depression (2), discuss the nature of reward processing impairments in depression, (3) provide a summary of the sleep/reward relationship in healthy human subjects, (4) introduce a testable model of how sleep disturbance may differentially affect distinct reward functions leading to, maintaining, and/or exacerbating MDD symptoms, (5) present preliminary evidence of sleep and reward interaction in unipolar depression, (6) discuss applicability of the integrated model to leading theories and conceptualizations of MDD, and (7) discuss limitations and propose future directions for the study of the interplay of sleep disturbance and reward function in MDD.
Sleep Disturbance in Depression
Symptoms of sleep disturbance, reported in 50–90% of individuals with depression (Tsuno, Besset, & Ritchie, 2005) have long served as key diagnostic criteria of MDD, and include difficulty falling asleep (i.e., early insomnia), awakening after sleep onset (i.e., middle insomnia) and awakening earlier than intended (i.e., late insomnia). Other patients may report symptoms of hypersomnia, i.e., sleeping much more than intended, that occur in approximately 50% of individuals with depression (Geoffroy et al., 2018). For the purposes of this review, we operationalize “sleep disturbance” to reflect any of these commonly reported sleep problems. More recently, research has begun to focus on the ways in which sleep disturbance may reflect a transdiagnostic process that spans the spectrum of mood disorders (Harvey, Murray, Chandler & Soehner, 2011) and may be directly involved in their onset, maintenance, and course). Below, we will briefly describe the evidence supporting these now widely held views.
Sleep disturbance and depression risk
Although it is generally accepted that the relation of sleep disturbance and depression is bidirectional (Alvaro, Roberts, & Harris, 2013; Fang, Tu, Sheng, & Shao, 2019), there is considerable evidence that sleep disturbance frequently occurs prior to the onset of depression (Staner, 2010) and is also linked with a greater likelihood of depression onset (Franzen & Buysse, 2008). For example, short sleep, or duration of sleep less than six hours per night, predicted depression symptoms and diagnoses in a sample of 3,134 adolescents (Roberts & Duong, 2014). Specifically, a reciprocal effect was observed, such that reduced sleep duration increased the risk for MDD while MDD itself increased the risk of sleep loss (Roberts & Duong, 2014). In addition, adolescents with sleep disturbance had 2.3 times the odds of developing depression as young adults (Roane & Taylor, 2008). Sleep disturbance in childhood also has been associated with greater odds of developing depression in adolescence (Wiebe, Cassoff, & Gruber, 2012).
Similar findings have been obtained in adult and older adult samples. Among 1,125 training physicians, sleep duration of less than 6 hours combined with self-reported poor sleep was associated with subsequent depressive symptoms (Kalmbach, Arnedt, Song, Guille, & Sen, 2017). Controlling for depression history, a prior history of sleep disturbance in adults has been shown to significantly predict MDD (Baglioni et al., 2011; Riemann & Voderholzer, 2003), though the pathways underlying this association have yet to be elucidated. Similarly, in a recent meta-analysis of sleep disturbance in older adults, persistent sleep disturbances were associated with a significantly greater risk of developing MDD, as well as a risk of recurrence and worsening of depressive episodes (Bao et al., 2017). Studies employing objectively measured sleep disturbance as it may relate to new onset depression are comparatively rare, but also predict incident depression. Fernandez-Mendoza et al. (2015) reported associations of subjectively reported poor sleep and insomnia with increased odds of incident depression but found that insomnia symptoms combined with objectively measured short sleep conferred the highest odds (OR = 2.2) of incident depression in a general population sample of 1137 adults.
Taken together, these studies support the idea that sleep disturbance often precedes, and may possibly increase, the likelihood of depression onset. This enduring pattern of association between sleep disturbance and depression risk across the life span suggests that sleep disturbance may influence the pathogenesis of MDD developmentally (see Palagini et al., 2018 for an extensive review). Indeed, it has been proposed that early life stress and associated sleep disturbance may impact the developing brain, leading to a greater likelihood of depression onset (Palagini et al., 2018). This model has considerable associative support, however additional longitudinal studies are needed to establish causal relationships and uncover the mechanisms underlying these associations.
Sleep disturbance and depression treatment
Significant sleep disturbance has been repeatedly shown to predict poor clinical depression treatment outcome. For example, objective sleep disturbance, i.e., disturbance observed via polysomnography, has been related to both slower (Dew et al., 1997) and lower overall (Thase, Simons & Reynolds, 1996) rates of recovery from MDD episodes. Subjectively reported sleep disturbance, particularly greater latency of initial sleep onset, also has been associated with an increased risk of non-remission following either pharmacological or psychotherapeutic treatment of MDD (Troxel et al., 2012).
The evidence is mixed, but studies frequently show that sleep disturbance is related to an increased likelihood of depression relapse. For example, Dombrovski et al. (2007) reported that older adults who experienced residual sleep disturbance following acute phase Interpersonal Psychotherapy (IPT) and open pharmacological treatment had a greater risk of early recurrence than participants whose sleep disturbance was completely ameliorated during acute treatment. In a separate study of 131 women, those who experienced residual insomnia at the time of randomization to maintenance IPT were also more likely to experience a recurrence (Dombrovski et al., 2008). There is evidence, moreover, that sleep tends to worsen in the prodromal phase leading up to relapse (Perlis, Giles, Buysse, Tu & Kupfer, 1997).
The literature is not wholly consistent, as several studies failed to demonstrate significant relations between sleep disturbance and increased relapse risk. Taylor, Walters, Vittengl, Krebaum and Jarrett (2010) observed significant residual insomnia symptoms in 84 adults who completed acute phase cognitive therapy but saw no relationship between residual symptoms and subsequent relapse. Similar null findings have been observed in pharmacological trials (e.g., Iovieno, van Nieuwenhuizen, Clain, Baer & Nierenberg, 2011; Nierenberg et al., 2010). More work is needed to increase our understanding of depression relapse and the role that sleep disturbance may play in its development. Given associations between sleep disturbance and poor depression treatment response, an important question is whether sleep disturbance could impact the efficacy of traditional depression treatments.
Antidepressant effects of sleep treatment
Further evidence of the positive relation of sleep disturbance and depression symptoms can also be found in the insomnia treatment literature, where there is mounting evidence that reductions in insomnia symptoms, particularly following Cognitive Behavioral Therapy for Insomnia (CBT-I), are associated with reductions in depressive symptoms. Although CBT-I teaches some of the same skills as those learned in traditional CBT for depression (e.g., cognitive restructuring of negative automatic thoughts, some focus on improving sleep hygiene habits), it is distinguished by a significant focus on reducing conditioned hyperarousal to the bed (i.e., stimulus control), and strengthening the homeostatic sleep drive by restricting time in bed to match average total sleep time (i.e., sleep restriction therapy) (Edinger & Means, 2005). In fact, research has shown that sleep restriction and stimulus control strategies are the best predictors of clinical improvement of insomnia (Harvey, Inglis & Espie, 2002), and sleep restriction alone is effective as a stand-alone treatment for insomnia (Miller et al., 2014). In contrast, the sleep hygiene strategies often incorporated into CBT for depression are generally found to be ineffective as stand-alone interventions at ameliorating significant sleep disturbance (Irish, Kline, Gunn, Buysse & Hall, 2015; Morgenthaler et al., 2006). It is possible that the behavioral components of CBT-I may have an antidepressant effect. In a report on the national Veterans Health Administration (VHA) dissemination of CBT-I, treatment was associated with significant reductions in depressive symptoms in a sample of 182 patients (Karlin, Trockel, Barr, Gimeno & Manber, 2013), and a pilot study of CBT-I monotherapy in patients with mild depression demonstrated a significant antidepressant effect of the intervention (Taylor, Lichstein, Weinstock, Sanford & Temple, 2007). A study in which CBT-I was combined with escitalopram treatment for 30 individuals with MDD and insomnia yielded higher overall depression remission rates than either escitalopram alone or a control therapy (Manber et al., 2008), though the effect was not replicated in a larger, randomized controlled trial in 150 patients (Manber et al., 2016). Importantly, however, the authors found that mid-treatment improvements in insomnia severity in this larger study mediated depression response among individuals who were randomized to receive CBT-I (Manber et al., 2016).
Paradoxically, while improvement of sleep disturbance has been associated with antidepressant effects, total deprivation of sleep has likewise been shown to produce rapid reductions in depression symptoms. Therapeutic sleep deprivation is the clinical application of wakefulness wherein an individual remains awake beginning from their usual wake time and throughout the night and following day (roughly 36 hours of wakefulness, known as “total” sleep deprivation) or goes to bed at their normal bedtime and is awakened several hours earlier (e.g., 1:00 am) and required to remain awake until regular bedtime (referred to as “partial” sleep deprivation). Although not a widely used antidepressant strategy in clinical practice, sleep deprivation has been studied in earnest since the 1960s, and pooled analyses of response rates have demonstrated a roughly 50% antidepressant response rate, with no significant differences in response observed between total and partial modalities (Boland et al., 2017). After subsequent sleep, however, depression symptoms usually return; thus the effects, while rapid, tend to be transient. Although the mechanisms of this treatment effect remain unconfirmed, different hypotheses exist. Many studies suggest the involvement of circadian rhythms and clock genes, with the overarching theory that sleep deprivation somehow “resets” the circadian clock, leading to depression improvement (Bunney & Bunney, 2013). Another hypothesis is that sleep deprivation combats a depressogenic effect of sleep in some individuals with MDD, thought to be brought about by reductions in the activity of the dopamine system during sleep, likening the effects of sleep deprivation with those of psychostimulants such as cocaine and amphetamines (Ebert & Berger, 1998).
Up to this point, we have reported on literature supporting the idea that sleep disturbance is often associated with poor depression treatment outcome and may contribute to the development of MDD (Harvey et al., 2011, Palagini et al., 2019). As mentioned previously, however, sleep disturbance could influence another core component of MDD to produce poor treatment outcomes. Given that reward processing impairments are well-documented in depression and share significant neurobiological overlap with regions associated with sleep regulation (see Fang et al., 2019; Palagini et al., 2019), reward processing is an ideal candidate for exploration in this regard.
Reward Processing Impairment in Depression
As mentioned previously, a comprehensive model has been proposed demonstrating how sleep disturbance is associated with multiple facets of MDD, including reward function, broadly (Palagini et al., 2019). However, reward processing encompasses a wide range of functions that may be differentially impacted by sleep, and these specific differences may be integral to our understanding of how episodes of depression are maintained in the face of antidepressant treatment. To understand how sleep disturbance may impact these functions, we will briefly review the nature of reward dysfunction in individuals with MDD.
Definition of and circuits relevant to reward processing in MDD
Research across multiple levels of analysis has shown aberrant reward processing to be an integral component of mood disorder pathology (Admon & Pizzagalli, 2015; Whitton, Treadway & Pizzagalli, 2015). Reward processing in the context of mood disorders refers to the ways in which humans assign value to rewards, feel satisfied by rewarding stimuli, put forth effort to obtain rewards, or make decisions based on information they receive about reward availability (Whitton et al. 2015). To better understand reward processing as it relates to sleep disturbance and depressive symptoms, it is helpful to review the neural substrates of reward. Although multiple regions in the brain respond to rewarding stimuli, the central neural pathway involved in reward processing is the fronto-striatal neural circuit (Nusslock & Alloy, 2017). This circuit encompasses dopaminergic neurons that project from the ventral tegmental area to subcortical striatal regions such as the ventral striatum, which includes the nucleus accumbens, which is primarily responsible for the processing of rewarding stimuli. Neural projections from the nucleus accumbens to frontal cortical regions such as the orbitofrontal cortex, medial prefrontal cortex, and anterior cingulate cortex are involved in decision-making processes. Together, these regions coordinate the experience, assessment, and evaluation of rewarding stimuli, translating this information into reward-relevant decision-making (Nusslock & Alloy, 2017).
Neuroimaging and behavioral evidence of blunted reward processing
Depression is associated with blunted reward responsivity (Luking, Pagliaccio, Luby & Barch, 2016), reduced anticipation of reward (Smoski et al., 2009), reduced willingness to exert effort for rewards (Treadway, Bossaller, Shelton & Zald, 2012), and difficulties integrating information about reward from the environment to guide behavior (Treadway et al., 2012). These associations are evidenced by both functional imaging studies of the fronto-striatal reward circuit and behavioral assessments of reward functioning. For example, Pizzagalli and colleagues noted lower activity in the caudate and nucleus accumbens via functional magnetic resonance imaging (fMRI) following monetary gains (but not neutral or loss outcomes) among 30 participants with MDD than healthy controls (Pizzagalli et al., 2009). Reduced striatal activity in the context of reward anticipation has been observed in youth with diagnoses of MDD (Olino et al., 2011) and those at high familial risk for MDD (Olino et al., 2014); the latter finding suggesting that blunted reward anticipation may be a trait marker of MDD rather than merely an artifact of depressed mood.
There is also behavioral evidence that the willingness to put forth effort to obtain rewards is reduced in MDD. Based on Brehm’s motivation intensity theory (Brehm & Self, 1989), healthy individuals will expend effort in such a way as to avoid unnecessary expenditure of resources. Toward that end, individuals will expend greater effort for tasks perceived to be “worth it” in terms of expected return but will avoid exerting more effort than is needed. In other words, greater effort is expended when a successful outcome is considered possible and worthy of the effort, but lower, or even no effort would be expended for outcomes that are considered near impossible (Gendolla, Wright, & Richter, 2012). However, this expected pattern of response to tasks, particularly tasks attached to rewarding outcomes, is not observed in MDD. For example, Treadway and colleagues (2012) assessed 20 depressed subjects who participated in the Effort Expenditure for Rewards Task (EEfRT; Treadway, Buckholtz, Schwartzman, Lambert & Zald, 2009), which requires participants to choose between low effort/low reward and high effort/high reward trials after being given information about both the magnitude and likelihood of reward attainment. Participants with MDD who completed the EEfRT task exhibited less physical effort to obtain large rewards than non-depressed individuals (Treadway et al., 2012). Moreover, feedback that a large reward was particularly likely following a high effort choice did not appear to contribute to a greater likelihood of making that choice among participants with MDD. Thus, depressed participants were less able to integrate information about the size and likelihood of rewards into their decision-making behavior compared to non-depressed individuals (Treadway et al., 2012).
Reward processing impairments in the context of depression symptoms and remitted MDD
Lower activation of key components of the fronto-striatal reward network is also evident among individuals with depression symptoms who have not been diagnosed with MDD. Children at high risk for MDD demonstrated a dampened response to gain and reduced activation to loss within the ventral striatum and anterior insula as assessed with fMRI during a card-guessing game involving the gain and loss of candy. More recently, Rzepa, Frisk and McCabe (2017) investigated the neural response to reward-relevant effort, anticipation, and consummation in adolescents considered at low (n = 17) or high (n = 16) risk for depression based on responses to a mood questionnaire. Reward anticipation, effort, and consummation were associated with reduced activity in the pregenual cingulate cortex (part of the anterior cingulate cortex) among adolescents with depressive symptoms, and reward relevant effort and consummation were also associated with reduced activation of the ventral medial prefrontal cortex (Rzepa et al., 2017). In a separate study, participants scoring high on depression measures performed poorly on a probabilistic reversal learning task, exhibiting a diminished capacity for learning contingencies for cueresponse combinations that were infrequently rewarded (Umemoto & Holroyd, 2017).
In line with Treadway et al.’s (2012) findings regarding impaired effort mobilization in individuals with MDD, there is similar evidence in individuals with negative mood but without clearly defined MDD diagnoses. In a comprehensive review of effort mobilization, Gendolla, Wright & Richter (2012) argue that mood states serve as “task-relevant information” and influence the effort individuals put forth. They note that in previous studies (see Gendolla & Krüksen 2001, 2002 for the original work) cardiovascular reactivity, used as a proxy for effort mobilization, was stronger during easy tasks when negative mood was present, presumably because the demand was experienced as greater in the context of poor mood. In difficult tasks, however, cardiovascular reactivity was greater among individuals with positive mood, indicative of greater sense of ability and/or likelihood of mastery as a consequence of effort. Participants with negative mood did not engage with the task due to perceptions that the difficulty was too high (Gendolla et al., 2012).
Reward processing dysfunction has also been observed among individuals whose depression has remitted. Dichter and colleagues noted greater activation of neural reward circuitry among 19 participants with remitted MDD during the anticipation phase of a monetary incentive delay task, but the opposite effect during the outcome phase, where activation was decreased (Dichter, Kozink, McClernon & Smoski, 2012). Participants with remitted MDD also exhibited similar difficulties as those with MDD in modulating behavior in response to previous reward reinforcement. Using a probabilistic reward task in 47 participants with remitted MDD, Pechtel, Duta, Goetz and Pizzagalli (2013) reported a general failure among the MDD group to form a response bias toward frequently rewarded stimuli. In other words, relative to 37 subjects who had never had depression, individuals with remitted MDD did not use information about how previous behaviors had been rewarded to guide their future decision making. More recently, researchers noted differences among subtypes of individual with remitted depression, specifically those with melancholic depression, which is characterized by, among other things, loss of pleasure in daily activities and lack of reactivity to positive events (i.e., anhedonia). Using event-related potentials obtained while participants completed a gambling task for monetary rewards, participants with remitted melancholic depression (n=29), relative to remitted non-melancholic depression (n = 56) and healthy controls (n = 81), exhibited a blunted response to rewards, though no group differences were detected in response to losses (Weinberg & Shankman, 2017). This finding is consistent with research reporting negative associations between self-reported anhedonia symptoms, irrespective of MDD diagnoses, and willingness to work for rewards (Treadway et al., 2009).
Changes in reward processing following depression treatment
Improvements in depressive symptomatology are associated with changes in activation of the brain regions that regulate reward functioning, supporting the idea that reward-processing dysfunction is a core feature of MDD. For example, one study showed that deep-brain electrical stimulation of the ventral striatum, including the nucleus accumbens, reduced depressive symptoms among three individuals resistant to psychotherapy, pharmacotherapy, and electro-convulsive therapy (Schlaepfer, Cohen & Frick 2008). Another study showed that deep brain stimulation of the nucleus accumbens in 10 subjects with MDD was associated with reductions in depressive symptoms and an increase in rewarding activities (Bewernick et al., 2010). Importantly, they also noted metabolic changes via positron emission tomography (PET) scan in the subgenual cingulate and prefrontal areas that correlated with these antidepressant effects.
From a behavioral standpoint, there is evidence that the successful treatment of depression with Behavioral Activation, a psychotherapeutic intervention that encourages individuals to exert effort to schedule and participate in valued activities (Jacobson, Martell, & Dimidjian, 2006), is associated with changes in activation of reward-relevant brain circuitry (Dichter et al., 2009). Twelve participants with MDD and 15 controls participated in two fMRI sessions of a probabilistic decision-making task, with the MDD participants completing an average of 11 sessions of Behavioral Activation therapy in-between scans. Seventy-five percent of the MDD participants responded to treatment, which correlated with increased striatal activation during the anticipation phase (Dichter et al., 2009). More research is needed to improve our understanding of the ways in which antidepressant treatments and therapies may possibly impact neural reward circuitry; however, an important point from this emerging literature is that the reward system is malleable and thus may be open to influence by sleep disturbance.
Impact of Sleep Disturbance and Deprivation on Reward Processing
Our understanding of how sleep disturbance and reward processing may interact in depression is informed by an awareness of the ways in which sleep disturbances are associated with changes in reward processing in healthy human subjects. To begin, it is important to distinguish between experimental and/or therapeutic sleep deprivation and the type of sleep disturbance often observed in MDD. As we mentioned previously, sleep deprivation, when intended as an experimental intervention or applied as a therapeutic strategy, is defined as either total (e.g., 36 hours of wakefulness) or partial (e.g., time in bed reduced to about 4 hours). Sleep disturbance, on the other hand, is often reflected by poor quality sleep and shortened total duration, and can include increased sleep onset latency, mid-sleep awakenings, early morning awakenings and/or hypersomnia. Sleep deprivation represents continuous, uninterrupted periods of wakefulness resulting in shortened duration of sleep, whereas sleep disturbance may involve short bursts of sleep interspersed with varying degrees of wakefulness after sleep onset, resulting in a shortened overall duration of sleep despite average or longer time in bed. These terms are sometimes used interchangeably, but the distinctions could be important: sleep disturbance may be more characteristic of the type of sleep observed during episodes of MDD than sleep deprivation, and sleep disturbance and deprivation may have differential effects on reward processing depending on the length and/or consolidation of wakefulness.
Effects of total or partial sleep deprivation on reward processing
There is ample evidence that sleep deprivation is associated with enhanced reward function. Gujar, Yoo, Hu & Walker (2011) noted that healthy individuals who had been experimentally sleep deprived demonstrated both greater activation in reward regions, including the ventral tegmental area, in response to positive emotional stimuli and a behavioral response bias for pleasant images relative to nonsleep deprived participants. When presented with monetary rewards, healthy participants who underwent sleep deprivation demonstrated increased activation of the ventral striatum and decreased deactivation of the medial prefrontal cortex than they did under normal sleep conditions (Mullin et al., 2013). Additionally, those participants who had shorter total sleep time in the days leading up to sleep deprivation, as measured by actigraphy, also showed a reduced deactivation of the medial prefrontal cortex.
Behavioral manifestations of reward dysfunction are also apparent following sleep deprivation. McKenna, Dickinson, Orff and Drummond (2007) reported that when asked to make choices about two lottery gambles of equivalent pay-out but differential risk, individuals who had been experimentally sleep deprived demonstrated greater risk-taking when considering choices involving gains compared to when they made these decisions following normal sleep. However, when considering gambles involving losses, sleep deprivation was associated with less risky choices. Thus, sleep deprivation may differentially impact distinct facets of reward function. Indeed, sleep deprivation has also been shown to affect the effort put forth to obtain rewards, but not the time an individual is willing to wait to receive them. Libedinsky et al. (2013) demonstrated that healthy participants who underwent total sleep deprivation were willing to wait for larger monetary rewards, but at the same time showed a preference for rewards that were easier to obtain but of lesser value than more difficult to obtain rewards.
Insomnia-like sleep disturbance has also been associated with aberrant reward processing. For example, in adolescents, actigraphic reductions in sleep duration and sleep quality, as well as later sleep onset time, were associated with less activation in the caudate (part of the ventral striatum) during reward anticipation trials of a guessing task with monetary rewards, while reward outcome trials evidenced associations with less caudate activation and lower sleep quality and later sleep onset and offset (Holm et al., 2009). There is also some evidence that, when sleep is disrupted, the willingness to exert effort for rewards is diminished. In a study of adolescent figure skaters, for example, more awakenings and greater time spent awake at night were associated with the selection of less difficult skating maneuvers the next day (Engle-Friedman, Palencar, & Riela, 2010). Though this may appear to be a straightforward association of sleep disturbance leading to reduced effort, the reality is that the links between sleep disturbance and effort mobilization may be more complex. Schmidt, Richter, Gendolla and Van der Linden (2010) for example demonstrated that college students with greater self-reported insomnia severity demonstrated increases in systolic blood pressure, considered a proxy for effort, during the learning phase of an easy memory task, but no associations were observed between insomnia severity and immediate recall. This suggests that sleep disturbance may be connected to greater effort for “easy” tasks, perhaps, as the authors hypothesize, as a means of compensating for the effects of continual poor sleep. Other studies have demonstrated increases in effort following sleep disturbance. Engle-Friedman and Riela (2004) showed that students who reported less total sleep time prior to an exam, coupled with increased study time, reported increased subjective effort on the exam, while subjective sleepiness predicted the selection of lower difficulty exam tasks. Importantly, these patterns are similar to those noted in studies where negative mood appears to hasten disengagement of difficult tasks in favor of objectively “easy” tasks for which individuals exert increased effort than expected.
There is still considerable work to be done to improve our understanding of the relationship of sleep disturbance to reward processing. The literature to date appears to indicate that sleep disturbance and deprivation are not associated with a singular dysfunction in reward processing, but perhaps may differentially affect reward function in ways that, while appearing oppositional, may in fact make depression onset and maintenance more likely. This possibility is reflected in an Integrated Sleep and Reward Processing model of MDD.
Integrated Sleep and Reward Model of MDD
Up to this point, we have presented descriptive evidence demonstrating that 1) sleep disturbances are pervasive in MDD, 2) treatment of sleep disturbance is associated with improvement in depressive symptoms, 3) reward processing, specifically the domains of reward-relevant effort, motivation, and learning, is impaired in MDD, and 4) insomnia-like sleep disturbance is associated with perturbations in reward processing. It is logical, therefore, to hypothesize that these findings are not distinct, but rather may reflect the workings of an integrated process whereby sleep disturbance can influence reward-relevant effort, motivation, and learning functions and contribute to the maintenance of an episode of MDD when depression treatments are not adequately addressing sleep disturbance.
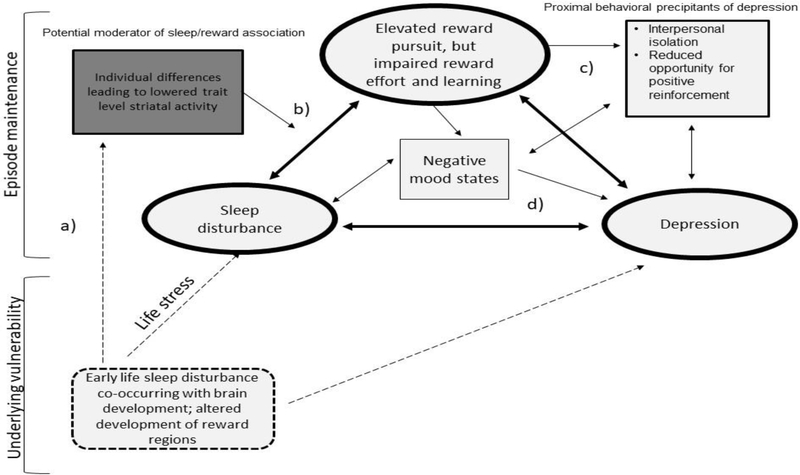
This theoretical framework is reflected in an Integrated Sleep and Reward Model of MDD, a moderated mediation model that attempts to explain the ways in which sleep disturbance may influence reward functions that either directly or indirectly maintain the disorder state. In this model, which we hereafter refer to as the ISR model (see Figure 1), we posit that among individuals with reduced trait-level reward responsivity, sleep disturbance may lead to enhanced reward seeking and increased salience of reward, but reduced effort and/or a failure to appropriately contextualize reward relevant information to guide effort allocation. These oppositional outcomes may lead directly to depressive symptoms or may increase the likelihood of negative mood states (e.g., feelings of loss, disappointment) or behavioral patterns that more proximally promote depression.
Figure 1.
Illustration of the Integrated Sleep and Reward (ISR) model.
Dashed lines indicate early developmental processes. Bolded ovals indicate primary sleep disturbance/reward processing pathways of the ISR model. Rectangles indicate moderators (dark shade) of the sleep/reward relationship and additional mediational pathways (light shade) through which the interaction of sleep disturbance and reward processing may lead to depression. In this model, an individual may experience sleep disturbance during early childhood, influencing concurrently developing neural pathways in the reward system (a). This may lead to reduced trait level striatal activity, which may strengthen the relationship between sleep disturbance and reward processing (b). Sleep mediated reward deficits may directly lead to depression, or may influence the development of negative mood states or behaviors that may more proximally lead to the development of MDD (c and d).
This chain of events can occur at various points throughout the course of illness and may operate on neurobiological and/or behavioral levels. There is evidence of neurotransmitter overlap in both the sleep and reward systems (e.g., orexin/hypocretin, dopamine, serotonin) and the mesolimbic dopaminergic system has been shown to be activated during sleep (Perogamvros & Schwartz, 2012). From a neuroanatomical perspective, dopaminergic pathways have been shown to interact with the suprachiasmatic nucleus – aka, the “biological clock,” which is central to sleep/wake regulation (Murray et al., 2009). There is also evidence that dopaminergic neurons in regions of the fronto-striatal circuit are implicated in REM sleep (Dahan et al., 2007; see Palagini et al., 2019 for additional discussion of these hypothesized neurobiological mechanisms). The association of sleep disturbance and reward dysfunction may also predispose individuals to more proximal behavioral precipitants. For example, sleep disturbance-related increases in reward seeking combined with preferences for low effort/low reward choices could make it more likely that an individual will isolate (i.e., staying at home watching TV as opposed to going out with friends) or experience negative mood as a consequence of the low reward value of the chosen activity failing to meet the demand of the increased reward desire. Feelings of frustration as well as negative rumination could also be possible outcomes.
The ISR model takes into account individual differences in the relationship between sleep disturbance and reward processing and posits that this relationship may not apply to all depressed individuals. In the ISR model, we propose that individual sensitivities to reward may make one more vulnerable to the reward-disrupting effects of sleep disturbance, a hypothesis developed on the basis of recent research demonstrating a potential protective effect of healthy striatal activity. Avinun et al. (2017) assessed sleep disturbance via the Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynold, Monk, Berman & Kupfer, 1989) in 1129 college students who also completed self-report measures of depression via the Mood and Anxiety Symptom Questionnaire (MASQ; Watson & Clark, 1991). Using fMRI while participants completed a monetarily-rewarded card-guessing game, greater activity in the ventral striatum was shown to protect against the effects of sleep disturbance on depressive symptoms (Avinun et al., 2017). The authors found that the association between depression and sleep disturbance was stronger when striatal activity was low, and this effect remained after the inclusion of demographic and clinical variables and life stress. Taking this recent finding into account in our model, we considered trait level reward sensitivity as a potential moderator of the insomnia-reward relationship. In other words, our framework represents a moderated mediation model, where the mediating effects of reward dysfunction on the insomnia-depression relationship may only be significant in individuals with low levels of reward sensitivity, either behaviorally or via evidence of reduced striatal activity in response to rewarding stimuli.
This ISR model also attempts to explain the antidepressant effects of insomnia treatment, as well as the increased risk of relapse and treatment non-response in the presence of significant sleep disturbance. The model suggests that ameliorating sleep disturbance may restore reward-related functionality, allowing for adaptive reward pursuit and realistic incorporation of reward-relevant information from the environment to guide more appropriate effort allocation. If sleep disturbance remains following depression treatment, it may continue to influence reward function, impairing the translation of reward valuation into adaptive reward-guided behaviors as well as increasing the likelihood of depression-promoting behaviors.
Evidence for the ISR model
There has been relatively little focus on the ways in which sleep disturbance and reward processing may interact in unipolar depression. To our knowledge, only three studies explicitly examine sleep disturbance in the context of reward circuitry in depression, including the above referenced study on the protective effects of greater ventral striatal activity. In a multi-year longitudinal study, Casement, Keenan, Hipwell, Guyer and Forbes (2016) assessed reward functioning in 123 adolescent girls using fMRI while they completed a guessing task where correct guesses were rewarded with $1.00. Sleep disturbance was measured using the sleep items from the depression module of the Schedule for Affective Disorders and Schizophrenia for School Aged Children (K-SADS; Kaufman et al., 1997). The authors found that non-restorative sleep during early adolescence was associated with elevated dorsal medial prefrontal cortex (DMPFC) response to reward anticipation in late adolescence (Casement et al., 2016). They also found that DMPFC response mediated associations between non-restorative sleep in early adolescence and future depression symptoms. This finding should be considered with the understanding that nonrestorative sleep is not synonymous with sleep loss; however, it is a signal that warrants further study. Perhaps most supportive of this burgeoning hypothesis is recent work by Burani et al. (2019) that used a prospective design to examine interactions of sleep, reward, and stress as they may relate to depression symptoms. In a sample of 317 adolescent girls ranging from 8 to 14, the authors utilized continuous EEG recording while participants completed a monetary reward task. Assessing sleep disturbance with the PSQI and stress with the Adolescent Life Events Questionnaire (ALEQ, Hankin & Abramson, 2002), the authors uncovered a three-way interaction where blunted reward processing, as evidenced by event-related potentials at baseline, predicted increased risk of depression symptoms at both high levels of stress and high levels of sleep problems. The authors note that increased depression risk was predicted by either sleep problems or stress among girls with blunted reward processing, but that girls who experienced both poor sleep and high stress combined with blunted reward processing exhibited the greatest overall risk (Burani et al., 2019).
Behavioral illustration of the ISR model
Although the research discussed thus far provides an academic overview of sleep disturbance and reward processing as interactive processes that underly MDD, it may be difficult to picture how this relationship may manifest outside of an MRI scanner or a sleep deprivation laboratory. To provide a better idea of how the interaction of sleep disturbance and reward processing may lead to and/or maintain depression symptoms, we can consider a hypothetical individual who has been experiencing sleep disturbance for approximately one month. According to the model, the individual’s sleep disturbance impacts her ability to adequately incorporate information about reward availability and reinforcement value and suppresses her willingness to pursue rewards. For example, she receives an invitation from a friend to attend a party about 15 minutes from her house. Not only will she be able to see friends she has not seen in a while, but some influential people in her field will be in attendance, which could be beneficial for her career. Following a month of disturbed sleep, she feels too tired and unmotivated to go through the hassle of getting ready for a party after a long day at work. Driving home and making herself dinner already felt like overwhelming tasks; the thought of preparing an “elevator pitch” for the purposes of networking feels equally taxing. She knows that even though she would have to put in considerable effort to get ready, spending time with friends would be fun (i.e., a high effort/high reward choice). However, in her sleep-deprived state, the draw to spend time with friends and socialize can’t compete with a night alone with her favorite television re-runs and ice cream (i.e., a low effort/low reward choice). She turns down the invitation, spends the night at home alone, flipping through channels and working her way through her kitchen pantry. She tries to go to bed early, hoping tomorrow she’ll be more rested and feel more like herself, but she still has difficulty falling and staying asleep. She ruminates about whether she made the wrong decision by staying home and feels isolated and guilty for how she chose to spend her evening. By turning down the invitation, she has removed herself from an environment where positive reinforcements would have been likely and fueled negative emotions and thoughts that are known to interfere with sleep. As her sleep disturbance progresses, she continues to engage in low-effort, lowreward behavior and begins to experience depressive symptoms. She feels more isolated and alone, struggles with rumination and guilt about her decisions, and feels sluggish and low on energy. As her depressive symptoms worsen, her sleep disturbance worsens, exerting continued influence on her reward system. Understanding that she is depressed, she seeks treatment from a psychiatrist. She is prescribed an antidepressant that, while effective for some of her feelings of sadness and guilt, does little for her sleep disturbance. If anything, she wonders if the medication is making her sleep worse. Despite objective improvement in some of her mood symptoms, her sleep disturbance remains, leading to an increasing number of scenarios like the one described previously, where she routinely favors low effort/low reward decisions that may actually increase the likelihood of a recurrence of her mood symptoms.
This hypothetical description is by no means the definitive way in which sleep disturbance and reward processing may interact to lead to MDD onset or maintenance. Indeed, the broad heterogeneity of MDD all but assures that these relationships will differ among individuals. With these variances in mind, it is important to discuss how the ISR model may fit with leading theories and/or other key components of MDD.
Applicability of the ISR model to the current understanding of MDD
It is important that emerging models of MDD reflect the current conceptualizations of depression. For example, any model of the pathogenesis of MDD would be incomplete without discussing mood and negative affect. Sad or depressed mood is one of the cardinal symptoms of MDD and has been shown to have a direct relationship with sleep disturbance. In a systematic review, Konjarski, Murray, Lee and Jackson (2018) report on 29 naturalistic prospective studies that explore day-to-day relationships of sleep and mood. In general, poor sleep has been shown to be associated with increased negative affect the following day in both healthy and depressed samples and, overall, the associations of poor sleep and next day negative affect were stronger in individuals who already were experiencing depressive symptoms at baseline. It is certainly possible that sleep disturbance can influence reward-relevant behaviors that could lead to poor mood the next day (e.g., isolation from social activities and opportunities for positive feedback; consumption of excess sugar (Knüppel, Shipley, Llewellyn & Brunner, 2017). Similarly, it is possible that the mismatch of increased reward pursuit and impaired mobilization of effort may lead to situations that promote negative emotions such as frustration and feelings of failure. Given that one of the emotion centers of the brain, the amygdala, is also implicated in reward function (Murray, 2007), it is possible that sleep disturbance affects these systems simultaneously on a neurobiological level. As research is conducted into the effects of sleep disturbance on reward processes in depression, a greater understanding of the complex relations of sleep, reward, and mood are necessary.
Equally important is the understanding that MDD is now widely conceptualized through a developmental lens, given that a spectrum of factors beginning from the prenatal period are thought to influence the onset and course of MDD (Kendler, Gardner & Prescott, 2002; 2006). In this light, sleep disturbance has been examined as a developmental process in MDD, with a recent review pointing to evidence of a mechanistic role of sleep disturbance in depression across the lifespan (Palagini et al., 2018). Thus, it is important that our model likewise incorporates this developmental perspective. As previously discussed, reward processing impairments are evident in children at high risk for MDD (Olino et al., 2014), suggesting they may represent a trait marker of MDD. Palagini et al. (2018) propose that sleep disturbance, also found to be present in offspring of depressed mothers (Meltzer & Mindell, 2007) impacts the developing brain, thus predisposing the individual to MDD. We include this developmental perspective in our model, and suggest the possibility that early sleep disturbance may impact the development of integral reward regions, potentially biasing the individual towards reward-based choices that can lead to depression symptoms. As relations between sleep disturbance and depression have been shown to be pronounced in individuals with blunted reward responsivity (Burani et al., 2019), this altered neural development may make the individual more susceptible to impaired reward processing in the face of sleep disturbance. This claim is supported by the aforementioned study by Avinun et al. (2011), where greater striatal activity buffered against the effects of sleep disturbance. This hypothesis is speculative at this point, and future prospective longitudinal studies that incorporate parallel assessments of sleep disturbance and reward processing across the developmental spectrum are integral to answering questions about how early childhood sleep disturbance may affect the development of reward relevant brain regions.
Limitations and Future Directions
Although we took an integrated approach to understanding the complex relations of sleep disturbance, reward processing, and depressive symptoms, there are limitations to this model that must be addressed with further research. For example, though we discuss that reward hyposensitivity has been found by several researchers to represent a trait marker of MDD (Olino et al., 2014), to assume that all deficits in reward anticipation are the direct result of pediatric sleep disturbance would not be valid. However, we were unable to identify any studies of reward response in children of depressed parents that concurrently examined their levels of sleep disturbance, thus limiting our ability to examine whether there are sleep-specific effects in these samples. As more research is conducted that assesses these domains in parallel, further refinement of the model can be undertaken. Consequently, another limitation of the model is that it implies that sleep disturbance precedes all episodes of depression. While sleep disturbance has been associated with greater likelihood of new depression onset (Baglioni et al., 2011), this sequence is not a ubiquitous phenomenon, and insomnia and depression are known to affect each other bidirectionally (Fang et al., 2019; Franzen & Buysse, 2008; Staner, 2010). Thus, we have focused on how the ISR model is more descriptive of disorder maintenance and recurrence than first onset and may best explain why sleep disturbance is associated with poorer depression treatment response and greater relapse risk. In this conceptualization, the ISR model does not require that initial reward processing impairments be the direct result of, but rather are able to be influenced by sleep disturbance. However, it is possible to determine the model’s applicability to first onset in future research. Longitudinal studies of children at high familial risk for depression that incorporate regular assessment of insomnia symptoms and neuroimaging and/or behavioral assessments of reward processing can help determine temporal precedence of emerging changes in sleep and reward function prior to depressive onset.
Additionally, the ISR model does not adequately address hypersomnia, a sleep complaint found in roughly 50% of individuals with MDD (Geoffroy et al., 2018). In general, the research demonstrating poor outcomes following depression treatment in the context of sleep disturbance, as well as studies demonstrating antidepressant effects with sleep improvement, have focused on insomnia-like sleep disturbance. However, it is possible that hypersomnia plays an important role in treatment outcome. To date, little work has been conducted exploring relations of hypersomnia and reward processing, none of which in depressed samples, limiting our ability to hypothesize how these interactions may lead to or exacerbate depression symptoms. There is some evidence, albeit slim, that hypersomnia may be related to reward dysfunction. In one study of patients with Kleine-Levin syndrome, a form of idiopathic hypersomnia, reduced activation in the nucleus accumbens was noted relative to controls during a cognitive effort task (Engström, Landtblom & Karlsson, 2013). A separate study by Rye and Freeman (2011) discusses the dopaminergic system as an underpinning of hypersomnia. Given the significant role of the dopaminergic system in reward processing, it is possible that there exists an interplay between hypersomnia and reward function. As there is a paucity of research in this domain, more work needs to be done to adequately address the role of hypersomnia in reward processes in depression.
Finally, MDD is a complex and highly heterogenous disorder, and it is very possible that a single moderated mediational model of sleep disturbance and reward processing may not adequately explain all symptoms and correlates of the disorder. Without more research to fill in gaps in understanding and explain differential treatment outcomes and trajectories, the model as it stands is highly preliminary. However, it is important to consider this theoretical framework as a starting point to advance future research that focuses on the complex interplay of sleep disturbance and reward processes, a relationship that has been given considerable attention in animal and healthy human subjects, but comparatively little focus in depressed samples. Given the complexity of MDD, studying these relationships using a network framework may be particularly useful (Borsboom & Cramer, 2013; McNally, 2016). Network approaches to the study of psychopathology posit that there are no singular common variables that underly a specific mental disorder; instead latent variables arise from the complex interplay of the various symptoms. As McNally (2016) succinctly explains, “…symptoms are not reflective of underlying mental disorders; they are constitutive of them.” Through this conceptualization, there are contexts (including environmental as well as genetic/biological) which, rather than cause the onset of a disorder, instead generate a network of symptoms that constitute, in that individual, the mental disorder as its currently understood (Borsboom & Cramer, 2013). Indeed, it is possible that sleep disturbance may represent a more severe symptom profile or phenotype, and that rather than sleep disturbance itself, it is this profile of symptoms that drives associations with poor treatment outcomes. Thus, a network approach may uncover a more complex working model of the interactions of sleep and reward function in depression.
Further refinement of this model can also be accomplished by examining the ways in which sleep disturbance and reward processes may possibly interact with other putative mechanisms of MDD, namely inflammatory processes, circadian rhythm disturbances, and monoamine regulation and communication (Fang et al., 2019). Research has already documented involvement of sleep disturbance in these areas (Fang et al., 2019; Palagini et al., 2019), however additional examination of how reward processes may be implicated within these systems is warranted. As previously stated, circadian rhythms and reward function share common neurobiological underpinnings (Murray at al., 2009). There is evidence that inflammation affects reward processing in depression (Felger et al., 2016), and research showing that the SSRI citalopram is associated with reduced activity in the ventral striatum in response to hedonic stimuli calls into question whether these effects may explain why antidepressants are not more universally effective for patients with depression, particularly those with significant anhedonia (McCabe, Mishor, Cowen & Harmer, 2010).
In essence, what we hope to accomplish with the presentation of the ISR model is to encourage parallel study of reward processes with analyses of other leading hypotheses of the mechanisms of MDD. Studies focused on reward processing impairments in depression can be enhanced by assessing both objective and subjective measures of sleep disturbance. Specific symptoms and profiles of sleep disturbance (e.g., sleep onset latency, wakefulness after sleep onset, short sleep duration, excessive sleep) may be differentially related to reward and depression outcomes, thus parsing some of the heterogeneity of MDD. It may be unrealistic for researchers to incorporate neuroimaging of reward pathways into existing protocols examining sleep disturbance in depression, however many behavioral tasks of reward processing (e.g., the EEfRT task (Treadway et al., 2009), as well as self-reports of reward sensitivity (e.g., the Behavioral Inhibition/Behavioral Activation Scales (BIS/BAS; Carver & White, 1994) may be more reasonably included and can help ascertain the degree to which sleep disturbance may influence, or be influenced by, behavioral or self-reported evidence of blunted reward processing. By introducing this model, we hope that the complexity and heterogeneity of MDD can be further parsed and bring us closer to more personalized treatments for those affected.
The need for future study notwithstanding, this model has potential clinical utility. As reviewed, amelioration of sleep disturbance can improve depression severity. Additionally, as mentioned previously, there is evidence that treatment with Behavioral Activation is associated with changes in activation of reward circuitry that correlates with depression improvement (Dichter et al., 2009). For those individuals with MDD and significant sleep disturbance, a combined intervention targeting sleep as well as increasing valued, goal-directed daytime activities may be a particularly potent treatment option. To some degree, some of the tenets of CBT-I include regulation of daytime behaviors (e.g., encouraging activity in the service of reducing daytime napping, getting regular exercise to promote healthy sleep), which may influence reward function; however, these claims must be tested. Investigating this combined treatment modality as well as specific mechanisms of action would be a worthy research enterprise as we search for more effective treatments for depression.
Conclusion
Individuals with depression characterized by significant sleep disturbance are vulnerable to negative treatment outcomes, including an increased likelihood of treatment failure and episode relapse. The ISR model of depression could help the field understand how the presence of sleep disturbance influences the course and treatment of depression. By examining the ways in which sleep disturbance impacts reward-relevant effort, motivation, and learning, we may be able to improve the specificity of treatments for individuals with depression and significant sleep disturbance.
Highlights.
Sleep disturbance is associated with poor depression treatment outcome.
The mechanisms underlying this relationship are poorly understood.
Reward processing is impaired in depression and may be affected by sleep disturbance.
The interaction of these two domains may impair depression recovery.
An integrated model may explain poor outcomes in the presence of sleep disturbance.
Acknowledgments
Funding: This work was supported by the United States Department of Veterans Affairs Clinical Science Research and Development Service (Boland; IK2 CX001501), the National Institute of Mental Health (Gehrman, MH107571; Nusslock, MH100117-01A1, MH077908-01A1; Goldschmied, K23 MH118580). The contents of this manuscript are solely the responsibility of the authors and do not represent the official views of the US government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Admon R, & Pizzagalli DA (2015). Dysfunctional reward processing in depression. Current Opinion in Psychology, 4, 114–118. 10.1016/j.copsyc.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvaro PK, Roberts RM, & Harris JK (2013). A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep, 36, 1059–1068. 10.5665/sleep.2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avinun R, Nevo A, Knodt AR, Elliott ML, Radtke SR, Brigidi BD, … Hariri AR (2017). Reward-Related Ventral Striatum Activity Buffers against the Experience of Depressive Symptoms Associated with Sleep Disturbances. Journal of Neuroscience, 37, 1734–17. 10.1523/JNEUROSCI [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, … Riemann D, (2011). Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders, 135, 10–19. 10.1016/j.jad.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Bao YP, Han Y, Ma J, Wang RJ, Shi L, Wang, … Lu L (2017). Co-occurrence and bidirectional prediction of sleep disturbances and depression in older adults: meta-analysis and systematic review. Neuroscience and Biobehavioral Reviews, 75, 257–273. 10.1016/j.neubiorev.2017.01.032 [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, … Brockmann H (2010). Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biological Psychiatry, 67, 110–116. 10.1210/jc.2011-2759 [DOI] [PubMed] [Google Scholar]
- Boland EM, Rao H, Dinges DF, Smith RV, Goel N, Detre JA, … & Gehrman PR (2017). Meta-analysis of the antidepressant effects of acute sleep deprivation. Journal of Clinical Psychiatry, 78, e1020–e1034. 10.4088/JCP.16r11332 [DOI] [PubMed] [Google Scholar]
- Borsboom D, & Cramer AO (2013). Network analysis: an integrative approach to the structure of psychopathology. Annual Review of Clinical Psychology, 9, 91–121. 10.1146/annurev-clinpsy-050212-185608 [DOI] [PubMed] [Google Scholar]
- Brehm JW, & Self EA (1989). The intensity of motivation. Annual Review of Psychology, 40(1), 109–131. 10.1146/annurev.ps.40.020189.000545 [DOI] [PubMed] [Google Scholar]
- Bunney BG, & Bunney WE (2013). Mechanisms of rapid antidepressant effects of sleep deprivation therapy: clock genes and circadian rhythms. Biological Psychiatry, 73(12), 1164–1171. 10.1016/j.biopsych.2012.07.020 [DOI] [PubMed] [Google Scholar]
- Burani K, Klawohn J, Levinson AR, Klein DN, Nelson BD, & Hajcak G (2019). Neural Response to Rewards, Stress and Sleep Interact to Prospectively Predict Depressive Symptoms in Adolescent Girls. Journal of Clinical Child & Adolescent Psychology, 1–10. 10.1080/15374416.2019.1630834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1989). Pittsburgh sleep quality index (PSQI). Psychiatry Research, 28, 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Carver CS, & White TL (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology, 67(2), 319–333. [Google Scholar]
- Casement MD, Keenan KE, Hipwell AE, Guyer AE, Forbes EE (2016). Neural reward processing mediates the relationship between insomnia symptoms and depression in adolescence. Sleep, 39, 439–447. 10.5665/sleep.5460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JE, & Shapiro CM (2018). Cognitive Behavioural Therapy for Insomnia (CBT-I) to treat depression: A systematic review. Journal of psychosomatic research, 106, 1–12. 10.1016/j.psychores.2017.12.012 [DOI] [PubMed] [Google Scholar]
- Dahan L, Asiter B, Vautrelle N, Urbain N, Kocsis B, Chouvet G (2007). Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology, 32, 1232–1241. 10.1038/sj.npp.1301251 [DOI] [PubMed] [Google Scholar]
- Dew MA, Reynolds CF, Houck PR, Hall M, Buysse DJ, Frank E, … Kupfer DJ (1997). Temporal profiles of the course of depression during treatment: predictors of pathways toward recovery in the elderly. Archives of General Psychiatry, 54, 1016–1024. 10.1001/archpsyc.1997.01830230050007 [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ (2009). The effects of psychotherapy on neural responses to rewards in major depression. Biological Psychiatry, 66, 886–697. 10.1016/j.biopsych.2009.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Kozink RV, McClernon FJ, & Smoski MJ (2012). Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. Journal of Affective Disorders, 136, 1126–1134. 10.1016/j.jad.2011.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Cyranowski JM, Mulsant BH, Houck PR, Buysse DJ, Andreescu C, … Frank E (2008). Which symptoms predict recurrence of depression in women treated with maintenance interpersonal psychotherapy? Depression and Anxiety, 25, 1060–1066. 10.1002/da.20467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Mulsant BH, Houck PR, Mazumdar S, Lenze EJ, Andreescu C, … Reynolds CF III (2007). Residual symptoms and recurrence during maintenance treatment of late-life depression. Journal of Affective Disorders, 103, 77–82. 10.1016/j.jad.2007.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert D, & Berger M (1998). Neurobiological similarities in antidepressant sleep deprivation and psychostimulant use: a psychostimulant theory of antidepressant sleep deprivation. Psychopharmacology, 140(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Edinger JD, & Means MK (2005). Cognitive–behavioral therapy for primary insomnia. Clinical psychology review, 25(5), 539–558. 10.1016/j.cpr.2005.04.003 [DOI] [PubMed] [Google Scholar]
- Engle-Friedman M, Palencar V, Riela S (2010). Sleep and effort in adolescent athletes. Journal of Child Health Care, 14, 131–141. 10.1177/1367493510362129 [DOI] [PubMed] [Google Scholar]
- Engle-Friedman M, & Riela S (2004). Self-imposed sleep loss, sleepiness, effort and performance. Sleep and Hypnosis, 6, 155–162. [Google Scholar]
- Engström M, Landtblom AM, & Karlsson T (2013). Brain and effort: brain activation and effort-related working memory in healthy participants and patients with working memory deficits. Frontiers in Human Neuroscience, 7, 140 10.3389/fnhum.2013.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Tu S, Sheng J, & Shao A (2019). Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. Journal of Cellular and Molecular Medicine, 23(4), 2324–2332. 10.1111/jcmm.14170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, & Miller AH (2016). Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Molecular Psychiatry, 21(10), 1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez Mendoza J, Shea S, Vgontzas AN, Calhoun SL, Liao D, & Bixler EO (2015). Insomnia and incident depression role of objective sleep duration and natural history. Journal of Sleep Research, 24(4), 390–398. 10.1111/jsr.12285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen PL, & Buysse DJ (2008). Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues in Clinical Neuroscience, 10, 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendolla GH, & Krüsken JAN (2001). The joint impact of mood state and task difficulty on cardiovascular and electrodermal reactivity in active coping. Psychophysiology, 38(3), 548–556. 10.1017/S0048577201000622 [DOI] [PubMed] [Google Scholar]
- Gendolla GH, & Krüsken J (2002). The joint effect of informational mood impact and performancecontingent consequences on effort-related cardiovascular response. Journal of Personality and Social Psychology, 83(2), 271 10.1037/0022-3514.83.2.271 [DOI] [PubMed] [Google Scholar]
- Gendolla GHE, Wright RA, Richter M (2012). Effort Intensity: Some Insights from the Cardiovascular System The Oxford Handbook of Human Motivation. Pages 420–438. Oxford University Press. [Google Scholar]
- Geoffroy PA, Hoertel N, Etain B, Bellivier F, Delorme R ….Peyre H (2018). Insomnia and hypersomnia in major depressive episode: Prevalence, sociodemographic characteristics and psychiatric comorbidity in a population-based study. Journal of Affective Disorders, 226, 132–141. 10.1016/j.jad.2017.09.032 [DOI] [PubMed] [Google Scholar]
- Gujar N, Yoo SS, Hu P, & Walker MP (2011). Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. Journal of Neuroscience, 31(12), 4466–4474. 10.1523/jneurosci.3220-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, & Abramson LY (2002). Measuring cognitive vulnerability to depression in adolescence: Reliability, validity, and gender differences. Journal of Clinical Child and Adolescent Psychology, 31(4), 491–504. [DOI] [PubMed] [Google Scholar]
- Harvey L, Inglis SJ, & Espie CA (2002). Insomniacs’ reported use of CBT components and relationship to long-term clinical outcome. Behaviour research and therapy, 40(1), 75–83. 10.1016/S0005-7967(01)00004-3 [DOI] [PubMed] [Google Scholar]
- Harvey AG (2001). Insomnia: symptom or diagnosis? Clinical Psychology Review, 21, 1037–1059. 10.1016/S0272-7358(00)00083-0 [DOI] [PubMed] [Google Scholar]
- Harvey AG (2011). Sleep and circadian functioning: critical mechanisms in the mood disorders? Annual Review of Clinical Psychology, 7, 297–319. 10.1146/annurev-clinpsy-032210-104550 [DOI] [PubMed] [Google Scholar]
- Harvey AG, Murray G, Chandler RA, & Soehner A (2011). Sleep disturbance as transdiagnostic: consideration of neurobiological mechanisms. Clinical Psychology Review, 31(2), 225–235. https://doi/org/10.1016/j.cpr.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm SM, Forbes EE, Ryan ND, Phillips ML, Tarr JA, Dahl RE (2009). Reward-related brain function and sleep in pre/early pubertal and mid/late pubertal adolescents. Journal of Adolescent Health, 45, 326–334. 10.1016/j.jadohealth.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish LA, Kline CE, Gunn HE, Buysse DJ, Hall MH (2015). The role of sleep hygiene in promoting public health: A review of empirical evidence. Sleep Medicine Reviews, 22, 23–36. 10.1016/j,smrv.2014.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovieno N, van Nieuwenhuizen A, Clain A, Baer L, Nierenberg AA (2011). Residual symptoms after remission of major depressive disorder with fluoxetine and risk of relapse. Depression and Anxiety, 28, 137–144. 10.1002/da.20768 [DOI] [PubMed] [Google Scholar]
- Jacobson NS, Martell CR, & Dimidjian S (2001). Behavioral activation treatment for depression: Returning to contextual roots. Clinical Psychology: Science and Practice, 8(3), 255–270. 10.1093/clipsy.8.3.255 [DOI] [Google Scholar]
- Kalmbach DA, Arnedt JT, Song PX, Guille C, Sen S (2017). Sleep disturbance and short sleep as risk factors for depression and perceived medical errors in first-year residents. Sleep, 40(3). 10.1093/sleep/zsw073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin BE, Trockel M, Barr TC, Gimeno J, Manber R (2013). National dissemination of cognitive behavioral therapy for insomnia in veterans: Therapist- and patient-level outcomes. Journal of Clinical and Consulting Psychology, 81, 912–917. 10.1037/a0032554 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, … & Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36(7), 980–988. 10.1097/00004583-1997017000-00021 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, & Prescott CA (2002). Toward a comprehensive developmental model for major depression in women. American Journal of Psychiatry, 159(7), 1133–1145. 10.1176/appi.ajp.159.7.1133 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, & Prescott CA (2006). Toward a comprehensive developmental model for major depression in men. American Journal of Psychiatry, 163(1), 115–124. 10.1176/appi.ajp.163.1.115 [DOI] [PubMed] [Google Scholar]
- Knüppel A, Shipley MJ, Llewellyn CH, & Brunner EJ (2017). Sugar intake from sweet food and beverages, common mental disorder and depression: prospective findings from the Whitehall II study. Scientific Reports, 7(1), 6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konjarski M, Murray G, Lee VV, & Jackson ML (2018). Reciprocal relationships between daily sleep and mood: A systematic review of naturalistic prospective studies. Sleep Medicine Reviews, 42, 47–58. 10.1016/j.smrv.2018.05.005 [DOI] [PubMed] [Google Scholar]
- Libedinsky C, Massar SA, Ling A, Chee W, Huettel SA, & Chee MW (2013). Sleep deprivation alters effort discounting but not delay discounting of monetary rewards. Sleep, 36(6), 899–904. 10.5665/sleep.2720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking KR, Pagliaccio D, Luby JL, & Barch DM (2016). Depression risk predicts blunted neural responses to gains and enhanced responses to losses in healthy children. Journal of the American Academy of Child & Adolescent Psychiatry, 55(4), 328–337. 10.1016/j.jaac.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manber R, Edinger JD, Gress JL, Pedro-Salcedo MGS, Kuo TF, Kalista T (2008). Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep, 31, 489–495. 10.1093/sleep/31.4.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manber R, Buysse DJ, Edinger J, Krystal A, Luther JF, Wisniewski SR, … & Thase ME (2016). Efficacy of Cognitive-Behavioral Therapy for Insomnia Combined with Antidepressant Pharmacotherapy in Patients with Comorbid Depression and Insomnia: A Randomized Controlled Trial. The Journal of Clinical Psychiatry, 77(10), e1316–e1323. 10.4088/JCP.15m10244 [DOI] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Cowen PJ, & Harmer CJ (2010). Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biological Psychiatry, 67(5), 439–445. 10.1016/j.biopsych.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna BS, Dickinson DL, Orff HJ, & Drummond SP (2007). The effects of one night of sleep deprivation on known risk and ambiguous risk decisions. Journal of Sleep Research, 16(3), 245–252. 10.1111/j.1365-2869.2007.00591.x [DOI] [PubMed] [Google Scholar]
- McNally RJ (2016). Can network analysis transform psychopathology? Behaviour Research and Therapy, 86, 95–104. 10.1016/j.brat.2016.06.006 [DOI] [PubMed] [Google Scholar]
- Meltzer LJ, & Mindell JA (2007). Relationship between child sleep disturbances and maternal sleep, mood, and parenting stress: a pilot study. Journal of Family Psychology, 21(1), 67 10.1037/0893-3200.21.1.67 [DOI] [PubMed] [Google Scholar]
- Miller CB, Espie CA, Epstein DR, Friedman L, Morin CM, Pigeon WR, … & Kyle SD (2014). The evidence base of sleep restriction therapy for treating insomnia disorder. Sleep Medicine Reviews, 18(5), 415–424. 10.1016/j.smrv.2014.01.006 [DOI] [PubMed] [Google Scholar]
- Morgenthaler T, Kramer M, Alessi C, Friedman L, Boehlecke B,…Swick T (2006). Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep, 29, 1415–9. 10.1093/sleep/29.11.1415 [DOI] [PubMed] [Google Scholar]
- Mullin BC, Phillips ML, Siegle GJ, Buysse DJ, Forbes EE, & Franzen PL (2013). Sleep deprivation amplifies striatal activation to monetary reward. Psychological Medicine, 43(10), 2215–2225. 10.1017/S0033291712002875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA (2007). The amygdala, reward and emotion. Trends in Cognitive Sciences, 11(11), 489–497. 10.1016/j.tics.2007.08.013 [DOI] [PubMed] [Google Scholar]
- Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, Allen NB … Trinder J (2009). Nature’s clocks and human mood: The circadian system modulated reward motivation. Emotion, 9, 705–716. 10.1037/a0017080 [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Husain MM, Trivedi MH, Fava M, Warden D, Wisniewski SR … Rush AJ (2010). Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR* D report. Psychological Medicine, 40, 41–50. 10.1017/S0033291709006011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, & Alloy LB (2017). Reward processing and mood-related symptoms: An RDoC and translational neuroscience perspective. Journal of Affective Disorders, 216, 3–16. 10.1016/j.jad.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, McMakin DL, Dahl RE, Ryan ND, Silk JS, Birmaher B, … Forbes EE (2011). “I won, but I’m not getting my hopes up”: Depression moderates the relationship of outcomes and reward anticipation. Psychiatry Research Neuroimaging, 194, 393–395. 10.1016/j.pscychresns.2011.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, McMakin DL, Morgan JK, Silk JS, Birmaher B, Axelson DA, … Forbes EE (2014). Reduced reward anticipation in youth at high-risk for unipolar depression: a preliminary study. Developmental Cognitive Neuroscience, 8, 55–64. 10.1016/j.dcn.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel J, Kessler RC, & Schoevers R (2019). Depression: more treatment but no drop in prevalence: how effective is treatment? And can we do better? Current Opinion in Psychiatry, 32(4), 348–354. 10.1097/YCO.00000000000000505 [DOI] [PubMed] [Google Scholar]
- Palagini L, Bastien CH, Marazziti D, Ellis JG, & Riemann D (2019). The key role of insomnia and sleep loss in the dysregulation of multiple systems involved in mood disorders: A proposed model. Journal of Sleep Research, e12841 10.1111/jsr/12841 [DOI] [PubMed] [Google Scholar]
- Palagini L, Domschke K, Benedetti F, Foster RG, Wulff K, & Riemann D (2018). Developmental pathways towards mood disorders in adult life: Is there a role for sleep disturbances? Journal of Affective Disorders, 243; 121–132. 10.1016/j.jad.2018.09.011 [DOI] [PubMed] [Google Scholar]
- Perlis ML, Giles DE, Buysse DJ, Tu X, Kupfer DJ (1997). Self-reported sleep disturbance as a prodromal symptom in recurrent depression. Journal of Affective Disorders, 42, 209–212. 10.1016/S0165-0327(96)01411-5 [DOI] [PubMed] [Google Scholar]
- Perogamvros L, Schwartz S (2012). The roles of the reward system in sleep and dreaming. Neuroscience and Biobehavioral Reviews, 36, 1934–1951. 10.1016/j.neubiorev.2012.05.010 [DOI] [PubMed] [Google Scholar]
- Pechtel P, Dutra SJ, Goetz EL, Pizzagalli DA (2013). Blunted reward responsiveness in remitted depression. Journal of Psychiatric Research, 47, 1864–1869. 10.1016/j.jpsychires.2013.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, … Fava M (2009). Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. American Journal of Psychiatry, 166, 702–710. 10.1176/appi.ajp.2008.08081201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann D, Voderholzer U (2003). Primary insomnia: a risk factor to develop depression? Journal of Affective Disorders, 76, 255–259. 10.1016/S0165-0327(02)00072-1 [DOI] [PubMed] [Google Scholar]
- Roane BM, Taylor DJ (2008). Adolescent insomnia as a risk factor for early adult depression and substance abuse. Sleep, 31, 1351–1356. 10.5665/sleep/31.10.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RE, Duong HT (2014). The prospective association between sleep deprivation and depression among adolescents. Sleep, 37, 239–244. 10.5665/sleep.3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rye DB, & Freeman AA (2011). Dopaminergic substrates underlying hypersomnia, sleepiness, and REM sleep expression In Narcolepsy (pp. 61–71). Springer, New York, NY. [Google Scholar]
- Rzepa E, Fisk J, McCabe C (2017). Blunted neural response to anticipation, effort and consummation of reward and aversion in adolescents with depression symptomatology. Journal of Psychopharmacology, 31, 303–311. 10.1177/0269881116681416 [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C (2008). Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology, 33, 368–377. 10.1038/sj.npp.1301408 [DOI] [PubMed] [Google Scholar]
- Schmidt RE, Richter M, Gendolla GH, & Van der Linden M (2010). Young poor sleepers mobilize extra effort in an easy memory task: Evidence from cardiovascular measures. Journal of Sleep Research, 19(3), 487–495. 10.1111/j.1365-5869.2010.00834.x [DOI] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, & Dichter GS (2009). fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. Journal of Affective Disorders, 118(1–3), 69–78. 10.1016/j.jad.2009.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staner L (2010). Comorbidity of insomnia and depression. Sleep Medicine Reviews, 14, 35–46. 10.1016/j.smrv.2009.09.003 [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Lichstein KL, Weinstock J, Sanford S, Temple JR (2007). A pilot study of cognitivebehavioral therapy of insomnia in people with mild depression. Behavior Therapy, 38, 49–57. 10.1016/j.beth.2006.04.002 [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Walters HM, Vittengl JR, Krebaum S, Jarrett RB (2010). Which depressive symptoms remain after response to cognitive therapy of depression and predict relapse and recurrence? Journal of Affective Disorders, 123, 181–187. 10.1016/j.jad.2009.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME, Simons AD, & Reynolds CF (1996). Abnormal electroencephalographic sleep profiles in major depression: association with response to cognitive behavior therapy. Archives of General Psychiatry, 53(2), 99–108. Doi: 10.1001/archpsyc.1996.01830020013003 [DOI] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH (2009). Worth the ‘EEfRT’? The Effort Expenditure for Rewards Task as an objective measure of motivation and anhedonia. PLoS ONE, 4, e6598 10.1371/journal.pone.0006598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH (2012). Effort-based decision-making in major depressive disorder: A translational model of motivational anhedonia. Journal of Abnormal Psychology, 121, 553–558. 10.1037/a0028813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxel WM, Kupfer DJ, Reynolds CF, Frank E, Thase M, Miewald J, … Buysse DJ (2012). Insomnia and objectively measured sleep disturbances predict treatment outcome in depressed patients treated with psychotherapy or psychotherapy-pharmacotherapy combinations. The Journal of Clinical Psychiatry, 73, 478–485. 10.4088/JCP.11m07184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuno N, Besset A, Ritchie K (2005). Sleep and depression. The Journal of Clinical Psychiatry, 66, 1254–1269. 10.4088/JCP.v66n1008 [DOI] [PubMed] [Google Scholar]
- Umemoto A, Holroyd CB (2017). Neural mechanisms of reward processing associated with depression-related personality traits. Clinical Neurophysiology, 128, 1184–1196. 10.1016/j.clinph.2017.03.049 [DOI] [PubMed] [Google Scholar]
- Watson D, & Clark LA (1991). The mood and anxiety symptom questionnaire (MASQ) Unpublished manuscript, University of Iowa, Iowa City. [Google Scholar]
- Weinberg A, Shankman SA (2017). Blunted reward processing in remitted melancholic depression. Clinical Psychological Science, 5, 14–25. 10.1177/2167702616633158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton AE, Treadway MT, Pizzagalli DA (2015). Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Current Opinion in Psychiatry, 28, 7–12. 10.1097/YCO.0000000000000122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe ST, Cassoff J, & Gruber R (2012). Sleep patterns and the risk for unipolar depression: a review. Nature and Science of Sleep, 4, 63 10.2147/NSS.S23490 [DOI] [PMC free article] [PubMed] [Google Scholar]