Summary
DNA methylation is a universal epigenetic mechanism involved in regulation of gene expression and genome stability. The DNA maintenance methylase DNMT1 ensures that DNA methylation patterns are faithfully transmitted to daughter cells during cell division. Because loss of DNMT1 is lethal, a pan-organismic analysis of DNMT1 function is lacking. We identified new recessive dnmt1 alleles in medaka and zebrafish and, guided by the structures of mutant proteins, generated a recessive variant of mouse Dnmt1. Each of the three missense mutations studied here distorts the catalytic pocket and reduces enzymatic activity. Because all three DNMT1 mutant animals are viable, it was possible to examine their phenotypes throughout life. The consequences of genome-wide hypomethylation of DNA of somatic tissues in the Dnmt1 mutants are surprisingly mild but consistently affect the development of the lymphoid lineage. Our findings indicate that developing lymphocytes in vertebrates are sensitive to perturbations of DNA maintenance methylation.
Subject Areas: Molecular Genetics, Phenotyping, Transcriptomics
Graphical Abstract
Highlights
-
•
Genetic screens identified recessive viable missense alleles of dnmt1 in teleosts
-
•
A viable mouse Dnmt1 mutant generated by structure-guided precision mutagenesis
-
•
Missense mutations distort the catalytic pocket and reduce enzymatic activity
-
•
DNA hypomethylation consistently affects development of the lymphoid lineage
Molecular Genetics; Phenotyping; Transcriptomics
Introduction
DNA methylation is a key mechanism of epigenetic control that is required for development and survival by regulating gene expression and genome stability (Bergman and Cedar, 2013; Smith and Meissner, 2013). Methylation of cytosines in DNA is established by de novo methylases Dnmt3a and Dnmt3b, whereas its propagation after DNA replication and repair depends on the maintenance of methylase Dnmt1. In the latter process, Dnmt1 recognizes the hemimethylated DNA duplex and copies the methylation pattern of the parental strand to the newly synthesized DNA strand. Considerable information is available concerning the structure and function of the various domains of the Dnmt1 protein, providing the basis to interpret the functional consequences of structural variants (Cheng et al., 2015; Song et al., 2012; Takeshita et al., 2011; Zhang et al., 2015). In accordance with its central cellular function (Smith and Meissner, 2013), mice lacking Dnmt1 die at around day 9.5 of embryonic development (Li et al., 1992). Similarly, mutations of zebrafish dnmt1 predicted to impair the function of the catalytic domain die at 8 days postfertilization (dpf) (Anderson et al., 2009). This early embryonic lethality conceals a possible tissue-specific function of this protein in the adult organism.
Lymphocytes represent the cellular underpinning of the adaptive immune system and are, as all blood lineages, descendants of hematopoietic stem cells. The molecular basis of their differentiation, proliferation, selection, and maturation has been extensively studied (Rothenberg et al., 2016; Cumano et al., 2019; Clark et al., 2014; Medvedovic et al., 2011), particularly with respect to the functional roles of certain transcription factors. By contrast, the intricacies of the epigenetic control of lymphopoiesis are only beginning to be explored. Nonetheless, previous work has indicated that DNA methylation is important for normal hematopoietic development (Jeong and Goodell, 2014; Guillamot et al., 2016; Cedar and Bergman, 2011); for instance, low levels of Dnmt1 protein are associated with failure of normal lymphocyte development (Broske et al., 2009) and leukemogenesis (Gaudet et al., 2003). However, so far, hypomorphic germline mutations compatible with a normal life span and selective failure of lymphoid differentiation have not been described in vertebrates.
Here, using a forward genetics approach with medaka and zebrafish, we have identified missense mutations in functionally important regions of dnmt1 that result in reduced enzymatic activities. The identification of the structural consequences of these missense mutations has allowed us to generate a mouse model of a recessive and viable Dnmt1 missense mutation that also gives rise to an enzyme of reduced activity. For the first time, these viable mutations provide a pan-organismic view of DNMT1 function in vertebrates that are separated by several million years of independent evolution. Remarkably, in all three species, DNMT1 mutations are associated with impaired lymphoid development. This demonstrates that hematopoietic precursors poised to feed the lymphoid lineages are uniquely sensitive to perturbations of the DNA methylation process. We thus conclude that an ancient epigenetic control mechanism was deployed to enable the development of lymphocytes, which represents a key innovation in the vertebrate immune system (Boehm and Swann, 2014).
Results
Previously, we have conducted large-scale forward genetic screens in zebrafish (Boehm et al., 2003; Iwanami et al., 2016; Schorpp et al., 2006), and medaka (Furutani-Seiki et al., 2004; Iwanami et al., 2004, 2008, 2009) aimed at identifying mutations affecting vertebrate T cell development. As a result, several dozens of mutants exhibiting a noticeable reduction of developing T cells in the thymus in early larval development were identified. Here, we describe the first two viable recessive alleles of teleost dnmt1, overcoming the previous limitations on phenotypic analyses imposed by lethal dnmt1 alleles.
A Zebrafish dnmt1 Mutation Specifically Affecting Lymphopoiesis
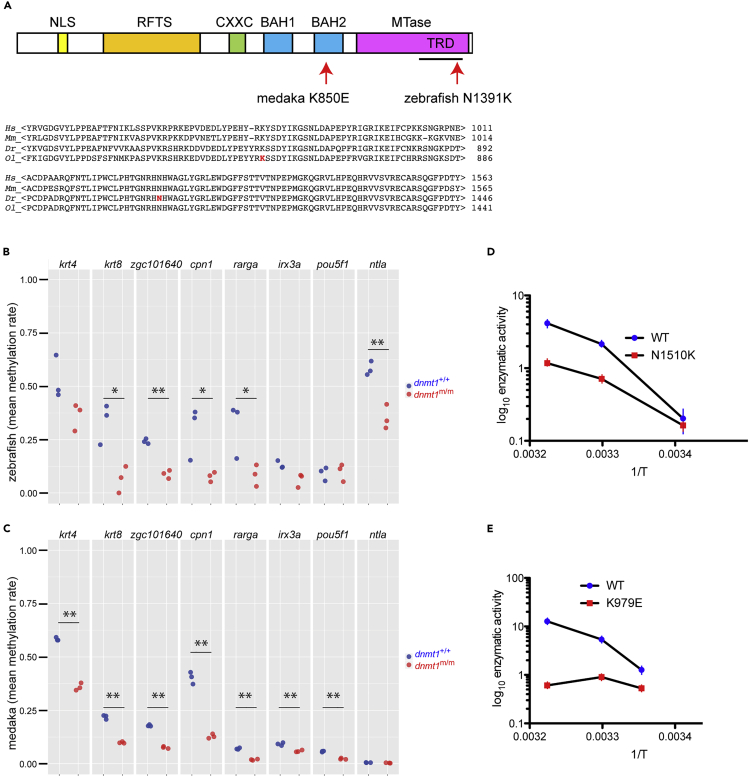
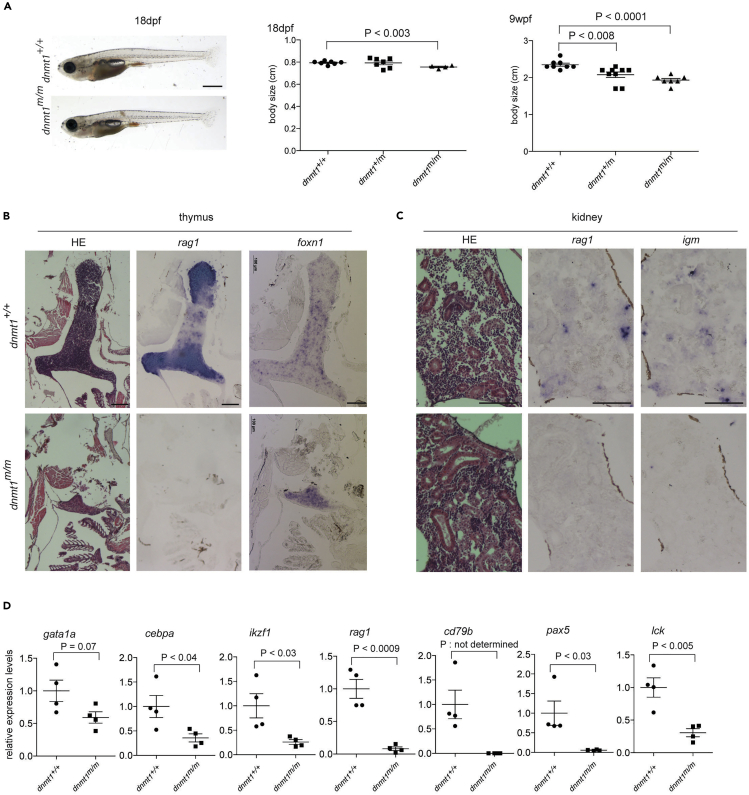
In line IY071, the extent of T cell development in the thymus at 5 days after fertilization (dpf) is severely reduced, as determined by whole mount RNA in situ hybridization (Iwanami et al., 2016). The mutation in the IY071 line was identified as a missense mutation (T to G at nucleotide position 3:54,337,492 [GRCz10]) in dnmt1 causing the substitution of the asparagine residue 1391 to lysine (N1391K) (Iwanami et al., 2016) (Figure 1A). The amino acid replacement occurred in a region exhibiting an exceptional degree of evolutionary conservation (Figure 1A). In the mouse protein, this residue is equivalent to N1510 and is situated in the target recognition domain (TRD) of Dnmt1 (Song et al., 2012). The mutants of both sexes reached adulthood, and apart from a slight size difference (Figure 2A), fish appeared grossly normal. Adult fish exhibited impaired development of T cells (Figure 2B) and B cells (Figure 2C), as determined by RNA in situ hybridization and subsequent qPCR-based gene expression analyses in whole kidney marrow cells; other hematopoietic cell lineages appear to be less affected (Figure 2D). Next, we examined whether the hematopoietic environment in dnmt1 mutants was capable of supporting normal blood cell development. To do this, we transplanted whole kidney marrow cells of wild-type ikzf1:egfp transgenic zebrafish into non-irradiated adult dnmt1 mutants as recipients; the reporter transgene marks the lymphocyte lineage in zebrafish (Bajoghli et al., 2009; Hess and Boehm, 2012). When assayed at 8 days after transplantation, donor cell engraftment was detectable in the thymus (data not shown) and kidney (Figure S1A) of 50% (7/14) of dnmt1 mutant recipients; by contrast, donor cells were not found in wild-type and heterozygous recipients (0/16; difference significant at p = 0.002, Χ2-test), presumably as a consequence of MHC mismatches between donor and recipient. Collectively, these results suggest that the non-hematopoietic microenvironment of dnmt1 mutants is capable of supporting the differentiation of wild-type lymphocytes. We attribute the failure of engraftment in some dnmt1 mutants to the residual presence of lymphocytes, which is evident from lymphocyte-specific transcripts in kidney marrow cells (Figure S1B); this indicates that the block of lymphocyte differentiation in the dnmt1 zebrafish mutant is incomplete. However, it is also possible that the variable outcome of the transplantation experiments is due to different fitness levels of mutant recipient and wild-type donor progenitor cells. When kidney marrow cells of successfully reconstituted dnmt1 mutants were secondarily transplanted into c-myb mutants (which lack both hematopoietic progenitor and mature blood cells and thus serve as universal recipients (Hess et al., 2013)), EGFP+ cells were found to be present in the kidney (Figure S1C) and thymus (Figure S1D) of secondary recipients. This finding suggests that long-term reconstituting hematopoietic precursor cells survive in the dnmt1 mutant recipients and supports the notion of the hematopoietic origin of failing lymphopoiesis in dnmt1 mutants.
Figure 1.
Identification of Missense Mutations in Medaka and Zebrafish dnmt1 Genes
(A) Schematic structure of the DNMT1 protein with relevant protein domains indicated; NLS, nuclear localization signal; RFTS, replication foci targeting site; CXXC, cysteine-rich domain; BAH, bromo-adjacent homology domains 1 and 2; MTase, catalytic domain. (Arrow) Approximate position of the amino acid replacement in the target recognition domain, TRD (top panel). Alignments of amino acid sequences of human (Hs), mouse (Mm), zebrafish (Dr), and medaka (Ol) dnmt1 proteins at the region close to the medaka (red, middle panel) and zebrafish (red, bottom panels) missense mutations.
(B) Average methylation levels of CpGs in indicated CpG islands of total body DNA in zebrafish dnmt1 mutants and siblings at 18 dpf. ∗p < 0.05, ∗∗p < 0.01; t test, two-tailed.
(C) Average methylation levels of CpGs in indicated CpG islands of total body DNA in medaka dnmt1 mutants and siblings at 18 dpf. ∗p < 0.05, ∗∗p < 0.01; t test, two-tailed.
(D) Enzymatic activities (mol CH3 incorporated/h/mol of Dnmt1) of mouse wild-type and N1510K variant Dnmt1 proteins as a function of temperature T measured in Kelvin displayed as Arrhenius plots; mean ± SEM (n = 3).
(E) Enzymatic activities (mol CH3 incorporated/h/mol of Dnmt1) of mouse wild-type and K979E variant Dnmt1 proteins as a function of temperature T measured in Kelvin displayed as Arrhenius plots; mean ± SEM (n = 3).
See also Figures S1, 2, and 3.
Figure 2.
Both T Cell and B Cell Development Are Predominantly Affected in Zebrafish Adult dnmt1 Mutant
(A) Gross appearances of dnmt1 mutants at 18 dpf. Scale bar, 1mm (left panel). Standard lengths of dnmt1 mutant fish at 18dpf. dnmt1+/+ (n = 7), dnmt1+/m (n = 7), dnmt1m/m (n = 4) (middle panel). Standard lengths of dnmt1 mutant fish at 9 wpf. dnmt1+/+ (n = 8), dnmt1+/m (n = 9), dnmt1m/m (n = 7) (right panel).
(B) Hematoxylin/eosin (HE) staining and RNA in situ hybridization using rag1 and foxn1 probes of paraffin sections of thymic region of dnmt1 mutant at 9 wpf. Scale bars, 100 μm.
(C) H&E staining and RNA in situ hybridization using rag1 and igm probes of paraffin sections of kidney region of dnmt1 mutant at 9 wpf. Scale bars, 100 μm.
(D) Gene expression levels for indicated genes in whole kidney marrow samples of dnmt1+/+ and dnmt1m/m at 18 wpf as assessed by qPCR; mean ± SEM. The values for wild-type controls are set to 1.0.
See also Figures S1, 2, and 3.
A Medaka dnmt1 Mutation Specifically Affecting Embryonic Lymphopoiesis
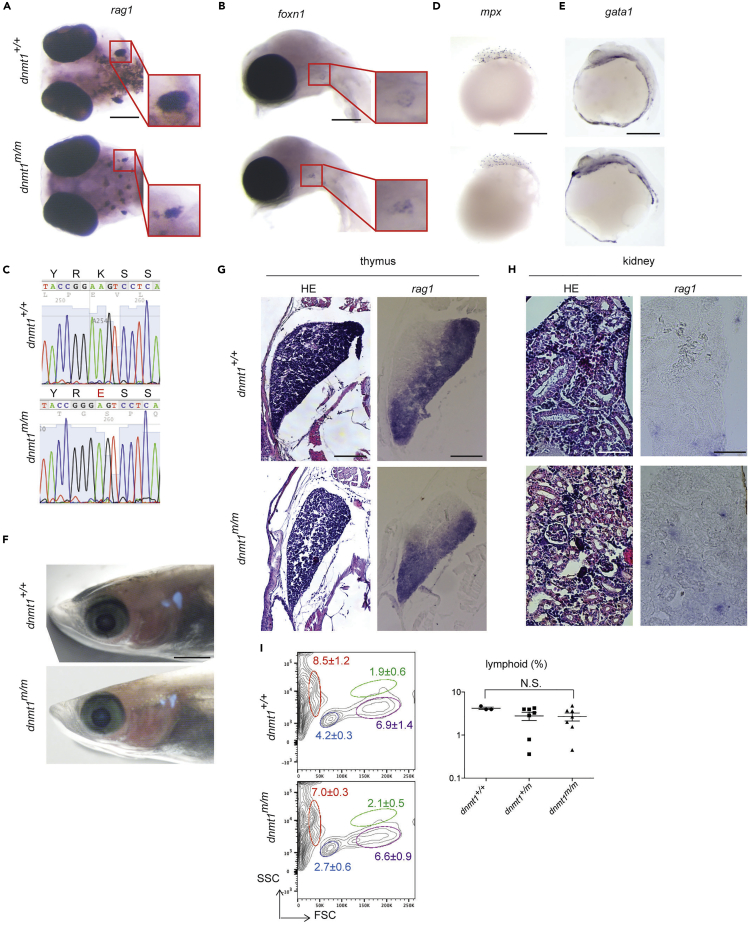
The gyokuro (gkr; strain j48-12B) mutant line exhibited a reduction of rag1-expressing immature thymocytes at stage 35 (equivalent to 5 days after fertilization [dpf]) (Iwamatsu, 2004) in whole mount RNA in situ hybridization assays (Iwanami et al., 2004) (Figure 3A); this contrasts with normal expression of foxn1, a marker of thymic epithelium (Schorpp et al., 2002) (Figure 3B). A combinatorial approach consisting of linkage analysis and genome sequencing (Table S1) identified a missense mutation (Figure 3C) in the second BAH domain of dnmt1, K850E (Figure 1A). This region is well conserved in vertebrate DNMT1 enzymes; in the mouse protein, this residue is equivalent to K979E (Figure 1A). Detailed characterization of the gkr mutant suggested that the dnmt1 missense mutation predominantly affects the lymphoid lineage of mutant embryos, whereas myeloid and erythroid differentiation pathways—as determined by RNA in situ hybridization using mpx- and gata1-specific probes—appear to be unimpaired (Figures 3D and 3E). By contrast, adult hematopoiesis was found to be essentially normal in the gkr mutants. This is evident from the normal cellularity of the thymus as revealed by analysis of gkr mutants transgenic for a rag1:egfp reporter (Li et al., 2007) (Figure 3F) and also in histological studies and RNA in situ hybridizations of thymus (Figure 3G) and kidney (Figure 3H) with a rag1-specific probe; moreover, analysis of kidney marrow cells by flow cytometry (Figure 3I) showed no abnormalities. Collectively, these results suggest that aberrations in the larval stages of T cell development are followed by an appreciable recovery of lymphopoiesis in adult medaka dnmt1 mutants, which exhibit a normal life-span.
Figure 3.
Impaired Lymphocyte Development in Medaka dnmt1 Mutant
(A) Whole mount RNA in situ hybridization pattern in gkr mutant using a rag1 probe at stage 35 (5 dpf). Ventral view. Right panels display enlarged thymic areas marked in left panels. Scale bar, 200 μm.
(B) Expression of the thymic epithelia cell marker foxn1 in dnmt1 mutant at stage 35. Right panels display enlarged thymic areas of left panels. Scale bar, 200 μm.
(C) Representative sequence traces indicating the A > G transversion at nucleotide position 8,624,154 (MEDAKA1 in Ensembl release 72) on chromosome 8. The conceptual translation in three-letter code is also shown.
(D) Expression of macrophage marker mpx in dnmt1 mutants at stage 22 (1.5 dpf). Scale bar, 500 μm.
(E) Expression of erythroid marker gata1 in dnmt1 mutant at stage 25 (2 dpf). Scale bar, 500 μm.
(F) Expression of rag1:egfp transgene in dnmt1 mutant at 7 wpf. Scale bar, 2 mm.
(G) H&E staining and RNA in situ hybridization using a rag1 probe on paraffin sections of the thymic region of dnmt1 mutant at 7 wpf. Scale bars, 100 μm.
(H) H&E staining and RNA in situ hybridization using a rag1 probe on paraffin sections of the kidney region of dnmt1 mutant at 7 wpf. Scale bars, 50 μm. For (F)-(H), figures are representative of 1 fish per genotype.
(I) Flow cytometric profiles of whole kidney marrow cells of dnmt1 mutant at 7 wpf. Light scatter profiles with percentages of erythroid (red), lymphoid (light blue), myeloid (green), and precursor (purple) indicated; mean ± SEM is shown; dnmt1+/+ (n = 3), dnmt1m/m (n = 7) (left panels). Percentages of lymphoid population, mean ± SEM is shown; dnmt1+/+ (n = 3), dnmt1+/m (n = 7), dnmt1m/m (n = 7) (right panel). N.S., not significant.
Panels A, B, D, and E representative of at least 8 fish per genotype. See also Figure S3.
DNA Hypomethylation in dnmt1 Mutants
The non-conservative amino acid replacements in zebrafish and medaka dnmt1 mutants (Figure 1A) predict reduced enzymatic activities for the altered proteins. This was examined in two ways. First, we used bisulfite sequencing using DNA extracted from the bodies of 18 dpf wild-type and mutant zebrafish and medaka fish. We focused our analysis on eight CpG islands, which were previously shown to be differentially methylated between sperm and oocyte DNA of zebrafish (five CpG islands were hypermethylated and three were hypomethylated in sperm) (Potok et al., 2013). In zebrafish, the average methylation levels of six CpG islands were substantially lower in mutants, whereas little change was observed in two CpG islands already exhibiting low methylation levels (Figure 1B). When the eight orthologous CpG islands in the medaka genome were examined, we found that the average methylation levels of seven CpG islands were also significantly lower in medaka mutants (Table S2; Figure 1C). These results suggest that the dnmt1 missense mutations in zebrafish and medaka identified here affect the extent of maintenance of DNA methylation in similar ways and eventually cause hypomethylation in somatic tissues. Second, we studied the consequences of replacing the two evolutionarily conserved residues on the in vitro activities of the mouse Dnmt1 enzyme. To this end, we mutated the mouse Dnmt1 gene to generate the orthologous mutants (K979E for the medaka mutation and N1510K for the zebrafish mutation); we then expressed the wild-type and the two mutant forms in a baculovirus system and used the purified proteins for in vitro methylation assays using a hemimethylated DNA substrate. Both mutants exhibited drastically reduced maintenance methylation activities; in addition, the K979E mutant exhibited a notable temperature sensitivity at 37°C (Figures 1D and 1E).
Structural Basis of Reduced Enzymatic Activities of Dnmt1 Mutants
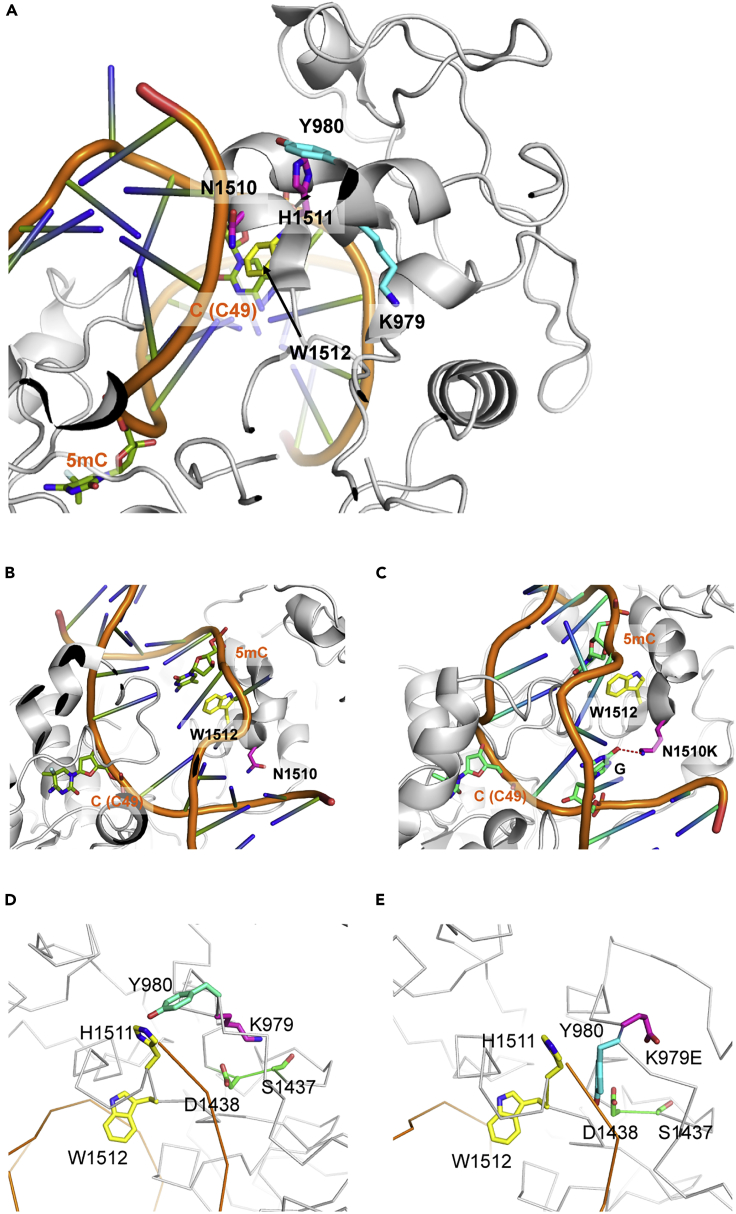
Since the recessive missense mutations in zebrafish and medaka dnmt1 genes identified here represent the only viable vertebrate mutant dnmt1 alleles so far described, we set out to determine the structural basis of impaired maintenance methylation. We first examined the available information on the structure of the mouse Dnmt1 protein (Cheng et al., 2015; Song et al., 2012; Takeshita et al., 2011; Zhang et al., 2015) to locate the corresponding residues that are mutated in zebrafish and medaka dnmt1 proteins. This analysis was facilitated by the high degree of evolutionary conservation of the regions containing the two mutations (Figure 1A). Remarkably, we found that the medaka mutation in the BAH2 domain is positioned in close proximity to the zebrafish mutation in the TRD of the enzyme. In the mouse Dnmt1 protein, residues Y980 and H1511 (both situated very close to the altered residues, i.e., equivalent to mouse residues K979 and N1510, respectively) directly interact with each other (Figure 4A) (Song et al., 2012; Takeshita et al., 2011). These structural features suggested a common mechanism leading to the impairment of DNMT1s enzymatic activity and prompted us to explore the structural consequences of the two mutations by molecular dynamics simulation. When the zebrafish mutation is modeled by replacing asparagine 1510 with a lysine residue, new hydrogen bonds are formed with bases of the substrate DNA near the hemimethylated site (Figures 4B and 4C); we propose that these changes interfere with the processivity of the Dnmt1 enzyme. When the structure of zebrafish dnmt1 protein is modeled on the basis of the mouse Dnmt1 crystal structure (Figure S2A), the emergence of new hydrogen bonds between K1391 and the substrate DNA is again observed (Figure S2B), suggesting that the model of the zebrafish protein calculated from molecular dynamics simulation is an accurate representation of its three-dimensional structure. Further simulations aimed at examining the structural consequences of the medaka mutation indicated that replacing lysine 979 with glutamic acid in mouse Dnmt1 causes a structural rearrangement that repositions Y980 next to D1438; as a result, the substrate DNA is dislodged from the catalytic cleft (Figures 4D and 4E). These structural changes are mirrored in the model of the medaka K850E mutant protein (Figures S3A and S3B). Collectively, the structural studies explain the hypomorphic nature of the two dnmt1 missense mutations through specific structural rearrangements of the catalytic site of the enzyme.
Figure 4.
Structure of Wild-type and Variant Dnmt1 Proteins
(A) Structure of the catalytic pocket of the mouse Dnmt1 protein (Song et al., 2012), highlighting the spatial proximity of the two mutated residues; zebrafish missense mutation corresponding to mouse residue N1510 and medaka missense mutation corresponding to mouse residue K979. The hemimethylated DNA substrate is indicated in orange, with the position of the 5-methylcytosine (5mC) indicated.
(B and C) Comparison of the catalytic pockets of mouse wild-type (B) and N1510K mutant (C) Dnmt1 proteins as determined by molecular dynamics simulation; note the formation of hydrogen bonds between the lysine residue and the guanine (G) base (red dotted line).
(D and E) Comparison of the catalytic pockets of mouse wild-type (D) and K979E mutant (E) Dnmt1 proteins as determined by molecular dynamics simulation; note the distortion of the catalytic pocket in the mutant variant.
See also Figure S3.
Embryonic Lethality of N1510K and K979E Mouse Mutants
The functional and structural studies reported above implied that the introduction of the N1510K and K979E mutations into the mouse germline should cause an impaired activity of the mutant Dnmt1 proteins in vivo. To confirm this, we generated the appropriate nucleotide changes in the mouse Dnmt1 gene by CRISPR/Cas9-mediated nucleotide replacement and examined the phenotype of the resulting mutant lines. Mice homozygous for either mutation exhibited embryonic lethality, similar to Dnmt1-deficient mice (Li et al., 1992). The phenotype of heterozygous mutants was found to be indistinguishable from that of wild-type siblings, indicating the recessive nature of the two mutant alleles. Based on the non-linear temperature-dependent loss of enzymatic activities of mutant proteins (Figures 1D and 1E), we propose that owing to the higher body temperature of mice (∼37°) compared with fish (∼28°C), the DNA methylase activities of both mutants fall below the threshold of activities required to sustain normal mouse development.
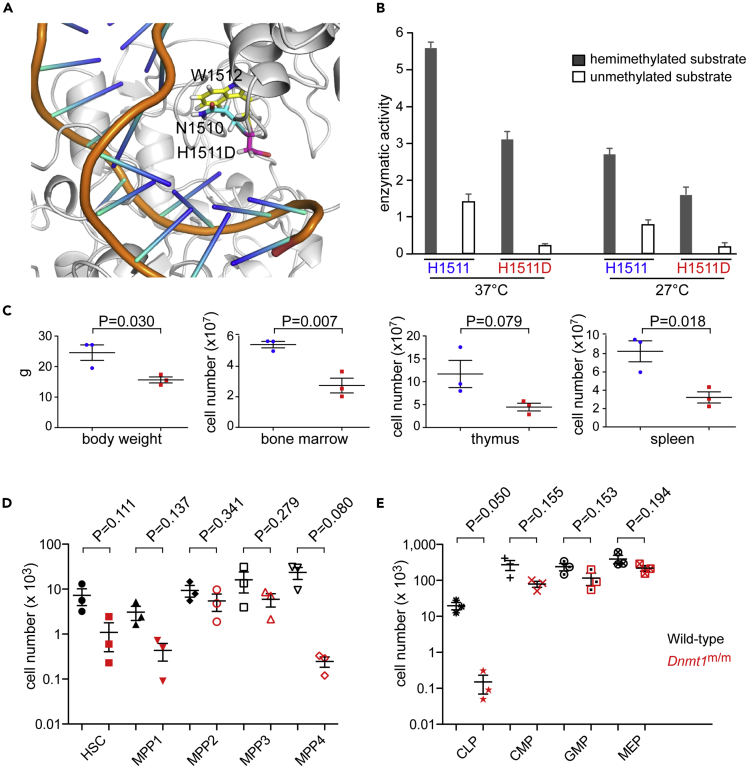
Structure-Guided Generation of a Viable Dnmt1 Mutant
Although the orthologous zebrafish and medaka mutations in Dnmt1 were not compatible with extended survival of homozygous mutant mice, our structural studies nevertheless provided a useful starting point to explore other mutations in Dnmt1. To this end, we focused on the H1511 residue, which was shown to be important in configuring the catalytic site of Dnmt1 (Song et al., 2012; Takeshita et al., 2011) (Figure 4A). Here, we report on a mutation that converts histidine 1511 to aspartic acid, H1511D. The outcome of molecular dynamics simulation indicated that the H1511D mutation causes the side chain of N1510 to reposition near to the indol group of tryptophane 1512 (Figure 5A). As expected, this structural rearrangement in the catalytic center is accompanied by reduced enzymatic activity in vitro; the H1511D mutation reduces maintenance activity to about 50% of the wild-type version (Figure 5B). The residual activity of the H1511D mutant is higher than those observed for the N1510K and K979E mutations (about 30% and 5%, respectively) (Figures 1D and 1E); this result indicates that we achieved our goal of generating a hypomorphic version of the enzyme with intermediate level of activity. Next, we used CRSPR/Cas9 mutagenesis to introduce the H1511D mutation into the germline of mice. Mice homozygous for the H1511D mutation are viable, providing in vivo evidence that the phenotypic consequences of the H1511D mutation are less severe than those of the N1510K and K979E mutations. Several phenotypic features of H1511D homozygous mutant mice are notable. The animals are smaller and have a reduced body mass (2/3 of normal weight at 7 weeks of age); these findings hint at pleiotropic effects of the mutation that do, however, not compromise viability. Considering the observations made in the two teleost models, we focused on the hematopoietic system of H1511D/H1511D mice. With respect to the cellularity in lymphoid organs, we found that the number of bone marrow cells is reduced to half, whereas the numbers of thymocytes and splenocytes are reduced even further to about 40% of wild-type levels (Figure 5C). In stark contrast to the previously described model of low Dnmt1 activity (Broske et al., 2009), the Dnmt1H1511D/H1511D mice did not develop hematological malignancies. Both male and female homozygous mutants proved to be fertile, indicating that differentiation of gametes in the mutants is unimpaired. We conclude that the H1511D mutation represents a unique mouse model with which enables us to examine the physiological role of Dnmt1 in development and in adult organ function.
Figure 5.
A Mouse Model of Impaired Dnmt1 Activity
(A) Structure of the distorted catalytic pocket of the mouse H1511D Dnmt1 variant. The hemimethylated DNA substrate is indicated in orange.
(B) Enzymatic activities (mol CH3 incorporated/h/mol of Dnmt1) of mouse wild-type and H1511D variant Dnmt1 proteins as a function of temperature in degree Celsius.
(C) Phenotypic features of H1511D homozygous mutant in 6-week-old mice. Body weight (left panel); absolute cell numbers of bone marrow, thymus, and spleen.
(D) Absolute cell numbers of HSC and MPP precursor populations in bone marrow.
(E) Absolute cell numbers of CLP, CMP, GMP, and MEP precursor populations in bone marrow. Note the dramatic loss of the common lymphoid progenitor (CLP).
(C–E) Wild-type (blue data points) and Dnmt1-mutant (red data points). See also Figures S4, 5, and 7.
Hematopoietic Abnormalities in Dnmt1H1511D Mutant Mice
Among the individual subsets of hematopoietic precursors in the bone marrow (Figures S4A, S5A, and S5B), we noted several-fold reductions in the numbers of HSC, MPP1, and MPP4 subsets but little change of MPP2 and MPP3 fractions (Figure 5D); interestingly, whereas near-normal numbers of CMP, GMP, and MEP precursors were found, CLPs were reduced about 100-fold (Figures 5E and S5A). Collectively, these findings indicate that the H1511D mutation predominantly affects the differentiation of the lymphoid lineage, reminiscent of the observations in the medaka and zebrafish mutants.
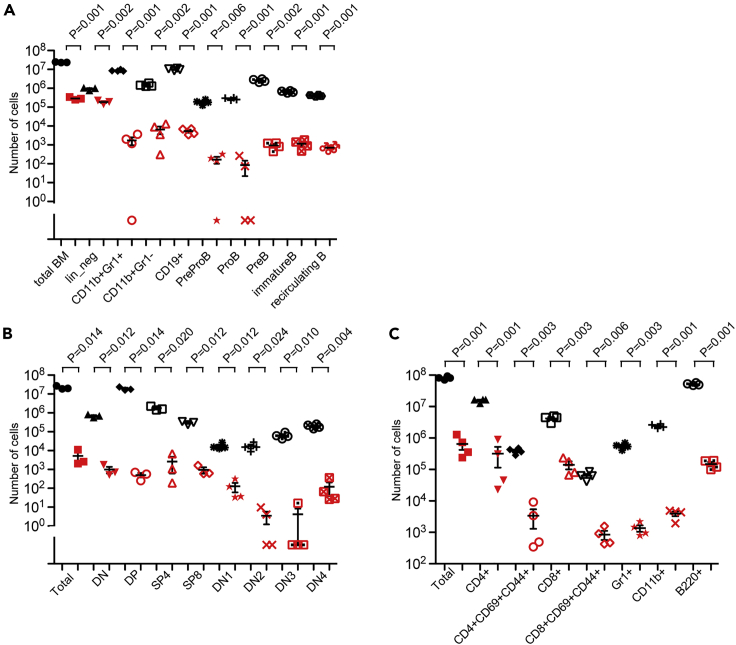
In order to examine the cell-intrinsic nature of the hematopoietic abnormalities in Dnmt1H1511D mutant mice, we carried out competitive bone marrow transplantation experiments. Bone marrow cells of wild-type and Dnmt1-mutant cells calibrated to contain 100 HSCs each were mixed and transplanted into irradiated wild-type recipients and their hematopoietic compartment analyzed after 12 weeks. The results indicated a poor contribution of mutant cells to the reconstituted hematopoietic compartment of the host animals. In the bone marrow of reconstituted mice, we found that wild-type cells outnumbered mutant cells by three orders of magnitude; prepro-B cells, pro-B cells, and pre-B cells were particularly affected (Figure 6A), in line with the differential reductions seen in the marrow of the mutant donors (Figures S4A and S5B). In the thymus of reconstituted mice, CD4/CD8 double-positive (DP) cells were almost entirely of wild-type origin, whereas DN1 thymocytes (Figure 6D) and mature CD4+ and CD8+ single-positive (SP) cells (Figure 6B) exhibited greater competitive potential; again, this outcome is reminiscent of the situation of the thymus in mutant donors (Figure S5C). In the spleen of reconstituted mice, the particular sensitivity of the B cell lineage to the H1511D mutation was readily detectable; of note, mutant CD4+ cells were less competitive than CD8+ cells (Figure 6C). In splenocytes of mutant donors, lymphocyte subsets are reduced to equal extent (Figure S5D). Interestingly, the contribution of mutant granulocytes and neutrophils to the reconstituted peripheral compartment was also small (Figure 6C); this suggests that although the lymphoid lineage is particularly sensitive to the effects of the H1511D mutation, the hematopoietic aberrations are not entirely lymphoid specific. Indeed, in secondary transplantation experiments no mutant cell engraftment was detectable (data not shown), suggesting that the H1511D mutation has detrimental effects also on the maintenance and/or the fitness of HSCs, at least when subjected to the transplantation procedure and/or when they are under competitive pressure in vivo.
Figure 6.
Impaired Fitness of Hematopoietic Precursor Populations of H1511D Homozygous Mice
The outcome of competitive transfer of allotype-tagged precursor populations was examined after 12 weeks. Results for wild-type donor cells are shown in black symbols and those for H1511D mutant hematopoietic cells in red symbols.
(A) Numbers of different cell types in the bone marrow.
(B) Numbers of different cell types in the thymus.
(C) Numbers of different cell types in the spleen.
See also Figures S4, 5, and 7.
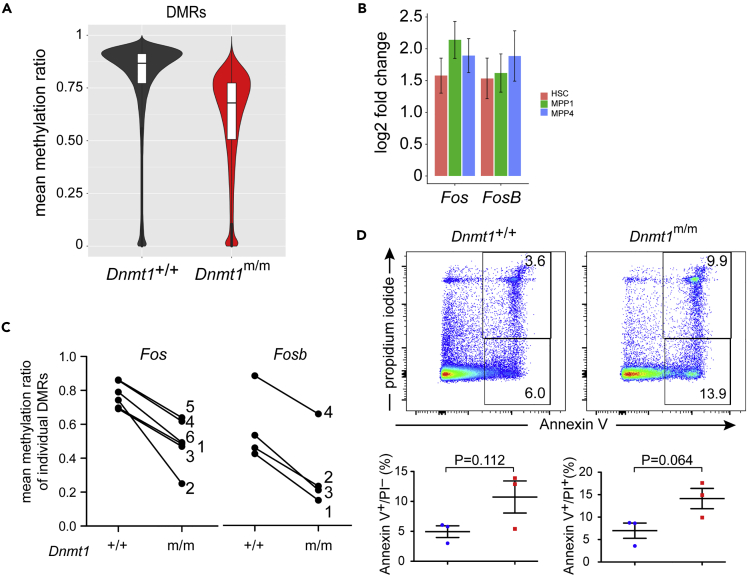
Next, we examined the methylation status of hematopoietic precursor cells. Given the pervasive effects of the H1511D mutation, we compared the extent of methylation in Lin– cells of the bone marrow. Significantly reduced methylation levels of CpG dinculeotides were detected (Figure 7A), clustered within 38,000 differentially methylated regions (DMRs), spread across the entire genome (Table S3). We then determined gene expression profiles of HSCs, and the MPP1 and MPP4 precursor populations between wild-type and Dnmt1H1511D mutant mice by RNA_seq (Table S4). The data indicated surprisingly few significant changes of generally modest degree; however, overexpression of Fos and FosB genes was consistently found in mutant progenitor subsets (Figure 7B; Table S4). The higher expression levels of these two genes were associated with hypomethylation of several locus-associated DMRs in Lin– cells (Figures 7C, S6A and S6B). The DMRs in the Fos and Fosb genes coincide neither with active chromatin marks nor with signatures of open chromatin that are found in HSCs, MPPs, and CLP, respectively (Sun et al., 2014a; Lara-Astiaso et al., 2014; Yoshida et al., 2019) (see Figure S6 for H3K4me1), indicating a complex relationship between hypomethylation and overexpression of these genes in mutant progenitor cells. Overexpression of Fos is known to selectively induce apoptosis in B cell precursors (Imoto et al., 1996; Hu et al., 1996); in the H1511D mutant, we found that pre-B cells of mutant mice exhibit moderately increased death rates (Figure 7D). This effect may contribute to reduced B cell numbers, because Lin– cells in the bone marrow proliferate normally (Figures S7A and S7B). By contrast, overexpression of Fos was shown to have no effect on T cell differentiation (Ruther et al., 1988; Fridkis-Hareli et al., 1992); indeed, despite higher expression levels of Fos and Fosb in thymocytes (Figure 7C), no changes in proliferation (Figure S7D) and levels of apoptosis were observed (Figure S7E). Collectively, these findings suggest that the drastic reduction of CLPs is the main reason for the severe cytopenia in the downstream lymphocyte differentiation pathways in mutant mice; in addition, the detrimental effect of the mutation in the B cell lineage is magnified by increased B cell apoptosis.
Figure 7.
Characterization of the Hematopoietic Compartment of H1511D Mutant Mice
(A) Extent of methylation in DMRs in lineage-negative bone marrow precursor cells.
(B) Expression levels of Fos and Fosb genes in hematopoietic precursor populations of H1511D mutants relative to controls.
(C) Mean methylation ratios of gene-associated DMRs in Lin– bone marrow cells of the indicated genotypes. The numbers refer to the map positions in Figure S6.
(D) Elevated levels of apoptosis in pro-B cells of Dnmt1-mutant animals. Flow cytometric analysis (top panels); AnnexinV+/PI− cells are in early apoptosis, whereas AnnexinV+/PI+ cells are in late stages of apoptosis. Quantification of results (bottom panels); wild-type (blue data points) and Dnmt1-mutant (red data points).
See also Figure S6.
Discussion
After cell division, multiple mechanisms ensure the faithful transfer of epigenetic information from the parental to the daughter cell. The DNA maintenance methylase, DNMT1, reestablishes the pattern of cytosine methylation on the newly synthesized strand after DNA replication and repair synthesis (Bergman and Cedar, 2013; Smith and Meissner, 2013). Hence, loss of DNMT1 leads to epigenetic aberrations culminating in cellular lethality. From an experimental perspective, the absolute requirement of DNMT1 for cell viability hampers studies on the role of DNA maintenance methylation during development and cell differentiation and thus precludes an assessment of tissue function in adults. This is well illustrated by the early lethality associated with deletion of DNMT1 in mice (Li et al., 1992) and apparent null alleles of dnmt1 in zebrafish (Anderson et al., 2009). In the latter case, maternal contributions of the enzyme to the developing embryo and larva can buffer the lack of zygotic activity only up to a certain developmental stage, highlighting the continuous requirement of (at least some level of) enzyme activity for development and differentiation. Harnessing the power of forward genetic screens, we identified two viable hypomorphic recessive alleles of DNMT1 in teleost fish. Orthologous amino acid replacements at the two critical positions in the catalytic site of mouse Dnmt1 protein conferred temperature sensitivity to the enzyme in vitro; this observation suggests an explanation for why the corresponding mouse alleles exhibited embryonic lethality. However, because these studies had identified the physiologically relevant region in the catalytic pocket, it became possible by structure-guided mutation of the adjacent histidine residue to generate a novel mouse Dnmt1 allele (H1511D) with properties amenable to organismic studies. In humans, dominant missense mutations in DNMT1 result in premature protein degradation and are associated with neuronal disorders (Klein et al., 2011; Winkelmann et al., 2012; Sun et al., 2014b). Our zebrafish mutant exhibits mild haploinsufficiency with respect to body mass, but this effect is not seen in the Dnmt1+/H1511D mice; moreover, no gross behavioral abnormalities were detectable in our mutant animals. In mice, low levels of wild-type Dnmt1 protein are also associated with defects in lymphoid differentiation (Broske et al., 2009), a phenotype that partially overlaps with the one observed here. However, an important distinction is the fact that the low levels of Dnmt1 lead to leukemic transformation and death at 12 weeks of age (Broske et al., 2009; Gaudet et al., 2003). Neither in our zebrafish and medaka mutants nor in the H1511D mutant mice, leukemia development has yet been detected. This feature represents an important distinction to the previous model, because it enables the analysis of hematopoietic abnormalities without the complication of concomitant malignant transformation. Collectively, the outcomes observed with the different DNMT1 alleles support the notion of a complex genotype-phenotype relationship, encouraging further site-specific mutagenesis studies.
The identification of viable alleles in two fish species, and the subsequent structure-guided development of a viable mouse allele, allowed us to examine the physiological consequences of impaired DNA methylation during the development of species that are separated by several hundred million years of independent evolution. It is remarkable that, among the myriad cellular differentiation events underlying the development and reproduction of the vertebrate body, and the many evolutionary innovations in organismal physiology distinguishing fish and mammals, lymphoid differentiation appears to be a common target of aberrations in DNA methylation. Although this general conclusion is well supported by our present study, there are certain notable differences among the three species. For instance, with respect to adult lymphopoiesis, we find that the phenotype in zebrafish is more severe than that in medaka; however, at present, we cannot distinguish between different degrees of functional impairment of the enzyme and divergent biological features of the two species. Nonetheless, inter-individual differences in the severity of lymphopenia in adult zebrafish are a sign of (albeit insufficient) recovery of lymphopoiesis, reminiscent of the more complete restoration in medaka mutants. It is known that larval and adult phases of zebrafish T cell development are genetically separable (Schorpp et al., 2006; Hess and Boehm, 2012; Tian et al., 2017); hence, it is possible that adult lymphoid progenitors undergo at least partial restoration of aberrant DNA methylation patterns. In some cases, this recovery process may be sufficient to rescue a certain degree of adult lymphopoiesis. On the other hand, the compensatory process often fails, such that a considerable fraction of mutant zebrafish accepts allogeneic transplants without the requirement of prior conditioning. Hence, from a practical perspective, the dnmt1 mutants can be added to the growing list of immunodeficient fish lines, such as those carrying mutations in c-myb, rag2, prkdc, and zap70 (Hess and Boehm, 2016: Hess et al., 2013; Moore et al., 2016a; Moore et al., 2016b) that have been used for transplantation experiments in diverse settings (Yan et al., 2019). Mice homozygous for the H1511D mutation are phenotypically more similar to zebrafish than to medaka, as indicated by the reduced lymphoid progenitor cells in the bone marrow and the thymus and the sustained peripheral cytopenia. Although our mutants are viable and grossly normal except for a smaller body size, it is well possible that they exhibit subtle abnormalities other than those of the hematopoietic system studied here; however, whatever their nature may be, they do not interfere with viability and fertility.
With respect to the molecular mechanism underlying the pathology in dnmt1 mutants, we propose that subtle changes in the interaction of the DNMT1 enzyme with its hemimethylated target sites underlie the observed reduced levels of DNA cytosine methylation, as implied by the crystal structure of the mouse DNMT1 protein (Song et al., 2012; Takeshita et al., 2011) and the molecular dynamics simulations reported herein. It is conceivable that the alterations in the catalytic site observed in the mutants change the mode and/or the efficiency with which the enzyme engages certain CpG dinucleotides. One obvious confounding factor is the sequence context of the CpG dinucleotide; other variables include chromatin context and altered interactions with accessory proteins in the methylase complex. Additional contributions to the altered methylation pattern could arise from compensatory de novo methylation processes. The structure-function relationships reported here should guide the generation of further mutants that may exhibit a different phenotypic spectrum than those uncovered in the present study.
In conclusion, our study establishes a set of three animal models with which to examine the physiological consequences of impaired maintenance methylation throughout life. The opportunity to study this in the organismal context is unprecedented and should therefore be applicable to many biological questions. Indeed, although we have focused here on hematological features of the mutant phenotype, in-depth analyses of other organ systems and physiological responses may reveal other so far undetected abnormalities. When viewed from an evolutionary angle, our study suggests that DNA methylation is required to protect the development of lymphocytes within the hematopoietic system of teleosts and mammals, which have independently evolved for several hundred millions years. Hence, it appears that an ancient epigenetic mechanism was repurposed to enable the establishment of an evolutionarily new cell type in vertebrates, underlying their adaptive immune systems.
Limitations of the Study
Our work demonstrates that the lymphoid lineage is consistently affected in three animal models homozygous for hypomorphic mutations in DNMT1. However, although viability is not compromised, the mutation also affects other organ systems, such as growth rate etc. Further studies are required to examine these additional features of the phenotypes. With respect to the mouse model described here, hematopoietic precursors should be examined in more detail, in particular with respect to possible signs of haploinsufficiency. Moreover, it will be important to determine whether the consistent upregulation of Fos and FosB genes is causal for the observed lymphoid defects or whether it is an indirect consequence of DNA hypomethylation in this lineage.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Thomas Boehm (boehm@ie-freiburg.mpg.de).
Materials Availability
All unique reagents generated in this study are available from the lead Contact with a completed Materials Transfer Agreement.
Data and Code Availability
The R code necessary to reproduce statistical analyses and results presented here is reported in Supplemental Code available at https://github.com/katsikora/Iwanami2019_SupplementaryCodeAndData_B. The methylation and RNA_seq data have been deposited in the GEO database under accession number GSE98648.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We are grateful to NBRP Medaka (https://shigen.nig.ac.jp/medaka/) for providing Kaga and gyokuro medaka strains and T. Sasado for help with their characterization. We thank U. Bönisch, S. Diehl, and A. Richter for help with next-generation sequencing, Sagar for help with RNA isolation, Benoît Kanzler for help with generating the mouse Dnmt1 mutants, and T. Clapes and E. Trompouki for help with competitive bone marrow transplantations. The members of the Boehm laboratory are gratefully acknowledged for help during various stages of the project. This project has received funding from the Max Planck Society, the Deutsche Forschungsgemeinschaft, and the European Research Council under the European Union's Seventh Framework Program (FP7/2007–2013) ERC grant agreement no. 323126. This research was partially supported by Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED under Grant Number JP19am0101070 (support number 1563). This work was performed in part under the International Collaborative Research Program of Institute for Protein Research, Osaka University, ICR-16-17.
Author Contributions
All authors designed experiments and analyzed data. N.I., D.-F.L., I.T., C.O'M., and I.S. performed experiments. Y. T., M.F.-S, and H.K. developed the medaka mutants; K.S. carried out the bioinformatic analyses; K.T. and Y.Y. carried out protein structure analysis; I.S. and S.T. carried out in vitro functional analysis; T.C. and E.T. established bone marrow chimeras; N.I. and T.B. directed the study and wrote the paper with input from all authors.
Declaration of Interests
The authors declare no competing interests.
Published: July 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101260.
Contributor Information
Norimasa Iwanami, Email: iwanami@cc.utsunomiya-u.ac.jp.
Thomas Boehm, Email: boehm@ie-freiburg.mpg.de.
Supplemental Information
References
- Anderson R.M., Bosch J.A., Goll M.G., Hesselson D., Dong P.D., Shin D., Chi N.C., Shin C.H., Schlegel A., Halpern M. Loss of Dnmt1 catalytic activity reveals multiple roles for DNA methylation during pancreas development and regeneration. Dev. Biol. 2009;334:213–223. doi: 10.1016/j.ydbio.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajoghli B., Aghaallaei N., Hess I., Rode I., Netuschil N., Tay B.-H., Venkatesh B., Yu J.-K., Kaltenbach S.L., Holland N.D. Evolution of genetic networks underlying the emergence of thymopoiesis in vertebrates. Cell. 2009;138:186–197. doi: 10.1016/j.cell.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Bergman Y., Cedar H. DNA methylation dynamics in health and disease. Nat. Struct. Mol. Biol. 2013;20:274–281. doi: 10.1038/nsmb.2518. [DOI] [PubMed] [Google Scholar]
- Boehm T., Bleul C.C., Schorpp M. Genetic dissection of thymus development in mouse and zebrafish. Immunol. Rev. 2003;195:15–27. doi: 10.1034/j.1600-065x.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- Boehm T., Swann J.B. Origin and evolution of adaptive immunity. Annu. Rev. Anim. Biosci. 2014;2:259–283. doi: 10.1146/annurev-animal-022513-114201. [DOI] [PubMed] [Google Scholar]
- Broske A.M., Vockentanz L., Kharazi S., Huska M.R., Mancini E., Scheller M., Kuhl C., Enns A., Prinz M., Jaenisch R. DNA methylation protects hematopoietic stem cell multipotency from myeloerythroid restriction. Nat. Genet. 2009;41:1207–1215. doi: 10.1038/ng.463. [DOI] [PubMed] [Google Scholar]
- Cedar H., Bergman Y. Epigenetics of haematopoietic cell development. Nat. Rev. Immunol. 2011;11:478–488. doi: 10.1038/nri2991. [DOI] [PubMed] [Google Scholar]
- Cheng J., Yang H., Fang J., Ma L., Gong R., Wang P., Li Z., Xu Y. Molecular mechanism for USP7-mediated DNMT1 stabilization by acetylation. Nat. Commun. 2015;6:7023. doi: 10.1038/ncomms8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M.R., Mandal M., Ochiai K., Singh H. Orchestrating B cell lymphopoiesis through interplay of IL-7 receptor and pre-B cell receptor signalling. Nat. Rev. Immunol. 2014;14:69–80. doi: 10.1038/nri3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumano A., Berthault C., Ramond C., Petit M., Golub R., Bandeira A., Pereira P. New molecular insights into immune cell development. Annu. Rev. Immunol. 2019;37:497–519. doi: 10.1146/annurev-immunol-042718-041319. [DOI] [PubMed] [Google Scholar]
- Fridkis-Hareli M., Abel L., Eisenbach L., Globerson A. Differentiation patterns of CD4/CD8 thymocyte subsets in cocultures of fetal thymus and lymphohemopoietic cells from c-fos transgenic and normal mice. Cell Immunol. 1992;141:279–292. doi: 10.1016/0008-8749(92)90148-i. [DOI] [PubMed] [Google Scholar]
- Furutani-Seiki M., Sasado T., Morinaga C., Suwa H., Niwa K., Yoda H., Deguchi T., Hirose Y., Yasuoka A., Henrich T. A systematic genome-wide screen for mutations affecting organogenesis in Medaka, Oryzias latipes. Mech. Dev. 2004;121:647–658. doi: 10.1016/j.mod.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Gaudet F., Hodgson J.G., Eden A., Jackson-Grusby L., Dausman J., Gray J.W., Leonhardt H., Jaenisch R. Induction of tumors in mice by genomic hypomethylation. Science. 2003;300:489–492. doi: 10.1126/science.1083558. [DOI] [PubMed] [Google Scholar]
- Guillamot M., Cimmino L., Aifantis I. The impact of DNA methylation in hematopoietic malignancies. Trends Cancer. 2016;2:70–83. doi: 10.1016/j.trecan.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess I., Boehm T. Intravital imaging of thymopoiesis reveals dynamic lympho-epithelial interactions. Immunity. 2012;36:298–309. doi: 10.1016/j.immuni.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Hess I., Boehm T. Stable multilineage xenogeneic replacement of definitive hematopoiesis in adult zebrafish. Sci. Rep. 2016;6:19634. doi: 10.1038/srep19634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess I., Iwanami N., Schorpp M., Boehm T. Zebrafish model for allogeneic hematopoietic cell transplantation not requiring preconditioning. Proc. Natl. Acad. Sci. U S A. 2013;110:4327–4332. doi: 10.1073/pnas.1219847110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Hatano M., Ruther U., Tokuhisa T. Overexpression of c-Fos induces apoptosis of CD43+ pro-B cells. J. Immunol. 1996;157:3804–3811. [PubMed] [Google Scholar]
- Imoto S., Hu L., Tomita Y., Phuchareon J., Ruther U., Tokuhisa T. A regulatory role of c-Fos in the development of precursor B lymphocytes mediated by interleukin-7. Cell Immunol. 1996;169:67–74. doi: 10.1006/cimm.1996.0092. [DOI] [PubMed] [Google Scholar]
- Iwamatsu T. Stages of normal development in the medaka Oryzias latipes. Mech. Dev. 2004;121:605–618. doi: 10.1016/j.mod.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Iwanami N., Takahama Y., Kunimatsu S., Li J., Takei R., Ishikura Y., Suwa H., Niwa K., Sasado T., Morinaga C. Mutations affecting thymus organogenesis in Medaka, Oryzias latipes. Mech. Dev. 2004;121:779–789. doi: 10.1016/j.mod.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Iwanami N., Higuchi T., Sasano Y., Fujiwara T., Hoa V.Q., Okada M., Talukder S.R., Kunimatsu S., Li J., Saito F. WDR55 is a nucleolar modulator of ribosomal RNA synthesis, cell cycle progression, and teleost organ development. PLoS Genet. 2008;4:e1000171. doi: 10.1371/journal.pgen.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanami N., Okada M., Hoa V.Q., Seo Y., Mitani H., Sasaki T., Shimizu N., Kondoh H., Furutani-Seiki M., Takahama Y. Ethylnitrosourea-induced thymus-defective mutants identify roles of KIAA1440, TRRAP, and SKIV2L2 in teleost organ development. Eur. J. Immunol. 2009;39:2606–2616. doi: 10.1002/eji.200939362. [DOI] [PubMed] [Google Scholar]
- Iwanami N., Sikora K., Richter A.S., Mönnich M., Guerri L., Soza-Ried C., Lawir D.-F., Mateos F., Hess I., O'Meara C.P. Forward genetic screens in zebrafish identify pre-mRNA-processing pathways regulating early T cell development. Cell Rep. 2016;17:2259–2270. doi: 10.1016/j.celrep.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong M., Goodell M.A. New answers to old questions from genome-wide maps of DNA methylation in hematopoietic cells. Exp. Hematol. 2014;42:609–617. doi: 10.1016/j.exphem.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C.J., Botuyan M.V., Wu Y., Ward C.J., Nicholson G.A., Hammans S., Hojo K., Yamanishi H., Karpf A.R., Wallace D.C. Mutations in DNMT1 cause hereditary sensory neuropathy with dementia and hearing loss. Nat. Genet. 2011;43:595–600. doi: 10.1038/ng.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Astiaso D., Weiner A., Lorenzo-Vivas E., Zaretsky I., Jaitin D.A., David E., Keren-Shaul H., Mildner A., Winter D., Jung S. Immunogenetics. Chromatin state dynamics during blood formation. Science. 2014;345:943–949. doi: 10.1126/science.1256271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E., Bestor T.H., Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Li J., Iwanami N., Hoa V.Q., Furutani-Seiki M., Takahama Y. Noninvasive intravital imaging of thymocyte dynamics in medaka. J. Immunol. 2007;179:1605–1615. doi: 10.4049/jimmunol.179.3.1605. [DOI] [PubMed] [Google Scholar]
- Medvedovic J., Ebert A., Tagoh H., Busslinger M. Pax5: a master regulator of B cell development and leukemogenesis. Adv. Immunol. 2011;111:179–206. doi: 10.1016/B978-0-12-385991-4.00005-2. [DOI] [PubMed] [Google Scholar]
- Moore J.C., Mulligan T.S., Yordan N.T., Castranova D., Pham V.N., Tang Q., Lobbardi R., Anselmo A., Liwski R.S., Berman J.N. T cell immune deficiency in zap70 mutant zebrafish. Mol. Cell. Biol. 2016;36:2868–2876. doi: 10.1128/MCB.00281-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.C., Tang Q., Yordan N.T., Moore F.E., Garcia E.G., Lobbardi R., Ramakrishnan A., Marvin D.L., Anselmo A., Sadreyev R.I. Single-cell imaging of normal and malignant cell engraftment into optically clear prkdc-null SCID zebrafish. J. Exp. Med. 2016;213:2575–2589. doi: 10.1084/jem.20160378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potok M.E., Nix D.A., Parnell T.J., Cairns B.R. Reprogramming the maternal zebrafish genome after fertilization to match the paternal methylation pattern. Cell. 2013;153:759–772. doi: 10.1016/j.cell.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E.V., Ungerback J., Champhekar A. Forging T-lymphocyte identity: intersecting networks of transcriptional control. Adv. Immunol. 2016;129:109–174. doi: 10.1016/bs.ai.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruther U., Muller W., Sumida T., Tokuhisa T., Rajewsky K., Wagner E.F. c-fos expression interferes with thymus development in transgenic mice. Cell. 1988;53:847–856. doi: 10.1016/s0092-8674(88)90289-9. [DOI] [PubMed] [Google Scholar]
- Schorpp M., Leicht M., Nold E., Hammerschmidt M., Haas-Assenbaum A., Wiest W., Boehm T. A zebrafish orthologue (whnb) of the mouse nude gene is expressed in the epithelial compartment of the embryonic thymic rudiment. Mech. Dev. 2002;118:179–185. doi: 10.1016/s0925-4773(02)00241-1. [DOI] [PubMed] [Google Scholar]
- Schorpp M., Bialecki M., Diekhoff D., Walderich B., Odenthal J., Maischein H.-M., Zapata A.G., Tübingen 2000 Screen Consortium, Freiburg Screening Group, Boehm T. Conserved functions of Ikaros in vertebrate lymphocyte development: genetic evidence for distinct larval and adult phases of T cell development and two lineages of B cells in zebrafish. J. Immunol. 2006;177:2463–2476. doi: 10.4049/jimmunol.177.4.2463. [DOI] [PubMed] [Google Scholar]
- Smith Z.D., Meissner A. DNA methylation: roles in mammalian development. Nat. Rev. Genet. 2013;14:204–220. doi: 10.1038/nrg3354. [DOI] [PubMed] [Google Scholar]
- Song J., Teplova M., Ishibe-Murakami S., Patel D.J. Structure-based mechanistic insights into DNMT1-mediated maintenance DNA methylation. Science. 2012;335:709–712. doi: 10.1126/science.1214453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Luo M., Jeong M., Rodriguez B., Xia Z., Hannah R., Wang H., Le T., Faull K.F., Chen R. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell Stem Cell. 2014;14:673–688. doi: 10.1016/j.stem.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Wu Y., Ordog T., Baheti S., Nie J., Duan X., Hojo K., Kocher J.P., Dyck P.J., Klein C.J. Aberrant signature methylome by DNMT1 hot spot mutation in hereditary sensory and autonomic neuropathy 1E. Epigenetics. 2014;9:1184–1193. doi: 10.4161/epi.29676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita K., Suetake I., Yamashita E., Suga M., Narita H., Nakagawa A., Tajima S. Structural insight into maintenance methylation by mouse DNA methyltransferase 1 (Dnmt1) Proc. Natl. Acad. Sci. U S A. 2011;108:9055–9059. doi: 10.1073/pnas.1019629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Xu J., Feng S., He S., Zhao S., Zhu L., Jin W., Dai Y., Luo L., Qu J.Y. The first wave of T lymphopoiesis in zebrafish arises from aorta endothelium independent of hematopoietic stem cells. J. Exp. Med. 2017;214:3347–3360. doi: 10.1084/jem.20170488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann J., Lin L., Schormair B., Kornum B.R., Faraco J., Plazzi G., Melberg A., Cornelio F., Urban A.E., Pizza F. Mutations in DNMT1 cause autosomal dominant cerebellar ataxia, deafness and narcolepsy. Hum. Mol. Genet. 2012;21:2205–2210. doi: 10.1093/hmg/dds035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C., Brunson D.C., Tang Q., Do D., Iftimia N.A., Moore J.C., Hayes M.N., Welker A.M., Garcia E.G., Dubash T.D. Visualizing engrafted human cancer and therapy responses in immunodeficient zebrafish. Cell. 2019;177:1903–1914.e14. doi: 10.1016/j.cell.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Lareau C.A., Ramirez R.N., Rose S.A., Maier B., Wroblewska A., Desland F., Chudnovskiy A., Mortha A., Dominguez C. The cis-regulatory atlas of the mouse immune system. Cell. 2019;176:897–912. doi: 10.1016/j.cell.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.M., Liu S., Lin K., Luo Y., Perry J.J., Wang Y., Song J. Crystal structure of human DNA methyltransferase 1. J. Mol. Biol. 2015;427:2520–2531. doi: 10.1016/j.jmb.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The R code necessary to reproduce statistical analyses and results presented here is reported in Supplemental Code available at https://github.com/katsikora/Iwanami2019_SupplementaryCodeAndData_B. The methylation and RNA_seq data have been deposited in the GEO database under accession number GSE98648.