Abstract
We have previously shown that a severely energy-restricted diet leads to greater loss of weight, fat, lean mass and bone mineral density (BMD) at 12 months in postmenopausal women with obesity than a moderately energy-restricted diet. We now aim to evaluate whether these effects are sustained longer term (ie, at 36 months). 101 postmenopausal women were randomized to either 12 months of moderate (25 to 35%) energy restriction with a food-based diet (moderate intervention), or 4 months of severe (65 to 75%) energy restriction with a total meal replacement diet followed by moderate energy restriction for 8 months (severe intervention). Body weight and composition were measured at 0, 24 and 36 months. Participants in the severe intervention lost ~1.5 to 1.7 times as much weight, waist circumference, whole-body fat mass and visceral adipose tissue compared to those in the moderate intervention, and were 2.6 times more likely (42% versus 16%) to have lost 10% or more of their initial body weight at 36 months (P < 0.01 for all). However, those in the severe versus moderate intervention lost ~1.4 times as much whole-body lean mass (P < 0.01), albeit this was proportional to total weight lost and there was no greater loss of handgrip strength, and they also lost ~2 times as much total hip BMD between 0 and 36 months (P < 0.05), with this bone loss occurring in the first 12 months. Thus, severe energy restriction is more effective than moderate energy restriction for reducing weight and adiposity in postmenopausal women in the long term (3 years), but attention to BMD loss in the first year is required.
Trial registration
Australian New Zealand Clinical Trials Registry Reference Number: 12612000651886, anzctr.org.au.
Keywords: Nutrition, Diet, Musculoskeletal system, Endocrinology, Clinical research, Weight loss, Body composition, Diet-reducing, Obesity, Magnetic resonance imaging
Nutrition; Diet; Musculoskeletal System; Endocrinology; Clinical Research; weight loss; Body composition; diet-reducing; Obesity; Magnetic Resonance Imaging.
1. Introduction
Overweight and obesity are major contributors to morbidity and mortality globally [1]. The greater the degree of excess weight, the greater the risk of disease [2]. Encouragingly, loss of excess weight has been shown to reduce the risk of disease in people with obesity, with greater weight losses (eg, 10% compared to 5% of initial body weight) producing greater reductions in disease risk [3, 4, 5]. Some health guidelines from around the world therefore recommend a minimum weight loss of 10% for people with obesity [6].
The most effective and accessible way for people with obesity to achieve 10% weight loss is with severely energy-restricted diets [7], where energy intake is restricted to less than 35% of energy requirements [8]. These diets are often implemented by replacing all or almost all meals and snacks with nutritionally replete meal replacement products (eg, shakes and bars), in meal replacement diets. In the short-term (weeks to months), severely energy-restricted meal replacement diets induce significantly greater average weight losses than food-based diets involving moderate energy restriction [9, 10, 11]. In the long-term (months to years), and contrary to expectations, severely energy-restricted diets do not result in faster weight regain than moderately energy-restricted diets [7, 12], and in fact result in greater average weight losses at 3 years following completion of the diet [11]. Besides greater short- and long-term weight loss, severely energy-restricted meal replacement diets offer additional advantages over moderately energy-restricted food-based diets: ease of use (eg, no need to prepare meals [13], and appetite is reduced during the diet, facilitating adherence [14]); and low cost (it is cheaper to purchase meal replacement products than the national average expenditure on food [15, 16], and meal replacement diets are 3 times cheaper than food-based diets in terms of dietetics support [7], while also being cheaper than bariatric surgery or anti-obesity medications).
Despite the above-mentioned advantages of severely energy-restricted meal replacement diets, there are concerns about potential long-term effects, notably on body composition [17]. Some clinical practice guidelines from around the world either recommend against the use of severely energy-restricted meal replacement diets for the treatment of obesity in general [18, 19], or recommend against their use in older adults specifically [20]. Some support for these guidelines comes from our randomised controlled clinical trial in postmenopausal women with obesity, the TEMPO Diet Trial (Type of Energy Manipulation for Promoting optimum metabolic health and body composition in Obesity). We showed that while a severely energy-restricted meal replacement diet led to approximately 2-fold greater losses of weight as well as whole-body fat mass and abdominal adipose tissue volume (subcutaneous and visceral) compared to a moderately energy-restricted food-based diet at 12 months, it also led to an approximately 1.5-fold greater loss of whole-body lean mass (albeit this was proportional to the greater weight loss and there was no loss of handgrip strength), and an approximately 2.5-fold greater loss of hip bone mineral density at 12 months [9]. While severely energy-restricted meal replacement diets clearly have benefits for cardiometabolic health [3], an outstanding question is what happens to body composition in the long-term (years)? In this paper, we thus report on body composition outcomes from our 2-year and 3-year follow-up of the postmenopausal women with obesity who participated in the TEMPO Diet Trial.
2. Methods
2.1. Study design and participants
The rationale, design, and methods of this trial, including details on sample size calculation, recruitment, inclusion, randomization, assessment, and intervention during the first year, have previously been reported [9, 21]. Briefly, the TEMPO Diet Trial was a single center, randomized clinical trial. Ethical approval was obtained from the Sydney Local Health District, Royal Prince Alfred Hospital Human Research Ethics Committee. It was conducted at the Charles Perkins Centre Royal Prince Alfred Clinic on the University of Sydney campus in Camperdown, New South Wales, Australia, with magnetic resonance imaging (MRI) scans performed at I-Med Radiology (Camperdown). Reporting in this article is aligned with the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline, and the full trial protocol can be found in Supplement 1 of our previous publication [9].
Key inclusion criteria were postmenopausal women aged 45 to 65 years with body mass index (calculated as weight in kilograms divided by height in meters squared) from 30 to 40 kg/m2, at least 5 years after menopause, with less than 3 h of structured physical activity per week (ie, sedentary) and living in the Sydney metropolitan area of New South Wales, Australia. Participants with osteoporosis, diabetes mellitus, or taking medication affecting body composition, were excluded. The full inclusion and exclusion criteria and our rationale for these have been detailed in our published protocol [21]. The trial was conducted in accordance with the Declaration of Helsinki [22] and Good Clinical Practice guidelines [23]. All participants provided written informed consent prior to participation.
2.2. Procedures
The moderate intervention involved a moderate energy restriction of 25 to 35% relative to estimated energy expenditure for a total of 12 months (52 weeks). This was achieved using a food-based diet, with recommendations based on the Australian Guide to Healthy Eating [24]. The severe intervention involved a severe energy restriction of 65 to 75% relative to estimated energy expenditure for 4 months (16 weeks) or until a body mass index of no lower than 20 kg/m2 was reached, whichever came first. This was achieved using a total meal replacement diet (KicStart meal replacement shakes and soups from Prima Health Solutions) supplemented with a whey protein isolate (Beneprotein from Nestlé Healthcare Nutrition) to achieve the prescribed protein target (described later). This was followed by moderate energy restriction (ie, as in the moderate intervention) for the remaining period to 12 months (52 weeks). For both interventions, a protein intake of 1.0 g/kg of actual body weight per day was prescribed, and physical activity was encouraged but not supervised. After 12 months, participants were given the option of attending monthly group support meetings, each of 60 to 90 minutes in duration, facilitated on a rotating basis by different members of the research team (R.V.S., S.M., C.H., A.A.G, and A.S.), sometimes in association with a guest facilitator (F.Q.d.L., A.L.P., R.A.H., M.B., M.S.H.S., C.K.S., C.D.M., E.S., and V.M.S.). The development process and rationale behind the dietary interventions for the TEMPO Diet Trial, as well as full details of the dietary interventions, have been published previously [8].
2.3. Outcomes
This article reports the 24- and 36-month body composition outcomes for the TEMPO Diet Trial. All body composition outcomes were measured at 0, 24 and 36 months following randomization. Height was measured at 0 and 36 months only. All data were collected with participants lightly clothed (ie, in a close-fitting sports bra and leggings or, for MRI scans, in a gown and underpants only), without shoes, and with all metal jewellery, accessories, and electronic devices removed.
Dual-energy x-ray absorptiometry (DXA), using a Discovery W bone densitometer (Hologic), was used to assess whole-body lean mass, whole-body fat mass, and BMD of the total left hip and anterior posterior lumbar spine (L1-L4) [25]. A hydraulic hand dynamometer (Jamar, Model 5030J1; Patterson Medical) was used to assess handgrip strength of the dominant and nondominant hands. For the 2 participants who were ambidextrous, the right and left hand were designated the dominant and nondominant hand, respectively. Waist and hip circumferences were measured to the nearest 0.1 cm using a narrow, flexible, and inelastic steel tape (Lufkin W606PM; Apex Tool Group). Waist circumference was measured in the midaxillary line at the halfway point between the bony landmarks of the lowest rib and the top of the iliac crest, while the hip circumference measurement was taken at the point of greatest protuberance of the participant's buttocks when viewed from the side. The ratio of waist to hip circumference was calculated by dividing waist circumference by hip circumference. A 3-T MRI scanner (Discovery MR750; GE Healthcare) was used to assess abdominal fat volume (subcutaneous and visceral adipose tissue from the diaphragm to the pelvis, with a slice thickness of 10 mm and an interslice gap of 10 mm). Accelerometers (SenseWear Pro Armband; BodyMedia Inc) were used to assess physical activity [26]. Physical activity data was included as a covariate in our analyses if participants wore the accelerometer for a minimum of 5 of 7 designated days for at least 85% of each 24-hour day. Mean physical activity across the days the accelerometer was worn was then entered into analyses as metabolic equivalents of task (equal to total energy expenditure divided by resting energy expenditure; calculated by SenseWear Professional Software version 7.0 [BodyMedia Inc]).
2.4. Statistical analysis
Statistical analyses were performed using SPSS statistical software version 24 for Windows (IBM Corp). Statistical significance was accepted as P < 0.05. Fisher exact tests were used to compare attrition between groups (ie, severe versus moderate). All continuous variables were assessed for normality before analysis using histograms and P–P plots. To compare continuous variables between groups at baseline (0 months), Mann-Whitney tests were used.
To compare longitudinal changes between groups, intention-to-treat analysis was performed using all available follow-up data from all participants originally randomized, using random-effects linear mixed models. While data from all time points was used in these models, this article reports on the differences between groups at 24 and 36 months, and the differences within each group between 24 or 36 months and baseline, because the data to 12 months has previously been published [9]. Intervention group and time were included as fixed effects, and participant was included as a random effect. For all outcomes, baseline values of the relevant variable were added as a covariate in the analysis. As the degree of physical activity is known to influence measures of body composition, physical activity (expressed in metabolic equivalents of task) was added as a covariate, as measured at each time point. When the overall P value for the interaction between group and time was less than 0.05, comparisons between groups at each of the 2 time points relevant to this paper (ie, at 24 and 36 months) were made, using Bonferroni adjustments to correct for multiple comparisons. For within-group changes, intervention group and time were included in the model as fixed effects and time as a repeated measure, with physical activity (metabolic equivalents of task) added as a covariate. Maximum-likelihood estimation was used, and an unstructured covariance matrix was specified. Comparisons between time points (ie, 24 or 36 months) and baseline (0 months) within each group were made using Bonferroni adjustments to correct for multiple comparisons.
3. Results
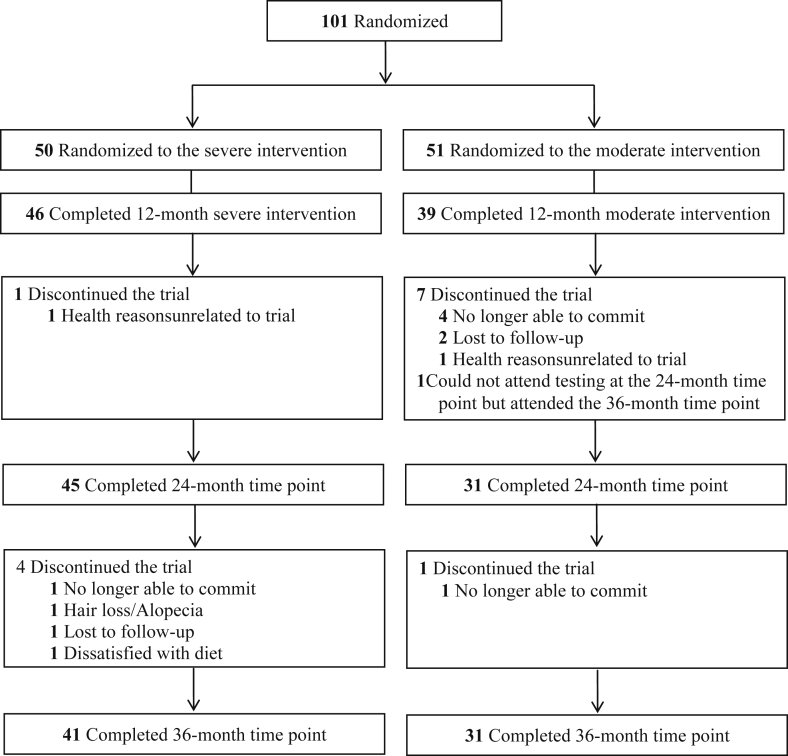
Participants were recruited between March 2013 and July 2016, and 101 participants were randomized to the trial (Figure 1). Participants had a mean (SD) age of 58.0 (4.2) years, a mean (SD) weight of 90.8 (9.1) kg, and a mean (SD) body mass index of 34.4 (2.5) kg/m2 at baseline (0 months, Table 1). There were no differences between groups at baseline in any of these variables or race (severe: 47 of 50 [94.0%] white participants; moderate: 48 of 51 [94.1%] white participants), or outcome variables (Table 1). Overall, 76 of 101 (75.2%) and 72 of 101 (71.3%) of participants completed the 24-month and 36-month time points respectively (45 of 50 [90.0%] in the severe group versus 31 of 51 [60.8%] in the moderate group at 24 months, and 41 of 50 [82.0%] in the severe group versus 31 of 51 [60.8%] in the moderate group at 36 months). There were 3.5-fold fewer participants discontinuing the trial in the severe group compared with the moderate group at 24 months (6 versus 21 participants; P = 0.0001), and 2.2-fold fewer discontinuations at 36 months (9 versus 20 participants; P = 0.05) (Figure 1).
Figure 1.
Flow of participants through the TEMPO Diet Trial (Type of Energy Manipulation for Promoting optimum metabolic health and body composition in Obesity). The moderate intervention involved a food-based diet with a 25 to 35% energy restriction for a total of 12 months (52 weeks). The severe intervention involved total diet replacement with 65 to 75% energy restriction for 4 months (16 weeks) or until a body mass index (calculated as weight in kilograms divided by height in meters squared) of no lower than 20 kg/m2 was reached, whichever came first, followed by moderate energy restriction until 12 months (52 weeks). Participants were then followed up at 24 and 36 months. Reasons for discontinuing the trial between 0 and 12 months have been published previously, along with outcomes to 12 months [9].
Table 1.
Baseline characteristics for all participants, completers and noncompleters, in the TEMPO Diet Trial.
|
Characteristic |
Severe Group |
Moderate Group |
||||
|---|---|---|---|---|---|---|
| All participants (n = 50) | Completers (n = 41) | Noncompleters (n = 9) | All participants (n = 51) | Completers (n = 31) | Noncompleters (n = 20) | |
| Age, y | 58.0 (4.4) | 58.2 (4.4) | 57.1 (4.4) | 58.0 (4.2) | 57.7 (4.2) | 58.3 (4.2) |
| Height, m | 1.62 (0.06) | 1.62 (0.06) | 1.61 (0.08) | 1.63 (0.05) | 1.63 (0.06) | 1.63 (0.04) |
| Weight, kg | 90.1 (9.4) | 89.2 (8.9) | 94.5 (11.1) | 92.4 (8.3) | 93.3 (9.0) | 91.0 (6.9) |
| Body mass index, kg/m2a | 34.3 (2.5) | 33.9 (2.4) | 36.2 (1.8) | 34.6 (2.5) | 35.0 (2.7) | 34.1 (2.2) |
|
Lean tissues | ||||||
| Whole-body lean mass, kgb | 44.3 (4.9) | 44.0 (4.5) | 45.5 (6.6) | 44.8 (4.0) | 45.0 (4.4) | 44.6 (3.3) |
| Muscle strength, kg | ||||||
| Dominant handc | 29.80 (6.31) | 29.25 (6.20) | 32.22 (6.55) | 30.00 (4.70) | 30.37 (4.79) | 29.45 (4.64) |
| Non-dominant hand | 27.90 (6.00) | 27.17 (5.51) | 31.22 (7.34) | 27.90 (4.42) | 28.48 (4.82) | 27.00 (3.64) |
|
Bone mineral density, g/cm2 | ||||||
| Total hipd | 0.988 (0.097) | 0.979 (0.091) | 1.028 (0.117) | 0.972 (0.107) | 0.971 (0.105) | 0.974 (0.114) |
| Femoral neckd | 0.810 (0.089) | 0.800 (0.080) | 0.853 (0.118) | 0.815 (0.097) | 0.818 (0.090) | 0.810 (0.110) |
| Lumbar spine | 1.001 (0.112) | 0.991 (0.114) | 1.045 (0.097) | 1.019 (0.125) | 0.995 (0.124) | 1.057 (0.120) |
| Whole-bodyb | 1.093 (0.077) | 1.093 (0.079) | 1.091 (0.069) | 1.100 (0.088) | 1.087 (0.082) | 1.118 (0.094) |
|
Fat mass and distribution | ||||||
| Waist circumference, cm | 108.3 (7.3) | 108.0 (7.5) | 109.8 (6.5) | 108.8 (7.0) | 109.7 (8.2) | 107.4 (4.3) |
| Hip circumference, cm | 118.6 (7.0) | 117.6 (6.6) | 123.4 (7.2) | 121.3 (6.6) | 122.2 (6.1) | 119.9 (7.2) |
| Ratio of waist to hip circumferencee | 0.915 (0.061) | 0.920 (0.061) | 0.891 (0.057) | 0.898 (0.060) | 0.899 (0.065) | 0.898 (0.053) |
| Whole-body fat mass, kgb | 42.2 (5.6) | 41.5 (5.5) | 45.5 (5.2) | 43.5 (5.9) | 44.2 (6.2) | 42.5 (5.4) |
| Abdominal adipose tissue, cm3 | ||||||
| Subcutaneousf,g | 12006 (3028) | 11767 (3088) | 13175 (2567) | 12176 (2508) | 12174 (2540) | 12181 (2527) |
| Visceralf | 4544 (1702) | 4549 (1734) | 4526 (1654) | 5123 (1954) | 5168 (1976) | 5049 (1969) |
Abbreviation: TEMPO, Type of Energy Manipulation for Promoting optimum metabolic health and body composition in Obesity.
Data are presented as means (standard deviations).
Calculated as weight in kilograms divided by height in meters squared.
Data for 2 participants in the moderate group who completed the study are missing because of machine failure.
Data for 1 participant in the severe group who completed the study are missing because the participant underwent thumb tendon surgery, and data for 1 participant in the moderate group who completed the study are missing because the participant had a scaphoid fracture.
Data for 1 participant in the moderate group who completed the study are missing because the scan could not be analyzed.
Calculated as waist circumference divided by hip circumference.
Data for 2 participants in the severe group who completed the study and 1 in the moderate group who did not complete the study are missing because they did not undergo magnetic resonance imaging scan.
Data for 1 participant in the severe group who did not complete the study and 3 participants in the moderate group (2 completers, 1 noncompleter) are missing because the scan was outside the window of analysis.
3.1. Weight
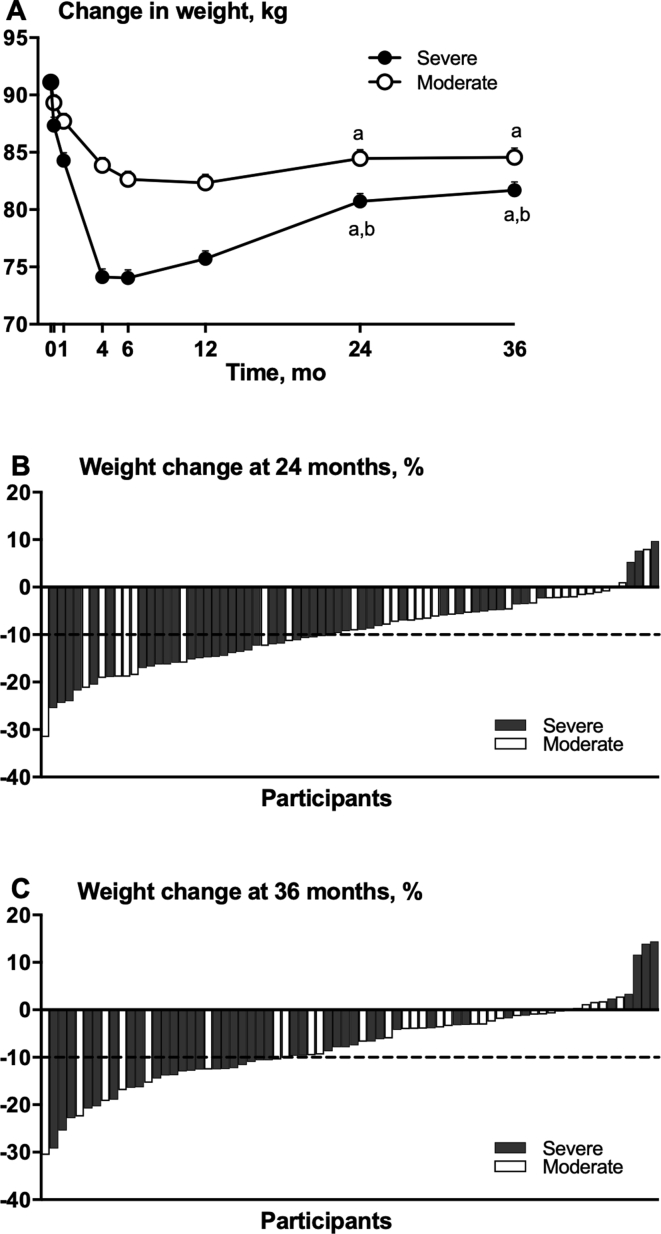
The severe group had a significantly lower weight than the moderate group at both 24 and 36 months (effect size at 36 months, −2.9 [95% CI, −5.0 to −0.8] kg; estimated marginal means at 36 months, −8.0 [95% CI, −11.7 to −4.4] kg versus −4.9 [95% CI, −8.9 to −0.9] kg; P = 0.008) (Figure 2A and Table 2). Both groups had significant decreases in weight at 24 and 36 months compared with baseline, although there was weight regain between 12 to 36 months in participants in both the severe group (effect size 7.3 [95% CI, 4.5 to 10.2] kg, P < 0.001) and the moderate group (effect size 3.2 [95% CI, -0.1 to 6.4] kg, P = 0.065). At 24 months, 27 of 45 participants (60.0%) in the severe group who remained in the trial had lost at least 10% of their baseline weight compared with 9 of 31 participants (29.0%) in the moderate group who remained in the trial (P < 0.001) (Figure 2B). Assuming participants who dropped out of the trial did not achieve at least 10% weight loss, then 27 of 50 participants (54.0%) in the severe group had lost at least 10% of their baseline weight compared with 9 of 51 participants (17.6%) in the moderate group (P < 0.001) at 24 months. This represents a 3.1-fold greater likelihood of losing a clinically significant amount of weight (ie, 10%) with the severe versus moderate intervention at 24 months. At 36 months, 21 of 41 participants (51.2%) in the severe group who remained in the trial had lost at least 10% of their baseline weight compared with 8 of 31 participants (25.8%) in the moderate group who remained in the trial (P < 0.01) (Figure 2C). Assuming participants who dropped out of the trial did not achieve at least 10% weight loss, then 21 of 50 participants (42.0%) in the severe group had lost at least 10% of their baseline weight compared with 8 of 51 participants (15.7%) in the moderate group (P < 0.01) at 36 months. This represents an approximately 2.6-fold (42% versus 16%) greater likelihood of losing a clinically significant amount of weight (ie, 10%) with the severe versus moderate intervention at 36 months.
Figure 2.
Weight changes in postmenopausal women with obesity during the TEMPO Diet Trial (Type of Energy Manipulation for Promoting optimum metabolic health and body composition in Obesity). Weight data presented as estimated marginal means (A), ie, group means after controlling for covariates. Whiskers (mostly obscured by the data points) indicate SEs of the means. Weight change at 24 (B) and 36 months (C) as percentage change from baseline for each participant in the severe and moderate groups. The dotted lines indicate 10% weight loss. aP < 0.001 versus baseline value within group. bP < 0.01 versus the moderate group at that time point. Statistical comparisons between groups in the first 12 months of the trial have been published previously [9].
Table 2.
Changes from baseline for body composition with the severe and moderate energy restriction interventions of the TEMPO Diet Trial.
| Measurement | No. | Severe Group, Estimated Marginal Mean (95% CI) |
No. | Moderate Group, Estimated Marginal Mean (95% CI) |
P Valuea |
|---|---|---|---|---|---|
| Weight change, kg | |||||
| 24 mo | 45 | -10.0 (-13.3 to -6.6)b | 31 | -6.0 (-9.7 to -2.4)b | <0.001 |
| 36 mo | 41 | -8.0 (-11.7 to -4.4)b | 31 | -4.9 (-8.9 to -0.9)b | 0.008 |
| Weight change, % of baseline | |||||
| 24 mo | 45 | -11.2 (-14.9 to -7.6)b | 31 | -6.7 (-10.7 to -2.7)b | <0.001 |
| 36 mo | 41 | -9.0 (-12.9 to -5.1)b | 31 | -5.5 (-9.8 to -1.2)f | 0.012 |
| Body mass index change, kg/m2c | |||||
| 24 mo | 45 | -3.79 (-5.08 to -2.49)b | 31 | -2.25 (-3.67 to -0.84)b | 0.001 |
| 36 mo | 41 | -2.94 (-4.37 to -1.51)b | 31 | -1.61 (-3.17 to -0.04)e | 0.007 |
|
Lean tissues | |||||
| Whole-body lean mass change, kgd | |||||
| 24 mo | 45 | -2.9 (-3.9 to -1.9)b | 31 | -1.9 (-3.1 to -0.8)b | 0.02 |
| 36 mo | 41 | -2.8 (-3.9 to -1.6)b | 31 | -2.0 (-3.2 to -0.7)b | 0.04 |
| Muscle strength change, kg | |||||
| Dominant hand | |||||
| 24 mo | 44 | -1.91 (-3.70 to -0.13)e | 31 | -3.21 (-5.24 to -1.18)b | 0.16 |
| 36 mo | 41 | -2.25 (-4.02 to -0.47)f | 31 | -4.20 (-6.12 to -2.28)b | 0.03 |
| Non-dominant hand | |||||
| 24 mo | 45 | -2.33 (-4.09 to -0.58)f | 31 | -3.07 (-5.04 to -1.11)b | 0.30 |
| 36 mo | 41 | -2.80 (-4.68 to -0.91)b | 31 | -2.81 (-4.84 to -0.77)f | 0.92 |
|
Bone mineral density, g/cm2d | |||||
| Total hip change | |||||
| 24 mo | 45 | -0.030 (-0.046 to -0.015)b | 30 | -0.007 (-0.025 to 0.011) | <0.001 |
| 36 mo | 41 | -0.038 (-0.054 to -0.021)b | 30 | -0.019 (-0.038 to -0.001)e | 0.02 |
| Femoral neck change | |||||
| 24 mo | 45 | -0.046 (-0.067 to -0.025)b | 30 | -0.039 (-0.063 to 0.015)b | 0.36 |
| 36 mo | 41 | -0.063 (-0.085 to -0.040)b | 30 | -0.045 (-0.070 to -0.020)b | 0.047 |
| Lumbar spine change | |||||
| 24 mo | 45 | -0.046 (-0.065 to -0.028)b | 31 | -0.042 (-0.063 to -0.021)b | 0.31 |
| 36 mo | 41 | -0.044 (-0.065 to -0.022)b | 31 | -0.048 (-0.073 to -0.024)b | 0.77 |
| Whole-body change | |||||
| 24 mo | 45 | -0.015 (-0.041 to 0.011) | 31 | -0.005 (-0.035 to 0.026) | 0.06 |
| 36 mo | 41 | -0.021 (-0.051 to 0.008) | 31 | 0.002 (-0.031 to 0.036) | 0.007 |
|
Fat mass and distribution | |||||
| Waist circumference change, cm | |||||
| 24 mo | 45 | -9.4 (-12.7 to -6.2)b | 31 | -5.1 (-8.8 to -1.5)f | <0.001 |
| 36 mo | 41 | -7.4 (-10.8 to -3.9)b | 31 | -4.3 (-8.1 to -0.4)e | 0.04 |
| Hip circumference change, cm | |||||
| 24 mo | 45 | -6.6 (-9.1 to -4.2)b | 31 | -4.7 (-7.4 to -1.9)b | 0.005 |
| 36 mo | 41 | -5.9 (-8.5 to -3.2)b | 31 | -4.4 (-7.3 to -1.5)b | 0.046 |
| Ratio of waist to hip circumference changeg | |||||
| 24 mo | 45 | -0.020 (-0.062 to 0.022) | 31 | 0.003 (-0.043 to 0.050) | 0.02 |
| 36 mo | 41 | -0.030 (-0.075 to 0.014) | 31 | 0.006 (-0.042 to 0.054) | 0.005 |
| Whole-body fat mass change, kgd | |||||
| 24 mo | 45 | -5.9 (-8.1 to -3.7)b | 31 | -3.3 (-5.8 to -0.9)f | <0.001 |
| 36 mo | 41 | -4.8 (-6.9 to -2.6)b | 31 | -3.1 (-5.6 to -0.7)f | 0.001 |
| Abdominal adipose tissue change, cm3 | |||||
| Subcutaneous | |||||
| 24 mo | 40 | -2341 (-3161 to -1520)b | 29 | -1551 (-2454 to -647)b | 0.01 |
| 36 mo | 35 | -1986 (-2900 to -1072)b | 26 | -1477 (-2504 to -451)f | 0.16 |
| Visceral | |||||
| 24 mo | 40 | -1647 (-2199 to -1094)b | 30 | -999 (-1594 to -403)b | 0.001 |
| 36 mo | 35 | -1287 (-1868 to -705)b | 26 | -866 (-1502 to -229)f | 0.03 |
Abbreviations: CI, confidence interval; mo, months; TEMPO, Type of Energy Manipulation for Promoting optimum metabolic health and body composition in Obesity.
Bolded P values in the right-hand column represent P values that are less than 0.05.
P values for comparison between the severe and moderate interventions at each time point.
P < 0.001 versus baseline for that group. For within-group comparisons between follow-up and baseline values, a repeated-measures linear mixed model was used.
Calculated as weight in kilograms divided by height in meters squared.
Measured by dual-energy x-ray absorptiometry.
P < 0.05 versus baseline for that group. For within-group comparisons between follow-up and baseline values, a repeated-measures linear mixed model was used.
P < 0.01 versus baseline for that group. For within-group comparisons between follow-up and baseline values, a repeated-measures linear mixed model was used.
Calculated as waist circumference divided by hip circumference.
3.2. Lean tissues
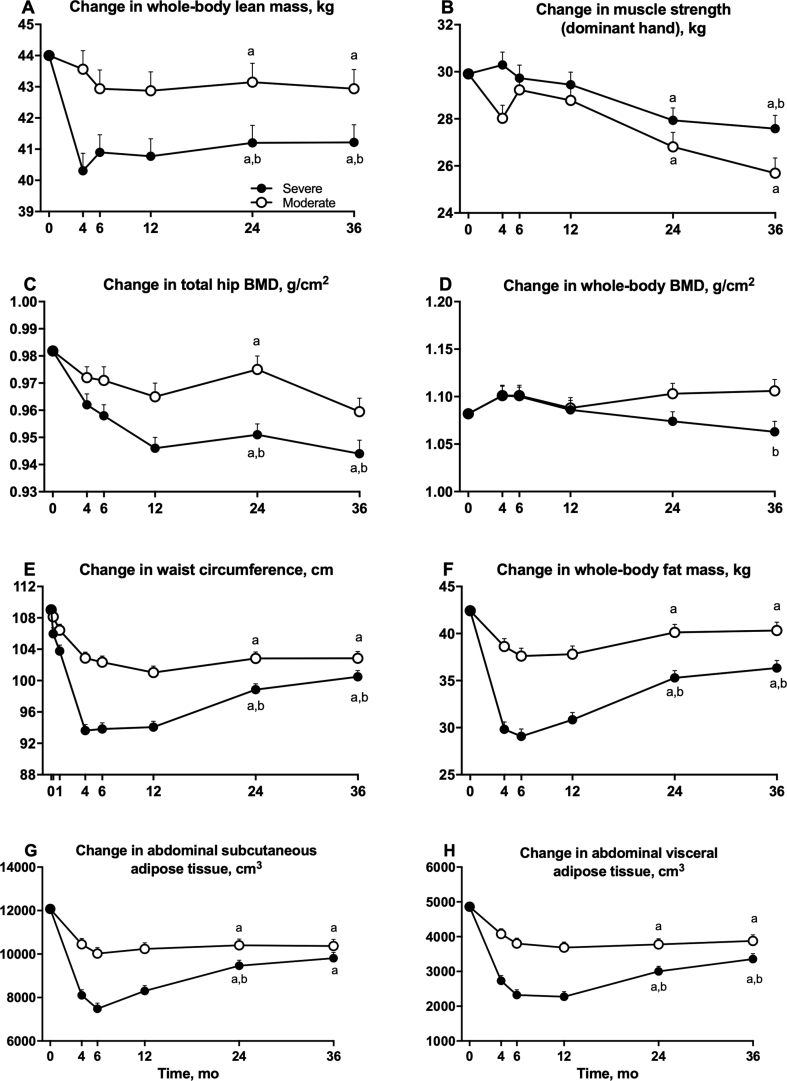
The severe group had significantly lower values of whole-body lean mass compared with the moderate group at both 24 and 36 months (effect size at 36 months, −1.7 [95% CI, −3.4 to −0.1] kg; estimated marginal means at 36 months, −2.8 [95% CI, −3.9 to −1.6] kg versus −2.0 [95% CI, −3.2 to −0.7] kg; P = 0.004) (Figure 3A and Table 2). After adjusting for body weight at each time point, there was no significant difference in whole-body lean mass between the severe and moderate group at 24 or 36 months. Both groups had significant decreases in whole-body lean mass at 24 and 36 months compared with baseline, although whole-body lean mass remained unchanged between 12 and 36 months in participants in both the moderate (0.20 [-0.81 to 1.21] kg, P = 1.00) and severe groups (0.55 [-0.35 to 1.44] kg, P = 1.00).
Figure 3.
Effect of severe versus moderate energy restriction on body composition in postmenopausal women with obesity. Estimated marginal means (ie, group means after controlling for covariates) of lean tissues: whole-body lean mass (A), muscle strength of the dominant hand (B); bone mineral density: total hip (C), whole-body (D); and fat mass and distribution: waist circumference (E), whole-body fat mass (F), abdominal subcutaneous adipose tissue (G), and abdominal visceral adipose tissue (H) in the severe and moderate groups during the 36 months of the TEMPO Diet Trial. Baseline values were the covariates in the statistical analysis model. Whiskers indicate SEs of the means. Abbreviation: BMD, bone mineral density. aP < 0.05 versus baseline value within group. bP < 0.05 versus the moderate group at that time point. Statistical comparisons between groups in the first 12 months of the trial have been published previously [9].
Apart from a significantly greater handgrip strength in the dominant hand of the severe compared to the moderate group at 36 months, there was no difference between groups in handgrip strength of the dominant or nondominant hand at 24 or 36 months (effect size at 36 months for the dominant and non-dominant hands: 1.89 [95% CI, 0.22 to 3.56] kg and −0.08 [95% CI, −1.51 to 1.68] kg, respectively; estimated marginal mean at 36 months for the dominant hand: −2.25 [95% CI, −4.02 to -0.47] kg versus −4.20 [95% CI, −6.12 to -2.28]; nondominant hand: −2.80 [95% CI, −4.68 to -0.91] kg versus −2.81 [95% CI, −4.84 to -0.77] kg) (Figure 3B and Table 2). Both groups had significant decreases in dominant and nondominant handgrip strength at 24 and 36 months compared with baseline.
3.3. Bone mineral density
The severe group had significantly lower total hip BMD than the moderate group at both 24 and 36 months (effect size at 36 months, −0.016 [95% CI, −0.029 to -0.002] g/cm2; estimated marginal means at 36 months, −0.038 [95% CI, −0.054 to −0.021] g/cm2 versus −0.019 [95% CI, −0.038 to −0.001] g/cm2; P < 0.05) (Figure 3C and Table 2). After adjusting for body weight at each time point, there was still a significant difference in total hip BMD between the severe and moderate group at 24 (P < 0.01), but not at 36 months (P = 0.09). The severe group had significantly lower femoral neck (Table 2) and whole-body BMD (Figure 3D) than the moderate group at 36 but not at 24 months, but there were no significant differences between groups in lumbar spine BMD (Table 2). The severe group had significant decreases from baseline in total hip, femoral neck and lumbar spine (but not whole-body) BMD at both 24 and 36 months, while the moderate group had significant decreases from baseline in total hip BMD at 36 but not at 24 months, and significant decreases from baseline in femoral neck and lumbar spine (but not whole-body) BMD at both 24 and 36 months (Figures 3C and 3D, Table 2). Further, there was no change in total hip BMD between 12 and 36 months in either the severe (-0.004 [95% CI, -0.019 to 0.010] g/cm2; P = 1.00) or moderate group (-0.004 [95% CI, -0.021 to 0.013], g/cm2; P = 1.00).
There was no difference in the number of participants with osteopenia (defined as a T-score between -1 and -2.5) [27] at the femoral neck between groups at 36 months, although there was a significant increase from baseline in the number of participants with osteopenia at the femoral neck in both the severe and moderate group (severe: 0 months, 8/50 [16.0%]; 36 months, 25/41 [60.9%]; P < 0.001; moderate: 0 months, 12/50 [24.0%]; 36 months, 17/30 [56.6%]; P < 0.01). In the total hip, there was also no difference between the severe or moderate groups in the number of participants with osteopenia at 36 months, but in contrast to the femoral neck there was no difference from baseline to 36 months in either group (severe: 0 months, 0/50; 36 months, 3/41 [7.3%]; moderate: 0 months, 3/50 [6.0%]; 36 months, 3/30 [10.0%]). There were no participants with osteoporosis of the hip (defined as a T-score of -2.5 or less) [27] at 0 or 36 months in the severe or moderate group.
3.4. Fat mass and distribution
The severe group had significantly lower waist and hip circumferences, and significantly lower values of waist to hip ratio compared with the moderate group at both 24 and 36 months (effect size for waist circumference at 36 months, −2.4 [95% CI, -4.7 to -0.1] cm; estimated marginal means of waist circumference at 36 months, −7.4 [95% CI, −10.8 to −3.9] cm versus −4.3 [95% CI, −8.1 to −0.4] cm; P < 0.05; effect size for hip circumference at 36 months, −1.88 [95% CI, −0.05 to -0.03] cm; estimated marginal means of hip circumference at 36 months, −5.9 [95% CI, −8.5 to −3.2] cm versus −4.4 [95% CI, −7.3 to −1.5] cm; P < 0.05) (Figure 3E, Table 2). Both groups had significant decreases from baseline in waist and hip circumference (but not in the ratio of waist to hip circumference) at 24 and 36 months. Compared with the moderate group, the severe group had significantly lower whole-body fat mass at both 24 and 36 months (effect size at 36 months, −5.5 [95% CI, −7.1 to −3.9] kg; estimated marginal means at 36 months, −10.2 [95% CI, −12.1 to −8.4] kg versus −5.5 [95% CI, −7.5 to −3.4] kg; P < 0.001) (Figure 3F and Table 2). The severe group also had significantly lower values for abdominal subcutaneous adipose tissue volumes than the moderate group at 24, but not at 36 months (effect size at 36 months, −561 [95% CI, −1352 to -229] cm3; estimated marginal means at 36 months, −1986 [95% CI, −2900 to −1072] cm3 versus −1477 [95% CI, −2504 to −451] cm3; P = 0.16) (Figure 3G and Table 2), and significantly lower values for abdominal visceral adipose tissue volume at both 24 and 36 months (effect size at 36 months, −521 [95% CI, −978 to -65] cm3; estimated marginal means at 36 months, −1287 [95% CI, −1868 to −705] cm3 versus −866 [95% CI, −1502 to −229] cm3; P < 0.05) (Figure 3H and Table 2). Both groups had significant decreases from baseline at 24 and 36 months in whole-body fat mass, abdominal subcutaneous adipose tissue and abdominal visceral adipose tissue (Figures 3F, 3G and 3H, Table 2). In the severe group, between 12 to 36 months, there was an increase in whole-body fat mass (effect size 5.5 [95% CI, 3.5 to 7.6] kg, P < 0.001), subcutaneous (effect size 1421 [95% CI, 563 to 2280] cm3, P < 0.001) and visceral (effect size 1135 [95% CI, 689 to 1580] cm3, P < 0.001) abdominal adipose tissue volumes, while in the moderate group between 12 to 36 months there was a trend for an increase in whole-body fat mass (effect size 2.2 [95% CI, -0.1 to 4.5] kg, P = 0.065), but no change in subcutaneous (effect size 163 [95% CI, -795 to 1121] cm3, P = 1.00) or visceral (effect size 202 [95% CI, -299 to 702]) cm3, P = 1.00) abdominal adipose tissue volumes.
3.5. Adverse events
There were 8 adverse events reported in 8 participants between 12 and 36 months, all in the severe group. Of these 8 adverse events, 5 were related or possibly related to the intervention, and 3 were not related to the intervention. The adverse events were gallstones (2 participants, which represents 4.0% of participants [2 of 50] in the severe intervention), breast cancer (2 participants [4.0%]), hemorrhoids (1 participant [2.0%]), hair loss/alopecia (1 participant [2.0%]), type 2 diabetes (1 participant [2.0%]), and osteoporosis of the spine (1 participant [2.0%], who had seen a specialist who made the diagnosis). We had also observed osteoporosis of the spine in this participant in our DXA scans at 24 and 36 months, where her T-score for the spine was -2.5 and -2.6, respectively. However, as her T-score for the hip was greater than the threshold for osteoporosis (-2.5 or less) [27], and as we had decided a priori to assess osteoporosis based on the hip rather than the spine due to measurement artifacts being common in the spine, we did not classify this participant as having osteoporosis.
4. Discussion
This 3-year follow up on a randomized clinical trial showed that postmenopausal women with obesity who had undergone severe energy restriction lost approximately 1.5 to 1.7 times as much net weight, waist circumference, whole-body fat mass and visceral adipose tissue compared to those who had undergone moderate energy restriction, and were 2.6 times more likely (42% versus 16%) to have lost 10% or more of their initial body weight at 3 years. However, those in the severe versus moderate intervention lost approximately 1.4 times as much whole-body lean mass (albeit this was proportional to total weight lost and there was no greater loss of handgrip strength), and approximately 2 times as much total hip bone mineral density between 0 and 3 years, with this bone loss occurring in the first year.
As with all weight loss trials, we observed weight regain in participants in both the severe and moderate groups by 36 months. Interestingly, the only participants who gained more than 5% of their initial body weight were from the severe group, with 3/50 of these participants (6.0%) regaining 11 to 14% of their initial body weight at 36 months. Such weight gain was not seen in any individuals in the moderate group. Although there is insufficient data to say if this difference is statistically significant, it is important to be aware that some individuals may need close monitoring following a severely energy-restricted diet, such as the total meal replacement diet used in this trial, and if rapid weight regain is evident then more intensive strategies to assist with weight maintenance could be deployed.
In terms of composition, it appears that weight regains in both the severe and moderate groups between 12 to 36 months were largely due to increases in fat mass, with whole-body lean mass remaining largely unchanged during this time. These results are in line with other studies suggesting that fat mass is regained to a greater degree than lean mass in postmenopausal women after weight loss [28]. In our study, muscle strength did not decrease from baseline during the 12-month intervention (although it did decrease between 12 and 36 months), which suggests that muscle quality was maintained during the intervention despite an apparent loss of muscle quantity (as indicated by reduced whole-body lean mass). This is important because muscle quality has been shown to be a more important predictor of health risk than muscle quantity [29, 30, 31]. The overall decrease in handgrip strength seen in both groups between 12 and 36 months (approximately 2 to 4 kg) was on par with the approximately 6 kg decrease in handgrip strength observed in women in the 2 decades of life between the ages of 45 and 65 years [32].
Despite weight and fat regain between 12 and 36 months in participants in both interventions, at 36 months those in the severe intervention still weighed significantly less and had significantly lower waist and hip circumference, waist to hip ratio, whole-body fat mass and visceral abdominal adipose tissue volume than those in the moderate intervention. This finding counters the theory that the fast weight loss associated with severe energy restriction may be associated with greater fat regain, especially abdominal fat regain [17]. Therefore, severe energy restriction (with a meal replacement diet) is more effective than moderate energy restriction (with a food-based diet) in terms of reducing body weight and fat – including the more pathogenic visceral adipose tissue – in the long term (3 years), at least in postmenopausal women with obesity.
The greater long-term weight and fat loss during severe versus moderate energy restriction must be considered in light of a greater loss of BMD. In this trial, participants in the moderate group exhibited an approximately 1.9% reduction in total hip BMD over the 36-month trial. This was under what was expected within 3 years, considering that the annual rate of BMD loss at the hip in the early postmenopausal years is approximately 1.0 to 1.4% (0.013 g/cm2) [33]. On the other hand, participants in the severe group exhibited an approximately 3.7% reduction in total hip BMD over the 36 months, which was still within the expected loss of BMD over 3 years based on the annual rate of BMD loss at the hip in this population, but total hip BMD loss at 36 months was still 2 times higher in the severe than the moderate group. Interestingly, there was no change in total hip BMD between 12 and 36 months in either the moderate or severe groups, despite weight regain during this period. It appears that total hip BMD loss only occurs in the first 12 months of a weight loss intervention, after which time it remains low and is not regained, even if weight is regained. These findings are consistent with other research suggesting that BMD loss during weight reduction may not be fully recovered with weight regain in postmenopausal women [34, 35].
Although weight variability has been shown to increase the risk of hip fractures in adults [36], the long-term impact of energy restriction on fractures is not clear, particularly when it is used for intentional weight loss [37, 38]. For instance, a study of 120,566 postmenopausal women (50 to 79 years of age at baseline) showed that those who unintentionally lost 5% or more of their body weight over 3 years – compared to those who remained weight stable – had 33% higher incidence rates of hip fracture and 16% higher incidence rates of vertebral fracture, while those who intentionally lost 5% or more of their body weight had 15% lower incidence rates of hip fracture and 11% higher incidence rates of lower limb fracture [39]. It is possible that one bout on a severely energy-restricted diet may not induce an increase in osteoporotic fracture risk, but the effects of repeated bouts of a severely energy-restricted diet, done over many years as a way of maintaining a lower weight [40], is not known.
In light of the effect of severe energy restriction to reduce BMD more than moderate energy restriction, at least in postmenopausal women with obesity, as well as the currently unknown impact of diet-induced BMD loss on long-term fracture risk, it could be important to monitor BMD in people who undertake severely energy-restricted diets for weight management. It would also seem prudent to recommend strategies to protect bone health during adherence to a severely energy-restricted diet. One such strategy would be to ensure adequate dietary intake of calcium and vitamin D during the diet. While it is unlikely that dietary deficiencies in these micronutrients could explain the greater BMD loss observed in the severe versus moderate intervention, because the meal replacement products used here met the estimated needs for calcium and vitamin D for women of the ages recruited for this trial (45 to 65 years) [53], dietary deficiencies could potentially worsen bone loss during a severely energy-restricted total meal replacement diet. Of note, while the currently-used brand of total meal replacement product met the prevailing Australian recommended dietary intake for calcium for women aged 19 to 70 years, 2 of the 7 other brands available on the local market did not [53]. Moreover, for people aged over 70 years, none of the 8 locally-available brands – including the brand used in this trial – met the recommended dietary intake of calcium [53]. For Vitamin D, only 3 of the 8 locally-available brands of total meal replacement products – including the brand used in this trial – provided adequate intake for adults aged 51 years and over [53]. Therefore, it would be important to check the nutritional content of the total meal replacement brand to be used, and consider whether another brand may be more suitable, or whether supplementation with calcium and vitamin D may be required. Another strategy to protect bone health during adherence to a severely energy-restricted diet could be to strongly recommend muscle strengthening exercises. Although it is not clear that strength training would have prevented the greater BMD loss in the severe versus the moderate intervention in participants in the current trial, as is the case for other trials that administered either moderate [41, 42, 43, 44] or severe [45] energy restriction, there is strong evidence that strength training at least twice per week has numerous health benefits for men and women of all ages [46, 47, 48]. As such, there seems little harm in recommending regular muscle strengthening exercises during a severely energy-restricted diet, subject to approval from the healthcare professional.
Over the course of this trial, ie, between baseline and 36 months, the two most common intervention-related (or possibly intervention-related) side-effects noted were haemorrhoids and gallstones. Haemorrhoids were noted by 3 of the 50 participants (ie, 6.0%) that underwent the severely energy-restricted diet. Two participants (4.0%) reported haemorrhoids during the first 12 months, reported in our publication of outcomes to 12 months [9], and 1 (2.0%) reported haemorrhoids between 12 and 36 months, which is not likely due to the intervention. Further, 4 of the 50 participants (8.0%) in the severe group reported gallstones over the course of the trial. Therefore, people undergoing severely energy-restricted diets could benefit from being informed about these side-effects so that strategies to prevent or remedy them can be taken. For example, after these 2 cases of haemorrhoids were reported within the first 12 months, we started giving participants a 1-page information sheet about avoiding and managing constipation by being aware of bowel movements, eating the prescribed 2 cups of non-starchy vegetables every day (which provide approximately 3 g of additional dietary fibre), drinking at least 2 L of water every day, being physically active, and taking laxatives (eg, fibre supplements) if necessary (Supplement 1). We also reiterated the importance of these instructions every 2 weeks while participants were on the diet, and provided samples of suitable laxatives to help allay constipation when indicated, such that no further cases of haemorrhoids were reported during the intervention. These instructions are particularly important given that none of the 8 locally-available brands of total meal replacement products provided more than 12.4 g of dietary fibre per day (assuming use of 3 products per day) [53], which is less than half the adequate intake of fibre for adults of all ages (25 g per day for women and 30 g per day for men in Australia) [49]. Further to recommending strategies to prevent constipation, it may be important for healthcare professionals to monitor for any signs of gallstones during and after a severely energy-restricted diet so that suitable actions can be taken if required.
4.1. Strengths and limitations
Strengths of this study include the randomized clinical trial design, with long-term follow-up at 24 and 36 months, as well as the fact that data variability was reduced by analysis of all data by a single researcher (ie, S.M. for DXA and A.L.W.-T. for MRI). A limitation of our trial is that participants were predominantly white, which limits the generalizability of the findings to populations of other races. An additional limitation is that changes in BMD observed using DXA during large weight losses might be exaggerated because of the varying amounts of soft tissue surrounding bone over time, which can result in unpredictable errors of up to 20% in BMD measurements [50, 51]. However, DXA remains the gold standard and the only available test for measuring BMD in clinical practice [52].
5. Conclusions
Severe energy restriction is more effective than moderate energy restriction for reducing weight and adiposity in the long term (3 years), but attention to bone mineral density loss in the first year is required in postmenopausal women.
Declarations
Author contribution statement
R. Seimon: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
A. Gibson: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
I. Caterson, N. Byrne and A. Sainsbury: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
S. McClintock, C. Harper and H. Fernando: Performed the experiments; Wrote the paper.
A. Wild-Taylor: Analyzed and interpreted the data.
N. Johnson, T. Markovic, J. Center, J. Franklin, P. Liu, S. Grieve and J. Lagopoulos: Analyzed and interpreted the data; Wrote the paper.
Funding statement
The TEMPO Diet Trial was supported by a Project Grant (1026005) from the National Health and Medical Research Council of Australia, awarded to A. Sainsbury, N. Byrne and I. Caterson. The Rebecca L. Cooper Medical Research Foundation and the University of Sydney/National Health and Medical Research Council of Australia provided grants that contributed to the purchase of equipment used for this trial. Prima Health Solutions (Brookvale, New South Wales, Australia) provided in-kind support for this trial in the form of below-cost KicStart meal replacement products (shakes) and a gift of associated adherence tools (shakers). This relationship with Prima Health Solutions was established after the dietary protocol for the TEMPO Diet Trial had been established. R. Seimon was supported by a National Health and Medical Research Council of Australia Early Career Research Fellowship (1072771), and a Postdoctoral Research Award from the Endocrine Society of Australia. A. Gibson was supported by an Australian Postgraduate Award from the Australian Government Department of Education and Training. H. Fernando was supported by an International Postgraduate Research Scholarship from The Australian Government Department of Education and Training. S. Grieve was supported by the Sydney Medical School Foundation, the Heart Research Institute, The Frecker Family Trust, the Parker Hughes Bequest, and the University of Sydney. P. Liu was supported in part by the National Heart, Lung, and Blood Institute (K24HL13632). A. Sainsbury was supported by Senior Research Fellowships (1042555 and 1135897), and a Sydney Outstanding Academic Researcher Fellowship from the University of Sydney.
Competing interest statement
The authors declare the following conflict of interests:
R. Seimon reported serving on the Nestlé Health Science Optifast VLCD advisory board.
A. Gibson reported receiving payment from the Pharmacy Guild of Australia and from Nestlé Health Science for presentations at conferences.
H. Fernando reported being employed by the University of Sydney as a tutor.
T. Markovic reported serving on the NovoNordisk Obesity advisory board and the Nestlé Health Science Optifast VLCD advisory board; receiving funds for performing a clinical trial from the Australian Egg Corporation, and giving talks on obesity for NovoNordisk.
J. Center reported receiving support and honoraria for educational talks and/or advisory meetings from Amgen, Teva Pharmaceutical Industries, and Bayer and receiving an investigator-sponsored grant from Amgen.
I. Caterson reported being a past president of the World Obesity Federation; receiving funds for performing clinical trials from SFI, the Australian Egg Corporation, Novo Nordisk, Bristol-Myers Squibb, and Pfizer; giving talks on obesity for Novo Nordisk; chairing the independent steering committee for the ACTION IO Study; and receiving grants from Rhythm Pharmaceuticals.
A. Sainsbury reported owning 50% of the shares in Zuman International, which receives royalties for books she has written and payments for presentations at industry conferences; receiving presentation fees and travel reimbursements from Eli Lilly and Co, the Pharmacy Guild of Australia, Novo Nordisk, the Dietitians Association of Australia, Shoalhaven Family Medical Centres, the Pharmaceutical Society of Australia, and Metagenics; and serving on the Nestlé Health Science Optifast VLCD advisory board from 2016 to 2018.
Additional information
The clinical trial described in this paper was registered at the Australian New Zealand Clinical Trials Registry under the registration number ACTRN12612000651886.
Acknowledgements
We thank all our participants for their time and cooperation in this trial. Michelle SH Hsu, MNurtDiet, and John McBride, BSc, assisted with recruitment of participants; Elisia Manson, with planning the dietary intervention; the staff at I-Med Radiology (Camperdown, New South Wales, Australia), namely Ron Shnier, MD, Lynette Masters, MD, David Rowan, MD, and Domenic Soligo, PGradDipMedRadSc (magnetic resonance imaging), conducted the magnetic resonance imaging for this trial; and Pia Wikstrom (I-Med Radiology) helped schedule our participants for these scans. They were not compensated for their time. We are grateful to the following people who volunteered as guest facilitators for group support meeting(s) with participants: Felipe Q. da Luz, PhD, Andrea L. Pattinson, MNurtDiet, Rebecca A. Harris, BSc (Hons), Manal Bardough, MNurtDiet, Michelle S.H. Hsu, MNurtDiet, Carmen Khung-Smith, BSc, Claire D. Madigan, PhD, Eunike Santoso, MNurtDiet, and Veronica M. Smith, BSc (Hons).
Contributor Information
Radhika V. Seimon, Email: radhika.seimon@sydney.edu.au.
Amanda Sainsbury, Email: amanda.salis@uwa.edu.au.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Online Supplemental Material
References
- 1.Ng M. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Australian Institute of Health and Welfare . 2017. A Picture of Overweight and Obesity in Australia 2017. [Google Scholar]
- 3.Lean M.E.J. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabet. Endocrinol. 2019 doi: 10.1016/S2213-8587(19)30068-3. [DOI] [PubMed] [Google Scholar]
- 4.Atukorala I. Is there a dose-response relationship between weight loss and symptom improvement in persons with knee osteoarthritis? Arthritis Care Res. (Hoboken) 2016;68(8):1106–1114. doi: 10.1002/acr.22805. [DOI] [PubMed] [Google Scholar]
- 5.Schrepf A. Improvement in the spatial distribution of pain, somatic symptoms, and depression after a weight loss intervention. J. Pain. 2017;18(12):1542–1550. doi: 10.1016/j.jpain.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Heart, Lung and Blood Institute . NIH Publication No. 98-4083; 1998. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: the Evidence Report. [Google Scholar]
- 7.Purcell K. The effect of rate of weight loss on long-term weight management: a randomised controlled trial. Lancet Diabet. Endocrinol. 2014;2(12):954–962. doi: 10.1016/S2213-8587(14)70200-1. [DOI] [PubMed] [Google Scholar]
- 8.Gibson A.A. Fast versus slow weight loss: development process and rationale behind the dietary interventions for the TEMPO Diet Trial. Obes. Sci. Pract. 2016;2(2):162–173. doi: 10.1002/osp4.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seimon R.V. Effect of weight loss via severe vs moderate energy restriction on lean mass and body composition among postmenopausal women with obesity: the TEMPO diet randomized clinical trial. JAMA Netw Open. 2019;2(10) doi: 10.1001/jamanetworkopen.2019.13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheskin L.J. Efficacy of meal replacements versus a standard food-based diet for weight loss in type 2 diabetes: a controlled clinical trial. Diabetes Educat. 2008;34(1):118–127. doi: 10.1177/0145721707312463. [DOI] [PubMed] [Google Scholar]
- 11.Franz M.J. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J. Am. Diet Assoc. 2007;107(10):1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Vink R.G. The effect of rate of weight loss on long-term weight regain in adults with overweight and obesity. Obesity (Silver Spring). 2016;24(2):321–327. doi: 10.1002/oby.21346. [DOI] [PubMed] [Google Scholar]
- 13.Harper C. Experiences of using very low energy diets for weight loss by people with overweight or obesity: a review of qualitative research. Obes. Rev. 2018;19(10):1412–1423. doi: 10.1111/obr.12715. [DOI] [PubMed] [Google Scholar]
- 14.Gibson A.A. Do ketogenic diets really suppress appetite? A systematic review and meta-analysis. Obes. Rev. 2015;16(1):64–76. doi: 10.1111/obr.12230. [DOI] [PubMed] [Google Scholar]
- 15.Lee A.J. Testing the price and affordability of healthy and current (unhealthy) diets and the potential impacts of policy change in Australia. BMC Publ. Health. 2016;16:315. doi: 10.1186/s12889-016-2996-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Australian Beurea of Statistics. Household expenditure survey, Australia: summary of results. Available online: https://www.abs.gov.au/household-expenditure (accessed on 3 February 2020).
- 17.Sainsbury A., Zhang L. Role of the hypothalamus in the neuroendocrine regulation of body weight and composition during energy deficit. Obes. Rev. 2012;13(3):234–257. doi: 10.1111/j.1467-789X.2011.00948.x. [DOI] [PubMed] [Google Scholar]
- 18.Jensen M.D. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart association task force on practice guidelines and the obesity society. Circulation. 2014;129(25 Suppl 2):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Clinical Guideline Centre . National Clinical Guideline Centre; London, UK: 2010. Obesity: Identification, Assessment and Management of Overweight and Obesity in Children, Young People and Adults. [Google Scholar]
- 20.National Health and Medical Research Council . Australian Government; Canberra, Australia: 2013. Clinical Practice Guidelines for the Management of Overweight and Obesity in Adults, Adolescents and Children in Australia ACT Canberra. [Google Scholar]
- 21.Seimon R.V. Rationale and protocol for a randomized controlled trial comparing fast versus slow weight loss in postmenopausal women with obesity-the TEMPO diet trial. Healthcare (Basel) 2018;6(3) doi: 10.3390/healthcare6030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J. Am. Med. Assoc. 2000:3043–3045. [PubMed] [Google Scholar]
- 23.International Conference on Harmonisation Expert Working Group, ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice
- 24.Australian Government National Health and Medical Research Council Healthy eating for adults. 2013. http://www.eatforhealth.gov.au/sites/default/files/files/ Available from.
- 25.Rachner T.D., Khosla S., Hofbauer L.C. Osteoporosis: now and the future. Lancet. 2011;377(9773):1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.BaHammam A. Circadian pattern of sleep, energy expenditure, and body temperature of young healthy men during the intermittent fasting of Ramadan. Appetite. 2010;54(2):426–429. doi: 10.1016/j.appet.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization . World Health Organization; Geneva, Switzerland: 2007. WHO Scientific Group on the Assessment of Osteoporosis at the Primary Health Care Level: Summary Meeting Report. Brussels, Belgium; May 5–7, 2004. [Google Scholar]
- 28.Beavers K.M. Is lost lean mass from intentional weight loss recovered during weight regain in postmenopausal women? Am. J. Clin. Nutr. 2011;94(3):767–774. doi: 10.3945/ajcn.110.004895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Visser M. Reexamining the sarcopenia hypothesis. Muscle mass versus muscle strength. Health, aging, and body composition study research group. Ann. N. Y. Acad. Sci. 2000;904:456–461. [PubMed] [Google Scholar]
- 30.Visser M. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60(3):324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 31.Newman A.B. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61(1):72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 32.Mathiowetz V. Grip and pinch strength: normative data for adults. Arch. Phys. Med. Rehabil. 1985;66(2):69–74. [PubMed] [Google Scholar]
- 33.Finkelstein J.S. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J. Clin. Endocrinol. Metab. 2008;93(3):861–868. doi: 10.1210/jc.2007-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villalon K.L. A losing battle: weight regain does not restore weight loss-induced bone loss in postmenopausal women. Obesity (Silver Spring) 2011;19(12):2345–2350. doi: 10.1038/oby.2011.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kammire D.E. Effect of weight change following intentional weight loss on bone health in older adults with obesity. Obesity (Silver Spring) 2019;27(11):1839–1845. doi: 10.1002/oby.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer H.E., Tverdal A., Selmer R. Weight variability, weight change and the incidence of hip fracture: a prospective study of 39,000 middle-aged Norwegians. Osteoporos. Int. 1998;8(4):373–378. doi: 10.1007/s001980050077. [DOI] [PubMed] [Google Scholar]
- 37.Villareal D.T. Effect of two-year caloric restriction on bone metabolism and bone mineral density in non-obese younger adults: a randomized clinical trial. J. Bone Miner. Res. 2016;31(1):40–51. doi: 10.1002/jbmr.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zibellini J. Does diet-induced weight loss lead to bone loss in overweight or obese adults? A systematic review and meta-analysis of clinical trials. J. Bone Miner. Res. 2015;30(12):2168–2178. doi: 10.1002/jbmr.2564. [DOI] [PubMed] [Google Scholar]
- 39.Crandall C.J. Postmenopausal weight change and incidence of fracture: post hoc findings from Women's Health Initiative Observational Study and Clinical Trials. BMJ. 2015;350:h25. doi: 10.1136/bmj.h25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christensen P. Long-term weight-loss maintenance in obese patients with knee osteoarthritis: a randomized trial. Am. J. Clin. Nutr. 2017;106(3):755–763. doi: 10.3945/ajcn.117.158543. [DOI] [PubMed] [Google Scholar]
- 41.Armamento-Villareal R. Changes in thigh muscle volume predict bone mineral density response to lifestyle therapy in frail, obese older adults. Osteoporos. Int. 2014;25(2):551–558. doi: 10.1007/s00198-013-2450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villareal D.T. Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: a one-year randomized controlled trial. J. Clin. Endocrinol. Metab. 2008;93(6):2181–2187. doi: 10.1210/jc.2007-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villareal D.T. Weight loss, exercise, or both and physical function in obese older adults. N. Engl. J. Med. 2011;364(13):1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beavers K.M. Effect of exercise modality during weight loss on bone health in older adults with obesity and cardiovascular disease or metabolic syndrome: a randomized controlled trial. J. Bone Miner. Res. 2018;33(12):2140–2149. doi: 10.1002/jbmr.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haywood C.J. Very low calorie diets for weight loss in obese older adults-A randomized trial. J. Gerontol. A Biol. Sci. Med. Sci. 2017;73(1):59–65. doi: 10.1093/gerona/glx012. [DOI] [PubMed] [Google Scholar]
- 46.Brown W.J. Report prepared for the Australian Government Department of Health; 2012. Development of evidence-based physical activity recommendations for adults (18-64 years) [Google Scholar]
- 47.U.S. Department of Health and Human Services. 2018 Physical Activity Guidelines Advisory Committee . 2018. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC. [Google Scholar]
- 48.Villareal D.T. Aerobic or resistance exercise, or both, in dieting obese older adults. N. Engl. J. Med. 2017;376(20):1943–1955. doi: 10.1056/NEJMoa1616338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Australian National Health and Medical Research Council (NHMRC), The New Zealand Ministry of Health (MoH) Nutrient reference values for Australia and New Zealand. 2019. https://www.nrv.gov.au/nutrients/dietary-fibre Available from:
- 50.Yu E.W. Bone metabolism after bariatric surgery. J. Bone Miner. Res. 2014;29(7):1507–1518. doi: 10.1002/jbmr.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bolotin H.H. DXA in vivo BMD methodology: an erroneous and misleading research and clinical gauge of bone mineral status, bone fragility, and bone remodelling. Bone. 2007;41(1):138–154. doi: 10.1016/j.bone.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 52.Yu E.W. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J. Bone Miner. Res. 2012;27(1):119–124. doi: 10.1002/jbmr.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gibson A.A. Comparison of Very Low Energy Diet products available in Australia and how to tailor them to optimise protein content for younger and older adult males and females. Healthcare. 2016;4(3):71. doi: 10.3390/healthcare4030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplemental Material