Abstract
There is a growing body of evidence for the effects of vitamin D on intestinal host-microbiome interactions related to gut dysbiosis and bowel inflammation. This brief review highlights the potential links between vitamin D and gut health, emphasizing the role of vitamin D in microbiological and immunological mechanisms of inflammatory bowel diseases. A comprehensive literature search was carried out in PubMed and Google Scholar using combinations of keywords “vitamin D,” “intestines,” “gut microflora,” “bowel inflammation”. Only articles published in English and related to the study topic are included in the review. We discuss how vitamin D (a) modulates intestinal microbiome function, (b) controls antimicrobial peptide expression, and (c) has a protective effect on epithelial barriers in the gut mucosa. Vitamin D and its nuclear receptor (VDR) regulate intestinal barrier integrity, and control innate and adaptive immunity in the gut. Metabolites from the gut microbiota may also regulate expression of VDR, while vitamin D may influence the gut microbiota and exert anti-inflammatory and immune-modulating effects. The underlying mechanism of vitamin D in the pathogenesis of bowel diseases is not fully understood, but maintaining an optimal vitamin D status appears to be beneficial for gut health. Future studies will shed light on the molecular mechanisms through which vitamin D and VDR interactions affect intestinal mucosal immunity, pathogen invasion, symbiont colonization, and antimicrobial peptide expression.
Keywords: vitamin D, intestine, gut microflora, bowel inflammation
I. Introduction
The human body is subjected to constant exposure to foreign antigens and pathogens. This has resulted in formation of several powerful protective systems that are necessary to maintain control over endogenous opportunistic microorganisms and to prevent harmful effects of exogenous microbial organisms. One of the main elements in this series of subtle mechanisms of anti-infective protection is the healthy microbiome (microflora, microbiota), formation of which begins immediately after birth and continues throughout the lifespan. Recent evidence suggests that the fetus is exposed to bacterial DNA and metabolites even before birth [77].
The role of the symbiotic microbiota is not limited to antagonistic functions. In many species, the microbiome collectively acts as an “organ” to regulate physiological processes such as metabolic and immunomodulatory effects, and biosynthesis of a variety compounds, and thus is an integral part of the host [34, 52]. Changes or imbalance in the qualitative and quantitative composition of autochthonous microorganisms, which are normally in a positive commensal relationship with the host, has unsafe consequences for human health in general and directly affects the quality and expectancy of life [45, 86]. The functional parameters of the gut microbiota are controlled by inherent immune and non-immune systems in an individual, and have the ability of physiological adaptation.
Vitamin D is mostly regarded as a transcriptional regulator of genes controlling mineral ion homeostasis of bone. A broad range of skeletal and non-skeletal diseases are related to vitamin D inadequacy [92], whereas hypervitaminosis D may occur when supplemental vitamin D is taken excessively [46]. Several studies of the effect of vitamin D and the vitamin D receptor (VDR) on the intestinal microbiota have shown essential roles of vitamin D in maintaining a healthy gut microenvironment. Thus, vitamin D may be useful as an adjuvant in prevention and treatment of certain gastrointestinal diseases.
II. Biogeography of the Gut Microbiota
The human digestive tract [gastrointestinal tract (GIT), gut] is in constant contact with the external microenvironment, which contains various microbes. On average throughout life, about 60 tons of food pass through the human digestive tract, along with numerous environmental microorganisms that could potentially disrupt gut integrity [6, 80]. The mucosal surface area of the GIT that engages with microorganisms is quite large; with one study suggesting an area about the size of a tennis court (260–300 m2) [58]. The microbial biomass inhabiting the digestive tract of an adult weighs up to 2 kg [51] and is composed of approximately 100 trillion (1014) microbes, which outnumber host cells by 10 times [44]. A total of 2776 different prokaryotic species have been isolated from the human body, including the GIT, and classified into 11 different phyla [7]. Anaerobic species compose >90% of the human microbiota, while aerobes and facultative anaerobes make up ~5% [40] (Fig. 1).
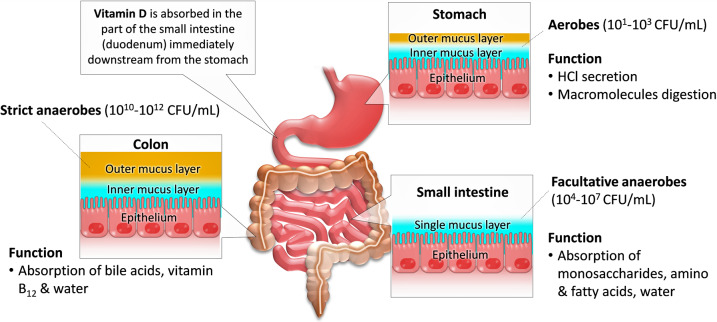
Fig. 1.
Distribution and predominance of the gut microbiota in the digestive tract. The total amount of microbiota increases gradually along the gastrointestinal tract (GIT), with low concentrations in the stomach and higher levels in the colon. The stomach harbors around 101–3 CFU/ml. An increasing abundance and diversity are found in the small intestine (duodenum, jejunum, ileum), and colon. The predominant members of the gut microbiota are anaerobic. Dietary or supplemental vitamin D is absorbed through the small intestine (especially through the duodenum) as a fat-soluble vitamin.
The microbiota of a healthy individual is diverse and includes mainly symbiotic and commensal microbial communities, as well as pathogenic microbial populations that have been accidentally introduced from the environment [21]. However, these transient microbes do not persist for a long time in the GIT due to the barrier mechanisms of host immune and non-immune defense systems that prevent excessive reproduction of undesirable microbes. In terms of intestinal microbial ecology, the normal microbiota is divided into autochthonous (resident) and allochthonous (transient) species [56]. The former group includes indigenous microbes that play an important role in metabolism in the host organism and in protecting it from pathogens of infectious diseases. The latter group are quite common in a healthy population, but the composition is not constant and changes over time [98].
Reproduction of most microbes in the stomach occurs slowly due to the acidic environment. The estimated number of bacteria in the gastric juice is <103 CFU/ml [37]. Almost all microbial species detected in the stomach come from the oral cavity and pharynx. The healthy human stomach is dominated by Prevotella, Streptococcus, Veillonella, Rothia, Haemophilus, and Helicobacter pylori; however, the composition of the microbiota is dynamic and is influenced by factors such as diet, drugs, and diseases [55] (Fig. 2).
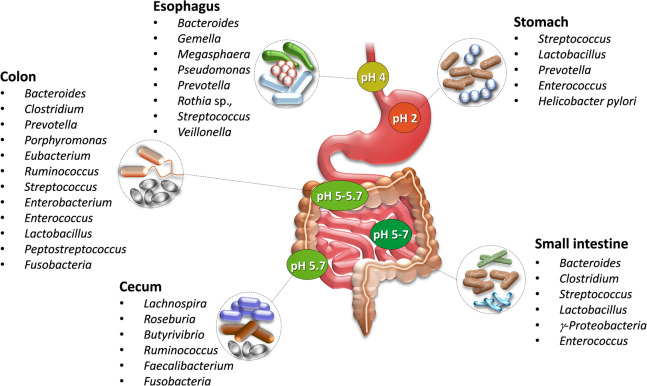
Fig. 2.
Microbial diversity and composition across gut sections (adapted and modified from [29]). The gut microbiota varies with pH and oxygen tension, flow rates of digestive fluids, substrate availability, and host secretions. pH plays a key role in microbial community dynamics. The stomach and proximal small intestine are particularly unfavorable for bacterial residence, and very few bacteria are resistant to the acidic microenvironment. The highest biodiversity is in the colon, in which the pH is around 5.
The duodenum and jejunum of the small intestine in healthy adults usually contain a small population of bacteria, from 100–4 CFU/ml. Peristalsis and large pH gradients in the small intestine prevent excessive growth of microorganisms. The bacterial concentration reaches 105 CFU/ml in the proximal ileum, 105–8 CFU/ml in the terminal ileum, and 1010–12 CFU/ml in the cecum [61, 70]. The number of bacteria in the lumen of the distal colon gradually increases, reaching 1012–1013 CFU/ml. The most numerous and diverse microflora is found in the colon, with 400 to 1500 species of microorganisms [22]. The anaerobic microflora mainly comprises Bifidobacterium, Bacteroides, Lactobacillus, Fusobacterium, Propionibacterium, Veillonella, and Clostridium. The facultative anaerobic and aerobic microorganisms of the colon include enterococci, streptococci, staphylococci, Escherichia, and yeast-like fungi of the genus Candida [14, 27].
Qualitative and quantitative changes in the gut microbiome may occur due to a wide variety of external and internal factors, including diet, environmental pollution, stressful conditions, medications, physical inactivity, reduced immune status, malnutrition, development of gastrointestinal pathological conditions, and inflammatory responses induced by infectious and non-infectious causes [71, 91, 93]. Disruption of the balance of the normal microbiome leads to a decrease in colonization resistance and changes in the metabolic activity of the microbiota. Development of intestinal dysbiosis of various etiologies aggravates the course of underlying diseases, leading to pronounced local and systemic disorders, including inflammatory bowel disease (IBD), celiac disease, irritable bowel syndrome, allergy, metabolic syndrome, obesity, asthma, and cardiovascular disease [11, 45, 60].
III. Functional Value of the Microbiota
The gut microbiota is considered to be an “extra organ” of the host because it plays a crucial role in good health by maintaining essential functions [59]. The main functions of the normal gut microbiota are presented in Table 1 [13, 19, 29, 32, 41, 72, 76]. Primarily, it has a trophic (digestive) effect, represented by symbiotic digestion, which is carried out by microbial enzymes. This is based on the energy supply of epithelial cells, which relies on utilization of low-molecular-weight (LMW) metabolites [69, 84]. Another important function is stimulation of local immunity by generating secretory IgA. LMW metabolites of saccharolytic microflora, which are primarily short-chain fatty acids (SCFAs), lactate, and other compounds, have a noticeable bacteriostatic effect [53, 87], and can suppress growth of pathogens such as Salmonella, Shigella and some fungi. At the same time, the bacteriostatic effects do not extend to the resident microbiota. LMW metabolites can also block receptors of epithelial cells, interfere with adhesion of pathogens to the epithelium [68], and induce chemotaxis of bacteria [16].
Table 1. .
Likely functions of gut microbiota
| Function |
|---|
| • Trophic functions |
| • Energy supply for epithelial cells |
| • Regulation of gastrointestinal motility |
| • Regulation of differentiation and regeneration of various tissues, primarily epithelial |
| • Maintenance of ionic homeostasis |
| • Detoxification and elimination of endogenous and exogenous toxic compounds |
| • Formation of signaling molecules, including neurotransmitters |
| • Immune stimulation |
| • Cytoprotection |
| • Increased resistance of epithelial cells to mutagens or carcinogens |
| • Antimicrobial activity |
| • Inhibition of adhesion of pathogens |
| • Removal of viruses |
| • Regulation of gluconeogenesis and lipogenesis |
| • Involvement in protein metabolism |
| • Involvement in recycling of bile acids, steroids, and other macromolecules |
| • Storage of microbial plasmid and chromosomal genes |
| • Regulation of the gas composition of cavities |
| • Synthesis and supply of B-group vitamins, pantothenic acid, etc. |
The systemic functions of the microbiota are carried out through exchange of LMW metabolites and “signaling molecules” of microbiotic origin (mono- and dicarboxylic acids and their salts, cyclic nucleotides, hydroxy acids, amino acids, amines, etc.) [39, 47]. The microbiota also has the important function of synthesis of vitamins (vitamin B complex, vitamin K) [26, 42] and SCFAs (acetic, propionic, and butyric acids). The level and ratio of SCFAs are key parameters of gut health (microbiome and mucosa) and should be maintained in a given range [12, 53]. Regardless of individual differences in the gut microbiota composition, intestinal microbiocenosis provides the host ecosystem with the required amount and composition of essential metabolites to regulate host metabolism.
IV. Immunoregulation of the “Microbiota-gastrointestinal Tract”
The basic principle of protective mechanisms that control the microbial community in the GIT is the ability to distinguish non-pathogenic (commensal) bacteria from enteropathogens. A growing number of studies suggest that the GIT is part of the human immune network, and refer to it as the mucosal immune system [1, 50, 75]. Three key interrelated components are involved in immune regulation in the GIT: normal flora; gut-associated lymphoid tissue (GALT); and cytokines secreted by immunocompetent and phagocytic cells that serve as mediators of intercellular communication. In Paneth epithelial cells, NOD2 transcription is promoted by 1,25(OH)2D3 and VDR interactions, resulting in expression of DEFB2/HBD2 (β-defensin-2 and cathelicidin). Loss of VDR functions causes changes in the microbiota and lowers host defense by reducing production of cathelicidin, lysozyme, and autophagy-related protein, ATG16L1 [4].
Normal flora and immunoregulation
Maintenance of the relative levels of different groups of microbiota is important for the typical functions of the normal microflora. The microbiota is involved in immune mechanisms; i.e., it contributes to the normal function of the immune system in the GIT through immunomodulating effects. Early microbial colonization of the gut in infants is important in eventual gut-mediated immune responses. Formation of personal immunity takes place in conditions of successful microbial colonization of the GIT, from initial “primary immunodeficiency” up to the development of immunological competence [8].
Gut-associated lymphoid tissue (GALT)
The intestinal lymphoid tissue contains inductive and effector sites with different anatomical and functional properties. An inductive site is characterized by stages of the immune response of antigen presentation and recognition, as well as formation of antigen-specific clones of lymphocytes. An effector site is characterized by synthesis of immunoglobulins via B-cells, manifestation of cellular cytotoxicity by T-killer cells, and production of cytokines by T-lymphocytes, macrophages and NK-cells [30].
Cytokines and regulation of the immune response in the GIT
Cells involved in immune responses to microbial antigens secrete soluble mediator cytokines that regulate innate and acquired immunity. These molecules include interleukins (IL), interferons (IFN), tumor necrosis factors (TNF), chemokines, growth factors, and colony-stimulating factors. Dysregulation of cytokines may cause uncontrolled activation of immune responses, and the biological effect of cytokines takes the form of an immune response of tolerance or immunopathology [74, 79]. In general, immunoregulation mechanisms play a crucial role in shaping the immune system of the GIT and maintaining an optimal balance of the interaction of the microbiota with the multi-functional GIT of the organism (Fig. 3).
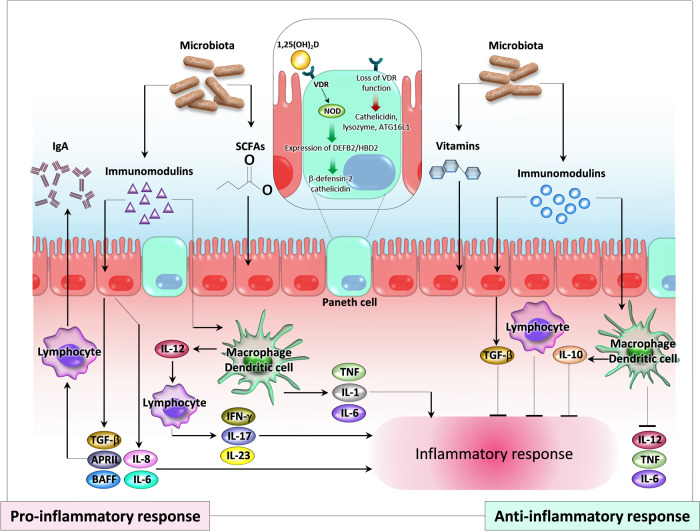
Fig. 3.
Immunomodulation by the gut microbiota and potential participation of vitamin D in the pathogenesis of inflammatory bowel disease (IBD) (adapted and modified from [4, 62]). Members of the gut microbiota and their metabolites modulate mucosal immunity. The gut microbiota may produce bioactive “immunomodulins” and regulatory cytokines that have both pro- and anti-inflammatory functions. Immunostimulation can occur due to release of proinflammatory cytokines from various cells (epithelial, mononuclear, and lymphocytes). Anti-inflammatory responses can occur through production of transforming growth factor (TGF-β) and interleukin 10 (IL-10) from epithelial and mononuclear cells. In Paneth cells, NOD2 transcription is stimulated by vitamin D/VDR and signaling through NOD2 promotes expression of DEFB2/HBD2 (β-defensin 2 and cathelicidin). Loss of VDR function causes changes in the microbiota and reduces host defense by inhibiting production of cathelicidin, lysozyme, and ATG16L1. SCFA, short-chain fatty acids; APRIL, a proliferation-inducing ligand; BAFF, B cell-activating factor; TNF, tumor necrosis factor; VDR, vitamin D receptor; NOD, nucleotide-binding oligomerization domain.
V. Interaction between Vitamin D and the Gut Microbiota
An appropriate balance of vitamins, micronutrients, and minerals, including vitamin D, is important for maintaining good health and controlling disease [2, 9, 17, 18, 63, 65, 66, 81–83]. Dysregulation of vitamin D induces infantile rickets and adult osteomalacia, and inadequate vitamin D has been linked to a higher risk of diabetes, hypertension, cardiovascular diseases, peripheral artery disease, certain cancers, and autoimmune and inflammatory diseases [28, 88]. Vitamin D deficiency is widespread [54], particularly in regions with low sunlight exposure [64].
Vitamin D historically belongs to a group of fat-soluble vitamins, but it is not a true vitamin: it is not biologically active by itself; it is not a cofactor of enzymes, like most other vitamins; it can be synthesized independently in the body, and can exert autocrine, paracrine and endocrine functions; and it exerts a wide range of biological functions through interaction with specific receptors in target tissues. 25(OH)D binds to the vitamin D binding protein in plasma, liver and adipose tissue, and has a biological half-life of about 19 days, whereas 1,25(OH)2D3 has higher affinity for VDR and a much shorter half-life of a few hours. Therefore, 25(OH)D is considered to be a storage form of vitamin D, while 1,25(OH)2D3 is considered to be the active form. The level of 25(OH)D usually reflects the status of vitamin D, and determining the level of 1,25(OH)2D3 may provide a clue to overall calcium homeostasis [85]. Given the diverse functions of vitamin D, an inadequate level may impair normal intestinal homeostasis and barrier functions, since vitamin D through interaction with VDR can influence bacterial colonization, affect tight junction architecture, and exert anti-inflammatory responses.
Vitamin D supplementation modulates the gut microbiota
Significant associations between vitamin D and the gut microbiota have been noted in various studies, as summarized in a systemic review [90]. A shift in microbial composition was observed during vitamin D supplementation in 4 out of 5 studies [5, 10, 33, 73]. Wang et al. [89] presented a comprehensive analysis of genome-wide host-microbiota associations, and showed that variation of the human VDR gene “generates” the gut microbiome. VDR is also involved in immunoregulation of non-gastrointestinal infections, such as chlamydiosis, and reduces the risk of prolonged infection caused by Chlamydia muridarum through regulation of several secretable proteins [24]. The results obtained to date warrant further in-depth studies to determine the underlying mechanisms through which vitamin D status influences the composition of the gut microbiome.
Vitamin D controls expression of antimicrobial peptides (AMPs)
Synthesis of extrarenal 1,25(OH)2D3 occurs in cells in the mucosal lining of the colon and lung, as well as in bone tissue, skin epithelium, and parathyroid glands. Activation of extrarenal expression of 1α-hydroxylase generates 1,25(OH)2D3, which then interacts with VDR to induce production of AMPs. As a result, the healthy commensal gut microbiota may be shaped via inhibition of certain sets of pathogenic bacteria [25, 90]. Krutzik et al. [38] described a vitamin-D-dependent pathway in a TLR2/1-associated intracellular process to initiate synthesis of AMPs in human monocytes, with the finding that the effects of TLR1/2 activation depend on the expression levels of VDR and 1α-hydroxylase. Vitamin D triggers AMP production when Gram-negative bacteria induce TLR4 with stimulation by lipopolysaccharide (LPS) [35].
The bioactive 1,25(OH)2D3 can induce expression of multiple β-defensin genes in cattle [49] and the cathelicidin (LL-37) antimicrobial peptide gene in humans [20]. AMPs can eradicate a wide range of pathogens and are expressed in both immune cells and epithelial cells [43] (Fig. 4). Edfeldt et al. showed that Th1-and Th2-associated cytokines differentially affect production of AMPs [15]. The main cytokines that affect TLR2/1 induction of cathelicidin and DEFB4 are IFN-γ and IL-4. In response to stimulation of IFN-γ, normal human macrophages synthesize 1,25(OH)2D3, and activation of monocytes and macrophages then occurs through activation of VDR.
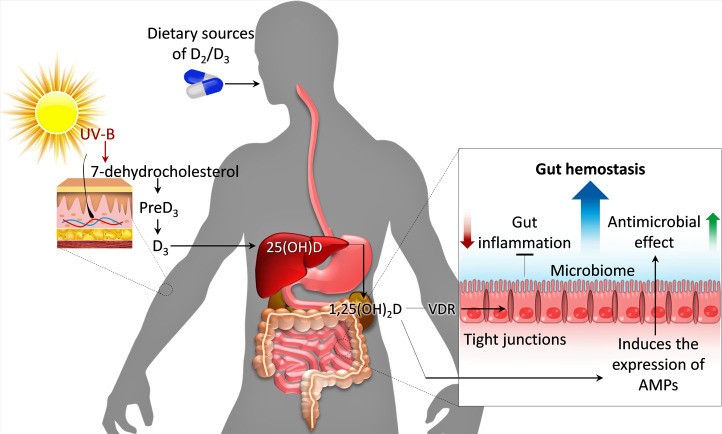
Fig. 4.
The role of vitamin D and the microbiota in gut homeostasis and inflammation (adapted and modified from [78]). The two major pathways to generate vitamin D are: 1) exposing the skin to sunlight, and 2) consuming vitamin D in the diet or as a supplement. Vitamin D and VDR interactions maintain the gut microbiota by regulating expression of antimicrobial peptides (AMPs) and maintaining the barrier functions of the gut mucosa.
Vitamin D/VDR has a protective effect on the epithelial barrier in the gut mucosa
Vitamin D regulates homeostasis of the gut mucosa by maintaining the integrity of the epithelial barrier and through healing of the epithelium [57]. Vitamin D supports the integrity of the epithelial barrier by increasing expression of VDR-associated intracellular junction proteins [occludin, claudin, vinculin, and zonula occludens (ZO-1, ZO-2)] that constitute tight junctions between epithelial cells [99]. Vitamin D inadequacy leads to increased susceptibility of the mucous membrane to damage, and significantly increases the risk of IBD [36]. Numerous studies have shown that patients with IBD often have vitamin D deficiency, even during remission [48, 67], and a reduced level of vitamin D is an external risk factor for exacerbation of IBD [3].
Bacteria in the gut microbiota and their fermentation products regulate VDR expression
As mentioned above, vitamin D and VDR interactions contribute to maintaining gut homeostasis by preventing pathogen invasion, suppressing inflammation and providing cell integrity [36]. In turn, both commensal and pathogenic gut microbiota may regulate VDR expression and location [94]. Probiotic treatment can increase VDR expression and activity in the host, thus inhibiting intestinal inflammation [97]. Yoon et al. observed an increase in VDR expression in human epithelial colonic cells treated with probiotics Lactobacillus rhamnosus strain GG and Lactobacillus plantarum, and also found an increase in intestinal VDR of a single probiotic-treated (mono-associated) pig after probiotic colonization, compared to the ex-germ-free pig in vivo [96]. This association may be mediated through bile acid metabolism, since bile acids (lithocholic acid (LCA), glycine-conjugated LCA, 3-keto-LCA) are transformed by gut commensal microbes and can serve as ligands and regulators of VDR expression [23, 89].
The complex relationships among dysbiosis, innate immune function, and genetic susceptibility through the intestinal epithelial VDR have been described by Wu et al. [95]. Of note, intestinal epithelial VDR regulates autophagy and innate immune functions through the autophagy gene ATG16L1, which could change the microbiota profile. Furthermore, low levels of VDR correlate with a decrease in intestinal ATG16L1 in patients with IBD and in an experimental model of colitis. Introduction of butyrate (a fermented product of the gut microbiota) increases expression of VDR and ATG16L1 and suppresses inflammation in an experimental model of colitis. This evidence raises the possibility of using microbial fermentation products as therapeutic agents to restore VDR-dependent functions in patients with IBD.
Probiotic bacteria increase the serum 25(OH)D level
Low serum 25(OH)D levels have been linked to gut inflammatory diseases [100]. In subjects consuming probiotic Lactobacillus reuteri NCIMB 30242, Jones et al. [31] found significantly increased levels of circulating 25(OH)D, and concluded that oral administration of bile salt hydrolase (BSH)-active L. reuteri NCIMB 30242 may increase serum 25(OH)D through expanding intraluminal lactic acid production or increasing synthesis of 7-dehydrocholesterol (7-DHC), or both.
VI. Conclusion
Vitamin D possesses anti-inflammatory and immune-modulating effects in the GIT. Many of these functions occur through complex ligand-receptor communication between vitamin D and VDR, and have an influence on the human microbiome. Vitamin D also has important functions in innate and adaptive immunity, intestinal barrier integrity, and gut homeostasis. Vitamin D regulates the gut microbiota, since dysregulation of vitamin D alters microbial imbalance (maladaptation) in the GIT. The antibacterial effect of vitamin D is associated with expression of AMPs. The gut microbiota is responsive to exogenous vitamin D, and some fermentation products of the microbiota can induce expression of VDR. A detailed study of the functional, immunological, biochemical and genetic interactions between vitamin D and the human gut microbiota will shed additional light on understanding of complex gut function.
VII. Conflicts of Interest
The authors declare that there are no conflicts of interest.
VIII. Acknowledgments
We are grateful to Ms. Rufsa H. Afroze and Ms. Peace Uwambaye for careful reading of the manuscript and providing useful suggestions. Dr. Razzaque is a Visiting Professor at the Harvard School of Dental Medicine, Boston (USA), and an Honorary Professor at the University of Rwanda College of Medicine and Health Sciences, Kigali (Rwanda).
IX. References
- 1.Ahluwalia B., Magnusson M. K. and Ohman L. (2017) Mucosal immune system of the gastrointestinal tract: maintaining balance between the good and the bad. Scand. J. Gastroenterol. 52; 1185–1193. [DOI] [PubMed] [Google Scholar]
- 2.Akimbekov N. S., Ortoski R. A. and Razzaque M. S. (2020) Effects of sunlight exposure and vitamin D supplementation on HIV patients. J. Steroid Biochem. Mol. Biol. 200; 105664. [DOI] [PubMed] [Google Scholar]
- 3.Ananthakrishnan A. N., Cheng S.-C., Cai T., Cagan A., Gainer V. S., Szolovits P., et al. (2014) Association between reduced plasma 25-hydroxy vitamin D and increased risk of cancer in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 12; 821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ardesia M., Ferlazzo G. and Fries W. (2015) Vitamin D and inflammatory bowel disease. Biomed. Res. Int. 2015; 470805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bashir M., Prietl B., Tauschmann M., Mautner S. I., Kump P. K., Treiber G., et al. (2016) Effects of high doses of vitamin D3 on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. Eur. J. Nutr. 55; 1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bengmark S. (1998) Ecological control of the gastrointestinal tract. The role of probiotic flora. Gut 42; 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilen M., Dufour J.-C., Lagier J.-C., Cadoret F., Daoud Z., Dubourg G., et al. (2018) The contribution of culturomics to the repertoire of isolated human bacterial and archaeal species. Microbiome 6; 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boirivant M., Amendola A. and Butera A. (2008) Intestinal microflora and immunoregulation. Mucosal Immunol. 1 Suppl 1; S47–49. [DOI] [PubMed] [Google Scholar]
- 9.Brown R. B. and Razzaque M. S. (2018) Phosphate toxicity and tumorigenesis. Biochim. Biophys. Acta Rev. Cancer 1869; 303–309. [DOI] [PubMed] [Google Scholar]
- 10.Cantarel B. L., Waubant E., Chehoud C., Kuczynski J., DeSantis T. Z., Warrington J., et al. (2015) Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J. Investig. Med. 63; 729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carding S., Verbeke K., Vipond D. T., Corfe B. M. and Owen L. J. (2015) Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health. Dis. 26; 26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chambers E. S., Preston T., Frost G. and Morrison D. J. (2018) Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 7; 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claus S. P., Guillou H. and Ellero-Simatos S. (2016) The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ. Biofilms. Microbiomes 2; 16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen-Poradosu, R. and Kasper, D. L. (2015) 244—Anaerobic Infections: General Concepts. In “Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases”, ed. by J. E. Bennett, R. Dolin, M. J. Blaser, Content Repository Only!, Philadelphia, pp. 2736–2743.e1. [Google Scholar]
- 15.Edfeldt K., Liu P. T., Chun R., Fabri M., Schenk M., Wheelwright M., et al. (2010) T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc. Natl. Acad. Sci. U S A 107; 22593–22598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egbert M. D., Barandiaran X. E. and Di Paolo E. A. (2010) A minimal model of metabolism-based chemotaxis. PLoS Comput. Biol. 6; e1001004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erem S. and Razzaque M. S. (2018) Dietary phosphate toxicity: an emerging global health concern. Histochem. Cell Biol. 150; 711–719. [DOI] [PubMed] [Google Scholar]
- 18.Erem S., Atfi A. and Razzaque M. S. (2019) Anabolic effects of vitamin D and magnesium in aging bone. J. Steroid Biochem. Mol. Biol. 193; 105400. [DOI] [PubMed] [Google Scholar]
- 19.Galland L. (2014) The gut microbiome and the brain. J. Med. Food 17; 1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo C., Sinnott B., Niu B., Lowry M. B., Fantacone M. L. and Gombart A. F. (2014) Synergistic induction of human cathelicidin antimicrobial peptide gene expression by vitamin D and stilbenoids. Mol. Nutr. Food. Res. 58; 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haque S. Z. and Haque M. (2017) The ecological community of commensal, symbiotic, and pathogenic gastrointestinal microorganisms—an appraisal. Clin. Exp. Gastroenterol. 10; 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harmsen, H. J. M. and de Goffau, M. C. (2016) The Human Gut Microbiota. In “Microbiota of the Human Body: Implications in Health and Disease”, ed. by A. Schwiertz, Springer International Publishing, Cham, pp. 95–108. [Google Scholar]
- 23.Haussler M. R., Haussler C. A., Bartik L., Whitfield G. K., Hsieh J.-C., Slater S., et al. (2008) Vitamin D receptor: molecular signaling and actions of nutritional ligands in disease prevention. Nutr. Rev. 66; S98–112. [DOI] [PubMed] [Google Scholar]
- 24.He Q., Ananaba G. A., Patrickson J., Pitts S., Yi Y., Yan F., et al. (2013) Chlamydial infection in vitamin D receptor knockout mice is more intense and prolonged than in wild-type mice. J. Steroid Biochem. Mol. Biol. 135; 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hewison M. (2011) Antibacterial effects of vitamin D. Nat. Rev. Endocrinol. 7; 337–345. [DOI] [PubMed] [Google Scholar]
- 26.Hill M. J. (1997) Intestinal flora and endogenous vitamin synthesis. Eur. J. Cancer Prev. 6 Suppl 1; S43–45. [DOI] [PubMed] [Google Scholar]
- 27.Hillman E. T., Lu H., Yao T. and Nakatsu C. H. (2017) Microbial Ecology along the Gastrointestinal Tract. Microbes. Environ. 32; 300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holick M. F. (2004) Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 80; 1678S–1688S. [DOI] [PubMed] [Google Scholar]
- 29.Jandhyala S. M., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M. and Nageshwar Reddy D. (2015) Role of the normal gut microbiota. World J. Gastroenterol. 21; 8787–8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jepson, M. A. (2012) Chapter 12—Gastrointestinal Tract. In “Adverse Effects of Engineered Nanomaterials”, ed. by B. Fadeel, A. Pietroiusti and A. A. Shvedova, Academic Press, Boston, pp. 209–224. [Google Scholar]
- 31.Jones M. L., Martoni C. J. and Prakash S. (2013) Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25-hydroxyvitamin D: a post hoc analysis of a randomized controlled trial. J. Clin. Endocrinol. Metab. 98; 2944–2951. [DOI] [PubMed] [Google Scholar]
- 32.Jones R. M., Desai C., Darby Trevor M., Luo L., Wolfarth Alexandra A., Scharer C. D., et al. (2015) Lactobacilli Modulate Epithelial Cytoprotection through the Nrf2 Pathway. Cell Rep. 12; 1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanhere M., He J., Chassaing B., Ziegler T. R., Alvarez J. A., Ivie E. A., et al. (2018) Bolus Weekly Vitamin D3 Supplementation Impacts Gut and Airway Microbiota in Adults With Cystic Fibrosis: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial. J. Clin. Endocrinol. Metab. 103; 564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kataoka K. (2016) The intestinal microbiota and its role in human health and disease. J. Med. Invest. 63; 27–37. [DOI] [PubMed] [Google Scholar]
- 35.Kempker J. A., Han J. E., Tangpricha V., Ziegler T. R. and Martin G. S. (2012) Vitamin D and sepsis: An emerging relationship. Dermatoendocrinol. 4; 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kong J., Zhang Z., Musch M. W., Ning G., Sun J., Hart J., et al. (2008) Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 294; G208–216. [DOI] [PubMed] [Google Scholar]
- 37.Korecka A. and Arulampalam V. (2012) The gut microbiome: scourge, sentinel or spectator? J. Oral Microbiol. 4; doi: 10.3402/jom.v4i0.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krutzik S. R., Hewison M., Liu P. T., Robles J. A., Stenger S., Adams J. S., et al. (2008) IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J. Immunol. 181; 7115–7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lach G., Schellekens H., Dinan T. G. and Cryan J. F. (2018) Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 15; 36–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lagier J.-C., Million M., Hugon P., Armougom F. and Raoult D. (2012) Human gut microbiota: repertoire and variations. Front. Cell. Infect. Microbiol. 2; 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazar V., Ditu L.-M., Pircalabioru G. G., Gheorghe I., Curutiu C., Holban A. M., et al. (2018) Aspects of Gut Microbiota and Immune System Interactions in Infectious Diseases, Immunopathology, and Cancer. Front. Immunol. 9; 1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LeBlanc J. G., Milani C., de Giori G. S., Sesma F., van Sinderen D. and Ventura M. (2013) Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr. Opin. Biotechnol. 24; 160–168. [DOI] [PubMed] [Google Scholar]
- 43.Lehrer R. I. and Ganz T. (2002) Cathelicidins: a family of endogenous antimicrobial peptides. Curr. Opin. Hematol. 9; 18–22. [DOI] [PubMed] [Google Scholar]
- 44.Ley R. E., Peterson D. A. and Gordon J. I. (2006) Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell 124; 837–848. [DOI] [PubMed] [Google Scholar]
- 45.Liang D., Leung R. K.-K., Guan W. and Au W. W. (2018) Involvement of gut microbiome in human health and disease: brief overview, knowledge gaps and research opportunities. Gut Pathog. 10; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maji D. (2012) Vitamin D toxicity. Indian J. Endocrinol. Metab. 16; 295–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manes N. P., Shulzhenko N., Nuccio A. G., Azeem S., Morgun A. and Nita-Lazar A. (2017) Multi-omics Comparative Analysis Reveals Multiple Layers of Host Signaling Pathway Regulation by the Gut Microbiota. mSystems 2; e00107–00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meeker S., Seamons A., Maggio-Price L. and Paik J. (2016) Protective links between vitamin D, inflammatory bowel disease and colon cancer. World J. Gastroenterol. 22; 933–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merriman K. E., Kweh M. F., Powell J. L., Lippolis J. D. and Nelson C. D. (2015) Multiple β-defensin genes are upregulated by the vitamin D pathway in cattle. J. Steroid Biochem. Mol. Biol. 154; 120–129. [DOI] [PubMed] [Google Scholar]
- 50.Million M., Tomas J., Wagner C., Lelouard H., Raoult D. and Gorvel J.-P. (2018) New insights in gut microbiota and mucosal immunity of the small intestine. Human Microbiome J. 7–8; 23–32. [Google Scholar]
- 51.Mischke, M. and Plösch, T. (2016) The Gut Microbiota and their Metabolites: Potential Implications for the Host Epigenome. In “Microbiota of the Human Body: Implications in Health and Disease”, ed. by A. Schwiertz, Springer International Publishing, Cham, pp. 33–44. [DOI] [PubMed] [Google Scholar]
- 52.Mohajeri M. H., Brummer R. J. M., Rastall R. A., Weersma R. K., Harmsen H. J. M., Faas M., et al. (2018) The role of the microbiome for human health: from basic science to clinical applications. Eur. J. Nutr. 57; 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrison D. J. and Preston T. (2016) Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7; 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nair R. and Maseeh A. (2012) Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharmacother. 3; 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nardone G. and Compare D. (2015) The human gastric microbiota: Is it time to rethink the pathogenesis of stomach diseases? United European Gastroenterol. J. 3; 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nava G. M. and Stappenbeck T. S. (2011) Diversity of the autochthonous colonic microbiota. Gut Microbes 2; 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicholson I., Dalzell A. M. and El-Matary W. (2012) Vitamin D as a therapy for colitis: a systematic review. J. Crohns. Colitis. 6; 405–411. [DOI] [PubMed] [Google Scholar]
- 58.Niess J. H. and Reinecker H.-C. (2006) Dendritic cells in the recognition of intestinal microbiota. Cell. Microbiol. 8; 558–564. [DOI] [PubMed] [Google Scholar]
- 59.O’Hara A. M. and Shanahan F. (2006) The gut flora as a forgotten organ. EMBO Rep. 7; 688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pascal M., Perez-Gordo M., Caballero T., Escribese M. M., Lopez Longo M. N., Luengo O., et al. (2018) Microbiome and Allergic Diseases. Front. Immunol. 9; 1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Posserud I., Stotzer P.-O., Björnsson E. S., Abrahamsson H. and Simrén M. (2007) Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut 56; 802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Preidis G. A. and Versalovic J. (2009) Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era. Gastroenterology 136; 2015–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Razzaque M. S. (2018) Magnesium: are we consuming enough? Nutrients 10; 1863. doi: 10.3390/nu10121863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Razzaque M. S. (2018) Sunlight exposure: Do health benefits outweigh harm? J. Steroid Biochem. Mol. Biol. 175; 44–48. [DOI] [PubMed] [Google Scholar]
- 65.Razzaque M. S. (2020) Overconsumption of sugar-sweetened beverages: why is it difficult to control? J. Popul. Ther. Clin. Pharmacol. 27; e62–e68. doi: 10.15586/jptcp.v27i2.678. [DOI] [PubMed] [Google Scholar]
- 66.Razzaque M. S. (2020) Can excessive dietary phosphate intake influence oral diseases? Adv. Hum. Biol. 10; 35–37. doi: 10.4103/AIHB.AIHB_3_20. [Google Scholar]
- 67.Reich K. M., Fedorak R. N., Madsen K. and Kroeker K. I. (2014) Vitamin D improves inflammatory bowel disease outcomes: basic science and clinical review. World J. Gastroenterol. 20; 4934–4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reid G. and Burton J. (2002) Use of Lactobacillus to prevent infection by pathogenic bacteria. Microbes Infect. 4; 319–324. [DOI] [PubMed] [Google Scholar]
- 69.Rowland I., Gibson G., Heinken A., Scott K., Swann J., Thiele I., et al. (2018) Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 57; 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rubio-Tapia A., Barton S. H., Rosenblatt J. E. and Murray J. A. (2009) Prevalence of small intestine bacterial overgrowth diagnosed by quantitative culture of intestinal aspirate in celiac disease. J. Clin. Gastroenterol. 43; 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sadykov R., Digel I., Artmann A., uuml l, T., Porst D., et al. (2009) Oral Lead Exposure Induces Dysbacteriosis in Rats. J. Occup. Health. 51; 64–73. [DOI] [PubMed] [Google Scholar]
- 72.Scarpellini E., Ianiro G., Attili F., Bassanelli C., De Santis A. and Gasbarrini A. (2015) The human gut microbiota and virome: Potential therapeutic implications. Dig. Liver Dis. 47; 1007–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schaffler H., Herlemann D. P., Klinitzke P., Berlin P., Kreikemeyer B., Jaster R., et al. (2018) Vitamin D administration leads to a shift of the intestinal bacterial composition in Crohn’s disease patients, but not in healthy controls. J. Dig. Dis. 19; 225–234. [DOI] [PubMed] [Google Scholar]
- 74.Schirmer M., Smeekens S. P., Vlamakis H., Jaeger M., Oosting M., Franzosa E. A., et al. (2016) Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 167; 1125–1136.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi N., Li N., Duan X. and Niu H. (2017) Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 4; 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shoaie S. and Nielsen J. (2014) Elucidating the interactions between the human gut microbiota and its host through metabolic modeling. Front. Genet. 5; 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stinson L. F., Boyce M. C., Payne M. S. and Keelan J. A. (2019) The Not-so-Sterile Womb: Evidence That the Human Fetus Is Exposed to Bacteria Prior to Birth. Front. Microbiol. 10; 1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun J. (2018) Dietary vitamin D, vitamin D receptor, and microbiome. Curr. Opin. Clin. Nutr. Metab. Care. 21; 471–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun M., He C., Cong Y. and Liu Z. (2015) Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol. 8; 969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thursby E. and Juge N. (2017) Introduction to the human gut microbiota. Biochem. J. 474; 1823–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Uwitonze A. M. and Razzaque M. S. (2018) Role of magnesium in vitamin D activation and function. J. Am. Osteopath. Assoc. 118; 181–189. [DOI] [PubMed] [Google Scholar]
- 82.Uwitonze A. M., Ojeh N., Murererehe J., Atfi A. and Razzaque M. S. (2020) Zinc adequacy is essential for the maintenance of optimal oral health. Nutrients 12; doi: 10.3390/nu12040949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Uwitonze A. M., Rahman S., Ojeh N., Grant W. B., Kaur H., Haq A., et al. (2020) Oral manifestations of magnesium and vitamin D inadequacy. J. Steroid Biochem. Mol. Biol. 200; 105636. [DOI] [PubMed] [Google Scholar]
- 84.Valdes A. M., Walter J., Segal E. and Spector T. D. (2018) Role of the gut microbiota in nutrition and health. BMJ 361; k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Veldurthy V., Wei R., Oz L., Dhawan P., Jeon Y. H. and Christakos S. (2016) Vitamin D, calcium homeostasis and aging. Bone Res. 4; 16041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang B., Yao M., Lv L., Ling Z. and Li L. (2017) The Human Microbiota in Health and Disease. Engineering 3; 71–82. [Google Scholar]
- 87.Wang G., Huang S., Wang Y., Cai S., Yu H., Liu H., et al. (2019) Bridging intestinal immunity and gut microbiota by metabolites. Cell. Mol. Life Sci. 76; 3917–3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang H., Chen W., Li D., Yin X., Zhang X., Olsen N., et al. (2017) Vitamin D and Chronic Diseases. Aging Dis. 8; 346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang J., Thingholm L. B., Skiecevičienė J., Rausch P., Kummen M., Hov J. R., et al. (2016) Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat. Genet. 48; 1396–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Waterhouse M., Hope B., Krause L., Morrison M., Protani M. M., Zakrzewski M., et al. (2019) Vitamin D and the gut microbiome: a systematic review of in vivo studies. Eur. J. Nutr. 58; 2895–2910. [DOI] [PubMed] [Google Scholar]
- 91.Wen L. and Duffy A. (2017) Factors Influencing the Gut Microbiota, Inflammation, and Type 2 Diabetes. J. Nutr. 147; 1468S–1475S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wimalawansa S. J., Razzaque M. S. and Al-Daghri N. M. (2018) Calcium and vitamin D in human health: Hype or real? J. Steroid Biochem. Mol. Biol. 180; 4–14. [DOI] [PubMed] [Google Scholar]
- 93.Wu G. D., Chen J., Hoffmann C., Bittinger K., Chen Y.-Y., Keilbaugh S. A., et al. (2011) Linking long-term dietary patterns with gut microbial enterotypes. Science 334; 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu S., Liao A. P., Xia Y., Li Y. C., Li J.-D., Sartor R. B., et al. (2010) Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am. J. Pathol. 177; 686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu S., Zhang Y.-G., Lu R., Xia Y., Zhou D., Petrof E. O., et al. (2015) Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut 64; 1082–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoon S., Wu S., Zhang Y.-g., Lu R., Petrof E. O., Yuan L., et al. (2011) Probiotic Regulation of Vitamin D Receptor in Intestinal Inflammation. Gastroenterology 140; S-19. [Google Scholar]
- 97.Yoon S. S. and Sun J. (2011) Probiotics, nuclear receptor signaling, and anti-inflammatory pathways. Gastroenterol. Res. Pract. 2011; 971938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang C., Derrien M., Levenez F., Brazeilles R., Ballal S. A., Kim J., et al. (2016) Ecological robustness of the gut microbiota in response to ingestion of transient food-borne microbes. ISME J. 10; 2235–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Y.-G., Wu S. and Sun J. (2013) Vitamin D, Vitamin D Receptor, and Tissue Barriers. Tissue Barriers 1; e23118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao J., Wang Y., Gu Q., Du Z. and Chen W. (2019) The association between serum vitamin D and inflammatory bowel disease. Medicine 98; e15233. [DOI] [PMC free article] [PubMed] [Google Scholar]