Summary
Polarized exocytosis is a fundamental process by which membranes and cargo proteins are delivered to the cell surface with precise spatial control. Although the need for the octameric exocyst complex is conserved from yeast to humans, what imparts spatial control is known only in yeast, i.e., a polarity scaffold called Bem1p. We demonstrate here that the mammalian scaffold protein, GIV/Girdin, fulfills the key criteria and functions of its yeast counterpart Bem1p; both bind Exo70 proteins via similar short-linear interaction motifs, and each prefers its evolutionary counterpart. Selective disruption of the GIV⋅Exo-70 interaction derails the delivery of the metalloprotease MT1-MMP to invadosomes and impairs collagen degradation and haptotaxis through basement membrane matrix. GIV's interacting partners reveal other components of polarized exocytosis in mammals. Findings expose how the exocytic functions aid GIV's pro-metastatic functions and how signal integration via GIV may represent an evolutionary advancement of the exocytic process in mammals.
Subject Areas: Biological Sciences, Cancer, Cell Biology
Graphical Abstract
Highlights
-
⋅
GIV (human) and Bem1p (yeast) bind Exo70 and are required for exocytosis
-
⋅
GIV binds and aids PM localization of Exo70 via a conserved short linear motif
-
⋅
Binding facilitates MT1-MMP delivery to invadosomes, ECM degradation, and invasion
-
⋅
Regulatory control over polarized exocytosis is enriched during evolution
Biological Sciences; Cancer; Cell Biology
Introduction
Exocytosis is an essential process in all eukaryotic cells, defined as the delivery of intracellular contents such as hormones, mRNAs, and proteins between secretory vesicles and the plasma membrane (PM). The spatially controlled fusion of these secretory vesicles with the PM requires SNARE proteins. However, before vesicle fusion with the PM, vesicles must first be anchored to the membrane by the octameric exocyst complex. First identified in S. cerevisiae, using a variety of biochemical and genetic approaches (Terbush et al., 1996, Terbush and Novick, 1995, Novick et al., 1980), the exocyst complex is an evolutionarily conserved macromolecular complex composed of Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84. Evidence suggests that it is this exocyst complex that imparts spatial and temporal control of exocytosis and that such precision control is critical for a myriad of cellular processes, such as morphogenesis, cell cycle progression, primary ciliogenesis, cell migration, and tumor invasion (Wu and Guo, 2015).
When it comes to spatial control, it appears that the process of exocytosis is highly polarized. Yeast genetics, and biochemical (Boyd et al., 2004) and structural studies using cryoelectron microscope and 3D molecular modeling (Mei et al., 2018) in yeast have suggested that the exocyst complex is assembled in a hierarchical manner and that several such complexes may cooperatively ensure the fidelity in recruiting the vesicle to the appropriate exocytic site on the PM (Picco et al., 2017). For example, in the budding yeast, the exocyst is localized to the emerging bud tip, where it mediates exocytosis for the asymmetric expansion of daughter cell surfaces during polarized cell growth. During cytokinesis, the exocyst is localized to the mother-daughter cell junction to mediate abscission (Terbush and Novick, 1995, Finger et al., 1998, Guo et al., 1999, Zhang et al., 2008). Exo70p and Sec3p were identified as the key subunits of the octameric complex, which plays a pivotal role in targeting of the exocyst to specific sites on the PM (Boyd et al., 2004). Such polarized localization of Exo70p and Sec3p cannot be attributed to t-SNARE (target-Soluble NSF-Attachment Protein Receptor) proteins, because the latter distributed along the entire PM (Brennwald et al., 1994). Who or what imparts polarity to the process of exocytosis remained unknown until systematic studies employing Exo70p mutants and yeast genetics pinpointed that a specific stretch of Exo70p (i.e., domain C) must interact with some other actin-independent polarity determinant (Hutagalung et al., 2009), which was subsequently identified as the multidomain polarity scaffold and bud emergence protein, Bem1p (Liu and Novick, 2014). This Exo70p⋅Bem1p interaction was implicated in polarizing the process of vesicle exocytosis in yeast; binding-deficient mutants of Exo70p were incapable of tethering secretory vesicles to cortical sites specified by Bem1p (Liu and Novick, 2014).
Although the fundamentals of exocytosis were discovered first in yeast, its existence and importance in mammals is widely accepted as a driver in multiple disease states in humans (Martin-Urdiroz et al., 2016), and insights into how the process is regulated in mammals remains unknown to date. As in yeast, the mammalian Exo70 has been the most studied subunit; these studies have revealed that although the composition of the canonical octameric exocyst component is essentially invariant (Boehm and Field, 2019), there is, however, a remarkable degree of evolutionary flexibility to allow for specialization through diversity in exocytic mechanism and building more regulatory control along the way. For example, unlike yeast Exo70p, mammalian Exo70 interacts directly with Arp2/3 complex during cell migration and enhances actin cytoskeleton remodeling (Zuo et al., 2006). Mammalian Exo70 is also under the control of growth factors—epidermal growth factor stimulation triggers phosphorylation of Exo70 by ERK1/2 leading to enhanced octameric complex formation, matrix metalloproteinase (MMP) secretion, and cell invasion (Zhao et al., 2013, He et al., 2007, Lu et al., 2013, Hertzog et al., 2012, Liu et al., 2009). In addition, a switch between Exo70 isoforms has been reported in breast cancer that correlates with a switch from epithelial to mesenchymal transition (Lu et al., 2013); the former is engaged in exocytosis, whereas the latter modulates actin dynamics. The fact that Exo70 was the only exocyst subunit found to correctly localize when overexpressed in mammalian epithelial cells (He et al., 2007, Yeaman et al., 2004) suggested that Exo70 might be the critical component that recognizes a polarity determinant at the cell cortex. In the absence of any clear mammalian counterpart of yeast polarity scaffold Bem1p, it has been speculated that other mammalian polarity determinants may fulfill that role to facilitate Exo70 recruitment in mammals. The identity of such Bem1p counterpart in humans remains unknown.
Here we reveal that the multi-modular polarity scaffold Gα-interacting vesicle-associated protein (GIV, a.k.a, Girdin) (Ohara et al., 2012, Sasaki et al., 2015, Aznar et al., 2016b) fulfills the criteria to serve as the mammalian functional homolog of the yeast polarity determinant, Bem1p. The scaffolding function of GIV has been shown to mediate the purposeful dynamic “glue”-ing of diverse signaling pathways: GIV is a guanine nucleotide exchange factor (GEF) for trimeric GTPases, Gi (Garcia-Marcos et al., 2009, Kalogriopoulos et al., 2019) and Gs (Gupta et al., 2016), a polarity scaffold that binds and modulates via G protein intermediates the aPKC/Par complexes (Sasaki et al., 2015, Ohara et al., 2012); it is a substrate of and major signaling conduit for multiple receptor and non-receptor tyrosine kinases (Lin et al., 2011, Mittal et al., 2011, Midde et al., 2018); serves as a signaling adaptor for growth factor (Lin et al., 2014), integrins (Lopez-Sanchez et al., 2015a, Leyme et al., 2015), and other classes of receptors (reviewed in Ghosh et al., 2017); and is an actin remodeler (Enomoto et al., 2005) and a scaffold for polymerized microtubules (Nechipurenko et al., 2016, Aznar et al., 2016b). Most of the work on GIV thus far has revealed its ability to serve as an integrator of multiple signaling pathways that enhances multiple fundamental cellular processes and how its dysregulation (overexpression and hyperactivation) favors cancer invasion and metastasis (reviewed in Aznar et al., 2016a). This work not only sheds light into GIV's ability to enhance a key process that aids metastasis (i.e., polarized exocytosis of matrix metalloproteases) but also reveals how the modular makeup and functional diversity of GIV imparts the process of exocytosis features of polarity and context-dependent regulation by diverse signaling pathways.
Results and Discussion
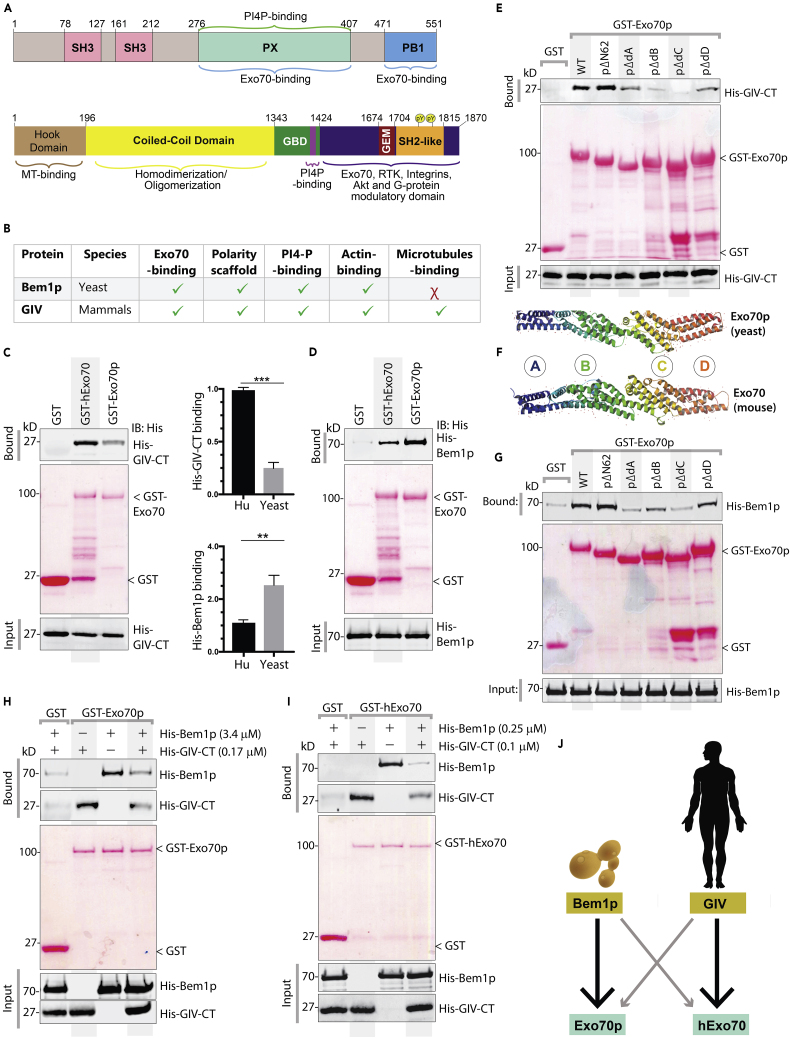
GIV and Bem1p Share Key Properties, Conserved Modes of Binding to Exo70
We first began by looking for polarity scaffold proteins with the mammalian Exo70 interactome (Figure S1) using Biological General Repository for Interaction Datasets (BioGRID; thebiogrid.org). A systematic analysis of all physical interactions of Exo70 revealed that among many coiled coil multi-modular proteins, it binds the mammalian polarity scaffold GIV (encoded by the gene CCDC88A), as revealed initially in a yeast 2-hybrid screen (Camargo et al., 2007) and validated later by us as a protein that colocalizes with (as determined by confocal immunofluorescence and in situ proximity ligation assays) and directly binds Exo70 (Lopez-Sanchez et al., 2015b). We noted that although the modular makeup of GIV and Bem1p had very little in common (Figure 1A), GIV fulfilled some of the key functional criteria that were previously deemed as necessary for Bem1p to polarize exocytosis in yeast (Figure 1B). Furthermore, besides its ability to bind and modulate Par-polarity complexes, GIV presented numerous additional modules, functional features, and interesting interactions (Figures 1A and 1B). We hypothesized that GIV may be the long-sought evolutionary counterpart of Bem1p. Because Exo70 was proposed as the exocyst subunit that interacts with polarity determinants in mammalian cells (He et al., 2007, Yeaman et al., 2004), we began first by comparing the abilities of Bem1p and GIV to bind yeast and human Exo70. We found that both GST-tagged human (hExo70) and yeast (Exo70p) Exo70 could bind full-length endogenous GIV (Figure S2A). Using recombinant His-tagged proteins in similar pull-down assays with GST-Exo70, we confirmed that the GIV⋅Exo70 interaction is direct and that the C terminus of GIV is sufficient (GIV-CT; Figure 1C). Although both Bem1p and GIV-CT could bind both yeast and human Exo70 proteins, each preferentially bound to its evolutionary counterpart (Figures 1C and 1D): GIV-CT binds four times as much to human Exo70 compared with yeast Exo70p (Figure 1C), whereas equimolar amounts of Bem1p binds two times as much to yeast Exo70p compared with human Exo70 (Figure 1D). These protein concentrations were chosen based on the published Kd of Bem1p with Exo70p (Liu and Novick, 2014); concentrations of all human proteins (GIV and Exo70) were adjusted to molar equivalents to yeast counterparts.
Figure 1.
Polarity Scaffolds GIV and Bem1p Share Key Properties and Display Conserved Modes of Binding to Exo70
(A) Domain maps of the yeast (Bem1p [top]) and human (GIV/Girdin [bottom]) polarity scaffolds. Major interactions are annotated.
(B) Table listing key properties of Bem1p that have been implicated in its ability to impart polarity to the process of exocytosis.
(C) Recombinant His-GIV-CT (~3 μg) was used in GST pull-down assays with GST, GST-hExo70 (human), and GST-Exo70p (yeast). Bound GIV was visualized by immunoblotting using anti-His mAb. Equal loading of GST proteins was confirmed by Ponceau S staining. Top right: bar graph displays fold change in binding. Error bars represent standard error of mean; ± SEM; n = 3; ∗∗∗p value = 0.001.
(D) Recombinant His-Bem1p (~3 μg) was used in GST pull-down assays with GST proteins as in (C). Bound Bem1p was visualized by immunoblotting using anti-His mAb. Equal loading of GST proteins was confirmed by Ponceau S staining. Bottom left: Bar graph displays fold change in binding. Error bars represent standard error of mean; ± SEM; n = 3; ∗∗p value = 0.01.
(E–G) Pull-down assays as in (C) were carried out using either recombinant His-GIV-CT (E) or His-Bem1p (G) and various GST constructs (Liu and Novick, 2014) lacking the indicated domains of Exo70 (yeast versus mouse comparison; (F) immobilized on glutathione beads. Bound GIV (E) or Bem1p (G) was visualized by immunoblotting using anti-His mAb. Equal loading of GST proteins was confirmed by Ponceau S staining.
(H–J) Competition between GIV and Bem1p for binding to either Exo70p (yeast; H) or hExo70 (human; I) was assessed by carrying out pull-down assays adding (+) or not (−) the soluble His-GIV-CT and His-Bem1p proteins alone or simultaneously to GST-Exo70 proteins immobilized on glutathione beads. Bound GIV or Bem1p proteins were visualized by immunoblotting using anti-His mAb. (J) Schematic summarizing the cross-species binding of Bem1p and GIV to Exo70. The size and darkness of arrows indicate binding preference.
Prior work showed that although Bem1p forms an extended interface with Exo70p, domain C is the most critical part of Exo70 that is responsible for the interaction (Liu and Novick, 2014). Using a series of previously validated Exo70p mutants, we confirmed that both Bem1p and GIV-CT form an extensive and similar interface with Exo70—although domain C is the major contributor, domains A, B, and D are also relevant (Figures 1E–1G). Despite the overall similarity in domain contributions, combination of multiple single point mutations on the surface of Exo70p that disrupts the Bem1p⋅Exo70 interaction (Liu and Novick, 2014) did not seem to affect binding to GIV-CT (Figure S2B). These findings are in keeping with the fact that mammalian and yeast Exo70 differ in both conformation and surface charge (Figures 1F and S2C) and share low level of sequence conservation, which has been suggested as a basis for species-specific functions of the exocyst (Moore et al., 2007). We also believe that these findings using Exo70p truncations and multi-point mutations are possibly real and not confounded by truncation/mutation-related artifacts (protein misfolding, denaturing, etc.) because the same constructs when used in earlier studies bound Rho3-GTP (Liu and Novick, 2014).
In the absence of conserved amino acids across species, or any other structural clues, generating corresponding mutations in human Exo70 was not attempted. When we carried out competition assays with recombinant proteins to determine if the Bem1p⋅Exo70p and GIV⋅Exo70 interactions are mutually exclusive or inclusive, we found that both GIV and Bem1p could compete for yeast (Figure 1H) and human (Figure 1I) Exo70. These findings suggest that Bem1p and GIV may share the mechanism of binding and that their binding interfaces with Exo70 are either significantly overlapping or non-overlapping but pose significant steric hindrance. Regardless, these findings demonstrate that despite divergent modular makeup, both polarity scaffolds Bem1p (yeast) and GIV (mammals) can bind Exo70 via mechanisms that are shared (i.e., use of Domains C of Exo70 as major contributor). They both may use either same or distinct interfaces for binding Exo70, but nonetheless bind in a mutually exclusive manner. Although each can bind both yeast and human Exo70 proteins, they demonstrate species preference (Figure 1J).
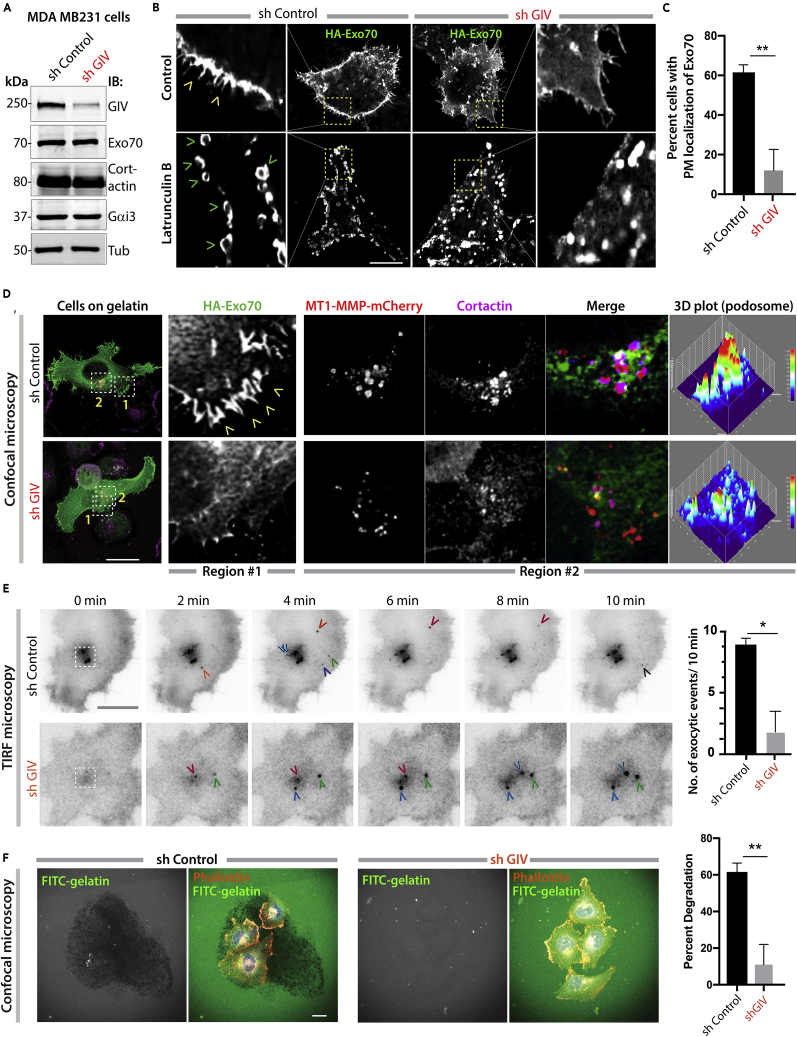
GIV Is Required for Exo70 Localization, Polarized Exocytosis
Because GIV's role in tumor cell invasion and its ability to promote cancer metastasis has been demonstrated in vitro (Leyme et al., 2015, Rahman-Zaman et al., 2018, Jiang et al., 2008) and in vivo (Jiang et al., 2008) using MDA MB231 human breast cancer cells, we used the same cells and asked if GIV is required for the polarized delivery of cargo proteins, e.g., matrix metalloproteases that aid in tumor dissemination by degrading extracellular matrix (ECM). We first confirmed that depletion of GIV (∼85% depletion by small hairpin RNA [shRNA]; Figure 2B) does not change the levels of Exo70 or cortactin; the latter is a marker of invadosomes, which are subcellular actin-rich landmarks that are specialized for matrix degradation because of polarized exocytosis of proteinases (Castro-Castro et al., 2016, Hoshino et al., 2013). We found that hemagglutinin (HA)-tagged Exo70 localized to the PM in control but not GIV-depleted cells (Figures 2B and S3A, top panel). In control cells, disruption of actin cytoskeleton with latrunculin B, a chemical that has been widely used for its ability to sequester G-actin and prevent F-actin assembly (Morton et al., 2000), prevented fusion of Exo70-positive vesicles but did not impact their docking at specific sites on the PM; in GIV-depleted cells the vesicles were in disarray (Figures 2B and S3A, bottom panel; Figure 2C). These findings indicate that GIV is required for the localization of Exo70 to the PM and that such localization is actin independent.
Figure 2.
GIV Is Required for Exo70 Localization and Polarized Exocytosis
(A) Immunoblots of lysates of control (sh Control) and GIV-depleted (sh GIV) MDA MB231 cells.
(B) Control (sh Control) and GIV-depleted (sh GIV) MDA MB231 cells expressing HA-Exo70 were treated (bottom) or not (top) with 25 μM latrunculin B for 24 h before fixation with PFA. Fixed cells were stained for HA and analyzed by confocal microscopy. Representative deconvolved images are shown. Scale bar, 10 μm. Arrowheads, PM localization.
(C) Bar graphs show the % cells with PM localization of Exo70-HA. Error bars represent standard error of mean; ± SEM; n = ∼ 30 cells/assay, 3 independent assays. ∗∗p value <0.01.
(D) Cells in (B) exogenously expressing HA-Exo70 (green) and MT1-MMP-mCherry (red) were plated on gelatin-coated coverslips and fixed and stained with the invadosome marker cortactin (magenta). Representative deconvolved images are shown. Boxed area with grey interrupted line represents cell's center, likely invadosomes. Colored arrowheads track single exocytic events. Scale bar, 10 μm. Insets were analyzed by rendering 3D surface plots using ImageJ. Cortactin/MT1-MMP colocalization was determined by Pearson's correlation coefficient reaching a value of 0.71 ± 0.05 and 0.15 ± 0.04 in control and GIV-depleted cells, respectively, from four different experiments.
(E) Cells in (B) exogenously expressing MT1-MMP-pHLuorin were plated on a layer of unlabeled gelatin and imaged by TIRF microscopy. Left: Still frames from a 10-min-long movie (Videos S1 and S2). Each colored arrowhead marks one successfully exocytosed vesicle. Scale bar, 2.5 μm. Right: Bar graph shows the number of exocytic events that were encountered during the 10-min-long movie in (D). N = ∼ 5–10 cells/assay. Error bars represent standard error of mean; ± SEM. ∗p value <0.05.
(F) Cells in (B) were plated for 5 h on fluorescein isothiocyanate (FITC)-conjugated cross-linked gelatin (green) and then fixed and stained for F-actin (Phalloidin; red). (Left) Representative images are shown. Scale bar, 10 μm. (Right) Bar graphs display the % of cells that showed degradation of gelatin. N = ∼100–200 cells/experiment × 3. Error bars represent standard error of mean; ± SEM. ∗∗p value = 0.001.
Cells transiently expressing MT1-MMP-pHluorin show real-time exocytic events. Images were acquired over 10 min. The movie was acquired at exposure time of 100 ms and with 1 pixel corresponding to 160 nm
GIV-depleted cells transiently expressing MT1-MMP-pHluorin shows real-time exocytic events. Images were acquired over 10 min. The movie was acquired at exposure time of 100 ms and with 1 pixel corresponding to 160 nm
Next, to analyze how Exo70 localization may impact polarized exocytosis of proteases to invadosomes, we co-transfected the MDA MB231 cells with HA-Exo70 and a previously validated MT1-MMP-mCherry construct (Steffen et al., 2008), plated them on gelatin, and looked for the co-localization of MT1-MMP, Exo70, and cortactin. Again, HA-Exo70 localized to the PM in the presence of GIV, but significantly less in its absence (Figure 2D; Region #1). Colocalization between MT1-MMP-mCherry and cortactin was easily detected in control cells, but significantly reduced in cells without GIV (Figures 2D and S3B; Region #2 and 3D plot of invadosome). These findings indicate that GIV is required for efficient localization of proteases near invadosomes. We also noted that despite similar levels of expression of cortactin in the GIV-depleted cells (Figure 2A), the localization of cortactin at the sub-nuclear plane at the center of these cells was consistently impaired (Figure 2D). To assess the dynamics of polarized exocytosis in real time, we used a pH-sensitive MT1-MMP-pHluorin construct (Monteiro et al., 2013), which is fluorescent only at an extracellular pH of 7.4. Prior studies using this construct and total internal reflection fluorescence (TIRF) live-cell microscopy have demonstrated that MT1-MMP-pHluorin demonstrates dot-like localization at the substrate-attached cell side, reminiscent of invadosomes (El Azzouzi et al., 2016, Planchon et al., 2018). When we carried out TIRF microscopy on control and GIV-depleted MDA MB231 cells, we found that the number of exocytic events were significantly lower in the latter (Figure 2E; Videos S1 and S2), and that they were not clustered near the center of the cell (Figure 2E; interrupted white square). Consistent with these findings, compared with controls cells fewer % of GIV-depleted cells demonstrated gelatin degradation (Figure 2F). Together, these findings suggest that GIV is required for the localization of Exo70 to specific sites at the PM, and for the polarized exocytosis of MT1-MMP at the invadosomes. Because the polarized exocytosis of MT1-MMP and the formation, maturation, and functions of the invadosome (which is marked by cortactin) are known to be intertwined (Juin et al., 2012, Monteiro et al., 2013, Castro-Castro et al., 2016, Castagnino et al., 2018, Artym et al., 2006), our findings also suggest that GIV-dependent exocytosis of the protease may impact the dynamics of invadosomes and efficient degradation of ECM.
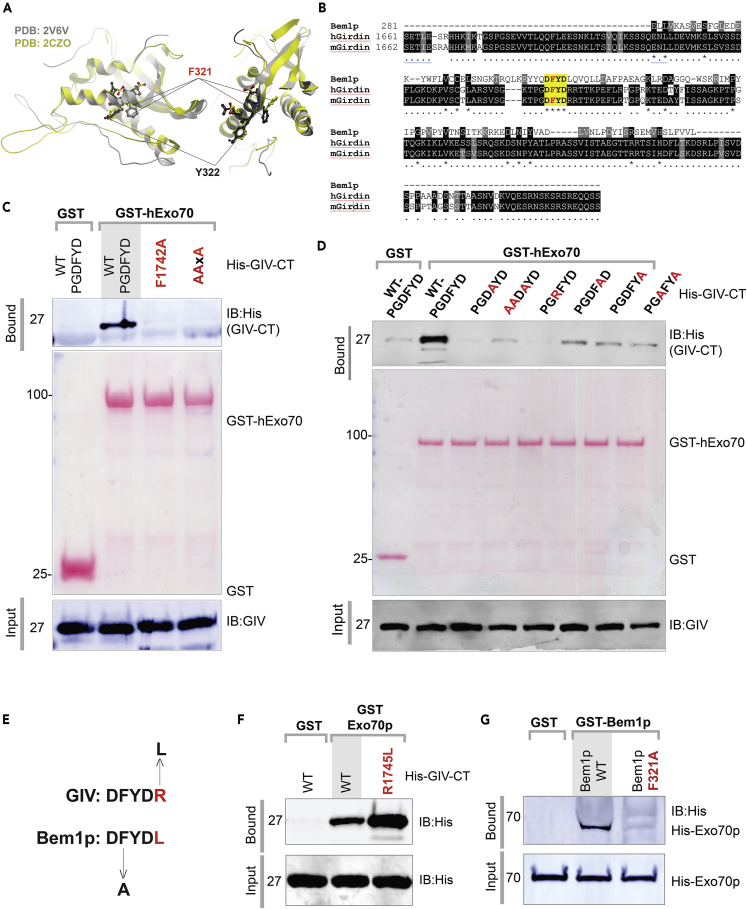
A Short Linear Motif on C Terminus Mediates GIV's Interaction with Exo70
To pinpoint the role of GIV in polarized exocytosis, we set out to identify mutant(s) of GIV that may not bind Exo70. Previously, using domain mapping studies the Exo70p-binding domain of Bem1p was narrowed down to amino acids 309–510, which includes three-quarters of the PX domain, half of the PB1 domain, and the region connecting them (see Figure 1A) as both necessary and sufficient (Liu and Novick, 2014). Because Bem1p and GIV appeared to share the mechanism of binding to Exo70, via what appears to be extensive binding interfaces (Figures 1H and 1I), we hypothesized that the interactions could be sensitive to some key residues. When Bem1p and GIV aligned, we noticed that they have very little similarity in sequences, except for a short stretch of sequence within the PX domain of Bem1p, 320DFYD323 (Figures 3A and 3B). When we superimposed two previously solved structures of Bem1p, we noted that the phenylalanine (F) within this sequence exhibits flexibility (Figure 3A). We targeted this putative short linear interaction motif (SLIM, 1741DFYD1744) in GIV either by selectively replacing the Phe > Ala (F1742A) or by replacing the motif with Ala at three of the four positions (AAxA). Both mutants did not bind GST-Exo70 (Figure 3C). Next we carried out a series of mutations within and flanking the SLIM and noted that several of them impaired binding of GIV to Exo70 (Figure 3D), demonstrating that regardless of potentially extensive contact sites, the GIV⋅Exo70 interaction is sensitive to point-directed mutagenesis.
Figure 3.
A Short Linear Motif on C Terminus Mediates GIV's Interaction with Exo70
(A) Two views of the two solved crystal structures of PX domain of Bem1p (PDB:2V6V, Stahelin et al., 2007; PDB:2CZO, unpublished), showing the position and orientation of key residues Phe (F)321 and Tyr (Y)322 on Bem1p that are within the previously identified stretch of amino acids 309–510 that is both necessary and sufficient for binding to Exo70p.
(B) Sequence alignment showing a short linear “DFYD” motif that is conserved between GIV and Bem1p.
(C and D) Recombinant WT and mutant His-GIV-CT (∼3 μg) proteins were used in GST pull-down assays with GST or GST-hExo70 (human). Two mutants in panel C are used throughout the remaining study to investigate the role of the GIV-Exo70 interaction. Other mutants in panel D were used to characterize the Exo-70-binding motif on GIV. Bound GIV was visualized by immunoblotting (IB) using anti-His monoclonal antibody (mAb). Equal loading of GST proteins was confirmed by Ponceau S staining.
(E) Sequence-based prediction of mutations in GIV (top; R1742»L) and Bem1p (bottom; F312»A) that should enhance and reduce binding to GST-Exo70p (yeast), respectively.
(F) Recombinant His-GIV-CT WT or R1742»L mutant was used in GST pull-down assays with GST or GST-hExo70 (human). Bound GIV was visualized by immunoblotting using anti-His mAb.
(G) Recombinant His-Exo70p was used in GST pull-down assays with GST or GST-Bem1p WT or F312»A mutant. Bound Exo70p was visualized by immunoblotting using anti-His mAb.
The sequence alignment also predicted mutations that would improve GIV's ability to bind yeast Exo70p (i.e., R1745L) or those that might impair Bem1p′s ability to bind Exo70p (F321A) (Figure 3E). We found both predictions to be true—a mutant GIV (R1745L) bound Exo70p stronger than GIV-wild-type (WT) (Figures 3F and S4A). Similarly, the F321A mutant Bem1p did not bind Exo70p (Figure 3G). These findings provide further evidence that although the interactions between GIV or Bem1p is complex and may occur via an extensive interface, and that the mechanisms of these interactions in yeast and mammals are divergent for the most part, the SLIM we identified in this work may represent an evolutionary conserved mechanism (Figure S4B). This Exo70-binding SLIM, localized within the C terminus of GIV, adds to the gathering catalog of SLIMs used by GIV to scaffold diverse signaling components (Figure S4C).
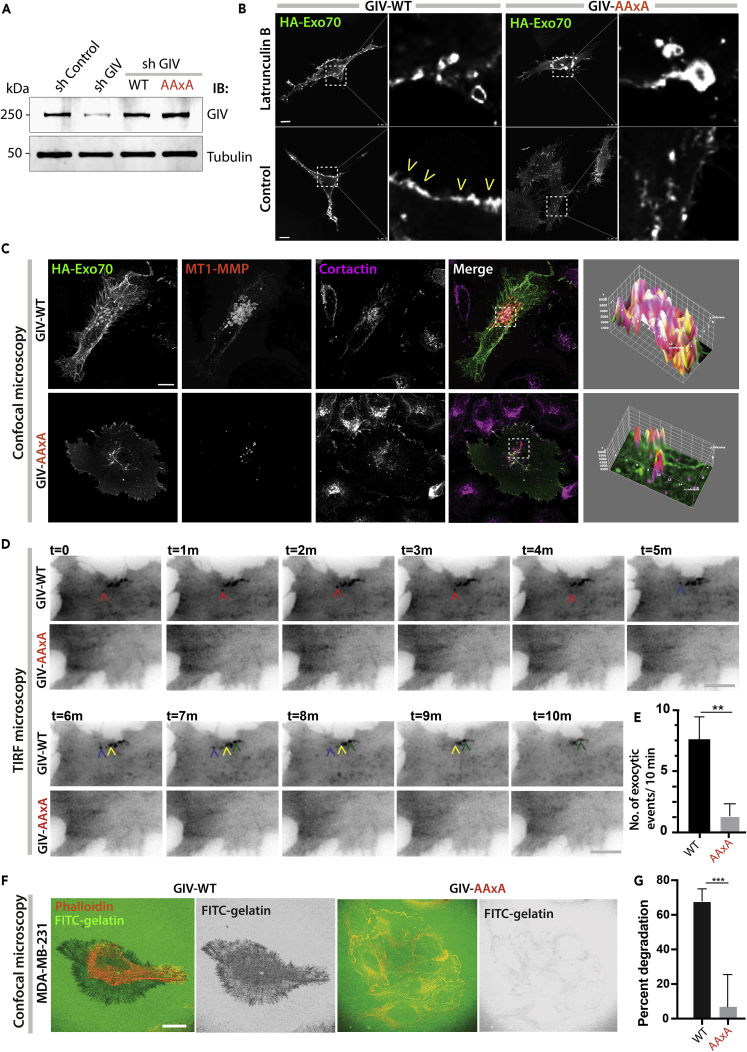
Exo70 Binding-Deficient GIV Mutant Fails to Deliver MT1-MMP to Invadosomes
We next asked if the phenotypes we observed previously (Figure 2) were because of GIV's ability to bind Exo70. To this end, we generated MDA MB231 cell lines in which endogenous GIV was depleted by shRNA before being restored with full-length GIV either WT or the Exo70 binding-deficient GIV mutant AAxA (Figure 4A) and used them in similar assays as before. We found that compared with cells expressing GIV-WT, those expressing the binding-deficient AAxA GIV mutant showed impaired localization of HA-Exo70 at the PM (Figure 4B); colocalization of both Exo70 and MT1-MMP-mCherry to cortactin-positive invadosomes (Figure 4C); rate of exocytic events, as determined by TIRF microscopy on cells expressing MT1-MMP-pHluorin (Figures 4D and 4E; Videos S3, S4, and S5); and gelatin degradation (Figures 4F and 4G). These results were also validated using another Exo70-binding deficient mutant, F1742A (Figures S5A–S5C and S6). Key findings were also reproduced in HeLa cells (Figures S7A–S7C), which have been widely used in the past decade as the cellular model system of choice to study GIV's functions. These findings indicate that the GIV⋅Exo70 interaction may be critical for the localization of Exo70 to vesicle tethering sites at the PM and for the polarized exocytosis of MT1-MMP proteases at or near the invadosomes of cells.
Figure 4.
Exo70-Binding-Deficient Mutant GIV Fails to Deliver MT1-MMP to Invadosomes
(A) Immunoblots of lysates of control (sh Control) and GIV-depleted (sh GIV) MDA MB231 cells stably expressing GIV-FLAG WT and exo70-binding deficient AAxA mutant constructs. See also Figure S5A.
(B) GIV-depleted (sh GIV) MDA-MB-231 cells stably expressing GIV-WT or GIV-AAxA mutant were transfected with HA-Exo70 and were either treated (top) or not (bottom) with 25 μM latrunculin B for 24 h before fixation with PFA. Fixed cells were stained for HA and analyzed by confocal microscopy. Representative deconvolved images are shown. Arrowheads, PM localization. Unusually large membrane vesicles (see inset) were noted in mutant cells, but PM localization was impaired. Scale bar, 10 μm.
(C) Cells in (B) transfected with HA-Exo70 (green) and MT1-MMP-mCherry (red) were plated on gelatin-coated coverslips, fixed, and stained with the invadosome marker cortactin (magenta). Representative deconvolved images are shown. Insets were analyzed by rendering 3D surface plots using ImageJ. See also Figure S5B for F1742A mutant GIV. Cortactin/MT1-MMP colocalization was determined by Pearson's correlation coefficient reaching a value of 0.87 ± 0.07 and 0.35 ± 0.02 in GIV-WT and GIV-AAxA cells, respectively, from three different experiments. Scale bar, 10 μm.
(D) Cells in (B) were transfected with MT1-MMP-pHLuorin and were plated on a layer of unlabeled gelatin and imaged by TIRF microscopy. Each colored arrowhead tracks one vesicle. Still frames from a 10-min-long movie (Videos S3 and S4). Scale bar, 2.5 μm. See also Figure S5C for F1742A mutant GIV.
(E) Bar graph shows the number of exocytic events that were encountered during the 10-min-long movie in (D). N = ~ 5–10 cells/assay. Error bars represent standard error of mean; ± SEM. ∗∗p value = 0.01.
(F) Cells in (B) were plated for 5 h on FITC-conjugated cross-linked gelatin (green) and then fixed and stained for F-actin (Phalloidin; red). Representative images are shown. Scale bar, 10 μm. See also Figure S6 for F1742A mutant GIV.
(G) Bar graphs display the % of cells that showed degradation of gelatin. N = ~100–200 cells/experiment × 3. Error bars represent standard error of mean; ± SEM. ∗∗∗p values = 0.001.
GIV-depleted cells rescued with GIV-WT (stably) and transiently expressing MT1-MMP-pHluorin shows real-time exocytic events. Images were acquired over 10 min. The movie was acquired
at exposure time of 100 ms and with 1 pixel corresponding to 160 nm
GIV-depleted cells rescued with GIV-mutant and transiently expressing MT1-MMP-pHluorin show real-time exocytic events. Images were acquired over 10 min. The movie was acquired at exposure time of 100 ms and with 1 pixel corresponding to 160 nm
GIV-depleted cells rescued with GIV-mutant and transiently expressing MT1-MMP-pHluorin show real-time exocytic events. Images were acquired over 10 min. The movie was acquired at exposure time of 100 ms and with 1 pixel corresponding to 160 nm.
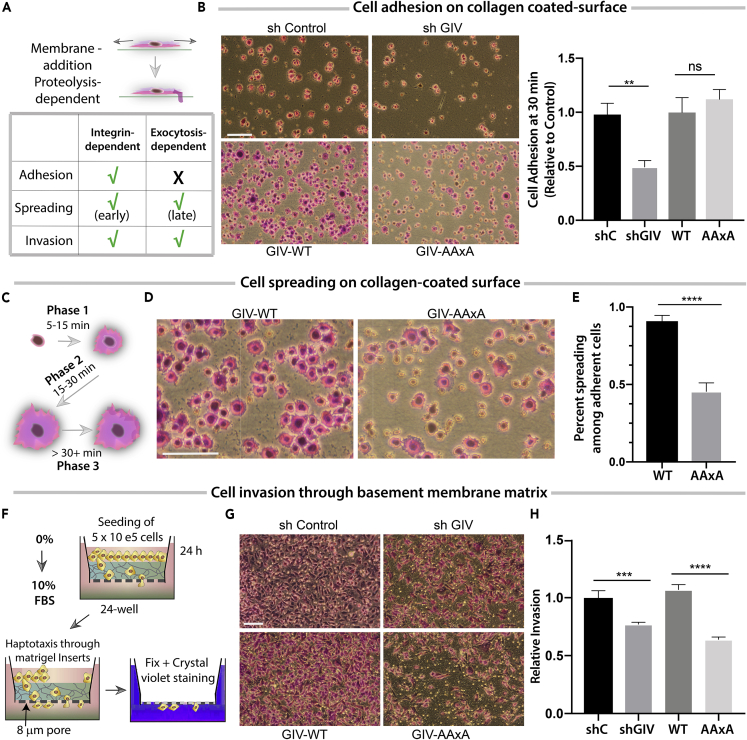
Cells Expressing the Exo70-Binding-Deficient Mutant GIV Fails to Spread, Degrade Matrix, and Invade
Next we asked how the GIV⋅Exo70 interaction impacts cellular phenotypes that have been previously attributed to exocytosis of either membrane or proteases, e.g., cell spreading and invasion through the basement membrane ECM, but not impact adhesion; the latter is primarily mediated by integrins and is independent of exocytosis (Figure 5A). We hypothesized that cells expressing a mutant GIV that cannot bind Exo70 may adhere normally but may not spread or invade efficiently through the ECM. Cell adhesion assays confirmed that depletion of GIV impairs adhesion on collagen-coated surface (Figure 5B), which is in keeping with prior work by others and us showing that GIV modulates integrin signaling (Leyme et al., 2015, Leyme et al., 2016, Lopez-Sanchez et al., 2015a). Cells expressing either GIV-WT or the binding-deficient GIV-AAxA mutant adhered to the collagen to similar extents as control cells (Figure 5B), indicating that the cell adhesion occurs independent of the GIV⋅Exo70 interaction. As for cell spreading, we analyzed spreading at or beyond ∼30 min, a phase of spreading that requires membrane exocytosis and is proteolysis dependent (Figure 5C). We found that although both GIV-WT and GIV-AAxA cells adhered equally well to collagen-coated surfaces (Figure 5B), the latter showed significant impairment in cell spreading (Figures 5D and 5E), indicating that cell spreading requires an intact GIV⋅Exo70 interaction. Haptotaxis assays carried out across a 0% to 10% serum gradient (Figure 5F) showed that depletion of GIV impairs cell invasion; however, such impairment is rescued by GIV-WT, but not the Exo70 binding-deficient GIV-AAxA mutant (Figures 5G and 5H). These findings demonstrate that the GIV⋅Exo70 interaction is required for key phenotypes of cancer cells that require efficient polarized exocytosis of proteases that degrade the ECM and aid in tumor dissemination.
Figure 5.
Cells Expressing Exo70-Binding-Deficient Mutant GIV Adhere, but Fail to Spread, Degrade Matrix, and Invade
(A) Schematic summarizing how polarized exocytosis of membrane and cargo (in this case MT1-MMP) impacts cell adhesion, spreading, and invasion. Check, dependent; cross, independent.
(B) MDA-MB 231 cell lines were analyzed for cell adhesion by growing on poly-D-lysine-coated surface, resuspending, and then plating on 12-well collagen-coated plates for 30 min before fixing with 4% PFA and staining with crystal violet. Cells were visualized and imaged by light microscopy. (Left) Representative images are shown. Scale bar, 250 μm. (Right) Bar graphs display the relative numbers of adherent cells per field, as determined using ImageJ. Error bars represent standard error of mean; ± SEM (n = 4); ∗∗p value < 0.01; ns, not significant.
(C) Schematic summarizing the phases of cell spreading during adhesion.
(D) Adherent MDA-MB 231 cells in (B) were further analyzed for attachment-induced cell spreading at higher magnification. Representative images are shown. Scale bar, 250 μm.
(E) Bar graphs display the quantification of % spreading in (D). Error bars represent standard error of mean; ± SEM (n = 4); ∗∗∗∗p value < 0.0001.
(F) Schematic diagram showing the serum gradient-induced haptotaxis assay conditions used in (G and H).
(G) MDA-MB 231 cell lines in (A) were analyzed for the ability to invade through Matrigel-coated transwells. The number of cells that successfully invaded within 24 h was imaged. Representative images are shown. Scale bar, 250 μm.
(H) Bar graphs display the number of invading cells in (G), as determined using ImageJ. Error bars represent standard error of mean; ± SEM (n = 4); ∗∗∗p value < 0.001, ∗∗∗∗p value < 0.0001.
Components of Polarized Exocytosis Synergize during Tumor Metastasis
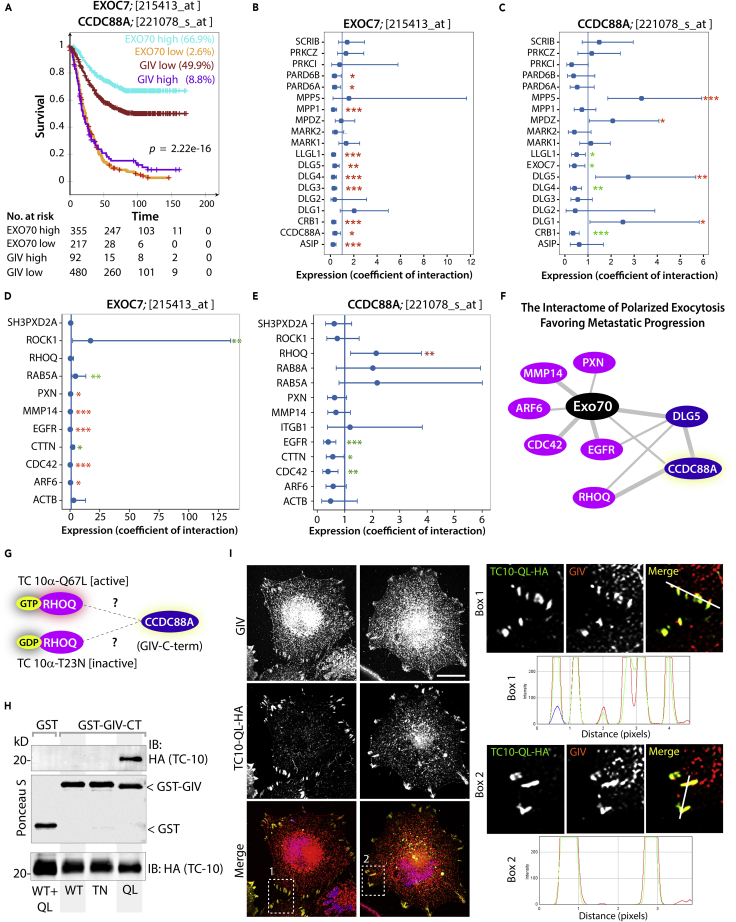
We next asked if the interaction between GIV and Exo70 we report here and the impact of such interaction on the invasive properties of tumor cells can be meaningful when assessing tumor behavior and/or prognosticating clinical outcome. To discern if the levels of expression of Exo70 or GIV alone could impact one of the most important readouts of cancer aggressiveness, i.e., metastasis-free patient survival, we chose to study a pooled cohort of patients (GSE2034, GSE2603 and GSE12276; Minn et al., 2005, Bos et al., 2009, Wang et al., 2005) with breast cancers. These cohorts are unique because the patients did not receive adjuvant chemotherapy, and hence, metastatic progression in them reflects natural disease progression and not resistance/selection under treatment. Samples were divided into “low” and “high” subgroups with regard to Exo70 (EXOC7) and GIV (CCDC88A) gene expression levels using the StepMiner algorithm (Sahoo et al., 2007), implemented within the hierarchical exploration of gene expression microarrays online (HEGEMON) software (Dalerba et al., 2011, Volkmer et al., 2012). Kaplan-Meier analyses of the disease-free survival showed that Exo70 and GIV had opposing impact on outcome (Figure 6A)—high levels of GIV expression carried a grave prognosis, i.e., a shorter metastasis-free survival, which is consistent with numerous prior studies (reviewed in Ghosh, 2015). By contrast, high expression of Exo70 carried a better prognosis, i.e., a longer metastasis-free survival (Figure 6A); this is also consistent with prior findings that an epithelial isoform of Exo70, which is the isoform that is primarily responsible for exocytic secretions, inhibits tumor metastasis in mice (Lu et al., 2013).
Figure 6.
Polarity Scaffold GIV (CCDC88A), Matrix Metalloprotease MT1-MMP, and Exocyst Complexes Synergize during Metastasis
(A) An overlay of Kaplan-Meier curves for metastasis-free survival over time among 572 patients (3 independent cohorts, pooled, Minn et al., 2005, Bos et al., 2009, Wang et al., 2005) who did not receive adjuvant therapy segregated into high versus low levels of exo70 (EXOC7) or GIV (CCDC88A) in primary tumors.
(B and C) Statistical interaction (synergy between variables) is measured in the Cox proportional hazards regression model for EXOC7 (exo70) (B), CCDC88A (GIV) (C), and all mammalian polarity scaffold proteins, including CCDC88A (GIV). Coefficient of the interaction term in Cox regression models is plotted with 95% confidence interval that demonstrates the significance of the statistical interaction. ∗p = 0.05, ∗∗p = 0.01. Red and green asterisks denote shorter or longer metastasis-free survival, respectively.
(D and E) Statistical interaction (synergy between variables) is measured in the Cox proportional hazards regression model as in (B and C) for EXOC7 (exo70) (D), CCDC88A (GIV) (E), and mammalian proteins known to be involved in or influence the exocytosis of MT1-MMP. Red and green asterisks denote shorter and longer metastasis-free survival, respectively.
(F) Schematic summarizing only those interactions with EXOC7 that reduced metastasis-free survival. Purple, genes that encode polarity proteins; pink, others. Network-based prediction of RHOQ (TC10) as the RHO GTPase counterpart in mammals that synergistically interacts with CCDC88A (GIV).
(G and H) (G) Schematic summarizing the constructs used in (H) to assess nucleotide state-dependent interaction between TC10 and GIV. GST pull-down assays were carried out using lysates of COS7 cells as source of TC10-HA WT or constitutively active (QL) or inactive (TN) mutants with GST or GST-GIV-CT. Bound proteins were assessed with anti-HA mAb.
(I) MDA MB231 cells exogenously expressing TC10-WT-QL (green) were fixed and stained for endogenous GIV (red). Representative images of transfected cells are shown. Scale bar, 10 μm. Red and green panels are shown in gray scale. Insets (Box1, 2) were analyzed for colocalization by line scans to generate RGB plots using ImageJ. TC-10/GIV colocalization was determined by Pearson's correlation coefficient reaching a value of 0.86 ± 0.06 from three different cultures.
We next sought to determine if the levels of expression of either GIV or Exo70 and other genes that encode major polarity scaffolds (Lin et al., 2015) interact synergistically as independent variables to impact survival of patients. To this end, we carried out a Cox proportional hazards model (Cox, 1972), which is a regression model that is commonly used as a statistical method for investigating the effect of several variables upon the time it takes for a specified event to happen, in this case, metastasis. We found that besides GIV/CCDC88A, Exo70 significantly and negatively interacts with multiple members of the Par, Scribble, and Crumbs family of polarity scaffolds (Figure 6B), in that, high levels of expression of each of these scaffolds in the setting of high Exo70 expression was sufficient in shortening metastasis-free survival. Of all those polarity scaffolds that interacted with Exo70, only DLG5 continued to show positive synergistic interactions with CCDC88A (GIV) (Figure 6C), in that, high levels of expression of DLG5 in the setting of high GIV expression further shortened metastasis-free survival. Next we asked how Exo70 or GIV interacts with other components of the exocytic machinery, i.e., core trafficking components (i.e., monomeric GTPases Rabs, Arf6), focal adhesions cytoskeleton and Rho GTPases (Paxillin, PXN; Actin, ACTB; TC10, RHOQ; Rho-associated kinase, ROCK1), cargo (MT1-MMP, MMP14), and receptors (ITGB1 and EGFR). We found that whereas many components may negatively interact with Exo70 (Figure 6D), only TC10 GTPase (RHOQ) positively and synergistically interacts with GIV to shorten metastasis-free survival (Figure 6E).
Based on all gene pairs tested, we computed the interactome for Exo70 that shortens metastasis-free survival (Figure 6F). The interactome enabled two major predictions. (1) Polarized exocytosis during metastasis may require Exo70 to synergize with both GIV and DLG5. Because GIV binds DLG5 and their interaction regulates the formation of invadopodia (Ke et al., 2017), and DLG5 in turn engages with tSNARE syntaxin-4 (Nechiporuk et al., 2007) to enable the exocytosis (Wang et al., 2014), it is possible that DLG5 also impacts polarized exocytosis. (2) The interactome predicted that TC10, a close relative of Cdc42 is a likely candidate Rho-superfamily GTPase that may cooperate with GIV during the exocytosis of MT1-MMP.
We asked if GIV's C terminus interacts with TC10 and if such interaction is regulated by its nucleotide-bound (activity) state. Using previously validated TC10 constructs (Chiang et al., 2002) that mimic active (QL) or inactive (TN) conformations (Figure 6G) in pull-down assays with GST-GIV-CT we first confirmed that GIV exclusively binds active, but not the inactive, GTPase (Figure 6H). To translate the relevance of these in vitro findings to cells, we tested the impact of TC10 on the localization of endogenous full-length GIV, as determined by confocal immunofluorescence on fixed cells. Transfection of active QL mutant, but not WT TC10, was sufficient to enhance the localization of GIV to focal adhesion and/or invadosome-like peripherally located structures (Figures 6I and S8), where they colocalized (Figure 6I, RGB plots). These findings are in keeping with the fact that mammalian Exo70 has been shown to directly interact with active TC10 (Inoue et al., 2003) and that activation of TC10 has been linked with the formation and functions of the invadosome. Together, our findings suggest that TC10 may be the evolutionary counterpart that binds GIV, and in doing so, may fulfill the role that cdc42p has been shown to perform alongside the Exo70p⋅Bem1p complex in yeast (Liu and Novick, 2014).
Conclusions
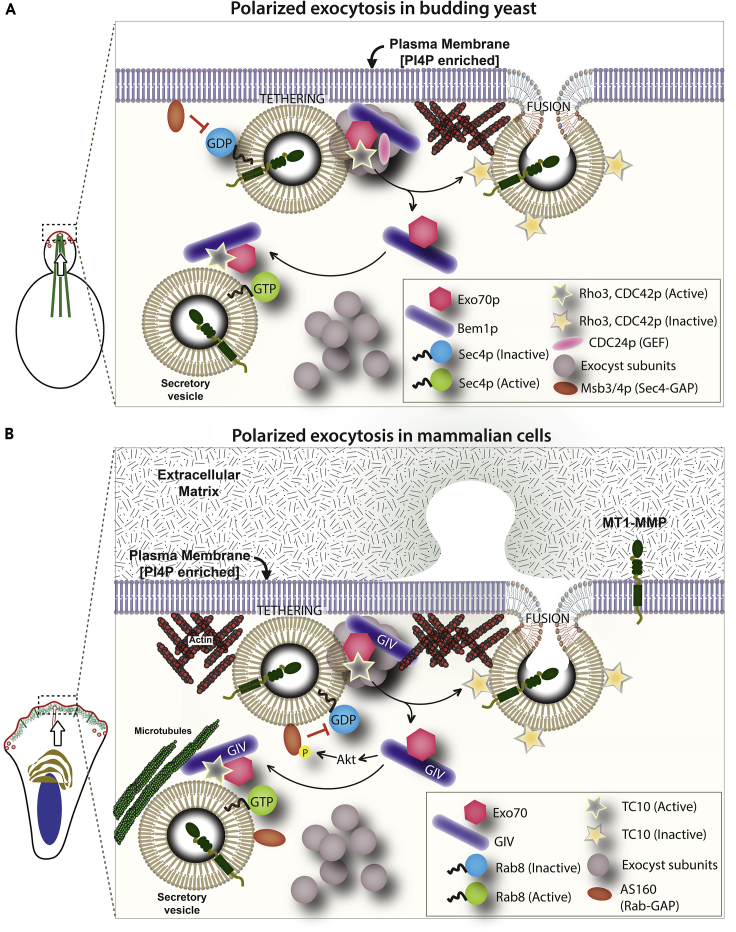
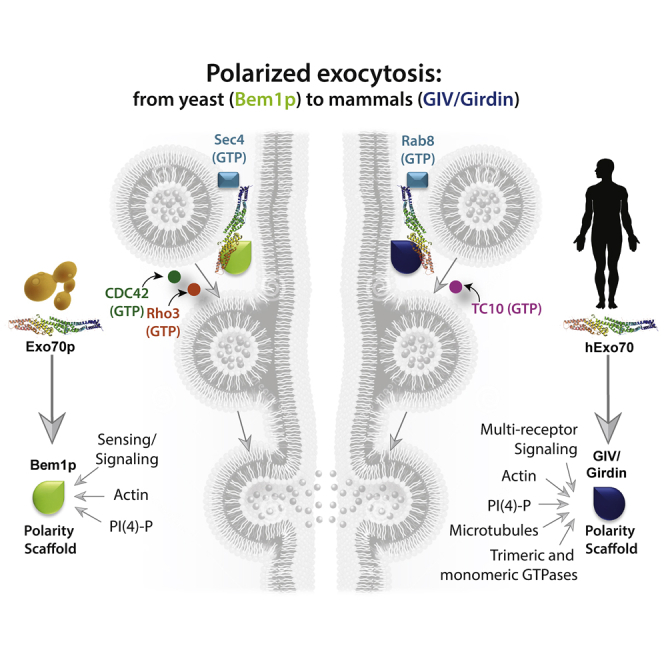
The major discovery we report herein is the mechanism and consequences of a direct interaction between GIV and Exo70, and its impact on polarized exocytosis of matrix metalloprotease MT1-MMP. Using selective single point mutants of GIV that are incapable of binding Exo70, we chart the two major consequences of this GIV⋅Exo70 interaction. First, the GIV⋅Exo70 interaction impacts our understanding of exocytosis in mammalian cells. Findings reveal that GIV imparts polarization to the process of exocytosis. How does GIV do so? Previously, we showed that GIV's ability to activate trimeric GTPase Gαi is critical for its ability to enhance insulin-stimulated exocytosis of glucose transporter, GLUT4-storage vesicles (Lopez-Sanchez et al., 2015b), which in part accounts for GIV's ability to maintain insulin sensitivity in skeletal muscle cells (Ma et al., 2015). Others have demonstrated that the same G protein regulatory module is essential for GIV's ability to activate Par3/6/aPKC polarity complexes (Sasaki et al., 2015). Because the Par3/6/aPKC complexes are highly versatile in their ability to orchestrate all forms of cell polarity, e.g., front-rear, apical-basal, planar cell, and polarity during migration (Suzuki and Ohno, 2006), and positive feedback loop signals are essential for the amplification of stochastically arising clusters of polarity factors that create and maintain such forms of polarity (Mccaffrey and Macara, 2012), we propose that GIV enables polarized exocytosis in a bipartite manner: its ability to bind Exo70 imparts polarity and its ability to activate the Par3/6/aPKC polarity complexes via G protein intermediates (i.e., “free” Gβˠ dimers) serves as the positive feedback loop. Furthermore, GIV has been shown to use “free” Gβˠ intermediates to activate Rho-GTPases (Leyme et al., 2015) and to terminate Arf-GTPase signaling on endomembranes (Lo et al., 2015), which may serve as additional positive and negative feedback loops. Such feedback loops have been shown to be critical for imparting robustness to the process of polarized exocytosis in yeast (Howell et al., 2012): a positive feedback occurs wherein Cdc42p⋅GTP at the membrane can recruit the cytoplasmic Bem1p complex, which generates more Cdc42p⋅GTP from local Cdc42p⋅GDP by GEF-catalyzed exchange; a negative feedback loop enables dispersal of the polarity complexes by affecting the duration of activation of Cdc42p⋅GTP or phosphoinhibition of Bem1p. Whether GIV is at the crossroads of these regulatory loops with the Rho-GTPase TC10 or Rab/Arf-GTPases and what might be the exact mechanism(s) that impart regulatory control and robustness to the mammalian polarized exocytic machinery remain unknown. Regardless, GIV's multi-modular makeup (see Figure 1A), its functional diversity (Aznar et al., 2016a), its growing catalog of phosphomodifications (including the “Y” within the Exo70-binding DFY1743D motif, see Figure 3; phosphosite.org), and its ability to integrate signals at the cross-roads of diverse types of signaling pathways (reviewed in Ghosh et al., 2017) tempts us to speculate that its association with Exo70 may impose intricate regulatory control over the mammalian polarized exocytic machinery making it more precise, context-dependent, versatile, and adapted to the needs of multi-cellular organisms. In keeping with this assumption, it is noteworthy that the yeast polarity scaffold Bem1p has no known homologs in any multicellular organisms (Liu and Novick, 2014), whereas GIV (Coleman et al., 2016) and its associated aPKC/Par3/6 complexes (Thompson, 2013) are absent in yeast and co-evolved only in worms and flies and highly conserved in mammals. Thus, our findings reveal the extent of evolutionary divergence in the polarized exocytic machinery from yeast to mammals (see legend, Figure 7); although the octameric exocyst complex remained constant, each component in yeast is replaced by its functional counterpart in mammals, beginning with an abrupt change in the polarity determinant coinciding with the onset of multicellular life forms. Although our findings suggest that the GIV⋅Exo70 co-complex may constitute the core component that imparts polarity to exocytosis, these may not be sufficient. It is noteworthy that just like Bem1p is not the only component affecting Exo70 localization in yeast (PI4,5P2, Cdc42, Rho1 and actin all play significant roles), GIV may not be the only component affecting polarized exocytosis in mammalian cells and Par/aPKC complexes, TC10, actin, and others may contribute significantly.
Figure 7.
Components of Polarized Exocytosis Display an Evolutionary Advancement from Yeast to Mammals
(A and B) Schematics summarizing the components of exocytosis in yeast (A) and mammals (B). Mammalian counterparts of all yeast components are represented using the same symbols. The octameric exocyst complex is conserved, whereas the polarity scaffolds Bem1p and GIV are divergent, alongside the previously annotated key components of the exocytic machinery. sec4p in yeast is replaced by Rabs in mammals, whereas the sec4p GAP (Msb2/3p) is replaced by the corresponding Rab-GAP, AS160, which has been previously shown to be activated by the GIV-dependent Class 1 PI3K » Akt signaling cascade (Ma et al., 2015).
Second, the GIV⋅Exo70 interaction impacts our understanding of the pro-metastatic role of GIV in diverse cancers and how it impacts key tumor cell behaviors that are dependent on proteolysis. Because GIV modulates integrin/FAK signaling via G protein intermediates (Leyme et al., 2015, Leyme et al., 2016, Lopez-Sanchez et al., 2015b), and because integrins and MT1-MMPs regulate each other's activity, trafficking, and signaling to integrate events in the extracellular milieu (Gonzalo et al., 2010, Das et al., 2017, Baciu et al., 2003, Galvez et al., 2002, Zigrino et al., 2001, Shi and Sottile, 2011), it is possible that GIV's C terminus may help in such process of integration. However, to begin to entertain that possibility or test it, an in-depth insight into how GIV binds integrins and how such binding impacts the GIV⋅Exo70 interaction is required first. Another issue that remains unexplored here is how the GIV⋅Exo70 complex may be dysregulated in cancers. Increased tyrosine-based signaling and hyperphosphorylation of GIV at Y1743 may impact the GIV⋅Exo70 interaction directly. Alternatively, because both GIV (Puseenam et al., 2009, Ghosh et al., 2010) and Exo70 (Lu et al., 2013) have been shown to have alternative isoforms, pre-mRNA splicing may derail the GIV⋅Exo70 interaction or alter its functions indirectly. For example, in the case of Exo70, unlike the epithelial isoform that inhibits tumor metastasis, a mesenchymal isoform aids in tumor metastasis in an exocytosis-independent manner (Lu et al., 2013); the latter promotes tumor cell invasion by interacting with the Arp2/3 complex and stimulating actin branching in lamelipodia and invadopodia. Because GIV is a bona fide actin remodeler that impacts actin dynamics (Jiang et al., 2008, Enomoto et al., 2005), it is possible that the GIV⋅(mesenchymal)Exo70 complex may collaboratively enhance these actin-based processes.
Limitations of the Study
As for the limitations of this study, although we used MT1-MMP as an example of a cargo in our experiments, GIV's ability to regulate such a fundamental component of exocytosis (Exo70) may affect the exocytosis of other cargo proteins, which extend beyond transmembrane proteases, such as receptors (e.g., RTKs and integrins) and other soluble cargo proteins. Although defining the complete inventory of the impacted cargo proteins is beyond the scope of this work, ongoing proteomics/metabolomics-based studies are expected to unravel that list. Similarly, we have not delineated the exact mechanism and dynamics of the interplay between MT1-MMP, TC10, cortactin, and GIV and how that impacts invadosome formation, function, and turnover; because GIV is an actin remodeler (Jiang et al., 2008, Enomoto et al., 2005), it is likely that GIV may have a significant effect on the functions of multiple components that coordinately regulate this critical structure used by invasive cancer cells.
In conclusion, this work not only sheds light into GIV's ability to enhance a key process that aids metastasis (i.e., polarized exocytosis of matrix metalloproteases) but also reveals how the modular makeup and functional diversity of GIV imparts the process of exocytosis features beyond polarity; it represents an evolutionary advancement of the process by adding layers of regulatory control by diverse signaling pathways.
Resource Availability
Lead Contact
Pradipta Ghosh, M.D. can be contacted for all correspondence related to this manuscript via email (prghosh@ucsd.edu).
Materials Availability
Any materials generated and used in this study are available for dissemination to others.
Data and Code Availability
This study did not generate datasets.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This paper was supported by NIH CA238042, AI141630, CA100768, and CA160911 (to P.G). C.R was supported by an NCI/NIH-funded Cancer Therapeutics Training (CT2) Training Program (T32 CA121938) and an NIDDK/NIH-funded training grant (T32 DK007202). We thank Ying Dunkel and Nina Sun for technical support in this work.
Author Contributions
C.R., N.R., I.-C.L., and P.G. designed, executed, and analyzed most of the experiments in this work. P.N. provided critical tools (Bem1p and Exo70p) and guidance on experimental design. D.S. carried out multivariate analysis on the impact of gene expression on metastatic progression. C.R. and P.G. conceived the project, wrote materials and methods, and edited the manuscript. P.G. wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: July 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101246.
Supplemental Information
References
- Artym V.V., Zhang Y., Seillier-Moiseiwitsch F., Yamada K.M., Mueller S.C. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–3043. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- Aznar N., Kalogriopoulos N., Midde K.K., Ghosh P. Heterotrimeric G protein signaling via GIV/Girdin: breaking the rules of engagement, space, and time. Bioessays. 2016;38:379–393. doi: 10.1002/bies.201500133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aznar N., Patel A., Rohena C.C., Dunkel Y., Joosen L.P., Taupin V., Kufareva I., Farquhar M.G., Ghosh P. AMP-activated protein kinase fortifies epithelial tight junctions during energetic stress via its effector GIV/Girdin. Elife. 2016;5:e20795. doi: 10.7554/eLife.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baciu P.C., Suleiman E.A., Deryugina E.I., Strongin A.Y. Membrane type-1 matrix metalloproteinase (MT1-MMP) processing of pro-alphav integrin regulates cross-talk between alphavbeta3 and alpha2beta1 integrins in breast carcinoma cells. Exp. Cell Res. 2003;291:167–175. doi: 10.1016/s0014-4827(03)00387-2. [DOI] [PubMed] [Google Scholar]
- Boehm C., Field M.C. Evolution of late steps in exocytosis: conservation, specialization. Wellcome Open Res. 2019;4:112. doi: 10.12688/wellcomeopenres.15142.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos P.D., Zhang X.H., Nadal C., Shu W., Gomis R.R., Nguyen D.X., Minn A.J., van de Vijver M.J., Gerald W.L., Foekens J.A., Massague J. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd C., Hughes T., Pypaert M., Novick P. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J. Cell Biol. 2004;167:889–901. doi: 10.1083/jcb.200408124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennwald P., Kearns B., Champion K., Keranen S., Bankaitis V., Novick P. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 1994;79:245–258. doi: 10.1016/0092-8674(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Camargo L.M., Collura V., Rain J.C., Mizuguchi K., Hermjakob H., Kerrien S., Bonnert T.P., Whiting P.J., Brandon N.J. Disrupted in Schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol. Psychiatry. 2007;12:74–86. doi: 10.1038/sj.mp.4001880. [DOI] [PubMed] [Google Scholar]
- Castagnino A., Castro-Castro A., Irondelle M., Guichard A., Lodillinsky C., Fuhrmann L., Vacher S., Aguera-Gonzalez S., Zagryazhskaya-Masson A., Romao M. Coronin 1C promotes triple-negative breast cancer invasiveness through regulation of MT1-MMP traffic and invadopodia function. Oncogene. 2018;37:6425–6441. doi: 10.1038/s41388-018-0422-x. [DOI] [PubMed] [Google Scholar]
- Castro-Castro A., Marchesin V., Monteiro P., Lodillinsky C., Rosse C., Chavrier P. Cellular and molecular mechanisms of MT1-MMP-dependent cancer cell invasion. Annu. Rev. Cell Dev. Biol. 2016;32:555–576. doi: 10.1146/annurev-cellbio-111315-125227. [DOI] [PubMed] [Google Scholar]
- Chiang S.H., Hou J.C., Hwang J., Pessin J.E., Saltiel A.R. Cloning and functional characterization of related TC10 isoforms, a subfamily of Rho proteins involved in insulin-stimulated glucose transport. J. Biol. Chem. 2002;277:13067–13073. doi: 10.1074/jbc.M109471200. [DOI] [PubMed] [Google Scholar]
- Coleman B.D., Marivin A., Parag-Sharma K., Digiacomo V., Kim S., Pepper J.S., Casler J., Nguyen L.T., Koelle M.R., Garcia-Marcos M. Evolutionary conservation of a GPCR-independent mechanism of trimeric G protein activation. Mol. Biol. Evol. 2016;33:820–837. doi: 10.1093/molbev/msv336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D.R. Regression models and life tables (with discussion) J. R. Statist. Soc. B. 1972;34:187–220. [Google Scholar]
- Dalerba P., Kalisky T., Sahoo D., Rajendran P.S., Rothenberg M.E., Leyrat A.A., Sim S., Okamoto J., Johnston D.M., Qian D. Single-cell dissection of transcriptional heterogeneity in human colon tumors. Nat. Biotechnol. 2011;29:1120–1127. doi: 10.1038/nbt.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Monteiro M., Barai A., Kumar S., Sen S. MMP proteolytic activity regulates cancer invasiveness by modulating integrins. Sci. Rep. 2017;7:14219. doi: 10.1038/s41598-017-14340-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Azzouzi K., Wiesner C., Linder S. Metalloproteinase MT1-MMP islets act as memory devices for podosome reemergence. J. Cell Biol. 2016;213:109–125. doi: 10.1083/jcb.201510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto A., Murakami H., Asai N., Morone N., Watanabe T., Kawai K., Murakumo Y., Usukura J., Kaibuchi K., Takahashi M. Akt/PKB regulates actin organization and cell motility via Girdin/APE. Dev. Cell. 2005;9:389–402. doi: 10.1016/j.devcel.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Finger F.P., Hughes T.E., Novick P. Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell. 1998;92:559–571. doi: 10.1016/s0092-8674(00)80948-4. [DOI] [PubMed] [Google Scholar]
- Galvez B.G., Matias-Roman S., Yanez-Mo M., Sanchez-Madrid F., Arroyo A.G. ECM regulates MT1-MMP localization with beta1 or alphavbeta3 integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells. J. Cell Biol. 2002;159:509–521. doi: 10.1083/jcb.200205026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Marcos M., Ghosh P., Farquhar M.G. GIV is a nonreceptor GEF for G alpha i with a unique motif that regulates Akt signaling. Proc. Natl. Acad. Sci. U S A. 2009;106:3178–3183. doi: 10.1073/pnas.0900294106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. Heterotrimeric G proteins as emerging targets for network based therapy in cancer: end of a long futile campaign striking heads of a Hydra. Aging (Albany NY) 2015;7:469–474. doi: 10.18632/aging.100781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P., Beas A.O., Bornheimer S.J., Garcia-Marcos M., Forry E.P., Johannson C., Ear J., Jung B.H., Cabrera B., Carethers J.M., Farquhar M.G. A G{alpha}i-GIV molecular complex binds epidermal growth factor receptor and determines whether cells migrate or proliferate. Mol. Biol. Cell. 2010;21:2338–2354. doi: 10.1091/mbc.E10-01-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P., Rangamani P., Kufareva I. The GAPs, GEFs, GDIs and...now, GEMs: new kids on the heterotrimeric G protein signaling block. Cell Cycle. 2017;16:607–612. doi: 10.1080/15384101.2017.1282584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo P., Moreno V., Galvez B.G., Arroyo A.G. MT1-MMP and integrins: hand-to-hand in cell communication. Biofactors. 2010;36:248–254. doi: 10.1002/biof.99. [DOI] [PubMed] [Google Scholar]
- Guo W., Grant A., Novick P. Exo84p is an exocyst protein essential for secretion. J. Biol. Chem. 1999;274:23558–23564. doi: 10.1074/jbc.274.33.23558. [DOI] [PubMed] [Google Scholar]
- Gupta V., Bhandari D., Leyme A., Aznar N., Midde K.K., Lo I.C., Ear J., Niesman I., Lopez-Sanchez I., Blanco-Canosa J.B. GIV/Girdin activates Galphai and inhibits Galphas via the same motif. Proc. Natl. Acad. Sci. U S A. 2016;113:E5721–E5730. doi: 10.1073/pnas.1609502113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., Xi F., Zhang X., Zhang J., Guo W. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J. 2007;26:4053–4065. doi: 10.1038/sj.emboj.7601834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog M., Monteiro P., le Dez G., Chavrier P. Exo70 subunit of the exocyst complex is involved in adhesion-dependent trafficking of caveolin-1. PLoS One. 2012;7:e52627. doi: 10.1371/journal.pone.0052627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino D., Branch K.M., Weaver A.M. Signaling inputs to invadopodia and podosomes. J. Cell Sci. 2013;126:2979–2989. doi: 10.1242/jcs.079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell A.S., Jin M., Wu C.F., Zyla T.R., Elston T.C., Lew D.J. Negative feedback enhances robustness in the yeast polarity establishment circuit. Cell. 2012;149:322–333. doi: 10.1016/j.cell.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutagalung A.H., Coleman J., Pypaert M., Novick P.J. An internal domain of Exo70p is required for actin-independent localization and mediates assembly of specific exocyst components. Mol. Biol. Cell. 2009;20:153–163. doi: 10.1091/mbc.E08-02-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Chang L., Hwang J., Chiang S.H., Saltiel A.R. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature. 2003;422:629–633. doi: 10.1038/nature01533. [DOI] [PubMed] [Google Scholar]
- Jiang P., Enomoto A., Jijiwa M., Kato T., Hasegawa T., Ishida M., Sato T., Asai N., Murakumo Y., Takahashi M. An actin-binding protein Girdin regulates the motility of breast cancer cells. Cancer Res. 2008;68:1310–1318. doi: 10.1158/0008-5472.CAN-07-5111. [DOI] [PubMed] [Google Scholar]
- Juin A., Billottet C., Moreau V., Destaing O., Albiges-Rizo C., Rosenbaum J., Genot E., Saltel F. Physiological type I collagen organization induces the formation of a novel class of linear invadosomes. Mol. Biol. Cell. 2012;23:297–309. doi: 10.1091/mbc.E11-07-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogriopoulos N.A., Rees S.D., Ngo T., Kopcho N.J., Ilatovskiy A.V., Sun N., Komives E.A., Chang G., Ghosh P., Kufareva I. Structural basis for GPCR-independent activation of heterotrimeric Gi proteins. Proc. Natl. Acad. Sci. U S A. 2019;116:16394–16403. doi: 10.1073/pnas.1906658116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y., Bao T., Zhou Q., Wang Y., Ge J., Fu B., Wu X., Tang H., Shi Z., Lei X. Discs large homolog 5 decreases formation and function of invadopodia in human hepatocellular carcinoma via Girdin and Tks5. Int. J. Cancer. 2017;141:364–376. doi: 10.1002/ijc.30730. [DOI] [PubMed] [Google Scholar]
- Leyme A., Marivin A., Garcia-Marcos M. GIV/Girdin (Galpha-interacting, vesicle-associated protein/girdin) creates a positive feedback loop that potentiates outside-in integrin signaling in cancer cells. J. Biol. Chem. 2016;291:8269–8282. doi: 10.1074/jbc.M115.691550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyme A., Marivin A., Perez-Gutierrez L., Nguyen L.T., Garcia-Marcos M. Integrins activate trimeric G proteins via the nonreceptor protein GIV/Girdin. J. Cell Biol. 2015;210:1165–1184. doi: 10.1083/jcb.201506041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Ear J., Midde K., Lopez-Sanchez I., Aznar N., Garcia-Marcos M., Kufareva I., Abagyan R., Ghosh P. Structural basis for activation of trimeric Gi proteins by multiple growth factor receptors via GIV/Girdin. Mol. Biol. Cell. 2014;25:3654–3671. doi: 10.1091/mbc.E14-05-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Ear J., Pavlova Y., Mittal Y., Kufareva I., Ghassemian M., Abagyan R., Garcia-Marcos M., Ghosh P. Tyrosine phosphorylation of the Galpha-interacting protein GIV promotes activation of phosphoinositide 3-kinase during cell migration. Sci. Signal. 2011;4:ra64. doi: 10.1126/scisignal.2002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W.H., Asmann Y.W., Anastasiadis P.Z. Expression of polarity genes in human cancer. Cancer Inform. 2015;14:15–28. doi: 10.4137/CIN.S18964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Novick P. Bem1p contributes to secretory pathway polarization through a direct interaction with Exo70p. J. Cell Biol. 2014;207:59–72. doi: 10.1083/jcb.201404122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Yue P., Artym V.V., Mueller S.C., Guo W. The role of the exocyst in matrix metalloproteinase secretion and actin dynamics during tumor cell invadopodia formation. Mol. Biol. Cell. 2009;20:3763–3771. doi: 10.1091/mbc.E08-09-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo I.C., Gupta V., Midde K.K., Taupin V., Lopez-Sanchez I., Kufareva I., Abagyan R., Randazzo P.A., Farquhar M.G., Ghosh P. Activation of galphai at the golgi by GIV/girdin imposes finiteness in Arf1 signaling. Dev. Cell. 2015;33:189–203. doi: 10.1016/j.devcel.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Sanchez I., Kalogriopoulos N., LO I.C., Kabir F., Midde K.K., Wang H., Ghosh P. Focal adhesions are foci for tyrosine-based signal transduction via GIV/Girdin and G proteins. Mol. Biol. Cell. 2015;26:4313–4324. doi: 10.1091/mbc.E15-07-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Sanchez I., Ma G.S., Pedram S., Kalogriopoulos N., Ghosh P. GIV/girdin binds exocyst subunit-Exo70 and regulates exocytosis of GLUT4 storage vesicles. Biochem. Biophys. Res. Commun. 2015;468:287–293. doi: 10.1016/j.bbrc.2015.10.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Liu J., Liu S., Zeng J., Ding D., Carstens R.P., Cong Y., Xu X., Guo W. Exo70 isoform switching upon epithelial-mesenchymal transition mediates cancer cell invasion. Dev. Cell. 2013;27:560–573. doi: 10.1016/j.devcel.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G., Lopez-Sanchez I., Aznar N., Kalogriopoulos N., Midde K., Ciaraldi T.P., Henry H.R., Ghosh P. Activation of G proteins by GIV-GEF is a pivot point for insulin resistance and sensitivity. Mol. Biol. Cell. 2015;26:4209–4223. doi: 10.1091/mbc.E15-08-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Urdiroz M., Deeks M.J., Horton C.G., Dawe H.R., Jourdain I. The exocyst complex in health and disease. Front. Cell Dev. Biol. 2016;4:24. doi: 10.3389/fcell.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccaffrey L.M., Macara I.G. Signaling pathways in cell polarity. Cold Spring Harb. Perspect. Biol. 2012;4:a009654. doi: 10.1101/cshperspect.a009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei K., Li Y., Wang S., Shao G., Wang J., Ding Y., Luo G., Yue P., Liu J.J., Wang X. Cryo-EM structure of the exocyst complex. Nat. Struct. Mol. Biol. 2018;25:139–146. doi: 10.1038/s41594-017-0016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midde K., Sun N., Rohena C., Joosen L., Dhillon H., Ghosh P. Single-cell imaging of metastatic potential of cancer cells. iScience. 2018;10:53–65. doi: 10.1016/j.isci.2018.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn A.J., Gupta G.P., Siegel P.M., Bos P.D., Shu W., Giri D.D., Viale A., Olshen A.B., Gerald W.L., Massague J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal Y., Pavlova Y., Garcia-Marcos M., Ghosh P. Src homology domain 2-containing protein-tyrosine phosphatase-1 (SHP-1) binds and dephosphorylates G(alpha)-interacting, vesicle-associated protein (GIV)/Girdin and attenuates the GIV-phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway. J. Biol. Chem. 2011;286:32404–32415. doi: 10.1074/jbc.M111.275685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro P., Rosse C., Castro-Castro A., Irondelle M., Lagoutte E., Paul-Gilloteaux P., Desnos C., Formstecher E., Darchen F., Perrais D. Endosomal WASH and exocyst complexes control exocytosis of MT1-MMP at invadopodia. J. Cell Biol. 2013;203:1063–1079. doi: 10.1083/jcb.201306162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B.A., Robinson H.H., Xu Z. The crystal structure of mouse Exo70 reveals unique features of the mammalian exocyst. J. Mol. Biol. 2007;371:410–421. doi: 10.1016/j.jmb.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton W.M., Ayscough K.R., Mclaughlin P.J. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat. Cell Biol. 2000;2:376–378. doi: 10.1038/35014075. [DOI] [PubMed] [Google Scholar]
- Nechiporuk T., Fernandez T.E., Vasioukhin V. Failure of epithelial tube maintenance causes hydrocephalus and renal cysts in Dlg5-/- mice. Dev. Cell. 2007;13:338–350. doi: 10.1016/j.devcel.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechipurenko I.V., Olivier-Mason A., Kazatskaya A., Kennedy J., Mclachlan I.G., Heiman M.G., Blacque O.E., Sengupta P. A conserved role for girdin in basal body positioning and ciliogenesis. Dev. Cell. 2016;38:493–506. doi: 10.1016/j.devcel.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Ohara K., Enomoto A., Kato T., Hashimoto T., Isotani-Sakakibara M., Asai N., Ishida-Takagishi M., Weng L., Nakayama M., Watanabe T. Involvement of Girdin in the determination of cell polarity during cell migration. PLoS One. 2012;7:e36681. doi: 10.1371/journal.pone.0036681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picco A., Irastorza-Azcarate I., Specht T., Boke D., Pazos I., Rivier-Cordey A.S., Devos D.P., Kaksonen M., Gallego O. The in vivo architecture of the exocyst provides structural basis for exocytosis. Cell. 2017;168:400–412.e18. doi: 10.1016/j.cell.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Planchon D., Rios Morris E., Genest M., Comunale F., Vacher S., Bieche I., Denisov E.V., Tashireva L.A., Perelmuter V.M., Linder S. MT1-MMP targeting to endolysosomes is mediated by upregulation of flotillins. J. Cell Sci. 2018;131:jcs218925. doi: 10.1242/jcs.218925. [DOI] [PubMed] [Google Scholar]
- Puseenam A., Yoshioka Y., Nagai R., Hashimoto R., Suyari O., Itoh M., Enomoto A., Takahashi M., Yamaguchi M. A novel Drosophila Girdin-like protein is involved in Akt pathway control of cell size. Exp. Cell Res. 2009;315:3370–3380. doi: 10.1016/j.yexcr.2009.06.019. [DOI] [PubMed] [Google Scholar]
- Rahman-Zaman A., Shan S., Reinhart-King C.A. Cell migration in microfabricated 3D collagen microtracks is mediated through the prometastatic protein girdin. Cell Mol. Bioeng. 2018;11:1–10. doi: 10.1007/s12195-017-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo D., Dill D.L., Tibshirani R., Plevritis S.K. Extracting binary signals from microarray time-course data. Nucleic Acids Res. 2007;35:3705–3712. doi: 10.1093/nar/gkm284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K., Kakuwa T., Akimoto K., Koga H., Ohno S. Regulation of epithelial cell polarity by PAR-3 depends on Girdin transcription and Girdin-Galphai3 signaling. J. Cell Sci. 2015;128:2244–2258. doi: 10.1242/jcs.160879. [DOI] [PubMed] [Google Scholar]
- Shi F., Sottile J. MT1-MMP regulates the turnover and endocytosis of extracellular matrix fibronectin. J. Cell Sci. 2011;124:4039–4050. doi: 10.1242/jcs.087858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahelin R.V., Karathanassis D., Murray D., Williams R.L., Cho W. Structural and membrane binding analysis of the Phox homology domain of Bem1p: basis of phosphatidylinositol 4-phosphate specificity. J. Biol. Chem. 2007;282:25737–25747. doi: 10.1074/jbc.M702861200. [DOI] [PubMed] [Google Scholar]
- Steffen A., Le Dez G., Poincloux R., Recchi C., Nassoy P., rottner K., Galli T., Chavrier P. MT1-MMP-dependent invasion is regulated by TI-VAMP/VAMP7. Curr. Biol. 2008;18:926–931. doi: 10.1016/j.cub.2008.05.044. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Ohno S. The PAR-aPKC system: lessons in polarity. J. Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- Terbush D.R., Maurice T., Roth D., Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Terbush D.R., Novick P. Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. J. Cell Biol. 1995;130:299–312. doi: 10.1083/jcb.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B.J. Cell polarity: models and mechanisms from yeast, worms and flies. Development. 2013;140:13–21. doi: 10.1242/dev.083634. [DOI] [PubMed] [Google Scholar]
- Volkmer J.P., Sahoo D., Chin R.K., Ho P.L., Tang C., Kurtova A.V., Willingham S.B., Pazhanisamy S.K., Contreras-Trujillo H., Storm T.A. Three differentiation states risk-stratify bladder cancer into distinct subtypes. Proc. Natl. Acad. Sci. U S A. 2012;109:2078–2083. doi: 10.1073/pnas.1120605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.H., Celic I., Choi S.Y., Riccomagno M., Wang Q., Sun L.O., Mitchell S.P., Vasioukhin V., Huganir R.L., Kolodkin A.L. Dlg5 regulates dendritic spine formation and synaptogenesis by controlling subcellular N-cadherin localization. J. Neurosci. 2014;34:12745–12761. doi: 10.1523/JNEUROSCI.1280-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Klijn J.G., Zhang Y., Sieuwerts A.M., Look M.P., Yang F., Talantov D., Timmermans M., Meijer-van Gelder M.E., Yu J. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- Wu B., Guo W. The exocyst at a glance. J. Cell Sci. 2015;128:2957–2964. doi: 10.1242/jcs.156398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C., Grindstaff K.K., Nelson W.J. Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells. J. Cell Sci. 2004;117:559–570. doi: 10.1242/jcs.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Orlando K., He B., Xi F., Zhang J., Zajac A., Guo W. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J. Cell Biol. 2008;180:145–158. doi: 10.1083/jcb.200704128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Liu J., Yang C., Capraro B.R., Baumgart T., Bradley R.P., Ramakrishnan N., XU X., Radhakrishnan R., Svitkina T., Guo W. Exo70 generates membrane curvature for morphogenesis and cell migration. Dev. Cell. 2013;26:266–278. doi: 10.1016/j.devcel.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigrino P., Drescher C., Mauch C. Collagen-induced proMMP-2 activation by MT1-MMP in human dermal fibroblasts and the possible role of alpha2beta1 integrins. Eur. J. Cell Biol. 2001;80:68–77. doi: 10.1078/0171-9335-00134. [DOI] [PubMed] [Google Scholar]
- Zuo X., Zhang J., Zhang Y., Hsu S.C., Zhou D., Guo W. Exo70 interacts with the Arp2/3 complex and regulates cell migration. Nat. Cell Biol. 2006;8:1383–1388. doi: 10.1038/ncb1505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cells transiently expressing MT1-MMP-pHluorin show real-time exocytic events. Images were acquired over 10 min. The movie was acquired at exposure time of 100 ms and with 1 pixel corresponding to 160 nm
GIV-depleted cells transiently expressing MT1-MMP-pHluorin shows real-time exocytic events. Images were acquired over 10 min. The movie was acquired at exposure time of 100 ms and with 1 pixel corresponding to 160 nm
GIV-depleted cells rescued with GIV-WT (stably) and transiently expressing MT1-MMP-pHluorin shows real-time exocytic events. Images were acquired over 10 min. The movie was acquired
at exposure time of 100 ms and with 1 pixel corresponding to 160 nm
GIV-depleted cells rescued with GIV-mutant and transiently expressing MT1-MMP-pHluorin show real-time exocytic events. Images were acquired over 10 min. The movie was acquired at exposure time of 100 ms and with 1 pixel corresponding to 160 nm
GIV-depleted cells rescued with GIV-mutant and transiently expressing MT1-MMP-pHluorin show real-time exocytic events. Images were acquired over 10 min. The movie was acquired at exposure time of 100 ms and with 1 pixel corresponding to 160 nm.
Data Availability Statement
This study did not generate datasets.