Abstract
At the present time, there is no successful synthetic, off-the-shelf small-caliber vascular graft (<6 mm) for the repair or bypass of the coronary or carotid arteries. This stimulates on-going investigations to fabricate an artificial vascular graft that has both sufficient mechanical properties as well as superior biological performance. Collagen has long been considered as a viable material to encourage cell recruitment, tissue regeneration, and revascularization, but its use has been limited by its inferior mechanical properties. In this study, novel electrochemically aligned collagen filaments were used to engineer a bilayer small-caliber vascular graft, by circular knitting the collagen filaments and electrospinning collagen nanofibers. The collagen prototype grafts showed significantly greater bursting strength under dry and hydrated conditions to that of autografts such as the human internal mammary artery and the saphenous vein (SV). The suture retention strength was sufficient under dry condition, but that under hydrated condition needs to be further improved. The radial dynamic compliance of the collagen grafts was similar to that of the human SV. During in vitro cell culture assays with human umbilical vein endothelial cells, the prototype collagen grafts also encouraged cell adhesion and promoted cell proliferation compared to the synthetic poly(lactic acid) grafts. In conclusion, this study demonstrated the feasibility of the use of novel collagen filaments for fabricating small caliber tissue-engineered vascular grafts that provide both sufficient mechanical properties and superior biological performance.
Keywords: small caliber vascular graft, endothelialization, knitting, electrospinning, electrochemically aligned collagen (ELAC) filament, collagen nanofibers, mechanical properties
1. Introduction
Cardiovascular diseases (CVDs) are the number one cause of death worldwide. According to the World Health Organization, 17.9 million people died from CVDs in 2016, which is 31% of all deaths globally, and this number is predicted to reach 23.4 million by 2030 [1]. Percutaneous intervention is commonly used to reopen occluded blood vessels, but when the stenosis reaches 70% or more [2], impacts several vessels [3], or involves the left coronary artery leading to a reduced cardiac ejection fraction [4], then bypass surgery is the treatment of choice.
Autologous grafts with their superior patency rates are considered as the ‘gold standard’ when bypassing small diameter blood vessels [5]. However, their availability and qualities are often limited due to the need for invasive harvesting, prior use, potential complications at the donor site [5], their variations from batch to batch and their potential for contamination make them hard to be off-shelf products [6].
While synthetic vascular prostheses have long been used as an alternative to autografts for replacing and bypassing large caliber arteries (>6 mm), they are known to fail in small caliber vessels (<6 mm) due to thrombosis, narrowing or blockage caused by plaque deposition and mechanical mismatch which leads to intimal hyperplasia [7].
To address these issues, tissue-engineered vascular grafts (TEVGs) with the abilities to grow and integrate with native tissue presents a promising alternative path for graft development. In order to fabricate a viable scaffold for a TEVG, synthetic materials, such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA), poly (caprolactone) (PCL) and natural polymers, like collagen, elastin, gelatin and have been studied extensively alone and in blends [8-10].
Reendothelialization is of vital importance in preventing the grafts from experiencing restenosis [11, 12]. So, an important part of the TEVG design is to encourage endothelial cell (EC) proliferation. Collagen is a viable material for the fabrication of the TEVGs because it is the most abundant protein component of native blood vessel walls [13], and it has significantly superior in vitro and in vivo cell recruitment performance compared to the synthetic polymers mentioned above [14, 15]. Collagen also activates platelet aggregation which is the first step in the healing process [16]. However, the inferior mechanical property is one of the limitations that restricted its utilization in fabricating vascular grafts and tissue scaffolds regardless of whether the fabrication method is bioprinting [17], electrospinning [18], molding [19], or freeze drying [6]. To the best of our knowledge, electrochemically-aligned collagen (ELAC) filaments [20] invented by Dr Ozan Akkus and colleagues were the first engineered fiber made from 100% type I collagen filaments. Since June 2018, these collagen filaments have been commercially available through CollaMedix Inc., Cleveland, Ohio. The appearance of the continuous collagen filaments makes it possible to combine this natural material with a range of textile technologies such as knitting, weaving, and braiding. This favors the fabrication of tissue-engineered vascular scaffolds that not only mimic the components and structure of the extracellular matrix (ECM) of native blood vessels to improve the biological performance, but also provide superior mechanical support and integrity.
This study had two sequential objectives. Firstly, because continuous collagen filaments have only recently become available, the mechanical properties of these filaments were measured under dry and hydrated conditions in order to design the desired structure and determine the appropriate processing methods for this vascular application.
The second objective was to design, fabricate and evaluate a bilayer small caliber vascular scaffold by knitting the collagen filaments into a tubular structure and electrospinning a luminal layer of collagen nanofibers so as to mimic the multilayer structure of ECM in the native blood vessel. The mechanical properties and biological performance were characterized experimentally under both dry and hydrated conditions because the grafts are fabricated and handled in the dry state while exposed to hydrated conditions in vivo. And the mechanical properties of the prototype collagen grafts in the hydrated state were compared to that of the autologous reference grafts. In addition, it was necessary to characterize the morphology and average pore size so as to regulate the hemodynamic performance and encourage cell adhesion, proliferation, and re-endothelialization.
2. Materials and methods
2.1. Preparation of collagen filaments
The type I collagen used to make the ELAC filaments was extracted from rat tail tendons. The sacrifice of the mice was approved by North Carolina State University Laboratory Animal Resources Institutional Animal Care and Use Committee. Briefly, the sheaths and skin of the rat tails were removed, and the tendon was peeled out and washed in 1X phosphate buffered saline (PBS) solution (Sigma-Aldrich), dissolved in 0.5M acetic acid for 48 h and precipitated by adding 10% sodium chloride (NaCl) (Fisher Scientific) into the supernatant. The precipitated collagen was re-dissolved in 0.25 M acetic acid by stirring for 24 h and dialyzed to 3 mg ml−1 in 17.5mM acetic acid. The whole extraction process was conducted at 4 °C.
The solution was loaded into a syringe and injected in the spacing between the anode and cathode strips of a rotation electrode. A constant 40 V electrical voltage potential was applied between the electrodes. Following electrocompaction of the molecules, the thread was collected in an 80% isopropanol solution. The threads were dried and reeled in a fume hood, then they were vacuum-sealed and stored in a fridge at 4 °C.
The principle of electrochemical compaction involves the creation of a pH gradient between two linear electrodes. Collagen molecules become similarly charged with the electrodes that they are closer to which results in molecular repulsion by both electrodes. The net effect is that the collagen molecules are compacted and aligned at a location between the two electrodes [21].
Two single collagen filaments were plied and twisted manually at 100 twistsm−1 in order to increase the mechanical strength of the filaments. Then they were crosslinked using 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC) (Oakwood Chemical) and N-hydroxysuccinimide (NHS) (Thermo Scientific). The molar ratio of collagen: EDC: NHS was 1:100:250, and 80% ethanol (Fisher Scientific) was added to the crosslinking reaction for two hours at room temperature, followed by an overnight crosslinking treatment in the refreshed reagent. The crosslinked filaments were rinsed in 0.1M sodium phosphate (ChemCenter) for 30 min to hydrolyze any unreacted o-isoacylurea intermediates and washed 3–4 times in PBS. The filaments were dried at room temperature and left in a desiccator overnight. The dried collagen filaments were sealed and stored at 4 °C before use.
2.2. Characterization of collagen filaments
The two-ply collagen filaments were evaluated in both dry and hydrated states. The filaments were knitted and handled under dry conditions, so their processability in the dry state needed to be evaluated. For example, the strength and modulus of the dry filaments decide the appropriate fabrication technology and parameters to manufacture the collagen scaffolds. On the other hand, their properties under hydrated conditions reflect the graft’s performance after implantation. The dry collagen filaments were conditioned in a desiccator for 24 h before the evaluation, and the hydrated collagen filaments were immersed in PBS for 1 h before the test.
The morphology and the diameters of the cross-linked 2-ply collagen filaments were measured with a Nikon Eclipse 50i POL optical microscope. Ten filaments were measured with 5 measurements on each specimen. The tensile properties of the 2-ply collagen filaments were measured on an Instron Model 5584 mechanical tester using a 2 kN load cell, a gauge length of 10 mm and a crosshead speed of 50mmmin−1. The maximum load (N), extension at maximum load (mm), extension at the yield point (mm) and Young’s modulus (N mm−1) were reported.
The swelling test was conducted on the uncrosslinked and crosslinked 2-ply collagen filaments over 24 h. The uncrosslinked and crosslinked collagen filaments were immersed in the 1X PBS for 0.5 h, 1 h, 2 h, 24 h and weighed after each time period. Three 10 cm long replicates were included for repeatability.
The swelling ratio was calculated following the equation below [22,23]
where
Wd = Weight of the sample in the dry state;
Wh = Weight of the sample in the hydrated state.
2.3. Fabricating the single-layer knitted grafts
A Model ST3AH/ZA single feed circular weft knitting machine (Lamb Knitting Machine Corporation) (figure 1(A)) with 16 needles was used to fabricate the dry 2-ply collagen filaments into a weft knitted tube (figure 1(B)) using a manual working speed of approximately 60 rpm. The tubes were then stored at 4 °C before further processing and testing.
Figure 1.
The collagen filaments were directly knitted into a tubular structure (B) on the lamb circular weft knitting machine (A).
Since polylactic acid has been widely used in various LDA-approved medical devices and products, for comparison purpose, a group of reference grafts was fabricated from 150 denier/92 multifilament PLA yarns (Xinxiang sunshine Textiles Co Ltd, Henan, China) under the same knitting and storage conditions. The PLA multifilaments share a similar size to the 2-ply collagen filaments.
2.4. Engineering the bilayer grafts by adding an electrospun layer of collagen nanofibers to the luminal surface of the knitted collagen scaffold
The bilayer graft was engineered to resemble the multilayer structure of the blood vessel. The electrospun layer was added to reduce the pore size so as to regulate hemodynamics and promote EC proliferation. The knitted collagen tube was mounted on a 3 mm in-diameter rotating mandrel to collect a thin layer of electrospun collagen fibers on its exterior surface.
The collagen powder used for electrospinning was lyophilized from the collagen solution as described in section 2.1. The collagen powder was used for electrospinning without further purification.
The electrospinning process was modified from thatin [24]. Briefly, the collagen concentration was 18-wt% and the solvent was 1:1 v/v 20X PBS : ethanol. A 10 ml syringe was filled with the solution which was pumped through a 20 gauge stainless steel needle for approximately 30 min, at a pumping rate of 0.1–0.5 ml h−1. The rotating collector covered with the knitted collagen tube was set at a horizontal tip-tocollector distance of 10 cm and the collector rotation rate was 200 rpm. A high voltage power supply of 20 kV was connected to the needle, and the collector was grounded.
After collection of a thin layer of the collagen fiber web, the bilayer grafts were crosslinked in 0.2M EDC and NHS in 80% ethanol [24]. The knitted and coated tube was then turned inside out to achieve a structure with a knitted external and an electrospun internal layer. To achieve this, one end of the tube was attached to a needle and a thread, which were pulled carefully through the lumen. The needle and thread were then removed.
The electrospun fibers were examined by scanning electron microscopy (JEOL, Model JSM-5900LV) and analyzed using ISCapture imaging analysis (Fuzhou Tucsen Photonics Co., Ltd, Fujian, China) to determine the average and standard deviation of the diameter of nanofibers. Ten measurements were made on each of the three images.
2.5. Morphological and mechanical properties of the collagen grafts
The dimensions and morphology of the prototype collagen grafts were analyzed using an SEM under an accelerating voltage of 20 kV [25]. The scaffolds were cut into square specimens measuring 5mm × 5mm and sputter coated with platinum.
The microscopic dimensions of the pores in the scaffold were measured by determining the distance (μm) between the edges of the filaments or fibers, according to ISO 7198: 2016. The pore size was measured at six locations on each image, with three images for each sample.
The probe bursting test was performed according to the standard test method in ISO 7198: 2016 on an Instron Model 5584 mechanical tester using a 2 kN load cell and a crosshead speed of 50mmmin−1. A compressional cage was attached to the crosshead with a 3mm in-diameter probe on the top that burst through the fixed flat sample on the bottom. The tubular specimen was cut open, flattened and mounted as a single layer, 1 cm × 1 cm flat square specimen that had either been dried in a desiccator overnight or immersed in PBS for 30 min prior to testing. Three specimens were tested under each condition. The test was run until failure. The maximum load (N) was recorded, and the bursting strength (MPa) of the samples was calculated following the equation below, where rwas the radius of the probe [25]
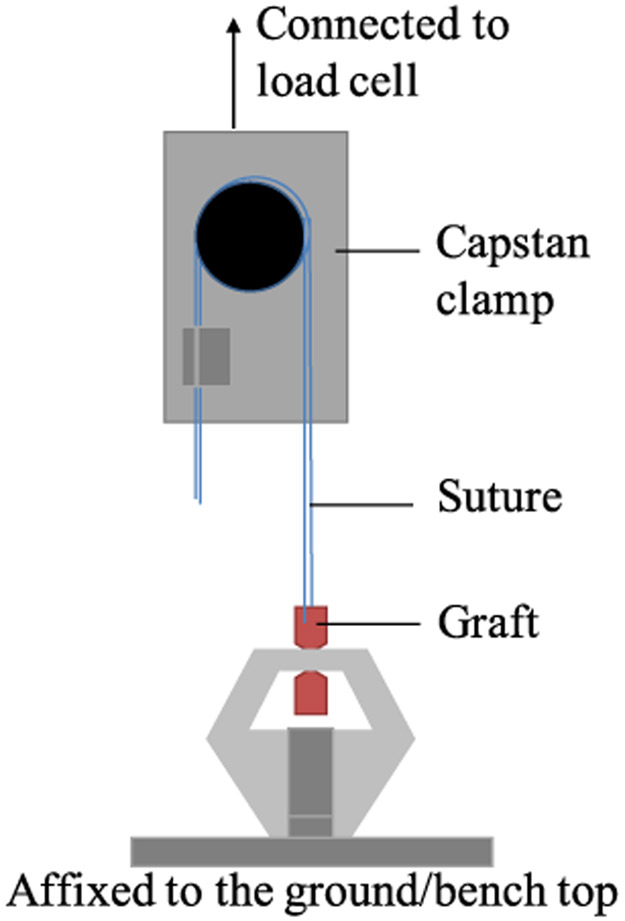
The suture retention test was conducted on the Instron using a 2 kN load cell. The ISO 7198: 2016 test procedure was followed on dry and hydrated samples conditioned as described previously. The set-up is shown in figure 2. The tubular graft specimens were cut into 20mm lengths with the lower end clamped between flat clamps in the bottom jaw. A 3-0 braided nylon suture was passed through the graft wall 2mm from the cut edge at the top end, which was clamped in a capstan clamp. The suture was pulled out at a crosshead speed of 50 mm min−1 until failure and the maximum load (N) was reported.
Figure 2.
Diagram of the suture retention test set-up.
The radial dynamic compliance of the collagen grafts was characterized according to ISO 7198: 2016. It was determined by measuring the diameter change during pressure cyclic loading on an Electroforce Biodynamic Mechanical Test System Model 5175 (TA Instruments, New Castle, DE, USA).
A 5 cm long sample was mounted on the test machine with a 4mm diameter highly deformable polyurethane (PU) tube inserted inside, which transferred the pressure wave to the vascular grafts [9]. The samples were immersed in 1X PBS solution prior to testing to imitate the hydrated state in vivo. Three replicates were included for each type of graft.
The water flow of 100 ml min−1 and pulsatile frequency of 1 Hz was applied. Three pressure ranges were used, which were 50–90 mmHg (hypotensive), 80–120 mmHg (normotensive) and 110–150 mmHg (hypertensive). The changes in the external diameter of the grafts in response to these pressures were measured by a laser micrometer. The internal radius was calculated as:
where
Rp = the pressurized internal radius, in mm;
Dp = the measured pressurized external diameter, in mm;
t = the wall thickness of the graft, in mm.
The circumferential compliance was obtained as follows:
where
p1 = the diastolic pressure value, in mmHg;
p2 = the systolic pressure value, in mmHg.
The β stiffness index is an indicator of the vascular stiffness [26]. It was calculated from the compliance test recordings of the outer diameters in response to the changing intraluminal pressure as described in [27,28]
where
p1 = the diastolic pressure value, in mmHg;
p2 = the systolic pressure value, in mmHg;
Dp1 = the measured pressurized external diameter under diastolic pressure value, in mm;
Dp2 = the measured pressurized external diameter under systolic pressure value, in mm.
2.6. Comparing the cell adhesion and proliferation on the collagen and PLA grafts
The biological performance of the collagen scaffolds was compared to that of the PLA scaffolds. The tubular scaffolds were cut open in the longitudinal direction and made into flat square specimens measuring 1 cm × 1 cm. The square samples were sterilized in an Anprolene AN74i ethylene oxide sterilizer (Andersen Products Inc.) over a period of 12 h, followed by at least a 2 h aeration, and then immersed in culture media for 8 h in a 24-well cell culture plate prior to cell seeding.
The culture media was composed of 95% human mammary epithelial cell medium (HuMEC) with 5ml HuMEC supplement, 25 mg bovine pituitary extract, 5% fetal bovine serum (Corning) and 1% penicillin/ streptomycin. All the media and reagents for cell culture were purchased from Gibco unless otherwise specified.
Human umbilical vein endothelial cells (HUVECs) (UNC Tissue Culture Core, Chapel Hill, NC) were seeded at a density of 200 000 cells per sample by adding dropwise 50 μl cell suspension with a concentration of 4 million cells ml−1. The cells were then incubated for 30 min at 37 °C, with 5% CO2 to allow for attachment. Then 1ml culture media was added to each well on the 24-well cell culture plates. The culture media was changed every two days.
The Day 3 samples were fixed in 3% glutaraldehyde (Ladd Research Industries) on ice and coated with palladium for morphology examination by SEM.
The alamarBlue® assay was conducted on Day 1, 3, 9 to quantify the viable HUVECs on the scaffolds by measuring the metabolic activity of the living cells. The samples were moved to an unused plate carefully before the assay so as to exclude any unattached cells. Then 1ml of media containing 10% alamarBlue® reagent (Invitrogen) was added dropwise to each sample and left in the dark for 4 h.
After 4 h of incubation in the dark, the media was transferred to 96-well plate, and the fluorescence was read using a Tecan GENios microplate reader (Tecan Trading AG) with an excitation wavelength of 550 nm, an emission wavelength at 590 nm and a gain of 40. The cell number was quantified by calculating the percentage reduction of the alamarBlue® reagent and comparing it to a standard curve. JMP Pro 13 software was used to analyze the relationship between the relative fluorescence unit and the known number of cells (p < 0.0001).
The negative controls were acquired by adding the mixture of 1 ml media containing 10% alamarBlue® reagent to the scaffold with no cells seeded, while the positive control was obtained by autoclaving the same mixture for 15 min in a liquid cycle and adding it to the cell-free scaffold. Three replicates for each group of samples and controls were included for repeatability.
2.7. Statistics
Statistical analysis in this study was performed using JMP Pro 13 (JMP, Cary, NC, USA). All the reported results were expressed in terms of the mean±standard deviation. Significant differences were analyzed by one-way analysis of variance followed by pair-wise post-hoc analysis with a Bonferroni correction. Significant differences were identified when p < 0.05.
3. Results
3.1. Characterization of the collagen filaments
The morphology, mechanical properties and swelling ratio of the experimental 2-ply collagen filaments were determined so as to demonstrate their knitting processability under dry conditions and predict their performance upon contact with the fluid environment. It was important to confirm that they were able to withstand the tension and friction due to the movement of the needles, the rubbing against the tension guides, and the tension due to the pull-down mechanism.
The collagen filaments behaved differently under dry and hydrated conditions. The dry collagen filaments were significantly thinner than the hydrated ones as shown in figures 3(A), (B) and table 1. The average thickness of the dry collagen filaments was 209.6 ± 33.0 μm and that of the hydrated filaments increased to 303.9 ± 65.3 μm.
Figure 3.
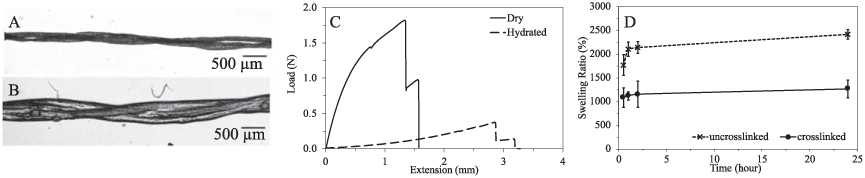
Characterization of the 2-ply collagen filaments under dry (A) and hydrated (B) conditions. (C) Representative loadextension curves of dry and hydrated collagen filaments. (D) Swelling ratio of the uncrosslinked and crosslinked collagen filaments over 24 h.
Table 1.
Physical properties of 2-ply collagen filaments.
| Dry | Hydrated | PLA | |
|---|---|---|---|
| Thickness (μm) | 209.6 ± 33.0 | 303.9 ± 65.3 | 206.0 |
| Breaking load (N) | 1.68 ± 0.37 | 0.31 ± 0.10 | 5.75 |
| Extension at break (mm) | 1.35 ± 0.56 | 2.13 ± 0.61 | 10.91 |
| Young’s modulus (N mm−1) | 3.43 ± 1.09 | 0.21 ± 0.07 | 1.68 |
Note: The data for the PLA multifilaments have been obtained and recalculated from [29] for comparison.
Collagen filaments were stronger and stiffer in the dry state compared to that in the hydrated condition, making the filaments easy to knit under dry conditions. Figure 3(C) illustrates the representative loadextension curves of the dry and hydrated 2-ply collagen filaments, and their tensile properties are listed in table 1. The dry collagen filaments had a significantly greater breaking force (1.68 ± 0.37 N) than the hydrated ones (0.31 ± 0.10 N).
The hydrated collagen filaments presented better flexibility and extensibility than dry filaments. The Young’s modulus of the hydrated filament (0.21± 0.07 N mm−1) was significantly lower than that of the dry filament (3.43 ± 1.09 N mm−1). The hydrated filament had a greater extension at break (2.13 ± 0.61mm) than the dry one (1.35 ± 0.56 mm).
The swelling ratios of the crosslinked and uncrosslinked collagen filaments are shown in figure 3(D). The swelling behavior of the uncrosslinked collagen filaments was significantly greater and more rapid than for the crosslinked filaments. This verifies that the EDC-NHS crosslinking agent was able to crosslink the structure and stabilize it against liquid imbibition in the fluid environment.
After 1 h of immersion, the swelling ratio of the uncrosslinked collagen filaments was approximately two times that of the cross-linked sample and they continued to swell during the 24 h exposure to PBS buffer solution.
In contrast, the swelling ratio of the crosslinked collagen filaments remained constant after 0.5 h immersion, indicating the crosslinked collagen filaments remain stable in PBS solution after the first 30 min.
As shown in table 1, the thickness of the PLA yarns used to fabricate the reference grafts was similar to the dry collagen filaments, but the collagen filaments had a significantly lower maximum load and extension at maximum load compared to the PLA yarns. The Young’s modulus of the PLA yarn lay in between the values for the dry and hydrated collagen filaments, indicating that the PLA yarn was stiffer than the hydrated collagen filament, but more flexible than the dry collagen filament. The tensile properties of the PLA yarns were obtained from one of the studies in our lab by Tang [29].
3.2. Morphology and dimension of the collagen grafts
Two pure collagen graft prototypes were successfully engineered. The single-layer collagen grafts were weft-knitted directly into a tubular structure to serve as the backbone of the graft (figures 1(B), 4(a)). The bilayer grafts were engineered by adding a second layer of electrospun collagen nanofibers on the internal surface of the knitted tubes, as shown in figure 4(c). The single-layer knitted collagen graft is referred to as COL-K, and the bilayer graft referred to as COL-KE.
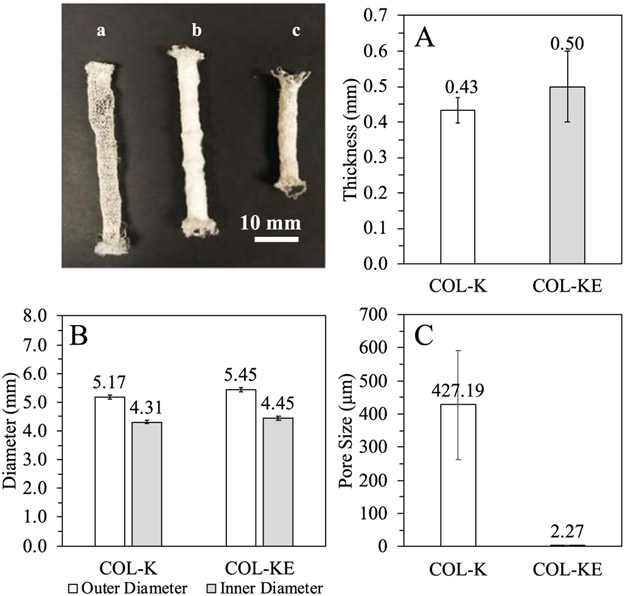
Figure 4.
Dimensions of the collagen prototype grafts. (a) The single-layer knitted collagen graft (COL-K); (b) the bilayer collagen graft with the electrospun layer on the outer surface of the knitted layer; (c) the bilayer knitted and electrospun collagen graft (COL-KE). (A) Wall thickness of the prototype grafts; (B) Outer and inner diameter of the grafts; (C) pore size of the grafts.
The wall thickness of the grafts was measured and compared (figure 4(A)). The thickness of the wall of the COL-K graft was 0.43 ± 0.09 mm, and that of the COL-KE graft was 0.50 ± 0.14 mm, between which there was no significant difference.
Based on measurements of the flat width and thickness, the outer diameter and inner diameter of the grafts were determined experimentally as shown in figure 4(B). The outer diameter of COL-K was 5.17 ± 0.17 mm, and the inner diameter was 4.30 ± 0.17 mm. The outer diameter of COL-KE was 5.45 ± 0.19 mm, and the inner diameter was 4.45 ±0.19 mm. There was no significant difference between the thickness and diameter of the single layerand bilayercollagengrafts.
Figure 5 shows the microscopic morphology of the single layer knitted collagen graft (COL-K), the bilayer knitted-electrospun collagen graft (COL-KE), and the single layer knitted PLA reference graft (PLA-K). The average pore size of the COL-K and COL-KE grafts was found to be significantly different, with the former measuring 427 ± 164 μm and the latter 2.27 ± 0.94 μm (figure 4(C)).
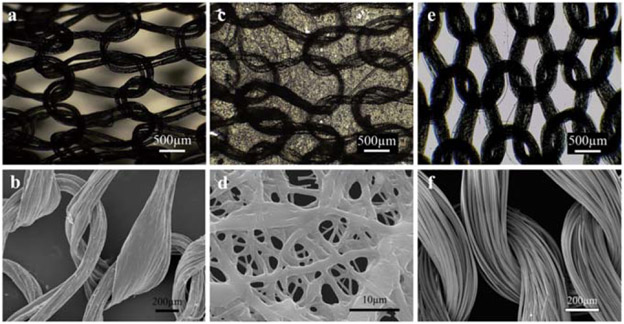
Figure 5.
Microscopic images of COL-K (a) and (b), COL-KE (c) and (d), and PLA-K (e) and (f) (scale bar: a, c, e = 500 μm; b, f = 200 μm; d = 10 μm).
The morphology and thickness of the collagen fibers electrospun from the 1:1 PBS: ethanol solution was confirmed by SEM as shown in figure 5(d). The electrospun collagen fibers were randomly aligned with some parts fused together and the average fiber thickness was 0.94 ± 0.81 μm.
3.3. Bursting strength of the collagen grafts
Bursting strength is one of the most critical mechanical properties of vascular grafts. According to ISO 7198: 2016, the probe bursting test determines the strength of an area on the wall of the graft to withstand a certain amount of force. As illustrated in figure 6(A), the bursting strength was reported in megapascals (MPa), which is calculated by dividing the maximum load (N) by the contact area (cm2) of the sample and the probe.
Figure 6.
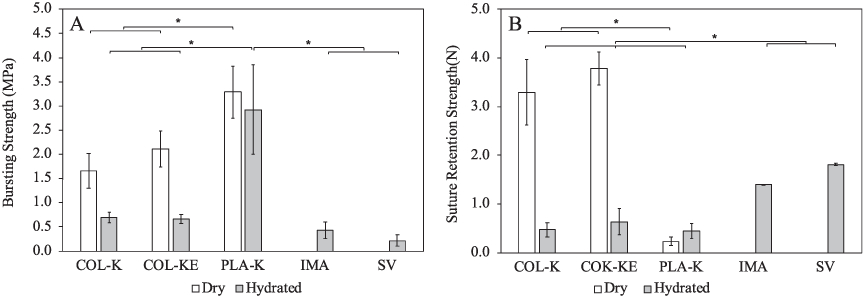
(A) Bursting strength and (B) suture retention strength of the prototype grafts compared with the hydrated human internal mammary artery (IMA) and the human saphenous vein (SV) and the PLA-K reference graft. The data of the reference autologous grafts were obtained from [30]. * indicates statistical significant difference, p < 0.05, n = 3.
The bursting strength of the dry COL-K prototype graft was 1.66 ±0.36 MPa and, and that of the dry COL-KE graft was marginally greater at 2.11 ± 0.37 MPa, between which there was no significant difference. In the hydrated state, the COL-K and COL-KE grafts have significantly lower bursting strengths of 0.70 ± 0.10 MPa and 0.66 ± 0.09 MPa, respectively. There was no statistical significance between these two values (p < 0.05). Under both dry and hydrated conditions, the bursting strength of both COL-K and COL-KE are significantly greater than those of the human internal mammary artery (IMA) (0.42 ± 0.17 MPa) [30], the human saphenous vein (SV) (0.21 ± 0.12 MPa) [30] and the typical requirement for TEVGs (0.26 MPa) [31], but significantly lower than that of the PLA-K reference graft (dry: 3.29 ± 0.36 MPa; hydrated 2.92 ± 0.10 MPa).
3.4. Suture retention strength
Suture retention strength is another mechanical property of vital importance for the successful implantation of a vascular graft. As shown in figure 6(B), the suture retention strength was expressed in terms of the maximum load (N) and is compared to that of the human IMA and the human SV [30], which are commonly used as autologous bypass grafts.
The suture retention strength of the dry COL-K graft was 3.30 ± 1.35 N and that of the dry COL-KE graft was 3.79 ± 0.67 N. There was no statistically significant difference between these two values. Both of them were greater than those of the human IMA (1.40 ± 0.01 N) and the human SV (1.81 ± 0.02 N) [30]. However, the suture retention strength of the hydrated samples was significantly lower (p < 0.05). The hydrated COL-K graft measurement was 0.47 ± 0.26 N, and the hydrated COL-KE graft was 0.64 ± 0.53 N. No significant difference was found between them. While the suture retention strengths of the hydrated collagen prototypes were weaker than the autologous grafts, their values were similar to the hydrated PLA-K grafts, which was 0.44 ± 0.26 N.
3.5. Radial dynamic compliance
Compliance is the most consequential mechanical property associated with the success of vascular grafts. It refers to the diameter change of the vascular graft in response to changes in intraluminal pressure. By utilizing a circular knitting technique, we rendered the graft with compliance approaching that of native blood vessels, as shown in table 2 and figure 7(A). Under normal blood pressure between 80 and 120 mmHg, the single layer knitted collagen grafts (COL-K) had a compliance of 2.81 ± 0.28%/ 100 mmHg, and the bilayer knitted/electrospun collagen grafts had 3.06 ± 0.96%/100 mmHg. There was no significant difference between these two values. Both of them fell in the range of the compliance of the SV (0.7–3.7%/100 mmHg) [19]. The single layer knitted PLA grafts (PLA-K) showed significantly higher compliance of 5.42 ± 0.17%/100 mmHg, which was similar to that of a human artery (4.7–17.0%/100 mmHg) [19].
Table 2.
Radial dynamic compliance of the prototype vascular grafts.
| 50–90 mmHg | 80–120 mmHg | 110–150 mmHg | |
|---|---|---|---|
| COL-K | 3.17 ± 0.68 | 2.81 ± 0.28 | 2.96 ± 0.55 |
| COL-KE | 2.66 ± 0.51 | 3.06 ± 0.96 | 2.63 ± 0.45 |
| PLA-K | 4.24 ± 0.26 | 5.42 ± 0.17 | 5.87 ± 0.69 |
Figure 7.

(A) Radial dynamic compliance and (B) β stiffness index of the prototype grafts. * indicates statistical significant difference, p < 0.05, n = 3.
As shown in figure 7(B) and table 3, the β stiffness indices of the prototype grafts were also calculated. Compared to the stiffness of the human SV (45.0 ± 12.5) under a normotensive pressure range [28], both COL-K (43.35 ± 4.95) and COL-KE (43.82 ± 17.06) showed similar stiffness. Along with the increase of pressure, the stiffness of all the singlelayered knitted grafts (COL-K and PLA-K) decreased significantly. However, with the electrospun layer added, the bilayer graft (COL-KE) showed no significant increase in stiffness when the intraluminal pressure increased from normotensive to hypertensive. As expected, the stiffness was inversely related to the compliance.
Table 3.
β stiffness index of the prototype vascular grafts.
| 50–90 mmHg | 80–120 mmHg | 110–150 mmHg | |
|---|---|---|---|
| COL-K | 58.10 ± 13.20 | 43.35 ± 4.95 | 32.10 ± 6.32 |
| COL-KE | 54.36 ± 10.32 | 43.82 ± 17.06 | 49.23 ± 8.81 |
| PLA-K | 39.96 ± 5.28 | 20.82 ± 5.28 | 15.10 ± 1.85 |
3.6. Cell adhesion and proliferation on collagen and PLA scaffolds
The microscopic structure and morphology of the single-layer collagen (COL-K), bilayer collagen (COL-KE) and PLA (PLA-K) scaffolds are shown in figures 8(a)-(c), respectively. The COL-K and PLA-K samples shared a similar knitting structure and size, but the surface morphology was different. The collagen filaments had a rough and thicker surface, while PLA scaffolds had a smoother and thinner surface with a greater surface area due to the feature of multifilaments. The bilayer COL-KE prototype presented a different morphology than the other two, with randomly oriented nanofibers and a significantly smaller average pore size.
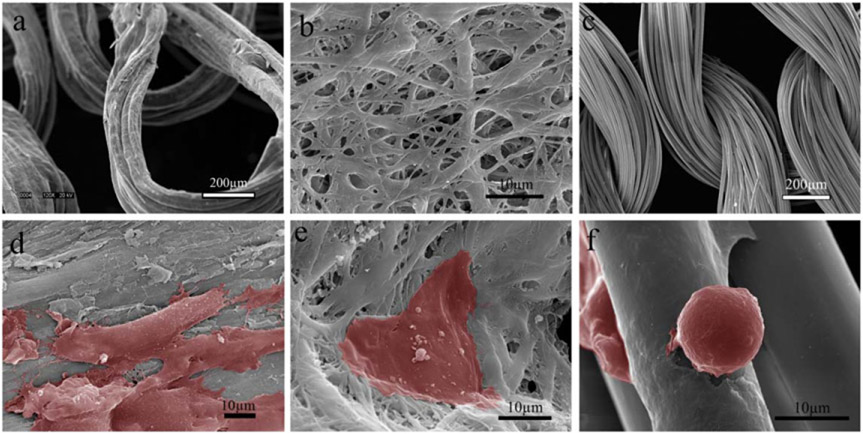
Figure 8.
Representative microscopic images of the collagen and PLA substrates and HUVECs attached at Day 3. (a) COL-K shares a similar structure with (c) PLA-K, but PLA-K has a smoother surface and larger surface area compared to COL-K due to the multifilament feature; (b) COL-KE, on the other hand, had a different morphology. A larger number of well-attached HUVECs grew on the collagen substrate (d), (e) compared to the PLA scaffold (f). Cells have been highlighted in red.
It is evident in figures 8(d)-(f) that more cells were spindle-shaped and well-attached to the collagen substrates as shown in (d) and (e), compared to those on the PLA substrate as in (f). Besides, the intracytoplasmic granules were visible on the cell surface in (d) and (e), which suggests the cells there were alive and active on the collagen substrate. On the contrary, the individual granules were not visible on the PLA scaffold at a similar magnification, indicating that the cells were not as active as on the collagen scaffolds.
The metabolic activities of HUVECs on the scaffold were measured by alamarBlue® assay, and the cell number was quantified by comparing the reduction of alamarBlue® reagent to the standard curve created with the known numbers of cells. In figure 9(A), the COL-K scaffolds showed significantly superior initial cell attachment and overall cell growth compared to the COL-KE and PLA-K grafts (p < 0.05). This agrees with the SEM observations. COL-KE showed the least initial cell attachment, but the most significant fold change in cell numbers over 9 d of cell culture, indicating active and rapid cell proliferation. As illustrated in figure 9(B), the cells expanded 4.5 fold on the COK-KE grafts from day 1 to day 9, which was significantly faster than that on the COL-K and PLA-K grafts (p < 0.05).
Figure 9.

Cell attachment and proliferation determined by alamarBlue® assay over 9 days. *Indicates significant difference, p < 0.05, n = 3.
4. Discussion
Tissue engineering has introduced a new path for vascular graft research and development. However, it is still a challenge to fabricate a TEVG with both sufficient mechanical properties and advanced biological performance in the same structure. Collagen is the predominant protein in the ECM of blood vessels that provides mechanical support. It has long been considered an advanced material for cell recruitment and tissue reconstruction, but until recently it has been restricted in use because of its inferior mechanical properties.
In this study, we have successfully engineered a bilayer collagen vascular graft by knitting innovative collagen filaments into a tubular structure and adding collagen nanofibers to the luminal surface. The engineered collagen vascular graft had comparable mechanical properties to native blood vessels and superior biological performance compared to synthetic grafts.
The novel collagen grafts described in this study have similar components and the multilayer structure as the ECM of native blood vessels. The exterior layer of knitted collagen filaments is composed of type I collagen, the predominant protein in the blood vessel wall, to provide mechanical support similar to the ECM in native blood vessels. It also promotes smooth muscle cell infiltration and proliferation by providing a three-dimensional porous structure [32] and encourages vascular remodeling by interacting with fibroblasts in the tunica adventitia [33]. The interior layer of electrospun collagen nanofibers provides the ECs with a bioactive substrate to attach to and interact with [34]. It resembles the morphology of fibrillar collagen and provides exposure to the preferred biological motifs so as to encourage EC adhesion and proliferation [35].
The knitted structure has long been utilized in fabricating vascular devices to provide the structure with good compliance. Matsumura et al [36] reported the composite vascular scaffold of knitted PGA fibers and copolymer P(LA/CL) sponge with the P(GA/CL) reinforcement which had shown long-term patency in a canine inferior vena cava model. Xie et al [9] also reported the mechanical properties of a bilayer tubular vascular graft made from a knitted external PLA layer and internal electrospun PLCL layer. However, as reported in our study, the cells were only loosely attached to the PLA fibers which adversely affected the formation of an intact endothelium on the PLA surface. Moreover, PLA has been reported to have the issue of long degradation time and non-degradable residual fragments that will last for years [37].
The utilization of collagen in this study makes it possible to offer better biological performance. It also degrades within a shorter period of time than the PLA yarn that allows for tissue remodelling.
As mentioned in the introduction, most of the collagen grafts have been made by freeze-drying [6], molding [19], electrospinning [18], electrocompacting [38] or bioprinting [17], which provided inferior mechanical properties that limited practical handling. A recent study by Nguyen et al [38] reported the successful fabrication of an electrocompacted collagen vascular graft showing improved mechanical properties. Furthermore, we combined this advanced material with knitting technology to improve the strength and compliance of the collagen graft.
To the best of our knowledge, the bilayer collagen graft described in this study is the first knitted collagen graft with both sufficient mechanical properties and superior biological performance. The single jersey knit design has been widely used in fabrics requiring good stretch and recovery performance. The availability of novel collagen filaments makes it possible to fabricate pure collagen grafts using a knitted structure. Fabricating the backbone of the vascular grafts by knitting provides the graft with good compliance. Circular weft knitting technology allows customization of the size of the vascular graft by changing the gauge and the number of needles, and by controlling the tension of the yarn feed and the takedown of the tubular fabric.
The collagen filaments behave significantly differently in the dry and hydrated states. Under dry conditions, the collagen filaments are thinner, stronger and stiffer, enabling the yarn to be easily processed by the circular knitting technology. At the same time, the hydrated collagen filaments had a greater extension at break and flexibility, which provides the collagen grafts with superior compliance.
While the tubular knitted backbone provides the main mechanical support for the graft, the large pores on the surface, which were more than ten times larger than ECs, adversely affected reendothelization and remodeling of the tissue in the graft wall. By adding a luminal layer of electrospun collagen nanofibers, with a pore size significantly smaller than ECs, it facilitated the migration of a monolayer of ECs and limited blood leakage. In addition, the electrospun collagen nanofibers share similar size, fibrous morphology and biological motifs to native collagen fibers and thus encourage EC adhesion, migration and proliferation by eliciting collagen recognition receptors such as integrin α1β1 and α2β1 and signaling pathways [28, 34, 39]. Re-endothelialization is of vital importance so as to preclude the activation of blood coagulation and stimulation of intimal hyperplasia. With a pre-cultured, intact endothelium on the luminal surface of the collagen grafts, the activation of the coagulation cascade by collagen will be significantly reduced to the normal level [31]. An intact endothelium is also important in regulating homeostasis to promote the remodeling of the blood vessel [16].
One of the main reasons for restenosis of small diameter vascular grafts after implantation is the mismatch in mechanical properties between the graft and the adjoining native vessel. In this study, the knitted collagen grafts were designed and fabricated to provide comparable mechanical properties to the native blood vessels. The thickness of the bilayer collagen graft prototype was 0.50 ± 0.14 mm, close to that of the normal human coronary artery wall, which is on average 0.75 ± 0.17mm [40]. The diameter of the human coronary arteries varies from one location to another and from person to person. The left coronary artery (LCA) has been found to measure 4.5 ± 0.5 mm, the left anterior descending (LAD) artery 3.7 ± 0.4 mm, and the distal LAD artery 1.9 ± 0.4 mm. The diameter of the right coronary artery varies from 2.8 ± 0.5 to 3.9 ± 0.6 mm, and for the left circumflex artery varies between 3.4 ± 0.5 and 4.2 ± 0.6 mm [41]. The bilayer collagen grafts knitted in this study have an outer diameter of 5.45 ± 0.19 mm and an inner diameter of 4.45 ± 0.19 mm, similar to the LCA and are recommended for use in the reconstruction of coronary arteries of the same size. However, with the use of circular weft knitting technology, the diameter of the final tubular product is adjustable by controlling the gauge size, the number of needles, the pulldown rate and tension on the knitted fabric, making it possible to knit the grafts to different sizes to fulfill various needs and applications.
Bursting strength is one of the most important mechanical properties of small diameter vascular grafts. The minimum performance requirement for the bursting strength is 0.26 MPa [42]. Autologous grafts such as the human IMA and SV, have long been utilized in bypass surgery with bursting strength of 0.43 ± 0.17 MPa and 0.21 ± 0.12 MPa, respectively [30]. In this study, both the single and bilayer prototypes had greater bursting strength than that of the autologous vessels under both dry and hydrated conditions. Since autologous grafts are commonly and successfully implanted as coronary artery bypass grafts, the collagen prototype grafts developed in this study should be safe and effective in withholding the systolic pressure at the time of implantation. Another degradation study in our lab [43] has proved that the crosslinked collagen filaments were stable in PBS at 37 °C without losing strength for up to 6 weeks, which is sufficient time for crucial tissue reconstruction [36].
Suture retention strength is crucial to the success of an implanted vascular graft. In the case of coronary artery bypass surgery, one end of the graft is anastomosed to the aorta, and the other end is anastomosed to the coronary artery. This requires the graft to have sufficient suture retention strength to withstand the pulsatile tension at the anastomosis. The generally accepted minimum requirement for the suture retention strength for a small diameter vascular graft is 2N [28]. Although the collagen grafts developed in this study have more than adequate suture retention strength under dry conditions, when hydrated, the suture retention strength decreases significantly. Such results indicate that the collagen grafts experience a significant loss of suture retention strength due to hydration, which is probably attributed to the significant loss of tensile strength, the increase in the flexibility of the collagen filaments and/or the decrease of friction that facilitated slippage of the yarn in the knitted loop. Given this significant loss in suture retention strength, further studies are proposed to secure the edge by modifying the structure. Instead of leaving a cut edge with free ends, the structure on the edge can be modified by sewing a reinforcing edge seam on both ends of the graft to prevent the structure from unraveling.
Compliance is one of the most pivotal properties of vascular grafts. The compliance mismatch between grafts and native blood vessels can lead to stress concentration at the anastomosis, disturbance to blood flow [44], and low wall shear stress at the endothelium [45], all of which can stimulate intimal hyperplasia [46]. These conditions are considered deleterious to long-term patency. Human arteries typically have compliance within the range of 4.7–17.0%/100 mmHg, and that of the saphenous veins is typically0.7–3.7%/100 mmHg [19, 47, 48]. At the anastomosis, the closer the compliance of the graft is to that of the native blood vessel, the greater the chance of extending long-term patency [45]. However, the compliance of commercially available synthetic grafts can only reach the range of 0.2–1.9%/100 mmHg [19]. In this study, graft prototypes with compliance similar to native blood vessels were fabricated by utilizing circular knitting technology. The single-layer and bilayer collagen grafts achieved compliances comparable to the human saphenous vein. Further study is underway to improve the compliance to the level of the human IMA.
Cell attachment and proliferation of HUVECs on the collagen and PLA substrates were quantified using the alamarBlue® assay. Consistent with previous studies [49], the grafts made from collagen filaments had significantly superior initial cell attachment compared to the PLA grafts. The likely reason was the collagenbinding receptors on the cell membrane, such as the integrins, recognize the collagen surface thus regulating cell adhesion [50, 51]. On the contrary, the cells do not have specific receptors that can recognize synthetic polymers like PLA, and thus the cells bind to such substrates through an indirect and non-specific mechanism that maybe less effective.
The SEM images showed consistently that the HUVECs appeared to be flat and well attached to the collagen surfaces. On the collagen substrate, there were intracytoplasmic granules observed on the cell surface and intercellular connections between cells, indicating that they are viable (figures 8(d), (e)). On the contrary, the cells on the PLA surface appeared to be round and loosely attached, with no interaction with other cells being observed. This suggests that the cells on the PLA surface were less likely to be alive and functioning.
Although the collagen scaffold showed significantly better cell attachment and overall growth over 9 d, the proportional increase (fold change) in cell numbers was significantly lower than the other two groups. This was possibly ascribed to the high number of HUVECs that attached initially at day 1 to the scaffold, and subsequently contact inhibition suppressed further cell growth of the HUVEC monolayer.
It was also found that the initial number of cells attached to the knitted collagen filaments (COL-K) was six-fold as much as that on the electrospun collagen nanofibers (COL-KE). However, the fold change in cell numbers was significantly faster on the surface of the electrospun collagen nanofibers compared to knitted collagen filaments. This agrees with the findings of Kishore et al [52] who demonstrated that aligned collagen encourages cell adhesion compared to randomly oriented collagen, but cell proliferation is promoted by randomly oriented collagen. A possible reason for the promising proliferation rate on the electrospun surface was that the smaller average pore size of 2.27 ± 0.94 μm on the electrospun surface may facilitate EC migration and intracellular interaction [53, 54]. However, the small pore size of electrospinning layer also decreases the water permeability, which can be one of the reasons that lead to the poor initial cell attachment on the bilayer scaffold.
Although HUVECs were observed to have poor attachment and inactive morphology on the PLA-K reference grafts, the alamarBlue® assay indicated that the cell growth on the PLA substrate was not as bad as predicted from the SEM images. This can be accredited to the multifilament feature of the PLA yarns. A single PLA multifilament yarn was composed of 92 finer filaments, which provided the PLA filaments with a greater surface area and a three-dimensional structure. This enabled the PLA samples to trap more cells in between the filaments instead of only on the external surface, resulting in a considerable number of cells being detected on days 1, 3 and 9.
5. Conclusion and future work
In this study, pure collagen grafts have been successfully fabricated, for the first time, using both circular knitting and electrospinning technologies. The prototype grafts have sufficient bursting strength, suture retention strength and radial dynamic compliance to provide dimensional stability and mechanical support during the post-implantation period.
The prototype collagen grafts had comparable bursting strength to that of the human IMA and the human SV, which are currently considered the ‘golden standard’ for autologous coronary artery bypass surgery. The suture retention strength of the collagen grafts at the anastomotic site 2mm away from the edge was strong enough under dry conditions, but when hydrated, the strength decreased significantly making it hard for the graft to withstand the pulsatile tension at the anastomosis. To secure the anastomosis, the edges need to be reinforced by sewing the bound seam to prevent the structure from unraveling. The compliance and stiffness of the collagen grafts were similar to those of the human saphenous vein, and the compliance of the PLA knitted graft was close to that of human arteries.
Cell attachment to the knitted collagen grafts was significantly superior to that for the PLA grafts, which affirms that collagen promotes cell attachment and growth better than PLA. It was also demonstrated that the improved rate of cell proliferation occurred on the bilayer knitted/electrospun collagen graft. This is believed to be due to the smaller average pore size that promotes the migration and proliferation of ECs.
This initial development was concentrated on the overall physical properties and biological performance of the bilayer collagen graft, and in future studies, the security and integrity will be investigated further. To build on the progress achieved in this study, the security at the anastomosis will be further reinforced by sewing an edge seam.
Regarding the biological performance, although we have demonstrated that the bilayer graft fabricated from collagen filaments and nanofibers is able to promote EC attachment, which is due to the collagenbinding receptors on ECs, platelets are also known to respond to collagen through the activity of collagenbinding receptors, such as integrin α2β1 and GPVI/FCRγ complex [55]. Thus, the hemocompatibility of the collagen graft is of great interest and the improvement of the anticoagulation properties also needs to be studied.
The rate of degradation of the collagen also needs to be tracked so as to predict the behavior of the graft after implantation. A fluorescence assay to validate the proliferation and tight-junction formation between ECs and an in vivo evaluation in an animal model are also planned.
Acknowledgments
This work was supported by funding from Donghua University, Shanghai, China. We also gratefully acknowledge the financial support from China Scholarship Council, Beijing, China. We appreciate Dr Xiangwu Zhang, Dr Stephen Michielsen and Dr Zhen Gu for providing their laboratory facilities and equipment. And we appreciate Dr Ke Cheng, Dr Duncan X. Lascelles and Morika Williams for donating the rat tails. The technical support of William Barnes and Eric Lawrence in the knitting lab is appreciated. We are also grateful for the help from Dr Lu Wang and Dr Laijun Liu in the compliance test at Donghua University College of Textiles, Shanghai, China.
References
- [1].WHO 2018. Cardiovascular Dis. (https://who.int/newsroom/fact-sheets/detail/cardiovascular-diseases-(cvds))
- [2].Ahmed M, George H and Seifalian AM 2014. The performance of a small-caliber graft for vascular reconstructions in a senescent sheep model Biomaterials 35 9033–40 [DOI] [PubMed] [Google Scholar]
- [3].Habib RH et al. 2015. CABG Versus PCI J. Am. Coll. Cardiol 66 1417–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yahagi K. et al. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat. Rev. Cardiol. 2016;13:79. doi: 10.1038/nrcardio.2015.164. [DOI] [PubMed] [Google Scholar]
- [5].Pashneh-Tala S, MacNeil S and Claeyssens F 2015. The tissue-engineered vascular graft-past, present, and future TissueEng. B 22 68–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Koens MJW et al. 2010. Controlled fabrication of triple layered and molecularly defined collagen/elastin vascular grafts resembling the native blood vessel Acta Biomater. 6 4666–74 [DOI] [PubMed] [Google Scholar]
- [7].Hibino N et al. 2015. The innate immune system contributes to tissue-engineered vascular graft performance FASEB J. 29 2431–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ju Y M, Choi J S, Atala A, Yoo JJ and Lee SJ 2010. Bilayered scaffold for engineering cellularized blood vessels Biomaterials 314313–21 [DOI] [PubMed] [Google Scholar]
- [9].Xie Y, Guan Y, Kim S and King MW 2016. The mechanical performance of weft-knitted/electrospun bilayer small diameter vascular prostheses J. Mech. Behav. Biomed. Mater 61 410–8 [DOI] [PubMed] [Google Scholar]
- [10].Bertram U. et al. Vascular tissue engineering: effects of integrating collagen into a PCL based nanofiber material. BiomedRes. Int. 2017;2017:9616939. doi: 10.1155/2017/9616939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Henry JJD, Yu J, Wang A, Lee R, Fang J and Li S 2017. Engineering the mechanical and biological properties of nanofibrous vascular grafts for in situ vascular tissue engineering Biofabrication 9 035007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Radke D. et al. Tissue engineering at the blood-contacting surface: a review of challenges and strategies in vascular graft development. Adv. Healthcare Mater. 2018;7:1701461. doi: 10.1002/adhm.201701461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Barrientos IJH et al. 2017. Electrospun collagen-based nanofibres: a sustainable material for improved antibiotic utilisation in tissue engineering applications Int. J. Pharm 531 67–79 [DOI] [PubMed] [Google Scholar]
- [14].Catto V, Farè S, Freddi Gand Tanzi MC 2014. Vascular tissue engineering: recent advances in small diameter blood vessel regeneration ISRN VascularMed. 2014 923030 [Google Scholar]
- [15].Brown JH, Das P, DiVito MD, Ivancic D, Tan LP and Wertheim JA 2018. Nanofibrous PLGA electrospun scaffolds modified with type I collagen influence hepatocyte function and support viability in vitro Acta Biomater. 73 217–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Manon-Jensen T, Kjeld NG and Karsdal MA 2016. Collagenmediated hemostasis J. Thrombosis Haemostasis 14 438–48 [DOI] [PubMed] [Google Scholar]
- [17].Norotte C, Marga FS, Niklason LE and Forgacs G 2009. Scaffold-free vascular tissue engineering using bioprinting Biomaterials 30 5910–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].HaghjooyJavanmard S, Anari J, Zargar Kharazi A and Vatankhah E 2016. In vitro hemocompatibility and cytocompatibility of a three-layered vascular scaffold fabricated by sequential electrospinning of PCL, collagen, and PLLA nanofibers J. Biomater. Appl 31 438–49 [DOI] [PubMed] [Google Scholar]
- [19].Kumar VA, Caves JM, Haller CA, Dai E, Liu L, Grainger S and Chaikof EL 2013. Acellular vascular grafts generated from collagen and elastin analogs Acta Biomater. 9 8067–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Younesi M, Islam A, Kishore V, Anderson JM and Akkus O 2014. Tenogenic induction of human MSCs by anisotropically aligned collagen biotextiles Adv. Funct. Mater 24 5762–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kishore V et al. 2012. In vivo response to electrochemically aligned collagen bioscaffolds J. Biomed. Mater. Res. B 100 400–8 [DOI] [PubMed] [Google Scholar]
- [22].Montoro SR, Medeiros SF and Alves GM 2014. Nanostructured hydrogels Nanostructured Polymer Blends ed Thomas S, Shanks R and Chandrasekharakurup S (Oxford: William Andrew Publishing; ) ch 10 pp 325–55 [Google Scholar]
- [23].Ma L, Gao C, Mao Z, Zhou J, Shen J, Hu X and Han C 2003. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering Biomaterials 24 4833–41 [DOI] [PubMed] [Google Scholar]
- [24].Dong B, Arnoult O, Smith ME and Wnek GE 2009. Electrospinning of collagen nanofiber scaffolds from benign solvents Macromol. Rapid Commun. 30 539–42 [DOI] [PubMed] [Google Scholar]
- [25].Yang X. et al. Mechanical and biocompatibility performance of bicomponent polyester/silk fibroin smalldiameter arterial prostheses. J. Appl. Biomater. Funct. Mater. 2015;13:201. doi: 10.5301/jabfm.5000225. [DOI] [PubMed] [Google Scholar]
- [26].Kim SY, Yang HS, Lee YW, Choe YBand Ahn KJ 2015. Evaluation of the beta stiffness index and carotid intima–media thickness in asian patients with psoriasis Angiology 66 889–95 [DOI] [PubMed] [Google Scholar]
- [27].Soletti L et al. 2010. A bilayered elastomeric scaffold for tissue engineering of small diameter vascular grafts Acta Biomater. 6 110–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang Y et al. 2017. A compliant and biomimetic three-layered vascular graft for small blood vessels (vol 9, 025010, 2017) Biofabrication 9 029501. [DOI] [PubMed] [Google Scholar]
- [29].Tang X. Master’s Thesis. North Carolina State University; 2016. The mechanical performance of 3D warp knitted spacer scaffold fabrics using uniaxial and biaxial measurements. [Google Scholar]
- [30].Konig G et al. 2009. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery Biomaterials 30 1542–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hasan A, Memic A, Annabi N, Hossain M, Paul A, Dokmeci MR, Dehghani F and Khademhosseini A 2014. Electrospun scaffolds for tissue engineering of vascular grafts Acta Biomater. 10 11–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yan J et al. 2018. Preparation of recombinant human-like collagen/fibroin scaffold and its promoting effect on vascular cells biocompatibility J. Bioact. Compat. Polym 33 416–25 [Google Scholar]
- [33].Rouillard AD and Holmes JW 2012. Mechanical regulation of fibroblast migration and collagen remodelling in healing myocardial infarcts J. Physiol 590 4585–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Johanna J et al. 2004. Integrin-mediated cell adhesion to type I collagen fibrils J. Biol. Chem 279 31956–63 [DOI] [PubMed] [Google Scholar]
- [35].Davidenko N et al. 2016. Evaluation of cell binding to collagen and gelatin: a study of the effect of 2D and 3D architecture and surface chemistry J. Mater. Sci., Mater. Med 27 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Matsumura G. et al. Long-term results of cell-free biodegradable scaffolds for in situ tissue-engineering vasculature: in a canine inferior vena cava model. PLoS One. 2012;7:e35760. doi: 10.1371/journal.pone.0035760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Farah S, Anderson DG and Langer R 2016. Physical and mechanical properties of PLA, and their functions in widespread applications—a comprehensive review Adv. Drug Deliv. Rev 107 367–92 [DOI] [PubMed] [Google Scholar]
- [38].Nguyen T, Shojaee M, Bashur CA and Kishore V 2018. Electrochemical fabrication of a biomimetic elastin-containing bi-layered scaffold for vascular tissue engineering Biofabrication 11 015007. [DOI] [PubMed] [Google Scholar]
- [39].Koohestani F, Braundmeier AG, Mahdian A, Seo J, Bi JJ and Nowak RA 2013. Extracellular matrix collagen alters cell proliferation and cell cycle progression of human uterine leiomyoma smooth muscle cells PLoS One 8 e75844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fayad ZA et al. 2000. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging Circulation 102 506–10 [DOI] [PubMed] [Google Scholar]
- [41].Dodge JT, Brown BG, Bolson EL and Dodge HT 1992. Lumen diameter of normal human coronary arteries. influence of age, sex, anatomicvariation, and left ventricular hypertrophy or dilation Circulation 86 232–46 [DOI] [PubMed] [Google Scholar]
- [42].Hasan A et al. 2014. Electrospun scaffolds for tissue engineering of vascular grafts Acta Biomater. 10 11–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Xie Y. Doctoral dissertation. North Carolina State University; 2018. An advanced collagen yarn based textile patch for rotator cuff tendon repair. [Google Scholar]
- [44].Goins A, Webb AR and Allen JB 2019. Multi-layer approaches to scaffold-based small diameter vessel engineering: a review Mater. Sci. Eng. C 97 896–912 [DOI] [PubMed] [Google Scholar]
- [45].Nezarati RM, Eifert MB, Dempsey DKand Cosgriff-Hernandez E 2015. Electrospun vascular grafts with improved compliance matching to native vessels J. Biomed. Mater. Res. B 103 313–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].van Uden S et al. 2019. A novel hybrid silk-fibroin/polyurethane three-layered vascular graft: towards in situ tissue-engineered vascular accesses for haemodialysis Biomed. Mater. 14 025007. [DOI] [PubMed] [Google Scholar]
- [47].Kumar VA, Brewster LP, Caves JM and Chaikof EL 2011. Tissue engineering of blood vessels: functional requirements, progress, and future challenges Cardiovascular Eng.Technol. 2 137–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].L’Heureux N et al. 2006. Human tissue-engineered blood vessels for adult arterial revascularization Nat. Med 12 361–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tambe N, Di J, Zhang Z, Bernacki S, El-Shafei A and King MW 2015. Novel genipin-collagen immobilization of polylactic acid (PLA) fibers for use as tissue engineering scaffolds J. Biomed. Mater. Res. B 103 1188–97 [DOI] [PubMed] [Google Scholar]
- [50].Whelan MC and Senger D 2003. Collagen I initiates endothelial cell morphogenesis by inducing actin polymerization through suppression of cyclicAMP and protein kinase a J. Biol. Chem 278 327–34 [DOI] [PubMed] [Google Scholar]
- [51].Lampugnani MG, Resnati M, Dejana E and Marchisio PC 1991. The role of integrins in the maintenance of endothelial monolayer integrity J. Cell Biol 112 479–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kishore V, Bullock W, Sun X, Van Dyke W S and Akkus O 2012. Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads Biomaterials 33 2137–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ju YM, Chio JS, Atala A, Yoo JJ and Lee SJ 2010. Bilayered scaffold for engineering cellularized blood vessels Biomaterialia 31 4313–21 [DOI] [PubMed] [Google Scholar]
- [54].Soliman S et al. 2010. Multiscale three-dimensional scaffolds for soft tissue engineering via multimodal electrospinning Acta Biomater. 6 1227–37 [DOI] [PubMed] [Google Scholar]
- [55].Marjoram RJ. et al. α2β1 integrin, GPVI receptor, and common FcRγ chain on mouse platelets mediate distinct responses to collagen in models of thrombosis. PLoS One. 2014;9:e114035. doi: 10.1371/journal.pone.0114035. [DOI] [PMC free article] [PubMed] [Google Scholar]