Summary
De novo skin regeneration with human keratinocytes amplified in culture is a life-saving procedure for patients with extensive skin loss and chronic wounds. It also provides a valuable platform for gene function and therapeutic assessments. Nevertheless, tissues generated in this manner lack hair follicles that are important for skin homeostasis, barrier function, and repair. In this study, we generated skin tissues with human keratinocytes combined with dermal papilla (DP) cells isolated from mouse whisker hair. For this, cultured keratinocytes and mouse DP cells were mixed at 10:1 ratio, and seeded onto devitalized human dermal matrix derived from surgically discarded human abdominoplasty skin. After one week in submerged culture, the cell/matrix composites were grafted onto the skin wound beds of immunocompromised NSG.SCID mice. Histological analysis of 6-weeks old skin grafts showed that tissues generated with the addition of mDP cells contained Sox2-positive dermal condensates and well-differentiated folliculoid structures that express human keratinocyte markers. These results indicate that cultured mouse DP cells can induce hair follicle neogenesis in the de novo regenerated skin tissues. Our method offers a new experimental system for mechanistic studies of hair follicle morphogenesis and tissue regeneration, and provide insights to solving an important clinical challenge-generation of fully functional skin with a limited source of donor cells.
Keywords: De novo, skin regeneration, keratinocyte, hair follicle, dermal papilla, dermal matrix, devitalized
1. Introduction
Hair follicles are distributed throughout the body surface, and function as a dynamic mini-organ (Fuchs, 2007; Schneider, Schmidt-Ullrich, & Paus, 2009). They are responsible for hair growth, and also house stem cells that give rise to all epidermal lineages (hair follicle, skin, and sebaceous gland) (Horsley et al., 2006; Jaks, Kasper, & Toftgard, 2010; Morris et al., 2004; P. Rompolas & Greco, 2014; Snippert et al., 2010; Taylor, Lehrer, Jensen, Sun, & Lavker, 2000) (Nowak, Polak, Pasolli, & Fuchs, 2008). In addition, hair follicle stem cells can migrate out to the epidermis, and contribute to epidermal renewal and re-epithelialization after wounding (Levy, Lindon, Zheng, Harfe, & Morgan, 2007) (Ito et al., 2005; Park et al., 2017). Although hair follicle bulge stem cells are dispensable for the acute phase of wound re-epithelialization (I. Driskell, Oeztuerk-Winder, Humphreys, & Frye, 2015; Garcin, Ansell, Headon, Paus, & Hardman, 2016; Panteleimon Rompolas, Mesa, & Greco, 2013), their absence delays wound healing (Langton, Herrick, & Headon, 2008; Liang et al., 2012). Moreover, hair follicles control skin resident immune cell function (Adachi et al., 2015), and are, in turn, regulated by immune cells (Ali et al., 2017). Thus, hair follicles are not only critical for skin appendage formation, but also involved in epidermal homeostasis, wound healing, barrier function, and immunity (Fuchs, 2007; Schneider et al., 2009).
Epidermis and hair follicles undergo constant and life-long self-renewal involving cell-autonomous processes and crosstalk with the dermal compartment (Ryan R. Driskell, Clavel, Rendl, & Watt, 2011; Sennett & Rendl, 2012; Toyoshima et al., 2012). The self-renewing capacity of keratinocytes enables de novo skin regeneration in 3-D organoid cultures and in vivo skin grafts (Dajee et al., 2003; Jin, Ke, Hall, & Zhang, 2011; Ratushny, Gober, Hick, Ridky, & Seykora, 2012)(McHeik et al., 2014; Metcalfe & Ferguson, 2007) (Siprashvili et al., 2016) (Hirsch et al., 2017). The latter involves seeding of human keratinocytes (hKC) onto synthetic or devitalized human dermal matrices followed by grafting onto the wound beds prepared in humans or mice. This de novo skin regeneration technique is a life-saving procedure for patients with extensive skin losses caused by severe burn injury and non-healing chronic venous leg ulcer (McHeik et al., 2014; Metcalfe & Ferguson, 2007), as well as those suffering from genetic disorders, most notably recessive and junctional epidermal bullosa (Siprashvili et al., 2016) (Hirsch et al., 2017). Additionally, this technique has provided a valuable experimental platform for gene function and mechanistic studies for oncogenes and tumor suppressors (Dajee et al., 2003; Jin et al., 2011; Ratushny et al., 2012). However, tissues generated in this manner lack hair follicles and the associated processes and functions.
Hair follicle morphogenesis and cycling requires mesenchymal-epithelial interactions (Ryan R. Driskell et al., 2011; Toyoshima et al., 2012). In particular, dermal papilla (DP) cells that exist at the base of hair follicle are essential for the induction of hair morphogenesis during embryogenesis and tissue regeneration during wound healing (Higgins, Chen, Cerise, Jahoda, & Christiano, 2013; Ohyama, Zheng, Paus, & Stenn, 2010; Sennett & Rendl, 2012). These cells are encaged by an extracellular matrix containing basement membrane-like components (Couchman, 1986), and control hair follicle morphogenesis via induction of BMP, SHH and β-catenin/WNT signaling pathways (Enshell-Seijffers, Lindon, Kashiwagi, & Morgan, 2010; Hu et al., 2010; Myung, Takeo, Ito, & Atit, 2013; Rendl, Polak, & Fuchs, 2008; Tsai et al., 2014; Woo, Zhen, & Oro, 2012). In recent years, several groups have used rat, mouse, or human dermal papilla (DP) cells to induce hair morphogenesis in organotypic skin cultures or skin grafts (Higgins et al., 2013; Plikus et al., 2008; Qiao et al., 2009; Robinson, Reynolds, Gharzi, & Jahoda, 2001; Sriwiriyanont, Lynch, McFarland, Supp, & Boyce, 2013; Thangapazham et al., 2014). These techniques are exciting, but their use could be cumbersome owing to the limited availability of human DP cells, the need of feeder cells for culturing mouse keratinocytes, and the variable quality of synthetic and cadaver dermal matrices (Jensen, Driskell, & Watt, 2010) (Segrelles et al., 2011) (Lucich, Rendon, & Valerio, 2018).
In this study, we report modified methods for the isolation and expansion of mouse dermal papilla (mDP) cells, preparation of human dermis from surgically discarded human skins, and de novo skin regeneration on immunodeficient mice. We seeded cultured human keratinocytes and mDP cells together onto the devitalized human dermis, and grafted the skin composite onto NSG.SCID mice. We found that skin grafts generated in this manner developed folliculoid structures consisted of human keratinocytes. Our results demonstrate the feasibility of co-seeding cultured mDP cells and human keratinocytes onto devitalized human dermis for hair follicle and skin regeneration, providing an in vivo experimental system for mechanistic studies of hair follicle morphogenesis and epidermal homeostasis.
2. Materials and Methods
2.1. Human keratinocyte cell culture
All cell cultures were maintained at 37°C in a humidified atmosphere of 5% CO2. Cell culture reagents were purchased from (Invitrogen, Grand Island, NY, USA), and tissue culture supplies were obtained from (Fisher Scientific, Pittsburgh, PA). For human keratinocyte culture, surgically discarded foreskin tissues were obtained from Duke Children’s hospital through an institutionally approved IRB protocol. Tissues were washed in phosphate buffered saline (PBS) containing ampicillin/streptomycin, and then incubated with 1 unit/mL dispase at 4 °C overnight. The epidermal pieces were then peeled and collected using sterile forceps, and digested with 0.25% trypsin for 10–15 minutes at 37°C with gentle shaking. Cell pellets were collected through centrifugation at 500 g force, resuspended, and cultured with keratinocyte serum free (KSF) culture medium. Keratinocytes were passaged at around 60% confluence, and used for skin regeneration at passage 3.
2.2. Mouse dermal papilla (mDP) cell culture
Mouse dermal papilla (mDP) cells were isolated from the whisker hair of 2–3 months old adult CB57/BL6 mice through a modified microdissection procedure adapted from a previous report (Gledhill, Gardner, & Jahoda, 2013). As depicted in (Supplementary Figure S1A–B), this procedure requires a dissection microscope, autoclaved surgical tools and paper towers and sterile petri dishes containing dissection medium (minimal essential medium supplemented with 100 μg/mL penicillin/streptomycin and 0.5 μg/mL amphotericin B). Animals were obtained and euthanized from Duke Animal facility in accordance to protocols approved by institutional Animal Care and Use Committee. After careful cleaning with 0.1 N iodine solution (Fisher Scientific, Pittsburgh, PA) and 70% ethanol swap, the animal was placed under a micro-dissection scope. The upper jaw skin of the mouse was cut with sterile blade along the depicted blue lines (Supplementary Figure S1C), and turned upside down with forceps to expose the dermal side and hair follicle end bulbs (Supplementary Figure S1D). The anagen hair follicles were identified by the densely pigmented end bulbs (Muller-Rover et al., 2001), pulled out with forceps, and then cut off with a blade along the schematic blue line (Supplementary Figure S1E). The detached hair follicles (about 15–20 per jaw) were washed in dish 1, and the end bulbs were cut off the shaft (Supplementary Figure S1F), and transferred with a 10-μL pipettor to a fresh drop of dissection medium placed in dish 2 (Supplementary Figure S1G). The DP condensates were squeezed out of the end bulb connective tissues using two 221/2-gauge dissection needles with one pinching down the DP and the other one tearing apart the matrix of the end bulb (Supplementary Fig. S1H). The released DP condensates were immediately transferred to a 6-cm tissue culture dish containing 1mL attachment medium (dissection medium plus 20% fetal bovine serum), and gently pushed down with a 221/2-gauge needle (Supplementary Figure S1H). The needle was immediately removed, leaving DPs remained at the bottom of the dish. About 20–30 DPs were placed in the same dish. Large DPs may be teared into 2–3 parts with needles, and seeded apart from each other. As soon as all DPs were transferred and settled down on the bottom of the dish, another 4 mL attachment medium was added the dish which was then moved to the tissue culture incubator (37 °C, 5% CO2). About 3–5 days later, cells and DP condensates were detached via incubation with 0.05% trypsin/EDTA. The detached cells and DP condensates were transferred to a 15-mL tube, pelleted by centrifugation at 500 g centrifugal force for 5 minutes, and reseeded to a new dish with 5 mL KGM medium [338 mL low-glucose Dulbecco’s modified Eagle medium (DMEM) mixed with 112 mL F12 medium containing L-glutamine, and supplemented with 5% fetal bovine serum, 0.18 mM adenine, 1x penicillin/streptomycin, 8 mM HEPES, 0.5 μg/mL hydrocortisone, 10−10 M cholera toxin, 10 ng/mL EGF, and 5 μg/mL insulin] for continued incubation at 37°C, 5% CO2. After 1–2 weeks in culture, DP cells growing out of the explant reached approximately 80% confluence, and were detached again via incubation with 0.05% trypsin/EDTA, and further expanded with KGM medium. At the third passage, the majority of DP cells were used for skin grafting and small aliquots were seeded on tissue culture treated cover slips for alkaline phosphatase staining and immunostaining.
2.3. Preparation of split thickness human dermis
Split thickness human dermis was prepared and devitalized using a similar protocol as previously described for cadaver skin (Medalie & Morgan, 1999) (Li & Sen, 2015). For this, surgically discarded human abdominal skin samples were obtained within 4 hours after abdominoplasty from Duke Plastic Surgery Units in accordance with an institutionally approved IRB protocol. The bulky skin samples were laid flat on a sturdy bench with epidermis facing up, and cut with a sterilized Air Dermatome Zimmer (DNOR 80 1185–01). The split-thickness skin pieces were washed 3 times with sterile PBS and then incubated at 37 °C with 2X concentrated PBS supplemented with 2X antibiotic and anti-mycotic solution (100X stock containing 10,000 units/mL of penicillin, 10,000 μg/mL of streptomycin, and 25 μg/mL of Amphotericin B) for about 1 week until the epidermis and dermis can be easily separated with forceps. The dermal pieces were transferred to a sterile 50-mL conical cell culture centrifugation tube containing 20 mL PBS, and then subject to 10 cycles of freeze-thaw by moving the tube between a 2-liter liquid nitrogen tank and a running tap water tank, processes known to induce cell death (Medalie & Morgan, 1999). The devitalized dermal pieces were kept at 4 °C in PBS supplemented with 2X antibiotic/antimycotic solution or frozen at −80 °C freezer.
2.4. Skin grafting on immunodeficient mice
Animal studies are performed in accordance to a protocol approved by institutional Animal Care and Use Committee. The NSG SCID mice (n=3 per group) were purchased from Duke Animal Isolation Facility. For graft preparation, trypsinized mDP cells (1×105) were mixed with cultured human keratinocytes (hKC, 1×106), and then seeded onto about 1 cm2 size split thickness human dermis prepared as described above. The skin composites were kept in tissue culture with KGM medium for about 1 week, and fed with fresh medium every other day.
Animal surgeries were performed on anesthetized animals in a ventilated hood provided by Duke Division of Laboratory Animal Resource (DLAR). After anesthesia, animals were shaved on the back skin, placed on a pile of warm and sterilize towel, and then cleaned with iodine and alcohol swabs. Appropriately 1 cm2 area of mouse back skin was removed with sterile tools. The skin composites were then placed to the wound beds, sutured to the mouse skin, and then dressed with polymoxin B antibiotic ointment, adaptic, telfa pad, and coban wrap (obtained from DLAR). The bandages were removed 2–3 weeks after surgery.
2.5. Histology and immunostaining
Skin grafts were collected from euthanized animals at 4 or 6 weeks after grafting. For H&E staining, tissues were fixed in 10% formalin and embedded in paraffin at Duke Pathology Lab (Durham, NC). For immunostaining, frozen tissue sections and cell cultures seeded on cover slips were fixed in cold methanol, blocked with 10% horse serum, and then incubated with various primary antibodies (Supplementary Table S1). Tissue sections were then detected with secondary antibodies conjugated with Dylight 555 or Alexa 488 dye (ThermoFisher Scientific), and counterstained with Hoechst 33258 (Sigma-Aldrich, St Louis, MO).
2.6. Alkaline phosphatase staining
For alkaline phosphatase (ALP) staining, cells and frozen tissue sections were fixed in 100% methanol for 10–15 minutes, rinsed with PBS followed by Tris-NaCl buffer (0.1 M Tris-HCl, 0.1 M NaCl, pH 9.5), and then incubated in 120 μg/mL 4-nitroblue tetrazolium and 60 μg/mL BCIP (5-bromo-4-chloro-3-indolylphophate) (Sigma) in Tris-NaCl buffer for 15 minutes. The reaction was stopped by washing with double distilled water.
2.7. Real-time RT-PCR
Total RNA samples were isolated from cultured mDP cells and dermal fibroblasts using the Direct-zol RNA Miniprep kit (Zymo Research, Irvine, CA). cDNA was generated by HiScript II qPCR superMixII (Vazyme, China) and real time PCR was carried out using the SYBR FAST Universal mix (KAPA, Wilmington, MA) in Bio-Rad CFX96 Touch Real time systems (Bio-Rad, Hercules, CA) and PCR primers specific for mouse Sox2 (forward primer sequence: 5’-tagagctagactccgggcgatga-3’ and reverse: 5’-ttgccttaaacaagaccacgaaa-3’). Glyceraldehyde-3-phosphate dehydrogenase (forward primer sequence: 5’-ctgacttcaacagcgacacc-3’ and reverse: 5’-tagccaaattcgttgtcatacc-3’) was used for internal control.
3. Results
3.1. mDP cell isolation and expansion in culture
The isolation of mDP cells followed an amended procedure (Gledhill et al., 2013), as schematized in (Supplementary Figure S1). The whisker hair follicles were pulled out of the dermis and the end bulbs were cut off from the hair shafts, and then transferred to a sterile petri dish containing dissection medium. After cleaning in the first petri dish, each end bulb was transferred to a drop of dissection medium placed in another dish. The surrounding connective tissues were gently teared apart with two 221/2-gauge needles to release the mDP condensates which were then transferred to the 1 mL attachment medium preplaced in a 6-cm tissue culture dish. A 221/2-gauge needle was used to gently push down mDP to the bottom of the dish. Once all mDP condensates were transferred and settled down on the bottom of the dish, an additional 4-mL attachment medium was added to the dish followed by incubation at 37 °C, 5% CO2 incubator. Cells were readily visible around the condensates by day 3. The confluent mDP cells and condensates were then detached via incubation with 0.05% trypsin/EDTA, and further expanded using KGM medium. By day 14 the passaged mDP cells reached over 90% confluence (Figure 1A). These cells were free of keratinocyte contamination as verified by the negative detection of the keratinocyte marker cytokeratin 14 (Figure 1B).
Figure 1. Passaged mouse dermal papilla (mDP) cells retained DP cell characteristics.
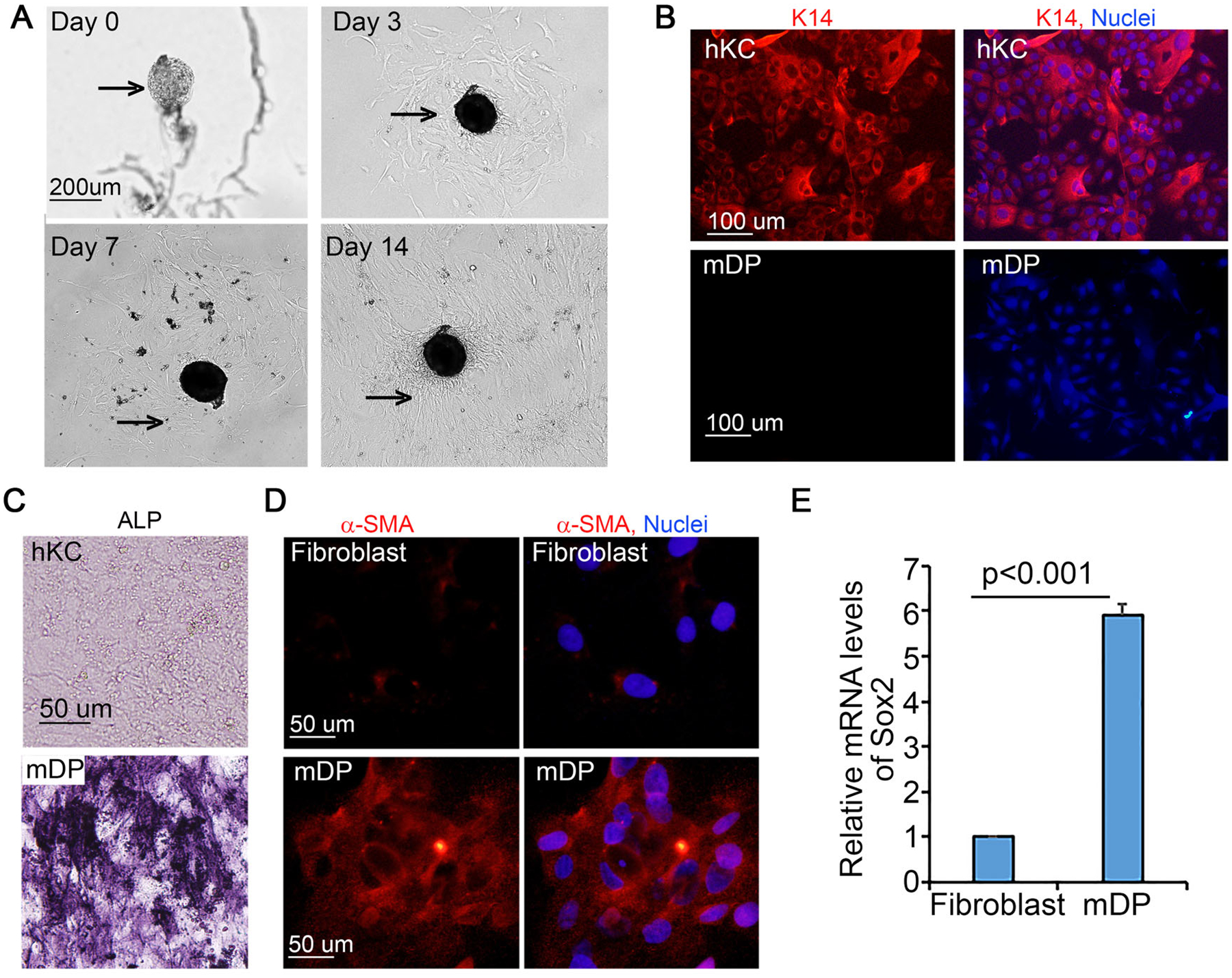
(A) Growth of mDP cells from DP condensates. Bright-field images were taken on day 1, 3, 7 and 14 after DP isolation. Arrows depict isolated DP condensates. (B) Immunostaining of human keratinocytes (hKC) and mDP cells with a primary antibody against cytokeratin 14 (K14) followed by detection with a Dylight 555-conjugated donkey anti-rabbit secondary antibody [orange]. Nuclei [blue, Hoechst 33258]. (C) Alkaline phosphatase staining of passaged mDP cells and hKC cells. (D) Immunostaining of passaged mDP cells and dermal fibroblasts for α-smooth muscle actin (a-SMA) [orange], Nuclei [Hoechst 33258, blue]. (E) Quantitative RT-PCR. Total RNA samples were isolated from cultured mDP and mouse dermal fibroblasts. Graph represents relative Sox2 mRNA levels of triplicate samples +/−SD. GAPDH was used for internal control.
Generally, a dish seeded with 20–30 mDPs can produce an estimate of 5×104 cells at the first passage (P1, day 7–12), 5×105 cells at P2 (day 16–20) and 1.5×106 cells at P3 (day 24–30). To test whether the passaged cells retained DP characteristics, we reseeded P3 cells for analysis of DP cell markers, including alkaline phosphatase (ALP), α-smooth muscle actin, and Sox2 (Clavel et al., 2012; R. R. Driskell, Giangreco, Jensen, Mulder, & Watt, 2009; Handjiski, Eichmuller, Hofmann, Czarnetzki, & Paus, 1994; Ohyama et al., 2010). Compared with hKC and dermal fibroblasts, cultured mDP cells were readily stained for ALP and α-smooth muscle actin (Figure 1C–D). Further, Sox2 expression was about 6-fold higher in mDP cells than that of dermal fibroblasts, as shown by real-time RT-PCR (Figure 1E). These results indicate that passaged mDP cells retain DP characteristics.
3.2. Skin regeneration with human keratinocytes and cultured mDP cells
To test the capacity of culture-amplified mDP cells in skin regeneration, we first prepared devitalized dermis from surgically discarded fresh human abdominal skin samples. This involved dermatome processing to obtain split-thickness skin pieces which were further subject to a devitalization process that included 1-week incubation with hypertonic salt buffer at 37 °C and 10 cycles of freeze-thaw of the dermis that had already detached from the epidermis. By H&E staining and immunostaining, we showed that tissues processed in this manner were depleted of eosin-positive cells, but retained base membrane proteins such as collagen VII (Supplementary Figure S2A–B). Consistently, no live cells were detected by live/dead cell co-staining with propidium iodide/Hoechst and calcein-AM/ethidium homodimer-1 (Supplementary Figure S2C–D). Further, the devitalized dermal tissues were free of keratinocytes and proliferative cells, as shown by the absence of cytokeratin 5 (K5) and Ki-67 positive cells (Supplementary Figure S2E–F), respectively.
For skin grafting, the devitalized dermal matrix were cut into 1-cm2 pieces, placed into 35-mm dishes, and then seeded with primary human keratinocytes either with or without the P3 mDP cells. The cell/matrix composite were submerged in KGM medium, kept in the tissue culture incubator, and fed with fresh KGM medium every other day. After 1 week in culture, 1 to 3 layers of cells were observed on top of the scaffold (Supplementary Figure S3A). By immunostaining, we found that that cells expressing the epidermal stem cell marker K15 or the fibroblast marker vimentin were scattered in the basal layer (Supplementary Figure S3B–D). In contrast, the basal cell marker K14 was expressed throughout the epidermis and the suprabasal differentiation marker K10 was detected in the top layer (Supplementary Figure S3E–F), indicating that epidermal stratification had occurred in culture. For in vivo skin regeneration, 1-week old cell/matrix composites were transferred and grafted onto the wound beds of NSG.SCID mice. At 4-weeks after the surgery, the regenerated skins appeared hairless and dark, and were readily distinguishable from the white and hairy mouse skin (Supplementary Figure 4A). Consistent with the dark appearance, the epidermis of the regenerated tissues contained a high number of cells that expressed the melanocyte marker Melan-A (Figure 2B) and were strongly pigmented (Figure 2C). About 4 weeks after grafting, ALP and Sox2-positive cells were detectable, and appeared scattered in the tissues generated with hKC and mDP cells (Supplementary Figure S4B–C). By the 6th week after grafting, tissues generated with hKC and mDP cells contained hair-follicle-like structures with typical DP condensates in the dermis and hair shaft-like structures emerging from the epidermis (Figure 2C). To verify that the regenerated tissues were of human cell origin, we performed immunostaining with an antibody specific for human cytokeratin (K1), a protein commonly expressed in the suprabasal layer of the epidermis. As expected, the epidermis of the grafted skin, but not that of the adjacent mouse skin, was readily stained for human K1 (Figure 2D). Additionally, the regenerated epidermis and hair follicles expressed human collagen VII in the basement membrane zone (Figure 2E), further confirming that the regenerated tissues were derived from human cells.
Figure 2. Cultured mDP cells induced hair follicle neogenesis in skin grafts regenerated with human keratinocytes.
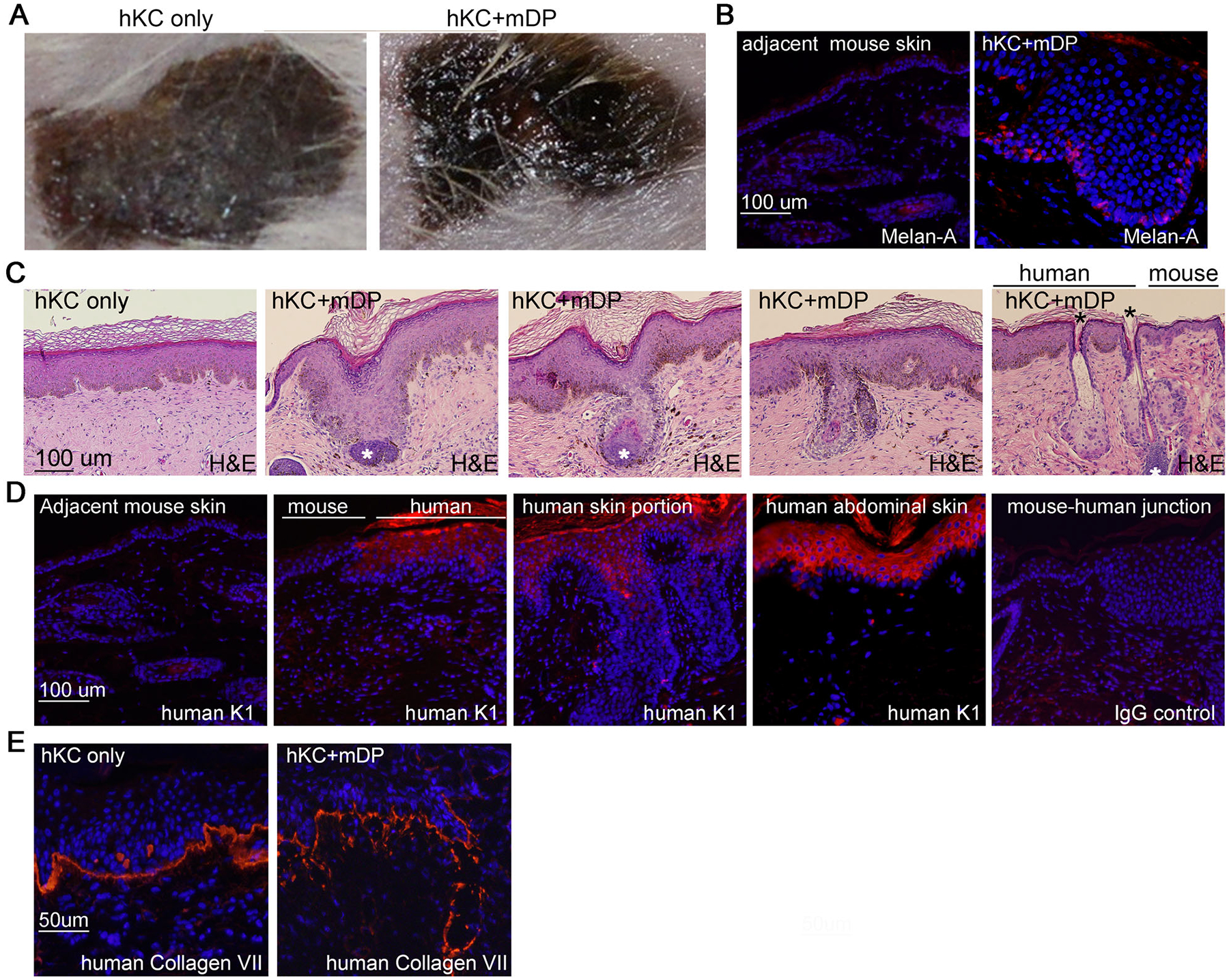
(A) Clinical pictures of 6-weeks old skin grafts generated on NSG.SCID mice with human keratinocytes (hKC) either alone or together with mDP cells. (B) Immunostaining of cryosections of skin grafts with antibodies specific for Melan-A. (C) H&E staining of paraffin sections of skin grafts. White asterisk (*) marks dermal papilla condensates and black asterisk denotes emerging hair shafts. (D-F) Immunostaining of cryosections of skin grafts with antibodies specific for human cytokeratin 1 (K1), human collagen VII, and Sox2. Immunostained sections were detected with a Dylight 555 dye-conjugated secondary antibody [orange], Nuclei [Hoechst 33258, blue].
The regenerated skin grafts were highly proliferative, as indicated by the abundance of Ki-67-positive cells in the epidermis and the folliculoid structure (Figure 3A). Similar to normal human epidermis, the basal to suprabasal layers of the epidermis expressed cytokeratin 5 (K5) and 17 (K17) (Figure 3B–C); the suprabasal layer expressed K10 and involucrin (Figure 3D–E and Supplementary Figure S4D–E) and the corneum layer expressed filaggrin (Figure 3F and Supplementary Figure S4F). Additionally, the folliculoid structures expressed the inner root sheath maker K25 and the outer root sheath marker K75 (Figure 3F–G) and the dermal condensates of the skin grafts retained Sox2 expression (Figure 4). These results indicate that mixed populations of passaged mDP cells and human keratinocytes are able to regenerate epidermis and hair follicles.
Figure 3. Regenerated hair follicles and epidermis were proliferative, and expressed relevant differentiation markers.
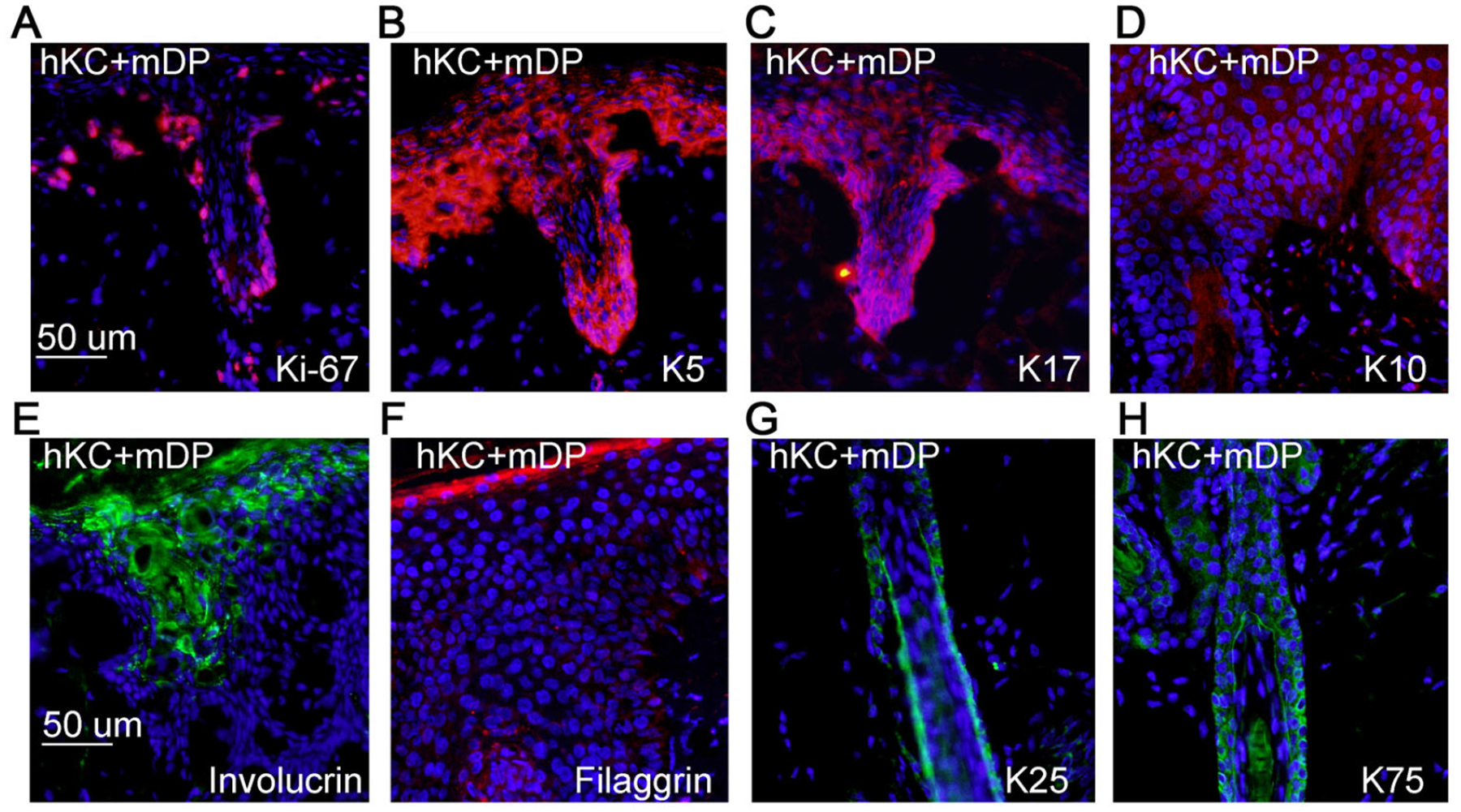
(A-E) Frozen tissue sections of 6-weeks old skin grafts regenerated with hKC and mDP cells were incubated with primary antibodies against Ki-67, K5, K17, K10, or filaggrin, and then detected with a Dylight 555-conjugated secondary antibody [orange], Nuclei [blue]. (F-G) Paraffin-embedded tissue sections of 6-weeks old skin grafts were incubated with primary antibodies against cytokeratin 25 (K25) and 75 (K75), and then detected with a Dylight 488-conjugated secondary antibody [green], Nuclei [blue].
Figure 4. Dermal papilla cells of the regenerated skins expressed Sox2.
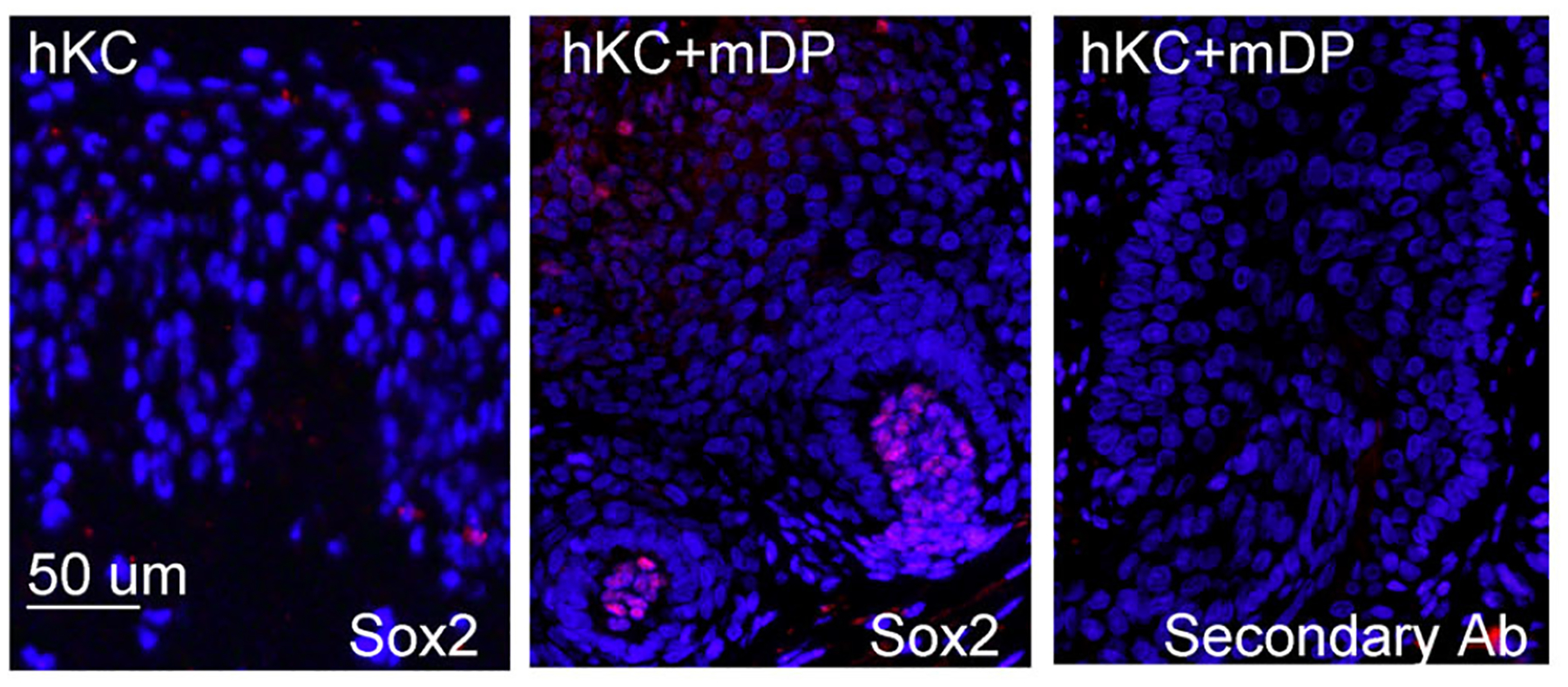
Paraffin-embedded tissue sections of 6-weeks old skin grafts were incubated with a primary antibody against SOX2, and then detected with the Dylight 555-conjugated secondary antibody [orange], Nuclei [blue].
Beside the Sox2-positive dermal condensates, there were plenty of Sox2-negative cells in the dermis of the 6-week old skin grafts. Since the dermal matrix was devitalized and the recipient SCID mice lacked lymphocytes, we predicted that cells present in the dermis of the skin grafts were of myeloid origin. Consistent with this idea, the skin sections showed positive detection by the antibodies specific for the mouse macrophage marker F4/80 and the leukocyte marker CD45 (Supplementary Figure S5), indicating that the grafts contain innate immune cells derived from the host.
4. Discussion
This study encompasses several techniques. These include 1) surgical isolation of mDP and in vitro propagation of mDP cells, 2) human keratinocyte isolation and amplification in culture, 3) preparation and devitalization of dermal matrices from surgically discarded abdominoplasty skin tissues, and 4) de novo skin regeneration with cultured mDP cells and human keratinocytes on immunodeficient mice. We demonstrate that cultured mDP cells have the capacity to induce human keratinocytes to undergo hair follicle neogenesis in regenerated skin tissues. Our methods are distinct from the previous reports in several aspects. First, these earlier studies used freshly isolated mDP cells or cultured human scalp DP (hDP) cells, and required a 2-step seeding of DP cells and keratinocytes onto the reticular and the basement membrane sides of the dermis, respectively. Earlier studies also required over 1-week air-liquid raft culture (Leiros et al., 2014; Sriwiriyanont et al., 2012; Thangapazham et al., 2014). In our protocol, DP cells and human keratinocytes were seeded as a mixture on top of the dermal scaffold, and maintained as one-dimensional submerged culture, avoiding the risks and complexities associated with the air-liquid interface culture. Thus, the experimental system reported in the current study represents a technical improvement.
Given the flexibility of isolating mDP cells from various genetic animal models and the ease of human keratinocyte culture and gene transduction, the chimeric skin model may be used to address following specific questions: 1) How do mDP cells sort out from keratinocytes and form dermal condensates? 2) Do the de novo regenerated follicular structures contain stem cell niches? And 3) if yes, do these niches contribute to epidermal renewal and repair in the same manner as those of native skin does? Also of interest, the regenerated skin grafts contained F4/80 and CD45 murine leukocytes, indicating that these skin tissues have innate immune reaction. On the other hand, due to the lack of T and B lymphocytes in the recipient animals, the chimeric skin grafts are not suitable for studies concerning adaptive immune cell function.
DP growth and function are regulated by a number of signaling pathways and accordingly, extracellular ligands such as FGF2, WNT10b, and WNT1a can enhance DP cell proliferation in culture (Dong et al., 2014; Osada, Iwabuchi, Kishimoto, Hamazaki, & Okochi, 2007; Ouji, Ishizaka, & Yoshikawa, 2012). Additionally, hair growth is regulated by dermal adipose tissues (Festa et al., 2011)(Huang et al., 2016). Taken together, animal model studies have been fruitful in understanding epidermal and hair follicle morphogenesis and function. On the other hand, mouse and human epidermal tissues display species specificity, as marked by their architectural differences. As such, animal data often require validation in human tissue models. The chimeric skin model reported in this study contains human epidermis and therefore, offers an alternative genetic skin model suitable for molecular mechanism studies and therapeutic discovery.
5. Conclusion
We report a technique that utilizes culture-amplified mDP cells to induce hair follicle neogenesis in skin tissues regenerated with primary human keratinocytes and devitalized human dermal matrix. This method offers an experimental system for mechanistic studies of human hair follicle morphogenesis and tissue regeneration. Our results may also provide insights to solving an important clinical challenge-de novo regeneration of architecturally and functionally complete skin.
Supplementary Material
ACKNOWLEDGEMENT
This work was funded by grants from NIH/NIAMS (R01-AR057746) to JZ and (P30-AR066527) to RH, Beijing Higher Education Young Elite Teacher Project (YETP0072) to WHW and Peking University Third Hospital Scientific Research Foundation for the Returned Overseas Scholars (77434-01) to LZ. We thank Julie Kent of Duke Laboratory Animal Resources for assistance in animal work and Wen-Bo Wu and Ting Chen of National Institute of Biological Sciences of Beijing for helpful discussions.
Footnotes
CONFLICT OF INTEREST
We declare non conflict of interest
REFERENCE
- Adachi T, Kobayashi T, Sugihara E, Yamada T, Ikuta K, Pittaluga S, … Nagao K (2015). Hair follicle-derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat Med, 21(11), 1272–1279. doi: 10.1038/nm.3962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong HA, Lai K, … Rosenblum MD (2017). Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell, 169(6), 1119–1129 e1111. doi: 10.1016/j.cell.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel C, Grisanti L, Zemla R, Rezza A, Barros R, Sennett R, … Rendl M (2012). Sox2 in the dermal papilla niche controls hair growth by fine-tuning BMP signaling in differentiating hair shaft progenitors. Dev Cell, 23(5), 981–994. doi: 10.1016/j.devcel.2012.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchman JR (1986). Rat hair follicle dermal papillae have an extracellular matrix containing basement membrane components. J Invest Dermatol, 87(6), 762–767. [DOI] [PubMed] [Google Scholar]
- Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, … Khavari PA (2003). NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature, 421(6923), 639–643. doi: 10.1038/nature01283 nature01283 [pii] [DOI] [PubMed] [Google Scholar]
- Dong L, Hao H, Xia L, Liu J, Ti D, Tong C, … Han W (2014). Treatment of MSCs with Wnt1a-conditioned medium activates DP cells and promotes hair follicle regrowth. Scientific Reports, 4, 5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell I, Oeztuerk-Winder F, Humphreys P, & Frye M (2015). Genetically induced cell death in bulge stem cells reveals their redundancy for hair and epidermal regeneration. Stem cells (Dayton, Ohio), 33(3), 988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Clavel C, Rendl M, & Watt FM (2011). Hair follicle dermal papilla cells at a glance. Journal of Cell Science, 124(Pt 8), 1179–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Giangreco A, Jensen KB, Mulder KW, & Watt FM (2009). Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development, 136(16), 2815–2823. doi: 10.1242/dev.038620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enshell-Seijffers D, Lindon C, Kashiwagi M, & Morgan BA (2010). beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell, 18(4), 633–642. doi: 10.1016/j.devcel.2010.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, & Horsley V (2011). Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell, 146(5), 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E (2007). Scratching the surface of skin development. Nature, 445(7130), 834–842. doi: 10.1038/nature05659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcin CL, Ansell DM, Headon DJ, Paus R, & Hardman MJ (2016). Hair Follicle Bulge Stem Cells Appear Dispensable for the Acute Phase of Wound Re-epithelialization. Stem Cells, 34(5), 1377–1385. doi: 10.1002/stem.2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gledhill K, Gardner A, & Jahoda CA (2013). Isolation and establishment of hair follicle dermal papilla cell cultures. Methods Mol Biol, 989, 285–292. doi: 10.1007/978-1-62703-330-5_22 [DOI] [PubMed] [Google Scholar]
- Handjiski BK, Eichmuller S, Hofmann U, Czarnetzki BM, & Paus R (1994). Alkaline phosphatase activity and localization during the murine hair cycle. Br J Dermatol, 131(3), 303–310. [DOI] [PubMed] [Google Scholar]
- Higgins CA, Chen JC, Cerise JE, Jahoda CA, & Christiano AM (2013). Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc Natl Acad Sci U S A, 110(49), 19679–19688. doi: 10.1073/pnas.1309970110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch T, Rothoeft T, Teig N, Bauer JW, Pellegrini G, De Rosa L, … De Luca M (2017). Regeneration of the entire human epidermis using transgenic stem cells. Nature, 551(7680), 327–332. doi: 10.1038/nature24487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, O’Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, … Fuchs E (2006). Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell, 126(3), 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Lefort K, Qiu W, Nguyen BC, Rajaram RD, Castillo E, … Dotto GP (2010). Control of hair follicle cell fate by underlying mesenchyme through a CSL-Wnt5a-FoxN1 regulatory axis. Genes Dev, 24(14), 1519–1532. doi: 10.1101/gad.1886910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-F, Chang Y-J, Hsueh Y-Y, Huang C-W, Wang D-H, Huang T-C, … Wu C-C (2016). Assembling Composite Dermal Papilla Spheres with Adipose-derived Stem Cells to Enhance Hair Follicle Induction. Scientific Reports, 6, 26436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, & Cotsarelis G (2005). Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med, 11(12), 1351–1354. doi: 10.1038/nm1328 [DOI] [PubMed] [Google Scholar]
- Jaks V, Kasper M, & Toftgard R (2010). The hair follicle-a stem cell zoo. Exp Cell Res, 316(8), 1422–1428. doi: 10.1016/j.yexcr.2010.03.014 [DOI] [PubMed] [Google Scholar]
- Jensen KB, Driskell RR, & Watt FM (2010). Assaying proliferation and differentiation capacity of stem cells using disaggregated adult mouse epidermis. Nature Protocols, 5(5), 898–911. doi: 10.1038/nprot.2010.39 [DOI] [PubMed] [Google Scholar]
- Jin JY, Ke H, Hall RP, & Zhang JY (2011). c-Jun promotes whereas JunB inhibits epidermal neoplasia. J Invest Dermatol, 131(5), 1149–1158. doi: 10.1038/jid.2011.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton AK, Herrick SE, & Headon DJ (2008). An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J Invest Dermatol, 128(5), 1311–1318. doi: 10.1038/sj.jid.5701178 [DOI] [PubMed] [Google Scholar]
- Leiros GJ, Kusinsky AG, Drago H, Bossi S, Sturla F, Castellanos ML, … Balana ME (2014). Dermal papilla cells improve the wound healing process and generate hair bud-like structures in grafted skin substitutes using hair follicle stem cells. Stem cells translational medicine, 3(10), 1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy V, Lindon C, Zheng Y, Harfe BD, & Morgan BA (2007). Epidermal stem cells arise from the hair follicle after wounding. Faseb J, 21(7), 1358–1366. doi: 10.1096/fj.06-6926com [DOI] [PubMed] [Google Scholar]
- Li JT, & Sen GL (2015). Generation of Genetically Modified Organotypic Skin Cultures Using Devitalized Human Dermis. Jove-Journal of Visualized Experiments(106). doi:ARTN e53280 10.3791/53280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Bhattacharya S, Bajaj G, Guha G, Wang Z, Jang H-S, … Ganguli-Indra G (2012). Delayed cutaneous wound healing and aberrant expression of hair follicle stem cell markers in mice selectively lacking Ctip2 in epidermis. PLoS One, 7(2), e29999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucich EA, Rendon JL, & Valerio IL (2018). Advances in addressing full-thickness skin defects: a review of dermal and epidermal substitutes. Regenerative Medicine, 13(4), 443–456. doi: 10.2217/rme-2017-0047 [DOI] [PubMed] [Google Scholar]
- McHeik JN, Barrault C, Levard G, Morel F, Bernard FX, & Lecron JC (2014). Epidermal healing in burns: autologous keratinocyte transplantation as a standard procedure: update and perspective. Plast Reconstr Surg Glob Open, 2(9), e218. doi: 10.1097/GOX.0000000000000176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalie DA, & Morgan JR (1999). Preparation and transplantation of a composite graft of epidermal keratinocytes on acellular dermis. Methods in Molecular Medicine, 18, 407–421. doi: 10.1385/0-89603-516-6:407 [DOI] [PubMed] [Google Scholar]
- Metcalfe AD, & Ferguson MW (2007). Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface, 4(14), 413–437. doi: 10.1098/rsif.2006.0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, … Cotsarelis G (2004). Capturing and profiling adult hair follicle stem cells. Nature Biotechnology, 22(4), 411–417. [DOI] [PubMed] [Google Scholar]
- Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, … Paus R (2001). A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol, 117(1), 3–15. doi:jid1377 [pii] 10.1046/j.0022-202x.2001.01377.x [DOI] [PubMed] [Google Scholar]
- Myung PS, Takeo M, Ito M, & Atit RP (2013). Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J Invest Dermatol, 133(1), 31–41. doi: 10.1038/jid.2012.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, & Fuchs E (2008). Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell, 3(1), 33–43. doi: 10.1016/j.stem.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama M, Zheng Y, Paus R, & Stenn KS (2010). The mesenchymal component of hair follicle neogenesis: background, methods and molecular characterization. Exp Dermatol, 19(2), 89–99. doi: 10.1111/j.1600-0625.2009.00935.x [DOI] [PubMed] [Google Scholar]
- Osada A, Iwabuchi T, Kishimoto J, Hamazaki TS, & Okochi H (2007). Long-term culture of mouse vibrissal dermal papilla cells and de novo hair follicle induction. Tissue Engineering, 13(5), 975–982. [DOI] [PubMed] [Google Scholar]
- Ouji Y, Ishizaka S, & Yoshikawa M (2012). Dermal papilla cells serially cultured with Wnt-10b sustain their hair follicle induction activity after transplantation into nude mice. Cell Transplantation, 21(10), 2313–2324. [DOI] [PubMed] [Google Scholar]
- Park S, Gonzalez DG, Guirao B, Boucher JD, Cockburn K, Marsh ED, … Greco V (2017). Tissue-scale coordination of cellular behaviour promotes epidermal wound repair in live mice. Nat Cell Biol, 19(2), 155–163. doi: 10.1038/ncb3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, & Chuong C-M (2008). Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature, 451(7176), 340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Zawadzka A, Philips E, Turetsky A, Batchelor S, Peacock J, … Teumer J (2009). Hair follicle neogenesis induced by cultured human scalp dermal papilla cells. Regenerative Medicine, 4(5), 667–676. [DOI] [PubMed] [Google Scholar]
- Ratushny V, Gober MD, Hick R, Ridky TW, & Seykora JT (2012). From keratinocyte to cancer: the pathogenesis and modeling of cutaneous squamous cell carcinoma. J Clin Invest, 122(2), 464–472. doi: 10.1172/JCI57415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendl M, Polak L, & Fuchs E (2008). BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes & Development, 22(4), 543–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M, Reynolds AJ, Gharzi A, & Jahoda CA (2001). In vivo induction of hair growth by dermal cells isolated from hair follicles after extended organ culture. J Invest Dermatol, 117(3), 596–604. doi: 10.1046/j.0022-202x.2001.01461.x [DOI] [PubMed] [Google Scholar]
- Rompolas P, & Greco V (2014). Stem cell dynamics in the hair follicle niche. Seminars in Cell and Developmental Biology, 25–26, 34–42. doi: 10.1016/j.semcdb.2013.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Mesa KR, & Greco V (2013). Spatial organization within a niche as a determinant of stem-cell fate. Nature, 502(7472), 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MR, Schmidt-Ullrich R, & Paus R (2009). The hair follicle as a dynamic miniorgan. Current biology : CB, 19(3), R132–142. [DOI] [PubMed] [Google Scholar]
- Segrelles C, Holguin A, Hernandez P, Ariza JM, Paramio JM, & Lorz C (2011). Establishment of a murine epidermal cell line suitable for in vitro and in vivo skin modelling. BMC Dermatol, 11, 9. doi: 10.1186/1471-5945-11-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennett R, & Rendl M (2012). Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Seminars in Cell and Developmental Biology, 23(8), 917–927. doi: 10.1016/j.semcdb.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siprashvili Z, Nguyen NT, Gorell ES, Loutit K, Khuu P, Furukawa LK, … Marinkovich MP (2016). Safety and Wound Outcomes Following Genetically Corrected Autologous Epidermal Grafts in Patients With Recessive Dystrophic Epidermolysis Bullosa. JAMA, 316(17), 1808–1817. doi: 10.1001/jama.2016.15588 [DOI] [PubMed] [Google Scholar]
- Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, … Clevers H (2010). Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science (New York, N Y), 327(5971), 1385–1389. [DOI] [PubMed] [Google Scholar]
- Sriwiriyanont P, Lynch KA, Maier EA, Hahn JM, Supp DM, & Boyce ST (2012). Morphogenesis of chimeric hair follicles in engineered skin substitutes with human keratinocytes and murine dermal papilla cells. Exp Dermatol, 21(10), 783–785. doi: 10.1111/exd.12003 [DOI] [PubMed] [Google Scholar]
- Sriwiriyanont P, Lynch KA, McFarland KL, Supp DM, & Boyce ST (2013). Characterization of hair follicle development in engineered skin substitutes. PLoS One, 8(6), e65664. doi: 10.1371/journal.pone.0065664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G, Lehrer MS, Jensen PJ, Sun TT, & Lavker RM (2000). Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell, 102(4), 451–461. [DOI] [PubMed] [Google Scholar]
- Thangapazham RL, Klover P, Wang JA, Zheng Y, Devine A, Li S, … Darling TN (2014). Dissociated human dermal papilla cells induce hair follicle neogenesis in grafted dermal-epidermal composites. J Invest Dermatol, 134(2), 538–540. doi: 10.1038/jid.2013.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima KE, Asakawa K, Ishibashi N, Toki H, Ogawa M, Hasegawa T, … Tsuji T (2012). Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat Commun, 3, 784. doi: 10.1038/ncomms1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Sennett R, Rezza A, Clavel C, Grisanti L, Zemla R, … Rendl M (2014). Wnt/beta-catenin signaling in dermal condensates is required for hair follicle formation. Dev Biol, 385(2), 179–188. doi: 10.1016/j.ydbio.2013.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo WM, Zhen HH, & Oro AE (2012). Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes Dev, 26(11), 1235–1246. doi: 10.1101/gad.187401.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


