Abstract
Background
Posttraumatic stress disorder (PTSD), a mental disorder induced by traumatic stress and often accompanied by depression and/or anxiety, may involve an imbalance in the neurotransmitters associated with the fear response. Korean Red Ginseng (KRG) has long been used as a traditional medicine and is known to be involved in a variety of pharmacological activities. We used the open field test and forced swimming test to examine the effects of KRG on the depression-like response of rats after exposure to single prolonged stress (SPS), leading to activation of the serotonergic system.
Methods
Male rats received KRG (30, 50, and 100 mg/kg, intraperitoneal injection) once daily for 14 days after exposure to SPS.
Results
Daily KRG administration significantly improved depression-like behaviors in the forced swimming test, increased the number of lines crossed and time spent in the central zone in the open field test, and decreased freezing behavior in contextual and cued fear conditioning. KRG treatment attenuated SPS-induced decreases in serotonin (5-HT) tissue concentrations in the hippocampus and medial prefrontal cortex. The increased 5-HT concentration during KRG treatment may be partially attributable to the 5-hydroxyindoleacetic acid/5-HT ratio in the hippocampus of rats with PTSD. These effects may be caused by the activation of hippocampal genes encoding tryptophan hydroxylase-1 and 2 mRNA levels.
Conclusion
Our findings suggest that KRG has an antidepressant effect in rats subjected to SPS and may represent an effective use of traditional medicine for the treatment of PTSD.
Keywords: depression, Korean Red Ginseng, posttraumatic stress disorder, single prolonged stress, serotonin
1. Introduction
Posttraumatic stress disorder (PTSD) is a serious psychiatric condition characterized by negative cognitions, avoidance, re-experiencing, mood, and hyperarousal that can develop after experiencing a life-threatening situation [1]. The characteristic symptoms of PTSD, including fear, numbing, nightmares, and hyperarousal, may result in a substantial social burden owing to higher rates of suicide and depression, as well as both occupational and psychosocial deficits [2].
Single prolonged stress (SPS) causing hypothalamic-pituitary-adrenal (HPA) axis dysregulation may lead to profound maladaptive changes that are manifested in behaviors that resemble anxiety and depression [3]. As these responses also resemble many of the clinical symptoms observed in PTSD patients, the SPS paradigm may be an appropriate experimental model with which to investigate PTSD [4]. Several investigations have shown that maladaptation of the HPA axis to stress can lead to pathological conditions, such as PTSD, resulting in serious changes in mental and emotional behavior that are indicative of depression-like symptoms [5].
Selective serotonin (5-HT) reuptake inhibitors, such as fluoxetine (FLX), are widely used to treat PTSD [6], as well as comorbidities of PTSD, such as anxiety and clinical depression [7]. Although selective 5-HT reuptake inhibitors are effective for many patients with PTSD, a significant proportion do not respond to these drugs, allowing for persistent re-experiencing of the traumatic event [8]. Identification of new therapies for the treatment of PTSD is therefore necessary, leading to renewed interest in traditional medicine, which has long been regarded as safe for long-term use [9].
Red ginseng (RG) (Panax ginseng Meyer) is widely used in traditional medicine. RG is made of ginseng root cultured for 4 to 6 years and modified through a process of repeated steaming and drying [10]. Among the saponins extracted as part of this process are a variety of ginsenosides [11]. Emerging data have revealed several physiological and pharmacological effects associated with Korean Red Ginseng (KRG, Ginseng Radix Rubra) [12], including antiinflammatory, antioxidative, antidiabetic, antihypertensive, antistress, and antiamnesic activities [10,13,14]. For example, the saponin fraction of KRG has been shown to improve scopolamine-induced memory deficits in rats [15], whereas other KRG extracts have not proven effective for preventing neurodegenerative disorders, such as Alzheimer's disease [11]. Other properties of KRG include attenuation of ischemia/reperfusion brain injury by decreasing the level of lipid peroxidation and enhancing endogenous antioxidant enzymatic activity [16,17]. Aside from the neuroprotective effects of KRG, crude saponin extracts have shown a range of antiobesity effects, including decreased food consumption, fat storage, and body weight in rats fed high-fat diets [12].
Taken together, this growing body of evidence highlights the value of traditional medicine, such as KRG, as a potential therapeutic option for the prevention of stress-associated or trauma-associated psychiatric conditions such as PTSD. Here, we evaluated the effects of KRG on depression in a PTSD-mediated psychiatric condition by exposing rats to SPS. We examined the impact of KRG on depression-like behaviors in rats exposed to SPS using the forced swim test (FST) and open field test (OFT), which measure the symptoms of PTSD-associated dysregulation. Moreover, we also investigated the activation of the serotonergic nervous system as a potential mechanism underlying the behavioral effects of SPS-triggered stress.
2. Materials and methods
2.1. Preparation of ginseng
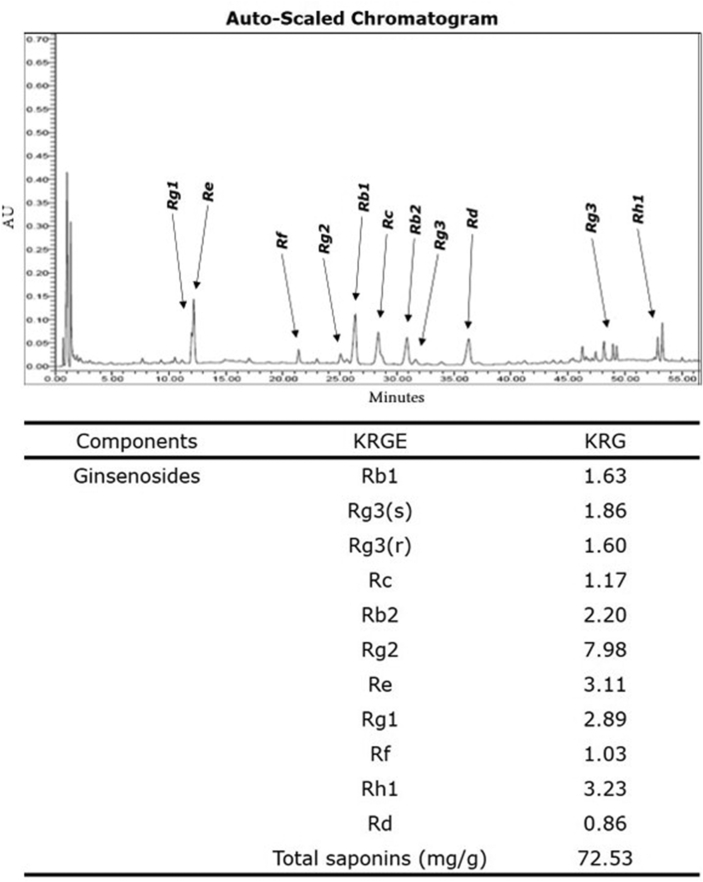
KRG was obtained from Korean Ginseng Corporation (KT&G, Daejeon, Korea). Briefly, KRG was steamed at 90∼100°C under no pressure for 3 h, dried at 50∼80°C, and then extracted three times with circulating hot water at 85∼90°C for 8 h each. The water content of the pooled extract was 36 % of the total weight. The KRG tablets were produced via Good Manufacturing Practices by Korea Ginseng Corporation, South Korea. HPLC analysis showed that KRG contained the following ginsenosides: Rb1, 1.63 mg/g; Rg3, 3.46 mg/g; Rc, 1.17 mg/g; Rb2, 2.20 mg/g; Rg2, 7.98 mg/g; Re, 3.11 mg/g; Rg1, 2.89 mg/g; Rf, 1.03 mg/g; Rh1, 3.23 mg/g; Rd, 0.86 mg/g. Their compositions and HPLC chromatograms of KRG are shown in Fig. 1.
Fig. 1.
HPLC chromatograms of KRG and its compositions. Analyzed extracts were generated from 1 g Korean ginseng. KRG, Korean Red Ginseng.
2.2. Animals and Korean Red Ginseng administration
Eight week-old male Sprague-Dawley (SD) rats (Samtako Animal Co., Seoul, Korea), weighing 200-230 g, were used for all examinations. Rats were pair-housed for 1 week before the start of the experiment. Rats were maintained on a 12/12 h light/dark cycle and were fed ad libitum throughout the duration of testing. All methods and procedures were approved by the Animal Care and Use Committee of Kyung Hee University (KHUASP(SE)-15-115). All procedures were executed according to the Guide for the Care and Use of Laboratory Animals.
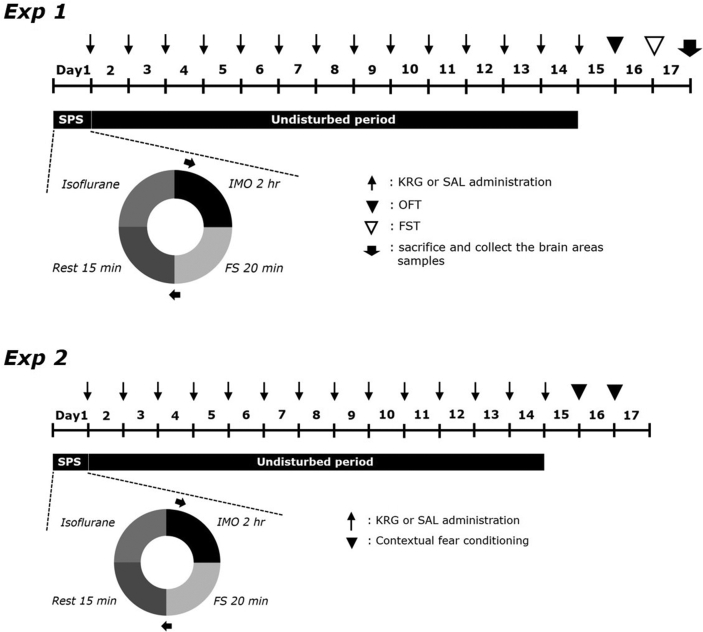
After exposure to SPS, KRG (30, 50, and 100 mg/kg, body weight) and FLX (10 mg/kg, FLX, fluoxetine hydrochloride; Sigma-Aldrich Chemical Co., St. Louis, MO, USA, positive control) were intraperitoneally (i.p.) injected once daily for 14 days. The standard doses of KRG and FLX were determined based on previous studies, with consideration of its use as a long-term therapeutic option [18,19]. KRG and FLX were dissolved in 0.9% saline before use. The entire experimental schedule is shown in Fig. 2.
Fig. 2.
Experimental protocols for single prolonged stress (SPS)-triggered depression-like behaviors and Korean Red Ginseng (KRG) treatment in rats. Different groups of rats (n = 6 or 7 per group) were used for each experimental condition. SAL, saline-treated; OFT, open field test; FST, forced swimming test; IMO: immobilization, FS: forced swim.
Rats were randomly divided into six groups of 6-7 individual rats as follows: saline-treated (SAL)-induced control group (SAL group, n = 7), SPS-triggered rats treated with saline (SPS group as a negative control, n = 6), SPS-triggered rats treated with 30 mg/kg KRG (SPS+KRG30 group, n = 6), SPS-triggered rats treated with 50 mg/kg KRG (SPS+KRG50 group, n = 6), SPS-triggered rats with 100 mg/kg KRG (SPS+KRG100 group, n = 7), and SPS-triggered rats treated with 10 mg/kg FLX (SPS+FLX group as a positive control, n = 6). The SAL group and SPS group also received 0.9% physiological saline instead of KRG as a vehicle control in a volume of 10 mL/kg for a period of 14 days.
This study consisted of two experiments. First, we examined the effects of selected KRG and the mechanism associated with the serotonergic nervous system. Second, we examined the contextual freezing behavior in the contextual fear conditioning tests after SPS procedure for confirmed KRG effect.
2.3. Single prolonged stress
Rats were exposed to SPS as previously described [18]. Briefly, the rats were immobilized for 2 hours, followed instantly by a 20 minute forced swim test. Rats were then allowed to recover for 15 min in their home cage and then exposed to ether until unconscious. For sensitization testing, rats were left untouched in their home cages for 7 days without disturbance other than routine checks to ensure proper sanitation and to replenish food and water if necessary, to allow PTSD-like symptoms to manifest [18].
2.4. Measurement of sucrose intake
Sucrose intake was measured as described previously [18]. A sucrose preference test was performed in which rats were housed in isolated cages and given free access to two bottles containing either 100 mL of water or 100 mL of sucrose solution (1%, w/v) beginning at 9:00 a.m. After 3 h, the remaining volumes of water and sucrose solution were measured, and the sucrose intake was calculated.
2.5. Forced swimming test
The modified FST described by Lee et al [18] was used in this study. Rats were forced to swim for 6 min. A rat was considered immobile when it floated without attempting to swim. During the 6 min test session, the last 4 min spent immobile were measured by two trained observers who were blinded to the treatment.
2.6. Open field test
Before completion of the FST trial, rats were exposed to the OFT as previously described [18]. Individually, each rat was allowed to explore in a rectangular container (60 × 60 × 30 cm) in the room. The locomotor activity was measured in terms of total distance traveled in the area, and exploratory activity was measured based on the evaluation of the total number of line crossings during 5 min. The number of grooming events was also measured during 5 min. Grooming behavior is reportedly a response to novelty and may vary as a function of stress intensity [3].
2.7. Contextual fear conditioning and extinction
The contextual fear conditioning tests were conducted as previously described [18]. Animals were exposed to situational reminders (i.e., the fear memory was activated by placing the rats in the same container and presenting a tone without the shock) for 5 min on days 7 and 14. The percentage of freezing responses was calculated by dividing the freezing time by the total time [20,21].
2.8. Corticosterone, serotonin, norepinephrine, dopamine, tryptophan, and 5-hydroxyindoleacetic acid measurements
Following the 14 day rest period, corticosterone (CORT) concentrations in the plasma, 5-HT concentrations in the brain tissue, and norepinephrine (NE), dopamine (DA), tryptophan (TRP), homovanillic acid (HVA), and 5-hydroxyindoleacetic acid (5-HIAA) concentrations in the hippocampus were assayed as described previously [18]. Four rats from each group were anesthetized through inhalation of isoflurane (1.2 %) and sacrificed 1 day after behavioral testing. Plasma was quickly collected via the abdominal aorta, after which the hippocampus, medial prefrontal cortex, striatum, and amygdala were rapidly removed from the brain. CORT, 5-HT, NE, DA, TRP, HVA, and 5-HIAA concentrations were evaluated by competitive enzyme-linked immunoassays (ELISAs) using antibodies against CORT (Novus Biologicals, LLC., Littleton, CO, USA), 5-HT (Abcam, Cambridge, UK), NE (Novus Biologicals), DA (Abcam), TRP (Biocompare, San Francisco, CA, USA), HVA (Abcam), and 5-HIAA (Abcam).
2.9. Total RNA preparation and reverse transcription-polymerase chain reaction
Expression of tryptophan hydroxylase-1 (TPH-1) and tryptophan hydroxylase-2 (TPH-2) mRNA was measured by reverse transcription-polymerase chain reaction as described previously [18]. Total RNA was extracted from the hippocampus of each rat using TRIzol reagent (Life Technologies, Carlsbad, CA, USA) and used as a template for cDNA synthesis using 2 μg total RNA and reverse transcriptase (TaKaRa Bio, Otsu, Japan) with random hexamers (COSMO Genetech, Seoul, Korea). cDNA was then amplified by PCR at 60°C for 28 cycles to produce TPH-1 and 58°C for 28 cycles to produce TPH-2 using Taq DNA polymerase (TaKaRa) on a thermal cycler (MJ Research, Watertown, MA, USA). PCR cycle numbers for each gene were sufficient for the production of TPH-1 and TPH-2 without overamplification. The following primer sequences were used for PCR: GAPDH (409 bp), (forward) 5′-ATT CCT TCA CCA TCT TCC AG-3′ and (reverse) 5′-CCT GCT TCA CCA CCT TCT TG-3′; TPH-1 (189 bp), (forward) 5′-ATT CCT CAG AAA GGG GGA GA-3′ and (reverse) 5′-TCA GCT GTT CTC GGT TGA TG-3′; TPH-2 (204 bp), (forward) 5′-CGT CTA TCG ACA GAG GA-3′ and (reverse) 5′-CTG TAG CCG CAG TAG TTG GT-3′. Data were normalized against glyceraldehyde 3-phosphate dehydrogenase expression in the corresponding sample.
2.10. Immunohistochemistry
Immunohistochemistry was also conducted to evaluate the tyrosine hydroxylase (TH) level in the ventral tegmental area (VTA) and the 5-HT level in the hippocampus, as described previously [19]. Briefly, three rats from each group were anesthetized deeply via inhalation of isoflurane (4 %), and their brain tissues were collected. Free-floating tissue sections were incubated overnight with primary rabbit anti-5-TH antibody (1:200 dilution, Abcam) and primary sheep anti-TH antibody (1:2000 dilution, Chemicon International Inc. Temecula, CA, USA), and the sections were then incubated for 2 hours at room temperature with secondary antibodies (1:200 dilution; Vector Laboratories Co., Burlingame, CA, USA). Next, the sections were incubated with avidin-biotin-peroxidase complex (Vector Laboratories) for 1 hour at room temperature and then in a solution containing 3,3′-diaminobenzidine (DAB; Sigma-Aldrich) and 0.03% hydrogen peroxide for 1 minute. The slides were viewed at 200× magnification, and the number of 5-HT–labeled cells in the hippocampus and TH-labeled cells in the VTA was determined.
2.11. Statistical analysis
Data are presented as the means ± SEM. Statistical differences between groups were identified using analysis of variance (ANOVA) in SPSS software (version 23.0; SPSS, Inc., Chicago, IL, USA), with corrections performed using Tukey's post hoc test. A p values < 0.05 were considered statistically significant.
3. Results
3.1. Effects of Korean Red Ginseng on single prolonged stress-triggered body weight gain, sucrose intake, and plasma corticosterone levels
The effect of KRG administration on physiological symptoms in PTSD, expressed as an increase in body weight and CORT levels in plasma, was investigated in the course of the experiments. The monitoring of the physiological symptoms is important as these can be an indicator of the side-effects on the organs in the treated groups.
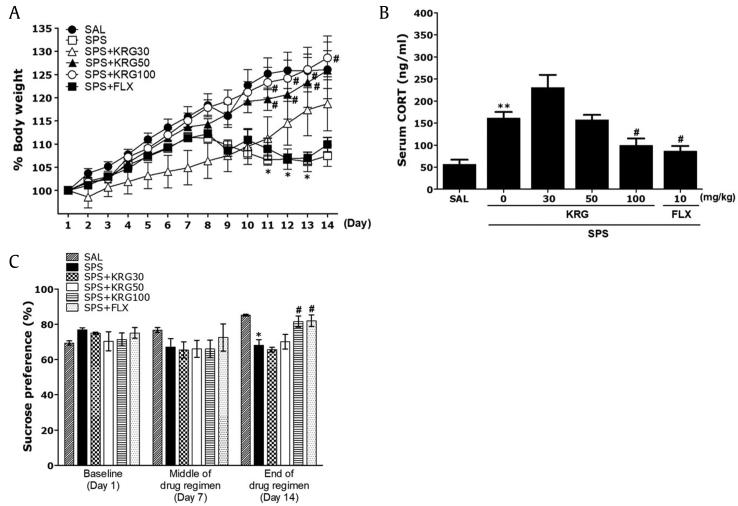
The body weight of each rat was monitored before initiating the SPS regimen and then daily for 14 days until the end of the SPS procedure (Fig. 3A). One-way ANOVA with repeated measures revealed significant differences among experimental groups: group effect [F(5,36) = 6.453, p < 0.05], time effect [F(13,468) = 87.387, p < 0.05], and group and time interaction effect [F(65,468) = 3.986, p < 0.05]. The rats in the SPS group had a significantly lower body weight at the end of the 2nd week [SPS group (F(2,23) = 17.41; Day 1 vs. Day 14, p < 0.05]. Rats exposed to SPS procedure began to lose weight on Day 8, and the rate of this stress-triggered reduction in body weight was sustained and in some cases increased, without a return to baseline. The body weight of rats in the SPS group decreased significantly compared with that of the SAL group from Day 11 to Day 13 (p < 0.05). Also, in the SPS+RG30 group, there was no significant difference between body weight for 14 days. However, the body weight of the SPS+KRG50 group and the SPS+KRG100 group showed a significant attenuation of weight loss compared with that in the SPS group from Day 11 to Day 14 (p < 0.05), indicating recovery of body weight in the SPS+KRG50 group and the SPS+KRG100 group. However, FLX-treated groups did not recover their body weight.
Fig. 3.
(A) Effects of KRG on body weight in rats exposed to SPS. (B) Plasma corticosterone (CORT) levels in rats exposed to SPS. (C) Sucrose intake in rats exposed to SPS. Body weights were significantly lower in SPS-exposed rats than in saline-treated (SAL) rats (significant main effect of SPS exposure vs. control handling (n = 6∼7/group). *p < 0.05, **p < 0.01 vs. SAL group; #p < 0.05 vs. SPS group. SPS, single prolonged stress; KRG, Korean Red Ginseng; FLX, fluoxetine.
Additionally, serum CORT levels were measured immediately after the behavioral testing, and an increase in serum CORT levels was observed following the SPS procedure. ELISA analysis showed that SPS rats exhibited significantly increased plasma CORT concentrations (284.96±25.22 %) after 14 days compared with the SAL group (p < 0.01; Fig. 3B). These data show that the SPS procedure triggered stress response in rats, consistent with that seen in PTSD. Therefore, the SPS group elicited depression-like symptoms. Administration of 100 mg/kg of KRG significantly inhibited these SPS-triggered increases in plasma CORT levels (p < 0.05). The SPS+KRG100 group as well as the SPS+FLX group (p < 0.05) not elicited stress-induced increase in CORT from baseline but had significant recovery from stress CORT levels, which are likely reflecting recovery from the SPS procedure.
We examined the sucrose consumption, anhedonia, and effects of KRG in SPS rats. Anhedonia is a diagnostic criterion for depression. Anhedonia was quantified using the sucrose intake test on 1 day before SPS (baseline), in the middle of the post-SPS period (7 days after SPS), and at the end of the drug regimen (14 days after SPS). Analysis of sucrose intake revealed a gradual but significant decrease in the rate of sucrose intake over the 14 days in the SPS group compared with the SAL group (p < 0.05; Fig. 3C). During this period, the sucrose intake of rats treated with 100 mg/kg KRG showed a significant increase of sucrose intake compared with that in the SPS group (p < 0.05). The results also revealed that recovery of sucrose preference in the SPS+KRG100 group was almost comparable to that in the SPS+FLX group (p < 0.05).
3.2. Effects of Korean Red Ginseng on single prolonged stress-triggered depression-like behavior
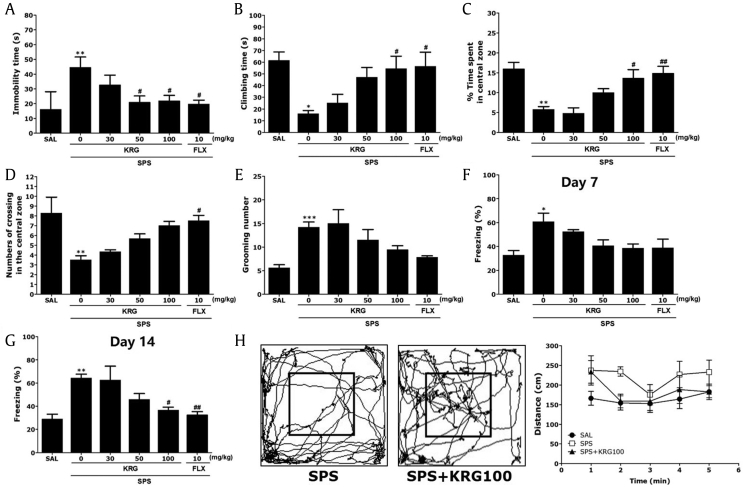
The effect of KRG administration on depression-like behavior, characterized by increased in immobility time during the FST compared with SPS-treated rats was investigated. A significant effect of KRG administration was observed in the FST, with post hoc tests showing that KRG administration significantly decreased immobility time in a dose-dependent manner (p < 0.05; Fig. 4A). Similarly, rats in the SPS+KRG100 group exhibited significant recovery in the time spent climbing in the FST (p < 0.05; Fig. 4B). Both test results indicate that KRG administration significantly reversed depression-like behaviors. FLX (positive control) also markedly decreased immobility time in the FST; however, SPS did not induce significant differences in swimming behaviors between groups during the FST (p = 0.674; data not shown).
Fig. 4.
(A) Effects of KRG on immobility time in the forced swimming test (FST) following exposure to SPS. (B) Effects of KRG on climbing behavior in the FST following exposure to SPS. Effects of KRG on locomotion and exploratory behavior in the open field test (OFT) in rats exposed to SPS. (C) Changes in time spent in the central zones; (D) the number of lines crossed in the central zone; (E) the number of grooming bouts. Effects of KRG on freezing behavior after exposure to SPS in rats. (F) The percentages of time spent freezing were determined on Days 7. (G) The percentages of time spent freezing were determined on Day 14. (H) Motion trails of SPS and SPS+KRG100 groups and distance traveled were recorded in the open field test. *p < 0.05, **p < 0.01, ***p < 0.001 vs. SAL group; #p < 0.05, ##p < 0.01 vs. SPS group. SAL, saline-treated; SPS, single prolonged stress; KRG, Korean Red Ginseng; FLX, fluoxetine.
3.3. Effects of Korean Red Ginseng on single prolonged stress-triggered locomotion and grooming behavior
We examined the anxiolytic effects of KRG administration in SPS rats. This anxiolytic effect can be expressed by a decrease in the time spent in the central zone in the OFT. Rats exposed to SPS spent significantly less time compared with the SAL group (p < 0.01; Fig. 4C). There was also a significant reduction in the number of central zone crossings following the SPS procedure (p < 0.01; Fig. 4D). In contrast, 100 mg/kg of KRG-treated rats spent significantly more time compared with the SPS group in the central zone (p < 0.05). The exploratory-like behaviors of the SPS+KRG100 group were similar to those of the SPS+FLX group. Grooming behavior was observed primarily in the open field area. The numbers of grooming behaviors in the OFT are shown in Fig. 4E. Changes in grooming behavior were only observed among rats treated with 100 mg/kg of KRG, although this result was only marginally significant. Also, comparisons performed using a parametric one-way ANOVA revealed no stress-associated differences between control, FLX-treated, and KRG-treated rats in terms of locomotor activity in the OFT [F(5,37) = 2.403, p = 0.059]. Locomotor activity measured by the distance traveled 5 min after saline-treated rats is shown in Fig. 4H. The locomotor activity in the SPS group was not significantly different that in the SAL group during 5 min (p = 0.061).
3.4. Effects of Korean Red Ginseng on single prolonged stress-triggered contextual freezing behavior
The effect of KRG administration on another PTSD-like or anxiety-like behavior, expressed by an increase in freezing behavior in the open field area, was investigated. The difference in freezing behavior between all groups during the SPS procedure was significant. Freezing time was significantly enhanced after exposure to SPS (p < 0.05 and p < 0.01 on Days 7 and 14, respectively; Fig. 4F and G). The difference in freezing behavior between SPS group and SPS+KRG100 group during the 7 day period was not significant (p = 0.75). Although 30 mg/kg and 50 mg/kg of KRG administration failed to reverse this increase in freezing time on Day 14, the percentage of time spent exhibiting freezing behavior was significantly reduced in the group treated with 100 mg/kg of KRG (p < 0.05). Our results also show that the freezing time of rats receiving FLX (p < 0.01) was decreased. These results showed that a persistent fear response was related to the initial trauma but was attenuated in response to KRG treatment.
3.5. Effects of Korean Red Ginseng on single prolonged stress-triggered serotonin, norepinephrine, and dopamine concentrations in the brain
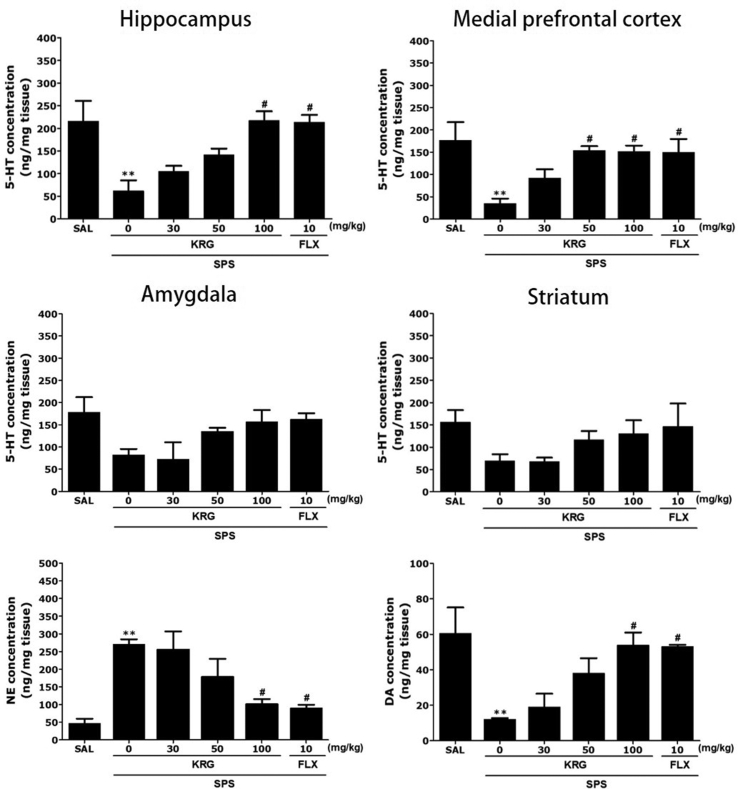
The serotonergic system, specifically that pertaining to 5-HT transmission, is critically implicated in mood regulation. Following 2 weeks of KRG treatment, we first measured the contents of 5-HT and their metabolites in rat brain regions (i.e. hippocampus, prefrontal cortex, amygdala, and striatum) that closely related to mood regulation.
Fig. 5 shows differences in the regional levels of 5-HT among the groups. One-way ANOVA showed significant difference in 5-HT levels in the hippocampus [F(5,20) = 6.288, p < 0.01], as well as DA levels [F(5,20) = 5.322, p < 0.01]. Post hoc test results revealed significantly lower 5-HT levels in the hippocampus of the SPS group than in the SAL group (p < 0.01; Fig. 5). Daily administration of 100 mg/kg of KRG significantly attenuated SPS-triggered decreases in 5-HT in the hippocampus and medial prefrontal cortex, relative to the SPS group compared with the SAL (p < 0.05).
Fig. 5.
Effects of KRG on serotonin (5-HT) concentration in the brain. Norepinephrine (NE) and dopamine (DA) concentrations in the hippocampus of rats exposed to SPS for 14 consecutive days are shown. **p < 0.01 vs. SAL group; #p < 0.05 vs. SPS group. SAL, saline-treated; SPS, single prolonged stress; KRG, Korean Red Ginseng; FLX, fluoxetine.
Treatment with 100 mg/kg of KRG significantly rescued 5-HT levels in the amygdala and striatum by 190.24 % and 188.4 %, respectively, relative to SPS group, although this result was only marginally significant. Furthermore, 5-HT levels in the brains of rats treated with 10 mg/kg of FLX were similar to those of rats treated with 100 mg/kg of KRG.
ELISA analysis revealed a 574.47 % increase in NE concentrations in the hippocampus of SPS rats after 14 days relative to those in the untreated SAL group (p < 0.01). These changes were significantly attenuated in rats treated with KRG in a dose-dependent manner (p < 0.05). DA concentrations in the hippocampus were also significantly decreased (50.0 %) in SPS rats after 14 days compared with those in the untreated SAL group (p < 0.01). Daily administration of 100 mg/kg of KRG significantly attenuated the SPS-triggered decrease in DA concentrations in the hippocampus (p < 0.05).
3.6. Effects of Korean Red Ginseng on single prolonged stress-triggered 5-HT system in the hippocampus
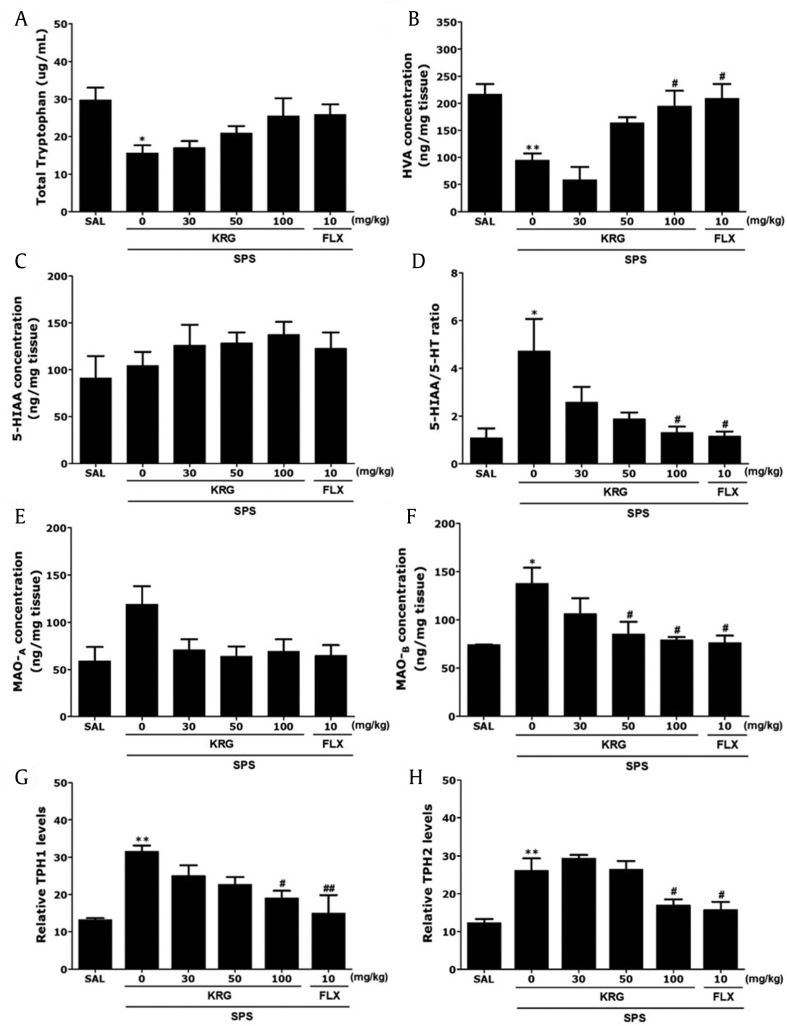
ELISA analysis found that daily SPS exposure for 14 days resulted in significant decreases in the TRP (51.72 %) and HVA (43.98 %) concentrations in the hippocampus compared with the levels in rats in the SAL group (p < 0.05 and p < 0.01, respectively; Fig. 6A and B). Administration of 100 mg/kg of KRG significantly inhibited these SPS-triggered decreases in the HVA levels in the hippocampus (p < 0.01), but not the TRP levels. However, in all groups, there was no significant difference between 5-HIAA concentrations in rats after the SPS procedure (Fig. 6C). With regard to the 5-HIAA level, there was only a marginal increase in the hippocampus following the highest dose of KRG treatment (100 mg/kg). As shown in Fig. 6D, chronic KRG treatment (100 mg/kg) significantly decreased the 5-HIAA/5-HT ratio in the hippocampus (p < 0.05). Also, we evaluated the effect of chronic KRG treatment on the Monoamine oxidase A (MAO-A) and MAO-B activities in the hippocampus of rats. Chronic treatment of rats with KRG (50 and 100 mg/kg) dose-dependently inhibited the MAO-B activity in the hippocampus (p < 0.05; Fig. 6E and F). However, the same KRG regimen hardly influenced the MAO-A activity in the hippocampus, and the results were not considered to be significant.
Fig. 6.
Effects of KRG on plasma tryptophan (TRP), homovanillic acid (HVA), 5-hydroxyindoleacetic acid (5-HIAA), and the 5-HIAA/5-HT ratios in the hippocampus, and expression of tryptophan hydroxylase-1 (TPH-1) and tryptophan hydroxylase-2 (TPH-2) mRNA in the hippocampus of rats exposed to SPS for 14 consecutive days. PCR bands on agarose gels and relative intensities are shown. *p < 0.05, **p < 0.01 vs. SAL group; #p < 0.05, ##p < 0.01 vs. SPS group. SAL, saline-treated; SPS, single prolonged stress; KRG, Korean Red Ginseng; FLX, fluoxetine.
3.7. Effects of Korean Red Ginseng on single prolonged stress-triggered tryptophan hydroxylase 1 and tryptophan hydroxylase 2 mRNA in the hippocampus
To examine the effects of KRG treatment on the expression of TPH-1 and TPH-2 in rat hippocampus damaged by SPS, the mRNA expressions of TPH-1 and TPH-2 were analyzed using reverse transcription-polymerase chain reaction (Fig. 6G and H). TPH-1 and TPH-2 mRNA levels were significantly increased in the SPS group than in the SAL group (p < 0.01). Treatment with 100 mg/kg KRG restored these levels to those observed in the SPS group (p < 0.05). Both TPH-1 and TPH-2 mRNA expression levels in the hippocampus of rats treated with 100 mg/kg KRG were similar to those in rats treated with 10 mg/kg FLX.
3.8. Effects of Korean Red Ginseng on single prolonged stress-triggered 5-HT expression in the hippocampus and TH expression in the VTA
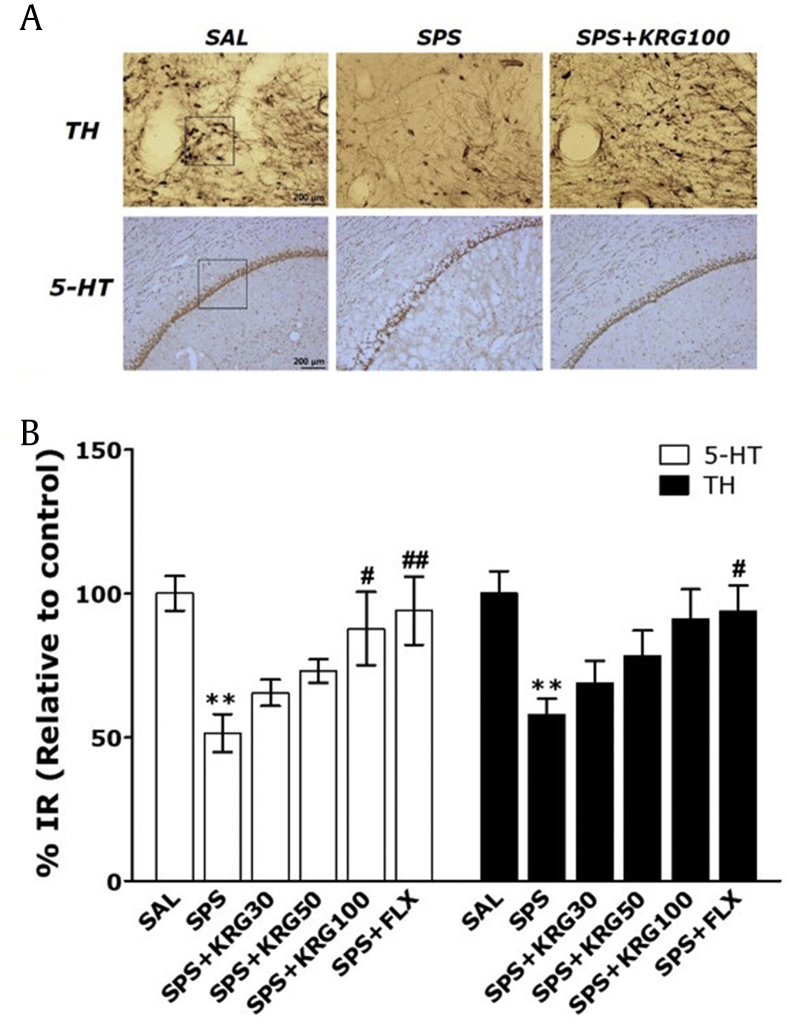
5-HT–like immunoreactivity was primarily observed in the bodies of hippocampal cells (Fig. 7A and B). The density of 5-HT immunoreactive fibers in the hippocampus of the SPS group decreased by 51.41 %, compared with the SAL group. The SPS-treated rats showed a gradual reduction in 5-HT expression in the hippocampus compared with the controls (p < 0.01). However, the 5-HT-reactive neuronal activity in the hippocampus associated with SPS-induced depression-like behavior was significantly restored in the SPS+KRG100 group compared with the SPS group (p < 0.05). Also, compared with the SPS group, there was an increase in the number of TH-immunoreactive neuronal cells in the VTA in the SPS+KRG100 group, although this result was only marginally significant. These results indicated that the numbers of 5-HT-reactive neuronal cells in the hippocampus in rats receiving 100 mg/kg KRG were similar to those in rats receiving 10 mg/kg FLX.
Fig. 7.
Effects of KRG administration on mean 5-HT expression in the hippocampus and tyrosine hydroxylase (TH) expression in the ventral tegmental area (VTA). (A) Representative images are shown. (B) The relative percentage values are shown. **p < 0.01 vs. SAL group; #p < 0.05, ##p < 0.01 vs. SPS group. SAL, saline-treated; SPS, single prolonged stress; KRG, Korean Red Ginseng; FLX, fluoxetine.
4. Discussion
The data presented here highlight the strong antidepressant-like effects of KRG in a rat model of depression and provide evidence of the potential mechanisms underlying these effects. Treatment with KRG reduced immobility time in the FST following SPS; however, this effect was only significant at the 100 mg/kg dose. Furthermore, our findings clearly show that KRG significantly increased the amount of time spent at the center of the open field in the OFT. As the hippocampus and medial prefrontal cortex are components of the primary neural pathway underlying major depressive disorders, the stabilizing effects of KRG on 5-HT levels in the brain, driven by the modulation of TPH and the 5-HIAA/5-HA ratio, was responsible for the behavioral changes. Accordingly, we found that KRG had antidepressant-like effects against SPS-triggered PTSD by inhibiting decreases in hippocampal 5-HT, consistent with the pathogenesis of clinical psychological disorders. Thus, our findings support a role for KRG as an antidepressant in a rat model of SPS-triggered PTSD.
Following numerous studies [22], we decided to use intraperitoneal administration in the present study. We examined general symptoms, clinical signs, and mortality at a given KRG dose and then monitored these phenomena on a daily basis for 14 days. None of the groups treated with KRG showed abnormal clinical signs, and the mortality rate was zero during the study period, indicating that KRG administration had a negligible toxic effect on the animals.
In the SPS model of depression, high plasma CORT concentrations modulate depression by regulating depression-like behavior and enhancing HPA axis negative feedback, which may be comparable to the progression of traumatic stress in humans [23]. Following SPS, we observed a reduction in body weight gain, increased plasma CORT concentrations, and a reduction in sucrose intake, indicating that the SPS model was successfully established [24]. The antidepressant activity of KRG suggests that the flavonoid inhibits HPA axis dysfunction-associated psychological disorders by reducing plasma CORT concentrations and increasing sucrose preference, thus restoring behavioral and neurochemical responses. Recent evidence has suggested that SPS-induced activation of the HPA axis leads to a significant decrease in body weight [25]. In present study, found such a decrease starting Day 8 after the SPS procedure. Specifically, after the SPS procedure, rats were socially isolated for 7 days. This method is known to exacerbate the physiological symptoms of PTSD. Therefore, there was a sharp decrease in body weight starting on Day 8; this contrasts with the normal pattern, reflected in our results, of a decrease starting 2 days after the SPS procedure.
Several studies have predicted that abnormal social behavior and increased depression-related symptoms will initiate other unstable behaviors [26]. Rats that have been exposed to unpredictable mild stress for three consecutive weeks exhibit behavioral disturbances and are likely to develop depression. These behavioral disturbances include reduced sucrose intake, decreased crossing, and rearing behavior in the OFT and increased immobility time in the FST [27]. Changes in the behavior of rats after exposure to SPS suggest that depression-like behaviors were successfully triggered in this study. Therefore, our results show that KRG treatment can help SPS-triggered rats recover from depression-like behaviors, as was manifested by their decreased rearing frequency in the OFT.
PTSD can affect the neuronal circuitry and induce dysregulation in monoamines, strengthening depression [28]. Indeed, monoamine neurotransmitters including DA, NE, and 5-HT in the Central nervous system (CNS) play key roles in the pathophysiology of depression [29]. Some studies have suggested that 5-HT performs inhibitory actions in the brain and that it is systematically involved in the modulation of behavior and emotions, including inhibition of aggression [30]. Here, we found that KRG inhibited SPS-triggered decreases in hippocampal NE levels, suggesting that KRG may regulate the central adrenergic system, as well as indirectly altering monoamine synthesis in the brain.
We hypothesized that SPS-triggered depression-like symptoms were related to impaired 5-HT signaling. We found a significant increase in the hippocampal 5-HT levels of rats treated with KRG after exposure to SPS. Levels were restored to near-baseline levels, consistent with previous findings [1]. These results suggest that KRG may be able to regulate aspects of the serotonergic nervous system within the brain as well as FLX, rescuing both behavioral and neurochemical reactions related to depression [31]. Also, KRG treatment normalized hippocampal TPH-1 mRNA levels. Some studies have suggested that genetic elimination of TPH1 and TPH2 strongly decreases the amounts of 5-HT and 5-HIAA in the brain [31,32]. This is consistent with evidence that TPH2 is the main primary synthetic enzyme in the brain, whereas TPH1 is responsible for 5-HT synthesis in the brain periphery. TPH catalyzes the rate-limiting step of 5-HT biosynthesis and plays a crucial role in 5-HT metabolism [32]. Our results demonstrated that TPH1 mRNA contributes to the pathogenesis of PTSD, providing strong evidence that the 5-HT system is a viable target for antidepressant development.
Therefore, we examined the levels of both monoamine neurotransmitters and their metabolites. KRG increased 5-HT and DA levels, but no obvious selectivity was found. In addition, KRG had no significant effect on 5-HT (5-HIAA) or DA (HVA) metabolites. Our results provide a rationale for examining the serotonergic mechanisms underlying the antidepressant effects of KRG. Specifically, following chronic KRG treatment, the increased 5-HT level and decreased 5-HIAA/5-HT ratio in brain regions indicate a change in 5-HT metabolism. First, the neurochemical evidence showed that chronic KRG treatment increased 5-HT levels in the brain and decreased the 5-HIAA/5-HT ratio in rats. Second, biochemical evidence demonstrated the suppression of MAO-B by KRG in the same brain regions (hippocampus), which provides a biochemical basis for the enhanced serotoninergic tone induced by chronic KRG administration. It cannot be excluded that the observed increases in the 5-HIAA levels and 5-HIAA/5-HT ratio resulted from MAO-B activation.
Numerous studies have suggested that chronic Ginseng total saponin (GTS) treatment has anxiolytic-like effects in models of depression [33,34]. Recent studies have shown that ginsenoside-Rb1 can also increase the neural 5-HT concentration and decease the immobility time in the FST under conditions of chronic unpredictable mild stress [35,36]. Additionally, ginsenoside-Rg3 reduces immobility time in the FST and TST in rats with depression-like symptoms caused by chronic social defeat stress [37]. Many ginsenosides are present in KRG, particularly high amounts of Rb1 and Rg3. In our study, KRG administration significantly decreased immobility time in the FST and increased the amount of time spent at the center of the OFT in rats with chronic PTSD-induced depression-like symptoms. This suggests that KRG acts as an antidepressant or as an anxiolytic by preventing a reduction in 5-HT levels in the brain. Therefore, KRG, especially Rb1 and Rg3, may have an antidepressant or anxiolytic effect.
Consequently, the data presented here suggest that KRG administration reduced depression-like behaviors in the FST and OFT, possibly via modulation of the serotonergic system. These findings suggest that KRG may improve the psychologically rooted behaviors and neurochemical changes seen in depression. KRG may be a useful alternative therapeutic agent for the treatment of trauma-related diseases, such as PTSD.
Conflicts of interest
The authors declare no potential conflicts of interests.
Acknowledgments
This research was supported by a grant from the Korean Society of Ginseng and the Korean Ginseng Cooperation (2017).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2019.09.005.
Contributor Information
Bombi Lee, Email: bombi@khu.ac.kr.
Seikwan Oh, Email: skoh@eaha.ac.kr.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lin C.C., Tung C.S., Liu Y.P. Escitalopram reversed the traumatic stress-induced depressed and anxiety-like symptoms but not the deficits of fear memory. Psychopharmacology (Berl) 2016;233:1135–1146. doi: 10.1007/s00213-015-4194-5. [DOI] [PubMed] [Google Scholar]
- 2.Ji L.L., Tong L., Xu B.K., Fu C.H., Shu W., Peng J.B., Wang Z.Y. Intra-hippocampal administration of ZIP alleviates depressive and anxiety-like responses in an animal model of posttraumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;10:28–38. doi: 10.1186/1744-9081-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serova L.I., Laukova M., Alaluf L.G., Sabban E.L. Intranasal infusion of melanocortin receptor four (MC4R) antagonist to rats ameliorates development of depression and anxiety related symptoms induced by single prolonged stress. Behav Brain Res. 2013;250:139–147. doi: 10.1016/j.bbr.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Pitman R.K., Rasmusson A.M., Koenen K.C., Shin L.M., Orr S.P., Gilbertson M.W., Milad M.R., Liberzon I. Biological studies of posttraumatic stress disorder. Nat Rev Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harvey B.H., Naciti C., Brand L., Stein D.J. Endocrine, cognitive and hippocampal/cortical 5HT 1A/2A receptor changes evoked by a time-dependent sensitisation (TDS) stress model in rats. Brain Res. 2003;983:97–107. doi: 10.1016/s0006-8993(03)03033-6. [DOI] [PubMed] [Google Scholar]
- 6.Ravindran L.N., Stein M.B. Pharmacotherapy of post-traumatic stress disorder. Curr Top Behav Neurosci. 2010;2:505–525. doi: 10.1007/7854_2009_15. [DOI] [PubMed] [Google Scholar]
- 7.Escalona R., Canive J.M., Calais L.A., Davidson J.R. Fluvoxamine treatment in veterans with combat-related post-traumatic stress disorder. Depression Anxiety. 2002;15:29–33. doi: 10.1002/da.1082. [DOI] [PubMed] [Google Scholar]
- 8.Stein D.J., Pedersen R., Rothbaum B.O., Baldwin D.S., Ahmed S., Musgnung J., Davidson J. Onset of activity and time to response on individual CAPS-SX17 items in patients treated for post-traumatic stress disorder with venlafaxine ER: a pooled analysis. Int J Neuropsychopharmacol (CINP) 2006;12:23–31. doi: 10.1017/S1461145708008961. [DOI] [PubMed] [Google Scholar]
- 9.Nie H., Peng Z., Lao N., Wang H., Chen Y., Fang Z., Hou W., Gao F., Li X., Xiong L. Rosmarinic acid ameliorates PTSD-like symptoms in a rat model and promotes cell proliferation in the hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:16–22. doi: 10.1016/j.pnpbp.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Kim S., Lee Y., Cho J. Korean red ginseng extract exhibits neuroprotective effects through inhibition of apoptotic cell death. Biol Pharm Bull. 2014;37:938–946. doi: 10.1248/bpb.b13-00880. [DOI] [PubMed] [Google Scholar]
- 11.Kho M.C., Lee Y.J., Park J.H., Cha J.D., Choi K.M., Kang D.G., Lee H.S. Combination with Red ginseng and Polygoni Multiflori ameliorates highfructose diet induced metabolic syndrome. BMC Complement Altern Med. 2016;16:98. doi: 10.1186/s12906-016-1063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J.H., Hahm D.H., Yang D.C., Kim J.H., Lee H.J., Shim I. Effect of crude saponin of Korean red ginseng on high-fat diet-induced obesity in the rat. J Pharmacol Sci. 2005;97:124–131. doi: 10.1254/jphs.fp0040184. [DOI] [PubMed] [Google Scholar]
- 13.Lee J., Cho J.Y., Kim W.K. Anti-inflammation effect of Exercise and Korean red ginseng in aging model rats with diet-induced atherosclerosis. Nutr Res Pract. 2014;8:284–291. doi: 10.4162/nrp.2014.8.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J.K., Shim J.Y., Cho A.R., Cho M.R., Lee Y.J. Korean red ginseng protects against mitochondrial damage and intracellular inflammation in an animal model of type 2 diabetes mellitus. J Med Food. 2018;21:544–550. doi: 10.1089/jmf.2017.4059. [DOI] [PubMed] [Google Scholar]
- 15.Jin S.H., Park J.K., Nam K.Y., Park S.N., Jung N.P. Korean red ginseng saponins with low ratios of protopanaxadiol and protopanaxatriol saponin improve scopolamine-induced learning disability and spatial working memory in mice. J Ethnopharmacol. 1999;66:123–129. doi: 10.1016/s0378-8741(98)00190-1. [DOI] [PubMed] [Google Scholar]
- 16.Lee J.S., Choi H.S., Kang S.W., Chung J.H., Park H.K., Ban J.Y., Kwon O.Y., Hong H.P., Ko Y.G. Therapeutic effect of Korean red ginseng on inflammatory cytokines in rats with focal cerebral ischemia/reperfusion injury. Am J Chin Med. 2011;39:83–94. doi: 10.1142/S0192415X1100866X. [DOI] [PubMed] [Google Scholar]
- 17.Ban J.Y., Kang S.W., Lee J.S., Chung J.H., Ko Y.G., Choi H.S. Korean red ginseng protects against neuronal damage induced by transient focal ischemia in rats. Exp Ther Med. 2012;3:693–698. doi: 10.3892/etm.2012.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee B., Lee H. Systemic administration of curcumin affect anxiety-related behaviors in a rat model of posttraumatic stress disorder via activation of serotonergic systems. Evid Based Complement Alternat Med. 2018:9041309. doi: 10.1155/2018/9041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee B., Sur B., Yeom M., Shim I., Lee H., Hahm D.H. L-tetrahydropalmatine ameliorates development of anxiety and depression-related symptoms induced by single prolonged stress in rats. Biomol Ther. 2014;22:213–222. doi: 10.4062/biomolther.2014.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji M.H., Jia M., Zhang M.Q., Liu W.X., Xie Z.C., Wang Z.Y., Yang J.J. Dexmedetomidine alleviates anxiety-like behaviors and cognitive impairments in a rat model of post-traumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:284–288. doi: 10.1016/j.pnpbp.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Qiu Z.K., Zhang L.M., Zhao N., Chen H.X., Zhang Y.Z., Liu Y.Q., Mi T.Y., Zhou W.W., Li Y., Yang R.F. Repeated administration of AC-5216, a ligand for the 18 kDa translocator protein, improves behavioral deficits in a mouse model of post-traumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:40–46. doi: 10.1016/j.pnpbp.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Nam S.J., Han Y.J., Lee W., Kang B., Choi M.K., Han Y.H., Song I.S. Effect of red ginseng extract on the pharmacokinetics and efficacy of metformin in streptozotocin-induced diabetic rats. Pharmaceutics. 2018;3:79–88. doi: 10.3390/pharmaceutics10030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serova L.I., Tillinger A., Alaluf L.G., Laukova M., Keegan K., Sabban E.L. Single intranasal neuropeptide Y infusion attenuates development of PTSD-like symptoms to traumatic stress in rats. Neuroscience. 2013;236:298–312. doi: 10.1016/j.neuroscience.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 24.Patki G., Li L., Allam F., Solanki N., Dao A.T., Alkadhi K., Salim S. Moderate treadmill exercise rescues anxiety and depression-like behavior as well as memory impairment in a rat model of posttraumatic stress disorder. Physiol Behav. 2014;130:47–53. doi: 10.1016/j.physbeh.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh J.Y., Kim Y.K., Kim S.N., Lee B., Jang J.H., Kwon S., Park H.J. Acupuncture modulates stress response by the mTOR signaling pathway in a rat post-traumatic stress disorder model. Sci Rep. 2018;8:11864. doi: 10.1038/s41598-018-30337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez L.E., File S.E., Overstreet D.H. Selectively bred lines of rats differ in social interaction and hippocampal 5-HT1A receptor function: a link between anxiety and depression? Pharmacol Biochem Behav. 1998;59:787–792. doi: 10.1016/s0091-3057(97)00525-x. [DOI] [PubMed] [Google Scholar]
- 27.Sun J., Wang F., Hong G., Pang M., Xu H., Li H., Tian F., Fang R., Yao Y., Liu J. Antidepressant-like effects of sodium butyrate and its possible mechanisms of action in mice exposed to chronic unpredictable mild stress. Neurosci Lett. 2016;618:159–166. doi: 10.1016/j.neulet.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Geracioti T.D., Jr., Baker D.G., Kasckow J.W., Strawn J.R., Jeffrey M.J., Dashevsky B.A., Horn P.S., Ekhator N.N. Effects of trauma-related audiovisual stimulation on cerebrospinal fluid norepinephrine and corticotropin-releasing hormone concentrations in post-traumatic stress disorder. Psychoneuroendocrinology. 2008;33:416–424. doi: 10.1016/j.psyneuen.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Lin C.C., Tung C.S., Lin P.H., Huang C.L., Liu Y.P. Traumatic stress causes distinctive effects on fear circuit catecholamines and the fear extinction profile in a rodent model of posttraumatic stress disorder. Eur Neuropsychopharmacol. 2016;26:1484–1495. doi: 10.1016/j.euroneuro.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Davidson R.J., Putnam K.M., Larson C.L. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 31.Clark J.A., Alves S., Gundlah C., Rocha B., Birzin E.T., Cai S.J., Flick R., Hayes E., Ho K., Warrier S. Selective estrogen receptor-beta (SERM-beta) compounds modulate raphe nuclei tryptophan hydroxylase-1 (THP-1) mRNA expression and cause antidepressant-like effects in the forced swim test. Neuropharmacology. 2012;63:1051–1063. doi: 10.1016/j.neuropharm.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Shishkina G.T., Kalinina T.S., Dygalo N.N. Up-regulation of tryptophan hydroxylase-2 mRNA in the rat brain by chronic fluoxetine treatment correlates with its antidepressant effect. Neuroscience. 2007;150:404–412. doi: 10.1016/j.neuroscience.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Cha H.Y., Park J.H., Hong J.T., Yoo H.S., Song S., Hwang B.Y., Eun J.S., Oh K.W. Anxiolytic-like effects of ginsenosides on the elevated plus-maze model in mice. Biol Pharm Bull. 2005;28:1621–1625. doi: 10.1248/bpb.28.1621. [DOI] [PubMed] [Google Scholar]
- 34.Dang H., Chen Y., Liu X., Wang Q., Wang L., Jia W., Wang Y. Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1417–1424. doi: 10.1016/j.pnpbp.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 35.Hao K., Gong P., Sun S.Q., Hao H.P., Wang G.J., Dai Y., Liang Y., Xie L., Li F.Y. Beneficial estrogen-like effects of ginsenoside Rb1, an active component of Panax ginseng, on neural 5-HT disposition and behavioral tasks in ovariectomized mice. Eur J Pharmacol. 2011;659:15–25. doi: 10.1016/j.ejphar.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Wang G.L., He Z.M., Zhu H.Y., Gao Y.G., Zhao Y., Yang H., Zhang L.X. Involvement of serotonergic, noradrenergic and dopaminergic systems in the antidepressant-like effect of ginsenoside Rb1, a major active ingredient of Panax ginseng C. A. Meyer. J Ethnopharmacol. 2017;204:118–124. doi: 10.1016/j.jep.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 37.You Z., Yao Q., Shen J., Gu Z., Xu H., Wu Z., Chen C., Li L. Antidepressant-like effects of ginsenoside Rg3 in mice via activation of the hippocampal BDNF signaling cascade. J Nat Med. 2017;71:367–379. doi: 10.1007/s11418-016-1066-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.