Abstract
Lumbar spine stenosis represents a complex degenerative pathology that has been a subject of significant dispute when it comes to fusion. A review of the literature from 2008 to 2019 was performed on the role of fusion in the treatment of lumbar spinal stenosis using PubMed, Ovid Medline, Cochrane Central Register of Controlled Trials, and the Cochrane Database of Systematic Reviews. Using the key words “lumbar spinal stenosis,” “lumbar fusion,” “lumbar decompression,” and “lumbar pedicle screw fixation,” the search revealed 490 papers. Of these, only Level 1 or Level 2 evidence papers were selected, leading to only 3 randomized controlled trials (RCTs) that were analyzed. None of the good-quality studies (RCTs) performed so far have proven any clinical benefit of adding fusion to degenerative lumbar spine decompression. The effect of spinal instability on the outcome following decompression remains controversial. At present, no unanimous criteria exist among the RCTs to identify what constitutes true instability. Fusion for instability or stenosis alone remains controversial, and the results are unconvincing. At this point, the issue expands to not only lumbar degenerative diseases but spinal fractures and lumbar isthmic spondylolisthesis. We thereby present the consensus of the World Federation of Neurosurgical Societies Spine Committee, which formulated the indications for lumbar spine fusion in degenerative lumbar stenosis.
Key words: Decompression, Fusion, Lumbar instability, Lumbar spinal stenosis, Spondylolisthesis
Abbreviations and Acronyms: LS, Linkert scale; LSS, Lumbar spinal stenosis; ODI, Oswestry Disability Index; RCT, Randomized controlled trial; Sedsign, Sedimentation sign; SF-36, Short Form-36
Introduction
Lumbar spinal stenosis (LSS) is defined as a degenerative pathology leading to anatomical narrowing of the spinal canal, foramen, or lateral recess. This may produce a constellation of signs and symptoms known as neurogenic claudication.1 It also represents the most common indication for neurosurgical consultation and spinal surgery in patients older than 65 years of age.2 The estimated prevalence of LSS has been reported as ranging from 1.7% to 13.1%.3 Several factors have been known to contribute to LSS, of which congenital factors play a dominant role. Kalichman et al.,4 in the Framingham Study, found that congenital relative LSS was 4.7% and absolute LSS was 2.6%, whereas acquired relative and absolute LSS was 22.5% and 7.3%, respectively. Due to a lack of high-quality clinical evidence, the latest systematic reviews are unable to conclude whether surgical treatment or a conservative approach is better for patients with LSS.4
However, common practice is to advise conservative treatment to all patients with mild-to-moderate symptoms followed by surgery for patients with intractable symptoms. Currently, the standard surgical management for LSS is single- or multilevel decompressive laminectomy.
Biomechanical studies have shown a correlation between the extent of decompression and postoperative instability.5 It has been postulated that degenerative spondylolisthesis may worsen following decompressive surgery, which is theoretically true, as we are taking away one of the supporting walls. However, the effect of spinal instability on the outcome, following decompression, is still controversial.6,7 As of now, no conclusive guidelines are indicating whether treating these patients would be more effective with or without fusion. This paper aims to create recommendations for when fusion surgery in LSS is needed, the different types of fusion techniques that can be applied, and their outcomes.
Methods
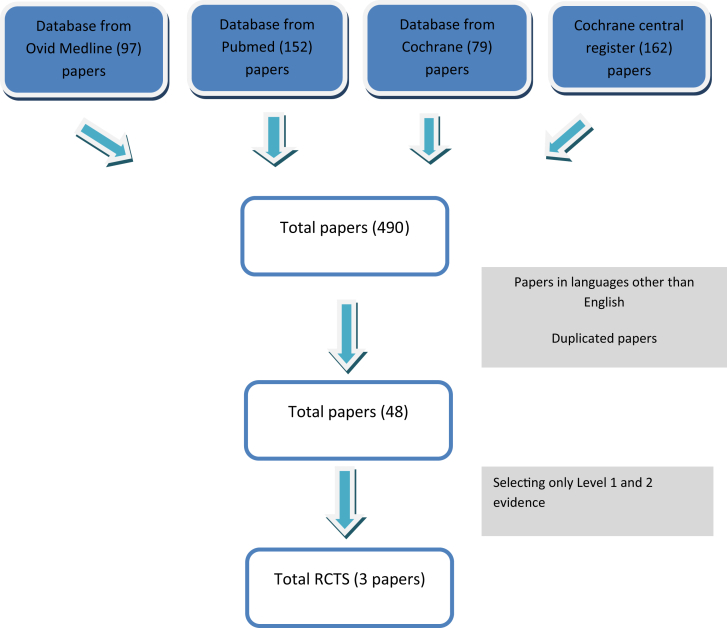
Three expert spinal surgeons (S.S., A.B., M.B.) reviewed the literature from 2008 to 2019 on the role of fusion in the treatment of LSS using PubMed, Ovid Medline, Cochrane Central Register of Controlled Trials, and the Cochrane Database of Systematic Reviews (Figure 1).
Figure 1.
Flowchart for manuscript selection of the last 10 years. LSS, lumbar spinal stenosis.
Using the key words “lumbar spinal stenosis,” “lumbar fusion,” “lumbar decompression,” and “lumbar pedicle screw fixation,” the search revealed 490 papers. Papers that were not in English were omitted. A total of 48 studies were shortlisted for comparison between cases with lumbar decompression alone and those with decompression with fusion. Of these, only Level 1 and 2 evidence studies were included with the following proposed selection criteria: 1) greater than 50 patients, 2) a randomized controlled (RCT) trial, and 3) a minimum of 24 months follow-up to assess outcome. Studies not meeting these criteria were excluded. Based on these characteristics, only 3 RCTs were identified, with Försth et al. performing the largest RCT. On the basis of the most significant literature, the panel drafted 10 statements that were presented in Milan in November 2018. After a preliminary voting session, 3 statements were excluded due to a lack of evidence. The remaining statements were then presented and voted on, at the committee meeting in Belgrade in March 2019.
During the second round of review, the questions to answer were as follows: 1) Is facet joint effusion an independent marker of instability? 2) Is it predominant complaint of back pain sufficient to justify fusion? 3) Does a bilateral facetectomy of more than 50% and discectomy warrant fusion? 4) Does stable spondylolisthesis with no back pain warrant fusion? During the second round of the review, due to the paucity of Level 1 evidence studies, we isolated the same 3 RCTs, which were then compared.
LSS: Radiologic Diagnostic Criteria
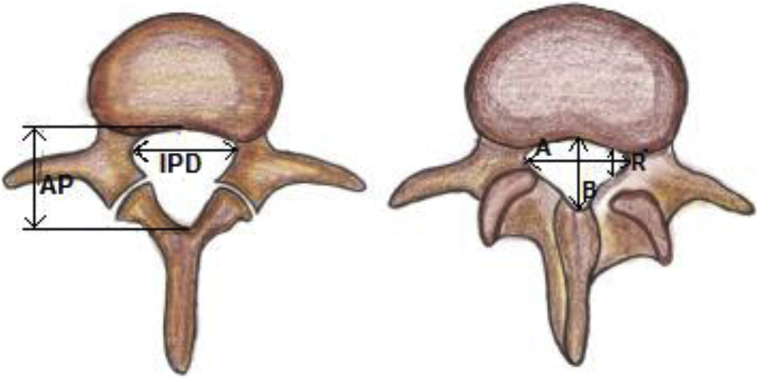
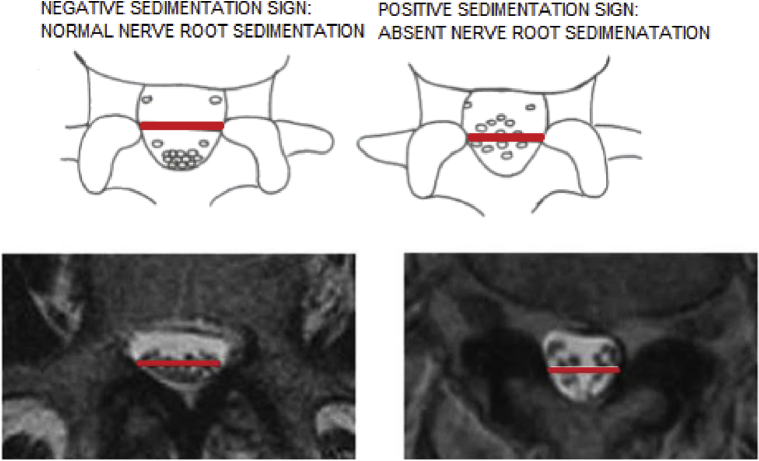
Prompt diagnoses are important to classify LSS and determine the confounding factors in the selection of surgical interventions. Although there are no standard criteria on magnetic resonance imaging to diagnose LSS, some studies consider that an anteroposterior canal diameter <10–15 mm, cross-sectional area <75–145 mm2 as the cut-off values to define central stenosis. It is reported that the height/depth of lateral recess ≤2–5 mm, and angle of lateral recess <30° are required to define lateral stenosis.8 In a large-scale study with standardized measurements to determine the magnetic resonance imaging criteria for developmental LSS, the results suggest that developmental LSS can be defined if the anteroposterior canal diameter was at L1 <20 mm, L2 <19 mm, L3 <19 mm, L4 <17 mm, L5 <16 mm, and at S1 <16 mm (Figure 2).8 Several studies also reported the importance of “sedimentation sign” (SedSign). A positive SedSign is defined as nerve roots being located in the ventral or central part of the dural sac, as seen in patients with severe LSS, whereas a negative SedSign is defined as all nerve roots being located in the dorsal part of the dural sac (Figure 3).8 Barz et al.9 concluded that the reversibility of a preoperative positive SedSign was demonstrated after the decompression of the affected segmental level and was associated with an improved clinical outcome. A persisting positive SedSign could be the result of incomplete decompression or surgical complications. A new positive SedSign after sufficient decompression surgery could be used as an indicator of new stenosis in previously operated patients.
Figure 2.
Shown is the lumbar canal diameter (normal vs. stenosis) A = transverse diameter of spinal canal, B = anteroposterior (AP) diameter of spinal cord, IPD = interpedicular distance, and R = lateral recess diameter.
Figure 3.
Positive and negative segmentation signs.
Evolution of LSS
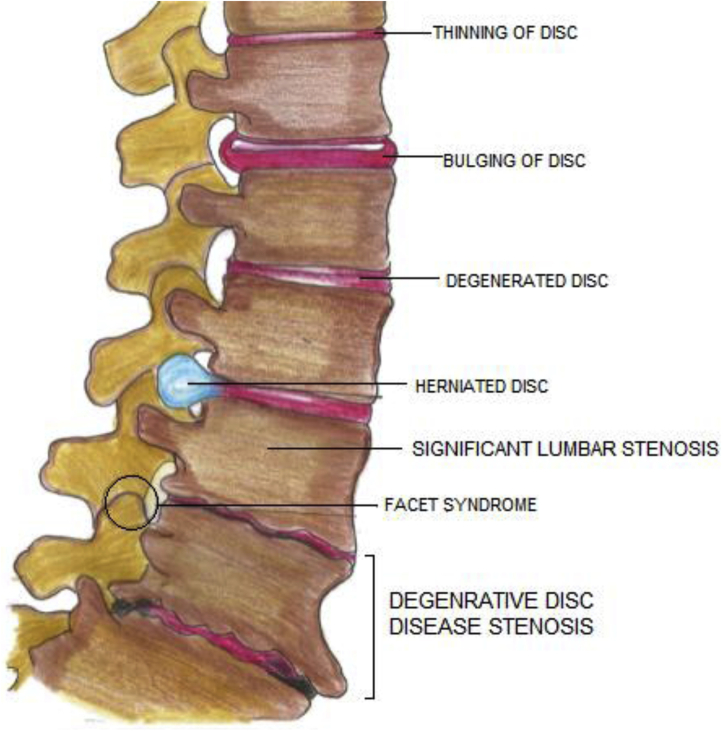
The initial event is disc degeneration, which leads to narrowing of the disc space and settling of the motion segment. This is followed by a buckling of the ligamentum flavum, which leads to a state of microinstability (Figure 4).10 Depending on the anatomic predisposing factors, the vertebra develops either anterolisthesis or retrolisthesis. With the development of listhesis, an abnormal motion leads to the formation of bony spurs, subchondral sclerosis, and hypertrophy of facets and ligaments. The combined effects of mechanical static compression along with dynamic compression aided by the movement and buckling of ligamentum flavum lead to a variable degree of stenosis and manifest clinically in terms of pain or neurogenic claudication.
Figure 4.
Pathophysiology of degenerative lumbar stenosis.
Surgical Management in LSS
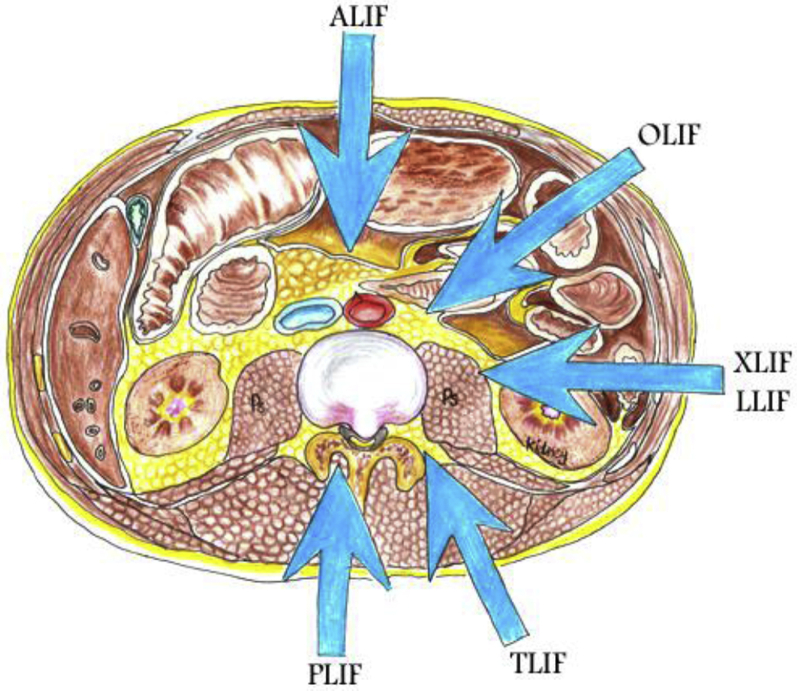
The primary goal of surgical intervention in LSS is to decompress the neural structures that are being encroached upon, to relieve the symptoms, and improve the function. The surgical approach may vary according to the location of the stenosis, the number of segments affected, associated deformity or spinal instability, history of previous surgery, patient's general condition, and the surgeon's preferences. The various approaches to achieve decompression include traditional laminectomy, bilateral laminotomies, bilateral decompression through unilateral laminotomy, and different forms of laminoplasty (Figure 5).11 Decompression of the neural structures generally focuses on relieving the leg symptoms (claudication or radiculopathy) associated with LSS and less on improving any accompanying back pain. Therefore, although back pain does improve, the improvement in leg pain is usually greater. Patients with LSS and predominant leg pain have better surgical outcomes and a greater relative improvement than do patients with predominant back pain or equally bothersome pain in the legs and the back.11 To treat the LSS by decompression alone or decompression with fusion is an old controversy. Many kinds of lumbar fusion techniques via different approaches have been described, including posterior/posterolateral lumbar fusion, posterior lumbar interbody fusion, transforaminal lumbar interbody fusion, and oblique lumbar interbody fusion.12 Since there is a lack of evidence for advantages of fusion, the fusion surgery should be restricted to those with spinal instability, spinal deformities, or neuroforaminal stenosis with compressed exiting nerves caused by postsurgical disk collapse.13 One of the major controversies about surgery for spinal stenosis is the role of spinal fusion. Spinal arthrodesis to achieve spinal fusion has generally been recommended for spinal stenosis associated with degenerative spondylolisthesis, recurrent stenosis after previous decompression, instability, or scoliosis. A recent clinical practice guideline by Resnick et al.14 recommended that “in the absence of deformity or instability, lumbar fusion has not been shown to improve outcomes in patients with isolated stenosis,” and therefore it is not recommended (grade C recommendation). Similarly, guidelines from the North American Spine Society recommend that in the absence of associated scoliosis or spondylolisthesis, “decompression alone is suggested for patients with predominant leg symptoms without instability” (grade B).15
Figure 5.
Various stabilization procedures for lumbar spine. ALIF, anterior lumbar interbody fusion; OLIF, oblique lumbar interbody fusion; XLIF, extreme lateral interbody fusion; LLIF, lateral lumbar interbody fusion; TLIF, transforaminal lumbar interbody fusion; PLIF, posterior lumbar interbody fusion.
Surgical Rationale: Decompression with or without Fusion
The rationale of the management of LSS is to regulate intraspinal pressure, blood flow, and metabolic status of neural structures by decompression of the neural elements and reducing the inflammatory process.16 The options for conservative management in LSS include drugs, physiotherapy, spinal injection, lifestyle modification, and multidisciplinary rehabilitation. These may be beneficial in the early stages of the disease with mild symptoms.17 When the patient's symptoms progress to moderate and severe, surgery may prevent a further decrease in function. However, it is important to emphasize that if a patient presents with true motor weakness, sphincter dysfunction, or other signs of acute cauda equina syndrome, prompt surgical evaluation and consideration of urgent decompression is the appropriate first step. Zaina et al.18 in the Cochrane database systematically reviewed the surgical versus nonsurgical treatment for LSS. They concluded that there were still no updated recommendations and no superiority between the various treatment options, which may suggest that the clinician should be very careful in informing their patients. Another meta-analysis conducted by Ma et al.19 concluded that surgically treated patients had better long-term clinical outcome, despite a greater complication rate. Stenosis may present in isolation, with or without a disc bulge or herniation, or can be associated with degenerative spondylolisthesis or degenerative scoliosis. The presence or absence of spondylolisthesis with stenosis makes a significant difference in the management of such patients. Weinstein et al.20 reported that in patients with concomitant spondylolisthesis and stenosis, surgery showed substantially greater improvement in the pain and function during a period of 2 years. Pearson et al.21 also showed similar results, with the surgical approach being superior to conservative management irrespective of listhesis grade, disc height, or mobility. Also, a systematic review conducted by Carreon et al.22 showed that spondylolisthesis treated with fusion had a better improvement in the Oswestry Disability Index (ODI) and Short Form-36 (SF-36) scores irrespective of the fusion technique, provided that the fusion was performed for an established cause like spondylolisthesis and predominant back pain.
Based on these studies, careful selection of patients is an important step in the management of these patients.22 It is also important to recognize and prevent iatrogenic instability by preserving at least 50% of the facet joint. Sun et al.23 followed 42 patients with decompression with multisegment LSS with single-segment degenerative spondylolisthesis. They demonstrated no iatrogenic instability. They stated that adequate and effective decompression of the affected nerve root can be achieved by hemilaminectomy, or undercutting decompression of the lateral recess, with care taken to preserve at least 50% of the facet joint, minimizing disruption of biomechanical integrity. They also took care to avoid injuring the joint capsule during exposure or screw insertion to protect the facet joint. Guha et al.24 did a systematic review of 24 studies involving 2496 patients, assessing both open laminectomy and minimally invasive bilateral canal enlargement. Instability was seen more frequently in patients with pre-existing spondylolisthesis (12.6%) and in those treated with open laminectomy (12%). Reoperation for instability was required in 1.8% of all patients and was greater for patients with preoperative spondylolisthesis (9.3%) and for those treated with open laminectomy (4.1%).
The historical indications proposed in 1995 by Hanley25 for lumbar spinal fusion were isthmic spondylolisthesis, unstable spinal stenosis syndromes (degenerative spondylolisthesis, degenerative scoliosis), and in patients with objective segmental instability. However, apart from a better understanding of the pathophysiology, little has changed in the indications of fusion. Detwiler et al.26 concluded that clear indications for fusion include iatrogenic instability, isthmic spondylolisthesis, kyphosis, stenosis that develops at a previously decompressed segment, stenosis adjacent to a previously fused lumbar segment, radiographically proven dynamic instability with pain or neurologic findings, adult scoliosis, and mechanical back pain. Relative indications for the use of spinal instrumentation in the setting of spinal stenosis include correction of deformity, recurrent spinal stenosis with instability, degenerative spondylolisthesis, adjacent-segment stenosis with instability, and multiple level fusions. Fusion is rarely indicated in the setting of routine discectomy, abnormal radiographs without appropriate findings (such as degenerative disc disease), facet joint syndrome, failed back surgery, or stable spinal stenosis.26
To Fuse or Not
Shen et al.27 performed a comprehensive meta-analysis that included the 5 most recent and cited RCTs to compare fusion with simple decompression, enrolling 438 patients. Pooled analysis showed no significant differences between decompression alone and fusion groups for the ODI scores at the baseline (P = 0.50) and 2 years' follow-up (P = 0.71), and the satisfaction rate of operations was also similar for the groups (P = 0.53). However, operation time (P = 0.002), blood loss (P < 0.00001), and length of hospital stay (P = 0.007) were remarkably greater in the fusion group. Furthermore, there was no difference in the reoperation rate between these 2 groups (P = 0.49).27 In our study, the only 3 recent RCTs included are shown for comparison in Table 1.28, 29, 30 The baseline issue of enrolled patients should not be neglected, which comprises the demographic and commodity hallmarks characterized by the Charlson comorbidity index.31 An increasing amount of evidence suggests that obesity and overweight, increasing age, cigarette smoking, anemia, dependent functional status, and malnutrition are determinant factors affecting the clinical outcome or adverse effects of lumbar surgery.32 Furthermore, it should be stressed that the notion of microstability, as well as the status of adjacent facet degeneration, are critical preoperative considerations to minimize the reoperation rate due to adjacent-segment disease.33 The comparison of clinical outcomes in randomized clinical trials might be more convincing if we eliminate the bias, by embracing the patient's baseline. Peul and Moojen15 point out that ODI is a more of a disease-specific index for evaluating the clinical outcome of LSS than SF-36. Apart from these 2 indexes, there are a variety of commonly used parameters, including a visual analog scale for back and leg pain, the EuroQol-5 questionnaires,34 novel lumbar stiffness disability index,35 and Roland–Morris Disability Questionnaire.36 Given the great clinical significance determining the welfare of millions of patients with LSS, adding clinical indexes might enhance the power of the evidence and address doubts in the terms of differences in SF-36 and ODI. Table 2 shows the comparative qualitative assessment of studies. Apart from the strong evidence provided by Försth et al.30 and Ghogawala et al.,28 there are inferior levels of evidence supporting the newly formed consensus. Sigmundsson et al.34 analyzed the impact of adding fusion to decompression on the clinical outcome of 1624 patients with LSS using the VAS, EuroQol-5 questionnaires, ODI, and SF-36 as measurable variables. Their conclusion supplemented the results proposed by Försth et al.30 Even in a setting in which fusion is indicated for patients with LSS, long-term follow-up studies indicate that fusion without instrumentation is linked with decreased costs and similar clinical outcomes. At present, no unanimous criteria exist among the RCTS to identify what constitutes true instability. Hence, whether fusion was performed for actual instability or not remains controversial and the results unconvincing. At this point, the overcautious issue expands to not only lumbar degenerative diseases but spinal fractures and lumbar isthmic spondylolisthesis. The spinal community seems to have evolved into the non-instrumentation trend. This can probably be explained by the fact that it took 3 decades to accept pedicle screws as a standard technique in the 1990s. Thereafter, there has been a rapid increase in the global instrumentation industry. Consequently, the issue of overtreatment attracted more and more attention in the scientific community. According to these literature reviews, the World Federation of Neurosurgical Societies Spine Committee proposed and voted upon the statements as follows: Statement 1: In patients with LSS and no sign or symptoms of instability and predominant leg pain, decompression alone is recommended. All expressed a positive vote to this statement with a strong consensus (88% voted 5 of the Linkert scale [LS], 12% voted 4 of LS). Statement 2: In patients with stenosis and stable spondylolisthesis, fusion is not mandatory and decompression alone is suggested. This statement reached a strong positive consensus (38% voted 5 of LS; 50% voted 4 of LS and 12% voted 2). Statement 3: Unstable spondylolisthesis with symptoms may require fusion. All expressed a positive vote to this statement with a strong consensus (63% voted 5 of LS; 12% voted 4 of LS and 25% voted 3). Statement 4: If the main complaint is mechanical axial low back pain, more than leg pain, this is suggestive of spondylolisthesis and the patient may benefit from a fusion surgery. This statement did not reach a consensus (25% voted 5 of LS; 25% voted 4 and 12% voted 3 of LS; 12% voted 1 of LS; 25% voted 2 of LS). Statement 5: Patients with LSS and loss of sagittal balance, if symptomatic, may benefit from decompression, fixation, and deformity correction surgery. This statement reached positive consensus (25% voted 5 of LS; 25% voted 4 of LS and 25% voted 3 of LS; 12% voted 1 of LS; 13% voted 2 of LS). Statement 6: Fusion may be advisable in patients who undergo bilateral facetectomy of more than 50% and bilateral discectomy. This statement reached positive consensus (38% voted 5 of LS; 25% voted 4 of LS; 12% voted 3 and 25% voted 2 of LS). Statement 7: Facet joint effusion alone is not proven to correlate with stability. This statement reached a strong positive consensus (25% voted 5 of LS; 38% voted 4 of LS and 25% voted 3 of LS; 12% voted 2 of LS).
Table 1.
Comparison of 3 RCTS (Patients, Procedures, and Outcome)
| Characteristics | Study |
||
|---|---|---|---|
| Försth et al., 201630 | Ghogawala et al., 201628 | Inose et al., 201829 | |
| Study design | RCT | RCT | RCT |
| Patients | 233 | 66 | 85 |
| Intervention | D = 120, F = 133 | D = 35, F =31 | D = 29, D + F = 56 |
| Follow-up | 5 years | 4 years | 5 years |
| Outcome | ODI score, EQ-5D score, VAS score for back and leg pain, ZCQ score, operation time, blood loss | SF-36, ODI score, blood loss, hospital stays, operation time | JOA, blood loss, hospital stay, VAS for leg pain and back pain |
| Result | No difference between fusion and decompression | No difference | No difference |
RCT, randomized controlled trial; ODI, Oswestry Disability Index; EQ-5D, EuroQol-5 questionnaire; VAS, visual analog scale; ZCQ, Zurich Claudication Questionnaire; SF-36, Short Form-36; JOA, Japanese Orthopaedic Association; D, decompression; F, fixation.
Table 2.
Quality of Studies
World Federation of Neurosurgical Societies Recommendations for Fusion Surgery for LSS
-
•
In patients with LSS and no sign or symptoms of instability and predominant leg pain, decompression alone is recommended.
-
•
In patients with stenosis and stable spondylolisthesis, fusion is not mandatory and decompression alone is suggested.
-
•
Unstable spondylolisthesis with symptoms may require fusion.
-
•
There is no consensus if the main complaint is mechanical axial low back pain, which is more than leg pain, the patient may benefit from a fusion surgery.
-
•
Patients with LSS and loss of sagittal balance, if symptomatic, may benefit from decompression, fixation, and deformity correction surgery.
-
•
Fusion may be advisable in patients who undergo bilateral facetectomy of more than 50% and bilateral discectomy.
-
•
Facet joint effusion alone is not proven to correlate with stability.
Declaration of Competing Interest
The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Illustrations were drawn by Ms. Aaliya Javed.
References
- 1.Genevay S., Atlas S.J. Lumbar spinal stenosis. Best Pract Res Clin Rheumatol. 2010;24:253–265. doi: 10.1016/j.berh.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amundsen T., Weber H., Nordal H.J. Lumbar spinal stenosis: conservative or surgical management? A prospective 10-year study. Spine (Phila Pa 1976) 2000;25:1424–1435. doi: 10.1097/00007632-200006010-00016. [DOI] [PubMed] [Google Scholar]
- 3.Fanuele J.C., Birkmeyer N.J., Abdu W.A., Tosteson T.D., Weinstein J.N. The impact of spinal problems on the health status of patients: have we underestimated the effect? Spine (Phila Pa 1976) 2000;25:1509–1514. doi: 10.1097/00007632-200006150-00009. [DOI] [PubMed] [Google Scholar]
- 4.Kalichman L., Cole R., Kim D.H. Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine J. 2009;9:545–550. doi: 10.1016/j.spinee.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abumi K., Panjabi M.M., Kramer K.M. Biomechanical evaluation of lumbar spinal stability after graded facetectomies. Spine (Phila Pa 1976) 1990;15:1142–1147. doi: 10.1097/00007632-199011010-00011. [DOI] [PubMed] [Google Scholar]
- 6.Johnsson K.E., Redlund-Johnell I., Udén A., Willner S. Preoperative and postoperative instability in lumbar spinal stenosis. Spine (Phila Pa 1976) 1989;14:591–593. doi: 10.1097/00007632-198906000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Fox M.W., Onofrio B.M., Hanssen A.D. Clinical outcomes and radiological instability following decompressive lumbar laminectomy for degenerative spinal stenosis: a comparison of patients undergoing concomitant arthrodesis versus decompression alone. J Neurosurg. 1996;85:793–802. doi: 10.3171/jns.1996.85.5.0793. [DOI] [PubMed] [Google Scholar]
- 8.Wu A., Zou F., Cao Y. Lumbar spinal stenosis: an update on the epidemiology, diagnosis and treatment. AME Medical J. 2017;2:63. [Google Scholar]
- 9.Barz C., Melloh M., Staub L., Lord S., Merk H., Barz T. Reversibility of nerve root sedimentation sign in lumbar spinal stenosis patients after decompression surgery. Eur Spine J. 2017;26:2573–2580. doi: 10.1007/s00586-017-4962-5. [DOI] [PubMed] [Google Scholar]
- 10.Herkowitz H.N. Spine update: degenerative lumbar spondylolisthesis. Spine (Phila Pa 1976) 1995;20:1084–1090. doi: 10.1097/00007632-199505000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Lurie J., Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ. 2016;352:h6234. doi: 10.1136/bmj.h6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mobbs R.J., Phan K., Malham G., Seex K., Rao P.J. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg. 2015;1:2–18. doi: 10.3978/j.issn.2414-469X.2015.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu A.M., Tong T.J., Wang X.Y. A rethink of fusion surgery for lumbar spinal stenosis. J Evid Based Med. 2016;9:166–169. doi: 10.1111/jebm.12215. [DOI] [PubMed] [Google Scholar]
- 14.Resnick D.K., Watters W.C., 3rd, Mummaneni P.V. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 10: lumbar fusion for stenosis without spondylolisthesis. J Neurosurg Spine. 2014;21:62–66. doi: 10.3171/2014.4.SPINE14275. [DOI] [PubMed] [Google Scholar]
- 15.Peul W.C., Moojen W.A. Fusion for lumbar spinal stenosis—safeguard or superfluous surgical implant? N Engl J Med. 2016;374:1478–1479. doi: 10.1056/NEJMe1600955. [DOI] [PubMed] [Google Scholar]
- 16.Ainslie P.N., Brassard P. Why is the neural control of cerebral autoregulation so controversial? F1000Prime Rep. 2014;6:14. doi: 10.12703/P6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ammendolia C., Stuber K.J., Rok E. Nonoperative treatment for lumbar spinal stenosis with neurogenic claudication. Cochrane Database Syst Rev. 2013;8:CD010712. doi: 10.1002/14651858.CD010712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaina F., Tomkins-Lane C., Carragee E., Negrini S. Surgical versus non-surgical treatment for lumbar spinal stenosis. Cochrane Database Syst Rev. 2016:CD010264. doi: 10.1002/14651858.CD010264.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma X., Zhao X., Ma J., Li F., Wang Y., Lu B. Effectiveness of surgery versus conservative treatment for lumbar spinal stenosis: a system review and meta-analysis of randomized controlled trials. Int J Surg. 2017;44:329–338. doi: 10.1016/j.ijsu.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein J., Lurie J., Tosteson T. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356:2257–2270. doi: 10.1056/NEJMoa070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearson A.M., Lurie J.D., Blood E.A. Spine patient outcomes research trial: radiographic predictors of clinical outcomes after operative or nonoperative treatment of degenerative spondylolisthesis. Spine (Phila Pa 1976) 2008;33:2759–2766. doi: 10.1097/BRS.0b013e31818e2d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carreon L., Glassman S., Howard J. Fusion and nonsurgical treatment for symptomatic lumbar degenerative disease: a systematic review of Oswestry Disability Index and MOS Short Form-36 outcomes. Spine J. 2008;8:747–755. doi: 10.1016/j.spinee.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Sun W., Xue C., Tang X. Selective versus multi-segmental decompression and fusion for multi-segment lumbar spinal stenosis with single-segment degenerative spondylolisthesis. J Orthop Surg Res. 2019;14:46. doi: 10.1186/s13018-019-1092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guha D., Heary R.F., Shamji M.F. Iatrogenic spondylolisthesis following laminectomy for degenerative lumbar stenosis: systematic review and current concepts. Neurosurg Focus. 2015;39:E9. doi: 10.3171/2015.7.FOCUS15259. [DOI] [PubMed] [Google Scholar]
- 25.Hanley E. The indications for lumbar spinal fusion with and without instrumentation. Spine (Phila Pa 1976) 1995;20:154S. [PubMed] [Google Scholar]
- 26.Detwiler P., Marciano F., Porter R., Sonntag V. Lumbar stenosis: indications for fusion with and without instrumentation. Neurosurgical Focus. 1997;3:E6. doi: 10.3171/foc.1997.3.2.7. [DOI] [PubMed] [Google Scholar]
- 27.Shen J., Xu S., Xu S., Ye S., Hao J. Fusion or not for degenerative lumbar spinal stenosis: a meta-analysis and systematic review. Pain Physician. 2018;21:1–8. [PubMed] [Google Scholar]
- 28.Ghogawala Z., Dziura J., Butler W.E. Laminectomy plus fusion versus laminectomy alone for lumbar spondylolisthesis. N Engl J Med. 2016;374:1424–1434. doi: 10.1056/NEJMoa1508788. [DOI] [PubMed] [Google Scholar]
- 29.Inose H., Kato T., Yuasa M. Comparison of decompression, decompression plus fusion, and decompression plus stabilization for degenerative spondylolisthesis. Clin Spine Surg. 2018;31:E347–E352. doi: 10.1097/BSD.0000000000000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Försth P., Olafsson G., Carlsson T. A randomized, controlled trial of fusion surgery for lumbar spinal stenosis. N Engl J Med. 2016;374:1413–1423. doi: 10.1056/NEJMoa1513721. [DOI] [PubMed] [Google Scholar]
- 31.Sundararajan V., Handerson T., Perry C., Muggivan A., Quan H., Ghali W.A. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57:1288–1294. doi: 10.1016/j.jclinepi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Knutsson B., Sanden B., Sjoden G., Jarvholm B., Michaelsson K. Body mass index and risk for clinical lumbar spinal stenosis: a cohort study. Spine (Phila Pa 1976) 2015;40:1451–1456. doi: 10.1097/BRS.0000000000001038. [DOI] [PubMed] [Google Scholar]
- 33.Heo Y., Park J.H., Seong H.Y. Symptomatic adjacent segment degeneration at the L3–4 level after fusion surgery at the L4–5 level: evaluation of the risk factors and 10-year incidence. Eur Spine J. 2015;24:2474–2480. doi: 10.1007/s00586-015-4188-3. [DOI] [PubMed] [Google Scholar]
- 34.Sigmundsson F.G., Jonsson B., Stromqvist B. Outcome of decompression with and without fusion in spinal stenosis with degenerative spondylolisthesis in relation to preoperative pain pattern: a register study of 1,624 patients. Spine J. 2015;15:638–646. doi: 10.1016/j.spinee.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 35.Sciubba D.M., Scheer J.K., Smith J.S. Which daily functions are most affected by stiffness following total lumbar fusion: comparison of upper thoracic and thoracolumbar proximal endpoints. Spine (Phila Pa 1976) 2015;40:1338–1344. doi: 10.1097/BRS.0000000000000968. [DOI] [PubMed] [Google Scholar]
- 36.Faldini C., Di Martino A., Borghi R. Long vs. short fusions for adult lumbar degenerative scoliosis: does balance matters? Eur Spine J. 2015;24(suppl 7):887–892. doi: 10.1007/s00586-015-4266-6. [DOI] [PubMed] [Google Scholar]