To the Editor,
The novel coronavirus SARS‐CoV‐2 is currently spreading around the world, causing one of the largest pandemics in history. Infections with SARS‐CoV‐2 in at risk populations, especially patients with chronic diseases, represent a major healthcare challenge. 1 Of these, severe asthma is of special concern, not only because of the underlying airway disease, but also because of the use of immunomodulatory medications. Over the last years, several types of highly potent immunomodulatory antibodies (biologics) have been approved for the treatment of severe asthma which can improve asthma control and reduce exacerbations and the need for treatments with side effects prone systemic corticosteroids. 2 However, the impact and safety of a treatment with biologics during SARS‐CoV‐2 infections is currently unknown. Here, we report, for the first time, a case of COVID‐19 during treatment with the anti‐IgE antibody omalizumab.
A 52‐year‐old man from Germany (federal province of Mecklenburg‐Western Pomerania) was assessed in our outpatient clinic for the first time in May 2019, with severe, early‐onset allergic asthma (main allergen: house dust mites). He had been treated with a fixed combination of the inhaled corticosteroid (ICS) fluticasone furoate (184 µg daily) and the long‐acting beta‐agonist (LABA) vilanterol (22 µg daily), and the long‐acting muscarinic antagonist (LAMA) tiotropium (18 µg daily). The patient did not suffer from other chronic diseases. Due to recurrent exacerbations and poor asthma control, treatment with the anti‐IgE antibody omalizumab 450 mg q4w was initiated, based on body weight (80 kg) and total IgE serum concentration (253 kU/L). After 6 months of omalizumab treatment (November 2019) (Figure 1), despite persistent airflow limitation, asthma control was improved with no further exacerbations in the last 6 months. Omalizumab treatment was continued, and at home, self‐administration was started. In 2020, he self‐administered omalizumab on January 21st and February 18th.
FIGURE 1.
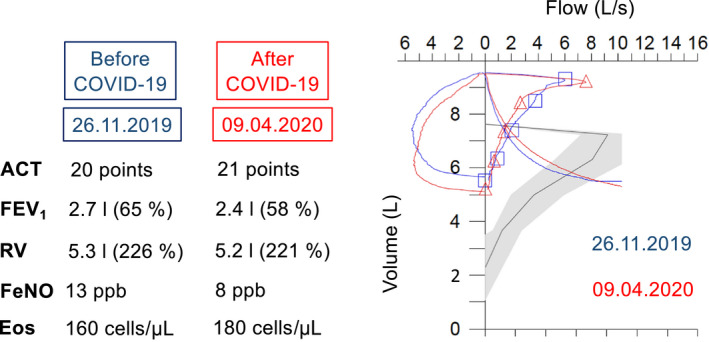
Lung function (measured using body plethysmography), asthma control and biomarkers before and after the SARS‐CoV‐2 infection. The y‐axis of the flow‐volume curve shows the volume (in liters) and the x‐axis the flow (in liters per second). ACT, Asthma Control Test, FeNO, Fraction of exhaled nitric oxide, ppb: parts per billion, Eos, Eosinophils in peripheral blood, FEV1, Forced expiratory volume in the first second of expiration, RV, Residual volume
On March 6, 2020, 4 friends (men between 37 and 52 years of age) and the patient went skiing in Soelden (Austria, federal province of Tyrol). On March 9th, a dry cough developed (Figure 2). The patient reported that he never experienced such a dry cough before. He continued skiing and was not limited in his physical activities. They returned home on March 11th, after a 9 hour car drive. Chills, myalgia, and headache developed in the night from the 11th to the 12th of March, which was followed by fever, fatigue, and a loss of appetite and sense of smell (Figure 2). His local GP ordered a test for SARS‐CoV‐2 which was reported positive on March 13th (during the following days, the 4 other skiers also became ill and were tested positive for SARS‐CoV‐2). Because there was neither shortness of breath nor dyspnea nor any evidence of pneumonia or worsening asthma, he was sent for home quarantine. There was no need for short‐acting bronchodilator (reliever) therapy at any time during the infection.
FIGURE 2.
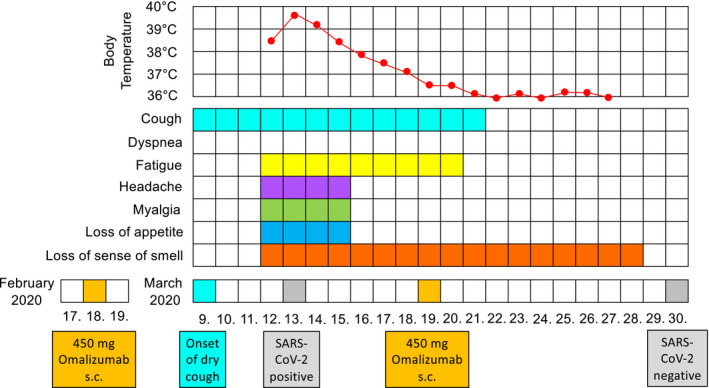
Timeline of symptoms and events before and during the SARS‐CoV‐2 infection
On March 16th, his clinical condition started to improve, although the loss of smell persisted for another 12 days (Figure 2). On the same day, the local physician contacted our asthma treatment center about further administration of omalizumab which was scheduled for March 17th. It was arbitrarily decided to postpone this for another 2 days. Following further clinical improvement, omalizumab was self‐administered at home on March 19th. The patient remained symptom‐free since March 29th and tested negative for SARS‐CoV‐2 on the following day (Figure 2). The patient was reassessed in our outpatient clinic on April 9th (Figure 1). He remained free of symptoms, and there were no significant differences in asthma control or biomarkers, as compared to November 2019 (Figure 1). There was a small decrease in FEV1 (−300 mL; −7% predicted) after COVID‐19, as compared to November 2019 (Figure 1), which might reflect the normal variability of lung function in this patient. However, a minor deterioration in lung function after COVID‐19 cannot be excluded.
There is evidence that patients with asthma might generally be at a lower risk for severe forms of COVID‐19. It has been reported that the proportion of patients with asthma among hospitalized patients with COVID‐19 is low and that hospitalized patients with asthma are not at an increased risk for severe COVID‐19 disease. 1 , 3 , 4 This is supported by data showing that angiotensin‐converting enzyme‐2 (ACE2), the receptor responsible for the uptake of SARS‐CoV‐2, is downregulated in epithelial cells of the respiratory tract of patients with asthma, especially in patients with allergies 5 and in patients treated with ICS. 6 On the other hand, treatment with the anti‐IgE antibody Omalizumab might protect from severe forms of COVID‐19. Omalizumab was shown to enhance anti‐viral immunity via a downregulation of the high‐affinity IgE receptor on plasmacytoid dendritic cells (pDCs), which are essential for anti‐viral immune responses. 7 The PROSE (Preventative Omalizumab or Step‐up Therapy for Severe Fall Exacerbations) study confirmed that the strong reduction of viral exacerbations in children with asthma following Omalizumab treatment is primarily mediated by a downregulation of the high‐affinity IgE receptor on pDCs. 8 , 9
Thus, we hypothesize that the patient described in this case report might have been protected from an asthma exacerbation or pneumonia during COVID‐19, either because of the underlying disease (allergic asthma) or because of the antibody used for treatment (Omalizumab), or both. Therefore, studies are needed to characterize the precise interaction of chronic airway diseases (such as asthma) and of biologics (such as Omalizumab) with SARS‐CoV‐2 infections in humans.
CONCLUSION
Circumstantial evidence suggests that patients with allergic asthma might have a lower risk to develop severe forms of COVID‐19. In addition, the anti‐IgE antibody Omalizumab was shown to enhance anti‐viral immunity. We report a case of a 52‐year‐old man with severe allergic asthma treated with Omalizumab with no evidence of an asthma exacerbation, loss of asthma control or pneumonia during symptomatic COVID‐19 disease. We hypothesize that the underlying disease (allergic asthma) or the antibody used for treatment (Omalizumab), or both, might have exerted protective effects.
CONFLICTS OF INTEREST
ML, PS, and JCV report no conflicts of interest regarding this case report.
Funding information
University of Rostock, Germany.
REFERENCES
- 1. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid‐19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Papadopoulos NG, Barnes P, Canonica GW, et al. The evolving algorithm of biological selection in severe asthma. Allergy. 2020. 10.1111/all.14256. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020. 10.1111/all.14238. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid‐19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. 10.1183/13993003.00547-2020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jackson DJ, Busse WW, Bacharier LB, et al. Association of respiratory allergy, asthma and expression of the SARS‐CoV‐2 Receptor, ACE2. J Allergy Clin Immunol. 2020. 10.1016/j.jaci.2020.04.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peters MC, Sajuthi S, Deford P, et al. COVID‐19 related genes in sputum cells in asthma: relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gill MA, Liu AH, Calatroni A, et al. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J Allergy Clin Immunol. 2018;141(5):1735‐1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Teach SJ, Gill MA, Togias A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136(6):1476‐1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Esquivel A, Busse WW, Calatroni A, et al. Effects of omalizumab on rhinovirus infections, illnesses, and exacerbations of asthma. Am J Respir Crit Care Med. 2017;196(8):985‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]


