Abstract
Zika virus (ZIKV) and nonhuman primates have been inextricably linked since the virus was first discovered in a sentinel rhesus macaque in Uganda in 1947. Soon after ZIKV was epidemiologically associated with birth defects in Brazil late in 2015, researchers capitalized on the fact that rhesus macaques are commonly used to model viral immunity and pathogenesis, quickly establishing macaque models for ZIKV infection. Within months, the susceptibility of pregnant macaques to experimental ZIKV challenge and ZIKV-associated abnormalities in fetuses were confirmed. This review discusses key unanswered questions in ZIKV immunity and in the pathogenesis of the congenital Zika virus syndrome. We focus on those questions that can be best addressed in pregnant nonhuman primates and lessons learned from developing macaque models for ZIKV in the midst of an active epidemic.
Keywords: ZIKV, macaque, arbovirus, flavivirus, Zika virus, pregnant, congenital Zika syndrome
Introduction
Writing a review article on Zika virus (ZIKV) in nonhuman primates is somewhat akin to releasing a greatest hits album after having only one notable song and two studio albums (1). There simply isn’t a large body of knowledge to review. ZIKV was originally discovered causing a febrile illness in a sentinel rhesus macaque in Uganda (2). Macaques are not native to Africa, so acquisition of ZIKV in this sentinel animal does not necessarily reflect dynamics of ZIKV transmission among native African nonhuman primates. Following this initial discovery, ZIKV was a neglected tropical pathogen until the 2015 outbreak in the Americas (3). Responding to newfound concern about ZIKV, several groups, including ours, moved rapidly to study the natural history of infection in macaque monkeys and other nonhuman primates (4–9). But as of this writing, there have only been approximately 30 peer-reviewed manuscripts describing ZIKV infection in rhesus, cynomolgus, and pig-tailed macaques, with most utilizing rhesus macaques. These papers show that ZIKV typically causes brief plasma viremia in macaques and that infections are often clinically inapparent, consistent with the natural history of ZIKV infection in humans. Also similar to humans, congenital ZIKV infection in macaques can cause adverse fetal outcomes, impacts that are collectively termed congenital Zika syndrome (CZS) (10). Meanwhile, scientific reviews of the primary literature have proliferated. More than 780 have been written to date (11), all but seven of which were written after 2016. With this in mind, we refer readers to a selection of recent review articles (see Reference Annotations) that we believe provide an overview of pathogenesis, vaccine prospects, treatment approaches, and animal models for ZIKV. The current review, instead, presents key unanswered questions and the potential value, and limitations, of using pregnant macaque models to address them.
1. Do infected macaques recapitulate key features of human ZIKV infections and CZS?
Human congenital ZIKV infections share many characteristics with the congenital diseases caused by a group of pathogens commonly referred to as TORCH pathogens (Toxoplasma gondii, other agents, rubella virus, cytomegalovirus, and herpes simplex virus). These similarities include brain anomalies, neurologic disease, ocular disease, hearing deficits, preterm delivery, and/or pregnancy loss (10, 12). However, congenital ZIKV infection can be distinguished from infection with the TORCH agents based on the pattern, frequency, and severity of the observed developmental defects such as severe microcephaly, extensive brain calcifications and brain volume loss, hypertonia, and congenital contractures (10, 13) that collectively define CZS. ZIKV-associated birth defects and/or CZS are estimated to occur in approximately 1 in 7 infants born to women infected with ZIKV during pregnancy (14). To date, both rhesus and pigtailed macaques have been used to study vertical ZIKV transmission and model CZS (5, 15–20). While many adverse outcomes have been reported in these macaque pregnancies, fetal demise is the only outcome for which there is sufficient data to support a significantly elevated risk associated with ZIKV infection (21). But this does not mean that macaques are a perfect proxy for human CZS; understanding how macaque and human ZIKV infections in pregnancy are both similar and different is essential to using this model appropriately.
Similar to human ZIKV infections, viral RNA (vRNA) is commonly detected in blood and in other body fluids of infected macaques (15, 16, 20, 22). Interestingly, ZIKV viremia can be substantially prolonged in pregnant women and macaques relative to males or non-pregnant females: viremia can persist >50 days in pregnant hosts, while it is resolved within ~10 days in non-pregnant humans and macaques (8, 23–26). The mechanism(s) of this prolonged viremia are unknown; it is impossible at this time to know whether prolonged viremia is more or less common in macaques than in people, whether the few strains that have been used to model ZIKV infection in macaques are representative of other strains in their ability to cause prolonged viremia, or if the duration of prolonged viremia varies between humans and macaques. Since macaque pregnancies are shorter than human pregnancies, the macaque fetoplacental unit may be exposed to virus for a greater proportion of pregnancy than human fetuses, if the duration of viremia in number of days is the same (Figure 1).
Figure 1.
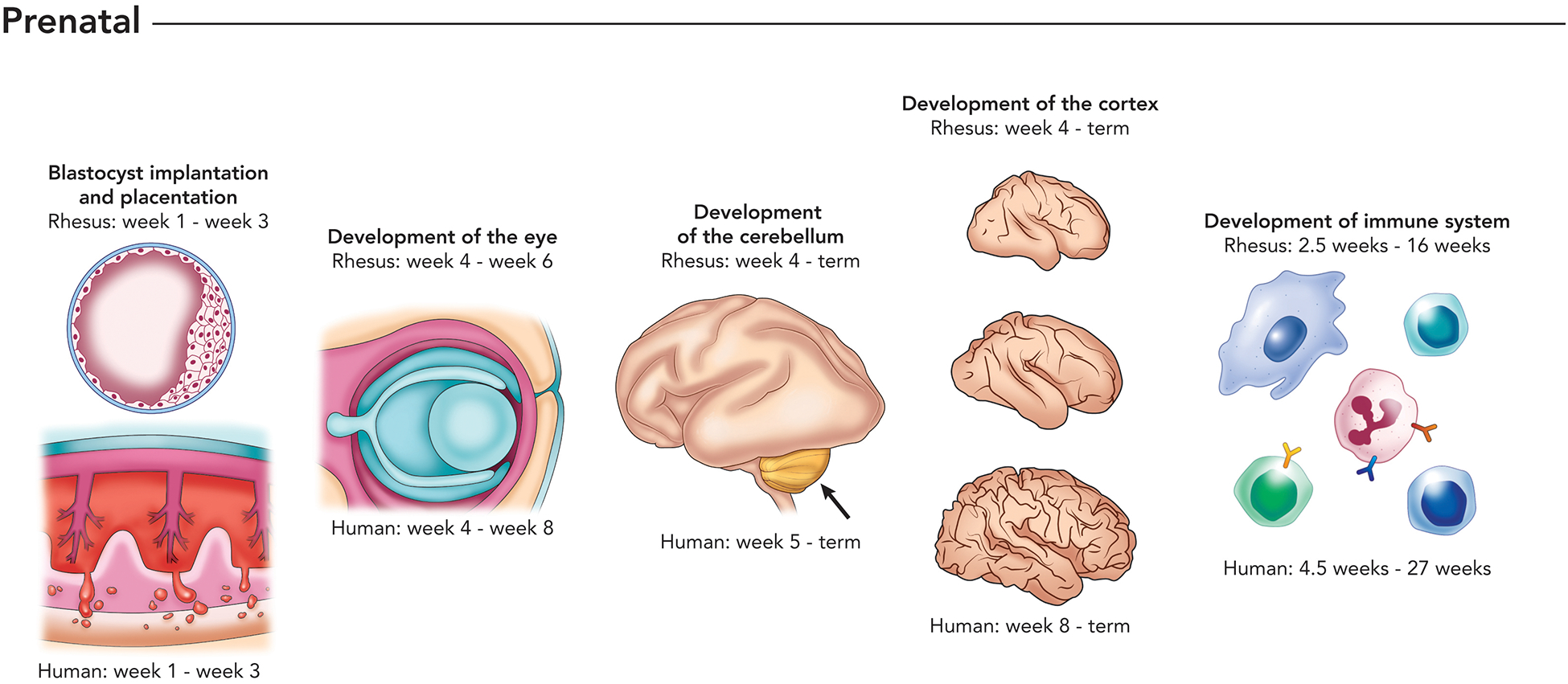
Depiction of stages of prenatal development that have been shown to be impacted by maternal infection with ZIKV, comparing the timeline of rhesus macaque and human fetal development. Developmental milestones include embryo implantation and placenta formation (e.g., placental insufficiency), development of the eye (e.g., choroidal coloboma), cerebellum (e.g., vermis agenesis), cerebral cortex (cortical simplification), and development of the immune system. The macaque and human immune systems are well-developed at birth and are able to mount immune responses to pathogens.
Because overt birth defects occur in a minority of both human and macaque congenital ZIKV infections, data on the manifestations and potential mechanisms of CZS in both humans and macaques remain sparse. Studies in macaques have used a variety of virus strains, as well as timing, route, and dose of inoculation (5, 15, 19, 20). This heterogeneity in design has made it difficult to compare results across macaque studies. Studies focusing on the natural history of congenital ZIKV infection typically use infectious doses in the range thought to be delivered by mosquitoes (104–105 plaque forming units, PFU) administered subcutaneously, either in a single dose or divided among multiple doses (15, 16, 18, 20). Other groups aim to maximize the likelihood of maternal-fetal transmission and fetal injury, using multiple inoculations delivering a combined dose of over 107 PFU (5, 19). Still others have utilized intravenous and intra-amniotic inoculation to ensure systemic maternal infection and/or direct fetal exposure by bypassing the maternal-fetal interface respectively (22). Each of these modes of inoculation has consistently yielded productive infections of macaque dams, congenital transmission, and virologic or histopathologic evidence of ZIKV infection at the maternal-fetal interface and/or the fetus (5, 15, 19, 20).
Subcutaneous ZIKV inoculation of rhesus macaques during the first or late second/early third trimester of pregnancy with 104 PFU of Zika virus/H.sapiens-tc/FRA/2013/FrenchPolynesia-01_v1c1 apparently led to vertical transmission in all cases, with vRNA detected in the maternal-fetal interface tissues, as well as in fetal and maternal lymph nodes, fetal ocular tissues, and other sites (20). In addition, a reduced velocity of head growth relative to femur length was observed for those fetuses late in gestation; however, none of the fetuses met the clinical definition for microcephaly (head circumference 2–3 standard deviations below the mean for gestational age) at delivery, nor were other physiologic anomalies consistent with CZS observed (20). In another study, subcutaneous inoculation with Zika virus PRVABC59 (Human/2015/Puerto Rico) infection in the first trimester led to fetal demise at 49 days post-infection with ocular anomalies including a choroidal coloboma, an abnormality consistent with ZIKV pregnancy outcomes described in humans (15, 27, 28). Conversely, in experimental infection of a pigtailed macaque with a high dose of Cambodian Zika virus/H.sapiens-tc/KHM/2010/FSS13025 (5 × 107 PFU), fetal brain lesions, brain volume loss, and neuronal progenitor cell loss were observed, as well as a lag in the growth of biparietal diameter that fell 3 standard deviations below the published mean (5). These results are especially striking given the late gestational timing of infection (119 days gestation for the animal with the reduced biparietal diameter), as human case reports have suggested that ZIKV infection during the first trimester represents the greatest risk for fetal injury (5, 29, 30).
Intra-amniotic and intravenous inoculation of rhesus macaque dams with ZIKV PRVABC59 was also associated with fetal brain calcifications, loss of neural progenitor cells, and fetal demise (22). In this study it was impossible to determine the extent to which direct intra-amniotic inoculation, bypassing the maternal-fetal interface, contributed to the observed outcomes. Nonetheless, the fetal brain injuries described in these studies are consistent with those described for CZS in humans (5, 13, 19, 22). In particular, the presence of calcifications, loss of neural progenitor cells, and increased fluid spaces in the brain have been noted in both macaques and humans (5, 13, 19, 22). Only one study using subcutaneous inoculation of rhesus macaques with a biologically relevant dose of a Brazilian ZIKV strain (105 PFU) has described extensive fetal brain pathology including smaller brain size, microcalcifications, and hemorrhage (18). The relatively infrequent detection of fetal brain injury with lower ZIKV challenge doses in macaques is consistent with relatively low incidence of overt brain malformations in human congenital infection and suggests that consistent induction of frank fetal neurologic disease in macaques may require artificially high doses of ZIKV or intra-amniotic inoculation (5, 19, 22). How this relates to the dose exposure of human fetuses remains to be determined.
Pregnancy loss and stillbirth is another aspect of congenital ZIKV infection that has been noted in both macaque studies and, less frequently, in humans (15, 17, 21, 31, 32). A meta-analysis showed that ZIKV infection of pregnant rhesus macaques before 55 days of gestation resulted in an almost four-fold increase in the likelihood of pregnancy loss or stillbirth (21). In an examination of human pregnancy outcomes in the French Territories, 11 miscarriages (defined as in utero death before 20 weeks gestation) and 6 stillbirths were reported out of 546 pregnancies with known outcomes for symptomatic ZIKV infections (33). Clinical case reports have also identified fetal loss in ZIKV infection, including one stillbirth during the 32nd week of pregnancy concurrent with severe microcephaly (34–36). However, a 2018 study of surveillance data encompassing all Brazilian states noted that a decrease in births in 2016 was not associated with an increase in miscarriage, stillbirths or abortions, but rather a decrease in the number of pregnancies (37).
Would a normal rate of pregnancies in Brazil during 2016 have led to an observable increase in the number of miscarriages and stillbirths? Likewise, in places where reporting of miscarriage and stillbirth is inconsistent, would an increase in stillbirths or miscarriages even be identified, let alone attributed to infection with ZIKV during pregnancy? 75% of stillbirths globally occur in south Asia and sub-Saharan Africa, with rates in sub-Saharan Africa approximately 10 times higher than in developed countries (38). One study recently estimated that stillbirth rates in south Asia may be 2 times higher than in sub-Saharan Africa (39). In addition to myriad healthcare challenges in resource-constrained African and Asian countries (e.g., limited healthcare access), malaria, syphilis, and HIV infection are also associated with stillbirth, so ZIKV may not necessarily be considered as the cause of a maternal infection leading to stillbirth or miscarriage even in cases when infections are thought to contribute to pregnancy loss (40). Pregnancy outcome data from pregnant macaques infected with ZIKV strains from different parts of the world (e.g., sub-Saharan Africa, south Asia) could serve as sentinel indicators to understand the dimensions of this risk before lengthy and expensive human studies are complete.
2. What is the mechanism of CZS pathogenesis?
Perhaps the most significant outstanding question that can be addressed in macaques is how and under what circumstances ZIKV infection causes CZS. The earliest reports of the impact of maternal ZIKV infection in human pregnancy included information about presence of ZIKV not only in the fetus, but also in the placenta (31, 41–45) These reports also noted ultrasound evidence of increased placental calcifications (43, 46) and substantial histopathology. Subsequently, extensive histopathological analyses of ZIKV-infected humans and nonhuman primates (15, 16, 20, 22), and in-vitro studies have confirmed that the placenta (47, 48) and other components of the maternal-fetal interface (MFI; consisting of the decidua, the uterine endometrium of pregnancy (16, 49–51), and the fetal membranes (49)) are permissive for ZIKV infection. A key unresolved question is whether adverse pregnancy outcomes require direct infection of the fetus itself (i.e., vertical transmission), or whether pathophysiology at the maternal-fetal interface (MFI) without vertical transmission is sufficient to cause adverse outcomes.
Data from mouse studies suggest that pregnancy loss is not solely driven in this model by fetal infection, but rather by immune responses and pathology in the placenta (52, 53). There is precedent for such a mechanism: placental insufficiency in human maternal-fetal medicine is a recognized contributor to intrauterine growth restriction, birth defects, preterm birth, and stillbirth (54–57). Placental insufficiency is a general term for situations in which the placenta cannot meet the needs of the fetus; if the insufficiency is severe this results in fetal loss. Unfortunately, mice do not recapitulate human placentation or gestation so addressing these same questions in macaques will provide insight into the role of placental pathology in human CZS. As in mice, investigators studying pregnant macaques know with certainty the moment of inoculation, the infectious dose, and have the ability to obtain frequent samples to monitor maternal viremia and serially sample the amniotic fluid (22) for ascertaining if/when vertical transmission has occurred. Moreover, advanced imaging techniques to monitor placental function serially and with sufficient frequency to define changes in placental function can be used in macaques (16, 58–60). In particular, MRI for assessing uteroplacental flow and perfusion and oxygenation of the placental cotyledons, the functional units of maternal-fetal oxygen and nutrient exchange, is valuable for assessing MFI competence.
With these tools, there is evidence for significant pathology at the MFI in pregnant macaques infected with ZIKV. In all published studies in pregnant macaques, there has been moderate to severe placental pathology (15, 16, 20–22). The significant histopathological lesions observed in the macaque MFI are remarkably consistent: vasculitis, placental lesions indicating chronic inflammation, including placental villous necrosis, thrombotic lesions and villous infarcts (15, 16, 20–22). Consistent with this, ZIKV RNA has been frequently detected in decidua and placenta (15, 16, 20–22, 61). Overall, macaques infected with ZIKV in the first trimester of pregnancy show a significantly increased risk of adverse pregnancy outcomes across a range of experimental designs, doses, and viral isolates used in macaque studies (21).
Placental insufficiency in human pregnancy is diagnosed by altered umbilical cord blood flow and fetal growth restriction. In the single macaque study in which careful assessment of the placenta was made (16), altered oxygen transport was associated with uteroplacental vasculitis and inflammatory responses. Most studies of ZIKV in pregnant macaques have not revealed a dramatic impact on overall fetal growth, although several studies have noted an apparently selective reduction in the trajectory of fetal head circumference in the final weeks of gestation (15, 20). Longitudinal assessment of placental perfusion monitored by MRI promises to provide greater insight into the ontogeny of placental impact of ZIKV at the MFI and the subsequent fetal outcomes.
3. Could therapeutic interventions fully protect fetuses from CZS?
Interventions that completely protect women from ZIKV infection during pregnancy would be ideal and sufficient for protecting fetuses from CZS. It is encouraging that a relatively low neutralizing antibody titer appears sufficient to confer protection (defined as a lack of plasma viremia) against ZIKV infection (62), as is true for other flaviviruses (63–65). In macaques, primary infection with ZIKV elicits antibodies that are protective against both homologous and heterologous ZIKV rechallenge for at least two years ((8, 66) and unpublished data).
The demonstration that natural infection elicits substantial protective immunity has galvanized efforts to develop ZIKV vaccines, with several promising candidates already in clinical trials (9, 9, 62, 67–71). Yet it is important to note that achieving “real-world” vaccine-mediated protection is not as straightforward as having a product that elicits ZIKV-specific antibodies. There may be challenges associated with deploying ZIKV vaccines in areas of DENV endemicity. Moreover, a vaccine might be optimally effective if used prior to reproductive maturity, but there may be a reluctance to vaccinate children due to lack of education about new vaccines by both healthcare providers and parents, perceived risks associated with vaccines, and health system issues including cost and access (72–74). Since ZIKV is a mild illness rarely associated with prolonged morbidity or mortality in non-pregnant adults, a lack of perceived risk associated with ZIKV could perpetuate vaccine hesitancy prior to pregnancy. Public confidence in vaccines against mosquito-borne viruses has also been shaken by the discovery that the risk of severe disease was paradoxically increased among dengue-naive children receiving a dengue virus (DENV) vaccine (75). Targeting a vaccine program to pregnant women would be difficult given that many pregnancies are unplanned and unrecognized throughout the first trimester, when the risk of CZS is thought to be highest. Given these potential challenges with ZIKV vaccines, alternative prophylaxis and treatment options are also necessary.
One such complementary approach is passive antibody infusion. Theoretically, antibodies could be administered to at-risk women during pregnancy to prevent or treat ZIKV infection when their motivation for preventing CZS would be highest. Would such antibody infusions need to completely prevent maternal ZIKV infection in order to have an impact on CZS? Studies in mice and nonhuman primates show monoclonal antibodies inconsistently prevent ZIKV infection when administered immediately prior to infection (73, 76, 77). In one case, a single monoclonal antibody directed against the envelope domain III (EDIII) of ZIKV selected for escape variant viruses in treated macaques; resistance could be mitigated by co-delivering a second monoclonal antibody targeting a different EDIII epitope (77). In another study, the monoclonal antibody B10, which targets the highly conserved envelope dimer epitope (EDE) region, also failed to eliminate ZIKV from immune privileged sites when used as treatment two days post-infection (73). In a third study (17), a cocktail of monoclonal antibodies recognizing EDII and EDIII given 3 days after infection reduced viral loads but did not prevent vertical transmission, though this same cocktail protected macaques from ZIKV infection when given prophylactically (78). Taken together, these results suggest that additional development of combination mAb therapies is warranted, but that these therapies will require significant optimization and validation in a pregnancy model before being ready for clinical use to prevent CZS after ZIKV infection. Currently there is one phase I clinical trial testing the monoclonal antibody Tyzivumab (79). Monoclonal antibodies can be combined to provide responses against a breadth of epitopes, reducing the risk of selecting escape variants. Another notable advantage to monoclonal antibodies is that they can be altered to abolish interactions with the Fcγ receptor (FcR; for example, by using so-called LALA mutations) (78, 80). This may minimize the risk of enhancing the severity of infection with related flaviviruses; such antibody-dependent enhancement is well described for DENV and is thought to require Fc-FcR interactions on target cells (81).
An alternative polyclonal antibody-based approach is to administer intravenous immunoglobulin (IVIG, purified from ZIKV-infected humans (ZIKV-IVIG), to pregnant women. IVIG is approved for treatment of other viruses such as varicella zoster virus (VZV) during pregnancy to reduce the risk of congenital defects associated with primary VZV infection (82). IVIG also reduces mortality associated with CMV infection during pregnancy in macaques (83). Therefore, there is precedent for IVIG treatment specifically in pregnancy, where many other modalities are frequently not tested given the challenges of ensuring safety to both the mother and the fetus. Since IVIG contains a heterogeneous mix of antibodies, the barrier to the development of resistance should be higher than when using a single monoclonal antibody. Moreover, IVIG contains the complex mix of neutralizing and binding antibodies that are elicited by natural infection. ZIKV-IVIG is currently in phase I clinical trials (84).
A possible complication of antibody-based therapies is that these would likely not cross the placenta into the fetus until late in pregnancy. If it is necessary to restrict fetal ZIKV replication, antiviral drugs that are delivered transplacentally to the fetus might be required to effectively eliminate or reduce CZS (85). Special consideration is also required for treatments used during pregnancy. Nonetheless, screens of known and/or FDA approved compounds have found many drugs with activity against Zika virus in tissue culture and/or mouse studies (reviewed in (85)). Multiple modes of action targeting either the virus replication cycle or host cells have been identified (86, 87). Drug screens have found compounds with anti-ZIKV activity for specific classes of nucleoside analogues/derivatives, the nucleotide analogue Sofosubuvir (FDA approved to treat the flavivirus hepatitis C virus), pyrimidine synthesis inhibitors, anti-cancer drugs, virucidal compounds, methyltransferase inhibitors, and protease inhibitors (88–99). Compounds that target host proteins include antimalarial drugs, drugs that interfere with the endocytic pathway, compounds that inhibit nucleoside or lipid biosynthesis, and kinase inhibitors (86, 100–103). Only two of these drugs have been tested in nonhuman primate models (25-Hydroxycholesterol and favipiravir, an RNA-dependent RNA polymerase inhibitor) but neither drug dramatically reduced peak viremia nor has any work been done in pregnant macaques (104, 105). While many of these drugs with anti-ZIKV activity are FDA approved to treat other viruses, none of them have entered clinical trials as treatments for Zika virus. Overall, we still do not understand what a therapeutic intervention would need to accomplish in order to prevent CZS. The pregnant macaque model will play a critical role in resolving the many remaining unanswered questions in this area.
4. Are fetal impacts limited to infections with Asian/American lineage viruses?
Because ZIKV-associated birth defects were first observed in the Americas, it is reasonable to hypothesize that viruses circulating in this region acquired one or more mutations conferring the ability to cause fetal harm. In accord with this hypothesis, one group found evidence that a single mutation causing a serine-to-asparagine substitution at position 139 of the viral polyprotein (S139N, in the prM protein) arose around the time ZIKV appeared in French Polynesia and persisted in lineages circulating in the Americas (106). ZIKV variants encoding S139N showed enhanced virulence in mouse and human neural progenitor cells and in an exvivo mouse brain model (106). It is tempting to infer from these results that S139N is associated with microcephaly. Indeed, the absence of this mutation in viruses from a recent ZIKV outbreak in India led health officials to downplay the risk of CZS (107).
We argue it is premature to conclude that S139N is unambiguously associated with CZS pathogenesis in humans, or to discount the risk of African and ancestral Asian ZIKV strains causing CZS (108). An Asian ZIKV lacking S139N was associated with a case of microcephaly in a Thai infant (109); ZIKV-associated microcephaly has also been reported in a Vietnamese infant, though no sequence information is available for that case (110). Consistent with these findings, a 2010 ZIKV isolate from Cambodia encoding serine at polyprotein position 139 caused brain lesions in the fetus of a macaque infected during pregnancy (5). There is also evidence that African ZIKV strains have at least a theoretical capacity to cause fetal harm; African-lineage ZIKV was found to be highly cytopathic to human neural progenitor cells, resulting in more extensive cell death than Asian-lineage ZIKV (111). This provides an additional rationale for challenging pregnant macaques with diverse global ZIKV strains under controlled laboratory conditions.
5. Do antigenic interactions between Zika and dengue viruses modulate the risk of CZS?
We have focused so far on potential mechanisms of CZS pathogenesis, but it is important to recall that a substantial proportion of women infected with ZIKV during pregnancy give birth to babies without apparent defects. Is this simply stochastic, or could there be other factors that influence ZIKV pathogenicity? One provocative hypothesis is that interactions between dengue virus (DENV) and ZIKV may increase the risk of severe birth defects. It has long been recognized that pre-existing immunity to DENV can be agathokakological, i.e., potentially helpful or harmful (112). For example, primary infection with one of the four DENV serotypes confers apparently lifelong protection against reinfection with the same serotype, but can predispose individuals to enhanced disease when they are infected with a heterologous serotype (113–115). This phenomenon, called antibody-dependent enhancement, occurs when cross-reactive anti-DENV antibodies facilitate entry of DENV into target cells bearing the Fcγ receptor (116, 117). Antibody-dependent enhancement increases the number of infected cells and may contribute to the higher viremia associated with severe disease (117–119).
It has been proposed that ZIKV could be essentially a “fifth serotype” of DENV (120), since it is transmitted by the same mosquito vectors, is found in overlapping geographic areas, has serological cross-reactivity, and is genetically similar. All global ZIKV isolates sequenced to date appear to circulate as a single serotype (121). ZIKV and DENV share ~30–50% amino acid identity within the three envelope (E) protein domains (122), which mediate fusion and are the main target of neutralizing antibodies (nAb). There are several lines of laboratory evidence supporting the possibility that DENV antibodies can enhance ZIKV replication (123, 123–129) and vice versa (130). However, the majority of these findings are from in vitro assays, mice, or other model systems; few were conducted in the specific pathophysiologic context of pregnancy. Large, case-control studies of pregnant women (e.g., (131)) may prove to be extremely useful for determining whether past DENV infection affects the pathogenesis of ZIKV infection during pregnancy (120). Such studies, however, are observational and are complicated by participant heterogeneity, including history of infection with other flaviviruses, and the precise time, dose, and genetic makeup of the infecting virus. They also may be further complicated by the challenge of finding and enrolling eligible participants, especially as ZIKV incidence wanes. For example, microcephaly was not detected during the French Polynesia outbreak, in which approximately 30,000 people were thought to be infected (132), though suspected cases of microcephaly were identified retrospectively (41). Therefore, it may be difficult to achieve experimental stringency necessary to show that pre-existing immunity to DENV or ZIKV is a risk factor for more severe infection with the other virus during pregnancy unless or until there is another large-scale outbreak of ZIKV.
In the interim, macaque models are well suited to address questions about DENVZIKV immune interactions. Rhesus macaques are susceptible to infection with all four DENV serotypes as well as ZIKV, and infection with both viruses induces a robust nAb response and cellular immune responses that parallel the human immune response (reviewed in (133)). Antibody-dependent enhancement of DENV infection also has been observed in macaques, with higher viremia during secondary infection (134) or when animals are pre-sensitized with an intravenous dose of DENV-immune human cord blood (135). To date, studies in rhesus macaques have demonstrated that DENV-specific antibodies can enhance ZIKV replication in vitro, but there is little evidence that DENV antibodies enhanced ZIKV viremia in vivo in macaques (136, 137), however, this was in nonpregnant animals. Notably, there are no data so far regarding the impact of pre-existing DENV immunity on ZIKV infection during pregnancy. We cannot simply extrapolate results from non-pregnant individuals—pregnancy is associated with major changes to the immune response that are likely related to the maintenance of an allogeneic fetus, including changes in the systemic cytokine milieu, immunoglobulin levels, impaired B cell lymphopoiesis, and high numbers of tolerogenic and regulatory T and B cells (138–140).
6. Can long-term monitoring of macaques exposed to ZIKV in utero anticipate developmental concerns throughout the healthspan?
The oldest children born to mothers infected with ZIKV in 2015 are only three to four years old as of this writing. What will the impacts of congenital ZIKV be as these children reach adolescence and adulthood? The accelerated postnatal development of macaques relative to humans may be useful for anticipating the impacts of congenital ZIKV years before today’s children reach the same developmental milestones (Figure 2). Macaques have been used for decades to model postnatal neurobehavioral development. They provide a translational platform in which the complexity of human brain development can be modeled in another species with similar neurobiology, complex social groups, and the kinds of sophisticated social and communication systems that are lacking in murine models (141, 142). As reviewed elsewhere (141), early social development in infant macaques mirrors human infants in many ways, with the caveat that the macaque infant matures at a rate four times faster than the human infant. However, this caveat may allow us to predict long-term outcomes of CZS much faster using the macaque model. Key parameters of human development that have established macaque model systems include: teratogenesis, autism spectrum disorder, and the development of age-related diseases in the elderly.
Figure 2.
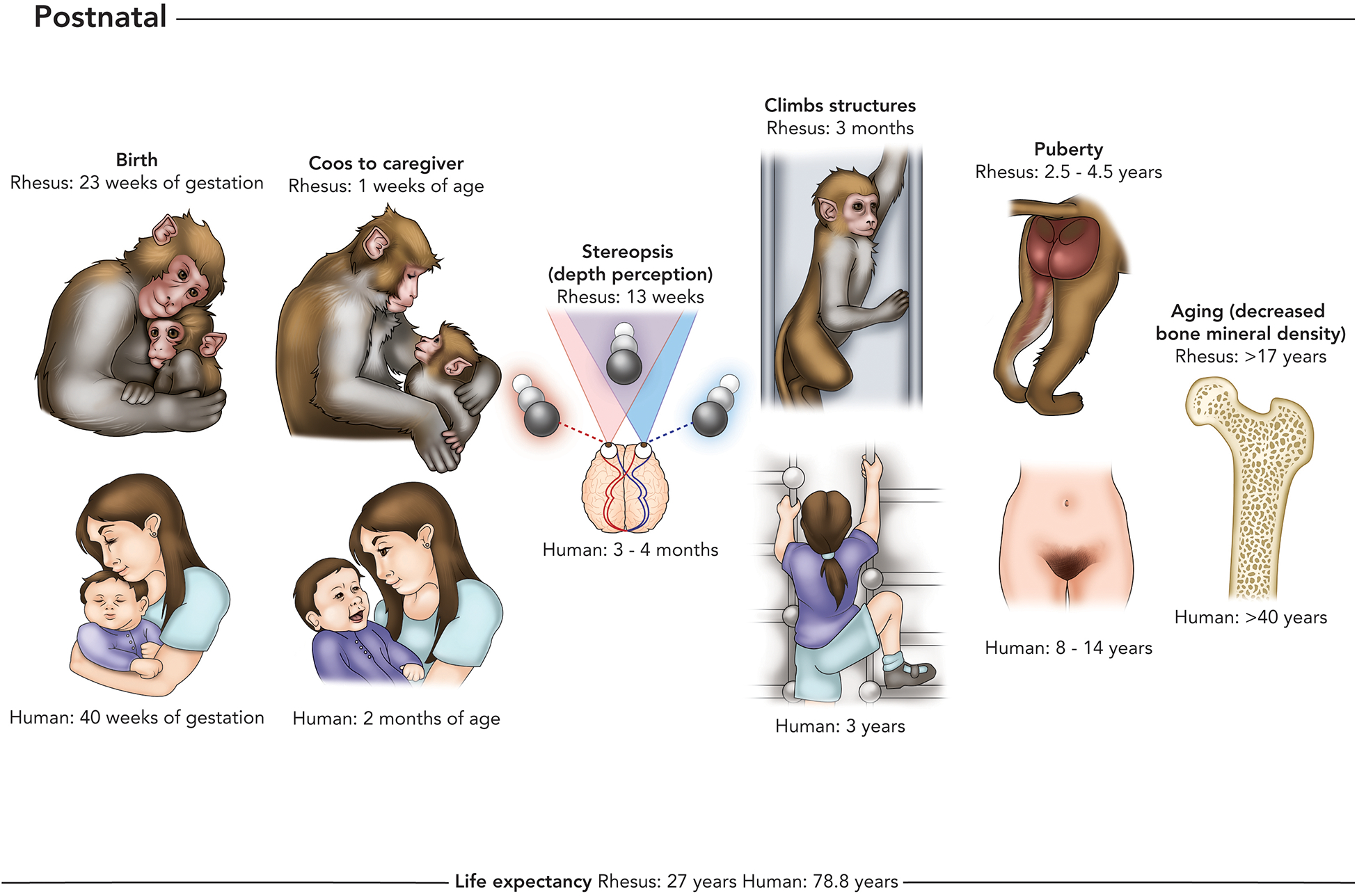
Stages of postnatal development that have been shown to be impacted by maternal infection with ZIKV, or may be identified as affected by congenital ZIKV exposure in long term clinical studies. Postnatal milestones include birth (e.g., higher miscarriage and stillbirth rates in ZIKV-exposed pregnancies), neurological milestones such as caregiver interactions, vision, and gross motor function (e.g. abnormal neurological or neuromuscular development in CZS), and life stage milestones including puberty and aging (these life stages have not been studied in ZIKV congenitally-exposed humans yet). Some children with severe CZS have decreased life expectancy compared with their unaffected human counterparts and it is remains unclear whether less affected infants with CZS will have altered life expectancies compared with their unaffected human counterparts.
Macaque models have been at the forefront of research studies evaluating the connections between teratogens and long-term neurodevelopment (teratogenesis). The link between ZIKV and congenital malformations lead the CDC to declare ZIKV a teratogen (143), therefore much could be learned from the more mature macaque teratology field. One such example is the mechanisms of pathogenesis of fetal alcohol exposure and its effect on neurodevelopmental outcomes. Maternal alcohol consumption during pregnancy by pregnant macaques results in attention and neuromotor functioning deficits in macaque infants, similar to what is seen in human infants (144). Because macaques models are able to integrate behavioral and repeated advanced neuroimaging which is not possible in humans of the same age given the risks of repeated sedation, the neuropathogenesis of alcohol exposure is being defined at a level not possible in human research. Recent advanced imaging studies in macaque infants with fetal alcohol exposure have identified long-term increases in a prefrontal dopamine receptor that are more prevalent in males, which could explain gender differences in the prevalence of neurodevelopmental disorders consequent to prenatal alcohol exposure (145). Determining the mechanism of sex differences in neurodevelopmental outcomes, like gross motor skills, are important in to study children affected by congenital ZIKV infection into the future because there is already a sex difference in the attainment of gross motor skills in early cohort studies in children with congenital ZIKV infection (146).
Another advantage of the macaque model is the capacity to evaluate pharmacologic interventions not possible in humans during childhood. The field of autism spectrum disorder has shown significant advancement by using its complementary NHP model. Autism spectrum disorder (ASD) is a collection of neurodevelopmental disorders characterized by early onset deficits in social behavior and communication caused by a complex interplay of genetic and environmental factors (141). Oxytocin is a possible pharmacologic intervention for autism spectrum disorder that showed enhanced social attachment in rodent models (141). However, given the significant differences between social communication between rodents and humans, it was unclear how this would work in humans (141). Initial studies in macaques have helped to bridge this gap, finding that oxytocin administration to adults amplifies social motivation and attenuates social vigilance. In addition, oxytocin administration increased the number of facial gestures given by the infant macaques to their human caregivers when administered to infants (141). As we begin to understand the pathogenesis of congenital ZIKV infection and its impact on in utero and neonatal brain development, the macaque model will be instrumental in evaluating pharmacologic interventions.
Macaque models are also critical to understand the aging process and identifying interventions that decrease incidence of diseases of the elderly; for example, they are being used to evaluate therapeutic interventions, such as caloric restriction, that increase life expectancy and decrease the incidence of age-related diseases (cancer, cardiovascular disease and diabetes) (147). The application of a macaque model of aging may seem like a stretch when considering the long term outcomes of congenital ZIKV infection. However, research studies in geriatrics are beginning to study the effects of viral infections on morbidity and mortality in the elderly, and are finding that seropositivity with viruses such as cytomegalovirus (CMV) increases the risk of frailty, disability and mortality (148). Subclinical lifelong infection with CMV results in long-lasting pro-inflammatory modulation and an increased risk for type 2 diabetes, atherosclerotic cardiovascular complications, autoimmune diseases and frailty (148). CMV causes a congenital viral infection that can lead to a similar profile of birth defects and adverse neurodevelopmental outcomes as congenital ZIKV infection. Similar research in the area of congenital ZIKV infection should begin evaluating the chronicity of in utero ZIKV infection in immunoprivileged sites to better understand whether a chronic infection occurs and begin understanding whether congenital ZIKV exposure impacts the immune system in a way that results in long-lasting pro-inflammatory modulation and an increased risk for diseases of the elderly.
The lifetime cost of care for a baby born with ZIKV-associated birth defects is expected to exceed $3m USD (Scott Grosse, personal communication). Similarly, long-term macaque experiments are extremely challenging. Neither individual investigator grants nor primate centers are resourced to provide care that is this expensive. Indeed, simply providing routine care and medical management of otherwise healthy macaques for years can be prohibitively expensive, leading most investigators to design studies that can be completed in weeks to months. Nonetheless, the unique opportunities afforded by studying the impact of ZIKV throughout the lifespan might merit this investment. In the event that development is largely uneventful, this would be useful for determining what sort of resourcing and support would be necessary for ZIKV-affected children. If, however, some impacts of ZIKV are not evident until later in life, it would be invaluable to understand this before children reach the same milestones as it would provide an opportunity for clinicians and caregivers to design interventions that could mitigate the severity of these impacts.
7. What lessons can be learned from studying CZS in macaques during the 2016 outbreak?
Studies of pregnant macaques and their offspring are extremely expensive. Consequently, most studies are constrained to using only a small number of animals. This makes federation of data across multiple studies vitally important. This was vividly demonstrated by the aggregation of data from multiple investigators to demonstrate that pregnancy loss is common following infection with ZIKV in the first trimester of pregnancy (21). Such aggregation, however, requires close coordination between investigators. At least some of the challenges in coordinating studies between centers are due to insufficient communication; until recently, data sharing between investigators was slow and cumbersome. Fortuitously, the internet now makes such data sharing easy and straightforward.
Our experience demonstrates the value of real-time data sharing from macaque studies. We posted protocols and data from our study, as it was acquired, to a website, http://zika.labkey.com. Almost immediately, other investigators planning or starting their own macaque studies contacted us for follow-up and additional details. Indeed, some other investigators similarly shared their macaque data in real time. The benefits of data sharing were tangible. Information about the susceptibility of macaques to Asian-lineage and African-lineage ZIKV infections, the biological fluids in which vRNA could be detected, and in-vivo virus replication dynamics were available by the end of March, 2016. The first manuscript describing these results was not published until June, three months later (8). In the setting of a rapidly developing unexpected disease outbreak, these three months were critical as the scientific response to the virus accelerated.
Why is such data sharing not typical in macaque studies? As noted above, macaque experiments are expensive and time-consuming, so there is a fear that making information available to others raises the specter of being “scooped.” Recognizing this concern, we suggest a different perspective. The primary reason to do macaque research urgently during an outbreak is to generate data that can be used to inform the response to the epidemic. Given this, there is a scientific imperative to make data available to the greatest number of people who could use it, as quickly as possible. Secondarily, the close genetic and physiological relationship between nonhuman primates and people is a reason why they are excellent models for human viral diseases, including ZIKV, but it also carries an obligation to learn as much as possible from as few animals as possible. This is codified in the 3Rs (reduce, replace, refine)(149) that are promulgated by the American Academy for the Advancement of Laboratory Animal Science (AAALAS). During future unexpected disease outbreaks, we propose that making datasets from nonhuman primate studies available online in real time should be a precondition for receiving federal funds for research. Finally, there is a concern that sharing research data freely would present those who lobby against macaque research with information that could be weaponized. While this is a legitimate concern, we believe that the most important obligation of researchers is to do the best science possible using the fewest number of macaques, and this necessitates transparency. Siloing research findings to protect them from those who are against macaque research has the unequivocal side-effect of making this same research invisible to researchers whose studies could benefit from it until it is published or presented publicly. It is therefore our strongly held belief that concerns about misappropriation of publicly available data should not be used as a pretext to avoid data sharing.
Conclusion
Even though the immediate risk of ZIKV has receded in the Americas, we believe that large populations of women throughout the world who are immunologically naive remain at significant risk of acquiring ZIKV during pregnancy and having infants with CZS, both in the event of future outbreaks, and in regions where ZIKV may be endemic and CZS is not (yet) ascertained. Pregnant macaques infected with ZIKV provide a translational model system that complements mouse and human studies and will help answer critical questions about CZS risk and pathogenesis.
Acknowledgements
The authors thank Drs. Sallie Permar, Koen Van Rompay, Rob Lipinski, and Esper Kallas for helpful discussions and advice, and Katie Stanley in the UW-Madison School of Medicine and Public Health Medical Illustration service for creative assistance with figure preparation.
Financial disclosures
This review was prepared by authors receiving NIH funding to study ZIKV in macaques (AI116382, AI138647, AI132132 to DHO; HD091163, AI129308, AI136014 and AI132519 to TGG; AI132563 to MTA and TCF). DHO is a consultant on macaque ZIKV projects for Battelle Memorial Institute and has recently served as a biosafety consultant for Takeda Pharmaceutical Company Limited. He has also served as an ad hoc consultant to Insight Strategy Advisors on ZIKV-related issues. None of these relationships impact the views presented herein and all have been reported to and reviewed by the University of Wisconsin-Madison Conflict of Interest Program.
Terms and Definitions
- CZS
Congenital Zika Syndrome. The CDC defines this by the presence of five features: “Severe microcephaly in which the skull has partially collapsed; Decreased brain tissue with a specific pattern of brain damage, including subcortical calcifications; Damage to the back of the eye, including macular scarring and focal pigmentary retinal mottling; Congenital contractures, such as clubfoot or arthrogryposis; Hypertonia restricting body movement soon after birth” (150). Throughout this review we use a more expansive definition of CZS that also includes pregnancy loss.
- MFI
Maternal-fetal interface
Literature Cited
- 1.Andersen K 2009. The Ten Worst Greatest-Hits Albums of All Time. http://www.mtv.com/news/2575998/the-ten-worst-greatest-hits-albums-of-all-time/
- 2.DICK GW, KITCHEN SF, HADDOW AJ 1952. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 46:509–520 [DOI] [PubMed] [Google Scholar]
- 3.Zanluca C, Melo VC, Mosimann AL, Santos GI, Santos CN, Luz K 2015. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz 110:569–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, Omange RW, Best K, Luo M, Hraber PT, Andersen-Elyard H, Ojeda EF, Huang S, Vanlandingham DL, Higgs S, Perelson AS, Estes JD, Safronetz D, Lewis MG, Whitney JB 2016. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med 22:1448–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RP, Studholme C, Boldenow E, Vornhagen J, Baldessari A, Dighe MK, Thiel J, Merillat S, Armistead B, Tisoncik-Go J, Green RR, Davis MA, Dewey EC, Fairgrieve MR, Gatenby JC, Richards T, Garden GA, Diamond MS, Juul SE, Grant RF, Kuller L, Shaw DW, Ogle J, Gough GM, Lee W, English C, Hevner RF, Dobyns WB, Gale M, Rajagopal L 2016. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med 22:1256–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koide F, Goebel S, Snyder B, Walters KB, Gast A, Hagelin K, Kalkeri R, Rayner J 2016. Development of a Zika Virus Infection Model in Cynomolgus Macaques. Front Microbiol 7:2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li XF, Dong HL, Huang XY, Qiu YF, Wang HJ, Deng YQ, Zhang NN, Ye Q, Zhao H, Liu ZY, Fan H, An XP, Sun SH, Gao B, Fa YZ, Tong YG, Zhang FC, Gao GF, Cao WC, Shi PY, Qin CF 2016. Characterization of a 2016 Clinical Isolate of Zika Virus in Non-human Primates. EBioMedicine 12:170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley DM, Aliota MT, Mohr EL, Weiler AM, Lehrer-Brey G, Weisgrau KL, Mohns MS, Breitbach ME, Rasheed MN, Newman CM, Gellerup DD, Moncla LH, Post J, Schultz-Darken N, Schotzko ML, Hayes JM, Eudailey JA, Moody MA, Permar SR, O’Connor SL, Rakasz EG, Simmons HA, Capuano S, Golos TG, Osorio JE, Friedrich TC, O’Connor DH 2016. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun 7:12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, Kirilova M, Li Z, Ng’ang’a D, Nanayakkara O, Nityanandam R, Mercado NB, Borducchi EN, Agarwal A, Brinkman AL, Cabral C, Chandrashekar A, Giglio PB, Jetton D, Jimenez J, Lee BC, Mojta S, Molloy K, Shetty M, Neubauer GH, Stephenson KE, Peron JP, Zanotto PM, Misamore J, Finneyfrock B, Lewis MG, Alter G, Modjarrad K, Jarman RG, Eckels KH, Michael NL, Thomas SJ, Barouch DH 2016. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 353:1129–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miranda-Filho DB, Martelli CM, Ximenes RA, Araújo TV, Rocha MA, Ramos RC, Dhalia R, França RF, Marques Júnior ET, Rodrigues LC 2016. Initial Description of the Presumed Congenital Zika Syndrome. Am J Public Health 106:598–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US National Library of Medicine National Institutes of Health 2018. Pubmed.gov. https://www.ncbi.nlm.nih.gov/pubmed?term=(zika)%20AND%20%22review%22%5BPublication%20Type%5D
- 12.Pereira L 2011. Have we overlooked congenital cytomegalovirus infection as a cause of stillbirth. J Infect Dis 203:1510–1512 [DOI] [PubMed] [Google Scholar]
- 13.Moore CA, Staples JE, Dobyns WB, Pessoa A, Ventura CV, Fonseca EB, Ribeiro EM, Ventura LO, Neto NN, Arena JF, Rasmussen SA 2017. Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA Pediatr 171:288–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice ME, Galang RR, Roth NM, Ellington SR, Moore CA, Valencia-Prado M, Ellis EM, Tufa AJ, Taulung LA, Alfred JM, Pérez-Padilla J, Delgado-López CA, Zaki SR, Reagan-Steiner S, Bhatnagar J, Nahabedian JF, Reynolds MR, Yeargin-Allsopp M, Viens LJ, Olson SM, Jones AM, Baez-Santiago MA, Oppong-Twene P, VanMaldeghem K, Simon EL, Moore JT, Polen KD, Hillman B, Ropeti R, Nieves-Ferrer L, Marcano-Huertas M, Masao CA, Anzures EJ, Hansen RL, Pérez-Gonzalez SI, Espinet-Crespo CP, Luciano-Román M, Shapiro-Mendoza CK, Gilboa SM, Honein MA 2018. Vital Signs: Zika-Associated Birth Defects and Neurodevelopmental Abnormalities Possibly Associated with Congenital Zika Virus Infection - U.S. Territories and Freely Associated States, 2018. MMWR Morb Mortal Wkly Rep 67:858–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohr EL, Block LN, Newman CM, Stewart LM, Koenig M, Semler M, Breitbach ME, Teixeira LBC, Zeng X, Weiler AM, Barry GL, Thoong TH, Wiepz GJ, Dudley DM, Simmons HA, Mejia A, Morgan TK, Salamat MS, Kohn S, Antony KM, Aliota MT, Mohns MS, Hayes JM, Schultz-Darken N, Schotzko ML, Peterson E, Capuano S, Osorio JE, O’Connor SL, Friedrich TC, O’Connor DH, Golos TG 2018. Ocular and uteroplacental pathology in a macaque pregnancy with congenital Zika virus infection. PLoS One 13:e0190617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch AJ, Roberts VHJ, Grigsby PL, Haese N, Schabel MC, Wang X, Lo JO, Liu Z, Kroenke CD, Smith JL, Kelleher M, Broeckel R, Kreklywich CN, Parkins CJ, Denton M, Smith P, DeFilippis V, Messer W, Nelson JA, Hennebold JD, Grafe M, Colgin L, Lewis A, Ducore R, Swanson T, Legasse AW, Axthelm MK, MacAllister R, Moses AV, Morgan TK, Frias AE, Streblow DN 2018. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat Commun 9:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnani DM, Rogers TF, Maness NJ, Grubaugh ND, Beutler N, Bailey VK, Gonzalez-Nieto L, Gutman MJ, Pedreño-Lopez N, Kwal JM, Ricciardi MJ, Myers TA, Julander JG, Bohm RP, Gilbert MH, Schiro F, Aye PP, Blair RV, Martins MA, Falkenstein KP, Kaur A, Curry CL, Kallas EG, Desrosiers RC, Goldschmidt-Clermont PJ, Whitehead SS, Andersen KG, Bonaldo MC, Lackner AA, Panganiban AT, Burton DR, Watkins DI 2018. Fetal demise and failed antibody therapy during Zika virus infection of pregnant macaques. Nat Commun 9:1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinot AJ, Abbink P, Afacan O, Prohl AK, Bronson R, Hecht JL, Borducchi EN, Larocca RA, Peterson RL, Rinaldi W, Ferguson M, Didier PJ, Weiss D, Lewis MG, De La Barrera RA, Yang E, Warfield SK, Barouch DH 2018. Fetal Neuropathology in Zika Virus-Infected Pregnant Female Rhesus Monkeys. Cell 173:1111–1122.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams Waldorf KM, Nelson BR, Stencel-Baerenwald JE, Studholme C, Kapur RP, Armistead B, Walker CL, Merillat S, Vornhagen J, Tisoncik-Go J, Baldessari A, Coleman M, Dighe MK, Shaw DWW, Roby JA, Santana-Ufret V, Boldenow E, Li J, Gao X, Davis MA, Swanstrom JA, Jensen K, Widman DG, Baric RS, Medwid JT, Hanley KA, Ogle J, Gough GM, Lee W, English C, Durning WM, Thiel J, Gatenby C, Dewey EC, Fairgrieve MR, Hodge RD, Grant RF, Kuller L, Dobyns WB, Hevner RF, Gale M, Rajagopal L 2018. Congenital Zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat Med 24:368–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen SM, Antony KM, Dudley DM, Kohn S, Simmons HA, Wolfe B, Salamat MS, Teixeira LBC, Wiepz GJ, Thoong TH, Aliota MT, Weiler AM, Barry GL, Weisgrau KL, Vosler LJ, Mohns MS, Breitbach ME, Stewart LM, Rasheed MN, Newman CM, Graham ME, Wieben OE, Turski PA, Johnson KM, Post J, Hayes JM, Schultz-Darken N, Schotzko ML, Eudailey JA, Permar SR, Rakasz EG, Mohr EL, Capuano S, Tarantal AF, Osorio JE, O’Connor SL, Friedrich TC, O’Connor DH, Golos TG 2017. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog 13:e1006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dudley DM, Van Rompay KK, Coffey LL, Ardeshir A, Keesler RI, Bliss-Moreau E, Grigsby PL, Steinbach RJ, Hirsch AJ, MacAllister RP, Pecoraro HL, Colgin LM, Hodge T, Streblow DN, Tardif S, Patterson JL, Tamhankar M, Seferovic M, Aagaard KM, Martín CS, Chiu CY, Panganiban AT, Veazey RS, Wang X, Maness NJ, Gilbert MH, Bohm RP, Adams Waldorf KM, Gale M, Rajagopal L, Hotchkiss CE, Mohr EL, Capuano SV, Simmons HA, Mejia A, Friedrich TC, Golos TG, O’Connor DH 2018. Miscarriage and stillbirth following maternal Zika virus infection in nonhuman primates. Nat Med 24:1104–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coffey LL, Keesler RI, Pesavento PA, Woolard K, Singapuri A, Watanabe J, Cruzen C, Christe KL, Usachenko J, Yee J, Heng VA, Bliss-Moreau E, Reader JR, von Morgenland W, Gibbons AM, Jackson K, Ardeshir A, Heimsath H, Permar S, Senthamaraikannan P, Presicce P, Kallapur SG, Linnen JM, Gao K, Orr R, MacGill T, McClure M, McFarland R, Morrison JH, Van Rompay KKA 2018. Intraamniotic Zika virus inoculation of pregnant rhesus macaques produces fetal neurologic disease. Nat Commun 9:2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jääskeläinen AJ, Smura T, Rosenberg A, Hill DA, DeBiasi RL, Vezina G, Timofeev J, Rodriguez FJ, Levanov L, Razak J, Iyengar P, Hennenfent A, Kennedy R, Lanciotti R, du Plessis A, Vapalahti O 2016. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N Engl J Med 374:2142–2151 [DOI] [PubMed] [Google Scholar]
- 24.Suy A, Sulleiro E, Rodó C, Vázquez É, Bocanegra C, Molina I, Esperalba J, Sánchez-Seco MP, Boix H, Pumarola T, Carreras E 2016. Prolonged Zika Virus Viremia during Pregnancy. N Engl J Med 375:2611–2613 [DOI] [PubMed] [Google Scholar]
- 25.Oliveira DB, Almeida FJ, Durigon EL, Mendes ÉA, Braconi CT, Marchetti I, Andreata-Santos R, Cunha MP, Alves RP, Pereira LR, Melo SR, Neto DF, Mesquita FS, Araujo DB, Favoretto SR, Sáfadi MA, Ferreira LC, Zanotto PM, Botosso VF, Berezin EN 2016. Prolonged Shedding of Zika Virus Associated with Congenital Infection. N Engl J Med 375:1202–1204 [DOI] [PubMed] [Google Scholar]
- 26.Baud D, Van Mieghem T, Musso D, Truttmann AC, Panchaud A, Vouga M 2016. Clinical management of pregnant women exposed to Zika virus. Lancet Infect Dis 16:523. [DOI] [PubMed] [Google Scholar]
- 27.de Paula Freitas B, de Oliveira Dias JR, Prazeres J, Sacramento GA, Ko AI, Maia M, Belfort R 2016. Ocular Findings in Infants With Microcephaly Associated With Presumed Zika Virus Congenital Infection in Salvador, Brazil. JAMA Ophthalmol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guevara JG, Agarwal-Sinha S 2018. Ocular abnormalities in congenital Zika syndrome: a case report, and review of the literature. J Med Case Rep 12:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin HZ, Tambyah PA, Yong EL, Biswas A, Chan SY 2017. A review of Zika virus infections in pregnancy and implications for antenatal care in Singapore. Singapore Med J 58:171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shapiro-Mendoza CK, Rice ME, Galang RR, Fulton AC, VanMaldeghem K, Prado MV, Ellis E, Anesi MS, Simeone RM, Petersen EE, Ellington SR, Jones AM, Williams T, Reagan-Steiner S, Perez-Padilla J, Deseda CC, Beron A, Tufa AJ, Rosinger A, Roth NM, Green C, Martin S, Lopez CD, deWilde L, Goodwin M, Pagano HP, Mai CT, Gould C, Zaki S, Ferrer LN, Davis MS, Lathrop E, Polen K, Cragan JD, Reynolds M, Newsome KB, Huertas MM, Bhatangar J, Quiñones AM, Nahabedian JF, Adams L, Sharp TM, Hancock WT, Rasmussen SA, Moore CA, Jamieson DJ, Munoz-Jordan JL, Garstang H, Kambui A, Masao C, Honein MA, Meaney-Delman D, Zika PAIRWG 2017. Pregnancy Outcomes After Maternal Zika Virus Infection During Pregnancy - U.S. Territories, January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep 66:615–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brasil P, Pereira JP, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai UA, Salles TS, Zin AA, Horovitz D, Daltro P, Boechat M, Raja Gabaglia C, Carvalho de Sequeira P, Pilotto JH, Medialdea-Carrera R, Cotrim da Cunha D, Abreu de Carvalho LM, Pone M, Machado Siqueira A, Calvet GA, Rodrigues Baião AE, Neves ES, Nassar de Carvalho PR, Hasue RH, Marschik PB, Einspieler C, Janzen C, Cherry JD, Bispo de Filippis AM, Nielsen-Saines K 2016. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N Engl J Med 375:2321–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaub B, Monthieux A, Najihoullah F, Harte C, Césaire R, Jolivet E, Voluménie JL 2016. Late miscarriage: another Zika concern. Eur J Obstet Gynecol Reprod Biol 207:240–241 [DOI] [PubMed] [Google Scholar]
- 33.Hoen B, Schaub B, Funk AL, Ardillon V, Boullard M, Cabié A, Callier C, Carles G, Cassadou S, Césaire R, Douine M, Herrmann-Storck C, Kadhel P, Laouénan C, Madec Y, Monthieux A, Nacher M, Najioullah F, Rousset D, Ryan C, Schepers K, Stegmann-Planchard S, Tressières B, Voluménie JL, Yassinguezo S, Janky E, Fontanet A 2018. Pregnancy Outcomes after ZIKV Infection in French Territories in the Americas. N Engl J Med 378:985–994 [DOI] [PubMed] [Google Scholar]
- 34.van der Eijk AA, van Genderen PJ, Verdijk RM, Reusken CB, Mögling R, van Kampen JJ, Widagdo W, Aron GI, GeurtsvanKessel CH, Pas SD, Raj VS, Haagmans BL, Koopmans MP 2016. Miscarriage Associated with Zika Virus Infection. N Engl J Med 375:1002–1004 [DOI] [PubMed] [Google Scholar]
- 35.Sarno M, Sacramento GA, Khouri R, do Rosário MS, Costa F, Archanjo G, Santos LA, Nery N, Vasilakis N, Ko AI, de Almeida AR 2016. Zika Virus Infection and Stillbirths: A Case of Hydrops Fetalis, Hydranencephaly and Fetal Demise. PLoS Negl Trop Dis 10:e0004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goncé A, Martínez MJ, Marbán-Castro E, Saco A, Soler A, Alvarez-Mora MI, Peiro A, Gonzalo V, Hale G, Bhatnagar J, López M, Zaki S, Ordi J, Bardají A 2018. Spontaneous Abortion Associated with Zika Virus Infection and Persistent Viremia. Emerg Infect Dis 24:933–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castro MC, Han QC, Carvalho LR, Victora CG, França GVA 2018. Implications of Zika virus and congenital Zika syndrome for the number of live births in Brazil. Proc Natl Acad Sci U S A 115:6177–6182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization 2018. Maternal, newborn, child and adolescent health: Stillbirths. https://www.who.int/maternal_child_adolescent/epidemiology/stillbirth/en/
- 39.Alliance for Maternal and Newborn Health Improvement (AMANHI) mortality study group 2018. Population-based rates, timing, and causes of maternal deaths, stillbirths, and neonatal deaths in south Asia and sub-Saharan Africa: a multi-country prospective cohort study. Lancet Glob Health 6:e1297–e1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldenberg RL, McClure EM, Saleem S, Reddy UM 2010. Infection-related stillbirths. Lancet 375:1482–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, Salje H, Van Kerkhove MD, Abadie V, Garel C, Fontanet A, Mallet HP 2016. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet 387:2125–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, Doriqui MJ, Neri JI, Neto JM, Wanderley HY, Cernach M, El-Husny AS, Pone MV, Serao CL, Sanseverino MT, Brazilian MGSETF 2016. Possible Association Between Zika Virus Infection and Microcephaly - Brazil, 2015. MMWR Morb Mortal Wkly Rep 65:59–62 [DOI] [PubMed] [Google Scholar]
- 43.Martines RB, Bhatnagar J, de Oliveira Ramos AM, Davi HP, Iglezias SD, Kanamura CT, Keating MK, Hale G, Silva-Flannery L, Muehlenbachs A, Ritter J, Gary J, Rollin D, Goldsmith CS, Reagan-Steiner S, Ermias Y, Suzuki T, Luz KG, de Oliveira WK, Lanciotti R, Lambert A, Shieh WJ, Zaki SR 2016. Pathology of congenital Zika syndrome in Brazil: a case series. Lancet 388:898–904 [DOI] [PubMed] [Google Scholar]
- 44.Noronha L, Zanluca C, Azevedo ML, Luz KG, Santos CN 2016. Zika virus damages the human placental barrier and presents marked fetal neurotropism. Mem Inst Oswaldo Cruz 111:287–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM 2016. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg. Ultrasound Obstet Gynecol 47:6–7 [DOI] [PubMed] [Google Scholar]
- 46.Soares de Oliveira-Szejnfeld P, Levine D, Melo AS, Amorim MM, Batista AG, Chimelli L, Tanuri A, Aguiar RS, Malinger G, Ximenes R, Robertson R, Szejnfeld J, Tovar-Moll F 2016. Congenital Brain Abnormalities and Zika Virus: What the Radiologist Can Expect to See Prenatally and Postnatally. Radiology 281:203–218 [DOI] [PubMed] [Google Scholar]
- 47.Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V, Vizjak A, Pizem J, Petrovec M, Avsic Zupanc T 2016. Zika Virus Associated with Microcephaly. N Engl J Med 374:951–958 [DOI] [PubMed] [Google Scholar]
- 48.Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, Harris E, Pereira L 2016. Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host Microbe 20:155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Platt DJ, Smith AM, Arora N, Diamond MS, Coyne CB, Miner JJ 2018. Zika virus-related neurotropic flaviviruses infect human placental explants and cause fetal demise in mice. Sci Transl Med 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weisblum Y, Oiknine-Djian E, Vorontsov OM, Haimov-Kochman R, Zakay-Rones Z, Meir K, Shveiky D, Elgavish S, Nevo Y, Roseman M, Bronstein M, Stockheim D, From I, Eisenberg I, Lewkowicz AA, Yagel S, Panet A, Wolf DG 2017. Zika Virus Infects Early- and Midgestation Human Maternal Decidual Tissues, Inducing Distinct Innate Tissue Responses in the Maternal-Fetal Interface. J Virol 91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El Costa H, Gouilly J, Mansuy JM, Chen Q, Levy C, Cartron G, Veas F, Al-Daccak R, Izopet J, Jabrane-Ferrat N 2016. ZIKA virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Sci Rep 6:35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yockey LJ, Jurado KA, Arora N, Millet A, Rakib T, Milano KM, Hastings AK, Fikrig E, Kong Y, Horvath TL, Weatherbee S, Kliman HJ, Coyne CB, Iwasaki A 2018. Type I interferons instigate fetal demise after Zika virus infection. Sci Immunol 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szaba FM, Tighe M, Kummer LW, Lanzer KG, Ward JM, Lanthier P, Kim IJ, Kuki A, Blackman MA, Thomas SJ, Lin JS 2018. Zika virus infection in immunocompetent pregnant mice causes fetal damage and placental pathology in the absence of fetal infection. PLoS Pathog 14:e1006994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.American College of Obstetricians and Gynecologists 2013. ACOG Practice bulletin no. 134: fetal growth restriction. Obstet Gynecol 121:1122–1133 [DOI] [PubMed] [Google Scholar]
- 55.Morgan TK 2016. Role of the Placenta in Preterm Birth: A Review. Am J Perinatol 33:258–266 [DOI] [PubMed] [Google Scholar]
- 56.Salafia CM, Charles AK, Maas EM 2006. Placenta and fetal growth restriction. Clin Obstet Gynecol 49:236–256 [DOI] [PubMed] [Google Scholar]
- 57.Silver RM 2018. Examining the link between placental pathology, growth restriction, and stillbirth. Best Pract Res Clin Obstet Gynaecol 49:89–102 [DOI] [PubMed] [Google Scholar]
- 58.Lo JO, Roberts VHJ, Schabel MC, Wang X, Morgan TK, Liu Z, Studholme C, Kroenke CD, Frias AE 2018. Novel Detection of Placental Insufficiency by Magnetic Resonance Imaging in the Nonhuman Primate. Reprod Sci 25:64–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ludwig KD, Fain SB, Nguyen SM, Golos TG, Reeder SB, Bird IM, Shah DM, Wieben OE, Johnson KM 2018. Perfusion of the placenta assessed using arterial spin labeling and ferumoxytol dynamic contrast enhanced magnetic resonance imaging in the rhesus macaque. Magn Reson Med 6:e1297–e1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Macdonald JA, Corrado PA, Nguyen SM, Johnson KM, Francois CJ, Magness RR, Shah DM, Golos TG, Wieben O 2018. Uteroplacental and Fetal 4D Flow MRI in the Pregnant Rhesus Macaque. J Magn Reson Imaging 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seferovic M, Sánchez-San Martín C, Tardif SD, Rutherford J, Castro ECC, Li T, Hodara VL, Parodi LM, Giavedoni L, Layne-Colon D, Tamhankar M, Yagi S, Martyn C, Reyes K, Suter MA, Aagaard KM, Chiu CY, Patterson JL 2018. Experimental Zika Virus Infection in the Pregnant Common Marmoset Induces Spontaneous Fetal Loss and Neurodevelopmental Abnormalities. Sci Rep 8:6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abbink P, Larocca RA, Visitsunthorn K, Boyd M, De La Barrera RA, Gromowski GD, Kirilova M, Peterson R, Li Z, Nanayakkara O, Nityanandam R, Mercado NB, Borducchi EN, Chandrashekar A, Jetton D, Mojta S, Gandhi P, LeSuer J, Khatiwada S, Lewis MG, Modjarrad K, Jarman RG, Eckels KH, Thomas SJ, Michael NL, Barouch DH 2017. Durability and correlates of vaccine protection against Zika virus in rhesus monkeys. Sci Transl Med 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.World Health Organization 2012. Recommendations to assure the quality, safety and efficacy of live attenuated yellow fever vaccines. https://www.who.int/biologicals/areas/vaccines/TRS_978_Annex_5.pdf?ua=1 [DOI] [PMC free article] [PubMed]
- 64.World Health Organization 2012. Recommendations to assure the quality, safety and efficacy of Japanese encephalitis vaccines (live, attenuated) for human use. https://www.who.int/biologicals/vaccines/JE-Recommendations_TRS_980_Annex_7.pdf?ua=1
- 65.Leonova GN, Pavlenko EV, Maistrovskaya OS, Chausov EV 2011. Protective antibody titer for patients vaccinated against tickborne encephalitis virus. Procedia in Vaccinology 4:84–91 [Google Scholar]
- 66.Aliota MT, Dudley DM, Newman CM, Mohr EL, Gellerup DD, Breitbach ME, Buechler CR, Rasheed MN, Mohns MS, Weiler AM, Barry GL, Weisgrau KL, Eudailey JA, Rakasz EG, Vosler LJ, Post J, Capuano S, Golos TG, Permar SR, Osorio JE, Friedrich TC, O’Connor SL, O’Connor DH 2016. Heterologous Protection against Asian Zika Virus Challenge in Rhesus Macaques. PLoS Negl Trop Dis 10:e0005168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Larocca RA, Abbink P, Peron JP, Zanotto PM, Iampietro MJ, Badamchi-Zadeh A, Boyd M, Ng’ang’a D, Kirilova M, Nityanandam R, Mercado NB, Li Z, Moseley ET, Bricault CA, Borducchi EN, Giglio PB, Jetton D, Neubauer G, Nkolola JP, Maxfield LF, De La Barrera RA, Jarman RG, Eckels KH, Michael NL, Thomas SJ, Barouch DH 2016. Vaccine protection against Zika virus from Brazil. Nature 536:474–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, Dowd KA, Sutherland LL, Scearce RM, Parks R, Wagner W, Granados A, Greenhouse J, Walker M, Willis E, Yu JS, McGee CE, Sempowski GD, Mui BL, Tam YK, Huang YJ, Vanlandingham D, Holmes VM, Balachandran H, Sahu S, Lifton M, Higgs S, Hensley SE, Madden TD, Hope MJ, Karikó K, Santra S, Graham BS, Lewis MG, Pierson TC, Haynes BF, Weissman D 2017. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 543:248–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Modjarrad K, Lin L, George SL, Stephenson KE, Eckels KH, De La Barrera RA, Jarman RG, Sondergaard E, Tennant J, Ansel JL, Mills K, Koren M, Robb ML, Barrett J, Thompson J, Kosel AE, Dawson P, Hale A, Tan CS, Walsh SR, Meyer KE, Brien J, Crowell TA, Blazevic A, Mosby K, Larocca RA, Abbink P, Boyd M, Bricault CA, Seaman MS, Basil A, Walsh M, Tonwe V, Hoft DF, Thomas SJ, Barouch DH, Michael NL 2018. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: phase 1, randomised, double-blind, placebo-controlled clinical trials. Lancet 391:563–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tebas P, Roberts CC, Muthumani K, Reuschel EL, Kudchodkar SB, Zaidi FI, White S, Khan AS, Racine T, Choi H, Boyer J, Park YK, Trottier S, Remigio C, Krieger D, Spruill SE, Bagarazzi M, Kobinger GP, Weiner DB, Maslow JN 2017. Safety and Immunogenicity of an Anti-Zika Virus DNA Vaccine - Preliminary Report. N Engl J Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gaudinski MR, Houser KV, Morabito KM, Hu Z, Yamshchikov G, Rothwell RS, Berkowitz N, Mendoza F, Saunders JG, Novik L, Hendel CS, Holman LA, Gordon IJ, Cox JH, Edupuganti S, McArthur MA, Rouphael NG, Lyke KE, Cummings GE, Sitar S, Bailer RT, Foreman BM, Burgomaster K, Pelc RS, Gordon DN, DeMaso CR, Dowd KA, Laurencot C, Schwartz RM, Mascola JR, Graham BS, Pierson TC, Ledgerwood JE, Chen GL, VRC, VRC, S. T. 2018. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: randomised, open-label, phase 1 clinical trials. Lancet 391:552–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Omer SB, Salmon DA, Orenstein WA, deHart MP, Halsey N 2009. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med 360:1981–1988 [DOI] [PubMed] [Google Scholar]
- 73.Abbink P, Larocca RA, Dejnirattisai W, Peterson R, Nkolola JP, Borducchi EN, Supasa P, Mongkolsapaya J, Screaton GR, Barouch DH 2018. Therapeutic and protective efficacy of a dengue antibody against Zika infection in rhesus monkeys. Nat Med 24:721–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leask J, McIntyre PB 2009. Vaccine refusal and the risks of vaccine-preventable diseases. N Engl J Med 361:723; author reply 723–4 [PubMed] [Google Scholar]
- 75.Larson HJ, Hartigan-Go K, de Figueiredo A 2018. Vaccine confidence plummets in the Philippines following dengue vaccine scare: why it matters to pandemic preparedness. Hum Vaccin Immunother 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fernandez E, Dejnirattisai W, Cao B, Scheaffer SM, Supasa P, Wongwiwat W, Esakky P, Drury A, Mongkolsapaya J, Moley KH, Mysorekar IU, Screaton GR, Diamond MS 2017. Human antibodies to the dengue virus E-dimer epitope have therapeutic activity against Zika virus infection. Nat Immunol 18:1261–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keeffe JR, Van Rompay KKA, Olsen PC, Wang Q, Gazumyan A, Azzopardi SA, Schaefer-Babajew D, Lee YE, Stuart JB, Singapuri A, Watanabe J, Usachenko J, Ardeshir A, Saeed M, Agudelo M, Eisenreich T, Bournazos S, Oliveira TY, Rice CM, Coffey LL, MacDonald MR, Bjorkman PJ, Nussenzweig MC, Robbiani DF 2018. A Combination of Two Human Monoclonal Antibodies Prevents Zika Virus Escape Mutations in Non-human Primates. Cell Rep 25:1385–1394.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Magnani DM, Rogers TF, Beutler N, Ricciardi MJ, Bailey VK, Gonzalez-Nieto L, Briney B, Sok D, Le K, Strubel A, Gutman MJ, Pedreño-Lopez N, Grubaugh ND, Silveira CGT, Maxwell HS, Domingues A, Martins MA, Lee DE, Okwuazi EE, Jean S, Strobert EA, Chahroudi A, Silvestri G, Vanderford TH, Kallas EG, Desrosiers RC, Bonaldo MC, Whitehead SS, Burton DR, Watkins DI 2017. Neutralizing human monoclonal antibodies prevent Zika virus infection in macaques. Sci Transl Med 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tychan Pte Ltd 2018. Safety and Tolerability of an Antibody Against Zika Virus (Tyzivumab) in Humans. https://clinicaltrials.gov/ct2/show/NCT03443830
- 80.Hezareh M, Hessell AJ, Jensen RC, van de Winkel JG, Parren PW 2001. Effector function activities of a panel of mutants of a broadly neutralizing antibody against human immunodeficiency virus type 1. J Virol 75:12161–12168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Whitehead SS, Blaney JE, Durbin AP, Murphy BR 2007. Prospects for a dengue virus vaccine. Nat Rev Microbiol 5:518–528 [DOI] [PubMed] [Google Scholar]
- 82.Centers for Disease Control and Prevention 2013. Updated recommendations for use of VariZIG--United States, 2013. MMWR Morb Mortal Wkly Rep 62:574–576 [PMC free article] [PubMed] [Google Scholar]
- 83.Nelson CS, Cruz DV, Tran D, Bialas KM, Stamper L, Wu H, Gilbert M, Blair R, Alvarez X, Itell H, Chen M, Deshpande A, Chiuppesi F, Wussow F, Diamond DJ, Vandergrift N, Walter MR, Barry PA, Cohen-Wolkowiez M, Koelle K, Kaur A, Permar SR 2017. Preexisting antibodies can protect against congenital cytomegalovirus infection in monkeys. JCI Insight 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Emergent BioSolutions 2018. Study in Healthy Volunteers Evaluating Safety and Pharmacokinetics of Zika Virus Immune Globulin (ZIKV-IG). https://clinicaltrials.gov/ct2/show/NCT03624946
- 85.Saiz JC, Martín-Acebes MA 2017. The Race To Find Antivirals for Zika Virus. Antimicrob Agents Chemother 61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alves MP, Vielle NJ, Thiel V, Pfaender S 2018. Research Models and Tools for the Identification of Antivirals and Therapeutics against Zika Virus Infection. Viruses 10:1385–1394. e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.da Silva S, Oliveira Silva Martins D, Jardim ACG 2018. A Review of the Ongoing Research on Zika Virus Treatment. Viruses 10:89–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zmurko J, Marques RE, Schols D, Verbeken E, Kaptein SJ, Neyts J 2016. The Viral Polymerase Inhibitor 7-Deaza-2’-C-Methyladenosine Is a Potent Inhibitor of In Vitro Zika Virus Replication and Delays Disease Progression in a Robust Mouse Infection Model. PLoS Negl Trop Dis 10:e0004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eyer L, Nencka R, Huvarová I, Palus M, Joao Alves M, Gould EA, De Clercq E, Ruzek D 2016. Nucleoside Inhibitors of Zika Virus. J Infect Dis 214:707–711 [DOI] [PubMed] [Google Scholar]
- 90.Hercík K, Kozak J, Sála M, Dejmek M, Hrebabecky H, Zborníková E, Smola M, Ruzek D, Nencka R, Boura E 2017. Adenosine triphosphate analogs can efficiently inhibit the Zika virus RNA-dependent RNA polymerase. Antiviral Res 137:131–133 [DOI] [PubMed] [Google Scholar]
- 91.Julander JG, Siddharthan V, Evans J, Taylor R, Tolbert K, Apuli C, Stewart J, Collins P, Gebre M, Neilson S, Van Wettere A, Lee YM, Sheridan WP, Morrey JD, Babu YS 2017. Efficacy of the broad-spectrum antiviral compound BCX4430 against Zika virus in cell culture and in a mouse model. Antiviral Res 137:14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Onorati M, Li Z, Liu F, Sousa AMM, Nakagawa N, Li M, Dell’Anno MT, Gulden FO, Pochareddy S, Tebbenkamp ATN, Han W, Pletikos M, Gao T, Zhu Y, Bichsel C, Varela L, Szigeti-Buck K, Lisgo S, Zhang Y, Testen A, Gao XB, Mlakar J, Popovic M, Flamand M, Strittmatter SM, Kaczmarek LK, Anton ES, Horvath TL, Lindenbach BD, Sestan N 2016. Zika Virus Disrupts Phospho-TBK1 Localization and Mitosis in Human Neuroepithelial Stem Cells and Radial Glia. Cell Rep 16:2576–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bullard-Feibelman KM, Govero J, Zhu Z, Salazar V, Veselinovic M, Diamond MS, Geiss BJ 2017. The FDA-approved drug sofosbuvir inhibits Zika virus infection. Antiviral Res 137:134–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sacramento CQ, de Melo GR, de Freitas CS, Rocha N, Hoelz LV, Miranda M, Fintelman-Rodrigues N, Marttorelli A, Ferreira AC, Barbosa-Lima G, Abrantes JL, Vieira YR, Bastos MM, de Mello Volotão E, Nunes EP, Tschoeke DA, Leomil L, Loiola EC, Trindade P, Rehen SK, Bozza FA, Bozza PT, Boechat N, Thompson FL, de Filippis AM, Brüning K, Souza TM 2017. The clinically approved antiviral drug sofosbuvir inhibits Zika virus replication. Sci Rep 7:40920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Adcock RS, Chu YK, Golden JE, Chung DH 2017. Evaluation of anti-Zika virus activities of broad-spectrum antivirals and NIH clinical collection compounds using a cell-based, high-throughput screen assay. Antiviral Res 138:47–56 [DOI] [PubMed] [Google Scholar]
- 96.Pascoalino BS, Courtemanche G, Cordeiro MT, Gil LH, Freitas-Junior L 2016. Zika antiviral chemotherapy: identification of drugs and promising starting points for drug discovery from an FDA-approved library. F1000Res 5:2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carneiro BM, Batista MN, Braga ACS, Nogueira ML, Rahal P 2016. The green tea molecule EGCG inhibits Zika virus entry. Virology 496:215–218 [DOI] [PubMed] [Google Scholar]
- 98.Coutard B, Barral K, Lichière J, Selisko B, Martin B, Aouadi W, Lombardia MO, Debart F, Vasseur JJ, Guillemot JC, Canard B, Decroly E 2017. Zika Virus Methyltransferase: Structure and Functions for Drug Design Perspectives. J Virol 91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee H, Ren J, Nocadello S, Rice AJ, Ojeda I, Light S, Minasov G, Vargas J, Nagarathnam D, Anderson WF, Johnson ME 2017. Identification of novel small molecule inhibitors against NS2B/NS3 serine protease from Zika virus. Antiviral Res 139:49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Delvecchio R, Higa LM, Pezzuto P, Valadão AL, Garcez PP, Monteiro FL, Loiola EC, Dias AA, Silva FJ, Aliota MT, Caine EA, Osorio JE, Bellio M, O’Connor DH, Rehen S, de Aguiar RS, Savarino A, Campanati L, Tanuri A 2016. Chloroquine, an Endocytosis Blocking Agent, Inhibits Zika Virus Infection in Different Cell Models. Viruses 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Balasubramanian A, Teramoto T, Kulkarni AA, Bhattacharjee AK, Padmanabhan R 2017. Antiviral activities of selected antimalarials against dengue virus type 2 and Zika virus. Antiviral Res 137:141–150 [DOI] [PubMed] [Google Scholar]
- 102.Watterson D, Modhiran N, Young PR 2016. The many faces of the flavivirus NS1 protein offer a multitude of options for inhibitor design. Antiviral Res 130:7–18 [DOI] [PubMed] [Google Scholar]
- 103.Saiz JC, Oya NJ, Blázquez AB, Escribano-Romero E, Martín-Acebes MA 2018. Host-Directed Antivirals: A Realistic Alternative to Fight Zika Virus. Viruses 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li C, Deng YQ, Wang S, Ma F, Aliyari R, Huang XY, Zhang NN, Watanabe M, Dong HL, Liu P, Li XF, Ye Q, Tian M, Hong S, Fan J, Zhao H, Li L, Vishlaghi N, Buth JE, Au C, Liu Y, Lu N, Du P, Qin FX, Zhang B, Gong D, Dai X, Sun R, Novitch BG, Xu Z, Qin CF, Cheng G 2017. 25-Hydroxycholesterol Protects Host against Zika Virus Infection and Its Associated Microcephaly in a Mouse Model. Immunity 46:446–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Best K, Guedj J, Madelain V, de Lamballerie X, Lim SY, Osuna CE, Whitney JB, Perelson AS 2017. Zika plasma viral dynamics in nonhuman primates provides insights into early infection and antiviral strategies. Proc Natl Acad Sci U S A 114:8847–8852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yuan L, Huang XY, Liu ZY, Zhang F, Zhu XL, Yu JY, Ji X, Xu YP, Li G, Li C, Wang HJ, Deng YQ, Wu M, Cheng ML, Ye Q, Xie DY, Li XF, Wang X, Shi W, Hu B, Shi PY, Xu Z, Qin CF 2017. A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science 358:933–936 [DOI] [PubMed] [Google Scholar]
- 107.Pulla P 2018. Ground Zero | Stopping the virus — muddled science, poor public health communication mar India’s response to Zika outbreak. The Hindu [Google Scholar]
- 108.Nutt C, Adams P 2017. Zika in Africa-the invisible epidemic. Lancet 389:1595–1596 [DOI] [PubMed] [Google Scholar]
- 109.Wongsurawat T, Athipanyasilp N, Jenjaroenpun P, Jun SR, Kaewnapan B, Wassenaar TM, Leelahakorn N, Angkasekwinai N, Kantakamalakul W, Ussery DW, Sutthent R, Nookaew I, Horthongkham N 2018. Case of Microcephaly after Congenital Infection with Asian Lineage Zika Virus, Thailand. Emerg Infect Dis 24:e0201495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moi ML, Nguyen TTT, Nguyen CT, Vu TBH, Tun MMN, Pham TD, Pham NT, Tran T, Morita K, Le TQM, Dang DA, Hasebe F 2017. Zika virus infection and microcephaly in Vietnam. Lancet Infect Dis 17:805–806 [DOI] [PubMed] [Google Scholar]
- 111.Goodfellow FT, Willard KA, Wu X, Scoville S, Stice SL, Brindley MA 2018. Strain-Dependent Consequences of Zika Virus Infection and Differential Impact on Neural Development. Viruses 10:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rey FA, Stiasny K, Vaney MC, Dellarole M, Heinz FX 2018. The bright and the dark side of human antibody responses to flaviviruses: lessons for vaccine design. EMBO Rep 19:206–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kouri GP, Guzmán MG, Bravo JR 1987. Why dengue haemorrhagic fever in Cuba? 2. An integral analysis. Trans R Soc Trop Med Hyg 81:821–823 [DOI] [PubMed] [Google Scholar]
- 114.Sangkawibha N, Rojanasuphot S, Ahandrik S, Viriyapongse S, Jatanasen S, Salitul V, Phanthumachinda B, Halstead SB 1984. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol 120:653–669 [DOI] [PubMed] [Google Scholar]
- 115.Halstead SB 1989. Antibody, macrophages, dengue virus infection, shock, and hemorrhage: a pathogenetic cascade. Rev Infect Dis 11 Suppl 4:S830–9 [DOI] [PubMed] [Google Scholar]
- 116.Halstead SB, O’Rourke EJ 1977. Antibody-enhanced dengue virus infection in primate leukocytes. Nature 265:739–741 [DOI] [PubMed] [Google Scholar]
- 117.Halstead SB 2003. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res 60:421–467 [DOI] [PubMed] [Google Scholar]
- 118.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, Nisalak A 2000. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 181:2–9 [DOI] [PubMed] [Google Scholar]
- 119.Libraty DH, Endy TP, Houng HS, Green S, Kalayanarooj S, Suntayakorn S, Chansiriwongs W, Vaughn DW, Nisalak A, Ennis FA, Rothman AL 2002. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J Infect Dis 185:1213–1221 [DOI] [PubMed] [Google Scholar]
- 120.Halstead SB 2017. Biologic Evidence Required for Zika Disease Enhancement by Dengue Antibodies. Emerg Infect Dis 23:569–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dowd KA, DeMaso CR, Pelc RS, Speer SD, Smith ARY, Goo L, Platt DJ, Mascola JR, Graham BS, Mulligan MJ, Diamond MS, Ledgerwood JE, Pierson TC 2016. Broadly Neutralizing Activity of Zika Virus-Immune Sera Identifies a Single Viral Serotype. Cell Rep 16:1485–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, Foglierini M, Pedotti M, Simonelli L, Dowall S, Atkinson B, Percivalle E, Simmons CP, Varani L, Blum J, Baldanti F, Cameroni E, Hewson R, Harris E, Lanzavecchia A, Sallusto F, Corti D 2016. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 353:823–826 [DOI] [PubMed] [Google Scholar]
- 123.Bardina SV, Bunduc P, Tripathi S, Duehr J, Frere JJ, Brown JA, Nachbagauer R, Foster GA, Krysztof D, Tortorella D, Stramer SL, García-Sastre A, Krammer F, Lim JK 2017. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 356:175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Charles AS, Christofferson RC 2016. Utility of a Dengue-Derived Monoclonal Antibody to Enhance Zika Infection In Vitro. PLoS Curr 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, Sakuntabhai A, Cao-Lormeau VM, Malasit P, Rey FA, Mongkolsapaya J, Screaton GR 2016. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol 17:1102–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kawiecki AB, Christofferson RC 2016. Zika Virus-Induced Antibody Response Enhances Dengue Virus Serotype 2 Replication In Vitro. J Infect Dis 214:1357–1360 [DOI] [PubMed] [Google Scholar]
- 127.Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, Pattanapanyasat K, Chokephaibulkit K, Mulligan MJ, Wilson PC, Ahmed R, Suthar MS, Wrammert J 2016. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci U S A 113:7852–7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Swanstrom JA, Plante JA, Plante KS, Young EF, McGowan E, Gallichotte EN, Widman DG, Heise MT, de Silva AM, Baric RS 2016. Dengue Virus Envelope Dimer Epitope Monoclonal Antibodies Isolated from Dengue Patients Are Protective against Zika Virus. MBio 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zimmerman MG, Quicke KM, O’Neal JT, Arora N, Machiah D, Priyamvada L, Kauffman RC, Register E, Adekunle O, Swieboda D, Johnson EL, Cordes S, Haddad L, Chakraborty R, Coyne CB, Wrammert J, Suthar MS 2018. Cross-Reactive Dengue Virus Antibodies Augment Zika Virus Infection of Human Placental Macrophages. Cell Host Microbe 24:731–742.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.George J, Valiant WG, Mattapallil MJ, Walker M, Huang YS, Vanlandingham DL, Misamore J, Greenhouse J, Weiss DE, Verthelyi D, Higgs S, Andersen H, Lewis MG, Mattapallil JJ 2017. Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Viremia in Rhesus Macaques. Sci Rep 7:10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.International RTI 2018. Zika in Infants and Pregnancy (ZIP). https://clinicaltrials.gov/ct2/show/NCT02856984
- 132.He D, Gao D, Lou Y, Zhao S, Ruan S 2017. A comparison study of Zika virus outbreaks in French Polynesia, Colombia and the State of Bahia in Brazil. Sci Rep 7:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sariol CA, White LJ 2014. Utility, limitations, and future of non-human primates for dengue research and vaccine development. Front Immunol 5:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Halstead SB, Shotwell H, Casals J 1973. Studies on the pathogenesis of dengue infection in monkeys. II. Clinical laboratory responses to heterologous infection. J Infect Dis 128:15–22 [DOI] [PubMed] [Google Scholar]
- 135.Halstead SB 1979. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J Infect Dis 140:527–533 [DOI] [PubMed] [Google Scholar]
- 136.Pantoja P, Pérez-Guzmán EX, Rodríguez IV, White LJ, González O, Serrano C, Giavedoni L, Hodara V, Cruz L, Arana T, Martínez MI, Hassert MA, Brien JD, Pinto AK, de Silva A, Sariol CA 2017. Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat Commun 8:15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McCracken MK, Gromowski GD, Friberg HL, Lin X, Abbink P, De La Barrera R, Eckles KH, Garver LS, Boyd M, Jetton D, Barouch DH, Wise MC, Lewis BS, Currier JR, Modjarrad K, Milazzo M, Liu M, Mullins AB, Putnak JR, Michael NL, Jarman RG, Thomas SJ 2017. Impact of prior flavivirus immunity on Zika virus infection in rhesus macaques. PLoS Pathog 13:e1006487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Erlebacher A 2013. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol 13:23–33 [DOI] [PubMed] [Google Scholar]
- 139.Munoz-Suano A, Hamilton AB, Betz AG 2011. Gimme shelter: the immune system during pregnancy. Immunol Rev 241:20–38 [DOI] [PubMed] [Google Scholar]
- 140.Moffett A, Loke C 2006. Immunology of placentation in eutherian mammals. Nat Rev Immunol 6:584–594 [DOI] [PubMed] [Google Scholar]
- 141.Bauman MD, Schumann CM 2018. Advances in nonhuman primate models of autism: Integrating neuroscience and behavior. Exp Neurol 299:252–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chang SW, Brent LJ, Adams GK, Klein JT, Pearson JM, Watson KK, Platt ML 2013. Neuroethology of primate social behavior. Proc Natl Acad Sci U S A 110 Suppl 2:10387–10394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR 2016. Zika Virus and Birth Defects--Reviewing the Evidence for Causality. N Engl J Med 374:1981–1987 [DOI] [PubMed] [Google Scholar]
- 144.Schneider ML, Roughton EC, Lubach GR 1997. Moderate Alcohol Consumption and Psychological Stress during Pregnancy Induce Attention and Neuromotor Impairments in Primate Infants. Child Dev 68:747–759 [DOI] [PubMed] [Google Scholar]
- 145.Converse AK, Moore CF, Holden JE, Ahlers EO, Moirano JM, Larson JA, Resch LM, DeJesus OT, Barnhart TE, Nickles RJ, Murali D, Christian BT, Schneider ML 2014. Moderate-level prenatal alcohol exposure induces sex differences in dopamine d1 receptor binding in adult rhesus monkeys. Alcohol Clin Exp Res 38:2934–2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wheeler AC, Ventura CV, Ridenour T, Toth D, Nobrega LL, Silva de Souza Dantas LC, Rocha C, Bailey DB, Ventura LO 2018. Skills attained by infants with congenital Zika syndrome: Pilot data from Brazil. PLoS One 13:e0201495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, de Cabo R, Anderson RM 2017. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun 8:14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Aiello AE, Chiu YL, Frasca D 2017. How does cytomegalovirus factor into diseases of aging and vaccine responses, and by what mechanisms. Geroscience 39:261–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Russell WMS, Burch RL 1959. The Principles of Humane Experimental Technique.
- 150.Centers for Disease Control and Prevention 2018. Congenital Zika Syndrome & Other Birth Defects. https://www.cdc.gov/pregnancy/zika/testing-follow-up/zika-syndrome-birth-defects.html
Reference Annotations
- Caine EA, Jagger BW, Diamond MS 2018. Animal Models of ZIKV Infection during Pregnancy. Viruses 10 [DOI] [PMC free article] [PubMed] [Google Scholar]; Excellent comparison of mouse and macaque models for ZIKV
- Abbink P, Stephenson KE, Barouch DH 2018. ZIKV vaccines. Nat Rev Microbiol 16:594–600 [DOI] [PMC free article] [PubMed] [Google Scholar]; Overview of preclinical and clinical ZIKV vaccines, current as of mid-2018
- Musso D, Gubler DJ 2016. Zika virus. Clin Microbiol Rev 29:487–524 [DOI] [PMC free article] [PubMed] [Google Scholar]; Encyclopedic review of ZIKV epidemiology and laboratory studies prior to the outbreak in the Americas.
- Pierson TC, Diamond MS 2018. The emergence of Zika virus and its new clinical syndromes. Nature 560:573–581 [DOI] [PubMed] [Google Scholar]; Concise overview of scientific understanding of ZIKV
- Alves MP, Vielle NJ, Theil V, Pfaender S 2018. Research Models and Tools for the Identificationof Antivirals and Therapeutics against Zika Virus Infection. Viruses. 10(11):593. [DOI] [PMC free article] [PubMed] [Google Scholar]; Overview of animal models and therapeutic approaches against ZIKV
- Saiz JC, Marin-Acebes MA The Race To Find Antiviral for Zika Virus. Antimicrob Agents Chemother. 61(6). [DOI] [PMC free article] [PubMed] [Google Scholar]; Thorough overview of ZIKV antivirals


