Abstract
Background
The coronavirus disease 2019 (COVID‐19) has evolved into a pandemic infectious disease transmitted by the severe acute respiratory syndrome coronavirus (SARS‐CoV‐2). Allergists and other healthcare providers (HCPs) in the field of allergies and associated airway diseases are on the front line, taking care of patients potentially infected with SARS‐CoV‐2. Hence, strategies and practices to minimize risks of infection for both HCPs and treated patients have to be developed and followed by allergy clinics.
Method
The scientific information on COVID‐19 was analysed by a literature search in MEDLINE, PubMed, the National and International Guidelines from the European Academy of Allergy and Clinical Immunology (EAACI), the Cochrane Library, and the internet.
Results
Based on the diagnostic and treatment standards developed by EAACI, on international information regarding COVID‐19, on guidelines of the World Health Organization (WHO) and other international organizations, and on previous experience, a panel of experts including clinicians, psychologists, IT experts, and basic scientists along with EAACI and the “Allergic Rhinitis and its Impact on Asthma (ARIA)” initiative have developed recommendations for the optimal management of allergy clinics during the current COVID‐19 pandemic. These recommendations are grouped into nine sections on different relevant aspects for the care of patients with allergies.
Conclusions
This international Position Paper provides recommendations on operational plans and procedures to maintain high standards in the daily clinical care of allergic patients while ensuring the necessary safety measures in the current COVID‐19 pandemic.
Keywords: allergen immunotherapy, allergy clinic, anaphylaxis, asthma, clinical trials, COVID‐19, Position Paper, psychological impact, SARS‐CoV‐2
Abbreviations
- AAAAI
American Academy of Allergy, Asthma and Immunology
- ACT
asthma control test
- AD
atopic dermatitis
- AIT
allergen immunotherapy
- AR
allergic rhinitis
- ARIA
Allergic Rhinitis and its Impact on Asthma
- BAD
British Association of Dermatologists
- BAL
bronchoalveolar lavages
- BSL
biosafety level
- COVID‐19
coronavirus disease 2019
- CRF
case report form
- CRS
chronic rhinosinusitis
- CRSwNP
chronic rhinosinusitis with nasal polyps
- CRU
clinical research unit
- DMZ
demilitarized zones
- EAACI
European Academy of Allergy and Clinical Immunology
- ECDC
European Centre for Disease Control
- EHR
Electronic Health record
- EMA
European Medicines Agency
- ENT
ear, nose and throat
- EPIT
epicutaneous immunotherapy
- ERS
European Respiratory Society
- ETFAD
Task Force on Atopic Dermatitis
- FDA
Food and Drug Administration
- FFP
filtering face‐piece particles
- GCP
good clinical practice
- GDPR
General Data Protection Regulation
- GINA
Global Initiative for Asthma
- HCP
healthcare providers
- ICS
inhaled corticosteroids
- ICU
intensive care unit
- IEC
institutional ethic committee
- INCS
intranasal corticosteroids
- IP
investigational product
- IRB
institutional review board
- ISOA
isolated onset of anosmia
- IT
information technology
- MDM
multidisciplinary meetings
- OCS
oral corticosteroids
- OIT
oral immunotherapy
- PAPRs
powered air‐purifying respirators
- PBMC
peripheral blood mononuclear cells
- PEF
peak expiratory flow
- pMDI
pressurized metered‐dose inhaler
- SCIT
subcutaneous AIT
- SCS
systemic corticosteroids
- SLIT
sublingual AIT
- SP
standard precautions
- WHO
World Health Organization
1. INTRODUCTION
On March 11, 2020, the World Health Organization (WHO) declared the “coronavirus disease 2019 (COVID‐19)” as a pandemic viral disease. Since the first transmission dynamics reported in China, 1 the number of infected patients and fatalities have been increasing worldwide. 2 Typical symptoms of COVID‐19 include general malaise, fever, respiratory problems and especially cough and shortness of breath. The clinical pattern differs somewhat from other airway diseases (Table 1).
TABLE 1.
Differences and similarities in the clinical pattern of COVID‐19, common cold, flu, allergic rhinitis, chronic rhinosinusitis and allergic asthma (modified from 3 )
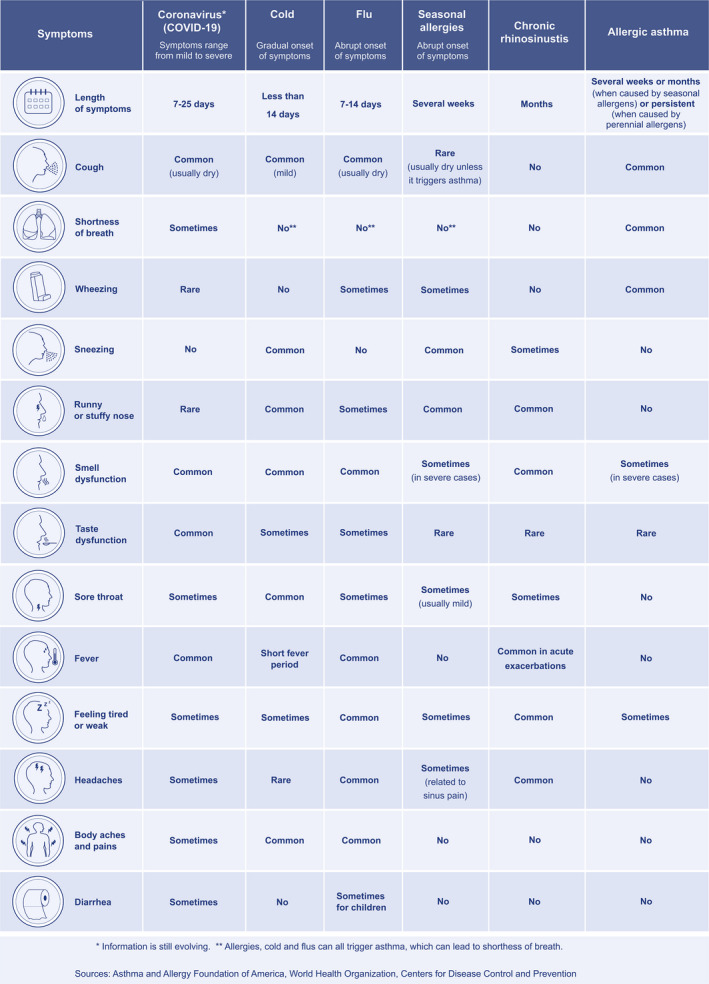
Other symptoms include muscle and joint pain, sore throat, headache, nausea or vomiting, diarrhoea, nasal symptoms and especially smell and taste dysfunction. In about 80% of the registered cases, the disease shows a milder and transient course. However, in about 5% of patients, admission to the intensive care unit (ICU) is necessary due to hypoxaemia and extensive pneumonia, frequently resulting in respiratory failure due to severe acute respiratory syndrome, frequently accompanied by coagulopathy and pulmonary embolism and the involvement of other organs including kidney, heart and the central nervous system. 2 , 4 , 5 Preventive measures have been implemented worldwide to adjust ambulatory health services and decrease direct patient contacts to a minimum. However, until now, there is no clear advice on how to manage allergic patients with co‐morbid COVID‐19 or non‐SARS‐CoV‐2–infected allergic patients during the ongoing pandemic. 6 The European Academy of Allergy and Clinical Immunology (EAACI), in alliance with the global initiative “Allergic Rhinitis and Its Impact on Asthma” (ARIA), has published several recommendations and assessments in the field of allergic diseases such as allergic rhinoconjunctivitis (ARC), allergic asthma and others 7 regarding pharmacotherapy, allergen immunotherapy (AIT), biological treatment and others. 5 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18
As allergists and other healthcare providers (HCPs) with a focus on allergic diseases frequently treat patients with manifestations of atopic disease in the upper and lower airways, they are on the front line in caring for patients potentially infected with SARS‐CoV‐2. As such, the clinical setting in an allergy outpatient clinic or hospital must ensure optimal care for the patients as well as sufficient prophylactic measures to minimize risks of infection for both the medical personnel and the patients requiring treatment, as reported in an academic allergy centre initiative. 19 Therefore, clinical procedures in allergy clinics and outpatient practices must be optimized and standardized, within the contextual considerations regarding national regulations. 20
The aim of this Position Paper—prepared by EAACI in collaboration with ARIA—is to provide allergy clinics, specialized centres and practices with practical recommendations on measures for daily practice and optimal care for allergic patients during the current COVID‐19 pandemic. These recommendations are grouped into nine sections (key conclusions in Table 2) as elaborated by experts including clinicians, psychologists, information technology (IT) experts and basic scientists in the field of allergy. These recommendations are conditional and should be adapted regularly on the basis of evolving clinical evidence.
TABLE 2.
Key conclusions on the practical considerations on the organization of an allergy clinic during the current COVID‐19 pandemic
| Section | Key conclusions |
|---|---|
| COVID‐19: general considerations for HCPs | Protective measures should be taken following the general recommendations from the European Centre for Disease Control and the World Health Organization, and current rules must comply with the national responsible government agencies. |
| COVID‐19: clinical course in allergic patients | Viral infections, including infections with coronaviruses, are associated with aggravation of allergies such as asthma exacerbations. Limited knowledge is available on the differences in the course of COVID‐19 infection in allergic compared with nonallergic patients, and further clinical evidence is needed. |
| Care of allergic patients: preclinical setting and triage of patients | Many clinics and medical offices already use remote healthcare tools to triage and manage patients outside the consultation hours and as part of usual practice. These measures can ideally be used to prioritize and triage allergic patients on the basis of the severity of the allergic disease, the need for in‐person consultation and the differentiation of allergic symptoms from clinical symptoms of COVID‐19. |
| Challenges and chances of information technology (IT) | Digital health solutions, especially the use of telemedicine, have been previously proposed as a useful tool to provide medical advice remotely when physical presence is impossible or should be limited to a strict minimum, such as in the current COVID‐19 pandemic. However, certain limitations of this technology need to be considered and special emphasis should be placed on data security and data protection. |
| Clinical setting | General hygiene rules should be followed, especially in the preclinical and clinical setting. The entrance, which is the first point of contact, patient traffic and the triage of allergic patients should be organized to minimize the risks of viral infection. Moreover, the organization of staff should be optimized and regular training of procedures should be provided. Any physical contact with the patient should be minimized, and effective preventive measures carried out for any further examination and diagnostic. |
| Specific considerations in diagnostic procedures in allergic patients | Specific considerations in a clinical setting are necessary for the diagnostic procedures of different allergic diseases during the current pandemic. As SARS‐CoV‐2 spreads primarily through respiratory aerosols, airways but also other allergy‐related organs are affected, and preventive measures should be ensured. These comprise ENT examinations (including endoscopy), bronchoscopy, nasal or bronchial allergen provocation tests, tissue sampling, lung function tests, skin testing, blood sample collection, drug provocation tests, oral food challenges and oesophageal examinations. |
| Specific considerations in the management of different allergic diseases | Though avoidance measures during the COVID‐19 pandemic are similar in different allergic diseases, specific aspects should also be followed with optimal care for allergic rhinoconjunctivitis, asthma, atopic dermatitis, chronic rhinosinusitis, drug allergy, food allergy, urticaria and venom allergy. Different recommendations can be provided for patients with suspected SARS‐CoV‐2 infection or diagnosed COVID‐19 disease versus noninfected individuals or patients having recovered from COVID‐19 infection. After recovery from COVID‐19, allergy care has to be resumed, but an interdisciplinary consultation is recommended before any further diagnostic or therapeutic procedure. |
| Socio‐psychological considerations for allergic patients and optimal care during and after the pandemic | Socio‐psychological mechanisms play a major role in terms of symptom development, symptom exacerbation and perception in allergic patients. Besides, the general population is highly sensitive to the perception of people showing respiratory symptoms during the COVID‐19 pandemic. This increases the risk of stigmatization of patients with allergies, further enhancing the psychosocial stress of patients. Therefore, optimal medical and psychological care for patients with allergies during the COVID‐19 pandemic is essential. |
| Considerations for performing non‐COVID‐19–related clinical trials | Clinical trials to combat the COVID‐19 pandemic currently have top priority. However, a number of non‐COVID‐19 trials are also essential and should be continued if they can be conducted in a safe manner. Safety measures and new guidelines need to be established for participants, and research/laboratory staff dealing with non‐COVID‐19–related clinical trials, to ensure the continuation of essential and critical non‐COVID‐19 trials. |
2. COVID‐19: GENERAL CONSIDERATIONS FOR HCPs
Viral respiratory infections such as COVID‐19 are most often transmitted by direct contact with the virus from the nose, mouth or coughed/sneezed in droplets from an infected individual. 2 , 21 Hand‐to‐hand contact, inhaling particles from the air after an infected person has coughed or sneezed and touching an infected surface are also common transmission mechanisms. Recommendations for allergic patients—just as for all subjects—include thorough handwashing with soap and water, frequent use of hand sanitizers, avoidance of people with cold‐like symptoms and taking prescribed medications to optimally control upper and/or lower airway disease.
Patients with a suspected or confirmed diagnosis of COVID‐19 should wear a face mask 22 and be treated and examined in an individual room with the door closed. Ideally, an isolation room should be used and equipped with technical measures to protect against airborne infectious agents. 2 , 21
HCPs are at risk of contracting COVID‐19, primarily through droplet spread, with the droplets containing a high reservoir of viral load. 23 If any procedures involving the airway are strictly necessary for COVID‐19–positive patients, it must be ensured that staff are supplied with the necessary personal protective equipment in order to avoid infection and possible fatalities. Filtering face‐piece (FFP2/FFP3) masks, full eye protection or PAPRs (powered, air‐purifying respirators) and further measures are recommended. 24
In accordance with WHO 25 and the European Centre for Disease Control (ECDC 26 ), the following preventive measures are recommended:
keep a distance of at least 1.5‐2 m from other people (social distancing)
promote compliance with general hygiene measures such as regular hand disinfection (using an effective disinfectant)/handwashing for at least 30 seconds, avoid touching your face and mucous membranes with your hands
minimize social contacts (social distancing)
limit direct patient contacts to a strict minimum
wear personal protective clothing
encourage regular surface disinfection, especially door handles, etc.
2.1. Conclusions
Protective measures should be taken following the general recommendations from the European Centre for Disease Control and the World Health Organization, and current rules must comply with the national responsible government agencies.
3. COVID‐19: CLINICAL COURSE IN ALLERGIC PATIENTS
Coronaviruses, just like the common cold viruses, may be associated with aggravation of asthma exacerbations 27 by stimulation of type 2 immune response–associated cytokine production in infected epithelial cells. 28 Allergic diseases might predispose to viral infections or a deferred viral clearance due to delayed and deficient production of the innate type I and type III interferons and/or deficient epithelial barrier function. 29 , 30
Until now, limited knowledge has been available on the differences in the course of COVID‐19 infections in allergic compared with nonallergic patients. Three studies from Wuhan reported allergic diseases (asthma, allergic rhinitis, atopic dermatitis, urticaria or drug hypersensitivity) as co‐morbidities of COVID‐19 patients. 5 , 8 , 31 In a study from Lombardy including over 1500 patients, 13% of COVID‐19 patients admitted to intensive care had asthma. 4 In none of these studies, a prolonged or aggravated disease course was reported for patients with allergic disease compared with the included nonallergic cases. In COVID‐19 patients from the Seattle area, 3 out of 24 patients developed severe respiratory failure after systemic glucocorticoid treatment due to asthma exacerbation. 32 In the United States, higher asthma rates were found in COVID‐19–hospitalized adult patients, especially those between 18 and 49 years. However, no information was available as to whether the asthma was caused by allergies. 33 A recent preliminary report suggested modulation of ACE‐2 receptor levels by type 2 inflammation, which might shed new light on the role of type 2 immunity in SARS‐CoV‐2 infections and the COVID‐19 disease course. 34 Without any doubt, more scientific evidence is needed to answer the question as to whether allergic diseases or the treatment of allergic diseases might predispose patients to COVID‐19 development and disease course.
3.1. Conclusions
Viral infections, including infections with coronaviruses, are associated with aggravation of allergies such as asthma exacerbations. Limited knowledge is available on the differences in the course of COVID‐19 infection in allergic compared with nonallergic patients, and further clinical evidence is needed.
4. CARE OF ALLERGIC PATIENTS: PRECLINICAL SETTING AND TRIAGE OF PATIENTS
Provision of the appropriate level of necessary medical care for patients with allergic diseases and asthma needs to be based on the following principles:
-
Delay elective ambulatory provider visits
Assess the patient's ability and resources to engage in home monitoring
Select the patients needing direct consultation via proper screening protocols
Reschedule any nonessential procedures that might impact on the safety of the patient and the HCP (eg, lung function testing, airway samplings, rhinoscopy, surgery, drug/food/venom provocation tests)
Implement remote healthcare tools
Many clinics and medical offices already use these remote healthcare tools to triage and manage patients outside the consultation hours and as part of usual practice. However, there are several core procedures that need to be taken into account. Primarily, sufficient numbers of healthcare providers (HCPs) should be identified to conduct telephone and telehealth interactions with patients to identify COVID‐19 symptoms. They should be assigned and followed by proper advice on the basis of the differentiation of COVID‐19 symptoms from those due to the common cold, flu, allergic rhinitis, chronic rhinosinusitis and allergic asthma (Table 1). For this purpose, telemedicine is a useful instrument, but should be provided by a dedicated HCP 35 , 36 strictly following the general prerequisites of information technology (Section 5). The triage of urgent cases is highly important to determine those needing in‐person consultation and diagnostic procedures and those who can be managed effectively via telemedicine or scheduled for later dates. However, this important triage should not be undertaken by a medically untrained call centre operator. Individual demands for allergy testing need to be evaluated by experienced nurses or doctors with dedicated skills in taking an allergological history. 36 The technique of documenting allergological history in telemedicine follows generally accepted principles of medical conversation. Standardized and validated questionnaires for quantifying symptoms can complement the analysis of the history, be prefilled by the patient before telemedicine consultation, document the collected data and facilitate computer‐assisted evaluation. 37 Patients with a clear need for in‐person consultation in the allergy clinic (Figure 1) should be identified, triaged to the clinic and instructed on the procedures to be followed in the clinic.
FIGURE 1.
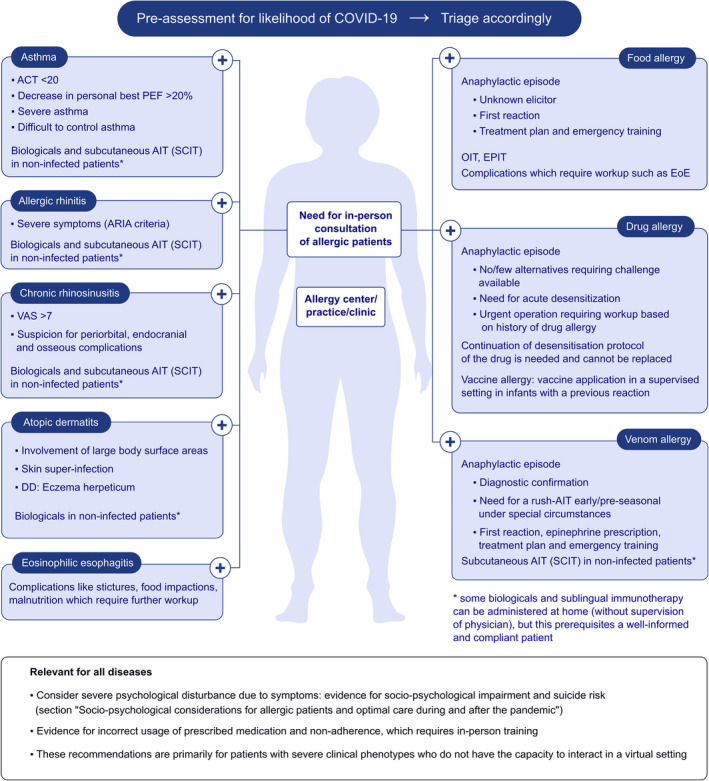
Proposed criteria for in‐person consultation of allergic patients. These recommendations should be considered as general guidelines that always need to be adapted to suit the needs of individual patients, the capabilities of the facility itself and must comply with the relevant and current rules from the responsibility government agency. ACT, asthma control test; AD, atopic dermatitis; ARIA, Allergic Rhinitis and its Impact on Asthma; AR, allergic rhinitis; CRS, chronic rhinosinusitis; EOE, eosinophilic esophagitis; EPIT, epicutaneous immunotherapy; PEF, peak expiratory flow; OIT, oral immunotherapy; SCIT, subcutaneous AIT
For milder forms, patients should be offered alternative solutions whenever possible to avoid any unnecessary risks associated with a real‐life consultation, for example shipping electronic inhalation monitoring devices, peak‐flow metres and providing prescriptions for medication (see section “clinical setting”). In particular, allergic patients with mild symptoms regularly attending the outpatient clinic should be instructed to continue their medication as prescribed (inhaled corticosteroids (ICS), intranasal corticosteroids (INCS), AIT and biologicals targeting the T2 immune response). Whenever possible, on‐site administration in clinics for sublingual AIT and biologicals should be replaced by (self)‐administration at home with close monitoring, and sufficient supplies of medication should be provided. Local community organizations and health services could be engaged to assist patients who are treated at home and who may need support services in order to ensure optimal care. In some cases, trained clinic nurses can assist by telephone. Additionally, these patients should be well‐instructed regarding proper prevention measures for allergen exposure control, for example using peak‐flow measurements or apps, 38 , 39 , 40 and food avoidance 41 (especially with online ordering and shipping where no individual selection of food is feasible). They should also be motivated to notify their HCPs in the case of exacerbation or deterioration of symptoms which cannot be appropriately managed at home.
4.1. Conclusions
Many clinics and medical offices already use these remote healthcare tools to triage and manage patients outside the consultation hours and as part of usual practices. These measures can ideally be used to prioritize and triage allergic patients on the basis of the severity of the allergic disease, the need for in‐person consultation and the differentiation of allergic symptoms from clinical symptoms of COVID‐19.
5. CHALLENGES AND CHANCES OF INFORMATION TECHNOLOGY (IT)
Combining the need for regular consultations and the highest degree of protection for both patients with allergic conditions and healthcare workers is a significant challenge in the current pandemic. Digital health solutions, especially the use of telemedicine, have been previously proposed as a useful tool to provide medical advice remotely when physical presence is impossible. 36 , 42 , 43 , 44 These technologies are now significantly gaining momentum. 45 However, certain limitations need to be considered.
As such, electronic health records (EHRs) have now been adopted by most major healthcare organizations. They facilitate remote access and offer greater flexibility than paper‐based medical records, a factor that is particularly important during the major clinic restructuring that is occurring during the current COVID‐19 pandemic. Here, we discuss some of the online tools and apps that can aid researchers, clinicians and other healthcare staff in working with each other (team communications) and with patients (clinical encounters) while working at different locations.
5.1. Cybersecurity
Every Internet connection to other communication partners also involves a certain risk. Normally, clinical and company IT networks are secured from external networks—for example the Internet—by a complex security infrastructure such as firewalls, separate security zones (DMZ = demilitarized zones), web content filters, intrusion detection systems and virus scanners. These systems protect internal clinical networks and also protect from hacker attacks and insecure processes from the Internet.
If a videoconferencing system has security gaps, the “own network” can become compromised, up to the installation of malware. A few weeks ago, the US Federal Bureau of Investigation (FBI) issued a warning on the security gaps in videoconferencing systems. 46 Security gaps were discovered in the popular communication platform ZOOM ("Zoom vulnerability would have allowed hackers to eavesdrop on calls" 47 ). The use of dedicated computers only for this purpose ‐ operated in a separately secured DMZ ‐is recommended for videoconferencing.
5.2. Data protection and legal regulations
The use of messenger and/or video services in the healthcare sector is particularly worrying from a data protection perspective. It is a classic area handling particularly "sensitive" and protected data. The processing of healthcare data is even prohibited for the time being according to Art. 9 Para. (a) of the General Data Protection Regulation (GDPR). 48 Exceptions to this may be for data processing, insofar as processing is necessary for medical diagnostics, care or treatment in the health or social field. Exceptions may be possible according to Art. 9 Para. (b) of the GDPR. 48
In this context, it should be noted that:
When using videoconferencing apps, the terms and conditions and privacy policies of the providers must be observed.
Usage should be conducted in compliance with institution‐specific policies and country‐specific laws.
Most of these apps prohibit commercial use of the service without a separate agreement. This may call into question the lawful use of these apps for healthcare communications.
If the user agrees to the terms and conditions of the apps, he/she often grants the manufacturers of the apps the rights of use of the transferred data, images, etc. When transmitting personal data, this means a violation of the basic data protection regulation and, if applicable, of medical confidentiality, since unauthorized third parties have access to these data. 49
5.3. Team communication
Commonly used team communication applications include Microsoft Teams, 50 Zoom, 51 Box, 52 WhatsApp 53 and Slack 54 (Table 3). However, these services should not be used for talking about sensitive health data of identifiable persons, but only for team organizational matters.
TABLE 3.
Commonly used team communication applications
| Communication Activity | Technology |
|---|---|
| Team communications | Microsoft Teams, Zoom, Box, WhatsApp, Slack |
| Educational forums | Zoom, Microsoft Teams |
| Patient encounters/communication | Telephone, messenger services a , secure email, Telemedicine (Amwell, Doctor on Demand, Healthtap, MDLive, Teladoc, Zocdoc, Video visits via EHR and others) |
| Research/quality initiatives | REDCap, Box, R markdown |
The use of these services for patient communication is only lawful in the case of very well‐informed written consent of the patient who must be aware of any risks.
Easy accessibility, reliability, videoconferencing, private messaging, creation of distinct channels for relevant discussion and privacy are all important considerations. There exist popular tools that enable online videoconferencing, screen sharing, chats and file sharing. Only the apps approved by the hospitals’ legal department should be used. They can be used for small teams or for larger and more formal meetings, such as weekly unit meetings covering inpatient activity and consultations with relevant educational background. Postclinic multidisciplinary meetings (MDM) remain achievable and desirable to ensure that best practice standards are met and can be hosted on “Teams.” Another popular tool is “Box,” where private health information can be stored and the password encrypted. Folders and documents can be shared, and different levels of access can be provided. Document versions can be managed and archived in an easily accessible and convenient manner. There are also a number of cell phone apps that can be used to communicate with smaller groups. In addition to individually‐owned cell phones, additional cell phones can be shared by members of nursing and administration staff. Office phones can be forwarded to these “hot” phones. Call forwarding between cell phones should be utilized based on a roster for receiving incoming calls.
Many of these tools allow the generation of distinct channels to capture relevant discussions and to ensure patient follow‐up. These can include clinic‐specific channels to capture the logistics of billing, scheduling, rebooking and deferring patients, as well as patient‐specific channels to capture follow‐up required by nurses and doctors. For all channels, the ability to “tag” staff and reply to comments can be utilized.
5.4. Clinical encounters
Virtual doctor consultations as an alternative to on‐site clinical encounters are increasing amid the coronavirus pandemic. Patient communications can take place via telephone, telemedicine/video, secure email and texting apps (Figure 2).
FIGURE 2.
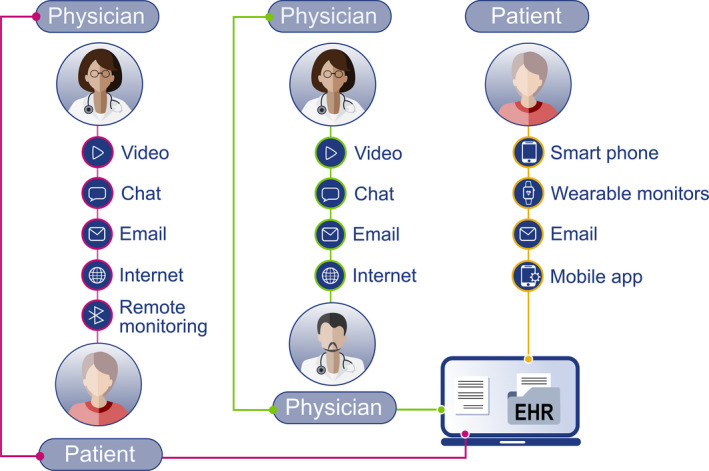
Remote communication between the HCP and the patient. EHR, electronic health record
Video visits with a virtual care environment within the EHR platform are also being offered. 55 It is recommended that an approved, certified procedure should be used for the patient‐related video consultation. These are subject to the respective legal regulations in the individual countries. It is important that the patient should be informed and should give his/her consent for the video consultation. This must be documented in order to have legal security in the case of complaint by the patient. Furthermore, it is important to have a direct "peer‐to‐peer" connection between the doctor's video workplace and the patient's workplace in order to prevent unauthorized third parties from recording the video stream. Corresponding security aspects must be taken into account (see also “cybersecurity”).
5.5. Conclusions
Digital health solutions, especially the use of telemedicine, have been previously proposed as a useful tool to provide medical advice remotely when physical presence is impossible or should be limited to a strict minimum, such as in the current COVID‐19 pandemic. However, certain limitations of this technology need to be considered and special emphasis should be placed on data security and data protection.
6. CLINICAL SETTING
6.1. Public transport to and from a COVID‐19 hospital
Transportation to and from the hospital should follow common healthcare recommendations. Public transport should have clear signs that it is going to the COVID‐19 specialty hospital. At the final stop, the bus should be thoroughly disinfected. The driver's seat should be protected by a transparent plastic wall, and the patients should be at least 2 metres away. 56 The front door of the bus should not open for passengers; only the middle and back doors should be used.
6.2. General hygiene rules
SARS‐CoV‐2 is an enveloped virus, which means it is susceptible to common hard‐surface disinfectants such as soap and alcohol. Hand sanitizers should be provided in every patient room (ideally both inside and outside), in other patient care and common areas, and at all entrances to the building. Sinks should be well‐stocked with soap and paper towels. High‐touch surfaces, such as door handles, need to be regularly cleaned with hospital grade‐approved disinfectant solutions or diluted bleach.
6.3. Entrance to the clinic
A hospital should have only two entrances for the patients: one for emergencies and one for nonemergencies. The emergency entrance should have full patient isolation equipment. The triage doctor should immediately determine the need for full isolation of the patient. Patients who present with an acute respiratory illness and require hospital admission should be tested for SARS‐CoV‐2 RNA expression by RT‐PCR on admission and placed in isolation until the results are ready. 57 Health care, cleaning and kitchen staff should all use a separate entrance. The entrance should be near the dressing rooms with two doors. At the nonemergency entrance to the hospital, masks and gloves should be provided for anyone who enters and it should be obligatory to wear these items. Visitors should be actively discouraged from entering the hospital if possible and in line with local recommendations. Outpatients should be pre‐assessed and triaged accordingly (see Section 4). Patients should wear a surgical mask and gloves, and perform strict and repeated hand hygiene. A separate toilet/bathroom/commode should be used for virus RNA RT‐PCR–positive patients.
6.4. Staff organization
In order to reduce the risk of the healthcare workers becoming infected, which would result in service disruption, new forms of staff deployment need to be elaborated. The personnel in the triage area should wear a FFP2/3 mask as well as a face shield, goggles, gown, gloves and closed shoes.
A two‐team approach has been adopted in many large hospital‐based departments with no or minimal contact between the two teams. A popular method involves:
“Team 1”: inpatient COVID‐19 deployment including consultant, senior registrar and junior medical officer. Several such groups could form a roster depending on unit and hospital size.
“Team 2”: outpatient allergy/asthma/immunology predominantly operating a virtual call centre approach and ideally remotely operating in staff homes.
A designated deputy head of department and subleads for allergy/asthma/immunology provide a backup framework for key personnel in the case of staff infection requiring substantial self‐isolation and resulting in workforce disruption. In COVID‐19 patient–overloaded clinics, day and night shifts can be decreased to 6 hours in order to avoid extreme fatigue and thus reduce the risk of HCP errors and infection. Moreover, there is an urgent need for staff training resources and mechanisms to ensure a constant re‐training of the most important policies. This regular training has to involve all of the staff members.
6.5. Patient organization
Any service that does not require a diagnostic or on‐site therapeutic procedure should be undertaken via telemedicine consultation. 58
For regular nonacute care: only patients requiring a timely diagnostic or therapeutic procedure should be seen in the hospital. A history of identifying potentially infected contacts, recent travel and early symptoms such as anosmia and dysgeusia 59 , 60 should be obtained from all patients before any in‐person consultation in the clinical setting. 61 There is now ample evidence that COVID‐19 may be contagious before the onset of the classical symptoms of cough and high fever. 8 Therefore, identifying the early symptoms of COVID‐19 is of particular importance and is a health system priority. Recently, a probable association between COVID‐19 and altered olfactory and gustatory function has been reported by several groups, often as the presenting symptom. 60 , 62 To evaluate whether this could be a first symptom of COVID‐19 can be particularly challenging when treating allergic and rhinosinusitis patients.
Establishing COVID‐19–free zones in the hospital includes a strict screening protocol to ensure that patients who are entering the clinic are not infected by COVID‐19.
Patients who have indications of potential COVID‐19 infection (classical COVID‐19 symptoms, fever, but also anosmia, and/or recent contact with a COVID‐19–positive subject) should not be admitted to these areas. 63 These patients should be guided to the COVID‐19 clinic for further screening evaluation.
To decrease the density of patients, the waiting area should be separated from the treatment area and the number of appointments reduced. Appointments should be scheduled with ample time intervals and online consulting services should be available whenever possible. 64
Precise appointment times are particularly important during the COVID‐19 pandemic as this can greatly reduce patient‐patient and patient‐HCP hospital‐acquired cross‐infections. Also, precise appointments can ensure that patients stay in the hospital for a minimum amount of time and that the medical staff are fully prepared with personal protective equipment. Moreover, the patient should be admitted to a consulting room with good ventilation, and, at the same time, there should not be more than one patient in the waiting area for visiting or for postimmunotherapy observation.
6.6. In the consultation room
All physical contact with the patient should be limited to a strict minimum. When caring for a patient with suspected COVID‐19, staff are recommended to wear a gown, gloves and either an FFP/FFP2 (or local equivalent) respirator plus face shield and goggles, or a powered air‐purifying respirator. However, in hospital areas outside potential SARS‐CoV‐2–contaminated zones, there is conflicting advice on the necessary level of personal protective equipment (even when available), and evidence‐based recommendations are scarce. Gloves and face masks 22 may be considered for use near entrances and in common areas. Personal protective equipment should be available at the entrance of patient rooms and in other areas where patient care is provided. Signs at the entrance of patient rooms should clearly indicate the level of personal protective equipment required.
Diagnostic procedures involving upper airway manipulation such as anterior rhinoscopy and, even more, nasal endoscopy, or nasal provocation, should be considered as high risk of viral transmission. These procedures should therefore be limited to patients with an urgent need of examination during the initial phase of the COVID‐19 outbreak. For further details, see nasal endoscopy during COVID‐19. 24
6.7. Conclusions
General hygiene rules should be followed, especially in the preclinical and clinical setting. The entrance, which is the first point of contact, patient traffic and the triage of allergic patients should be organized to minimize the risks of viral infection. Moreover, the organization of staff should be optimized and regular training of procedures should be provided. Any physical contact with the patient should be minimized, and effective preventive measures carried out for any further examination and diagnostic.
7. SPECIFIC CONSIDERATIONS IN DIAGNOSTIC PROCEDURES IN ALLERGIC PATIENTS
The following sections overview the specific considerations for diagnostic procedures in different allergic diseases in a clinical setting during the current pandemic. The indication and the urgency for these tests should be taken into account and can be confirmed, for example, by an initial visit performed via telemedicine. Contraindications for skin tests, provocation tests and lung function tests can be clarified, and this can help to avoid unnecessary in‐person consultations with patients during the COVID‐19 pandemic.
7.1. ENT examination, nasal provocation testing and sampling procedures
SARS‐CoV‐2 spreads primarily through respiratory aerosols, and higher viral loads have been detected in nasal swabs compared with other locations. 65 Thus, rhinoscopy, nasal endoscopy, nasal provocation testing, smell and taste testing and samplings are high‐risk procedures. Nasal provocation tests should be avoided, whereas rhinoscopy, endoscopy and nasal samplings should be limited to patients with an urgent need for examination. 64 A tower with camera, screen and light source can maximize the examiner‐patient distance during endoscopy. 24 The use of anaesthetic spray can be replaced by a soaked pledget, thus avoiding virus atomization. 24 The examiner should wear the adequate personal protective equipment recommended for HCPs: FFP2 or FFP3 face mask, goggles or disposable face shield covering the front and sides of the face, clean gloves and clean isolation gown. 66
7.2. Lung function testing, bronchoprovocation tests and lower airway sampling
Aerosols can be generated during spirometry, bronchoprovocation testing, fractionated exhaled nitric oxide (FeNO) measurement and other lower airway sampling procedures. 67 , 68 , 69 Therefore, routine lung function testing and related procedures should be generally suspended during the current pandemic. In cases of extreme need, the personnel should use personal protective equipment and follow the other safety measures as described above. 66 , 70
7.3. Skin testing and blood sample collection for diagnostic use
Skin testing should be generally suspended during the current pandemic. Nevertheless, exceptions can be considered after a careful/proper risk‐benefit assessment or may be replaced by laboratory tests. When collecting biological samples or conducting skin testing, the personnel must use the recommended personal protective equipment 66 and also follow the standard precautions (SP) when handling clinical specimens, all of which may contain potentially infectious materials. 71 In this case, a laboratory gown and a single‐use waterproof apron may replace the isolation gown. 72 , 73 After collection, samples should be placed in a leakproof primary container and inserted into watertight secondary packaging with absorbent material. This package should be placed in a rigid outer packaging with appropriate labelling. 74 Sample processing should be performed following biosafety level 2 (BLS‐2) practices, the current standard in clinical laboratories. Aerosols can originate from centrifugation, pipetting, vortexing, mixing, decanting liquids, loading and spilling samples or cleaning up spills. Therefore, these procedures should be performed inside a biological safety cabinet and using centrifuge safety cups and sealed rotors. 71 Work surfaces and equipment should be appropriately decontaminated and laboratory waste should be handled as biohazardous agents. 75 The inactivation of serum samples suspected to be contaminated with SARS‐CoV‐2 should be carried out by following the procedure recommended by WHO for serum samples for ELISA‐based analysis. 76
7.4. Sample collection for research use
Research procedures involving virus isolation and propagation in cell culture should be conducted in a BSL‐3 laboratory. 77 The appropriate minimum containment measures for research procedures other than virus propagation (eg, flow cytometry) are currently unclear. The addition of a virus‐neutralizing agent to research samples might be considered to ensure safe processing under BSL‐2 conditions. 77
7.5. Drug provocation tests
Cough, sneezing or rhinorrhoea may occur during drug provocation tests. 78 , 79 Therefore, these procedures should not be generally conducted during the current pandemic. 79 Nevertheless, exceptions can be considered after a proper risk‐benefit assessment. Examples of these include chemotherapy in oncologic patients, perioperative drugs or radiocontrast media in subjects needing urgent procedures, or antibiotics in infected individuals without any alternative effective drug. 80 , 81
7.6. Oral food challenges and oesophageal examination
Oral food challenge may induce respiratory symptoms with aerosol‐generating potential, together with vomiting and diarrhoea. 82 Importantly, the virus can persist in gastrointestinal fluids for a longer period than in the respiratory specimens. 83 Therefore, oral food challenges should be avoided during the current pandemic, as they lack urgency. 84 The diagnosis of eosinophilic oesophagitis requires a gastroscopy‐guided oesophageal biopsy. 85 The performance of a gastroscopy is not recommended during the current pandemic, due to the possible persistence of virus in biological fluids. 86 In the case of extreme need (eg, frequent food impaction), a proper risk‐benefit assessment should be conducted. 87
7.7. Conclusions
Specific considerations in a clinical setting are necessary for the diagnostic procedures of different allergic diseases during the current pandemic. As SARS‐CoV‐2 spreads primarily through respiratory aerosols, airways but also other allergy‐related organs are affected, and preventive measures should be ensured. These comprise ENT examinations (including endoscopy), bronchoscopy, nasal or bronchial allergen provocation tests, tissue sampling, lung function tests, skin testing, blood sample collection, drug provocation tests, oral food challenges and oesophageal examinations.
8. SPECIFIC CONSIDERATIONS IN THE MANAGEMENT OF DIFFERENT ALLERGIC DISEASES
8.1. General treatment recommendations for selected allergic diseases during COVID‐19
According to WHO, patients at risk of or with diagnosed COVID‐19 should continue their treatment for any other disease (this includes allergic disease) in line with current guidelines. Special consideration should be given to the interference of drugs with COVID‐19 or vice versa. 25 It is generally recommended that patients should have a supply of medication for at least a 14‐day quarantine. Where more stringent or lengthy measures of isolation are enforced, consideration must be given to availability of medicines and potential substitutes for current treatments, if particular medications cannot be obtained. Patients should have an action plan to ensure that these issues are covered.
Telemedicine visits cannot replace all personal consultations, notably those mandatory for the administration of subcutaneous allergen immunotherapy (SCIT). Nevertheless, prior to the consultation, questions identifying actual contraindications can be clarified by a telemedicine consultation. Many of the biologics used for the treatment of allergic diseases (eg, omalizumab, benralizumab, mepolizumab and dupilumab) are registered for self‐application if the patients are adequately trained in the injection technique and in the assessment and management of allergic side effects. During telemedicine visits, injection techniques may be rechecked, and patients’ questions answered regarding their treatment. Peak‐flow protocols can be discussed during a telemedicine visit, and treatments can be adapted.
In general, patients can be instructed on allergen avoidance measures and treatment modalities. They can show the drugs they have at home and can be instructed on the use and especially on the application techniques of inhalers and topical nasal sprays. Patients suffering from anaphylaxis may be trained to use adrenaline autoinjectors for self‐administration; this improves safety and may also improve the patient's quality of life.
As a general rule, patients with severe allergic disease who are on biologicals and have a SARS‐CoV‐2 infection should pause the biologicals. Proper management and background controller treatment (topical steroids or other controller medications as recommended by current guidelines) should be continued as prescribed. If resolution of the disease is established (eg, via a negative SARS‐CoV‐2 test) at a minimum of 2 weeks postonset/positive testing, the re‐administration of the biological should be re‐initiated. 9
8.2. Avoidance measures
Importantly, self‐identified and physician‐diagnosed (via skin prick test, blood test, provocation testing) triggers of asthma exacerbation and allergies (seasonal, food allergies, etc.) need to be understood for each patient. Targeted messages to patients who have a known allergen sensitivity may be a meaningful way of connecting with patients during a time of limited in‐person clinic visits (eg, reminder alerts that the spring season has arrived). Educational outreach messages or televisits can include instructions on allergen avoidance, indoor air purifiers and proper medication use (eg, reviewing the appropriate use of inhalers with spacers). Food scarcity during pandemic operations may adversely impact food‐allergic families, and strategies for planning and stocking safe foods should be discussed. Medication supply should also be addressed in conversations with patients to plan for adequate controller and rescue medication with the possibility of substituting or switching medications as needed.
8.3. COVID‐19 in the light of different allergic diseases
The following section overviews recommendations for selected allergic diseases (Table 4).
TABLE 4.
Key recommendations from recently published EAACI/ARIA statements
| Disease | Recommendations for COVID‐19–diagnosed individuals or for cases with suspected SARS‐CoV‐2 infection | Recommendations for noninfected individuals during the COVID‐19 pandemic or for patients having recovered from COVID‐19 infection |
|---|---|---|
| Allergic rhinoconjunctivitis 10 , 88 |
Continue INCS Continue second‐generation H1 antihistamines Stop SCIT until resolution of the disease is established Stop SLIT until resolution of the disease is established Biologicals a |
Continue INCS Continue second‐generation H1 antihistamines Continue SCIT and SLIT Consider supplying patient with a sufficient amount of SLIT medication for home self‐administration (for a 14‐day quarantine at least) Biologicalsa |
| Asthma 13 |
For severe attacks, a pressurized metered‐dose inhaler (pMDI) via a spacer is the preferred treatment instead of nebulizers While a patient is being treated for a severe asthma attack, his/her maintenance inhaled asthma treatment should be continued (at home and at hospital) For acute asthma attacks, patients should take a short course of oral corticosteroids (if instructed in their asthma action plan or by their healthcare provider), to prevent serious consequences. Additional treatment should be based on the individual patient and on the underlying disease. Biologicalsa |
Continue all inhaled medication, including ICS (containing therapies), as prescribed by the physician and in line with the personal asthma action plan. If needed, OCS should be continued at the lowest possible dose in patients at risk of severe attacks/exacerbations. Routine spirometry testing should be suspended to reduce the risk of viral transmission, and, if absolutely necessary, adequate infection control measures should be taken. Biologicalsa |
| Atopic dermatitis |
Continue topical treatment Systemic immune‐modulating therapy may be paused based on interdisciplinary risk assessment. Optimize the topical treatment after pausing systemic treatment. Biologicalsa |
Continue topical treatment Continue systemic immune‐modulating treatment Biologicalsa |
| Chronic rhinosinusitis 11 |
Like in other upper airway viral infections (common cold or flu), the loss of smell is a frequent symptom in COVID‐19 patients. But a sudden and severe loss of smell (anosmia) and/or taste may also be present in COVID‐19 patients who are otherwise asymptomatic 59 , 62 Surgery for CRS should be avoided unless patients are proven COVID‐19–negative Patients with CRS should continue to use their INCS Biologicalsa |
Anosmia in COVID‐19 patients often improves within 14 days Patients with CRS should continue using their INCS Biologicalsa |
| Drug allergy 89 | Quick and accurate diagnostic and therapeutic decisions are mandatory in the case of DHRs induced by COVID‐19 drugs |
Severe allergic reactions must be treated immediately. Diagnostic testing may be urgently indicated in the case of suspicion of allergic reaction to highly necessary drugs. When validated and reliable, in vitro testing may be preferred for diagnosis. If not immediately required, drug allergy diagnostic must be postponed until the pandemic is locally under control, and alternative drugs should be used until then. |
| Food allergy |
Severe allergic reactions must be treated immediately. Diagnostic testing should be postponed. In vitro diagnostic tests can be preferred for diagnosis in severe anaphylaxis cases. Strict avoidance measures must be taken, and an adrenaline autoinjector must be carried. OIT or EPIT: adapt dosing as indicated in the dosing plan and in coordination with the treating physician. |
Severe allergic reactions must be treated immediately. Diagnostic testing should be postponed. In vitro diagnostic tests can be preferred for diagnosis in severe anaphylaxis cases. Strict avoidance measures must be taken and an adrenaline autoinjector carried. Continue OIT or EPIT |
| Urticaria |
Continue second‐generation H1 antihistamines. Systemic immune‐modulating therapy may be paused based on interdisciplinary risk assessment. Biologicalsa |
Continue second‐generation H1 antihistamines. Continue systemic immune‐modulating treatment. Biologicalsa |
| Venom allergy |
Severe allergic reactions must be treated immediately. Diagnostic testing is postponed. Strict avoidance measures must be taken and an adrenaline autoinjector carried. Stop SCIT until resolution of the disease is established. |
Mastocytosis and grade 3 or 4 anaphylaxis patients need to be diagnosed and venom IT initiated. Strict avoidance measures must be taken and an adrenaline autoinjector carried. Continue SCIT. |
| Biologicalsa | Recommendations for COVID‐19‐diagnosed individuals or for cases with suspected SARS‐CoV‐2 infection | Recommendations for noninfected individuals during the COVID‐19 pandemic or for patients having recovered from COVID‐19 infection |
|---|---|---|
|
Stop until resolution of the disease is established (eg, via a negative SARS‐CoV‐2 test in connection with clinical recovery), but for a minimum of 2 weeks from onset/positive testing. Ensure re‐administration after recovery |
Continue the application of biologicals—if possible as home self‐administration |
These recommendations are conditional and should be adapted regularly on the basis of more clinical data.
Recommendation applies for biologicals in the context of all diseases 9
Abbreviations: EPIT, Epicutaneous immunotherapy; CRS, chronic rhinosinusitis; INCS, intranasal corticosteroids; OCS, oral corticosteroids, OIT, oral immunotherapy; SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy
8.4. Atopic dermatitis
Atopic dermatitis is one of the most common skin disorders. The lifetime prevalence varies between 0.2% and 25% worldwide, the most affected area being the northern part of Europe. 90 The disease most often starts in early childhood and persists into adult life in up to 50% of affected patients. 91 Co‐morbidities with other atopic diseases including asthma, allergic rhinitis and food allergy are common. 92 Most with mild‐to‐moderate atopic dermatitis can be controlled on topical treatment. However, in the severe cases, systemic immune‐modulating treatments including immunosuppressive therapy are needed. 93 Conventional systemic immunosuppressive treatment, such as cyclosporine, may interact with the human body's defence mechanisms against viral disease, while dupilumab, which is registered in many countries for the treatment of moderate‐to‐severe atopic dermatitis, selectively interferes with type 2 inflammation and is in general not considered to increase the risk of viral infections.
It is well known that viral and bacterial infection may complicate and exacerbate atopic dermatitis including infections with Staphylococcus aureus (impetigo), poxvirus (molluscum contagiosum) and herpes simplex virus (eczema herpeticum). 92 Severe and untreated atopic dermatitis is a known risk factor for disseminated viral skin disease. 94
In the current SARS‐CoV‐2 pandemic, the European Task Force on Atopic Dermatitis (ETFAD) recommends the continuity of all immune‐modulating treatment since exacerbations of underlying diseases can have a large negative impact on the patient's immunity. 30 However, patients at risk are advised to strictly follow the recommendations issued by the local health authorities in each European country. 30 The British Association of Dermatologists (BAD) has addressed potential issues regarding the COVID‐19 infection of patients undergoing immune‐modulating treatment. 95 Other countries will follow. A thorough hygienic procedure is recommended with handwashing and disinfectants. Nonirritant soap substitutes should be used following the same instructions as those for regular soap. Moisturizers should be applied afterwards. In the case of COVID‐19–infected atopic dermatitis patients, interdisciplinary risk assessment should be carried out, and, in accordance with current guidelines on active infections and systemic therapy, the immune‐modulating therapy may or may not be paused afterwards. 30 If systemic treatment is paused, it is important to optimize the topical treatment. Furthermore, if the paused systemic treatment also has an effect on co‐morbidity such as asthma, then the co‐morbidity also has to be treated by other drugs. According to a letter from Italy regarding 245 patients on therapy with dupilumab, only two developed COVID‐19. 96 An abnormal course of COVID‐19 was not observed in these 2 patients. More clinical data are needed for this specific treatment.
8.5. Urticaria
Urticaria is characterized by the development of wheals (hives), angio‐oedema or both. 97 Acute urticaria is defined as the occurrence of wheals, angio‐oedema or both for less than 6 weeks. Chronic urticaria is defined as wheals, angio‐oedema or both for 6 weeks or more. 97 Viral infection has been found as a potential trigger—and sometimes as the main aetiologic agent—in causing acute or chronic urticaria. 98
In Italy, 88 patients with COVID‐19 were studied by a group of dermatologists. 20% developed cutaneous symptoms including erythematous rash and urticaria. It was concluded that the skin manifestations related to the COVID‐19 infection are similar to those occurring during common viral infections. 99 In France, among 103 out‐ and inpatients with confirmed COVID‐19 infection, two had urticaria. 100 In a study from China, 1.4% of the COVID‐19 patients reported an underlying urticaria. However, skin symptoms during the infection were not described. 5 The manifestation of urticaria could appear before the onset of fever or respiratory symptoms. 101
As a consequence of these observations, the manifestation of acute urticaria could be an indication to test for SARS‐CoV‐2. According to the guidelines, second‐generation H1 antihistamines are the base of urticaria treatment 97 and should be continued during the pandemic. If urticaria cannot be controlled on antihistamines in a 4‐fold dose, omalizumab is recommended as an add‐on treatment. Omalizumab is registered for self‐administration after patients have received training on the injection technique and on the assessment of allergic side effects. Only the first two injections need to be administered in hospital, due to the risk of anaphylaxis. Therefore, especially during the COVID pandemic, treatment at home is favourable. By telemedical visits, the efficacy of the treatment can be evaluated and patients’ questions regarding their treatment may be reviewed. This is currently recommended by the BAD. 95 As for all COVID‐19–infected patients, interdisciplinary risk assessment should be performed, and, in accordance with current guidelines on active infections and systemic therapy, the immune‐modulating therapy may or may not be paused afterwards.
8.6. Food allergy
Many reactions to foods are mild‐to‐moderate and can be self‐managed by the patient, given the availability of an up‐to‐date action plan and adequate rescue medication. During periods of isolation, it is vital that children and adults with food allergy have access to suitable foods according to their dietary recommendations. 102 Patients with a history of severe anaphylactic reaction urgently need an emergency health card providing information on the diagnosis, eliciting (causative) allergens and necessary treatment in the case of a severe reaction and/or an unexpected hospital admission due to COVID‐19. 103 Oral (OIT) and epicutaneous immunotherapy (EPIT) for food allergy should follow the general rules of EAACI/ARIA for AIT during the COVID‐19 pandemic (description in subchapter “allergic rhinoconjunctivitis”).
8.7. Venom allergy
In the case of an anaphylactic reaction due to an actual insect sting, patients should be treated according to the guidelines. Especially in high‐risk patients (eg, high risk of subsequent stings, patient suffering from mastocytosis, patient with grade 3 or 4 anaphylaxis), the diagnosis of insect venom allergy must be proved urgently. Venom immunotherapy should be initiated without any delay in order to prevent severe reactions in the case of further future stings. 104 The treatment should follow the general rules of EAACI/ARIA for AIT during COVID‐19 pandemic (description in subchapter “allergic rhinoconjunctivitis”). Patients must be informed of avoidance strategies and provided with drugs for self‐administration. Adrenaline autoinjectors must be prescribed, and patients must be trained to use these devices. Prior to the initiation of venom immunotherapy, contraindications and requirements for treatment can be discussed with the patient in a telemedicine consultation.
8.8. Drug allergy
Severe allergic reactions to drugs must be treated immediately. Diagnostic testing may be urgently indicated in the case of a suspicion of allergic reaction to highly necessary drugs. This may be the case for example in patients suffering from reactions to antibiotics which may be necessary for treating bacterial superinfection in COVID‐19 pneumonia. In the case of an immediate need for treatment with a drug that is suspected to be responsible for immediate type systemic reactions, drug desensitization or temporary tolerance induction is an option. This is a therapeutic procedure with the aim to induce a temporary state of unresponsiveness to a drug in a patient with confirmed immediate type reaction. During drug desensitization, respiratory and gastrointestinal symptoms occur commonly 105 , 106 , with a subsequent risk of spreading infectious aerosols. Therefore, the decision to conduct the procedure during the current pandemic must consider both the expected benefits obtained from the drug administration, as well as the potential risks of severe reaction and spread of infection. 89 Absolute indications for desensitization/tolerance induction may include chemotherapeutic agents in oncologic patients, aspirin in subjects with ischaemic diseases and antibiotics in infected individuals when no effective alternative is available. 107
8.9. Chronic rhinosinusitis
Chronic rhinosinusitis (CRS) affects approximately 12% of the general population worldwide and is regarded as a chronic airway disease that, according to WHO recommendations, may be a risk factor for COVID‐19 patients. 6 , 11 The inflammatory changes affecting the nasal and paranasal mucous membranes in CRS with nasal polyps (CRSwNP) are, in most cases, of the type 2 (T2) inflammation endotype. They are typically associated with epithelial damage and tissue destruction, 108 which can promote viral infections. 109 Asthma often co‐exists with CRSwNP, and it is known that deterioration in the control of CRSwNP can promote asthma exacerbations. 109
Symptoms of nasal obstruction, rhinorrhoea, facial pressure and smell dysfunction regularly occur in CRS. Recently, a number of reports have been indicating that a sudden and severe (anosmia) isolated onset of loss of smell (ISOA) and/or loss of taste may also be present in COVID‐19 patients who are otherwise asymptomatic. This is considered a marker symptom in screening for SARS‐CoV‐2 infection, 62 , 110 , 111 but may also interfere with loss of smell in CRS. 11 CRS is treated with intranasal corticosteroids (INCS), systemic corticosteroids or specific T2 endotype–driven anti‐inflammatory therapies according to the severity of disease. 112 INCS remain the standard treatment for CRS in the COVID‐19 pandemic and also for patients with SARS‐CoV‐2 infection. 11 Surgical treatments should be reduced to a minimum, and surgery preserved only for patients with local complications and those for whom no other treatment options exist. Systemic corticosteroids should be avoided. Treatment of severe uncontrolled CRSwNP patients 112 with biologicals can be continued with careful monitoring in noninfected patients. However, it should be temporarily discontinued in patients having tested positive for SARS‐CoV‐2 (RT‐PCR), until recovery. We suggest that physicians assess the risks vs. benefits in low‐risk patients before initiating biologics therapy on a case‐by‐case basis. However, it should not be initiated in high‐risk patients. 9 , 11
8.10. Allergic rhinoconjunctivitis
There have been reports that topical applied "corticosteroid preparations" may increase the risk of developing COVID‐19 or may cause a more severe course of the disease. This opinion massively unsettled numerous patients suffering from allergic rhinitis (AR), CRS or asthma. A current Position Paper by ARIA and EAACI on AR treatment 88 states that INCS is the therapeutic standard for the treatment of AR, regardless of symptoms and inflammation, thus avoiding mucosal damage. In approved doses (see package leaflet), INCS do not increase the risk of infections in patients with SARS‐CoV‐2, nor do they trigger a more severe course of COVID‐19 disease. 88 Reducing allergic mucosal inflammation by INCS may even shorten the duration and decrease the severity of symptoms in upper respiratory tract virus infections of AR patients, 113 but seems not to have any marked effects on the common cold symptoms of patients without AR. Therefore, it is recommended that patients with AR should continue to regularly use their INCS at the individually‐prescribed dose. Hence, it is not advised that they change or stop their treatment without consulting their doctor. 88 Discontinuation of INCS may worsen AR symptoms with increased secretion and sneezing which may promote viral droplet transmission from SARS‐CoV‐2–infected patients to healthy individuals. 88 In addition, worsening of AR can trigger an exacerbation of asthma, 88 which is regarded by WHO as a risk factor for severe COVID‐19 courses. Systemic glucocorticosteroids in general have several adverse effects if given long term. 114 For AR, they should be used with even more caution during the current COVID‐19 pandemic and only when no therapeutic alternatives are available 88 because of a potential temporary immunosuppression and a possible increased risk of contracting a SARS‐CoV‐2 infection or progression to severe disease. 114
Allergen immunotherapy (AIT) is the only disease‐modifying treatment option for patients with allergic diseases and it is administered through the subcutaneous (SCIT) or sublingual route (SLIT). 115 , 116 During the current pandemic, special considerations should be introduced for the management of AIT. 10 , 117 All in‐person consultations may be preceded and prepared by a telemedicine visit, during which information is obtained on the patient's health status and possible contraindications for AIT. Since SLIT is self‐administered at home during the maintenance phase, telemedicine visits may be helpful for advising patients and increasing their adherence to treatment.
In noninfected individuals during the COVID‐19 pandemic or in patients having recovered from COVID‐19 infection, it is recommended to continue SCIT in potentially life‐threatening allergies, such as venom allergy. 10 The possibility of expanding injection intervals in the continuation phase should be evaluated and may be beneficial. SLIT should also be continued, and the patient must be supplied with sufficient medication.
For both application routes, a continuation of AIT is possible in principle under the following prerequisites 10 : (a) asymptomatic patients without suspicion of SARS‐CoV‐2 infection and/or contact with SARS‐CoV‐2–positive individuals, (b) patients with a negative test result (RT‐PCR), (c) patients after an adequate quarantine or (iv) patients with detection of serum IgG to SARS‐CoV‐2 without virus‐specific IgM. In COVID‐19–diagnosed cases (positive RT‐PCR), in patients suspected of SARS‐CoV‐2 infection, or in symptomatic patients with exposure or contact to SARS‐CoV‐2‐positive individuals, EAACI recommends interrupting SCIT and SLIT until recovery. 10
If AIT is stopped due to signs of a potential SARS‐CoV‐2 infection (such as fever, cough, dyspnoea), or due to other signs of ill health, or due to local restrictions on clinic operations, it should be resumed after recovery but with proper dosage adjustment and under medical supervision when appropriate. To maintain social distancing procedures in the clinic, the following methods could be considered: stretching out the interval of IT or organizing different clinic hours to limit the number of patients attending for IT. 10 , 20
8.11. Asthma
To date, there is very little information available on patients with asthma who have COVID‐19. In general, viruses, including rhinoviruses and respiratory syncytial viruses, have been shown to induce asthma episodes or exacerbations. 118 , 119 Mounting evidence implicates that particular viral pathogens, namely the human rhinovirus and respiratory syncytial virus, are among the most likely culprits in asthma inception. 119 Bacterial infections and colonization have also been associated with exacerbation and recurrent wheeze, an effect that may be independent, or a cofactor with viruses. In addition, certain individuals may have a genetic predisposition towards viral‐induced wheezing and the development of asthma. 119 Whether this also applies to SARS‐CoV‐2 infection remains to be seen. Interestingly, according to initial reports, allergic airway disease including asthma did not appear to be a risk factor for COVID‐19 or for a severe clinical course. 5 , 8 , 120 However, more recent reports from the United States show asthma as an underlying condition in 13%‐27% of patients hospitalized with COVID‐19, within the COVID‐NET hospitals. 33 Additionally, immunocompromised patients—including the elderly, those with diabetes mellitus or those on (systemic) corticosteroids in conjunction with the underlying immune disorders—may be at an increased risk of being infected and more severely affected by SARS‐CoV‐2. 5 , 121
Optimal disease control is the first defence against respiratory triggers including infections in patients with inflammatory airway disease such as allergic rhinitis and asthma. Inhaled maintenance therapy with bronchodilators and ICS should not be stopped during the COVID‐19 pandemic. 13 The termination of inhaled treatment may in fact imply an increased risk for asthma symptom worsening and acute asthma exacerbations. Furthermore, the risk of asthma deteriorating in a threatening manner and necessitating (otherwise unscheduled) doctor visits or hospital stays—potentially responsible for contact with COVID‐19 patients—is far more dangerous for asthmatic patients than a possible increased risk of SARS‐CoV‐2 infection due to a theoretic local immunodepression induced by ICS. Given the lack of current evidence that ICS negatively affects the COVID‐19 outcome, experts and professional societies within the respiratory and allergy field—including the Global Initiative for Asthma (GINA), the American Academy of Allergy, Asthma and Immunology (AAAAI), the European Respiratory Society (ERS) and EAACI—all stress the importance of disease control, especially since many countries are now entering the spring pollen season. 13 Apart from the generally applicable avoidance measures issued by the governments, the societies recommend that patients continue to take their corticosteroid‐containing controller medications and other controllers (including biologicals), as detailed in their personal asthma plan and that they should seek medical help if disease control deteriorates. 88 , 122 , 123 , 124 , 125 This applies both for adults and children with chronic inflammatory airway disease and with COVID‐19 or a suspicion of having the infection. Contact with healthcare providers should be digital as much as possible.
Treatment of severe asthma with biologicals should also be continued. 9 A discontinuation of biologicals can lead to a worsening of the underlying disease, which in turn could have a negative impact on the course of a COVID‐19 infection. 9 Virus‐related asthma exacerbations occur less frequently or are less severe under biologicals, as demonstrated for omalizumab, but not in the context of COVID‐19. 126 , 127 However, when authorized by international regulatory bodies (eg, the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA)), self‐administration should always be advised in order to reduce exposure to high‐risk environments such as hospitals and primary care settings.
In the absence of biological therapy, patients would have to be switched to therapy with systemic glucocorticosteroids, especially those with severe asthma. This can have negative effects on the immune defence against SARS‐CoV‐2. In a study performed on patients from the Seattle area, severe respiratory failure was observed in 3 of 24 COVID‐19 patients after systemic glucocorticoid treatment due to asthma exacerbation. 32
8.12. Post–COVID‐19 routine care
For patients who have recovered from moderate‐to‐severe COVID‐19 infection, particular attention should focus on lung, kidney, cardiac and liver recovery before re‐instituting usual medications 128 , 129 , 130 , 131 (Figure 3). Some patients infected with SARS‐CoV‐2 have a severe systemic inflammatory response, with marked elevation in CRP, D‐dimer and ESR in addition to multi‐organ dysfunction. Patients may have participated in COVID‐19 clinical trials, and it is important to note which medication was potentially administered (see section “Considerations for performing clinical trials”). When resuming allergy care, clinical judgement should determine whether additional laboratories (complete blood count, liver function test, kidney function) or lung function testing are necessary before restarting the treatment of allergic diseases. This decision should be based on a multidisciplinary consultation. In some cases, a close follow‐up to assess pulmonary rehabilitation may be warranted.
FIGURE 3.
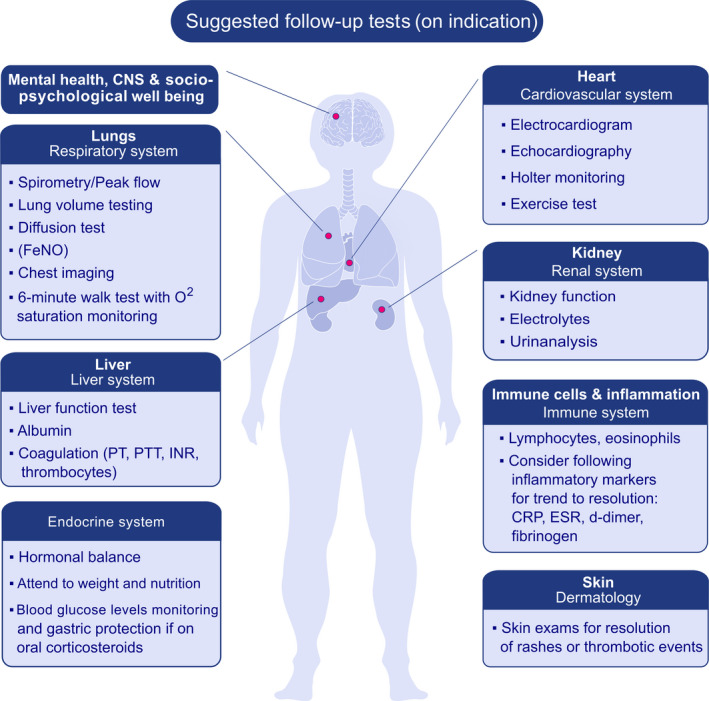
General diagnostic measures in post–COVID‐19 routine care. The decision on the diagnostic tests or additional laboratories before restarting allergy care should be based on individual patients and/or interdisciplinary consultation.
8.13. Conclusions
Though avoidance measures during the COVID‐19 pandemic are similar in different allergic diseases, specific aspects should also be followed with optimal care for allergic rhinoconjunctivitis, asthma, atopic dermatitis, chronic rhinosinusitis, drug allergy, food allergy, urticaria and venom allergy. Different recommendations can be provided for patients with suspected SARS‐CoV‐2 infection or diagnosed COVID‐19 disease versus noninfected individuals or patients having recovered from COVID‐19 infection.
After recovery from COVID‐19, allergy care has to be resumed, but an interdisciplinary consultation is recommended before any further diagnostic or therapeutic procedure.
9. SOCIO‐PSYCHOLOGICAL CONSIDERATIONS FOR ALLERGIC PATIENTS AND OPTIMAL CARE DURING AND AFTER THE PANDEMIC
Allergic responses are affected by psychological factors such as stress and anxiety and can be modulated by interventions other than conventional drug therapy. 132 These psychological mechanisms play a role in terms of symptom development, symptom exacerbation and perception. 133 , 134 The reactions of other people to patients showing allergic respiratory symptoms during the COVID‐19 pandemic are amplified. These reactions, along with the governmental regulations (eg, social distancing) for dealing with the pandemic, induce further stigmatization and thus enhance psychosocial stress for allergic patients.
Symptom development and symptom perception are only partially caused by the biological mechanisms of the allergy, and many patients report bodily symptoms that are mainly developed via psychological effects (nocebo effects). Relevant psychological mechanisms for symptom development include negative expectations, increased self‐observation of somatic reactions, catastrophizing of perceived symptom (dysfunctional appraisal), fears and negative affect. 135 For many patients (and sometimes even for their physician), the reported symptoms are a conglomerate of potential allergic symptoms, potential symptoms of COVID‐19 and correlates of concern that are almost impossible to disentangle. These nocebo symptoms can account for up to 80% of patients with medical conditions. 136
During the COVID‐19 pandemic, the general population is highly sensitive to the perception of people showing respiratory symptoms. This increases the risk of stigmatization of patients with allergies, further increasing the psychosocial stress of the patients. The neuroendocrine and immunological consequences of stress exposure are in turn able to amplify the development of allergic symptoms. 133 , 137 Negative effects on the willingness to expose oneself to those contacts (eg, at work, in private social networks) are further potential consequences with negative impact on health conditions. This is even more problematic, because social contact and social support can dampen negative stress effects and reduce disease symptoms. 138 Several recommendations to improve medical care for patients with allergies during the COVID‐19 pandemic can be given (Table 5).
TABLE 5.
Improving medical care for patients with allergies during the COVID‐19 pandemic
| Manage the increased potential for the development of nocebo symptoms: patients should be informed about the potential detrimental effects of nocebo mechanisms, such as increased self‐observation or negative expectations. Patients should be encouraged to work against them and to disentangle stress effects from symptoms of clinical conditions |
| Despite public encouragement for social distancing and increased social stigmatization in the public, patients should be encouraged to maintain an active social network employing the available communication channels. Social support is a crucial factor for improving health in general. |
| Encourage patients to do regular physical exercise. Regular physical activities induce anti‐inflammatory responses. |
| An empathetic, reliable and predictable doctor‐patient relationship guarantees patient compliance with medical recommendations and also lowers nocebo effects |
| Encourage engagement in stress reduction activities such as relaxation techniques, mindfulness and yoga |
9.1. Conclusions
Socio‐psychological mechanisms play a major role in terms of symptom development, symptom exacerbation and perception in allergic patients. Besides, the general population is highly sensitive to the perception of people showing respiratory symptoms during the COVID‐19 pandemic. This increases the risk of stigmatization of patients with allergies, further enhancing the psychosocial stress of patients. Therefore, optimal medical and psychological care for patients with allergies during the COVID‐19 pandemic is essential.
10. CONSIDERATIONS FOR PERFORMING NON‐COVID‐19–RELATED CLINICAL TRIALS
For safety and feasibility reasons, many of the ongoing non‐COVID‐19–related clinical trials have been suspended during the current COVID‐19 pandemic, as recommended by guidance from the US Food and Drug Agency as well as the European Medicines Agency. 139 , 140 While it is imperative that clinical trials on COVID‐19 be prioritized and quickly implemented following local regulatory policies and fulfilling highest quality standards, 141 , 142 it is also critical that other essential clinical research programmes, such as those involving study drugs or clinical assessments, should continue safely as much as possible. However, the social distancing measures put into place by different governments (eg, close‐down of clinical units and public transportation) complicate the implementation of certain protocol‐related procedures, scheduled visits or hospital/clinic procurement of study medication. Nevertheless, with proper adjustments, some studies can be continued safely without loss of data integrity. In the following, we discuss safety and regulatory measures as well as logistical issues that should be taken into consideration when amending non‐COVID‐19–related clinical trial protocols during the COVID‐19 pandemic (Table 6).
TABLE 6.
General consideration when amending non‐COVID‐19– related clinical trial protocols during the COVID‐19 pandemic
| Provide written and oral instructions on disease symptoms and signs and for the prevention of disease spread |
| Study participants, research and laboratory staff may need to monitor their temperature and check for symptoms and signs of the pandemic during participation in the trial and if entering the clinical research unit/workplace |
| Study participants, research and laboratory staff should frequently wash their hands with disinfectants, wear PPE (eg, laboratory garments, gloves, face masks, eye protection) and clean work surfaces and equipment with appropriate disinfectants |
| Consider which visits can be conducted via remote solutions (phone check‐ups, teleconsulting and monitoring) 36 |
| Provide specific instructions on clinical trial unit procedures, particularly those that generate aerosols/droplets (eg, sputum and nasal fluid collection, nasal and bronchial provocation testing). All isolations of peripheral blood mononuclear cells (PBMC) and bronchoalveolar lavages (BAL) should be performed in a BSL‐2 bench. For centrifugation steps, the use of closed beakers should be mandatory |
| Provide specific instructions for the collecting, handling and processing/testing of specimens from clinical trial participants |
Immediate contact is indicated with all involved parties—sponsor, institutional review board (IRB), institutional ethics committee (IEC), participants, investigators and staff—as well as with all enrolled participants, to inform them of the changes related to the pandemic. All immediate actions should take place as long as the pandemic‐related policies are in place and documented. Otherwise, conventional amendments are required.
Essential non‐COVID‐19–related research can be continued safely during the COVID‐19 pandemic while maintaining data integrity with appropriate amendments to the protocols. These adjustments should take into consideration modern technological communication tools (between hospital staff and patients), IP delivery, appropriate laboratory safety guidelines, proper data source (case report form (CRF)), statistical analysis plans and regulatory documentation (Figure 4).
FIGURE 4.
Ensuring data integrity in non‐COVID‐19–related research
10.1. Participant interactions
In conducting non‐COVID‐19–related trials (eg, in apparently noninfected individuals), the safety and well‐being of the participants and hospital/research staff during physical interactions and specimen handling should be considered and risks evaluated. When possible and appropriate, remote options for participant visits using telemedicine or video consulting may facilitate the conduct of the clinical trial. Contact scripts to standardize messages are recommended. In general, these scripts do not require approval during lockdown. Study procedures that generate aerosols or droplets, such as spirometry or nebulization of agents or medication, may require special considerations on how to perform similar assessments safely or may need to be reconsidered. Staffing considerations should include the minimal essential personnel required to carry out study visits, and staff should be cross‐trained and added to Delegation of Authority logs, as appropriate.
10.2. Regulatory guidance
Regulatory aspects of protocol amendments need to be considered. 139 , 140 Changes to protocols after Institutional Review Board (IRB) approval are only allowed without prior approval in cases where there is a need to eliminate immediate hazards for the participants. Regulations allow protocol adjustments for participant safety and minimization of risks for data integrity in line with good clinical practice (GCP). In the light of the COVID‐19 pandemic, certain study visits or procedures may need to be paused or redesigned. This could facilitate ongoing research while maintaining safety, and also allow time for evaluating the benefits of continuing investigational products for enrolled participants versus the disadvantages (harm incurred) of discontinuing. New strategies for safety assessments, monitoring and data collection should be considered. Proper and detailed documentation of updates to procedures and operations is critical, and protocol amendments should be communicated to research staff, participants, sponsors and regulatory agencies as soon as possible. Changes that may impact efficacy assessments, data management and/or statistical analysis plans should be discussed, when necessary, with the appropriate EMA‐/FDA‐division review. Records of all deferred procedures need to be kept and documented.
10.3. Investigational product
Depending on the clinical trial, adjustments to investigational product (IP) administration may be possible with the use of telemedicine. These decisions must involve conversations with protocol chairs, sponsors, medical monitors and regulatory authorities as applicable. If participants are not able to go to the clinical research unit (CRU), other options to continue IP intake include curbside dispensing or direct shipping of pharmaceuticals. The research pharmacy staff should ensure that all local and federal regulations are followed with regard to the documentation and transport of study medication. In parallel, any necessary rescue medications should be provided to study participants and documented with prior communication to relevant parties. Further guidance for the investigational product should be followed (Table 7).
TABLE 7.
General considerations on investigational products when performing a non‐COVID‐19–related clinical trial during the COVID‐19 pandemic
| Treatment initiation and dose increases only performed in clinic; levels maintained at a stable dosage (eg, for oral immunotherapy) when clinic visits not possible |
| Training of at‐home administration of biologics and injectables, where applicable 9 |
| Ensuring participants maintain adequate IP supply to continue at‐home dosing as needed without disclosing identity via research pharmacy (direct‐to‐participant shipments or curbside dispensing) |
| Ensuring integrity between pharmacy and participant in the case of shipping (secure chain of custody and monitoring of storage conditions in transit) |
| Necessary rescue medication provision with written instructions and emergency phone numbers |
10.4. Laboratory safety and precautions
Laboratory staff should follow standard precautions (SP) when handling clinical specimens, all of which may contain potentially infectious materials (see also Section 6) 71 ). SP include hand hygiene and the use of PPE, such as laboratory coats or gowns, gloves and eye protection. All new arriving samples can be potentially infected. Isolations have to be performed with closed centrifuge beakers which have to be loaded and unloaded in a biosafety cabinet. Materials should be carried in aerosol tight transport boxes. The usage of BSL‐2 benches for all arriving patient materials is mandatory during and after the pandemic. Handling of open vessels may only be performed inside of class II biosafety cabinets.
Antibody tests and procedures that utilize samples that are formaldehyde‐fixed or virus‐inactivated, or those that concentrate viruses (via precipitation or membrane filtration), may be performed in a BSL‐2 laboratory. BSL‐3 facilities and procedures are recommended for virus isolation in cell culture, initial characterization of viral agents recovered in cultures of SARS‐CoV‐2 specimens, and virus‐neutralizing antibody assays (Table 8). All work surfaces and equipment should be decontaminated with appropriate disinfectants.
TABLE 8.
Laboratory procedures that may require different biosafety level precautions 71
| Biosafety level 2 (BSL‐2) precautions | Biosafety level 3 (BSL‐3) precautions |
|---|---|
| Flow cytometry of formaldehyde‐fixed specimens | Procedures with human or animal primary specimens to intentionally concentrate or isolate SARS‐CoV‐2 for research purposes (eg, ultracentrifugation of a sample) |
| Cell sorting with FACS Sorter has to be performed in the closed tube system. If a plate sort is necessary, the aerosol protection has to be used | Culturing specimens (eg, propagated virus) |
| Assays with virus‐inactivated specimens | Preparatory work for in vivo activities |
| Concentration of samples prior to inactivation | Processing a culture (eg, propagated or cultivated) known to contain SARS‐CoV‐2 for packaging and distribution to laboratories |
| Sample preparation for nucleic acid extraction, flow cytometry analysis, molecular testing of nucleic acids | Preparing inoculum, inoculating animals and collecting specimens from experimentally infected animals |
| Antigen and antibody assays | Virus neutralization tests for blocking activity against SARS‐CoV‐2 (with live virus) |
10.5. Conclusion
Clinical trials to combat the COVID‐19 pandemic currently have top priority. However, a number of non‐COVID‐19 trials are also essential and should be continued if they can be conducted in a safe manner. Safety measures and new guidelines need to be established for participants and research/laboratory staff dealing with non‐COVID‐19–related clinical trials, to ensure the continuation of essential and critical non‐COVID‐19 trials.
11. DISCUSSION
In view of the ongoing and emerging novel coronavirus (COVID‐19) pandemic spreading worldwide, 2 the safety and well‐being of our patients, personnel and colleagues globally are of primary importance. EAACI and ARIA are closely monitoring the situation. They recommend aligning any diagnostic and treatment operations with guidance from WHO 25 and ECDC, 26 in accordance with all applicable national/regional/local government and public health authority requirements.
Allergists and other healthcare providers (HCPs) in the field of allergies and associated airway diseases are on the front line in taking care of patients potentially infected with SARS‐CoV‐2. Hence, strategies and practices to minimize risks of infection of both HCPs and the treated patients have to be developed and followed by allergy clinics. 20 This is especially important in high‐risk patients for the course of the SARS‐CoV‐2 infection, for example in the elderly or in those with co‐morbidities.
If patients are diagnosed with COVID‐19 or are suspected to have COVID‐19, they should follow the local area treatment and quarantine guidance. In general, most medications should be continued. 10 , 88 Patients may be unable to attend clinic visits, have examinations and/or receive prescriptions. E‐Health and telemedicine can assess the value of specialized treatments, provide education for self‐management without the risk of infection 44 and triage patients for urgent in‐person consultations. Examples for the latter are diagnostic testing in drug allergy in the case of suspicion of allergic reaction to highly necessary drugs 89 or the application of medication (eg, SCIT or biologicals) through a HCP.
Clinic staff should keep in contact with the patient, preferably through telephone calls or videoconferences, to maintain awareness of their status in the case of symptom exacerbations. Dispensing a sufficient amount of medication is a way of enabling the patient to self‐treat. In treatment with biologicals, the decision to continue or discontinue a treatment should be made on a case‐by‐case basis by the attending physician, since the safety and efficacy of the mentioned biologicals in COVID‐19 patients are currently unknown. 9 Besides, psychological care for patients with allergies during the COVID‐19 pandemic is essential.
Non‐COVID trials may be able to be continued according to regional regulations. However, special emphasis should continue to be placed on the safety of the participants and research/laboratory staff. The same safety precautions used in clinical routine for aerosol‐generating procedures and handling of samples should also be applied in the non‐COVID trials.
12. CONCLUSION
According to current WHO and ECDC guidelines, patients at risk or with diagnosed COVID‐19 should continue to be treated for all other diseases in line with current guidelines, if there are no interferences of treatment with COVID‐19 or vice versa. Preparedness of the allergy clinic, specialized centre or practice is imperative in order to cope with COVID‐19 correctly. The recommendations in this Position Paper are conditional since there is a paucity of data. They should be revised regularly with new incoming information on COVID‐19.
As doctors, scientists and specialist societies, we are required to observe our patients, to provide optimal advice and treatment based on the current state of medical knowledge, and to inform them accordingly when new evidence is available, making it possible to adapt the therapies. EAACI has a prevailing requirement to protect the safety and welfare of our patients, allergists and staff and is working diligently to ensure responses to new recommendations as quickly as possible.
CONFLICT OF INTEREST
Dr Agache reports grants from Associate Editor Allergy. Dr Akdis reports grants from Allergopharma, Idorsia, Swiss National Science Foundation, Christine Kühne‐Center for Allergy Research and Education, European Commission's Horizon's 2020 Framework Programme, Cure, Novartis Research Institutes, AstraZeneca and Scibase, and plays advisory role in Sanofi/Regeneron, outside the submitted work. Dr Ansotegui reports personal fees from Mundipharma, ROXALL, Sanofi, MSD, Faes Farma, Hikma, UCB, AstraZeneca, Stallergenes and Abbott, outside the submitted work. Dr Bosnic Anticevich reports grants from TEVA, and personal fees from TEVA, AstraZeneca, Boehringer Ingelheim, GSK, Sanofi and Mylan, outside the submitted work. Dr Bousquet reports personal fees from Chiesi, Cipla, Hikma, Menarini, Mundipharma, Mylan, Novartis, Purina, Sanofi‐Aventis, Takeda, Teva and Uriach, and other from KYomed INNOV, outside the submitted work. Dr Brehler reports personal fees from ALK, Allergopharma, AstraZeneca, Bancard, GSK, HAL, LETI, Merck, Novartis, Stallergenes, Takeda, Thermo Fischer and Novartis, and other from ALK, Allergopharma, Novartis, Takeda, Bencard, Biotech Tools, Circassia, Genentech and LETI, outside the submitted work. Dr Brough reports speaker fees from DBV Technologies and Sanofi and research support from Thermo Fisher Scientific. Dr Canonica reports having received research grants as well as being lecturer or having received advisory board fees from A.Menarini, Alk‐Abello’, Allergy Therapeutics, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Genentech, Guidotti‐Malesci, Glaxo Smith Kline, Hal Allergy, Mylan, Merck, Merck Sharp & Dome, Mundipharma, Novartis, Regeneron, Roche, Sanofi‐Aventis, Sanofi‐Genzyme, Stallergenes‐Greer, UCB Pharma, Uriach Pharma, Valeas, Vibor‐Pharma. Dr Cardona reports personal fees from ALK, Allergopharma, Allergy Therapeutics, Diater, LETI, Thermo Fisher and Stallergenes, outside the submitted work. Dr Chinthrajah reports grants from NIAID, CoFAR, Aimmune, DBV Technologies, Astellas and Regeneron, other from Alladapt, Genentech and Novartis, and personal fees from Before Brands, outside the submitted work. Dr Cruz reports grants and personal fees from GSK, AstraZeneca, Boehringer Ingelheim, Novartis and MYLAN, grants from Sanofi, and nonfinancial support from CHIESI, outside the submitted work. Dr del Giacco reports personal fees from AstraZeneca, Chiesi, CSL‐Behring, Grifols and Menarini; and grants and personal fees from GSK and Novartis, outside the submitted work. Apart from academic affiliations and assignments, Dr Diamant acts as Executive and Scientific Medical Director at a phase I/II pharmacological unit (QPS‐NL), which performs clinical studies for pharmaceutical companies. In the past 3 years, she received honoraria, consultancy and speaker fees from AcuCort, AstraZeneca, ALK, Aquilon, Boehringer Ingelheim, CSL, HAL Allergy, MSD and Sanofi Genzyme. Dr Eiwegger reports other from DBV and Regeneron; and grants from Innovation fund Denmark, CIHR. He is the Co‐I or scientific lead in three investigator‐initiated oral immunotherapy trials including the usage of biologicals supported by the Allergy and Anaphylaxis Program SickKids and CIHR. He serves as an associate editor for Allergy. He is on advisory boards for ALK. Dr Hox reports personal fees from consultant work for ALK, outside the submitted work. Dr Knol reports speaker fees from Sanofi and Thermo Fisher Scientific. Dr Ivancevich reports personal fees from Faes Farma, Abbott Colombia and Eurofarma Argentina, and other from Laboratory Casasco Argentina, outside the submitted work. Dr Jutel reports personal fees from ALK‐Abello, Allergopharma, Stallergenes, Anergis, Allergy Therapeutics, Circassia, LETI, Biomay and HAL, during the conduct of the study; and from AstraZeneca, GSK, Novartis, Teva, Vectura, UCB, Takeda, Roche, Janssen, MedImmune and Chiesi, outside the submitted work. Dr Klimek reports grants and personal fees from Allergopharma, MEDA/Mylan, LETIPharma and Sanofi; and from HAL Allergie and Allergy Therapeut.; grants from ALK Abelló, AstraZeneca, GSK, Inmunotek, Stallergenes, Quintiles, ASIT Biotech and Lofarma, outside the submitted work; and is member of AeDA, DGHNO, Deutsche Akademie fürAllergologie und Klinische Immunologie, HNO‐BV, GPA, and EAACI. Dr Larenas‐Linnemann reports personal fees from Allakos, Armstrong, AstraZeneca, Boehringer Ingelheim, Chiesi, DBV Technologies, Grunenthal, GSK, MEDA, Menarini, MSD, Novartis, Pfizer, Novartis, Sanofi, Siegfried, UCB and Gossamer, and grants from Sanofi, AstraZeneca, Novartis, UCB, GSK, TEVA, Boehringer Ingelheim, Chiesi and Purina institute, outside the submitted work. Dr Mortz reports grants from Novartis, outside the submitted work. Dr Naclerio reports personal fees from Sanofi, Regeneron, GSK, AstraZeneca, American Chemistry Council, Lyra and TASC, outside the submitted work. Dr Nadeau reports grants and other from NIAID, FARE, Novartis, AnaptysBio, Adare Pharmaceuticals, Stallergenes Greer, NHLBI, NIEHS, EPA, WAO Center of Excellence, IgGenix, ProBio, Vedanta, Centocor, Seed, Immune Tolerance Network, NIH, Sanofi, Astellas, Nestle, Before Brands, Alladapt, Fortra, Genentech, Aimmune Therapeutics and DBV Technologies; personal fees and other from Regeneron; grants from EAT, AllerGenis and Ukko Pharma; and personal fees from AstraZeneca, ImmuneWorks and Cour Pharmaceuticals, outside the submitted work; in addition, Dr Nadeau has a patent inhibition of allergic reaction to peanut allergen using an IL‐33 Inhibitor pending, a patent Special Oral Formula for Decreasing Food Allergy Risk and Treatment for Food Allergy pending, a patent Basophil Activation‐Based Diagnostic Allergy Test pending, a patent Granulocyte‐based methods for detecting and monitoring immune system disorders pending, a patent Methods and Assays for Detecting and Quantifying Pure Subpopulations of White Blood Cells in Immune System Disorders pending, a patent Mixed Allergen Compositions and Methods for Using the Same pending and a patent Microfluidic Device and Diagnostic Methods for Allergy Testing Based on Detection of Basophil Activation pending. Dr O'Mahony reports personal fees from AHL, and grants from GSK, outside the submitted work. Dr Palomares received research grants from Inmunotek SL and Novartis and has received fees for giving scientific lectures from Allergy Therapeutics, Amgen, AstraZeneca, Diater, GlaxoSmithKline, S.A, Inmunotek S.L, Novartis, Sanofi Genzyme and Stallergenes and participated in advisory boards from Novartis and Sanofi Genzyme. Dr Schwarze reports speakers fees from MYLAN, personal fees from F2F events, outside the submitted work; and Industry support to educational activities of the Scottish Allergy and Respiratory Academy and of the Children's and Young people's Allergy Networtk Scotland. Industry support to EAACI, he is EAACI Secretary General 2019‐2021. Dr Papadopoulos reports personal fees from Novartis, Nutricia, HAL, Menarini/Faes Farma, Sanofi, Mylan/MEDA, Biomay, AstraZeneca, GSK, MSD, ASIT Biotech and Boehringer Ingelheim, and grants from Gerolymatos International SA, Capricare, outside the submitted work. Dr Pfaar reports grants and personal fees from ALK‐Abelló, Allergopharma, Stallergenes Greer, HAL Allergy Holding BV/HAL Allergie GmbH, Bencard Allergie GmbH/Allergy Therapeutics, Lofarma, ASIT Biotech Tools SA, Laboratorios LETI/LETIPharma, MEDA Pharma/MYLAN and Anergis SA, grants from Biomay, Circassia and GlaxoSmith Kline; personal fees from Mobile Chamber Experts (a GA2LEN Partner), Indoor Biotechnologies, Astellas Pharma Global, EUFOREA, ROXALL, Novartis, Sanofi‐Aventis, Med Update Europe GmbH, streamedup! and GmbH, outside the submitted work. Dr Regateiro reports personal fees from AstraZeneca, Novartis, Lusomedicamenta, Sanofi and GSK, outside the submitted work. Dr Toppila‐Salmi reports personal fees from Roche, personal fees from Novartis, personal fees from ERT, personal fees from Sanofi, grants from GSK, outside the submitted work. Dr Torres reports grants from the Spanish and Andalusian Ministry of Health, Horizon 2020, Diater, ALK‐Abello, Astra‐Zeneca, GSK and Leti. Dr. Torres reports grants from the Spanish and Andalusian Ministry of Health, Horizon 2020, Diater, ALK‐Abello, Astra‐Zeneca, GSK and Leti. Dr Traidl‐Hoffmann reports grants and personal fees from Töpfer GmbH and Danone Nutricia, personal fees from Sanofi, La Roche‐Posay, Lilly Pharma and Novartis, and grants from Sebapharma and Thermo Fischer, outside the submitted work. Dr Zuberbier reports grants from Organizational affiliations: Committee member: WHO‐Initiative "Allergic Rhinitis and Its Impact on Asthma" (ARIA); Member of the Board: German Society for Allergy and Clinical Immunology (DGAKI); Head: European Centre for Allergy Research Foundation (ECARF); President: Global Allergy and Asthma European Network (GA2LEN); and Member: Committee on Allergy Diagnosis and Molecular Allergology, World Allergy Organization (WAO). The remaining authors have nothing to declare.
Pfaar O, Klimek L, Jutel M, et al. COVID‐19 pandemic: Practical considerations on the organization of an allergy clinic—An EAACI/ARIA Position Paper. Allergy.2021;76:648–676. 10.1111/all.14453
Pfaar, Klimek, Jutel, Akdis and Bousquet participated equally in the paper.
REFERENCES
- 1. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med. 2020;382(13):1199‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coronavirus disease (COVID‐2019) situation reports. Weekly Epidemiological Update and Weekly Operational Update. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/situation‐reports. Accessed 21 Apr, 2020.
- 3. Asthma and Allergy Foundation of America (aafa). https://www.aafa.org/media/2631/respiratory‐illness‐symptoms‐chart‐coronavirus‐flu‐cold‐allergies.png. Accessed April 21, 2020.
- 4. Grasselli G, Zangrillo A, Zanella A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS‐CoV‐2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75(7):1730‐1741. [DOI] [PubMed] [Google Scholar]
- 6. WHO . Coronavirus disease (COVID‐19) technical guidance: Patient management. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/technical‐guidance/patient‐management. Accessed April 21, 2020. [Google Scholar]
- 7. EAACI resource center COVID‐19. https://www.eaaci.org/4702. Accessed 16 May, 2020.
- 8. Dong X, Cao YY, Lu XX, et al. Eleven faces of coronavirus disease 2019. Allergy. 2020;75(7):1699‐1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vultaggio A, Agache I, Akdis CA, et al. Considerations on Biologicals for Patients with allergic disease in times of the COVID‐19 pandemic: an EAACI Statement. Allergy. 2020;75:2764‐2774. 10.1111/all.14407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klimek L, Jutel M, Akdis C, et al. Handling of allergen immunotherapy in the COVID‐19 pandemic: An ARIA‐EAACI statement. Allergy. 2020;75(7):1546‐1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klimek L, Jutel M, Bousquet J, et al. Management of patients with chronic rhinosinusitis during the COVID‐19 pandemic ‐ An EAACI Position Paper. Allergy. 2021;76:677‐688. 10.1111/all.14629 [DOI] [PubMed] [Google Scholar]
- 12. Bousquet J, Anto JM, Iaccarino G, et al. group A, Is diet partly responsible for differences in COVID‐19 death rates between and within countries? Clin Transl Allergy. 2020;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bousquet J, Jutel M, Akdis CA, et al. ARIA‐EAACI statement on asthma and COVID‐19 (June 2, 2020). Allergy. 2021;76:689‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cai Q, Huang D, Ou P, et al. COVID‐19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75(7):1742‐1752. [DOI] [PubMed] [Google Scholar]
- 15. Zhang JJ, Cao YY, Dong X, et al. Distinct characteristics of COVID‐19 patients with initial rRT‐PCR‐positive and rRT‐PCR‐negative results for SARS‐CoV‐2. Allergy. 2020;75(7):1809‐1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sotgiu G, Gerli AG, Centanni S, et al. Advanced forecasting of SARS‐CoV‐2‐related deaths in Italy, Germany, Spain, and New York State. Allergy. 2020;75(7):1813‐1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gursel M, Gursel I. Is global BCG vaccination‐induced trained immunity relevant to the progression of SARS‐CoV‐2 pandemic? Allergy. 2020;75(7):1815‐1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ozdemir C, Kucuksezer UC, Tamay ZU. Is BCG vaccination affecting the spread and severity of COVID‐19? Allergy. 2020;75(7):1824‐1827. [DOI] [PubMed] [Google Scholar]
- 19. Malipiero G, Paoletti G, Puggioni F, et al. An academic allergy unit during COVID‐19 pandemic in Italy. J Allergy Clin Immunol. 2020;146(1):227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaker MS, Oppenheimer J, Grayson M, et al. COVID‐19: Pandemic Contingency Planning for the Allergy and Immunology Clinic. J Allergy Clin Immunol Pract. 2020;8(5):1477‐1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. CDC.gov . Coronavirus Disease 2019; 2020. Available from: https://www.cdc.gov/. Accessed 21 Apr, 2020.
- 22. Liang M, Gao L, Cheng C, et al. Efficacy of face mask in preventing respiratory virus transmission: A systematic review and meta‐analysis. Travel Med Infect Dis 2020;36:101751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. https://www.europeanrhinologicsociety.org. Accessed 21 Apr, 2020.
- 24. Van Gerven L, Hellings PW, Cox T, et al. Personal protection and delivery of rhinologic and endoscopic skull base procedures during the COVID‐19 outbreak. Rhinology 2020;58(3):289‐294. [DOI] [PubMed] [Google Scholar]
- 25. WHO . Coronavirus disease (COVID‐19) pandemic; 2020. Available from https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019. Accessed 21 Apr, 2020. [Google Scholar]
- 26. European Centre for Disease Prevention and Control . Guidelines for the use of non‐pharmaceutical measures to delay and mitigate the impact of 2019‐nCoV. Stockholm: ECDC; 2020. https://www.ecdc.europa.eu/sites/default/files/documents/novel-coronavirus-guidelines-non-pharmaceutical-measures_0.pdf. Accessed 21 Apr, 2020. [Google Scholar]
- 27. Papadopoulos NG, Christodoulou I, Rohde G, et al. Viruses and bacteria in acute asthma exacerbations–a GA2 LEN‐DARE systematic review. Allergy. 2011;66(4):458‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beale J, Jayaraman A, Jackson DJ, et al. Rhinovirus‐induced IL‐25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med. 2014;6(256):256ra134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Edwards MR, Strong K, Cameron A, Walton RP, Jackson DJ, Johnston SL. Viral infections in allergy and immunology: How allergic inflammation influences viral infections and illness. J Allergy Clin Immunol. 2017;140(4):909‐920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wollenberg A, Flohr C, Simon D, et al. European Task Force on Atopic Dermatitis statement on severe acute respiratory syndrome coronavirus 2 (SARS‐Cov‐2)‐infection and atopic dermatitis. J Eur Acad Dermatol Venereol. 2020;34(6):e241‐e242. [DOI] [PubMed] [Google Scholar]
- 31. Du Y, Tu L, Zhu P, et al. Clinical Features of 85 Fatal Cases of COVID‐19 from Wuhan. A Retrospective Observational Study. Am J Respir Crit Care Med. 2020;201(11):1372‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid‐19 in Critically Ill Patients in the Seattle Region ‐ Case Series. N Engl J Med. 2020;382(21):2012‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garg S, Kim L, Whitaker M, et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory‐Confirmed Coronavirus Disease 2019 ‐ COVID‐NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jackson DJ, Busse WW, Bacharier LB, et al. Association of respiratory allergy, asthma, and expression of the SARS‐CoV‐2 receptor ACE2. J Allergy Clin Immunol. 2020;146(1):203‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Waibel KH, Bickel RA, Brown T. Outcomes From a Regional Synchronous Tele‐Allergy Service. J Allergy Clin Immunol Pract. 2019;7(3):1017‐1021. [DOI] [PubMed] [Google Scholar]
- 36. Matricardi PM, Dramburg S, Alvarez‐Perea A, et al. The role of mobile health technologies in allergy care: An EAACI position paper. Allergy. 2020;75(2):259‐272. [DOI] [PubMed] [Google Scholar]
- 37. Waller M, Stotler C. Telemedicine: a Primer. Curr Allergy Asthma Rep. 2018;18(10):54. [DOI] [PubMed] [Google Scholar]
- 38. Pereira AM, Jácome C, Almeida R, Fonseca JA. How the Smartphone is Changing allergy Diagnostics. Curr Allergy Asthma Rep. 2018;18:69. [DOI] [PubMed] [Google Scholar]
- 39. Bousquet J, Caimmi DP, Bedbrook A, et al. Pilot study of mobile phone technology in allergic rhinitis in European countries: the MASK‐rhinitis study. Allergy. 2017;72(6):857‐865. [DOI] [PubMed] [Google Scholar]
- 40. Cuervo‐Pardo L, Barcena‐Blanch MA, Gonzalez‐Estrada A, Schroer B. Apps for food allergy: A critical assessment. J Allergy Clin Immunol Pract. 2015;3(6):980‐981. [DOI] [PubMed] [Google Scholar]
- 41. Worm M, Reese I, Ballmer‐Weber B, et al. Guidelines on the management of IgE‐mediated food allergies: S2k‐Guidelines of the German Society for Allergology and Clinical Immunology (DGAKI) in collaboration with the German Medical Association of Allergologists (AeDA), the German Professional Association of Pediatricians (BVKJ), the German Allergy and Asthma Association (DAAB), German Dermatological Society (DDG), the German Society for Nutrition (DGE), the German Society for Gastroenterology, Digestive and Metabolic Diseases (DGVS), the German Society for Oto‐Rhino‐Laryngology, Head and Neck Surgery, the German Society for Pediatric and Adolescent Medicine (DGKJ), the German Society for Pediatric Allergology and Environmental Medicine (GPA), the German Society for Pneumology (DGP), the German Society for Pediatric Gastroenterology and Nutrition (GPGE), German Contact Allergy Group (DKG), the Austrian Society for Allergology and Immunology (AE‐GAI), German Professional Association of Nutritional Sciences (VDOE) and the Association of the Scientific Medical Societies Germany (AWMF). Allergo J Int. 2015;24:256‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alvarez‐Perea A, Sánchez‐García S, Muñoz Cano R, Antolín‐Amérigo D, Tsilochristou O, Stukus DR. Impact Of "eHealth" in Allergic Diseases and Allergic Patients. J Investig Allergol Clin Immunol. 2019;29(2):94‐102. [DOI] [PubMed] [Google Scholar]
- 43. Taylor L, Waller M, Portnoy JM. Telemedicine for allergy services to rural communities. J Allergy Clin Immunol Pract. 2019;7(8):2554‐2559. [DOI] [PubMed] [Google Scholar]
- 44. Krishna MT, Knibb RC, Huissoon AP. Is there a role for telemedicine in adult allergy services? Clin Exp Allergy. 2016;46(5):668‐677. [DOI] [PubMed] [Google Scholar]
- 45. Portnoy J, Waller M, Elliott T. Telemedicine in the Era of COVID‐19. J Allergy Clinical Immunol Pract. 2020;8(5):1489‐1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. https://www.theverge.com/2020/1/28/21082331/zoom‐vulnerability‐hacker‐eavesdrop‐security‐google‐hangouts‐skype‐checkpoint. Accessed 21 Apr, 2020.
- 47. https://www.fbi.gov/contact‐us/field‐offices/boston/news/press‐releases/fbi‐warns‐of‐teleconferencing‐and‐online‐classroom‐hijacking‐during‐covid‐19‐pandemic. Accessed 21 Apr, 2020.
- 48. https://eur‐lex.europa.eu/legal‐content/EN/TXT/PDF/?uri=CELEX:32016R0679&from=EN. Accessed 21 Apr, 2020.
- 49. https://www.whatsapp.com/legal/?lang=en#terms‐of‐service. Accessed 21 Apr, 2020.
- 50. https://products.office.com/en‐us/microsoft‐teams/group‐chat‐software. Accessed 21 Apr, 2020.
- 51. https://zoom.us. Accessed 21 Apr, 2020.
- 52. https://www.box.com/collaboration/document‐management. Accessed 21 Apr, 2020.
- 53. https://www.whatsapp.com. Accessed 21 Apr 2020.
- 54. https://slack.com. Accessed 21 Apr, 2020.
- 55. https://www.healthline.com/health/best‐telemedicine‐iphone‐android‐apps. Accessed 21 Apr, 2020.
- 56. Xie X, Li Y, Chwang ATY, Ho PL, Seto WH. How far droplets can move in indoor environments–revisiting the Wells evaporation‐falling curve. Indoor Air. 2007;17(3):211‐225. [DOI] [PubMed] [Google Scholar]
- 57. Siegel JD, Rhinehart E, Jackson M, Chiarello L. Health Care Infection Control Practices Advisory Committee. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am J Infect Control. 2007;35(10 Suppl 2):S65‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cancer Care Goes Virtual in Response to COVID‐19. Cancer Discov. 2020;10(6):755. [DOI] [PubMed] [Google Scholar]
- 59. Hopkins C, Surda P, Kumar N. Presentation of new onset anosmia during the COVID‐19 pandemic. Rhinology. 2020;58(3):295‐298. [DOI] [PubMed] [Google Scholar]
- 60. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Glauser W. Proposed protocol to keep COVID‐19 out of hospitals. CMAJ. 2020;192(10):E264‐E265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The Prevalence of Olfactory and Gustatory Dysfunction in COVID‐19 Patients: A Systematic Review and Meta‐analysis. Otolaryngol Head Neck Surg. 2020;163(1):3‐11. [DOI] [PubMed] [Google Scholar]
- 63. Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID‐19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 64. Lu D, Wang H, Yu R, Yang H, Zhao Y. Integrated infection control strategy to minimize nosocomial infection of coronavirus disease 2019 among ENT healthcare workers. J Hospital Infect. 2020;104(4):454‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med. 2020;382(12):1177‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. World Health Organization . Rational Use of Personal Protective Equipment (PPE) for Coronavirus Disease 2019 (COVID‐19); 2020. https://apps.who.int/iris/bitstream/handle/10665/331498/WHO‐2019‐nCoV‐IPCPPE_use‐2020.2‐eng.pdf. Accessed 21 Apr, 2020. [Google Scholar]
- 67. Tran K, Cimon K, Severn M, Pessoa‐Silva C, Conly J. Aerosol‐generating procedures and risk of transmission of acute respiratory infections: a systematic review. CADTH Technol Overv. 2013;3(1):e3201. [PMC free article] [PubMed] [Google Scholar]
- 68. Diamant Z, Gauvreau GM, Cockcroft DW, et al. Inhaled allergen bronchoprovocation tests. J Allergy Clin Immunol. 2013;132(5):1045–1055. [DOI] [PubMed] [Google Scholar]
- 69. Alving K, Diamant Z, Lucas S, et al. Point‐of‐care biomarkers in asthma management: Time to move forward. Allergy. 2020;75(4):995‐997. [DOI] [PubMed] [Google Scholar]
- 70. Meselson M. Droplets and Aerosols in the Transmission of SARS‐CoV‐2. N Engl J Med. 2020;382(21):2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Centers for Disease Control and Prevention . Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Coronavirus Disease 2019 (COVID‐19). https://www.cdc.gov/coronavirus/2019‐ncov/lab/lab‐biosafety‐guidelines.html. Accessed April 19, 2020. [Google Scholar]
- 72. Practical information from Robert Koch Institute. https://www.rki.de/DE/Home/homepage_node.html. Accessed 21 Apr, 2020.
- 73. Practical information from World Health Organization. https://www.who.int/. Accessed 21 Apr, 2020.
- 74. Practical information from International Air Transport Association (IATA). https://www.iata.org/en/programs/cargo/dgr/. Accessed 21 Apr, 2020.
- 75. World Health Organization . Guidance on regulations for the transport of infectious substances 2019–2020. https://www.who.int/ihr/publications/WHO‐WHE‐CPI‐2019.20/en/. Accessed 21 Apr, 2020. [Google Scholar]
- 76. World Health Organization (WHO) . General procedures for inactivation of potentially infectious samples with ebola virus and other highly pathogenic viral agents. https://www.paho.org/hq/dmdocuments/2014/2014‐cha‐procedures‐inactivation‐ebola.pdf. Accessed 01 May, 2020. [Google Scholar]
- 77. Gangadharan D, Smith J, Weyant R. Biosafety Recommendations for Work with Influenza Viruses Containing a Hemagglutinin from the A/goose/Guangdong/1/96 Lineage. MMWR Recomm Rep. 2013;62(RR‐06):1‐7. [PubMed] [Google Scholar]
- 78. Aberer W, Bircher A, Romano A, et al. European Network for Drug Allergy (ENDA); EAACI interest group on drug hypersensitivity. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003;58(9):854‐863. [DOI] [PubMed] [Google Scholar]
- 79. Romano A, Atanaskovic‐Markovic M, Barbaud A, et al. Towards a more precise diagnosis of hypersensitivity to beta‐lactams ‐ an EAACI position paper. Allergy. 2020;75(6):1300‐1315. [DOI] [PubMed] [Google Scholar]
- 80. Kowalski ML, Agache I, Bavbek S, et al. Diagnosis and management of NSAID‐Exacerbated Respiratory Disease (N‐ERD)‐a EAACI position paper. Allergy. 2019;74(1):28‐39. [DOI] [PubMed] [Google Scholar]
- 81. Muraro A, Lemanske RF Jr, Castells M, et al. Precision medicine in allergic disease‐food allergy, drug allergy, and anaphylaxis‐PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma and Immunology. Allergy. 2017;72(7):1006‐1021. [DOI] [PubMed] [Google Scholar]
- 82. Eiwegger T, Hung L, San Diego KE, O'Mahony L, Upton J. Recent developments and highlights in food allergy. Allergy. 2019;74(12):2355‐2367. [DOI] [PubMed] [Google Scholar]
- 83. Chen Y, Chen L, Deng Q, et al. The presence of SARS‐CoV‐2 RNA in the feces of COVID‐19 patients. J Med Virol. 2020;92(7):833‐840. [DOI] [PubMed] [Google Scholar]
- 84. Pettersson ME, Koppelman GH, Flokstra‐de Blok BMJ, Kollen BJ, Dubois AEJ. Prediction of the severity of allergic reactions to foods. Allergy. 2018;73(7):1532‐1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Greuter T, Straumann A. Medical algorithm: Diagnosis and treatment of eosinophilic esophagitis in adults. Allergy. 2020;75(3):727‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bonato G, Dioscoridi L, Mutignani M. Faecal‐oral transmission of SARS‐COV‐2: practical implications. Gastroenterology. 2020. 10.1053/j.gastro.2020.03.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cianferoni A, Warren CM, Brown‐Whitehorn T, Schultz‐Matney F, Nowak‐Wegrzyn A, Gupta RS. Eosinophilic esophagitis and allergic comorbidities in a US‐population‐based study. Allergy. 2020;75(6):1466‐1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bousquet J, Akdis C, Jutel M, et al. Intranasal corticosteroids in allergic rhinitis in COVID‐19 infected patients: An ARIA‐EAACI statement. Allergy. 2020;75:2440‐2444. 10.1111/all.14302 [DOI] [PubMed] [Google Scholar]
- 89. Gelincik A, Brockow K, Çelik G, et al. Diagnosis and management of the drug hypersensitivity reactions in Coronavirus disease 19. Allergy. 2020;75:2775‐2793. 10.1111/all.14439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Williams H, Robertson C, Stewart A, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999;103(1 Pt 1):125‐138. [DOI] [PubMed] [Google Scholar]
- 91. Mortz CG, Andersen KE, Dellgren C, Barington T, Bindslev‐Jensen C. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy. 2015;70(7):836‐845. [DOI] [PubMed] [Google Scholar]
- 92. Akdis CA, Akdis M, Bieber T, et al. Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL Consensus Report. Allergy. 2006;61(8):969‐987. [DOI] [PubMed] [Google Scholar]
- 93. Wollenberg A, Barbarot S, Bieber T, et al. Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32(5):657‐682. [DOI] [PubMed] [Google Scholar]
- 94. Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum ‐ a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients. J Eur Acad Dermatol Venereol. 2020;34(5):1074‐1079. [DOI] [PubMed] [Google Scholar]
- 95. Advice for Dermatology HCPs during Covid‐19 Pandemic; 2020. Available from: https://www.bad.org.uk/. Accessed 22 Apr, 2020.
- 96. Ferrucci S, Romagnuolo M, Angileri L, Berti E, Tavecchio S. Safety of dupilumab in severe atopic dermatitis and infection of Covid‐19: two case reports. J Eur Acad Dermatol Venereol. 2020;34(7):e303‐e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zuberbier T, Aberer W, Asero R, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73(7):1393‐1414. [DOI] [PubMed] [Google Scholar]
- 98. Imbalzano E, Casciaro M, Quartuccio S, et al. Association between urticaria and virus infections: A systematic review. Allergy Asthma Proc. 2016;37(1):18‐22. [DOI] [PubMed] [Google Scholar]
- 99. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34(5):e212‐e213. [DOI] [PubMed] [Google Scholar]
- 100. Hedou M, Carsuzaa F, Chary E, Hainaut E, Cazenave‐Roblot F, Masson Regnault M. Comment on "Cutaneous manifestations in COVID‐19: a first perspective " by Recalcati S. J Eur Acad Dermatol Venereol. 2020;34(7):e299‐e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. van Damme C, Berlingin E, Saussez S, Accaputo O. Acute urticaria with pyrexia as the first manifestations of a COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34(7):e300‐e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Venter C, Groetch M, Netting M, Meyer R. A patient‐specific approach to develop an exclusion diet to manage food allergy in infants and children. Clin Exp Allergy. 2018;48(2):121‐137. [DOI] [PubMed] [Google Scholar]
- 103. Pajno GB, Fernandez‐Rivas M, Arasi S, et al. EAACI Guidelines on allergen immunotherapy: IgE‐mediated food allergy. Allergy. 2018;73(4):799‐815. [DOI] [PubMed] [Google Scholar]
- 104. Sturm GJ, Varga EM, Roberts G, et al. EAACI guidelines on allergen immunotherapy: Hymenoptera venom allergy. Allergy. 2018;73(4):744‐764. [DOI] [PubMed] [Google Scholar]
- 105. Cernadas JR, Brockow K, Romano A, et al. General considerations on rapid desensitization for drug hypersensitivity ‐ a consensus statement. Allergy. 2010;65(11):1357‐1366. [DOI] [PubMed] [Google Scholar]
- 106. Alvarez‐Cuesta E, Madrigal‐Burgaleta R, Angel‐Pereira D, et al. Delving into cornerstones of hypersensitivity to antineoplastic and biological agents: value of diagnostic tools prior to desensitization. Allergy. 2015;70(7):784‐794. [DOI] [PubMed] [Google Scholar]
- 107. Scherer K, Brockow K, Aberer W, et al. Desensitization in delayed drug hypersensitivity reactions – an EAACI position paper of the Drug Allergy Interest Group. Allergy. 2013;68(7):844‐852. [DOI] [PubMed] [Google Scholar]
- 108. Soyka MB, Wawrzyniak P, Eiwegger T, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN‐γ and IL‐4. J Allergy Clin Immunol. 2012;130(5):1087‐1096. [DOI] [PubMed] [Google Scholar]
- 109. Hirsch AG, Stewart WF, Sundaresan AS, et al. Nasal and sinus symptoms and chronic rhinosinusitis in a population‐based sample. Allergy. 2017;72(2):274‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID‐19 infection. A novel syndrome? Rhinology. 2020;58(3):299‐301. [DOI] [PubMed] [Google Scholar]
- 111. Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and Ageusia: Common Findings in COVID‐19 Patients. Laryngoscope. 2020;130(7):1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Fokkens WJ, Lund VJ, Hopkins C, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology. 2020;58(Suppl S29):1‐464. [DOI] [PubMed] [Google Scholar]
- 113. Puhakka T, Mäkelä MJ, Malmström K, et al. The common cold: effects of intranasal fluticasone propionate treatment. J Allergy Clin Immunol. 1998;101(6 Pt 1):726‐731. [DOI] [PubMed] [Google Scholar]
- 114. Hox V, Lourijsen E, Jordens A, et al. Benefits and harm of systemic steroids for short‐ and long‐term use in rhinitis and rhinosinusitis: an EAACI position paper. Clin Transl Allergy. 2020;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Muraro A, Roberts G, Halken S, et al. EAACI guidelines on allergen immunotherapy: Executive statement. Allergy. 2018;73(4):739‐743. [DOI] [PubMed] [Google Scholar]
- 116. Pfaar O, Angier E, Muraro A, Halken S, Roberts G. Algorithms in allergen immunotherapy in allergic rhinoconjunctivitis. Allergy. 2020;75(9):2411‐2414. [DOI] [PubMed] [Google Scholar]
- 117. Pfaar O, Worm M. AIT und COVID‐19. German Society of Allergy and Clinical Immunology (DGAKI). https://dgaki.de/gefaehrliche‐defizite‐in‐der‐allergologie‐bleibt‐der‐patient‐auf‐der‐strecke/. Accessed April 21, 2020.
- 118. Bourdin A, Bjermer L, Brightling C, et al. ERS/EAACI statement on severe exacerbations in asthma in adults: facts, priorities and key research questions. Eur Respir J. 2019;54(3):1900900. [DOI] [PubMed] [Google Scholar]
- 119. Darveaux JI, Lemanske RF Jr. Infection‐related asthma. J Allergy Clin Immunol Pract. 2014;2(6):658‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Halpin DMG, Faner R, Sibila O, Badia JR, Agusti A. Do chronic respiratory diseases or their treatment affect the risk of SARS‐CoV‐2 infection? Lancet Respir Med. 2020;8(5):436‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kaiser UB, Mirmira RG, Stewart PM. Our Response to COVID‐19 as Endocrinologists and Diabetologists. J Clin Endocrinol Metab. 2020;105(5):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. American Academy of Allergy Asthma, and Immunology . COVID‐19 and asthma: What patients need to know; 2020. https://www.aaaai.org/conditions‐and‐treatments/library/asthma‐library/covid‐asthma. Accessed April 17, 2020. [Google Scholar]
- 123. Global Initiative for Asthma . Reccomendations for inhaled asthma controller medications; 2020. https://ginasthma.org/recommendations‐for‐inhaled‐asthma‐controller‐medications/. Accessed April 17, 2020. [Google Scholar]
- 124. European Respiratory Society . COVID‐19: Guidelines and recommendations directory; 2020. https://www.ersnet.org/covid‐19‐guidelines‐and‐recommendations‐directory. Accessed April 17, 2020. [Google Scholar]
- 125. National Institute for Health and Care Excellence. Coronavirus (COVID‐19); 2020. https://www.nice.org.uk/covid‐19. Accessed April 17, 2020.
- 126. Esquivel A, Busse WW, Calatroni A, et al. Effects of omalizumab on rhinovirus infections, illnesses, and exacerbations of asthma. Am J Respir Crit Care Med. 2017;196(8):985‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Teach SJ, Gill MA, Togias A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136(6):1476‐1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Resp Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Singhal T. A review of coronavirus disease‐2019 (COVID‐19). Indian J Pediatr. 2020;87(4):281‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zheng YY, Ma YT, Zhang JY, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Pfaar O, Agache I, Bergmann KC, et al. Placebo effects in allergen immunotherapy ‐ an EAACI Task Force Position Paper. Allergy. 2021;76:629‐647. 10.1111/all.14331 [DOI] [PubMed] [Google Scholar]
- 133. Kiecolt‐Glaser JK, Heffner KL, Glaser R, et al. How stress and anxiety can alter immediate and late phase skin test responses in allergic rhinitis. Psychoneuroendocrinology. 2009;34(5):670‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Vits S, Cesko E, Benson S, et al. Cognitive factors mediate placebo responses in patients with house dust mite allergy. PLoS One. 2013;8(11):e79576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Petrie KJ, Rief W. Psychobiological Mechanisms of Placebo and Nocebo Effects: Pathways to Improve Treatments and Reduce Side Effects. Annu Rev Psychol. 2019;70:599‐625. [DOI] [PubMed] [Google Scholar]
- 136. Schedlowski M, Enck P, Rief W, Bingel U. Neuro‐Bio‐Behavioral Mechanisms of Placebo and Nocebo Responses: Implications for Clinical Trials and Clinical Practice. Pharmacol Rev. 2015;67(3):697‐730. [DOI] [PubMed] [Google Scholar]
- 137. Kemeny ME, Schedlowski M. Understanding the interaction between psychosocial stress and immune‐related diseases: a stepwise progression. Brain Behav Immun. 2007;21(8):1009‐1018. [DOI] [PubMed] [Google Scholar]
- 138. Dantzer R, Cohen S, Russo SJ, Dinan TG. Resilience and immunity. Brain Behav Immun. 2018;74:28‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Food and Drug Administration . Clinical Trial Conduct During the COVID‐19 Pandemic. https://www.fda.gov/drugs/coronavirus‐covid‐19‐drugs/clinical‐trial‐conduct‐during‐covid‐19‐pandemic. Accessed April 19, 2020. [Google Scholar]
- 140. European Medicines Agency . Guidance on the Management of Clinical Trials during the COVID‐19 (Coronavirus) pandemic. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol‐10/guidanceclinicaltrials_covid19_en.pdf. Accessed April 19, 2020. [Google Scholar]
- 141. Shrestha GS, Paneru HR, Vincent JL. Precision medicine for COVID‐19: a call for better clinical trials. Crit Care. 2020;24(1):282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Bonini S, Maltese G. COVID‐19 Clinical trials: quality matters more than quantity. Allergy. 2020;75:2542‐2547. 10.1111/all.14409 [DOI] [PMC free article] [PubMed] [Google Scholar]


