Abstract
A high prevalence rate of macrolide-resistant Mycoplasma pneumoniae (MRMP) has been reported in Asia. We performed a systematic review and meta-analysis to investigate the effect of macrolide resistance on the manifestations and clinical judgment during M. pneumoniae infections. We found no difference in clinical severity between MRMP and macrolide-sensitive Mycoplasma pneumoniae (MSMP) infections. However, in the pooled data, patients infected with MRMP had a longer febrile period (1.71 days), length of hospital stay (1.61 day), antibiotic drug courses (2.93 days), and defervescence time after macrolide treatment (2.04 days) compared with patients infected with MSMP. The risk of fever lasting for >48 hours after macrolide treatment was also significantly increased (OR 21.24), and an increased proportion of patients was changed to second-line treatment (OR 4.42). Our findings indicate diagnostic and therapeutic challenges after the emergence of MRMP. More precise diagnostic tools and clearly defined treatment should be appraised in the future.
Keywords: Mycoplasma pneumoniae, macrolide resistance, children, pneumonia, bacteria, Asia, antimicrobial resistance
Mycoplasma pneumoniae is a common causative pathogen in community-acquired pneumonia (CAP) during childhood. In the post–pneumococcal conjugate vaccine (PCV) 13 era, the epidemiology of pediatric pneumonia has changed. In some countries where PCV13 is already included in national immunization program, M. pneumoniae has become the leading pathogen in pediatric CAP (1,2).
The clinical manifestations of M. pneumoniae infection are usually mild and self-limited. However, life-threatening pneumonia or even acute respiratory distress syndrome requiring extracorporeal membrane oxygen has been reported (3). Furthermore, some extrapulmonary symptoms, such as mucositis, hepatitis, encephalitis, hemolysis, or erythema multiforme, have linked M. pneumoniae infection to the formation of autoimmunity or immune complexes. The association between M. pneumoniae and refractory asthma has also been mentioned (4).
Macrolides are the first-line therapy for M. pneumoniae. Because of high oral bioavailability and once-daily formulation, macrolides have been widely used in outpatient settings. During the past 10 years, however, macrolide-resistant Mycoplasma pneumoniae (MRMP) has emerged worldwide. The most prevalent area is Asia, where prevalence rates are 13.6%–100% (5). In Japan and China, resistance rates are >90% in some epidemic years (5,6).
The treatment of MRMP has become challenging. Although 1 report showed more complications in managing MRMP infections (7), the association between severe disease and resistance remains inconsistent and unclear. We conducted a systematic review and meta-analysis to examine the effect of macrolide resistance on the manifestations, outcomes, and clinical judgment of M. pneumoniae infection.
Methods
Search Strategy
We conducted a systematic literature search in PubMed, Embase, and the Cochrane Library database using the keywords Mycoplasma pneumoniae, macrolide, antibiotic resistance, and drug resistance. There was no language restriction in our search. We reviewed eligible full texts and the reference lists of the relevant studies. The last update of the study was on December 1, 2019.
Two independent reviewers (Y.-C.C. and T.-H.C.) screened all titles and abstracts for eligibility. Studies were eligible for inclusion if the study population was restricted to children (<18 years of age) with community-acquired pneumonia; macrolide resistance was detected by PCR including the 2 common point mutations, positions 2063 and 2064; and a direct comparator was used in the same cohorts (macrolide-sensitive M. pneumoniae [MSMP] group). We excluded review articles, editorial comments, case reports, and posters but included correspondence or letters that fulfilled these criteria.
Data Extraction and Quality Assessment
After full-text screening for eligibility and review, the 3 authors extracted data independently of one another. We resolved disagreements by consensus or review by another reviewer. We extracted the following variables from each study, if available: author, journal, year of publication, study design, study country, time period, detected point mutations, clinical symptoms, total febrile days, length of hospital stay, defervescence days after macrolide, antibiotic history, laboratory results, and chest radiographic findings. We also extracted pediatric data from studies with both children and adults, if available. We assessed the quality of nonrandomized studies included in the meta-analysis using the Newcastle-Ottawa Scale and excluded articles with poor quality (score 0–3).
Data Analysis
We used Review Manager software version 5.3 (Cochrane Collaboration, https://training.cochrane.org) and Comprehensive Meta-Analysis version 3 (Biostat, https://www.meta-analysis.com) for the analysis and conducted meta-analysis when >3 studies with available data reported the same outcome. We calculated heterogeneity (I2) to examine statistical heterogeneity across the included studies. We considered I2 >50% and p<0.05 to indicate substantial heterogeneity. We used random effects models to calculate odds ratios for binary outcomes and mean differences for continuous outcomes. We used Egger precision weighted linear regression tests and funnel plots to test potential publication bias. If publication bias was present, we used the trim-and-fill method and calculated Rosenthal’s fail-safe N to evaluate the effect.
Results
Study Characteristics
We identified 1,100 articles in the initial search (Figure 1). After removing duplicates, we screened 892 articles by titles and abstracts. We excluded obviously irrelevant articles and retrieved the remaining 151 for full text assessment. We then excluded epidemiologic or in vitro studies without clinical data. We included 27 full-text studies in the qualitative synthesis. We identified 3 records through manual search of the reference lists of retrieved articles. Finally, we included 24 full-text articles in the meta-analysis. The studies were conducted in the Asia-Pacific region, except for 1 in Italy. The range of resistance rates was 10%–88%. The A2063G transition mutation was detected in all studies (Table).
Figure 1.
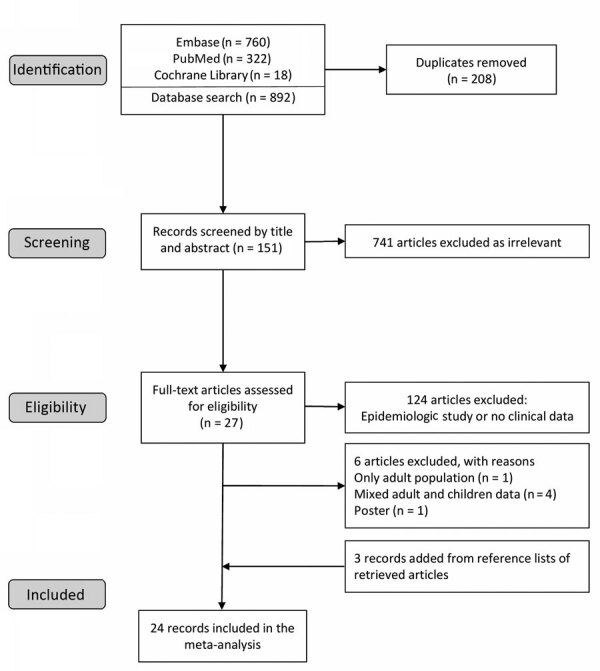
Flow diagram of selection process for meta-analysis of macrolide-resistant Mycoplasma pneumoniae infections in pediatric community-acquired pneumonia.
Table. Characteristics of the eligible studies of macrolide resistance and Mycoplasma pneumoniae infections.
| Author | Study period | Country | Mutations detected | Disease entity | Case no. | Resistance |
|---|---|---|---|---|---|---|
| Chen (8) | 2014–2016 | China | A2063G, A2064G | CAP, inpatient | 136 | 60% |
| Ma (9) | 2010–2011 | China | A2063G, A1290G | CAP, inpatient | 57 | 63% |
| Xin (10) | 2004–2005 | China | A2063G, A2064G | CAP, inpatient | 64 | 59% |
| Yuan (11) | 2016 | China | A2063G, A2064G, C2617G | CAP, inpatient | 120 | 82% |
| Zhou (7) | 2009–2010 | China | A2063G, A2063T, A2064G | CAP, inpatient | 235 | 88% |
| Cheong (12) | 2011–2013 | HK | A2063G | CAP, inpatient | 93 | 27% |
| Lung (13) | 2010–2013 | HK | A2063G | CAP, inpatient | 48 | 71% |
| Cardinale (14) | 2010 | Italy | A2063G, A2064G | CAP, inpatient | 46 | 17% |
| Akashi (15) | 2016–2017 | Japan | A2063G, A2064G | CAP, mixed | 222 | 65% |
| Ishiguro (16) | 2013–2015 | Japan | A2063G | CAP, mixed | 109 | 54% |
| Kawai (17) | 2005–2010 | Japan | A2063G, A2064G | CAP, mixed | 29 | 72% |
| Kawai (18) | 2005–2012 | Japan | A2063G, A2064G | CAP, mixed | 188 | 80% |
| Matsubara (19) | 2002–2006 | Japan | A2063G, A2064G | CAP, NS | 69 | 32% |
| Miyashita (20) | 2008–2011 | Japan | A2063G, A2064G | CAP, NS | 71 | 59% |
| Okada (21) | 2011 | Japan | A2063G, A2064G | CAP, mixed | 202 | 87% |
| Kim JH (22) | 2011–2015 | Korea | A2063G | CAP, inpatient | 250 | 74% |
| Kim YJ (23) | 2010–2015 | Korea | A2063G | CAP, inpatient | 107 | 10% |
| Lee (24) | 2015 | Korea | A2063G | CAP, mixed | 94 | 13% |
| Seo (25) | 2011 | Korea | A2063G | CAP, inpatient | 95 | 52% |
| Yoo (26) | 2011 | Korea | A2063G | CAP, mixed | 91 | 30% |
| Yoon (27) | 2010–2015 | Korea | A2063G | CAP, inpatient | 116 | 71% |
| Wu HM (28) | 2011 | Taiwan | A2063G | CAP, inpatient | 73 | 12% |
| Wu PS (29) | 2010–2011 | Taiwan | A2063G | CAP, inpatient | 60 | 23% |
| Yang (30) | 2010–2017 | Taiwan | A2063G, A2063T, A2064G | CAP, mixed | 471 | 24% |
*CAP, community acquired pneumonia; HK, Hong Kong; NS, not specified.
Effect on Clinical Features and Outcomes
The duration of fever was longer in patients with MRMP than in patients with MSMP (mean difference [MD] 1.71, 95% CI 1.34–2.09; p<0.001) (Figure 2). The result was stable and consistent within studies (I2 = 0%; p = 0.54). MRMP infections were also associated with prolonged hospitalization compared with MSMP infections (MD 1.61, 95% CI 1.08–2.13; p<0.001) (Appendix Figure 1). We found no significant heterogeneity in the studies included (I2 = 28%; p = 0.18).
Figure 2.
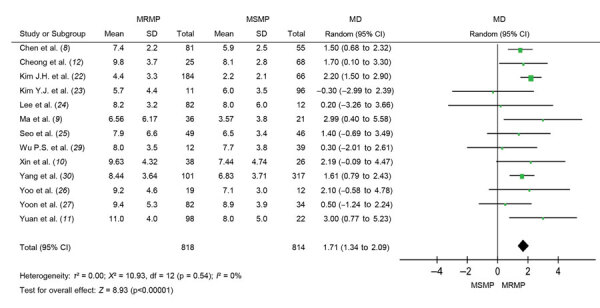
Forest plots of difference in total febrile days between MRMP and MSMP in meta-analysis of MRMP infections in pediatric community-acquired pneumonia. MD, mean difference; MRMP, macrolide-resistant Mycoplasma pneumoniae; MSMP, macrolide-sensitive Mycoplasma pneumoniae.
We examined the effect of macrolide resistance on work of breath and extrapulmonary symptoms. We found a slight trend toward MRMP patients with more extensive disease (Appendix Figure 2). Nevertheless, we found no difference in clinical features, such as dyspnea (OR 1.71, 95% CI 0.69–4.24; p = 0.24) or extrapulmonary manifestations (OR 1.31, 95% CI 0.85–2.02; p = 0.22), in patients with MRMP infections.
Laboratory Results
We assessed inflammatory markers commonly examined during M. pneumoniae infection (Appendix Figure 3). Eleven studies provided data on leukocyte count; we found no significant difference between MRMP and MSMP patients (MD 0.09, 95% CI −0.31 to 0.50; p = 0.65). Nine studies assessed C-reactive protein (mg/L) during infection; again, we found no significant differences between MRMP and MSMP patients (MD −2.79, 95% CI −8.33 to 2.76; p = 0.32).
Figure 3.
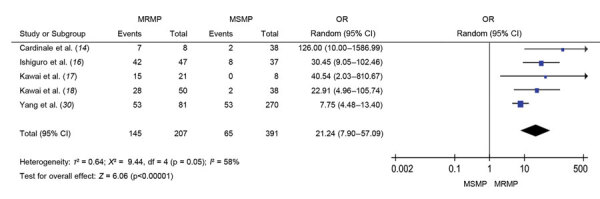
Forest plots comparing the pooled odds ratio of fever lasting for 48 hours after macrolide treatment between MRMP and MSMP in meta-analysis of MRMP infections in pediatric community-acquired pneumonia. MRMP, macrolide-resistant Mycoplasma pneumoniae; MSMP, macrolide-sensitive Mycoplasma pneumoniae; OR, odds ratio.
Chest Radiographic Findings
We assessed the difference in chest radiographic findings in MRMP and MSMP patients (Appendix Figure 4). Neither consolidation ratio (OR 1.06, 95% CI 0.88–1.27; p = 0.52) nor pleural effusion (OR 1.19, 95% CI 0.70–2.03; p = 0.51) was influenced by macrolide resistance.
Figure 4.
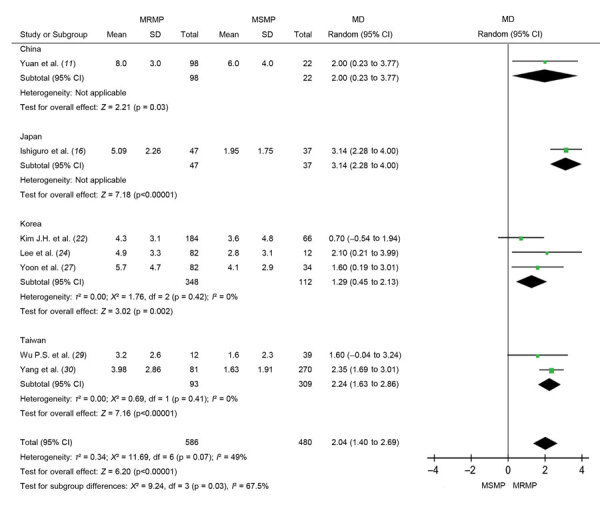
Forest plots depicting the defervescence time (days) after macrolide treatment in meta-analysis of MRMP infections in pediatric community-acquired pneumonia. Subgroup analysis was performed according to country. MD, mean difference; MRMP, macrolide-resistant Mycoplasma pneumoniae; MSMP, macrolide-sensitive Mycoplasma pneumoniae.
Effect on Macrolide Efficacy and Antibiotic Prescription
In children with MRMP infection, fever may persist for >48 hours despite macrolide use. Figure 3 illustrates significantly increased odds of poor response to macrolide in patients with MRMP infections (OR 21.24, 95% CI 7.90–57.09; p<0.001).
Because efficacy of macrolides was reduced in patients with MRMP infections, we further investigated the exact effect of macrolide resistance on defervescence. The pooled results show significantly longer febrile duration (days) after ineffective treatment (MD 2.04, 95% CI 1.40–2.69; p<0.001). However, we observed an overall low-to-moderate heterogeneity within studies (I2 = 49%; p = 0.07). Considering different treatment policies (timing to initiate second-line antibiotic or corticosteroid) for M. pneumoniae among regions, we performed a subgroup analysis according to country (Figure 4).
During macrolide treatment, some patients with M. pneumoniae infection would be switched to other classes of antibiotic drugs, such as the most commonly used fluoroquinolones and tetracyclines, that have different mechanisms of action (Figure 5). Increased proportions of patients were changed to second-line treatment when infected with MRMP (OR 4.42, 95% CI 2.32–8.41; p<0.001). Subgroup analysis divided by countries reveals that the heterogeneities were still high in Japan and Hong Kong.
Figure 5.
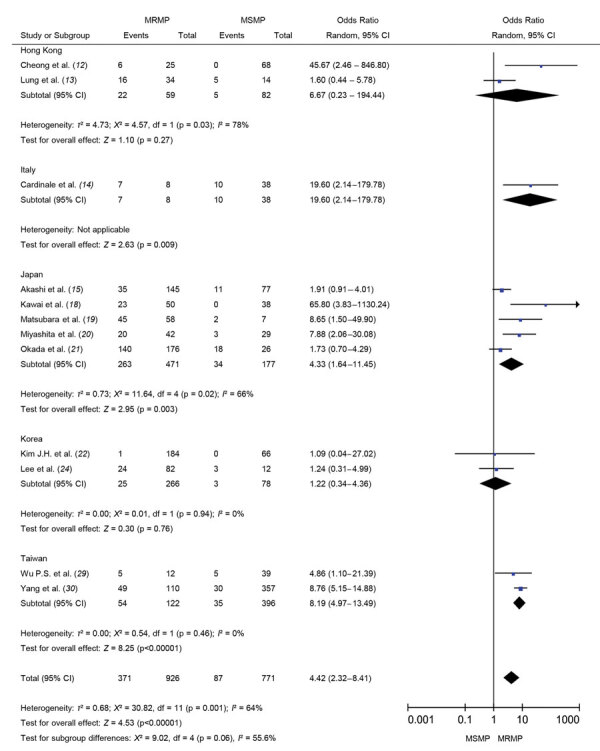
Forest plots comparing MRMP and MSMP by the pooled odds ratio of changing antibiotics in meta-analysis of MRMP infections in pediatric community-acquired pneumonia. MRMP, macrolide-resistant Mycoplasma pneumoniae; MSMP, macrolide-sensitive Mycoplasma pneumoniae; OR, odds ratio.
The total duration of antibiotic drug treatment was longer when used to treat MRMP infections than when used to treat MSMP infections (MD 2.93, 95% CI 1.97–3.89; p<0.001) (Figure 6). There was no substantial heterogeneity (I2 = 0%; p = 0.48).
Figure 6.
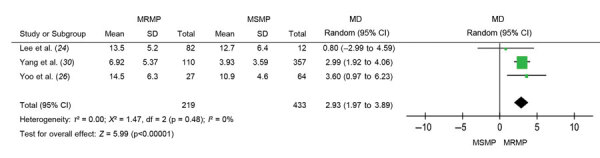
The duration difference (days) of antibiotic use between MRMP and MSMP infections in meta-analysis of MRMP infections in pediatric community-acquired pneumonia. MD, mean difference; MRMP, macrolide-resistant Mycoplasma pneumoniae; MSMP, macrolide-sensitive Mycoplasma pneumoniae.
Discussion
This systematic review and meta-analysis summarized currently available studies to compare the difference between MRMP and MSMP infections. The resistance rates varied within the studies, even in the same country (5). The overall resistance rate in large cohort studies in South Korea and Taiwan (30,31) increased over time; in contrast, the rate has gradually decreased in Japan since 2012 (32,33).
We applied multiple molecular methods to explain the spread of MRMP in Asia. Some reports using multilocus variable-number tandem-repeat analysis as the molecular typing method showed that the spread of M. pneumoniae seemed to be polyclonal (34,35). However, 2 recently published reports in South Korea and Japan that used multilocus sequence typing as the diagnostic method revealed that the wide spread of MRMP was associated with clonal expansion of the resistant ST3 clone (31,36). Whole-genome sequencing might be a better and more comprehensive tool for solving inconsistency and investigating M. pneumoniae evolutionary trends in the future.
The clinical manifestations, chest radiographic findings, and laboratory data were not altered by macrolide resistance. Although some studies showed more severe radiological findings (30) and more complications (7) after MRMP infections, there appeared to be no significant difference in the pooled data. M. pneumoniae presents a unique virulence factor in humans, an ADP-ribosyl transferase known as the community-acquired respiratory distress syndrome toxin (CARDS toxin). Lluch-Senar et al. (37) performed sequence analysis of the P1 adhesin gene and stated that type 2 strain produced more CARDS toxin. However, Zhao et al. (38) and Eshaghi et al. (39) failed to demonstrate this difference between MRMP and MSMP. Currently, no evidence supports the causal relationship between macrolide resistance and disease severity.
The efficacy of macrolide is significantly decreased during MRMP treatment compared to MSMP treatment. The most common point mutation in the domain V 23S rRNA is A2063G, which will cause great MIC increase to all macrolide drugs. Other than A2063G, some studies also reported A2064G mutation, which could result in decreased macrolide affinity and elevation of MIC (21). Based on these results, we expected to see much longer fever duration from ineffective treatment. However, the pooled data revealed only an interval difference of 1.71 days between fever durations in MRMP and MSMP infections. We further examined the exact days of patients being afebrile after macrolide treatment and the clinical judgment on antibiotic drug use. The study results showed significant heterogeneity.
We then performed subgroup analysis by country. The results reflected different treatment policies among countries, even among institutions. Treatment selection for MRMP might modify the effect of macrolide resistance on clinical course. For instance, a report in South Korea (22) demonstrated less effect of resistance on macrolide efficacy. The possible reason is that steroids were given to 18.5% of patients with MRMP in this cohort, but not to MSMP patients (3%; p = 0.002). The initiation of corticosteroid treatment is early in South Korea (40) but reserved for refractory cases in Japan (41). Another study in China (7) noted that all patients in the report received only macrolides, given that the antimicrobial drug options are limited for preschool-age children. Therefore, more extrapulmonary complications (encephalitis, myocarditis, or hepatitis) occurred.
To treat or not to treat M. pneumoniae is still a dilemma to be resolved. MRMP treatment has raised another problem. Our meta-analysis identified 2 knowledge gaps. The first is the diagnostic gap. Macrolide resistance detection in most institutions relied on in-house PCR. Weighing the costs and benefits, it usually takes time to provide formal reports. Physicians usually base their suspicions of MRMP infections on clinical judgment of patients’ response to treatment. In Japan and Taiwan, if fever persists for 48–72 hours after macrolide treatment, second-line antimicrobial drugs, such as fluoroquinolone or tetracycline, would be considered (42,43). Delayed defervescence of 2 days after macrolide (Figure 4) could be explained by this clinical practice. Timeliness of diagnostic tests after disease onset can be a factor in confirming macrolide resistance. Real-time or point-of-care testing should be used to make the diagnosis more precisely and quickly.
The second challenge is the therapeutic gap. In Japan, the therapeutic efficacy of tosufloxacin and minocycline has been demonstrated in several studies (16,18,21). However, because of side effects and the development of new resistant strains, empirical treatment for MRMP, especially in endemic areas, is the subject of an ongoing debate. In addition, delayed effective antimicrobial treatment for M. pneumoniae has been found to be related to immune reaction, which may lead to prolonged or extrapulmonary disease (30). Macrolide resistance is one of the significant risk factors for delayed effective treatment. This finding partially explains why patients with MRMP infections showed a trend of more extrapulmonary manifestations or consolidation in 2 studies (7,30). Some randomized controlled trials indicated a positive effect on early corticosteroid treatment whether or not there was macrolide resistance (44,45). However, a retrospective study of a large database in Japan did not support this viewpoint (46). A well-designed randomized trial or meta-analysis should be considered to clarify the role of corticosteroids. In conclusion, the management of M. pneumoniae infection might need to be reappraised.
In addition to prolonged clinical courses, our study indicates the effect of macrolide resistance on antibiotic drug consumption and imprecision. Increased macrolide usage in primary healthcare settings, as well as unnecessary and inappropriate prescriptions to treat acute respiratory tract infections, are common in countries in Asia (47–49). Continuous selective pressure of routinely used antibiotic drugs and high population density can possibly explain the emergence of MRMP. The extent of M. pneumoniae simultaneously increased with rising resistance, further resulting in increased consumption of antibiotic drugs. Antibiotic stewardship should be promoted to reduce macrolide resistance.
Our meta-analysis has limitations. First, not all reported mutations (such as C2617G) were described or checked in the included studies. Because positions 2063 and 2064 accounted for most of the mutations and have been reported in all articles included in this analysis, this influence could be minimized. Second, co-infection was not excluded in all studies. The co-infection rate with M. pneumoniae is low in some studies (1,12,30). Nevertheless, how to discriminate between carriage and infection is still a key issue. A combination of PCR and serologic tests, such as measurement of M. pneumoniae–specific IgM-secreting cells, would be a better way to determine the role of macrolide resistance in the future (50). Third, the natural course of MRMP infection is modified because in institutions where physicians are alert to MRMP, second-line therapy or corticosteroids will be administered promptly. Although this bias existed in the initial selection process, it reflected the current clinical practice in MRMP-prevalent areas and the dilemma in management of MRMP.
In summary, our analysis found that MRMP infections are associated with longer febrile duration than MSMP infections. Decreased macrolide efficacy and increased ineffective antimicrobial drug use have also been found. The effect of macrolide resistance on disease severity is inconclusive, and there are still diagnostic and therapeutic gaps in the management of MRMP. Reappraisal of precise diagnostic tools and clearly defined treatment are needed.
Additional information about the study of macrolide-resistant pediatric Mycoplasma pneumoniae infections.
Biography
Dr. Chen is a pediatrician in Chi-Mei Medical Center, Chiali, Tainan, Taiwan. Her research interests include microbiology, neonatology, and vaccinology.
Footnotes
Suggested citation for this article: Chen Y-C, Hsu W-Y, Chang T-H. Macrolide-resistant Mycoplasma pneumoniae infections in pediatric community-acquired pneumonia. Emerg Infect Dis. 2020 Jul [date cited]. http://dx.doi.org/10.3201/eid2607.200017
All authors contributed equally to this article.
References
- 1.Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, et al. ; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372:835–45. 10.1056/NEJMoa1405870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin EJ, Kim Y, Jeong J-Y, Jung YM, Lee M-H, Chung EH. The changes of prevalence and etiology of pediatric pneumonia from National Emergency Department Information System in Korea, between 2007 and 2014. Korean J Pediatr. 2018;61:291–300. 10.3345/kjp.2017.06100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsieh YC, Tsao KC, Huang CG, Tong S, Winchell JM, Huang YC, et al. Life-threatening pneumonia caused by macrolide-resistant Mycoplasma pneumoniae. Pediatr Infect Dis J. 2012;31:208–9. 10.1097/INF.0b013e318234597c [DOI] [PubMed] [Google Scholar]
- 4.Wood PR, Hill VL, Burks ML, Peters JI, Singh H, Kannan TR, et al. Mycoplasma pneumoniae in children with acute and refractory asthma. Ann Allergy Asthma Immunol. 2013;110:328–34.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereyre S, Goret J, Bébéar C. Mycoplasma pneumoniae: current knowledge on macrolide resistance and treatment. Front Microbiol. 2016;7:974. 10.3389/fmicb.2016.00974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer Sauteur PM, Unger WW, Nadal D, Berger C, Vink C, van Rossum AM. Infection with and carriage of Mycoplasma pneumoniae in children. Front Microbiol. 2016;7:329. 10.3389/fmicb.2016.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y, Zhang Y, Sheng Y, Zhang L, Shen Z, Chen Z. More complications occur in macrolide-resistant than in macrolide-sensitive Mycoplasma pneumoniae pneumonia. Antimicrob Agents Chemother. 2014;58:1034–8. 10.1128/AAC.01806-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Tian WM, Chen Q, Zhao HY, Huang P, Lin ZQ, et al. [Clinical features and treatment of macrolide-resistant Mycoplasma pneumoniae pneumonia in children]. Zhongguo Dang Dai Er Ke Za Zhi. 2018;20:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Z, Zheng Y, Deng J, Ma X, Liu H. Characterization of macrolide resistance of Mycoplasma pneumoniae in children in Shenzhen, China. Pediatr Pulmonol. 2014;49:695–700. 10.1002/ppul.22851 [DOI] [PubMed] [Google Scholar]
- 10.Xin D-L, Wang S, Han X, Ma S-J, Chen X-G. Clinical characteristics of children with macrolide-resistant Mycoplasma pneumoniae pneumonia [in Chinese]. J Appl Clin Pediatr. 2010;16:1213–5. [Google Scholar]
- 11.Yuan C, Min FM, Ling YJ, Li G, Ye HZ, Pan JH, et al. Clinical characteristics and antibiotic resistance of Mycoplasma pneumoniae pneumonia in hospitalized Chinese children. Comb Chem High Throughput Screen. 2018;21:749–54. 10.2174/1386207322666190111112946 [DOI] [PubMed] [Google Scholar]
- 12.Cheong KN, Chiu SS, Chan BW, To KK, Chan EL, Ho PL. Severe macrolide-resistant Mycoplasma pneumoniae pneumonia associated with macrolide failure. J Microbiol Immunol Infect. 2016;49:127–30. 10.1016/j.jmii.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 13.Lung DC, Yip EK, Lam DS, Que TL. Rapid defervescence after doxycycline treatment of macrolide-resistant Mycoplasma pneumoniae-associated community-acquired pneumonia in children. Pediatr Infect Dis J. 2013;32:1396–9. 10.1097/INF.0b013e3182a25c71 [DOI] [PubMed] [Google Scholar]
- 14.Cardinale F, Chironna M, Chinellato I, Principi N, Esposito S. Clinical relevance of Mycoplasma pneumoniae macrolide resistance in children. J Clin Microbiol. 2013;51:723–4. 10.1128/JCM.02840-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akashi Y, Hayashi D, Suzuki H, Shiigai M, Kanemoto K, Notake S, et al. Clinical features and seasonal variations in the prevalence of macrolide-resistant Mycoplasma pneumoniae. J Gen Fam Med. 2018;19:191–7. 10.1002/jgf2.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishiguro N, Koseki N, Kaiho M, Ariga T, Kikuta H, Togashi T, et al. ; Hokkaido Pediatric Respiratory Infection Study Group. Therapeutic efficacy of azithromycin, clarithromycin, minocycline and tosufloxacin against macrolide-resistant and macrolide-sensitive Mycoplasma pneumoniae pneumonia in pediatric patients. PLoS One. 2017;12:e0173635. 10.1371/journal.pone.0173635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawai Y, Miyashita N, Yamaguchi T, Saitoh A, Kondoh E, Fujimoto H, et al. Clinical efficacy of macrolide antibiotics against genetically determined macrolide-resistant Mycoplasma pneumoniae pneumonia in paediatric patients. Respirology. 2012;17:354–62. 10.1111/j.1440-1843.2011.02102.x [DOI] [PubMed] [Google Scholar]
- 18.Kawai Y, Miyashita N, Kubo M, Akaike H, Kato A, Nishizawa Y, et al. Therapeutic efficacy of macrolides, minocycline, and tosufloxacin against macrolide-resistant Mycoplasma pneumoniae pneumonia in pediatric patients. Antimicrob Agents Chemother. 2013;57:2252–8. 10.1128/AAC.00048-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsubara K, Morozumi M, Okada T, Matsushima T, Komiyama O, Shoji M, et al. A comparative clinical study of macrolide-sensitive and macrolide-resistant Mycoplasma pneumoniae infections in pediatric patients. J Infect Chemother. 2009;15:380–3. 10.1007/s10156-009-0715-7 [DOI] [PubMed] [Google Scholar]
- 20.Miyashita N, Kawai Y, Akaike H, Ouchi K, Hayashi T, Kurihara T, et al. ; Atypical Pathogen Study Group. Macrolide-resistant Mycoplasma pneumoniae in adolescents with community-acquired pneumonia. BMC Infect Dis. 2012;12:126. 10.1186/1471-2334-12-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okada T, Morozumi M, Tajima T, Hasegawa M, Sakata H, Ohnari S, et al. Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin Infect Dis. 2012;55:1642–9. 10.1093/cid/cis784 [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Kim JY, Yoo CH, Seo WH, Yoo Y, Song DJ, et al. Macrolide resistance and its impacts on M. pneumoniae pneumonia in children: comparison of two recent epidemics in Korea. Allergy Asthma Immunol Res. 2017;9:340–6. 10.4168/aair.2017.9.4.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YJ, Shin KS, Lee KH, Kim YR, Choi JH. Clinical characteristics of macrolide-resistant Mycoplasma pneumoniae from children in Jeju. J Korean Med Sci. 2017;32:1642–6. 10.3346/jkms.2017.32.10.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee E, Cho HJ, Hong SJ, Lee J, Sung H, Yu J. Prevalence and clinical manifestations of macrolide resistant Mycoplasma pneumoniae pneumonia in Korean children. Korean J Pediatr. 2017;60:151–7. 10.3345/kjp.2017.60.5.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seo YH, Kim JS, Seo SC, Seo WH, Yoo Y, Song DJ, et al. Predictive value of C-reactive protein in response to macrolides in children with macrolide-resistant Mycoplasma pneumoniae pneumonia. Korean J Pediatr. 2014;57:186–92. 10.3345/kjp.2014.57.4.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoo SJ, Kim HB, Choi SH, Lee SO, Kim SH, Hong SB, et al. Differences in the frequency of 23S rRNA gene mutations in Mycoplasma pneumoniae between children and adults with community-acquired pneumonia: clinical impact of mutations conferring macrolide resistance. Antimicrob Agents Chemother. 2012;56:6393–6. 10.1128/AAC.01421-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoon IA, Hong KB, Lee HJ, Yun KW, Park JY, Choi YH, et al. Radiologic findings as a determinant and no effect of macrolide resistance on clinical course of Mycoplasma pneumoniae pneumonia. BMC Infect Dis. 2017;17:402. 10.1186/s12879-017-2500-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu HM, Wong KS, Huang YC, Lai SH, Tsao KC, Lin YJ, et al. Macrolide-resistant Mycoplasma pneumoniae in children in Taiwan. J Infect Chemother. 2013;19:782–6. 10.1007/s10156-012-0523-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu PS, Chang LY, Lin HC, Chi H, Hsieh YC, Huang YC, et al. Epidemiology and clinical manifestations of children with macrolide-resistant Mycoplasma pneumoniae pneumonia in Taiwan. Pediatr Pulmonol. 2013;48:904–11. 10.1002/ppul.22706 [DOI] [PubMed] [Google Scholar]
- 30.Yang TI, Chang TH, Lu CY, Chen JM, Lee PI, Huang LM, et al. Mycoplasma pneumoniae in pediatric patients: Do macrolide-resistance and/or delayed treatment matter? J Microbiol Immunol Infect. 2019;52:329–35. 10.1016/j.jmii.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 31.Lee JK, Lee JH, Lee H, Ahn YM, Eun BW, Cho EY, et al. Clonal expansion of macrolide-resistant sequence type 3 Mycoplasma pneumoniae, South Korea. Emerg Infect Dis. 2018;24:1465–71. 10.3201/eid2408.180081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka T, Oishi T, Miyata I, Wakabayashi S, Kono M, Ono S, et al. Macrolide-resistant Mycoplasma pneumoniae infection, Japan, 2008–2015. Emerg Infect Dis. 2017;23:1703–6. 10.3201/eid2310.170106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katsukawa C, Kenri T, Shibayama K, Takahashi K. Genetic characterization of Mycoplasma pneumoniae isolated in Osaka between 2011 and 2017: Decreased detection rate of macrolide-resistance and increase of p1 gene type 2 lineage strains. PLoS One. 2019;14:e0209938. 10.1371/journal.pone.0209938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki Y, Seto J, Shimotai Y, Itagaki T, Katsushima Y, Katsushima F, et al. Polyclonal spread of multiple genotypes of Mycoplasma pneumoniae in semi-closed settings in Yamagata, Japan. J Med Microbiol. 2019;68:785–90. 10.1099/jmm.0.000969 [DOI] [PubMed] [Google Scholar]
- 35.Pereyre S, Charron A, Hidalgo-Grass C, Touati A, Moses AE, Nir-Paz R, et al. The spread of Mycoplasma pneumoniae is polyclonal in both an endemic setting in France and in an epidemic setting in Israel. PLoS One. 2012;7:e38585. 10.1371/journal.pone.0038585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ando M, Morozumi M, Adachi Y, Ubukata K, Iwata S. Multilocus sequence typing of Mycoplasma pneumoniae, Japan, 2002–2016. Emerg Infect Dis. 2018;24:1895–901. 10.3201/eid2410.171194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lluch-Senar M, Cozzuto L, Cano J, Delgado J, Llórens-Rico V, Pereyre S, et al. Comparative “-omics” in Mycoplasma pneumoniae clinical isolates reveals key virulence factors. PLoS One. 2015;10:e0137354. 10.1371/journal.pone.0137354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao F, Liu G, Wu J, Cao B, Tao X, He L, et al. Surveillance of macrolide-resistant Mycoplasma pneumoniae in Beijing, China, from 2008 to 2012. Antimicrob Agents Chemother. 2013;57:1521–3. 10.1128/AAC.02060-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eshaghi A, Memari N, Tang P, Olsha R, Farrell DJ, Low DE, et al. Macrolide-resistant Mycoplasma pneumoniae in humans, Ontario, Canada, 2010-2011. Emerg Infect Dis. 2013;19:1525. 10.3201/eid1909.121466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang EA, Kang HM, Rhim JW, Kang JH, Lee KY. Early corticosteroid therapy for Mycoplasma pneumoniae pneumonia irrespective of used antibiotics in children. J Clin Med. 2019;8:726. 10.3390/jcm8050726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Izumikawa K. Clinical features of severe or fatal Mycoplasma pneumoniae pneumonia. Front Microbiol. 2016;7:800. 10.3389/fmicb.2016.00800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamazaki T, Kenri T. Epidemiology of Mycoplasma pneumoniae infections in Japan and therapeutic strategies for macrolide-resistant M. pneumoniae. Front Microbiol. 2016;7:693. 10.3389/fmicb.2016.00693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chou CC, Shen CF, Chen SJ, Chen HM, Wang YC, Chang WS, et al. ; Infectious Diseases Society of Taiwan; Taiwan Society of Pulmonary and Critical Care Medicine; Medical Foundation in Memory of Dr. Deh-Lin Cheng; Foundation of Professor Wei-Chuan Hsieh for Infectious Diseases Research and Education; CY Lee’s Research Foundation for Pediatric Infectious Diseases and Vaccines; 4th Guidelines Recommendations for Evidence-based Antimicrobial agents use in Taiwan (GREAT) working group. Recommendations and guidelines for the treatment of pneumonia in Taiwan. J Microbiol Immunol Infect. 2019;52:172–99. 10.1016/j.jmii.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 44.Huang L, Gao X, Chen M. Early treatment with corticosteroids in patients with Mycoplasma pneumoniae pneumonia: a randomized clinical trial. J Trop Pediatr. 2014;60:338–42. 10.1093/tropej/fmu022 [DOI] [PubMed] [Google Scholar]
- 45.Yang E-A, Kang H-M, Rhim J-W, Kang J-H, Lee K-Y. Early corticosteroid therapy for Mycoplasma pneumoniae pneumonia irrespective of used antibiotics in children. J Clin Med. 2019;8:726. 10.3390/jcm8050726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okubo Y, Michihata N, Morisaki N, Uda K, Miyairi I, Ogawa Y, et al. Recent trends in practice patterns and impact of corticosteroid use on pediatric Mycoplasma pneumoniae-related respiratory infections. Respir Investig. 2018;56:158–65. 10.1016/j.resinv.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 47.Teratani Y, Hagiya H, Koyama T, Adachi M, Ohshima A, Zamami Y, et al. Pattern of antibiotic prescriptions for outpatients with acute respiratory tract infections in Japan, 2013-15: a retrospective observational study. Fam Pract. 2019;36:402–9. 10.1093/fampra/cmy094 [DOI] [PubMed] [Google Scholar]
- 48.Uda K, Kinoshita N, Morisaki N, Kasai M, Horikoshi Y, Miyairi I. Targets for optimizing oral antibiotic prescriptions for pediatric outpatients in Japan. Jpn J Infect Dis. 2019;72:149–59. 10.7883/yoken.JJID.2018.374 [DOI] [PubMed] [Google Scholar]
- 49.Park J, Han E, Lee SO, Kim D-S. Antibiotic use in South Korea from 2007 to 2014: A health insurance database-generated time series analysis. PLoS One. 2017;12:e0177435. 10.1371/journal.pone.0177435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meyer Sauteur PM, Trück J, van Rossum AMC, Berger C. Circulating antibody-secreting cell response during Mycoplasma pneumoniae childhood pneumonia. J Infect Dis. 2020;jiaa062. 10.1093/infdis/jiaa062 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information about the study of macrolide-resistant pediatric Mycoplasma pneumoniae infections.


