Abstract
Over the past decades, a variety of PET tracers have been used for the evaluation of patients with brain tumors. For clinical routine, the most important clinical indications for PET imaging in patients with brain tumors are the identification of neoplastic tissue including the delineation of tumor extent for the further diagnostic and therapeutic management (ie, biopsy, resection, or radiotherapy planning), the assessment of response to a certain anticancer therapy including its (predictive) effect on the patients’ outcome and the differentiation of treatment-related changes (eg, pseudoprogression and radiation necrosis) from tumor progression at follow-up. To serve medical professionals of all disciplines involved in the diagnosis and care of patients with brain tumors, this review summarizes the value of PET imaging for the latter-mentioned 3 clinically relevant indications in patients with glioma, meningioma, and brain metastases.
Keywords: DOTATOC, DOTATATE, FDOPA, FDG, FET, FLT, MRI, positron emission tomography
Key Points.
In gliomas, radiolabeled amino acids provide important diagnostic information regarding the delineation of tumor extent for treatment planning, diagnosis of treatment-related changes and for the assessment of treatment response.
PET ligands for somatostatin receptors may add valuable diagnostic information to standard MRI in meningiomas, especially concerning differential diagnosis and detection of meningioma tissue.
Amino acid PET is of great value in distinguishing posttherapeutic reactive changes following radiotherapy from recurrent brain metastases.
Contrast-enhanced conventional MRI is the diagnostic method of choice for patients with primary and secondary (metastatic) brain tumors and is related to its excellent soft-tissue contrast, high spatial resolution, and widespread availability.1,2 MRI is also an essential component of many brain tumor treatment trials, based on its ability to generate surrogate endpoints (eg, MRI findings consistent with tumor progression) that can be correlated with patient outcomes. However, its specificity for neoplastic tissue is low, resulting in challenges regarding the distinction between cancer and nonneoplastic lesions, the delineation of tumor extent, especially of nonenhancing tumor portions, and the differentiation of treatment-related changes from tumor relapse.1,3–8 Besides a continuously expanding repertoire of advanced MRI techniques, PET with numerous radiolabeled molecules has been evaluated over the past decades to overcome these limitations of conventional MRI. For example, it has been emphasized by the PET task force of the Response Assessment in Neuro-Oncology (RANO) working group that for gliomas the additional clinical value of radiolabeled amino acids (amino acid PET) compared with standard MRI is outstanding and justifies the widespread clinical use.9,10
In the recent past, a high number of diagnostic challenges have been addressed using PET techniques (eg, noninvasive grading in primary brain tumors, prediction of molecular markers, detection of tumor portions with malignant progression, and evaluation of prognosis in newly diagnosed and untreated brain tumors). Nevertheless, for neuro-oncologists and medical professionals involved in the diagnosis and care of patients with brain tumors, the following 3 indications for PET imaging are of particular clinical interest: the identification of neoplastic tissue including the delineation of tumor extent for the further diagnostic and therapeutic management, the differentiation of treatment-related changes from tumor progression at follow-up, and the assessment of response to a certain anticancer therapy including its (predictive) effect on the patients’ outcome.
This work summarizes the value of PET imaging for the latter-mentioned 3 clinically highly relevant indications in patients with the 3 most common types of brain tumors—gliomas, meningiomas, and brain metastases.
PET Tracers
For cancer diagnostics in general oncology, PET imaging using [18F]-2-fluoro-2-deoxy-d-glucose (FDG) has evolved over the last decades into the most important clinical PET modality.11 Increased glucose metabolism indicated by an increased FDG uptake is commonly seen in proliferating tumors due to the increased glucose transporter expression and the enzyme hexokinase, converting FDG to a phosphorylated product. However, the physiological high FDG uptake in the healthy brain parenchyma hampers the delineation of brain tumors,10 and cerebral inflammatory processes may also exhibit high FDG uptake, thereby diminishing its diagnostic performance.1
Radiolabeled amino acids are of particular interest for brain tumor imaging using PET because of their increased uptake in neoplastic tissue but low uptake in normal brain parenchyma, resulting in an improved tumor-to-brain contrast.1,9,10,12–14 Within the group of amino acid PET tracers, [11C]-methyl-l-methionine (MET), 3,4-dihydroxy-6-[18F]-fluoro-l-phenylalanine (FDOPA), and O-(2-[18F]-fluoroethyl)-l-tyrosine (FET) are frequently used.1,15,16 In both gliomas and brain metastases, increased uptake of MET, FET, and FDOPA is related to an increased transport via certain amino acid carriers (amino acid transporters of the L-type (LAT); LAT subtypes 1 and 2), which are overexpressed in neoplastic tissue.17–20 Thus, imaging of the amino acid transport in these tumor types using this group of PET tracers is a compelling target.20
Owing to the overexpression of somatostatin receptors (SSTR) in meningiomas,21–23 radiolabeled SSTR ligands allow the visualization of meningiomas using PET. It has been observed that the SSTR subtype 2 is the most abundant isoform, with approximately 100% expression in meningioma tissue.21 For PET imaging, SSTR ligands are typically labeled with 68Ga (half-life, 68 min). The 68Ga-labeled tracers DOTA-D-Phe1-Tyr3-octreotate (DOTATATE) and DOTA-Tyr3-octreotide (DOTATOC) are the most common applied tracers in the clinical management of meningioma patients14 and can also be used for the imaging of patients with neuroendocrine tumors, which as well express high levels of SSTR.24 Furthermore, these tracers provide an excellent lesion-to-background contrast, which is related to a low uptake in bony structures and healthy brain parenchyma.25,26
In patients with gliomas and brain metastases, a few studies have also used non-FDG and non-amino acid PET imaging. For example, the PET tracer 3’-deoxy-3’-[18F]-fluorothymidine (FLT) is an analog to the nucleoside thymidine and was developed to assess cellular proliferation by tracking the thymidine salvage pathway.27–30
PET Imaging in Glioma Patients
Glioma Detection and Delineation of Glioma Spread
In terms of brain tumor detection, an increased amino acid accumulation in PET images is highly predictive for a brain tumor such as a glioma31–33 or a brain metastasis.34 For example, a meta-analysis including more than 400 patients on the diagnostic value of MET PET yielded a high pooled sensitivity and specificity of 91% and 86% for neoplastic tissue, whereas the diagnostic performance of FDG PET was only moderate with a sensitivity and specificity of 71% and 77%, respectively.35 A meta-analysis of 13 FET PET studies including more than 450 patients yielded a pooled sensitivity and specificity of around 80% for the diagnosis of primary brain tumors.33
However, although this is much less common, it should be kept in mind that (usually mild) increased amino acid tracer uptake may also occur in nonneoplastic lesions or processes (eg, ischemic stroke, local infections related to a brain abscess, inflammatory processes such as multiple sclerosis, status epilepticus).32,36–40 Importantly, 20%–30% of isocitrate dehydrogenase (IDH)-mutated gliomas of the World Health Organization (WHO) grade II show no amino acid uptake; thus, negative amino acid PET scans do not necessarily exclude a low-grade glioma.32,37,41
Concerning the correct delineation of the glioma extent, conventional MRI sequences are particularly limited in their ability to identify nonenhancing glioma subregions.1 Radiolabeled amino acids for PET imaging have the ability to cross the intact blood–brain barrier,42,43 and a number of studies have spatially compared histological findings in predominantly nonenhancing gliomas obtained by stereotactic biopsy with amino acid tracer uptake and provided evidence that this technique identifies the glioma extent more reliably than standard MRI.44,45 Furthermore, in patients with an MRI-based suspicion of a WHO grade II glioma (ie, glioma-like lesions without contrast enhancement), it has been demonstrated that FET PET findings obtained from static and dynamic acquisition were histologically correlated with malignant anaplastic foci,46,47 which has highly relevant implications for prognostic evaluation and treatment planning.
In terms of the volumetric comparison of contrast enhancement with the tumor volume obtained by amino acid PET, previous studies in both newly diagnosed and recurrent IDH-wild-type glioblastomas suggest that there were significant differences in size, overlap, and spatial correlation of tumor volumes,3,48 indicating that conventional contrast-enhanced MRI substantially underestimates the metabolically active tumor volume. However, it remains to be determined whether an amino acid PET-guided treatment (eg, amino acid PET-based resection or radiotherapy planning) significantly affects patient survival.
Differentiation of Treatment-Related Changes From Glioma Progression
Following treatment for brain tumors, the differentiation of treatment-related changes from progression remains challenging1,4,8,49,50 and is of pivotal clinical relevance. The erroneous interpretation of treatment-related changes as tumor progression may lead to a premature cessation of an effective treatment with a potentially negative impact on survival and an overestimation of the efficacy of the subsequent treatment.51 The latter may also generate misleading results in studies evaluating salvage therapies.52
A high diagnostic accuracy of amino acid PET using the tracers FET and FDOPA in differentiating between tumor progression and treatment-related changes with early (ie, pseudoprogression following chemoradiation plus temozolomide within the first 3 months) and late occurrence (eg, radiation necrosis, onset usually > 6 months after radiotherapy completion; Figure 1) in patients with predominantly IDH-wild-type malignant glioma has repeatedly been shown.49,53–60 In these studies, the diagnostic accuracy for a correct differentiation was in the range of 80%–90%. For MET PET, the diagnostic performance seems to be slightly lower with an accuracy of approximately 75%,61,62 most probably related to a higher affinity of MET for inflammatory processes.63
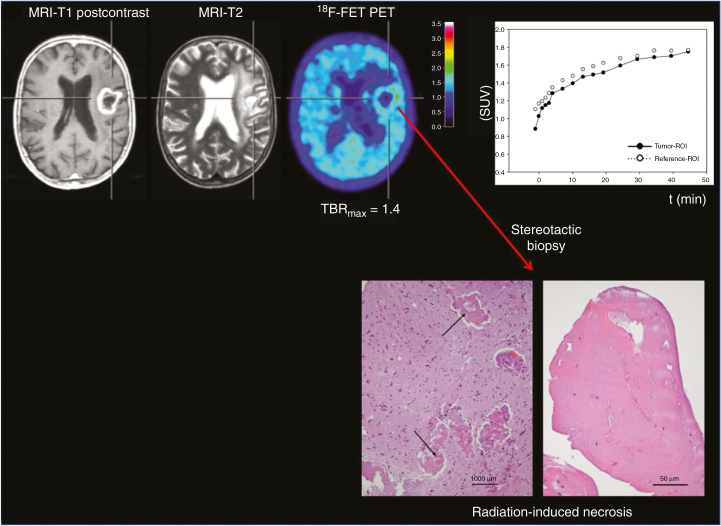
Fig. 1.
A 70-year-old patient with an anaplastic astrocytoma. Contrast-enhanced MRI 31 months after radiation therapy suggests tumor progression. In contrast, O-(2-[18F]-fluoroethyl)-l-tyrosine (FET) PET shows only slight metabolic activity, and the time–activity curve shows a constantly increasing FET uptake, consistent with treatment-related changes. After a stereotactic biopsy, histological examination yielded signs of radiation-induced necrosis (hematoxylin and eosin staining, original magnification ×200; scale bar, 50 mm). Brain parenchyma shows reactive changes and blood vessels with thickened hyalinized walls (arrows; hematoxylin and eosin staining, original magnification ×100; scale bar, 1000 mm; reproduced from Galldiks et al.,59 with permission from Oxford University Press).
Assessment of Response to Systemic Glioma Treatment Options
In patients with brain tumor, changes in the MRI contrast enhancement extent are typically used as an indicator of treatment response or tumor relapse.51,64 Furthermore, changes of the T2 or fluid attenuated inversion recovery (FLAIR) hyperintensity following antiangiogenic therapy were also used for the diagnosis of “non-enhancing tumor progression.”51 However, these changes are nonspecific and may not always be a reliable parameter for treatment effects.4,8,50 In addition, both T2 and FLAIR signal hyperintensity may be related to perifocal tumor edema, radiation injury, demyelination, ischemia, or inflammation, thereby hampering the distinction from nonenhancing tumor.50 Consequently, alternative diagnostic methods such as PET have been evaluated to improve treatment response assessment. In gliomas, frequently used systemic treatment options are conventional (alkylating) chemotherapy and antiangiogenic therapy.
Using MET PET, a reliable response assessment to temozolomide and nitrosourea-based chemotherapy has been demonstrated in patients with high-grade glioma at recurrence.29,65–67 Importantly, metabolic responders in MET PET had a significantly improved outcome compared with metabolic nonresponders.65 Subsequently, FET PET has been used to evaluate the effects of temozolomide in patients with low-grade glioma (according to the European Organization of Research and Treatment of Cancer (EORTC) protocol 22033-26033).68 In responders, a FET PET tumor volume reduction after treatment initiation could be observed significantly earlier than volume reductions on FLAIR MRI. These findings were confirmed by subsequent FET PET studies with a higher number of patients.69,70
In newly diagnosed patients with IDH-wild-type glioblastoma, prospective studies assessed the predictive value of early FET uptake changes 6–8 weeks after postoperative chemoradiation with temozolomide.71,72 FET PET responders with a decrease of metabolic activity as assessed by tumor/brain ratios (>10%) had a significantly longer survival than patients with stable or increasing tracer uptake after chemoradiation.
Furthermore, amino acid PET has been investigated as an alternative imaging method for the assessment of treatment response to antiangiogenic therapy such as bevacizumab.73 In addition, it has been demonstrated that FDOPA and FET PET are useful for the identification of pseudoresponse.74–78 Moreover, FDOPA and FET PET seem also to be useful to predict a favorable outcome in bevacizumab responders.77–79 A recent prospective study suggests that FET PET appears to be useful for identifying metabolic responders to the combination of bevacizumab and lomustine in newly diagnosed IDH-wild-type glioblastoma patients early after treatment initiation.80 In that study, MRI changes (according to the RANO criteria51) were not predictive for a favorable outcome, whereas FET PET parameters significantly predicted an overall survival of more than 9 months.80
Regarding PET tracers that assess cellular proliferation, previous studies in glioma patients suggest that FLT is able to predict favorable survival after bevacizumab therapy.28,81 Unfortunately, FLT tracer uptake is necessarily related to a disrupted blood–brain barrier, hampering its routine use in the field of neuro-oncology.4
PET Imaging in Patients With Meningioma
Detection of Meningioma Tissue and Meningioma Delineation
Although PET plays only a minor role in the initial diagnosis of meningiomas, SSTR imaging may be of value regarding meningioma detection. Clinically, this is highly relevant because meningiomas located at the skull base or nearby the falx cerebri, with transosseous extension, or equivocal imaging findings related to artifacts or calcifications were difficult to detect by anatomical MRI alone.14 Similar to gliomas,82 the tracer FDG is not suitable for precise meningioma delineation, which is related to high glucose levels in the cerebrum causing a poor tumor-to-background contrast. In contrast, particularly SSTR PET ligands generally elicit higher tumor-to-background ratios. A study comparing contrast-enhanced MRI and SSTR PET before radiotherapy observed that all meningiomas could be detected by DOTATOC PET. In contrast, only 90% (171 of 190) meningiomas were detected by contrast-enhanced anatomical MRI indicating an improved sensitivity for DOTATOC PET in meningioma detection when compared with MRI.26 In a comparative study with histological confirmation of imaging findings using neuro-navigated tissue sampling, DOTATATE PET revealed a more precise delineation of tumor extent in various tumor locations than contrast-enhanced MRI.25 Especially in meningiomas located in regions such as the skull base, orbita, and cavernous sinus or with transosseous extension, DOTATATE, and DOTATOC PET were superior than anatomical MRI in terms of tumor delineation.83–85 Furthermore, DOTATATE PET helps to differentiate optic nerve sheath meningiomas from other lesions being associated with a nontumoral optic nerve affection.86 In summary, SSTR PET may provide important additional information in meningioma patients with unclear MRI findings or may help to confirm the diagnosis of meningioma based on MRI alone.
Diagnosis of Meningioma Recurrence
Even if a meningioma is considered neuropathologically benign (WHO grade I), the 10-year recurrence rate is in the range of 20%–40% despite complete resection.87 Importantly, the recurrence rates of WHO grade I meningiomas are substantially higher if only a subtotal resection could be achieved (clearly more than 50% in a 10-year interval).87 In these cases with incomplete resection, adjuvant radiation therapy is frequently being administered to lower the recurrence rate. At suspicion of recurrence, contrast-enhanced MRI is the imaging modality of choice for both diagnostic evaluation and treatment planning. However, the diagnostic accuracy of standard MRI is limited, especially in complex anatomic situations in which bone infiltration or scar tissue is present. Additional imaging modalities to detect tumor remnants or recurrence more precisely are therefore needed.
It has been demonstrated that SSTR PET adds important clinical information in discriminating meningioma tissue from posttherapeutic reactive changes (eg, scars related to pretreatment), usually presenting as equivocal radiological findings on contrast-enhanced MRI.25,26,88 For the differentiation of scar tissue from active tumor, it has been demonstrated that SSTR PET using DOTATATE has a high sensitivity, outperforming standard MRI 90%–79%.25 Accordingly, a subsequent DOTATATE PET study with focus on the detection of transosseous meningiomas after therapy showed a better diagnostic performance than standard MRI in terms of sensitivity (97% vs. 54%) and specificity (100% vs. 83%).89
Radiotherapy Planning and Assessment of Response to Radiotherapy in Meningiomas
Regarding the assessment of treatment response to radiotherapy using PET in meningioma patients, however, only a limited number of studies is currently available. For example, serial MET PET scans were used to prospectively assess the effects of proton radiotherapy.90 No significant tumor size reduction but an average decrease of tumor-to-brain ratios in the range of 20% was observed, suggesting that MET PET may enable an earlier evaluation of treatment effects than MRI. Throughout the long-term follow-up of these patients over 10 years, MET tumor-to-brain ratios showed a further decrease in the majority of patients, whereas the tumor size was predominantly unchanged.91
To date, PET studies using SSTR ligands in for radiotherapy monitoring are not available. Notwithstanding, in the field of radiation oncology, SSTR PET has been predominantly used for radiotherapy planning. The definition of the target volume is crucial for the planning of radiosurgery or fractionated radiotherapy. In meningiomas, target volumes are frequently delineated based on coregistered contrast-enhanced tomographic images (MRI and CT). However, in meningiomas located at the skull base (approximately one-third of cases), it is difficult to differentiate between meningioma tissue and dura and/or bone, because of a high contrast enhancement of these structures. Moreover, in transosseous meningiomas, it is difficult to exactly define the degree of infiltration, despite using the bone window on CT images. In the setting of the identification of meningioma remnants after incomplete resection (Figure 2), PET imaging seems to be helpful for adjuvant radiotherapy planning.
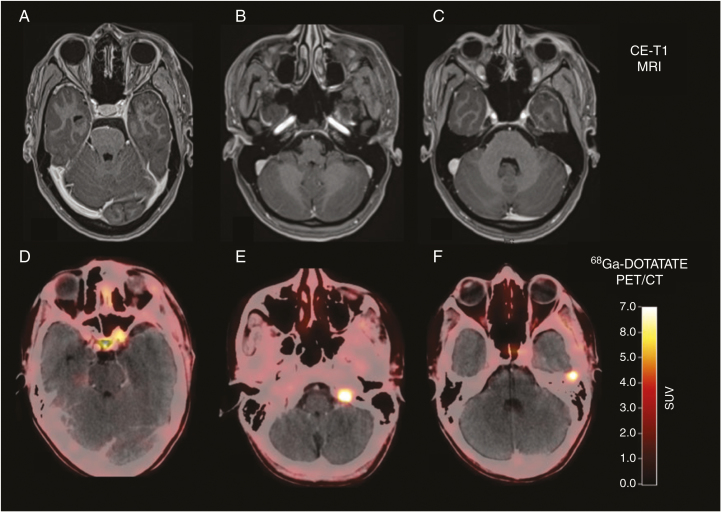
Fig. 2.
Postoperative contrast-enhanced MRI and DOTATATE PET/CT of a 32-year-old patient after resection of a World Health Organization grade I meningioma show residual tumor located at the left internal carotid artery and at tumor at the tip of the left orbit (A and D). Surprisingly, 2 additional meningiomas were also visible on the DOTATATE PET/CT (E and F), without corresponding contrast enhancement on MRI (B and, C) (reproduced from Galldiks et al.,14 with permission from Oxford University Press).
It has been demonstrated that an optimized target volume delineation for fractionated radiation therapy in patients with benign, atypical and even anaplastic meningiomas (WHO grades I–III) can be obtained by DOTATOC PET.83 Similar findings could be confirmed in subsequent DOTATOC PET studies.85,92,93
Amino acid PET can also be helpful for radiation therapy target volume delineation in patients with meningioma. Astner et al.94 demonstrated that in the WHO grade I skull base meningiomas (n = 32) the addition of MET PET changed the gross tumor volume almost in all patients. As a consequence, areas without tumor infiltration could be excluded from the gross tumor volume and critical anatomical structures such as the optic chiasm, optic nerves, and pituitary gland could be preserved more effectively.94,95 Subsequently, these findings could be confirmed using other radiolabeled amino acids such as FET.96
PET Imaging in Patients With Brain Metastases
Value of PET for the Identification of Brain Metastases
Contrary to transosseous meningiomas and gliomas, the vast majority of brain metastases (including brain metastases with a lesion size <5 mm) can be easily delineated by contrast-enhanced standard MRI. A recent meta-analysis included more than 900 patients and observed that contrast-enhanced MRI has a clearly higher cumulative sensitivity than FDG PET (77% vs. 21%) for the diagnosis of brain metastases secondary to lung cancer.97 Notwithstanding, the increased expression of amino acid transporters in brain metastases is a compelling target for PET imaging using radiolabeled amino acids.20 Accordingly, it has been demonstrated that the sensitivity of amino acid PET using FET to depict brain metastases larger than 1 cm in diameter seems to be superior than that of FDG PET.34 In that study, approximately 90% of brain metastases had a FET uptake of 1.6 or more (compared with the healthy contralateral hemisphere). Nevertheless, the most commonly used imaging modality for brain metastases detection with the highest sensitivity remains thin-slice contrast-enhanced MRI.
Differentiation of Treatment-Related Changes From Brain Metastasis Recurrence
Depending on the performance status of the patient and the number of brain metastases, radiotherapy is an effective treatment option, either as whole-brain radiotherapy or stereotactic radiosurgery.12 Furthermore, resection is frequently combined with postoperative radiotherapy, especially in patients with single brain metastasis or oligometastases.98 Importantly, depending on the irradiated brain volume and radiation dose, in patients with brain metastases treated by radiosurgery a radiation necrosis rate of 25%–50% has been reported.99
Several FDG PET studies with considerable differences in methodology evaluated the value of this tracer to differentiate brain metastasis relapse from radiation-induced changes. Importantly, the diagnostic performance of FDG PET spanned a wide range (range of sensitivity and specificity, 40%–100%),100–105 indicating an inferior value for clinical applicability.
In contrast, PET using FDOPA and MET has consistently demonstrated a higher sensitivity and specificity of approximately 80% for the correct diagnosis of brain metastasis recurrence.61,106–109 Similarly, FET PET parameters derived from static and dynamic acquisition showed a high a sensitivity and specificity in the range of 80%–90% for distinguishing radiation-induced changes (especially after radiosurgery) from recurrent brain metastases.110–112
Furthermore, reactive changes may also occur following systemic treatment and can also be difficult to distinguish from brain metastases relapse. Pseudoprogression may occur in brain metastases treated with immune checkpoint inhibitors using CTLA-4 (eg, ipilimumab) or PD-1 (eg, nivolumab or pembrolizumab) inhibitors. A pilot study highlighted the potential of amino acid PET using FET to identify pseudoprogression in patients with brain metastases secondary to malignant melanoma treated with ipilimumab.113
Assessment of Treatment Response
The advent of immunotherapy using immune checkpoint inhibitors and targeted therapy has dramatically improved the treatment of extracranial cancer, especially in patients with skin, lung, or breast cancers. Moreover, recent trials have shown that patients with brain metastases may also benefit from these agents.
In patients with melanoma brain metastases undergoing immune checkpoint blockade or targeted therapy, a pilot study observed that metabolic responders may show a proliferative reduction on FLT PET despite unchanged findings on standard MRI.114 The pilot data suggest that FLT PET has also the potential to detect a reduction proliferative tumor activity despite apparent morphological progression on conventional MRI (ie, pseudoprogression).
Studies evaluating amino acid PET for the assessment of treatment response remain scarce. Single reports suggest that amino acid PET has the potential to add valuable information to standard MRI for the assessment of treatment response. Similar to FLT PET, a reduction of metabolic activity in patients with brain metastases secondary to melanoma or non-small cell lung cancer treated with targeted therapy could be identified by FET PET, whereas findings on standard MRI remained unchanged (Figure 3).12,115
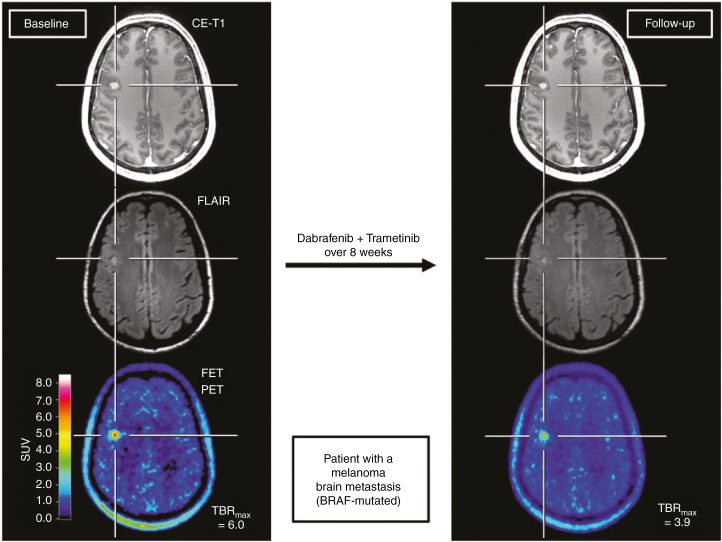
Fig. 3.
A 45-year-old female patient with a brain metastasis secondary to a v-Raf murine sarcoma viral oncogene homolog B (BRAF)-mutated malignant melanoma treated with dabrafenib and trametinib. Comparison of contrast-enhanced MR and O-(2-[18F]-fluoroethyl)-l-tyrosine (FET) PET images at baseline (left column) and follow-up 8 weeks later (right column). At follow-up, a clear decrease of the tumor/brain ratios (–35%) is observed, whereas the MRI shows no significant change of both the contrast enhancement and FLAIR signal defined as stable disease according to RANO criteria for brain metastases. The metabolic response was associated with an overall survival of 9 months after treatment initiation (reproduced from Galldiks et al.,12 with permission from Oxford University Press).
Limitations
It has to be pointed out that a considerable number of studies were performed in single centers only or were based on a retrospective PET data collection. Thus, the clinical value and the additional biological information of these methods warrants further investigation including neuropathological validation, preferentially in prospective multicenter clinical trials.
Summary and Outlook
The present literature provides strong evidence that PET can be of great clinical value for the most important diagnostic indications in the field of neuro-oncology (Table 1). Especially PET using amino acid tracers and SSTR ligands offers a variety of insights for the assessment of brain tumors with the potential to overcome the limitations of conventional MRI. The diagnostic improvement probably results in relevant benefits for patients with brain tumor and justifies a more widespread use of this diagnostic tool.9,10 Furthermore, the necessary PET infrastructure is widely available, and the production of radiolabeled amino acids and SSTR ligands is well established with comparable costs to FDG. Moreover, additional costs of this method can be potentially saved by the incurred costs of less reliable diagnostic imaging techniques.116–119
Table 1.
Summary of Recommendations
| Gliomas | Meningiomas | Brain metastases | |
|---|---|---|---|
| Identification of neoplastic tissue including the delineation of tumor extent | AA PET ++ | SSTR PET ++ AA PET + FDG PET − |
MRI method of choice AA PET + FDG PET − |
| Assessment of treatment response | AA PET ++ FLT + |
AA PET (++) SSTR PET n.a. |
AA PET (++) FLT PET (++) |
| Differentiation of treatment-related changes from tumor progression at follow-up | AA PET ++ | SSTR PET (++) | AA PET ++ |
++ high diagnostic accuracy; (++) high diagnostic accuracy, but limited data available; + limited diagnostic accuracy; − not helpful; AA PET = amino acid PET; FDG = [18F]-2-fluoro-2-deoxy-d-glucose PET; FLT = 3’-deoxy-3’-[18F]-fluorothymidine PET; n.a. = data not available; SSTR PET = PET using radiolabeled somatostatin receptor ligands.
Especially amino acid PET is a robust and attractive approach for clinicians for many indications including easy scan reading. Importantly, most studies using amino acid PET provide comparable results across different scanners, which is also a consequence of national and international efforts concerning the standardization of amino acid PET acquisition and evaluation in brain tumor imaging.13 At present, joint practice guidelines were developed by major European and American medical societies for Nuclear Medicine and Neuro-Oncology (ie, Society of Nuclear Medicine and Molecular Imaging, European Association of Nuclear Medicine, European Association of Neuro-Oncology, and the RANO group).13
The addition of advanced MRI techniques (eg, MR spectroscopic imaging, perfusion- and diffusion-weighted imaging) to amino acid or SSTR PET has the potential for a more profound evaluation of biological characteristics in patients with primary or secondary brain cancer. The complementary information derived from these imaging techniques suggests differential biological information, which therefore warrants further evaluation.120 A methodological innovation that potentially alleviates research in patients with brain tumors is the increasing availability of hybrid PET/MR systems, which enables the simultaneous acquisition of PET and advanced MRI. In the light of emerging high-throughput analysis methods such as radiomics and machine learning, this is also of great clinical interest; for example, it has been demonstrated that combined PET and MRI radiomics encodes more important diagnostic information than either modality alone.121,122
Furthermore, the combination of diagnostics and therapy (theranostics) has been introduced to meningioma treatment. The so-called peptide receptor radionuclide therapy can easily be provided by exchanging the radionuclide, that is, the exchange of the short-lived positron emitter 68Ga used for PET with the longer-lived β-emitters such as 90Y or 177Lu allows for receptor-targeted therapy. In patients with progressive, treatment-refractory meningiomas, a disease stabilization has been reported in a considerable number of patients,123 suggesting that this therapy modality is a promising treatment alternative, which should be evaluated in further studies to determine its role in meningioma management.
Funding
The Wilhelm-Sander Stiftung, Germany, supported this work.
Authorship statement:
Study design, writing of manuscript drafts: NG. Revising manuscript, approving final content of the manuscript: All.
References
- 1. Langen KJ, Galldiks N, Hattingen E, Shah NJ. Advances in neuro-oncology imaging. Nat Rev Neurol. 2017;13(5):279–289. [DOI] [PubMed] [Google Scholar]
- 2. Pope WB, Brandal G. Conventional and advanced magnetic resonance imaging in patients with high-grade glioma. Q J Nucl Med Mol Imaging. 2018;62(3):239–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lohmann P, Stavrinou P, Lipke K, et al. . FET PET reveals considerable spatial differences in tumour burden compared to conventional MRI in newly diagnosed glioblastoma. Eur J Nucl Med Mol Imaging. 2019;46(3):591–602. [DOI] [PubMed] [Google Scholar]
- 4. Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010;9(9):906–920. [DOI] [PubMed] [Google Scholar]
- 5. Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. [DOI] [PubMed] [Google Scholar]
- 6. Hygino da Cruz LC Jr., Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol. 2011;32( 11):1978–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang I, Aghi MK. New advances that enable identification of glioblastoma recurrence. Nat Rev Clin Oncol. 2009;6(11):648–657. [DOI] [PubMed] [Google Scholar]
- 8. Kumar AJ, Leeds NE, Fuller GN, et al. . Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217(2):377–384. [DOI] [PubMed] [Google Scholar]
- 9. Langen KJ, Watts C. Neuro-oncology: amino acid PET for brain tumours—ready for the clinic? Nat Rev Neurol. 2016;12(7):375–376. [DOI] [PubMed] [Google Scholar]
- 10. Albert NL, Weller M, Suchorska B, et al. . Response assessment in neuro-oncology working group and European association for neuro-oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18(9):1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herholz K, Langen KJ, Schiepers C, Mountz JM. Brain tumors. Semin Nucl Med. 2012;42(6):356–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Galldiks N, Langen KJ, Albert NL, et al. . PET imaging in patients with brain metastasis-report of the RANO/PET group. Neuro Oncol. 2019;21(5):585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Law I, Albert NL, Arbizu J, et al. . Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46(3):540–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galldiks N, Albert NL, Sommerauer M, et al. . PET imaging in patients with meningioma-report of the RANO/PET group. Neuro Oncol. 2017;19(12):1576–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galldiks N, Langen KJ, Pope WB. From the clinician’s point of view - what is the status quo of positron emission tomography in patients with brain tumors? Neuro Oncol. 2015;17(11):1434–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Langen KJ, Stoffels G, Filss C, et al. . Imaging of amino acid transport in brain tumours: positron emission tomography with O-(2-[18F]fluoroethyl)-L-tyrosine (FET). Methods. 2017;130:124–134. [DOI] [PubMed] [Google Scholar]
- 17. Okubo S, Zhen HN, Kawai N, Nishiyama Y, Haba R, Tamiya T. Correlation of L-methyl-11C-methionine (MET) uptake with L-type amino acid transporter 1 in human gliomas. J Neurooncol. 2010;99(2):217–225. [DOI] [PubMed] [Google Scholar]
- 18. Wiriyasermkul P, Nagamori S, Tominaga H, et al. . Transport of 3-fluoro-L-α-methyl-tyrosine by tumor-upregulated L-type amino acid transporter 1: a cause of the tumor uptake in PET. J Nucl Med. 2012;53(8):1253–1261. [DOI] [PubMed] [Google Scholar]
- 19. Youland RS, Kitange GJ, Peterson TE, et al. . The role of LAT1 in (18)F-DOPA uptake in malignant gliomas. J Neurooncol. 2013;111(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Papin-Michault C, Bonnetaud C, Dufour M, et al. . Study of LAT1 expression in brain metastases: towards a better understanding of the results of positron emission tomography using amino acid tracers. PLoS One. 2016;11(6):e0157139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dutour A, Kumar U, Panetta R, et al. . Expression of somatostatin receptor subtypes in human brain tumors. Int J Cancer. 1998;76(5):620–627. [DOI] [PubMed] [Google Scholar]
- 22. Reubi JC, Maurer R, Klijn JG, et al. . High incidence of somatostatin receptors in human meningiomas: biochemical characterization. J Clin Endocrinol Metab. 1986;63(2):433–438. [DOI] [PubMed] [Google Scholar]
- 23. Menke JR, Raleigh DR, Gown AM, Thomas S, Perry A, Tihan T. Somatostatin receptor 2a is a more sensitive diagnostic marker of meningioma than epithelial membrane antigen. Acta Neuropathol. 2015;130(3):441–443. [DOI] [PubMed] [Google Scholar]
- 24. Johnbeck CB, Knigge U, Kjær A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: current status and review of the literature. Future Oncol. 2014;10(14):2259–2277. [DOI] [PubMed] [Google Scholar]
- 25. Rachinger W, Stoecklein VM, Terpolilli NA, et al. . Increased 68Ga-DOTATATE uptake in PET imaging discriminates meningioma and tumor-free tissue. J Nucl Med. 2015;56(3):347–353. [DOI] [PubMed] [Google Scholar]
- 26. Afshar-Oromieh A, Giesel FL, Linhart HG, et al. . Detection of cranial meningiomas: comparison of 68Ga-DOTATOC PET/CT and contrast-enhanced MRI. Eur J Nucl Med Mol Imaging. 2012;39(9):1409–1415. [DOI] [PubMed] [Google Scholar]
- 27. Shields AF, Grierson JR, Dohmen BM, et al. . Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4(11):1334–1336. [DOI] [PubMed] [Google Scholar]
- 28. Chen W, Delaloye S, Silverman DH, et al. . Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J Clin Oncol. 2007;25(30):4714–4721. [DOI] [PubMed] [Google Scholar]
- 29. Galldiks N, Kracht LW, Burghaus L, et al. . Patient-tailored, imaging-guided, long-term temozolomide chemotherapy in patients with glioblastoma. Mol Imaging. 2010;9(1):40–46. [PubMed] [Google Scholar]
- 30. Jacobs AH, Thomas A, Kracht LW, et al. . 18F-fluoro-L-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J Nucl Med. 2005;46(12):1948–1958. [PubMed] [Google Scholar]
- 31. Rapp M, Heinzel A, Galldiks N, et al. . Diagnostic performance of 18F-FET PET in newly diagnosed cerebral lesions suggestive of glioma. J Nucl Med. 2013;54(2):229–235. [DOI] [PubMed] [Google Scholar]
- 32. Pichler R, Dunzinger A, Wurm G, et al. . Is there a place for FET PET in the initial evaluation of brain lesions with unknown significance? Eur J Nucl Med Mol Imaging. 2010;37(8):1521–1528. [DOI] [PubMed] [Google Scholar]
- 33. Dunet V, Rossier C, Buck A, Stupp R, Prior JO. Performance of 18F-fluoro-ethyl-tyrosine (18F-FET) PET for the differential diagnosis of primary brain tumor: a systematic review and metaanalysis. J Nucl Med. 2012;53(2):207–214. [DOI] [PubMed] [Google Scholar]
- 34. Unterrainer M, Galldiks N, Suchorska B, et al. . 18F-FET PET uptake characteristics in patients with newly diagnosed and untreated brain metastasis. J Nucl Med. 2017;58(4):584–589. [DOI] [PubMed] [Google Scholar]
- 35. Zhao C, Zhang Y, Wang J. A meta-analysis on the diagnostic performance of (18)F-FDG and (11)C-methionine PET for differentiating brain tumors. AJNR Am J Neuroradiol. 2014;35(6):1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Floeth FW, Pauleit D, Sabel M, et al. . 18F-FET PET differentiation of ring-enhancing brain lesions. J Nucl Med. 2006;47(5):776–782. [PubMed] [Google Scholar]
- 37. Hutterer M, Nowosielski M, Putzer D, et al. . [18F]-fluoro-ethyl-L-tyrosine PET: a valuable diagnostic tool in neuro-oncology, but not all that glitters is glioma. Neuro Oncol. 2013;15(3):341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sala Q, Metellus P, Taieb D, Kaphan E, Figarella-Branger D, Guedj E. 18F-DOPA, a clinically available PET tracer to study brain inflammation? Clin Nucl Med. 2014;39(4):e283–e285. [DOI] [PubMed] [Google Scholar]
- 39. Hutterer M, Ebner Y, Riemenschneider MJ, et al. . Epileptic activity increases cerebral amino acid transport assessed by 18F-fluoroethyl-l-tyrosine amino acid PET: a potential brain tumor mimic. J Nucl Med. 2017;58(1):129–137. [DOI] [PubMed] [Google Scholar]
- 40. Ito K, Matsuda H, Kubota K. Imaging spectrum and pitfalls of (11)C-methionine positron emission tomography in a series of patients with intracranial lesions. Korean J Radiol. 2016;17(3):424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jansen NL, Graute V, Armbruster L, et al. . MRI-suspected low-grade glioma: is there a need to perform dynamic FET PET? Eur J Nucl Med Mol Imaging. 2012;39(6):1021–1029. [DOI] [PubMed] [Google Scholar]
- 42. Pauleit D, Stoffels G, Schaden W, et al. . PET with O-(2-18F-fluoroethyl)-L-tyrosine in peripheral tumors: first clinical results. J Nucl Med. 2005;46(3):411–416. [PubMed] [Google Scholar]
- 43. Pirotte B, Goldman S, Massager N, et al. . Comparison of 18F-FDG and 11C-methionine for PET-guided stereotactic brain biopsy of gliomas. J Nucl Med. 2004;45(8):1293–1298. [PubMed] [Google Scholar]
- 44. Kracht LW, Miletic H, Busch S, et al. . Delineation of brain tumor extent with [11C]L-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res. 2004;10(21):7163–7170. [DOI] [PubMed] [Google Scholar]
- 45. Pauleit D, Floeth F, Hamacher K, et al. . O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128(Pt 3):678–687. [DOI] [PubMed] [Google Scholar]
- 46. Kunz M, Thon N, Eigenbrod S, et al. . Hot spots in dynamic (18)FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro Oncol. 2011;13(3):307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stockhammer F, Plotkin M, Amthauer H, van Landeghem FK, Woiciechowsky C. Correlation of F-18-fluoro-ethyl-tyrosin uptake with vascular and cell density in non-contrast-enhancing gliomas. J Neurooncol. 2008;88(2):205–210. [DOI] [PubMed] [Google Scholar]
- 48. Galldiks N, Ullrich R, Schroeter M, Fink GR, Jacobs AH, Kracht LW. Volumetry of [(11)C]-methionine PET uptake and MRI contrast enhancement in patients with recurrent glioblastoma multiforme. Eur J Nucl Med Mol Imaging. 2010;37(1):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Galldiks N, Dunkl V, Stoffels G, et al. . Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur J Nucl Med Mol Imaging. 2015;42(5):685–695. [DOI] [PubMed] [Google Scholar]
- 50. Ahluwalia MS, Wen PY. Antiangiogenic therapy for patients with glioblastoma: current challenges in imaging and future directions. Expert Rev Anticancer Ther. 2011;11(5):653–656. [DOI] [PubMed] [Google Scholar]
- 51. Wen PY, Macdonald DR, Reardon DA, et al. . Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 52. Reardon DA, Weller M. Pseudoprogression: fact or wishful thinking in neuro-oncology? Lancet Oncol. 2018;19(12):1561–1563. [DOI] [PubMed] [Google Scholar]
- 53. Pöpperl G, Götz C, Rachinger W, Gildehaus FJ, Tonn JC, Tatsch K. Value of O-(2-[18F]fluoroethyl)-L-tyrosine PET for the diagnosis of recurrent glioma. Eur J Nucl Med Mol Imaging.. 2004;31(11):1464–1470. [DOI] [PubMed] [Google Scholar]
- 54. Rachinger W, Goetz C, Pöpperl G, et al. . Positron emission tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery. 2005;57(3):505–511; discussion 505. [DOI] [PubMed] [Google Scholar]
- 55. Mihovilovic MI, Kertels O, Hänscheid H, et al. . O-(2-(18F)fluoroethyl)-L-tyrosine PET for the differentiation of tumour recurrence from late pseudoprogression in glioblastoma. J Neurol Neurosurg Psychiatry. 2019;90(2):238–239. [DOI] [PubMed] [Google Scholar]
- 56. Mehrkens JH, Pöpperl G, Rachinger W, et al. . The positive predictive value of O-(2-[18F]fluoroethyl)-L-tyrosine (FET) PET in the diagnosis of a glioma recurrence after multimodal treatment. J Neurooncol. 2008;88(1):27–35. [DOI] [PubMed] [Google Scholar]
- 57. Jena A, Taneja S, Gambhir A, et al. . Glioma recurrence versus radiation necrosis: single-session multiparametric approach using simultaneous O-(2-18F-fluoroethyl)-L-tyrosine PET/MRI. Clin Nucl Med. 2016;41(5):e228–e236. [DOI] [PubMed] [Google Scholar]
- 58. Pyka T, Hiob D, Preibisch C, et al. . Diagnosis of glioma recurrence using multiparametric dynamic 18F-fluoroethyl-tyrosine PET-MRI. Eur J Radiol. 2018;103:32–37. [DOI] [PubMed] [Google Scholar]
- 59. Galldiks N, Stoffels G, Filss C, et al. . The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro Oncol. 2015;17(9):1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Herrmann K, Czernin J, Cloughesy T, et al. . Comparison of visual and semiquantitative analysis of 18F-FDOPA-PET/CT for recurrence detection in glioblastoma patients. Neuro Oncol. 2014;16(4):603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Minamimoto R, Saginoya T, Kondo C, et al. . Differentiation of brain tumor recurrence from post-radiotherapy necrosis with 11C-methionine PET: visual assessment versus quantitative assessment. PLoS One. 2015;10(7):e0132515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nihashi T, Dahabreh IJ, Terasawa T. Diagnostic accuracy of PET for recurrent glioma diagnosis: a meta-analysis. AJNR Am J Neuroradiol. 2013;34(5):944–50, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Salber D, Stoffels G, Pauleit D, et al. . Differential uptake of O-(2-18F-fluoroethyl)-L-tyrosine, L-3H-methionine, and 3H-deoxyglucose in brain abscesses. J Nucl Med. 2007;48(12):2056–2062. [DOI] [PubMed] [Google Scholar]
- 64. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 65. Galldiks N, Kracht LW, Burghaus L, et al. . Use of 11C-methionine PET to monitor the effects of temozolomide chemotherapy in malignant gliomas. Eur J Nucl Med Mol Imaging. 2006;33(5):516–524. [DOI] [PubMed] [Google Scholar]
- 66. Herholz K, Kracht LW, Heiss WD. Monitoring the effect of chemotherapy in a mixed glioma by C-11-methionine PET. J Neuroimaging. 2003;13(3):269–271. [PubMed] [Google Scholar]
- 67. Galldiks N, Langen KJ. Amino acid PET—an imaging option to identify treatment response, posttherapeutic effects, and tumor recurrence? Front Neurol. 2016;7:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wyss M, Hofer S, Bruehlmeier M, et al. . Early metabolic responses in temozolomide treated low-grade glioma patients. J Neurooncol. 2009;95(1):87–93. [DOI] [PubMed] [Google Scholar]
- 69. Roelcke U, Wyss MT, Nowosielski M, et al. . Amino acid positron emission tomography to monitor chemotherapy response and predict seizure control and progression-free survival in WHO grade II gliomas. Neuro Oncol. 2016;18(5):744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Suchorska B, Unterrainer M, Biczok A, et al. . 18F-FET-PET as a biomarker for therapy response in non-contrast enhancing glioma following chemotherapy. J Neurooncol. 2018;139(3):721–730. [DOI] [PubMed] [Google Scholar]
- 71. Piroth MD, Pinkawa M, Holy R, et al. . Prognostic value of early [18F]fluoroethyltyrosine positron emission tomography after radiochemotherapy in glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2011;80(1):176–184. [DOI] [PubMed] [Google Scholar]
- 72. Galldiks N, Langen KJ, Holy R, et al. . Assessment of treatment response in patients with glioblastoma using O-(2-18F-fluoroethyl)-L-tyrosine PET in comparison to MRI. J Nucl Med. 2012;53(7):1048–1057. [DOI] [PubMed] [Google Scholar]
- 73. Reithmeier T, Lopez WO, Spehl TS, et al. . Bevacizumab as salvage therapy for progressive brain stem gliomas. Clin Neurol Neurosurg. 2013;115(2):165–169. [DOI] [PubMed] [Google Scholar]
- 74. Galldiks N, Rapp M, Stoffels G, Dunkl V, Sabel M, Langen KJ. Earlier diagnosis of progressive disease during bevacizumab treatment using O-(2-18F-fluorethyl)-L-tyrosine positron emission tomography in comparison with magnetic resonance imaging. Mol Imaging. 2013;12(5):273–276. [PubMed] [Google Scholar]
- 75. Galldiks N, Filss CP, Goldbrunner R, Langen KJ. Discrepant MR and [(18)F]fluoroethyl-L-tyrosine PET imaging findings in a patient with bevacizumab failure. Case Rep Oncol. 2012;5(3):490–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Morana G, Piccardo A, Garrè ML, Nozza P, Consales A, Rossi A. Multimodal magnetic resonance imaging and 18F-L-dihydroxyphenylalanine positron emission tomography in early characterization of pseudoresponse and nonenhancing tumor progression in a pediatric patient with malignant transformation of ganglioglioma treated with bevacizumab. J Clin Oncol. 2013;31(1):e1–e5. [DOI] [PubMed] [Google Scholar]
- 77. Hutterer M, Nowosielski M, Putzer D, et al. . O-(2-18F-fluoroethyl)-L-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J Nucl Med. 2011;52(6):856–864. [DOI] [PubMed] [Google Scholar]
- 78. Galldiks N, Rapp M, Stoffels G, et al. . Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]fluoroethyl-L-tyrosine PET in comparison to MRI. Eur J Nucl Med Mol Imaging. 2013;40(1):22–33. [DOI] [PubMed] [Google Scholar]
- 79. Schwarzenberg J, Czernin J, Cloughesy TF, et al. . Treatment response evaluation using 18F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin Cancer Res. 2014;20(13):3550–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Galldiks N, Dunkl V, Ceccon G, et al. . Early treatment response evaluation using FET PET compared to MRI in glioblastoma patients at first progression treated with bevacizumab plus lomustine. Eur J Nucl Med Mol Imaging. 2018;45(13):2377–2386. [DOI] [PubMed] [Google Scholar]
- 81. Wardak M, Schiepers C, Cloughesy TF, Dahlbom M, Phelps ME, Huang SC. 18F-FLT and 18F-FDOPA PET kinetics in recurrent brain tumors. Eur J Nucl Med Mol Imaging. 2014;41(6):1199–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Delbeke D, Meyerowitz C, Lapidus RL, et al. . Optimal cutoff levels of F-18 fluorodeoxyglucose uptake in the differentiation of low-grade from high-grade brain tumors with PET. Radiology. 1995;195(1):47–52. [DOI] [PubMed] [Google Scholar]
- 83. Milker-Zabel S, Zabel-du Bois A, Henze M, et al. . Improved target volume definition for fractionated stereotactic radiotherapy in patients with intracranial meningiomas by correlation of CT, MRI, and [68Ga]-DOTATOC-PET. Int J Radiat Oncol Biol Phys. 2006;65(1):222–227. [DOI] [PubMed] [Google Scholar]
- 84. Henze M, Schuhmacher J, Hipp P, et al. . PET imaging of somatostatin receptors using [68Ga]DOTA-D-phe1-tyr3-octreotide: first results in patients with meningiomas. J Nucl Med. 2001;42(7):1053–1056. [PubMed] [Google Scholar]
- 85. Nyuyki F, Plotkin M, Graf R, et al. . Potential impact of (68)Ga-DOTATOC PET/CT on stereotactic radiotherapy planning of meningiomas. Eur J Nucl Med Mol Imaging. 2010;37(2):310–318. [DOI] [PubMed] [Google Scholar]
- 86. Klingenstein A, Haug AR, Miller C, Hintschich C. Ga-68-DOTA-TATE PET/CT for discrimination of tumors of the optic pathway. Orbit. 2015;34(1):16–22. [DOI] [PubMed] [Google Scholar]
- 87. Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62(1):18–24. [DOI] [PubMed] [Google Scholar]
- 88. Johnson MD, Powell SZ, Boyer PJ, Weil RJ, Moots PL. Dural lesions mimicking meningiomas. Hum Pathol. 2002;33(12):1211–1226. [DOI] [PubMed] [Google Scholar]
- 89. Kunz WG, Jungblut LM, Kazmierczak PM, et al. . Improved detection of transosseous meningiomas using 68Ga-DOTATATE PET/CT compared with contrast-enhanced MRI. J Nucl Med. 2017;58(10):1580–1587. [DOI] [PubMed] [Google Scholar]
- 90. Gudjonsson O, Blomquist E, Lilja A, Ericson H, Bergström M, Nyberg G. Evaluation of the effect of high-energy proton irradiation treatment on meningiomas by means of 11C-L-methionine PET. Eur J Nucl Med. 2000;27(12):1793–1799. [DOI] [PubMed] [Google Scholar]
- 91. Ryttlefors M, Danfors T, Latini F, Montelius A, Blomquist E, Gudjonsson O. Long-term evaluation of the effect of hypofractionated high-energy proton treatment of benign meningiomas by means of (11)C-L-methionine positron emission tomography. Eur J Nucl Med Mol Imaging. 2016;43(8):1432–1443. [DOI] [PubMed] [Google Scholar]
- 92. Gehler B, Paulsen F, Oksüz MO, et al. . [68Ga]-DOTATOC-PET/CT for meningioma IMRT treatment planning. Radiat Oncol. 2009;4:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Graf R, Nyuyki F, Steffen IG, et al. . Contribution of 68Ga-DOTATOC PET/CT to target volume delineation of skull base meningiomas treated with stereotactic radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(1):68–73. [DOI] [PubMed] [Google Scholar]
- 94. Astner ST, Dobrei-Ciuchendea M, Essler M, et al. . Effect of 11C-methionine-positron emission tomography on gross tumor volume delineation in stereotactic radiotherapy of skull base meningiomas. Int J Radiat Oncol Biol Phys. 2008;72(4):1161–1167. [DOI] [PubMed] [Google Scholar]
- 95. Grosu AL, Weber WA, Astner ST, et al. . 11C-methionine PET improves the target volume delineation of meningiomas treated with stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66(2):339–344. [DOI] [PubMed] [Google Scholar]
- 96. Rutten I, Cabay JE, Withofs N, et al. . PET/CT of skull base meningiomas using 2-18F-fluoro-L-tyrosine: initial report. J Nucl Med. 2007;48(5):720–725. [DOI] [PubMed] [Google Scholar]
- 97. Li Y, Jin G, Su D. Comparison of gadolinium-enhanced MRI and 18FDG PET/PET-CT for the diagnosis of brain metastases in lung cancer patients: A meta-analysis of 5 prospective studies. Oncotarget. 2017;8(22):35743–35749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Thon N, Kreth FW, Tonn JC. The role of surgery for brain metastases from solid tumors. Handb Clin Neurol. 2018;149:113–121. [DOI] [PubMed] [Google Scholar]
- 99. Minniti G, Clarke E, Lanzetta G, et al. . Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chao ST, Suh JH, Raja S, Lee SY, Barnett G. The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int J Cancer. 2001;96(3):191–197. [DOI] [PubMed] [Google Scholar]
- 101. Belohlávek O, Simonová G, Kantorová I, Novotný J Jr, Liscák R. Brain metastases after stereotactic radiosurgery using the leksell gamma knife: can FDG PET help to differentiate radionecrosis from tumour progression? Eur J Nucl Med Mol Imaging. 2003;30(1):96–100. [DOI] [PubMed] [Google Scholar]
- 102. Chernov M, Hayashi M, Izawa M, et al. . Differentiation of the radiation-induced necrosis and tumor recurrence after gamma knife radiosurgery for brain metastases: importance of multi-voxel proton MRS. Minim Invasive Neurosurg. 2005;48(4):228–234. [DOI] [PubMed] [Google Scholar]
- 103. Lai G, Mahadevan A, Hackney D, et al. . Diagnostic accuracy of PET, SPECT, and arterial spin-labeling in differentiating tumor recurrence from necrosis in cerebral metastasis after stereotactic radiosurgery. AJNR Am J Neuroradiol. 2015;36(12):2250–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hatzoglou V, Yang TJ, Omuro A, et al. . A prospective trial of dynamic contrast-enhanced MRI perfusion and fluorine-18 FDG PET-CT in differentiating brain tumor progression from radiation injury after cranial irradiation. Neuro Oncol. 2016;18(6):873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tomura N, Kokubun M, Saginoya T, Mizuno Y, Kikuchi Y. Differentiation between treatment-induced necrosis and recurrent tumors in patients with metastatic brain tumors: comparison among 11C-methionine-PET, FDG-PET, MR permeability imaging, and MRI-ADC-preliminary results. AJNR Am J Neuroradiol. 2017;38(8):1520–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Terakawa Y, Tsuyuguchi N, Iwai Y, et al. . Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med. 2008;49(5):694–699. [DOI] [PubMed] [Google Scholar]
- 107. Tsuyuguchi N, Sunada I, Iwai Y, et al. . Methionine positron emission tomography of recurrent metastatic brain tumor and radiation necrosis after stereotactic radiosurgery: is a differential diagnosis possible? J Neurosurg. 2003;98(5):1056–1064. [DOI] [PubMed] [Google Scholar]
- 108. Lizarraga KJ, Allen-Auerbach M, Czernin J, et al. . (18)F-FDOPA PET for differentiating recurrent or progressive brain metastatic tumors from late or delayed radiation injury after radiation treatment. J Nucl Med. 2014;55(1):30–36. [DOI] [PubMed] [Google Scholar]
- 109. Cicone F, Minniti G, Romano A, et al. . Accuracy of F-DOPA PET and perfusion-MRI for differentiating radionecrotic from progressive brain metastases after radiosurgery. Eur J Nucl Med Mol Imaging. 2015;42(1):103–111. [DOI] [PubMed] [Google Scholar]
- 110. Galldiks N, Stoffels G, Filss CP, et al. . Role of O-(2-(18)F-fluoroethyl)-L-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J Nucl Med. 2012;53(9):1367–1374. [DOI] [PubMed] [Google Scholar]
- 111. Ceccon G, Lohmann P, Stoffels G, et al. . Dynamic O-(2-18F-fluoroethyl)-L-tyrosine positron emission tomography differentiates brain metastasis recurrence from radiation injury after radiotherapy. Neuro Oncol. 2017;19(2):281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Romagna A, Unterrainer M, Schmid-Tannwald C, et al. . Suspected recurrence of brain metastases after focused high dose radiotherapy: can [18F]FET- PET overcome diagnostic uncertainties? Radiat Oncol. 2016;11(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kebir S, Rauschenbach L, Galldiks N, et al. . Dynamic O-(2-[18F]fluoroethyl)-L-tyrosine PET imaging for the detection of checkpoint inhibitor-related pseudoprogression in melanoma brain metastases. Neuro Oncol. 2016;18(10):1462–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Nguyen NC, Yee MK, Tuchayi AM, Kirkwood JM, Tawbi H, Mountz JM. Targeted therapy and immunotherapy response assessment with F-18 fluorothymidine positron-emission tomography/magnetic resonance imaging in melanoma brain metastasis: A pilot study. Front Oncol. 2018;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Abdulla DSY, Scheffler M, Brandes V, et al. . Monitoring treatment response to erlotinib in EGFR-mutated non-small-cell lung cancer brain metastases using serial O-(2-[18F]fluoroethyl)-L-tyrosine PET. Clin Lung Cancer. 2019;20(2):e148–e151. [DOI] [PubMed] [Google Scholar]
- 116. Heinzel A, Müller D, Langen KJ, et al. . The use of O-(2-18F-fluoroethyl)-L-tyrosine PET for treatment management of bevacizumab and irinotecan in patients with recurrent high-grade glioma: a cost-effectiveness analysis. J Nucl Med. 2013;54(8):1217–1222. [DOI] [PubMed] [Google Scholar]
- 117. Heinzel A, Müller D, Yekta-Michael SS, et al. . O-(2-18F-fluoroethyl)-L-tyrosine PET for evaluation of brain metastasis recurrence after radiotherapy: an effectiveness and cost-effectiveness analysis. Neuro Oncol. 2017;19(9):1271–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Heinzel A, Stock S, Langen KJ, Müller D. Cost-effectiveness analysis of amino acid PET-guided surgery for supratentorial high-grade gliomas. J Nucl Med. 2012;53(4):552–558. [DOI] [PubMed] [Google Scholar]
- 119. Heinzel A, Stock S, Langen KJ, Müller D. Cost-effectiveness analysis of FET PET-guided target selection for the diagnosis of gliomas. Eur J Nucl Med Mol Imaging. 2012;39(7):1089–1096. [DOI] [PubMed] [Google Scholar]
- 120. Lohmann P, Werner JM, Shah NJ, Fink GR, Langen KJ, Galldiks N. Combined amino acid positron emission tomography and advanced magnetic resonance imaging in glioma patients. Cancers (Basel). 2019;11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lohmann P, Kocher M, Ceccon G, et al. . Combined FET PET/MRI radiomics differentiates radiation injury from recurrent brain metastasis. Neuroimage Clin. 2018;20:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lohmann P, Kocher M, Steger J, Galldiks N. Radiomics derived from amino-acid PET and conventional MRI in patients with high-grade gliomas. Q J Nucl Med Mol Imaging. 2018;62(3):272–280. [DOI] [PubMed] [Google Scholar]
- 123. Seystahl K, Stoecklein V, Schüller U, et al. . Somatostatin receptor-targeted radionuclide therapy for progressive meningioma: benefit linked to 68Ga-DOTATATE/-TOC uptake. Neuro Oncol. 2016;18(11):1538–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]