Abstract
Purpose
Confirm that the corneal endothelial pump uses a lactate-coupled water efflux mechanism.
Methods
Corneal thickness, lactate efflux, and stromal [lactate] were measured in de-epithelialized swollen and nonswollen ex vivo-mounted rabbit corneas perfused with bicarbonate-rich and bicarbonate-free Ringers, ouabain, or acetazolamide to determine if the relationships among these parameters were similar to previous data using intact corneas. The role of barrier function was tested by perfusion with calcium-free EGTA. Predictions of [lactate] in endothelial dystrophy were examined in the Slc4a11 knock out mouse.
Results
De-epithelialized corneal swelling, lactate efflux, and stromal [lactate] in response to bicarbonate-free Ringers, ouabain, and acetazolamide perfusion had the same relationship as in intact corneas. The absolute amount of lactate efflux and stromal [lactate] in the de-epithelialized corneas was about half of intact corneas. De-epithelialized, swollen corneas deswelled fully with bicarbonate-rich, partially in the presence of acetazolamide, but continued to swell with bicarbonate-free or ouabain. The relationship among corneal thickness, lactate efflux, and [lactate] was the same as with nonswollen de-epithelialized corneas. In intact corneas swollen by perfusion with calcium-free EGTA, the relationship between swelling and lactate flux was the inverse of control corneas. The relationship between corneal swelling and [lactate] of intact corneas exposed to ouabain, but perfused with 7 mM lactate to simulate aqueous humor, was the same as without lactate. Corneal [lactate] in Slc4a11 knock out was twice that of wild type.
Conclusions
The corneal endothelial pump works via a lactate efflux mechanism that requires an intact osmotic barrier.
Keywords: corneal endothelial pump, lactate flux, active transport, Slc4a11 mouse
Maintenance of corneal hydration and transparency depends on the active ion transport function of the corneal endothelium, often called the endothelial pump. Numerous studies indicate that the pump requires primary active transport (Na+-K+ ATPase) and works best in the presence of bicarbonate, which suggested that the pump mechanism is a bicarbonate secretory process.1–4 Although there is compelling evidence for robust bicarbonate uptake via the sodium bicarbonate cotransporter, NBCe1, on the basolateral (stromal side) of corneal endothelial cells,5–8 efforts to measure net transendothelial bicarbonate fluxes have been equivocal,1,9,10 most likely because of a lack of apical (anterior chamber side) bicarbonate transporters or channels, which is consistent with apical bicarbonate permeability being three times smaller than basolateral.11,12 Therefore we sought alternate mechanisms for the pump that required active transport and used bicarbonate.
Studies by Doughty and Maurice13 suggested that the corneal endothelial pump can function in the absence of bicarbonate if the perfusing solution was supplied with a high concentration of Good's (HEPES and MES) buffers. Recently, we confirmed this observation and showed that the pump functions best in the presence of solutions with high buffering power (β), for example, 28 mM bicarbonate or 60 mM HEPES.14 Lactic acid is the most prevalent metabolic byproduct of the glycolytic cornea15,16 and is a prime candidate for being buffered. We found that lactate efflux across the endothelium was directly proportional to Ringers β, whereas corneal edema was inversely related to β.14 Inhibition of active transport, carbonic anhydrase activity, or lactate:H+ cotransport all decreased lactate efflux while swelling the cornea. We concluded that the corneal endothelial pump is a lactate efflux–linked osmotic process rather than a bicarbonate secretory process, where bicarbonate has an essential supporting role owing to its robust β.
This new model of the endothelial pump consists of primary and secondary active transport mechanisms and carbonic anhydrase activity that regulate intracellular pH and intracellular buffering power and, together with extracellular buffering, facilitates lactate:H+ cotransport via monocarboxylate cotransporters (MCT1, MCT2, and MCT4) on the basolateral and apical membranes of endothelial cells. The model14 hypothesizes that robust lactate uptake at the basolateral membrane creates a low [lactate] in the basolateral space relative to the nearby stroma, whereas lactate efflux from the cells at the apical membrane creates a relatively higher [lactate] in the unstirred layer of the apical surface. This transendothelial [lactate] gradient then drives water via aquaporin 1 across the endothelium by osmosis. The model is also supported by studies that showed corneal edema in rabbits when MCT1 and MCT4 expression were reduced in vivo,17 and in vitro experiments18–20 demonstrating that endothelial lactate fluxes were decreased by inhibiting secondary active transport or removal of bicarbonate.
The lactate flux model of the corneal endothelial pump elicits several questions and predictions. One critical observation is that de-epithelialized corneas can maintain stromal hydration,4,21,22 but this process removes a significant source of lactate. Is decreased lactate production and therefore decreased efflux compatible with the lactate flux model? Second, the model requires an intact osmotic membrane for a transendothelial [lactate] gradient to produce osmosis. Does disruption of the osmotic membrane, for example, by breaking tight junctions, disrupt the relationship among corneal thickness (CT) and lactate efflux? Endothelial pump function studies customarily used perfusing Ringers that had zero lactate. However, aqueous humor has between 5 and 10 mM lactate.23,24 So, we also ask if the coupling of lactate to water flux could be an artifact of perfusing with zero lactate by measuring this relationship while inhibiting active transport in the presence of a lactate perfusing Ringers. Last, the lactate flux model predicts that lactate efflux will be diminished in endothelial dystrophies and therefore the steady-state corneal [lactate] will be elevated. We tested this prediction using the Slc4a11 knock out mouse model25 of congenital hereditary endothelial dystrophy.
Methods
Ethical Approval
Animal procedures were approved by the Indiana University Bloomington Institutional Animal Care and Use Committee. New Zealand White rabbits were from Charles River (Wilmington, MA). Both males and females, age 8 to 12 weeks, weighing approximately 2.2 kg were used for these studies. Animals were fed standard rabbit chow ad libitum. Slc4a11−/− mice25 were generously provided by Eranga N. Vithana, Singapore Eye Research Institute. All mice were housed, bred, and maintained in pathogen-free conditions. All animals were used in experiments in accordance with institutional guidelines and the current regulations of the National Institutes of Health, the United States Department of Health and Human Services, the United States Department of Agriculture, and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Cornea Mounting
To collect corneas, rabbits were first sedated via intramuscular injection of ketamine HCl (60 mg/kg) and xylazine (5 mg/kg), followed by an intracardiac injection of pentobarbital sodium, 100 mg/kg (Euthasol; Henry Schein, Columbus, OH). A circular incision was made in the skin around the palpebral aperture of the rabbit and the eyeball was enucleated complete with conjunctiva and lids. The rabbit cornea was mounted for superfusion of the endothelial surface essentially as described by Dikstein and Maurice26 with some modification.14 Briefly, a rabbit eyeball with lids was placed ocular surface facing down on a plastic mounting ring that was pressed onto a cannulated methacrylate rod, held by a metal scaffold. The lids and conjunctiva were everted over the globe and pulled down onto the rod. The eye was anchored in place by a suture tied around a circular groove on the ring. The conjunctiva was then trimmed up to the suture. The posterior part of the globe was cut away a few millimeters from the corneal–scleral junction, and the scleral rim was reflected down over the ring. The vitreous body was wiped away, and the ciliary body was gripped with forceps and dissected away together with the lens and iris in one piece without causing any wrinkling of the corneal surface. A metal platform was then passed over the plastic rod and was pressed over the outside of the ring. A plastic cap, serving as an artificial anterior chamber, tightly sealed the posterior surface of the scleral rim. The plastic cap has three holes fitted with 23-gauge tubing. The central tube provided inflow and the two side tubes provided outflow. The cornea chamber was fitted into a metal jacket with the epithelium facing up. Anterior chamber perfusion was started and the rod was pulled free. The metal jacket has an embedded electric heater to maintain the chamber and the fluid at 37°C. A thermistor embedded in the posterior plastic cap indicates temperature equilibration within about 10 minutes.
Cornea De-Epithelialization
For a number of experiments in this study, the corneal epithelium was removed before mounting the cornea. The epithelium was scrapped off with a scalpel blade held perpendicular to the ocular surface until a dull sheen of the basement membrane was seen. The cornea was then mounted, endothelium perfused, and anterior surface covered with silicone oil [XIAMETER PMX-200] (Dow Inc, Midland, MI) to prevent drying. In one experimental series, the de-epithelialized surface was exposed to glutathione bicarbonate Ringers (GBR) and the stroma allowed to swell for 30 minutes, GBR removed, and then silicone oil applied.
Perfusion and Solutions
The perfusion fluid was driven into the corneal chamber by a micro-pump (P720 Peristaltic Pump; Instech, Plymouth Meeting PA) at a constant speed of 50 µL/min. The two outflow tubes were joined behind the chamber to form one outflow tube that is placed 20 cm above the epithelium to simulate an IOP of 15 mm Hg. Each cornea was perfused for a period of 5 hours. The perfusate was collected for 30 minutes (1.5-mL aliquots), and frozen for measuring [lactate] at a later time.
Perfusion solutions include GBR and bicarbonate-free (BF) Ringers. GBR contains (in mmol/L): 110 NaCl, 2 KCl, 1 K2HPO4, 0.6 MgCl2, 1.4 Ca-gluconate, 5 glucose, 0.3 reduced glutathione, 15 Na-gluconate, and 28.5 NaHCO3−, equilibrated with 5% CO2, pH 7.5. BF Ringers was the same as in GBR, except 28.5 NaHCO3− was substituted with 10 Na-HEPES and 18.5 Na-gluconate. BF Ringers was equilibrated with air and pH adjusted to 7.5 with NaOH. The osmolarity of all solutions was measured with a vapor pressure osmometer (Wescor, Logan, UT), and adjusted to 295 mOsm/L with sucrose.
Rabbit corneas were mounted in pairs to maximize the usefulness of the tissue and minimize animal use. The endothelium of both corneas were perfused with GBR for the first 90 minutes, Corneas with intact epithelium were covered with GBR (approximately 0.6 mL). After the 90-minute equilibration, the control cornea perfusion was continued with GBR for 210 minutes, and the paired cornea perfusion was switched to an experimental Ringers.
Corneal Thickness
During perfusion, the central CT was measured every 15 minutes by OCT using an iVue instrument (Optovue, Inc., Fremont, CA) that was mounted above the perfusion chamber.14 For intact corneas, the liquid covering the epithelial surface was removed to make the OCT measurement and then fresh GBR added back. Three measurements of the CT were taken every time and the average used for that time point.
Corneal Lactate Retention
After the 5-hour perfusion, the cornea was removed and trephined to a 10-mm button that was snap frozen in liquid N2 and pulverized to powder using a ceramic mortar and pestle. The cornea powder was collected in a preweighed microcentrifuge tube and 0.5 mL PBS was added, vortexed for 1 minute, and centrifuged at 13,000×g for 15 minutes. The supernatant was saved for measuring lactate content. The remaining pellet was dried at 60°C within a vacuum centrifuge for 2 hours and weighed. Lactate (in nanomoles) was measured by an assay kit from BioVision Research Products (Milpitas, CA). The same procedure was used for mouse corneas, except the corneal button had a 5-mm diameter.
Statistical Analysis
Each experimental condition included a minimum of three corneas. The results are expressed as mean ± SD and compared using the t-test or regression analysis, as appropriate. CT changes are reported as maximal rates (micrometers per hour), which typically occurred between the 120- and 180-minute perfusion period, or as total thickness change from 90 to 300 minutes.
Results
A major criticism of the lactate flux model14 of the endothelial pump is that de-epithelialized corneas, which retain all the pump functions, produce significantly less lactate and could therefore be incompatible with the model. The lactate flux model, however, does not rely on an absolute amount of lactate production; rather, it depends on the endothelium generating a small apical to basolateral [lactate] gradient with the lactate that is available. Whereas methods to measure small concentration gradients across cell layers are unavailable, we can ask if the relationship between water and lactate efflux observed in intact corneas14 is similar in de-epithelialized corneas producing less lactate. De-epithelialized corneas were mounted and perfused on the endothelial side with GBR. The perfusate was collected over 30-minute intervals and later analyzed for lactate. After 90 minutes, either GBR was continued or the perfusing solution was switched to one containing 100 µM Ouabain, 100 µM acetazolamide (ACTZ) in GBR, or BF Ringers. Figure 1A shows that ouabain produced the most corneal swelling relative to GBR alone, then BF, and a modest amount of swelling was produced with ACTZ, the same rank order as found in intact corneas.14 Figure 1B shows the lactate efflux over the course of the experiment, indicating that flux was greatest in GBR, slightly less in ACTZ, much less in BF, and least with ouabain. This finding is summarized in Figure 1C showing the total lactate efflux from 120 to 300 minutes. Last, Figure 1D shows that corneas that swelled the least and had the greatest lactate efflux had the lowest amount of lactate retained in the cornea at the end of the experiment, similar to what was observed in intact corneas.14 However, the absolute lactate efflux and the amount retained in the cornea of de-epithelialized corneas was approximately 50% of that in intact corneas, for example, the lactate efflux was 1300 nanomoles in intact corneas14 and 700 nanomoles in de-epithelialized corneas (Fig. 1C) during GBR perfusion.
Figure 1.
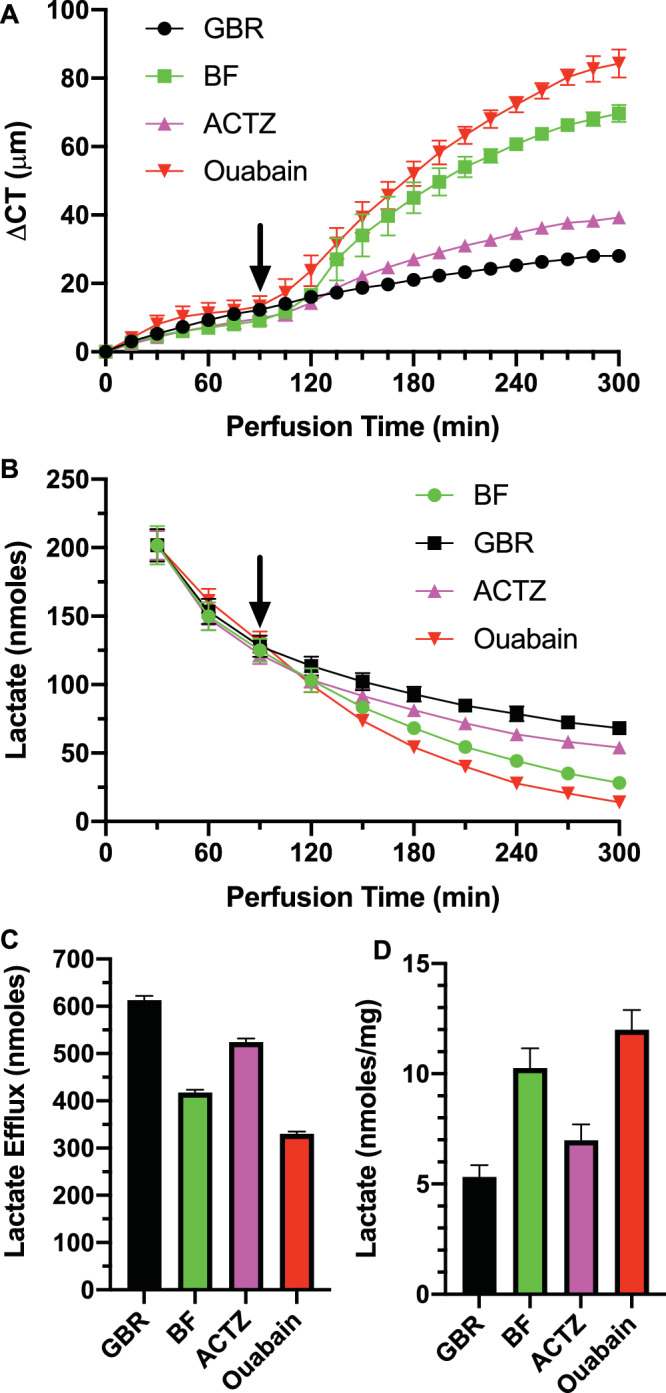
Corneal swelling and lactate efflux in de-epithelialized (De-epi) rabbit corneas. (A) Change in CT (ΔCT) over time in GBR and effects of changing endothelial perfusion (at arrow) to 100 µM Ouabain, 100 µmol/L ACTZ, and BF Ringers. (B) Lactate content in perfusate collected over 30-minute intervals. (C) Total lactate efflux over the 120- to 300-minute period. (D) [Lactate] in corneas at end of the experiment (n = 3 corneas per condition).
An alternate approach to examining endothelial function is to swell the cornea and then observe de-swelling. Corneas were de-epithelialized, mounted, and perfused with GBR on the endothelial side and the bare stromal surface exposed to GBR for 30 minutes. Then the GBR was removed and replaced with silicone oil. This procedure swelled the corneas by approximately 75 µm. Figure 2A shows that corneas perfused with GBR de-swelled significantly, whereas those exposed to ACTZ starting at 90 minutes de-swelled significantly less, and those exposed to BF and ouabain continued to swell. Figure 2B shows the lactate efflux over time and Figures 2C and 2D summarize the total efflux and lactate retained in the cornea. Lactate flux was similar to nonswollen de-epithelialized corneas, but again approximately 50% less than that observed in intact corneas.14 Using the de-swelling approach with de-epithelialized corneas, we see the same relationship between CT changes and lactate efflux as in nonswollen de-epithelialized and intact corneas. Figure 3 shows the total changes in CT as a function of lactate efflux in swollen and nonswollen de-epithelialized corneas. The regression line (ΔCT= ae−b * efflux + c; r2 = 0.97), is similar to that for intact corneas,14 except it is shifted to the left by a factor of approximately 2, reflecting the 50% lower amount of lactate efflux. In sum, the relationship between corneal swelling and lactate efflux was similar in de-epithelialized and intact corneas, even though the absolute amount of lactate efflux was much different.
Figure 2.
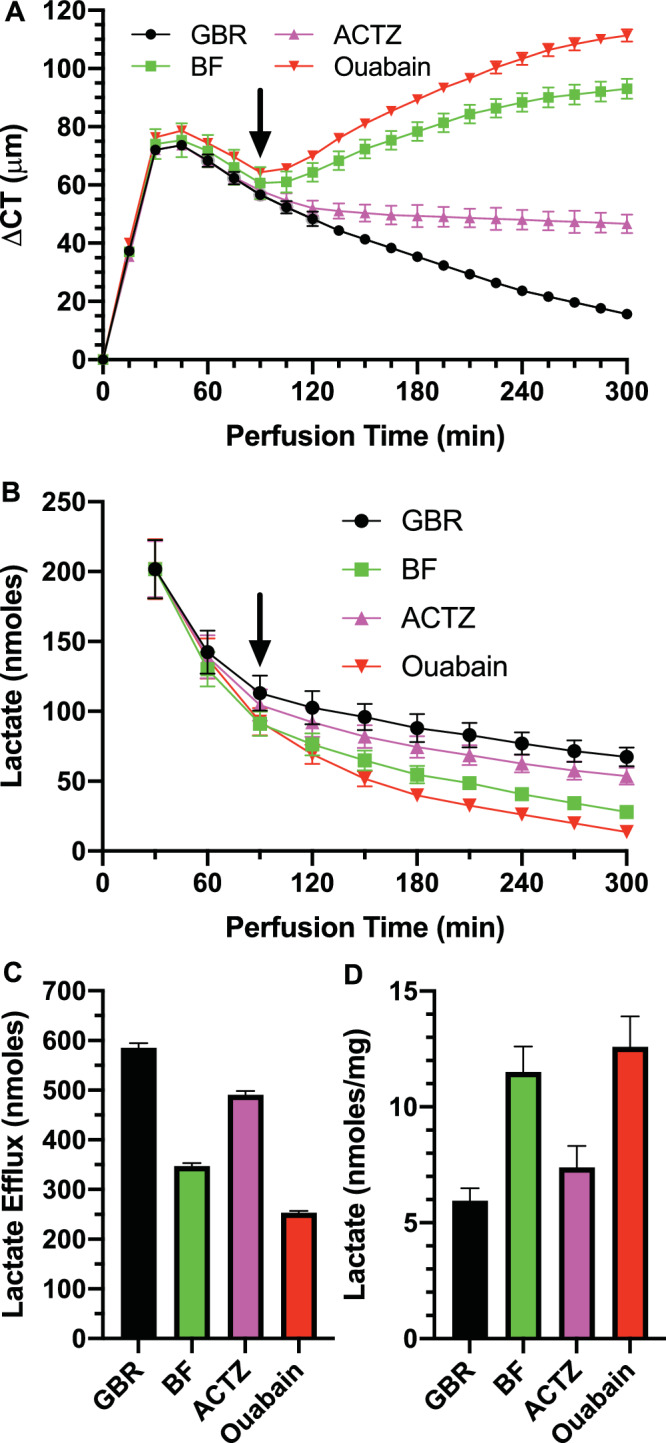
CT and lactate flux in de-epithelialized preswollen corneas. (A) Change in CT (ΔCT) over time in GBR and effects of changing endothelial perfusion (at arrow) to 100 µM Ouabain, 100 µM ACTZ, and BF Ringers. (B) Lactate content in perfusate collected over 30-minute intervals. (C) Total lactate efflux over the 120- to 300-minute period. (D) [Lactate] in cornea at end of experiment (n = 3 corneas per condition).
Figure 3.
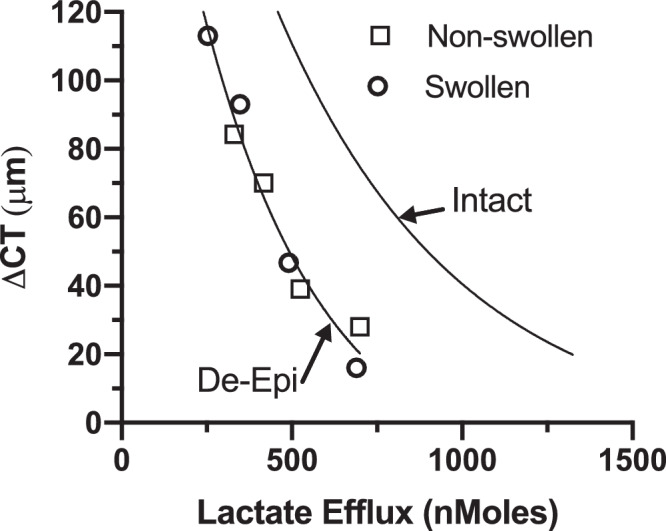
Relationship between CT changes and lactate efflux. Data from de-epithelialized corneas that were preswollen (Figure 2; circles) or not preswollen (Figure 1; squares) with regression curve (ΔCT= ae−b * efflux + c, r2 = 0.97, n = 24). For comparison, regression curve of ΔCT versus lactate efflux in intact corneas.14
All secretory models, including the lactate efflux model, require an intact osmotic membrane or barrier function to establish the basolateral to apical [osmotic] gradient. To test that disruption of endothelial barrier function will also decouple lactate from water flux, we perfused intact corneas with calcium-free EGTA, which disrupts tight junctions. Figure 4A shows that this produced significant corneal swelling. However, in contrast with all other mechanisms that cause corneal swelling coupled to a decrease in lactate efflux, lactate efflux increased relative to control (Fig. 4C), yet the stromal lactate retained in the cornea was only slightly lower (Fig. 4D). These data show that an intact osmotic membrane is required for the coupling of lactate flux to water and that simple passive diffusive efflux of lactate is not associated with water flux.
Figure 4.
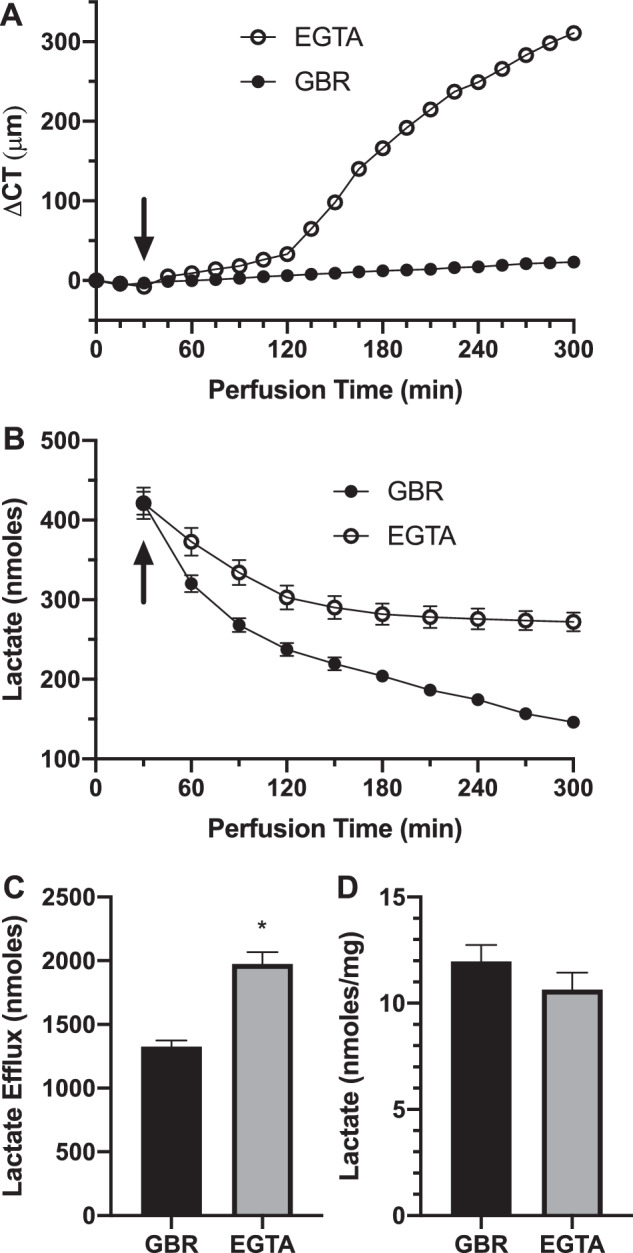
Effect of calcium-free perfusion on CT and lactate flux. (A) CT change of intact corneas perused in GBR and switched (at arrow) to zero calcium 100 µmol/L EGTA in GBR. (B) Lactate content in perfusate collected over 30-minute intervals. (C) Total lactate efflux over the 120- to 300-minute period. (D) [Lactate] in cornea at end of experiment. *P < 0.05, significantly different from GBR, n = 3 corneas per condition.
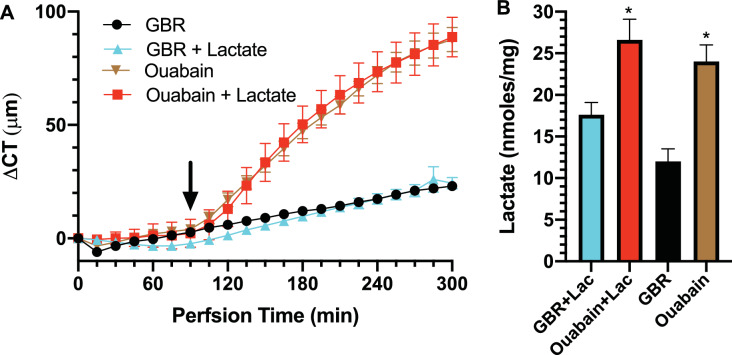
Corneal endothelial physiology studies have customarily used GBR perfusion without lactate. However, the aqueous humor has 5 to 10 mM lactate. To show that the data that led to the lactate flux model is not an artifact of zero lactate perfusion, we perfused intact corneas with GBR containing 7 mM lactate. Figure 5A shows that there was no difference in CT changes over time between GBR with and without lactate or ouabain with or without lactate. When perfusing with lactate, we could not measure lactate efflux because the increment of efflux over the 7mM lactate is not detectable. However, the lactate retained in the cornea always has the same relative relationship with efflux, that is, with more efflux, there is less retained. Figure 5B shows that perfusion with lactate caused an increase in lactate retained, as would be expected, and that ouabain significantly increased lactate retained indicating that lactate efflux was decreased by ouabain whether perfused with 0 or 7 mM lactate.
Figure 5.
Effect of lactate perfusion on corneal swelling and lactate retention during active transport inhibition. (A) Change in CT (ΔCT) over time in corneas perfused with GBR ± 7 mM acetate and effects of changing endothelial perfusion (at arrow) to 100 µM Ouabain. (B) [Lactate] in cornea at end of experiment. *P < 0.05, significantly higher [lactate] with ouabain; n = 3 corneas per condition.
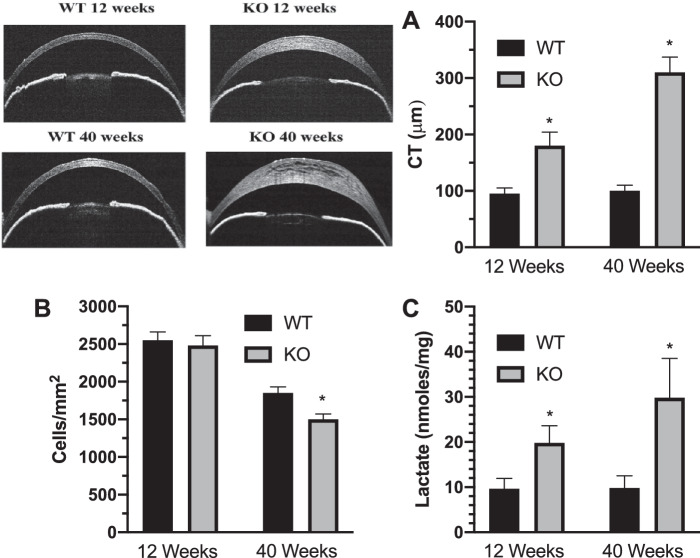
Last, the lactate efflux model predicts that corneal edema is due to diminished lactate efflux and thus [lactate] should be increased in corneas with endothelial dystrophy. We tested this prediction in a model of congenital hereditary endothelial dystrophy, the Slc4a11 knock out mouse. Figure 6A shows that at 12 and 40 weeks of age there is significant corneal edema in the knock out relative to wild-type mouse; however, cell density is not significantly different at 12 weeks (Fig. 6B). Corneas were collected from 3 wild-type and 3 knock out mice (6 corneas each) and subjected to lactate content analysis. Figure 6C shows that the [lactate] in the knock out mice was significantly greater than wild-type mice at 12 and 40 weeks.
Figure 6.
Corneal [lactate] is increased in a mouse model of congenital hereditary endothelial dystrophy. (A) OCT images of corneal cross-sections illustrating differences in CT plotted for 12- and 40-week-old wild-type and Slc4a11 knock out mice (n = 9 mice per condition). (B) Endothelial cell density (n = 3 mice per condition). (C) Corneal [lactate], n = 3 mice per condition. *P < 0.05, significantly from wild-type mice.
Discussion
The current study follows up our previous work that tested a new hypothesis for the mechanism of the corneal endothelial pump. We previously found that corneal swelling (i.e., water influx) was inversely related to corneal lactate efflux, and that lactate efflux varied directly with perfusing solution buffering power (β).14 In fact, the endothelial pump was supported by highly buffered HEPES Ringers in the absence of bicarbonate, confirming the findings of Doughty and Maurice.13 High intra- and extracellular β facilitates lactate:H+ cotransport at basolateral and apical membranes by providing or consuming transported protons, thereby maintaining robust proton gradients and the coupled lactate transport. Primary active transport, that is, the Na+-K+ ATPase, produces Na gradients that drive Na+:2HCO3− cotransport and Na+/H+ exchange, which control intracellular pH and intracellular buffering power. Extracellular and intracellular carbonic anhydrase activity speeds the hydration and dehydration of CO2, thus increasing the speed and depth of bicarbonate buffering. Therefore, BF perfusion of low β, the carbonic anhydrase inhibitor ACTZ, and ouabain all cause corneal swelling, decreased lactate efflux, and increased retention of corneal lactate consistent with the hypothesis.
Several investigators have shown that corneal hydration and endothelial function are maintained in de-epithelialized corneas, which provided evidence that the endothelium is primarily responsible for corneal hydration control.21 Because the epithelium is a major source of lactate, there was concern that the reduced efflux would be incompatible with the lactate flux model. Therefore, we asked if the relationship between lactate efflux and corneal hydration held up in de-epithelialized corneas. Our results show that, whereas total lactate production is decreased by approximately 50% in de-epithelialized corneas, corneal water efflux still varies directly with lactate efflux. Our interpretation is that while the [lactate] is decreased in the de-epithelialized corneas, the gradient between basolateral (low [lactate]) and apical (higher [lactate]) surfaces is maintained. This interpretation is also supported by the lactate perfusion data (Fig. 5). Previously, we showed that switching endothelial perfusion from standard GBR to GBR with added lactate caused transient corneal swelling followed by a new steady-state CT, consistent with briefly slowing lactate efflux.14 In the current study, we perfused the endothelium of intact corneas continuously with GBR plus 7 mM lactate to simulate the aqueous humor environment. Ouabain induced CT changes with lactate in the Ringers were identical to those without lactate. Adding lactate to the Ringers increases the total corneal [lactate], the converse of de-epithelialization, yet does not alter the relationship between water flux and lactate flux. Moreover, this demonstrates that our findings are not an artifact of an infinite lactate gradient that is the case when the endothelium is perfused with zero lactate.
Importantly, all osmosis-dependent water flux mechanisms require an intact osmotic membrane (the cell layer). We found that this is also true for water flux coupled to lactate. When the endothelial tight junctions are disrupted, barrier function is lost and there is increased passive diffusion of lactate out of the cornea; however, simultaneously the cornea swells, indicating that the endothelial pump is not related to passive diffusion of lactate, but requires active transport with an intact osmotic barrier.
Last, the lactate flux model predicts that, in the early stages of endothelial dystrophies, that is, before cell density is so low that barrier function is lost, corneal edema should be associated with decreased lactate efflux and as such increased corneal [lactate]. Ideally, to test this prediction in humans, penetrating keratoplasty specimens from Fuchs patients and controls (keratoconus specimens?) would be plunged into liquid nitrogen immediately after surgical dissection and then analyzed for lactate. Given the advent of non-PK transplants, this method is not tenable. Therefore, we used the Slc4a11 knock out model for congenital hereditary dystrophy.25 An advantage of a mouse model is that the conditions under which the corneas are harvested are very tightly controlled and the control group is normal, not representing any corneal disorder. In this model, corneal edema is apparent very early in the knock out and cell density has not reached a critically low level. We found that corneal [lactate] was significantly elevated at both 12 and 40 weeks of age, consistent with the model prediction.
Our model proposes that the endothelial layer creates a [lactate] gradient, low in the basolateral space and high at the apical surface. This is an example of the standing gradient model27 for transcellular fluid transport. Direct evidence for standing gradients are generally lacking and are quite a formidable experimental measure. Another possible mechanism for coupling lactate flux to water flux that has been suggested for the retinal pigment epithelium is a direct coupling within the MCTs.28 Interestingly, the Fischbarg laboratory had reported water flux by cultured endothelial cells.29,30 This measure is technically difficult and has not been replicated, but if true it implies that lactate production by the endothelium itself could be driving the pump and that epithelial and stromal lactate is not needed. Future studies are required to determine if the endothelium generates [lactate] gradients, whether it can do so by itself, and/or whether lactate transport via MCTs is coupled to water flux. Moreover, the interpretation of these data and our previous studies are limited to the rabbit,14,17 bovine,19,20 and mouse corneal endothelia. Therefore, extension of the model to human corneal endothelium cannot be made without direct experimentation.
Acknowledgments
Supported by the National Institute of Health Grant RO1EY08834 (JAB).
Disclosure: S. Li, None; E. Kim, None; D.G. Ogando, None; J.A. Bonanno, None
References
- 1. Mayes KR, Hodson S. Local osmotic coupling to the active trans-endothelial bicarbonate flux in the rabbit cornea. Biochim Biophys Acta. 1978; 514: 286–293. [DOI] [PubMed] [Google Scholar]
- 2. Fischbarg J, Hernandez J, Leibovitch LS, Koniarek JP. The mechanism of fluid and electrolyte transport across corneal endothelium: critical revision and update of a model. Curr Eye Res. 1985; 4: 351, 360. [DOI] [PubMed] [Google Scholar]
- 3. Baum J, Maurice D, McCarey B. The active and passive transport of water across the corneal endothelium. Exp Eye Res. 1984; 39: 335–342. [DOI] [PubMed] [Google Scholar]
- 4. Riley MV, Winkler BS, Peters MI, Czajkowski CA. Relationship between fluid transport and in situ inhibition of Na(+)-K+ adenosine triphosphatase in corneal endothelium. Invest Ophthalmol Vis Sci. 1994; 35: 560–567. [PubMed] [Google Scholar]
- 5. Sun XC, Bonanno JA, Jelamskii S, Xie Q. Expression and localization of Na(+)-HCO(3)(-) cotransporter in bovine corneal endothelium. Am J Physiol Cell Physiol. 2000; 279: C1648–1655. [DOI] [PubMed] [Google Scholar]
- 6. Usui T, Seki G, Amano S, et al.. Functional and molecular evidence for Na(+)-HCO3- cotransporter in human corneal endothelial cells. Pflugers Arch. 1999; 438: 458–462. [DOI] [PubMed] [Google Scholar]
- 7. Diecke FP, Wen Q, Sanchez JM, Kuang K, Fischbarg J. Immunocytochemical localization of Na+-HCO3- cotransporters and carbonic anhydrase dependence of fluid transport in corneal endothelial cells. Am J Physiol Cell Physiol. 2004; 286: C1434–1442. [DOI] [PubMed] [Google Scholar]
- 8. Jentsch T, Korbmacher C, Janicke I, et al.. Regulation of cytoplasmic pH of cultured bovine corneal endothelial cells in the absence and presence of bicarbonate. J Memb Biol. 1988; 103: 29–40. [DOI] [PubMed] [Google Scholar]
- 9. Fischbarg J, Jin LJ. Fluid and electrolyte transports across corneal endothelium. Curr Topics Res. 1984; 4: 201–223. [PubMed] [Google Scholar]
- 10. Maurice DM. Passive ion fluxes cross the corneal endothelium. Curr Eye Res. 1985; 4: 339–349. [DOI] [PubMed] [Google Scholar]
- 11. Li J, Sun XC, Bonanno JA. Role of NBC1 in apical and basolateral HCO3- permeabilities and transendothelial HCO3- fluxes in bovine corneal endothelium. Am J Physiol Cell Physiol. 2005; 288: C739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonanno J, Guan Y, Jelamskii S, Kang X. Apical and basolateral CO2-HCO3− permeability in cultured bovine corneal endothelial cells. Am J Physiol. 1999; 277: C545–553. [DOI] [PubMed] [Google Scholar]
- 13. Doughty MJ, Maurice D.. Bicarbonate sensitivity of rabbit corneal endothelium fluid pump in vitro. Invest Ophthalmol Vis Sci. 1988; 29: 216–223. [PubMed] [Google Scholar]
- 14. Li S, Kim E, Bonanno JA. Fluid transport by the cornea endothelium is dependent on buffering lactic acid efflux. Am J Physiol Cell Physiol. 2016; 311: C116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klyce S. Stromal lactate accumulation can account for corneal oedema osmotically following epithelial hypoxia in the rabbit. J Physiol (Lond). 1981; 321: 49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riley M. Glucose and oxygen utilization by the rabbit cornea. Exp Eye Res. 1969; 8: 193–200. [DOI] [PubMed] [Google Scholar]
- 17. Li S, Nguyen TT, Bonanno JA. CD147 required for corneal endothelial lactate transport. Invest Ophthalmol Vis Sci. 2014; 55: 4673–4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu C, Cheng Q, Nguyen T, Bonanno JA. Knockdown of NBCe1 in vivo compromises the corneal endothelial pump. Invest Ophthalmol Vis Sci. 2010; 51: 5190–5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nguyen TT, Bonanno JA. Bicarbonate, NBCe1, NHE, and carbonic anhydrase activity enhance lactate-H+ transport in bovine corneal endothelium. Invest Ophthalmol Vis Sci. 2011; 52: 8086–8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nguyen TT, Bonanno JA. Lactate-H(+) transport is a significant component of the in vivo corneal endothelial pump. Invest Ophthalmol Vis Sci. 2012; 53: 2020–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maurice D. The location of the fluid pump in the cornea. J Physiol. 1972; 221: 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fischbarg J. Active and passive properties of the rabbit corneal endothelium. Exp Eye Res. 1973; 15: 615–638. [DOI] [PubMed] [Google Scholar]
- 23. Bergmanson JP, Johnsson J, Soderberg PG, Philipson BT. Lactate levels in the rabbit cornea and aqueous humor subsequent to non-gas permeable contact lens wear. Cornea. 1985; 4: 173–176. [PubMed] [Google Scholar]
- 24. Reim M, Boeck H, Krug P, Venske G. Aqueous humour and cornea stroma metabolite levels under various conditions. Ophthalmol Res. 1972; 3: 241–250. [Google Scholar]
- 25. Han SB, Ang HP, Poh R, et al.. Mice with a targeted disruption of Slc4a11 model the progressive corneal changes of congenital hereditary endothelial dystrophy. Invest Ophthalmol Vis Sci. 2013; 54: 6179–6189. [DOI] [PubMed] [Google Scholar]
- 26. Dikstein S, Maurice DM. The active control of corneal hydration. Isr J Med Sci. 1972; 8: 1523–1528. [PubMed] [Google Scholar]
- 27. Diamond JM. Osmotic water flow in leaky epithelia. J Membr Biol. 1979; 51: 195–216. [DOI] [PubMed] [Google Scholar]
- 28. Hamann S, Kiilgaard JF, la Cour M, Prause JU, Zeuthen T. Cotransport of H+, lactate, and H2O in porcine retinal pigment epithelial cells. Exp Eye Res. 2003; 76: 493–504. [DOI] [PubMed] [Google Scholar]
- 29. Narula P, Xu M, Kuang K, Akiyama R, Fischbarg J. Fluid transport across cultured bovine corneal endothelial cell monolayers. Am J Physiol. 1992; 262: C98–103. [DOI] [PubMed] [Google Scholar]
- 30. Kuang K, Yiming M, Wen Q, et al.. Fluid transport across cultured layers of corneal endothelium from aquaporin-1 null mice. Exp Eye Res. 2004; 78: 791–798. [DOI] [PubMed] [Google Scholar]