Abstract
Cormorant fishing is a traditional Japanese fishing method using captive Japanese cormorants (Phalacrocorax capillatus). Between June and July 2017, an avian pox outbreak was reported in captive cormorant populations throughout several distant cities in Japan. We examined the lesions obtained from two such affected cormorants, which were raised in distant cities. The affected cormorants were grossly characterized by the development of cutaneous nodules around the base of the beak. Histopathologically, these nodules consisted of marked epidermal hyperplasia with ballooning degeneration of spinous cells and eosinophilic intracytoplasmic inclusions (Bollinger bodies). The lesions displayed 4b core protein (P4b) of Avipoxvirus (APV) and DNA polymerase genes, which were detected by PCR. Moreover, the nucleotide sequences detected from both cormorants were found to be identical. No identical sequence was found in any international database. These findings suggest that both examined cormorants were infected with an identical APV, which has never been previously reported. According to the phylogenetic analysis, the detected sequences were observed to cluster in subclade A3, which consists mainly of the sequences detected from several marine birds, including other cormorant species. This observation suggests that the viruses might be maintained in Japanese cormorants in nature.
Keywords: avian pox, avipoxvirus, Japanese cormorant, pathology, phylogenetic analysis
Avian pox is an infectious disease of the avian species caused by the double-stranded DNA viruses of the genus Avipoxvirus [14]. Avipoxviruses (APVs), in the genus, have currently been classified into ten species primarily based on host species according to the International Committee on Taxonomy of Viruses [14], but the exact number of species that indeed exist within the genus is unknown. Additionally, APV infections have been found in more than 278 bird species worldwide, including wild, captive, and domestic birds [1, 16].
APV infections cause two forms of diseases, cutaneous and diphtheritic. In the cutaneous form, after a given incubation period, papular and nodular lesions occur mainly in the featherless areas of the body, such as the legs, feet, base of the beak, and eyelids [1, 12, 19]. In the diphtheritic form, nodular lesions develop on the mucous membrane of the mouth, oesophagus, and trachea [1, 12, 19]. The routes of infection for APVs include direct contact with infected birds, aerosol infection due to feather dust and droppings contaminated with the virus, oral infection by ingestion of contaminated feed and water, and mechanical transmission by blood-sucking arthropods such as mosquitoes [4]. Histopathologically, both forms of the APV infection are characterized by epithelial hyperplasia and hypertrophy with intracytoplasmic inclusions known as Bollinger bodies [4, 12, 17, 19]. In recent years, due to the APV P4b gene being highly conserved in APVs, it is used as a pan-genus marker in the molecular diagnosis of APV infections [7, 8]. In addition, nucleotide sequence variations in the gene were reported as useful markers for genotyping of APV, and the phylogenetic analysis of the gene is proposed as a novel classification method of the APV [3, 5, 8, 18].
With approximately 1,300 years of Japanese history to back up its longstanding tradition, cormorant fishing is a method in which fishers use trained Japanese cormorants (Phalacrocorax capillatus) to catch fish in rivers. Currently, cormorant fishing takes place in thirteen cities across Japan from early summer to autumn, mainly for tourism and preservation of traditional culture. The Japanese cormorant is a migratory seabird belonging to the family Phalacrocoracidae, and lives on the rocky coastline of the open sea in south-eastern Russia, the Korean Peninsula, eastern China, and Japan [13]. Since scheduled breeding of the Japanese cormorant remains to be a challenge, young wild cormorants are historically captured on the Pacific coast at Ishihama beach in Ibaraki Prefecture, Japan by a special national permit. Then subsequently transported to cities where cormorant fishing takes place.
During June and July 2017, an outbreak of the APV infection in the captive Japanese cormorants occurred in several distant cities in Japan. Between one and ten cormorants with facial nodules were found in each city, with most of the affected cormorants being young birds captured in the spring. In the present report, we described the pathological and molecular findings in two affected cormorants, which were found in Miyoshi City in Hiroshima Prefecture and Iwakuni City in Yamaguchi prefecture.
MATERIALS AND METHODS
Samples
In Case No. 1, a surgically excised lesion was submitted to the Laboratory of Veterinary Pathology at Gifu University for histopathological examination. A portion of the lesion was frozen and sent to the Laboratory of Veterinary Hygiene at Tottori University for molecular analysis. In Case No. 2, pieces of the facial nodule were manually excised, and frozen samples were sent to the laboratories for histopathological and molecular examinations.
Histopathological examination
The excised lesions were fixed with 10% phosphate-buffered formalin, followed by routine processing, and then embedded in paraffin. Sections were stained with haematoxylin and eosin (H&E).
Molecular detection and characterization of APV
A section of P4b and DNA polymerase genes of APVs were detected by PCR using the following primers: P1X (5′-TCAGCAGGTGCTAAACAACA-3′) and P2 (5′-CGGTAGCTTAACGCCGAATA-3′) for APV P4b gene [2] and PoPr1 (5′-CGCCGCATCATCTACTTATC-3′) and PoPr2 (5′-CCACACAGCGCCATTCATTA-3′) for DNA polymerase gene of APV [3]. The expected sizes of the PCR products were 578 and 642 bp, respectively. DNA was extracted from the frozen samples using the Sepagene® kit (Sanko Junyaku Co., Ltd., Tokyo, Japan) following the manufacturer’s instructions. PCR was performed with GoTaq® Green Master Mix (Promega, Tokyo, Japan). PCR of the P4b gene was performed with initial denaturation for 5 min at 94°C, followed by 35 amplification cycles at 94°C for 1 min, 59°C for 1 min, and 72°C for 1 min. The final extension step was performed at 72°C for 7 min, as previously described [2]. PCR carried out on the DNA polymerase gene was performed with initial denaturation for 5 min at 95°C, followed by 35 amplification cycles at 95°C for 30 sec, 53°C for 30 sec, and 72°C for 1 min. The final extension step was performed for 5 min at 72°C, as previously described [3].
After PCR amplification, the PCR products were purified by QIAquick® Gel Extraction Kit (Qiagen, Tokyo, Japan) and subjected to sequencing analysis using an ABI PRISM® 3100 Genetic Analyzer with a BigDye™ Terminator v3.1 Cycle Sequencing Ready Kit (Applied Biosystems, Foster City, CA, USA). The nucleotide sequences of the PCR products were subjected to a BLAST search against the nucleotide collection consisting of GenBank, EMBL, DDBJ, PDB, and RefSeq sequences. Subsequently, the nucleotide sequence of each gene and the concatenated sequences of both genes were employed for multiple sequence alignment using the CLUSTAL W 2.1 alignment program in the DNA Data Bank of Japan (DDBJ). Construction of the phylogenetic trees used 444 bp and 555 bp long sequences of the P4b and DNA polymerase genes, respectively. The phylogenetic trees were constructed by the Neighbor-Joining method [11], and the topological accuracy of the tree was estimated using the bootstrap method, with 1,000 replicates. The phylogenetic trees were drawn with Njplot® (http://doua.prabi.fr/software/njplot) [9].
RESULTS
Gross appearance, course of the disease, and spread of the disease within flocks
Case No. 1 was one of the affected birds found in captive flocks of Japanese cormorants in Miyoshi City, Hiroshima Prefecture, Japan. On June 16th, 2017, a total of six cormorants were introduced into two different flocks in a rearing facility. All of the cormorants were young birds, which were caught on Ishihama beach between May and early June 2017. Approximately two weeks after the introduction, five birds of the newly introduced cormorants developed facial nodules. Although the facial nodules did not cause deterioration in their general condition, one of the affected birds (Case No. 1) underwent surgical resection of the nodules under anesthesia for therapeutic and diagnostic purposes. Case No. 1 developed multiple coalescent nodules around the base of the upper beak (Fig. 1). The total size of the coalescent nodules was 3 × 5 cm, and the nodules were black or reddish-black on the surface (Fig. 1). The cut surface of the resected nodules was black with some white patches and streaks. After surgery, the bird was given antibiotics; the surgical wound healed within a few weeks. In the remaining four affected cormorants, facial nodules spontaneously healed without any treatment. While Case No. 1 and another affected young bird were reared together with five resident cormorants, the disease did not spread within the flock. Similarly, while the remaining young cormorants were introduced into another flock, the facial nodules were found only in the three new cormorants.
Fig. 1.

Gross appearance of coalescent nodules around the base of the upper beak of a Japanese cormorant (Case No. 1).
During approximately the same time, an affected cormorant (Case No. 2) was also found in a flock in Iwakuni City, Yamaguchi Prefecture, which is located over 90 km away from Miyoshi City. Case No. 2 developed a nodular lesion on the face that was 2 × 1 cm in size and black in colour. Pieces of the facial lesion were manually excised for diagnosis in late July 2017, and after, the lesion spontaneously healed without additional treatment. Notably, this bird was also a young cormorant introduced from Ishihama beach during the same spring.
Over the next year, the cormorants, including Cases No. 1 and 2, did not show any signs of recurrence.
Histopathological findings
The histopathological examination of Case No. 1 revealed that the facial nodules were composed of severe epidermal hyperplasia, with ballooning degeneration of the spinous cells containing large ring-shaped eosinophilic intracytoplasmic inclusions known as Bollinger bodies (Fig. 2A and 2B). The hyperplastic epidermis was thrown into papillary folds (Fig. 2A). In large parts of the nodules, the surface area was covered by thick coagulative necrotic tissue of the hyperplastic epidermis with bleeding. In the upper spinous layer of the hyperplastic epidermis, the epidermal cells with Bollinger bodies had dense eosinophilic cytoplasm, as observed in healthy epidermis but became necrotic before full keratinization (Fig. 2B). Bacterial colonies were frequently found at the surface of the necrotic tissue, and moderate infiltration of heterophils and macrophages was observed in the dermis, at the base of the lesion.
Fig. 2.
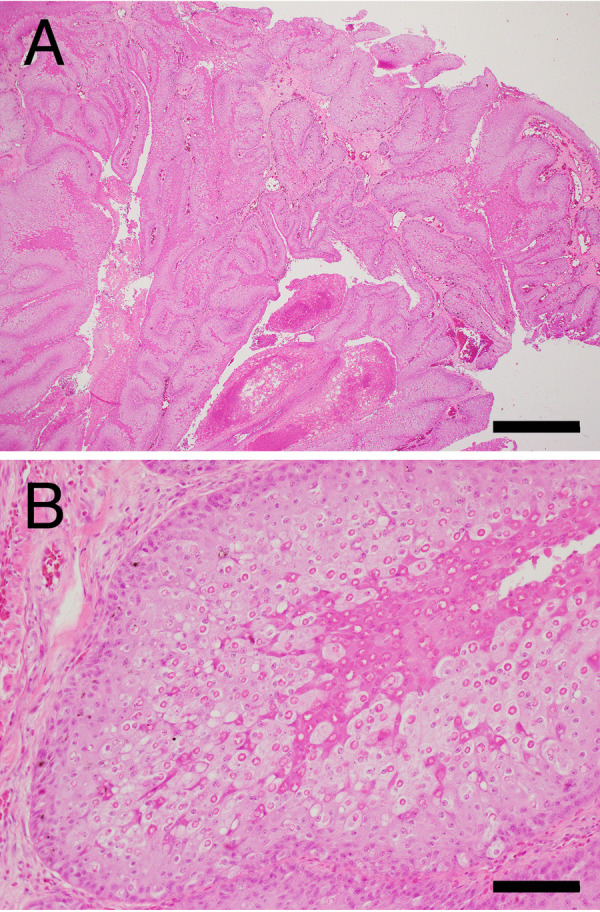
Histopathology of the cutaneous nodules of Case No. 1. hematoxylin and eosin staining. (A) Low-magnification. Bar=1 mm. (B) Higher magnification of (A). Bar=100 µm.
Although the lesion in Case No. 2 showed advanced autolysis, possibly due to freezing preservation before formalin fixation, we found similar histological changes characterized by epidermal hyperplasia with Bollinger bodies. A secondary bacterial infection was also observed.
Molecular detection and characterization of APV
Products of the expected sizes were obtained from the lesions of both cases by PCR for APV P4b and DNA polymerase genes. Excluding the primer sequences used for PCR, subsequent nucleotide sequences of PCR products were determined (accession numbers LC481450–LC481453). It is worth noting that the nucleotide sequence of each detected gene from both samples were identical to one another. Furthermore, the BLAST search revealed that there were no sequences identical to those of the P4b and DNA polymerase genes detected from both cormorants in the database.
Additionally, the BLAST search revealed several sequences with a high degree of similarity. In the case of the P4b gene, the sequence of the Flamingopox virus (accession number: MF678796.1) showed 99% similarity. Phylogenetic analyses using the concatenated sequences of APV P4b and DNA polymerase genes showed that detected APV in Japanese cormorants clustered in subclade A3 (Fig. 3), which consists mainly of APVs from marine birds [3]. Both sequences clustered with detected sequences from common murre (Uria aalge), Magellanic penguin (Spheniscus magellanicus), southern giant petrel (Macronectes giganteus), Laysan albatross (Diomedea immutabilis), and pelagic cormorant (Phalacrocorax pelagicus). Moreover, nearly identical phylogenic relationships were also detected in phylogenetic trees constructed with the sequences of the P4b gene or DNA polymerase gene (Supplementary Figs. 1 and 2). On the contrary, the detected sequences differed from the sequences of the Fowlpox virus, Pigeonpox virus, Sparrowpox virus, and other APV species.
Fig. 3.
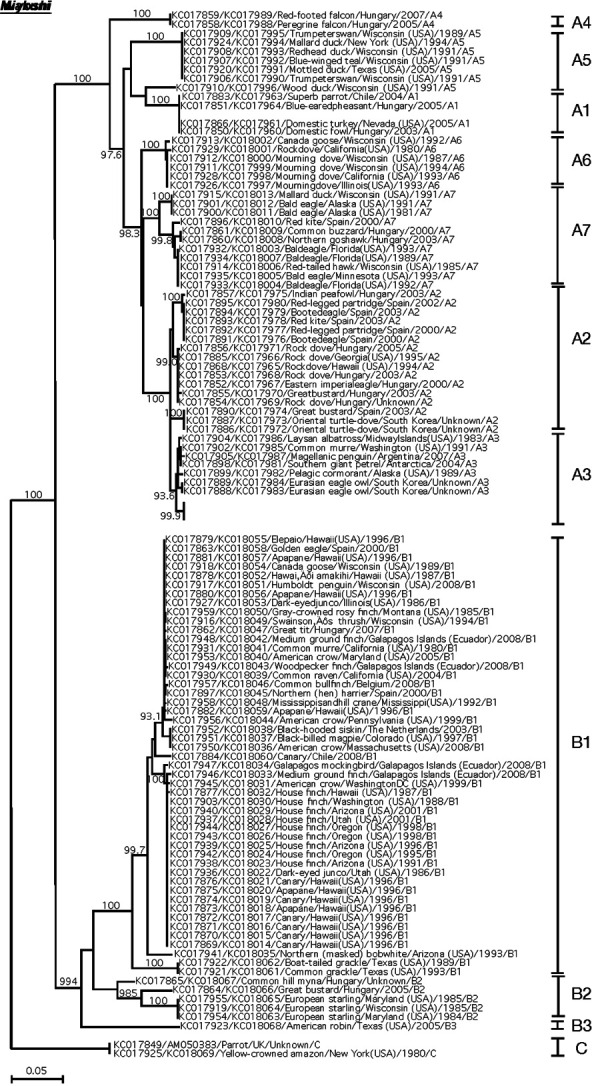
Neighbor-joining phylogenetic tree based on concatenated DNA sequences encoding 4b core protein and DNA polymerase of avipoxviruses (APVs). APV clades A to C and subclades are labeled according to the nomenclature of Gyuranecz et al. [3]. The sequences obtained from Japanese cormorants in this study are indicated in italicized underlined bold face. Both sequences are clustered in subclade A3, which represents APVs of marine birds [3]. All other sequences are indicated as accession number/host species/country/year/clade and subclade number. The bootstrap probabilities, as determined for 1,000 resampling, larger than 900 are given.
DISCUSSION
The pathological and molecular findings of the present cases were consistent with previously reported cases on avipoxvirus infection [2, 6, 10, 20]. To the best of our knowledge, these were the first cases of avian pox in Japanese cormorants. Whereas APV infection is rarely fatal in most species, mortality can reach up to 50% in cases of some species [2, 4, 17]. In the present report, all of the affected Japanese cormorants recovered from the avian pox and did not show any signs of recurrence, which suggests that the detected APV would not result in a highly lethal infection in Japanese cormorants. On the other hand, the lethal potential of an APV infection might depend primarily on the affected sites rather than on the virulence of the virus; for example, facial lesions can lead to debilitation and death by interference with sight, breathing, and eating [12, 15, 16, 19]. In cases of potentially harmful lesions, surgical resection could be a therapeutic option for cormorants affected by the avian pox, as performed in Case No. 1. In this study, avian pox did not spread within the flocks. Although we cannot exclude the possibility that the resident cormorants had acquired immunity against APV by previous infection, this finding suggests that the APV detected in Japanese cormorants may not be highly infectious.
PCR amplification and direct sequencing of APV P4b and DNA polymerase genes revealed that there were no differences in the partial nucleotide sequences of both genes between cases, indicating that both of the affected cormorants were infected with an identical APV near the same time. Notably, all of the affected cormorants found in Miyoshi and Iwakuni were young birds captured on Ishihama beach in the spring of that year. It is reasonable to assume that the cormorants had been infected with APV before transportation, and the infection became clinically apparent in the respective cities. In fact, the captured cormorants were reared together in a single cage until transportation. Our hypothesis is also supported by the fact that suspected cases of avian pox were also found in Japanese cormorants, which were captured on Ishihama beach and transported to other cities in that year. Although detailed information on the individual cases was not available, the suspected cases were found in at least four cities other than Miyoshi and Iwakuni.
A large-scale phylogenetic analysis of APVs based on gene sequences of APV P4b and DNA polymerase showed avian family-specific grouping within clades, indicating a specific role of host adaptation in APV evolution [3]. Consistently, in this study, the APV detected in Japanese cormorants was included in subclade A3 together with pelagic cormorants in the phylogenetic tree. The sequences of P4b and DNA polymerase genes of the detected APV were not identical to those of any other APVs in the database, including the Fowlpox virus, Pigeonpox virus, Sparrowpox virus, and other APV species, which may be distributed in the region where the outbreaks occurred. Clinically, the infected cormorants developed lesions of substantial size (Fig. 1), and APVs are generally assumed to be more pathogenic in their natural hosts [17]. The clinical signs observed in the present cases and the phylogenetic analyses implied that the Japanese cormorant is the natural host of the APV detected in this study. However, as some APV strains can infect several groups or species of birds [4], we cannot rule out the possibility that a previously unreported APV derived from other marine birds was introduced into the flocks of Japanese cormorant. However, judging from the results of the phylogenetic analyses, it is unlikely that Japanese cormorants were infected with APVs derived from terrestrial birds during and/or after transportation.
In the current report, we described pathological and molecular findings of the first cases of APV infection in Japanese cormorants, providing useful information in the diagnosis and prevention of avian pox in cormorants. It would be necessary to carefully monitor the avian pox in both captive and wild Japanese cormorants.
Supplementary Material
Acknowledgments
We thank Mr. Bungo Hisaka and the Miyoshi City Tourism Association for providing an opportunity to examine the present cases.
REFERENCES
- 1.Bolte A. L., Meurer J., Kaleta E. F.1999. Avian host spectrum of avipoxviruses. Avian Pathol. 28: 415–432. doi: 10.1080/03079459994434 [DOI] [PubMed] [Google Scholar]
- 2.Fukui D., Nakamura M., Yamaguchi T., Takenaka M., Murakami M., Yanai T., Fukushi H., Yanagida K., Bando G., Matsuno K., Nagano M., Tsubota T.2016. An Epizootic of emerging novel avian pox in carrion crows (corvus corone) and large-billed crows (corvus macrorhynchos) in Japan. J. Wildl. Dis. 52: 230–241. doi: 10.7589/2015-07-172 [DOI] [PubMed] [Google Scholar]
- 3.Gyuranecz M., Foster J. T., Dán Á., Ip H. S., Egstad K. F., Parker P. G., Higashiguchi J. M., Skinner M. A., Höfle U., Kreizinger Z., Dorrestein G. M., Solt S., Sós E., Kim Y. J., Uhart M., Pereda A., González-Hein G., Hidalgo H., Blanco J. M., Erdélyi K.2013. Worldwide phylogenetic relationship of avian poxviruses. J. Virol. 87: 4938–4951. doi: 10.1128/JVI.03183-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen W.1999. Avian pox. pp. 163–170. In: Field Manual of Wildlife Diseases: General Field Procedures and Diseases of Birds (Friend, M. and Franson, J. C. eds.), US Geological Survey, Madison. [Google Scholar]
- 5.Jarmin S., Manvell R., Gough R. E., Laidlaw S. M., Skinner M. A.2006. Avipoxvirus phylogenetics: identification of a PCR length polymorphism that discriminates between the two major clades. J. Gen. Virol. 87: 2191–2201. doi: 10.1099/vir.0.81738-0 [DOI] [PubMed] [Google Scholar]
- 6.Lecis R., Secci F., Antuofermo E., Nuvoli S., Scagliarini A., Pittau M., Alberti A.2017. Multiple gene typing and phylogeny of avipoxvirus associated with cutaneous lesions in a stone curlew. Vet. Res. Commun. 41: 77–83. doi: 10.1007/s11259-016-9674-5 [DOI] [PubMed] [Google Scholar]
- 7.Lüschow D., Hoffmann T., Hafez H. M.2004. Differentiation of avian poxvirus strains on the basis of nucleotide sequences of 4b gene fragment. Avian Dis. 48: 453–462. doi: 10.1637/7111 [DOI] [PubMed] [Google Scholar]
- 8.Manarolla G., Pisoni G., Sironi G., Rampin T.2010. Molecular biological characterization of avian poxvirus strains isolated from different avian species. Vet. Microbiol. 140: 1–8. doi: 10.1016/j.vetmic.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 9.Perrière G., Gouy M.1996. WWW-query: an on-line retrieval system for biological sequence banks. Biochimie 78: 364–369. doi: 10.1016/0300-9084(96)84768-7 [DOI] [PubMed] [Google Scholar]
- 10.Saito K., Kodama A., Yamaguchi T., Gotoh Y., Sakai H., Fukushi H., Masegi T., Yanai T.2009. Avian poxvirus infection in a white-tailed sea eagle (Haliaeetus albicilla) in Japan. Avian Pathol. 38: 485–489. doi: 10.1080/03079450903349246 [DOI] [PubMed] [Google Scholar]
- 11.Saitou N., Nei M.1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4: 406–425. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt R. E., Reavill D. R., Phalen D. N.2015. Pathology of Pet and Aviary Birds, 2nd ed., John Wiley & Sons, Hoboken. [Google Scholar]
- 13.Shimba T.2008. A Photographic Guide to the Birds of Japan and North-east Asia, Yale University Press, New Haven. [Google Scholar]
- 14.Skinner M. A., Buller R. M., Damon I. K., Lefkowitz E. J., McFadden G., McInnes C. J., Mercer A. A., Moyer R. W., Upton C.2011. Family Poxviridae. In: Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses (King, A. M., Lefkowitz, E., Adams, M. J. and Carstens, E. B. eds.), Academic Press, London. [Google Scholar]
- 15.Tarello W.2008. Prevalence and clinical signs of avipoxvirus infection in falcons from the Middle East. Vet. Dermatol. 19: 101–104. doi: 10.1111/j.1365-3164.2008.00656.x [DOI] [PubMed] [Google Scholar]
- 16.van Riper C., III, Forrester D. J.2007. Avian pox. pp. 131–176. In: Infectious Diseases of Wild Birds (Thomas, N. J., Hunter, D. B. and Atkinson, C. T. eds.), Blackwell Publishing, Ames. [Google Scholar]
- 17.Weli S. C., Tryland M.2011. Avipoxviruses: infection biology and their use as vaccine vectors. Virol. J. 8: 49. doi: 10.1186/1743-422X-8-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weli S. C., Traavik T., Tryland M., Coucheron D. H., Nilssen O.2004. Analysis and comparison of the 4b core protein gene of avipoxviruses from wild birds: evidence for interspecies spatial phylogenetic variation. Arch. Virol. 149: 2035–2046. [DOI] [PubMed] [Google Scholar]
- 19.Wernery U.2008. Viral diseases. pp. 358–392. In: Avianedicine (Samour, J. ed.), Mosby, Edinburgh. [Google Scholar]
- 20.Yu M. H. H., Tsuyoshi Y., Miyano N., Shimizu H., Murai A., Yanai T., Masegi T., Ohya K., Fukushi H.2007. Fowlpox virus infection in a captive Japanese Rock Ptarmigan (Lagopus mutus japonicus). Jpn. J. Zoo Wildl. Med. 12: 77–80. doi: 10.5686/jjzwm.12.77 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


