Abstract
The past decade has witnessed a proliferation of studies aimed at characterizing the human connectome. These projects map the brain regions comprising large-scale systems underlying cognition using non-invasive neuroimaging approaches and advanced analytic techniques adopted from network science. While the idea that the human brain is composed of multiple macro-scale functional networks has been gaining traction in cognitive neuroscience, the field has yet to reach consensus on several key issues regarding terminology. What constitutes a functional brain network? Are there “core” functional networks, and if so, what are their spatial topographies? What naming conventions, if universally adopted, will provide the most utility and facilitate communication amongst researchers? Can a taxonomy of functional brain networks be delineated? Here we survey the current landscape to identify six common macro-scale brain network naming schemes and conventions utilized in the literature, highlighting inconsistencies and points of confusion where appropriate. As a minimum recommendation upon which to build, we propose that a scheme incorporating anatomical terminology should provide the foundation for a taxonomy of functional brain networks. A logical starting point in this endeavor might delineate systems that we refer to here as “occipital”, “pericentral”, “dorsal frontoparietal”, “lateral frontoparietal”, “midcingulo-insular”, and “medial frontoparietal” networks. We posit that as the field of network neuroscience matures, it will become increasingly imperative to arrive at a taxonomy such as that proposed here, that can be consistently referenced across research groups.
Keywords: coactivation, functional connectivity, human connectome, network neuroscience
Introduction
As fields of science mature, they formalize by adopting standardized terminology. In biology, for example, the taxonomic categories of kingdom, phylum, class, order, family, genus and species are universally accepted and utilized to communicate new research findings. Such classification systems are grounded in accepted principles specific to a given field, and their consistent usage greatly facilitates discovery and progress in scientific inquiry. In the imaging neurosciences, the adoption of standardized 3-dimensional coordinate systems such as those utilized in the Talairach atlas (Talairach and Tournoux 1988) and later the Montreal Neurological Institute (MNI) atlas (Collins et al. 1994) revolutionized neuroimaging by providing a means for researchers to compare results across different studies using common reference points.
The emerging field of network neuroscience aims to understand the principles and mechanisms underlying cognition and behavior by studying structural and functional brain networks (Bassett and Sporns 2017). Improved neuroimaging data acquisition protocols, computational advances, and population neuroscience data sharing initiatives have contributed significant insights over the past decade (Van Essen et al. 2013; Zhou et al. 2019). Yet, theoretical advances have not always kept pace with these methodological innovations and achievements. As an example, the notion of “large-scale neurocognitive networks” describing the neural architecture subserving cognition and behavior has persisted for nearly thirty years. Even before the widespread use of non-invasive neuroimaging, neurologists theorized based on lesion studies that cognitive processes including attention, language, and memory rely on distributed processing within “multi-focal neural systems” rather than specific anatomical sites (Mesulam 1990). However, we have yet to arrive at a clear definition of what precisely constitutes a large-scale neurocognitive network. Contemporary network neuroscience is fragmented due to the lack of consistent naming conventions. Consider the three statements below:
“The cingulo-opercular network includes the anterior prefrontal cortex, anterior insula/frontal operculum, dorsal anterior cingulate cortex and thalamus” (Dosenbach et al. 2008).
“The anterior insular cortex is thought to be a key node of a salience network that also includes the dorsal anterior cingulate cortex and other subcortical and limbic structures” (Uddin 2015).
“Core regions of the ventral [attention] network include temporoparietal junction…and ventral frontal cortex, including parts of the middle frontal gyrus, inferior frontal gyrus, frontal operculum, and anterior insula” (Corbetta et al. 2008).
In all of these cases, the authors refer to a functional brain network that includes the anterior insula. In these three instances, the authors use specific terms to refer to the networks of interest (“cingulo-opercular network”, “salience network”, and “ventral attention network”) and go on to ascribe different - if partially overlapping - functions to them. This proliferation of terminology is particularly problematic when one attempts to integrate information across multiple empirical investigations. Indeed, one can imagine a scenario in which a researcher might search for studies investigating the role of the anterior insula in the ventral attention network but be completely unaware of relevant publications using the salience or cingulo-opercular network terminology.
If we were to synthesize the claims implicit in these descriptions, we might posit that a single brain region, the anterior insula, participates in multiple functional networks. The anterior insula example highlights the ubiquitous “many-to-many mapping” dilemma which arises when we consider structure-function mapping in the brain. The fact that the anterior insula is thought to participate in multiple large-scale brain networks is perhaps not surprising considering that this region shows diverse patterns of co-activation (Uddin et al. 2014), dynamic functional connectivity (Nomi et al. 2016), and structural connectivity (Nomi et al. 2018) consistent with this capacity. Yet, network naming conventions that have been widely adopted by researchers in the field have yet to sufficiently capture this complexity. Some have acknowledged this explicitly, conceding both that individual brain regions participate in many functions, and that many functions are carried out by multiple brain regions (Pessoa 2014). Others have posited the existence of domain general, distributed structure-function mappings that account for a range of cognitive phenomena (Barrett and Satpute 2013). Alongside these conceptualizations, the “neural context” hypothesis - the idea that the functional relevance of a brain area depends on the status of other connected areas (McIntosh 2004) - provides another illustration of the difficulties inherent to structure-function mapping in the brain. The neural context hypothesis has been extended to the whole-brain level, where it has been shown that static localized networks are superordinate approximations of underlying dynamic states (Ciric et al. 2017). In light of these considerations, any attempt to derive a universal taxonomy of functional brain networks must balance the need for communication amongst researchers investigating similar phenomena with the desire to accurately represent the dynamic, hierarchical nature of the brain.
While there are complex dynamics at play when we observe large-scale neurocognitive systems, a remarkable degree of consensus has been obscured by disparate network characterizations. We begin by briefly surveying how functional brain networks are currently (inconsistently and incompletely) defined. We explore the question of how many networks are thought to exist at the macro-scale, as well as their purported anatomical configurations and dynamic properties. Finally, we outline a proposal for a suggested universal network naming scheme, or taxonomy, that should facilitate future cross-study comparisons, meta-analyses, and empirical investigations.
How is a functional brain network defined? How many functional brain networks are there?
A fundamental construct in neuroscience is the definition of a brain area. Brain areas are defined by their functional specificity, connectivity, architectonics and topographic organization (Felleman and Van Essen 1991; Van Essen and Glasser 2018; Eickhoff et al. 2018b). Not all four criteria are met in defining brain areas. Substantial effort by neuropsychologists and cognitive neuroscientists has delineated the putative functions of many regions of the brain. Interconnected brain areas form large-scale networks, observable at the macro-scale. What constitutes a connection between brain regions for a functional network is typically a statistical dependency, such as a correlation or covariance (Friston 1994). Stable functional networks are likely underpinned by mono- or poly-synaptic white matter connections (Lu et al. 2011). Critically, the functional interactions of the brain regions comprising a network, both within the network and with the rest of the brain, lead to the emergence of complex behavior that is likely more than additive of the discrete computations of each region alone (Mesulam 1990).
Brain networks are characterized in graph theory as comprising nodes (brain regions) and edges (connections). By examining patterns of pairwise associations and network-level properties, graph theory has been extremely successful in characterizing the architecture of the brain. However, not all graphs, or brain networks characterized by network statistics, are equal. A common approach to studying the functional network architecture of the brain is to examine the functional connectivity between approximately equally-sized segments of cortex. However, nodes (parcels, vertices or voxels) rarely constitute brain areas as defined above (Wig et al. 2011). Network neuroscientists have been successful in delineating large-scale systems, as well as some of their functional attributes (Betzel and Bassett 2017). Regrettably, much of the fine-grained information related to the function of specific areas is lost as the pieces are broken up and put back together agnostically without consideration of any of their relevant functional or structural properties. A critical way forward in determining the cognitive network neuroscience architecture of the human brain will be to assemble networks from brain regions. For this reason, our proposed solution to the number of networks will be low. There is structure within every level of analysis in the human brain, and solutions depend upon the unit of measurement and analysis, as we discuss next. This “resolution issue” will account for some of the variability in characterizing the number of brain networks based on analytic approaches. Here we focus on functional brain networks, though similar issues of node and edge definition arise when considering structural properties (Eickhoff et al. 2018a, b).
It is important to note that different network definitions and node selection procedures can create confusion in the literature. Different naming conventions for brain areas and idiosyncratic seed (region-of-interest, ROI) selection during network construction can further contribute to apparent inconsistencies in the network neuroscience literature.
The question How many functional brain networks are there? is ultimately ill-posed given the hierarchies inherent to the network architecture of the brain. Organization is observed at multiple levels of analysis in neuroscience (Sejnowski et al. 1988). A multi-resolution decomposition of large-scale functional networks into functional areas with hierarchical ordering has recently been demonstrated (Urchs et al. 2019). Coarse- and fine-grained network parcellations both provide valid solutions for network analysis. Nevertheless, low model order independent component analysis (ICA), meta-analysis of task-fMRI (Smith et al. 2009), and whole-brain parcellation studies (Yeo et al. 2011) provide the basis for our claim that six networks represent a reasonable starting point for taxonomy building. Rather than continue with a proliferation of network names based on idiosyncratic findings, we suggest that the field should embrace a common nomenclature to provide a basis for integration of findings across a fractionated literature.
Some of the earliest resting state fMRI studies to delineate multiple macro-scale networks arrived at 5 (De Luca et al. 2006) and 10 (Damoiseaux et al. 2006) networks, respectively. These earlier works, combined with evidence from parcellation studies for seven (Yeo et al. 2011) and five (Doucet et al. 2019) networks observable at the macro-scale, were considered in our current proposal. As it will likely be easier for the community to agree upon a small set of core networks rather than a larger number, our proposal here centers around six functional brain networks that appear ubiquitously in both task and resting state fMRI investigations. In an effort towards standardization, here we call these the occipital network (ON), pericentral network (PN), dorsal frontoparietal network (D-FPN), lateral frontoparietal network (L-FPN), midcingulo-insular network (M-CIN), and medial frontoparietal network (M-FPN) (Figure 1).
Figure 1. Taxonomy of functional brain networks.
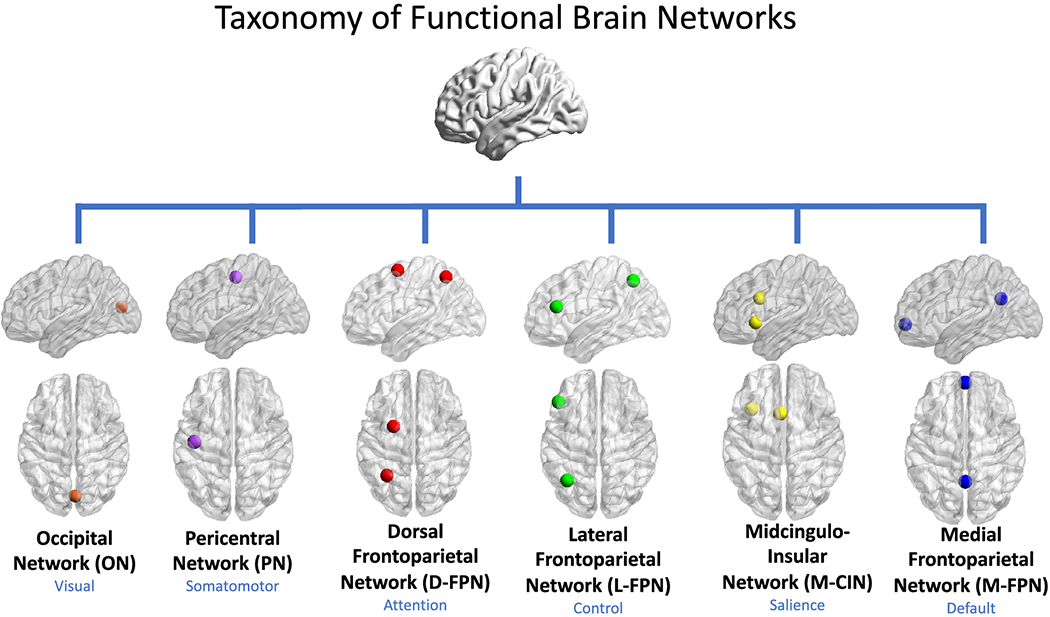
In our proposed taxonomy, networks are referred to by anatomical names that best describe six ubiquitous large-scale functional systems. The names in blue refer to the broad cognitive domains with which a given anatomical system is most commonly associated. Only 1-2 core nodes of each network are depicted here, though it is understood that multiple additional cortical, subcortical, and cerebellar nodes may be affiliated with a given network.
Our proposed taxonomy is cortico-centric at present, though subcortical and cerebellar structures associated with each network are delineated where adequate information permits. Subcortical and cerebellar nodes are clearly associated with each of the networks we discuss. In the interest of focusing on the issue of network nomenclature, rather than network composition, we refer the reader to several previous works that have carefully delineated these components (Buckner et al. 2011; Choi et al. 2012; Ji et al. 2019).
At higher resolution, the six networks identified here will fractionate into subsystems (for example, the dissociation of the language network from the default network, and primary from secondary visual regions (Ji et al. 2019)). It is important to remember that fractionated systems will likely show greater functional affiliation within the broader macro-scale network than between macro-scale networks, though time-varying analysis may reveal dynamic affiliations of brain regions with areas outside their core networks (Uddin 2014).
Resting-state functional connectivity
Large-scale brain networks have been successfully delineated using an approach referred to as resting-state functional connectivity (RSFC). This approach examines synchronized patterns of spontaneous oscillations in blood-oxygen level dependent (BOLD) signal measured at rest with MRI (Biswal et al. 1995)(see (Fox and Raichle 2007) for review). Some of the earliest studies using RSFC to delineate macro-scale functional brain networks used ICA. ICA is a model-free approach that decomposes neuroimaging datasets into a set of independent onedimensional time series and associated three-dimensional spatial maps that describe the temporal and spatial characteristics of the underlying signals (Beckmann et al. 2005). Many investigators using ICA label the derived components by letter (Damoiseaux et al. 2006) or number (De Luca et al. 2006) in publication figures, while speculating on possible functional interpretations of these coherent systems in the text. In practice, ‘naming’ of networks is often an ad hoc process by the investigators, who may or may not choose to label networks derived from resting state fMRI data on the basis of spatial similarity with activation patterns seen in task fMRI datasets (Smith et al. 2009).
Importantly, the dimensionality of ICA, or the number of networks, can be set by the user or estimated from the data. Therefore, ICA cannot be used in isolation to determine the absolute number of large-scale functional networks. ICA is useful, however, for producing data-driven components constituted by functionally connected brain regions. Investigators often select lower model order ICA (eg. 20 components or fewer) when attempting to recover macro-scale functional networks (Ray et al. 2013), and higher model order ICA (eg. 100 components or greater) when aiming to achieve brain parcellation (Kiviniemi et al. 2009) or delineate ROIs to be used in subsequent analyses (Allen et al. 2014). An interesting point to note is that as higher model orders effectively break down larger components into smaller ones, ICA can provide information regarding network hierarchies (Smith et al. 2009).
One network identification scheme derived from resting state fMRI data that has been very influential is that proposed by Yeo and colleagues (Yeo et al. 2011). Yeo and colleagues used a clustering algorithm to parcellate the cortex into networks of functionally coupled brain regions using two large samples (n=500 each). The assumption here and in similar resting state fMRI parcellation work (e.g. (Power et al. 2011)) is that cortical networks can be defined as sets of regions with similar profiles of cortico-cortical functional connectivity. Here, the authors struggle with the question regarding the number of networks that need to be specified, ultimately deciding that none of their conclusions depend on a strong assumption that there is one correct answer. They go on to examine the stability of the derived clusters, to arrive at a coarse (7-network) and finer (17-network) solution. Still, they are cautious to state that the focus on 7- and 17-network solutions should not be taken to imply that meaningful properties are absent in alternative schemes (Yeo et al. 2011). Indeed, in that same work, they demonstrate that multiple network solutions exhibit similar levels of stability, underscoring the point that there is often no one correct solution or number of networks.
With regards to the question of what to call these networks, the authors are again careful. While they provide common names associated with each network (eg. visual, somatomotor, dorsal attention, ventral attention, limbic, frontoparietal, default), in a figure caption they state: “This should not be taken to mean that our estimated networks correspond exactly to those in the literature or that the networks code solely for functions associated with their assigned name. As examples of limitations of heuristic reference labels, the violet ventral attention network is likely an aggregate of (or closely adjacent to) multiple networks in the literature variably referred to as the salience (Seeley et al. 2007) and cingulo-opercular networks (Dosenbach et al. 2007), and the red default network can be fractionated (e.g., (Andrews-Hanna et al. 2010)).”
Similar caveats can also be seen in other work. For example Farrant and Uddin (Farrant and Uddin 2015) note in their work that while some investigators see the high degree of functional and anatomical overlap between the ventral attention network and salience network as evidence that they are part of the same system (Kucyi et al. 2012), others have conceptualized these networks as distinct entities (Power et al. 2011; Cole et al. 2013). Unfortunately, this type of nuance is not always evident in the broad network neuroscience literature. As no universally accepted network naming convention currently exists, researchers continue to adopt their own preferred nomenclature in publications, contributing to a greater proliferation of network naming schemes.
Task-activation and meta-analysis
Another way to define functional networks is by examining patterns of task co-activation, and amalgamating these results through meta-analyses to discover reliable network nodes. The first such successful meta-analytic approach led to the discovery of the ubiquitous medial frontoparietal network. Reliable decreases in blood flow were found during active visual tasks in posterior cingulate, inferior parietal cortex, medial prefrontal cortex, and other regions (Shulman et al. 1997). It was only later that this constellation of regions was described as active “by default” (Raichle et al. 2001), and subsequently referred to as the “default mode network” upon demonstration of functional connectivity between its key nodes (Greicius et al. 2003). This network has been reliably observed to be suppressed during many tasks that require visuospatial attention and has been referred to as the “task-negative network” for its antiphase and largely antagonistic relationship with the dorsal frontoparietal attention network (Fox et al. 2005) and other lateral frontoparietal networks (Sridharan et al. 2008) (see also (Dixon et al. 2017)). This unfortunate “task-negative” nomenclature has obscured the active role of the default network in numerous forms of cognition. Meta-analytic evidence suggests that this network is involved in memory processes, such as recollection, as well as social reasoning (Spreng et al. 2009). However, inquiry into cognition is often siloed into discrete domains of research, and a common set of co-active brain regions have been named for discrete cognitive functions, with limited cross-talk and enriched understanding of how these seemingly diverse set of functions may rely on core mechanisms. For example:
“Recollection - retrieval of qualitative information about a past event - is associated with enhanced neural activity in a consistent set of neural regions (the ‘core recollection network’)…including the hippocampus, angular gyrus, medial prefrontal cortex, retrosplenial/posterior cingulate cortex, and middle temporal gyrus” (Thakral et al. 2017).
“The mentalizing system - consisting of the temporo-parietal junction, the medial prefrontal cortex and the precuneus - is activated when behavior that enables inferences to be made about goals, beliefs or moral issues presented in abstract terms” (Van Overwalle and Baetens 2009).
“The neural systems specialized for storage and retrieval of semantic knowledge are widespread and occupy a large proportion of the cortex in the human brain. The areas implicated in these processes can be grouped into 3 broad categories: posterior heteromodal association cortex (AG, MTG, and fusiform gyrus), specific subregions of heteromodal prefrontal cortex (dorsal, ventromedial, and inferior prefrontal cortex), and medial paralimbic regions with strong connections to the hippocampal formation (parahippocampus and posterior cingulate gyrus)” (Binder et al. 2009).
Similar to our earlier example centered on the anterior insula, a common nomenclature for the underlying network architecture could enrich the cognitive characterization of these systems (e.g. (Spreng and Andrews-Hanna 2015)). Some of the imprecision in the field is explained by an incomplete correspondence between RSFC networks and task coactivation patterns. While there is a broad convergence between task-evoked networks (Smith et al. 2009; Laird et al. 2011; Yeo et al. 2016) and resting-state fMRI derived networks (Yeo et al. 2011), there is not a perfect match. In many cases, resting state networks appear to be more broadly distributed across the cortex, whereas task-evoked networks often appear more circumscribed (Yeo et al. 2016). One speculation based on this observation is that resting state networks might represent the full functional repertoire of brain modes, from which tasks engage subsets of regions as revealed by subtraction of tightly-matched control conditions. Projects such as the “cognitive atlas” (Poldrack et al. 2011) and the “Cognitive Paradigm Ontology” (Turner and Laird 2012) that aim to systematically characterized mental processes provide critical empirical data with which one can begin to delineate task-evoked networks. Of note, the ubiquitous antagonistic brain activation/deactivation pattern between dorsal frontoparietal attention and medial frontoparietal default network brain regions, discussed above, can be recapitulated using such meta-analytic approaches (Bolt et al. 2017b; Toro et al. 2008). While an in depth discussion of this issue is beyond the scope of the current work, it is worth bearing in mind that the degree of correspondence between rest and task functional network configurations is a topic of ongoing investigation (Cole et al. 2014; Krienen et al. 2014; Bolt et al. 2017a).
Complex cognition may also evoke multiple, and interacting, networks. For example, working memory for visuospatial information will engage both the lateral frontoparietal control and dorsal frontoparietal attention networks; whereas working memory for mnemonic information will evoke activity in both the lateral frontoparietal control network and medial frontoparietal default networks (Spreng et al. 2010, 2012). Inter-regional patterns of RSFC have revealed that particular regions within the lateral frontoparietal control network are more functionally aligned with either the medial default or dorsal frontopariatal attention networks (Spreng et al. 2013). These observations are consistent with a fractionation of the extended lateral frontoparietal control network into subsystems, with differing functional alignment depending upon task demands (Dixon et al. 2018). Depending on the spatial scale, it is likely that all large-scale neurocognitive networks will fractionate, revealing both network hierarchies and dissociable cognitive functions.
Partially owing to these complexities, network neuroscience has largely sidestepped several key issues with regards to terminology. A common nomenclature based upon shared neuroanatomy would greatly facilitate the integration of novel discoveries within a cognitive network neuroscience framework. Such integration has the potential to deeply enrich our understanding of the macro-scale network architecture of the human brain and ensure that findings from disparate subdisciplines can be more readily accessed and incorporated into theory.
Outline of a universal taxonomy of functional brain networks
As reviewed above, the emerging field of network neuroscience currently suffers from the lack of a consistent network taxonomy. This is particularly problematic in that it hinders successful interfacing with decades of findings from cognitive neuroscience. The only way we see to remedy this is to formally propose a consensus nomenclature, closely tied to human neuroanatomy. This proposal synthesizes observations from RSFC MRI, reliable patterns of functional coactivation from task-based fMRI, and cross-modal convergence where available. We suggest common nomenclature for six reliable macro-scale brain networks composed of specific core brain regions. For each, we provide a primary anatomical label, as well as a secondary, and necessarily broad, cognitive label. We note that these cognitive labels may need to be continuously revised as newer investigations suggesting previously unidentified functionality emerge. For this reason, we emphasize a priority to the anatomical network label. We name the core regions which comprise each network, noting that additional brain regions may participate in any given network through processes including dynamic affiliation (Pessoa 2014).
Because most networks are spatially distributed across cortical regions, anatomical labels reflect core regions for each network. Across studies, the extent of connectivity and coactivation can vary for many regions as a function of analytic approach, temporal signal-to-noise, and idiosyncratic task-dependent coactivation patterns. We denote these as zones that are less reliably characterized where appropriate. In addition, we point out cases in which networks appear to break down further into separable subsystems. In some instances, the nodes we delineate as central to a given network can be characterized as “core” or “hub” nodes as defined in the graph theoretical sense (van den Heuvel and Sporns 2011). Our proposed taxonomy includes brief summaries of the cognitive functions associated with each network, and previously used terms for the network to aid in organization of prior observations.
The proposal is that going forward, network neuroscientists and cognitive neuroscientists should endeavor to use the following nomenclature whenever possible in order to provide a common reference point for other investigators interested in similar questions. As discussed, the core networks we describe can often fractionate into multiple subsystems that may not yet be fully described or agreed upon. In the interest of parsimony, we recommend that researchers may benefit from using the broad anatomical network names suggested here, before further elaborating on the extent to which any given set of findings warrants the usage of additional nomenclature to more completely describe the network structure observed. For illustration purposes, we show examples from the literature of networks derived from resting-state fMRI parcellations (Yeo et al. 2011; Gordon et al. 2017c; Ji et al. 2019) and task-based fMRI (Toro et al. 2008; Smith et al. 2009; Corbetta and Shulman 2011; Niendam et al. 2012) that guide our taxonomy building project.
Anatomical name: Occipital network (ON)
Cognitive Domain: Visual network.
Core regions are the occipital lobe, including striate and extrastriate cortex (Figure 2). This network also likely includes the lateral geniculate nucleus of the thalamus. The cognitive label “visual” is applied to this network, as the system is robustly observed to be involved in visual processing.
Figure 2. Occipital network.

A) Medial (120), occipital pole (220), and lateral (320) visual areas (Smith et al. 2009). RSN = resting state network, BM = BrainMap meta-analytic activation maps. B) Purple and red visual networks in 17-network parcellation (Yeo et al. 2011). C) Medial (tan) and lateral (blue) visual networks (Gordon et al. 2017c).
Figure 2 illustrates several examples of occipital networks. In searching for correspondence between task-activation and ICA-derived resting state networks, Smith and colleagues observe three maps corresponding to medial, occipital pole, and lateral visual areas. Parcellations derived solely based on RSFC provide evidence for two visual networks, medial and lateral (Yeo et al. 2011; Gordon et al. 2017c). Taken together, these parcellation studies provided evidence for at least two subsystems associated with the ON, one situated more medially, associated with primary visual cortex along the calcarine sulcus, and another more laterally encompassing extrastriate areas involved in visual processing (Haxby et al. 1994).
Note that the dorsal and ventral visual streams (Goodale and Milner 1992) likely originate from this core occipital network. These streams have been referred to as the “where” and “what” pathways for visual object perception (Ungerleider and Haxby 1994).
Anatomical name: Pericentral network (PN)
Cognitive Domain: Somatomotor network.
Core regions are motor and somatomotor cortices, anterior and posterior to the central sulcus. Regions of the pericentral network additionally include the juxtapositional lobule (supplementary motor area) (Figure 3). Less well characterized zones include auditory cortex of the superior temporal gyrus, which is often encapsulated within this network in studies using RSFC. The cognitive label “somatomotor” is applied to this network for the system’s well-documented involvement in motor processes and somatosensory processing.
Figure 3. Pericentral network.
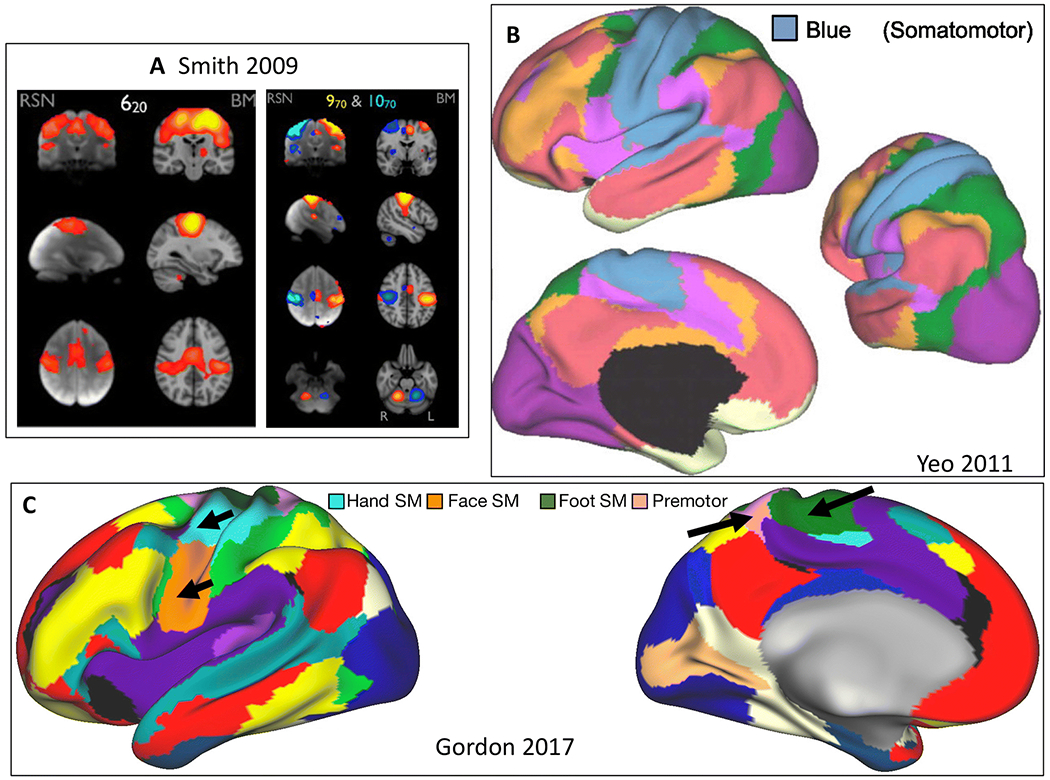
A) Sensorimotor areas in 20 (left) and 70 (right) component ICA solutions (Smith et al. 2009). RSN = resting state network, BM = BrainMap meta-analytic activation maps. B) Blue network in 7-network parcellation (Yeo et al. 2011). C) Hand (light blue), face (orange), and foot (green) somatomotor comprise three networks. Another network labeled auditory/premotor/parietal memory is also included (Gordon et al. 2017c).
At least two subsystems are likely associated with the PN. Left and right separation can be observed using high model order ICA (Smith et al. 2009), and dorsal (hand) and ventral (face) subsystems appear in some parcellations (Yeo et al. 2011) 17-network; (Gordon et al. 2017c). At higher resolution MRI, auditory and somatosensory face areas can also be separated (Kong et al. 2019). Note that the PN serves as the cortical component of both primary sensory and motor pathways.
Anatomical name: Dorsal frontoparietal network (D-FPN)
Cognitive Domain: Attention network.
Core regions include the superior parietal lobule extending into the intraparietal sulcus, middle temporal complex (MT+) and the putative frontal eye fields (BA8) (Figure 4). The dorsal frontoparietal network additionally includes ventral premotor cortex. Less well characterized zones are: 1) right-lateralized dorsolateral prefrontal cortex; 2) superior colliculus.
Figure 4. Dorsal frontoparietal network.
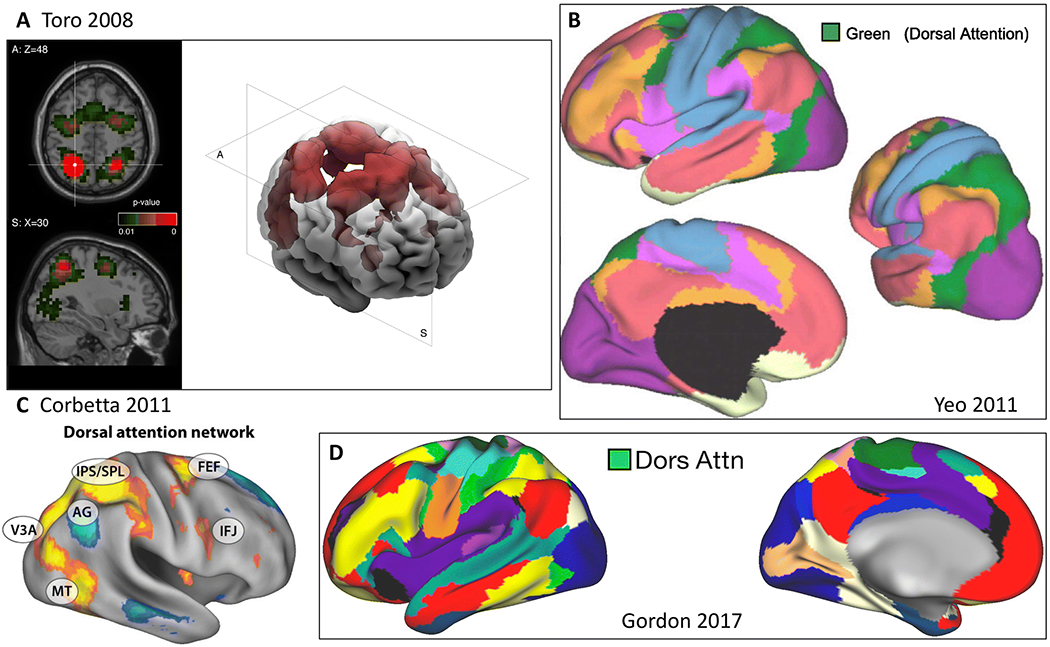
A) Coactivation map based on coordinates in left intraparietal cortex (Toro et al. 2008). B) Green network in 7-network parcellation (Yeo et al. 2011). C) Dorsal attention network (yellow) (Corbetta and Shulman 2011). IPS/SPL = intraparietal sulcus/superior parietal lobule, FEF = frontal eye fields, IFJ = inferior frontal junction. D) Dorsal attention network (green) (Gordon et al. 2017c).
Note that the proposed anatomical name for this network is the same as that originally proposed by Corbetta and Shulman (Corbetta and Shulman 2002). The cognitive label “attention” is applied to this network for the system’s broad role in visuospatial attention. The functions of this system include the previously identified processes of the “dorsal frontoparietal network” (Corbetta and Shulman 2002) which are to prepare and apply top-down selection for stimuli and responses. Interestingly, more recent findings have shown that the inferior frontal junction (IFJ), along the ventrolateral aspect of prefrontal cortex, also displays strong functional coupling with D-FPN regions during the voluntary deployment and maintenance of visuospatial attention. However, this pattern of IFJ connectivity shifts from the D-FPN to more ventrolateral regions during more stimulus-driven attention (Tamber-Rosenau et al. 2018). While this provides some evidence that the D-FPN may demonstrate a putative subnetwork architecture based on functional connectivity profiles, there remains little evidence to date that the D-FPN is composed of distinct subsystems. In resting state fMRI work, the D-FPN is commonly referred to as the “dorsal attention system” (Fox et al. 2006) or “dorsal attention network” (Yeo et al. 2011).
Anatomical name: Lateral frontoparietal network (L-FPN)
Cognitive Domain: Control network.
Core regions are lateral prefrontal cortex along the middle frontal gyrus (including rostral and dorsolateral prefrontal cortex) and the anterior inferior parietal lobule, into the intraparietal sulcus. Regions of the lateral frontoparietal network additionally include midcingulate gyrus (Figure 5). Less well characterized zones are: 1) dorsal precuneus; 2) posterior inferior temporal lobe, anterior to MT+; 3) dorsomedial thalamus and head of the caudate. This network can sometimes be separated into right and left lateralized systems, particularly with ICA (Smith et al. 2009). The cognitive label “control” is applied to the L-FPN for the system’s broad role in the executive, goal-directed, control of information flow in the brain. The functions of this system include executive functions, such as goal-oriented cognition, working-memory, inhibition and task switching.
Figure 5. Lateral frontoparietal network.
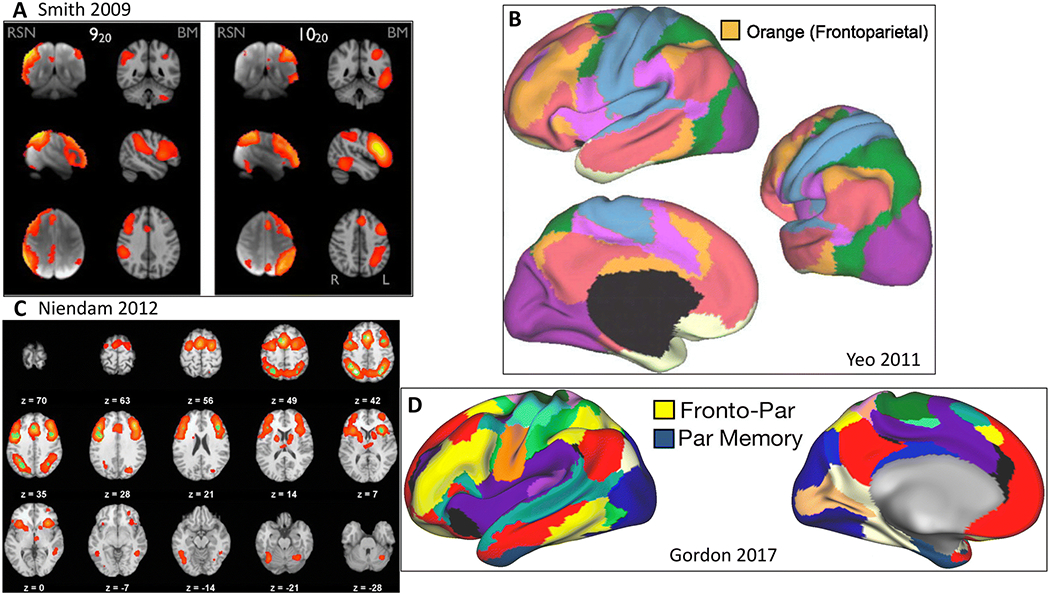
A) “Left and right frontoparietal” (920 and 1020)(Smith et al. 2009). RSN = resting state network, BM = BrainMap meta-analytic activation maps. B) Orange network in 7-network parcellation (Yeo et al. 2011). C) Cognitive control/executive function network from meta-analysis (Niendam et al. 2012). D) Fronto-parietal network (yellow) (Gordon et al. 2017c).
Subsystems of the L-FPN have also been identified based upon their functional affiliation with other systems. One of these subsystems displays preferential connectivity with the M-FPN, whereas a second subsystem is preferentially connected to regions of the D-FPN (Dixon et al. 2018). Regions of the L-FPN showing preferential connections to the M-FPN have been implicated in the control of internally-directed, cognitive control and attentional processes (Kam et al. 2019). In contrast, regions connected to D-FPN have been implicated in the control of stimulus-driven, or externally-directed, cognitive processes (Murphy et al. 2019). Further evidence for this subsystem architecture comes from recent evidence that these subsystems show differentiated patterns of gene expression (Murphy et al. 2019).
Versions of the L-FPN have also been called the central executive (or executive control) network (Seeley et al. 2007), the multiple demand system (Duncan 2010), the extrinsic mode network (Hugdahl et al. 2015), the domain general system (Fedorenko et al. 2013), the frontoparietal control network (Dosenbach et al. 2008; Vincent et al. 2008) and the cognitive control network (Niendam et al. 2012).
Anatomical name: Midcingulo-insular network (M-CIN)
Cognitive Domain: Salience network.
Core regions are bilateral anterior insula and anterior midcingulate cortex. Regions of the midcingulo-insular network additionally include less well characterized areas such as inferior parietal cortex (Yeo et al. 2011), right temporal parietal junction (Corbetta and Shulman 2002) and lateral prefrontal cortex (Gordon et al. 2017c), as well as subcortical structures, including the substantia nigra/ventral tegmental area, periaqueductal grey, central nucleus of the amygdala, hypothalamus, parabrachial nucleus, and basal ventromedial nucleus of the thalamus (Seeley et al. 2007; Uddin 2015)(Figure 6).
Figure 6. Midcingulo-insular network.
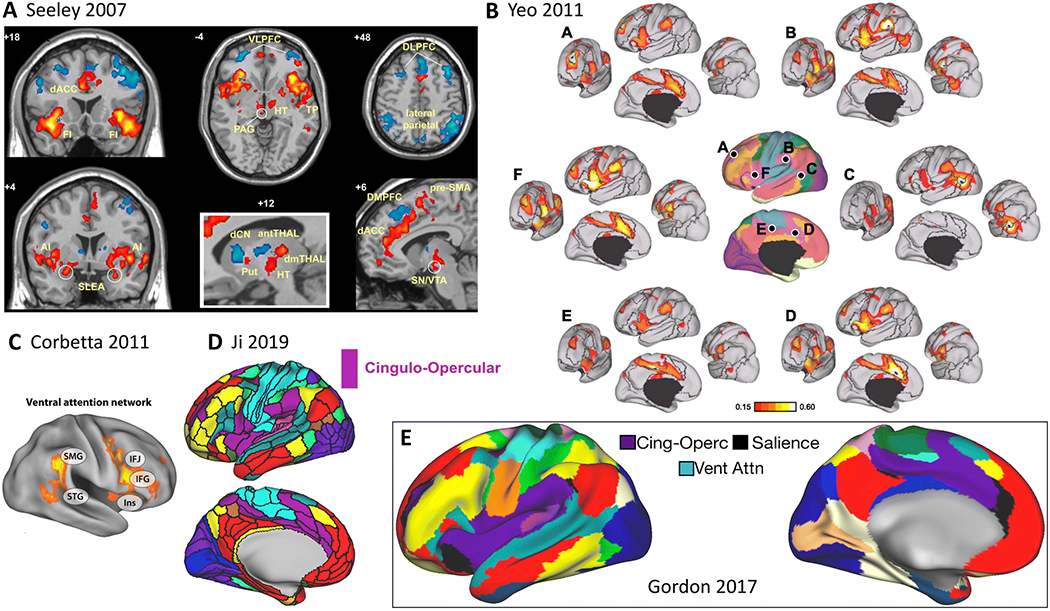
A) Salience network (Seeley et al. 2007). B) Functional connectivity of different nodes of the ventral attention network (Yeo et al. 2011). C) Ventral attention network (Corbetta and Shulman 2011). SMG = supramarginal gyrus, STG = superior temporal gyrus, IFJ = inferior frontal junction, IFG = inferior frontal gyrus, Ins = insula. D) Cingulo-opercular network (violet) from cortical-subcortical atlas (Ji et al. 2019). E) Cingulo-opercular, salience, and ventral attention networks (Gordon et al. 2017c).
The cognitive label “salience” is applied to this network for its broad role in identifying important, or salient, information. Salience processing involves the detection of behaviorally relevant environmental stimuli and may include internally generated (i.e. remembered) information. While the term “salience network” originated from analysis of resting state fMRI data, this descriptor is consistent with findings from task fMRI of homeostatic, emotional, and cognitive factors associated with subjective salience (Uddin 2015).
The midcingulo-insular network in our proposed taxonomy includes the previously characterized “ventral attention network” and “cingulo-opercular network”. Variously referred to as the “ventral frontoparietal network” (Corbetta and Shulman 2002)/“ventral attention system” (Fox et al. 2006)/“ventral attention network” (Yeo et al. 2011; Rueter et al. 2018), this right lateralized system directs attention to spatial locations of salient stimuli (Corbetta and Shulman 2002). We propose that the ventral attention network represents an instantiation of the larger, bilateral midcingulo-insular network. The ventral attention network appears to function mainly during exogenous salience detection, whereas the midcingulo-insular salience network plays a broader role, engaging across domains during processing of personally relevant inputs.
The M-CIN also encapsulates the “cingulo-opercular network”, which was originally described as a system involved in set-maintenance activities (Dosenbach et al. 2008). The salience network nomenclature, in contrast, comes from studies demonstrating a transient role for the anterior insula in detection of salient stimuli and initiation of control signals (Menon and Uddin 2010). A study demonstrating that increased demands on moment-to-moment adjustments are associated with phasic activity in midcingulate and anterior insula (Wilk et al. 2012) is consistent with the conceptualization of the midcingulo-insular salience network as a system for rapid transmission of important information (Seeley et al. 2007; Uddin 2016).
In the social neuroscience literature, the M-CIN has been referred to as the “empathy network” (Kennedy and Adolphs 2012), as both nociceptive and empathic pain produce activation in the insula and midcingulate cortices (Zaki et al. 2016). In other work, this network has been referred to as the “goal priority network”, and related to individual differences in conscientiousness (Rueter et al. 2018).
Parcellations based on repeated measurements from a small number of subjects describe dissociations between cingulo-opercular, salience, and ventral attention networks (Gordon et al. 2017c), whereas those based on hundreds of subjects combine salience and ventral attention networks (Yeo et al. 2011). These findings may represent a case where individual-connectome and group averaging approaches diverge. Extensive further investigation is warranted to ascribe function to possible discrete subsystems within the broader M-CIN.
Anatomical name: Medial frontoparietal network (M-FPN)
Cognitive Domain: Default network.
Core regions are medial prefrontal cortex, posterior cingulate cortex and the posterior extent of the inferior parietal lobule. Regions of the M-FPN also include the inferior frontal gyrus, middle temporal gyrus and superior temporal sulcus, and parahippocampal cortex. Less well characterized zones are: 1) areas dorsal and ventral to the posterior cingulate, the precuneus and retrosplenial cortex, respectively; 2) hippocampus; 3) superior/middle frontal gyrus; 4) ventral frontal cortex and anterior temporal lobes; 5) temporoparietal junction (Buckner et al. 2008; Yeo et al. 2011; Spreng et al. 2013; Andrews-Hanna et al. 2014) (Figure 7).
Figure 7. Medial frontoparietal network.

a) Functional connectivity of posterior cingulate seed (Greicius et al. 2003). b) Default mode network (420) (Smith et al. 2009). RSN = resting state network, BM = BrainMap meta-analytic activation maps. c) Functional connectivity of different nodes of the default network (Yeo et al. 2011). d) Medial temporal subsystem (green), dorsal medial subsystem (blue) and core (yellow) of the default network (Andrews-Hanna et al. 2014). e) Default network (red) and adjacent language network (teal) from cortical-subcortical atlas (Ji et al. 2019). f) Default network (red) (Gordon et al. 2017c).
The cognitive label “default” is retained due to the continued lack of consensus regarding even the broad central functions of the M-FPN. A primary difficulty in identifying the cognitive functions of this network lies in the relative remoteness of its regions from motor and perceptual inputs in terms of topographical organization (Margulies et al. 2016). The network likely involves the formation, temporal binding, and dynamic reconfiguration of associative representations based on current goal-states. The network also detects the associative relevance of internal and external stimuli, providing value coding (Roy et al. 2012) and elaboration to perceived events (Bar et al. 2007; Spreng et al. 2014). Other accounts suggest M-FPN function accommodates predictive coding, semantic associations, and plays a role continuously monitoring the environment (Dohmatob et al. 2018). Significant work clearly remains to delineate the core functions of this system.
At the macro-scale, the M-FPN includes regions previously identified as the semantic system (Binder et al. 2009) and language network (e.g (Ji et al. 2019)) for its role in semantic cognition (Ralph et al. 2017) and narrative comprehension and construction (Mar 2004, 2011). The anterior temporal lobes and orbitofrontal cortex, sometimes referred to as a “limbic network” (Yeo et al. 2011) are also subsumed by the M-FPN.
Functional subsystems of the M-FPN have been identified with RSFC and task fMRI (Figure 6D, (Ngo et al.; Andrews-Hanna et al. 2010, 2014). One subsystem has been neuro-anatomically referred to as the medial temporal lobe subsystem (Andrews-Hanna et al. 2010, 2014). This subsystem corresponds to cognitive processes including recollection, thereby earning the label of the “core recollection network” (Hayama et al. 2012; Thakral et al. 2017), but is also involved in imagination, future-thinking, counterfactual reasoning (Schacter et al. 2012), and contextual associative processing (Bar et al. 2007) central to mind-wandering and spontaneous thought (Christoff et al. 2016). The dorsomedial prefrontal subsystem (Andrews-Hanna et al. 2010, 2014) has also been referred to as the mentalizing system (Van Overwalle and Baetens 2009; Spunt and Lieberman 2012) for its role in the inference of other people’s mental states. Note that in social neuroscience, particularly in the realm of research investigating self-related cognition, brain areas comprising the M-FPN are also referred to as “cortical midline structures” (Uddin et al. 2007).
Earlier RSFC work characterized the M-FPN as a “task-negative” network based on observations that regions within the network can exhibit deactivations during attention demanding tasks (Fox et al. 2005). However, overwhelming empirical evidence has demonstrated that the medial frontoparietal network is functionally not a task-negative network and is in fact engaged during goal-directed cognition, depending on the nature of the task (Spreng 2012).
Outstanding Issues and Future Directions
Several important considerations and outstanding issues should be acknowledged with regards to our proposed network taxonomy. Here we focus on macro-scale functional networks, with a strong emphasis on converging evidence from RSFC and task-activation fMRI studies. In this final section, we note that continued development in functional connectivity dynamics, accounting for inter-individual variability, and incomplete delineation remain significant challenges as we move forward in the development and adoption of a universal taxonomy.
As we have alluded to throughout, a simplifying assumption is that static macro-scale human brain networks can be delineated and described. However, recent work emphasizes the time-varying nature of functional connectivity and the importance of considering temporal properties of brain networks (Hutchison et al. 2013). Early observations of this phenomenon include work by Chang and Glover, who demonstrated that the posterior cingulate cortex, a primary node of the M-FPN, exhibits variable functional connectivity with the rest of the brain such that commonly observed negative correlations between the M-FPN and other frontoparietal networks should not be viewed as static (Chang and Glover 2010). Some have proposed methods for leveraging time-varying properties of functional networks for parcellation, uncovering “representative dominant patterns” (Preti and Van De Ville 2017), though these approaches await further validation. Controversies surrounding the interpretation of dynamic functional connectivity notwithstanding (Hindriks et al. 2016; Laumann et al. 2017; Liegeois et al. 2017), consideration of brain dynamics remains an intriguing direction for future research aimed at network taxonomy delineation.
There is substantial variability across humans in the precise spatial location of functional brain areas (Stevens et al. 2015). Subject-specific functional localization of brain regions using task fMRI provides one solution to determining broader network affiliation, which can meaningfully predict individual differences in behavior (Stevens et al. 2017). Several researchers have noted that the size, location, and spatial arrangement of individual-specific brain networks vary substantially across participants (Wang et al. 2015; Harrison et al. 2015; Laumann et al. 2015; Glasser et al. 2016; Gordon et al. 2017a, b, c; Braga and Buckner 2017) Recent studies have suggested that the spatial arrangement (eg. topography) and size of individual-specific networks can be predictive of demographics (e.g., sex) and behavior (Bijsterbosch et al. 2018; Salehi et al. 2018; Cui et al. 2019; Kong et al. 2019; Li et al. 2019b, a; Seitzman et al. 2019). Whole brain approaches to better estimate and delineate inter-subject variability in functional brain regions comprising large-scale brain networks are being developed in earnest. These whole brain approaches determine individual locations of functional brain areas from patterns of RSFC (Chong et al. 2017), and may be more sensitive to detecting RSFC associations with individual differences in behavior (Mwilambwe-Tshilobo et al. 2019). Furthermore, some have suggested that network subsystems might be fully dissociable, rather than simply overlapping, when examined within-subject and with high-resolution data (Braga and Buckner 2017; Braga et al. 2019). In one such example, the dorsal, lateral and medial frontoparietal networks were found to each represent two fully dissociable networks when examined within-subject (Braga and Buckner 2017), an idea that warrants further investigation. Finally, analysis of task fMRI reveals that inter-subject and task-condition variability can be influenced by the resolution of the data, such that when moving from lower to higher resolution, variance in activation maps explained by between-person differences increases while variance explained by task conditions decreases (Bolt et al. 2019). All of these considerations surrounding inter-subject variability must be considered in future iterations of the taxonomy proposed here.
Within the proposed six-network taxonomy, many regions of cortex are not classified, and subcortical regions are not fully incorporated at this time. Our classification most prominently excludes ventral temporal cortex. These regions have been characterized as “ventral multi-modal” zones in a recent parcellation scheme (Ji et al. 2019). Future work further incorporating whole brain characterization, including subcortical, cerebellar and brainstem structures will be essential.
While the task of creating a universally accepted taxonomy of human brain networks is daunting, we are optimistic that it will be realized in the coming decade. The six-network scheme outlined here is based on a synthesis of practices and assumptions that are already in place. The critical contribution of the current proposal is the introduction of a consistent, anatomically-grounded naming convention that will enable researchers investigating the same brain systems to communicate more effectively. If we can agree on a basic set of regions, their rough boundaries, and the conditions under which they interact to form a network, then we may move forward as a field with a consensus on the macro-scale neurocognitive networks of the human brain.
There are multiple approaches for defining large-scale networks (Eickhoff et al. 2018a, b). In order to create a taxonomy that has a broad impact and is universally adopted, a larger group within the community of researchers must be engaged. Inspiration for this endeavor could come from similar efforts within the neuorimaging community, such as the Time Varying Working Group, which has worked towards consensus with regards to issues surrounding the measurement and interpretation of dynamic functional connectivity (Lurie et al. 2018). Other groups have worked towards standardized definitions of functional and effective connectivity (Reid et al. 2019), and best practices in MRI data analysis and sharing (Nichols et al. 2017). A working group devoted to standardization of network naming conventions could be assembled to follow up the initial effort presented here, potentially drawing upon more varied network characterization, such as structural measures.
As it is unlikely that these naming conventions will immediately replace those that have been used up to this point, we propose a simple process by which a transition to the taxonomy proposed here might be adopted. In future studies, some researchers may still wish to name their networks of interest using their favorite nomenclature for the sake of continuity with previous work. However, we would urge that they should also include the terminology we introduce here. For example, in future papers using the “salience network” term, we would hope that the authors include “midcingulo-insular network” as a keyword in the publication so that their work will be accessible in the future. Moving forward, we suggest that networks not be named for a favored cognitive function, particularly when based solely on task-evoked activation patterns. The principle of many-to-many mapping suggests that ascribing a singular function to a brain region or network is likely erroneous, and serves to deepen the silos of an already fractionated literature. We hope that the proposal outlined here is adopted in the fields of network neuroscience and cognitive neuroscience, and that we will see many more studies examining functional properties of the occipital, pericentral, dorsal frontoparietal, lateral frontoparietal, midcingulo-insular, and medial frontoparietal networks in the years to come.
Acknowledgments
LQU is supported by the National Institute of Mental Health (R01MH107549), the Canadian Institute for Advanced Research, and a University of Miami Gabelli Senior Scholar Award. RNS is supported by the Natural Sciences and Engineering Research Council of Canada and Canadian Institutes of Health Research, and is a Research Scholar supported by Fonds de recherche du Québec – Santé. BTTY is supported by the Singapore National Research Foundation (NRF) Fellowship (Class of 2017). The authors gratefully acknowledge Roberto Toro and Evan Gordan for assistance with figures.
References
- Allen EA, Damaraju E, Plis SM, et al. (2014) Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 24:663–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, et al. (2010) Functional-anatomic fractionation of the brain’s default network. Neuron 65:550–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, Spreng RN (2014) The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci 1316:29–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Aminoff E, Mason M, Fenske M (2007) The units of thought. Hippocampus 17:420–428 [DOI] [PubMed] [Google Scholar]
- Barrett LF, Satpute AB (2013) Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Curr Opin Neurobiol. https://doi.org/S0959-4388(13)00017-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Sporns O (2017) Network neuroscience. NatNeurosci 20:353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM (2005) Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360:1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel RF, Bassett DS (2017) Multi-scale brain networks. Neuroimage 160:73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijsterbosch JD, Woolrich MW, Glasser MF, et al. (2018) The relationship between spatial configuration and functional connectivity of brain regions. eLife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL (2009) Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex 19:2767–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541 [DOI] [PubMed] [Google Scholar]
- Bolt T, Nomi JS, Bainter SA, et al. (2019) The situation or the person? Individual and task-evoked differences in BOLD activity. Hum Brain Mapp 40:2943–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolt T, Nomi JS, Rubinov M, Uddin LQ (2017a) Correspondence between evoked and intrinsic functional brain network configurations. Hum Brain Mapp 38:1992–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolt T, Nomi JS, Yeo BTT, Uddin LQ (2017b) Data-Driven Extraction of a Nested Model of Human Brain Function. J Neurosci 37:7263–7277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga RM, Buckner RL (2017) Parallel Interdigitated Distributed Networks within the Individual Estimated by Intrinsic Functional Connectivity. Neuron 95:457–471.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga RM, Van Dijk KRA, Polimeni JR, et al. (2019) Parallel distributed networks resolved at high resolution reveal close juxtaposition of distinct regions. J Neurophysiol 121:1513–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, et al. (2011) The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106:2322–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH (2010) Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage 50:81–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EY, Yeo BTT, Buckner RL (2012) The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol 108:2242–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong M, Bhushan C, Joshi AA, et al. (2017) Individual parcellation of resting fMRI with a group functional connectivity prior. Neuroimage 156:87–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Irving ZC, Fox KCR, et al. (2016) Mind-wandering as spontaneous thought: a dynamic framework. Nat Rev Neurosci 17:718–731 [DOI] [PubMed] [Google Scholar]
- Ciric R, Nomi JS, Uddin LQ, Satpute AB (2017) Contextual connectivity: A framework for understanding the intrinsic dynamic architecture of large-scale functional brain networks. Sci Rep 7:6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, et al. (2014) Intrinsic and task-evoked network architectures of the human brain. Neuron 83:238–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, et al. (2013) Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 16:1348–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC (1994) Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18:192–205 [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL (2008) The reorienting system of the human brain: from environment to theory of mind. Neuron 58:306–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2011) Spatial neglect and attention networks. Annu Rev Neurosci 34:569–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3:201–215 [DOI] [PubMed] [Google Scholar]
- Cui Z, Li H, Xia CH, et al. (2019) Individual Variation in Control Network Topography Supports Executive Function in Youth. bioRxiv [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, et al. (2006) Consistent resting-state networks across healthy subjects. ProcNatl Acad Sci U S A 103:13848–13853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, et al. (2006) fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage 29:1359–1367 [DOI] [PubMed] [Google Scholar]
- Dixon ML, Andrews-Hanna JR, Spreng RN, et al. (2017) Interactions between the default network and dorsal attention network vary across default subsystems, time, and cognitive states. Neuroimage 147:632–649 [DOI] [PubMed] [Google Scholar]
- Dixon ML, De La Vega A, Mills C, et al. (2018) Heterogeneity Within the Frontoparietal Control Network and its Relationship to the Default and Dorsal Attention Networks. Proc Natl Acad Sci U S A 115(7):E1598–E1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmatob E, Dumas G, Bzdok D (2018) Dark Control: Towards a Unified Account of Default Mode Function by Markov Decision Processes. bioRxiv 148890 [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, et al. (2008) A dual-networks architecture of top-down control. Trends Cogn Sci 12:99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, et al. (2007) Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A 104:11073–11078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet GE, Lee WH, Frangou S (2019) Evaluation of the spatial variability in the major resting-state networks across human brain functional atlases. Hum Brain Mapp 40:4577–4587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J (2010) The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci 14:172–179 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Constable RT, Yeo BTT (2018a) Topographic organization of the cerebral cortex and brain cartography. Neuroimage 170:332–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Yeo BTT, Genon S (2018b) Imaging-based parcellations of the human brain. Nat Rev Neurosci 19:672–686 [DOI] [PubMed] [Google Scholar]
- Farrant K, Uddin LQ (2015) Asymmetric development of dorsal and ventral attention networks in the human brain. Dev Cogn Neurosci 12:165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N (2013) Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci U S A 110:16616–16621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC (1991) Distributed Hierarchical Processing in the Primate Cerebral Cortex. Cerebral Cortex 1:1–47 [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, et al. (2006) Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A 103:10046–10051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711 [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, et al. (2005) The human brain is intrinsically organized into dynamic, anti correlated functional networks. Proc Natl Acad Sci U S A 102:9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K (1994) Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp 2:56–78 [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, et al. (2016) A multi-modal parcellation of human cerebral cortex. Nature 536:171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale MA, Milner AD (1992) Separate visual pathways for perception and action. Trends Neurosci 15:20–25 [DOI] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Adeyemo B, et al. (2017a) Individual-specific features of brain systems identified with resting state functional correlations. Neuroimage 146:918–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Adeyemo B, Petersen SE (2017b) Individual Variability of the System-Level Organization of the Human Brain. Cereb Cortex 27:386–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Gilmore AW, et al. (2017c) Precision Functional Mapping of Individual Human Brains. Neuron 95:791–807.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003) Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SJ, Woolrich MW, Robinson EC, et al. (2015) Large-scale probabilistic functional modes from resting state fMRI. Neuroimage 109:217–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Horwitz B, Ungerleider LG, et al. (1994) The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. J Neurosci 14:6336–6353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama HR, Vilberg KL, Rugg MD (2012) Overlap between the neural correlates of cued recall and source memory: evidence for a generic recollection network? J Cogn Neurosci 24:1127–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindriks R, Adhikari MH, Murayama Y, et al. (2016) Can sliding-window correlations reveal dynamic functional connectivity in resting-state fMRI? Neuroimage 127:242–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K, Raichle ME, Mitra A, Specht K (2015) On the existence of a generalized non-specific task-dependent network. Front Hum Neurosci 9:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, et al. (2013) Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage 80:360–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji JL, Spronk M, Kulkarni K, et al. (2019) Mapping the human brain’s cortical-subcortical functional network organization. Neuroimage 185:35–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam JWY, Lin JJ, Solbakk A-K, et al. (2019) Default network and frontoparietal control network theta connectivity supports internal attention. Nat Hum Behav. 10.1038/s41562-019-0717-0 [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Adolphs R (2012) The social brain in psychiatric and neurological disorders. Trends Cogn Sci 16:559–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi V, Starck T, Remes J, et al. (2009) Functional segmentation of the brain cortex using high model order group PICA. Hum Brain Mapp 30:3865–3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong R, Li J, Orban C, et al. (2019) Spatial Topography of Individual-Specific Cortical Networks Predicts Human Cognition, Personality, and Emotion. Cerebral Cortex 29:2533–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Yeo BTT, Buckner RL (2014) Reconfigurable task-dependent functional coupling modes cluster around a core functional architecture. Philosophical Transactions of the Royal Society B: Biological Sciences 369:20130526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Hodaie M, Davis KD (2012) Lateralization in intrinsic functional connectivity of the temporoparietal junction with salience- and attention-related brain networks. J Neurophysiol 108:3382–3392 [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, et al. (2011) Behavioral interpretations of intrinsic connectivity networks. J CognNeurosci 23:4022–4037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann TO, Gordon EM, Adeyemo B, et al. (2015) Functional System and Areal Organization of a Highly Sampled Individual Human Brain. Neuron 87:657–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann TO, Snyder AZ, Mitra A, et al. (2017) On the Stability of BOLD fMRI Correlations. Cereb Cortex 27:4719–4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegeois R, Laumann TO, Snyder AZ, et al. (2017) Interpreting temporal fluctuations in resting-state functional connectivity MRI. Neuroimage 163:437–455 [DOI] [PubMed] [Google Scholar]
- Li J, Bolt T, Bzdok D, et al. (2019a) Topography and behavioral relevance of the global signal in the human brain. Sci Rep 9:14286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Wang D, Ren J, et al. (2019b) Performing group-level functional image analyses based on homologous functional regions mapped in individuals. PLoS Biol 17:e2007032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Liu H, Zhang M, et al. (2011) Focal pontine lesions provide evidence that intrinsic functional connectivity reflects polysynaptic anatomical pathways. J Neurosci 31:15065–15071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie D, Kessler D, Bassett D, et al. (2018) On the nature of resting fMRI and time-varying functional connectivity. PsyArXiv Preprints [Google Scholar]
- Margulies DS, Ghosh SS, Goulas A, et al. (2016) Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc Natl Acad Sci U S A 113:12574–12579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar RA (2004) The neuropsychology of narrative: story comprehension, story production and their interrelation. Neuropsychologia 42:1414–1434 [DOI] [PubMed] [Google Scholar]
- Mar RA (2011) The neural bases of social cognition and story comprehension. Annu Rev Psychol 62:103–134 [DOI] [PubMed] [Google Scholar]
- McIntosh AR (2004) Contexts and catalysts: a resolution of the localization and integration of function in the brain. Neuroinformatics 2:175–182 [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214:655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM (1990) Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol 28:597–613 [DOI] [PubMed] [Google Scholar]
- Murphy AC, Bertolero MA, Papadopoulos L, et al. (2019) Multiscale and multimodal network dynamics underpinning working memory. arXiv [q-bio.NC] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwilambwe-Tshilobo L, Ge T, Chong M, et al. (2019) Loneliness and meaning in life are reflected in the intrinsic network architecture of the brain. Soc Cogn Affect Neurosci 14:423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo GH, Eickhoff SB, Nguyen M, et al. Beyond Consensus: Embracing Heterogeneity in Curated Neuroimaging Meta-Analysis [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Das S, Eickhoff SB, et al. (2017) Best practices in data analysis and sharing in neuroimaging using MRI. Nat Neurosci 20:299–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, et al. (2012) Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci 12:241–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi JS, Farrant K, Damaraju E, et al. (2016) Dynamic functional network connectivity reveals unique and overlapping profiles of insula subdivisions. Hum Brain Mapp 37:1770–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi JS, Schettini E, Broce I, et al. (2018) Structural Connections of Functionally Defined Human Insular Subdivisions. Cereb Cortex 28:3445–3456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L (2014) Understanding brain networks and brain organization. Phys Life Rev [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Kittur A, Kalar D, et al. (2011) The cognitive atlas: toward a knowledge foundation for cognitive neuroscience. Front Neuroinform 5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, et al. (2011) Functional network organization of the human brain. Neuron 72:665–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preti MG, Van De Ville D (2017) Dynamics of functional connectivity at high spatial resolution reveal long-range interactions and fine-scale organization. Scientific Reports 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, et al. (2001) A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MAL, Lambon Ralph MA, Jefferies E, et al. (2017) The neural and computational bases of semantic cognition. Nature Reviews Neuroscience 18:42–55 [DOI] [PubMed] [Google Scholar]
- Ray KL, McKay DR, Fox PM, et al. (2013) ICA model order selection of task co-activation networks. Front Neurosci 7:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid AT, Headley DB, Mill RD, et al. (2019) Advancing functional connectivity research from association to causation. Nature Neuroscience [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD (2012) Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci 16:147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueter AR, Abram SV, MacDonald AW 3rd, et al. (2018) The goal priority network as a neural substrate of Conscientiousness. Hum Brain Mapp 39:3574–3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi M, Karbasi A, Shen X, et al. (2018) An exemplar-based approach to individualized parcellation reveals the need for sex specific functional networks. Neuroimage 170:54–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, et al. (2012) The future of memory: remembering, imagining, and the brain. Neuron 76:677–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, et al. (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitzman BA, Gratton C, Laumann TO, et al. (2019) Trait-like variants in human functional brain networks. Proc Natl Acad Sci USA. 10.1073/pnas.1902932116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejnowski TJ, Koch C, Churchland PS (1988) Computational neuroscience. Science 241:1299–1306 [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, et al. (1997) Common Blood Flow Changes across Visual Tasks: II. Decreases in Cerebral Cortex. J Cogn Neurosci 9:648–663 [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, et al. (2009) Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A 106:13040–13045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN (2012) The fallacy of a “task-negative” network. Front Psychol 3:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Andrews-Hanna JR (2015) The Default Network and Social Cognition. Brain Mapping 165–169 [Google Scholar]
- Spreng RN, DuPre E, Selarka D, et al. (2014) Goal-congruent default network activity facilitates cognitive control. J Neurosci 34:14108–14114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS (2009) The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci 21:489–510 [DOI] [PubMed] [Google Scholar]
- Spreng RN, Nathan Spreng R, Schacter DL (2012) Default Network Modulation and Large-Scale Network Interactivity in Healthy Young and Old Adults. Cerebral Cortex 22:2610–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, et al. (2013) Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci 25:74–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, et al. (2010) Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage 53:303–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt RP, Lieberman MD (2012) Dissociating modality-specific and supramodal neural systems for action understanding. J Neurosci 32:3575–3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V (2008) A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A 105:12569–12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WD, Dale Stevens W, Kravitz DJ, et al. (2017) Privileged Functional Connectivity between the Visual Word Form Area and the Language System. The Journal of Neuroscience 37:5288–5297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WD, Dale Stevens W, Tessler MH, et al. (2015) Functional connectivity constrains the category-related organization of human ventral occipitotemporal cortex. Human Brain Mapping 36:2187–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain. 1988 New York: Theime [Google Scholar]
- Tamber-Rosenau BJ, Asplund CL, Marois R (2018) Functional dissociation of the inferior frontal junction from the dorsal attention network in top-down attentional control. J Neurophysiol 120:2498–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Wang TH, Rugg MD (2017) Decoding the content of recollection within the core recollection network and beyond. Cortex 91:101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T (2008) Functional coactivation map of the human brain. Cereb Cortex 18:2553–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JA, Laird AR (2012) The cognitive paradigm ontology: design and application. Neuroinformatics 10:57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ (2015) Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci 16:55–61 [DOI] [PubMed] [Google Scholar]
- Uddin LQ (2014) Dynamic connectivity and dynamic affiliation. Comment on “Understanding brain networks and brain organization” by L. Pessoa. Phys Life Rev 11:460–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ (2016) Salience Network of the Human Brain. Academic Press [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP (2007) The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn Sci 11:153–157 [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kinnison J, Pessoa L, Anderson ML (2014) Beyond the tripartite cognition-emotion-interoception model of the human insular cortex. J Cogn Neurosci 26:16–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV (1994) “What”and “where”in the human brain. Curr Opin Neurobiol 4:157–165 [DOI] [PubMed] [Google Scholar]
- Urchs S, Armoza J, Moreau C, et al. (2019) MIST: A multi-resolution parcellation of functional brain networks. MNI Open Research 1: [Google Scholar]
- van den Heuvel MP, Sporns O (2011) Rich-club organization of the human connectome. J Neurosci 31:15775–15786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Glasser MF (2018) Parcellating Cerebral Cortex: How Invasive Animal Studies Inform Noninvasive Mapmaking in Humans. Neuron 99:640–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Smith SM, Barch DM, et al. (2013) The WU-Minn Human Connectome Project: an overview. Neuroimage 80:62–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K (2009) Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage 48:564–584 [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, et al. (2008) Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100:3328–3342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Buckner RL, Fox MD, et al. (2015) Parcellating cortical functional networks in individuals. Nature Neuroscience 18:1853–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig GS, Schlaggar BL, Petersen SE (2011) Concepts and principles in the analysis of brain networks. Ann NY Acad Sci 1224:126–146 [DOI] [PubMed] [Google Scholar]
- Wilk HA, Ezekiel F, Morton JB (2012) Brain regions associated with moment-to-moment adjustments in control and stable task-set maintenance. Neuroimage 59:1960–1967 [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, et al. (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Eickhoff SB, Yaakub SN, Fox PT, Buckner RL, Asplund CL, Chee MWL (2016) Functional specialization and flexibility in human association cortex. Cerebral Cortex 25:3654–3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Wager TD, Singer T, et al. (2016) The Anatomy of Suffering: Understanding the Relationship between Nociceptive and Empathic Pain. Trends Cogn Sci 20:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhao L, Zhou N, et al. (2019) Predictive Big Data Analytics using the UK Biobank Data. Sci Rep 9:6012. [DOI] [PMC free article] [PubMed] [Google Scholar]


