Abstract
Background
We investigated the association between sleep symptoms, which cause sleep disorder, and quality of life (QoL) among people with type 2 diabetes (T2D).
Methods
In this cross-sectional study of 342 people with T2D, the Japan National Health and Wellness Survey (NHWS) database 2016 were used. We treated the respondents who reported experiencing any of the sleep symptoms as having sleep disorders. To examine health-related QoL (HRQoL), we used the physical component summary (PCS) and the mental component summary (MCS) from the 36-Item short-form and the EuroQol 5 Dimension (EQ-5D) survey instruments. Overall activity impairment was used for assessment of the effect on the individual’s ability to perform regular daily activities. We used t-test and one-way ANOVA test for comparison QoL scores between the participants with and without sleep disorders.
Results
66.4% of the participants with T2D reported having a sleep disorder. The PCS, MCS, EQ-5D, and overall activity impairment of people with sleep disorder was significantly poorer than those of the people without. Specific sleep symptoms, such as waking up to go to the bathroom, daytime sleepiness, and waking up too early (before the alarm clock), had high prevalence (35.4, 27.8 and 20.2%). The participants who experienced waking up to go to the bathroom or daytime sleepiness demonstrated significantly poorer QoL on all scores related to QoL, but those who experienced waking up too early only demonstrated significantly poorer QoL on the EQ-5D.
Conclusions
Two-thirds of people with T2D in this study suffer from sleep disorders. The people who experience waking up to go to the bathroom or daytime sleepiness had significantly poorer QoL than those without these symptoms. Thus, sleep disorders, especially the symptoms of waking up to go to the bathroom or daytime sleepiness, might be the treatment targets for QOL of people with T2DM.
Keywords: Type 2 diabetes, Quality of life, Sleep disorder, Sleep quality, Sleep quantity, Sleep fragmentation
Background
The number of people with type 2 diabetes (T2D) is now increasing. The overall treatment goal of diabetes is to prevent diabetic complications, while maintaining quality of life (QoL). Reports show that the QoL of people with diabetes is lower than that of people without diabetes [1]. Health-related QoL (HRQoL) includes aspects of QoL that may affect physical or mental health [2]. Furthermore, there is a close association between HRQoL values and glycemic control in people with T2D [3, 4]. Thus, to prevent a decline in the QoL of people with T2D is treatment target for the management of diabetes.
Sleep disorder, which many people with T2D suffer, [5] is one of the factors impairing QoL in people with T2D. According to an internet survey of over 7 thousands people in the United States with T2D, three-quarters of participants suffered from sleep symptoms, and one-quarter of them were actually diagnosed with a sleep disorder [6]. Also, reports show that about half of people with T2D in Japan have sleep disorder [7]. Furthermore, in a study of almost 1 thousand Chinese people with T2D, 33.6% suffered from poor sleep quality, and there was an association between the pittsburgh sleep quality index and QoL [8]. These studies were based on the sleep disorder scores. These scores and sleep duration significantly correlate with QoL values in people with T2D [9, 10]. Generally, these sleep disorder scores were meaning the summary of symptoms in previous studies. We couldn’t specify which symptoms impact on patient’s QoL. Thus, we investigated the relationship between specific sleep-related symptoms and QoL in people with T2D.
Methods
Study design and participants
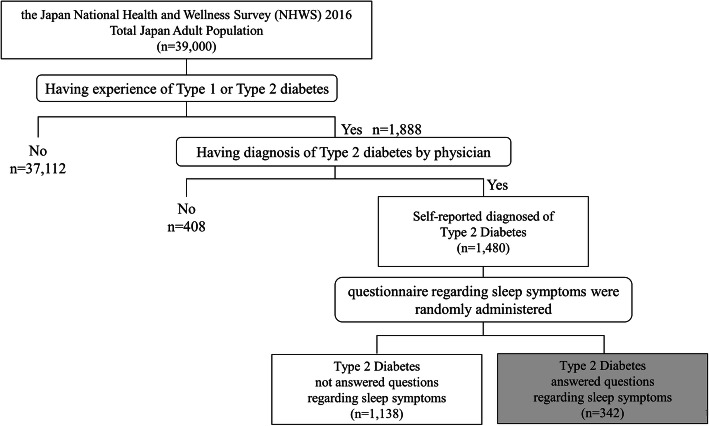
This cross-sectional study is based on the data of the Japan National Health and Wellness Survey (NHWS) 2016 (N = 39,000), which is a self-administered, internet-based questionnaire from a nationwide sample of adults (aged 18 years or older). In this survey, participants, were recruited through a random sampling approach, stratified by age and gender, are required to provide various information [11, 12]. The survey received Institutional Review Board approval and all respondents provided written informed consent prior to participating. Potential respondents for this study were identified through the general panel of Lightspeed Research (LSR). Panel members are general people who explicitly agreed to join this panel. The LSR members were recruited through a variety of means, including co-registration with other internet panels, e-newsletter campaigns, and banner placements. The NHWS sample is generally comparable to the greater population concerning these characteristics [11–13]. We extracted the data including self-reported diagnosis of type 2 diabetes from the Japan NHWS 2016. At first, all respondents were asked if they have ever experienced Type 1 or Type 2 Diabetes. For respondents who responded “yes” to this question, they were asked if their Type 2 Diabetes has been diagnosed by a physician. Respondents who answered “yes” to the second question were classified as T2D patients in the study. Among this T2D patients, we selected the individuals who answered questions regarding sleep symptoms. In addition, the questions about sleep symptoms were randomly administrated from all participants in the Japan NHWS 2016 (Fig. 1).
Fig. 1.
Data extraction from the Japan NHWS 2016
Clinical and sociodemographic profiles
Using the questionnaire, age, sex, marital status (married/living with partner, or not married), education (university degree or all others), household income (< 3,000,000 JPY, 3,000,000 to < 5,000,000 JPY, 5,000,000 to < 8,000,000 JPY, 8,000,000 JPY or more, or decline to answer), health insurance (national health insurance, social insurance, late stage elderly insurance, or other/no insurance), and employment status (currently employed or not). Additional questions include: smoking (current, former, or never smoker), exercise (currently exercise or not), alcohol use (currently consume alcohol or not), body mass index (BMI) category (underweight; BMI < 18.5 kg/m2, normal weight; 18.5 kg/m2 ≤ BMI < 25 kg/m2, overweight; 25 kg/m2 ≤ BMI < 30 kg/m2, obese; 30 kg/m2 ≤ BMI, or decline to answer), taking steps to lose weight (yes or no), Charlson comorbidity index (CCI), HbA1c level (HbA1c < 7, HbA1c ≥7, or don’t know), use of injectable medicines (yes or no), and ever experienced hypoglycemia (yes, no, or don’t know). BMI (kg/m2) was calculated as person’s weight (kg) divided by the square of height (m2).
Definition of sleep disorders
This study treats respondents who reported experiencing any of the sleep symptoms as having sleep disorders [6]. Sleep symptoms were made referencing the Diagnostic and Statistical Manual of Mental Disorders [14]”.
Definition of QoL
HRQoL was assessed using the 36-Item short-form (SF-36v2) and the EuroQol 5 Dimension (EQ-5D) survey instruments. The SF-36v2 is a multipurpose, generic HRQoL instrument comprising 36 questions that map onto 8 health domains, and these relevant scores are summarized into the physical component summary (PCS) and the mental component summary (MCS) [15–17]. Higher scores indicate a better QoL. Validation of these score was confirmed in Japanese population [16]. The EQ-5D is an instrument that evaluates the generic QoL, which is a standardized measure of health status to provide a simple, generic measure of health [18]. EQ-5D were widely used in various countries and diseases [19–23]. This questionnaire and descriptive system comprising five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) with five levels of severity [24] and then responses were converted into a single health index by applying a formula [24]. Higher scores indicate a better health status. Overall activity impairment was assessed using the Work Productivity and Activity Impairment (WPAI) questionnaire [25]. The WPAI was validated in number of disease states [25, 26] This is a 6-item validated instrument that consists of four metrics: presenteeism, absenteeism, overall work productivity loss, and overall activity impairment. Overall activity impairment is one of the WPAI’s metrics and is showing the percentage of impairment in daily activities because of one’s health in the past 7 days. Overall activity impairment subscales are expressed as percentages and higher percentages indicate greater impairment.
Statistical analysis
We compared demographic and general health characteristics with and without sleep disorders. We also compared QoL, including HRQoL and overall activity impairment, based on the presence or absence of sleep disorders and of specific sleep symptoms. T-test and one-way ANOVA test were used for continuous variables and the Chi-square test for categorical variables.
Subsequently, the QoL associated with the presence or absence of sleep disorders or of specific sleep symptoms were compared by multivariable analysis (generalized linear model), adjusting for age, sex, marital status, level of education, Charlson comorbidity index, smoking status, alcohol use, BMI category, taking steps to lose weight, duration of diabetes, HbA1c level, use of injectable medications, and experience with hypoglycemia. We selected the specific sleep symptoms of waking up to go to the bathroom, daytime sleepiness, and waking up too early (such as before the alarm clock) for multivariable analysis because the prevalence rates of these symptoms were over 20%.
There was a significant difference when the p-value was less than 0.05. All data analyses were performed using IBM SPSS Statistics Version 22.
Results
According to the Japan NHWS database 2016 (N = 39,000), 1480 participants were identified as having T2D. Among these T2D, the questionnaire about condition of sleep were randomly applied. Three hundred-forty two participants responded the questionnaire, and we analyzed the data of these respondents.
Table 1 shows the clinical characteristics of the 342 participants. The mean (SD) age and duration of diabetes were 63.25 (10.21) and 13.54 (10.59) years, and 82.5% of the participants were men. Out of the 342 participants, 15.5% used injectables and 27.8% had experienced hypoglycemia.
Table 1.
Clinical and sociodemographic profile of study participants
| N = 342 | |
|---|---|
| Age (years) | 63.25 (10.21) |
| Male | 82.5% (282) |
| Marital status | |
| Married/living with partner | 74.9% (256) |
| Divorced/separated/widowed | 25.1% (86) |
| Education | |
| University degree | 46.5% (159) |
| All others | 53.5% (183) |
| Household income | |
| < 3,000,000JPY | 17.0% (58) |
| 3,000,000 to < 5,000,000 JPY | 28.1% (96) |
| 5,000,000 to < 8,000,000 JPY | 24.0% (82) |
| 8,000,000 JPY or more | 18.7% (64) |
| Decline to answer | 12.3% (42) |
| Insurance | |
| National health insurance | 57.0% (195) |
| Social insurance | 30.7% (105) |
| Late stage elderly insurance | 8.8% (30) |
| Other/No insurance | 3.5% (12) |
| Worker | 46.2% (158) |
| Charlson comorbidity index | 0.45 (0.91) |
| Smoking status | |
| Current smoker | 26.3% (90) |
| Former smoker | 38.9% (133) |
| Never smoker | 34.8% (119) |
| Currently exercisers | 55.0% (188) |
| Alcohol drinker | 69.3% (237) |
| BMI category | |
| Underweight (< 18.5) | 4.1% (14) |
| Normal weight (18.5 to < 25) | 55.3% (189) |
| Overweight (25 to < 30) | 27.2% (93) |
| Obese (≥30) | 11.1% (38) |
| Decline to answer | 2.3% (8) |
| Taking steps to lose weight | 35.4% (121) |
| Duration of type 2 diabetes (years) | 13.54 (10.59) |
| HbA1c level | |
| HbA1c < 7 | 37.1% (127) |
| HbA1c ≥7 | 41.8% (143) |
| Don’t know/Decline to answer | 21.1% (72) |
| Use of injectable medications | 15.5% (53) |
| Ever experienced hypoglycemia | |
| Experienced hypoglycemia | 27.8% (95) |
| Not experienced hypoglycemia | 63.5% (217) |
| Do not know | 8.8% (30) |
Continuous variables are expressed as mean (SD) and categorical variables are expressed as % (number)
Table 2 shows the prevalence of sleep symptoms in the participants. 66.4% reported some sleep symptoms. For individual symptoms, 35.4% wake up to go to the bathroom, 27.8% suffer daytime sleepiness, and 20.2% wake up too early (such as before the alarm clock).
Table 2.
The prevalence of sleep symptoms
| N = 342 | |
|---|---|
| Experience any sleep symptoms | 66.4% (227) |
| Difficulty falling asleep | 12.6% (43) |
| Waking during the night and not being able to get back to sleep | 12.3% (42) |
| Waking up several times during the night | 13.2% (45) |
| Waking up too early (such as before the alarm clock) | 20.2% (69) |
| Sleep apnea (temporary absence of breathing) | 9.1% (31) |
| Leg cramps/leg problems | 9.9% (34) |
| Waking up to go to the bathroom | 35.4% (121) |
| Night sweats/hot flashes | 5.8% (20) |
| Pain | 0.9% (3) |
| Poor quality of sleep | 12.0% (41) |
| Daytime sleepiness | 27.8% (95) |
| Difficulty staying awake | 2.3% (8) |
| Other | 4.1% (14) |
The data were expressed as % (number)
Table 3 shows the clinical characteristics difference between participants with/without sleep disorders. The proportion of current smoker, worse HbA1c, and experience with hypoglycemia were higher in the participants with sleep disorders than those in the participants without.
Table 3.
Clinical and sociodemographic profile according to the experience of any sleep symptoms
| Any sleep symptoms (−) (n = 115) | Any sleep symptoms (+) (n = 227) | p-value | |
|---|---|---|---|
| Age (years) | 63.6 (9.61) | 63.08 (10.52) | 0.66 |
| Male | 81.7% (94) | 82.8% (188) | 0.804 |
| Marital status | 0.068 | ||
| Married/living with partner | 80.9 (93) | 71.8% (163) | |
| Divorced/separated/widowed | 19.1% (22) | 28.2% (64) | |
| Education | 0.306 | ||
| University degree | 42.6% (49) | 48.5% (110) | |
| All others | 57.4% (66) | 51.5% (117) | |
| Household income | 0.018 | ||
| < 3,000,000JPY | 18.3% (21) | 16.3% (37) | |
| 3,000,000 to < 5,000,000 JPY | 21.7% (25) | 31.3% (71) | |
| 5,000,000 to < 8,000,000 JPY | 24.3% (28) | 23.8% (54) | |
| 8,000,000 JPY or more | 15.7% (18) | 20.3% (46) | |
| Decline to answer | 20.0% (23) | 8.4% (19) | |
| Insurance | 0.813 | ||
| National health insurance | 59.1% (68) | 55.9% (127) | |
| Social insurance | 27.8% (32) | 32.2% (73) | |
| Late stage elderly insurance | 8.7% (10) | 8.8% (20) | |
| Other/No insurance | 4.3% (5) | 3.1% (7) | |
| Worker | 40.0% (46) | 49.3% (112) | 0.102 |
| Charlson comorbidity index | 0.27 (0.61) | 0.55 (1.01) | 0.008 |
| Smoking status | 0.007 | ||
| Current smoker | 22.6% (26) | 28.2% (64) | |
| Former smoker | 31.3% (36) | 42.7% (97) | |
| Never smoker | 46.1% (53) | 29.1% (66) | |
| Currently exerciser | 55.7% (64) | 54.6% (124) | 0.857 |
| Alcohol drinker | 65.2% (75) | 71.4% (162) | 0.244 |
| BMI category | 0.131 | ||
| Underweight (< 18.5) | 6.1% (7) | 3.1% (7) | |
| Normal weight (18.5 to < 25) | 60.9% (70) | 52.4% (119) | |
| Overweight (25 to < 30) | 20.9% (24) | 30.4% (69) | |
| Obese (≥30) | 8.7% (10) | 12.3% (28) | |
| Decline to answer | 3.5% (4) | 1.8% (4) | |
| Taking steps to lose weight | 28.7% (33) | 38.8% (88) | 0.066 |
| Duration of T2DM (years) | 13.83 (10.63) | 13.39 (10.59) | 0.721 |
| HbA1c level | 0.008 | ||
| HbA1c < 7 | 34.8% (40) | 38.3% (87) | |
| HbA1c ≥7 | 34.8% (40) | 45.4% (103) | |
| Don’t know/Decline to answer | 30.4% (35) | 16.3% (37) | |
| Use of injectable medications | 11.3% (13) | 17.6% (40) | 0.127 |
| Ever experienced hypoglycemia | 0.001 | ||
| Experienced hypoglycemia | 14.8% (17) | 34.4% (78) | |
| Not experienced hypoglycemia | 74.8% (86) | 57.7% (131) | |
| Do not know | 10.4% (12) | 7.9% (18) |
Continues variables were expressed as mean (SD) and categorical variables were expressed as % (number). T-test was used for continuous variables, and Chi-square test was used for categorical variables
Table 4 shows the QoL difference between participants with/without sleep disorders. The PCS, MCS, EQ-5D of the participants with sleep disorders were lower than those of the participants without. In addition, overall activity impairment of participants with sleep disorders was significantly higher than that of participants without.
Table 4.
QoL scores according to the experience of any sleep symptoms
| All (n = 342) | Any sleep symptoms (−) (n = 115) | Any sleep symptoms (+) (n = 227) | p-value | |
|---|---|---|---|---|
| QoL score | ||||
| PCS | 48.71 (7.40) | 50.59 (6.71) | 47.76 (7.56) | < 0.001 |
| MCS | 48.15 (10.59) | 51.90 (8.36) | 46.25 (11.10) | < 0.001 |
| EQ-5D | 0.79 (0.17) | 0.86 (0.15) | 0.76 (0.18) | < 0.001 |
| Overall activity impairment, % | 26.23 (26.42) | 18.52 (22.53) | 30.13 (27.42) | < 0.001 |
QoL quality of life, PCS Physical component summary, MCS Mental Component Summary, EQ-5D EuroQol 5 Dimension. Continues variables were expressed as mean (SD). T-test was used for continuous variables
Table 5 shows the adjusted means examining the effect of having sleep symptoms on QoL scores after controlling for clinical characteristics. The adjusted PCS, MCS, EQ-5D of the participants with any sleep disorders were lower than those of the participants without and adjusted overall activity impairment of participants with sleep disorders was significantly higher than that of participants without. This result was the same for the symptoms of waking up to go to the bathroom and daytime sleepiness. On the other hand, there was no difference in the adjusted means PCS, MCS, and overall activity impairment between participants with and without the symptom of waking up too early.
Table 5.
Adjusted means examining the effect of having sleep symptoms on QoL scores after controlling for clinical characteristics
| Sleep symptoms | Health outcomes | Adjusted mean (95% CI) | Adjusted mean (95% CI) | p-value |
|---|---|---|---|---|
| Any sleep symptoms | No (n = 115) | Yes (n = 227) | ||
| PCS | 50.14 (48.86–51.41) | 47.99 (47.10–48.88) | 0.009 | |
| MCS | 51.32 (49.56–53.08) | 46.54 (45.31–47.76) | < 0.001 | |
| EQ-5D | 0.84 (0.81–0.87) | 0.77 (0.75–0.79) | < 0.001 | |
| Overall activity impairment, % | 18.87 (15.16–23.48) | 27.26 (23.46–31.68) | 0.009 | |
| Waking up to go to the bathroom | No (n = 221) | Yes (n = 121) | ||
| PCS | 49.67 (48.78–50.57) | 46.96 (45.73–48.19) | < 0.001 | |
| MCS | 49.29 (48.03–50.54) | 46.06 (44.33–47.79) | 0.004 | |
| EQ-5D | 0.81 (0.79–0.84) | 0.76 (0.73–0.78) | 0.002 | |
| Overall activity impairment, % | 20.73 (17.81–24.14) | 31.24 (25.35–38.5) | 0.003 | |
| Daytime sleepiness | No (n = 247) | Yes (n = 95) | ||
| PCS | 49.21 (48.36–50.06) | 47.41 (45.99–48.83) | 0.038 | |
| MCS | 49.51 (48.34–50.68) | 44.6 (42.64–46.55) | < 0.001 | |
| EQ-5D | 0.82 (0.80–0.84) | 0.73 (0.70–0.77) | < 0.001 | |
| Overall activity impairment, % | 22.30 (19.39–25.64) | 29.92 (23.73–37.71) | 0.038 | |
| Waking up too early (such as before the alarm clock) | No (n = 273) | Yes (n = 69) | ||
| PCS | 48.94 (48.14–49.75) | 47.8 (46.13–49.46) | 0.233 | |
| MCS | 48.51 (47.39–49.64) | 46.7 (44.37–49.03) | 0.178 | |
| EQ-5D | 0.80 (0.79–0.82) | 0.75 (0.71–0.79) | 0.02 | |
| Overall activity impairment, % | 23.83 (20.88–27.19) | 26.58 (20.19–34.99) | 0.493 |
QoL quality of life, PCS Physical component summary, MCS Mental Component Summary, EQ-5D EuroQol 5 Dimension. The QoL scores of the presence or absence of specific sleep symptoms were compared by multivariable analysis adjusting for age, sex, marital status, level of education, charlson comorbidity index, smoking status, alcohol use, BMI category, taking steps to lose weight, duration of diabetes, HbA1c level, use of injectable medications and experience of hypoglycemia. We selected specific sleep symptoms of waking up to go to the bathroom, daytime sleepiness and waking up too early (such as before the alarm clock) for multivariable analysis, because prevalence rates of these symptoms were over 20%
Discussion
This cross-sectional study showed the association between sleep disorders or specific sleep symptoms and QoL in patients with T2D. It revealed that 66.4% people with T2D suffer from sleep symptoms and that their QoL, including HRQoL and overall activity impairment, was worse than those of respondents without symptoms. This finding correlates with the results of previous studies [6–10, 27]. Also, this study revealed that waking up to go to the bathroom and daytime sleepiness are associated with worse QoL in people with T2D. Because to maintain and improve the QoL of people with diabetes is important goal, it should be recognized that the patients may have sleep disorders, especially the specific sleep symptoms discussed here.
Sleep disorders affect sleep quantity and quality. Sleep quantity is connected to the risk of lifestyle-related diseases, such as diabetes [28] and nonalcoholic fatty liver disease [29]. Also, sleep quantity affects QoL; a survey of more than 200,000 people in the United States with chronic illnesses has confirmed that the U-shape relationship between sleep time and the prevalence of decreased HRQoL, suggesting that decrease in sleep time leads to a decline in HRQoL [30]. This relationship has been observed similarly in other countries in people with various diseases [31–34]. Relatedly, sleep quality is also an important matter, and one of the problems of sleep quality is sleep fragmentation. Sleep fragmentation leads to metabolic disorders through activating autonomic sympathetic nerves [35]. The activation of autonomic sympathetic nerves leads to cardiovascular disease through changes in hemodynamics, vasoconstriction, and blood coagulation status [36]. Sleep fragmentation is also associated with psychological symptoms, such as depression [37]. Furthermore, the frequency of sleep disturbance is closely linked with the severity of self-reported symptoms in healthy individuals [38].
In this study, waking up to go to the bathroom is associated with poor QoL, including PCS, MCS, EQ-5D, and overall activity impairment, whereas waking up too early (such as before the alarm clock) is not associated with poor QoL, including PCS, MCS, and overall activity impairment. These results might be because of the difference in the type of sleep disorder. Because of the interruption of sleep, sleep fragmentation occurs when waking up to go to the bathroom. Waking up to go to the bathroom has close association with nocturia. Many people with T2DM are suffered from nocturia [39]. Many risk factors, including male sex, hypertension, high B-type natriuretic peptide level, low vegetable intake and early bedtime are reported [39, 40]. Dietary education [41] and medication intervention might be useful to reduce these risk factors. On the other hand, a decrease in sleep time occurs when waking up too early (such as before the alarm clock). Previous studies revealed that the effect of sleep fragmentation on mental and physical health is more severe than that of sleep quantity [42, 43].
This study also revealed that daytime sleepiness is associated with poor QoL. This correlates with studies that show that elderly people with hypertension and daytime sleepiness have a reduced QoL [44]. The decline of sleep quantity, which reduces the performance of higher cognitive processes, [45] might cause HRQoL to decline through daytime sleepiness.
This study has some limitations. Data from the NHWS are self-reported and did not include medical records or physician’s reports, and so no verification of diagnoses can be conducted. Moreover, the representativeness of each subsample is unknown. However, these data have been used in various publications, including data of T2D [46, 47]. Causal relationships between sleep symptoms and health outcomes cannot be assumed. The Japan NHWS 2016 had been completed when we started to analyze it. Therefore, the sample size was not predefined.
Conclusion
In this study, about two-thirds of the people with T2D suffered from sleep disorders, and there is a relationship between sleep-related symptoms, especially waking up to go to the bathroom and daytime sleepiness, and HRQoL or labor productivity. It is, therefore, important to focus on sleep disorders, especially on symptoms of waking up to go to the bathroom or daytime sleepiness, in order to maintain and improve QoL of people with diabetes. Further studies focusing on sleep symptoms are needed for improvement QoL of people with diabetes.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Abbreviations
- QoL
Quality of life
- T2D
Type 2 diabetes
- NHWS
National Health and Wellness Survey
- HRQoL
Health-related QoL
- PCS
Physical component summary
- MCS
Mental component summary
- EQ-D
EuroQol 5 Dimension
- LSR
Lightspeed Research
- BMI
Body mass index
- CCI
Charlson comorbidity index
- SF-36
36-Item short-form
- WPAI
Work Productivity and Activity Impairment
Authors’ contributions
Y.H. designed the study, interpretation data and wrote manuscript, R.S. designed the study and contributed to discussion. K.I. designed the study, interpretation data and wrote manuscript. M.F. designed the study and revised the discussion. All authors approved the final version of the manuscript, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
Kowa Pharma Co. Ltd. provided funds to Kantar Health for analysis of the data. The funder had no role in the data collection and analysis.
Availability of data and materials
The data that support the findings of this study are available from Kantar Health but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from corresponding authors, Michiaki Fukui, upon reasonable request and with permission of Kantar Health.
Ethics approval and consent to participate
The survey was conducted with approval from Pearl IRB (IRB Study Number: 16-KAN-124). Written informed consent was obtained from all participants. The authors were granted access to NHWS data from Kantar Health for this research.
Consent for publication
Not applicable.
Competing interests
K.I. is an employee of Kowa Pharma Co. Ltd., M.F. received research supports and honorarium from Kowa Pharma Co. Ltd. In addition, Y.H. received honoraria from Mitsubishi Tanabe Pharma Corp, and Novo Nordisk Pharma Ltd., and M.F. received grants from Takeda Pharma Co. Ltd., Sanofi K.K., Kissei Phama Co. Ltd., Mitsubishi Tanabe Pharma Corp, Astellas Pharma Inc., Nippon Boehringer Ingelheim Co. Ltd., Daiichi Sankyo Co. Ltd., MSD K.K., Sanwa Kagagu Kenkyusho CO., LtD., Kowa Pharma Co. Ltd., Kyowa Kirin Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharma Co. Ltd., Eli Lilly Japan K.K., Taisho Pharma Co., Ltd., Tejin Pharma LtD., Nippon Chemiphar Co., Ltd., Johnson & Johnson k.k. Medical Co., Abbott japan Co. Ltd., and Terumo Corp., and received honoraria from Teijin Pharma Ltd., Arkray Inc., Kissei Pharma Co., Ltd., Novo Nordisk Pharma Ltd., Mitsubishi Tanabe Pharma Corp., Sanofi K.K., Takeda Pharma Co. Ltd., Astellas Pharma Inc., MSD K.K., Kyowa Kirin Co. Ltd., Sumitomo Dainippon Pharma Co. Ltd., Daiichi Sankyo Co. Ltd., Ono Pharma Co. Ltd., Sanwa Kagaku Kenkyusho Co. Ltd., Nippon Boehringer Ingelheim Co., Ltd., Taisho Pharma Co., Ltd., Bayer Yakuhin, Ltd., AstraZeneca K.K., Mochida Pharma Co. Ltd., Abbott japan Co. Ltd., Eli Lilly Japan K.K., Medtronic Japan Co. Ltd., and Nipro Corp. outside the submitted work. R.S. has no potential conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev. 1999;15(3):205–218. doi: 10.1002/(sici)1520-7560(199905/06)15:3<205::aid-dmrr29>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.Gandek B, Sinclair SJ, Kosinski M, et al. Psychometric evaluation of the SF-36 health survey in Medicare managed care. Health Care Financ Rev. 2004;25(4):5–25. [PMC free article] [PubMed] [Google Scholar]
- 3.Kuznetsov L, Griffin SJ, Davies MJ, et al. Diabetes-specific quality of life but not health status is independently associated with glycaemic control among patients with type 2 diabetes: a cross-sectional analysis of the ADDITION-Europe trial cohort. Diabetes Res Clin Pract. 2014;104(2):281–287. doi: 10.1016/j.diabres.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 4.Svedbo EM, Leksell J, Johansson UB, et al. Health-related quality of life and glycaemic control among adults with type 1 and type 2 diabetes: a nationwide cross-sectional study. Health Qual Life Outcomes. 2019;17(1):141. doi: 10.1186/s12955-019-1212-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barakat S, Abujbara M, Banimustafa R, et al. Sleep quality in patients with type 2 diabetes mellitus. J Clin Med Res. 2019;11(4):261–266. doi: 10.14740/jocmr2947w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S, Wang Z. Predictors of sleep disorders among patients with type 2 diabetes mellitus. Diab Metab Syndr. 2016;10(4):213–220. doi: 10.1016/j.dsx.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Sakamoto R, Yamakawa T, Takahashi K, et al. Association of usual sleep quality and glycemic control in type 2 diabetes in Japanese: a cross sectional study. Sleep and Food Registry in Kanagawa (SOREKA) PLoS One. 2018;13(1):e0191771. doi: 10.1371/journal.pone.0191771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lou P, Qin Y, Zhang P, et al. Association of sleep quality and quality of life in type 2 diabetes mellitus: a cross-sectional study in China. Diabetes Res Clin Pract. 2015;107(1):69–76. doi: 10.1016/j.diabres.2014.09.060. [DOI] [PubMed] [Google Scholar]
- 9.Zeng Y, Wu J, Yin J, et al. Association of the combination of sleep duration and sleep quality with quality of life in type 2 diabetes patients. Qual Life Res. 2018;27(12):3123–3130. doi: 10.1007/s11136-018-1942-0. [DOI] [PubMed] [Google Scholar]
- 10.Bani-Issa W, Al-Shujairi AM, et al. Association between quality of sleep and health-related quality of life in persons with diabetes mellitus type 2. J Clin Nurs. 2018;27(7–8):1653–1661. doi: 10.1111/jocn.14221. [DOI] [PubMed] [Google Scholar]
- 11.Bolge SC, Doan JF, Kannan H, Baran RW. Association of insomnia with quality of life, work productivity, and activity impairment. Qual Life Res. 2009;18(4):415–422. doi: 10.1007/s11136-009-9462-6. [DOI] [PubMed] [Google Scholar]
- 12.DiBonaventura MD, Wagner J-S, Yuan Y, et al. Humanistic and economic impacts of hepatitis C infection in the United States. J Med Econ. 2010;13(4):709–718. doi: 10.3111/13696998.2010.535576. [DOI] [PubMed] [Google Scholar]
- 13.Finkelstein EA, Allaire BT, DiBonaventura MD, et al. Direct and indirect costs and potential cost savings of laparoscopic adjustable gastric banding among obese patients with diabetes. J Occup Environ Med. 2011;53(9):1025–1029. doi: 10.1097/JOM.0b013e318229aae4. [DOI] [PubMed] [Google Scholar]
- 14.American-Psychiatric-Association: Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association Publishing; 2014.
- 15.Maruish ME (ed.) User’s Manual for the SF-36v2 Health Survey (3rd Ed.). Lincoln, RI: QualityMetric Incorporated 2011.
- 16.Suzukamo Y, Fukuhara S, Green J, et al. Validation testing of a three-component model of short Form-36 scores. J Clin Epidemiol. 2011;64(3):301–308. doi: 10.1016/j.jclinepi.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Pawaskar M, Witt EA, Engel SS, et al. Severity of hypoglycaemia and health-related quality of life, work productivity and healthcare costs in patients with type 2 diabetes in Europe. Endocrinol Diab Metab. 2018;1(2):e00011. [DOI] [PMC free article] [PubMed]
- 18.EuroQol Group EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 19.Bekairy AM, Bustami RT, Almotairi M, et al. Validity and reliability of the Arabic version of the the EuroQOL (EQ-5D). A study from Saudi Arabia. Int J Health Sci (Qassim) 2018;12(2):16–20. [PMC free article] [PubMed] [Google Scholar]
- 20.Wang HM, Patrick DL, Edwards TC, et al. Validation of the EQ-5D in a general population sample in urban China. Qual Life Res. 2012;21(1):155–160. doi: 10.1007/s11136-011-9915-6. [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Jo MW, Lee JW, et al. Validity and reliability of EQ-5D-3L for breast cancer patients in Korea. Health Qual Life Outcomes. 2015;23(13):203. doi: 10.1186/s12955-015-0399-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakthong P, Sonsa-Ardjit N, Sukarnjanaset P, et al. Psychometric properties of the EQ-5D-5L in Thai patients with chronic diseases. Qual Life Res. 2015;24(12):3015–3022. doi: 10.1007/s11136-015-1038-z. [DOI] [PubMed] [Google Scholar]
- 23.Golicki D, Niewada M, Karlińska A, et al. Comparing responsiveness of the EQ-5D-5L, EQ-5D-3L and EQ VAS in stroke patients. Qual Life Res. 2015;24(6):1555–63. [DOI] [PMC free article] [PubMed]
- 24.Tsuchiya A, Ikeda S, Ikegami N, et al. Estimating an EQ-5D population value set: the case of Japan. Health Econ. 2002;11(4):341–353. doi: 10.1002/hec.673. [DOI] [PubMed] [Google Scholar]
- 25.Reilly, Margaret C., Gooch, K. L., Wong, R. L., et al. (2010). Validity, reliability and responsiveness of the Work Productivity and Activity Impairment Questionnaire in ankylosing spondylitis. Rheumatology (Oxford, Engl), 2010;49(4):812–819. [DOI] [PubMed]
- 26.Reilly MC, Bracco A, Ricci J-F, et al. The validity and accuracy of the work productivity and activity impairment questionnaire—irritable bowel syndrome version (WPAI:IBS) Aliment Pharmacol Ther. 2004;20(4):459–467. doi: 10.1111/j.1365-2036.2004.02091.x. [DOI] [PubMed] [Google Scholar]
- 27.Luyster FS, Dunbar-Jacob J. Sleep quality and quality of life in adults with type 2 diabetes. Diabetes Educ. 2011;37(3):347–355. doi: 10.1177/0145721711400663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cappuccio FP, D’Elia L, Strazzullo P, et al. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okamura T, Hashimoto Y, Hamaguchi M, et al. Short sleep duration is a risk of incident nonalcoholic fatty liver disease: a population-based longitudinal study. J Gastrointestin Liver Dis. 2019;28(1):73–81. doi: 10.15403/jgld.2014.1121.281.alc. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Wheaton AG, Croft JB, et al. Relationship between sleep duration and self-reported health-related quality of life among US adults with or without major chronic diseases, 2014. Sleep Health. 2018;4(3):265–272. doi: 10.1016/j.sleh.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai H, Mei Z, An A, et al. Association between sleep problems and health-related quality of life in Canadian adults with chronic diseases. Sleep Med. 2019;61:26–30. doi: 10.1016/j.sleep.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Reis C, Dias S, Rodrigues AM, et al. Sleep duration, lifestyles and chronic diseases: a cross-sectional population-based study. Sleep Sci. 2018;11(4):217–230. doi: 10.5935/1984-0063.20180036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rafie C, Ning Y, Wang A, et al. Impact of physical activity and sleep quality on quality of life of rural residents with and without a history of cancer: findings of the day and night study. Cancer Manag Res. 2018;10:5525–5535. doi: 10.2147/CMAR.S160481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JH, Kim KR, Cho KH, et al. The association between sleep duration and self-rated health in the Korean general population. J Clin Sleep Med. 2013;9(10):1057–1064. doi: 10.5664/jcsm.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12(3):197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151–161. doi: 10.2147/NSS.S134864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finan PH, Quartana PJ, Smith MT. The effects of sleep continuity disruption on positive mood and sleep architecture in healthy adults. Sleep. 2015;38(11):1735–1742. doi: 10.5665/sleep.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tkachenko O, Olson EA, Weber M, et al. Sleep difficulties are associated with increased symptoms of psychopathology. Exp Brain Res. 2014;232(5):1567–1574. doi: 10.1007/s00221-014-3827-y. [DOI] [PubMed] [Google Scholar]
- 39.Furukawa S, Sakai T, Niiya T, et al. Dietary intake habits and the prevalence of nocturia in Japanese patients with type 2 diabetes mellitus. J Diab Investig. 2018;9(2):279–285. doi: 10.1111/jdi.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabara Y, Matsumoto T, Murase K, et al. Lifestyle habits associated with nocturnal urination frequency: the Nagahama study. Neurourol Urodyn. 2019;38(8):2359–2367. doi: 10.1002/nau.24156. [DOI] [PubMed] [Google Scholar]
- 41.Kaji A, Hashimoto Y, Sakai R, et al. Frequent Usage of Convenience Stores is Associated with Low Diet Quality. Nutrients 2019;11(6): pii: E1212. [DOI] [PMC free article] [PubMed]
- 42.Tiemeier H, Pelzer E, Jönck L, et al. Plasma catecholamines and selective slow wave sleep deprivation. Neuropsychobiology. 2002;45(2):81–86. doi: 10.1159/000048681. [DOI] [PubMed] [Google Scholar]
- 43.Ekstedt M, Akerstedt T, Söderström M. Microarousals during sleep are associated with increased levels of lipids, cortisol, and blood pressure. Psychosom Med. 2004;66(6):925–931. doi: 10.1097/01.psy.0000145821.25453.f7. [DOI] [PubMed] [Google Scholar]
- 44.Uchmanowicz I, Markiewicz K, Uchmanowicz B, et al. The relationship between sleep disturbances and quality of life in elderly patients with hypertension. Clin Interv Aging. 2019;14:155–165. doi: 10.2147/CIA.S188499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCoy JG, Strecker RE. The cognitive cost of sleep lost. Neurobiol Learn Mem. 2011;96(4):564–582. doi: 10.1016/j.nlm.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terauchi Y, Ozaki A, Zhao X, et al. Humanistic and economic burden of cardiovascular disease related comorbidities and hypoglycaemia among patients with type 2 diabetes in Japan. Diabetes Res Clin Pract. 2019;149:115–125. doi: 10.1016/j.diabres.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 47.Meneghini LF, Lee LK, Gupta S, Preblick R. Association of hypoglycaemia severity with clinical, patient-reported and economic outcomes in US patients with type 2 diabetes using basal insulin. Diabetes Obes Metab. 2018;20(5):1156–1165. doi: 10.1111/dom.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from Kantar Health but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from corresponding authors, Michiaki Fukui, upon reasonable request and with permission of Kantar Health.