Abstract
Background
Liver biopsy is the reference standard to diagnose nonalcoholic steatohepatitis (NASH) but is invasive with potential complications.
Purpose
To evaluate molecular MRI with type 1 collagen–specific probe EP-3533 and allysine-targeted fibrogenesis probe Gd-Hyd, MR elastography, and native T1 to characterize fibrosis and to assess treatment response in a rat model of NASH.
Materials and Methods
MRI was performed prospectively (June–November 2018) in six groups of male Wistar rats (a) age- and (b) weight-matched animals received standard chow (n = 12 per group); (c) received choline-deficient, l-amino acid–defined, high-fat diet (CDAHFD) for 6 weeks or (d) 9 weeks (n = 8 per group); (e) were fed 6 weeks of CDAHFD and switched to standard chow for 3 weeks (n = 12); (f) were fed CDAHFD for 9 weeks with daily treatment of elafibranor beginning at week 6 (n = 14). Differences in imaging measurements and tissue analyses among groups were tested with one-way analysis of variance. The ability of each imaging measurement to stage fibrosis was quantified by using area under the receiver operating characteristic curve (AUC) with quantitative digital pathology (collagen proportionate area [CPA]) as reference standard. Optimal cutoff values for distinguishing advanced fibrosis were used to assess treatment response.
Results
AUC for distinguishing fibrotic (CPA >4.8%) from nonfibrotic (CPA ≤4.8%) livers was 0.95 (95% confidence interval [CI]: 0.91, 1.00) for EP-3533, followed by native T1, Gd-Hyd, and MR elastography with AUCs of 0.90 (95% CI: 0.83, 0.98), 0.84 (95% CI: 0.74, 0.95), and 0.65 (95% CI: 0.51, 0.79), respectively. AUCs for discriminating advanced fibrosis (CPA >10.3%) were 0.86 (95% CI: 0.76, 0.97), 0.96 (95% CI: 0.90, 1.01), 0.84 (95% CI: 0.70, 0.98), and 0.74 (95% CI: 0.63, 0.86) for EP-3533, Gd-Hyd, MR elastography, and native T1, respectively. Gd-Hyd MRI had the highest accuracy (24 of 26, 92%; 95% CI: 75%, 99%) in identifying responders and nonresponders in the treated groups compared with MR elastography (23 of 26, 88%; 95% CI: 70%, 98%), EP-3533 (20 of 26, 77%; 95% CI: 56%, 91%), and native T1 (14 of 26, 54%; 95% CI: 33%, 73%).
Conclusion
Collagen-targeted molecular MRI most accurately detected early onset of fibrosis, whereas the fibrogenesis probe Gd-Hyd proved most accurate for detecting treatment response.
© RSNA, 2020
Summary
Collagen-targeted molecular MRI probe most accurately detected early onset of liver fibrosis, whereas fibrogenesis probe proved most accurate at detecting treatment response compared with MR elastography and native T1 methods.
Key Results
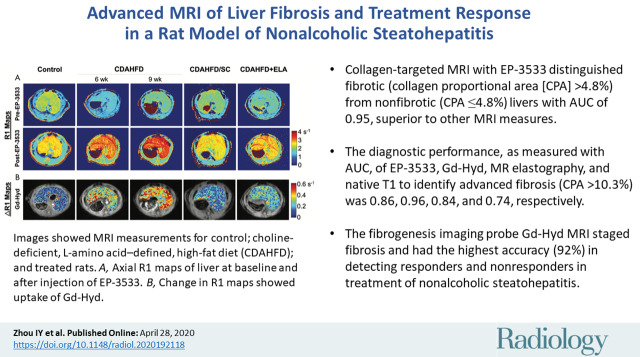
■ Collagen-targeted MRI with EP-3533 distinguished fibrotic (collagen proportional area [CPA] >4.8%) from nonfibrotic (CPA ≤4.8%) livers with an area under the receiver operating characteristic curve (AUC) of 0.95, superior to other MRI measures.
■ The diagnostic performance, as measured with the AUC, of EP-3533, Gd-Hyd, MR elastography, and native T1 to identify advanced fibrosis (CPA >10.3%) was 0.86, 0.96, 0.84, and 0.74, respectively.
■ The fibrogenesis imaging probe Gd-Hyd MRI can stage fibrosis and had the highest accuracy (92%) in detecting responders and nonresponders in treatment of nonalcoholic steatohepatitis.
Introduction
Nonalcoholic steatohepatitis (NASH) is a major form of chronic liver disease in the Western world, with an estimated prevalence of 5% in the general population (1). Closely associated with obesity, diabetes, and the metabolic syndrome (2), NASH is recognized as a major cause of liver-related morbidity and mortality. NASH is associated with steatosis, inflammation, and fibrosis. However, fibrosis is the only histologic feature of disease that is associated with worse liver-related outcomes in NASH (3). Therefore, accurate assessment of fibrosis stage and early detection of fibrosis are essential for effective management of NASH (4). Currently, disease management focuses on diet and other lifestyle modifications. However, a large number of drug candidates targeting different metabolic pathways are under evaluation in clinical trials. It is critical to identify effective approaches for monitoring therapeutic response in NASH (5).
Liver biopsy is the reference standard for diagnosing NASH but it is invasive, has limited sampling, has risk of complication, and cannot be used for serial monitoring (6). Noninvasive imaging techniques that can repeatedly measure disease state throughout the entire organ hold great promise for a more accurate assessment of disease burden, progression, and treatment response. For example, molecular MRI with the type 1 collagen–specific probes EP-3533 or CM-101 was shown to help detect and stage liver fibrosis in different animal models (7–9). Another molecular MRI probe, Gd-Hyd, targeting the allysine residues formed on oxidized collagen, was reported for imaging liver fibrogenesis (10). MR elastography measures stiffness and other mechanoelastic properties of tissue by imaging the propagation of mechanical waves. MR elastography has been most widely applied to assess liver fibrosis, particularly for reliable detection of advanced fibrosis and cirrhosis (11–13). Native T1 has also been proposed for the diagnosis and staging of liver diseases (14,15).
In this study, we used a protocol that combined these four methods to noninvasively characterize disease progression in a choline-deficient, l-amino acid–defined, high-fat diet (CDAHFD) rat model of NASH. To assess treatment response, we used both a diet reversal model and drug treatment with elafibranor (ELA), a dual peroxisome proliferator–activated receptor agonist currently in phase III clinical trials for the treatment of NASH (16).
Materials and Methods
Animal Model
All experiments were performed prospectively (June−November 2018) in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by the institution’s animal care and use committee. A total of 66 adult male Wistar rats (Charles River Laboratories, Wilmington, Mass) were split into six groups and fed with standard chow for (a) 0 weeks; or (b) 9 weeks to serve as weight- and age-matched controls; (c) or with CDAHFD consisting of 60% of kilocalories from fat and 0.1% methionine (A06071302; Research Diets, New Brunswick, NJ) by weight for 6 weeks; or (d) 9 weeks to induce a NASH phenotype; (e) or CDAHFD for 6 weeks followed by standard chow for 3 weeks; (f) or CDAHFD for 9 weeks with daily treatment of ELA (30 mg/kg oral gavage) beginning at week 6. Experimental design, animal numbers, and group classification are shown in Figure 1a. No differences in ex vivo tissue analyses were found between the age- and weight-matched controls (Table E1 [online]), so these two control groups were combined for subsequent analyses.
Figure 1a:
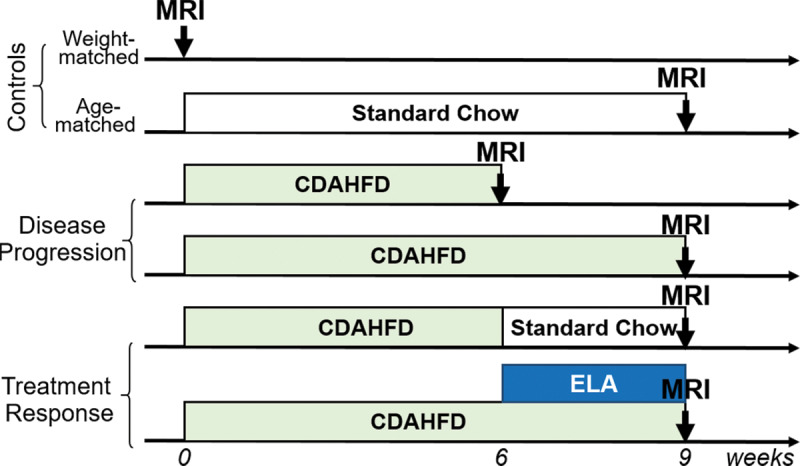
Diagram shows experimental design, animal group classification, and in vivo multiparametric MRI protocol. (a) Adult male Wistar rats receiving standard chow served as weight-matched (n = 12) and age-matched (n = 12) controls. Rats fed with choline-deficient, l-amino acid–defined, high-fat diet (CDAHFD) for 6 or 9 weeks (n = 8 per group) were used to study disease progression of nonalcoholic steatohepatitis (NASH). Rats that received 6 weeks of CDAHFD subsequently either switched back to standard chow for 3 weeks (n = 12) or underwent 3 weeks of daily oral gavage of elafibranor (ELA) (30 mg/kg) while continuing on CDAHFD (n = 14) were used to study treatment effect. (b) MRI protocol involved inversion-recovery sequence for liver R1 quantification and T1-weighted imaging at baseline, followed by Gd-Hyd probe injection, dynamic contrast material–enhanced (DCE) imaging to validate probe delivery to liver, and R1 estimation after Gd-Hyd administration. EP-3533 probe was injected 20 minutes after Gd-Hyd injection, followed by DCE imaging, MR elastography, and R1 quantification after EP-3533 administration. Rats were then killed and liver samples were harvested for biochemical and histologic analyses.
Molecular MRI Probes
EP-3533 is a peptide-based probe containing three gadolinium chelates, binds specifically to type 1 collagen, and has a relaxivity of 48.3 L · mmol–1 · sec–1 at 1.4 T (17,18). Gd-Hyd is a single gadolinium chelate with an allysine-targeting hydrazide moiety and has a relaxivity of 4.1 L · mmol–1 · sec–1 at 1.4 T (10). Both probes were synthesized as reported.
In Vivo MRI
Animals were anesthetized with isoflurane (1%–2%) and imaged supine at 1.5 T (Siemens Healthcare, Malvern, Pa) by using a transmit-receive solenoid coil. Respiration rate was monitored with a pneumatic bellows and maintained 60 breaths per minute ± 5 (standard deviation). The tail vein was cannulated for intravenous delivery of molecular probe. A sterile silver acupuncture needle (48 × 0.20 mm; Asahi Iryoki, Japan) was inserted into the liver tissue through the anterior body wall (19). The needle position was confirmed by using a T1-weighted three-dimensional spoiled gradient-echo scan ensuring no abdominal organs (stomach or duodenum) interfered with the needle trajectory.
The imaging workflow is shown in Figure 1b, and imaging sequences and parameters are summarized in Table 1. A two-point Dixon sequence was performed in six animals from each group to estimate liver fat prior to probe administration (20). Inversion recovery–based R1 mapping and three-dimensional T1-weighted imaging were performed before a bolus injection of Gd-Hyd (65 nmol per total body surface area or total body surface area of 9.83 W2/3, where W is rat weight in grams [21]), followed by dynamic contrast material–enhanced MRI. Then the T1-weighted sequence and the inversion recovery sequence using a single inversion time that nulled the liver signal before Gd-Hyd injection were repeated and interleaved for 20 minutes. EP-3533 was administered (6.5 nmol per total body surface area) as a bolus with dynamic contrast-enhanced MRI and immediately followed with MR elastography. A gradient-echo MR elastography sequence with one pair of 20 mT/m trapezoidal motion-encoding gradients at 200 Hz was used. Forty-five minutes after EP-3533 injection, R1 mapping was performed. Full descriptions of MRI methods are detailed in Appendix E1 (online).
Figure 1b:

Diagram shows experimental design, animal group classification, and in vivo multiparametric MRI protocol. (a) Adult male Wistar rats receiving standard chow served as weight-matched (n = 12) and age-matched (n = 12) controls. Rats fed with choline-deficient, l-amino acid–defined, high-fat diet (CDAHFD) for 6 or 9 weeks (n = 8 per group) were used to study disease progression of nonalcoholic steatohepatitis (NASH). Rats that received 6 weeks of CDAHFD subsequently either switched back to standard chow for 3 weeks (n = 12) or underwent 3 weeks of daily oral gavage of elafibranor (ELA) (30 mg/kg) while continuing on CDAHFD (n = 14) were used to study treatment effect. (b) MRI protocol involved inversion-recovery sequence for liver R1 quantification and T1-weighted imaging at baseline, followed by Gd-Hyd probe injection, dynamic contrast material–enhanced (DCE) imaging to validate probe delivery to liver, and R1 estimation after Gd-Hyd administration. EP-3533 probe was injected 20 minutes after Gd-Hyd injection, followed by DCE imaging, MR elastography, and R1 quantification after EP-3533 administration. Rats were then killed and liver samples were harvested for biochemical and histologic analyses.
Table 1:
Imaging Parameters for Multiparametric MRI in Liver
Image Analysis
Images were analyzed by using a custom-written Matlab R2015a (MathWorks, Natick, Mass) program. Briefly, R1 maps before and after EP-3533 administration were generated by using a voxelwise nonlinear least-squares three-parameter fit of signal intensity as a function of inversion time and repetition time. R1 maps after Gd-Hyd administration were estimated from the single inversion recovery images repeated after Gd-Hyd injection by using the fitted maximum signal intensity and preexponential parameters from the R1 map before Gd-Hyd administration. A threshold-based approach was applied to the R1 maps to generate a region of interest encompassing the liver parenchyma while excluding major blood vessels. Region-of-interest analysis was performed on the R1 maps to obtain change in R1, or ΔR1, for Gd-Hyd and EP-3533, respectively. For MR elastography, a phase gradient reconstruction method (22) was used to extract shear stiffness values from the unwrapped phase images. Fat fraction was calculated from Dixon MRI images (20). Image analysis was performed by I.Y.Z. (with more than 11 years of experience), V.C.J. (with more than 10 years of experience), and N.J.R. (with more than 5 years of experience).
Tissue Analyses
After imaging, the animals were killed and the liver tissue harvested. Pieces of each lobe were fixed in neutral 10% formalin saline, embedded in paraffin, and sectioned into 5-μm-thick slices for staining with Sirius red and hematoxylin and eosin. Collagen proportionate area (CPA), defined as the percentage of the area stained positive by Sirius red, was measured with ImageJ (version 1.52a; National Institutes of Health, Bethesda, Md) as previously described (8,18,23). CPA was shown to be superior to other histologic scoring methods in predicting outcomes in patients with hepatitis C and alcoholism (24). In nonalcoholic fatty liver disease (25), CPA greater than 4.8% correlated with a fibrosis stage greater than or equal to F1, and a CPA greater than 10.3% correlated with fibrosis stage F4. Accordingly, all animals were separated based on their CPA values (no fibrosis, CPA <4.8%; early fibrosis, CPA 4.8%–10.3%; advanced fibrosis, CPA >10.3%). Similarly, morphometric quantitation of hepatic steatosis, expressed as percentage of lipid vacuolization, was performed on the slides stained with hematoxylin and eosin (26). Additional liver samples were analyzed for hydroxyproline expressed as micrograms of hydroxyproline per gram wet weight of liver as previously described (8,23,27).
Statistical Analysis
Results are reported as means ± standard deviation. All statistical analyses were performed by using Prism 6 (GraphPad Software, La Jolla, Calif). Pairwise differences in group means were evaluated by using one-way analysis of variance followed by the Tukey-Kramer posthoc test with P < .05 considered to indicate statistical significance. A multiplicity-adjusted P value was reported. To quantify the discriminatory ability of each imaging measurement between different fibrosis stages, area under the receiver operating characteristic (AUC) was calculated with CPA-defined fibrosis stage as reference standard. The optimal cutoff value was determined from the value that maximized sensitivity and specificity.
Results
Fibrosis progressed with duration on CDAHFD as determined by Sirius red staining (Fig 2, A), which revealed multiple portal fibrotic expansions with occasional bridging fibrosis in the livers of 6-week CDAHFD rats and then complete bridging fibrosis with appearance of some regenerative nodules in the livers of 9-week CDAHFD rats. CPA (Fig 2, B) was elevated in the 6-week CDAHFD rats (7.1% ± 1.9; P = .03) compared with controls (3.5% ± 1.4) and further increased in the 9-week CDAHFD rats (15.7% ± 5.4; P < .001) as disease progressed. Switching to standard chow or undergoing ELA treatment resulted in a reduction in fibrosis (diet reversal: 6.5% ± 2.1, P < .001; ELA: 8.6% ± 1.3, P < .001) compared with 9-week CDAHFD animals that were not treated. Hydroxyproline, as a measure of total tissue collagen (Fig 2, C), was higher in rats that received CDAHFD for 9 weeks compared with the control group and to rats on CDAHFD for 6 weeks (control: 218 µg/g ± 52; CDAHFD 6 weeks: 341 µg/g ± 66; CDAHFD 9 weeks: 1052 µg/g ± 329; P < .001). Consistent with CPA, hydroxyproline levels were reduced in diet reversal and ELA-treated groups compared with the 9-week CDAHFD group (diet reversal: 310 µg/g ± 88; ELA: 359 µg/g ± 77; P < .001). CDAHFD also resulted in rapid formation of liver steatosis as evidenced by the white lipid droplets in images stained with hematoxylin and eosin (Fig 2, A). Histologic quantitation of steatosis, the percentage of lipid vacuolization (Fig 2, D), was increased in livers of rats fed CDAHFD (CDAHFD 6 weeks: 35% ± 4, P < .001; CDAHFD 9 weeks: 24% ± 6, P < .001) compared with livers from control animals (5% ± 2). Steatosis was higher after 6 weeks of CDAHFD than after 9 weeks of diet (P < .001) where steatosis was replaced by fibrosis. Compared with rats fed CDAHFD for 9 weeks (24% ± 6), steatosis was largely resolved after switching to normal diet (13% ± 3; P < .001) but not in animals that underwent ELA treatment (23% ± 8; P > .99). Together, these results demonstrated that the model successfully induced fibrosis and steatosis in the rat liver. Diet reversal markedly alleviated both fibrosis and steatosis, while ELA treatment resulted in a reduction in fibrosis compared with untreated animals but no reduction in steatosis.
Figure 2:
Images show ex vivo tissue characterization of changes in fibrosis and steatosis in choline-deficient, l-amino acid–defined, high-fat diet (CDAHFD) model of nonalcoholic steatohepatitis (NASH) and after treatment. A, Representative images of Sirius red–stained (top row) and hematoxylin and eosin (H&E)–stained (bottom row) liver tissue from each experimental group. Marked hepatic fibrosis and steatosis were observed in rats treated with CDAHFD for 6 weeks (CDAHFD 6wk) or 9 weeks (CDAHFD 9 wk). Fibrosis and steatosis were largely resolved 3 weeks after switching to standard chow (CDAHFD/SC). Reduced fibrosis but not steatosis was found in CDAHFD rats treated with 3 weeks of elafibranor (CDAHFD+ELA). B, Collagen proportionate area (CPA) obtained from analyzing tissue sections stained with Sirius red. C, Total collagen as assessed with liver hydroxyproline (Hyp) content. D, Hepatic steatosis expressed as percentage of lipid vacuolization (LV) obtained from analyzing sections stained with H&E. One-way analysis of variance followed by Tukey posthoc test was performed. * = P < .05, ** = P < .01, and *** = P < .001.
Representative R1 maps of each group acquired before and 45 minutes after the type 1 collagen–targeted probe EP-3533 are shown in Figure 3, A. Liver ΔR1 is higher following EP-3533 administration to the CDAHFD rats compared with the control or treated animals, indicative of greater probe uptake as a result of collagen binding. Figure 3, B shows representative ΔR1 maps averaged over 20 minutes after the injection of fibrogenesis probe Gd-Hyd. Similar to EP-3533, larger ΔR1 changes were seen in animals receiving CDAHFD.
Figure 3:
Images show in vivo MRI measurements for control; choline-deficient, l-amino acid–defined, high-fat diet (CDAHFD) and treated rats. A, Representative axial R1 maps of liver for each group at baseline and after injection of EP-3533. B, Representative change in R1 (ΔR1) maps from each group show uptake of Gd-Hyd. C, EP-3533–induced ΔR1 in liver tissue. D, Gd-Hyd–induced ΔR1. E, Tissue stiffness assessed with MR elastography (MRE). F, Longitudinal T1 prior to injection of any probe. G, Fat fraction (FF) in liver tissue measured with Dixon MRI protocol. One-way analysis of variance followed by Tukey posthoc test was performed. * = P < .05, ** = P < .01, and *** = P < .001. CDAHFD 6wk = rats treated with CDAHFD for 6 weeks, CDAHFD 9wk = rats treated with CDAHFD for 9 weeks, CDAHFD/SC = CDAHFD rats switched to standard chow for 3 weeks, CDAHFD+ELA = CDAHFD rats treated with 3 weeks of elafibranor.
Summary plots from quantitative analysis of each imaging measurement evaluated to detect disease progression and treatment are provided for EP-3533–induced ΔR1 (Fig 3, C), Gd-Hyd–induced ΔR1 (Fig 3, D), shear stiffness values extracted from the MR elastography data (Fig 3, E), native liver tissue T1 measurement (Fig 3, F), and fat fraction measured by using Dixon MRI (Fig 3, G). Summary of in vivo MRI and ex vivo tissue analyses measurements can be found in Table E2 (online). The difference between the control and 6-week CDAHFD animals could be detected with EP-3533–induced ΔR1, Gd-Hyd–induced ΔR1, and native T1 and Dixon fat fraction with P < .001 but not MR elastography stiffness (P = .97). All five imaging measurements revealed a difference between the control and 9-week CDAHFD animals with P < .001. Additionally, Gd-Hyd–induced ΔR1 and MR elastography stiffness increased (both with P < .001) as disease progressed from 6-week to 9-week CDAHFD while native T1 decreased (P = .03). After reversal to standard chow, all imaging measurements could identify differences compared with animals that remained on CDAHFD (9-week CDAHFD) with P < .001. The treatment effect of ELA could be detected with molecular MRI with EP-3533 (P < .001; ELA vs 9-week CDAHFD) and Gd-Hyd (P = .002) as well as MR elastography stiffness (P < .001) but not with native T1 measurement (P = .23) or Dixon (P = .65).
We performed receiver operating characteristic analysis to evaluate the effectiveness of each imaging technique to discriminate different stages of fibrosis determined by using CPA values (Fig 4). The AUC for distinguishing fibrotic (CPA >4.8%) from nonfibrotic (CPA ≤4.8%) livers was 0.95 (95% confidence interval [CI]: 0.91, 1.00) for EP-3533 (Fig 4a), 0.84 (95% CI: 0.74, 0.95) for Gd-Hyd (Fig 4b), and 0.90 (95% CI: 0.83, 0.98) for native T1 elastography (Fig 4d), whereas MR elastography (Fig 4c) had the smallest AUC of 0.65 (95% CI: 0.51, 0.79; P < .001). Molecular imaging with EP-3533, Gd-Hyd, and MR elastography have similar AUCs of 0.86 (95% CI: 0.76, 0.97), 0.96 (95% CI: 0.90, 1.01), and 0.84 (95% CI: 0.70, 0.98), respectively, for discriminating advanced fibrosis (CPA >10.3%). Native T1 AUC was much lower 0.74 (95% CI: 0.63, 0.86; P = .01).
Figure 4a:
Graphs show receiver operating characteristic (ROC) analysis of liver fibrosis staging by (a) type 1 collagen MRI with EP-3533, (b) oxidized collagen MRI with Gd-Hyd, (c) shear stiffness with MR elastography, and (d) native T1 over collagen proportionate area (CPA) values. ROC curves were grouped to assess ability of each imaging technique to distinguish nonfibrotic group (CPA ≤4.8%) from fibrotic groups (CPA >4.8%) and to distinguish no disease or moderate fibrosis (CPA ≤10.3%) from severe fibrosis (CPA >10.3%). Area under ROC curve (AUROC), 95% confidence interval (CI), and P value are given for each curve.
Figure 4b:
Graphs show receiver operating characteristic (ROC) analysis of liver fibrosis staging by (a) type 1 collagen MRI with EP-3533, (b) oxidized collagen MRI with Gd-Hyd, (c) shear stiffness with MR elastography, and (d) native T1 over collagen proportionate area (CPA) values. ROC curves were grouped to assess ability of each imaging technique to distinguish nonfibrotic group (CPA ≤4.8%) from fibrotic groups (CPA >4.8%) and to distinguish no disease or moderate fibrosis (CPA ≤10.3%) from severe fibrosis (CPA >10.3%). Area under ROC curve (AUROC), 95% confidence interval (CI), and P value are given for each curve.
Figure 4d:
Graphs show receiver operating characteristic (ROC) analysis of liver fibrosis staging by (a) type 1 collagen MRI with EP-3533, (b) oxidized collagen MRI with Gd-Hyd, (c) shear stiffness with MR elastography, and (d) native T1 over collagen proportionate area (CPA) values. ROC curves were grouped to assess ability of each imaging technique to distinguish nonfibrotic group (CPA ≤4.8%) from fibrotic groups (CPA >4.8%) and to distinguish no disease or moderate fibrosis (CPA ≤10.3%) from severe fibrosis (CPA >10.3%). Area under ROC curve (AUROC), 95% confidence interval (CI), and P value are given for each curve.
Figure 4c:
Graphs show receiver operating characteristic (ROC) analysis of liver fibrosis staging by (a) type 1 collagen MRI with EP-3533, (b) oxidized collagen MRI with Gd-Hyd, (c) shear stiffness with MR elastography, and (d) native T1 over collagen proportionate area (CPA) values. ROC curves were grouped to assess ability of each imaging technique to distinguish nonfibrotic group (CPA ≤4.8%) from fibrotic groups (CPA >4.8%) and to distinguish no disease or moderate fibrosis (CPA ≤10.3%) from severe fibrosis (CPA >10.3%). Area under ROC curve (AUROC), 95% confidence interval (CI), and P value are given for each curve.
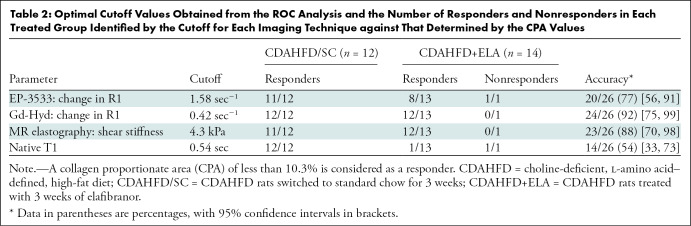
For the diet reversal group, all animals met the treatment response criteria of CPA less than 10.3%, whereas for the ELA group, 13 of 14 rats showed positive treatment response. Table 2 shows the optimal cutoff value for each imaging technique and corresponding accuracy to predict treatment response. Overall, Gd-Hyd had the highest accuracy (24 of 26, 92%; 95% CI: 75%, 99%) in detecting responders and nonresponders in the treatment groups, followed by MR elastography (23 of 26, 88%; 95% CI: 70%, 98%) and EP-3533 (20 of 26, 77%; 95% CI: 56%, 91%), with native T1 being the least accurate (14 of 26, 54%; 95% CI: 33%, 73%). Native T1 correlated positively with measures of steatosis (r = 0.88 vs percentage of lipid vacuolization, r = 0.94 vs Dixon fat fraction) but less with fibrosis (r = 0.46 vs CPA, r = 0.31 vs hydroxyproline) (Figs E3–E5 [online]), indicating that native T1 is a much better predictor of steatosis in our model.
Table 2:
Optimal Cutoff Values Obtained from the ROC Analysis and the Number of Responders and Nonresponders in Each Treated Group Identified by the Cutoff for Each Imaging Technique against That Determined by the CPA Values
Discussion
Collagen-targeted MRI with EP-3533 was the most sensitive of the four methods for detecting initial fibrosis in our model and further confirms the robustness of this noninvasive technique in assessing fibrosis across animal species and liver injury models (7,8,19,23). EP-3533–enhanced MRI showed heterogeneity in the choline-deficient, l-amino acid–defined, high-fat diet (CDAHFD) liver, highlighting the benefit of a complete assessment of liver fibrosis compared with the potential for sampling error in biopsy-based diagnosis (28). In addition, as suggested by the large interquartile range of the 9-week CDAHFD group, interindividual variations were high in this fibrotic group. This was consistent with variations in collagen proportionate area and hydroxyproline levels, indicating that the extent of fibrosis varied among animals after this duration on diet.
EP-3533 targets the end result of fibrosis, collagen accumulation, whereas Gd-Hyd binds to oxidized collagen, a marker of disease activity (fibrogenesis). Gd-Hyd MRI was shown to robustly detect and stage pulmonary and hepatic fibrogenesis, as well as monitor therapeutic response in mouse models (10,26). In our rat CDAHFD model, Gd-Hyd–enhanced MRI provides robust detection of both early onset of disease and more advanced fibrosis, as well as the ability to distinguish intermediate fibrosis from early or late disease.
Elastography methods using US or MRI are increasingly used to help detect liver fibrosis (29–31). These methods assess tissue stiffness, which increases exponentially with histologic stage of fibrosis, and are weighted toward more advanced disease (32,33). Using MR elastography, we observed a similar effect in our model where much larger changes in tissue stiffness occurred in advanced fibrosis. Whereas liver fibrosis is consistently associated with increased hepatic parenchymal stiffness, the reverse is not always true. Acute inflammation, severe steatosis, or increased postprandial portal blood flow have been found to independently elevate hepatic stiffness (30,34,35).
Native T1 measurement with iron content correction was used to stage liver fibrosis (36,37). However, NASH is characterized by increased hepatic fat, which can complicate T1 measurement for staging fibrosis. At 1.5 T and an echo time of 2.4 msec in our T1 mapping protocol, the spins of water and fat protons go out of phase. This phase cancellation effect has been used to identify and even quantify fat content in the liver (38). We found a strong positive correlation between the native T1 measurement and fat fraction measured by using histologic analysis or Dixon MRI. Despite a relatively large AUC of 0.90 for native T1 to detect early stage fibrosis, the T1 difference is dominated by the contribution from marked steatosis. Therefore, the effect of fat content on T1 should be accounted for when using T1 measurement to stage liver fibrosis.
Having assessed their ability to detect and stage fibrosis, we then showed how these imaging techniques can be used to monitor treatment response. Switching CDAHFD-fed rats to standard chow resulted in resolved fibrosis and improved steatosis. This disease regression can be robustly captured with individual imaging measurements. In our NASH model, ELA treatment had a beneficial effect in improving fibrosis but less so steatosis. Gd-Hyd–enhanced MRI and MR elastography both sensitively detected the antifibrotic effects of both diet reversal and of ELA treatment. Conversely, native T1 detected no significant difference associated with ELA treatment, further confirming the major contribution from fat content in the T1 measurement.
Our study had limitations. EP-3533 uses linear gadopentetate dimeglumine chelates and is not suitable for clinical translation due to risk of gadolinium retention (9). A similar collagen-binding probe CM-101 (23) using the more stable macrocyclic Gd-DOTA chelate could replace the use of EP-3533, possibly offering comparable sensitivity and specificity. Our CDAHFD model lacks the metabolic derangements typically observed in patients with NASH. However, simultaneously establishing metabolic dysregulation and steatohepatitis that leads to progressive liver failure has proven difficult, and most models accentuate either the metabolic or the hepatotoxic components (39). While the Western diet most accurately captures the dietary habits of humans with obesity and insulin resistance, fibrosis progression is very slow. Therefore, we added choline deficiency in an effort to capture a more complete spectrum of disease despite the acknowledged metabolic limitations. Another limitation was our use of only male animals. It is well documented that female rodents are relatively protected from fibrosis progression (40). Future endeavors are needed to better address sex discrepancy. Finally, both treatments resulted in a marked improvement in fibrosis such that we were only able to assess the overall accuracy of the techniques to measure response, but not sensitivity and specificity.
The advanced MRI techniques applied here differ in their capture of the fibrotic process. All techniques were performed in a single protocol, highlighting the strength of MRI for characterizing liver disease in the context of nonalcoholic steatohepatitis (NASH). Although not the purpose of our study, a multiparametric composite readout could be created that may be more accurate than the individual measurements given the complementary nature of the techniques. The rich information provided by this protocol raises new possibilities for clinical imaging and for assessing treatment response for new NASH therapies in development.
APPENDIX
SUPPLEMENTAL FIGURES
Disclosures of Conflicts of Interest: I.Y.Z. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution received a grant from Pfizer. Other relationships: disclosed no relevant relationships. V.C.J. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution received a grant from Pfizer. Other relationships: disclosed no relevant relationships. N.J.R. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution received a grant from Pfizer. Other relationships: disclosed no relevant relationships. E.A. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution received a grant from Pfizer. Other relationships: disclosed no relevant relationships. S.K. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is employed by Crispr. Other relationships: disclosed no relevant relationships. G.A. disclosed no relevant relationships. H.K. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution received a grant from Pfizer. Other relationships: disclosed no relevant relationships. H.S. disclosed no relevant relationships. N.W. disclosed no relevant relationships. N.M. disclosed no relevant relationships. C.T.F. disclosed no relevant relationships. J.W. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is employed by and holds stock/stock options in Pfizer. Other relationships: disclosed no relevant relationships. R.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is employed by and holds stock/stock options in Pfizer. Other relationships: disclosed no relevant relationships. F.S. disclosed no relevant relationships. K.K.T. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: has grants/grants pending with Blade Pharmaceuticals, Enanta Pharmaceuticals, and Zafgen; has patents (planned, pending, or issued). Other relationships: disclosed no relevant relationships. B.C.F. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a board member of Mediar Therapeutics; is a consultant for Collagen Medical and Gilead; has grants/grants pending with Blade Pharmaceuticals, Enanta Pharmaceuticals, and Collagen Medical. Other relationships: disclosed no relevant relationships. P.C. Activities related to the present article: is a consultant for Collagen Medical. Activities not related to the present article: is a consultant for Bayer; has grants/grants pending with Indalo Therapeutics, National Institutes of Health, Pfizer, and Pliant Therapeutics; holds stock/stock options in Reveal Pharmaceuticals and Collagen Medical. Other relationships: disclosed no relevant relationships.
B.C.F. supported by National Institute of Diabetes and Digestive and Kidney Diseases (R01DK104956). P.C. supported by Pfizer, National Institute for Biomedical Imaging and Bioengineering (R01EB009062), National Institute of Diabetes and Digestive and Kidney Diseases (R01DK121789), and Office of the Director (S10OD010650, S10OD025234).
Abbreviations:
- AUC
- area under the receiver operating characteristic curve
- CDAHFD
- choline-deficient
- l-amino acid–defined
- high-fat diet
- CI
- confidence interval
- CPA
- collagen proportionate area
- ELA
- elafibranor
- NASH
- nonalcoholic steatohepatitis
References
- 1.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34(3):274–285. [DOI] [PubMed] [Google Scholar]
- 2.Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 2001;50(8):1844–1850. [DOI] [PubMed] [Google Scholar]
- 3.Hagström H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol 2017;67(6):1265–1273. [DOI] [PubMed] [Google Scholar]
- 4.Serfaty L. Management of patients with non-alcoholic steatohepatitis (NASH) in real life. Liver Int 2018;38(Suppl 1):52–55. [DOI] [PubMed] [Google Scholar]
- 5.Motola DL, Caravan P, Chung RT, Fuchs BC. Noninvasive Biomarkers of Liver Fibrosis: Clinical Applications and Future Directions. Curr Pathobiol Rep 2014;2(4):245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 2014;20(2):475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polasek M, Fuchs BC, Uppal R, et al. Molecular MR imaging of liver fibrosis: a feasibility study using rat and mouse models. J Hepatol 2012;57(3):549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuchs BC, Wang H, Yang Y, et al. Molecular MRI of collagen to diagnose and stage liver fibrosis. J Hepatol 2013;59(5):992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrar CT, Gale EM, Kennan R, et al. CM-101: Type I Collagen-targeted MR Imaging Probe for Detection of Liver Fibrosis. Radiology 2018;287(2):581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen HH, Waghorn PA, Wei L, et al. Molecular imaging of oxidized collagen quantifies pulmonary and hepatic fibrogenesis. JCI Insight 2017;2(11):91506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huwart L, Peeters F, Sinkus R, et al. Liver fibrosis: non-invasive assessment with MR elastography. NMR Biomed 2006;19(2):173–179. [DOI] [PubMed] [Google Scholar]
- 12.Rouvière O, Yin M, Dresner MA, et al. MR elastography of the liver: preliminary results. Radiology 2006;240(2):440–448. [DOI] [PubMed] [Google Scholar]
- 13.Loomba R, Wolfson T, Ang B, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology 2014;60(6):1920–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee R, Pavlides M, Tunnicliffe EM, et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol 2014;60(1):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavlides M, Banerjee R, Sellwood J, et al. Multiparametric magnetic resonance imaging predicts clinical outcomes in patients with chronic liver disease. J Hepatol 2016;64(2):308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phase 3 Study to Evaluate the Efficacy and Safety of Elafibranor Versus Placebo in Patients With Nonalcoholic Steatohepatitis (NASH).
- 17.Helm PA, Caravan P, French BA, et al. Postinfarction myocardial scarring in mice: molecular MR imaging with use of a collagen-targeting contrast agent. Radiology 2008;247(3):788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caravan P, Das B, Dumas S, et al. Collagen-targeted MRI contrast agent for molecular imaging of fibrosis. Angew Chem Int Ed Engl 2007;46(43):8171–8173. [DOI] [PubMed] [Google Scholar]
- 19.Zhu B, Wei L, Rotile N, et al. Combined magnetic resonance elastography and collagen molecular magnetic resonance imaging accurately stage liver fibrosis in a rat model. Hepatology 2017;65(3):1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon WT. Simple proton spectroscopic imaging. Radiology 1984;153(1):189–194. [DOI] [PubMed] [Google Scholar]
- 21.Gouma E, Simos Y, Verginadis I, Lykoudis E, Evangelou A, Karkabounas S. A simple procedure for estimation of total body surface area and determination of a new value of Meeh’s constant in rats. Lab Anim 2012;46(1):40–45. [DOI] [PubMed] [Google Scholar]
- 22.Yin M, Woollard J, Wang X, et al. Quantitative assessment of hepatic fibrosis in an animal model with magnetic resonance elastography. Magn Reson Med 2007;58(2):346–353. [DOI] [PubMed] [Google Scholar]
- 23.Farrar CT, DePeralta DK, Day H, et al. 3D molecular MR imaging of liver fibrosis and response to rapamycin therapy in a bile duct ligation rat model. J Hepatol 2015;63(3):689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsochatzis E, Bruno S, Isgro G, et al. Collagen proportionate area is superior to other histological methods for sub-classifying cirrhosis and determining prognosis. J Hepatol 2014;60(5):948–954. [DOI] [PubMed] [Google Scholar]
- 25.Buzzetti E, Hall A, Ekstedt M, et al. Collagen proportionate area is an independent predictor of long-term outcome in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2019;49(9):1214–1222. [DOI] [PubMed] [Google Scholar]
- 26.Erstad DJ, Farrar CT, Ghoshal S, et al. Molecular magnetic resonance imaging accurately measures the antifibrotic effect of EDP-305, a novel farnesoid X receptor agonist. Hepatol Commun 2018;2(7):821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutson PR, Crawford ME, Sorkness RL. Liquid chromatographic determination of hydroxyproline in tissue samples. J Chromatogr B Analyt Technol Biomed Life Sci 2003;791(1-2):427–430. [DOI] [PubMed] [Google Scholar]
- 28.Marcellin P, Asselah T, Boyer N. Fibrosis and disease progression in hepatitis C. Hepatology 2002;36(5 Suppl 1):S47–S56. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Talwalkar JA, Yin M, Glaser KJ, Sanderson SO, Ehman RL. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology 2011;259(3):749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salameh N, Larrat B, Abarca-Quinones J, et al. Early detection of steatohepatitis in fatty rat liver by using MR elastography. Radiology 2009;253(1):90–97. [DOI] [PubMed] [Google Scholar]
- 31.Nobili V, Vizzutti F, Arena U, et al. Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology 2008;48(2):442–448. [DOI] [PubMed] [Google Scholar]
- 32.Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003;29(12):1705–1713. [DOI] [PubMed] [Google Scholar]
- 33.Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005;41(1):48–54. [DOI] [PubMed] [Google Scholar]
- 34.Arena U, Vizzutti F, Corti G, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 2008;47(2):380–384. [DOI] [PubMed] [Google Scholar]
- 35.Petta S, Maida M, Macaluso FS, et al. The severity of steatosis influences liver stiffness measurement in patients with nonalcoholic fatty liver disease. Hepatology 2015;62(4):1101–1110. [DOI] [PubMed] [Google Scholar]
- 36.Eddowes PJ, McDonald N, Davies N, et al. Utility and cost evaluation of multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2018;47(5):631–644. [DOI] [PubMed] [Google Scholar]
- 37.Pavlides M, Banerjee R, Tunnicliffe EM, et al. Multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease severity. Liver Int 2017;37(7):1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Outwater EK, Blasbalg R, Siegelman ES, Vala M. Detection of lipid in abdominal tissues with opposed-phase gradient-echo images at 1.5 T: techniques and diagnostic importance. RadioGraphics 1998;18(6):1465–1480. [DOI] [PubMed] [Google Scholar]
- 39.Haczeyni F, Yeh MM, Ioannou GN, et al. Mouse models of non-alcoholic steatohepatitis: A reflection on recent literature. J Gastroenterol Hepatol 2018;33(7):1312–1320. [DOI] [PubMed] [Google Scholar]
- 40.Yasuda M, Shimizu I, Shiba M, Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology 1999;29(3):719–727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.