Once defined in rhetorical but ultimately meaningless terms as “the conscientious, judicious and explicit use of current best evidence in making decisions about the care of individual patients” [1], evidence-based medicine rests on certain philosophical assumptions: a singular truth, ascertainable through empirical enquiry; a linear logic of causality in which interventions have particular effect sizes; rigour defined primarily in methodological terms (especially, a hierarchy of preferred study designs and tools for detecting bias); and a deconstructive approach to problem-solving (the evidence base is built by answering focused questions, typically framed as ‘PICO’—population-intervention-comparison-outcome) [2].
The trouble with pandemics is that these assumptions rarely hold. A pandemic-sized problem can be framed and contested in multiple ways. Some research questions around COVID-19, most notably relating to drugs and vaccines, are amenable to randomised controlled trials (and where such trials were possible, they were established with impressive speed and efficiency [3, 4]). But many knowledge gaps are broader and cannot be reduced to PICO-style questions. Were care home deaths avoidable [5]? Why did the global supply chain for personal protective equipment break down [6]? What role does health system resilience play in controlling the pandemic [7]? And so on.
Against these—and other—wider questions, the neat simplicity of a controlled, intervention-on versus intervention-off experiment designed to produce a definitive (i.e. statistically significant and widely generalisable) answer to a focused question rings hollow. In particular, upstream preventive public health interventions aimed at supporting widespread and sustained behaviour change across an entire population (as opposed to testing the impact of a short-term behaviour change in a select sample) rarely lend themselves to such a design [8, 9]. When implementing population-wide public health interventions—whether conventional measures such as diet or exercise, or COVID-19 related ones such as handwashing, social distancing and face coverings—we must not only persuade individuals to change their behavior but also adapt the environment to make such changes easier to make and sustain [10–12].
Population-wide public health efforts are typically iterative, locally-grown and path-dependent, and they have an established methodology for rapid evaluation and adaptation [9]. But evidence-based medicine has tended to classify such designs as “low methodological quality” [13]. Whilst this has been recognised as a problem in public health practice for some time [11], the inadequacy of the dominant paradigm has suddenly become mission-critical.
Whilst evidence-based medicine recognises that study designs must reflect the nature of question (randomized trials, for example, are preferred only for therapy questions [13]), even senior scientists sometimes over-apply its hierarchy of evidence. An interdisciplinary group of scholars from the UK’s prestigious Royal Society recently reviewed the use of face masks by the general public, drawing on evidence from laboratory science, mathematical modelling and policy studies [14]. The report was criticised by epidemiologists for being “non-systematic” and for recommending policy action in the absence of a quantitative estimate of effect size from robust randomized controlled trials [15].
Such criticisms appear to make two questionable assumptions: first, that the precise quantification of impact from this kind of intervention is both possible and desirable, and second, that unless we have randomized trial evidence, we should do nothing.
It is surely time to turn to a more fit-for-purpose scientific paradigm. Complex adaptive systems theory proposes that precise quantification of particular cause-effect relationships is both impossible (because such relationships are not constant and cannot be meaningfully isolated) and unnecessary (because what matters is what emerges in a particular real-world situation). This paradigm proposes that where multiple factors are interacting in dynamic and unpredictable ways, naturalistic methods and rapid-cycle evaluation are the preferred study design. The 20th-century logic of evidence-based medicine, in which scientists pursued the goals of certainty, predictability and linear causality, remains useful in some circumstances (for example, the drug and vaccine trials referred to above). But at a population and system level, we need to embrace 21st-century epistemology and methods to study how best to cope with uncertainty, unpredictability and non-linear causality [16].
In a complex system, the question driving scientific inquiry is not “what is the effect size and is it statistically significant once other variables have been controlled for?” but “does this intervention contribute, along with other factors, to a desirable outcome?”. Multiple interventions might each contribute to an overall beneficial effect through heterogeneous effects on disparate causal pathways, even though none would have a statistically significant impact on any predefined variable [11]. To illuminate such influences, we need to apply research designs that foreground dynamic interactions and emergence. These include in-depth, mixed-method case studies (primary research) and narrative reviews (secondary research) that tease out interconnections and highlight generative causality across the system [16, 17].
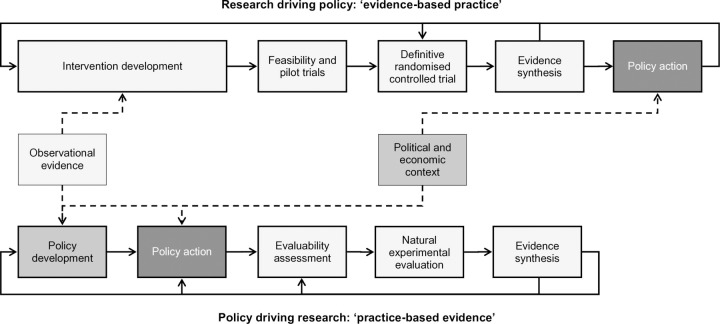
Table 1 lists some philosophical contrasts between the evidence-based medicine and complex-systems paradigms. Ogilvie et al have argued that rather than pitting these two paradigms against one another, they should be brought together [9]. As illustrated in (Fig 1), these authors depict randomized trials (what they call the “evidence-based practice pathway”) and natural experiments (the “practice-based evidence pathway”) in a complementary and recursive relationship rather than a hierarchical one. They propose that “…intervention studies [e.g. trials] should focus on reducing critical uncertainties, that non-randomised study designs should be embraced rather than tolerated and that a more nuanced approach to appraising the utility of diverse types of evidence is required.” (page 203) [9].
Table 1. Evidence-based medicine versus complex systems research paradigms.
Adapted under Creative Commons licence from Greenhalgh and Papoutsi [16].
| Evidence-based medicine paradigm | Complex systems paradigm | |
|---|---|---|
| Perspective on scientific truth | Singular, independent of the observer, ascertainable through empirical inquiry | Multiple, influenced by mode of inquiry and perspective taken |
| Goal of research | Establishing the truth; finding more or less universal and generalisable solutions to well-defined problems | Exploring tensions; generating insights and wisdom; exposing multiple perspectives; viewing complex systems as moving targets |
| Assumed model of causality | Linear, cause-and-effect causality (perhaps incorporating mediators and moderators) | Emergent causality: multiple interacting influences account for a particular outcome but none can be said to have a fixed ‘effect size’ |
| Typical format of research question | “What is the effect size of the intervention on the predefined outcome, and is it statistically significant?” | “What combination of influences has generated this phenomenon? What does the intervention of interest contribute? What happens to the system and its actors if we intervene in a particular way? What are the unintended consequences elsewhere in the system?” |
| Mode of representation | Attempt to represent science in one authoritative voice | Attempt to illustrate the plurality of voices inherent in the research and phenomena under study |
| Good research is characterised by | Methodological ‘rigour’, i.e. strict application of structured and standardised design, conventional approaches to generalisability and validity | Strong theory, flexible methods, pragmatic adaptation to emerging circumstances, contribution to generative learning and theoretical transferability |
| Purpose of theorising | Disjunctive: simplification and abstraction; breaking problems down into analysable parts | Conjunctive: drawing parts of the problem together to produce a rich, nuanced picture of what is going on and why |
| Approach to data | Research should continue until data collection is complete | Data will never be complete or perfect; decisions often need to be made despite incomplete or contested data |
| Analytic focus | Dualisms: A versus B; influence of X on Y | Dualities: inter-relationships and dynamic tensions between A, B, C and other emergent aspects |
Fig 1. Ogilvie et al’s model of two complementary modes of evidence generation: evidence-based practice and practice-based evidence.
Reproduced under CC-BY-4.0 licence from authors’ original [9].
In the current fast-moving pandemic, where the cost of inaction is counted in the grim mortality figures announced daily, implementing new policy interventions in the absence of randomized trial evidence has become both a scientific and moral imperative. Whilst it is hard to predict anything in real time, history will one day tell us whether adherence to “evidence-based practice” helped or hindered the public health response to Covid-19—or whether an apparent slackening of standards to accommodate “practice-based evidence” was ultimately a more effective strategy.
Funding Statement
TG’s research is funded from the following sources: National Institute for Health Research (BRC-1215-20008), UK Research and Innovation (COVID-19 Emergency Fund), and Wellcome Trust (WT104830MA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sackett DL, Rosenberg WM, Gray JM, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn't. Bmj. 1996;312(7023):71–2. 10.1136/bmj.312.7023.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenhalgh T. How to Read a Paper: The basics of evidence-based medicine and healthcare (6th edition). Oxford: John Wiley and Sons Ltd; 2019. [Google Scholar]
- 3.Baden LR, Rubin EJ. Covid-19—the search for effective therapy. New Engl J Med. 2020;382:1851–2. 10.1056/NEJMe2005477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 vaccines at pandemic speed. New Engl J Med. 2020;382:1969–73. 10.1056/NEJMp2005630 [DOI] [PubMed] [Google Scholar]
- 5.Gordon AL, Goodman C, Achterberg W, Barker RO, Burns E, Hanratty B, et al. COVID in Care Homes—Challenges and Dilemmas in Healthcare Delivery. Age and Ageing. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armani AM, Hurt DE, Hwang D, McCarthy MC, Scholtz A. Low-tech solutions for the COVID-19 supply chain crisis. Nature Reviews Materials. 2020:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Legido-Quigley H, Asgari N, Teo YY, Leung GM, Oshitani H, Fukuda K, et al. Are high-performing health systems resilient against the COVID-19 epidemic? The Lancet. 2020;395(10227):848–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.West R, Michie S, Rubin GJ, Amlôt R. Applying principles of behaviour change to reduce SARS-CoV-2 transmission. Nature Human Behaviour. 2020:1–9. [DOI] [PubMed] [Google Scholar]
- 9.Ogilvie D, Adams J, Bauman A, Gregg EW, Panter J, Siegel KR, et al. Using natural experimental studies to guide public health action: turning the evidence-based medicine paradigm on its head. J Epidemiol Community Health. 2020;74(2):203–8. 10.1136/jech-2019-213085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glass TA, McAtee MJ. Behavioral science at the crossroads in public health: extending horizons, envisioning the future. Social science & medicine. 2006;62(7):1650–71. [DOI] [PubMed] [Google Scholar]
- 11.Rutter H, Savona N, Glonti K, Bibby J, Cummins S, Finegood DT, et al. The need for a complex systems model of evidence for public health. The Lancet. 2017;390(10112):2602–4. [DOI] [PubMed] [Google Scholar]
- 12.Jefferson T, Del Mar CB, Dooley L, Ferroni E, Al‐Ansary LA, Bawazeer GA, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane database of systematic reviews. 2011;(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj. 2008;336(7650):924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DELVE. Face Masks for the General Public. London: Royal Society DELVE (Data Evaluation and Learning for Viral Epidemics) initiative; 2020. Accessed 4th May 2020 at https://rs-delve.github.io/reports.html. [Google Scholar]
- 15.Science Media Centre. Expert reaction to review of evidence on face masks and face coverings by the Royal Society DELVE Initiative. London: Science Media Centre; 2020. (4th May). Accessed 7th May 2020 at https://www.sciencemediacentre.org/expert-reaction-to-review-of-evidence-on-face-masks-and-face-coverings-by-the-royal-society-delve-initiative/. [Google Scholar]
- 16.Greenhalgh T, Papoutsi C. Studying complexity in health services research: desperately seeking an overdue paradigm shift. BioMed Central; 2018. p. 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenhalgh T, Thorne S, Malterud K. Time to challenge the spurious hierarchy of systematic over narrative reviews? European journal of clinical investigation. 2018;48(6):e12931 10.1111/eci.12931 [DOI] [PMC free article] [PubMed] [Google Scholar]