Abstract
Context
According to the carbohydrate-insulin model of obesity, an elevated insulin-to-glucagon ratio in response to a high-carbohydrate diet directs metabolic fuels toward storage, resulting in lower circulating energy.
Objective
To determine differences in total circulating energy post-meal related to dietary carbohydrate.
Design
Ancillary study within the Framingham State Food Study.
Setting
University community.
Participants
29 adults (aged 20 to 65 years) with overweight or obesity (body mass index ≥25 kg/m2)
Intervention
After achieving 10% to 14% weight loss on a run-in diet, participants were randomized to weight-loss-maintenance test diets varying in carbohydrate content (high-carbohydrate, 60% of total energy, n = 11; moderate-carbohydrate, 40%, n = 8; low-carbohydrate, 20%, n = 10) and controlled for protein (20%). During 24-hour metabolic ward admissions between 10 and 15 weeks on the test diets, metabolic fuels and hormones were measured.
Main Outcome Measure
Energy availability (EA) based on energy content of blood glucose, beta-hydroxybutyrate, and free fatty acids, in the late postprandial period (180 to 300 minutes). Insulin at 30 minutes into the test meal (Meal Insulin-30) was measured as an effect modifier.
Results
Insulin-to-glucagon ratio was 7-fold higher in participants on the high- vs low-carbohydrate diet (2.5 and 0.36, respectively). Late postprandial EA was 0.58 kcal/L lower on the high- vs low-carbohydrate diet (P < 0.0001), primarily related to suppression of free fatty acids. Early postprandial EA (30 to 180 minutes) declined fastest in the high-carbohydrate group, and Meal Insulin-30 modified this diet effect.
Conclusions
During weight-loss maintenance on a high-carbohydrate diet, late postprandial EA is reduced, consistent with the carbohydrate-insulin model.
Keywords: carbohydrate, obesity, insulin, beta-hydroxybutyrate, glucose, fatty acids
Many people with excess weight have difficulty maintaining long-term weight loss [1]. Physiological adaptations to weight loss, such as a decline in energy expenditure and increase in hunger [2, 3], may be among the factors driving weight regain. Although the conventional approach to weight loss has focused on energy restriction, an alternative strategy aims to reduce total or high–glycemic index carbohydrate intake, based on the carbohydrate-insulin model of obesity [4, 5].
Insulin secretion in response to dietary carbohydrate is known to promote storage of circulating glucose and fatty acids into tissues (liver, muscle, and adipose), and to suppress ketone formation. By lowering the insulin-to-glucagon ratio, a diet low in glycemic load (the arithmetic product of glycemic index and carbohydrate amount) may improve metabolic fuel availability in the late postprandial phase, thereby attenuating hunger [6, 7] and increasing energy expenditure [8-10]. Conversely, a high glycemic load may increase hunger and stimulate brain centers controlling reward and craving 4 hours after a meal [11]. In a preliminary study of 1-month duration, total energy from circulating metabolic fuels was observed to be tightly controlled, in the range of 4 to 6 kcal/L, and lower in the late postprandial period following a high- vs low-carbohydrate meal [12]. However, the longer-term effects of glycemic load on metabolic fuel availability during weight loss maintenance, controlled from dietary protein, have not been studied.
The primary aim of this study was to evaluate the effect of 3 diets varying widely in carbohydrate content on postprandial energy availability (EA), defined as circulating fuel level multiplied by relative energy content for glucose, beta-hydroxybutyrate (BOHB), and free fatty acids (FFA) in a large-scale, relatively long-term feeding trial. Specifically, we hypothesized that during weight-loss-maintenance, total EA (EAGlucose + EABOHB + EAFFA) during the late postprandial period (180-300 minutes) would be lowest on a high-carbohydrate diet compared to a moderate- or low-carbohydrate diet. The carbohydrate-insulin model has focused on the late postprandial period as critical to understanding the control of energy intake at the subsequent meal, and possibly also energy expenditure [6, 7, 9, 11, 12].
Materials and Methods
Overview
This study was performed as an ancillary study to the Framingham State Food Study, (FS)2, with participants in the first 2 of 3 cohorts invited to opt-in [13]. Adults with body mass index (BMI) ≥25 kg/m2 underwent a 10% to 14% weight loss on a run-in diet with energy intake restricted to 60% of estimated needs (minimum 1200 kcal/day) and comprising 45% of energy from carbohydrate, 30% from fat, and 25% from protein. Following achievement of target weight loss, body weight was stabilized by adjusting energy intake (energy intake during weight loss maintenance = energy intake during weight loss [kcal/day] + (rate of weight loss [kg/day] × 7700 kcal/kg). Participants were subsequently randomized to 1 of 3 weight-loss-maintenance test diets controlled for protein content (20% of total energy) but varying in carbohydrate-to-fat ratio as follows: high-carbohydrate diet ([HC]: 60% of total energy from carbohydrate, 20% from fat), moderate-carbohydrate diet ([MC]: 40% carbohydrate, 40% fat) or low-carbohydrate diet ([LC]: 20% carbohydrate, 60% fat). Relative to the high-carbohydrate diet, energy targets for the moderate- and low-carbohydrate diets were obtained by decreasing the quantity of grains, fruits, and legumes and by adding more foods containing fat. Saturated fat content was maintained at 35% of total fat across all 3 diets, and no high-fructose corn syrup was used. During the weight-maintenance phase, participants were weighed daily and energy intake was adjusted to maintain weight loss within 2 kg of post–weight loss anchor weight; that is, weight (g) was regressed on time (days); slope of ≥15 g per day over 14 days indicated need to adjust energy intake [13]. Between weeks 10 and 15 on the test diet, participants were admitted to a metabolic unit at Brigham and Women’s Hospital, Boston for 24 hours.
Participants
All those in the first 2 (of 3) cohorts of the parent study were invited to participate in this ancillary study. Interested participants underwent a telephone interview to screen for additional eligibility criteria and attended an in-person informational session. Because participants in this ancillary study also underwent biopsies of subcutaneous adipose tissue for other purposes, additional exclusionary criteria included history of allergy or prior reaction to lidocaine, and any medical issue or medication that may increase risk of bleeding, infection, or skin reaction following adipose tissue biopsy. Additional inclusionary criteria included willingness and ability to travel to Boston for the 24-hour admission. Participants were enrolled after they achieved the target weight loss for the run-in phase, but prior to randomization to the test diets in the (FS)2 [13], to prevent selection bias. Financial compensation of $1000 and reimbursement for travel expenses were provided.
Ethics
The study was approved by the Institutional Review Board at Boston Children’s Hospital using reliance agreements with Brigham and Women’s Hospital and Framingham State University and registered at www.ClinicalTrials.gov (NCT02235038). We obtained written informed consent from study participants.
Meals
Subjects within each diet group consumed a variable menu of diet meals based on food production schedules for the parent study [13]. This approach was chosen both for logistical reasons and also to provide a broader test of macronutrient effects, beyond those of a specific test meal. Participants consumed their usual dinner and evening snack together at 6 pm on the day of admission, and then breakfast at 7:30 am and lunch at 12:30 pm on the following day. Daily energy content was distributed as 22.5% for breakfast, 32.5% for lunch, 32.5% for dinner, and 12.5% for snack [13]. Participants were instructed to eat at a steady pace, aiming to fully consume all meals in 20 to 30 minutes. Meal trays were collected, and leftovers weighed to document full consumption. Participants were allowed up to 1 serving (8 fl oz) of noncaloric artificially sweetened and/or caffeinated beverage (e.g., coffee, tea) with breakfast only.
Assessments
Intravenous (IV) catheters were placed in the hand, forearm, or antecubital fossa at least 30 minutes prior to initial blood sampling. Normal saline was infused at a rate of 20 to 50 mL/hour to keep the IV patent for blood draws. Blood samples were drawn prior to (time 0) and then at 30, 60, 120, 180, 240, and 300 minutes after each meal. An additional sample was drawn during the night, 9.5 hours after dinner. A heated hand-box was utilized to “arterialize” samples according to recommended procedures [14], to minimize variability related to differential metabolic extraction across the capillary bed. Immediately after being drawn, blood samples were centrifuged, and the plasma extracted and frozen on dry ice. Participants rated hunger and satiety levels at all times of blood sampling, except during the night, with a 10-cm visual analog scale, answering the prompt “How hungry/full are you right now?’’ with verbal anchors ranging from ‘‘Not at all hungry/full’’ to ‘‘Extremely hungry/full’’. To minimize the metabolic effects of inactivity, participants were allowed to freely walk around the inpatient unit in between assessments.
Outcome Measures
We measured glucose, BOHB, FFA, lactate, insulin (0, 30, 60, 120, 180, 240, 300 minutes), glucagon (0, 120, and 240 minutes), and epinephrine (300 minutes) at the specified time points. Glucagon was not measured in 8 subjects (4 in HC, 2 in MC, 2 in LC). Glucose, BOHB, FFA, lactate and insulin were measured on Roche Cobas 6000 system (glucose by hexokinase method using Roche Diagnostics reagents [Indianapolis, IN] [15], BOHB by enzymatic colorimetric assay with reagents and calibrators from Genzyme [Cambridge, MA], FFA by in-vitro enzymatic colorimetric assay using reagents from Wako Chemicals USA [Richmond, VA], lactate by enzymatic colorimetric assay using Roche Diagnostics reagents; and insulin by electrochemiluminescence immunoassay). Epinephrine was measured by a high-sensitivity competitive ELISA kit from Eagle Biosciences (Nashua, NH). Samples for the measurement of glucagon were drawn in BD P800 tubes which contain protease inhibitors that stabilize glucagon from degradation from the time of collection.
Energy availability (EA) from the metabolic fuels was calculated as previously described [12], as the sum of EA from glucose, BOHB, and FFA in kcal/L. EA from glucose was obtained from the measured level (mg/dL) assuming energy content of 4 kcal/g. EA from BOHB was calculated from the measured level (mmol/L) using a molecular weight of 104 g/mol and assuming energy content of 4.2 kcal/g [16]. EA from FFA was calculated from the measured level (mmol/L) assuming an average molecular weight of 270 g/mol (based on estimated average chain length of 17) and energy content of 9 kcal/g [12, 17, 18]. This calculation takes into account metabolic fuels that are directly available for oxidation in extrahepatic tissues, but not other energy-containing compounds (e.g., triglycerides or amino acids), that typically require metabolic transformation (into FFA or glucose, respectively) prior to oxidation. Lactate also requires metabolic transformation to glucose by the liver prior to oxidation. However, because of the acute changes in lactate following carbohydrate consumption [19], and possible direct effects in the liver, we performed an additional analysis including the energy contribution of lactate in our calculation (using a molecular weight of 90 g/mol and energy content of 3.6 kcal/g). Plasma acetone and acetoacetate are both unstable and not easily measured by standard laboratory methodologies [20]. Although we attempted to measure acetoacetate via an enzymatic assay (MAK134, SigmaAldrich, St. Louis MO), these results were later invalidated due to evidence of an interfering substance. However, these ketone species make a relatively minor contribution to total ketone concentration [20, 21] and any error caused by their exclusion would bias toward the null hypothesis (as they would be higher on the low-carbohydrate diet). Per a priori hypothesis [7, 9, 22-26], we also investigated possible effect modification related to insulin secretion, measured as insulin 30 minutes post-meal (Meal Insulin-30).
Statistical design and analysis
Because subjects were randomized as part of the parent study procedures [13], we did not have control over the number of subjects assigned to the 3 diet groups. We chose an enrollment goal of 30 participants to allow for dropout and to provide a buffer against imbalance among diet groups, which we calculated was unlikely to be severe enough to cause more than 5% loss of precision. Based on the observed residual standard deviation of 0.32 kcal/L for postprandial EA in the preliminary study [12], 8 to 10 participants per diet group gave us 80% power with a Bonferroni-corrected type I error rate of 5% to detect a diet difference between 0.42 and 0.48 kcal/L in postprandial energy availability at 180 to 300 minutes.
Each outcome was analyzed by repeated-measures analysis of variance, accounting for joint effects and potential interactions of diet, meal, and time and adjusted for age, sex, baseline BMI, weight loss, calories prescribed, and time since start of test diet. Intra-subject and intra-meal correlation were accounted for by a random subject effect and spatial power covariance structure respectively. Adjusted diet-specific means for each time point and for the early and late postprandial periods were constructed from parameters of the fitted model and compared among diet groups, overall by F-test (2 df) and pairwise by t test. For insulin and glucagon, incremental area under the curve (AUC, mean excursion from baseline averaged over the 3 meals) was likewise constructed from parameters of the fitted model and compared among diet groups. We employed the principle of closed testing [27] to control the familywise type I error rate at 5%, using P < 0.05 as the criterion for statistical significance in each pairwise comparison conditional on rejection of the 2-df hypothesis of 3 equal means.
An additional model was constructed to assess the influence of insulin concentration at 30 minutes into each meal (Meal Insulin-30) on the initial rate of decline in total EA (30-180 minutes) and the late mean (180-300 minutes), potentially varying by diet. Insulin concentrations showed a right-skewed distribution and were log-transformed for this analysis. The model included log insulin as both a covariate and an effect modifier, as well as adjustments for systematic differences among the 3 meals and for intra-subject correlation.
The primary outcome prespecified 150 to 300 minutes as the postprandial time interval of interest but, due to logistical difficulties, we obtained blood samples no more frequently than hourly, precluding the 150-minute time point. Therefore, we used the 180 to 300 minute timeframe for our primary outcome. However, we also conducted a sensitivity analysis with interpolated data to approximate the 150 to 300 minute interval.
SAS software (version 9.4, Cary, NC) was used for all computations.
Results
Participant flow and characteristics
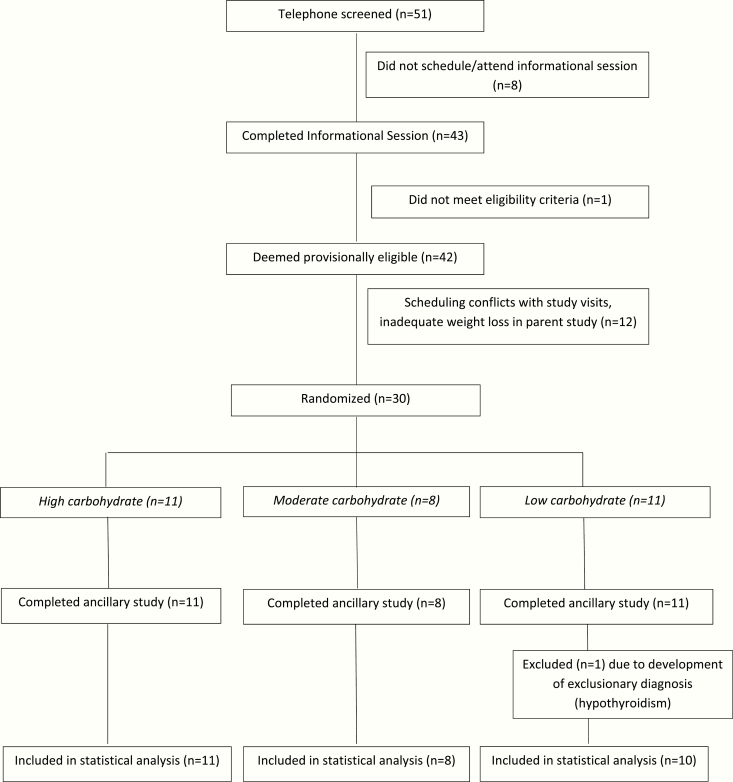
Of 51 individuals who began the screening process, 30 were enrolled and provided informed consent, as depicted in the flow diagram (Fig. 1). Following randomization, sample sizes by test diet group were 11 in HC, 8 in MC, and 11 in LC. All 30 subjects completed the hospital admission; however, 1 participant from the LC group was excluded from statistical analysis due to development of an exclusionary diagnosis (hypothyroidism). Participant characteristics are detailed in Table 1, showing comparable baseline covariates between test diet groups. At the time of the hospital admission, the mean number of days on the test diet was similar between groups (83, 82, and 84 days for HC, MC, and LC respectively). The mean daily calorie prescription was highest for LC (consistent with the higher energy requirement in this diet group in adjusted models in the parent study [26]), although the raw differences between diet groups were not significant here.
Figure 1:
Participant flow diagram.
Table 1.
Participant Characteristics in High-Carbohydrate, Moderate-Carbohydrate, and Low-Carbohydrate Test Diets
| CATEGORY | HC | MC | LC |
|---|---|---|---|
| N [male:female] | 11[8:3] | 8[4:4] | 10[5:5] |
| Mean age [range] at hospital admission in years | 46 [20-66] | 42 [20-64] | 38 [22-62] |
| % white ethnicity | 91 | 100 | 90 |
| % non-Hispanic | 82 | 100 | 90 |
| Baseline BMI [SD] in kg/m2 | 30.6 [3.4] | 32.6 [4.1] | 30.9 [3.6] |
| BMI at 24-hour admission [SD] in kg/m2 | 26.7 [2.9] | 28.8 [3.6] | 26.9 [3.1] |
| % weight loss [SD] | 12.7 [3.9] | 11.7 [2.2] | 12.6 [3.3] |
| Daily calorie prescription at the time of hospital admission in kcal [SD]a | 2359 [545] | 2337 [580] | 2424 [709] |
| Days from start of test diet to hospital admission [SD] | 83 [8] | 82 [8] | 84 [10] |
Abbreviations: BMI, body mass index; HC, high-carbohydrate diet; LC, low-carbohydrate diet; MC, moderate-carbohydrate diet; SD, standard deviation.
aNo significant interaction between daily calorie prescription and diet group
Process data
Of 87 meals provided throughout the protocol (29 participants × 3 meals per participant), 4 had a small amount of plate waste, ranging from 5% to 10% of calories in the meal. Two protocol deviations occurred involving deviation in calorie consumption by >10%: one participant in the HC group did not consume 12% of calories at dinner due to fullness; one participant in the LC group consumed an extra 254 calories at breakfast due to a tray error.
Energy availability
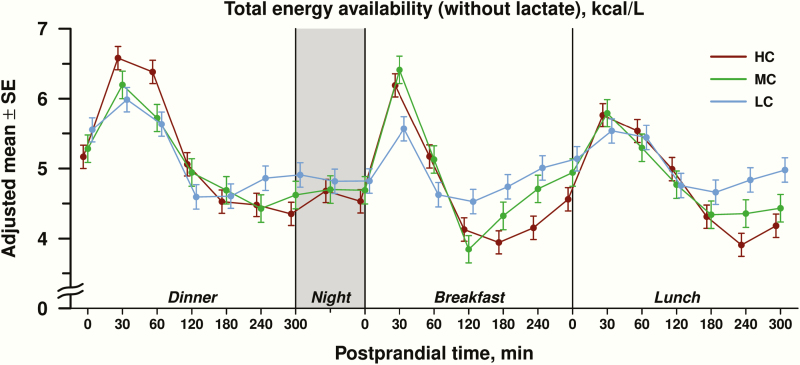
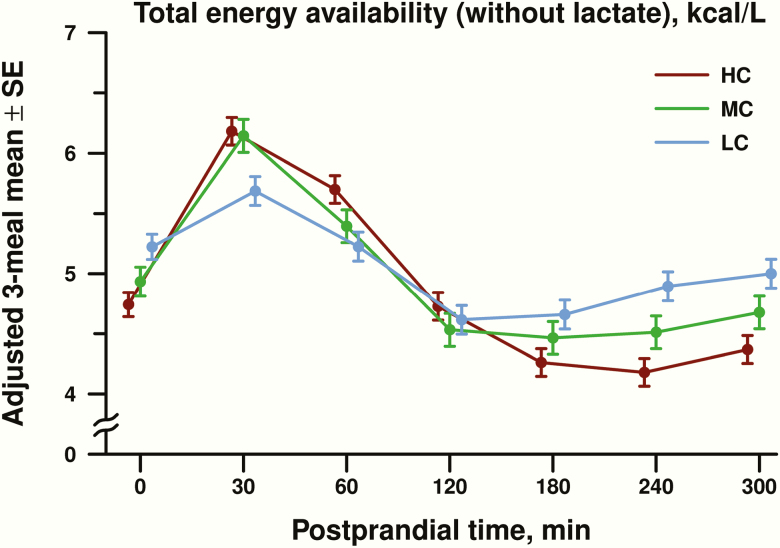
EA over the 24-hour inpatient period is shown in Fig. 2. The 3-way interaction among diet, meal, and time was nonsignificant, providing a rationale to combine data for all 3 meals into a covariate-adjusted diet-specific mean at each postprandial time point (Fig. 3). For postprandial EA, there was a significant diet × time interaction (P < 0.0001). In the early postprandial period (30-120 minutes after the meal), EA was higher in the HC compared with the LC group (0.36 ± 0.14 kcal/L, adjusted difference ± standard error, P = 0.01), with no significant difference between HC and MC groups (0.18 ± 0.15, P = 0.25) or between MC and LC groups (−0.18 ± 0.16, P = 0.25). In the late postprandial period (180-300 minutes after the meal), EA was 4.27 ± 0.09 kcal/L (adjusted mean ± standard error) in the HC group, 4.55 ± 0.11 kcal/L in the MC group, and 4.85 ± 0.10 kcal/L in the LC group. Late postprandial EA was lower in the HC compared with the LC group by 0.58 ± 0.14 kcal/L (P < 0.0001) and the MC group by 0.28 ± 0.15 kcal/L (P = 0.06). The difference in late postprandial EA between the MC and LC group was −0.30 ± 0.15 kcal/L (P = 0.05).
Figure 2:
Total energy availability of metabolic fuels (glucose, beta-hydroxybutyrate, free fatty acids) excluding lactate, expressed as adjusted mean ± standard error (SE) in kcal/L, depicted over the 24-hour study period for high carbohydrate (HC), moderate carbohydrate (MC) and low carbohydrate (LC) diets. Postprandial time points (in minutes) and overnight periods are highlighted on the horizontal axis.
Figure 3:
Postprandial total energy availability of metabolic fuels (glucose, beta-hydroxybutyrate, free fatty acids) excluding lactate, expressed as adjusted mean ± standard error (SE) in kcal/L. Postprandial energy availability for all 3 meals (dinner, breakfast, and lunch) were combined into a covariate-adjusted diet-specific mean for high carbohydrate (HC), moderate carbohydrate (MC), and low carbohydrate (LC) at each postprandial time point in minutes.
The overall diet effect on late postprandial EA using data interpolated from the 150-minute timepoint, as considered in “Materials and Methods,” remained strongly significant (difference between LC and HC group of 0.50 ± 0.13 kcal/L, P = 0.0002). In sensitivity analyses, the difference in EA between HC and LC did not change materially when we omitted hemolyzed samples (−0.58 ± 0.14 kcal/L) or omitted the 2 participants with deviations in calorie consumption (−0.56 ± 0.15 kcal/L). When EA was calculated with inclusion of lactate, comparisons between diet groups were similar for the overall group effect and HC vs LC diet comparison in the late postprandial phase [28].
Dynamic changes in EA and effect modification by Meal Insulin-30
Mean Meal Insulin-30 was 1.64-fold higher in the HC than in the LC group (95% confidence interval [CI], 1.25-2.14, P = 0.0004) and 1.72-fold higher in the MC than in the LC group (95% CI, 1.29-2.30, P = 0.0004) but did not differ significantly between the HC and MC diet groups (0.95-fold, 95% CI, 0.72-1.27, P = 0.73); these differences were accounted for by inclusion of Meal Insulin-30 as a covariate in the analytical model of dynamic changes in EA. The average decline in total EA from 30 to 180 minutes after each meal was 0.620 ± 0.023 kcal/L/h (rate ± standard error), varying significantly according to diet: 0.784 ± 0.038 for the HC group, 0.661 ± 0.044 for the MC group, and 0.414 ± 0.040 for the LC group (P < 0.0001). Meal Insulin-30 modified the diet differences in the early decline (interaction P = 0.048). Each 50% increase in Meal Insulin-30 accelerated the rate of EA decline by 0.061 ± 0.025 kcal/L/h in the HC group (P = 0.02) and 0.095 ± 0.051 kcal/L/h in the MC group (P = 0.07), whereas the impact was not significant in the LC group (0.035 ± 0.034 kcal/L/h, P = 0.30). The late postprandial mean EA (180-300 minutes) was not modified by Meal Insulin-30 (interaction P = 0.82). The results for decline in total EA with lactate were similar [28].
Individual metabolic fuels
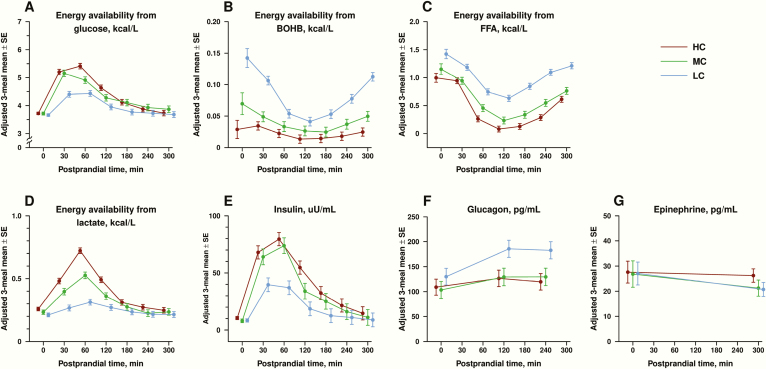
Mean postprandial EA of individual fuels (glucose, BOHB, FFA, lactate) are shown in Fig. 4, panels A-D, with further details available in supplemental materials [28]. In the early postprandial period, EA from glucose was highest in the HC group and lowest in the LC group. EA from BOHB and FFA were also lower in the HC and MC groups compared with the LC group. In the late postprandial period, there were no group differences in EA from glucose; however, EA from FFA and BOHB remained lowest in the HC group, intermediate in the MC group and highest in the LC group. EA from lactate was significantly higher in the HC vs LC group in the early postprandial period and marginally higher in the late postprandial period.
Figure 4:
Mean postprandial energy availability of individual fuels are shown in panels A-D (A for glucose, B for beta hydroxybutyrate [BOHB], C for free fatty acids [FFA] and D for lactate). Postprandial energy availability is expressed as the adjusted mean in kcal/L ± standard error (SE) of values at dinner, breakfast, and snack, separated by test diet assignment (high carbohydrate [HC], moderate carbohydrate [MC], low carbohydrate [LC]). Mean concentrations for postprandial insulin, glucagon, and epinephrine are shown in panels E-G (E for insulin in uU/mL, F for glucagon in pg/mL, G for epinephrine in pg/mL). Hormonal concentrations are expressed as the adjusted mean ± standard error (SE) of values at dinner, breakfast, and snack, separated by test diet assignment.
Hormones
Mean concentrations for postprandial insulin, glucagon, and epinephrine are shown in Fig. 4, panels E-G. The area under the curve (AUC) for insulin increased with dietary carbohydrate (160 ± 15 uU/mL × h in the HC group, 129 ± 17uU/mL × h in the MC group and 54 ± 15 uU/mL × h in the LC group; P < 0.0001). AUC for glucagon varied inversely with dietary carbohydrate (61 ± 20 pg/mL × h in the HC group, 82 ± 22 pg/mL × h in the MC group, and 148 ± 19 pg/mL × h in the LC group; P = 0.006). The ratio of mean AUC for insulin to mean AUC for glucagon was 2.5 in the HC group and 0.36 in the LC group, a 7-fold difference between the ratios. There was no difference in postprandial epinephrine among diet groups (P = 0.33).
Hunger and satiety
Hunger scores increased while satiety scores decreased in all 3 diet groups during the postprandial period [28]. There were no significant differences in hunger scores among groups (P = 0.47). Satiety scores were lower in the LC compared to the HC group during the early and late postprandial periods, respectively (−2.0 ± 0.6, P = 0.001; −1.7 ± 0.6, P = 0.007).
Discussion
This study investigated the impact of dietary carbohydrate on postprandial metabolic fuels and hormonal responses in adults with overweight or obesity between 10 and 15 weeks of weight-loss maintenance. We examined a novel parameter, total EA, reflecting the combined energy content of circulating metabolic fuels immediately available for oxidation. We found that total EA in the late postprandial period was significantly lower (0.58 kcal/L) in participants on the high-carbohydrate compared to the low-carbohydrate diet. Our results are consistent with previous 1-day [6] and 4-week [12] crossover studies, suggesting persistence of this effect during weight-loss maintenance and with control for protein content.
We did not see evidence of a late-postprandial counter-regulatory hormone response involving epinephrine as in a prior single-day study [6]. This difference may relate to inclusion of a high-carbohydrate meal with a notably higher glycemic load in the prior study versus the current study; or to the younger age (adolescents), more extreme obesity, or lack of prior weight loss among participants in the prior study.
Insulin promotes anabolism by suppressing lipolysis, ketogenesis, and gluconeogenesis, among other actions, whereas glucagon has opposing, catabolic effects [7]. Drugs that raise the insulin-to-glucagon ratio are associated with hypoglycemia and weight gain, whereas those that decrease the ratio are associated with the opposite effects [29]. In our study, the postprandial insulin-to-glucagon AUC was approximately 7-fold higher in the HC vs LC group, arising from the recognized effects of carbohydrate on insulin secretion and the suppressive effects of glucose and insulin on glucagon secretion [30]. Given that protein content was controlled across diets, increased glucagon secretion in the LC group likely reflects diminished inhibition of alpha cells. We also observed a steeper decline in early postprandial energy availability associated with higher insulin secretion in the high-carbohydrate group, consistent with an a priori hypothesis [7, 9, 22-26]. These findings provide support for the carbohydrate-insulin model of obesity, wherein the persistent effects of high insulin-to-glucagon ratio soon after eating alter substrate partitioning away from oxidative organs (primarily muscle) and toward deposition in adipose, promoting weight gain [5].
Participants reported no difference in hunger between diet groups, although reported satiety was lower in the low-carbohydrate group, opposite to the hypothesized effect. However, the validity of visual analog scale ratings for inter-individual comparisons following several months on the test diets remains unclear. These scales are designed to identify changes during the postprandial period within individuals following a single meal; habituation with chronic exposure to a diet may confound inter-individual comparisons. Furthermore, self-reported scales may not reliably predict feeding behavior, which may be better assessed by ad libitum food intake [31-33]. In addition, we were not able to distinguish homeostatic hunger (motivation to eat based on declining energy stores) versus hedonic hunger (desire to eat palatable foods) [34], with the former presumably more directly relevant to our hypothesis. In the parent feeding trial, the “hunger hormone” ghrelin was significantly lower on the low- versus high-carbohydrate diet [9]. In another study, PYY (a hormone that promotes satiety) was higher after 12 months on a low-carbohydrate versus a low-fat diet [35]. In any event, further research is needed to clarify the chronic effects of dietary carbohydrate during weight-loss maintenance on hunger, satiety, and voluntary food intake.
Our study has several strengths. Utilizing the infrastructure of a large-scale feeding trial, we carefully controlled test meal production and ensured that the meals served for this ancillary study were consumed as intended in the setting of a metabolic ward (with only 2 minor deviations). The diets were designed to reflect realistic and healthful examples of their respective macronutrient compositions (i.e., with no attempt to inflate glycemic load, such as by including only highly processed carbohydrates in the high-carbohydrate diet). By design, participants within each group consumed a variable menu of diet meals. Despite the potential for this approach to increase within-group variability, we still found differences between groups with a high degree of statistical confidence. Participants were studied after substantial weight loss, a physiological state with relevance to the long-term success of obesity treatment. Outcomes were obtained throughout a 24-hour period, allowing for control of diurnal influences. In addition, we were able to examine effect modification by insulin secretion, a trait that may contribute to a biologically informed choice of weight-loss diets.
One study limitation is generalizability. Although diets were constructed to be as similar as possible with the exception of macronutrient composition, other dietary factors could have contributed to the findings. In addition, translatability to the general public (with or without prior weight loss) remains to be determined. Logistical issues precluded control for menstrual phase in premenopausal women, but any resulting imprecision did not appreciably affect our ability to test the primary hypothesis.
Several methodological issues also warrant consideration. Although our calculation of energy in FFA employs an assumption of average fatty acid chain length, physiological variations in this parameter would have little consequence to our effect estimates. Due to technical difficulties, we were not able to measure acetoacetic acid or acetone. However, the concentration of these 2 ketone bodies, like BOHB, are inversely related to carbohydrate intake; therefore, our findings may underestimate to a small degree the true effects of the diets. In addition, we did not include other circulating energy-containing species, such as amino acids or triglycerides, in our calculation of energy availability, as these are generally not considered directly available for oxidation by extrahepatic tissue. Some evidence suggests that fatty acids may be released to a limited degree from triglycerides directly in muscle capillary beds [36]. However, according to Frayn, muscle is primarily dependent on FFA (as released from adipose) under usual conditions, and direct update of energy from triglycerides may only become physiologically relevant at higher levels of physical activity [37]. In any event, this effect would not likely inflate our estimate of the primary outcome. Whereas triglycerides are typically lower on a low-carbohydrate diet in the fasting state, they increase proportionately more in the postprandial state due to the higher fat content of that meal. Thus, inclusion of energy in triglycerides into our models may tend to increase the magnitude of effect.
Finally, we recognize that the concentrations of metabolic fuels and their energetic equivalents are proxy measures of actual substrate utilization and oxidation [38]; these were not assessed, representing a major study limitation. In addition, inter-individual comparisons may be confounded by differences in insulin resistance or other factors relating to metabolic efficiency. Even so, the concentration of individual substrates has clear relevance to metabolism, as exemplified by the metabolic consequence of hypoglycemia. Moreover, an integrated view of substrate availability provides a broader view of metabolism than can be achieved by considering substrates in isolation (e.g., symptomatic severity of hypoglycemia is greater in the absence versus presence of ketosis). Thus, a novelty of the current study is use of an integrated measure of fuel availability measured dynamically, throughout the postprandial period. Future studies directly examining tissue-specific metabolic rate would be informative.
In conclusion, this study suggests a high-carbohydrate diet during weight-loss maintenance lowers energy availability in the late postprandial period, due primarily to suppression of fat metabolism, likely resulting from higher insulin-to-glucagon ratio. These physiological changes could reflect a redirection of metabolic fuels toward energy storage, consistent with the carbohydrate-insulin model of obesity. The relationship between changes in metabolic fuel availability, eating behavior, and weight regain warrants further study.
Acknowledgments
We thank the study participants for their time and commitment to advancing science and the faculty and staff of Boston Children’s Hospital, Framingham State University, and Sodexo who provided support with the design and execution of the parent study. We specifically thank personnel who worked on this ancillary study: Kelly Thompson (participant recruitment), Sheila Driscoll and Judith Lauerman (nursing assistance with coordinating and implementing the study protocol), Silvia Sumale (processing timed blood specimens), Lauren Holmes (meal preparation and delivery), Leigh Keating (meal service and documentation), Gary Bradwin (analysis of blood specimens), Tram Tran (storage of biospecimens), Mark Kellogg (development of methods for ketone measurements), and Michael Agus (data and safety monitoring).
Financial Support: This work was conducted with grants from Nutrition Science Initiative (made possible by gifts from the Laura and John Arnold Foundation and Robert Lloyd Corkin Charitable Foundation), New Balance Foundation, Many Voices Foundation, and Blue Cross Blue Shield. KJS was supported by a Pediatric Endocrine Society fellowship. DSL was supported by a mid-career mentoring award from the National Institute of Diabetes and Digestive and Kidney Diseases (K24DK082730). Nutrition Science Initiative monitored study progress and was given an opportunity to comment on the manuscript. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; approval of the manuscript; and decision to submit the manuscript for publication. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the study sponsors.
Clinical Trial Information: ClinicalTrials.gov registration no. NCT02235038
Glossary
Abbreviations
- AUC
area under the curve
- BMI
body mass index
- BOHB
beta-hydroxybutyrate
- EA
energy availability
- FFA
free fatty acid
- HC
high-carbohydrate
- IV
intravenous
- LC
low-carbohydrate
- MC
moderate-carbohydrate
Additional Information
Disclosure Summary: D.S.L. received royalties for books on obesity and nutrition that recommend a low-glycemic load diet. No other author has relevant disclosures.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Kraschnewski JL, Boan J, Esposito J, et al. Long-term weight loss maintenance in the United States. Int J Obes. 2010;34(11):1644-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621-628. [DOI] [PubMed] [Google Scholar]
- 3. Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597-1604. [DOI] [PubMed] [Google Scholar]
- 4. Ludwig DS, Friedman MI. Increasing adiposity: consequence or cause of overeating? JAMA. 2014;311(21):2167-2168. [DOI] [PubMed] [Google Scholar]
- 5. Ludwig DS, Ebbeling CB. The carbohydrate-insulin model of obesity: beyond “Calories In, Calories Out”. JAMA Intern Med. 2018;178(8):1098-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ludwig DS, Majzoub JA, Al-Zahrani A, Dallal GE, Blanco I, Roberts SB. High glycemic index foods, overeating, and obesity. Pediatrics. 1999;103(3):E26. [DOI] [PubMed] [Google Scholar]
- 7. Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287(18):2414-2423. [DOI] [PubMed] [Google Scholar]
- 8. Ebbeling CB, Swain JF, Feldman HA, et al. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. 2012;307(24):2627-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ebbeling CB, Feldman HA, Klein GL, et al. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial. BMJ. 2018;363:k4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ludwig DS, Lakin PR, Wong WW, Ebbeling CB. Scientific discourse in the era of open science: a response to Hall et al. regarding the carbohydrate-insulin model. Int J Obes. 2019;43(12):2355-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lennerz BS, Alsop DC, Holsen LM, et al. Effects of dietary glycemic index on brain regions related to reward and craving in men. Am J Clin Nutr. 2013;98(3):641-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walsh CO, Ebbeling CB, Swain JF, Markowitz RL, Feldman HA, Ludwig DS. Effects of diet composition on postprandial energy availability during weight loss maintenance. Plos One. 2013;8(3):e58172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ebbeling CB, Klein GL, Luoto PK, et al. A randomized study of dietary composition during weight-loss maintenance: Rationale, study design, intervention, and assessment. Contemp Clin Trials. 2018;65:76-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu D, Moberg E, Kollind M, Lins PE, Adamson U, Macdonald IA. Arterial, arterialized venous, venous and capillary blood glucose measurements in normal man during hyperinsulinaemic euglycaemia and hypoglycaemia. Diabetologia. 1992;35(3):287-290. [DOI] [PubMed] [Google Scholar]
- 15. Sacks D. Carbohydrates. In: Burtis C, Ashwood E, eds. Tietz Textbook of Clinical Chemistry. 3rd ed. Saunders; 1999:750-808. [Google Scholar]
- 16. Rich AJ. Ketone bodies as substrates. Proc Nutr Soc. 1990;49(3):361-373. [DOI] [PubMed] [Google Scholar]
- 17. Costa CG, Dorland L, Holwerda U, et al. Simultaneous analysis of plasma free fatty acids and their 3-hydroxy analogs in fatty acid beta-oxidation disorders. Clin Chem. 1998;44(3):463-471. [PubMed] [Google Scholar]
- 18. Abdelmagid SA, Clarke SE, Nielsen DE, et al. Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults. Plos One. 2015;10(2):e0116195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koutsari C, Malkova D, Hardman AE. Postprandial lipemia after short-term variation in dietary fat and carbohydrate. Metabolism. 2000;49(9):1150-1155. [DOI] [PubMed] [Google Scholar]
- 20. Carmant L. Assessing ketosis: approaches and pitfalls. Epilepsia. 2008;49(Suppl 8):20-22. [DOI] [PubMed] [Google Scholar]
- 21. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999;15(6):412-426. [DOI] [PubMed] [Google Scholar]
- 22. Pawlak DB, Kushner JA, Ludwig DS. Effects of dietary glycaemic index on adiposity, glucose homoeostasis, and plasma lipids in animals. Lancet. 2004;364(9436):778-785. [DOI] [PubMed] [Google Scholar]
- 23. Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297(19):2092-2102. [DOI] [PubMed] [Google Scholar]
- 24. Chaput JP, Tremblay A, Rimm EB, Bouchard C, Ludwig DS. A novel interaction between dietary composition and insulin secretion: effects on weight gain in the Quebec Family Study. Am J Clin Nutr. 2008;87(2):303-309. [DOI] [PubMed] [Google Scholar]
- 25. Hron BM, Ebbeling CB, Feldman HA, Ludwig DS. Relationship of insulin dynamics to body composition and resting energy expenditure following weight loss. Obesity. 2015;23(11):2216-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ebbeling CB, Bielak L, Lakin PR, et al. Higher energy requirement during weight-loss maintenance on a low- versus high-carbohydrate diet: secondary analyses from a randomized controlled feeding study. J Nutr. 2020; nxaa150 [Online First]. doi: 10.1093/jn/nxaa150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bender R, Lange S. Adjusting for multiple testing–when and how? J Clin Epidemiol. 2001;54(4):343-349. [DOI] [PubMed] [Google Scholar]
- 28. Shimy KJ, Feldman HA, Klein GL, Bielak LB, Ebbeling CB, Ludwig DS. Supplemental Tables, Full Dataset and Statistical Codes. Data from: A Mechanistic Examination of Dietary Composition on Metabolic Fuels Availability. OSF Repository. Deposited May 26, 2020 and June 3, 2020. https://osf.io/3j58k/. [Google Scholar]
- 29. Kalra S, Gupta Y. The insulin:glucagon ratio and the choice of glucose-lowering drugs. Diabetes Ther. 2016;7(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rorsman P, Ashcroft FM, Berggren PO. Regulation of glucagon release from pancreatic A-cells. Biochem Pharmacol. 1991;41(12):1783-1790. [DOI] [PubMed] [Google Scholar]
- 31. Stratton R, Rowley E, Blundell JE, et al. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr. 2000;84:405-415. [DOI] [PubMed] [Google Scholar]
- 32. Mattes RD. Hunger and thirst: issues in measurement and prediction of eating and drinking. Physiol Behav. 2010;100(1):22-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Green SM, Delargy HJ, Joanes D, Blundell JE. A satiety quotient: a formulation to assess the satiating effect of food. Appetite. 1997;29(3):291-304. [DOI] [PubMed] [Google Scholar]
- 34. Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr. 2009;139(3):629-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hu T, Yao L, Reynolds K, et al. The effects of a low-carbohydrate diet on appetite: A randomized controlled trial. Nutr Metab Cardiovasc Dis. 2016;26(6):476-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bickerton AS, Roberts R, Fielding BA, et al. Preferential uptake of dietary Fatty acids in adipose tissue and muscle in the postprandial period. Diabetes. 2007;56(1):168-176. [DOI] [PubMed] [Google Scholar]
- 37. Frayn KN. Fat as a fuel: emerging understanding of the adipose tissue-skeletal muscle axis. Acta Physiol. 2010;199(4):509-518. [DOI] [PubMed] [Google Scholar]
- 38. Friedman MI, Stricker EM. The physiological psychology of hunger: a physiological perspective. Psychol Rev. 1976;83(6):409-431. [PubMed] [Google Scholar]