Abstract
Breast cancer is the most commonly diagnosed malignancy in female worldwide, over 70% of which are estrogen receptor α (ERα) positive. ERα has a crucial role in the initiation and progression of breast cancer and is an indicator of endocrine therapy, while endocrine resistance is an urgent problem in ER-positive breast cancer patients. In the present study, we identify a novel E3 ubiquitin ligase TRIM11 function to facilitate ERα signaling. TRIM11 is overexpressed in human breast cancer, and associates with poor prognosis. The protein level of TRIM11 is highly correlated with ERα. RNA-seq results suggest that ERα signaling may be an underlying target of TRIM11. Depletion of TRIM11 in breast cancer cells significantly decreases cell proliferation and migration. And the suppression effects can be reversed by overexpressing ERα. In addition, ERα protein level, ERα target genes expression and estrogen response element activity are also dramatically decreased by TRIM11 depletion. Further mechanistic analysis indicates that the RING domain of TRIM11 interacted with the N terminal of ERα in the cytoplasm and promotes its mono-ubiquitination, thus enhances ERα protein stability. Our study describes TRIM11 as a modulating factor of ERα and increases ERα stability via mono-ubiquitination. TRIM11 could be a promising therapeutic target for breast cancer treatment.
Keywords: Breast cancer, ERα, TRIM11, Mono-ubiquitination, Polyubiquitination
Introduction
Breast cancer is the most frequent malignancy in female worldwide and results in the second leading cause of woman cancer death. It is a major heath burden in female [1]. Based on the absence or presence of molecular markers of estrogen receptor α (ERα), progesterone receptor (PR) and human epidermal growth factor 2 (HER2), breast cancer is categorized into 3 major subtypes: Luminal (hormone receptor positive), HER2-enriched and triple-negative (lacking the 3 molecular markers) [2]. ERα is a nuclear receptor composed of several domains: Activation function domain-1 (AF1) domain at the N-terminus and activation function domain-2 (AF2) at the C-terminus, which recruit transcriptional coregulators, and DNA-binding domain (DBD) that binds to the estrogen response elements of its target genes [3].
ERα has a crucial role in the initiation and progression of breast cancer, over 70% of all breast cancer cases are driven by ERα [4]. The activity of ERα is mainly regulated by estrogen and is essential for the transition of cell cycle. Overexpression of ERα increases the oncogenic proteins expression levels, such as cyclin D1 and c-myc, which promote breast cancer cell growth via accelerating the G1–S phase transition [5]. Since ERα and its signaling pathways are necessary for the progression of luminal type of breast cancers, it is important to detect ERα for the administration of endocrine therapy: including hormone depletion and ERα antagonists. Previous studies demonstrated that application of anti-estrogen treatment can benefit ER‐positive breast cancer patients, while half of the patients will develop acquired resistance and suffer from relapse or even death [6]. Thus, understanding the underlying mechanisms and insight into the novel modulatory factors of estrogen signaling might identify promising treatment strategies in dealing with ER‐positive breast cancer patients with endocrine resistance.
Previous studies demonstrated that estrogen signaling in breast cancer cell could be facilitated by a group of ubiquitin ligases. The ubiquitination of ERα does not necessary lead to proteasomal degradation. RNF8, RNF31 and SHARPIN increases ERα stability via triggering ERα mono-ubiquitination [7], [8], [9]. TRIM56 increases ERα protein stability and enhances ERα signaling activity through targeting ERα K63-linked ubiquitination [10]. In the current study, we identify the E3 ubiquitin ligase TRIM11 as a novel ERα modulation factor which can promote ERα signaling activity. Recently, TRIM11 was found elevated in several tumor, including glioma, lung cancer and hepatocellular carcinoma cancer, which acts as an oncogene [11], [12], [13], [14]. However, the function of TRIM11 in breast cancer remains largely unclear. Our present study reveals TRIM11 as a novel modulatory factor of ERα, which could control the ubiquitination and stability of ERα, and thereby regulated estrogen-dependent gene expression and tumor progression.
Materials and methods
Breast cancer tissue microarray and immunohistochemistry
Commercial tissue microarrays were purchased from Shanghai Outdo Biotech (Shanghai, China). Specific primary antibodies against TRIM11 (Proteintech, China) and ERα (Proteintech, China) were used for IHC. The immunohistochemical score were assessed as we previously described [15].
Cell culture
HEK293T cells and ERα-positive breast cancer cell lines MCF-7, and T47D were obtained from the American Type Culture Collection (ATCC; Rockville, MD, USA). HEK293T and MCF7 were maintained in DMEM (Irvine Scientific, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS). T47D cells were cultured in RPMI/1640 medium (Gibco, Carlsbad, CA) supplemented with 10% FBS. All cells were cultured at 37 °C in a humidified 5% CO2 incubator.
Plasmids and RNA inference
The wild type TRIM11 and its mutant constructs were obtained from Hanbio Biotechnology Co. Ltd. (Shanghai, China). The ERα full- and deletion constructs were kindly presented from Pro. Ting Zhuang and were previously described [16]. The HA-K6, -K11, -K27, -K29, -K33, -K48, -K63, -K0 and -Ub plasmids were acquired from Addgene. The Estrogen-Response-Element (ERE)-TK reporter and renilla plasmids were gifted from Pro. Ting Zhuang and were described in previous study [16]. Plasmids were transfected by Lipofectamin 2000 (Invitrogen, Carlsbad, CA, USA). Cells were transfected with 50 nM small interfering RNAs using Lipofectamin RNAiMAX (Invitrogen, Carlsbad, CA, USA). The TRIM11 siRNA sequences used were: siRNA #1: 5′-CCAACCGCCCGCUUGCUAA-3′; siRNA #2: 5′- GGGUGAGUUCGAGCGUCUU-3′.
RNA extraction and qPCR analysis
The total RNA was extracted from the cancer cells using the RNeasy Mini Kit (Qiagen, Germany). Reverse transcription was performed using the HiScript II 1st Strand cDNA Synthesis Kit (Vazyme, China). qRT-PCR was performed using the ChamQ SYBR qPCR Master Mix (Vazyme) with the CFX96TM Real-time PCR Detection System (Bio-Rad, USA). Relative expression was calculated using the 2−ΔΔCt method, which was normalized to 36B4. All assays were performed in triplicates.
Proliferation, cell cycle and colony formation assay
MCF7 and T47D cells were transfected with SiTRIM11 or SiControl in 6-well plates. Forty-eight hours after transfection, the cells were seeded (2 × 103 cells/well) in 96-well plates and cell viability was measured using Cell Counting Kit-8 (CCK8) every 24 h. Cells transfected with SiTRIM11 or SiControl for 48 h were stained with propidium iodide (Multisciences, China) and analyzed by flow cytometer (Beckman, USA), The cell cycle phases were determined by relative DNA content. For the colony formation assay, cells were treated with 50 nM TRIM11 siRNA or 50 nM siControl, after 48 hours, the cells were typsinized and seeded (1–1.5 × 103 cells/well) in 6-well plates and maintained in complete medium for 2 weeks. The cells were fixed with 4% paraformaldehyde for 2 h, and stained with 1% crystal violet.
Wound-healing assay
Cells were seeded into 6-well plates and transfected with TRIM11 siRNA or siControl. When full confluent, the cell layer was scratched with a 200 μl sterile pipette tip and washed with PBS. Cells were maintained in the medium containing 1% FBS and wound distance was measured every 24 h.
Luciferase assay
The Dual-Luciferase Reporter kit (Promega, Germany) was used to measure the luciferase activity of ERE luciferase reporter. The ERE luciferase reporter and Renilla plasmid were transfected together into the cells. Luciferase activity was detected after 24 h.
Co-immunoprecipitation assay
Total cell lysis of MCF7 were precleared with rabbit IgG for 2 h and then immunoprecipitated with ERα (Cell Signaling Technology, #8644) or TRIM11 (Proteintech, 10851-1-AP) antibody overnight, while rabbit IgG (Santa Cruz) was used as the negative control. The bounded protein was analyzed by Anti- ERα or Anti-TRIM11 antibody.
Immunofluorescence assay
MCF7 cells cultured on 14 mm slides in 24-well plates were fixed in 4% paraformaldehyde at room temperature for 30 min. After washing with PBS for 3 times, the cells were blocked with 10% goat serum and incubated with primary antibodies against ERα (mouse, Santa Cruz), and TRIM11 (rat, Proteintech) at 4 °C overnight. Followed by incubating with FITC- and Cy3-conjugated secondary antibodies. The images were examined with EVOS™ M5000 Imaging System.
Protein stability assays
MCF7 and T47D cells were seeded in 24-well plates and transfected with siTRIM11 or siControl. After 24 h, cells were treated with 100 μM cycloheximide (MCE) for indicated time points. Western blot was performed to detect ERα degradation.
Western blot analysis
The breast cells were lysed with RIPA extraction reagent (Beyotime, China) supplemented with protease inhibitors (Sigma-Aldrich, USA). Total protein was separated using 10–12.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to 0.45 μm PVDF membrane (Millipore, USA). Primary antibodies were ERα (Cell Signaling Technology, #8644), TRIM11 (Proteintech, 10851-1-AP), HA (Proteintech, 51064-2-AP), Myc (Proteintech, 60003-2-Ig), GAPDH (Proteintech, 60004-1-Ig) antibodies. Bands were visualized using an enhanced chemiluminescence (ECL) kit (Boster, China) and detected by ChemiDoc XRS + Imaging System (Bio-Rad).
RNA sequence analysis
The RNA sequence analysis (siControl and siTRIM11) was performed by Beijing Genomic Institute (BGI). The RNA sequence data are deposited in the SRA database. The RNA sequence data are available at www.ncbi.nlm.nih.gov/bioproject/PRJNA609245/.
Statistical analysis
Student’s t test and one-way ANOVA were used to compare 2 and more groups respectively. Multiple comparison with Bonferroni correction was performed when appropriate. A P value <0.05 was considered as statistically significant and all tests were two-tailed. All statistical tests were performed with Prism 7.0 (GraphPad, USA).
Results
TRIM11 is associated with ERα protein levels in human breast cancer samples and poor prognosis
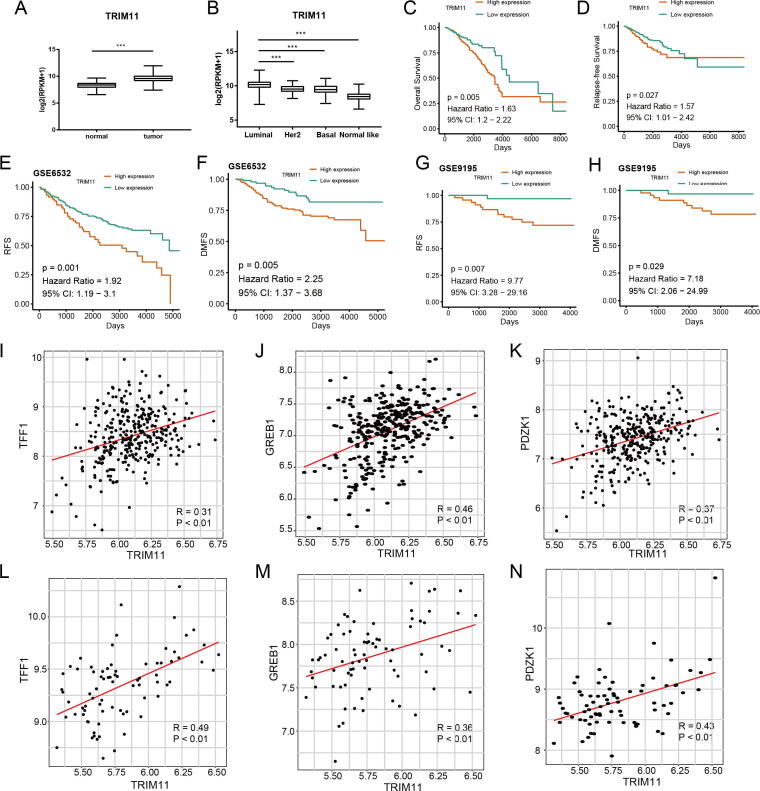
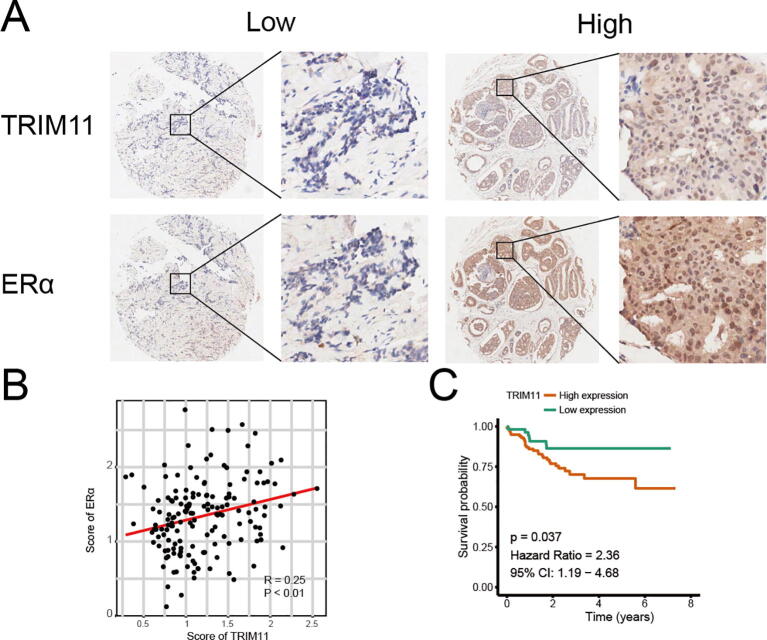
Based on the analysis of publicly available data from TCGA, we found that TRIM11 expression was elevated in breast cancer, especially in the luminal subtype (Fig. S1A, B). Survival analysis of TCGA, GSE6532 (ER-positive breast cancer patients) and GSE9195 (ER-positive breast cancer patients treated with tamoxifen) revealed that high expression of TRIM11 was associated with poor prognosis of breast cancer patients (Fig. S1C–H). We then analyzed the correlation between TRIM11 and ERα target genes expression, our results indicated expression of TRIM11 was positively correlated with TFF1, GREB1 and PDZK1 (Fig. S1I–M). We performed IHC analysis by using two tissue microarrays (TMA) collaborated with Shanghai Outdo Biotech (Shanghai, China). The results demonstrated that TRIM11 staining was positively associated with ERα and high expression of TRIM11 correlated with worse clinical outcome (Fig. S2).
TRIM11 promotes ERα-positive breast cancer cell proliferation
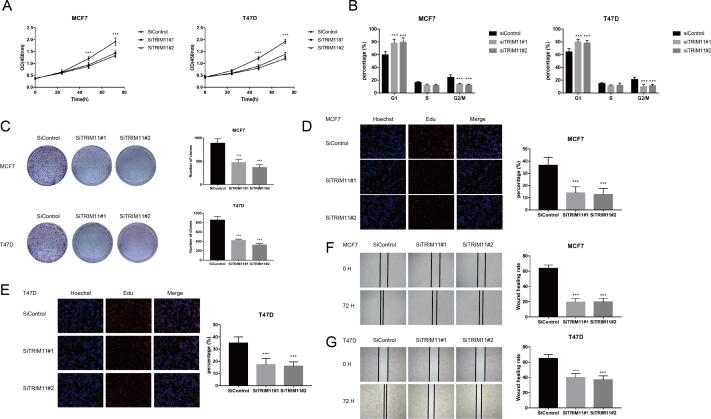
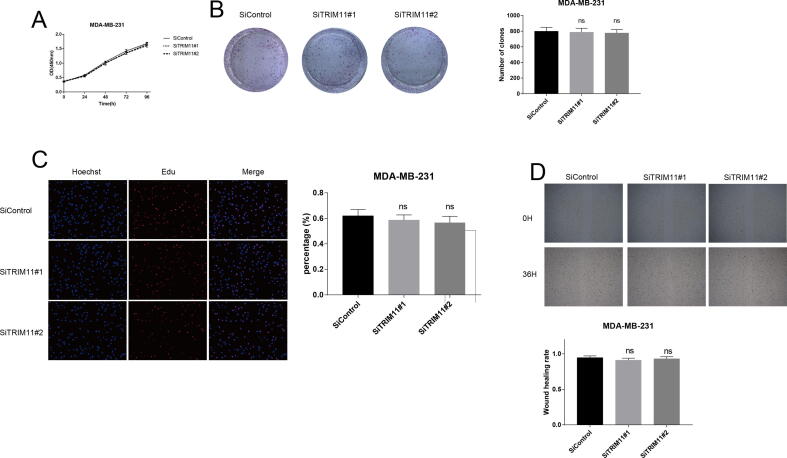
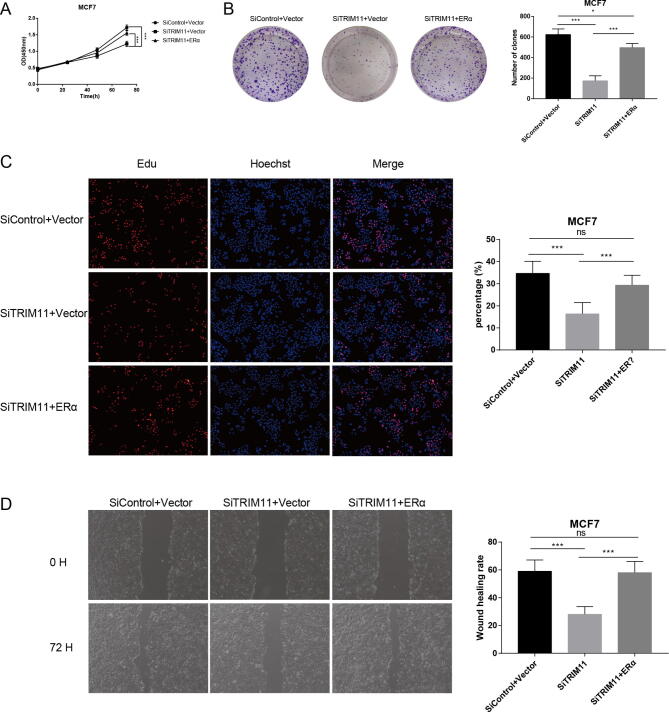
We explored the potential role of TRIM11 using two ERα-positive breast cancer cell lines, MCF7 and T47D. Depletion of TRIM11 significantly inhibited cell proliferation and induced G1 phase arrest (Fig. 1A, B). Clone formation capability was also decreased by TRIM11 knockdown (Fig. 1C). In agreement, as evaluated by EdU incorporation assay, DNA synthesis was inhibited by TRIM11 depletion (Fig. 1D, E). Besides, wound-healing assay showed that TRIM11 knockdown significantly decreased cell migration capacity of MCF7 and T47D cells (Fig. 1F, G). We then depleted TRIM11 in MDA-MB-231 cells (ER-negative breast cancer cell line), our results demonstrated TRIM11 depletion had little effect on the proliferation and migration capabilities of MDA-MB-231 cells, suggesting the phenotypic dependence is specific to ER-positive cell lines (Fig. S3). Furthermore, we performed a rescue experiment by overexpressing ERα in TRIM11-knockdown cells to verify whether the functions of TRIM11 in cell proliferation and migration require ERα. Increased ERα expression recovered the effect of TRIM11 knockdown (Fig. S4), indicating that TRIM11 promotes breast cancer cell proliferation and migration via the regulation of ERα.
Fig. 1.
TRIM11 depletion inhibits ERα-positive breast cancer cell proliferation and migration. (A). TRIM11 depletion inhibits the cell proliferation in breast cancer cells. (B). TRIM11 depletion induces G1 cell cycle arrest in breast cancer cells. (C). TRIM11 depletion decreases clone formation capability of breast cancer cells. (D, E). Representative images of EdU assay of breast cancer cells. (F, G). Wound-healing assay of breast cancer cells. *, P value < 0.05; **, P value < 0.01; ***, P value < 0.001.
TRIM11 knockdown inhibits ERα signaling activity
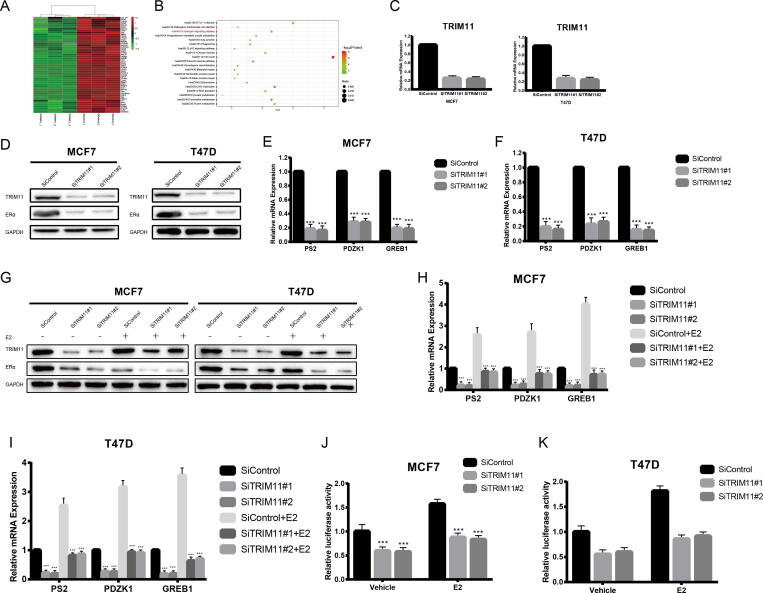
The RNA sequence analysis (siTRIM11 and siControl) was performed to approach the function of TRIM11. The results demonstrated that TRIM11 knockdown significantly decreased ERα target genes expression in MCF7 cells. And we noticed that estrogen signaling pathway was significantly suppressed upon TRIM11 depletion. (Fig. 2A, B). We used two different individual siRNAs which could significantly decrease TRIM11 expression to further addressed the function of TRIM11 (Fig. 2C). It was shown that TRIM11 knockdown significantly decreased ERα protein level and ERα target genes expression (PS2, GREB1 and PDZK1) in both MCF7 and T47D cells (Fig. 2D–F). In addition, depletion of TRIM11 could decrease ERα protein level and target genes expression in both estrogen and vehicle conditions (Fig. 2G–I). Consistently, ERα reporter gene activity was inhibited in the presence or absence of estrogen when TRIM11 knocked-down (Fig. 2J, K). These results indicated that TRIM11 might be a potential regulator of ERα signaling pathway.
Fig. 2.
TRIM11 depletion decreases ERα signaling activity in breast cancer cells. (A). Heatmap shows the ERα regulating genes are significantly inhibited by TRIM11 depletion in MCF7 cells. (B). Suppressed pathways in MCF7 cells upon TRIM11 depletion (C). TRIM1 depletion effect by two different siRNA oligos. Breast cancer cells are transfected with two independent TRIM11 siRNAs or siControl. After 48 h, TRIM11 mRNA levels are determined by qRT-PCR. 36B4 was used as internal control. (D). TRIM11 depletion decreases ERα protein level. (E, F). TRIM11 depletion decreases ERα target genes using two different siRNA oligos. (G). TRIM11 depletion decreases ERα protein level in the presence of estrogen. Breast cancer cells were transfected with siTRIM11 or siControl. After 48 h, cells were treated with either ethanol or 10 nM estrogen for 6 h. TRIM11 and ERα protein levels were determined by the western blot analysis. (H, I). TRIM11 depletion decreases ERα target genes in the presence of estrogen. Breast cancer cells were transfected with siTRIM11 or siControl. After 48 h, cells were treated with either ethanol or 10 nM estrogen for 6 h. Total RNA was prepared and the expression of the endogenous ERα target genes, PS2, GREB1, and PDZK1 were determined by qRT-PCR. (J, K). TRIM11 depletion affects ERE-luciferase activity. Breast cancer cells were transfected with siTRIM11 or siControl together with ERE luciferase reporter plasmid. Cells were treated with 10 nM estrogen or vehicle. Luciferase activity was measured 48 h after transfection. *, P value < 0.05; **, P value < 0.01; ***, P value < 0.001.
TRIM11 associates with ERα and enhances ERα stability
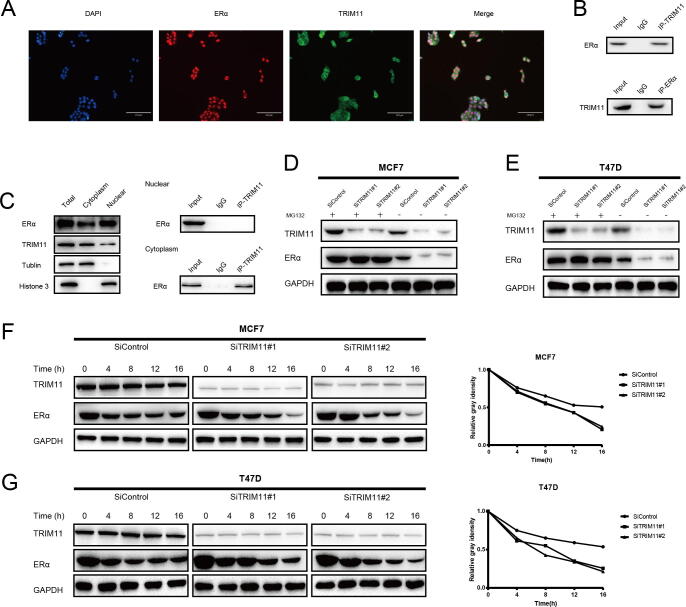
The results of immunostaining indicated that ERα mainly localized in the nucleus, while TRIM11 localized in both the nuclear and cytoplasmic. Co-immunoprecipitation showed that TRIM11 could interact with ERα (Fig. 3A, B). Nuclear and cytoplasmic separation assay demonstrated that TRIM11 interacted with ERα in the cytoplasm (Fig. 3C). TRIM11 depletion significantly decreased ERα protein level, while in the presence of the proteasome inhibitor MG132, ERα protein level was not further regulated by TRIM11 (Fig. 3D, E). In the protein stability assay, TRIM11 depletion significantly decreased ERα protein stability in MCF7 and T47D cells upon inhibition of protein synthesis by cycloheximide (Fig. 3F, G).
Fig. 3.
TRIM11 associates with ERα and increases its stability. (A). Intracellular localization analysis of TRIM11 and ER alpha by immunofluorescence assay. (B). Co-IP assay reveals association between endogenous TRIM11 and ERα in MCF7 cells. MCF7 cells were harvested with RIPA lysis buffer. Co-IP was performed using antibody as indicated. (C). TRIM11 is mainly localized in the cytoplasm and associates with ERα in the cytosol. The subcellular protein fractionation kit (Thermo Scientific, 78840) was used for cytoplasm and nuclear separation. Tubulin and Histone-3 were used for cytoplasm and nuclear control. (D, E). In the presence of the proteasome inhibitor MG132, depletion of TRIM11 did not further decrease ERα protein levels. Breast cancer cells were transfected with siTRIM11 or siControl. After 48 h, cells were treated with 10 µM MG132/vehicle for 6 h, cell lysates were prepared for western blot analysis. (F, G). TRIM11 depletion decreases ERα half-life in breast cancer cells. Breast cancer cells were transfected with siTRIM11 or siControl. After 48 h, cells were treated with 100 µM cycloheximide/vehicle for indicated times. Cell lysates were prepared for western blot analysis.
Mapping of the binding region between TRIM11 and ERα
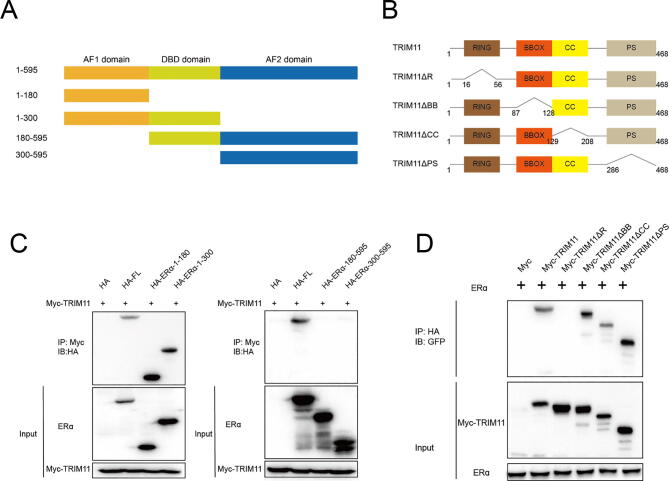
ERα has the three functional domains: AF1, DBD and AF2. The deletion mutants were constructed as follows: ΔAF1 domain (ERα-180–595), ΔAF1 + ΔDBD domain (ERα-300–595), ΔAF2 domain (ERα-1–300) and ΔAF2 + ΔDBD domain (ERα-1–180). As a member of the tripartite motif (TRIM) family, TRIM11 is composed of a RING, a B-box type 1 and a B-box type 2, a coiled-coil region, and a C-terminal PRY-SPRY (PS) motif. We constructed TRIM11 deletion mutants lacking each of the individual domains (ΔR, ΔBB, ΔCC and ΔPS) to identify the domain(s) of TRIM11 that mediates the interaction with ERα (Fig. 4A, B). Co-IP assay indicated that ΔAF1 domain was necessary to interact with TRIM11 (Fig. 4C). TRIM11-ΔBB, ΔCC and -ΔPS retained the ability to interact with ERα. However, TRIM11-ΔR completely lost this ability (Fig. 4D).
Fig. 4.
TRIM11 associates with ERα AF1 domain through its RING domain. (A, B). ERα and TRIM11 domain structure and deletion mutants used in the study. (C). TRIM11 interacts with ERα through its AF1 domain. HEK293 cells were transfected with 2 µg Myc-TRIM11 together with HA-ER alpha full length or mutants. After 24 h, cells were harvested with NP-40 lysis buffer. Co-IP was performed using Myc antibody. The possible interacted ERα domains were detected by HA antibody. (D). RING domain is required for TRIM11 to interaction with ERα. HEK293 cells were transfected with 2 µg HA-ER alpha together with Myc-TRIM11 full length or mutants. After 24 h, cells were harvested with NP-40 lysis buffer. Co-IP was performed using HA antibody. The possible interacted TRIM11 domains were detected by Myc antibody.
TRIM11 stabilizes ERα possibly through mono-ubiquitination
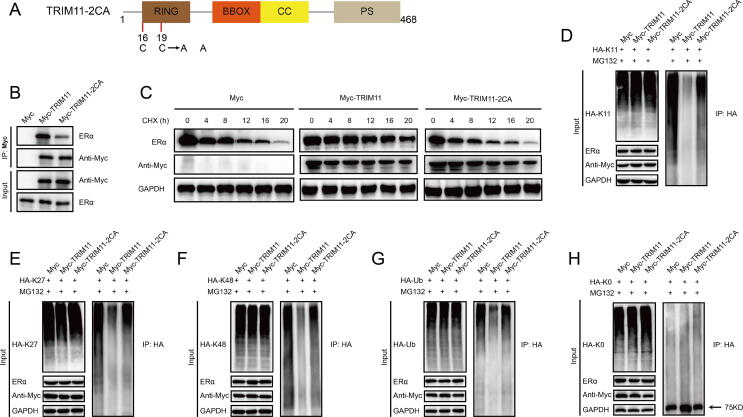
We also performed ubiquitination assay with a series of mutant ubiquitin. We found that TRIM11 significantly increased the mono-ubiquitinated ERα while decreased K11-, K27- and K48-dependent polyubiquitination on ERα protein. In addition, depletion of TRIM11 dramatically decreased mono-ubiquitination of endogenous ERα in MCF7 cells (Fig. 5). We then transfected TRIM11 and its deletion constructs together with ERα into HEK293 cells to identify the functional domain of TRIM11 to modulate ERα ubiquitination. TRIM11-ΔR completely lost the ability to promote ERα mono-ubiquitination and inhibit K11-, K27- and K48-linked polyubiquitination of ERα. While TRIM11-ΔBB, ΔCC and -ΔPS retained this ability (Fig. 6). We used a mutant of TRIM11 to further examine the role of the RING domain, two conserved Cys resides involved in Zn2+ binding (Cys16 and Cys19) in the RING domain were changed to Ala (TRIM11-2CA) (Fig. S5A). TRIM11-2CA exhibited a significantly reduced ability to interact with ERα (Fig. S5B). It also lost the ability to enhance ERα stability (Fig. S5C). Ubiquitination assay indicated that TRIM11-2CA did not promote ERα mono-ubiquitination and inhibit K11-, K27- and K48-linked ERα polyubiquitination (Fig. S5D–H). Collectively, these results demonstrate that TRIM11 regulates ERα via a direct protein–protein interaction that involves the AF1 domain of ERα and the RING domain of TRIM11.
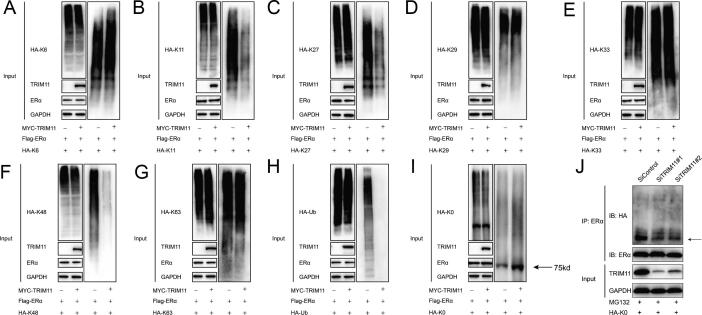
Fig. 5.
TRIM11 increases the mono-ubiquitinated ERα while decreases K11-, K27- and K48-dependent polyubiquitination on ERα protein. (A-I). HEK293 cells were transfected with 2 µg Flag- ERα plasmid, 0.5 µg Myc-TRIM11 plasmids, and 0.5 µg HA-K6, -K11, -K27, -K29, -K33, -K48, -K63, -K0 or -Ub plasmids. The cell extracts were immunoprecipitated with HA antibody. The specific polyubiquitinated and mono-ubiquitinated ERα were detected via western blotting analysis. (J). TRIM11 depletion decreases ERα mono-ubiquitination in MCF7 cells. MCF7 cells were transfected with siTRIM11 or siControl. After 48 h, cells were treated with 10 µM MG132 for 6 h, cell lysates were prepared for western blot analysis and anti-HA antibody was used to detect mono-ubiquitylated ERα.
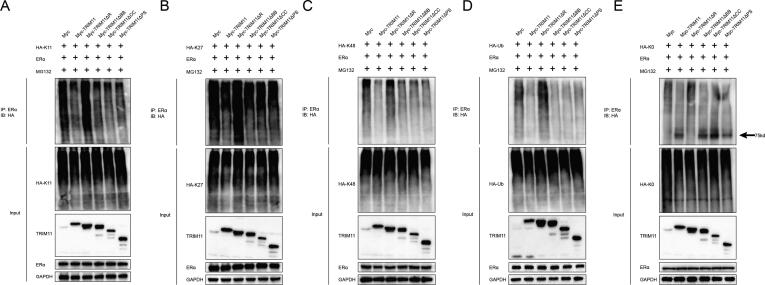
Fig. 6.
TRIM11 increases the mono-ubiquitinated ERα and decreases K11-, K27- and K48-dependent polyubiquitination on ERα protein via its RING domain. (A-E). HEK293 cells were transfected with 2 µg Flag-ERα plasmid, 0.5 µg Myc-TRIM11 or its deletion mutant plasmids, and 0.5 µg HA-K11, -K27, -K48, -K0 or -Ub plasmids. The cell extracts were immunoprecipitated with HA antibody. The specific polyubiquitinated and mono-ubiquitinated ERα were detected via western blotting analysis.
Discussion
ERα plays a central role both inside and outside the nucleus during the signal transduction which was firstly cloned from MCF-7 cell in 1985 [17]. Over 70% of breast cancers are ERα positive, which depends on ERα signaling for cell growth. ERα is a suitable target for breast cancer therapy. For ERα positive breast cancer patients, selective estrogen receptor modulators, such as tamoxifen, are standard endocrine treatment. However, endocrine resistance is one important issue in breast cancer therapy. The activity of ERα is mainly regulated by E2 hormone, however, growth factors including IGF and EGF could also promote its activity [18]. ERα is recruited on the enhancer and promoter sequences of its target genes in response to E2 hormone in breast cancer cells to facilitate their transcription. ERα co-regulators are also important during the development of breast cancer. Coactivator complexes facilitate transcription and corepressor complexes inhibit gene expression through opening or compacting chromatin [19], [20], [21]. In addition to the coregulators of ERα, other mechanisms such as posttranslational modifications which regulate the stability of ERα, also act as important regulatory factors of ERα function [22], [23]. Modulating ERα protein stability could be one plausible strategy to overcome endocrine resistance. In general, there are 4 main types of E3 ligases: HECT, RING-finger, U-box and PHD-finger families [24]. Previous studies reported several ubiquitin ligases such as CHIP, BRCA1, BARD1, E6AP, MDM2, and SKP2 were involved ERα protein degradation [25], [26], [27], [28], [29]. Interestingly, the ubiquitination of ERα does not necessary lead to proteasomal degradation. For example, TRIM56 is found to promote ERα stability via K63-linked ubiquitination [10]. Mono-ubiquitination could confer ERα stability by inhibiting its polyubiquitination and degradation [30]. RNF8 stabilize ERα through inducing its mono-ubiquitination [7]. RNF31 and SHARPIN also acts as E3-ubiquitin ligases that associate with ERα and induce its mono-ubiquitination to block the polyubiquitination, thus increasing its protein stability [8], [9]. Polyubiquitination and mono-ubiquitination compete to modify to ERα to regulate its stability, and mono-ubiquitination of ERα is more likely to confer its stability and enhance estrogen signaling in breast cancer cells.
TRIM11 is an E3 ubiquitin ligase and belongs to the TRIM family. The oncogene role of TRIM11 has been reported in a variety of human cancers, including glioma, lung cancer and hepatocellular carcinoma cancer [11], [12], [13], [14]. In breast cancer, TRIM11 is also a crucial proto-oncogene, depletion of TRIM11 dramatically reduced the proliferation in different types of breast cancer cells [31]. While the underlying mechanisms are poorly investigated. Here, by analyzing the commercial tissue microarrays, we found positive correlation between TRIM11 and ERα protein levels. Kaplan–Meier survival curves with log-rank test demonstrated that high expression of TRIM11 was associated with poor prognosis. Analysis of online available datasets (TCGA, GSE6532 and GSE9195) also indicated that TRIM11 was an unfavourable prognostic marker. We then investigated the biologic functions of TRIM11 in breast cancer. Depletion of TRIM11 in ER-positive breast cancer cell lines (MCF7 and T47D) significantly suppressed cell growth and migration. And the suppression effects were reversed by overexpressing ERα. RNA-seq results suggested that TRIM11 may play an oncogenic role through ERα signaling pathway. Silencing TRIM11 could significantly decrease ERα protein level. As TRIM11 is an E3 ubiquitin ligase, we further analyzed whether TRIM11 could directly bind to ERα and modulate its protein stability. Upon inhibition of protein synthesis by cycloheximide, TRIM11 depletion significantly decreased ERα protein stability in MCF7 and T47D cells. Co-IP and ubiquitination assay indicated that the RING domain of TRIM11 interacted with the AF1 domain of ERα, and promoted ERα mono-ubiquitination. TRIM11-2CA exhibited a significantly reduced ability to interact with ERα and lost the ability to enhance ERα stability. Collectively, TRIM11 regulates ERα via a direct protein–protein interaction that involves the AF1 domain of ERα and the RING domain of TRIM11 and this stabilization effect may depend on its mono-ubiquitination modification.
ERα can be modified by both mono-and polyubiquitination, while the two modification are dissimilar. Polyubiquitination is often associated with the degradation of ERα, However, mono-ubiquitination of ERα modulates its DNA-binding activity, its stability, and its interactions [32], [33], [34]. Competition between mono-ubiquitination and polyubiquitination may exist, as the sites for these modifications appear to be the same. Thus, mono-ubiquitination confers ERα stability by inhibiting its polyubiquitination [34]. Besides, acetylation and methylation of the lysine residues responsible for mono/polyubiquitination may affect its stability as well [35], [36], [37], [38]. Some ER-positive breast cancer patients develop acquired resistance to endocrine therapy, while underlying mechanisms have not been completely defined yet. ERα stability may promote endocrine resistance in ER-positive breast cancer. When ERα stability is enhanced, its turnover is affected and levels of ERα are sustained. Sustained levels of ERα may stimulate persistent extranuclear and nuclear signaling associated with endocrine resistance and alter crosstalk between pathways, generating new signaling routes that promote the progression of this cancer [30]. In this case, a better understanding of potential mechanisms that promote as well as inhibit polyubiquitination and degradation of ERα may be crucial for preventing resistance to endocrine therapy. Hence, mechanisms underlying ERα stability may be targeted in the development of new therapeutic strategies as well as indicators of endocrine therapy selection.
Conclusion
In the present study, we noticed that TRIM11 was possibly to enhance ERα stability. The RING domain of TRIM11 interacted with the N terminal of ERα in the cytoplasm and promoted its mono-ubiquitination, while inhibited K11-, K27- and K48-linked polyubiquitination. Due to the central role of ERα signaling in breast cancer proliferation, post-translational modification of ERα protein could be an approach to restore endocrine resistance and inhibit breast cancer cell progression. In all, TRIM11 could be a promising therapeutic target for breast cancer treatment.
Competing interests
The authors declare that they have no competing of interest.
Author’s contribution
JT performed most of the bench work. ZT, QY and XL participated in the modification and prognosis analysis of the manuscript. GW supervised the process of the study and wrote the manuscript. All authors read and approved the final manuscript.
Funding
This project was funded by the Research and Development Fund of Zhongnan Hospital of Wuhan University (Wu, No.20170101).
Acknowledgements
We thank Pro. Ting Zhuang and Jian Zhu (Henan Key Laboratory of immunology and targeted therapy, Xinxiang Medical University) for kindly providing ERα full-/deletion constructs plasmids and Estrogen-Response-Element (ERE)-TK reporter plasmids.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neo.2020.06.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Supplementary Fig. 2.
Supplementary Fig. 3.
Supplementary Fig. 4.
Supplementary Fig. 5.
Data available statement
The datasets generated and during the current study are available in the SRA database repository, https://www.ncbi.nlm.nih.gov/sra/PRJNA609245
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Waks A.G., Winer E.P. Breast cancer treatment: a review. JAMA. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 3.Kumar R., Zakharov M.N., Khan S.H., Miki R., Jang H., Toraldo G., Singh R., Bhasin S., Jasuja R. The dynamic structure of the estrogen receptor. J Amino Acids. 2011;2011 doi: 10.4061/2011/812540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker J.S., Mullins M., Cheang M.C., Leung S., Voduc D., Vickery T., Davies S., Fauron C., He X., Hu Z., Quackenbush J.F., Stijleman I.J., Palazzo J. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giulianelli S., Vaque J.P., Wargon V., Soldati R., Vanzulli S.I., Martins R., Zeitlin E., Helguero L., Lamb C., Molinolo A.A., Gutkind J.S., Lanari C. The role of estrogen receptor alpha in breast cancer cell proliferation mediated by progestins. Medicina (B Aires) 2012;72:315–320. [PubMed] [Google Scholar]
- 6.Musgrove E.A., Sutherland R.L. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 7.Wang S., Luo H., Wang C., Sun H., Sun G., Sun N., Zeng K., Song H., Zou R., Zhou T., Cong R., Liu W., Yang L. RNF8 identified as a co-activator of estrogen receptor alpha promotes cell growth in breast cancer. Biochim Biophys Acta, Mol Basis Dis. 2017;1863:1615–1628. doi: 10.1016/j.bbadis.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Zhuang T., Yu S., Zhang L., Yang H., Li X., Hou Y., Liu Z., Shi Y., Wang W., Yu N., Li A., Li X., Li X. SHARPIN stabilizes estrogen receptor alpha and promotes breast cancer cell proliferation. Oncotarget. 2017;8:77137–77151. doi: 10.18632/oncotarget.20368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu J., Zhao C., Kharman-Biz A., Zhuang T., Jonsson P., Liang N., Williams C., Lin C.Y., Qiao Y., Zendehdel K., Stromblad S., Treuter E., Dahlman-Wright K. The atypical ubiquitin ligase RNF31 stabilizes estrogen receptor alpha and modulates estrogen-stimulated breast cancer cell proliferation. Oncogene. 2014;33:4340–4351. doi: 10.1038/onc.2013.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue M., Zhang K., Mu K., Xu J., Yang H., Liu Y., Wang B., Wang Z., Li Z., Kong Q., Li X., Wang H., Zhu J. Regulation of estrogen signaling and breast cancer proliferation by an ubiquitin ligase TRIM56. Oncogenesis. 2019;8:30. doi: 10.1038/s41389-019-0139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y., Li L., Qian X., Ge Y., Xu G. High expression of TRIM11 correlates with poor prognosis in patients with hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2017;41:190–196. doi: 10.1016/j.clinre.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Di K., Linskey M.E., Bota D.A. TRIM11 is overexpressed in high-grade gliomas and promotes proliferation, invasion, migration and glial tumor growth. Oncogene. 2013;32:5038–5047. doi: 10.1038/onc.2012.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X., Shi W., Shi H., Lu S., Wang K., Sun C., He J., Jin W., Lv X., Zou H., Shu Y. TRIM11 overexpression promotes proliferation, migration and invasion of lung cancer cells. J Exp Clin Cancer Res. 2016;35:100. doi: 10.1186/s13046-016-0379-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Zhang Z., Xu C., Zhang X., Huang L., Zheng C., Chen H., Wang Y., Ju H., Yao Q. TRIM11 Upregulation contributes to proliferation, invasion, and EMT of hepatocellular carcinoma cells. Oncol Res. 2017;25:691–699. doi: 10.3727/096504016X14774897404770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong D., Li A., Liu Y., Cui Q., Wang K., Zhang D., Tang J., Du Y., Liu Z., Wu G., Wu K. SIX1 activates STAT3 signaling to promote the proliferation of thyroid carcinoma via EYA1. Front Oncol. 2019;9:1450. doi: 10.3389/fonc.2019.01450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang H., Yu N., Xu J., Ding X., Deng W., Wu G., Li X., Hou Y., Liu Z., Zhao Y., Xue M., Yu S., Wang B. SMURF1 facilitates estrogen receptor a signaling in breast cancer cells. J Exp Clin Cancer Res. 2018;37:24. doi: 10.1186/s13046-018-0672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter P., Green S., Greene G., Krust A., Bornert J.M., Jeltsch J.M., Staub A., Jensen E., Scrace G., Waterfield M. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci U S A. 1985;82:7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato S. Estrogen receptor-mediated cross-talk with growth factor signaling pathways. Breast Cancer. 2001;8:3–9. doi: 10.1007/BF02967472. [DOI] [PubMed] [Google Scholar]
- 19.Hah N., Kraus W.L. Hormone-regulated transcriptomes: lessons learned from estrogen signaling pathways in breast cancer cells. Mol Cell Endocrinol. 2014;382:652–664. doi: 10.1016/j.mce.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hah N., Murakami S., Nagari A., Danko C.G., Kraus W.L. Enhancer transcripts mark active estrogen receptor binding sites. Genome Res. 2013;23:1210–1223. doi: 10.1101/gr.152306.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manavathi B., Samanthapudi V.S., Gajulapalli V.N. Estrogen receptor coregulators and pioneer factors: the orchestrators of mammary gland cell fate and development. Front Cell Dev Biol. 2014;2:34. doi: 10.3389/fcell.2014.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tecalco-Cruz A.C., Perez-Alvarado I.A., Ramirez-Jarquin J.O., Rocha-Zavaleta L. Nucleo-cytoplasmic transport of estrogen receptor alpha in breast cancer cells. Cell Signal. 2017;34:121–132. doi: 10.1016/j.cellsig.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Tecalco-Cruz A.C., Ramirez-Jarquin J.O. Mechanisms that increase stability of estrogen receptor alpha in breast cancer. Clin Breast Cancer. 2017;17:1–10. doi: 10.1016/j.clbc.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Metzger M.B., Hristova V.A., Weissman A.M. HECT and RING finger families of E3 ubiquitin ligases at a glance. J Cell Sci. 2012;125:531–537. doi: 10.1242/jcs.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatt S., Xiao Z., Meng Z., Katzenellenbogen B.S. Phosphorylation by p38 mitogen-activated protein kinase promotes estrogen receptor alpha turnover and functional activity via the SCF(Skp2) proteasomal complex. Mol Cell Biol. 2012;32:1928–1943. doi: 10.1128/MCB.06561-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan M., Park A., Nephew K.P. CHIP (carboxyl terminus of Hsc70-interacting protein) promotes basal and geldanamycin-induced degradation of estrogen receptor-alpha. Mol Endocrinol. 2005;19:2901–2914. doi: 10.1210/me.2005-0111. [DOI] [PubMed] [Google Scholar]
- 27.Hashizume R., Fukuda M., Maeda I., Nishikawa H., Oyake D., Yabuki Y., Ogata H., Ohta T. The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J Biol Chem. 2001;276:14537–14540. doi: 10.1074/jbc.C000881200. [DOI] [PubMed] [Google Scholar]
- 28.Saji S., Okumura N., Eguchi H., Nakashima S., Suzuki A., Toi M., Nozawa Y., Saji S., Hayashi S. MDM2 enhances the function of estrogen receptor alpha in human breast cancer cells. Biochem Biophys Res Commun. 2001;281:259–265. doi: 10.1006/bbrc.2001.4339. [DOI] [PubMed] [Google Scholar]
- 29.Sun J., Zhou W., Kaliappan K., Nawaz Z., Slingerland J.M. ERalpha phosphorylation at Y537 by Src triggers E6-AP-ERalpha binding, ERalpha ubiquitylation, promoter occupancy, and target gene expression. Mol Endocrinol. 2012;26:1567–1577. doi: 10.1210/me.2012-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tecalco-Cruz A.C., Ramirez-Jarquin J.O., Cruz-Ramos E. Estrogen receptor alpha and its ubiquitination in breast cancer cells. Curr Drug Targets. 2019;20:690–704. doi: 10.2174/1389450119666181015114041. [DOI] [PubMed] [Google Scholar]
- 31.Song W., Wang Z., Gu X., Wang A., Chen X., Miao H., Chu J., Tian Y. TRIM11 promotes proliferation and glycolysis of breast cancer cells via targeting AKT/GLUT1 pathway. Onco Targets Ther. 2019;12:4975–4984. doi: 10.2147/OTT.S207723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eakin C.M., Maccoss M.J., Finney G.L., Klevit R.E. Estrogen receptor alpha is a putative substrate for the BRCA1 ubiquitin ligase. Proc Natl Acad Sci U S A. 2007;104:5794–5799. doi: 10.1073/pnas.0610887104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.La Rosa P., Acconcia F. Signaling functions of ubiquitin in the 17beta-estradiol (E2):estrogen receptor (ER) alpha network. J Steroid Biochem Mol Biol. 2011;127:223–230. doi: 10.1016/j.jsbmb.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Ma Y., Fan S., Hu C., Meng Q., Fuqua S.A., Pestell R.G., Tomita Y.A., Rosen E.M. BRCA1 regulates acetylation and ubiquitination of estrogen receptor-alpha. Mol Endocrinol. 2010;24:76–90. doi: 10.1210/me.2009-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berry N.B., Fan M., Nephew K.P. Estrogen receptor-alpha hinge-region lysines 302 and 303 regulate receptor degradation by the proteasome. Mol Endocrinol. 2008;22:1535–1551. doi: 10.1210/me.2007-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim S.H., Kang H.J., Na H., Lee M.O. Trichostatin A enhances acetylation as well as protein stability of ERalpha through induction of p300 protein. Breast Cancer Res. 2010;12:R22. doi: 10.1186/bcr2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang C., Fu M., Angeletti R.H., Siconolfi-Baez L., Reutens A.T., Albanese C., Lisanti M.P., Katzenellenbogen B.S., Kato S., Hopp T., Fuqua S.A., Lopez G.N., Kushner P.J. Direct acetylation of the estrogen receptor alpha hinge region by p300 regulates transactivation and hormone sensitivity. J Biol Chem. 2001;276:18375–18383. doi: 10.1074/jbc.M100800200. [DOI] [PubMed] [Google Scholar]
- 38.Subramanian K., Jia D., Kapoor-Vazirani P., Powell D.R., Collins R.E., Sharma D., Peng J., Cheng X., Vertino P.M. Regulation of estrogen receptor alpha by the SET7 lysine methyltransferase. Mol Cell. 2008;30:336–347. doi: 10.1016/j.molcel.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and during the current study are available in the SRA database repository, https://www.ncbi.nlm.nih.gov/sra/PRJNA609245