Abstract
Despite the well-documented capacity of embryonic stem cells (ESCs) to differentiate into cardiomyocytes, transplantation of ESCs or ESC-derived cells is plagued by several formidable problems, including graft rejection, arrhythmias, and potential risk of teratomas. Life-long immunosuppression is a disease in itself. Transplantation of human ESC-derived cells in primates causes life-threatening arrhythmias, and the doses used to show efficacy are not clinically relevant. In contemporary clinical research, the margin of tolerance for such catastrophic effects as malignancies is zero, and although the probability of tumours can be reduced by ESC differentiation, it is unlikely to be completely eliminated, particularly when billions of cells are injected. Although ESCs and ESC-derived cells were touted as capable of long-term regeneration, these cells disappear rapidly after transplantation and there is no evidence of long-term engraftment, let alone regeneration. There is, however, mounting evidence that they act via paracrine mechanisms—just like adult cells. To date, no controlled clinical trial of ESC-derived cells in cardiovascular disease has been conducted or even initiated. In contrast, adult cells have been used in thousands of patients with heart disease, with no significant adverse effects and with results that were sufficiently encouraging to warrant Phase II and III trials. Furthermore, induced pluripotent stem cells offer pluripotency similar to ESCs without the need for lifelong immunosuppression. After two decades, the promise that ESC-derived cells would regenerate dead myocardium has not been fulfilled. The most reasonable interpretation of current data is that ESC-based therapies are not likely to have clinical application for heart disease.
Keywords: Embryonic stem cells, Heart failure, Cell therapy, Fibrosis, Cardiomyocytes, Regeneration
Stem cells are defined as undifferentiated cells that are self-renewing and able to give rise to mature cells.1,2 Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) are pluripotent—they can differentiate into any cell type. In contrast, stem cells obtained from adult tissues are multipotent or unipotent—they can give rise only to one or few cell types.1–4 All controlled clinical trials of stem cells performed to date in cardiovascular medicine have used adult cells, with results that were encouraging enough to warrant ongoing Phase III trials.5,6 Here, we will address the question: is there a sound rationale for pursuing clinical trials of hESCs or hESC-derived cells?
When cell therapy emerged as a novel approach to heart disease almost two decades ago, the hope was that it would enable one, for the first time, to regenerate cardiac muscle after myocardial infarction (MI), replacing dead tissue with new contracting myocytes.7–9 In the ensuing years, various types of adult cells (obtained from bone marrow, heart, adipose tissue, or other tissues) were found to be effective in improving left ventricular (LV) function in animal models of ischaemic cardiomyopathy but none has been shown to regenerate new bona fide myocytes, due to failure of the cells to engraft and their inability to differentiate into mature cardiomyocytes (CMs).5,10–19 In fact, most of these cell types have not been shown to be self-renewing and multi/unipotent, and thus do not meet the strict definition of ‘stem cells’ mentioned above and should not be referred to as ‘stem cells’ (the term ‘progenitor cells’ is more appropriate). Despite this, the evidence that adult cells improve LV function in preclinical models of MI or heart failure (HF) is overwhelming,5,10–19 and although conclusive Phase III data are still lacking, many Phase I and II clinical trials have demonstrated safety and provided initial evidence of efficacy of adult cells in patients with HF or refractory angina.5,6 It is now widely agreed that adult cells work via paracrine mechanisms5,19–21 and that their salubrious effects on LV function probably do not reflect formation of new myocytes, but rather a panoply of reparative actions, e.g., anti-inflammatory, anti-fibrotic, anti-apoptotic, and pro-angiogenic actions, modulation of contractile function, etc.5,19–21 Thus, when speaking of adult cell-based therapies, the terms ‘cardiac regeneration’ and ‘regenerative therapy’ are incorrect and potentially misleading, and should be replaced by ‘cardiac repair’ and ‘reparative therapy’.
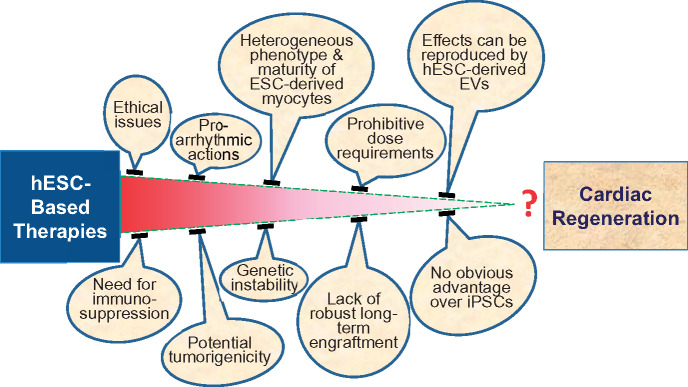
ESCs and iPSCs, on the other hand, constitute a population of stem cells with clear multilineage potential, including cardiomyogenesis. Numerous studies have documented that ESCs can differentiate into mature, contracting myocytes in vitro,22–28 raising the possibility that these cells could be used to regenerate dead myocardium. However, the same pluripotency of ESCs that paves the way to regeneration is also a major concern, because these cells can form teratomas in the host. One possible solution to prevent tumourigenicity is to differentiate ESCs into cardiovascular progenitor cells (CVPCs) or CMs and eliminate pluripotent cells from the pool of transplanted cells. In the past several years, numerous studies have addressed the safety and efficacy of ESC-derived CVPCs and CMs in small and large animal models.22,26,27,29–36 Based on these studies, it is becoming clear that while these cells can improve cardiac function, they are unlikely to have any clinical applicability, for a host of reasons (Table 1), including prohibitive dose requirements, strong pro-arrhythmic actions, lack of robust evidence for safety, no evidence of long-term engraftment, and inadequate evidence of regeneration of CMs after in vivo administration. Surprisingly, despite these enormous problems, ESCs and ESC-derived cells have continued to be enthusiastically heralded for two decades as a major breakthrough in medicine that will usher in unprecedented opportunities for the treatment of human disease.37–44 A reality check is therefore in order. The purpose of this review is to provide a critical appraisal of the current evidence regarding the potential use of ESCs or ESC-derived cells as a clinical therapy for MI or HF. The major problems with the development of these therapies are discussed below and summarized in Table 1 and Figure 1.
Table 1.
Problems with human embryonic stem cell-based therapies
| Ethical and regulatory issues |
| Risk of tumourigenicity after transplantation of a large number (billions) of hESC-derived cells |
| Prolonged culture (including scale-up and differentiation) provides opportunities for genetic abnormalities (potentially tumorigenic) |
| Need for long-term immunosuppression |
| Pro-arrhythmic actions |
| No evidence of long-term engraftment of transplanted ESC-derived cells (most studies had a follow-up of <1 month, with <1% cell survival; when follow-up was >1 month, survival was even less) |
| Failure of ESC-CMs and ESC-CVPCs to form non-myocytes |
| Prohibitive dose requirements |
| Heterogeneous phenotypes and maturity of ESC-derived cells (nodal, atrial, ventricular phenotypes, and immature electrophysiological properties) |
| No obvious advantages over iPSC-derived cells |
| Effects may be recapitulated by hESC-derived extracellular vesicles |
Figure 1.
Obstacles to the clinical application off embryonic stem cell-based therapies for the treatment of heart disease.
Doses of hESC-derived cells
Can hESC-derived cells be delivered to humans in doses comparable to those used in animal studies to show a beneficial effect? The most relevant studies addressing this question are those performed in non-human primates. Chong et al.36 injected an enormous number of hESC-CMs (1 billion) into the infarct and border zone of four pigtail macaques. Despite this massive transplantation, infarct size was not significantly reduced. Considering that the body weight of pigtail macaques (9.2–12.3 kg) was ∼7 times less than that of a 75-kg human, an equivalent human dose would be ∼7 billion hESC-CMs, which is obviously not a feasible possibility for clinical use, for several reasons. Interestingly, this study36 was proposed by the authors as a proof-of-concept test for the use of hESC-CMs in clinical trials.
In a subsequent study in the same primate species, Liu et al.34 injected 750 million hESC-CMs intramyocardially. In a recent editorial,45 we wrote: ‘Liu et al.34 injected an astonishing number of hESC-CMs (750 million) in a total of 1.5 mL. (Incidentally, it is unclear how 750 million cells can be suspended in 1.5 mL of solution.) The weight of the five-treated macaques averaged 8.6 kg, ∼9 times smaller than the average 75-kg human. Heart weight was not reported. However, since in the average 75-kg human the LV contains ∼5 billion CMs, the number of CMs in the macaque LV, which should be ∼9 times smaller, can be estimated at ∼5000 million/9 = ∼560 million. Thus, the number of hESC-CMs injected was greater than the total number of CMs in the macaque heart! The authors administered the cells in 15 injections in the border zone and infarcted region. Since, as stated above, in the average 75-kg human the weight of the heart is ≈ 9 times greater than in these macaques, the equivalent human dose of hESC-CMs would be 750 × 9 = 6.750 billion cells. Administration of 6.75 billion cells in a human heart is not possible, because it would require an inordinate number of injections, an inordinate volume of injections, or both. Given that this enormous cell load increased LV mass by just ∼2%, the treatment described by Liu et al.34 is not applicable to humans, and thus its clinical relevance is unclear.’
Do hESC-derived cells engraft and form mature cardiomyocytes?
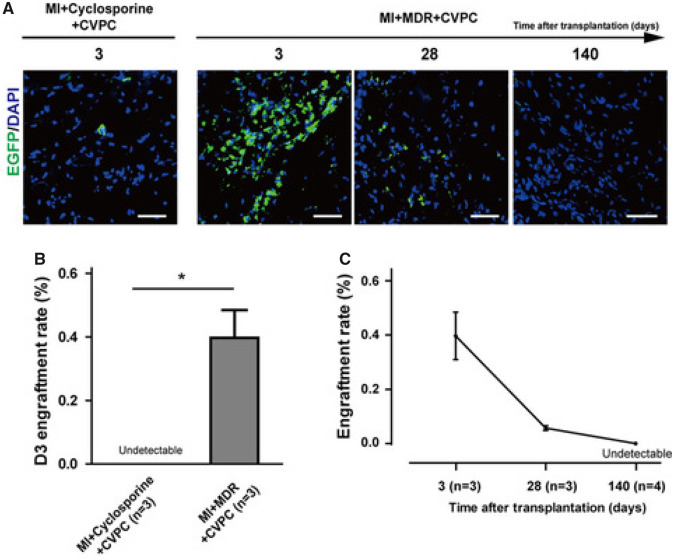
Studies in pigs suggest that at 1 month after transplantation, <1% of injected human iPSCs survive.46 In a landmark study, Zhu et al.47 reported that hESC (line H9)-derived CVPCs (hESC-CVPCs), injected in the hearts of non-human primates after MI, improved LV function; however, the transplanted cells did not engraft and did not contribute to the formation of new CMs. In this important investigation, the authors injected 1 × 107 EGFP+ hESC-CVPCs in the hearts of cynomolgus monkeys 4 days after MI. To avoid immune rejection, two immunosuppression protocols were applied and compared: cyclosporine alone and cyclosporine in combination with Simulect and methylprednisolone. Regardless of the immunosuppressive protocol used, hESC-CVPCs in the heart declined markedly at 28 days after transplantation and disappeared completely at 140 days (Figure 2). Therefore, despite aggressive immunosuppressive treatment, the transplanted cells did not engraft and did not contribute to new contractile myocytes. Echocardiographic analysis showed a significant increase in left ventricular ejection fraction (LVEF) in hESC-CVPC-treated animals at 28 days after cell injection compared with controls. Thus, transplanted cells produced an improvement in LV function despite failure to engraft and differentiate into new myocytes, indicating that they did not regenerate dead myocardium but instead released signals or factors that affected the host tissue in a manner that was conducive to tissue repair and improved contractile performance. Because echocardiographic studies were not performed at 140 days after cell transplantation, it is unknown whether the functional improvement persisted in the long term. With a total of 42 animals used, this is the largest non-human primate study of ESC-derived CVPCs or CMs reported to date. It provides compelling evidence that, similar to adult cells, transplanted ESC-derived cells do not engraft long-term, do not remuscularize the heart, and act via paracrine mechanisms.
Figure 2.
Negligible long-term engraftment of human embryonic stem cell-derived cardiovascular progenitor cells in immunosuppressed non-human primates. Cynomolgus monkeys were subjected to myocardial infarction by permanent ligation of the left anterior descending coronary artery. Thirty minutes after ligation, animals were treated with cyclosporine or multiple-drug regimen (cyclosporine in combination with Simulect and methylprednisolone) and intramyocardially injected with 1 × 107 EGFP labelled human embryonic stem cell-derived cardiovascular progenitor cells. Engraftment of transplanted cells was evaluated with immunofluorescence in heart tissue collected at 3, 28, and 140 days after cell injection (A). At 3 days, the engraftment rate was nil with cyclosporine and 0.4% with MDR (B). By 140 days, cells became undetectable (C). Bar = 50 μm. *P < 0.05. Reprinted with permission from Zhu et al.47
One could argue that the immunosuppressive regimen used in this study was not sufficient to completely prevent rejection of the transplanted cells. However, similar studies were performed by Bellamy et al.29 in immunodeficient rats with a similar outcome. The animals were transplanted with a fibrin patch loaded with hESC-CVPCs (700 000 cells per patch) or cell-free patch as a control 5–7 weeks after permanent coronary artery ligation. At 4 months after transplantation, LV function was significantly improved (echocardiography); however, no transplanted cells were detectable at this time point, suggesting that even in the absence of immune rejection and despite the fact that cells were embedded in a fibrin patch to increase their survival, the cells did not engraft long term and did not contribute to new functional myocytes.29 Therefore, the only explanation for the functional improvement is that the transplanted cells improved myocardial repair through a paracrine mechanism, which is consistent with the study by Zhu et al.47
A similar study was performed by Blin et al.30 in immunosuppressed Rhesus monkeys (Macaca Mulatta) subjected to a 90-min coronary occlusion followed by 2 weeks of reperfusion. Injection of unpurified hESC-CVPCs (which included SSEA-1neg undifferentiated pluripotent stem cells) resulted in formation of a microteratoma. In further experiments, 2 × 107 SSEA-1+ ESC-CVPCs were injected in eight monkeys. Two months later, no teratoma was detected in any organ. The injected hearts contained clusters of GFP+ cells that expressed CM markers but did not appear to have a fully mature mophology. Although surviving GFP+ cells occupied ∼20% of the scar area, their number was not quantified. Moreover, cell survival was evaluated only at 2 months after transplantation, a follow-up that may be too short to determine long-term engraftment. LV function was not assessed.30
The same group also performed ESCORT, the first clinical study of hESC-CVPCs,48,49 in which a fibrin scaffold containing SSEA-1+ sorted hESC-CVPCs (median dose, 8.2 million cells; range 5–10 million) was implanted on the epicardium of the infarcted LV region of six patients with ischaemic HF undergoing coronary artery bypass surgery; no control patients were enrolled. All patients received immunosuppressive drugs to prevent graft rejection. No teratoma formation was detected at 6 and 12 months after implantation, and there was no evidence of arrhythmias during the follow-up period. Three patients developed clinically silent allorejecion reactions, demonstrated by the presence of alloantibodies at low titre, which resolved over time, most likely due to immunosuppression. During a 1-year follow-up in four patients, regional wall motion improved significantly. However, the lack of a control group, the minuscule sample size, and the fact that patients received both revascularization and stem cells at the same time make it impossible to draw any conclusions regarding the effects of hESC-CVPCs on LV function. The small sample size (only six patients) and the small number of hESC-CVPCs used (relative to the doses tested in monkeys) also make it difficult to evaluate the safety of this therapy.48,49 Ultimately, the trial was stopped because of the growing evidence that transplanted cells most likely work via paracrine mechanism, not by engrafting and forming new contractile units. Indeed, recently it has been demonstrated that hESC-CVPC-derived exosomes injected in the infarcted heart can reproduce the beneficial effects of hESC-CVPCs.50
Studies using hESC-CVPCs have revealed numerous potential issues that would have to be resolved before moving forward with clinical trials. One of the common problems is that transplanted cells may not have proper cues to fully differentiate into adult myocytes that would electrically couple with the host cardiac muscle and contribute to synchronized LV contractility. This problem could be resolved with transplantation of mature hESC-derived myocytes (hESC-CMs). However, a series of studies conducted in an immunodeficient NOD-SCID mouse model of HF demonstrated that injection of 1–3 × 106 hESC-CMs led to only a transient improvement in LV function [measured with magnetic resonance imaging (MRI)], which was evident at 4 weeks after transplantation but was not sustained and disappeared at 12 weeks.32,51–53 It could be speculated that the transient reparative effects were caused by the progressive loss of transplanted cells. However, transplantation of larger amounts of hESC-CMs, which increased graft size, did not result in sustained functional improvement at 12 weeks.52 Detailed histological analysis revealed that the functional improvement was most likely related to enhanced vasculogenesis in the tissue surrounding the graft rather than to the actual graft size.51 Moreover, the graft was surrounded by fibrous tissue that precluded electrical coupling of the surviving transplanted myocytes with the host myocytes.53 Taken together, these observations32,51–53 indicate that the transient improvement in LV function was most likely related to paracrine factors released by hESC-CMs, not to regeneration of contractile cardiac muscle by the transplanted cells. The lack of electrical coupling of transplanted cells with host myocytes could potentially lead to arrhythmias, which could be missed in the mouse because of the high heart rate of this species (∼600 b.p.m.). These studies32,51–53 also suggest that long-term follow-up (>12 weeks) is necessary to evaluate the efficacy of transplantation of hESC-CMs.
In a similar investigation,31 SCID-beige mice with MI received an intramyocardial injection of hESC-CMs (0.5 million). After 2 and 4 weeks, LVEF (measured with MRI) was improved in cell-treated mice, and manganese-enhanced MRI analysis demonstrated more viable myocardium compared with control mice. However, microscopic analysis revealed rather minimal cell engraftment, suggesting that the transplanted cells improved survival of endogenous myocytes rather than contributing to de novo cardiomyogenesis. Consistent with this hypothesis, the hESC-CM secretome improved CM survival and promoted expression of the promigratory marker CXCR4 and angiogenesis in vitro. Taken together, these observations corroborate (again) the view that hESC-CMs preserve LV function not by engrafting but by secreting paracrine factors that exert a favourable influence on the myocardium. No teratoma was observed, suggesting no contamination with pluripotent stem cells, but the study follow-up (4 weeks) was too short to definitely exclude that possibility.31
Experiments in rats have also failed to document full maturation and integration of transplanted hESC-CMs with host myocytes. Laflamme et al.54 subjected nude rats to a reperfused MI and, 4 days later, transplanted 10 million hESCs which were differentiated into CMs by incubation with Activin A and BMP4, along with a cocktail of pro-survival factors aimed at limiting CM death after transplantation. Four weeks later, LV dilation was attenuated and regional and global LV function (measured with MRI and echocardiography) were improved in rats treated with hESC-CMs compared with controls. Infarct size did not differ between the groups. The engrafted cells, however, did not resemble the adult phenotype; CMs were smaller and irregularly shaped, with irregular sarcomeric organization.54 Therefore, despite the expression of adult cardiac markers, their contribution to LV function is rather questionable. Similar to the observations in the murine models described above,31,32,51–53 the hESC-CMs were separated from the host myocardium by scar tissue, suggesting that the graft was not electrically coupled with the host CMs and, therefore, could not contribute to synchronized LV contractile activity. Similar to the mouse studies,31,32,51–53 hESC-CMs induced angiogenesis of host-derived endothelial cells, resulting in an increased number of host capillaries. Given the short follow-up (4 weeks),54 it is unknown whether the presence of hESC-CMs and the functional improvement would persist in the long-term in the rat heart.
In a second rat study, performed by an independent group, Caspi et al.55 injected 1.5 million hESC-CMs intramyocardially in immunosuppressed rats 7–10 days after permanent coronary artery ligation and examined the hearts 30 and 60 days later. The authors confirmed the observations of Laflamme et al.54 that the donor cells present in the scar and the border zone expressed markers of adult CMs but lacked adult CM morphology.55 Echocardiographic analysis showed that fractional shortening was greater in hESC-CM-treated rats than in controls, but when the changes were analysed within the treated group, the improvement at 30 and 60 days after cell injection vs. baseline was modest and did not achieve statistical significance.55 No quantitative analysis of hESC-CMs was reported. Again, given the 60-day follow-up and the absence of quantitative data on hESC-CMs, no conclusions are possible regarding long-term engraftment.
Studies in non-human primate models of ischaemic cardiomyopathy have provided even less evidence for long-term engraftment and ‘remuscularization’, and stronger evidence for dangerous, potentially lethal, adverse effects of transplanting ESC-derived cells. Chong et al.36 attempted to overcome the issue of poor engraftment by injecting, 14 days after reperfusion, an extraordinary number (1 billion) of hESC-CMs (suspended in a 1.5-mL volume) into the hearts of pigtail macaques with MI induced by a 90-min coronary occlusion. It is unclear how one billion cells were suspended in 1.5 mL. Immunosuppressive therapy consisting of methylprednisolone, cyclosporine, and Abatacept (CTLA4 immunoglobulin) was started 5 days before cell delivery and continued until euthanasia. The entire study consisted of four cell-treated and two-vehicle treated monkeys which, for unclear reasons, were euthanized after different (but short) follow-up periods ranging from 14 to 84 days; thus, the study was predicated on two monkeys euthanized at 14 days (one control and one treated), three at 28 days (two treated and one control), and one at 84 days (treated) after cell injection. At these time-points, pathologic examination of the hearts showed that some of the injected cells were still present, but the number of cells was not quantified; furthermore, although surviving human cells expressed markers of CMs, their small size and irregular shape suggest that they were not fully mature.36 Although the grafted cells were shown to be electrically coupled with host myocytes, all animals exhibited life-threatening arrhythmias.36 Even at these early time-points, the graft area was only ∼2% of the left ventricle.
As pointed out by Anderson et al.,56 this study36 all but failed to show that transplantation of hESC-CMs is a safe and promising strategy for the treatment of patients with HF, for multiple reasons: (i) the study was anecdotal, involving only four-treated animals that were euthanized at different time-points; (ii) because of the short follow-up, the possibility that any latent minor population of pluripotent cells remained in the injected animals and eventually led to teratomas cannot be excluded; (iii) for the same reason, long-term engraftment of hESC-CMs cannot be evaluated; (iv) the occurrence of ventricular arrhythmias indicates that transplantation of hESC-CMs may potentially be life-threatening; (v) no evidence that hESC-CMs exerted beneficial effects on LV function was provided; and (vi) the persistence of some hESC-CMs at 14–84 days could simply reflect the long time necessary for the death of the enormous number of cells transplanted (1 billion) to be complete.
In conclusion, although ESC-based therapies have been touted as a means to achieve cardiac regeneration,37–44 long-term engraftment, let alone regeneration, has not been clearly demonstrated thus far.
Are hESC-derived cells safe?
Safety is a sine qua non for moving forward with translational studies from animals to humans. The preclinical studies of hESC-derived cells reviewed above raise two major safety concerns: formation of teratomas and life-threatening arrhythmias, either of which, if not properly addressed, would preclude clinical use of these cells.
Teratomas
The possibility of teratomas is a risk if any pluripotent cells are injected along with more differentiated cells. This concern is particularly germane when very large numbers of cells (billions) are injected.34,36 Partial differentiation of hESCs to CVPCs or terminal differentiation to CMs could potentially eliminate the pluripotent cells from the pool of injected cells, but obtaining a pure population of billions of partially differentiated cells is difficult. Therefore, even with these products, there may still be a risk of tumours. For safe clinical use, the purity of partially differentiated cells needs to approach 100%. However, the studies of hESC-CVPCs or hESC-CMs in small and large animal models discussed above23–39 failed to demonstrate that the purity of their products was close to 100% (e.g. it was ∼73% in the study by Chong et al.36 and 86–99% in the study by Liu et al.34) even more concerning, none of these studies has developed rigorous protocols to test pluripotent stem cell contamination in the injected products.
The studies discussed above23–39 did not report formation of teratomas after hESC-CVPC or hESC-CM injection, but because of the short follow-up (1–2 months) and the limited number of animals, the possibility that a small population of pluripotent cells contaminating the injected cells may survive long term and eventually form tumours cannot be excluded. For example, if the incidence of teratomas is 1%, hundreds of animals would have to be studied to detect this problem—numbers that are orders of magnitude greater than those reported heretofore.23–39 Development of teratomas in patients with HF would be disastrous. In the current medico-legal environment, the tolerance for this complication is close to zero: even one instance of tumourigenesis may suffice to halt all clinical trials of hESC-derived cells, particularly because alternative (adult) cell types are available that have been amply proven to be safe in thousands of patients. Rigorous animal studies with large sample sizes (at least 100 animals) and long-term follow-up (at least 1 year) are necessary to eliminate the possibility of teratoma formation. No such studies have been reported thus far or, to our knowledge, are planned.
Arrhythmias
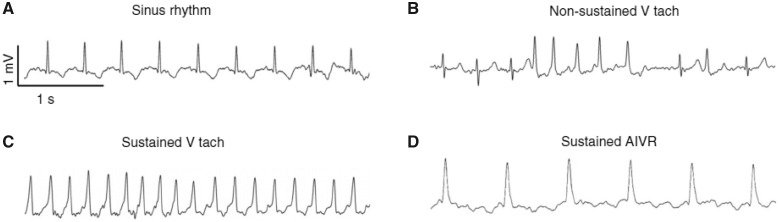
Another safety issue is related to the arrhythmogenic effects of ESC-CMs. In the studies by Chong et al.36 and Liu et al.,34 ESC-CMs transplanted in non-human primates caused life-threatening arrhythmias (ventricular tachycardia) that would preclude the use of these cells in humans (Figure 3). Since arrhythmias of similar severity were not observed in control animals, the most plausible conclusion is that they were induced by the ESC-CM graft.34 The arrhythmogenic effects of ESC-CMs could be accounted for by the absence of terminal differentiation of these cells, as in most of the studies discussed above there was no clear evidence that the transplanted cells resemble an adult phenotype or that they possess the functional properties of adult myocytes, i.e. proper calcium handling. This could be a result of the artificial in vitro conditions of expansion and differentiation of ESCs that may produce an immature and heterogeneous population of CMs.
Figure 3.
Arrhythmias in non-human primates subjected to a 3-h coronary occlusion followed by reperfusion. After intramuscular injection of human embryonic stem cell-derived cardiac myocytes or vehicle, cardiac rhythms were recorded continuously for 24-h periods at 3-day intervals. (A–D) Electrocardiograms from telemetry analysis of four biologically independent control animals and five human embryonic stem cell-cardiomyocyte-treated animals, demonstrating normal sinus rhythm (A), non-sustained ventricular tachycardia (B), sustained ventricular tachycardia (C), and sustained accelerated idioventricular rhythm (D). Reprinted with permission from Liu et al.34
Genomic instability and phenotypic heterogeneity of ESCs
Prolonged culture (including scale-up and differentiation) provides opportunities for genetic abnormalities due to stochastically arising new mutations that unpredictably change the composition of the ESCs in culture.57In vitro, ESCs are heterogenous cell populations composed of different subsets of clones.58–60 Each clone is derived from a single cell that was expanded but because of the accumulation of mutations during the expansion process, each single cell-derived clone is heterogenous.57 Some of the mutations are particularly dangerous, e.g. mutations in the tumour suppressor gene p53 can cause the cells to proliferate faster and become dominant in undifferentiated ESCs. These cells can accumulate further mutations that eventually could lead to cancerous transformation both in vitro and after transplantation in patients.61,62 Furthermore, ESC-CMs have heterogeneous phenotypes and maturity (nodal, atrial, and ventricular phenotypes are observed, with immature electrophysiological properties).63 It is unclear whether this heterogeneity occurs because of suboptimal in vitro differentiation protocols or lack of uniformity of ESCs resulting from accumulating mutations. When discussing the use of ESCs for regenerative purposes, these issues are largely ignored in the literature. Importantly, to date, there are no in vitro tests to evaluate potential mutations in the ESC-derived cell products that are injected in animals or humans.
Evidence that ESCs work via paracrine mechanisms
Despite no evidence of long-term survival of transplanted ESC-CVPCs or ESC-CMs, one common finding in the studies discussed above was the improvement in LV function. This suggests that similar to adult cells, transplanted ESC-CVPCs or ESC-CMs contribute to cardiac repair by producing short-lived paracrine factors such as cytokines or exosomes. Additional studies support this concept. In immunodeficient nude mice, Kervadec et al.50 found that hESC-CVPCs transplanted 2–3 weeks after MI disappeared quickly and were cleared completely after 6 weeks, yet the functional benefits were maintained. Echocardiographic analysis performed 6 weeks after cell injection showed that mice in both cell-treated and vehicle groups had significantly reduced end-systolic and end-diastolic volumes compared to baseline; however, there were no significant intergroup differences. No surviving human-derived cells were detected at the end of the protocol. Transcriptomic analysis showed that 927 genes were up-regulated in the cell-treated group. Pathway analysis indicated that these genes are involved in cell survival, proliferation, DNA repair, as well as fibrosis, suggesting that injected cell do not have to survive long term to have an impact on the gene expression in the infarcted hearts. Importantly, the functional benefits of hESC-derived progenitors were recapitulated by the delivery of EVs derived from these cells.50 The authors concluded that compared with cell grafts, EVs ‘may be safer, more reproducible, more scalable, and more controllable, obviate the need for immunosuppression, and reduce manufacturing, regulatory, and cost issues associated with hESC transplantation’.50
Bellamy et al.29 induced MI in nude rats by permanent coronary ligation. After 5–7 weeks, rats received a cell-free fibrin patch (control group), a fibrin patch populated with 700 000 hESC-CVPCs (cell treated), or no treatment (sham-operated group). The fibrin patches were implanted in the infarcted regions of the LV with obvious dyskinesis. At 4 months after implantation, cell-treated animals exhibited better functional outcomes (LVEF and ESV) vs. sham-operated rats (but not vs. fibrin-implanted rats). Since no hESC-CVPCs were detected at the end of the study (4 months), the results support paracrine mechanisms.
Unclear advantages of ESCs over iPSCs
Similarly to ESCs, iPCS form embryonic bodies that have pluripotent stem cell characteristics including self-renewal and trilineage potential.64,65 Direct transcriptional comparisons have demonstrated that iPSCs are nearly identical to ESCs, with a few exceptions suggesting that perhaps the reprogramming is never 100% efficient. However, the genes that were differentially expressed between ESCs and iPSCs could not be clustered in a logical pathway or group of genes that clearly differentiate the two types of cells.66 Based on these data, it was speculated that perhaps the small discrepancy in gene expression between ESCs and iPSCs could be due to genetic differences of the donors for ESCs and somatic cells for iPSC reprogramming, or to discrepancies in the protocols for propagation of these cells.67–69 This issue was addressed in elegant experiments in which ESCs and iPSCs were isolated from donor mice with identical genetic background: transcriptomic analysis showed only two differentially expressed transcripts—non-coding RNA Gtl2 and small nuclear RNA Rian. They localize to the imprinted Dlk-Dio3 gene cluster on mouse chromosome 12 and are maternally expressed.52 These findings confirm that there are transcriptomic discrepancies between ESCs and iPSCs but they are most likely related to genetic differences in the cell donors, not to their pluripotency.67
To further test whether iPSCs share their stemness with ESCs, controlled in vitro differentiation to CMs was performed. In general, both cells types showed the potential to differentiate into functional CMs, but there are some discrepancies in the published data.70–76 In some cases, iPSCs showed differentiation potential equivalent to that of ESCs,70,76,77 whereas other studies showed that iPSCs have delayed or lower efficiency of differentiation.71–73,75 These discrepancies could be explained by numerous factors. In some studies, there was no validation of iPSC purity; further, the differentiation protocols used in these studies were adopted from ESCs, and perhaps need further optimization. The newly-established protocols are more specific for iPSCs and show high efficiency of cardiomyogenic differentiation.78 Together with the transcriptional profiling data, these studies suggest that ESCs and iPSC are virtually identical in their stemness and commitment to the cardiovascular lineage.
There are still concerns regarding the safety of iPSCs and iPSC-derived CM progenitor cells or CMs for cardiac regeneration related to the genetic manipulations of somatic cells with integrating viruses and to potential contamination with pluripotent cells that can form teratomas. Some of these safety concerns have been partially addressed by the use of non-integrating viruses, viral free constructs, or even recombinant proteins,79–82 all of which make iPSCs safer and more suitable for clinical use. Taken together, the considerations reviewed herein indicate that ESCs have negligible advantages over iPSCs in regenerative medicine.
What is the rationale for using hESC-derived cells?
It is apparent from the above discussion that the use of ESCs and ESC-derived cells is plagued by a host of formidable problems that seemingly preclude their use as a therapy for heart disease (Table 1 and Figure 1). Clearly, given that extensive work has already been done with a variety of adult cell types, it is important that new cells, such as ESC-derived cells, be shown to offer advantages over those tested thus far in order to provide a rationale for clinical trials of these cells. Have ESC-derived cells been shown to offer advantages over adult cells? A concise answer to this question (based on the detailed discussion provided above in this essay) can be formulated as follows (Table 2):
Table 2.
Embryonic stem cell-derived cells vs. adult cells
| Evidence that ESC-derived cells engraft better than adult cells? | No |
| Preclinical evidence that ESC-derived cells are more effective at cardiac repair than adult cells? | No |
| Clinical evidence that ESC-derived cells are more effective at cardiac repair than adult cells? | No |
| ESC-derived cells safer than adult cells? | No |
| ESC-derived cells more cost-effective than adult cells? | No |
-
Is there evidence that ESC-derived cells engraft better than adult cells?
The answer is no. Thus, the promise that ESC-derived cells promote cardiac regeneration (formation of new mature and long-lasting myocytes) remains unfulfilled.
-
Is there preclinical evidence that ESC-derived cells are more effective at cardiac repair than adult cells?
The answer is no.
-
Is there clinical evidence that ESC-derived cells are more effective at cardiac repair than adult cells?
The answer is no. In fact, there are no controlled clinical data on the efficacy of ESC-derived cells, whereas there are abundant clinical data (Phase I and II trials) supporting the efficacy of adult cells such as mesenchymal stromal cells (MSCs).
-
Are ESC-derived cells safer than adult cells?
The answer is a resounding no. As expounded above, ESCs and ESC-derived cells are plagued by genetic instability, rejection, life-threatening arrhythmias, and their proclivity to produce malignancies. On the contrary, adult cells have an excellent record of safety: no adverse effects directly ascribable to adult cells have been reported in clinical trials in thousands of patients with cardiovascular disease.
-
Are ESC-derived cells more cost-effective than adult cells?
The answer is definitely no. Allogeneic adult cells (e.g. bone marrow MSCs) are not more expensive to produce, particularly if one envisions billions of ESC-derived cells being necessary for one patient as the monkey studies34,36 suggest. The number of adult cells necessary for one patient would be at least two orders of magnitude less. Not to mention the expenses involved in differentiating and ensuring optimal purification of ESC-derived cells in order to arrive at a clinical-grade product.
Conclusions
In conclusion, given the (i) moral problems associated with destruction of human embryos, (ii) lack of evidence of long-term engraftment, (iii) lack of preclinical evidence of greater therapeutic effectiveness, (iv) lack of preclinical evidence that clinically relevant doses are effective, (v) lack of any clinical data suggesting effectiveness, (vi) lack of greater cost-effectiveness, and (vii) greater risks (tumours, immunosuppression, arrhythmias, and genomic instability) (Tables 1and2; Figure 1), what is the rationale for using hESC-derived cells instead of iPSCs or adult cells? Why would patients want to receive hESC-derived cells in lieu of adult cells? And would the FDA approve trials of hESC-derived cells? The available evidence indicates that ESCs and ESC-derived cells are unlikely to become a clinical therapy for cardiovascular disease, at least in the near future.
Funding
This work was supported by the National Institutes of Health (P20 GM103492, P01 HL078825, UM1 HL113530, and R01 HL141191).
Conflict of interest: none declared.
References
- 1. Yilmaz A, Benvenisty N.. Defining human pluripotency. Cell Stem Cell 2019;25:9–22. [DOI] [PubMed] [Google Scholar]
- 2. De Los Angeles A, Ferrari F, Xi R, Fujiwara Y, Benvenisty N, Deng H, Hochedlinger K, Jaenisch R, Lee S, Leitch HG, Lensch MW, Lujan E, Pei D, Rossant J, Wernig M, Park PJ, Daley GQ.. Hallmarks of pluripotency. Nature 2015;525:469–478. [DOI] [PubMed] [Google Scholar]
- 3.Blau HM, Daley GQ. Stem cells in the treatment of disease. N Engl J Med 2019;380:1748–1760. [DOI] [PubMed] [Google Scholar]
- 4. Trounson A, DeWitt ND.. Pluripotent stem cells progressing to the clinic. Nat Rev Mol Cell Biol 2016;17:194–200. [DOI] [PubMed] [Google Scholar]
- 5. Wysoczynski M, Khan A, Bolli R.. New paradigms in cell therapy: repeated dosing, intravenous delivery, immunomodulatory actions, and new cell types. Circ Res 2018;123:138–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banerjee MN, Bolli R, Hare JM.. Clinical studies of cell therapy in cardiovascular medicine: recent developments and future directions. Circ Res 2018;123:266–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Welt FG, Losordo DW.. Cell therapy for acute myocardial infarction: curb your enthusiasm? Circulation 2006;113:1272–1274. [DOI] [PubMed] [Google Scholar]
- 8. Honold J, Assmus B, Lehman R, Zeiher AM, Dimmeler S.. Stem cell therapy of cardiac disease: an update. Nephrol Dial Transplant 2004;19:1673–1677. [DOI] [PubMed] [Google Scholar]
- 9. Ott HC, Davis BH, Taylor DA.. Cell therapy for heart failure–muscle, bone marrow, blood, and cardiac-derived stem cells. Semin Thorac Cardiovasc Surg 2005;17:348–360. [DOI] [PubMed] [Google Scholar]
- 10. Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ.. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 2004;428:664–668. [DOI] [PubMed] [Google Scholar]
- 11. Guo Y, Wysoczynski M, Nong Y, Tomlin A, Zhu X, Gumpert AM, Nasr M, Muthusamy S, Li H, Book M, Khan A, Hong KU, Li Q, Bolli R.. Repeated doses of cardiac mesenchymal cells are therapeutically superior to a single dose in mice with old myocardial infarction. Basic Res Cardiol 2017;112:18.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hong KU, Guo Y, Li QH, Cao P, Al-Maqtari T, Vajravelu BN, Du J, Book MJ, Zhu X, Nong Y, Bhatnagar A, Bolli R.. c-kit+ Cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS One 2014;9:e96725.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tokita Y, Tang XL, Li Q, Wysoczynski M, Hong KU, Nakamura S, Wu WJ, Xie W, Li D, Hunt G, Ou Q, Stowers H, Bolli R.. Repeated administrations of cardiac progenitor cells are markedly more effective than a single administration: a new paradigm in cell therapy. Circ Res 2016;119:635–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wysoczynski M, Dassanayaka S, Zafir A, Ghafghazi S, Long BW, Noble C, DeMartino AM, Brittian KR, Bolli R, Jones SP.. A new method to stabilize C-kit expression in reparative cardiac mesenchymal cells. Front Cell Dev Biol 2016;4:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharp TE 3rd, Schena GJ, Hobby AR, Starosta T, Berretta RM, Wallner M, Borghetti G, Gross P, Yu D, Johnson J, Feldsott E, Trappanese DM, Toib A, Rabinowitz JE, George JC, Kubo H, Mohsin S, Houser SR.. Cortical bone stem cell therapy preserves cardiac structure and function after myocardial infarction. Circ Res 2017;121:1263–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wysoczynski M, Guo Y, Moore JBt, Muthusamy S, Li Q, Nasr M, Li H, Nong Y, Wu W, Tomlin AA, Zhu X, Hunt G, Gumpert AM, Book MJ, Khan A, Tang XL, Bolli R.. Myocardial reparative properties of cardiac mesenchymal cells isolated on the basis of adherence. J Am Coll Cardiol 2017;69:1824–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM.. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation 2013;127:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanganalmath SK, Bolli R.. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res 2013;113:810–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keith MC, Bolli R.. “String theory” of c-kit(pos) cardiac cells: a new paradigm regarding the nature of these cells that may reconcile apparently discrepant results. Circ Res 2015;116:1216–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bolli R, Ghafghazi S.. Cell therapy needs rigorous translational studies in large animal models. J Am Coll Cardiol 2015;66:2000–2004. [DOI] [PubMed] [Google Scholar]
- 21. Bolli R, Ghafghazi S.. Stem cells: cell therapy for cardiac repair: what is needed to move forward? Nat Rev Cardiol 2017;14:257–258. [DOI] [PubMed] [Google Scholar]
- 22. Bai F, Ho Lim C, Jia J, Santostefano K, Simmons C, Kasahara H, Wu W, Terada N, Jin S.. Directed differentiation of embryonic stem cells into cardiomyocytes by bacterial injection of defined transcription factors. Sci Rep 2015;5:15014.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boheler KR, Czyz J, Tweedie D, Yang HT, Anisimov SV, Wobus AM.. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res 2002;91:189–201. [DOI] [PubMed] [Google Scholar]
- 24. Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L.. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest 2001;108:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, Huber I, Satin J, Itskovitz-Eldor J, Gepstein L.. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol 2004;22:1282–1289. [DOI] [PubMed] [Google Scholar]
- 26. Mummery CL, Ward D, Passier R.. Differentiation of human embryonic stem cells to cardiomyocytes by coculture with endoderm in serum-free medium. Curr Protoc Stem Cell Biol 2007;Chapter 1:Unit 1F 2. [DOI] [PubMed] [Google Scholar]
- 27. Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ.. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res 2012;111:344–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu C, Police S, Rao N, Carpenter MK.. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res 2002;91:501–508. [DOI] [PubMed] [Google Scholar]
- 29. Bellamy V, Vanneaux V, Bel A, Nemetalla H, Emmanuelle Boitard S, Farouz Y, Joanne P, Perier M-C, Robidel E, Mandet C, Hagège A, Bruneval P, Larghero J, Agbulut O, Menasché P.. Long-term functional benefits of human embryonic stem cell-derived cardiac progenitors embedded into a fibrin scaffold. J Heart Lung Transplant 2015;34:1198–1207. [DOI] [PubMed] [Google Scholar]
- 30. Blin G, Nury D, Stefanovic S, Neri T, Guillevic O, Brinon B, Bellamy V, Rücker-Martin C, Barbry P, Bel A, Bruneval P, Cowan C, Pouly J, Mitalipov S, Gouadon E, Binder P, Hagège A, Desnos M, Renaud J-F, Menasché P, Pucéat M.. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest 2010;120:1125–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tachibana A, Santoso MR, Mahmoudi M, Shukla P, Wang L, Bennett M, Goldstone AB, Wang M, Fukushi M, Ebert AD, Woo YJ, Rulifson E, Yang PC.. Paracrine effects of the pluripotent stem cell-derived cardiac myocytes salvage the injured myocardium. Circ Res 2017;121:e22–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, den Ouden K, Ward-van Oostwaard D, Korving J, Tertoolen LG, van Echteld CJ, Doevendans PA, Mummery CL.. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res 2007;1:9–24. [DOI] [PubMed] [Google Scholar]
- 33. Fernandes S, Naumova AV, Zhu WZ, Laflamme MA, Gold J, Murry CE.. Human embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats. J Mol Cell Cardiol 2010;49:941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu YW, Chen B, Yang X, Fugate JA, Kalucki FA, Futakuchi-Tsuchida A, Couture L, Vogel KW, Astley CA, Baldessari A, Ogle J, Don CW, Steinberg ZL, Seslar SP, Tuck SA, Tsuchida H, Naumova AV, Dupras SK, Lyu MS, Lee J, Hailey DW, Reinecke H, Pabon L, Fryer BH, MacLellan WR, Thies RS, Murry CE.. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol 2018;36:597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, Murry CE, Laflamme MA.. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 2012;489:322–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE.. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014;510:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM.. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145–1147. [DOI] [PubMed] [Google Scholar]
- 38. Gearhart J. New human embryonic stem-cell lines—more is better. N Engl J Med 2004;350:1275–1276. [DOI] [PubMed] [Google Scholar]
- 39. Daley GQ. Missed opportunities in embryonic stem-cell research. N Engl J Med 2004;351:627–628. [DOI] [PubMed] [Google Scholar]
- 40. Okie S. Stem-cell politics. N Engl J Med 2006;355:1633–1637. [DOI] [PubMed] [Google Scholar]
- 41. Schwartz RS. The politics and promise of stem-cell research. N Engl J Med 2006;355:1189–1191. [DOI] [PubMed] [Google Scholar]
- 42. Cohen IG, Adashi EY.. Human embryonic stem-cell research under siege–battle won but not the war. N Engl J Med 2011;364:e48.. [DOI] [PubMed] [Google Scholar]
- 43. Chong JJ, Murry CE.. Cardiac regeneration using pluripotent stem cells–progression to large animal models. Stem Cell Res 2014;13:654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miller LW. Trial of embryonic stem cell-derived cardiac progenitor cells: an encouraging start. J Am Coll Cardiol 2018;71:439–442. [DOI] [PubMed] [Google Scholar]
- 45. Bolli R, Wysoczynski M.. Human embryonic stem cell-derived cardiomyocytes. Circ Res 2019;124:1157–1159. [DOI] [PubMed] [Google Scholar]
- 46. Templin C, Zweigerdt R, Schwanke K, Olmer R, Ghadri JR, Emmert MY, Muller E, Kuest SM, Cohrs S, Schibli R, Kronen P, Hilbe M, Reinisch A, Strunk D, Haverich A, Hoerstrup S, Luscher TF, Kaufmann PA, Landmesser U, Martin U.. Transplantation and tracking of human-induced pluripotent stem cells in a pig model of myocardial infarction: assessment of cell survival, engraftment, and distribution by hybrid single photon emission computed tomography/computed tomography of sodium iodide symporter transgene expression. Circulation 2012;126:430–439. [DOI] [PubMed] [Google Scholar]
- 47. Zhu K, Wu Q, Ni C, Zhang P, Zhong Z, Wu Y, Wang Y, Xu Y, Kong M, Cheng H, Tao Z, Yang Q, Liang H, Jiang Y, Li Q, Zhao J, Huang J, Zhang F, Chen Q, Li Y, Chen J, Zhu W, Yu H, Zhang J, Yang HT, Hu X, Wang J.. Lack of remuscularization following transplantation of human embryonic stem cell-derived cardiovascular progenitor cells in infarcted nonhuman primates. Circ Res 2018;122:958–969. [DOI] [PubMed] [Google Scholar]
- 48. Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Cacciapuoti I, Parouchev A, Benhamouda N, Tachdjian G, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Guillemain R, Suberbielle Boissel C, Tartour E, Desnos M, Larghero J.. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J 2015;36:2011–2017. [DOI] [PubMed] [Google Scholar]
- 49. Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Parouchev A, Cacciapuoti I, Al-Daccak R, Benhamouda N, Blons H, Agbulut O, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Charron D, Tartour E, Tachdjian G, Desnos M, Larghero J.. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J Am Coll Cardiol 2018;71:429–438. [DOI] [PubMed] [Google Scholar]
- 50. Kervadec A, Bellamy V, El Harane N, Arakelian L, Vanneaux V, Cacciapuoti I, Nemetalla H, Perier MC, Toeg HD, Richart A, Lemitre M, Yin M, Loyer X, Larghero J, Hagege A, Ruel M, Boulanger CM, Silvestre JS, Menasche P, Renault NK.. Cardiovascular progenitor-derived extracellular vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic heart failure. J Heart Lung Transplant 2016;35:795–807. [DOI] [PubMed] [Google Scholar]
- 51. van Laake LW, Passier R, den Ouden K, Schreurs C, Monshouwer-Kloots J, Ward-van Oostwaard D, van Echteld CJ, Doevendans PA, Mummery CL.. Improvement of mouse cardiac function by hESC-derived cardiomyocytes correlates with vascularity but not graft size. Stem Cell Res 2009;3:106–112. [DOI] [PubMed] [Google Scholar]
- 52. van Laake LW, Passier R, Doevendans PA, Mummery CL.. Human embryonic stem cell-derived cardiomyocytes and cardiac repair in rodents. Circ Res 2008;102:1008–1010. [DOI] [PubMed] [Google Scholar]
- 53. van Laake LW, van Donselaar EG, Monshouwer-Kloots J, Schreurs C, Passier R, Humbel BM, Doevendans PA, Sonnenberg A, Verkleij AJ, Mummery CL.. Extracellular matrix formation after transplantation of human embryonic stem cell-derived cardiomyocytes. Cell Mol Life Sci 2010;67:277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE.. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 2007;25:1015–1024. [DOI] [PubMed] [Google Scholar]
- 55. Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L.. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol 2007;50:1884–1893. [DOI] [PubMed] [Google Scholar]
- 56. Anderson ME, Goldhaber J, Houser SR, Puceat M, Sussman MA.. Embryonic stem cell-derived cardiac myocytes are not ready for human trials. Circ Res 2014;115:335–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sverdlov ED, Mineev K.. Mutation rate in stem cells: an underestimated barrier on the way to therapy. Trends Mol Med 2013;19:273–280. [DOI] [PubMed] [Google Scholar]
- 58. Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA.. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005;122:947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Torres-Padilla ME, Chambers I.. Transcription factor heterogeneity in pluripotent stem cells: a stochastic advantage. Development 2014;141:2173–2181. [DOI] [PubMed] [Google Scholar]
- 60. Young RA. Control of the embryonic stem cell state. Cell 2011;144:940–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Olivos DJ, Mayo LD.. Emerging non-canonical functions and regulation by p53: p53 and stemness. Int J Mol Sci 2016;17:E1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Koifman G, Shetzer Y, Eizenberger S, Solomon H, Rotkopf R, Molchadsky A, Lonetto G, Goldfinger N, Rotter V.. A mutant p53-dependent embryonic stem cell gene signature is associated with augmented tumorigenesis of stem cells. Cancer Res 2018;78:5833–5847. [DOI] [PubMed] [Google Scholar]
- 63. Singh AM, Terada N.. Bypassing heterogeneity: the road to embryonic stem cell-derived cardiomyocyte specification. Trends Cardiovasc Med 2007;17:96–101. [DOI] [PubMed] [Google Scholar]
- 64. Takahashi K, Yamanaka S.. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- 65. Yamanaka S. Elite and stochastic models for induced pluripotent stem cell generation. Nature 2009;460:49–52. [DOI] [PubMed] [Google Scholar]
- 66. Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, Ambartsumyan G, Aimiuwu O, Richter L, Zhang J, Khvorostov I, Ott V, Grunstein M, Lavon N, Benvenisty N, Croce CM, Clark AT, Baxter T, Pyle AD, Teitell MA, Pelegrini M, Plath K, Lowry WE.. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 2009;5:111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, Natesan S, Kono T, Shioda T, Hochedlinger K.. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature 2010;465:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Guenther MG, Frampton GM, Soldner F, Hockemeyer D, Mitalipova M, Jaenisch R, Young RA.. Chromatin structure and gene expression programs of human embryonic and induced pluripotent stem cells. Cell Stem Cell 2010;7:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Newman AM, Cooper JB.. Lab-specific gene expression signatures in pluripotent stem cells. Cell Stem Cell 2010;7:258–262. [DOI] [PubMed] [Google Scholar]
- 70. Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, Hasenfuss G, Martin U.. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation 2008;118:507–517. [DOI] [PubMed] [Google Scholar]
- 71. Kuzmenkin A, Liang H, Xu G, Pfannkuche K, Eichhorn H, Fatima A, Luo H, Saric T, Wernig M, Jaenisch R, Hescheler J.. Functional characterization of cardiomyocytes derived from murine induced pluripotent stem cells in vitro. FASEB J 2009;23:4168–4180. [DOI] [PubMed] [Google Scholar]
- 72. Kaichi S, Hasegawa K, Takaya T, Yokoo N, Mima T, Kawamura T, Morimoto T, Ono K, Baba S, Doi H, Yamanaka S, Nakahata T, Heike T.. Cell line-dependent differentiation of induced pluripotent stem cells into cardiomyocytes in mice. Cardiovasc Res 2010;88:314–323. [DOI] [PubMed] [Google Scholar]
- 73. Martinez-Fernandez A, Nelson TJ, Ikeda Y, Terzic A.. c-MYC independent nuclear reprogramming favors cardiogenic potential of induced pluripotent stem cells. J Cardiovasc Transl Res 2010;3:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, Conway S, Orkin SH, Yoder MC, Mikkola HK.. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell 2008;2:252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ.. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res 2009;104:e30–e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gupta MK, Illich DJ, Gaarz A, Matzkies M, Nguemo F, Pfannkuche K, Liang H, Classen S, Reppel M, Schultze JL, Hescheler J, Saric T.. Global transcriptional profiles of beating clusters derived from human induced pluripotent stem cells and embryonic stem cells are highly similar. BMC Dev Biol 2010;10:98.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schenke-Layland K, Rhodes KE, Angelis E, Butylkova Y, Heydarkhan-Hagvall S, Gekas C, Zhang R, Goldhaber JI, Mikkola HK, Plath K, MacLellan WR.. Reprogrammed mouse fibroblasts differentiate into cells of the cardiovascular and hematopoietic lineages. Stem Cells 2008;26:1537–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Burridge PW, Zambidis ET.. Highly efficient directed differentiation of human induced pluripotent stem cells into cardiomyocytes. Methods Mol Biol 2013;997:149–161. [DOI] [PubMed] [Google Scholar]
- 79. Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K.. Induced pluripotent stem cells generated without viral integration. Science 2008;322:945–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA.. Human induced pluripotent stem cells free of vector and transgene sequences. Science 2009;324:797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S.. Generation of mouse induced pluripotent stem cells without viral vectors. Science 2008;322:949–953. [DOI] [PubMed] [Google Scholar]
- 82. Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, Siuzdak G, Scholer HR, Duan L, Ding S.. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 2009;4:381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]