Abstract
Background
Breast cancer (BRCA) is the leading cause of cancer‐related death in women worldwide. Pre‐ and postoperative radiotherapy play a pivotal role in BRCA treatment but its efficacy remains limited and plagued by the emergence of radiation resistance, which aggravates patient prognosis. The long noncoding RNA (lncRNA)‐implicated mechanisms underlying radiation resistance are rarely reported. The aim of this study was to determine whether lncRNA HOX transcript antisense RNA (HOTAIR) modulated the radiosensitivity of breast cancer through HSPA1A.
Methods
A Gammacell 40 Exactor was used for irradiation treatment. Bioinformatic tools and luciferase reporter assay were adopted to explore gene expression profile and demonstrate the interactions between lncRNA, miRNA and target mRNA 3′‐untranslated region (3′‐UTR). The expression levels of certain genes were determined by real‐time PCR and western‐blot analyses. in vitro and in vivo functional assays were conducted by cell viability and tumorigenicity assays.
Results
The levels of oncogenic lncRNA HOTAIR were positively correlated with the malignancy of BRCA but reversely correlated with the radiosensitivity of breast cancer cells. Moreover, the expression levels of HOTAIR were positively associated with those of heat shock protein family A (Hsp70) member 1A (HSPA1A) in clinical BRCA tissues and HOTAIR upregulated HSPA1A at the mRNA and protein levels in irradiated BRCA cells. Mechanistically, miR‐449b‐5p restrained HSPA1A expression through targeting the 3′‐UTR of HSPA1A mRNA, whereas HOTAIR acted as a competing sponge to sequester miR‐449b‐5p and thereby relieved the miR‐449b‐5p‐mediated HSPA1A repression. Functionally, HOTAIR conferred decreased radiosensitivity on BRCA cells, while miR‐449b‐5p overexpression or HSPA1A knockdown abrogated the HOTAIR‐enhanced BRCA growth under the irradiation exposure both in vitro and in vivo.
Conclusions
LncRNA HOTAIR facilitates the expression of HSPA1A by sequestering miR‐449b‐5p post‐transcriptionally and thereby endows BRCA with radiation resistance.
Key points
Therapeutically, HOTAIR and HSPA1A may be employed as potential targets for BRCA radiotherapy. Our findings shed new light into the mechanism by which lncRNAs modulate the radiosensitivity of tumors.
Keywords: Breast cancer, HOTAIR, HSPA1A, miR‐449b‐5p, radioresistance
Introduction
Breast cancer (BRCA) is the most frequent type of malignancy in women,1, 2 with a global burden of more than two million new cases (11.6% of all new cancer cases) and over 626 thousand deaths (6.6% of all cancer deaths) in 2018.3, 4 With regard to the molecular typing of BRCA, it is categorized into four intrinsic subtypes based on the presence or absence of molecular markers for estrogen or progesterone receptors (ER or PR) and human epidermal growth factor 2 (ERBB2; formerly HER2): luminal A (ER+, PR+/−, HER2–), luminal B (ER+, PR+/−, HER2+), HER2‐enriched (ER–, PR–, HER2+), and basal‐like plus normal‐like subtype (including triple‐negative breast cancer: ER–, PR–, HER2–).5, 6 To eradicate tumor from the breast (nonmetastatic breast cancer) or prolong life and symptom palliation (metastatic breast cancer), local therapy and systemic therapy are typical therapeutic approaches for breast cancer treatment.6 As a kind of local therapy, pre‐/postoperative radiotherapy plays a pivotal role in BRCA treatment but its efficacy remains limited and plagued by the emergence of radiation resistance, which aggravates prognosis.7 A wide spectrum of obstacles, such as cancer stem cells, tumor heterogeneity, angiogenesis and expression alterations of various tumor‐promoting/suppressing proteins and non‐coding RNAs (ncRNAs) contribute to the resistant phenotypes.8
A surprising finding about the ENCODE project is that ncRNAs corresponds to >97% of the human genome.9 At present, ncRNAs are divided into two groups according to their sizes: small ncRNAs (<200 nt) and long ncRNAs (lncRNAs) (>200 nt).10, 11 It is acknowledged that many lncRNAs have been defined as oncogenes and tumor suppressors in a wide variety of solid tumors and hematological malignancies.12 The lncRNA HOX transcript antisense RNA (HOTAIR) which was first found to be highly expressed in breast cancer (BRCA) is a prime example of an oncogenic trans‐acting lncRNA. HOTAIR reprograms chromatin state to promote invasion and metastasis via recruitment of histone modification complexes polycomb repressive complex 2 (PRC2) and lysine specific demethylase 1 (LSD1) to its target genes in breast cancer.13, 14, 15 It has been reported that knockdown of HOTAIR inhibited breast cancer cell proliferation, increased apoptosis and inhibited cell cycle progression in vitro.16 However, the mechanisms underlying the radioresistant function mediated by HOTAIR in breast cancer are far from clear.
MicroRNAs (miRNAs) are a class of small ncRNAs containing approximately 19–25 nucleotides. MiRNAs post‐transcriptionally modulate gene expression through binding to the 3′‐untranslated region (3′‐UTR) of target mRNAs.17 Numerous reports show that miR‐449b‐5p plays a crucial role in multiple diseases such as cancer, reproductive system diseases, viral infection, Parkinson’s disease, diabetes and development.18, 19, 20, 21, 22, 23, 24 It acts as a significant tumor suppressive gene in various cancer types.25 However, the role of miR‐449b‐5p in HOTAIR‐mediated radiation resistance of breast cancer remains unclear.
Heat shock protein family A (Hsp70) member 1A (HSPA1A), the major stress‐inducible member of the 70 kDa stress protein family, is found in nearly all subcellular compartments of nucleated cells where they fulfil chaperoning functions.26, 27, 28 It facilitates protein folding, translocation, and the assembly of intracellular protein, which may protect against various ambient challenges such as an increase in temperature as well as other environmental stresses.29 Another remarkable signature is that HSPA1A is overexpressed in a large variety of tumor types.30, 31 Hsp70 and Hsp90 mediate the conformation regulation of p53 DNA binding domain and p53 cancer variants.32 HSPA1A can serve as a theranostic target for cancer therapy.28, 33 Nevertheless, whether lncRNA HOTAIR is implicated in the elevation of HSPA1A in radioresistance of breast cancer is poorly understood.
The aim of the present study was to determine whether lncRNA HOTAIR modulated the radiosensitivity of breast cancer through HSPA1A. Expectedly, our data indicated that HOTAIR post‐transcriptionally increases HSPA1A expression by sequestering miR‐449b‐5p and thereby confers the radiation resistance on breast cancer. Our findings provide new insights into the mechanism by which lncRNAs modulate tumor radiosensitivity.
Methods
Patient specimens
In order to test the expression levels of HOTAIR, miR‐449b‐5p as well as HSPA1A mRNA, a total of 20 paired breast carcinoma and their adjacent normal breast specimens were collected from patients receiving excision of breast tumors in Tianjin Tumor Hospital (Tianjin, China). The basic patient information derived from the medical records is provided in Table S1. The informed consent for the study purposes was obtained from each patient, and the study was approved by the Institute Research Ethics Committee at Institute of Radiation Medicine, Chinese Academy of Medical Sciences and Peking Union Medical College (IRM‐PUMC).
Cell lines and cell culture
Human breast cancer cell lines, MCF‐7, T47D, LM‐MCF‐7,34 BT‐474, SKBR‐3 and MDA‐MB‐231 were maintained in RPMI medium 1640 (Gibco, USA). Media were supplemented with 10% fetal bovine serum (FBS, Gibco, USA), 100 U/mL penicillin and 100 mg/mL streptomycin in 5% CO2 at 37°C.
Irradiation
An Exposure Instrument Gammacell‐40 137Cs‐irradiator (Atomic Energy of Canadian Inc., Mississauga, Canada) at a dose rate of 0.88 Gy/minute was used for all experiments.
Statistical analysis
Each experiment was repeated at least three times. Statistical significance was assessed by comparing mean values (± S.D.) using a Student's t‐test for independent groups and was assumed for *, P < 0.05; **, P < 0.01; ***, P < 0.001, and no significance (NS). The expression levels of HOTAIR, miR‐449b‐5p and HSPA1A in BRCA tumor tissues and their adjacent normal breast tissues were compared through the Wilcoxon's signed‐rank test. Associations between expression levels of the two of HOTAIR, miR‐449b‐5p and HSPA1A in BRCA tumor tissues were assayed by Pearson's correlation assay.
Results
HOTAIR endows breast cancer with radiation resistance
Using the Gene Expression Profiling Interactive Analysis (GEPIA) (http://gepia.cancer-pku.cn/) database, we determined that oncogenic long non‐coding RNA HOTAIR, representing high expression level in the vast majority of cancers, was most highly expressed in BRCA among all cancer types and its expression level in tumor tissues was much higher than that in normal tissues (Fig 1a,b), but it also prompted poor prognosis of BRCA patients (Fig 1c). Moreover, qRT‐PCR analysis confirmed that expression levels of HOTAIR in 20 cases of breast tumor tissues were much higher than those in the adjacent normal tissues (P < 0.001, Wilcoxon's signed‐rank test, Fig S1a). These findings implied that HOTAIR might be a key initiator of the malignant phenotype of BRCA. Accordingly, we supposed that HOTAIR might modulate the radiosensitivity of BRCA. Taking the molecular subtypes into account, we first examined the endogenous HOTAIR expression in BRCA cell lines, including MCF‐7, T47D, LM‐MCF‐7 (luminal A), BT‐474 (luminal B), SKBR‐3 (HER2‐enriched) and MDA‐MB‐231 (triple‐negative breast cancer), by qRT‐PCR. The data showed that T47D harbored the highest levels of HOTAIR while SKBR‐3 harbored the lowest of that (Fig 1d). Next, we evaluated the impact of HOTAIR on the radiosensitivity of the two cell lines using CCK‐8 assay. The data revealed that cell viability of T47D displayed no significant difference with or without a 10 Gy γ‐irradiation exposure, whereas that of SKBR‐3 dropped markedly after the same irradiation treatment (Fig 1e,f). Colony formation assay showed the same results (Figs 1g and S1b). CCK‐8 assay also indicated that the radiosensitivities of BRCA cells belonging to different subtypes were SKBR‐3 > BT‐474 > MCF‐7 > MDA‐MA‐231 under the time‐ and dose‐dependent irradiation treatment condition, which was negatively associated with the internal HOTAIR levels (Fig S1c,d). We further explored the relationship between HOTAIR and the radiosensitivity of BRCA cells in MDA‐MB‐231 and MCF‐7 which harbor the moderate levels of HOTAIR. Not surprisingly, CCK‐8 assay showed that HOTAIR overexpression significantly enhanced the proliferation capacity of the two cells in a time‐dependent manner after the exposure to 10 Gy γ‐irradiation (Fig 1h,i). On the contrary, silencing HOTAIR in MDA‐MB‐231 and MCF‐7 cells led to the decrease of cell proliferation ability time‐dependent under the same conditions (Fig 1j,k), indicating that HOTAIR expression positively relates to the radioresistance of BRCA cells. Furthermore, CCK‐8 assay under dose‐dependent irradiation condition and colony formation assay under a 6 Gy irradiation treatment showed similar outcomes (Figs 1l–n and S1e–g). Collectively, our findings demonstrated that oncogenic lncRNA HOTAIR favors the radioresistance of BRCA cells.
Figure 1.
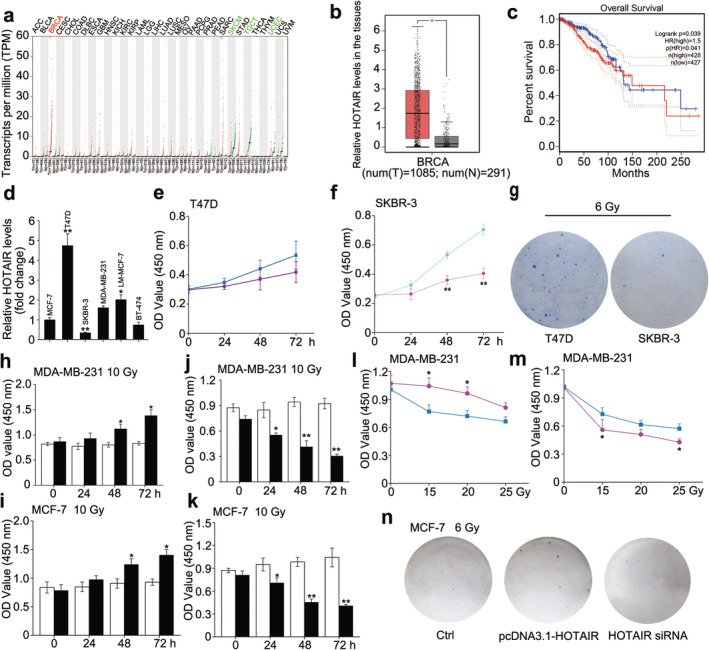
HOTAIR endows breast cancer with radiation resistance. (a–c) The expression profile of HOTAIR in different cancer types and the corresponding normal tissues (a), the relative HOTAIR levels in breast cancer tissues and normal breast tissues ( ) Tumour, (
) Tumour, ( ) Normal (b) and the relationship between HOTAIR levels and overall survival of BRCA patients (
) Normal (b) and the relationship between HOTAIR levels and overall survival of BRCA patients ( ) Tumour, (
) Tumour, ( ) Normal (c) were obtained from the GEPIA database. (
) Normal (c) were obtained from the GEPIA database. ( ) Low HOTAIR TPM, (
) Low HOTAIR TPM, ( ) High HOTAIR TPM (d) The relative abundance of HOTAIR in different BRCA cells was determined by real‐time PCR. (e and f) CCK‐8 assay was used to measure the cell viability of T47D (e) (
) High HOTAIR TPM (d) The relative abundance of HOTAIR in different BRCA cells was determined by real‐time PCR. (e and f) CCK‐8 assay was used to measure the cell viability of T47D (e) ( ) 0 Gy, (
) 0 Gy, ( ) 10 Gy and SKBR‐3 (f) after 0 or 10 Gy irradiation treatment. (
) 10 Gy and SKBR‐3 (f) after 0 or 10 Gy irradiation treatment. ( ) 0 Gy, (
) 0 Gy, ( ) 10 Gy (g) Colony formation assay was used to measure the proliferative ability of T47D and SKBR‐3 after 6 Gy irradiation treatment. (h and i) MDA‐MB‐231 and MCF‐7 cells were transfected with pcDNA3.1/pcDNA3.1‐HOTAIR 12 hours before the irradiation treatment, CCK‐8 assay was used to measure the cell viability after 10 Gy irradiation treatment. (
) 10 Gy (g) Colony formation assay was used to measure the proliferative ability of T47D and SKBR‐3 after 6 Gy irradiation treatment. (h and i) MDA‐MB‐231 and MCF‐7 cells were transfected with pcDNA3.1/pcDNA3.1‐HOTAIR 12 hours before the irradiation treatment, CCK‐8 assay was used to measure the cell viability after 10 Gy irradiation treatment. ( ) pcDNA3.1, (
) pcDNA3.1, ( ) pcDNA3.1‐HOTAIR (j and k) MDA‐MB‐231 and MCF‐7 cells were transfected with siRNA Ctrl/si‐HOTAIR 12 hours before the irradiation treatment, CCK‐8 assay was used to measure the cell viability after 10 Gy irradiation treatment. ; (
) pcDNA3.1‐HOTAIR (j and k) MDA‐MB‐231 and MCF‐7 cells were transfected with siRNA Ctrl/si‐HOTAIR 12 hours before the irradiation treatment, CCK‐8 assay was used to measure the cell viability after 10 Gy irradiation treatment. ; ( ) siRNA Ctrl, (
) siRNA Ctrl, ( ) HOTAIR siRNA; (l and m) MDA‐MB‐231 cells were transfected with pcDNA3.1/pcDNA3.1‐HOTAIR (l) or siRNA Ctrl/si‐HOTAIR (
) HOTAIR siRNA; (l and m) MDA‐MB‐231 cells were transfected with pcDNA3.1/pcDNA3.1‐HOTAIR (l) or siRNA Ctrl/si‐HOTAIR ( ), pcDNA3.1, (
), pcDNA3.1, ( ) pcDNA3.1‐HOTAIR (m) 12 hours before the irradiation treatment, CCK‐8 assay was used to measure the cell viability after 0, 15, 20 and 25 Gy irradiation treatment. (
) pcDNA3.1‐HOTAIR (m) 12 hours before the irradiation treatment, CCK‐8 assay was used to measure the cell viability after 0, 15, 20 and 25 Gy irradiation treatment. ( ) siRNA Ctrl, (
) siRNA Ctrl, (
 ) HOTAIR siRNA (n) MCF‐7 cells were transfected with pcDNA3.1 + siRNA Ctrl/pcDNA3.1‐HOTAIR/si‐HOTAIR 12 hours before the irradiation treatment, then colony formation assay was used to measure the proliferative ability after 6 Gy irradiation treatment. Data are shown as mean ± SD of three independent experiments. Statistical significant differences are indicated: *, P < 0.05; **, P < 0.01; Student's t‐test.
) HOTAIR siRNA (n) MCF‐7 cells were transfected with pcDNA3.1 + siRNA Ctrl/pcDNA3.1‐HOTAIR/si‐HOTAIR 12 hours before the irradiation treatment, then colony formation assay was used to measure the proliferative ability after 6 Gy irradiation treatment. Data are shown as mean ± SD of three independent experiments. Statistical significant differences are indicated: *, P < 0.05; **, P < 0.01; Student's t‐test.
HOTAIR upregulates the stress‐inducible oncogene HSPA1A in BRCA cells
Considering that radiation exposure is a typical stress‐inducing event to organisms,35 we supposed that the HOTAIR‐mediated radiation resistant effect might work through the stress response signaling pathways in cells. There is growing evidence that heat shock protein family plays crucial roles in countering with environmental challenges.29 Given that HSPA1A is the major stress‐inducible member of the 70 kDa stress protein family and it is overexpressed in a large variety of tumor types,30, 31 we searched the GEPIA and Kaplan‐Meier Plotter database (http://gepia.cancer-pku.cn/; http://kmplot.com/analysis/index.php?p = service&cancer = breast&tdsourcetag = s_pcqq_aiomsg) for the expression profile of HSPA1A. Intriguingly, HSPA1A was highly expressed in BRCA and its expression level in tumor tissues was much higher than that in normal tissues (Fig 2a,b). In parallel, BRCA patients carrying high level of HSPA1A also experienced poor prognosis (Fig 2c). Therefore, we investigated the expression of HSPA1A in clinical BRCA specimens. As expected, qRT‐PCR analysis displayed that the mRNA levels of HSPA1A were markedly increased in 20 cases of BRCA tissues relative to their corresponding normal tissues (P < 0.001, Wilcoxon's signed‐rank test, Fig 2d) and the levels of HOTAIR were positively correlated with those of HSPA1A in clinical BRCA tissues (R = 0.5391, P < 0.05, Pearson's correlation test, Fig 2e), suggesting that HOTAIR may upregulate HSPA1A in BRCA cells. Accordingly, we evaluated the relationship between HOTAIR and HSPA1A in BRCA cells. Interestingly, overexpression of HOTAIR led to upregulation of HSPA1A mRNA in MDA‐MB‐231 and MCF‐7 cells, particularly, the protein levels of HSPA1A elevated in a dose‐dependent manner following 10 Gy irradiation treatment (Fig 2f–h). Inversely, inhibition of HOTAIR by siRNA caused the reduction at the mRNA level and a dose‐dependent decrease at the protein level of HSPA1A in MDA‐MB‐231 and MCF‐7 cells (Fig 2i–k). Thus, we concluded that HOTAIR upregulates the stress‐induced oncogene HSPA1A in irradiated BRCA cells.
Figure 2.
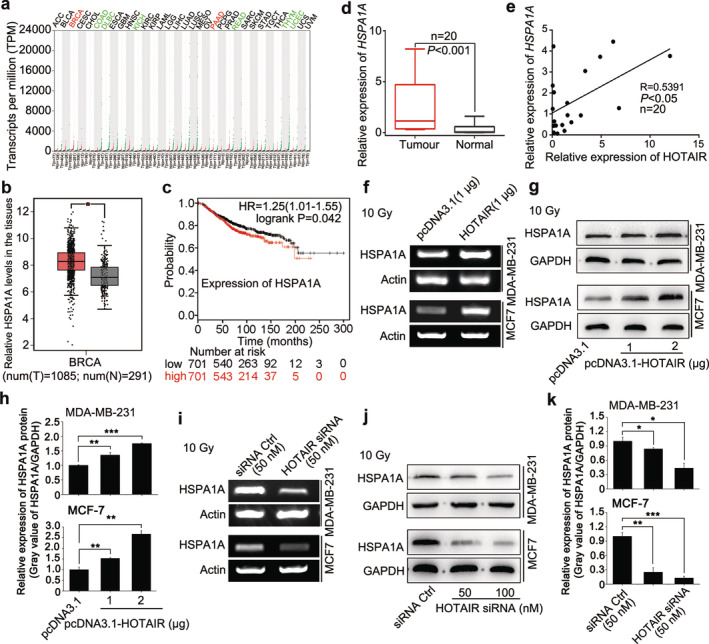
HOTAIR upregulates the stress‐inducible oncogene HSPA1A in BRCA cells. (a and b) The abundance of HSPA1A in different cancer types and the corresponding normal tissues (a) and the relative HSPA1A levels in breast cancer tissues and normal breast tissues ( ) Tumour, (
) Tumour, ( ) Normal (b) were obtained from the GEPIA database. (
) Normal (b) were obtained from the GEPIA database. ( ) Tumour, (
) Tumour, ( ) Normal (c) The relationship between HSPA1A levels and overall survival of BRCA patients was explored by searching the Kaplan‐Meier Plotter database. (
) Normal (c) The relationship between HSPA1A levels and overall survival of BRCA patients was explored by searching the Kaplan‐Meier Plotter database. ( ) Low, (
) Low, ( ) High (d) The expression of HSPA1A at mRNA level in 20 cases of clinical BRCA tissues and their paired peritumor tissues (P < 0.001, Wilcoxon's signed‐rank test) was examined by real‐time PCR analysis. (e) The correlation of HSPA1A and HOTAIR mRNA levels in 20 cases of clinical BRCA tissues was measured by real‐time PCR and analyzed by Pearson's correlation (R = 0.5391, P < 0.05). (f–h) The levels of HSPA1A mRNA (f) and protein (g and h) in irradiated BRCA cell lines MDA‐MB‐231 and MCF‐7 after the transfection of pcDNA3.1/pcDNA3.1‐HOTAIR were tested by RT‐PCR and western blot analysis, respectively. The relative expression levels of HSPA1A in three independent experiments were statistically analyzed by measuring the gray value of each band using Image J software. (i–k) The levels of HSPA1A mRNA (i) and protein (j and k) in irradiated BRCA cell lines MDA‐MB‐231 and MCF‐7 after the transfection of siRNA Ctrl/si‐HOTAIR were tested by RT‐PCR and western blot analysis, respectively. The relative expression levels of HSPA1A in three independent experiments were statistically analyzed by measuring the gray value of each band using Image J software. Data are shown as mean ± SD of three independent experiments. Statistical significant differences are indicated: *, P < 0.05; **, P < 0.01; ***, P < 0.001; Student's t‐test.
) High (d) The expression of HSPA1A at mRNA level in 20 cases of clinical BRCA tissues and their paired peritumor tissues (P < 0.001, Wilcoxon's signed‐rank test) was examined by real‐time PCR analysis. (e) The correlation of HSPA1A and HOTAIR mRNA levels in 20 cases of clinical BRCA tissues was measured by real‐time PCR and analyzed by Pearson's correlation (R = 0.5391, P < 0.05). (f–h) The levels of HSPA1A mRNA (f) and protein (g and h) in irradiated BRCA cell lines MDA‐MB‐231 and MCF‐7 after the transfection of pcDNA3.1/pcDNA3.1‐HOTAIR were tested by RT‐PCR and western blot analysis, respectively. The relative expression levels of HSPA1A in three independent experiments were statistically analyzed by measuring the gray value of each band using Image J software. (i–k) The levels of HSPA1A mRNA (i) and protein (j and k) in irradiated BRCA cell lines MDA‐MB‐231 and MCF‐7 after the transfection of siRNA Ctrl/si‐HOTAIR were tested by RT‐PCR and western blot analysis, respectively. The relative expression levels of HSPA1A in three independent experiments were statistically analyzed by measuring the gray value of each band using Image J software. Data are shown as mean ± SD of three independent experiments. Statistical significant differences are indicated: *, P < 0.05; **, P < 0.01; ***, P < 0.001; Student's t‐test.
miR‐449b‐5p suppresses the expression of HSPA1A by targeting the mRNA 3′‐UTR of HSPA1A
Given that lncRNAs can serve as molecular sponges to sequester miRNAs,10, 36 we assumed that HOTAIR might regulate the expression of HSPA1A through miRNAs. Therefore, we predicted the potential targeting miRNAs of HSPA1A by bioinformatic analysis (Targetscan: http://www.targetscan.org/vert_72/, miRDB: http://www.mirdb.org/ and miRWalk: http:// mirwalk. Umm.uni‐heidelberg.de/). Fortunately, we noticed that miR‐449b‐5p, a miRNA playing tumor‐suppressive roles in various cancer types, was one of the candidates with the ability to bind with the 3′‐UTR of HSPA1A mRNA (Fig 3a). Exhilaratingly, the predicted interaction between HOTAIR and miR‐449b‐5p on Bielefeld Bioinformatics Service (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/submission.html) gave a cue that HOTAIR could potentially bind with miR‐449b‐5p through complementary base‐paring reactions (Fig 3b). Thus, we preliminarily selected miR‐449b‐5p as the target miRNA for subsequent investigations. We then assayed the expression levels of miR‐449b‐5p in clinical BRCA samples and their paired normal breast tissues, and found that miR‐449b‐5p represented a lower expression in BRCA tissues than that in normal breast tissues (P < 0.001, Wilcoxon's signed‐rank test, Fig 3c). The expression relationship between HSPA1A and miR‐449b‐5p showed a converse correlation in BRCA tissues (R = − 0.6204, P < 0.01, Pearson's correlation, Fig 3d), indicating that miR‐449b‐5p may restrain the expression of HSPA1A. Based on the bioinformatics prediction, we synthesized the fragments harboring the miR‐449b‐5p binding site in the 3′‐UTR of HSPA1A mRNA and the corresponding mutant, and inserted them into the luciferase reporter gene vector pGL3‐control to create the pGL‐HSPA1A and pGL‐HSPA1A‐mut constructs respectively (Fig 3a). Luciferase reporter gene assays revealed that miR‐449b‐5p decreased the luciferase activities of pGL‐HSPA1A in MDA‐MB‐231 and MCF‐7 cells after the stimulation of γ‐irradiation in a dose‐dependent fashion, but not for pGL‐HSPA1A‐mut (Fig 3e,f). On the contrary, transfection of miR‐449b‐5p inhibitors increased the luciferase activities of pGL‐HSPA1A in above cells dose‐dependently, rather than pGL‐HSPA1A‐mut (Fig 3g,h), suggesting that miR‐449b‐5p indeed bind to the 3′‐UTR of HSPA1A mRNA. Moreover, real‐time PCR and western blot analysis revealed that miR‐449b‐5p was able to repress the expression of HSPA1A at the mRNA and protein levels dose‐dependently in MDA‐MB‐231 and MCF‐7 cells after exposed to γ‐irradiation (Fig 4a,b), whereas the converse result was achieved when the cells were treated with anti‐miR‐449b‐5p (Fig 4c,d). Collectively, miR‐449b‐5p suppresses the expression of HSPA1A by targeting the 3′‐UTR of HSPA1A mRNA.
Figure 3.
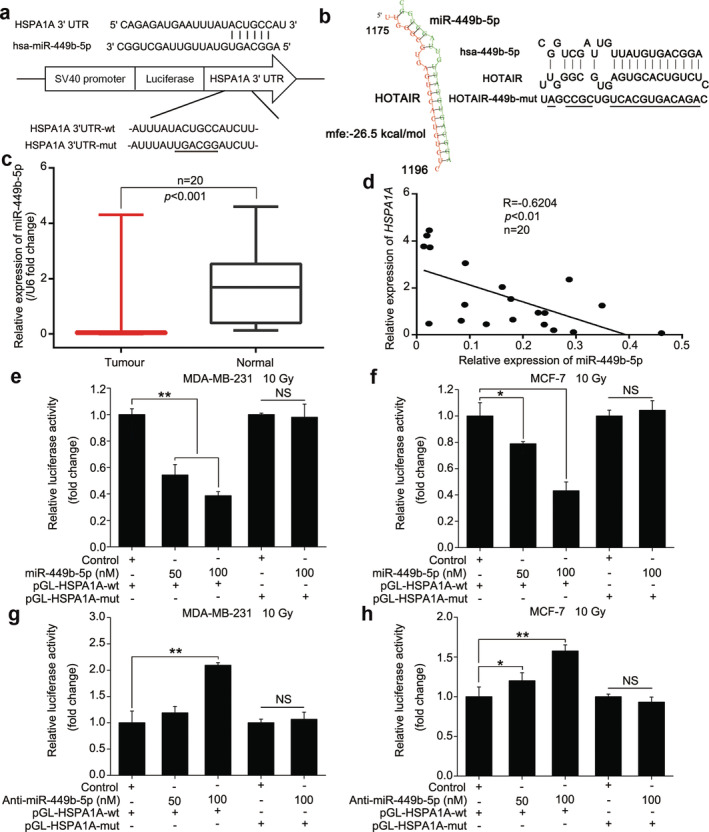
miR‐449b‐5p suppresses the expression of HSPA1A by targeting the mRNA 3′‐UTR of HSPA1A. (a) A schematic representation for the predicted binding site of miR‐449b‐5p in the 3′‐UTR of HSPA1A mRNA. The HSPA1A mRNA 3′‐UTR fragment containing the wild‐type or mutant of miR‐449b‐5p binding site was synthetized and inserted into the downstream of the luciferase reporter gene in pGL3‐control vector. (b) A schematic model depicting the interaction between HOTAIR and miR‐449b‐5p via complementary base‐pairing was obtained from the bioinformatic prediction of Bielefeld Bioinformatics Service. The mutant sites were indicated. (c) Real‐time PCR analysis was performed to evaluate the expression levels of miR‐449b‐5p in 20 paired of clinical BRCA tissues and the corresponding normal tissues (P < 0.001, Wilcoxon's signed‐rank test). (d) The correlation between HSPA1A mRNA and miR‐449b‐5p levels was determined by real‐time PCR in 20 clinical BRCA tissues (P < 0.01, R = −0.6204, Pearson's correlation coefficient). (e and f) The effects of miR‐449b‐5p on reporters of pGL‐HSPA1A‐wt and pGL‐HSPA1A‐mut in irradiated MDA‐MB‐231 (e) and MCF‐7 (f) cell lines were measured by luciferase reporter gene assays. (g and h) The effects of anti‐miR‐449b‐5p on reporters of pGL‐HSPA1A‐wt and pGL‐HSPA1A‐mut in irradiated MDA‐MB‐231 (g) and MCF‐7 (h) cell lines were examined by luciferase reporter gene assays. Data are shown as mean ± SD of three independent experiments. Statistical significant differences are indicated: *, P < 0.05; **, P < 0.01; Student's t‐test.
Figure 4.
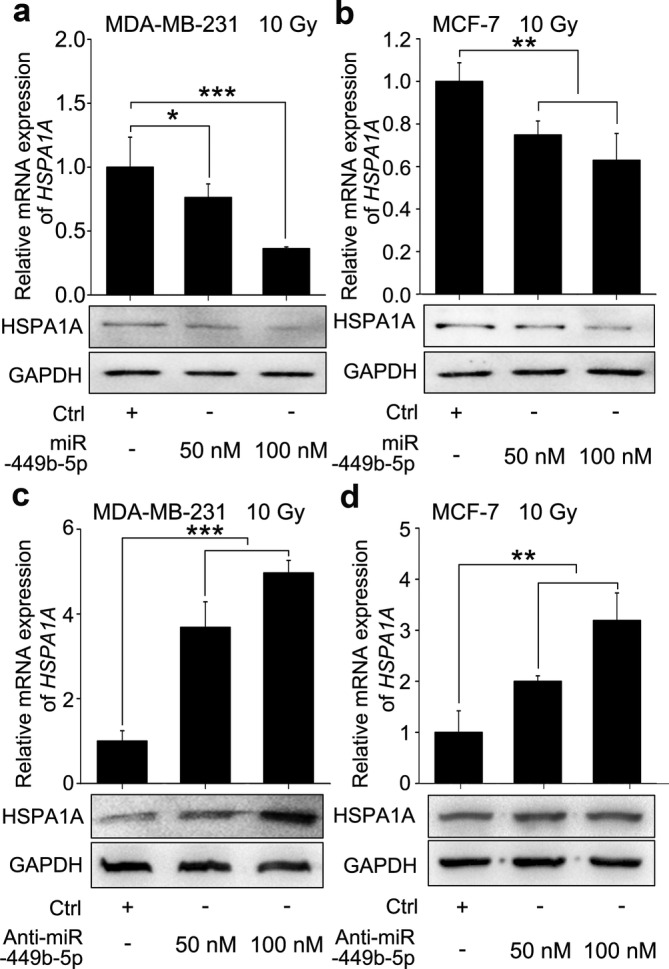
miR‐449b‐5p suppresses the expression of HSPA1A by targeting the mRNA 3′‐UTR of HSPA1A. (a and b) The BRCA cell lines MDA‐MB‐231 (a) and MCF‐7 (b) were transfected with miR‐449b‐5p or control 12 hours before the irradiation treatment. Then, 36 hours after the irradiation, total RNA and protein were extracted. The mRNA and protein levels of HSPA1A were detected by real‐time PCR and western blot analysis, respectively. (c and d) The BRCA cell lines MDA‐MB‐231 (c) and MCF‐7 (d) were transfected with the miR‐449b‐5p inhibitor or control 12 hours before the irradiation treatment. A total of 36 hours after the irradiation, total RNA and protein were extracted. The mRNA and protein levels of HSPA1A were detected by real‐time PCR and western blot analysis, respectively. Data are shown as mean ± SD of three independent experiments. Statistical significant differences are indicated: *, P < 0.05; **, P < 0.01; ***, P < 0.001; Student's t‐test.
HOTAIR facilitates expression of HSPA1A by sequestering miR‐449b‐5P
On the basis of the aforementioned bioinformatics prediction, we further assessed the sponging effect of HOTAIR on miR‐449b‐5p in irradiated BRCA cells. Initially, we assayed the data of qRT‐PCR and found that the expression levels of HOTAIR were inversely associated with those of miR‐449b‐5p in 20 cases of clinical BRCA tissues (R = −0.5407, P < 0.05, Pearson's correlation, Fig 5a), implying a potential interaction between HOTAIR and miR‐449b‐5p in BRCA cells. Further, real‐time PCR analysis demonstrated that overexpression of HOTAIR inhibited the levels of miR‐449b‐5p dose‐dependently in 10 Gy‐treated MDA‐MB‐231 and MCF‐7 cells (Fig 5b,c). Likewise, the overexpression of miR‐449b‐5p restrained the expression of HOTAIR dose‐dependently, whereas the interference with miR‐449b‐5p by its inhibitors achieved the opposite effect in 10 Gy‐irradiated MDA‐MB‐231 cells (Fig 5d,e), suggesting that the interaction between HOTAIR and miR‐449b‐5p cause the downregulation of each other. To confirm whether HOTAIR promotes the expression of HSPA1A by sequestering miR‐449b‐5p, we constructed the mutant of HOTAIR (HOTAIR‐449b‐mut) by introducing a substitution of the complementary pairing nucleotides into the binding region of miR‐449b‐5p and performed luciferase reporter gene assays (Fig 3b). The data showed that while miR‐449b‐5p impaired the luciferase activities of pGL‐HSPA1A, overexpression of HOTAIR rescued it. Reversely, HOTAIR‐449b‐mut failed to work (Fig 5f). Additionally, the effect of HOTAIR or HOTAIR‐449b‐mut on the expression of HSPA1A at the protein level in irradiated MDA‐MB‐231 cells was similar to above (Fig 5g). Collectively, we concluded that HOTAIR was capable of upregulating the expression of HSPA1A by sequestering miR‐449b‐5p post‐transcriptionally.
Figure 5.
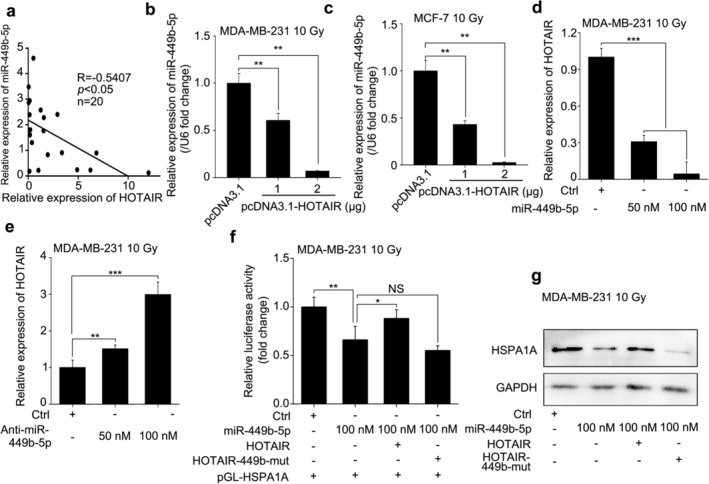
HOTAIR facilitates the expression of HSPA1A by sequestering miR‐449b‐5P. (a) The correlation between the levels of miR‐449b‐5p and HOTAIR in 20 cases of clinical BRCA tissues were tested by real‐time PCR and assayed by Pearson's correlation (R = −0.5407, P < 0.05). (b and c) The BRCA cell lines MDA‐MB‐231 (b) and MCF‐7 (c) were transfected with pcDNA3.1 or pcDNA3.1‐HOTAIR 12 hours before 10 Gy irradiation treatment. Total RNA was extracted and polyadenylated by poly (A) polymerase 36 hours after the irradiation. The effects of HOTAIR on the expression of miR‐449b‐5p were monitored by real‐time PCR analysis. (d and e) The BRCA cell line MDA‐MB‐231 was transfected with Ctrl/50 nM of miR‐449b‐5p/ 100 nM of miR‐449b‐5p (d) or Ctrl/50 nM of miR‐449b‐5p inhibitor/100 nM of miR‐449b‐5p inhibitor (e) 12 hours before 10 Gy irradiation treatment. Total RNA was extracted 36 hours after the irradiation. The effects of miR‐449b‐5p on the expression of HOTAIR were monitored by real‐time PCR analysis. (f) The BRCA cell line MDA‐MB‐231 was cotransfected with pGL‐HSPA1A, pRL‐TK plus Ctrl/miR‐449b‐5p/miR‐449b‐5p + HOTAIR/miR‐449b‐5p + HOTAIR‐449b‐mut 12 hours before 10 Gy irradiation treatment. The luciferase activities of pGL‐HSPA1A were measured 36 hours later by luciferase reporter gene assays. (g) The BRCA cell line MDA‐MB‐231 was transfected with Ctrl/miR‐449b‐5p/miR‐449b‐5p + HOTAIR/miR‐449b‐5p + HOTAIR‐449b‐mut 12 hours before 10 Gy irradiation treatment. Total proteins were extracted 36 hours later. The protein levels of HSPA1A were detected by western blot analysis, respectively. Data are shown as mean ± SD of three independent experiments. Statistical significant differences are indicated: *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, no significance; Student's t‐test.
HOTAIR enhances growth of irradiated‐BRCA cells through miR‐449b‐5p/HSPA1A axis in vitro and in vivo
To examine whether HSPA1A is implicated in the HOTAIR‐mediated radioresistance of BRCA cells, we performed a CCK‐8 assay in MDA‐MB‐231 cells. The expression of HSPA1A at mRNA and protein levels was determined by qRT‐PCR and western blot analysis after 10 Gy ionizing radiation (IR) treatment (Fig 6a). Overexpression of HOTAIR fought against the cytotoxicity induced by irradiation; however, transfection of miR‐449b‐5p or si‐HSPA1A suppressed HOTAIR‐derived radioprotection of BRCA cells (Fig 6b). In line with the results of the CCK‐8 assay, colony formation and EdU assays confirmed the effects (Fig 6c,d).
Figure 6.
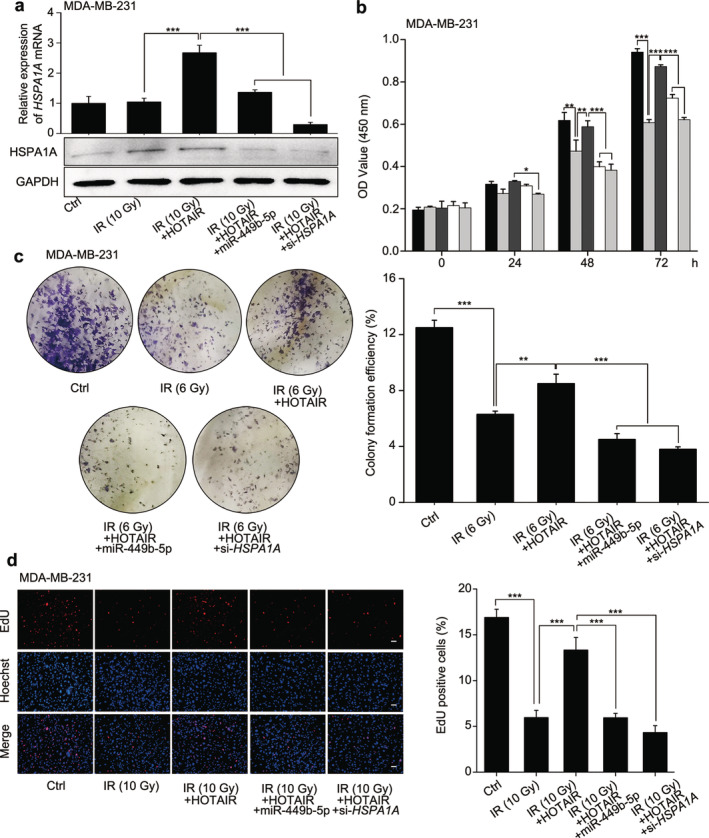
HOTAIR enhances the proliferation of irradiated‐BRCA cells through HSPA1A in vitro. The grouping of the test was as follows: Ctrl, IR (10 or 6 Gy), IR (10 or 6 Gy) + HOTAIR, IR (10 or 6 Gy) + HOTAIR+miR‐449b‐5p and IR (10 or 6 Gy) + HOTAIR+si‐HSPA1A. (a) The expression of HSPA1A at mRNA and protein levels in MDA‐MB‐231 cells from each group was assessed by real‐time PCR and western blot analysis, respectively. (b) The cell viability of MDA‐MB‐231 cells from each group was determined by CCK‐8 assay. ( ) Ctrl, (
) Ctrl, ( ) IR 10 Gy, (
) IR 10 Gy, ( ) IR 10 Gy + HOTAIR, (
) IR 10 Gy + HOTAIR, ( ) IR 10 Gy + HOTAIR+miR‐449b‐5p, (
) IR 10 Gy + HOTAIR+miR‐449b‐5p, ( ) IR 10 Gy + HOTAIR + si‐HSPA1A (c and d) The proliferative ability of MDA‐MB‐231 cells from each group was validated by colony formation assay (c) and EdU incorporation assay (d, scale bar, 30 μM), respectively. The colony formation efficiency and percentage of EdU positive cells were calculated. Data are shown as mean ± SD of three independent experiments. Statistical significant differences are indicated: *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, no significance; Student's t‐test.
) IR 10 Gy + HOTAIR + si‐HSPA1A (c and d) The proliferative ability of MDA‐MB‐231 cells from each group was validated by colony formation assay (c) and EdU incorporation assay (d, scale bar, 30 μM), respectively. The colony formation efficiency and percentage of EdU positive cells were calculated. Data are shown as mean ± SD of three independent experiments. Statistical significant differences are indicated: *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, no significance; Student's t‐test.
On the basis of the in vitro data, we further verified the conclusion in vivo using a tumor xenograft model. Consistently, we found that the growth curve, terminal volume and weight of the xenografts receiving IR treatment decreased markedly compared with the control group, but overexpression of HOTAIR erased irradiation‐induced tumor inhibition. In addition, overexpression of miR‐449b‐5p or silence of HSPA1A abolished the tumorous radioresistance strengthened by HOTAIR (Fig 7a–c). Meanwhile, we validated the expression of HSPA1A and miR‐449b‐5p in tumor tissues. As expected, the expression levels of HSPA1A, both at the mRNA and protein levels, and HOTAIR, elevated in the HOTAIR‐overexpression group, rather than in HOTAIR+miR‐449b‐5p or HOTAIR+si‐HSPA1A group, which might be the consequence of the impaired tumorigenicity (Fig 7d–f). The expression trend of miR‐449b‐5p was opposite to HSPA1A (Fig 7g), suggesting that HOTAIR enhances the growth of BRCA cells under irradiation‐stress through miR‐449b‐5p/HSPA1A signaling. In addition, we tested the expression of Ki‐67, a cell proliferative marker, in the tumor tissues from each group. Results of IHC assay manifested that HOTAIR overexpression reversed the IR‐caused Ki‐67 inhibition effect, HOTAIR+miR‐449b‐5p or HOTAIR+si‐HSPA1A restored it instead, implying that miR‐449b‐5p and HSPA1A participate in the HOTAIR‐enhanced growth of BRCA cells with IR‐treatment (Fig 7h). To summarize, we concluded that HOTAIR contributed to the radioresistance of BRCA cells involving miR‐449b‐5p and HSPA1A in vitro and in vivo.
Figure 7.
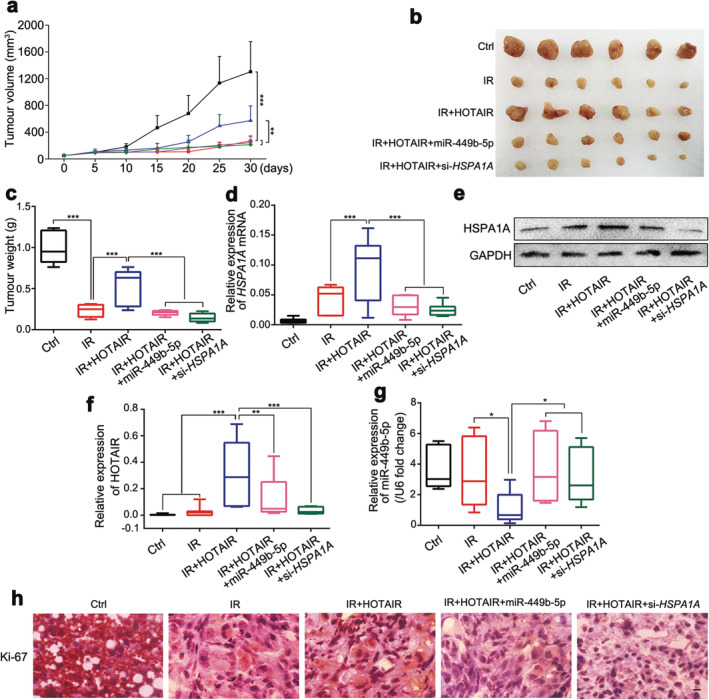
HOTAIR enhances the growth of irradiated‐BRCA cells through HSPA1A in vivo. MDA‐MB‐231 cells were grouped as the following treatment: Ctrl‐1, Ctrl‐2, HOTAIR, HOTAIR+miR‐449b‐5p and HOTAIR+si‐HSPA1A. The aforementioned cells were injected subcutaneously into the first mammary fat pad in the armpit of nude mice. Five days of consecutive 2 Gy IR‐treatment was applied to the tumors from Ctrl‐2, HOTAIR, HOTAIR+miR‐449b‐5p and HOTAIR+si‐HSPA1A groups when the tumors reached 100 mm3. The tumor volume was recorded during the tumorigenicity process every five days. (a) The growth curves of tumors from each group are presented. ( ) Ctrl, (
) Ctrl, ( ) IR, (
) IR, ( ) IR + HOTAIR, (
) IR + HOTAIR, ( ) IR + HOTAIR + miR‐449b‐5p, (
) IR + HOTAIR + miR‐449b‐5p, ( ) IR + HOTAIR + si‐HSPA1A (b and c) The image of excised tumors and tumor weights of each group are presented. (d–g) The expression levels of HSPA1A mRNA and protein (d and e), HOTAIR (f) and miR‐449b‐5p (g) were quantified by real‐time PCR or western blot analysis. (h) The expression levels of Ki‐67 in tumor tissues were examined by IHC staining. Scale bar, 20 μM. Data are shown as mean ± SD of three independent experiments. Statistical significant differences are indicated: *, P < 0.05; **, P < 0.01; ***, P < 0.001; Student's t‐test.
) IR + HOTAIR + si‐HSPA1A (b and c) The image of excised tumors and tumor weights of each group are presented. (d–g) The expression levels of HSPA1A mRNA and protein (d and e), HOTAIR (f) and miR‐449b‐5p (g) were quantified by real‐time PCR or western blot analysis. (h) The expression levels of Ki‐67 in tumor tissues were examined by IHC staining. Scale bar, 20 μM. Data are shown as mean ± SD of three independent experiments. Statistical significant differences are indicated: *, P < 0.05; **, P < 0.01; ***, P < 0.001; Student's t‐test.
Discussion
Ionizing radiation which plays a central part in cancer therapy often debases its curative effects when radiation resistance emerges.37, 38 The expression alteration of various biomolecules including tumor‐promoting/suppressing proteins and non‐coding RNAs contributes to the resistant phenotypes. LncRNAs are involved in a series of physiologic and pathologic processes. It has been previously reported that the dysregulation of lncRNAs is associated with the development, metastasis as well as prognosis of cancer.39, 40 In this study, we were curious about the role of lncRNA HOTAIR in the radioresistance of breast cancer.
To understand the significance of HOTAIR in breast cancer, we first searched the GEPIA dataset for the expression profile of HOTAIR and the relationship between HOTAIR and breast cancer malignancy. Results showed that the expression level of HOTAIR was higher in BRCA than that in normal breast tissues and it was positively related to BRCA malignancy. In parallel, we evaluated the expression pattern of HOTAIR in clinical BRCA and normal breast samples and verified the online data. Meanwhile, we validated that the expression level of HOTAIR was inversely correlated with the radiosensitivity of BRCA cells by experimental approaches. Thus, the data indicate that HOTAIR endows breast cancer with radiation resistant ability. Next, we tried to clarify the underlying mechanism by which HOTAIR causes radioresistance of BRCA. Given that IR is a typical stress stimuli to the organisms, we speculated that HOTAIR might exert the radioresistant function through targeting the hot shock proteins which respond to various ambient challenges.29 HSPA1A, a major stress‐inducible member of this family, attracted us by its overexpression in a large variety of tumor types. Intriguingly, we affirmed that HSPA1A was also positively related to BRCA malignancy from both online and experimental data. Moreover, the expression levels of HSPA1A and HOTAIR exhibited a positive correlation in clinical BRCA tissues and HOTAIR promoted the expression of HSPA1A in irradiated BRCA cells, suggesting that HSPA1A mediates the radioresistance property triggered by HOTAIR. On the basis that lncRNAs can act as miRNA sponges to accelerate the progression of cancers, we identified miRNA (miR‐449b‐5p) targeted the 3′‐UTR of HSPA1A mRNA by bioinformatic and experimental approaches. Of note, HOTAIR could interact with miR‐449b‐5p through complementary pairing fashion. The interaction between HOTAIR and miR‐449b‐5p led to the downregulation of each other in irradiated BRCA cells. Importantly, the HOTAIR‐mediated downregulation of miR‐449b‐5p resulted in the elevation of HSPA1A expression. Thus, we illustrate the mechanism by which HOTAIR modulates the expression of the stress‐induced protein HSPA1A under IR condition. Functionally, we revealed that HOTAIR was capable of facilitating the acquisition of radioresistant phenotype of BRCA cells through upregulating HSPA1A in vitro and in vivo, in which miR‐449b‐5p was involved. Therapeutically, HOTAIR and HSPA1A may serve as potential targets in the radioactive treatment of BRCA.
In this study, we identified the regulation axis of HOTAIR/miR‐449b‐5p/HSPA1A implicated in BRCA radioresistance occurrence, and confirmed it from the online bioinformatics, the clinic, and last the in vitro/in vivo experimental aspects, which make our study solid. To find the eventual executive protein of HOTAIR‐mediated radiation resistance, we set out from the stress stimulation effects of irradiation and chose HSPA1A as the target protein for its stress‐responsive efficacy with definite oncogenic role, and fortunately we were able to affirm the modulating association between HOTAIR and HSPA1A in multiple ways. Then, in the process of identifying the miRNA sponge which mediates the connection of HOTAIR and HSPA1A, four miRNAs (miR‐449a‐5p, miR‐449b‐5p, miR‐34a‐5p and miR‐34c‐5p) attracted us initially both for their potent complementary base pairing‐based binding to the 3′‐UTR of HSPA1A mRNA as well as to HOTAIR and the clearly reported tumor‐suppressive function. Nevertheless, the other three except miR‐449b‐5p were excluded because the experimental exploration did not show a consistent result with the prediction data in this project. Therefore, we selected miR‐449b‐5p as the link miRNA between HOTAIR and HSPA1A. Intriguingly, the following data verified that. An intricate modulating network, consisting of other miR‐449b‐5p target genes and HOTAIR‐sponging miRNAs as well as other lncRNAs, is intertwined in the radiation sensitivity regulation of breast cancer.41, 42 Given miR‐449b‐5p targets FOXP1,25 E2F3,43 TGF‐β,44 HMGB145 and SOX4,46 et al. and HOTAIR sponges miR‐29b,47 miR‐126,48 miR‐520b,49 miR‐148b50 and miR‐196a,51 et al. we only focused on the stress effects of γ‐irradiation and identified hot shock protein HSPA1A as the terminal target of HOTAIR, then miR‐449b‐5p connecting HOTAIR and HSPA1A was discovered. Together, we just illustrated HOTAIR/miR‐449b‐5p/HSPA1A signaling as one of novel axes among the network regulating radiosensitivity of BRCA, and giving a partial view of HOTAIR‐implicated BRCA radiation resistance in this study. Of note, other underlying mechanisms need to be clarified further.
The lncRNA HOTAIR plays pivotal roles in the development of BRCA. For example, it has been reported that HOTAIR promotes invasion of breast cancer cells through chondroitin sulfotransferase CHST15.52 HOTAIR can act as “scaffold” between HBXIP and LSD1 to mediate transcriptional activation function of c‐Myc.53 Clinically, HOTAIR enhances estrogen receptor signaling and confers tamoxifen resistance in breast cancer.54 TGF‐β1/HOTAIR axis can serve as a target in breast cancer treatment.55 Accordingly, the oncogenic function of HOTAIR in other reports is consistent with our findings here, which supports our conclusion that HOTAIR confers the radiation resistance on breast cancer.
Numerous studies have shown that miR‐449b‐5p acts as a significant tumor suppressor gene in various cancer types, including breast cancer, liver cancer, prostate cancer, cervical cancer, nasopharyngeal carcinoma and pediatric acute lymphoblastic leukemia.25, 56, 57, 58, 59, 60, 61, 62, 63 Notably, it is implicated in the occurrence of chemotherapy and radiotherapy.59 In this study, we clarify the role of miR‐449b‐5p in HOTAIR‐mediated radiation resistance of breast cancer, making a supplement for the tumor‐suppressive function of miR‐449b‐5P.
Previous studies have demonstrated that HSPA1A participates in the cancerous networks. It is involved in the lethal oxidative and mitochondria toxic stress of the apoptotic elimination of malignant melanoma cells.64 Extracellular HSPA1A promotes the growth of hepatocarcinoma by augmenting tumor cell proliferation and apoptosis‐resistance.65 It interacts with KIAA0100 gene to modulate cancer cell aggression behavior of breast cancer cell MDA‐MB‐231.66 Here, we first report that HSPA1A partakes in the HOTAIR‐radioresistance axis in breast cancer.
As the first‐line treatment option for BRCA, the efficacy of radiotherapy remains limited and plagued by the emergence of radiation resistance, which aggravates prognosis.67 Breast cancer is heterogeneous and is usually divided into four molecular subtypes based on the ER/PR/HER2 status clinically. Studies have synthetically showed that HOTAIR is responsible for the malignancy of HER2‐subtypes,68, 69, 70, 71 coincidentally, our data showed that the expression level of HOTAIR in SKBR‐3, which is affiliated to the HER2+ subtype, is the lowest among the six BRCA cell lines covering the four types. Therefore, we selected another two cell lines, MCF‐7 and MDA‐MB‐231, which harbor the relative higher levels of HOTAIR to SKBR‐3, as the main vehicle in the subsequent study. To evaluate the in vivo effect of HOTAIR/miR‐449b‐5p/HSPA1A signal on radiosensitivity of BRCA, we established a xenograft mouse model by injecting cells into the fat pad, which is the alternative model used in breast cancer tumorigenicity assessment,72, 73, 74 and performed local irradiation treatment simulating the clinical regimen when a tumor lump is initially formed. Yet, given the immune deficiency of the athymic nude mice, utilizing this model for radiation study may just partially retain the impact of radiation on immune system and thereby influence the evaluation of the real tumorous radiosensitivity existing in human body. It is still necessary to exploit other breast cancer animal models, such as the chemical induced model, patient‐derived xenograft (PDX) model and transgenic mouse model, develop novel animal models, or even acquire clinical support to make the conclusions obtained in this study more robust. Collectively, we conclude that HOTAIR endows breast cancer with radioresistance in which miR‐449b‐5p and HSPA1A is involved.
In summary, our findings demonstrate that HOTAIR can boost the radioresistance of breast cancer by upregulating HSPA1A in vitro and in vivo. Mechanistically, miR‐449b‐5p is able to hinder the expression of HSPA1A via targeting the mRNA 3′‐UTR of HSPA1A, whereas HOTAIR is capable of sponging miR‐449b‐5p, resulting in the recovery of HSPA1A expression under the irradiation condition. LncRNA HOTAIR and HSPA1A may serve as therapeutic targets in the treatment of breast cancer. Thus, our findings shed new light on the mechanism by which lncRNAs modulate tumorous radiosensitivity.
Disclosure
The authors declare no competing interests.
Supporting information
Appendix S1: HOTAIR endows breast cancer with radiation resistance.
Figure S1 HOTAIR endows breast cancer with radiation resistance. (a) The expression levels of HOTAIR in 20 cases of breast tumor tissues and the adjacent normal tissues were detected by qRT‐PCR analysis (P < 0.001, Wilcoxon's signed‐rank test) (b) The colony formation efficiency of T47D and SKBR‐3 receiving a 6 Gy γ‐irradiation treatment were calculated. (c and d) The time‐ (c) or dose‐ (d) dependent radiosensitivities of BRCA cell lines affiliated to different molecular subtypes (MDA‐MB‐231, triple‐negative breast cancer; MCF‐7, Luminal A; BT‐474, Luminal B; and SKBR‐3, HER2‐enriched) were tested by CCK‐8 assay after the cells were exposed to γ‐irradiation. The relative radiosensitivity differences were compared by the ratio of (OD (IR)/OD (No‐IR) % at each time or dose point. (e and f) MCF‐7 cells were transfected with pcDNA3.1/pcDNA3.1‐HOTAIR (e) or siRNA Ctrl/si‐HOTAIR (f) 12 hours before the irradiation treatment, CCK‐8 assay was used to measure the cell viability after 0, 15, 20 and 25 Gy irradiation treatment. (g) The colony formation efficiency of MCF‐7 transfected with pcDNA3.1 + siRNA Ctrl/pcDNA3.1‐HOTAIR/si‐HOTAIR 12 hours before the 6 Gy irradiation treatment were calculated. Data are shown as mean ± SD of three independent experiments. Statistical significant differences are indicated: *, P < 0.05, ***, P < 0.001; Student's t‐test
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81803062, 81872555, 81730086 and 81572969), China Postdoctoral Science Foundation (2018M631391), CAMS Innovation Fund for Medical Sciences (CIFMS, 2016‐I2M‐1‐017 and 2016‐I2M‐B&R‐13), the Technology and Development and Research Projects for Research Institutes, Ministry of Science and Technology (2014EG150134), the Tianjin Science and Technology Support Plan Project (TJKJZC, 14ZCZDSY00001), the Drug Innovation Major Project of China (2018ZX09711001‐007‐008).
Contributor Information
Ming Cui, Email: cuiming0403@bjmu.edu.cn.
Saijun Fan, Email: fansaijun@irm-cams.ac.cn.
References
- 1. Harbeck N, Penault‐Llorca F, Cortes J et al Breast cancer. Nat Rev Dis Primers 2019; 5: 66. [DOI] [PubMed] [Google Scholar]
- 2. Semmler L, Reiter‐Brennan C, Klein A. BRCA1 and breast cancer: A review of the underlying mechanisms resulting in the tissue‐specific tumorigenesis in mutation carriers. J Breast Cancer 2019; 22: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freddie B, Jacques F, Isabelle S et al GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. A Cancer Journal for Clinicians, CA: 2018. [Google Scholar]
- 4. Benvenuto M, Focaccetti C, Izzi V, Masuelli L, Modesti A, Bei R. Tumor antigenS heterogeneity and immune response‐targeting neoantigens in breast cancer. Semin Cancer Biol 2019. 10.1016/j.semcancer.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 5. Llombart‐Cussac A, Cortes J, Pare L et al HER2‐enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early‐stage HER2‐positive breast cancer (PAMELA): An open‐label, single‐group, multicentre, phase 2 trial. Lancet Oncol 2017; 18: 545–54. [DOI] [PubMed] [Google Scholar]
- 6. Waks AG, Winer EP. Breast cancer treatment: A review. JAMA 2019; 321: 288–300. [DOI] [PubMed] [Google Scholar]
- 7. Rajitha B, Malla RR, Vadde R et al Horizons of nanotechnology applications in female specific cancers. Semin Cancer Biol 2019. 10.1016/j.semcancer.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 8. Wang H, Mu X, He H, Zhang X‐D. Cancer Radiosensitizers. Trends Pharmacol Sci 2018; 39: 24–48. [DOI] [PubMed] [Google Scholar]
- 9. Djebali S, Davis CA, Merkel A et al Landscape of transcription in human cells. Nature 2012; 489: 101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khanduja JS, Calvo IA, Joh RI, Hill IT, Motamedi M. Nuclear noncoding RNAs and genome stability. Mol Cell 2016; 63: 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem 2012; 81: 145–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov 2011; 1: 391–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta RA, Shah N, Wang KC et al Long non‐coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010; 464: 1071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Y, Ren Y, Wang Y. et al A compound AC1Q3QWB selectively disrupts HOTAIR‐mediated recruitment of PRC2 and enhances cancer therapy of DZNep. Theranostics 2019;9:4608–4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu Y, Zhang L, Zhang L et al Long non‐coding RNA HOTAIR promotes tumor cell invasion and metastasis by recruiting EZH2 and repressing E‐cadherin in oral squamous cell carcinoma. Int J Oncol 2015; 46: 2586–94. [DOI] [PubMed] [Google Scholar]
- 16. Xu S, Kong D, Chen Q, Ping Y, Pang D. Oncogenic long noncoding RNA landscape in breast cancer. Mol Cancer 2017; 16: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell 2009; 136: 215–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buggele WA, Krause KE, Horvath CM. Small RNA profiling of influenza a virus‐infected cells identifies miR‐449b as a regulator of histone deacetylase 1 and interferon beta. PLOS One 2013; 8: e76560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen W, Peng R, Sun Y et al The topological key lncRNA H2k2 from the ceRNA network promotes mesangial cell proliferation in diabetic nephropathy via the miR‐449a/b/Trim11/Mek signaling pathway. FASEB J 2019; 33: 11492–506. [DOI] [PubMed] [Google Scholar]
- 20. Lv J, Zhang Z, Pan L, Zhang Y. MicroRNA‐34/449 family and viral infections. Virus Res 2019; 260: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Noveski P, Popovska‐Jankovic K, Kubelka‐Sabit K et al MicroRNA expression profiles in testicular biopsies of patients with impaired spermatogenesis. Andrology 2016; 4: 1020–7. [DOI] [PubMed] [Google Scholar]
- 22. Tolosa E, Botta‐Orfila T, Morato X et al MicroRNA alterations in iPSC‐derived dopaminergic neurons from Parkinson disease patients. Neurobiol Aging 2018; 69: 283–91. [DOI] [PubMed] [Google Scholar]
- 23. Wang M, Gao Y, Qu P et al Sperm‐borne miR‐449b influences cleavage, epigenetic reprogramming and apoptosis of SCNT embryos in bovine. Sci Rep 2017; 7: 13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu J, Bao J, Kim M. et al Two miRNA clusters, miR‐34b/c and miR‐449, are essential for normal brain development, motile ciliogenesis, and spermatogenesis. Proc Natl Acad Sci U S A 2014; 111: 2851–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheng L, Shi X, Huo D, Zhao Y, Zhang H. MiR‐449b‐5p regulates cell proliferation, migration and radioresistance in cervical cancer by interacting with the transcription suppressor FOXP1. Eur J Pharmacol 2019; 856: 172399. [DOI] [PubMed] [Google Scholar]
- 26. Hartl FU, Hayer‐Hartl M. Molecular chaperones in the cytosol: From nascent chain to folded protein. Science 2002; 295: 1852–8. [DOI] [PubMed] [Google Scholar]
- 27. Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. Intracellular and extracellular functions of heat shock proteins: Repercussions in cancer therapy. J Leukoc Biol 2007; 81: 15–27. [DOI] [PubMed] [Google Scholar]
- 28. Shevtsov M, Huile G, Multhoff G. Membrane heat shock protein 70: A theranostic target for cancer therapy. Philos Trans R Soc Lond B Biol Sci 2018; 373: 20160526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim YK, Suarez J, Hu Y et al Deletion of the inducible 70‐kDa heat shock protein genes in mice impairs cardiac contractile function and calcium handling associated with hypertrophy. Circulation 2006; 113: 2589–97. [DOI] [PubMed] [Google Scholar]
- 30. Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: Chaperones of tumorigenesis. Trends Biochem Sci 2006; 31: 164–72. [DOI] [PubMed] [Google Scholar]
- 31. Wu J, Liu T, Rios Z, Mei Q, Lin X, Cao S. Heat shock proteins and cancer. Trends Pharmacol Sci 2017; 38: 226–56. [DOI] [PubMed] [Google Scholar]
- 32. Boysen M, Kityk R, Mayer MP. Hsp70‐ and Hsp90‐mediated regulation of the conformation of p53 DNA binding domain and p53 cancer variants. Mol Cell 2019; 74: 831–43 e834. [DOI] [PubMed] [Google Scholar]
- 33. Kaur P, Hurwitz MD, Krishnan S, Asea A. Combined hyperthermia and radiotherapy for the treatment of cancer. Cancers 2011; 3: 3799–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li H, Liu Q, Wang Z et al The oncoprotein HBXIP modulates the feedback loop of MDM2/p53 to enhance the growth of breast cancer. J Biol Chem 2015; 290: 22649–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mishra B, Luderer U. Reproductive hazards of space travel in women and men. Nat Rev Endocrinol 2019; 15: 713–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell 2009; 136: 629–41. [DOI] [PubMed] [Google Scholar]
- 37. Lee SY, Jeong EK, Ju MK. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cancer 2017; 16: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Song G, Cheng L, Chao Y, Yang K, Liu Z. Emerging nanotechnology and advanced materials for cancer radiation therapy. Adv Mater 2017; 29: 996–1022. [DOI] [PubMed] [Google Scholar]
- 39. Huang JL, Zheng L, Hu YW, Wang Q. Characteristics of long non‐coding RNA and its relation to hepatocellular carcinoma. Carcinogenesis 2014; 35: 507–14. [DOI] [PubMed] [Google Scholar]
- 40. Liu XH, Sun M, Nie FQ et al Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR‐331‐3p in gastric cancer. Mol Cancer 2014; 13: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bracken CP, Scott HS, Goodall GJ. A network‐biology perspective of microRNA function and dysfunction in cancer. Nat Rev Genet 2016; 17: 719–32. [DOI] [PubMed] [Google Scholar]
- 42. Chan JJ, Tay Y. Noncoding RNA:RNA regulatory networks in cancer. Int J Mol Sci 2018; 19: 1310–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martinez LA, Goluszko E, Chen HZ et al E2F3 is a mediator of DNA damage‐induced apoptosis. Mol Cell Biol 2010; 30: 524–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vanpouille‐Box C, Diamond JM, Pilones KA et al TGFbeta is a master regulator of radiation therapy‐induced antitumor immunity. Cancer Res 2015; 75: 2232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shrivastava S, Mansure JJ, Almajed W et al The role of HMGB1 in radioresistance of bladder cancer. Mol Cancer Ther 2016; 15: 471–9. [DOI] [PubMed] [Google Scholar]
- 46. Yoon TM, Kim SA, Cho WS et al SOX4 expression is associated with treatment failure and chemoradioresistance in oral squamous cell carcinoma. BMC Cancer 2015; 15: 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li G, Zhao J, Peng X, Liang J, Deng X, Chen Y. The mechanism involved in the loss of PTEN expression in NSCLC tumor cells. Biochem Biophys Res Commun 2012; 418: 547–52. [DOI] [PubMed] [Google Scholar]
- 48. Liu W, Chen H, Wong N, Haynes W, Baker CM, Wang X. Pseudohypoxia induced by miR‐126 deactivation promotes migration and therapeutic resistance in renal cell carcinoma. Cancer Lett 2017; 394: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lu YC, Cheng AJ, Lee LY et al MiR‐520b as a novel molecular target for suppressing stemness phenotype of head‐neck cancer by inhibiting CD44. Sci Rep 2017; 7: 2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lai Y, Chen Y, Lin Y, Ye L. Down‐regulation of LncRNA CCAT1 enhances radiosensitivity via regulating miR‐148b in breast cancer. Cell Biol Int 2018; 42: 227–36. [DOI] [PubMed] [Google Scholar]
- 51. Fang X, Jeong JH, Long X et al IKKalpha‐mediated biogenesis of miR‐196a through interaction with Drosha regulates the sensitivity of cancer cells to radiotherapy. Cell Death Differ 2016; 23: 1471–82. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52. Liu LC, Wang YL, Lin PL et al Long noncoding RNA HOTAIR promotes invasion of breast cancer cells through chondroitin sulfotransferase CHST15. Int J Cancer 2019; 145: 2478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li Y, Wang Z, Shi H et al HBXIP and LSD1 Scaffolded by lncRNA Hotair mediate transcriptional activation by c‐Myc. Cancer Res 2016; 76: 293–304. [DOI] [PubMed] [Google Scholar]
- 54. Xue X, Yang YA, Zhang A et al LncRNA HOTAIR enhances ER signaling and confers tamoxifen resistance in breast cancer. Oncogene 2016; 35: 2746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ren Y, Jia HH, Xu YQ et al Paracrine and epigenetic control of CAF‐induced metastasis: The role of HOTAIR stimulated by TGF‐ss1 secretion. Mol Cancer 2018; 17: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jiang J, Yang X, He X et al MicroRNA‐449b‐5p suppresses the growth and invasion of breast cancer cells via inhibiting CREPT‐mediated Wnt/beta‐catenin signaling. Chem Biol Interact 2019; 302: 74–82. [DOI] [PubMed] [Google Scholar]
- 57. Sandbothe M, Buurman R, Reich N et al The microRNA‐449 family inhibits TGF‐beta‐mediated liver cancer cell migration by targeting SOX4. J Hepatol 2017; 66: 1012–21. [DOI] [PubMed] [Google Scholar]
- 58. Ji C, Xu Q, Guo L et al eEF‐2 kinase‐targeted miR‐449b confers radiation sensitivity to cancer cells. Cancer Lett 2018; 418: 64–74. [DOI] [PubMed] [Google Scholar]
- 59. Yin W, Shi L, Mao Y. MicroRNA‐449b‐5p suppresses cell proliferation, migration and invasion by targeting TPD52 in nasopharyngeal carcinoma. J Biochem 2019; 166: 433–40. [DOI] [PubMed] [Google Scholar]
- 60. Yang X, Feng M, Jiang X et al miR‐449a and miR‐449b are direct transcriptional targets of E2F1 and negatively regulate pRb‐E2F1 activity through a feedback loop by targeting CDK6 and CDC25A. Genes Dev 2009; 23: 2388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gutierrez‐Camino A, Lopez‐Lopez E, Martin‐Guerrero I et al Noncoding RNA‐related polymorphisms in pediatric acute lymphoblastic leukemia susceptibility. Pediatr Res 2014; 75: 767–73. [DOI] [PubMed] [Google Scholar]
- 62. Ostling P, Leivonen SK, Aakula A et al Systematic analysis of microRNAs targeting the androgen receptor in prostate cancer cells. Cancer Res 2011; 71: 1956–67. [DOI] [PubMed] [Google Scholar]
- 63. Dzikiewicz‐Krawczyk A, Macieja A, Maly E et al Polymorphisms in microRNA target sites modulate risk of lymphoblastic and myeloid leukemias and affect microRNA binding. J Hematol Oncol 2014; 7: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hua AB, Justiniano R, Perer J et al Repurposing the electron transfer reactant phenazine methosulfate (PMS) for the apoptotic elimination of malignant melanoma cells through induction of lethal oxidative and mitochondriotoxic stress. Cancers 2019; 11: 590–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wu FH, Yuan Y, Li D et al Extracellular HSPA1A promotes the growth of hepatocarcinoma by augmenting tumor cell proliferation and apoptosis‐resistance. Cancer Lett 2012; 317: 157–64. [DOI] [PubMed] [Google Scholar]
- 66. Zhong Z, Pannu V, Rosenow M, Stark A, Spetzler D. KIAA0100 modulates cancer cell aggression behavior of MDA‐MB‐231 through microtubule and heat shock proteins. Cancers 2018; 10: 180–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rajitha B, Malla RR, Vadde R et al Horizons of Nanotechnology Applications in Female Specific Cancers. Seminars in Cancer Biology 2019. [DOI] [PubMed]
- 68. Collina F, Aquino G, Brogna M et al LncRNA HOTAIR up‐regulation is strongly related with lymph nodes metastasis and LAR subtype of triple negative breast cancer. J Cancer 2019; 10: 2018–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Muller V, Oliveira‐Ferrer L, Steinbach B, Pantel K, Schwarzenbach H. Interplay of lncRNA H19/miR‐675 and lncRNA NEAT1/miR‐204 in breast cancer. Mol Oncol 2019; 13: 1137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Olgun G, Sahin O, Tastan O. Discovering lncRNA mediated sponge interactions in breast cancer molecular subtypes. BMC Genomics 2018; 19: 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhuang Y, Nguyen HT, Burow ME et al Elevated expression of long intergenic non‐coding RNA HOTAIR in a basal‐like variant of MCF‐7 breast cancer cells. Mol Carcinog 2015; 54: 1656–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bartoschek M, Oskolkov N, Bocci M et al Spatially and functionally distinct subclasses of breast cancer‐associated fibroblasts revealed by single cell RNA sequencing. Nat Commun 2018; 9: 5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lan J, Lu H, Samanta D, Salman S, Lu Y, Semenza GL. Hypoxia‐inducible factor 1‐dependent expression of adenosine receptor 2B promotes breast cancer stem cell enrichment. Proc Natl Acad Sci U S A 2018; 115: E9640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Li CW, Lim SO, Chung EM et al Eradication of triple‐negative breast cancer cells by targeting glycosylated PD‐L1. Cancer Cell 2018; 33: 187–201 e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: HOTAIR endows breast cancer with radiation resistance.
Figure S1 HOTAIR endows breast cancer with radiation resistance. (a) The expression levels of HOTAIR in 20 cases of breast tumor tissues and the adjacent normal tissues were detected by qRT‐PCR analysis (P < 0.001, Wilcoxon's signed‐rank test) (b) The colony formation efficiency of T47D and SKBR‐3 receiving a 6 Gy γ‐irradiation treatment were calculated. (c and d) The time‐ (c) or dose‐ (d) dependent radiosensitivities of BRCA cell lines affiliated to different molecular subtypes (MDA‐MB‐231, triple‐negative breast cancer; MCF‐7, Luminal A; BT‐474, Luminal B; and SKBR‐3, HER2‐enriched) were tested by CCK‐8 assay after the cells were exposed to γ‐irradiation. The relative radiosensitivity differences were compared by the ratio of (OD (IR)/OD (No‐IR) % at each time or dose point. (e and f) MCF‐7 cells were transfected with pcDNA3.1/pcDNA3.1‐HOTAIR (e) or siRNA Ctrl/si‐HOTAIR (f) 12 hours before the irradiation treatment, CCK‐8 assay was used to measure the cell viability after 0, 15, 20 and 25 Gy irradiation treatment. (g) The colony formation efficiency of MCF‐7 transfected with pcDNA3.1 + siRNA Ctrl/pcDNA3.1‐HOTAIR/si‐HOTAIR 12 hours before the 6 Gy irradiation treatment were calculated. Data are shown as mean ± SD of three independent experiments. Statistical significant differences are indicated: *, P < 0.05, ***, P < 0.001; Student's t‐test


