Abstract
Background
The efficacy and safety of neoadjuvant treatment over surgery alone and that of neoadjuvant chemoradiotherapy (NCRT) over neoadjuvant chemotherapy (NCT) in resectable esophageal carcinoma remains inconclusive. This study (NewEC) used global data to comprehensively evaluate these comparisons and to provide a preferable strategy for patient subsets.
Methods
This study included a meta-analysis of randomized controlled trials (RCTs) identified from inception to May 2019 from PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, ClinicalTrials.gov, and congresses and a registry-based cohort study with patients from Massachusetts General Hospital (Massachusetts, USA) and Guangdong Provincial People's Hospital (Guangzhou, China) recruited from November 2000 and June 2017, to cross-validate the comparisons among NCRT versus NCT versus surgery. The GRADE approach was used to assessed quality of evidence in meta-analysis. Neural network machine learning propensity score–matched analysis was used to account for confounding by patient-level characteristics in the cohort study. The primary endpoint was overall survival (OS). The study was registered with PROSPERO CRD42017072242 and ClinicalTrials.gov NCT04027543.
Findings
Of 22,070 studies assessed, there were 38 (n = 6,993 patients) eligible RCTs. Additionally, 423 out of 467 screened patients were included in the cohort study. The results from trials showed that NCT had a better OS than surgery alone (hazard ratio [HR] 0·88, 95% confidence interval [CI] 0·79–0·98; high quality) and was only favorable for adenocarcinoma (HR 0·83, 95% CI 0·72–0·96; moderate quality). High-quality evidence showed a significantly better OS for NCRT than surgery alone (HR 0·74, 95% CI 0·66–0·82) for both adenocarcinoma (HR 0·73, 95% CI 0·62–0·86) and squamous cell carcinoma (SCC) (HR 0·73, 95% CI 0·65–0·83). The OS benefit of NCRT over NCT was seen in the pairwise (HR 0·78, 95% CI 0·62–0·99; high quality) and network (HR 0·82, 95% CI 0·72–0·93; high quality) meta-analyses, with similar results before (HR 0·60, 95% CI 0·40–0·91) and after (HR 0·44, 95% CI 0·25–0·77) matching in the cohort study, leading to a significantly increased 5-year OS rate in both adenocarcinoma and SCC before and after matching. The increased benefits from NCT or NCRT were not associated with the risk of 30-day or in-hospital mortality.
Interpretation
NewEC Study provided high-quality evidence supporting the survival benefits of NCRT or NCT over surgery alone, with NCRT presenting the greatest benefit for resectable esophageal carcinoma.
Funding
National Science and Technology Major Project, the National Natural Science Foundation of China, the Natural Science Foundation of Guangdong Province, the Guangzhou Science and Technology Major Program, the Medical artificial intelligence project of Sun Yat-Sen Memorial Hospital, the Guangdong Science and Technology Department, the Guangdong Province Medical Scientific Research Foundation, and Guangdong Provincial People's Hospital Intermural Program.
Keywords: Resectable esophageal carcinoma, Neoadjuvant chemotherapy, Neoadjuvant, Chemoradiotherapy, Surgery, Clinical evidence
Research in context.
Evidence before this study
We searched for “esophageal cancer”, “chemotherapy”, “surgery”, “chemoradiotherapy”, “neoadjuvant therapy”, and "randomized clinical trials" in public databases published from the inception to May 2019. Neoadjuvant chemotherapy (NCT) or chemoradiotherapy (NCRT) has been shown to be better than surgery alone in patients with resectable esophageal carcinoma, but higher quality evidence is needed as multiple new findings have emerged regarding this issue. Additionally, previous evidence-based findings and current guidelines have not established an advantage of NCRT over NCT and the feasibility to use short-term outcomes such as disease-free survival (DFS) as a surrogate for overall survival (OS).
Added value of this study
This updated meta-analysis involved 6,993 patients with resectable esophageal carcinoma, representing an estimated sample size increase of 40·1% compared with the 4,188-patient sample size in a previous meta-analysis. Compared with surgery, either NCRT or NCT was associated with longer OS. Compared with NCT, NCRT resulted in a significantly improved OS; treatment effects were shown in both adenocarcinoma and squamous cell carcinoma (SCC), and in both patients with high-risk scores and low-risk scores. There was no significant difference among NCRT, NCT, and surgery alone in terms of the 30-day postoperative or in-hospital mortality. Additionally, the effect on DFS was strongly associated with that on OS in trials investigating NCRT or NCT versus surgery alone.
Implications of all the available evidence
First, this study provided the most comprehensive high-quality evidence to date to strengthen the recommendation for both NCT and NCRT as better choices than surgery alone in patients with resectable oesophagus carcinoma. Second, we showed a substantial improvement of survival as well as a favorable safety profile with NCRT compared with NCT, for either adenocarcinoma or SCC, and for either patients with high or low risk of death. These findings further support the superiority and generalizability of the use of NCRT in resectable esophagus carcinoma. Third, this is the first study to clarify that DFS could serve as a potential surrogate endpoint for OS when investigating NCRT or NCT versus surgery alone in resectable esophageal carcinoma.
Alt-text: Unlabelled box
1. Introduction
Esophageal carcinoma is the sixth leading cause of cancer-related mortality worldwide and is more prevalent in developing nations, such as China [1]. Even after surgery, survival remains poor in patients with resectable esophageal cancer, and the 5-year survival rate is only 25–35% [2]. Previous randomized clinical trials (RCTs) have reported that neoadjuvant chemoradiotherapy (NCRT) and neoadjuvant chemotherapy (NCT) have greater clinical benefit than surgery alone among patients with resectable esophageal carcinoma [3,4]. However, as additional findings have emerged from recent studies investigating neoadjuvant interventions [5], [6], [7], for instance, the regimen comprising cisplatin and vinorelbine [5], an updated analysis is needed to confirm the beneficial role of NCRT or NCT.
Moreover, previous evidence-based findings and the current guidelines have established neither a clear survival advantage of NCRT over NCT nor an acceptable safety profile of the addition of radiotherapy to NCT [8], [9], [10], [11]. As to esophageal adenocarcinoma, a meta-analysis revealed no significant survival difference between NCRT and NCT in direct comparison based on only two trials with no more than 100 patients in each group; [8] furthermore, a similar treatment effect was observed between NCRT and NCT in recent trials carried out by Klevebro et al [12] and Stahl et al. [13] Although squamous cell carcinoma (SCC) patients seem to be more sensitive to NCRT, the definitive superiority of NCRT in SCC is still unclear due to inconsistent results found in recent trials [3,7,12]. Therefore, high-quality studies are warranted to further clarify whether NCRT has superior clinical benefits over NCT in resectable esophageal carcinoma, specifically adenocarcinoma or SCC of the esophagus.
This NewEC Study aimed to perform an updated comprehensive meta-analysis of RCTs to evaluate the efficacy and safety of NCRT versus NCT versus surgery in resectable esophageal carcinoma. Next, we would further investigate the comparison between NCRT and NCT using cohort data.
2. Methods
2.1. Study design and patients
2.1.1. Meta-analysis
This study included a meta-analysis of RCTs and an individual patient analysis of cohorts. The meta-analysis was conducted according to the Cochrane Collaboration recommendations and PRISMA statement. We searched PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov for randomized clinical trials (RCTs) up to May 2019 using the following terms: “esophageal cancer”, “chemotherapy”, “surgery”, “chemoradiotherapy”, and “neoadjuvant therapy”. The proceedings of the American Society of Clinical Oncology, European Society for Medical Oncology and American Society for Therapeutic Radiology and Oncology as well as the references in the included RCTs and relevant meta-analyses were also reviewed manually.
For inclusion, RCTs had to evaluate the efficacy and safety of NCRT or NCT followed by surgery versus surgery alone or NCRT versus NCT as the primary schedule among patients with esophageal carcinoma or gastroesophageal junction carcinoma. Trials involving patients who had histologically proven adenocarcinoma or SCC of the stomach or lower third of the esophagus but did not separate the available data for esophageal cancer patients were excluded. We excluded studies whose abstracts or full texts were not in English and studies that did not have available data. Three investigators (S-PZ, A-LL, Y-YF) screened the titles and abstracts to choose relevant studies, and the eligibility of the studies that seemed to meet the inclusion criteria was confirmed by a full-text review. The data collected included the recruitment period, sample size, follow-up time, treatment group allocation, details regarding the chemotherapy and radiotherapy regimens, and patient and tumor characteristics. Full details methods are described in Supplemental Methods 1.
2.1.2. Cohort study
The retrospective cohort was reported according to the CONSORT guideline, STROBE statement and TRIPOD guideline. The cohort analyzed 423 individual patients at Massachusetts General Hospital (Massachusetts, USA) or Guangdong Provincial People's Hospital (Guangzhou, China) who underwent NCRT or NCT between November 2000 and June 2017 to estimate the benefit of NCRT versus NCT in patients with resectable esophageal carcinoma. Full description of cohort study is provided in Supplemental Methods 2.
2.2. End point definitions
The primary endpoint was overall survival (OS). The secondary end points included disease-free survival (DFS), R0 resection rate, pathologic complete response (pCR) and 30-day postoperative or in-hospital mortality. OS was calculated as the time from the date of the histologically documented diagnosis to the date of death or final follow-up. DFS was calculated from the date of R0 resection to the date of disease recurrence or death from any cause. R0 resection was defined as gross disease removed with negative margins (tumor-free resection margin). Incomplete resection (R1) was defined as residual gross disease or positive surgical margins (tumor removal ≤1 mm from any margin). pCR was defined as no evidence of residual tumor cells in the primary tumor site or resected lymph nodes. The clinical response rate after neoadjuvant treatment was evaluated by a CT of the chest and abdomen based on the modified Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 guidelines.
2.3. Statistical analysis
2.3.1. Meta-analysis
We initially conducted a pairwise meta-analysis of all direct treatment comparisons, and next we conducted a random-effects Bayesian network meta-analysis at a frequentist setting to further estimate the benefit of NCRT compared with that of NCT [14,15]. The network meta-analysis assumed that treatment effects were consistent across all included trials and the heterogeneity was common within networks, which meant the relative treatment effect of treatment A versus treatment B can be indirectly obtained from the comparisons of treatment A with treatment C and treatment B with treatment C [16]. We evaluated network consistency by comparing the direct and indirect estimates for each comparison [17]. The level of loop and design inconsistencies were addressed using a global inconsistency test in the design-by-treatment interaction model [18]. The treatment effect on the time-to-event outcome was estimated by the hazard ratio (HR) with 95% confidence interval (CI), and the dichotomous outcomes were evaluated by the risk ratio (RR) and risk difference (RD). Prespecified subgroup analyses were performed to examine the effects of NCT or NCRT according to tumor histology (SCC or adenocarcinoma), the timing of chemotherapy and radiotherapy (concurrent or sequential), and the chemotherapy regimen (platinum plus taxanes or platinum plus fluorouracil). The I2 statistic was used to assess the heterogeneity across the trials, and I2≥50% indicated substantial heterogeneity. All analyses were performed on the basis of the intention-to-treat principle when possible. A trial sequential analysis (TSA) was used to decide whether a trial could be terminated early, and indicates whether a P value is sufficient to indicate a reliable effect for the benefit, harm, or futility before the required information size is reached. Pearson correlation coefficients (ρ) and the coefficient of determination (R2) of a weighted linear regression model was used to estimate the strength of the potential correlation between different clinical outcomes. Full methodologies are described in Supplemental Methods 3.
2.3.2. Cohort study
We first used Power Analysis and Sample Size software version 15 to conduct an a priori power analysis based on the 5-year survival rate to estimate the sample size, approximating a sample ratio of patients in the NCRT group to patients in the NCT group of 3:1, an α significance level of 0·05, a power of 80%, a 5-year survival rate of 45% in the NCRT cohort, and a 15% difference between the NCRT and NCT groups (5-year survival, 45% versus 30%) pooled from three trials [7,13,19]. This estimation was based on the results of the meta-analysis of RCTs comparing NCRT with NCT in resectable esophageal carcinoma patients. Using these parameters, at least 409 patients (307 in the NCRT group and 102 in the NCT group) were required.
Propensity score matching based on neural network machine learning was performed using R package MatchIt, which was used to optimally reduce the treatment assignment bias caused by effects of potential confounders by making baseline characteristics more comparable between the NCRT and NCT groups [20,21]. Unlike conventional propensity score matching approach using logistic regression, neural network has no causal interpretation, presents a mixed function of the input data, and could achieve less bias or greater accuracy [21]. We built a propensity score model based on covariates including gender, post-neoadjuvant pathologic TNM stage, tumor location, and histology type, and calculated a propensity score for each patient. The NCRT and NCT individuals within each stratum of the data were matched using 2:1 matching protocol without replacement, using a nearest-neighbor algorithm with caliper width equal to 0.2. We constructed a model to predict the OS following post-neoadjuvant interventions, with the aim of evaluating NCRT versus NCT in patient subset with high-risk or that with death or low-risk of death. The prognostic accuracy was assessed by using operating characteristic curve analysis and by calculating the area under the curves. Decision curve analysis was performed to assess the clinical utility of the prediction model by quantifying the net benefits when different threshold probabilities were considered.
The x2 or Fisher's exact test was used to compare categorical variables. The Kaplan-Meier method was used to estimate median OS and generate survival curves, and survival was compared using the log-rank test and the restricted mean survival time ratio (RMSTR) of NCRT arm to NCT arm [22]. The HRs with their 95% CIs and the corresponding P-values from these analyses were estimated with a Cox regression analysis. Univariate and multivariate analyses were performed with the Cox proportional hazards model to investigate the effect of different factors on survival. All statistical tests were two-sided, and P values less than 0·05 were considered statistically significant. The statistical analyses were performed using R version 3.4.3. Full methodologies are described in Supplemental Methods 4.
The meta-analysis is registered with PROSPERO CRD42017072242 and the cohort study is registered with ClinicalTrials.gov. NCT04027543.
3. Results
3.1. Trials and patients’ characteristics
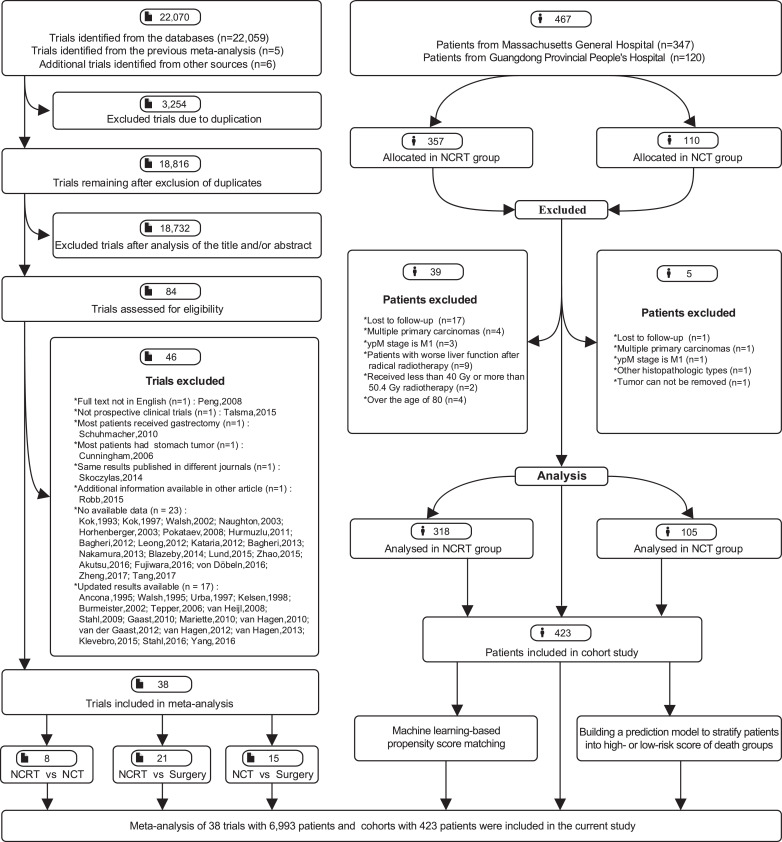
Of 22,070 studies assessed, 38 RCTs [[3], [4], [5], [6], [7],12,13,19,[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52]] comprising 6,993 patients were eligible to be included in meta-analysis (Fig. 1); 18 RCTs compared NCRT with surgery alone, 12 RCTs compared NCT with surgery alone, 5 RCTs compared NCRT with NCT, and 3 RCTs (two 2 × 2 factorial studies and one three-arm study) simultaneously compared the effects of NCRT, NCT and surgery. Twenty and seven RCTs included patients with SCC and adenocarcinoma, respectively, eleven enrolled patients with either SCC or adenocarcinoma, and one had no histological data. Most trials had a low risk of bias (Supplemental Figures 1–2). The characteristics of each trial are summarized in Supplemental Table 1.
Fig. 1.
Flowchart of study selection and design. NCRT, neoadjuvant chemoradiotherapy; NCT, neoadjuvant chemotherapy.
Of 467 patients screened, 423 patients were eligible to be recruited in cohort study (Fig. 1), including 318 (75·2%) patients who underwent NCRT and 105 (24·8%) patients who received NCT; the total number exceeded the estimated required sample size. We identified 192 patients, including 113 individuals from the NCRT group (48 [42·5%] adenocarcinoma and 65 [57·5%] SCC) and 79 individuals from the NCT group (25 [31·6%] adenocarcinoma and 54 [68·4%] SCC) via machine learning-based propensity score matching. The demographic features are detailed in Supplemental Table 2; the baseline bias was largely reduced after matching.
3.2. NCT with higher efficacy than surgery alone in RCTs
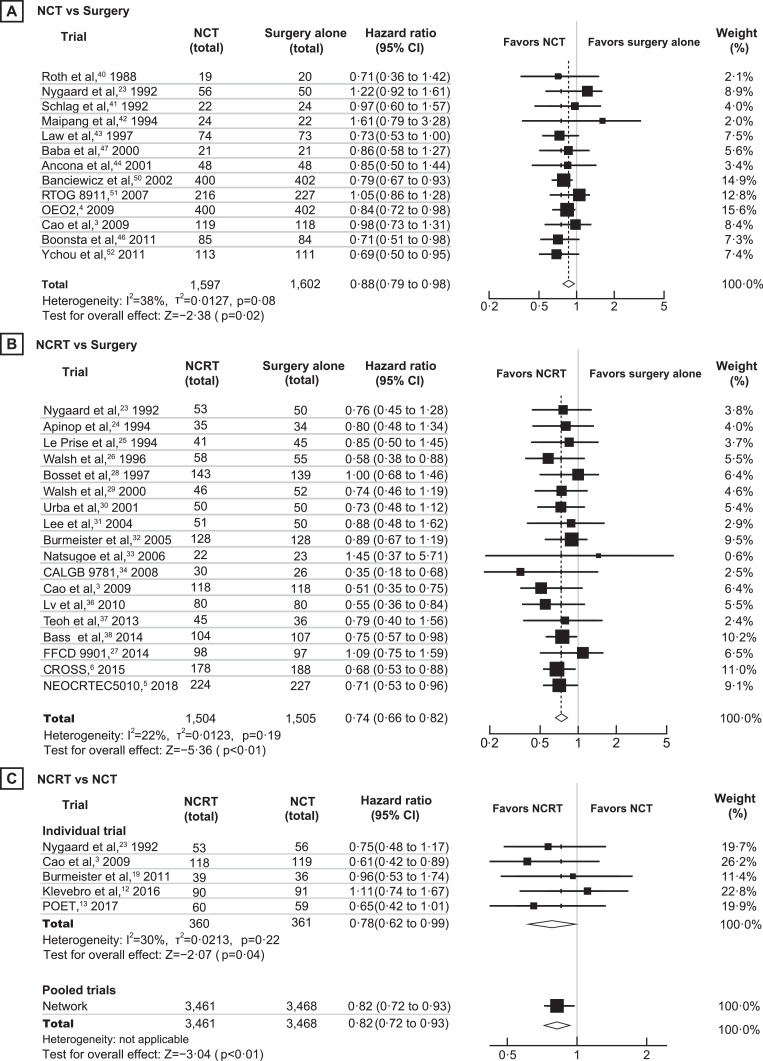
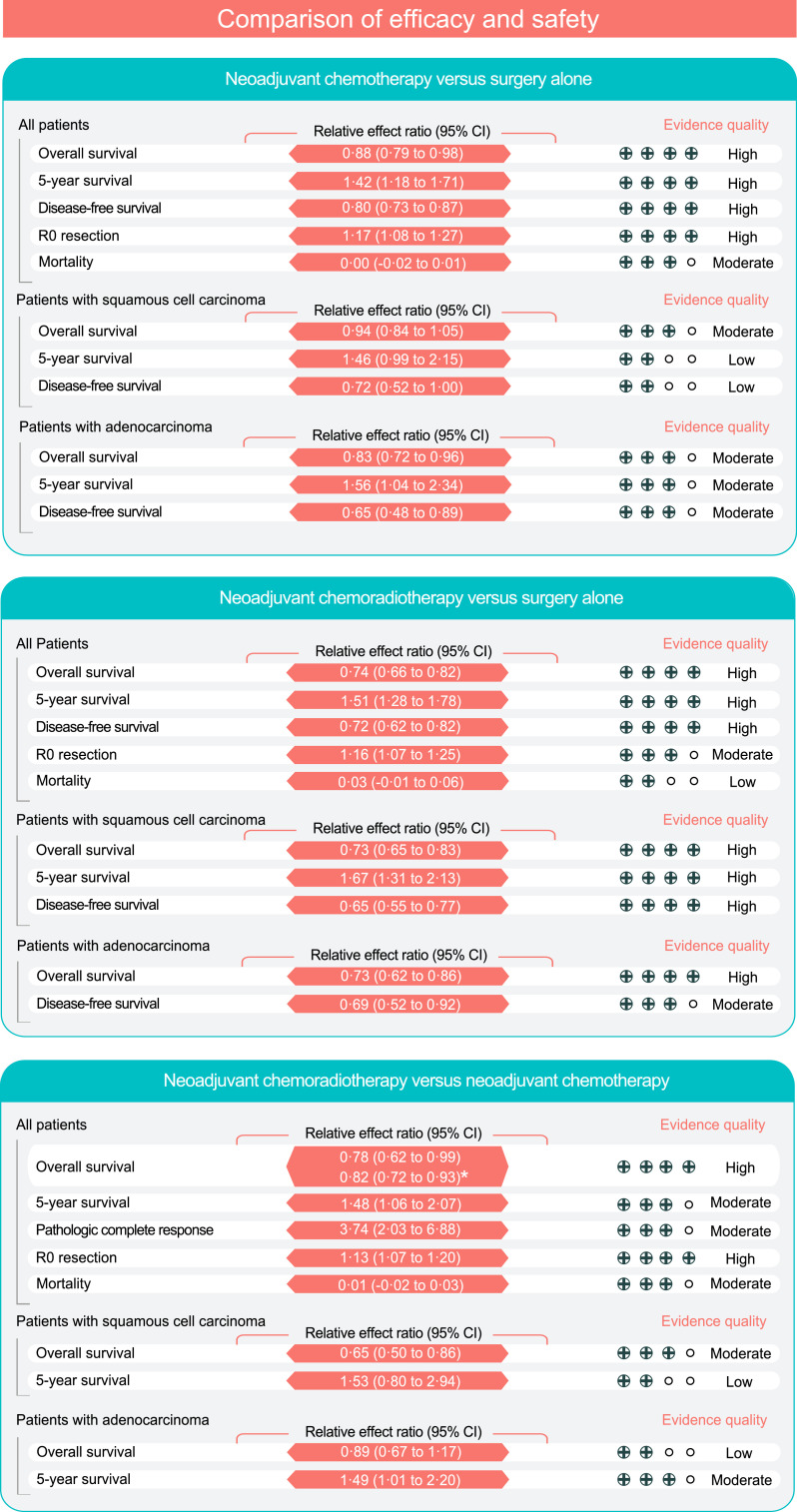
Fifteen RCTs [3,4,7,23,[40], [41], [42], [43], [44], [45], [46], [47],[50], [51], [52]] involving 3,343 patients were included in the comparison of NCT with surgery. Compared with surgery, NCT significantly improved OS (HR 0·88, 95% CI 0·79–0·98, P=0·02; high quality), DFS (HR 0·80, 95% CI 0·73–0·87, P<0·01; high quality) and the R0 resection rate (RR 1·17, 95% CI 1·08–1·27, P<0·01; high quality) (Figs. 2A, 3 and Supplemental Figure 3). The 5-year OS rate was 27·9% with NCT and 19·7% with surgery (RR 1·42, 95% CI 1·18–1·71, P<0·01; high quality). TSA indicated that additional trials were unlikely to alter the outcomes of the R0 resection and 5-year OS rates (Supplemental Figure 4A and 5A). Adenocarcinoma patients who received NCT showed significantly better OS (HR 0·83; 95% CI 0·72–0·96, P=0·012) and 5-year OS rate (RR 1·56, 95% CI 1·04–2·34, P=0·030) than those who underwent surgery alone, but there was no clear difference between the two treatments in patients with SCC (Fig. 3 and Supplemental Table 3).
Fig. 2.
Meta-analysis results of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy versus surgery alone for overall survival. A, Neoadjuvant chemotherapy versus surgery alone. B, Neoadjuvant chemoradiotherapy versus surgery alone. C, Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy. The total number shown in the figure referred to number of patients with valid OS data. NCRT, neoadjuvant chemoradiotherapy; NCT, neoadjuvant chemotherapy; CI, confidence interval.
Fig. 3.
Summary of the pooled estimates and GRADE of efficacy and safety in the meta-analysis. GRADE indicates Grading of Recommendations, Assessment, Development, and Evaluation Evidence. Mortality indicates 30-day postoperative or in-hospital mortality. CI, confidence interval. *The results of Bayesian network meta-analysis.
3.3. NCRT with higher efficacy than surgery alone in RCTs
Twenty-one RCTs [3,[5], [6], [7],[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39]] involving 3,138 patients were included in the comparison of NCRT with surgery. Compared with surgery alone, NCRT was associated with increased OS (HR 0·74, 95% CI 0·66–0·82, P<0·01; high quality), DFS (HR 0·72, 95% CI 0·62–0·82, P<0·01; high quality), 3-year OS (RR 1·28, 95% CI 1·09–1·49, P<0·01; moderate quality), 5-year OS (RR 1·51, 95% CI 1·28–1·78, P<0·01; high quality), and R0 resection rate (RR 1·16, 95% CI 1·07 to 1·25, P<0·01; moderate quality) (Fig. 2B, Fig. 3 and Supplemental Figure 6). The TSA analysis suggested conclusive evidence of the R0 resection and 5-year OS rates (Supplemental Figures 4B and 5B).
Among patients with adenocarcinoma, NCRT resulted in significant improvements in OS (HR 0·73, 95% CI 0·62–0·86, P=0·000), the 3- year OS rate (RR 6·01, 95% CI 1·88–19·17, P=0·003), and DFS (HR 0·69, 95% CI 0·52–0·92, P=0·011). The advantage of NCRT among SCC was seen with respect to OS (HR 0·73, 95% CI 0·65–0·83, P=0·000), the 3-year OS rate (RR 1·24, 95% CI 1·12–1·38, P=0·000), the 5-year OS rate (RR 1·67, 95% CI 1·31–2·13, P=0·000), and DFS (HR 0·65, 95% CI 0·55–0·77, P=0·000) (Fig. 3 and Supplemental Table 4).
Concurrent radiotherapy resulted in significant improvements in OS (HR 0·72, 95% CI 0·63–0·81, P=0·000) and the 5-year OS rate (RR 1·51, 95% CI 1·29–1·77, P=0·000) in NCRT versus surgery alone, but there was no significant benefit of NCRT with sequential radiotherapy. The OS benefits were observed in regimens containing platinum plus taxanes (HR 0·64, 95% CI 0·52–0·80, P=0·000), with an increased 5-year OS rate (RR 1·39, 95% CI 1·12–1·72, P=0·003), and in regimens containing platinum plus fluorouracil (HR 0·79, 95% CI 0·69–0·90, P=0·000), with an increased 5-year OS rate (RR 1·65, 95% CI 1·32–2·08, P=0·000) (Supplemental Table 4).
3.4. NCRT with higher efficacy than NCT in RCTs
Eight RCTs [3,7,12,13,19,23,48,49] involving 1,030 patients were included in the comparison of NCRT with NCT. Compared with NCT, NCRT was associated with increased OS (HR 0·78, 95% CI 0·62–0·99, P=0·04; high quality), 5-year OS rate (RR 1·48, 95% CI 1·06–2·07, P=0·02; moderate quality), R0 resection rate (RR 1·13, 95% CI 1·07–1·20, P<0·01; high quality), and pCR (RR 3·74, 95% CI 2·03–6·88, P<0·01; moderate quality) (Figs. 2C, 3 and Supplemental Figure 7). The benefit on the R0 resection rate was robust in the TSA (Supplemental Figure 4C). NCRT significantly improved OS in SCC (HR 0·65, 95% CI 0·50–0·86, P=0·002) and 5-year OS rate in adenocarcinoma (RR 1·49, 95% CI 1·01–2·20, P=0·047) (Fig. 3 and Supplemental Table 5).
Next, we conducted a network meta-analysis to compare 3,461 patients who received NCRT with 3,468 patients who received NCT. The pooled estimate yielded a significant enhancement in OS in favor of NCRT (HR 0·82, 95% CI 0·72–0·93, P=0·002; high quality; Fig. 3 and Supplemental Table 5).
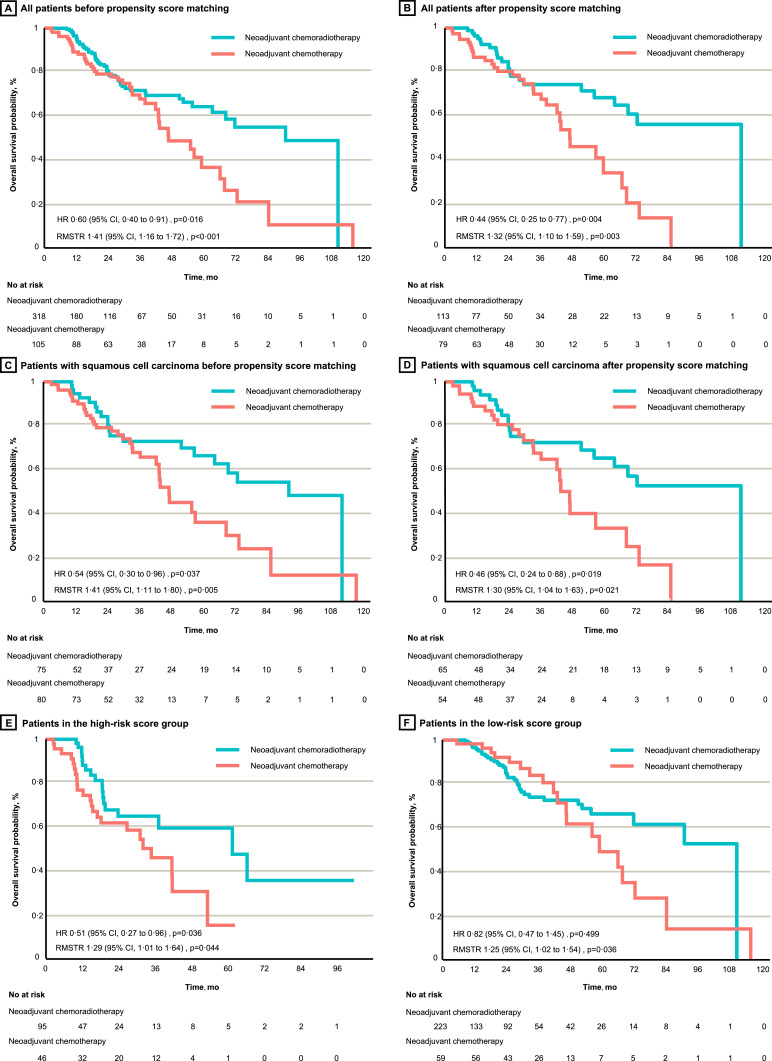
3.5. NCRT with higher OS than NCT before and after matching in cohort study
In the cohort study, NCRT was associated with a significant improvement in OS (HR 0·60, 95% CI 0·40–0·91, P=0·016; RMSTR 1·41, 95% CI 1·16–1·72, P<0·001; Fig. 4A) compared with NCT before matching. Significant improvements were found in the 1-year (RR 1·08, 95% CI 1·01–1·06, P=0·024) and 5-year (RR 1·77, 95% CI 1·36–2·31, P<0·001) OS rates (Table 1). In the matched cohort, the OS advantage of NCRT over NCT reached a greater extent (HR 0·44, 95% CI 0·25–0·77, P=0·004; RMSTR 1·32, 95% CI 1·10–1·59, P=0·003; Fig. 4B). The differences in the 1-year and 5-year OS rates were also significant after matching (Table 1).
Fig. 4.
Overall survival of neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy in individual patient-level cohort study. A and B, All patients before and after propensity score matching, respectively. C and D, Patients with squamous cell carcinoma before and after propensity score matching, respectively. E and F, Patients with high-risk and low-risk scores for death, respectively. HR, hazard ratio; CI, confidence interval; RMSTR, restricted mean survival time ratio.
Table 1.
Outcomes of neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy groups in the cohort study.
| Variable | Before matchinga |
After matchingb |
High-risk score groupc |
Low-risk score groupd |
||||
|---|---|---|---|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | RR (95% CI) | P value | RR (95% CI) | P value | |
| All patients | ||||||||
| R0 resection: | 1·00 (0·95 to 1·05) | 1·000 | 0·99 (0·92 to 1·06) | 0·788 | 1·04 (0·95 to 1·13) | 0·396 | 0·98 (0·93 to 1·04) | 0·450 |
| ypN0 | 1·50 (1·15 to 1·95) | 0·003 | 1·31 (0·94 to 1·82) | 0·111 | 2·42 (1·17 to 5·03) | 0·017 | 1·17 (0·91 to 1·51) | 0·221 |
| ypN+ | 0·68 (0·55 to 0·84) | <0·001 | 0·76 (0·57 to 1·02) | 0·062 | 0·77 (0·61 to 0·96) | 0·028 | 0·73 (0·51 to 1·04) | 0·085 |
| Survival rate: | ||||||||
| 1-year | 1·08 (1·01 to 1·16) | 0·024 | 1·12 (1·02 to 1·23) | 0·018 | 1·26 (1·06 to 1·49) | 0·009 | 0·98 (0·94 to 1·02) | 0·342 |
| 3-year | 1·03 (0·89 to 1·19) | 0·692 | 1·07 (0·89 to 1·28) | 0·472 | 1·31 (0·94 to 1·80) | 0·111 | 0·87 (0·76 to 1·00) | 0·044 |
| 5-year | 1·77 (1·36 to 2·31) | <0·001 | 1·99 (1·43 to 2·78) | <0·001 | 3·94 (1·95 to 7·95) | <0·001 | 1·35 (1·02 to 1·78) | 0·036 |
| Patients with adenocarcinoma | ||||||||
| R0 resection: | 1·08 (0·93 to 1·25) | 0·313 | 1·66 (1·16 to 2·39) | 0·006 | 1·25 (0·88 to 1·78) | 0·213 | 1·00 (0·88 to 1·15) | 1·000 |
| ypN0 | 1·78 (0·96 to 3·32) | 0·067 | 1·22 (0·70 to 2·11) | 0·483 | 7·04 (0·47 to 106·34) | 0·158 | 1·40 (0·79 to 2·46) | 0·249 |
| ypN+ | 0·63 (0·46 to 0·88) | 0·004 | 2·61 (1·21 to 5·64) | 0·014 | 0·78 (0·52 to 1·16) | 0·230 | 0·66 (0·39 to 1·13) | 0·122 |
| Survival rate: | ||||||||
| 1-year | 1·21 (1·00 to 1·48) | 0·050 | 1·19 (1·04 to 1·36) | 0·011 | 3·80 (1·31 to 11·01) | 0·014 | 0·97 (0·94 to 0·99) | 0·057 |
| 3-year | 0·88 (0·72 to 1·09) | 0·212 | 0·96 (0·80 to 1·15) | 0·661 | 2·98 (0·87 to 10·23) | 0·082 | 0·73 (0·66 to 0·80) | <0·001 |
| 5-year | 1·99 (1·12 to 3·56) | 0·019 | 2·35 (1·58 to 3·50) | <0·001 | 2·60 (0·75 to 8·96) | 0·132 | 1·52 (0·86 to 2·67) | 0·150 |
| Patients with squamous cell carcinoma | ||||||||
| R0 resection: | 0·98 (0·93 to 1·04) | 0·450 | 0·97 (0·91 to 1·04) | 0·350 | 0·99 (0·89 to 1·09) | 0·853 | 0·98 (0·92 to 1·06) | 0·531 |
| ypN0 | 1·56 (1·13 to 2·14) | 0·007 | 0·74 (0·51 to 1·10) | 0·113 | 1·98 (0·85 to 4·61) | 0·113 | 1·25 (0·92 to 1·69) | 0·154 |
| ypN+ | 0·61 (0·43 to 0·88) | 0·006 | 1·25 (0·86 to 1·80) | 0·242 | 0·74 (0·51 to 1·09) | 0·113 | 0·60 (0·32 to 1·10) | 0·111 |
| Variable | RR (95% CI) | P value | RR (95% CI) | P value | RR (95% CI) | P value | RR (95% CI) | P value |
| Survival rate: | ||||||||
| 1-year | 1·04 (0·95 to 1·13) | 0·396 | 1·22 (1·00 to 1·49) | 0·050 | 1·04 (0·85 to 1·28) | 0·703 | 1·00 (0·95 to 1·06) | 1·000 |
| 3-year | 1·09 (0·89 to 1·33) | 0·405 | 0·99 (0·78 to 1·26) | 0·934 | 1·16 (0·75 to 1·78) | 0·505 | 0·97 (0·78 to 1·20) | 0·784 |
| 5-year | 1·84 (1·32 to 2·56) | <0·001 | 2·47 (1·37 to 4·46) | 0·003 | 3·85 (1·74 to 8·54) | <0·001 | 1·41 (0·98 to 2·01) | 0·064 |
The x2 exact test was used to obtain the P values. RR=risk ratio; CI=confidence interval; pCR=pathologic complete response; yp=postneoadjuvant pathologic; N0=no regional lymph node metastasis; N+=metastasis in regional lymph nodes; NR=not reached.
Study patients before machine learning-based propensity score matching.
Study patients after machine learning-based propensity score matching.
Study patients with a high-risk score in our prediction model.
Study patients with a low-risk score in our prediction model.
Adenocarcinoma patients treated with NCRT had significantly a higher 5-year OS rate than those who received NCT both before matching (RR 1·99, 95% CI 1·12–3·56, P=0·019) and after matching (RR 2·35, 95% CI 1·58–3·50, P<0·001) (Table 1). The OS advantage of NCRT was identified in SCC patients before matching (HR 0·54, 95% CI 0·30–0·96, P=0·037; RMSTR 1·41, 95% CI 1·11–1·80, P=0·005; Fig. 4C) and after matching (HR 0·46, 95% CI 0·24–0·88, P=0·019; RMSTR 1·30, 95% CI 1·04–1·63, P=0·021; Fig. 4D), leading to an increased 5-year OS rate both before matching (RR 1·84, 95% CI 1·32–2·56, P<0·001) and after matching (RR 2·47, 95% CI 1·37–4·46, P=0·003) (Table 1).
Before matching, NCRT was associated with higher OS rate than NCT in various subgroups, including patients aged over 60 years (HR 0·49, 95% CI 0·26–0·92), male patients (HR 0·59, 95% CI 0·38–0·93), patients who underwent R0 resection (HR 0·61, 95% CI 0·40–0·93), patients with an objective response to treatment (HR 0·38, 95% CI 0·22–0·67), and patients with an ECOG-PS higher than 2 (HR 0·15, 95% CI 0·04–0·55). These findings were consistent with the results after matching (Supplemental Figure 8).
3.6. NCRT with higher OS than NCT of high and low-risk score of death group in cohort study
We built a prediction model incorporating post-neoadjuvant pathologic tumor-node-metastasis (TNM) stage, age, best response to treatment, tumor location, and ECOG-PS prognostic factors to categorize patients into groups with high- or low- risk score of death, which could better predict OS after neoadjuvant intervention than the TNM staging system. The construction and validation of the prediction model are detailed in Section 2, Supplemental Figures 9-10 and Tables 6-7.
Patients with high-risk scores obtained significant OS benefits from NCRT over NCT (HR 0·51, 95% CI 0·27–0·96, P=0·036; RMSTR 1·29, 95% CI 1·01–1·64, P=0·044; Fig. 4E). The OS HR of NCRT versus NCT in the low-risk score group was not significant (0·82, 95% CI 0·47–1·45, P=0·499; Fig. 4F); however, after adopting RMSTR, which is a more robust quantitative method, a significant difference was detected (1·25, 95% CI 1·02–1·54, P=0·036). The NCRT group had a larger proportion of low-risk patients than did the NCT group (70·1% versus 56·2%; Supplemental Figure 11A). Compared with NCT, NCRT resulted in an increased 5-year OS rate in both the high-risk (HR 3·94, 95% CI 1·95–7·95, P<0·001) and low-risk (HR 1·35, 95% CI 1·02–1·78, P=0·036) groups, and difference in the 5-year OS rate was specifically seen among SCC patients with high-risk scores (RR 3·85, 95% CI 1·74–8·54, P<0·001 (Table 1). As expected, the high-risk group had a larger proportion of SCC patients than did the low-risk group (43·3% versus 33·3%; Supplemental Figure 11B).
3.7. Benefit of NCRT or NCT was not offset by higher mortality
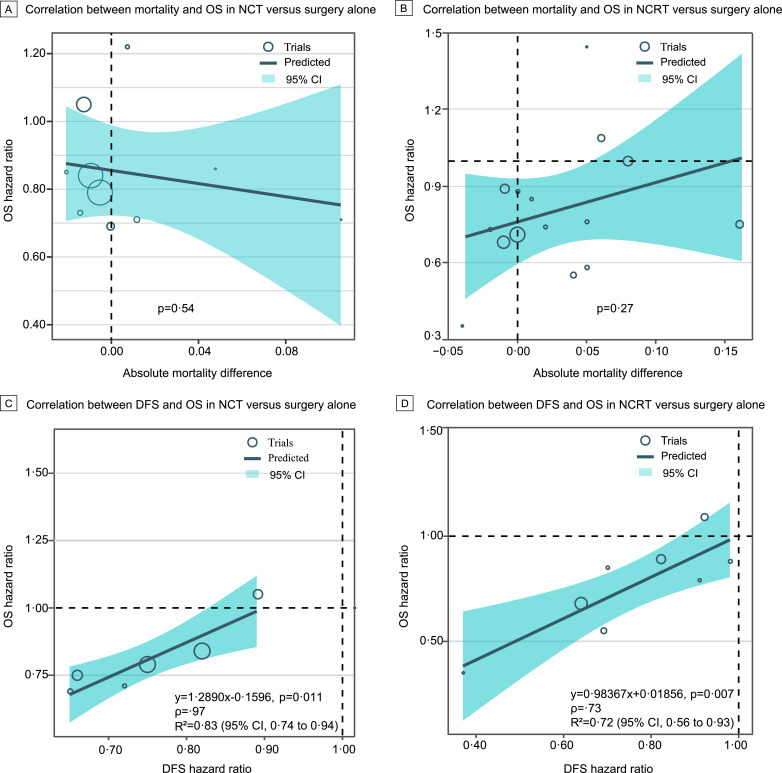
In the meta-analysis, we examined whether the benefit of NCRT or NCT was offset by a higher mortality rate. The 30-day postoperative or in-hospital mortality rate was 7·7% with NCT versus 8·0% with surgery alone, with an RD of -0·00 (95% CI -0·02–0·01, P=0·73); 7·3% with NCRT versus 3·9% with surgery alone, with an RD of 0·03 (95% CI -0·01–0·06, P=0·11); and 4·5% with NCRT versus 2·4% with NCT, with an RD of 0·01 (95% CI -0·02–0·03, P=0·44) (Fig. 3 and Supplemental Figure 12). There was no association between the OS benefits of the neoadjuvant treatments and the risk of 30-day postoperative or in-hospital mortality (Fig. 5A–B).
Fig. 5.
Association of overall survival with mortality and disease-free survival. A and B, Weighted linear correlation between overall survival and 30-day postoperative or in-hospital mortality for neoadjuvant chemotherapy versus surgery alone and neoadjuvant chemoradiotherapy versus surgery alone, respectively. C and D, Same as A and B but describing the correlation between overall survival and disease-free survival. OS, overall survival; DFS, disease-free survival; CI, confidence interval. Mortality indicates 30-day postoperative or in-hospital mortality.
3.8. DFS was associated with OS in NCRT or NCT versus surgery alone
We further explored the potential surrogate endpoints for OS in the meta-analysis. The association between DFS and OS in NCT versus surgery alone gave rise to a high R2 value of 0·83 (95% CI 0·74–0·94; Fig. 5C), which yielded the regression equation as follow: OS=1·2890 × DFS-0·1596. DFS was also strongly associated with OS in NCRT (based on regimens comprising platinum plus fluorouracil or taxanes) versus surgery alone (R2=0·72, 95% CI 0·56–0·93; Fig. 5D), which yielded the regression equation as follow: OS=0·98367 × DFS+0·01856. However, we did not observe any significant correlation between the R0 resection rate and the other outcomes (Supplemental Figure 13).
4. Discussion
This study performed a meta-analysis of 38 RCTs involving 6,993 patients, which is based on the largest sample size to date, with an estimated sample size increase of 40·1% compared with the sample size in a previous meta-analysis (4,188 patients) [8]. The GRADE system indicated high-quality evidence in favor of both NCRT and NCT over surgery alone and indicated moderate to high evidence showing that compared with NCT, NCRT significantly prolonged survival, and increased the R0 and pCR rates among the patients with resectable esophageal carcinoma. The survival advantage of NCRT over NCT was further confirmed in cohort study at the individual patient level.
In patients with esophageal adenocarcinoma, our meta-analysis showed a trend towards longer OS following NCRT than following NCT, and the HR of NCRT versus surgery alone (HR 0·73, 95% CI 0·62–0·86; high quality) was better than that of NCT versus surgery alone (0·83, 0·72–0·96; moderate quality). Moreover, the meta-analysis, coupled with cohort data demonstrated that compared to NCT, NCRT improves the 5-year OS rate in adenocarcinoma. Researchers designing new studies involving neoadjuvant treatments for resectable esophageal carcinoma should guarantee an increased recruitment of adenocarcinoma patients to further validate our findings.
Among the SCC patients, our meta-analysis provided high-quality evidence of a strikingly improved OS associated with NCRT compared with surgery alone, but this benefit was not observed among patients receiving NCT. The superiority of NCRT in SCC was further supported in the direct comparison of NCRT with NCT, which provided moderate quality evidence of the survival benefit in favor of NCRT. The cohort study suggested that in the SCC patients, NCRT significantly enhanced both OS and the 5-year OS rate compared with those of NCT, and this survival advantage was specifically identified in those who were concurrently graded as a high-risk score of death. Our study strengthens the evidence of the superior efficacy of NCRT in patients with SCC and highlights that clinicopathologic prediction model would aid in the selection of SCC patients.
We considered the possibility that any survival gains from adding neoadjuvant intervention to surgery or adding radiotherapy to preoperative chemotherapy might be offset by postoperative mortality. A previous meta-analysis revealed a negligible association between the risk of postoperative mortality and neoadjuvant interventions in resectable esophageal carcinoma [8]. Our updated meta-analysis further confirmed no significant difference among NCRT, NCT, and surgery alone in terms of the 30-day postoperative or in-hospital mortality, and the increased survival benefit conferred by NCRT or NCT was not associated with the risk of 30-day postoperative or in-hospital mortality in a linear regression model. Recent work has suggested rather than 30-day mortality, 90-day mortality might be more robust and firmer in the evaluation of the safety of trimodal therapy, particularly in the elderly; [53,54] therefore, future researches are needed to focus on different safety outcomes and more specific age-based population. Moreover, ongoing randomized trials comparing NCRT and NCT (NCT04138212 and NCT03366883) and those comparing different radiation dose of NCRT (NCT03381651) in esophageal cancer would help to explore NCRT with an ideal safety profile over NCT.
Identification of appropriate chemotherapy strategy is essential to improve the treatment effect and to minimize the incidence of adverse events. The phase III FLOT4-AIO trial demonstrated that compared with fluorouracil or capecitabine plus cisplatin and epirubicin, a combination consisting of fluorouracil, leucovorin, oxaliplatin, and docetaxel improved OS, DFS and the R0 resection rate and had no significant difference in treatment-related adverse events among resectable gastroesophageal junction or gastric adenocarcinoma [55]. By contrast, the OEO5 randomized trial investigating NCT in patients with esophageal or gastroesophageal junction adenocarcinoma demonstrated that compared with cisplatin plus fluorouracil, neoadjuvant capecitabine plus either epirubicin or cisplatin was associated with significantly higher toxicity with no meaningful difference in survival [56]. Moreover, the phase III ESO-Shanghai 1 trial evaluated NCRT for IIA-IVA esophageal SCC and showed a similar OS benefit but distinct aspects of side effects between paclitaxel plus fluorouracil and cisplatin plus fluorouracil [57], suggesting that personalized chemotherapy delivery should also provide insight into the difference in safety profile among chemotherapy regimens. In addition, immunotherapy has achieved promising clinical benefits in second-line patients with inoperable esophageal carcinoma or non-small-cell lung cancer; [58,59] whether the combination of immunotherapy and chemotherapy could be an effective neoadjuvant strategy for resectable esophageal cancer is being investigated in ongoing trials (NCT03544736 and NCT04225364).
The strengths of our study come from its comprehensive assessment and validation of efficacy and safety outcomes. First, this study is the first to provide reliable evidence-based findings indicating the survival advantage of NCRT over NCT in resectable esophageal carcinoma, which has not previously been a consensus in current guidelines. Second, we provided a novel prediction model characterizing the risk of death, which was distinctively superior to TNM staging system and demonstrated both patients in the high-risk group and low-risk group could derive significant OS benefits from NCRT versus NCT. These findings further extended the superiority and generalizability of the use of NCRT. Third, in a previous meta-analysis [60], DFS was not confirmed as a good surrogate endpoint for OS in studies investigating neoadjuvant treatment for gastroesophageal cancers. However, that analysis described a highly heterogeneous group of patients treated with multiple neoadjuvant regimens. In our study, which included a larger sample size, we restricted the chemotherapy regimen of NCRT to platinum plus fluorouracil or taxanes and identified a strong correlation between DFS and OS when investigating NCRT versus surgery alone and NCT versus surgery alone. Therefore, we suggest that DFS could be an appropriate surrogate endpoint for OS in such circumstances. We additionally confirmed that the R0 resection was not a valid surrogate for both DFS and OS.
Several limitations affected our interpretation. First, this study had a retrospective nature and was limited by the heterogeneity of patient characteristics and treatments. Diverse patient region, surgical approaches, radiation dosages, and lack of standardization of imaging and staging modalities might obscure the impact of the neoadjuvant treatments, but we were unable to perform corresponding subgroup analyses owing to the limited data. Second, some potential confounders such as patient region were not considered in the construction of the propensity score model. The close similarity in results of the matched and unmatched analyses raised concern that the correlation due to matching may not have been optimally modeled. Third, our data were derived from limited centers; therefore, the generalizability of the findings required extensive validation in boarder populations in future prospective trials. Additionally, our prediction model lacked tumor microenvironment-based variables. Hence, an integrated predictive biomarker based on more signatures, such as the methylation signature and tumor mutation burden should be considered to better guide appropriate neoadjuvant therapy.
In summary, our findings recommended that both NCRT and NCT had greater benefits on survival than does surgery alone and that, compared with NCT, NCRT significantly improved efficacy among patients with resectable esophageal carcinoma. We believe that the results of our study could guide future research and are important for policy and guideline revisions when choosing a treatment for patients with resectable esophageal carcinoma.
Declaration of Competing Interest
We declare no conflicts of interest.
Acknowledgments
Contributors
H-YZ, S-PZ, A-LL, Q-LG, Q-YO, ML, H-RY, and Y-FY conceived and designed the study. All authors analyzed and interpreted the data, and did the statistical analysis. H-YZ and S-PZ had full access to the data in the cohort study and take responsibility for the integrity and accuracy of the cohort data. A-LL, Q-LG, and Y-FY had full access to the data of meta-analysis and take responsibility for the integrity and accuracy of the meta-analysis data. H-YZ, S-PZ, A-LL, Q-LG, Q-YO, D-GL, ML, H-RY, and Y-FY drafted the manuscript, and all authors revised the manuscript. H-YZ, S-PZ, ML, H-RY, and Y-FY provided administrative, technical, and material support. H-YZ, ML, H-RY, and Y-FY provided study supervision. All authors reviewed the manuscript and approved the final version
Consent for publication
Not applicable.
Acknowledgments
This study was supported by grants from the National Science and Technology Major Project (2020ZX09201021), the National Natural Science Foundation of China (81572596, 81972471, U1601223), the Natural Science Foundation of Guangdong Province (2017A030313828, 2018A0303130113), the Guangzhou Science and Technology Major Program (201704020131, 201903010028), the Medical artificial intelligence project of Sun Yat-Sen Memorial Hospital (YXRGZN201902), the Guangdong Science and Technology Department (2017B030314026), the Guangdong Province Medical Scientific Research Foundation (A2015333, B2018148), and Guangdong Provincial People's Hospital Intermural Program (KJ012019447).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100422.
Contributor Information
Michael Lanuti, Email: mlanuti@mgh.harvard.edu.
He-Rui Yao, Email: yaoherui@mail.sysu.edu.cn.
Yun-Fang Yu, Email: yuyf9@mail.sysu.edu.cn.
Appendix. Supplementary materials
References
- 1.Bray F., Ferlay J., Soerjomataram I. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Herskovic A., Russell W., Liptay M. Esophageal carcinoma advances in treatment results for locally advanced disease: review. Ann Oncol. 2012;23:1095–1103. doi: 10.1093/annonc/mdr433. [DOI] [PubMed] [Google Scholar]
- 3.Cao X.F., He X.T., Ji L. Effects of neoadjuvant radiochemotherapy on pathological staging and prognosis for locally advanced esophageal squamous cell carcinoma. Dis Esophagus. 2009;22:477–481. doi: 10.1111/j.1442-2050.2008.00910.x. [DOI] [PubMed] [Google Scholar]
- 4.Allum W.H., Stenning S.P., Bancewicz J. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–5067. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 5.Yang H., Liu H., Chen Y. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018;36:2796–2803. doi: 10.1200/JCO.2018.79.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shapiro J., van Lanschot J.J.B., Hulshof M. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 7.Skoczylas T., Wallner G., Dabrowski A. The impact of neoadjuvant chemotherapy and chemoradiotherapy on long-term outcome in squamous cell carcinoma of the thoracic esophagus: an analysis of the prospective randomized multicenter trial. J Am Coll Surg. 2014;219:e46. [Google Scholar]
- 8.Sjoquist K.M., Burmeister B.H., Smithers B.M. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–692. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network . National Comprehensive Cancer Network; 2019. NCCN clinical practice guidelines in oncology. Esophageal and Esophagogastric Junction Cancers Version 1. [DOI] [PubMed] [Google Scholar]
- 10.Lordick F., Mariette C., Haustermans K. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v50–v57. doi: 10.1093/annonc/mdw329. [DOI] [PubMed] [Google Scholar]
- 11.National Institute for Health and Care Excellence . National Institute for Health and Care Excellence; 2018. Oesophago-gastric cancer: assessment and management in adults. [PubMed] [Google Scholar]
- 12.Klevebro F., von Dobeln G.A., Wang N. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol. 2016;27:660–667. doi: 10.1093/annonc/mdw010. [DOI] [PubMed] [Google Scholar]
- 13.Stahl M., Walz M.K., Riera-Knorrenschild J. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): long-term results of a controlled randomised trial. Eur J Cancer. 2017;81:183–190. doi: 10.1016/j.ejca.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Hong H., Carlin B.P., Shamliyan T.A. Comparing Bayesian and frequentist approaches for multiple outcome mixed treatment comparisons. Med Decis Making. 2013;33:702–714. doi: 10.1177/0272989X13481110. [DOI] [PubMed] [Google Scholar]
- 16.Donegan S., Williamson P., D'Alessandro U. Assessing key assumptions of network meta-analysis: a review of methods. Res Synth Methods. 2013;4:291–323. doi: 10.1002/jrsm.1085. [DOI] [PubMed] [Google Scholar]
- 17.Puhan M.A., Schünemann H.J., Murad M.H. GRADE Working Group. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 18.Dias S., Welton N.J., Caldwell D.M. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29:932–944. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 19.Burmeister B.H., Thomas J.M., Burmeister E.A. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eu J Cancer. 2011;47:354–360. doi: 10.1016/j.ejca.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Ho D., Imai K., King G., Stuart E.A. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42(8):28. [Google Scholar]
- 21.Westreich D., Lessler J., Funk M.J. Propensity score estimation: neural networks, support vector machines, decision trees (CART), and meta-classifiers as alternatives to logistic regression. J Clin Epidemiol. 2010;63:826–833. doi: 10.1016/j.jclinepi.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pak K., Uno H., Kim D.H. Interpretability of cancer clinical trial results using restricted mean survival time as an alternative to the Hazard ratio. JAMA Oncol. 2017;3(12):1692–1696. doi: 10.1001/jamaoncol.2017.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nygaard K., Hagen S., Hansen H.S. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg. 1992;16:1104–1109. doi: 10.1007/BF02067069. [DOI] [PubMed] [Google Scholar]
- 24.Apinop C., Puttisak P., Preecha N. A prospective study of combined therapy in esophageal cancer. Hepatogastroenterology. 1994;41:391–393. [PubMed] [Google Scholar]
- 25.Le Prise E., Etienne P.L., Meunier B. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer. 1994;73:1779–1784. doi: 10.1002/1097-0142(19940401)73:7<1779::aid-cncr2820730702>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 26.Walsh T.N., Noonan N., Hollywood D. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–467. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 27.Mariette C., Dahan L., Mornex F. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32:2416–2422. doi: 10.1200/JCO.2013.53.6532. [DOI] [PubMed] [Google Scholar]
- 28.Bosset J.F., Gignoux M., Triboulet J.P. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med. 1997;337:161–167. doi: 10.1056/NEJM199707173370304. [DOI] [PubMed] [Google Scholar]
- 29.Walsh T.N., McDonnell C.O., Mulligan E.D. Multimodal therapy versus surgery alone for squamous cell carcinoma of the esophagus: a prospective randomized trial. Gastroenterology. 2000;118:A177. [Google Scholar]
- 30.Urba S.G., Orringer M.B., Turrisi A. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305–313. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 31.Lee J.L., Park S.I., Kim S.B. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol. 2004;15:947–954. doi: 10.1093/annonc/mdh219. [DOI] [PubMed] [Google Scholar]
- 32.Burmeister B.H., Smithers B.M., Gebski V. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659–668. doi: 10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 33.Natsugoe S., Okumura H., Matsumoto M. Randomized controlled study on preoperative chemoradiotherapy followed by surgery versus surgery alone for esophageal squamous cell cancer in a single institution. Dis Esophagus. 2006;19:468–472. doi: 10.1111/j.1442-2050.2006.00615.x. [DOI] [PubMed] [Google Scholar]
- 34.Tepper J., Krasna M.J., Niedzwiecki D. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–1092. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanazono K. Preoperative chemoradiation therapy in potentially resectable esophageal cancer - comparison with surgery alone. Dis Esophagus. 2010;23:119A. [Google Scholar]
- 36.Lv J., Cao X.F., Zhu B. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J Gastroenterol. 2010;16:1649–1654. doi: 10.3748/wjg.v16.i13.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teoh A.Y., Chiu P.W., Yeung W.K. Long-term survival outcomes after definitive chemoradiation versus surgery in patients with resectable squamous carcinoma of the esophagus: results from a randomized controlled trial. Ann Oncol. 2013;24:165–171. doi: 10.1093/annonc/mds206. [DOI] [PubMed] [Google Scholar]
- 38.Bass G.A., Furlong H., O'Sullivan K.E. Chemoradiotherapy, with adjuvant surgery for local control, confers a durable survival advantage in adenocarcinoma and squamous cell carcinoma of the oesophagus. Eur J Cancer. 2014;50:1065–1075. doi: 10.1016/j.ejca.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 39.Dash N., Pal S., Sharma A. Preoperative chemoradiation and surgery versus surgery alone in gastroesophageal junction adenocarcinoma of oesophagus: Interim analysis of a closed level randomized control trial. o206. 07. Dis Esophagus. 2014;27:53A. [Google Scholar]
- 40.Roth J.A., Pass H.I., Flanagan M.M. Randomized clinical trial of preoperative and postoperative adjuvant chemotherapy with cisplatin, vindesine, and bleomycin for carcinoma of the esophagus. J Thorac Cardiovasc Surg. 1988;96:242–248. [PubMed] [Google Scholar]
- 41.Schlag P.M. Randomized trial of preoperative chemotherapy for squamous cell cancer of the esophagus. The chirurgische arbeitsgemeinschaft fuer onkologie der deutschen gesellschaft fuer chirurgie study group. Arch Surg. 1992;127:1446–1450. doi: 10.1001/archsurg.1992.01420120080015. [DOI] [PubMed] [Google Scholar]
- 42.Maipang T., Vasinanukorn P., Petpichetchian C. Induction chemotherapy in the treatment of patients with carcinoma of the esophagus. J Surg Oncol. 1994;56:191–197. doi: 10.1002/jso.2930560314. [DOI] [PubMed] [Google Scholar]
- 43.Law S., Fok M., Chow S. Preoperative chemotherapy versus surgical therapy alone for squamous cell carcinoma of the esophagus: a prospective randomized trial. J Thorac Cardiovasc Surg. 1997;114:210–217. doi: 10.1016/S0022-5223(97)70147-8. [DOI] [PubMed] [Google Scholar]
- 44.Ancona E., Ruol A., Santi S. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer. 2001;91:2165–2174. [PubMed] [Google Scholar]
- 45.Bokhyan V., Stilidi I., Malikhova O. 6553 Neoadjuvant chemotherapy followed by transthoracic resection for locally advanced carcinoma of the esophagus: a randomized study. EJC Supplements. 2009;2:377. [Google Scholar]
- 46.Boonstra J.J., Kok T.C., Wijnhoven B.P. Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: long-term results of a randomized controlled trial. BMC Cancer. 2011;11:181. doi: 10.1186/1471-2407-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baba M., Natsugoe S., Shimada M. Prospective evaluation of preoperative chemotherapy in resectable squamous cell carcinoma of the thoracic esophagus. Dis Esophagus. 2000;13:136–141. doi: 10.1046/j.1442-2050.2000.00101.x. [DOI] [PubMed] [Google Scholar]
- 48.Cormack O.M., Burmeister B., Baker P. Longitudinal health related quality of life following preoperative chemotherapy or chemoradiotherapy for adenocarcinoma of the esophagus. Results from a randomised trial. Dis Esophagus. 2014;27:51A. [Google Scholar]
- 49.Lindblad M., Klevebro F., Johnsen E. Neoadjuvant chemoradiotherapy Vs chemotherapy in cancer of the esophagus and gastric cardia. Effect on postoperative morbidity and mortality. Dis Esophagus. 2014;27:49A. [Google Scholar]
- 50.Banciewicz J., Clark P., Smith D. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727–1733. doi: 10.1016/S0140-6736(02)08651-8. [DOI] [PubMed] [Google Scholar]
- 51.Kelsen D.P., Winter K.A., Gunderson L.L. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25:3719–3725. doi: 10.1200/JCO.2006.10.4760. [DOI] [PubMed] [Google Scholar]
- 52.Ychou M., Boige V., Pignon J.P. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 53.Jiang W., Verma V., Haque W. Post-treatment mortality after definitive chemoradiotherapy versus resection for esophageal cancer. Dis Esophagus. 2020;33(4) doi: 10.1093/dote/doz073. pii: doz073. [DOI] [PubMed] [Google Scholar]
- 54.Rahmani R., Koffler D., Haisley K.R. Stop hedging your bets: reasons for non-adherence to a tri-modality regimen in the treatment of esophageal cancer in a multidisciplinary setting. J Gastrointest Oncol. 2019;10(3):387–390. doi: 10.21037/jgo.2019.01.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Batran S.E., Homann N., Pauligk C. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;11(393(10184)) doi: 10.1016/S0140-6736(18)32557-1. [DOI] [PubMed] [Google Scholar]
- 56.Alderson D., Cunningham D., Nankivell M. Neoadjuvant cisplatin and fluorouracil versus epirubicin, cisplatin, and capecitabine followed by resection in patients with oesophageal adenocarcinoma (UK MRC OE05): an open-label, randomised phase 3 trial. Lancet Oncol. 2017;18:1249–1260. doi: 10.1016/S1470-2045(17)30447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y., Ye J., Zhu Z. Comparing paclitaxel plus fluorouracil versus cisplatin plus fluorouracil in chemoradiotherapy for locally advanced esophageal squamous cell cancer: a randomized, multicenter, Phase III Clinical Trial. J Clin Oncol. 2019;37(20):1695–1703. doi: 10.1200/JCO.18.02122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kojima T., Muro K., Francois E. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: Phase III KEYNOTE-181 study. J Clin Oncol. 2019;37(4_suppl):2. -2. [Google Scholar]
- 59.Yu Y., Zeng D., Ou Q. Association of survival and immune-related biomarkers with immunotherapy in patients with non-small cell lung cancer: a meta-analysis and individual patient-level analysis. JAMA Netw Open. 2019;2(7) doi: 10.1001/jamanetworkopen.2019.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petrelli F., Tomasello G., Barni S. Surrogate end-points for overall survival in 22 neoadjuvant trials of gastro-oesophageal cancers. Eur J Cancer. 2017;05:76. doi: 10.1016/j.ejca.2017.01.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.