Abstract
Living organisms are surrounded with heavy metals such as methylmercury, manganese, cobalt, cadmium, arsenic, as well as pesticides such as deltamethrin and paraquat, or atmospheric pollutants such as quinone. Extensive studies have demonstrated a strong link between environmental pollutants and human health. Redox toxicity is proposed as one of the main mechanisms of chemical-induced pathology in humans. Acting as both a sensor of oxidative stress and a positive regulator of antioxidants, the nuclear factor erythroid 2-related factor 2 (NRF2) has attracted recent attention. However, the role NRF2 plays in environmental pollutant-induced toxicity has not been systematically addressed. Here, we characterize NRF2 function in response to various pollutants, such as metals, pesticides and atmospheric quinones. NRF2 related signaling pathways and epigenetic regulations are also reviewed.
Keywords: NRF2, Heavy metals, Pesticides, Air pollutants, Epigenetic modifications, Redox signaling pathways
Graphical abstract
Highlights
-
•
Environmental chemicals cause redox toxicity via oxidative stress.
-
•
NRF2 acts as a sensor of oxidative stress and a positive regulator of antioxidants.
-
•
NRF2 responds to metals, pesticides and atmospheric pollutants.
-
•
NRF2 and related pathways can be epigenetically regulated.
Abbreviations
- 9,10-PQ
9,10- phenanthraquinone
- ARE
antioxidant response elements
- As
Arsenic
- Cd
Cadmium
- Co
cobalt
- CoNPs
cobalt nanoparticles
- DEP
diesel exhaust particles
- GSH
glutathione
- GSK-3
glycogen synthase kinase-3
- HO-1
heme oxygenase 1
- HSPs
heat shock proteins
- KEAP1
kelch-like ECH-associated protein 1
- LncRNA
long noncoding RNA
- MeHg
methylmercury
- miRNA
microRNAs
- Mn
manganese
- NOS
nitric oxide synthase
- NQO-1
NAD(P)H dehydrogenase (quinone 1)
- NRF2
nuclear factor erythroid 2-related factor 2
- PD
Parkinson's disease
- PI3K
phosphatidyl inositol 3′ kinase
- PTPs
protein tyrosine phosphatases
- ROS
reactive oxygen species
1. Introduction
We are exposed on a daily basis to a variety of environmental chemicals, such as pesticides, heavy metals and air pollutants in our living environment. Evidence from epidemiological findings, cultured cells and animal studies has indicated strong association among many of these toxicants and the onset of a plethora of pathological diseases, such as neurodegenerative/psychiatric disorders and cardiovascular diseases [[1], [2], [3], [4], [5]]. Informative reviews on health impact of environmental chemicals are available, including metals [[6], [7], [8]], pesticides [9], endocrine-disrupting chemicals [10,11] and air pollutants [12]. However, intracellular mechanisms associated with the toxic effects of these compounds have yet to be fully understood. Yet, generation of reactive oxygen/nitrogen species (ROS/RNS) and imbalance in the antioxidant defense system represent a common toxic mechanism for many compounds [[13], [14], [15], [16], [17]]. Under homeostatic conditions, free radicals are produced in a controlled manner to fine-tune signaling pathways associated with cell division, inflammation, autophagy and stress response [18,19]. However, exacerbated production of oxidants and induction of oxidative stress impaire a wide range of biochemical pathways, leading to pathological consequences [20,21]. Environmental toxicants such as heavy metals, pesticides and air pollutants have been demonstrated to generate ROS/RNS, in turn, disrupting circadian rhythms with ensuing pathogenesis [[22], [23], [24], [25]].
The concept of exposome has been recently introduced, referring to lifelong total exposures one receives, both externally (such as environmental toxicants from air or water, life-style related factors) and internally, first proposed by Wild in 2005 [26]. Oxidative stress along with other endogenous processes, such as inflammation, gut flora and epigenetic modifications, are considered to be the internal expoures [27]. Using population-based cohort studies, starting with blood samples and in combination with biomarker technologies, the exposome framework has been advanced, aiming to provide an integrated approach to epidemiological data [28]. In practice, by undertaking exposome-wide association studies (EWAS), the relationship between external exposures and biological samples (molecular features) can be linked to decipher disease risks [29]. Here, we discuss the link between external environmental toxicants and internal biological responses, focusing on the oxidative stress aspect.
The nuclear transcription factor erythroid 2-related factor 2 (NRF2 or NFE2L2) has emerged as an important regulator of oxidative stress and as a target for various intracellular signaling pathways involved in cellular response to a wide range of toxicants [21,30]. Accordingly, the present review provides state-of-the-art synopsis on the mechanisms associated to NRF2 activation in response to environmental toxicants. Considerable attention is directed to the effects of heavy metals, pesticides and air pollutant exposures on NRF2 activation, as well as its epigenetic modifications.
2. Activation of redox signaling pathways by environmental pollutants
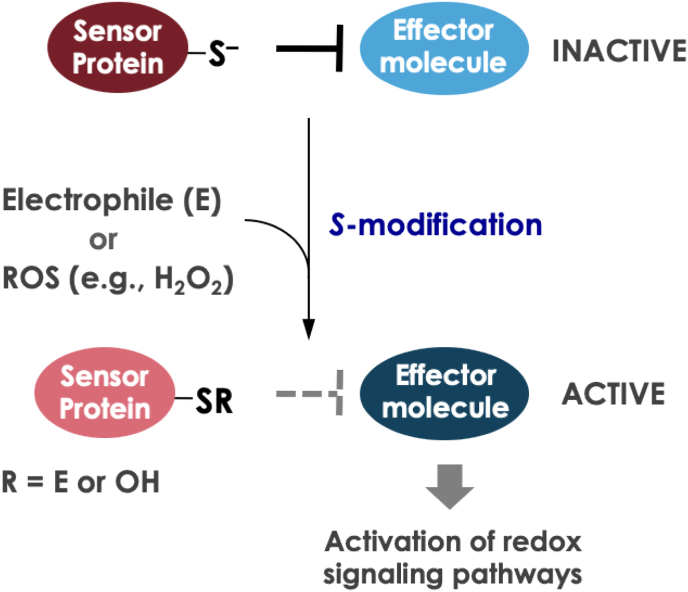
In cells, a variety of redox signaling pathways consist of effector molecules (e.g., kinases and transcription factors) that are negatively regulated by sensor proteins with thiolate ions under normal conditions [[31], [32], [33], [34]]. The current consensus is that such cellular signaling can be activated through modification of the thiol groups of sensor proteins under oxidative stress [[31], [32], [33], [34]] (Fig. 1). It should be noted that not only oxidant stress but also electrophilic stress is categorized as “oxidative stress” because of the modification of protein thiols in either case. First, we would like to provide well-known examples of the signaling activated by ROS and electrophiles besides NRF2.
Fig. 1.
Scheme illustration of the activation of redox signaling pathways. Redox signaling pathways are composed of sensor proteins with deprotonated thiol groups (S) and their targeted effector molecules. Under basal condition, the effector molecules are suppressed by the sensor proteins. When electrophiles (E) or reactive oxygen species (ROS) are present, the sensor proteins are inhibited by S-modification, resulting in activation of the effector molecules. This activation of effector molecules such as transcription factors or kinases leads to the activation of redox signaling pathways.
2.1. PTP1B/EGFR signaling
The protein tyrosine phosphatase 1B (PTP1B) is a member of the family of Cys-based protein tyrosine phosphatases (PTPs), which have HCX5R(S/T) motifs in their active sites [35]. Because PTPs have a reactive thiol with low pKa at their active site [35], the thiol in PTPs is readily oxidized by endogenous H2O2 generated by epidermal growth factor (EGF)-mediated activation of NADPH oxidases (Fig. 1) [36]. Once the PTP activity is inhibited by S-modification at the active site, the EGF receptor (EGFR) will escape dephosphorylation by PTPs such as PTP1B and phosphorylate, leading to activation of EGFR downstream cascades [37,38]. These findings suggest that ROS derived from environmental pollutants are able to oxidize PTP1B and thereby activate EGFR, which is involved in cell survival and proliferation. A recent study shows that 9,10-PQ-derived H2O2 activates PTP1B/EGFR/ERK signaling through S-oxidation at Cys121, which is the same modification as that performed by endogenous H2O2, in A431 cells (Luong et al., unpublished). 1,2-Naphthoquinone (1,2-NQ, Fig. 2), also an environmental pollutant especially common in the vapor phase of ambient air samples [39], covalently modifies Cys121 and Cys215 on the 37-kDa recombinant human PTP1B [40]. In vitro experiments with its C121S mutant indicate that 1,2-NQ is covalently bound to Cys121, leading to inhibition of its enzymatic activity. Cys121 is not in the active site of PTP1B, but is located only about 8 Å from Cys215, which is in the active site, and allosterically inhibits the enzyme activity [41,42]. This finding is consistent with the hypothesis that other environmental electrophiles may also modify, not only Cys215, but also Cys121, resulting in diminished PTP activity, thereby activating substantial EGFR signaling. Yoshida et al. reports that an environmental electrophile, methylmercury, causes inhibition of PTP1B activity and induction of COX-2 through activation of EGFR/p38 MAPK signaling in human brain microvascular endothelial cells [43], suggesting that covalent modification of PTP1B Cys215 and/or Cys121 may participate in the EGFR activation mediated by MeHg exposure because of its high association constant for thiols (1015–1016) [44].
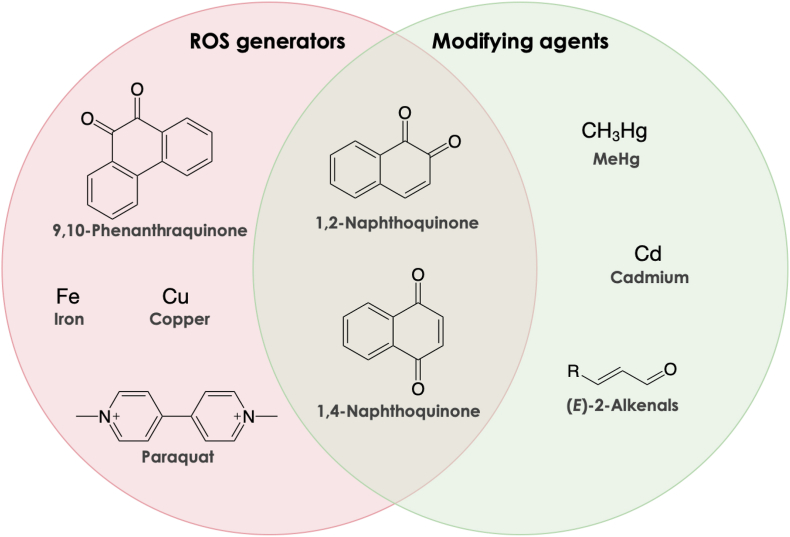
Fig. 2.
Structures of a variety of chemicals that serve as ROS generators and modifying agents in the environment. The ROS generators in pink circle are redox active and thus cause redox cycle reaction associated with ROS production. The modifying agents cause covalent binding to nucleophiles such as protein cysteine residues. 1,2-Naphthoquinone and 1,4-naphthoquinone, however, can generate ROS by both redox cycling and direct protein modification.
2.2. PTEN/Akt signaling
The phosphatase and tensin homolog deletion from chromosome 10 (PTEN) is associated with PTEN hamartoma tumor syndrome, many types of tumor and Cowden's disease [45]. PTEN dephosphorylates phosphatidylinositol 3,4,5-triphosphate, which is formed from phosphatidylinositol 4,5-diphosphate by the kinase activity of PI3K (phosphatidyl inositol 3′ kinase), back to phosphatidylinositol 4,5-diphosphate [46]. Akt binds to phosphatidylinositol 3,4,5-triphosphate on the cellular membrane through the N-terminal pleckstrin homology (PH) domain and is then activated by phosphorylation of Ser473 or Thy308 by mTORC2 or PDK1, respectively, leading to activation of the downstream cascade [[47], [48], [49], [50]]. In basal conditions, PTEN negatively regulates PI3K/Akt signaling through dephosphorylation of phosphatidylinositol 3,4,5-triphosphate, resulting in reduced cell survival [51]. PTEN has 10 Cys residues in both mice and humans, including reactive Cys124 in its phosphatase domain, like PTPs [46], and Cys71 and Cys83 located near basic amino acids [52] (suggesting that the thiols of these cysteine residues potentially undergo deprotonation by the basic amino acid effect, leading to formation of thiolate ions). Through these cysteines, the phosphatase may be a target for chemical modification. Supporting this concept, a recent in vitro experiment has demonstrated that H2O2 reversibly inactivates PTEN by oxidation to form a disulfide bond between Cys71 and Cys124 [53]. This result suggests that H2O2-derived from redox cycling of environmental chemicals such as 9,10-PQ or paraquat (Fig. 2), inhibits PTEN, leading to activation of its downstream cascade (see Fig. 1). Mild oxidants such as NO are reported to activate Akt signaling by S-nitrosylation at PTEN Cys83, while the alcohol-induced 4-hydroxy-2-nonenal (HNE) phosphorylates Akt, presumably through covalent modification of PTEN Cys71 in vivo [54,55]. Kumagai and his colleague have reported that 1,4-NQ, a structural isomer of 1,2-NQ (Fig. 2), covalently modifies Cys71 and Cys81 of recombinant human PTEN and activates PTEN/Akt/cAMP response element-binding protein (CREB) signaling in mouse primary hepatocytes, while an excess concentration of 1,4-NQ disrupts the signal cascade via non-specific protein modification [56]. Interestingly, the 1,4-NQ-mediated activation of Akt/CREB signaling at lower concentration, and disruption of the signaling at higher concentration, are markedly suppressed by simultaneous exposure to a model of a highly nucleophilic polysulfide, Na2S4, through formation of 1,4-NQ–sulfur adducts without electrophilicity [56]. 2-NQ, is also classified as a class III inducer, modified KEAP1 through not only Cys151, Cys273, and Cys288, but also Cys257 and Cys489 [57,58]. It is also found that MeHg (up to 2 μM) activates Akt/CREB signaling and upregulates BCL-2, at least in part through S-mercuration of PTEN in human neuroblastoma SH-SY5Y cells; however, exposure to over 5 μM of MeHg modifies not only PTEN, but also CREB, resulting in disruption of the signaling [59].
2.3. HSP90/HSF1 signaling
Heat shock proteins (HSPs), also known as chaperons, are upregulated in response to various stresses such as heat, oxidants, or electrophiles [60]. In basal conditions, HSP90 and HSP70 interact with a transcriptional factor, heat shock factor 1 (HSF1), to negatively regulate the transactivation capacity [61]. An endogenous electrophile, HNE, modifies Cys572 of HSP90α [62] and Cys267 of HSP72, which is a cytosolic variant of HSP70 [63]. HSP90α and HSP90β show high homology (although at least 99 of the 732 amino acids in hHSP90α are different from hHSP90β) [64]. Using recombinant hHSP90β, Cd or 1,4-NQ binds to both Cys412 and Cys564 (which correspond respectively to Cys420 and Cys572 of hHSP90α), rather than to the other four Cys residues of hHSP90β (Cys366, Cys512, Cys589, and Cys590) [56,65,66]. Exposure of bovine aortic endothelial cells (BAECs) or A431 cells to Cd or 1,4-NQ, respectively, also modifies cellular HSP90 and disrupted the interaction of HSP90 with HSF1, leading to nuclear translocation of HSF1 to upregulate HSPs such as HSP90 and HSP70 [56,65]. Sulfoxythiocarbamate inhibits the chaperon activity of HSP90 through binding at Cys412, Cys564, and Cys589 or Cys590, resulting in destabilization of client oncoproteins, such as CDK1, CHK1, HER2, mutant p53, and RAF1 and inhibition of cell proliferation [67]. Because Cys412 and Cys564 are next to Lys411 and Lys565, the pKa of these Cys residues is lowered by the proximal basic amino acids, and these Cys can be preferable targets for electrophiles. 6-Methylsulfinylhexyl isothiocyanate (6-HITC), contained in edible plants such as wasabi, appears to modify Cys521, but not Cys412 and Cys564, suggesting that the hydrophobic pocket near Cys521 provides a suitable environment for 6-HITC binding [68]. Taken together, it is suggested that electrophilic modification of HSPs such as HSP90 leads to activation of HSF1 (see Fig. 1).
3. NRF2 signaling pathway
NRF2 is a transcription factor encoded by the nfe2l2 gene in humans, that belongs to the cap n collar family due the basic leucine zipper in its structure [13,30,69]. NRF2 is first described as a homolog of the nuclear factor-erythroid 2 p45 (NF-E2), which is associated to the expression of the β-globin gene [70,71]. However, no alterations related to hematopoiesis nor an anemic phenotype are noted in NRF2 knockout mice [72,73]. These results have raised the hypothesis that this transcription factor regulates a different set of genes. Later, it is discovered that NRF2 binds to the antioxidant responsive element (ARE) in the DNA inducing the expression of phase-II detoxing enzymes and antioxidants proteins/enzymes such as glutamate cysteine ligase, heme oxygenase-1 (HO-1) and NAD(P)H dehydrogenase (quinone 1) (NQO-1) [[74], [75], [76], [77]]. Knockout of NRF2 in mice markedly increases susceptibility to a wide-range of toxicants and worsens pathological conditions associated with oxidative stress [21,[78], [79], [80], [81]]. In contrast, pharmacological or genetic increment of NRF2 activity is able to induce protection against oxidative damage in diverse in vitro and in vivo models [79,[82], [83], [84]]. In addition to NRF2's well-known role as a master regulator of antioxidant responses, it plays an important role in the regulation of inflammatory response, autophagy, and cell proliferation [[85], [86], [87], [88]].
NRF2 exhibits a dual role in the context of tumor biology and cancer. NRF2-deficient rodents are more prone to develop cancer in response to diverse carcinogenic agents such as benzopyrene, ultraviolet and ionizing radiation, highlighting the role of this transcription factor in cancer prevention [[89], [90], [91]]. In contrast, NRF2 can cause cancer progression. NRF2 activation is associated with increased cancer cell survival, senescence, and also increases resistance to several chemotherapeutic agents such as cisplatin [88,92,93]. Collectively, those findings suggest NRF2 acts as a “double-sword” in cellular processes and pathogenesis.
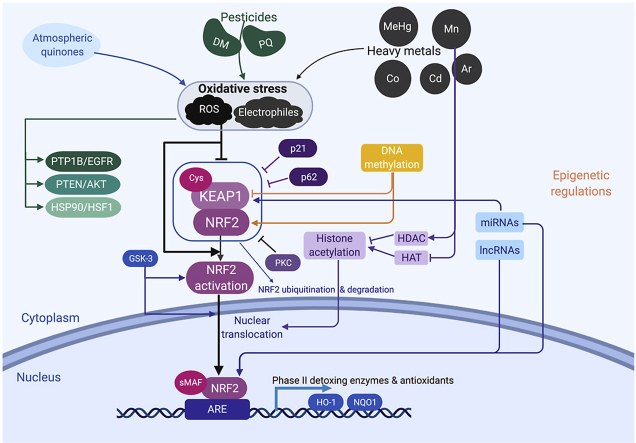
The cellular and biochemical events that lead to NRF2-related gene expression are not yet fully understood. Under basal conditions NRF2 is associated with the Kelch-like ECH-associated protein 1 (KEAP1), promoting NRF2 ubiquitination by E3 ubiquitin ligase, and consequent degradation through 26S proteasome. Two KEAP1 molecules are associated with one molecule of NRF2. Each of the Kelch domains in the KEAP1 structure binds to one of two motifs in the NRF2 protein allowing the attachment of ubiquitin-ligase enzymes, leading to NRF2 polyubiquitination and subsequent degradation by the proteasome [[94], [95], [96]]. Upon cellular stress, the KEAP1/NRF2 association is disrupted and NRF2 is degraded, causing de novo NRF2 to build up within the cell. Those events lead to increased NRF2 translocation into the nucleus where it heterodimerizes with other nuclear factors (Jun and small MAF protein) and binds to the antioxidant responsive elements (ARE) located in the regulatory regions of many response genes, including the ones related to the antioxidant response [30,97]. After its nuclear import, NRF2 also recruits a complex transcriptional machinery in order to transactivate the ARE-driven genes. This machinery involves others co-activators such as the receptor associated co-activator (RAC3) which starts the transactivation domain of NRF2, while other co-regulators such as CREB binding protein, the coactivator-associated arginine methyltransferase and protein arginine methyl-transferase, further enhance the ability of RAC3 to initiate the transactivation domain [98,99].
4. Regulation of NRF2 activity
In addition to the regulatory role of KEAP1 on NRF2 activity, several intracellular signaling pathways may induce post-translational modifications in the structure of this transcription factor, in turn, regulating its function. The following sections will describe some of the main mechanisms associated with NRF2 activation.
4.1. KEAP1-dependent mechanism
As mentioned above, the negative regulation of KEAP1 on NRF2 activation has been extensively described. KEAP1 acts as an intracellular sensor for oxidative and/or electrophilic stress. The KEAP1 structure consists of an N-terminal region (residues 1–49), a BTB domain (residues 50–179), and a C-terminal Kelch domain (residues 327–611) as well as an intervening region (IVR, residues 180–314) [94]. The BTB/POZ domain is essential for the ubiquitin-protein ligase complex interaction, sustaining the basal NRF2 levels, while the IVR domain can act as a functional regulatory region of the protein, controlling the interaction between KEAP1 and NRF2, and consequently NRF2 ubiquitination [30,100].
NRF2 activation is induced by various activators via divergent mechanisms, for example, the negative regulation of NRF2 by KEAP1 which interacts with Cul-3 through the BTB and IVR domain [101]. Human and mouse KEAP1 have 27 and 25 Cys residues among their 624 amino acids, respectively (PRO_0000119,093 and PRO_0000119,094). Using some of its reactive Cys residues, KEAP1 can sense ROS and electrophiles to change its conformation, resulting in escape of NRF2 from KEAP1-dependent degradation by the Ub-proteasome pathway. Especially, Cys151, Cys273, Cys288, Cys297, Cys434, and Cys613 are known to be highly reactive Cys residues, involved in sensing electrophiles. Suzuki et al. classified NRF2 inducers into five classes: class I, Cys151-preferring inducers (e.g., sulforaphane, diethyl maleate, nitric oxide, and ebselen); class II, Cys288-preferring inducers (e.g., 15-deoxy-Δ12,14-prostaglandin J2); class III, Cys151/273/288-selective inducers (e.g., HNE, sodium meta-arsenite); class IV, Cys226/613/622/624-preferring inducers (e.g., H2O2); and class V, non-electrophilic inducers [102,103]. Various inducers can modify specific combinations of cysteines in order to tightly control the KEAP1/NRF2 association and stress-sensing response [94].
The stability of the complex KEAP1/NRF2 can be controlled by other intracellular processes as well. p21 is a cyclin dependent kinase inhibitor that regulates some cellular process like differentiation, senescence and also is able to inhibit gene transcription and apoptosis [104]. p21 competes with KEAP1 for NRF2 binding, lessening KEAP1-dependent NRF2 ubiquitination, leading to increased NRF2 transcriptional activity [[105], [106], [107]]. The role of p62 on the cellular metabolism extends beyond regulation of autophagy [108]. p62 processes a KEAP1‐interacting region that binds to the KEAP1 domain associated to NRF2 interaction. Under basal conditions, the interaction between p62 and KEAP1 is too weak to affect KEAP1/NRF2 interactions. However, under cellular stress, mTORC1 and TGF‐β‐activated kinase can phosphorylate p62 at S349, resulting in the phosphorylated form of p62, which exhibits high affinity for KEAP1, disrupting the KEAP1/NRF2 complex and also decreasing KEAP1 levels through autophagy [86,108]. In addition, oxidative stress increases the expression of a splicing variation of the p62 pre-mRNA, which lacks the domain responsible for its interactions with KEAP1. This variant is associated with proteasomal NRF2 degradation, allowing for the fine tunning of NRF2 activation, counteracting the positive-feedback loop executed by full-length p62 [109]. Moreover, NRF2 phosphorylation at Ser40 by Protein Kinase C induces the dissociation of NRF2 from the complex with KEAP1, increasing the transcription of ARE-driven genes [110,111]. Several phosphorylation sites on NRF2 are proposed to be targets for mitogen-activated protein kinases (MAPKs) mediate phosphorylation, but the role of MAPK in NRF2 activation has yet to be fully investigated [112,113].
In addition to these mechanisms, some toxicants, such as S-(1,1,2,2-tetrafluoroethyl)-l-cysteine, a metabolite of industrial gas, can induce NRF2 activation in the absence of oxidative-stress [114]. The complicated mechanisms of the ROS-independent NRF2 activation are not yet fully understood. It appears that the endoplasmic reticular stress response is able to induce NRF2 phosphorylation through ER-mediated protein kinases such as PKR-like endoplasmic reticular kinase (PERK). The PERK mediated phosphorylation appears to destabilize the KEAP1/NRF2 complex inducing NRF2 activation [115].
4.2. KEAP1-independent mechanism
In addition to the KEAP1 negative regulation on NRF2 activation, NRF2 stability and nuclear localization can be regulated by signaling pathways involving the activation of glycogen synthase kinase3 (GSK3). This enzyme is constitutively active but can be inactivated by phosphorylation at S9 or S21 (in GSK3β and GSK3α, respectively) by protein kinase B (PKB)/Akt. Thus, GSK3 plays a crucial role in cellular process such as cell growth differentiation and survival [116,117]. In addition, a positive correlation between Akt and NRF2 activation has been described [[118], [119], [120]].
McMahon et al. have demonstrated that the Neh6 domain of NRF2 contains a KEAP1-independent degradation region with a putative sequence for GSK3 phosphorylation. This is in line with other reports associating the inhibition of this enzyme and increased NRF2 activity. Within this context, it has been already demonstrated that this kinase phosphorylates specific serine residues in the Neh6 domain of NRF2 creating a degradation domain that is recognized by the ubiquitin ligase adapter β-TrCP and labeled for proteasome degradation by a Cullin1/Rbx1 complex [121,122].
The nuclear localization of NRF2 can also be modulated by GSK3. To date, no phosphorylation site on the NRF2 structure that can be targeted by GSK3 has been described. However, this kinase is able to phosphorylate and activate another kinase, named Fyn. Once activated, Fyn is translocated to the nucleus and phosphorylates NRF2 at Y568, inducing its nuclear export [122,123]. Taken together, these findings allow us to speculate that the modulation of GSK-3 and/or Fyn might connect the activation of NRF2 after the upregulation of the Akt pathway, but additional evidence is still necessary to confirm this hypothesis.
5. Heavy metals activate the redox sensor NRF2 and its signaling pathways
As stated above, NRF2 is a master regulator of cellular responses against environmental stresses. In this section, we discuss the NRF2 pathway as it relates to the effects of heavy metals.
5.1. Methylmercury (MeHg)
Mercury (Hg) is a heavy metal ubiquitously present in the environment. Hg is released to the atmosphere by natural sources such as forest fire and volcanic activity or by anthropogenic sources (mining, coal combustion and other industrial activities). Once in the environment, Hg deposits into aquatic systems and is biomethylated by aquatic sulfate-reducing bacteria forming methylmercury [124] where it undergoes biomagnification, accumulating across the food chain and reaching the highest concentrations in large predatory fish [125]. Thus, the consumption of fish contaminated with MeHg is the most relevant exposure source in human populations [8].
MeHg, as an organometallic cation with the chemical formula [CH3Hg]+, is a potent electrophilic substance, possessing high affinity for thiol and selenol groups. MeHg is readily absorbed by the gastrointestinal tract, and accumulates preferably in the central nervous system (CNS). Rodents exposed to MeHg in drinking water exhibit similar deficits as the humans exposed to this toxicant, such as impairment in motor coordination, disturbances in learning and memory processing and depressive like behavior [8,[126], [127], [128], [129]]. The intracellular mechanisms associated to MeHg exposure are not yet fully understood, but depletion of reduced glutathione levels, induction of oxidative stress and mitochondrial dysfunction play important roles in its cellular toxicity [8,13]. NRF2 nuclear translocation has been increased after exposure to MeHg in vivo [130] and also in vitro [79,118,131,132]. NRF2-related gene expression (such as: ho-1, nqo-1, cglc and nfe2l2) is also increased upon metal exposure [79,118,131,132]. Thus, this data provides strong evidence of MeHg-induced increase in NRF2 activation.
The role of NRF2 in MeHg-induced cellular toxicity has been demonstrated by Kumagai and his colleague [84]. NRF2-deficient (NRF2−/−) mice exposed to MeHg show increased sensitivity to this toxicant and higher mercury accumulation in the brain and liver when compared to wild type animals. In addition, isolated hepatocytes obtained from NRF2−/− mice exhibit increased sensibility to MeHg. Furthermore, pretreatment with the NRF2 activators isothiocyanate and sulforaphane suppresses cellular accumulation of mercury and cytotoxicity in wild-type, but not in NRF2−/− primary mouse hepatocytes. Taken together, this data supports the hypothesis that the upregulation of NRF2 is associated with cell defense mechanisms, and the upregulation of NRF2 decreases MeHg accumulation, ameliorating its toxic consequences.
MeHg can increase NRF2 activation in KEAP1-dependent and -independent ways. Due its electrophilic properties, the cysteine residues on KEAP1 are an important target for MeHg. MeHg can directly bind to the Cys151, Cys319, Cys368 and Cys489 on KEAP1 [79,[133], [134], [135]]. As mentioned previously, the Cys151 residue is essential to NRF2 upregulation after electrophile stress and may play an important role on the NRF2 upregulation upon MeHg exposure. The Cys319 residue seems also important for the ubiquitin E3 ligase activity and consequent NRF2 degradation, and can be also associated to these effects [79,101,136,137].
Aschner et al. have shown inhibition of GSK3β by phosphorylation after in vitro MeHg exposure in primary cortical astrocytes. MeHg also decreases Fyn nuclear localization and mRNA expression, concomitant with increased NRF2 activity [118]. These results corroborate previous reports, demonstrating that MeHg-induced NRF2 activation is linked to PI3K/Akt activation [132]. Taken together, it appears that MeHg promotes Akt activation leading to consequent inhibitory phosphorylation of GSK3β, decreasing of Fyn phosphorylation and Fyn-mediated NRF2 nuclear export. Further experiments are necessary to confirm this hypothesis, addressing KEAP1-independent mechanisms associated with MeHg-induced increase in NRF2 activation.
5.2. Manganese (Mn)
Mn is an essential trace element for human normal developments and functions [138]. As an enzyme co-factor or activator for metabolic reactions such as Mn-superoxide dismutase (Mn-SOD), Mn is also required [139]. However, excessive Mn causes primarily neurotoxicity, characterized by Parkinsonism-like symptoms with degeneration of dopaminergic neurons in the basal ganglia. The similarities and differences between PD and manganism have been extensively reviewed elsewhere [140,141]. Li et al. have demonstrated that MnCl2 upregulates NRF2 protein level and increases the binding activity of NRF2 with ARE. This upregulation is accompanied with promotion of HO-1 expression and reduction in levels of reduced GSH [142]. In aquatic organisms, analogous upregulation of NRF2 is found. In the gill of grass carp, excessive Mn leads to upregulation of NRF2 [143]. In contrast, Mn deficiency causes depression of intestinal immunity, induction of inflammation and dysfunction of the intestinal physical barrier secondary to NRF2 activation in grass carp [144]. Reproductive toxicology studies have shown that Mn exposure inhibits NRF2 expression, unexpectedly, disrupting gonadotropin-releasing hormone (GnRH) synthesis both in vitro and in vivo [145,146].
Mn-induced injury can be antagonized by various chemicals. For example, tert-butylhydroquinone (tBHQ) pretreatment activates NRF2/ARE pathway and attenuates both the cytotoxicity and apoptosis in PC12 cells by MnCl2 [147]. Similarly, melatonin, quercetin and 15 d-PGJ2 exert protective effects in astrocytes against Mn-induced toxicity (inflammation and oxidative stress) by modulating the activation of the NRF2/ARE signaling pathways in various organisms [[146], [147], [148], [149]].
5.3. Cadmium (Cd) and Arsenic (As)
Cadmium and Arsenic are widespread pollutants of concern [[150], [151], [152]]. Exposure to Cd and As from cigarette smoke, rice, water or other environmental and occupational conditions may lead to deleterious consequences, including defects on the liver, bones, kidneys, lungs testes, brain, immunological and cardiovascular systems [[153], [154], [155], [156], [157], [158], [159]]. NRF2, as an ROS sensor, can be activated by Cd and As [[160], [161], [162]]. It is also subsequently found that covalent modification of KEAP1 through Cys249, Cys368 and/or Cys613 are involved in Cd-mediated activation of NRF2 [163]. NRF2 has also been implicated in responses to lung injuries caused by Cd originating from cigarettes [164]. For example, NRF2 knockdown enhances Cd cytotoxicity, whereas KEAP1 knockdown diminishes such a heavy-metal toxicity in the vascular endothelial cells [163]. Pretreatment with sulforaphane, an NRF2 activator, represses As cytotoxicity through reduced intracellular As levels due to increase in NRF2-related protein expressions such as GCSh, GCSl, GSTs MRP1 in primary mouse hepatocytes [165]. Human keratinocytes overexpressing NRF2 are hyper-resistant to arsenic toxicity, an effect that is abolished by NRF2 stable knockdown [166].
Curcumin and lycopene attenuate Cd-induced lipid peroxidation, GSH depletion and alternations in antioxidant enzymes via induction of the NRF2/KEAP1/ARE pathway [[167], [168], [169]]. Attenuation of As2O3 -induced toxicity in cardiomyocytes is noted in response to l-ascorbic acid and α-tocopherol treatment, secondary to the activation of NRF2 and BCL-2 [170]. In male mice, lutein activates the NRF2 signaling pathway, alleviating As induced reproductive defects [171].
5.4. Cobalt (Co) and cobalt nanoparticles (CoNPs)
As a component of vitamin B12, cobalt (Co) is an essential metal. However, high levels of cobalt lead to metallosis, characterized by sensorineural hearing loss, visual and cognitive impairment and peripheral neuropathy [172,173]. Excessive serum cobalt may arise from a variety of sources, including diet, exposure from industrial fabrication, or from alloys used in tooth and hip joint replacements (see reviews [[174], [175], [176]] for details).
As a well-known hypoxia inducer, cobalt is directly linked with oxidative stress and cytotoxicity in varying cell types. Previously, Zheng et al. have shown that cobalt and its nanoparticles overdose result in defects in rat neural cells, including lethality, hypoxia and the loss of learning, memory and spatial-exploration abilities [177,178]. Tungsten carbide-cobalt particles activate NRF2 and its downstream targets such as GST and NQO-1 initiated by ROS and contribute to adverse effects in lymphoma cells JB6 [179]. Compared with CoO NPs and Co3O4 NPs, CoNPs are highly cytotoxic and induce NRF2 signaling related to oxidative stress and DNA strand breaks [180,181]. Microarray and mass spectrometry analysis study have shown that exposure to cobalt causes transcriptomic and proteomic changes in rat liver cells, including HIF-1α signaling, NRF2-mediated response, protein degradation and GSH production [182]. In human keratinocytes, NRF2 has been shown to be involved in the antioxidant response triggered by cobalt chloride [183]. Yuan et al. have demonstrated that overexpression of NRF2 in mesenchymal stem cells prevents apoptosis caused by cobalt, maintaining the stemness [184]. Thymoquinone and mfat-1 transgene expression (which overproduces omega-3 polyunsaturated fatty acid) both exhibit neuroprotective effects against cobalt chloride by upregulating NRF2 [185,186]. Andrographolide, the main bioactive component generated from plant Andrographis paniculate, suppresses cobalt induced inflammation by activating NRF2/HO-1 and inhibiting p38 MAPK signaling [187].
In summary, heavy metals induce oxidative stress and upregulate the ROS sensor NRF2. By further induction of NRF2 and its downstream antioxidant compounds or scavenging excess ROS, the metal induced-defects are likely to be rescued.
6. Pesticide exposures stimulate NRF2-induced cellular defense mechanisms
Pesticides are heavily used in agriculture for crop protection, but excessive use of them contaminates soil, water and atmosphere, harming the non-targeted organisms.
6.1. Deltamethrin (DM)
Deltamethrin ((S)a-cyano-3-phenoxybenzyl-(1R)-cis-3- (2.2-dibromovinyl)-2,2-dimethylcyclopropane carboxy-late, DM) is one of the most potent pyrethroid insecticides [188,189]. Pyrethroid pesticides are highly selective as nerve poisons for mammals in agriculture and urban settings. DM produces specific damages to dopaminergic nerve terminals of the striatum in mice [190]. DM inhibits dopamine biosynthesis and causes increased apoptosis in brain [[191], [192], [193], [194], [195]]. Li et al. have further demonstrated that DM activates NRF2 at multiple levels including enhancing cellular expression, increasing nuclear accumulation as well as activating HO-1 and gamma-glutamylcysteine synthetase catalytic heavy subunit (GCSh). When pretreated with ROS scavenger NAC, the DM-induced NRF2 signaling pathway is suppressed, suggesting that ROS is one of the mediators of this process [196]. Moreover, tert-butyl- hydroquinone (tBHQ), a cytoprotective compound that functions via a mechanism involving NRF2 activation and ARE-dependent genes expression, protects cells from DM-induced oxidative stress and regulates DM-mediated adaptive responses in PC12 cells and rat brain via the translocation of NRF2 [[196], [197], [198], [199]]. The Artemisia campestris essential oil can also protect DM-induced oxidative stress in kidney and brain of rats [200]. It has been also shown that sitagliptin and curcumin limit the poisonous impact of DM by ROS scavenging and activating NRF2 pathway [201]. Similar results were found in zebrafish cell lines of DM induced NRF2 activity, upon treatment with pesticides such as diazinon, diuron and metazachlor [202]. A bioinformatic analysis study has shown the importance of KEAP1-NRF2-ARE pathway in DM stress in Drosophila melanogaster upon RNASeq analysis [203]. Collectively, DM induced-toxicity is accompanied with NRF2 activation involved cellular defense system, which can be ameliorated with ROS scavengers.
6.2. Paraquat (PQ)
Paraquat (1,10-dimethyl-4,40-bipyridilium dichloride, PQ), a widely used nonselective herbicide, has been strongly linked to Parkinson's diseases (PD) [198,[204], [205], [206], [207]]. Studies have demonstrated that PQ treatment results in several of the hallmarks of dopaminergic pathogenesis associated with PD, including decreased dopamine levels, reduction of dopaminergic neurons in the substantia nigra and increased α-synuclein expression [[208], [209], [210]]. The NRF2-ARE pathway has been shown to play a role in neuroprotection against PQ-associated neurodegeneration in the CNS, in vitro and in nigrostriatal dopaminergic neurons [4,120,198,204,[211], [212], [213], [214], [215], [216]]. Moreover, the NRF2-ARE pathway has been shown to be activated by inhibition of 26S proteasome in PC12 cells [217]. As stated above, PQ exerts its toxic effects through the production of superoxide anions in organisms and contribution to mitochondrial dysfunction leading to cell death. Though no treatment for PQ poisoning is currently available, an antioxidant therapy might be a viable alternative. In fact, antioxidants such as naringin, sylimarin, edaravone, Bathysa cuspidata extracts, alpha-lipoic acid, pirfenidone, lysine acetylsalicylate, selenium, quercetin, C-phycocyanin, bacosides, and vitamin C may be useful in the treatment against PQ toxicity ([218] and references herein). Li et al. have shown that PQ neurotoxicity can be ameliorated by tBHQ. tBHQ pretreatment restored motor performance, protected against the reduction in nigral tyrosine hydroxylase -positive neurons, increased TUNEL-positive cells, and led to upregulation of NRF2 and HO-1, which were impaired by PQ exposure [198]. Moreover, the JAW gene, also known as ARL6IP5, exerts a protective effect on PQ-induced PD through the regulation of the MEK/PI3K-NRR2 axis [219].
In addition to PQ, Betarbet et al. first proposed that the insecticide rotenone is able to reproduce the pathological hallmarks of PD [220]. The rotenone model of PD has been recently reviewed [221]. Rotenone causes mitochondrial dysfunction by specifically inhibiting complex I of the mitochondrial electron transport chain (NADH:ubiquinone oxidoreductase activity) [222]. In addition to this inhibition, rotenone also induces mitochondrial membrane depolarization, which in turn, leads to oxidative stress by generating ROS and RNS [223,224]. In response to ROS, signaling pathways such as c-Jun N-terminal kinase 3 and p38 mitogen-activated kinases/p53 signaling pathway as well as NRF2 were activated [[225], [226], [227]]. Rapamycin is a natural anti-fungal product originally isolated from a soil sample from Easter Island [228]. Later, rapamycin demonstrates immunosuppressive, antitumor, neuroprotective, and neuro-regenerative properties (see reviews for details [229,230]). Concurrently, the target of rapamycin (TOR) was identified in several animal models [231,232]. The mammalian target of rapamycin (mTOR) is composed of two functionally distinct complexes, mTORC1 and mTORC2; the first one exhibiting greater sensitivity to rapamycin [230]. Dysregulated mTOR and its signaling pathway have been observed in malignant and neurodegenerative diseases. Therefore, rapamycin, a drug that selectively targets mTORC1, might be potentially efficacious as a potential anti-cancer treatment. However, recent clinal trails have shown that rapalogs show only modest therapeutic efficacy in solid tumors. This is likely due to mTORC1-regulated negative feedback loops. Thus, alternatives such as the inhibition of rictor/mTORC2 have been proposed in cancer treatment [233].
Links between NRF2 and other pesticides have been proposed. Mancozeb, a broad-spectrum fungicide, induces oxidative damage and modulated NRF2 levels in Drosophila melanogaster [234]. Another fungicide prochloraz, used as a post-harvest anti-mold treatment, induces NRF2 activity in cultured human cells and DNA damages in non-toxic concentrations [235]. An oxidizing organophosphate pesticide methyl parathion can generate ROS and modulates NRF2 methylation level in germ cells rather than its expression level [236]. Atrazine has been shown to associated with environmental nephrosis [237], which can be alleviated by lycopene via the activation of autophagy and NRF2 signaling pathway [238].
7. Redox cycling (in the content of NRF2) of atmospheric quinones and its related diseases
Air pollution from industrial activities (e.g., power plants, combustion of fossil fuels, or traffic emissions) is a health risk causing adverse medical effects (Table 1). Air pollutants contain various compounds such as aldehydes, nitrogen oxides, aromatic hydrocarbons, trace metals and particulate matter (PM) [252]. An average increase of 10 μg/m3 of PM, less than 2.5 μm in diameter (PM2.5), is associated with an approximately 8% increase in the risk of mortality by lung cancer [253]. Tobacco smoke typically contains electrophilic or redox-active metals such as Cd (65 ng/cig.) and Pb (32 ng/cig.), or Cu (13 ng/cig.) and Fe (16.8 ng/cig.) [254]. A case-series approach by Kresovich et al. and a cohort-study by White et al. reveal that the airborne concentrations of metals such as Cd and Co, or Hg, Cd, and Pb in residential areas are associated with developing estrogen receptor/progesterone receptor-negative breast cancer or a higher risk of postmenopausal breast cancer, respectively, suggesting that airborne metals affect the breast cancer risk [255,256]. An atmospheric sample collected at the University of Birmingham contains about 12 ng/m3 and 3.9 ng/m3 of redox-active quinones such as phenanthraquinone, naphthoquinones, and benzoquinones in the vapor and particle phase, respectively, and 17 ng/m3 of phenanthrene in the vapor phase, which is a parent compound of 9,10- phenanthraquinone (9,10-PQ, Figs. 2 and 3) [257]. These air contaminants and the ROS derived from such components can injure lung tissue [258]. Sagai et al. demonstrates that diesel exhaust particles (DEP) generate ROS such as superoxide (O2•−) and hydroxyl radicals (•OH), and intratracheal administration of DEP to mice caused lung edema formation that is blocked by pretreatment with polyethylene glycerol-conjugated superoxide dismutase (PEG-SOD), suggesting that the pulmonary toxicity caused by DEP is attributable to large amounts of superoxide production. The production of excess ROS in DEP is caused by NADPH-cytochrome P450 reductase-mediated metabolic activation based on one-electron reduction of quinoid compounds in DEP [259]. Notably, redox-active metals such as Fe and Cu in DEP and PM2.5 are also associated with ROS generation, causing oxidative stress.
Table 1.
Examples of sources of air pollutants and their related diseases.
| Exposure source | Disease | Refs |
|---|---|---|
| Ambient particulate matter | Lower respiratory infections, cancers (trachea, bronchus, and lung), Ischaemic heart disease, cerebrovascular disease, COPD, coronary events | [[239], [240], [241]] |
| Household air pollution from solid fuels | Lower respiratory infections, cancers (trachea, bronchus, and lung), Ischaemic heart disease, cerebrovascular disease, COPD, cataract | [239] |
| Second hand smoke | Stroke, bronchitis, bronchiolitis, lower respiratory infections, asthma, lung cancer | [[242], [243], [244], [245]] |
| Traffic-related air pollutant | Cardiovascular mortality, deep vein thrombosis, coronary athteroclerosis, bronchitic symptom, asthma, stroke | [[246], [247], [248], [249]] |
| Diesel engine exhaust (occupational exposure) | Lung cancers | [250,251] |
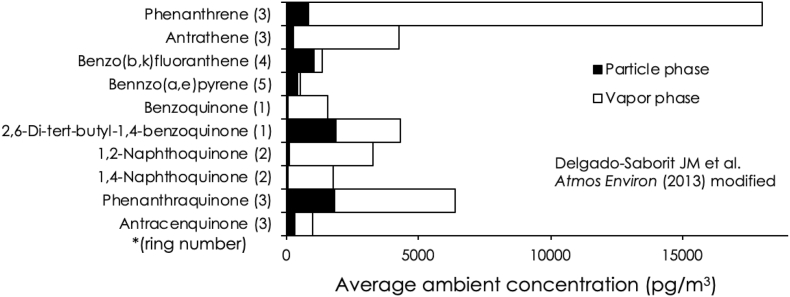
Fig. 3.
Average ambient concentrations of various toxicants in an atmospheric sample at the University of Birmingham. The data is derived from Delgado-Saborit JM et al. [257]. An ambient air sample, taken for 5 days in Birmingham, is analyzed by GC-MS. The black and white bars indicate average ambient concentrations of aromatic hydrocarbons in the particle phase and vapor phase, respectively.
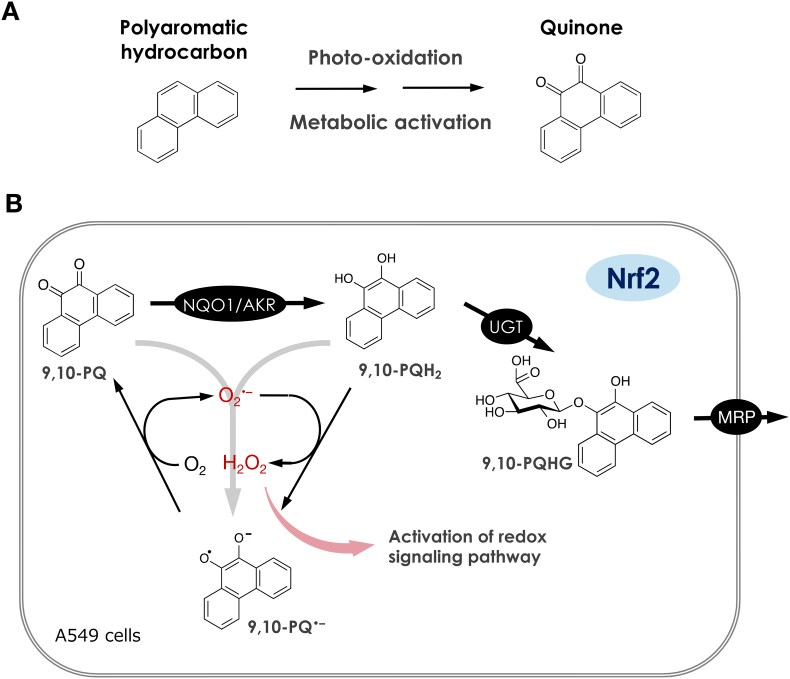
Quinones can be generated by autoxidation of their parent aromatic hydrocarbons in the atmosphere (Fig. 4A) [260,261]. Kumagai et al. have established a GC-MS assay and quantitated four aromatic hydrocarbon quinones, including 9,10-PQ, in DEP and PM2.5 and found that 9,10-PQ is abundant in these particles [262]. Intratracheal administration of DEP or 9,10-PQ to ICR mice upregulates of IL-5 and other inflammatory response-related genes [263,264], and 9,10-PQ exposure recruits inflammatory cells to the murine airways [265]. In A549 or MOLT-4 cells, 9,10-PQ exposure causes apoptotic cell death [266,267]. Quinone-mediated toxicity has been proposed to be caused by at least two mechanisms: (1) excess production of ROS through redox cycling as mentioned below, and (2) direct covalent modification to nucleophiles such as thiols by Michael addition chemistry. While 9,10-PQ has α,β-unsaturated carbonyl groups, this quinoid undergoes redox cycling, and does not serve as a Michael acceptor [[268], [269], [270]]. This indicates that 9,10-PQ is a risk factor in the atmosphere specifically through excess ROS generation. Cd, which is detected in ambient air, enhances the pro-inflammatory responses induced by 9,10-PQ in airway epithelial cells, specifically the BEAS-2B cell line [271].
Fig. 4.
The generation of quinones and the activation of redox signaling pathway. (A) Polyaromatic hydrocarbons, such as phenanthrene undergoes photo-oxidation in the environment or metabolic activation by enzymes, yielding its quinone. (B) 9,10-Phenanthraquinone (9,10-PQ) is reduced by enzymes such as NAD(P)H quinone dehydrogenase (NQO1) or aldo-keto reductase (AKR) to 9,10-dihydroxyphenanthrene (9,10-PQH2), which is conjugated with glucuronic acid by glucuronosyltransferases (UGT) and then excreted viamultidrug-associated proteins (MRP). 9,10-PQH2 is also able to react with superoxide (O2•–) to form its semiquinone radical (9,10-PQ•–) and hydrogen peroxide (H2O2). Alternatively, 9,10-PQ•– is generated by disproportionation reaction of 9,10-PQ with 9,10-PQH2. The 9,10-PQ•– produced interacts with molecular oxygen (O2) to form 9,10-PQ and O2•–. Thus, H2O2 during these redox cycle reactions of 9,10-PQ could activate redox signaling pathways mimicking endogenous H2O2.
Nitric oxide synthase (NOS), which synthesizes NO from l-arginine, plays an important role in neurotransmission, vasorelaxation, and immune response [272]. A C-terminal reductase domain in NOS is able to transfer electrons from NADPH to other acceptors [273]. A variety of quinones inhibit neuronal NO synthase (nNOS) activity; in one study, among 21 quinones, 9,10-PQ (with a one-electron reduction potential of −124 mV) is the most potent inhibitor of nNOS. Using purified nNOS, Kumagai et al. have found that 9,10-PQ interacts with the NADPH-P450 reductase domain on nNOS and thus inhibits NO production (IC50 = 10 μM) by shunting electrons away from the normal catalytic activity [274]. 9,10-PQ is also a potent inhibitor of endothelial NOS activity (IC50 = 0.6 μM), thereby suppressing acetylcholine-dependent vasorelaxation of the aortic rings of rats and increasing their blood pressure [275]. 9,10-PQ also undergoes one-electron reduction by cytochrome P450 reductase in the presence of NADPH and by dithiols or persulfides such as dihydrolipoic acid, DTT, or Na2S2 to yield the semiquinone radical of 9,10-PQ (9,10-PQ•−), which reacts with oxygen to form 9,10-PQ and superoxide (Fig. 4B) [259,268,276,277]. From these observations, O2 is evidently a key factor to initiate 9,10-PQ-mediated toxicity through ROS generation. Consistent with this, the toxicity of this quinone to the marine bacterium Vibrio fischeri is found to be lower under anaerobic conditions [278]. Furthermore, aldo-keto reductase (AKR) isozymes, NQO1, and l-xylulose reductase can catalyze two-electron reduction of 9,10-PQ to produce 9,10-dihydroxyphenanthrene (9,10-PQH2), which reacts with parent 9,10-PQ through a disproportionation reaction forming 9,10-PQ•− as well [279]. However, the monoglucuronide of 9,10-PQH2 (9,10-PQHG) produced by UDP-glucuronosyltransferase (UGT) isoforms is no longer redox-active and is excreted into extracellular space through multidrug-associated proteins (MRPs) (Fig. 4B) [280]. Such a glucuronide of 9,10-PQ has been also detected in the urine of residents of suburban Kanazawa, Japan [281]. The important thing is that the gene expressions of the AKRs, NQO1, UGTs, and MRPs involved in biotransformation of 9,10-PQ to 9,10-PQHG are cooperatively regulated by transcription factor NRF2 [279,280,282,283]. Therefore, NRF2 deletion is found to markedly enhance 9,10-PQ toxicity [280]. Interestingly, ambient air samples collected in California activates NRF2 through oxidative and electrophilic stresses [284,285]. Suzuki et al. recently reports that H2O2 oxidizes KEAP1 to form disulfide bonds between Cys226 and Cys613, Cys226 and Cys622/624, or Cys613 and Cys622/624 [103], leading to NRF2 activation. Collectively, it appears that NRF2 activation mediated by exposure to air pollutants is, at least in part, caused by ROS generators such as 9,10-PQ through oxidation of KEAP1.
8. Epigenetic regulations of NRF2 linked to cancer and other diseases by environmental chemicals
Epigenetic regulations such as DNA methylation, histone modifications, microRNA (miRNA) and long noncoding RNA (lncRNA) degradation have been emergingly reported as NRF2 modulations.
8.1. DNA methylation
DNA methylation is widely observed from prokaryotes to vertebrates. In mammalian cells, DNA methylation is established by three types of DNA methyltransferases (DNMTs), DNMT3a, DNMT3b and DNMT1. DNA methylation can be reversed by the ten eleven translocation (TET) enzymes [286]. CpG islands are GC-rich DNA stretches mainly found in promoter regions of genes, and are predominantly nonmethylated [287]. Methylation of the CpG islands is associated with alterations in chromatin structure, genomic imprinting, transposon and chromosome X inactivation, differentiation, and cancer [288]. The effect of DNA methylation is achieved by proteins containing methyl-CpG binding domains (MBDs). Such MBD-containing proteins appear to act as structural proteins, which recruit a variety of histone deacetylase complexes and chromatin remodelling factors, leading to chromatin condensation and thus resulting in transcriptional repression [289].
DNA methylation on both KEAP1 and NRF2 can regulate the NRF2 signaling pathway. In solid tumors and cancers, the hypermethylation of KEAP1 promoter causes NRF2 nuclear accumulation in lung, breast, renal cell carcinoma, colorectal cancer, head and neck cancer tissues [290]. On the other hand, the hypermethylation of KEAP1 promoter is mainly playing protective roles in oxidative stress related diseases, such as diabetes and aging [[291], [292], [293]]. Epigenetic modifications on NRF2 are investigated to a lesser extent. Yu and colleagues have identified the CpG islands in the promoter region of NRF2; moreover, the repression of NRF2 in prostate tumors is due to the hypermethylation of those CpG islands. The addition of DNMT inhibitor restores NRF2 expression and dissociation of MBD2 and methylated histones [294]. Suppression of NRF2 hypermethylation by γ-tocopherol–rich mixture of tocopherols (γ-TmT) in mice appears to be associated with higher NRF2 and NQO-1 protein levels, which is found to be involved in the action of many dietary chemo-preventive chemicals [295]. Furthermore, 5-fluorouracil- (5-FU-) induced ROS activates DNA demethylases and produces a hypomethylated NRF2 promoter, which consequently activates the translocation of NRF2 and enhances the resistance to 5-FU in cancer cells [296].
8.2. Histone modifications
Four core histone proteins wrap up eukaryotic DNA and fold into chromatin fibers. This highly organized protein-DNA complex consists of two configurations: condensed heterochromatin and easily transcribed euchromatin [297,298]. The post-translational modifications of histone residues modify chromatin structure and further regulate the transcriptional activity of various genes on the chromatin. Histone modifications consist of histone acetylation, methylation and modification on readers.
Histone acetylation destabilizes the nucleosome structure and promotes the accessibility of transcriptional factors to a genetic locus, thereby activating gene transcription, supported by convincing evidence [299]. Histone acetylation is achieved by histone acetyltransferases (HATs), while histone deacetylases (HDACs) remove the acetyl groups. The mammalian sirtuins (SIRT1-7) belong to NAD+ dependent HDACs. As the most studied sirtuin, SIRT1 can deacetylate both histone and non-histone targets, thus is deeply involved in cellular processes, such as apoptosis, autophagy, aging and tumorigenesis [300]. Amongst the various deacetylate targets of SIRT1, NRF2 deacetylation by SIRT1 enhances the activity of KEAP1/NRF2/ARE pathway in glomerular mesangial cells under oxidative stress [301]. Huang et al. further noted that NRF2 positively regulates SIRT1 protein expression and deacetylase activity, forming a positive feedback loop to inhibit the development of diabetic nephropathy (DN) [302]. This protective effect is further supported by a recent study adopting a potent SIRT1 activator, SRT2104 [303]. Besides DN, SIRT1-induced NRF2 activation and related protective effects have been noted in acute lung injury [304], optic neuritis [305], oocytes aging [306], and vascular calcification [307], to name a few. The major roles of SIRT1 in response to environmental stress and DNA damage have been extensively reviewed elsewhere [308]. Mn significantly suppresses the acetylation of histone H3 and H4 in PC12 and SHSY5Y cells by increasing HDAC3 and HDAC4 activities and inactivating HATs [309]. Down-regulation of histone acetylation by HAT inhibitor anacardic acid causes inhibition of NRF2 nuclear translocation and suppresses Mn-activated NRF2/HO-1 pathway. This histone deacetylation also increases the ROS production and decreases GSH in neurons [310]. Nevertheless, HDAC inhibition does not always lead to NRF2 activation, especially in airway epithelial cells or lung inflammation conditions [311]. Furthermore, NRF2-mediated oxidative stress responses can have an epigenetic impact by modulations of HDAC activity conversely [312,313].
EZH2, a methyltransferase involved in histone methylation, can trimethylate lysine 27 on histone H3 (H3K27Me3) in the NFR2 promoter region and leads to transcription repression [289,314]. In addition, histone-lysine N-methyltransferase (SetD7) monomethylates histone H3 lysine 4 (H3K4me1) and increases the binding of transcription factor [315]. The findings suggest that histone methylation can lead to either gene activation or suppression depending on which residue is methylated and the degree of methylation.
H2O2 induced ROS production can be ameliorated by the inhibition of BET (bromodomain and extraterminal) proteins, which are known as acetyl-lysine readers and act as negative regulators of NRF2 signaling pathway [316,317]. However, in the toxicology field, not much study has been carried out yet.
8.3. Noncoding RNAs
miRNAs are single stranded non-coding RNA molecules of approximately 22 nucleotides. miRNAs are able to regulate gene expression by complementarily binding to the 3′-untranslated regions (3′-UTRs) of target mRNAs. The changes in miRNA levels can be induced by exposure to persistent chemicals, endocrine disrupting chemicals, heavy metals and pesticides. Next to redox stress, the toxicity of these environmental chemicals can also be mediated by the alteration of miRNAs, which participate in cellular processes by regulating target gene expressions. miRNAs are also able to “fine-tune” redox signaling by interacting with NRF2 [318]. The first documented miRNA that negatively regulates NRF2 expression is miR-28, found in MCF-7 breast cancer cells [319]. This modulation of NRF2 is then found in miR155 [320], miR144 [321], miR93 [322], miR153, miR27a, miR-142-5p [323], miR340 [324], miR-507, miR-634, miR-450a and miR-129-5p [325]. miR-140-5p attenuates oxidative stress in Cisplatin induced acute kidney injury by activating NRF2/ARE pathway through a KEAP1-independent mechanism. miR-140-5p directly targets the 3′-UTR of NRF2 mRNA and plays a positive role in the regulation of NRF2 expression [326]. PQ and MPTP alter microRNA expression profiles and downregulate expression of miR‐17‐5p, which contributes to PQ‐ induced dopaminergic neurodegeneration [327]. Moreover, Li et al. have found that miR-380-3p blocks the translation of Sp3 in the presence of PQ-induced neurotoxicity [328].
Not only the direct binding of miRNAs to NRF2 mRNA alters its activity, binding to KEAP1 mRNA also plays a role in NRF2 activity regulation. The first miRNA found to induce NRF2 nuclear translocation is miR-141 in ovarian carcinoma cell lines [329].
Other than miRNAs, lncRNAs larger than 200 nucleotides are also involved in regulating the KEAP1/NRF2 pathway. The smoke and cancer-associated lncRNA1 (SCAL1) is the first characterized lncRNA activated by NRF2, and it functions as a downstream mediator against oxidative stress [330].
9. Summary and conclusions
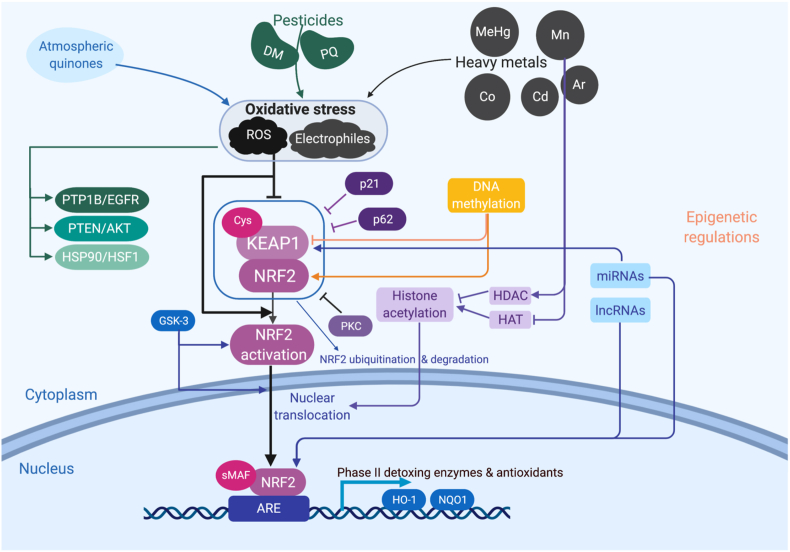
As shown in Fig. 5, KEAP1 binds to NRF2 under normal conditions, followed by NRF2 ubiquitination and degradation. When exposed to environmental chemicals such as atmospheric quinones, pesticides or heavy metals, oxidative stress is induced including ROS or electrophiles, causing disassociation of KEAP1 and NRF2. NRF2 then translocates from cytoplasm to nucleus and binds to ARE, following interaction with a partner protein small MAF (sMAF), activating the transcription of downstream detoxing enzymes and antioxidants such as HO-1 and NQO-1. Besides KEAP1/NRF2, redox cycling can activate other signaling pathways including PTP1B/EGFR, PTEN/Akt, HSP90/HSF1, etc. On the other hand, NRF2 signaling can be regulated by p21 and p62 induced KEAP1 inhibition or GSK-3 related NRF2 activation. Moreover, epigenetic modifications of NRF2 have drawn increasing attention, including DNA methylation, histone acetylation and noncoding RNAs-involved regulations.
Fig. 5.
NRF2 signaling pathway in the context of environmental pollutants induced redox toxicity and its regulation. Exposure of environmental toxicants such as atmospheric pollutants, pesticides and heavy metals could cause oxidative stress, including ROS and electrophiles. Besides the well-known PTP1B/EGFR, PTEN/AKT and HSP90/HSF1 pathways, NRF2 can be activated. This NRF2 activation can be induced by the negative regulation of KEAP1 through Cys residues or by the inhibition of KEAP1/NRF2 complex stability via p21, p62 and PKC. Upon the dissociation of KEAP1, NRF2 no longer proceeds to the ubiquitination and degradation, resulting in the translocation to the nucleus. The translocated NRF2 heterodimerizes with other nuclear factors such as sMAF and binds to AREs, driven the activation of detoxing enzymes and antioxidants. This process is also epigenetically regulated. For instance, the hypermethylation of KEAP1 promoter results in NRF2 nuclear translocation, while the hypermethylation of NRF2 promoter represses NRF2. Environmental pollutants could modulate histone acetylation through histone acetyltransferases (HATs) or histone deacetylases (HDACs). Finally, miRNAs and LncRNAs also involve in this regulation process.
The regulation of NRF2 in response to various environmental toxicants is closely linked to human diseases. First of all, genes targeted by NRF2 protect cells from age-related pathologies by either neutralizing free radicals to prevent damages from occurring or fight against the damages. The lose of NRF2 results in neurodegenerative disorders such as PD, AD and age-related cancers [331]. Morover, NRF2 activators have been proposed to be a therapeutic target in AD [332]. In diabetic cardiomyopathy and hypertension, the protective role of NRF2 is also suggested [333]. However, this activation of NRF2 has to be undertaken with cation. Some studies suggested that hyperactivation of NRF2 and its target gene HO-1 has a detrimental effect in atherosclerosis and vascular clacification owing to NRF2-mediated inflammation [334,335]. Moreover, constitutive NRF2 activation and its subsequent upregulation of iron storage protein can lead to enhanced proliferation of cancer and thus therapy resistance [336]. Besides NRF2, other redox cycling activated pathways play a role in diseases. The activation PTP1B/EGFR pathway participates in endothelial dysfunction, resulting in cardiovascular disorders and diabetes [337]. PTEN, the tumor suppressor which negatively regulates Akt, plays a key role in cell proliferation and migration, glucose uptake and ovarian dysfunction. Dysregulation of the signaling pathway may contribute to cancer, obesity, cardiovascular dysfunction and infertility [338,339]. HSP90 and HSF1 are also highly associated with neurodegenerative diseases and cancer owing to their role in protein misfolding [340,341]. In skin aging and skin carcinogenesis, however, a star transcription factor has to be pointed out, the aryl hydrocarbon receptor (AHR). AHR activated by environmental stressors such as organic compounds and UV radiation is of considerable importance in regulating keratinocyte proliferation, epidermal barrier function, immunity and melanogenesis. While long-term activation of AHR could result in premature aging [342].
Worth-pointing out, redox signaling impacts gut microbiome, metabolome and proteome. Thanks to the development of high-throughput techniques in defining and analyzing massive components, concepts such as microbiome, metabolome and proteome emerged. The gut microbiome is considered as an “organ” consisting of thousands of microoganisms and 150 times more genes than the human genome, The disturbance of microbiome by oxidative stress can affect health status by inducing pathophysiological responses in the host [343]. The metabolic products from gut microbes can regulate host metabolism. The alterations in the metabolome can both be affected by and lead to redox toxicity, associating to various dietary disorders and diseases [344,345]. Similarly, proteome alterations and proteostasis disturbance are cellular responses to environmental exposure in a redox-dependent manner [346].
Although NRF2 has been discussed and studied extensively, as additional environmental issues emerge and (epi)genetic mechanisms are characterized, further studies will be required. For instance, the unresolved mechanisms involved in the activation of NRF2 pathway independent of oxidative stress nor KEAP1 need further investigations. Moreover, deeper understanding of pesticides or metals associated NRF2 regulations should be addressed on both upstream and downstream of NRF2-centered signaling pathways. Finally, given that several studies have shown correlative effects between toxicants and epigenetic regulation, more research is needed to draw a connective network of cellular responses to environmental chemicals.
In conclusion, our review summarizes the activation mechanisms of NRF2 and related pathways upon exposure to environmental toxicants, including metals, pesticides and atmospheric pollutants. This regulation of NRF2 can be achieved both genetically and epigenetically. The adverse redox signaling by environmental chemicals impacts multiple clinical disorders, such as cancer, cardiovascular and neurodegenerative diseases. Nonetheless, addtional research on the topic is needed to better characterize up- and down-stream regulators of the pathway.
Funding
This work is supported by the National Institutes of Health [grant numbers R01ES07331, R01ES10563], the National Natural Science Foundation of China [grant number 81573195, 81903352, 81973083], the Joint Funds for the Innovation of Science and Technology, Fujian province [grant number 2017Y9105] and grant-in-aids for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan [grant number 18H05293, 17K15489].
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled “Redox Toxicology of Environmental Chemicals Causing Oxidative Stress”.
Acknowledgments
We thank Leo Holroyd, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a part of this manuscript.
Contributor Information
Fuli Zheng, Email: f.zheng@fjmu.edu.cn.
Huangyuan Li, Email: lhy@fjmu.edu.cn.
Yoshito Kumagai, Email: yk-em-tu@md.tsukuba.ac.jp.
Michael Aschner, Email: michael.aschner@einsteinmed.org.
References
- 1.Ye B.S., Leung A.O.W., Wong M.H. The association of environmental toxicants and autism spectrum disorders in children. Environ. Pollut. 2017;227:234–242. doi: 10.1016/j.envpol.2017.04.039. [DOI] [PubMed] [Google Scholar]
- 2.Kahn L.G., Trasande L. Environmental toxicant exposure and hypertensive disorders of pregnancy: recent findings. Curr. Hypertens. Rep. 2018;20(10):87. doi: 10.1007/s11906-018-0888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riancho J. The increasing importance of environmental conditions in amyotrophic lateral sclerosis. Int. J. Biometeorol. 2018;62(8):1361–1374. doi: 10.1007/s00484-018-1550-2. [DOI] [PubMed] [Google Scholar]
- 4.Drechsel D.A., Patel M. Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson's disease. Free Radic. Biol. Med. 2008;44(11):1873–1886. doi: 10.1016/j.freeradbiomed.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosselman K.E., Navas-Acien A., Kaufman J.D. Environmental factors in cardiovascular disease. Nat. Rev. Cardiol. 2015;12(11):627. doi: 10.1038/nrcardio.2015.152. [DOI] [PubMed] [Google Scholar]
- 6.Navas-Acien A. Lead exposure and cardiovascular disease—a systematic review. Environ. Health Perspect. 2007;115(3):472–482. doi: 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tellez-Plaza M. Cadmium exposure and clinical cardiovascular disease: a systematic review. Curr. Atherosclerosis Rep. 2013;15(10):356. doi: 10.1007/s11883-013-0356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ke T. Post-translational modifications in MeHg-induced neurotoxicity. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2019;1865(8):2068–2081. doi: 10.1016/j.bbadis.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao F. Elucidating conserved transcriptional networks underlying pesticide exposure and Parkinson's disease: a focus on chemicals of epidemiological relevance. Front. Genet. 2019;9:701. doi: 10.3389/fgene.2018.00701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lind P.M., Lind L. Endocrine-disrupting chemicals and risk of diabetes: an evidence-based review. Diabetologia. 2018;61(7):1495–1502. doi: 10.1007/s00125-018-4621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humblet O. Dioxins and cardiovascular disease mortality. Environ. Health Perspect. 2008;116(11):1443–1448. doi: 10.1289/ehp.11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourdrel T. Cardiovascular effects of air pollution. Arch. Cardiovasc. Dis. 2017;110(11):634–642. doi: 10.1016/j.acvd.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antunes Dos Santos A. Oxidative stress in methylmercury-induced cell toxicity. Toxics. 2018;6(3) doi: 10.3390/toxics6030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H., Reynolds M. Cadmium exposure in living organisms: a short review. Sci. Total Environ. 2019;678:761–767. doi: 10.1016/j.scitotenv.2019.04.395. [DOI] [PubMed] [Google Scholar]
- 15.Mohammadi H. Pyrethroid exposure and neurotoxicity: a mechanistic approach. Arh. Hig. Rada. Toksikol. 2019;70(2):74–89. doi: 10.2478/aiht-2019-70-3263. [DOI] [PubMed] [Google Scholar]
- 16.Lushchak V.I. Pesticide toxicity: a mechanistic approach. EXCLI J. 2018;17:1101–1136. doi: 10.17179/excli2018-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim C.C., Thurston G.D. Air pollution, oxidative stress, and diabetes: a life course epidemiologic perspective. Curr. Diabetes Rep. 2019;19(8):58. doi: 10.1007/s11892-019-1181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo Conte M., Carroll K.S. The redox biochemistry of protein sulfenylation and sulfinylation. J. Biol. Chem. 2013;288(37):26480–26488. doi: 10.1074/jbc.R113.467738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finkel T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011;194(1):7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Q. Transcriptional responses to oxidative stress: pathological and toxicological implications. Pharmacol. Ther. 2010;125(3):376–393. doi: 10.1016/j.pharmthera.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H. Influence of mental stress and environmental toxins on circadian clocks - implications for redox regulation of the heart and cardioprotection. Br. J. Pharmacol. 2019 doi: 10.1111/bph.14949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haberzettl P. Circadian toxicity of environmental pollution. Inhalation of polluted air to give a precedent. Curr. Opin. Physiol. 2018;5:16–24. doi: 10.1016/j.cophys.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parmalee N.L., Aschner M. Metals and circadian rhythms. Adv. Neurotoxicol. 2017;1:119–130. doi: 10.1016/bs.ant.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim F.L. Emerging evidence for the interrelationship of xenobiotic exposure and circadian rhythms: a review. Xenobiotica. 2006;36(10–11):1140–1151. doi: 10.1080/00498250600861819. [DOI] [PubMed] [Google Scholar]
- 26.Wild C.P. AACR; 2005. Complementing the Genome with an “Exposome”: the Outstanding Challenge of Environmental Exposure Measurement in Molecular Epidemiology. [DOI] [PubMed] [Google Scholar]
- 27.Rappaport S.M. Implications of the exposome for exposure science. J. Expo. Sci. Environ. Epidemiol. 2011;21(1):5–9. doi: 10.1038/jes.2010.50. [DOI] [PubMed] [Google Scholar]
- 28.Vrijheid M. The exposome: a new paradigm to study the impact of environment on health. Thorax. 2014;69(9):876–878. doi: 10.1136/thoraxjnl-2013-204949. [DOI] [PubMed] [Google Scholar]
- 29.Vineis P. The exposome in practice: design of the EXPOsOMICS project. Int. J. Hyg Environ. Health. 2017;220(2):142–151. doi: 10.1016/j.ijheh.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva-Islas C.A., Maldonado P.D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 2018 doi: 10.1016/j.phrs.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Winterbourn C.C., Hampton M.B. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 2008;45(5):549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Kumagai Y., Abiko Y. Environmental electrophiles: protein adducts, modulation of redox signaling, and interaction with persulfides/polysulfides. Chem. Res. Toxicol. 2017;30(1):203–219. doi: 10.1021/acs.chemrestox.6b00326. [DOI] [PubMed] [Google Scholar]
- 33.Jones D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008;295(4):C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudolph T.K., Freeman B.A. Transduction of redox signaling by electrophile-protein reactions. Sci. Signal. 2009;2(90):re7. doi: 10.1126/scisignal.290re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z.Y. Protein-tyrosine phosphatases: biological function, structural characteristics, and mechanism of catalysis. Crit. Rev. Biochem. Mol. Biol. 1998;33(1):1–52. doi: 10.1080/10409239891204161. [DOI] [PubMed] [Google Scholar]
- 36.Bae Y.S. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 1997;272(1):217–221. [PubMed] [Google Scholar]
- 37.Lee S.R. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J. Biol. Chem. 1998;273(25):15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 38.Tiganis T. Protein tyrosine phosphatases: dephosphorylating the epidermal growth factor receptor. IUBMB Life. 2002;53(1):3–14. doi: 10.1080/15216540210811. [DOI] [PubMed] [Google Scholar]
- 39.Kumagai Y. The chemical biology of naphthoquinones and its environmental implications. Annu. Rev. Pharmacol. Toxicol. 2012;52:221–247. doi: 10.1146/annurev-pharmtox-010611-134517. [DOI] [PubMed] [Google Scholar]
- 40.Iwamoto N. Chemical knockdown of protein-tyrosine phosphatase 1B by 1,2-naphthoquinone through covalent modification causes persistent transactivation of epidermal growth factor receptor. J. Biol. Chem. 2007;282(46):33396–33404. doi: 10.1074/jbc.M705224200. [DOI] [PubMed] [Google Scholar]
- 41.Andersen J.N. Structural and evolutionary relationships among protein tyrosine phosphatase domains. Mol. Cell Biol. 2001;21(21):7117–7136. doi: 10.1128/MCB.21.21.7117-7136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansen S.K. Allosteric inhibition of PTP1B activity by selective modification of a non-active site cysteine residue. Biochemistry. 2005;44(21):7704–7712. doi: 10.1021/bi047417s. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida E. Methylmercury promotes prostacyclin release from cultured human brain microvascular endothelial cells via induction of cyclooxygenase-2 through activation of the EGFR-p38 MAPK pathway by inhibiting protein tyrosine phosphatase 1B activity. Toxicology. 2017;392:40–46. doi: 10.1016/j.tox.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 44.Simpson R.B. Association constants of methylmercury with sulfhydryl and other bases. J. Am. Chem. Soc. 1961;83(23):4711–&. [Google Scholar]
- 45.Yehia L., Eng C. 65 years OF the double helix: one gene, many endocrine and metabolic syndromes: PTEN-opathies and precision medicine. Endocr. Relat. Canc. 2018;25(8):T121–T140. doi: 10.1530/ERC-18-0162. [DOI] [PubMed] [Google Scholar]
- 46.Maehama T., Dixon J.E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998;273(22):13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 47.Marte B.M., Downward J. PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem. Sci. 1997;22(9):355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 48.Ebner M. PI(3,4,5)P3 engagement restricts Akt activity to cellular membranes. Mol. Cell. 2017;65(3):416–431 e6. doi: 10.1016/j.molcel.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 49.Sarbassov D.D. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 50.Alessi D.R. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr. Biol. 1997;7(4):261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 51.Stambolic V. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95(1):29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 52.Lee J.O. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99(3):323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- 53.Lee S.R. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 2002;277(23):20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 54.Numajiri N. On-off system for PI3-kinase-Akt signaling through S-nitrosylation of phosphatase with sequence homology to tensin (PTEN) Proc. Natl. Acad. Sci. U. S. A. 2011;108(25):10349–10354. doi: 10.1073/pnas.1103503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shearn C.T. Increased carbonylation of the lipid phosphatase PTEN contributes to Akt2 activation in a murine model of early alcohol-induced steatosis. Free Radic. Biol. Med. 2013;65:680–692. doi: 10.1016/j.freeradbiomed.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abiko Y. Polysulfide Na2S4 regulates the activation of PTEN/Akt/CREB signaling and cytotoxicity mediated by 1,4-naphthoquinone through formation of sulfur adducts. Sci. Rep. 2017;7(1):4814. doi: 10.1038/s41598-017-04590-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miura T. Initial response and cellular protection through the Keap1/Nrf2 system during the exposure of primary mouse hepatocytes to 1,2-naphthoquinone. Chem. Res. Toxicol. 2011;24(4):559–567. doi: 10.1021/tx100427p. [DOI] [PubMed] [Google Scholar]
- 58.Kobayashi M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol. Cell Biol. 2009;29(2):493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Unoki T. Methylmercury, an environmental electrophile capable of activation and disruption of the Akt/CREB/Bcl-2 signal transduction pathway in SH-SY5Y cells. Sci. Rep. 2016;6:28944. doi: 10.1038/srep28944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lindquist S., Craig E.A. The heat-shock proteins. Annu. Rev. Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 61.Bjork J.K., Sistonen L. Regulation of the members of the mammalian heat shock factor family. FEBS J. 2010;277(20):4126–4139. doi: 10.1111/j.1742-4658.2010.07828.x. [DOI] [PubMed] [Google Scholar]
- 62.Carbone D.L. Modification of heat shock protein 90 by 4-hydroxynonenal in a rat model of chronic alcoholic liver disease. J. Pharmacol. Exp. Therapeut. 2005;315(1):8–15. doi: 10.1124/jpet.105.088088. [DOI] [PubMed] [Google Scholar]
- 63.Carbone D.L. Inhibition of Hsp72-mediated protein refolding by 4-hydroxy-2-nonenal. Chem. Res. Toxicol. 2004;17(11):1459–1467. doi: 10.1021/tx049838g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hickey E. Sequence and regulation of a gene encoding a human 89-kilodalton heat shock protein. Mol. Cell Biol. 1989;9(6):2615–2626. doi: 10.1128/mcb.9.6.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shinkai Y. Cadmium-mediated activation of the HSP90/HSF1 pathway regulated by reactive persulfides/polysulfides. Toxicol. Sci. 2017;156(2):412–421. doi: 10.1093/toxsci/kfw268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen B. The HSP90 family of genes in the human genome: insights into their divergence and evolution. Genomics. 2005;86(6):627–637. doi: 10.1016/j.ygeno.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y. Sulphoxythiocarbamates modify cysteine residues in HSP90 causing degradation of client proteins and inhibition of cancer cell proliferation. Br. J. Canc. 2014;110(1):71–82. doi: 10.1038/bjc.2013.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shibata T. Transthiocarbamoylation of proteins by thiolated isothiocyanates. J. Biol. Chem. 2011;286(49):42150–42161. doi: 10.1074/jbc.M111.308049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fuse Y., Kobayashi M. Conservation of the keap1-nrf2 system: an evolutionary journey through stressful space and time. Molecules. 2017;22(3) doi: 10.3390/molecules22030436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moi P. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. U. S. A. 1994;91(21):9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Itoh K. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol. Cell Biol. 1995;15(8):4184–4193. doi: 10.1128/mcb.15.8.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chan K. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc. Natl. Acad. Sci. U. S. A. 1996;93(24):13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuroha T. Ablation of Nrf2 function does not increase the erythroid or megakaryocytic cell lineage dysfunction caused by p45 NF-E2 gene disruption. J. Biochem. 1998;123(3):376–379. doi: 10.1093/oxfordjournals.jbchem.a021947. [DOI] [PubMed] [Google Scholar]
- 74.Itoh K. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236(2):313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 75.Alam J. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999;274(37):26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 76.Wild A.C., Moinova H.R., Mulcahy R.T. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J. Biol. Chem. 1999;274(47):33627–33636. doi: 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- 77.Tanigawa S., Fujii M., Hou D.X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Biol. Med. 2007;42(11):1690–1703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 78.Tarantini S. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood-brain barrier disruption, neuroinflammation, amyloidogenic gene expression, and cognitive decline in mice, mimicking the aging phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2018;73(7):853–863. doi: 10.1093/gerona/glx177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Toyama T. Cytoprotective role of Nrf2/Keap1 system in methylmercury toxicity. Biochem. Biophys. Res. Commun. 2007;363(3):645–650. doi: 10.1016/j.bbrc.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 80.Chu C. Ambient PM2.5 caused depressive-like responses through Nrf2/NLRP3 signaling pathway modulating inflammation. J. Hazard Mater. 2019;369:180–190. doi: 10.1016/j.jhazmat.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 81.Bouvier E. Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol. Psychiatr. 2017;22(12):1701–1713. doi: 10.1038/mp.2016.144. [DOI] [PubMed] [Google Scholar]
- 82.Zolnourian A., Galea I., Bulters D. Neuroprotective role of the Nrf2 pathway in subarachnoid haemorrhage and its therapeutic potential. Oxid. Med. Cell. Longev. 2019;2019:6218239. doi: 10.1155/2019/6218239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dai P. The roles of Nrf2 and autophagy in modulating inflammation mediated by TLR4 - NFkappaB in A549cell exposed to layer house particulate matter 2.5 (PM2.5) Chemosphere. 2019;235:1134–1145. doi: 10.1016/j.chemosphere.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 84.Toyama T. Isothiocyanates reduce mercury accumulation via an Nrf2-dependent mechanism during exposure of mice to methylmercury. Environ. Health Perspect. 2011;119(8):1117–1122. doi: 10.1289/ehp.1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hou Y. Nrf2 inhibits NLRP3 inflammasome activation through regulating Trx1/TXNIP complex in cerebral ischemia reperfusion injury. Behav. Brain Res. 2018;336:32–39. doi: 10.1016/j.bbr.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 86.Jiang T. p62 links autophagy and Nrf2 signaling. Free Radic. Biol. Med. 2015;88(Pt B):199–204. doi: 10.1016/j.freeradbiomed.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kahroba H. The Role of Nrf2 signaling in cancer stem cells: from stemness and self-renewal to tumorigenesis and chemoresistance. Life Sci. 2019:116986. doi: 10.1016/j.lfs.2019.116986. [DOI] [PubMed] [Google Scholar]
- 88.Rojo de la Vega M., Chapman E., Zhang D.D. NRF2 and the hallmarks of cancer. Canc. Cell. 2018;34(1):21–43. doi: 10.1016/j.ccell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ramos-Gomez M. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2001;98(6):3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Knatko E.V. Nrf2 activation protects against solar-simulated ultraviolet radiation in mice and humans. Canc. Prev. Res. 2015;8(6):475–486. doi: 10.1158/1940-6207.CAPR-14-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sekhar K.R., Freeman M.L. Nrf2 promotes survival following exposure to ionizing radiation. Free Radic. Biol. Med. 2015;88(Pt B):268–274. doi: 10.1016/j.freeradbiomed.2015.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Homma S. Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin. Canc. Res. 2009;15(10):3423–3432. doi: 10.1158/1078-0432.CCR-08-2822. [DOI] [PubMed] [Google Scholar]
- 93.Menegon S., Columbano A., Giordano S. The dual roles of NRF2 in cancer. Trends Mol. Med. 2016;22(7):578–593. doi: 10.1016/j.molmed.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 94.Dinkova-Kostova A.T., Kostov R.V., Canning P. Keap1, the cysteine-based mammalian intracellular sensor for electrophiles and oxidants. Arch. Biochem. Biophys. 2017;617:84–93. doi: 10.1016/j.abb.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Canning P. Structural basis for Cul3 protein assembly with the BTB-Kelch family of E3 ubiquitin ligases. J. Biol. Chem. 2013;288(11):7803–7814. doi: 10.1074/jbc.M112.437996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tong K.I. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol. Cell Biol. 2006;26(8):2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Canning P., Sorrell F.J., Bullock A.N. Structural basis of Keap1 interactions with Nrf2. Free Radic. Biol. Med. 2015;88(Pt B):101–107. doi: 10.1016/j.freeradbiomed.2015.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin W. Regulation of Nrf2 transactivation domain activity by p160 RAC3/SRC3 and other nuclear co-regulators. J. Biochem. Mol. Biol. 2006;39(3):304–310. doi: 10.5483/bmbrep.2006.39.3.304. [DOI] [PubMed] [Google Scholar]
- 99.Bryan H.K. The Nrf2 cell defence pathway: keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 2013;85(6):705–717. doi: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 100.Krajka-Kuzniak V., Paluszczak J., Baer-Dubowska W. The Nrf2-ARE signaling pathway: an update on its regulation and possible role in cancer prevention and treatment. Pharmacol. Rep. 2017;69(3):393–402. doi: 10.1016/j.pharep.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 101.Taguchi K., Motohashi H., Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Gene Cell. 2011;16(2):123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 102.Suzuki T., Yamamoto M. Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress. J. Biol. Chem. 2017;292(41):16817–16824. doi: 10.1074/jbc.R117.800169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Suzuki T. Molecular mechanism of cellular oxidative stress sensing by Keap1. Cell Rep. 2019;28(3):746–758 e4. doi: 10.1016/j.celrep.2019.06.047. [DOI] [PubMed] [Google Scholar]
- 104.Al Bitar S., Gali-Muhtasib H. The role of the cyclin dependent kinase inhibitor p21(cip1/waf1) in targeting cancer: molecular mechanisms and novel therapeutics. Cancers. 2019;11(10) doi: 10.3390/cancers11101475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen W. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol. Cell. 2009;34(6):663–673. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Villeneuve N.F. Nrf2 and p21 regulate the fine balance between life and death by controlling ROS levels. Cell Cycle. 2009;8(20):3255–3256. doi: 10.4161/cc.8.20.9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shan Y. Prophylaxis of diallyl disulfide on skin carcinogenic model via p21-dependent Nrf2 stabilization. Sci. Rep. 2016;6:35676. doi: 10.1038/srep35676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sanchez-Martin P., Komatsu M. p62/SQSTM1 - steering the cell through health and disease. J. Cell Sci. 2018;131(21) doi: 10.1242/jcs.222836. [DOI] [PubMed] [Google Scholar]
- 109.Kageyama S. Negative regulation of the keap1-nrf2 pathway by a p62/sqstm1 splicing variant. Mol. Cell Biol. 2018;38(7) doi: 10.1128/MCB.00642-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Numazawa S. Atypical protein kinase C mediates activation of NF-E2-related factor 2 in response to oxidative stress. Am. J. Physiol. Cell Physiol. 2003;285(2):C334–C342. doi: 10.1152/ajpcell.00043.2003. [DOI] [PubMed] [Google Scholar]
- 111.Huang H.C., Nguyen T., Pickett C.B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 2002;277(45):42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 112.Huang Y. The complexity of the Nrf2 pathway: beyond the antioxidant response. J. Nutr. Biochem. 2015;26(12):1401–1413. doi: 10.1016/j.jnutbio.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun Z., Huang Z., Zhang D.D. Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PloS One. 2009;4(8):e6588. doi: 10.1371/journal.pone.0006588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ho H.K. Nrf2 activation involves an oxidative-stress independent pathway in tetrafluoroethylcysteine-induced cytotoxicity. Toxicol. Sci. 2005;86(2):354–364. doi: 10.1093/toxsci/kfi205. [DOI] [PubMed] [Google Scholar]
- 115.Cullinan S.B. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell Biol. 2003;23(20):7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Duda P. Targeting GSK3 signaling as a potential therapy of neurodegenerative diseases and aging. Expert Opin. Ther. Targets. 2018;22(10):833–848. doi: 10.1080/14728222.2018.1526925. [DOI] [PubMed] [Google Scholar]
- 117.Hayes J.D. Dual regulation of transcription factor Nrf2 by Keap1 and by the combined actions of beta-TrCP and GSK-3. Biochem. Soc. Trans. 2015;43(4):611–620. doi: 10.1042/BST20150011. [DOI] [PubMed] [Google Scholar]
- 118.Culbreth M., Zhang Z., Aschner M. Methylmercury augments Nrf2 activity by downregulation of the Src family kinase Fyn. Neurotoxicology. 2017;62:200–206. doi: 10.1016/j.neuro.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang L. Nrf2-mediated liver protection by esculentoside A against acetaminophen toxicity through the AMPK/Akt/GSK3beta pathway. Free Radic. Biol. Med. 2016;101:401–412. doi: 10.1016/j.freeradbiomed.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 120.Calkins M.J. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxidants Redox Signal. 2009;11(3):497–508. doi: 10.1089/ars.2008.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McMahon M. Redox-regulated turnover of Nrf2 is determined by at least two separate protein domains, the redox-sensitive Neh2 degron and the redox-insensitive Neh6 degron. J. Biol. Chem. 2004;279(30):31556–31567. doi: 10.1074/jbc.M403061200. [DOI] [PubMed] [Google Scholar]
- 122.Cuadrado A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/beta-TrCP. Free Radic. Biol. Med. 2015;88(Pt B):147–157. doi: 10.1016/j.freeradbiomed.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 123.Jain A.K., Jaiswal A.K. GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J. Biol. Chem. 2007;282(22):16502–16510. doi: 10.1074/jbc.M611336200. [DOI] [PubMed] [Google Scholar]
- 124.Compeau G.C., Bartha R. Sulfate-reducing bacteria: principal methylators of mercury in anoxic estuarine sediment. Appl. Environ. Microbiol. 1985;50(2):498–502. doi: 10.1128/aem.50.2.498-502.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hintelmann H. Organomercurials. Their formation and pathways in the environment. Met. Ions Life Sci. 2010;7:365–401. doi: 10.1039/BK9781847551771-00365. [DOI] [PubMed] [Google Scholar]
- 126.Bisen-Hersh E.B. Behavioral effects of developmental methylmercury drinking water exposure in rodents. J. Trace Elem. Med. Biol. 2014;28(2):117–124. doi: 10.1016/j.jtemb.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Albores-Garcia D. Early developmental low-dose methylmercury exposure alters learning and memory in periadolescent but not young adult rats. BioMed Res. Int. 2016;2016:6532108. doi: 10.1155/2016/6532108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Farina M., Aschner M., Rocha J.B. Oxidative stress in MeHg-induced neurotoxicity. Toxicol. Appl. Pharmacol. 2011;256(3):405–417. doi: 10.1016/j.taap.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Karpova N.N. TrkB overexpression in mice buffers against memory deficits and depression-like behavior but not all anxiety- and stress-related symptoms induced by developmental exposure to methylmercury. Front. Behav. Neurosci. 2014;8:315. doi: 10.3389/fnbeh.2014.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Feng S. Sulforaphane prevents methylmercury-induced oxidative damage and excitotoxicity through activation of the nrf2-ARE pathway. Mol. Neurobiol. 2017;54(1):375–391. doi: 10.1007/s12035-015-9643-y. [DOI] [PubMed] [Google Scholar]
- 131.Ni M. Comparative study on the response of rat primary astrocytes and microglia to methylmercury toxicity. Glia. 2011;59(5):810–820. doi: 10.1002/glia.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang L. Methylmercury toxicity and Nrf2-dependent detoxification in astrocytes. Toxicol. Sci. 2009;107(1):135–143. doi: 10.1093/toxsci/kfn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kumagai Y. The role of the Keap1/Nrf2 pathway in the cellular response to methylmercury. Oxid. Med. Cell. Longev. 2013;2013:848279. doi: 10.1155/2013/848279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Toyama T. Convenient method to assess chemical modification of protein thiols by electrophilic metals. J. Toxicol. Sci. 2013;38(3):477–484. doi: 10.2131/jts.38.477. [DOI] [PubMed] [Google Scholar]
- 135.Yoshida E., Abiko Y., Kumagai Y. Glutathione adduct of methylmercury activates the Keap1-Nrf2 pathway in SH-SY5Y cells. Chem. Res. Toxicol. 2014;27(10):1780–1786. doi: 10.1021/tx5002332. [DOI] [PubMed] [Google Scholar]
- 136.Levonen A.L. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem. J. 2004;378(Pt 2):373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Unoki T. Molecular pathways associated with methylmercury-induced Nrf2 modulation. Front. Genet. 2018;9:373. doi: 10.3389/fgene.2018.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Underwood E. Trace metals in human and animal health. J. Human Nutr. 1981 doi: 10.3109/09637488109143484. [DOI] [PubMed] [Google Scholar]
- 139.Stephenson A.P. Manganese-induced oxidative DNA damage in neuronal SH-SY5Y cells: attenuation of thymine base lesions by glutathione and N-acetylcysteine. Toxicol. Lett. 2013;218(3):299–307. doi: 10.1016/j.toxlet.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guilarte T.R. Manganese and Parkinson's disease: a critical review and new findings. Environ. Health Perspect. 2010;118(8):1071–1080. doi: 10.1289/ehp.0901748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guilarte T.R., Gonzales K.K. Manganese-induced parkinsonism is not idiopathic Parkinson's disease: environmental and genetic evidence. Toxicol. Sci. 2015;146(2):204–212. doi: 10.1093/toxsci/kfv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li H. Nrf2/HO‐1 pathway activation by manganese is associated with reactive oxygen species and ubiquitin–proteasome pathway, not MAPKs signaling. J. Appl. Toxicol. 2011;31(7):690–697. doi: 10.1002/jat.1654. [DOI] [PubMed] [Google Scholar]
- 143.Jiang W.-D. Impairment of gill structural integrity by manganese deficiency or excess related to induction of oxidative damage, apoptosis and dysfunction of the physical barrier as regulated by NF-κB, caspase and Nrf2 signaling in fish. Fish Shellfish Immunol. 2017;70:280–292. doi: 10.1016/j.fsi.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 144.Jiang W.-D. Manganese deficiency or excess caused the depression of intestinal immunity, induction of inflammation and dysfunction of the intestinal physical barrier, as regulated by NF-κB, TOR and Nrf2 signalling, in grass carp (Ctenopharyngodon idella) Fish Shellfish Immunol. 2015;46(2):406–416. doi: 10.1016/j.fsi.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 145.Qi Z. The effect of manganese exposure on GnRH secretion via Nrf2/mGluR5/COX-2/PGE2/signaling pathway. Toxicol. Ind. Health. 2019;35(3):211–227. doi: 10.1177/0748233719825720. [DOI] [PubMed] [Google Scholar]
- 146.Deng Y. Melatonin antagonizes Mn-induced oxidative injury through the activation of keap1–Nrf2–ARE signaling pathway in the striatum of mice. Neurotox. Res. 2015;27(2):156–171. doi: 10.1007/s12640-014-9489-5. [DOI] [PubMed] [Google Scholar]
- 147.Li H. NF-E2-related factor 2 activation in PC12 cells: its protective role in manganese-induced damage. Arch. Toxicol. 2011;85(8):901–910. doi: 10.1007/s00204-010-0625-6. [DOI] [PubMed] [Google Scholar]
- 148.Lee E. 15-Deoxy-Δ12, 14-prostaglandin J2 modulates manganese-induced activation of the NF-κB, Nrf2, and PI3K pathways in astrocytes. Free Radic. Biol. Med. 2012;52(6):1067–1074. doi: 10.1016/j.freeradbiomed.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bahar E., Kim J.-Y., Yoon H. Quercetin attenuates manganese-induced neuroinflammation by alleviating oxidative stress through regulation of apoptosis, iNOS/NF-κB and HO-1/Nrf2 pathways. Int. J. Mol. Sci. 2017;18(9):1989. doi: 10.3390/ijms18091989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Anetor J.I. Rising environmental cadmium levels in developing countries: threat to genome stability and health. Niger. J. Physiol. Sci. 2012;27(2):103–115. [PubMed] [Google Scholar]
- 151.Shimada H. Strain difference of cadmium accumulation by liver slices of inbred Wistar-Imamichi and Fischer 344 rats. Toxicol. Vitro. 2008;22(2):338–343. doi: 10.1016/j.tiv.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 152.Erbanova L. Export of arsenic from forested catchments under easing atmospheric pollution. Environ. Sci. Technol. 2008;42(19):7187–7192. doi: 10.1021/es800467j. [DOI] [PubMed] [Google Scholar]
- 153.Patra R., Rautray A.K., Swarup D. Oxidative stress in lead and cadmium toxicity and its amelioration. Vet. Med. Int. 2011;2011 doi: 10.4061/2011/457327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu J., Qu W., Kadiiska M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009;238(3):209–214. doi: 10.1016/j.taap.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Thevenod F., Lee W.K. Cadmium and cellular signaling cascades: interactions between cell death and survival pathways. Arch. Toxicol. 2013;87(10):1743–1786. doi: 10.1007/s00204-013-1110-9. [DOI] [PubMed] [Google Scholar]
- 156.Bernhoft R.A. Cadmium toxicity and treatment. Sci. World J. 2013;2013 doi: 10.1155/2013/394652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cancer I.A.f.R.o. Dry cleaning, some chlorinated solvents and other industrial chemicals. Vol. 63. 1995. IARC monographs on the evaluation of carcinogenic risks to humans; pp. 443–465. [PMC free article] [PubMed] [Google Scholar]
- 158.Hettick B.E. Arsenic: a review of the element's toxicity, plant interactions, and potential methods of remediation. J. Agric. Food Chem. 2015;63(32):7097–7107. doi: 10.1021/acs.jafc.5b02487. [DOI] [PubMed] [Google Scholar]
- 159.Jomova K. Arsenic: toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011;31(2):95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- 160.He X., Chen M.G., Ma Q. Activation of Nrf2 in defense against cadmium-induced oxidative stress. Chem. Res. Toxicol. 2008;21(7):1375–1383. doi: 10.1021/tx800019a. [DOI] [PubMed] [Google Scholar]
- 161.Chen J., Shaikh Z.A. Activation of Nrf2 by cadmium and its role in protection against cadmium-induced apoptosis in rat kidney cells. Toxicol. Appl. Pharmacol. 2009;241(1):81–89. doi: 10.1016/j.taap.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 162.Fu J. Low-level arsenic impairs glucose-stimulated insulin secretion in pancreatic beta cells: involvement of cellular adaptive response to oxidative stress. Environ. Health Perspect. 2010;118(6):864–870. doi: 10.1289/ehp.0901608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shinkai Y. Partial contribution of the Keap1-Nrf2 system to cadmium-mediated metallothionein expression in vascular endothelial cells. Toxicol. Appl. Pharmacol. 2016;295:37–46. doi: 10.1016/j.taap.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 164.Tuder R.M., Petrache I. Pathogenesis of chronic obstructive pulmonary disease. J. Clin. Invest. 2012;122(8):2749–2755. doi: 10.1172/JCI60324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shinkai Y. Sulforaphane, an activator of Nrf2, suppresses cellular accumulation of arsenic and its cytotoxicity in primary mouse hepatocytes. FEBS Lett. 2006;580(7):1771–1774. doi: 10.1016/j.febslet.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 166.Wu X. NRF2 is a potential modulator of hyperresistance to arsenic toxicity in stem-like keratinocytes. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/7417694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mohajeri M., Rezaee M., Sahebkar A. Cadmium‐induced toxicity is rescued by curcumin: a review. Biofactors. 2017;43(5):645–661. doi: 10.1002/biof.1376. [DOI] [PubMed] [Google Scholar]
- 168.Wu K.C., Liu J.J., Klaassen C.D. Nrf2 activation prevents cadmium-induced acute liver injury. Toxicol. Appl. Pharmacol. 2012;263(1):14–20. doi: 10.1016/j.taap.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 169.Chen D., Huang C., Chen Z. A review for the pharmacological effect of lycopene in central nervous system disorders. Biomed. Pharmacother. 2019;111:791–801. doi: 10.1016/j.biopha.2018.12.151. [DOI] [PubMed] [Google Scholar]
- 170.Vineetha R.C. L-ascorbic acid and α-tocopherol attenuate arsenic trioxide-induced toxicity in H9c2 cardiomyocytes by the activation of Nrf2 and Bcl2 transcription factors. Toxicol. Mech. Methods. 2018;28(5):353–360. doi: 10.1080/15376516.2017.1422578. [DOI] [PubMed] [Google Scholar]
- 171.Li S. Lutein alleviates arsenic-induced reproductive toxicity in male mice via Nrf2 signaling. Hum. Exp. Toxicol. 2016;35(5):491–500. doi: 10.1177/0960327115595682. [DOI] [PubMed] [Google Scholar]
- 172.Clark M. Brain structure and function in patients after metal-on-metal hip resurfacing. Am. J. Neuroradiol. 2014;35(9):1753–1758. doi: 10.3174/ajnr.A3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pelclova D. Severe cobalt intoxication following hip replacement revision: clinical features and outcome. Clin. Toxicol. 2012;50(4):262–265. doi: 10.3109/15563650.2012.670244. [DOI] [PubMed] [Google Scholar]
- 174.Cheung A. Systemic cobalt toxicity from total hip arthroplasties: review of a rare condition Part 1-history, mechanism, measurements, and pathophysiology. Bone Joint J. 2016;98(1):6–13. doi: 10.1302/0301-620X.98B1.36374. [DOI] [PubMed] [Google Scholar]
- 175.Zywiel M. Systemic cobalt toxicity from total hip arthroplasties: review of a rare condition Part 2. measurement, risk factors, and step-wise approach to treatment. Bone Joint J. 2016;98(1):14–20. doi: 10.1302/0301-620X.98B1.36712. [DOI] [PubMed] [Google Scholar]
- 176.Leyssens L. Cobalt toxicity in humans—a review of the potential sources and systemic health effects. Toxicology. 2017;387:43–56. doi: 10.1016/j.tox.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 177.Zheng F. Comparison of the neurotoxicity associated with cobalt nanoparticles and cobalt chloride in Wistar rats. Toxicol. Appl. Pharmacol. 2019;369:90–99. doi: 10.1016/j.taap.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 178.Akhtar M.J. Nanotoxicity of cobalt induced by oxidant generation and glutathione depletion in MCF-7 cells. Toxicol. Vitro. 2017;40:94–101. doi: 10.1016/j.tiv.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 179.Zhang X.D. Tungsten carbide-cobalt particles activate Nrf2 and its downstream target genes in JB6 cells possibly by ROS generation. J. Environ. Pathol. Toxicol. Oncol. 2010;29(1):31–40. doi: 10.1615/jenvironpatholtoxicoloncol.v29.i1.60. [DOI] [PubMed] [Google Scholar]
- 180.Cappellini F. Mechanistic insight into reactivity and (geno) toxicity of well-characterized nanoparticles of cobalt metal and oxides. Nanotoxicology. 2018;12(6):602–620. doi: 10.1080/17435390.2018.1470694. [DOI] [PubMed] [Google Scholar]
- 181.Zheng F., Li H. Nanotoxicity. Springer; 2019. Evaluation of Nrf2 with exposure to nanoparticles; pp. 229–246. [DOI] [PubMed] [Google Scholar]
- 182.Permenter M.G. Exposure to cobalt causes transcriptomic and proteomic changes in two rat liver derived cell lines. PloS One. 2013;8(12) doi: 10.1371/journal.pone.0083751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yang B. Protective roles of NRF2 signaling pathway in cobalt chloride-induced hypoxic cytotoxicity in human HaCaT keratinocytes. Toxicol. Appl. Pharmacol. 2018;355:189–197. doi: 10.1016/j.taap.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 184.Yuan Z. NRF2 overexpression in mesenchymal stem cells induces stem-cell marker expression and enhances osteoblastic differentiation. Biochem. Biophys. Res. Commun. 2017;491(1):228–235. doi: 10.1016/j.bbrc.2017.07.083. [DOI] [PubMed] [Google Scholar]
- 185.Jiang L., Li H., Zhao N. Thymoquinone protects against cobalt chloride-induced neurotoxicity via Nrf2/GCL-regulated glutathione homeostasis. J. Biol. Regul. Homeost. Agents. 2017;31(4):843–853. [PubMed] [Google Scholar]
- 186.Yu J. mfat‐1 transgene protects cultured adult neural stem cells against cobalt chloride‐mediated hypoxic injury by activating Nrf2/ARE pathways. J. Neurosci. Res. 2018;96(1):87–102. doi: 10.1002/jnr.24096. [DOI] [PubMed] [Google Scholar]
- 187.Lin H.C. Andrographolide inhibits hypoxia‐induced HIF‐1α‐driven endothelin 1 secretion by activating Nrf2/HO‐1 and promoting the expression of prolyl hydroxylases 2/3 in human endothelial cells. Environ. Toxicol. 2017;32(3):918–930. doi: 10.1002/tox.22293. [DOI] [PubMed] [Google Scholar]
- 188.Casida J.E. Mechanisms of selective action of pyrethroid insecticides. Annu. Rev. Pharmacol. Toxicol. 1983;23(1):413–438. doi: 10.1146/annurev.pa.23.040183.002213. [DOI] [PubMed] [Google Scholar]
- 189.Soderlund D.M. Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology. 2002;171(1):3–59. doi: 10.1016/s0300-483x(01)00569-8. [DOI] [PubMed] [Google Scholar]
- 190.Kirby M.L., Castagnoli K., Bloomquist J.R. In vivo effects of deltamethrin on dopamine neurochemistry and the role of augmented neurotransmitter release. Pestic. Biochem. Physiol. 1999;65(3):160–168. [Google Scholar]
- 191.Wu A., Liu Y. Deltamethrin induces delayed apoptosis and altered expression of p53 and bax in rat brain. Environ. Toxicol. Pharmacol. 2000;8(3):183–189. doi: 10.1016/s1382-6689(00)00039-9. [DOI] [PubMed] [Google Scholar]
- 192.Wu A., Liu Y. Apoptotic cell death in rat brain following deltamethrin treatment. Neurosci. Lett. 2000;279(2):85–88. doi: 10.1016/s0304-3940(99)00973-8. [DOI] [PubMed] [Google Scholar]
- 193.Wu A. Deltamethrin induces altered expression of P53, Bax and Bcl-2 in rat brain. Neurosci. Lett. 2000;284(1–2):29–32. doi: 10.1016/s0304-3940(00)00952-6. [DOI] [PubMed] [Google Scholar]
- 194.Liu G.-p., Ma Q., Shi N. Tyrosine hydroxylase as a target for deltamethrin in the nigrostriatal dopaminergic pathway. Biomed. Environ. Sci. 2006;19(1):27. [PubMed] [Google Scholar]
- 195.Liu G.-P., Shi N. The inhibitory effects of deltamethrin on dopamine biosynthesis in rat PC12 cells. Toxicol. Lett. 2006;161(3):195–199. doi: 10.1016/j.toxlet.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 196.Li H.-Y., Wu S.-Y., Shi N. Transcription factor Nrf2 activation by deltamethrin in PC12 cells: involvement of ROS. Toxicol. Lett. 2007;171(1–2):87–98. doi: 10.1016/j.toxlet.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 197.Li H. The pesticide deltamethrin increases free radical production and promotes nuclear translocation of the stress response transcription factor Nrf2 in rat brain. Toxicol. Ind. Health. 2011;27(7):579–590. doi: 10.1177/0748233710393400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li H. Neuroprotective effects of tert-butylhydroquinone on paraquat-induced dopaminergic cell degeneration in C57BL/6 mice and in PC12 cells. Arch. Toxicol. 2012;86(11):1729–1740. doi: 10.1007/s00204-012-0935-y. [DOI] [PubMed] [Google Scholar]
- 199.Li H.Y. NF-E2 related factor 2 activation and heme oxygenase-1 induction by tert-butylhydroquinone protect against deltamethrin-mediated oxidative stress in PC12 cells. Chem. Res. Toxicol. 2007;20(9):1242–1251. doi: 10.1021/tx700076q. [DOI] [PubMed] [Google Scholar]
- 200.Saoudi M. Deltamethrin induced oxidative stress in kidney and brain of rats: protective effect of Artemisia campestris essential oil. Biomed. Pharmacother. 2017;94:955–963. doi: 10.1016/j.biopha.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 201.Shi W. Curcumin synergistically potentiates the protective effect of sitagliptin against chronic deltamethrin nephrotoxicity in rats: impact on pro‐inflammatory cytokines and Nrf2/Ho‐1 pathway. J. Biochem. Mol. Toxicol. 2019;33(10) doi: 10.1002/jbt.22386. [DOI] [PubMed] [Google Scholar]
- 202.Lungu-Mitea S., Oskarsson A., Lundqvist J. Development of an oxidative stress in vitro assay in zebrafish (Danio rerio) cell lines. Sci. Rep. 2018;8(1):12380. doi: 10.1038/s41598-018-30880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Liu Y. Exploring the correlation between deltamethrin stress and keap1-nrf2-are pathway from drosophila melanogaster RNASeq Data. Genomics. 2019 doi: 10.1016/j.ygeno.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 204.Kang M.J., Gil S.J., Koh H.C. Paraquat induces alternation of the dopamine catabolic pathways and glutathione levels in the substantia nigra of mice. Toxicol. Lett. 2009;188(2):148–152. doi: 10.1016/j.toxlet.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 205.Li A.A. Evaluation of epidemiologic and animal data associating pesticides with Parkinson's disease. J. Occup. Environ. Med. 2005;47(10):1059–1087. doi: 10.1097/01.jom.0000174294.58575.3e. [DOI] [PubMed] [Google Scholar]
- 206.Betarbet R. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat. Neurosci. 2000;3(12):1301. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 207.Liou H. Environmental risk factors and Parkinson's disease: a case‐control study in Taiwan. Neurology. 1997;48(6):1583–1588. doi: 10.1212/wnl.48.6.1583. [DOI] [PubMed] [Google Scholar]
- 208.McCormack A.L. Environmental risk factors and Parkinson's disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol. Dis. 2002;10(2):119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- 209.Peng J. The herbicide paraquat induces dopaminergic nigral apoptosis through sustained activation of the JNK pathway. J. Biol. Chem. 2004;279(31):32626–32632. doi: 10.1074/jbc.M404596200. [DOI] [PubMed] [Google Scholar]
- 210.Manning-Bog A.B. The herbicide paraquat causes up-regulation and aggregation of α-synuclein in mice paraquat and α-synuclein. J. Biol. Chem. 2002;277(3):1641–1644. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- 211.Jin W. Protective effect of tert-butylhydroquinone on cerebral inflammatory response following traumatic brain injury in mice. Injury. 2011;42(7):714–718. doi: 10.1016/j.injury.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 212.Nouhi F. Dietary supplementation with tBHQ, an Nrf2 stabilizer molecule, confers neuroprotection against apoptosis in amyloid β-injected rat. Neurochem. Res. 2011;36(5):870–878. doi: 10.1007/s11064-011-0417-2. [DOI] [PubMed] [Google Scholar]
- 213.Toulouse A., Sullivan A.M. Progress in Parkinson's disease—where do we stand? Prog. Neurobiol. 2008;85(4):376–392. doi: 10.1016/j.pneurobio.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 214.Dauer W., Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 215.Yang W. Paraquat induces cyclooxygenase-2 (COX-2) implicated toxicity in human neuroblastoma SH-SY5Y cells. Toxicol. Lett. 2010;199(3):239–246. doi: 10.1016/j.toxlet.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 216.Ishii T. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000;275(21):16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 217.Izumi Y. Compensatory role of the Nrf2–ARE pathway against paraquat toxicity: relevance of 26S proteasome activity. J. Pharmacol. Sci. 2015;129(3):150–159. doi: 10.1016/j.jphs.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 218.Blanco-Ayala T., Andérica-Romero A., Pedraza-Chaverri J. New insights into antioxidant strategies against paraquat toxicity. Free Radic. Res. 2014;48(6):623–640. doi: 10.3109/10715762.2014.899694. [DOI] [PubMed] [Google Scholar]
- 219.Zhao X. JWA antagonizes paraquat-induced neurotoxicity via activation of Nrf2. Toxicol. Lett. 2017;277:32–40. doi: 10.1016/j.toxlet.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 220.Betarbet R. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat. Neurosci. 2000;3(12):1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 221.Johnson M.E., Bobrovskaya L. An update on the rotenone models of Parkinson's disease: their ability to reproduce the features of clinical disease and model gene-environment interactions. Neurotoxicology. 2015;46:101–116. doi: 10.1016/j.neuro.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 222.Talpade D.J. In vivo labeling of mitochondrial complex I (NADH:ubiquinone oxidoreductase) in rat brain using [(3)H]dihydrorotenone. J. Neurochem. 2000;75(6):2611–2621. doi: 10.1046/j.1471-4159.2000.0752611.x. [DOI] [PubMed] [Google Scholar]
- 223.Nisticò R. SAGE Publications Sage UK; London, England: 2011. Paraquat-and Rotenone-Induced Models of Parkinson's Disease. [DOI] [PubMed] [Google Scholar]
- 224.Circu M.L., Aw T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010;48(6):749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zagoura D. Evaluation of the rotenone-induced activation of the Nrf2 pathway in a neuronal model derived from human induced pluripotent stem cells. Neurochem. Int. 2017;106:62–73. doi: 10.1016/j.neuint.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 226.Wu F. p38MAPK/p53-Mediated Bax induction contributes to neurons degeneration in rotenone-induced cellular and rat models of Parkinson's disease. Neurochem. Int. 2013;63(3):133–140. doi: 10.1016/j.neuint.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 227.Choi W.-S. JNK3 mediates paraquat-and rotenone-induced dopaminergic neuron death. J. Neuropathol. Exp. Neurol. 2010;69(5):511–520. doi: 10.1097/NEN.0b013e3181db8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Vezina C., Kudelski A., Sehgal S. Rapamycin (AY-22, 989), a new antifungal antibiotic. J. Antibiot. 1975;28(10):721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 229.Yoo Y.J. An overview of rapamycin: from discovery to future perspectives. J. Ind. Microbiol. Biotechnol. 2017;44(4–5):537–553. doi: 10.1007/s10295-016-1834-7. [DOI] [PubMed] [Google Scholar]
- 230.Li J., Kim S.G., Blenis J. Rapamycin: one drug, many effects. Cell Metabol. 2014;19(3):373–379. doi: 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Laplante M., Sabatini D.M. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Loewith R., Hall M.N. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189(4):1177–1201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zou Z. Targeted inhibition of rictor/mTORC2 in cancer treatment: a new era after rapamycin. Curr. Cancer Drug Targets. 2016;16(4):288–304. doi: 10.2174/1568009616666151113120830. [DOI] [PubMed] [Google Scholar]
- 234.Saraiva M.A. Exposure of Drosophila melanogaster to mancozeb induces oxidative Damage and modulates Nrf2 and HSP70/83. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/5456928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lundqvist J., Hellman B., Oskarsson A. Fungicide prochloraz induces oxidative stress and DNA damage in vitro. Food Chem. Toxicol. 2016;91:36–41. doi: 10.1016/j.fct.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 236.Hernandez-Cortes D. Epigenetic modulation of Nrf2 and Ogg1 gene expression in testicular germ cells by methyl parathion exposure. Toxicol. Appl. Pharmacol. 2018;346:19–27. doi: 10.1016/j.taap.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 237.Lebov J.F. Pesticide use and risk of end-stage renal disease among licensed pesticide applicators in the Agricultural Health Study. Occup. Environ. Med. 2016;73(1):3–12. doi: 10.1136/oemed-2014-102615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lin J. Lycopene triggers nrf2–AMPK cross talk to alleviate atrazine-induced nephrotoxicity in mice. J. Agric. Food Chem. 2018;66(46):12385–12394. doi: 10.1021/acs.jafc.8b04341. [DOI] [PubMed] [Google Scholar]
- 239.Lim S.S. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Cesaroni G. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ. 2014;348:f7412. doi: 10.1136/bmj.f7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Miller K.A. Long-term exposure to air pollution and incidence of cardiovascular events in women. N. Engl. J. Med. 2007;356(5):447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 242.Oono I.P., Mackay D.F., Pell J.P. Meta-analysis of the association between secondhand smoke exposure and stroke. J. Public Health. 2011;33(4):496–502. doi: 10.1093/pubmed/fdr025. [DOI] [PubMed] [Google Scholar]
- 243.Jones L.L. Parental smoking and the risk of middle ear disease in children: a systematic review and meta-analysis. Arch. Pediatr. Adolesc. Med. 2012;166(1):18–27. doi: 10.1001/archpediatrics.2011.158. [DOI] [PubMed] [Google Scholar]
- 244.Tinuoye O., Pell J.P., Mackay D.F. Meta-analysis of the association between secondhand smoke exposure and physician-diagnosed childhood asthma. Nicotine Tob. Res. 2013;15(9):1475–1483. doi: 10.1093/ntr/ntt033. [DOI] [PubMed] [Google Scholar]
- 245.Hori M. Secondhand smoke exposure and risk of lung cancer in Japan: a systematic review and meta-analysis of epidemiologic studies. Jpn. J. Clin. Oncol. 2016;46(10):942–951. doi: 10.1093/jjco/hyw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Chen H. Long-term exposure to traffic-related air pollution and cardiovascular mortality. Epidemiology. 2013;24(1):35–43. doi: 10.1097/EDE.0b013e318276c005. [DOI] [PubMed] [Google Scholar]
- 247.Baccarelli A. Living near major traffic roads and risk of deep vein thrombosis. Circulation. 2009;119(24):3118–3124. doi: 10.1161/CIRCULATIONAHA.108.836163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Hoffmann B. Residential exposure to traffic is associated with coronary atherosclerosis. Circulation. 2007;116(5):489–496. doi: 10.1161/CIRCULATIONAHA.107.693622. [DOI] [PubMed] [Google Scholar]
- 249.Korek M.J. Traffic-related air pollution exposure and incidence of stroke in four cohorts from Stockholm. J. Expo. Sci. Environ. Epidemiol. 2015;25(5):517–523. doi: 10.1038/jes.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lipsett M., Campleman S. Occupational exposure to diesel exhaust and lung cancer: a meta-analysis. Am. J. Publ. Health. 1999;89(7):1009–1017. doi: 10.2105/ajph.89.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Silverman D.T. The Diesel Exhaust in Miners study: a nested case-control study of lung cancer and diesel exhaust. J. Natl. Cancer Inst. 2012;104(11):855–868. doi: 10.1093/jnci/djs034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Farmer S.A. Ambient and household air pollution: complex triggers of disease. Am. J. Physiol. Heart Circ. Physiol. 2014;307(4):H467–H476. doi: 10.1152/ajpheart.00235.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Pope C.A., 3rd Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. J. Am. Med. Assoc. 2002;287(9):1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Schneider G., Krivna V. Multi-element analysis of tobacco and smoke condensate by instrumental neutron activation analysis and atomic absorption spectrometry. Int. J. Environ. Anal. Chem. 1993;53(2):87–100. [Google Scholar]
- 255.Kresovich J.K. Metallic air pollutants and breast cancer heterogeneity. Environ. Res. 2019;177:108639. doi: 10.1016/j.envres.2019.108639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.White A.J. Metallic air pollutants and breast cancer risk in a nationwide cohort study. Epidemiology. 2019;30(1):20–28. doi: 10.1097/EDE.0000000000000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Delgado-Saborit J.M. Analysis of atmospheric concentrations of quinones and polycyclic aromatic hydrocarbons in vapour and particulate phases. Atmos. Environ. 2013;77:974–982. [Google Scholar]
- 258.Doelman C.J., Bast A. Oxygen radicals in lung pathology. Free Radic. Biol. Med. 1990;9(5):381–400. doi: 10.1016/0891-5849(90)90015-b. [DOI] [PubMed] [Google Scholar]
- 259.Kumagai Y. Generation of reactive oxygen species during interaction of diesel exhaust particle components with NADPH-cytochrome P450 reductase and involvement of the bioactivation in the DNA damage. Free Radic. Biol. Med. 1997;22(3):479–487. doi: 10.1016/s0891-5849(96)00341-3. [DOI] [PubMed] [Google Scholar]
- 260.Kautzman K.E. Chemical composition of gas- and aerosol-phase products from the photooxidation of naphthalene. J. Phys. Chem. 2010;114(2):913–934. doi: 10.1021/jp908530s. [DOI] [PubMed] [Google Scholar]
- 261.Chu S.N. Ozone oxidation of surface-adsorbed polycyclic aromatic hydrocarbons: role of PAH-surface interaction. J. Am. Chem. Soc. 2010;132(45):15968–15975. doi: 10.1021/ja1014772. [DOI] [PubMed] [Google Scholar]
- 262.Cho A.K. Determination of four quinones in diesel exhaust particles, SRM 1649a, an atmospheric PM2.5. Aerosol. Sci. Technol. 2004;38:68–81. [Google Scholar]
- 263.Takano H. Diesel exhaust particles enhance antigen-induced airway inflammation and local cytokine expression in mice. Am. J. Respir. Crit. Care Med. 1997;156(1):36–42. doi: 10.1164/ajrccm.156.1.9610054. [DOI] [PubMed] [Google Scholar]
- 264.Hiyoshi K. Effects of phenanthraquinone on allergic airway inflammation in mice. Clin. Exp. Allergy. 2005;35(9):1243–1248. doi: 10.1111/j.1365-2222.2005.02297.x. [DOI] [PubMed] [Google Scholar]
- 265.Hiyoshi K. Effects of a single intratracheal administration of phenanthraquinone on murine lung. J. Appl. Toxicol. 2005;25(1):47–51. doi: 10.1002/jat.1017. [DOI] [PubMed] [Google Scholar]
- 266.Sugimoto R. 9,10-Phenanthraquinone in diesel exhaust particles downregulates Cu,Zn-SOD and HO-1 in human pulmonary epithelial cells: intracellular iron scavenger 1,10-phenanthroline affords protection against apoptosis. Free Radic. Biol. Med. 2005;38(3):388–395. doi: 10.1016/j.freeradbiomed.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 267.Matsunaga T. L-Xylulose reductase is involved in 9,10-phenanthrenequinone-induced apoptosis in human T lymphoma cells. Free Radic. Biol. Med. 2008;44(6):1191–1202. doi: 10.1016/j.freeradbiomed.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 268.Kumagai Y. Oxidation of proximal protein sulfhydryls by phenanthraquinone, a component of diesel exhaust particles. Chem. Res. Toxicol. 2002;15(4):483–489. doi: 10.1021/tx0100993. [DOI] [PubMed] [Google Scholar]
- 269.Rodriguez C.E. An examination of quinone toxicity using the yeast Saccharomyces cerevisiae model system. Toxicology. 2004;201(1–3):185–196. doi: 10.1016/j.tox.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 270.Rodriguez C.E. The interactions of 9,10-phenanthrenequinone with glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a potential site for toxic actions. Chem. Biol. Interact. 2005;155(1–2):97–110. doi: 10.1016/j.cbi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 271.Honda A. Synergic effects of 9,10-phenanthrenequinone and cadmium on pro-inflammatory responses in airway epithelial cells. Environ. Toxicol. Pharmacol. 2017;52:276–279. doi: 10.1016/j.etap.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 272.Moncada S., Palmer R.M., Higgs E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43(2):109–142. [PubMed] [Google Scholar]
- 273.Stuehr D.J. Structure-function aspects in the nitric oxide synthases. Annu. Rev. Pharmacol. Toxicol. 1997;37:339–359. doi: 10.1146/annurev.pharmtox.37.1.339. [DOI] [PubMed] [Google Scholar]
- 274.Kumagai Y. Inhibition of nitric oxide formation by neuronal nitric oxide synthase by quinones: nitric oxide synthase as a quinone reductase. Chem. Res. Toxicol. 1998;11(6):608–613. doi: 10.1021/tx970119u. [DOI] [PubMed] [Google Scholar]
- 275.Kumagai Y. Phenanthraquinone inhibits eNOS activity and suppresses vasorelaxation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281(1):R25–R30. doi: 10.1152/ajpregu.2001.281.1.R25. [DOI] [PubMed] [Google Scholar]
- 276.Koizumi R. Interaction of 9,10-phenanthraquinone with dithiol causes oxidative modification of Cu,Zn-superoxide dismutase (SOD) through redox cycling. J. Toxicol. Sci. 2013;38(3):317–324. doi: 10.2131/jts.38.317. [DOI] [PubMed] [Google Scholar]
- 277.Abiko Y. Interaction of quinone-related electron acceptors with hydropersulfide Na2S2: evidence for one-electron reduction reaction. Chem. Res. Toxicol. 2019;32(4):551–556. doi: 10.1021/acs.chemrestox.8b00158. [DOI] [PubMed] [Google Scholar]
- 278.Wang W.X. Examination of the mechanism of phenanthrenequinone toxicity to Vibrio fischeri: evidence for a reactive oxygen species-mediated toxicity mechanism. Environ. Toxicol. Chem. 2009;28(8):1655–1662. doi: 10.1897/08-463.1. [DOI] [PubMed] [Google Scholar]
- 279.Taguchi K. An approach to evaluate two-electron reduction of 9,10-phenanthraquinone and redox activity of the hydroquinone associated with oxidative stress. Free Radic. Biol. Med. 2007;43(5):789–799. doi: 10.1016/j.freeradbiomed.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 280.Taguchi K. Redox cycling of 9,10-phenanthraquinone to cause oxidative stress is terminated through its monoglucuronide conjugation in human pulmonary epithelial A549 cells. Free Radic. Biol. Med. 2008;44(8):1645–1655. doi: 10.1016/j.freeradbiomed.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 281.Asahi M. Identification and quantification of in vivo metabolites of 9,10-phenanthrenequinone in human urine associated with producing reactive oxygen species. Chem. Res. Toxicol. 2014;27(1):76–85. doi: 10.1021/tx400338t. [DOI] [PubMed] [Google Scholar]
- 282.Cho H.Y. Role of NRF2 in protection against hyperoxic lung injury in mice. Am. J. Respir. Cell Mol. Biol. 2002;26(2):175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 283.Maher J.M. Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology. 2007;46(5):1597–1610. doi: 10.1002/hep.21831. [DOI] [PubMed] [Google Scholar]
- 284.Iwamoto N. Biochemical and cellular effects of electrophiles present in ambient air samples. Atmos. Environ. 2010;44(12):1483–1489. [Google Scholar]
- 285.Shinkai Y. Ambient vapor samples activate the nrf2-ARE pathway in human bronchial epithelial BEAS-2B cells. Environ. Toxicol. 2014;29(11):1292–1300. doi: 10.1002/tox.21860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Schübeler D. Function and information content of DNA methylation. Nature. 2015;517(7534):321–326. doi: 10.1038/nature14192. [DOI] [PubMed] [Google Scholar]
- 287.Edwards C.A. The origins of genomic imprinting in mammals. Reprod. Fertil. Dev. 2019 doi: 10.1071/RD18176. [DOI] [PubMed] [Google Scholar]
- 288.Jones P.A., Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293(5532):1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- 289.Li Z. The polycomb group protein EZH2 inhibits lung cancer cell growth by repressing the transcription factor Nrf2. FEBS Lett. 2014;588(17):3000–3007. doi: 10.1016/j.febslet.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 290.Fabrizio F.P. Epigenetic versus genetic deregulation of the KEAP1/NRF2 Axis in solid tumors: focus on methylation and noncoding RNAs. Oxid. Med. Cell. Longev. 2018;2018:2492063. doi: 10.1155/2018/2492063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Elanchezhian R. Age-related cataracts: homocysteine coupled endoplasmic reticulum stress and suppression of Nrf2-dependent antioxidant protection. Chem. Biol. Interact. 2012;200(1):1–10. doi: 10.1016/j.cbi.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Palsamy P. Promoter demethylation of Keap1 gene in human diabetic cataractous lenses. Biochem. Biophys. Res. Commun. 2012;423(3):542–548. doi: 10.1016/j.bbrc.2012.05.164. [DOI] [PubMed] [Google Scholar]
- 293.Gao Y., Yan Y., Huang T. Human age-related cataracts: epigenetic suppression of the nuclear factor erythroid 2-related factor 2-mediated antioxidant system. Mol. Med. Rep. 2015;11(2):1442–1447. doi: 10.3892/mmr.2014.2849. [DOI] [PubMed] [Google Scholar]
- 294.Yu S. Nrf2 expression is regulated by epigenetic mechanisms in prostate cancer of TRAMP mice. PloS One. 2010;5(1):e8579. doi: 10.1371/journal.pone.0008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Huang Y. A γ-tocopherol-rich mixture of tocopherols maintains Nrf2 expression in prostate tumors of TRAMP mice via epigenetic inhibition of CpG methylation. J. Nutr. 2012;142(5):818–823. doi: 10.3945/jn.111.153114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Kang K. Epigenetic modification of Nrf2 in 5-fluorouracil-resistant colon cancer cells: involvement of TET-dependent DNA demethylation. Cell Death Dis. 2014;5(4):e1183. doi: 10.1038/cddis.2014.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Kornberg R.D., Lorch Y. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell. 1999;98(3):285–294. doi: 10.1016/s0092-8674(00)81958-3. [DOI] [PubMed] [Google Scholar]
- 298.Huisinga K.L., Brower-Toland B., Elgin S.C. The contradictory definitions of heterochromatin: transcription and silencing. Chromosoma. 2006;115(2):110–122. doi: 10.1007/s00412-006-0052-x. [DOI] [PubMed] [Google Scholar]
- 299.Ura K. Histone acetylation: influence on transcription, nucleosome mobility and positioning, and linker histone‐dependent transcriptional repression. EMBO J. 1997;16(8):2096–2107. doi: 10.1093/emboj/16.8.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Alves-Fernandes D.K., Jasiulionis M.G. The role of SIRT1 on DNA damage response and epigenetic alterations in cancer. Int. J. Mol. Sci. 2019;20(13) doi: 10.3390/ijms20133153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Huang K. Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-β1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free Radic. Biol. Med. 2013;65:528–540. doi: 10.1016/j.freeradbiomed.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 302.Huang K., Gao X., Wei W. The crosstalk between Sirt1 and Keap1/Nrf2/ARE anti-oxidative pathway forms a positive feedback loop to inhibit FN and TGF-beta1 expressions in rat glomerular mesangial cells. Exp. Cell Res. 2017;361(1):63–72. doi: 10.1016/j.yexcr.2017.09.042. [DOI] [PubMed] [Google Scholar]
- 303.Ma F. P53/NRF2 mediates SIRT1's protective effect on diabetic nephropathy. Biochim. Biophys. Acta Mol. Cell Res. 2019;1866(8):1272–1281. doi: 10.1016/j.bbamcr.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 304.Chai D. Nrf2 activation induced by Sirt1 ameliorates acute lung injury after intestinal ischemia/reperfusion through NOX4-mediated gene regulation. Cell. Physiol. Biochem. 2018;46(2):781–792. doi: 10.1159/000488736. [DOI] [PubMed] [Google Scholar]
- 305.McDougald D.S. SIRT1 and NRF2 gene transfer mediate distinct neuroprotective effects upon retinal ganglion cell survival and function in experimental optic neuritis. Invest. Ophthalmol. Vis. Sci. 2018;59(3):1212–1220. doi: 10.1167/iovs.17-22972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Ma R. Sirt1/Nrf2 pathway is involved in oocyte aging by regulating Cyclin B1. Aging. 2018;10(10):2991–3004. doi: 10.18632/aging.101609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Zhang P. Resveratrol ameliorated vascular calcification by regulating sirt-1 and Nrf2. Transplant. Proc. 2016;48(10):3378–3386. doi: 10.1016/j.transproceed.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 308.Ong A.L.C., Ramasamy T.S. Role of Sirtuin1-p53 regulatory axis in aging, cancer and cellular reprogramming. Ageing Res. Rev. 2018;43:64–80. doi: 10.1016/j.arr.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 309.Guo Z. Manganese chloride induces histone acetylation changes in neuronal cells: its role in manganese-induced damage. Neurotoxicology. 2018;65:255–263. doi: 10.1016/j.neuro.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 310.Zhang Z. Role of histone acetylation in activation of nuclear factor erythroid 2-related factor 2/heme oxygenase 1 pathway by manganese chloride. Toxicol. Appl. Pharmacol. 2017;336:94–100. doi: 10.1016/j.taap.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 311.Mercado N. Decreased histone deacetylase 2 impairs Nrf2 activation by oxidative stress. Biochem. Biophys. Res. Commun. 2011;406(2):292–298. doi: 10.1016/j.bbrc.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Adenuga D. Nrf2 deficiency influences susceptibility to steroid resistance via HDAC2 reduction. Biochem. Biophys. Res. Commun. 2010;403(3–4):452–456. doi: 10.1016/j.bbrc.2010.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Lam H.C. Histone deacetylase 6–mediated selective autophagy regulates COPD-associated cilia dysfunction. J. Clin. Invest. 2013;123(12):5212–5230. doi: 10.1172/JCI69636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Chase A., Cross N.C. Aberrations of EZH2 in cancer. Clin. Canc. Res. 2011;17(9):2613–2618. doi: 10.1158/1078-0432.CCR-10-2156. [DOI] [PubMed] [Google Scholar]
- 315.Ruthenburg A.J., Allis C.D., Wysocka J. Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Mol. Cell. 2007;25(1):15–30. doi: 10.1016/j.molcel.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 316.Sanchez R., Zhou M.-M. The role of human bromodomains in chromatin biology and gene transcription. Curr. Opin. Drug Discov. Dev. 2009;12(5):659. [PMC free article] [PubMed] [Google Scholar]
- 317.Michaeloudes C. Bromodomain and extraterminal proteins suppress NF-E2–Related factor 2–mediated antioxidant gene expression. J. Immunol. 2014;192(10):4913–4920. doi: 10.4049/jimmunol.1301984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Miguel V. The role of MicroRNAs in environmental risk factors, noise-induced hearing loss, and mental stress. Antioxidants Redox Signal. 2018;28(9):773–796. doi: 10.1089/ars.2017.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Yang M. MiR-28 regulates Nrf2 expression through a Keap1-independent mechanism. Breast Canc. Res. Treat. 2011;129(3):983–991. doi: 10.1007/s10549-011-1604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Chen C. MicroRNA-155 regulates arsenite-induced malignant transformation by targeting Nrf2-mediated oxidative damage in human bronchial epithelial cells. Toxicol. Lett. 2017;278:38–47. doi: 10.1016/j.toxlet.2017.07.215. [DOI] [PubMed] [Google Scholar]
- 321.Zhou C. MicroRNA-144 modulates oxidative stress tolerance in SH-SY5Y cells by regulating nuclear factor erythroid 2-related factor 2-glutathione axis. Neurosci. Lett. 2017;655:21–27. doi: 10.1016/j.neulet.2017.06.045. [DOI] [PubMed] [Google Scholar]
- 322.Singh B. MicroRNA-93 regulates NRF2 expression and is associated with breast carcinogenesis. Carcinogenesis. 2013;34(5):1165–1172. doi: 10.1093/carcin/bgt026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Narasimhan M. Identification of novel microRNAs in post-transcriptional control of Nrf2 expression and redox homeostasis in neuronal, SH-SY5Y cells. PloS One. 2012;7(12):e51111. doi: 10.1371/journal.pone.0051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Shi L. miR-340 reverses cisplatin resistance of hepatocellular carcinoma cell lines by targeting Nrf2-dependent antioxidant pathway. Asian Pac. J. Cancer Prev. APJCP. 2015;15(23):10439–10444. doi: 10.7314/apjcp.2014.15.23.10439. [DOI] [PubMed] [Google Scholar]
- 325.Qaisiya M. Bilirubin mediated oxidative stress involves antioxidant response activation via Nrf2 pathway. Cell. Signal. 2014;26(3):512–520. doi: 10.1016/j.cellsig.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 326.Liao W. MicroRNA-140-5p attenuated oxidative stress in Cisplatin induced acute kidney injury by activating Nrf2/ARE pathway through a Keap1-independent mechanism. Exp. Cell Res. 2017;360(2):292–302. doi: 10.1016/j.yexcr.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 327.Wang Q. Paraquat and MPTP alter microRNA expression profiles, and downregulated expression of miR‐17‐5p contributes to PQ‐induced dopaminergic neurodegeneration. J. Appl. Toxicol. 2018;38(5):665–677. doi: 10.1002/jat.3571. [DOI] [PubMed] [Google Scholar]
- 328.Cai Z. Nrf2-regulated miR-380-3p blocks the translation of Sp3 protein and its mediation of paraquat-induced toxicity in mouse neuroblastoma N2a cells. Toxicol. Sci. 2019;171(2):515–529. doi: 10.1093/toxsci/kfz162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Van Jaarsveld M. miR-141 regulates KEAP1 and modulates cisplatin sensitivity in ovarian cancer cells. Oncogene. 2013;32(36):4284. doi: 10.1038/onc.2012.433. [DOI] [PubMed] [Google Scholar]
- 330.White N.M. Transcriptome sequencing reveals altered long intergenic non-coding RNAs in lung cancer. Genome Biol. 2014;15(8):429. doi: 10.1186/s13059-014-0429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Schmidlin C.J. Redox regulation by NRF2 in aging and disease. Free Radic. Biol. Med. 2019;134:702–707. doi: 10.1016/j.freeradbiomed.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Bahn G., Jo D.G. Therapeutic approaches to alzheimer's disease through modulation of NRF2. NeuroMolecular Med. 2019;21(1):1–11. doi: 10.1007/s12017-018-08523-5. [DOI] [PubMed] [Google Scholar]
- 333.Satta S. The role of Nrf2 in cardiovascular function and disease. Oxid. Med. Cell. Longev. 2017;2017:9237263. doi: 10.1155/2017/9237263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Sim H.-J. Glucose oxidase facilitates osteogenic differentiation and mineralization of embryonic stem cells through the activation of Nrf2 and ERK signal transduction pathways. Mol. Cell. Biochem. 2016;419(1–2):157–163. doi: 10.1007/s11010-016-2760-8. [DOI] [PubMed] [Google Scholar]
- 335.Freigang S. Nrf2 is essential for cholesterol crystal‐induced inflammasome activation and exacerbation of atherosclerosis. Eur. J. Immunol. 2011;41(7):2040–2051. doi: 10.1002/eji.201041316. [DOI] [PubMed] [Google Scholar]
- 336.Kerins M.J., Ooi A. The roles of NRF2 in modulating cellular iron homeostasis. Antioxidants Redox Signal. 2018;29(17):1756–1773. doi: 10.1089/ars.2017.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Abdelsalam S.S. The role of protein tyrosine phosphatase (PTP)-1B in cardiovascular disease and its interplay with insulin resistance. Biomolecules. 2019;9(7) doi: 10.3390/biom9070286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Li A. PTEN, insulin resistance and cancer. Curr. Pharmaceut. Des. 2017;23(25):3667–3676. doi: 10.2174/1381612823666170704124611. [DOI] [PubMed] [Google Scholar]
- 339.Makker A., Goel M.M., Mahdi A.A. PI3K/PTEN/Akt and TSC/mTOR signaling pathways, ovarian dysfunction, and infertility: an update. J. Mol. Endocrinol. 2014;53(3):R103–R118. doi: 10.1530/JME-14-0220. [DOI] [PubMed] [Google Scholar]
- 340.Hoter A., El-Sabban M.E., Naim H.Y. The HSP90 family: structure, regulation, function, and implications in health and disease. Int. J. Mol. Sci. 2018;19(9) doi: 10.3390/ijms19092560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Gomez-Pastor R., Burchfiel E.T., Thiele D.J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018;19(1):4–19. doi: 10.1038/nrm.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Vogeley C. Role of the aryl hydrocarbon receptor in environmentally induced skin aging and skin carcinogenesis. Int. J. Mol. Sci. 2019;20(23) doi: 10.3390/ijms20236005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Rosenfeld C.S. Gut dysbiosis in animals due to environmental chemical exposures. Front. Cell. Infect. Microbiol. 2017;7:396. doi: 10.3389/fcimb.2017.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Jin Q. Metabolomics and microbiomes as potential tools to evaluate the effects of the mediterranean diet. Nutrients. 2019;11(1) doi: 10.3390/nu11010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Hirata S.I., Kunisawa J. Gut microbiome, metabolome, and allergic diseases. Allergol. Int. 2017;66(4):523–528. doi: 10.1016/j.alit.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 346.Zhang T. Mass spectrometry-based proteomics for system-level characterization of biological responses to engineered nanomaterials. Anal. Bioanal. Chem. 2018;410(24):6067–6077. doi: 10.1007/s00216-018-1168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]