Abstract
Cardiovascular disease remains the leading cause of death in the United States, and cardiopulmonary bypass is a cornerstone in the surgical management of many related disease states. Pathophysiologic changes associated both with extracorporeal circulation and shock can beget a syndrome of low systemic vascular resistance paired with relatively preserved cardiac output, termed vasoplegia. While increased vasopressor requirements accompany vasoplegia, related pathophysiologic mechanisms may also lead to true catecholamine resistance, which is associated with further heightened mortality. The introduction of a second non-catecholamine vasopressor, angiotensin II, and non-specific nitric oxide scavengers offers potential means by which to manage this challenging phenomenon. This narrative review addresses both the definition, risk factors, and pathophysiology of vasoplegia and potential therapeutic interventions.
Keywords: Vasoplegia, cardiopulmonary bypass, surgical shock, vasoconstrictor agents, methylene blue, hydroxocobalamin
Introduction
Despite improvements in the overall mortality from cardiovascular disease over the past 35 years, it remains the leading cause of death in the United States.1 While the volume of minimally invasive cardiac procedures has recently increased, the overall volume of open heart surgeries has also increased.2 As such, cardiopulmonary bypass (CPB) remains a cornerstone in the surgical management for our country’s deadliest disease. A relatively common complication of CPB is vasoplegia with an estimated incidence of 5%–25%.3 Outcomes associated with vasoplegia after CPB include renal failure, prolonged intensive care unit and hospital length of stay, and increased mortality.3–5 Catecholamine-resistant vasoplegia is particularly lethal with mortality rates as high as 25%.6
This narrative review will address the pathophysiology of post-CPB vasoplegia and summarize emerging treatment modalities with a focus on two non-vasopressor-targeted therapies: methylene blue and hydroxocobalamin.
Post-CPB vasoplegia: definition, risk factors, and pathophysiology
Vasoplegia, also known as vasoplegic shock or distributive shock, is the syndrome of low systemic vascular resistance (SVR) in the presence of normal or high cardiac output. Criteria in the published literature have been variable, but broadly a mean arterial pressure of less than 65 mmHg with a cardiac index of greater than 2.2 L/min/m2 is consistent with vasoplegia.7 High-dose vasopressor drugs are typically necessary to maintain adequate mean arterial blood pressure. Beyond the effects of CPB, vasoplegia may occur in many disease states, such as septic shock, end-stage liver disease, and glucocorticoid deficiency. Risk factors for post-CPB vasoplegia include the use of preoperative angiotensin-converting enzyme inhibitors (ACEi) or beta-blockers, higher comorbid disease burden, low preoperative ejection fraction, need for vasopressors before or during CPB, warmer core temperatures while on bypass, and relatively longer durations of aortic cross-clamping and CPB.8,9
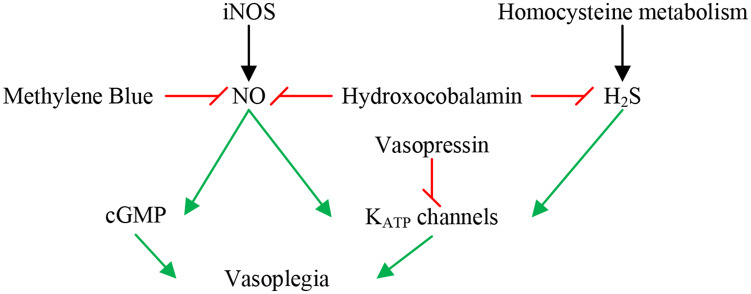
The pathophysiology of vasoplegia has been relatively well elucidated at the cellular level. A myriad of interactions cause the associated impaired vascular smooth muscle contraction resulting in vasoplegia: derangements in receptor signaling, metabolic changes, the depletion of endogenous vasoactive hormones, and the alteration of the endothelial glycocalyx (Figure 1).
Figure 1.
Biochemical pathways and therapies for vasoplegia.
Red lines denote inhibition; green arrows denote stimulation. iNOS: inducible nitric oxide synthase; NO: nitric oxide; cGMP: cyclic guanosine monophosphate; KATP: potassium-adenosine triphosphate; H2S: hydrogen sulfide.
Inducible nitric oxide synthase (iNOS), triggered by inflammatory cytokines, is likely a major contributor to inappropriate vasodilation in vasoplegia. Notably, CPB is associated with increased iNOS levels proportional to the total time on CPB.5 iNOS produces nitric oxide (NO), which increases vascular levels of cyclic guanosine monophosphate (cGMP), resulting in vasodilation.10 In addition, in vascular smooth muscle cells, adenosine triphosphate-sensitive potassium (KATP) channels prevent calcium entry, thus preventing vasoconstriction. NO is an activator of KATP channels thereby providing another pathophysiologic role in vasoplegia.5
The downstream effects of increased NO concentrations in vasoplegia are exacerbated by low levels of serum vasopressin, typical of prolonged shock. Of interest, in post-CPB patients that develop vasoplegia, vasopressin levels have been found to be even more depressed than in septic states.5 Vasopressin normally induces vasoconstriction through vasopressin 1 (V1) and oxytocin receptors by way of increased intracellular calcium levels.11 In addition, vasopressin also modulates KATP channels, blunting the NO-induced increase in cGMP5 and enhancing the vascular response to catecholamines.12
Another pathophysiologic mediator of vasoplegia is hydrogen sulfide (H2S), a by-product of the vitamin B6-dependent pathway of homocysteine metabolism. At high concentrations, such as in inflammatory states, H2S directly activates and hyperpolarizes KATP channels, therefore reducing the vascular tone.13,14 This mechanism is similar to the NO-mediated pathway of vasoplegia, as mentioned above. A small proportion of its vasodilatory effects may also be attributed to its synergistic effect with NO.14
Other specific mechanisms of post-CPB vasoplegia are likely related to the pathologic response secondary to surgical trauma, ischemia-reperfusion injury, transfusion, and/or exposure of blood to the foreign surfaces of CPB circuitry.15 All of these processes result in increased oxygen-free radicals, endothelins, NO, platelet-activating factors, thromboxane A2, prostaglandins, a variety of cytokines, and other vasoactive substances. These factors likely lead to a systemic inflammatory response syndrome, further contributing to the derangement of vascular reactivity. It has been posited that this inflammatory response might explain why pre-existing heart failure is a risk factor for post-CPB vasoplegia, as chronic heart failure patients have high levels of inflammatory mediators.5
Endothelial glycocalyx alterations after CPB have been reported after CPB.16 It is postulated that elements of the glycocalyx regulate vascular tone, making the glycocalyx a potential target for therapy in the setting of post-CPB vasoplegia. A recent study revealed that lower preoperative levels of a proteoglycan, syndecan 1, were associated with postoperative vasoplegia for patients exposed to CPB.17 Currently, there are no commercially available therapeutic interventions which target the endothelial glycocalyx.
Catecholamine resistance in vasoplegia
Vasoplegia, by definition, involves vascular hyporeactivity, which is typically combatted clinically through the administration of vasopressors, many of which are catecholamines. Vasopressor needs are, therefore, typically elevated in vasoplegic states. However, a subset of patients develops true catecholamine resistance, which is associated with significant mortality after CPB.6 Catecholamine-resistant vasoplegia may be defined as a low SVR state with normal or increased CO with an inability to maintain a mean arterial pressure of 60 mmHg despite high-dose vasopressors, typically 0.5 mcg/kg/min of norepinephrine (or equivalent) or greater.10 Some underlying pathologic mechanisms are shared between vasoplegia and true catecholamine resistance.18 For example, KATP under the influence of NO, vasopressin, and (indirectly) endotoxins modulates the endothelial response to catecholamines.19,20 Oxidative stress during shock may be such that the typical physiologic reduction of superoxide anions to hydrogen peroxide by superoxide dismutase can be overwhelmed, leading to their proliferation. Superoxide has been shown to deactivate exogenous norepinephrine, which can be reversed by synthetic analogues of superoxide dismutase.21 In extrapolating experimental evidence from animal models of septic shock, it is also conceivable that altered alpha-1 adrenergic receptor expression may contribute to catecholamine resistance.22
Therapies for post-CPB vasoplegia
Vasopressors
Vasopressors are typically the first-line treatment for vasoplegia after a fluid challenge has been performed without success (Table 1). At this time, there is no established first-line vasopressor for vasoplegia following CPB.12,23–27 Sympathomimetic agents, such as norepinephrine, epinephrine, and phenylephrine, are commonly used. Norepinephrine is an alpha-1 and beta-1 adrenergic receptor agonist. Epinephrine is an alpha-1, beta-1, and beta-2 adrenergic receptor agonist. Phenylephrine is an alpha-1 adrenergic receptor agonist. These agents have variable but equally unwanted side effects at high doses, including dysrhythmias, increased myocardial oxygen demand, hyperglycemia, and lactic acidosis.28,29 Regardless, these agents are very familiar to clinicians owing to their routine perioperative utilization for cardiothoracic procedures. As previously discussed, catecholamine resistance may accompany vasoplegia, and minimal or absent response to vasopressor up-titration is a common but alarming scenario for clinicians caring for patients after prolonged exposure to CPB.
Table 1.
Pharmacologic options for the treatment of post-CPB vasoplegia.
| Class | Drug | Cautions | |
|---|---|---|---|
| Treatment | Vasopressor | Catecholamines | Well studied and familiar, however, clear risks (end-organ damage) exist with prolonged infusion. |
| Vasopressin | Second line for vasoplegia. Risk for mesenteric malperfusion. | ||
| Angiotensin II | Newest agent for high-output shock. Yet to be studied in the cardiac surgery population. | ||
| Non-vasopressor | Corticosteroids | Extensively studied in septic shock, less so in cardiac surgery. May result in hyperglycemia, GI bleeding. | |
| Ascorbic acid | Dearth of high-quality evidence for its use both in septic shock and cardiac surgery. | ||
| Methylene blue | May decrease norepinephrine requirement but can increase PVR. Monoamine oxidase inhibitory effects represent a contraindication in patients taking selective serotonin reuptake inhibitors or other serotonergic medications. | ||
| Hydroxocobalamin | Under investigation for post-CPB vasoplegia. May cause dialysis alarms due to chromaturia. |
GI: gastrointestinal; PVR: pulmonary vascular resistance.
As such, there is an established role for non-catecholamine vasopressors in these scenarios. Vasopressin, one such agent, has an established track record for the management of catecholamine-resistant shock. Its release from the posterior pituitary gland normally occurs in humans in response to increased plasma osmolality or hypotension. It binds to V1a, V1b, and V2 receptors, which cause vasoconstriction, water reabsorption at the renal collecting ducts, and increased secretion of cortisol and insulin, respectively.30 Agonism of V1 receptors also augments baroreflex inhibition of efferent sympathetic nerve activity, which explains the mild bradycardia and lack of blood pressure effect seen for healthy adults receiving a vasopressin infusion.31 However, vasopressin infusions can be quite effective in the shock state with depletion of endogenous sympathetic activity. As discussed above, post-CPB patients may have low serum vasopressin levels, and therefore, vasopressin may be used with some success in the treatment of post-CPB vasoplegia.12,25,27 However, it may also have unwanted side effects at higher doses, including renal and gastrointestinal malperfusion.32–34
Angiotensin II is a new vasopressor used for the treatment of vasodilatory shock. As a component of the renin–angiotensin–aldosterone system, angiotensin II acts on angiotensin type I receptors throughout the body to cause vasoconstriction, sympathetic nervous system activation, secretion of aldosterone, and renal sodium and water retention.35 Its utility has been demonstrated with persistent hypotension unresponsive to high-dose vasopressors. It has been shown to decrease other vasopressor requirements but with a mortality improvement only in specific subgroups, including those with an Acute Physiology and Chronic Health Evaluation II (APACHE II) score greater than 30 and in those with kidney injury receiving renal replacement therapy.36–38 It has not been exclusively studied in cardiac surgery patients, though case reports of successful use in this population exist.39,40 Undesirable side effects may include its association with a reduction in glomerular filtration rate, increased pulmonary vascular resistance, and asthma exacerbations.35 Although the underlying evidence is inconclusive, some concern persists about the potential prothrombotic effects of angiotensin II.41,42 Additional investigational and clinical experience is needed to establish its safety in this domain, namely in patients exposed to extracorporeal circulation.
Non-vasopressor therapies
Corticosteroids are often used to treat vasodilatory shock with the assumption that they may supplement a depleted adrenal axis in critical illness. Several randomized controlled trials have shown that steroids may reduce the duration of vasoplegia in septic shock. Two of these studies demonstrated a mortality benefit with steroids, but three other large studies failed to replicate that mortality benefit.43–47 In all of these trials, adverse events occurred similarly in the treatment groups, though hyperglycemia was unsurprisingly more frequent in those that received steroids. There are some data that prophylactic dexamethasone administration to cardiac surgery patients may reduce the composite outcome of death and other major morbidities.48 The use of corticosteroids for the treatment of vasoplegia after CPB has not been studied, but their adverse effects in this population should be closely considered, including delayed wound healing, hyperglycemia, and an increased risk of gastrointestinal bleeding.5
Ascorbic acid (i.e. vitamin C) is a novel non-vasopressor agent used in the treatment of vasodilatory shock, currently being evaluated for its role (if any) in septic shock. The rationale for its use stems from its anti-inflammatory properties and its role as an electron donor in the synthesis of norepinephrine from dopamine by dopamine-beta-hydroxylase.49,50 Ascorbic acid also mediates non-enzymatic metabolism of superoxide, albeit modestly.51 In small studies of septic patients, it has been shown to decrease the duration and dose of norepinephrine infusion and improve mortality.49,52 The trial that prompted enthusiasm for using vitamin C in septic shock was a retrospective before-after study with 47 patients, and therefore, these original results should be interpreted with great caution pending the forthcoming results of large clinical trials. A recent trial examined the role of high-dose vitamin C in patients with sepsis and acute respiratory distress syndrome and found that it did not significantly improve SOFA scores or biomarkers at 1 week.53 An additional recent publication revealed that in concert with hydrocortisone and thiamine, vitamin C did not lead to a shorter time-to-shock-resolution when compared to hydrocortisone alone.54 Case reports exist of vitamin C’s successful use in cardiac surgery patients,55 but proof of its efficacy in all vasoplegic states is lacking.
Targeted therapies
While many of the above therapies have been studied in a variety of vasoplegic conditions, limited attention has been focused on targeted therapies for vasoplegia after CPB. As discussed, NO is likely a key modulator of vasoplegia after CPB, and two therapies which target NO overproduction hold promise for cardiac surgery patients: methylene blue and hydroxocobalamin.
Methylene blue: everything old is new again?
Methylene blue directly competes with guanylyl cyclase, thereby interrupting the production of cGMP, which leads to vasodilation. It also inhibits iNOS, therefore decreasing the production of NO.56 The use of methylene blue in cardiac surgery patients was first described over 20 years ago.57
Several case reports have described the use of methylene blue in the postoperative period following cardiac surgery with vasoplegic syndrome, which resulted in decreased vasopressor requirements.58–62 A retrospective review of methylene blue administration in vasoplegia after CPB found improved survival and a reduced rate of major adverse events (as defined by the Society of Thoracic Surgeons major morbidities) when administered early versus late in the course of vasoplegia.63 Notably, this study is observational, and the decision for early versus late administration of methylene blue was made under the discretion of the physicians managing the case without any protocol or randomization.
Two randomized trials prophylactically administered methylene blue to patients undergoing surgery with CPB and found a decreased norepinephrine requirement post-CPB.64,65 In one such study, the sample size was small (N = 30) and only included patients currently taking ACEi medications.64 Similarly, another study used a small sample size (N = 100) and only included patients taking ACEi medications, calcium channel blockers, or heparin.65 Another small (N = 56) randomized trial showed a decrease in mortality and duration of vasoplegic syndrome in patients receiving methylene blue compared to placebo.66
In contrast, a retrospective analysis found that cardiac surgery patients who had received methylene blue had higher rates of mortality and a higher composite morbidity measure.67 Of note, after propensity-matching, only the morbidity association remained significant, which may suggest that in this retrospective study, patients at risk for complications of vasoplegic syndrome may have been more likely to receive methylene blue.68
Mild side effects of methylene blue include nausea and vomiting, chest pain, hypertension, and interference with pulse oximetry readings.69 A more serious adverse effect is impaired hypoxic pulmonary vasoconstriction and impaired gas exchange which may limit its use in patients with concomitant impaired respiratory function.70 High doses of methylene blue may compromise splanchnic perfusion.71 As methylene blue can paradoxically act as an oxidant, at high doses, it may cause hemolysis (particularly in patients with glucose-6-phosphate dehydrogenase deficiency), methemoglobinemia, and hyperbilirubinemia.72 In patients also exposed to serotonergic medications (e.g. selective serotonin reuptake inhibitors (SSRIs), fentanyl), methylene blue’s monoamine oxidase inhibitory effect can precipitate serotonergic excess or serotonin syndrome.73 As patients with heart failure may be on SSRIs for the treatment of depression, this is an important consideration.
While there is some evidence for the use of methylene blue in the treatment of post-CPB vasoplegia, more research is needed. Methylene blue offers another treatment option for this condition, particularly when patients have failed traditional vasopressor therapy and face high mortality risk. Serious side effects of the drug must be weighed with its potential benefits. Certain patient populations, such as those already taking serotonergic drugs and patients with glucose-6-phosphate dehydrogenase deficiency, should be thoughtfully considered before administering methylene blue. It should also be used with caution in patients with lung injury or those who cannot tolerate further increases in pulmonary vascular resistance.
Hydroxocobalamin: an emerging therapeutic option
Hydroxocobalamin, a precursor of vitamin B12, is a novel compound increasingly utilized for the treatment of vasoplegia. It is worth noting that the use of hydroxocobalamin for vasoplegia is an off-label use of the medication. Hydroxocobalamin is approved in the United States for the treatment of cyanide poisoning under the trade name Cyanokit. Similar to methylene blue, B12 has the ability to inhibit guanylate cyclase and is a scavenger for NO (see Figure 1).74,75 It binds H2S attenuating its downstream effects leading to decreased vascular tone whereby leading to hypotension.76
There are several case reports on the use of hydroxocobalamin for postoperative vasoplegia in cardiac surgery, liver transplant surgery, and vascular surgery.77–84 In these reports, hydroxocobalamin was used for refractory vasoplegia and authors reported increased mean arterial blood pressure and decreased vasopressor needs after infusion. The degree to which hydroxocobalamin affects mean arterial blood pressure is unclear as demonstrated in a retrospective case series of 33 patients. This series showed significant heterogeneity in response to hydroxocobalamin infusion for vasoplegic cardiac surgery patients. Of relevance, 27% of patients had no change in blood pressure or vasopressor requirements, and the remainder of the patients had variable positive responses to hydroxocobalamin.85
Another study retrospectively analyzed a small cohort of vasoplegic post-CPB patients who received treatment of only methylene blue (N = 14) or treatment of methylene blue and hydroxocobalamin (N = 6). The authors found that both groups had no significant difference in increased mean arterial blood pressure at 1 h after treatment; however, they did find that the dual therapy group required significantly less vasopressors at that same time point.86 The retrospective nature of this study should be noted with variable-dosing regimens among patients and disparate, small sample sizes in the treatment groups.
As clinical experience with hydroxocobalamin grows, more rigorous studies are necessary to determine its utility and safety.87 There are several pending clinical trials, including the comparison of methylene blue versus hydroxocobalamin in cardiac surgical patients.88,89 Another study will randomize patients with septic shock to receive hydroxocobalamin versus placebo.90 An additional trial will examine the use of hydroxocobalamin versus methylene blue for vasoplegic patients undergoing liver transplant.91
Adverse effects associated with hydroxocobalamin are generally mild and rare. These include chromaturia, erythema, headache, and photosensitivity. More serious side effects include allergic reactions and acute renal failure.92 Other side effects include its ability to falsely activate the blood leak alarm during dialysis owing to chromaturia, transient hypokalemia if used in patients with anemia secondary to B12 deficiency, and interference with various laboratory values.80,92,93 Elevated serum cobalt levels have also been reported after multiple doses of hydroxocobalamin, which can be associated with myocardial dysfunction, polyneuropathy, thyroid dysfunction, and cognitive dysfunction.78
Conclusion
Vasoplegia after cardiac surgery with CPB remains a serious and relatively frequent occurrence. Patients that do not respond to traditional vasopressor therapy are at risk for complications associated with high-dose vasopressors and death. Targeted therapies which address the multifaceted causes of post-CPB vasoplegia will be important for improving outcomes. Methylene blue and hydroxocobalamin are two options in the clinician’s armamentarium for treating vasoplegia, however, further investigation of their utility and safety is unequivocally needed.
This review has limitations. While vasoplegia may affect numerous patient populations, such as those undergoing liver transplantation, we have focused on those requiring CPB. While the underlying pathophysiology may be similar, vasoplegia in other clinical settings may be best approached differently; care should be taken when extrapolating lessons from post-CPB vasoplegia. This narrative review was further intended to maximize clinical utility at the bedside. As discussed previously, scant evidence remains to guide clinicians when confronted with vasoplegia, and our conception of its underlying pathophysiology and management remains dynamic. Many of the therapeutic options reviewed herein are not grounded in firm investigational evidence, which highlights the need for continued research. This need for greater scrutiny cannot be understated, as previous targeted therapies, such as a nitric oxide synthase inhibitor for vasoplegia, showed promise until a randomized trial demonstrated harm.94
The era of tailored medicine with the ability to target specific cellular level pathophysiology, rather than broad treatments with unintended consequences, holds promise. Given the mortality burden conferred by various vasoplegic etiologies, namely post-CPB and septic shock, if future trials show success with targeted therapies, perhaps findings can be applied across disease states.
Footnotes
Author contributions: T.J.B. helped to acquire and interpret the primary source information, drafted the work, and approved the final version to be published. M.H. helped to acquire and interpret the primary source information, revised the work critically for important intellectual content, and approved the final version to be published. C.S.J. helped conceive the work, helped interpret the primary source information, revised the work critically for important intellectual content, and approved the final version to be published.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Theresa J Barnes  https://orcid.org/0000-0002-5279-5373
https://orcid.org/0000-0002-5279-5373
Craig S Jabaley  https://orcid.org/0000-0001-6687-3953
https://orcid.org/0000-0001-6687-3953
References
- 1. Wang H, Naghavi M, Allen C, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the global burden of disease study 2015. Lancet 2016; 388(10053): 1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. D’Agostino RS, Jacobs JP, Badhwar V, et al. The society of thoracic surgeons adult cardiac surgery database: 2019 update on outcomes and quality. Ann Thorac Surg 2019; 107(1): 24–32. [DOI] [PubMed] [Google Scholar]
- 3. Fischer GW, Levin MA. Vasoplegia during cardiac surgery: current concepts and management. Semin Thorac Cardiovasc Surg 2010; 22(2): 140–144. [DOI] [PubMed] [Google Scholar]
- 4. Carrel T, Englberger L, Mohacsi P, et al. Low systemic vascular resistance after cardiopulmonary bypass: incidence, etiology, and clinical importance. J Card Surg 2000; 15(5): 347–353. [DOI] [PubMed] [Google Scholar]
- 5. Shaefi S, Mittel A, Klick J, et al. Vasoplegia after cardiovascular procedures—pathophysiology and targeted therapy. J Cardiothorac Vasc Anesth 2018; 32(2): 1013–1022. [DOI] [PubMed] [Google Scholar]
- 6. Gomes WJ, Carvalho AC, Palma JH, et al. Vasoplegic syndrome after open heart surgery. J Cardiovasc Surg (Torino) 1998; 39(5): 619–623. [PubMed] [Google Scholar]
- 7. Dayan V, Cal R, Giangrossi F. Risk factors for vasoplegia after cardiac surgery: a meta-analysis. Interact Cardiovasc Thorac Surg 2019; 28(6): 838–844. [DOI] [PubMed] [Google Scholar]
- 8. Weis F, Kilger E, Beiras-Fernandez A, et al. Association between vasopressor dependence and early outcome in patients after cardiac surgery. Anaesthesia 2006; 61(10): 938–942. [DOI] [PubMed] [Google Scholar]
- 9. Levin MA, Lin HM, Castillo JG, et al. Early on-cardiopulmonary bypass hypotension and other factors associated with vasoplegic syndrome. Circulation 2009; 120(17): 1664–1671. [DOI] [PubMed] [Google Scholar]
- 10. Jentzer JC, Vallabhajosyula S, Khanna AK, et al. Management of refractory vasodilatory shock. Chest 2018; 154(2): 416–426. [DOI] [PubMed] [Google Scholar]
- 11. Barrett LK, Singer M, Clapp LH. Vasopressin: mechanisms of action on the vasculature in health and in septic shock. Crit Care Med 2007; 35(1): 33–40. [DOI] [PubMed] [Google Scholar]
- 12. Hajjar LA, Vincent JL, Barbosa Gomes Galas FR, et al. Vasopressin versus norepinephrine in patients with vasoplegic shock after cardiac surgery. Anesthesiology 2017; 126(1): 85–93. [DOI] [PubMed] [Google Scholar]
- 13. Tang G, Wu L, Liang W, et al. Direct stimulation of KATP channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol Pharmacol 2005; 68(6): 1757–1764. [DOI] [PubMed] [Google Scholar]
- 14. Koenitzer JR, Isbell TS, Patel HD, et al. Hydrogen sulfide mediates vasoactivity in an O2-dependent manner. Am J Physiol Heart Circ Physiol 2007; 292(4): H1953–H1960. [DOI] [PubMed] [Google Scholar]
- 15. Omar S, Zedan A, Nugent K. Cardiac vasoplegia syndrome: pathophysiology, risk factors and treatment. Am J Med Sci 2015; 349(1): 80–88. [DOI] [PubMed] [Google Scholar]
- 16. Myers GJ, Wegner J. Endothelial glycocalyx and cardiopulmonary bypass. J Extra Corpor Technol 2017; 49(3): 174–181. [PMC free article] [PubMed] [Google Scholar]
- 17. Abou-Arab O, Kamel S, Beyls C, et al. Vasoplegia after cardiac surgery Is associated with endothelial glycocalyx alterations. J Cardiothorac Vasc Anesth 2020; 34(4): 900–905. [DOI] [PubMed] [Google Scholar]
- 18. Levy B, Collin S, Sennoun N, et al. Vascular hyporesponsiveness to vasopressors in septic shock: from bench to bedside. Intensive Care Med 2010; 36(12): 2019–2029. [DOI] [PubMed] [Google Scholar]
- 19. Landry DW, Oliver JA. The ATP-sensitive K+ channel mediates hypotension in endotoxemia and hypoxic lactic acidosis in dog. J Clin Invest 1992; 89(6): 2071–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buckley JF, Singer M, Clapp LH. Role of KATP channels in sepsis. Cardiovasc Res 2006; 72(2): 220–230. [DOI] [PubMed] [Google Scholar]
- 21. Macarthur H, Westfall TC, Riley DP, et al. Inactivation of catecholamines by superoxide gives new insights on the pathogenesis of septic shock. Proc Natl Acad Sci U S A 2000; 97(17): 9753–9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kalkoff M, Chan-Dominy A, Sleigh JW, et al. Alpha1-adrenergic receptor mRNA and inflammatory mediator expression in circulating leucocytes after cardiac surgery. Anaesth Intensive Care 2008; 36(4): 535–543. [DOI] [PubMed] [Google Scholar]
- 23. Egi M, Bellomo R, Langenberg C, et al. Selecting a vasopressor drug for vasoplegic shock after adult cardiac surgery: a systematic literature review. Ann Thorac Surg 2007; 83(2): 715–723. [DOI] [PubMed] [Google Scholar]
- 24. Cheng Y, Pan T, Ge M, et al. Evaluation of vasopressin for vasoplegic shock in patients with preoperative left ventricular dysfunction after cardiac surgery. Shock 2018; 50(5): 519–524. [DOI] [PubMed] [Google Scholar]
- 25. Papadopoulos G, Sintou E, Siminelakis S, et al. Perioperative infusion of low- dose of vasopressin for prevention and management of vasodilatory vasoplegic syndrome in patients undergoing coronary artery bypass grafting-A double-blind randomized study. J Cardiothorac Surg 2010; 5(1): 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elgebaly AS, Sabry M. Infusion of low-dose vasopressin improves left ventricular function during separation from cardiopulmonary bypass: a double-blind randomized study. Ann Card Anaesth 2012; 15(2): 128–133. [DOI] [PubMed] [Google Scholar]
- 27. Argenziano M, Chen JM, Choudhri AF, et al. Management of vasodilatory shock after cardiac surgery: identification of predisposing factors and use of a novel pressor agent. J Thorac Cardiovasc Surg 1998; 116(6): 973–980. [DOI] [PubMed] [Google Scholar]
- 28. Dünser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med 2009; 24(5): 293–316. [DOI] [PubMed] [Google Scholar]
- 29. Schmittinger CA, Torgersen C, Luckner G, et al. Adverse cardiac events during catecholamine vasopressor therapy: a prospective observational study. Intensive Care Med 2012; 38(6): 950–958. [DOI] [PubMed] [Google Scholar]
- 30. Demiselle J, Fage N, Radermacher P, et al. Vasopressin and its analogues in shock states: a review. Ann Intensive Care 2020; 10(1): 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Treschan TA, Peters J. The vasopressin system: physiology and clinical Strategies. Anesthesiology 2006; 105(3): 599–612. [DOI] [PubMed] [Google Scholar]
- 32. Porhomayon J, Davari-Farid S, Li CM, et al. Intraoperative administration of vasopressin during coronary artery bypass surgery is associated with acute postoperative kidney injury. J Crit Care 2015; 30(5): 963–968. [DOI] [PubMed] [Google Scholar]
- 33. Bragadottir G, Redfors B, Nygren A, et al. Low-dose vasopressin increases glomerular filtration rate, but impairs renal oxygenation in post-cardiac surgery patients. Acta Anaesthesiol Scand 2009; 53(8): 1052–1059. [DOI] [PubMed] [Google Scholar]
- 34. Nygren A, Thorén A, Ricksten SE. Vasopressin decreases intestinal mucosal perfusion: a clinical study on cardiac surgery patients in vasodilatory shock. Acta Anaesthesiol Scand 2009; 53(5): 581–588. [DOI] [PubMed] [Google Scholar]
- 35. Wakefield BJ, Busse LW, Khanna AK. Angiotensin II in vasodilatory shock. Crit Care Clin 2019; 35(2): 229–245. [DOI] [PubMed] [Google Scholar]
- 36. Khanna A, English SW, Wang XS, et al. Angiotensin II for the treatment of vasodilatory shock. N Engl J Med 2017; 377(5): 419–430. [DOI] [PubMed] [Google Scholar]
- 37. Szerlip H, Bihorac A, Chang S, et al. Effect of disease severity on survival in patients receiving angiotensin II for vasodilatory shock. Crit Care Med 2018; 46: 3. [Google Scholar]
- 38. Tumlin JA, Murugan R, Deane AM, et al. Outcomes in patients with vasodilatory shock and renal replacement therapy treated with intravenous angiotensin II. Crit Care Med 2018; 46(6): 949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Evans A, McCurdy MT, Weiner M, et al. Use of angiotensin II for post cardiopulmonary bypass vasoplegic syndrome. Ann Thorac Surg 2019; 108(1): e5–e7. [DOI] [PubMed] [Google Scholar]
- 40. Wieruszewski PM, Radosevich MA, Kashani KB, et al. Synthetic human Angiotensin II for Postcardiopulmonary Bypass Vasoplegic Shock. J Cardiothorac Vasc Anesth 2019; 33(11): 3080–3084. [DOI] [PubMed] [Google Scholar]
- 41. Ekholm M, Kahan T, Jörneskog G, et al. Angiotensin II infusion in man is proinflammatory but has no short-term effects on thrombin generation in vivo. Thromb Res 2009; 124(1): 110–115. [DOI] [PubMed] [Google Scholar]
- 42. La Jolla Pharmaceutical Company. Giapreza (angiotensin II) [package insert]. US. Food and Drug Administration website, https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209360s000lbl.pdf (accessed 29 March 2020).
- 43. Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med 2008; 358(2): 111–124. [DOI] [PubMed] [Google Scholar]
- 44. Annane D, Sébille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. J Am Med Assoc 2002; 288(7): 862–871. [DOI] [PubMed] [Google Scholar]
- 45. Annane D, Renault A, Brun-Buisson C, et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med 2018; 378(9): 809–818. [DOI] [PubMed] [Google Scholar]
- 46. Keh D, Trips E, Marx G, et al. Effect of hydrocortisone on development of shock among patients with severe sepsis the HYPRESS randomized clinical trial. JAMA—J Am Med Assoc 2016; 316(17): 1775–1785. [DOI] [PubMed] [Google Scholar]
- 47. Venkatesh B, Finfer S, Cohen J, et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med 2018; 378(9): 797–808. [DOI] [PubMed] [Google Scholar]
- 48. Dieleman JM, Nierich AP, Rosseel PM, et al. Intraoperative high-dose dexamethasone for cardiac surgery. JAMA 2012; 308(17): 1761–1767. [DOI] [PubMed] [Google Scholar]
- 49. Marik PE, Khangoora V, Rivera R, et al. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest 2017; 151(6): 1229–1238. [DOI] [PubMed] [Google Scholar]
- 50. Rodwell VW, Bender DA, Botham KM, et al. Harper’s illustrated biochemistry. Pennsylvania, New York: McGraw-Hill Medical, 2012. [Google Scholar]
- 51. Jackson TS, Xu A, Vita JA, et al. Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res 1998; 83(9): 916–922. [DOI] [PubMed] [Google Scholar]
- 52. Zabet MH, Mohammadi M, Ramezani M, et al. Effect of high-dose ascorbic acid on vasopressor’s requirement in septic shock. J Res Pharm Pract 2016; 5(2): 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fowler AA, Truwit JD, Hite RD, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA 2019; 322: 1261–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fujii T, Luethi N, Young PJ, et al. Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the VITAMINS randomized clinical trial. JAMA 2020; 323: 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wieruszewski PM, Nei SD, Maltais S, et al. Vitamin C for vasoplegia after cardiopulmonary bypass: a case series. A A Pract 2018; 11(4): 96–99. [DOI] [PubMed] [Google Scholar]
- 56. Mayer B, Brunner F, Schmidt K. Inhibition of nitric oxide synthesis by methylene blue. Biochem Pharmacol 1993; 45(2): 367–374. [DOI] [PubMed] [Google Scholar]
- 57. Evora PR, Ribeiro PJ, de Andrade JC. Methylene blue administration in SIRS after cardiac operations. Ann Thorac Surg 1997; 63(4): 1212–1213. [DOI] [PubMed] [Google Scholar]
- 58. Manghelli J, Brown L, Tadros HB, et al. A reminder of methylene blue’s effectiveness: in treating vasoplegic syndrome after on-pump cardiac surgery. Texas Hear Inst J 2015; 42(5): 491–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Carley M, Schaff J, Lai T, et al. Methylene blue for vasoplegia when on cardiopulmonary bypass during double-lung transplantation. A A Case Rep 2015; 5(8): 127–130. [DOI] [PubMed] [Google Scholar]
- 60. Yiu P, Robin J, Pattison CW. Reversal of refractory hypotension with single-dose methylene blue after coronary artery bypass surgery. J Thorac Cardiovasc Surg 1999; 118(1): 195–196. [DOI] [PubMed] [Google Scholar]
- 61. Pagni S, Austin EH. Use of intravenous methylene blue for the treatment of refractory hypotension after cardiopulmonary bypass. J Thorac Cardiovasc Surg 2000; 119(6): 1297–1298. [DOI] [PubMed] [Google Scholar]
- 62. Kofidis T, Strüber M, Wilhelmi M, et al. Reversal of severe vasoplegia with single-dose methylene blue after heart transplantation. J Thorac Cardiovasc Surg 2001; 122(4): 823–824. [DOI] [PubMed] [Google Scholar]
- 63. Mehaffey JH, Johnston LE, Hawkins RB, et al. Methylene blue for vasoplegic syndrome after cardiac operation: early administration improves survival. Ann Thorac Surg 2017; 104(1): 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maslow AD, Stearns G, Butala P, et al. The hemodynamic effects of methylene blue when administered at the onset of cardiopulmonary bypass. Anesth Analg 2006; 103(1): 2–8. [DOI] [PubMed] [Google Scholar]
- 65. Ozal E, Kuralay E, Yildirim V, et al. Preoperative methylene blue administration in patients at high risk for vasoplegic syndrome during cardiac surgery. Ann Thorac Surg 2005; 79(5): 1615–1619. [DOI] [PubMed] [Google Scholar]
- 66. Levin RL, Degrange MA, Bruno GF, et al. Methylene blue reduces mortality and morbidity in vasoplegic patients after cardiac surgery. Ann Thorac Surg 2004; 77(2): 496–499. [DOI] [PubMed] [Google Scholar]
- 67. Weiner MM, Lin H-M, Danforth D, et al. Methylene blue is associated with poor outcomes in vasoplegic shock. J Cardiothorac Vasc Anesth 2013; 27(6): 1233–1238. [DOI] [PubMed] [Google Scholar]
- 68. McCartney SL, Duce L, Ghadimi K. Intraoperative vasoplegia. Curr Opin Anaesthesiol 2018; 31(1): 43–49. [DOI] [PubMed] [Google Scholar]
- 69. Faber P, Ronald A, Millar BW. Methylthioninium chloride: pharmacology and clinical applications with special emphasis on nitric oxide mediated vasodilatory shock during cardiopulmonary bypass. Anaesthesia 2005; 60(6): 575–587. [DOI] [PubMed] [Google Scholar]
- 70. Gachot B, Bedos JP, Veber B, et al. Short-term effects of methylene blue on hemodynamics and gas exchange in humans with septic shock. Intensive Care Med 1995; 21(12): 1027–1031. [DOI] [PubMed] [Google Scholar]
- 71. Juffermans NP, Vervloet MG, Daemen-Gubbels CRG, et al. A dose-finding study of methylene blue to inhibit nitric oxide actions in the hemodynamics of human septic shock. Nitric Oxide Biol Chem 2010; 22(4): 275–280. [DOI] [PubMed] [Google Scholar]
- 72. Provepharm SAS. Povayblue (methylene blue) [package insert]. US. Food and Drug Administration website, https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/204630s000lbl.pdf. Revised (April 2016, accessed 9 November 2019).
- 73. Schumacher Blumer V, Chaparro SV. Methylene blue–induced serotonin syndrome after left ventricular assist device implantation: a case report and literature review. J Thorac Cardiovasc Surg 2017; 154(3): e39–e43 [DOI] [PubMed] [Google Scholar]
- 74. Weinberg JB, Chen Y, Jiang N, et al. Inhibition of nitric oxide synthase by cobalamins and cobinamides. Free Radic Biol Med 2009; 46(12): 1626–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gerth K, Ehring T, Braendle M, et al. Nitric oxide scavenging by hydroxocobalamin may account for its hemodynamic profile. Clin Toxicol (Phila) 2006; 44(Suppl 1): 29–36. [DOI] [PubMed] [Google Scholar]
- 76. Haouzi P, Chenuel B, Sonobe T. High-dose hydroxocobalamin administered after H2S exposure counteracts sulfide-poisoning-induced cardiac depression in sheep. Clin Toxicol (Phila) 2015; 53(1): 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cai Y, Mack A, Ladlie BL, et al. The use of intravenous hydroxocobalamin as a rescue in methylene blue-resistant vasoplegic syndrome in cardiac surgery. Ann Card Anaesth 2017; 20(4): 462–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Seelhammer TG, Charnin J, Zhao Y, et al. Elevated serum cobalt concentrations associated with hydroxocobalamin administration for refractory vasoplegia. J Cardiothorac Vasc Anesth 2019; 33: 3402–3405. [DOI] [PubMed] [Google Scholar]
- 79. Burnes ML, Boettcher BT, Woehlck HJ, et al. Hydroxocobalamin as a rescue treatment for refractory vasoplegic syndrome after prolonged cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2017; 31(3): 1012–1014. [DOI] [PubMed] [Google Scholar]
- 80. Cheungpasitporn W, Hui J, Kashani KB, et al. High-dose hydroxocobalamin for vasoplegic syndrome causing false blood leak alarm. Clin Kidney J 2017; 10(3): 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Roderique JD, VanDyck K, Holman B, et al. The use of high-dose hydroxocobalamin for vasoplegic syndrome. Ann Thorac Surg 2014; 97(5): 1785–1786. [DOI] [PubMed] [Google Scholar]
- 82. Warner MA, Mauermann WJ, Armour S, et al. Red urinary discolouration following hydroxocobalamin treatment for vasoplegic syndrome. Can J Anaesth 2017; 64(6): 673–674. [DOI] [PubMed] [Google Scholar]
- 83. An SS, Henson CP, Freundlich RE, et al. Case report of high-dose hydroxocobalamin in the treatment of vasoplegic syndrome during liver transplantation. Am J Transplant 2018; 18(6): 1552–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Woehlck HJ, Boettcher BT, Lauer KK, et al. Hydroxocobalamin for vasoplegic syndrome in liver transplantation: restoration of blood pressure without vasospasm. A A Case Rep 2016; 7(12): 247–250. [DOI] [PubMed] [Google Scholar]
- 85. Shah PR, Reynolds PS, Pal N, et al. Hydroxocobalamin for the treatment of cardiac surgery-associated vasoplegia: a case series. Can J Anaesth 2018; 65(5): 560–568. [DOI] [PubMed] [Google Scholar]
- 86. Feih JT, Rinka JRG, Zundel MT. Methylene blue monotherapy compared with combination therapy with hydroxocobalamin for the treatment of refractory vasoplegic syndrome: aRetrospective cohort study. J Cardiothorac Vasc Anesth 2019; 33(5): 1301–1307. [DOI] [PubMed] [Google Scholar]
- 87. Armour S, Armour TK, Joppa WR, et al. Use of hydroxocobalamin (Vitamin B12a) in patients with vasopressor refractory hypotension after cardiopulmonary bypass: a case series. Anesth Analg 2019; 129(1): e1–e4. [DOI] [PubMed] [Google Scholar]
- 88. ClinicalTrials. gov. Hemodynamic effects of methylene blue vs hydroxocobalamin in patients at risk of vasoplegia during cardiac surgery, https://clinicaltrials.gov/ct2/show/NCT03446599 (accessed 17 December 2019).
- 89. ClinicalTrials.gov. Vitamin B12a vasoplegic syndrome, https://clinicaltrials.gov/ct2/show/NCT03735316?term=hydroxocobalamin&cond=vasoplegia&draw=2&rank=3 (accessed 17 December 2019).
- 90. ClinicalTrials.gov. A trial of vitamin B12 and in Septic Shock, https://clinicaltrials.gov/ct2/show/NCT03783091 (accessed 17 December 2019).
- 91. ClinicalTrials.gov. Methylene blue vs cyanokit for intraoperative vasoplegic syndrome in liver transplant patients, https://clinicaltrials.gov/ct2/show/NCT04054999?term=hydroxocobalamin&draw=2&rank=9 (accessed 17 December 2019).
- 92. Meridian Medical Technologies. Cyanokit (hydroxocobalamin) [package insert]. US. Food and Drug Administration website, https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/022041lbl.pdf (2006, accessed 9 November 2019).
- 93. Shapeton AD, Mahmood F, Ortoleva JP. Hydroxocobalamin for the treatment of vasoplegia: a review of current literature and considerations for use. J Cardiothorac Vasc Anesth 2019; 33(4): 894–901. [DOI] [PubMed] [Google Scholar]
- 94. López A, Lorente JA, Steingrub J, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med 2004; 32(1): 21–30. [DOI] [PubMed] [Google Scholar]