Abstract
The ocular lens is a unique tissue that contains an age gradient of cells and proteins ranging from newly differentiated cells containing newly synthesized proteins to cells and proteins that are as old as the organism. Thus, the ocular lens is an excellent model for studying long-lived proteins (LLPs) and the effects of aging and post-translational modifications on protein structure and function. Given the architecture of the lens, with young fiber cells in the outer cortex and the oldest cells in the lens nucleus, spatially-resolved studies provide information on age-specific protein changes. In this review, experimental strategies and proteomic methods that have been used to examine age-related and cataract-specific changes to the human lens proteome are described. Measured spatio-temporal changes in the human lens proteome are summarized and reveal a highly consistent, time-dependent set of modifications observed in transparent human lenses. Such measurements have led to the discovery of cataract-specific modifications and the realization that many animal systems are unsuitable to study many of these modifications. Mechanisms of protein modifications such as deamidation, racemization, truncation, and protein-protein crosslinking are presented and the implications of such mechanisms for other long-lived proteins in other tissues are discussed in the context of age-related neurological diseases. A comprehensive understanding of LLP modifications will enhance our ability to develop new therapies for the delay, prevention or reversal of age-related diseases.
Keywords: Lens, Aging, Proteome, Proteoform, Post-translational modification, Mass spectrometry, Cataract, Long-lived protein
1. Introduction
The term “proteome”, coined in 1994 by Marc Wilkins (Wasinger et al., 1995) to define the protein complement of the genome, is commonly used to describe the entire protein composition of a cell, tissue, or organism. A proteome is dynamic since proteins are involved in all aspects of cellular function including: cell signaling, cell architecture, cell differentiation, cell proliferation, etc. In addition, most cells regulate their proteome, in part, through post-translational modification as well as through protein degradation and synthesis of new proteins. Each specific modified form of a protein is called a proteoform (Smith and Kelleher, 2013). Thus, from a genome containing ~20,000 protein coding genes, the human proteome contains hundreds of thousands of proteoforms (Aebersold et al., 2018).
The lens proteome is complex, made up of >5,000 proteins (Zhao et al., 2019); however, it is dominated by a few very abundant soluble proteins, lens crystallins, that serve to create a long-lived transparent tissue responsible for focusing light onto the retina via the establishment of a refractive index gradient. What makes the lens proteome so fascinating is the fact that mature lens fiber cells are fully differentiated cells and these mature fiber cells lose their ability to catabolize old proteins and to synthesize new proteins. Thus, the proteins in individual fiber cells are as old as that cell and protein modifications accumulate resulting in a highly complex mixture of proteoforms. This phenomenon creates the situation where age-related protein modifications are available for study and provides a model tissue for examining protein aging. Indeed, early proteomic analyses of human lens tissue focused on characterization of post-translational modifications (PTMs) (Takemoto, 1995a; Colvis and Garland, 2002; MacCoss et al., 2002; Wilmarth et al., 2006). Within the structure of the human lens (Figure 1), the age of fiber cells increases from outer cortex to embryonic nucleus; therefore, age-related changes to proteins can be mapped within a single lens using spatially-resolved proteomic methods. Furthermore, within the lens, multiple different sub-proteomes can be defined and characterized. For example, a lens proteome can be defined based on solubility (water soluble, urea soluble or urea insoluble) or based on a specific posttranslational modification (e.g. phosphoproteome, ubiquitinated proteome, or palmitoylated proteome) or based on spatial localization (outer cortex, inner cortex, barrier, outer nucleus, or inner nucleus). All such sub-proteomes can provide information on aging effects on lens proteins and potentially lead to a mechanistic understanding of aging and to therapeutic strategies to alleviate its consequences. In this review, we describe methods used for measuring spatiotemporal proteomes of the lens and what these studies have revealed about age effects on long-lived proteins (LLPs), lens biochemistry, and cataractogenesis. In addition, we discuss how these revelations can inform studies of other age-related diseases, e.g. Alzheimer’s disease and other neurodegenerative disorders.
Figure 1. Diagram of human lens regions.
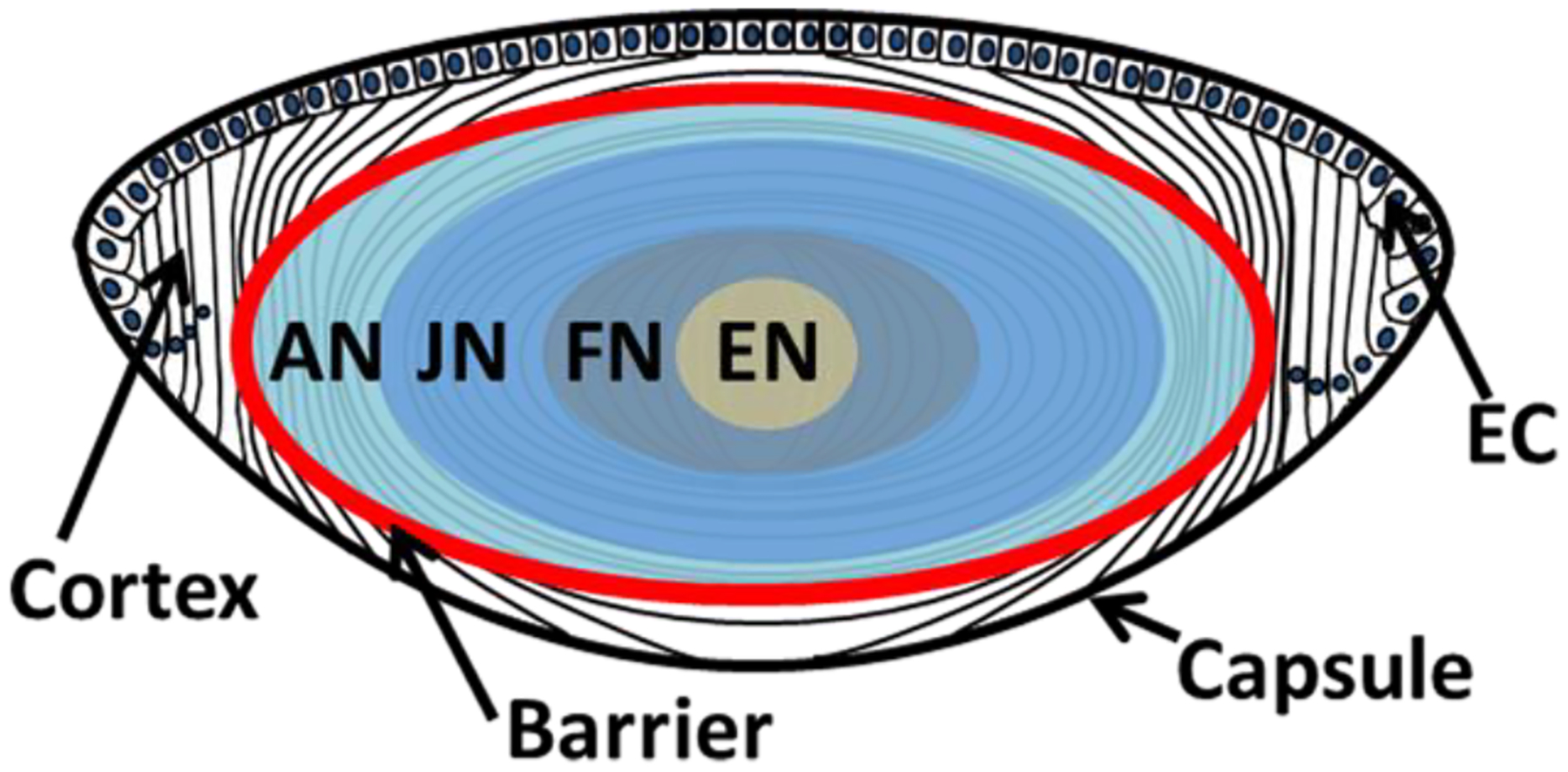
Schematic diagram of the human lens showing capsule, anterior layer of epithelial cells (EC), cortex, adult nucleus (AN), juvenile nucleus (JN), fetal nucleus (FN) and embryonic nucleus (EN). The red line indicates the barrier region where transport of water and glutathione are impeded in aged lenses.
2. The Lens as a Model Tissue for Protein Aging
It took many years for it to become apparent that the myriad of physical and biochemical changes that take place within the human lens with age, and with cataract, stem inexorably from post-translational modification of the long-lived molecules of which the lens is composed (Truscott and Friedrich, 2016). Once this was recognized, several important consequences inevitably flowed. Two are particularly noteworthy. Firstly, animal models would be of little use in discovering mechanisms responsible for the age-related clinical manifestations of human vision, such as presbyopia and cataract. Put simply, commonly used experimental animals do not live sufficiently long lives. This fact means that an experimental approach commonly utilized by researchers interested in other human diseases, is not relevant for scientists interested in uncovering the molecular processes responsible for human lens aging. Secondly, it might be possible that the age-dependent modification of lens proteins may also be mirrored by other proteins in the body if they, too, were long-lived. This second hypothesis appears to be correct, since there are many long-lived proteins in the human body (Toyama et al., 2013; Truscott et al., 2016b) and these LLPs undergo similar processes of age-dependent modification. This aspect will be discussed in greater detail later in this review. At this point, it is instructive to note that some protein modifications such as deamidation, racemization and truncation appear to be common to all long-lived proteins. Other modifications, for example those that depend on enzymes, are not observed to any great extent in the lens because there is no turnover of proteins in the lens nucleus and, thus, after some years, enzymes that were active at birth undergo inactivation (Zhu et al., 2010). Such enzyme-mediated reactions can; however, occur in other tissues and, conceivably, in young metabolically active fiber cells. The conversion of arginine to citrulline is one example, since this is catalysed by the enzyme arginine deiminase (Witalison et al., 2015). It needs to be emphasized that the study of LLPs outside of the lens is a relatively new field and that much remains to be discovered. However, for age-related protein changes, the human lens is a valuable model.
2.1. Long-lived cells
The human body has many long-lived cells some with rates of renewal from 39 days up to 30 yrs (Arrojo et al., 2019). The human lens is comprised of two types of long-lived cells and a long-lived capsule of extracellular matrix. A single layer of specialized cuboidal epithelial cells cover the anterior surface of the lens. The central epithelial cells are mitotically quiescent. The presence of a low level of progenitor cells in the central epithelial zone that have the capacity to divide has been suggested from studies in mouse (Zhou et al., 2006). The epithelial cells near the lens equator (germinative/transition zone) have the capacity to continue to divide and differentiate into lens fiber cells throughout an individual’s life. The lens fiber cells are not only continually formed but remain throughout the life of the individual. Thus, a human lens has long-lived cells of ages that span the life of an individual, from newly formed fiber cells in the outer cortex to those that are the age of the individual in the embryonic nucleus (Figure 1).
The functions of both the epithelial cells and the fiber cells are to maintain lens transparency albeit by different mechanisms. Epithelial cells are metabolically active and require energy production and transport function to maintain lens transparency. The cells also provide nutritional support for the underlying fiber cells, for example, through maintenance of a lens microcirculation system (Mathias et al., 2007) and the synthesis of high concentrations of glutathione (Giblin, 2000). It is noteworthy that the lens capsule is made up of protein secreted by the epithelial cells and is long-lived. Advanced glycation end products have been identified on capsule proteins and such modifications affected the levels of proteins associated with epithelial-to-messenchymal transition (EMT); a process involved in secondary cataract formation through posterior capsule opacification (PCO) (Raghavan et al., 2016).
For lens fiber cells, mechanisms have evolved to generate the continual formation of shells of long-lived fiber cells for up to ten or more decades with an organization of those cells that minimizes light scattering and establishes the light focusing properties of the tissue. These include a cytoskeleton that maintains cell shape (Quinlan et al., 1999), membranes with cell-cell connections allowing for ion flux/fluid dynamics, the loss of organelles to prevent light scattering, membrane remodeling (Lim et al., 2009), cell connections or suture patterns at the poles of the lens that minimize light scattering, mechanisms for packing the cells to maintain a lens size and shape for function, and expression of highly abundant fiber cell-specific proteins in a gradient to form a refractive index gradient (Wride, 2011; Bassnett, 2009; Kuszak et al., 1984; Costello et al., 2013; Kuszak et al., 2004; Taylor et al., 1996; Michael and Bron, 2011; Trokel, 1962; Bassnett and Costello, 2017). While the fiber cells in the cortex are hexagonally shaped, during the formation of the lens nucleus the fiber cells undergo a morphological change to more rounded cells (Taylor et al., 1996). There is no protein movement from the new fiber cells to the center of the lens (Lynnerup et al., 2008); however, some evidence of protein turnover in the human lens nucleus was reported based on soluble protein 14C/12C ratios, but this result has not been replicated (Stewart et al., 2013).
2.2. Long-lived proteins (LLPs)
The lifetimes of proteins, even within a single cell, also vary widely and can range from minutes to years. LLPs are typically defined as those that have life-times from months to years. They can be present in cells that asymmetrically divide or in long-lived cells that do not divide. The long-lived proteins can remain as full length proteins in these cells or can be truncated and/or modified proteoforms (Thayer et al., 2014).
While the factors that determine the lifespan of a protein are not known, the evolution of LLPs may provide a functional advantage. LLPs have evolved for use in phenomena of maximal importance to long term human function such as vision, long term memory (Heo et al., 2018), nuclear pore function and the cytoskeleton (Toyama et al., 2013). For long-lived proteins, functional stability and resistance to age-related changes are likely to be critical factors. Thus, it is reasonable to assume that protein sequence and, thus, structure have also evolved to provide the stability and resistance to age-related changes that are needed to maintain important function during the life time of a protein. It is possible that long-lived protein sequences and structures may also possess the flexibility to alter function through post-translational modification as the cells and/or the environment change.
In tissues where long-lived cells and long-lived proteins reside, age is a major risk factor for disease. Santos and Lindner characterized aging as “the progressive decline of biochemical and physiological function in an individual” (Santos and Lindner, 2017). Nine hallmarks of aging as defined by Lopez-Otin et al., include “genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion and altered intercellular communication” (Lopez-Otin et al., 2013). Of these, the loss of proteostasis and the fate of LLPs through posttranslational modification have been a major focus to understand aging and disease mechanisms particularly in cataracts (Santos and Lindner, 2017; Toyama and Hetzer, 2013; Michael and Bron, 2011).
One of the most important age-related modifications of the human lens occurs at about age 40, when the lens barrier develops. This functional change occurs after free αcrystallin in the center of the lens has been consumed (McFall-Ngai et al., 1985) by binding to other proteins as they denature. This internal barrier has been demonstrated by following the diffusion of both labelled GSH (Sweeney and Truscott, 1998) and D2O (Moffat et al., 1999). Subsequent to barrier development after middle age, the center of the lens becomes partially isolated from the metabolically active cortical region. Barrier formation impairs the movement of GSH, resulting in a situation where proteins in the lens nucleus become susceptible to oxidative damage (Truscott, 2005). The exact molecular changes that occur to form this barrier remain unknown.
Although lens hardness increases with age (Heys et al., 2004), a decline in lens function occurs with the development of presbyopia past age 45y and then with the increase in cataract prevalence past age 50y. Yet crystallins undergo a consistent process of post-translational modification beginning at birth in already formed fiber cells and almost immediately with all layers of newly formed fiber cells. These modifications result in the formation of the stable proteoform pattern typical of the lens nucleus by sexual maturity or by about age 20y (Zhao et al., 2011). Thus, there are (1) spatial and temporal/age-related PTMs that are not associated with loss of lens function and may contribute to long term lens function and (2) PTMs that are the result of aging that may lead to the loss of lens function. The challenge is to determine how to distinguish between these two types of modifications and how to prevent the latter one from occurring.
3. Proteomic Methods
The ability to characterize the protein content or the proteome of cells and tissues has evolved as technology has evolved, primarily due to advances in mass spectrometry. Original studies of the lens proteome utilized two dimensional gel electrophoresis (2DE) where distinct proteoforms were spatially separated. Subsequent identification of proteins in spatially resolved gel spots relies on mass spectrometric methods. Although potentially thousands of proteoforms can be separated, individual gel spots often contain multiple proteins. Furthermore, identification of protein modifications requires those modifications to be directly detected in the mass spectrometry experiments. Nevertheless, lens proteome changes have been mapped by 2D gels including age-related, diabetic, and cataractogenic changes (discussed below).
High performance liquid chromatography – mass spectrometry (HPLC-MS) has become the key technology that has allowed nearly the entire human proteome to be characterized (Kim et al., 2014) (https://www.hupo.org/). Multiple dimensions of separation (LCn), enrichment of specifically modified peptides (e.g. phosphopeptides (Aebersold and Mann, 2016; Sharma et al., 2014)), and newer, faster mass spectrometers have enhanced our ability to define a specific proteome, even down to a single cell level (Budnik et al., 2018). Given the complexity of age-related modifications present in a human lens (discussed below), modern instrumentation is required for comprehensive proteome analysis and new information continues to emerge from studies of the lens proteome. Furthermore, given the quantitative nature of HPLC-MS data, a common objective of HPLC-MS studies is to not only characterize PTMs, but to quantify their abundances with age and with cataract formation.
3.1. Spatially-Resolved Proteomics
Spatially-resolved proteomics is required to identify age-related changes in the lens proteome within a single lens and typically relies on physical separation of regions of interest prior to proteomic analysis. Methods to achieve such separation include: manual dissection (discussed below) and laser capture microdissection (Wang et al., 2008). Newer methods, including hydrogel digestion/extraction (Rizzo et al., 2017), liquid extraction for surface analysis (LESA) (Ryan et al., 2019), and imaging mass spectrometry (IMS, discussed below) have been used to extract proteomic information directly from tissue sections in a spatially-resolved manner.
3.1.1. Human Lens Dissection
In the absence of spatially-resolved proteomics techniques, tissue dissection followed by standard proteomic analysis is a commonly employed strategy to generate spatial information on protein expression. For human lens studies, as shells of fiber cells are generated during the growth of the lens, the ends of fiber cells make connections with other fiber cells forming suture patterns (Kuszak et al., 1985; Kuszak et al., 2004). Since mature fiber cell lengths are constant at about 12mm, as the lens grows the suture patterns necessarily become more complex. Thus, suture patterns are characteristic for the age at which cell layers are formed and these patterns are typically visible throughout the lens as overlying layers are removed. In order to define the spatial and temporal post-translational modifications of crystallins, Garland et al., peeled successive shells of fiber cells from lenses of all ages using the suture patterns as a guide to approximate the age of the fiber cell layers (Figure 2) (Garland et al., 1996). This method was possible because there were positions at which the layers could be removed as a shell of cells. It is not clear if this was the result of the formation of suture planes, the presence of a fused stratum of cells, changes in the cell connections at different development stages, or changes related to zones of refractive index (Kuszak et al., 2004; Shi et al., 2009; Bahrami et al., 2014).
Figure 2. Dissection of human lens.
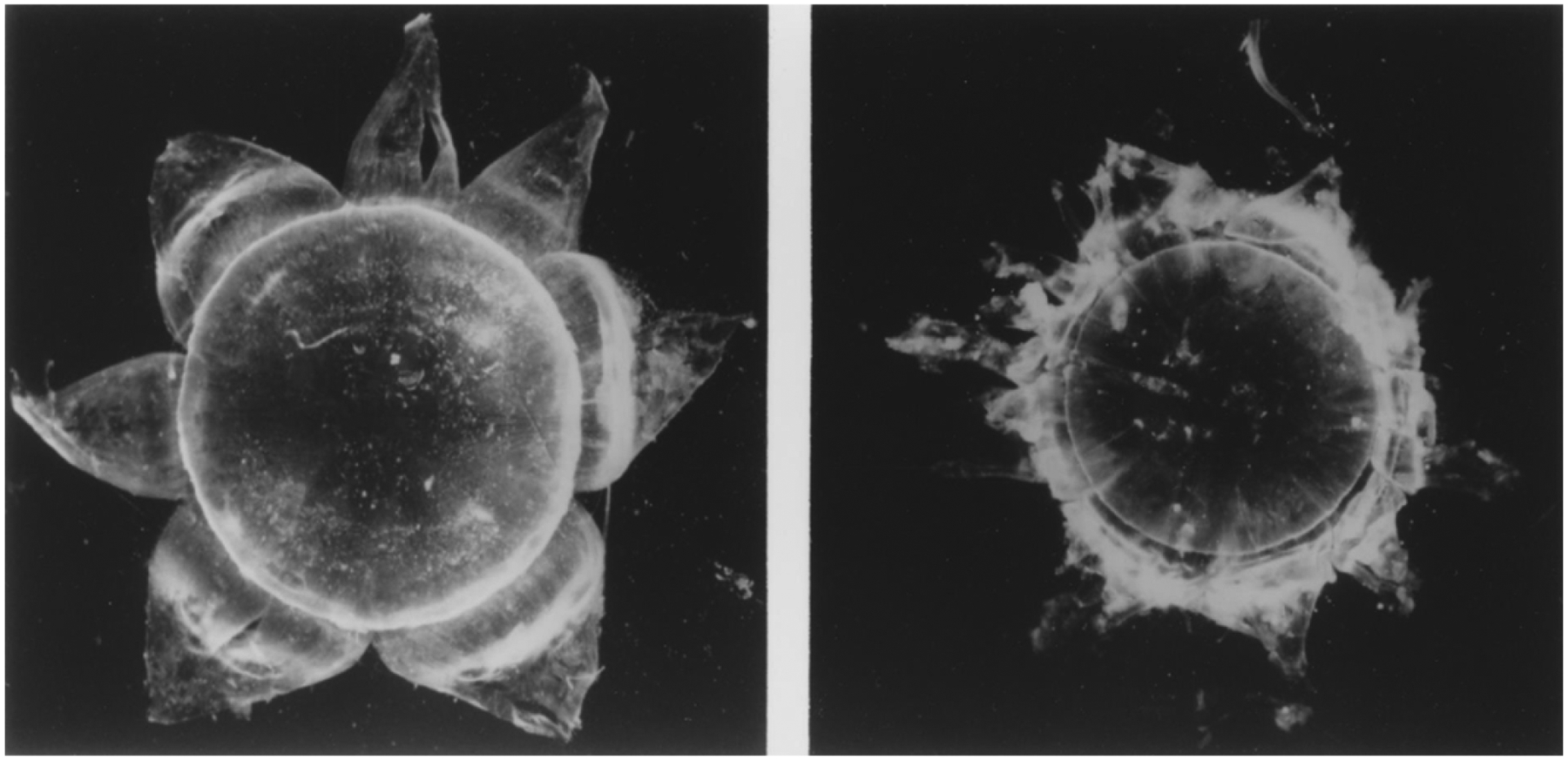
Photographs of a partially dissected human lens showing the outer shell of fiber cells of the lens cortex (left) and the adult nucleus (right). Figure from (Garland et al., 1996).
The dissected adult human lens nucleus seen in Figure 2 (right panel) has a well-defined surface and appears to be a separate and unique structure with a diameter of about 7mm. This may not be surprising as the human lens nucleus has a unique cell structure, unique protein composition and concentration, and exhibits barrier properties (Kuszak et al., 1985; Sweeney and Truscott, 1998; Zhao et al., 2011; Vendra et al., 2016; Augusteyn, 2010). The adult lens nucleus forms the majority of the optical axis and comprises about 80% of the lens at the equatorial region as measured by dissection. The adult lens nucleus contains the primary fiber cells (embryonic nucleus), secondary fiber cells formed up to birth (fetal nucleus) and fiber cells formed up to about sexual maturity or 20y. The lens cortex includes a continuum of fiber cell layers formed after an individual reaches 20y. With the continual formation of fiber cells, the lens continues to grow throughout life (Augusteyn, 2010). Given the constant size of the adult lens nucleus and continuous lens growth, the cortical cells become compacted to maintain the size of the adult lens (Bassnett and Costello, 2017).
3.1.2. Imaging Mass Spectrometry
A newer technology that is particularly relevant for spatially-resolved proteomics is imaging mass spectrometry (IMS) (Caprioli et al., 1997); the workflow for which is shown in Figure 3. In IMS, thin tissue sections are scanned, typically by a laser raster, and a mass spectrum or protein profile is acquired at equally spaced locations (pixels) on the tissue. Matrix-assisted laser desorption ionization (MALDI) is commonly used for protein IMS and spatial resolutions of 5 microns have been demonstrated for protein images (Yang et al., 2018). Protein IMS results are typically limited to abundant, soluble proteins of molecular weight <30kDa and therefore, lens crystallins are highly amenable to imaging by IMS. IMS methods have been developed to image integral membrane proteins (Grey et al., 2009) and tryptic peptides (as surrogates for larger or insoluble proteins) (Groseclose et al., 2007). This “molecular microscope” allows differences in proteoform expression to be spatially mapped to a tissue and this technology is highly appealing in the analysis of spatiotemporal changes in lens proteins (see results below).
Figure 3. MALDI imaging mass spectrometry workflow.
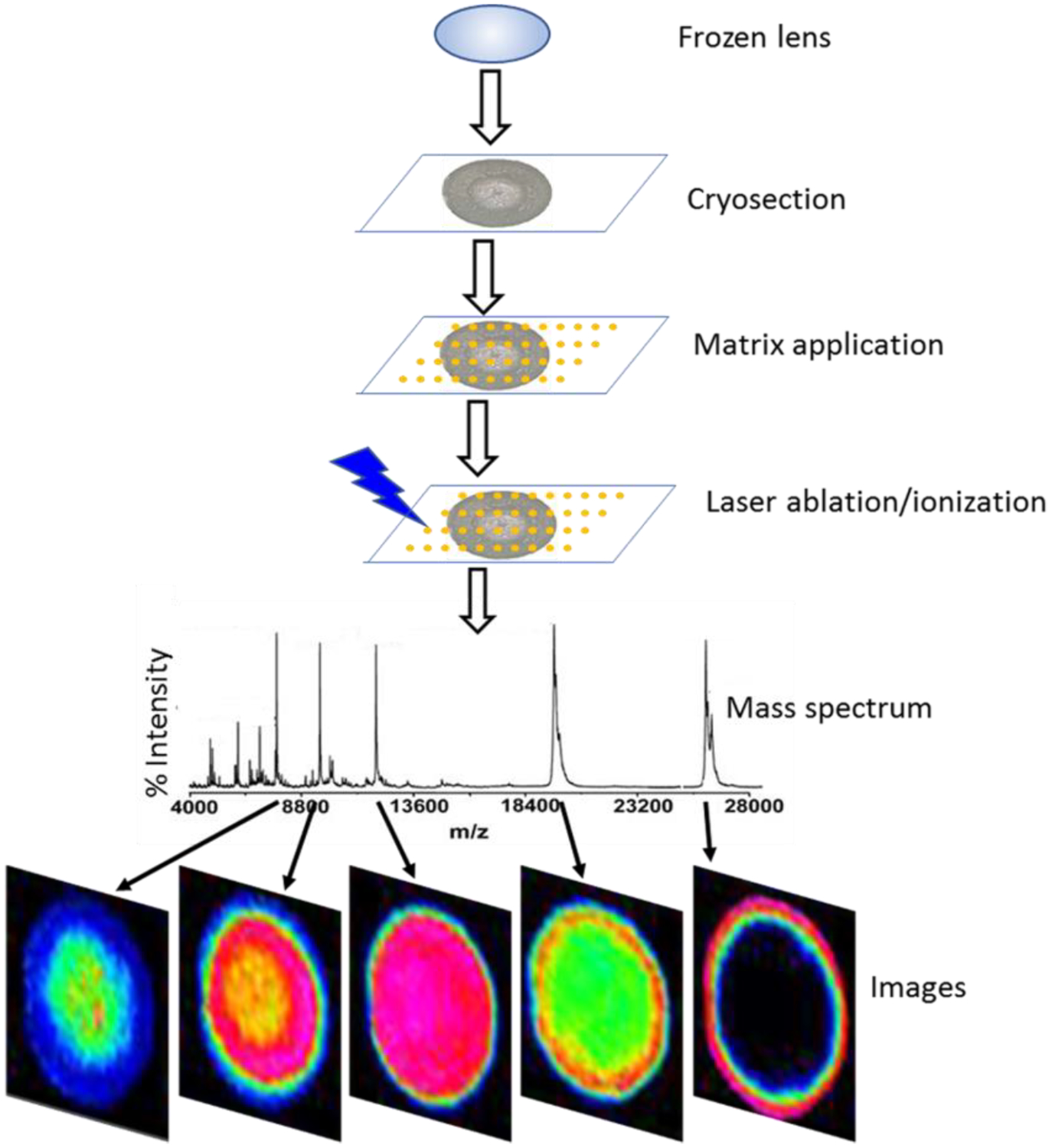
Schematic diagram of workflow for imaging MALDI imaging mass spectrometry of frozen lens tissue showing major steps in the procedure including: cryosectioning, matrix application, laser ablation/ionization, data acquisition, and image generation.
4. The human lens proteome
As mentioned above, the lens proteome is crystallin dominant where a relatively small number of crystallin (CRY) genes encode more than 90% of the lens cytoplasmic proteins (Hejtmancik et al., 2015). Due to their extraordinarily high concentrations (up to 450 mg/mL), this small number of crystallins dominates most forms of proteomic analysis and limits the ability to detect low-abundance components. Thus, many age-related changes that have been reported are of crystallin modifications. Fractionation into subproteomes, e.g. into soluble and membrane fractions, or enrichment, e.g. of phosphopeptides, provides increased depth of proteome coverage and of age-related modifications.
4.1. Spatial and temporal crystallin modifications in normal human lenses
Early proteomic analysis of lens samples almost exclusively employed 2-dimensional electrophoresis combined with mass spectrometry analysis. This approach allowed not only a view of the total protein composition and the sizes and relative concentrations of each protein but also provided an indication as to whether the protein had been post-translationally modified. Nearly all 2DE studies of lens proteins have identified only crystallins (Garland et al., 1996; Jungblut et al., 1998; Lampi et al., 2002; Wang-Su et al., 2003; Wilmarth et al., 2004; Zhang et al., 2007; Simpanya et al., 2008).
The 2DE separation pattern of crystallins from cortical fibers in a normal 42y lens is contrasted in Figure 4 (Garland et al., 1996) to the pattern from each of the three nuclear regions of the same lens. Each image represents total protein, water soluble and water insoluble, for all four samples (A-D). The individual crystallins are present in the cortical fibers as largely unmodified forms. In contrast, the original forms of each crystallin are not visible in the nuclear regions; however, the patterns for the adult, fetal and embryonic nuclear regions are comparable. The pattern in the nucleus represents modified β- and γ-crystallins. Only small amounts of truncated αA-crystallin are visible. The results shown in Figure 5 provide a global view of how dramatically the crystallins become post-translationally modified spatially and therefore temporally, within a normal human lens (Garland, unpublished). The 2DE crystallin protein patterns of 5 successive layers of cortical fiber cells (Panels A-E) inward from the newest fiber cells to the oldest fiber cells in the lens nucleus (Panel F) are shown for a normal 45y lens. Each image represents total protein, water soluble and water insoluble, for all samples including the lens nucleus. The pattern in Panel A is comparable to the 2DE crystallin patterns from 3 day (Lampi et al., 1997) and 22 day (Figure 16, left panel) lenses suggesting the pattern reflects newly synthesized forms of the crystallins. With successive layers of fiber cells inward there is a continuing decrease in the concentrations of the original forms of each of the major crystallins and an increase in new protein species which are modified forms of crystallins. The typical pattern of the lens nucleus in adult lenses in Panel F is comparable to Panels B-D in Figure 4.
Figure 4. 2DE analysis of human lens regions.
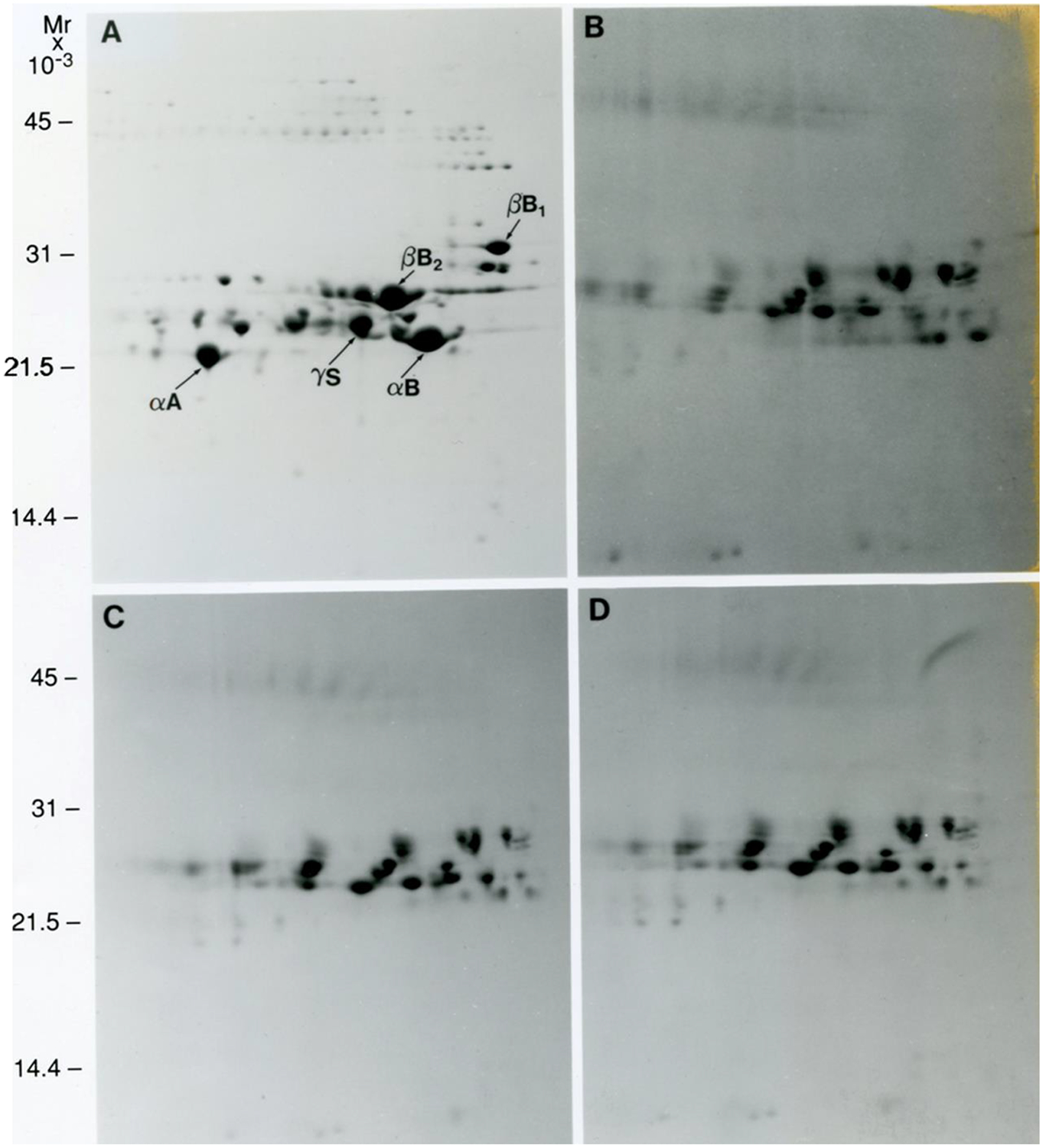
Images of 2DE gels showing separation of proteins from a 42y human lens dissected into cortex (A), adult nucleus (B), fetal nucleus (C), and embryonic nucleus (D) regions. Figure from (Garland et al., 1996). Samples were solubilized in 9M urea, 2% NP40, 10mM DTT, 2% ampholytes (Resolyte pH 3.5–10). Proteins were separated based on isoelectric point using 18 cm non-linear, immobilized pH 3–10 gradients for 1st dimension isoelectric focusing (separation from acidic on the left to basic on the right) and based on size using SDS-PAGE in the second, vertical, dimension (18 × 25 cm gels). Each image represents total protein, water soluble and water insoluble, for all samples including the lens nucleus. Crystallin species migrated between pIs 8.6 and 5.
Figure 5. 2DE analysis of human lens regions.
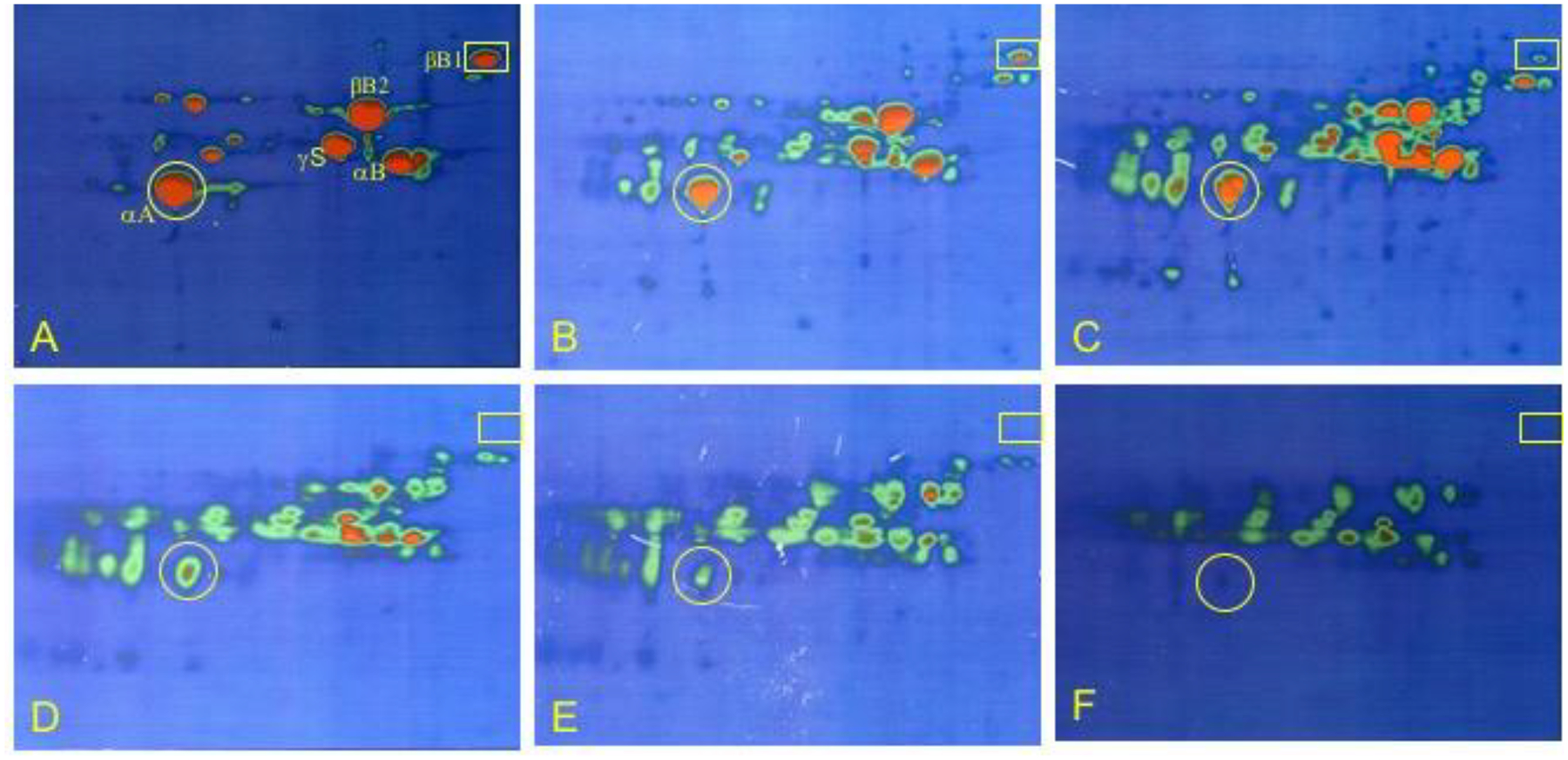
Psuedo colored images of 2DE separation of 5 cortical shells of fiber cells and the nuclear region of a 45y lens. Left to right: outer cortical layers (panels A-E) with successive cortical fibers inward until the lower right image is of the lens nucleus (panel F). Protein concentration is from high (red) to low (green). The original locations of αA- and βB1-crystallin are indicated by yellow circles and rectangles, respectively. Samples were solubilized in 9M urea, 2% NP40, 10mM DTT, 2% ampholytes (Resolyte pH 3.5–10). Proteins were separated based on isoelectric point using 18 cm non-linear, immobilized pH 3–10 gradients for 1st dimension isoelectric focusing (separation from acidic on the left to basic on the right) and based on size using SDS-PAGE in the second, vertical, dimension (18 × 25 cm gels). Each image represents total protein, water soluble and water insoluble, for all samples including the lens nucleus. Crystallin species migrated between pI 8.6 and 5.
Figure 16. 2DE analysis of a 22d human cataractous lens.
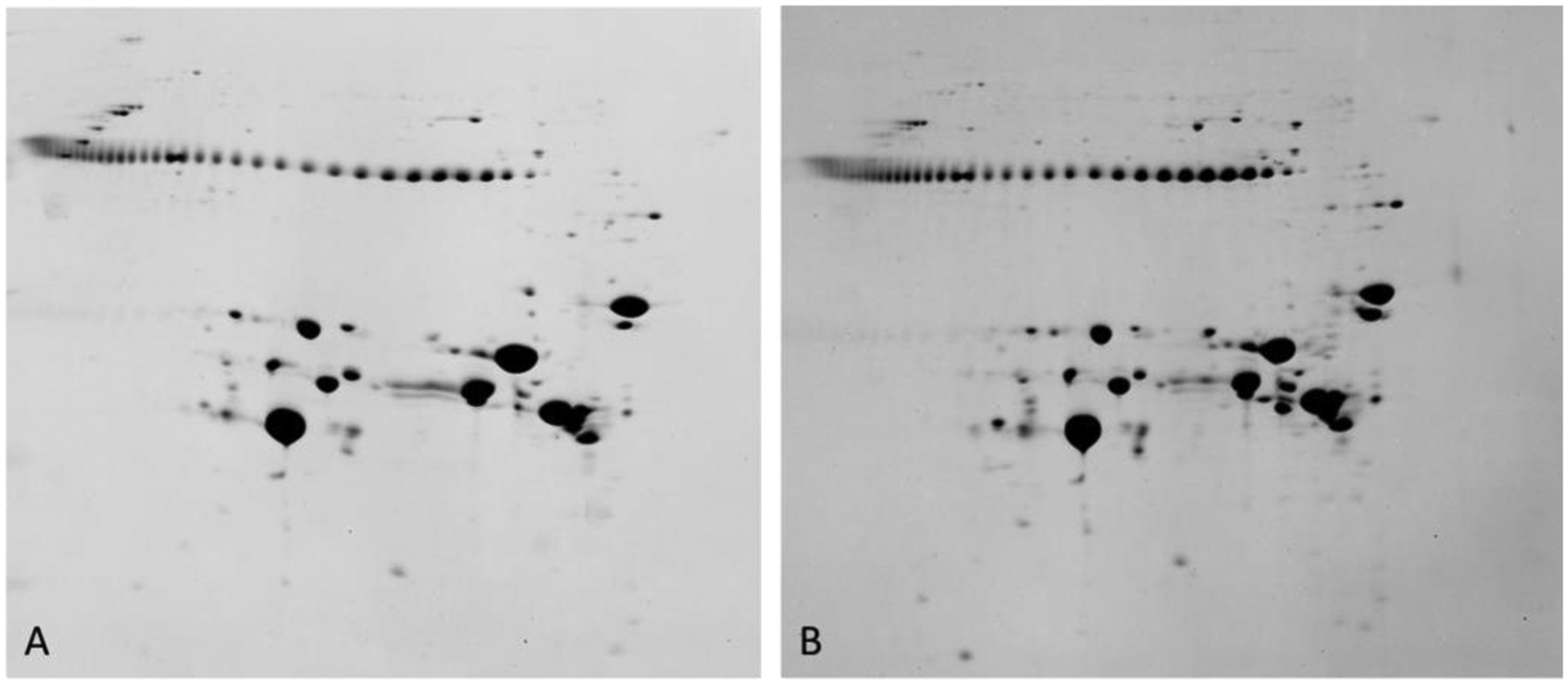
2DE patterns of human lens proteins observed from the clear region (left) and cortical cataract region (right) of a 22 day old human lens. These data are for total protein including water soluble and water insoluble proteins. The string of protein spots at Mr 42k are pI markers. Proteins on the gel range from about pI 3 (left) to about pI 8.9 (right). Samples were solubilized in 9M urea, 2% NP40, 10mM DTT, 2% ampholytes (Resolyte pH 3.5–10). (Garland, unpublished data).
Random patterns of crystallin modifications are not observed in normal lenses from birth to 80y of age. In other words, the 2DE patterns are characteristic, spatially and temporally, within normal human lenses over the same age range. In fact, the patterns for the center of lenses from birth to 20y show similar crystallin modifications resulting, ultimately, in the formation of the nuclear pattern shown here (Figure 4B–D and Figure 5F). These consistent patterns of protein modification suggest a constant time/age-dependent process of protein modification. Small differences observed were not unexpected considering the technical difficulties in the dissections.
The intensity of the protein spot of each major crystallin observed in outer cortical samples diminishes until none are visible in the inner cortex and nuclear regions (Figure 4B–D). Upon post-translational modification a protein will migrate at a new position on electrophoresis that is dependent on whether it leads to a change in protein charge or size. Even in a normal 17 year old lens at least 20 different proteoforms of αA-crystallin were identified in the outer 3 cortical layers (Colvis and Garland, 2002). These included the full-length protein that was phosphorylated, acetylated and/or deamidated. In addition, several truncated forms were identified including several that were truncated after Ser162 and Ser172. The mechanism of truncation at serine residues has subsequently been determined (Lyons et al., 2016a). Numerous studies have identified post-translationally modified forms of each human lens crystallin (Wang et al., 2013; Lampi et al., 2014; MacCoss et al., 2002; Wilmarth et al., 2006; Hains and Truscott, 2007, 2010; Bakthisaran et al., 2016a; Zhang et al., 2003). The identification of all proteins visible on gels of a 3 year-old human lens was accomplished and showed that the majority of crystallins were present as multiple proteoforms indicating that modifications start early in life (Nakajima et al., 2009).
Importantly, although full length nascent forms of crystallins are only present at birth or in newly synthesized fiber cells, lenses can remain transparent for decades after the crystallins have undergone multiple modifications and the original forms of the major crystallins are no longer visible in the lens nucleus. An argument could be made that the litany of crystallin modifications provides advantage to the lens. They are non-random, predictable, and appear to be biologically/biochemically determined. This further suggests that different regions of the lens require different forms of each crystallin for function or stability. In the outer cortex the newly formed fiber cells undergo maturation as they move centrally. As the cells move toward the nucleus, they experience compaction. The lens nucleus, which has the highest protein concentration, makes up the majority of the optical axis. The highest concentration of proteins with high cysteine content (γ-crystallins) is in the lens nucleus to establish the gradient of refractive index (GRIN) necessary for lens focusing and minimization of spherical aberrations (Zhao et al., 2011).
4.2. Human lens sub-proteomes
With the interference from highly abundant proteins and the degradation of many cellular organelles, proteomic analysis of adult lens samples normally yields fewer numbers of proteins identified than in other tissues; however, the rapid advances in mass spectrometry instrumentation, chromatography, and bioinformatics have enabled more in-depth analysis of the lens proteome (MacCoss et al., 2002; Wilmarth et al., 2006). One strategy that has expanded our understanding of the human lens proteome is the fractionation of proteins into sub-proteomes. Subcellular proteomic analysis decreases the complexity of the samples and provides valuable information for proteins present in low copy numbers.
For example, by removing highly abundant lens crystallins and cytoskeletal proteins, lens membrane fractions have been prepared and the lens membrane proteome characterized in mouse (Bassnett et al., 2009) and human (Wang et al., 2013) lenses. A total of 575 proteins were detected in young mouse lenses by separate analysis of soluble and membrane fractions using offline strong cation exchange fractionation together with reverse phase LC-MS/MS (Bassnett et al., 2009). In this study, 232 proteins were found enriched in the membrane fraction (Bassnett et al., 2009). An additional human lens membrane proteome study identified 951 proteins in whole human lenses including 379 membrane proteins (Wang et al., 2013). This study revealed important components of biological pathways that are associated with fiber cell membranes including glycolysis/gluconeogenesis, glutathione metabolism, regulation of actin cytoskeleton and cell-cell communication and signaling. Other than well-known highly abundant lens proteins, several other proteins that may have an important role in the human lens were detected. These proteins include: lactase-like protein (LCTL) which has a role in lens suture formation; lengsin (LGSN) that serves as a chaperone for the reorganization of intermediate filament proteins during terminal differentiation; and tudor domain-containing protein 7 (TDRD7) that has been reported to play a role in cataractogenesis (Lachke et al., 2011). Many proteins that were thought to be neuron-specific in their expression were detected in the lens membrane fraction such as limbic system-associated membrane protein (LSAMP), brain acid soluble protein 1 (BASP1), neurofascin (NFASC), kinesin-like protein (KIF1A) and synaptophysin-like protein 1 (SYPL1) supporting the previous observation of extensive parallels between neurons and fiber cells (Frederikse et al., 2012).
The lens membrane proteome can be further fractionated into membrane microdomains that have a variety of different lipid compositions. Cholesterol- and sphingolipid-enriched microdomains, called lipid rafts, exist as distinct liquid-ordered regions of the membrane that are important for signal transduction. A further fractionation of fiber cell membranes results in a lipid raft-like detergent resistant membrane (DRM) fraction and the DRM proteome has been studied (Wang and Schey, 2015). A total of 506 proteins were detected in the high-buoyancy lipid raft fraction and 359 of these proteins are present in the high-confidence list of the lipid raft protein database (Shah et al., 2015). Over 50% of the proteins (330 proteins) detected in the DRM fraction were not detected in our previous analysis of whole membrane fractions (Wang et al., 2013) demonstrating the increased dynamic range afforded by fractionating samples into subproteomes.
Human lens fiber cell membranes differ from cell membranes of other tissues and other experimental models in that they contain high concentrations of long-chain saturated fatty acids, cholesterol, and sphingomyelin (Borchman and Yappert, 2010; Deeley et al., 2008). Indeed, cholesterol levels are such that cholesterol bilayers form in the plasma membrane (Jacob et al., 1999; Widomska et al., 2017). Based on this lipid composition of lens membranes, it is expected that lipid raft domains are highly abundant in lens fiber cells. In bovine lenses, not only were significant numbers of proteins detected in the lipid raft-like DRM fraction, but also a significant fraction of highly abundant proteins were detected in the DRM fraction such as AQP0, MP20, and flotillins (Figure 6). As previously reported (Tong et al., 2009) only small amounts of αA-crystallin and connexin 50 were found in the DRM fraction, but the presence of connexin 50 in this fraction is highly sensitive to cholesterol removal by methyl-β-cyclodextrin (MβCD). Many lipid raft marker proteins were abundant in the DRM fraction from lens fiber cells such as flotillins, paralemmins, cadherin-2, caveolins, coxsackievirus and adenovirus receptor homolog, and erlins. This result suggests that lipid rafts play important roles in lens fiber cells such as subcellular membrane transport, trafficking, and signaling as suggested by the list of proteins identified in the DRM fraction. We anticipate that similar results would be found in human lens samples. Distinct membrane domains are present in the complex lens membrane interdigitations such as ball-and-sockets and protrusions (Bagchi et al., 2003; Biswas et al., 2010; Lo et al., 2014). Consistent with the results from bovine lenses described above, ball-and-socket structures in chicken and monkey lenses have gap junctions and low cholesterol content, but protrusions have high cholesterol content and no gap junctions (Biswas et al., 2010). In mouse and chicken lenses, AQP0 (Lo et al., 2014) and paralemmins (Bagchi et al., 2003) localize particularly in protrusions.
Figure 6. Identification of bovine lens lipid raft proteins.
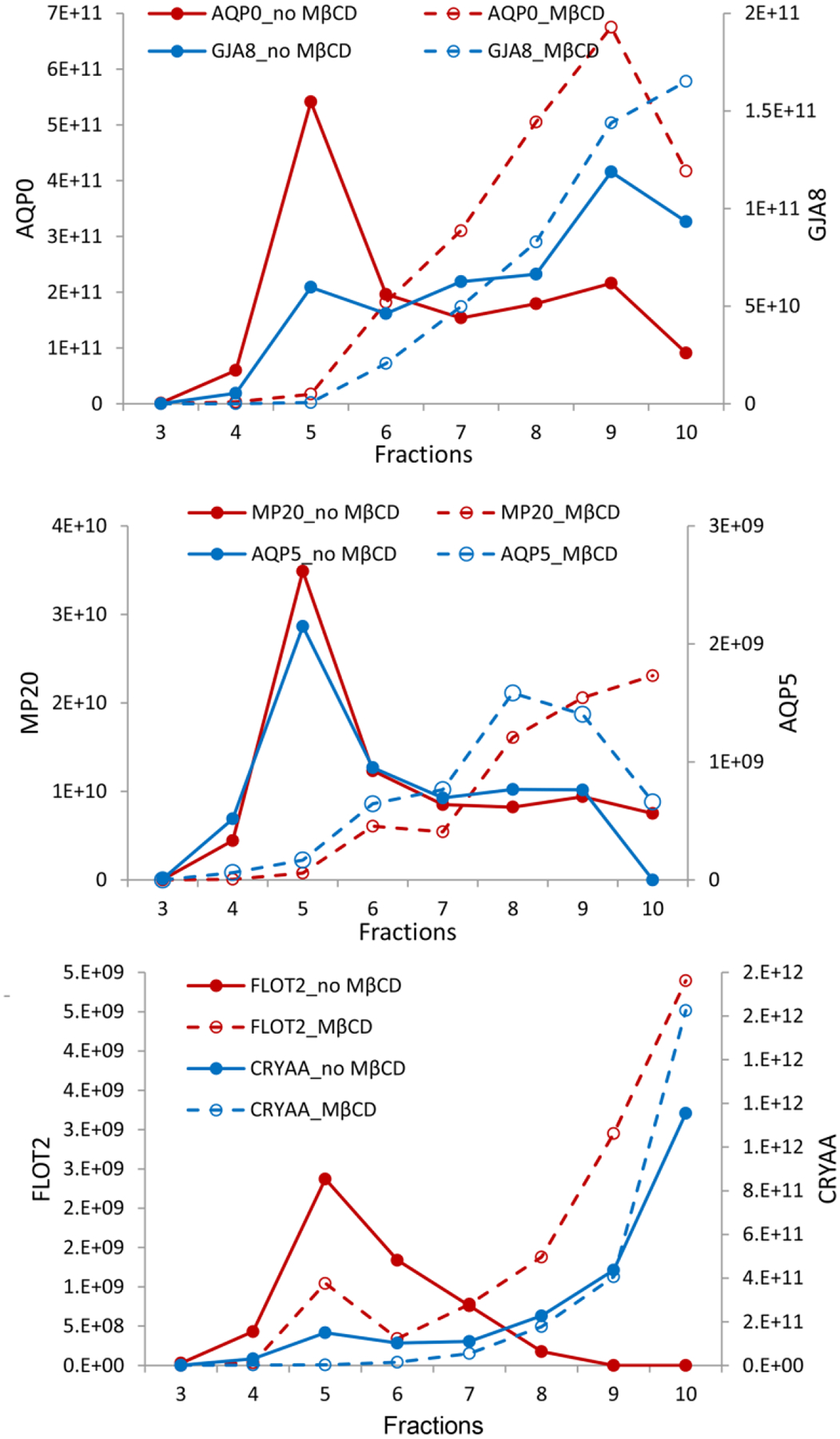
Fractionation of lens membrane proteins in a sucrose density gradient indicating lipid raft domains (fractions 3–6) containing AQP0 and connexin 50 (GJA8) (top), MP20 and AQP5 (middle) and Flotillin-2 and αA-crystallin (bottom). The addition of methyl-β-cyclodextrin reduces lipid raft domains and shifts contents to later eluting fractions (dashed lines). The y-axes represent peak area intensities for corresponding peptides from each protein in arbitrary units. Figure from (Wang and Schey, 2015).
Alterations of lipid composition that lead to abnormal lipid raft organization have been reported to be associated with neurodegenerative diseases including Alzheimer’s disease and Parkinson’s disease (Sonnino et al., 2014; Paladino et al., 2004). Alteration of lipid raft organization in human lenses has not been studied as a function of age or during cataractogenesis. Considering the increased affinity of lipid raft domains for oligomeric proteins reported in age-related brain diseases, it is important to study the lens lipid raft composition changes during aging which could help our understanding of protein aggregation and membrane protein binding and assist in developing effective nonsurgical treatments.
4.3. Post-translational modifications (PTMs)
Post-translational modifications can arise through enzymatic or non-enzymatic processes. To a large extent, enzyme-mediated modifications are likely to be beneficial and indeed many are essential for normal metabolism. For example, phosphorylation regulates numerous cellular processes including protein-protein interactions (Betts et al., 2017) and cell signaling (Tanimura and Takeda, 2017) and others, such as lipidation, are involved in cell signaling, targeting of proteins to subcellular locations and altering protein function (Chen et al., 2018). The lens outer cortex contains fiber cells in different stages of differentiation from precursor epithelial cells; therefore, some PTMs in the outer cortex likely play important regulatory roles in cell differentiation and elongation. These PTMs, such as phosphorylation, acetylation, methylation, ubiquitination and lipidation, are typically enzymatic and reversible. Given the long-lived nature of lens fiber cells and their proteins, many lens protein modifications are likely to be non-enzymatically formed and to accumulate in the lens with age. Such modifications including: oxidation, deamidation, truncation, racemization, and crosslinking, are largely irreversible, are thought to be generally deleterious to protein function, and have been linked to cataract and presbyopia. Here, we briefly discuss the major and most studied lens protein PTMs and what is known about how they change with age.
4.3.1. Phosphorylation
Phosphorylation, a reversible process, is one of the most common and important cellular regulatory PTMs. Although protein phosphorylation has been studied for decades, identification of thousands of phosphorylation sites in a single experiment was not possible until recent advances in mass spectrometry and the development of methods for phosphopeptide enrichment. Initial phosphorylation studies in the lens were focused on a single or a few highly abundant proteins (Moroni and Garland, 2001; Kamei et al., 2001; Aquilina et al., 2004; Schaefer et al., 2003; Shearer et al., 2008; Wang and Schey, 2009; Wang et al., 2010). Later, several phosphoproteomic studies were performed using immobilized metal affinity chromatography (IMAC) for phosphopeptide enrichment and less than 100 phosphorylation sites were identified in human or porcine lenses (Huang et al., 2011; Chiou et al., 2010). Combining TiO2 phosphopeptide enrichment and MudPIT analysis allowed 855 phosphorylation sites on 271 proteins to be identified in the human lens fiber cell membrane fraction, including many new phosphorylation sites (Wang et al., 2013). These results also indicated that many lens proteins are highly phosphorylated, including 54 proteins with at least five phosphorylation sites.
The functional role of lens protein phosphorylation has not been widely studied; therefore, it is unclear whether all of these sites are physiologically relevant. This is particularly true for those proteins with many different phosphorylation sites. Phosphorylation on at least some lens proteins has been linked with cataract. Phosphorylation of αA- and αB-crystallins has been reported to increase in cataract lenses (Kamei et al., 2004) and is stimulated by oxidative stress (Wang et al., 1995). Phosphorylation was found to reduce chaperone activity of αB-crystallin in some studies (Kamei et al., 2001; Ito et al., 2001), but not others (Wang et al., 1995). As demonstrated by proteomic analysis, αB-crystallin has many phosphorylation sites and the inconsistency of results may indicate different functional consequences of different phosphorylation sites or extent of phosphorylation. For example, Bakthisaran et al. report that low levels of αB-crystallin phosphorylation may be beneficial whereas hyperphosphorylation may be deleterious (Bakthisaran et al., 2016b). Spatial analysis of singly and doubly phosphorylated αB-crystallin in human lenses by IMS showed the highest abundance in the nucleus of a 7y lens and in the outer cortex of older human lenses (Grey and Schey, 2009).
Another important protein that is regulated by phosphorylation is AQP0. The regulatory role of three conserved AQP0 C-terminal phosphorylation sites (Ser229, Ser231, Ser235) on AQP0 water permeability has been studied and phosphorylation regulates AQP0 water permeability through calcium dependent calmodulin (CaM)-AQP0 gating. Phosphorylation was first found to reduce CaM and AQP0 binding when a C-terminal AQP0 peptide was used to interact with CaM (Reichow and Gonen, 2008; Rose et al., 2008). Later, Fields et al. reported AQP0 phosphorylation did not significantly affect CaM binding with the full length AQP0 but, instead, phosphorylation modified the AQP0-CaM interaction interface, particularly at an arginine rich cytoplasmic loop (Fields et al., 2017). Phosphorylation of AQP0 on Ser6, Ser8, Thr120 and Thr199 was also detected; however, the functional importance of these phosphorylation sites has not been established.
High throughput quantitative phosphoproteomics has not been done in the lens; however, spatially-resolved, absolute quantification of phosphorylation in AQP0 and MP20 detected in different lens regions revealed that phosphorylation of Ser235 on AQP0 and Thr170 on MP20 was found to reach a maximum at the permeability barrier region (r/a of ~0.9); a region defined by Moffat et al. (Moffat et al., 1999) and Sweeney and Truscott (Sweeney and Truscott, 1998). Importantly, in older lenses where such a barrier exists, AQP0 phosphorylation was significantly decreased interior to the barrier region (Figure 7) (Gutierrez et al., 2016). This result led us to speculate that regulation of lens membrane protein function via phosphorylation may play a significant role in establishing and maintaining the lens microcirculation system and perhaps contribute to the formation of the age-related permeability barrier.
Figure 7. Spatial quantification of human lens AQP0 and MP20 phosphorylation.
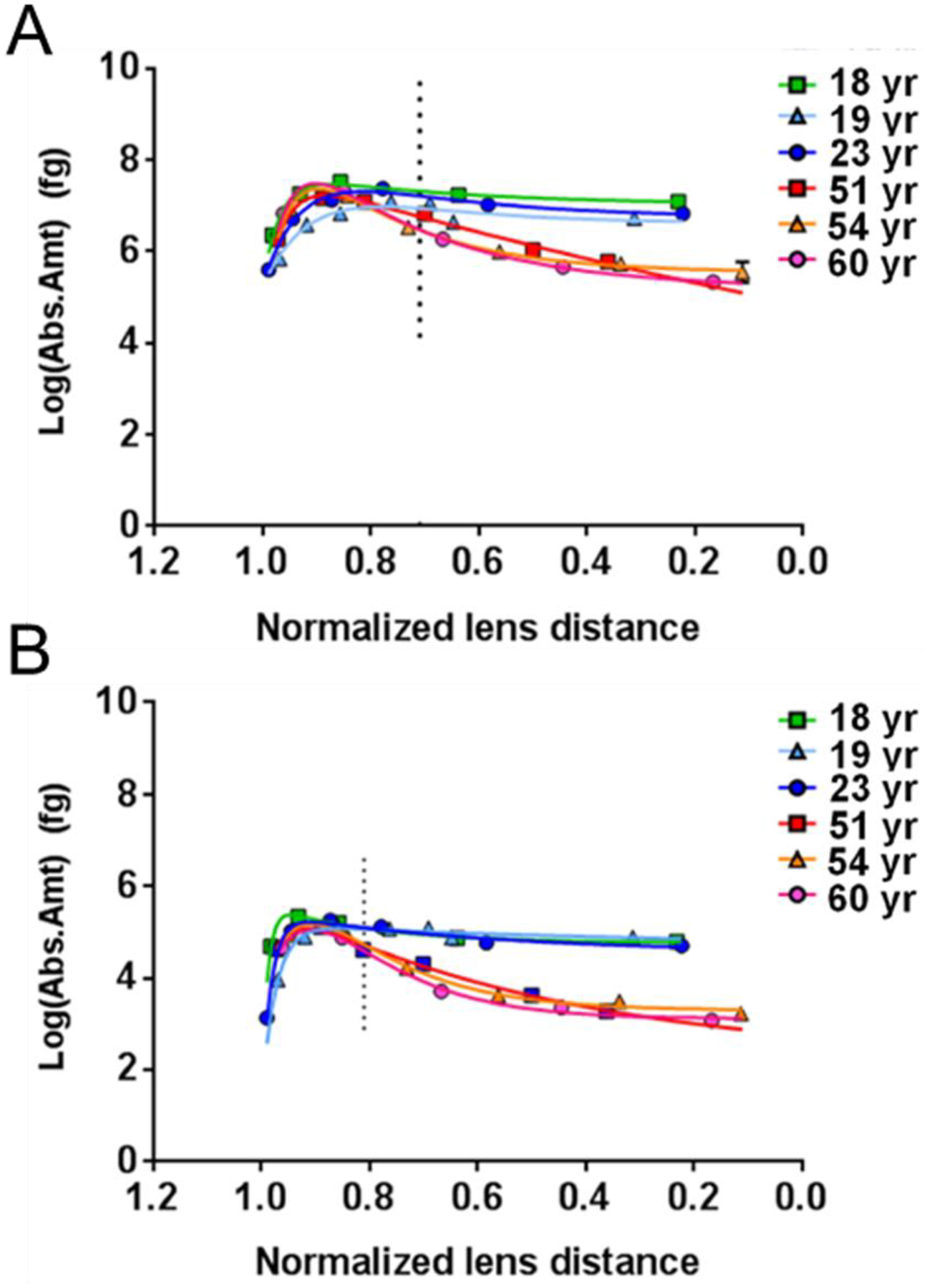
Abundance of phosphorylated Ser235 of AQP0 (A) and phosphorylated Ser170 of MP20 (B) is shown as a function of lens age and normalized distance from the lens center. Lens surface (1.0) and lens center (0.0). The vertical dotted lines indicate the lens distance at which the phosphorylation levels are significantly different between young and old lenses. Figure from (Gutierrez et al., 2016).
4.3.2. Truncation
Truncation occurs by removing part of the sequence from the N- or C-terminus of a protein, or cleavage of the protein resulting in multiple peptide fragments. Due to the long-lived nature of lens proteins, truncation is one of the most commonly observed protein modifications in the lens. The reasons for this are elaborated below.
Truncation in the lens was first detected in highly abundant lens crystallins and all three types of lens crystallins undergo truncation with age. Truncation of αA-crystallin starts from early age and mainly occurs at the C-terminus. Truncation starts through removal of the C-terminal Ser173 residue and continues with removal of additional residues with age (Takemoto, 1995b; Colvis and Garland, 2002; Grey and Schey, 2009). The major truncation sites include: Ser172, Ser162, Asn101, Asp58 and Leu40 in human lenses. Truncation of αB-crystallin also starts from the C-terminus and the major truncation sites include Lys174, Thr170, Thr40, Glu34 (Takemoto, 1995b; Grey and Schey, 2009; Colvis et al., 2000). Unlike α-crystallins, truncation of β-crystallins mainly occurs through removing certain residues from the N-terminus of the proteins (Lampi et al., 1998; Ma et al., 1998; Srivastava and Srivastava, 2003a) with βB2-crystallin exhibiting increased resistance to proteolysis compared to βB1- and βA3/A1-crystallin (Zhang et al., 2001). In contrast to α- and β-crystallins, γ–crystallins undergo less proteolysis during lens maturation as shown by several studies (Lampi et al., 1998); however, γ–crystallins still undergo truncation. Truncation of γD crystallin was detected in human lenses as a product of removing N-terminal 87 residues (Srivastava et al., 1992). Cleavage of γS-crystallin generates 12-residue peptide (SPAVQSFRRIVE) that was found bound tightly to lens cell membranes (Friedrich et al., 2012). Peptides from β-crystallins and γ–crystallins that contain a double basic residue motif are detected in aged or cataract lenses and could lead to membrane binding (Friedrich et al., 2012)(Schey, unpublished results).
In addition to crystallins, truncation has been detected in many other lens proteins. The lens major intrinsic protein, AQP0, exhibits extensive truncation in its C-terminal region at residues Asn259 and Asn246 (Ball et al., 2004; Korlimbinis et al., 2009; Gutierrez et al., 2011) (Figure 8). By age 21y, the only intact AQP0 present in the human lens is in the lens outer cortex. Truncation of both connexin 50 and connexin 46 starts from early stage of fiber cell differentiation and truncation occurs in multiple regions of these proteins (Wang and Schey, 2009; Slavi et al., 2016; Shearer et al., 2008). Lens beaded filament proteins filensin and phakinin are extensively processed during lens fiber cell differentiation (Sandilands et al., 1995; Wang et al., 2010) by removal of about 40 residues from the N-terminus (Wang et al., 2010). The new N-terminus of phakinin is not modified, but the new N-terminus of filensin is acetylated (Wang et al., 2010). In addition, the major truncation site for filensin is Asp431 in bovine lens and Asp433 in human lens and, after truncation, the newly formed N-terminus is myristoylated (Wang et al., 2010).
Figure 8. Quantification and spatial localization of human AQP0 truncation products.
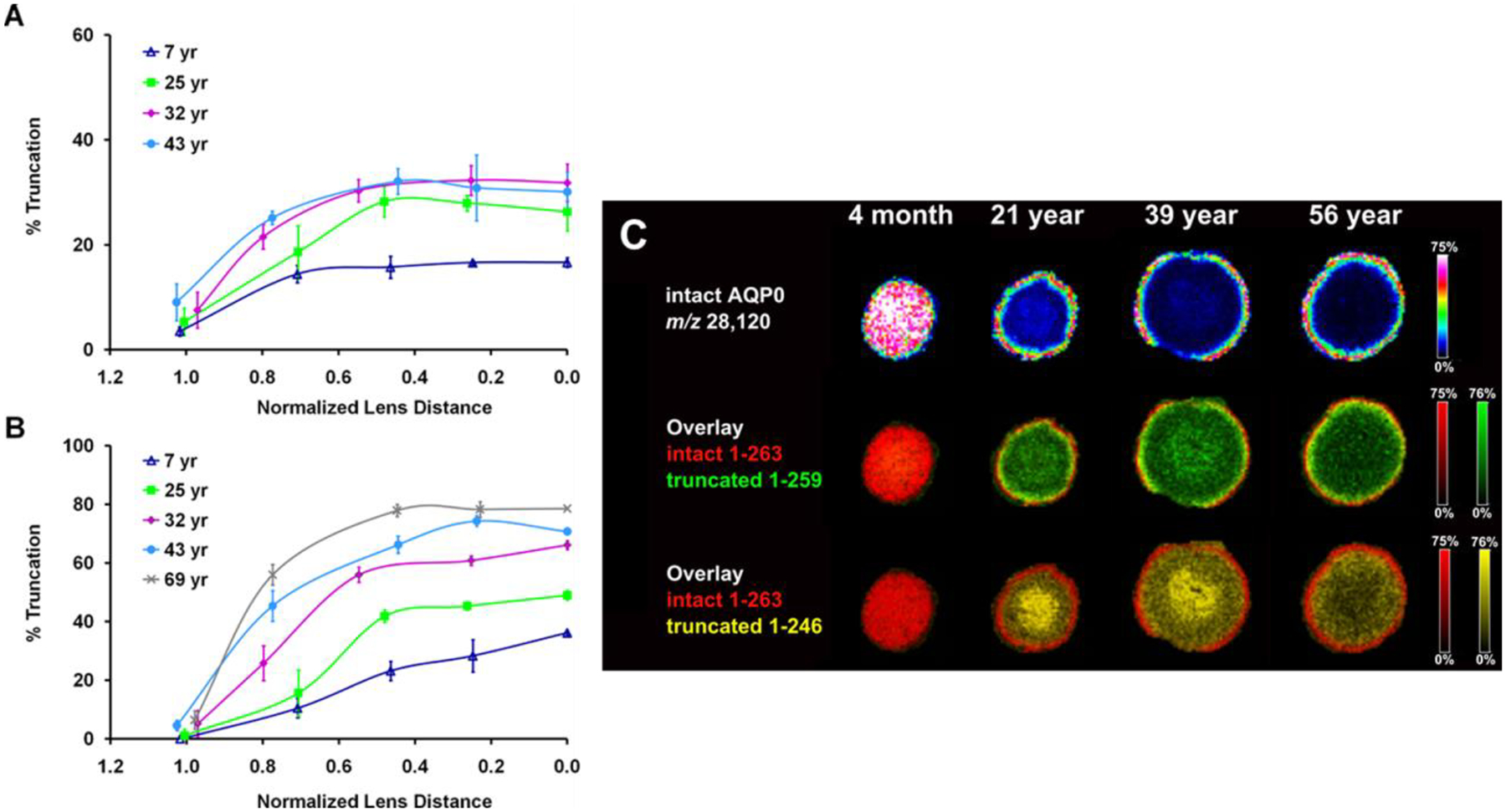
The abundance of human AQP0 truncation at residues 259 (A) and 246 (B) is shown as a function of lens age and lens region measured in manually dissected human lenses and (C) imaged by imaging mass spectrometry. Figure adapted from (Gutierrez et al., 2011) and (Wenke et al., 2015).
Peptide bond cleavages are believed to occur either non-enzymatically or catalyzed by the activation of lens proteolytic enzymes and are likely to have significant biological consequences. Although a number of proteases have been identified in lens fiber cells (Sharma and Santhoshkumar, 2009), the majority of protein truncation in the human lens is spontaneous and results from reactions of the side chains of Asn (Wang et al., 2019; Ball et al., 2004) and Ser (Su et al., 2012). Spontaneous deamidation, isomerization, and racemization of Asn residues occur through formation of a succinimide ring intermediate. Alternatively, and also through a succinimide intermediate, attack on the peptide bond by the Asn side chain can result in peptide bond cleavage (Voorter et al., 1988). This mechanism probably explains truncation at many different Asn residues especially the ones that are followed by a small, flexible residue such as glycine. Peptide bond cleavage also occurs on the N-terminal side of Ser (Takemoto, 1995b; Lyons et al., 2016a). In addition, proteins can also undergo truncation at Asp residues, again by attack of the side chain and the formation of succinic anhydrides followed by hydrolysis (Wang et al., 2019). Side chain-mediated cleavage at these sites is just the first step and, from this initial peptide bond hydrolysis, a series of additional steps take place that result in progressive removal of one or two amino acids at a time from the N-terminus (Su et al., 2012). Similar, but less well understood cleavage reactions take place at the C-terminus. The overall result is a complex series of “laddered peptides”. Examination of low molecular weight endogenous crystallin peptides revealed peptides with Lys and Arg situated at the C-terminus with significantly higher frequency compared to other residues, suggesting that trypsin-like proteolysis may take place in the lens cortical fiber cells (Su et al., 2011). Analysis of aged human lenses revealed a large mixture of peptides derived by truncation of crystallins (Su et al., 2012). Some of these peptides such as the C-terminal peptide of γS-crystallin (Friedrich et al., 2012) and some α-crystallin peptides (Santhoshkumar et al., 2008) could themselves play important biological roles in the lens. The spatial distribution of major αA-crystallin truncation products in human lenses can be seen visually via imaging mass spectrometry as shown in Figure 9. Successive truncation from the C-terminus of αA-crystallin occurs and small peptide products accumulate in the older fiber cells of the lens nucleus. Thus, the only intact, and presumably functional, αA-crystallin in older human lenses is present in the outer cortical fiber cells.
Figure 9. Spatial distribution of human αA-crystallin and its truncation products.
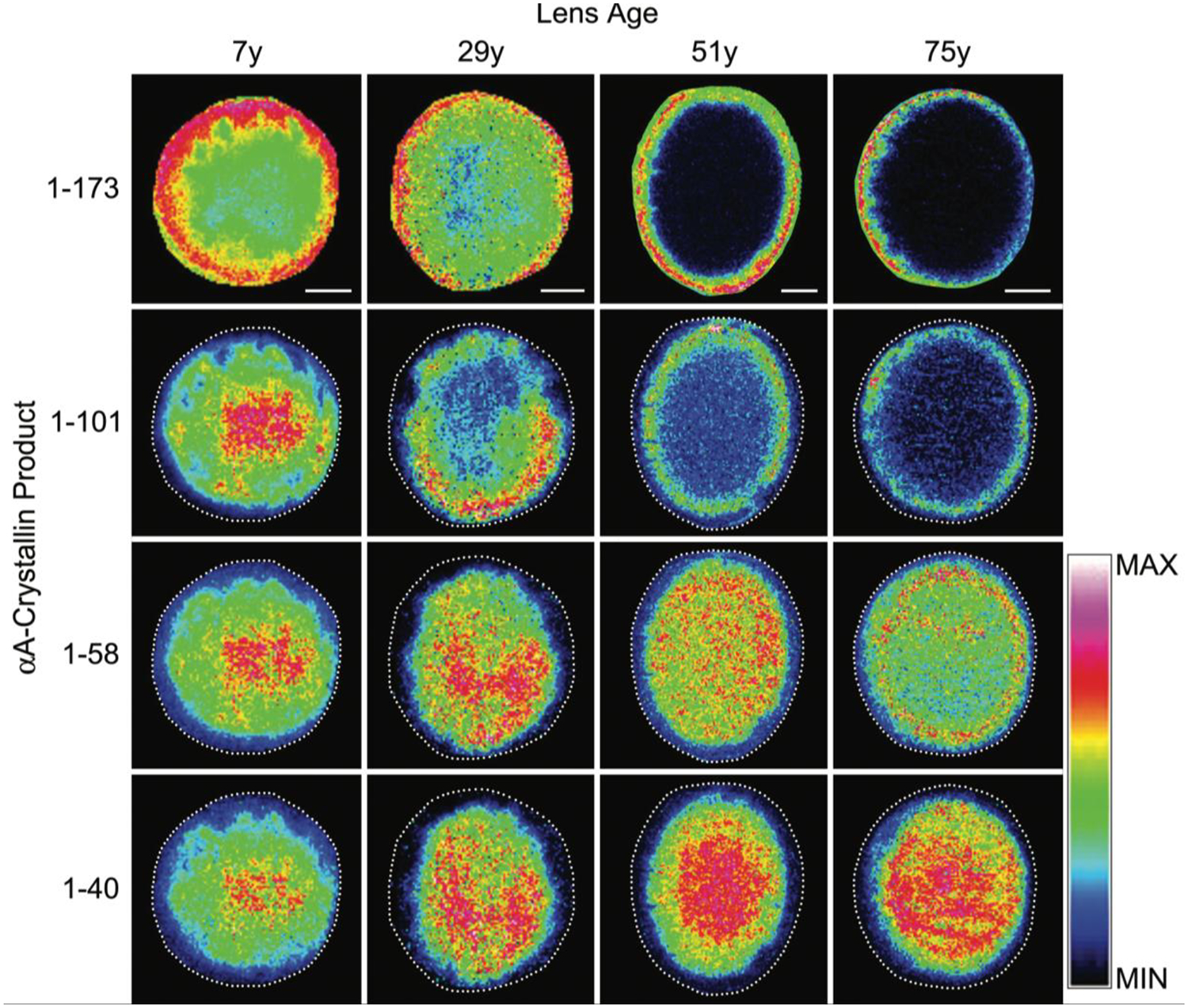
Imaging mass spectrometry of full length αA-crystallin (top row) and truncation products (rows 2–4) in human lenses as a function of age. Figure from (Grey and Schey, 2009).
Some evidence exists for enzymatic truncation of lens proteins. The major truncation site in filensin is likely formed by action of caspase (Tapodi et al., 2019). Calpain related proteolysis has been well-studied in rodents (David et al., 1994; Ueda et al., 2002) but is deemed less important in human lenses due to a low level of calpain activity and an excess of the calpain inhibitor, calpistatin, in human lenses (David et al., 1989). A putative role for calpain in human cortical cataract formation has been reported (Andersson et al., 1996).
LLPs, such as those found in lens fiber cells, may use PTMs to expand protein functions. For example, post-translational N-myristoylation of filensin C-terminal fragment is believed to facilitate the transition of filensin C-terminal fragment from cytoplasmic localization to membrane domains and may regulate AQP0 permeability (Wang et al., 2010; Wang and Schey, 2017; Tapodi et al., 2019). Another example of lens protein truncation affecting function was shown for connexin50 where C-terminal truncation by calpain significantly reduced the pH sensitivity of gap junction gating (Lin et al., 1997; Lin et al., 1998). Truncation of many proteins during aging may also have detrimental biological consequences. It is interesting that αA-crystallin chaperone activity was found to be improved after removal of the C-terminal serine 173, but its chaperone activity significantly dropped after further truncation at C-terminus (Aziz et al., 2007). Truncation of crystallins has been associated with protein aggregation and insolubilization of crystallins (Asomugha et al., 2010; Srivastava and Srivastava, 2003b; Srivastava et al., 2017). Further, low molecular weight crystallin peptides that accumulate with age (Santhoshkumar et al., 2008; Su et al., 2010) have been shown to have anti-chaperone, pro-aggregatory properties (Raju et al., 2015; Kannan et al., 2013) and to even generate hydrogen peroxide and induce apoptosis (Raju et al., 2017). Recently, as discussed below, we discovered a new mechanism of protein-protein crosslinking in the aged lenses after a terminal Asp residue is formed by protein truncation (Wang et al., 2019). This mechanism of crosslinking explains at least partially why truncated crystallins may be more prone to be found in insoluble or membrane fractions with age. Distinguishing which age-related PTMs impart novel functions to lens proteins and which adversely affect protein function remains a challenge to the field of lens protein research.
4.3.3. Racemization, deamidation, and isomerization
Racemization, the process where an amino acid is converted from its native L-form to a D-form, appears to be the most abundant PTM in aged human lenses lenses (Fujii et al., 2001; Fujii et al., 1999) and it is estimated that by age 60y, on average, EVERY protein in the lens contains two or three D-amino acids (Hooi and Truscott, 2011). When one considers that replacement of just one L-amino acid by a D-amino acid can have major consequences on the structure of peptides and proteins, it is likely that racemization on this scale has significant implications for the properties of aged human lenses.
The amino acids most affected by racemization are Asp, Asn and Ser, and in each case the reactions are spontaneous. Thr is affected to a smaller degree. In the case of Ser, it appears that simple abstraction of the alpha proton and re-protonation are responsible for conversion of L- to D-Ser. This is difficult to prove in the lens, but the relative ease with which this process occurs for Ser peptides in vitro suggests that it also may occur in proteins in a biological environment. For Asn, the pathway is more complicated and racemization is linked inexorably with deamidation since a shared cyclic intermediate is involved (Figure 10). It is thought that more facile racemization of the succinimide intermediate is a key factor in converting an L-Asn to a D-Asp residue. It should be emphasized that for each site of deamidation of Asn, a negatively charged Asp residue has been introduced. Transformation of a neutral site in a protein, to one that is negative, will also affect structure. It is likely that a similar process underpins racemization of Asp residues although this has not been as well studied. The scheme commonly used to illustrate this process is shown in Figure 10 and indicates that each step is an equilibrium and one could be forgiven for believing that each step might be readily reversible under biological conditions. It seems that this is not, in fact, the case. The stability of succinimides varies greatly and there is a marked effect of pH. For example, D-isoAsp residues in some peptides appear to show negligible reversion to the other Asp isomers despite extended incubation (Hooi et al., 2013b). Flanking residues also significantly affect stability. Much remains to be discovered about this seemingly straightforward process. It has been observed that the major product of Asn racemization in adult lens proteins is D-isoAsp (Hooi et al., 2012c). Proteomic investigations also revealed that in most cases a mixture of the four Asp isomers of each tryptic peptide were obtained with the D-isoAsp peak being the largest in aged lenses. This finding itself proves that the mechanism responsible for Asp and Asn racemization involves the cyclic intermediate and is not the result of simple proton abstraction as noted for racemization of Ser.
Figure 10. Mechanism of Asn deamidation and racemization.
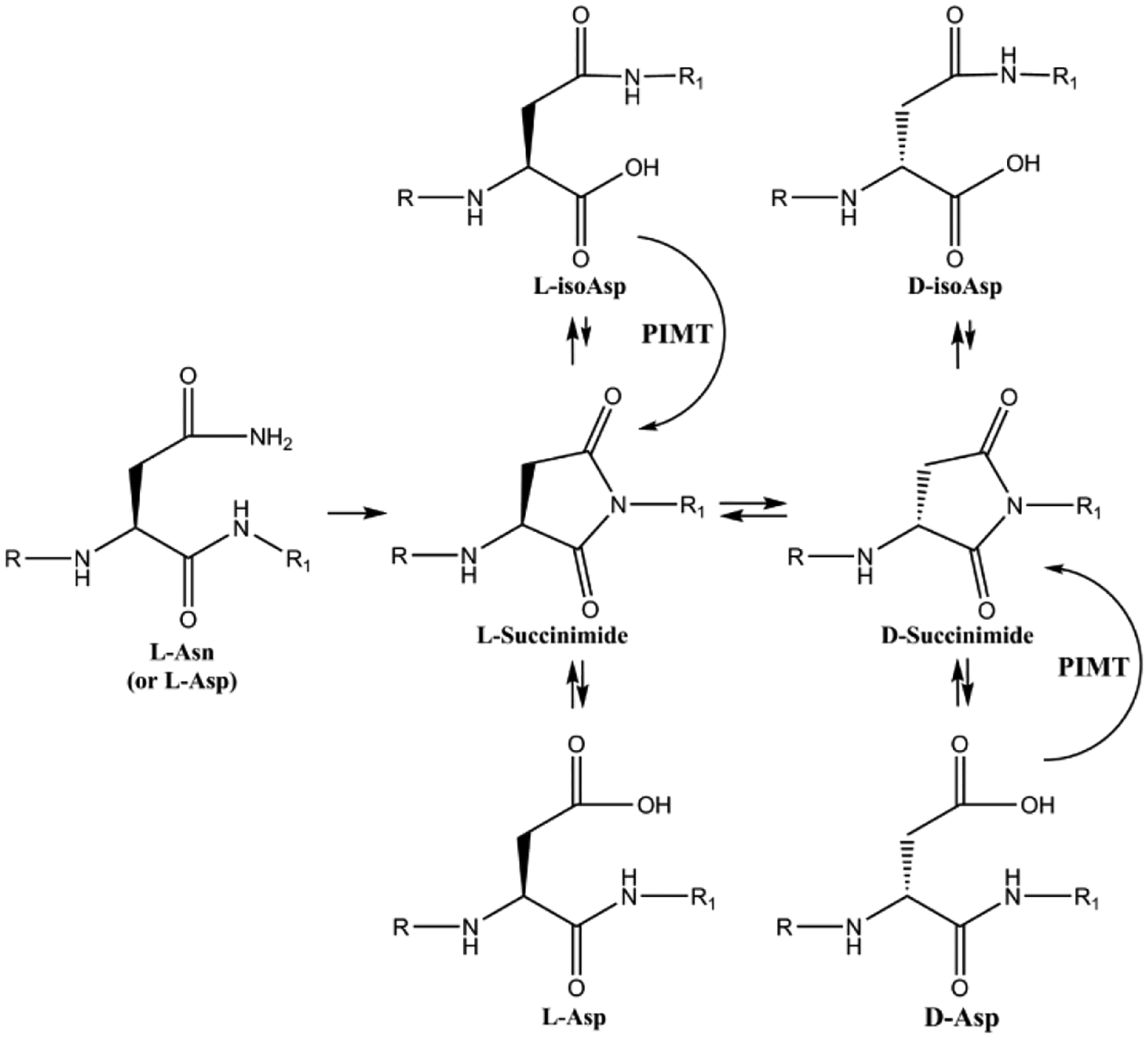
Scheme showing the mechanism of deamidation and racemization of asparagine residues. Adapted from (Truscott et al., 2016a).
Deamidation, the process where Asn and Gln residues are converted to Asp and Glu residues, can occur at a very young age. Imaging mass spectrometry analysis of human AQP0 deamidation (Figure 11) showed that significant amounts of deamidation occur as early as 4 months of age. This finding is consistent with whole lens protein analysis which revealed significant racemisation occurring within the first decade of life (Hooi and Truscott, 2011).
Figure 11. Spatial distribution of deamidated AQP0 in human lenses.
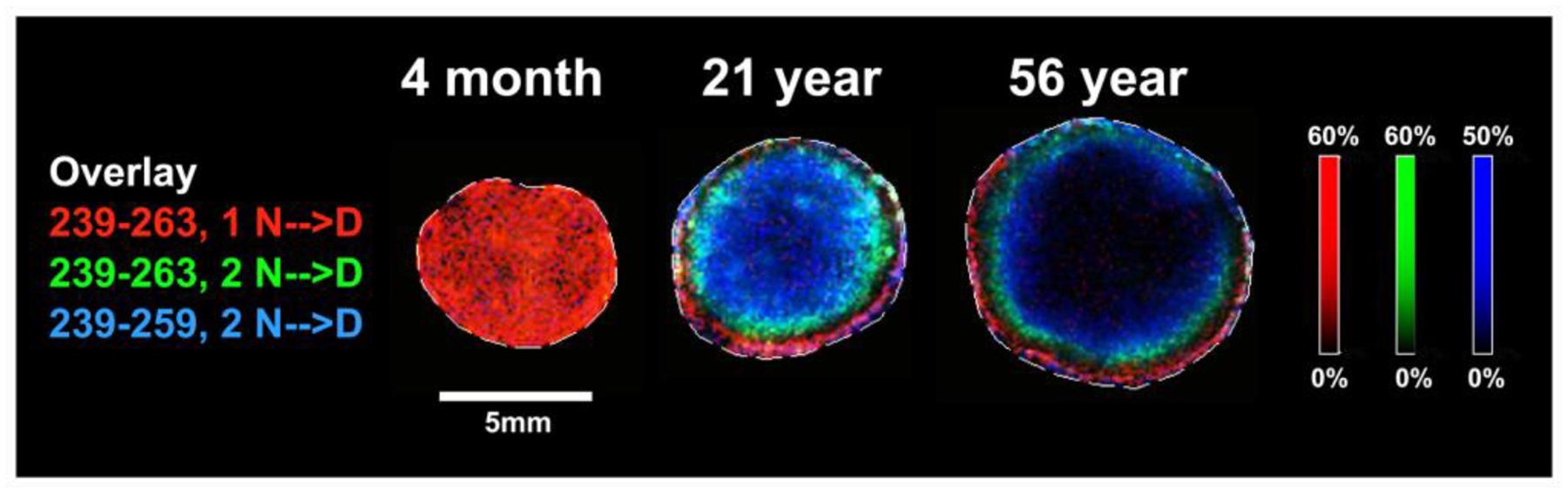
Imaging mass spectrometry of human lenses of various ages showing the spatial distributions of singly deamidated (red), doubly deamidated (green), and doubly deamidated and truncated (blue) C-terminal peptides of AQP0. Figure from (Wenke et al., 2015).
In other tissues and organs such as the brain, protein isoaspartate methyl transferase (PIMT) is present to help reverse the major process of protein racemization (Murray and Clarke, 1984; Shimizu et al., 2005). This protective enzyme methylates D-Asp and L-isoAsp residues in proteins and in this way, shifts the equilibrium back towards the original L-Asp form. PIMT is however inactive towards D-isoAsp and it is also important to note that once Asn residues undergo cyclisation and ring opening to form Asp, there can be no reversion to Asn. PIMT is active in the lens cortex but its activity is very low or absent in the lens nucleus past the age of 40y (Lyon et al., 2018). Recently, Warmack et al. (Warmack et al., 2019) suggested PIMT activity was reduced with age in the nucleus by limited amounts of the cofactor AdoMet and the inability to access L-isoAsp residues in aggregated proteins. Therefore, the spontaneous processes that occur in the lens center do so in the absence of enzymatic surveillance.
Isomerization and racemization of Asp and Ser residues, respectively, have been shown to have significant functional consequences on lens proteins. Both modifications disrupt the native oligomeric structure of α-crystallin (Lyon et al., 2019) and isomerization of Asp in structurally ordered regions lead to reduced solubility of α-crystallin (Lyon et al., 2017). Deamidation of β-crystallin has been shown to reduce solubility as well (Lampi et al., 2014).
4.3.4. Lipidation
Lipidation is a co- or post-translational modification in which lipid moieties are covalently attached to proteins; a modification that can regulate protein targeting to membranes via protein-lipid interactions and protein-protein interactions. Lens protein lipidation had only been inferred (Cenedella, 1990; Manenti et al., 1990) until direct detection of lipidation of AQP0 (Schey et al., 2010). AQP0 was found to be significantly modified by oleic acid and palmitic acid on Lys 238 and on its N-terminus through the formation of an unusual amide bond (Schey et al., 2010). Later, many other fatty acids were also found to be involved; the relative abundances of which mirror the fatty acid composition of lens phosphatidylethanolamine lipids (Ismail et al., 2016) suggesting fatty acylation of AQP0 is a non-enzymatic process. However, lipidation enzymyes such as palmitoyltransferases are known to use multiple fatty acids as substrates (Muszbek et al., 1999) and therefore enzymatic lipidation cannot be ruled out. MALDI imaging data suggests this modification starts at a relatively young fiber cell age in the inner cortex region of the lens (Schey et al., 2010). The function of this modification remains unknown; however, lipidated AQP0 was found to be highly enriched in the lipid raft domain of fiber cell membranes (Figure 12) (Schey et al., 2010; Wang and Schey, 2015). AQP0 present in lipid raft and non-lipid raft domains, presumably trafficked by its lipidation status, is likely to have a different permeability given that the local lipid bilayer environment influences AQP0 permeability (Tong et al., 2013). Based on these results, it would not be surprising to discover that many long-lived integral membrane proteins experience non-enzymatic lipidation.
Figure 12. Distribution of lipidated AQP0 in lipid raft and non-lipid raft domains of bovine lens membranes.
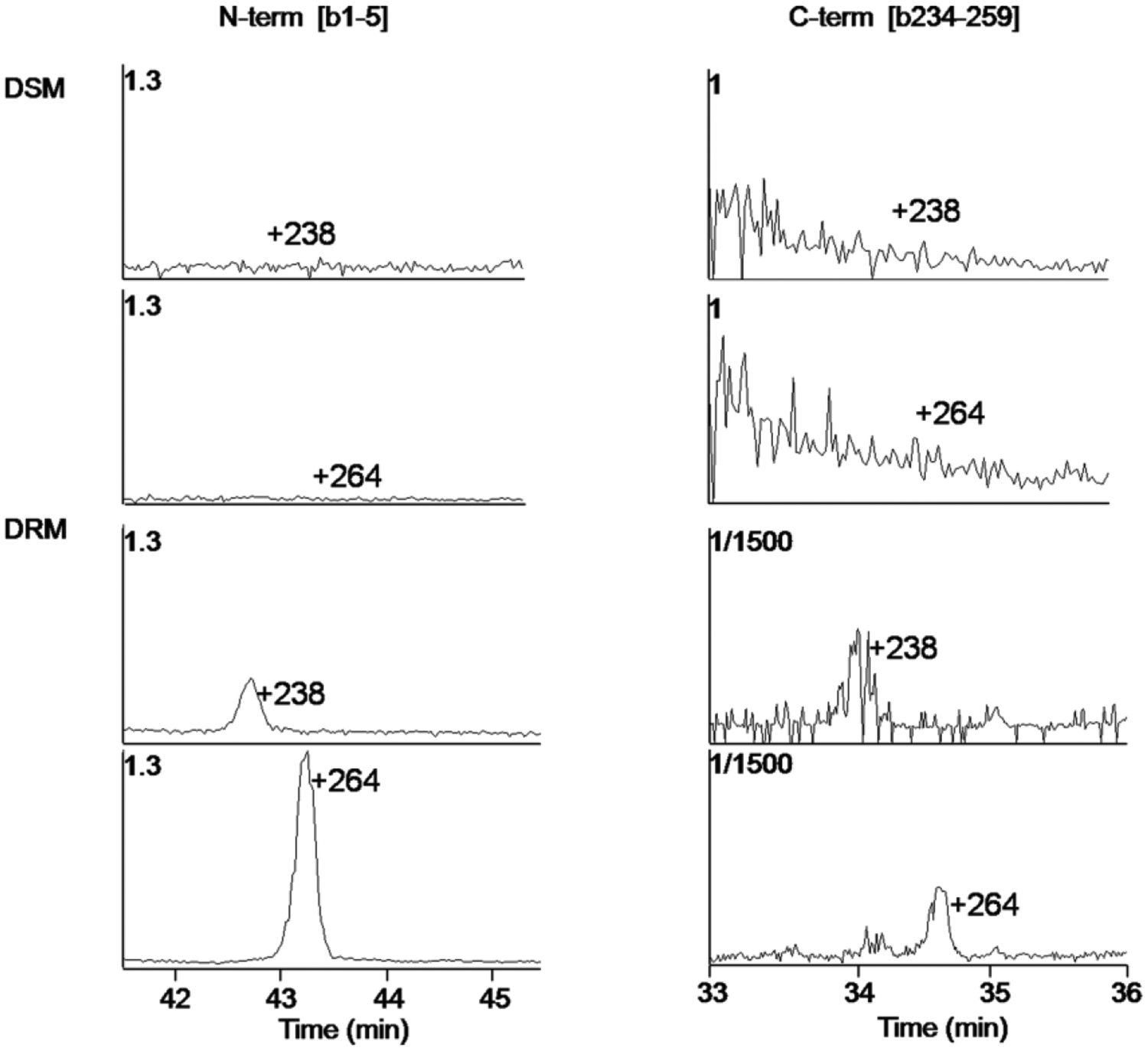
Selected ion chromatograms of lipid modified bovine AQP0 1–5 (left) and AQP0 234–259 (right) from detergent soluble (top panels) and detergent resistant (bottom panels) fractions. Figure from (Schey et al., 2010). DSM = detergent soluble membrane fraction. DRM = detergent resistant membrane fraction.
Canonical palmitoylation occurs on cysteine residues, is reversible, and is believed to regulate protein-membrane and protein-protein interactions. A survey of the lens “palmitoylome” revealed 174 proteins in the bovine lens indicating palmitoylation is prevalent in lens fiber cells (Wang and Schey, 2018a). Several biological processes including cell adhesion, regulation of cell shape, vesicle fusion, and establishment of protein localization to plasma membrane were enriched in the list of potential palmitoylated proteins. Important lens membrane proteins AQP5 and MP20 were found to be palmitoylated (Wang and Schey, 2018a) and, during this analysis, a series of lipidated proteins was also detected (Table 1). Recent global profiling of N-myristoylated proteomes suggests that close to 100 proteins are myristoylated in human cells (Thinon et al., 2014). With direct detection, 19 proteins were found to be myristoylated in the lens (Schey, unpublished results). This list also confirms that all detected Rab proteins are geranylgeranylated and all detected G-proteins, critical for receptor mediated signaling and cellular trafficking, are myristoylated or both myristoylated and palmitoylated. As for other enzymes in the lens, the activity of lipid transferases is expected to be low in older fiber cells.
Table 1.
List of lipidated proteins identified in human lens (data from Wang and Schey, (Wang and Schey, 2018a).
| Gene Name | Protein Description | Peptide | Sites | Modification |
|---|---|---|---|---|
| GNAI2 | Guanine nucleotide- binding protein G(i) subunit alpha-2 | G*C#TVSAEDKAAAER | G2, C3 | Myristoylation and palmitoylation |
| GNAI3 or GNAI1 | Guanine nucleotide- binding protein G(k) or G(i) subunit alpha | G * C#TLSA E D KAAV E R | G2, C3 | Myristoylation and palmitoylation |
| ERICH5 | Glutamate-rich protein 5 | G*C#SSSALNK | G2, C3 | Myristoylation and palmitoylation |
| FLOT1 | flotillin-1 | VFVLPC#IQQIQR | C34 | Palmitoylation |
| LIM2 | MP20 | RFGDWRFSWSYILGWVALLMTFFAGIF YMC#AYRMHECR | C159 | Palmitoylation |
| AQP5 | Aquaporin-5 | EVC#SVAFLK | C6 | Palmitoylation |
| GNAI2 | Guanine nucleotide- binding protein G(i) subunit alpha-2 | G*CTVSAEDKAAAER | G2 | Myristoylation |
| GNAI1 or GNAI3 | Guanine nucleotide- binding protein G(i) or G(k) subunit alpha | G*CTLSAEDKAAVER | G2 | Myristoylation |
| GNAI1 | Guanine nucleotide- binding protein G(i) subunit alpha-1 | G*CTLSAEDKAAVER | G2 | Myristoylation |
| ERICH5 | Glutamate-rich protein 5 | G*CSSSALNK | G2 | Myristoylation |
| BASP1 | Brain acid soluble protein 1 | G*GKLSKK | G2 | Myristoylation |
| MARC2 | Mitochondrial amidoxime reducing component 2 | G*AAGSSALAR | G2 | Myristoylation |
| TOMM4 0L | Mitochondrial import receptor subunit TOM40B | G*NTLGLAPMGALPR | G2 | Myristoylation |
| PSMC1 | 26S protease regulatory subunit 4 | G*QSQSGGHGPGGK | G2 | Myristoylation |
| PPM1A or PPM1B | Protein phosphatase 1A or 1B | G*AFLDKPK | G2 | Myristoylation |
| EEPD1 | Endonuclease/exonuclease/phosphatase family domain-containing protein 1 | G*STLGCHR | G2 | Myristoylation |
| NDUFB7 | NADH dehydrogenase 1 beta subcomplex subunit 7 | G*AHLAR | G2 | Myristoylation |
| GORASP2 | Golgi reassembly-stacking protein 2 | G*SSQSVEIPGGGTEGYHVLR | G2 | Myristoylation |
| MGC127919 | Putative uncharacterized protein MGC127919 | G*SFVSQPLEPVK | G2 | Myristoylation |
| CHMP6 | Charged multivesicular body protein 6 | G*NLFGRK, G*NLFGR | G2 | Myristoylation |
| ARF4 | ADP-ribosylation factor 4 | G*LTISSLFSRLFGR | G2 | Myristoylation |
| ARF2 | ADP-ribosylation factor 2 | G*NVFEKLFKSLFGKK | G2 | Myristoylation |
| ARF1 | ADP-ribosylation factor 1 | G*NIFANLFKGLFGK | G2 | Myristoylation |
| CYB5R3 | NADH-cytochrome b5 reductase 3 | G*AQLSTLGHVVLSPVWFLYSLIMKLFQR | G2 | Myristoylation |
| MIP | Aquaporin 0 | M#WELR | M1 | N-fatty acylation |
| LSILK#GSRPSESNGQPEVTGEPVELK | K238 | N-fatty acylation | ||
| BFSP1 | Filensin | G*GQISK | G432 | Posttranslational N-myristoylation |
| RAB5A | Ras-related protein Rab-5A | GVDLTEPTQPTRSQC$C$SN | C212, C213 | Geranyl-geranyl |
| RAB1A | RAB1A, member RAS oncogene family | IQSTPVKQSGGGC$C$ | C204, C205 | Geranyl-geranyl |
| RAB14 | RAB14 protein | LTSEPQPQREGC$GC$ | C213, C215 | Geranyl-geranyl |
| RAB6A | Ras-related protein Rab-6A | LEKPQEQPVSEGGC$SC$ | C206, C208 | Geranyl-geranyl |
| RAB5C | Ras-related protein Rab-5C | GVDLQENNPASRSQC$C$SN | C213, C214 | Geranyl-geranyl |
| RAB2A | RAB2A, member RAS oncogene family | IGPQHAATNATHAGSQGGQQAGGGC$C$ | C188, C189 | Geranyl-geranyl |
Indicates sites of myristoylation
indicates sites of palmitoylation
indicates sites of geranylgeranylation
4.3.5. Protein-protein crosslinking
It is well accepted that protein aggregation, crosslinking, and insolubilization processes are associated with aging and cataract formation (Dilley and Pirie, 1974; Harrington et al., 2004). One mechanism of protein-protein crosslinking formed in the lens is through the formation of reversible disulfide bonds. Oxidative stress and reversible disulfide bond formation during aging and cataract formation has been widely studied (Lou et al., 1990; Lou and Dickerson, 1992; Wang et al., 2017; Yu et al., 1985; Takemoto, 1996; Simpanya et al., 2005; David LL, 2011). Irreversible non-disulfide protein-protein crosslinking is known to occur in the lens because at least a certain proportion of high molecular weight complexes do not dissociate under denaturing and reducing conditions (Truscott and Augusteyn, 1977). Several crosslinking mechanisms have been proposed such as through advanced glycation end products (Nagaraj et al., 1991), oxidized ascorbate (Ortwerth and Olesen, 1988; Linetsky et al., 2008), and dehydroalanine (DHA) formation (Linetsky et al., 2004; Linetsky and LeGrand, 2005). In a recent series of studies, three distinct crosslinking mechanisms have been elucidated: 1) via DHA (Wang et al., 2014), 2) via an Asn succinimide intermediate (Friedrich et al., 2018) and 3) via truncation and a C-terminal succinic anhydride intermediate (Wang et al., 2019). Crosslinked peptides that are detected in aged human lenses are summarized in Table 2.
Table 2.
List of Crosslinked Proteins/Peptides Discovered in Human Lenses
| Crosslinked proteins | Detected crosslinked peptide | Potential mechanism of crosslinking | Reference |
|---|---|---|---|
| βA4 crystallin C5-βA4 crystallin C166 | βA4 2–7:acetyl-T LQC*TK βA4 159–174: GFQYVLEC* DHHSGDYK |
DHA-mediated | Wang et al., 2014 |
| βA4 crystallin C5-βA3 crystallin S59 | βA4 2–7: acetyl-TLQC*TK βA3 46–64: MEFTSSCPNV SERS * FDNVR |
||
| βB2 crystallin K76-βB2 crystallin S204 | βB2 69–81: GEQFVFEK* GEYPR βB2 199–205: GAFHPS*Da |
||
| βB1 crystallin S77-γS crystallin C130 | βB1 74–86: AEFS*GECSNLADR βS 126–131: EIHSC*K |
||
| AQP0 N246-AQP0 K228 | AQP0 239–263: GAKPDV SN*GQPEVTGEPVELDTQAL AQP0 227–233: LK*SISER |
Succinimide | Friedrich et al., 2018 |
| AQP0 N259-Lys | AQP0 239–263: GAKPDV SNGQPEVTGEPVELN * TQAL Unknown (maybe αB): K |
||
| βB1 crystallin K118-Spectrin D1207 | βB1 111–123: GEMFILEK*GEYPR Spectrin 1207–1210: D*LR |
||
| βB2 crystallin K76-Spectrin D1207 | βB2 69–81: GEQFVFEK*GEYPR Spectrin 1207–1210: D*LR |
||
| αA crystallin D151-αB crystallin K150 | αA 146–157: IQTGLD*ATHAER αB 150–157: K*QVSGPER |
||
| AQP0 D246-AQP0 K228 | AQP0 227–233: LK*SISER AQP0 239–246: GAKPDVSD* |
succinic anhydride | unpublished |
| AQP0 K238-AQP0 D246 | AQP0 234–243: LSVLK*GAKPD AQP0 239–246:GAKPDVSD* |
||
| αA crystallin D101-αB crystallin K174 | αA:HD* αB: EEKPAVTAAPK*K |
||
| αA crystallin D101-αB crystallin K150 | αA:HD* αB: K*QVSGPER |
||
| AQP0 M1-AQP0 D243 | AQP0 1–5: *MWELR AQP0 239–243: GAKPD* |
succinic anhydride | Wang et al., 2019 |
| AQP0 D243-AQP0 K228 | AQP0 227–233: LK*SISER AQP0 239–243: GAKPD* |
||
| AQP0 D243-γS crystallin K14 | AQP0 239–243: GAKPD* γS 8–19: ITFYEDK*NFQGR |
||
| αA crystallin D151-αB crystallin K150 | αA: IQTGLD* αB:K*QVSGPER |
||
| αA crystallin K78-αA crystallin D58 | αA:FVIFLDVK*HPSPEDLTVK αA: TVLD* |
||
| αB crystallin D129 - αB crystallin K90 | αB: IPADVD* αB: HFSPEELK*VK |
||
| αB crystallin D129 - αB crystallin K82 | αB: IPADVD* αB: VK*VLGDVIEVHGK |
||
| αB crystallin D129 - αB crystallin K121 | αB: IPADVD* αB: K*YR |
||
| αA crystallin D151 - αB crystallin K82 | αA: IQTGLD* αB: VK*VLGDVIEVHGK |
||
| αA crystallin D151 – γC or yD crystallin G2 | αA: IQTGLD* γC or γD: *GK |
||
| Filensin K467-βA4 Q195 or βA3 Q214 | Filensin: SPKEPETPTELYTK#ER βA4 or βA3: IQ#Q |
transglutaminase | unpublished |
| Filensin Q245-Phakinin K342 | Filensin: VELQAQ* TTTLEQAIK Phakinin: ALK*R |
||
| Filensin K12-Phakinin Q361 | Filensin: K#EQYEHADEASR Phakinin: HWHDMELQ#NLGAVVGR | ||
| αA crystallin K99 -αB crystallin K150 | αA: VQDDFVEIHGK#HNER αB: KQ#VSGPER |
||
| βB1 crystallin Q236-Filensin K451 | βB1: Q*WHLEGSFPVLATEPPK Filensin: EK*VR |
||
| Phakinin Q329-Phakinin C65 | Phakinin: VELHNTS CQV Q#SLQAETESLR Phakinin: #CIGGLGAR |
?? | |
| γD crystallin E8-γDcrystallin K3 | γD: HYE*CSSDHPNLQPYLSR γD: G(carbamyl)K*ITLYEDR |
unknown | unpublished |
| αB crystallin E99 -αB crystallin K121 | αB: VLGDVIE*VHGK αB: K*YR |
||
| βB3 crystallin E177- γS K154 | βB3: GE*YR γS: QYLLDK*K |
||
bolded residues indicate an Asp residue resulting from deamidation of an Asn residue in the nascent protein sequence.
Irreversible crosslinking through DHA or through dehydrobutyrine (DHB) involves β-elimination of phosphorylated serine or threonine, respectively. Additionally, disulfide linked cysteine residues can form DHA. Once formed, DHA residues can then undergo nucleophilic addition by thiol or amine groups in proteins to form irreversible thioether or amine linkages. The overall mechanism is shown in Figure 13. Although protein-protein crosslinks through DHA intermediates have been identified (Table 2), glutathione (GSH) was also found to be irreversibly crosslinked with many lens proteins (52 sites on 18 lens proteins) through DHA or DHB intermediates (Friedrich et al., 2017; Wang et al., 2014; Wang and Schey, 2018b). Certain lens protein residues are extensively modified such as Cys5 on βA4-crystallin, Cys117 on βA3-crystallin, and S235 on AQP0 (Friedrich et al., 2017; Wang and Schey, 2018b). This crosslinking of GSH with lens proteins may represent a protective mechanism whereby high concentrations of GSH prevent irreversible protein-protein crosslink formation. Quantification of irreversible GSH crosslinks in human lenses shows some clear increases with age and in cataract lenses; however, not all crystallins show the same pattern (Wang and Schey, 2018b). This could be due to additional modifications to GSH-modified peptides that evaded detection.
Figure 13. Mechanisms of DHA formation and reactions.
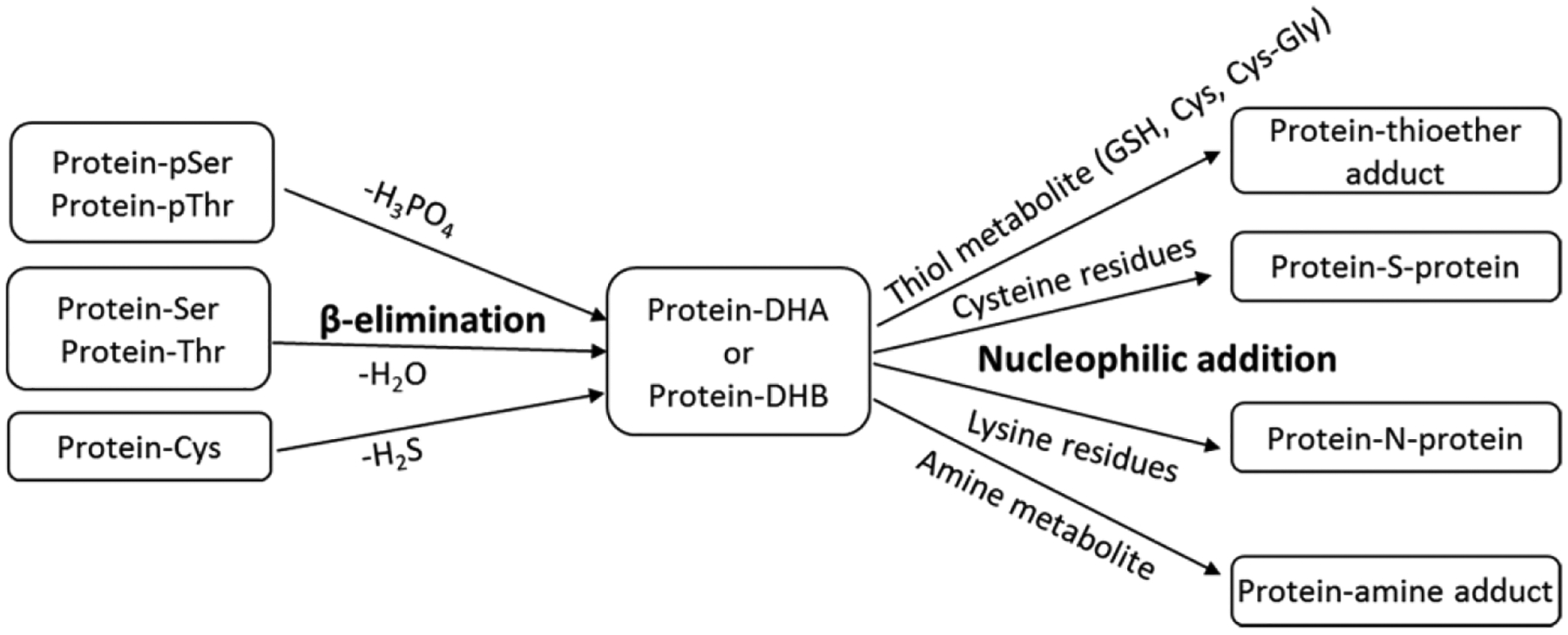
Scheme showing dehydroalanine (DHA) formation and subsequent reaction with a thiol containing metabolite, a protein thiol, an amine containing metabolite, or a protein amine to form protein modifications and crosslinks.
In addition to racemization and deamidation that occur via a succinimide intermediate, we have also found protein-protein crosslinks to form through this intermediate (Friedrich et al., 2018). In these cases, nucleophilic addition of an amine from lysine side chains or from the protein N-terminus occurs to a succinimide intermediate. In a distinctly different mechanism, protein-protein crosslinks can occur via succinic anhydride intermediate after truncation occurs on the C-terminal side of aspartic acid residues (Wang et al., 2019). Many crosslinked peptides were detected to form via this mechanism and the major lens proteins involved include αA-crystallin, αB-crystallin, and AQP0. Crosslinking of AQP0 was increased in aged or cataract lenses compared with young lenses (Wang et al., 2019). Truncated crystallins have been associated with protein aggregation and insolubilization (Asomugha et al., 2010; Srivastava and Srivastava, 2003a; Srivastava et al., 2017) and these crosslinking mechanisms demonstrate a direct relationship between three important processes in the aged lens: deamidation/racemization, truncation, and irreversible protein-protein crosslinking. These results, at least partially, explain key mechanisms of long-lived protein aggregation.
Enzymatic crosslinking mechanisms, including via γ-transglutaminase, have been proposed to catalyze protein crosslinking in the lens (Lorand et al., 1981) and several peptide crosslinks between glutamine and lysine residues have been detected in human lenses. This group of crosslinked peptides mainly involves beaded filament protein filensin and phakanin; however, other crosslinked peptides have been detected between lysine and glutamic acid residues. Whether these crosslinks occur non-enzymatically through the formation of glutamic acid anhydride or glutarimide intermediates in analogous mechanisms to the aforementioned succinic acid and succinimide pathways is not known.
4.3.6. Other modifications
In addition to the aforementioned PTMs, many other modifications can be detected in the lens such as acetylation, carbamylation, oxidation, methylation and glycation. Acetylation has been well studied in other tissues, but it has not been globally studied in the lens. Acetylation is either a co-translational or a post-translational process and a majority of lens major proteins such as α-crystallins, β-crystallins, and lens beaded filament proteins are acetylated at the N-terminus, most likely through a co-translational process. Another interesting finding is posttranslational acetylation of newly formed protein N-terminus after truncation, which has been reported in filensin (Wang et al., 2010). Even though it has not been reported for other proteins in the lens, this phenomenon can be detected in many lens proteins. The acetylation on the N-terminus probably stabilizes the protein N-terminus to prevent further degradation. In addition, acetylation on lysine residues has been detected and has been shown to affect α-crystallin chaperone activity (Nagaraj et al., 2012). Similar to acetylation, many proteins undergo carbamylation on the original protein N-terminus and on lysine residues. Lens protein carbamylation appears to be more age-related than acetylation based on its increased abundance in the lens nucleus compared to the cortex; a pattern not observed for acetylation (Schey, unpublished results), although others have speculated that N-terminal carbamylation is developmentally related (Lapko et al., 2003). Carbamylation has been shown to be highly associated with aging and life expectancy (Gorisse et al., 2016).
4.4. Effects of age
Clearly, PTMs in the human lens accumulate with age starting from birth. It is difficult to accurately quantify many of the changes that occur to lens proteins with age primarily because each polypeptide chain is subject to so many different PTMs. As one illustration, size-exclusion chromatography separates the soluble proteins from human fetal lenses beautifully into α, β and γ−crystallin peaks. However, it is not possible to quantify age-related changes to crystallins using gel filtration because some crystallins become truncated and move to later elution times whereas others aggregate or crosslink and elute earlier. To complicate matters further, lens proteins become progressively insoluble over time, so analysis of soluble proteins from older lenses represents a sub-fraction of the whole proteome. A typical strategy to examine age-related changes to lens proteins is to fractionate the sub-proteomes into soluble and insoluble protein fractions. Additionally, age-related changes can be examined within a single lens via dissection of specific regions (e.g. outer cortex, inner cortex, and nucleus) or by dissection and examination of the nucleus from different aged lenses. Using such strategies, selected PTMs have been measured across the age range of human lenses (Truscott et al., 2011).
As discussed above, one remarkable result of analyses of many scores of human lenses is the lack of deviation of the results. For example, from a graph of the degree of protein modification, it is possible to estimate the age of the individual. Quite simply, there is little individual variation. This could be the outcome of spontaneous processes driven by heat and time. Further evidence of minimal diversity among human lens proteoforms with age appears in both 2DE results (Figures 4 and 5) and in imaging mass spectrometry results (discussed above). Such constant patterns of modification suggest a mechanism whereby specific protein modifications are formed in specific lens regions as a function of protein age. A corollary to this hypothesis is that such modifications are important for maintenance of lens function and that only when protein processing deviates from the average age-dependency observed in clear tissues do cataract-causing modifications occur. Indeed, the levels of modification on some protein residues in cataract lenses were found to differ consistently from the levels observed in age-matched clear lenses (discussed below). It was proposed that such cataract-specific modifications may indeed be responsible for causing lens opacification.
Interestingly, in most cases, graphs of PTM abundance versus age are often non-linear particularly in younger individuals (Figures 14 and 15). As noted earlier, racemization is the most abundant PTM in adult human lenses (Hooi and Truscott, 2011) but surprisingly, the most rapid rate of increase of racemization occurs in the first decade of life (Figure 14). Why this is the case, is unclear. Could it be that proteins within the lens nucleus, or regions within the proteins, during the first 10 years of life are more mobile? This hypothesis would be consistent with the observation that flexible, unstructured regions of crystallins are more susceptible to spontaneous PTMs (Hooi et al., 2013c) than comparable residues located in β sheets or α helixes. Proteomic analysis of whole tissue samples is the best way to quantify changes since there is no selective purification of modified or un-modified proteins prior to analysis. Proteomics was therefore used to delve more deeply into racemization at specific sites in crystallins. As had been found with whole lens digests, when the relative extent of racemization at one site in each crystallin was monitored as a function of age, the profiles were non-linear (Hooi et al., 2012c) (Figure 15). Profiles for individual crystallins and sites within each crystallin were distinctive. Some sites, for example Asp151 in αA-crystallin, are highly prone to racemization and showed a large degree of change even at young ages. Others showed little or no evidence of this change up to age 100y. It was notable that while most crystallins exhibit age-related PTMs, others such as βB2-crystallin, were little affected.
Figure 14. Conversion of L-Asn and L-Asp to D-Asp in human lens proteins as a function of age.
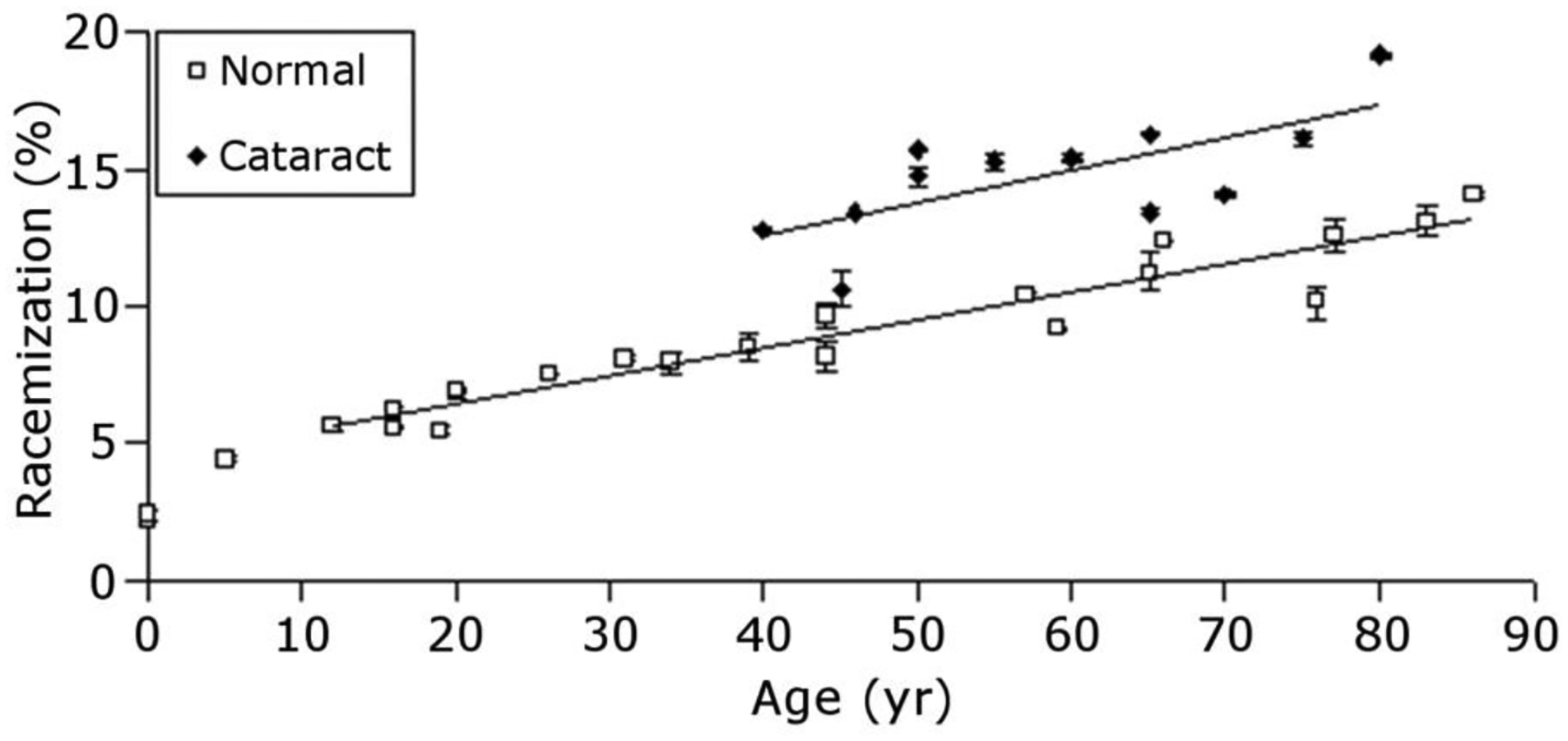
Quantification of racemization in human lens protein content showed that cataract lenses were found to display consistently greater amounts of racemization at every age, suggesting that racemization may be responsible for cataract formation. Total protein (water soluble + water insoluble) was analyzed. Figure from (Hooi and Truscott, 2011).
Figure 15. γS-Crystallin racemization as a function of age.
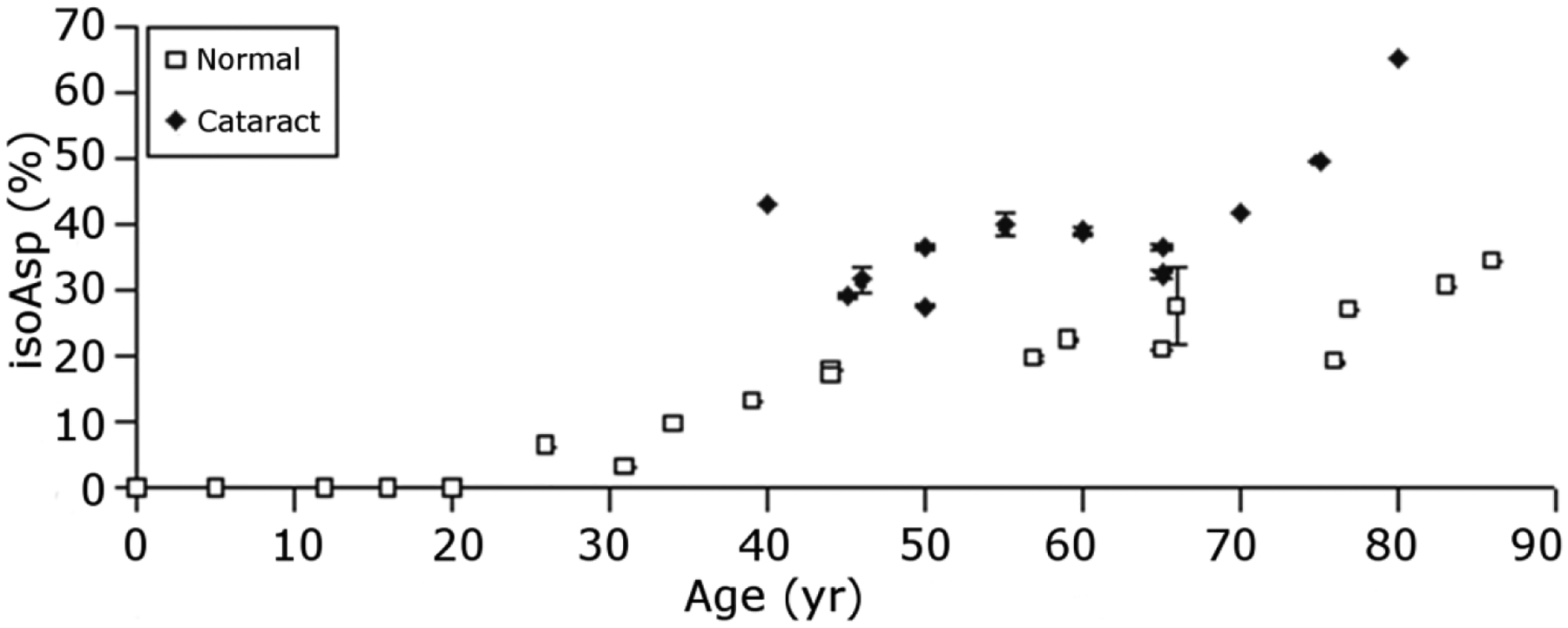
This graph illustrates that Asn76 of γS-crystallin is consistently more racemized in cataract lenses compared to clear lenses. Total protein (water soluble + water insoluble) was analyzed. Open squares = clear lenses; filled diamonds=cataract lenses. Figure from (Hooi et al., 2012a).
4.5. Cataract-specific protein modifications
A cataract is defined as a lens that has a light scattering center that interferes significantly with vision. The light scattering center can be a cell or groups of cells located in an area of the lens such as the cortex, nucleus, the posterior or anterior capsular region or the entire lens and each has a different etiology. Age is the biggest risk factor for cataract and by age 75y about 50% of the white U.S. population has cataract (National Eye Institute (2010)). Two observations to consider regarding the etiology of nuclear cataracts are:1) the lens density increase was reported to be homogeneous across the nuclear region (Michael and Bron, 2011) and 2) cataract lenses are stiffer than comparable age-matched normal lenses (Heys and Truscott, 2008).
In an effort to determine the cause of cataract there has been a major focus on the crystallins and their modifications with age and in cataract. In early work, 2D electrophoresis was used to determine if there were modifications or expression differences that correlated with types of cataracts. The following are results of a survey of at least 80 cataracts and 70 donor lenses.
4.5.1. Modification of αB-crystallin
Human αB-crystallin is a protein of 175 amino acids. One of the first cataract-associated modifications identified was C-terminal truncation of αB-crystallin through loss of the C-terminal lysine generating αB-crystallin (1–174) (Jimenez-Asensio et al., 1999). The truncated protein migrated one charge more acidic and at a slightly lower molecular weight, 22.4kDa relative to 23kDa. The removal of the C-terminal lysine was the only modification identified in this spot on the 2D gel and the entire sequence was accounted for by mass spectrometry. This form of αB-crystallin was present in amounts that varied up to 90% of the total αB-crystallin present in cortical fiber cells of cataractous lenses. It was also present in Soemmering’s rings, a form of posterior capsular opacity, in amounts up to 30% of the total αB-crystallin (Colvis et al., 2000). So far this modified αB-crystallin has been observed in cortical/nuclear cataracts from India but it is not known if it is restricted to cortical/nuclear cataracts. Eleven of seventeen cataracts from India had this form of αB-crystallin in the lens cortex. Seven control non-cataractous lenses also from India did not contain this form of αB-crystallin. It is also not known if high concentrations of αB-crystallin (1–174) are sufficient to cause cataracts. The chaperone function of alpha crystallin is thought to be important in preventing cataracts. The chaperone function of αB-crystallin, however, was not diminished by the removal of the C-terminal lysine (Jimenez-Asensio et al., 1999).
The mechanism leading to the removal of the C-terminal lysine residue of αB-crystallin is not known. Carboxypeptidase E/H, a zinc metalloprotease, catalyzes the removal of C-terminal lysine residues from proteins, but it is not clear that this enzyme is present in the lens (Nakajima et al., 2009; Ji et al., 2017). Incubation of human lenses with a calcium ionophore did lead to the removal of the C-terminal lysine residue from αB-crystallin, but not to the formation of the 16.4 kDa form present in the cataractous lenses with high concentrations of αB-crystallin (1–174) (Jimenez-Asensio et al., 1999; Nakajima et al., 2009). The 16.4 kDa truncated forms of αB-crystallin had N-termini that resulted from cleavage before residues 41, 43, 45 and 47 suggesting cleavage by a dipeptidase. Alternatively, this pattern of truncation could be the result of non-enzymatic cleavage at serine residues 41, 43, and 45 (Lyons et al., 2016a) and/or dipeptide loss through a diketopiperzine intermediate (Lyons et al., 2016c).
Based on the previously described 2DE results, it seems reasonable that the cataractous region of a lens may reveal clues as to the why it scatters light. To this end the opaque portion of a 22 day old lens was dissected and the 2DE gels of that region were compared with that of the transparent region of the same lens. As seen in Figure 16 (Garland, unpublished) the crystallin patterns were remarkably similar suggesting that the opacity was likely not caused by a significant change in the expression or the post-translational modification of any crystallin. It is important to note that the cataract in this 22d lens is not age-related and that the mechanism(s) leading to light scatter may be different than those involved in age-related nuclear cataract formation.
4.5.2. Cataracts – analysis of the lens nucleus
The nuclear region of lenses is of particular interest since nuclear cataracts are the most common form of age-related cataract (Congdon et al., 2004). In Figure 17 the 2DE protein patterns of cataracts are compared to those of normal adult human lenses (Figures 4 and 5; Figure 17A & G). β- and γS-Crystallins are the major proteins present and are present as a series of increasingly acidic species. The top and bottom rows show β-crystallins that elute together on a size exclusion column and the middle row shows γS-crystallin that elutes as the monomer form. As described above, multiple sites of both deamidation and phosphorylation of β- and γS-crystallins have been reported (Hains and Truscott, 2010; Hooi et al., 2012b; Wang et al., 2013) and deamidation and phosphorylation would lead to more acidic protein species. In fact, the deamidation of Gln92 in γS-crystallin follows the temporal formation of the protein pattern typical of the nuclear region in normal lenses (Figures 4 and 5) (Hooi et al., 2012b).
Figure 17. 2DE analysis of human cataractous lenses.
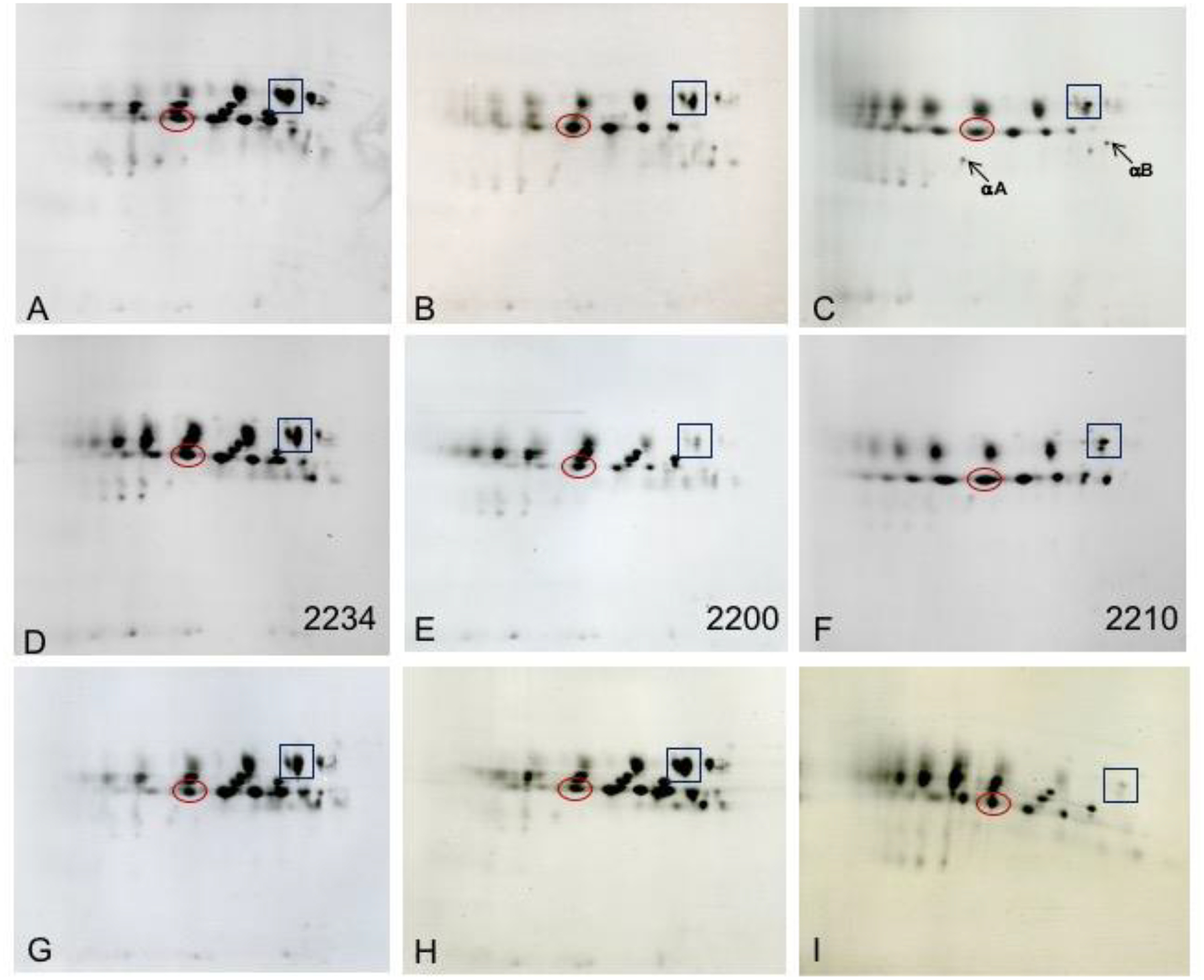
2DE patterns of human lens proteins observed from clear and cataractous lenses. Panel A: 57 y clear lens; Panel B: 64 y cataract; Panel C: 50 y cataract from India; Panel D: mixed cataract with LOCS 2234; Panel E: nuclear cataract with LOCS 2200; Panel F: nuclear cataract with LOCS 2210; Panel G: 35 y clear lens; Panel H: 44 y diabetic clear lens; Panel I: 81 y cataract. LOCS scores are for nuclear opalescence, nuclear color, cortical, PSC. On these gels the original form of αA-crystallin focuses at about pI 5.8, βB-crystallin focuses at about pI 6.8, and γS-crystallin focuses at about pI 6.4. The protein spots at the bottom of the gels migrate at Mr 10k. The boxes and circles in each image indicate the respective locations of 2 different crystallin species. The locations of the original forms of αA- and βB-crystallins are also indicated in Panel C. Proteins were solubilized in 8M urea/ 2% NP40/10 mM DTT, 2% ampholytes (Resolyte pH 3.5–10). (Garland and Datiles, unpublished).
The 2DE protein patterns of the nuclear regions of cataracts show the presence of the same three rows of protein species but the patterns vary quantitatively. The extremes of the 2DE protein patterns obtained in the nucleus of cataracts (from 35 analyzed) are shown in Figure 17B–F & I. All samples were prepared in 10 mM DTT thus disulfide crosslinks are not a likely explanation for the variation in patterns. Panels A and G show the typical patterns observed in normal adult lenses. In panel F, γS-crystallin species are not visible and in panel C they are diminished. If any γS-crystallin is present it is very acidic or may be present in the very acidic low molecular weight material or protein migrating at a higher molecular weight. In panel B there is diminished γS-crystallin and what was present migrated as acidic species. In panels E and I, typical forms of γS-crystallin are present but β-crystallins are diminished. In most panels, low molecular weight fragments of α-crystallins are present. Of particular interest is the comparison of panels D-F. All three of the patients had identical Lens Opacities Classification System (LOCS) scores (Chylack et al., 1993) for nuclear opalescence of 2 and nuclear color of 2, yet, they show three distinct 2DE patterns. The pattern in panel D is similar to that of the 57y clear lens, whereas panel E shows diminished β-crystallins and panel F shows no visible γS-crystallins. Panel H shows the crystallin pattern from a clear lens of a 44y donor with diabetes. The pattern is comparable to those of clear lenses (panels A & G). The boxes and circles in each image indicate the respective locations of 2 different crystallin species. The locations of the original forms of αA- and αB-crystallins are also indicated in Panel C. Using these as a guide it is possible to compare the extent to which the pI of the crystallins becomes altered in the cataracts. In Panel C there are β- and γ-crystallins species that migrate close to pI 3.
The variation in the protein patterns and the modifications of crystallins identified in the nucleus of cataractous lenses suggest different mechanisms and/or different post-translational modifications are responsible for the migrational differences of the γ- and β-crystallins. It has not been possible to demonstrate that protein insolubilization is responsible for the differences. The 2DE gel patterns include both water soluble and water insoluble proteins and in these samples urea insoluble protein was not detected. In the 2DE image from a more severe cataract sample (panel C) there is, however, evidence of protein smeared at higher and lower molecular weights in the most acidic positions indicating the presence of urea insoluble protein in severe, dark colored cataracts.
There are likely multiple biochemical pathways that can lead to protein aggregation and opacification. The data published to date suggest deamidation, racemization, and oxidative processes leading to protein crosslinking can all lead to insolubilization of lens proteins (Lyon et al., 2017; Lampi et al., 2014; Warmack et al., 2019). Specifically, cataract-abundant PTMs include: tryptophan oxidation and racemization (Hains and Truscott, 2007; Hooi and Truscott, 2011), cysteine oxidation in γS-crystallin and deamidation at three Asn residues in γS-crystallin (Hains and Truscott, 2008, 2010; Hooi et al., 2012a), deamidation of Asn143 in γS crystallin (Takemoto and Boyle, 2000), racemization and isomerization of Asp58 in αA crystallin (Hooi et al., 2013a), and racemization of Ser59/62 in αA-crystallin (Hooi et al., 2013a).
4.6. Presbyopia
Presbyopia is a condition associated with the aging of the eye that results in a progressively worsening ability to focus clearly on nearby objects (Truscott and Zhu, 2010). Presbyopia typically affects people in the fifth decade of life and is due to a hardening of the lens such that light is focussed behind the retina rather than on the retina itself, causing close objects to appear blurred.
Our information as to the biochemical processes that are responsible for presbyopia are largely correlative. This is inevitable for reasons that will be outlined below. In order to understand presbyopia, it is necessary to first understand the normal process of accommodation (Glasser and Campbell, 1998). Focussing of light on the retina in young adults relies on the plasticity of the lens. The lens must be deformable and this change in shape is mediated by the ciliary muscles that surround the lens and attach via the zonules to the capsule at the lens periphery. When close vision is needed, the ciliary muscles contract and as a consequence the tension on the ciliary processes is released. The natural elasticity of the lens and its capsule, causes the lens to bulge and to assume a shape that refracts light to a greater degree.
4.6.1. Can presbyopia be explained by the age-dependent loss of α crystallin?
If the lens became stiffer with age such that it no longer changed shape when tension was released then this, of itself, would be sufficient to explain the origin of presbyopia. This is precisely what occurs. Much of the elasticity of the lens can be traced to the lens capsule, but if the body of the lens which is made up of fiber cells, cannot be altered, then visual function will be compromised. This would be true, even if the properties of the capsule remained unchanged with age.
Biophysical measurements on human lenses of different ages showed a massive increase in stiffness particularly in the center of the lens (Heys et al., 2004). This increase in lens stiffness could be replicated by heating whole human lenses (Heys et al., 2007) and under such heat stress, α−crystallin levels decreased. Thus, it is likely that the loss of free α-crystallin that occurs in all human lenses by age 40y (Roy and Spector, 1976; McFall-Ngai et al., 1985) may have a physical consequence. Although the exact processes responsible for the huge increase in stiffness are not precisely known, an intriguing hypothesis is that the massive binding of crystallins to lens membranes that takes place in the decade after age 40y may play a part (Friedrich and Truscott, 2009, 2010). This process could occur together with a decrease in the fluidity of the cytosol within the lens fiber cells. Cytosolic fluidity may also be modulated by α−crystallin. In young lenses, such fluidity is necessary for a change in shape of the lens fiber cells and ultimately the lens as a whole. Either or both phenomena could provide the basis for the inability to clearly read this text by elderly readers who lack reading glasses or replacement lenses.
5. LLP changes in other tissues
It will be quite remarkable if historical analysis confirms that the lessons on protein deterioration learned during biochemical forays into the molecular mechanism of human cataract formation turned out to have much wider ramifications for other diseases of old age. Discovering the molecular basis for human cataract could then be viewed as the starting point for a much wider, and ultimately more important, study with implications for most, if not all, age-related diseases, particularly those associated with protein aggregation. This includes Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, and amyotrophic lateral sclerosis (ALS). If this eventuates, one irony will be that, whereas an aged lens filled with highly modified proteins becomes opaque, it can be readily removed and a plastic lens used to restore vision. Such replacement is unlikely to ever be available for other tissues, such as the brain, which also contain many long-lived proteins. This realization awaits comprehensive analyses of other human tissues. In the meantime there are tantalizing clues that such a vista may represent an important future research area for such diseases. Two age-related diseases will be discussed briefly: multiple sclerosis and Alzheimer’s disease.
5.1. Multiple sclerosis
Multiple sclerosis (MS) is a common autoimmune disease that affects the central nervous system. In MS, the myelin sheath that surrounds nerve axons is attacked. There is no cure for MS. It typically affects people in their thirties and is 2–3 times more common in women than in men (Duquette et al., 1992).
5.1.1. Human myelin is long-lived
When human myelin that had been extracted from the brains of unaffected adults was examined it was discovered that a major protein, myelin basic protein (MBP) was degraded. Surprisingly tryptic digests of MBP showed features in common with those from adult lens proteins (Friedrich et al., 2016). For example, most Asp sites in MBP were found to exist as a mixture of Asp isomers including isoAsp residues (Friedrich et al., 2016). These data suggested that MBP, and the other proteins of myelin, may be very long-lived. The total degree of racemization in MBP was also similar to that of lens proteins. The extent of MBP modification was such that very little unmodified MBP (i.e. as it was originally translated), exists in the cerebellum of an adult. Another way of viewing this is that the human brain of an adult contains a large amount of a novel protein – in the sense that it was not present when myelin was formed during childhood. Thus, it may not be surprising that the body mounts an immune response to a protein it regards as ‘foreign’.
5.1.2. Degradation of MBP and its putative role in the genesis of multiple sclerosis
Even more remarkably, when the MBP from MS brains was analysed, it was found to be significantly more highly modified at some specific sites (Friedrich et al., 2016). An hypothesis was formulated that MS may be the result of the body’s immune response to this more highly modified MBP.
Although lens proteins and myelin proteins both appear to be long-lived and to suffer major age-dependent degradation, two important differences are apparent. Firstly, many Arg residues in adult MBP have become modified to citrulline (Friedrich et al., 2016).This reaction is catalysed by a deiminase enzyme. As noted earlier the center of the adult human lens lacks active enzymes, so this loss of Arg and formation of citrulline is not a feature associated with crystallins. Secondly, and of most importance for the possible induction of MS, proteins in the lens are under immune privilege, meaning that the introduction (or formation) of novel antigens will not elicit an inflammatory immune response; however, this is not the case in the brain. An illustration of this is experimental autoimmune encephalomyelitis (EAE) that is often used as an animal model of multiple sclerosis where proteins or peptides derived from myelin proteins are injected to induce symptoms that closely resemble those of MS (‘t Hart et al., 2011).
The knowledge of extensively degraded myelin in the brain may lead to a completely new way of looking at the origin of MS; one that is quite different from traditional views. Orthodox proposals envisage MS as developing due to an aberrant immune response to a self-protein that is triggered by exposure to some unknown environmental antigen.
5.2. Alzheimer’s disease
Alzheimer’s disease (AD) is the most common form of dementia. The defining feature of AD is the presence of amyloid deposits and this is the characteristic that Alois Alzheimer first noticed in a histological examination of an AD brain. Its relationship to lens proteins can be appreciated when one compares aged crystallins with β-amyloid. Amyloid is more abundant in brains from AD patients than in normal non-affected individuals and the general view is that it is composed of highly aggregated peptides, Aβ 1–40 and Aβ 1–42. Detailed analysis of amyloid from AD brains by Japanese researchers revealed however that AD amyloid was not composed of Aβ 1–40 and Aβ 1–42, but rather it was made up from highly modified Aβ (Kubo et al., 2002; Shimizu et al., 2000). This is not just a semantic point. Human AD amyloid was composed predominantly of Aβ that had been degraded from the N-terminal end, or had an N-terminal Asp that was present as isoAsp. Other Asp residues and Ser residues were also racemized.
All of these features are consistent with AD amyloid being present for a long period of time within the brain since these are the changes associated with aged long-lived proteins. Furthermore, extended incubation of synthetic Aβ peptides yielded the same PTMs as found in authentic AD brains (Lyons et al., 2016b). The ramifications of these findings are as yet unknown. One aspect seems clear, that generating Aβ amyloid in the brains of experimental animals by Aβ overexpression may not be the most appropriate model for human AD. It is therefore unsurprising that Aβ antibodies that clear amyloid from these experimental models of AD have been found not to be efficacious in human trials.
6. Conclusions and Future Directions
The spatial and temporal modifications in human lens proteomes appear to follow a time-dependent path of modification as evidenced by little variation in the examined proteomes with age. An important consequence of this finding is that cataract-specific modifications should exist. Although some cataract-specific modifications have been discovered, there are clearly multiple pathways that can lead to opacification and multiple different modifications may be involved. The results also accentuate the point that the lens can tolerate a large number of modifications while maintaining transparency. In fact, we speculate based on specific examples of filensin and connexin truncation, that specific modifications confer new functionality to lens proteins, including abundant lens crystallins. It is also likely that some modifications can be tolerated up to a threshold concentration that, when surpassed, causes opacification. It is also clear that subtle changes in protein structure, e.g. racemization or deamidation, can lead to protein insolubility.
Key questions that arise from the existing body of literature on lens proteome changes with age include: what controls the rate of lens protein modification? Can such processes be modulated? Which lens protein modifications are beneficial and which are deleterious/cataractous? What are the functional consequences of each modification? What modifications increase lens stiffness and play a role in presbyopia? What molecular changes lead to a permeability barrier in middle age? Are there cataract-specific modifications? Answers to these questions will undoubtedly assist in the development of therapies to delay, prevent, or potentially reverse cataractogenesis.
With regard to cataract and potential therapeutic intervention, recent articles suggest that treatment with pharmacological chaperones such as sterols and α-crystallin mini-chaperones offer some potential as evidenced by prevention of protein aggregation, disaggregation of crystallins in vitro, increases in soluble crystallins, and reversal of cataracts in vitro in rabbit lenses and in vivo in mice and dogs upon treatment (Makley et al., 2015; Zhao et al., 2015; Raju et al., 2018). The promise of these approaches is tempered by recent publications that failed to reproduce the original findings (Shanmugam et al., 2015; Daszynski et al., 2019). To be successful in reversing mature nuclear cataracts, drugs developed for prevention or treatment of nuclear cataract would need to penetrate into the center of the lens, crossing the inner cortical barrier in the process. In addition, while these treatments may be effective in breaking up non-covalent protein aggregates, the underlying covalent modifications that may have initiated aggregation such as deamidation, racemization, truncation and protein-protein crosslinking will not be reversed and will still be present in the lens.
Future technological advances are likely to provide new methods for discovery and to provide answers to some of these key questions. Proteomic technologies are advancing at a rapid pace where single cell proteomics (Zhu et al., 2018; Budnik et al., 2018) and comprehensive coverage of a specific proteome (Kim et al., 2014; Mann et al., 2013; Aebersold and Mann, 2016) are now within reach. Such comprehensive coverage will allow previously unknown PTMs to be discovered. Fractionation of the lens proteome into subproteomes has led to enhanced detection of lens PTMs. In the future, examination of sub-cellular organelles will provide organelle-specific proteome information and lead to a new understanding of lens fiber cell differentiation. Improvements in mass spectrometry will facilitate study of protein-protein interactions and protein-protein crosslinking in lens cells. Lastly, advances in imaging mass spectrometry will provide higher spatial resolution and more in-depth coverage of spatially-resolved proteomes. Such advances will allow direct viewing and correlation of lens protein (and lipid and metabolite) distributions in clear and cataractous lenses allowing correlation of specific lens proteoforms with opacification.
Most exciting is the opportunity to utilize discoveries from lens research to understand age-related diseases such as multiple sclerosis and Alzheimer’s disease. Initial work suggests that similar age-related protein modifications can be detected in aged neurological tissues suggesting that any therapeutic strategies developed for presbyopia and/or cataract may be useful for other age-related diseases.
Acknowledgements
This work was supported by National Institutes of Health grants R01EY013462 (KLS), R01EY024258 (KLS), R21EY019728 (KLS), R01EY013570 (RJWT), and P30EY008126.
References
- ‘t Hart BA, Gran B, and Weissert R, 2011. EAE: imperfect but useful models of multiple sclerosis. Trends in molecular medicine 17, 119–25. [DOI] [PubMed] [Google Scholar]
- Aebersold R and Mann M, 2016. Mass-spectrometric exploration of proteome structure and function. Nature 537, 347–55. [DOI] [PubMed] [Google Scholar]
- Aebersold R, Agar JN, Amster IJ, Baker MS, Bertozzi CR, Boja ES, Costello CE, Cravatt BF, Fenselau C, Garcia BA, Ge Y, Gunawardena J, Hendrickson RC, Hergenrother PJ, Huber CG, Ivanov AR, Jensen ON, Jewett MC, Kelleher NL, Kiessling LL, Krogan NJ, Larsen MR, Loo JA, Ogorzalek Loo RR, Lundberg E, MacCoss MJ, Mallick P, Mootha VK, Mrksich M, Muir TW, Patrie SM, Pesavento JJ, Pitteri SJ, Rodriguez H, Saghatelian A, Sandoval W, Schluter H, Sechi S, Slavoff SA, Smith LM, Snyder MP, Thomas PM, Uhlen M, Van Eyk JE, Vidal M, Walt DR, White FM, Williams ER, Wohlschlager T, Wysocki VH, Yates NA, Young NL, and Zhang B, 2018. How many human proteoforms are there? Nat Chem Biol 14, 206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M, Sjostrand J, and Karlsson JO, 1996. Calpains in the human lens: relations to membranes and possible role in cataract formation. Ophthalmic Res. 28 Suppl 1, 51–4. [DOI] [PubMed] [Google Scholar]
- Aquilina JA, Benesch JL, Ding LL, Yaron O, Horwitz J, and Robinson CV, 2004. Phosphorylation of alphaB-crystallin alters chaperone function through loss of dimeric substructure. J. Biol. Chem 279, 28675–80. [DOI] [PubMed] [Google Scholar]
- Arrojo EDR, Lev-Ram V, Tyagi S, Ramachandra R, Deerinck T, Bushong E, Phan S, Orphan V, Lechene C, Ellisman MH, and Hetzer MW, 2019. Age Mosaicism across Multiple Scales in Adult Tissues. Cell Metab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asomugha CO, Gupta R, and Srivastava OP, 2010. Identification of crystallin modifications in the human lens cortex and nucleus using laser capture microdissection and CyDye labeling. Mol. Vis 16, 476–94. [PMC free article] [PubMed] [Google Scholar]
- Augusteyn RC, 2010. On the growth and internal structure of the human lens. Exp. Eye Res 90, 643–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz A, Santhoshkumar P, Sharma KK, and Abraham EC, 2007. Cleavage of the C-terminal serine of human alphaA-crystallin produces alphaA1–172 with increased chaperone activity and oligomeric size. Biochemistry (Mosc). 46, 2510–9. [DOI] [PubMed] [Google Scholar]
- Bagchi M, Katar M, Lo WK, and Maisel H, 2003. Paralemnin of the lens. J. Cell. Biochem 89, 917–21. [DOI] [PubMed] [Google Scholar]
- Bahrami M, Hoshino M, Pierscionek B, Yagi N, Regini J, and Uesugi K, 2014. Optical properties of the lens: an explanation for the zones of discontinuity. Exp. Eye Res 124, 93–9. [DOI] [PubMed] [Google Scholar]
- Bakthisaran R, Akula KK, Tangirala R, and Rao Ch M, 2016a. Phosphorylation of alphaB-crystallin: Role in stress, aging and patho-physiological conditions. Biochim. Biophys. Acta 1860, 167–82. [DOI] [PubMed] [Google Scholar]
- Bakthisaran R, Akula KK, Tangirala R, and Rao Ch M, 2016b. Phosphorylation of alphaB-crystallin: Role in stress, aging and patho-physiological conditions. Biochim. Biophys. Acta 1860, 167–82. [DOI] [PubMed] [Google Scholar]
- Ball LE, Garland DL, Crouch RK, and Schey KL, 2004. Post-translational modifications of aquaporin 0 (AQP0) in the normal human lens: spatial and temporal occurrence. Biochemistry (Mosc). 43, 9856–65. [DOI] [PubMed] [Google Scholar]
- Bassnett S, 2009. On the mechanism of organelle degradation in the vertebrate lens. Exp. Eye Res 88, 133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett S and Costello MJ, 2017. The cause and consequence of fiber cell compaction in the vertebrate lens. Exp. Eye Res 156, 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett S, Wilmarth PA, and David LL, 2009. The membrane proteome of the mouse lens fiber cell. Mol. Vis 15, 2448–63. [PMC free article] [PubMed] [Google Scholar]
- Betts MJ, Wichmann O, Utz M, Andre T, Petsalaki E, Minguez P, Parca L, Roth FP, Gavin AC, Bork P, and Russell RB, 2017. Systematic identification of phosphorylation-mediated protein interaction switches. PLoS computational biology 13, e1005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SK, Lee JE, Brako L, Jiang JX, and Lo WK, 2010. Gap junctions are selectively associated with interlocking ball-and-sockets but not protrusions in the lens. Mol. Vis 16, 2328–41. [PMC free article] [PubMed] [Google Scholar]
- Borchman D and Yappert MC, 2010. Lipids and the ocular lens. J. Lipid Res 51, 2473–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik B, Levy E, Harmange G, and Slavov N, 2018. SCoPE-MS: mass spectrometry of single mammalian cells quantifies proteome heterogeneity during cell differentiation. Genome Biol 19, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli RM, Farmer TB, and Gile J, 1997. Molecular imaging of biological samples: localization of peptides and proteins using MALDI-TOF MS. Anal. Chem 69, 4751–60. [DOI] [PubMed] [Google Scholar]
- Cenedella RJ, 1990. Palmitoylation of ocular lens membrane proteins. Invest. Ophthalmol. Vis. Sci 31, 368–73. [PubMed] [Google Scholar]
- Chen B, Sun Y, Niu J, Jarugumilli GK, and Wu X, 2018. Protein Lipidation in Cell Signaling and Diseases: Function, Regulation, and Therapeutic Opportunities. Cell chemical biology 25, 817–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou SH, Huang CH, Lee IL, Wang YT, Liu NY, Tsay YG, and Chen YJ, 2010. Identification of in vivo phosphorylation sites of lens proteins from porcine eye lenses by a gel-free phosphoproteomics approach. Mol. Vis 16, 294–302. [PMC free article] [PubMed] [Google Scholar]
- Chylack LT Jr., Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, Friend J, McCarthy D, and Wu SY, 1993. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch. Ophthalmol 111, 831–6. [DOI] [PubMed] [Google Scholar]
- Colvis C and Garland D, 2002. Posttranslational modification of human alphaA-crystallin: correlation with electrophoretic migration. Arch. Biochem. Biophys 397, 319–23. [DOI] [PubMed] [Google Scholar]
- Colvis CM, Duglas-Tabor Y, Werth KB, Vieira NE, Kowalak JA, Janjani A, Yergey AL, and Garland DL, 2000. Tracking pathology with proteomics: identification of in vivo degradation products of alphaB-crystallin. Electrophoresis 21, 2219–27. [DOI] [PubMed] [Google Scholar]
- Congdon N, Vingerling JR, Klein BE, West S, Friedman DS, Kempen J, O’Colmain B, Wu SY, and Taylor HR, 2004. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch. Ophthalmol 122, 487–94. [DOI] [PubMed] [Google Scholar]
- Costello MJ, Brennan LA, Basu S, Chauss D, Mohamed A, Gilliland KO, Johnsen S, Menko S, and Kantorow M, 2013. Autophagy and mitophagy participate in ocular lens organelle degradation. Exp. Eye Res 116, 141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszynski DM, Santhoshkumar P, Phadte AS, Sharma KK, Zhong HA, Lou MF, and Kador PF, 2019. Failure of Oxysterols Such as Lanosterol to Restore Lens Clarity from Cataracts. Scientific reports 9, 8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LL, Azuma M, and Shearer TR, 1994. Cataract and the Acceleration of Calpain-Induced Beta-Crystallin Insolubilization Occurring during Normal Maturation of Rat Lens. Invest. Ophthalmol. Vis. Sci 35, 785–93. [PubMed] [Google Scholar]
- David LL, Varnum MD, Lampi KJ, and Shearer TR, 1989. Calpain II in human lens. Invest. Ophthalmol. Vis. Sci 30, 269–75. [PubMed] [Google Scholar]
- David LL WP, 2011. Identification of crystallin disulfide cross-links in cataractous human lens by electron transfer dissociation. Invest. Ophthalmol. Vis. Sci 52, 2083. [Google Scholar]
- Deeley JM, Mitchell TW, Wei X, Korth J, Nealon JR, Blanksby SJ, and Truscott RJ, 2008. Human lens lipids differ markedly from those of commonly used experimental animals. Biochim. Biophys. Acta 1781, 288–98. [DOI] [PubMed] [Google Scholar]
- Dilley KJ and Pirie A, 1974. Changes to the proteins of the human lens nucleus in cataract. Exp. Eye Res 19, 59–72. [DOI] [PubMed] [Google Scholar]
- Duquette P, Pleines J, Girard M, Charest L, Senecal-Quevillon M, and Masse C, 1992. The increased susceptibility of women to multiple sclerosis. Can. J. Neurol. Sci 19, 466–71. [PubMed] [Google Scholar]
- Fields JB, Nemeth-Cahalan KL, Freites JA, Vorontsova I, Hall JE, and Tobias DJ, 2017. Calmodulin Gates Aquaporin 0 Permeability through a Positively Charged Cytoplasmic Loop. J. Biol. Chem 292, 185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederikse PH, Kasinathan C, and Kleiman NJ, 2012. Parallels between neuron and lens fiber cell structure and molecular regulatory networks. Dev. Biol 368, 255–60. [DOI] [PubMed] [Google Scholar]
- Friedrich MG and Truscott RJW, 2009. Membrane Association of Proteins in the Aging Human Lens: Profound Changes Take Place in the Fifth Decade of Life. Investigative Ophthalmology & Visual Science 50, 4786–93. [DOI] [PubMed] [Google Scholar]
- Friedrich MG and Truscott RJW, 2010. Large-Scale Binding of α-Crystallin to Cell Membranes of Aged Normal Human Lenses: A Phenomenon That Can Be Induced by Mild Thermal Stress. Investigative Ophthalmology & Visual Science 51, 5145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MG, Lam J, and Truscott RJ, 2012. Degradation of an old human protein: age-dependent cleavage of gammaS-crystallin generates a peptide that binds to cell membranes. J. Biol. Chem 287, 39012–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MG, Hancock SE, Raftery MJ, and Truscott RJW, 2016. Isoaspartic acid is present at specific sites in myelin basic protein from multiple sclerosis patients: could this represent a trigger for disease onset? Acta Neuropathologica Communications 4, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MG, Wang Z, Schey KL, and Truscott RJW, 2018. Spontaneous cross-linking of proteins at aspartate and asparagine residues is mediated via a succinimide intermediate. Biochem. J 475, 3189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MG, Wang Z, Oakley AJ, Schey KL, and Truscott RJW, 2017. Hotspots of age-related protein degradation: the importance of neighboring residues for the formation of non-disulfide crosslinks derived from cysteine. Biochem. J 474, 2475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii N, Matsumoto S, Hiroki K, and Takemoto L, 2001. Inversion and isomerization of Asp-58 residue in human alphaA-crystallin from normal aged lenses and cataractous lenses. Biochim Biophys Acta 1549, 179–87. [DOI] [PubMed] [Google Scholar]
- Fujii N, Takemoto LJ, Momose Y, Matsumoto S, Hiroki K, and Akaboshi M, 1999. Formation of Four Isomers at the Asp-151 Residue of Aged Human αA-Crystallin by Natural Aging. Biochemical and Biophysical Research Communications 265, 746–51. [DOI] [PubMed] [Google Scholar]
- Garland DL, Duglas-Tabor Y, Jimenez-Asensio J, Datiles MB, and Magno B, 1996. The nucleus of the human lens: demonstration of a highly characteristic protein pattern by two-dimensional electrophoresis and introduction of a new method of lens dissection. Exp. Eye Res 62, 285–91. [DOI] [PubMed] [Google Scholar]
- Giblin FJ, 2000. Glutathione: a vital lens antioxidant. J. Ocul. Pharmacol. Ther 16, 121–35. [DOI] [PubMed] [Google Scholar]
- Glasser A and Campbell MC, 1998. Presbyopia and the optical changes in the human crystalline lens with age. Vision Res. 38, 209–29. [DOI] [PubMed] [Google Scholar]
- Gorisse L, Pietrement C, Vuiblet V, Schmelzer CE, Kohler M, Duca L, Debelle L, Fornes P, Jaisson S, and Gillery P, 2016. Protein carbamylation is a hallmark of aging. Proc. Natl. Acad. Sci. U. S. A 113, 1191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey AC and Schey KL, 2009. Age-related changes in the spatial distribution of human lens alpha-crystallin products by MALDI imaging mass spectrometry. Invest. Ophthalmol. Vis. Sci 50, 4319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey AC, Chaurand P, Caprioli RM, and Schey KL, 2009. MALDI imaging mass spectrometry of integral membrane proteins from ocular lens and retinal tissue. J Proteome Res 8, 3278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groseclose MR, Andersson M, Hardesty WM, and Caprioli RM, 2007. Identification of proteins directly from tissue: in situ tryptic digestions coupled with imaging mass spectrometry. J. Mass Spectrom 42, 254–62. [DOI] [PubMed] [Google Scholar]
- Gutierrez DB, Garland D, and Schey KL, 2011. Spatial analysis of human lens aquaporin-0 post-translational modifications by MALDI mass spectrometry tissue profiling. Exp. Eye Res 93, 912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez DB, Garland DL, Schwacke JH, Hachey DL, and Schey KL, 2016. Spatial distributions of phosphorylated membrane proteins aquaporin 0 and MP20 across young and aged human lenses. Exp. Eye Res 149, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains PG and Truscott RJ, 2007. Post-translational modifications in the nuclear region of young, aged, and cataract human lenses. J Proteome Res 6, 3935–43. [DOI] [PubMed] [Google Scholar]
- Hains PG and Truscott RJ, 2008. Proteome analysis of human foetal, aged and advanced nuclear cataract lenses. Proteomics Clin Appl 2, 1611–9. [DOI] [PubMed] [Google Scholar]
- Hains PG and Truscott RJ, 2010. Age-dependent deamidation of lifelong proteins in the human lens. Invest. Ophthalmol. Vis. Sci 51, 3107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington V, McCall S, Huynh S, Srivastava K, and Srivastava OP, 2004. Crystallins in water soluble-high molecular weight protein fractions and water insoluble protein fractions in aging and cataractous human lenses. Mol. Vis 10, 476–89. [PubMed] [Google Scholar]
- Hejtmancik JF, Riazuddin SA, McGreal R, Liu W, Cvekl A, and Shiels A, 2015. Lens Biology and Biochemistry. Prog Mol Biol Transl Sci 134, 169–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo S, Diering GH, Na CH, Nirujogi RS, Bachman JL, Pandey A, and Huganir RL, 2018. Identification of long-lived synaptic proteins by proteomic analysis of synaptosome protein turnover. Proc. Natl. Acad. Sci. U. S. A 115, E3827–E36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heys KR and Truscott RJ, 2008. The stiffness of human cataract lenses is a function of both age and the type of cataract. Exp. Eye Res 86, 701–3. [DOI] [PubMed] [Google Scholar]
- Heys KR, Cram SL, and Truscott RJ, 2004. Massive increase in the stiffness of the human lens nucleus with age: the basis for presbyopia? Mol. Vis 10, 956–63. [PubMed] [Google Scholar]
- Heys KR, Friedrich MG, and Truscott RJ, 2007. Presbyopia and heat: changes associated with aging of the human lens suggest a functional role for the small heat shock protein, alpha-crystallin, in maintaining lens flexibility. Aging cell 6, 807–15. [DOI] [PubMed] [Google Scholar]
- Hooi MY and Truscott RJ, 2011. Racemisation and human cataract. D-Ser, D-Asp/Asn and D-Thr are higher in the lifelong proteins of cataract lenses than in age-matched normal lenses. Age (Dordr) 33, 131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooi MY, Raftery MJ, and Truscott RJ, 2012a. Racemization of two proteins over our lifespan: deamidation of asparagine 76 in gammaS crystallin is greater in cataract than in normal lenses across the age range. Invest. Ophthalmol. Vis. Sci 53, 3554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooi MY, Raftery MJ, and Truscott RJ, 2012b. Age-dependent deamidation of glutamine residues in human gammaS crystallin: deamidation and unstructured regions. Protein Sci. 21, 1074–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooi MY, Raftery MJ, and Truscott RJ, 2013a. Accelerated aging of Asp 58 in alphaA crystallin and human cataract formation. Exp. Eye Res 106, 34–9. [DOI] [PubMed] [Google Scholar]
- Hooi MYS, Raftery MJ, and Truscott RJW, 2012c. Racemization of Two Proteins over Our Lifespan: Deamidation of Asparagine 76 in γS Crystallin Is Greater in Cataract than in Normal Lenses across the Age RangeRacemization and Cataract Formation. Investigative Ophthalmology & Visual Science 53, 3554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooi MYS, Raftery MJ, and Truscott RJW, 2013b. Interconversion of the peptide isoforms of aspartate: Stability of isoaspartates. Mechanisms of Ageing and Development 134, 103–09. [DOI] [PubMed] [Google Scholar]
- Hooi MYS, Raftery MJ, and Truscott RJW, 2013c. Age-dependent racemization of serine residues in a human chaperone protein. Protein Science 22, 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CH, Wang YT, Tsai CF, Chen YJ, Lee JS, and Chiou SH, 2011. Phosphoproteomics characterization of novel phosphorylated sites of lens proteins from normal and cataractous human eye lenses. Mol. Vis 17, 186–98. [PMC free article] [PubMed] [Google Scholar]
- Institute, N.E. ‘Cataract Data and Statistics’, <https://nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/cataract-data-and-statistics>.
- Ismail VS, Mosely JA, Tapodi A, Quinlan RA, and Sanderson JM, 2016. The lipidation profile of aquaporin-0 correlates with the acyl composition of phosphoethanolamine lipids in lens membranes. Biochim. Biophys. Acta 1858, 2763–68. [DOI] [PubMed] [Google Scholar]
- Ito H, Kamei K, Iwamoto I, Inaguma Y, Nohara D, and Kato K, 2001. Phosphorylation-induced change of the oligomerization state of alpha B-crystallin. J. Biol. Chem 276, 5346–52. [DOI] [PubMed] [Google Scholar]
- Jacob RF, Cenedella RJ, and Mason RP, 1999. Direct evidence for immiscible cholesterol domains in human ocular lens fiber cell plasma membranes. J. Biol. Chem 274, 31613–8. [DOI] [PubMed] [Google Scholar]
- Ji L, Wu HT, Qin XY, and Lan R, 2017. Dissecting carboxypeptidase E: properties, functions and pathophysiological roles in disease. Endocr Connect 6, R18–R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Asensio J, Colvis CM, Kowalak JA, Duglas-Tabor Y, Datiles MB, Moroni M, Mura U, Rao CM, Balasubramanian D, Janjani A, and Garland D, 1999. An atypical form of alphaB-crystallin is present in high concentration in some human cataractous lenses. Identification and characterization of aberrant N- and C-terminal processing. J. Biol. Chem 274, 32287–94. [DOI] [PubMed] [Google Scholar]
- Jungblut PR, Otto A, Favor J, Lowe M, Muller EC, Kastner M, Sperling K, and Klose J, 1998. Identification of mouse crystallins in 2D protein patterns by sequencing and mass spectrometry. Application to cataract mutants. FEBS Lett. 435, 131–7. [DOI] [PubMed] [Google Scholar]
- Kamei A, Hamaguchi T, Matsuura N, and Masuda K, 2001. Does post-translational modification influence chaperone-like activity of alpha-crystallin? I. Study on phosphorylation. Biol. Pharm. Bull 24, 96–9. [DOI] [PubMed] [Google Scholar]
- Kamei A, Takamura S, Nagai M, and Takeuchi N, 2004. Phosphoproteome analysis of hereditary cataractous rat lens alpha-crystallin. Biol. Pharm. Bull 27, 1923–31. [DOI] [PubMed] [Google Scholar]
- Kannan R, Santhoshkumar P, Mooney BP, and Sharma KK, 2013. The alphaA66–80 peptide interacts with soluble alpha-crystallin and induces its aggregation and precipitation: a contribution to age-related cataract formation. Biochemistry (Mosc). 52, 3638–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Pinto SM, Getnet D, Nirujogi RS, Manda SS, Chaerkady R, Madugundu AK, Kelkar DS, Isserlin R, Jain S, Thomas JK, Muthusamy B, Leal-Rojas P, Kumar P, Sahasrabuddhe NA, Balakrishnan L, Advani J, George B, Renuse S, Selvan LD, Patil AH, Nanjappa V, Radhakrishnan A, Prasad S, Subbannayya T, Raju R, Kumar M, Sreenivasamurthy SK, Marimuthu A, Sathe GJ, Chavan S, Datta KK, Subbannayya Y, Sahu A, Yelamanchi SD, Jayaram S, Rajagopalan P, Sharma J, Murthy KR, Syed N, Goel R, Khan AA, Ahmad S, Dey G, Mudgal K, Chatterjee A, Huang TC, Zhong J, Wu X, Shaw PG, Freed D, Zahari MS, Mukherjee KK, Shankar S, Mahadevan A, Lam H, Mitchell CJ, Shankar SK, Satishchandra P, Schroeder JT, Sirdeshmukh R, Maitra A, Leach SD, Drake CG, Halushka MK, Prasad TS, Hruban RH, Kerr CL, Bader GD, Iacobuzio-Donahue CA, Gowda H, and Pandey A, 2014. A draft map of the human proteome. Nature 509, 575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korlimbinis A, Berry Y, Thibault D, Schey KL, and Truscott RJ, 2009. Protein aging: truncation of aquaporin 0 in human lens regions is a continuous age-dependent process. Exp. Eye Res 88, 966–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Nishimura S, Kumagae Y, and Kaneko I, 2002. In vivo conversion of racemized beta-amyloid ([D-Ser 26]A beta 1–40) to truncated and toxic fragments ([D-Ser 26]A beta 25–35/40) and fragment presence in the brains of Alzheimer’s patients. J. Neurosci. Res 70, 474–83. [DOI] [PubMed] [Google Scholar]
- Kuszak JR, Zoltoski RK, and Tiedemann CE, 2004. Development of lens sutures. Int. J. Dev. Biol 48, 889–902. [DOI] [PubMed] [Google Scholar]
- Kuszak JR, Bertram BA, Macsai MS, and Rae JL, 1984. Sutures of the crystalline lens: a review. Scan. Electron Microsc. 1369–78. [PubMed] [Google Scholar]
- Kuszak JR, Macsai MS, Bloom KJ, Rae JL, and Weinstein RS, 1985. Cell-to-cell fusion of lens fiber cells in situ: correlative light, scanning electron microscopic, and freeze-fracture studies. J. Ultrastruct. Res 93, 144–60. [DOI] [PubMed] [Google Scholar]
- Lachke SA, Alkuraya FS, Kneeland SC, Ohn T, Aboukhalil A, Howell GR, Saadi I, Cavallesco R, Yue Y, Tsai AC, Nair KS, Cosma MI, Smith RS, Hodges E, Alfadhli SM, Al-Hajeri A, Shamseldin HE, Behbehani A, Hannon GJ, Bulyk ML, Drack AV, Anderson PJ, John SW, and Maas RL, 2011. Mutations in the RNA granule component TDRD7 cause cataract and glaucoma. Science 331, 1571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampi KJ, Wilmarth PA, Murray MR, and David LL, 2014. Lens beta-crystallins: the role of deamidation and related modifications in aging and cataract. Prog. Biophys. Mol. Biol 115, 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampi KJ, Shih M, Ueda Y, Shearer TR, and David LL, 2002. Lens proteomics: analysis of rat crystallin sequences and two-dimensional electrophoresis map. Invest. Ophthalmol. Vis. Sci 43, 216–24. [PubMed] [Google Scholar]
- Lampi KJ, Ma Z, Shih M, Shearer TR, Smith JB, Smith DL, and David LL, 1997. Sequence analysis of betaA3, betaB3, and betaA4 crystallins completes the identification of the major proteins in young human lens. J. Biol. Chem 272, 2268–75. [DOI] [PubMed] [Google Scholar]
- Lampi KJ, Ma Z, Hanson SR, Azuma M, Shih M, Shearer TR, Smith DL, Smith JB, and David LL, 1998. Age-related changes in human lens crystallins identified by two-dimensional electrophoresis and mass spectrometry. Exp. Eye Res 67, 31–43. [DOI] [PubMed] [Google Scholar]
- Lapko VN, Smith DL, and Smith JB, 2003. Methylation and carbamylation of human gamma-crystallins. Protein Sci. 12, 1762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JC, Walker KL, Sherwin T, Schey KL, and Donaldson PJ, 2009. Confocal microscopy reveals zones of membrane remodeling in the outer cortex of the human lens. Invest. Ophthalmol. Vis. Sci 50, 4304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JS, Eckert R, Kistler J, and Donaldson P, 1998. Spatial differences in gap junction gating in the lens are a consequence of connexin cleavage. Eur. J. Cell Biol 76, 246–50. [DOI] [PubMed] [Google Scholar]
- Lin JS, Fitzgerald S, Dong Y, Knight C, Donaldson P, and Kistler J, 1997. Processing of the gap junction protein connexin50 in the ocular lens is accomplished by calpain. Eur. J. Cell Biol 73, 141–9. [PubMed] [Google Scholar]
- Linetsky M and LeGrand RD, 2005. Glutathionylation of lens proteins through the formation of thioether bond. Mol. Cell. Biochem 272, 133–44. [DOI] [PubMed] [Google Scholar]
- Linetsky M, Hill JM, LeGrand RD, and Hu F, 2004. Dehydroalanine crosslinks in human lens. Exp. Eye Res 79, 499–512. [DOI] [PubMed] [Google Scholar]
- Linetsky M, Shipova E, Cheng R, and Ortwerth BJ, 2008. Glycation by ascorbic acid oxidation products leads to the aggregation of lens proteins. Biochim. Biophys. Acta 1782, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo WK, Biswas SK, Brako L, Shiels A, Gu S, and Jiang JX, 2014. Aquaporin-0 targets interlocking domains to control the integrity and transparency of the eye lens. Invest. Ophthalmol. Vis. Sci 55, 1202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, and Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L, Hsu LK, Siefring GE Jr., and Rafferty NS, 1981. Lens transglutaminase and cataract formation. Proc. Natl. Acad. Sci. U. S. A 78, 1356–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou MF and Dickerson JE Jr., 1992. Protein-thiol mixed disulfides in human lens. Exp. Eye Res 55, 889–96. [DOI] [PubMed] [Google Scholar]
- Lou MF, Dickerson JE Jr., and Garadi R, 1990. The role of protein-thiol mixed disulfides in cataractogenesis. Exp. Eye Res 50, 819–26. [DOI] [PubMed] [Google Scholar]
- Lynnerup N, Kjeldsen H, Heegaard S, Jacobsen C, and Heinemeier J, 2008. Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout life. PLoS One 3, e1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon YA, Sabbah GM, and Julian RR, 2017. Identification of Sequence Similarities among Isomerization Hotspots in Crystallin Proteins. J Proteome Res 16, 1797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon YA, Sabbah GM, and Julian RR, 2018. Differences in alpha-Crystallin isomerization reveal the activity of protein isoaspartyl methyltransferase (PIMT) in the nucleus and cortex of human lenses. Exp. Eye Res 171, 131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon YA, Collier MP, Riggs DL, Degiacomi MT, Benesch JLP, and Julian RR, 2019. Structural and functional consequences of age-related isomerization in alpha-crystallins. J. Biol. Chem 294, 7546–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons B, Kwan AH, and Truscott RJ, 2016a. Spontaneous cleavage of proteins at serine and threonine is facilitated by zinc. Aging cell 15, 237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons B, Friedrich M, Raftery M, and Truscott R, 2016b. Amyloid Plaque in the Human Brain Can Decompose from Aβ(1–40/1–42) by Spontaneous Nonenzymatic Processes. Analytical Chemistry 88, 2675–84. [DOI] [PubMed] [Google Scholar]
- Lyons B, Friedrich M, Raftery M, and Truscott R, 2016c. Amyloid Plaque in the Human Brain Can Decompose from Abeta(1–40/1–42) by Spontaneous Nonenzymatic Processes. Anal. Chem 88, 2675–84. [DOI] [PubMed] [Google Scholar]
- Ma Z, Hanson SR, Lampi KJ, David LL, Smith DL, and Smith JB, 1998. Age-related changes in human lens crystallins identified by HPLC and mass spectrometry. Exp. Eye Res 67, 21–30. [DOI] [PubMed] [Google Scholar]
- MacCoss MJ, McDonald WH, Saraf A, Sadygov R, Clark JM, Tasto JJ, Gould KL, Wolters D, Washburn M, Weiss A, Clark JI, and Yates JR 3rd, 2002. Shotgun identification of protein modifications from protein complexes and lens tissue. Proc. Natl. Acad. Sci. U. S. A 99, 7900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makley LN, McMenimen KA, DeVree BT, Goldman JW, McGlasson BN, Rajagopal P, Dunyak BM, McQuade TJ, Thompson AD, Sunahara R, Klevit RE, Andley UP, and Gestwicki JE, 2015. Pharmacological chaperone for alpha-crystallin partially restores transparency in cataract models. Science 350, 674–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manenti S, Dunia I, and Benedetti EL, 1990. Fatty acid acylation of lens fiber plasma membrane proteins. MP26 and alpha-crystallin are palmitoylated. FEBS Lett. 262, 356–8. [DOI] [PubMed] [Google Scholar]
- Mann M, Kulak NA, Nagaraj N, and Cox J, 2013. The coming age of complete, accurate, and ubiquitous proteomes. Mol. Cell 49, 583–90. [DOI] [PubMed] [Google Scholar]
- Mathias RT, Kistler J, and Donaldson P, 2007. The lens circulation. J. Membr. Biol 216, 1–16. [DOI] [PubMed] [Google Scholar]
- McFall-Ngai MJ, Ding LL, Takemoto LJ, and Horwitz J, 1985. Spatial and temporal mapping of the age-related changes in human lens crystallins. Exp. Eye Res 41, 745–58. [DOI] [PubMed] [Google Scholar]
- Michael R and Bron AJ, 2011. The ageing lens and cataract: a model of normal and pathological ageing. Philos. Trans. R. Soc. Lond. B. Biol. Sci 366, 1278–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat BA, Landman KA, Truscott RJ, Sweeney MH, and Pope JM, 1999. Age-related changes in the kinetics of water transport in normal human lenses. Exp. Eye Res 69, 663–9. [DOI] [PubMed] [Google Scholar]
- Moroni M and Garland D, 2001. In vitro dephosphorylation of alpha-crystallin is dependent on the state of oligomerization. Biochim. Biophys. Acta 1546, 282–90. [DOI] [PubMed] [Google Scholar]
- Murray ED and Clarke S, 1984. Synthetic peptide substrates for the erythrocyte protein carboxyl methyltransferase. Detection of a new site of methylation at isomerized L-aspartyl residues. Journal of Biological Chemistry 259, 10722–32. [PubMed] [Google Scholar]
- Muszbek L, Haramura G, Cluette-Brown JE, Van Cott EM, and Laposata M, 1999. The pool of fatty acids covalently bound to platelet proteins by thioester linkages can be altered by exogenously supplied fatty acids. Lipids 34 Suppl, S331–7. [DOI] [PubMed] [Google Scholar]
- Nagaraj RH, Sell DR, Prabhakaram M, Ortwerth BJ, and Monnier VM, 1991. High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proc. Natl. Acad. Sci. U. S. A 88, 10257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj RH, Nahomi RB, Shanthakumar S, Linetsky M, Padmanabha S, Pasupuleti N, Wang B, Santhoshkumar P, Panda AK, and Biswas A, 2012. Acetylation of alphaA-crystallin in the human lens: effects on structure and chaperone function. Biochim. Biophys. Acta 1822, 120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima E, David LL, Riviere MA, Azuma M, and Shearer TR, 2009. Human and monkey lenses cultured with calcium ionophore form alphaB-crystallin lacking the C-terminal lysine, a prominent feature of some human cataracts. Invest. Ophthalmol. Vis. Sci 50, 5828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortwerth BJ and Olesen PR, 1988. Glutathione inhibits the glycation and crosslinking of lens proteins by ascorbic acid. Exp. Eye Res 47, 737–50. [DOI] [PubMed] [Google Scholar]
- Paladino S, Sarnataro D, Pillich R, Tivodar S, Nitsch L, and Zurzolo C, 2004. Protein oligomerization modulates raft partitioning and apical sorting of GPI-anchored proteins. J. Cell Biol 167, 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan RA, Sandilands A, Procter JE, Prescott AR, Hutcheson AM, Dahm R, Gribbon C, Wallace P, and Carter JM, 1999. The eye lens cytoskeleton. Eye (Lond) 13 ( Pt 3b), 409–16. [DOI] [PubMed] [Google Scholar]
- Raghavan CT, Smuda M, Smith AJ, Howell S, Smith DG, Singh A, Gupta P, Glomb MA, Wormstone IM, and Nagaraj RH, 2016. AGEs in human lens capsule promote the TGFbeta2-mediated EMT of lens epithelial cells: implications for age-associated fibrosis. Aging cell 15, 465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju M, Santhoshkumar P, and Sharma KK, 2017. Lens Endogenous Peptide alphaA66–80 Generates Hydrogen Peroxide and Induces Cell Apoptosis. Aging and disease 8, 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju M, Santhoshkumar P, and Sharma KK, 2018. Cell-penetrating Chaperone Peptide Prevents Protein Aggregation And Protects Against Cell Apoptosis. Advanced biosystems 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju M, Mooney BP, Thakkar KM, Giblin FJ, Schey KL, and Sharma KK, 2015. Role of alphaA-crystallin-derived alphaA66–80 peptide in guinea pig lens crystallin aggregation and insolubilization. Exp. Eye Res 132, 151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichow SL and Gonen T, 2008. Noncanonical binding of calmodulin to aquaporin-0: implications for channel regulation. Structure 16, 1389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo DG, Prentice BM, Moore JL, Norris JL, and Caprioli RM, 2017. Enhanced Spatially Resolved Proteomics Using On-Tissue Hydrogel-Mediated Protein Digestion. Anal. Chem 89, 2948–55. [DOI] [PubMed] [Google Scholar]
- Rose KM, Wang Z, Magrath GN, Hazard ES, Hildebrandt JD, and Schey KL, 2008. Aquaporin 0-calmodulin interaction and the effect of aquaporin 0 phosphorylation. Biochemistry (Mosc). 47, 339–47. [DOI] [PubMed] [Google Scholar]
- Roy D and Spector A, 1976. Absence of low-molecular-weight alpha crystallin in nuclear region of old human lenses. Proceedings of the National Academy of Sciences of the United States of America 73, 3484–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan DJ, Patterson NH, Putnam NE, Wilde AD, Weiss A, Perry WJ, Cassat JE, Skaar EP, Caprioli RM, and Spraggins JM, 2019. MicroLESA: Integrating Autofluorescence Microscopy, In Situ Micro-Digestions, and Liquid Extraction Surface Analysis for High Spatial Resolution Targeted Proteomic Studies. Anal. Chem 91, 7578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandilands A, Prescott AR, Hutcheson AM, Quinlan RA, Casselman JT, and FitzGerald PG, 1995. Filensin is proteolytically processed during lens fiber cell differentiation by multiple independent pathways. Eur. J. Cell Biol 67, 238–53. [PubMed] [Google Scholar]
- Santhoshkumar P, Udupa P, Murugesan R, and Sharma KK, 2008. Significance of interactions of low molecular weight crystallin fragments in lens aging and cataract formation. J. Biol. Chem 283, 8477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos AL and Lindner AB, 2017. Protein Posttranslational Modifications: Roles in Aging and Age-Related Disease. Oxid Med Cell Longev 2017, 5716409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer H, Marcus K, Sickmann A, Herrmann M, Klose J, and Meyer HE, 2003. Identification of phosphorylation and acetylation sites in alphaA-crystallin of the eye lens ( mus musculus) after two-dimensional gel electrophoresis. Anal Bioanal Chem 376, 966–72. [DOI] [PubMed] [Google Scholar]
- Schey KL, Gutierrez DB, Wang Z, Wei J, and Grey AC, 2010. Novel fatty acid acylation of lens integral membrane protein aquaporin-0. Biochemistry (Mosc). 49, 9858–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Chen D, Boda AR, Foster LJ, Davis MJ, and Hill MM, 2015. RaftProt: mammalian lipid raft proteome database. Nucleic Acids Res 43, D335–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam PM, Barigali A, Kadaskar J, Borgohain S, Mishra DK, Ramanjulu R, and Minija CK, 2015. Effect of lanosterol on human cataract nucleus. Indian J. Ophthalmol 63, 888–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, D’Souza RC, Tyanova S, Schaab C, Wisniewski JR, Cox J, and Mann M, 2014. Ultradeep human phosphoproteome reveals a distinct regulatory nature of Tyr and Ser/Thr-based signaling. Cell Rep 8, 1583–94. [DOI] [PubMed] [Google Scholar]
- Sharma KK and Santhoshkumar P, 2009. Lens aging: effects of crystallins. Biochim. Biophys. Acta 1790, 1095–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer D, Ens W, Standing K, and Valdimarsson G, 2008. Posttranslational modifications in lens fiber connexins identified by off-line-HPLC MALDI-quadrupole time-of-flight mass spectrometry. Invest. Ophthalmol. Vis. Sci 49, 1553–62. [DOI] [PubMed] [Google Scholar]
- Shi Y, Barton K, De Maria A, Petrash JM, Shiels A, and Bassnett S, 2009. The stratified syncytium of the vertebrate lens. J. Cell Sci 122, 1607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Matsuoka Y, and Shirasawa T, 2005. Biological significance of isoaspartate and its repair system. Biol. Pharm. Bull 28, 1590–6. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Watanabe A, Ogawara M, Mori H, and Shirasawa T, 2000. Isoaspartate formation and neurodegeneration in Alzheimer’s disease. Arch. Biochem. Biophys 381, 225–34. [DOI] [PubMed] [Google Scholar]
- Simpanya MF, Ansari RR, Suh KI, Leverenz VR, and Giblin FJ, 2005. Aggregation of lens crystallins in an in vivo hyperbaric oxygen guinea pig model of nuclear cataract: dynamic light-scattering and HPLC analysis. Invest. Ophthalmol. Vis. Sci 46, 4641–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpanya MF, Wistow G, Gao J, David LL, Giblin FJ, and Mitton KP, 2008. Expressed sequence tag analysis of guinea pig (Cavia porcellus) eye tissues for NEIBank. Mol. Vis 14, 2413–27. [PMC free article] [PubMed] [Google Scholar]
- Slavi N, Wang Z, Harvey L, Schey KL, and Srinivas M, 2016. Identification and Functional Assessment of Age-Dependent Truncations to Cx46 and Cx50 in the Human Lens. Invest. Ophthalmol. Vis. Sci 57, 5714–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LM and Kelleher NL, 2013. Proteoform: a single term describing protein complexity. Nat Methods 10, 186–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnino S, Aureli M, Grassi S, Mauri L, Prioni S, and Prinetti A, 2014. Lipid rafts in neurodegeneration and neuroprotection. Mol. Neurobiol 50, 130–48. [DOI] [PubMed] [Google Scholar]
- Srivastava OP and Srivastava K, 2003a. BetaB2-crystallin undergoes extensive truncation during aging in human lenses. Biochem. Biophys. Res. Commun 301, 44–9. [DOI] [PubMed] [Google Scholar]
- Srivastava OP and Srivastava K, 2003b. Crosslinking of human lens 9 kDa gammaD-crystallin fragment in vitro and in vivo. Mol. Vis 9, 644–56. [PubMed] [Google Scholar]
- Srivastava OP, McEntire JE, and Srivastava K, 1992. Identification of a 9 kDa gamma-crystallin fragment in human lenses. Exp. Eye Res 54, 893–901. [DOI] [PubMed] [Google Scholar]
- Srivastava OP, Srivastava K, Chaves JM, and Gill AK, 2017. Post-translationally modified human lens crystallin fragments show aggregation in vitro. Biochem Biophys Rep 10, 94–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DN, Lango J, Nambiar KP, Falso MJ, FitzGerald PG, Rocke DM, Hammock BD, and Buchholz BA, 2013. Carbon turnover in the water-soluble protein of the adult human lens. Mol. Vis 19, 463–75. [PMC free article] [PubMed] [Google Scholar]
- Su S, Liu P, Zhang H, Li Z, Song Z, Zhang L, and Chen S, 2011. Proteomic analysis of human age-related nuclear cataracts and normal lens nuclei. Invest. Ophthalmol. Vis. Sci 52, 4182–91. [DOI] [PubMed] [Google Scholar]
- Su SP, McArthur JD, and Andrew Aquilina J, 2010. Localization of low molecular weight crystallin peptides in the aging human lens using a MALDI mass spectrometry imaging approach. Exp. Eye Res 91, 97–103. [DOI] [PubMed] [Google Scholar]
- Su SP, Lyons B, Friedrich M, McArthur JD, Song X, Xavier D, Truscott RJ, and Aquilina JA, 2012. Molecular signatures of long-lived proteins: autolytic cleavage adjacent to serine residues. Aging cell 11, 1125–7. [DOI] [PubMed] [Google Scholar]
- Sweeney MH and Truscott RJ, 1998. An impediment to glutathione diffusion in older normal human lenses: a possible precondition for nuclear cataract. Exp. Eye Res 67, 587–95. [DOI] [PubMed] [Google Scholar]
- Takemoto L, 1995a. Quantitation of C-terminal modification of alpha-A crystallin during aging of the human lens. Exp. Eye Res 60, 721–4. [PubMed] [Google Scholar]
- Takemoto L, 1996. Increase in the intramolecular disulfide bonding of alpha-A crystallin during aging of the human lens. Exp. Eye Res 63, 585–90. [DOI] [PubMed] [Google Scholar]
- Takemoto L and Boyle D, 2000. Increased deamidation of asparagine during human senile cataractogenesis. Mol. Vis 6, 164–8. [PubMed] [Google Scholar]
- Takemoto LJ, 1995b. Identification of the in vivo truncation sites at the C-terminal region of alpha-A crystallin from aged bovine and human lens. Curr. Eye Res 14, 837–41. [DOI] [PubMed] [Google Scholar]
- Tanimura S and Takeda K, 2017. ERK signalling as a regulator of cell motility. J Biochem 162, 145–54. [DOI] [PubMed] [Google Scholar]
- Tapodi A, Clemens DM, Uwineza A, Goldberg MW, Thinon E, Heal WP, Tate EW, Nemeth-Cahalan K, Vorontsova I, Jarrin M, Hall JE, and Quinlan RA, 2019. BFSP1 C-terminal domains released by post-translational processing events can alter significantly the calcium regulation of AQP0 water permeability. Exp. Eye Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VL, al-Ghoul KJ, Lane CW, Davis VA, Kuszak JR, and Costello MJ, 1996. Morphology of the normal human lens. Invest. Ophthalmol. Vis. Sci 37, 1396–410. [PubMed] [Google Scholar]
- Thayer NH, Leverich CK, Fitzgibbon MP, Nelson ZW, Henderson KA, Gafken PR, Hsu JJ, and Gottschling DE, 2014. Identification of long-lived proteins retained in cells undergoing repeated asymmetric divisions. Proc. Natl. Acad. Sci. U. S. A 111, 14019–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinon E, Serwa RA, Broncel M, Brannigan JA, Brassat U, Wright MH, Heal WP, Wilkinson AJ, Mann DJ, and Tate EW, 2014. Global profiling of co- and post-translationally N-myristoylated proteomes in human cells. Nat Commun 5, 4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Canty JT, Briggs MM, and McIntosh TJ, 2013. The water permeability of lens aquaporin-0 depends on its lipid bilayer environment. Exp. Eye Res 113, 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Briggs MM, Mlaver D, Vidal A, and McIntosh TJ, 2009. Sorting of lens aquaporins and connexins into raft and nonraft bilayers: role of protein homo-oligomerization. Biophys. J 97, 2493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama BH and Hetzer MW, 2013. Protein homeostasis: live long, won’t prosper. Nat Rev Mol Cell Biol 14, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama Brandon H., Savas Jeffrey N., Park Sung K., Harris Michael S., Ingolia Nicholas T., Yates John R., and Hetzer Martin W., 2013. Identification of Long-Lived Proteins Reveals Exceptional Stability of Essential Cellular Structures. Cell 154, 971–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trokel S, 1962. The physical basis for transparency of the crystalline lens. Invest. Ophthalmol 1, 493–501. [PubMed] [Google Scholar]
- Truscott RJ, 2005. Age-related nuclear cataract-oxidation is the key. Exp. Eye Res 80, 709–25. [DOI] [PubMed] [Google Scholar]
- Truscott RJ and Augusteyn RC, 1977. The state of sulphydryl groups in normal and cataractous human lenses. Exp. Eye Res 25, 139–48. [DOI] [PubMed] [Google Scholar]
- Truscott RJ and Zhu X, 2010. Presbyopia and cataract: a question of heat and time. Prog. Retin. Eye Res 29, 487–99. [DOI] [PubMed] [Google Scholar]
- Truscott RJW and Friedrich MG, 2016. The etiology of human age-related cataract. Proteins don’t last forever. Biochim Biophys Acta 1860, 192–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott RJW, Schey KL, and Friedrich MG, 2016a. Old Proteins in Man: A Field in its Infancy. Trends Biochem. Sci 41, 654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott RJW, Schey KL, and Friedrich MG, 2016b. Old Proteins in Man: A Field in its Infancy. Trends Biochem Sci 41, 654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott RJW, Comte-Walters S, Ablonczy Z, Schwacke JH, Berry Y, Korlimbinis A, Friedrich MG, and Schey KL, 2011. Tight binding of proteins to membranes from older human cells. Age (Dordr) 33, 543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Duncan MK, and David LL, 2002. Lens proteomics: the accumulation of crystallin modifications in the mouse lens with age. Invest. Ophthalmol. Vis. Sci 43, 205–15. [PubMed] [Google Scholar]
- Vendra VP, Khan I, Chandani S, Muniyandi A, and Balasubramanian D, 2016. Gamma crystallins of the human eye lens. Biochim. Biophys. Acta 1860, 333–43. [DOI] [PubMed] [Google Scholar]
- Voorter CE, de Haard-Hoekman WA, van den Oetelaar PJ, Bloemendal H, and de Jong WW, 1988. Spontaneous peptide bond cleavage in aging alpha-crystallin through a succinimide intermediate. J. Biol. Chem 263, 19020–3. [PubMed] [Google Scholar]
- Wang-Su ST, McCormack AL, Yang S, Hosler MR, Mixon A, Riviere MA, Wilmarth PA, Andley UP, Garland D, Li H, David LL, and Wagner BJ, 2003. Proteome analysis of lens epithelia, fibers, and the HLE B-3 cell line. Invest. Ophthalmol. Vis. Sci 44, 4829–36. [DOI] [PubMed] [Google Scholar]
- Wang B, Hom G, Zhou S, Guo M, Li B, Yang J, Monnier VM, and Fan X, 2017. The oxidized thiol proteome in aging and cataractous mouse and human lens revealed by ICAT labeling. Aging cell 16, 244–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Ma W, and Spector A, 1995. Phosphorylation of alpha-crystallin in rat lenses is stimulated by H2O2 but phosphorylation has no effect on chaperone activity. Exp. Eye Res 61, 115–24. [DOI] [PubMed] [Google Scholar]
- Wang Z and Schey KL, 2009. Phosphorylation and truncation sites of bovine lens connexin 46 and connexin 50. Exp. Eye Res 89, 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z and Schey KL, 2015. Proteomic Analysis of Lipid Raft-Like Detergent-Resistant Membranes of Lens Fiber Cells. Invest. Ophthalmol. Vis. Sci 56, 8349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z and Schey KL, 2017. Identification of a direct Aquaporin-0 binding site in the lens-specific cytoskeletal protein filensin. Exp. Eye Res 159, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z and Schey KL, 2018a. Proteomic Analysis of S-Palmitoylated Proteins in Ocular Lens Reveals Palmitoylation of AQP5 and MP20. Invest. Ophthalmol. Vis. Sci 59, 5648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z and Schey KL, 2018b. Quantification of thioether-linked glutathione modifications in human lens proteins. Exp. Eye Res 175, 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Han J, and Schey KL, 2008. Spatial differences in an integral membrane proteome detected in laser capture microdissected samples. J Proteome Res 7, 2696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Obidike JE, and Schey KL, 2010. Posttranslational modifications of the bovine lens beaded filament proteins filensin and CP49. Invest. Ophthalmol. Vis. Sci 51, 1565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Han J, David LL, and Schey KL, 2013. Proteomics and phosphoproteomics analysis of human lens fiber cell membranes. Invest. Ophthalmol. Vis. Sci 54, 1135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Lyons B, Truscott RJ, and Schey KL, 2014. Human protein aging: modification and crosslinking through dehydroalanine and dehydrobutyrine intermediates. Aging cell 13, 226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Friedrich MG, Truscott RJW, and Schey KL, 2019. Cleavage C-terminal to Asp leads to covalent crosslinking of long-lived human proteins. Biochim Biophys Acta Proteins Proteom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmack RA, Shawa H, Liu K, Lopez K, Loo JA, Horwitz J, and Clarke SG, 2019. The L-isoaspartate modification within protein fragments in the aging lens can promote protein aggregation. J. Biol. Chem [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasinger VC, Cordwell SJ, Cerpa-Poljak A, Yan JX, Gooley AA, Wilkins MR, Duncan MW, Harris R, Williams KL, and Humphery-Smith I, 1995. Progress with gene-product mapping of the Mollicutes: Mycoplasma genitalium. Electrophoresis 16, 1090–4. [DOI] [PubMed] [Google Scholar]
- Wenke JL, Rose KL, Spraggins JM, and Schey KL, 2015. MALDI Imaging Mass Spectrometry Spatially Maps Age-Related Deamidation and Truncation of Human Lens Aquaporin-0. Invest. Ophthalmol. Vis. Sci 56, 7398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widomska J, Subczynski WK, Mainali L, and Raguz M, 2017. Cholesterol Bilayer Domains in the Eye Lens Health: A Review. Cell Biochem. Biophys 75, 387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmarth PA, Taube JR, Riviere MA, Duncan MK, and David LL, 2004. Proteomic and sequence analysis of chicken lens crystallins reveals alternate splicing and translational forms of beta B2 and beta A2 crystallins. Invest. Ophthalmol. Vis. Sci 45, 2705–15. [DOI] [PubMed] [Google Scholar]
- Wilmarth PA, Tanner S, Dasari S, Nagalla SR, Riviere MA, Bafna V, Pevzner PA, and David LL, 2006. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility? J Proteome Res 5, 2554–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witalison EE, Thompson PR, and Hofseth LJ, 2015. Protein Arginine Deiminases and Associated Citrullination: Physiological Functions and Diseases Associated with Dysregulation. Curr Drug Targets 16, 700–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wride MA, 2011. Lens fibre cell differentiation and organelle loss: many paths lead to clarity. Philos. Trans. R. Soc. Lond. B. Biol. Sci 366, 1219–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Norris JL, and Caprioli R, 2018. Novel vacuum stable ketone-based matrices for high spatial resolution MALDI imaging mass spectrometry. J. Mass Spectrom 53, 1005–12. [DOI] [PubMed] [Google Scholar]
- Yu NT, DeNagel DC, Pruett PL, and Kuck JF Jr., 1985. Disulfide bond formation in the eye lens. Proc. Natl. Acad. Sci. U. S. A 82, 7965–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Liu P, Wang N, Li Y, and Wang L, 2007. Comparison of two tandem mass spectrometry-based methods for analyzing the proteome of healthy human lens fibers. Mol. Vis 13, 1873–7. [PubMed] [Google Scholar]
- Zhang Z, Smith DL, and Smith JB, 2003. Human beta-crystallins modified by backbone cleavage, deamidation and oxidation are prone to associate. Exp. Eye Res 77, 259–72. [DOI] [PubMed] [Google Scholar]
- Zhang Z, David LL, Smith DL, and Smith JB, 2001. Resistance of human betaB2-crystallin to in vivo modification. Exp. Eye Res 73, 203–11. [DOI] [PubMed] [Google Scholar]
- Zhao H, Brown PH, Magone MT, and Schuck P, 2011. The molecular refractive function of lens gamma-Crystallins. J. Mol. Biol 411, 680–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Chen XJ, Zhu J, Xi YB, Yang X, Hu LD, Ouyang H, Patel SH, Jin X, Lin D, Wu F, Flagg K, Cai H, Li G, Cao G, Lin Y, Chen D, Wen C, Chung C, Wang Y, Qiu A, Yeh E, Wang W, Hu X, Grob S, Abagyan R, Su Z, Tjondro HC, Zhao XJ, Luo H, Hou R, Jefferson J, Perry P, Gao W, Kozak I, Granet D, Li Y, Sun X, Wang J, Zhang L, Liu Y, Yan YB, and Zhang K, 2015. Lanosterol reverses protein aggregation in cataracts. Nature 523, 607–11. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wilmarth PA, Cheng C, Limi S, Fowler VM, Zheng D, David LL, and Cvekl A, 2019. Proteome-transcriptome analysis and proteome remodeling in mouse lens epithelium and fibers. Exp. Eye Res 179, 32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Leiberman J, Xu J, and Lavker RM, 2006. A hierarchy of proliferative cells exists in mouse lens epithelium: implications for lens maintenance. Invest. Ophthalmol. Vis. Sci 47, 2997–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Korlimbinis A, and Truscott RJ, 2010. Age-dependent denaturation of enzymes in the human lens: a paradigm for organismic aging? Rejuvenation Res 13, 553–60. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Clair G, Chrisler WB, Shen Y, Zhao R, Shukla AK, Moore RJ, Misra RS, Pryhuber GS, Smith RD, Ansong C, and Kelly RT, 2018. Proteomic Analysis of Single Mammalian Cells Enabled by Microfluidic Nanodroplet Sample Preparation and Ultrasensitive NanoLC-MS. Angew. Chem. Int. Ed. Engl 57, 12370–74. [DOI] [PMC free article] [PubMed] [Google Scholar]


