Abstract
Calcium signaling is critical to neuronal function and regulates highly diverse processes such as gene transcription, energy production, protein handling, and synaptic structure and function. Because there are many common underlying calcium-mediated pathological features observed across several neurological conditions, it has been proposed that neurodegenerative diseases have an upstream underlying calcium basis in their pathogenesis. With certain diseases such as Alzheimer's, Parkinson's, and Huntington's, specific sources of calcium dysregulation originating from distinct neuronal compartments or channels have been shown to have defined roles in initiating or sustaining disease mechanisms. Herein, we will review the major hallmarks of these diseases, and how they relate to calcium dysregulation. We will then discuss neuronal calcium handling throughout the neuron, with special emphasis on channels involved in neurodegeneration.
Calcium is one of the most ubiquitous signaling messengers in the brain, and as such it has fundamental roles in maintaining neuronal health and function (Berridge 1998; Berridge et al. 2000; Clapham 2007; Bootman 2012). In the extracellular space between cells in the brain, calcium levels are typically maintained between 1.1 and 1.4 mm, whereas resting cytosolic levels within neurons is tightly regulated and maintained around the 100 nm range (Kawamoto et al. 2012). This substantial calcium gradient allows for the initiation of signaling cascades that underlie neuronal functions, including gene transcription, synaptic transmission, memory encoding, apoptosis, and many others (Berridge 1998). Calcium enters neurons primarily through plasma membrane–localized channels and is quickly sequestered by buffers, calcium-binding proteins, and internal organelles, such as the endoplasmic reticulum (ER), mitochondria, and lysosomes. Upon activation of plasma membrane channels or release from internal stores, the calcium concentration may rise by 2–3 orders of magnitude near the mouth of the channel, cueing complex signaling cascades, the nature of which is highly dependent upon cellular localization, calcium concentration, and metabolic state (Berridge 1998). With the intricate regulatory strategies dedicated to maintaining specific calcium parameters, abnormalities in intracellular calcium handling and homeostasis can contribute to, and sustain, a variety of neurodegenerative diseases (Khachaturian 1987) such as Alzheimer's disease (AD), Huntington's disease (HD), and Parkinson's disease (PD). Subsequent years of research have detailed the mechanisms by which neuronal calcium dysregulation contributes to motor and/or cognitive dysfunction in these diseases (Mattson et al. 1999; Stutzmann 2007; Surmeier et al. 2017c; Pchitskaya et al. 2018). With the increased understanding of the role of calcium dysregulation as an early or upstream mechanism in neurodegenerative disease, targeting specific calcium channels is increasingly considered as a viable strategy for therapeutic development (Chakroborty et al. 2012; Oules et al. 2012; Chami and Checler 2014; Surmeier et al. 2017a).
In this review, we will first introduce the most predominant neurodegenerative diseases (AD, PD, and HD) and provide the context by which calcium signaling plays a role in their pathogenesis. Following this, we will walk through the major calcium channels and sources to expand on how their deficiencies contribute to symptoms or features of specific neurodegenerative diseases.
ALZHEIMER'S DISEASE (AD)
First described in 1907 by Dr. Alois Alzheimer, sporadic AD (SAD) is the most common form of dementia, affecting 1 in 10 people over the age of 65 (Alzheimer's Association 2019). In SAD, age is the greatest risk factor and the disease is typically diagnosed in people over 65 years of age. The most prominent and defining feature is the progressive and irreversible impairment in memory and cognitive functions resulting from defects in vulnerable brain regions critical for memory encoding and storage, such as the hippocampus and associated cortices. As AD advances, other brain functions become impaired such as speech, motor function, and affect (Wolk and Dickerson 2019). The etiology of AD is unknown, and no treatment currently exists that cures or prevents the disease progression. FDA-approved therapies, such as acetylcholinesterase inhibitors and N-methyl-d-aspartate receptor (NMDAR) antagonists, can provide temporary symptom support, but do not stop the inevitable decline. Life expectancy of a patient postdiagnosis is typically 8–10 years, albeit with a high degree of variability depending upon presentation, comorbidities, etc. (Alzheimer's Association 2019; Wolk and Dickerson 2019).
From a histopathological perspective, AD features two prominent hallmarks: accumulation of extracellular plaques composed of β-amyloid (Aβ), and intracellular neurofibrillary tangles composed of hyperphosphorylated microtubule-associated protein tau (tau). The vast majority of AD cases are sporadic (>95%), with no known cause although several genetic risk factors have been identified. The highest risk factor associated with SAD is expression of E4 alleles of apolipoprotein E (ApoE), which increases the likelihood of developing AD by threefold for one allele, and 12-fold for two alleles (Mahley et al. 2007; Kim et al. 2009). ApoE has three variants, with ApoE4 expression conferring increased risk for AD, ApoE3 considered the “normal” allele, and ApoE2 considered protective against AD (Holtzman et al. 2012). ApoE functions in lipid homeostasis and cholesterol transport, and it is thought that ApoE4 has increased affinity for the pathogenic forms of Aβ thus increasing amyloid burden in the central nervous system (CNS) (Carter 2005).
Familial AD (FAD), which accounts for 1%–5% of cases, is caused by mutations in presenilin 1 (PS1), presenilin 2 (PS2), or amyloid precursor protein (APP) genes. These autosomal-dominant mutations are 100% penetrant, and result in a more aggressive, earlier onset (as early as 30–40 years of age) form of AD than SAD, although the symptoms and disease phenotypes are nearly identical. Because the ER-localized PS1 protein is part of an enzymatic complex that cleaves APP into Aβ fragments, this association in FAD has helped spur the “amyloid cascade hypothesis,” which posits that formation and aggregation of Aβ is the cause of AD (Hardy and Higgins 1992; Selkoe 2000; Hardy and Selkoe 2002; Shirwany et al. 2007; Selkoe and Hardy 2016). As such, targeting APP processing and Aβ plaques have been prioritized for clinical development. These therapeutic strategies have shown varying degrees of success in clearing plaques from brains of AD patients, but to date, the more than 15 phase III clinical trials have failed to alter the progression of cognitive decline in early–late-stage AD patients in a positive manner (Kametani and Hasegawa 2018; Makin 2018). These consistently negative outcomes, in combination with the lack of correlation between brain amyloid levels and cognitive status (rather, it is synaptic loss that correlates with memory loss in AD; DeKosky and Scheff 1990; Lassmann et al. 1993; Scheff et al. 2006, 2014), support the need for considering alternative mechanisms driving AD-related memory impairment. Further studies exploring upstream pathogenic mechanisms identified that FAD mutations lead to calcium dysregulation prior to the emergence of plaques and tangles. The initial sources of the aberrant calcium originated from the ER via inositol 1,4,5-trisphosphate receptor (IP3R)- and ryanodine receptor (RyR)-evoked calcium release (Etcheberrigaray et al. 1998; Leissring et al. 2000; Mattson and Chan 2001; LaFerla 2002). Calcium signaling/homeostasis defects may occur at several points throughout the neuronal calcium-handling system such as through plasma membrane channels, possibly through a feedforward modification by Aβ (Texidó et al. 2011). Additionally, Aβ oligomers alone can form calcium-permeable pores on the plasma membrane (Arispe et al. 1993), which leads to increased cytosolic calcium concentrations (Demuro et al. 2011). These and other sources of calcium dyshomeostasis will be discussed in further detail below (see Fig. 1; Stutzmann et al. 2004, 2006; Nelson et al. 2007; Shilling et al. 2014).
Figure 1.
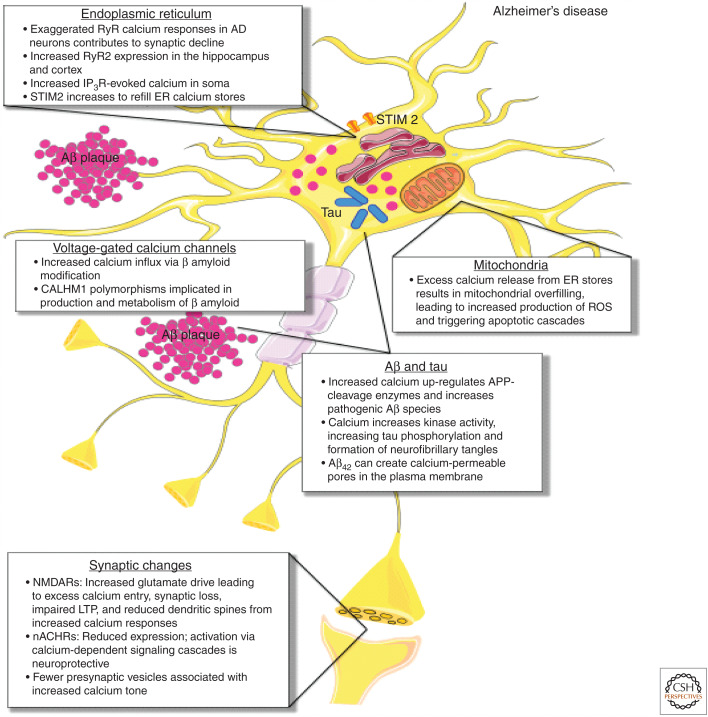
Calcium-handling defects in Alzheimer's disease (AD). In AD, exaggerated ryanodine receptor (RyR) calcium responses in AD neurons contribute to synaptic decline, with increased RyR2 expression seen in the hippocampus and cortex. Increased inositol-1,4,5-trisphosphate receptor (IP3R)-evoked calcium is seen in the soma, and stromal interaction molecule 2 (STIM2) expression and activation increases to refill ER calcium stores. As a result of excess calcium release from ER stores, mitochondrial calcium overload occurs, leading to metabolic dysfunction, increased production of reactive oxygen species (ROS), and apoptotic engagement. Increased calcium also up-regulates APP-cleavage enzymes and increases pathogenic β-amyloid species. Aβ42 in particular can create calcium-permeable pores in the plasma membrane, contributing to calcium dysregulation. β-amyloid modification also increases calcium influx through voltage-gated calcium channels (VGCCs). Increased cytosolic calcium enhances the activity of kinases that phosphorylate tau, leading to increased tau phosphorylation and ultimately the formation of neurofibrillary tangles. At the synapse, expression of neuronal nicotinic acetylcholine receptors (nACHRs) is reduced through neuronal loss, while activation of nACHRs via calcium-dependent signaling cascades that are neuroprotective. Increased glutamate drive on N-methyl-d-aspartate receptors (NMDARs) leads to excess calcium entry, synaptic loss, and impaired long-term potentiation (LTP), and reduced dendritic spines from increased calcium responses. Fewer presynaptic vesicles result in increased calcium tone at terminals.
HUNTINGTON'S DISEASE (HD)
First described as an inheritable disease by George Huntington in 1872 (Huntington 1872; Lanska 2000), HD is an autosomal-dominant disease that affects about 1 in 7500 people worldwide. HD presents with choreiform motor movements: uncontrolled abrupt and “jerky” movements of the face, body, and limbs. This lack of motor control progresses rapidly, and leaves patients unable to walk or balance, and progresses to difficulty breathing and death. HD can also present with cognitive and psychological impairments consistent with deterioration of frontostriatal motor control pathways (Suchowersky 2019). The age of onset is typically in one's 40s, although this varies inversely with the number of CAG repeats (see below). There are no cures or effective treatments for HD, and the life expectancy of a person with HD is typically 10–20 years after initial diagnosis (Suchowersky 2019).
Unlike the other neurodegenerative diseases discussed here, the genetic basis of HD is fairly well understood. The Huntingtin gene (Htt) is on chromosome 4 and the nonmutant Htt protein interacts with over 200 other proteins, and has roles in development, cellular trafficking, and apoptosis (MacDonald et al. 1993; Nasir et al. 1995; Duyao et al. 1995; Zeitlin et al. 1995; Dragatsis et al. 2000; Caviston et al. 2007). What makes mutant Htt pathogenic is the increased number of CAG repeats, which results in abnormally long polyglutamine chains at the amino-terminal end of Htt, placing HD in the polyglutamine family of disorders such as spinocerebellar ataxia (MacDonald et al. 1993). Mutant Htt is proteolytically cleaved and the amino-terminal fragments aggregate to form inclusion bodies within neurons (Arrasate and Finkbeiner, 2012). The age of onset and severity of HD is strongly influenced by the number of CAG repeats in the Htt gene (Penney et al. 1997; Langbehn et al. 2010; Keum et al. 2016), where repeats of 10–35 are considered normal and CAG repeats of 40 or above (termed mHtt) result in HD pathology. Interestingly, Htt is expressed throughout the body but the GABAergic medium spiny neurons (MSNs) within the neostriatum are most affected in the disease.
The mHtt gene interferes with a variety of transcriptional targets involved in calcium homeostasis, including up-regulation of calretinin, presenilin 2, calmyrin 1, and down-regulation of calmodulin, ORAI2, and septin 4 (Czeredys et al. 2013, 2018). In line with these transcriptional changes, MSNs in the R6/2 HD mouse model (Li et al. 2005) display abnormally high-resting calcium levels (Hannson 2001). This is influenced by the mutant Htt protein interaction with IP3Rs, which results in exaggerated calcium release from ER stores (Tang et al. 2003), leading to overactivation of store-operated calcium entry (SOCE) (Wu et al. 2011, 2016; Vigont et al. 2015). In total, this increase in cytosolic calcium concentration contributes to mitochondrial calcium-handling defects driving reactive oxygen species (ROS) production, cellular stress responses, and apoptotic engagement (see Fig. 2; Giacomello et al. 2013).
Figure 2.
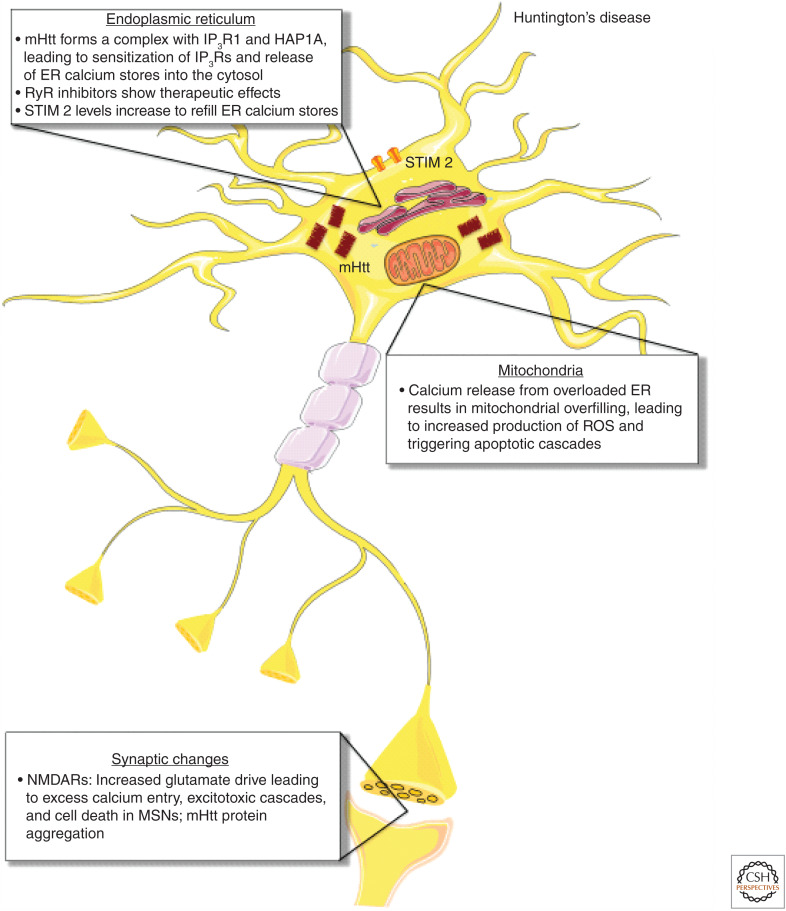
Calcium-handling defects in Huntington's disease (HD). In HD, the mHtt protein forms a complex with inositol-14,5-trisphosphate receptor 1 (IP3R1) and Huntingtin-associated protein 1 (HAP1A), leading to sensitization of IP3Rs and release of ER calcium stores into the cytosol. RyR inhibitors also show therapeutic effects in HD, suggesting that RyR-mediated calcium release is involved in neurodegeneration. As ER calcium stores are released and become depleted, stromal interaction molecule 2 (STIM2) increases to refill ER calcium stores by traveling to the plasma membrane and recruiting ORAI channels to allow calcium entry. Much like AD, calcium release from the overloaded ER results in mitochondrial overfilling, which in turn leads to increased production of reactive oxygen species (ROS) and triggers apoptotic cascades. At the synapse, increased glutamate drive at N-methyl-d-aspartate receptors (NMDARs) leads to excess calcium entry, excitotoxic death in medium spiny neurons (MSNs), and mHtt protein aggregation.
PARKINSON'S DISEASE (PD)
First described by James Parkinson in 1817 (see Parkinson 2002), PD presents with distinct motor impairments and may later incorporate cognitive decline and psychiatric conditions (Barone 2009). Specific motor symptoms include slowed, delayed, or loss of voluntary movement (akinesia), tremors, rigidity, and postural instability (Chou 2019). The incidence of dementia in later-stage PD is also high with deficits in executive function and visuospatial recognition (Litvan et al. 2012; Chou 2019). These symptoms arise from the selective death of midbrain dopamine neurons in the substantia nigra pars compacta (SNpc), dramatically reducing dopamine (DA) levels in target brain regions important for controlling movement such as the striatum. Levodopa, which is converted to dopamine in the brain and thus acutely boosts CNS dopamine levels, is an effective but temporary intervention for PD that can ameliorate both motor and cognitive deficits, but can also lead to dyskinesia or irregular movements. The average age of diagnosis ranges from 60 to 70 years, with a mean survival time of 6–22 years (Chou 2019). Like AD, PD exists in both a familial form (FPD) with four known mutations inherited in an autosomal-dominant pattern, and the more common idiopathic form (IPD), which accounts for >80% of cases (Schiesling et al. 2008).
In the familial forms, polymorphisms, duplications, and triplications in the SNCA gene (previously identified as Park1) have been identified (Rajput et al. 1984). SNCA encodes α-synuclein, which is highly expressed in presynaptic compartments and plays a role in synaptic function by maintaining and clustering supplies of synaptic vesicles and regulating their release; thus, SNCA mutations are associated with decreased nigrostriatal DA transmission in PD (Vargas et al. 2017). One of the major histological features of PD is aggregation of mutant α-synuclein into structures known as Lewy bodies, which are associated with synaptic decline and calcium dyshomeostasis (Maroteaux et al. 1988; Maroteaux and Scheller 1991).
Additional sources of calcium dyshomeostasis in PD include the Cav1.2 and Cav1.3 voltage-gated calcium channels (Surmeier et al. 2017b). SNpc DA neurons rely on the pace-making activity of these calcium channels to regulate DA release, and in PD, there is thought to be a shift in the relative roles of these channels from Cav1.2 to Cav1.3, which leads to increases in cytosolic calcium, mitochondrial stress, and eventually neuronal death (Hurley et al. 2013; Berger and Bartsch 2014; Dragicevic et al. 2015). Furthermore, aberrant ER calcium flux has been observed through RyRs (Bruzzone et al. 2003) and GWAS identified calcium homeostasis genes in Parkinson’s disease (Saad et al. 2011), indicating that intracellular calcium-handling and voltage-gated calcium entry may be working concurrently to mediate mitochondrial stress (see Fig. 3).
Figure 3.
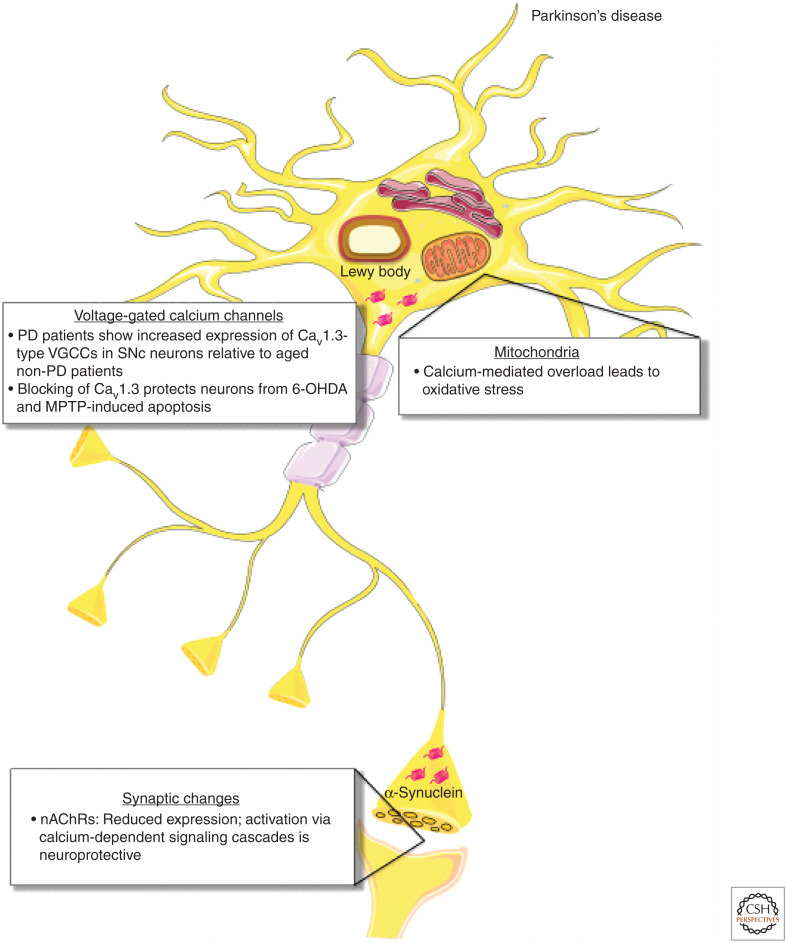
Calcium-handling defects in Parkinson's disease (PD). In PD, patients show increased expression of Cav1.3-type voltage-gated calcium channels (VGCCs) in SNc neurons compared to aged non-PD patients, leading to increases in cytosolic calcium. Blocking Cav1.3-type VGCCs has been shown to protect neurons from 6-OHDA (synthetic neurotoxin selectively targeting dopaminergic and noradrenergic neurons) and MPTP (mitochondrial toxin that produces PD-like pathology)-induced apoptosis. In mitochondria, calcium-mediated overload leads to oxidative stress. At the synapse, expression of nicotinic acetylcholine receptors (nAChRs) is reduced, while activation of nAChRs via calcium-dependent signaling cascades resulting in up-regulation of antiapoptotic proteins is neuroprotective as in AD.
SOURCES OF CELLULAR CALCIUM DYSREGULATION DRIVING NEURODEGENERATIVE DISEASE
Here we selected the major contributing calcium pathways implicated in neurodegenerative disease, beginning with sites at the plasma membrane and continuing through to internal stores, and discuss the mechanisms by which they contribute to a disease state.
Ionotropic Glutamate Receptors
Ionotropic glutamate receptors (iGluRs) include the NMDA, AMPA, and kainate receptors (Sobolevsky 2015), which serve as the primary mediators of excitatory synaptic transmission in the brain. Of these, the NMDARs are often implicated in cellular pathophysiology based upon their relatively high calcium permeability (Vyklicky et al. 2014). NMDARs are heterotetramers composed of subunits NR1-3. Typically, NMDARs consist of two NR1 and two NR2 subunits: NR1 is required for channel function, and additional cation conductance variability is introduced with NR2 (NR2A-D) and NR3 (A-B) subunits (Papadia and Hardingham 2007; Henson et al. 2010; Sanz-Clemente et al. 2013). Notably, among the isoforms, NR2B has the highest calcium conductance and its expression is enhanced during early stages of development. NR3 containing NMDARs are understudied, but as NR3 subunits display reduced calcium conductance, they act as dominant-negative inhibitors of NMDAR activity (Cavara and Hollmann 2008; Henson et al. 2010). Unlike AMPARs, which can be activated solely by glutamate binding, NMDARs require both glutamate and membrane depolarization to displace bound Mg2+ to conduct. This tightly regulated strategy of calcium entry functions as a coincidence detector, such that calcium-dependent signaling pathways in the postsynaptic compartment are not activated unless there is sufficient glutamatergic input, allowing for the selective strengthening of synapses.
NMDAR-mediated calcium dysregulation is thought to contribute to AD through several means. One proposal is through NMDAR interaction with Aβ42 peptides, which potentiates NMDAR activity and contributes to synapse loss (Lacor et al. 2004; DeFelice and Goswami 2007; Bezprozvanny 2008; Ronicke 2011; Zhang et al. 2016). Under normal conditions, sustained NMDAR activation results in dendritic spine remodeling through calcium-dependent signaling cascades involving kinase activation, protein synthesis, and actin polymerization (Bosch et al. 2014). However, in AD mouse models and human AD samples, there are fewer mature or stable spines, possibly through aberrant NMDAR-generated and/or RyR-evoked calcium signals, providing a mechanism by which calcium dysregulation leads to synaptic decay (Spires-Jones et al. 2007; Spires-Jones and Hyman 2014; Chakroborty et al. 2019). Notably, while NMDAR-mediated calcium entry appears normal in AD mouse models, calcium entry through this channel triggers a large RyR-evoked calcium response within dendritic spines, demonstrating a cooperative pathogenic signaling cascade that underlies spine loss in AD (Goussakov et al. 2010, 2011). Preventing the NMDAR-triggered calcium-induced calcium response (CICR) with the RyR negative allosteric modulator, dantrolene, normalized the overall calcium response, and prevented spine and synaptic loss in the AD models (Chakroborty et al. 2019).
Aβ potentiation of NMDARs, likely through the enhanced calcium response, has been shown to increase localization of the phosphatase calcineurin (PP2B) in synapses, which counteracts long-term potentiation (LTP) encoding and promotes synaptic depression. Indeed, the amyloid-induced calcium dysregulation may activate molecular pathways more consistent with long-term depression (LTD), resulting in synapse shrinkage and eventual synapse loss (Snyder et al. 2005; Roselli et al. 2009; Yamin et al. 2009).
The contribution of NMDAR-calcium dysregulation in AD can also be inferred through the therapeutic effects achieved when targeting the channel. Selectively down-regulating the NR2B subunit mitigates the deleterious effects of Aβ on synaptic structure and function (Danysz and Parsons 2003; Rogawski and Wenk 2003; Costa 2012; Bazzari et al. 2019), and the weak NMDAR antagonist memantine is one of two FDA-approved drug classes for AD. The effectiveness is limited, but memantine provides moderate and short-lived improvements to memory and cognitive performance in AD patients (Lipton 2004; Kishi et al. 2017).
NMDARs also play a role in HD pathology primarily by sensitizing MSNs to excitotoxic cascades and cell death (Fernandes and Raymond 2009). Increased NMDAR currents (Starling et al. 2005), as well as enhanced cellular swelling in response to NMDAR activation (Cepeda et al. 2001), have been observed in MSNs from HD models. This may reflect the enhanced NR2B-containing NMDARs in MSNs in HD (Li et al. 2003), resulting in larger calcium influx and vulnerability to NMDAR-mediated cell death (Zeron et al. 2002). The pathological cascade can become cyclical in that NMDAR activation facilitates mHtt protein aggregation (Okamoto et al. 2009), thus escalating until cell death. Again, use of memantine leads to reduction of NMDAR activation, curtailing excess calcium influx, and neuronal death in HD (Levine et al. 2010). Both AD and HD exhibit forms of “excitotoxicity,” where disruptions in synaptic regulation, or local injury, result in increased glutamate release, excessive NMDAR activation, and subsequent excess calcium entry. If sustained, this leads to mitochondrial depolarization, organelle failure, apoptosis, and neuronal death (Dong et al. 2009; Zhou et al. 2013). The notable differences are the cellular specificity and brain regions affected; however, in these and other neurodegenerative disorders, it is still unclear what exactly is driving the anatomical and cell-specific nature of the diseases.
NMDAR-mediated excitotoxicity is not thought to initiate the degeneration observed in PD (Ambrosi 2014). However, as SNpc dopaminergic neurons are often depolarized through their pace-making activity, the Mg2+ block of NMDARs is often relieved, increasing NMDAR open probability and calcium current, which then likely contributes to calcium dyshomeostasis, synaptic dysfunction, and excitotoxicity (Surmeier and Schumacker 2013). Indeed, weak NMDAR antagonists have demonstrated neuroprotection in MPTP or 6-OHDA lesioned rodent models, where use of memantine or MK-801 reduced the loss of dopaminergic neurons in the SNpc and reduced rotational behavior, suggesting therapeutic value in humans (Greenamyre et al. 1991; Blandini et al. 2001; Wild et al. 2013). Furthermore, NR2B levels are dramatically reduced in PD denervation models (a late-stage PD model), suggesting that loss of dopamine alters NDMAR composition (Picconi et al. 2012). In humans, the role of NMDA activity and glutamatergic transmission in PD remains to be determined. NMDAR antagonists have shown mixed results in treating PD-associated dementia (Aarsland et al. 2009; Emre et al. 2010), and limited success in treating both PD dyskinesias and Levodopa-induced dyskinesias (LIDs) (Greenamyre et al. 1991; Verhagen Metman et al. 1998; Johnson et al. 2009).
Nicotinic Acetylcholine Receptors
Neuronal nicotinic acetylcholine receptors (nAchRs) are in the cys-loop family of receptors that form pentameric structures, and are composed of two families of subunit isomers (α2-α7, α9, α10, and β2-β4; Dani 2015). Cholinergic projections are vastly distributed throughout the brain and as such are involved in a variety of processes, although they are often associated with attention and arousal. Ionotropic nAchRs are permeable to Na+, K+, and calcium, and will display differing cation conductances based on subunit composition. The α7 subunit expressing nAchRs have the highest calcium conductance of the nAchRs and are found in brain regions vulnerable in AD, including the hippocampus (Philie and Choremis 1997; Broide and Leslie 1999; Albuquerque et al. 2009; Cheng and Yakel 2015).
Interest in nAchRs as a neurodegenerative disease mechanism gained favor in the 1990s with an epidemiological study finding a relationship between long-term smokers and reduced incidence of PD, and suggested that cigarette smoking offered protection (Morens et al. 1995). The neuroprotective mechanisms are not completely clear, but nicotine remains under consideration as a therapeutic agent for PD. Long-term nicotine use paradoxically increases nAchRs expression, which may counter the reduced nAchRs levels observed in PD patients (Wonnacott 1990; Maggio et al. 1997; Quik et al. 2008; Mehta et al. 2012). In vitro studies demonstrated that nicotine treatment on cultured neurons prevents MPTP (a mitochondrial toxin that produces profound PD-like pathology in humans and animal models) from inducing apoptosis, further confirming that nAchR activation protects against otherwise devastating insults (Jeyarasasingam et al. 2002). The protective mechanism for nicotine on nigrostriatal neurons may be through a calcium-mediated signaling cascade initiated through α7-containing nAchRs. Here, their stimulation is coupled to activation of PI3K through calmodulin, and the subsequent up-regulation of antiapoptotic protein Bcl2. This pathway offers an intrinsic mechanism by which nAchR activation protects against neuronal loss in PD (Séguéla et al. 1993; Toulorge et al. 2011; Quik et al. 2015).
In AD, acetylcholinesterase inhibitors are used to treat early-to-moderate AD. Though unable to stop the progression of AD, they provide symptom support in the early stages of the disease. The “cholinergic hypothesis of AD” was born of research in the 1980s, when it was discovered that AD patients display an early loss of cholinergic neurons, reduced levels of AchR and nAchRs, and increased butyryl-cholinesterase (Bartus et al. 1982). Further work revealed that neurons expressing the α7 and α4 subunits (Nagele et al. 2002; D'Andrea and Nagele 2006) are uniquely vulnerable in AD, with those neurons being most highly associated with amyloid plaques (Wevers et al. 1999). Degeneration of cholinergic neurons in AD has been widely reported in the cortex, locus coeruleus, and cholinergic nucleus basalis (Lyness et al. 2003). Boosting acetylcholine levels delays the progression of neurodegeneration in AD through similar calcium-dependent mechanisms as proposed in PD (e.g., activation of α7 containing nAchRs), recruiting calcium-dependent cascades resulting in the up-regulation of antiapoptotic proteins such as Bcl2 and Bcl-xl (Robertson et al. 2000; Geerts et al. 2005; Karlnoski et al. 2007; Xu et al. 2019).
Voltage-Gated Calcium Channels (VGCCs)
VGCCs consist of two main subtypes: high-voltage activated (HVA) and low-voltage activated (LVA) channels based upon their activation threshold. As suggested by their name, HVAs, such as L-type VGCCs, activate quickly in response to large changes in membrane potential, such as action potentials, and display slower, calcium-dependent inactivation properties (Simms and Zamponi 2014). In contrast, LVAs, such as the T-type VGCCs are sensitive to subthreshold changes in membrane potential and exhibit rapid voltage-dependent inactivation. HVAs are composed of multiple subunits consisting of Cavα, Cavβ, and Cavδ, while LVAs are only composed of Cavα and β subunits. As the main determinant of calcium conductance of either HVAs or LVAs is the Cavα subunit, VGCCs are organized into three families, Cav1–3. Each family has specialized roles based upon their conductance and localization, and have diverse functions ranging from activating gene expression through neurotransmitter release. Their complexity underscores their role in regulating calcium signaling, and are therefore important in our discussion of calcium in neurodegeneration (Simms and Zamponi 2014).
In PD, it has been demonstrated that the pace-making activity of the SNpc neurons is vulnerable to VGCC-mediated calcium dysregulation. SNpc neurons are autonomous pace-makers (Guzman et al. 2009), and release dopamine through rhythmic depolarizations dependent on Cav1 family VGCCs. As SNpc neurons age, their pace-making activity becomes more dependent on Cav1.3, and is thought to contribute to their vulnerability. Dopaminergic SNpc neurons primarily depend on Cav1.2 for their pace-making activity early in life, and shift to Cav1.3 as they mature. Notably, PD patients have increased Cav1.3 expression relative to aged non-PD patients (Hurley et al. 2013). Upon blocking Cav1.2 and Cav1.3 with israpidine, a VGCC blocker in the dihydropyridine class, neurons are protected from both 6-OHDA- and MPTP-induced apoptosis, suggesting that the observed shift in Cav1.3 dependence in PD results in increased sensitivity to insults (Ilijic et al. 2011). To this end, new relatively selective Cav1.3 inhibitors are being explored as a potential therapeutic in PD (Zaichick et al. 2017; Surmeier 2018).
In AD, Aβ potentiates calcium influx through VGCCs, possibly through inducing or changing surface expression of Cav1.2 and Cav1.3, or directly interacting with the β3 subunits of L-type VGCCs to increase channel activity (Yang et al. 2009; Kim and Rhim 2011; Pourbadie et al. 2015). These observations were largely made from studies in cultured neurons in which exogenous application of Aβ increases calcium influx, and is inhibited by calcium channel inhibitors such as nimodipine (Pourbadie et al. 2015). However, in brain slice preparations from adult AD mouse models, there have been little or no observed changes in VGCC responses from either an electrophysiological or calcium signaling standpoint (Stutzmann et al. 2006; Goussakov et al. 2010; Chakroborty et al. 2012). The discrepancy may reflect the model systems used, and acute effects of supraphysiological levels of Aβ on cultured cells rather than a sustained pathogenic mechanism in AD brains. Furthermore, clinical trials and epidemiological studies searching for cognitive benefits in AD in patients taking L-type channel inhibitors for hypertension or cardiac conditions have not revealed any positive relationships (Lawlor et al. 2018); although there is evidence that amyloid levels may be reduced in patients taking VGCC inhibitors, they did not have a significantly reduced risk of AD or AD-related cognitive impairment (Yasar et al. 2005). Notably, the use of dihydropyridines was associated with a significant reduction in the risk of developing dementia in elderly hypertensive patients (Hussain et al. 2018). Thus, the dissociation between amyloid load and cognitive status in AD is suggested in these observations, with a different functional relationship existing between VGCC activity and memory retention in hypertension-related pathology.
Purinergic Receptors
Purinergic receptors are a family of plasma membrane localized channels and are widely distributed throughout most mammalian tissues. They are divided into three families: the P1 and P2Y, which are G-protein-coupled receptors (GPCRs), and the P2X, which are ligand-gated ionotropic channels permeable to calcium, K+, and Na+ (Takenouchi et al. 2010; Burnstock 2017, 2018). The seven subunit subtypes of the P2X receptors (P2X1-7) can form both heterotrimeric and homotrimeric receptors each sensitive to ATP. The distribution of receptor subtype is varied by cell type and region throughout the CNS, but the P2X2, P2X4, and P2X6 are most highly expressed in neurons, with evidence for both pre- and postsynaptic localization. Common to several neurodegenerative diseases are the overactivation of P2X receptors, leading to cell death by way of sustained membrane depolarization, mitochondrial stress, and/or ROS production. Interestingly, P2X receptors are present on microglia, where they have been implicated in neurodegeneration through inflammation.
Consistent with the variety and range of P2X receptors, their roles in pathology can be quite varied. In AD, elevated P2X7 levels in microglia have been measured in postmortem brain tissue from AD patients, and are thought to contribute to enhanced inflammatory responses in early AD (McLarnon et al. 2006; Sanz et al. 2009; Lee et al. 2011; Illes et al. 2019). Subsequent in vitro studies indicate that P2X7 receptor activation triggers the NLRP3 inflammasome on microglia, leading to the production of proinflammatory cytokines. This suggests that P2X7 receptors on microglia are critical in generating the innate immune response in AD. Where initially this response may be helpful in clearing debris from dying neurons, the chronic immune stimulation through continued neuronal death may result in unconstrained inflammation and ultimately be harmful. Microglia harvested from P2X7 KO mice stimulated with Aβ produced significantly less IL-1β compared to the wild-type (WT) controls, suggesting an interactive relationship with pathogenic peptide species (Sanz et al. 2009). Likewise, pharmacological inhibitors of P2X7 receptors, such as Brilliant Blue G (BBG), have demonstrated neuroprotective effects. Studies of primary hippocampal neurons suggest that BBG reduces Aβ-induced dendritic spine loss (Jana et al. 2016), and promotes protective mechanisms, including activation of α-secretase to reduce Aβ peptide production (Diaz-Hernandez et al. 2012) and resistance to apoptotic engagement (Kong et al. 2005; Woods et al. 2016). Reporter animals expressing EGFP-P2X7R crossed with the J20 AD model demonstrate that Aβ-induced neuroinflammation increases P2X7R expression on microglia throughout neuroinflammation, and reduces neuronal expression in early and advanced, but not late AD (Martínez-Frailes et al. 2019). In line with this discovery, P2XR7 genetic knockdown resulted in reduced plaque size, and improved behavior scores (Martin et al. 2019), suggesting P2XR7 as a potential therapeutic target. P2X4 has also been implicated in AD, with putative roles in unregulated calcium influx resulting in apoptosis (Varma et al. 2009).
In PD, P2X7 receptor signaling also contributes to ROS production and apoptotic engagement (Belarbi et al. 2017; Miras-Portugal et al. 2017; Munoz et al. 2017). In animal models of hemi-parkinsonism generated by 6-OHDA lesioning of the basal ganglia, P2X7 antagonists increase dopamine production, reduce rotational behavior, and suppress cell death (Carmo et al. 2014; Ferrazoli et al. 2017). P2X7 is not the only ATP receptor implicated in PD, as P2X1 receptor involvement has been reported as well. ATP was shown to induce lysosome dysfunction and α-synuclein aggregation in vitro, where P2X1 antagonism or genetic knockout improved lysosome function and reduced aggregation (Gan et al. 2015). Indeed, the purinergic receptors may provide a link between neurodegeneration and deleterious inflammatory processes.
CALHM1
The calcium homeostasis modulator 1 channel (CALHM1) is a recently identified voltage-gated channel that increases cytosolic calcium influx in response to decreases in extracellular calcium (Dreses-Werringloer et al. 2008; Ma et al. 2012). The channel is composed of six subunits, each containing four TMDs with an intracellular ATD and CTD (Siebert et al. 2013). Multiple cellular kinase signaling cascades are activated by CALHM1 activity in neurons (Dreses-Werringloer et al. 2013). The role of CALHM1 in the development of neurodegenerative disease is currently debated, with the controversy based around the discovery of polymorphisms in the genomes of AD patients. The frequency of a CALHM1 polymorphism (P86L) was initially shown to be increased in five independent SAD cohorts (Dreses-Werringloer et al. 2008). A competing study demonstrated no association between CALHM1 and the risk of AD in the analysis of more than 8100 subjects (Feher et al. 2011). However, studies comparing the WT protein and a P86L polymorphism in transfected cells have shown that the mutant resulted in increased levels of Aβ1-40 and Aβ1-42, indicating a role in the development of AD (Dreses-Werringloer et al. 2008). Additional studies of CALHM1 function have suggested roles in the production and metabolism of Aβ, as well as links of protein polymorphisms to early-onset AD (Koppel et al. 2011; Rubio-Moscardo et al. 2013; Vingtdeux et al. 2014).
INTRACELLULAR CALCIUM HOMEOSTASIS
Endoplasmic Reticulum
Several channels that regulate the influx and efflux of calcium between the ER and the cytosol (Berridge 1998) have been implicated in neurodegenerative disease. IP3Rs, RyRs, and sarcoplasmic reticulum (SR)-ER (calcium)-ATPase pumps (SERCA) are the main contributors to ER–cytosol homeostasis (Berridge 1998). Defects in stromal interaction molecule 2 (STIM2) proteins, which are part of the STIM-Orai complex that allows for refilling of ER calcium stores, have also been implicated (Pascual-Caro et al. 2018; Secondo et al. 2018).
IP3R
The IP3R is a tetrameric calcium channel found in the ER membrane (Maeda et al. 1991; Berridge 1995; Yamazaki and Mikoshiba 2009). The IP3R agonist, InsP3, is generated through activation of Gq- or certain tyrosine kinase-coupled receptors and subsequent hydrolysis of membrane-bound phosphatidylinositol 4,5-bisphosphate (Berridge 2005, 2009). There are three IP3Rs isoforms distributed throughout the body, particularly within muscle cells, secretory cells, and neurons, and their expression levels differ depending on location and developmental stage (Berridge 2009). Regulation of IP3Rs function is complex, involving numerous cofactors and binding proteins as well as calcium itself (Mak et al. 1998; Shilling et al. 2014).
Altered IP3R-mediated calcium signal was one of the first pathways implicated in AD and HD pathogenesis in both human cells and animal models (Etcheberrigaray et al. 1998; Tang et al. 2003; Stutzmann et al. 2004; Bezprozvanny 2011). In AD, early studies demonstrated that exogenous expression of mutant PS1 in oocyte models potentiated IP3R-calcium responses relative to WT PS1 (Leissring et al. 1999a,b). Just prior to this, human cells obtained from presymptomatic FAD patients demonstrated enhanced calcium responses to IP3-generating stimuli relative to age-matched non-AD cohorts and non-FAD family members (Etcheberrigaray et al. 1998). This phenomenon was again confirmed in PS1 knockin mice and other presymptomatic AD mouse models (Stutzmann et al. 2004, 2006; Goussakov et al. 2010), suggesting that the FAD mutations can drive calcium dyshomeostasis and emerges prior to other salient features of AD, such as protein aggregation and cognitive impairments. Following this discovery, further research found a variety of calcium signaling abnormalities in presenilin mutants (Tu et al. 2006; Nelson et al. 2007; Cheung et al. 2010; Kipanyula et al. 2012; Ryazantseva et al. 2018) and APP mutations (Pchitskaya et al. 2015). Complementary studies have demonstrated that reduction in IP3R expression can normalize exaggerated calcium responses, and restore hippocampal LTP in two AD mouse models (Shilling et al. 2014).
In HD, IP3Rs have been implicated in pathology. Using a yeast 2-hybrid screen, and later confirmed in a high-throughput unbiased screen, it was discovered that the mutant Htt protein is capable of forming a complex with IP3R1 and Huntington's associated protein 1 (HAP1) (Tang et al. 2003; Kaltenbach et al. 2007). Lipid bilayer reconstructions revealed that not only do these proteins interact, but their interaction promotes ER calcium release. mHtt dramatically increases the open probability of IP3R1, and this is further increased when HAP1 is present, supporting a direct role for IP3R regulation in HD pathology. This potentiation contributes to neuronal dysfunction and death through a variety of mechanisms, including caspase activation, calpain activation, and apoptotic engagement (Tang et al. 2005).
RyRs
RyRs are homotetramer proteins localized in the ER membrane of neurons, and with a total molecular mass of more than 2 MDa (each subunit is >550 kDa), it is one of the largest and most complex calcium channels (Inui et al. 1987; Lai et al. 1988; Meissner 2017). RyRs are found in many tissues and cell types, particularly in muscle, secretory cells, and the CNS; the three isoforms (RyR1–3) show tissue-type specificity, with RyR1 largely in skeletal muscle, RyR2 in cardiac muscle and CNS, and RyR3 predominantly in smooth muscle and the brain (Meissner 2017). The primary activation mechanism in neurons is via calcium-induced calcium release, with the evoked calcium signal regulating a broad range of downstream effects, the nature of which depends upon cellular localization, amount of calcium released, and temporal patterns of release (Hamilton and Serysheva 2009; Del Prete et al. 2014; Arias-Cavieres et al. 2018). The RyR channel properties are also tuned by several phosphorylation, nitrosylation, and oxidation sites on the channel, and, thus, alterations in cellular state can change the activation and inactivation properties of RyR-calcium release (Marx et al. 2000; Wehrens et al. 2006; Niggli et al. 2013; Lacampagne et al. 2017; Nikolaienko et al. 2018). Because RyRs are found in synaptic compartments as well as dendrites and soma, their actions on cellular physiology are broad and include modulation of synaptic plasticity, activation of kinase/phosphatase/enzymatic cascades, and mitochondrial and protein-handling responses, among others (McPherson et al. 1991; Sukhareva et al. 2002; Abu-Omar et al. 2018; Mustaly-Kalimi et al. 2018). Thus, the RyR is subject to multiple means of regulation and dysregulation, and is implicated in several neurodegenerative disease mechanisms.
Following the identification of potentiated IP3R-mediated calcium responses in AD models, it was discovered that increased RyR calcium signaling is evident prior to the emergence of histopathology and cognitive decline (Chan et al. 2000; Stutzmann et al. 2006, 2007). In AD, aberrant posttranslational modifications of the RyR have been implicated in the excessive release, including oxidation, phosphorylation, and nitrosylation on specific residues (SanMartín et al. 2017; More et al. 2018). In addition to these modifications, increased RyR2 expression within vulnerable brain regions such as the hippocampus and cortex have been identified in animal models and in human brains from AD patients (Chakroborty et al. 2009; Bruno et al. 2012). The resulting exaggerated RyR calcium responses in AD neurons are linked to impaired neurophysiology and synaptic signaling events, such as increased calcium-dependent K+ currents, decreased synaptic vesicle stores, and reduced postsynaptic spines, all of which may contribute to memory impairments (Stutzmann et al. 2006; Chakroborty et al. 2009, 2012; Briggs et al. 2017). Particularly notable in terms of synaptic pathology are the supraphysiological RyR-evoked calcium responses within dendritic spines and synaptic compartments in AD neurons, which appear to drive synaptic depression and morphological defects, and thus impede proper memory-encoding processes (Fifková et al. 1983; Chakroborty et al. 2009, 2012, 2019; Segal and Korkotian 2014). Also, RyR-mediated calcium release up-regulates secretases that increases APP cleavage, resulting in increased Aβ fragments and plaque load in AD (Oules et al. 2012). In parallel, calcium-regulated kinases that hyperphosphorylate tau also appear to be up-regulated by increased RyR-dependent calcium deregulation, resulting in increased phospho-tau pathology.
Recently, several studies independently have focused on the RyR as a therapeutic target for neurodegenerative disorders. In AD mouse models, subchronic treatment with negative allosteric modulators such as dantrolene, and CNS penetrant versions such as Ryanodex, have resulted in broad therapeutic outcomes. These include a reduction in amyloid and tau pathology, normalized intracellular calcium responses, restored synaptic structure and synaptic density, normalized synaptic plasticity, and enhanced behavioral performance on cognitive and memory tasks (Stutzmann et al. 2006; Chakroborty et al. 2012, 2019; Oules et al. 2012; Peng et al. 2012). Not only do these outcomes support a central and upstream role of dysregulated ER calcium signaling in AD pathogenesis, but also serves as proof of principle for therapeutic strategies. Dantrolene has also been shown to have beneficial effects in HD models, further suggesting a role of RyR-mediated calcium release in neurodegeneration (Chen et al. 2011)
Store-Operated Calcium Entry (SOCE)
The release of calcium from the ER is balanced by a complex refilling process through STIM mobilization and SERCA pumps. In the brain, two forms of STIM are expressed (STIM1 and STIM2), with a predominance of STIM1 in the cerebellum, and STIM2 in the hippocampus and cortex (Kraft, 2015; Secondo et al. 2018). Upon depletion of ER calcium stores, the low calcium concentration is detected by STIM, where it then aligns with the plasma membrane. Once in proximity to the plasma membrane, STIM triggers calcium-permeable Orai channels or transient receptor potential channels (TRPCs), allowing calcium entry and ER refilling through the SERCA pumps. This mechanism for maintaining ER calcium has been found to contribute to calcium dysregulation in neurodegeneration (Hogan and Rao 2015; Bollimuntha et al. 2017; Secondo et al. 2018). STIM2 overexpression in the AD models resulted in a restoration of spine morphology, strongly implicating SOCE in AD pathology (Sun et al. 2014; Popugaeva et al. 2015).
In AD, presenilin mutations leave neurons vulnerable to synaptic decay and dendritic spine loss, and targeting SOCE may help restore function. In AD mouse models, hippocampal neurons have altered spine morphology in addition to their physiological deficits. Additionally, synaptic SOCE is impaired in hippocampal neurons, resulting in decreases in calcium entry at the synapse, which inhibits critical signaling pathways for spine function.
SOCE is dysregulated in HD through mutant Htt (Wu et al. 2011, 2016; Vigont et al. 2015). In the YAC128 HD model, mutant Htt protein and HAP1 sensitize IP3Rs, leading to lowered ER calcium stores, and a near-continuous SOCE activation (Czeredys et al. 2018). This excess calcium influx contributes to MSN loss. Of note, shRNA silencing of HAP1 normalized SOCE currents presumably through normalization of STIM 2 expression, suggesting that targeting SOCE may be a viable strategy for treating HD.
Mitochondrial Calcium Handling
Mitochondria calcium handling oversees several complex roles in neurons, ranging from signal transduction to energy production. The electrochemical gradient produced by the electron transport chain (ETC) allows mitochondria to take up calcium from the cytosol through the mitochondrial calcium uniporter (MCU) complex, and exported through the Na+/calcium antiporter (Nicholls and Crompton 1980; De Stefani et al. 2011; Tarasov et al. 2012; Marchi and Pinton 2014). This ability allows mitochondria to synchronize calcium-signaling events in neurons with upticks in energetic demands. This process depends heavily on mitochondrial subcellular localization, and through mitochondrial-associated ER membranes (MAMs), the mitochondria receive calcium through specific microdomains associated with the ER, Golgi, and plasma membrane. This association is important, as it provides mechanistic links for defined processes such as exaggerated ER calcium release that results in mitochondrial damage, or the associated loss of mitochondrial calcium buffering at synapses in AD. When this process runs awry, mitochondrial calcium overload (MCO) results in decreased mitochondrial function, and eventual formation of the mitochondrial to permeability transition pore (mPTP), which initiates apoptosis. As apoptosis is a common mechanism of cell death in AD, PD, and HD, it is important to consider whether mitochondrial calcium dysregulation is unique to any one disease, or a consequence of neuronal stress. In AD, the case is clear that mitochondrial calcium dysregulation is a culprit in pathology (DiMauro and Schon 2008; Calì et al. 2012; Müller et al. 2018).
In several models, Aβ has been shown to induce cell death. This process may reflect increased ER calcium levels, leading to exaggerated calcium release and associated mitochondrial stress. This is consistent with several electron microscopy (EM) studies, suggesting that mitochondrial expression, shape, and turnover rates are negatively affected in AD, all of which could contribute to disturbed signaling and apoptotic engagement. More recent studies suggest a dual role for Aβ in both ROS generation and increases in ER calcium, which culminate in sensitizing RyRs on the ER, leading to MCO and further ROS production. This feedforward cycle may represent a means by which calcium dysregulation, in any form, ultimately leads to synaptic dysfunction and neuronal death (Hirai et al. 2001; Ferreiro et al. 2008; Wang et al. 2009; SanMartín et al. 2017).
In PD, the link to mitochondrial stress appears to be mediated through Cav1.3 activity, as described earlier. An additional mitochondrial connection to PD has also been inferred through the recreational use of synthetic heroin contaminated with MPTP, now known to be a mitochondrial complex I inhibitor. Recounted by Langston and originally published in 1983, he was confronted with a modern “medical mystery” when a patient in his 40s was presented with catatonic schizophrenia, with symptoms emerging overnight. Langston's observations fit with advanced PD, and through communication with the local hospitals and law enforcement uncovered several other patients with “rapid-onset severe PD” who had taken the contaminated drug. MPTP has since become a tool in PD modeling. Further research will reveal the full role of mitochondrial calcium dysregulation in PD (Furukawa et al. 2006; Danzer et al. 2007; Hettiarachchi et al. 2009; Calì et al. 2012; Langston 2017).
The linkage between HD and mitochondrial dysfunction has long been hypothesized through the observation that HD patients exhibit weight loss throughout the progression of the disease. Though the complete functions of the Htt protein is still debated, there is evidence to suggest that mHtt leads to mitochondrial calcium dysregulation, decreased threshold for mPTP, and apoptotic engagement. The mechanism by which this occurs is still debated, but emphasizes the link between calcium and degeneration in HD. This may also tie neatly into the observation that Htt potentiates IP3Rs responses, which, if the mitochondria are at a lower threshold for mPTP, could readily lead to MCO (Choo et al. 2004; Milakovic et al. 2006; Gellerich et al. 2008; Lim et al. 2008).
CONCLUSIONS
Calcium is a ubiquitous secondary messenger, which requires precise spatial and temporal regulation. While cells possess the means to maintain physiological homeostasis in the short term if calcium levels are perturbed, sustained dysregulation of cellular calcium homeostasis leads to a more global breakdown in cellular function and structure. In neurodegenerative diseases, vulnerable brain regions or specific neuronal subtypes exhibit unique patterns of calcium signaling alterations that are closely tied to the disease symptomology and cellular pathophysiology. Whether it is the memory-encoding hippocampal circuitry in AD, dopaminergic cell bodies in PD, or striatal neurons in HD, each of these devastating diseases exhibit early calcium signaling abnormalities that can initiate or sustain the pathological cascades that define the disease. It is hopeful to consider that targeting the respective sources of calcium dysregulation could serve as an effective therapeutic strategy, and provide a more complete understanding of disease etiology.
Footnotes
Editors: Geert Bultynck, Martin D. Bootman, Michael J. Berridge, and Grace E. Stutzmann
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
- Aarsland D, Ballard C, Walker Z, Bostrom F, Alves G, Kossakowski K, Leroi I, Pozo-Rodriguez F, Minthon L, Londos E. 2009. Memantine in patients with Parkinson's disease dementia or dementia with Lewy bodies: A double-blind, placebo-controlled, multicentre trial. Lancet Neurol 8: 613–618. 10.1016/S1474-4422(09)70146-2 [DOI] [PubMed] [Google Scholar]
- Abu-Omar N, Das J, Szeto V, Feng ZP. 2018. Neuronal ryanodine receptors in development and aging. Mol Neurobiol 55: 1183–1192. 10.1007/s12035-016-0375-4 [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EFR, Alkondon M, Rogers SW. 2009. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol Rev 89: 73–120 10.1152/physrev.00015.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer's Association. 2019. 2019 Alzheimer's Disease Facts and Figures. Special Report on Alzheimer's Detection in the Primary Care Setting: Connecting Patients and Physicians. https://www.alz.org/alzheimers-dementia/facts-figures
- Ambrosi G, Cerri S, Blandini F. 2014. A further update on the role of excitotoxicity in the pathogenesis of Parkinson's disease. J Neural Transm 121: 849–859. 10.1007/s00702-013-1149-z [DOI] [PubMed] [Google Scholar]
- Arias-Cavieres A, Barrientos GC, Sánchez G, Elgueta C, Muñoz P, Hidalgo C. 2018. Ryanodine receptor-mediated calcium release has a key role in hippocampal LTD induction. Front Cell Neurosci 12: 403 10.3389/fncel.2018.00403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe N, Rojas E, Pollard HB. 1993. Alzheimer disease amyloid β protein forms calcium channels in bilayer membranes: Blockade by tromethamine and aluminum. Proc Natl Acad Sci 90: 567–571. 10.1073/pnas.90.2.567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrasate M, Finkbeiner S. 2012. Protein aggregates in Huntington's disease. Exp Neurol 238: 1–11. 10.1016/j.expneuro.2011.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone P. 2009. Medline Abstract for Reference 45 of “Clinical Manifestations of Parkinson Disease.” UpToDate https://www.uptodate.com/contents/clinical-manifestations-of-parkinson-disease/abstract/45
- Bartus RT, Dean RL, Beer B, Lippa AS. 1982. The cholinergic hypothesis of geriatric memory dysfunction. Science 217: 408–414. 10.1126/science.7046051 [DOI] [PubMed] [Google Scholar]
- Bazzari FH, Abdallah DM, El-Abhar HS. 2019. Pharmacological interventions to attenuate Alzheimer's disease progression: The story so far. Curr Alzheimer Res 16: 261–277. 10.2174/1567205016666190301111120 [DOI] [PubMed] [Google Scholar]
- Belarbi K, Cuvelier E, Destée A, Gressier B, Chartier-Harlin MC. 2017. NADPH oxidases in Parkinson's disease: A systematic review. Mol Neurodegener 12: 84 10.1186/s13024-017-0225-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SM, Bartsch D. 2014. The role of L-type voltage-gated calcium channels Cav1.2 and Cav1.3 in normal and pathological brain function. Cell Tissue Res 357: 463–476. 10.1007/s00441-014-1936-3 [DOI] [PubMed] [Google Scholar]
- Berridge MJ. 1995. Calcium signalling and cell proliferation. Bioessays 17: 491–500. 10.1002/bies.950170605 [DOI] [PubMed] [Google Scholar]
- Berridge MJ. 1998. Neuronal calcium signaling review. Neuron 21: 13–26. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. 2005. Unlocking the secrets of cell signaling. Annu Rev Physiol 67: 1–21. 10.1146/annurev.physiol.67.040103.152647 [DOI] [PubMed] [Google Scholar]
- Berridge MJ. 2009. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta 1793: 933–940. 10.1016/j.bbamcr.2008.10.005 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. 2000. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21. 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I. 2009. Calcium signaling and neurodegenerative diseases. Trends Mol Med 15: 89–100. 10.1016/j.molmed.2009.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I. 2011. Role of inositol 1,4,5-trishosphate receptors in pathogenesis of Huntington's disease and spinocerebellar ataxias. Neurochem Res 36: 1186–1197. 10.1007/s11064-010-0393-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Mattson MP. 2008. Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease. Trends Neurosci 31: 454–463. 10.1016/j.tins.2008.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandini F, Nappi G, Greenamyre JT. 2001. Subthalamic infusion of an NMDA antagonist prevents basal ganglia metabolic changes and nigral degeneration in a rodent model of Parkinson's disease. Ann Neurol 49: 525–529. 10.1002/ana.104 [DOI] [PubMed] [Google Scholar]
- Bollimuntha S, Pani B, Singh BB. 2017. Neurological and motor disorders: Neuronal store-operated Ca2+ signaling: An overview and its function. Adv Exp Med Biol 993: 535–556. 10.1007/978-3-319-57732-6_27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD. 2012. Calcium signaling. Cold Spring Harb Perspect Biol 4: a011171 10.1101/cshperspect.a011171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Koutouzis TK, Sanberg PR. 1997. 3-Nitropropionic acid animal model and Huntington's disease. Neurosci Biobehav Rev 21: 289–293. 10.1016/S0149-7634(96)00027-9 [DOI] [PubMed] [Google Scholar]
- Bosch M, Castro J, Saneyoshi T, Matsuno H, Sur M, Hayashi Y. 2014. Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron 82: 444–459. 10.1016/j.neuron.2014.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs CA, Chakroborty S, Stutzmann GE. 2017. Emerging pathways driving early synaptic pathology in Alzheimer's disease. Biochem Biophys Res Commun 483: 988–997. 10.1016/j.bbrc.2016.09.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broide RS, Leslie FM. 1999. The α7 nicotinic acetylcholine receptor in neuronal plasticity. Mol Neurobiol 20: 1–16. 10.1007/BF02741361 [DOI] [PubMed] [Google Scholar]
- Brooks SP, Dunnett SB. 2015. Mouse models of Huntington's disease. Curr Top Behav Neurosci 22: 101–133. 10.1007/7854_2013_256 [DOI] [PubMed] [Google Scholar]
- Bruno A, Huang J, Bennett DA, Marr R, Hastings ML, Stutzmann GE. 2012. Altered ryanodine receptor expression in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging 33: 1001.e1–1001.e6. 10.1016/j.neurobiolaging.2011.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone S, Kunerth S, Zocchi E, De Flora A, Guse AH. 2003. Spatio-temporal propagation of Ca2+ signals by cyclic ADP-ribose in 3T3 cells stimulated via purinergic P2Y receptors. J Cell Biol 163: 837–845. 10.1083/jcb.200307016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. 2017. Purinergic signalling: Therapeutic developments. Front Pharmacol 8: 661 10.3389/fphar.2017.00661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. 2018. Purine and purinergic receptors. Brain Neurosci Adv 2 10.1177/2398212818817494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calì T, Ottolini D, Brini M. 2012. Mitochondrial Ca2+ and neurodegeneration. Cell Calcium 52: 73–85. 10.1016/j.ceca.2012.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo MR, Menezes AP, Nunes AC, Pliássova A, Rolo AP, Palmeira CM, Cunha RA, Canas PM, Andrade GM. 2014. The P2X7 receptor antagonist Brilliant Blue G attenuates contralateral rotations in a rat model of Parkinsonism through a combined control of synaptotoxicity, neurotoxicity and gliosis. Neuropharmacology 81: 142–152. 10.1016/j.neuropharm.2014.01.045 [DOI] [PubMed] [Google Scholar]
- Carter DB. 2005. The interaction of amyloid-β with APoE. Subcell Biochem 38: 255–272. 10.1007/0-387-23226-5_13 [DOI] [PubMed] [Google Scholar]
- Cavara NA, Hollmann M. 2008. Shuffling the deck anew: How NR3 tweaks NMDA receptor function. Mol Neurobiol 38: 16–26. 10.1007/s12035-008-8029-9 [DOI] [PubMed] [Google Scholar]
- Caviston JP, Ross JL, Antony SM, Tokito M, Holzbaur ELF. 2007. Huntingtin facilitates dynein/dynactin-mediated vesicle transport. Proc Natl Acad Sci 104: 10045–10050. 10.1073/pnas.0610628104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Ariano MA, Calvert CR, Flores-Hernández J, Chandler SH, Leavitt BR, Hayden MR, Levine MS. 2001. NMDA receptor function in mouse models of Huntington disease. J Neurosci Res 66: 525–539. 10.1002/jnr.1244 [DOI] [PubMed] [Google Scholar]
- Chakroborty S, Stutzmann GE. 2011. Early calcium dysregulation in Alzheimer's disease: Setting the stage for synaptic dysfunction. Sci China Life Sci 54: 752–762. 10.1007/s11427-011-4205-7 [DOI] [PubMed] [Google Scholar]
- Chakroborty S, Goussakov I, Miller MB, Stutzmann GE. 2009. Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3xTg-AD mice. J Neurosci 29: 9458–9470. 10.1523/jneurosci.2047-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakroborty S, Briggs C, Miller MB, Goussakov I, Schneider C, Kim J, Wicks J, Richardson JC, Conklin V, Cameransi BG, et al. 2012a. Stabilizing ER Ca2+ channel function as an early preventative strategy for Alzheimer's disease. PLoS ONE 7: e52056 10.1371/journal.pone.0052056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakroborty S, Kim J, Schneider C, Jacobson C, Molgó J, Stutzmann GE. 2012b. Early presynaptic and postsynaptic calcium signaling abnormalities mask underlying synaptic depression in presymptomatic Alzheimer's disease mice. J Neurosci 32: 8341–8353. 10.1523/jneurosci.0936-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakroborty S, Hill ES, Christian DT, Helfrich R, Riley S, Schneider C, Kapecki N, Mustaly-Kalimi S, Seiler FA, Peterson DA, et al. 2019. Reduced presynaptic vesicle stores mediate cellular and network plasticity defects in an early-stage mouse model of Alzheimer's disease. Mol Neurodegen 14: 7 10.1186/s13024-019-0307-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chami M, Checler F. 2014. Ryanodine receptors: DUAL contribution to Alzheimer disease? Channels (Austin) 8: 168 10.4161/chan.29000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP. 2000. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol Chem 275: 18195–18200. [DOI] [PubMed] [Google Scholar]
- Chen X, Wu J, Lvovskaya S, Herndon E, Supnet C, Bezprozvanny I. 2011. Dantrolene is neuroprotective in Huntington's disease transgenic mouse model. Mol Neurodegener 6: 81 10.1186/1750-1326-6-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Yakel JL. 2015. The effect of α7 nicotinic receptor activation on glutamatergic transmission in the hippocampus. Biochem Pharmacol 97: 439–444. 10.1016/j.bcp.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K, Mei L, Mak DD, Hayashi I, Iwatsubo T, Kang DE, Foskett JK. 2010. Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer's disease-linked presenilin mutants in human cells and mouse neurons. Sci Signal 3: ra22 10.1126/scisignal.2000818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo YS, Johnson GVW, MacDonald M, Detloff PJ, Lesort M. 2004. Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Hum Mol Genet 13: 1407–1420. 10.1093/hmg/ddh162 [DOI] [PubMed] [Google Scholar]
- Chou K. 2019. Clinical manifestations of Parkinson disease. UpToDate https://www.uptodate.com/contents/clinical-manifestations-of-parkinson-disease
- Clapham DE. 2007. Calcium signaling. Cell 131: 1047–1058. 10.1016/j.cell.2007.11.028 [DOI] [PubMed] [Google Scholar]
- Czeredys M, Gruszczynska-Biegala J, Schacht T, Methner A, Kuznicki J, Hasan G, Muma NA. 2013. Expression of genes encoding the calcium signalosome in cellular and transgenic models of Huntington's disease. Front Mol Neurosci 6: 42 10.3389/fnmol.2013.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeredys M, Vigont VA, Boeva VA, Mikoshiba K, Kaznacheyeva EV, Kuznicki J. 2018. Huntingtin-associated protein 1A regulates store-operated calcium entry in medium spiny neurons from transgenic YAC128 mice, a model of Huntington's disease. Front Cell Neurosci 12: 381 10.3389/fncel.2018.00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea MR, Nagele RG. 2006. Targeting the α7 nicotinic acetylcholine receptor to reduce amyloid accumulation in Alzheimer's disease pyramidal neurons. Curr Pharm Des 12: 677–684. 10.2174/138161206775474224 [DOI] [PubMed] [Google Scholar]
- Dani JA. 2015. Neuronal nicotinic acetylcholine receptor structure and function and response to nicotine. Int Rev Neurobiol 124: 3–19. 10.1016/bs.irn.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danysz W, Parsons CG. 2003. The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer's disease: Preclinical evidence. Int J Geriatr Psychiatry 18(Suppl 1): S23–S32. 10.1002/gps.938 [DOI] [PubMed] [Google Scholar]
- Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M. 2007. Different species of α-synuclein oligomers induce calcium influx and seeding. J Neurosci 27: 9220–9232. 10.1523/jneurosci.2617-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelice LJ, Goswami T. 2007. Transporters as channels. Annu Rev Physiol 69: 87–112. 10.1146/annurev.physiol.69.031905.164816 [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. 1990. Synapse loss in frontal cortex biopsies in Alzheimer's disease: Correlation with cognitive severity. Ann Neurol 27: 457–464. 10.1002/ana.410270502 [DOI] [PubMed] [Google Scholar]
- Del Prete D, Checler F, Chami M. 2014. Ryanodine receptors: Physiological function and deregulation in Alzheimer disease. Mol Neurodegener 9: 21 10.1186/1750-1326-9-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuro A, Smith M, Parker I. 2011. Single-channel Ca2+ imaging implicates Aβ1-42 amyloid pores in Alzheimer's disease pathology. J Cell Biol 195: 515 10.1083/jcb201104133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. 2011. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476: 336–340. 10.1038/nature10230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Hernandez J, Gomez-Villafuertes R, León-Otegui M, Hontecillas-Prieto L, Del Puerto A, Trejo JL, Lucas JJ, Garrido JJ, Gualix J, Miras-Portugal MT, et al. 2012. In vivo P2X7 inhibition reduces amyloid plaques in Alzheimer's disease through GSK3β and secretases. Neurobiol Aging 33: 1816–1828. 10.1016/j.neurobiolaging.2011.09.040 [DOI] [PubMed] [Google Scholar]
- DiMauro S, Schon EA. 2008. Mitochondrial disorders in the nervous system. Annu Rev Neurosci 31: 91–123. 10.1146/annurev.neuro.30.051606.094302 [DOI] [PubMed] [Google Scholar]
- Dong X, Wang Y, Qin Z. 2009. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin 30: 379–387. 10.1038/aps.2009.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragatsis I, Levine MS, Zeitlin S. 2000. Inactivation of Hdh in the brain and testis results in progressive neurodegeneration and sterility in mice. Nat Genet 26: 300–306. 10.1038/81593 [DOI] [PubMed] [Google Scholar]
- Dragicevic E, Schiemann J, Liss B. 2015. Dopamine midbrain neurons in health and Parkinson's disease: Emerging roles of voltage-gated calcium channels and ATP-sensitive potassium channels. Neuroscience 284: 798–814. 10.1016/j.neuroscience.2014.10.037 [DOI] [PubMed] [Google Scholar]
- Dreses-Werringloer U, Lambert JC, Vingdeux V, Zhao H, Vais H, Siebert A, Jain A, Koppel J, Rovelet-Lecrux A, Hannequin D, et al. 2008. A polymorphism in CALHM1 influences Ca2+ homeostasis, Aβ levels, and Alzheimer's disease risk. Cell 133: 1149–1161. 10.1016/j.cell.2008.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreses-Werringloer U, Vingtdeux V, Zhao H, Chandakkar P, Davies P, Marambaud P. 2013. CALHM1 controls the Ca2+-dependent MEK, ERK, RSK and MSK signaling cascade in neurons. J Cell Sci 126: 1199–1206. 10.1242/jcs.117135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyao MP, Auerach AB, Ryan A, Persichetti F, Barnes GT, McNeil SM, Ge P, Vonsattel JP, Gusessa JF, Joyner AL, et al. 1995. Inactivation of the mouse Huntington's disease gene homolog Hdh. Science 269: 407–410. 10.1126/science.7618107 [DOI] [PubMed] [Google Scholar]
- Emre M, Tsolaki M, Bonuccelli U, Destée A, Tolosa E, Kutzelnigg A, Ceballas-Baumann A, Zdravkovic S, Bladström A, Jones R, et al. 2010. Memantine for patients with Parkinson's disease dementia or dementia with Lewy bodies: A randomised, double-blind, placebo-controlled trial. Lancet Neurol 9: 969–977. 10.1016/S1474-4422(10)70194-0 [DOI] [PubMed] [Google Scholar]
- Etcheberrigaray R, Hirashima N, Nee L, Prince J, Govani S, Racchi M, Tanzi RE, Alkon DL. 1998. Calcium responses in fibroblasts from asymptomatic members of Alzheimer's disease families. Neurobiol Dis 5: 37–45. 10.1006/nbdi.1998.0176 [DOI] [PubMed] [Google Scholar]
- Fehér A, Juhász A, Rimanóczy A, Pákáski M, Kálmán J, Janka Z. 2011. No association between CALHM1 polymorphism and Alzheimer's disease risk in a Hungarian population. Psychiatr Genet 21: 249–252. 10.1097/YPG.0b013e3283457bcc [DOI] [PubMed] [Google Scholar]
- Fernandes HB, Raymond LA. 2009. NMDA receptors and Huntington's disease. In Biology of the NMDA receptor (ed. Van Dongen AM), Chap. 2. CRC, Boca Raton, FL. [Google Scholar]
- Ferrazoli EG, De Souza HDN, Nascimento IC, Oliveira-Giacomelli Á, Schwindt TT, Britto LR, Ulrich H. 2017. Brilliant Blue G, but not Fenofibrate, treatment reverts hemiparkinsonian behavior and restores dopamine levels in an animal model of Parkinson's disease. Cell Transplant 26: 669–677. 10.3727/096368917X695227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreiro E, Oliveira CR, Pereira CMF. 2008. The release of calcium from the endoplasmic reticulum induced by amyloid-β and prion peptides activates the mitochondrial apoptotic pathway. Neurobiol Dis 30: 331–342. 10.1016/j.nbd.2008.02.003 [DOI] [PubMed] [Google Scholar]
- Fifková E, Markham JA, Delay RJ. 1983. Calcium in the spine apparatus of dendritic spines in the dentate molecular layer. Brain Res 266: 163–168. 10.1016/0006-8993(83)91322-7 [DOI] [PubMed] [Google Scholar]
- Foster TC. 2007. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell 6: 319–325. 10.1111/j.1474-9726.2007.00283.x [DOI] [PubMed] [Google Scholar]
- Furukawa K, Matsuzaki-Kobayashi M, Hasegawa T, Kikuchi A, Sugeno N, Itoyama Y, Wang Y, Yao PJ, Bushlin I, Takeda A. 2006. Plasma membrane ion permeability induced by mutant α-synuclein contributes to the degeneration of neural cells. J Neurochem 97: 1071–1077. 10.1111/j.1471-4159.2006.03803.x [DOI] [PubMed] [Google Scholar]
- Gan M, Moussaud S, Jiang P, McLean PJ. 2015. Extracellular ATP induces intracellular α-synuclein accumulation via P2X1 receptor-mediated lysosomal dysfunction. Neurobiol Aging 36: 1209–1220. 10.1016/j.neurobiolaging.2014.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts H, Trojanowski JQ, Lee VM. 2005. Drug discovery in neurodegenerative diseases. Sci Aging Knowledge Environ 2005: pe4 10.1126/sageke.2005.6.pe4 [DOI] [PubMed] [Google Scholar]
- Gellerich FN, Gizatullina Z, Nguyen HP, Trumbeckaite S, Vielhaber S, Seppet E, Zierz S, Landwehreyer B, Riess O, von Horsten S, et al. 2008. Impaired regulation of brain mitochondria by extramitochondrial Ca2+ in transgenic Huntington disease rats. J Biol Chem 283: 30715–30724. 10.1074/jbc.M709555200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomello M, Oliveros JC, Naranjo JR, Carafoli E. 2013. Neuronal Ca2+ dyshomeostasis in Huntington disease. Prion 7: 76–84. 10.4161/pri.23581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goussakov I, Miller MB, Stutzmann GE. 2010. NMDA-mediated Ca2+ influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer's disease mice. J Neurosci 30: 12128–12137. 10.1523/jneurosci.24747-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goussakov I, Chakroborty S, Stutzmann GE. 2011. Generation of dendritic Ca2+ oscillations as a consequence of altered ryanodine receptor function in AD neurons. Channels 5: 9–13. 10.4161/chan.5.1.14124 [DOI] [PubMed] [Google Scholar]
- Greenamyre JT, O'Brien CF. 1991. N-methyl-d-aspartate antagonists in the treatment of Parkinson's disease. Arch Neurol 48: 977–981. 10.1001/archneur.1991.00530210109030 [DOI] [PubMed] [Google Scholar]
- Guzman JN, Sánchez-Padilla J, Chan CS, Surmeier DJ, James D. 2009. Robust pacemaking in substantia nigra dopaminergic neurons. J Neurosci 29: 11011–11019. 10.1523/jneurosci.2519-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SL, Serysheva II. 2009. Ryanodine receptor structure: Progress and challenges. J Biol Chem 284: 4047–4051. 10.1074/jbc.R800054200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. 1992. Alzheimer's disease: The amyloid cascade hypothesis. Science 256: 184–185. 10.1126/science.1566067 [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. 2002. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science 297: 353–356. 10.1126/science.1072994 [DOI] [PubMed] [Google Scholar]
- Harjes P, Wanker EE. 2003. The hunt for huntingtin function: Interaction partners tell many different stories. Trends Biochem Sci 28: 425–433. 10.1016/S0968-0004(03)00168-3 [DOI] [PubMed] [Google Scholar]
- Hefter D, Draguhn A. 2017. APP as a protective factor in acute neuronal insults. Front Mol Neurosci 10: 22 10.3389/fnmol.2017.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson AM, Roberts AC, Pérez-Otaño I, Philpot BD. 2010. Influence of the NR3A subunit on NMDA receptor functions. Prog Neurobiol 91: 23–37. 10.1016/j.pneurobio.2010.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettiarachchi NT, Parker A, Dallas ML, Pennington K, Hung CC, Pearson HA, Boyle JP, Robinson P, Peers C. 2009. α-Synuclein modulation of Ca2+ signaling in human neuroblastoma (SH-SY5Y) cells. J Neurochem 111: 1192–1201. 10.1111/j.1471-4159.2009.06411.x [DOI] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, et al. 2001. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci 21: 3017–3023. 10.1523/jneurosci.21-09-03017.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan PG, Rao A. 2015. Store-operated calcium entry: Mechanisms and modulation. Biochem Biophys Res Commun 460: 40–49. 10.1016/j.bbrc.2015.02.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Herz J, Bu G. 2012. Apolipoprotein E and apolipoprotein E receptors: Normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med 2: a006312 10.1101/cshperspect.a006312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington G. 1872. On chorea. Med Surg Rep 26: 317–321. [Google Scholar]
- Hurley MJ, Brandon B, Gentleman SM, Dexter DT. 2013. Parkinson's disease is associated with altered expression of CaV1 channels and calcium-binding proteins. Brain 136: 2077–2097. 10.1093/brain/awt134 [DOI] [PubMed] [Google Scholar]
- Hussain S, Singh A, Rahman SO, Habib A, Najmi AK. 2018. Calcium channel blocker use reduces incident dementia risk in elderly hypertensive patients: A meta-analysis of prospective studies. Neurosci Lett 671: 120–127. 10.1016/j.neulet.2018.02.027 [DOI] [PubMed] [Google Scholar]
- Ilijic E, Guzman JN, Surmeier DJ. 2011. The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson's disease. Neurobiol Dis 43: 364–371. 10.1016/j.nbd.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes P, Rubini P, Huang L, Tang Y. 2019. The P2X7 receptor: A new therapeutic target in Alzheimer's disease. Expert Opin Ther Targets 23: 165–176. 10.1080/14728222.2019.1575811 [DOI] [PubMed] [Google Scholar]
- Inui M, Saito A, Fleischer S. 1987. Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. J Biol Chem 262: 1740–1747. [PubMed] [Google Scholar]
- Jana MK, Cappai R, Ciccotosto GD. 2016. Oligomeric amyloid-β toxicity can be inhibited by blocking its cellular binding in cortical neuronal cultures with addition of the triphenylmethane dye brilliant blue G. ACS Chem Neurosci 7: 1141–1147. 10.1021/acschemneuro.6b00108 [DOI] [PubMed] [Google Scholar]
- Jeyarasasingam G, Tompkins L, Quik M. 2002. Stimulation of non-α7 nicotinic receptors partially protects dopaminergic neurons from 1-methyl-4-phenylpyridinium-induced toxicity in culture. Neuroscience 109: 275–285. 10.1016/S0306-4522(01)00488-2 [DOI] [PubMed] [Google Scholar]
- Johnson KA, Conn PJ, Niswender CM. 2009. Glutamate receptors as therapeutic targets for Parkinsons disease. CNS Neurol Disord Drug Targets 8: 475–491. 10.2174/187152709789824606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach LS, Romero E, Becklin RR, Chettier R, Bell R, Phansalkar A, Strand A, Torcassi C, Savage J, Hurlburt A, et al. 2007. Huntingtin interacting proteins are genetic modifiers of neurodegeneration. PLoS Genet 3: e82 10.1371/journal.pgen.0030082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kametani F, Hasegawa M. 2018. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer's disease. Front Neurosci 12: 25 10.3389/fnins.2018.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Cooper G, Dunne SF, Luan CH, James Surmeier D, Silverman RB. 2013. Antagonism of L-type Ca2+ channels CaV1.3 and CaV1.2 by 1,4-dihydropyrimidines and 4H-pyrans as dihydropyridine mimics. Bioorg Med Chem 21: 4365–4373. 10.1016/j.bmc.2013.04.054 [DOI] [PubMed] [Google Scholar]
- Karlnoski R, Wilcock D, Dickey C, Ronan V, Gordon MN, Zhang W, Morgan D, Taglialatela G. 2007. Up-regulation of Bcl-2 in APP transgenic mice is associated with neuroprotection. Neurobiol Dis 25: 179–188. 10.1016/j.nbd.2006.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto EM, Vivar C, Camandola S. 2012. Physiology and pathology of calcium signaling in the brain. Front Pharmacol 3: 61 10.3389/fphar.2012.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keum JW, Shin A, Gillis T, Mysore JS, Elneel KA, Lucente D, Hadzi T, Homans P, Jones L, Orth M, et al. 2016. The HTT CAG-expansion mutation determines age at death but not disease duration in Huntington disease. Am J Hum Genet 98: 287–298. doi:10.1016/j.ajhg.2015.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachaturian ZS. 1987. Hypothesis on the regulation of cytosol calcium concentration and the aging brain. Neurobiol Aging 8: 345–346. 10.1016/0197-4580(87)90073-X [DOI] [PubMed] [Google Scholar]
- Kim S, Rhim H. 2011. Effects of amyloid-β peptides on voltage-gated l-type Cav1.2 and Cav1.3 Ca2+ channels. Mol Cells 32: 289–294. 10.1007/s10059-011-0075-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. 2009. The role of apolipoprotein E in Alzheimer's disease. Neuron 63: 287–303. 10.1016/j.neuron.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipanyula MJ, Contreras L, Zampese E, Lazzari C, Wong AK, Pizzo P, Fasolato C, Pozzan T. 2012. Ca2+ dysregulation in neurons from transgenic mice expressing mutant presenilin 2. Aging Cell 11: 885–893. 10.1111/j.1474-9726.2012.00858.x [DOI] [PubMed] [Google Scholar]
- Kishi T, Matsunaga S, Oya K, Nomura I, Ikuta T, Iwata N. 2017. Memantine for Alzheimer's disease: An updated systematic review and meta-analysis. J Alz Dis 60: 401–425. 10.3233/JAD-170424 [DOI] [PubMed] [Google Scholar]
- Kong Q, Wang M, Liao Z, Camden JM, Yu S, Simonyi A, Weisman GA. 2005. P2X7 nucleotide receptors mediate caspase-8/9/3-dependent apoptosis in rat primary cortical neurons. Purinergic Signal 1: 337–347. 10.1007/s11302-005-7145-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel J, Campagne F, Vingtdeux V, Dreses-Werringloer U, Ewers M, Rujescu D, Hampel H, Gordon ML, Christen E, Chapuis J, et al. 2011. CALHM1 P86L polymorphism modulates CSF Aβ levels in cognitively healthy individuals at risk for Alzheimer's disease. Mol Med 17: 974–979. 10.2119/molmed.2011.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft R. 2015. STIM and ORAI proteins in the nervous system. Channels 9: 245–252. 10.1080/19336950.2015.1071747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacampagne A, Liu X, Reiken S, Bussiere R, Meli AC, Lauritzen I, Teich AF, Zalk R, Saint N, Arancio O, et al. 2017. Post-translational remodeling of ryanodine receptor induces calcium leak leading to Alzheimer's disease-like pathologies and cognitive deficits. Acta Neuropathol 134: 749–767. 10.1007/s00401-017-1733-7 [DOI] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, et al. 2004. Synaptic targeting by Alzheimer's-related amyloid β oligomers. J Neurosci 24: 10191–10200. 10.1523/jneurosci.3432-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla FM. 2002. Calcium dyshomeostasis and intracellular signalling in alzheimer's disease. Nat Rev Neurosci 3: 862–872. 10.1038/nrn960 [DOI] [PubMed] [Google Scholar]
- Lai FA, Anderson K, Rousseau E, Liu QY, Meissner G. 1988. Evidence for a Ca2+ channel within the ryanodine receptor complex from cardiac sarcoplasmic reticulum. Biochem Biophys Res Commun 151: 441–449. 10.1016/0006-291X(88)90613-4 [DOI] [PubMed] [Google Scholar]
- Langbehn DR, Hayden M, Paulsen J. 2010. CAG-repeat length and the age of onset in Huntington disease: A review and validation study of statistical approaches. Am J Med Genet B: Neuropsychiatr Genet 153b: 397–408. 10.1002/amjg.b.30992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston JW. 2017. The MPTP story. J Parkinsons Dis 7: S11–S19. 10.3233/JPD-179006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanska DJ. 2000. George Huntington (1850–1916) and hereditary chorea. J Hist Neurosci 9: 76–89. 10.1076/0964-704X(200004)9:1;1-2;FT076 [DOI] [PubMed] [Google Scholar]
- Lassmann H, Fischer P, Jellinger K. 1993. Synaptic pathology of Alzheimer's disease. Ann NY Acad Sci 695: 59–64. 10.1111/j.1749-6632.1993.tb23028.x [DOI] [PubMed] [Google Scholar]
- Lawlor B, Segurado R, Kennelly S, Olde Rikkert MGM, Howard R, Pasquier F, Borjesson-Hanson A, Tsolaki M, Lucca U, Molloy DW, et al. 2018. Nilvadipine in mild to moderate Alzheimer disease: A randomised controlled trial. PLoS Med 15: e1002660 10.1371/journal.pmed.1002660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HG, Won SM, Gwag BJ, Lee YB. 2011. Microglial P2X₇ receptor expression is accompanied by neuronal damage in the cerebral cortex of the APPswe/PS1dE9 mouse model of Alzheimer's disease. Exp Mol Med 43: 7–14. 10.3858/emm.2011.43.1.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leissring MA, Parker I, LaFerla FM. 1999a. Presenilin-2 mutations modulate amplitude and kinetics of inositol 1,4,5-trisphosphate-mediated calcium signals. J Biol Chem 274: 32535–32538. 10.1074/jbc.274.46.32535 [DOI] [PubMed] [Google Scholar]
- Leissring MA, Paul BA, Parker I, Cotman CW, Laferla FM. 1999b. Alzheimer's presenilin-1 mutation potentiates inositol 1,4,5-trisphosphate-mediated calcium signaling in Xenopus oocytes. J Neurochem 72: 1061–1068. 10.1046/j.1471-4159.1999.0721061.x [DOI] [PubMed] [Google Scholar]
- Leissring MA, Yamasaki TR, Wasco W, Buxbaum JD, Parker I, LaFerla FM. 2000. Calsenilin reverses presenilin-mediated enhancement of calcium signaling. Proc Natl Acad Sci 97: 8590–8593. 10.1073/pnas.97.15.8590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MS, Cepeda C, André VM. 2010. Location, location, location: Contrasting roles of synaptic and extrasynaptic NMDA receptors in Huntington's disease. Neuron 65: 145–147. 10.1016/j.neuron.2010.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SH, Li XJ. 2004. Huntingtin–protein interactions and the pathogenesis of Huntington's disease. Trends Genet 20: 146–154. 10.1016/j.tig.2004.01.008 [DOI] [PubMed] [Google Scholar]
- Li L, Fan M, Icton CD, Chen N, Leavitt BR, Hayden MR, Murphy TH, Raymond LA. 2003. Role of NR2B-type NMDA receptors in selective neurodegeneration in Huntington disease. Neurobiol Aging 24: 1113–1121. 10.1016/j.neurobiolaging.2003.04.003 [DOI] [PubMed] [Google Scholar]
- Li JY, Popovic N, Brundin P. 2005. The use of the R6 transgenic mouse models of Huntington's disease in attempts to develop novel therapeutic strategies. NeuroRx 2: 447–464. 10.1602/neurorx.2.3.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D, Fedrizzi L, Tartari M, Zuccato C, Cattaneo E, Brini M, Carafoli E. 2008. Calcium homeostasis and mitochondrial dysfunction in striatal neurons of Huntington disease. J Biol Chem 283: 5780–5789. 10.1074/jbc.M704704200 [DOI] [PubMed] [Google Scholar]
- Lipton SA. 2004. Failures and successes of NMDA receptor antagonists: Molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRX 1: 101–110. 10.1602/neurorx.1.1.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams-Gray CH, et al. 2012. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement disorder society task force guidelines. Mov Disord 27: 349–356. 10.1002/mds.24893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. 2013. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat Rev Neurol 9: 106–118. 10.1038/nrneurol.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyness SA, Zarow C, Chui HC. 2003. Neuron loss in key cholinergic and aminergic nuclei in Alzheimer disease: A meta-analysis. Neurobiol Aging 24: 1–23. 10.1016/S0197-4580(02)00057-X [DOI] [PubMed] [Google Scholar]
- MacDonald ME, Ambrose CM, Duyao MP, Myers RH, Lakshmi-Srinidhi CL, Barnes G, Taylor SA, James M, Groot N, MacFarlane H, et al. 1993. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72: 971–983. 10.1016/0092-8674(93)90585-E [DOI] [PubMed] [Google Scholar]
- Maeda N, Kawasaki T, Nakade S, Yokota N, Taguchi T, Kasai M, Mikoshiba K. 1991. Structural and functional characterization of inositol 1,4,5-trisphosphate receptor channel from mouse cerebellum. J Biol Chem 266: 1109–1116. [PubMed] [Google Scholar]
- Maggio R, Riva M, Vaglini F, Fornai F, Racagni G, Corsini GU. 1997. Striatal increase of neurotrophic factors as a mechanism of nicotine protection in experimental parkinsonism. J Neural Transm (Vienna) 104: 1113–1123. 10.1007/BF01273324 [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y, Weisgraber KH. 2007. Detrimental effects of apolipoprotein E4: potential therapeutic targets in Alzheimers disease. Curr Alzheimer Res 4: 537–540. 10.2174/156720507783018334 [DOI] [PubMed] [Google Scholar]
- Mak DOD, McBride S, Foskett JK. 1998. Inositol 1,4,5-tris-phosphate activation of inositol tris-phosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc Natl Acad Sci 95: 15821–15825 10.1073/pnas.95.26.15821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin S. 2018. The amyloid hypothesis on trial. Nature 559: S4–S7. 10.1038/d41586-018-05719-4 [DOI] [PubMed] [Google Scholar]
- Marchi S, Pinton P. 2014. The mitochondrial calcium uniporter complex: Molecular components, structure and physiopathological implications. J Physiol 592: 829–839. 10.1113/jphysiol.2013.268235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux L, Scheller RH. 1991. The rat brain synucleins; family of proteins transiently associated with neuronal membrane. Brain Res Mol Brain Res 11: 335–343. 10.1016/0169-328X(91)90043-W [DOI] [PubMed] [Google Scholar]
- Maroteaux L, Campanelli JT, Scheller RH. 1988. Synuclein: A neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci 8: 2804–2815. 10.1523/jneurosci.08-08-02804.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E, Amar M, Dalle C, Youssef I, Boucher C, Le Duigou C, Bruckner M, Prigent A, Sazdovitch V, Halle A, et al. 2019. New role of P2X7 receptor in an Alzheimer's disease mouse model. Mol Psychiatry 24: 108–125. 10.1038/s41380-018-0108-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Frailes C, Di Lauro C, Bianchi C, de Diego-García L, Sebastián-Serrano Á, Boscá L, Díaz-Hernández M. 2019. Amyloid peptide induced neuroinflammation increases the P2X7 receptor expression in microglial cells, impacting on its functionality. Front Cell Neurosci 13: 143 10.3389/fncel.2019.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. 2000. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell 101: 365–376. 10.1016/S0092-8674(00)80847-8 [DOI] [PubMed] [Google Scholar]
- Mattson MP. 2004. Pathways towards and away from Alzheimer's disease. Nature 430: 631–639. 10.1038/nature02621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Chan SL. 2001. Dysregulation of cellular calcium homeostasis in Alzheimer's disease: Bad genes and bad habits. J Mol Neurosci 17: 205–224. 10.1385/JMN:17:2:205 [DOI] [PubMed] [Google Scholar]
- Mattson MP, Pedersen WA, Duan W, Culmsee C, Camandola S. 1999. Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer's and Parkinson's diseases. Ann NY Acad Sci 893: 154–175. 10.1111/j.1749-6632.1999.tb07824.x [DOI] [PubMed] [Google Scholar]
- McLarnon JG, Ryu JK, Walker DG, Choi HB. 2006. Upregulated expression of purinergic P2X7 receptor in Alzheimer disease and amyloid-β peptide-treated microglia and in peptide-injected rat hippocampus. J Neuropathol Exp Neurol 65: 1090–1097. 10.1097/01.jnen.0000240470.97295.d3 [DOI] [PubMed] [Google Scholar]
- McPherson PS, Kim YK, Valdivia H, Knudson CM, Takekura H, Franzini-Armstrong C, Coronado R, Campbell KP. 1991. The brain ryanodine receptor: A caffeine-sensitive calcium release channel. Neuron 7: 17–25. 10.1016/0896-6273(91)90070-G [DOI] [PubMed] [Google Scholar]
- Meissner G. 2017. The structural basis of ryanodine receptor ion channel function. J Gen Physiol 149: 1065–1089. 10.1085/jgp.201711878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milakovic T, Quintanilla RA, Johnson GVW. 2006. Mutant huntingtin expression induces mitochondrial calcium handling defects in clonal striatal cells: Functional consequences. J Biol Chem 281: 34785–34795. 10.1074/jbc.M603845200 [DOI] [PubMed] [Google Scholar]
- Miras-Portugal MT, Sebastian-Serrano A, de Diego Garcia L, Diaz-Hernandez M. 2017. Neuronal P2X7 receptor: Involvement in neuronal physiology and pathology. J Neurosci 37: 7063–7072. 10.1523/jneurosci.3104-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- More JY, Bruna BA, Lobos PE, Galaz JL, Figueroa PL, Namias S, Sánchez GL, Barrientos GC, Valdés JL, Paula-Lima AC, et al. 2018. Calcium release mediated by redox-sensitive RyR2 channels has a central role in hippocampal structural plasticity and spatial memory. Antioxid Redox Signal 29: 1125–1146. 10.1089/ars.2017.7277 [DOI] [PubMed] [Google Scholar]
- Morens DM, Grandinetti A, Reed D, White LR, Ross GW. 1995. Cigarette smoking and protection from Parkinson's disease: False association or etiologic clue? Neurology 45: 1041–1051. 10.1212/WNL.45.6.1041 [DOI] [PubMed] [Google Scholar]
- Müller M, Ahumada-Castro U, Sanhueza M, Gonzalez-Billault C, Court FA, Cárdenas C. 2018. Mitochondria and calcium regulation as basis of neurodegeneration associated with aging. Front Neurosci 12: 470 10.3389/fnins.2018.00470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz FM, Gao R, Tian Y, Henstenburg BA, Barrett JE, Hu H. 2017. Neuronal P2X7 receptor-induced reactive oxygen species production contributes to nociceptive behavior in mice. Sci Rep 7: 3539 10.1038/s41598-017-03813-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustaly-Kalimi S, Littlefield AM, Stutzmann GE. 2018. Calcium signaling deficits in glia and autophagic pathways contributing to neurodegenerative disease. Antioxid Redox Signal 29: 1158–1175. 10.1089/ars.2017.7266 [DOI] [PubMed] [Google Scholar]
- Nagele RG, D'Andrea MR, Anderson WJ, Wang HY. 2002. Intracellular accumulation of β-amyloid1–42 in neurons is facilitated by the α7 nicotinic acetylcholine receptor in Alzheimer's disease. Neuroscience 110: 199–211. 10.1016/S0306-4522(01)00460-2 [DOI] [PubMed] [Google Scholar]
- Nasir J, Floresco SB, O'Kusky JR, Diewert VM, Richman JM, Zeisler J, Borowski A, Marth JD, Phillips AG, Hayden MR. 1995. Targeted disruption of the Huntington's disease gene results in embryonic lethality and behavioral and morphological changes in heterozygotes. Cell 81: 811–823. 10.1016/0092-8674(95)90542-1 [DOI] [PubMed] [Google Scholar]
- Nelson O, Tu H, Lei T, Bentahir M, de Strooper B, Bezprozvanny I. 2007. Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J Clin Invest 117: 1230–1239. 10.1172/JCI30447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Crompton M. 1980. Mitochondrial calcium transport. FEBS Lett 111: 261–268. 10.1016/0014-5793(80)80806-4 [DOI] [PubMed] [Google Scholar]
- Niggli E, Ullrich ND, Gutierrez D, Kyrychenko S, Poláková E, Shirokova N. 2013. Posttranslational modifications of cardiac ryanodine receptors: Ca2+ signaling and EC-coupling. Biochim Biophys Acta 1833: 866–875. 10.1016/j.bbamcr.2012.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaienko R, Bovo E, Zima AV. 2018. Redox dependent modifications of ryanodine receptor: Basic mechanisms and implications in heart diseases. Front Physiol 9: 1775 10.3389/fphys.2018.01775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S, Pouladi MA, Talantova M, Yao D, Xia P, Ehrnhoefer DE, Zaidi R, Clemente A, Kaul M, Graham RK, et al. 2009. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat Med 15: 1407–1413. 10.1038/nm.2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oules B, Del Prete D, Greco B, Zhang X, Lauritzen I, Sevalle J, Moreno S, Paterlini-Brechot P, Trebak M, Checler F, et al. 2012. Ryanodine receptor blockade reduces amyloid-load and memory impairments in Tg2576 mouse model of Alzheimer disease. J Neurosci 32: 11820–11834. 10.1523/jneurosci.0875-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadia S, Hardingham GE. 2007. The dichotomy of NMDA receptor signaling. Neuroscientist 13: 572–579. 10.1177/1073858407305833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. 2002 (1817). An essay on the shaking palsy. J Neuropsychiatry Clin Neurosci 14: 223–236. 10.1176/jnp.14.2.223 [DOI] [PubMed] [Google Scholar]
- Pascual-Caro C, Espinosa-Bermejo N, Pozo-Guisado E, Martin-Romero FJ. 2018. Role of STIM1 in neurodegeneration. World J Biol Chem 9: 16–24. 10.4331/wjbc.v9.i2.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul BD, Snyder SH. 2019. Impaired redox signaling in Huntington's disease: Therapeutic implications. Front Mol Neurosci 12: 68 10.3389/fnmol.2019.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pchitskaya E, Bezprozvanny I, Zhang H, Saito T, Saido T, Zakharova O, Wu L. 2015. Neuronal store-operated calcium entry and mushroom spine loss in amyloid precursor protein knock-in mouse model of Alzheimer's disease. J Neurosci 35: 13275–13286. 10.1523/jneurosci.1034-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pchitskaya E, Popugaeva E, Bezprozvanny I. 2018. Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium 70: 87–94. 10.1016/j.ceca.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Liang G, Inan S, Wu Z, Joseph DJ, Meng Q, Peng Y, Eckenhoff MF, Wei H. 2012. Dantrolene ameliorates cognitive decline and neuropathology in Alzheimer triple transgenic mice. Neurosci Lett 516: 274–279. 10.1016/j.neulet.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney JB, Vonsattel JP, Macdonald ME, Gusella JF, Myers RH. 1997. CAG repeat number governs the development rate of pathology in Huntington's disease. Ann Neurol 41: 689–692. 10.1002/ana.410410521 [DOI] [PubMed] [Google Scholar]
- Picconi B, Piccoli G, Calabresi P. 2012. Synaptic dysfunction in Parkinson's disease. Adv Exp Med Biol 970: 553–572. 10.1007/978-3-7091-0932-8_24 [DOI] [PubMed] [Google Scholar]
- Pivovarova NB, Andrews SB. 2010. Calcium-dependent mitochondrial function and dysfunction in neurons. FEBS J 277: 3622–3636. 10.1111/j.1742-4658.2010.07754.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popugaeva E, Pchitskaya E, Speshilova A, Alexandrov S, Zhang H, Vlasova O, Bezprozvanny I. 2015. STIM2 protects hippocampal mushroom spines from amyloid synaptotoxicity. Mol Neurodegener 10: 37 10.1186/s13024-015-0034-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourbadie HG, Naderi N, Mehranfard N, Janahmadi M, Khodagholi F, Motamedi F. 2015. Preventing effect of L-type calcium channel blockade on electrophysiological alterations in dentate gyrus granule cells induced by entorhinal amyloid pathology. PLoS ONE 10: e0117555 10.1371/journal.pone.0117555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Philie J, Choremis J. 1997. Modulation of α7 nicotinic receptor-mediated calcium influx by nicotinic agonists. Mol Pharmacol 51: 499–506. [PubMed] [Google Scholar]
- Quik M, O'Leary K, Tanner CM. 2008. Nicotine and Parkinson's disease: Implications for therapy. Mov Disord 23: 1641–1652. 10.1002/mds.21900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Zhang D, McGregor M, Bordia T. 2015. α7 Nicotinic receptors as therapeutic targets for Parkinson's disease. Biochem Pharmacol 97: 399–407. 10.1016/j.bcp.2015.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput AH, Offord KP, Beard CM, Kurland LT. 1984. Epidemiology of parkinsonism: Incidence, classification, and mortality. Ann Neurol 16: 278–282. 10.1002/ana.410160303 [DOI] [PubMed] [Google Scholar]
- Robertson LS, Causton HC, Young RA, Fink GR. 2000. The yeast A kinases differentially regulate iron uptake and respiratory function. Proc Natl Acad Sci 97: 5984–5988. 10.1073/pnas.100113397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Wenk GL. 2003. The neuropharmacological basis for the use of memantine in the treatment of Alzheimer's disease. CNS Drug Rev 9: 275–308. 10.1111/j.1527-3458.2003.tb00254.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronicke R, Mikhaylova M, Meinhardt J, Schroder UH, Fandrich M, Reiser G, Kreutz MR, Reymann KG. 2011. Early neuronal dysfunction by amyloid β oligomers depends on activation of NR2B-containing NMDA receptors. Neurobiol Aging 32: 2219–2228. 10.1016/j.neurobiolaging.2010.01.011 [DOI] [PubMed] [Google Scholar]
- Roselli F, Hutzler P, Wegerich Y, Livrea P, Almeida OF. 2009. Disassembly of shank and homer synaptic clusters is driven by soluble β-amyloid (1-40) through divergent NMDAR-dependent signalling pathways. PLoS ONE 4: e6011 10.1371/journal.pone.0006011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Moscardo F, Setó-Salvia N, Pera M, Bosch-Morató M, Plata C, Belbin O, Gené G, Dols-Icardo O, Ingelsson M, Helisalmi S, et al. 2013. Rare variants in calcium homeostasis modulator 1 (CALHM1) found in early onset Alzheimer's disease patients alter calcium homeostasis. PLoS ONE 8: e74203 10.1371/journal.pone.0074203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryazantseva M, Goncharova A, Skobeleva K, Erokhin M, Methner A, Georgiev P, Kaznacheyeva E. 2018. Presenilin-1 delta E9 mutant induces STIM1-driven store-operated calcium channel hyperactivation in hippocampal neurons. Mol Neurobiol 55: 4667–4680. 10.1007/s12035-017-0674-4 [DOI] [PubMed] [Google Scholar]
- Saad M, Lesage S, Saint-Pierre A, Corvol JC, Zelenika D, Lambert JC, Vidailhet M, Mellick GD, Lohmann E, Durif F, et al. 2011. Genome-wide association study confirms BST1 and suggests a locus on 12q24 as the risk loci for Parkinson's disease in the European population. Hum Mol Genet 20: 615–627. 10.1093/hmg/ddq497 [DOI] [PubMed] [Google Scholar]
- SanMartín CD, Veloso P, Adasme T, Lobos P, Bruna B, Galaz J, García A, Hartel S, Paula-Lima AC. 2017. RyR2-mediated Ca2+ release and mitochondrial ROS generation partake in the synaptic dysfunction caused by amyloid β peptide oligomers. Front Mol Neurosci 10: 115 10.3389/fnmol.2017.00115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz JM, Chiozzi P, Ferrari D, Colaianna M, Idzko M, Falzoni S, Fellin R, Trabace L, Di Virgilio F. 2009. Activation of microglia by amyloid β requires P2X7 receptor expression. J Immunol 182: 4378–4385. 10.4049/jimmunol.0803612 [DOI] [PubMed] [Google Scholar]
- Sanz-Clemente A, Gray JA, Ogilvie KA, Nicoll RA, Roche KW. 2013. Activated CaMKII couples GluN2B and casein kinase 2 to control synaptic NMDA receptors. Cell Rep 3: 607–614. 10.1016/j.celrep.2013.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, Mufson EJ. 2006. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol Aging 27: 1372–1384. 10.1016/j.neurobiolaging.2005.09.012 [DOI] [PubMed] [Google Scholar]
- Scheff SW, Neltner JH, Nelson PT. 2014. Is synaptic loss a unique hallmark of Alzheimer's disease? Biochem Pharmacol 88: 517–528 10.1016/j.bcp.2013.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiesling C, Kieper N, Seidel K, Krüger R. 2008. Review: Familial Parkinson's disease—Genetics, clinical phenotype and neuropathology in relation to the common sporadic form of the disease. Neuropathol Appl Neurobiol 34: 255–271. 10.1111/j.1365-2990.2008.00952.x [DOI] [PubMed] [Google Scholar]
- Secondo A, Bagetta G, Amantea D. 2018. On the role of store-operated calcium entry in acute and chronic neurodegenerative diseases. Front Mol Neurosci 11: 87 10.3389/fnmol.2018.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M, Korkotian E. 2014. Endoplasmic reticulum calcium stores in dendritic spines. Front Neuroanat 8: 64 10.3389/fnana.2014.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. 1993. Molecular cloning, functional properties, and distribution of rat brain α7: A nicotinic cation channel highly permeable to calcium. J Neurosci 13: 596–604. 10.1523/jneurosci.13-02-00596.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. 2000. Toward a comprehensive theory for Alzheimer's disease. Hypothesis: Alzheimer's disease is caused by the cerebral accumulation and cytotoxicity of amyloid β-protein. Ann NY Acad Sci 924: 17–25. 10.1111/j.1749-6632.2000.tb05554.x [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Hardy J. 2016. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med 8: 595–608. 10.15252/emmm.201606210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilling D, Müller M, Takano H, Mak DOD, Abel T, Coulter DA, Foskett JK. 2014. Suppression of InsP3 receptor-mediated Ca2+ signaling alleviates mutant presenilin-linked familial Alzheimer's disease pathogenesis. J Neurosci 34: 6910–6923. 10.1523/jneurosci.5441-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirwany NA, Payette D, Xie J, Guo Q. 2007. The amyloid β ion channel hypothesis of Alzheimer's disease. Neuropsychiatr Dis Treat 3: 597–612. [PMC free article] [PubMed] [Google Scholar]
- Siebert AP, Ma Z, Grevet JD, Demuro A, Parker I, Foskett JK. 2013. Structural and functional similarities of calcium homeostasis modulator 1 (CALHM1) ion channel with connexins, pannexins, and innexins. J Biol Chem 288: 6140–6153. 10.1074/jbc.M112.409789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms BA, Zamponi GW. 2014. Neuronal voltage-gated calcium channels: Structure, function, and dysfunction. Neuron 82: 24–45. 10.1016/j.neuron.2014.03.016 [DOI] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. 2005. A role for adult neurogenesis in spatial long-term memory. Neuroscience 130: 843–852. 10.1016/j.neuroscience.2004.10.009 [DOI] [PubMed] [Google Scholar]
- Spires-Jones TL, Hyman BT. 2014. The intersection of amyloid β and tau at synapses in Alzheimer's disease. Neuron 82: 756–771. 10.1016/j.neuron.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones TL, Meyer-Luehmann M, Osetek JD, Jones PB, Stern EA, Bacskai BJ, Hyman BT. 2007. Impaired spine stability underlies plaque-related spine loss in an Alzheimer's disease mouse model. Am J Pathol 171: 1304–1311. 10.2353/ajpath.2007.070055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling A, Andre VM, Cepeda C, Lima M, Chandler SH, Levine MS. 2005. Alterations in N-methyl-d-aspartate receptor sensitivity and magnesium blockade occur early in development in the R6/2 mouse model of Huntington's disease. J Neurosci Res 82: 377–386. 10.1002/jnr.20651 [DOI] [PubMed] [Google Scholar]
- Sobolevsky AI. 2015. Structure and gating of tetrameric glutamate receptors. J Physiol 593: 29–38. 10.1113/jphysiol.2013.264911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann GE. 2007. The pathogenesis of Alzheimers disease—Is it a lifelong “calciumopathy”? Neuroscientist 13: 546–559. 10.1177/1073858407299730 [DOI] [PubMed] [Google Scholar]
- Stutzmann GE, Caccamo A, LaFerla FM, Parker I. 2004. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer's-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J Neurosci 24: 508–513. 10.1523/jneurosci.4386-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann GE, Smith I, Caccamo A, Oddo S, Laferla FM, Parker I. 2006. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer's disease mice. J Neurosci 26: 5180–5189. 10.1523/jneurosci.0739-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchowersky O. 2019. Huntington disease: Clinical features and diagnosis. UpToDate https://www.uptodate.com/contents/huntington-disease-clinical-features-and-diagnosis
- Sukhareva M, Smith SV, Maric D, Barker JL. 2002. Functional properties of ryanodine receptors in hippocampal neurons change during early differentiation in culture. J Neurophysiol 88: 1077–1087. 10.1152/jn.2002.88.3.1077 [DOI] [PubMed] [Google Scholar]
- Sun S, Zhang H, Liu J, Popugaeva E, Xu N, Feske S, White CL III, Bezprozvanny I. 2014. Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice. Neuron 82: 79–93. 10.1016/j.neuron.2014.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ. 2018. Determinants of dopaminergic neuron loss in Parkinson's disease. FEBS J 285: 3657–3668. 10.1111/febs.14607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Schumacker PT. 2013. Calcium, bioenergetics, and neuronal vulnerability in Parkinson's disease. J Biol Chem 288: 10736–10741. 10.1074/jbc.R112.410530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Halliday GM, Simuni T. 2017a. Calcium, mitochondrial dysfunction and slowing the progression of Parkinson's disease. Exp Neurol 298: 202–209. 10.1016/j.expneurol.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Obeso JA, Halliday GM. 2017b. Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci 18: 101–113. 10.1038/nrn.2016.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Schumacker PT, Guzman JD, Ilijic E, Yang B, Zampese E. 2017c. Calcium and Parkinson's disease. Biochem Biophys Res Commun 483: 1013–1019. 10.1016/j.bbrc.2016.08.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenouchi T, Sekiyama K, Sekigawa A, Fujita M, Waragai M, Sugama S, Iwamaru Y, Kitani H, Hashimoto M. 2010. P2X7 receptor signaling pathway as a therapeutic target for neurodegenerative diseases. Arch Immunol Ther Exp (Warsz) 58: 91–96. 10.1007/s00005-010-0069-y [DOI] [PubMed] [Google Scholar]
- Tang TS, Tu H, Chan EYW, Maximov A, Wang Z, Wellington CL, Hauden MR, Bezprozvanny I. 2003. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron 39: 227–239. 10.1016/S0896-6273(03)00366-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TS, Slow E, Lupu V, Stavrovskaya IG, Sugimori M, Llinás R, Kristal BS, Hayden MR, Bezprozvanny I. 2005. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington's disease. Proc Natl Acad Sci 102: 2602–2607. 10.1073/pnas.0409402102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasov AI, Griffiths EJ, Rutter GA. 2012. Regulation of ATP production by mitochondrial Ca2+. Cell Calcium 52: 28–35. 10.1016/j.ceca.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Texidó L, Martín-Satué M, Alberdi E, Solsona C, Matute C. 2011. Amyloid β peptide oligomers directly activate NMDA receptors. Cell Calcium 49: 184–190. 10.1016/j.ceca.2011.02.001 [DOI] [PubMed] [Google Scholar]
- Toulorge D, Guerreiro S, Hild A, Maskos U, Hirsch EC, Michel PP. 2011. Neuroprotection of midbrain dopamine neurons by nicotine is gated by cytoplasmic Ca2+. FASEB J 25: 2563–2573. 10.1096/fj.11-182824 [DOI] [PubMed] [Google Scholar]
- Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. 2006. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell 126: 981–993. 10.1016/j.cell.2006.06.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas KJ, Schrod N, Davis T, Fernandez-Busnadiego R, Taguchi YV, Laugks U, Lucic V, Chandra SS. 2017. Synucleins have multiple effects on presynaptic architecture. Cell Rep 18: 161–173. 10.1016/j.celrep.2016.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma R, Chai Y, Troncoso J, Gu J, Xing H, Stojilkovic SS, Mattson MP, Haughey NJ. 2009. Amyloid-β induces a caspase-mediated cleavage of P2X4 to promote purinotoxicity. Neuromolecular Med 11: 63–75. 10.1007/s12017-009-8073-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen Metman L, Del Dotto P, van den Munckhof P, Fang J, Mouradian MM, Chase TN. 1998. Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson's disease. Neurology 50: 1323–1326. 10.1212/WNL.50.5.1323 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. 2005. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev 85: 201–279. 10.1152/physrev.00004.2004 [DOI] [PubMed] [Google Scholar]
- Vingtdeux V, Tanis JE, Chandakkar P, Zhao H, Dreses-Werringloer U, Campagne F, Foskett JK, Marambaud P. 2014. Effect of the CALHM1 G330D and R154H human variants on the control of cytosolic Ca2+ and Aβ levels. PLoS ONE 9: e112484 10.1371/journal.pone.0112484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyklicky V, Korinek M, Smejkalova T, Balik A, Krausova B, Kaniakova M, Lichnerova K, Cerny J, Krusek J, Dittert I, et al. 2014. Structure, function, and pharmacology of NMDA receptor channels. Physiol Res 63: 191–203. 10.1113/jphysiol.2014.288209 [DOI] [PubMed] [Google Scholar]
- Wang X, Su B, Lee H, Li X, Perry G, Smith MA, Zhu X. 2009. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci 29: 9090–9103. 10.1523/jneurosci.1357-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens XHT, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. 2006. Ryanodine receptor/calcium release channel PKA phosphorylation: A critical mediator of heart failure progression. Proc Natl Acad Sci 103: 511–518. 10.1073/pnas.0510113103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wevers A, Monteggia L, Nowacki S, Bloch W, Schutz U, Lindstrom J, Pereira EF, Giacobini E, de Vos RA, et al. 1999. Expression of nicotinic acetylcholine receptor subunits in the cerebral cortex in Alzheimer's disease: Histotopographical correlation with amyloid plaques and hyperphosphorylated-tau protein. Eur J Neurosci 11: 2551–2565. 10.1046/j.1460-9568.1999.00676.x [DOI] [PubMed] [Google Scholar]
- Wild AR, Akyol E, Brothwell SLC, Kimkool P, Skepper JN, Gibb AJ, Jones S. 2013. Memantine block depends on agonist presentation at the NMDA receptor in substantia nigra pars compacta dopamine neurones. Neuropharmacology 73: 138–146. 10.1016/j.neuropharm.2013.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk A, Dickerson B. 2019. Clinical features and diagnosis of Alzheimer disease. UpToDate https://www.uptodate.com/contents/clinical-features-and-diagnosis-of-alzheimer-disease
- Wonnacott S. 1990. The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Pharmacol Sci 11: 216–219. 10.1016/0165-6147(90)90242-Z [DOI] [PubMed] [Google Scholar]
- Wu J, Shih H, Vignot V, Hrdlicka L, Diggins L, Singh C, Mahoney M, Chesworth R, Shapiro G, Zimina O, et al. 2011. Neuronal store-operated calcium entry pathway as a novel therapeutic target for Huntington's disease treatment. Chem Biol 18: 777–793. 10.1016/j.chembiol.2011.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Ryskamp DA, Liang X, Egorova P, Zakharova O, Hung G, Bezprozvanny I. 2016. Enhanced store-operated calcium entry leads to striatal synaptic loss in a Huntington's disease mouse model. J Neurosci 36: 125–141. 10.1523/jneurosci.1038-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods LT, Ajit D, Camden JM, Erb L, Weisman GA. 2016. Purinergic receptors as potential therapeutic targets in Alzheimer's disease. Neuropharmacology 104: 169–179. 10.1016/j.neuropharm.2015.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Fang T, Yan H, Jiang L. 2019. The protein kinase Cmk2 negatively regulates the calcium/calcineurin signalling pathway and expression of calcium pump genes PMR1 and PMC1 in budding yeast. Cell Commun Signal 17: 7 10.1186/s12964-019-0320-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H, Mikoshiba K. 2009. Structure of IP3 receptor. In Handbook of neurochemistry and molecular neurobiology (ed. Lajtha A, Mikoshiba K), pp. 441–461. Springer, New York. [Google Scholar]
- Yamin R, Zhao C, O'Connor PB, McKee AC, Abraham CR. 2009. Acyl peptide hydrolase degrades monomeric and oligomeric amyloid-β peptide. Mol Neurodegener 4: 33 10.1186/1750-1326-4-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang Z, Wang B, Justice NJ, Zheng H. 2009. Amyloid precursor protein regulates Cav1.2 L-type calcium channel levels and function to influence GABAergic short-term plasticity. J Neurosci 29: 15660–15668. 10.1523/jneurosci.4104-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasar S, Corrada M, Brookmeyer R, Kawas C. 2005. Calcium channel blockers and risk of AD: The Baltimore Longitudinal Study of Aging. Neurobiol Aging 26: 157–163. 10.1016/j.neurobiolaging.2004.03.009 [DOI] [PubMed] [Google Scholar]
- Zaichick SV, McGrath KM, Caraveo G. 2017. The role of Ca2+ signaling in Parkinson's disease. Dis Model Mech 10: 519–535. 10.1242/dmm.028738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin S, Liu JP, Chapman DL, Papaioannou VE, Efstratiadis A. 1995. Increased apoptosis and early embryonic lethality in mice nullizygous for the Huntington's disease gene homologue. Nat Genet 11: 155–163. 10.1038/ng1095-155 [DOI] [PubMed] [Google Scholar]
- Zeron MM, Hansson O, Chen N, Wellington CL, Leavitt BR, Brundin P, Hayden MR, Raymond LA. 2002. Increased sensitivity to N-methyl-d-aspartate receptor-mediated excitotoxicity in a mouse model of Huntington's disease. Neuron 33: 849–860. 10.1016/S0896-6273(02)00615-3 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li P, Feng J, Wu M. 2016. Dysfunction of NMDA receptors in Alzheimer's disease. Neurol Sci 37: 1039–1047. 10.1007/s10072-016-2546-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Hollern D, Liao J, Andrechek E, Wang H. 2013. NMDA receptor-mediated excitotoxicity depends on the coactivation of synaptic and extrasynaptic receptors. Cell Death Dis 4: e560 10.1038/cddis.2013.82 [DOI] [PMC free article] [PubMed] [Google Scholar]