Abstract
Objective
To examine whether lowering plasma glucose concentration with the sodium-glucose transporter-2 inhibitor empagliflozin improves β-cell function in patients with type 2 diabetes mellitus (T2DM).
Methods
Patients with T2DM (N = 15) received empagliflozin (25 mg/d) for 2 weeks. β-Cell function was measured with a nine-step hyperglycemic clamp (each step, 40 mg/dL) before and at 48 hours and at 14 days after initiating empagliflozin.
Results
Glucosuria was recorded on days 1 and 14 [mean ± standard error of the mean (SEM), 101 ± 10 g and 117 ± 11 g, respectively] after initiating empagliflozin, as were reductions in fasting plasma glucose levels (25 ± 6 mg/dL and 38 ± 8 mg/dL, respectively; both P < 0.05). After initiating empagliflozin and during the stepped hyperglycemic clamp, the incremental area under the plasma C-peptide concentration curve increased by 48% ± 12% at 48 hours and 61% ± 10% at 14 days (both P < 0.01); glucose infusion rate increased by 15% on day 3 and 16% on day 14, compared with baseline (both P < 0.05); and β-cell function, measured with the insulin secretion/insulin resistance index, increased by 73% ± 21% at 48 hours and 112% ± 20% at 14 days (both P < 0.01). β-cell glucose sensitivity during the hyperglycemic clamp was enhanced by 42% at 14 hours and 54% at 14 days after initiating empagliflozin (both P < 0.01).
Conclusion
Lowering the plasma glucose concentration with empagliflozin in patients with T2DM augmented β-cell glucose sensitivity and improved β-cell function.
This study demonstrates that inhibition of renal glucose uptake with empagliflozin improves β-cell function measured with the gold standard technique, the hyperglycemic clamp.
Progressive β-cell failure is the principal factor responsible for the development and progression of hyperglycemia in patients with type 2 diabetes mellitus (T2DM) (1–3). Multiple factors contribute to the development of β-cell failure, including genetic and environmental factors (1–3). Chronic elevation of the plasma free fatty acid concentration (i.e., lipotoxicity) markedly impairs insulin secretion in offspring of parents with T2DM (4). Similarly, chronic elevation of plasma glucose concentration (i.e., glucotoxicity) impairs β-cell function (5–7). Conversely, reducing the plasma glucose concentration improves insulin secretion in in vivo and in vitro studies in experimental animals (6, 7). Using insulin to lower plasma glucose concentration improves β-cell function in humans with T2DM (8–11). However, in addition to decreasing the plasma glucose concentration, insulin has multiple other metabolic effects, including reduction of the plasma free fatty acid concentration, which independently can improve β-cell function.
Sodium-glucose transporter-2 (SGLT2) inhibitors are a novel class of drugs that inhibit renal glucose absorption, thereby producing glucosuria and decreasing the plasma glucose concentration. We have shown that lowering plasma glucose concentration with an SGLT2 inhibitor reduces plasma glucose concentration and improves insulin-mediated glucose uptake in muscle (12). Because skeletal muscle does not express SGLT2, these results indicate that the improvement in insulin sensitivity caused by SGLT2 inhibition is secondary to amelioration of glucotoxicity. In preclinical studies in experimental animal models of diabetes, SGLT2 inhibitors reduced or normalized plasma glucose concentration and improved β-cell function (6). Recent studies using oral glucose tolerance tests (OGTTs) have demonstrated that, in patients with T2DM, 2 to 4 weeks of dapagliflozin and empagliflozin treatment improved β-cell function (13, 14).
The aim of the current study was to examine the effect of lowering plasma glucose concentration with empagliflozin on β-cell function, as measured with the gold standard stepped hyperglycemic-clamp technique.
Patients and Methods
Patients
A total of 15 patients (12 men, three women) with T2DM participated in the study. Patient characteristics were as follows (values reported as mean ± standard error of the mean): age, 55 ± 2 years; body mass index, 31.1 ± 2.1 kg/m2; diabetes duration, 8.2 ± 1.6 years; hemoglobin A1c (HbA1c) value, 7.8% ± 0.2%; fasting plasma glucose (FPG), 195 ± 9 mg/dL; and estimated glomerular filtration rate, 107 ± 7 mL/min × 1.73 m2. Other than diabetes, patients were in good general health as determined by medical history, physical examination, screening laboratory tests, and electrocardiogram. Body weight was stable (±3 pounds) in all patients for at least 3 months before the study and no patient participated in any excessively heavy exercise program. Patients were treated with metformin (n = 11), metformin/sulfonylureas (n = 2), sulfonylurea (n = 1), and diet (n = 1). Other than these oral antidiabetic agents, patients did not take any medication known to affect glucose metabolism.
This was an open-label study in which all patients were treated with empagliflozin 25 mg/d and received a stepped hyperglycemic clamp before and at 48 hours and 14 days after the starting empagliflozin treatment.
Research design
All studies were performed at the Clinical Research Center of the Texas Diabetes Institute, San Antonio, Texas, after patients had fasted overnight. On days 5, 4, 2, and 1 before initiating empagliflozin treatment, 24-hour urine samples were collected for measurement of baseline urinary glucose excretion. On day 3 before initiating empagliflozin treatment, a baseline stepped hyperglycemic clamp was performed after a 10-hour overnight fast. Patients reported to the Clinical Research Center at 6:00 am and a catheter was placed into an antecubital vein. A low-dose insulin infusion (0.1 to 0.2 mU/kg × minute) was started to reduce the FPG concentration to ∼100 mg/dL. The insulin infusion then was discontinued for 20 minutes, at which time the stepped hyperglycemic clamp (15) was performed. A second catheter was placed into a vein on the dorsum of the hand for blood withdrawal, and the hand was placed in a thermoregulated box heated to 60°C to obtain arterialized blood samples.
At 7:30 am (i.e., −90 minutes from time 0 of the study), patients were asked to void, the urine was discarded, and a volume of water equivalent to the voided volume of urine was consumed. At time 0 (9:00 am), patients voided and a stepped hyperglycemic clamp was performed. The plasma glucose concentration was acutely raised and maintained at 40 mg/dL above the fasting level (i.e., from ∼100 to 140 mg/dL) for 40 minutes, and urine was collected from 0 to 40 minutes for measurement of urinary glucose excretion. From 40 to 80, 80 to 120, 120 to 160, 160 to 200, 200 to 240, 240 to 280, 280 to 320, and 320 to 360 minutes, the plasma glucose concentration was acutely raised and maintained at 180, 220, 260, 300, 340, 380, 420, and 460 mg/dL, respectively, with a variable infusion of 20% dextrose solution. Urine was collected during each 40- minute period and the patients each consumed an amount of water equal to the voided urine volume to ensure spontaneous voiding. All urine samples were analyzed for glucose concentration. Plasma C-peptide and glucose concentrations were measured at 2, 4, 6, 8, and 10 minutes and every 5 to 10 minutes thereafter until 360 minutes had elapsed.
On the day after the stepped hyperglycemic clamp (day 0), patients initiated empagliflozin treatment, 25 mg/d, which they took in the morning for 14 days. On day 2 (i.e., 48 hours after starting empagliflozin) and on day 14, the stepped hyperglycemic clamp was repeated as described in the previous paragraph. The same insulin infusion rate (i.e., 0.1 to 0.2 mU/kg × minute) was used to reduce the FPG concentration to ∼100 mg/dL during both repeated hyperglycemic clamp studies. The 24-hour urine collections for measurement of glucose excretion were obtained during the 48 hours (i.e., on days 0 and 1) after the start of empagliflozin and on days 12 and 13.
Analytical techniques
Plasma and urine glucose concentrations were determined by glucose oxidation method (Analox; Analox Instruments, Middlebrough, UK). Plasma C-peptide and insulin concentrations (Linco Research, St. Louis, MO) were determined by radioimmunoassay.
Calculations and statistical analyses
Because the increment in plasma glucose concentration (+40 mg/dL every 40 minutes) was the same in all patients, insulin secretion during each step of the hyperglycemic clamp was calculated as the incremental area under the plasma C-peptide concentration during that step. Tissue glucose uptake during each step was calculated as the glucose infusion rate during the last 20 minutes of each hyperglycemic step minus urinary glucose excretion during the same period. Under the combined effects of hyperinsulinemia plus hyperglycemia, we previously have shown that endogenous glucose production is completely or near-completely suppressed (16). Insulin sensitivity index during the hyperglycemic clamp was calculated as the glucose infusion rate minus urinary glucose excretion at each step, divided by the plasma insulin concentration. β-Cell function was calculated as the insulin secretion/insulin resistance index (IS/IR; or disposition index)—that is, the product of the change in C-peptide concentration over 0 to 360 minutes [∆C-pep(0-360 min)] and insulin sensitivity index. β-Cell glucose sensitivity was calculated as the slope of the line relating C-peptide secretion and the plasma glucose concentration during each hyperglycemic clamp step.
Values are expressed as mean ± standard error of the mean. C-peptide secretion, β-cell function, glucose infusion rate, and β-cell glucose sensitivity on day 2 (i.e., 48 hours after the start of empagliflozin) and on day 14 were compared with baseline by paired t test. Statistical significance was set at P < 0.05.
To determine independent factors related to ∆C-pep0–360, we created a multivariate linear regression model with ∆C-pep0–360 as the dependent variable and age, body mass index, diabetes duration, HbA1c value, baseline FPG, decrement in FPG, and improvement in insulin sensitivity (i.e., glucose infusion rate minus urinary glucose excretion) as independent variables.
The study protocol was approved by the Institutional Review Board of University of Texas Health Science Center in San Antonio, Texas, and all patients gave written informed voluntary consent before participation.
Results
Before the start of empagliflozin treatment, urinary glucose excretion (the mean of four determinations before initiating empagliflozin) was 20 ± 3 g/d; this value rose to 95 ± 13 and 97 ± 10 g/d (P < 0.0001 vs baseline) on days 0 and 1 after empagliflozin treatment and remained elevated (117 ± 11g/d) on day 13. The FPG concentration (the mean of four determinations performed on days 5, 4, 2, and 1 before the start of empagliflozin) was 195 ± 9 mg/dL; this decreased to 169 ± 11 mg/dL (difference from baseline mean, 25 ± 6 mg/dL) and to 165 ± 7 mg/dL (difference from baseline mean, 29 ± 6 mg/dL) at 24 hours and 48 hours after starting empagliflozin (both P < 0.001). The FPG decreased to 157 ± 8 mg/dL (difference from baseline mean, 38 ± 8 mg/dL) on day 14 (P = 0.0005). The fasting plasma C-peptide concentration declined slightly, but not significantly, during empagliflozin treatment from 3.5 ± 0.3 ng/mL at baseline to 3.2 ± 0.3, 3.2 ± 0.3, and 3.2 ± 0.3 ng/mL at 24 hours, 48 hours, and 14 days, respectively, after the start of empagliflozin.
Plasma glucose and C-peptide concentration
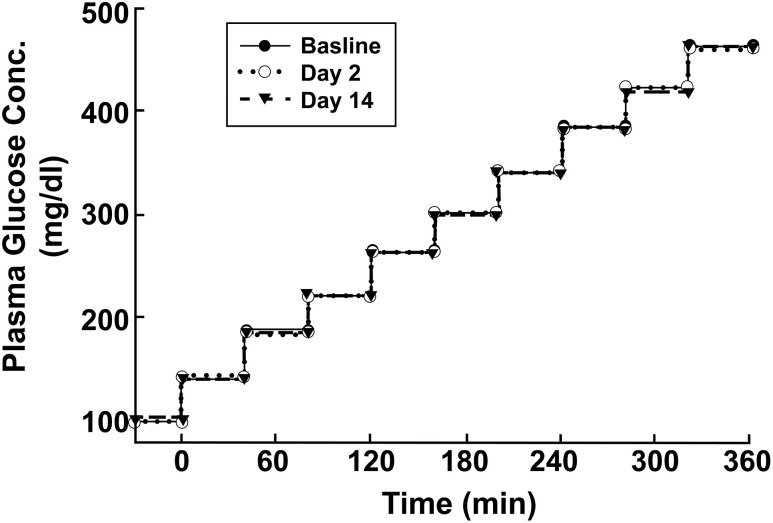
The increment in plasma glucose concentration during each of the three hyperglycemic clamp studies was similar (Fig. 1).
Figure 1.
Plasma glucose concentrations during the three hyperglycemic clamp studies performed at baseline and on days 2 and 14 after the start of empagliflozin therapy. Conc., concentration.
C-peptide secretion
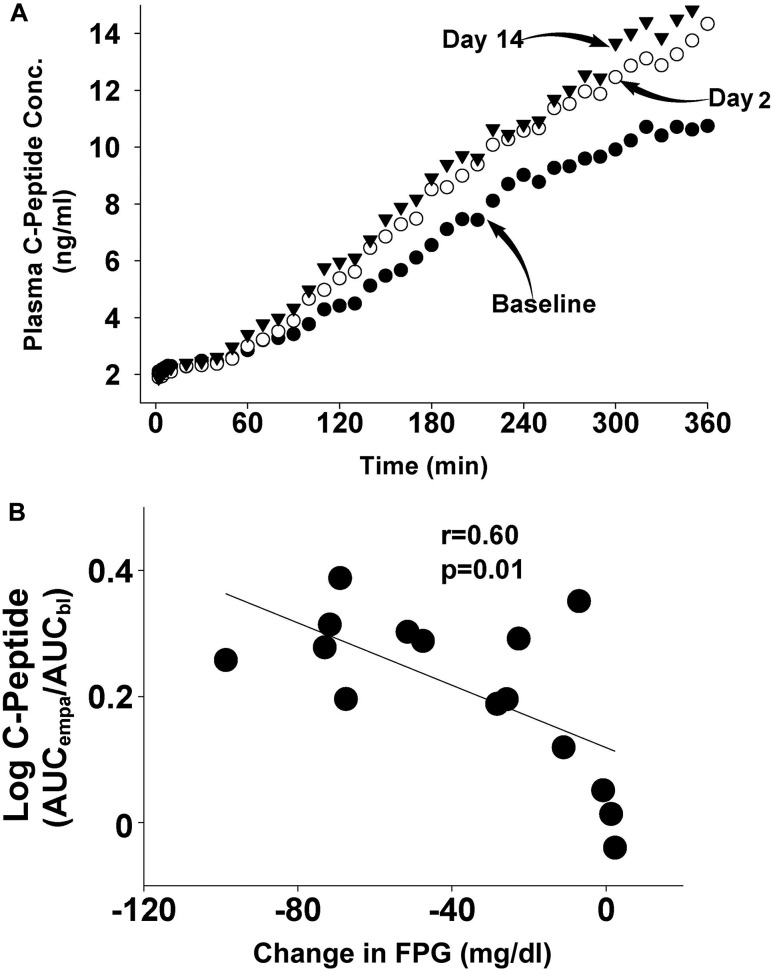
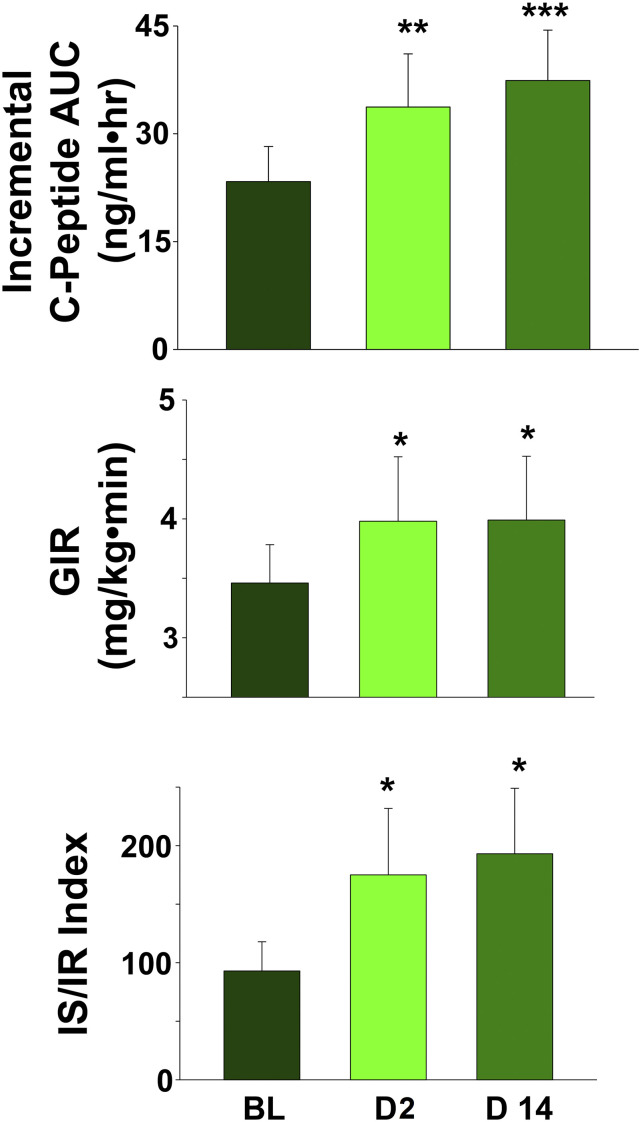
Fig. 2 depicts the plasma C-peptide concentration during the hyperglycemic clamp performed at baseline and on day 2 (i.e., 48 hours after the start of empagliflozin) and on day 14. C-peptide concentration increased significantly on day 2 after initiation of empagliflozin therapy and the increase persisted on day 14. The incremental area under the plasma C-peptide concentration curve [i.e., ∆C-Pep(0-360)] during the baseline hyperglycemic clamp was 23 ± 5 ng/mL × hour and increased to 32 ± 7 and 37 ± 8 ng/mL × hour on days 2 and 14, respectively (P = 0.02 and < 0.005, respectively; Fig. 3). The incremental area under the plasma C-peptide concentration curve during the first 10 minutes of the hyperglycemic clamp (which represents first-phase insulin secretion) was not altered by empagliflozin [0.61 ± 0.36, 0.21 ± 0.35 ng/mL × hour (P = 0.30) and 1.2 ± 0.44 ng/mL × hour (P = 0.10) at baseline, day 2, and day 14, respectively).
Figure 2.
(A) Plasma C-peptide response during the stepped hyperglycemic clamp performed at baseline and on days 2 and 14 after the start of empagliflozin therapy. (B) Correlation between the decrease in the FPG concentration and the log of the ratio of the plasma C-peptide response after 14 days of empagliflozin to the plasma C-peptide response at baseline. AUCbl, plasma C-peptide response at baseline; AUCempa, area under the curve after 14 days of empagliflozin; Conc, concentration.
Figure 3.
(A) Incremental AUC for the plasma C-peptide response at baseline and on days 2 and day 14 after the start of empagliflozin treatment. (B) GIR at baseline and on days 2 and 14 after the start of empagliflozin. (C) The IS/IR index at baseline and on days 2 and 14 after the start of empagliflozin treatment. *P < 0.05. AUC, area under the curve; BL, baseline; D2, day 2; D14, day 14; GIR, glucose infusion rate.
β-Cell function
Tissue glucose uptake (measured as the mean glucose infusion rate minus urinary glucose excretion) during the hyperglycemic clamp was 3.36 ± 0.29 mg/kg/min and increased by 14% and 19% (to 3.84 ± 0.44 mg/kg/min and 4.01 ± 0.43 mg/kg/min) on days 2 and 14, respectively (P < 0.05; Fig. 3). However, when divided by the mean plasma insulin concentration during the hyperglycemic clamp, tissue glucose uptake was not significantly increased. β-Cell function, measured with the IS/IR index, increased by 73% ± 20% and 106% ± 20% on days 2 and 14, respectively (from 88% ± 23% at baseline to 155% ± 46% and 187% ± 58% on days 2 and 14, respectively; both P < 0.01; Fig. 3).
β-cell glucose sensitivity
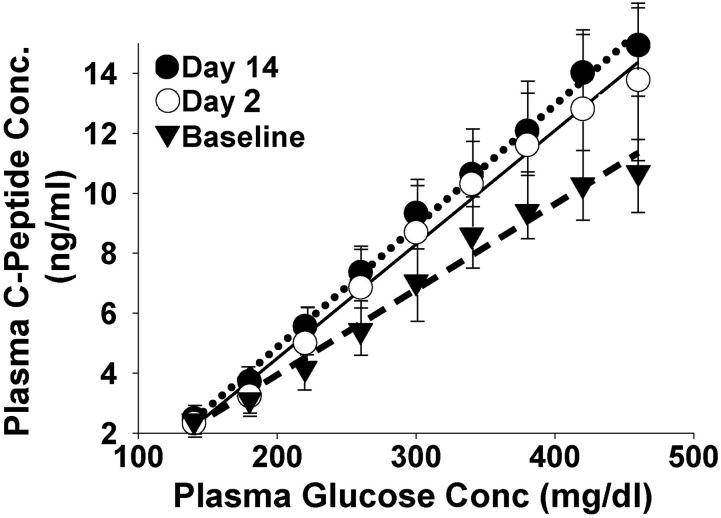
The slope of the line relating the area under the plasma C-peptide concentration curve and the plasma glucose concentration at each step of the hyperglycemic clamp represents β-cell glucose sensitivity. During the baseline hyperglycemic clamp, the slope was 0.024 ± 0.005 ng/mL per milligram per deciliter. Empagliflozin caused a 42% (to 0.034 ± 0.007ng/mL per milligram per deciliter) and 54% (to 0.037 ± 0.007ng/mL per milligram per deciliter) increase in β-cell glucose sensitivity on day 2 (i.e., 48 hours after the start of empagliflozin) and on day 14, respectively (both P < 0.05; Fig. 4).
Figure 4.
Slope of the line relating the increment in plasma C-peptide concentration and the increment in plasma glucose concentration during the stepped hyperglycemic clamp. Conc, concentration.
Relationship between increase in β-cell function and decrease in FPG concentration
The increments in plasma C-peptide concentration during the hyperglycemic clamp and in the IS/IR index strongly correlated with the decrease in FPG concentration on days 2 and 14 [r = 0.60 and 0.61, respectively; P = 0.01; Fig. 2(B)]. No significant relationship was observed between the decrease in FPG concentration and the increase in glucose infusion rate during the hyperglycemic clamp. In a multivariate linear regression model, only the decrease in FPG was a significant predictor of the increase in ∆C-pep0–360 at 14 days (standardized β = 0.862; P = 0.002; r2 = 0.65).
Discussion
The current study assessed the acute (48 hours) and chronic (14 days) effect of SGLT2 inhibition on β-cell function in patients with T2DM; two findings, in particular, are highlighted. First, by using the gold standard hyperglycemic clamp, a marked increase in β-cell sensitivity to glucose was observed after a reduction in the plasma glucose concentration. Second, we were able to delineate the rapid (48 hours), time-related effect of glucotoxicity reversal on β-cell function.
Progressive β-cell failure is the principal factor responsible for the development and progression of hyperglycemia (1, 17–19). Thus, therapies that improve or halt β-cell failure should be effective in producing a durable reduction in HbA1c. Although several classes of antidiabetic drugs (e.g., sulfonylureas, glinides, incretins, thiazolidinediones) augment insulin secretion, only the GLP-1 receptor agonists (20) and thiazolidinediones (21, 22) improve β-cell function on a long-term basis. DPP4 inhibitors improve insulin secretion, but their effect on the β-cell is weak (23) and, not surprisingly, they do not produce a durable reduction in HbA1c (24–26).
SGLT2 inhibitors lower the plasma glucose concentration via a mechanism independent of insulin secretion and insulin action. By inhibiting renal glucose reabsorption, they cause glucosuria, leading to a decline in fasting and postprandial plasma glucose concentration. Despite the lack of a direct effect on the β-cell, preclinical studies in animal models of diabetes have demonstrated improved β-cell function secondary to reduction of the plasma glucose concentration and amelioration of glucotoxicity (6). Using mathematical modeling, both canagliflozin (27) and empagliflozin (14) have been shown to improve β-cell function during the OGTT in patients with T2DM. Ipragliflozin also improves insulin secretion indices during the OGTT in patients with T2DM (28), and we have shown an improvement in the IS/IR index during the OGTT in a small group of patients with T2DM treated with dapagliflozin (13).
To our knowledge, no previous study has used the hyperglycemic clamp to quantitate insulin secretion acutely and more chronically after treatment with an SGLT2 inhibitor. A 29 mg/dL decrement (at 48 hours after the start of empagliflozin) and 38 mg/dL decrement (at 14 days after starting empagliflozin treatment) in FPG resulted in a 43% and 74% increase, respectively, in C-peptide secretion during the hyperglycemic clamp; β-cell function (measured using the IS/IR index) increased by 73% and 106%, respectively. Remarkably, the improvement in C-peptide secretion observed with empagliflozin was evident 48 hours after starting treatment with the SGLT2 inhibitor, indicating onset of the beneficial effect of glucotoxicity reversal on β-cell function is rapid. Insulin secretion and insulin sensitivity are inversely related (29). We previously have shown that lowering the FPG concentration with an SGLT2 inhibitor in T2DM patients improves insulin sensitivity. If anything, this would be expected to reduce C-peptide secretion. Thus, the increase in C-peptide secretion caused by empagliflozin, as observed in the current study, represents a primary effect on the β-cell, most likely secondary to reversal of glucotoxicity, and not a secondary effect due to change in insulin sensitivity. One other factor that could explain the improvement in β-cell function relates to the observation that glucose lowering improves the effect of incretins on insulin secretion (30). Although this was not examined in the current study, the possibility is worthy of exploration in future studies.
Although SGLT2 inhibitors do not exert a direct effect on the β-cell, we (31) and others (14) have demonstrated that this class of drugs alters fuel metabolism, causing a reduction in whole-body glucose oxidation and a reciprocal increase in lipid oxidation. If the increase in lipid oxidation were to occur in the β-cell, this could lead to a decrease in β-cell lipid content and reversal of lipotoxicity (31). During the hyperglycemic clamp, empagliflozin caused a modest increase in glucose infusion rate, which was not significant when divided by plasma insulin concentration.
We (31) and others (14) have shown that glucosuria produced by SGLT2 inhibition stimulates a compensatory increase in the rate of hepatic glucose production. Because the total body rate of glucose appearance was not measured with labeled glucose in the current study, we could not quantitate the residual rate of hepatic glucose production during the hyperglycemic clamp. Therefore, it is not possible to reach any definitive conclusion about the effect of empagliflozin on insulin sensitivity in the current study.
In summary, lowering the plasma glucose concentration with empagliflozin causes a robust increase in β-cell function in patients with T2DM. This effect of empagliflozin occurs rapidly and lasts for the entire treatment period (i.e., 14 days).
Acknowledgments
Financial Support: This work was supported by Boehringer-Ingelheim and Eli Lilly. R.A.D.’s salary is supported, in part, by the South Texas Veterans Health Care System.
Author Contributions: R.A.D. and M.A.-G. wrote the initial draft of the manuscript. All authors reviewed and revised the manuscript.
Disclosure Summary: E.C. receives research support from Astra Zeneca and Janssen, and is on the speaker's bureaus of Janssen, Lily, Boehringer-Ingelheim, Astra Zeneca, and Sanofi. C.T. receives research support from Astra Zeneca and Janssen, and is on the speaker's bureaus of Janssen, Lily, Boehringer-Ingelheim, Astra Zeneca, and Sanofi. R.A.D. is on advisory boards of Astra Zeneca, Novo Nordisk, Janssen, Boehringer-Ingelheim, Intarcia, and Elcelyx; receives research support from Boehringer-Ingelheim, Takeda, Astra Zeneca and Janssen; and is on the speaker's bureaus of Novo-Nordisk and Astra Zeneca. The remaining authors have nothing to disclose.
Abbreviations:
- FPG
fasting plasma glucose
- HbA1C
hemoglobin A1c
- IS/IR
insulin secretion/insulin resistance index
- OGTT
oral glucose tolerance test
- SEM
standard error of the mean
- SGLT2
sodium-glucose transporter-2
- T2DM
type 2 diabetes mellitus
- ∆C-pep(0–360 min)
change in C-peptide concentration over 0 to 360 minutes.
References
- 1. DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Halban PA, Polonsky KS, Bowden DW, Hawkins MA, Ling C, Mather KJ, Powers AC, Rhodes CJ, Sussel L, Weir GC. β-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care. 2014;37(6):1751–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Talchai C, Lin HV, Kitamura T, Accili D. Genetic and biochemical pathways of beta-cell failure in type 2 diabetes. Diabetes Obes Metab. 2009;11(Suppl 4):38–45. [DOI] [PubMed] [Google Scholar]
- 4. Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, Bajaj M, Mandarino L, DeFronzo R, Cusi K. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 2003;52(10):2461–2474. [DOI] [PubMed] [Google Scholar]
- 5. Giaccari A, Sorice G, Muscogiuri G. Glucose toxicity: the leading actor in the pathogenesis and clinical history of type 2 diabetes - mechanisms and potentials for treatment. Nutr Metab Cardiovasc Dis. 2009;19(5):365–377. [DOI] [PubMed] [Google Scholar]
- 6. Rossetti L, Shulman GI, Zawalich W, DeFronzo RA. Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J Clin Invest. 1987;80(4):1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zini E, Osto M, Franchini M, Guscetti F, Donath MY, Perren A, Heller RS, Linscheid P, Bouwman M, Ackermann M, Lutz TA, Reusch CE. Hyperglycaemia but not hyperlipidaemia causes beta cell dysfunction and beta cell loss in the domestic cat. Diabetologia. 2009;52(2):336–346. [DOI] [PubMed] [Google Scholar]
- 8. Garvey WT, Olefsky JM, Griffin J, Hamman RF, Kolterman OG. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985;34(3):222–234. [DOI] [PubMed] [Google Scholar]
- 9. Wang D, Sun L, Song G, Chen S. Effects of intensive insulin therapy upon pancreatic β cell function in patients newly diagnosed with type II diabetes. Int J Clin Exp Med. 2015;8(1):1391–1395. [PMC free article] [PubMed] [Google Scholar]
- 10. Hu Y, Li L, Xu Y, Yu T, Tong G, Huang H, Bi Y, Weng J, Zhu D. Short-term intensive therapy in newly diagnosed type 2 diabetes partially restores both insulin sensitivity and β-cell function in subjects with long-term remission. Diabetes Care. 2011;34(8):1848–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weng J, Li Y, Xu W, Shi L, Zhang Q, Zhu D, Hu Y, Zhou Z, Yan X, Tian H, Ran X, Luo Z, Xian J, Yan L, Li F, Zeng L, Chen Y, Yang L, Yan S, Liu J, Li M, Fu Z, Cheng H. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371(9626):1753–1760. [DOI] [PubMed] [Google Scholar]
- 12. Abdul-Ghani MA, Norton L, DeFronzo RA. Renal sodium-glucose cotransporter inhibition in the management of type 2 diabetes mellitus. Am J Physiol Renal Physiol. 2015;309(11):F889–F900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Merovci A, Mari A, Solis C, Xiong J, Daniele G, Chavez-Velazquez A, Tripathy D, Urban McCarthy S, Abdul-Ghani M, DeFronzo RA. Dapagliflozin lowers plasma glucose concentration and improves β-cell function. J Clin Endocrinol Metab. 2015;100(5):1927–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, Broedl UC, Woerle HJ. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124(2):499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. [DOI] [PubMed] [Google Scholar]
- 16. DeFronzo RA, Ferrannini E, Hendler R, Felig P, Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes. 1983;32(1):35–45. [DOI] [PubMed] [Google Scholar]
- 17. Bergman RN, Finegood DT, Kahn SE. The evolution of beta-cell dysfunction and insulin resistance in type 2 diabetes. Eur J Clin Invest. 2002;32(Suppl 3)35–45. [DOI] [PubMed] [Google Scholar]
- 18. Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA; San Antonio metabolism study . Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia. 2004;47(1):31–39. [DOI] [PubMed] [Google Scholar]
- 19. Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29(5):1130–1139. [DOI] [PubMed] [Google Scholar]
- 20. Bunck MC, Cornér A, Eliasson B, Heine RJ, Shaginian RM, Taskinen MR, Smith U, Yki-Järvinen H, Diamant M. Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care. 2011;34(9):2041–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2007;292(3):E871–E883. [DOI] [PubMed] [Google Scholar]
- 22. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, Viberti G; ADOPT Study Group . Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–2443. [DOI] [PubMed] [Google Scholar]
- 23. Mari A, Sallas WM, He YL, Watson C, Ligueros-Saylan M, Dunning BE, Deacon CF, Holst JJ, Foley JE. Vildagliptin, a dipeptidyl peptidase-IV inhibitor, improves model-assessed beta-cell function in patients with type 2 diabetes. J Clin Endocrinol Metab. 2005;90(8):4888–4894. [DOI] [PubMed] [Google Scholar]
- 24. Esposito K, Chiodini P, Maiorino MI, Bellastella G, Capuano A, Giugliano D. Glycaemic durability with dipeptidyl peptidase-4 inhibitors in type 2 diabetes: a systematic review and meta-analysis of long-term randomised controlled trials. BMJ Open. 2014;4(6):e005442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Foley JE, Sreenan S. Efficacy and safety comparison between the DPP-4 inhibitor vildagliptin and the sulfonylurea gliclazide after two years of monotherapy in drug-naïve patients with type 2 diabetes. Horm Metab Res. 2009;41(12):905–909. [DOI] [PubMed] [Google Scholar]
- 26. Ahrén B, Johnson SL, Stewart M, Cirkel DT, Yang F, Perry C, Feinglos MN; HARMONY 3 Study Group . HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care. 2014;37(8):2141–2148. [DOI] [PubMed] [Google Scholar]
- 27. Polidori D, Mari A, Ferrannini E. Canagliflozin, a sodium glucose co-transporter 2 inhibitor, improves model-based indices of beta cell function in patients with type 2 diabetes. Diabetologia. 2014;57(5):891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takahara M, Shiraiwa T, Matsuoka TA, Katakami N, Shimomura I. Ameliorated pancreatic β cell dysfunction in type 2 diabetic patients treated with a sodium-glucose cotransporter 2 inhibitor ipragliflozin. Endocr J. 2015;62(1):77–86. [DOI] [PubMed] [Google Scholar]
- 29. Diamond MP, Thornton K, Connolly-Diamond M, Sherwin RS, DeFronzo RA. Reciprocal variations in insulin-stimulated glucose uptake and pancreatic insulin secretion in women with normal glucose tolerance. J Soc Gynecol Investig. 1995;2(5):708–715. [DOI] [PubMed] [Google Scholar]
- 30. Højberg PV, Vilsbøll T, Rabøl R, Knop FK, Bache M, Krarup T, Holst JJ, Madsbad S. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52(2):199–207. [DOI] [PubMed] [Google Scholar]
- 31. Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, Xiong J, Perez Z, Norton L, Abdul-Ghani MA, DeFronzo RA. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124(2):509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011;54(10):2506–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]