Abstract
Approximately half of individuals with Social Anxiety Disorder (SAD) treated with psychological intervention do not achieve clinically significant improvement or retain long-term gains. Neurobiological models of SAD propose that disruptions in functioning of amygdala-prefrontal circuitry is implicated in short-term treatment response. However, whether treatment-related changes in functional connectivity predict long-term well-being after psychotherapy is unknown. Patients with SAD completed an incidental emotion regulation task during fMRI before and after treatment with cognitive behavioral therapy or acceptance and commitment therapy (n=23, collapsed across groups). Psychophysiological interaction analyses using amygdala seed regions were conducted to assess changes in functional connectivity from pre- to post-treatment that predicted symptom change from 6 to 12-month follow-up. Negative change (i.e., greater inverse/weaker positive) in amygdala connectivity with the dorsomedial prefrontal cortex (dmPFC) and dorsal anterior cingulate cortex (dACC) predicted greater symptom reduction during follow-up. Positive change in amygdala connectivity with the cerebellum, fusiform gyrus, and pre-central and post-central gyri predicted less symptom reduction (e.g., no change or worsening). Results suggest that strengthened amygdala connectivity with regulatory regions may promote better long-term outcomes, whereas changes with visual and sensorimotor regions may represent sensitization to emotion-related cues, conferring poorer outcomes. Clinical implications for treatment personalization are discussed, should effects replicate in larger samples.
Keywords: fMRI, long-term treatment response, amygdala, prefrontal cortex, emotion regulation
Introduction
Social Anxiety Disorder (SAD), characterized by persistent and excessive fear of being scrutinized in social situations, is one of the most common anxiety disorders (Kessler et al., 2005). SAD is associated with significant impairment in social and occupational functioning, and often precedes the development of other comorbid psychiatric disorders including depression (Beesdo et al., 2007). Although extant psychological treatments can be efficacious in reducing social anxiety symptoms, approximately forty-five percent of individuals do not achieve clinically significant improvement in the long-term (Loerinc et al., 2015), making it important to investigate factors that contribute to sustained well-being. Neurobiological factors that alter through treatment provide insight into potential mechanisms of therapeutic change, both in the short-term and long-term. Knowledge of mechanisms can pave the way for treatment development and refinement, leading to more targeted and effective interventions.
Emotion regulation refers to flexible management of emotions in response to affective stimuli. Neurobiological models suggest that regions of the prefrontal cortex (PFC) are central to regulation of emotional reactivity in the amygdala through top-down connectivity (Berkman & Lieberman, 2009; Brühl, Delsignore, Komossa, & Weidt, 2014). Compared to healthy individuals, individuals with SAD demonstrate aberrant activity in and connectivity between the amygdala and prefrontal regions (Freitas-Ferrari et al., 2010). In response to social or emotional stimuli, amygdala hyper-reactivity has been consistently implicated in the pathophysiology of SAD (Davies et al., 2017), the degree of which also corresponds with symptom severity (Goldin, Manber, Hakimi, Canli, & Gross, 2009). Moreover, aberrant recruitment of cortical regions in SAD, including dorsolateral, ventrolateral, and medial prefrontal cortex (dl/vl/mPFC) and anterior cingulate cortex (ACC), may be linked to deficits in emotion regulation (Brühl, Delsignore, et al., 2014; Picó-Pérez, Radua, Steward, Menchón, & Soriano-Mas, 2017; Zilverstand, Parvaz, & Goldstein, 2017). However, findings have been inconsistent regarding the specific prefrontal sub-regions as well as direction of effects (both relatively increased (Brühl, Delsignore, et al., 2014; Goldin et al., 2009) or decreased activation (Zilverstand et al., 2017) compared to healthy individuals).
Discrepancies may in part be due to examination of activation rather than patterns of functional connectivity between limbic and prefrontal regions, which may serve as a more reliable biomarker (Nord et al., 2019). Functional connectivity studies have found that compared to healthy individuals, individuals with SAD demonstrate disrupted communication between the amygdala and prefrontal regions during resting state (vmPFC (Hahn et al., 2011)), as well as during implicit (vmPFC (Young et al., 2017)) and explicit emotion regulation tasks (dl/vlPFC (Goldin et al., 2009)). Additionally, evidence from dynamic causal modeling suggests impaired bidirectional effective connectivity from amygdala to vmPFC while perceiving emotional cues (Sladky, Höflich, et al., 2015).
Changes in this circuitry have been implicated as mechanisms in treatment response (Goldin et al., 2013, 2014; Young et al., 2017). Individuals with SAD demonstrated strengthened inverse connectivity (i.e., negative correlation in activity between two regions) between the amygdala and the dmPFC while reappraising negative self-beliefs after cognitive behavioral therapy (Goldin et al., 2013). Similar patterns were observed in a randomized control trial of cognitive behavioral therapy and acceptance and commitment therapy for SAD (Craske et al., 2014), wherein anxiety symptom reduction in either treatment condition corresponded to increases in inverse functional connectivity between the left amygdala-to-vmPFC and right amygdala-to-vlPFC during implicit emotion regulation (Young et al., 2017). In addition to changes in inverse connectivity between amygdala and prefrontal regions, enhanced positive connectivity among prefrontal regions (medial PFC, vlPFC, dlPFC, and dACC) while reappraising social criticism has been found after cognitive behavioral therapy (Goldin et al., 2014), possibly indicating increased communication between regions implicated in regulation.
Mechanistic research informs questions about how treatments work and requires evidence for change in a purported mediator to predict subsequent change in measures of outcome. Very few studies have evaluated neural change as a predictor of subsequent clinical outcomes, which is important to consider when identifying what factors contribute to sustained well-being after the end of psychological treatment. Instead, by far the majority of studies have evaluated baseline neural predictors of short-term clinical outcomes in SAD (Burklund, Torre, Lieberman, Taylor, & Craske, 2017; Doehrmann et al., 2013; Klumpp et al., 2017; Young et al., 2018). A limited number of studies have examined whether patterns of neural activation predict long-term outcomes (Furmark et al., 2002; Månsson et al., 2015). For example, weaker baseline dorsal anterior cingulate cortex (dACC)-amygdala functional connectivity in response to self-referential criticism predicted treatment-responder status one year after receiving internet-delivered CBT with attention bias modification (Månsson et al., 2015, 2017).
As noted, baseline prediction studies do not speak to treatment mechanisms, which requires investigation of whether changes in neural functioning throughout treatment predict outcomes at follow-up assessments. To date, only one positron emission tomography (PET) study has taken this approach, finding that attenuation in bilateral amygdala, periaqueductal gray, and left thalamus regional cerebral blood flow (rCBF) during a public speaking task predicted responder status one year after treatment with either a selective serotonin reuptake inhibitor or cognitive behavioral group therapy (Furmark et al., 2002). To our knowledge ours is the first study to investigate the extent to which treatment-related changes in functional connectivity during emotion regulation predict long-term outcomes for social anxiety disorder. Understanding changes in brain networks during emotion regulation holds promise for elucidating how treatments mechanistically influence neural plasticity to promote lasting clinical improvements.
The current study examined whether changes in functional connectivity during implicit emotion regulation (affect labeling) predict changes in symptoms from 6 months to 12 months after treatment. Affect labeling, the process of naming emotions, has been demonstrated to be an effective emotion regulation strategy, resulting in reductions in the intensity of emotional reactions to labeled stimuli (Lieberman, Inagaki, Tabibnia, & Crockett, 2011). Previous fMRI studies have shown that affect labeling (compared to a gender labeling control condition) is typically associated with reduced amygdala and enhanced prefrontal activation, as well as enhanced connectivity between these regions in healthy controls (Lieberman et al., 2007; Torre & Lieberman, 2018). Short-term changes in functional connectivity during affect labeling by completion of cognitive behavioral therapy (CBT) or acceptance and commitment therapy (ACT) observed using data from the parent study have been reported elsewhere from (Young et al., 2017), with post-treatment symptom reduction corresponding to increased inverse connectivity between the right amygdala-right vlPFC and left amygdala-vmPFC, irrespective of treatment type. Young et al. (2017) analyzed pre- to post-treatment neural changes during affect labeling that corresponded with pre- to post-treatment symptom reduction (defined using one self-report measure, the Liebowitz Social Anxiety Scale (Fresco et al., 2001)). The current study builds upon Young et al. (2017) by examining pre- to post-treatment neural changes during affect labeling that predict changes in a composite score of self-reported social anxiety symptoms from 6 to 12-month follow-up (using two self-report measures to capture cognitive, behavioral, and affective facets; methods described below).
We hypothesized that greater strengthening of functional connectivity in emotion regulation circuitry during treatment would predict greater symptom reduction from 6 to 12-month follow-up periods. Based on literature and theory of implicit emotion regulation and treatment prediction, we expected strengthened inverse connectivity between the amygdala to prefrontal regulatory regions (vlPFC, vmPFC, and dACC) to relate to better long-term outcomes.
Methods and materials
Full details regarding method and clinical outcomes are reported elsewhere, as data in the current study were collected as part of an RCT of CBT and ACT for SAD (Craske et al., 2014).
Participants
Participants were recruited through the University of California, Los Angeles (UCLA) Anxiety and Depression Research Center from referrals, flyers, and Internet and newspaper advertisements. The UCLA Office for the Protection of Human Research Subjects approved all procedures, and participants provided written informed consent. Participants were 18–45 years old, right-handed, English-speaking, and had a principal or co-principal diagnosis of SAD (demographic details reported in Table 1). Exclusion criteria consisted of a history of bipolar disorder, substance abuse or dependence in the past six months, suicidality, psychosis or psychiatric hospitalizations; standard MRI contraindications (claustrophobia, pregnancy, non-removable metal, serious medical conditions or brain injury); and concurrent psychotherapy for an anxiety disorder or changes in other psychotherapy within the past 6 months. Additionally, participants were required to be stabilized on psychotropic medication (with no changes within the past three months for SSRIs, SNRIs, and heterocyclics, or within the past month for benzodiazepines).
Table 1.
Participant demographic and clinical information. M = male, F = female, 12MFU = 12-month follow-up, CSR = clinical severity rating. Independent samples t-tests confirmed no treatment group differences in demographic or clinical variables (all p > 0.25).
| CBT | ACT | Total | |
|---|---|---|---|
| N | 11 | 12 | 23 |
| Age | |||
| Mean years | 26.85 | 26.48 | 26.65 |
| (SD) | (7.79) | (4.44) | (6.12) |
| Gender (M/F) | 7/4 | 5/7 | 12/11 |
| Ethnicity | |||
| Asian | 4 | 1 | 5 |
| Hispanic | 0 | 2 | 2 |
| Caucasian | 5 | 7 | 12 |
| African American | 0 | 0 | 0 |
| Other | 2 | 2 | 4 |
| Medication | 2 | 3 | 5 |
| Mean pre-treatment symptom composite | −0.16 | 0.14 | 0.00 |
| (SD) | (0.88) | (0.79) | (0.83) |
| Mean 6MFU symptom composite | −0.16 | −0.14 | 0.00 |
| (SD) | (0.83) | (104) | (0.90) |
| Mean 12MFU symptom composite | −0.02 | 0.07 | 0.01 |
| (SD) | (0.87) | (1.01) | (0.93) |
| Mean 12MFU-6MFU symptom change score | −0.18 | 0.12 | 0.00 |
| (SD) | (0.68) | (145) | (0.20) |
| Mean pre-treatment SAD CSR | 5.54 | 5.42 | 5.47 |
| (SD) | (0.69) | (1.08) | (0.89) |
| Mean 6MFU SAD CSR | 3.73 | 2.92 | 3.30 |
| (SD) | (2.00) | (138) | (1.72) |
| Mean 12MFU SAD CSR | 4.0 | 3.75 | 3.87 |
| (SD) | (1.61) | (1.71) | (163) |
Clinical and self-report measures
Trained interviewers conducted diagnostic evaluations using the Anxiety Disorders Interview Schedule-IV (ADIS IV (Brown, Barlow, & DiNardo, 1994)) to assign diagnoses and clinical severity ratings (CSR), with ratings of 4 or higher indicating clinically significant severity. Participants completed two valid and reliable self-report measures of social anxiety symptoms including the Social Interaction Anxiety Scale (SIAS (Mattick & Clarke, 1998)) and the Social Phobia Scale (SPS (Mattick & Clarke, 1998)). A symptom composite score was calculated from the two self-report measures to create a valid and reliable index of symptoms that encompasses cognitive, affective, and behavioral facets of social anxiety. The symptom composite reflects the mean of Z-scores for each measure at the 6-month follow-up (6MFU) and 12-month follow-up (12MFU) time-points. In the one case in which SIAS data was missing, the symptom composite was based on the mean of SPS Z-scores. A follow-up symptom change score was computed by subtracting the 12MFU symptom composite from the 6MFU symptom composite score.
Procedure & treatment
Eligible patients were randomized to either receive one of two manualized psychotherapy treatments for anxiety (Cognitive Behavioral Therapy, n=40; or Acceptance and Commitment Therapy, n=34), which involved 12 weekly 1-hour individual therapy sessions, or to the wait list (WL; n=26). As described in more detail elsewhere (Craske et al., 2014), both treatments involved exposure to feared social situations, as well as an emotion regulation component. In CBT, the emotion regulation component focused on cognitive restructuring of negative thoughts and breathing retraining, whereas ACT focused on acceptance and mindfulness. Patients who completed all 12 sessions of treatment were included in the present study. Following the 12 sessions of psychotherapy, therapists conducted follow-up booster phone calls (20–35 minutes) once a month for 6 months consistent with the assigned therapy modality.
From post-treatment to 12MFU, two participants changed psychotropic medication (one in CBT and one in ACT). Three began new psychotherapy (all three in the ACT group between 6MFU and 12MFU).
All participants who underwent treatment were invited to complete both pre- and post-treatment fMRI scanning sessions (see Figure 1 for details of numbers of participants who completed each session). As in previous work, we excluded participants with noisy fMRI data at either pre- or post-treatment scans (defined as >10% of images with global signal intensity > 2.5 SD of the mean, or affected by motion more than 2.5mm in any direction; see Table 2). An additional inclusion criterion for this study was the completion of both the 6-month (6MFU) and 12-month follow-up (12MFU) assessments, which occurred approximately 3 and 9 months after treatment completion respectively (i.e., 6 and 12-months after the start of treatment). Follow-up assessments involved symptom self-report measures and a diagnostic interview. The resulting sample of 23 patients was included in the present analysis. Given the lack of treatment group differences in both immediate and follow-up clinical outcomes (Craske et al., 2014), as well as functional connectivity in the affect labeling task (Young et al., 2017), the current analysis collapsed across treatment modality to create a combined sample. Independent samples t-tests on the connectivity estimates were used to explore potential group differences in the current sample.
Figure 1.
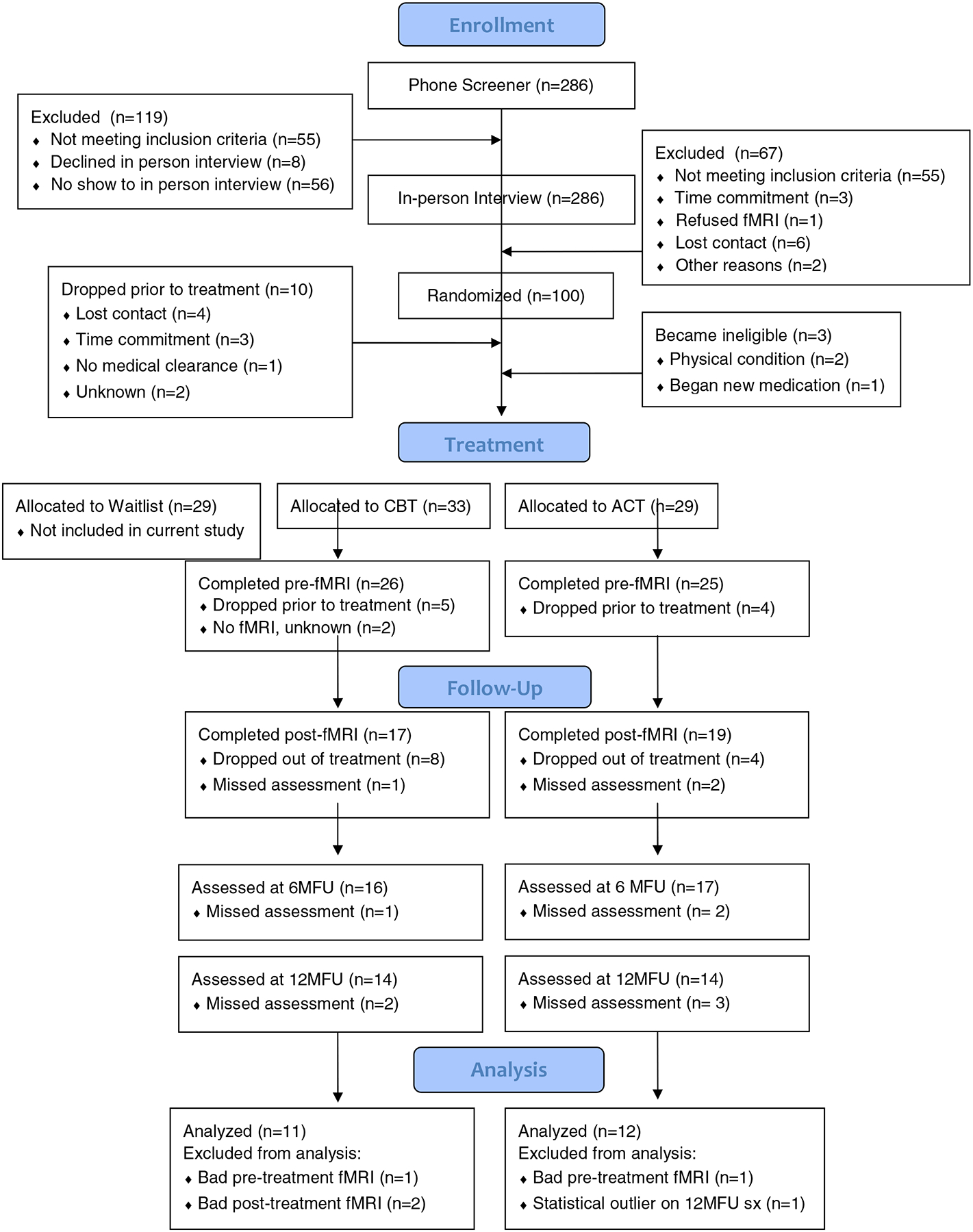
Patient flow chart with reasons for exclusion at each time point. CBT = cognitive behavioral therapy; ACT = acceptance and commitment therapy; 6MFU = 6-month follow-up; 12MFU = 12-month follow-up.
Table 2.
Pre- to post-treatment changes in functional connectivity from whole-brain PPI analysis using left and right amygdala seed regions (contrast: ‘Post [Affect Label – Gender Label] – Pre [Affect Label – Gender Label]’) correlated with change in symptom composite scores from 6 to 12-month follow-up, controlling for pre-treatment symptom composite scores. Peak Montreal Neurological Institute (MNI) coordinates and respective Broadmann’s Areas (BA) are reported for each cluster. All p < 0.05, corrected (see Methods).
| Amygdala seed (L/R) | Anatomical Region | BA | MNI Coordinates | Cluster size (voxels) | Peak t value |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Changes in functional connectivity (Post-Pre) correlation with 12 mo. Symptom Composite | |||||||
| Affect Label > Gender Label | |||||||
| L | dmPFC / R dACC | 9/10 | 0 | 53 | 25 | 152 | 4.288 |
| L | mid/posterior cingulate, L calcarine, L/R precuneus | 23/29/31 | 3 | −40 | 19 | 295 | 5.170 |
| L | L/R precuneus | 7 | −3 | −52 | 52 | 129 | 5.039 |
| L | L cerebellum, L lingual gyrus, L fusiform | 37 | −33 | −49 | −26 | 150 | −4.473 |
| L | L post-central gyrus, L pre-central gyrus | 2/3/4 | −45 | −10 | 46 | 217 | −4.348 |
Incidental emotion regulation task
Participants completed the affect labeling task (Lieberman et al., 2007), commonly used in the laboratory as a measure of implicit emotion regulation independent of effort or intentionality (for a review see Torre & Lieberman, 2018). Affect labeling is thought to serve as a form of incidental emotion regulation that reduces negative affect by “putting feelings into words.” While cognitive behavioral therapy and acceptance and commitment therapy train explicit emotion regulation through different strategies, implicit regulation via affect labeling may tap into processes that are common across treatment types. In the task (see Figure 2), participants viewed images of emotional facial expressions (anger, fear, disgust) or geometric shapes while instructed to complete labeling or matching tasks (for full details of the affect labeling task see Burklund et al., 2015). Consistent with prior research, we focused on the contrast between the Affect Label (choosing which of two words matches the facial expression) and Gender Label (choosing which name matches the gender of the face) conditions to isolate incidental emotion regulation processes. This contrast examines emotional verbal-linguistic processing specifically while controlling for response selection, verbal processing, and emotion perception (Lieberman et al., 2007).
Figure 2.
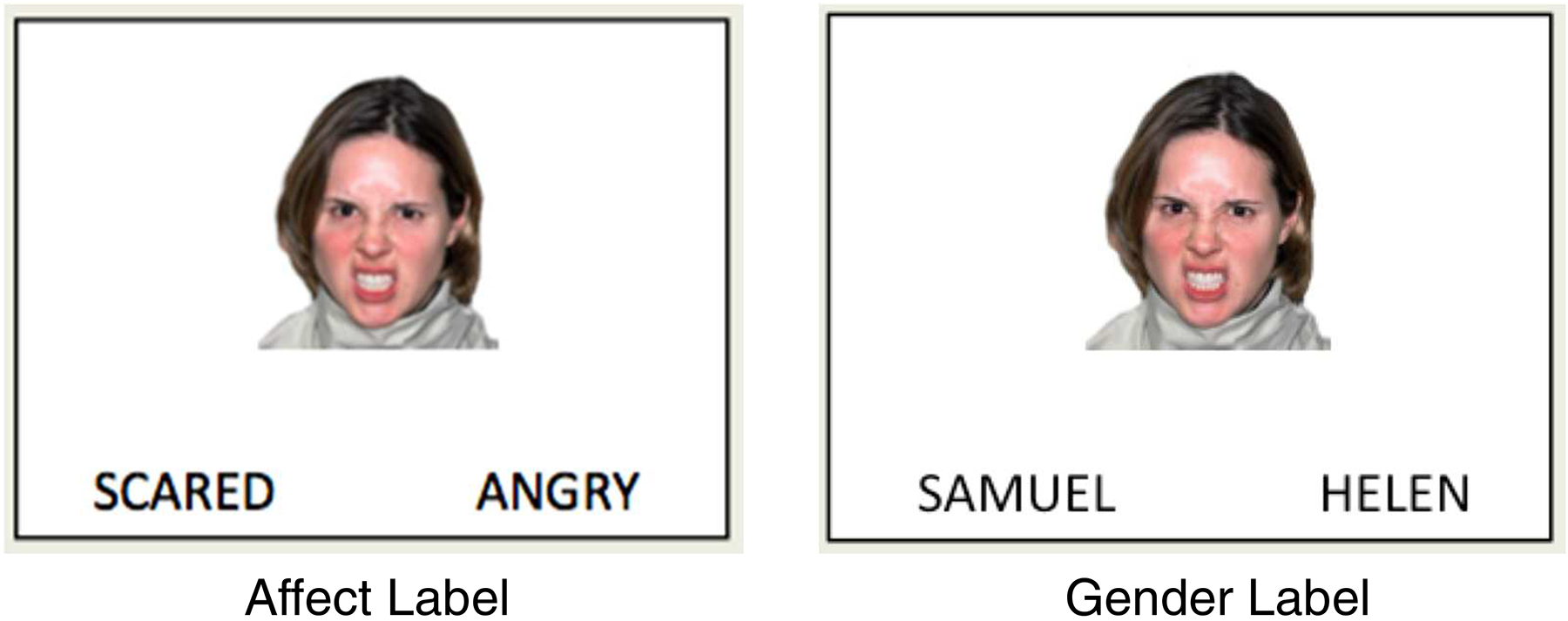
Example stimuli from the contrast of interest (Affect Label > Gender Label) in the affect labeling task.
The task consisted of four blocks of each condition type with six trials per block, with condition order counterbalanced across participants. Each block began with a 3 second instruction cue corresponding with the condition type. Each trial lasted for 5 seconds, during which the stimulus was presented for the entire duration, followed by a 10 second inter-trial-interval, during which a fixation cross was displayed.
fMRI acquisition
Magnetic resonance images were acquired at the UCLA Ahmanson-Lovelace Brain Mapping Center on a Trio 3.0 Tesla MRI scanner. A high-resolution structural T2-weighted echo-planar imaging volume (spin-echo, TR=5000 ms, TE=34 ms, matrix size=128×128, resolution=1.6mm×1.6mm×3mm, FOV=200 mm, 36 slices, 3mm thick, flip angle=90°, bandwidth=1302 Hz/Px) was acquired for each participant coplanar with functional scans. A total of 344 volumes were acquired from four functional runs (gradient-echo, TR=3000 ms, TE=25 ms, flip angle=90°, matrix size=64×64, resolution=3.1mmx3.1mmx3.0 mm, FOV=200 mm, 36 axial slices, 3mm thick, bandwidth=2604 Hz/Px).
fMRI data analysis
Imaging data were analyzed using SPM8 (Wellcome Trust Center for Neuroimaging, University College London, UK: http://www.fil.ion.ucl.ac.uk). Functional images for each participant were realigned to correct for head motion, co-registered to the high-resolution structural images, normalized into a standard stereotactic space as defined by the Montreal Neurological Institute and smoothed with an 8mm Gaussian kernel full width at half maximum. Experimental blocks were modeled using a boxcar function convolved with the canonical hemodynamic response. Motion parameters were included in the model as regressors of no interest.
Generalized psychophysiological interaction (gPPI) analyses were conducted in SPM8 (McLaren, Ries, Xu, & Johnson, 2012) using the left and right amygdala as seed regions (defined with Automated Anatomical Labeling atlas, Tzourio-Mazoyer et al., 2002) to assess functional connectivity (for Affect Label > Gender Label contrast) for each individual at pre and post-treatment. Cortical regions of interest (ROIs) were functionally-defined using the search term ‘emotion regulation’ in the meta-analytic tool Neurosynth (www.neurosynth.org; Yarkoni et al., 2012). The resulting forward inference map was parsed into clusters to the left and right vlPFC, vmPFC, and dACC (Figure 3). The ‘dACC’ cluster was additionally constrained to include only voxels within the cingulate gyrus using the probabilistic mask of the anterior cingulate gyrus (thresholded at >50%) from the Harvard-Oxford anatomical atlas. The Marsbar toolbox in SPM (Brett et al., 2002) was used to extract parameter estimates (beta values) for each ROI, which were used to create neural change scores [Post (Affect Label – Gender Label) – Pre (Affect Label – Gender Label)]. Connectivity estimate outliers, defined as greater than 2.5 standard deviations from the mean, were removed from analyses (n=1). To investigate the relationship between treatment-related change in functional connectivity and symptom change from 6 to 12-month follow-up, we conducted linear regression in SPSS 25 by regressing the follow-up symptom change score (12MFU – 6MFU symptom composite scores) onto the neural change scores for each of the four ROIs.
Figure 3.
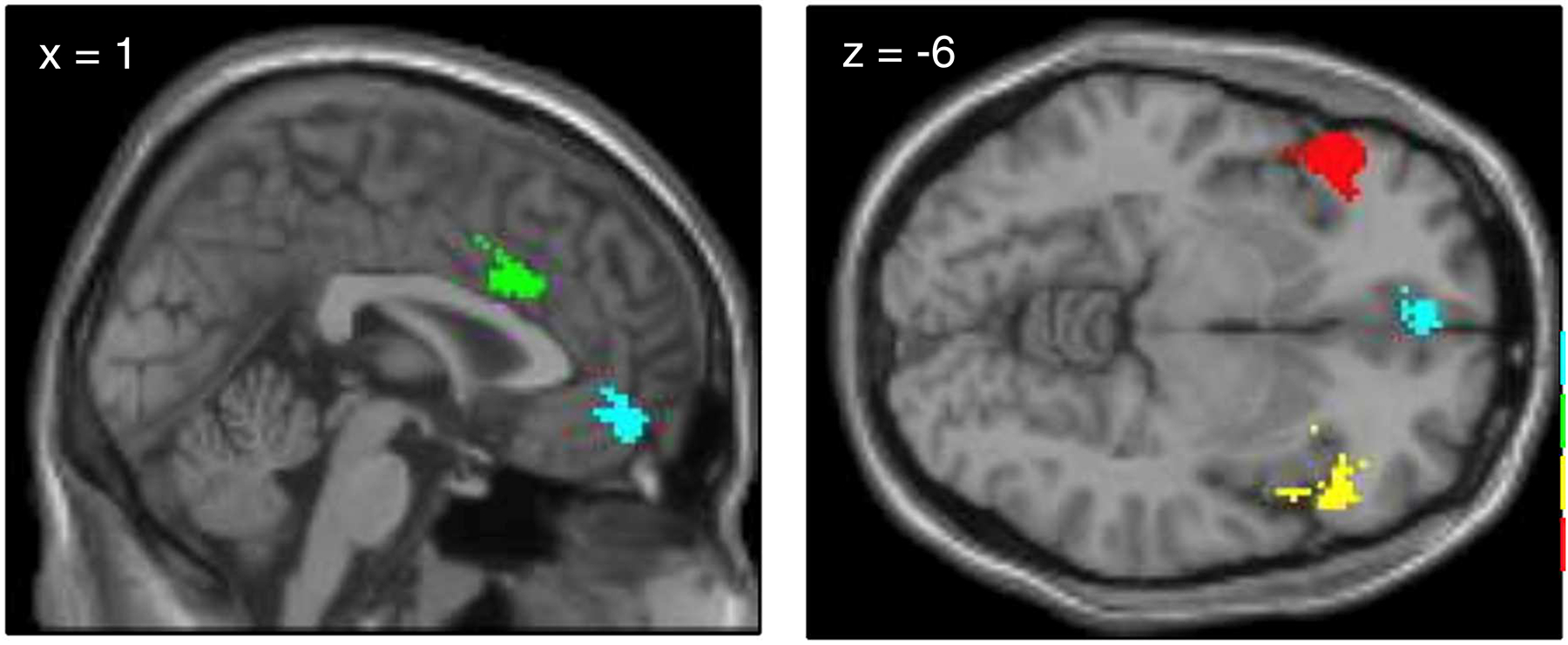
Regions of interest for connectivity analyses, defined using Neurosynth displaying left and right vlPFC (red and yellow), vmPFC (blue), and dACC (green).
In addition to ROI analyses, we conducted exploratory whole-brain analyses using the left and right amygdala seed regions. Images reflecting change in functional connectivity from pre- to post-treatment were computed [Post (Affect Label > Gender Label) – Pre (Affect Label> Gender Label)], using imcalc (SPM8). A one sample t-test was computed on the neural change scores with a covariate of interest (follow-up symptom change score) and a covariate of no interest (pre-treatment symptom composite score) to control for pre-treatment severity. Analyses were constrained within a whole-brain standardized grey matter mask (derived from SPM’s grey.nii). Whole-brain analyses were corrected for multiple comparisons using the autocorrelation (-acf) function in 3dFWHMx and 3dClustSim (two-tailed tests; AFNI version 19.0.15: http://afni.nimh.nih.gov/afni/) to account for the non-Gaussian nature of fMRI data (Eklund, Nichols, & Knutsson, 2016). To achieve an alpha level of .05, the voxel-wise threshold was set to p<.005 combined with a minimum cluster size of k > 67 voxels.
Results
Behavioral task performance and pre-treatment neural activity during the affect labeling task is reported in another paper (see Burklund et al., 2015). In brief, there were no significant differences in response times or error rates for the Affect Label or Gender Label conditions in the SAD compared to the healthy control (HC) group. Compared to the HC group, the SAD group demonstrated greater activity in the rostral ventrolateral prefrontal cortex (rvlPFC) and amygdala. A direct comparison revealed that only amygdala activity was significantly greater in the SAD than HC group. In the current sample, a one-sample t-test revealed that there was not a significant difference in amygdala activation between the Affect Label versus Gender Label conditions (right amygdala: t(22)=0.61, p=0.55: left amygdala: t(22)=0.04, p=0.96), although the effect was in the same direction as Burklund et al. (2015) with greater amygdala activation during Affect Label compared to Gender Label. While amygdala activation was not significantly different between conditions, amygdala activation during both conditions was significantly greater than implicit baseline: one-sample t-tests confirmed significant activation at pre-treatment in the left and right amygdala ROI’s during the Affect Label (left amygdala: t(22)= 2.88, p = 0.009; right amygdala: t(22) = 4.25, p < 0.001) and Gender Label (left amygdala: t(22)=3.99, p < 0.001; right amygdala: t(22) = 3.97, p <0.001) conditions.
Region of interest analyses
Simple linear regressions revealed no significant relationships between 6 to 12-month follow-up symptom change and pre- to post-changes in right amygdala connectivity with any of the ROIs (left vlPFC: β = 0.84, p = 0.13; right vlPFC: β = 0.67, p = 0.22; vmPFC: β = 0.55, p = 0.07; dACC; β = 0.29, p = 0.31). The marginal relationship between follow-up symptom change and right amygdala-vmPFC connectivity was weakened when pre-treatment symptoms were controlled for in the model (β = 0.38, p = 0.18). Similarly, no significant relationships were found between follow-up symptom change and changes in left amygdala connectivity with the ROIs (left vlPFC: β = −0.03, p = 0.96; right vlPFC: β = 0.07, p = 0.92; vmPFC: β = 0.20, p = 0.66; dACC; β = 0.39, p = 0.31).
Whole-brain analyses
A one-sample t-test was conducted on the neural change scores with the 6 to 12-month follow-up symptom change scores entered as a covariate of interest and pre-treatment symptom composite scores entered as a covariate of no interest (Table 2, Figure 4). Greater follow-up symptom reduction was associated with more negative change (i.e., reduced positive connectivity/enhanced negative connectivity) between the left amygdala and the dmPFC, dACC, midcingulate and posterior cingulate cortex, left calcarine sulcus, and left and right precuneus. Less follow-up symptom reduction was associated with positive change (i.e., enhanced positive connectivity/reduced negative connectivity) between the left amygdala and the left cerebellum, left lingual gyrus, left fusiform gyrus, and left pre- and post-central gyri connectivity.
Figure 4.
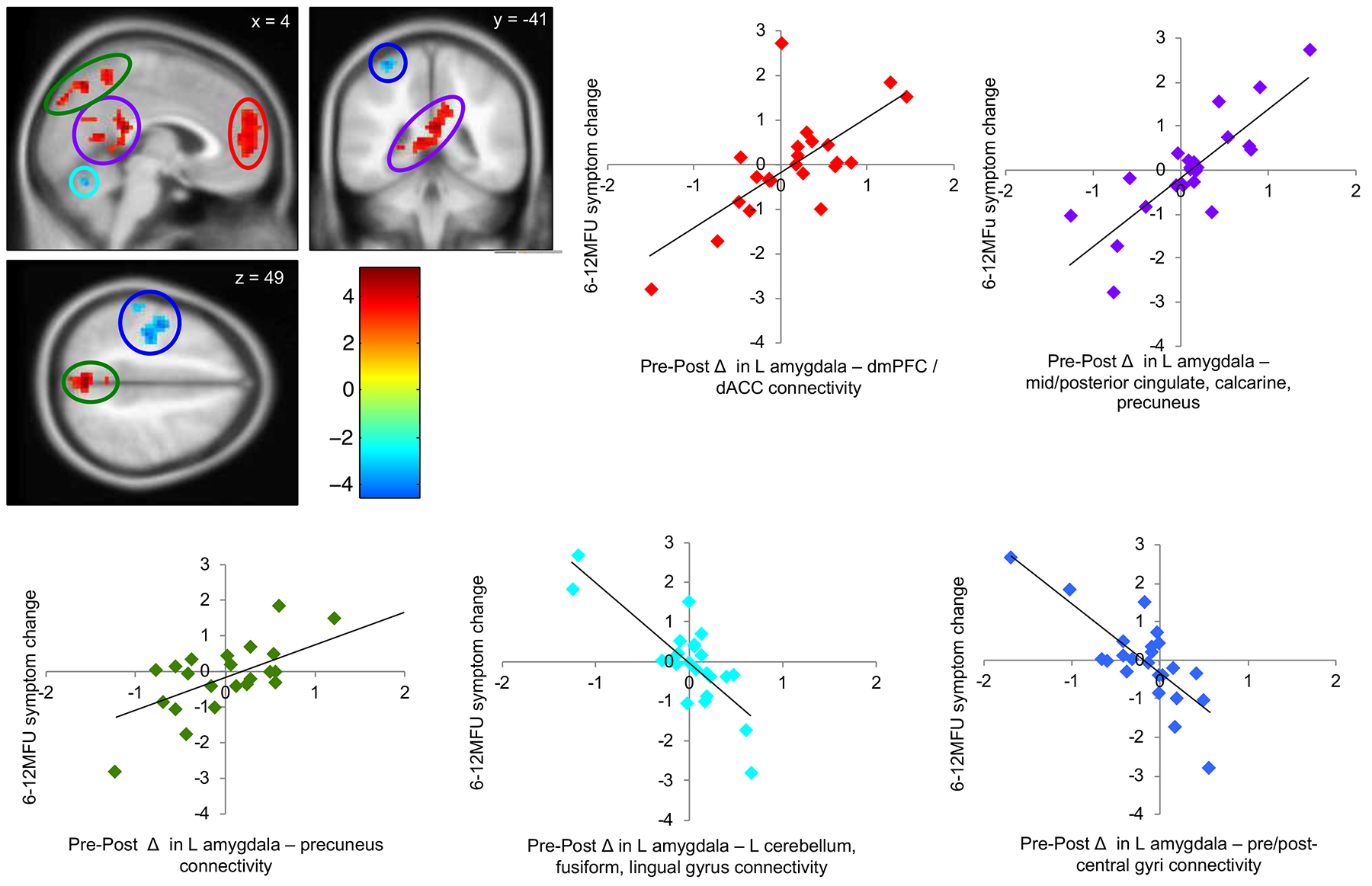
Pre- to post-treatment changes in left amygdala functional connectivity during affect labeling were associated with changes in symptoms from 6 to 12-month follow-up, controlling for pre-treatment symptom severity. Lower symptom change scores reflect greater improvement in social anxiety symptom severity from 6 to 12-month follow-up. Red indicates changes in left amygdala connectivity with dmPFC and dACC; purple indicates left amygdala connectivity with mid- to posterior cingulate cortex, calcarine and precuneus; green indicates left amygdala connectivity with precuneus; light blue indicates left amygdala connectivity with the cerebellum; dark blue indicates left amygdala connectivity with the pre- and post-central gyrus. All p < 0.05, corrected (see Methods).
Independent samples t-tests on the connectivity estimates from the five resulting clusters confirmed that there were no treatment group differences in functional connectivity (all p > 0.20). Similarly, there were no group differences on the 6 to 12-month follow-up symptom change scores in the present sample (t(21) = 0.62, p = 0.54). Interaction plots (see Supplementary Materials, Figures S1 and S2) demonstrate that the direction of the relationship between functional connectivity and 6 to 12-month follow-up symptoms changes from pre- to post-treatment for both AL and GL conditions. Specifically, the association between amygdala connectivity with several regions (dmPFC/dACC, mid-posterior cingulate cortex, and precuneus) and follow-up symptoms changed from a negative correlation at pre to a positive correlation at post. The association between amygdala connectivity with other regions (cerebellum/fusiform/lingual gyrus and pre/post-central gyri) and follow-up symptoms switched from a positive correlation at pre to a negative correlation at post.
Discussion
Changes in functional connectivity from pre to post cognitive behavioral therapy or acceptance and commitment therapy for social anxiety disorder significantly predicted clinical outcomes during 6-month to 12-month follow-up. Specifically, negative change in connectivity (i.e., enhanced inverse or attenuated positive connectivity) between the left amygdala and the dorsomedial PFC, dorsal anterior cingulate cortex, mid- to posterior cingulate cortex, calcarine sulcus, and precuneus during implicit emotion regulation was associated with greater social anxiety symptom reduction from 6 to 12-month follow-up. Conversely, positive change in left amygdala connectivity with the cerebellum, lingual gyrus, fusiform gyrus, pre-central gyrus and post-central gyrus was associated with less symptom reduction (no change or worsening) from 6 to 12-month follow-up. Notably, pre- to post-treatment neural change predicted follow-up symptom change over and above pre-treatment symptom severity. Further, we found that the direction of the relationship between functional connectivity and symptoms changed as a result of therapy, suggesting that pre-treatment functional connectivity, or changes in functional connectivity during Gender Labeling alone, are not solely driving the effects, but that change over time is also an important factor.
Emotion Regulation Circuitry
Our findings suggest that change in the strength of neural connectivity during psychological treatment significantly impacts long-term treatment outcomes. By identifying patterns of neural connectivity that may indicate lasting therapeutic success after treatment, this study begins to address mechanisms of therapeutic change. In particular, strengthened inverse amygdala connectivity with the dorsomedial prefrontal cortex and anterior cingulate from pre- to post-treatment predicted superior long-term outcomes. This pattern of results points to changes in emotion regulation circuitry as a potential mechanism of effective psychotherapy for social anxiety disorder. The results are consistent with neurobiological models of prefrontal cortical down-regulation of affective regions such as the amygdala (Ochsner, Silvers, & Buhle, 2012).
The dmPFC, implicated in social cognition (Bzdok et al., 2013; Lieberman, Straccia, Meyer, Du, & Tan, 2019), mentalizing (Zaki & Ochsner, 2012), and monitoring one’s own and others’ emotional states (Ochsner et al., 2012), is thought to play a role in emotion regulation (Buhle et al., 2014; Ochsner & Gross, 2005). In particular, when using perspective-taking as an emotion regulation strategy, healthy individuals engaged the same anterior portion of the dmPFC found in the present study, which modulated amygdala activation (Gilead et al., 2016). Further, greater dmPFC activation and connectivity with the amygdala corresponded to reappraisal success in both healthy individuals (Banks, Eddy, Angstadt, Nathan, & Phan, 2007; Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008) and patients with social anxiety disorder (Goldin et al., 2009). Our results are consistent with treatment studies, which have found strengthened dmPFC activation as well as inverse connectivity with the amygdala during reappraisal after cognitive behavioral therapy (Goldin et al., 2013). Although the region of the dmPFC found in this study is partially overlapping with those mentioned above (e.g., Banks et al., 2007; Gilead et al., 2016; Wager et al., 2008), relatively more dorsal and posterior regions of the medial PFC (i.e., closer to the pre-supplementary motor area) are typically implicated in meta-analyses of emotion regulation (Buhle et al., 2014). Future research should clarify the role of distinct subregions of the dorsomedial prefrontal cortex, especially with respect to amygdala connectivity.
The ACC lies at the nexus of large scale networks involved in regulating attention to internal and external stimuli, and has been implicated in modulating amygdala activation in the presence of emotional distractors (Etkin, Egner, Peraza, Kandel, & Hirsch, 2006). Historically, the ACC has been associated with a multitude of psychological functions; however, a reverse-inference meta-analysis indicated that the dACC specifically is related to pain and distress-related emotions rather than conflict, salience, or executive processes (Jahn, Nee, Alexander, & Brown, 2016; Lieberman & Eisenberger, 2015). Relatedly, the dACC may relate to survival goals, making it sensitive to social rejection, a central component of social anxiety disorder (Lieberman & Eisenberger, 2015).
While the ROI-specific analyses with the dACC region did not yield significant relationships, we found in the whole-brain analyses that negative change in amygdala connectivity with relatively more rostral regions of the dACC predicted greater social anxiety symptom improvement during follow-up. These findings are consistent with prior work implicating amygdala-ACC connectivity for prediction of short-term treatment response (Burklund et al., 2017; Fitzgerald et al., 2017; Månsson et al., 2015). It is worth noting that these results could mean either weakened positive connectivity or strengthened inverse connectivity at post-treatment. Changes in amygdala-dACC connectivity through treatment may reflect less coupling between threat-detection (Phelps & LeDoux, 2005) and social distress (Lieberman & Eisenberger, 2015) regions respectively during implicit emotion regulation, which in turn relate to superior outcomes at follow-up. Reduced coupling between these regions might mean that the amygdala does not trigger the dACC subjective distress response as easily or often, thus conferring better long-term outcomes. Alternatively, our results may reflect strengthened inverse amygdala-dACC connectivity. Given that a similar pattern of inverse connectivity has been observed during successful emotion regulation in healthy controls (Banks et al., 2007; Ochsner et al., 2012), normalization of this circuitry during treatment for social anxiety disorder may be responsible for long-term treatment gains. As the PPI analyses used here do not allow inference of directionality within neural circuitry, it is not possible to state which of these two interpretations is more likely. Future work might therefore examine directionality within this circuitry more closely to determine the functional role of these regions in short- and long-term clinical response.
In our prior study, social anxiety symptom improvement immediately after cognitive behavioral therapy or acceptance and commitment therapy corresponded to strengthened inverse amygdala connectivity with the vlPFC and vmPFC during affect labeling (Young et al., 2017). Contrary to these short-term effects, changes in amygdala connectivity with ventral and lateral prefrontal regions were not associated with long-term outcomes. Instead, we found that enhanced inverse amygdala connectivity with medial regions including dmPFC, rmPFC, dACC, and pgACC predicted better outcomes from 6 to 12-month follow-up.
Our findings can be conceptualized within a recent framework of emotion regulation along two dimensions: the goal, ranging from implicit to explicit, and the change process, ranging from automatic to controlled (Braunstein, Gross, & Ochsner, 2017). Affect labeling is considered a hybrid “implicit-controlled” strategy, characterized by an implicit regulatory goal (i.e. individuals do not intend to regulate when labeling) and engagement of controlled processes (i.e. the labeling itself is likely a controlled process). Neural regions implicated in this class of emotion regulation strategies include the posterior mPFC, dACC, and vlPFC. Our findings were consistent with the exception of the vlPFC, which is traditionally found during affect labeling in healthy controls (Lieberman et al., 2007; Torre & Lieberman, 2018) and was implicated in short-term treatment response in our previous analyses (Young et al., 2017). It is currently unknown why vlPFC-amygdala connectivity was not implicated in long-term symptom reduction. It may be that whereas vlPFC regulation of amygdala responses may be important in immediate responses to treatment, long-term benefit may rely on a different set of neural mechanisms. For example, it is possible for controlled processes to become more habitual/automatic through practice, which could correspond to shifts in regulatory control from lateral to medial PFC regions (Braunstein et al., 2017). Future work should further investigate changes in prefrontal functional connectivity over time, across the various domains of emotion regulation, including implicit vs. explicit goals, and automatic vs. controlled processes.
Other Regions
Strengthened inverse amygdala connectivity with several other regions was predictive of long-term outcomes. Left amygdala connectivity with the midcingulate and posterior cingulate cortex, precuneus, and calcarine sulcus corresponded to changes in follow-up symptoms in a similar direction. The extent to which these regions contribute to effective regulation is unknown. Individuals with social anxiety have been found to exhibit decreased amygdala-posterior cingulate/precuneus connectivity while viewing facial expressions (Hahn et al., 2011). Change in amygdala connectivity with visual regions, such as the precuneus and calcarine sulcus, may suggest altered emotional reactivity. Our findings suggest that normalization of this circuitry through treatment may relate to superior long-term outcomes. Functioning of this circuitry as well as other important processes beyond emotion regulation should be further investigated, especially as it relates to sustained long-term treatment gains.
Strengthened positive connectivity
Positive change in amygdala connectivity predicted poorer long-term outcomes, such that left amygdala connectivity with the cerebellum, lingual gyrus, fusiform gyrus, and pre- and post-central gyri was related to less symptom improvement from 6 to 12-month follow-up.
The cerebellum, traditionally associated with motor coordination and behavior (Stein & Glickstein, 1992), has been theorized to play a role in emotion (Schutter & Van Honk, 2005). There is some evidence that individuals with social anxiety demonstrate cerebellum hypo-activation during passive viewing (Nakao et al., 2011) and reappraisal of social stimuli (Ziv, Goldin, Jazaieri, Hahn, & Gross, 2013) compared to healthy controls, although studies have not investigated functional connectivity with the amygdala. Further research is needed to clarify the role of cerebellar functioning as it relates to emotion regulation in anxiety disorders.
The lingual gyrus, involved in visual word processing, is also activated while verbalizing high-emotion words compared to neutral-emotion words (Isenberg et al., 1999). In the context of affect labeling, a strategy that down-regulates emotions by “putting feelings into words” (Lieberman et al., 2007), strengthened positive connectivity between the amygdala’s threat response and the lingual gyrus’ visual word representation may represent sensitization to emotion-related cues, thus conferring poorer long term outcomes.
The fusiform gyrus plays a central role in visual face perception (McCarthy, Puce, Gore, & Allison, 1997), and has been shown in a meta-analyses to be hyper-reactive to negative emotional stimuli in social anxiety disorder (Brühl, Delsignore, et al., 2014; Etkin & Wager, 2007). Compared to healthy controls, patients with social anxiety disorder have demonstrated greater fusiform gyrus activation in response to fearful faces, as well as greater fusiform-amygdala connectivity, which corresponded with greater social anxiety severity (Frick, Howner, Fischer, Kristiansson, & Furmark, 2013). Our results are complementary, such poorer outcomes at follow up (no change or worsening of symptoms) related to strengthened positive amygdala-fusiform connectivity, perhaps reflecting sensitization to aversive stimuli (faces) across treatment.
In the context of the emotional experience, the pre-central gyrus may relate to preparation of motor responses to affective material (Hardee et al., 2017). Strengthened positive connectivity between the pre- and post-central gyri and amygdala across the course of treatment may reflect persistent physiological reactivity or motoric responding to emotional faces, in turn predicting poorer outcomes. However, we did not find changes in amygdala connectivity with the insula, which would be expected, given its implication in interoception and pain processing in anxiety disorders (Paulus & Stein, 2010). Future work should clarify the role of these regions during emotion regulation as it relates to social anxiety.
Limitations
One limitation of this study is the small sample size, in part due to missing clinical data at follow-up and excluded fMRI data due to motion, which prevented us from examining the effects of medication, additional psychotherapy, and comorbidities. As psychotropic medications, such as selective serotonin reuptake inhibitors, have been shown to enhance prefrontal down-regulation of the amygdala in healthy controls (Sladky, Spies, et al., 2015) and in depressed patients (Heller et al., 2013; Maslowsky et al., 2010), medication effects are an important consideration for future research on emotion regulation. Furthermore, three patients in the current study (all in the ACT group) began a new psychotherapy between the 6 and 12-month follow-up, which may have contributed to continued symptom improvement. On the other hand, the lack of group differences in symptom change from 6 to 12-month follow-up suggests that that additional psychotherapy did not have a significant impact. It is also important to note that the potential impact of booster phone calls between follow-up periods also cannot be ruled out as an explanation for symptom improvement. In addition, the effect of comorbidities on emotion regulation circuitry is also worthy of further examination, given evidence of differential neural activity during affect labeling depending on depression comorbidity status in SAD (Burklund et al., 2015).
Further, although our findings fit well within a framework of prefrontal down-regulation of amygdala, the functional connectivity analysis used in the present study cannot conclude the direction of communication between regions. Indeed, evidence from dynamic causal modeling has demonstrated bidirectional connectivity between the amygdala and orbitofrontal cortex and ventral PFC during emotional processing (Sladky, Höflich, et al., 2015). Future work should use these approaches to investigate specific patterns of communication between regions that relate to clinically meaningful change.
Finally, as the waitlist control group did not complete follow-up assessments, we did not have the necessary temporal precedence in the control group as well as the treatment group to conduct a formal mediational model testing whether neural change was responsible for follow-up symptoms as an effect of treatment. While our results suggest that changes in emotion regulation circuitry may be a mechanism associated with lasting treatment response, future research should apply mediational analyses to examine this possibility.
Conclusion
Our results suggest that treatment-related changes in functional connectivity predict long-term therapeutic success for social anxiety disorder. Changes in amygdala connectivity with the dorsomedial prefrontal cortex and dorsal anterior cingulate through treatment may reflect strengthened emotion regulation, in turn promoting lasting therapeutic success. Conversely, changes in amygdala connectivity with sensorimotor and visual word/face processing areas may relate to sensitization to emotion-related cues, conferring poorer long-term outcomes. However, it should be noted that effects were observed in a limited sample size and investigation of the replicability of these findings in a larger sample is warranted. Should the effects be found to generalize, these findings would have several clinical implications. For instance, training in cognitive bias modification might be a targeted way of achieving patterns of neural change that overlap with our findings (Wiers & Wiers, 2017). Further, this knowledge could be applied to treatment personalization by using patterns of neuroimaging to evaluate whether a treatment is working, and to guide how long (i.e., the number of therapy sessions) a patient needs to stay in treatment in order to achieve a sustained response. Knowledge of key neural circuitry may also help guide treatment for affective disorders with neurofeedback to promote efficient emotion regulation (Brühl, Scherpiet, et al., 2014; Johnston et al., 2011; Zotev, Phillips, Young, Drevets, & Bodurka, 2013). These clinical implications have the potential to improve the cost-effectiveness of treatments by enhancing the efficiency, potency, and longevity of psychotherapeutic interventions for anxiety disorders.
Supplementary Material
Highlights.
We assessed functional connectivity after psychotherapy for social anxiety disorder in relation to long-term outcomes.
Treatment-related change in amygdala connectivity with the dACC and dmPFC related to symptom reduction during follow-up.
Change in amygdala connectivity with visual and sensorimotor regions predicted poorer long-term outcomes.
Strengthened emotion regulation circuitry may be a mechanism that contributes to long-term well-being outcomes after treatment.
Acknowledgements
The authors would like to thank the participants in this study as well as the staff of the Anxiety and Depression Research Center (ADRC) at UCLA, especially Bita Mesri, who contributed to data collection and management of this study.
Role of the funding source
This project was funded by the National Institute of Mental Health 1 R21 MH081299 (PIs: Craske, Lieberman & Taylor) and the UCLA Psychology Department Graduate Fellowship. This funding source had no role in the execution of this study, analyses, interpretation of data, or decision to submit results. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, & Phan KL (2007). Amygdala–frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience, 2(4), 303–312. Retrieved from http://scan.oxfordjournals.org/content/2/4/303.short [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Bittner A, Pine DS, Stein MB, Höfler M, Lieb R, & Wittchen H-U (2007). Incidence of Social Anxiety Disorder and the Consistent Risk for Secondary Depression in the First Three Decades of Life. Archives of General Psychiatry, 64(8), 903 10.1001/archpsyc.64.8.903 [DOI] [PubMed] [Google Scholar]
- Berkman ET, & Lieberman MD (2009). Using Neuroscience to Broaden Emotion Regulation: Theoretical and Methodological Considerations. Social and Personality Psychology Compass, 3(4), 475–493. 10.1111/j.1751-9004.2009.00186.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein LM, Gross JJ, & Ochsner KN (2017). Explicit and implicit emotion regulation: A multi-level framework. Social Cognitive and Affective Neuroscience, 12(10), 1545–1557. 10.1093/scan/nsx096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Barlow DH, & DiNardo PA (1994). Anxiety Disorders Interview Schedule for DSM-IV (ADIS-IV): Client Interview Schedule/. Graywind Publications Incorporated; Retrieved from https://books.google.com/books?hl=en&lr=&id=NSsWKR5tG8AC&oi=fnd&pg=PP2&dq=dinardo+brown+barlow+&ots=Y-8NzWz9Of&sig=lIrAsNOpCaMF356-j4ZSrMcvYT0 [Google Scholar]
- Brühl AB, Delsignore A, Komossa K, & Weidt S (2014). Neuroimaging in social anxiety disorder—A meta-analytic review resulting in a new neurofunctional model. Neuroscience & Biobehavioral Reviews, 47, 260–280. 10.1016/J.NEUBIOREV.2014.08.003 [DOI] [PubMed] [Google Scholar]
- Brühl AB, Scherpiet S, Sulzer J, Stämpfli P, Seifritz E, & Herwig U (2014). Real-time neurofeedback using functional MRI could improve down-regulation of amygdala activity during emotional stimulation: A proof-of-concept study. Brain Topography, 27(1), 138–148. 10.1007/s10548-013-0331-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, … Ochsner KN (2014). Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cerebral Cortex, 24(11), 2981–2990. 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burklund LJ, Craske MG, Taylor SE, & Lieberman MD (2015). Altered emotion regulation capacity in social phobia as a function of comorbidity. Social Cognitive and Affective Neuroscience, 10(2), 199–208. 10.1093/scan/nsu058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burklund LJ, Torre JB, Lieberman MD, Taylor SE, & Craske MG (2017). Neural responses to social threat and predictors of cognitive behavioral therapy and acceptance and commitment therapy in social anxiety disorder. Psychiatry Research - Neuroimaging, 261(August 2015), 52–64. 10.1016/j.pscychresns.2016.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Schilbach L, Engemann DA, Laird AR, Fox PT, & Eickhoff SB (2013). Segregation of the human medial prefrontal cortex in social cognition. Frontiers in Human Neuroscience, 7, 232 10.3389/fnhum.2013.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Niles AN, Burklund LJ, Wolitzky-Taylor KB, Vilardaga JCP, Arch JJ, … Lieberman MD (2014). Randomized controlled trial of cognitive behavioral therapy and acceptance and commitment therapy for social phobia: Outcomes and moderators. Journal of Consulting and Clinical Psychology, 82(6), 1034–1048. 10.1037/a0037212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CD, Young K, Torre JB, Burklund LJ, Goldin PR, Brown LA, … Craske MG (2017). Altered time course of amygdala activation during speech anticipation in social anxiety disorder. Journal of Affective Disorders, 209(November 2016), 23–29. 10.1016/j.jad.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehrmann O, Ghosh SS, Polli FE, Reynolds GO, Horn F, Keshavan A, … Gabrieli JD (2013). Predicting Treatment Response in Social Anxiety Disorder From Functional Magnetic Resonance Imaging. JAMA Psychiatry, 70(1), 87 10.1001/2013.jamapsychiatry.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, & Knutsson H (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113(28), 7900–7905. 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, & Hirsch J (2006). Resolving Emotional Conflict: A Role for the Rostral Anterior Cingulate Cortex in Modulating Activity in the Amygdala. Neuron, 51(6), 871–882. 10.1016/J.NEURON.2006.07.029 [DOI] [PubMed] [Google Scholar]
- Etkin A, & Wager TD (2007). Functional neuroimaging of anxiety: A meta-ana lysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry, 164(10), 1476–1488. 10.1176/appi.ajp.2007.07030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JM, Phan KL, Kennedy AE, Shankman SA, Langenecker SA, & Klumpp H (2017). Prefrontal and amygdala engagement during emotional reactivity and regulation in generalized anxiety disorder. Journal of Affective Disorders, 218, 398–406. 10.1016/j.jad.2017.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas-Ferrari MC, Hallak JEC, Trzesniak C, Filho AS, Machado-de-Sousa JP, Chagas MHN, … Crippa JAS (2010). Neuroimaging in social anxiety disorder: A systematic review of the literature. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 34(4), 565–580. 10.1016/J.PNPBP.2010.02.028 [DOI] [PubMed] [Google Scholar]
- Fresco DM, Coles ME, Heimberg RG, Liebowitz MR, Hami S, Stein MB, & Goetz D (2001). The Liebowitz Social Anxiety Scale: A comparison of the psychometric properties of self-report and clinician-administered formats. Psychological Medicine, 31(6), 1025–1035. 10.1017/S0033291701004056 [DOI] [PubMed] [Google Scholar]
- Frick A, Howner K, Fischer H, Kristiansson M, & Furmark T (2013). Altered fusiform connectivity during processing of fearful faces in social anxiety disorder. Translational Psychiatry, 3(June), 1–6. 10.1038/tp.2013.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furmark T, Tillfors M, Marteinsdottir I, Fischer H, Pissiota A, Långström B, & Fredrikson M (2002). Common Changes in Cerebral Blood Flow in Patients With Social Phobia Treated With Citalopram or Cognitive-Behavioral Therapy. Archives of General Psychiatry, 59(5), 425 10.1001/archpsyc.59.5.425 [DOI] [PubMed] [Google Scholar]
- Gilead M, Boccagno C, Silverman M, Hassin RR, Weber J, & Ochsner KN (2016). Self-regulation via neural simulation. Proceedings of the National Academy of Sciences, 113(36), 10037–10042. 10.1073/pnas.1600159113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, & Gross JJ (2009). Neural bases of social anxiety disorder: Emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry, 66(2), 170–180. 10.1001/archgenpsychiatry.2008.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Ziv M, Jazaieri H, Hahn K, Heimberg R, & Gross JJ (2013). Impact of Cognitive Behavioral Therapy for Social Anxiety Disorder on the Neural Dynamics of Cognitive Reappraisal of Negative Self-beliefs. JAMA Psychiatry, 70(10), 1048 10.1001/jamapsychiatry.2013.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Ziv M, Jazaieri H, Weeks J, Heimberg RG, & Gross JJ (2014). Impact of cognitive-behavioral therapy for social anxiety disorder on the neural bases of emotional reactivity to and regulation of social evaluation. Behaviour Research and Therapy, 62, 97–106. 10.1016/J.BRAT.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, … Lanzenberger R (2011). Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. NeuroImage, 56(3), 881–889. 10.1016/J.NEUROIMAGE.2011.02.064 [DOI] [PubMed] [Google Scholar]
- Hardee JE, Cope LM, Munier EC, Welsh RC, Zucker RA, & Heitzeg MM (2017). Sex differences in the development of emotion circuitry in adolescents at risk for substance abuse: a longitudinal fMRI study. Social Cognitive and Affective Neuroscience, 12(6), 965–975. 10.1093/scan/nsx021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, van Reekum CM, Schaefer SM, Lapate RC, Radler BT, Ryff CD, & Davidson RJ (2013). Sustained Striatal Activity Predicts Eudaimonic Well-Being and Cortisol Output. Psychological Science, 24(11), 2191–2200. 10.1177/0956797613490744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg N, Silbersweig D, Engelien A, Emmerich S, Malavade K, Beattie B, … Stern E (1999). Linguistic threat activates the human amygdala. Proceedings of the National Academy of Sciences of the United States of America, 96(18), 10456–10459. 10.1073/PNAS.96.18.10456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn A, Nee DE, Alexander WH, & Brown JW (2016). Distinct regions within medial prefrontal cortex process pain and cognition. Cite as: J. Neurosci 10.1523/JNEUROSCI.2180-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S, Linden DEJ, Healy D, Goebel R, Habes I, & Boehm SG (2011). Upregulation of emotion areas through neurofeedback with a focus on positive mood. Cognitive, Affective, & Behavioral Neuroscience, 11(1), 44–51. 10.3758/s13415-010-0010-1 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. Retrieved from http://jamanetwork.com/journals/jamapsychiatry/fullarticle/208678 [DOI] [PubMed] [Google Scholar]
- Klumpp H, Fitzgerald JM, Kinney KL, Kennedy AE, Shankman SA, Langenecker SA, & Phan KL (2017). Predicting cognitive behavioral therapy response in social anxiety disorder with anterior cingulate cortex and amygdala during emotion regulation. NeuroImage: Clinical, 15, 25–34. 10.1016/j.nicl.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, & Eisenberger NI (2015). The dorsal anterior cingulate cortex is selective for pain: Results from large-scale reverse inference. Proceedings of the National Academy of Sciences, 112(49), 15250–15255. 10.1073/pnas.1515083112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, & Way BM (2007). Putting Feelings Into Words. Psychological Science, 18(5), 421–428. 10.1111/j.1467-9280.2007.01916.x [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Inagaki TK, Tabibnia G, & Crockett MJ (2011). Subjective Responses to Emotional Stimuli During Labeling, Reappraisal, and Distraction. Emotion, 11(3), 468–480. 10.1037/a0023503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Straccia MA, Meyer ML, Du M, & Tan KM (2019). Social, Self, (Situational), and Affective Processes in Medial Prefrontal Cortex (MPFC): Causal, Multivariate, and Reverse Inference Evidence. Neuroscience & Biobehavioral Reviews. 10.1016/J.NEUBIOREV.2018.12.021 [DOI] [PubMed] [Google Scholar]
- Loerinc AG, Meuret AE, Twohig MP, Rosenfield D, Bluett EJ, & Craske MG (2015). Response rates for CBT for anxiety disorders: Need for standardized criteria. Clinical Psychology Review, 42, 72–82. 10.1016/J.CPR.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Månsson KNT, Frick A, Boraxbekk C-J, Marquand AF, Williams SCR, Carlbring P, … Furmark T (2015). Predicting long-term outcome of Internet-delivered cognitive behavior therapy for social anxiety disorder using fMRI and support vector machine learning. Translational Psychiatry, 5(3), e530 10.1038/tp.2015.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Månsson KNT, Salami A, Carlbring P, Boraxbekk CJ, Andersson G, & Furmark T (2017). Structural but not functional neuroplasticity one year after effective cognitive behaviour therapy for social anxiety disorder. Behavioural Brain Research, 318, 45–51. 10.1016/j.bbr.2016.11.018 [DOI] [PubMed] [Google Scholar]
- Maslowsky J, Mogg K, Bradley BP, McClure-Tone E, Ernst M, Pine DS, & Monk CS (2010). A Preliminary Investigation of Neural Correlates of Treatment in Adolescents with Generalized Anxiety Disorder. Journal of Child and Adolescent Psychopharmacology, 20(2), 105–111. 10.1089/cap.2009.0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, & Clarke JC (1998). Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behaviour Research and Therapy, 36(4), 455–470. 10.1016/S0005-7967(97)10031-6 [DOI] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Gore JC, & Allison T (1997). Face-specific processing in the human fusiform gyrus. Journal of Cognitive Neuroscience, 9(5), 605–610. 10.1162/jocn.1997.9.5.605 [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, & Johnson SC (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage, 61(4), 1277–1286. 10.1016/J.NEUROIMAGE.2012.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Sanematsu H, Yoshiura T, Togao O, Murayama K, Tomita M, … Kanba S (2011). FMRI of patients with social anxiety disorder during a social situation task. Neuroscience Research, 69(1), 67–72. 10.1016/j.neures.2010.09.008 [DOI] [PubMed] [Google Scholar]
- Nizamani ZA, de Almeida RS, Albina E, Parveen F, & Libeau G (2014). In vitro study of lymphotropic and immunomodulatory properties of the peste des petits ruminants virus (PPRV). Journal of Animal and Plant Sciences, 24(5), 1380–1387. 10.1111/j.1467-9280.2007.01916.x [DOI] [Google Scholar]
- Nord C, Gray A, Robinson O, Roiser J, Nord CL, Gray A, … Roiser JP (2019). Reliability of Fronto–Amygdala Coupling during Emotional Face Processing. Brain Sciences, 9(4), 89 10.3390/brainsci9040089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, & Gross JJ (2005, May). The cognitive control of emotion. Trends in Cognitive Sciences. 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, & Buhle JT (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–24. 10.1111/j.1749-6632.2012.06751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, & Stein MB (2010). Interoception in anxiety and depression. Brain Structure & Function, 214(5–6), 451–463. 10.1007/s00429-010-0258-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters L (2000). Discriminant validity of the social phobia and anxiety inventory (SPAI), the social phobia scale (SPS) and the social interaction anxiety scale (SIAS). Behaviour Research and Therapy, 38(9), 943–950. [DOI] [PubMed] [Google Scholar]
- Phelps EA, & LeDoux JE (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron, 48(2), 175–187. Retrieved from http://www.sciencedirect.com/science/article/pii/S0896627305008238 [DOI] [PubMed] [Google Scholar]
- Picó-Pérez M, Radua J, Steward T, Menchón JM, & Soriano-Mas C (2017). Emotion regulation in mood and anxiety disorders: A meta-analysis of fMRI cognitive reappraisal studies. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 79, 96–104. 10.1016/J.PNPBP.2017.06.001 [DOI] [PubMed] [Google Scholar]
- Schutter DJLG, & Van Honk J (2005). The cerebellum on the rise in human emotion. Cerebellum, 4(4), 290–294. 10.1080/14734220500348584 [DOI] [PubMed] [Google Scholar]
- Sladky R, Höflich A, Küblböck M, Kraus C, Baldinger P, Moser E, … Windischberger C (2015). Disrupted effective connectivity between the amygdala and orbitofrontal cortex in social anxiety disorder during emotion discrimination revealed by dynamic causal modeling for fMRI. Cerebral Cortex, 25(4), 895–903. 10.1093/cercor/bht279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladky R, Spies M, Hoffmann A, Kranz G, Hummer A, Gryglewski G, … Kasper S (2015). (S)-citalopram influences amygdala modulation in healthy subjects: a randomized placebo-controlled double-blind fMRI study using dynamic causal modeling. NeuroImage, 108, 243–250. 10.1016/J.NEUROIMAGE.2014.12.044 [DOI] [PubMed] [Google Scholar]
- Stein JF, & Glickstein M (1992). Role of the cerebellum in visual guidance of movement. Physiological Reviews, 72(4), 967–1017. 10.1152/physrev.1992.72.4.967 [DOI] [PubMed] [Google Scholar]
- Torre JB, & Lieberman MD (2018). Putting Feelings Into Words: Affect Labeling as Implicit Emotion Regulation. Emotion Review. 10.1177/1754073917742706 [DOI] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, … Joliot M (2002). Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage, 15(1), 273–289. 10.1006/NIMG.2001.0978 [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, & Ochsner KN (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59(6), 1037–1050. Retrieved from http://www.sciencedirect.com/science/article/pii/S0896627308007538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers CE, & Wiers RW (2017). Imaging the neural effects of cognitive bias modification training. NeuroImage, 151, 81–91. 10.1016/j.neuroimage.2016.07.041 [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Essen D. C. Van, Tor D, & Group WM (2012). Large-scale automated synthesis of human functional neuroimaging data. Nature Methods, 8(8), 665–670. 10.1038/nmeth.1635.Large-scale [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KS, Burklund LJ, Torre JB, Saxbe D, Lieberman MD, & Craske MG (2017). Treatment for social anxiety disorder alters functional connectivity in emotion regulation neural circuitry. Psychiatry Research, 261(January), 44–51. 10.1016/j.pscychresns.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KS, LeBeau RT, Niles AN, Hsu KJ, Burklund LJ, Mesri B, … Craske MG (2018). Neural connectivity during affect labeling predicts treatment response to psychological therapies for social anxiety disorder. Journal of Affective Disorders. 10.1016/J.JAD.2018.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, & Ochsner KN (2012). The neuroscience of empathy: progress, pitfalls and promise. Nature Neuroscience, 15(5), 675–680. 10.1038/nn.3085 [DOI] [PubMed] [Google Scholar]
- Zilverstand A, Parvaz MA, & Goldstein RZ (2017). Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. NeuroImage, 151, 105–116. 10.1016/j.neuroimage.2016.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv M, Goldin PR, Jazaieri H, Hahn KS, & Gross JJ (2013). Emotion regulation in social anxiety disorder: behavioral and neural responses to three socio-emotional tasks. Biology of Mood & Anxiety Disorders, 3(1), 20 10.1186/2045-5380-3-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V, Phillips R, Young KD, Drevets WC, & Bodurka J (2013). Prefrontal control of the amygdala during real-time fMRI neurofeedback training of emotion regulation. PLoS ONE, 8(11), e79184 10.1371/journal.pone.0079184 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


