Abstract
Objectives:
Osteoarthritis (OA) and pain are both made more severe by low-grade inflammation. We examined whether visceral fat, a major source of inflammatory cytokines and adipokines, was associated with an increased risk of knee OA or of musculoskeletal pain.
Methods:
Member of the Multicenter Osteoarthritis Study cohort, age 50–79 with or at high risk of knee osteoarthritis had whole body DEXA scans at baseline. At baseline, 30, and 60 months they obtained knee radiographs and MRIs, were asked to score the severity of their knee pain and using a body homunculus, to identify sites of joint pain. We used DEXA scans to measure total body fat and in the torso, visceral fat and subcutaneous fat. We assessed the association of fat depot size with structural outcomes (incident radiographic OA, MRI cartilage loss and synovitis) and with pain outcomes (worsening knee pain, the number of painful joints and widespread pain). Regression analyses were adjusted for age, sex, race, education, smoking, physical activity, BMI and depressive symptoms.
Results:
At baseline, of 2,961 participants, 60.7% were women; mean age was 62.5 years and BMI 30.5 kg/m2. After adjustment for covariates, no fat measures were associated with any structural outcomes. However, total and visceral but not subcutaneous fat were positively associated with worsening knee pain and widespread pain and the amount of visceral fat was associated with the number of painful joints.
Conclusion:
Visceral fat was associated with an increased risk of musculoskeletal and widespread pain.
Keywords: Obesity, body composition, osteoarthritis, pain
INTRODUCTION
When fat tissue in the body is overwhelmed by positive energy balance and becomes dysfunctional, ectopic fat depots form. Among these is visceral fat, whose accumulation is accompanied by low-grade systemic inflammation and abnormal production of adipokines(1). The accumulation of visceral fat increases the risk of diabetes, dyslipidemia, insulin resistance and adverse cardiovascular outcomes(2). The unique pathogenic properties of visceral fat, beyond its contributions to overall adiposity, may be due to its role as an endocrine organ secreting adipokines and serving as a home for circulating inflammatory macrophages(3). Further, visceral fat secretes a more pro-inflammatory cytokine profile than subcutaneous fat, characterized by higher levels of C-reactive protein and other pro-inflammatory molecules(4).
Increasingly recognized as a disease with an inflammatory component(5), osteoarthritis (OA) is the most common form of arthritis and a leading cause of disability(6). Given the paucity of effective treatments and prevention opportunities and its burgeoning prevalence, there is a desperate need for new insights into OA that may offer prevention or treatment opportunities. There are two weakly related components of OA, each of which may be affected by inflammation, structural damage to the joint and joint pain. While evidence (predominantly from animal models of posttraumatic OA) has indicated a prominent role of inflammation in causing structural damage including cartilage loss, it has been extremely challenging to find evidence for the contribution of systemic inflammation in chronic knee OA in humans(7–10). For example, in studies reporting an association of metabolic syndrome with knee OA, the association vanishes when analyses adjust for body mass index (BMI), suggesting that the increased load conferred by obesity accounts for most of this association(8, 9). Other studies examining measures of systemic inflammation with knee OA show no association with disease when analyses adjust for BMI(11–14). Identifying a source of inflammation associated with knee OA in humans independent of body weight will provide clues as to what elements of inflammation may induce or contribute to disease and may point to treatment opportunities.
While an association of systemic inflammation with structural features of OA such as cartilage loss has been difficult to identify, another line of inquiry has uncovered an association of inflammation with localized and generalized pain(15–19), the other component of OA. C-reactive protein has been linked more strongly to painful OA than to structural disease(7). Further, in animal models of OA, inflammatory cytokines provoke central pain sensitization(19–21). Low-grade chronic systemic inflammation might contribute to central pain augmentation in the joint(22). Many persons with painful knee OA have pain in many joints, and systemic inflammation may contribute to generalized pain(23).
We are unaware of previous studies of the relation of visceral adiposity to risk of knee OA and its related symptoms. To address the association of visceral adiposity with structural features of OA, and with pain, we leveraged a unique study – the Multicenter Osteoarthritis Study (MOST), a large prospective cohort study of older adults with or at high risk of knee OA. To our knowledge, MOST is the only large-scale cohort study of OA that includes a measure of visceral adiposity. Like studies that have addressed the role of visceral adiposity in cardiometabolic diseases, we included an evaluation of subcutaneous fat depots to see if the relationship of fat depots with OA differed by the type of fat depot.
PATIENTS AND METHODS
Study sample:
MOST is a large NIH-funded longitudinal observational study focused on symptomatic and radiographic knee OA in a cohort of community dwelling older adults with or at high risk for knee OA(24). The study enrolled 3,026 participants age 50–79 years from 2003–2006 at two clinical sites (Iowa City, Iowa and Birmingham, Alabama). Information regarding the participants’ demographic, medical, and lifestyle information as well as imaging were collected at baseline. Participants were followed with repeated examinations at 30 and 60 months.
Weight-bearing, semi-flexed posteroanterior (PA) and lateral views of the knees were obtained at baseline and each exam according to the MOST radiograph protocol(25). Each of two readers interpreted and graded all radiographs according to Kellgren-Lawrence (KL) grade and if they disagreed, readings were adjudicated by a panel of three readers(26). MRIs of the knee were acquired at each visit using a 1.0 T magnet (OrthOne, ONI Inc., Wilmington, MA, USA) and a circumferential extremity coil. All images were acquired without contrast. As in previous work(27) we read one randomly selected knee MRI per person. This was done for budgetary reasons and because of the high rate of symmetry in knee MRIs. The MRIs were read by two experienced musculoskeletal radiologists using the Whole Organ MRI Score (WORMS)(28). Synovitis and cartilage morphology were scored in MRIs at baseline, 30, and 60 months. There was good interobserver agreement for each of the features reported(29).
At each examination, participants completed the WOMAC questionnaire(30), reporting on the amount of pain experienced during activities in each knee. In addition, participants were presented with a homunculus at each examination (see Supplement Figure 1) on which they noted the joint sites that were painful on most days of the month.
Anthropometric measurements—BMI:
Weight was measured to the nearest 0.1 kg on a standard medical balance beam scale, and height was measured on full inspiration to the nearest 1 mm with a wall-mounted Harpenden stadiometer by certified MOST personnel following a written protocol. BMI was calculated as weight in kilograms divided by the height in meters squared.
Dual-energy radiographic absorptiometry (DEXA)-derived abdominal visceral and subcutaneous adipose tissue:
In the MOST study, a whole-body DEXA scan using Hologic scanners was obtained at baseline using standard positioning. In Birmingham, AL, this was a QDR 4500 and in Iowa City, it was a Discovery).
Scans were analyzed locally with central quality control provided by the University of California, San Francisco (UCSF) including certification of local DEXA operators, review of selected participant scans, and monitoring of scanner quality control. Standard whole-body DEXA outcomes included total fat mass, total mass and percent total fat calculated as (total fat mass/total mass) x 100%. Since our study began, Hologic developed software to permit the measurement of the visceral fat depot. In 2017, the baseline whole body scans were re-analyzed centrally at UCSF using Hologic software 13.5 (Apex 3.5) to obtain estimates of visceral (VAT) and subcutaneous abdominal adipose tissue (SAT). VAT and SAT were determined from an abdominal region of interest (31). Initial placement of the regions of interest was provided by an automated algorithm. Each scan was then reviewed and regions of interest were adjusted manually as needed. Hologic VAT area results are calibrated to, and highly correlated with, VAT area results provided by a computed tomography slice at the L4-L5 level. DEXA VAT measurement has been shown to have excellent validity compared with VAT based on CT scan, with r=0.89 to 0.93 (31–33).
Assessment of OA structure and pain symptoms:
We defined three structural knee outcomes: incident radiographic OA, MRI assessed cartilage loss, and synovitis assessed on MRI. The first structural outcome was incident radiographic knee OA up to 5 years after baseline among the subset of participants who had no radiographic OA in either knee (both knees with Kellgren and Lawrence grade <2) at baseline. Those who developed either radiographic knee OA (KL≥2) or had a knee arthroplasty in either knee by follow-up were defined as having incident radiographic knee OA.
Based on MRI readings(28), we studied cartilage loss and change in synovitis. Within each of 14 subregions in each knee, cartilage morphology was scored 0–6 using the WORMS scale. We defined worsening cartilage morphology by analyzing each subregion and characterizing each as having worsening when the score increased by 1 point on the 0–6 scale. Subregions with baseline scores of 6 were excluded. Second, we examined change in synovitis. Each region (infrapatellar, intercondylar and suprapatellar) was scored 0–3 based on volume at each timepoint, and the score was then summed (0–9). We defined worsening synovitis as an increase in that score by at least one excluding knees with synovitis scores of 9 at baseline(34).
We assessed one knee pain outcome (change in WOMAC pain) and two broader musculoskeletal pain outcomes, the presence of widespread pain and the number of painful joints. We calculated changes in pain as the difference of WOMAC pain score from baseline to the end of follow-up in each knee(35).
Using the homunculus, we counted the number of painful joint sites identified by participants at each exam. First, we used the homunculus to define whether a participant had widespread pain (defined as present when the participant identified painful joint sites above and below the waist on both sides of the body and in the axial spine). We have found in previous work(36) that those with widespread pain almost always had pain at multiple sites at exams before they met the threshold for widespread pain, suggesting that we could not reasonably examine incidence. We therefore focused on the proportion of the three exams that participants met criteria for widespread pain. To gauge the effect of visceral adiposity on the number of painful sites, we examined number of painful sites at baseline, 30 and 60 months and tested whether visceral adiposity led to an increase in the number of painful joints from baseline.
Potential confounders:
For our main analyses, we adjusted for participants’ demographic, lifestyle, and medical history reported on the baseline questionnaire including age, sex (men, women), education (college or above, yes vs. no), physical activity (Physical Activity Scale for the Elderly (PASE), continuous), smoking (never, past, current), and BMI (kg/m2, continuous). We used an indicator variable to adjust for race (white vs. non-white). For all knee outcomes, we included as covariates mechanical alignment (varus, neutral, valgus), history of knee injury or surgery and Kellgren and Lawrence grade for the contralateral knee. For all pain outcomes, we included depressive symptom score (Center for Epidemiologic Studies Depression (CES-D) scale score ≥16, yes vs. no as a covariate).
Statistical analyses:
Our analytic sample consisted of 2,961 MOST participants (1,797 women and 1,164 men) with baseline dual-energy radiographic absorptiometry (DEXA) measurement and who were followed from baseline through at least 30 months.
Analyses of radiographic OA and MRI findings and of WOMAC pain were at the level of the knee, or knee subregion for cartilage loss and to adjust for the correlation between knees (or subregions of knees), we performed generalized estimating equations (GEEs). Analyses of body wide pain outcomes were conducted at the person level. We modeled each adiposity measure in quintiles and estimated the relative risk with quintile 1 as reference group. For the GEE model, we specified a log-binomial distribution for binary outcomes and a negative binomial for count data. Results of sex-specific analyses were similar to sex-adjusted results presented here, and we found no statistically significant interactions by sex. For WOMAC pain and body wide pain outcomes, we used the score at each follow-up time point as our outcome, adjusting for baseline score in each model. We adjusted the correlation between outcomes at different times using GEE.
Mechanical forces play a large role in knee osteoarthritis and excess body weight is a well-known risk factor. To identify the independent effect of fat depots and their products on OA and pain outcomes, our primary analyses adjusted for BMI. We carried out secondary analyses substituting weight for BMI and found no difference in results. In addition, we created visceral fat residuals of BMI (in which we used the residuals of the equation of VAT predicting BMI) and substituted this residual and BMI in equations and found no difference in results.
Institutional review board approvals were obtained from University of California, San Francisco, Boston University, University of Alabama at Birmingham and The University of Iowa. All participants provided written consent for study participation.
RESULTS
At baseline, mean age was 62.5 years with average BMI 30.5 kg/m2. Among 3026 subjects at baseline, 65 (2.2%) had no DEXA scans or had scans that were of insufficient quality to measure fat depots. This left 2,961 MOST participants. Follow-up examinations included a clinic visit with x-rays but, for those subjects who did not come to this visit, we obtained WOMAC and pain data over the phone. While 247 (8.3%) had no radiographic follow-up, all but 3 subjects (0.1%) had WOMAC pain assessed at at least one follow-up visit. Half of the study participants (51%) reported widespread pain at baseline, with mean number of painful sites=6 (see Table 1).
Table 1.
Baseline characteristics of 2961 MOST participants
| Mean age at baseline in years (+/− s.d.) | 62.5 ± 8.08 |
| Mean body mass index (Kg/m2) (+/− s.d.) | 30.5 ± 5.65 |
| Mean Charlson comorbidity score, modified (+/−s.d.) | 0.52 ± 0.96 |
| Mean CES-D Score * (+/− s.d.) | 7.59 ± 7.80 |
| Mean PASE Score** (+/− s.d.) | 174.6 ± 88.0 |
| Never smokers | 1646 (55.6%) |
| Current smokers | 194 (6.5%) |
| Former smokers | 1121 (37.9%) |
| Caucasians | 2481 (83.8%) |
| Number with some college education or above | 1306 (44.1%) |
| Mean WOMAC Pain Score (0–20) (+/− s.d.) | 3.38 ± 3.75 |
| Number with widespread pain (%) | 1486 (50.7%) |
| Mean number of painful sites (+/−s.d.) | 5.95 ± 4.58 |
| Study site: Iowa | 1506 (50.9%) |
| Mean total body fat (%) (+/− s.d.) | 34.4 ± 8.53 |
| Mean abdominal visceral adipose tissue (cm2) (+/− s.d.) | 165.7 ± 72.0 |
| Mean abdominal subcutaneous adipose tissue (cm2) (+/− s.d.) | 373.2 ± 135.1 |
CES-D: Center for Epidemiologic Studies Depression Scale
PASE: Physical Activity Scale for the Elderly
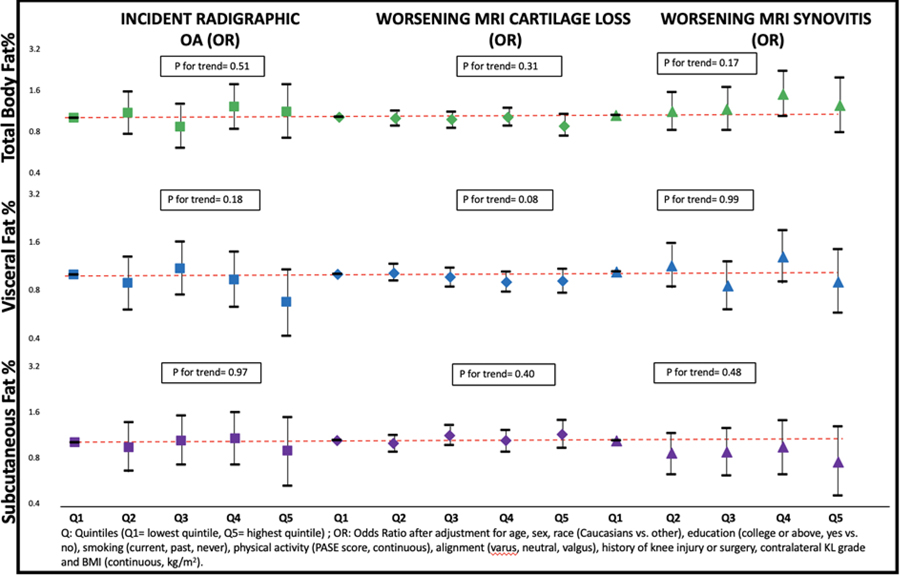
While fat depot was associated with structural outcomes in knee OA when analyses were not adjusted for BMI, these associations became null when we further adjusted for BMI (Supplement Table 1). For example, 584/3032 (19.3%) knees developed incident radiographic OA at either the 30- or 60-month follow-up, and we found no relation of the size of any of the fat depots with OA incidence (Figure 1, Supplement Table 1). Further, on MRI, 11738/35864 (32.7%) cartilage subregions experienced cartilage loss during follow-up, but we found no association of fat depot size with an increase in cartilage loss (Figure 1, Supplement Table 2). Similarly, we found no relation between fat depot size and worsening synovitis (Figure 1, Supplement Table 3).
Figure 1.
Association between fat depots and structural outcomes
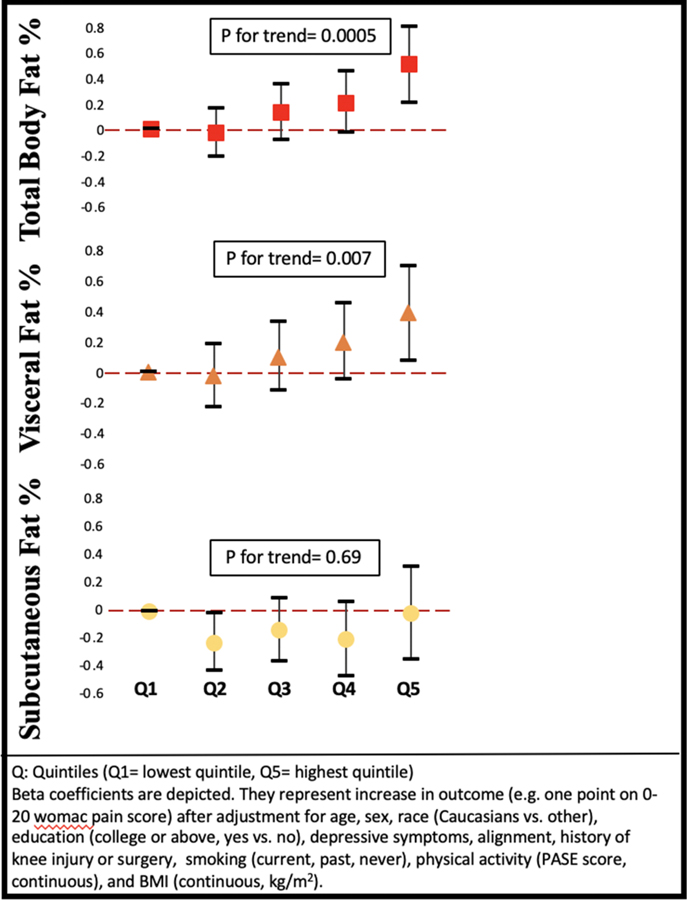
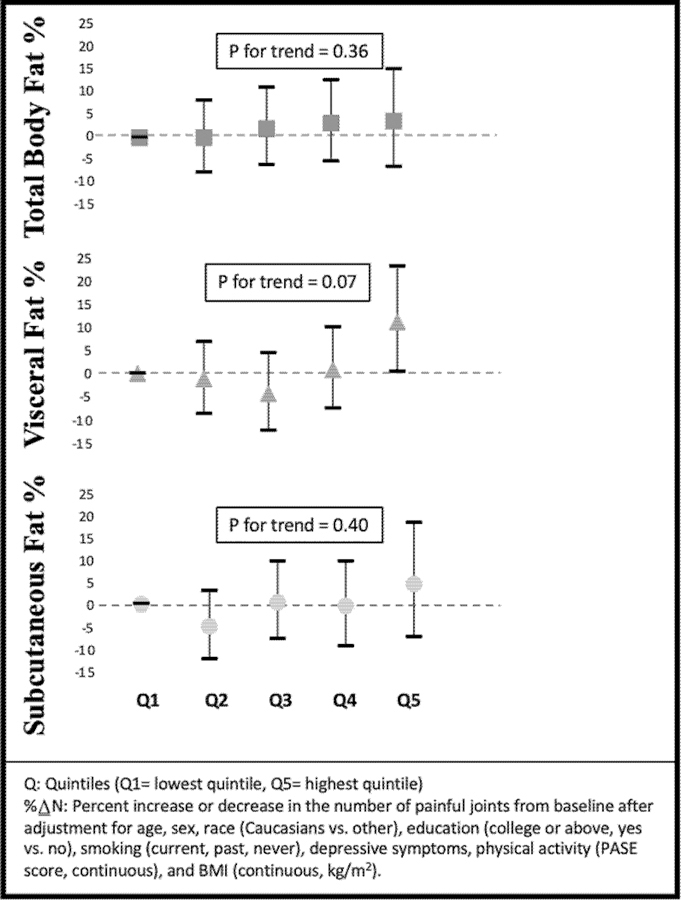
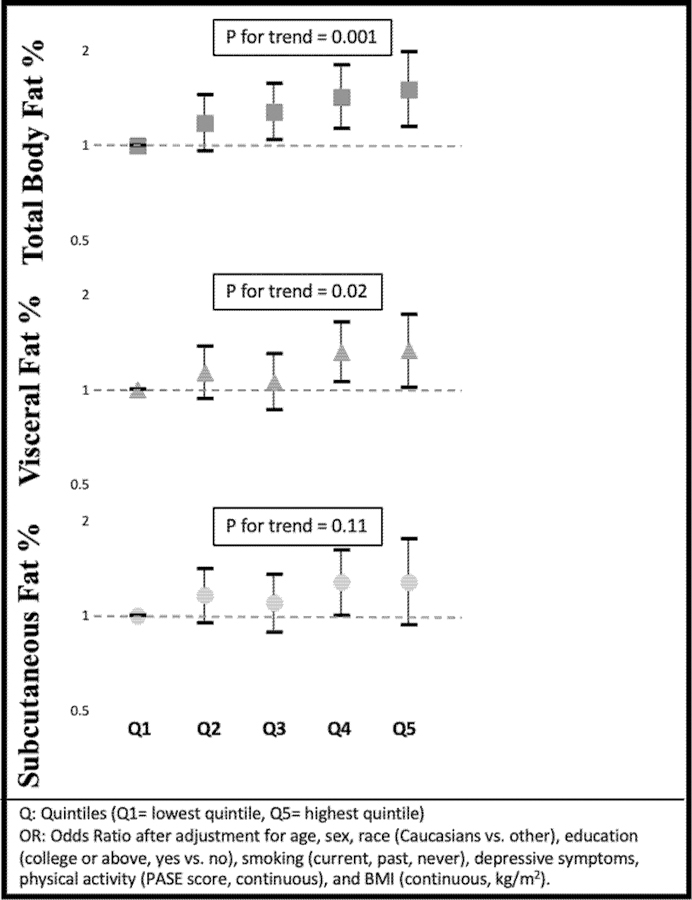
On the other hand, we found a consistent association of total fat and the visceral fat depot both before and after adjustment for BMI with knee pain and with widespread pain outcomes. For example, those in the highest quintile of total body fat and visceral adiposity had more worsening of their WOMAC pain score than those in the lowest quintile (Figure 2, Supplement Table 4). The result was consistent when we examined repeated widespread pain (Figure 3, Supplement Table 5). There was a modest association between visceral fat and the number of painful joint sites (Figure 4, Supplement Table 6), but number of painful joints was not significantly associated with total body fat. In contrast, after adjustment for BMI, subcutaneous fat depot size was unassociated with any pain outcomes.
Figure 2.
Association between fat depots and worsening WOMAC knee pain (β)
Figure 3.
Association between fat depots and widespread pain (%∆N)
Figure 4.
Association between fat depots and number of painful joint sites (OR)
DISCUSSION
We did not find evidence that visceral or total fat was associated with the occurrence of radiographic OA, of cartilage loss or synovitis. However, we found a consistent association of body fat, especially total and visceral fat, with worsening knee pain outcomes and with widespread pain, and this association was independent of body mass index. Visceral fat was associated with an increase over time in the number of painful joints.
Ectopic fat deposition including visceral fat is characterized by an infiltration of macrophages and is a major source of pro-inflammatory cytokines including interleukin-6 and tumor necrosis factor-α (TNFα)(37, 38). Further, it is a site for conversion of local macrophages to a pro-inflammatory M1 phenotype, which then enter the systemic circulation (39) and have been implicated in OA pathogenesis(40). Visceral fat accumulation has a range of other pro-inflammatory effects including suppression of the transcription of adiponectin(41), an anti-inflammatory cytokine found to have articular effects(42). The production of leptin, which has deleterious effects on chondrocyte metabolism(43), increases with visceral fat accumulation(44).
Given the range of consistently pro-inflammatory effects of visceral fat with consequences for the risk of type 2 diabetes, cardiovascular disease and other cardiometabolic conditions, it is surprising that we were unable to detect an association of this accumulation on OA, an inflammatory disorder. A likely explanation is that while there is no blood synovial barrier, the systemic concentrations of these pro-inflammatory effectors do not translate into injurious levels in the synovial fluid(22, 45). The few studies examining the correlations between blood and synovial fluid concentrations of the salient molecules have not found strong or consistent associations(46, 47). Our findings have implications for efforts to modify the course of OA using systemic anti-inflammatory treatment.
Other explanations for our null findings are that while inflammation affects OA risk, visceral adiposity may not be the inflammatory phenotype of greatest relevance. After all, we (and others) have also found no association of metabolic syndrome with OA occurrence(9).
On the other hand, our findings suggest that the inflammatory environment created by the accumulation visceral fat may aggravate pain independent of the loading effect of weight. These data suggest that pain needs to be added to the list of conditions affected by the accumulation of visceral fat. Musculoskeletal pain including back pain, osteoarthritic pain and pain from other musculoskeletal disorders is the world’s leading cause of disability(48). This study is important in providing new insights into potential treatable causes of this pain. Literature on body composition and pain is mainly cross-sectional and has focused on foot and lower back pain(49, 50). A study of knee pain has reported an association with systemic markers of inflammation(18). One study also suggested a dose-response relationship between painful joint burden and systemic inflammation among OA patients(51). Widespread pain, a common condition, has not, to our knowledge, been evaluated.
Our study had a large sample size with long-term follow up and detailed data on both structural pathology and pain symptoms of the knee. However, our study has limitations. Among them is the absence of data on products of visceral fat that may mediate the relationship we uncovered including adipokines. Further work is needed to identify the active product (s). Another limitation is that the majority of our study participants (84%) were Caucasian. Further studies on other race/ethnic groups are needed. The complex relationship between weight, visceral and total body fat, activity and pain are worth examining further. While our sample was large and results testing the association of visceral fat and structural OA outcomes were nonsignificant and hovered around the null value, the confidence bounds we report are compatible with modest associations of visceral fat with these outcomes. The high prevalence of widespread pain in our cohort suggests that results may not be generalizable to other groups. Also, the lack of association we report of central fat depots with structural changes of OA may differ in less developed societies (52). The associations we report need to be replicated.
In conclusion, in this prospective cohort study of a community dwelling population, we found no association independent of BMI between the size of the visceral fat depot and the occurrence of radiographic OA, of cartilage loss or of synovitis. However, we found a positive and consistent independent association between visceral adiposity and pain outcomes including knee pain severity, widespread pain and the number of painful joints. These findings suggest that visceral adiposity may be an important source of biological mediators for musculoskeletal pain.
Supplementary Material
ACKNOWLEDGEMENT
We appreciate the contributions all participants in the MOST study and of study staff at the clinical sites and the coordinating center.
Grants and Financial Support: The MOST study was supported by the National Institutes of Health (Felson, U01 AG18820; Lewis, U01 AG18947; Nevitt, U01 AG19069; Torner, U01 AG18832). This analysis was supported by a supplement to U01 AG18820. This work was also supported by NIH AR P30 072571. Dr. Felson receives support from the National Institute for Health Research (NIHR), as part of the Manchester Musculoskeletal NIHR Biomedical Research Centre Grant.
Contributor Information
Shanshan Li, Boston University School of Medicine, Slone Epidemiology Center.
Ann V. Schwartz, University of California, San Francisco.
Michael P. Lavalley, Boston University School of Public Health, Department of Biostatistics.
Na Wang, Section of Rheumatology.
Nancy Desai, Section of Rheumatology.
Xianbang Sun, Section of Rheumatology.
Tuhina Neogi, Boston University School of Medicine, Slone Epidemiology Center.
Michael Nevitt, University of California, San Francisco.
Cora E. Lewis, University of Alabama at Birmingham, Department of Epidemiology.
Ali Guermazi, Department of Radiology.
Frank Roemer, Department of Radiology.
Neil Segal, University of Kansas Medical Center, Orthopaedics and Rehabilitation.
David Felson, Section of Rheumatology, University of Manchester Centre for Epidemiology, and the NIHR Manchester BRC, Manchester University NHS Trust.
REFERENCES
- 1.Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA, et al. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int J Mol Sci. 2019;20(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015;132(17):1639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu JY, Choi WJ, Lee HS, Lee JW. Relationship between inflammatory markers and visceral obesity in obese and overweight Korean adults: An observational study. Medicine (Baltimore). 2019;98(9):e14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013;21(1):16–21. [DOI] [PubMed] [Google Scholar]
- 6.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–30. [DOI] [PubMed] [Google Scholar]
- 7.Jin X, Beguerie JR, Zhang W, Blizzard L, Otahal P, Jones G, et al. Circulating C reactive protein in osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(4):703–10. [DOI] [PubMed] [Google Scholar]
- 8.Kerkhof HJ, Bierma-Zeinstra SM, Castano-Betancourt MC, de Maat MP, Hofman A, Pols HA, et al. Serum C reactive protein levels and genetic variation in the CRP gene are not associated with the prevalence, incidence or progression of osteoarthritis independent of body mass index. Ann Rheum Dis. 2010;69(11):1976–82. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Felson DT. What is the evidence to support the association between metabolic syndrome and osteoarthritis? - A systematic review. Arthritis Care Res (Hoboken). 2018. [DOI] [PMC free article] [PubMed]
- 10.Vlad SC, Neogi T, Aliabadi P, Fontes JD, Felson DT. No association between markers of inflammation and osteoarthritis of the hands and knees. J Rheumatol. 2011;38(8):1665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toussirot E, Michel F, Bereau M, Dehecq B, Gaugler B, Wendling D, et al. Serum adipokines, adipose tissue measurements and metabolic parameters in patients with advanced radiographic knee osteoarthritis. Clinical rheumatology. 2017;36(11):2531–9. [DOI] [PubMed] [Google Scholar]
- 12.Suh DH, Han KD, Hong JY, Park JH, Bae JH, Moon YW, et al. Body composition is more closely related to the development of knee osteoarthritis in women than men: a cross-sectional study using the Fifth Korea National Health and Nutrition Examination Survey (KNHANES V-1, 2). Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2016;24(4):605–11. [DOI] [PubMed] [Google Scholar]
- 13.King LK, Henneicke H, Seibel MJ, March L, Anandacoomarasmy A. Association of adipokines and joint biomarkers with cartilage-modifying effects of weight loss in obese subjects. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2015;23(3):397–404. [DOI] [PubMed] [Google Scholar]
- 14.Richter M, Trzeciak T, Rybka JD, Suchorska W, Augustyniak E, Lach M, et al. Correlations between serum adipocytokine concentrations, disease stage, radiological status and total body fat content in the patients with primary knee osteoarthritis. International orthopaedics. 2017;41(5):983–9. [DOI] [PubMed] [Google Scholar]
- 15.Eitner A, Pester J, Vogel F, Marintschev I, Lehmann T, Hofmann GO, et al. Pain sensation in human osteoarthritic knee joints is strongly enhanced by diabetes mellitus. Pain. 2017;158(9):1743–53. [DOI] [PubMed] [Google Scholar]
- 16.Eitner A, Hofmann GO, Schaible HG. Mechanisms of Osteoarthritic Pain. Studies in Humans and Experimental Models. Frontiers in molecular neuroscience. 2017;10:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roux CH, Foltz V, Maheu E, Baron G, Gandjbakhch F, Lukas C, et al. MRI and serum biomarkers correlate with radiographic features in painful hand osteoarthritis. Clinical and experimental rheumatology. 2016;34(6):991–8. [PubMed] [Google Scholar]
- 18.Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann Rheum Dis. 2013;72(4):535–40. [DOI] [PubMed] [Google Scholar]
- 19.Schaible HG. Mechanisms of chronic pain in osteoarthritis. Curr Rheumatol Rep. 2012;14(6):549–56. [DOI] [PubMed] [Google Scholar]
- 20.Fan YX, Qian C, Liu B, Wang C, Liu H, Pan X, et al. Induction of suppressor of cytokine signaling 3 via HSF-1-HSP70-TLR4 axis attenuates neuroinflammation and ameliorates postoperative pain. Brain, behavior, and immunity. 2018;68:111–22. [DOI] [PubMed] [Google Scholar]
- 21.Choi JI, Svensson CI, Koehrn FJ, Bhuskute A, Sorkin LS. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. PAIN®. 2010;149(2):243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rezus E, Cardoneanu A, Burlui A, Luca A, Codreanu C, Tamba BI, et al. The Link Between Inflammaging and Degenerative Joint Diseases. International journal of molecular sciences. 2019;20(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Generaal E, Vogelzangs N, Macfarlane GJ, Geenen R, Smit JH, Dekker J, et al. Basal inflammation and innate immune response in chronic multisite musculoskeletal pain. Pain. 2014;155(8):1605–12. [DOI] [PubMed] [Google Scholar]
- 24.Segal NA, Nevitt MC, Gross KD, Hietpas J, Glass NA, Lewis CE, et al. The Multicenter Osteoarthritis Study (MOST): Opportunities for Rehabilitation Research. PM & R : the journal of injury, function, and rehabilitation. 2013;5(8): 10.1016/j.pmrj.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheehy L, Culham E, McLean L, Niu J, Lynch J, Segal NA, et al. Validity and sensitivity to change of three scales for the radiographic assessment of knee osteoarthritis using images from the Multicenter Osteoarthritis Study (MOST). Osteoarthritis Cartilage. 2015;23(9):1491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felson DT, Niu J, Neogi T, Goggins J, Nevitt MC, Roemer F, et al. Synovitis and the risk of knee osteoarthritis: the MOST Study. Osteoarthritis Cartilage. 2016;24(3):458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–90. [DOI] [PubMed] [Google Scholar]
- 29.Lynch JA, Roemer FW, Nevitt MC, Felson DT, Niu J, Eaton CB, et al. Comparison of BLOKS and WORMS scoring systems part I. Cross sectional comparison of methods to assess cartilage morphology, meniscal damage and bone marrow lesions on knee MRI: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2010;18(11):1393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellamy N. A health status instrument for measuring clinically-important patient relevant outcomes following hip or knee arthroplasty in osteoarthritis. Journal of Orthopaedic Rheumatology. 1988;1:95–108. [PubMed] [Google Scholar]
- 31.Bredella MA, Gill CM, Keating LK, Torriani M, Anderson EJ, Punyanitya M, et al. Assessment of abdominal fat compartments using DXA in premenopausal women from anorexia nervosa to morbid obesity. Obesity (Silver Spring, Md). 2013;21(12):2458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity (Silver Spring, Md). 2012;20(5):1109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fourman LT, Kileel EM, Hubbard J, Holmes T, Anderson EJ, Looby SE, et al. Comparison of visceral fat measurement by dual-energy X-ray absorptiometry to computed tomography in HIV and non-HIV. Nutr Diabetes. 2019;9(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guermazi A, Eckstein F, Hayashi D, Roemer FW, Wirth W, Yang T, et al. Baseline radiographic osteoarthritis and semi-quantitatively assessed meniscal damage and extrusion and cartilage damage on MRI is related to quantitatively defined cartilage thickness loss in knee osteoarthritis: the Multicenter Osteoarthritis Study. Osteoarthritis Cartilage. 2015;23(12):2191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellamy N, Wells G, Campbell J. Relationship between severity and clinical importance of symptoms in osteoarthritis. Clinical rheumatology. 1991;10(2):138–43. [DOI] [PubMed] [Google Scholar]
- 36.Felson DT, Niu J, Quinn EK, Neogi T, Lewis CL, Lewis CE, et al. Multiple Nonspecific Sites of Joint Pain Outside the Knees Develop in Persons With Knee Pain. Arthritis Rheumatol. 2017;69(2):335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samaras K, Botelho NK, Chisholm DJ, Lord RV. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity (Silver Spring, Md). 2010;18(5):884–9. [DOI] [PubMed] [Google Scholar]
- 38.Villaret A, Galitzky J, Decaunes P, Esteve D, Marques MA, Sengenes C, et al. Adipose tissue endothelial cells from obese human subjects: differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes. 2010;59(11):2755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghigliotti G, Barisione C, Garibaldi S, Fabbi P, Brunelli C, Spallarossa P, et al. Adipose tissue immune response: novel triggers and consequences for chronic inflammatory conditions. Inflammation. 2014;37(4):1337–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kraus VB, McDaniel G, Huebner JL, Stabler TV, Pieper CF, Shipes SW, et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2016;24(9):1613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302(2):179–88. [DOI] [PubMed] [Google Scholar]
- 42.Tang Q, Hu ZC, Shen LY, Shang P, Xu HZ, Liu HX. Association of osteoarthritis and circulating adiponectin levels: a systematic review and meta-analysis. Lipids in health and disease. 2018;17(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su YP, Chen CN, Huang KC, Chang HI, Lee KC, Lo CM, et al. Leptin induces MMP1/13 and ADAMTS 4 expressions through bone morphogenetic protein-2 autocrine effect in human chondrocytes. Journal of cellular biochemistry. 2018;119(4):3716–24. [DOI] [PubMed] [Google Scholar]
- 44.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. European heart journal. 2008;29(24):2959–71. [DOI] [PubMed] [Google Scholar]
- 45.Vicenti G, Bizzoca D, Carrozzo M, Solarino G, Moretti B. Multi-omics analysis of synovial fluid: a promising approach in the study of osteoarthritis. Journal of biological regulators and homeostatic agents. 2018;32(6 Suppl. 1):9–13. [PubMed] [Google Scholar]
- 46.Gandhi R, Takahashi M, Syed K, Davey JR, Mahomed NN. Relationship between body habitus and joint leptin levels in a knee osteoarthritis population. J Orthop Res. 2010;28(3):329–33. [DOI] [PubMed] [Google Scholar]
- 47.Zhang W, Likhodii S, Aref-Eshghi E, Zhang Y, Harper PE, Randell E, et al. Relationship between blood plasma and synovial fluid metabolite concentrations in patients with osteoarthritis. The Journal of rheumatology. 2015;42(5):859–65. [DOI] [PubMed] [Google Scholar]
- 48.Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walsh TP, Arnold JB, Gill TK, Evans AM, Yaxley A, Hill CL, et al. Foot pain severity is associated with the ratio of visceral to subcutaneous fat mass, fat-mass index and depression in women. Rheumatology international. 2017;37(7):1175–82. [DOI] [PubMed] [Google Scholar]
- 50.Hussain SM, Urquhart DM, Wang Y, Shaw JE, Magliano DJ, Wluka AE, et al. Fat mass and fat distribution are associated with low back pain intensity and disability: results from a cohort study. Arthritis research & therapy. 2017;19(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perruccio AV, Chandran V, Power JD, Kapoor M, Mahomed NN, Gandhi R. Systemic inflammation and painful joint burden in osteoarthritis: a matter of sex? Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2017;25(1):53–9. [DOI] [PubMed] [Google Scholar]
- 52.Wallace IJ, Felson DT, Worthington S, Duryea J, Clancy M, Aliabadi P, et al. Knee osteoarthritis risk in non-industrial societies undergoing an energy balance transition: evidence from the indigenous Tarahumara of Mexico. Ann Rheum Dis. 2019;78(12):1693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.