Abstract
Background: Childhood maltreatment is a global public health issue linked to a vast mortality and morbidity burden. This study builds on current literature to explore the risk of developing central sensitivity syndromes (CSS) (consisting of somatic and visceral pain syndromes) subsequent to childhood maltreatment exposure.
Methods: A retrospective population based open cohort study using the UK primary care database, ‘The Health Improvement Network,’ between 1st January 1995-31st December 2018. 80,657 adult patients who had experienced childhood maltreatment or maltreatment related concerns (exposed patients) were matched to 161,314 unexposed patients by age and sex. Outcomes of interest were the development of CSS: either somatic (Fibromyalgia, chronic fatigue syndrome, temporomandibular joint disorder, chronic lower back pain, chronic headache, myofascial pain syndrome and restless leg syndrome) or visceral (Interstitial cystitis, vulvodynia, chronic prostatitis and irritable bowel syndrome) in nature. Effect sizes are presented as adjusted incidence rate ratios (aIRR) with confidence intervals (CI). Models were adjusted for the following covariates at cohort entry: age, sex, deprivation, anxiety, depression and serious mental ill health.
Results: The average age at cohort entry was 23.4 years and the median follow was 2.2 years. There was an increased risk of developing fibromyalgia (aIRR 2.06; 95% CI 1.71–2.48), chronic fatigue syndrome (1.47; 1.08–2.00), chronic lower back pain (1.99; 1.68–2.35), restless leg syndrome (1.82; 1.41–2.35) and irritable bowel syndrome (1.15; 1.08–1.22) when compared to the unexposed group, whereas no statistical association was seen with the development of temporomandibular joint disorder (1.00; 0.88–1.13), chronic headache (1.04; 0.59–1.86), interstitial cystitis (1.19; 0.51–2.74), vulvodynia (0.65; 0.34–1.26), chronic prostatitis (0.34; 0.07–1.77) and myofascial pain syndrome (0.88; 0.36–2.14). Outcome numbers were low, most likely, due to the rarity of visceral conditions (aside from irritable bowel syndrome). The association between a history of childhood maltreatment and CSS were mainly observed in somatic CSS.
Interpretation: The debilitating effects of CSS carry a substantial physical, psychological and economic burden to both the individuals who are diagnosed with them and the health services who serve them. Primary prevention approaches targeting childhood maltreatment as well as secondary preventative approaches should be considered to minimise the associated burden of CSS.
Keywords: Childhood maltreatment, Central sensitivity syndromes, Epidemiology, Primary care
Introduction
Childhood maltreatment (defined as any form of physical, sexual or emotional abuse (including witnessing or being a survivor of domestic abuse (includes intimate partner violence)) and neglect (both emotional and physical in nature) experienced by those under the age of 18 years old) [1] is a global public health issue and violation of the Human Rights Act [2]. The negative downstream social, psychological and physical health effects of childhood maltreatment or living household where there is adversity, are extensive and bear a substantial societal cost [3], [4], [5], [6], [7], [8], [9], [10], [11], [71]. In 2017, it was estimated globally that 5.2 million disability adjusted life years (DALY) are attributable to childhood maltreatment [12].
Exposure to childhood maltreatment, in similar fashion to other forms of abuse often consists of experiences leading to acute and chronic stress responses, which some suggest results in the dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis [13,14]. Biochemical effects of the stress experienced in some childhood maltreatment survivors have been hypothesised to alter normal functioning of the immune, metabolic, neuroendocrine and autonomic systems [15]. Due to disruption of these systems, some survivors of abuse have been shown to have higher levels of markers relating to sustained inflammation (such as increased circulating interleukins, tumour necrosis factor and C-reactive protein levels) [16], [17], [18]. To further exacerbate these biochemical effects, some survivors of abuse may adopt poor lifestyle choices (physical inactivity, substance misuse and poor diet) [5,15,[19], [20], [21]] as well as developing psychopathological changes as a result of the abuse leading to mental ill health [7,8]. One such psychological hypothesis thought to be demonstrated in abuse survivors is the ‘fear avoidance model’, where individuals who have experienced traumatic abuse episodes may be unable to regulate the severity of pain responses precipitating a cycle of ‘pain-related fear’ [22,23]. Another important neurobiological change seen in some subgroups of maltreated children are changes in the structural and functional organisation of their developing brain network which may be associated with an increased risk of psychopathology [24]. Functional and traditional magnetic resonance imaging techniques on such children in some studies show that those exposed to maltreatment when compared to unexposed controls have changes in their amygdala (leading to changes in emotion processing), corticol thickness, centrality (connectedness) within regions involved in emotional regulation and increased neural path lengths, all of which may be responsible for later-life psychopathology [24], [25], [26]. Changes in these pathways has been associated with the development of function neurological conditions (including non-epileptic seizures, voicer disorders and other mixed disorders) explored through observational study design and functional imaging [27,28]. Of interest, these brain networks affected by childhood maltreatment are thought to be involved in an individual's third order networks within their central pain matrix (a group of brain structures jointly activated by painful stimuli), thereby suggesting alternations in this region may go onto affect an individual's ability to perceive and respond to pain [29]. These numerous pathways (physiological, psychopathological and sociological) are being proposed to associate exposure to experiences such as childhood maltreatment and the development of chronic pain, however more research is required to confirm this [30].
The global prevalence of chronic pain (often non-specific in nature) in lower and middle income countries is thought to be 33% [31], and even in countries of higher economic status such as the UK it is thought to affect more than one third of the adult population [32]. However, the field of chronic pain medicine is evolving as our understanding of the involvement of central nervous system (CNS) sensitisation (otherwise known as central sensitisation) has improved. CNS sensitisation may provide a rationale for the pain severity and disability in a subgroup of patients experiencing cases of ‘unexplained’ chronic musculoskeletal pain [33,34]. Although there is not yet an agreed upon terminology for CNS sensitisation conditions, the umbrella term ‘central sensitisation/sensitivity syndromes’ (CSS) is accepted in the literature. CSS includes both somatic (fibromyalgia, chronic fatigue syndrome, temporomandibular joint disorder, chronic lower back pain, chronic headache, myofascial pain syndrome and restless leg syndrome) and visceral (Interstitial cystitis, vulvodynia, chronic prostatitis and irritable bowel syndrome) pain syndromes [33,35,36]. Although somatic and visceral nociceptive pathways may be largely similar and can both lead to CNS sensitisation, visceral pathways have important specific clinical characteristics not often seen in somatic disorders viz. 1) they are not evoked from all viscera 2) they are not always linked to a specific visceral injury 3) the pain experienced is diffuse and poorly localised 4) they often have referred pain and 5) are accompanied with motor and autonomic reflexes [37]. Functional imaging demonstrates differences in the processing of visceral and somatic pain (visceral compared to somatic activation has greater activation of the periaqueductal grey matter and nucleus cuneiformis) which may explain some of these differences [38].
CNS sensitisation itself refers to chronic upregulation of the peripheral nociceptive receptors which may occur as a result of injury, illness or iatrogenic causes resulting in hyperexcitation experienced by the somatosensory cortex and spinal pain processing pathways (sensitisation) [39,40]. This results most commonly in allodynia and hyperalgesia [39]. An established theory is that the excess nociception may lead to increased levels of substance P being released into the peripheral c-fibres lowering thresholds for perceived pain [40]. Although the exact role traumatic experiences and abuse play in the development of CNS sensitisation is not fully understood, it is clear that dysregulation of the HPA axis, particularly downstream effects, may affect nociceptive processing in the peripheral nervous system predisposing individuals to a state of sensitisation [41]. Additionally, detrimental lifestyle choices and mental ill health caused by abuse are often independent risk factors mediating the subsequent development of CSS such as fibromyalgia and chronic fatigue syndrome [18].
The evidence base associating traumatic experiences such as childhood maltreatment with the development of CSS is growing [35]. Published systematic reviews have demonstrated positive associations between childhood maltreatment with the subsequent development of fibromyalgia, chronic fatigue syndrome and irritable bowel syndrome [18,42]. However, the studies included in those reviews were; mostly either case-control or cross-sectional in design; unable to control for important covariates; low in participant numbers; or had self-reported exposures/outcomes leading to the possibility of recall bias [43]. In addition, few studies were generalisable to a UK population in both participant characteristics and exposure definition. With regards to evidence associating childhood maltreatment to other CSS, the literature following a systematic search[35] is either non-existent (Myofascial pain syndrome and restless leg syndrome) or is present with similar limitations or limited to exposure of sexual abuse (temporomandibular joint disorders, chronic lower back pain, chronic headaches, interstitial cystitis, vulvodynia and chronic prostatitis) [44], [45], [46], [47], [48], [49], [50], [51], [52]. Population based matched cohort study evidence is urgently needed to confirm these associations to identify whether traumatic events such as childhood maltreatment predispose individuals to CSS.
Therefore, we have conducted the first UK retrospective cohorts using ‘The Health Improvement Network’ (THIN) dataset to explore the association between childhood maltreatment and subsequent development of CSS (consisting of somatic and visceral pain syndromes).
Methods
Study design and data source
A retrospective cohort study derived from the THIN database to explore exposure to childhood maltreatment or maltreatment related concerns with the subsequent development of CSS. This study is a population based, retrospective open cohort, allowing for patients to enter and exit the study at different time points. The study period was set between 1st January 1995 and 31st December 2018. Each individual patient contributed person years of follow-up from the time of cohort entry (index date) to the time they leave the cohort (exit date).
During the study period, THIN consisted of electronic medical records taken from 787 general practices, deemed to be representative of the UK population in terms of demographic structure and prevalence of key comorbidities [53]. Symptoms, examinations, and diagnoses in THIN are recorded using a hierarchical clinical coding system called Read codes [54]. General practices were eligible for inclusion 12 months following their instalment of electronic practice records or from the practice's acceptable mortality recording date [55]. During the study period this left 9,588,734 patients eligible to contribute.
Exposure and outcome definition
The purpose of this study was to compare adult (>18 years at index date) exposed patients (those with a code identifying officially confirmed childhood maltreatment or a maltreatment related concern code which has been inputted whilst they were <18 years old) to unexposed patients (those without such codes) and then calculate their risk of developing CSS (either somatic (fibromyalgia, chronic fatigue syndrome, temporomandibular joint disorder, chronic lower back pain, chronic headache, myofascial pain syndrome and restless leg syndrome) or visceral (Interstitial cystitis, vulvodynia, chronic prostatitis and irritable bowel syndrome)) defined through Read codes.
These CSS were selected due to the presence of read codes relating to their diagnosis. Examples of CSS which have no associated Read code are chronic pelvic pain, chronic neck pain and chronic whiplash pain, which therefore were unavailable for inclusion in this study [33]. Sex specific outcomes such as vulvodynia and chronic prostatitis were only examined in female and male patients respectively.
Exposure code selection relating to confirmed childhood maltreatment and maltreatment related concerns have been described in our previous work [7,56,71]. Confirmed childhood maltreatment relates to recorded episodes of maltreatment against the child, whereas maltreatment related concerns includes episodes of suspected or possible childhood maltreatment. As there is likely a hidden burden of childhood maltreatment not identified by healthcare professionals, in this study (similar to an approach in our previous work) our exposure definition includes codes for both confirmed and suspected/possible childhood maltreatment to ensure inclusion of cases where childhood maltreatment may actually occur but is not brought to the attention of the healthcare professional [7]. However, a sensitivity analysis has also been conducted to explore the outcomes in the confirmed cases only.
Although there are currently no validated code lists for CSS outcomes, the musculoskeletal and chronic pain burden of GP consultations [57] is extensive so we anticipate reasonable coding. Outcome code lists relating to CSS were selected with the assistance of co-authors who had expertise in primary care and Read code selection, in conjunction with comparison of published code lists. Where relevant (e.g. back pain) it is important to note that the incidence of chronic pain outcomes is low in comparison to non-specific or acute pain codes. However, to ensure we are including conditions relating to CNS sensitisation we have not included non-specific/acute pain codes.
Read code lists relating to exposure terms and outcomes are provided (Appendix p2).
Selection of unexposed group
Each exposed patient was matched with up to two unexposed patients, who had no previously documented Read code relating to the exposure. Unexposed patients were matched by age at index date (+/- one year) and sex within the THIN database.
Follow-up period
The index date for those in the exposed group was the date at which they reached 18 years of age, a year after registration with the general practice or the date the general practice was eligible to contribute to the database, whichever was the latest. To mitigate immortality time bias[58], the same index date was assigned to the corresponding unexposed patient. The follow-up period for each patient was from the index date until the exit date. Exit date is defined as the earliest of the following dates: study end date, last date of data collection from a given general practice, date patient transferred from general practice, date of death or date the outcome of interest (CSS diagnosis) occurred. The median follow-up time in this study was 2.2 years (Interquartile range (IQR) 0.8–4.9 years [the maximum range was up to 22.2 years].
Co-variates
All data on co-variates were also extracted from the patient's records present on THIN.
Co-variates used in our modelling were selected due to their independent relationship with CSS development: age, sex, Townsend deprivation score (a measure of material deprivation within a locality, incorporating information on unemployment, household overcrowding and car/home ownership; a higher score indicates a greater level of socioeconomic deprivation)[59] as well as depression, anxiety and serious mental ill health were captured at baseline. Mental ill health, a prevalent factor in the childhood maltreatment cohort, has been shown in numerous studies to be associated with the development of a variety of chronic pain and central sensitisation syndromes and so we have accounted for its confounding effect in the statistical analysis [7,60]. In addition, data on body mass index (BMI), alcohol drinking status and smoking status are reported however, in young cohorts there is substantial missing data [7].
Statistical analysis
Categorical baseline data were described using proportions and continuous data were described using means or median with standard deviations or inter quartile range. Missing data are highlighted in relevant baseline characteristic tables. Where there were missing data in our covariates (Townsend score), it was treated as a separate missing category and included in the final analysis. Across the cohort, 16.8% of Townsend score data were missing. Other approaches such as complete case analysis, a missing dummy indicator approach and multiple imputations methods were considered, however the results were largely similar and a missing category was continued in line with previously published work in this field [7].
In order to calculate an incidence rate (IR) per 100,000 person years for each of the outcomes of interest, patients with pre-existing illness (defined as a CSS code) were excluded to ensure the IR reflected incident outcomes. Poisson regression offsetting for person years of follow-up was then used to calculate an incidence rate ratio (IRR) for each outcome of interest during the study period. Following adjustment for the co-variates (Models were adjusted for the following covariates at cohort entry: age, sex, Townsend score, anxiety, depression and serious mental ill health), we calculated and present an adjusted IRR (aIRR). IRRs are presented with 95% confidence intervals (CI) with statistical significance set at p<0·05. Outcomes which occurred less than 5 times are presented as <5.
A subgroup analysis was conducted to display the results by somatic (Fibromyalgia, chronic fatigue syndrome, temporomandibular joint disorder, chronic lower back pain, chronic headache, myofascial pain syndrome and restless leg syndrome) or visceral (Interstitial cystitis, vulvodynia, chronic prostatitis and irritable bowel syndrome) pain in nature.
A further sensitivity analysis was conducted to explore if findings differed when only looking at officially confirmed maltreatment codes (excluding possible/suspected maltreatment). Additionally, as the literature suggests older exposure to childhood maltreatment may correlate with worse outcomes [61]. In order to assess the differential impact of age at exposure to child maltreatment, a second sensitivity analysis was conducted. In the second analysis the exposed cohort was stratified into the age groups (based on age of exposure to childhood maltreatment) aged 0–4 years, 5–9 years, 10–15 and 16–17 years (age group boundaries comparable with national education data) [62]. The risks of developing outcomes in each group were compared against their respective controls and an aIRR was calculated for each outcome per age group. Due to the low number of comparable outcomes of chronic headache, myofascial pain syndrome, interstitial cystitis, vulvodynia and chronic prostatitis, they were excluded from this subgroup analysis.
STATA version 15.1 MP/4 software (StataCorp 2017) was used to conduct all analyses.
Ethical approval
Anonymised data were used throughout the study provided by the data provider to the University of Birmingham. Studies using The Health Improvement Network (THIN) database have had initial ethical approval from the NHS South-East Multicentre Research Ethics Committee, subject to prior independent scientific review. The Scientific Review Committee (IQVIA) approved the study protocol (SRC Reference Number: SRC18THIN034) prior to its undertaking. As the data held within THIN is anonymised and does not contain sensitive or identifiable information and therefore the need for informed consent was waived.
Results
Baseline characteristics
Of the total database cohort of 9,588,734 eligible patients, we identified 80,657 (0.8%) adult patients exposed to childhood maltreatment or maltreatment related concerns during childhood (<18 years) who were matched to 161,314 (1.7%) adult unexposed patients. The median follow-up was similar between the groups (exposed 2.3 years: unexposed 2.2 years). Due to matching characteristics mean age (23 years) and sex proportions (42% male) were similar between the groups. Where BMI and alcohol drinking status were recorded it was similar between the groups however there was substantial missing data both groups (BMI; exposed 53%: unexposed 46%; Drinking status; exposed 53%: unexposed: 49%). A greater proportion of the exposed group were current smokers (38%) compared to the unexposed group (20%). There was a greater proportion of socio-economic deprivation, mental ill health and baseline CSS comorbidity in the exposed group compared to the unexposed group. Further details can be seen in Table 1.
Table. 1.
Baseline characteristics of those exposed and unexposed to childhood maltreatment.
| Baseline Characteristics (Standard Deviation, Interquartile range or Percentage) |
||
|---|---|---|
| Childhood maltreatment |
||
| Exposed Group | Unexposed Group | |
| Number of patients | 80,657 | 161,314 |
| Median follow-up period (person years) | 2.3 (IQR 0.9–4.9) | 2.2 (IQR 0.8–4.8) |
| Age at cohort entry (years) | 23.3 (SD 7.3) | 23.4 (SD 7.2) |
| Age when maltreatment occurred (years) | 9.6 (5.2) | - |
| Sex; Male (%) | 33,614 (41.7%) | 67,228 (41.7%) |
| Body mass index | ||
| <25 kg/m2 | 24,091 (29.9%) | 56,633 (35.1%) |
| 25–30 kg/m2 | 7642 (9.5%) | 18,667 (11.6%) |
| >30 kg/m2 | 6322 (7.8%) | 11,254 (7.0%) |
| Not available | 42,602 (52.9%) | 74,760 (46.3%) |
| Smoking status | ||
| Current smoker | 30,462 (37.8%) | 31,577 (19.6%) |
| Non-current smoker | 28,998 (36.0%) | 102,302 (63.4%) |
| Not available | 21,197 (26.3%) | 27,435 (17.0%) |
| Drinking Status | ||
| Non-drinker | 13,990 (17.4%) | 24,164 (15.0%) |
| Drinker | 23,990 (29.7%) | 58,473 (36.3%) |
| Not available | 42,677 (52.9%) | 78,677 (48.8%) |
| Townsend index | ||
| (Least deprived) 1 | 6296 (7.8%) | 27,339 (17.0%) |
| 2 | 7925 (9.8%) | 24,915 (15.5%) |
| 3 | 13,469 (16.7%) | 29,599 (18.4%) |
| 4 | 19,116 (23.7%) | 29,947 (18.6%) |
| 5 | 19,860 (24.6%) | 22,881 (14.2%) |
| Not available | 13,991 (17.4%) | 26,633 (16.5%) |
| Mental ill health at baseline | ||
| Depression | 13,874 (17.2%) | 9063 (5.6%) |
| Anxiety | 6927 (8.6%) | 5858 (3.6%) |
| Serious mental ill health | 1777 (2.2%) | 676 (0.4%) |
| Central sensitivity syndrome at baseline | ||
| Fibromyalgia | 181 (0.2%) | 161 (0.1%) |
| Chronic fatigue syndrome | 171 (0.2%) | 342 (0.2%) |
| Temporomandibular joint disorder | 620 (0.8%) | 1358 (0.8%) |
| Chronic lower back pain | 279 (0.4%) | 289 (02%) |
| Interstitial cystitis | 12 (0.0%) | 19 (0.0%) |
| Vulvodynia (female) | 9 (0.0%) | 14 (0.0%) |
| Chronic prostatitis (male) | <5 (0.0%) | 14 (0.0%) |
| Chronic headache | 24 (0.0%) | 22 (0.0%) |
| Myofascial pain syndrome | 7 (0.0%) | 7 (0.0%) |
| Irritable bowel syndrome | 2768 (3.4%) | 4219 (2.6%) |
| Restless leg syndrome | 57 (0.1%) | 98 (0.1%) |
| All somatic syndromes | 1293 (1.6%) | 2240 (1.4%) |
| All visceral syndromes | 2783 (3.5%) | 4263 (2.6%) |
Cohort findings
Somatic CSS
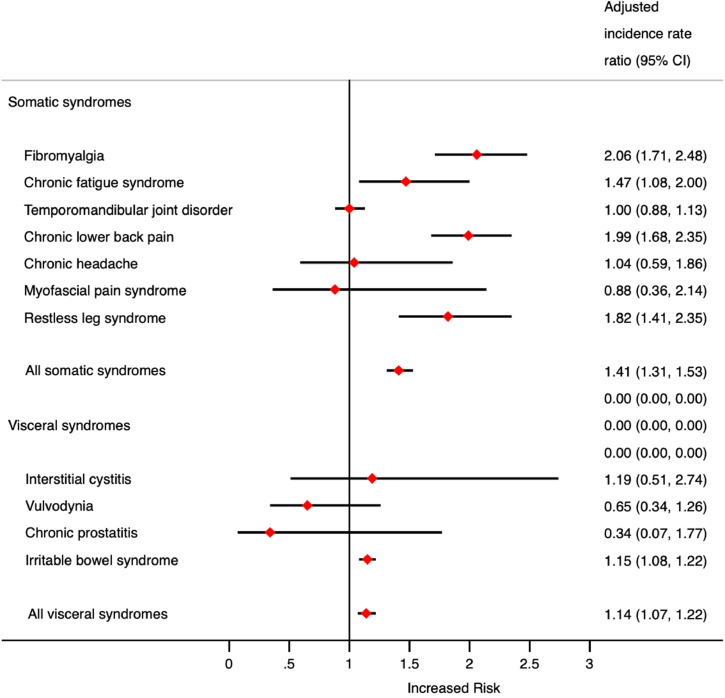
When examining the association between childhood maltreatment and the subsequent development of somatic CSS outcomes, there were notable increased risks (presented as aIRR with 95% CI) in the development of fibromyalgia (2.06; 1.71–2.48), chronic fatigue syndrome (1.47; 1.08–2.00), chronic lower back pain (1.99; 1.68–2.35) and restless leg syndrome (1.82; 1.41–2.35). However, there was no significant association seen in the development of temporomandibular joint disorder (1.00; 0.88–1.13), chronic headache (1.04; 0.59–1.86) and myofascial pain syndrome (0.88; 0.36–2.14). The combined risk of developing somatic CSS was 1.41; 1.31–1.53.
Visceral CSS
When examining visceral CSS outcomes, childhood maltreatment was only associated with the development of irritable bowel syndrome (1.15; 1.08–1.22). Childhood maltreatment was not statistically associated with the development of interstitial cystitis (1.19; 0.51–2.74), vulvodynia (0.65; 0.34–1.26) or chronic prostatitis (0.34; 0.07–1.22). However, the number of outcomes of interstitial cystitis, vulvodynia and chronic prostatitis were low during the study period. The combined risk of developing visceral CSS was 1.14; 1.07–1.22).
Table 2 displays further details of the risk of developing CSS following exposure to childhood maltreatment. It is important to note that amongst all of the findings the difference between the adjusted (adjusted for age, sex, Townsend score, anxiety, depression and serious mental ill health) and unadjusted values was low, suggesting the association may not be driven mainly by the role of mental ill health and demographic factors. Fig. 1 demonstrates the aIRR for developing CSS following exposure to childhood maltreatment displayed by either somatic or visceral CSS nature.
Table 2.
The risk of developing a central sensitivity syndrome in those exposed and unexposed to childhood maltreatment.
| Childhood maltreatment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fibromyalgia |
Chronic fatigue syndrome |
Temporomandibular joint disorder |
Chronic lower back pain |
Chronic headache |
Myofascial pain syndrome |
|||||||
| Exposed | Unexposed | Exposed | Unexposed | Exposed | Unexposed | Exposed | Unexposed | Exposed | Unexposed | Exposed | Unexposed | |
| Number of Patients | 80476 | 161150 | 80486 | 160972 | 80037 | 159956 | 80378 | 161025 | 80633 | 161292 | 80650 | 161307 |
| Numbers of Outcomes | 300 | 209 | 88 | 97 | 445 | 787 | 361 | 261 | 21 | 31 | 8 | 16 |
| Person-years | 292791 | 553912 | 293326 | 553628 | 290371 | 547522 | 292265 | 553294 | 294175 | 554996 | 294234 | 555031 |
| Incidence Rate (per 100000 person years) | 102.46 | 37.73 | 30.00 | 17.52 | 153.25 | 143.74 | 123.52 | 47.17 | 7.14 | 5.59 | 2.72 | 2.88 |
| Incidence Rate Ratio (95% Confidence intervals)* | 2.72 (2.28-3.24) | 1.71 (1.28-2.29) | 1.07 (0.95-1.20) | 2.62 (2.23-3.07) | 1.28 (0.73-2.22) | 0.94 (0.40-2.20) | ||||||
| P value | <0.001 | <0.001 | 0.280 | <0.001 | 0.385 | 0.893 | ||||||
| Adjusted Incidence Rate Ratio (95% Confidence intervals)⁎⁎ | 2.06 (1.71 -2.48) | 1.47 (1.08-2.00) | 1.00 (0.88-1.13) | 1.99 (1.68-2.35) | 1.04 (0.59-1.86) | 0.88 (0.36-2.14) | ||||||
| P value | <0.001 | 0.015 | 0.953 | <0.001 | 0.888 | 0.775 | ||||||
| Restless leg syndrome |
Interstitial cystitis |
Vulvodynia |
Chronic prostatitis |
Irritable bowel syndrome |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exposed | Unexposed | Exposed | Unexposed | Exposed | Unexposed | Exposed | Unexposed | Exposed | Unexposed | |
| Number of Patients | 80600 | 161216 | 80645 | 161295 | 47034 | 94072 | 33612 | 67214 | 77889 | 157095 |
| Numbers of Outcomes | 149 | 122 | 10 | 15 | 14 | 33 | <5 | 9 | 1750 | 2643 |
| Person-years | 293552 | 554412 | 294199 | 555030 | 171833 | 303854 | 122371 | 251109 | 277110 | 530415 |
| Incidence Rate (per 100000 person years) | 50.76 | 22.01 | 0.34 | 0.27 | 8.15 | 10.86 | 1.63 | 3.58 | 631.52 | 498.29 |
| Incidence Rate Ratio (95% Confidence intervals)* | 2.30 (1.82-2.93) | 1.26 (0.57-2.80) | 0.75 (0.40-1.40) | 0.46 (0.10-2.11) | 1.27 (1.19-1.35) | |||||
| P value | <0.001 | 0.574 | 0.368 | 0.315 | <0.001 | |||||
| Adjusted Incidence Rate Ratio (95% Confidence intervals)⁎⁎ | 1.82 (1.41-2.35) | 1.19 (0.51-2.74) | 0.65 (0.34-1.26) | 0.34 (0.07-1.77) | 1.15 (1.08-1.22) | |||||
| P value | <0.001 | 0.691 | 0.203 | 0.201 | <0.001 | |||||
| All somatic syndromes |
All visceral syndromes |
|||
|---|---|---|---|---|
| Exposed | Unexposed | Exposed | Unexposed | |
| Number of Patients | 79364 | 159074 | 77874 | 157051 |
| Numbers of Outcomes | 1250 | 1432 | 1768 | 2689 |
| Person-years | 285536 | 542495 | 277014 | 530210 |
| Incidence Rate (per 100000 person years) | 437.77 | 263.97 | 638.23 | 507.16 |
| Incidence Rate Ratio (95% Confidence intervals)* | 1.66 (1.54-1.79) | 1.26 (1.19-1.34) | ||
| P value | <0.001 | <0.001 | ||
| Adjusted Incidence Rate Ratio (95% Confidence intervals)⁎⁎ | 1.41 (1.31-1.53) | 1.14 (1.07-1.22) | ||
| P value | <0.001 | <0.001 | ||
Unadjusted incidence rate ratio
Adjusted Incidence rate ratio: adjusted for age, sex, depression, anxiety, serious mental ill health and Townsend deprivation score at baseline
Fig. 1.
The risk of developing a central sensitivity syndrome in those exposed and unexposed to childhood maltreatment broken down by somatic or visceral subtype.
Sensitivity analysis
When conducting the sensitivity analysis, 22,078 (27% of the total exposed cohort) exposed cases were identified as exposed to confirmed childhood maltreatment who were matched to 44,156 (27% of the total unexposed cohort) unexposed patients. The baseline characteristics were similar to the main cohort (see appendix p9). However, the average age was older at 27 years and the median follow was 2.4 years for the exposed group compared to 2.2 years in the unexposed group.
When isolating confirmed codes only, the risk of developing conditions previously seen as positively associated continued to remain persistent: fibromyalgia (3.25; 2.39–4.41), chronic fatigue syndrome (1.64; 1.00–2.67), chronic lower back pain (2.52; 1.90–3.34), restless leg syndrome (2.60; 1.72–3.95) and irritable bowel syndrome (1.30; 1.16–1.46). Of interest, when isolating confirmed cases of childhood maltreatment only, the risk of developing temporomandibular joint disorder became positively associated with exposure to childhood maltreatment (1.32; 1.06–1.65) which was not in the main analysis. The combined risk of developing somatic CSS (1.96; 1.71–2.24) continued to be higher than the risk of developing visceral CSS (1.29; 1.15–1.44) Further details can be seen on appendix p11.
When conducting a second sensitivity analysis examining the differential impact of age of childhood maltreatment exposure, there were four separate cohorts (defined by age at exposure): 1) 0–4 years of age (22.3% of total cohort): exposed cohort (n = 17,970); unexposed cohort (n = 35,940), 2) 5–9 years of age (23.7% of total cohort): exposed cohort (n = 19,088); unexposed cohort (n = 38,176), 3) 10–15 years of age (40.0% of total cohort): exposed cohort (n = 32,223); unexposed group (n = 64,446) and 4) 16–17 years of age (14.1% of total cohort): exposed cohort (11,376); unexposed (n = 22,752). The most notable difference in the baseline characteristics across the groups was the difference in sex ratio across the four groups, as the percentage of female exposed survivors was more prevalent than males in the older age groups. Otherwise, the groups were relatively similar as seen in appendix p12.
Although, there were differential impacts of age group, there was no clear trend of risk dependent on increasing or decreasing age group. For example, the risk of developing fibromyalgia was significant in the younger age groups (0–4-year group: 2.14; 1.33–3.43, 5–9-year group; 2.62; 1.81–3.78, 10–15-year group; 2.06; 1.54–2.76) compared to a non-significant result in the eldest group of those aged 16–17-year group (1.31; 0.83–2.07). By contrast, the eldest age group showed the greatest risk in developing chronic back pain (2.99; 1.76–5.09). In comparison, for restless leg syndrome and chronic fatigue syndrome the greatest risk groups were those who had experienced childhood maltreatment in the 5–9-year group and 10–15-year group respectively.
Discussion
To our knowledge, this is the first attempt to synthesise data taken from UK primary care records exploring the relationship between childhood maltreatment and the subsequent development of CSS. The key findings were that after adjusting for important covariates such as the presence of mental ill health, exposure to childhood maltreatment leads to up to a doubling in risk of developing of certain somatic syndromes (fibromyalgia, chronic fatigue syndrome, chronic lower back pain and restless leg syndrome) and when isolated to confirmed cases, childhood maltreatment is also associated with temporomandibular joint disorder. In addition to somatic disorders, exposure to childhood maltreatment is associated with the development of irritable bowel syndrome. However, childhood maltreatment was not clearly associated with other CSS; somatic (chronic headaches and myofascial pain syndrome) or visceral (interstitial cystitis, vulvodynia and chronic prostatitis). However, it must be noted that the number of outcomes during the study period for non-significant findings were low. Overall it appeared as though the risk of developing somatic CSS were higher than visceral CSS following exposure to childhood maltreatment, which was further emphasised in the confirmed cases only sensitivity analysis.
As the authors believe this study was the first to assess the incidence of CSS outcomes using UK definitions of childhood maltreatment, it is difficult to compare our incidence rates with other international study findings. Despite this, the results of our study have been able to build on the existing observational data linking childhood maltreatment to the subsequent development of a variety of CSS [18,42,[44], [45], [46], [47], [48], [49], [50], [51], [52]]. To the authors knowledge there have been no reported studies examining the outcomes of myofascial pain syndrome or restless leg syndrome in cohorts of childhood maltreatment patients, therefore these findings are novel.
Also, of note is that some of our non-significant findings are in support of existing literature. In a cross-sectional survey of male participants (n = 5506) based in Boston, US, there was no association between chronic prostatitis with one experience of childhood abuse (Odds ratio (OR) 1.05; 0.55–2.01) [52]. Similarly, although evidence exists suggesting experiences of childhood abuse may be more prevalent in cohorts of individuals experiencing interstitial cystitis, there is no evidence to suggest the risk of developing interstitial cystitis in higher in those who have experienced childhood abuse [49,63,64]. The current evidence on the development of chronic headaches is contrasting. One cross-sectional study of 41 patients with chronic headache identified no significant association with the presence of childhood neglect or abuse [65]. However, an alternative cross-sectional study including 18,303 adults was able to adjust for mental health disorders and identified an increased risk of developing chronic headaches [66]. Although in this study, exposure and outcomes were self-reported. Also, in contrast to our findings, a single case-control study (n = 215 pairs) identified severe childhood abuse survivors were three times (aOR 2.9;1.5–4.8) more likely to have gone onto present with VD. However, in that case-control study, when cases were isolated to moderate abuse, the odds ratio became non-significant (aOR 1.2;0.8–1.8) more in line with our findings. However, it is important to note, for the conditions which showed non-significance in our study, there is little available literature examining their association with childhood maltreatment.
The authors also believe this is the first study which examines the differential impact of age of exposure in the presentation of CSS. Despite previous suggestions relating to poorer outcomes associated with an older age of first exposure to childhood maltreatment [61], our findings do not demonstrate the relationship such a clear-cut relationship. There appears to be a differential risk suggesting the impact of stress at different points in childhood may affect the development of different CSS through differing aetiological pathways.
Our findings are important in both a UK and a global setting as the morbidity burden of conditions included within CSS such as chronic lower back pain on disability adjusted life years are substantial [67]. Although, there are no internationally agreed upon guidelines for the current management of patients experiencing these syndromes, it has been shown that pharmacotherapeutic agents have shown little promise in symptom management [68]. Instead more promising approaches consider the complex pathology of the condition and in turn deliver a multimodal treatment plan combining bio-psycho-social elements such as management of stress [68].
The use of UK primary care records for epidemiological research relies upon the accuracy of documenting by the healthcare professionals contributing to the dataset. A limitation of the study is the exposure and outcome Read codes have not yet been validated against patient notes [69]. To try and mitigate this, for the childhood maltreatment exposure (under-recorded compared to national estimates), [70], [72] we also included maltreatment related codes. Under-recording of exposure is likely to lead to misclassification bias where the unexposed group may be incorrectly coded as unexposed when they are in fact exposed. Therefore, it is possible our findings are an underestimate of the true effect size. On the other hand, it is possible that if childhood maltreatment were recorded by a healthcare practitioner, it could mean the traumatic experience was particularly severe. The granularity of the codes did not allow for more in-depth investigation based on severity of childhood maltreatment. However, the sensitivity analysis exploring confirmed childhood maltreatment cases gave an indication that confirmation of childhood maltreatment led to an increased risk of CSS outcomes. Despite this increased risk seen in the sensitivity analysis, future research should consider case validation approaches to examine the definition of “confirmed” childhood maltreatment in electronic health records research. When examining the validity of coding it is also important to note that the number of some outcomes (interstitial cystitis, vulvodynia, myofascial pain syndrome, chronic headaches and chronic prostatitis) was very low in this study. It is not clear as to the potential reason for the low number of these outcomes which may include; an under-recording/reporting of the condition, diagnostic delay or expected low incidence in the young cohort we have examined here. Future work must be done to examine the epidemiology of these in community based studies. Additionally, although in this study we were able to adjust for some important confounders, there are others which may play an important role in the development of CSS not included due to limitations in the dataset. Future research should explore this relationship in datasets which hold complete information on these additional confounders e.g. ethnicity and educational status.
Our results may appear to demonstrate a greater affiliation towards the development of somatic CSS subtypes, this must be taken into consideration with the likelihood that the outcomes in the visceral groups were underpowered due to their rarity in primary care recording, young age of the cohort and limited follow up time.
In conclusion, our study showed increased risk of many types of CSS following exposure to childhood maltreatment. Although this association has been demonstrated, further work needs to be done to explore the mechanistic pathways between childhood maltreatment and the development of CSS.
Despite the need for further work to examine the explanatory pathways, it is clear the increased risk demonstrates the need to consider primary and secondary preventative approaches to improve the prevention and detection of childhood maltreatment as well as mitigation of subsequent negative effects. Clinicians can consider this by exploring a past history of either childhood maltreatment in patients presenting with CSS, and then subsequently consider therapeutic options which could support either 1) the maladaptive psychological coping strategy from the traumatic maltreatment experience, 2) the lifestyle choices made by patients as result of the childhood maltreatment or 3) the necessity of medical therapies to deal with the psychological or physical comorbidities, all of which could mediate or relate to symptom presentation in CSS.
Contributors
This study contributed to the PhD thesis for the main author JSC. JSC, JT, SB and KN were responsible for initial conception of the study and supervision. JSC was responsible for data extraction, analysis and first draft of the manuscript. These stages were supported by DK, DZ, KO and KM. The final manuscript was authorised by all of the authors with JT providing expert knowledge on childhood maltreatment, KR providing expert knowledge on functional pain syndromes/study design whilst SB and KN provided methodological expertise.
Declaration of Competing Interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work, no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work.
Funding
There is no funding to declare in this study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100392.
Contributor Information
Joht Singh Chandan, Email: Joht.chandan@nhs.net.
Deepiksana Keerthy, Email: DXK763@student.bham.ac.uk.
Dawit Tefra Zemedikun, Email: D.T.Zemedikun@bham.ac.uk.
Kelvin Okoth, Email: KOO657@student.bham.ac.uk.
Krishna Margadhamane Gokhale, Email: k.m.gokhale@bham.ac.uk.
Karim Raza, Email: k.raza@bham.ac.uk.
Siddhartha Bandyopadhyay, Email: s.bandyopadhyay@bham.ac.uk.
Julie Taylor, Email: j.taylor.1@bham.ac.uk.
Krishnarajah Nirantharakumar, Email: k.nirantharan@bham.ac.uk.
Appendix. Supplementary materials
References
- 1.HM Government. Working Together to Safeguard Children: a guide to inter-agency working to safeguard and promote the welfare of children. 2018.
- 2.Human Rights Act1998.
- 3.Bellis M.A., Hughes K., Ford K., Ramos Rodriguez G., Sethi D., Passmore J. Life course health consequences and associated annual costs of adverse childhood experiences across Europe and North America: a systematic review and meta-analysis. Lancet Public Heal. 2019 doi: 10.1016/S2468-2667(19)30145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert R., Widom C.S., Browne K., Fergusson D., Webb E., Janson S. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373:68–81. doi: 10.1016/S0140-6736(08)61706-7. [DOI] [PubMed] [Google Scholar]
- 5.Bacchus L.J., Ranganathan M., Watts C., Devries K. Recent intimate partner violence against women and health: a systematic review and meta-analysis of cohort studies. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-019995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krug EG, Dahlberg LL, Mercy JA, Zwi AB, Lozano R. World report on violence and health. http://apps.who.int/iris/bitstream/handle/10665/42495/9241 545615_eng.pdf;jsessionid=38974668595BA31895E557DC1CB1EFEC?sequence=1 (accessed 4 Jul2018).
- 7.Chandan J.S., Thomas T., Gokhale K.M., Bandyopadhyay S., Taylor J., Nirantharakumar K. The burden of mental ill health associated with childhood maltreatment in the UK, using The Health Improvement Network database: a population-based retrospective cohort study. Lancet Psychiatry. 2019;6:926–934. doi: 10.1016/S2215-0366(19)30369-4. [DOI] [PubMed] [Google Scholar]
- 8.Chandan J.S., Thomas T., Bradbury-Jones C., Russell R., Bandyopadhyay S., Nirantharakumar K. Female survivors of intimate partner violence and risk of depression, anxiety and serious mental illness. Br J Psychiatry. 2019:1–6. doi: 10.1192/bjp.2019.124. [DOI] [PubMed] [Google Scholar]
- 9.Chandan J.S., Thomas T., Bradbury-Jones C., Taylor J., Bandyopadhyay S., Nirantharakumar K. Intimate partner violence and temporomandibular joint disorder. J Dent. 2019;82:98–100. doi: 10.1016/j.jdent.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Chandan J.S., Thomas T., Raza K., Bradbury-Jones C., Taylor J., Bandyopadhyay S. Intimate partner violence and the risk of developing fibromyalgia and chronic fatigue syndrome. J Interpers Viol. 2019 doi: 10.1177/0886260519888515. 088626051988851. [DOI] [PubMed] [Google Scholar]
- 11.Chandan J.S., Thomas T., Bradbury-Jones C., Taylor J., Bandyopadhyay S., Nirantharakumar K. Risk of cardiometabolic disease and all-cause mortality in female survivors of domestic abuse. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afshin A., Gakidou E., Lim S.S., Abate D., Abate K.H., GBD 2017 Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study. Lancet (Lond, Engl) 2017;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pico-Alfonso M.A., Garcia-Linares M.I., Celda-Navarro N., Herbert J., Martinez M. Changes in cortisol and dehydroepiandrosterone in women victims of physical and psychological intimate partner violence. Biol Psychiatry. 2004;56:233–240. doi: 10.1016/j.biopsych.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez A. The impact of childhood maltreatment on biological systems: implications for clinical interventions. Paediatr Child Health. 2013;18:415–418. [PMC free article] [PubMed] [Google Scholar]
- 15.Suglia S.F., Koenen K.C., Boynton-Jarrett R., Chan P.S., Clark C.J., Danese A. Childhood and adolescent adversity and cardiometabolic outcomes: a scientific statement from the American heart association. Circulation. 2018;137 doi: 10.1161/CIR.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasmussen L.J.H., Moffitt T.E., Eugen-Olsen J., Belsky D.W., Danese A., Harrington H. Cumulative childhood risk is associated with a new measure of chronic inflammation in adulthood. J Child Psychol Psychiatry. 2018 doi: 10.1111/jcpp.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Out D., Hall R.J., Granger D.A., Page G.G., Woods S.J. Assessing salivary C-reactive protein: longitudinal associations with systemic inflammation and cardiovascular disease risk in women exposed to intimate partner violence. Brain Behav Immun. 2012;26:543–551. doi: 10.1016/j.bbi.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borsini A., Hepgul N., Mondelli V., Chalder T., Pariante C.M. Childhood stressors in the development of fatigue syndromes: a review of the past 20 years of research. Psychol. Med. 2014;44:1809–1823. doi: 10.1017/S0033291713002468. [DOI] [PubMed] [Google Scholar]
- 19.Crane C.A., Hawes S.W., Weinberger A.H. Intimate partner violence victimization and cigarette smoking: a meta-analytic review. Trauma Violence Abuse. 2013;14:305–315. doi: 10.1177/1524838013495962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afifi T.O., Henriksen C.A., Asmundson G.J.G., Sareen J. Victimization and perpetration of intimate partner violence and substance use disorders in a nationally representative sample. J Nerv Ment Dis. 2012;200:684–691. doi: 10.1097/NMD.0b013e3182613f64. [DOI] [PubMed] [Google Scholar]
- 21.Davies R., Lehman E., Perry A., McCall-Hosenfeld J.S. Association of intimate partner violence and health-care provider-identified obesity. Women Health. 2016;56:561–575. doi: 10.1080/03630242.2015.1101741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turk D.C., Wilson H.D. Fear of pain as a prognostic factor in chronic pain: conceptual models, assessment, and treatment implications. Curr Pain Headache Rep. 2010;14:88–95. doi: 10.1007/s11916-010-0094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicol A.L., Sieberg C.B., Clauw D.J., Hassett A.L., Moser S.E., Brummett C.M. The Association between a history of lifetime traumatic events and pain severity, physical function, and affective distress in patients with chronic pain. J Pain. 2016;17:1334–1348. doi: 10.1016/j.jpain.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Teicher M.H., Anderson C.M., Ohashi K., Polcari A. Childhood maltreatment: altered network centrality of cingulate, precuneus, temporal pole and insula. Biol Psychiatry. 2014;76:297–305. doi: 10.1016/j.biopsych.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dannlowski U., Kugel H., Huber F., Stuhrmann A., Redlich R., Grotegerd D. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Hum Brain Mapp. 2013;34:2899–2909. doi: 10.1002/hbm.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puetz V.B., Parker D., Kohn N., Dahmen B., Verma R., Konrad K. Altered brain network integrity after childhood maltreatment: a structural connectomic DTI-study. Hum Brain Mapp. 2017;38:855–868. doi: 10.1002/hbm.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludwig L., Pasman J.A., Nicholson T., Aybek S., David A.S., Tuck S. Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and meta-analysis of case-control studies. The Lancet Psychiatry. 2018;5:307–320. doi: 10.1016/S2215-0366(18)30051-8. [DOI] [PubMed] [Google Scholar]
- 28.Diez I, Larson AG, Nakhate V, Dunn EC, Fricchione GL, Nicholson TR. et al. Early-life trauma endophenotypes and brain circuit-gene expression relationships in functional neurological (conversion) disorder. doi: 10.1038/s41380-020-0665-0. [DOI] [PMC free article] [PubMed]
- 29.Garcia-Larrea L, Peyron R. Pain matrices and neuropathic pain matrices: a review. 2013. doi: 10.1016/j.pain.2013.09.001. [DOI] [PubMed]
- 30.Davis D.A., Luecken L.J., Zautra A.J. Are reports of childhood abuse related to the experience of chronic pain in adulthood. Clin J Pain. 2005;21:398–405. doi: 10.1097/01.ajp.0000149795.08746.31. [DOI] [PubMed] [Google Scholar]
- 31.Jackson T., Thomas S., Stabile V., Han X., Shotwell M., McQueen K. Prevalence of chronic pain in low-income and middle-income countries: a systematic review and meta-analysis. Lancet. 2015;385:S10. doi: 10.1016/S0140-6736(15)60805-4. [DOI] [PubMed] [Google Scholar]
- 32.Fayaz A., Croft P., Langford R.M., Donaldson L.J., Jones G.T. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nijs J., Polli A., Willaert W., Malfliet A., Huysmans E., Coppieters I. Central sensitisation: another label or useful diagnosis. Drug Ther Bull. 2019;57:60–63. doi: 10.1136/dtb.2018.000035. [DOI] [PubMed] [Google Scholar]
- 34.Nijs J., Paul van Wilgen C., Van Oosterwijck J., van Ittersum M., Meeus M. How to explain central sensitization to patients with ‘unexplained’ chronic musculoskeletal pain: practice guidelines. Man Ther. 2011;16:413–418. doi: 10.1016/j.math.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Chandan J.S., Thomas T., Raza K., Bandyopadhyay S., Nirantharakumar K., Taylor J. Association between child maltreatment and central sensitivity syndromes: a systematic review protocol. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-025436. bmjopen-2018-025436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moshiree B., Zhou Q., Price D.D., Verne G.N. Central sensitisation in visceral pain disorders. Gut. 2006;55:905–908. doi: 10.1136/gut.2005.078287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cervero F., Laird J.M. Visceral pain. Lancet. 1999;353:2145–2148. doi: 10.1016/S0140-6736(99)01306-9. [DOI] [PubMed] [Google Scholar]
- 38.Dunckley P., Wise R.G., Fairhurst M., Hobden P., Aziz Q., Chang L. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci. 2005;25:7333–7341. doi: 10.1523/JNEUROSCI.1100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woolf C.J. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Latremoliere A., Woolf C.J. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eller-Smith O.C., Nicol A.L., Christianson J.A. Potential mechanisms underlying centralized pain and emerging therapeutic interventions. Front Cell Neurosci. 2018;12:35. doi: 10.3389/fncel.2018.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chitkara D.K., van Tilburg M.A.L., Blois-Martin N., Whitehead W.E. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103:765–774. doi: 10.1111/j.1572-0241.2007.01722.x. quiz 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sedgwick P. What is recall bias. BMJ. 2012;344 e3519–e3519. [Google Scholar]
- 44.Fillingim R.B., Maixner W., Sigurdsson A., Kincaid S. Sexual and physical abuse history in subjects with temporomandibular disorders: relationship to clinical variables, pain sensitivity, and psychologic factors. J Orofac Pain. 1997;11:48–57. [PubMed] [Google Scholar]
- 45.Linton S.J. A population-based study of the relationship between sexual abuse and back pain: establishing a link. Pain. 1997;73:47–53. doi: 10.1016/s0304-3959(97)00071-7. [DOI] [PubMed] [Google Scholar]
- 46.Leisner S., Gerhardt A., Tesarz J., Janke S., Seidler G.H., Eich W. Frühe missbrauchserlebnisse bei chronischem Kreuzschmerz. Der Schmerz. 2014;28:600–606. doi: 10.1007/s00482-014-1487-2. [DOI] [PubMed] [Google Scholar]
- 47.You D.S., Albu S., Lisenbardt H., Meagher M.W. Cumulative childhood adversity as a risk factor for common chronic pain conditions in young adults. Pain Med. 2019;20:486–494. doi: 10.1093/pm/pny106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schrepf A., Naliboff B., Williams D.A., Stephens-Shields A.J., Landis J.R., Gupta A. Adverse childhood experiences and symptoms of urologic chronic pelvic pain syndrome: a multidisciplinary approach to the study of chronic pelvic pain research network study. Ann Behav Med. 2018;52:865–877. doi: 10.1093/abm/kax060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiu C.-.D., Lee M.-.H., Chen W.-.C., Ho H.L., Wu H.-.C. Childhood trauma perpetrated by close others, psychiatric dysfunction, and urological symptoms in patients with interstitial cystitis/bladder pain syndrome. J Psychosom Res. 2017;93:90–95. doi: 10.1016/j.jpsychores.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 50.Edwards L., Mason M., Phillips M., Norton J., Boyle M. Childhood sexual and physical abuse. Incidence in patients with vulvodynia. J Reprod Med. 1997;42:135–139. [PubMed] [Google Scholar]
- 51.Harlow B.L., Stewart E.G. Adult-onset vulvodynia in relation to childhood violence victimization. Am J Epidemiol. 2005;161:871–880. doi: 10.1093/aje/kwi108. [DOI] [PubMed] [Google Scholar]
- 52.Hu J.C., Link C.L., McNaughton-Collins M., Barry M.J., McKinlay J.B. The association of abuse and symptoms suggestive of chronic prostatitis/chronic pelvic pain syndrome: results from the boston area community health survey. J Gen Intern Med. 2007;22:1532–1537. doi: 10.1007/s11606-007-0341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blak B.T., Thompson M., Dattani H., Bourke A. Generalisability of the health improvement network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19:251–255. doi: 10.14236/jhi.v19i4.820. [DOI] [PubMed] [Google Scholar]
- 54.Booth N. What are the read codes. Health Libr Rev. 1994;11:177–182. doi: 10.1046/j.1365-2532.1994.1130177.x. [DOI] [PubMed] [Google Scholar]
- 55.Maguire A., Blak B.T., Thompson M. The importance of defining periods of complete mortality reporting for research using automated data from primary care. Pharmacoepidemiol Drug Saf. 2009;18:76–83. doi: 10.1002/pds.1688. [DOI] [PubMed] [Google Scholar]
- 56.Woodman J., Freemantle N., Allister J., de Lusignan S., Gilbert R., Petersen I. Variation in recorded child maltreatment concerns in UK primary care records: a cohort study using The Health Improvement Network (THIN) database. PLoS ONE. 2012;7:e49808. doi: 10.1371/journal.pone.0049808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phillips C.J. The cost and burden of chronic pain. Rev Pain. 2009;3:2–5. doi: 10.1177/204946370900300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lévesque L.E., Hanley J.A., Kezouh A., Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087. doi: 10.1136/bmj.b5087. [DOI] [PubMed] [Google Scholar]
- 59.Townsend P., Phillimore P., Beattie A. Health and deprivation: inequality and the North. London Croom Helm Google Sch 1988. 1988 [Google Scholar]
- 60.Osterweis M. Pain and disability. National Academies Press: Washington, DC; 1987. Kleinman A MD. [DOI] [PubMed] [Google Scholar]
- 61.Dunn E.C., Busso D.S., Raffeld M.R., Smoller J.W., Nelson C.A., Doyle A.E. Does developmental timing of exposure to child maltreatment predict memory performance in adulthood? Results from a large, population-based sample. Child Abuse Negl. 2016;51:181–191. doi: 10.1016/j.chiabu.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Department for Education. Children looked after in England including adoption: 2018 to 2019 - GOV.UK. 2019. https://www.gov.uk/government/statistics/children-looked-after-in-england-including-adoption-2018-to-2019 (accessed 15 Dec 2019).
- 63.Mayson B.E., Teichman J.M.H. The relationship between sexual abuse and interstitial cystitis/painful bladder syndrome. Curr Urol Rep. 2009;10:441–447. doi: 10.1007/s11934-009-0070-3. [DOI] [PubMed] [Google Scholar]
- 64.Link C.L., Pulliam S.J., Hanno P.M., Hall S.A., Eggers P.W., Kusek J.W. Prevalence and psychosocial correlates of symptoms suggestive of painful bladder syndrome: results from the boston area community health survey. J Urol. 2008;180:599–606. doi: 10.1016/j.juro.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yücel B., Özyalcin S., HÖ Sertel, Çamlica H., Ketenci A., Talu G.K. Childhood traumatic events and dissociative experiences in patients with chronic headache and low back pain. Clin J Pain. 2002;18:394–401. doi: 10.1097/00002508-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 66.Scott K.M., Von Korff M., Angermeyer M.C., Benjet C., Bruffaerts R., de Girolamo G. Association of childhood adversities and early-onset mental disorders with adult-onset chronic physical conditions. Arch Gen Psychiatry. 2011;68:838–844. doi: 10.1001/archgenpsychiatry.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators SL. Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nijs J., Leysen L., Vanlauwe J., Logghe T., Ickmans K., Polli A. Treatment of central sensitization in patients with chronic pain: time for change. Expert Opin Pharmacother. 2019;20:1961–1970. doi: 10.1080/14656566.2019.1647166. [DOI] [PubMed] [Google Scholar]
- 69.McBrien K.A., Souri S., Symonds N.E., Rouhi A., Lethebe B.C., Williamson T.S. Identification of validated case definitions for medical conditions used in primary care electronic medical record databases: a systematic review. J Am Med Informatics Assoc. 2018;25:1567–1578. doi: 10.1093/jamia/ocy094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Radford L, Corral S, Bradley C, Fisher H, Bassett C, Howat N. et al. Child abuse and neglect in the UK today.
- 71.Chandan J.S, Okoth K, Gokhale KM, Bandyopadhyay S, Taylor J, Nirantharakumar K. Increased cardiometabolic and mortality risk following childhood maltreatment in the United Kingdom. J Am Heart Assoc. 2020 doi: 10.1161/JAHA.119.015855. https://www.ahajournals.org/doi/10.1161/JAHA.119.015855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chandan J.S, Gokhale K.M, Bradbury-Jones C, Nirantharakumar K, Bandyopadhyay S, Taylor J. An exploration of trends in the incidence and prevalence of childhood maltreatment and domestic abuse recording in UK primary care: a retrospective cohort study using ‘The Health Improvement Network’ database. BMJ Open. 2020;10:10:e036949. doi: 10.1136/bmjopen-2020-036949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.