Abstract
The year 2017 marked the 20th anniversary of the first publication describing Klotho. This single protein was and is remarkable in that its absence in mice conferred an accelerated aging, or progeroid, phenotype with a dramatically shortened life span. On the other hand, genetic overexpression extended both health span and life span by an impressive 30%. Not only has Klotho deficiency been linked to a number of debilitating age-related illnesses but many subsequent reports have lent credence to the idea that Klotho can compress the period of morbidity and extend the life span of both model organisms and humans. This suggests that Klotho functions as an integrator of organ systems, making it both a promising tool for advancing our understanding of the biology of aging and an intriguing target for interventional studies. In this review, we highlight advances in our understanding of Klotho as well as key challenges that have somewhat limited our view, and thus translational potential, of this potent protein.
Keywords: Klotho, Longevity, Geroscience, Health span, Biomarker
It was six men of Indostan
To learning much inclined
Who went to see the Elephant?
Though all of them were blind,
That each by observation
Might satisfy his mind.
[…]
And so these men of Indostan
Disputed loud and long,
Each in his own opinion
Exceeding stiff and strong.
Though each was partly in the right,
They all were partly wrong.
—John Godfrey Saxe
In the parable of the Blind Men and the Elephant, a series of blind men happen upon a mysterious and unknown object—an elephant. Having never before encountered an elephant, the men endeavor to describe their finding. However, each one’s experience is limited to a single part of the elephant’s body (e.g., the tail, the tusk, or the trunk), resulting in a contracted, though not incorrect, interpretation of the animal in its entirety. The challenge, then, becomes for the men to compile and integrate their experiences toward a more complete vision of the animal as a whole.
Geroscience in many ways embodies this philosophy. The overarching premise of Geroscience is that a unifying formalism, such as the Hallmarks of Aging (1), is needed to better understand and address the contribution of organismal aging to disease pathogenesis over time (2,3). Arguably, the longevity protein, Klotho, provides a useful framework for such a holistic understanding of the aging process. Recent years have enjoyed many noteworthy advances in our mechanistic understanding of the antiaging effects of Klotho, and recognition of the significant impact of Klotho on a number of individual diseases and disease states abounds. As such, there is much reason for optimism in the development of Klotho-based clinical therapeutics to counteract the deleterious effects of aging on organismal health. Yet, despite a number of promising basic science discoveries over the last 20 years since Klotho’s discovery, there are currently no clinical trials investigating Klotho administration. This review seeks to coalesce the current understanding of the opportunities and barriers to the clinical translation of Klotho-based therapeutics.
Overview of the Biology of Klotho
There have been a number of comprehensive and excellent reviews that describe our current understanding of the biology of the Klotho family of endocrine proteins, to which the reader is referred (4–9). The family members α- and β-Klotho owe most, but not all, of their actions to serving as essential co-receptors for specific fibroblast growth factors (FGF) at FGF receptors (FGFRs) (6,9). Here, we focus specifically on α-Klotho (hereafter referred to as “Klotho”) and briefly summarize key aspects of the history and known functions to set a foundation for discussion of knowledge gaps relevant for the future of translationally-oriented studies.
Mouse Models
The Klotho mouse was fortuitously generated as a byproduct of a standard pronuclei microinjection in an attempt to produce a transgenic mouse (rabbit Na+–H+ exchanger) (10). To obtain fertilized eggs, superovulated female mice (C3H×C57BL/6 F1) were mated with C3H males, and male pronuclei were microinjected with the transgene. The surviving microinjected eggs were transferred into the oviducts of pseudopregnant Swiss Albino female mice, which were then mated with vasectomized Swiss Albino males. Only 10% of the generated transgenic mice expressed the transgene. Therefore, the remaining mice were independently mated in the quest of homozygotes of the exogenous transgene (10,11). One set of homozygous mutant offspring from these matings unexpectedly exhibited multiple aging-related phenotypes, including growth retardation, gait disturbance, arteriosclerosis, and, most strikingly, a markedly shortened life span. It was determined that the ectopic insertion of the transgene had caused an 8 kb deletion in the 5′ upstream promoter, a region of the now-identified Klotho gene locus.
The aged phenotype of these “Klotho” (kl/kl) mice became manifest at 3 weeks of age, at which time the mice stopped growing. Most of these mice died prematurely by the age of 8–9 weeks, with average life spans of 15 weeks and none surviving more than 25 weeks. This was in stark contrast to wild-type counterparts, which have average life spans approximately 2.5–3 years. The Klotho-hypomorphic (kl/kl) phenotype was subsequently reproduced by a Klotho null mutant mouse (kl−/−) on the same background and then again on a BALB/c background (11). Since then, many different mice with varying levels of Klotho expression have been engineered and used for testing of therapeutic interventions testing and mechanistic studies of tissue pathogenesis (11–25). It is important to note, however, that loss of Klotho does not always fully recapitulate the underlying mechanisms driving the phenotype that typifies normal aging. For instance, Klotho mutation on a 129S1/SvImJ X C3H/HeJ X C57BL/6J background resulted in a decreased retinal function but no decrease in photoreceptor number (12), as is typical of aged wild-type mice (12,26).
In addition to full knockouts, models possessing varying levels of Klotho expression also provide valuable information regarding Klotho’s effects on tissue health. Despite the more subtly aged phenotype of heterozygous Klotho mutants, these mice possess distinct pathophysiological features from their knockout and kl/kl mice counterparts, making them a powerful research tool. Consider the effect of Klotho on immune system function, for example. Increased numbers of monocytes, macrophages, and T cells have been reported in the kidneys of heterozygous Klotho mice (27). In contrast, this immune response was not observed in kl/kl mice (11,28). This discrepancy is probably due to the abnormal leukocyte cell number, abnormal definitive hematopoiesis, thymus atrophy, decreased osteoclast cell number, decreased B-cell number, abnormal spleen morphology, and lung inflammation of knockout mice. Along these lines, the CRISPR/Cas9 system to generate mice with point mutations is another experimental approach that may be useful for evaluating the roles of specific nucleotides or amino acids in the function of Klotho polypeptides (29). By microinjecting guide RNA, hCas9 messenger RNA (mRNA) and single-stranded donor oligonucleotides into mouse zygotes, specific genomic modifications in Klotho can be generated with a low cost and in a short time.
Although a major impetus for the development of Klotho mouse strains has been to study premature aging, the phenotypes have not consistently been compared across different backgrounds. Instead, the systemic phenotypes are often referenced with respect to the characterization of the original klotho (kl/kl) mouse (11), though it is not entirely clear to what extent they manifest in other backgrounds or models. It was recently shown that the engineering of hypomorphic Klotho mice on a pure C57BL/6 mouse background results in mice that have a health span and life span similar to wild-type controls (30). An interesting area of future studies will be to better understand how pathophysiological responses to Klotho deficiency may be influenced by the background mouse strain, thereby providing a unique lens into genetic influences on Klotho-mediated tissue aging.
Another important consideration is the chronology of the onset of pathogenic comorbidities that has been observed within specific organs (11). Because the progeroid phenotype manifests throughout the organism by approximately 2 months of age, whether loss of Klotho in one tissue or organ may be a driving force for the onset of an aged phenotype across the system is of great interest. A good example of such a phenomenon has been elegantly demonstrated in Caenorhabditis elegans, where mutation of genes encoding the electron transport chain of mitochondria was shown to delay aging and extend life span (31). It was subsequently demonstrated that inactivation of the mitochondrial electron transport chain in just the intestine similarly increased life span, whereas inactivation in the muscle, for example, had no effect on longevity (32). These findings suggest that the onset of an aged phenotype may be cell nonautonomous in nature and that dysfunctional communication between organ systems may be a driver of organismal aging (32). Likewise, it was recently shown that loss of Klotho exclusively in the kidney completely recapitulates the Klotho hypomorphic phenotype (20). On the other hand, deletion of Klotho within the parathyroid, an organ also known to have high levels of Klotho, did not alter the gross phenotype or survival (33). Such studies are important, as they may pave the way for a refined understanding of the systemic aged phenotype accompanying a loss of Klotho, ultimately guiding the development of targeted and specific Klotho-based therapeutic interventions.
Klotho Gene and Protein Structure
The Klotho gene (KL) is composed of five exons in mice, rats, and humans (34–36). The gene was elucidated by screening human kidney and hippocampus complementary DNA (cDNA) libraries with a mouse Klotho cDNA fragment and subsequent isolation of human Klotho cDNA clones. Sequencing of these clones revealed two alternative RNA splice variants, a membrane-bound full length, and a truncated “secreted” form. Both human and mouse Klotho promoters, which are 67% identical, are TATA-less and or CAAT-less and are situated 500 bp and 300 bp upstream of their respective transcriptional initiation sites (34–36). The absence of the TATA-box has been attributed to its replacement by five potential binding sites for Sp1, which may enhance the downstream Klotho expression (36). KL promoter hypermethylation, histone deacetylation, and associated Klotho transcriptional repression play essential pathological roles in human and murine tissues (37–41). Thus, expression levels of Klotho are fine-tuned through multilayered epigenetic processes that we are only beginning to understand.
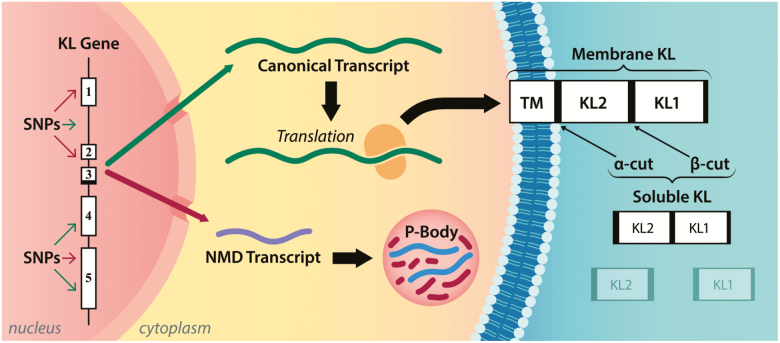
Alternative splicing of human Klotho produces a shorter mRNA transcript that harbors a premature termination codon positioned 50 intronic base pairs after exon 3 that precludes the complete KL gene translation (36). Despite over 90% cDNA and protein homology (42), the mouse KL gene is also subjected to alternative mRNA splicing (34–36), whereas the rat KL gene is not (43). The existence of the alternative Klotho transcript in mice and humans is well established, but recent evidence suggests that the transcripts are marked for rapid degradation without translation (Figure 1) (44,45). Indeed, analysis of diseased human kidney biopsies and oxidatively stressed human kidney cells revealed an abnormal increase in Klotho transcripts nonsense-mediated decay (45), implying that a translation-coupled RNA surveillance mechanism controls Klotho potential abundance through the regulation of alternative transcripts containing nonsense mutations, such as a premature translation-termination codon (46). Notably, alternative KL mRNAs have been colocalized within the membraneless organelles called processing bodies (Figure 1) (45). Processing bodies are cytoplasmic ribonucleoprotein granules primarily composed of translationally repressed mRNAs and proteins related to mRNA decay, and their primary role is posttranscriptional regulation (47). As the total KL mRNA is a balance between full-length and alternatively spliced transcripts, Klotho alternative splicing may function as an additional mechanism to control the levels of membrane-bound and secreted Klotho protein (45).
Figure 1.
The Klotho gene consists of five exons and four introns with several hundreds of genomic variants in both coding and non-coding sequences that are predicted to affect the splicing of the gene transcript. Two Klotho messenger RNAs (mRNAs) messages are produced: a canonical transcript encoding the full-length membrane Klotho protein or a processed transcript harboring premature termination codons that undergo nonsense mRNA decay in processing bodies. Klotho possesses two homologous domains KL1 and KL2. Shedding α-cut (after the membrane portion of Klotho) generates a “full-length” soluble Klotho, whereas shedding at both β-cut and α-cut (between KL1 and KL2) results in KL1 and KL2, respectively. NMD = nonsense-mediated decay; TM = transmembrane.
The full-length human KL transcript encodes a single-pass membrane protein of 1,012 amino acids. Its murine counterpart encodes for 1,014 amino acids. The translated Klotho protein is constituted of three domains, an N-terminal signal sequence, an extracellular domain with two internal repeats (ie two homologous extracellular domains KL1 and KL), and a short intracellular cytosolic domain of 10 amino acids (34) (Figure 1). The full-length membrane-bound Klotho protein is subject to cleavage by different “sheddases,” notably the A disintegrin and metalloproteinase 10 and 17 (48) as well as the β‐secretase, BACE1 (49). These proteases target a first site of cleavage that is situated directly above the membrane (α-cut), and a second site lies between the KL1 and KL2 domains (β-cut) (50–52), generating soluble Klotho, or KL1 and KL2 fragments of soluble Klotho, respectively (Figure 1). The remnant membrane-bound form of the Klotho protein is processed by the γ-secretases (49). Attempts to purify the peptide corresponding to the small intracellular domain have proven to be challenging, most likely because of its very short half-life (49). Thus, the physiological function of this peptide remains currently uncertain.
The Klotho family of proteins shares one or two glycoside hydrolase motifs homologous to glycoside hydrolase family 1 (53). Proteins that are structurally related to Klotho (α-Klotho) are β-Klotho, which features two glycoside hydrolase domains like Klotho, KLPH and Klotho-related protein (KlrP), which, like KLPH, has only one glycoside hydrolase motif. β-Klotho, KLPH, and KLrP share 41%, 36%, and 41% amino acid sequence homology with Klotho, respectively (54–56). However, it is not clear whether Klotho’s homologs are functionally linked. A better understanding of such a relationship may provide a more complete vision of Klotho’s role in organismal aging. For example, loss of Klotho has been associated with loss of skeletal muscle mass in both mice and humans (11,57). At present, loss of β-Klotho was similarly implicated as playing an important role in the onset of sarcopenia via FGF19 signaling (58). An interesting area of future study will be to better understand whether regulation of these two homologs is systemically coordinated and whether the onset of an aged muscle phenotype is a function of a disrupted interaction between the two.
Tissue Distribution
Murine studies originally revealed that Klotho is expressed mainly in the distal convoluted tubule cells and to a lesser extent in the proximal convoluted tubule cells of the kidney (11,59). Elimination of renal Klotho expression reduces circulating levels by 80% (20), confirming that the kidney is the primary source of the soluble form. This is consistent with the finding that unilateral nephrectomy in humans results in approximately 30% reduction in circulating Klotho (60). Soluble Klotho is the most abundant form in all tissues and is found in blood, urine, and cerebrospinal fluid (7,51,61).
Within the central nervous system, Klotho is produced predominantly by the choroid plexus, with lower levels of expression seen in specific brain regions, particularly in the hippocampus (62). Klotho within the cerebrospinal fluid may be involved in baroreflex sensitivity and protection of myelin integrity (63,64). However, its functions in the brain and involvement in neurological pathologies are still poorly understood.
Query of the publicly available RNA sequencing data from four databases (the FANTOM5 project, the Human Protein Atlas, the GTEx Consortium, and Illumina Body Map) revealed that the Klotho transcripts are pervasive and expressed in many tissues from multiple organ systems (65). Klotho expression in humans appears to be similar to that seen in rodents (11,36,43,65) and is particularly enhanced in kidney, parathyroid gland, and placenta, and to a lesser extent, in the lung, adipose tissue, and certain brain regions. Klotho expression is much less abundant, though still present, in many other tissues, such as skeletal muscle, heart, smooth muscles, pancreas, digestive tissues, bone marrow, lymph node, and adrenal and thyroid glands. At the protein level, mass spectrometry, western blotting, and immunohistochemistry confirmed Klotho’s expression in a variety of tissues (66).
Cellular Roles and Functions
The cellular actions of membrane-bound and soluble Klotho are primarily mediated by their actions as obligate co-receptor for FGF23 (fibroblast growth factor 23) in activating several FGFRs throughout the body (67,68). Although the molecular mechanisms of these biological roles are not fully understood, basic mineral homeostasis, expression of key metabolic regulatory genes, and essential metabolic signaling pathways have been shown to be affected by Klotho expression.
The Klotho/FGF23/FGFR complexes bind and downregulate renal proximal tubule type-II sodium phosphate co-transports to maintain phosphate and mineral balance (67,69). The complexes also regulate renal TRPV5 and TRPV6 calcium channels and renal outer medullary potassium channel 1. In addition, Klotho/FGF23 complexes downregulate vitamin D metabolizing enzymes to control active vitamin D hormone levels (68,69). Paradoxically, both Klotho-deficient and overexpressing mice express higher levels of serum FGF23 and exhibit hypervitaminosis D, resulting in hyperphosphatemia, increased calcification in the kidney and premature aging-like phenotype (70). Andrukhova and colleagues showed that FGF23 knockout mice phenocopy Klotho knockout mice and FGF23/Klotho knockout mice (71), indicating complementary roles of FGF23 and Klotho in the regulation of phosphaturia and renal calcium handling. Further, triple knockout FGF23/Klotho/VDR (vitamin D receptor) mice were protected from the pathologies of the FGF23/Klotho mice, as were FGF23/VDR and Klotho/VDR knockout mice (71). These results suggest that the coincidence of these phenotypes is largely dependent on vitamin D.
Systemic mineral and non-mineral effects of Klotho have been primarily attributed to the circulating form. Soluble Klotho has been shown to modulate a number of evolutionarily conserved longevity pathways, including insulin and insulin-like growth factor 1 (50), target of rapamycin (72), FGF23 (73), cyclic adenosine monophosphate (74), Protein kinase C (75), transforming growth factor-β (76), p53/p21 (77), and Wnt signaling (78). Further, in the vasculature, it has been shown that Klotho increases nitric oxide production, mediates anti-inflammatory actions, and maintains calcium balance (79–82). Mechanistically, however, it is not well-understood why Klotho overexpressing mice live 20%–30% longer compared to their wild-type counterparts (22).
Klotho as a Biomarker of Aging
Just as genetic manipulation in murine models have linked increased Klotho expression with improved health span and life span (22,83), a number of epidemiological studies have associated elevated Klotho levels to better health outcomes and even extended life span in humans (84–88). Specifically, studies have associated higher circulating Klotho levels with a decreased risk for cardiovascular disease, decreased macrovascular complications in patients with type 2 diabetes, and improved grip strength (84,87,89). Recent studies have included Klotho among a panel of recommended biomarkers that may serve as promising predictors of frailty in elderly individuals (86,90–92). It is, therefore, not surprising that Klotho is emerging as a biomarker for healthy aging. According to the clinical trials transformation initiative (https://www.ctti-clinicaltrials.org/; Accessed August 16, 2018), there are 48 ongoing clinical studies involving Klotho, including 20 observational and 28 interventional studies that collect data on Klotho levels as part of the outcome measurements. Among these studies, three included Klotho as a primary outcome variable. Interestingly, all three of these studies evaluated the Klotho response to physical activity.
Although circulating Klotho levels are emerging as a novel biomarker for progression of a number of pathological conditions (93–95), there are still conflicting reports regarding the relationship between circulating Klotho levels and clinical outcomes. For instance, epidemiological studies have causally linked the imbalance in the FGF23-Klotho axis with adverse cardiovascular outcomes (96,97). Left ventricular myopathy has been the major cardiovascular morbidity and mortality linked to aberrant FGF23/Klotho signaling in chronic kidney disease, as it leads to congestive heart failure, arrhythmias, and ischemic cardiomyopathy (97,98). Circulating Klotho levels are inversely related to atrial fibrillation in individuals receiving hemodialysis (99), and a recent large prospective epidemiological study found a similar inverse relationship between Klotho levels and left ventricular hypertrophy (98). There is, however, uncertainty as to whether this is a direct effect of Klotho, or if the phenotype results from an excessive increase in FGF23 levels, which occurs as Klotho falls and phosphatemia increases (reviewed in ref. (97)). It is possible that increased cardiac FGF23 enhances Klotho-independent FGF23 signaling through FGFR4 to promote cardiomyopathy (97). However, in a model of heterozygous Klotho mice with chronic kidney disease, cardiomyopathies were reduced only when ectopic Klotho was expressed and not when FGF23 or phosphate levels were reduced (100). Thus, this uncertainty in the distinction of Klotho as a driver of disease versus an indicator of disease highlights the need to better resolve tissue-specific signaling mechanisms underlying the Klotho/FGF23 axis, as well as Klotho- and FGF23-independent effects.
Taken together, these examples highlight the potential value of Klotho as a biomarker of age-related tissue dysfunction. At the same time, the fact that a loss of Klotho is likely not only an indicator but also a driver of tissue aging, paves the way for the development of Klotho-based therapeutic strategies to counteract age-related declines.
Toward Clinical Translation
Preclinical Intervention Studies
Several preclinical studies have demonstrated that elevation of circulating Klotho levels may be a promising therapeutic strategy. Given that the kidney is the primary organ associated with functional Klotho expression and that kidney dysfunction has been tightly linked to a loss of Klotho (11,20), interest in Klotho-based therapies for the treatment of renal pathologies is not surprising (101). At present, overexpression of exogenous Klotho was shown to be effective in preventing renal fibrosis, even at late time points corresponding to kidney lesions (101).
Klotho replacement has also been proven to be of prophylactic value (59). For instance, the administration of recombinant Klotho post-acute kidney injury has been suggested to be reno-protective by blunting the extent of tissue damage and progression to chronic kidney disease in murine models (59,102). Klotho administration also reduced uremic cardiopathy in the post-acute kidney injury model, although the beneficial therapeutic effects of Klotho did not preserve the normal renal function and morphology, nor did it prevent cardiac remodeling (102). Intriguingly, atrial fibrillation, a condition that is intertwined with end-stage renal disease, was more strongly associated with Klotho than the presence of traditional cardiovascular risk factors, such as age and complete loss of kidney function (99).
Interest in Klotho-replacement therapies has also been reported in the context of the central nervous system. Dubal and colleagues demonstrated that genetic overexpression of Klotho improved cognition and neural resistance in young and aged mice (103,104). Building on these encouraging findings, Leon and colleagues (105) asked whether systemic (intraperitoneal) administration of a Klotho protein fragment enhances cognitive performance in young, aged, and α-synuclein transgenic mice. Investigators chose to study α-synuclein transgenic mice because they recapitulate the cognitive and motor declines typical of neurodegenerative diseases (106). They found that a single peripheral injection of Klotho was sufficient to significantly improve cognitive performance in both young and old mice (105). Intriguingly, no evidence was found that the Klotho fragment crossed the blood–brain barrier, raising a number of questions as to the mechanisms by which systemically administered Klotho exerts beneficial effects on the brain (105). Adeno-associated viral-based therapy to increase the levels of Klotho in the brain has been shown to similarly prevent the cognitive decline associated with aging in old animals (107). The cognitive benefit for old animals that resulted from Klotho administration at a younger age suggests gene therapy as a potential therapy for the prevention of neurodegenerative conditions such as Alzheimer’s disease (107). These findings are particularly encouraging given that adeno-associated viral has been shown in clinical trials to be safe and effective for targeting the central nervous system (108).
At present, it has been shown that Klotho is an important mediator of brain immune functions (109). This suggests that that age-related Klotho declines within the choroid plexus may contribute to neuropathologies with an immune component, including Parkinson’s disease, Alzheimer’s disease, and multiple sclerosis disorder, and that these pathologies may be particularly amenable to therapeutic targeting by Klotho. Although the overexpression of Klotho strongly supports a neuroprotective role against brain aging and neurodegenerative disorders (22), further research is needed to better understand neuronal Klotho functions and to identify the most efficacious means to modulate Klotho levels at ages when intervention would be most relevant.
Clinical Trial Considerations
As murine models increasingly demonstrate the potential value of Klotho administration as a promising method to promote healthy aging and protect against a number of age-related diseases, the next logical question is whether Klotho-based therapies may have therapeutic potential in humans. Though a long-term goal may be the use of Klotho supplementation to reverse organismal tissue aging, near-term clinical implementation of a Klotho-derived therapeutic will likely be in the context of specific age-related diseases. High-throughput screening to search for small molecules that can be used as a starting point (or lead compound) for a new drug to manipulate Klotho under both physiological and pathological conditions has been performed (110–112), although none of these have yet directly translated to clinical applications to the best of our knowledge. On the other hand, the first significant round of venture capital was launched in 2017 to finance the Food and Drug Administration application for the use of recombinant Klotho in human studies to treat kidney disease and other diseases associated with aging (https://www.klotho.com/news/). Others (eg http://www.klogene.com/) are focusing on enhancing Klotho expression through a range of small molecules as a potential novel therapeutic for age-related and other neurodegenerative diseases (112).
As research progresses and likelihood of translation of Klotho-based therapeutics increases, a key challenge will be the identification of who qualifies as a good candidate for such interventions. To interrogate a relationship between circulating Klotho levels and disease state or predisposition, epidemiological studies often stratify individuals according to their levels of circulating Klotho (57,86,87). Though physical activity and kidney disease have been shown to affect Klotho expression (23,59,83,113), the inherent diversity in Klotho levels within the population is presumably largely a function of genetic variability. This is important, as emerging precision medicine concepts suggest that naturally occurring genetic variants may help determine individual responsiveness to therapeutic intervention (114,115).
In humans, genome-wide association and case studies have revealed the presence of single nucleotide polymorphisms (SNPs) in the KL gene on exons 2, 3, and 4, as well as in non-coding regions including the promoter region and introns (116). Klotho variants have been associated with a host of pathological conditions affecting bone mineral density, osteoarthritis, systolic blood pressure, atherosclerotic coronary artery disease, cardioembolic stroke, and fasting glucose (117–128).
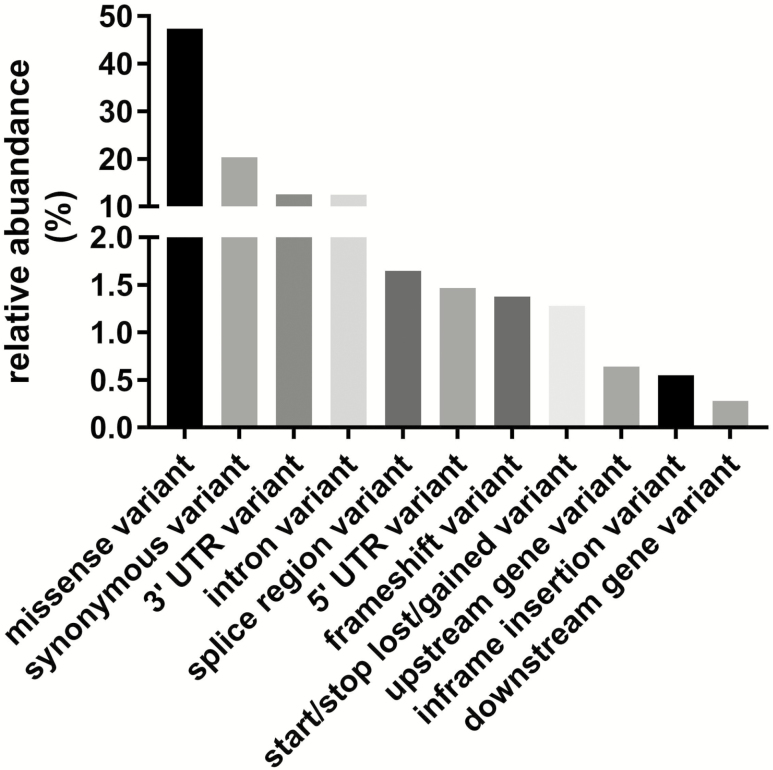
Recent advances in next-generation sequencing technologies have given an impetus to identify causality for the variance in circulating Klotho levels across the population. However, benchmarking the potential consequences of hundreds of Klotho variants on gene expression changes and proteomic products has not been systemically addressed, making it challenging to define a causal narrative around Klotho-associated genotypes and phenotypes in health and disease. To overcome such challenges, there is an urgent need for systematic functional genetic variation studies to understand the molecular mechanisms and pathways that link genotype to phenotype. We accessed a publicly available global “reference set” sequencing data spanning 123,136 exomes and 15,496 whole-genomes from unrelated individuals as part of various disease-specific and population genetic studies (https://console.cloud.google.com/storage/browser/gnomad-public/release). Analysis revealed the presence of between 1,287 and 1,508 Klotho variants, including 1,090 annotated variants, of which the effects are predicted to include changes in splice donors and regions, introns and 3′UTRs, gain or loss of stop codon, frameshifts, missenses, in-frame deletions, loss of start codon, as well as synonym variants (Figure 2). The potential impact of KL SNPs on physiological function suggests that the genomic profile of the individual may be an important consideration for Klotho administration in a clinical population. The next challenge will be to demonstrate tractable links between KL SNPs and differential physiological responses that may have translational implications, such as the development of genomic tests that can guide future Klotho-based therapeutic strategies (129).
Figure 2.
Annotated Klotho variants (n = 1,090) using Gencode, version 19 with variant effect prediction, version 85 from the Genome Aggregation Database (gnomAD). This database spans 123,136 exomes and 15,496 genomes from unrelated individuals sequenced as part of various international disease-specific and population genetic projects, including the 1,000 genomes, Alzheimer’s Disease Sequencing Project, Atrial Fibrillation Genetics Consortium, T2D-SEARCH, and the Cancer Genome Atlas.
The most studied of the Klotho polymorphisms is the functional variant known as KL-VS. KL-VS contains six SNP sequence variants in complete linkage disequilibrium (130). Three of these SNPs are located in the intron surrounding exon 2 and do not alter splicing. One SNP in exon 2 does not alter the amino acid sequence, and two SNPs encode for amino acid changes at phenylalanine 352 (V) and cysteine 370 (S). These latter two have been implicated in the alteration of Klotho function (130). Although KL-VS is observed in only some populations (131), a recent meta-analysis summarizing the evidence of pioneering Klotho epidemiological studies indicated a significant association of the Klotho variant with healthy aging (132). Intriguingly, the KL-VS allele was associated with a heterozygous survival advantage to the age of 75, but a homozygous disadvantage after 75 years due to occult coronary artery disease, high-density lipoprotein cholesterol, blood pressure, and stroke (85,121,130). KL-VS homozygosity was also associated with an increased risk of age-related decline of memory, though this may be related to Klotho’s effects on baseline intelligence quotient levels (122,130,133). Furthermore, lower incidence of KL-VS genotype in type 1 diabetes was also associated with retinopathy as well as low levels of inflammatory markers, pro-antigenic factors, and adhesion molecules (134).
It is currently not understood why SNPs of the human KL gene are associated with stroke, early-onset coronary artery disease, and longevity (121). Variances in KL-VS dimerization and protein–protein interaction, notably with FGFR1c, have been suggested to play an important role in disease risk33. KL-VS heterozygotes display an increase in serum Klotho and Klotho function, although it is not clear why there is a paradoxical lower Klotho level and cognitive connectivity in KL-VS homozygotes (133). At the molecular level, dysregulation of alternative splicing or splicing efficiency from alternative SNP intron/exon borders may be involved33.
Elucidation of Klotho’s Therapeutic Window
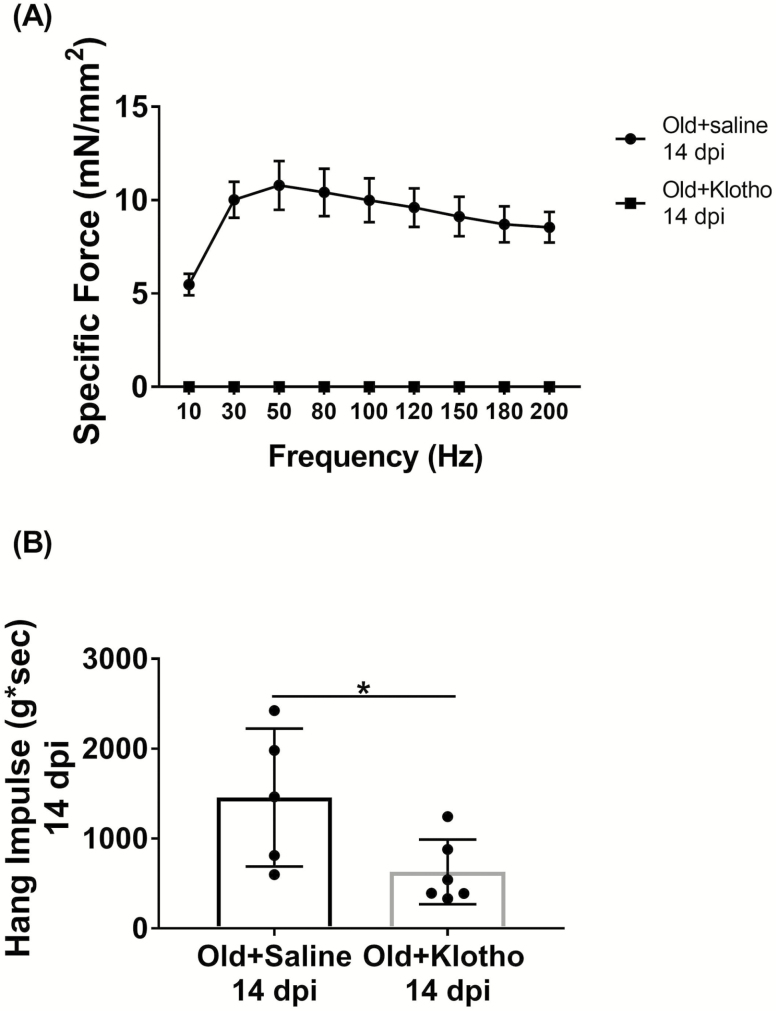
Another important consideration with the translation of Klotho therapeutics is the identification of Klotho’s therapeutic window, as there is evidence to suggest that higher levels of Klotho are not always associated with improved health (127). At the cellular level, it has been shown that apoptotic cells in models of retinal degeneration exhibited high levels of the Klotho protein (135). Moreover, exposure of organotypic explant cultures of wild-type retina to recombinant Klotho displayed greater nuclear disorganization when compared to their control counterparts, indicating retinal degeneration (135). Along these lines, it was recently demonstrated that systemic administration of mouse recombinant Klotho significantly enhanced skeletal muscle force recovery after injury in aged mice (136). However, the timing of administration was shown to be crucial, and administration at a time point that mimicked upregulation of KL in young mice (ie on days 3–5 post-injury) yielded the most improvement (136). On the other hand, daily administration of mouse recombinant Klotho from days 1–6 after injury severely impaired functional recovery when compared to saline-injected controls (Figure 3). These examples highlight the need for caution when considering the timing and dosing of Klotho delivery so as to avoid tipping the balance from beneficial to harmful effects.
Figure 3.
Aged animals (22–24 mo old) received intraperitoneal injections of recombinant mouse α-Klotho (1.05 μg/200 μL injection; R&D Systems, 1819-KL, aa 35-982) or isotonic saline for 6 consecutive days immediately after injury. (A) Force-frequency curves obtained via in situ contractile testing of anterior compartment strength (n = 5–6/group; two-way repeated measures analysis of variance, *p < .05; one animal removed from the saline group for analysis due to electrode misplacement). (B) Hang impulse (calculated as hanging time (s) × mouse bodyweight [g]) at 14 d post-injury (n = 5–6/group; *p < .05, one-tailed Student’s t-test).
Individual characteristics, including comorbidities, are also potentially important in the development and, ultimately, prescription of Klotho interventions. Even age itself is likely to be an important consideration, as Klotho’s effects may not necessarily be equivalent throughout a person’s lifetime. As an example, it has been demonstrated that the survival advantage of the heterozygous KL-VS genotype is dependent on the individual’s age. That is, regardless of sex, individuals who are heterozygous for the KL-VS allele are more likely to live beyond 70 years of age but display no advantage beyond the age of 90 when compared to the rest of the population (137). These findings suggest that Klotho may exert its effect in an age-specific manner, which may ultimately play an important role in determining therapeutic efficacy.
Experimental Challenges for Klotho Research
Considerable variability in the reliability and validity of the reagents and resources available for Klotho research can introduce major challenges to reproducing key findings across laboratories or even across experimental replicates within the same laboratory. In the light of the fact that an unexpectedly high number of landmark papers failed to be reproducible by independent laboratories (138), here, we highlight areas warranting careful attention in the context of Klotho-based studies.
Recombinant Klotho Protein
The notion that Klotho deficiency is not only a biomarker but also a driving pathogenic factor of aging phenotypes has understandably fostered a growing interest in the therapeutic—and potentially prophylactic—potential of recombinant Klotho protein administration (24,102,105). Unfortunately, variable and unpredictable quality of commercially available recombinant protein may contribute to lot-to-lot fluctuations in its activity, which in turn will affect the reproducibility of reported effects.
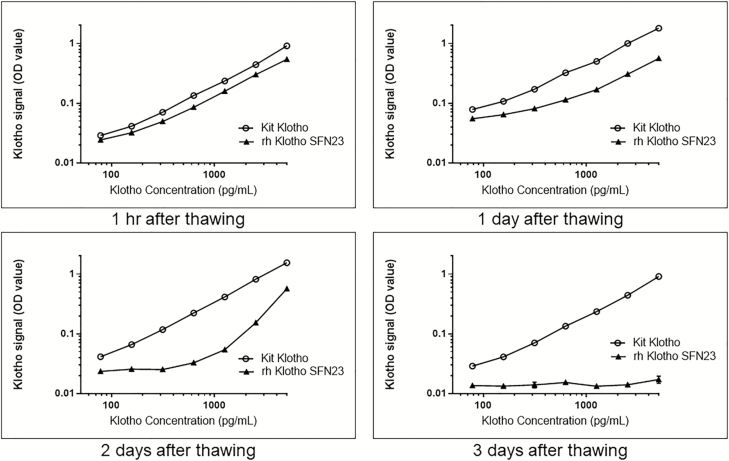
One way to assess consistency of Klotho activity across lots is to compare the EC50 values (and corresponding uncertainty bounds of the dose–response curves). The EC50 represents the amount of protein required to elicit a 50% maximum response in the bioassay (139). We evaluated the EC50 values for specific lots of recombinant Klotho from multiple vendors. R&D Systems, for example, validates Klotho activity in a cell proliferation assay using BaF3 mouse proB cells transfected with human FGF RIIIc in the presence of 1 μg/mL recombinant human FGF23 and 10 μg/mL heparin. To reconfirm recombinant Klotho activity in our laboratory, we used an FGF23-dependent Klotho proliferation assay on NIH-3T3 cells. Briefly, cells were plated in growth media overnight at 37°C, 5% CO2. The next day, media was removed and replaced with a reduced serum containing media along with serial diluted Klotho and FGF23 at 1 µg/mL and 10 μg/mL of heparin. Cells were incubated at 37°C, 5% CO2 for 3 days before adding 20 μL/well of Promega Aqueous One Substrate. The plate was incubated for 2 hours at 37°C, 5% CO2 at which time the absorbance A490 nm was determined and EC50 determinations made. From these studies, it was determined that recombinant Klotho is fairly unstable and that even minimal freeze/thaw cycles can significantly influence Klotho activity. Accordingly, repeat freeze/thaw cycles decrease the detection of recombinant Klotho, as determined by an enzyme-linked immunosorbent assay (ELISA); Figure 4). These findings stress the importance of rigorous, in-house validation to ascertain the activity of the recombinant Klotho protein used for therapeutic applications. Moreover, given the observed instability of the Klotho protein, alternative methods to enhance Klotho expression, such as adeno-associated viral delivery (though not without its own limitations), may prove to be more efficacious in the long term when considering clinical applications (107,140,141).
Figure 4.
Recombinant human Klotho (SFN23) stability after thawing was evaluated over 3 d. Enzyme-linked immunosorbent assay (ELISA) was performed at 1 hr, 1, 2, and 3 d after Klotho was stored at +4°C from a –80°C freezer. The recombinant human Klotho ELISA optical density (OD) values were compared with the provided kit standard reference OD values. Results reveal that the activity of recombinant human Klotho rapidly decreases when stored at +4°C.
Immunoassays
It has been estimated that only a staggering 0.5%–5% of antibodies in a polyclonal reagent bind to their intended target (138). Of course, even monoclonal antibodies can bind nonspecifically. Testing of 6,000 commercial antibodies from 26 suppliers revealed that more than 75% of the antibodies evaluated were nonspecific or did not work at all (142). Likewise, in another study that evaluated 5,000 antibodies, only 50% were shown to be reliable in their anticipated applications (138,143,144). The production of high-quality Klotho antibodies is a particularly challenging task given heightened tolerance to antibody induction against secreted proteins (145). Yet, the number of studies presenting a rigorous validation of the reagents used in Klotho research is unacceptably low.
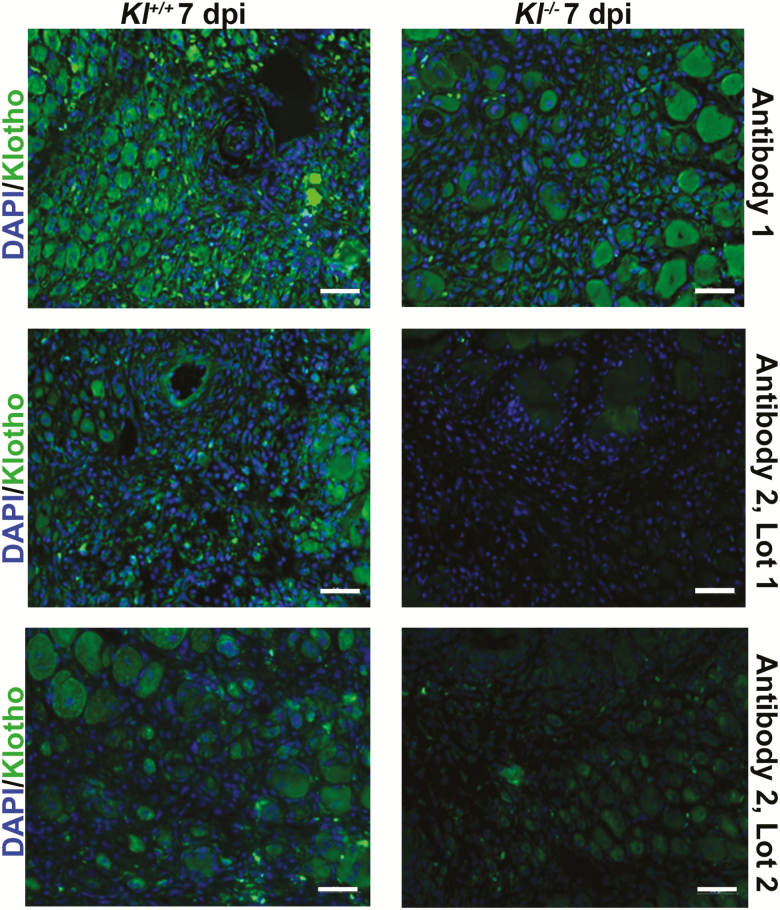
We compared two commercially available Klotho antibodies to validate their specificity and sensitivity for use in immunofluorescent cell and tissue assays. Using these antibodies, both reported by the vendor to be applicable for immunohistochemistry, we compared Klotho expression in the injured skeletal muscle of wild-type and Klotho hypomorphs. We selected injured skeletal muscle as it was recently demonstrated that young muscle displays a robust Klotho response to an acute injury (136). Marked differences in the extent of nonspecific binding according to both vendor and lot number were observed (Figure 5), further supporting the need for thorough antibody validation before investigation.
Figure 5.
Immunohistochemistry staining validation of two commercially available Klotho antibodies from two different vendors (Antibody #1 and Antibody #2). Each of the antibodies were diluted at 1:400 in an antibody solution (3% bovine serum albumin + 0.1% Triton-X + 5% goat serum) made in phosphate-buffered saline. Tibialis anterior muscle of Klotho wild-type (Kl+/+) and hypomorphs (Kl-/-) 7 d post-injury were incubated with one of the two antibodies overnight at 4°C, and images obtained were thresholded to negative control images.
Given the fact that soluble Klotho is present in the circulation and has been shown to exert endocrine and paracrine effects (146), quantification of the Klotho protein using immunoassays of biological samples such as plasma, serum, cells, conditioned media, tissues, and tissue homogenates is a potentially powerful and essential analytical tool. The ELISA is one of the most sensitive, rapid, simple to perform, and easily automated quantifiers of Klotho expression. However, as with any assay, the reproducibility and reliability of ELISAs can be challenging and costly due to false-positive and false-negative reactions, regardless of the target antigen(s). Such technical confounds may dramatically affect interpretations and conclusions of research findings. For example, it was initially reported that murine and human circulating Klotho as measured by immunoprecipitation and western blot analysis concentrations were similar (51). However, a subsequent study using radioimmunoassay estimated murine Klotho concentrations to be approximately 20-fold greater relative to the normal human average concentration (22). Although this discrepancy was acknowledged in the subsequent report, no explanation was offered. Problems in comparability of measures by different Klotho immunoassays highlight the need for standardization. Assaying even identical samples on different days may result in large intraassay variability (intraassay coefficient of variation range for Klotho of X–Y) and high interassay variability (coefficient of variation range for Klotho Z–W), suggesting plate-to-plate variability. A recent study rigorously evaluated the intra- and interassay precision of a commercially available ELISA kit for the detection of soluble Klotho (147,148) and serves as an example of steps needed to thoroughly validate the kit used.
Taken all of these considerations together, the importance of identifying and reporting on good antibodies and reliable validation systems cannot be stressed enough. Current immunoassays involve probes that convert analyte concentrations to signal intensities by linking antibodies to fluorescent or luminescent reporter molecules (149,150). The main disadvantage of this approach is non-specificity. As an alternative, linkage of engineered recombinant antibody fragments as direct conjugates to a reporter protein has the potential to yield higher quality probes for immunoassays (151). Antibody validation not only will help reproduce findings and define reliable reference values of Klotho protein expression levels but also will allow for a more realistic insight into Klotho biology.
Outlook for the Future
Our population’s average life span has nearly doubled in the past century. This life-span extension is ascribed largely to reduced age-specific mortality, resulting from environmental improvements, such as better health care and other improved standards of living. As our population ages, the development of strategies to enhance the number of “healthy” years becomes more important than ever.
The role of Klotho in modulating evolutionarily conserved life extension mechanisms, such as the insulin/insulin-like growth factor 1 signaling pathway, suggests that age-related phenotypes may be amenable to intervention using Klotho-based therapeutics. However, despite over 20 years of multidisciplinary research efforts and the wealth of knowledge gained from the utilization of different model organisms, there are still no Klotho-derived products that are being tested for efficacy in human trials. And yet, the outlook is promising. A significant objective in the coming years will be to adopt a systems-based approach that seeks to better understand the integrative role of Klotho in the optimization of organismal physiology over time through systemic organ communications. From a methodological perspective, challenges in the use of the Klotho protein and antibodies stress the need for replicative and rigorously designed studies. Taken together, the aspiration is that a holistic view of this “elephant” will aid in the development of targeted and specific therapeutics that exploit Klotho’s promise into clinically meaningful interventions for a wide range of pathological outcomes.
Funding
The authors are grateful for support from the National Institute on Aging under award number 1R01AG052978 (F.A.) as well as the Pittsburgh Claude D. Pepper Older Americans Independence Center P30 AG024827 (A.C.).
Conflict of Interest
Drs. H.L., C.A.K., J.H., and M.F. are employees of Boehringer Ingelheim Pharmaceuticals Inc.
References
- 1. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14:61–70. doi: 10.1038/nrc3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pawelec G, McElhaney JE, Aiello AE, Derhovanessian E. The impact of CMV infection on survival in older humans. Curr Opin Immunol. 2012;24:507–511. doi: 10.1016/j.coi.2012.04.002 [DOI] [PubMed] [Google Scholar]
- 4. Razzaque MS. The role of Klotho in energy metabolism. Nat Rev Endocrinol. 2012;8:579–587. doi: 10.1038/nrendo.2012.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dërmaku-Sopjani M, Kolgeci S, Abazi S, Sopjani M. Significance of the anti-aging protein Klotho. Mol Membr Biol. 2013;30:369–385. doi: 10.3109/09687688.2013.837518 [DOI] [PubMed] [Google Scholar]
- 6. Patrikios I. Klotho: the protein of faith. EC Neurology. 2017;7:189–223. [Google Scholar]
- 7. Dalton GD, Xie J, An SW, Huang CL. New insights into the mechanism of action of soluble klotho. Front Endocrinol (Lausanne). 2017;8:323. doi: 10.3389/fendo.2017.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuro OM. Molecular mechanisms underlying accelerated aging by defects in the FGF23-klotho system. Int J Nephrol. 2018;2018:9679841. doi: 10.1155/2018/9679841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuro-o M. Klotho in health and disease. Curr Opin Nephrol Hypertens. 2012;21:362–368. doi: 10.1097/MNH.0b013e32835422ad [DOI] [PubMed] [Google Scholar]
- 10. Kuro-o M, Hanaoka K, Hiroi Y, et al. Salt-sensitive hypertension in transgenic mice overexpressing Na(+)-proton exchanger. Circ Res. 1995;76:148–153. doi: 10.1161/01.RES.76.1.148 [DOI] [PubMed] [Google Scholar]
- 11. Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285 [DOI] [PubMed] [Google Scholar]
- 12. Reish NJ, Maltare A, McKeown AS, et al. The age-regulating protein klotho is vital to sustain retinal function. Invest Ophthalmol Vis Sci. 2013;54:6675–6685. doi: 10.1167/iovs.13-12550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagai T, Yamada K, Kim HC, et al. Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J. 2003;17:50–52. doi: 10.1096/fj.02-0448fje [DOI] [PubMed] [Google Scholar]
- 14. Yamashita T, Okada S, Higashio K, Nabeshima Y, Noda M. Double mutations in klotho and osteoprotegerin gene loci rescued osteopetrotic phenotype. Endocrinology. 2002;143:4711–4717. doi: 10.1210/en.2002-220602 [DOI] [PubMed] [Google Scholar]
- 15. Utsugi T, Ohno T, Ohyama Y, et al. Decreased insulin production and increased insulin sensitivity in the klotho mutant mouse, a novel animal model for human aging. Metabolism. 2000;49:1118–1123. doi: 10.1053/meta.2000.8606 [DOI] [PubMed] [Google Scholar]
- 16. Kawaguchi H, Manabe N, Miyaura C, Chikuda H, Nakamura K, Kuro-o M. Independent impairment of osteoblast and osteoclast differentiation in klotho mouse exhibiting low-turnover osteopenia. J Clin Invest. 1999;104:229–237. doi: 10.1172/JCI5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takeshita K, Fujimori T, Kurotaki Y, et al. Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation. 2004;109:1776–1782. doi: 10.1161/01.CIR.0000124224.48962.32 [DOI] [PubMed] [Google Scholar]
- 18. Mori K, Yahata K, Mukoyama M, et al. Disruption of klotho gene causes an abnormal energy homeostasis in mice. Biochem Biophys Res Commun. 2000;278:665–670. doi: 10.1006/bbrc.2000.3864 [DOI] [PubMed] [Google Scholar]
- 19. Fukino K, Suzuki T, Saito Y, et al. Regulation of angiogenesis by the aging suppressor gene klotho. Biochem Biophys Res Commun. 2002;293:332–337. doi: 10.1016/S0006-291X(02)00216-4 [DOI] [PubMed] [Google Scholar]
- 20. Lindberg K, Amin R, Moe OW, et al. The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol. 2014;25:2169–2175. doi: 10.1681/ASN.2013111209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olauson H, Lindberg K, Amin R, et al. Targeted deletion of Klotho in kidney distal tubule disrupts mineral metabolism. J Am Soc Nephrol. 2012;23:1641–1651. doi: 10.1681/ASN.2012010048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Avin KG, Coen PM, Huang W, et al. Skeletal muscle as a regulator of the longevity protein, Klotho. Front Physiol. 2014;5:189. doi: 10.3389/fphys.2014.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pavlatou MG, Remaley AT, Gold PW. Klotho: a humeral mediator in CSF and plasma that influences longevity and susceptibility to multiple complex disorders, including depression. Transl Psychiatry. 2016;6:e876. doi: 10.1038/tp.2016.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kurosu H, Kuro-O M. The Klotho gene family as a regulator of endocrine fibroblast growth factors. Mol Cell Endocrinol. 2009;299:72–78. doi: 10.1016/j.mce.2008.10.052 [DOI] [PubMed] [Google Scholar]
- 26. Gresh J, Goletz PW, Crouch RK, Rohrer B. Structure–function analysis of rods and cones in juvenile, adult, and aged C57BL/6 and Balb/c mice. Vis Neurosci. 2003;20:211–220. doi: 10.1017/S0952523803202108 [DOI] [PubMed] [Google Scholar]
- 27. Zhou X, Chen K, Lei H, Sun Z. Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation. J Am Soc Nephrol. 2015;26:121–132. doi: 10.1681/ASN.2013101033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu L, Stein LR, Kim D, et al. Klotho controls the brain-immune system interface in the choroid plexus. Proc Natl Acad Sci USA. 2018;115:E11388–E11396. doi: 10.1073/pnas.1808609115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prykhozhij SV, Fuller C, Steele SL, et al. Optimized knock-in of point mutations in zebrafish using CRISPR/Cas9. Nucleic Acids Res. 2018;46:9252. doi: 10.1093/nar/gky674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Phelps M, Pettan-Brewer C, Ladiges W, Yablonka-Reuveni Z. Decline in muscle strength and running endurance in klotho deficient C57BL/6 mice. Biogerontology. 2013;14:729–739. doi: 10.1007/s10522-013-9447-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dillin A, Crawford DK, Kenyon C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science. 2002;298:830–834. doi: 10.1126/science.1074240 [DOI] [PubMed] [Google Scholar]
- 32. Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Olauson H, Lindberg K, Amin R, et al. Parathyroid-specific deletion of Klotho unravels a novel calcineurin-dependent FGF23 signaling pathway that regulates PTH secretion. PLoS Genet. 2013;9:e1003975. doi: 10.1371/journal.pgen.1003975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu Y, Sun Z. Molecular basis of Klotho: from gene to function in aging. Endocr Rev. 2015;36:174–193. doi: 10.1210/er.2013-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shiraki-Iida T, Aizawa H, Matsumura Y, et al. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424:6–10. doi: 10.1016/S0014-5793(98)00127-6 [DOI] [PubMed] [Google Scholar]
- 36. Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626–630. [DOI] [PubMed] [Google Scholar]
- 37. Lee J, Jeong DJ, Kim J, et al. The anti-aging gene KLOTHO is a novel target for epigenetic silencing in human cervical carcinoma. Mol Cancer. 2010;9:109. doi: 10.1186/1476-4598-9-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jin SL, Zhang Y, Chen ZH, et al. Epigenetic changes of the Klotho gene in age-related cataracts. Eur Rev Med Pharmacol Sci. 2015;19:2544–2553. [PubMed] [Google Scholar]
- 39. Seo M, Kim MS, Jang A, et al. Epigenetic suppression of the anti-aging gene KLOTHO in human prostate cancer cell lines. Anim Cells Syst (Seoul). 2017;21:223–232. doi: 10.1080/19768354.2017.1336112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang Q, Yin S, Liu L, Liu Z, Cao W. Rhein reversal of DNA hypermethylation-associated Klotho suppression ameliorates renal fibrosis in mice. Sci Rep. 2016;6:34597. doi: 10.1038/srep34597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Young GH, Wu VC. KLOTHO methylation is linked to uremic toxins and chronic kidney disease. Kidney Int. 2012;81:611–612. doi: 10.1038/ki.2011.461 [DOI] [PubMed] [Google Scholar]
- 42. Wang Y, Sun Z. Current understanding of klotho. Ageing Res Rev. 2009;8:43–51. doi: 10.1016/j.arr.2008.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ohyama Y, Kurabayashi M, Masuda H, et al. Molecular cloning of rat klotho cDNA: markedly decreased expression of klotho by acute inflammatory stress. Biochem Biophys Res Commun. 1998;251:920–925. [DOI] [PubMed] [Google Scholar]
- 44. Isken O, Kim YK, Hosoda N, Mayeur GL, Hershey JW, Maquat LE. Upf1 phosphorylation triggers translational repression during nonsense-mediated mRNA decay. Cell. 2008;133:314–327. doi: 10.1016/j.cell.2008.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mencke R, Harms G, Moser J, et al. Human alternative Klotho mRNA is a nonsense-mediated mRNA decay target inefficiently spliced in renal disease. JCI Insight. 2017;2. doi: 10.1172/jci.insight.94375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Karousis ED, Mühlemann O. Nonsense-mediated mRNA decay begins where translation ends. Cold Spring Harb Perspect Biol. 2019;11:a032862. doi: 10.1101/cshperspect.a032862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Luo Y, Na Z, Slavoff SA. P-bodies: composition, properties, and functions. Biochemistry. 2018;57:2424–2431. doi: 10.1021/acs.biochem.7b01162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bloch L, Sineshchekova O, Reichenbach D, et al. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett. 2009;583:3221–3224. doi: 10.1016/j.febslet.2009.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yamamoto M, Clark JD, Pastor JV, et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280:38029–38034. doi: 10.1074/jbc.M509039200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Imura A, Iwano A, Tohyama O, et al. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090 [DOI] [PubMed] [Google Scholar]
- 52. Kato Y, Arakawa E, Kinoshita S, et al. Establishment of the anti-Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem Biophys Res Commun. 2000;267:597–602. doi: 10.1006/bbrc.1999.2009 [DOI] [PubMed] [Google Scholar]
- 53. Hayashi Y, Ito M. Klotho-related protein KLrP: structure and functions. Vitam Horm. 2016;101:1–16. doi: 10.1016/bs.vh.2016.02.011 [DOI] [PubMed] [Google Scholar]
- 54. Ito S, Fujimori T, Hayashizaki Y, Nabeshima Y. Identification of a novel mouse membrane-bound family 1 glycosidase-like protein, which carries an atypical active site structure. Biochim Biophys Acta. 2002;1576:341–345. doi: 10.1016/S0167-4781(02)00281-6 [DOI] [PubMed] [Google Scholar]
- 55. Ito S, Kinoshita S, Shiraishi N, et al. Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech Dev. 2000;98:115–119. doi: 10.1016/S0925-4773(00)00439-1 [DOI] [PubMed] [Google Scholar]
- 56. Hayashi Y, Okino N, Kakuta Y, et al. Klotho-related protein is a novel cytosolic neutral beta-glycosylceramidase. J Biol Chem. 2007;282:30889–30900. doi: 10.1074/jbc.M700832200 [DOI] [PubMed] [Google Scholar]
- 57. Semba RD, Ferrucci L, Sun K, et al. ; Health ABC Study Low plasma Klotho concentrations and decline of knee strength in older adults. J Gerontol A Biol Sci Med Sci. 2016;71:103–108. doi: 10.1093/gerona/glv077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Benoit B, Meugnier E, Castelli M, et al. Fibroblast growth factor 19 regulates skeletal muscle mass and ameliorates muscle wasting in mice. Nat Med. 2017;23:990–996. doi: 10.1038/nm.4363 [DOI] [PubMed] [Google Scholar]
- 59. Hu MC, Shi M, Zhang J, Quiñones H, Kuro-o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78:1240–1251. doi: 10.1038/ki.2010.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kakareko K, Rydzewska-Rosolowska A, Brzosko S, et al. The effect of nephrectomy on Klotho, FGF-23 and bone metabolism. Int Urol Nephrol. 2017;49:681–688. doi: 10.1007/s11255-017-1519-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vo HT, Laszczyk AM, King GD. Klotho, the key to healthy brain aging? Brain Plast. 2018;3:183–194. doi: 10.3233/BPL-170057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen CD, Sloane JA, Li H, et al. The antiaging protein Klotho enhances oligodendrocyte maturation and myelination of the CNS. J Neurosci. 2013;33:1927–1939. doi: 10.1523/JNEUROSCI.2080-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen LJ, Cheng MF, Ku PM, Cheng JT. Cerebral klotho protein as a humoral factor for maintenance of baroreflex. Horm Metab Res. 2015;47:125–132. doi: 10.1055/s-0034-1375689 [DOI] [PubMed] [Google Scholar]
- 65. Olauson H, Mencke R, Hillebrands JL, Larsson TE. Tissue expression and source of circulating αKlotho. Bone. 2017;100:19–35. doi: 10.1016/j.bone.2017.03.043 [DOI] [PubMed] [Google Scholar]
- 66. Lim K, Groen A, Molostvov G, et al. α-Klotho expression in human tissues. J Clin Endocrinol Metab. 2015;100:E1308–E1318. doi: 10.1210/jc.2015-1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Erben RG. α-Klotho’s effects on mineral homeostasis are fibroblast growth factor-23 dependent. Curr Opin Nephrol Hypertens. 2018;27:229–235. doi: 10.1097/MNH.0000000000000415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hum JM, O’Bryan L, Smith RC, White KE. Novel functions of circulating Klotho. Bone. 2017;100:36–40. doi: 10.1016/j.bone.2016.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kuro-o M. Molecular mechanisms underlying accelerated aging by defects in the FGF23-klotho system. 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Razzaque MS. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5:611–619. doi: 10.1038/nrendo.2009.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Andrukhova O, Bayer J, Schüler C, et al. Klotho lacks an FGF23-independent role in mineral homeostasis. J Bone Miner Res. 2017;32:2049–2061. doi: 10.1002/jbmr.3195 [DOI] [PubMed] [Google Scholar]
- 72. Zhao Y, Zhao MM, Cai Y, et al. Mammalian target of rapamycin signaling inhibition ameliorates vascular calcification via Klotho upregulation. Kidney Int. 2015;88:711–721. doi: 10.1038/ki.2015.160 [DOI] [PubMed] [Google Scholar]
- 73. Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393–2403. doi: 10.1210/me.2003-0048 [DOI] [PubMed] [Google Scholar]
- 74. Rakugi H, Matsukawa N, Ishikawa K, et al. Anti-oxidative effect of Klotho on endothelial cells through cAMP activation. Endocrine. 2007;31:82–87. doi: 10.1007/s12020-007-0016-9 [DOI] [PubMed] [Google Scholar]
- 75. Imai M, Ishikawa K, Matsukawa N, et al. Klotho protein activates the PKC pathway in the kidney and testis and suppresses 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression. Endocrine. 2004;25:229–234. doi: 10.1385/ENDO:25:3:229 [DOI] [PubMed] [Google Scholar]
- 76. Doi S, Zou Y, Togao O, et al. Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286:8655–8665. doi: 10.1074/jbc.M110.174037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ikushima M, Rakugi H, Ishikawa K, et al. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun. 2006;339:827–832. doi: 10.1016/j.bbrc.2005.11.094 [DOI] [PubMed] [Google Scholar]
- 78. Liu H, Fergusson MM, Castilho RM, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578 [DOI] [PubMed] [Google Scholar]
- 79. Saito Y, Yamagishi T, Nakamura T, et al. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998;248:324–329. doi: 10.1006/bbrc.1998.8943 [DOI] [PubMed] [Google Scholar]
- 80. Maekawa Y, Ishikawa K, Yasuda O, et al. Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine. 2009;35:341–346. doi: 10.1007/s12020-009-9181-3 [DOI] [PubMed] [Google Scholar]
- 81. Huang CL, Moe OW. Klotho: a novel regulator of calcium and phosphorus homeostasis. Pflugers Arch. 2011;462:185–193. doi: 10.1007/s00424-011-0950-5 [DOI] [PubMed] [Google Scholar]
- 82. Hui H, Zhai Y, Ao L, et al. Klotho suppresses the inflammatory responses and ameliorates cardiac dysfunction in aging endotoxemic mice. Oncotarget. 2017;8:15663–15676. doi: 10.18632/oncotarget.14933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Matsubara T, Miyaki A, Akazawa N, et al. Aerobic exercise training increases plasma Klotho levels and reduces arterial stiffness in postmenopausal women. Am J Physiol Heart Circ Physiol. 2014;306:H348–H355. doi: 10.1152/ajpheart.00429.2013 [DOI] [PubMed] [Google Scholar]
- 84. Semba RD, Cappola AR, Sun K, et al. Plasma klotho and mortality risk in older community-dwelling adults. J Gerontol A Biol Sci Med Sci. 2011;66:794–800. doi: 10.1093/gerona/glr058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Arking DE, Atzmon G, Arking A, Barzilai N, Dietz HC. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res. 2005;96:412–418. doi: 10.1161/01.RES.0000157171.04054.30 [DOI] [PubMed] [Google Scholar]
- 86. Shardell M, Semba RD, Rosano C, et al. Plasma klotho and cognitive decline in older adults: findings from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2016;71:677–682. doi: 10.1093/gerona/glv140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Semba RD, Cappola AR, Sun K, et al. Plasma klotho and cardiovascular disease in adults. J Am Geriatr Soc. 2011;59:1596–1601. doi: 10.1111/j.1532-5415.2011.03558.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Semba RD, Cappola AR, Sun K, et al. Relationship of low plasma klotho with poor grip strength in older community-dwelling adults: the InCHIANTI study. Eur J Appl Physiol. 2012;112:1215–1220. doi: 10.1007/s00421-011-2072-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pan HC, Chou KM, Lee CC, Yang NI, Sun CY. Circulating Klotho levels can predict long-term macrovascular outcomes in type 2 diabetic patients. Atherosclerosis. 2018;276:83–90. doi: 10.1016/j.atherosclerosis.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 90. Cardoso AL, Fernandes A, Aguilar-Pimentel JA, et al. Towards frailty biomarkers: candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res Rev. 2018;47:214–277. doi: 10.1016/j.arr.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 91. Shardell M, Semba RD, Kalyani RR, et al. Plasma klotho and frailty in older adults: findings from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2019;74: 1052–1058. doi: 10.1093/gerona/glx202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Shardell M, Semba RD, Kalyani RR, Hicks GE, Bandinelli S, Ferrucci L. Serum 25-hydroxyvitamin D, plasma klotho, and lower-extremity physical performance among older adults: findings from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2015;70:1156–1162. doi: 10.1093/gerona/glv017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dąbrowska AM, Tarach JS. Soluble Klotho protein as a novel serum biomarker in patients with acromegaly. Arch Med Sci. 2016;12:222–226. doi: 10.5114/aoms.2014.45050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lee EY, Kim SS, Lee JS, et al. Soluble α-klotho as a novel biomarker in the early stage of nephropathy in patients with type 2 diabetes. PLoS One. 2014;9:e102984. doi: 10.1371/journal.pone.0102984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lu X, Hu MC. Klotho/FGF23 Axis in chronic kidney disease and cardiovascular disease. Kidney Dis (Basel). 2017;3:15–23. doi: 10.1159/000452880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Olauson H, Vervloet MG, Cozzolino M, Massy ZA, Torres PU, Larsson TE. New insights into the FGF23-Klotho axis. Semin Nephrol: Elsevier. 2014;. 34: 586–597. doi: 10.1016/j.semnephrol.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 97. Stohr R, Schuh A, Heine GH, Brandenburg V. FGF23 in cardiovascular disease: innocent bystander or active mediator? Front Endocrinol (Lausanne). 2018;9:351. doi: 10.3389/fendo.2018.00351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kim HJ, Kang E, Oh YK, et al. The association between soluble klotho and cardiovascular parameters in chronic kidney disease: results from the KNOW-CKD study. BMC Nephrol. 2018;19:51. doi: 10.1186/s12882-018-0851-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Nowak A, Friedrich B, Artunc F, et al. Prognostic value and link to atrial fibrillation of soluble Klotho and FGF23 in hemodialysis patients. PLoS One. 2014;9:e100688. doi: 10.1371/journal.pone.0100688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Xie J, Yoon J, An SW, Kuro-o M, Huang CL. Soluble klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol. 2015;26:1150–1160. doi: 10.1681/ASN.2014040325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sanchez-Niño MD, Sanz AB, Ortiz A. Klotho to treat kidney fibrosis. J Am Soc Nephrol. 2013;24:687–689. doi: 10.1681/ASN.2013030294 [DOI] [PubMed] [Google Scholar]
- 102. Hu MC, Shi M, Gillings N, et al. Recombinant α-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int. 2017;91:1104–1114. doi: 10.1016/j.kint.2016.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Prather AA, Epel ES, Arenander J, et al. Longevity factor klotho and chronic psychological stress. Transl Psychiatry. 2015;5:e585. doi: 10.1038/tp.2015.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dubal DB, Yokoyama JS, Zhu L, et al. Life extension factor klotho enhances cognition. Cell Rep. 2014;7:1065–1076. doi: 10.1016/j.celrep.2014.03.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Leon J, Moreno AJ, Garay BI, et al. Peripheral elevation of a klotho fragment enhances brain function and resilience in young, aging, and α-synuclein transgenic mice. Cell Rep. 2017;20:1360–1371. doi: 10.1016/j.celrep.2017.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fleming SM, Salcedo J, Fernagut PO, et al. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci. 2004;24:9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Massó A, Sánchez A, Bosch A, Giménez-Llort L, Chillón M. Secreted αKlotho isoform protects against age-dependent memory deficits. Mol Psychiatry. 2017;23:1–11. doi: 10.1038/mp.2017.211 [DOI] [PubMed] [Google Scholar]
- 108. Hocquemiller M, Giersch L, Audrain M, Parker S, Cartier N. Adeno-associated virus-based gene therapy for CNS diseases. Hum Gene Ther. 2016;27:478–496. doi: 10.1089/hum.2016.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhu L, Stein LR, Kim D, et al. Klotho controls the brain–immune system interface in the choroid plexus. Proc Natl Acad Sci USA. 2018;115:E11388–E11396. doi: 10.1073/pnas.1808609115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Xu ZL, Gao H, Ou-Yang KQ, Cai SX, Hu YH. Establishment of a cell-based assay to screen regulators for Klotho gene promoter. Acta Pharmacol Sin. 2004;25:1165–1170. [PubMed] [Google Scholar]
- 111. King GD, Chen C, Huang MM, et al. Identification of novel small molecules that elevate Klotho expression. Biochem J. 2012;441:453–461. doi: 10.1042/BJ20101909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Abraham CR, Chen C, Cuny GD, Glicksman MA, Zeldich E. Small-molecule Klotho enhancers as novel treatment of neurodegeneration. Future Med Chem. 2012;4:1671–1679. doi: 10.4155/fmc.12.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Liu QF, Ye JM, Yu LX, et al. Plasma s-Klotho is related to kidney function and predicts adverse renal outcomes in patients with advanced chronic kidney disease. J Investig Med. 2018;66:669–675. doi: 10.1136/jim-2017-000560 [DOI] [PubMed] [Google Scholar]
- 114. Smart A, Martin P. The promise of pharmacogenetics: assessing the prospects for disease and patient stratification. Stud Hist Philos Biol Biomed Sci. 2006;37:583–601. doi: 10.1016/j.shpsc.2006.06.002 [DOI] [PubMed] [Google Scholar]
- 115. Gat-Viks I, Chevrier N, Wilentzik R, et al. Deciphering molecular circuits from genetic variation underlying transcriptional responsiveness to stimuli. Nat Biotechnol. 2013;31:342–349. doi: 10.1038/nbt.2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Shimoyama Y, Nishio K, Hamajima N, Niwa T. KLOTHO gene polymorphisms G-395A and C1818T are associated with lipid and glucose metabolism, bone mineral density and systolic blood pressure in Japanese healthy subjects. Clin Chim Acta. 2009;406:134–138. doi: 10.1016/j.cca.2009.06.011 [DOI] [PubMed] [Google Scholar]
- 117. Kawano K, Ogata N, Chiano M, et al. Klotho gene polymorphisms associated with bone density of aged postmenopausal women. J Bone Miner Res. 2002;17:1744–1751. doi: 10.1359/jbmr.2002.17.10.1744 [DOI] [PubMed] [Google Scholar]
- 118. Yamada Y, Ando F, Niino N, Shimokata H. Association of polymorphisms of the androgen receptor and klotho genes with bone mineral density in Japanese women. J Mol Med (Berl). 2005;83:50–57. doi: 10.1007/s00109-004-0578-4 [DOI] [PubMed] [Google Scholar]
- 119. Tsezou A, Furuichi T, Satra M, Makrythanasis P, Ikegawa S, Malizos KN. Association of KLOTHO gene polymorphisms with knee osteoarthritis in Greek population. J Orthop Res. 2008;26:1466–1470. doi: 10.1002/jor.20634 [DOI] [PubMed] [Google Scholar]
- 120. Rhee EJ, Oh KW, Yun EJ, et al. Relationship between polymorphisms G395A in promoter and C1818T in exon 4 of the KLOTHO gene with glucose metabolism and cardiovascular risk factors in Korean women. J Endocrinol Invest. 2006;29:613–618. doi: 10.1007/BF03344160 [DOI] [PubMed] [Google Scholar]
- 121. Arking DE, Becker DM, Yanek LR, et al. KLOTHO allele status and the risk of early-onset occult coronary artery disease. Am J Hum Genet. 2003;72:1154–1161. doi: 10.1086/375035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Deary IJ, Harris SE, Fox HC, et al. KLOTHO genotype and cognitive ability in childhood and old age in the same individuals. Neurosci Lett. 2005;378:22–27. doi: 10.1016/j.neulet.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 123. Houlihan LM, Harris SE, Luciano M, et al. Replication study of candidate genes for cognitive abilities: the Lothian Birth Cohort 1936. Genes Brain Behav. 2009;8:238–247. doi: 10.1111/j.1601-183X.2008.00470.x [DOI] [PubMed] [Google Scholar]
- 124. Imamura A, Okumura K, Ogawa Y, et al. Klotho gene polymorphism may be a genetic risk factor for atherosclerotic coronary artery disease but not for vasospastic angina in Japanese. Clin Chim Acta. 2006;371:66–70. doi: 10.1016/j.cca.2006.02.021 [DOI] [PubMed] [Google Scholar]
- 125. Rhee EJ, Oh KW, Lee WY, et al. The differential effects of age on the association of KLOTHO gene polymorphisms with coronary artery disease. Metabolism. 2006;55:1344–1351. doi: 10.1016/j.metabol.2006.05.020 [DOI] [PubMed] [Google Scholar]
- 126. Kim Y, Kim JH, Nam YJ, et al. Klotho is a genetic risk factor for ischemic stroke caused by cardioembolism in Korean females. Neurosci Lett. 2006;407:189–194. doi: 10.1016/j.neulet.2006.08.039 [DOI] [PubMed] [Google Scholar]
- 127. Brownstein CA, Adler F, Nelson-Williams C, et al. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci USA. 2008;105:3455–3460. doi: 10.1073/pnas.0712361105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ichikawa S, Imel EA, Kreiter ML, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117:2684–2691. doi: 10.1172/JCI31330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Tucker Zhou TB, King GD, Chen C, Abraham CR. Biochemical and functional characterization of the klotho-VS polymorphism implicated in aging and disease risk. J Biol Chem. 2013;288:36302–36311. doi: 10.1074/jbc.M113.490052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Arking DE, Krebsova A, Macek M Sr, et al. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci USA. 2002;99:856–861. doi: 10.1073/pnas.022484299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Shimoyama Y, Taki K, Mitsuda Y, Tsuruta Y, Hamajima N, Niwa T. KLOTHO gene polymorphisms G-395A and C1818T are associated with low-density lipoprotein cholesterol and uric acid in Japanese hemodialysis patients. Am J Nephrol. 2009;30:383–388. doi: 10.1159/000235686 [DOI] [PubMed] [Google Scholar]
- 132. Di Bona D, Accardi G, Virruso C, Candore G, Caruso C. Association of Klotho polymorphisms with healthy aging: a systematic review and meta-analysis. Rejuvenation Res. 2014;17:212–216. doi: 10.1089/rej.2013.1523 [DOI] [PubMed] [Google Scholar]
- 133. Yokoyama JS, Marx G, Brown JA, et al. Systemic klotho is associated with KLOTHO variation and predicts intrinsic cortical connectivity in healthy human aging. Brain Imaging Behav. 2017;11:391–400. doi: 10.1007/s11682-016-9598-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Słomiński B, Ryba-Stanisławowska M, Skrzypkowska M, Myśliwska J, Myśliwiec M. The KL-VS polymorphism of KLOTHO gene is protective against retinopathy incidence in patients with type 1 diabetes. Biochim Biophys Acta Mol Basis Dis. 2018;1864:758–763. doi: 10.1016/j.bbadis.2017.12.015 [DOI] [PubMed] [Google Scholar]
- 135. Farinelli P, Arango-Gonzalez B, Völkl J, et al. Retinitis Pigmentosa: over-expression of anti-ageing protein Klotho in degenerating photoreceptors. J Neurochem. 2013;127:868–879. doi: 10.1111/jnc.12353 [DOI] [PubMed] [Google Scholar]
- 136. Sahu A, Mamiya H, Shinde SN, et al. Age-related declines in α-Klotho drive progenitor cell mitochondrial dysfunction and impaired muscle regeneration. Nat Commun. 2018;9:4859. doi: 10.1038/s41467-018-07253-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Invidia L, Salvioli S, Altilia S, et al. The frequency of Klotho KL-VS polymorphism in a large Italian population, from young subjects to centenarians, suggests the presence of specific time windows for its effect. Biogerontology. 2010;11:67–73. doi: 10.1007/s10522-009-9229-z [DOI] [PubMed] [Google Scholar]
- 138. Bradbury A, Pluckthun A. Standardize antibodies used in research: to save millions of dollars and dramatically improve reproducibility, protein-binding reagents must be defined by their sequences and produced as recombinant proteins, say Andrew Bradbury, Andreas Pluckthun and 110 co-signatories. Nature. 2015;518:27–30. [DOI] [PubMed] [Google Scholar]
- 139. Shalhoub V, Ward SC, Sun B, et al. Fibroblast growth factor 23 (FGF23) and alpha-klotho stimulate osteoblastic MC3T3.E1 cell proliferation and inhibit mineralization. Calcif Tissue Int. 2011;89:140–150. doi: 10.1007/s00223-011-9501-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009;54:810–817. doi: 10.1161/HYPERTENSIONAHA.109.134320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Hum JM, O’Bryan LM, Tatiparthi AK, et al. Chronic hyperphosphatemia and vascular calcification are reduced by stable delivery of soluble klotho. J Am Soc Nephrol. 2017;28:1162–1174. doi: 10.1681/ASN.2015111266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Weller MG. Quality issues of research antibodies. Anal Chem Insights. 2016;11:21–27. doi: 10.4137/ACI.S31614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature. 2014;505:612–613. doi: 10.1038/505612a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Berglund L, Björling E, Oksvold P, et al. A genecentric human protein atlas for expression profiles based on antibodies. Mol Cell Proteomics. 2008;7:2019–2027. doi: 10.1074/mcp.R800013-MCP200 [DOI] [PubMed] [Google Scholar]
- 145. Maltare A, Nietz AK, Laszczyk AM, et al. Development and characterization of monoclonal antibodies to detect klotho. Monoclon Antib Immunodiagn Immunother. 2014;33:420–427. doi: 10.1089/mab.2014.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Erben RG. Update on FGF23 and Klotho signaling. Mol Cell Endocrinol. 2016;432:56–65. doi: 10.1016/j.mce.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 147. Devaraj S, Syed B, Chien A, Jialal I. Validation of an immunoassay for soluble Klotho protein: decreased levels in diabetes and increased levels in chronic kidney disease. Am J Clin Pathol. 2012;137:479–485. doi: 10.1309/AJCPGPMAF7SFRBO4 [DOI] [PubMed] [Google Scholar]
- 148. Heijboer AC, Blankenstein MA, Hoenderop J, de Borst MH, Vervloet MG; NIGRAM consortium Laboratory aspects of circulating α-Klotho. Nephrol Dial Transplant. 2013;28:2283–2287. doi: 10.1093/ndt/gft236 [DOI] [PubMed] [Google Scholar]
- 149. Kobayashi N, Oyama H. Antibody engineering toward high-sensitivity high-throughput immunosensing of small molecules. Analyst. 2011;136:642–651. doi: 10.1039/c0an00603c [DOI] [PubMed] [Google Scholar]
- 150. Hermanson GT. Bioconjugate Techniques. Academic Press; 2013. [Google Scholar]
- 151. Kobayashi N, Iwakami K, Kotoshiba S, et al. Immunoenzymometric assay for a small molecule,11-deoxycortisol, with attomole-range sensitivity employing an scFv-enzyme fusion protein and anti-idiotype antibodies. Anal Chem. 2006;78:2244–2253. doi: 10.1021/ac051858f [DOI] [PubMed] [Google Scholar]